Abstract
Oligonucleotide microarrays or oDNA chips are effective decoding and analytical tools for genomic sequences and are useful for a broad range of applications. Therefore, it is desirable to have synthesis methods of DNA chips that are highly flexible in sequence design and provide high quality and general adoptability. We report herein, DNA microarray synthesis based on a flexible biochip method. Our method simply uses photogenerated acid (PGA) in solution to trigger deprotection of the 5′-OH group in conventional nucleotide phosphoramidite monomers (i.e. PGA-gated deprotection), with the rest of the reactions in the synthesis cycle the same as those used for routine synthesis of oligonucleotides. The complete DNA chip synthesis process is accomplished on a regular DNA synthesizer that is coupled with a UV-VIS projection display unit for performing digital photolithography. Using this method, oDNA chips containing probes of newly discovered genes can be quickly and easily synthesized at high yields in a conventional laboratory setting. Furthermore, the PGA-gated chemistry should be applicable to microarray syntheses of a variety of combinatorial molecules, such as peptides and organic molecules.
INTRODUCTION
DNA sequence microarrays (DNA chips) have gained increased importance as powerful tools for researchers in biological and biomedical sciences, who seek to understand the correlation of gene sequences with their functions (1–5). In many applications, DNA chips containing surface-bound oligonucleotides (probes) are used to interrogate sample (target) sequence information through complementary recognition (hybridization) in a highly parallel fashion (6–11). Many reports have established the applications of DNA chips in monitoring gene expression and gene mutation through hybridization. To meet the demands of these genetic analyses at unprecedented large numbers and diversity, it is important to have DNA chips that are flexible in sequence design, are easy to manufacture and have high sequence fidelity. Presently, the two major types of DNA chips available are cDNA chips containing long DNA sequences (from hundreds to several thousand residues per strand) and oDNA chips containing oligonucleotides (synthetic sequences of less than 100 residues). Although oDNA chips have the advantage of being flexible in sequence design and choices of chain lengths (compared with cDNA chips) and oDNA chips made from direct on-chip synthesis offer higher density and more homogeneous surface distributions (compared with spotted oDNA chips), the fabrication of directly synthesized oDNA chips is not a routine laboratory practice (4,12). Additionally, oDNA chips are suitable for the detection of single-nucleotide variation(s) in DNA sequences, because the shorter probes used are more sensitive to defective hybridization (3,13–17). These attractive features of oDNA chips have stimulated intense interest in alternative methods of oDNA chip synthesis (18–20).
Our efforts have been focused on the development of a novel solution photochemistry that is suitable for parallel synthesis of oligonucleotides, peptides and other types of molecular microarrays (18,21,22). We established on-chip optimization of synthesis conditions and demonstrated synthesis of a single sequence on bulk solid supports, such as CPG (controlled porous glass) (18,21–23). This chemistry is based on photogeneration of reagents that participate in otherwise conventional solution chemical reactions (Fig. 1A). These applications represent a significant advancement over the photoresist chemical amplification reactions (24,25) that have been widely used for solid-phase processing of microelectronic devices and recently for polymer phase synthesis of oligonucleotide arrays (26,27).
Figure 1.
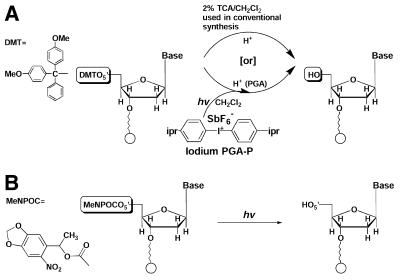
Chemical reactions used in light-directed parallel synthesis of oDNA chips. (A) Solution photogeneration of an acid for 5′-DMT deprotection of nucleotides versus what takes place in conventional synthesis. (B) Light deprotection of 5′-photolabile group-protected nucleotides (12).
In a parallel synthesis of oligonucleotides or peptides, the reaction of solution photogenerated acids (PGAs) (18,21,22) would produce the same net effect at specific light-irradiated sites as that of conventional acids, such as trichloroacetic acid (TCA) and trifluoroacetic acid (TFA), for the removal of acid-labile groups (such as 5′-O-DMT of nucleotides/oligonucleotides and N-t-Boc of amino acids/peptides) (DMT, 4,4′-dimethoxytrityl; t-Boc, t-butyloxycarbonyl). Figure 1A shows the deprotection of the 5′-DMT-protected nucleotide. In this reaction, the OH group forms and couples with incoming monomers in the subsequent chain growth reactions. This article will describe the synthesis of oDNA chips using an integrated, programmable synthesis system and conventional chemical supplies (i.e. 5′-DMT-protected nucleophosphoramidites) and the experimental validation of the oDNA chips thus synthesized. This work demonstrates the usefulness of our chemistry for parallel synthesis using photogenerated reagents and its application in synthesis of oligonucleotide microarrays.
