ABSTRACT
Based on studies over the last several decades, the self-renewing skeletal lineages derived from bone marrow stroma could be an ideal source for skeletal tissue engineering. However, the markers for osteogenic precursors; i.e., bone marrowderived skeletal stem cells (SSCs), in association with other cells of the marrow stroma (bone marrow stromal cells, BMSCs) and their heterogeneous nature both in vivo and in vitro remain to be clarified. This review aims to highlight: i) the importance of distinguishing BMSCs/SSCs from other “mesenchymal stem/stromal cells”, and ii) factors that are responsible for their heterogeneity, and how these factors impact on the differentiation potential of SSCs towards bone. The prospective role of SSC enrichment, their expansion and its impact on SSC phenotype is explored. Emphasis has also been given to emerging single cell RNA sequencing approaches in scrutinizing the unique population of SSCs within the BMSC population, along with their committed progeny. Understanding the factors involved in heterogeneity may help researchers to improvise their strategies to isolate, characterize and adopt best culture practices and source identification to develop standard operating protocols for developing reproducible stem cells grafts. However, more scientific understanding of the molecular basis of heterogeneity is warranted that may be obtained from the robust high-throughput functional transcriptomics of single cells or clonal populations.
Key Words: bone marrow stromal cells, clonal analysis, heterogeneity, single cell analysis, skeletal stem cells
Introduction
Bone marrow stromal cell (BMSC) populations contain a sub-population of bona fide skeletal stem cells (SSCs).1, 2 Owing to their ability to differentiate into multiple lineages (cartilage, bone, haematopoiesis supportive stroma, marrow adipocytes) these BMSCs/SSCs are considered to be promising candidates for skeletal tissue engineering.3 In the quest for developing and refining skeletal tissue engineering, utilization of BMSCs/SSCs has been explored for decades. Categorically, in many bone tissue engineering studies, this specific subset of the population has been the central focus, and has been proven to be essential for successful regeneration of bone and re-establishment of its marrow by the cells themselves. The emergence of strategies based on cell sorting using unique combinations of cell surface markers has identified different forms of SSCs in different locations (periosteum, growth plate, marrow) (reviewed in Ambrosi et al.4). With increasing understanding of the role of different SSCs in growth (bone shape) and maintenance (lifelong bone turnover) of the skeletal system, keen interest in their precise characteristics, potency (e.g., progenitors of bone, cartilage, and stroma) is growing. SSCs are reported to be highly clonogenic in vitro, display multipotency when transplanted in vivo, and are able to self-renew5 (reviewed in Bianco and Robey2). However, due to the heterogeneity of BMSCs/SSCs at different developmental and maturational stages and locations, and distinguishing them appropriately from non-skeletal populations of cells with similar cell surface characteristics, has posed a great challenge in their application.
In this review, we will discuss the essential need for appropriate nomenclature and characterization of similar but physiologically distinctive populations of skeletal cells in different tissues. The plausible causative factors underlying single cell heterogeneity will also be discussed in the context of BMSCs/SSCs. We will explore up-to-date information to describe single cell heterogeneity and its biological basis in bone marrow-resident SSCs. We will also address the potential role of high throughput single cell RNA sequencing tools to unravel the inherent variations at the single cell level and identification of consistent subsets amid supposedly homogenous populations, that have hitherto been unnoticed.
What are Bone Marrow Stromal Cells/Skeletal Stem Cells?
Based on the seminal work of Friedenstein et al.6 starting in the late 1960s, and later with Owen and coworkers,7 it is now known that bone marrow is the home to two different post-natal stem cells: the haematopoietic stem cell that gives rise to all cell types found in blood, and the skeletal stem cell that can reform all skeletal tissues (cartilage, bone, haematopoiesis-supportive stroma and marrow adipocytes). Importantly, this finding was based on clonal analyses, whereby single cell suspensions of bone marrow were plated at low density into tissue culture plastic dishes. As human haematopoietic cells generally do not adhere, the rapidly adherent cells fraction was referred to as colony forming units-fibroblasts (CFU-Fs) by Friedenstein et al.6 These cells are initially quiescent, but begin to proliferate within 24-48 hours to form a colony in a density-independent fashion.6, 8, 9 When these colonies are individually expanded (single-colony-derived strains), and tested for their differentiation capacity by the cartilage pellet culture in vitro,10 and by in vivo transplantation with an appropriate scaffold, it was determined by Friedenstein,8 and later by others, that ∼10- 20% of the single colony-derived strains are multipotent; i.e., they were able to make bone, stroma and marrow adipocytes of donor origin, and importantly, support haematopoiesis of recipient origin. Of the remaining single colony-derived strains, ∼50% made only bone, and the remainder only made fibrous tissue.11-13 Outcomes of cartilage formation in pellet cultures10 were variable, depending on the age of the donor, and the length of time in culture. These results highlight that ∼1:5 of the original CFU-Fs is a multipotent SSC, whereas the remainder are transiently amplifying progenitor cells; i.e., they can proliferate, but are not SSCs based on their loss of potency (the ability to make a complete bone/marrow organ).
Bone Marrow Stromal Cells/Skeletal Stem Cells Aka Bone Marrow-Derived “Mesenchymal Stem Cells”: What’s in A Name?
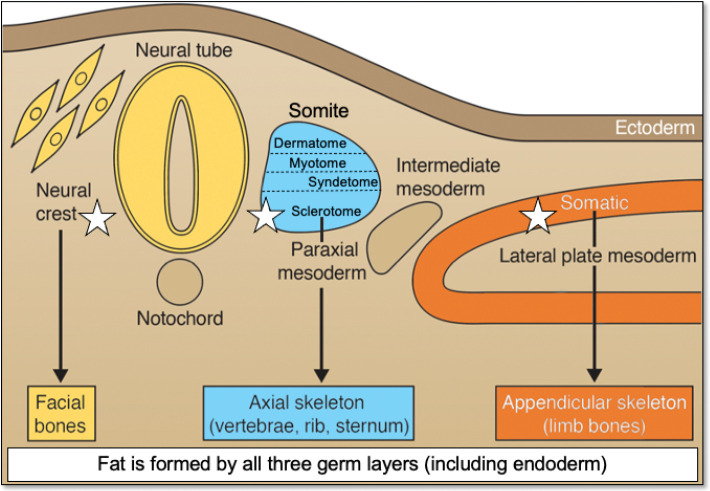
Based on these clonal analyses, it is clear that there is a subset of cells in the BMSC population that are multipotent cells, and later, they were determined to be self-renewing based on serial transplantation studies,5 qualifying them as bona fide stem cells. Friedenstein and Owen called them “bone marrow stromal stem cells”,9 later to be called “SSCs”.1 However, these cells have also been named “mesenchymal stem cells,” a term first coined in the 1990s,14 and later changed to “mesenchymal stromal cells” by the International Society for Cell Therapy.15 However, neither of these terms is scientifically accurate. Mesenchyme, as classically defined by developmental biologists,16 is an embryonic connective tissue that forms not only connective tissues, but also blood and blood vessels (primarily mesodermal in origin). There is no report so far where post-natal stem cells have been shown to give rise to all three tissues based on rigorous and appropriate differentiation assays. Of note, bone and associated tissues derive from at least three (and possibly four) different embryonic specifications. The sclerotome of somites (paraxial mesoderm) gives rise to bones of the posterior cranial vault and the axial skeleton, somatic lateral plate mesoderm gives rise to the appendicular skeleton, neural crest (ectoderm) forms the facial bones,2, 17 and it has been suggested that cells from the dorsal root of the developing aorta (mesoangioblasts) also contribute to skeletal tissue formation.18 On the other hand, dermis, skeletal muscle and tendon derive from different specifications of the somitic paraxial mesoderm (dermatotome, myotome, syndetome, respectively) in the axial skeleton, and from the somatopleure of somatic lateral plate mesoderm, and from paraxial mesodermal somitomeres and neural crest in the craniofacial skeleton. While bone marrow adipose tissue develops from mesoderm and neural crest, other forms of adipose tissue are derived from all three germ layers19 (Figure 1). Consequently, there is no common embryonic source for skeletal tissues, and there is no reason to believe that there would be one in the post-natal organism. In other words, “mesenchymal stem/ stromal cells” (“MSCs”) are not a lineage.20
Figure 1. Developmental origins of different connective tissues that have been reported to contain “MSCs”. Of note, bone originates from three different embryonic specifications (noted by a white star). Skin, muscle, tendons and ligaments, and bone arise from different specifications of the sclerotome. Bone marrow adipose tissue originates from paraxial mesoderm, lateral plate mesoderm and neural crest. While bone marrow adipose tissue arises from mesoderm and neural crest, other forms of fat originate from all three germ layers. “MSCs” are not a lineage. MSC: mesenchymal stem/stromal cell. Adapted from Bianco and Robey.2.
In spite of these developmental facts, it has been reported, and it continues to be reported, that “MSCs” can be isolated from virtually any tissue in the body,21 based on the expression of certain cell surface markers such as CD29, CD73 and CD90 (to name just a few).15 However, these CD markers are not specific. They are expressed by almost all fibroblastic cells, and they cannot be used, in and of themselves, to prove the stem cell nature of a given population or even of a single cell.22 These are the cell surface proteins that give fibroblastic cells the ability to interact with the extracellular environment and with other cells in the tissue. Of note, many of these markers not only change with time in culture, but also change due cell-cell contact, plastic adherence and its quality, growth factors and enzymatic manipulations.12, 23-25
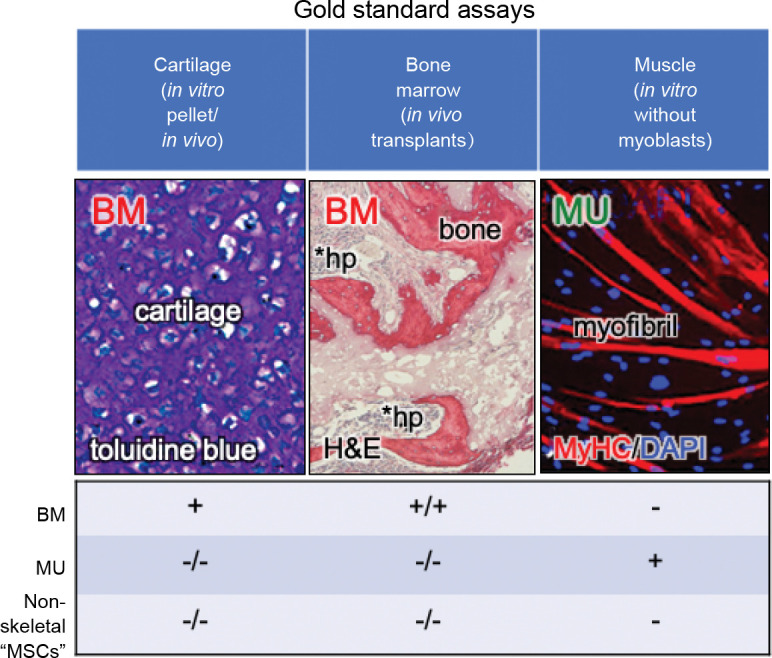
In addition to the lack of specificity of the cell surface markers, many of the in vitro assays that have been used to determine differentiation of “MSCs” are highly prone to artifact.20 For example, the in vitro osteogenic assay relies on culturing cells either with bone morphogenetic protein or with medium that contains high levels (10 mM) of β-glycerophosphate and supraphysiological doses of dexamethasone (10-7 M). Bone morphogenetic proteins will temporarily induce osteogenic differentiation of fibroblastic cells as has long been known from the pioneering work of Urist.26 But that does make the fibroblast inherently osteogenic (they are what Friedenstein termed an “inducible” osteoprogenitor27), and when bone morphogenetic protein-induced Smad signaling dissipates, often so does the bone. With respect to β-glycerophosphate/ dexamethasone differentiation, if the cells make tissue non-specific alkaline phosphatase (which many types of cells do), it cleaves β-glycerophosphate to form free phosphate, and when the phosphate product becomes high enough, it precipitates with calcium in the medium to form calcium phosphate. In addition, when cultured in this type of medium long enough, cells begin to die, and mitochondria in dead and dying cells serve as an efficient nidus for the formation of dystrophic calcification. Both calcium phosphate and dystrophic calcification stain positively with alizarin red S or von Kossa, but neither are indicative of matrix mineralization.28 In the adipogenic assay, there are a few cocktails that will induce differentiation into multilocular adipocytes. However, many cell types will accumulate lipid from serum in the medium (in particular, from horse serum), but they do not synthesize lipids de novo.29 The gold standard for osteogenic and adipogenic differentiation is by in vivo transplantation of the cells in conjunction with an appropriate scaffold, and detection of extracellular matrix, osteocytes, osteoblasts, stroma and marrow adipocytes of donor origin. For cartilage formation, the in vitro pellet culture is currently the gold standard (Figure 2).30 In this assay, one must be able to see chondrocytes lying in lacunae, surrounded by matrix that stains purple with toluidine blue (metachromasia).3 It is the use of the highly artifactual in vitro assays, and the misinterpretation of the chondrogenic assay that has led to the widely held, but inaccurate belief that “MSCs” with chondrogenic, osteogenic and adipogenic capacities are found almost everywhere.31
Figure 2. Gold standard assays by which to assess differentiation capacity. Many of the currently used “standard” assays of differentiation are prone to artifact or misinterpretation. However, there are assays that can faithfully report differentiation capacity: 1) the in vitro cartilage pellet assay, whereby one can see chondrocytes lying in lacunae surrounded by extracellular matrix that stains purple with toluidine blue, 2) the in vivo transplantation assay whereby donor cells are able to make bone matrix, osteocytes, osteoblasts, and in some cases, support haematopoiesis and formation of marrow adipocytes (the latter two properties are not shared by all forms of skeletal stem cells), and 3) the in vitro myogenic assay, whereby myotubes are formed in the absence of exogenous myoblasts (which will spontaneously fuse with any fibroblastic population). Adapted in part from Sacchetti et al.30 BM: bone marrow; DAPI: diamidino-2-phenylindole; H&E: hematoxylin and eosin; hp: haematopoiesis; MSC: mesenchymal stem/stromal cell; MU: muscle; MyHC: myosin heavy chain.
The Biological Truth about “Mesenchymal Stem/Stromal Cells”
In stem cell biology, the term “MSCs” is being used as a designation for all types of populations of stromal and fibroblastic cells. However, based on fundamental knowledge of developmental biology, the application of rigorous cell and biochemical assays in vitro and in vivo, coupled with high throughput tools for extensive and thorough characterization of various populations labeled as “MSCs”, it is now apparent that multiple and distinct populations of stem/progenitor cells exist in different tissues30 (tissue-specific stem/progenitor cells), and even within bone itself (reviewed in Ambrosi et al.4). Although these cell populations have similar cell surface characteristics due to their fibroblastic/stromal nature, their differentiation potential is not identical, and is rooted in their tissue of origin. Thus, the issue of scientifically accurate representation of these unique subsets of cells within different tissues, and within bone, based on their embryonic origins and lineages, has been raised and remains controversial to date.
While several of the assays described above have proven to be ineffective in determining the differentiation capacity of a given fibroblastic population of cells, there are assays that can faithfully predict the presence of tissue specific stem/progenitor cells in a number of connective tissues. For example, by using techniques that were developed for the analysis of the SSCs within the BMSC population, dental pulp stem cells have been identified within the adherent dental pulp cell population of permanent teeth,32 and stem cells from exfoliated deciduous teeth (SHED) have been identified within the dental pulp cell population of primary teeth.33 These cells are CFU-Fs, but instead of making a bone/marrow organ upon in vivo transplantation, they make dentin and a pulp-like complex, and SHED also make something that looks like a combination of bone and dentin (osteodentin). Likewise, clonogenic cells can be isolated from periodontal ligament that make cementum along with a periodontal ligament-like structure upon in vivo transplantation.34 In addition, isolation of cells with similar cell surface markers as BMSCs/SSCs from muscle yields cells that are inherently myogenic (form myotubes) when switched to an appropriate substrate and culture medium, but in the absence of myoblasts.30 This in vitro assay is currently the gold standard by which to demonstrate myogenesis,35 based on the fact that in vivo, myoblasts are promiscuous and will fuse with just about any fibroblastic cell, but that does make the cell inherently myogenic (Figure 2). These findings highlight the fact that rigorous differentiation assays are not “one size fits all”. They must be tailored to faithfully recapitulate differentiation at play in different tissues.
Why Do We Care About Heterogeneity?
In the field of regenerative medicine, the selection of appropriate cells is indispensable, as the ultimate quality and outcome of cellular therapy rely on the potency and biological functions of the cells (the critical quality attributes). The spectrum of variations present in any cell population can have a significant impact on the successful creation of functional regenerative therapy products.
Many published in vitro and in vivo results have raised concerns about BMSC/SSC heterogeneity,36 and so the question arises, why do we care? The answer is varied, depending on what we want to do with the cells, and is related to the nature and the biological basis of the heterogeneity; e.g., heterogeneity due to the presence of extraneous cell types as compared with heterogeneity within the cell itself.
First and foremost, the most obvious concern is that the population is contaminated with irrelevant and/or unwanted cell types. For example, it is well known that rodent BMSC cultures are heavily contaminated with haematopoietic cells (primarily macrophages), sometimes up to 90%, based on their tight association with rodent BMSCs,37 and their residence within large mountains of hyaluronic acid that is secreted by rodent BMSCs. Obviously, this level of contamination can alter experimental results and their interpretation significantly, and could significantly impact tissue engineering procedures.
Other reasons for heterogeneity are less overt as described below and may or may not impact on the goal of a particular experiment. While CD markers cannot be used to identify a population as “stem cells,” populations of cells that do not have near > 90% expression of markers such as CD29, CD73, and CD90 are most likely contaminated with cell types other than BMSCs.3 However, in the absence of contamination, one may be less concerned about other forms of heterogeneity for tissue engineering purposes if the existence of the SSC within the BMSC population can be documented and is maintained. In order to regenerate large quantities of bone, it is essential that the SSC remains in the transplanted population in order to support bone turnover. In this context, one would not use clones for this purpose, based on the labour-intensive nature of generating clones, and the high number of population doublings that a single CFU-F goes through to generate a colony that is subsequently expanded. For this purpose, multi-colony derived strains (generated by plating single cell suspensions at non-clonal densities) can be used along with in vivo transplants to determine if the population can generate a complete bone/marrow organ, which is a surrogate marker for the SSC based on the complete dependence on the SSC to generate a complete organoid.
In terms of regenerative medicine, there are a plethora of studies that suggest the notion that BMSCs/SSCs (and other “MSCs”) do not regenerate cartilage or bone themselves, but rather that they exert a paracrine, immunomodulatory and/or immunosuppressive effect that encourages endogenous cells to begin the repair process.38, 39 However, to date, it has not been established that it is the subset of stem cells that is responsible for potential improvements in a long list of diseases and disorders, and it is unlikely to be, based on their rarity. The putative effects are based on the population as a whole, and cannot rightly be called “stem” cell therapy. It is possible that subpopulations other than the stem cell subpopulation are responsible for this effect, and perhaps if they were more highly enriched, results would be more remarkable and reproducible. More study of these potential sub-populations is needed. Nonetheless, these purported properties have led to another change in terminology to the “medicinal signalling cell”.40 But it must be noted that all cells in the body secrete a variety of factors. In many pre-clinical and clinical applications, “MSCs” from one tissue are interchanged with “MSCs” from another tissue, but it is not clear whether their secretomes are similar or not. Furthermore, it has been suggested that skin fibroblasts may be equally as effective as “MSCs”.41 Rigorous pre-clinical and clinical trials are needed to determine: 1) the benefits of cells from one tissue source versus another, 2) whether there is a need to identify and isolate subpopulations that are more effective in exerting these effects, and finally, 3) if there is a real, substantial and long-lasting effect of such treatments. Currently, efficacy has been limited due to a number of factors such as poor experimental/trial design, lack of appropriate power calculations and sample size, and inadequate primary outcome measures.42
In terms of SSC biology, it is of great importance to independently track the SSC in order to follow its fate and biological activity in vitro and in vivo. This knowledge would enable determination of how to best maintain and manipulate the SSC. As discussed below, a recognition of the nature of, and an understanding of, the biological basis of heterogeneity of BMSCs/SSCs is emerging.
Nature of Bone Marrow Stromal Cell/Skeletal Stem Cell Heterogeneity
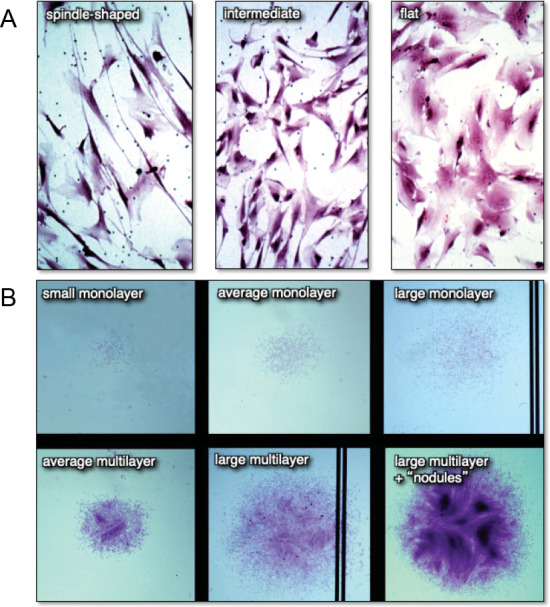
Setting aside contamination by non-BMSC cell types, heterogeneity of BMSCs/SSCs can be noted at different levels. When plated at clonal density and growth is initiated 24-48 hours later, cells exhibit a variety of cell shapes and sizes, ranging from long spindle-shaped cells, to smaller, polygonal cells, to large, flat cells43, 44 (Figure 3A). As growth continues, colonies (counted as those having > 50 cells) of different sizes ranging from very small to very large are noted, with some colonies being composed of cells that are widely separated from each other by migration (“loose”), and others where cells remain very closely associated with each other (“dense”). Some colonies remain as a monolayer, while others begin to multilayer (Figure 3B). However, the morphology of the cells, and the size and growth habit of colonies do not correlate with their potency; i.e., the ability to form a complete bone/marrow organ upon in vivo transplantation with an appropriate scaffold.43
Figure 3. Differences in cell morphology (A) and colony size and habit (B) of freshly isolated bone marrow stromal cell suspensions plated at clonal density. When individual colonies with different cell shapes and colony habits are expanded ex vivo and transplanted in vivo, neither parameter correlated with the formation of a bone/marrow organ (a measure of multipotency). Adapted from Satomura et al.43.
Once established, colonies can also spontaneously differentiate, indicating their commitment to a particular phenotype (Figure 4A). When stained histochemically with alkaline phosphatase, the vast majority of the colonies contain cells that are alkaline phosphatase positive, indicative of a pre-osteogenic and osteogenic, stromagenic, and pre-adipogenic phenotype. Approximately 50% of the colonies begin to deposit mineralized matrix as detected by inverted light microscopy as phase bright material, or by alizarin red S in fixed cultures, and ∼10% of the colonies have cells that have multi-locular lipid accumulation that can again be seen inverted light microscopy, or by oil red O staining in fixed cultures (Figure 4A). This spontaneous in vitro differentiation is somewhat congruent with the results obtained when single-colony-derived strains were attached to hydroxyapatite/tricalcium phosphate ceramic particles and transplanted into immunocompromised mice (∼10-20% were multipotent, ∼50% formed bone and the remainder formed fibrous tissue) (Figure 4B).12, 13
Figure 4. Clonal analysis - an essential step in the determination of stem cell potency. (A) When colonies are allowed to grow beyond the 10-14 days usually used for colony forming efficiency, the individual colonies begin to spontaneously differentiate. The vast majority of the colonies are alkaline phosphatase positive (right panel), indicative of osteogenic and pre-adipogenic cells. Approximately 50% of the colonies were Alizarin Red positive (centre panel) and approximately 10% stained with oil red O (right panel, unpublished data). (B) When individual colonies were isolated, expanded ex vivo, and transplanted subcutaneously into immunocompromised mice with an hydroxyapatite/tricalcium phosphate scaffold, ∼10% of the single colony-derived strains made a complete bone/marrow organ (multipotent), whereas ∼50% formed only bone (unipotent), and the remainder formed only fibrous tissue. Adapted from Sworder et al.13 (C) In studies where colonies were first incubated with adipogenic medium, colonies that accumulated fat identifiable by inverted light microscopy were marked with a blue circle. When the medium was changed to an osteogenic medium, a number of the adipogenic colonies also became alizarin red positive, indicating that the original CFU-F was able to give rise to adipogenic cells, and then osteogenic cells; an indication of “flexibility” (unpublished data). CFU-F: colony forming units-fibroblast.
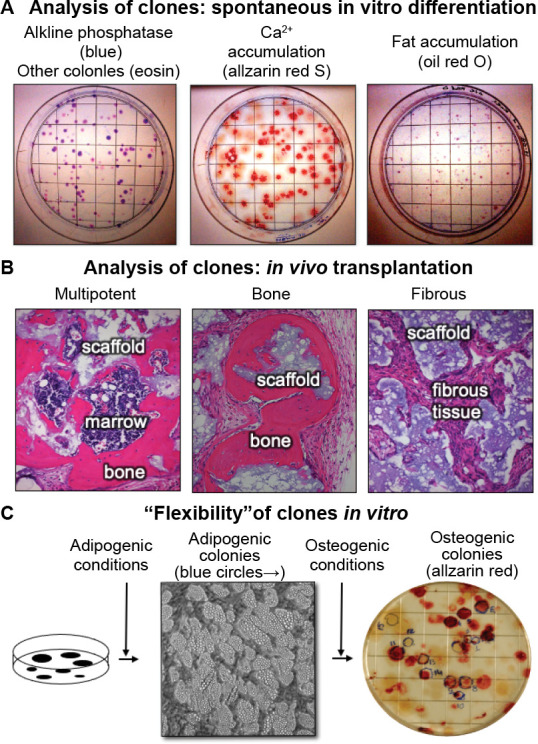
Interestingly, unlike many cell types, clonal populations of BMSCs can shift from one phenotype to another (“flexibility”). This was demonstrated by first culturing cells in adipogenic conditions to identify adipogenic colonies by microscopic methods, then switching to osteogenic medium. It was found that a small number of colonies that were first identified as adipogenic colonies, were then able to become osteogenic colonies (Figure 4C). While it cannot be said that a single cell was first an adipocyte, and then became an osteoblast, it can be said that a single CFU-F was able to self-renew during establishment of the colony able to give rise to cells capable of differentiating into adipogenic cells first, and then osteogenic cells.
Causes of heterogeneity
In determining the reasons for BMSC/SSC heterogeneity, factors can be categorized as being intrinsic to the cells themselves, or extrinsic to the cell (the microenvironment in which they reside).
Intrinsic factors
These sources of heterogeneity include the developmental origin of cartilage and bone, which are multiple as indicated above (paraxial and somatic lateral plate mesoderm, neural crest and dorsal root of the aorta). Bone made from lateral plate mesoderm and from neural crest has been shown to exhibit different levels of CFU-Fs within their bone marrow, different rates of proliferation and response to osteogenic inducers, and different marker genes.45 These differences were also reflected in the nature of the transplants that were generated from lateral plate mesodermal BMSCs and neural crest-derived BMSCs/ SSCs, with neural crest-derived cells establishing fewer and smaller regions of haematopoietic marrow, but large regions of dense bone, reminiscent of the structure of jawbone.45 Recently, directed differentiation of induced pluripotent stem cells first into paraxial and lateral plate mesoderm, and neural crest, and then into osteoprogenitor cells revealed significant differences in the transcriptomes of each type of osteoprogenitor.46
In addition to developmental differences, it is now recognized that different compartments of the same bone have SSCs that are similar, but not identical to one another. The resting zone of the growth plate has been shown to be the home of an SSC that can make cartilage, bone and haematopoiesis-supportive stroma, but not marrow adipocytes. Similar, but not identical, cells have also been found in the periosteum (reviewed in Ambrosi et al.4). Both of these populations have a higher propensity to make cartilage in comparison with bone marrow-derived SSCs, whereas bone marrow-derived SSCs are routinely able to make cartilage, bone, haematopoiesis supportive stroma and marrow adipocytes, based on clonal analysis and in vivo transplantation assays.2
The characteristics of a discrete periosteal stem cells owing to: (i) distinct transcriptional signatures (e.g., high Nanog and Wnt 5α levels), (ii) clonal multipotency, (iii) increased bone formation capacity per cell, and (iv) self-renewal properties, indicates that a multiple but distinct pools of stem cell progenitors exists in bone.47 Sivaraj and coworkers48 have also reported heterogenous subpopulations in the bone-derived skeletal progenitors. These subpopulations have divergence in their physiological functions and platelet-derived growth factor receptor β signaling plays an important role in their fate decision. In fact, it has now been established that in postnatal long bones in mice, two types of bone progenitors (i.e., early osteochondral and perivascular) exist and they have the characteristics similar to bona fide skeletal stem cells.49 This study further showed that the osteochondral stem cell is responsible for long bone growth and repair, while the perivascular stem cell is part of the haematopoietic stem cell niche and supports haematopoiesis, and the formation of marrow adipocytes. Thus, multiple (heterogenous) types of stem cells exist in bone that has different abilities to perform different functions during development as well as in repairment of bone. Endosteal surfaces may also harbour SSCs that vary from the other three types.50 One might ask as to why there are so many variations on a theme; however, it is not difficult to imagine that there would be different subtypes of SSCs based on differences in their developmental history that put them in specific locations to perform explicit functions during development and modeling, growth, homeostasis and remodelling, and repair.
Second only to tissue source, donor variability has a major impact on BMSC/SSC heterogeneity. The skeleton is primarily the sum product of the coordinated action of osteoclastic and osteogenic cell types, but is highly influenced by numerous other organ systems. As such, skeletal variation is enormous. It has been reported that 50-85% of the variation in a key parameter, bone mineral density, is based on genetic factors.51 Consequently, it is understandable that such genetic variability would impact on the biological activity of BMSCs/SSCs. The precise genetic factors are beginning to emerge, but are far from being completely identified. Donor origin has been shown to impact on growth and the transcriptomic profile of BMSCs/SSCs,52 which may relate to genomic differences, but would also be influenced by the health status of the donor. Yet in spite of this variability, transcriptome analysis shows that BMSCs/SSCs isolated from different donors do have distinctive features that clearly identify them as being BMSCs,20 but variations, much like there are variations between different types of apples; an apple is an apple, but MacIntosh apples are different from Granny Smiths.
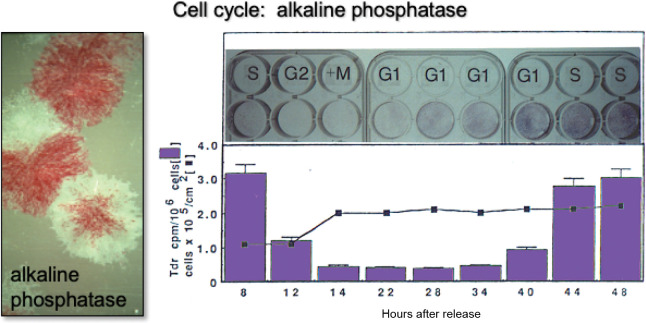
Although not often recognized, position of the cell within the cell cycle impacts on the character of BMSCs/SSCs. For example, it is now known that alkaline phosphatase, a cell membrane-anchored enzyme expressed by osteogenic and pre-adipogenic cells, is expressed in a cell-cycle dependent fashion. In synchronous cultures induced by a thymidine-aphidicolin protocol, alkaline phosphatase activity dropped precipitously at G2 + M phase and returned during G1 and S phase (Figure 5). A majority of the alkaline phosphatase activity lost from the cell surface at mitosis was recovered in the medium due to cleavage of the enzyme by a GPI transamidase.53 Interestingly, the master osteogenic transcription factor, Runx2, shows an even more complex pattern. Runx2 levels oscillate from maximal levels during early G1 to minimal levels during early S phase and mitosis during osteoprogenitor expansion, and are highly up-regulated with cessation of cell growth at the G0/G1 transition during differentiation.54
Figure 5. Changes in alkaline phosphatase based on position on the cell cycle. It has long been recognized that BMSC/ SSC colonies are heterogeneous with respect to alkaline phosphatase activity (left panel, slide given to PGR by Alexander Friedenstein). Later, it was determined that when cultures of osteogenic cells are synchronized using an amphidicolin protocol, cells in S phase have high levels of activity. During G2 + M phase, alkaline phosphatase is cleaved from the cell surface and released into the medium. Cell surface activity is restored during the following G1 and S phases. Adapted in part from Fedarko et al.53 BMSC: bone marrow stromal cells; SSC: skeletal stem cell.
Development and aging of stem cells is intricately controlled by many factors including: i) telomere lengthening, ii) reactive oxygen species generation, iii) transcriptional regulation of the genome, iv) epigenetic dysregulation, (v) miRNAs changes (vi) mitochondrial dysfunctions (vii) modifications of DNA, RNA and the proteome.55-57 Various studies have reported that stem cell intrinsic/extrinsic pathways of aging (senescence) and their cross-talk could play an important role in the determination of stem cell functions and their regenerative (repair/healing) capacity.57-61. Previously, it has been shown that with aging, the osteochondrogenic potential in human SSC populations is gradually decreased (via upregulation of senescence-related pathways and loss of Sirtuin1 expression) and effects the fracture healing process. However, the decline in in vitro clonogenicity with aging has not been confirmed.56 By using a genetic mouse model (Cdkn2aLUC), the role of senescent cells in fracture repair has been established, whereby with elimination of senescent cells (by drugs), improvement in the fracture healing process was shown.61 Mechanistically, it has been revealed that aging SSCs can affect the signaling pathways of the bone marrow niche and alters the differentiation potential of haematopoietic lineages, which could also be related to fragile bone.58 Josephson and coworkers59 have shown that age-associated inflammation plays an important role in SSC dysfunction where NF-κB plays a crucial role. Epigenetic errors could also lead to stem cell senescence and cause homeostasis alterations.60
Epigenetic regulation of chromatin has brought to light additional mechanisms of gene regulation that can affect self-renewal and lineage commitment potential of adult BMSCs/SSCs. With the advent of genome-wide ChIPseq and DNAseq analysis, our understanding of how epigenetic regulations are involved in stem cell self-renewal and commitment has increased.62 Targeting of epigenetic regulatory mechanisms has previously been attempted to restore the potency of haematopoietic stem cells by delaying aging.63 The process of aging in BMSCs/SSCs is also affected by epigenetic changes such as histone modifications and DNA methylation.64 These age-associated epigenetic changes may contribute to intrinsic heterogeneity of clonal stem cell populations, perhaps due to their tendency to introduce differential growth potential in cells of a population. During ex vivo expansion of BMSCs/SSCs, epigenetic and transcriptional alterations have been observed to increase the expression of osteogenic genes [such as TNAP (tissue non-specific alkaline phosphatase), Runx2, BGLAP (osteocalcin), and Spp1 (osteopontin)] with a concomitant decrease in stemness-related genes [e.g., Tert (telomerase reverse transcriptase)]. A decrease in H3K9 and K14 acetylation in the promoter region of stemness-controlling genes,65 and increase in acetylation in the promoter region of osteogenic genes has frequently been observed.66 These observations may explain, in part, why stemness is frequently lost over the sub-culturing in ex vivo cultures of stromal stem cells. As BMSCs/ SSCs age, the expression of some DNA methyltransferases (such as DNA methyltransferase 1 and DNA methyltransferase 3B has been noted to decrease, and accompanied with global hypomethylation.67 Such epigenetic modifications regulate the fate of adult stem cells and studying these mechanisms of gene regulation may improve our understanding of self-renewal, growth and differentiation potential of BMSCs/SSCs during their lifetime. To understand cellular heterogeneity, is it of paramount importance to consider single cell epigenomics in addition to transcriptome profiling.
Extrinsic factors
Extrinsic factors establish changes in the BMSC/SSC microenvironment, in response to which BMSCs/SSCs can dramatically alter their biological activity. Microenvironmental changes are related to changes in the composition and structure of the extracellular matrix, changes in levels of paracrine and endocrine factors, and changes in mechanical forces. All of these external influences can converge on a single cell, bestowing it with a unique in vivo history that will likely influence its future activity. Given their potential for use in tissue engineering to replace segments of bone (and perhaps even cartilage), lost due to trauma or disease, there has been a considerable amount of effort to develop methods for generating clinical grade BMSCs/SSCs by ex vivo expansion.68
Heterogeneity may arise due to culture conditions or cell manufacturing.69 Many attributes for heterogeneity have been described as intrinsic factors such as tissue source, donor age, sex, clonal variation, inconsistency in their phenotypes, including proliferation capacity, expression of cell surface markers and ability to secrete cytokines, or extrinsic factors like cell sorting, seeding densities, medium growth conditions and cell expansion. Such factors greatly affect osteogenic and adipogenic differentiation frequencies in vivo post transplantation. Much of the consideration has been given to heterogeneity in a clonal population of stem cells, yet there remains some pertinent concern in our quest to better understand single cell heterogeneity in stem cell biology. Is the absolute characterization of a population down to the single cell really needed? What are the sources of variability in genomically similar cells and plausible reasons for their altered biological behavior? Why does an isolated population loose its potential for differentiation or function? How can we target single cell heterogeneity to bring about the success of regenerative medicine? BMSCs/SSCs are manufactured using many different methods, but the spectrum of manufacturing methods used and their effects on BMSC/SSC characteristics and function has not been well characterized to date.
Several publications have clearly highlighted the importance of bone marrow harvesting protocols and culture conditions to successfully generate cell products for regenerative medicine.70, 71 Often, the variations in cell isolation procedures (bone marrow aspiration vs. lavage of marrow-containing bone fragments, density gradient centrifugation), and in culture conditions (plastic adherent materials, growth medium, culture period, etc.) have been vividly reflected in poor outcomes of such efforts in some cases. We determined a number of years ago that optimal growth of murine and human BMSCs/SSCs is critically dependent on the amount of foetal bovine serum used (usually 20%), and that the serum be lot-selected based on the results of colony forming efficiency assays,12 and on proliferation rate. It was furthermore determined that the serum should not be heat-inactivated, based on colony forming efficiency and cell numbers upon subsequent passage These studies highlight how even common parameters of cell culture medium composition can impact cellular activity, and points to the need for continued optimization of culture medium for expansion of BMSCs/SSCs.
In a recent study, eight centres using Good Laboratory or Manufacturing Practices were surveyed as to their production methods.72 The transcriptomes from products of each centre were analyzed, and in vivo transplantation into immunocompromised mice was performed. All of the centres used marrow aspirates as the starting material, but some isolated mononuclear cells by density gradient centrifugation while others isolated cells by direct cell adhesion to tissue culture plastic. The basal culture medium and additives (e.g., heat inactivated foetal bovine serum vs. non-heat inactivated foetal bovine serum vs. human platelet lysate) also varied from centre to centre, along with the manufacturing methods. Some centres used standard flasks, others used cell factories, while one used a commercially available bioreactor. Twenty-four different BMSC lots were analysed, and variability was low between lots from the same centre, but significant variability between all centres was noted based on principal component analysis of transcriptomic profiles. BMSCs/SSCs from six centres were tested by in vivo transplantation for their ability to form bone and support haematopoiesis, a defining feature of BMSCs/SSCs. Cells from all six centres tested formed bone, but the quantity was highly variable. BMSCs/SSCs from only two centres substantially supported haematopoiesis. These results show that differences in manufacturing resulted in variable BMSC/SSC characteristics including their ability to form bone and support haematopoiesis, their defining characteristics.
In addition to suitable cell populations, bone tissue engineering with BMSCs/SSCs essentially requires a biocompatible matrix/scaffold to construct a tissue microenvironment that is conducive to bone/marrow formation. Biomaterials are a critical part of many tissue engineering-based products based on their mimicry of the microenvironment required by certain cell types; e.g., bone-forming cells. For tissue engineering, fine tuning between cells and biomaterials is generally required. Any inconsistency in the microenvironment due to an unsupportive biomaterial may compromise the formation of the desired tissue. There are a number of reports that have demonstrated that biomaterials play an influential role in BMSC/SSC-based bone regeneration.73-75
“Homogeneity Means a Single Cell” (Mickie Bhatia, ISSCR, 2011, Toronto, Canada): Advent of Single Cell RNA-sequencing Analysis
Heterogeneity or cell-to-cell variation is a commonly observed phenomenon in virtually all populations of cells, including stem cell populations. The heterogeneous nature of BMSCs/ SSCs is problematic in terms of comparing cells that are ex vivo expanded in one laboratory setting or another, but also is an inherent property of the cell population that needs to be recognized, embraced, and most importantly, to be understood. Very few biological processes are synchronized; e.g., all cells going through different stages of maturation at the same time. While we have some markers in the SSC lineage that identify particular stages of commitment and maturation (e.g., Sox9 (SRY-box transcription factor 9) for chondrogenesis, Osx/Sp7 (osterix, Sp7 transcription factor) for osteogenesis, PPARγ (peroxisome proliferator activated receptor gamma) for adipogenesis), differentiation is more like a wave rather than a staircase with distinct steps.
In order to create a cellular consensus, concerted attempts to build comprehensive molecular reference databases of all cell populations have recently been created. In this effort, Hay et al.76 have developed an interactive and comprehensive web portal that has facilitated researchers to mine and curate data from the Human Cell Atlas. Differences in cell populations within and between donors can be easily probed by this portal, along with the classification of cellular populations based on progenitor stages and reference markers. This strategy is in line with other interactive web resource-based studies that also aim to investigate the diversity of haematopoietic cells in healthy and diseased states of mouse and human bone marrow.77, 78 With the use of advanced computational modeling and library preparation algorithms (e.g., droplet-based sequencing.), thousands of cells can be subjected to sequencing and explored for their reference surface marker profiles, transcription factor genes, multilineage progenitor frequencies and variations in their gene regulatory networks. These studies have revealed much about the heterogeneity and confounding factors that exist within BMSC/SSC populations (e.g., Chan et al.,79 Tikhonova et al.,80 Baryawno et al.,81 Wolock et al.,82 Liu et al.83).
The transcriptome of a single cell is highly influenced by multiple external signals as well as internal factors (such as “noises,” mRNA synthesis and decay).84, 85 This dynamic behavior of the transcriptome poses a great challenge in understanding the heterogeneity within a clonal population as the signature landscape varies significantly at different time points. Moreover, technical errors introduced by sample preparation are also a source of variation in transcriptome-based studies.86 In comparison with single-step procedures and assays, multistep processing has always been challenging in terms of defining the yield, viability, and inter-step variations. This could significantly affect the ultimate results of RNA sequence analysis, therefore, leading to a substantial ex vivo heterogeneity in addition to what lineage commitment and differentiation may contribute. Preparation of bone marrow single cell population is a multi-step procedure that involves: i) bone marrow acquisition, ii) mechanical and/or enzymatic dissociation, iii) differential centrifugation, iv) fluorescense-activated cell sorting and/or cell adherence, and v) direct analysis vs. clone/population selection, etc. In particular, recovery and viability of freshly isolated BMSCs/SSCs through these steps is a major problem that is not well recognized by many. By tracking colony forming efficiency of the freshly isolated single cell suspension of bone marrow and at each step of the way, major losses due to either loss of viability or adherence of the cells to the various vessels is a huge problem. The initial fractionation steps must include the removal of haematopoietic cells due to the fact that the BMSCs/SSCs represent only a minute fraction of the marrow cell population (∼1/105 cells). ACK (ammonium-chloride-potassium) lysis and/or density gradient centrifugation removes most erythroid cells, however there are major losses of BMSCs/SSCs, and many haematopoietic cells remain. Fluorescense-activated cell sorting is also used to further remove CD45+ haematopoietic cells and CD31+ endothelial cells, but again, there are major loses of BMSC/SSCs with this depletion strategy, and the losses continue with positive selection strategies (manuscript in preparation). Given these losses, and the time that it takes to go through all of these steps, it is doubtful that the transcriptome that is generated is an accurate depiction of the state of the cells in situ. Methods for using cells fixed immediately after procurement and prior to all subsequent steps are sorely needed to alleviate this problem, and such methods are under development.87 Thus, use of simple, effective and selective strategies for isolation and enrichment of rare cell populations is highly desirable to gain confidence in the outcomes of any RNA profiling experiment.
A number of transcriptome-based studies have revealed that expansion of rare BMSC/SSC populations, which is an unavoidable step for clinical manufacturing to produce sufficient numbers of cells, can cause inconsistency in the ultimate biological outcomes.52 These steps can lead to significant perturbations in BMSC/SSC differentiation and functional potentials. A significant decrease in bone formation efficiency (∼36-fold) of singly passaged BMSCs has been observed when compared with fresh bone marrow cells.88 Correspondingly, the morphological and functional behavior of plastic-adherent human BMSCs/SSCs have also been reported to be compromised when compared between different passages.12, 89, 90 It has been determined that after bone marrow dissociation, limiting cell culture procedures and the length of culture are really crucial for BMSCs/SSCs which could affect: their cellular attributes (such as cell cycle, senescence and apoptosis), progenitor properties, immune response, molecular and functional genotypes as well as phenotypes.90, 91
In addition to ascertaining the elements of cellular and functional heterogeneity of BMSCs/SSCs, scRNA-sequencing reveals transcriptomic diversity between haematopoietic and non- haematopoietic cell types. The characterization of hierarchical organization and unique populations (stem cells) with defined potency and lineage differentiation has been the subject of many studies.79, 82, 92 Unfortunately, each paper has its own nomenclature and utilizes different markers to establish potential hierarchical schemes, and harmonization is needed in the future. As an example of such a scheme, in Chan et al.79 the population that is PDPN+ (podoplanin)/CD146- (melanoma cell adhesion molecular)/CD73+ (ecto-5′-nucleotidase/CD164+ (endolyn), isolated from adult femur, has been proposed to be a self-renewing, multipotent human SSC. These cells display characteristic features such as: i) a higher frequency of colony forming efficiency even after secondary and tertiary serial transfers in vitro, ii) ability of Lin-/PDPN+/CD164+/CD73+/ CD146- (skeletal stem cells) to form multilineage ossicles upon sub-renal transplantation into mice, then to form Lin-/ PDPN+/CD146+ (bone, cartilage, and stromal progenitors) prior to their terminal differentiation into cartilage (Lin-/ PDPN+/CD146-), and bone/stroma (Lin-/PDPN+/CD146+). Using transcriptomic profiling and microarray technology with single BMSCs/SSCs (microfluidics separated), Liu et al.83 have identified the existence of three distinct subpopulations in BMSCs: skeletal stem cells (fibroblast growth receptor 2), skeletal stem cell progenitors (fibroblast growth receptor 5), angiogenesis-promoting cells (plasminogen activator, tissue type and vascular cell adhesion molecular 1). Based on the scRNA-sequencing gene expression profiling, Wolock et al.93 have further elaborated and validated the transcriptional hierarchy of stromal cell phenotypes, along with their differentiation fate to osteoblasts, chondrocytes, and adipocytes.
With a great potential for application in studying gene expression pattern, and identification of novel transcripts, the high throughput single cell RNA sequencing tool has offered us a sensitive and specific platform to underpin molecular characterization of transcriptomic heterogeneity at the single cell level. Moreover, identification of unique stem cell populations or sub-populations that differ significantly in their differentiation potency predicted by their transcriptome has been attempted. However, the molecular basis of prevailing heterogeneity among individual stem cells in a clonal population is still poorly understood and requires more comprehensive and multiple approaches to better understand cellular heterogeneity. This may result in fruitful efforts in the clinic by using a closely similar cell population to generate functional and reproducible grafts for tissue engineering.
Conclusions
Stem cell-based skeletal tissue regeneration is one of the promising areas that may afford clinical solutions for musculoskeletal grafts that are needed to treat tissue loss due to trauma or disease. Employing a suitable stem cell population remains central to the success of bone regeneration and re-establishment of its marrow. BMSCs contain a subpopulation of SSCs with the potential to differentiate into multiple lineages of the skeletal system. Lack of recognition of the differences between BMSCs/SSCs and other “MSCs,” and heterogeneity among BMSCs/SSCs remain as significant issues in the field. The isolation of a population of cells that includes extraneous cell types (i.e., those from non-skeletal tissues that do not contribute to reformation of skeletal tissues), or a population with a limited number of SSCs results in the failure of tissue engineering approaches. With the advent in the characterization and sorting of a stem cell population with a specific phenotype, and subsequent amplification to enrich populations of desired SSCs has become achievable in the laboratory settings. However, technical and procedural issues that decrease viability and recovery remain to be resolved. Furthermore, utilizing an appropriate scaffolding compatible with the microenvironment also contributes greatly to the success of stem cell-based regenerative strategies. High throughput single cell transcriptomics has emerged as a powerful tool for the phenotypic characterization of a given population (be it freshly isolated or ex vivo expanded) to identify the genetic basis of the inherent heterogeneity. Such information can be utilized in the characterization of pure and desired stem cell populations to be used for a given function. Overall, BMSCs/SSCs represent a suitable cell population for the desired outcome of creating a regenerative bone graft; however, the success remains critically reliant on knowing the characteristics and the true functional nature of the cell population being used. Future advancements in the isolation and characterization of different subsets of SSCs would enable us to engineer successful bone or other skeletal tissue grafts for clinical applications.
Footnotes
Author contributions: Review design by DA and PGR, 1st draft of review by DA, 2nd draft of review by PGR, final edits of review by DA and PGR, graphical abstract by DA, figures by PGR. Both authors read and approved the final version of the manuscript.
Financial support: This work was supported in part by the Fulbright-Nehru Postdoctoral Research Fellowship, United States-India Educational Foundation, the Biosystem and Biomaterials Division, National Institute of Standards and Technology, Department of Commerce, and the Shobhit Institute of Engineering and Technology, Meerut, India (to DA), and by the Division of Intramural Research, National Institute of Dental and Craniofacial Research, a part of the Intramural Research Program, the National Institute of Health, Department of Health and Human Services (1ZIA DE000380 to PGR).
Acknowledgement: The authors wish to acknowledge members of the Skeletal Biology Section, National Institute of Dental and Craniofacial Research, past and present, that have contributed to the body of knowledge presented in this review.
Conflicts of interest statement: The authors have no conflicts of interest to declare.
References
- 1.Bianco P., Robey P. G. Skeletal stem cells. In: Lanza R. P., editor. Handbook of adult and fetal stem cells. Academic Press; San Diego: 2004. pp. 415–424. [Google Scholar]
- 2.Bianco P., Robey P. G. Skeletal stem cells. Development. 2015;142:1023–1027. doi: 10.1242/dev.102210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robey P. G. Cell sources for bone regeneration: the good, the bad, and the ugly (but promising) Tissue Eng Part B Rev. 2011;17:423–430. doi: 10.1089/ten.teb.2011.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrosi T. H., Longaker M. T., Chan C. K. F. A revised perspective of skeletal stem cell biology. Front Cell Dev Biol. 2019;7:189. doi: 10.3389/fcell.2019.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacchetti B., Funari A., Michienzi S., Di Cesare S., Piersanti S., Saggio I., Tagliafico E., Ferrari S., Robey P. G., Riminucci M., Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 6.Friedenstein A. J., Chailakhjan R. K., Lalykina K. S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 7.Owen M. E., Cavé J., Joyner C. J. Clonal analysis in vitro of osteogenic differentiation of marrow CFU-F. J Cell Sci. 1987;87(Pt 5):731–738. doi: 10.1242/jcs.87.5.731. [DOI] [PubMed] [Google Scholar]
- 8.Friedenstein A. J. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- 9.Owen M., Friedenstein A. J. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 10.Johnstone B., Hering T. M., Caplan A. I., Goldberg V. M., Yoo J. U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238:265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 11.Gronthos S., Zannettino A. C., Hay S. J., Shi S., Graves S. E., Kortesidis A., Simmons P. J. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 12.Kuznetsov S. A., Krebsbach P. H., Satomura K., Kerr J., Riminucci M., Benayahu D., Robey P. G. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–1347. doi: 10.1359/jbmr.1997.12.9.1335. [DOI] [PubMed] [Google Scholar]
- 13.Sworder B. J., Yoshizawa S., Mishra P. J., Cherman N., Kuznetsov S. A., Merlino G., Balakumaran A., Robey P. G. Molecular profile of clonal strains of human skeletal stem/progenitor cells with different potencies. Stem Cell Res. 2015;14:297–306. doi: 10.1016/j.scr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan A. I. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 15.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F., Krause D., Deans R., Keating A., Prockop D., Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 16.MacCord K. “Mesenchyme”. Embryo Project Encyclopedia (2012-09-14) http://embryo.asu.edu/handle/10776/3941 ISSN: 1940-5030.
- 17.Olsen B. R., Reginato A. M., Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 18.Minasi M. G., Riminucci M., De Angelis L., Borello U., Berarducci B., Innocenzi A., Caprioli A., Sirabella D., Baiocchi M., De Maria R., Boratto R., Jaffredo T., Broccoli V., Bianco P., Cossu G. The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development. 2002;129:2773–2783. doi: 10.1242/dev.129.11.2773. [DOI] [PubMed] [Google Scholar]
- 19.Gilbert S. F. Developmental Biology. 10th ed. Sinauer Associates, Inc.; Sunderland: 2014. [Google Scholar]
- 20.Ren J., Jin P., Sabatino M., Balakumaran A., Feng J., Kuznetsov S. A., Klein H. G., Robey P. G., Stroncek D. F. Global transcriptome analysis of human bone marrow stromal cells (BMSC) reveals proliferative, mobile and interactive cells that produce abundant extracellular matrix proteins, some of which may affect BMSC potency. Cytotherapy. 2011;13:661–674. doi: 10.3109/14653249.2010.548379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.da Silva Meirelles L., Chagastelles P. C., Nardi N. B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 22.Bianco P., Robey P. G., Simmons P. J. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y. K., Ogando C. R., Wang See C., Chang T. Y., Barabino G. A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res Ther. 2018;9:131. doi: 10.1186/s13287-018-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rojewski M. T., Weber B. M., Schrezenmeier H. Phenotypic characterization of mesenchymal stem cells from various tissues. Transfus Med Hemother. 2008;35:168–184. doi: 10.1159/000129013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sotiropoulou P. A., Perez S. A., Salagianni M., Baxevanis C. N., Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- 26.Urist M. R. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 27.Friedenstein A. J., Lalykina K. S. Thymus cells are inducible to osteogenesis. Eur J Immunol. 1972;2:602–603. doi: 10.1002/eji.1830020624. [DOI] [PubMed] [Google Scholar]
- 28.Bonewald L. F., Harris S. E., Rosser J., Dallas M. R., Dallas S. L., Camacho N. P., Boyan B., Boskey A. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int. 2003;72:537–547. doi: 10.1007/s00223-002-1057-y. [DOI] [PubMed] [Google Scholar]
- 29.Diascro D. D., Jr, Vogel R. L., Johnson T. E., Witherup K. M., Pitzenberger S. M., