Abstract
Many different intrinsically disordered proteins and proteins with intrinsically disordered regions are associated with neurodegenerative diseases. These types of proteins including amyloid-β, tau, α-synuclein, CHCHD2, CHCHD10, and G-protein coupled receptors are increasingly becoming evaluated as potential drug targets in the pharmaceutical-based treatment approaches. Here, we focus on the neurobiology of this class of proteins, which lie at the center of numerous neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases, Huntington’s disease, amyotrophic lateral sclerosis, frontotemporal dementia, Charcot–Marie–Tooth diseases, spinal muscular atrophy, and mitochondrial myopathy. Furthermore, we discuss the current treatment design strategies involving intrinsically disordered proteins and proteins with intrinsically disordered regions in neurodegenerative diseases. In addition, we emphasize that although the G-protein coupled receptors are traditionally investigated using structural biology-based models and approaches, current studies show that these receptors are proteins with intrinsically disordered regions and therefore they require new ways for their analysis.
Keywords: Intrinsically disordered proteins, Proteins with intrinsically disordered regions, Neurodegeneration, Pathology, Drug design
Introduction
The physiologically active proteins that are intrinsically disordered (IDPs) or those exhibiting intrinsically disordered protein regions (IDPRs) are known for their absence of a stable three-dimensional structure (Coskuner and Uversky 2019). Their extended state, conformational flexibility, and structural plasticity go beyond the ordinary and challenge multiple principles of natural sciences. In fact, the majority of their structural and functional characteristics are not compatible with the long-established considerations of classical physicists or structural biologists disciplined with the “lock-and-key” model (Uversky 2016). Structural studies of IDPs/IDPRs require the use of integrated experimental techniques, such as nuclear magnetic resonance (NMR)–based chemical shifts, paramagnetic relaxation enhancement (PRE) distance restraints, pulsed-field gradient (PGF)–derived Rh values, 15N R2 relaxation rates, and 1H-15N heteronuclear nuclear Overhauser effects, and various sophisticated computational techniques, such as molecular dynamics (MD) simulations integrated with machine learning (ML) treatments (Uversky 2018; Best 2017; Coskuner and Uversky 2017; Coskuner and Wise-Scira 2013).
It is rare that IDPs/IDPRs are completely describable as unstructured random coils (Zhang et al. 2013; Uversky and Keith Dunker 2010; Uversky 2013a; Park et al. 1988; DeForte and Uversky 2016; Uversky 2016). IDPs/IDPRs can often exhibit transiently populated (but distinct) secondary structural elements that may be targeted by their interacting partners to promote binding in a more kinetically efficient fashion (Wright and Jane Dyson 2015). It is thought that these secondary structural elements of IDPs/IDPRs may be highly dynamic in the sense that they not only undergo secondary structure interconversions but also rapidly sample conformational space within the unfolded form (Milles et al. 2018; Oldfield et al. 2005a; Szöllosi et al. 2014; Oldfield et al. 2008; Uversky 2015a; Oldfield and Dunker 2014; Uversky 2011; Kim and Han 2018; Uversky 2015b). From more familiar maladies, such as Parkinson’s and Alzheimer’s diseases (PD and AD), to relatively less known ones, such as Charcot–Marie–Tooth disease along with other examples, such as spinal muscular atrophy (SMA), and Huntington’s and Lewy body diseases, which together are referred as neurodegenerative diseases, IDPs/IDPRs represent integral components in their pathogenesis (Coskuner-Weber and Uversky 2018; Burstein et al. 2018; Liu et al. 2020; Auranen et al. 2015; Ajroud-Driss et al. 2015). To illustrate this point, the amyloid-β and α-synuclein proteins are IDPs, and yet they are both capable of adopting β-sheet structures necessary for participating in the amyloid polymerization process that is pivotal to neurodegenerative diseases (Oldfield et al. 2005b; Sawaya et al. 2007; Nelson et al. 2005). Likewise, in terms of the presence of IDPRs, coiled-coil-helix-coiled-coil-helix domain containing 10 (CHCH10) and coiled-coil-helix-coiled-coil-helix domain containing 2 (CHCHD2) proteins are at the center of many diseases, such as mitochondrial myopathy, frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), SMA, Charcot–Marie–Tooth disease, PD, and AD, in a similar manner with aforementioned IDPs, as they assume β-sheet structure at certain residues, which are in charge of their oligomerization (see, for example, Huang et al. 2018, 2). Table 1 summarizes the peculiarities of tissue expression, cellular localization, function, relation to different diseases, pathological mutation, and pathological mechanisms of IDPs/IDPRs discussed in the present study.
Table 1.
Peculiarities of tissue expression, cellular localization, function, relation to the various diseases, pathological mutations, and pathological mechanisms of disordered proteins discussed in the present study
| Protein (UniProt ID) | Tissue expression: cellular localization | Function | Disease and associated mutation | Pathological mechanism |
|---|---|---|---|---|
| α-Synuclein (P37840) | Tissue-enhanced expression in bone marrow and brain (substantia nigra, cerebellum, and prefrontal cortex): cytoplasm, nucleus, synapse, axon, can be membrane-bound, can be secreted | Involved in synaptic activity, acts as molecular chaperone, regulates dopamine neurotransmission | Sporadic Parkinson’s disease (PD): wild type; | Aggregation and amyloid fibril formation; Lewy bodies, Lewy neurites (Kao et al. 2009) |
| Familial early-onset PD: A30P, E46K, H50Q, A53T, gene duplication and triplication; | ||||
| Dementia with Lewy bodies (DLB): E46K | ||||
| Multiple other synucleinopathies: wild type | ||||
| Aβ peptides (derived from APP, P05067) | Expressed with low tissue specificity | APP: Cell surface receptor, regulates neurite outgrowth, acts as protease inhibitor | Sporadic Alzheimer disease (AD): wild type; | Aggregation and amyloid fibril formation; extracellular amyloid plaques; vascular amyloid deposits; promotes tau phosphorylation and fibrillation (Sadigh-Eteghad et al. 2015) |
| Familial early-onset AD: D678N, A692G, E693G, A713T, T714A/I, V516M, I716V, V717F/G/I/L, L73P | ||||
| APP: Cell surface single-pass type I transmembrane protein, cytoplasmic vesicles, Golgi apparatus, early endosome, can be secreted | Aβ peptides: Lipophilic metal chelators with metal-reducing activity, bind to lipoproteins and apolipoproteins E and J, activate mononuclear phagocytes | |||
| Cerebral amyloid angiopathy, APP-related (CAA-APP): E693K/Q, D694N, L705V | ||||
| Aβ peptides: cell surface | ||||
| Microtubule-associated protein tau (P10636) | Tissue-enhanced expression in brain, skeletal muscle: cell membrane, cell projection, cytoplasm, cytoskeleton, membrane (cytoplasmic site of peripheral membrane), microtubule, secreted | Regulates microtubule assembly and stability, as well as neuronal polarity, acts as linker between axonal microtubules and plasma membrane components | Sporadic AD: wild type; | Disruption of the neuronal cytoskeleton; |
| Frontotemporal dementia (FTD): R5H, L583V, G589V, G590R, N596K, ΔK597, N613H, P618L/S, S622N, K634M, V654M, E659V | ||||
| tau aggregation and formation of paired helical filaments (PHF) and straight filaments (Liu and Gong 2008) | ||||
| Pick disease of the brain (PIDB): K574T, S637F, K686I, G706R | ||||
|
Progressive supranuclear palsy 1 (PSNP1): R5L, G620V | ||||
| Parkinson-dementia syndrome (PARDE): wild type | ||||
| Multiple other taupathies: wild type | ||||
| Coiled-coil-helix-coiled-coil-helix domain containing protein-10 CHCHD10 (Q8WYQ3) | Tissue-enhanced expression in heart muscle and skeletal muscle: mitochondrion intermembrane space | Maintenance of mitochondrial organization and mitochondrial cristae structure | Amyotrophic lateral sclerosis (ALS): R11G, P12S, R15L, G66V, P80L, Y92C, Q102H, Q108P | Loss of function, mitochondrial/synaptic damage and cytoplasmic TDP-43 accumulation (Woo et al. 2017) |
| FTD: H22Y, P23T, P23S, P23L, A32D, A35D, V57E, Q82X | ||||
|
PD: S30L Mitochondrial myopathy: R15S, G58R, S59L | ||||
| Charcot–Marie–Tooth disease: G66V | ||||
| Jokela type spinal muscular atrophy (SMAJ): G66V | ||||
| Coiled-coil-helix-coiled-coil-helix domain containing protein-2 CHCHD2 (Q8WYQ3) | Expressed with low tissue specificity: mitochondrion intermembrane space, nucleus | Transcription factor; bi-organellar mediator of oxidative phosphorylation; regulates cell migration and differentiation, mitochondrial cristae structure, and apoptosis (Kee et al. 2021) | PD, AD, DLB, FTD, multiple system atrophy (MSA): P2L, G4R, S5R, R8H, P14S, R18Q, A32T, P34L, A37V, A49V, T61I, V66M, A71P, A79S, I80V, S85R, A93V, Q126X, R145Q | Mitochondrial dysfunction (Kee et al. 2021) |
| G-Protein coupled receptors (GPCRs; more than 800) | Multi-pass membrane protein | Membrane receptors that utilize trimeric G proteins to transduce information from the extracellular environment to intracellular signals; can recognize a wide spectrum of extracellular ligands and trigger a large variety of intracellular signaling cascades; can interact with (and be activated by) more than a 1000 natural and artificial extracellular ligands; play a role in multiple physiological functions, such as sight, taste, smell, neurotransmission, pain perception, and immune responses | Over 600 inactivating and almost 100 activating mutations in GPCRs have been identified, which are responsible for more than 30 different human diseases (Schöneberg et al. 2004). For example, GPCR mutations can cause acquired and inherited diseases, such as retinitis pigmentosa (RP), hypo- and hyperthyroidism, nephrogenic diabetes insipidus, fertility disorders, carcinomas (Schöneberg et al. 2004), and neurodegeneration | Loss of function, pathological gain of function (Schöneberg et al. 2004) |
Most of information is retrieved from UniProt
Neurodegeneratıon and the usual suspects
α-Synuclein and Parkinson’s disease
A group of neurodegenerative diseases including multiple system atrophy, PD, and Lewy body dementia are denoted as synucleinopathies due to the accumulation of Lewy bodies and neurites, which are principally formed by the aggregated α-synuclein (αS) protein in the central nervous system (CNS) (Kao et al. 2009). The 140-amino-acid-long αS is a product of the SNCA gene, which is located in the q21 region of the chromosome 4 in humans (Stefanis 2012). Based on its amino acid sequence, three distinct regions of αS were identified: the positively charged N-terminal domain (1–65) that contains four 11-amino-acid imperfect repeats with a highly conservative hexamer motif (KTKEGV) that can form amphipathic α-helices at interaction with lipid membranes; the NAC domain (Non-Amyloid-β Component) of plaques, spanning the residues from 66 to 95, contains two additional KTKEGV repeats, which play a role in promoting fibrillation and aggregation due to their hydrophobicity and capability of β-sheet formation; and the negatively charged, acidic C-terminal domain, which contains three highly conserved tyrosine residues, and is typically involved in the protein–protein and protein–small molecule interactions (Bartels et al. 2010).
The existence of lipid-associated αS in the plasma membrane of synaptic vesicles and terminals indicates a role for this protein in neurotransmitter release (Man et al. 2021). αS was considered to have chaperone activity based on its interaction with the elements of the SNARE complex, which renders in its ability to dilate the exocytotic fusion pore (Wu et al. 2017). The misfolding of lipid-associated αS in synucleinopathies results in β-sheet structure in fibrils, which are found as the fundamental element of Lewy neurites and Lewy bodies (Fanning et al. 2020). In addition to phosphorylation of aggregated αS at S87 and S129, further posttranslational modifications including truncation, nitration, oxidation (possible contribution of dopamine), and transition metal coordination occur as well (Chen et al. 2019a). The nitration and oxidation were suggested to reduce fibril formation and stabilize oligomer protofibrils of αS, which may be toxic (Meade et al. 2019). Truncation or metal ion coordination of αS, which mostly occurs at its C-terminal region, demonstrated an elevated inclination for fibrillation (Hong et al. 2011). Of note, in some familial cases of the early-onset PD, it was found that single point mutations in αS (e.g., A18T, A29S, A30P, E46K, H50Q, G51D, A53E, and A53T) result in alterations in its disordered structure and its fibrillation propensity (Coskuner-Weber and Uversky 2018; Coskuner and Wise-Scira 2013; Wise-Scira et al. 2013a, b).
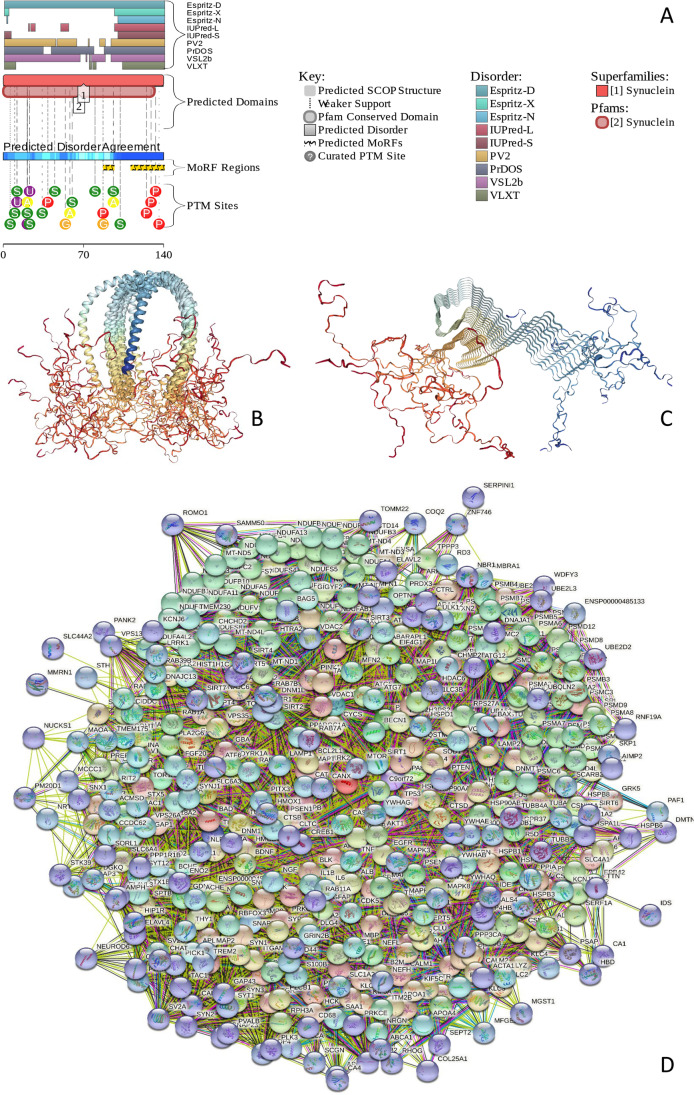
Figure 1 describes information relating to the functional disorder of human αS. Figure 1A shows the functional disorder profile for this protein generated by the D2P2 platform (https://d2p2.pro/) (Oates et al. 2013), indicating high disorder level, as well as the presence of multiple potential sites for posttranlational modifications (PTMs) and two disorder-based protein binding sites, molecular recognition features (MoRFs), which are disordered regions capable of disorder-to-order transition at binding to specific partners (Oldfield et al. 2005a; Uversky et al. 2005; Mohan et al. 2006; Vacic et al. 2007; Ng et al. 2007; Cheng et al. 2007; Mészáros et al. 2009; Dosztányi et al. 2009; Disfani et al. 2012; Yan et al. 2016; Mészáros et al. 2018). Figure 1B describes the NMR-determined structures of this protein bound to a small vesicle (Rao et al. 2010), whereas Fig. 1C describes the NMR-derived structure of αS within an amyloid-like fibril (Tuttle et al. 2016). It is clear that in both structural cases, significant parts of this protein possess highly disordered nature. Figure 1D illustrates the binding promiscuity of αS by showing its protein–protein interaction network (PPIN) generated using the web-tool STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) (http://string-db.org/) (Snel et al. 2000). This αS-centered PPIN includes 461 binding partners connected by 11,489 interactions. This network is characterized by the average node degree of 49.7 (i.e., each member of this network is involved in interaction with almost 50 partners).
Fig. 1.
A D2P2-generated functional profile of human αS (UniProt ID: P37840). D2P2 (http://d2p2.pro/) is a database of predicted disorder for a large library of proteins from completely sequenced genomes that uses outputs of IUPred, PONDR VLXT, PrDOS, PONDR VSL2B, PV2, and Espritz and is further supplemented by data concerning location of various curated posttranslational modifications and predicted disorder-based protein binding sites, known as molecular recognition features, MoRFs. B NMR solution structure of human αS bound to a small micelle of the detergent sodium lauroyl sarcosinate (SLAS) (PDB ID: 2KKW) (Rao et al. 2010). C NMR structure of a pathogenic fibril of full-length human αS (PDB ID: 2N0A) (Tuttle et al. 2016). D αS-Centric PPIN generated by STRING (http://string-db.org/). In this network, nodes correspond to proteins, whereas the edges show predicted or known functional associations. Seven types of evidence are used to build the corresponding network, where they are indicated by the differently colored lines: a green line represents neighborhood evidence; a red line—the presence of fusion evidence; a purple line—experimental evidence; a blue line—co-occurrence evidence; a light blue line—database evidence; a yellow line—text mining evidence; and a black line—co-expression evidence. This network was generated using the minimum required interaction score of 0.4 (medium confidence)
Amyloid-β and tau in the pathogenesis of Alzheimer’s disease
AD is one of the most widespread neurodegenerative diseases, which affects the cognitive processes and is diagnosed through the identification of senile plaques made of amyloid-β (Aβ) peptides and neurofibrillary tangles (NFTs) of tau protein aggregates (Sadigh-Eteghad et al. 2015). Aβ aggregates consist mainly of the 39–43-residue-long peptides derived as a result of the proteolytic cleavage of amyloid precursor protein (APP) encoded in human by the APP gene, which is located on chromosome 21 and contains 18 exons spanning 290 kilobases (Khanahmadi et al. 2015). There have been studies reporting that one of the early events of neuronal dysfunction is the accumulation of Aβ42 peptide that seeds the formation of amyloid plaques (Selkoe and Hardy 2016). Moreover, it is believed that genetic mutations in APP/Aβ play a part in the pathology of early-onset AD (Coskuner-Weber and Uversky 2018; Coskuner et al. 2013). The toxicity caused by Aβ aggregates is correlated with the interactions between Aβ and membranes (Niu et al. 2018). Earlier studies proposed that Aβ interacts with phospholipid membranes. Two types of Aβ and membrane interactions were detected; in the first type, Aβ is inserted into the membrane, forming a pore-like structure (Niu et al. 2018). This insertion may cause leakage in the membrane and may trigger cell death. In the second type, Aβ binds onto the membrane surface, compressing the membrane and makes it thinner (Niu et al. 2018). We should mention here that these interactions may not be completely separate from each other. For instance, depending on the ratio of lipids to proteins, Aβ can bind to membrane surface and can form an oligomer with a pore-like structure as the lipid to protein ratio decreases (Niu et al. 2018). Aβ and the membrane may interact during the Aβ aggregation process within the membrane environment. These interactions may impact membrane permeability (Niu et al. 2018). In parallel, the composition of the membrane and the lipid-to-protein ratios may regulate Aβ association (Niu et al. 2018). Aβ and membrane interactions and neurotoxicity are still poorly understood.
