Abstract
As far the current severe coronavirus disease 2019 (COVID-19), respiratory disease is still the biggest threat to human health. In addition, infectious respiratory diseases are particularly prominent. In addition to killing and clearing the infection pathogen directly, regulating the immune responses against the pathogens is also an important therapeutic modality. Sirtuins belong to NAD+-dependent class III histone deacetylases. Among 7 types of sirtuins, silent information regulator type-1 (SIRT1) played a multitasking role in modulating a wide range of physiological processes, including oxidative stress, inflammation, cell apoptosis, autophagy, antibacterial and antiviral functions. It showed a critical effect in regulating immune responses by deacetylation modification, especially through high-mobility group box 1 (HMGB1), a core molecule regulating the immune system. SIRT1 was associated with many respiratory diseases, including COVID-19 infection, bacterial pneumonia, tuberculosis, and so on. Here, we reviewed the latest research progress regarding the effects of SIRT1 on immune system in respiratory diseases. First, the structure and catalytic characteristics of SIRT1 were introduced. Next, the roles of SIRT1, and the mechanisms underlying the immune regulatory effect through HMGB1, as well as the specific activators/inhibitors of SIRT1, were elaborated. Finally, the multitasking roles of SIRT1 in several respiratory diseases were discussed separately. Taken together, this review implied that SIRT1 could serve as a promising specific therapeutic target for the treatment of respiratory diseases.
Keywords: Silent information regulator type-1 (SIRT1), Deacetylation, Respiratory diseases, Modulate, Promising target
INTRODUCTION
At present, respiratory diseases, especially infectious respiratory diseases, are still the biggest threat to human health. Infection-induced inflammation is a physiological response of the immune system to harmful infectious stimuli. In response to such stimuli, immune cells, such as macrophages and neutrophils cells take concerted actions to recover and maintain immune homeostasis. Immune imbalance has been implicated to be the pathogenesis of various respiratory diseases, including coronavirus disease 2019 (COVID-19), bacterial pneumonia, tuberculosis, and so on (1,2,3). Actively restoring the balance of the immune system will be an effective treatment strategy. High-mobility group box 1 (HMGB1) as the core molecule of the immune regulatory network has become a research hotspot. Silent information regulator type-1 (SIRT1) has received particular attention based on its specific modulatory role on HMGB1.
SIRT1 can regulate the immune system and restore immune homeostasis through its NAD+ dependent histone deacetylase (HDAC) activity. It shares a generic catalytic core domain with other sirtuins family members, but it has its unique N-terminal and C-terminal sequences. Substrate and catalytic core domain binding plays an essential role in the deacetylation activity of SIRT1. Base on the structure characteristics, SIRT1 was originally identified as a critical enzyme to increase life expectancy in yeast, worm, fly and mice (4). More importantly, it also has other vital functions such as anti-apoptosis, anti-oxidation, anti-inflammation, and regulating autophagy and mitochondrial biogenesis (5). At present, the mechanisms by which SIRT1 and HMGB1 participate in inflammation are still not fully defined. However, there is no doubt that SIRT1 is involved in a variety of respiratory diseases, including COVID-19, bacterial pneumonia, tuberculosis, etc., bringing a heavy burden to patients and society. It is of great significance to validate SIRT1 as a promising therapeutic target. Hence, the focus of this review is to summarize the latest advances regarding the potential beneficial roles of SIRT1 in regulating respiratory inflammation.
MOLECULE STRUCTURE AND NAD+ DEPENDENT CATALYTIC CHARACTERISTICS OF SIRT1
Sirtuins belong to the NAD+ dependent class III HDAC family, and are highly conserved during the process of evolution (6,7). Currently, 7 Sirtuin members including SIRT1–7 have been identified. SIRT1, SIRT6, and SIRT7 are mainly localized in the nucleus, while most SIRT3, SIRT4, and SIRT5 are localized in mitochondria (8). Residues 41–46 of SIRT1 protein constitute a nuclear localization signal (KRKKRK). Moreover, SIRT1 is also expressed in the cytoplasm, and the nuclear import and export sequences on N-terminal region are responsible for nucleocytoplasmic shuttling of SIRT1 (9). SIRT1 gene is located on human chromosome 10q22.1, containing 9 exons and 8 introns. Its exons encode 747 amino acids, including about 270 deacetylated amino acids in the core domain (10). The gene sequence is about 33kb in length, and consists of an untranslated region of 53 bp and 1,793 bp at the 5' and 3' ends, respectively (11).
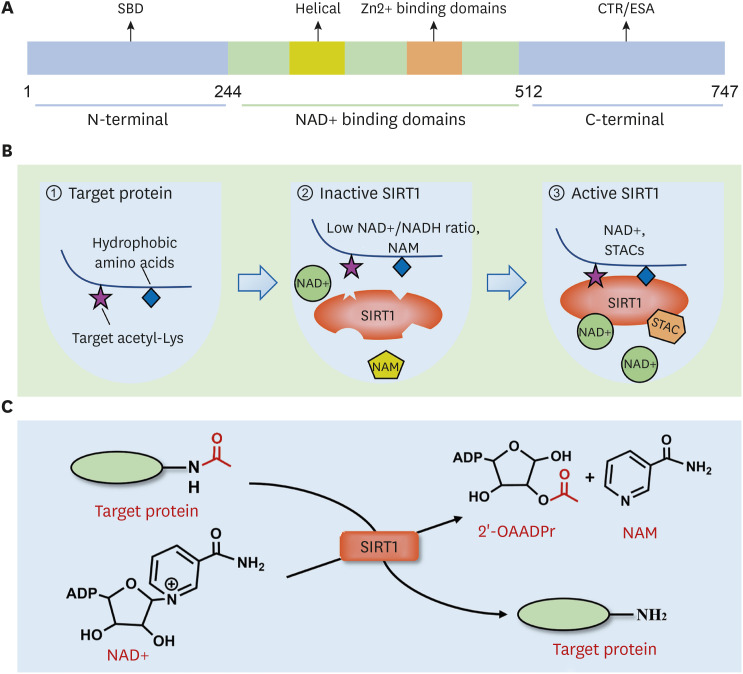
All sirtuins share an evolutionarily conserved NAD+-dependent catalytic core domain. Moreover, each of them has an unique N-terminal and C-terminal sequence that is related to its specific cellular localization and function (12,13). The generic catalytic core domain of about 270 amino acids (spanning residues 244–512) folds into 2 subdomains. The larger subdomain adopts a Rossmann fold conformation that is typical for NAD+ binding proteins, comprising 6 parallel β-strands forming a central β-sheet surrounded by α-helices. The small subdomain forms a Zn2+ binding module in which 4 cysteine residues serve as Zn2+ binding ligands (13). A groove is shaped between the large and small subdomains, which can bind the substrate. Among SIRT1–7, SIRT1 has the largest terminal region extensions. Its unique N-terminal (the NH2-terminal region) contains 513–747 residues, and its unique C-terminal (the COOH-terminal region) includes 1–180 residues. SIRT1 protein is characterized by a N-terminal triple-helix bundle representing the sirtuins-activating compounds binding domain, and a C-terminal regulatory segment (9,12,14) (Fig. 1).
Figure 1. Molecular structure and biological functional characteristics of SIRT1. (A) SIRT1 has an evolutionarily conserved NAD+-dependent catalytic core domain (244–512 residues), unique N-terminal (513–747 residues), and C-terminal (1–180 residues) sequences. SBD locates on N-terminal, and CTR/ESA locates on C-terminal. (B) SIRT1 prefers specific hydrophobic amino acids near the target Lys residue for substrate recognition (panel 1). Low NAD+/NADH ratio and NAM weaken the activity of SIRT1 (panel 2). STACs activate SIRT1 by combing with the SBD, increasing the catalytic activity of SIRT1 (panel 3). (C) The deacetylation mechanism is mediated by SIRT1. The acetyl group of the substrate is transferred to the ADP ribosyl part of NAD+, while an NAD+ molecule is split into 1 NAM and 1 2-OAADPr.
SBD, sirtuins-activating compounds binding domain; STAC, sirtuins-activating compound; CTR, C-terminal regulatory segment.
In Sirtuin-mediated deacetylation reaction, the acetyl group of the substrate is transferred to the ADP ribosyl moiety of NAD+. At the same time, 1 NAD+ molecule splits into 1 nicotinamide (NAM) and 1 2′-O-acylated ADP-ribose (15) (Fig. 1). NAD+ participates in the deacetylation of target proteins, and a low NAD+/NADH ratio will weaken SIRT1 activity. Meanwhile, as a product of the deacetylation process, NAM can suppress the activity of SIRT1 through a negative feedback mechanism (16) (Fig. 1). SIRT1 recognizes NAD+ through the active sites located on the Rossmann fold. One study showed that SIRT1 first approached specific hydrophobic amino acids near target lysine (Lys) residue for substrate recognition (17). Once recognized, SIRT1 deacetylates the substrate using the same catalytic mechanism as other members of the Sirtuin family. When the acetylated substrate binds to the enzyme, the conformation of NAD+ changes, which allows the NAM group to be easily cleaved. Through the active site on the Rossmann fold of SIRT1, the carbonyl oxygen group of the acetylated Lys residue in the substrate contacts the anomeric carbon of the NAD+ NAM nucleoside. This combination promotes cleavage of the NAD+ NAM moiety and transfer of ADP-ribose, resulting in deacetylation of the substrate (18). The process of deacetylation of acetylated substrates catalyzed by SIRT1 is as follows: Step one, SIRT1 deacetylase recognizes NAD+ and acetylated substrate to form a ternary complex. Then, NAD+ is hydrolyzed, releasing NAM from the 1′-ribose carbon of NAD+, which concomitantly forms a new covalent bond through binding with the acyl oxygen of the acylated Lys residue on the substrate. Step 2, the resulting 1′-O-alkylimidate intermediate is converted to a second bicyclic 1′-2′-acetal intermediate. The last step, in the presence of water molecules, the bicyclic intermediate is hydrolyzed to yield the deacetylated substrate and 2′-O-acylated ADP-ribose (2-OAADPr). Under physiological conditions, 2-OAADPr can be reversibly transformed to 3-OAADPr (13).
