Abstract
Purpose
In males, testosterone levels have been implicated in various diseases. Recently, the influence of gut microbial-derived compounds on host metabolism has become evident, and it has been suggested that some gut bacteria may be involved in testosterone metabolism. In the present study, we examined the relationship between testosterone levels and gut microbiota in elderly Japanese men.
Materials and Methods
We collected samples from Japanese male subjects suspected of having prostate cancer and underwent prostate biopsies and excluded patients with positive biopsies to avoid the effect of prostate cancer on the gut microbiota. In total, 54 Japanese males with negative biopsy results were included in our study. The gut microbiota was analyzed by 16S rRNA gene sequencing of bacterial DNA extracted from rectal swabs. Gut microbiota compositions were compared between the two groups according to the level of serum testosterone (above or below 3.5 ng/mL).
Results
The median age of the cohort was 71 years, and the quartile range was 67 to 73 years. We observed no significant difference in alpha or beta diversity, but some bacteria belonging to the phylum Firmicutes (Clostridiales, Turicibacter, and Gemella) were increased in the high testosterone group. Serum testosterone levels positively correlated with the relative amount of Firmicutes (rS=0.3323, p=0.0141), and the amount of Firmicutes affected serum testosterone levels independent of host factors (age, body mass index, triglyceride, and total cholesterol; β=0.770, p=0.0396).
Conclusions
Some intestinal bacteria belonging to the phylum Firmicutes were associated with testosterone levels in elderly males. Therefore, the gut microbiota could affect testosterone metabolism in elderly males.
Keywords: Firmicutes, Gastrointestinal microbiome, Metagenomics, Testosterone
INTRODUCTION
Microorganisms are present on every surface of the human body and are known to play important and diverse roles in human health and disease [1]. In particular, the gut microbiota, which is composed of 1013 to 1014 microorganisms [2], is the largest and most investigated human flora. Gut microbiota critically impacts the local condition of the intestinal tract, as well as distant organs and systemic diseases, such as liver or neurological diseases [3,4,5].
Recently, intestinal bacteria-derived metabolites, including testosterone, have been the focus of attention. Testosterone is produced mainly by Leydig cells in the testis and adrenal glands of males. Certain bacteria promote testosterone metabolism and reabsorption in the colon [6]. Therefore, it has been suggested that the gut microbiota may be associated with host testosterone levels. Several studies have evaluated the relationship between the microbiota and polycystic ovarian syndrome (PCOS), a disease caused by high testosterone production in female patients [7,8]. However, few reports are available on testosterone levels and the gut microbiota in males. As testosterone plays an important role in several diseases in male patients, such as metabolic syndrome, prostate cancer, and late-onset hypogonadism syndrome [9,10], it would be worthwhile to investigate the relationship between the gut microbiota and host testosterone levels in elderly male subjects.
In the present study, we examined the gut microbiota composition and blood testosterone levels in samples derived from elderly Japanese males. The objective of this study was to elucidate the relationship between the gut microbiota composition and testosterone levels in elderly males.
MATERIALS AND METHODS
1. Study design
We collected rectal swab samples from 177 Japanese male subjects suspected of having prostate cancer based on screening procedures and underwent prostate biopsies from December 2018 to October 2020 at Osaka University Hospital. We analyzed 54 samples of males with negative biopsy; samples of patients with positive biopsies were excluded to avoid the effect of prostate cancer on the gut microbiota. Among the 54 samples, 37 were included in the cohort of our previous report on the gut microbiota profile associated with prostate cancer [11]. Patients treated with antibiotics within six months of sample collection were omitted from the analysis. Other detailed exclusion criteria have been listed in our previous report [11].
2. Rectal swab collection and bacterial DNA extraction
The swab samples were collected during a sterile digital rectal examination before the administration of pre-biopsy antibiotics. The swabs were maintained at -80℃ until DNA extraction. Bacterial DNA was extracted from the samples using the DNeasy Power Soil Kit (Qiagen, Hilden, Germany).
