Abstract
Despite declines in total cardiovascular mortality rates in the United States, heart failure (HF) mortality rates as well as hospitalizations and readmissions have increased in the past decade. Increases have been relatively higher among young and middle‐aged adults (<65 years). Therefore, identification of individuals HF at‐risk (Stage A) or with pre‐HF (Stage B) before the onset of overt clinical signs and symptoms (Stage C) is urgently needed. Multivariate risk models (e.g., Pooled Cohort Equations to Prevent Heart Failure [PCP‐HF]) have been externally validated in diverse populations and endorsed by the 2022 HF Guidelines to apply a risk‐based framework for the prevention of HF. However, traditional risk factors included in the PCP‐HF model only account for half of an individual's lifetime risk of HF; novel risk factors (e.g., adverse pregnancy outcomes, impaired lung health, COVID‐19) are emerging as important risk‐enhancing factors that need to be accounted for in personalized approaches to prevention. In addition to determining the role of novel risk‐enhancing factors, integration of social determinants of health (SDoH) in identifying and addressing HF risk is needed to transform the current clinical paradigm for the prevention of HF. Comprehensive strategies to prevent the progression of HF must incorporate pharmacotherapies (e.g., sodium glucose co‐transporter‐2 inhibitors that have also been termed the “statins” of HF prevention), intensive blood pressure lowering, and heart‐healthy behaviors. Future directions include investigation of novel prediction models leveraging machine learning, integration of risk‐enhancing factors and SDoH, and equitable approaches to interventions for risk‐based prevention of HF.
Keywords: heart failure, machine learning, primary prevention, risk prediction, social determinants
1. INTRODUCTION
Heart failure (HF) affects about 26 million people worldwide and is an important cause of cardiovascular morbidity and mortality. 1 , 2 , 3 In the United States, about 6.2 million people are affected by HF with a projected prevalence to exceed eight million by 2030. 4 In addition, healthcare expenditures related to HF are expected to rise to $69.7 billion by 2030. 5 Due to the increasing prevalence of cardiovascular risk factors (e.g., obesity, hypertension, diabetes), 6 , 7 more people are now living at risk of HF. Recent trends show that cardiovascular mortality related to HF has increased in the past decade with the greatest increases among younger adults. 8 Similarly, increases in HF hospitalizations and readmissions have been observed. Therefore, targeted approaches based on risk assessment are urgently needed to prevent or slow the rise in HF‐related burden. The recent universal classification of HF classifies the stages of HF as stage A: patients at risk of HF but without clinical signs or symptoms of HF; stage B: patients without HF but with abnormal heart structural and function; stage C: patients with signs or symptoms of HF; and stage D: patients with severe advanced signs or symptoms of HF refractory to therapy. 9 Based on this classification (Figure 1), the population at‐risk (prevalence of stage A or B HF) is much larger than those with clinical signs or symptoms (stage C or D). 10 , 11 , 12 In addition, once a patient progresses from stage A or B to stages C and D, the best outcome is to achieve remission despite optimal guideline‐directed medical treatment. 9 It is therefore important to focus on primary prevention of HF in patients at stages A and B, where more interventions can prevent or delay progression to later stages.
Figure 1.
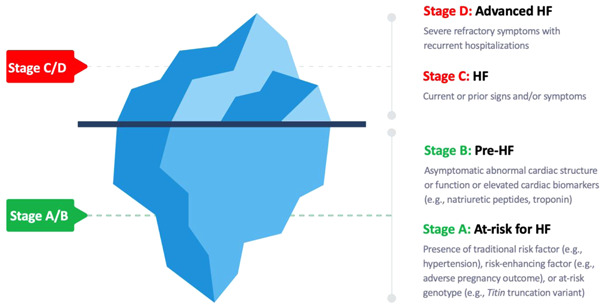
Refocusing on the primary prevention of heart failure. The large proportion of patients with Stage A/B below the surface who are at risk for progression to Stage C/D represents an important target of prevention strategies at the population‐ and individual level. HF, heart failure.
Strategies to prevent HF are critical to lower the prevalence of HF. From a population‐level perspective, the most effective prevention interventions identify and target high‐risk populations before they develop a disease condition. The four main levels of prevention for public health (Figure 2) include (1) primordial prevention, which focuses on preventing the development of risk factors; (2) primary prevention, which focuses on preventing the onset of disease in people at high risk; (3) secondary prevention, which focuses on preventing recurrence of disease‐related events in people with known disease condition; and (4) tertiary prevention, which focuses on preventing the progression of clinical disease or development of complications in people with a known disease condition. 13 Contemporary secondary and tertiary HF prevention efforts focus on the reduction of residual risk in patients with stages C and D HF, respectively, who represent a smaller proportion of patients compared to those with stage A and B HF. 14 Although guideline‐directed medical therapies are increasingly available for HF with reduced ejection fraction (HFrEF), prognosis remains dismal with 50% survival at 5 years. 15 Further, few effective disease‐modifying therapies currently exist for patients with HF with preserved ejection fraction (HFpEF), which is becoming the most common HF subtype. Therefore, prevention efforts urgently need to also shift upstream to primary and primordial prevention of HF, before irreversible myocardial changes that cause symptomatic HF begin. By focusing on primary and primordial prevention, more people at risk of HF may be prevented from progression to Stages C and D, which may be the “point of no return.” While we often discuss curative therapy in managing malignancy, once signs and symptoms of HF develop (Stage C and D), the target becomes remission and stabilization, and no curative therapies for HF are currently available. As a result, risk prediction to target prevention of HF, particularly for HFpEF, is a critical next step to improve outcomes.
Figure 2.
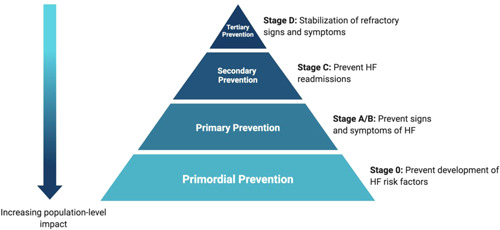
The public health pyramid of stages of prevention applied to heart failure. With each subsequent upstream step in prevention from tertiary to primordial, there is increasing impact at the population level. HF, heart failure.
