Abstract
The energy cost of information processing is thought to be chiefly neuronal, with a minor fraction attributed to glial cells. However, there is compelling evidence that astrocytes capture synaptic K+ using their Na+/K+ ATPase, and not solely through Kir4.1 channels as was once thought. When this active buffering is taken into account, the cost of astrocytes rises by >200%. Gram-per-gram, astrocytes turn out to be as expensive as neurons. This conclusion is supported by 3D reconstruction of the neuropil showing similar mitochondrial densities in neurons and astrocytes, by cell-specific transcriptomics and proteomics, and by the rates of the tricarboxylic acid cycle. Possible consequences for reactive astrogliosis and brain disease are discussed.
Keywords: Energy metabolism, K+ buffering, ATP turnover, Na+/K+ ATPase, Kir4.1, reactive astrocyte
Introduction
The notion that neurons are the major energy sink in brain tissue appears solid enough. Neurons spend considerable energy on the recovery of cation gradients challenged by excitatory postsynaptic potentials (EPSPs) and action potentials, while the neuronal signaling suppression by deep anesthesia or by pharmacological inhibition of the Na+/K+ ATPase pump (NKA) reduces brain metabolism by 50–60%. 1 Accordingly, a seminal bottom-up analysis based on the microscopic properties of ion channels, transporters and pumps concluded that 95% of the energy expenditure of gray matter signaling is neuronal and 5% is glial. 2 When action potentials were later found to be more efficient than anticipated, 3 the budget was modified, with 70% ascribed to dendrites, 15% to axons, and 7% to astrocytes. 4 In line with the energetic prominence of dendrites, glucose consumption in gray matter is several times higher than that in white matter. 5
There is however evidence that astrocytes use the NKA to capture the K+ that is released by neurons during neurotransmission. 6 Here I discuss why this active process, together with fresh insights on astrocytic oxidative metabolism, prompts a revision of the energy budget of gray matter.
K+ buffering is chiefly mediated by the astrocytic NKA
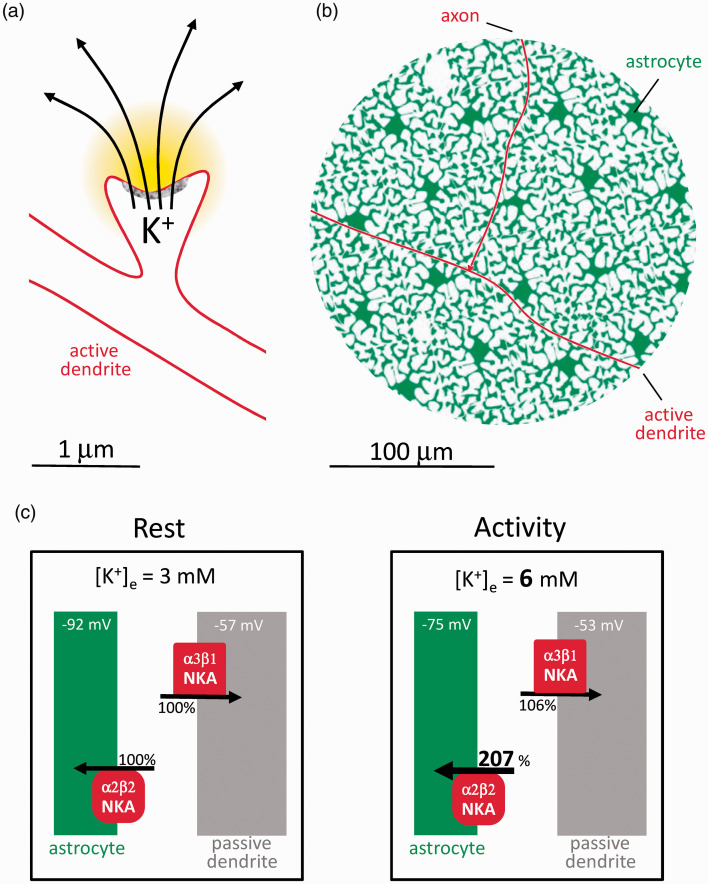
The EPSP is the transient dendritic depolarization caused by Na+ and Ca2+ entry through AMPA and NMDA receptors. Less widely appreciated is that these ionotropic glutamate receptors are permeable to K+, and that during neurotransmission the inward movement of cations is mirrored by the release of K+.2,7 The journey of Na+ in the aftermath of the EPSP is straightforward. It diffuses along the dendritic shaft to be pumped out later on by the NKA. 8 The EPSP lasts for a few milliseconds, whereas the extrusion of Na+ by the sluggish NKA takes seconds to minutes. 8 Most of the Ca2+ load is transformed by the Na+/Ca2+ exchanger NCX into a Na+ load, which is then dealt with by the NKA. Hence, the energy burden of the Na+ and Ca2+ load is carried entirely by the postsynaptic neuron. The fate of the K+ is more convoluted. After leaving the dendrite, K+ enters an intricate space bounded by many different cells, mostly neurons and astrocytes. Assuming a square law for the escalation of cell surface and a typical diffusional distance of 100 micrometers,8,9 it can be calculated that <0.01% of the surface screened by the K+ belongs to the neuron that released it. In fact, it is well established that most of the K+ released by synaptic activity is not cleared by neurons, but by astrocytes, a phenomenon known as K+ buffering.6,10
