Abstract
Purpose
The purpose of this study was to evaluate failed implants and reimplantation survival and to identify the relative risk factors for implant re-failure.
Methods
Ninety-one dental implants were extracted between 2006 and 2020 at the National Health Insurance Service Ilsan Hospital, including 56 implants in the maxilla and 35 implants in the mandible that were removed from 77 patients. Patient information (e.g., age, sex, and systemic diseases) and surgical information (e.g., the date of surgery and location of the implants and bone grafts) were recorded. If an implant prosthesis was used, prosthesis information was also recorded.
Results
In total, 91 first-time failed dental implants in 77 patients were analyzed. Of them, 69 implants in 61 patients received reimplantation after failure. Sixteen patients (22 implants) refused reimplantation or received reimplantation at a different site. Eight of the 69 reimplants failed again. The 1-year survival rate of the 69 reimplants was 89.4%. Age at reimplantation and smoking significantly increased the risk of reimplantation failure. However, a history of taking anti-thrombotic agents showed a statistically significant negative association with reimplantation failure. Of the failed implants, 66% showed early failure and 34% showed late failure of the initial implantation. All 8 re-failed implants showed early failure. Only 3 of these 8 failed reimplants were re-tried and the second reimplants all survived.
Conclusions
The total survival rate of implants, which included reimplants and second reimplants was 99.2%, although the survival rate of the initial implantations was 96.3%. Previous failure did not affect the success of the next trial. Reimplantation failure was more strongly affected by patient factors than by implant factors. Therefore, each patient’s specific factors need to be meticulously controlled to achieve successful reimplantation.
Keywords: Dental implants, Risk factors, Survival rate
Graphical Abstract
INTRODUCTION
Dental implants are increasingly used as restorative therapy for partially or completely edentulous patients. Various studies have shown that implants are predictable substitutes for missing teeth, with the 5-year survival rate of implant-supported prostheses reported to be 97.1% by Pjetursson et al. [1]. Furthermore, a systematic review [2] reported that the survival rate in 12–74 months of follow-up was 91.5%. Although the overall survival rate is high, implant failure remains a concern for clinicians.
Multiple etiological factors contribute to implant failure, which can be divided into early and late according to the timing of implant loss. Early implant failure refers to implant loss before occlusal loading [3]. It occurs mainly due to the lack of osseointegration [4]. Late implant failure occurs after functional loading, and it is caused by biological or mechanical complications [4,5].
Systematic reviews have reported that dental implant survival is associated with smoking, systemic disease, and a history of periodontitis [6,7]. The size, length, and surface of the dental implant can also affect implant failure [8,9]. Peri-implantitis is a major biological complication that can lead to implant failure with symptoms of marginal bone loss, suppuration, and implant mobility [7]. Timely identification of peri-implantitis can provide an opportunity to treat and save implants using non-surgical or surgical methods [10,11,12]. Nevertheless, if the treatment outcomes are not predictable, the clinician and the patient may consider removing the implant.
Mechanical complications are associated with bruxism, heavy occlusal force, and cantilever-type prostheses [13]. Off-axis forces and mechanical overloading contribute to implant fixture fractures or de-osseointegration [13], which can also lead to implant removal.
In most cases of dental implant failure, reimplantation is the first choice. If the cause of failure is that the first implant was not assessed properly, the reimplant could fail again for similar reasons. However, few studies have investigated reimplantation outcomes with consideration of factors that can increase the survival rate of reimplants [14,15].
Thus, the purpose of this study was to evaluate failed implants and the survival of reimplants. In addition, the relative risk factors for implant re-failure were identified.
MATERIALS AND METHODS
Subjects
In total, 91 failed dental implants in 77 patients, out of 2,442 dental implants placed in 1,751 patients between June 2006 and March 2020 at the Department of Periodontology at National Health Insurance Service Ilsan Hospital (Korea), were evaluated. The data were collected through clinical chart reviews.
The inclusion criteria for reimplantation were: 1) patients aged 20 to 80 years who received reimplantation; 2) no implant was previously placed into the site where the initial implant was placed; and 3) both the initial implantation and the reimplantation were performed at the Department of Periodontology at National Health Insurance Service Ilsan Hospital.
The exclusion criteria for reimplantation were: 1) the patient refused reimplantation; 2) the implantation site was changed at the time of reimplantation; and 3) insufficient surgical details were recorded in the chart.
The study protocol was reviewed and approved by the Institutional Review Board of National Health Insurance Service Ilsan Hospital (approval number: NHIMC 2019-07-021).
Retrospective data collection
Data such as patient factors, surgical information, and other factors were recorded retrospectively.
The patient factors were sex, age at reimplantation, hypertension (HTN), diabetes mellitus (DM), history of taking an anti-thrombotic agent, smoking status, and single-site failure or multiple-site failure.
