Abstract
Glutamatergic synapses harbor abundant amounts of the multifunctional Ca2+/calmodulin-dependent protein kinase type II (CaMKII). Both in the postsynaptic density as well as in the cytosolic compartment of postsynaptic terminals, CaMKII plays major roles. In addition to its Ca2+-stimulated kinase activity, it can also bind to a variety of membrane proteins at the synapse and thus exert spatially restricted activity. The abundance of CaMKII in glutamatergic synapse is akin to scaffolding proteins although its prominent function still appears to be that of a kinase. The multimeric structure of CaMKII also confers several functional capabilities on the enzyme. The versatility of the enzyme has prompted hypotheses proposing several roles for the enzyme such as Ca2+ signal transduction, memory molecule function and scaffolding. The article will review the multiple roles played by CaMKII in glutamatergic synapses and how they are affected in disease conditions.
Keywords: Ca2+/calmodulin-dependent protein kinase type II (CaMKII), glutamatergic synapse, LTP, LTD, synaptic plasticity, CaMKII genetic models, CaMKII mutations
Introduction
Glutamatergic synapses are the main excitatory synapses in the brain particularly in the cerebral cortex and hippocampus. More than 80% of synapses in the cortex are glutamatergic (Micheva et al., 2010). Glutamatergic transmission plays a major role in neuronal functions in the brain. Imbalances in glutamatergic signaling can lead to several neurodegenerative and psychiatric conditions (Moretto et al., 2018).
Calcium (Ca2+) signaling is an essential component in signal transduction at glutamatergic synapses. Calcium signals are tightly regulated since sustained elevation in Ca2+ levels can lead to toxicity. In glutamatergic synapses, the spike patterns of Ca2+ signals are thought to encode information. Decoding these signals requires the participation of efficient protein machineries that convert them into long-lasting biochemical and cellular changes representing memories. Calcium (Ca2+)/calmodulin (CaM)-dependent protein kinase II (CaMKII) at synapses plays a significant role in decoding Ca2+ spike patterns and in converting them to corresponding biochemical states. Thus, CaMKII has gained the status of a “memory molecule” by being the initiator of biochemical memory in the brain. However, the multiple isoforms and splice variants of CaMKII that assemble in varying combinations to give rise to several holoenzyme subtypes, makes it so versatile that it is involved in several other functions both in the brain and in other tissues. The phylogenetic relations of CaMKII with other kinases, its structure, its different isoforms and splice variants, biochemical and physiological functions, especially in long-term potentiation (LTP) and long-term depression (LTD), and its role in various diseases have been reviewed recently (Bayer and Schulman, 2019; Giese, 2021; Sloutsky and Stratton, 2021). Its functions specifically in the glutamatergic postsynaptic compartment have also been previously described (Hell, 2014). This article covers the basics on CaMKII including the recent advances in structure, isoforms, activation mechanisms, role in LTP and LTD, regulation of its translation, role in synapse morphology regulation, role in presynaptic mechanisms and role in various pathological conditions with emphasis on its functioning at glutamatergic synapses. In vivo models of CaMKII mutants with the associated behavioral phenotypes and CaMKII mutations reported in neurodevelopmental disorders and learning disabilities in humans have also been included in the present review.
Ca2+/Calmodulin-Dependent Protein Kinase Type II Isoforms and Their Function in Glutamatergic Synapses
Even though CaMKII has four distinct isoforms (α, β, γ, and δ) encoded by four different genes with molecular weight ranging from 52 to 83 kDa, α and β are the predominant ones in neurons. CaMKIIα has distinct roles in neuronal plasticity and memory. It is predominant in the hippocampal and in the neocortical areas of the brain. CaMKIIβ is enriched in cerebellum and is involved in neuronal development. While both CaMKIIα and CaMKIIβ are expressed in excitatory pyramidal neurons in the cortex and hippocampus, only CaMKIIβ is found in inhibitory interneurons in these regions (Nicole and Pacary, 2020). CaMKIIδ isoform participates in long-lasting memory storage in the hippocampus (Zalcman et al., 2018, 2019). CaMKIIγ isoform is attributed with the main function of synapse-to-nucleus communication, conveying Ca2+ signals to the nucleus and regulating gene expression that is essential for neural plasticity involved in memory (Ma et al., 2014; Cohen et al., 2018).
Ca2+/Calmodulin-Dependent Protein Kinase Type II Structure in Relation to Its Function
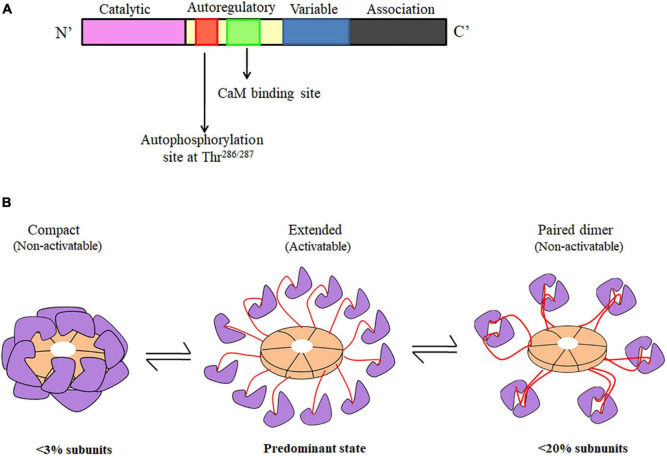
CaMKII forms large homo or hetero oligomeric assemblies of either single or multiple isoforms (Hoelz et al., 2003; Bayer and Schulman, 2019). The core sequence for the CaMKII isoforms includes an N-terminal catalytic domain, followed by a CaM binding autoregulatory domain containing Thr286/Thr287, a variable domain that is subject to alternative splicing and a C-terminal self-association domain. A linear representation of a CaMKII subunit is shown in Figure 1A.
FIGURE 1.
(A) Linear representation of CaMKII structure showing catalytic, autoregulatory, variable and association domains. (B) CaMKII holoenzyme structure in three different forms-CaMKII can exist predominantly in the activatable state with an extended conformation along with some non-activatable states, which are represented as both compact form and kinase domain paired form. The different subunits of a single CaMKII holoenzyme can exist in any combination of the three forms. Purple color indicates kinase domain, peach color denotes association domain, and red color indicates regulatory domain (Myers et al., 2017).
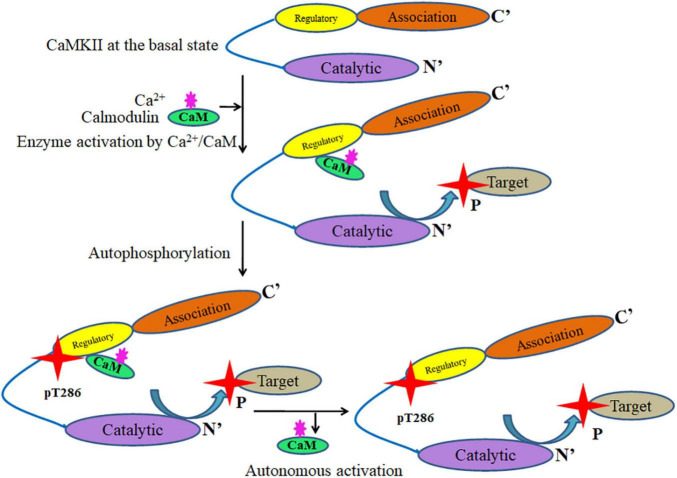
Under basal state, the enzyme is present in an autoinhibited state with the regulatory segment bound to the substrate-docking groove on each kinase domain. It can be activated by the binding of Ca2+/CaM to the autoregulatory domain which releases the regulatory segment off the kinase domain. Activation of adjacent subunits can result in trans-autophosphorylation at Thr286 site (Rich and Schulman, 1998) in the regulatory segment which generates ‘autonomous’ kinase activity even after the initial Ca2+-stimulus subsides (Miller and Kennedy, 1986) by preventing the regulatory segment binding on the kinase domain. This inter-subunit autophosphorylation mechanism enables Ca2+-spike frequency-detection by CaMKII (De Koninck and Schulman, 1998). The autophosphorylation at Thr286 can also increase the affinity of the enzyme for CaM by about 1000-fold, a process termed as CaM trapping. A single autophosphorylated subunit can also rapidly phosphorylate its neighbors. Thus, a brief Ca2+ stimulus in the synapse can lead to the persistence of Thr286-autophosphorylated CaMKII that represents molecular memory (Figure 2). Autophosphorylation at Thr286 is an essential event in the induction of LTP that underlies memory formation.
FIGURE 2.
Basic activation mechanism of CaMKII that leads to autonomy resulting from Thr286 autophosphorylation. Under basal conditions, the enzyme is present in an autoinhibited state with the regulatory segment bound to the catalytic domain. This can be activated by the binding of Ca2+/CaM to the regulatory domain which releases the regulatory segment from the catalytic domain. The activated enzyme can autophosphorylate at Thr286 or any other substrates. The autonomous CaMKII thus generated can be catalytically active even in the absence of Ca2+/CaM.
Once Ca2+/CaM dissociates from the kinase, cis-autophosphorylation occurs at the CaM binding domain of CaMKII at the Thr305/306 position. Phosphorylation at these sites, termed as “inhibitory” or “burst” autophosphorylation, prevents the binding of Ca2+/CaM and hence kinase cannot be further stimulated. Autophosphorylation at Thr305 and Thr306 before phosphorylation of Thr286 makes the kinase non-responsive to Ca2+/CaM stimulus and such a kinase cannot be activated. On the other hand, if Thr286 gets autophosphorylated first, it leads to a holoenzyme in which Thr305 and Thr306 are protected by Ca2+/CaM and cannot be phosphorylated (Bhattacharyya et al., 2020). It is also reported that CaMKII phosphorylation at Thr305/306 is selectively promoted by LTD inducing stimuli and not by LTP inducing stimuli, and phosphorylation at Thr305/306 directs CaMKII movement during LTD from excitatory to inhibitory synapses. This phosphorylation can also reduce the activity of phospho-Thr286 CaMKII in the absence of Ca2+ (Cook et al., 2021).
The first snapshot of the 3D structure of this enzyme was an electron microscopy (EM) image of CaMKII purified from rabbit skeletal muscle (Woodgett et al., 1983) that revealed a symmetrical hexagonal structure, composed of two stacked 6-membered rings. Since then, several hypotheses have been proposed about its structure in relation to its function. The catalytic/autoregulatory domains of each subunit are attached to the hexameric ring by a stalk-like appendage that presumably allows subunits to behave independently of one another for activity and Ca2+/CaM binding, but in concert with one another for the intra-holoenzyme autophosphorylation reaction (Figure 1B). Most of the crystallographic studies provided structures at atomic resolution of truncated forms having single or multiple domains (Hoelz et al., 2003; Rosenberg et al., 2006) giving insights on the mechanism of catalytic activity and atomic level details of the interactions holding the 3D structure and interactions between domains.
The recent studies based on single-particle EM (Myers et al., 2017; Bhattacharyya et al., 2020) in combination with biochemical and live-cell imaging experiments (Buonarati et al., 2021) further substantiated the multimeric structure of CaMKII holoenzyme having a rigid central hub complex formed by the association domains. The kinase domains are linked to the hub by the intrinsically disordered and highly flexible linker regions (residues 301–344) so that they can readily perform inter-subunit autophosphorylation. The holoenzymes range from 15–35 nm in diameter. This model also predicts that CaMKII holoenzymes can exist in three different conformations. Among these three conformations, <3% of the holoenzymes are in the compact conformation, ∼20% appears to form kinase dimers and most of the kinase domains are ordered independently both in vitro and inside the cells. CaMKII holoenzymes which appear as either compact or kinase dimers are inactive, whereas the fraction with fully extended kinase domains is in the activatable state (Figure 1B; Myers et al., 2017; Bayer and Schulman, 2019).
The formation of extended intra-holoenzyme kinase dimers could enable cooperative activation by CaM in both α and β isoforms (Myers et al., 2017; Bhattacharyya et al., 2020; Buonarati et al., 2021) but there could be distinct steric positioning of kinase domains in the CaMKIIα versus β holoenzyme due to differences in the linker length. This explains the differences in the autophosphorylation states of both the isoforms; CaMKIIα acquires Thr286 phosphorylation more readily than Thr305/306 phosphorylation whereas inhibitory autophosphorylation at Thr306/307 in CaMKIIβ occurs more readily. Inter-holoenzyme kinase dimer formation is thought to involve a high order clustering among CaMKII holoenzymes and is present in minimal quantities under normal physiological conditions for both the isoforms. But it is enhanced in both excitotoxic and ischemic conditions and the high-order CaMKII clustering formed by inter-holoenzyme kinase domain dimerization is reduced for the β isoform for both basal and excitotoxicity-induced clusters, both in vitro and in neurons (Buonarati et al., 2021). Much of the studies on holoenzyme structure have been carried out using homomers of either α or β isoforms. However, heteromultimeric CaMKII formed by α and β is known to play key functions in the brain. Structural insights into heteromultimeric forms of CaMKII would help in further advancing the understanding of the physiological functioning of this enzyme. It has been also noted that a small percentage (<4%) of holoenzymes of CaMKIIα were found as 14-mers even with full-length kinase domains (Myers et al., 2017) whereas CaMKIIβ can even exist in 16-mers (Buonarati et al., 2021). The existence of a full-length 14-mer is thought to be an intermediate state in which the exchange of subunits is possible (Myers et al., 2017) and it entails the exchange of activated subunits between two activated, or an activated and a non-activated holoenzyme (Bhattacharyya et al., 2020). This hypothesis was supported by the finding that proteolytic cleavage of the kinase domains from a 12-meric holoenzyme preparation results in the subsequent formation of 14-meric hub domain assemblies (Rosenberg et al., 2006). The function of this kind of subunit exchange is currently unknown, but it is speculated that it can be a part of repair mechanisms of individual subunits and/or synaptic plasticity mechanisms (Bayer and Schulman, 2019).
Ca2+/Calmodulin-Dependent Protein Kinase Type II Activation in Response to Ca2+ Influx Through N-Methyl-D-Aspartate Receptor
N-Methyl-D-aspartate receptor (NMDAR) is an ionotropic glutamate receptor with high Ca2+ permeability that plays an important role in excitatory neurotransmission in the central nervous system (CNS). Glutamate binding to α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs) can induce depolarization in the postsynaptic membrane of glutamatergic synapses. The binding of glutamate and glycine and the depolarization-induced removal of Mg2+ block causes NMDAR to open and conduct Ca2+ and Na+ into the cell. This Ca2+ influx activates several important signaling pathways involving different protein kinases including CaMKII and phosphatases. Activated CaMKII can bind to various membrane proteins as listed in Table 1. The enzyme can interact with each of these proteins either in the Ca2+/CaM activated form or in the autophosphorylated form. It can bind with high affinity at the GluN2B subunit of NMDAR and phosphorylate GluN2B-Ser1303 (Omkumar et al., 1996). GluN2B-binding can also happen at the T-site of CaMKII (site where Thr286 is bound in the inactive state) making the enzyme permanently active even after the Ca2+ stimulus subsides (Bayer et al., 2001). In addition, the kinetic parameters of CaMKII activity and its affinity for ATP are altered in an allosteric manner upon binding to GluN2B (Pradeep et al., 2009; Cheriyan et al., 2011; Madhavan et al., 2020) and this regulation is limited only to the subunit of the enzyme that binds GluN2B (Cheriyan et al., 2012). CaMKII activated in the cytosol can translocate to the postsynaptic membrane where the NMDAR complex is embedded in the postsynaptic density (PSD). CaMKII reversibly translocates to synaptic sites in response to brief stimuli, but its resident time at the synapse increases after longer stimulation (Bayer et al., 2006). It is also reported that the phosphorylation status of GluN2B at Ser1303 also regulates GluN2B-CaMKII interaction (Raveendran et al., 2009), whereas the phosphorylation status of Ser1303, in turn, is regulated by the action of kinases and phosphatases (Ramya et al., 2012). In the GluN2B-bound state, the enzyme becomes resistant to the action of phosphatases (Cheriyan et al., 2011) indicating GluN2B-induced structural changes which can be abolished by specific mutations in CaMKII (Mayadevi et al., 2016). This could be a possible reason for the resistance of phospho-Thr286-CaMKIIα to phosphatases in the PSD (Mullasseril et al., 2007). Autonomy of CaMKII due to GluN2B-binding can be terminated only by dissociation of CaMKII from GluN2B. Repeated Ca2+ influx through NMDAR promotes the persistent binding of CaMKII to GluN2B which occurs during LTP (Bayer et al., 2006).
TABLE 1.
Protein ligands of CaMKII in the postsynaptic compartment of glutamatergic synapses.
| Sl. No. | Protein ligand to which CaMKII binds | Region of binding | Functional implications of this binding | Reference(s) |
| 1 | NMDAR subunit GluN2B | 839–1120 | The binding requires auto phosphorylated CaMKII; tethering at the synaptic membrane; LTP | Lisman et al., 2012 |
| 2 | NMDAR subunit GluN2B | 1289–1310 | Activated CaMKII can bind; tethering at the synaptic membrane; LTP | Lisman et al., 2012 |
| 3 | NMDAR subunit GluN2A | 1349–1464 | Synaptic targeting | Gardoni et al., 1999 |
| 4 | NMDAR subunit GluN1 | 845–863 | Synaptic targeting | Leonard et al., 2002 |
| 5 | Cav1.2 | 1589–1690 | Tethering at the synaptic membrane | Hudmon et al., 2005 |
| 6 | Densin-180 | 1247–1495 | Membrane localization | Strack et al., 2000b; Robison et al., 2005 |
| 7 | Tiam 1 | 1540–1560 | Constitutive CaMKII activation; LTP | Saneyoshi et al., 2019 |
| 8 | Ether-a-go-go (Eag) | 731–803 | Constitutive CaMKII activation; LTP | Sun et al., 2004 |
Long Term Potentiation Induction by the Activation of N-Methyl-D-Aspartate Receptors-Role of Ca2+/Calmodulin-Dependent Protein Kinase Type II in N-Methyl-D-Aspartate Receptor-Dependent Long Term Potentiation
LTP is a process in which brief periods of synaptic activity produces long-lasting increase in the strength of a synapse, as shown by an increase in the size of the excitatory postsynaptic current (EPSC) (Lisman et al., 2012; Bliss and Collingridge, 2019). Several studies have shown that LTP has the essential characteristics of a cellular mechanism that could underlie memory and can serve as an excellent cellular model of memory. Impairment in LTP formation predicts memory impairment in human subjects (Di Lorenzo et al., 2020). LTP occurring at CA3-CA1 synapses (between Schaffer collateral (SC) terminals and CA1 pyramidal neurons) of the hippocampal region is mainly mediated through NMDARs and occurs predominantly by postsynaptic modifications (MacDonald et al., 2006). This model of LTP is a suitable model for associative learning (Baltaci et al., 2019).
LTP has an early phase which is independent of protein synthesis, called early-LTP (E-LTP), and a late phase (L-LTP) which involves the activation of transcription factors and is dependent on protein synthesis, during which structural changes are observed. Single brief tetanus leads to E-LTP that lasts up to 1–3 h and intermittent and repetitive stimulations (or single stronger tetanus) produce L-LTP that lasts at least 24 h (Baltaci et al., 2019). During the induction of LTP, Ca2+ influx through NMDARs activates signaling pathways that lead to synaptic modifications (Malenka et al., 1989). NMDAR-dependent LTP requires one or more trains of 100 Hz stimulations (Baltaci et al., 2019).
Over three decades of study suggests that CaMKII is one of the key players in LTP (Zalcman et al., 2018). Inhibition of CaMKII activity blocks the induction as well as maintenance of LTP (Malenka et al., 1989; Malinow et al., 1989; Tao et al., 2021). In response to sufficient influx of Ca2+ into the postsynaptic neuron, CaMKII gets activated by the binding of Ca2+/CaM and autophosphorylated at Thr286. Both these forms of CaMKII can translocate to PSD and bind to GluN2B. Autonomously active nature of Thr286 phosphorylated CaMKII as well as GluN2B-bound CaMKII is proposed to contribute toward molecular memory. But Thr286 autophosphorylation does not have an essential role in NMDAR dependent synaptic potentiation in early postnatal development and in adult dentate gyrus, where neurogenesis occurs (Giese, 2021). Persistent nature of GluN2B-CaMKII interaction could also contribute towards its role in maintaining synaptic strength (Sanhueza et al., 2011). If this interaction is impaired by mutations on the binding sites on CaMKII and/or GluN2B (Yang and Schulman, 1999; Strack et al., 2000a; Mayadevi et al., 2002; Pradeep et al., 2009), then LTP gets impaired (Barria and Malinow, 2005). The binding of GluN2B locks CaMKII in an active conformation and the enzyme can phosphorylate its substrates present in the PSD. The protein substrates of CaMKII in the PSD and the physiological consequences of their phosphorylation status are listed out in Supplementary Table 1 (McGlade-McCulloh et al., 1993; Inagaki et al., 1997; Gardoni et al., 2003, 2006; Oh et al., 2004; Chen and Roche, 2007; Shin et al., 2012; Zhang et al., 2019; Zybura et al., 2020). One of the main effectors of LTP is AMPAR. CaMKII that is localized in PSD through interaction with GluN2B can phosphorylate Ser831 residue of the GluA1 subunit of AMPAR causing potentiation of the single channel conductance of AMPAR (Figure 3; Barria et al., 1997a,b). As part of LTP, more AMPARs are recruited to the synapses and this process is called AMPAfication (Malenka and Nicoll, 1999). The process of AMPAfication makes the transmission even stronger (Zhu and Malinow, 2002). It is also reported that the interaction of CaMKII with GluN2B effects a liquid-liquid phase separation with co-segregation of AMPA receptors and the synaptic adhesion molecule neuroligin into a phase-in-phase assembly indicating the formation of functional nanodomains in the synapse (Hosokawa et al., 2021).
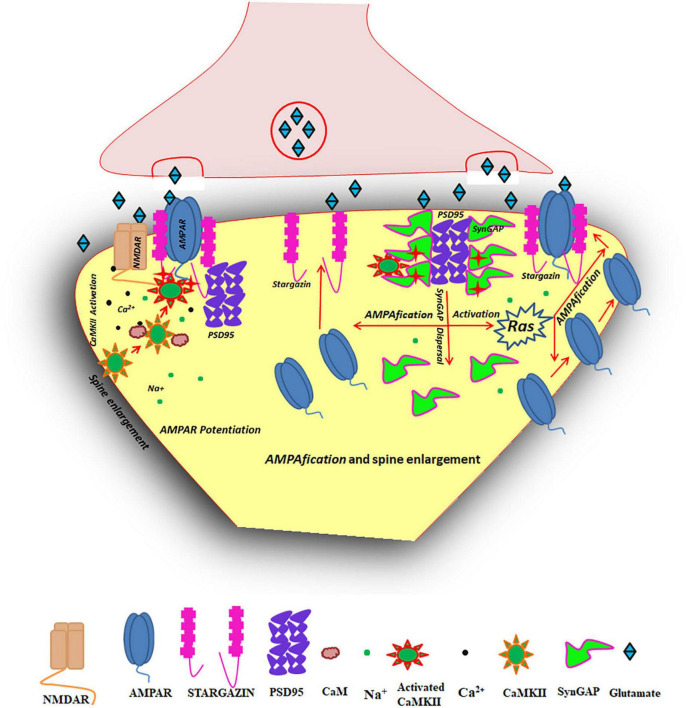
FIGURE 3.
Schematic diagram shows the role of CaMKII in LTP. CaMKII activity at the PSD is essential for the induction and maintenance of LTP, either through (i) enhancement of AMPAR conductance or through (ii) AMPAfication of the postsynaptic site. In either of these functions, activation of CaMKII along with its translocation to its own adapters at the PSD, especially to the GluN2B subunit of NMDAR is essential. The translocated CaMKII can phosphorylate its substrates involved in the induction and maintenance of LTP. (i) AMPAR potentiation-The phosphorylation at Ser831 of GluA1 of AMPAR by CaMKII enhances the single channel conductance of AMPAR especially AMPAR formed by GluA1 homomers (Derkach et al., 1999). (ii) AMPAfication (conversion of silent synapses to active synapses)- AMPARs are positioned in the PSD by interaction with many proteins, especially stargazin. Phosphorylation of stargazin by CaMKII results in its dissociation from lipid rafts and binding to PSD95 to make more AMPAR slots on the membrane (slot hypothesis for AMPAfication). In addition to this, CaMKII can phosphorylate SynGAP which results in its elimination from the synapse followed by the activation of Ras/ERK signaling which mediates AMPAfication or AMPAR recruitment to the PSD. These signaling cascades finally lead to spine enlargement.
Other than AMPAR, CaMKII has other downstream targets such as transmembrane AMPAR-regulatory proteins (TARPs). TARPs are auxiliary proteins that help in AMPAR functions and trafficking (Jackson and Nicoll, 2011). They have several phosphorylation sites for CaMKII which are implicated in the positioning and trapping of AMPAR in PSD (Supplementary Table 1). The C-tail of the TARP family member, stargazin (TARPγ-2) can be phosphorylated by CaMKII which disrupts the interaction of stargazin with phospholipids in the membrane and eventually allows stargazin to bind with PSD95, a major scaffolding protein in PSD to which many other proteins can bind. In this way, stargazin can trap AMPAR in the PSD (Opazo et al., 2010; Hafner et al., 2015). It is also known that the hippocampally enriched TARPγ-8, but not TARPγ-2/3/4, is a critical CaMKII substrate necessary for LTP induction. The residues of TARPγ-8, Ser277 and Ser281 are major phosphorylation sites for CaMKII, which sufficiently enhances AMPAR transmission. Mutations of these residues impair LTP, without affecting AMPAR-mediated basal transmission and protein levels of AMPAR in PSD or extrasynaptic regions (Park et al., 2016).
CaMKII can also trap AMPAR in the postsynaptic site through other pathways such as Ras/ERK signaling. In the postsynaptic site, SynGAP (synaptic Ras/Rap GTPase-activating protein) is highly enriched and harbors phosphorylation sites for CaMKII. SynGAP contains C-terminal PDZ binding domain which interacts with PSD95 under basal conditions. During LTP induction, CaMKII can phosphorylate this protein. This phosphorylation decreases the affinity of SynGAP toward PSD95, which in turn dissociates away from the same. The massive removal of SynGAP makes more PSD95 available for binding of TARPs and thereby AMPAR trapping in the postsynaptic site (Figure 3; Gamache et al., 2020).
The synaptic SynGAP dispersion also decreases its RasGAP activity, leading to the activation of Ras/ERK signaling crucial for AMPAR delivery (Walkup et al., 2016). The phosphorylation of SynGAP by CaMKII leads to activation of Ras/ERK signaling and inactivation of Rap. The activation of Ras/ERK signaling drives AMPAR delivery to the postsynaptic site whereas Rap mediates AMPAR removal upon its activation. Thus, SynGAP phosphorylation by CaMKII can enhance AMPAR recruitment during LTP (Zhu et al., 2002; Rumbaugh et al., 2006; Wang C.C. et al., 2013; Araki et al., 2015; Walkup et al., 2015).
LTP is also accompanied by increase in spine volume mediated by activation of CaMKII. Activated CaMKII can influence the activity of Rho GTPase–regulatory proteins [e.g., RhoGEFs (guanine nucleotide exchange factors that activate Rho GTPases) and/or RhoGAPs (GTPase-activating proteins that inhibit Rho GTPases)] to promote actin polymerization in the head and neck region of dendritic spines (Herring and Nicoll, 2016). This results in an increase in size of the spine head and diameter of the neck. Increased actin polymerization also results in the reorganization of PSD proteins in such a way that more AMPARs can be incorporated. SynGAP dispersion from PSD resulting from CaMKII phosphorylation is also related to spine enlargement (Araki et al., 2015).