DEACETYLATION EFFECTS AND MOMENTOUS SUBSTRATES
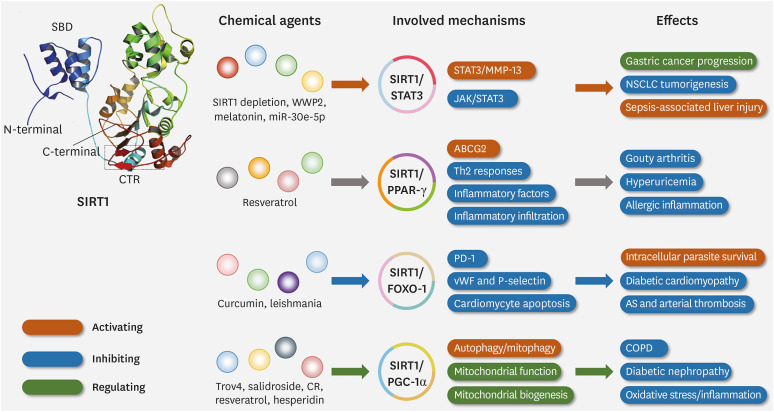
Truly, as a HDAC, SIRT1 has been reported to indeed catalyze the deacetylation of histone H1 at Lys 26 (K26), histone H3 at Lys 9 (K9), and histone H4 at Lys 16 (K16) (19). However, it also plays an important role in regulating the deacetylation of non-histone proteins, including p53, HMGB1, forkhead box Os (FoxOs), STAT3, peroxisome proliferator-activated receptor-γ (PPAR-γ), NF-κB, peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), B-cell lymphoma 2-associated X protein (Bax), etc. (8,20). Recently, many studies have reported the emerging roles of SIRT1 in many diseases, especially in the occurrence, development, and prognosis of respiratory diseases (Fig. 2).
Figure 2. Three-dimensional structure, effects of SIRT1 and the underlying mechanism. SIRT1 has obvious effects in regulating a variety of respiratory diseases by acting on different momentous substrates. SIRT1 can regulate gastric cancer, NSCLC, and sepsis-associated liver injury by activating the STAT3/MMP-13 pathway or inhibiting the JAK/STAT3 pathway, especially via STAT3. SIRT1 affects gouty arthritis, hyperuricemia, and allergic inflammation by activating ABCG2, inhibiting Th2 response and inflammatory cell infiltration, especially through binding with PPAR-γ. SIRT1 can modulate diabetic cardiomyopathy, atherosclerosis, and arterial thrombosis through inhibiting PD-1, cardiomyocyte apoptosis, vWF, and P-selectin, especially acting on FOXO-1. SIRT1 can control COPD, diabetic nephropathy, oxidative stress, and inflammation by activating autophagy, mitophagy, or regulating mitochondrial function, mitochondrial biogenesis, especially via PGC-1α.
vWF, von Willebrand factor; CTR, C-terminal regulatory segment; CR, caloric restriction.
STAT3 is a member of the STATs, and is described as a cytoplasmic transcription factor. It participates in regulating many biological events, including cell proliferation, differentiation, apoptosis, angiogenesis, inflammation, and immune responses (21,22). STAT3 has been reported as a downstream target of SIRT1, which catalyzes the deacetylation of STAT3 Lys residue to control its function (23). Recently, some posttranslational modifications related to the transcriptional function of STAT3 have been mentioned, such as acetylation and methylation (24). Some studies have shown that SIRT1/STAT3 plays an important role in the development of some diseases, including influencing the expression of IL-4 in T-lymphocytes, inhibiting T-cell differentiation into Th7 and Th17 cells, suppressing the proliferation and metastasis of gastric cancer cells, and preventing hyperglycemia in sepsis, etc. (23,25). Moreover, the role of SIRT1 in regulating respiratory diseases has also been revealed. For example, a recent study has found that microRNA (miR)-30e-5p can inhibit the development of non-small cell lung cancer (NSCLC) through downregulating ubiquitin-specific peptidase 22-mediated SIRT1/JAK/STAT3 signaling (26).
Transcriptional coactivator PGC-1α is regarded as a main regulator of oxidative phosphorylation and ROS detoxification, and it is also described as an essential factor in tying metabolic regulation, redox control, and inflammatory pathways (27). The post-transcriptional activity of PGC-1α was modulated by several post-translational modifications in cells, including phosphorylation, acetylation, and ubiquitination (28). PGC-1α is considered as a substrate of SIRT1, which means that the transcriptional activity of PGC-1α is influenced by SIRT1-mediated deacetylation (29). A number of studies have suggested the protective role of SIRT1/PGC-1α against some diseases, including protecting the intestinal mucosal barrier from free oxygen radical damage, coping with oxidative stress caused by hyperglycemia, and protecting the diabetic heart (30,31). Specifically, one study indicated that resveratrol could exert a therapeutic effect on the rat chronic obstructive pulmonary disease (COPD) model. This study also pointed out that the efficacy of resveratrol was interrelated to the inhibition of oxidative stress and inflammatory responses, and the possible underlying mechanism might involve the activation and upgrading of the SIRT1/PGC-1α pathway (32). Another strong evidence showed that hesperidin mitigated inflammatory responses and oxidative stress in cigarette smoke extract-induced COPD mice, which was reported to be associated with the SIRT1/PGC-1α/NF-κB signaling axis (33). These findings demonstrate that SIRT1 may have a therapeutic effect for some diseases, including respiratory diseases, through targeting its specific substrates, such as PGC-1α.
FoxOs are a subgroup family of forkhead box transcription factors and play a crucial role in cell proliferation, differentiation, and apoptosis. In mammals, this family consisted of 4 members, including FOXO1 (also named FKHR), FOXO3 (also named FKHRL1), FOXO4 (also named AFX1), and FOXO6 (34,35). The activity of FoxOs is dependent on its phosphorylation modification and nuclear localization. In addition, the transcriptional activity of FOXOs can be regulated by other post-translational modifications, such as acetylation and deacetylation (36). FOXO1 is one target of SIRT1. Some studies have reported the role of SIRT1/FOXO1 in some diseases, such as decreasing osteoclast and increasing osteoblast number in bone, preventing the progression of atherosclerosis and arterial thrombosis, and inhibiting oxidative stress and apoptosis in cardiomyocytes (37,38). In a recent study, SIRT1 has been demonstrated to be a major factor to mediate the deacetylation of FOXO1, thus inhibiting apoptosis. This view was further confirmed by the fact that knockdown and inhibition of SIRT1 could maintain the acetylated state of FOXO1. Moreover, this study also showed that Leishmania negatively regulated the production of inflammatory TNFα, ROS, and nitric oxide via the SIRT1/FOXO1 axis (39). A recent study has shown that the expression levels of SIRT1 and FOXO1 in PBMCs of COPD outpatients are positively correlated with the duration of physical activity (40). Although the longitudinal relationship among physical activity, SIRT1, and FOXO1 in COPD is unclear, the results may provide a novel strategy in controlling COPD. According to these evidences, we can infer that SIRT1/FOXO1 may be a potential therapeutic target for the treatment of respiratory diseases, although there are no definite reports on the mechanism underlying the action of SIRT1/FOXO1 in respiratory diseases.
PPAR-γ is a member of the PPAR family, which includes 3 members, PPAR-α, PPAR-β/δ, and PPAR-γ (41). Recent studies have shown that PPAR-γ mediates part of the SIRT1 reactions, and the SIRT1/PPAR-γ signaling plays a crucial role in anti-hyperuricemia and anti-inflammatory function (42,43). On one hand, a recent study demonstrated that SIRT1 and RSV (the activator of SIRT1) inhibited inflammatory cell infiltration and secretion of inflammatory factors through mediating PPAR-γ, which could control the acute onset of gouty arthritis (43). On the other hand, another study showed that SIRT1 inhibited the activity of PPAR-γ in dendritic cells, thus facilitating a Th2 response (44). Based on the substrate profile of SIRT1, it can be indicated that PPAR-γ is an emerging target of SIRT1 in controlling some diseases, including respiratory diseases.
DOWNSTREAM PATHWAYS AND MECHANISMS THROUGH HMGB1
HMGB1 is a nuclear protein that is considered a key component involved in the late inflammatory responses (45). The functions of HMGB1 rely on its localization and post-translational modifications. In addition, translocation and secretion of HMGB1 are important processes influencing inflammation (46). Recently, TLRs have been demonstrated to be of great significance in the innate immune system, and HMGB1 could control the inflammatory responses through binding with other cellular receptors such as TLR2 and TLR4 (47). NF-κB refers to a family of transcription factors that exist in numerous cell types (48). Many studies have shown the key effect of NF-κB on regulating immunity and inflammatory responses. In the process of inflammation, extracellular HMGB1 activates some receptors such as advanced glycation end products and TLRS via medullary differentiation factor 88 (MyD88)-dependent signaling and non-MyD88-dependent signaling pathways (49). These pathways contribute to the activation of NF-κB. Phosphorylation of NF-κB can promote the release of a large number of inflammatory cytokines to initiate inflammation (50). The activation of HMGB1/NF-κB pathway is regulated by post-translational modifications, among which acetylation catalyzed by HDACs and histone acetyltransferases is a common post-translational modification form. Translocation and secretion of HMGB1 are also regulated by these enzymes, including SIRT1 (51).
HMGB1/TLR4/NF-κB pathway plays a critical role in regulating the occurrence, development, and prognosis of many diseases, such as the diseases of the digestive system, respiratory system, and nervous system, through acting on NLRP3 and absent in melanoma 2 inflammasomes in macrophages (52). In vivo mouse neonatal hypoxic-ischaemic brain injury model and in vitro experiments have shown that HMGB1 released from microglia can activate the TLR4/MyD88/NF-κB signaling in microglia, which contributes to high expression of neuroinflammatory mediators, leading to neuroinflammation (53,54). Moreover, another study also reported that ω-3 PUFA enhanced the SIRT1 activity to inhibit acetylation of HMGB1, leading to direct interactions between SIRT1 and HMGB1. This kind of interaction can inhibit the translocation and secretion of HMGB1, and hold back the activation of the NF-κB pathway mediated by HMGB1 after TBI-induced microglia activation, thus controlling the subsequent inflammatory responses (49). In summary, SIRT1 can modulate the development of some diseases via the HMGB1/TLR4/NF-κB pathway.
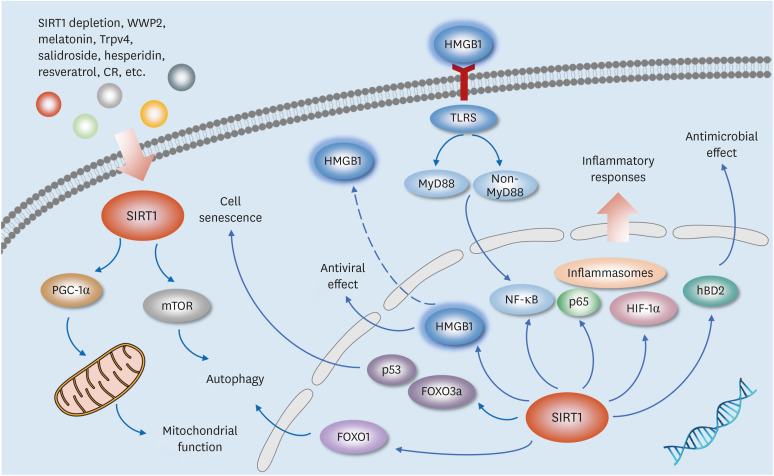
Other SIRT1-related pathways also play an important role in regulating respiratory diseases, and the mechanisms may involve influencing autophagy, anti-inflammation, anti-apoptosis, and so on (55,56,57,58,59). Recent reports have shown that regulating SIRT1/NF-κB pathway can inhibit TNF-α-induced pro-inflammatory responses (60). SIRT1/GATA-3 signaling could decrease the expression of IL-4 in patients with severe asthma (61). SIRT1/Akt/NF-κB signaling can play an anti-inflammatory role in asthma, while SIRT1/HIF-1α signaling exhibits a pro-inflammatory effect (62). In addition, SIRT1/TAK1 and SIRT1/Bax pathways are implicated in tuberculosis (63), SIRT1/COX-2 signaling is involved in the development of bacterial pneumonia (64), and SIRT1/MAPK signaling is important in regulating lung cancer progression (65) (Fig. 3).