The surgical information collected included surgery date (for the initial implantation, reimplantation, and second reimplantation), site (maxilla or mandible, anterior or posterior), submerged implant (for the initial implantation, reimplantation, and second reimplantation), and use of bone graft (for the initial implantation, reimplantation, and second reimplantation).
Other factors collected included failure time (early or late failure), time of reimplantation (immediate, early, or late), fixture change between the first implantation and second implantation (design, diameter, and length), and prosthodontic details (single or splinted, or bridge, cement or screw).
In the category of other factors, early failure referred to implant failure before connection of the prosthesis and late failure referred to failure after connection of the prosthesis to the implant. The timing of reimplantation was divided into immediate, early, and late because each reimplantation case had a different interval after the initial implant removal. Immediate reimplantation referred to reimplantation immediately after removal of the initial implant. Early reimplantation was performed within 16 weeks after the removal of the initial implant, and late reimplantation referred to reimplantation after 16 weeks. All fixtures in this study had moderately rough surfaces (Straumann [Basel, Switzerland], Dentium [Suwon, Korea], Osstem [Busan, Korea], Zimmer Biomet [Palm Beach Gardens, FL, USA] and Shinhung [Seoul, Korea]) and it was recorded whether there was a change in the fixture design (tissue level type or bone level type), diameter, or length.
Sixteen patients refused reimplantation. Thus, 69 reimplants were placed for 91 failed implants. The characteristics of the reimplants were recorded. Of the 69 reimplants in 61 patients, 8 reimplants failed again in 7 patients. These failed reimplants were evaluated and compared with the previously failed implants.
Statistical analysis
Statistical analyses were performed using SPSS version 23 (IBM Corp., Armonk, NY, USA) with a significance level of 5%. Survival analysis was performed using the Kaplan-Meier method. Different cross-analysis statistical methods for failure rates were used due to differences in the number of subjects between the groups. Failure rates were compared between initial implantation and reimplantation using the Fisher exact test. Separately, the failure rate of implants placed with a bone graft was compared using the χ2 test. Factors affecting implant failure were determined using Cox regression analysis.
RESULTS
Study population
Patient-related information for the study is summarized in Table 1. The mean age of the patients at the first failure was 60 years (range, 20–85 years). Of 77 patients who lost implants, 21 (27%) had HTN and 7 (9%) had DM. Fifteen (19%) patients were smokers, 7 patients were taking anti-thrombotic agents, and 13 patients had implant failures at multiple sites. After the removal of the failed implants, only 61 patients received reimplantation at the same site. Of them, 7 patients had reimplant failures. Of these 7 patients, 1 patient had failures of the reimplants at multiple sites.
Table 1. Demographics of patients with failed implants and failed reimplants.
| Variables | First failure | Second failure | |
|---|---|---|---|
| Sex | |||
| Male | 43 (55.8) | 4 (57.1) | |
| Female | 34 (44.2) | 3 (42.9) | |
| Age at reimplantation (yr) | 60 (range, 20–80) | 61 (range, 40–76) | |
| Systemic disease | |||
| Hypertension | 21 (27.2) | 4 (57.1) | |
| Diabetes mellitus | 7 (9.1) | 1 (14.3) | |
| Taking an anti-thrombotic agent | 7 (9.1) | 2 (25.0) | |
| Smoking | 15 (19.4) | 3 (42.8) | |
| Multiple site failure | 13 (16.9) | 1 (14.3) | |
Data shown are number (%) not otherwise specified.
The associations between the first reimplantation failure and patient factors (age, systemic disease, and smoking status) were analyzed using a univariate Cox regression model (Table 2). The age at reimplantation and a history of taking antithrombotic agents showed statistically significant associations with reimplant failure, although reimplant failure showed no statistically significant association with sex, HTN, DM, or smoking status. However, in multiple regression analysis (Table 2), smoking significantly increased the risk for reimplant failure.
Table 2. Cox regression model of factors related to reimplant failure.