MATERIALS AND METHODS
Microwell microarray plates
Microarray glass plates (22 × 75 mm) containing n × n (n = 12, 24 or 48) of d diameter (d = 600, 300 or 150 µm) and 30 µm deep reaction wells confined in a 1.4 × 1.4 cm2 area were prepared according to standard photolithographic methods. Briefly, 4 inch borofloat glass wafers (Schott) were coated with a thin film of Cr (50 nm)/Au (300 nm) by electron beam deposition. A layer of photoresist (PR 1827, Hoechst Celanese) was applied by spinning coating at 2000 r.p.m. for 30 s, soft-baked at 90°C in an oven for 30 min and then patterned using a first mask and UV irradiation (404.7 nm, 5 mW/cm2, 70 s). After removal of activated photoresist by developer solution (MF 319, Hoechst Celanese) for 60 s, the wafers were hard-baked at 110°C for 30 min. The exposed Au/Cr layer was etched by a gold etchant solution (TFA, Transene Co.) for 120 s and by a chromium etchant solution (CR-14, Cyantek Inc.) for 120 s. The exposed glass surface was further etched in HF for 4 min to obtain 30 µm deep microwells as measured by surface profilometry. The remaining photoresist was removed by resist stripper (PRS-2000, J. T. Baker Inc.). The exposed Au/Cr layers were removed successively by gold etchant solution and chromium etchant solution. Microwell wafers were protected by spin coating a layer of photoresist (AZ 4620, Clariant) at 500 r.p.m. for 30 s, soft-baked at 90°C for 1 h, patterned by a second mask under UV exposure (404.7 nm, 5 mW/cm2, 150 s) to generate activated photoresist patterns inside the wells and then dipped into a developer solution (1:4 AZ 400K:H2O, Clariant) for 3 min. The surface outside the wells was derivatized with a solution of (heptadecafluoror-1,1,2,2-tetrahydrodecyl) triethoxysilane (Gelest) in cyclohexane (20 mM, 20 ml) overnight at room temperature under gentle shaking. After curing for 1 h at 100°C under an N2 atmosphere, individual plates were diced from the wafers using a diamond saw. The photoresist inside the wells was removed by successive washing in acetone (5 ml), 2-propanol (5 ml), 1:1 H2O2:H2SO4 for 15 min (300 ml), H2O (3× 20 ml) and EtOH (3× 20 ml). The well surfaces were then derivatized in a solution of N-(3-triethoxysilylpropyl)-4-hydroxybutyramide (Lacaster) 1% v/v in 95% EtOH overnight at room temperature under gentle shaking. Upon completion of the reaction, glass plates were rinsed thoroughly with 95% EtOH and cured at 100°C under N2 for 1 h. The derivatized plates were stored in a clean, dry container. For flat microarrays patterned with a non-wetting film, a similar procedure without prior etching of the glass was used.
Reaction apparatus
The apparatus consists of projection optics, a reactor assembly, a reagent manifold and a computer control system. A 500 W mercury lamp (Oriel Instruments) was used as the light source. A water infrared filter (Oriel) was used to eliminate the heating effect to the chemical reactions. A dichroic mirror (Oriel) having a reflection wavelength range from 350 to 450 nm was used to select adequate wavelengths for the excitation of PGA-Ps. A 480 × 640 digital micromirror device (DMD) was used to generate light patterns. A 100 mm lens was used to project images onto the substrate surface held by a flow-through microarray reactor. At the substrate surface, each projected pixel, which corresponds to a single mirror plate (16 × 16 µm with 17 µm center-to-center distance) in DMD, measures 30 × 30 µm. A flux density of 30 mW/cm2 was obtained at the substrate position in a wavelength range from 400 to 450 nm. A beam splitter and a CCD video camera (SONY) were used to assist projector-microarray plate alignment. A commercial DNA/RNA synthesizer (Expedite 8909, PerSeptive) was used, without any alternation, as a reagent manifold. A microarray reactor was flow-cell constructed using 316-stainless steel. The reactor was mounted on a xyz translation stage and a tilt platform. Computer software, written in C++, was developed in-house to optimize synthesis steps and generate light patterns based on predetermined oDNA chip sequence layouts.