2. HF RISK PREDICTION TOOLS
2.1. Short‐term multivariable risk prediction models
Although early identification of risk factors is key for primary prevention of HF, patients in Stages A and B are heterogeneous, and therefore identification may be difficult. Despite well‐established data on causal risk factors for HF (e.g., obesity, hypertension, diabetes, tobacco use), 16 , 17 models using single (or individual) risk factors may fail to discriminate between many patients who have different combinations of risk factors, and therefore markedly different absolute risk for HF. For example, not all patients with diabetes have a similarly high risk of HF. To address this heterogeneity in risk, several risk prediction tools have been developed that combine risk factor levels to facilitate preventive interventions based on absolute risk levels. Whereas risk‐based prevention (matching the intensity of prevention with the absolute risk of the individual) is widely accepted in the primary prevention of atherosclerotic cardiovascular disease, no such prevention paradigm currently exists for HF in clinical practice. Earlier risk prediction models developed in single cohorts, such as the Framingham Heart Study, Atherosclerosis Risk in Communities Study, the Health ABC, and the Atherosclerosis Risk in Communities Study included demographic and clinical risk factors for HF. 18 However, each of these models had variable performance in external validation studies. 6 , 19
More recently, the Pooled Cohort Equations to Prevent HF (PCP‐HF) tool was developed using routinely collected risk factor data (age, body mass index, systolic blood pressure, treatment for hypertension, fasting glucose, treatment for diabetes, total cholesterol, high‐density lipoprotein cholesterol, smoking status, and QRS duration on electrocardiography [ECG]) from 23 541 participants enrolled in five cohort‐based studies with at least 12 years follow‐up. 20 The model derivation sample included adults aged 30–79 years and performed well in internal and external validation in identifying and discriminating risk of HF. 20 In external validation, the model had good discrimination and calibration in diverse samples. 20 Furthermore, the PCP‐HF model has also been validated in real‐world data from electronic health records (with and without ECG QRS as a predictor to enhance generalizability), 21 and has been extended to large, nationally representative samples in the United States, Europe, and in Israel. 22 , 23
Based on the available data, the 2022 American College of Cardiology/American Heart Association/Heart Failure Society of America (ACC/AHA/HFSA) Guideline for the Management of HF recommended, for the first time, consideration of validated multivariable scores (e.g., PCP‐HF) to estimate the risk of incident HF in the general population with a class of recommendation 2a and level of evidence B‐NR. 24 This advances the paradigm of prevention of HF moving beyond focus on individual risk factor control and towards absolute risk. 25 , 26 , 27
Patients in Stage A may have a lower risk of HF compared to those at Stage B. However, the association between HF risk factors and HF is variable (i.e., different patients at Stage A may have different risks of HF because of the different risk factors that they have). Risk prediction models have also been developed to calculate risk in specific high‐risk populations that are known to have significantly increased risk of HF. 26 Risk prediction models have been developed for the elderly, 6 , 28 patients with hypertension, 29 patients with diabetes, 30 , 31 and patients with chronic kidney disease. 32 For example, Sahle et al. 29 developed a tool for 10‐year prediction of incident HF in elderly people with hypertension using routinely collected demographic and clinical data. However, the model was not externally validated. 29 Additionally, risk prediction models have been developed to discriminate between risk of HFpEF and HFrEF. 33
Traditional HF risk prediction models 6 , 18 , 19 , 20 , 28 have largely been based on traditional statistical modeling with Cox proportional hazard regression, which faces limitations, including the inability to utilize nonlinear outcomes. Advances in machine learning allows for the inclusion of large amounts of multidimensional data, are well equipped to handle missing data on covariates, and can adjust for nonlinear interactions between covariates. Machine learning models also offer the ability to continuously update models as new covariates and data become available in additional cohorts. Machine learning‐derived models have shown the ability to predict adverse events in population‐based studies 34 , 35 ; one such machine learning‐derived model developed by Segar et al. 36 using clinical, laboratory, and biomarker data demonstrated superior performance to multiple traditional HF risk prediction models. A key future step for machine learning‐derived risk assessment of groups at high risk of HF will be the integration of machine learning‐based models into the electronic health records to allow for better data capture and, ideally, to enable models to provide real‐time estimates of a patient's HF risk. As a result, these models could enable prospective identification of individuals at increased risk for HF (and HF subtypes) for targeted screening (e.g., biomarkers, echocardiography), prevention strategies (e.g., sodium glucose co‐transporter 2 inhibitors [SGLT2i]), and recruitment of high‐risk phenotypes for randomized clinical trials focused on HF prevention.
2.2. Long‐term HF risk prediction
The majority of risk prediction tools have focused only on short‐term (5–10 year) risk of HF. 20 , 21 , 22 , 29 However, because risk of HF across the lifetime is substantial (ranging between 20% and 46% at age 45 years), increases with age, and varies based on the prevalence of cardiovascular risk factors, long‐term risk prediction tools are needed. Importantly, an individual who may be at low short‐term risk but high long‐term risk for HF may not be identified with short‐term risk prediction alone. Therefore, focusing only on short‐term risk represents a key opportunity for prevention to intervene on long‐term risk. Recently, our group developed the first long‐term risk prediction model to estimate 30‐year risk of HF. 37 The models had strong predictive ability in women and men with all C‐statistics greater than 0.80. 37
2.3. Laboratory and imaging biomarkers for risk prediction
Contemporary HF risk prediction tools primarily rely on well‐studied traditional risk factors for the development of incident HF, including the presence or absence of hypertension, coronary artery disease, and diabetes; body mass index; and tobacco use. However, there has been growing interest in incorporating blood‐, electrocardiographic (ECG)‐, and echocardiographic (TTE)‐based biomarkers that may enhance risk prediction, particularly among individuals without established cardiovascular disease. 38 , 39 Elevated levels of routinely collected biomarkers of neurohormonal stress, myocardial injury, systemic inflammation, as well as the presence of left ventricular hypertrophy indicate the presence of systemic derangement early in the course of HF disease progression, and have been associated with a higher risk of incident HF among healthy community‐dwelling adults. 38 , 40 , 41 , 42
Cardiac troponin (cTn) is a marker of myocardial injury and N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) and B‐type natriuretic peptide (BNP) are biomarkers of myocardial neurohormonal stress that are detectable in the general population and associated with risk of adverse cardiovascular events. 41 , 43 These biomarkers have demonstrated some ability to predict incident HF, and currently comprise the most commonly utilized biomarkers in risk models. 19 , 28 , 31 , 44 , 45 However, routine use of these biomarkers is not currently widespread in clinical practice to identify HF risk despite guideline recommendations for BNP‐based prevention. Additional biomarkers that have shown predictive value for the development of incident HF include high‐sensitivity C‐reactive protein (hs‐CRP), 31 , 40 , 44 a nonspecific marker of systemic inflammation; plasminogen activator inhibitor (PAI)‐1, d‐dimer, and fibrinogen 44 which represent thrombotic and fibrinolytic pathways; galectin‐3 and soluble interleukin‐1 receptor‐like 1 (sST2) 44 , 46 which indicate tissue fibrosis; and cystatin‐C 44 which reflects renal dysfunction. Commonly measured biomarkers, including cTn, BNP, and hs‐CRP appear to be more strongly correlated with the development of HFrEF than HFpEF. 47 A substantial evidence base exists supporting the role of BNP/NT‐proBNP and cTn as diagnostic and prognostic markers of acute and chronic HF; 48 , 49 , 50 , 51 , 52 , 53 however, the recent emergence of sST2 and galectin‐3 as prognostic markers led to their inclusion in the 2017 HF guidelines for additive risk stratification. 25 Routine use of blood‐based biomarkers to assess risk of incident HF is complicated by changes in circulating levels of cTn and BNP/NT‐proBNP across the lifespan—these markers are influenced by age, 54 renal function, 55 body composition, 56 , 57 menopause, 58 and pregnancy. 59 , 60 Further research is needed to elucidate specific patient populations for whom routine use of blood‐based biomarkers in the use of HF risk prediction may be of most benefit that account for cost and scalability.
Several ECG findings have been associated with incident HF in adults without existing CAD, including QRS prolongation >120 ms 61 and left ventricular hypertrophy 62 , 63 (defined using a variety of criteria). These ECG findings represent underlying changes to myocardial structure, which may reflect left ventricular systolic dysfunction, and suggest patients who have transitioned from Stage A to Stage B. A prolonged QRS, in particular, has also been associated with increased mortality in Stage C HF with improved outcomes with cardiac resynchronization therapy in this subset of symptomatic patients. 25 , 64 As a result, QRS prolongation and ECG criteria for left ventricular hypertrophy have been integrated into several HF predictive risk scores. 19 , 20 However, routine ECG is not recommended in all adults by the United States Preventive Services Task Force (USPSTF), which leads to barriers in implementation of such risk scores.
Similarly, TTE provides noninvasive measures of subclinical systolic and diastolic dysfunction that may allow early detection of patients transitioning from Stage A to Stage B. Left ventricular hypertrophy, particularly when accompanied by systolic dysfunction or diastolic dysfunction, has been associated with increased risk of incident HF. 65 , 66 Global longitudinal strain can detect subtle systolic dysfunction before a reduction in ejection fraction occurs, and has been used to predict progression of HF in Stage A/B individuals across the lifespan. 67 , 68 , 69 Future outcome studies are needed to better define the role of TTE and advanced cardiac imaging in the prevention of progression of HF in high‐risk individuals, both as a screening mechanism and as tools to assess the impact of risk factor modification and medical therapy on incident HF, particularly with cost‐effectiveness in mind.
3. RISK‐ENHANCING FACTORS ASSOCIATED WITH HF
Although multivariable HF risk prediction models discriminate HF risk and stratify individuals based on estimates of risk, they may underestimate risk in people without traditional HF risk factors. The use of risk‐enhancing factors to personalize atherosclerotic cardiovascular disease risk may similarly be applied to HF risk prediction to personalize HF risk. About 50% of population attributable fraction of HF is associated with the presence of traditional risk factors, specifically obesity, hypertension, diabetes, hyperlipidemia, and smoking. 70 Risk‐enhancing factors are nontraditional risk factors that have not classically been included in HF prediction tools, but may provide an opportunity to personalize the individual‐level approach to HF prevention (Figure 3). Some representative examples are discussed here, which include hypertensive disorders of pregnancy, breast cancer, chronic lung disease, and Covid‐19.
