Abstract
Background
The relationships among small fiber neuropathy, age, sex and pain intensity in the context of Fabry's disease remain unclear. We aim to study the correlations of small fiber neuropathy, age, sex and pain intensity in Fabry patients.
Methods
We evaluated C-fiber function by recording the withdrawal latencies to painful heat stimulus (WLPHS) when each subject's right hand was immersed in a 50 °C hot water bath and correlated this parameter with the patient's perceived pain intensity and quality of life assessed by the short-form McGill Pain Questionnaire (SF-MPQ) in a large Taiwanese Fabry family and normal controls.
Results
Male Fabry patients showed a significantly increased WLPHS compared to that of normal controls. Furthermore, male Fabry patients showed a positive correlation of increased WLPHS with patient age. The SF-MPQ of male Fabry patients showed a bell distribution with age, and maximal pain scores were detected between the ages of the early 20s and late 40s. In contrast, the female Fabry patients had variable associations of WLPHS and SF-MPQ with age.
Conclusions
We proposed a probable mechanism by which globotriaosylceramide (Gb3) or globotriaosylsphingosine (lyso-Gb3) is gradually deposited into the small nerve bundles with increasing age, which induces continuous damage and produces injury discharges to sustain neuropathic pain in young male Fabry patients. However, once the small fibers are reduced to a certain degree, they no longer produce enough noxious discharges to sustain neuropathic pains in older male Fabry patients, which leads these patients to have lower SF-MPQ scores. In contrast, female Fabry patients had less and variable small fiber damage, pain intensity and clinical signs/symptoms.
Keywords: Fabry's disease, Neuropathic pain, Small fiber neuropathy, Acroparesthesia, Unmyelinated fiber
At a glance commentary
Scientific background on the subject
Fabry's disease is a rare X-linked metabolic disorder, and Fabry patients experience episodes of excruciating pain (Fabry crises) caused by small, unmyelinated nerve fibers damages. However, the relationships among small fiber neuropathy, age, sex, and pain intensity in Fabry patients remain unclear.
What this study adds to the field
The WLPHS increased linearly with age in male Fabry patients, but the SF-MPQ scores decreased at old ages and demonstrated a bell shape distribution. Those findings suggest once the small-fiber damage reaches a certain degree, the small fibers can no longer transmit enough noxious discharges to sustain neuropathic pains in older male Fabry patients, which leads these patients to have lower pain scores.
Fabry's disease is a rare X-linked metabolic disorder associated with deficiency of the lysosomal enzyme α-galactosidase A (AGA). AGA deficiencies lead to the accumulation of globotriaosylceramide (Gb3) or globotriaosylsphingosine (lyso-Gb3) and α-galactosyl breakdown products in affected males and, to a lesser extent, in females, who are carriers of this disorder. The process leads to selective damage of neurons of the dorsal root ganglia and autonomic nervous system, renal glomerular and tubular epithelial cells, myocardial cells and valvular fibrocytes, endothelial, and smooth muscle cells of blood vessels [1,2]. In patients with Fabry's disease, episodic Fabry crises and constant acroparesthesias are both debilitating symptoms and are probably related to the storage of Gb3 in dorsal root ganglia and peripheral autonomic ganglia [1,3,4]. The current findings indicate that these symptoms are due to axonal neuropathy, which damages small myelinated and unmyelinated fibers but spares large myelinated fibers [[5], [6], [7], [8]]. Those small fiber neuropathies in Fabry patients have been shown by skin biopsies, various electrophysiology studies, and quantitative sensory testing [6,[9], [10], [11], [12], [13]]. Our previous studies also showed peripheral microcirculatory dysfunction caused by small autonomic fiber neuropathy in Fabry patients [14]. These small fiber losses are related to pain intensity in Fabry patients, and the older Fabry patient were found to have a lower intraepidermal nerve fiber density in Uceyler et al. study [13]. However, some studies surprisingly found that there was no correlation between pain score, quantitative sensory testing and intraepidermal nerve fiber density in Fabry patients [12,15]. In conclusion, the relationships among small fiber neuropathy, age, sex and pain intensity in Fabry patients remain unclear [9,16]. The purpose of this study was to observe the relationships among small fiber neuropathy, age, sex and pain intensity in Fabry patients in a large Taiwanese family by evaluating the Short Form McGill Pain Questionnaire (SF-MPQ) and the withdrawal latencies to painful heat (50 °C) stimuli, which can provide evidence of unmyelinated fiber dysfunction [17,18].
Materials and method
Patients
Sixteen patients of different ages with Fabry's disease, including eight hemizygous men and eight heterozygous women, who were identified through family history, enzymatic assay and polymorphism linkage and mutation analysis were enrolled. All of them were members of a large Taiwanese family described in our previous report [7,19] [Fig. 1]. Those patients were diagnosed as Fabry patients by enzymatic and genetic studies since 1995 and received regular follow-up in the next few years. All patients were required to cease medications for pain treatment at least one week before the study, and those tests were performed before the approbation of enzyme replacement therapy for Fabry's disease in Taiwan. One hundred normal subjects aged between 8 and 75 years with a normal neurological examination and without any known peripheral neuropathy were included as normal controls in the painful heat stimuli tests. The normal subjects in these studies were carefully screened before entry to ensure that they were indeed normal. Informed consent was obtained from each subject, and the study was approved by the institutional review board of Chang Gung Memorial Hospital and University (No: 94-1005B).
Fig. 1.
Pedigree of the large Taiwanese Fabry family in this study.
Evaluation of severity of pain and quality of life (SF-MPQ) and withdrawal latencies to painful heat stimulus (WLPHS)
The severity of pain and quality of life were evaluated by the Short Form McGill Pain Questionnaire (SF-MPQ) [20] as described in our previous report [19]. The Fabry patients were evaluated using the SF-MPQ at the beginning of each test. We further measured each subject's latency (in seconds) to evoke a withdrawal reaction when the subject sensed pain by immersing each subject's right hand into a hot water bath with a temperature of 50 °C in the same laboratory, which was kept at a constant temperature of 22 °C–23 °C. Before the test, all of the subjects were asked to keep the tested hand at approximately 30 ± 1 °C using a heater or cooler. A cut-off time of 180 s was imposed to avoid tissue injury. Three readings of the right-hand withdrawal time were taken and averaged for the latency (allowing 10-min intervals between the hand withdrawals to prevent sensitization). Similar to the heat withdrawal latencies in an animal model [21], we supposed that a shorter latency in the test was indicative of hyperalgesia, while a longer latency was indicative of hypoalgesia due to C-fiber injuries or losses. The cut-off level for WLPHS in normal controls (n = 50 males and females, respectively) was defined as the mean ± 2 SD.
Statistical analysis
Prism software (version 8; GraphPad Software, USA) was used for statistical analysis. To compare the WLPHS of Fabry patients and controls and the SF-MPQ of male and female Fabry patients, the Shapiro–Wilk test was used to check if the data were normally distributed. Unpaired t tests or Mann–Whitney tests were performed, when appropriate. Simple linear regression was performed to quantify the relationship between the age and SF-MPQ/WLPHS of Fabry patients. Multiple linear regressions were performed to identify the differences between the linear regression line of WLPHS and age in male Fabry patients, female Fabry patients, and normal controls. The p value indicating significance was set at 0.05. All P values were two-sided.
Results
Demographic characteristics of Fabry patients and normal controls
The mean age of the eight male Fabry patients was 41.00 ± 16.31 years (range: 14 to 62), and that of the eight female Fabry patients was 42.63 ± 18.98 years (range: 12 to 67). The normal control group included 50 men and 50 women. The mean age of the men was 33.71 ± 15.78 years (range: 8 to 75), and that of the women was 32.72 ± 16.40 years (range: 9 to 74) [Table 1].
Table 1.
The demographic data and results of SF-MPQ/WLPHS.