Figure 3. Multitasking roles of SIRT1 through the downstream signaling pathways mediated by HMGB1. The multitasking roles of SIRT1 are shown in regulating antimicrobial effect, inflammatory responses, antiviral effect, autophagy, and mitochondrial function through the downstream signaling pathways mediated by HMGB1.
CR, caloric restriction.
ACTIVATORS AND INHIBITORS OF SIRT1
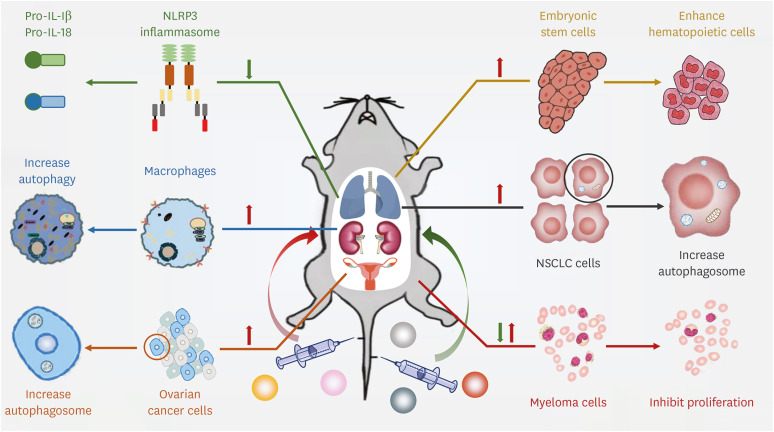
SIRT1 regulates its downstream targets through deacetylation, influencing the occurrence, development, and prognosis of various diseases (63,66,67,68). Therefore, SIRT1 may become a novel therapeutic target in treating many diseases, especially respiratory diseases. In addition, more efforts have been put into developing the modulators of the emerging attractive therapeutic target. The modulators of SIRT1 included 2 forms, the activators and inhibitors (Table 1). The former contained resveratrol, berberine, quercetin, metformin, SRT1720, SRT1460, SRT2183, and so on (69,70,71,72,73,74,75,76). The later comprised EX-527, tenovin-1, tenovin-6, cambinol, sirtinol, salermide, splitomicin, and NAM (77,78,79,80,81,82,83,84). These extensively investigated activators and inhibitors can modulate SIRT1 in cardiovascular diseases, inflammatory diseases, diabetes, and obesity (Fig. 4).
Table 1. List of activators and inhibitors of SIRT1.
| Type | Modulator | Function | Model | Reference |
|---|---|---|---|---|
| Activator | Resveratrol | Increase mitophagy | Osteoporosis rats | (69) |
| Quercetin | Suppress NLRP3 inflammasome | COVID-19 | (70) | |
| Berberine | Promote autophagy of peritoneal macrophages | Atherosclerosis | (73) | |
| Metformin | Activate autophagy, mitigates cartilage degradation | Mouse osteoarthritis | (74) | |
| Melatonin | Regulate apoptosis and autophagy | Sepsis-induced cardiac dysfunction | (75) | |
| SRT1720 | Partially attenuate fibrosis and apoptosis | Fibrotic kidney disease | (72) | |
| SRT1460 | Weaken oxidative stress | Myocardial ischemia/reperfusion injury | (71) | |
| SRT2183 | Induce autophagy | Ovarian cancer cells | (76) | |
| Inhibitor | EX-527 | Induced cell apoptosis | Glioma | (77) |
| Tenovin-1 | Induce a nonlinear apoptosis-inducing factor-dependent cell death | p53 null Ewing’s sarcoma cell line | (78) | |
| Tenovin-6 | Induced apoptosis and cell cycle arrest | Primary effusion lymphoma | (79) | |
| Cambinol | Inhibit proliferation and induce apoptosis | Myeloma cell lines | (80) | |
| Sirtinol | Protect the allograft from inflammatory cell infiltration | Mouse cervical heterotopic heart transplantation | (81) | |
| Salermide | Induce autophagy in human NSCLC cells | NSCLC | (82) | |
| Splitomicin | Enhance the yield of specific hematopoietic lineage cells from embryonic stem cells | Hematopoietic differentiation of embryonic stem cells | (83) | |
| NAM | Increase the sensitivity of chronic myeloid leukemia to doxorubicin | Chronic myeloid leukemia | (84) |
Figure 4. Regulatory effects verification of SIRT1 by activators or inhibitors injection. The regulatory effects of several SIRT1 activators and inhibitors are shown. Quercetin (an activator of SIRT1) suppresses NLRP3 inflammasome in COVID-19. Berberine (an activator of SIRT1) promotes the autophagy of peritoneal macrophages. SRT2183 (an activator of SIRT1) induces autophagy in ovarian cancer cells. SRT1720 (an activator of SIRT1) notably decreases collagen deposition in the mice kidneys. Salermide (an inhibitor of SIRT1) induces autophagy in human NSCLC cells. EX-527 (an inhibitor of SIRT1) suppresses the proliferation and colony formation ability of human glioma cells.
Activators
Resveratrol is a natural polyphenol with anti-inflammatory properties that has been deeply studied and can activate sirtuins (85). One study demonstrated that resveratrol could protect osteoblasts in osteoporosis rats by promoting mitophagy, and this effect was realized by mediating SIRT1 and PI3K/AKT/mTOR signaling pathways (69). Another study showed that resveratrol inhibited oxidative stress and apoptosis through SIRT1/FOXO3a and PI3K/AKT signaling pathways, which alleviated radiation-induced intestinal injury (86). Similarly, resveratrol exhibited a protective role against respiratory diseases. For example, resveratrol can inhibit oxidative stress and inflammatory responses on a rat COPD model, probably through activating the SIRT1/PGC-1α signaling (32).
Quercetin is a kind of abundant flavonoid compound present in plants and shows a variety of biological activities. It is reported that quercetin has powerful antioxidant, anti-inflammatory, and anti-tumor effects, offering significant prospects in the clinical application (87). In a study on diabetic encephalopathy, quercetin up-regulated the expression of SIRT1 protein and inhibited the expression of endoplasmic reticulum (ER)-associated proteins, which meant that quercetin may participate in diabetic encephalopathy through the SIRT1/ER pathway (88). In addition, quercetin could increase the expression of SIRT1 and suppress the content of NLRP3 inflammasome in COVID-19 patients, showing therapeutic potential to treat COVID-19 (70).
SRT1720 and SRT1460 are structurally diverse synthetic compounds and are also used as small molecular modulators of SIRT1. It was found that SRT1460 could activate SIRT1, which played a protective role in myocardial ischemia/reperfusion (I/R) injury (71). This finding may provide a new treatment strategy for myocardial I/R injury. Moreover, another study showed that SRT1720 improved the level of SIRT1 and partly alleviated unilateral ureteral obstruction (UUO)-induced renal fibrosis and apoptosis (72). This study concluded that SRT1720 as a SIRT1 activator had clinical significance in treating UUO-induced tubulointerstitial fibrosis.
Inhibitors
EX-527 is a SIRT1 inhibitor with great effectiveness and selectivity compared with other SIRT1 inhibitors (89). A recent study has shown that SIRT1 promotes tumorigenesis in glioma, and EX-527 induces cell apoptosis through activating p53, suggesting that EX-527 might be a potential target in the treatment of glioma (77). Moreover, it was reported that small interfering RNA (siRNA) or EX-527 could inhibit SIRT1 activity, which notably strengthened MK-1775-induced apoptosis and growth inhibition in human lung cancer cells (90).
Tenovin-6 is a potent class III-specific HDAC inhibitor and is also known as a p53 activator (91). It was confirmed in NSCLC cell lines with different liver kinase B1 status that the combination of metformin and tenovin-6 was more effectual in inhibiting cell growth compared with either drug alone (92). In addition, it was demonstrated that knockdown of SIRT1 by specific short hairpin RNAs or using SIRT1 inhibitor tenovin-6 induced apoptosis and cell cycle arrest in primary effusion lymphoma cells (79).
Sirtinol is another SIRT1 inhibitor discovered among more than 1,000 compounds through a high throughput cell-based screen (93). Some data from a recent study revealed the oncogenic role of SIRT1. In this study, it was also found that sirtinol could reduce cell proliferation of adrenocortical cancer and formation of colony and spheroids, as well as activate the intrinsic apoptotic pathway (94). Another evidence showed that SIRT1 played a key role in the immune responses after organ transplantation. However, inhibiting SIRT1 through sirtinol could protect the allograft from inflammatory cell infiltration then lengthen allograft survival time (81).
Salermide is a reverse amide with a strong inhibitory effect on SIRT1 (95). Some results have shown that SIRT1 inhibitors (such as salermide, NAM, sirtinol, and EX-527) significantly increase the survival rate of taste bud organoids after irradiation (96). Moreover, Mu et al. (82) found that the expression of SIRT1/2 was blocked by salermide or siRNAs, which could induce autophagy in human NSCLC cells.
MULTITASKING ROLES IN RESPIRATORY DISEASES
The COVID-19 broke out in December 2019. It is a kind of respiratory disease with a highly destructive effect, which has brought a heavy burden to patients, medical systems, and societies worldwide (97). According to recent data, including those on COVID-19, respiratory diseases seriously affected physical and mental health, as well as social development. Among respiratory diseases, infectious diseases account for a large proportion. In addition to acting directly on viruses and bacteria, regulating immunity is also an important therapeutic strategy in the treatment of infectious respiratory diseases and other respiratory diseases. When targeting different substrates, SIRT1 may show different effects in regulating the immunity. Thus, SIRT1 can exhibit multitasking roles in the development and treatment of respiratory diseases (Table 2).