The molecular mechanism responsible for K+ buffering is paramount to this article’s main point. Over the years, three candidates were considered: the Na+-K+-2Cl− co-transporter NKCC, the K+ channel Kir4.1 and the NKA. The NKCC hypothesis, based on culture experiments, lost impetus when it was realized that the NKCC is not significantly expressed in adult gray matter, although the NKCC may play a role in development, in white matter oligodendrocytes and in glioma cells.6,11 The second candidate, Kir4.1, mediates a form of K+ buffering termed spatial buffering. According to this hypothesis, K+ can be captured by astrocytes via constitutively open Kir4.1 channels and at the same time an identical amount of K+ leaves distant astrocytes, also via Kir4.1. This long-range electrotonic phenomenon requires gap junction coupling between astrocytes. 10 Because permeation across ion channels does not involve ATP hydrolysis, spatial buffering is costless, which explains the minor role attributed to astrocytes in various energy budgets.2,4,12
However, spatial buffering and Kir4.1 channels are no longer regarded as the main mechanisms of K+ buffering, a conclusion reached from anatomical and physiological considerations and the outcome of pharmacological and genetic manipulation of gap junctions and Kir4.1 9,.11,13–18 The participation of Kir4.1 in K+ buffering has been analized in depth by Nanna MacAulay. 6 Revised roles for Kir4.1 in astrocytes and oligodendrocytes are in setting the membrane potential, limiting the amplitude of the K+ rise at high frequency stimulation, and mediating the delayed release of astrocytic K+, which is then captured by neurons to complete the cycle. Thus, Kir4.1 remains an integral element of the K+ cycle. Spatial buffering is still thought to be dominant in the highly polarized retina. 19
The lion’s share of K+ buffering in brain tissue is nowadays attributed to the astrocytic NKA. 6 The NKA is an oligomeric enzyme consisting of one catalytic subunit α (four isoforms) and one regulatory subunit β (three isoforms). The preferential role of astrocytes over neurons in K+ buffering is explained by NKA subtype allocation.16,20–22 At 3 mM resting K+, the neuronal NKA (α3β1) is saturated with K+ and therefore primed to respond to variations in intracellular Na+, for which it has low affinity. The neuronal NKA behaves as a true Na+ pump, that is, driven by Na+, with K+ playing a permissive role. Conversely, the major astrocytic NKA (α2β2) is primed by intracellular Na+ and ready to respond to extracellular K+, for which it has low affinity. Moreover, α2β2 is dormant at resting membrane potential and is activated by depolarization, which is not the case for the neuronal pump.16,22 Because of parallel sodium and chloride conductances, the membrane potential of neurons is four times less sensitive to [K+]e than the astrocytic membrane potential. 23 The compounded effect of NKA sensitivity to K+, NKA sensitivity to membrane potential, and membrane potential sensitivity to K+, is that a physiological rise in K+ from 3 to 6 mM will stimulate the astrocytic NKA by 107% but the neuronal NKA only by 6% (Figure 1), an 18-fold difference. Driven by intracellular Na+, the NKA of the stimulated dendrite would be more efficient at buffering K+ than the non-stimulated neurons, but this uptake occurs in delayed fashion and far from the active zone. 8
Figure 1.