| Factors | Survival (n=61) | Failure (n=8) | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|---|
| HR | P value | HR | P value | ||||
| Sex | 1.329 | 0.697 | |||||
| Male (ref.) | 34 | 5 | |||||
| Female | 27 | 3 | |||||
| Age at reimplantation | 26 (younger, ref.) to 76 | 1.083 | 0.020a) | 1.097 | 0.008a) | ||
| HTN | 0.502 | 0.330 | |||||
| No (ref.) | 42 | 4 | |||||
| Yes | 19 | 4 | |||||
| DM | 0.380 | 0.366 | 8.134 | 0.083 | |||
| No (ref.) | 58 | 7 | |||||
| Yes | 3 | 1 | |||||
| Antithrombotic agents taking | 0.172 | 0.032a) | |||||
| No (ref.) | 58 | 6 | |||||
| Yes | 3 | 2 | |||||
| Smoking habit | 0.297 | 0.087 | 4.789 | 0.042a) | |||
| No (ref.) | 48 | 4 | |||||
| Yes | 13 | 4 | |||||
| Anterior-posterior | 0.701 | 0.739 | |||||
| Anterior (ref.) | 10 | 1 | |||||
| Posterior | 51 | 7 | |||||
| Maxilla-mandible | 3.842 | 0.208 | |||||
| Maxilla (ref.) | 38 | 7 | |||||
| Mandible | 23 | 1 | |||||
| Bone graft at initial implantation | 0.456 | 0.463 | |||||
| No (ref.) | 16 | 1 | |||||
| Yes | 45 | 7 | |||||
| Nonsubmerged or submerged at initial implantation | 0.646 | 0.550 | |||||
| Nonsubmerged (ref.) | 31 | 2 | |||||
| Submerged | 30 | 6 | |||||
| Early-late failure of initial implantation | 3.967 | 0.197 | |||||
| Early (ref.) | 18 | 7 | |||||
| Late | 43 | 1 | |||||
| Timing of reimplantation | |||||||
| Immediate (ref.) | 12 | 2 | |||||
| Early | 24 | 4 | 1.041 | 0.963 | |||
| Late | 25 | 2 | 0.521 | 0.515 | |||
| Fixture length change | 2.007 | 0.340 | |||||
| No (ref.) | 31 | 5 | |||||
| Yes | 30 | 3 | |||||
| Bone graft at reimplantation | 0.211 | 0.057 | |||||
| No (ref.) | 39 | 2 | |||||
| Yes | 22 | 6 | |||||
| Nonsubmerged or submerged at reimplantation | 1.684 | 0.523 | |||||
| Nonsubmerged (ref.) | 23 | 2 | |||||
| Submerged | 38 | 6 | |||||
All HRs listed are with respect to the reference categories. The multivariate analysis of this model included age at reimplantation, HTN, DM, taking an antithrombotic agent, smoking, a bone graft at initial implantation, the implantation site (maxilla or mandible), failure time of initial implantation (early or late), and a bone graft at reimplantation, which were thought to be highly relevant. Finally, 3 variables (DM, smoking, and age at reimplantation) remained. A HR >1 suggests an increased risk for reimplantation failure.
HR: hazard ratio, HTN: hypertension, DM: diabetes mellitus.
a)Statistically significant difference compared to the reference, P<0.05.
Survival rate and failure rate
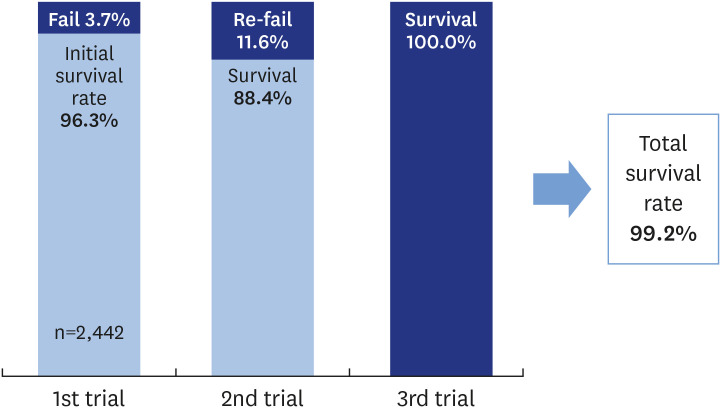
Figure 1 demonstrates the overall failure rate. Implant failure was observed in 3.7% of the implants and 4.4% of the patients, while 11.6% of reimplants in 11.5% of the patients failed again. The difference in failure rates between the initial implants and the reimplants was statistically significant (P<0.05, Fisher exact test). However, the failure rate of reimplantation at the previously failed site was 11.6% due to spontaneous falling out or explanation, with 8 instances of implant re-failure. Three of them received a second reimplantation, and all of the second reimplants survived.
Figure 1. Overall failure rate.