Table 2. Summary of the related mechanism of SIRT1 in regulating several respiratory diseases.
| Disease | Receptor/Pathway | Activation | Inhibition | Function | Reference |
|---|---|---|---|---|---|
| COPD | FOXO3a/p53 | + | Protect against ACEII senescence in rats | (127) | |
| PGC-1α/NF-κB | + | Alleviate inflammation and oxidative stress responses | (33) | ||
| NF-κB/p65 | + | Suppress COPD inflammation | (66) | ||
| PGC-1α | + | Inhibit oxidative stress and inflammatory response | (32) | ||
| Asthma | IL-6 | + | Affect pulmonary function | (56) | |
| mTOR | + | Inhibit allergic airway inflammation by suppressing autophagy | (57) | ||
| Akt/NF-κB | + | Inhibit the development of airway inflammation | (62) | ||
| HIF-1α/VEGF | + | Increase the secretion of proinflammatory cytokines | (62) | ||
| PPAR-γ | + | Inhibit anti-inflammatory actions | (62) | ||
| Tuberculosis | TAK1/p65/p38/JNK/ERK | + | Enhance the secretion of IL-6 and TNF-α | (63) | |
| RelA/p65 | + | Dampen Mtb-mediated persistent inflammatory responses | (114) | ||
| GSK3β | + | Inhibit M. tuberculosis-induced apoptosis in macrophage | (58) | ||
| Bacterial pneumonia | hBD-2 | + | Antimicrobial effect | (110) | |
| IL-8 | + | Reduce inflammatory response | (110) | ||
| COX-2 | + | Reduce the bacterial load in different organs | (64) | ||
| Lung cancer | NF-κB/Smac | + | Reduce radiosensitivity | (59) | |
| NF-κB | + | Attenuate cell proliferation, migration and invasion | (67) | ||
| ATF4 and DDIT4 | + | Induce pro-survival autophagy in NSCLC cells | (82) | ||
| COVID-19 | NLRP3 | + | Inhibit inflammation | (70) | |
| K63 | + | Boost virally mediated induction of type 1 interferons | (102) | ||
| HMGB1 | + | Enhance the antiviral efficacy of type 1 interferons | (102) | ||
| ARDS | p65 | + | Ameliorate inflammatory response and oxidative stress | (58) | |
| MAPK | + | Alleviate ARDS | (132) |
COVID-19
The COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) erupted in December 2019 and was declared a pandemic by the World Health Organization (WHO) (98). COVID-19 infections are related to respiratory dysfunctions that can cause substantial alterations in clinical manifestation and have become a significant public health concern (99). COVID-19 may have 3 clinical phases, including the incipient upper respiratory tract infection phase, the pneumonia phase, and the hyperinflammatory phase that can lead to death (100). It has also been reported that imbalance of inflammatory responses, defectiveness in immune responses, and lymphopenia are annotated as critical factors of the pathogenesis of SARS-CoV-2 infection (100). In addition, high expression of p53 is associated with significantly decreased expression of SIRT1 and is related to higher expression of p21 in COVID-19 patients (101). A study showed that SIRT1 might improve the antiviral efficacy of type 1 interferons by preventing hyperacetylation of HMGB1 (102). Moreover, the inhibition of SIRT1 can reduce cytotoxicity of CD8 T cells in patients with systemic erythematosus lupus who were susceptible to SARS-CoV-2 infections (103). Given the above findings, SIRT1 may be an important factor regulating inflammatory responses, which may be necessary for the fight against COVID-19.
Bacterial pneumonia
Pneumonia can be caused by bacteria infection, such as Streptococcus pneumoniae, Klebsiella pneumoniae, Staphylococcus aureus, and Pseudomonas aeruginosa (104), which is also named bacterial pneumonia. The common complications of bacterial pneumonia include respiratory failure, sepsis, multiorgan failure, coagulopathy, etc. Many studies have shown that drug resistance of bacteria is increasing for some reasons, for example, the abuse of antibiotics (105). Because of the diversity of pathogenic bacteria, the severity of complications, and increased drug resistance, it is critical to develop a better treatment strategy for bacterial pneumonia. Streptococcus pneumoniae is a Gram-positive bacterium, and pneumococcal infection is a pathogenic factor for some diseases, including pneumonia, meningitis, and bacteremia/sepsis (106). Recent studies have shown that SIRT1 plays an important role in infection and inflammation (107,108). Previous in vivo and in vitro studies have demonstrated that celecoxib, a non-antibiotic agent, induced SIRT1 expression, which controlled the expressions of COX-2 and NF-κB, thus causing decreased expressions of pro-inflammatory cytokines (64). Similar effects were shown in a study on the effect of the SRT3025 compound (a SIRT1 activator) in treating pneumococcal pneumonia. The results showed that SRT3025 promoted the elimination of bacteria and reduced inflammatory cytokines in tissues of animals infected with Streptococcus pneumoniae, and this effect was most pronounced in the lung (109). Moreover, another study showed similar findings and reported that recombinant human β-defensin-2 (hBD-2) had an antimicrobial effect on Streptococcus pneumoniae in human pulmonary epithelial cells. IL-8 is a kind of CXC chemokine that can promote the recruitment of neutrophils and maintain inflammatory responses in the airway. It was found that enhancing expression of SIRT1 through activators could increase mRNA expression of hBD-2 but decrease IL-8 mRNA expression, and this effect could be reversed by SIRT1 inhibitors (110). Furthermore, chlorogenic acid could alleviate Klebsiella pneumoniae-induced pneumonia via SIRT1, concretely, chlorogenic acid inhibited the acetylation level and nuclear translocation of HMGB1 by activating SIRT1, thereby promoting M2 polarization and alleviating Klebsiella pneumoniae-induced pneumonia (111). Thus, we can deduce that SIRT1 plays an important role in bacteria pneumonia, especially Streptococcus pneumoniae.
Tuberculosis
Tuberculosis, a special kind of bacterial infectious disease caused by Mycobacterium tuberculosis (Mtb), is one of the deadliest infectious diseases around the world and has become a global health issue (112). A study reported that the increase in autophagy could promote innate host defense against multiple intracellular pathogens, especially Mtb (113). Thus, regulating autophagy is of great significance in controlling tuberculosis. In addition, it was found that post-translational activation of autophagy was realized by deacetylating autophagy-related genes ATG5, BECN1, and ATG7 through activating SIRT1 (114). In another study on SIRT1 and its activators in cells and animals infected by Mtb, some data such as bacillary loads and SIRT1 mRNA expression indicated that the activation of SIRT1 could inhibit the growth of Mtb and increase the clearance rate of Mtb with anti-tuberculosis drugs (115). Resveratrol can activate SIRT1, and in Mtb-infected macrophages, it can inhibit the activation of MAPK, TAK1, and NF-κB signaling pathways, as well as the levels of inflammatory cytokines, indicating that resveratrol may be used to treat tuberculosis through targeting SIRT1 (63). Therefore, we reasonably speculate that SIRT1 may become a potential target for the treatment of tuberculosis.
COPD and asthma
COPD is a prevalent and severe disease with high health and social care costs (116). Because of its complicated pathogenesis, there is no curative treatment currently. It has been reported that more than 3 million people die of COPD worldwide, accounting for 6% of all deaths (55). Moreover, COPD can further develop into pulmonary heart disease and respiratory failure (117), so it is of great significance to find a better treatment strategy for COPD. Zhou et al. (118) reported that oxidative stress, inflammation, and apoptosis were considered the most important influential factors for COPD occurrence. It was found that SIRT1 exerted anti-inflammatory, anti-apoptotic, and antioxidant roles in the pathogenesis of COPD (119). A recent study has demonstrated that LINC00987 regulates LPS-induced oxidative stress, cell apoptosis, inflammation, and autophagy through promoting the binding of let-7b-5p with SIRT1, which ameliorated COPD. Moreover, this regulation could be reduced by the knockdown of SIRT1 gene (120). A similar protective role of SIRT1 against COPD was also shown in another study. The results showed that melatonin could alleviate apoptosis and ER stress by upregulating the expression of SIRT1 in rats, which played a positive role in controlling COPD. However, this positive role would be abolished by the addition of EX-527 (an inhibitor of SIRT1) (121). There is also much other evidence showing that SIRT1 plays an important role in regulating COPD (66,122,123), which indicates that SIRT1 might be a key target in the treatment of COPD.
Asthma is a chronic airway inflammatory disease, featured as aberrant immune-inflammatory responses and increased mucus exudation and airway remodeling (124). Epidemiological statistics have shown the increased prevalence of asthma during the past few decades, meaning that asthma poses a great threat to human health (125). There are about 235 million asthma patients, and more than 20 million people in China are suffering from asthma, with an incidence rate of 1.24% (56). Many recent studies have reported that the activity and expression level of SIRT1 are related to asthma conditions (126,127). However, it seems that SIRT1 has dual roles in asthma development, including pro-inflammatory and anti-inflammatory effects. It was reported that increased SIRT1 activity contributed to the suppression of NF-κB p65 acetylation and inhibited the production of IL-6 and IL-8, which could reduce the inflammatory responses in asthma (60). However, the proinflammatory function of SIRT1 may be related to repressed PPAR-γ activity (62). It was found that the expression of SIRT1 inhibited the activity of PPAR-γ in dendritic cells (44). Meanwhile, in mice dendritic cells with a shortage of SIRT1, the development of airway inflammation was notably reduced with the increased activity of PPAR-γ (62). Other evidences also demonstrated that SIRT1 exhibited a vital role in regulating asthma (128,129,130). Thus, the inhibition of PPAR-γ activity by SIRT1 may exert a pro-inflammatory effect in the pathological process of asthma. Since SIRT1 exhibits dual roles in asthma development, it is important to explore the precise effect of SIRT1 in asthma progression, which has a great significance for the treatment of asthma.
Acute respiratory distress syndrome (ARDS)
ARDS, a syndrome of acute respiratory failure, is mainly caused by direct lung injuries and indirect systemic diseases, such as sepsis and severe trauma. It is featured as a systemic inflammatory response to infection, which can cause multiple-organ dysfunction and death (131,132). The high incidence rate, mortality, and medical costs of ARDS have brought a heavy burden to patients and society. Although some measures have been taken, such as lung-protective ventilation and the establishment of an overall intensive care unit, the fatality rate of ARDS patients is still high (133). Recently, some studies have indicated that SIRT1 may become a novel target in regulating ARDS. It was reported that in alveolar macrophages, SIRT1 was a direct target of miR-199a, and the level of SIRT1 was negatively related to miR-199a. The results also suggested that downregulating miR-199a could suppress excessive inflammatory responses and cellular apoptosis through upregulating SIRT1, which prevented lung tissue from sepsis-induced ARDS (134). Moreover, another study showed that the pretreatment with SRT1720 (the SIRT1 activator) decreased the levels of IL-6, TNF-α, and IFN-γ, while increasing the level of IL-10 and attenuating lung injury. It also exhibited that activation of SIRT1 alleviated inflammatory reaction and oxidative stress in ARDS induced by LPS (68). In addition, a present study has illustrated that 3,5,4'-tri-O-acetylresveratrol (AC-Rsv) plays a protective role against LPS exposure-induced ARDS in mice by regulating the expression of SIRT1 (135). Another study showed that low expression of mir-138-5p induced by metformin might increase the expression of SIRT1 and inhibit MAPK signaling, which alleviated ARDS (136). According to these reports, it is indicated that regulating SIRT1 may be a promising treatment strategy for ARDS.