3. Analysis of the gut microbiota
Bacterial DNA was analyzed using amplicon sequencing targeting the V1–V2 variable regions of the 16S rRNA gene. The QIIME pipeline version 1.9.1 was used to process raw sequencing data. A detailed analysis was performed as described in our previous report [11].
4. Human testosterone measurement
Human blood samples used for serum testosterone measurements were collected prior to the prostate biopsy. In accordance with the Endocrine Society’s guidelines [12], the blood collection time for testosterone measurement was restricted between 8:00 am and 9:00 am to reduce errors owing to diurnal variation. Total testosterone (TT) levels in human blood were measured using Lumipulse Presto Testosterone (Fujirebio Inc., Tokyo, Japan).
5. Statistical analysis
The Mann–Whitney U-test was used to compare characteristics between the two TT groups. Alpha diversity was assessed by rarefaction analysis of values for evenness and richness, and we statistically compared each value at 10,000 sequences. Beta diversity was evaluated by principal coordinate analysis (PCoA) and analysis of similarities (ANOSIM). ANOSIM was calculated using the R version 4.0.2 package ‘Vegan.’ Comparison of each bacterial taxa was performed using linear discriminant analysis (LDA) effect size (LEfSe) with the Galaxy web application (https://huttenhower.sph.harvard.edu/galaxy/). Correlations between bacterial taxon abundance and TT levels were evaluated using Spearman’s rank correlation coefficient. Univariate and multivariate analyses were performed using multiple regression analysis. No outliers were excluded from the analysis. Statistical significance was set at p<0.05. Except for ANOSIM and LEfSe, all statistical tests were performed using JMP Pro 14 (SAS Institute, Cary, NC, USA).
6. Ethics statement
This study was approved by the Institutional Review Board of Osaka University (IRB #13397-16), and written informed consent was obtained from all patients.
RESULTS
1. Patient characteristics
The cohort was divided into two groups according to blood TT levels. The cut-off point was set at 3.5 ng/mL, which is close to the normal threshold in EAU recommendations [13]. TT high group for subjects exhibiting levels >3.5 ng/mL and TT low group for all other subjects. The TT high group comprised 28 subjects, while the TT low group was composed of 26 subjects. Only five subjects exhibited TT levels below the cut-off point for hypogonadism (2.3 ng/mL) [13]. Patient characteristics of the two groups are summarized in Table 1. We observed no significant difference in age (p=0.106) and prostate-specific antigen (p=0.782), but the TT low group showed a significantly higher body mass index (BMI; p=0.0402). The serum triglyceride (TG) level was significantly higher in the TT low group than the TT high group (p=0.0449); however, total cholesterol (T-Chol) levels did not significantly differ between groups (p=0.197).
Table 1. Background characteristics of the cohort.
| Parameter | All | TT high groupa | TT low groupb | p-valuec |
|---|---|---|---|---|
| Case | 54 | 28 | 26 | |
| Age (y) | 71.0 (67.0–73.0) | 72.0 (68.0–74.3) | 70.0 (65.3–71.8) | 0.106 |
| BMI (kg/m2) | 23.2 (21.6–25.5) | 22.5 (21.0–24.3) | 24.9 (22.0–26.2) | 0.0402 |
| TT (ng/mL) | 3.59 (2.80–4.74) | 4.63 (3.95–5.65) | 2.72 (2.43–3.12) | <0.0001 |
| T-Chol (mg/dL) | 206 (180–220) | 204 (180–216) | 208 (195–229) | 0.197 |
| TG (mg/dL) | 138 (103–210) | 111 (89–171) | 171 (125–234) | 0.0449 |
| PSA (mg/dL) | 7.0 (4.6–9.3) | 6.7 (4.6–9.5) | 7.1 (4.5–7.9) | 0.782 |
Values are presented as number only or median (interquartile range).
aBlood TT level >3.5 ng/mL. bBlood TT level ≤3.5 ng/mL. cTT high group vs. TT low group.
BMI: body mass index, TT: total testosterone, T-Chol: total cholesterol, TG: triglyceride, PSA: prostate-specific antigen.