| Patient No. | Age (y/o)/Sex | SF−MPQ | WLPHS (secs) |
|---|---|---|---|
| F1 | 67/F | 11 | 24 |
| F2 | 65/F | 6 | 4 |
| F3 | 46/F | 5 | 22 |
| F4 | 44/F | 15 | 7 |
| F5 | 41/F | 1 | 17 |
| F6 | 45/F | 12 | 28 |
| F7 | 12/F | 2 | 4 |
| F8 | 21/F | 10 | 9 |
| Mean ± SD | 42.63 ± 18.98 (P = 0.18) |
7.75 ± 5.01 | 14.38 ± 9.58 (p = 0.06) |
| M1 | 62/M | 5 | 103 |
| M2 | 57/M | 6 | 81 |
| M3 | 48/M | 22 | 51 |
| M4 | 45/M | 18 | 130 |
| M5 | 42/M | 20 | 75 |
| M6 | 38/M | 17 | 72 |
| M7 | 22/M | 13 | 43 |
| M8 | 14/M | 6 | 9 |
| Mean ± SD | 41 ± 16.31 (P = 0.51) |
13.38 ± 6.89 | 70.5 ± 37.13 (p < 0.01) |
| Normal ref. | Normal ref. | ||
| Female | 32.72 ± 16.40 (n = 50) |
8.15 ± 6.39 (n = 50) |
|
| Male | 33.71 ± 15.78 (n = 50) | 8.23 ± 4.85 (n = 50) | |
| ∗p = 0.94 | ∗p = 0.08 | ∗p < 0.01 |
Abbreviations: Ref.: Reference; SF-MPQ: short form McGill pain questionnaire; WLPHS: withdrawal latencies to painful heat stimulus; ∗p: refers to the difference between hemizygotes and heterozygotes, unpaired t test or Mann–Whitney test, if appropriate; p: Differences between Fabry patients and normal controls, unpaired t test or Mann–Whitney test, if appropriate.
Severity of pain and quality of life (SF-MPQ)
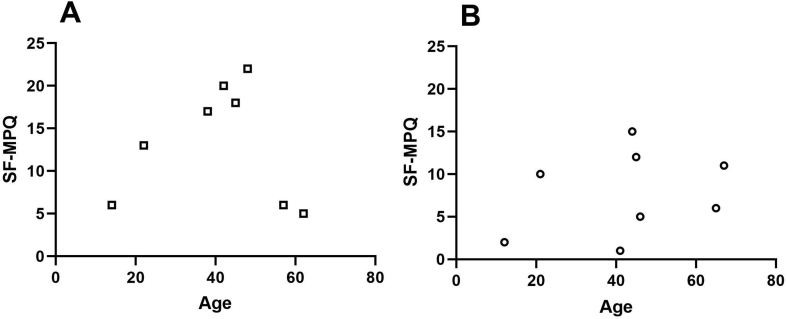
In the male Fabry patients, there was a bell distribution of pain scores with age, and maximal pain scores were detected between the ages of the early 20s and late 40s. In contrast, there was a variable association between the degree of pain and age in the female Fabry patients [Fig. 2A and B]. Neither male nor female Fabry patients demonstrated a correlation between the SF-MPQ and age (r2 < 0.01 and r2 = 0.08 for the male and female Fabry patients, respectively).
Fig. 2.
Evaluation of the pain severity and quality of life of Fabry patients by the Short Form McGill Pain Questionnaire (SF-MPQ). On this scale, higher scores indicate greater pain. (A) In male Fabry patients (□), there was a tendency of increased pain severity with age from the teens to late 40s, and maximal pain scores were noted between the ages of the early 20s to late 40s. The pain severity gradually declined after the age of 48 years. (B) However, there was variability in the degree of pain associated with age in female Fabry patients (○).
Withdrawal latencies to painful heat stimuli
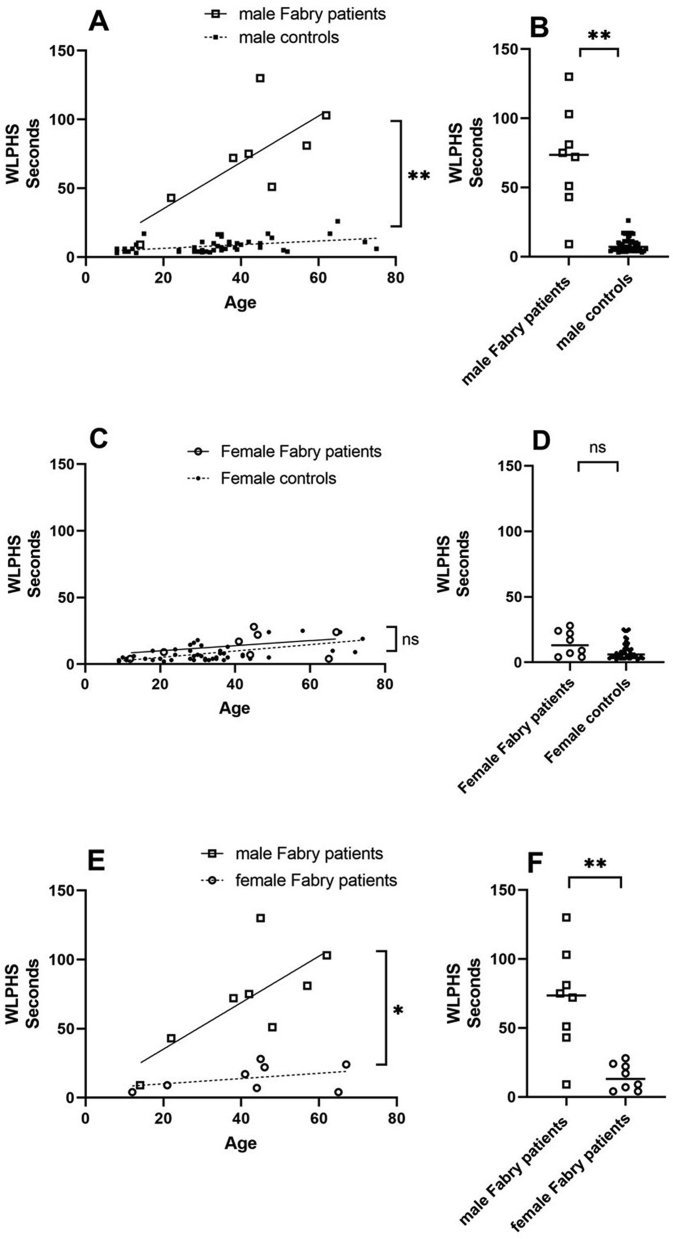
In Fabry patients and normal controls, both age and sex influenced the withdrawal latencies to painful heat stimuli (WLPHS), as shown by a multiple linear regression analysis. To reduce the confounding effect of sex, we used a stratified analysis by dividing normal controls into male and female groups.
The mean WLPHS of the female controls (8.15 ± 6.39 s) was similar to that of the male controls (8.23 ± 4.85 s) (p > 0.05). In the control subjects, there was a weak positive linear relationship between heat pain perception time and age (simple linear regression. r2 = 0.19 and r2 = 0.38 for the male and female controls, respectively) [Fig. 3A and C].