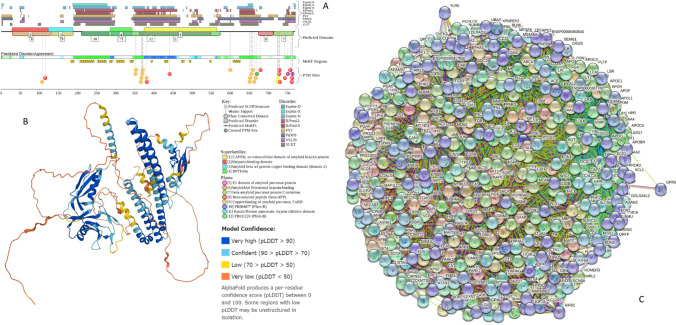
Basic disorder-related information pertaining to APP is summarized in Fig. 2. Here, functional disorder profile generated by D2P2 (Oates et al. 2013) illustrates the presence of multiple long IDPRs, and various PTMs and MoRFs in this protein (Fig. 2A). The highly disordered nature of human APP is further illustrated by the structural model generated for the full-length protein by AlphaFold (Jumper et al. 2021) (Fig. 2B), whereas Fig. 2C shows the APP-centered PPIN generated by STRING (Snel et al. 2000) that includes 433 proteins connected by 7489 interactions and is characterized by the average node degree of 34.4.
Fig. 2.
A D2P2-generated functional profile of human APP (UniProt ID: P05067). B AlphaFold-generated model (Jumper et al. 2021) of human APP. C APP-centered PPIN generated by STRING (http://string-db.org/) (Snel et al. 2000). This network was generated using the minimum required interaction score of 0.5
Six tau isoforms are produced in the mature human brain by alternative mRNA splicing of exons 2, 3, and 10 of the microtubule associated protein tau (MAPT) gene, which is positioned on chromosome 17q21 (Liu and Gong 2008). Tau is diversely and extensively post-translationally modified, including phosphorylation, SUMOylation, nitration, and methylation (Alquezar et al. 2021). This modification bar-core has been proposed to regulate functionality of this important protein. However, abnormal post-translational modifications (PTMs) of tau (e.g., hyperphosphorylation) render it with an increased tendency toward aggregation (Schaffert and Carter 2020). Curiously, tau aggregation and subsequent formation of intracellular deposits are at the root of multiple neurodegenerative diseases, known collectively as taupathies (Mandelkow and Mandelkow 2012). Acetylation and/or glycosylation of tau is a determinant for its phosphorylation pattern (Trzeciakiewicz et al. 2017). By virtue of its disordered structure, interactions between tau and intracellular molecules such as nucleic acids, proteins, and metal ions occur, which impact its structural and biological functions. The interaction with Aβ is one of them, which strengthens toxicity in tau (Medeiros et al. 2011). Studies have shown that the lipid membrane causes tau misfolding and aggregation (Jones et al. 2012). Tau has a tendency to associate with or intercalate into anionic lipid monolayers and bilayers. Negatively charged membranes have been presented to impact tau fibrillization, and this may render the otherwise soluble tau aggregation competent, or proaggregant and seed tau into fibrils (Jones et al. 2012). Moreover, lipid packing is disrupted when tau interacts with negatively charged lipid membranes (Jones et al. 2012).
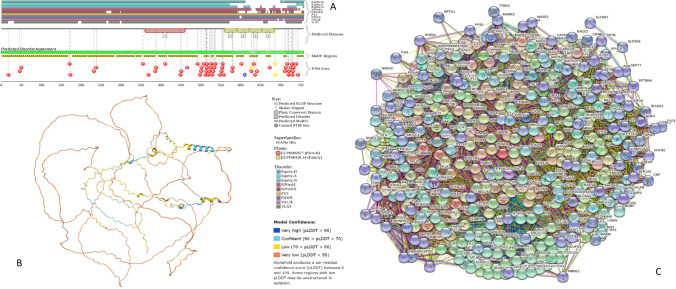
To get a better appreciation of the highly disordered nature of this multifunctional protein, Fig. 3 represents some basic disorder-related information. Here, the functional disorder profile generated by D2P2 (Oates et al. 2013) clearly shows that this protein is disordered almost through its entire length and contains multiple phosphorylation sites and several other PTMs. Furthermore, almost the entire tau can serve as disorder-based interactor since more than 80% of its residues are predicted to exist as part of the 14 MoRFs (Fig. 3A). The highly disordered nature of human tau is further illustrated by the structural model generated for the full-length protein by AlphaFold (Jumper et al. 2021) (Fig. 3B), where there are almost no elements of stable structure. Finally, Fig. 3C represents the PPIN generated for human tau (MAPT) by STRING (Snel et al. 2000) and shows that this PPIN includes 401 proteins connected by 7736 interactions and is characterized by the average node degree of 38.3.
Fig. 3.
A D2P2-generated functional profile (Oates et al. 2013) of human tau protein (UniProt ID: P10636). B AlphaFold-generated model (Jumper et al. 2021) of human tau protein. C Tau/MAPT-centered PPIN generated by STRING (http://string-db.org/) (Snel et al. 2000). This network was generated using minimum required interaction score of 0.45
Neurodegeneratıon and some less usual suspects
CHCHD10 in neurodegenerative diseases
Some of the less commonly known proteins associated with neurodegeneration are coiled-coil-helix-coiled-coil-helix domain containing proteins 10 and 2 (CHCHD10 and CHCHD2) (Zhou et al. 2020). These mitochondrial proteins are at the center of many diseases, such as mitochondrial myopathy, FTD, ALS, SMA, Marie–Tooth disease, PD, and AD (Zhou et al. 2017). Considering the important role of mitochondria in the metabolism and in lethal signaling processes for both physiological and pathological cell death, it is indisputable that mutations in mitochondrial proteins are associated with neurodegenerative diseases (Zhou et al. 2017).
CHCHD10 is a mitochondrial protein found in the intermembrane space and is abundant at cristae junctions (Genin et al. 2016). CHCHD10 is encoded by the CHCHD10 gene, which is 2138 base pairs and is located at 11.23 position on the q arm of chromosome 22 (Genin et al. 2016). The predicted structure for the CHCHD10 protein includes an irregular N-terminal domain, a hydrophobic helix, and a C-terminal domain that contains a Cx(9)C motif (Alici et al. 2022), as well as two additional cysteines, where totally four cysteines were proposed to build two disulfide bonds (Imai et al. 2019). Although the function of CHCHD10 is not known, it is considered to be involved in maintaining oxidative phosphorylation and/or cristae morphology (Ajroud-Driss et al. 2015). Recent studies identified clustered heterozygous mutations in exon 2 of CHCHD10 to possess clinically heterogeneous phenotypes (Ranganathan et al. 2020). Mutations in CHCHD10 protein have confirmed the long-suspected involvement of mitochondrial dysfunction in neurodegenerative disease etiology (Johnson et al. 2014). Although the exact mechanism still remains elusive, investigations of wild-type and S59L mutant proteins in the context of mutational effects in their thermodynamic and structural properties have been revealed (Alici et al. 2022). A broad range of neurological symptoms associated with the defects related to CHCHD10 are briefly outlined below.
Amyotrophic lateral sclerosis (ALS) disease, characterized by the degeneration of upper and lower motor neurons (UMNs and LMNs, respectively), is a progressive disease that causes impairment in communication between neurons and muscles. R11G, P12S, R15L, G66V, P80L, Y92C, Q102H, and Q108P genetic mutations of CHCHD10 have been linked to ALS (Keith et al. 2020).
Frontotemporal dementia (FTD) disease: a slowly progressive disease, causing behavioral changes, language disorders, loss of cognitive skills, and extrapyramidal symptoms. Thus far, several mutant CHCHD10 proteins including H22Y, P23T, P23S, P23L, A32D, A35D, V57E, and Q82X have been identified in patients with sporadic FTD (Keith et al. 2020).
Mitochondrial myopathy: this mitochondrial disease causes prominent muscular problems, such as weakness, amyotrophy, and exercise intolerance. R15S, G58R, and S59L mutations of CHCHD10 have been linked to mitochondrial myopathy (Keith et al. 2020).
Parkinson`s disease: this neurodegeneration is characterized by slowness of movements, tremor, rigidity, and postural instability. S30L mutation of CHCHD10 has been linked to PD (Keith et al. 2020).
Charcot–Marie–Tooth disease: this group of inherited disorders is also known as hereditary motor and sensory neuropathy. It causes nerve damage and is characterized by motor and emotional polyneuropathy, smaller muscles, muscle weakness, numbness, and skeletal problems. G66V mutant form of CHCHD10 has been linked to Charcot–Marie–Tooth disease (Keith et al. 2020).
Jokela type spinal muscular atrophy (SMAJ): This disease is also known as late-onset spinal motor neuronopathy (LOSMoN). It is a rare neuromuscular disorder characterized by cramps and muscle twitches. The G66V mutant form of CHCHD10 has been linked to SMAJ (Keith et al. 2020).
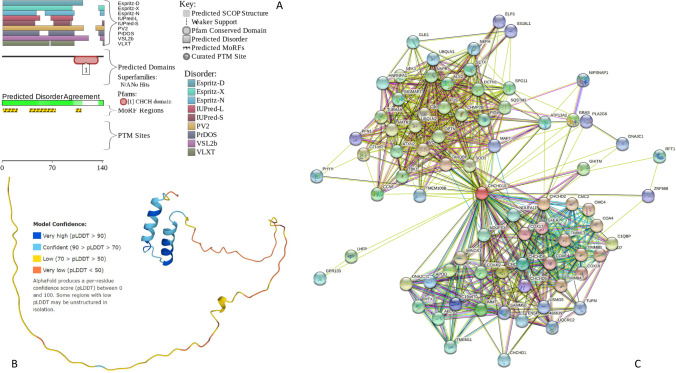
Figure 4 assembles basic information on the prevalence and potential functionality of intrinsic disorder in the human CHCHD10 protein and shows that more than half of this protein is expected to be disordered, and that the N-terminal disordered domain contains three MoRFs.
Fig. 4.
A D2P2-generated functional profile of human CHCHD10 protein (UniProt ID: Q8WYQ3) (Oates et al. 2013). B AlphaFold-generated model of human CHCHD10 protein (Jumper et al. 2021). C CHCHD10-centered PPIN generated by STRING (http://string-db.org/) (Snel et al. 2000). This network was generated using the minimum required interaction score of 0.4 (medium confidence). In this PPIN, which is characterized by the average node degree of 17.6, there are 78 proteins connected by 687 interactions
CHCHD2 and neurodegeneration
CHCHD2 is another member of the CHCHD family. CHCHD2 and CHCHD10 are bound to membrane in a way that resists alkaline carbonate extraction and sonication (Burstein et al. 2018). Because digitonin which is a steroidal saponin, which in turn disrupts lipid membranes enriched in sterols, it was proposed that CHCHD2 and CHCHD10 are located in the mitochondrial inner membrane regions where cholesterol is rich (Burstein et al. 2018). However, the interaction mechanisms between CHCHD2 and/or CHCHD10 with membranes are unknown. Mutations in this transcription factor, i.e., P2L, G4R, S5R, R8H, P14S, R18Q, A32T, P34L, A37V, A49V, T61I, V66M, A71P, A79S, I80V, S85R, A93V, Q126X, and R145Q, are related to various neurodegenerative diseases, such as PD, AD, dementia with Lewy bodies, FTD, and multiple system atrophy (Kee et al. 2021). The structures of both wild-type and mutant forms of CHCHD2 remain unknown. Figure 5 suggests that the highly disordered nature of human CHCHD2 (see Fig. 5A and B) could be one of the reasons for the lack of structural characterization of this protein. In fact, it is expected that only the coiled coil-helix-coiled coil-helix (CHCH) domain of this protein (residues 111–151) can gain structure known as CHCH fold, which is stabilized by two disulfide bonds formed by the four cysteines in the twin CX9C motifs (i.e., two pairs of cysteines each spaced by nine residues). On the other hand, based on data shown in Fig. 5C, the highly disordered status of CHCHD2 is likely to be responsible for its observed high binding promiscuity.
Fig. 5.
A D2P2-generated functional profile of human CHCHD2 protein (UniProt ID: Q9Y6H1) (Oates et al. 2013). B AlphaFold-generated model of human CHCHD2 protein (Jumper et al. 2021). C CHCHD2-centered PPIN generated by STRING (http://string-db.org/) (Snel et al. 2000). This network was generated using the minimum required interaction score of 0.4 (medium confidence). In this PPIN, which is characterized by the average node degree of 21.2, there are 133 proteins connected by 1409 interactions
Several studies have found a link between mitochondrial dysfunction and αS (Chen et al. 2019b). It was discovered in 2016 that αS interacts with TOM20, a mitochondrial outer membrane protein, emphasizing the importance of pathologically correlated forms of αS aggregating in mitochondria (Di Maio et al. 2016). Despite the numerous links between αS and mitochondrial dysfunction, the molecular basis of the involvement of CHCHD2 and CHCHD10 in αS-associated mitochondrial dysfunction remains unknown.
GPCRs and neurodegeneration
GPCR universe
G proteins and G-protein coupled receptors (GPCRs) constitute a complex signaling system that is capable of recognizing a wide spectrum of extracellular ligands with subsequent triggering of a large variety of intracellular signaling cascades (Syrovatkina et al. 2016). This GPCR-G protein signaling machinery includes more than 800 various GPCRs which represent one of the largest families of protein receptors and a very large set of heterotrimeric G proteins (Fonin et al. 2019). GPCRs are classified as A-F (or 1–6) based on their sequence and functional similarities. Rhodopsin-class (class A) comprises neurotransmitters, hormones, and light receptors, accounting for more than 80% of all GPCRs. Secretin-receptor family is known as class B. Class C includes the metabotropic glutamate family, GABA receptors, calcium-sensing receptors, and taste receptors. Class D involves fungal mating pheromone receptors, and cyclic AMP (cAMP) receptors are classified as class E. Frizzled (FZD) and smoothened (SMO) receptors are members of class F.
GPCRs can interact with (and be activated by) more than a 1000 natural and artificial extracellular ligands, ranging from photons to amines, lipids, nucleotides, organic odorants, peptides, and proteins (Fredriksson et al. 2003; Flock et al. 2017). GPCR-G protein system mediates most cellular responses to hormones, neurotransmitters, ions, photons, and other environmental stimuli, and they are responsible for vision, olfaction, and taste (Fonin et al. 2019). Most of the signal transduction machinery is associated with membranes and therefore lipid related (Kiriakidi et al. 2019). The important role of lipids in the efficient and correct extracellular signal propagation has attracted scientific interest (Escribá et al. 2007). Lipid–GPCR interactions have a significant influence on GPCR activity (Oates and Watts 2011). The direct and indirect interactions between GPCRs and bilayers are proposed to control various essential aspects of GPCR function (Kiriakidi et al. 2019). In fact, the interaction with cholesterol has been investigated in various recent studies (Gimpl 2016). GPCRs possess specific binding sites for cholesterol (Kiriakidi et al. 2019). In fact, the abundant cholesterol within the plasma membrane of eukaryotic cells is close to all integral membrane proteins (Kiriakidi et al. 2019). Therefore, the function of GPCRs depends on cholesterol. The curvature and fluidity of the membrane, membrane thickness, and lateral pressure influence the cholesterol approach as well as GPCR interactions with drugs (Kiriakidi et al. 2019). Cholesterol itself is an amphoteric compound and the steroid skeletal and isooctyl alkyl chain increase its lipophilic character while the 3β-hydroxyl group affects its polarity and amphiphilicity (Gimpl 2016). The β surface embraces two methyl groups while its α surface does not embrace substitutions and thus, it is a smooth surface (Kiriakidi et al. 2019). Its high chirality and structural characteristics play a role in the interactions with lipid bilayers and proteins. Computational studies and experiments have shown binding sites of cholesterol, and their ability to modulate GPCR function and GPCR–drug interactions (Kiriakidi et al. 2019; Gimpl 2016). However, these computational and experimental studies have not considered the disordered nature of GPCRs.
As a general note, the prevalence and potential functionality of intrinsic disorder in the GPCR-G protein signaling system was a subject of a recent focused study (Fonin et al. 2019). Based on the results of this comprehensive analysis, it was concluded that the “presence of intrinsic disorder and associated with it high conformational flexibility plays an important role in regulation and controlling this GPCR–G protein signaling system and also contributes to the multifunctionality and binding promiscuity of proteins involved in the GPCR and G protein signaling” (Fonin et al. 2019).
Structural organization and functional intrinsic disorder of GPCRs
GPCRs are prime examples of proteins possessing ordered and disordered regions which exhibit different and complementary activities (Venkatakrishnan et al. 2014). They feature a well-folded core domain with 3D structures determinable by X-ray and cryo-EM (García-Nafría and Tate 2021), as well as unfolded domains that are poorly described because electron densities are lacking (Akbayrak et al. 2020). The disordered domains of class A (rhodopsin-class) GPCRs are the N- and C-terminal domains, as well as extracellular and intracellular loops (Wheatley et al. 2012). When a GPCR binds a ligand, the N-terminal region folds, triggering a variety of physiological actions (Martin et al. 2005). This disorder-to-order transition initiates signal transmission via transmembrane core rearrangement (Hilger et al. 2018).
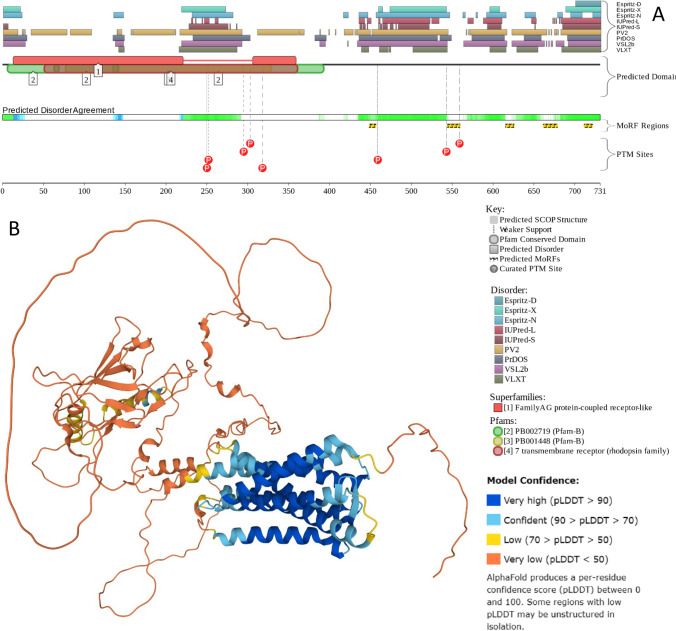
C-terminal domains of class A GPCRs vary in length (30–325 amino acid residues) and primary sequence and are classified as fully disordered. The C-terminal domains of GPCRs are prone to many various PTMs (Pal and Chattopadhyay 2019). Together with these PTM sites, the highly flexible regions of GPCRs contain SLiM and/or MoRF regions (van der Lee et al. 2014). Their combination increases diversity in their binding sites. These tendencies are illustrated by Fig. 6 representing the D2P2-generated functional profile (Oates et al. 2013) of human GPCR149 and a structural model of this protein generated by AlphaFold (Jumper et al. 2021) and clearly showing massive disorder in the C-terminal half of protein, where residues 364–731 (i.e., 368 of 731 residues, 50.3%) are expected to be mostly disordered.