2. Diversity of gut microbiota is not affected by testosterone levels
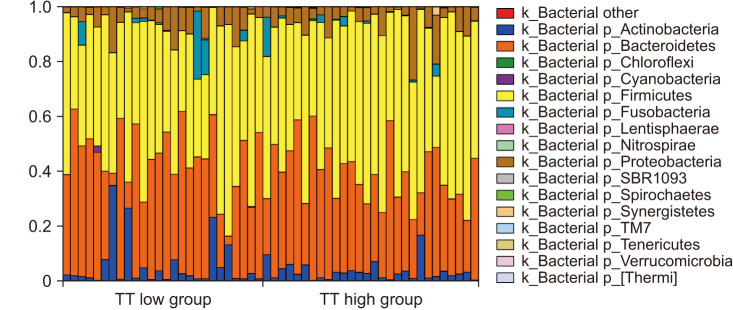
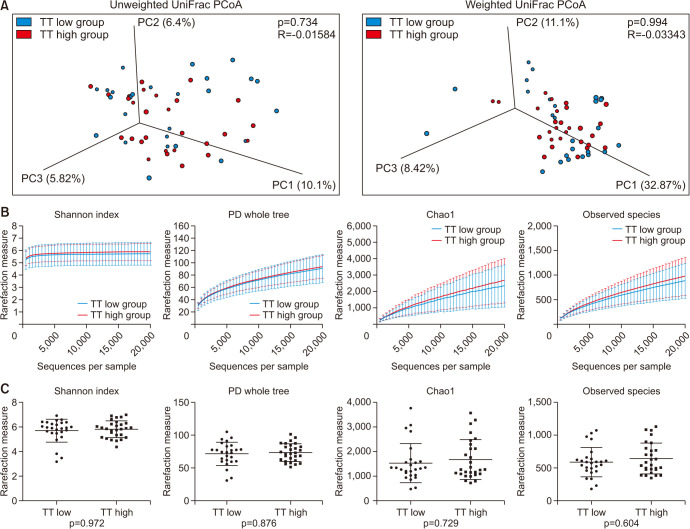
The composition of the gut microbiota in all samples at the phylum level is shown in Fig. 1. In most examined samples, the gut microbiota was dominated by Firmicutes (median 50.2%) and Bacteroides (median 38.4%). For beta diversity, weighted and unweighted UniFrac distances within and between both groups did not differ significantly (unweighted UniFrac distance, p=0.734; weighted UniFrac distance, p=0.994), and PCoA revealed that samples were not separated into groups (Fig. 2A). Alpha diversity was assessed using four types of values (Shannon index, phylogenetic diversity (PD) whole tree, Chao 1, and Observed species); however, none differed significantly between the TT high and TT low groups (p=0.972, 0.876, 0.729, and 0.604, respectively; Fig. 2B, 2C).
Fig. 1. The composition of the gut microbiota in 54 samples at the phylum level. Bars are arranged in the order of serum TT levels. k_: kingdom, p_: phylum, TT: total testosterone.
Fig. 2. The diversity of the gut microbiota. (A) PCoA plots based on unweighted (top) and weighted UniFrac distances (bottom) showing the gut microbiota composition. Blue dots represent the TT low group and red dots represent the TT high group. Rarefaction analysis (B) and boxplots at 10,000 sequences (C) of Shannon index, PD whole tree, Chao1 index, and observed species. Data are presented as the mean±standard deviation. PCoA: principal coordinate analysis, TT: total testosterone, PD: phylogenetic diversity.