Astrocytes are poised to capture K+ while neurons are not. (a) The postsynaptic neuron releases K+ during excitatory neurotransmission. (b) Compared to astrocytes, the active postsynaptic neuron represents a minor fraction of the cellular surface to be screened by K+. Passive neurons are omitted for simplicity. (c) According to the Goldman-Hodgkin-Katz equation, membrane potential Vm = 59 x log(PK x [K+]e + PNa x [Na+]e + PCl x [Cl−]I/PK x [K+]I + PNa x [Na+]I + PCl x [Cl−]e) where PK, PNa and PCl (permeabilities) are assumed to be 1.0, 0.04 and 0.45 for neurons and 1.0, 0 and 0 for astrocytes, 23 with resting concentrations (mM; e, extracellular; i. intracellular) of [K+]e = 3, [Na+]e = 140, [Cl−]I = 20 mM, [K+]I = 110 mM, [Na+]I =10 mM and [Cl−]e = 110 mM. Under these conditions, a [K+]e rise from 3 to 6 mM will depolarize astrocytes by 17 mV (−92 mV to −75 mV) and neurons by 4 mV (- 57 mV to −53 mV). These depolarizations will double the K+ affinity of α2β2 without affecting α3β1 . 16 With the compounded effects of higher K+ and higher affinity, α2β2 occupancy rises from 35 to 73% (a 107% increase), whereas α3β1 occupancy rises only from 88 to 94% (a 6% increase).
The astrocytic NKA should perhaps be best understood as a K+ pump, whose permissive co-factor is intracellular Na+. The NKA exchanges three Na+ ions per two K+ ions and therefore leads to cell shrinkage. Yet, synaptic activity is accompanied by astrocytic swelling, 24 which is partly explained by parallel influx of Na+ and bicarbonate via the NBCe1, and partly by metabolic stimulation.25,26 The Na+/glutamate cotransport is deemed a minor contributor, as only 3 Na+ ions are imported per glutamate, compared with the 150 Na+ ions per glutamate exported by the NKA. Additional Na+ influx is provided by voltage-sensitive Na+ channels, TRP channels and the Na+/Ca2+ exchanger.11,27–29
Astrocytes as major energy spenders
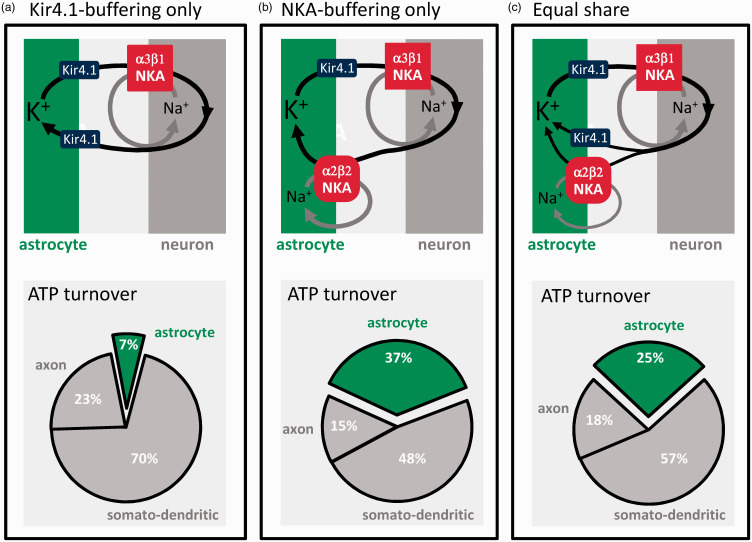
How much energy is consumed by astrocytes? According to the current budget, most K+ is passively buffered via Kir4.1, and astrocytes use just 7% of the signaling budget, for glutamate recycling and membrane potential generation (Figure 2(a)). At the opposite end of the possibility spectrum, if all synaptic K+ were cleared by the NKA, astrocytes would spend 37% of the ATP (Figure 2(b)). With the NKA and Kir4.1 contributing equally, a conservative estimate, astrocytes would use 25% of the ATP (Figure 2(c)), plus a minor contribution from the astrocytic Ca2+ pumps. 30 In light of the experimental evidence cited above showing that the NKA surpasses Kir4.1 in the buffering of K+, it seems safe to state that astrocytes account for at least 25% of the ATP spent in gray matter signaling, i.e. more than 200% higher than previously thought. In this hybrid model, the NKA would possibly cater for low and moderate activity and be supplemented by Kir4.1-mediated K+ buffering during high frequency stimulation, 6 thus limiting maximum energy demand. Brain tissue must also spend energy for protein and lipid turnover, membrane trafficking, amino acid and nucleotide metabolism, pH regulation, cytoskeleton remodeling, etc, collectively estimated at 20–30% of the total tissue ATP turnover.2,4,30 Several of these housekeeping functions have been evolutionary outsourced from neurons to astrocytes, including control of interstitial fluid composition, glycogen storage and mobilization, supply of energy substrates and precursors for biosynthesis, and the recycling of neurotransmitters, oxidized lipids and scavengers, and other waste products.31–33 The astrocytic share of the housekeeping cost might be even larger than its share of the signaling cost, further supporting the case for the expensive astrocyte.