Fig. 6.
A D2P2-generated functional profile of human GPCR149 (UniProt ID: Q86SP6). B AlphaFold-generated model of human GPCR149
In GPCRs, these C-terminal regions were assigned as functional semi-foldons and inducible foldons (Uversky 2015a). These conformational changes complement the dynamical assembly of foldons represented by the GPCR’s central core. The functional unfoldons are exemplified in GPCRs’ interaction with arrestin in which the flexible cytosolic domain of GPCRs releases the C-terminal tail of arrestin which in turn exposes specific interaction regions that may trigger various downstream effectors in the cell (Jean-Charles et al. 2017). The conformational changes enhance the GPCR’s central core’s dynamical assembly of foldons. In the interaction of GPCRs with arrestin, the flexible cytosolic domain of GPCRs releases the C-terminal tail of arrestin, exposing particular interaction areas that may activate multiple downstream effectors in the cell (Jean-Charles et al. 2017).
Modes of regulation of the GPCR functionality
GPCRs transmit information by a pattern that requires agonist activation and subsequent connection to intracellular signaling partners (Hilger et al. 2018). The first of these partners to be detected were G proteins, which gave rise to the name GPCRs. G proteins are αβγ heterodimers made up of various combinations of subunit types. Coupling to GPCRs causes a GDP to GTP exchange in the subunit, which culminates in G protein activation (Oldham and Hamm 2008). Activated G proteins induce signaling by activating downstream effectors (Tuteja 2009). GPCRs can activate signaling via cytoplasmic partners such as arrestins (Gurevich and Gurevich 2019). There are four arrestin isoforms, including arrestin and four isoforms that are involved in signaling and receptor desensitization (Peterson and Luttrell 2017). Activated GPCRs are identified by G-protein coupled receptor kinases (GRKs), which leads to phosphorylation at particular sites on the receptor (Bünemann and Hosey 1999). These phosphorylated receptors are capable of interacting with arrestins. Furthermore, arrestin recruitment to the phosphorylated receptor may result in the initiation of an additional intracellular signal (Jean-Charles et al. 2017). However, the impact of this extra signal correlation on G protein activation is still being contested and needs to be researched further.
In general, GPCRs can interact with a wide range of cytoplasmic proteins, including scaffolding proteins that govern signal transmission (Bockaert et al. 2004). The GPCR family has around 800 members divided into five classes: glutamate, rhodopsin, frizzled/taste2, adhesion, and secretin (Fredriksson et al. 2003). Despite the fact that these five classes have distinct structures and purposes, they all have common structural components. GPCRs are made up of two distinct domains: extramembrane and intramembrane segments (Parrill and Bautista 2010). The membrane domain of all classes is made up of seven transmembrane helices labeled TM1–TM7. The extramembrane area changes depending on the GPCR class under consideration. Despite the transmembrane sequence’s highly conserved patterns, the extracellular and cytoplasmic portions of GPCRs are significantly more diverse in fundamental structure (Mirzadegan et al. 2003). As previously stated, the N and C termini of GPCRs, as well as the extracellular and intracellular loops that connect the TM segments, are located outside the membrane (Moro et al. 1999). Furthermore, these areas are engaged in GPCR functions and are modulated by PTMs (Moro et al. 1999). These areas exhibit characteristics of IDPs. However, we should note that their structural arrangement remains difficult to determine since truncation of the N and C termini, as well as fusion of protein partners in intracellular loops or the N terminus, prevented their identification in the majority of the measured crystal structures. Because of this dynamic nature, the application of modeling tools is likewise unclear.
Large amplitude motion of TM helices and unique preserved microswitches are among the alterations. This process, however, cannot be properly explained by the limited conformational states specified by either cryo-EM or X-ray data. Computational studies, which have revealed conformational flexibility for GPCRs, supplement investigations such as NMR and fluorescence. According to current thinking, receptors have complex conformational landscapes and adapt their structure to the environment via a series of conformational changes that are critical to the selective activation of downstream signaling partners (Heldin et al. 2016). The extramembrane areas are required for the central core architecture to be maintained. These areas have functional features as well as PTMs that influence the function. These areas exhibit some of the features of IDPs/IDPRs. The presence of repetitions in the area encoding the third intracellular loops of the dopamine receptor, for example, causes this region to have a high disorder index (Woods 2010). IDPs/IDPRs are the preferred locus for alternative splicing and PTMs in eukaryotes (Zhou et al. 2018; Romero et al. 2006; Iakoucheva et al. 2004; Pejaver et al. 2014).
GPCRs are susceptible to PTMs that largely impact their unstructured extramembranous domains, particularly the intracellular loops and the C-terminal domain (Jaakola et al. 2005). PTMs are essential for GPCR function and regulation (Patwardhan et al. 2021). GPCR PTMs include glycosylation, ubiquitination, phosphorylation, lipidation, and sulfation (Goth et al. 2020). Because the majority of alteration sites are deleted in crystallization tests to increase uniformity, the effect of PTMs on GPCR structures is unclear. Glycosylation, particularly N-glycosylation, has an effect on the N terminus of GPCRs and is associated with their folding and trafficking to the cell surface (Chen et al. 2010). As a result, it may have an effect on signaling via changing receptor numbers at the cell surface. Glycosylation might have an immediate impact on receptor activity (Goth et al. 2020). Pathogen activation of 2-adrenergic receptors, for example, has been shown to exert traction pressures on the receptor’s N terminus via N-glycan chains (Marullo et al. 2020). Ubiquitination has been shown to modulate GPCR signaling by degrading cell surface receptors (Dores and Trejo 2012). Ubiquitination can also influence signaling, as demonstrated by CXCR-4-dependent MAPK activation (Skieterska et al. 2017). The attachment of a palmitoyl chain to a conserved Cys residue at the receptor’s C terminus is the process by which GPCRs get lapidated (Naumenko and Ponimaskin 2018). This PTM is associated with anchoring this area to the membrane, resulting in an extra intracellular loop. As a result, lipidation is postulated to modify the geometrical properties of this area, which may affect internalization and signaling. In the scope of GPCR-dependent signal regulation, phosphorylation is one of the most important PTMs (Alfonzo-Méndez et al. 2016).
Analytical methods, particularly mass spectrometry (MS) and specialized antibodies, have been developed to detect residues that undergo phosphorylation under various circumstances (Chen et al. 2014). Phosphorylation happens as a result of agonist-mediated receptor activation and is associated with several kinase classes (PKA, PKC) as well as GRKs (Tobin 2008). These phosphorylate Ser and Thr amino acid residues on a receptor’s intracellular extramembrane regions (Cargnello and Roux 2011). Phosphorylation occurs specifically in the third intracellular loop and the C-terminal region, both of which are critical in signaling partner coupling activities (Tobin 2008). Phosphorylation of the C terminus may potentially influence the process by which it interacts with the membrane, as well as its structural and dynamic properties. Finally, extracellular loops (particularly ECL2) can influence ligand binding properties by acting as a lid that prevents compounds from dissociating from their binding location inside the transmembrane domain (Sykes et al. 2019). For the intracellular regions of GPCRs, the existence of a potential binding platform role for related partners may be important. First, the C terminus is involved in the assembly of a series of proteins (scaffolding proteins), particularly GPCR interacting proteins (GIPs), by recognition of tiny motifs in the C terminus of GPCRs (Walther and Ferguson 2015). As a result, massive protein–protein complexes important in signaling are formed. GPCR intracellular loops also interact with G proteins (Tuteja 2009). Contacts between the three intracellular loops and the G protein’s α and β subunits have been discovered in all investigated structures, despite the fact that the intracellular third loop is poorly resolved (Hollenstein et al. 2014). Interactions with arrestins are also mediated via intracellular loops and the C-terminal region of the receptors (Peterson and Luttrell 2017).
Nearly all pharmaceutical investigations targeting GPCRs have so far focused on ligand binding sites, either allosteric or orthosteric (Conn et al. 2009). Because various disorders have been associated to mutations in the extracellular and/or intracellular (unstructured sections) of GPCRs, these unstructured areas of GPCRs may be responsible for signaling system failure. We cannot rule out the possibility that this effect is related to the effects of mutations on the 3D structures of GPCRs by changing the arrangement of their transmembrane helices. Because these mutations are discovered in locations where protein–protein interactions occur during signaling processes, they may also affect the interaction routes with signaling partners. Extramembrane domains are less conserved than transmembrane domains (Cvicek et al. 2016). As a result, this might pave the way for targeted therapies altering protein–protein interactions in the signaling cascade. For addressing physiological activities, the peptudin technique was introduced, which is based on peptide mimicking to intracellular loops of GPCRs (Tressel et al. 2011). Except for this method, the areas beyond the membrane have been largely ignored in the search for remedies. Protein–protein interaction-based inhibitor design, which aims to examine the sequence and structural diversity of these areas in order to modulate signaling complexes, might possibly be a future cornerstone for treatment development.
PCRs and cognitive deficits in AD
AD patients exhibit decreased levels of acetylcholine in their brain (Ferreira-Vieira et al. 2016). As a result of this finding, acetylcholinesterase inhibitors were trialed as a potential treatment and were shown to reduce AD symptoms by decreasing acetylcholine breakdown, resulting in enhanced cholinergic neurotransmission and a minor improvement in cognitive function (Stanciu et al. 2019). Triggered excitotoxicity due to overstimulation of glutamatergic neurotransmission has also been connected to the etiology of AD (Dong et al. 2009).
An antagonist for N-methyl-D-aspartate (NMDA) receptor is memantine, which blocks the NMDA-orchestrated calcium influx into neurons, while simultaneously enhancing cognitive skills and shielding neurons from glutamate-induced neuronal death and excitotoxicity (Olivares et al. 2012). In addition to memantine, acetylcholinesterase inhibitors are symptomatic therapies that delay the deterioration in cognitive performance of patients with AD (Parsons et al. 2013).
The majority of excitatory neurotransmission in the brain is mediated by glutamate receptors, which are members of a large family of G-protein coupled receptors (GPCRs) (Niswender and Jeffrey Conn 2010). Glutamate neurotransmission is mediated by the metabotropic glutamate receptor (mGluR) family (Niswender and Conn, 2010). mGluR5 has been demonstrated to be involved in cognitive function and Aβ generation (Danysz and Parsons 2012). In an APPswelPSEN1E9 AD mouse model that overexpresses human APP with the Swedish mutation and human presenilin 1 lacking exon 9, genetic deletion of mGluR5 was demonstrated to reduce cognitive impairment and Aβ production (Poon et al. 2020). Furthermore, in the same mouse model, pharmacological inhibition of mGluR5 with the 3-[(2-methyl-1,3-thiazol-4yl)ethynyl]-pyridine (MTEP) antagonist has been demonstrated to alleviate cognitive impairments (Poon et al. 2020).
Excessive levels of serotonergic denervation were detected in the neocortex and hippocampus of patients diagnosed with AD, where 5-hydroxytryptamine (5-HT, serotonin), 5-HT1A, 5-HT2A, 5-HT4, as well as 5-HT6 receptor levels were lower in the hippocampus and/or prefrontal cortex (King et al. 2008). Activation of 5-HT2A and 5-HT4 receptors improves hippocampal-dependent learning and memory in mouse models either by G protein- or β-arrestin reliant activation of the extracellular signal-regulated kinase (ERK) (Barthet et al. 2007). Antagonism of the 5-HT1A and 5-HT5A receptors, on the other hand, has been found to lessen memory problems in a rat AD model, presumably by inhibiting the heterotrimeric Gαi protein signaling and activating protein kinase A (PKA), resulting in activation of the NMDA receptors (Zhou et al. 2019). Antagonists and agonists of 5-HT6 receptor improve memory and learning via separate processes (Woods et al. 2012).
It was found that the expression of brain-derived neurotrophic factor (BDNF) mRNA is increased upon activation of the activated 5-HT6 receptor (de Foubert et al. 2007). Furthermore, a connection between the Fyn-kinase-dependent activation of ERK1/2 and cognitive performance has been proposed (Chin et al. 2005). However, agonists of 5-HT6 receptor have been demonstrated to enhance acetylcholine and glutamate secretion in rodent brains, which has been proven to alleviate scopolamine- and MK-801-induced associative learning impairments (Ramírez 2013). These findings lend credence to the potential for targeted regulation of 5-HT receptors in therapy of AD.
Increased expression levels for adenosine receptors A1 and A2A (A1R and A2AR) were identified in the frontal cortex of the AD brain (Stockwell et al. 2017). The nonselective A1 inhibitor, caffeine, was reported to strengthen memory reinforcement in humans, whereas reduced Aβ levels led to improved cognitive performance observed in mouse models (Reneerkens et al. 2013). Likewise, the A2A antagonist SCH58261 and caffeine have also been demonstrated to protect against cognitive damage caused by Aβ (Batalha et al. 2016). Intriguingly, selective ablation of astrocytic A2ARs were shown to increase memory impairment in mutant AD mouse models via Gs-coupled signaling, while stimulation of Gi-coupled A1 and inhibition of PKA have been shown to alleviate long-term depression (Zhao et al. 2016).
Furthermore, one of the orphan GPCRs, GPR3, was found to control Aβ production and cognitive performance in vivo (Watkins and Orlandi 2020). It has been shown that genetic deletion of GPR3 improves learning and memory problems in mice, as well as diminishes amyloid pathology (Huang et al. 2015). GPR3 levels are higher in AD brains (Huang et al. 2015). Regardless of the Gs-coupling, the impact of GPR3 on amyloid pathology involves recruiting β-arrestin (Thathiah et al. 2013). Approaches for the treatment of cognitive impairments can be developed with GPCRs such as 5-HT and adenosine receptors, which are frequently found in the neurochemical pathways affected in AD.
GPCRs and neuropsychiatric symptoms in AD
In the hippocampus of AD patients co-diagnosed with major depression, the density of amyloid plaques is reported to be higher in comparison with AD patients without depression (Rapp et al. 2006). GPCRs, designated CRHR1 and CRH2 (named in the reference to their corticotropin secretions), have been associated with depression (Bu et al. 2019). Furthermore, genetic deletion of CRHR1 has been reported to reduce amyloid pathology in mouse models of AD (Campbell et al. 2015). Pharmacological research on the Tg2576 AD mouse model, which carries Swedish mutation and exhibits overexpression of human APP, using antalarmin, a CRHR1 antagonist, revealed an increase in Aβ production involving the Gs-signaling pathway, whereas pre-treatment with antalarmin was ineffective, as no increase in Aβ production has been observed (Luo et al. 2012). Furthermore, in vitro research utilizing other CRHR1 antagonists including NBI-27914, astressin, but also antalarmin with cell-free γ-secretase activity assays demonstrated regulated Aβ production in the absence of CRHR1, which indicates a CRHR1-independent modulatory effect on the activity of γ-secretase (Futch et al. 2017). In vivo studies suggested that using selective antagonists that are specific for CRHR1 could help with the depressive symptoms in AD (Zorrilla and Koob 2010). Furthermore, wild-type mouse models treated with antalarmin showed lower depression, whereby genetically induced CRHR2 impairments increased depression (Bale and Vale 2003).
GPCRs and cognitive deficits in PD
It is known that two dopamine receptors D1R and D2R, which are highly expressed in many regions of the brain such as striatum, nucleus accumbens (NAc), and substantia nigra, are at higher levels in PD patients, and this is linked to the occurrence of dopamine denervation hypersensitivity (Yang et al. 2020). Studies in which a partial D1R agonist, SKF38393, without the D2R agonist quinpirole, was infused into the NAc of wild-type mouse models showed an increase in their visuospatial ability, whereas treatment with a D1R antagonist, SCH23390, resulted in a decrease in the visuospatial ability (Ahmadian et al. 2020). Furthermore, deactivation of D2R or the use of a D2R antagonist, sulpiride, in the NAc has shown impaired attention (Mehta et al. 2004). Taken together, these studies provide evidence for the role of D1 and D2 receptors in the visuospatial and attentional impairments in PD.
Motor symptoms of PD reflect the dopamine deficit in the basal ganglia (Magrinelli et al. 2016). At present, the most effective pharmaceutical-based medication treatment for PD symptoms is based on replenishing dopamine with a chemical precursor of dopamine, namely, levodopa (L-DOPA) (Fahn 2015). However, the decline in clinical efficacy as a result of long-term treatment with L-DOPA necessitates the use of higher doses, resulting in undesirable effects on motor activities (Poewe et al. 2010). Several D1R and D2R agonists, such as bromocroptine, lisuride, and rotigoin, have been developed to overcome these side effects, and, indeed, the agonists outperform L-DOPA in terms of pharmacodynamic and pharmacokinetic characteristics either being utilized as monotherapy or as supplementary therapy to L-DOPA medication (De Leeuw Van Weenen et al. 2010; Bonuccelli et al. 2009). Nonetheless, these agonists show a long-term reduction in their efficacy as well.
Non-dopaminergic approaches are also alternatives for the treatment of the PD motor symptoms, such as istradefylline, an A2AR antagonist licensed in Japan for use in combination with L-DOPA (Fox 2013). A balance between the dopaminergic and cholinergic systems is critical. It is known that the increase in dopamine in the striatum triggers the excessive release of acetylcholine by overactivation of cholinergic interneurons (Aosaki et al. 2010).
Anticholinergics that are specific for the muscarinic receptor subtype 1 (M1R), such as trihexyphenidyl and biperiden, are useful in suppressing tremors (Katzenschlager et al. 2002). Anticholinergics demonstrate only minor effects on bradykinesia and stiffness, indicating that M1Rs play a function in tremor (Marjama-Lyons and Koller 2000). In studies with mutant mouse models in which muscarinic acetylcholine receptor 4 (M4R) is deleted, the observed decrease in catalepsy indicates the role of M4R in motor symptoms (Fink-Jensen et al. 2011). Nonetheless, dopamine agonists may affect cognition in PD and hence complicate therapy choices in people with dementia who have Parkinson’s disease (PDD, Parkinson’s disease dementia).
Although the knowledge of orphan GPCRs (oGPCRs), a term used for GPCRs whose ligands currently have not been detected, is quite limited, they are of intense interest as therapeutic targets (Watkins and Orlandi 2020). There are studies reporting that oGPCRs have key roles in AD and schizophrenia (Bakker and Leurs 2003). The expression map of the mouse brain revealed the involvement of 78 oGPCRs in a range of important mechanisms ranging from mood regulation to cognitive activities (Ehrlich et al. 2018). GPR37 and GPR55 are two orphan GPCRs that are involved in motor coordination, as indicated by studies using drug-associated Parkinsonian tremor models, where the genetic deletion of GPR37 resulted in attenuation of tremulous jaw movements (TJMs) induced by pilocarpine, a non-selective muscarinic cholinergic receptor (Gandía et al. 2015). Similarly, a A2AR antagonist, SCH58261, reduces TJMs arising from pilocarpine, which, however, was not detected in GPR37-lacking mouse (Collins et al. 2010). Overall, these findings suggest that novel techniques targeting GPR37 and GPR55 may offer alternative therapeutics for PD. The GPCRs mentioned here have the potential to be used in the discovery of disease-modifying medicines. Given the multiplicity of GPCRs implicated in disease development and the complexity of the corresponding disease pathways, combinatorial treatment methods targeting several GPCRs may be effective in slowing and maybe even halting the pathological processes (Table 2).