LTP induction is also associated with the rapid formation of a positive feedback loop, formed by a reciprocally activating kinase-effector complex (RAKEC) in dendritic spines, which consist of CaMKII and Tiam1, a Rac1-specific guanine nucleotide exchange factor (Rac-GEF). Activated CaMKII can persistently interact with Tiam1, in stimulated spines enabling the persistence and confinement of a molecular memory (Saneyoshi et al., 2019). The constitutive activation of CaMKII by occupation of its T-site would help to maintain Tiam1 phosphorylation even after Ca2+ concentration subsides. This mechanism can therefore convert transient Ca2+ signaling into a persistent activation of Rac1 (protein required for spine formation and enlargement) and its downstream actin regulators. This pathway helps in the maintenance of the enlarged spine and thereby contributes to structural LTP (Kojima et al., 2019).
NMDAR activation in pyramidal neurons causes CaMKII-dependent phosphorylation of the guanine-nucleotide exchange factor (GEF), kalirin-7 at residue Thr95, regulating its GEF activity, leading to activation of Rac1 and rapid enlargement of existing spines. Kalirin-7 also interacts with AMPA receptors and controls their synaptic expression (Xie et al., 2007).
During LTP maintenance, the levels of protein kinase M zeta (PKMζ), a constitutively active protein kinase C (PKC) isoform, are elevated. PKMζ maintains synaptic potentiation by preventing AMPAR endocytosis and promoting stabilization of dendritic spine growth. Inhibition of PKMζ, with zeta-inhibitory peptide (ZIP), can reverse LTP and impair established long-term memories (LTMs). Activated CaMKII can release the translational block on PKMζ mRNA, thereby helping in long-term maintenance of LTP (Patel and Zamani, 2021). It has been shown by direct evidence that CaMKII is essential for memory storage (Rossetti et al., 2017) by using a kinase-dead mutant (K42M) in the hippocampus where the mutant disrupted CaMKII signaling in vivo.
Putative Mechanisms of Memory Storage by Ca2+/Calmodulin-Dependent Protein Kinase Type II
While considerable insights have been obtained on the mechanisms by which LTP-inducing tetanic stimuli are converted to enhanced AMPAR activity at the postsynaptic side, the mechanisms by which the potentiated state is maintained has been intensively debated (Giese et al., 1998; Buard et al., 2010; Coultrap et al., 2012; Chang et al., 2017; Giese, 2021; Tao et al., 2021). Even long-lasting structural changes such as spine enlargement are maintained by dynamic molecular mechanisms (Gamache et al., 2020). Among the several molecular systems that were proposed to sustain altered biochemical states, the bistable switch model involving CaMKII (Lisman and Zhabotinsky, 2001) has attracted considerable attention, in which the unphosphorylated and Thr286-phosphorylated states of CaMKII represented the “OFF” and “ON” states respectively. The ability of the CaMKII oligomer to sustain its autophosphorylated state by autonomous activity has initially been proposed to convert information encoded in Ca2+-spikes into stable biochemical traces (Miller and Kennedy, 1986; Hudmon and Schulman, 2002). However, rigorous computational modeling studies showed that successful functioning of the switch requires the participation of protein phosphatase 1 (PP1) and GluN2B (Miller et al., 2005; Michalski, 2013; Urakubo et al., 2014; Lisman and Raghavachari, 2015). The switch was predicted to function in an energy-efficient manner and remain active despite protein turnover (Lisman and Zhabotinsky, 2001). In the unpotentiated synapse, the switch will be in the “OFF” state with CaMKII mostly unphosphorylated. Any phosphorylation supported by resting Ca2+ concentration will be successfully annihilated by PP1–mediated dephosphorylation thereby preventing a slow drift to the autophosphorylated “ON” state thus providing stability to the “OFF” state.
LTP-inducing stimulus causes extensive CaMKII autophosphorylation at Thr286 due to high Ca2+ levels. Autophosphorylated CaMKII that translocates to the PSD will be more than sufficient to saturate the available PP1 activity. Thus, autophosphorylated CaMKII would compete out PP1 activity and thus the high level of autophosphorylation and autonomous activity will be maintained thereby giving stability to the “ON” state. Continued phosphorylation required to negate the effect of PP1 activity while maintaining the “ON” state, leads to consumption of energy in the form of ATP. The model predicted the switch to function in an energy-efficient manner with minimal consumption of ATP and remain active despite protein turnover (Lisman and Zhabotinsky, 2001). Evidence obtained later was in accordance with these predictions on the final functional outcome of the switch, although it involved additional mechanisms than the predicted ones. Accordingly, the revised model (Lisman and Raghavachari, 2015) predicts that energy efficiency is achieved by the reduced dephosphorylation rate of the GluN2B-bound CaMKII (Cheriyan et al., 2011; Mayadevi et al., 2016). Stability against protein turnover is possible since protein turnover operates by subunit exchange between holoenzymes. Thus, replacement of a phosphorylated subunit with a new, unphosphorylated subunit will be followed by phosphorylation of the newly recruited subunit by adjacent autonomous subunits (Stratton et al., 2014; Lisman and Raghavachari, 2015).
In its “ON” state, the switch can initiate and maintain long-term strengthening of the synapse by the multiple mechanisms described above (see section entitled “LTP Induction by the Activation of NMDARs-Role of CaMKII in NMDAR-Dependent LTP”). But later studies indicated that the autophosphorylation of CaMKIIα was required only for rapid learning especially induced by a single stimulus but was not essential for memory formed by multiple trial learning (Irvine et al., 2005, 2011). This was further supported by the evidence that autophosphorylation at Thr286 lowers the stimulation frequency required to induce synaptic plasticity and permits CaMKII to better integrate Ca2+ signals at physiologically relevant frequencies that would happen only in LTP induction and not in maintenance (Chang et al., 2017). These findings are not consistent with the bistable switch model in which Thr286 autophosphorylation is an essential element. These studies suggest that Thr286 autophosphorylation might have a major role in the initial capture of information encoded in the synaptic Ca2+ spikes with more efficiency. However, inhibition of CaMKII activity can erase LTP showing the involvement of CaMKII in LTP maintenance, further suggesting that CaMKII acts as a molecular storage device (Tao et al., 2021).
CaMKII activity necessary for LTP maintenance at resting Ca2+ concentrations could be arising from the autonomous forms of CaMKII, Thr286-phosphorylated or GluN2B-bound. If Thr286 is dispensable (Irvine et al., 2005, 2011; Chang et al., 2017) as mentioned above, the GluN2B-bound form of CaMKII could provide the autonomous activity. However, in the PSD, all the CaMKII subunits in a holoenzyme need not be bound by GluN2B unlike the in vitro experiments (Bayer et al., 1999) in which all CaMKII subunits could be bound by GluN2B. Whether the autonomous activity of the GluN2B-bound subunits of CaMKII in the PSD would be sufficient to maintain LTP needs further investigation, since GluN2B-binding does not spread to other subunits of a holoenzyme of CaMKII like Thr286 autophosphorylation.
Regulation of Translation of Ca2+/Calmodulin-Dependent Protein Kinase Type II in Synaptic Plasticity
Gene expression needed for long-lasting synaptic plasticity is tightly regulated. In particular, protein synthesis, regulation of mRNA transport and mRNA stability contribute to the control of gene expression. mRNA translation happens in synaptic locations - dendrites and dendritic spines, which are filled with polyribosomes, translation factors, and mRNAs (Steward and Levy, 1982; Crino and Eberwine, 1996; Job and Eberwine, 2001; Steward and Schuman, 2001).
Regulation of Ca2+/Calmodulin-Dependent Protein Kinase Type II by Cytoplasmic Polyadenylation Element-Binding Protein in Long Term Potentiation
Cytoplasmic polyadenylation element (CPE) present in the 3′ untranslated region (UTR) of mRNAs plays a major role in the regulation of translation in response to cellular signals (Klann and Dever, 2004). CPE sequence present in CaMKIIα mRNA helps in its rapid translation during LTP (Ouyang et al., 1997; Giovannini et al., 2001).
The neuronal CPE-binding protein (CPEB) protein from Aplysia has an amino-terminal extension, which can be converted into a prion-like molecule and this mechanism will aid in sustained protein synthesis. Thus, this process would play crucial roles during synaptic plasticity (Si et al., 2003). CPEB blocks translation when it is bound to CPE. Upon phosphorylation, CPEB can dissociate from CPE thereby triggering a series of molecular events leading to initiation of translation. CPEB can be phosphorylated by CaMKII (Wu et al., 1998). CPE-mediated translation following membrane depolarization is also CaMKII-dependent (Lisman et al., 2002). The 3′UTR of CaMKII and other specific mRNAs bind CPEB and polyadenylation specificity factor (CPSF) leading to translational arrest. With NMDAR activation, aurora kinase and CaMKII get activated leading to phosphorylation of CPEB. This is followed by CPEB-CPSF interaction which allows poly(A) polymerase (PAP) recruitment to this complex. PAP initiates the poly(A) tail elongation. This in turn activates poly(A)-binding protein (PABP) which binds to poly(A) tail and initiates interaction with elongation factor eIF4G and thereby activates translation.
Hence, CaMKII activation after LTP activates the CPE-dependent translation which in turn translates CaMKIIα mRNA. This feedforward mechanism is very important for maintaining sustained protein synthesis in LTP and memory (Klann and Dever, 2004).
Regulation of Ca2+/Calmodulin-Dependent Protein Kinase Type II by Elongation Factors in Long Term Potentiation
Translation can be regulated even at the elongation level via phosphorylation of the eukaryotic elongation factor 2 (eEF2), which is a GTP binding protein (Moldave, 1985). eEF2 kinase is regulated by mammalian target of rapamycin (mTOR) activation, which phosphorylates the eEF2 kinase near the CaM binding site, resulting in decreased kinase activity (Browne and Proud, 2004).
In dendrites of cultured cortical neurons (Marin et al., 1997) and tadpole tecta (Scheetz et al., 1997), NMDAR activation leads to phosphorylation of the eEF2 factor thus leading to elongation becoming a rate-limiting step in translation. This is correlated with increased CaMKIIα synthesis but decrease in overall protein synthesis (Scheetz et al., 2000). Similarly, chemically-induced LTP also leads to increased eEF2 phosphorylation with decreased protein synthesis, but with increase in Arc and Fos protein levels (Chotiner et al., 2003). So, phosphorylation of eEF2 leads to overall decrease in protein synthesis but with exceptions of increased translation like that of CaMKIIα (Scheetz et al., 2000).
Regulation of Neuromodulator Release by Ca2+/Calmodulin-Dependent Protein Kinase Type II
The neurotrophins (NTs) are involved as major players in synaptic development and synaptic plasticity (Poo, 2001). Among the NTs – Neuregulin (NRG), BDNF, NT-3 and NT-4, extensive research has been done on BDNF and its role in synaptic plasticity. Postsynaptic NMDAR gating is regulated by BDNF signaling (Levine et al., 1995, 1998). BDNF is important in LTP, as seen by lack of proper establishment of LTP in BDNF knockout (KO) mouse models (Korte et al., 1995; Patterson et al., 1996). BDNF supports high-frequency transmitter release, which is required for LTP induction (Figurov et al., 1996; Gottschalk et al., 1998; Pozzo-Miller et al., 1999; Abidin et al., 2006).
Moro et al. (2020) reported reduced BDNF secretion in mouse deficient in α and β CaMKII [αβCaMKII double-knockout (DKO)] hippocampal neurons. These neurons had drastically reduced levels of BDNF and fewer BDNF containing dense core vesicles (DCV) targeted to the axon, leading to fewer DCVs per synapse and thus reduced BDNF secretion upon stimulation. CaMKIIβ is crucial for increasing the amount of secreted BDNF by CaMKIV and phospho-cAMP-response element binding protein (CREB) pathway. Interestingly, active CaMKIIβ and not CaMKIIα or inactive CaMKIIβ/CaMKIIα could restore the reduced levels of BDNF expression (Moro et al., 2020). BDNF binds to TrkB and this activates CaMKIIβ further leading to a series of downstream signaling events. Subsequently, Ca2+/CaM enters into the nucleus and CaMKIV gets activated, phosphorylating CREB at Ser133 position, along with nuclear-localized neurogranin. Phosphorylated CREB promotes BDNF transcription (Wheeler et al., 2008; Ma et al., 2014; Wang et al., 2017). Thus, BDNF-mediated activation of CaMKIIβ acts as a positive feedback loop to initiate the expression of the neuromodulator (Moro et al., 2020).
Ca2+/Calmodulin-Dependent Protein Kinase Type II in Axonal/Dendritic Growth Regulation Promoting Synaptic Strength
Role of Ca2+/Calmodulin-Dependent Protein Kinase Type II α
Alterations in synaptic strength are brought about majorly through post-translational modifications such as phosphorylation or dephosphorylation of synapse associated proteins (Davis and Squire, 1984; Yan-You Huang et al., 1996). Miller et al. showed that mutating the targeting signal at the 3′UTR of CaMKIIα mRNA caused significant reduction in the level of CaMKIIα in PSDs and impairments in L-LTP and LTM. The 3′UTR mutants in BL6 mice showed poor behavioral performances in fear conditioning, water maze and object recognition indicating cognitive alterations (Miller et al., 2002).
Wu et al. (1998) and Wells et al. (2001) demonstrated that dendritic CaMKIIα is inducible by showing an increase in CaMKIIα in synaptosomes prepared from the visual cortex of dark-reared rat pups that were transferred to light. Tetanic stimulation was found to increase CaMKIIα levels in stratum radiatum of CA1 (Ouyang et al., 1999), which suggests that CaMKIIα present in PSDs, might arise from the activity-dependent translation of dendritic mRNAs. Assembly of CaMKII holoenzymes occur after the translation of the subunits. The β subunit facilitates the association of the holoenzyme with actin cytoskeleton and thereby localization to the synapses (Shen et al., 1998). Since the mRNA of β subunit is located only in the soma (Burgin et al., 1990), some of the CaMKIIα might be transported into dendrites as pre-assembled holoenzyme (Miller et al., 2002).
Miller et al. (2002) also showed that disrupting the dendritic localization of CaMKIIα mRNA disrupted LTM but not short-term memory (STM) formation. Hence, dendritic CaMKIIα might be a requirement for memory consolidation. Local CaMKIIα synthesis might facilitate transmission by regulating AMPAR phosphorylation (Barria et al., 1997b) or by inserting additional AMPARs into the synapse (Hayashi et al., 2000). CaMKIIα has also been reported to be stabilizing the dendritic arbors and thus regulating synapse shape and density (Wu and Cline, 1998; Koh et al., 1999; Rongo and Kaplan, 1999). Filopodia-like extensions and movements in the dendritic arbors play an important role for neurons in order to determine new contact sites, which can then evolve into nascent synapses and mature into functional synaptic connections (Vaughn, 1989; Jontes and Smith, 2000; Wong and Wong, 2000; Ahmari and Smith, 2002). For all these mechanisms, continued supply of CaMKIIα is mandatory and this might be supported via the dendritic translation of CaMKIIα.
Role of Ca2+/Calmodulin-Dependent Protein Kinase Type II β
Motility and plasticity of axonal and dendritic arbors, leading to alterations in synaptic contacts (Fischer et al., 1998; Zou and Cline, 1999; Jontes et al., 2000; Colicos et al., 2001), play significant roles in developing and mature neurons. Shen et al. (1998) showed localization of CaMKIIβ to the actin cytoskeleton, thus demonstrating its role in actin-related morphology modifications. CaMKIIβ overexpression increased the number of synapses whereas inhibiting CaMKIIβ caused significant reduction in motility of filopodia as well as in small dendritic branches with long-term decrease in the degree of dendritic arborization (Fink et al., 2003). In developing hippocampal neurons, CaMKIIβ promotes arborization of the dendritic tree whereas in mature neurons, it has a strong morphogenic effect, leading to dendritic remodeling rather than overall arborization. CaMKIIβ, and not CaMKIIα is expressed in early development when the neurons build the dendritic arbor (Bayer et al., 1999). Even in the hippocampal region where CaMKIIα expression is exceedingly high, CaMKIIβ dominates during the first postnatal week, thus implying its direct role in morphogenic activity. A small insert in CaMKIIβ is responsible for its F-actin localization and for selective upregulation of dendritic motility. Wang Q. et al. reported that CaMKIIβ that has a longer linker of 93 amino acids (aa) binds more strongly and efficiently to F-actin than does CaMKIIα which has only a 30 aa linker (Wang Q. et al., 2019). They show that peptides derived from the regulatory, linker and association domains of CaMKIIβ can bind F-actin. Based on simulations, they calculated that about 20% of free energy of binding is contributed by the regulatory domain. The remaining energy is derived from the linker and association domains with nearly equal contribution. The linker domain is flexible (Myers et al., 2017) and contributes to the thermodynamics of binding unlike the association domain which has higher rigidity and thus helps in maintaining strict geometry between CaMKIIβ and the bound actin filaments. Thus, the formation of the CaMKII/F-actin complex requires the linker, regulatory and association domains of CaMKIIβ (Wang Q. et al., 2019).
When a short sequence of the variable region of CaMKIIβ was inserted in CaMKIIα, a partial colocalization and partial effect on the dendritic morphology was observed. Thus, neurons high in β isoform would have higher degree of arborization with larger numbers of synapses, an example being the cerebellar neurons having persistently high CaMKIIβ levels than in neurons in the forebrain (Miller and Kennedy, 1985). This is reflected in the highly branched morphology of cerebellar neurons when compared to neurons present in the forebrain.
Another important question is how CaMKIIβ is activated. One report suggested that actin and Ca2+/CaM involve in competitive binding to CaMKIIβ (Shen and Meyer, 1999). Fink et al. (2003) reported the involvement of Ca2+/CaM binding to CaMKII for dendritic mobility. Ca2+/CaM levels present in the unstimulated neurons were sufficient to induce CaMKIIβ-dependent dendritic extension/motility. Hence, Ca2+/CaM stimulus provided by basal neuronal activity in cultures is sufficient for the morphogenic function of CaMKIIβ. Since autophosphorylation at Thr287, which requires Ca2+/CaM binding, was possible at basal conditions (25% of CaMKII phosphorylation) (Molloy and Kennedy, 1991), sufficient Ca2+/CaM should be present during basal neuronal activity leading to partial CaMKIIβ activation. In contrast, CaMKIIα requires stronger stimulation to activate AMPA receptors. Thus, differential expression of the two CaMKII isoforms leads to either strengthening of the synapse if CaMKIIα function dominates or filopodia extension with synapse formation if CaMKIIβ dominates.
The mRNA of CaMKIIα, and not β is present in the dendrites and hence the protein translated in dendrites would have CaMKIIα homomers which would not be actin localized. The mixed population of both the isoforms, translated in the cell body would create α/β hetero-oligomers that might bind to actin and regulate filopodia extension and synapse formation (Mori et al., 2000; Aakalu et al., 2001).
Protein kinase C-mediated phosphorylation of CaMKIIβ is required for maintenance of spine morphology. PKC phosphorylates CaMKIIβ at Ser315 during group I metabotropic glutamate receptor (mGluR1) signaling which results in CaMKIIβ/F-actin complex dissociation thereby repressing formation and elongation of spines in mature Purkinje cells (Sugawara et al., 2017).
Puram et al. (2011) found a centrosomal targeting sequence (CTS) within the variable region of CaMKIIβ. The CTS mediates the required CaMKII - pericentriolar material 1 (PCM1, a centrosomal targeting protein) interaction which is required for CaMKII localization to the centrosome. In the centrosome, CaMKIIβ phosphorylates the E3 ubiquitin ligase Cdc20-APC (cell division cycle 20–anaphase promoting complex) at Ser51, thereby inducing Cdc20 dispersion from the centrosome and thus inhibiting centrosomal Cdc20-APC activity. This triggers the switch to retraction mode from growth of the dendrites. This CaMKIIβ function at the centrosome is independent of CaMKIIα.
Ca2+/Calmodulin-Dependent Protein Kinase Type II Phosphorylation States in Spine Size and Regulation
Spine size and synaptic strength were shown to covary in experiments involving photolysis of caged glutamate, which is present in individual spines (Matsuzaki et al., 2004; Zhang et al., 2008). The spines present on dendrites can vary in size (Lisman and Harris, 1993), which might correlate with postsynaptic strength of the synapse at that particular spine (Matsuzaki et al., 2001; Asrican et al., 2007). It is known that by overexpressing autonomous (T286D)-CaMKIIα in CA1 hippocampal cells, there is enhancement in the synaptic strength with Thr305/Thr306 sites not being phosphorylated. But there is a decrease in synaptic strength when Thr305/Thr306 sites are phosphorylated (Lisman et al., 2012). Interestingly, Pi et al. (2010) showed that CaMKII and its various phosphorylation states can regulate spine size. They found that all autonomous forms of CaMKII can increase spine size. In other words, CaMKII leads to spine enlargement irrespective of Thr305/Thr306 phosphorylation. Also, the T286D/T305D/T306D form can increase spine size but at the same time decrease synaptic strength. Thus, the mechanisms through which CaMKII regulates spine structure and synaptic strength have different levels of dependence on the phosphorylation state of the enzyme. A T286D form with an additional mutation, K42R, that inhibits enzymatic activity, could actually enhance spine size, with no effect on synaptic strength, thus showing the importance of the structural (non-enzymatic) role of CaMKIIα in this postsynaptic process. Thus, the overall process might involve two steps in which initial enzymatic activity is required for initiating autophosphorylation at Thr286 followed by spine enlargement that does not require enzymatic activity. This explains why the kinase-dead T286D mutant (K42R/T286D) can support spine enlargement but not the T286A mutant (Pi et al., 2010).
Role of Presynaptic Ca2+/Calmodulin-Dependent Protein Kinase Type II in Axon Terminal Growth
Extensive structural remodeling on the presynaptic and postsynaptic sides of the synapse is important for synaptogenesis. The axon growth cone is very dynamic as it responds to its surrounding signals ultimately growing toward the target region forming the synapse (Nesler et al., 2016). Alterations in axon terminals occur very fast and also at distant sites from the cell body. To enable these changes, the local machinery should be active and working in the growth cone and presynaptic boutons.
Ca2+ is an important secondary messenger in axon growth and guidance (Sutherland et al., 2014). Increased intracellular Ca2+ levels can activate even enzymes such as protein kinase A (PKA) through S100A1, a Ca2+-binding protein (Melville et al., 2017). Ca2+ influx results in activating Ca2+/CaM-dependent enzymes like calcineurin (CaN) and CaMKII (Faas et al., 2011). Activation of CaMKII and PKA promotes attraction of the growth cone toward external cues and dual inhibition of both the enzymes leads to repulsion (Wen et al., 2004). Synapsin is an important target for phosphorylation by CaMKII in the presynaptic nerve terminals. The association of synapsin with synaptic vesicles is reversible and it facilitates vesicle clustering and presynaptic plasticity. This mechanism is regulated by phosphorylation at specific sites by CaMKII and PKA (Stefani et al., 1997; Hosaka et al., 1999). Synapsin gets redistributed to sites of activity-dependent axon terminal growth and thus regulates outgrowth via a PKA-dependent pathway (Vasin et al., 2014).
CaMKII expression is post-transcriptionally regulated at the level of translation by the microRNA (miRNA) containing RNA-induced silencing complex (RISC) (Ashraf et al., 2006). Nesler et al. (2013) observed that growth of new synaptic boutons in response to spaced depolarization requires the function of activity-regulated neuronal miRNAs including miR-8, miR-289 and miR-958 in Drosophila larval ventral ganglia. This suggests that mRNAs encoding synaptic proteins might be regulated by these miRNAs. The fly CaMKII 3′UTR has two putative binding sites for activity-regulated miR-289 (Ashraf et al., 2006). It is also reported that miR-148a/b can target CaMKIIα through bioinformatics analysis and luciferase assay (Liu et al., 2010). In animal models of schizophrenia wherein the levels of miR-148b were significantly upregulated, increased levels of CaMKIIα transcript did not lead to a concomitant increase in protein levels (Gunasekaran et al., 2022), implying miR-148b involvement in regulation of CaMKIIα in vivo. Knockdown of CaMKII in the presynaptic compartment using transgenic RNAi, disrupted activity-dependent presynaptic growth as it prevented the formation of new ghost boutons in response to spaced stimulus. Abundant levels of phosphorylated CaMKII were found at the presynaptic axon terminal. Spaced stimulation leads to accumulation of a significant amount of total CaMKII protein in the axon terminals. This increase was blocked by treatment with either the translational inhibitor cycloheximide or presynaptic overexpression of miR-289 suggesting a translation-dependent mechanism. Similarly, presynaptic CaMKII has been implicated in controlling both bouton number and morphology during development of the larval neuromuscular junction (NMJ) (Nesler et al., 2016). Presynaptic CaMKII has also been shown to be involved in axon pathfinding in cultured neurons of Xenopus (Wen et al., 2004).
Activation in Response to Voltage Gated Calcium Channels
Voltage gated calcium channels (VGCCs) are present throughout the neuronal membrane and are a major source of Ca2+. especially in dendritic spines after a depolarization of the membrane. Different subtypes of VGCCs are known with distinct functions; mainly involved in Ca2+ influx into the cell as well as in regulating gene transcription. Activation of dendritic VGCCs can generate LTP, STP (short-term potentiation) or LTD. Perhaps because of the distinct subcellular localization of VGCCs, LTP induced due to their activation may use mechanisms distinct from NMDAR-dependent LTP (Malenka and Nicoll, 1999). With aging, LTP induction through NMDAR becomes lesser compared to VGCC-dependent LTP, as shown by the limited sensitivity of LTP generated in slices from older rats to NMDAR antagonists and increased sensitivity to antagonists of L-type VGCC (Izumi and Zorumski, 1998). Studies have also shown that repetitive activation of VGCCs is involved in LTD (Pöschel and Manahan-Vaughan, 2007) in a Ca2+-dependent manner. Among the various categories of VGCCs, L-type VGCCs are mainly involved in synaptic plasticity mechanisms.
In the CA1 area of hippocampus, an LTP component has been found that is dependent only on the activation of VGCCs without NMDAR (Grover and Teyler, 1990; Alkadhi, 2021) which was later termed as VDCC LTP. Ca2+ entry through VGCCs mediates LTP at thalamic input synapses to the lateral nucleus of amygdala, which may be mechanistically different from the NMDAR-dependent form of plasticity found in the hippocampus but is still dependent on activated CaMKII (Weisskopf et al., 1999). The conditional hippocampus/neocortex Cav1.2 (L-type VGCCs) KO mouse demonstrates an essential role of Cav1.2 in CREB signaling during LTP and spatial learning (Moosmang et al., 2005). In the cortical neurons, activation of T-type VGCCs enhanced LTP and CaMKII autophosphorylation (Moriguchi et al., 2012a). Even in the NMDAR-dependent mechanisms of LTP and LTD (Di Biase et al., 2008), Cav channels are involved (Zhao et al., 2021) by enhancing Ca2+ influx into the synaptic site and through CREB mediated events.
Upon aging, the expression of NMDAR diminishes and its subunit composition also changes (Zhao et al., 2009), whereas VGCCs, especially the L-type channels, increase in expression (Thibault and Landfield, 1996; Wang and Mattson, 2014) and can majorly involve in LTP or LTD mechanisms. Activation of L-type VGCCs, especially Cav1.2 localized in the postsynaptic membrane (Patriarchi et al., 2018) leads to Ca2+ influx into the spine, which can activate CaMKII. Even if the expression levels of GluN2B are lower, CaMKII can still tether to the postsynaptic site by binding with the C-terminus of Cav1.2 (Hudmon et al., 2005). This binding, however, does not lead to constitutively active CaMKII and hence, cannot support molecular memory. The enzyme tethered at the membrane can easily get activated with the trains of depolarization stimulus and can facilitate further Ca2+ influx through these channels (Ca2+-dependent facilitation).