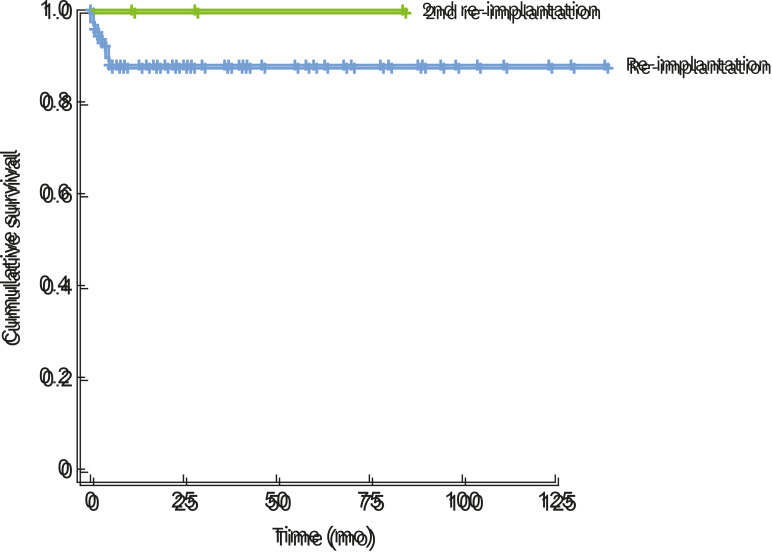
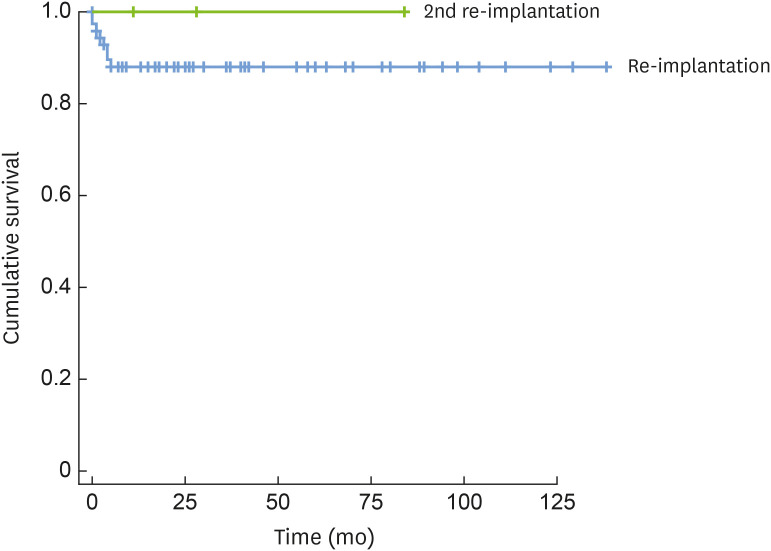
The cumulative survival rate of reimplants was calculated using Kaplan-Meier curve analysis. The 1-year survival rate of the 69 reimplants was 89.4% (Figure 2).
Figure 2. Cumulative survival rate of reimplantation (Kaplan-Meier curve analysis).
The blue line indicates the cumulative survival of reimplantation and the green line indicates cumulative survival of the second reimplantation.
Failure time and reasons for failure
The early failure rate was 66% and the late failure rate was 34% for failed implants. The reasons for the 91 failed implants were analyzed separately for early and late failures (Table 3). Most early failures had an unspecified cause (35%). Inflammation and infection accounted for 32% and 22%, respectively, of the early failures. Other causes included iatrogenic problems (malposition and nerve damage) and fixture problems. The reasons for late failures included biological problems (i.e., peri-implantitis) in 19 (61%) of a total of 31 cases, accounting for more than half of the late failures. The other causes of late failures included unspecified reasons, overloading, infections, and fixture problems.
Table 3. Reasons for early and late failures of the initial implantation, reimplantation, and second reimplantation.
| Reasons | Failures | |||||
|---|---|---|---|---|---|---|
| Initial implantation | Reimplantation | Second reimplantation | ||||
| Early | Late | Early | Late | Early | Late | |
| Unspecified | 21 (35) | 4 (12.9) | 6 (75) | 0 | 0 | 0 |
| Inflammation | 19 (31.7) | 19 (61.3) | 1 (12.5) | 0 | 0 | 0 |
| Infection | 13 (21.7) | 1 (3.2) | 1 (12.5) | 0 | 0 | 0 |
| Iatrogenic | 6 (10) | 0 | 0 | 0 | 0 | 0 |
| Fixture problem | 1 (1.7) | 3 (9.7) | 0 | 0 | 0 | 0 |
| Overloading | 0 | 4 (12.9) | 0 | 0 | 0 | 0 |
| Total number | 60 (66) | 31 (33) | 8 (100) | 0 | 0 | 0 |
Data shown are number (%) not otherwise specified.
Eight reimplantation failures were seen in 0–5 months after the reimplant was placed and they were all early failures (Table 3). In the univariate Cox regression model, the failure time of the initial implantation was not significantly associated with reimplant failure (Table 2). There were no cases of second reimplant failure by the last follow-up date.
Surgical site
Of the 91 failed implants included in this study, 56 were in the maxilla (11 anterior and 45 posterior) and 35 were in the mandible (2 anterior and 33 posterior) (Table 4). Thirty-one initial implants failed at the maxillary first-molar area, which was the most common failure site (34%). Regarding the site of the 8 reimplantation failures, there were 7 reimplants in the maxilla (1 anterior and 6 posterior) and 1 reimplant in the posterior mandible. In the univariate Cox regression model, the surgical site (anterior or posterior, maxilla or mandible) was not significantly associated with reimplant failure (Table 2).