Figure 3.
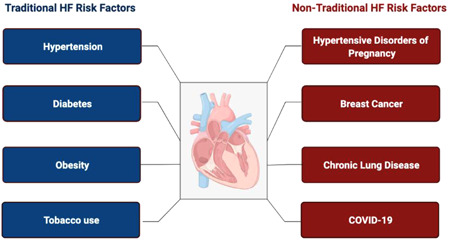
Traditional and nontraditional risk factors for heart failure. Only half of lifetime risk of heart failure is captured by traditional risk factors. Emerging risk‐enhancing factors should be incorporated into personalized strategies for prevention of heart failure and include (but are not limited to) hypertensive disorders of pregnancy, breast cancer, chronic lung disease, and Covid‐19. HF, heart failure.
3.1. Hypertensive disorders of pregnancy
Pre‐eclampsia, which affects about 2%–8% of all pregnancies, is associated with a fourfold higher maternal risk of future HF. 71 , 72 Women who develop pre‐eclampsia during pregnancy are at higher risk of future hypertension and diabetes, 73 , 74 which are known risk factors of HF. Although the mechanism by which pre‐eclampsia increases the risk of HF is not well‐established, the factors that predispose women to pre‐eclampsia are similar to risk factors for HF, 16 , 75 , 76 and therefore may be targeted for pre‐eclampsia prevention, and subsequent HF prevention (e.g., optimizing dietary quality, increasing physical activity, maintaining a healthy body mass index).
3.2. Breast cancer
Breast cancer is the most common malignancy among women in the United States, 77 and associated with an increased risk for HF. 78 , 79 Additionally, several chemotherapy agents for breast cancer such as doxorubicin, cyclophosphamide, anastrozole, and trastuzumab increase risk for HF. 80 , 81 The 5‐year survival after initial breast cancer diagnosis is about 90%, with about three million breast cancer survivors in the United States who are at increased risk of HF. 82 Measurement of left ventricular ejection fraction before, during, or after chemotherapy for breast cancer may allow for early detection of cardiovascular toxicity related to treatment for early intervention. 81 Since risk factors for breast cancer and HF are similar (e.g., smoking, obesity, sedentary lifestyle) and shared mechanisms may increase risk for both cancer recurrence and HF (e.g., inflammation, angiogenesis, clonal hematopoiesis of indeterminate potential), 82 incorporation of breast cancer and cardiotoxic chemotherapy regimens as risk‐enhancing factors in HF risk prediction may help individualize and optimize prevention strategies for both HF and cancer.
3.3. Chronic lung diseases
In 2010, about 384 million people were affected by chronic obstructive pulmonary disease (COPD), 83 and by 2030, COPD is projected to be the fourth leading cause of death worldwide. 84 COPD and HF commonly coexist and are associated with significantly higher morbidity and mortality, particularly among older adults. 85 , 86 , 87 Beyond the concurrence of COPD and HF that is attributable to shared upstream risk factors (e.g., smoking, aging), COPD is independently associated with an increased risk of HF that may be, in part, mediated by inflammation. 88 , 89 Even in the absence of symptomatic lung disease, subclinical impairments in lung health are associated with adverse cardiac remodeling. Therefore, it may be important to include chronic lung disease as a risk‐enhancing factor to improve personalization of HF risk prediction. However, data are needed on the timing and associated mechanisms of the transition from heart and lung health to disease across the life course.
3.4. Covid‐19 infection
In the United States, about 63 million cases of Covid‐19 and about 840 000 deaths related to Covid‐19 were recorded as of January 2022. 90 Although Covid‐19 was initially thought to predominantly affect the respiratory system, it rapidly became evident that acute and postacute infection with Covid‐19 affects multiple organs, including the heart with evidence of biomarker elevation in many hospitalized patients. 91 Among 243 hospitalized patients with acute respiratory distress syndrome (ARDS) due to Covid‐19 who required intubation and mechanical ventilation from a single academic center, 51% had troponin levels above the upper limit of normal. 92 When compared with patients with ARDS without Covid‐19, rates of biomarker elevation were lower after adjustment for age and comorbidities suggesting observed myocardial injury reflects critical illness rather than direct viral injury. In patients with pre‐existing HF (either with reduced or preserved ejection fraction), Covid‐19 is associated with an increased risk for HF hospitalization 93 and in‐hospital death. 94 Emerging data also suggest increased short‐ and long‐term risk of incident HF after Covid‐19 infection. One study from Germany of 100 individuals who were at least 2 weeks post‐Covid‐19 diagnosis (median [interquartile range] 71 [64–92] days) demonstrated evidence of myocardial inflammation on cardiac magnetic resonance imaging in 60% of people. 95 Furthermore, recent evidence suggests that Covid‐19 increases the risk of long‐term cardiovascular outcomes, including HF (even among patients who were not hospitalized during the acute phase of Covid‐19 infection). In a nationwide cohort study from the Department of Veteran Affairs, 153 760 individuals with Covid‐19 had a higher risk of HF at 12 months (HR: 1.72 [1.65, 1.80]) compared with 5 637 647 controls. 96 More studies are needed to improve understanding of both short‐ and long‐term complications of Covid‐19 on heart health., However, due to the high prevalence of Covid‐19 and higher risk of severe Covid‐19 infection among those with HF risk factors (e.g., obesity, hypertension), 90 incorporation of history or current Covid‐19 infection as a risk enhancing factor in HF risk prediction models may help to better stratify risk in HF prevention and inform patient management after surviving Covid‐19.
4. SOCIAL DETERMINANTS OF RISK FOR HF
In recent years a growing body of research has examined the association between a multitude of social, demographic, economic, and environmental domains, including race, ethnicity, access to health care and health insurance, public health infrastructure, neighborhood environment, air pollution, economic stability, and education and risk of incident HF. Racial disparities in both the prevalence and incidence of HF are well‐established; the prevalence of HF is greater among Black individuals than among non‐Hispanic White individuals, 97 and Black individuals develop HF at younger ages than White individuals. 98 However, additional social determinants have an established and consistent association with incident HF, including living in an area with few healthcare services, lack of health insurance, low educational attainment, increased exposure to particulate air matter, and low annual income. 17 , 99 Meta‐analysis of the independent contribution of socioeconomic status to risk of incident HF found a 62% increase in HF risk associated with socioeconomic deprivation by any measure of SES, including income, education, occupation, or area‐level measures of neighborhood deprivation. 100 Furthermore, there is also evidence to suggest that multiple socially determined vulnerabilities have a cumulative effect on HF risk within the same individual, particularly in individuals under 65 years of age independent of traditional risk factors. 101 A sum of socially determined vulnerabilities may substitute as an additional risk enhancer in patients who are at increased risk of incident HF, especially among younger individuals. Importantly, social and political systematic changes implemented with the aim of reducing social, environmental, and economic disparities provide a significant opportunity to address upstream risk factors before patients become adversely affected by downstream effects, including poverty, poor education, inadequate housing, decreased access to nutritious food, and inadequate access to healthcare resources. 99 Efforts to address social determinants of risk must simultaneously address structural and systemic barriers (e.g., racism) that contribute to HF risk and may provide the highest‐impact opportunities to conduct both primordial and primary HF prevention across the spectrum of risk.
5. GENETIC RISK MARKERS FOR HF: OVERLAP WITH CARDIOMYOPATHY
HF is a heterogeneous syndrome that represents the terminal clinical pathway of most cardiovascular diseases and manifests as an array of phenotypes. There is also significant diversity mirrored in the genetic architecture of HF, which can range from multiple genetic or epigenetic low‐penetrance loci that are modified by environmental factors to monogenic HF syndromes resulting from a single rare disease‐causing or pathogenic variant (e.g., Titin‐truncating variants). 102 Genetic cardiomyopathies comprise a small proportion of overall incident HF, although this varies by age and population. Among pediatric patients, a familial monogenic origin has been identified in 26%–40% of patients, while among adults with idiopathic dilated cardiomyopathy the proportion of familial disease is approximately 30%. 103 , 104 , 105 Susceptibility to HF is also heritable as a complex trait, with heritability estimated to be ~18%. 106 Cardiovascular research has made rapid advancements in elucidating the cardiac epigenome, and in the last several years epigenome‐wide associations have linked epigenetic loci with clinical HF in living patients utilizing a multiomics approach, in which genomic, epigenomic, transcriptomic, proteomic, and microbiome data are combined in analysis. 107 , 108 , 109 These methods offer significant potential for the development of precision medicine approaches to identification of risk and risk‐based prevention of HF. 110 Using an integrative approach, it may eventually be possible to combine an individual's multi‐OMIC data into an individual risk profile, which, when combined with phenotypic clinical and sociodemographic data incorporated from the electronic health record, may enhance prediction of incident HF as well as HF outcomes utilizing machine learning techniques and artificial intelligence.