CONCLUSIONS AND PERSPECTIVES
In this review, we summarize the latest evidence on the multitasking roles of SIRT1 in regulating respiratory diseases. As one of the most well-studied sirtuins, SIRT1 can modulate multiple biological functions, including oxidative stress, inflammation, cell apoptosis, autophagy, antibacterial and antiviral effects, through its NAD+-dependent deacetylation enzymatic activity.
The immune-balance regulatory effects of SIRT1 have been stated in vivo and in vitro models of infectious respiratory disease. Previous research has indicated that SIRT1 can modulate reactions in respiratory disease via many different signaling pathways and molecules. Here some potential modulatory pathways are summarized, especially the downstream signalings mediated by HMGB1. Moreover, specific SIRT1 activators and inhibitors are reviewed respectively, and the molecular functions of SIRT1 are inversely verified by relevant experiments. Some regulatory details of SIRT1 in the process of different respiratory diseases, including COVID-19 infection, bacterial pneumonia, tuberculosis, are further described.
Although numerous mechanisms underlying the action of SIRT1 have been elaborated, some questions still need to be answered. For example, due to the diversity and abundance of substrates, the intricacy of the regulatory mechanisms, and the involvement of multiple regulating pathways, it is complicated to determine the roles of SIRT1 during the pathogenesis and prognosis of different respiratory diseases. Therefore, further investigations on SIRT1 are still needed to estimate the promising targets for the treatment of respiratory diseases, especially the signaling pathways mediated by HMGB1, which is the core immunomodulatory molecule.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (grant numbers 81503399), Beijing Natural Science Foundation (grant numbers 7182071) and Beijing Construction Program for High-level Public Health of Technical Talents (grant numbers 2022-3-009). This work would not have been possible without the enormous efforts of our collaborators and scientific friends from over the years. We thank Beijing Institute of Chinese Medicine for support in the process.
Abbreviations
- COVID-19
coronavirus disease 2019
- HMGB1
high-mobility group box 1
- SIRT1
silent information regulator type-1
- NAM
nicotinamide
- Lys
lysine
- OAADPr
O-acylated ADP-ribose
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator-1α
- Bax
B-cell lymphoma 2-associated X protein
- miR
microRNA
- NSCLC
non-small cell lung cancer
- COPD
chronic obstructive pulmonary disease
- MyD88
medullary differentiation factor 88
- HDAC
histone deacetylase
- FoxOs
forkhead box Os
- ER
endoplasmic reticulum
- I/R
ischemia/reperfusion
- UUO
unilateral ureteral obstruction
- siRNA
small interfering RNA
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- hBD-2
human β-defensin-2
- Mtb
Mycobacterium tuberculosis
- ARDS
acute respiratory distress syndrome
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest.
- Conceptualization: Ding J.
- Formal analysis: Zhang F.
- Writing - original draft: Zhou Y, Zhang F.
- Writing - review & editing: Ding J.
References
- 1.Wu J, Tang Y. Revisiting the immune balance theory: a neurological insight into the epidemic of COVID-19 and its alike. Front Neurol. 2020;11:566680. doi: 10.3389/fneur.2020.566680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vyas SP, Goswami R. Striking the right immunological balance prevents progression of tuberculosis. Inflamm Res. 2017;66:1031–1056. doi: 10.1007/s00011-017-1081-z. [DOI] [PubMed] [Google Scholar]
- 3.Cigana C, Lorè NI, Bernardini ML, Bragonzi A. Dampening host sensing and avoiding recognition in Pseudomonas aeruginosa pneumonia. J Biomed Biotechnol. 2011;2011:852513. doi: 10.1155/2011/852513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan R, Cai Z, Wang J, Ding M, Li Z, Xu J, Li Y, Li J, Yao H, Liu W, et al. Hydrogen sulfide attenuates mitochondrial dysfunction-induced cellular senescence and apoptosis in alveolar epithelial cells by upregulating sirtuin 1. Aging (Albany NY) 2019;11:11844–11864. doi: 10.18632/aging.102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li WF, Yang K, Zhu P, Zhao HQ, Song YH, Liu KC, Huang WF. Genistein ameliorates ischemia/reperfusion-induced renal injury in a SIRT1-dependent manner. Nutrients. 2017;9:403. doi: 10.3390/nu9040403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Zhou M, Ge Y, Wang X. SIRT1 and aging related signaling pathways. Mech Ageing Dev. 2020;187:111215. doi: 10.1016/j.mad.2020.111215. [DOI] [PubMed] [Google Scholar]
- 7.D’Onofrio N, Servillo L, Balestrieri ML. SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Signal. 2018;28:711–732. doi: 10.1089/ars.2017.7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida M, Porter RM. Sirtuins and FoxOs in osteoporosis and osteoarthritis. Bone. 2019;121:284–292. doi: 10.1016/j.bone.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiao F, Gong Z. The beneficial roles of SIRT1 in neuroinflammation-related diseases. Oxid Med Cell Longev. 2020;2020:6782872. doi: 10.1155/2020/6782872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang W, Huang Q, Zeng Z, Wu J, Zhang Y, Chen Z. Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxid Med Cell Longev. 2017;2017:7543973. doi: 10.1155/2017/7543973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu G, Li J, Zhang H, Zhao X, Yan LJ, Yang X. Role and possible mechanisms of Sirt1 in depression. Oxid Med Cell Longev. 2018;2018:8596903. doi: 10.1155/2018/8596903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Q, Zhou Y, Sun Y, Luo Y, Shen Y, Shao A. Will sirtuins be promising therapeutic targets for TBI and associated neurodegenerative diseases? Front Neurosci. 2020;14:791. doi: 10.3389/fnins.2020.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suenkel B, Steegborn C. Recombinant preparation, biochemical analysis, and structure determination of sirtuin family histone/protein deacylases. Methods Enzymol. 2016;573:183–208. doi: 10.1016/bs.mie.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Davenport AM, Huber FM, Hoelz A. Structural and functional analysis of human SIRT1. J Mol Biol. 2014;426:526–541. doi: 10.1016/j.jmb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu CL, Liao MT, Hou YC, Fang YW, Zheng CM, Liu WC, Chao CT, Lu KC, Ng YY. Sirtuin-1 and its relevance in vascular calcification. Int J Mol Sci. 2020;21:1593. doi: 10.3390/ijms21051593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikas IP, Paschou SA, Ryu HS. The role of nicotinamide in cancer chemoprevention and therapy. Biomolecules. 2020;10:477. doi: 10.3390/biom10030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kane AE, Sinclair DA. Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ Res. 2018;123:868–885. doi: 10.1161/CIRCRESAHA.118.312498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alqarni MH, Foudah AI, Muharram MM, Labrou NE. The pleiotropic function of human sirtuins as modulators of metabolic pathways and viral infections. Cells. 2021;10:460. doi: 10.3390/cells10020460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carafa V, Altucci L, Nebbioso A. Dual tumor suppressor and tumor promoter action of sirtuins in determining malignant phenotype. Front Pharmacol. 2019;10:38. doi: 10.3389/fphar.2019.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farghali H, Kemelo MK, Canová NK. SIRT1 modulators in experimentally induced liver injury. Oxid Med Cell Longev. 2019;2019:8765954. doi: 10.1155/2019/8765954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, You S, Tian Y, Lu S, Cao L, Sun Y, Zhang N. WWP2 regulates SIRT1-STAT3 acetylation and phosphorylation involved in hypertensive angiopathy. J Cell Mol Med. 2020;24:9041–9054. doi: 10.1111/jcmm.15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee H, Jeong AJ, Ye SK. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. 2019;52:415–423. doi: 10.5483/BMBRep.2019.52.7.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, Yang Y, Huang S, Deng C, Zhou S, Yang J, Cao Y, Xu L, Yuan Y, Yang J, et al. SIRT1 inhibits gastric cancer proliferation and metastasis via STAT3/MMP-13 signaling. J Cell Physiol. 2019;234:15395–15406. doi: 10.1002/jcp.28186. [DOI] [PubMed] [Google Scholar]
- 24.Hillmer EJ, Zhang H, Li HS, Watowich SS. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016;31:1–15. doi: 10.1016/j.cytogfr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Xia H, Zhang L, Zhang H, Wang D, Tao X. Protective effects of melatonin on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through activating SIRT1/STAT3 pathway. Biomed Pharmacother. 2019;117:109150. doi: 10.1016/j.biopha.2019.109150. [DOI] [PubMed] [Google Scholar]
- 26.Xu G, Cai J, Wang L, Jiang L, Huang J, Hu R, Ding F. MicroRNA-30e-5p suppresses non-small cell lung cancer tumorigenesis by regulating USP22-mediated Sirt1/JAK/STAT3 signaling. Exp Cell Res. 2018;362:268–278. doi: 10.1016/j.yexcr.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 27.Rius-Pérez S, Torres-Cuevas I, Millán I, Ortega ÁL, Pérez S. PGC-1α, inflammation, and oxidative stress: an integrative view in metabolism. Oxid Med Cell Longev. 2020;2020:1452696. doi: 10.1155/2020/1452696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y, Fan H, Li X, Liu J, Qu X, Wu X, Liu M, Liu Z, Yao R. Trpv4 regulates Nlrp3 inflammasome via SIRT1/PGC-1α pathway in a cuprizone-induced mouse model of demyelination. Exp Neurol. 2021;337:113593. doi: 10.1016/j.expneurol.2020.113593. [DOI] [PubMed] [Google Scholar]