Table 4. Characteristics of failed implant and failed reimplant sites.
| Variables | First failure | Second failure | |
|---|---|---|---|
| Site | |||
| Maxilla | 56 (61.5) | 7 (87.5) | |
| Mandible | 35 (38.5) | 1 (12.5) | |
| Anterior | 13 (14.2) | 1 (12.5) | |
| Posterior | 78 (85.7) | 7 (87.5) | |
| Submerged | |||
| Nonsubmerged | 47 (51.6) | 2 (25.0) | |
| Submerged | 44 (48.4) | 6 (75.0) | |
| Bone graft | |||
| Yes | 23 (25.3) | 6 (75.0) | |
| No | 68 (74.7) | 2 (25.0) | |
Data shown are number (%) not otherwise specified.
Bone grafting
Among the 91 implants, 74.7% underwent simultaneous bone grafting with the initial implantation (Table 4). Bone grafting was categorized based on the method, as simple bone graft, guided bone regeneration (GBR), osteotome sinus floor elevation, bone-added osteotome sinus floor elevation, sinus elevation by the lateral approach, and 2 or more of the above. Sixty-nine reimplants were placed at the previously failed site and only 28 cases were reimplanted with a bone graft. Of the 8 failed reimplants, 6 (75%) sites received bone grafting with the reimplantation. Bone grafting in the initial implantation and reimplantation was not significantly associated with reimplantation failure in the Cox regression analysis (Table 2). However, the log-rank test in the Kaplan-Meier curve analysis showed that bone grafting with spontaneous reimplantation at the failed site had a statistically significant negative effect on the survival rate of the reimplant (P=0.035).
Nonsubmerged and submerged implants
Among 91 failed implants, 47 were nonsubmerged and 44 were submerged (Table 4). At reimplantation, 25 implants were nonsubmerged and 44 implants were submerged. Among the 69 reimplants, 2 nonsubmerged reimplants and 6 submerged reimplants failed. Cox regression analysis showed no significant differences in reimplantation failure between nonsubmerged or submerged initial implants and reimplants (Table 2).
Timing of reimplantation
The interval from the initial failure to reimplantation ranged from 0 and 12 months. The timing of reimplantation was divided into immediate, early, and late. Fourteen cases were immediately reimplanted among 69 reimplants. Twenty-eight cases involved early reimplantation and 26 cases were late reimplantation. In the Cox regression analysis, there was no significant association between the timing of reimplantation and reimplantation failure (Table 2).
Fixture change and prosthodontic details
There were 20 cases of fixture design changes, 19 fixture diameter changes, and 36 fixture length changes. Furthermore, 27 cases (17 single crowns and 10 multiple crowns) had prostheses at the initial implantation. However, due to the lack of cases for analysis, statistical results were only derived for fixture length change, and there was no significant association with reimplantation failure (Table 2).
Multivariate Cox regression analysis
The multivariate Cox regression model included age at reimplantation, HTN, DM, taking an antithrombotic agent, smoking, use of a bone graft at the first implantation, the surgical site (maxilla or mandible), time of the first implant failure (early or late), fixture length, and use of a bone graft at the implantation, which were thought to be relevant. Finally, three variables (DM, smoking, and age at reimplantation) remained (Table 2). Age at reimplantation and smoking significantly increased the risk of reimplant failure.
DISCUSSION
There was a clear discrepancy in the failure rate between the initial implantation and reimplantation at the failed site in the present study. Several studies have reported lower survival rates for reimplants, similar to this study. Systematic reviews have also reported survival rates of reimplantation from 71% to 100% [16] and 88.7% [17]. A higher failure rate was observed for reimplants than for the initial implants. Thus, additional precautions are required during reimplantation at a previously failed site. The purpose of the present study was to determine the outcomes of reimplantation and to identify the factors influencing survival.
Patient-related factors such as age, sex, HTN, DM, and smoking status were evaluated. Among these factors, age at reimplantation was positively associated with the failure rate of the reimplants. Aging jeopardizes proper bone healing because it negatively impacts the inflammatory phase [18]. Increasing age could be associated with an increased risk of tissue damage because of a dysregulated immune response and the persistence of inflammatory cells in the periodontal tissue [19]. There was also less plaque accumulation on implants, and the peri-implant mucosa showed a stronger response than the gingiva around the teeth in elderly patients [20]. For reimplant survival, oral hygiene, poor local bone quality, poor bone quantity, and the biological mechanism of bone remodeling, which leads to osseointegration, are more important risk factors than age [21]. Therefore, age should be considered a risk factor, not a contraindication.
Multiple researchers [22,23] have observed that smoking history did not affect the failure rate of reimplantation. However, Alsaadi et al. [24] reported a higher failure rate of implants in patients who smoked more than 20 cigarettes a day. A systematic review reported that smoking negatively influenced bone healing [25]. Smoking history was statistically significant in the multivariate analysis in the present study. In particular, reimplantation failure more frequently occurred in smokers (hazard ratio, 4.79) (Table 2). Exposure to nicotine has been reported to affect osteogenesis and angiogenesis and to be associated with an increased risk of implant failure [26]. The smokers who experienced reimplant failure did not quit smoking in the present study and showed a higher failure rate. Therefore, smoking could be a potent risk factor for reimplantation failure, especially heavy smoking.