Oligonucleotide microarray synthesis
The light-directed microarray reactions were carried out using apparatus described previously. The reactions used biaryl iodium PGA-P (Secant Chemicals) (3% in CH2Cl2) and photosensitizing thioxanthenone (Sigma-Aldrich). The solution was protected from light during preparation and storage. The derivatized microarray plate was held in a homebuilt flow reactor that was connected to the DNA synthesizer via lure-lock connectors. Standard DNA synthesizer protocols were modified for the use of the microarray reactor. In a PGA deprotection step, droplet formation was achieved by filling the reactor from bottom to top with PGA-P solution, and then draining the solution from the bottom of the reactor. The flow direction was controlled by a four-way electronic valve (Valco). The array plate was illuminated according to a computer-generated pattern. The light-directed acid deprotection steps lasted 0.5–2 min and were followed by a rapid pyridine/CH3CN (10/90 v/v) wash. The rest of the synthesis steps were coupling, capping and oxidation and they were the same as those performed in conventional synthesis of oligonucleotides (28).
The experiments designed for characterization of PGA deprotection as a function of digital light conditions (irradiation intensity and time) used substrates containing TTA, TTC, TTG and TTT microarrays, all sequences terminated with 5′-DMT. The deprotection involved several cycles of irradiation according to predetermined light patterns followed by pyridine/CH3CN wash and a coupling reaction using a mixture 1:10 of fluorescein:T phosphoramidites. The final deprotection of oDNA chips synthesized for hybridization purposes used EDA/EtOH (anhydrous, 1:1 v/v) at room temperature for 2 h. The chip was briefly rinsed with EtOH, and then a sodium phosphate buffer (5 mM, pH 7.5). The fluorescein dye was activated under these conditions and the chip was imaged.
Synthesis of target oligonucleotides
DNA synthesis reagents were purchased from Glenn Research (Sterling). Synthesis was performed using a DNA synthesizer (Expedite 8909) and standard protocols of phosphoramidite chemistry (28). Fluorescein phosphoramidite was synthesized from 9-amino-4,5-dicarboxyfluoroscein (Sigma-Aldrich) according to a modified literature procedure (29) and added in the last coupling step of oligonucleotide synthesis to give target sequences of 5′-(fluorescein)-CTGCCTCYCGTAGGAG (Y = A, C, G or T). The fluorescein-tagged sequences and the primers used in PCR reactions were purified using a procedure developed in house.
PCR reactions
Aliquots of 50 µl of PCR reaction mixture contained 1 pg template DNA strand (1 µl, 30 pM), 11 pmol of primers, 10 mM of each dNTP (4 µl) and 2 µl Taq enzyme (Promega) in storage buffer. Amplification was carried out for 30 cycles of 90°C for 30 s, 53°C for 30 s and 72°C for 30 s. Upon completion of the reaction, DNA was purified from the unincorporated dNTPs and the primer with a Clontech purification kit (chroma spin + TE-30) according to the manufacturer’s protocol.
Oligonucleotide and PCR sequence hybridization
The target sequences (50–200 nM) were dissolved in a 6× SSPE solution (100 µL, 1 M NaCl, 66 mM sodium phosphate, 6 mM EDTA, pH 7.4). The hybridization experiment was performed under a cover slip at room temperature for 2–18 h. After hybridization, the chip was washed twice with the 6× SSPE buffer, and the fluorescence emission (FRE) image was taken. The chip was then washed with low-salt buffer solution containing NaCl (5 mM) and NaH2PO4 (5 mM) pH 7.0, until the FRE reading was comparable with background in surrounding areas of the glass plate. The hybridization and image acquisition were repeated using different target sequences.
Imaging oligonucleotide microarray plates
An oDNA chip covered with a thin layer of 6× SSPE solution was placed under a homebuilt imaging system using a cooled CCD camera (Apogee Instruments). Fluorophore excitation and detection were at 475 and 535 nm, respectively. A 200 W Xenon lamp was used as the light source. The FRE image was processed and analyzed using the Image Pro (Media Cybernetics), ScanAlyze2 (http://rana.Stanford.EDU/software/) and the Excel (Microsoft) programs. FRE intensities were reported after baseline correction and averaging over redundant data points.
RESULTS
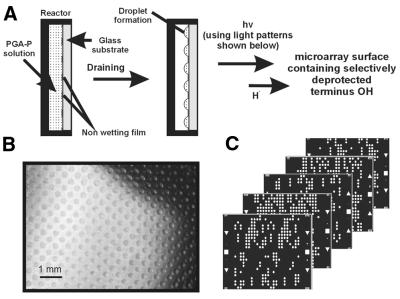
The parallel synthesis of DNA microarrays used a laboratory synthesizer, which consists of four major components: (i) a solvent/reagent manifold; (ii) a microarray reactor assembly; (iii) projection optics containing a light source and a digital micromirror device (DMD, Texas Instruments); and (iv) a controller computer. The chemistry used was mostly the same as that used in regular oligonucleotide synthesis (28) except that acid (H+) deprotection in CH2Cl2 employed light illumination and a PGA-P compound. The parallel synthesis of oDNA chips was performed on Si-based substrates containing isolated reaction sites. Figure 2A and B shows glass plates containing arrays of microreaction wells isolated by hydrofluorocarbon alkyl coating (30). The features of these microarray plates were n × n (n = 12, 24 or 48) with a diameter of d (d = 600, 300 or 150 µm) and a center-to-center separation of l (l = 900, 450 or 300 µm). Whereas smaller reaction site features are possible using the same fabrication methods as reported in ‘Materials and Methods’, other microfabrication methods could generate even fine features. The microarray glass plates had the same dimensions as 22 × 75 mm microscope slides and were held in a microreactor (a flow cell) that was connected to the synthesizer. The microreaction well surfaces were derivatized with alkyl silyl linkers coupled with nucleophosphoramidite terminated with the 5′-O-DMT group. During the deprotection step, the reaction cell was first filled with a PGA-P solution (diaryl iodium salt) (21) (Figs 1A and 2A), which was then drained to allow the formation of droplets as illustrated in Figure 2A and B. A digital light pattern (Fig. 2C) that was generated according to the predetermined oDNA chip layout and aligned to the droplets was projected onto the microarray plate. At the irradiated reaction sites, 5′-O-DMT groups were removed by in situ formed PGA (H+) and the OH group formed (Fig. 1A). At the unirradiated reaction sites, no chemical reaction took place. After deprotection, the reactor was flushed with a neutralizing solution (10% pyridine/CH3CN). A solution containing the appropriate nucleophosphoramidite (monomer) was then added, and the deprotected OH groups at the selected sites coupled with monomers to complete the addition of a new residue to the growing chain. The synthesis of an oligonucleotide array involved the use of predetermined digital light irradiating patterns in successive synthesis cycles (Fig. 2).
Figure 2.
Formation of isolated reaction sites and the microarray synthesis approach. (A) Droplet formation by filling and draining a PGA-P solution in a microarray reactor for the subsequent PGA-gated deprotection reaction. (B) A microscopic image of CH3OH droplets formed on a microarray glass plate patterned by a non-wetting film. (C) Examples of digital light projection patterns generated on a desktop computer for selective PGA deprotection. Four masks are required for the A, C, G or T residue per step growth in oligonucleotide array synthesis. At irradiated circles (white), light is controllable (in terms of irradiation time and strength). Alignment marks are on the left and right side of the digital masks.
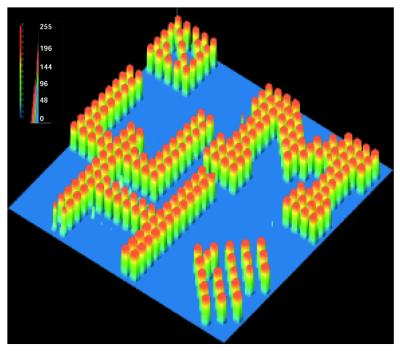
The gating step in the parallel synthesis of oligonucleotide microarrays described above involves the use of a display projector, DMD, for light activation of PGA-P in each synthesis cycle. The same projection device was also used for parallel synthesis of oDNA chips using the chemistry developed by Affymetrix (i.e. using photolabile group-protected monomers) (12,19). DMD was originally designed for use in making large screen displays. It contains a plurality of small (16 µm × 16 µm) and individually controllable rocking-mirrors (with 17 µm center-to-center distance, up to 1024 × 768 for a commercially available XVGA DMD) that can be programmed to tilt according to predetermined orientations. Through reflection, these mirror plates steer light beams to produce images or light patterns on a projection screen. DMD is fully automated and programmed to generate patterns based on array synthesis requirements. The optical properties of DMD, such as high optical contrast and wavelength tolerance (31), are superior for gated photoreactions. In our application, DMD projects digital light patterns onto microarray plates where photogeneration of reaction reagents takes place at discrete sites. The reflectance UV-VIS spectrum of the DMD was measured. It was found that the optimum operation wavelength was between 400 and 700 nm. Below λ = 400 nm reflectance decreased rapidly, and light reflectance efficiency was ∼50% at 350 nm. To improve light absorption efficiency, PGAs in combination with photosensitizers that can be activated >400 nm were used for this study (21,22). Longer wavelengths are also less likely to induce adverse photo-effects on synthesis reagents and DNA products (32). Specifically, a 500 W mercury lamp was used as the light source, and a dichroic mirror was used to allow wavelengths between 350 and 450 nm to pass to DMD. The reflective DMD was operated through a desktop computer using an in-house program (DIGI-SYN) for digital photolithography. For a given array of DNA probe sequences, the program minimized synthesis steps and produced a set of light projection patterns. Examples of these digital projections used for the synthesis of oligonucleotide arrays are shown in Figure 2C. To demonstrate the feasibility and the potential of our technology for flexible oDNA chip design, an oDNA chip displaying a graphic pattern is demonstrated in Figure 3. In this chip, a digital projection allowed irradiation of the selected reaction sites corresponding to UH (representing the University of Houston) and UM (representing the University of Michigan) letters. Coupling of the deprotected OH groups to fluorescein phosphoramidite produced the designed FRE image.
Figure 3.
An FRE intensity display of a fluorescein-labeled oDNA chip synthesized using solution generated PGA as the deprotection agent. The UH (representing the University of Houston) and UM (representing the University of Michigan) pattern is shown.
The reaction condition studies using the diaryl iodium and thioxanthenone system revealed non-linear photosensitivity of the PGA deprotection reaction as measured by FRE intensity variations (Fig. 4). These are desirable features for achieving high-spatial resolution during the deprotection of 5′-DMT. In the reactions shown in Figure 4, light intensities projected onto each individual reaction well and irradiation times were systematically varied using programmed digital light patterns. The PGA-deprotected OH groups were coupled with fluorescein phosphoramidite, and the resulting FRE intensities were proportional to the yield of PGA deprotection. For instance, Figure 4A shows the PGA-deprotection pattern when light irradiation intensities varied from 0 to 100% in 10% increments and light irradiation time was constant (60 s). Figure 4C shows the PGA-deprotection pattern when light irradiation times varied from 1 to 16 s and the light intensity was kept at 100%. These reactions involved all four nucleotides (A, C, G and T) in 5′-DMT-ATT, 5′-DMT-CTT, 5′-DMT-GTT and 5′-DMT-TTT. The plots of the image intensities as a function of light intensity and irradiation time are shown in Figure 4B and D, respectively. These results indicate that PGA detritylation is fast for all four nucleotides with the deprotection completed in <15 s. In comparison, the deprotection step in oligonucleotide synthesis normally takes at least 60 s. Thus, microarray synthesis is efficient and may require overall shorter reaction time than conventional reaction cycles. The reaction undergoes instantaneous formation of H+ upon light irradiation, the protonation of the 5′-O-DMT (a weak acid equilibrium), and the fast dissociation of the DMT group. Our UV experiments of PGA detritylation following the DMT+ absorption at 530 nm demonstrate that the formation of acid upon light irradiation is instantaneous and that detritylation is an acid-limited reaction. Under these conditions, a general acid catalyzed reaction displays a non-linear reaction rate with respect to solution pH. The non-linear relationship of the deprotection rate with light intensity is consistent with general acid catalyzed detritylation reactions (Fig. 4). Based on this information, non-selective deprotection was further eliminated using 10% pyridine in CH2Cl2 as the after deprotection wash. These measures, which include the use of non-wetting film barrier between reaction sites, suitable timing and intensity for light irradiation, and neutralizing wash, helped minimization of cross interactions among microwell reaction sites.
Figure 4.
Display of the non-linear relationship of FRE intensity as a function of light intensity and irradiation time. (A) Image of FRE as a function of light intensity varying from 0 to 100% with 100% the maximum light intensity. Irradiation time was constant (60 s). Fluorescein was coupled to four types of trimers (all terminated with 5′-DMT): ATT, CTT, GTT and TTT. (B) A plot of FRE (arbitrary unit) as a function of light intensity. Data were taken from the analysis of a total of 328 data points in the various regions of the oDNA chip. (C) Image of FRE as a function of irradiation time varying from 1 to 16 s. Light intensity was constant (100%). (D) A plot of FRE as a function of irradiation time. Data were taken from the analysis of a total of 108 data points in the various regions of the oDNA chip.