Figure 2.
The active buffering of K+ makes astrocytes energetically costly. Postsynaptic neurons release approx. 100 K+ ions for exocytosed glutamate. 2 The current energy budget of gray matter signaling 2 assumes that these ions are passively recycled via Kir4.1 channels without energy expenditure. In such case, astrocytes would use only 7% of the energy budget (a). If the contribution of Kir4.1 were negligible and all synaptic K+ were captured by the NKA, astrocytes would use 37% of the budget (b). In an intermediate scenario, K+ buffering is equally shared between Kir4.1 and the NKA, and astrocytes use 25% of the signaling budget (c).
Mitochondrial function and distribution also point to expensive astrocytes
Lack of immunological and histochemical detection of cytochrome oxidase led to the pervasive idea that astrocytes do not possess significant oxidative phosphorylation, OXPHOS, 34 a conclusion supported by the apparent absence of mitochondria in the astrocytic processes that surround synapses. 35 In those days, astrocytes were even thought to produce 100% of their ATP in glycolysis. 36 Later work has shown otherwise. Even in culture, a condition that promotes glycolysis via the Warburg effect, 75% of the astrocytic ATP is generated by mitochondria. 37 In vivo determinations have shown that 20–33% of the brain TCA cycle flux is glial.30,38–41 When these figures are fed into mathematical models, once again, over 75% of the astrocytic ATP is shown to be mitochondrial.12,42 Noticing that, relative to deep anesthesia, oxidative glial ATP synthesis in awake animals is well in excess of that required by glutamate recycling, Gulin Oz, Rolf Gruetter and colleagues advanced that “glial oxidative ATP synthesis sustains additional metabolic reactions in the astrocyte that are related to overall brain activity and not only those that maintain glutamatergic action”. 39 K+ buffering fits the bill. And the anatomy has also been revised. Refinements of electron microscopy and targeted fluorescent markers have detected abundant mitochondria in astrocytes.43–47 In fact, at 7–10%, the fraction of the cellular volume occupied by mitochondria is similar in astrocytes and neurons.48,49 Transcriptomics and proteomics concurred by showing similar expression of TCA cycle and OXPHOS enzymes in astrocytes and neurons.43,50,51 On top of that is the ATP production of glycolysis, for which astrocytes are better equipped.52–54
There are however qualitative differences in mitochondrial metabolism between the two cell types. In neurons, OXPHOS complex I is predominantly assembled into supercomplexes, whereas in astrocytes the abundance of free complex I is higher, associated with several-fold higher reactive oxygen species (ROS) production compared with neurons. 55 Neurons degenerate in response to partial genetic inhibition of OXPHOS, whereas astrocytes upregulate glycolysis and survive indefinitely, without ostensible functional deficit.56,57 Astrocytes express PDK4, a kinase that inhibits pyruvate dehydrogenase, and the anaplerotic enzyme pyruvate carboxylase,50,58 suggesting tonic deviation of pyruvate away from oxidation. Instead, astrocytic mitochondria are better endowed than those of neurons for fatty acid oxidation.59–61 Fatty acids can be transferred from neurons to astrocytes in an activity-dependent fashion32,62,63 and enter the TCA cycle beyond the PDH blockage, as do ketone bodies, fuels of pathophysiological and therapeutic relevance 64,65 that have a powerful inhibitory effect on astrocytic glucose metabolism. 66 Determining the relative weight of glucose, neuronal fatty acids, blood-borne fatty acids, and glutamate in fueling astrocytic respiration is a pending task.67–69 Another emerging aspect of astrocytic respiration is that it appears to be highly regulated. In vitro experiments have shown that astrocytic respiration is subject to short-term modulation by physiological levels of extracellular K+, NH4+ and NO.70–72 These signals, which are released by neurons and endothelial cells during local activity and reactive hyperemia, are proposed to generate glycolytic lactate and spare tissue oxygen for the benefit of active neurons. 73
In summary, there is a good correlation between the energy demand imposed by K+ buffering on astrocytes, and the mitochondrial endowment and speed of respiration of these cells. The volume occupied by astrocytes in the neuropil is less than half that occupied by neurons,12,42,49,74 which means that gram-per-gram, astrocytes are as expensive as neurons. This conclusion is not in conflict with the metabolic effects of anesthesia and NKA inhibition 1 nor with the faster metabolic rate of gray matter versus white matter. 5