Role of Ca2+/Calmodulin-Dependent Protein Kinase Type II in Long Term Depression
LTD is an activity-dependent reduction in the efficacy of neuronal synapses (Malenka and Nicoll, 1999) and is thought to be involved in learning and memory. It brings about a long-lasting decrease in synaptic strength or a reversal of LTP mechanisms. LTD is triggered by synaptic activation of either NMDARs or metabotropic glutamate receptors (mGluRs). A low frequency stimulation (LFS) of NMDARs (700–900 pulses at 1 Hz) can activate LTD mechanisms (Figure 4). If the Ca2+ influx is low in intensity (if the activation is only for a postsynaptic compartment), it will majorly activate phosphatases and result in LTD (Baltaci et al., 2019). Initially it was thought that protein kinases are required for LTP and phosphatases are involved in LTD. But recent findings suggest that kinases are involved in LTD mechanisms also. It has been noted that the bath application of CaMKII inhibitor KN-62 could block LTD during low-frequency SC collateral stimulation (1 Hz/15 min) (Stanton and Gage, 1996). Experiments with CaMKIIα KO mice also pointed to the role of CaMKII in LTD (Stevens et al., 1994). Even though these initial experiments indicated the role of CaMKII in LTD, the exact mechanism by which CaMKII participates in the process is unknown. In contrast to the previously accepted dogma, it has also been shown by using T286A mutant mouse that Thr286 autophosphorylation is a requisite for LTD (Coultrap et al., 2014). The most recent studies on CaMKII autophosphorylation indicates that the autophosphorylation at Thr305/306 is selectively induced by LTD stimuli and the mutation of these residues impairs LFS-induced LTD but not HFS-induced LTP (Cook et al., 2021). Both the autophosphorylations are necessary for LTD but the exact role of Thr286 with respect to Thr305/306 in LTD remains controversial. The death-associated protein kinase 1 (DAPK1) can regulate CaMKII-GluN2B interaction to facilitate LTD. DAPK1 is a CaM kinase family member and is enriched in excitatory synapses. They can bind to GluN2B at a site overlapping the CaMKII binding site. The enzyme gets activated by CaN, a Ca2+-activated protein phosphatase. LTD-stimuli can activate DAPK1 in hippocampal slices in a CaN-dependent manner. Inhibition of DAPK1 or CaN allowed the accumulation of CaMKII at excitatory synapses after LTD-stimuli (Goodell et al., 2017). This indicates that during LTD, DAPKI activated by phosphatases will compete for GluN2B binding and would reduce the binding of activated CaMKII generated by the low frequency stimuli.
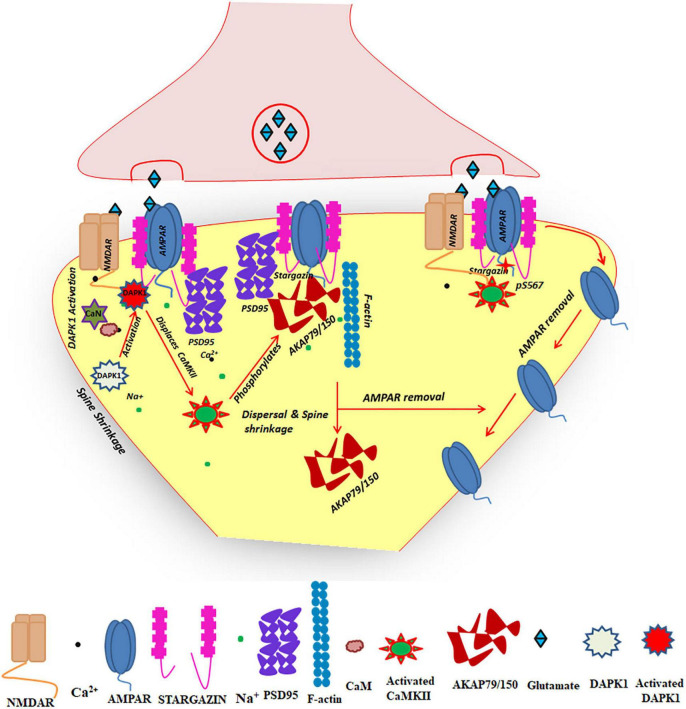
FIGURE 4.
The schematic representation showing the role of CaMKII in LTD. The low tetanic stimulation leading to LTD activates more phosphatases than kinases. Calcineurin thus activated can activate DAPK1 and it can translocate to GluN2B where CaMKII binds. The activation of DAPK1 can even displace activated CaMKII generated under minimal Ca2+ stimulus from its binding with GluN2B. The role of CaMKII in LTD involves inhibition of AMPAfication and facilitation of spine shrinkage. Phosphorylation of GluA1 of AMPAR at Ser567 obstructs AMPAfication of synapses; CaMKII mediated phosphorylation and depalmitoylation of AKAP79/150 results in its synaptic elimination. Since AKAP79/150 is a major adapter for many proteins required for LTP, its elimination due to dissociation from F-actin can result in AMPAR endocytosis and spine shrinkage.
CaMKII can phosphorylate Ser567 residue of GluA1 subunit of AMPAR, a unique phosphorylation site for CaMKII in the C-terminal loop of GluA1. The C-terminal tail of GluA1 is involved in AMPAR trafficking from extra-synaptic pool to the synapses. Phosphorylation of GluA1 at Ser567 by CaMKII inhibits AMPAR trafficking to the synapses (Lu et al., 2010). It has been noted that LTD-inducing stimulation of hippocampal slices produced a robust phosphorylation of Ser567 whereas LTP-inducing stimulus could yield only Ser831 phosphorylation. The differential phosphorylation of GluA1 by CaMKII under the two synaptic plasticity conditions underlies the role of CaMKII in LTD (Coultrap et al., 2014).
In contrast to spine enlargement in LTP, LTD is associated with spine shrinkage aided by the removal of the AMPA receptor regulatory scaffold protein, A-kinase anchoring protein (AKAP) 79/150. The synaptic removal of AKAP79/150 is brought about by the phosphorylation of the substrate sites within the AKAP79/150 N-terminal polybasic membrane-cytoskeletal targeting domain (residues 1–153) by CaMKII. Phosphorylation by CaMKII inhibits AKAP79/150 association with F-actin, thus facilitating AKAP79/150 removal from spines (Figure 4). In addition to the direct phosphorylation of AKAP79/150, CaMKII is also responsible for its depalmitoylation on two Cys residues within the N-terminal targeting domain. Depalmitoylation also promotes synaptic elimination of AKAP79/150. Since the protein harbors PKA and protein phosphatase 2B (PP2B) at the PSD, it can regulate both synaptic insertion and elimination of AMPARs. Under LTP stimulation, PKA can phosphorylate Ser845 of GluA1 of AMPAR and thereby more AMPAR trafficking to the synapse occurs, whereas in LTD conditions due to the elimination of AKAP79/150 along with activation of phosphatases, AMPAR dephosphorylation at Ser845 and its endocytosis is promoted which eventually leads to spine shrinkage (Woolfrey et al., 2018).
The stimulation pattern-dependent activation of NMDAR that yields either LTP or LTD, causes activation of CaMKII in either case. With the differing stimuli the enzyme targets different substrates and thereby activates specific signaling mechanisms to yield either form of synaptic plasticity.
Ca2+/Calmodulin-Dependent Protein Kinase Type II in Signaling Complexes in Glutamatergic Synapses
CaMKII plays an important role in several physiological pathways including synaptic plasticity and hence its localization in the cytosol and PSD are crucial determinants of its function. Immunoelectron microscopy studies show that CaMKIIα is significantly higher in dendritic shafts when compared to dendritic spines. When it gets any proper stimulus, it will abundantly translocate to the spines (Shen and Meyer, 1999; Shen et al., 2000; Ding et al., 2013). In the basal condition, more CaMKII will be available in the dendritic shaft than in spines. Whenever activation happens the activated CaMKII can translocate to the spines.
Translocated CaMKII can bind with various protein ligands in the PSD as indicated in Table 1. One such protein is densin-180, which is a core protein in the PSD that does not span the membrane. Though densin-180 is the only documented interaction partner for the association domain of CaMKII, it will not bind with CaMKII holoenzymes which contain β isoform (Penny and Gold, 2018). The PDZ domain of densin-180 contributes to its binding to α-actinin. A distinct domain of α-actinin interacts with the kinase domains of both α and β subunits of CaMKII. Thus, these three proteins can form a ternary complex in the PSD stabilized by multiple interactions (Walikonis et al., 2001). This ternary complex within the PSD is an additional mode of localization of CaMKII to PSD apart from its binding to GluN2B.
SAP97, a member of membrane-associated guanylate kinase protein family, has been implicated in the processes of targeting ionotropic glutamate receptors such as NMDARs and AMPARs at postsynaptic sites and is enriched in PSD. SAP97 shares its interaction with AKAP79/150 in addition to the C-terminal region of GluA1. AKAP79/150 in turn harbors PKC, PKA and PP2B. This molecular arrangement inside the PSD works in accordance with the stimuli received. The most important function of this complex is the regulation of AMPARs in synapses including both potentiation and trafficking. CaMKIIα displays a high degree of co-localization with SAP97. CaMKII phosphorylation of Ser39 in the N-terminus of SAP97 modulates trafficking of SAP97 (Mauceri et al., 2004) and the associated proteins; in contrast, CaMKII phosphorylation of Ser232 in the first PDZ domain of SAP97 may modulate binding of other proteins, such as NMDAR and AMPAR subunits (Nikandrova et al., 2010), especially GluA1 of AMPAR. SAP97 is in close association with AKAP 79/150, but the phosphorylation of SAP97 at Ser39 by CaMKII disengages AKAP79/150 from regulating GluA1-AMPARs.
Another complex associated with CaMKII in the PSD is the complex formed by SynGAP, MUPP1 and CaMKII. SynGAP and CaMKII are brought together by direct physical interaction with the PDZ domains of MUPP1, a multi-PDZ domain-containing protein (Krapivinsky et al., 2004). In this complex, SynGAP is phosphorylated by CaMKII which enhances its Ras GTPase activity which in turn promotes AMPAR trafficking as shown in Figure 3.
CaMKII has an important role in dendritic spine remodeling upon synaptic stimulation. Electron micrographic studies showed that at physiological molar ratios, single CaMKII holoenzymes cross-linked multiple F-actin filaments at random, whereas at higher CaMKII/F-actin ratios, filaments bundled. From this bundled state CaMKII is released upon Ca2+/CaM activation, triggering network disassembly and expansion leading to spine enlargement. Upon subsequent disappearance of Ca2+, compaction will occur (Khan et al., 2019).
Role of Ca2+/Calmodulin-Dependent Protein Kinase Type II in Calcium Overload-Induced Excitotoxicity
Excitotoxicity is a pathological condition triggered by excessive stimulation of receptors by excitatory neurotransmitters, primarily glutamate, causing Ca2+ overload in the cytosol and thereby resulting in neuronal dysfunction and cell death. Increased Ca2+ influx and high intracellular Ca2+ ([Ca2+]i) rise trigger gene expression (Ortuño-Sahagún et al., 2012) and long-lasting activation of CaMKIIα in hippocampal neurons (Otmakhov et al., 2015). Autophosphorylation of CaMKII at Thr253, Thr286 (Vest et al., 2010; Otmakhov et al., 2015; Rostas et al., 2017) and simultaneous S-nitrosylation at Cys280/Cys289 by nitric oxide (NO) (Coultrap and Bayer, 2014) generates autonomous activity of the kinase during excitotoxic cell death. Activated CaMKII redistributes to the spines (Otmakhov et al., 2015), promotes its interaction with synaptic GluN2B (Wang N. et al., 2014; Buonarati et al., 2020) and mediates the NMDA-induced caspase-3-dependent cell death pathway (Goebel, 2009). During a glutamate-induced excitotoxic event, CaMKII can also modulate the activity of neuronal nitric oxide synthase (nNOS) (Araki et al., 2020), can cause axonal degeneration via necroptosis (Hou et al., 2009; Arrazola and Court, 2019) and also contribute to the regulated necrosis (RN) pathway (Wang S. et al., 2019).
Contrastingly both overexpression (Vest et al., 2010) and sustained CaMKII inhibition during excitotoxicity can exacerbate cell death of cultured neurons (Ashpole and Hudmon, 2011; Ashpole et al., 2012). Loss of CaMKII activity in astrocytes results in dysregulated Ca2+ homeostasis and reduced glutamate uptake (Ashpole et al., 2013) by excitatory amino acid transporter 1 (EAAT1) (Chawla et al., 2017). On the whole, dysregulated CaMKII function upon excitotoxic insult shifts the tight homeostatic balance maintained between kinases and phosphatases in the cell, resulting in dysfunction of excitatory synaptic transmission (Farinelli et al., 2012). The following section reviews the role of CaMKII at glutamatergic synapses in a few diseases in which excitotoxicity is one of the causes.
Alzheimer’s Disease
Alzheimer’s disease (AD) is a progressive neurodegenerative condition characterized by loss of memory and cognitive function. The presence of amyloid β (Aβ) plaques and neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein, is the distinctive feature in AD neuropathology. CaMKII catalyzes the hyperphosphorylation of tau protein at multiple Ser/Thr sites in the AD brain (Yoshimura et al., 2003). Loss of synapses and cognitive decline associated with AD positively correlate to the accumulation of soluble Aβ (Lue et al., 1999; Näslund et al., 2000; Almeida et al., 2005), which leads to reduced CaMKII activation (Zeng et al., 2010; Ly and Song, 2011; Ghosh and Giese, 2015) and inhibition of LTP-induced CaMKII trafficking to excitatory synapses (Cook et al., 2019). A significant reduction in the density and number of synapses (Terry et al., 1991; Scheff and Price, 1993, 1998; Scheff et al., 2006) and altered expression of synaptic proteins (Masliah et al., 2001; Almeida et al., 2005) contributes to synaptic dysfunction and cognitive decline in the AD brain.
In amyloid precursor protein (APP) transgenic mice, Aβ-induced change in CaMKII subcellular distribution aids in the removal of AMPARs from the synaptic membrane (Gu et al., 2009). Opazo et al. (2018) showed that oligomeric forms of Aβ peptide engage in synaptic metaplasticity via aberrant activation of CaMKII, mediated through GluN2B-containing NMDARs, which leads to LTP deficits and destabilization of AMPARs in the early stages of AD.
Epilepsy
Epilepsy is a neurological disorder characterized by recurrent seizures, caused by abnormal brain activity. A strong epileptic stimulus can induce alterations in the composition of PSD proteins (Wyneken et al., 2001) and loss of CA3 cells in a kainic acid (KA)-induced seizure model, wherein hippocampal injury correlates with increased CaMKII activity (Lee et al., 2001). Activation of CaMKIIα is concomitant with a reduction in density of hippocampal dendritic spines and spine PSDs during epileptiform activity (Zha et al., 2009). Also, CaMKII activation via L-type VGCCs and NMDARs are essential for the development and maintenance of an in vitro kindling-like state and EPSP-spike potentiation in CA1 pyramidal cells (Semyanov and Godukhin, 2001).
However, a few studies have reported an NMDAR-dependent reduction in CaMKII activity with increased neuronal excitability (Kochan et al., 1999; Churn et al., 2000). Regulation of CaMKII activity during seizures either by the reversible formation of inactivated CaMKII (Yamagata and Obata, 2004; Yamagata et al., 2006) or by modulating different CaMKII isoforms (Murray et al., 2003; Savina et al., 2013), can prevent excessive CaMKII activation due to Ca2+ overload (Yamagata et al., 2006). Recently, Vieira et al. (2020) functionally characterized the epilepsy-associated de novo variant of GluN2A, S1459G. This mutation disrupts CaMKIIα phosphorylation of GluN2A resulting in defects in NMDAR trafficking and reduced synaptic function (Vieira et al., 2020).
Huntington’s Disease
Huntington’s disease (HD) is an autosomal, dominantly inherited disorder caused by the expansion of a polyglutamine repeat in the N-terminus of the huntingtin (htt) protein. Progressive and selective degeneration of the striatal medium spiny neurons (MSNs) in HD results in abnormalities of movement, cognition, personality and mood. Being an abundant protein in striatal MSNs (Erondu and Kennedy, 1985), reduced levels of both CaMKII and CaMKII-Thr286 phosphorylation have been reported in various mouse models of HD (Deckel et al., 2001, 2002a,b; Brito et al., 2014; Blum et al., 2015; Gratuze et al., 2015). Altered expression levels of CaMKII in the hippocampus can disrupt GluA1-Ser831 phosphorylation (Brito et al., 2014) and disturb AMPAR surface diffusion (Zhang et al., 2018). CaMKII inhibition in striatal MSNs causes a reduction in functional glutamatergic synapses and an enhancement in intrinsic excitability (Klug et al., 2012). Although the role of altered CaMKII function in HD is not extensively studied, it is evident that it could contribute to cognitive dysfunction observed in HD (Giralt et al., 2012; Zhang et al., 2018).
Parkinson’s Disease (PD)
Parkinson’s disease (PD) is a progressive neurodegenerative movement disorder caused by degeneration of dopaminergic neurons in the substantia nigra, that project to the striatum. At the molecular level, dopamine (DA) can modulate or gate the cortical glutamatergic inputs onto striatal MSNs (Freund et al., 1984; Gardoni and Bellone, 2015). Striatal DA depletion causes selective loss of dendritic spines and glutamatergic synapses on striatopallidal MSNs (Day et al., 2006) and differentially affects the expression and phosphorylation of glutamate receptor subunits and CaMKIIα (Brown et al., 2005; Gardoni et al., 2010; Koutsokera et al., 2014).
Dopamine denervation in vivo induces an increase in CaMKIIα-Thr286 phosphorylation in the striatum (Brown et al., 2005; Koutsokera et al., 2014), concurrent with increased recruitment of activated CaMKIIα to GluN2A-GluN2B subunits (Picconi et al., 2004). On the other hand, reduced levels of CaMKIIα autophosphorylation and GluA1-Ser831 phosphorylation in the hippocampus correlates with impaired CA1 LTP in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice (Moriguchi et al., 2012b). Overall, DA deficiency can induce deficits in synaptic plasticity and motor behavior by altering striatal glutamatergic signaling and CaMKII activity (Picconi et al., 2004; Brown et al., 2005; Deutch, 2006; Paillé et al., 2010; Moriguchi et al., 2012b; Koutsokera et al., 2014).
Cerebral Ischemia
Cerebral ischemia is a condition in which restricted blood supply to the brain causes tissue damage and cell death. Excess glutamate release and high [Ca2+]i trigger a range of downstream neurotoxic cascades leading to apoptosis or necrosis (Szydlowska and Tymianski, 2010). Ca2+ influx ensuing an ischemic insult significantly increases NMDAR-mediated activation of CaMKII (Meng et al., 2003) followed by its phosphorylation at Thr253 (Gurd et al., 2008) and Thr286 (Shamloo et al., 2000; Matsumoto et al., 2002). CaMKII-Thr253 autophosphorylation enhances its association with PSD (Migues et al., 2006) and induces the persistent activation of the enzyme (Rostas et al., 2017). Oxidation of Met281/282 (Cys281/Met282 in CaMKIIα) in the auto-regulatory domain of the enzyme, by reactive oxygen species (ROS) generated during glutamate excitotoxicity and oxidative stress, can also lead to autonomous activity of the kinase (Anderson, 2015), which in turn augments reperfusion injury in acute ischemic stroke (Gu et al., 2016; Qu et al., 2019; Zhang et al., 2021). Autophosphorylated CaMKII translocates to the synaptic membrane (Matsumoto et al., 2004), binds to synaptic GluN2B (Buonarati et al., 2020) and phosphorylates serine residue(s) of the GluN2B subunit (Meng and Zhang, 2002; Meng et al., 2003) to mediate ischemic cell death. However, a recent study by Tullis et al. (2021), reported that neuronal death in global cerebral ischemia in vivo is promoted by the binding of CaMKII to GluN2B and not by CaMKII-mediated GluN2B-Ser1303 phosphorylation (Kumar et al., 2019; Buonarati et al., 2020; Tullis et al., 2021). CaMKII activation dependent on NMDARs or L-type VGCCs can also phosphorylate serine residues of GluR6 subunit of kainate receptors via the assembly of GluR6-PSD95-CaMKII signaling module in cerebral ischemia injury (Hao et al., 2005; Xu et al., 2010).
The changes observed in expression levels and activity of CaMKII are dependent on the duration of ischemic insult (Gurd et al., 2008), which in turn can regulate NMDAR-mediated field excitatory postsynaptic potentials (fEPSPs) (Wang N. et al., 2014). Likewise, 10 min oxygen-glucose deprivation (OGD) treatment in vitro can induce NMDAR-mediated postischemic LTP, mediated by CaMKII-NMDAR interaction and NMDAR trafficking to the membrane (Wang N. et al., 2014).
Traumatic Brain Injury
Traumatic brain injury (TBI) is a disruption in the normal function of the brain caused by an external mechanical force. It is associated with the release of excitatory amino acids, particularly glutamate, in the extracellular space (Faden et al., 1989; Chamoun et al., 2010). Overactivation of glutamate receptors (Faden et al., 1989; Liu et al., 2017) and elevated levels of [Ca2+]i (Deshpande et al., 2008; Sun et al., 2008) transiently activates CaMKIIα (Atkins et al., 2006; Folkerts et al., 2007; Liu et al., 2017) and CaMKIIδ (Zhang et al., 2012). Alterations in NMDAR function, CaMKIIα expression and dendritic spine anatomy in the hippocampus prevent LTP induction after lateral fluid percussion injury (Schwarzbach et al., 2006), thereby causing cognitive impairment often associated with CNS trauma (Atkins et al., 2006; Schwarzbach et al., 2006; Folkerts et al., 2007; Deshpande et al., 2008). Long-term alterations in Ca2+ homeostasis mechanisms (Sun et al., 2008) contributes to morbidity and mortality following TBI.
Functional Implications of Ca2+/Calmodulin-Dependent Protein Kinase Type II Mutations in Synaptic Plasticity
CaMKII plays a versatile role in different regulatory processes involved in synaptic plasticity. This section reviews the different CaMKII mutant animal models generated to study the physiological role of the kinase in synaptic plasticity and its associated behavioral phenotype. Targeted disruption of CaMKIIα/β/γ function in vivo dysregulates different types of synaptic plasticity (Silva et al., 1992a; Stevens et al., 1994; Mayford et al., 1995; Giese et al., 1998; Elgersma et al., 2002; Miller et al., 2002; Cho et al., 2007; van Woerden et al., 2009; Yamagata et al., 2009; Yin et al., 2017; Cohen et al., 2018; Kool et al., 2019) and impairs learning (Silva et al., 1992b,1996; Bach et al., 1995; Giese et al., 1998; Elgersma et al., 2002; Irvine et al., 2005; Yamagata et al., 2009; Borgesius et al., 2011; Achterberg et al., 2014; Cohen et al., 2018), memory (Miller et al., 2002; von Hertzen and Giese, 2005; Cho et al., 2007) and the emotional state (Chen et al., 1994; Yamasaki et al., 2008; Hasegawa et al., 2009; Bachstetter et al., 2014). Although the behavior exhibited varies slightly with the genetic background of the mouse strain used (Gordon et al., 1996; Silva et al., 1996; Hinds et al., 1998; Need and Giese, 2003), the molecular and electrophysiological alterations remain largely unchanged.
Ca2+/Calmodulin-Dependent Protein Kinase Type II α
Ca2+/Calmodulin-Dependent Protein Kinase Type II α Global Knockout Mice
Silva et al. (1992a) reported the production of the first genetically altered mice lacking the α subunit of CaMKII. LTP, STP and LTD were either absent or significantly attenuated in the sensory neocortex and hippocampal slices from young homozygous CaMKIIα–/– KO mice (Silva et al., 1992a; Stevens et al., 1994; Kirkwood et al., 1997; Hinds et al., 1998; Elgersma et al., 2002). Long-term plasticity and reversal of LTP were normal in the CA1 hippocampal region of heterozygous CaMKIIα+/– mice (Silva et al., 1996; Elgersma et al., 2002); however, they exhibited impaired short-lived plasticity (SLP) and paired-pulse facilitation (PPF) and an enhanced post-tetanic potentiation (PTP) response expressed within seconds of stimulation (Silva et al., 1992a,1996; Chapman et al., 1995; Hojjati et al., 2007).
Plasticity deficits due to either partial or complete loss of CaMKIIα activity manifest as abnormalities in various behavioral paradigms. CaMKIIα null mutant mice have been reported to exhibit pronounced deficits in spatial learning (Silva et al., 1992b; Elgersma et al., 2002; Achterberg et al., 2014), working memory (Yamasaki et al., 2008) and Pavlovian fear conditioning (Chen et al., 1994; Silva et al., 1996; Elgersma et al., 2002; Achterberg et al., 2014). Dysregulated emotional states like increased aggression, decreased anxiety and depression-like behavior and an exaggerated infradian rhythm have also been observed in CaMKIIα+/– mice (Silva et al., 1992b; Chen et al., 1994; Yamasaki et al., 2008).
Dysfunction of the dentate gyrus (DG) due to the immaturity of DG neurons (Yamasaki et al., 2008; Matsuo et al., 2009) and ectopic projection of mossy fibers (Nakahara et al., 2015), causes suppressed induction of activity-dependent genes like c-fos and arc, resulting in altered behavior exhibited by CaMKIIα KO mice (Yamasaki et al., 2008; Matsuo et al., 2009). Disrupted regulation of Zif268 gene expression and growth associated protein 43 (GAP43), a synaptogenesis marker, by CaMKIIα+/– mutation can also impair the maturation of cortical circuits necessary for remote memory (Frankland et al., 2004).
Ca2+/Calmodulin-Dependent Protein Kinase Type II α-Thr286 Mutant Mice (T286A/T286D)
The Ca2+/CaM-independent, autonomous state of CaMKIIα, induced by autophosphorylation of Thr286, is required for NMDAR-dependent LTP and LTD at CA1 pyramidal cells (Giese et al., 1998), spatial learning (Giese et al., 1998; Need and Giese, 2003), fear learning (Irvine et al., 2005, 2011) and regulation of synapse development in vivo (Gustin et al., 2011). During induction of synaptic plasticity, CaMKIIα-Thr286 phosphorylation is essential for optimal integration of Ca2+ signals; however, it is dispensable for LTP maintenance and memory (Irvine et al., 2005; Chang et al., 2017). High-frequency synaptic stimulation can rescue impaired LTP induction in CA1 neurons from Camk2aT286A mice (Chang et al., 2017). Although L-LTP could not be induced at CA1 synapses of T286A mutants (Irvine et al., 2011), mTOR-mediated upregulation of PSD95 expression and a persistent generation of multi-innervated spines (MIS) can contribute to LTM formation in these mutant animals where functional strengthening of synapses is impaired (Radwanska et al., 2011).
The deficit in spatial learning of CaMKIIα-T286A mutant mice is due to decreased spatial selectivity, stability and experience-dependent tuning of CA1 hippocampal place cells (Cho et al., 1998; Cacucci et al., 2007) and an impaired precision of spatial memory (Śliwińska et al., 2020). Pre-adolescent KI mice had disruption in synaptic targeting of CaMKII and enhanced activity of GluN2B-containing-NMDARs at CA3-CA1 synapses along with impaired cognition and anxiety phenotypes (Gustin et al., 2011). The T286A knockin (KI) mutants have normal neurogenesis in their DG (Kee et al., 2007). Therefore, alternate signaling mechanisms involving either PKA or CaMKIIβ are activated in the absence of CaMKIIα autophosphorylation at excitatory synapses in the neonatal rodent hippocampus (Yasuda et al., 2003), hippocampal inhibitory interneurons (Lamsa et al., 2007) and the medial perforant path-granule cell synapses in adult mice (Cooke et al., 2006) to induce LTP.
Constitutive expression of the Ca2+-independent, autonomously active form of CaMKIIα (CaMKIIα-T286D) in vivo favors LTD at LTP-inducing θ frequencies (5–10 Hz) and consequently influences spatial learning and fear conditioning (Bach et al., 1995; Mayford et al., 1995, 1996; Wiedenmayer et al., 2000; Bejar et al., 2002; Yasuda and Mayford, 2006). The use of the tetracycline transactivator (tTA) system to limit the expression of CaMKIIα-T286D regionally and temporally, has shed light on the role of CaMKIIα signaling in synaptic plasticity during development, memory encoding and memory storage (Mayford et al., 1996; Glazewski et al., 2001; Bejar et al., 2002; Yasuda and Mayford, 2006). CA1 hippocampal place cells in these mutant animals are less common, less precise and less stable, thereby affecting spatial memory storage (Rotenberg et al., 1996).