3. Relative abundance of Firmicutes in gut microbiota correlates with testosterone levels
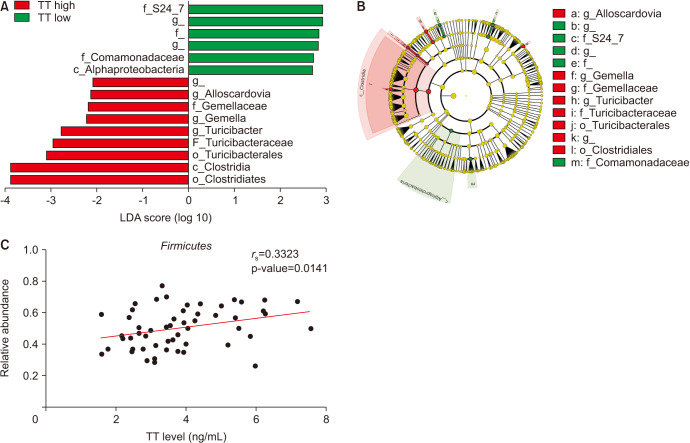
The LEfSe analysis revealed that nine bacterial taxa were significantly more abundant in the gut microbiota of the TT high group, and six taxa were significantly less abundant in the TT high group (p<0.05, LDA score ≥|2.0|) (Fig. 3A). Most taxa abundant in the TT high group belonged to the phylum Firmicutes, except for the genus Alloscardovia, which belonged to the phylum Actinobacteria (Fig. 3B). The relative amount of Firmicutes in the gut microbiota was significantly correlated with serum TT levels of hosts, as determined by Spearman’s rank correlation coefficient (Fig. 3C; rS=0.3323, p=0.0141). Five males with hypogonadism (TT <2.3 ng/mL) had a lower relative amount of Firmicutes than the TT high group but not significantly (0.55 vs. 0.43, p=0.075). The relative abundance of Firmicutes had a significantly positive effect on TT levels, independent of age, BMI, serum TG, and T-Chol levels (β=0.770, p=0.0396; Table 2).
Fig. 3. Comparison of the abundance of bacteria in the gut microbiota based on TT levels. Bar graph (A) and cladogram (B) of LEfSe analysis including OTUs that were significantly different in abundance between TT high and TT low groups (p<0.05, LDA score >|2.0|). Red bars represent OTUs associated with the TT high group, and green bars represent OTUs associated with the TT high group. Bars without bacterial names refer to OTUs that could not be identified. (C) Correlation between the relative abundance of Firmicutes in the gut microbiota and serum TT levels. c_: class, o_: order, f_: family, g_: genus, TT: total testosterone, LDA: linear discriminant analysis, LEfSe: LDA effect size, OTU: operational taxonomic unit.
Table 2. Univariate and multivariate analysis to determine factors that influence TT levels.
| Parameter | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| β | SE | p-value | β | SE | p-value | |
| Age | −0.345 | 0.444 | 0.441 | −0.896 | 0.404 | 0.0315 |
| BMI | −0.771 | 0.404 | 0.0615 | −0.628 | 0.390 | 0.1140 |
| T-Chol | −0.649 | 0.482 | 0.1843 | −0.593 | 0.422 | 0.1670 |
| TG | −1.191 | 0.373 | 0.0024 | −1.017 | 0.377 | 0.0097 |
| Firmicutes | 0.893 | 0.376 | 0.021 | 0.770 | 0.364 | 0.0396 |
TT: total testosterone, β: standardized partial regression coefficient, SE: standard error, BMI: body mass index, T-Chol: total cholesterol, TG: triglyceride.
DISCUSSION
In the present study, we examined the correlation between gut microbiota and testosterone levels in elderly male patients without prostate cancer. For the gut microbiota, alpha and beta diversities did not differ according to testosterone status. However, when bacterial taxa were compared individually, some genera belonging to the phylum Firmicutes were more common in male subjects with high TT levels. Furthermore, Firmicutes exhibited a positive correlation with serum TT levels, suggesting that Firmicutes affect the testosterone levels in their hosts.
Metabolites produced by the gut microbiome are absorbed by the host and may be associated in various diseases [14,15]. Previously, we have focused on the relationship between prostate cancer and the gut microbiota and reported that their metabolites, including short-chain fatty acids, promote cancer growth through the insulin-like growth factor-1 signaling pathway [16]. In addition, we examined the gut microbiota composition in Japanese males who were suspected of having prostate cancer and underwent prostate biopsy. We observed that patients with high-grade prostate cancer presented high levels of Alistipes and Lachnospira, and an index calculated from the number of several specific intestinal bacteria could identify patients with high-risk prostate cancer with high accuracy [11]. Moreover, Firmicutes/Bacteroidetes ratio was significantly higher in patients with enlarged prostates [17].