Table 2.
Some of the GPCR-targeting drugs utilized in various neurodegenerative diseases
| Agent | Target | Receptor family | Indication |
|---|---|---|---|
| Erenumab (Markham 2018) | CALCRL | Calcitocin | Migraine |
| Ubrogepant (Scott 2020) | CALCRL | Calcitocin | Migraine |
| Eptinezumab (Dhillon 2020) | CGRP | Calcitocin | Migraine |
| Cannabidivarin (Orphan, 2017) (Morano et al. 2016) | GPR119 | GPR119 | MS and epilepsy |
| Cannabidiol (Devinsky et al. 2014) | GPR55 | GPR55 | Epilepsy |
| Fingolimod (Pelletier and Hafler 2012) | Sphingosine 1-phospate (S1P) | Sphingosine 1-phosphate (S1P) receptor | MS |
| Leuprolide (Butler et al. 2021) | AchEI | Cholinergic | Synergies AChEI activities |
| Vortioxetine (Katona and Katona 2014) | 5-HT3, 5-HT7, 5-HT1D | Serotonin | Major depressive disorder |
| Haloperidol (Ulrich et al. 1998) | D2R | Dopamine | Schizophrenia |
| VCE-003.2 (orphan) (Aguareles et al. 2019) | CB2R | Cannabinoid | HD |
| DMXBA (GTS-21) (Kem et al. 2006) | Α7nAChR | Cholinergic | Schizophrenia |
| Galantamine (Scott and Goa 2000) | AchEI | Cholinergic | AD |
| Rivastigmine (Spencer and Noble 1998) | AchEI | Cholinergic | AD |
As mentioned, despite our limited knowledge of oGPCRS, among those identified ones, GPRc5c, GPR17, GPR27, GPR37, GPR39, GPR63, GPR85, GPR88, GPR123 (Adgra1), GPR125 (Adgra3), GPR153, and GPR176 have all been shown to be strongly expressed in mouse brain prefrontal cortex (PFC), which is important in learning and cognition (Ehrlich et al. 2018). Furthermore, GPR88, GPR123, GPR149, and GPR151 were shown to be strongly expressed in both human and mouse brains, although our understanding regarding normal and pathological activities of these oGPCRs is limited with the exception of GPR88 (Ehrlich et al. 2018).
GPR3, which is highly and consistently expressed in 11 distinct regions of the human brain, is associated with the emergence of AD via a mechanism based on the recruitment of β-arrestin 2 by GPR3 (Thathiah et al. 2013), through independent G-protein coupling, which results in boosted activity of γ-secretase that causes increased cleavage of APP, and thereby speeds up Aβ generation and accumulation (Laun et al. 2019). Furthermore, studies using AD mouse models with the GPR3 deletion have shown improvement in cognitive function (Huang et al. 2015).
With regard to memory, another orphan GPCR, GPCR158, has been shown to improve memory by binding osteocalcin (OC), a non-collagenous protein found in large amounts in bone, which is involved in many processes from the regulation of glucose and lipid metabolisms to testosterone synthesis (Patel et al. 2013). After crossing the blood–brain barrier (BBB), OC contributes to serotonin synthesis in the brainstem by binding to neurons in the dorsal and median raphe nuclei, further promoting dopamine synthesis in the midbrain by connecting to neurons in the ventral tegmental region. Furthermore, OC binds to neurons in the CA3 region of the hippocampus.
Turning back to GPR158, it has been reported that OC binds to GPR158, which modulates osteocalcin transduction, which, in turn, regulates the expression of RbAp48, a histone-binding protein that is a determinant of memory disorders due to aging, in hippocampal formation (Kosmidis et al. 2018). The signaling pathway of OC/GPR158/RbAp48 was reported to act on the dentate gyrus/A3c and CA3a regions in the brain to regulate contextual functions. It has been demonstrated that inhibition of RbAp48 blocks the cognition supporting functions of OC and results deficiencies in discrimination memory (Kosmidis et al. 2018). Indeed, transduction of OC by GPR158 regulates IP3 and BDNF in CA3 neurons, which, in turn, contributes to enhancement of memory recall and formation. However, its overexpression in the PFC has been shown to be correlated with depressive symptoms in patients clinically diagnosed with major depression and also in animal models of stress-dependent depression (Ménard et al. 2016). The underlying mechanism beyond GPR158-induced depression might be related to its capability of suppressing cAMP synthesis and thereby decreasing the activity of superficial cortical neurons (Sutton et al. 2018).
With regard to the orphan receptor GPCR52, studies in humans and animals have revealed enhanced expression levels in the striatum (Nishiyama et al. 2017). qRT-PCR-based studies in mice demonstrated expression of GPR52 in regions that regulate including the medial prefrontal complex, habenula, and amygdala (Nishiyama et al. 2017). An important finding of this work was the spatially separated co-expression of GPR52, with dopamine receptor D1 in medial PFC, and with dopamine receptor D2 in basal ganglia, which led to the suggestion that GPR52 activation of Gαs might boost the production of cAMP that mitigates the outcomes of Gαi activation by D2R. Based on this, numerous ligands were developed for implementation in pharmaceutical-based strategies for the treatment of schizophrenia (Nishiyama et al. 2017). In addition, histology-based studies have indicated that GPR52 promotes and regulates the Crelox system and is also associated with glutaminergic and dopaminergic transmission, which indicates a potential role in the regulation of cognitive skills and emotional behavior (Komatsu et al. 2014). Aggregation of the HTT protein, the product of the Huntingtin gene (HTT), has been suggested to trigger cytotoxicity that results in the emergence of Huntington’s disease (HD) (Finkbeiner 2011). Deactivation of GPR52 has been shown to decrease HTT protein levels in the striatum via stimulation of HTT clearance with concomitant suppression of HD phenotypes (Song et al. 2018). These findings indicate that striatal degradation of mutant HTT necessitates higher cAMP levels mediated by GPR52. In a recent study conducted by Wang et al. in 2020, several GPR52 agonists have been designed and synthesized, and were evaluated under consideration for use in the generation of structure–activity relationships ranked on pharmacokinetic properties (Wang et al. 2020). Molecules with greater cAMP signaling enhancing features were chosen for additional investigations, of which the so-called compound 12c reported to have better ClogP and PK properties was further investigated in vivo (Wang et al. 2020). These additional efforts have shown the great capability of compound 12c in inhibiting hyperactivity induced by amphetamine in mouse models (Wang et al. 2020).
Allosteric ligands typically modulate GPCR activity by binding to their endogenous ligand-binding sites (Yanamala and Klein-Seetharaman 2010). Allosteric ligands allow for the possible manipulation of GPCR function for possible therapeutic effects (Wang et al. 2009). However, their complicated functions make novel medication screening and development difficult. Several research investigations have studied the mechanism of interactions between GPCRs and their allosteric ligands with the intention of biasing signaling for potential advantage in drug development (Wacker et al. 2017).
As opposed to “allosteric ligands”, there are numerous types of “allosteric modulators” that include both small and large compounds, proteins, and ions (Klein et al. 2013). These different types might be useful therapeutic products if they are evolved into low molecular weight, non-peptidic compounds that can penetrate the blood–brain barrier (Bartfai and Wang 2013). Based on receptor signaling, allosteric modulators are classified into two types: positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs) (Klein et al. 2013). Unlike ligands, they do not activate or inhibit receptors. They attach to a location that is unique and extremely varied from the active site rather than the typical binding site. As a result, PAMs and NAMs may be able to decrease adverse effects and manage the strength and success of a medication response (Sulli et al. 2018).
Recent advances in the study of neurodegenerative diseases have revealed a possible disease-modifying therapy based on the regulation of allosterism (Nickols and Jeffrey Conn 2014). The mAChR subtypes M1 and M4 are critical targets for schizophrenia, AD, and PD (Foster et al. 2014). Although the M1/M4 agonist xanomeline enhanced cognitive performance in a phase III clinical study for schizophrenia, it is linked with gastrointestinal side effects; therefore a PAM might be a viable and safe option (Montani et al. 2021). Several pharmacological research studies in animal models have revealed active M1 PAMs, but the safety margin remains to be validated (Moran et al. 2019). M1 PAM MK-7622 was discontinued following a phase IIa/IIb trial (Voss et al. 2018). Despite this, various selected M4 PAMs, described as VU0152099, VU0152100, VU0467485, and LY2033298, have been tested in preclinical models of schizophrenia (Brady et al. 2008). A new crystallization method of M1 and M4 receptors gave a structural foundation for comprehending PAMs, allowing their possible development as for AD (Thal et al. 2016). Furthermore, in a preclinical investigation for AD, allosteric modulators of numerous mGluR members demonstrated symptomatic therapeutic promise (Dube et al. 2014).
To maximize the chance of success while developing a GPCR target for a CNS indication, many critical characteristics must be met:
-
(i)
The most evident is that the target is directly related to the disease. In rare circumstances, the aimed protein has a gain in functionality or a loss-mutation that results in a disease phenotype, as seen in a patient with mutant NaV1.7 sodium channel, which caused hypersensitivity or insensitivity to pain signals.
-
(ii)
That the expression of the targeted protein takes place in the brain and is connected to the disease via regulation at the circuit or cellular level.
-
(iii)
Druggability of the target protein to affect therapeutic response.
Whereas types 1 and 3 GPCRs exhibit excellent druggability with small compounds, type 2 GPCRs have been shown to be less effective (Hauser et al. 2017). Antibodies are relatively difficult for use as ligands for GPCR targets; however, some progress has lately been observed (Riemekasten et al. 2020). Because less than 0.1% of therapeutic antibodies can cross the blood–brain barrier, distribution to deep brain areas is a substantial issue, highlighting the need for specialized delivery technologies (Kouhi et al. 2021).
The traditional approach to small molecule drug development involves screening vast libraries of drug-like compounds affecting the protein target of interest (Hughes et al. 2011). In general, when employing automation in trials, selective antagonists present a considerably simpler discovery route than agonists and allosteric modulators (Korczynska et al. 2018). GPCR drug development is undergoing a transition due to the rising usage of small molecule allosteric modulators in customized ligand design and useful pharmacological complexity selection.
Mu opiate receptor-based ligands can provide therapeutically desired results if they are G protein biased, but they can also produce unwanted and perhaps deadly results if they are arrestin biased (Narayan et al. 2021). All such ligands can exhibit a partial bias, and assessment of this relative bias is reliant on the parameters of assays that may differ among laboratories (Luttrell et al. 2018). It remains to be seen if orthosteric and allosteric biased signaling will be therapeutically beneficial. The topic of how allosteric and orthosteric drugs could impact intracellular signaling in distinct ways remains unanswered. Rational drug design, in conjunction with ML, would aid the development of either partial or full NAMs or PAMs, whether or not they exhibit an inherently agonistic and antagonistic action.
Neuropeptides may potentially be employed as therapeutic targets for neurodegenerative diseases (Catalani et al. 2017). Neuropeptides are compounds that have the ability to influence neuronal function (Brain and Cox 2006). Neuropeptides play a function in pain perception and inflammation (Carniglia et al. 2017). Multiple neuropeptides, such as calcitonin gene-related peptide (CGRP), neuropeptide Y (NPY), somatostatin (SST), proopiomelanocortin (POMC), and vasoactive intestinal peptide (VIP), as well as cortistatin, have been linked to neuroinflammation (Carniglia et al. 2017; Sato 1998). Furthermore, one of the members of the nicotinamide adenine dinucleotide (NAD)–dependent deacetylase family, silent information regulator 1 (SIRT1), is linked to AD development, as indicated by the research, which reported SIRT1-mediated reduction of a serine/threonine Rho kinase, namely ROCK1, and regulatory effects of SIRT1 on senile plaques and Aβ oligomers via stimulation of APP synthesis in a nonamyloidogenic manner (Zhang et al. 2011).
Natural derivatives such as oxyresveratrol, resveratrol, and 2,3.4′,5-tetrahydroxystilbene-2-O-b-D-glucoside (TSG) have been shown to activate or increase SIRT1 and reduce the risk of neurodegenerative diseases (Karaman et al. 2020). Moreover, it was reported that upregulation of SIRT1 reduces the levels of accumulated amyloid plaque and improved behavioral patterns via deacetylation of retinoic acid receptor β (RARβ), which is a transcriptional stimulator of ADAM10 (disintegrin and metalloproteinase-domain-containing-protein 10) that is in charge of non-amyloidogenic processing of APP that reduces the production of toxic Aβ42 forms (Manjula et al. 2020). Expression of SIRT has been shown to deacetylate tau proteins, reduce NT formation, and decrease tau-induced pathologies (Min et al. 2018). In addition to the generation by γ-aminobutyric acid (GABA)-ergic interneurons in the hippocampus, NPY is found in the hypothalamus, thalamus, brainstem, cerebellum, and cerebral cortex, where it is involved in memory, learning, endocrine secretions, and nutrition (Li et al. 2019).
According to research, NPY expression decreases with the advancement of PD, AD, and HD (Li et al. 2019). By lowering Ca2+ influx in the presynaptic nerve terminal via the protein kinase Aβ and p38 pathways, NPY may buffer neuronal cell death caused by excessive GluR activation (Ambrósio et al. 2000). Through the impact of Y1 receptors on neuroblasts, NPY-dependent neurogenesis was found in the caudal subventricular zone (cSVZ), subcallosal zone, and dentate gyrus (Thiriet et al. 2011). In the substantia nigra of 6-OHDA-lesioned rats utilized as a PD model, NPY treatment protected neurons against microglia-induced inflammation (Parra et al. 2020). Furthermore, ghrelin, a 28 amino acid long peptide, can increase pituitary hormone (GH) secretion and is extensively expressed in the hypothalamus (Abdalla 2015). The ghrelin receptor (GHSR) forms a heterodimer with the D1R and D2R and may influence G-protein coupling and ligand binding (Damian et al. 2018). Ghrelin has been shown in studies to have neuroprotective action against neurodegeneration (Ibrahim and Mohamed 2015). Furthermore, leptin, a cytokine-like 167 amino acid long peptide, affects the hypothalamic synthesis of NYP and POMC and may modulate neuronal excitability, neurogenesis, and synaptogenesis (Cowley et al. 2001). The functions of numerous neuropeptides in neurodegeneration and cognitive enhancement, on the other hand, are complicated and not entirely understood.
Neuropeptides including pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal peptide that were shown to inhibit mitochondrial apoptosis and act on GPCRs involved in neuroprotection could be alternative therapeutics for targeting GPCRs involved in neuroprotection against oxidative stress and inflammation (Gábriel et al. 2019). Overall, evidence proposes that targeting either single or multiple GPCRs could potentially intervene against neurodegeneration (Azam et al. 2020). Neuropeptides and allosteric targets could be the next frontier in the development of treatments for neurodegenerative disorders. Moreover, the potential of natural active compounds in terms of acting on GPCRs needs to be studied. LRR, PYD domains containing protein 3 and NACHT as well as glutamate receptors have potential.
Metal trafficking and GPCRs
GPCRs are essential for cellular activity because they serve as a biochemical and physical gateway for many regulatory activities. GPCRs are required for the physiological function of about 80% of neurotransmitters and hormones (Rosenbaum et al. 2009). The receptor’s manner of regulation is provided via ligand binding, activation, internalization, recycling, or destruction (Goh and Sorkin 2013). Ligand binding to the extracellular domains of the receptor causes conformational changes that cause an intercellular reaction(s) (Lemmon and Schlessinger 2010). The selectivity and specificity of the stimuli on the receptor determine which signaling cascade is triggered. Metal binding or amino acid oxidation can be used to modify signaling by modifying ligand or receptor structures (Christofides et al. 2018). Despite the fact that GPCR structures and activities are becoming more understood, the cell biology underlying receptor deactivation, desensitization, degradation, or recycling remain unknown and need to be further explored because these processes might alter receptor signaling and cell function.
Metal binding to receptors is a well-known phenomenon (Zou et al. 2022). Zn2+ utilized by glutamatergic neurons, for example, is thought to operate as a neuromodulator by binding to receptors such as β-adrenoreceptors and dopamine and influencing their activity (Köles et al. 2016). The κ-opioid and tachykinin receptors contain a Zn2+ binding site, and zinc was shown to behave as an antagonist (Satała et al. 2018). Although a native di-histidine Zn2+ site in the tachykinin 3 receptor (NK3R) was discovered, Zn2+ binding to NK3R behaved as an agonist—most likely by creating a conformational shift in the receptor structure (Thakar et al. 2012). Copper (Cu) is excreted by hippocampus neurons and can interact with a variety of receptors, affecting synaptic transmission (Gaier et al. 2013). The Menkes ATPase transports copper into synaptic vesicles, where it is probably released as Cu+, where it can bind to the S atoms of Met and Cys (Otoikhian et al. 2012). The copper complex can resemble a disulfide bond, and it is known that the redox state of Cys influences receptor activation. It is unknown if copper that has been coupled to a receptor is endocytosed.
Another method a metal ion might interact with a receptor is by first binding to the cognate ligand (Ma et al. 2009). Metal ions bind to peptide neurotransmitters and neurohormones (Hui 2007). Metal interaction with these peptides can change their structural characteristics and have unpredictability in receptor activation. Cu binding to GnRH demonstrates these effects because it changes intracellular signaling cascades generated by the receptor (Pathan and Williams 2012). Cu binding to neurokinin B (NKB) occurs at the N termini (Russino et al. 2013), which may exhibit IDPR properties. The extracellular domain of NKB’s cognate receptor, NK3R, comprises a di-His Zn-binding site. NK3R may have a Cu affinity and may bind to Cu (Russino et al. 2013). It is uncertain if Cu-bound NKB can transfer Cu to NK3R, and more studies are needed in this area.
Intrinsic disorder and drug design
Because IDPs and IDPRs are discovered practically at all stages of a disease, ranging from GPCRs to a wide spectrum of specific proteins, IDPs/IDPRs are appealing therapeutic options. Finding compounds that bind to target proteins does not mean that these proteins are fully druggable because the molecule must also be able to access the target protein in the organism. Lipinski established five criteria for assessing bioavailability, namely “rule of five,” which contains constraints on the possible lead compound’s molecular weight (<500 Da), number of hydrogen bond acceptors (<10), number of hydrogen bond donors (<5), and octanol–water partition coefficient (<5) (Benet et al. 2016). These requirements should be paired with the basic need for small molecule drug design, which is the ability to create robust contacts with the protein of interest in order to overcome entropy loss during binding.