Expensive astrocytes and disease
A common pathogenic factor in stroke, traumatic brain injury and other acute diseases of the brain is a local energy deficit, which has been assumed to be neuronal. With astrocytes promoted to non-trivial energy spenders, their metabolism becomes more interesting. Neurons are more fragile than astrocytes. Brain disease, acute and chronic, causes the preferential death of neurons, while astrocytes survive and often thrive, a phenomenon termed reactive astrogliosis. Reactive astrocytes are pleiotropic and may differ from normal astrocytes in morphology, cytoskeleton, transcription factors, signaling, chaperones, secreted proteins, etc. 75 A common feature of these cells is a reduced ability to cater for the needs of neurons, i.e. they become “selfish”. 31 For example, astrocytes located near human tumors showed a 60-70% reduction in the mRNA expression of the three transport proteins that link the buffering of K+ to energy metabolism (α2β2 NKA, Kir4.1 and NBCe1). 76 Glial K+ buffering is also downregulated in a Drosophila seizure model, 77 whereas its reactivation by genetic means suppressed neuronal hyperactivity and seizures, and increased the life span of the fly. 77 The role of K+ buffering and associated energy demand in neuroprotection is complex. Reactive astrocytes are predicted to spend less energy and glucose during neural activity,78,79 contributing to the well-being of neurons, which use glucose for antioxidation; 52 but they would also fail to remove extracellular K+ and reduce their own oxygen consumption on demand,70,73 to the detriment of neurons. Supporting the latter, in a mouse model of Huntington’s disease Kir4.1 was found to be diminished in astrocytes, leading to K+ accumulation and neuronal hyperexcitability. Viral delivery of Kir4.1 to these astrocytes normalized extracellular K+, ameliorated the neuronal dysfunction and prolonged neuronal survival. 80 Huntington´s astrocytes switch from glucose to fatty acid metabolism, a phenomenon associated with reactive oxygen species-induced damage in the striatum but not in the cerebellum, a clue to the baffling regional damage of this genetic disease. 81 And in a mouse model of Alzheimer´s disease glycolysis was impaired in the early stages, leading to reduced production of serine and altered synaptic plasticity and memory, which was reverted by dietary serine. 33 In summary, reactive astrocytes may be protective or detrimental to neurons according to brain region, pathology, disease stage and experimental model.75,82 More research focusing on astrocytes, both normal and reactive, is needed to clarify the role of energy metabolism in brain disease.
Acknowledgements
My thanks to all members of my laboratory and to our collaborators for generous and fruitful discussions. I also thank Karen Everett for critical reading of the manuscript.
Funding: The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partially funded by Fondecyt Grant 1200029 and by ANID-BMBF Grant 180045.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: LF Barros https://orcid.org/0000-0002-6623-4833
References
- 1.Erecińska M, Silver IA. ATP and brain function. J Cereb Blood Flow Metab 1989; 9: 2–19. [DOI] [PubMed] [Google Scholar]
- 2.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 2001; 21: 1133–1145. [DOI] [PubMed] [Google Scholar]
- 3.Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science 2009; 325: 1405–1408. [DOI] [PubMed] [Google Scholar]
- 4.Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron 2012; 75: 762–777. [DOI] [PubMed] [Google Scholar]
- 5.Reivich M, Kuhl D, Wolf A, et al. The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ Res 1979; 44: 127–137. [DOI] [PubMed] [Google Scholar]
- 6.MacAulay N. Molecular mechanisms of K(+) clearance and extracellular space shrinkage-Glia cells as the stars. Glia. 2020; 68: 2192–2211. [DOI] [PubMed] [Google Scholar]
- 7.Yu SP, Yeh C, Strasser U, et al. NMDA receptor-mediated K+ efflux and neuronal apoptosis. Science 1999; 284: 336–339. [DOI] [PubMed] [Google Scholar]
- 8.Mondragao MA, Schmidt H, Kleinhans C, et al. Extrusion versus diffusion: mechanisms for recovery from sodium loads in mouse CA1 pyramidal neurons. J Physiol 2016; 594: 5507–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallraff A, Kohling R, Heinemann U, et al. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci 2006; 26: 5438–5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orkand RK, Nicholls JG, Kuffler SW. Effect of nerve impulses on the membrane potential of glial cells in the Central nervous system of amphibia. J Neurophysiol 1966; 29: 788–806. [DOI] [PubMed] [Google Scholar]