- 29.Xue H, Li P, Luo Y, Wu C, Liu Y, Qin X, Huang X, Sun C. Salidroside stimulates the Sirt1/PGC-1α axis and ameliorates diabetic nephropathy in mice. Phytomedicine. 2019;54:240–247. doi: 10.1016/j.phymed.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 30.Liang D, Zhuo Y, Guo Z, He L, Wang X, He Y, Li L, Dai H. SIRT1/PGC-1 pathway activation triggers autophagy/mitophagy and attenuates oxidative damage in intestinal epithelial cells. Biochimie. 2020;170:10–20. doi: 10.1016/j.biochi.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Waldman M, Cohen K, Yadin D, Nudelman V, Gorfil D, Laniado-Schwartzman M, Kornwoski R, Aravot D, Abraham NG, Arad M, et al. Regulation of diabetic cardiomyopathy by caloric restriction is mediated by intracellular signaling pathways involving ‘SIRT1 and PGC-1α’. Cardiovasc Diabetol. 2018;17:111. doi: 10.1186/s12933-018-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang XL, Li T, Li JH, Miao SY, Xiao XZ. The effects of resveratrol on inflammation and oxidative stress in a rat model of chronic obstructive pulmonary disease. Molecules. 2017;22:1529. doi: 10.3390/molecules22091529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, He N, Xing H, Sun Y, Ding J, Liu L. Function of hesperidin alleviating inflammation and oxidative stress responses in COPD mice might be related to SIRT1/PGC-1α/NF-κB signaling axis. J Recept Signal Transduct Res. 2020;40:388–394. doi: 10.1080/10799893.2020.1738483. [DOI] [PubMed] [Google Scholar]
- 34.Brown AK, Webb AE. Regulation of FOXO factors in mammalian cells. Curr Top Dev Biol. 2018;127:165–192. doi: 10.1016/bs.ctdb.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng Y, Wang F, Hughes T, Yu J. FOXOs in cancer immunity: knowns and unknowns. Semin Cancer Biol. 2018;50:53–64. doi: 10.1016/j.semcancer.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M, Zhang Q, Hu Y, Xu L, Jiang Y, Zhang C, Ding L, Jiang R, Sun J, Sun H, et al. miR-181a increases FoxO1 acetylation and promotes granulosa cell apoptosis via SIRT1 downregulation. Cell Death Dis. 2017;8:e3088. doi: 10.1038/cddis.2017.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Q, Hu Y, Jiang M, Wang F, Gong G. Effect of autophagy regulated by Sirt1/FoxO1 pathway on the release of factors promoting thrombosis from vascular endothelial cells. Int J Mol Sci. 2019;20:4132. doi: 10.3390/ijms20174132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren BC, Zhang YF, Liu SS, Cheng XJ, Yang X, Cui XG, Zhao XR, Zhao H, Hao MF, Li MD, et al. Curcumin alleviates oxidative stress and inhibits apoptosis in diabetic cardiomyopathy via Sirt1-Foxo1 and PI3K-Akt signalling pathways. J Cell Mol Med. 2020;24:12355–12367. doi: 10.1111/jcmm.15725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy S, Saha S, Gupta P, Ukil A, Das PK. Crosstalk of PD-1 signaling with the SIRT1/FOXO-1 axis during the progression of visceral leishmaniasis. J Cell Sci. 2019;132:jcs226274. doi: 10.1242/jcs.226274. [DOI] [PubMed] [Google Scholar]
- 40.Taka C, Hayashi R, Shimokawa K, Tokui K, Okazawa S, Kambara K, Inomata M, Yamada T, Matsui S, Tobe K. SIRT1 and FOXO1 mRNA expression in PBMC correlates to physical activity in COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:3237–3244. doi: 10.2147/COPD.S144969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korbecki J, Bobiński R, Dutka M. Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors. Inflamm Res. 2019;68:443–458. doi: 10.1007/s00011-019-01231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Zhu XX, Liu L, Xue Y, Yang X, Zou HJ. SIRT1 prevents hyperuricemia via the PGC-1α/PPARγ-ABCG2 pathway. Endocrine. 2016;53:443–452. doi: 10.1007/s12020-016-0896-7. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Chen G, Lu L, Zou H. Sirt1 inhibits gouty arthritis via activating PPARγ. Clin Rheumatol. 2019;38:3235–3242. doi: 10.1007/s10067-019-04697-w. [DOI] [PubMed] [Google Scholar]
- 44.Legutko A, Marichal T, Fiévez L, Bedoret D, Mayer A, de Vries H, Klotz L, Drion PV, Heirman C, Cataldo D, et al. Sirtuin 1 promotes Th2 responses and airway allergy by repressing peroxisome proliferator-activated receptor-γ activity in dendritic cells. J Immunol. 2011;187:4517–4529. doi: 10.4049/jimmunol.1101493. [DOI] [PubMed] [Google Scholar]
- 45.Andersson U, Yang H, Harris H. Extracellular HMGB1 as a therapeutic target in inflammatory diseases. Expert Opin Ther Targets. 2018;22:263–277. doi: 10.1080/14728222.2018.1439924. [DOI] [PubMed] [Google Scholar]
- 46.Xu S, Zeng Z, Zhao M, Huang Q, Gao Y, Dai X, Lu J, Huang W, Zhao K. Evidence for SIRT1 mediated HMGB1 release from kidney cells in the early stages of hemorrhagic shock. Front Physiol. 2019;10:854. doi: 10.3389/fphys.2019.00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao S, Yang J, Liu T, Zeng J, Mi L, Xiang K. Dexamethasone inhibits NF-κBp65 and HMGB1 expression in the pancreas of rats with severe acute pancreatitis. Mol Med Rep. 2018;18:5345–5352. doi: 10.3892/mmr.2018.9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Gregorio E, Colell A, Morales A, Marí M. Relevance of SIRT1-NF-κB axis as therapeutic target to ameliorate inflammation in liver disease. Int J Mol Sci. 2020;21:3858. doi: 10.3390/ijms21113858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen X, Chen C, Fan S, Wu S, Yang F, Fang Z, Fu H, Li Y. Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury. J Neuroinflammation. 2018;15:116. doi: 10.1186/s12974-018-1151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meng L, Li L, Lu S, Li K, Su Z, Wang Y, Fan X, Li X, Zhao G. The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB and PI3K/Akt/mTOR pathways. Mol Immunol. 2018;94:7–17. doi: 10.1016/j.molimm.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 51.Chi JH, Seo GS, Cheon JH, Lee SH. Isoliquiritigenin inhibits TNF-α-induced release of high-mobility group box 1 through activation of HDAC in human intestinal epithelial HT-29 cells. Eur J Pharmacol. 2017;796:101–109. doi: 10.1016/j.ejphar.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Li R, Peng Z, Hu B, Rao X, Li J. HMGB1 participates in LPS-induced acute lung injury by activating the AIM2 inflammasome in macrophages and inducing polarization of M1 macrophages via TLR2, TLR4, and RAGE/NF-κB signaling pathways. Int J Mol Med. 2020;45:61–80. doi: 10.3892/ijmm.2019.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo LT, Wang SQ, Su J, Xu LX, Ji ZY, Zhang RY, Zhao QW, Ma ZQ, Deng XY, Ma SP. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J Neuroinflammation. 2019;16:95. doi: 10.1186/s12974-019-1474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu X, Piao HN, Aosai F, Zeng XY, Cheng JH, Cui YX, Li J, Ma J, Piao HR, Jin X, et al. Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br J Pharmacol. 2020;177:5224–5245. doi: 10.1111/bph.15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gu C, Zhang Q, Ni D, Xiao QF, Cao LF, Fei CY, Ying Y, Li N, Tao F. Therapeutic effects of SRT2104 on lung injury in rats with emphysema via reduction of type II alveolar epithelial cell senescence. COPD. 2020;17:444–451. doi: 10.1080/15412555.2020.1797657. [DOI] [PubMed] [Google Scholar]
- 56.Zhang YZ, Wu QJ, Yang X, Xing XX, Chen YY, Wang H. Effects of SIRT1/Akt pathway on chronic inflammatory response and lung function in patients with asthma. Eur Rev Med Pharmacol Sci. 2019;23:4948–4953. doi: 10.26355/eurrev_201906_18085. [DOI] [PubMed] [Google Scholar]
- 57.Wu Y, Li W, Hu Y, Liu Y, Sun X. Suppression of sirtuin 1 alleviates airway inflammation through mTOR-mediated autophagy. Mol Med Rep. 2020;22:2219–2226. doi: 10.3892/mmr.2020.11338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang H, Chen J, Chen Y, Jiang Y, Ge B, Hong L. Sirtuin inhibits M. tuberculosis -induced apoptosis in macrophage through glycogen synthase kinase-3β. Arch Biochem Biophys. 2020;694:108612. doi: 10.1016/j.abb.2020.108612. [DOI] [PubMed] [Google Scholar]
- 59.Ji K, Sun X, Liu Y, Du L, Wang Y, He N, Wang J, Xu C, Liu Q. Regulation of apoptosis and radiation sensitization in lung cancer cells via the Sirt1/NF-κB/Smac pathway. Cell Physiol Biochem. 2018;48:304–316. doi: 10.1159/000491730. [DOI] [PubMed] [Google Scholar]
- 60.Chen M, Chen C, Gao Y, Li D, Huang D, Chen Z, Zhao X, Huang Q, Wu D, Lai T, et al. Bergenin-activated SIRT1 inhibits TNF-α-induced proinflammatory response by blocking the NF-κB signaling pathway. Pulm Pharmacol Ther. 2020;62:101921. doi: 10.1016/j.pupt.2020.101921. [DOI] [PubMed] [Google Scholar]
- 61.Colley T, Mercado N, Kunori Y, Brightling C, Bhavsar PK, Barnes PJ, Ito K. Defective sirtuin-1 increases IL-4 expression through acetylation of GATA-3 in patients with severe asthma. J Allergy Clin Immunol. 2016;137:1595–1597.e7. doi: 10.1016/j.jaci.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 62.Ma K, Lu N, Zou F, Meng FZ. Sirtuins as novel targets in the pathogenesis of airway inflammation in bronchial asthma. Eur J Pharmacol. 2019;865:172670. doi: 10.1016/j.ejphar.2019.172670. [DOI] [PubMed] [Google Scholar]