HTN was observed to be unrelated to reimplant failure. However, taking an antithrombotic agent had a significantly negative correlation with reimplantation failure in the univariate Cox regression analysis. Chrcanovic et al. [27] reported that reimplant failure was not related to HTN, although a higher implant failure rate was observed in patients taking antithrombotic agents. Conversely, Agari and Le [14] reported that patients taking antithrombotic agents had lower failure rates of reimplantation. They explained that these agents could protect bone regeneration and osseointegration during implant placement [14]. In addition, some studies reported that the use of an antithrombotic agent such as aspirin contributed to better bone healing by regulating bone remodeling factors [28,29].
Several studies reported that well-controlled DM was not a risk factor for dental implant survival [27,30,31]. However, DM still showed a tendency to affect the rate of reimplant failure in this study,, although the relationship was not statistically significant. Hyperglycemia can inhibit osteoblastic differentiation and affect proper bone remodeling [32]. Patients with poorly controlled DM tended to have delayed osseointegration compared to healthy patients [31]. In animal studies, rats with uncontrolled DM showed decreasing bone-to-implant contact with time [30]. Moreover, Monje et al. [33] reported that DM was a high-risk factor for peri-implantitis, which can lead to implant failure. Thus, clinicians should be aware that DM could be a risk factor for reimplantation failure. If it is not controlled, this risk can become more serious.
In this study, the reimplant failures were all early failures, before the implant prosthesis was connected. There was no statistically significant difference in reimplant failure according to the time of the previous failure (early or late), indicating that a previous failure did not affect the success of the next trial. A higher implant failure rate was observed when a bone graft was used at reimplantation than when a bone graft was not used. This may not be a problem inherently related to bone grafts; rather, once a dental implant fails, it may form a larger defect and create poorer bone quality, with a lack of bone volume, which increases the failure rate of reimplants [34]. The implant removal site is usually made to form a 3-wall defect [35]. For subsequent implant placement, GBR can give good results when the implant is removed [35]. Similar to this study, other studies have also observed a tendency for early failure in reimplants [14,23,36]. Some studies have suggested that site-specific conditions of the failed site can interfere with the osseointegration of reimplants and lead to early failure [14,27].
The second reimplantation (third trial) was observed to succeed in 3 out of 3 cases, with a failure rate of 0% as of the final follow-up in this study. The main focus of the second reimplantation was to eliminate the risk factors of previous trials. Patients with DM needed to control their glucose levels and smokers were advised to quit smoking. If the patient was unable to quit smoking, the third trial was abandoned based on a consideration of various site factors. Moreover, patients waited for a longer period than in previous attempts to compensate for the compromised bone quality and quantity of the re-failed site. The average waiting time for these 3 second reimplants for occlusal loading with a prosthesis after the second reimplant placement was 13 months, which was longer than the general waiting time [37]. Vayron et al. [38] performed animal studies and found that a longer waiting period after implantation was associated with a higher bone-to-implant contact ratio. As in this study, for implants placed in poor-quality bone, a higher bone-to-implant contact ratio was associated with better stress distribution for the contacted bone when masticatory pressure was applied to the implant [39]. Accordingly, the possibility of implant failure may decrease.
This study had some limitations. First, failed implants performed by 3 or more clinicians were collected and analyzed. There might have been differences in the technique of each clinician. Further analysis was also difficult due to the lack of implantation site information, such as bone quality and quantity. Moreover, some statistical analyses could not be performed because there were not many failed implants.
The 1-year survival rate of reimplantation was 89.4%. The failure rate of the reimplants was higher than that of the initial implants. To decrease the failure rate of reimplants, clinicians should make efforts to find and analyze the factors affecting previous failures and extend the submerged period after implantation. At the same site, the total survival rate of implants (including reimplants and second reimplants) was 99.2%, although the survival rate of the initial implants was 96.3%. A previous failure did not affect the success of the next trial. Failure of reimplantation was more strongly affected by patient factors than by implant factors. Therefore, each patient’s specific factors need to be meticulously controlled to achieve successful reimplantation.
Footnotes
Funding: This work was supported by a grant (NHIMC2018CR059) from National Health Insurance Service Ilsan Hospital.
- Conceptualization: Young-Taek Kim.
- Formal analysis: Yu-Seon Park.
- Funding acquisition: Young-Taek Kim Investigation.
- Methodology: Yu-Seon Park, Young-Taek Kim.
- Validation: Seong-Ho Choi.
- Supervision: Seong-Ho Choi, Young-Taek Kim.
- Writing - original draft: Yu-Seon Park, Bo-Ah Lee.