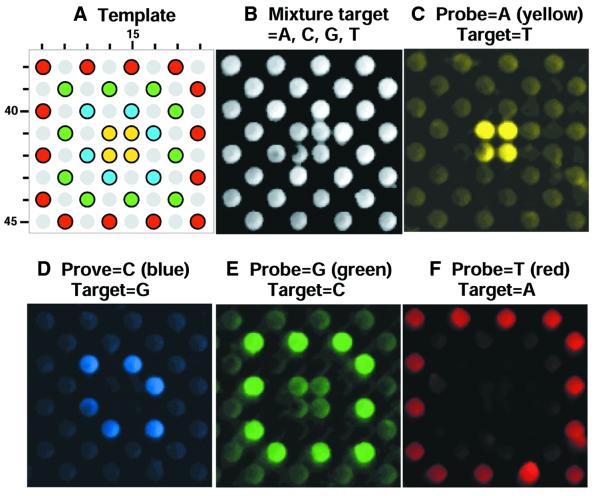
The oDNA chips were synthesized in high quality with an average >98% stepwise yield. The sequence fidelity was validated by means of hybridization using fluorescein-labeled target sequences and PCR products. A representative result is displayed in Figure 5. This DNA chip has a dimension of 48 × 48 rows (R1–R48) and columns (C1–C48) and contains oligomer sequences 5′-CTCCTAX1GNX2AX3GCAG (X1 = C or deletion; X2,3 = G or deletion; N = A, C, G, T or deletion). The synthesis was performed using the method described above. Figure 5A shows the sequence layout template corresponding to the region of the images shown in Figure 5B–F. These are the results of five experiments using the same oDNA chip. Hybridization experiments were carried out as follows. A solution sample containing the target sequence that was complementary to the probe with N = A, X1 = C and X2,3 = G was applied to the chip surface and incubated at room temperature for 2 h. The resulting image is shown in Figure 5C. The oDNA chip was washed using a lower salt buffer (dehybridization buffer) until all target sequences disappeared (null FRE) and then hybridized to a different target sequence complementary to the probe with N = C, X1 = C and X2,3 = G under the same hybridization conditions as described above. This result is shown in Figure 5D. The same oDNA chip was washed and hybridized to the target sequences complementary to the probes with N = G and N = T, respectively, and X1 = C and X2,3 = G. These results are shown in Figure 5E and F. Finally, the same oDNA chip was hybridized to a mixture of four target sequences complementary to the probes with N = A/C/G/T, X1 = C and X2,3 = G (Fig. 5B). The brightest spots in Figure 5 correspond to probe hybridization to the 5′-fluorescein-labeled complementary target sequences, whereas mismatch probes produced lower FRE intensity. Under the conditions used, the presence of some stable mismatches was also observed, such as A·C (Fig. 5E) and G·A (Fig. 5F) in the 16mer sequence containing GNG·CYC (Fig. 5). The oDNA chip also exhibited intra-chip homogeneous profiles because the variations between two sites of the same experiment are mostly ∼5%. In a separate experiment (Fig. 6), oDNA probes were hybridized to PCR products of more than 200 residues. Correct hybridization was achieved. These results confirm the success of the oDNA synthesis.
Figure 5.
FRE images of oDNA chip hybridization with fluorescein-labeled target sequences. A region of probes containing variation in a single-nucleotide position is shown (row and column coordinates are displayed). The site feature size is 150 µm. These hybridization experiments used the same oDNA chip. (A) The designed template of probes. The sites containing sequences with A, C, G or T in the variable position are color marked. Light gray circles are for probes of less than full length because of the deletion of 1–4 residues at certain residue positions in these sequences. (B) oDNA chip hybridization with a mixture of four target sequences which are complementary to all probe sequences in the region shown. (C–F) oDNA chip hybridization using a single-target sequence, which is complementary to the A, C, G or the T probe.
Figure 6.
oDNA chip hybridization to 212 residue PCR product.
DISCUSSION
Demonstrated herein are examples of a flexible chemistry applied for parallel synthesis of oDNA chip. While the concept of light-directed synthesis of microarrays has been demonstrated for several years (12), the majority of research users still face serious technical and cost barriers. Progress has been made for the maskless photolithographic synthesis of oDNA chips, but the method is limited by the use of photolabile group-protected monomers (19). Our work represents major advancement in its use of conventional chemistry and elimination of the need for photolabile group-protected monomers (12,19,33) (Fig. 1B). Oligonucleotide microarrays of any sequence design can be easily synthesized in an ordinary laboratory. This capability will bring ample opportunities for creative research with an increased level of versatility and complexity. Further, as the deprotection reaction is based on conventional chemistry (Fig. 1A), the quality of the sequences synthesized is high with stepwise yield approaching those achievable in a conventional synthesis (23). In the long term, the microarray synthesis method presented herein will have impact on biochip availability in general. The method is not limited to the syntheses of regular oDNA chips, but is also suitable for special oDNA chips, such as those containing oligonucleotides in a 5′ to 3′ direction (3′-OH is free for primer extension) and those containing analogs of nucleotides and RNA residues. These oDNA and oRNA chips are needed for a variety of studies, such as parallel analysis of oDNA chips for investigation of carcinogen or toxin modification of DNA sequences and interrogations of molecular interactions of RNA molecules with ligand or proteins.