6. PREVENTION STRATEGIES FOR HIGH‐RISK INDIVIDUALS
6.1. Heart‐healthy lifestyle modifications
Lifestyle factors are associated with the risk of HF. 111 , 112 Based on evidence from multiple clinical trials and observational studies, the AHA recommends seven health factors to define cardiovascular health: Life's Simple 7. 113 These factors include: (1) not smoking, (2) optimal levels of physical activity, (3) healthy diet quality, (4) normal body mass index, (5) optimize cholesterol, (6) control fasting blood glucose, and (7) optimize blood pressure. 113 Smoking increases risk for HF. 114 Moreover, people who have never smoked have a lower risk of HF compared with former smokers. 115 Engaging in >150 min/week of moderate‐intensity physical activity or >75 min/week of high‐intensity activity is associated with a lower risk of HF. 116 , 117 Similarly, Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets are associated with lower cardiovascular risk and better cardiovascular health. 118 , 119 , 120 Age‐specific interventions that target frailty in older adults may also have benefit in prevention of HF.
6.2. Intensive blood pressure lowering
Patients with hypertension have a higher risk for HF compared with normotensive patients. 121 In both patients with and without known cardiovascular disease, blood pressure lowering is associated with lower risk of adverse outcomes. 122 Based on evidence of greater benefits of lower blood pressure, ACC/AHA guidelines recommend blood pressure lower than 130/80 mmHg for optimal cardiovascular risk, but treatment is initiated when blood pressure >140/90 mmHg. 123 The systolic blood pressure intervention trial (SPRINT) trial documented that intensive blood pressure lowering to a systolic pressure <120 mmHg decreased risk for future HF compared with <140 mmHg, 124 , 125 While there is growing evidence for the effectiveness of intensive blood pressure lowering in the prevention of HF, intensive blood pressure lowering can also lead to an increase in serious adverse events. Therefore, maximizing the benefit and minimizing adverse effects by estimating HF risk may guide selection for intensive BP lowering. A recent post hoc analysis of the Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated greater risk reduction in those with the highest baseline risk (low risk: HR: 0.86 [95% CI: 0.29, 2.56]; intermediate risk: 0.54 [0.23, 1.30]; high risk: 0.45 [0.23, 0.86]). 126 Despite the benefits of intensive blood pressure lowering in HF prevention, there remains ongoing debate regarding the comparative effectiveness of various antihypertensive therapies. Emerging therapies for HF treatment, such as sacubitril‐valsartan, have not been studied in the prevention of HF but may offer benefits related to both BP lowering as well as reverse cardiac remodeling. A study by Sciarretta et al. 127 showed that diuretics, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers (ARBs) were more effective than calcium channel blockers for HF prevention. 127
6.3. Biomarker‐based prevention
Two landmark trials that focused on biomarker‐based prevention of HF: the STOP‐HF (St. Vincent Screening to Prevent Heart Failure) 128 and PONTIAC (NT‐proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease) 129 led to a guideline‐based recommendation for natriuretic peptide‐based prevention. Both of these trials focused on screening with either BNP or NT‐proBNP to identify patients at risk for HF. Participants randomized to the intervention were then referred for intensive preventive therapies, such as up‐titration of renin–angiotensin–aldosterone system antagonists and beta‐blockers, which led to lower risk of incident subclinical or clinical HF (systolic or diastolic). However, widespread implementation of BNP‐based screening continues to be limited given the cost and lack of clarity in which patients may benefit from biomarker testing. Alternative strategies include sequential biomarker testing with initial assessment of risk using multivariable risk prediction models that only require readily available clinical factors, such as the PCP‐HF. Among those at intermediate risk, biomarker‐based testing may improve reclassification. Future studies investigating the utility of combining risk factor‐based models with biomarker‐based models are needed.
6.4. Mineralocorticoid receptor antagonists (MRA)
Despite the beneficial effects of aldosterone in normal physiologic regulation of body sodium, potassium, and water regulation, aldosterone can also negatively affect the heart, including inducing inflammation, stiffening of vessels, and stimulation of fibrosis in myocardium. 130 , 131 , 132 Aldosterone may therefore play an important role in the pathogenesis of HF. As such, MRAs are associated with improved outcomes in patients with HFrEF. 133 , 134 , 135 In the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial, although spironolactone did not effectively reduce risk of the composite outcome of adverse cardiovascular events (death from cardiovascular causes, aborted cardiac arrest, or hospitalization for the management of HF), it reduced the risk of HF hospitalization. 136 Despite established benefits of MRAs in HF, there have been no large‐scale randomized control trials to study the effects of MRAs in primary prevention of HF.
6.5. Sodium‐glucose co‐Transporter‐2 inhibitors (SGLT2i)
SGLT2i are novel drugs that lower blood glucose by decreasing the rate of glucose reabsorption and increasing glucose excretion. 137 SGLT2i reduces risk of adverse cardiovascular events in people with diabetes. 138 , 139 , 140 SGLT2i also reduced the risk of cardiovascular death or HF hospitalizations in patients with and without diabetes with chronic kidney disease, HFrEF, and HFpEF. 141 , 142 , 143 , 144 The benefits of SGLT2i have also been observed in patients who are at high risk of HF but do not have a known history of HF and suggest a role for SGLT2i in the primary prevention of HF. As a result, SGLT2i's have been proposed for use in a risk‐based algorithm for the prevention of HF, 145 akin to the role of statins in atherosclerotic cardiovascular disease risk prevention.
6.6. Telemedicine in HF prevention
Significant rural–urban disparities exist in cardiovascular health and HF mortality. One potential approach to focus on equitable prevention strategies is telemedicine, which includes home telemonitoring and telephone‐supported monitoring. Telemedicine has been demonstrated to be effective in reducing mortality and hospital admission rates among people with HF. 146 , 147 The prevalence of telemedicine interventions has grown rapidly as a result of the Covid‐19 pandemic, when medical systems rapidly transitioned to noncontact care delivery methods for ambulatory care as social lockdowns, quarantine, and perceived risk of infection resulted in decreased contact between patients with HF and medical contacts. 148 , 149 Future studies are needed to evaluate the effect of telemedicine in HF prevention.
7. FUTURE DIRECTIONS AND CONCLUSIONS
Given the growing burden of HF hospitalization and mortality, strategies are urgently needed to focus on the primary prevention of HF before the onset of clinical signs and symptoms. The goal will be to reduce the number of people who develop Stage C or Stage D HF who have a dismal prognosis. Intervening earlier during the risk stage with preventive strategies may offer the greatest yield before irreversible damage has occurred. This will require an understanding of which populations are at highest risk of developing HF to match the intensity of the preventive intervention with the absolute risk of the individual. Several risk prediction tools have been developed, including the PCP‐HF model that is well‐validated in multiple international populations and endorsed by the 2022 multisociety HF Guidelines in the United States. 24 There is a wide array of genetic, biologic, clinical, and socioeconomic markers of enhanced risk, which may enhance personalized risk prediction when combined with traditional risk factor levels. Furthermore, machine learning and multi‐OMIC approaches offer further opportunities to identify novel mechanisms and refine risk estimates. Once individuals at risk are identified, implementation gaps that persist in achieving optimal risk factor levels need to be addressed. Lastly, future research is necessary to determine whether specific high‐risk individuals may benefit from medical therapy, including MRA or SGLT2i, for the primary prevention of incident HF.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
S.S.K. is supported by grants from the NHLBI (R01HL161514; R01HL159250; U01HL160279) and from the American Heart Association (#19TPA34890060). The funding sponsor did not contribute to design and conduct of the study, collection, management, analysis, or interpretation of the data or preparation, review, or approval of the manuscript. The authors take responsibility for decision to submit the manuscript for publication.