- Writing – review & Editing: Yu-Seon Park, Seong-Ho Choi, Young-Taek Kim.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Pjetursson BE, Asgeirsson AG, Zwahlen M, Sailer I. Improvements in implant dentistry over the last decade: comparison of survival and complication rates in older and newer publications. Int J Oral Maxillofac Implants. 2014;29 Suppl:308–324. doi: 10.11607/jomi.2014suppl.g5.2. [DOI] [PubMed] [Google Scholar]
- 2.Del Fabbro M, Testori T, Francetti L, Weinstein R. Systematic review of survival rates for implants placed in the grafted maxillary sinus. Int J Periodontics Restorative Dent. 2004;24:565–577. [PubMed] [Google Scholar]
- 3.Derks J, Håkansson J, Wennström JL, Tomasi C, Larsson M, Berglundh T. Effectiveness of implant therapy analyzed in a Swedish population: early and late implant loss. J Dent Res. 2015;94:44S–51S. doi: 10.1177/0022034514563077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montes CC, Pereira FA, Thomé G, Alves ED, Acedo RV, de Souza JR, et al. Failing factors associated with osseointegrated dental implant loss. Implant Dent. 2007;16:404–412. doi: 10.1097/ID.0b013e31815c8d31. [DOI] [PubMed] [Google Scholar]
- 5.Sakka S, Baroudi K, Nassani MZ. Factors associated with early and late failure of dental implants. J Investig Clin Dent. 2012;3:258–261. doi: 10.1111/j.2041-1626.2012.00162.x. [DOI] [PubMed] [Google Scholar]
- 6.Dreyer H, Grischke J, Tiede C, Eberhard J, Schweitzer A, Toikkanen SE, et al. Epidemiology and risk factors of peri-implantitis: a systematic review. J Periodontal Res. 2018;53:657–681. doi: 10.1111/jre.12562. [DOI] [PubMed] [Google Scholar]
- 7.Mombelli A, Müller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012;23 Suppl 6:67–76. doi: 10.1111/j.1600-0501.2012.02541.x. [DOI] [PubMed] [Google Scholar]
- 8.Steigenga JT, al-Shammari KF, Nociti FH, Misch CE, Wang HL. Dental implant design and its relationship to long-term implant success. Implant Dent. 2003;12:306–317. doi: 10.1097/01.id.0000091140.76130.a1. [DOI] [PubMed] [Google Scholar]
- 9.Chuang SK, Wei LJ, Douglass CW, Dodson TB. Risk factors for dental implant failure: a strategy for the analysis of clustered failure-time observations. J Dent Res. 2002;81:572–577. doi: 10.1177/154405910208100814. [DOI] [PubMed] [Google Scholar]
- 10.Prathapachandran J, Suresh N. Management of peri-implantitis. Dent Res J (Isfahan) 2012;9:516–521. doi: 10.4103/1735-3327.104867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claffey N, Clarke E, Polyzois I, Renvert S. Surgical treatment of peri-implantitis. J Clin Periodontol. 2008;35:316–332. doi: 10.1111/j.1600-051X.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 12.Renvert S, Roos-Jansåker AM, Claffey N. Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol. 2008;35:305–315. doi: 10.1111/j.1600-051X.2008.01276.x. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz MS. Mechanical complications of dental implants. Clin Oral Implants Res. 2000;11 Suppl 1:156–158. doi: 10.1034/j.1600-0501.2000.011s1156.x. [DOI] [PubMed] [Google Scholar]
- 14.Agari K, Le B. Successive reimplantation of dental implants into sites of previous failure. J Oral Maxillofac Surg. 2020;78:375–385. doi: 10.1016/j.joms.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Oh SL, Shiau HJ, Reynolds MA. Survival of dental implants at sites after implant failure: a systematic review. J Prosthet Dent. 2020;123:54–60. doi: 10.1016/j.prosdent.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Quaranta A, Perrotti V, Piattelli A, Piemontese M, Procaccini M. Implants placed in sites of previously failed implants: a systematic review. Implant Dent. 2014;23:311–318. doi: 10.1097/ID.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 17.Gomes GH, Misawa MYO, Fernandes C, Pannuti CM, Saraiva L, Huynh-Ba G, et al. A systematic review and meta-analysis of the survival rate of implants placed in previously failed sites. Braz Oral Res. 2018;32:e27. doi: 10.1590/1807-3107bor-2018.vol32.0027. [DOI] [PubMed] [Google Scholar]
- 18.Gibon E, Lu L, Goodman SB. Aging, inflammation, stem cells, and bone healing. Stem Cell Res Ther. 2016;7:44. doi: 10.1186/s13287-016-0300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Preshaw PM, Henne K, Taylor JJ, Valentine RA, Conrads G. Age-related changes in immune function (immune senescence) in caries and periodontal diseases: a systematic review. J Clin Periodontol. 2017;44 Suppl 18:S153–S177. doi: 10.1111/jcpe.12675. [DOI] [PubMed] [Google Scholar]
- 20.Meyer S, Giannopoulou C, Courvoisier D, Schimmel M, Müller F, Mombelli A. Experimental mucositis and experimental gingivitis in persons aged 70 or over. Clinical and biological responses. Clin Oral Implants Res. 2017;28:1005–1012. doi: 10.1111/clr.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ikebe K, Wada M, Kagawa R, Maeda Y. Is old age a risk factor for dental implants? Jpn Dent Sci Rev. 2009;45:59–64. [Google Scholar]