The chemistry principle illustrated in this paper has the potential to become the basis of a platform biochip technology for a broad range of applications. This platform technology will offer clear efficiency, versatility, flexibility, quality and economic advantages. Progress has been made in this laboratory in synthesizing peptide microarrays containing natural and synthetic amino acids. This quick extension of the synthesis is possible because our chemistry using photogenerated reagents in solution employs readily available starting materials and the alteration of the process can be accomplished. On the contrary, it is practically impossible to use photolabile protection-based chemistry to synthesize a variety of biochips, because of the unavailability of the various photolabile group-protected monomers and the difficulty, the time and cost associated with making these compounds. In the next decade, there will be an inevitable conversion in the manner in which many chemical and biochemical analyses are conducted. This will be a gradual change from macro- to micro-scale, miniaturized, highly parallel platforms. Therefore, there will be continuous efforts to provide enabling sciences and technologies. The work described herein represents our effort in this direction.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank Dr Woody T. Zhou for improvements on the imaging system. We acknowledge the supply of Digital Light Projector evaluation kits by Texas Instruments to the University of Michigan. This research is supported by grants from NIH/NCI, The National Foundation of Cancer Research, Texas Higher Education Coordinating Board ATP, and the R. A. Welch Foundation to X.G., from NSF to E.G., from NIH/HGRI to X.Z. and from the Merck Genome Research Institute to X.G. and E.G.
REFERENCES
- 1.Drmanc S., Kita,D., Labat,I., Hauser,B., Schmidt,C., Burczak,J.D. and Drmanac,R. (1998) Accurate sequencing by hybridization for DNA diagnostics and individual genomics. Nature Biotechol., 16, 54–58. [DOI] [PubMed] [Google Scholar]
- 2.Schena M., Shalon,D.D., Davis,R.W. and Brown,P.O. (1995) Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science, 270, 467–460. [DOI] [PubMed] [Google Scholar]
- 3.Yershov G., Barsky,V., Belgovskiy,A., Kirillov,E., Kreindlin,E., Ivanov,I., Parinov,S., Guschin,D., Drobishev,A., Dubiley,S. and Mirzabekov,A. (1996) DNA analysis and diagnostics on oligonucleotide microchips. Proc. Natl Acad. Sci. USA, 93, 4913–4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Southern E.M. (1996) DNA chips: analysing sequence by hybridization to oligonucleotides on a large scale. Trends Genet., 12, 110–115. [DOI] [PubMed] [Google Scholar]
- 5.Lockhart D.L. and Winzeler,E.A. (2000) Genomics, gene expression and DNA arrays. Nature, 405, 827–836. [DOI] [PubMed] [Google Scholar]
- 6.Southern E.M., Maskos,U. and Elder,J.K. (1992) Analyzing and comparing nucleic acid sequences by hybridization to arrays of oligonucleotides: evaluation using experimental models. Genomics, 13, 1008–1017. [DOI] [PubMed] [Google Scholar]
- 7.Khrapko K.R., Lysov,Y., Khorlyn,A.A., Shick,V.V., Florentiev,V.L. and Mirzabekov,A.D. (1989) An oligonucleotide hybridization approach to DNA sequencing. FEBS Lett., 256, 118–122. [DOI] [PubMed] [Google Scholar]
- 8.Drmanac R., Labat,I., Brukner,I. and Crkvenjakov,R. (1989) Sequencing of megabase plus DNA by hybridization: theory of the method. Genomics, 4, 114–128. [DOI] [PubMed] [Google Scholar]
- 9.Wetmur J.G. (1976) Hybridization and renaturation kinetics of nucleic acids. Annual Review of Biophysics and Bioengineering. Annual Reviews Inc., pp. 337–361. [DOI] [PubMed]
- 10.Wetmur J.G. and Davidson,N. (1968) Kinetics of renaturation of DNA. J. Mol. Biol., 31, 349–370. [DOI] [PubMed] [Google Scholar]
- 11.Gillam S., Waterman,K. and Smith,M. (1975) The base-pairing specificity of cellulose-pdT9. Nucleic Acids Res., 2, 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fodor S.P., Leighton,P.A.J., Pirrung,M.C., Stryer,L. and Solas,D. (1991) Light-directed spatially addressable parallel chemical synthesis. Science, 251, 767–773. [DOI] [PubMed] [Google Scholar]
- 13.Higgins G.S., Little,D.P. and Koster,H. (1997) Competitive oligonucleotide single-base extension combined with mass spectrometric detection for mutation screening. Biotechniques, 23, 710–714. [DOI] [PubMed] [Google Scholar]