Fig. 3.
(A and B) Withdrawal latencies to painful heat stimuli (WLPHS) in male Fabry patients (□) showed markedly increased withdrawal latencies with age compared with the latencies of controls (■) (70.50 ± 37.13 s vs 8.23 ± 4.85 s multiple linear regression, ∗∗p < 0.01, Mann–Whitney test, ∗∗p < 0.01). There was a positive linear relationship between WLPHS and age in male Fabry patients (simple linear regression, r2 = 0.55) but not in male normal controls (simple linear regression, r2 = 0.19). (C and D). In contrast, female Fabry patients (○) had variable withdrawal latencies but generally had mildly increased withdrawal latencies compared with those of controls (●) (14.38 ± 9.58 s vs 8.15 ± 6.39 s multiple linear regression, p = 0.70, Mann–Whitney test, p = 0.06, ns: not significant). There was a weak positive linear relationship between WLPHS and age in female normal controls (simple linear regression. r2 = 0.38) but not in female Fabry patients (simple linear regression. r2 = 0.14). (E and F) Male Fabry patients had significantly increased withdrawal latencies with age compared with female Fabry patients (multiple linear regression, ∗p < 0.05, unpaired t test, ∗∗p < 0.01). The black solid lines represent simple linear regression of WLPHS and age in male and female Fabry patients, respectively. The black dotted lines represent simple linear regression of WLPHS and age in male and female normal controls, respectively.
The mean WLPHS was 70.50 ± 37.13 s and 14.38 ± 9.58 s in the male and female Fabry patients, respectively. The male Fabry patients had significantly increased withdrawal latencies with age compared with those of controls (p < 0.01) [Fig. 3A]. The mean value of WLPHS was significantly different between Fabry patient group and the control group in male participants (p < 0.01) [Fig. 3B]. The female Fabry patients had similar increased withdrawal latencies with age compared with those of controls (p > 0.05) [Fig. 3C]. The mean value of WLPHS was not significantly different between Fabry patient group and the control group in female participants (p > 0.05) [Fig. 3D]. Neither male nor female Fabry patients showed a bell distribution of WLPHS with age. The male Fabry patients had significantly increased withdrawal latencies with age compared with those of female Fabry patients (p < 0.01) [Fig. 3E]. The mean value of WLPHS was significantly different between male and female Fabry patients (p < 0.01) [Fig. 3F]. Neither male nor female Fabry patients demonstrated a correlation between SF-MPQ and WLPHS (r2 = 0.02 and r2 = 0.04 for the male and female Fabry patients, respectively). The demographic data and results of the SF-MPQ/WLPHS are shown in Table 1.