IDPs/IDPRs are particularly difficult targets in this context because their flexibility suggests that they tend to interact with small molecules weaker in comparison with structured proteins, whereas the corresponding loss of entropy is larger (Uversky 2013). As previously stated, the great number and unique activities of IDPs/IDPRs in protein–protein interactions make them attractive therapeutic targets (Uversky 2013b). Efforts to produce small molecule medications, which disrupt interactions between proteins, on the other hand, have proven to be generally difficult. Protein–protein interactions contain complicated binding surfaces, discontinuous epitopes or several continuous epitopes, no pockets, huge interaction areas, and energetics, which are not dispersed equally across a wide interaction area, but rather concentrated at smaller areas termed hotspots (Uversky 2013b).
It is worth noting that there are attempts to develop an IDP ensemble database (Varadi et al. 2014). However, dealing with the corresponding conformational ensembles is problematic since it is not clear whether stationary entities comprising an ensemble persist, and, if so then, how long they should persist. Furthermore, IDPs/IDPRs might undergo liquid–liquid phase separation (LLPS) and the resulting liquid droplets might affect the bioavailability of treatment candidates (Uversky 2017). Therefore, while developing medications, the potential for undergoing LLPS must be considered as well. As previously stated, allostery must be considered as well. Furthermore, a thorough knowledge of the dynamics of an IDP/IDPR and drug target complex connection and dissociation can give critical insights in understanding of the molecular mode of action and assist in strategic planning in drug development. Chemical kinetic tests are commonly carried out using amyloidogenic IDPs to track their accumulation mechanisms and the effect of small molecules on the pace of fibrillation processes (Giorgetti et al. 2018). These might be critical for examining structure–activity relationships (SAR), which tie the chemical composition to its effect on a biological target. The “structure-kinetic-activity relationship” (SKAR) has been proposed as a more broad method for medication discovery in protein misfolding diseases (Chia et al. 2018). SKAR is built on the rates of change of distinct monomers, oligomers, and fibrils, rather than a simple generic description of the overall rate of fibril production.
Furthermore, the binding of a ligand to a protein is driven by entropic and enthalpic contributions, where the enthalpic contribution is defined by a wide range of interatomic forces such as van der Waals interactions, electrostatic interactions, as well as hydrogen-bonding-based interactions (Du et al. 2016). On the other hand, the changes in the number of conformations accessible to the whole system, comprising the protein, ligand, and solvent molecules, are expressed by the entropic contribution (Du et al. 2016). For most of the situations, enthalpic factors and solvent-driven entropic impacts, for example hydrophobic effects, promote ligand binding to structured proteins. In the case of IDPs/IDPRs, however, advantageous entropic involvements from the biological targets and the solvent may constitute an alternate drug development technique (Ambadipudi and Zweckstetter 2016). IDPs/IDPRs might be responsive to bind through “entropic expansion,” which implies that the ligand enhances the amount of conformations available to the IDP/IDPR, as proven by small compounds targeting disordered monomeric c-Myc (Heller et al. 2017).
Traditional docking techniques that target proteins folded into a specific 3D structure are widely used. Due to the highly dynamic structural ensembles of IDPs/IDPRs, direct search for the cavities capable of ligand binding is a difficult process. Yu et al. offered a standard two-step strategy, in which MD simulations of an IDP is first performed and followed by screening a chemical library using multiconformational docking (Yu et al. 2016). Another computational rational drug design technique is based on the “combined pharmacophore hypothesis,” which aims to engage as much of the dimerization surface between two proteins as feasible (Tsigelny et al. 2017). This strategy differs from the traditional approach of targeting protein–protein interactions, which relies on a hotspot to lead drug development efforts. To use this method, one must first describe the “parental” dimerization pharmacophore, which is a model of the whole range of interactions that would optimize the impact of the protein and medication (Mustata et al. 2009). Many “daughter” pharmacophores that incorporate subgroups of all those interactions must be defined. The pharmaceutical candidates are then sorted based on their capacity to contact the greatest number of “daughter” pharmacophores. This method produced the leading chemical SKOG12, which was demonstrated to interact with DNA-binding of the transcription factor OLIG12 (Inukai et al. 2017). Studies in vivo on glioblastoma showed that the computationally developed SKOG12 was able to inhibit tumor outgrowth (Sestito et al. 2018). Although this method was designed to address transcription factor protein–protein dimerization interfaces, it is also applicable to other vast interfaces, for which no pockets can be defined, such as those seen in IDPs/IDPRs and their complexes. This method necessitates comprehensive structural understanding of the interaction interface. The “joint pharmacophore model” (JPS) is a promising strategy that uses a ML algorithm to find the best compounds that fit any required criteria (Ranu and Singh 2011). This strategy may be appropriate for unbound IDPs/IDPRs. JPS produced a high-scoring chemical named AC0107, which was further investigated using MS and AFM measurements. AC0107 was shown to destabilize fibrils and counteract Aβ accumulation in a later study.
Conclusion
Here, we discussed the biological and pathological roles of various IDPs and IDPRs in neurodegenerative diseases, such as AD, PD, ALS, FTD, SMA, HD, Charcot–Marie–Tooth disease, and mitochondrial myopathy. The structures of GPCRs have been studied using traditional models, but these are IDPRs based on recent investigations. Furthermore, we discuss the importance of CHCHD2 and CHCHD10 proteins and their pathological variants in neurodegenerative diseases. Drug design studies using IDPs/IDPRs as models on these proteins in neurodegenerative diseases are discussed as well.
Acknowledgements
We are extremely thankful to the Biophysical Reviews Editorial Board for additional helpful comments that helped to strengthen our manuscript.
Abbreviations
- Aβ
Amyloid-β
- αS
α-Synuclein
- CHCHD10
Coiled-coil-helix-coiled-coil-helix domain containing protein 10, mitochondrial
- CHCHD2
Coiled-coil-helix-coiled-coil-helix domain containing protein 2, mitochondrial
- GPCR
G-Protein coupled receptor
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- ALS
Amyotrophic lateral sclerosis
- FTD
Frontotemporal dementia
- SMA
Spinal muscular atrophy
- SMAJ
Jokela type spinal muscular atrophy
- IDP
Intrinsically disordered protein
- IDPR
Intrinsically disordered protein region
- NMR
Nuclear magnetic resonance
- PRE
Paramagnetic relaxation enhancement
- PGF
Pulse-field gradient
- MD
Molecular dynamics
- ML
Machine learning
- CNS
Central nervous system
- SNCA
Synuclein alpha
- NAC
Non-amyloid-β component
- PTM
Posttranslational modification
- MoRF
Molecular recognition feature
- PPIN
Protein–protein interaction network
- STRING
Search Tool for the Retrieval of Interacting Genes/Proteins
- SLAS
Sodium lauroyl sarcosinate
- NFT
Neurofibrillary tangle
- APP
Amyloid precursor protein
- MAPT
Microtubule associated protein tau
- UMN
Upper motor neuron
- LMN
Lower motor neuron
- LOSMoN
Late-onset spinal motor neuronopathy
- SLiM
Short linear motif
- TM
Transmembrane
- MAPK
Mitogen-activated protein kinase
- MS
Mass spectrometry
- PKA
Protein kinase A
- PKC
Protein kinase C
- GRK
G protein-coupled receptor kinase
- Ser
Serine
- Thr
Threonine
- ECL2
Extracellular loop 2
- GIP
GPCR interacting protein
- NMDA
N-Methyl-D-aspartate
- mGluR
Metabotropic glutamate receptor
- MTEP
3-[(2-Methyl-1,3-thiazol-4yl)ethynyl]-pyridine
- 5-HT
5-Hydroxytryptamine
- ERK
Extracellular signal-regulated kinase
- BDNF
Brain-derived neurotrophic factor
- CRHR1
Corticotropin releasing hormone receptor 1
- CRH
Corticotropin releasing hormone
- DRD1
Dopamine receptor D1
- A2AR
A2A receptor
- M1R
Muscarinic receptor subtype 1
- M4R
Muscarinic acetylcholine receptor 4
- PDD
Parkinson’s disease dementia
- oGPCR
Orphan GPCR
- TJM
Tremulous jaw movement
- CALCRL
Calcitocin receptor-like
- CGRP
Calcitocin gene-related peptide
- S1P
Sphingosine 1-phospate
- AChEI
Acetylcholinesterase inhibitors
- CB2R
Cannabinoid 2 receptor
- OC
Osteocalcin
- IP3
Inositol triphosphate
- BDNF
Brain-derived neurotrophic factor
- CA3
Carbonic anhydrase 3
- PFC
Prefrontal cortex
- cAMP
Cyclic adenosine 3′,5′-monophosphate
- HTT
Huntingtin
- HD
Huntington’s disease
- PAM
Positive allosteric modulator
- NAM
Negative allosteric modulator
- mAChR
Muscarinic acetylcholine receptor
- mGluR
Metabotropic glutamate receptor
- NPY
Neuropeptide Y
- SST
Somatostatin
- POMC
Proopiomelanocortin
- VIP
Vasoactive intestinal peptide
- NAD
Nicotinamide adenine dinucleotide
- SIRT1
Silent information regulator 1
- TSG
2,3.4′,5-Tetrahydroxystilbene-2-O-b-D-glucoside
- RARβ
Retinoic acid receptor β
- ADAM10
Disintegrin and metalloproteinase domain-containing protein 10
- Y1
Neuropeptide Y
- 6-OHDA
6-Hydroxydopamine
- GH
Growth hormone
- GHSR
Growth hormone secretagogue receptor
- POMC
Proopiomelanocortin
- LRR
Leucine rich repeat
- PYD
Pyrin domain
- NACHT
Neuronal apoptosis inhibitor protein
- NK3R
Neurokinin 3 receptor
- GnRH
Gonadotropin-releasing hormone
- NKB
Neurokinin B
- LLPS
Liquid–liquid phase separation
- SAR
Structure–activity relationship
- SKAR
Structure–kinetic activity relationship
- AFM
Atomic force microscopy
- JPS
Joint pharmacophore model
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguareles J, Paraíso-Luna J, Palomares B, Bajo-Grañeras R, Navarrete C, Ruiz-Calvo A, García-Rincón D, et al. Oral administration of the cannabigerol derivative VCE-003.2 promotes subventricular zone neurogenesis and protects against mutant huntingtin-ınduced neurodegeneration. Transl Neurodegen. 2019;8(1):9. doi: 10.1186/s40035-019-0148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian SM, Ghahremani P, Alaei H. Microinjection of a dopamine-D1 receptor agonist into the ventral tegmental area reverses the blocked expression of morphine conditioned place preference by N-methyl-D-aspartate receptor antagonist. Adv Biomed Res. 2020;9:54. doi: 10.4103/abr.abr_11_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajroud-Driss S, Fecto F, Ajroud K, Lalani I, Calvo SE, Mootha VK, Deng H-X, et al. Mutation in the novel nuclear-encoded mitochondrial protein CHCHD10 in a family with autosomal dominant mitochondrial myopathy. Neurogenetics. 2015;16(1):1–9. doi: 10.1007/s10048-014-0421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbayrak, Ibrahim Y, Caglayan SI, Ozcan Z, Uversky VN, Coskuner-Weber O. Current challenges and limitations in the studies of ıntrinsically disordered proteins in neurodegenerative diseases by computer simulations. Curr Alzheimer Res. 2020;17(9):805–818. doi: 10.2174/1567205017666201109094908. [DOI] [PubMed] [Google Scholar]
- Alfonzo-Méndez MA, Alcántara-Hernández R, Adolfo García-Sáinz J. Novel structural approaches to study GPCR regulation. Int J Mol Sci. 2016;18(1):E27. doi: 10.3390/ijms18010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alici H, Uversky VN, Kang DE, Woo JA, Coskuner-Weber O. Structures of the wild-type and S59L mutant CHCHD10 proteins ımportant in amyotrophic lateral sclerosis-frontotemporal dementia. ACS Chem Neurosci. 2022;13(8):1273–1280. doi: 10.1021/acschemneuro.2c00011. [DOI] [PubMed] [Google Scholar]
- Alquezar C, Arya S, Kao AW. Tau post-translational modifications: dynamic transformers of tau function, degradation, and aggregation. Front Neurol. 2021;11(January):595532. doi: 10.3389/fneur.2020.595532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambadipudi S, Zweckstetter M. Targeting ıntrinsically disordered proteins in rational drug discovery. Expert Opin Drug Discovery. 2016;11(1):65–77. doi: 10.1517/17460441.2016.1107041. [DOI] [PubMed] [Google Scholar]
- Ambrósio AF, Silva AP, Malva JO, Mesquita JF, Carvalho AP, Carvalho CM. Role of desensitization of AMPA receptors on the neuronal viability and on the [Ca 2+ ]i changes in cultured rat hippocampal neurons: AMPA receptor desensitization in hippocampal neurons. Eur J Neurosci. 2000;12(6):2021–2031. doi: 10.1046/j.1460-9568.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Miura M, Suzuki T, Nishimura K, Masuda M. Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatr Gerontol Int. 2010;10(Suppl 1 (July)):S148–S157. doi: 10.1111/j.1447-0594.2010.00588.x. [DOI] [PubMed] [Google Scholar]
- Auranen M, Ylikallio E, Shcherbii M, Paetau A, Kiuru-Enari S, Toppila JP, Tyynismaa H. CHCHD10 variant p.(Gly66Val) causes axonal Charcot-Marie-Tooth disease. Neurology. Genetics. 2015;1(1):e1. doi: 10.1212/NXG.0000000000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam S, Haque ME, Jakaria M, Jo S-H, Kim I-S, Choi D-K. G-Protein-coupled receptors in CNS: a potential therapeutic target for ıntervention in neurodegenerative disorders and associated cognitive deficits. Cells. 2020;9(2):E506. doi: 10.3390/cells9020506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker RA, Leurs R. From the human genome to new drugs: the potential of orphan G-protein-coupled receptors. In: Dingermann T, Steinhilber D, Folkers G, editors. In: Methods and principles in medicinal chemistry. 1. Hoboken: Wiley; 2003. pp. 95–121. [Google Scholar]
- Bale TL, Vale WW. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. J Neurosci Off J Soc Neurosci. 2003;23(12):5295–5301. doi: 10.1523/JNEUROSCI.23-12-05295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels T, Ahlstrom LS, Leftin A, Kamp F, Haass C, Brown MF, Beyer K. The N-terminus of the ıntrinsically disordered protein α-synuclein triggers membrane binding and helix folding. Biophys J. 2010;99(7):2116–2124. doi: 10.1016/j.bpj.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartfai T, Wang M-w. Positive allosteric modulators to peptide GPCRs: a promising class of drugs. Acta Pharmacol Sin. 2013;34(7):880–885. doi: 10.1038/aps.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthet G, Framery B, Gaven F, Pellissier L, Reiter E, Claeysen S, Bockaert J, Dumuis A. 5-Hydroxytryptamine 4 receptor activation of the extracellular signal-regulated kinase pathway depends on Src activation but not on G protein or beta-arrestin signaling. Mol Biol Cell. 2007;18(6):1979–1991. doi: 10.1091/mbc.e06-12-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalha VL, Ferreira DG, Coelho JE, Valadas JS, Gomes R, Temido-Ferreira M, Shmidt T, et al. The caffeine-binding adenosine A2A receptor ınduces age-like HPA-axis dysfunction by targeting glucocorticoid receptor function. Sci Rep. 2016;6(1):31493. doi: 10.1038/srep31493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet LZ, Hosey CM, Ursu O, Oprea TI. BDDCS, the rule of 5 and drugability. Adv Drug Deliv Rev. 2016;101(June):89–98. doi: 10.1016/j.addr.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best RB. Computational and theoretical advances in studies of ıntrinsically disordered proteins. Curr Opin Struct Biol. 2017;42(February):147–154. doi: 10.1016/j.sbi.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Roussignol G, Bécamel C, Gavarini S, Joubert L, Dumuis A, Fagni L, Marin P. GPCR-ınteracting proteins (GIPs): nature and functions. Biochem Soc Trans. 2004;32(Pt 5):851–855. doi: 10.1042/BST0320851. [DOI] [PubMed] [Google Scholar]
- Bonuccelli U, Del Dotto P, Rascol O. Role of dopamine receptor agonists in the treatment of early Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(December):S44–S53. doi: 10.1016/S1353-8020(09)70835-1. [DOI] [PubMed] [Google Scholar]
- Brady AE, Jones CK, Bridges TM, Phillip Kennedy J, Thompson AD, Heiman JU, Breininger ML, et al. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine-ınduced hyperlocomotor activity in rats. J Pharmacol Exp Ther. 2008;327(3):941–953. doi: 10.1124/jpet.108.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brain SD, Cox HM. Neuropeptides and their receptors: ınnovative science providing novel therapeutic targets. Br J Pharmacol 147 Suppl. 2006;1(January):S202–S211. doi: 10.1038/sj.bjp.0706461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G, Fan J, Yang M, Lv C, Lin Y, Li J, Meng F, et al. Identification of a novel functional corticotropin-releasing hormone (CRH2) in chickens and ıts roles in stimulating pituitary TSHβ expression and ACTH secretion. Front Endocrinol. 2019;10(August):595. doi: 10.3389/fendo.2019.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bünemann M, Hosey MM. G-Protein coupled receptor kinases as modulators of G-protein signalling. J Physiol. 1999;517((Pt 1) (May)):5–23. doi: 10.1111/j.1469-7793.1999.0005z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein SR, Valsecchi F, Kawamata H, Bourens M, Zeng R, Zuberi A, Milner TA, et al. In vitro and in vivo studies of the ALS-FTLD protein CHCHD10 reveal novel mitochondrial topology and protein ınteractions. Hum Mol Genet. 2018;27(1):160–177. doi: 10.1093/hmg/ddx397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T, Goldberg JD, Galvin JE, Maloney T, Ravdin L, Glodzik L, de Leon MJ, Hochman T, Bowen RL, Atwood CS. Rationale, study design and ımplementation of the LUCINDA trial: leuprolide plus cholinesterase ınhibition to reduce neurologic decline in Alzheimer’s. Contemp Clin Trials. 2021;107(August):106488. doi: 10.1016/j.cct.2021.106488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SN, Zhang C, Roe AD, Lee N, Lao KU, Monte L, Donohue MC, Rissman RA. Impact of CRFR1 ablation on amyloid-β production and accumulation in a mouse model of Alzheimer’s disease. J Alzheimer’s Disease: JAD. 2015;45(4):1175–1184. doi: 10.3233/JAD-142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2011;75(1):50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carniglia L, Ramírez D, Durand D, Saba J, Turati J, Caruso C, Scimonelli TN, Lasaga M. Neuropeptides and microglial activation in ınflammation, pain, and neurodegenerative diseases. Mediat Inflamm. 2017;2017:5048616. doi: 10.1155/2017/5048616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalani E, De Palma C, Perrotta C, Cervia D. Current evidence for a role of neuropeptides in the regulation of autophagy. Biomed Res Int. 2017;2017:5856071. doi: 10.1155/2017/5856071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Miller LJ, Dong M. Role of N-linked glycosylation in biosynthesis, trafficking, and function of the human glucagon-like peptide 1 receptor. Am J Physiol Endocrinol Metab. 2010;299(1):E62–E68. doi: 10.1152/ajpendo.00067.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Bai B, Tian Y, Hui D, Chen J. Identification of serine 348 on the apelin receptor as a novel regulatory phosphorylation site in apelin-13-ınduced G protein-ındependent biased signaling. J Biol Chem. 2014;289(45):31173–31187. doi: 10.1074/jbc.M114.574020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Turnbull DM, Reeve AK. Mitochondrial dysfunction in Parkinson’s disease-cause or consequence? Biology. 2019;8(2):E38. doi: 10.3390/biology8020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Zhao Y-F, Chen Y-X, Li Y-M. Exploring the roles of post-translational modifications in the pathogenesis of Parkinson’s disease using synthetic and semisynthetic modified α-synuclein. ACS Chem Neurosci. 2019;10(2):910–921. doi: 10.1021/acschemneuro.8b00447. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Oldfield CJ, Meng J, Romero P, Uversky VN, Keith Dunker A. Mining alpha-helix-forming molecular recognition features with cross species sequence alignments. Biochemistry. 2007;46(47):13468–13477. doi: 10.1021/bi7012273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia S, Habchi J, Michaels TCT, Cohen SIA, Linse S, Dobson CM, Knowles TPJ, Vendruscolo M. SAR by kinetics for drug discovery in protein misfolding diseases. Proc Natl Acad Sci. 2018;115(41):10245–10250. doi: 10.1073/pnas.1807884115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Palop JJ, Puoliväli J, Massaro C, Bien-Ly N, Gerstein H, Scearce-Levie K, Masliah E, Mucke L. Fyn kinase ınduces synaptic and cognitive ımpairments in a transgenic mouse model of Alzheimer’s disease. J Neurosci Off J Soc Neurosci. 2005;25(42):9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christofides K, Menon R, Jones CE. Endocytosis of G protein-coupled receptors and their ligands: ıs there a role in metal trafficking? Cell Biochem Biophys. 2018;76(3):329–337. doi: 10.1007/s12013-018-0850-9. [DOI] [PubMed] [Google Scholar]