- 63.Yang H, Hu J, Chen YJ, Ge B. Role of Sirt1 in innate immune mechanisms against Mycobacterium tuberculosis via the inhibition of TAK1 activation. Arch Biochem Biophys. 2019;667:49–58. doi: 10.1016/j.abb.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 64.Annamanedi M, Varma GY, Anuradha K, Kalle AM. Celecoxib enhances the efficacy of low-dose antibiotic treatment against polymicrobial sepsis in mice and clinical isolates of ESKAPE pathogens. Front Microbiol. 2017;8:805. doi: 10.3389/fmicb.2017.00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J, Li J, Cao N, Li Z, Han J, Li L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. Onco Targets Ther. 2018;11:7777–7786. doi: 10.2147/OTT.S159095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun X, Dong Z, Li N, Feng X, Liu Y, Li A, Zhu X, Li C, Zhao Z. Nucleosides isolated from Ophiocordyceps sinensis inhibit cigarette smoke extract-induced inflammation via the SIRT1-nuclear factor-κB/p65 pathway in RAW264.7 macrophages and in COPD mice. Int J Chron Obstruct Pulmon Dis. 2018;13:2821–2832. doi: 10.2147/COPD.S172579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li X, Jiang Z, Li X, Zhang X. SIRT1 overexpression protects non-small cell lung cancer cells against osteopontin-induced epithelial-mesenchymal transition by suppressing NF-κB signaling. Onco Targets Ther. 2018;11:1157–1171. doi: 10.2147/OTT.S137146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhan Y, Yang C, Zhang Q, Yao L. Silent information regulator type-1 mediates amelioration of inflammatory response and oxidative stress in lipopolysaccharide-induced acute respiratory distress syndrome. J Biochem. 2021;169:613–620. doi: 10.1093/jb/mvaa150. [DOI] [PubMed] [Google Scholar]
- 69.Yang X, Jiang T, Wang Y, Guo L. The role and mechanism of SIRT1 in resveratrol-regulated osteoblast autophagy in osteoporosis rats. Sci Rep. 2019;9:18424. doi: 10.1038/s41598-019-44766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saeedi-Boroujeni A, Mahmoudian-Sani MR. Anti-inflammatory potential of Quercetin in COVID-19 treatment. J Inflamm (Lond) 2021;18:3. doi: 10.1186/s12950-021-00268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao S, Yu L. Sirtuin 1 activated by SRT1460 protects against myocardial ischemia/reperfusion injury. Clin Hemorheol Microcirc. 2021;78:271–281. doi: 10.3233/CH-201061. [DOI] [PubMed] [Google Scholar]
- 72.Ren Y, Du C, Shi Y, Wei J, Wu H, Cui H. The Sirt1 activator, SRT1720, attenuates renal fibrosis by inhibiting CTGF and oxidative stress. Int J Mol Med. 2017;39:1317–1324. doi: 10.3892/ijmm.2017.2931. [DOI] [PubMed] [Google Scholar]
- 73.Zheng Y, Kou J, Wang P, Ye T, Wang Z, Gao Z, Cong L, Li M, Dong B, Yang W, et al. Berberine-induced TFEB deacetylation by SIRT1 promotes autophagy in peritoneal macrophages. Aging (Albany NY) 2021;13:7096–7119. doi: 10.18632/aging.202566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang C, Yao Z, Zhang Y, Yang Y, Liu J, Shi Y, Zhang C. Metformin mitigates cartilage degradation by activating AMPK/SIRT1-mediated autophagy in a mouse osteoarthritis model. Front Pharmacol. 2020;11:1114. doi: 10.3389/fphar.2020.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang WX, He BM, Wu Y, Qiao JF, Peng ZY. Melatonin protects against sepsis-induced cardiac dysfunction by regulating apoptosis and autophagy via activation of SIRT1 in mice. Life Sci. 2019;217:8–15. doi: 10.1016/j.lfs.2018.11.055. [DOI] [PubMed] [Google Scholar]
- 76.Sun T, Hu Y, He W, Shang Y, Yang X, Gong L, Zhang X, Gong P, Yang G. SRT2183 impairs ovarian cancer by facilitating autophagy. Aging (Albany NY) 2020;12:24208–24218. doi: 10.18632/aging.104126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang T, Li X, Sun SL. EX527, a Sirt-1 inhibitor, induces apoptosis in glioma via activating the p53 signaling pathway. Anticancer Drugs. 2020;31:19–26. doi: 10.1097/CAD.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 78.Marx C, Marx-Blümel L, Lindig N, Thierbach R, Hoelzer D, Becker S, Wittig S, Lehmann R, Slevogt H, Heinzel T, et al. The sirtuin 1/2 inhibitor tenovin-1 induces a nonlinear apoptosis-inducing factor-dependent cell death in a p53 null Ewing’s sarcoma cell line. Invest New Drugs. 2018;36:396–406. doi: 10.1007/s10637-017-0541-1. [DOI] [PubMed] [Google Scholar]
- 79.He M, Tan B, Vasan K, Yuan H, Cheng F, Ramos da Silva S, Lu C, Gao SJ. SIRT1 and AMPK pathways are essential for the proliferation and survival of primary effusion lymphoma cells. J Pathol. 2017;242:309–321. doi: 10.1002/path.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu B, Zhang D, Wang X, Lin D, Chen Y, Xu X. Targeting SIRT1 to inhibit the proliferation of multiple myeloma cells. Oncol Lett. 2021;21:306. doi: 10.3892/ol.2021.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ye Q, Zhang M, Wang Y, Fu S, Han S, Wang L, Wang Q. Sirtinol regulates the balance of Th17/Treg to prevent allograft rejection. Cell Biosci. 2017;7:55. doi: 10.1186/s13578-017-0182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mu N, Lei Y, Wang Y, Wang Y, Duan Q, Ma G, Liu X, Su L. Inhibition of SIRT1/2 upregulates HSPA5 acetylation and induces pro-survival autophagy via ATF4-DDIT4-mTORC1 axis in human lung cancer cells. Apoptosis. 2019;24:798–811. doi: 10.1007/s10495-019-01559-3. [DOI] [PubMed] [Google Scholar]
- 83.Park JA, Park S, Park WY, Han MK, Lee Y. Splitomicin, a SIRT1 inhibitor, enhances hematopoietic differentiation of mouse embryonic stem cells. Int J Stem Cells. 2019;12:21–30. doi: 10.15283/ijsc18040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pan S, Leng J, Deng X, Ruan H, Zhou L, Jamal M, Xiao R, Xiong J, Yin Q, Wu Y, et al. Nicotinamide increases the sensitivity of chronic myeloid leukemia cells to doxorubicin via the inhibition of SIRT1. J Cell Biochem. 2020;121:574–586. doi: 10.1002/jcb.29303. [DOI] [PubMed] [Google Scholar]
- 85.Chung JY, Jeong JH, Song J. Resveratrol modulates the gut-brain axis: focus on glucagon-like peptide-1, 5-HT, and gut microbiota. Front Aging Neurosci. 2020;12:588044. doi: 10.3389/fnagi.2020.588044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qin H, Zhang H, Zhang X, Zhang S, Zhu S, Wang H. Resveratrol attenuates radiation enteritis through the SIRT1/FOXO3a and PI3K/AKT signaling pathways. Biochem Biophys Res Commun. 2021;554:199–205. doi: 10.1016/j.bbrc.2021.03.122. [DOI] [PubMed] [Google Scholar]
- 87.Yang D, Wang T, Long M, Li P. Quercetin: its main pharmacological activity and potential application in clinical medicine. Oxid Med Cell Longev. 2020;2020:8825387. doi: 10.1155/2020/8825387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu T, Shi JJ, Fang J, Wang Q, Chen YB, Zhang SJ. Quercetin ameliorates diabetic encephalopathy through SIRT1/ER stress pathway in db/db mice. Aging (Albany NY) 2020;12:7015–7029. doi: 10.18632/aging.103059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Broussy S, Laaroussi H, Vidal M. Biochemical mechanism and biological effects of the inhibition of silent information regulator 1 (SIRT1) by EX-527 (SEN0014196 or selisistat) J Enzyme Inhib Med Chem. 2020;35:1124–1136. doi: 10.1080/14756366.2020.1758691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen G, Zhang B, Xu H, Sun Y, Shi Y, Luo Y, Jia H, Wang F. Suppression of Sirt1 sensitizes lung cancer cells to WEE1 inhibitor MK-1775-induced DNA damage and apoptosis. Oncogene. 2017;36:6863–6872. doi: 10.1038/onc.2017.297. [DOI] [PubMed] [Google Scholar]
- 91.Moschos MM, Dettoraki M, Androudi S, Kalogeropoulos D, Lavaris A, Garmpis N, Damaskos C, Garmpi A, Tsatsos M. The role of histone deacetylase inhibitors in uveal melanoma: current evidence. Anticancer Res. 2018;38:3817–3824. doi: 10.21873/anticanres.12665. [DOI] [PubMed] [Google Scholar]
- 92.Lee BB, Kim Y, Kim D, Cho EY, Han J, Kim HK, Shim YM, Kim DH. Metformin and tenovin-6 synergistically induces apoptosis through LKB1-independent SIRT1 down-regulation in non-small cell lung cancer cells. J Cell Mol Med. 2019;23:2872–2889. doi: 10.1111/jcmm.14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu J, Jing H, Lin H. Sirtuin inhibitors as anticancer agents. Future Med Chem. 2014;6:945–966. doi: 10.4155/fmc.14.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chimento A, De Luca A, Nocito MC, Sculco S, Avena P, La Padula D, Zavaglia L, Sirianni R, Casaburi I, Pezzi V. SIRT1 is involved in adrenocortical cancer growth and motility. J Cell Mol Med. 2021;25:3856–3869. doi: 10.1111/jcmm.16317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kratz EM, Sołkiewicz K, Kubis-Kubiak A, Piwowar A. Sirtuins as important factors in pathological states and the role of their molecular activity modulators. Int J Mol Sci. 2021;22:630. doi: 10.3390/ijms22020630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guo Q, Chen S, Rao X, Li Y, Pan M, Fu G, Yao Y, Gao X, Tang P, Zhou Y, et al. Inhibition of SIRT1 promotes taste bud stem cell survival and mitigates radiation-induced oral mucositis in mice. Am J Transl Res. 2019;11:4789–4799. [PMC free article] [PubMed] [Google Scholar]
- 97.He M, Li X, Tan Q, Chen Y, Kong Y, You J, Lin X, Lin Y, Zheng Q. Disease burden from COVID-19 symptoms among inpatients at the temporary military hospitals in Wuhan: a retrospective multicentre cross-sectional study. BMJ Open. 2021;11:e048822. doi: 10.1136/bmjopen-2021-048822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kadkhoda K. COVID-19: an immunopathological view. MSphere. 2020;5:e00344-20. doi: 10.1128/mSphere.00344-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jit BP, Qazi S, Arya R, Srivastava A, Gupta N, Sharma A. An immune epigenetic insight to COVID-19 infection. Epigenomics. 2021;13:465–480. doi: 10.2217/epi-2020-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miller R, Wentzel AR, Richards GA. COVID-19: NAD+ deficiency may predispose the aged, obese and type2 diabetics to mortality through its effect on SIRT1 activity. Med Hypotheses. 2020;144:110044. doi: 10.1016/j.mehy.2020.110044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bordoni V, Tartaglia E, Sacchi A, Fimia GM, Cimini E, Casetti R, Notari S, Grassi G, Marchioni L, Bibas M, et al. The unbalanced p53/SIRT1 axis may impact lymphocyte homeostasis in COVID-19 patients. Int J Infect Dis. 2021;105:49–53. doi: 10.1016/j.ijid.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.DiNicolantonio JJ, McCarty M, Barroso-Aranda J. Melatonin may decrease risk for and aid treatment of COVID-19 and other RNA viral infections. Open Heart. 2021;8:e001568. doi: 10.1136/openhrt-2020-001568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huarachi Olivera RE, Lazarte Rivera A. Coronavirus disease (COVID-19) and sirtuins. Rev Fac Cien Med Univ Nac Cordoba. 2020;77:117–125. doi: 10.31053/1853.0605.v77.n2.28196. [DOI] [PubMed] [Google Scholar]