- 22.Mardinger O, Ben Zvi Y, Chaushu G, Nissan J, Manor Y. A retrospective analysis of replacing dental implants in previously failed sites. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114:290–293. doi: 10.1016/j.tripleo.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Machtei EE, Mahler D, Oettinger-Barak O, Zuabi O, Horwitz J. Dental implants placed in previously failed sites: survival rate and factors affecting the outcome. Clin Oral Implants Res. 2008;19:259–264. doi: 10.1111/j.1600-0501.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- 24.Alsaadi G, Quirynen M, Komárek A, van Steenberghe D. Impact of local and systemic factors on the incidence of oral implant failures, up to abutment connection. J Clin Periodontol. 2007;34:610–617. doi: 10.1111/j.1600-051X.2007.01077.x. [DOI] [PubMed] [Google Scholar]
- 25.Patel RA, Wilson RF, Patel PA, Palmer RM. The effect of smoking on bone healing: a systematic review. Bone Joint Res. 2013;2:102–111. doi: 10.1302/2046-3758.26.2000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chrcanovic BR, Albrektsson T, Wennerberg A. Smoking and dental implants: a systematic review and meta-analysis. J Dent. 2015;43:487–498. doi: 10.1016/j.jdent.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Chrcanovic BR, Kisch J, Albrektsson T, Wennerberg A. Survival of dental implants placed in sites of previously failed implants. Clin Oral Implants Res. 2017;28:1348–1353. doi: 10.1111/clr.12992. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y, Xiong J, Mei S, Wang F, Zhao Z, Wang S, et al. Aspirin promotes bone marrow mesenchymal stem cell-based calvarial bone regeneration in mini swine. Stem Cell Res Ther. 2015;6:210. doi: 10.1186/s13287-015-0200-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang W, Lu X, Yuan Z, Shen M, Song Y, Liu H, et al. Establishing an osteoimmunomodulatory coating loaded with aspirin on the surface of titanium primed with phase-transited lysozyme. Int J Nanomedicine. 2019;14:977–991. doi: 10.2147/IJN.S190766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Javed F, Romanos GE. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: a systematic literature review. J Periodontol. 2009;80:1719–1730. doi: 10.1902/jop.2009.090283. [DOI] [PubMed] [Google Scholar]
- 31.Naujokat H, Kunzendorf B, Wiltfang J. Dental implants and diabetes mellitus-a systematic review. Int J Implant Dent. 2016;2:5. doi: 10.1186/s40729-016-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sghaireen MG, Alduraywish AA, Srivastava KC, Shrivastava D, Patil SR, Al Habib S, et al. Comparative evaluation of dental implant failure among healthy and well-controlled diabetic patients-a 3-year retrospective study. Int J Environ Res Public Health. 2020;17:5253. doi: 10.3390/ijerph17145253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monje A, Catena A, Borgnakke WS. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: systematic review and meta-analysis. J Clin Periodontol. 2017;44:636–648. doi: 10.1111/jcpe.12724. [DOI] [PubMed] [Google Scholar]
- 34.Chrcanovic BR, Albrektsson T, Wennerberg A. Bone quality and quantity and dental implant failure: a systematic review and meta-analysis. Int J Prosthodont. 2017;30:219–237. doi: 10.11607/ijp.5142. [DOI] [PubMed] [Google Scholar]
- 35.Solderer A, Al-Jazrawi A, Sahrmann P, Jung R, Attin T, Schmidlin PR. Removal of failed dental implants revisited: questions and answers. Clin Exp Dent Res. 2019;5:712–724. doi: 10.1002/cre2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim YK, Park JY, Kim SG, Lee HJ. Prognosis of the implants replaced after removal of failed dental implants. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:281–286. doi: 10.1016/j.tripleo.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Whetman J, Mealey BL. Effect of healing time on new bone formation after tooth extraction and ridge preservation with demineralized freeze-dried bone allograft: a randomized controlled clinical trial. J Periodontol. 2016;87:1022–1029. doi: 10.1902/jop.2016.160139. [DOI] [PubMed] [Google Scholar]
- 38.Vayron R, Soffer E, Anagnostou F, Haïat G. Ultrasonic evaluation of dental implant osseointegration. J Biomech. 2014;47:3562–3568. doi: 10.1016/j.jbiomech.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Ohyama T, Uchida T, Shibuya N, Nakabayashi S, Ishigami T, Ogawa T. High bone-implant contact achieved by photofunctionalization to reduce periimplant stress: a three-dimensional finite element analysis. Implant Dent. 2013;22:102–108. doi: 10.1097/ID.0b013e31827b9415. [DOI] [PubMed] [Google Scholar]