Ca2+/Calmodulin-Dependent Protein Kinase Type II α-Thr305/Thr306 Mutant Mice
Inhibitory phosphorylation of CaMKIIα at Thr305/Thr306 is essential to modulate the association of the kinase with PSD, the threshold for induction of NMDAR-dependent LTP at SC-CA1 synapses, hippocampal-dependent spatial learning and fear conditioning, reversal learning and to induce LTP at inhibitory synapses (iLTP) (Elgersma et al., 2002; Cook et al., 2021). Phosphorylation of CaMKIIα-Thr305/Thr306 during an excitatory LTD stimulus blocks the translocation of CaMKIIα to glutamatergic excitatory synapses and directs CaMKIIα to GABAergic inhibitory synapses to induce iLTP. In this way, Thr305/Thr306 phosphorylation governs the fundamental LTP vs. LTD decision at excitatory synapses (Cook et al., 2021). Similar to CaMKIIα-T286D mutant, CaMKIIα-T305D favors LTD over LTP at weak tetanic stimulations (Elgersma et al., 2002).
Ca2+/Calmodulin-Dependent Protein Kinase Type II α-K42R Mutant Mice
Similar to the CaMKIIα mutant models reviewed above, the kinase-dead CaMKIIα (CaMKIIα-K42R) KI mouse also exhibited deficits in NMDAR-dependent LTP and hippocampus-dependent learning and memory (Yamagata et al., 2009, 2018). Although the levels of PSD associated CaMKIIα and activity-dependent postsynaptic translocation of CaMKIIα were intact in the mutants, the stimulus-induced increase in spine volume was severely impaired compared to WT mice (Yamagata et al., 2009). Amygdala-dependent fear memory is only partially affected by the loss of kinase activity (Yamagata et al., 2018). Stronger conditioning or multi-trial training could achieve slight or no improvement in the memory deficits of CaMKIIα-K42R mutant mice (Yamagata et al., 2009, 2018).
Conditional Mutant Models
Apart from the models described above, there are a few other transgenic (Tg) mouse models generated to study specific functions of CaMKII in synaptic plasticity. The CaMKIIα-3′UTR mutant has reduced expression of the kinase in the dendrites and its association to PSD (Miller et al., 2002), with no substantial alteration in other protein constituents of the synaptic membrane (Li et al., 2007). Disruption in the local translation of the protein causes a reduction in L-LTP, memory consolidation and LTM storage, with no change in E-LTP and STM formation (Miller et al., 2002).
Using an inducible and forebrain specific CaMKIIα-F89G Tg mouse model, Joe Z. Tsien and group have shown that the levels of CaMKIIα protein can affect the degree and direction of synaptic plasticity (Wang et al., 2003, 2008). A switch between the normal and higher activity state of CaMKIIα during the memory consolidation phase can severely disrupt LTM formation. The synaptic consolidation of LTMs requires the reactivation of CaMKIIα, during the first week after training, to the level present at the time of initial learning (Wang et al., 2003); on the other hand, a shift in CaMKIIα activation status within the immediate post-learning 10 min can alter STM formation (Wang et al., 2008).
In the study reported by Achterberg et al. (2014), conditional Camk2a mutant mice models were employed to achieve regional and temporal specific deletion of CaMKIIα. Telencephalon-specific deletion of the Camk2a gene (Camk2aflox/Emx–Cre) resulted in severe deficits in spatial and contextual learning and hippocampal LTP in adult mice, whereas mice with deletion specific to Purkinje cells in the cerebellum (Camk2aflox/L7–cre) learned normally (Achterberg et al., 2014).
At hippocampal synapses, CaMKIIα functions non-enzymatically by limiting the size of docked vesicles (Hojjati et al., 2007) and by regulating neurotransmitter release at glutamatergic synapses (Chapman et al., 1995; Hinds et al., 2003), thereby modulating short-term presynaptic plasticity. A few of the CaMKIIα Tg mice also exhibited seizures (Butler et al., 1995; Mayford et al., 1995; Elgersma et al., 2002; Yamagata et al., 2009). With a potential role for CaMKIIα in controlling the state of emotion, these models can also be exploited in the study of neuropsychiatric diseases (Yamasaki et al., 2008; Hasegawa et al., 2009; Matsuo et al., 2009; Nakahara et al., 2015; Yamagata et al., 2018).
Ca2+/Calmodulin-Dependent Protein Kinase Type II β
The first Tg mouse model of CaMKIIβ was generated by Cho et al. (2007), by selectively overexpressing CaMKIIβ-F90G in the DG. Elevated CaMKIIβ activity does not affect baseline glutamatergic neurotransmission but causes deficits in LTP (Cho et al., 2007) and in NMDAR-dependent LTD (Yin et al., 2017). The Tg mice displayed normal acquisition, retention and recall of 1-day-old LTM, but showed severe impairments in 10-day-old contextual fear memory (Cho et al., 2007) and behavioral flexibility (Yin et al., 2017). Overexpression of CaMKIIβ decreases the activity of PP1/protein phosphatase 2A (PP2A) and glycogen synthase kinase 3β (GSK3β), which can shift the direction of synaptic plasticity toward potentiation during LTD induction. This disrupts the regulation of synaptic stargazin and interrupts the internalization of AMPAR and dephosphorylation of Ser831 and Ser845 of GluA1 during NMDAR-LTD (Yin et al., 2017).
Global KO models of CaMKIIβ (Camk2b–/–) have been generated by deletion of exon sequences of the Camk2b gene (van Woerden et al., 2009; Bachstetter et al., 2014; Kool et al., 2016). Camk2b–/– mice exhibited cerebellar ataxia and severe deficits in locomotion (Kool et al., 2016), motor coordination (van Woerden et al., 2009), balance and cognition (Bachstetter et al., 2014). Interestingly, they showed reduced anxiety in a gene dose-dependent manner (Bachstetter et al., 2014).
Loss of CaMKIIβ, in Camk2b–/– mice, results in bidirectional inversion of postsynaptic plasticity at the parallel fiber (PF)-Purkinje cell (PC) synapse (van Woerden et al., 2009; Pinto et al., 2020). Failure of proper targeting of CaMKIIα to dendritic spines in the absence of CaMKIIβ in the Camk2b–/– mice results in impaired hippocampal NMDAR-dependent LTP and fear learning (Borgesius et al., 2011). This disrupted phenotype was absent in the Camk2bA303R/A303R KI model in which Ca2+/CaM-dependent kinase activation of CaMKIIβ is disabled but F-actin binding and bundling functions are preserved (Borgesius et al., 2011). During LTP induction, a transient detachment of CaMKIIβ from F-actin, triggered by Ca2+ influx through glutamate receptors and the associated autophosphorylation of the F-actin binding region, is necessary for spine enlargement and LTP maintenance (Kim et al., 2015). Persistent binding of CaMKIIβ to F-actin in the amygdala could be causing deficits in LTP (Kim et al., 2015, 2019). To study the regulation of CaMKIIβ-F-actin interaction by autophosphorylation, a KI mouse model was generated by substituting Thr and Ser residues with Ala at exon 13 of Camk2b (CaMKIIβexon13:TS/A). This KI mouse exhibited reduced freezing in fear conditioning tests (Kim et al., 2019). The absence of impairment in fear learning in the CaM-binding deficient mutant reported by Borgesius et al. (2011) might be due to phosphorylation of the F-actin binding domain in the non-activable CaMKIIβ-A303R mutant by neighboring α-subunits of the same oligomer (Kim et al., 2019).
Regardless of normal hippocampal plasticity, Camk2bA303R/A303R mice exhibited severe deficits in motor behavior. However, the autophosphorylation deficient Camk2b mice, Camk2bT287A/T287A, showed no significant change in locomotion compared to WT littermates, indicating a crucial role for Ca2+/CaM-dependent activity, but not autonomous activity in normal mouse locomotion (Kool et al., 2016). Among the different Camk2b conditional mutants generated (Kool et al., 2016), Camk2bf/f;L7-cre mice with specific loss of CaMKIIβ in cerebellar Purkinje cells showed impaired motor learning when tested for five consecutive days, indicating that cerebellar CaMKIIβ is essential for motor function (Kool et al., 2016).
Camk2a-Camk2b Double Mutants
The use of single mutants of Camk2a or Camk2b to study their function during development and in the mature brain can be inadequate when crucial functions are masked by compensation by the non-deleted form. For this purpose, double mutants of both isoforms (Camk2a–/–;Camk2b–/–) were generated (Kool et al., 2019). Germline or adult deletion of both CaMKIIα and CaMKIIβ in mice is lethal. Similarly, the Ca2+-dependent and -independent activities of CaMKIIα and CaMKIIβ are also essential for survival. Acute deletion of both CaMKII isoforms does not overtly affect the biochemical composition of PSD. Adult loss of CaMKIIα and CaMKIIβ also abolished LTP in the hippocampal CA3-CA1 SC pathway. This deficit was absent in mice containing a specific deletion of CaMKII isoforms in the CA3 region of the hippocampus (Camk2af/f;Camk2bf/f;CA3-Cre), indicating that presynaptic CaMKIIα and CaMKIIβ are dispensable for LTP at the CA3-CA1 synapses. However, deletion of CaMKII in the CA3 region resulted in significant reduction in LTP at the associational/commissural pathway (CA3-CA3 synapse) (Kool et al., 2019).
Ca2+/Calmodulin-Dependent Protein Kinase Type II γ
Similar to CaMKIIα and CaMKIIβ, global CaMKIIγ KO mice (CaMKIIγ–/–) displayed pronounced impairments in hippocampal-dependent memory tasks and avoidance behavior (Cohen et al., 2018). Training-induced increase in the expression of plasticity genes – BDNF, c-Fos and Arc – was prevented in CaMKIIγ–/– mice. While E-LTP was intact, L-LTP was strongly affected at SC-CA1 synapses of CaMKIIγ–/– mice, indicating deficits in LTM, but not STM. KO mice harboring a selective deletion of CaMKIIγ in excitatory neurons (CaMKIIγ-exc-KO), also exhibited impaired spatial learning and a decrease in training-induced nuclear translocation of CaM and c-Fos expression, suggesting a role for NMDAR activation upstream to CaMKIIγ-mediated cytonuclear signaling in CaMKIIγ–/– mice (Cohen et al., 2018). In vivo deletion of CaMKIIγ in parvalbumin (PV)-expressing inhibitory interneurons (CaMKIIγ PV-KO) eliminates NMDAR-induced synaptic potentiation of excitatory synapses onto inhibitory neurons (LTPE→I) and impairs experience-dependent neural oscillations, thereby disrupting memory consolidation and hippocampus-dependent LTM (He et al., 2021).
Functional Implications of Ca2+/Calmodulin-Dependent Protein Kinase Type II Mutations in Diseases
In humans, de novo mutations in CaMKII have been identified and reported majorly in cases of neurodevelopmental disorders (NDDs) (Study, 2017; Akita et al., 2018) and intellectual disability (ID) (Küry et al., 2017). The role of CaMKII and glutamatergic signaling in neuropsychiatric diseases has been reviewed by Robison (Robison, 2014; see also Nicole and Pacary, 2020). Supplementary Table 2 summarizes the different CaMKII variants reported with their functional implications and clinical manifestations if any. The type of mutation (synonymous, missense, splice region, frameshift, deletion), the specific CaMKII isoform (α, β and γ) that is mutated and the protein domain (catalytic, auto-regulatory or association) affected determine the disease phenotype. The zygosity of inheritance (heterozygous/homozygous) can also influence the pathogenicity of the variant (Chia et al., 2018); however, intrafamilial variations in the expression of disease symptoms by subjects carrying the same heterozygous variant, have also been reported (Heiman et al., 2021).
Clinical manifestations of the identified mutations range from global neurodevelopmental delay, seizures, mild to severe ID, hypotonia, delayed development of motor and speech/language skills, abnormal emotional behavior, cerebellar atrophy, facial dysmorphism, visual impairment and gastrointestinal issues. Dysfunction of CaMKIIα can cause seizure-associated activity in the forebrain (Akita et al., 2018) and pronounced motor delay (Küry et al., 2017), while individuals with CaMKIIβ variants exhibit severe ID accompanied with hypotonia (Küry et al., 2017) and cerebellar atrophy (Akita et al., 2018). Facial dysmorphisms along with severe ID and severe hypotonia has been reported in patients carrying a CAMK2G variant (Proietti Onori et al., 2018). The vast majority of the variants identified, affect amino acids conserved across species (Küry et al., 2017; Stephenson et al., 2017; Akita et al., 2018; Chia et al., 2018; Proietti Onori et al., 2018), which may explain the degree of severity of pathogenicity.
Stephenson et al. (2017) reported the first characterization of a de novo missense mutation in the CAMK2A gene, encoding for CaMKIIα, that was found in a patient with autism spectrum disorder (ASD) (Iossifov et al., 2014). Replacement of Glu with Val at 183rd position in the catalytic domain of CaMKIIα (CaMKIIαGlu183Val) disrupts the interaction of CaMKII with ASD-associated proteins, such as Shank3 (SH3 and multiple ankyrin repeat domains 3) (Jiang and Ehlers, 2013), GluN2B (Pan et al., 2015) and the metabotropic glutamate receptor mGlu5 (Chana et al., 2015), which can reduce targeting of CaMKIIα to spines (Stephenson et al., 2017). Neuronal expression of CaMKIIαGlu183Val disrupts AMPAR-mediated synaptic transmission, interferes with CaMKII autophosphorylation and reduces dendritic spine density. Heterozygous (Camk2aWT/E183V) and homozygous (Camk2aE183V/E183V) KI mice displayed enhanced repetitive behaviors and deficits in social interactions, which mimic symptoms of ASD (Stephenson et al., 2017). Decreased autoinhibition and increased Thr286 autophosphorylation of the CaMKIIαPro212Gln mutant, identified in an individual with NDD, affects the efficiency of excitatory synaptic transmission by enhancing K+ currents in dendrites in vitro (Akita et al., 2018).
A biallelic, germline, loss-of-function CAMK2A missense mutation, CAMK2Ap.(His477Tyr) in the association domain of CaMKIIα, was reported in two siblings displaying psychomotor retardation, frequent seizures and severe ID (Chia et al., 2018). Compared to the WT enzyme, the mutant form disrupts CaMKIIα self-oligomerization and holoenzyme assembly which in turn affects its subcellular localization in neurons and ability to support synaptic function in vivo (Chia et al., 2018). Recently, Brown et al. (2021) characterized six heterozygous variants of CAMK2A found in patients with schizophrenia. The p.(Arg396*) mutation in the association domain of CaMKIIα ablates holoenzyme formation, impairs GluN2B binding and consequently fails to accumulate at excitatory synapses in response to a LTP stimulus. While both p.(Arg396*) and p.(Arg8His) variants of CAMK2A exhibited impaired autophosphorylation at Thr286, only the p.(Arg8His) mutation in the kinase domain significantly affected the Ca2+/CaM-stimulated kinase activity (Brown et al., 2021). The absence of impaired function or expression for the remaining four mutants studied indicates that the mere occurrence of a mutation in a patient does not imply that the disease is caused by the mutation (Brown et al., 2021).
In addition to NDDs and ID, CAMK2A variants/single nucleotide polymorphisms (SNPs)/single nucleotide variants (SNVs) have been reported to be associated with risk for bipolar disorder (BD) in cohorts of European descent (Ament et al., 2015), in sporadic AD patients belonging to the Han Chinese population (Fang et al., 2019) and mild cognitive impairment (MCI) subjects in a Spanish population (Bufill et al., 2015). Deletion of the chromosome at 5q32, covering CAMK2A, might be responsible for mild ID observed in two patients diagnosed with mandibulofacial dysostosis (Vincent et al., 2014). Interestingly, CAMK2A genetic variants have been reported to be nominally associated with non-verbal communication in ASD cohorts (Chiocchetti et al., 2018) and logical memory performance in the elderly people (Rhein et al., 2020). CAMK2A polymorphisms can also influence spatial working memory in Caucasian adolescents (Easton et al., 2013) and cognitive ability in Taiwanese senior high school students (Lee et al., 2021). Recruitment of higher number of subjects from distinct populations is warranted to further validate the association of CAMK2A SNPs in genotype-phenotype association studies (Chiocchetti et al., 2018; Rhein et al., 2020).
Apart from mutations in CAMK2A, de novo mutations in CAMK2B have also been reported in 10 unrelated individuals exhibiting mild to severe ID (Küry et al., 2017). There are 19 rare variants of CAMK2A and CAMK2B that are heterozygous nonsense, missense or splice-site mutations affecting the catalytic or auto-regulatory domain of CaMKII. The identified variants could affect protein expression and autophosphorylation at Thr286/Thr287 when expressed in vitro, and cause deficits in neuronal migration in vivo (Küry et al., 2017). De novo mutations in CAMK2A and CAMK2B can also result in varying neurodevelopmental phenotypes (Akita et al., 2018). The missense variants disrupted the interaction between the catalytic domain and the regulatory segment, leading to increased Ca2+-independent activity (Akita et al., 2018).
A heterozygous c.85C>T, p.(Arg29*) mutation in CAMK2B was found in a patient with mild ID, delayed speech development and seizures (Küry et al., 2017). This mutation was also reported in a 3-year-old European girl with complex focal seizures and global neurodevelopmental delay (Heiman et al., 2021). This maternally inherited pathogenic variant of the CAMK2B gene only mildly affected the patient’s sibling, with the same variant, while the mother was phenotypically healthy and intellectually normal (Heiman et al., 2021). Similarly, a heterozygous c.416C>T, p.(Pro139Leu) variant of CAMK2B found in four Caucasian patients presented with severe ID, global developmental delay, hypotonia and microcephaly (Küry et al., 2017), was also reported in a 22-year-old South Asian woman (Rizzi et al., 2020) as well as in a MECP-2 (methyl-CpG binding protein 2) negative proband of Japanese origin (Iwama et al., 2019). The recurrence of a few pathogenic variants of CAMK2A and CAMK2B (Küry et al., 2017) calls for elaborate functional studies of the mutant proteins both in vitro and in vivo (Onori and van Woerden, 2021).
De novo mutations in CAMK2G have also been identified and reported in cases of NDDs and severe ID (De Ligt et al., 2012; Study, 2017; Proietti Onori et al., 2018). Whole-exome sequencing performed on two patients revealed c.875G>C, p.(Arg292Pro) mutation in the auto-regulatory domain of CaMKIIγ, which is a putative CaM trapping region. One research group showed that CAMK2Gp.(Arg292Pro) affects protein stability in vitro and functions as a pathogenic gain-of-function mutation by rendering it constitutively active and by blocking neuronal migration during development in vivo (Proietti Onori et al., 2018). The pathogenicity of the mutant is dependent on its catalytic activity (Proietti Onori et al., 2018). Cohen et al. (2018) reported that the ID observed in these patients might be due to the inability of CaMKIIγArg292Pro to effectively trap CaM and shuttle Ca2+/CaM complex to the nucleus, thereby disrupting a major link connecting activation of NMDARs and Cav1 channels to nuclear transcription of BDNF, c-Fos and Arc. This in turn adversely affects synaptic strengthening and LTM in vivo. Similar to CAMK2A SNPs reported, a genetic cluster containing CAMK2G polymorphisms has been identified to be associated with episodic memory performance (Dominique and Papassotiropoulos, 2006).
Although the CAMK2 variants reported so far shed light on the probable role of the kinase in mediating disease symptoms, the number of human subjects identified with the mutation is insufficient, compared to the samples tested, to correlate the variant to the disease with good statistical power. Neither is it mandatory for the identified variant(s) to be a causal factor in the diseased phenotype (Brown et al., 2021), nor can an indirect role by the mutant protein be overlooked. More detailed functional characterization of the identified and reported CaMKII mutations than what is already reported, both in vitro (Küry et al., 2017; Stephenson et al., 2017; Akita et al., 2018; Chia et al., 2018; Cohen et al., 2018; Proietti Onori et al., 2018; Brown et al., 2021) and in vivo (Stephenson et al., 2017; Chia et al., 2018), can further substantiate the critical role of CaMKII mutants in disease conditions. Nonetheless, screening for either sporadic or inherited CAMK2 variants in disorders majorly affecting cognition, can help in unraveling the theragnostic potential of CaMKII, if any.
Ca2+/Calmodulin-Dependent Protein Kinase Type II as a Druggable Target for Treating Glutamatergic Dysfunction
Antagonists against glutamate receptors, majorly NMDARs, have been designed, synthesized and evaluated for their efficacy in preventing excitotoxicity in CNS diseases (Liu et al., 2020; Chandran et al., 2021). Signaling molecules downstream to NMDARs, like CaMKII, can also be targeted to restore Ca2+ and glutamate homeostasis at synapses (Vest et al., 2010). Likewise, CaMKII has been exploited as a potential drug target in neuropsychiatric and neurodegenerative diseases (Sałaciak et al., 2021).
Based on the differential regulation of CaMKII function during neurotoxicity, the modulators either enhance (Yamamoto et al., 2009; Zeng et al., 2010; Wang D.M. et al., 2013; Wei et al., 2013; Wang S.Q. et al., 2014) or inhibit CaMKII activity (Wang D. et al., 2013; Jiang et al., 2019). Their modus operandi includes binding to Ca2+/CaM binding site of CaMKII (Brooks and Tavalin, 2011; Wong et al., 2019), targeting autonomous CaMKII activity (Coultrap et al., 2011; Wang D.M. et al., 2013; Wang et al., 2016; Deng et al., 2017), interacting with CaMKII hub domain (Leurs et al., 2021), preventing CaMKII translocation to the synaptic membrane (Matsumoto et al., 2008), inhibiting GluN2B-CaMKII binding (Tullis et al., 2021) or by modulating CaMKII-mediated signaling pathways (Liu et al., 2012; Matsumoto et al., 2013; Wei et al., 2013; Zhang et al., 2017; Islam et al., 2019; Wu et al., 2019; Izumi et al., 2020; Chen et al., 2021). The different CaMKII modulators reported from studies involving glutamatergic synapses in neurons are listed below:
-
1.
Synthetic small molecule inhibitors like KN-62 and KN-93 (Tokumitsu et al., 1990; Sumi et al., 1991; Vest et al., 2010; Ashpole and Hudmon, 2011; Brooks and Tavalin, 2011).
-
2.
Synthetic peptide inhibitors like AIP (autocamtide-2-related inhibitory peptide) (Fan et al., 2006; Goebel, 2009; Zha et al., 2009; Ashpole and Hudmon, 2011) and AC3-I (autocamtide-3 derived inhibitory peptide) (Leonard et al., 1999).
-
3.
The natural CaMKII inhibitor protein CaM-KIIN (CN) and its peptide derivatives, CaM-KIINtide (CN27) (Chang et al., 1998; Saha et al., 2006; Mayadevi et al., 2016), CN21 (Vest et al., 2007, 2010; Ashpole and Hudmon, 2011; Ahmed et al., 2017), CN19 (Coultrap and Bayer, 2011; Chalmers et al., 2020) and CN17β (Gomez-Monterrey et al., 2013).
-
4.
CaMKII antisense oligodeoxynucleotides (Liu et al., 2012).
-
5.
Long non-coding RNA CAMK2D-associated transcript 1 (C2dat1) (Xu et al., 2016).
-
6.
Analogs of γ-hydroxybutyrate (GHB) (Leurs et al., 2021).
-
7.
Volatile anesthetics like isoflurane (Matsumoto et al., 2008).
-
8.
Compounds isolated from natural sources like nobiletin (Yamamoto et al., 2009), curcumin (Mayadevi et al., 2012), β-asarone (Wei et al., 2013), paeoniflorin (Wang D. et al., 2013; Zhang et al., 2017), naringin (Wang D.M. et al., 2013), Ganoderma lucidum polysaccharides (GLP) (Wang S.Q. et al., 2014), baicalin (Wang et al., 2016), theobromine (Islam et al., 2019), tilianin (Jiang et al., 2019) and gastrodin (Chen et al., 2021).
-
9.
The histone deacetylase (HDAC) inhibitor, vorinostat (Matsumoto et al., 2013).
-
10.
SAK3, a T-type calcium channel enhancer (Izumi et al., 2020).
Although a majority of the modulators have been widely employed to combat neuronal glutamatergic dysfunction in vitro and in vivo, their clinical use will require extensive studies on possible side effects and toxicity to cardiac health too (Nassal et al., 2020).
Future Perspectives
Majority of the biochemical and structural studies on CaMKII have been performed on homomeric holoenzymes of one of the isoforms. However, under physiological conditions the enzyme can form hetero-multimers with different subunit stoichiometries. The relative abundance of different heteromeric subtypes under different developmental stages and in different regions would be an important determinant in the physiological functioning of CaMKII. Detailed studies on the functional variation among heteromers and their participation in specific cellular functions would be essential in making further progress in understanding the physiological functions of CaMKII. Further refinement of molecular genetic techniques for ectopic expression of isoforms and mutants of CaMKII with better control on heteromer formation would be essential for progress toward this goal.
The binding of CaMKII to GluN2B modulates the kinetic parameters of CaMKII enzyme activity and attenuates dephosphorylation of CaMKII (Pradeep et al., 2009; Cheriyan et al., 2011). These regulatory events strongly support the bistable switch model of molecular memory involving CaMKII and PP1. However, the physiological relevance of these regulatory events and the existence of the bistable switch in vivo needs to be demonstrated. Elucidation of the structure of the CaMKII-GluN2B complex could contribute significantly toward understanding the physiological functions of this complex.
CaMKII activation is a prerequisite for both LTP and LTD and recently it has been shown (Woolfrey et al., 2018) that two types of substrates (high autonomy, low autonomy) are preferred under each condition. If so, what are the exact signaling events with respect to the amount of Ca2+ entry into the postsynaptic site responsible for each of these events?
The recurrence of a few pathogenic variants of CAMK2A and CAMK2B (Küry et al., 2017) calls for elaborate functional studies of the mutant proteins both in vitro and in vivo (Onori and van Woerden, 2021). It also encourages more extensive screening for genetic variants of CaMKII in human populations.
Conclusion
CaMKII is an enzyme highly enriched in the brain. It has important roles to play in the functioning of glutamatergic synapses. Significant advances have been made in understanding the structure, function and physiological role of CaMKII. Its contribution to learning and memory has been investigated extensively with the help of most modern techniques. This has unequivocally established the integral part played by this enzyme in learning and memory. However, there is still more to be understood about the exact manner in which CaMKII participates in the underlying cellular mechanisms such as synaptic plasticity. Novel features of the structure and biochemical regulation of CaMKII are still being revealed by biophysical and biochemical experiments. Since the molecular properties of CaMKII holoenzyme are among the foundations on which most of the models of synaptic plasticity, learning and memory are built, progress in structural studies would continue to necessitate revisions in these models. Powerful molecular genetic techniques have permitted the controlled expression of isoforms and mutants of CaMKII in specific cell types in the brain of model organisms leading to important insights into its role in the cellular and systemic mechanisms. However, it has been difficult to dissect out all of its synaptic functions from cellular functions due to technical hurdles. The occurrence of heteromeric subtypes of CaMKII and the redundancy in the function among the isozymes also poses challenges to molecular genetic interrogation of its cellular functions. Progress in the understanding of CaMKII has prompted attempts to pursue it as a therapeutic target for pharmacological and genetic interventions since it is part of impaired Ca2+ signaling in many disease conditions. A limited number of genetic variants of CaMKII have been found associated with human neurological disease conditions. The central role of CaMKII in brain functions calls for large scale screening for CaMKII variants in human populations.