Discussion
Our study showed that male Fabry patients demonstrated remarkably increased withdrawal latencies to painful heat stimuli compared with that of controls, and those withdrawal latencies showed a positive correlation with patient age. Heat pain sensation is mainly conveyed by unmyelinated C-fibers [17,18,22]. Previous studies and theories have concluded selective C-fiber involvement in Fabry's disease [[5], [6], [7], [8],23]. Furthermore, recent findings also suggest that the symptoms of acroparesthesias in Fabry patients are due to small and unmyelinated fiber neuropathy [2,[6], [7], [8]]. Our findings provide physiological evidence suggesting that male Fabry patients actually have continuous C-fiber dysfunction and that the degree of C-fiber dysfunction increases with age. These findings were consistent with previous studies, which showed that older male Fabry patients had a much lower intraepidermal nerve fiber density (IENFD) [9,13].
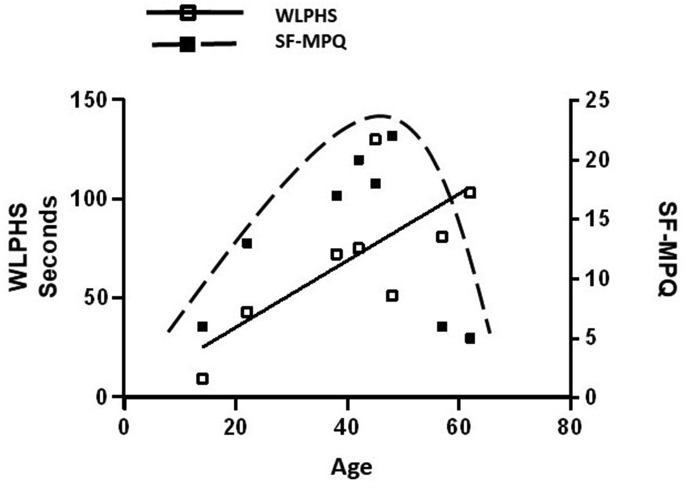
In addition to the peripheral small fiber neuropathy theories, Inagaki et al. proposed that burning pain in patients with Fabry disease might be related to abnormal perception by the central nervous system [24]. Central sensitization is a phenomenon that indicates that repeated noxious stimulation to peripheral C-fiber nociceptors may have generated prolonged poststimulus sensory disturbances and decreased threshold of the nociceptive system [25,26], and those alterations finally may lead to a hyperexcitable state, the consequences being intense nociceptive input to the dorsal horn [26]. Interestingly, our studies showed that the SF-MPQ score of male Fabry patients did not increase with patient age as WLPHS did. Instead, maximal pain scores according to the SF-MPQ in male Fabry patients were detected between the ages of the early 20s and late 40s rather than at old ages. We proposed a mechanism by which gradual Gb3 or lyso-Gb3 accumulation causes continuous C-fiber dysfunction that transmits persistent noxious discharges to the central nervous system, thereby resulting in central sensitization and playing an important role in the maintenance of acroparesthesias. As Gb3 is gradually deposited into the unmyelinated nerve fiber as age increases, the latency of WLPHS prolongs progressively as age increases. However, when patients are in old age, they have less or no acroparesthesias or painful crises because their C-fibers have gradually been damaged to such a degree that they can no longer provide sufficient injury discharges to sustain peripheral and central sensitization. Therefore, although the latency of WLPHS increased linearly with age in male Fabry patients, the SF-MPQ scores decreased at old ages and demonstrated a bell shape distribution [Fig. 4]. These findings are consistent with several previous reports [9,12], which showed that the acroparesthesias of Fabry patients were more severe in young patients [12].
Fig. 4.
Relationship between SF-MPQ and WLPHS in male Fabry patients. The WLPHS indicated that C-fiber dysfunction increased with age. However, the SF-MPQ, which indicated subjective pain sensation, had a bell shape distribution with age. We hypothesized that the damaged C-fibers cannot sustain peripheral and central sensitization in old age, which leads older patients to have lower SF-MPQ scores. (□): Withdrawal latencies to painful heat stimuli (WLPHS) in male Fabry patients (■): Short Form McGill Pain Questionnaire (SF-MPQ) in male Fabry patients. The black solid line represents the simple linear regression of WLPHS and age in male Fabry patients. The dashed line represents the bell shape distribution of the SF-MPQ with age in male Fabry patients.