Testosterone is a crucial hormone regulating health in males, and the lowering of testosterone levels with aging (late-onset hypogonadism) is closely associated with various health-related challenges, including sexual dysfunction, muscle weakness, and metabolic syndrome [9]. Furthermore, testosterone is involved in prostate cancer and benign prostatic hyperplasia, and its metabolism is considered a therapeutic target in these diseases [18].
Herein, we observed no significant change in alpha and beta diversity of the gut microbiota owing to testosterone levels, consistent with other reports in young male subjects under 15 years of age [19]. This finding could be attributed to the fact that there were no major differences in the gut microbiota composition of this cohort, as the cohort consisted only of Japanese males living in urban areas, whose lifestyle and genetic factors affecting bacterial flora were similar. Furthermore, our results revealed that serum testosterone levels within the normal range do not strongly impact intestinal bacteria.
In the present study, serum testosterone levels and the abundance of Firmicutes were positively correlated among older male subjects. Although testosterone levels were found to be affected by age, BMI, and lipoprotein levels [20,21,22], we found that the abundance of Firmicutes correlated positively with blood testosterone levels independent of these confounding factors. For the first time, our study observed a positive relationship between Firmicutes and testosterone in elderly Japanese males with unique gut microbiota compositions [23]; however, similar relationships have been reported in different countries and age groups. Shin et al [24] investigated the relationship between gut microbiota and serum sex steroid hormones in 57 male and female subjects aged 25 to 65 years in Korea. The study revealed that the abundances of several bacteria belonging to Firmicutes were positively correlated with TT levels in male participants. The authors revealed that the abundance of several bacteria belonging to Firmicutes positively correlated with TT levels in male participants [24]. Conversely, in females, the abundance of Firmicutes was reportedly lower, and that of Bacteroides was higher in the group presenting high estradiol levels [24]. Patients with PCOS producing markedly high testosterone levels demonstrate a higher abundance of Bacteroides and a lower abundance of Firmicutes in the gut microbiota [25]. In an animal experiment, supplementation with Lactobacillus plantarum HL2 belonging to the phylum Firmicutes reportedly decreased serum testosterone levels of female PCOS model rats [26]. On the other hand, supplementation with Lactobacillus reuteri to male mice increased testosterone levels [27]. Moreover, a human study showed that a synbiotic containing Lactobacillus paracasei can increase TT levels in males [28]. Interestingly, the relationship between sex hormones and the phylum Firmicutes differs between males and females, but the underlying cause remains unclear.
The gut microbiota profile is distinct depending on the testosterone status, attributed to the mutual influence of the following two factors. Testosterone levels were increased when feces from male mice were transplanted into female mice, suggesting that specific intestinal bacteria promote testosterone production [29]. Inflammation driven by intestinal bacteria-derived endotoxins has been reported to suppress testosterone production in Leydig cells [30,31]. In addition, Firmicutes synthesize testosterone or promote its reabsorption through unconjugation [6], and supplementation with some bacteria belonging to the phylum Firmicutes can boost testosterone in men [27,28]. These previous reports indicate that Firmicutes in the intestinal tract may affect testosterone levels. In contrast, testosterone alters the composition of gut microbiota in several animal studies. Harada et al. have reported that the Firmicutes/Bacteroidetes ratio and Lactobacillus increase the gut microbiota of castrated high-fat diet-fed mice [32]. Another report has shown that the gut microbiota composition of female rats administered high-dose androgens on early postnatal life shows lower Firmicutes abundance at early adulthood [33]. Testosterone itself may negatively impact bacteria belonging to the phylum Firmicutes. Mechanisms that mediate these opposite effects need to be investigated in both human and animal studies. Overall, our results, which show a positive correlation between testosterone level and Firmicutes abundance, suggest that the effect of Firmicutes on elevated testosterone is greater than the opposite effect of testosterone in elderly Japanese males.