Hammond MM, Everitt IK, Khan SS. New strategies and therapies for the prevention of heart failure in high‐risk patients. Clin Cardiol. 2022;45:S13‐S25. 10.1002/clc.23839
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63(12):1123‐1133. 10.1016/j.jacc.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 2. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22(8):1342‐1356. 10.1002/ejhf.1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18(8):891‐975. 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 4. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics‐2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139‐e596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 5. Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606‐619. 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1(2):125‐133. 10.1161/CIRCHEARTFAILURE.108.768457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ogden CL, Fryar CD, Martin CB, et al. Trends in obesity prevalence by race and Hispanic origin‐1999‐2000 to 2017‐2018. JAMA. 2020;324(12):1208‐1210. 10.1001/jama.2020.14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glynn P, Lloyd‐Jones DM, Feinstein MJ, Carnethon M, Khan SS. Disparities in cardiovascular mortality related to heart failure in the United States. J Am Coll Cardiol. 2019;73(18):2354‐2355. 10.1016/j.jacc.2019.02.042 [DOI] [PubMed] [Google Scholar]
- 9. Bozkurt B, Coats A, Tsutsui H. Universal definition and classification of heart failure. J Card Fail . 2021. 10.1016/j.cardfail.2021.01.022 [DOI] [PubMed]
- 10. Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115(12):1563‐1570. 10.1161/CIRCULATIONAHA.106.666818 [DOI] [PubMed] [Google Scholar]
- 11. Wagner M, Tiffe T, Morbach C, et al. Characteristics and Course of heart failure stages A‐B and determinants of progression—design and rationale of the STAAB cohort study. Eur J Prev Cardiol. 2017;24(5):468‐479. 10.1177/2047487316680693 [DOI] [PubMed] [Google Scholar]
- 12. Morbach C, Gelbrich G, Tiffe T, et al. Prevalence and determinants of the precursor stages of heart failure: results from the population‐based STAAB cohort study. Eur J Prev Cardiol. 2021;28(9):924‐934. 10.1177/2047487320922636 [DOI] [PubMed] [Google Scholar]
- 13. Mensah GA, Dietz WH, Harris VB, et al. Prevention and control of coronary heart disease and stroke—nomenclature for prevention approaches in public health: a statement for public health practice from the Centers for Disease Control and Prevention. Am J Prev Med. 2005;29(5 Suppl 1):152‐157. 10.1016/j.amepre.2005.07.035 [DOI] [PubMed] [Google Scholar]
- 14. Greene SJ, Fonarow GC, Butler J. Risk profiles in heart failure: baseline, residual, worsening, and advanced heart failure risk. Circ Heart Fail. 2020;13(6):e007132. 10.1161/CIRCHEARTFAILURE.120.007132 [DOI] [PubMed] [Google Scholar]
- 15. Writing Committee M, Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128(16):e240‐e327. 10.1161/CIR.0b013e31829e8776 [DOI] [PubMed] [Google Scholar]
- 16. Yang H, Negishi K, Otahal P, Marwick TH. Clinical prediction of incident heart failure risk: a systematic review and meta‐analysis. Open Heart. 2015;2(1):e000222. 10.1136/openhrt-2014-000222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Piepoli MF, Adamo M, Barison A, et al. Preventing heart failure: a position paper of the Heart Failure Association in collaboration with the European Association of Preventive Cardiology. Eur J Heart Fail. 2022;24(1):143‐168. 10.1002/ejhf.2351 [DOI] [PubMed] [Google Scholar]
- 18. Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159(11):1197‐1204. 10.1001/archinte.159.11.1197 [DOI] [PubMed] [Google Scholar]
- 19. Agarwal SK, Chambless LE, Ballantyne CM, et al. Prediction of incident heart failure in general practice: the Atherosclerosis Risk in Communities (ARIC) Study. Circ Heart Fail. 2012;5(4):422‐429. 10.1161/CIRCHEARTFAILURE.111.964841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khan SS, Ning H, Shah SJ, et al. 10‐year risk equations for incident heart failure in the general population. J Am Coll Cardiol. 2019;73(19):2388‐2397. 10.1016/j.jacc.2019.02.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bavishi A, Bruce M, Ning H, et al. Predictive accuracy of heart failure‐specific risk equations in an electronic health record‐based cohort. Circ Heart Fail. 2020;13(11):e007462. 10.1161/CIRCHEARTFAILURE.120.007462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Glynn PA, Ning H, Bavishi A, et al. Heart failure risk distribution and trends in the United States population, NHANES 1999‐2016. Am J Med. 2021;134(3):e153‐e164. 10.1016/j.amjmed.2020.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khan SS, Barda N, Greenland P, et al. Validation of heart failure‐specific risk equations in 1.3 million Israeli adults and usefulness of combining ambulatory and hospitalization data from a large integrated health care organization. Am J Cardiol. 2022;168:105‐109. 10.1016/j.amjcard.2021.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heidenreich PA, Bozkurt B, Aguilar D, et al. AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;2022:101161. 10.1161/CIR.0000000000001063 [DOI] [Google Scholar]
- 25. Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70(6):776‐803. 10.1016/j.jacc.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 26. Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117(19):2544‐2565. 10.1161/CIRCULATIONAHA.107.188965 [DOI] [PubMed] [Google Scholar]
- 27. Lloyd‐Jones DM, Braun LT, Ndumele CE, et al. Use of risk assessment tools to guide decision‐making in the primary prevention of atherosclerotic cardiovascular disease. A Special Report From the American Heart Association and American College of Cardiology. Am. J. Cardiol. 2018;73:3153‐3167. 10.1016/j.jacc.2018.11.005 [DOI] [Google Scholar]
- 28. Chahal H, Bluemke DA, Wu CO, et al. Heart failure risk prediction in the multi‐ethnic study of atherosclerosis. Heart. 2015;101(1):58‐64. 10.1136/heartjnl-2014-305697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sahle BW, Owen AJ, Wing LM, et al. Prediction of 10‐year risk of incident heart failure in elderly hypertensive population: The ANBP2 Study. Am J Hypertens. 2017;30(1):88‐94. 10.1093/ajh/hpw119 [DOI] [PubMed] [Google Scholar]
- 30. Hippisley‐Cox J, Coupland C. Development and validation of risk prediction equations to estimate future risk of heart failure in patients with diabetes: a prospective cohort study. BMJ Open. 2015;5(9):e008503. 10.1136/bmjopen-2015-008503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pandey A, Vaduganathan M, Patel KV, et al. Biomarker‐Based risk prediction of incident heart failure in pre‐diabetes and diabetes. JACC Heart Fail. 2021;9(3):215‐223. 10.1016/j.jchf.2020.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mehta R, Ning H, Bansal N, et al. Ten‐year risk‐prediction equations for incident heart failure hospitalizations in chronic kidney disease: findings from the chronic renal insufficiency cohort study and the multi‐ethnic study of atherosclerosis. J Card Fail. 2021;28:540‐550. 10.1016/j.cardfail.2021.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ho JE, Enserro D, Brouwers FP, et al. Predicting heart failure with preserved and reduced ejection fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail. Jun 2016;9(6). 10.1161/CIRCHEARTFAILURE.115.003116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Segar MW, Vaduganathan M, Patel KV, et al. machine learning to predict the risk of incident heart failure hospitalization among patients with diabetes: The WATCH‐DM Risk Score. Diabetes Care. 2019;42(12):2298‐2306. 10.2337/dc19-0587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ambale‐Venkatesh B, Yang X, Wu CO, et al. Cardiovascular event prediction by machine learning: the multi‐ethnic study of atherosclerosis. Circ Res. 2017;121(9):1092‐1101. 10.1161/CIRCRESAHA.117.311312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Segar MW, Jaeger BC, Patel KV, et al. Development and validation of machine learning‐based race‐specific models to predict 10‐year risk of heart failure: a multicohort analysis. Circulation. 2021;143(24):2370‐2383. 10.1161/CIRCULATIONAHA.120.053134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Khan SS, Ning H, Allen NB, et al. devopment and validation of a longterm incident heart failure risk model. Circ Res. 2022;130(2):200‐209. 10.1161/CIRCRESAHA.121.319595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. de Lemos JA, Ayers CR, Levine BD, et al. Multimodality strategy for cardiovascular risk assessment: performance in 2 population‐based cohorts. Circulation. 2017;135(22):2119‐2132. 10.1161/CIRCULATIONAHA.117.027272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pandey A, Patel KV, Vongpatanasin W, et al. Incorporation of biomarkers into risk assessment for allocation of antihypertensive medication according to the 2017 ACC/AHA high blood pressure guideline: a pooled cohort analysis. Circulation. 2019;140(25):2076‐2088. 10.1161/CIRCULATIONAHA.119.043337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kalogeropoulos A, Georgiopoulou V, Psaty BM, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55(19):2129‐2137. 10.1016/j.jacc.2009.12.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655‐663. 10.1056/NEJMoa031994 [DOI] [PubMed] [Google Scholar]