- 14.Hacia J.G., Brody,L.C., Chee,M.S., Fodor,S.P. and Collins,F.S. (1996) Detection of heterozygous mutations in BRCA1 using high density oligonucleotide arrays and two-colour fluorescence analysis. Nature Genet., 14, 441–447. [DOI] [PubMed] [Google Scholar]
- 15.Sosnowski R.G., Tu,E., Butler,W.F., O’Connell,J.P. and Heller,M.J. (1997) Rapid determination of single base mismatch mutations in DNA hybrids by direct electric field control. Proc. Natl Acad. Sci. USA, 94, 1119–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hacia J.G., Fan,J.B., Ryder,O., Jin,L., Edgemon,K., Ghandour,G., Mayer,R.A., Sun,B., Hsie,L., Robbins,C.M., Brody,L.C., Wang,D., Lander,E.S., Lipshutz,R., Fodor,S.P. and Collins,F.S. (1999) Determination of ancestral alleles for human single-nucleotide polymorphisms using high-density oligonucleotide arrays. Nature Genet., 2, 164–167. [DOI] [PubMed] [Google Scholar]
- 17.Gilles P.N., Wu,D.J., Foster,C.B., Dillon,P.J. and Chanock,S.J. (1999) Single nucleotide polymorphic discrimination by an electronic dot blot assay on semiconductor microchips. Nature Biotechol., 17, 365–370. [DOI] [PubMed] [Google Scholar]
- 18.Gao X., Yu,P., Leproust,E., Sonigo,L., Pellois,J.P. and Zhang,H. (1998) Oligonucleotide synthesis using solution photogenerated acids. J. Am. Chem. Soc., 120, 12698–12699. [Google Scholar]
- 19.Singh-Gasson S., Green,R.D., Yue,Y., Nelson,C., Blattner,F., Sussman,M.R. and Cerrina,F. (1999) Maskless fabrication of light-directed oligonucleotide microarrays using a digital micromirror array. Nature Biotechol., 17, 974–978. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto T., Suzuki,T. and Yamamoto,N. (2000) Microarray fabrication with covalent attachment of DNA using bubble jet technology. Nature Biotechol., 18, 438–441. [DOI] [PubMed] [Google Scholar]
- 21.LeProust E., Pellois,J.P., Yu,P., Zhang,H., Srivannavit,O., Gulari,E., Zhou,X. and Gao,X. (2000) Combinatorial screening method for synthesis optimization on a digital light-controlled microarray platform. J. Comb. Chem., 2, 349–354. [DOI] [PubMed] [Google Scholar]
- 22.Pellois J.P., Wang,W. and Gao,X. (2000) Peptide synthesis based on t-Boc chemistry and solution photogenerated acids. J. Comb. Chem., 2, 355–360. [DOI] [PubMed] [Google Scholar]
- 23.LeProust E., Zhang,H., Yu,P., Gao,X., Xiang,X. and Zhou,X. (2001) Characterization of oligodeoxyribonucleotide synthesis on glass plates. Nucleic Acids Res., 29, 2171–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald S.A., Willson,C.G. and Fréchet,J.M. (1994) Chemical amplification in high-resolution imaging systems. Acc. Chem. Res., 27, 151–157. [Google Scholar]
- 25.Thompson L.F., Willson,C.G. and Bowden,M.J. (1994) Introduction to Microlithography, 2nd Edn. ACS Professional Reference Book, American Chemical Society, Washington, DC, pp. 138–267.
- 26.Beecher J.E., McGall,G.H. and Goldberg,M. (1997) The application of photolithographic techniques for the fabrication of high density oligonucleotide arrays. Polym. Mater. Sci. Eng., 76, 394–395. [Google Scholar]
- 27.Beecher J.E., McGall,G.H. and Goldberg,M. (1997) Chemically amplified photolithography for the fabrication of high density oligonucleotide arrays. Polym. Mater. Sci. Eng., 76, 597–598. [Google Scholar]
- 28.McBride L.J. and Caruthers,M.H. (1983) An investigation of several deoxynucleotide phosphoramidites. Tetrahedron Lett., 24, 245–248. [Google Scholar]
- 29.Andrus A. (1992) Fluorescent dye phosphoramidite labeling of oligonucleotides. Tetrahedron Lett., 33, 5033–5036. [DOI] [PubMed] [Google Scholar]
- 30.Stenger D.A., Georger,J.H., Dulcey,C.S., Hickman,J.J., Rudolph,A.S., Nielsen,T.B., McCort,S.M. and Calvert,J.M. (1992) Coplanar molecular assemblies of amino- and perfluorinated alkylsilanes: characterization and geometric definition of mammalian cell adhesion and growth. J. Am. Chem. Soc., 114, 8435–8442. [Google Scholar]
- 31.Hornbeck L.J. (1996) Digital light processing and MEMS, reflecting the digital display needs of the networked society. SPIE Eur. Proc., 2783, 135–145. [Google Scholar]
- 32.Cadet J. and Vigny,P. (1990) In Morrison,H. (ed.), Bioorganic Photochemistry, Vol. 1, Wiley, New York, NY, pp. 1–272.
- 33.Beier M. and Hoheisel,J.D. (2000) Production by quantitative photolithographic synthesis of individually quality checked DNA microarrays. Nucleic Acids Res., 28, e11. [DOI] [PMC free article] [PubMed] [Google Scholar]