Author Contributions
AM and RO contributed to conception and design of the manuscript. AM, SG, RJ, and RO co-wrote the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This work was supported by Rajiv Gandhi Center for Biotechnology (RGCB) (Grant No: RGCB-LRF), Department of Science and Technology (DST) (Grant Nos: DST INSPIRE Fellowships, IF150643 and IF150638), Government of India and Science and Engineering Research Board, Government of India (Grant No. CRG/2018/004528).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnmol.2022.855752/full#supplementary-material
References
- Aakalu G., Smith W. B., Nguyen N., Jiang C., Schuman E. M. (2001). Dynamic visualization of local protein synthesis in hippocampal neurons. Neuron 30 489–502. 10.1016/s0896-6273(01)00295-1 [DOI] [PubMed] [Google Scholar]
- Abidin I., Köhler T., Weiler E., Zoidl G., Eysel U. T., Lessmann V., et al. (2006). Reduced presynaptic efficiency of excitatory synaptic transmission impairs LTP in the visual cortex of BDNF-heterozygous mice. Eur. J. Neurosci. 24 3519–3531. 10.1111/j.1460-9568.2006.05242.x [DOI] [PubMed] [Google Scholar]
- Achterberg K. G., Buitendijk G. H., Kool M. J., Goorden S. M., Post L., Slump D. E., et al. (2014). Temporal and region-specific requirements of αCaMKII in spatial and contextual learning. J. Neurosci. 34 11180–11187. 10.1523/JNEUROSCI.0640-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmari S. E., Smith S. J. (2002). Knowing a nascent synapse when you see it. Neuron 34 333–336. 10.1016/s0896-6273(02)00685-2 [DOI] [PubMed] [Google Scholar]
- Ahmed M. E., Dong Y., Lu Y., Tucker D., Wang R., Zhang Q. (2017). Beneficial effects of a CaMKIIα inhibitor TatCN21 peptide in global cerebral ischemia. J. Mole. Neurosci. 61 42–51. 10.1007/s12031-016-0830-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akita T., Aoto K., Kato M., Shiina M., Mutoh H., Nakashima M., et al. (2018). De novo variants in CAMK2A and CAMK2B cause neurodevelopmental disorders. Ann. Clin. Transl. Neurol. 5 280–296. 10.1002/acn3.528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkadhi K. A. (2021). NMDA receptor-independent LTP in mammalian nervous system. Prog. Neurob. 200:101986. 10.1016/j.pneurobio.2020.101986 [DOI] [PubMed] [Google Scholar]
- Almeida C. G., Tampellini D., Takahashi R. H., Greengard P., Lin M. T., Snyder E. M., et al. (2005). Beta-amyloid accumulation in APP mutant neurons reduces PSD-95 and GluR1 in synapses. Neurobiol. Dis. 20 187–198. 10.1016/j.nbd.2005.02.008 [DOI] [PubMed] [Google Scholar]
- Ament S. A., Szelinger S., Glusman G., Ashworth J., Hou L., Akula N., et al. (2015). Rare variants in neuronal excitability genes influence risk for bipolar disorder. Proc. Natl. Acad. Sci. 112 3576–3581. 10.1073/pnas.1424958112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. E. (2015). Oxidant stress promotes disease by activating CaMKII. J. Mole. Cell. Cardiol. 89 160–167. 10.1016/j.yjmcc.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki S., Osuka K., Takata T., Tsuchiya Y., Watanabe Y. (2020). Coordination between Calcium/Calmodulin-Dependent Protein Kinase II and Neuronal Nitric Oxide Synthase in Neurons. Internat. J. Mole. Sci. 21:7997. 10.3390/ijms21217997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y., Zeng M., Zhang M., Huganir R. L. (2015). Rapid dispersion of SynGAP from synaptic spines triggers AMPA receptor insertion and spine enlargement during LTP. Neuron 85 173–189. 10.1016/j.neuron.2014.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrazola M. S., Court F. A. (2019). Compartmentalized necroptosis activation in excitotoxicity-induced axonal degeneration: a novel mechanism implicated in neurodegenerative disease pathology. Neur. Regenerat. Res. 14:1385. 10.4103/1673-5374.253520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole N. M., Chawla A. R., Martin M. P., Brustovetsky T., Brustovetsky N., Hudmon A. (2013). Loss of calcium/calmodulin-dependent protein kinase II activity in cortical astrocytes decreases glutamate uptake and induces neurotoxic release of ATP. J. Biol. Chem. 288 14599–14611. 10.1074/jbc.M113.466235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashpole N. M., Hudmon A. (2011). Excitotoxic neuroprotection and vulnerability with CaMKII inhibition. Mole. Cell. Neurosci. 46 720–730. 10.1016/j.mcn.2011.02.003 [DOI] [PubMed] [Google Scholar]
- Ashpole N. M., Song W., Brustovetsky T., Engleman E. A., Brustovetsky N., Cummins T. R., et al. (2012). Calcium/calmodulin-dependent protein kinase II (CaMKII) inhibition induces neurotoxicity via dysregulation of glutamate/calcium signaling and hyperexcitability. J. Biol. Chem. 287 8495–8506. 10.1074/jbc.M111.323915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf S. I., McLoon A. L., Sclarsic S. M., Kunes S. (2006). Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell 124 191–205. 10.1016/j.cell.2005.12.017 [DOI] [PubMed] [Google Scholar]
- Asrican B., Lisman J., Otmakhov N. (2007). Synaptic strength of individual spines correlates with bound ca2+–calmodulin-dependent kinase ii. J. Neurosci. 27 14007–14011. 10.1523/JNEUROSCI.3587-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins C. M., Chen S., Alonso O. F., Dietrich W. D., Hu B. R. (2006). Activation of calcium/calmodulin-dependent protein kinases after traumatic brain injury. J. Cereb. Blood Flow Metab. 26 1507–1518. 10.1038/sj.jcbfm.9600301 [DOI] [PubMed] [Google Scholar]
- Bach M. E., Hawkins R. D., Osman M., Kandel E. R., Mayford M. (1995). Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the θ frequency. Cell 81 905–915. 10.1016/0092-8674(95)90010-1 [DOI] [PubMed] [Google Scholar]
- Bachstetter A. D., Webster S. J., Tu T., Goulding D. S., Haiech J., Watterson D. M., et al. (2014). Generation and behavior characterization of CaMKIIβ knockout mice. PLoS One 9:e105191. 10.1371/journal.pone.0105191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltaci S. B., Mogulkoc R., Baltaci A. K. (2019). Molecular mechanisms of early and late LTP. Neurochem. Res. 44 281–296. 10.1007/s11064-018-2695-4 [DOI] [PubMed] [Google Scholar]
- Barria A., Derkach V., Soderling T. (1997a). Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the α-amino-3-hydroxyl-5-methyl 4-isoxazole-propionate-type glutamate receptor. J. Biol. Chem. 272 32727–32730. 10.1074/jbc.272.52.32727 [DOI] [PubMed] [Google Scholar]
- Barria A., Muller D., Derkach V., Griffith L. C., Soderling T. R. (1997b). Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276 2042–2045. 10.1126/science.276.5321.2042 [DOI] [PubMed] [Google Scholar]
- Barria A., Malinow R. (2005). NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48 289–301. 10.1016/j.neuron.2005.08.034 [DOI] [PubMed] [Google Scholar]
- Bayer K. U., LeBel E., McDonald G. L., O’Leary H., Schulman H., De Koninck P. (2006). Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. J. Neurosci. 26 1164–1174. 10.1523/JNEUROSCI.3116-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer K. U., Löhler J., Schulman H., Harbers K. (1999). Developmental expression of the CaM kinase II isoforms: ubiquitous γ-and δ-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Mole. Brain Res. 70 147–154. 10.1016/s0169-328x(99)00131-x [DOI] [PubMed] [Google Scholar]
- Bayer K. U., Schulman H. (2019). CaM kinase: still inspiring at 40. Neuron 103 380–394. 10.1016/j.neuron.2019.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer K. U. D. K., De Koninck P., Leonard A. S., Hell J. W., Schulman H. (2001). Interaction with the NMDA receptor locks CaMKII in an active conformation. Nature 411 801–805. 10.1038/35081080 [DOI] [PubMed] [Google Scholar]
- Bejar R., Yasuda R., Krugers H., Hood K., Mayford M. (2002). Transgenic calmodulin-dependent protein kinase II activation: dose-dependent effects on synaptic plasticity, learning, and memory. J. Neurosci. 22 5719–5726. 10.1523/JNEUROSCI.22-13-05719.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya M., Karandur D., Kuriyan J. (2020). Structural insights into the regulation of Ca2+/calmodulin-dependent protein kinase II (CaMKII). Cold Spring Harb. Perspect. Biol. 12:a035147. 10.1101/cshperspect.a035147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V. P., Collingridge G. L. (2019). Persistent memories of long-term potentiation and the N-methyl-d-aspartate receptor. Brain Neurosci. Adv. 3:2398212819848213. 10.1177/2398212819848213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum D., Herrera F., Francelle L., Mendes T., Basquin M., Obriot H., et al. (2015). Mutant huntingtin alters Tau phosphorylation and subcellular distribution. Hum. Molec. Genet. 24 76–85. 10.1093/hmg/ddu421 [DOI] [PubMed] [Google Scholar]
- Borgesius N. Z., van Woerden G. M., Buitendijk G. H., Keijzer N., Jaarsma D., Hoogenraad C. C., et al. (2011). βCaMKII plays a nonenzymatic role in hippocampal synaptic plasticity and learning by targeting αCaMKII to synapses. J. Neurosci. 31 10141–10148. 10.1523/JNEUROSCI.5105-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito V., Giralt A., Enriquez-Barreto L., Puigdellívol M., Suelves N., Zamora-Moratalla A., et al. (2014). Neurotrophin receptor p75 NTR mediates Huntington’s disease-associated synaptic and memory dysfunction. J. Clin. Invest. 124 4411–4428. 10.1172/JCI74809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks I. M., Tavalin S. J. (2011). Ca2+/calmodulin-dependent protein kinase II inhibitors disrupt AKAP79-dependent PKC signaling to GluA1 AMPA receptors. J. Biol. Chem. 286 6697–6706. 10.1074/jbc.M110.183558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Deutch A. Y., Colbran R. J. (2005). Dopamine depletion alters phosphorylation of striatal proteins in a model of Parkinsonism. Eur. J. Neurosci. 22 247–256. 10.1111/j.1460-9568.2005.04190.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. N., Cook S. G., Allen H. F., Crosby K. C., Singh T., Coultrap S. J., et al. (2021). Characterization of six CaMKIIα variants found in patients with schizophrenia. Iscience 24:103184. 10.1016/j.isci.2021.103184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne G. J., Proud C. G. (2004). A novel mTOR-regulated phosphorylation site in elongation factor 2 kinase modulates the activity of the kinase and its binding to calmodulin. Molec. Cell. Biol. 24 2986–2997. 10.1128/MCB.24.7.2986-2997.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buard I., Coultrap S. J., Freund R. K., Lee Y. S., Dell’Acqua M. L., Silva A. J., et al. (2010). CaMKII “autonomy” is required for initiating but not for maintaining neuronal long-term information storage. J. Neurosci. 30 8214–8220. 10.1523/JNEUROSCI.1469-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufill E., Roura-Poch P., Sala-Matavera I., Antón S., Lleó A., Sánchez-Saudinós B., et al. (2015). Reelin signaling pathway genotypes and Alzheimer disease in a Spanish population. Alzheimer Dis. Assoc. Dis. 29 169–172. 10.1097/WAD.0000000000000002 [DOI] [PubMed] [Google Scholar]
- Buonarati O. R., Cook S. G., Goodell D. J., Chalmers N. E., Rumian N. L., Tullis J. E., et al. (2020). CaMKII versus DAPK1 binding to GluN2B in ischemic neuronal cell death after resuscitation from cardiac arrest. Cell Rep. 30 1–8. 10.1016/j.celrep.2019.11.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonarati O. R., Miller A. P., Coultrap S. J., Bayer K. U., Reichow S. L. (2021). Conserved and divergent features of neuronal CaMKII holoenzyme structure, function, and high-order assembly. Cell Rep. 37:110168. 10.1016/j.celrep.2021.110168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin K. E., Waxham M. N., Rickling S., Westgate S. A., Mobley W. C., Kelly P. T. (1990). In situ hybridization histochemistry of Ca2+/calmodulin-dependent protein kinase in developing rat brain. J. Neurosci. 10 1788–1798. 10.1523/JNEUROSCI.10-06-01788.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler L. S., Silva A. J., Abeliovich A., Watanabe Y., Tonegawa S., McNamara J. O. (1995). Limbic epilepsy in transgenic mice carrying a Ca2+/calmodulin-dependent kinase II alpha-subunit mutation. Proc. Natl. Acad. Sci. 92 6852–6855. 10.1073/pnas.92.15.6852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacucci F., Wills T. J., Lever C., Giese K. P., O’Keefe J. (2007). Experience-dependent increase in CA1 place cell spatial information, but not spatial reproducibility, is dependent on the autophosphorylation of the α-isoform of the calcium/calmodulin-dependent protein kinase II. J. Neurosci. 27 7854–7859. 10.1523/jneurosci.1704-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers N. E., Yonchek J., Steklac K. E., Ramsey M., Bayer K. U., Herson P. S., et al. (2020). Calcium/calmodulin-dependent kinase (CaMKII) inhibition protects against Purkinje cell damage following CA/CPR in mice. Mole. Neurob. 57 150–158. 10.1007/s12035-019-01765-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamoun R., Suki D., Gopinath S. P., Goodman J. C., Robertson C. (2010). Role of extracellular glutamate measured by cerebral microdialysis in severe traumatic brain injury. J. Neurosurg. 113 564–570. 10.3171/2009.12.JNS09689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chana G., Laskaris L., Pantelis C., Gillett P., Testa R., Zantomio D., et al. (2015). Decreased expression of mGluR5 within the dorsolateral prefrontal cortex in autism and increased microglial number in mGluR5 knockout mice: Pathophysiological and neurobehavioral implications. Brain Behav. Imm. 49 197–205. 10.1016/j.bbi.2015.05.009 [DOI] [PubMed] [Google Scholar]
- Chandran R., Vijayan D., Reddy E. K., Kumar M., Kesavan L., Jacob R., et al. (2021). Neuroprotective derivatives of tacrine that target NMDA receptor and acetylcholinesterase-Design, synthesis and biological evaluation. Comp. Struct. Biotechnol. J. 19 4517–4537. 10.1016/j.csbj.2021.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B. H., Mukherji S., Soderling T. R. (1998). Characterization of a calmodulin kinase II inhibitor protein in brain. Proc. Natl. Acad. Sci. 95 10890–10895. 10.1073/pnas.95.18.10890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. Y., Parra-Bueno P., Laviv T., Szatmari E. M., Lee S. J. R., Yasuda R. (2017). CaMKII autophosphorylation is necessary for optimal integration of Ca2+ signals during LTP induction, but not maintenance. Neuron 94 800–808. 10.1016/j.neuron.2017.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P. F., Frenguelli B. G., Smith A., Chen C. M., Silva A. J. (1995). The α-Ca2+/calmodulin kinase II: a bidirectional modulator of presynaptic plasticity. Neuron 14 591–597. 10.1016/0896-6273(95)90315-1 [DOI] [PubMed] [Google Scholar]
- Chawla A. R., Johnson D. E., Zybura A. S., Leeds B. P., Nelson R. M., Hudmon A. (2017). Constitutive regulation of the glutamate/aspartate transporter EAAT 1 by Calcium-Calmodulin-Dependent Protein Kinase II. J. Neurochem. 140 421–434. 10.1111/jnc.13913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B. S., Roche K. W. (2007). Regulation of NMDA receptors by phosphorylation. Neuropharmacology 53 362–368. 10.1016/j.neuropharm.2007.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Rainnie D. G., Greene R. W., Tonegawa S. (1994). Abnormal fear response and aggressive behavior in mutant mice deficient for alpha-calcium-calmodulin kinase II. Science 266 291–294. 10.1126/science.7939668 [DOI] [PubMed] [Google Scholar]
- Chen T. T., Zhou X., Xu Y. N., Li Y., Wu X. Y., Xiang Q., et al. (2021). Gastrodin ameliorates learning and memory impairment in rats with vascular dementia by promoting autophagy flux via inhibition of the Ca2+/CaMKII signal pathway. Aging 13:9542. 10.18632/aging.202667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyan J., Kumar P., Mayadevi M., Surolia A., Omkumar R. V. (2011). Calcium/calmodulin dependent protein kinase II bound to NMDA receptor 2B subunit exhibits increased ATP affinity and attenuated dephosphorylation. PLoS One 6:e16495. 10.1371/journal.pone.0016495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheriyan J., Mohanan A. G., Kurup P. K., Mayadevi M., Omkumar R. V. (2012). Effect of multimeric structure of CaMKII in the GluN2B-mediated modulation of kinetic parameters of ATP. PLoS one 7:e45064. 10.1371/journal.pone.0045064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia P. H., Zhong F. L., Niwa S., Bonnard C., Utami K. H., Zeng R., et al. (2018). A homozygous loss-of-function CAMK2A mutation causes growth delay, frequent seizures and severe intellectual disability. Elife 7:e32451. 10.7554/eLife.32451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocchetti A. G., Yousaf A., Bour H. S., Haslinger D., Waltes R., Duketis E., et al. (2018). Common functional variants of the glutamatergic system in Autism spectrum disorder with high and low intellectual abilities. J. Neural Trans. 125 259–271. 10.1007/s00702-017-1813-9 [DOI] [PubMed] [Google Scholar]
- Cho M. H., Cao X., Wang D., Tsien J. Z. (2007). Dentate gyrus-specific manipulation of β-Ca2+/calmodulin-dependent kinase II disrupts memory consolidation. Proc. Natl. Sci. 104 16317–16322. 10.1073/pnas.0703344104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y. H., Giese K. P., Tanila H., Silva A. J., Eichenbaum H. (1998). Abnormal hippocampal spatial representations in αCaMKIIT286A and CREBαΔ− mice. Science 279 867–869. 10.1126/science.279.5352.867 [DOI] [PubMed] [Google Scholar]
- Chotiner J. K., Khorasani H., Nairn A. C., O’dell T. J., Watson J. B. (2003). Adenylyl cyclase-dependent form of chemical long-term potentiation triggers translational regulation at the elongation step. Neuroscience 116 743–752. 10.1016/s0306-4522(02)00797-2 [DOI] [PubMed] [Google Scholar]
- Churn S. B., Sombati S., Jakoi E. R., Sievert L., DeLorenzo R. J. (2000). Inhibition of calcium/calmodulin kinase II alpha subunit expression results in epileptiform activity in cultured hippocampal neurons. Proc. Natl. Acad. Sci. 97 5604–5609. 10.1073/pnas.080071697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. M., Suutari B., He X., Wang Y., Sanchez S., Tirko N. N., et al. (2018). Calmodulin shuttling mediates cytonuclear signaling to trigger experience-dependent transcription and memory. Nat. Comm. 9 1–12. 10.1038/s41467-018-04705-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colicos M. A., Collins B. E., Sailor M. J., Goda Y. (2001). Remodeling of synaptic actin induced by photoconductive stimulation. Cell 107 605–616. 10.1016/s0092-8674(01)00579-7 [DOI] [PubMed] [Google Scholar]
- Cook S. G., Buonarati O. R., Coultrap S. J., Bayer K. U. (2021). CaMKII holoenzyme mechanisms that govern the LTP versus LTD decision. Sci. Adv. 7:eabe2300. 10.1126/sciadv.abe2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S. G., Goodell D. J., Restrepo S., Arnold D. B., Bayer K. U. (2019). Simultaneous live imaging of multiple endogenous proteins reveals a mechanism for Alzheimer’s-related plasticity impairment. Cell Rep. 27 658–665. 10.1016/j.celrep.2019.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke S. F., Wu J., Plattner F., Errington M., Rowan M., Peters M., et al. (2006). Autophosphorylation of αCaMKII is not a general requirement for NMDA receptor-dependent LTP in the adult mouse. J. Phys. 574 805–818. 10.1113/jphysiol.2006.111559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap S. J., Barcomb K., Bayer K. U. (2012). A significant but rather mild contribution of T286 autophosphorylation to Ca2+/CaM-stimulated CaMKII activity. PLoS One 7:e37176. 10.1371/journal.pone.0037176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap S. J., Bayer K. U. (2011). Improving a natural CaMKII inhibitor by random and rational design. PLoS One 6:e25245. 10.1371/journal.pone.0025245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap S. J., Bayer K. U. (2014). Nitric oxide induces Ca2+-independent activity of the Ca2+/calmodulin-dependent protein kinase II (CaMKII). J. Biol. Chem. 289 19458–19465. 10.1074/jbc.M114.558254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap S. J., Freund R. K., O’Leary H., Sanderson J. L., Roche K. W., Dell’Acqua M. L., et al. (2014). Autonomous CaMKII mediates both LTP and LTD using a mechanism for differential substrate site selection. Cell Rep. 6 431–437. 10.1016/j.celrep.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap S. J., Vest R. S., Ashpole N. M., Hudmon A., Bayer K. U. (2011). CaMKII in cerebral ischemia. Acta Pharm. Sin. 32 861–872. 10.1038/aps.2011.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crino P. B., Eberwine J. (1996). Molecular characterization of the dendritic growth cone: regulated mRNA transport and local protein synthesis. Neuron 17 1173–1187. 10.1016/s0896-6273(00)80248-2 [DOI] [PubMed] [Google Scholar]
- Davis H. P., Squire L. R. (1984). Protein synthesis and memory: a review. Psychol. Bull. 96:518. 10.1037/0033-2909.96.3.518 [DOI] [PubMed] [Google Scholar]
- Day M., Wang Z., Ding J., An X., Ingham C. A., Shering A. F., et al. (2006). Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat. Neurosci. 9 251–259. 10.1038/nn1632 [DOI] [PubMed] [Google Scholar]
- De Koninck P., Schulman H. (1998). Sensitivity of CaMKII to the frequency of Ca2+ oscillations. Science 279 227–230. [DOI] [PubMed] [Google Scholar]
- De Ligt J., Willemsen M. H., Van Bon B. W., Kleefstra T., Yntema H. G., Kroes T., et al. (2012). Diagnostic exome sequencing in persons with severe intellectual disability. New Engl. J. Med. 367 1921–1929. 10.1056/NEJMoa1206524 [DOI] [PubMed] [Google Scholar]
- Deckel A. W., Gordinier A., Nuttal D., Tang V., Kuwada C., Freitas R., et al. (2001). Reduced activity and protein expression of NOS in R6/2 HD transgenic mice: effects of L-NAME on symptom progression. Brain Res. 919 70–81. 10.1016/s0006-8993(01)03000-1 [DOI] [PubMed] [Google Scholar]
- Deckel A. W., Tang V., Nuttal D., Gary K., Elder R. (2002a). Altered neuronal nitric oxide synthase expression contributes to disease progression in Huntington’s disease transgenic mice. Brain Res. 939 76–86. 10.1016/s0006-8993(02)02550-7 [DOI] [PubMed] [Google Scholar]
- Deckel A. W, Elder R., Fuhrer G. (2002b). Biphasic developmental changes in Ca2+/calmodulin-dependent proteins in R6/2 Huntington’s disease mice. Neuroreport 13 707–711. 10.1097/00001756-200204160-00034 [DOI] [PubMed] [Google Scholar]
- Deng G., Orfila J. E., Dietz R. M., Moreno-Garcia M., Rodgers K. M., Coultrap S. J., et al. (2017). Autonomous CaMKII activity as a drug target for histological and functional neuroprotection after resuscitation from cardiac arrest. Cell Rep. 18 1109–1117. 10.1016/j.celrep.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V., Barria A., Soderling T. R. (1999). Ca2+/calmodulin-kinase II enhances channel conductance of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc. Natl. Acad. Sci. 96 3269–3274. 10.1073/pnas.96.6.3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande L. S., Sun D. A., Sombati S., Baranova A., Wilson M. S., Attkisson E., et al. (2008). Alterations in neuronal calcium levels are associated with cognitive deficits after traumatic brain injury. Neurosci. Lett. 441 115–119. 10.1016/j.neulet.2008.05.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch A. Y. (2006). Striatal plasticity in parkinsonism: dystrophic changes in medium spiny neurons and progression in Parkinson’s disease. Parkins. Dis. Relat. Dis. 2006 67–70. 10.1007/978-3-211-45295-0_12 [DOI] [PubMed] [Google Scholar]
- Di Biase V., Obermair G. J., Szabo Z., Altier C., Sanguesa J., Bourinet E., et al. (2008). Stable membrane expression of postsynaptic CaV1. 2 calcium channel clusters is independent of interactions with AKAP79/150 and PDZ proteins. J. Neurosci. 28 13845–13855. 10.1523/JNEUROSCI.3213-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo F., Motta C., Casula E. P., Bonnì S., Assogna M., Caltagirone C., et al. (2020). LTP-like cortical plasticity predicts conversion to dementia in patients with memory impairment. Brain Stimul. 13 1175–1182. 10.1016/j.brs.2020.05.013 [DOI] [PubMed] [Google Scholar]
- Ding J. D., Kennedy M. B., Weinberg R. J. (2013). Subcellular organization of camkii in rat hippocampal pyramidal neurons. J. Comp. Neurol. 521 3570–3583. 10.1002/cne.23372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominique J. F., Papassotiropoulos A. (2006). Identification of a genetic cluster influencing memory performance and hippocampal activity in humans. Proc. Natl. Acad. Sci. 103 4270–4274. 10.1073/pnas.0510212103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton A. C., Lourdusamy A., Loth E., Torro R., Giese K. P., Kornhuber J., et al. (2013). CAMK2A polymorphisms predict working memory performance in humans. Mole. Psych. 18 850–852. 10.1038/mp.2012.114 [DOI] [PubMed] [Google Scholar]
- Elgersma Y., Fedorov N. B., Ikonen S., Choi E. S., Elgersma M., Carvalho O. M., et al. (2002). Inhibitory autophosphorylation of CaMKII controls PSD association, plasticity, and learning. Neuron 36 493–505. 10.1016/s0896-6273(02)01007-3 [DOI] [PubMed] [Google Scholar]
- Erondu N. E., Kennedy M. B. (1985). Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J. Neurosci. 5 3270–3277. 10.1523/JNEUROSCI.05-12-03270.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas G. C., Raghavachari S., Lisman J. E., Mody I. (2011). Calmodulin as a direct detector of Ca 2+ signals. Nat. Neurosci. 14 301–304. 10.1038/nn.2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden A. I., Demediuk P., Panter S. S., Vink R. (1989). The role of excitatory amino acids and NMDA receptors in traumatic brain injury. Science 244 798–800. 10.1126/science.2567056 [DOI] [PubMed] [Google Scholar]
- Fan W., Agarwal N., Cooper N. G. (2006). The role of CaMKII in BDNF-mediated neuroprotection of retinal ganglion cells (RGC-5). Brain Res. 1067 48–57. 10.1016/j.brainres.2005.10.030 [DOI] [PubMed] [Google Scholar]
- Fang X., Tang W., Yang F., Lu W., Cai J., Ni J., et al. (2019). A comprehensive analysis of the CaMK2A gene and susceptibility to Alzheimer’s disease in the Han Chinese population. Front. Aging Neurosci. 11:84. 10.3389/fnagi.2019.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinelli M., Heitz F. D., Grewe B. F., Tyagarajan S. K., Helmchen F., Mansuy I. M. (2012). Selective regulation of NR2B by protein phosphatase-1 for the control of the NMDA receptor in neuroprotection. PLoS One 7:e34047. 10.1371/journal.pone.0034047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figurov A., Pozzo-Miller L. D., Olafsson P., Wang T., Lu B. (1996). Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature 381 706–709. 10.1038/381706a0 [DOI] [PubMed] [Google Scholar]