Uceyler et al. study found that older Fabry patients have a lower intraepidermal nerve fiber density, and there was a relationship between small fiber loss and pain intensity in Fabry patients [13]. However, their study only showed that 72% of Fabry patients never showing pain symptoms had normal IENFD; on the other hand, 17% of Fabry patients showing pain symptoms had normal IENFD. Further detailed correlations between pain score, intraepidermal nerve fiber density and patient age were not mentioned in their study [13]. MacDermot's large cohort studies from the UK registration also did not demonstrate the same bell shape distribution of Fabry patients' pain scores as age increased [27]. However, various genotypes and comorbidities, such as diabetes mellitus and alcoholism, in the Fabry patients were not mentioned in the cohort study. Multiple protein polymorphisms can modulate phenotypic expression in Fabry's disease. Thus, Fabry's disease patients with different genotypes may develop different phenotypic expressions [28,29]. In contrast, our patients are all from the same family and have the same genetic mutation, which can reduce phenotypic heterogeneity and provide more homogenous data of correlations between thermal sensory testing and pain symptoms or ages in Fabry patients.
Our studies also showed that female Fabry patients had a lower pain score and more variable responses to painful heat stimuli than male Fabry patients. However, female Fabry patients generally showed a tendency toward increased withdrawal latencies to painful heat stimuli compared to the latencies of normal controls. Previous studies have demonstrated that female Fabry patients also have clinical symptoms of Fabry's disease, although the clinical symptoms exhibit a later onset and a milder degree than those of male Fabry patients [1]. Female Fabry patients also have small fiber neuropathy that is detected by IENFD and quantitative sensory testing [15,30], although there was no correlation between pain scores, quantitative sensory testing and IENFD [15]. Our study had similar findings, which showed that female Fabry patients had a lesser degree of prolonged latencies of WLPHS. However, the WLPHS in female Fabry patients had a weaker positive correlation with patient age than that in male Fabry patients. There were significantly lower levels of AGA activity in the male Fabry patients than in the female Fabry patients in our previous study [19], which is consistent with the findings of lower IENFD in male Fabry patients than in female Fabry patients in Uceyler's study [13]. These results are probably due to the effect of random X chromosome inactivation [31].
Conclusion
In conclusion, our study confirms that male Fabry patients had more severe and continuous C-fiber dysfunction as age increased, which results in sufficient and persistent injury discharges to sustain peripheral and central sensitization. However, once the small-fiber damage reaches a certain degree, the small fibers can no longer transmit enough noxious discharges to sustain peripheral and central sensitization in their old ages, which leads the older Fabry patients to have lower pain scores. In contrast, female Fabry patients had less and variable small fiber damage and fewer clinical signs/symptoms. However, a major limitation in this study is that only a small number of Fabry patients were recruited and investigated, which is due to the rarity of Fabry disease, and we cannot generalize the results to all Fabry patients. Nevertheless, our study's advantage is that we recruited a large Taiwanese Fabry family with homogenous genetic inheritance, which can reduce some phenotypic heterogeneity. Enzyme replacement therapy can improve pain score [[32], [33], [34]], life quality [34] and C-fiber function [35] of Fabry patients. However, our cross-sectional study didn't have the follow-up SF-MPQ and WLPHS results after enzyme replacement treatment. A further large cohort of physiological studies in Fabry patients before and after enzyme replacement treatment to evaluate the underlying pathophysiology of Fabry disease are warranted.
Funding
This work was supported by Ministry of Science and Technology, Taiwan, ROC, under the grants (MOST108-2314-B-182A-051).
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors are grateful to Ms. Sin-Fan Wu and Ms. Chuen-Hui Chen for their technical assistance.
Footnotes
Peer review under responsibility of Chang Gung University.