- 11.Verkhratsky A, Nedergaard M. Physiology of astroglia. Physiol Rev 2018; 98: 239–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jolivet R, Coggan JS, Allaman I, et al. Multi-timescale modeling of activity-dependent metabolic coupling in the neuron-glia-vasculature ensemble. PLoS Comput Biol 2015; 11: e1004036. 101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Ambrosio R, Gordon DS, Winn HR. Differential role of KIR channel and Na(+)/K(+)-pump in the regulation of extracellular K(+) in rat hippocampus. J Neurophysiol 2002; 87: 87–102. [DOI] [PubMed] [Google Scholar]
- 14.Chever O, Djukic B, McCarthy KD, et al. Implication of Kir4.1 channel in excess potassium clearance: an in vivo study on anesthetized glial-conditional Kir4.1 knock-out mice. JNeurosci 2010; 30: 15769–15777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larsen BR, MacAulay N. Kir4.1-mediated spatial buffering of K(+): experimental challenges in determination of its temporal and quantitative contribution to K(+) clearance in the brain. Channels (Austin) 2014; 8: 544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen BR, Assentoft M, Cotrina ML, et al. Contributions of the Na(+)/K(+)-ATPase, NKCC1, and Kir4.1 to hippocampal K(+) clearance and volume responses. Glia 2014 2014; 62: 608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breithausen B, Kautzmann S, Boehlen A, et al. Limited contribution of astroglial gap junction coupling to buffering of extracellular K(+) in CA1 stratum radiatum. Glia 2020; 68: 918–931. [DOI] [PubMed] [Google Scholar]
- 18.Larson VA, Mironova Y, Vanderpool KG, et al. Oligodendrocytes control potassium accumulation in white matter and seizure susceptibility. Elife 2018; 7: 10–7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kofuji P, Newman EA. Potassium buffering in the central nervous system. Neuroscience 2004; 129: 1045–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanco G, Koster JC, Sanchez G, et al. Kinetic properties of the alpha 2 beta 1 and alpha 2 beta 2 isozymes of the Na,K-ATPase. Biochemistry 1995; 34: 319–325. [DOI] [PubMed] [Google Scholar]
- 21.Crambert G, Hasler U, Beggah AT, et al. Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J Biol Chem 2000; 275: 1976–1986. [DOI] [PubMed] [Google Scholar]
- 22.Stanley CM, Gagnon DG, Bernal A, et al. Importance of the voltage dependence of cardiac Na/K ATPase isozymes. Biophys J 2015; 109: 1852–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hille B. Ion channels of excitable membranes. Sunderland MA: Sinauer Associates, 2001. [Google Scholar]
- 24.Ransom BR, Yamate CL, Connors BW. Activity-dependent shrinkage of extracellular space in rat optic nerve: a developmental study. J Neurosci 1985; 5: 532–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Florence CM, Baillie LD, Mulligan SJ. Dynamic volume changes in astrocytes are an intrinsic phenomenon mediated by bicarbonate ion flux. Plos One 2012; 7: e5112410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsen BR, MacAulay N. Activity-dependent astrocyte swelling is mediated by pH-regulating mechanisms. Glia 2017 2017; 65: 1668–1681. [DOI] [PubMed] [Google Scholar]
- 27.Sontheimer H, Fernandez-Marques E, Ullrich N, et al. Astrocyte Na+ channels are required for maintenance of Na+/K(+)-ATPase activity. J Neurosci 1994; 14: 2464–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Black JA, Waxman SG. Noncanonical roles of voltage-gated sodium channels. Neuron 2013; 80: 280–291. [DOI] [PubMed] [Google Scholar]
- 29.Dunn KM, Hill-Eubanks DC, Liedtke WB, et al. TRPV4 channels stimulate Ca2+-induced Ca2+ release in astrocytic endfeet and amplify neurovascular coupling responses. Proc Natl Acad Sci U S A 2013; 110: 6157–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Y, Herman P, Rothman DL, et al. Evaluating the gray and white matter energy budgets of human brain function. J Cereb Blood Flow Metab 2018; 38: 1339–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber B, Barros LF. The astrocyte: Powerhouse and recycling center. Cold Spring Harb Perspect Biol 2015; 7: a020396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ioannou MS, Jackson J, Sheu SH, et al. Neuron-astrocyte metabolic coupling protects against activity-induced fatty acid toxicity. Cell 2019; 177: 1522–1535. [DOI] [PubMed] [Google Scholar]
- 33.Le Douce J, Maugard M, Veran J, et al. Impairment of glycolysis-derived l-serine production in astrocytes contributes to cognitive deficits in Alzheimer's disease. Cell Metab 2020; 31: 503–517. [DOI] [PubMed] [Google Scholar]
- 34.Wong-Riley MT. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci 1989; 12: 94–101. [DOI] [PubMed] [Google Scholar]