- Fink C. C., Bayer K. U., Myers J. W., Ferrell J. E., Jr., Schulman H., Meyer T. (2003). Selective regulation of neurite extension and synapse formation by the β but not the α isoform of CaMKII. Neuron 39 283–297. 10.1016/s0896-6273(03)00428-8 [DOI] [PubMed] [Google Scholar]
- Fischer M., Kaech S., Knutti D., Matus A. (1998). Rapid actin-based plasticity in dendritic spines. Neuron 20 847–854. 10.1016/s0896-6273(00)80467-5 [DOI] [PubMed] [Google Scholar]
- Folkerts M. M., Parks E. A., Dedman J. R., Kaetzel M. A., Lyeth B. G., Berman R. F. (2007). Phosphorylation of calcium calmodulin-dependent protein kinase II following lateral fluid percussion brain injury in rats. J. Neurotr. 24 638–650. 10.1089/neu.2006.0188 [DOI] [PubMed] [Google Scholar]
- Frankland P. W., Bontempi B., Talton L. E., Kaczmarek L., Silva A. J. (2004). The involvement of the anterior cingulate cortex in remote contextual fear memory. Science 304 881–883. 10.1126/science.1094804 [DOI] [PubMed] [Google Scholar]
- Freund T. F., Powell J. F., Smith A. (1984). Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience 13 1189–1215. 10.1016/0306-4522(84)90294-x [DOI] [PubMed] [Google Scholar]
- Gamache T. R., Araki Y., Huganir R. L. (2020). Twenty years of SynGAP research: from synapses to cognition. J. Neurosci. 40 1596–1605. 10.1523/JNEUROSCI.0420-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F., Bellone C. (2015). Modulation of the glutamatergic transmission by Dopamine: a focus on Parkinson, Huntington and Addiction diseases. Front. Cell. Neurosci. 9:25. 10.3389/fncel.2015.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardoni F., Ghiglieri V., Di Luca M., Calabresi P. (2010). Assemblies of glutamate receptor subunits with post-synaptic density proteins and their alterations in Parkinson’s disease. Prog. Brain Res. 183 169–182. 10.1016/S0079-6123(10)83009-2 [DOI] [PubMed] [Google Scholar]
- Gardoni F., Mauceri D., Fiorentini C., Bellone C., Missale C., Cattabeni F., et al. (2003). CaMKII-dependent phosphorylation regulates SAP97/NR2A interaction. J. Biol. Chem. 278 44745–44752. 10.1074/jbc.M303576200 [DOI] [PubMed] [Google Scholar]
- Gardoni F., Polli F., Cattabeni F., Di Luca M. (2006). Calcium–calmodulin-dependent protein kinase II phosphorylation modulates PSD-95 binding to NMDA receptors. Eur. J. Neurosci. 24 2694–2704. 10.1111/j.1460-9568.2006.05140.x [DOI] [PubMed] [Google Scholar]
- Gardoni F., Schrama L. H., Van Dalen J. J. W., Gispen W. H., Cattabeni F., Di Luca M. (1999). αCaMKII binding to the C-terminal tail of NMDA receptor subunit NR2A and its modulation by autophosphorylation. FEBS Lett. 456 394–398. 10.1016/s0014-5793(99)00985-0 [DOI] [PubMed] [Google Scholar]
- Ghosh A., Giese K. P. (2015). Calcium/calmodulin-dependent kinase II and Alzheimer’s disease. Mole. Brain 8 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese K. P. (2021). The role of CaMKII autophosphorylation for NMDA receptor-dependent synaptic potentiation. Neuropharmacology 193:108616. 10.1016/j.neuropharm.2021.108616 [DOI] [PubMed] [Google Scholar]
- Giese K. P., Fedorov N. B., Filipkowski R. K., Silva A. J. (1998). Autophosphorylation at Thr286 of the α calcium-calmodulin kinase II in LTP and learning. Science 279 870–873. 10.1126/science.279.5352.870 [DOI] [PubMed] [Google Scholar]
- Giovannini M. G., Blitzer R. D., Wong T., Asoma K., Tsokas P., Morrison J. H., et al. (2001). Mitogen-activated protein kinase regulates early phosphorylation and delayed expression of Ca2+/calmodulin-dependent protein kinase II in long-term potentiation. J. Neurosci. 21 7053–7062. 10.1523/JNEUROSCI.21-18-07053.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt A., Saavedra A., Alberch J., Pérez-Navarro E. (2012). Cognitive dysfunction in Huntington’s disease: humans, mouse models and molecular mechanisms. J. Hunting. Dis. 1 155–173. 10.3233/JHD-120023 [DOI] [PubMed] [Google Scholar]
- Glazewski S., Bejar R., Mayford M., Fox K. (2001). The effect of autonomous alpha-CaMKII expression on sensory responses and experience-dependent plasticity in mouse barrel cortex. Neuropharmacology 41 771–778. 10.1016/s0028-3908(01)00097-1 [DOI] [PubMed] [Google Scholar]
- Goebel D. J. (2009). Selective blockade of CaMKII-α inhibits NMDA-induced caspase-3-dependent cell death but does not arrest PARP-1 activation or loss of plasma membrane selectivity in rat retinal neurons. Brain Res. 1256 190–204. 10.1016/j.brainres.2008.12.051 [DOI] [PubMed] [Google Scholar]
- Gomez-Monterrey I., Sala M., Rusciano M. R., Monaco S., Maione A. S., Iaccarino G., et al. (2013). Characterization of a selective CaMKII peptide inhibitor. Eur. J. Med. Chem. 62 425–434. 10.1016/j.ejmech.2012.12.053 [DOI] [PubMed] [Google Scholar]
- Goodell D. J., Zaegel V., Coultrap S. J., Hell J. W., Bayer K. U. (2017). DAPK1 mediates LTD by making CaMKII/GluN2B binding LTP specific. Cell Rep. 19 2231–2243. 10.1016/j.celrep.2017.05.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. A., Cioffi D., Silva A. J., Stryker M. P. (1996). Deficient plasticity in the primary visual cortex of α-calcium/calmodulin-dependent protein kinase II mutant mice. Neuron 17 491–499. 10.1016/s0896-6273(00)80181-6 [DOI] [PubMed] [Google Scholar]
- Gottschalk W., Pozzo-Miller L. D., Figurov A., Lu B. (1998). Presynaptic modulation of synaptic transmission and plasticity by brain-derived neurotrophic factor in the developing hippocampus. J. Neurosci. 18 6830–6839. 10.1523/JNEUROSCI.18-17-06830.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratuze M., Noël A., Julien C., Cisbani G., Milot-Rousseau P., Morin F., et al. (2015). Tau hyperphosphorylation and deregulation of calcineurin in mouse models of Huntington’s disease. Hum. Mole. Genet. 24 86–99. 10.1093/hmg/ddu456 [DOI] [PubMed] [Google Scholar]
- Grover L. M., Teyler T. J. (1990). Two components of long-term potentiation induced by different patterns of afferent activation. Nature 347 477–479. 10.1038/347477a0 [DOI] [PubMed] [Google Scholar]
- Gu S. X., Blokhin I. O., Wilson K. M., Dhanesha N., Doddapattar P., Grumbach I. M., et al. (2016). Protein methionine oxidation augments reperfusion injury in acute ischemic stroke. JCI Insight 1:7. 10.1172/jci.insight.86460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Liu W., Yan Z. (2009). β-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J. Biol. Chem. 284 10639–10649. 10.1074/jbc.M806508200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasekaran S., Jacob R. S., Omkumar R. V. (2022). Differential expression of miR-148b, miR-129-2 and miR-296 in animal models of schizophrenia-Relevance to NMDA receptor hypofunction. Neuropharmacology 2022:109024. 10.1016/j.neuropharm.2022.109024 [DOI] [PubMed] [Google Scholar]
- Gurd J. W., Rawof S., Huo J. Z., Dykstra C., Bissoon N., Teves L., et al. (2008). Ischemia and status epilepitcus result in enhanced phosphorylation of calcium and calmodulin-stimulated protein kinase II on threonine 253. Brain Res. 1218 158–165. 10.1016/j.brainres.2008.04.040 [DOI] [PubMed] [Google Scholar]
- Gustin R. M., Shonesy B. C., Robinson S. L., Rentz T. J., Baucum A. J., II, Jalan-Sakrikar N. (2011). Loss of Thr286 phosphorylation disrupts synaptic CaMKIIα targeting, NMDAR activity and behavior in pre-adolescent mice. Mole. Cell. Neurosci. 47 286–292. 10.1016/j.mcn.2011.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner A. S., Penn A. C., Grillo-Bosch D., Retailleau N., Poujol C., Philippat A., et al. (2015). Lengthening of the stargazin cytoplasmic tail increases synaptic transmission by promoting interaction to deeper domains of PSD-95. Neuron 86 475–489. 10.1016/j.neuron.2015.03.013 [DOI] [PubMed] [Google Scholar]
- Hao Z. B., Pei D. S., Guan Q. H., Zhang G. Y. (2005). Calcium/calmodulin-dependent protein kinase II (CaMKII), through NMDA receptors and L-Voltage-gated channels, modulates the serine phosphorylation of GluR6 during cerebral ischemia and early reperfusion period in rat hippocampus. Molecular Brain Res. 140 55–62. 10.1016/j.molbrainres.2005.07.005 [DOI] [PubMed] [Google Scholar]
- Hasegawa S., Furuichi T., Yoshida T., Endoh K., Kato K., Sado M., et al. (2009). Transgenic up-regulation of alpha-CaMKII in forebrain leads to increased anxiety-like behaviors and aggression. Mole. Brain 2 1–11. 10.1186/1756-6606-2-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Shi S. H., Esteban J. A., Piccini A., Poncer J. C., Malinow R. (2000). Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287 2262–2267. 10.1126/science.287.5461.2262 [DOI] [PubMed] [Google Scholar]
- He X., Li J., Zhou G., Yang J., McKenzie S., Li Y., et al. (2021). Gating of hippocampal rhythms and memory by synaptic plasticity in inhibitory interneurons. Neuron 109 1013–1028. 10.1016/j.neuron.2021.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman P., Drewes S., Ghaloul-Gonzalez L. (2021). A familial case of CAMK2B mutation with variable expressivity. SAGE Open Med. Case Rep. 9:2050313X21990982. 10.1177/2050313X21990982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell J. W. (2014). CaMKII: claiming center stage in postsynaptic function and organization. Neuron 81 249–265. 10.1016/j.neuron.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring B. E., Nicoll R. A. (2016). Long-term potentiation: from CaMKII to AMPA receptor trafficking. Annu. Rev. Phys. 78 351–365. 10.1146/annurev-physiol-021014-071753 [DOI] [PubMed] [Google Scholar]
- Hinds H. L., Goussakov I., Nakazawa K., Tonegawa S., Bolshakov V. Y. (2003). Essential function of α-calcium/calmodulin-dependent protein kinase II in neurotransmitter release at a glutamatergic central synapse. Proc. Natl. Acad. Sci. 100 4275–4280. 10.1073/pnas.0530202100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds H. L., Tonegawa S., Malinow R. (1998). CA1 long-term potentiation is diminished but present in hippocampal slices from α-CaMKII mutant mice. Learn. Mem. 5 344–354. 10.1101/lm.5.4.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelz A., Nairn A. C., Kuriyan J. (2003). Crystal structure of a tetradecameric assembly of the association domain of Ca2+/calmodulin-dependent kinase II. Mole. Cell 11 1241–1251. 10.1016/s1097-2765(03)00171-0 [DOI] [PubMed] [Google Scholar]
- Hojjati M. R., Van Woerden G. M., Tyler W. J., Giese K. P., Silva A. J., Pozzo-Miller L., et al. (2007). Kinase activity is not required for αCaMKII-dependent presynaptic plasticity at CA3-CA1 synapses. Nat. Neurosci. 10 1125–1127. 10.1038/nn1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka M., Hammer R. E., Südhof T. C. (1999). A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron 24 377–387. 10.1016/s0896-6273(00)80851-x [DOI] [PubMed] [Google Scholar]
- Hosokawa T., Liu P. W., Cai Q., Ferreira J. S., Levet F., Butler C., et al. (2021). CaMKII activation persistently segregates postsynaptic proteins via liquid phase separation. Nature Neurosci. 24 777–785. 10.1038/s41593-021-00843-3 [DOI] [PubMed] [Google Scholar]
- Hou S. T., Jiang S. X., Aylsworth A., Ferguson G., Slinn J., Hu H., et al. (2009). CaMKII phosphorylates collapsin response mediator protein 2 and modulates axonal damage during glutamate excitotoxicity. J. Neurochem. 111 870–881. 10.1111/j.1471-4159.2009.06375.x [DOI] [PubMed] [Google Scholar]
- Hudmon A., Schulman H. (2002). Neuronal CA2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 71 473–510. 10.1146/annurev.biochem.71.110601.135410 [DOI] [PubMed] [Google Scholar]
- Hudmon A., Schulman H., Kim J., Maltez J. M., Tsien R. W., Pitt G. S. (2005). CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J. Cell Biol. 171 537–547. 10.1083/jcb.200505155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N., Goto H., Ogawara M., Nishi Y., Ando S., Inagaki M. (1997). Spatial patterns of Ca2+ signals define intracellular distribution of a signaling by Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 272 25195–25199. 10.1074/jbc.272.40.25195 [DOI] [PubMed] [Google Scholar]
- Iossifov I., O’roak B. J., Sanders S. J., Ronemus M., Krumm N., Levy D., et al. (2014). The contribution of de novo coding mutations to autism spectrum disorder. Nature 515 216–221. 10.1038/nature13908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine E. E., Danhiez A., Radwanska K., Nassim C., Lucchesi W., Godaux E., et al. (2011). Properties of contextual memory formed in the absence of αCaMKII autophosphorylation. Mole. Brain 4 1–10. 10.1186/1756-6606-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine E. E., Vernon J., Giese K. P. (2005). αCaMKII autophosphorylation contributes to rapid learning but is not necessary for memory. Nat. Neurosci. 8 411–412. 10.1038/nn1431 [DOI] [PubMed] [Google Scholar]
- Islam R., Matsuzaki K., Sumiyoshi E., Hossain M. E., Hashimoto M., Katakura M., et al. (2019). Theobromine improves working memory by activating the CaMKII/CREB/BDNF pathway in rats. Nutrients 11:888. 10.3390/nu11040888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwama K., Mizuguchi T., Takeshita E., Nakagawa E., Okazaki T., Nomura Y., et al. (2019). Genetic landscape of Rett syndrome-like phenotypes revealed by whole exome sequencing. J. Med. Genet. 56 396–407. 10.1136/jmedgenet-2018-105775 [DOI] [PubMed] [Google Scholar]
- Izumi H., Kawahata I., Shinoda Y., Helmstetter F. J., Fukunaga K. (2020). SAK3 administration improves spine abnormalities and cognitive deficits in appNL-GF/NL-GF knock-in mice by increasing proteasome activity through CaMKII/Rpt6 signalling. Internat. J. Mole. Sci. 21 3833. 10.3390/ijms21113833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y., Zorumski C. F. (1998). LTP in CA1 of the adult rat hippocampus and voltage-activated calcium channels. Neurorep. 9 3689–3691. 10.1097/00001756-199811160-00022 [DOI] [PubMed] [Google Scholar]
- Jackson A. C., Nicoll R. A. (2011). The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron 70 178–199. 10.1016/j.neuron.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Fang J., Xing J., Wang L., Wang Q., Wang Y., et al. (2019). Tilianin mediates neuroprotection against ischemic injury by attenuating CaMKII-dependent mitochondrion-mediated apoptosis and MAPK/NF-κB signalling. Life Sci. 216 233–245. 10.1016/j.lfs.2018.11.035 [DOI] [PubMed] [Google Scholar]
- Jiang Y. H., Ehlers M. D. (2013). Modeling autism by SHANK gene mutations in mice. Neuron 78 8–27. 10.1016/j.neuron.2013.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job C., Eberwine J. (2001). Localization and translation of mRNA in dentrites and axons. Nat. Rev. Neurosci. 2 889–898. 10.1038/35104069 [DOI] [PubMed] [Google Scholar]
- Jontes J. D., Buchanan J., Smith S. J. (2000). Growth cone and dendrite dynamics in zebrafish embryos: early events in synaptogenesis imaged in vivo. Nat. Neurosci. 3 231–237. 10.1038/72936 [DOI] [PubMed] [Google Scholar]
- Jontes J. D., Smith S. J. (2000). Filopodia, spines, and the generation of synaptic diversity. Neuron 27 11–14. 10.1016/s0896-6273(00)00003-9 [DOI] [PubMed] [Google Scholar]
- Kee N., Teixeira C. M., Wang A. H., Frankland P. W. (2007). Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat. Neurosci. 10 355–362. 10.1038/nn1847 [DOI] [PubMed] [Google Scholar]
- Khan S., Downing K. H., Molloy J. E. (2019). Architectural dynamics of CaMKII-actin networks. Biophys. J. 116 104–119. 10.1016/j.bpj.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Lakhanpal G., Lu H. E., Khan M., Suzuki A., Hayashi M. K., et al. (2015). A temporary gating of actin remodeling during synaptic plasticity consists of the interplay between the kinase and structural functions of CaMKII. Neuron 87 813–826. 10.1016/j.neuron.2015.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., Suzuki A., Kojima H., Kawamura M., Miya K., Abe M., et al. (2019). Autophosphorylation of F-actin binding domain of CaMKIIβ is required for fear learning. Neurobiol. Learn. Memory 157 86–95. 10.1016/j.nlm.2018.12.003 [DOI] [PubMed] [Google Scholar]
- Kirkwood A., Silva A., Bear M. F. (1997). Age-dependent decrease of synaptic plasticity in the neocortex of αCaMKII mutant mice. Proc. Natl. Acad. Sci. 94 3380–3383. 10.1073/pnas.94.7.3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann E., Dever T. E. (2004). Biochemical mechanisms for translational regulation in synaptic plasticity. Nat. Rev. Neurosci. 5 931–942. 10.1038/nrn1557 [DOI] [PubMed] [Google Scholar]
- Klug J. R., Mathur B. N., Kash T. L., Wang H. D., Matthews R. T., Robison A. J., et al. (2012). Genetic inhibition of CaMKII in dorsal striatal medium spiny neurons reduces functional excitatory synapses and enhances intrinsic excitability. PLoS One 7:e45323. 10.1371/journal.pone.0045323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan L. D., Churn S. B., Omojokun O., Rice A., DeLorenzo R. J. (1999). Status epilepticus results in an N-methyl-D-aspartate receptor-dependent inhibition of Ca2+/calmodulin-dependent kinase II activity in the rat. Neuroscience 95 735–743. 10.1016/s0306-4522(99)00462-5 [DOI] [PubMed] [Google Scholar]
- Koh Y. H., Popova E., Thomas U., Griffith L. C., Budnik V. (1999). Regulation of DLG localization at synapses by CaMKII-dependent phosphorylation. Cell 98 353–363. 10.1016/s0092-8674(00)81964-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H., Rosendale M., Sugiyama Y., Hayashi M., Horiguchi Y., Yoshihara T., et al. (2019). The role of CaMKII-Tiam1 complex on learning and memory. Neurobiol. Learn. Memory 166:107070. 10.1016/j.nlm.2019.107070 [DOI] [PubMed] [Google Scholar]
- Kool M. J., Onori M. P., Borgesius N. Z., van de Bree J. E., Elgersma-Hooisma M., Nio E., et al. (2019). CAMK2-dependent signaling in neurons is essential for survival. J. Neurosci. 39 5424–5439. 10.1523/JNEUROSCI.1341-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M. J., Van De Bree J. E., Bodde H. E., Elgersma Y., van Woerden G. M. (2016). The molecular, temporal and region-specific requirements of the beta isoform of Calcium/Calmodulin-dependent protein kinase type 2 (CAMK2B) in mouse locomotion. Scientif. Rep. 6 1–12. 10.1038/srep26989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M., Carroll P., Wolf E., Brem G., Thoenen H., Bonhoeffer T. (1995). Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. 92 8856–8860. 10.1073/pnas.92.19.8856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsokera M., Kafkalias P., Giompres P., Kouvelas E. D., Mitsacos A. (2014). Expression and phosphorylation of glutamate receptor subunits and CaMKII in a mouse model of Parkinsonism. Brain Res. 1549 22–31. 10.1016/j.brainres.2013.12.023 [DOI] [PubMed] [Google Scholar]
- Krapivinsky G., Medina I., Krapivinsky L., Gapon S., Clapham D. E. (2004). SynGAP-MUPP1-CaMKII synaptic complexes regulate p38 MAP kinase activity and NMDA receptor-dependent synaptic AMPA receptor potentiation. Neuron 43 563–574. 10.1016/j.neuron.2004.08.003 [DOI] [PubMed] [Google Scholar]
- Kumar M., John M., Madhavan M., James J., Omkumar R. V. (2019). Alteration in the phosphorylation status of NMDA receptor GluN2B subunit by activation of both NMDA receptor and L-type voltage gated calcium channel. Neurosci. Lett. 709:134343. 10.1016/j.neulet.2019.134343 [DOI] [PubMed] [Google Scholar]
- Küry S., van Woerden G. M., Besnard T., Onori M. P., Latypova X., Towne M. C., et al. (2017). De novo mutations in protein kinase genes CAMK2A and CAMK2B cause intellectual disability. Am. J. Hum. Genet. 101 768–788. 10.1016/j.ajhg.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamsa K., Irvine E. E., Giese K. P., Kullmann D. M. (2007). NMDA receptor-dependent long-term potentiation in mouse hippocampal interneurons shows a unique dependence on Ca2+/calmodulin-dependent kinases. J. Phys. 584 885–894. 10.1113/jphysiol.2007.137380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. C., Su M. T., Huang H. Y., Cho Y. C., Yeh T. K., Chang C. Y. (2021). Association of CaMK2A and MeCP2 signaling pathways with cognitive ability in adolescents. Mole. Brain 14 1–14. 10.1186/s13041-021-00858-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. C., Ban S. S., Woo Y. J., Kim S. U. (2001). Calcium/calmodulin kinase II activity of hippocampus in kainate-induced epilepsy. J. Korean Med. Sci. 16 643–648. 10.3346/jkms.2001.16.5.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard A. S., Bayer K. U., Merrill M. A., Lim I. A., Shea M. A., Schulman H., et al. (2002). Regulation of calcium/calmodulin-dependent protein Kinase II docking toN-Methyl-d-aspartate receptors by calcium/calmodulin and α-actinin. J. Biol. Chem. 277 48441–48448. 10.1074/jbc.M205164200 [DOI] [PubMed] [Google Scholar]
- Leonard A. S., Lim I. A., Hemsworth D. E., Horne M. C., Hell J. W. (1999). Calcium/calmodulin-dependent protein kinase II is associated with the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. 96 3239–3244. 10.1073/pnas.96.6.3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leurs U., Klein A. B., McSpadden E. D., Griem-Krey N., Solbak S. M., Houlton J., et al. (2021). GHB analogs confer neuroprotection through specific interaction with the CaMKIIα hub domain. Proc. Natl. Acad. Sci. 118:31. 10.1073/pnas.2108079118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine E. S., Crozier R. A., Black I. B., Plummer M. R. (1998). Brain-derived neurotrophic factor modulates hippocampal synaptic transmission by increasing N-methyl-D-aspartic acid receptor activity. Proc. Natl. Acad. Sci. 95 10235–10239. 10.1073/pnas.95.17.10235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine E. S,., Dreyfus C. F., Black I. B., Plummer M. R. (1995). Brain-derived neurotrophic factor rapidly enhances synaptic transmission in hippocampal neurons via postsynaptic tyrosine kinase receptors. Proc. Natl. Acad. Sci. 92 8074–8077. 10.1073/pnas.92.17.8074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K. W., Miller S., Klychnikov O., Loos M., Stahl-Zeng J., Spijker S., et al. (2007). Quantitative proteomics and protein network analysis of hippocampal synapses of CaMKIIα mutant mice. J. Prot. Res. 6 3127–3133. 10.1021/pr070086w [DOI] [PubMed] [Google Scholar]
- Lisman J., Raghavachari S. (2015). Biochemical principles underlying the stable maintenance of LTP by the CaMKII/NMDAR complex. Brain Res. 1621 51–61. 10.1016/j.brainres.2014.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J., Schulman H., Cline H. (2002). The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 3 175–190. 10.1038/nrn753 [DOI] [PubMed] [Google Scholar]
- Lisman J., Yasuda R., Raghavachari S. (2012). Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 13 169–182. 10.1038/nrn3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Harris K. M. (1993). Quantal analysis and synaptic anatomy—integrating two views of hippocampal plasticity. Trends Neurosci. 16 141–147. 10.1016/0166-2236(93)90122-3 [DOI] [PubMed] [Google Scholar]
- Lisman J. E., Zhabotinsky A. M. (2001). A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron 31 191–201. 10.1016/s0896-6273(01)00364-6 [DOI] [PubMed] [Google Scholar]
- Liu W., Jiang X., Zu Y., Yang Y., Liu Y., Sun X., et al. (2020). A comprehensive description of GluN2B-selective N-methyl-D-aspartate (n.d.) receptor antagonists. Eur. Opean J. Med. Chem. 200:112447. 10.1016/j.ejmech.2020.112447 [DOI] [PubMed] [Google Scholar]
- Liu X., Qiu J., Alcon S., Hashim J., Meehan W. P., III, Mannix R. (2017). Environmental enrichment mitigates deficits after repetitive mild traumatic brain injury. J. Neurotr. 34 2445–2455. 10.1089/neu.2016.4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Zhan Z., Xu L., Ma F., Li D., Guo Z., et al. (2010). MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKIIα. J. Immunol. 185 7244–7251. 10.4049/jimmunol.1001573 [DOI] [PubMed] [Google Scholar]
- Liu Z., Xu J., Shen X., Lv C., Xu T., Pei D. (2012). CaMKII antisense oligodeoxynucleotides protect against ischemia-induced neuronal death in the rat hippocampus. J. Neurol. Sci. 314 104–110. 10.1016/j.jns.2011.10.012 [DOI] [PubMed] [Google Scholar]
- Lu W., Isozaki K., Roche K. W., Nicoll R. A. (2010). Synaptic targeting of AMPA receptors is regulated by a CaMKII site in the first intracellular loop of GluA1. Proc. Natl. Acad. Sci. 107 22266–22271. 10.1073/pnas.1016289107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue L. F., Kuo Y. M., Roher A. E., Brachova L., Shen Y., Sue L., et al. (1999). Soluble amyloid β peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am. J. Pathol. 155 853–862. 10.1016/s0002-9440(10)65184-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly P. T., Song W. (2011). Loss of activated CaMKII at the synapse underlies Alzheimer’s disease memory loss. J. Neurochem. 119 673–675. 10.1111/j.1471-4159.2011.07473.x [DOI] [PubMed] [Google Scholar]
- Ma H., Groth R. D., Cohen S. M., Emery J. F., Li B., Hoedt E., et al. (2014). γCaMKII shuttles Ca2+/CaM to the nucleus to trigger CREB phosphorylation and gene expression. Cell 159 281–294. 10.1016/j.cell.2014.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F., Jackson M. F., Beazely M. A. (2006). Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit. Rev. Neurob. 18 1–2. 10.1615/critrevneurobiol.v18.i1-2.80 [DOI] [PubMed] [Google Scholar]
- Madhavan M., Mohanan A. G., Jacob R. S., Gunasekaran S., Nair R. R., Omkumar R. V. (2020). Glu60 of α-Calcium/calmodulin dependent protein kinase II mediates crosstalk between the regulatory T-site and protein substrate binding region of the active site. Archiv. Biochem. Biophys. 685:108348. 10.1016/j.abb.2020.108348 [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Mauk M. D., Kelly P. T., Nicoll R. A., et al. (1989). An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature 340 554–557. 10.1038/340554a0 [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Nicoll R. A. (1999). Long-term potentiation–a decade of progress? Science 285 1870–1874. 10.1126/science.285.5435.1870 [DOI] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. (1989). Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245 862–866. 10.1126/science.2549638 [DOI] [PubMed] [Google Scholar]