- Collins LE, Galtieri DJ, Brennum LT, Sager TN, Hockemeyer J, Müller CE, Hinman JR, Chrobak JJ, Salamone JD. Oral tremor ınduced by the muscarinic agonist pilocarpine ıs suppressed by the adenosine A2A antagonists MSX-3 and SCH58261, but not the adenosine A1 antagonist DPCPX. Pharmacol Biochem Behav. 2010;94(4):561–569. doi: 10.1016/j.pbb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov. 2009;8(1):41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskuner O, Uversky VN. Tyrosine regulates β-sheet structure formation in amyloid-β 42: a new clustering algorithm for disordered proteins. J Chem Inf Model. 2017;57(6):1342–1358. doi: 10.1021/acs.jcim.6b00761. [DOI] [PubMed] [Google Scholar]
- Coskuner O, Uversky VN. In Progress in Molecular Biology and Translational Science. 166. Netherlands: Elsevier; 2019. Intrinsically disordered proteins in various hypotheses on the pathogenesis of Alzheimer’s and Parkinson’s diseases; pp. 145–223. [DOI] [PubMed] [Google Scholar]
- Coskuner O, Wise-Scira O. Structures and free energy landscapes of the A53T mutant-type α-synuclein protein and ımpact of A53T mutation on the structures of the wild-type α-synuclein protein with dynamics. ACS Chem Neurosci. 2013;4(7):1101–1113. doi: 10.1021/cn400041j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskuner O, Wise-Scira O, Perry G, Kitahara T. The Structures of the E22Δ mutant-type amyloid-β alloforms and the ımpact of E22Δ mutation on the structures of the wild-type amyloid-β alloforms. ACS Chem Neurosci. 2013;4(2):310–320. doi: 10.1021/cn300149j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coskuner-Weber O, Uversky V. Insights into the molecular mechanisms of Alzheimer’s and Parkinson’s diseases with molecular simulations: understanding the roles of artificial and pathological missense mutations in ıntrinsically disordered proteins related to pathology. Int J Mol Sci. 2018;19(2):336. doi: 10.3390/ijms19020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411(6836):480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Cvicek V, Goddard WA, Abrol R. Structure-based sequence alignment of the transmembrane domains of all human GPCRs: phylogenetic, structural and functional ımplications. PLoS Comput Biol. 2016;12(3):e1004805. doi: 10.1371/journal.pcbi.1004805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damian M, Pons V, Renault P, M’Kadmi C, Delort B, Hartmann L, Kaya AI, et al. GHSR-D2R Heteromerization modulates dopamine signaling through an effect on G protein conformation. Proc Natl Acad Sci. 2018;115(17):4501–4506. doi: 10.1073/pnas.1712725115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danysz W, Parsons CG. Alzheimer’s disease, β-amyloid, glutamate, NMDA receptors and memantine—searching for the connections. Br J Pharmacol. 2012;167(2):324–352. doi: 10.1111/j.1476-5381.2012.02057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Foubert G, O’Neill MJ, Zetterström TSC. Acute Onset by 5-HT(6)-Receptor activation on rat brain brain-derived neurotrophic factor and activity-regulated cytoskeletal-associated protein MRNA expression. Neuroscience. 2007;147(3):778–785. doi: 10.1016/j.neuroscience.2007.04.045. [DOI] [PubMed] [Google Scholar]
- De Leeuw Van Weenen JE, Parlevliet ET, Maechler P, Havekes LM, Romijn JA, Ouwens DM, Pijl H, Guigas B. The dopamine receptor D2 agonist bromocriptine ınhibits glucose-stimulated ınsulin secretion by direct activation of the alpha2-adrenergic receptors in beta cells. Biochem Pharmacol. 2010;79(12):1827–1836. doi: 10.1016/j.bcp.2010.01.029. [DOI] [PubMed] [Google Scholar]
- DeForte S, Uversky VN. Order, disorder, and everything in between. Molecules (Basel, Switzerland) 2016;21(8):E1090. doi: 10.3390/molecules21081090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, Katz R, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802. doi: 10.1111/epi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon S. Eptinezumab: first approval. Drugs. 2020;80(7):733–739. doi: 10.1007/s40265-020-01300-4. [DOI] [PubMed] [Google Scholar]
- Disfani FM, Hsu W-L, Mizianty MJ, Oldfield CJ, Bin Xue A, Dunker K, Uversky VN, Kurgan L. MoRFpred, a Computational tool for sequence-based prediction and characterization of short disorder-to-order transitioning binding regions in proteins. Bioinformatics (Oxford, England) 2012;28(12):i75–i83. doi: 10.1093/bioinformatics/bts209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X-x, Wang Y, Qin Z-h. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009;30(4):379–387. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dores MR, Trejo JA. Ubiquitination of G protein-coupled receptors: functional ımplications and drug discovery. Mol Pharmacol. 2012;82(4):563–570. doi: 10.1124/mol.112.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosztányi Z, Mészáros B, Simon I. ANCHOR: web server for predicting protein binding regions in disordered proteins. Bioinformatics (Oxford, England) 2009;25(20):2745–2746. doi: 10.1093/bioinformatics/btp518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X, Li Y, Xia Y-L, Ai S-M, Liang J, Sang P, Ji X-L, Liu S-Q. Insights into protein–ligand ınteractions: mechanisms, models, and methods. Int J Mol Sci. 2016;17(2):144. doi: 10.3390/ijms17020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube A, Chaudhary S, Mengawade T, Upasani CD. Therapeutic potential of metabotropic glutamate receptor 4-positive allosteric modulator TAS-4 in rodent models of movement disorders. J Neurol Sci. 2014;S0022-510X(14):00452–00453. doi: 10.1016/j.jns.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Ehrlich AT, Maroteaux G, Robe A, Lydie Venteo M, Nasseef T, van Kempen LC, Mechawar N, Turecki G, Darcq E, Kieffer BL. Expression map of 78 brain-expressed mouse orphan GPCRs provides a translational resource for neuropsychiatric research. Commun Biol. 2018;1:102. doi: 10.1038/s42003-018-0106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribá PV, Wedegaertner PB, Goñi FM, Vögler O. Lipid–protein ınteractions in GPCR-associated signaling. Biochim et Biophysica Acta (BBA) -Biomembranes. 2007;1768(4):836–852. doi: 10.1016/j.bbamem.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Fahn S. The medical treatment of Parkinson disease from James Parkinson to George Cotzias. Mov Disorders: Official Journal of the Movement Disorder Society. 2015;30(1):4–18. doi: 10.1002/mds.26102. [DOI] [PubMed] [Google Scholar]
- Fanning S, Selkoe D, Dettmer U. Parkinson’s disease: proteinopathy or lipidopathy? NPJ Parkinson’s Disease. 2020;6:3. doi: 10.1038/s41531-019-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Vieira HT, Guimaraes IM, Silva FR, Ribeiro FM. Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol. 2016;14(1):101–115. doi: 10.2174/1570159X13666150716165726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S. Huntington’s disease. Cold Spring Harb Perspect Biol. 2011;3(6):a007476. doi: 10.1101/cshperspect.a007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink-Jensen A, Schmidt LS, Dencker D, Schülein C, Wess J, Wörtwein G, Woldbye DPD. Antipsychotic-ınduced catalepsy ıs attenuated in mice lacking the M4 muscarinic acetylcholine receptor. Eur J Pharmacol. 2011;656(1–3):39–44. doi: 10.1016/j.ejphar.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock T, Hauser AS, Lund N, Gloriam DE, Balaji S, Madan Babu M. Selectivity determinants of GPCR-G-protein binding. Nature. 2017;545(7654):317–322. doi: 10.1038/nature22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonin AV, Darling AL, Kuznetsova IM, Turoverov KK, Uversky VN. Multi-functionality of proteins ınvolved in GPCR and G protein signaling: making sense of structure-function continuum with ıntrinsic disorder-based proteoforms. Cell Mol Life Sci: CMLS. 2019;76(22):4461–4492. doi: 10.1007/s00018-019-03276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster DJ, Choi DL, Jeffrey Conn P, Rook JM. Activation of M1 and M4 muscarinic receptors as potential treatments for Alzheimer’s disease and schizophrenia. Neuropsychiatr Dis Treat. 2014;10:183–191. doi: 10.2147/NDT.S55104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SH. Non-dopaminergic treatments for motor control in Parkinson’s disease. Drugs. 2013;73(13):1405–1415. doi: 10.1007/s40265-013-0105-4. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerström MC, Lundin L-G, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63(6):1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Futch HS, Croft CL, Truong VQ, Krause EG, Golde TE. Targeting psychologic stress signaling pathways in Alzheimer’s disease. Mol Neurodegener. 2017;12(1):49. doi: 10.1186/s13024-017-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gábriel R, Pöstyéni E, Dénes V. Neuroprotective potential of pituitary adenylate cyclase activating polypeptide in retinal degenerations of metabolic origin. Front Neurosci. 2019;13(October):1031. doi: 10.3389/fnins.2019.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaier ED, Eipper BA, Mains RE. Copper signaling in the mammalian nervous system: synaptic effects. J Neurosci Res. 2013;91(1):2–19. doi: 10.1002/jnr.23143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandía J, Morató X, Stagljar I, Fernández-Dueñas V, Ciruela F. Adenosine A2A receptor-mediated control of pilocarpine-ınduced tremulous jaw movements ıs Parkinson’s disease-associated GPR37 receptor-dependent. Behav Brain Res. 2015;288(July):103–106. doi: 10.1016/j.bbr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- García-Nafría J, Tate CG. Structure determination of GPCRs: Cryo-EM compared with X-ray crystallography. Biochem Soc Trans. 2021;49(5):2345–2355. doi: 10.1042/BST20210431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genin EC, Plutino M, Bannwarth S, Villa E, Cisneros-Barroso E, Roy M, Ortega-Vila B, et al. CHCHD10 Mutations promote loss of mitochondrial cristae junctions with ımpaired mitochondrial genome maintenance and ınhibition of apoptosis. EMBO Mol Med. 2016;8(1):58–72. doi: 10.15252/emmm.201505496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G. Interaction of G protein coupled receptors and cholesterol. Chem Phys Lipids. 2016;199(September):61–73. doi: 10.1016/j.chemphyslip.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Giorgetti S, Greco C, Tortora P, Aprile FA. Targeting amyloid aggregation: an overview of strategies and mechanisms. Int J Mol Sci. 2018;19(9):E2677. doi: 10.3390/ijms19092677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh LK, Sorkin A. Endocytosis of receptor tyrosine kinases. Cold Spring Harb Perspect Biol. 2013;5(5):a017459. doi: 10.1101/cshperspect.a017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goth CK, Petäjä-Repo UE, Rosenkilde MM. G Protein-coupled receptors in the sweet spot: glycosylation and other post-translational modifications. ACS Pharmacol Transl Sci. 2020;3(2):237–245. doi: 10.1021/acsptsci.0c00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. GPCR Signaling regulation: the role of GRKs and arrestins. Front Pharmacol. 2019;10:125. doi: 10.3389/fphar.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and ındications. Nat Rev Drug Discov. 2017;16(12):829–842. doi: 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C-H, Benson L, Evans R, Silvio Gutkind J. Signals and receptors. Cold Spring Harb Perspect Biol. 2016;8(4):a005900. doi: 10.1101/cshperspect.a005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller G, Aprile F, Bonomi M, Camilloni C, De Simone A, Vendruscolo M (2017) Sequence specificity in the entropy-driven binding of a small molecule and a disordered peptide. J Mol Biol. 10.17863/CAM.13377 [DOI] [PubMed]
- Hilger D, Masureel M, Kobilka BK. Structure and dynamics of GPCR signaling complexes. Nat Struct Mol Biol. 2018;25(1):4–12. doi: 10.1038/s41594-017-0011-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenstein K, de Graaf C, Bortolato A, Wang M-W, Marshall FH, Stevens RC. Insights into the structure of class B GPCRs. Trends Pharmacol Sci. 2014;35(1):12–22. doi: 10.1016/j.tips.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong D-P, Xiong W, Chang J-Y, Jiang C. The role of the C-terminus of human α-synuclein: ıntra-disulfide bonds between the C-terminus and other regions stabilize non-fibrillar monomeric ısomers. FEBS Lett. 2011;585(3):561–566. doi: 10.1016/j.febslet.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Huang Y, Skwarek-Maruszewska A, Horré K, Vandewyer E, Wolfs L, Snellinx A, Saito T, et al. Loss of GPR3 reduces the amyloid plaque burden and ımproves memory in Alzheimer’s disease mouse models. Sci Transl Med. 2015;7(309):309ra164. doi: 10.1126/scitranslmed.aab3492. [DOI] [PubMed] [Google Scholar]
- Huang X, Wu BP, Nguyen D, Liu Y-T, Marani M, Hench J, Bénit P, et al. CHCHD2 accumulates in distressed mitochondria and facilitates oligomerization of CHCHD10. Hum Mol Genet. 2018;27(22):3881–3900. doi: 10.1093/hmg/ddy270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JP, Rees S, Kalindjian SB, Philpott KL. Principles of early drug discovery. Br J Pharmacol. 2011;162(6):1239–1249. doi: 10.1111/j.1476-5381.2010.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K-S. Neuropeptidases. In: Lajtha A, Banik N, editors. Handbook of neurochemistry and molecular neurobiology. Boston: Springer US; 2007. pp. 625–651. [Google Scholar]
- Iakoucheva LM, Radivojac P, Brown CJ, O’Connor TR, Sikes JG, Obradovic Z, Keith Dunker A. The ımportance of ıntrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004;32(3):1037–1049. doi: 10.1093/nar/gkh253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A, Mohamed M. Ghrelin – physiological functions and regulation. Eur Endocrinol. 2015;11(2):90–95. doi: 10.17925/EE.2015.11.02.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Meng H, Shiba-Fukushima K, Hattori N. Twin CHCH proteins, CHCHD2, and CHCHD10: key molecules of Parkinson’s disease, amyotrophic lateral sclerosis, and frontotemporal dementia. Int J Mol Sci. 2019;20(4):E908. doi: 10.3390/ijms20040908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai S, Kock KH, Bulyk ML. Transcription factor-DNA binding: beyond binding site motifs. Curr Opin Genet Dev. 2017;43(April):110–119. doi: 10.1016/j.gde.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaakola V-P, Prilusky J, Sussman JL, Goldman A. G Protein-coupled receptors show unusual patterns of ıntrinsic unfolding. Protein Eng Design Select: PEDS. 2005;18(2):103–110. doi: 10.1093/protein/gzi004. [DOI] [PubMed] [Google Scholar]
- Jean-Charles P-Y, Kaur S, Shenoy SK. G Protein-coupled receptor signaling through β-arrestin-dependent mechanisms. J Cardiovasc Pharmacol. 2017;70(3):142–158. doi: 10.1097/FJC.0000000000000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JO, Glynn SM, Raphael Gibbs J, Nalls MA, Sabatelli M, Restagno G, Drory VE, Chiò A, Rogaeva E, Traynor BJ. Mutations in the CHCHD10 gene are a common cause of familial amyotrophic lateral sclerosis. Brain: A. J Neurol. 2014;137(Pt 12):e311. doi: 10.1093/brain/awu265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EM, Dubey M, Camp PJ, Vernon BC, Biernat J, Mandelkow E, Majewski J, Chi EY. Interaction of tau protein with model lipid membranes ınduces tau structural compaction and membrane disruption. Biochemistry. 2012;51(12):2539–2550. doi: 10.1021/bi201857v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, Tunyasuvunakool K, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596(7873):583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao AW, Racine CA, Quitania LC, Kramer JH, Christine CW, Miller BL. Cognitive and neuropsychiatric profile of the synucleinopathies: Parkinson disease, dementia with Lewy bodies, and multiple system atrophy. Alzheimer Dis Assoc Disord. 2009;23(4):365–370. doi: 10.1097/WAD.0b013e3181b5065d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman M, Berin WS, Ntie-Kang F. Natural products as modulators of sirtuins. Molecules (Basel, Switzerland) 2020;25(14):E3287. doi: 10.3390/molecules25143287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona C, Katona C. New generation multi-modal antidepressants: focus on vortioxetine for major depressive disorder. Neuropsychiatr Dis Treat. 2014;349:349. doi: 10.2147/NDT.S39544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenschlager R, Sampaio C, Costa J, Lees A (2002) Anticholinergics for symptomatic management of Parkinson’s disease. Edited by Cochrane Movement Disorders Group. Cochrane Database Syst Rev. 10.1002/14651858.CD003735 [DOI] [PMC free article] [PubMed]
- Kee TR, Gonzalez PE, Wehinger JL, Bukhari MZ, Ermekbaeva A, Sista A, Kotsiviras P, Liu T, Kang DE, Woo JAA. Mitochondrial CHCHD2: disease-associated mutations, physiological functions, and current animal models. Front Aging Neurosci. 2021;13:660843. doi: 10.3389/fnagi.2021.660843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith JL, Swinkin E, Gao A, Alminawi S, Zhang M, McGoldrick P, McKeever P, Robertson J, Rogaeva E, Zinman L. Neuropathologic description of CHCHD10 mutated amyotrophic lateral sclerosis. Neurol Gen. 2020;6(1):e394. doi: 10.1212/NXG.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kem W, Soti F, Wildeboer K, LeFrancois S, MacDougall K, Wei D-Q, Chou K-C, Arias H. The nemertine toxin anabaseine and ıts derivative DMXBA (GTS-21): chemical and pharmacological properties. Mar Drugs. 2006;4(3):255–273. doi: 10.3390/md403255. [DOI] [Google Scholar]