- 42. Pandey A, Keshvani N, Ayers C, et al. Association of cardiac injury and malignant left ventricular hypertrophy with risk of heart failure in African Americans: The Jackson Heart Study. JAMA Cardiol. 2019;4(1):51‐58. 10.1001/jamacardio.2018.4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Daniels LB, Laughlin GA, Clopton P, Maisel AS, Barrett‐Connor E. Minimally elevated cardiac troponin T and elevated N‐terminal pro‐B‐type natriuretic peptide predict mortality in older adults: results from the Rancho Bernardo Study. J Am Coll Cardiol. 2008;52(6):450‐459. 10.1016/j.jacc.2008.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suthahar N, Lau ES, Blaha MJ, et al. Sex‐specific associations of cardiovascular risk factors and biomarkers with incident heart failure. J Am Coll Cardiol. 2020;76(12):1455‐1465. 10.1016/j.jacc.2020.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Glick D, deFilippi CR, Christenson R, Gottdiener JS, Seliger SL. Long‐term trajectory of two unique cardiac biomarkers and subsequent left ventricular structural pathology and risk of incident heart failure in community‐dwelling older adults at low baseline risk. JACC Heart Fail. 2013;1(4):353‐360. 10.1016/j.jchf.2013.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aimo A, Vergaro G, Passino C, et al. Prognostic value of soluble suppression of tumorigenicity‐2 in chronic heart failure: a meta‐analysis. JACC Heart Fail. 2017;5(4):280‐286. 10.1016/j.jchf.2016.09.010 [DOI] [PubMed] [Google Scholar]
- 47. de Boer RA, Nayor M, deFilippi CR, et al. Association of cardiovascular biomarkers with incident heart failure with preserved and reduced ejection fraction. JAMA Cardiol. 2018;3(3):215‐224. 10.1001/jamacardio.2017.4987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Booth RA, Hill SA, Don‐Wauchope A, et al. Performance of BNP and NT‐proBNP for diagnosis of heart failure in primary care patients: a systematic review. Heart Fail Rev. 2014;19(4):439‐451. 10.1007/s10741-014-9445-8 [DOI] [PubMed] [Google Scholar]
- 49. Zaphiriou A, Robb S, Murray‐Thomas T, et al. The diagnostic accuracy of plasma BNP and NTproBNP in patients referred from primary care with suspected heart failure: results of the UK natriuretic peptide study. Eur J Heart Fail. 2005;7(4):537‐541. 10.1016/j.ejheart.2005.01.022 [DOI] [PubMed] [Google Scholar]
- 50. Moe GW, Howlett J, Januzzi JL, Zowall H. Canadian Multicenter Improved Management of Patients With Congestive Heart Failure Study I. N‐terminal pro‐B‐type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE‐CHF study. Circulation. 2007;115(24):3103‐3110. 10.1161/CIRCULATIONAHA.106.666255 [DOI] [PubMed] [Google Scholar]
- 51. Mueller C, Scholer A, Laule‐Kilian K, et al. Use of B‐type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350(7):647‐654. 10.1056/NEJMoa031681 [DOI] [PubMed] [Google Scholar]
- 52. Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O'Connor CM, Felker GM. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol. 2010;56(14):1071‐1078. 10.1016/j.jacc.2010.06.016 [DOI] [PubMed] [Google Scholar]
- 53. Savarese G, Musella F, D'Amore C, et al. Changes of natriuretic peptides predict hospital admissions in patients with chronic heart failure: a meta‐analysis. JACC Heart Fail. 2014;2(2):148‐158. 10.1016/j.jchf.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 54. Forman DE, de Lemos JA, Shaw LJ, et al. Cardiovascular biomarkers and imaging in older adults: JACC council perspectives. J Am Coll Cardiol. 2020;76(13):1577‐1594. 10.1016/j.jacc.2020.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Das SR, Abdullah SM, Leonard D, et al. Association between renal function and circulating levels of natriuretic peptides (from the Dallas Heart Study). Am J Cardiol. 2008;102(10):1394‐1398. 10.1016/j.amjcard.2008.07.018 [DOI] [PubMed] [Google Scholar]
- 56. Neeland IJ, Winders BR, Ayers CR, et al. Higher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. J Am Coll Cardiol. 2013;62(8):752‐760. 10.1016/j.jacc.2013.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Das SR, Drazner MH, Dries DL, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112(14):2163‐2168. 10.1161/CIRCULATIONAHA.105.555573 [DOI] [PubMed] [Google Scholar]
- 58. Chang AY, Abdullah SM, Jain T, et al. Associations among androgens, estrogens, and natriuretic peptides in young women: observations from the Dallas Heart Study. J Am Coll Cardiol. 2007;49(1):109‐116. 10.1016/j.jacc.2006.10.040 [DOI] [PubMed] [Google Scholar]
- 59. Mayama M, Yoshihara M, Uno K, et al. Factors influencing brain natriuretic peptide levels in healthy pregnant women. Int J Cardiol. 2017;228:749‐753. 10.1016/j.ijcard.2016.11.111 [DOI] [PubMed] [Google Scholar]
- 60. Sheikh M, Ostadrahimi P, Salarzaei M, Parooie F. Cardiac Complications in pregnancy: a systematic review and meta‐analysis of diagnostic accuracy of BNP and N‐terminal Pro‐BNP. Cardiol Ther. 2021;10(2):501‐514. 10.1007/s40119-021-00230-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dhingra R, Pencina MJ, Wang TJ, et al. Electrocardiographic QRS duration and the risk of congestive heart failure: the Framingham Heart Study. Hypertension. May 2006;47(5):861‐867. 10.1161/01.HYP.0000217141.20163.23 [DOI] [PubMed] [Google Scholar]
- 62. Oseni AO, Qureshi WT, Almahmoud MF, et al. Left ventricular hypertrophy by ECG versus cardiac MRI as a predictor for heart failure. Heart. 2017;103(1):49‐54. 10.1136/heartjnl-2016-309516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rautaharju PM, Prineas RJ, Wood J, Zhang ZM, Crow R, Heiss G. Electrocardiographic predictors of new‐onset heart failure in men and in women free of coronary heart disease (from the Atherosclerosis in Communities [ARIC] Study). Am J Cardiol. 2007;100(9):1437‐1441. 10.1016/j.amjcard.2007.06.036 [DOI] [PubMed] [Google Scholar]
- 64. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539‐1549. 10.1056/NEJMoa050496 [DOI] [PubMed] [Google Scholar]
- 65. Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37(4):1042‐1048. 10.1016/s0735-1097(01)01110-x [DOI] [PubMed] [Google Scholar]
- 66. Verdecchia P, Angeli F, Gattobigio R, Sardone M, Porcellati C. Asymptomatic left ventricular systolic dysfunction in essential hypertension: prevalence, determinants, and prognostic value. Hypertension. 2005;45(3):412‐418. 10.1161/01.HYP.0000154822.37141.f6 [DOI] [PubMed] [Google Scholar]
- 67. Shah AM, Claggett B, Loehr LR, et al. Heart failure stages among older adults in the community: the atherosclerosis risk in communities study. Circulation. 2017;135(3):224‐240. 10.1161/CIRCULATIONAHA.116.023361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yang H, Negishi K, Wang Y, Nolan M, Saito M, Marwick TH. Echocardiographic screening for non‐ischaemic stage B heart failure in the community. Eur J Heart Fail. 2016;18(11):1331‐1339. 10.1002/ejhf.643 [DOI] [PubMed] [Google Scholar]
- 69. Biering‐Sørensen T, Biering‐Sørensen SR, Olsen FJ, et al. Global Longitudinal strain by echocardiography predicts long‐term risk of cardiovascular morbidity and mortality in a low‐risk general population: The Copenhagen City Heart Study. Circ Cardiovasc Imaging. 2017;10(3). 10.1161/CIRCIMAGING.116.005521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sinha A, Gupta DK, Yancy CW, et al. Risk‐based approach for the prediction and prevention of heart failure. Circ Heart Fail. 2021;14(2):e007761. 10.1161/CIRCHEARTFAILURE.120.007761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: a systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2). 10.1161/CIRCOUTCOMES.116.003497 [DOI] [PubMed] [Google Scholar]
- 72. Ahmed R, Dunford J, Mehran R, Robson S, Kunadian V. Pre‐eclampsia and future cardiovascular risk among women: a review. J Am Coll Cardiol. 2014;63(18):1815‐1822. 10.1016/j.jacc.2014.02.529 [DOI] [PubMed] [Google Scholar]
- 73. Wu P, Kwok CS, Haththotuwa R, et al. Pre‐eclampsia is associated with a twofold increase in diabetes: a systematic review and meta‐analysis. Diabetologia. 2016;59(12):2518‐2526. 10.1007/s00125-016-4098-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brown MC, Best KE, Pearce MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre‐eclampsia: systematic review and meta‐analysis. Eur J Epidemiol. 2013;28(1):1‐19. 10.1007/s10654-013-9762-6 [DOI] [PubMed] [Google Scholar]
- 75. O'Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology. 2003;14(3):368‐374. 10.1097/00001648-200305000-00020 [DOI] [PubMed] [Google Scholar]
- 76. Innes KE, Wimsatt JH, McDuffie R. Relative glucose tolerance and subsequent development of hypertension in pregnancy. Obstet Gynecol. 2001;97(6):905‐910. 10.1016/s0029-7844(01)01342-4 [DOI] [PubMed] [Google Scholar]
- 77. Noone A, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975‐2015; National Cancer Institute; 2018.