- Marin P., Nastiuk K. L., Daniel N., Girault J. A., Czernik A. J., Glowinski J., et al. (1997). Glutamate-dependent phosphorylation of elongation factor-2 and inhibition of protein synthesis in neurons. J. Neurosci. 17 3445–3454. 10.1523/JNEUROSCI.17-10-03445.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Mallory M., Alford M., DeTeresa R., Hansen L. A., McKeel D. W. (2001). Altered expression of synaptic proteins occurs early during progression of Alzheimer’s disease. Neurology 56 127–129. 10.1212/wnl.56.1.127 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Murozono M., Nagaoka D., Matsuoka S., Takeda A., Narita H., et al. (2008). Isoflurane Inhibits Protein Kinase Cγ and Calcium/Calmodulin Dependent Protein Kinase II-α Translocation to Synaptic Membranes in Ischemic Mice Brains. Neurochem. Res. 33 2302–2309. 10.1007/s11064-008-9727-4 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Shamloo M., Isshiki A., Wieloch T. (2002). Persistent phosphorylation of synaptic proteins following middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 22 1107–1113. 10.1097/00004647-200209000-00008 [DOI] [PubMed] [Google Scholar]
- Matsumoto S., Shamloo M., Matsumoto E., Isshiki A., Wieloch T. (2004). Protein kinase C-γ and calcium/calmodulin-dependent protein kinase II-α are persistently translocated to cell membranes of the rat brain during and after middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 24 54–61. 10.1097/01.WCB.0000095920.70924.F5 [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Morinobu S., Yamamoto S., Matsumoto T., Takei S., Fujita Y., et al. (2013). Vorinostat ameliorates impaired fear extinction possibly via the hippocampal NMDA-CaMKII pathway in an animal model of posttraumatic stress disorder. Psychopharmacology 229 51–62. 10.1007/s00213-013-3078-9 [DOI] [PubMed] [Google Scholar]
- Matsuo N., Yamasaki N., Ohira K., Takao K., Toyama K., Eguchi M., et al. (2009). Neural activity changes underlying the working memory deficit in alpha-CaMKII heterozygous knockout mice. Front. Behav. Neurosci. 3:20. 10.3389/neuro.08.020.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M., Ellis-Davies G. C., Nemoto T., Miyashita Y., Iino M., Kasai H. (2001). Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat. Neurosci. 4 1086–1092. 10.1038/nn736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M., Honkura N., Ellis-Davies G. C., Kasai H. (2004). Structural basis of long-term potentiation in single dendritic spines. Nature 429 761–766. 10.1038/nature02617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauceri D., Cattabeni F., Di Luca M., Gardoni F. (2004). Calcium/calmodulin-dependent protein kinase II phosphorylation drives synapse-associated protein 97 into spines. J. Biol. Chem. 279 23813–23821. 10.1074/jbc.M402796200 [DOI] [PubMed] [Google Scholar]
- Mayadevi M., Lakshmi K., Suma Priya S. D., John S., Omkumar R. V. (2016). Protection of α-CaMKII from dephosphorylation by GluN2B subunit of NMDA receptor is abolished by mutation of Glu96 or His282 of α-CaMKII. PLoS One 11:e0162011. 10.1371/journal.pone.0162011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayadevi M., Praseeda M., Kumar K. S., Omkumar R. V. (2002). Sequence determinants on the NR2A and NR2B subunits of NMDA receptor responsible for specificity of phosphorylation by CaMKII. Biochimica et Biophysica Acta 1598 40–45. 10.1016/s0167-4838(02)00315-1 [DOI] [PubMed] [Google Scholar]
- Mayadevi M., Sherin D. R., Keerthi V. S., Rajasekharan K. N., Omkumar R. V. (2012). Curcumin is an inhibitor of calcium/calmodulin dependent protein kinase II. Bioorg. Med. Chem. 20 6040–6047. 10.1016/j.bmc.2012.08.029 [DOI] [PubMed] [Google Scholar]
- Mayford M., Bach M. E., Huang Y. Y., Wang L., Hawkins R. D., Kandel E. R. (1996). Control of memory formation through regulated expression of a CaMKII transgene. Science 274 1678–1683. 10.1126/science.274.5293.1678 [DOI] [PubMed] [Google Scholar]
- Mayford M., Wang J., Kandel E. R., O’Dell T. J. (1995). CaMKII regulates the frequency-response function of hippocampal synapses for the production of both LTD and LTP. Cell 81 891–904. 10.1016/0092-8674(95)90009-8 [DOI] [PubMed] [Google Scholar]
- McGlade-McCulloh E., Yamamoto H., Tan S. E., Brickey D. A., Soderling T. R. (1993). Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature 362 640–642. 10.1038/362640a0 [DOI] [PubMed] [Google Scholar]
- Melville Z., Hernández-Ochoa E. O., Pratt S. J., Liu Y., Pierce A. D., Wilder P. T., et al. (2017). The activation of protein kinase A by the calcium-binding protein S100A1 is independent of cyclic AMP. Biochemistry 56 2328–2337. 10.1021/acs.biochem.7b00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F., Guo J., Zhang Q., Song B., Zhang G. (2003). Autophosphorylated calcium/calmodulin-dependent protein kinase IIα (CaMKIIα) reversibly targets to and phosphorylates N-methyl-D-aspartate receptor subunit 2B (NR2B) in cerebral ischemia and reperfusion in hippocampus of rats. Brain Res. 967 161–169. 10.1016/s0006-8993(02)04267-1 [DOI] [PubMed] [Google Scholar]
- Meng F., Zhang G. (2002). Autophosphorylated calcium/calmodulin-dependent protein kinase II α induced by cerebral ischemia immediately targets and phosphorylates N-methyl-D-aspartate receptor subunit 2B (NR2B) in hippocampus of rats. Neurosci. Lett. 333 59–63. 10.1016/s0304-3940(02)00961-8 [DOI] [PubMed] [Google Scholar]
- Michalski P. J. (2013). The delicate bistability of CaMKII. Biophys. J. 105 794–806. 10.1016/j.bpj.2013.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva K. D., Busse B., Weiler N. C., O’Rourke N., Smith S. J. (2010). Single-synapse analysis of a diverse synapse population: proteomic imaging methods and markers. Neuron 68 639–653. 10.1016/j.neuron.2010.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migues P. V., Lehmann I. T., Fluechter L., Cammarota M., Gurd J. W., Sim A. T., et al. (2006). Phosphorylation of CaMKII at Thr253 occurs in vivo and enhances binding to isolated postsynaptic densities. J. Neurochem. 98 289–299. 10.1111/j.1471-4159.2006.03876.x [DOI] [PubMed] [Google Scholar]
- Miller P., Zhabotinsky A. M., Lisman J. E., Wang X. J. (2005). The stability of a stochastic CaMKII switch: dependence on the number of enzyme molecules and protein turnover. PLoS Biol. 3:e107. 10.1371/journal.pbio.0030107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S., Yasuda M., Coats J. K., Jones Y., Martone M. E., Mayford M. (2002). Disruption of dendritic translation of CaMKIIα impairs stabilization of synaptic plasticity and memory consolidation. Neuron 36 507–519. 10.1016/s0896-6273(02)00978-9 [DOI] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. (1985). Distinct forebrain and cerebellar isozymes of type II Ca2+/calmodulin-dependent protein kinase associate differently with the postsynaptic density fraction. J. Biol. Chem. 260 9039–9046. 10.1016/s0021-9258(17)39454-1 [DOI] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. (1986). Regulation of brain Type II Ca2+ calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell 44 861–870. 10.1016/0092-8674(86)90008-5 [DOI] [PubMed] [Google Scholar]
- Moldave K. (1985). Eukaryotic protein synthesis. Ann. Rev. Biochem. 54 1109–1149. [DOI] [PubMed] [Google Scholar]
- Molloy S. S., Kennedy M. B. (1991). Autophosphorylation of type II Ca2+/calmodulin-dependent protein kinase in cultures of postnatal rat hippocampal slices. Proc. Natl. Acad. Sci. 88 4756–4760. 10.1073/pnas.88.11.4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosmang S., Haider N., Klugbauer N., Adelsberger H., Langwieser N., Müller J., et al. (2005). Role of hippocampal Cav1. 2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J. Neurosci. 25 9883–9892. 10.1523/JNEUROSCI.1531-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretto E., Murru L., Martano G., Sassone J., Passafaro M. (2018). Glutamatergic synapses in neurodevelopmental disorders. Prog. Neuro-Psychopharm. Biol. Psych. 84 328–342. 10.1016/j.pnpbp.2017.09.014 [DOI] [PubMed] [Google Scholar]
- Mori Y., Imaizumi K., Katayama T., Yoneda T., Tohyama M. (2000). Two cis-acting elements in the 3′ untranslated region of α-CaMKII regulate its dendritic targeting. Nat. Neurosci. 3 1079–1084. 10.1038/80591 [DOI] [PubMed] [Google Scholar]
- Moriguchi S., Shioda N., Yamamoto Y., Tagashira H., Fukunaga K. (2012a). The T-type voltage-gated calcium channel as a molecular target of the novel cognitive enhancer ST101: enhancement of long-term potentiation and CaMKII autophosphorylation in rat cortical slices. J. Neurochem. 121 44–53. 10.1111/j.1471-4159.2012.07667.x [DOI] [PubMed] [Google Scholar]
- Moriguchi S., Yabuki Y., Fukunaga K. (2012b). Reduced calcium/calmodulin-dependent protein kinase II activity in the hippocampus is associated with impaired cognitive function in MPTP-treated mice. J. Neurochem. 120 541–551. 10.1111/j.1471-4159.2011.07608.x [DOI] [PubMed] [Google Scholar]
- Moro A., Van Woerden G. M., Toonen R. F., Verhage M. (2020). CaMKII controls neuromodulation via neuropeptide gene expression and axonal targeting of neuropeptide vesicles. PLoS biol. 18:e3000826. 10.1371/journal.pbio.3000826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullasseril P., Dosemeci A., Lisman J. E., Griffith L. C. (2007). A structural mechanism for maintaining the ‘on-state’of the CaMKII memory switch in the post-synaptic density. J. Neurochem. 103 357–364. 10.1111/j.1471-4159.2007.04744.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K. D., Isackson P. J., Jones E. G. (2003). N-methyl-D-aspartate receptor-dependent transcriptional regulation of two calcium/calmodulin-dependent protein kinase type II isoforms in rodent cerebral cortex. Neuroscience 122 407–420. 10.1016/j.neuroscience.2003.07.015 [DOI] [PubMed] [Google Scholar]
- Myers J. B., Zaegel V., Coultrap S. J., Miller A. P., Bayer K. U., Reichow S. L. (2017). The CaMKII holoenzyme structure in activation-competent conformations. Nat. Comm. 8 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara S., Miyake S., Tajinda K., Ito H. (2015). Mossy fiber mis-pathfinding and semaphorin reduction in the hippocampus of α-CaMKII hKO mice. Neurosci. Lett. 598 47–51. 10.1016/j.neulet.2015.05.012 [DOI] [PubMed] [Google Scholar]
- Näslund J., Haroutunian V., Mohs R., Davis K. L., Davies P., Greengard P., et al. (2000). Correlation between elevated levels of amyloid β-peptide in the brain and cognitive decline. Jama 283 1571–1577. 10.1001/jama.283.12.1571 [DOI] [PubMed] [Google Scholar]
- Nassal D., Gratz D., Hund T. J. (2020). Challenges and opportunities for therapeutic targeting of calmodulin kinase II in heart. Front. Pharm. 11:35. 10.3389/fphar.2020.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Need A. C., Giese K. P. (2003). Handling and environmental enrichment do not rescue learning and memory impairments in αCamKIIT286A mutant mice. Genes Brain Behav. 2 132–139. 10.1034/j.1601-183x.2003.00020.x [DOI] [PubMed] [Google Scholar]
- Nesler K. R., Sand R. I., Symmes B. A., Pradhan S. J., Boin N. G., Laun A. E., et al. (2013). The miRNA pathway controls rapid changes in activity-dependent synaptic structure at the Drosophila melanogaster neuromuscular junction. PLoS One 8:e68385. 10.1371/journal.pone.0068385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesler K. R., Starke E. L., Boin N. G., Ritz M., Barbee S. A. (2016). Presynaptic CamKII regulates activity-dependent axon terminal growth. Mole. Cell. Neurosci. 76 33–41. 10.1016/j.mcn.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicole O., Pacary E. (2020). Camkiiβ in neuronal development and plasticity: an emerging candidate in brain diseases. Internat. J. Mole. Sci. 21:7272. 10.3390/ijms21197272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikandrova Y. A., Jiao Y., Baucum A. J., Tavalin S. J., Colbran R. J. (2010). Ca2+/calmodulin-dependent protein kinase II binds to and phosphorylates a specific SAP97 splice variant to disrupt association with AKAP79/150 and modulate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-type glutamate receptor (AMPAR) activity. J. Biol. Chem. 285 923–934. 10.1074/jbc.M109.033985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J. S., Manzerra P., Kennedy M. B. (2004). Regulation of the neuron-specific Ras GTPase-activating protein, synGAP, by Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 279 17980–17988. 10.1074/jbc.M314109200 [DOI] [PubMed] [Google Scholar]
- Omkumar R. V., Kiely M. J., Rosenstein A. J., Min K. T., Kennedy M. B. (1996). Identification of a phosphorylation site for calcium/calmodulin dependent protein kinase II in the NR2B subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 271 31670–31678. 10.1074/jbc.271.49.31670 [DOI] [PubMed] [Google Scholar]
- Onori M. P., van Woerden G. M. (2021). Role of calcium/calmodulin-dependent kinase 2 in neurodevelopmental disorders. Brain Res. Bull. 171 209–220. 10.1016/j.brainresbull.2021.03.014 [DOI] [PubMed] [Google Scholar]
- Opazo P., da Silva S. V., Carta M., Breillat C., Coultrap S. J., Grillo-Bosch D., et al. (2018). CaMKII metaplasticity drives Aβ oligomer-mediated synaptotoxicity. Cell Rep. 23 3137–3145. 10.1016/j.celrep.2018.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P., Labrecque S., Tigaret C. M., Frouin A., Wiseman P. W., De Koninck P., et al. (2010). CaMKII triggers the diffusional trapping of surface AMPARs through phosphorylation of stargazin. Neuron 67 239–252. 10.1016/j.neuron.2010.06.007 [DOI] [PubMed] [Google Scholar]
- Ortuño-Sahagún D., Rivera-Cervantes M. C., Gudiño-Cabrera G., Junyent F., Verdaguer E., Auladell C., et al. (2012). Microarray analysis of rat hippocampus exposed to excitotoxicity: reversal Na+/Ca2+ exchanger NCX3 is overexpressed in glial cells. Hippocampus 22 128–140. 10.1002/hipo.20869 [DOI] [PubMed] [Google Scholar]
- Otmakhov N., Gorbacheva E. V., Regmi S., Yasuda R., Hudmon A., Lisman J. (2015). Excitotoxic insult results in a long-lasting activation of CaMKIIα and mitochondrial damage in living hippocampal neurons. PLoS One 10:e0120881. 10.1371/journal.pone.0120881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y., Kantor D., Harris K. M., Schuman E. M., Kennedy M. B. (1997). Visualization of the distribution of autophosphorylated calcium/calmodulin-dependent protein kinase II after tetanic stimulation in the CA1 area of the hippocampus. J. Neurosci. 17 5416–5427. 10.1523/JNEUROSCI.17-14-05416.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Y., Rosenstein A., Kreiman G., Schuman E. M., Kennedy M. B. (1999). Tetanic stimulation leads to increased accumulation of Ca2+/calmodulin-dependent protein kinase II via dendritic protein synthesis in hippocampal neurons. J. Neurosci. 19 7823–7833. 10.1523/JNEUROSCI.19-18-07823.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillé V., Picconi B., Bagetta V., Ghiglieri V., Sgobio C., Di Filippo M., et al. (2010). Distinct levels of dopamine denervation differentially alter striatal synaptic plasticity and NMDA receptor subunit composition. J. Neurosci. 30 14182–14193. 10.1523/JNEUROSCI.2149-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Chen J., Guo H., Ou J., Peng Y., Liu Q., et al. (2015). Association of genetic variants of GRIN2B with autism. Sci. Rep. 5 1–5. 10.1038/srep08296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Chávez A. E., Mineur Y. S., Morimoto-Tomita M., Lutzu S., Kim K. S., et al. (2016). CaMKII phosphorylation of TARPγ-8 is a mediator of LTP and learning and memory. Neuron 92 75–83. 10.1016/j.neuron.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H., Zamani R. (2021). The role of PKMζ in the maintenance of long-term memory: a review. Rev. Neurosci. 32 481–494. 10.1515/revneuro-2020-0105 [DOI] [PubMed] [Google Scholar]
- Patriarchi T., Buonarati O. R., Hell J. W. (2018). Postsynaptic localization and regulation of AMPA receptors and Cav1. 2 by β2 adrenergic receptor/PKA and Ca2+/CaMKII signaling. EMBO J. 37:e99771. 10.15252/embj.201899771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson S. L., Abel T., Deuel T. A., Martin K. C., Rose J. C., Kandel E. R. (1996). Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron 16 1137–1145. 10.1016/s0896-6273(00)80140-3 [DOI] [PubMed] [Google Scholar]
- Penny C. J., Gold M. G. (2018). Mechanisms for localising calcineurin and CaMKII in dendritic spines. Cell. Signal. 49 46–58. 10.1016/j.cellsig.2018.05.010 [DOI] [PubMed] [Google Scholar]
- Pi H. J., Otmakhov N., El Gaamouch F., Lemelin D., De Koninck P., Lisman J. (2010). CaMKII control of spine size and synaptic strength: role of phosphorylation states and nonenzymatic action. Proc. Natl. Acad. Sci. 107 14437–14442. 10.1073/pnas.1009268107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picconi B., Gardoni F., Centonze D., Mauceri D., Cenci M. A., Bernardi G., et al. (2004). Abnormal Ca2+-calmodulin-dependent protein kinase II function mediates synaptic and motor deficits in experimental parkinsonism. J. Neurosci. 24 5283–5291. 10.1523/JNEUROSCI.1224-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto T. M., Schilstra M. J., Roque A. C., Steuber V. (2020). Binding of Filamentous Actin to CaMKII as Potential Regulation Mechanism of Bidirectional Synaptic Plasticity by β CaMKII in Cerebellar Purkinje Cells. Sci. Rep. 10 1–16. 10.1038/s41598-020-65870-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M. M. (2001). Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2 24–32. 10.1038/35049004 [DOI] [PubMed] [Google Scholar]
- Pöschel B., Manahan-Vaughan D. (2007). Persistent (> 24 h) long-term depression in the dentate gyrus of freely moving rats is not dependent on activation of NMDA receptors, L-type voltage-gated calcium channels or protein synthesis. Neuropharmacology 52 46–54. 10.1016/j.neuropharm.2006.07.019 [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller L. D., Gottschalk W., Zhang L., McDermott K., Du J., Gopalakrishnan R., et al. (1999). Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J. Neurosci. 19 4972–4983. 10.1523/JNEUROSCI.19-12-04972.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeep K. K., Cheriyan J., Suma Priya S. D., Rajeevkumar R., Mayadevi M., Praseeda M., et al. (2009). Regulation of Ca2+/calmodulin-dependent protein kinase II catalysis by N-methyl-D-aspartate receptor subunit 2B. Biochem. J. 419 123–136. 10.1042/BJ20081707 [DOI] [PubMed] [Google Scholar]
- Proietti Onori M., Koopal B., Everman D. B., Worthington J. D., Jones J. R., Ploeg M. A., et al. (2018). The intellectual disability-associated CAMK2G p. Arg292Pro mutation acts as a pathogenic gain-of-function. Hum. Mutat. 39 2008–2024. 10.1002/humu.23647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puram S. V., Kim A. H., Ikeuchi Y., Wilson-Grady J. T., Merdes A., Gygi S. P., et al. (2011). A CaMKIIβ signaling pathway at the centrosome regulates dendrite patterning in the brain. Nat. Neurosci. 14 973–983. 10.1038/nn.2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu J., Mei Q., Niu R. (2019). Oxidative CaMKII as a potential target for inflammatory disease. Mole. Med. Rep. 20 863–870. 10.3892/mmr.2019.10309 [DOI] [PubMed] [Google Scholar]
- Radwanska K., Medvedev N. I., Pereira G. S., Engmann O., Thiede N., Moraes M. F., et al. (2011). Mechanism for long-term memory formation when synaptic strengthening is impaired. Proc. Natl. Acad. Sci. 108 18471–18475. 10.1073/pnas.1109680108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramya R. P., Priya S. S., Mayadevi M., Omkumar R. V. (2012). Regulation of phosphorylation at Ser1303 of GluN2B receptor in the postsynaptic density. Neurochem. Internat. 61 981–985. 10.1016/j.neuint.2012.08.016 [DOI] [PubMed] [Google Scholar]
- Raveendran R., Devi Suma, Priya S., Mayadevi M., Steephan M., Santhoshkumar T. R., et al. (2009). Phosphorylation status of the NR2B subunit of NMDA receptor regulates its interaction with calcium/calmodulin-dependent protein kinase II. J. Neurochem. 110 92–105. 10.1111/j.1471-4159.2009.06108.x [DOI] [PubMed] [Google Scholar]
- Rhein C., Mühle C., Lenz B., Richter-Schmidinger T., Kogias G., Boix F., et al. (2020). Association of a CAMK2A genetic variant with logical memory performance and hippocampal volume in the elderly. Brain Res. Bull. 161 13–20. 10.1016/j.brainresbull.2020.05.001 [DOI] [PubMed] [Google Scholar]
- Rich R. C., Schulman H. (1998). Substrate-directed function of calmodulin in autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 273 28424–28429. 10.1074/jbc.273.43.28424 [DOI] [PubMed] [Google Scholar]
- Rizzi S., Spagnoli C., Salerno G. G., Frattini D., Caraffi S. G., Trimarchi G., et al. (2020). Severe intellectual disability, absence of language, epilepsy, microcephaly and progressive cerebellar atrophy related to the recurrent de novo variant p.(P139L) of the CAMK2B gene: a case report and brief review. Am. J. Med. Genet. Part A 182 2675–2679. 10.1002/ajmg.a.61803 [DOI] [PubMed] [Google Scholar]
- Robison A. J. (2014). Emerging role of CaMKII in neuropsychiatric disease. Trends Neurosci. 37 653–662. 10.1016/j.tins.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Robison A. J., Bass M. A., Jiao Y., MacMillan L. B., Carmody L. C., Bartlett R. K., et al. (2005). Multivalent interactions of calcium/calmodulin-dependent protein kinase II with the postsynaptic density proteins NR2B, densin-180, and α-actinin-2. J. Biol. Chem. 280 35329–35336. 10.1074/jbc.M502191200 [DOI] [PubMed] [Google Scholar]
- Rongo C., Kaplan J. M. (1999). CaMKII regulates the density of central glutamatergic synapses in vivo. Nature 402 195–199. 10.1038/46065 [DOI] [PubMed] [Google Scholar]
- Rosenberg O. S., Deindl S., Comolli L. R., Hoelz A., Downing K. H., Nairn A. C., et al. (2006). Oligomerization states of the association domain and the holoenyzme of Ca2+/CaM kinase II. FEBS J. 273 682–694. 10.1111/j.1742-4658.2005.05088.x [DOI] [PubMed] [Google Scholar]
- Rossetti T., Banerjee S., Kim C., Leubner M., Lamar C., Gupta P., et al. (2017). Memory erasure experiments indicate a critical role of CaMKII in memory storage. Neuron 96 207–216. 10.1016/j.neuron.2017.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostas J. A., Hoffman A., Murtha L. A., Pepperall D., McLeod D. D., Dicksosn P. W. (2017). Ischaemia-and excitotoxicity-induced CaMKII-Mediated neuronal cell death: the relative roles of CaMKII autophosphorylation at T286 and T253. Neurochem. Internat. 104 6–10. 10.1016/j.neuint.2017.01.002 [DOI] [PubMed] [Google Scholar]
- Rotenberg A., Mayford M., Hawkins R. D., Kandel E. R., Muller R. U. (1996). Mice expressing activated CaMKII lack low frequency LTP and do not form stable place cells in the CA1 region of the hippocampus. Cell 87 1351–1361. 10.1016/s0092-8674(00)81829-2 [DOI] [PubMed] [Google Scholar]
- Rumbaugh G., Adams J. P., Kim J. H., Huganir R. L. (2006). SynGAP regulates synaptic strength and mitogen-activated protein kinases in cultured neurons. Proceedings of the National Academy of Sciences 103 4344–4351. 10.1073/pnas.0600084103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S., Ramanathan A., Rangarajan P. N. (2006). Regulation of Ca2+/calmodulin kinase II inhibitor α (CaMKIINα) in virus-infected mouse brain. Biochem. Biophys. Res. Comm. 350 444–449. 10.1016/j.bbrc.2006.09.066 [DOI] [PubMed] [Google Scholar]
- Sałaciak K., Koszałka A., Żmudzka E., Pytka K. (2021). The Calcium/Calmodulin-Dependent Kinases II and IV as Therapeutic Targets in Neurodegenerative and Neuropsychiatric Disorders. Internat. J. Mole. Sci. 22:4307. 10.3390/ijms22094307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saneyoshi T., Matsuno H., Suzuki A., Murakoshi H., Hedrick N. G., Agnello E. (2019). Reciprocal activation within a kinase-effector complex underlying persistence of structural LTP. Neuron 102 1199–1210. 10.1016/j.neuron.2019.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhueza M., Fernandez-Villalobos G., Stein I. S., Kasumova G., Zhang P., Bayer K. U., et al. (2011). Role of the CaMKII/NMDA receptor complex in the maintenance of synaptic strength. J. Neurosci. 31 9170–9178. 10.1523/JNEUROSCI.1250-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savina T. A., Shchipakina T. G., Godukhin O. V. (2013). Effects of Convulsive Activity on the Subunit Composition of Ca 2+/Calmodulin-Dependent Protein Kinase II in the Hippocampus of Krushinskii–Molodkina Rats. Neurosci. Behav. Physiol. 43 267–274. 10.1007/s11055-013-9727-y [DOI] [Google Scholar]
- Scheetz A. J., Nairn A. C., Constantine-Paton M. (1997). N-methyl-D-aspartate receptor activation and visual activity induce elongation factor-2 phosphorylation in amphibian tecta: a role for N-methyl-D-aspartate receptors in controlling protein synthesis. Proc. Natl. Acad. Sci. 94 14770–14775. 10.1073/pnas.94.26.14770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheetz A. J., Nairn A. C., Constantine-Paton M. (2000). NMDA receptor-mediated control of protein synthesis at developing synapses. Nat. Neurosci. 3 211–216. 10.1038/72915 [DOI] [PubMed] [Google Scholar]
- Scheff S. W., Price D. A. (1993). Synapse loss in the temporal lobe in Alzheimer’s disease. Ann. Neurol. 33 190–199. 10.1002/ana.410330209 [DOI] [PubMed] [Google Scholar]
- Scheff S. W., Price D. A. (1998). Synaptic density in the inner molecular layer of the hippocampal dentate gyrus in Alzheimer disease. J. Neuropathol. Exp. Neurol. 57 1146–1153. 10.1097/00005072-199812000-00006 [DOI] [PubMed] [Google Scholar]
- Scheff S. W., Price D. A., Schmitt F. A., Mufson E. J. (2006). Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 27 1372–1384. 10.1016/j.neurobiolaging.2005.09.012 [DOI] [PubMed] [Google Scholar]
- Schwarzbach E., Bonislawski D. P., Xiong G., Cohen A. S. (2006). Mechanisms underlying the inability to induce area CA1 LTP in the mouse after traumatic brain injury. Hippocampus 16 541–550. 10.1002/hipo.20183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A., Godukhin O. (2001). Epileptiform activity and EPSP-spike potentiation induced in rat hippocampal CA1 slices by repeated high-K+: involvement of ionotropic glutamate receptors and Ca2+/calmodulin-dependent protein kinase II. Neuropharmacology 40 203–211. 10.1016/s0028-3908(00)00147-7 [DOI] [PubMed] [Google Scholar]
- Shamloo M., Kamme F., Wieloch T. (2000). Subcellular distribution and autophosphorylation of calcium/calmodulin-dependent protein kinase II-α in rat hippocampus in a model of ischemic tolerance. Neuroscience 96 665–674. 10.1016/s0306-4522(99)00586-2 [DOI] [PubMed] [Google Scholar]