- 35.Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci 1999; 19: 6897–6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sibson NR, Dhankhar A, Mason GF, et al. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci U S A 1998; 95: 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bouzier-Sore AK, Voisin P, Bouchaud V, et al. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci 2006; 24: 1687–1694. [DOI] [PubMed] [Google Scholar]
- 38.Bluml S, Moreno-Torres A, Shic F, et al. Tricarboxylic acid cycle of glia in the in vivo human brain. NMR Biomed 2002; 15: 1–5. [DOI] [PubMed] [Google Scholar]
- 39.Oz G, Berkich DA, Henry PG, et al. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci 2004; 24: 11273–11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonnay S, Poirot J, Just N, et al. Astrocytic and neuronal oxidative metabolism are coupled to the rate of glutamate-glutamine cycle in the tree shrew visual cortex. Glia 2018; 66: 477–491. [DOI] [PubMed] [Google Scholar]
- 41.Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab 2007; 27: 219–249. [DOI] [PubMed] [Google Scholar]
- 42.Aubert A, Costalat R, Magistretti PJ, et al. Brain lactate kinetics: modeling evidence for neuronal lactate uptake upon activation. Proc Natl Acad Sci U S A 2005; 102: 16448–16453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovatt D, Sonnewald U, Waagepetersen HS, et al. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci 2007; 27: 12255–12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Genda EN, Jackson JG, Sheldon AL, et al. Co-compartmentalization of the astroglial glutamate transporter, GLT-1, with glycolytic enzymes and mitochondria. J Neurosci 2011; 31: 18275–18288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephen TL, Higgs NF, Sheehan DF, et al. Miro1 regulates Activity-Driven positioning of mitochondria within astrocytic processes apposed to synapses to regulate intracellular calcium signaling. J Neurosci 2015; 35: 15996–16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Derouiche A, Haseleu J, Korf HW. Fine astrocyte processes contain very small mitochondria: Glial oxidative capability may fuel transmitter metabolism. Neurochem Res 2015; 40: 2402–2413. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal A, Wu PH, Hughes EG, et al. Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 2017; 93: 587–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santuy A, Turegano-Lopez M, Rodriguez JR, et al. A quantitative study on the distribution of mitochondria in the neuropil of the juvenile rat somatosensory cortex. Cereb Cortex 2018; 28: 3673–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cali C, Agus M, Kare K, et al. 3D cellular reconstruction of cortical glia and parenchymal morphometric analysis from serial Block-Face electron microscopy of juvenile rat. Prog Neurobiol 2019; 183: 101696. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, Chen K, Sloan SA, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci 2014; 34: 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharma K, Schmitt S, Bergner CG, et al. Cell type- and brain region-resolved mouse brain proteome. Nat Neurosci 2015; 18: 1819–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herrero-Mendez A, Almeida A, Fernandez E, et al. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nat Cell Biol 2009; 11: 747–752. [DOI] [PubMed] [Google Scholar]
- 53.Barros LF, Weber B. CrossTalk proposal: an important astrocyte-to-neuron lactate shuttle couples neuronal activity to glucose utilisation in the brain. J Physiol 2018; 596: 347–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Magistretti PJ, Allaman I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat Rev Neurosci 2018; 19: 235–249. [DOI] [PubMed] [Google Scholar]
- 55.Lopez-Fabuel I, Le DJ, Logan A, et al. Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc Natl Acad Sci U S A 2016; 113: 13063–13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernandez-Fernandez S, Almeida A, Bolanos JP. Antioxidant and bioenergetic coupling between neurons and astrocytes. Biochem J 2012; 443: 3–11. [DOI] [PubMed] [Google Scholar]
- 57.Supplie LM, Duking T, Campbell G, et al. Respiration-deficient astrocytes survive as glycolytic cells in vivo. J Neurosci 2017; 37: 4231–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu AC, Drejer J, Hertz L, et al. Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J Neurochem 1983; 41: 1484–1487. [DOI] [PubMed] [Google Scholar]
- 59.Fecher C, Trovo L, Muller SA, et al. Cell-type-specific profiling of brain mitochondria reveals functional and molecular diversity. Nat Neurosci 2019; 22: 1731–1742. [DOI] [PubMed] [Google Scholar]