- 104.Samadder S. Immunopathological changes in SARS-CoV-2 critical and non-critical pneumonia patients: a systematic review to determine the cause of co-infection. Front Public Health. 2021;8:544993. doi: 10.3389/fpubh.2020.544993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferri M, Ranucci E, Romagnoli P, Giaccone V. Antimicrobial resistance: a global emerging threat to public health systems. Crit Rev Food Sci Nutr. 2017;57:2857–2876. doi: 10.1080/10408398.2015.1077192. [DOI] [PubMed] [Google Scholar]
- 106.Dunne EM, Murad C, Sudigdoadi S, Fadlyana E, Tarigan R, Indriyani SA, Pell CL, Watts E, Satzke C, Hinds J, et al. Carriage of Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Staphylococcus aureus in Indonesian children: a cross-sectional study. PLoS One. 2018;13:e0195098. doi: 10.1371/journal.pone.0195098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Elesela S, Morris SB, Narayanan S, Kumar S, Lombard DB, Lukacs NW. Sirtuin 1 regulates mitochondrial function and immune homeostasis in respiratory syncytial virus infected dendritic cells. PLoS Pathog. 2020;16:e1008319. doi: 10.1371/journal.ppat.1008319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Singh V, Ubaid S. Role of silent information regulator 1 (SIRT1) in regulating oxidative stress and inflammation. Inflammation. 2020;43:1589–1598. doi: 10.1007/s10753-020-01242-9. [DOI] [PubMed] [Google Scholar]
- 109.Opal SM, Ellis JL, Suri V, Freudenberg JM, Vlasuk GP, Li Y, Chahin AB, Palardy JE, Parejo N, Yamamoto M, et al. Pharmacological SIRT1 activation improves mortality and markedly alters transcriptional profiles that accompany experimental sepsis. Shock. 2016;45:411–418. doi: 10.1097/SHK.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 110.Lin L, Wen SH, Guo SZ, Su XY, Wu HJ, Chong L, Zhang HL, Zhang WX, Li CC. Role of SIRT1 in Streptococcus pneumoniae-induced human β-defensin-2 and interleukin-8 expression in A549 cell. Mol Cell Biochem. 2014;394:199–208. doi: 10.1007/s11010-014-2095-2. [DOI] [PubMed] [Google Scholar]
- 111.Li QR, Tan SR, Yang L, He W, Chen L, Shen FX, Wang Z, Wang HF. Mechanism of chlorogenic acid in alveolar macrophage polarization in Klebsiella pneumoniae-induced pneumonia. J Leukoc Biol. 2021 doi: 10.1002/JLB.3HI0721-368R. [DOI] [PubMed] [Google Scholar]
- 112.Yang H, Chen J, Chen Y, Jiang Y, Ge B, Hong L. Sirt1 activation negatively regulates overt apoptosis in Mtb-infected macrophage through Bax. Int Immunopharmacol. 2021;91:107283. doi: 10.1016/j.intimp.2020.107283. [DOI] [PubMed] [Google Scholar]
- 113.Stek C, Allwood B, Walker NF, Wilkinson RJ, Lynen L, Meintjes G. The immune mechanisms of lung parenchymal damage in tuberculosis and the role of host-directed therapy. Front Microbiol. 2018;9:2603. doi: 10.3389/fmicb.2018.02603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kim SY, Yang CS, Lee HM, Kim JK, Kim YS, Kim YR, Kim JS, Kim TS, Yuk JM, Dufour CR, et al. ESRRA (estrogen-related receptor α) is a key coordinator of transcriptional and post-translational activation of autophagy to promote innate host defense. Autophagy. 2018;14:152–168. doi: 10.1080/15548627.2017.1339001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cheng CY, Gutierrez NM, Marzuki MB, Lu X, Foreman TW, Paleja B, Lee B, Balachander A, Chen J, Tsenova L, et al. Host sirtuin 1 regulates mycobacterial immunopathogenesis and represents a therapeutic target against tuberculosis. Sci Immunol. 2017;2:eaaj1789. doi: 10.1126/sciimmunol.aaj1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fernández-Villar A, Represas-Represas C, Mouronte-Roibás C, Ramos-Hernández C, Priegue-Carrera A, Fernández-García S, López-Campos JL. Reliability and usefulness of spirometry performed during admission for COPD exacerbation. PLoS One. 2018;13:e0194983. doi: 10.1371/journal.pone.0194983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y, Su NX, Pan SG, Ge XP, Dai XP. Fengbaisan suppresses endoplasmic reticulum stress by up-regulating SIRT1 expression to protect rats with chronic obstructive pulmonary diseases. Pharm Biol. 2020;58:878–885. doi: 10.1080/13880209.2020.1806335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhou X, Yi D, Wu Y, Pei X, Yu H, Chen Y, Jiang Y, Li W. Expression of diaphragmatic myostatin and correlation with apoptosis in rats with chronic obstructive pulmonary disease. Exp Ther Med. 2018;15:2295–2300. doi: 10.3892/etm.2018.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang L, Luo B, Ting Y, He S, Xie L, Sun S. SIRT1 attenuates endoplasmic reticulum stress and apoptosis in rat models of COPD. Growth Factors. 2020;38:94–104. doi: 10.1080/08977194.2020.1810029. [DOI] [PubMed] [Google Scholar]
- 120.Wang Y, Chen J, Chen W, Liu L, Dong M, Ji J, Hu D, Zhang N. LINC00987 ameliorates COPD by regulating LPS-induced cell apoptosis, oxidative stress, inflammation and autophagy through Let-7b-5p/SIRT1 axis. Int J Chron Obstruct Pulmon Dis. 2020;15:3213–3225. doi: 10.2147/COPD.S276429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.He B, Zhang W, Qiao J, Peng Z, Chai X. Melatonin protects against COPD by attenuating apoptosis and endoplasmic reticulum stress via upregulating SIRT1 expression in rats. Can J Physiol Pharmacol. 2019;97:386–391. doi: 10.1139/cjpp-2018-0529. [DOI] [PubMed] [Google Scholar]
- 122.Gu C, Li Y, Liu J, Ying X, Liu Y, Yan J, Chen C, Zhou H, Cao L, Ma Y. LncRNA-mediated SIRT1/FoxO3a and SIRT1/p53 signaling pathways regulate type II alveolar epithelial cell senescence in patients with chronic obstructive pulmonary disease. Mol Med Rep. 2017;15:3129–3134. doi: 10.3892/mmr.2017.6367. [DOI] [PubMed] [Google Scholar]
- 123.Shin NR, Ko JW, Kim JC, Park G, Kim SH, Kim MS, Kim JS, Shin IS. Role of melatonin as an SIRT1 enhancer in chronic obstructive pulmonary disease induced by cigarette smoke. J Cell Mol Med. 2020;24:1151–1156. doi: 10.1111/jcmm.14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang Y, Liang R, Xie A, Shi W, Huang H, Zhong Y. Antagonistic peptides that specifically bind to the first and second extracellular loops of CCR5 and Anti-IL-23p19 antibody reduce airway inflammation by suppressing the IL-23/Th17 signaling pathway. Mediators Inflamm. 2020;2020:1719467. doi: 10.1155/2020/1719467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin X, Ren X, Xiao X, Yang Z, Yao S, Wong GW, Liu Z, Wang C, Su Z, Li J. Important role of immunological responses to environmental exposure in the development of allergic asthma. Allergy Asthma Immunol Res. 2020;12:934–948. doi: 10.4168/aair.2020.12.6.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tang L, Chen Q, Meng Z, Sun L, Zhu L, Liu J, Hu J, Ni Z, Wang X. Suppression of Sirtuin-1 increases IL-6 expression by activation of the Akt pathway during allergic asthma. Cell Physiol Biochem. 2017;43:1950–1960. doi: 10.1159/000484119. [DOI] [PubMed] [Google Scholar]
- 127.Zhang H, Sun Y, Rong W, Fan L, Cai Y, Qu Q, Gao Y, Zhao H. miR-221 participates in the airway epithelial cells injury in asthma via targeting SIRT1. Exp Lung Res. 2018;44:272–279. doi: 10.1080/01902148.2018.1533051. [DOI] [PubMed] [Google Scholar]
- 128.Lin H, Wan N. Circular RNA has Circ 001372-reduced inflammation in ovalbumin-induced asthma through Sirt1/NFAT5 signaling pathway by miRNA-128-3p. Mol Biotechnol. 2022 doi: 10.1007/s12033-022-00480-6. [DOI] [PubMed] [Google Scholar]
- 129.Liu Y, Zhang M, Zhang H, Qian X, Luo L, He Z. Anthocyanins inhibit airway inflammation by downregulating the NF-κB pathway via the miR-138-5p/SIRT1 axis in asthmatic mice. Int Arch Allergy Immunol. 2022;183:539–551. doi: 10.1159/000520645. [DOI] [PubMed] [Google Scholar]
- 130.Lai T, Su G, Wu D, Chen Z, Chen Y, Yi H, Gao Y, Chen C, Zeng M, Chen M, et al. Myeloid-specific SIRT1 deletion exacerbates airway inflammatory response in a mouse model of allergic asthma. Aging (Albany NY) 2021;13:15479–15490. doi: 10.18632/aging.203104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huppert LA, Matthay MA, Ware LB. Pathogenesis of acute respiratory distress syndrome. Semin Respir Crit Care Med. 2019;40:31–39. doi: 10.1055/s-0039-1683996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lupu L, Palmer A, Huber-Lang M. Inflammation, thrombosis, and destruction: the three-headed cerberus of trauma- and SARS-CoV-2-induced ARDS. Front Immunol. 2020;11:584514. doi: 10.3389/fimmu.2020.584514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Banavasi H, Nguyen P, Osman H, Soubani AO. Management of ARDS – What works and what does not. Am J Med Sci. 2021;362:13–23. doi: 10.1016/j.amjms.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu Y, Guan H, Zhang JL, Zheng Z, Wang HT, Tao K, Han SC, Su LL, Hu D. Acute downregulation of miR-199a attenuates sepsis-induced acute lung injury by targeting SIRT1. Am J Physiol Cell Physiol. 2018;314:C449–C455. doi: 10.1152/ajpcell.00173.2017. [DOI] [PubMed] [Google Scholar]
- 135.Ma L, Zhao Y, Wang R, Chen T, Li W, Nan Y, Liu X, Jin F. 3,5,4′-Tri-O-acetylresveratrol attenuates lipopolysaccharide-induced acute respiratory distress syndrome via MAPK/SIRT1 pathway. Mediators Inflamm. 2015;2015:143074. doi: 10.1155/2015/143074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yu LL, Zhu M, Huang Y, Zhao YM, Wen JJ, Yang XJ, Wu P. Metformin relieves acute respiratory distress syndrome by reducing miR-138 expression. Eur Rev Med Pharmacol Sci. 2018;22:5355–5363. doi: 10.26355/eurrev_201808_15737. [DOI] [PubMed] [Google Scholar]