- 78. Abdel‐Qadir H, Thavendiranathan P, Austin PC, et al. The risk of heart failure and other cardiovascular hospitalizations after early stage breast cancer: a matched cohort study. J Natl Cancer Inst. 2019;111(8):854‐862. 10.1093/jnci/djy218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gernaat SAM, Boer JMA, van den Bongard DHJ, et al. The risk of cardiovascular disease following breast cancer by Framingham risk score. Breast Cancer Res Treat. 2018;170(1):119‐127. 10.1007/s10549-018-4723-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mehta LS, Watson KE, Barac A, et al. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137(8):e30‐e66. 10.1161/CIR.0000000000000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Barish R, Lynce F, Unger K, Barac A. Management of cardiovascular disease in women with breast cancer. Circulation. 2019;139(8):1110‐1120. 10.1161/CIRCULATIONAHA.118.039371 [DOI] [PubMed] [Google Scholar]
- 82. de Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22(4):561‐570. 10.1158/1055-9965.EPI-12-1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta‐analysis. J Glob Health. 2015;5(2):020415. 10.7189/jogh.05-020415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rutten FH, Cramer MJ, Grobbee DE, et al. Unrecognized heart failure in elderly patients with stable chronic obstructive pulmonary disease. Eur Heart J. 2005;26(18):1887‐1894. 10.1093/eurheartj/ehi291 [DOI] [PubMed] [Google Scholar]
- 86. Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, McMurray JJ. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11(2):130‐139. 10.1093/eurjhf/hfn013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kaszuba E, Odeberg H, Rastam L, Halling A. Impact of heart failure and other comorbidities on mortality in patients with chronic obstructive pulmonary disease: a register‐based, prospective cohort study. BMC Fam Pract. 2018;19(1):178. 10.1186/s12875-018-0865-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Curkendall SM, DeLuise C, Jones JK, et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16(1):63‐70. 10.1016/j.annepidem.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 89. Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice. Ther Adv Respir Dis. 2018;12:1753465817750524. 10.1177/1753465817750524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Centers for Disease Control and Prevention . Covid data tracker weekly review, Accessed January 17, 2022. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html
- 91. Tomasoni D, Italia L, Adamo M, et al. COVID‐19 and heart failure: from infection to inflammation and angiotensin II stimulation. Searching for evidence from a new disease. Eur J Heart Fail. 2020;22(6):957‐966. 10.1002/ejhf.1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Metkus TS, Sokoll LJ, Barth AS, et al. Myocardial injury in severe COVID‐19 compared with non–COVID‐19 acute respiratory distress syndrome. Circulation. 2021;143(6):553‐565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Italia L, Tomasoni D, Bisegna S, et al. COVID‐19 and heart failure: from epidemiology during the pandemic to myocardial injury, myocarditis, and heart failure sequelae. Front Cardiovasc Med. 2021;8:713560. 10.3389/fcvm.2021.713560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sokolski M, Trenson S, Sokolska JM, et al. Heart failure in COVID‐19: the multicentre, multinational PCHF‐COVICAV registry. ESC Heart Fail. Dec 2021;8(6):4955‐4967. 10.1002/ehf2.13549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(11):1265‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Xie Y, Xu E, Bowe B, Al‐Aly Z. Long‐term cardiovascular outcomes of COVID‐19. Nature Med. 2022;28(3):583‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sharma A, Colvin‐Adams M, Yancy CW. Heart failure in African Americans: disparities can be overcome. Cleve Clin J Med. 2014;81(5):301‐311. 10.3949/ccjm.81a.13045 [DOI] [PubMed] [Google Scholar]
- 98. Bibbins‐Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360(12):1179‐1190. 10.1056/NEJMoa0807265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. White‐Williams C, Rossi LP, Bittner VA, et al. Addressing social determinants of health in the care of patients with heart failure: a scientific statement from the American Heart Association. Circulation. 2020;141(22):e841‐e863. 10.1161/CIR.0000000000000767 [DOI] [PubMed] [Google Scholar]
- 100. Potter EL, Hopper I, Sen J, Salim A, Marwick TH. Impact of socioeconomic status on incident heart failure and left ventricular dysfunction: systematic review and meta‐analysis. Eur Heart J Qual Care Clin Outcomes. 2019;5(2):169‐179. 10.1093/ehjqcco/qcy047 [DOI] [PubMed] [Google Scholar]
- 101. Pinheiro LC, Reshetnyak E, Sterling MR, Levitan EB, Safford MM, Goyal P. Multiple vulnerabilities to health disparities and incident heart failure hospitalization in the REGARDS Study. Circ Cardiovasc Qual Outcomes. 2020;13(8):e006438. 10.1161/CIRCOUTCOMES.119.006438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cahill TJ, Ashrafian H, Watkins H. Genetic cardiomyopathies causing heart failure. Circ Res. 2013;113(6):660‐675. 10.1161/CIRCRESAHA.113.300282 [DOI] [PubMed] [Google Scholar]
- 103. Monserrat L, Hermida M, Bouzas B, et al. Miocardiopatia dilatada familiar en pacientes trasplantados por miocardiopatia dilatada idiopatica [Familial dilated cardiomyopathy in patients transplanted for idiopathic dilated cardiomyopathy]. Rev Esp Cardiol. 2002;55(7):725‐732. 10.1016/s0300-8932(02)76691-8 [DOI] [PubMed] [Google Scholar]
- 104. Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296(15):1867‐1876. 10.1001/jama.296.15.1867 [DOI] [PubMed] [Google Scholar]
- 105. Baig MK, Goldman JH, Caforio AL, Coonar AS, Keeling PJ, McKenna WJ. Familial dilated cardiomyopathy: cardiac abnormalities are common in asymptomatic relatives and may represent early disease. J Am Coll Cardiol. 1998;31(1):195‐201. 10.1016/s0735-1097(97)00433-6 [DOI] [PubMed] [Google Scholar]
- 106. Lee DS, Pencina MJ, Benjamin EJ, et al. Association of parental heart failure with risk of heart failure in offspring. N Engl J Med. 2006;355(2):138‐147. 10.1056/NEJMoa052948 [DOI] [PubMed] [Google Scholar]
- 107. Meder B, Haas J, Sedaghat‐Hamedani F, et al. Epigenome‐wide association study identifies cardiac gene patterning and a novel class of biomarkers for heart failure. Circulation. 2017;136(16):1528‐1544. 10.1161/CIRCULATIONAHA.117.027355 [DOI] [PubMed] [Google Scholar]
- 108. Wu J, Zhao M, Li T, et al. HFIP: an integrated multi‐omics data and knowledge platform for the precision medicine of heart failure. Database (Oxford). 2021;2021(2021). 10.1093/database/baab076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Santolini M, Romay MC, Yukhtman CL, et al. A personalized, multiomics approach identifies genes involved in cardiac hypertrophy and heart failure. NPJ Syst Biol Appl. 2018;4:12. 10.1038/s41540-018-0046-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cresci S, Pereira NL, Ahmad F, et al. Heart failure in the era of precision medicine: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2019;12(10):458‐485. 10.1161/HCG.0000000000000058 [DOI] [PubMed] [Google Scholar]