- 60.Ebert D, Haller RG, Walton ME. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy. J Neurosci 2003; 23: 5928–5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Edmond J, Robbins RA, Bergstrom JD, et al. Capacity for substrate utilization in oxidative metabolism by neurons, astrocytes, and oligodendrocytes from developing brain in primary culture. J Neurosci Res 1987; 18: 551–561. [DOI] [PubMed] [Google Scholar]
- 62.Ellis JM, Wong GW, Wolfgang MJ. Acyl coenzyme a thioesterase 7 regulates neuronal fatty acid metabolism to prevent neurotoxicity. Mol Cell Biol 2013; 33: 1869–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White CJ, Lee J, Choi J, et al. Determining the bioenergetic capacity for fatty acid oxidation in the mammalian nervous system. Mol Cell Biol 2020; 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Kuang Y, Xu K, et al. Ketosis proportionately spares glucose utilization in brain. J Cereb Blood Flow Metab 2013; 33: 1307–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Klepper J, Leiendecker B. Glut1 deficiency syndrome and novel ketogenic diets. J Child Neurol 2013; 28: 1045–1048. [DOI] [PubMed] [Google Scholar]
- 66.Valdebenito R, Ruminot I, Garrido-Gerter P, et al. Targeting of astrocytic glucose metabolism by beta-hydroxybutyrate. JCerebBlood Flow Metab 2016; 36: 1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schousboe A, Scafidi S, Bak LK, et al. Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol 2014; 11: 13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panov A, Orynbayeva Z, Vavilin V, et al. Fatty acids in energy metabolism of the central nervous system. Biomed Res Int 2014; 2014: 472459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schonfeld P, Reiser G. Why does brain metabolism not favor burning of fatty acids to provide energy? Reflections on disadvantages of the use of free fatty acids as fuel for brain. J Cereb Blood Flow Metab 2013; 33: 1493–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fernandez-Moncada I, Ruminot I, Robles-Maldonado D, et al. Neuronal control of astrocytic respiration through a variant of the crabtree effect. Proc Natl Acad Sci U S A 2018; 115: 1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lerchundi R, Fernandez-Moncada I, Contreras-Baeza Y, et al. NH4+ triggers the release of astrocytic lactate via mitochondrial pyruvate shunting. Proc Natl Acad Sci U S A 2015; 112: 11090–11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.San Martin A, Arce-Molina R, Galaz A, et al. Nanomolar nitric oxide concentrations quickly and reversibly modulate astrocytic energy metabolism. J Biol Chem 2017; 292: 9432–9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barros LF, Ruminot I, San Mart¡N A, et al. Aerobic glycolysis in the brain: Warburg and crabtree contra pasteur. NeurochemRes 2021; 46: 15–22. [DOI] [PubMed] [Google Scholar]
- 74.Williams V, Grossman RG, Edmunds SM. Volume and surface area estimates of astrocytes in the sensorimotor cortex of the cat. Neuroscience 1980; 5: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 75.Escartin C, Galea E, Lakatos A, et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 2021; 24: 312–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krawczyk MC, Haney JR, Caneda C, et al. Human astrocytes exhibit tumor microenvironment-, age-, and sex-related transcriptomic signatures. J Neurosci 2022. DOI: 10.1523/JNEUROSCI.0407-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li H, Lones L, DiAntonio A. Bidirectional regulation of glial potassium buffering – glioprotection versus neuroprotection. Elife 2021; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ruminot I, Schmalzle J, Leyton B, et al. Tight coupling of astrocyte energy metabolism to synaptic activity revealed by genetically encoded FRET nanosensors in hippocampal tissue. J Cereb Blood Flow Metab 2019; 39: 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernandez-Moncada I, Robles-Maldonado D, Castro P, et al. Bidirectional astrocytic GLUT1 activation by elevated extracellular K+. Glia 2021; 69: 1012–1021. [DOI] [PubMed] [Google Scholar]
- 80.Tong X, Ao Y, Faas GC, et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nat Neurosci 2014; 17: 694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Polyzos AA, Lee DY, Datta R, et al. Metabolic reprogramming in astrocytes distinguishes region-specific neuronal susceptibility in Huntington mice. Cell Metab 2019; 29: 1258–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li K, Li J, Zheng J, et al. Reactive astrocytes in neurodegenerative diseases. Aging Dis 2019; 10: 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]