- Shen K., Meyer T. (1999). Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science 284 162–167. 10.1126/science.284.5411.162 [DOI] [PubMed] [Google Scholar]
- Shen K., Teruel M. N., Connor J. H., Shenolikar S., Meyer T. (2000). Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nat. Neurosci. 3 881–886. 10.1038/78783 [DOI] [PubMed] [Google Scholar]
- Shen K., Teruel M. N., Subramanian K., Meyer T. (1998). CaMKIIβ functions as an F-actin targeting module that localizes CaMKIIα/β heterooligomers to dendritic spines. Neuron 21 593–606. 10.1016/s0896-6273(00)80569-3 [DOI] [PubMed] [Google Scholar]
- Shin S. M., Zhang N., Hansen J., Gerges N. Z., Pak D. T., Sheng M., et al. (2012). GKAP orchestrates activity-dependent postsynaptic protein remodeling and homeostatic scaling. Nat. Neurosci. 15 1655–1666. 10.1038/nn.3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si K., Lindquist S., Kandel E. R. (2003). A neuronal isoform of the aplysia CPEB has prion-like properties. Cell 115 879–891. 10.1016/s0092-8674(03)01020-1 [DOI] [PubMed] [Google Scholar]
- Silva A. J., Paylor R., Wehner J. M., Tonegawa S. (1992b). Impaired spatial learning in alpha-calcium-calmodulin kinase II mutant mice. Science 257 206–211. 10.1126/science.1321493 [DOI] [PubMed] [Google Scholar]
- Silva A. J., Rosahl T. W., Chapman P. F., Marowitz Z., Friedman E., Frankland P. W., et al. (1996). Impaired learning in mice with abnormal short-lived plasticity. Curr. Biol. 6 1509–1518. 10.1016/s0960-9822(96)00756-7 [DOI] [PubMed] [Google Scholar]
- Silva A. J., Stevens C. F., Tonegawa S., Wang Y. (1992a). Deficient hippocampal long-term potentiation in alpha-calcium-calmodulin kinase II mutant mice. Science 257 201–206. 10.1126/science.1378648 [DOI] [PubMed] [Google Scholar]
- Śliwińska M. A., Cały A., Borczyk M., Ziółkowska M., Skonieczna E., Chilimoniuk M., et al. (2020). Long-term memory upscales volume of postsynaptic densities in the process that requires autophosphorylation of αCaMKII. Cereb. Cortex 30 2573–2585. 10.1093/cercor/bhz261 [DOI] [PubMed] [Google Scholar]
- Sloutsky R., Stratton M. M. (2021). Functional implications of CaMKII alternative splicing. Eur. J. Neurosci. 54 6780–6794. 10.1111/ejn.14761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton P. K., Gage A. T. (1996). Distinct synaptic loci of Ca2+/calmodulin-dependent protein kinase II necessary for long-term potentiation and depression. J. Neurophys. 76 2097–2101. 10.1152/jn.1996.76.3.2097 [DOI] [PubMed] [Google Scholar]
- Stefani G., Onofri F., Valtorta F., Vaccaro P., Greengard P., Benfenati F. (1997). Kinetic analysis of the phosphorylation-dependent interactions of synapsin I with rat brain synaptic vesicles. J. Physiol. 504 501–515. 10.1111/j.1469-7793.1997.501bd.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Wang X., Perfitt T. L., Parrish W. P., Shonesy B. C., Marks C. R. (2017). A novel human CAMK2A mutation disrupts dendritic morphology and synaptic transmission and causes ASD-related behaviors. J. Neurosci. 37 2216–2233. 10.1523/JNEUROSCI.2068-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F., Tonegawa S., Wang Y. (1994). The role of calcium–calmodulin kinase II in three forms of synaptic plasticity. Curr. Biol. 4 687–693. 10.1016/s0960-9822(00)00153-6 [DOI] [PubMed] [Google Scholar]
- Steward O., Levy W. B. (1982). Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J. Neurosci. 2 284–291. 10.1523/JNEUROSCI.02-03-00284.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O., Schuman E. M. (2001). Protein synthesis at synaptic sites on dendrites. Ann. Rev. Neurosci. 24 299–325. 10.1146/annurev.neuro.24.1.299 [DOI] [PubMed] [Google Scholar]
- Strack S., McNeill R. B., Colbran R. J. (2000a). Mechanism and regulation of calcium/calmodulin-dependent protein kinase II targeting to the NR2B subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 275 23798–23806. 10.1074/jbc.M001471200 [DOI] [PubMed] [Google Scholar]
- Strack S., Robison A. J., Bass M. A., Colbran R. J. (2000b). Association of calcium/calmodulin-dependent kinase II with developmentally regulated splice variants of the postsynaptic density protein densin-180. J. Biol. Chem. 275 25061–25064. 10.1074/jbc.C000319200 [DOI] [PubMed] [Google Scholar]
- Stratton M., Lee I. H., Bhattacharyya M., Christensen S. M., Chao L. H., Schulman H., et al. (2014). Activation-triggered subunit exchange between CaMKII holoenzymes facilitates the spread of kinase activity. Elife 3:e01610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Study T. D. D. D. (2017). Prevalence and architecture of de novo mutations in developmental disorders. Nature 542:433. 10.1038/nature21062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara T., Hisatsune C., Miyamoto H., Ogawa N., Mikoshiba K. (2017). Regulation of spinogenesis in mature Purkinje cells via mGluR/PKC-mediated phosphorylation of CaMKIIβ. Proc. Natl. Acad. Sci. 114 E5256–E5265. 10.1073/pnas.1617270114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi M., Kiuchi K., Ishikawa T., Ishii A., Hagiwara M., Nagatsu T., et al. (1991). The newly synthesized selective Ca2+ calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem. Biophys. Res. Comm. 181 968–975. 10.1016/0006-291x(91)92031-e [DOI] [PubMed] [Google Scholar]
- Sun D. A., Deshpande L. S., Sombati S., Baranova A., Wilson M. S., Hamm R. J., et al. (2008). Traumatic brain injury causes a long-lasting calcium (Ca2+)-plateau of elevated intracellular Ca levels and altered Ca2+ homeostatic mechanisms in hippocampal neurons surviving brain injury. Eur. J. Neurosci. 27 1659–1672. 10.1111/j.1460-9568.2008.06156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. X., Hodge J. J., Zhou Y., Nguyen M., Griffith L. C. (2004). The eag potassium channel binds and locally activates calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 279 10206–10214. 10.1074/jbc.M310728200 [DOI] [PubMed] [Google Scholar]
- Sutherland D. J., Pujic Z., Goodhill G. J. (2014). Calcium signaling in axon guidance. Trends Neurosci. 37 424–432. 10.1016/j.tins.2014.05.008 [DOI] [PubMed] [Google Scholar]
- Szydlowska K., Tymianski M. (2010). Calcium, ischemia and excitotoxicity. Cell Cal. 47 122–129. 10.1016/j.ceca.2010.01.003 [DOI] [PubMed] [Google Scholar]
- Tao W., Lee J., Chen X., Díaz-Alonso J., Zhou J., Pleasure S., et al. (2021). Synaptic memory requires CaMKII. Elife 10:e60360. 10.7554/eLife.60360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry R. D., Masliah E., Salmon D. P., Butters N., DeTeresa R., Hill R., et al. (1991). Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30 572–580. 10.1002/ana.410300410 [DOI] [PubMed] [Google Scholar]
- Thibault O., Landfield P. W. (1996). Increase in single L-type calcium channels in hippocampal neurons during aging. Science 272 1017–1020. 10.1126/science.272.5264.1017 [DOI] [PubMed] [Google Scholar]
- Tokumitsu H., Chijiwa T., Hagiwara M., Mizutani A., Terasawa M., Hidaka H. (1990). KN-62, 1-[N, O-bis (5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazi ne, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 265 4315–4320. 10.1016/s0021-9258(19)39565-1 [DOI] [PubMed] [Google Scholar]
- Tullis J. E., Buonarati O. R., Coultrap S. J., Bourke A. M., Tiemeier E. L., Kennedy M. J., et al. (2021). GluN2B S1303 phosphorylation by CaMKII or DAPK1: no indication for involvement in ischemia or LTP. Iscience 24:103214. 10.1016/j.isci.2021.103214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakubo H., Sato M., Ishii S., Kuroda S. (2014). In vitro reconstitution of a CaMKII memory switch by an NMDA receptor-derived peptide. Biophys. J. 106 1414–1420. 10.1016/j.bpj.2014.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woerden G. M., Hoebeek F. E., Gao Z., Nagaraja R. Y., Hoogenraad C. C., Kushner S. A., et al. (2009). βCaMKII controls the direction of plasticity at parallel fiber–Purkinje cell synapses. Nat. Neurosci. 12 823–825. 10.1038/nn.2329 [DOI] [PubMed] [Google Scholar]
- Vasin A., Zueva L., Torrez C., Volfson D., Littleton J. T., Bykhovskaia M. (2014). Synapsin regulates activity-dependent outgrowth of synaptic boutons at the Drosophila neuromuscular junction. J. Neurosci. 34 10554–10563. 10.1523/JNEUROSCI.5074-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. E. (1989). Fine structure of synaptogenesis in the vertebrate central nervous system. Synapse 3 255–285. 10.1002/syn.890030312 [DOI] [PubMed] [Google Scholar]
- Vest R. S., Davies K. D., O’Leary H., Port J. D., Bayer K. U. (2007). Dual mechanism of a natural CaMKII inhibitor. Mole. Biol. Cell 18 5024–5033. 10.1091/mbc.e07-02-0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest R. S., O’Leary H., Coultrap S. J., Kindy M. S., Bayer K. U. (2010). Effective post-insult neuroprotection by a novel Ca2+/calmodulin-dependent protein kinase II (CaMKII) inhibitor. J. Biol. Chem. 285 20675–20682. 10.1074/jbc.M109.088617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira M. M., Nguyen T. A., Wu K., Badger J. D., II, Collins B. M., Anggono V., et al. (2020). An Epilepsy-Associated GRIN2A rare variant disrupts CaMKIIα phosphorylation of GluN2A and NMDA receptor trafficking. Cell Rep. 32:108104. 10.1016/j.celrep.2020.108104 [DOI] [PubMed] [Google Scholar]
- Vincent M., Collet C., Verloes A., Lambert L., Herlin C., Blanchet C., et al. (2014). Large deletions encompassing the TCOF1 and CAMK2A genes are responsible for Treacher Collins syndrome with intellectual disability. Eur. J. Hum. Genet. 22 52–56. 10.1038/ejhg.2013.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hertzen L. S., Giese K. P. (2005). Alpha-isoform of Ca2+/calmodulin-dependent kinase II autophosphorylation is required for memory consolidation-specific transcription. Neuroreport 16 1411–1414. 10.1097/01.wnr.0000175244.51084.bb [DOI] [PubMed] [Google Scholar]
- Walikonis R. S., Oguni A., Khorosheva E. M., Jeng C. J., Asuncion F. J., Kennedy M. B. (2001). Densin-180 forms a ternary complex with the α-subunit of Ca2+/calmodulin-dependent protein kinase II and α-actinin. J. Neurosci. 21 423–433. 10.1523/JNEUROSCI.21-02-00423.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup W. G., IV, Mastro T. L., Schenker L. T., Vielmetter J., Hu R., Iancu A., et al. (2016). A model for regulation by SynGAP-α1 of binding of synaptic proteins to PDZ-domain’Slots’ in the postsynaptic density. Elife 5:e16813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup W. G., Washburn L., Sweredoski M. J., Carlisle H. J., Graham R. L., Hess S., et al. (2015). Phosphorylation of synaptic GTPase-activating protein (synGAP) by Ca2+/calmodulin-dependent protein kinase II (CaMKII) and cyclin-dependent kinase 5 (CDK5) alters the ratio of its GAP activity toward Ras and Rap GTPases. J. Biol. Chem. 290 4908–4927. 10.1074/jbc.M114.614420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. C., Held R. G., Hall B. J. (2013). SynGAP regulates protein synthesis and homeostatic synaptic plasticity in developing cortical networks. PLoS One 8:e83941. 10.1371/journal.pone.0083941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Tan Q. R., Zhang Z. J. (2013). Neuroprotective effects of paeoniflorin, but not the isomer albiflorin, are associated with the suppression of intracellular calcium and calcium/calmodulin protein kinase II in PC12 cells. J. Mole. Neurosci. 51 581–590. 10.1007/s12031-013-0031-7 [DOI] [PubMed] [Google Scholar]
- Wang D. M., Yang Y. J., Zhang L., Zhang X., Guan F. F., Zhang L. F. (2013). Naringin enhances CaMKII activity and improves long-term memory in a mouse model of Alzheimer’s disease. Internat. J. Mole. Sci. 14 5576–5586. 10.3390/ijms14035576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Feng R., Wang L. P., Li F., Cao X., Tsien J. Z. (2008). CaMKII activation state underlies synaptic labile phase of LTP and short-term memory formation. Curr. Biol. 18 1546–1554. 10.1016/j.cub.2008.08.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Shimizu E., Tang Y. P., Cho M., Kyin M., Zuo W., et al. (2003). Inducible protein knockout reveals temporal requirement of CaMKII reactivation for memory consolidation in the brain. Proc. Natl. Acad. Sci. 100 4287–4292. 10.1073/pnas.0636870100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Chen L., Cheng N., Zhang J., Tian T., Lu W. (2014). Active calcium/calmodulin-dependent protein kinase II (CaMKII) regulates NMDA receptor-mediated postischemic long-term potentiation (i-LTP) by promoting the interaction between CaMKII and NMDA receptors in ischemia. Neur. Plast. 2014:827161. 10.1155/2014/827161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Cao Y., Yu J., Liu R., Bai B., Qi H., et al. (2016). Baicalin alleviates ischemia-induced memory impairment by inhibiting the phosphorylation of CaMKII in hippocampus. Brain Res. 1642 95–103. 10.1016/j.brainres.2016.03.019 [DOI] [PubMed] [Google Scholar]
- Wang Q., Chen M., Schafer N. P., Bueno C., Song S. S., Hudmon A., et al. (2019). Assemblies of calcium/calmodulin-dependent kinase II with actin and their dynamic regulation by calmodulin in dendritic spines. Proc. Natl. Acad. Sci. 116 18937–18942. 10.1073/pnas.1911452116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Liao L., Huang Y., Wang M., Zhou H., Chen D., et al. (2019). Pin1 is regulated by CaMKII activation in glutamate-induced retinal neuronal regulated necrosis. Front. Cell. Neurosci. 13:276. 10.3389/fncel.2019.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Q., Li X. J., Qiu H. B., Jiang Z. M., Simon M., Ma X. R., et al. (2014). Anti-epileptic effect of Ganoderma lucidum polysaccharides by inhibition of intracellular calcium accumulation and stimulation of expression of CaMKII α in epileptic hippocampal neurons. PLoS One 9:e102161. 10.1371/journal.pone.0102161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Marks C. R., Perfitt T. L., Nakagawa T., Lee A., Jacobson D. A., et al. (2017). A novel mechanism for Ca2+/calmodulin-dependent protein kinase II targeting to L-type Ca2+ channels that initiates long-range signaling to the nucleus. J. Biol. Chem. 292 17324–17336. 10.1074/jbc.M117.788331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Mattson M. P. (2014). L-type Ca2+ currents at CA1 synapses, but not CA3 or dentate granule neuron synapses, are increased in 3xTgAD mice in an age-dependent manner. Neurobiol. Aging 35 88–95. 10.1016/j.neurobiolaging.2013.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G., Chen Y. B., Chen D. F., Lai X. P., Liu D. H., Deng R. D., et al. (2013). β-Asarone inhibits neuronal apoptosis via the CaMKII/CREB/Bcl-2 signalling pathway in an in vitro model and AβPP/PS1 mice. J. Alzheimer’s Dis. 33 863–880. 10.3233/jad-2012-120865 [DOI] [PubMed] [Google Scholar]
- Weisskopf M. G., Bauer E. P., LeDoux J. E. (1999). L-type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J. Neurosci. 19 10512–10519. 10.1523/JNEUROSCI.19-23-10512.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells D. G., Dong X., Quinlan E. M., Huang Y. S., Bear M. F., Richter J. D., et al. (2001). A role for the cytoplasmic polyadenylation element in NMDA receptor-regulated mRNA translation in neurons. J. Neurosci. 21 9541–9548. 10.1523/JNEUROSCI.21-24-09541.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z., Guirland C., Ming G. L., Zheng J. Q. (2004). A CaMKII/calcineurin switch controls the direction of Ca2+-dependent growth cone guidance. Neuron 43 835–846. 10.1016/j.neuron.2004.08.037 [DOI] [PubMed] [Google Scholar]
- Wheeler D. G., Barrett C. F., Groth R. D., Safa P., Tsien R. W. (2008). CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation–transcription coupling. J. Cell Biol. 183 849–863. 10.1083/jcb.200805048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmayer C. P., Myers M. M., Mayford M., Barr G. A. (2000). Olfactory based spatial learning in neonatal mice and its dependence on CaMKII. Neuroreport 11 1051–1055. 10.1097/00001756-200004070-00030 [DOI] [PubMed] [Google Scholar]
- Wong M. H., Samal A. B., Lee M., Vlach J., Novikov N., Niedziela-Majka A., et al. (2019). The KN-93 molecule inhibits calcium/calmodulin-dependent protein kinase II (CaMKII) activity by binding to Ca2+/CaM. J. Mole. Biol. 431 1440–1459. 10.1016/j.jmb.2019.02.001 [DOI] [PubMed] [Google Scholar]
- Wong W. T., Wong R. O. (2000). Rapid dendritic movements during synapse formation and rearrangement. Curr. Opin. Neurob. 10 118–124. 10.1016/s0959-4388(99)00059-8 [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Davison M. T., Cohen P. (1983). The calmodulin-dependent glycogen synthase kinase from rabbit skeletal muscle: purification, subunit structure and substrate specificity. Eur. J. Biochem. 136 481–487. 10.1111/j.1432-1033.1983.tb07766.x [DOI] [PubMed] [Google Scholar]
- Woolfrey K. M., O’Leary H., Goodell D. J., Robertson H. R., Horne E. A., Coultrap S. J., et al. (2018). CaMKII regulates the depalmitoylation and synaptic removal of the scaffold protein AKAP79/150 to mediate structural long-term depression. J. Biol. Chem. 293 1551–1567. 10.1074/jbc.M117.813808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Y., Cline H. T. (1998). Stabilization of dendritic arbor structure in vivo by CaMKII. Science 279 222–226. 10.1126/science.279.5348.222 [DOI] [PubMed] [Google Scholar]
- Wu L., Wells D., Tay J., Mendis D., Abbott M. A., Barnitt A., et al. (1998). CPEB-mediated cytoplasmic polyadenylation and the regulation of experience-dependent translation of α-CaMKII mRNA at synapses. Neuron 21 1129–1139. 10.1016/s0896-6273(00)80630-3 [DOI] [PubMed] [Google Scholar]
- Wu S. P., Li D., Wang N., Hou J. C., Zhao L. (2019). YiQi tongluo granule against cerebral ischemia/reperfusion injury in rats by freezing GluN2B and CaMK II through NMDAR/ERK1/2 signalling. Chem. Pharm. Bull. 67 244–252. 10.1248/cpb.c18-00806 [DOI] [PubMed] [Google Scholar]
- Wyneken U., Smalla K. H., Marengo J. J., Soto D., De La Cerda A., Tischmeyer W., et al. (2001). Kainate-induced seizures alter protein composition and N-methyl-D-aspartate receptor function of rat forebrain postsynaptic densities. Neuroscience 102 65–74. 10.1016/s0306-4522(00)00469-3 [DOI] [PubMed] [Google Scholar]
- Xie Z., Srivastava D. P., Photowala H., Kai L., Cahill M. E., Woolfrey K. M., et al. (2007). Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron 56 640–656. 10.1016/j.neuron.2007.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Liu Z. A., Pei D. S., Xu T. J. (2010). Calcium/calmodulin-dependent kinase II facilitated GluR6 subunit serine phosphorylation through GluR6-PSD95-CaMKII signaling module assembly in cerebral ischemia injury. Brain Res. 1366 197–203. 10.1016/j.brainres.2010.09.087 [DOI] [PubMed] [Google Scholar]
- Xu Q., Deng F., Xing Z., Wu Z., Cen B., Xu S., et al. (2016). Long non-coding RNA C2dat1 regulates CaMKII δ expression to promote neuronal survival through the NF-κ B signalling pathway following cerebral ischemia. Cell Death Dis. 7 e2173–e2173. 10.1038/cddis.2016.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata Y., Imoto K., Obata K. (2006). A mechanism for the inactivation of Ca2+/calmodulin-dependent protein kinase II during prolonged seizure activity and its consequence after the recovery from seizure activity in rats in vivo. Neuroscience 140 981–992. 10.1016/j.neuroscience.2006.02.054 [DOI] [PubMed] [Google Scholar]
- Yamagata Y., Kobayashi S., Umeda T., Inoue A., Sakagami H., Fukaya M. (2009). Kinase-dead knock-in mouse reveals an essential role of kinase activity of Ca2+/calmodulin-dependent protein kinase IIα in dendritic spine enlargement, long-term potentiation, and learning. J. Neurosci. 29 7607–7618. 10.1523/JNEUROSCI.0707-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata Y., Obata K. (2004). Ca2+/calmodulin-dependent protein kinase II is reversibly autophosphorylated, inactivated and made sedimentable by acute neuronal excitation in rats in vivo. J. Neurochem. 91 745–754. 10.1111/j.1471-4159.2004.02753.x [DOI] [PubMed] [Google Scholar]
- Yamagata Y., Yanagawa Y., Imoto K. (2018). Differential involvement of kinase activity of Ca2+/calmodulin-dependent protein kinase IIα in hippocampus-and amygdala-dependent memory revealed by kinase-dead knock-in mouse. Eneuro 5 e133–e118. 10.1523/ENEURO.0133-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Shioda N., Han F., Moriguchi S., Nakajima A., Yokosuka A., et al. (2009). Nobiletin improves brain ischemia-induced learning and memory deficits through stimulation of CaMKII and CREB phosphorylation. Brain Res. 1295 218–229. 10.1016/j.brainres.2009.07.081 [DOI] [PubMed] [Google Scholar]
- Yamasaki N., Maekawa M., Kobayashi K., Kajii Y., Maeda J., Soma M., et al. (2008). Alpha-CaMKII deficiency causes immature dentate gyrus, a novel candidate endophenotype of psychiatric disorders. Mole. Brain 1 1–21. 10.1186/1756-6606-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E., Schulman H. (1999). Structural examination of autoregulation of multifunctional calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 274 26199–26208. 10.1074/jbc.274.37.26199 [DOI] [PubMed] [Google Scholar]
- Yan-You Huang P. V., Nguyen T. A., Kandel E. R. (1996). Long-lasting forms of synaptic potentiation in the mammalian hippocampus. Learn. Memory. 3 74–85. 10.1101/lm.3.2-3.74 [DOI] [PubMed] [Google Scholar]
- Yasuda H., Barth A. L., Stellwagen D., Malenka R. C. (2003). A developmental switch in the signaling cascades for LTP induction. Nat. Neurosci. 6 15–16. 10.1038/nn985 [DOI] [PubMed] [Google Scholar]
- Yasuda M., Mayford M. R. (2006). CaMKII activation in the entorhinal cortex disrupts previously encoded spatial memory. Neuron 50 309–318. 10.1016/j.neuron.2006.03.035 [DOI] [PubMed] [Google Scholar]
- Yin P., Xu H., Wang Q., Wang J., Yin L., Xu M., et al. (2017). Overexpression of βCaMKII impairs behavioral flexibility and NMDAR-dependent long-term depression in the dentate gyrus. Neuropharmacology 116 270–287. 10.1016/j.neuropharm.2016.12.013 [DOI] [PubMed] [Google Scholar]
- Yoshimura Y., Ichinose T., Yamauchi T. (2003). Phosphorylation of tau protein to sites found in Alzheimer’s disease brain is catalyzed by Ca2+/calmodulin-dependent protein kinase II as demonstrated tandem mass spectrometry. Neurosci. Lett. 353 185–188. 10.1016/j.neulet.2003.09.037 [DOI] [PubMed] [Google Scholar]
- Zalcman G., Federman N., Fiszbein A., de la Fuente V., Ameneiro L., Schor I., et al. (2019). Sustained CaMKII delta gene expression is specifically required for long-lasting memories in mice. Mole. Neurob. 56 1437–1450. 10.1007/s12035-018-1144-3 [DOI] [PubMed] [Google Scholar]
- Zalcman G., Federman N., Romano A. (2018). CaMKII isoforms in learning and memory: localization and function. Front. Mole. Neurosci. 11:445. 10.3389/fnmol.2018.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Zhao D., Xie C. W. (2010). Neurotrophins enhance CaMKII activity and rescue amyloid-β-induced deficits in hippocampal synaptic plasticity. J. Alzheimer’s Dis. 21 823–831. 10.3233/JAD-2010-100264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha X. M., Dailey M. E., Green S. H. (2009). Role of Ca2+/calmodulin-dependent protein kinase II in dendritic spine remodelling during epileptiform activity in vitro. J. Neurosci. Res. 87 1969–1979. 10.1002/jnr.22033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhang C., Vincent J., Zala D., Benstaali C., Sainlos M., et al. (2018). Modulation of AMPA receptor surface diffusion restores hippocampal plasticity and memory in Huntington’s disease models. Nat. Comm. 9 1–16. 10.1038/s41467-018-06675-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Shan H., Gu Z., Wang D., Wang T., Wang Z., et al. (2012). Increased expression of calcium/calmodulin-dependent protein kinase type II subunit delta after rat traumatic brain injury. J. Mole. Neurosci. 46 631–643. 10.1007/s12031-011-9651-y [DOI] [PubMed] [Google Scholar]
- Zhang X., Connelly J., Levitan E. S., Sun D., Wang J. Q. (2021). Calcium/Calmodulin–Dependent Protein Kinase II in Cerebrovascular Diseases. Transl. Stroke Res. 12 513–529. 10.1007/s12975-021-00901-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Qiao L., Xu W., Wang X., Li H., Xu W., et al. (2017). Paeoniflorin attenuates cerebral ischemia-induced injury by regulating Ca2+/CaMKII/CREB signalling pathway. Molecules 22:359. 10.3390/molecules22030359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. P., Holbro N., Oertner T. G. (2008). Optical induction of plasticity at single synapses reveals input-specific accumulation of αCaMKII. Proc. Natl. Acad. Sci. 105 12039–12044. 10.1073/pnas.0802940105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Chuang Y. A., Na Y., Ye Z., Yang L., Lin R., et al. (2019). Arc oligomerization is regulated by CaMKII phosphorylation of the GAG domain: an essential mechanism for plasticity and memory formation. Mol. Cell 75 13–25.e5. 10.1016/j.molcel.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N., Huang W., Cãtãlin B., Scheller A., Kirchhoff F. (2021). L-Type Ca2+ Channels of NG2 Glia Determine Proliferation and NMDA Receptor-Dependent Plasticity. Front. Cell Dev. Biol. 2021:9. 10.3389/fcell.2021.759477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Rosenke R., Kronemann D., Brim B., Das S. R., Dunah A. W., et al. (2009). The effects of aging on N-methyl-D-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory. Neuroscience 162 933–945. 10.1016/j.neuroscience.2009.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. J., Malinow R. (2002). Acute versus chronic NMDA receptor blockade and synaptic AMPA receptor delivery. Nat. Neurosci. 5 513–514. 10.1038/nn0602-850 [DOI] [PubMed] [Google Scholar]
- Zhu J. J., Qin Y., Zhao M., Van Aelst L., Malinow R. (2002). Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell 110 443–455. 10.1016/s0092-8674(02)00897-8 [DOI] [PubMed] [Google Scholar]
- Zou D. J., Cline H. T. (1999). Postsynaptic calcium/calmodulin-dependent protein kinase II is required to limit elaboration of presynaptic and postsynaptic neuronal arbors. J. Neurosci. 19 8909–8918. 10.1523/JNEUROSCI.19-20-08909.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zybura A. S., Baucum A. J., Rush A. M., Cummins T. R., Hudmon A. (2020). CaMKII enhances voltage-gated sodium channel Nav1. 6 activity and neuronal excitability. J. Biol. Chem. 295 11845–11865. 10.1074/jbc.RA120.014062 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.