- 111. Del Gobbo LC, Kalantarian S, Imamura F, et al. Contribution of major lifestyle risk factors for incident heart failure in older adults: The Cardiovascular Health Study. JACC Heart Fail. 2015;3(7):520‐528. 10.1016/j.jchf.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Djousse L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302(4):394‐400. 10.1001/jama.2009.1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lloyd‐Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586‐613. 10.1161/CIRCULATIONAHA.109.192703 [DOI] [PubMed] [Google Scholar]
- 114. Gopal DM, Kalogeropoulos AP, Georgiopoulou VV, et al. Cigarette smoking exposure and heart failure risk in older adults: the Health, Aging, and Body Composition Study. Am Heart J. 2012;164(2):236‐242. 10.1016/j.ahj.2012.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lee H, Son YJ. Influence of smoking status on risk of incident heart failure: a systematic review and meta‐analysis of prospective cohort studies. Int J Environ Res Public Health. 2019;16(15). 10.3390/ijerph16152697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ogunmoroti O, Oni E, Michos ED, et al. Life's Simple 7 and Incident heart failure: the multi‐ethnic study of atherosclerosis. J Am Heart Assoc. 2017;6(6). 10.1161/JAHA.116.005180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Uijl A, Koudstaal S, Vaartjes I, et al. Risk for heart failure: the opportunity for prevention with the American Heart Association's Life's Simple 7. JACC Heart Fail. 2019;7(8):637‐647. 10.1016/j.jchf.2019.03.009 [DOI] [PubMed] [Google Scholar]
- 118. Martinez‐Gonzalez MA, Gea A, Ruiz‐Canela M. The Mediterranean diet and cardiovascular health. Circ Res. 2019;124(5):779‐798. 10.1161/CIRCRESAHA.118.313348 [DOI] [PubMed] [Google Scholar]
- 119. Ros E, Martínez‐González MA, Estruch R, et al. Mediterranean diet and cardiovascular health: teachings of the PREDIMED study. Adv Nutr. 2014;5(3):330S‐336SS. 10.3945/an.113.005389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Appel LJ, Champagne CM, Harsha DW, et al. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289(16):2083‐2093. 10.1001/jama.289.16.2083 [DOI] [PubMed] [Google Scholar]
- 121. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275(20):1557‐1562. [PubMed] [Google Scholar]
- 122. Blood Pressure Lowering Treatment Trialists C. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant‐level data meta‐analysis. Lancet. 2021;397(10285):1625‐1636. 10.1016/S0140-6736(21)00590-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Flack JM, Adekola B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc Med. 2020;30(3):160‐164. 10.1016/j.tcm.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 124. Upadhya B, Stacey RB, Kitzman DW. Preventing heart failure by treating systolic hypertension: what does the SPRINT add? Curr Hypertens Rep. 2019;21(1):9. 10.1007/s11906-019-0913-3 [DOI] [PubMed] [Google Scholar]
- 125. SPRINT Research G, Wright JT Jr, Jr. , Williamson JD, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373(22):2103‐2116. 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Molsberry RJ, Rethy L, Wang MC, et al. Risk‐based intensive blood pressure lowering and prevention of heart failure: a SPRINT post hoc analysis. Hypertension. 2021;78(6):1742‐1749. 10.1161/HYPERTENSIONAHA.121.18315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Sciarretta S, Palano F, Tocci G, Baldini R, Volpe M. Antihypertensive treatment and development of heart failure in hypertension: a Bayesian network meta‐analysis of studies in patients with hypertension and high cardiovascular risk. Arch Intern Med. 2011;171(5):384‐394. 10.1001/archinternmed.2010.427 [DOI] [PubMed] [Google Scholar]
- 128. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide–based screening and collaborative care for heart failure: the STOP‐HF randomized trial. JAMA. 2013;310(1):66‐74. [DOI] [PubMed] [Google Scholar]
- 129. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT‐proBNP selected PreventiOn of cardiac eveNts in a populaTion of dIabetic patients without A history of Cardiac disease) A prospective randomized controlled trial. J Am Coll Cardiol. 2013;62(15):1365‐1372. [DOI] [PubMed] [Google Scholar]
- 130. Fraccarollo D, Galuppo P, Bauersachs J. Mineralocorticoid receptor antagonism and cardiac remodeling in ischemic heart failure. Curr Med Chem Cardiovasc Hematol Agents. 2004;2(4):287‐294. 10.2174/1568016043356219 [DOI] [PubMed] [Google Scholar]
- 131. Struthers AD. The clinical implications of aldosterone escape in congestive heart failure. Eur J Heart Fail. 2004;6(5):539‐545. 10.1016/j.ejheart.2004.04.013 [DOI] [PubMed] [Google Scholar]
- 132. Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345(23):1689‐1697. 10.1056/NEJMra000050 [DOI] [PubMed] [Google Scholar]
- 133. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348(14):1309‐1321. 10.1056/NEJMoa030207 [DOI] [PubMed] [Google Scholar]
- 134. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709‐717. 10.1056/NEJM199909023411001 [DOI] [PubMed] [Google Scholar]
- 135. Rogers JK, McMurray JJ, Pocock SJ, et al. Eplerenone in patients with systolic heart failure and mild symptoms: analysis of repeat hospitalizations. Circulation. 2012;126(19):2317‐2323. 10.1161/CIRCULATIONAHA.112.110536 [DOI] [PubMed] [Google Scholar]
- 136. Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383‐1392. 10.1056/NEJMoa1313731 [DOI] [PubMed] [Google Scholar]
- 137. Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences. Diab Vasc Dis Res. 2015;12(2):78‐89. 10.1177/1479164114561992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644‐657. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 139. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347‐357. 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 140. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117‐2128. 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 141. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31‐39. 10.1016/S0140-6736(18)32590-X [DOI] [PubMed] [Google Scholar]
- 142. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995‐2008. 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 143. Packer M, Anker SD, Butler J, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383(15):1413‐1424. 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 144. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385(16):1451‐1461. 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 145. Freaney PM, Lloyd‐Jones DM, Khan SS. Could flozins be the statins for risk‐based primary prevention of heart failure? JAMA Cardiol. 2021;6(7):741‐742. 10.1001/jamacardio.2021.1133 [DOI] [PubMed] [Google Scholar]
- 146. Lin MH, Yuan WL, Huang TC, Zhang HF, Mai JT, Wang JF. Clinical effectiveness of telemedicine for chronic heart failure: a systematic review and meta‐analysis. J Investig Med. 2017;65(5):899‐911. 10.1136/jim-2016-000199 [DOI] [PubMed] [Google Scholar]
- 147. Zhu Y, Gu X, Xu C. Effectiveness of telemedicine systems for adults with heart failure: a meta‐analysis of randomized controlled trials. Heart Fail Rev. 2020;25(2):231‐243. 10.1007/s10741-019-09801-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. DeFilippis EM, Reza N, Donald E, Givertz MM, Lindenfeld J, Jessup M. Considerations for heart failure care during the COVID‐19 pandemic. JACC Heart Fail. 2020;8(8):681‐691. 10.1016/j.jchf.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Reza N, DeFilippis EM, Jessup M. Secondary impact of the COVID‐19 pandemic on patients with heart failure. Circ Heart Fail. 2020;13(5):e007219. 10.1161/CIRCHEARTFAILURE.120.007219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