- Khanahmadi M, Farhud DD, Malmir M. Genetic of Alzheimer’s disease: a narrative review article. Iran J Public Health. 2015;44(7):892–901. [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Han KH. PreSMo Target-binding signatures in ıntrinsically disordered proteins. Mol Cell. 2018;41:889–899. doi: 10.14348/molcells.2018.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MV, Marsden CA, Fone KCF. A role for the 5-HT(1A), 5-HT4 and 5-HT6 receptors in learning and memory. Trends Pharmacol Sci. 2008;29(9):482–492. doi: 10.1016/j.tips.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Kiriakidi S, Kolocouris A, Liapakis G, Ikram S, Durdagi S and Mavromoustakos T. (2019). Effects of cholesterol on GPCR function: ınsights from computational and experimental studies. In: Direct mechanisms in cholesterol modulation of protein function. Edited by Avia Rosenhouse-Dantsker and Anna N. Bukiya, 1135, 89–103. Advances in Experimental Medicine and Biology. Cham: Springer International Publishing. 10.1007/978-3-030-14265-0_5. [DOI] [PubMed]
- Klein MT, Vinson PN, Niswender CM. Approaches for probing allosteric ınteractions at 7 transmembrane spanning receptors. Prog Mol Biol Transl Sci. 2013;115:1–59. doi: 10.1016/B978-0-12-394587-7.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köles L, Kató E, Hanuska A, Zádori ZS, Al-Khrasani M, Zelles T, Rubini P, Illes P. Modulation of excitatory neurotransmission by neuronal/glial signalling molecules: ınterplay between purinergic and glutamatergic systems. Purinergic Signal. 2016;12(1):1–24. doi: 10.1007/s11302-015-9480-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu H, Maruyama M, Yao S, Shinohara T, Sakuma K, Imaichi S, Chikatsu T, et al. Anatomical transcriptome of G protein-coupled receptors leads to the ıdentification of a novel therapeutic candidate GPR52 for psychiatric disorders. PLoS One. 2014;9(2):e90134. doi: 10.1371/journal.pone.0090134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczynska M, Clark MJ, Valant C, Jun X, Von Moo E, Albold S, Weiss DR, et al. Structure-based discovery of selective positive allosteric modulators of antagonists for the M2 muscarinic acetylcholine receptor. Proc Natl Acad Sci. 2018;115(10):E2419–E2428. doi: 10.1073/pnas.1718037115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosmidis S, Polyzos A, Harvey L, Youssef M, Denny CA, Dranovsky A, Kandel ER. RbAp48 Protein ıs a critical component of GPR158/OCN signaling and ameliorates age-related memory loss. Cell Rep. 2018;25(4):959–973.e6. doi: 10.1016/j.celrep.2018.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouhi A, Pachipulusu V, Kapenstein T, Peisheng H, Epstein AL, Khawli LA. Brain disposition of antibody-based therapeutics: dogma, approaches and perspectives. Int J Mol Sci. 2021;22(12):6442. doi: 10.3390/ijms22126442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laun AS, Shrader SH, Brown KJ, Song Z-H. GPR3, GPR6, and GPR12 as novel molecular targets: their biological functions and ınteraction with cannabidiol. Acta Pharmacol Sin. 2019;40(3):300–308. doi: 10.1038/s41401-018-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xiujuan W, Liu S, Zhao Y, Zhu J, Liu K. Roles of neuropeptide Y in neurodegenerative and neuroimmune diseases. Front Neurosci. 2019;13:869. doi: 10.3389/fnins.2019.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Gong C-X. Tau exon 10 alternative splicing and tauopathies. Mol Neurodegener. 2008;3(July):8. doi: 10.1186/1750-1326-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Jung-A A, Woo MZ, Bukhari PLP, Chacko A, Selenica M-LB, Yan Y, et al. CHCHD10-Regulated OPA1-mitofilin complex mediates TDP-43-ınduced mitochondrial phenotypes associated with frontotemporal dementia. FASEB J: Official Publication of the Federation of American Societies for Experimental Biology. 2020;34(6):8493–8509. doi: 10.1096/fj.201903133RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Rustay NR, Ebert U, Hradil VP, Cole TB, Llano DA, Mudd SR, Zhang Y, Fox GB, Day M. Characterization of 7- and 19-month-old Tg2576 mice using multimodal in vivo ımaging: limitations as a translatable model of Alzheimer’s disease. Neurobiol Aging. 2012;33(5):933–944. doi: 10.1016/j.neurobiolaging.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Luttrell LM, Maudsley S, Gesty-Palmer D. Translating in vitro ligand bias into in vivo efficacy. Cell Signal. 2018;41(January):46–55. doi: 10.1016/j.cellsig.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009;109(10):4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrinelli F, Picelli A, Tocco P, Federico A, Roncari L, Smania N, Zanette G, Tamburin S. Pathophysiology of motor dysfunction in Parkinson’s disease as the rationale for drug treatment and rehabilitation. Parkinson’s Disease. 2016;2016:9832839. doi: 10.1155/2016/9832839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio D, Roberto PJ, Barrett EK, Hoffman CW, Barrett AZ, Borah A, Xiaoping H, et al. α-Synuclein binds to TOM20 and ınhibits mitochondrial protein ımport in Parkinson’s disease. Sci Transl Med. 2016;8(342):342ra78. doi: 10.1126/scitranslmed.aaf3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man WK, Tahirbegi B, Vrettas MD, Preet S, Ying L, Vendruscolo M, De Simone A, Fusco G. The docking of synaptic vesicles on the presynaptic membrane ınduced by α-synuclein ıs modulated by lipid composition. Nat Commun. 2021;12(1):927. doi: 10.1038/s41467-021-21027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow E-M, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harbor Perspect Med. 2012;2(7):a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula R, Anuja K, Alcain FJ. SIRT1 and SIRT2 activity control in neurodegenerative diseases. Front Pharmacol. 2020;11:585821. doi: 10.3389/fphar.2020.585821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjama-Lyons J, Koller W. Tremor-predominant Parkinson’s disease. Approach Treatment Drugs Aging. 2000;16(4):273–278. doi: 10.2165/00002512-200016040-00003. [DOI] [PubMed] [Google Scholar]
- Markham A. Erenumab: first global approval. Drugs. 2018;78(11):1157–1161. doi: 10.1007/s40265-018-0944-0. [DOI] [PubMed] [Google Scholar]
- Martin B, Lopez R, de Maturana R, Brenneman TW, Mattson MP, Maudsley S. Class II G protein-coupled receptors and their ligands in neuronal function and protection. NeuroMolecular Med. 2005;7(1–2):3–36. doi: 10.1385/nmm:7:1-2:003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marullo S, Doly S, Saha K, Enslen H, Scott MGH, Coureuil M. Mechanical GPCR activation by traction forces exerted on receptor N-glycans. ACS Pharmacol Transl Sci. 2020;3(2):171–178. doi: 10.1021/acsptsci.9b00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade RM, Fairlie DP, Mason JM. Alpha-synuclein structure and Parkinson’s disease – lessons and emerging principles. Mol Neurodegener. 2019;14(1):29. doi: 10.1186/s13024-019-0329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros R, Baglietto-Vargas D, LaFerla FM. The role of tau in Alzheimer’s disease and related disorders. CNS Neurosci Ther. 2011;17(5):514–524. doi: 10.1111/j.1755-5949.2010.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Manes FF, Magnolfi G, Sahakian BJ, Robbins TW. Impaired set-shifting and dissociable effects on tests of spatial working memory following the dopamine D2 receptor antagonist sulpiride in human volunteers. Psychopharmacology. 2004;176(3–4):331–342. doi: 10.1007/s00213-004-1899-2. [DOI] [PubMed] [Google Scholar]
- Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: ınsights from human and rodent studies. Neuroscience. 2016;321(May):138–162. doi: 10.1016/j.neuroscience.2015.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mészáros B, Simon I, Dosztányi Z. Prediction of protein binding regions in disordered proteins. PLoS Comput Biol. 2009;5(5):e1000376. doi: 10.1371/journal.pcbi.1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mészáros B, Erdos G, Dosztányi Z. IUPred2A: context-dependent prediction of protein disorder as a function of redox state and protein binding. Nucleic Acids Res. 2018;46(W1):W329–W337. doi: 10.1093/nar/gky384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milles S, Salvi N, Blackledge M, Jensen MR. Characterization of ıntrinsically disordered proteins and their dynamic complexes: from in vitro to cell-like environments. Prog Nucl Magn Reson Spectrosc. 2018;109:79–100. doi: 10.1016/j.pnmrs.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Min S-W, Sohn PD, Li Y, Devidze N, Johnson JR, Krogan NJ, Masliah E, Mok S-A, Gestwicki JE, Gan L. SIRT1 deacetylates tau and reduces pathogenic tau spread in a mouse model of tauopathy. J Neurosci Off J Soc Neurosci. 2018;38(15):3680–3688. doi: 10.1523/JNEUROSCI.2369-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadegan T, Benkö G, Filipek S, Palczewski K. Sequence analyses of G-protein-coupled receptors: similarities to rhodopsin. Biochemistry. 2003;42(10):2759–2767. doi: 10.1021/bi027224+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan A, Oldfield CJ, Radivojac P, Vacic V, Cortese MS, Keith Dunker A, Uversky VN. Analysis of molecular recognition features (MoRFs) J Mol Biol. 2006;362(5):1043–1059. doi: 10.1016/j.jmb.2006.07.087. [DOI] [PubMed] [Google Scholar]
- Montani C, Canella C, Schwarz AJ, Li J, Gilmour G, Galbusera A, Wafford K, et al. The M1/M4 preferring muscarinic agonist xanomeline modulates functional connectivity and NMDAR antagonist-ınduced changes in the mouse brain. Neuropsychopharmacology: Official Publication of the American College of. Neuropsychopharmacology. 2021;46(6):1194–1206. doi: 10.1038/s41386-020-00916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran SP, Maksymetz J, Jeffrey Conn P. Targeting muscarinic acetylcholine receptors for the treatment of psychiatric and neurological disorders. Trends Pharmacol Sci. 2019;40(12):1006–1020. doi: 10.1016/j.tips.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morano A, Cifelli P, Nencini P, Antonilli L, Fattouch J, Ruffolo G, Roseti C, et al. Cannabis in epilepsy: from clinical practice to basic research focusing on the possible role of cannabidivarin. Epilepsia Open. 2016;1(3–4):145–151. doi: 10.1002/epi4.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro S, Hoffmann C, Jacobson KA. Role of the extracellular loops of G protein-coupled receptors in ligand recognition: a molecular modeling study of the human P2Y1 receptor. Biochemistry. 1999;38(12):3498–3507. doi: 10.1021/bi982369v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustata G, Follis AV, Hammoudeh DI, Metallo SJ, Wang H, Prochownik EV, Lazo JS, Bahar I. Discovery of novel Myc-Max heterodimer disruptors with a three-dimensional pharmacophore model. J Med Chem. 2009;52(5):1247–1250. doi: 10.1021/jm801278g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan A, Hunkele A, Jin X, Bassoni DL, Pasternak GW, Pan Y-X. Mu opioids ınduce biased signaling at the full-length seven transmembrane C-terminal splice variants of the mu opioid receptor gene, Oprm1. Cell Mol Neurobiol. 2021;41(5):1059–1074. doi: 10.1007/s10571-020-00973-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumenko VS, Ponimaskin E. Palmitoylation as a functional regulator of neurotransmitter receptors. Neural Plasticity. 2018;2018:5701348. doi: 10.1155/2018/5701348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R, Sawaya MR, Balbirnie M, Madsen AØ, Riekel C, Grothe R, Eisenberg D. Structure of the cross-beta spine of amyloid-like fibrils. Nature. 2005;435(7043):773–778. doi: 10.1038/nature03680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KP, Potikyan G, Savene ROV, Denny CT, Uversky VN, Lee KAW. Multiple aromatic side chains within a disordered structure are critical for transcription and transforming activity of EWS family oncoproteins. Proc Natl Acad Sci U S A. 2007;104(2):479–484. doi: 10.1073/pnas.0607007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols HH, Jeffrey Conn P. Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol Dis. 2014;61(January):55–71. doi: 10.1016/j.nbd.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama K, Suzuki H, Maruyama M, Yoshihara T, Ohta H. Genetic deletion of GPR52 enhances the locomotor-stimulating effect of an adenosine A2A receptor antagonist in mice: a potential role of GPR52 in the function of striatopallidal neurons. Brain Res. 2017;1670(September):24–31. doi: 10.1016/j.brainres.2017.05.031. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Jeffrey Conn P. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu Z, Zhang Z, Zhao W, Yang J. Interactions between amyloid β peptide and lipid membranes. Biochim et Biophysica Acta (BBA) -Biomembranes. 2018;1860(9):1663–1669. doi: 10.1016/j.bbamem.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Oates J, Watts A. Uncovering the ıntimate relationship between lipids, cholesterol and GPCR activation. Curr Opin Struct Biol. 2011;21(6):802–807. doi: 10.1016/j.sbi.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Oates ME, Romero P, Ishida T, Ghalwash M, Mizianty MJ, Xue B, Dosztányi Z, et al. D2P2: database of disordered protein predictions. Nucleic Acids Res. 2013;41(Database issue):D508–D516. doi: 10.1093/nar/gks1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield CJ, Dunker AK. Intrinsically disordered proteins and ıntrinsically disordered protein regions. Annu Rev Biochem. 2014;83:553–584. doi: 10.1146/annurev-biochem-072711-164947. [DOI] [PubMed] [Google Scholar]
- Oldfield CJ, Cheng Y, Cortese MS, Brown CJ, Uversky VN, Keith Dunker A. Comparing and combining predictors of mostly disordered proteins. Biochemistry. 2005;44(6):1989–2000. doi: 10.1021/bi047993o. [DOI] [PubMed] [Google Scholar]
- Oldfield CJ, Cheng Y, Cortese MS, Romero P, Uversky VN, Dunker AK. Coupled folding and binding with alpha-helix-forming molecular recognition elements. Biochemistry. 2005;44:12454–12470. doi: 10.1021/bi050736e. [DOI] [PubMed] [Google Scholar]
- Oldfield CJ, Meng J, Yang JY, Yang MQ, Uversky VN, Dunker AK. Flexible nets: disorder and ınduced fit in the associations of P53 and 14-3-3 with their partners. BMC Genomics. 2008;9(Suppl. 1):1–20. doi: 10.1186/1471-2164-9-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9(1):60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- Olivares D, Deshpande VK, Shi Y, Lahiri DK, Greig NH, Rogers JT, Huang X. N-Methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer’s disease, vascular dementia and Parkinson’s disease. Curr Alzheimer Res. 2012;9(6):746–758. doi: 10.2174/156720512801322564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otoikhian A, Barry AN, Mayfield M, Nilges M, Huang Y, Lutsenko S, Blackburn NJ. Lumenal loop M672-P707 of the Menkes protein (ATP7A) transfers copper to peptidylglycine monooxygenase. J Am Chem Soc. 2012;134(25):10458–10468. doi: 10.1021/ja301221s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal S, Chattopadhyay A. Extramembranous regions in G protein-coupled receptors: Cinderella in receptor biology? J Membr Biol. 2019;252(4–5):483–497. doi: 10.1007/s00232-019-00092-3. [DOI] [PubMed] [Google Scholar]
- Park MH, Wolff EC, Abbruzzese A, Folk JE. Biosynthesis of hypusine in EIF-4D precursors. Adv Exp Med Biol. 1988;250:435–447. doi: 10.1007/978-1-4684-5637-0_38. [DOI] [PubMed] [Google Scholar]
- Parra I, Martínez I, Ramírez-García G, Tizabi Y, Mendieta L. Differential effects of LPS and 6-OHDA on microglia’s morphology in rats: ımplications for ınflammatory model of Parkinson’s disease. Neurotox Res. 2020;37(1):1–11. doi: 10.1007/s12640-019-00104-z. [DOI] [PubMed] [Google Scholar]
- Parrill AL, Bautista DL. GPCR conformations: ımplications for rational drug design. Pharmaceuticals. 2010;4(1):7–43. doi: 10.3390/ph4010007. [DOI] [Google Scholar]
- Parsons CG, Danysz W, Dekundy A, Pulte I. Memantine and cholinesterase ınhibitors: complementary mechanisms in the treatment of Alzheimer’s disease. Neurotox Res. 2013;24(3):358–369. doi: 10.1007/s12640-013-9398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N, Itakura T, Gonzalez JM, Schwartz SG, Elizabeth Fini M. GPR158, an orphan member of G protein-coupled receptor family C: glucocorticoid-stimulated expression and novel nuclear role. Edited by Lucia R. Languino. PLoS One. 2013;8(2):e57843. doi: 10.1371/journal.pone.0057843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathan H, Williams J. Basic opioid pharmacology: an update. Br J Pain. 2012;6(1):11–16. doi: 10.1177/2049463712438493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan A, Cheng N, Trejo JA. Post-translational modifications of G protein-coupled receptors control cellular signaling dynamics in space and time. Pharmacol Rev. 2021;73(1):120–151. doi: 10.1124/pharmrev.120.000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pejaver V, Hsu W-L, Fuxiao Xin A, Dunker K, Uversky VN, Radivojac P. The structural and functional signatures of proteins that undergo multiple events of post-translational modification. Protein Sci: A Publication of the Protein Society. 2014;23(8):1077–1093. doi: 10.1002/pro.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier D, Hafler DA. Fingolimod for multiple sclerosis. N Engl J Med. 2012;366(4):339–347. doi: 10.1056/NEJMct1101691. [DOI] [PubMed] [Google Scholar]
- Peterson YK, Luttrell LM. The diverse roles of arrestin scaffolds in G protein-coupled receptor signaling. Pharmacol Rev. 2017;69(3):256–297. doi: 10.1124/pr.116.013367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W, Antonini A, Zijlmans JC, Burkhard PR, Vingerhoets F. Levodopa in the treatment of Parkinson’s disease: an old drug still going strong. Clin Interv Aging. 2010;5(September):229–238. doi: 10.2147/cia.s6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon CH, Wang Y, Fung M-L, Zhang C, Lim LW. Rodent models of amyloid-beta feature of Alzheimer’s disease: development and potential treatment ımplications. Aging Dis. 2020;11(5):1235–1259. doi: 10.14336/AD.2019.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez MJ. 5-HT6 Receptors and Alzheimer’s disease. Alzheimers Res Ther. 2013;5(2):15. doi: 10.1186/alzrt169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan R, Haque S, Coley K, Shepheard S, Cooper-Knock J, Kirby J. Multifaceted genes in amyotrophic lateral sclerosis-frontotemporal dementia. Front Neurosci. 2020;14(July):684. doi: 10.3389/fnins.2020.00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu S, Singh AK. Novel method for pharmacophore analysis by examining the joint pharmacophore space. J Chem Inf Model. 2011;51(5):1106–1121. doi: 10.1021/ci100503y. [DOI] [PubMed] [Google Scholar]