References
- 1.Clarke J.T. Narrative review: Fabry disease. Ann Intern Med. 2007;146:425–433. doi: 10.7326/0003-4819-146-6-200703200-00007. [DOI] [PubMed] [Google Scholar]
- 2.Zarate Y.A., Hopkin R.J. Fabry's disease. Lancet. 2008;372:1427–1435. doi: 10.1016/S0140-6736(08)61589-5. [DOI] [PubMed] [Google Scholar]
- 3.Cable W.J., Dvorak A.M., Osage J.E., Kolodny E.H. Fabry disease: significance of ultrastructural localization of lipid inclusions in dermal nerves. Neurology. 1982;32:347–353. doi: 10.1212/wnl.32.4.347. [DOI] [PubMed] [Google Scholar]
- 4.Hilz M.J. Evaluation of peripheral and autonomic nerve function in Fabry disease. Acta Paediatr Suppl. 2002;91:38–42. doi: 10.1111/j.1651-2227.2002.tb03108.x. [DOI] [PubMed] [Google Scholar]
- 5.Cable W.J., Kolodny E.H., Adams R.D. Fabry disease: impaired autonomic function. Neurology. 1982;32:498–502. doi: 10.1212/wnl.32.5.498. [DOI] [PubMed] [Google Scholar]
- 6.Dutsch M., Marthol H., Stemper B., Brys M., Haendl T., Hilz M.J. Small fiber dysfunction predominates in Fabry neuropathy. J Clin Neurophysiol. 2002;19:575–586. doi: 10.1097/00004691-200212000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Ro L.S., Chen S.T., Tang L.M., Hsu W.C., Chang H.S., Huang C.C. Current perception threshold testing in Fabry's disease. Muscle Nerve. 1999;22:1531–1537. doi: 10.1002/(sici)1097-4598(199911)22:11<1531::aid-mus7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Valeriani M., Mariotti P., Le Pera D., Restuccia D., De Armas L., Maiese T., et al. Functional assessment of A delta and C fibers in patients with Fabry's disease. Muscle Nerve. 2004;30:708–713. doi: 10.1002/mus.20174. [DOI] [PubMed] [Google Scholar]
- 9.Biegstraaten M., Hollak C.E., Bakkers M., Faber C.G., Aerts J.M., van Schaik I.N. Small fiber neuropathy in Fabry disease. Mol Genet Metabol. 2012;106:135–141. doi: 10.1016/j.ymgme.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Scott L.J., Griffin J.W., Luciano C., Barton N.W., Banerjee T., Crawford T., et al. Quantitative analysis of epidermal innervation in Fabry disease. Neurology. 1999;52:1249–1254. doi: 10.1212/wnl.52.6.1249. [DOI] [PubMed] [Google Scholar]
- 11.Luciano C.A., Russell J.W., Banerjee T.K., Quirk J.M., Scott L.J., Dambrosia J.M., et al. Physiological characterization of neuropathy in Fabry's disease. Muscle Nerve. 2002;26:622–629. doi: 10.1002/mus.10236. [DOI] [PubMed] [Google Scholar]
- 12.Biegstraaten M., Binder A., Maag R., Hollak C.E., Baron R., van Schaik I.N. The relation between small nerve fibre function, age, disease severity and pain in Fabry disease. Eur J Pain. 2011;15:822–829. doi: 10.1016/j.ejpain.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Uceyler N., He L., Schonfeld D., Kahn A.K., Reiners K., Hilz M.J., et al. Small fibers in Fabry disease: baseline and follow-up data under enzyme replacement therapy. J Peripher Nerv Syst. 2011;16:304–314. doi: 10.1111/j.1529-8027.2011.00365.x. [DOI] [PubMed] [Google Scholar]
- 14.Ro L.S., Liao M.F., Chen C.J., Lau Y.T., Lu K.T., Chen W.H. Peripheral microcirculation dysfunction evaluated by computed tomography perfusion study in Fabry patients. Eur J Neurol. 2012;19:e4–e6. doi: 10.1111/j.1468-1331.2011.03558.x. [DOI] [PubMed] [Google Scholar]
- 15.Torvin Moller A., Winther Bach F., Feldt-Rasmussen U., Rasmussen A., Hasholt L., Lan H., et al. Functional and structural nerve fiber findings in heterozygote patients with Fabry disease. Pain. 2009;145:237–245. doi: 10.1016/j.pain.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 16.Burlina A.P., Sims K.B., Politei J.M., Bennett G.J., Baron R., Sommer C., et al. Early diagnosis of peripheral nervous system involvement in Fabry disease and treatment of neuropathic pain: the report of an expert panel. BMC Neurol. 2011;11:61. doi: 10.1186/1471-2377-11-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LaMotte R.H., Campbell J.N. Comparison of responses of warm and nociceptive C-fiber afferents in monkey with human judgments of thermal pain. J Neurophysiol. 1978;41:509–528. doi: 10.1152/jn.1978.41.2.509. [DOI] [PubMed] [Google Scholar]