Although it has been reported that the ratio of Firmicutes increases in the gut microbiota of obese individuals [34], BMI was significantly higher in the TT low group with less Firmicutes than in the TT high group (24.9 kg/m2 vs. 22.5 kg/m2, p=0.0402). We suspect that this discrepancy was due to the small number of obese individuals (2 with BMI ≥30 or higher) in the cohort, and the difference in BMI below the range of obesity showed little alteration in the gut microbiota.
This study has some limitations. First, as samples were collected at a single hospital, the cohort size was small, and the residential area was limited to urban locations. The composition of gut microbiota varies depending on the region of residence [23,35]; therefore, it is desirable to include male participants from various regions to accurately assess the relationship between gut microbiota and testosterone metabolism in Japanese males. We are currently conducting a large-scale study, collecting samples from several hospitals nationwide in Japan (UMIN000043489). Second, the cohort for this study was predominantly composed of elderly male subjects, as we analyzed a sample of males with suspected prostate cancer who underwent prostate biopsy. As the composition of gut microbiota varies with age [35], it remains unclear whether similar relationships would be observed in younger groups. On the other hand, as testosterone synthesis is reduced in the elderly, the effect of bacteria-derived testosterone was possibly more apparent in the present study. A positive correlation has also been reported between fecal Firmicutes and blood testosterone levels in younger male subjects [24]. In addition, because males with negative biopsies may have benign prostatic hyperplasia and prostatitis which may influence their gut microbiota, the cohort in this study was strictly different from the healthy volunteer cohort. Third, although the time of blood sample collection was fixed in the morning, there was no consistent fasting time before sample collection. Since fasting status can affect the testosterone level [36], in future studies, the fasting time should be considered to provide a more accurate assessment. In addition, other factors that may be involved in TT and the gut microbiota, such as sex hormone-binding globulin and estrogen levels, should be measured. Lastly, as the gut microbiota composition was identified using 16S rRNA sequencing, we could not evaluate bacterial genomic functions or compare the composition at the species level. Detailed analysis using shotgun metagenomics would enable these evaluations.
CONCLUSIONS
The present study suggests that there is a positive association between the phylum Firmicutes, and testosterone metabolism in elderly Japanese males. Accordingly, probiotics and other bacterial agents may be successfully used to treat and prevent various testosterone-associated diseases in the future.
Acknowledgements
We thank our laboratory and hospital staff for their assistance in collecting, storing, and managing appropriate samples.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Funding: This work was supported by research grants from the Japanese Urological Association (Grant No. 21K09421), Yakult Bio-Science Foundation, and Project MEET of Osaka University Graduate School of Medicine.
- Conceptualization: KF, KH, JH, MN, EB, KT, SF, HK, TT, AT, SY, SN, WO, HU, NN.
- Data curation: MM, DM, YP.
- Formal analysis: MM, DM, YP.
- Funding acquisition: KF, NN.
- Investigation: MM, DM, EB, YP.
- Methodology: KF, KH, TT, AT.
- Supervision: WO, HU, NN.
- Writing – original draft: MM.
- Writing – review & editing: KF, DM, KH, JH, MN, EB, KT, SF, HK, YP, TT, AT, SY, SN, WO, HU, NN.
Data Sharing Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/ZJTFRM.