- Rao JN, Jao CC, Hegde BG, Langen R, Ulmer TS. A combinatorial NMR and EPR approach for evaluating the structural ensemble of partially folded proteins. J Am Chem Soc. 2010;132(25):8657–8668. doi: 10.1021/ja100646t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Grossman HT, Sano M, Perl DP, Purohit DP, Gorman JM, Haroutunian V. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63(2):161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- Reneerkens OAH, Rutten K, Bollen E, Hage T, Blokland A, Steinbusch HWM, Prickaerts J. Inhibition of phosphodiesterase type 2 or type 10 reverses object memory deficits ınduced by scopolamine or MK-801. Behav Brain Res. 2013;236(January):16–22. doi: 10.1016/j.bbr.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Riemekasten G, Petersen F, Heidecke H. What makes antibodies against G protein-coupled receptors so special? A novel concept to understand chronic diseases. Front Immunol. 2020;11:564526. doi: 10.3389/fimmu.2020.564526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero PR, Zaidi S, Fang YY, Uversky VN, Radivojac P, Oldfield CJ, Cortese MS, et al. Alternative splicing in concert with protein ıntrinsic disorder enables ıncreased functional diversity in multicellular organisms. Proc Natl Acad Sci U S A. 2006;103(22):8390–8395. doi: 10.1073/pnas.0507916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SGF, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459(7245):356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russino D, McDonald E, Hejazi L, Hanson GR, Jones CE. The tachykinin peptide neurokinin B binds copper forming an unusual [CuII(NKB)2] complex and ınhibits copper uptake into 1321N1 astrocytoma cells. ACS Chem Neurosci. 2013;4(10):1371–1381. doi: 10.1021/cn4000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J. Amyloid-beta: a crucial factor in Alzheimer’s disease. Medi Principles Pract: International Journal of the Kuwait University, Health Science Centre. 2015;24(1):1–10. doi: 10.1159/000369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satała G, Duszyńska B, Lenda T, Nowak G, Bojarski AJ. Allosteric ınhibition of serotonin 5-HT7 receptors by zinc ıons. Mol Neurobiol. 2018;55(4):2897–2910. doi: 10.1007/s12035-017-0536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N. Calcitonin gene-related peptide-, neuropeptide Y- and tyrosine hydroxylase-ımmunoreactive nerve fibers in the human umbilical cord. Kurume Med J. 1998;45(4):327–331. doi: 10.2739/kurumemedj.45.327. [DOI] [PubMed] [Google Scholar]
- Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, Apostol MI, Thompson MJ, et al. Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature. 2007;447(7143):453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- Schaffert L-N, Carter WG. Do post-translational modifications ınfluence protein aggregation in neurodegenerative diseases: a systematic review. Brain Sci. 2020;10(4):E232. doi: 10.3390/brainsci10040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöneberg T, Schulz A, Biebermann H, Hermsdorf T, Römpler H, Sangkuhl K. Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol Ther. 2004;104(3):173–206. doi: 10.1016/j.pharmthera.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Scott LJ. Ubrogepant: first approval. Drugs. 2020;80(3):323–328. doi: 10.1007/s40265-020-01264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LJ, Goa KL. Galantamine: a review of its use in Alzheimer’s disease. Drugs. 2000;60(5):1095–1122. doi: 10.2165/00003495-200060050-00008. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestito S, Runfola M, Tonelli M, Chiellini G, Rapposelli S. New multitarget approaches in the war against glioblastoma: a mini-perspective. Front Pharmacol. 2018;9:874. doi: 10.3389/fphar.2018.00874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skieterska K, Rondou P, Van Craenenbroeck K. Regulation of G protein-coupled receptors by ubiquitination. Int J Mol Sci. 2017;18(5):E923. doi: 10.3390/ijms18050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snel B, Lehmann G, Bork P, Huynen MA. STRING: a web-server to retrieve and display the repeatedly occurring neighbourhood of a gene. Nucleic Acids Res. 2000;28(18):3442–3444. doi: 10.1093/nar/28.18.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Li H, Guo S, Pan Y, Yuhua F, Zhou Z, Li Z, et al. Targeting Gpr52 lowers mutant HTT levels and rescues Huntington’s disease-associated phenotypes. Brain J Neurol. 2018;141(6):1782–1798. doi: 10.1093/brain/awy081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Noble S. Rivastigmine: a review of its use in Alzheimer’s disease. Drugs Aging. 1998;13(5):391–411. doi: 10.2165/00002512-199813050-00005. [DOI] [PubMed] [Google Scholar]
- Stanciu GD, Luca A, Rusu RN, Bild V, Chiriac SIB, Solcan C, Bild W, Ababei DC. Alzheimer’s disease pharmacotherapy in relation to cholinergic system ınvolvement. Biomolecules. 2019;10(1):E40. doi: 10.3390/biom10010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis L. α-Synuclein in Parkinson’s disease. Cold Spring Harbor Perspect Med. 2012;2(2):a009399. doi: 10.1101/cshperspect.a009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell J, Jakova E, Cayabyab FS. Adenosine A1 and A2A receptors in the brain: current research and their role in neurodegeneration. Molecules (Basel, Switzerland) 2017;22(4):E676. doi: 10.3390/molecules22040676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulli G, Manoogian ENC, Taub PR, Panda S. Training the circadian clock, clocking the drugs, and drugging the clock to prevent, manage, and treat chronic diseases. Trends Pharmacol Sci. 2018;39(9):812–827. doi: 10.1016/j.tips.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton LP, Orlandi C, Song C, Won Chan O, Muntean BS, Xie K, Filippini A, et al. Orphan receptor GPR158 controls stress-ınduced depression. ELife. 2018;7(February):e33273. doi: 10.7554/eLife.33273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes DA, Stoddart LA, Kilpatrick LE, Hill SJ. Binding kinetics of ligands acting at GPCRs. Mol Cell Endocrinol. 2019;485(April):9–19. doi: 10.1016/j.mce.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syrovatkina V, Alegre KO, Dey R, Huang X-Y. Regulation, signaling, and physiological functions of G-proteins. J Mol Biol. 2016;428(19):3850–3868. doi: 10.1016/j.jmb.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöllosi D, Horváth T, Han KH, Dokholyan NV, Tompa P, Kalmár L. Discrete molecular dynamics can predict helical prestructured motifs in disordered proteins. PLoS One. 2014;9:95795. doi: 10.1371/journal.pone.0095795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakar A, Sylar E, Flynn FW. Activation of tachykinin, neurokinin 3 receptors affects chromatin structure and gene expression by means of histone acetylation. Peptides. 2012;38(2):282–290. doi: 10.1016/j.peptides.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thal DM, Sun B, Feng D, Nawaratne V, Leach K, Felder CC, Bures MG, et al. Crystal structures of the M1 and M4 muscarinic acetylcholine receptors. Nature. 2016;531(7594):335–340. doi: 10.1038/nature17188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thathiah A, Horré K, Snellinx A, Vandewyer E, Huang Y, Ciesielska M, De Kloe G, Munck S, De Strooper B. β-Arrestin 2 regulates Aβ generation and γ-secretase activity in Alzheimer’s disease. Nat Med. 2013;19(1):43–49. doi: 10.1038/nm.3023. [DOI] [PubMed] [Google Scholar]
- Thiriet N, Agasse F, Nicoleau C, Guégan C, Vallette F, Cadet J-L, Jaber M, Malva JO, Coronas V. NPY promotes chemokinesis and neurogenesis in the rat subventricular zone: NPY, neurogenesis and chemokinesis of SVZ cells. J Neurochem. 2011;116(6):1018–1027. doi: 10.1111/j.1471-4159.2010.07154.x. [DOI] [PubMed] [Google Scholar]
- Tobin AB. G-Protein-coupled receptor phosphorylation: where, when and by whom. Br J Pharmacol. 2008;153(Suppl1 (March)):S167–S176. doi: 10.1038/sj.bjp.0707662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tressel SL, Koukos G, Tchernychev B, Jacques SL, Covic L, Kuliopulos A. Pharmacology, biodistribution, and efficacy of GPCR-based pepducins in disease models. Methods Mol Biol (Clifton, NJ) 2011;683:259–275. doi: 10.1007/978-1-60761-919-2_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trzeciakiewicz H, Tseng J-H, Wander CM, Madden V, Tripathy A, Yuan C-X, Cohen TJ. A dual pathogenic mechanism links tau acetylation to sporadic tauopathy. Sci Rep. 2017;7(March):44102. doi: 10.1038/srep44102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigelny IF, Mukthavaram R, Kouznetsova VL, Chao Y, Babic I, Nurmemmedov E, Pastorino S, et al. Multiple spatially related pharmacophores define small molecule ınhibitors of OLIG2 in glioblastoma. Oncotarget. 2017;8(14):22370–22384. doi: 10.18632/oncotarget.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuteja N. Signaling through G protein coupled receptors. Plant Signal Behav. 2009;4(10):942–947. doi: 10.4161/psb.4.10.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle MD, Comellas G, Nieuwkoop AJ, Covell DJ, Berthold DA, Kloepper KD, Courtney JM, et al. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nat Struct Mol Biol. 2016;23(5):409–415. doi: 10.1038/nsmb.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich S, Wurthmann C, Brosz M, Meyer FP. The relationship between serum concentration and therapeutic effect of haloperidol in patients with acute schizophrenia. Clin Pharmacokinet. 1998;34(3):227–263. doi: 10.2165/00003088-199834030-00005. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Multitude of binding modes attainable by ıntrinsically disordered proteins: a portrait gallery of disorder-based complexes. Chem Soc Rev. 2011;40:1623–1634. doi: 10.1039/c0cs00057d. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Intrinsic disorder-based protein ınteractions and their modulators. Curr Pharm Des. 2013;19:4191–4213. doi: 10.2174/1381612811319230005. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Unusual biophysics of ıntrinsically disordered proteins. Biochim Biophys Acta. 2013;1834(5):932–951. doi: 10.1016/j.bbapap.2012.12.008. [DOI] [PubMed] [Google Scholar]
- Uversky VN. A decade and a half of protein ıntrinsic disorder: biology still waits for physics: protein ıntrinsic disorder. Protein Sci. 2013;22(6):693–724. doi: 10.1002/pro.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN. Functional roles of transiently and ıntrinsically disordered regions within proteins. FEBS J. 2015;282:1182–1189. doi: 10.1111/febs.13202. [DOI] [PubMed] [Google Scholar]
- Uversky VN. The multifaceted roles of ıntrinsic disorder in protein complexes. FEBS Lett. 2015;589(19 Pt A):2498–2506. doi: 10.1016/j.febslet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Dancing protein clouds: the strange biology and chaotic physics of ıntrinsically disordered proteins. J Biol Chem. 2016;291(13):6681–6688. doi: 10.1074/jbc.R115.685859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN. Protein ıntrinsic disorder-based liquid–liquid phase transitions in biological systems: complex coacervates and membrane-less organelles. Adv Colloid Interf Sci. 2017;239(January):97–114. doi: 10.1016/j.cis.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Uversky VN. Conserved functional dynamics: ı like to move ıt, move ıt! Structure. 2018;26(3):371–373. doi: 10.1016/j.str.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Keith Dunker A. Understanding protein non-folding. Biochim Biophys Acta. 2010;1804(6):1231–1264. doi: 10.1016/j.bbapap.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uversky VN, Oldfield CJ, Keith Dunker A. Showing your ID: ıntrinsic disorder as an ID for recognition, regulation and cell signaling. J Mol Recogn: JMR. 2005;18(5):343–384. doi: 10.1002/jmr.747. [DOI] [PubMed] [Google Scholar]
- Vacic V, Oldfield CJ, Mohan A, Radivojac P, Cortese MS, Uversky VN, Keith Dunker A. Characterization of molecular recognition features, MoRFs, and their binding partners. J Proteome Res. 2007;6(6):2351–2366. doi: 10.1021/pr0701411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lee R, Buljan M, Lang B, Weatheritt RJ, Daughdrill GW, Keith Dunker A, Fuxreiter M, et al. Classification of ıntrinsically disordered regions and proteins. Chem Rev. 2014;114(13):6589–6631. doi: 10.1021/cr400525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi M, Kosol S, Lebrun P, Valentini E, Martin Blackledge A, Dunker K, Felli IC, et al. PE-DB: a database of structural ensembles of ıntrinsically disordered and of unfolded proteins. Nucleic Acids Res. 2014;42(D1):D326–D335. doi: 10.1093/nar/gkt960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatakrishnan AJ, Flock T, Prado DE, Oates ME, Gough J, Madan Babu M. Structured and disordered facets of the GPCR fold. Curr Opin Struct Biol. 2014;27(August):129–137. doi: 10.1016/j.sbi.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Voss T, Li J, Cummings J, Farlow M, Assaid C, Froman S, Leibensperger H, et al. Randomized, controlled, proof-of-concept trial of MK-7622 in Alzheimer’s disease. Alzheimer’s Dementia (New York, N Y) 2018;4:173–181. doi: 10.1016/j.trci.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker D, Stevens RC, Roth BL. How ligands illuminate GPCR molecular pharmacology. Cell. 2017;170(3):414–427. doi: 10.1016/j.cell.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther C, Ferguson SSG. Minireview: Role of ıntracellular scaffolding proteins in the regulation of endocrine G protein-coupled receptor signaling. Mol Endocrinol (Baltimore, Md) 2015;29(6):814–830. doi: 10.1210/me.2015-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Martin B, Brenneman R, Luttrell LM, Maudsley S. Allosteric modulators of G protein-coupled receptors: future therapeutics for complex physiological disorders. J Pharmacol Exp Ther. 2009;331(2):340–348. doi: 10.1124/jpet.109.156380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Felsing DE, Chen H, Stutz SJ, Murphy RE, Cunningham KA, Allen JA, Zhou J. Discovery of potent and brain-penetrant GPR52 agonist that suppresses psychostimulant behavior. J Med Chem. 2020;63(22):13951–13972. doi: 10.1021/acs.jmedchem.0c01498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Orlandi C. Orphan G protein coupled receptors in affective disorders. Genes. 2020;11(6):694. doi: 10.3390/genes11060694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley M, Wootten D, Conner MT, Simms J, Kendrick R, Logan RT, Poyner DR, Barwell J. Lifting the lid on GPCRs: the role of extracellular loops. Br J Pharmacol. 2012;165(6):1688–1703. doi: 10.1111/j.1476-5381.2011.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise-Scira O, Aloglu AK, Dunn A, Sakallioglu IT, Coskuner O. Structures and free energy landscapes of the wild-type and A30P mutant-type α-synuclein proteins with dynamics. ACS Chem Neurosci. 2013;4(3):486–497. doi: 10.1021/cn300198q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise-Scira O, Dunn A, Aloglu AK, Sakallioglu IT, Coskuner O. Structures of the E46K mutant-type α-synuclein protein and ımpact of E46K mutation on the structures of the wild-type α-synuclein protein. ACS Chem Neurosci. 2013;4(3):498–508. doi: 10.1021/cn3002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JA, Liu T, Trotter C, Fang CC, De Narvaez E, Le Pochat P, Maslar D, Bukhari A, Zhao X, Deonarine A, Westerheide SD, et al. Loss of function CHCHD10 mutations in cytoplasmic TDP-43 accumulation and synaptic ıntegrity. Nat Commun. 2017;8(June):15558. doi: 10.1038/ncomms15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AS. The Dopamine D(4) Receptor, the ultimate disordered protein. J Recept Signal Transduct Res. 2010;30(5):331–336. doi: 10.3109/10799893.2010.513842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods S, Clarke NN, Layfield R, Fone KCF. 5-HT(6) Receptor agonists and antagonists enhance learning and memory in a conditioned emotion response paradigm by modulation of cholinergic and glutamatergic mechanisms. Br J Pharmacol. 2012;167(2):436–449. doi: 10.1111/j.1476-5381.2012.02022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PE, Jane Dyson H. Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol. 2015;16(1):18–29. doi: 10.1038/nrm3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Thiyagarajan S, O’Shaughnessy B, Karatekin E. Regulation of exocytotic fusion pores by SNARE protein transmembrane domains. Front Mol Neurosci. 2017;10(October):315. doi: 10.3389/fnmol.2017.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Keith Dunker A, Uversky VN, Kurgan L. Molecular recognition features (MoRFs) in three domains of life. Mol BioSyst. 2016;12(3):697–710. doi: 10.1039/c5mb00640f. [DOI] [PubMed] [Google Scholar]
- Yanamala N, Klein-Seetharaman J. Allosteric modulation of G protein coupled receptors by cytoplasmic, transmembrane and extracellular ligands. Pharmaceuticals. 2010;3(10):3324–3342. doi: 10.3390/ph3103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Perlmutter JS, Benzinger TLS, Morris JC, Jinbin X. Dopamine D3 receptor: a neglected participant in Parkinson disease pathogenesis and treatment? Ageing Res Rev. 2020;57(January):100994. doi: 10.1016/j.arr.2019.100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Niu X, Jin F, Liu Z, Jin C, Lai L. Structure-based ınhibitor design for the ıntrinsically disordered protein c-Myc. Sci Rep. 2016;6(March):22298. doi: 10.1038/srep22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang S, Gan L, Vosler PS, Gao Y, Zigmond MJ, Chen J. Protective effects and mechanisms of sirtuins in the nervous system. Prog Neurobiol. 2011;95(3):373–395. doi: 10.1016/j.pneurobio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Faraggi E, Li Z, Zhou Y. Intrinsically semi-disordered state and ıts role in ınduced folding and protein aggregation. Cell Biochem Biophys. 2013;67(3):1193–1205. doi: 10.1007/s12013-013-9638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Deng Y, Jiang Z, Qing H. G Protein-coupled receptors (GPCRs) in Alzheimer’s disease: a focus on BACE1 related GPCRs. Front Aging Neurosci. 2016;8:58. doi: 10.3389/fnagi.2016.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z-D, Saw W-T, Tan E-K. Mitochondrial CHCHD-containing proteins: physiologic functions and link with neurodegenerative diseases. Mol Neurobiol. 2017;54(7):5534–5546. doi: 10.1007/s12035-016-0099-5. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhao S, Keith Dunker A. Intrinsically disordered proteins link alternative splicing and post-translational modifications to complex cell signaling and regulation. J Mol Biol. 2018;430(16):2342–2359. doi: 10.1016/j.jmb.2018.03.028. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhang R, Zhang S, Jihong W, Sun X. Activation of 5-HT1A receptors promotes retinal ganglion cell function by ınhibiting the CAMP-PKA pathway to modulate presynaptic GABA release in chronic glaucoma. J Neurosci Off J Soc Neurosci. 2019;39(8):1484–1504. doi: 10.1523/JNEUROSCI.1685-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Ma D, Tan E-K. Mitochondrial CHCHD2 and CHCHD10: roles in neurological diseases and therapeutic ımplications. Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2020;26(2):170–184. doi: 10.1177/1073858419871214. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010;15(9–10):371–383. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou R, Wang X, Shu Li HC, Chan S, Vogel H, Yuan S. The role of metal ıons in G protein-coupled receptor signalling and drug discovery. WIREs Comp Mol Sci. 2022;12(2):e1565. doi: 10.1002/wcms.1565. [DOI] [Google Scholar]