- 18.Tillman D.B., Treede R.D., Meyer R.A., Campbell J.N. Response of C fibre nociceptors in the anaesthetized monkey to heat stimuli: correlation with pain threshold in humans. J Physiol. 1995;485:767–774. doi: 10.1113/jphysiol.1995.sp020767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ro L.S., Chen C.M., Chang H.S., Lyu R.K., Wu Y.R., Hsu W.C., et al. Contribution of clinical screening to carrier detection in a large Chinese family with Fabry disease due to a novel alpha-galactosidase A gene deletion. Eur J Neurol. 2007;14:493–497. doi: 10.1111/j.1468-1331.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 20.Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 21.Mogil J.S. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 22.Fowler C.J., Sitzoglou K., Ali Z., Halonen P. The conduction velocities of peripheral nerve fibres conveying sensations of warming and cooling. J Neurol Neurosurg Psychiatry. 1988;51:1164–1170. doi: 10.1136/jnnp.51.9.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomes I., Nora D.B., Becker J., Ehlers J.A., Schwartz I.V., Giugliani R., et al. Nerve conduction studies, electromyography and sympathetic skin response in Fabry's disease. J Neurol Sci. 2003;214:21–25. doi: 10.1016/s0022-510x(03)00172-2. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki M., Ohno K., Ohta S., Sakuraba H., Takeshita K. Relief of chronic burning pain in Fabry disease with neurotropin. Pediatr Neurol. 1990;6:211–213. doi: 10.1016/0887-8994(90)90067-b. [DOI] [PubMed] [Google Scholar]
- 25.Woolf C.J. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 26.Woolf C.J., Mannion R.J. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 27.MacDermot K.D., Holmes A., Miners A.H. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001;38:769–775. doi: 10.1136/jmg.38.11.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shitrit D., Ollech J.E., Ollech A., Bakal I., Saute M., Sahar G., et al. Itraconazole prophylaxis in lung transplant recipients receiving tacrolimus (FK 506): efficacy and drug interaction. J Heart Lung Transplant. 2005;24:2148–2152. doi: 10.1016/j.healun.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Germain D.P., Oliveira J.P., Bichet D.G., Yoo H.W., Hopkin R.J., Lemay R., et al. Use of a rare disease registry for establishing phenotypic classification of previously unassigned GLA variants: a consensus classification system by a multispecialty Fabry disease genotype-phenotype workgroup. J Med Genet. 2020;57:542–551. doi: 10.1136/jmedgenet-2019-106467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laaksonen S.M., Roytta M., Jaaskelainen S.K., Kantola I., Penttinen M., Falck B. Neuropathic symptoms and findings in women with Fabry disease. Clin Neurophysiol. 2008;119:1365–1372. doi: 10.1016/j.clinph.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Maier E.M., Osterrieder S., Whybra C., Ries M., Gal A., Beck M., et al. Disease manifestations and X inactivation in heterozygous females with Fabry disease. Acta Paediatr Suppl. 2006;95:30–38. doi: 10.1111/j.1651-2227.2006.tb02386.x. [DOI] [PubMed] [Google Scholar]
- 32.Eng C.M., Guffon N., Wilcox W.R., Germain D.P., Lee P., Waldek S., et al. Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 33.Schiffmann R., Kopp J.B., Austin H.A., 3rd, Sabnis S., Moore D.F., Weibel T., et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. J Am Med Assoc. 2001;285:2743–2749. doi: 10.1001/jama.285.21.2743. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann B., Garcia de Lorenzo A., Mehta A., Beck M., Widmer U., Ricci R., et al. Effects of enzyme replacement therapy on pain and health related quality of life in patients with Fabry disease: data from FOS (Fabry Outcome Survey) J Med Genet. 2005;42:247–252. doi: 10.1136/jmg.2004.025791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hilz M.J., Brys M., Marthol H., Stemper B., Dutsch M. Enzyme replacement therapy improves function of C-, Adelta-, and Abeta-nerve fibers in Fabry neuropathy. Neurology. 2004;62:1066–1072. doi: 10.1212/01.wnl.0000118207.84514.40. [DOI] [PubMed] [Google Scholar]