References
- 1.Amato KR, Arrieta MC, Azad MB, Bailey MT, Broussard JL, Bruggeling CE, et al. The human gut microbiome and health inequities. Proc Natl Acad Sci U S A. 2021;118:e2017947118. doi: 10.1073/pnas.2017947118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill SR, Pop M, Deboy RT, Eckburg PB, Turnbaugh PJ, Samuel BS, et al. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–1359. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol. 2017;14:573–584. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suganya K, Koo BS. Gut-brain axis: role of gut microbiota on neurological disorders and how probiotics/prebiotics beneficially modulate microbial and immune pathways to improve brain functions. Int J Mol Sci. 2020;21:7551. doi: 10.3390/ijms21207551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou A, Tang L, Zeng S, Lei Y, Yang S, Tang B. Gut microbiota: a new piece in understanding hepatocarcinogenesis. Cancer Lett. 2020;474:15–22. doi: 10.1016/j.canlet.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Cross TL, Kasahara K, Rey FE. Sexual dimorphism of cardiometabolic dysfunction: gut microbiome in the play? Mol Metab. 2018;15:70–81. doi: 10.1016/j.molmet.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mammadova G, Ozkul C, Yilmaz Isikhan S, Acikgoz A, Yildiz BO. Characterization of gut microbiota in polycystic ovary syndrome: findings from a lean population. Eur J Clin Invest. 2021;51:e13417. doi: 10.1111/eci.13417. [DOI] [PubMed] [Google Scholar]
- 8.Jobira B, Frank DN, Pyle L, Silveira LJ, Kelsey MM, Garcia-Reyes Y, et al. Obese adolescents with PCOS have altered biodiversity and relative abundance in gastrointestinal microbiota. J Clin Endocrinol Metab. 2020;105:e2134–e2144. doi: 10.1210/clinem/dgz263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsujimura A. The relationship between testosterone deficiency and men's health. World J Mens Health. 2013;31:126–135. doi: 10.5534/wjmh.2013.31.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ujike T, Uemura M, Kawashima A, Nagahara A, Fujita K, Miyagawa Y, et al. A novel model to predict positive prostate biopsy based on serum androgen level. Endocr Relat Cancer. 2018;25:59–67. doi: 10.1530/ERC-17-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsushita M, Fujita K, Motooka D, Hatano K, Fukae S, Kawamura N, et al. The gut microbiota associated with high-Gleason prostate cancer. Cancer Sci. 2021;112:3125–3135. doi: 10.1111/cas.14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92:405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 13.Lunenfeld B, Saad F, Hoesl CE. ISA, ISSAM and EAU recommendations for the investigation, treatment and monitoring of late-onset hypogonadism in males: scientific background and rationale. Aging Male. 2005;8:59–74. doi: 10.1080/13685530500163416. [DOI] [PubMed] [Google Scholar]
- 14.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi K, Saigusa D, Kanemitsu Y, Matsumoto Y, Thanai P, Suzuki N, et al. Gut microbiome-derived phenyl sulfate contributes to albuminuria in diabetic kidney disease. Nat Commun. 2019;10:1835. doi: 10.1038/s41467-019-09735-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsushita M, Fujita K, Hayashi T, Kayama H, Motooka D, Hase H, et al. Gut microbiota-derived short-chain fatty acids promote prostate cancer growth via IGF1 signaling. Cancer Res. 2021;81:4014–4026. doi: 10.1158/0008-5472.CAN-20-4090. [DOI] [PubMed] [Google Scholar]
- 17.Takezawa K, Fujita K, Matsushita M, Motooka D, Hatano K, Banno E, et al. The Firmicutes/Bacteroidetes ratio of the human gut microbiota is associated with prostate enlargement. Prostate. 2021;81:1287–1293. doi: 10.1002/pros.24223. [DOI] [PubMed] [Google Scholar]
- 18.van der Sluis TM, Vis AN, van Moorselaar RJ, Bui HN, Blankenstein MA, Meuleman EJ, et al. Intraprostatic testosterone and dihydrotestosterone. Part I: concentrations and methods of determination in men with benign prostatic hyperplasia and prostate cancer. BJU Int. 2012;109:176–182. doi: 10.1111/j.1464-410X.2011.10651.x. [DOI] [PubMed] [Google Scholar]
- 19.Yuan X, Chen R, Zhang Y, Lin X, Yang X. Gut microbiota: effect of pubertal status. BMC Microbiol. 2020;20:334. doi: 10.1186/s12866-020-02021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derby CA, Zilber S, Brambilla D, Morales KH, McKinlay JB. Body mass index, waist circumference and waist to hip ratio and change in sex steroid hormones: the Massachusetts male ageing study. Clin Endocrinol (Oxf) 2006;65:125–131. doi: 10.1111/j.1365-2265.2006.02560.x. [DOI] [PubMed] [Google Scholar]
- 21.Tchernof A, Labrie F, Bélanger A, Prud'homme D, Bouchard C, Tremblay A, et al. Relationships between endogenous steroid hormone, sex hormone-binding globulin and lipoprotein levels in men: contribution of visceral obesity, insulin levels and other metabolic variables. Atherosclerosis. 1997;133:235–244. doi: 10.1016/s0021-9150(97)00125-1. [DOI] [PubMed] [Google Scholar]
- 22.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 23.Nishijima S, Suda W, Oshima K, Kim SW, Hirose Y, Morita H, et al. The gut microbiome of healthy Japanese and its microbial and functional uniqueness. DNA Res. 2016;23:125–133. doi: 10.1093/dnares/dsw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin JH, Park YH, Sim M, Kim SA, Joung H, Shin DM. Serum level of sex steroid hormone is associated with diversity and profiles of human gut microbiome. Res Microbiol. 2019;170:192–201. doi: 10.1016/j.resmic.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Chu W, Han Q, Xu J, Wang J, Sun Y, Li W, et al. Metagenomic analysis identified microbiome alterations and pathological association between intestinal microbiota and polycystic ovary syndrome. Fertil Steril. 2020;113:1286–1298.e4. doi: 10.1016/j.fertnstert.2020.01.027. [DOI] [PubMed] [Google Scholar]
- 26.He Y, Wang Q, Li X, Wang G, Zhao J, Zhang H, et al. Lactic acid bacteria alleviate polycystic ovarian syndrome by regulating sex hormone related gut microbiota. Food Funct. 2020;11:5192–5204. doi: 10.1039/c9fo02554e. [DOI] [PubMed] [Google Scholar]
- 27.Lee J, Yang W, Hostetler A, Schultz N, Suckow MA, Stewart KL, et al. Characterization of the anti-inflammatory Lactobacillus reuteri BM36301 and its probiotic benefits on aged mice. BMC Microbiol. 2016;16:69. doi: 10.1186/s12866-016-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maretti C, Cavallini G. The association of a probiotic with a prebiotic (Flortec, Bracco) to improve the quality/quantity of spermatozoa in infertile patients with idiopathic oligoasthenoteratospermia: a pilot study. Andrology. 2017;5:439–444. doi: 10.1111/andr.12336. [DOI] [PubMed] [Google Scholar]
- 29.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 30.Tremellen K. Gut endotoxin leading to a decline in gonadal function (GELDING) - a novel theory for the development of late onset hypogonadism in obese men. Basic Clin Androl. 2016;26:7. doi: 10.1186/s12610-016-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tremellen K, McPhee N, Pearce K, Benson S, Schedlowski M, Engler H. Endotoxin-initiated inflammation reduces testosterone production in men of reproductive age. Am J Physiol Endocrinol Metab. 2018;314:E206–E213. doi: 10.1152/ajpendo.00279.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harada N, Hanaoka R, Hanada K, Izawa T, Inui H, Yamaji R. Hypogonadism alters cecal and fecal microbiota in male mice. Gut Microbes. 2016;7:533–539. doi: 10.1080/19490976.2016.1239680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barroso A, Santos-Marcos JA, Perdices-Lopez C, Vega-Rojas A, Sanchez-Garrido MA, Krylova Y, et al. Neonatal exposure to androgens dynamically alters gut microbiota architecture. J Endocrinol. 2020;247:69–85. doi: 10.1530/JOE-20-0277. [DOI] [PubMed] [Google Scholar]
- 34.Amabebe E, Robert FO, Agbalalah T, Orubu ESF. Microbial dysbiosis-induced obesity: role of gut microbiota in homoeostasis of energy metabolism. Br J Nutr. 2020;123:1127–1137. doi: 10.1017/S0007114520000380. [DOI] [PubMed] [Google Scholar]
- 35.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearce KL, Tremellen K. The effect of macronutrients on reproductive hormones in overweight and obese men: a pilot study. Nutrients. 2019;11:3059. doi: 10.3390/nu11123059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed for this study have been deposited in HARVARD Dataverse and are available at https://doi.org/10.7910/DVN/ZJTFRM.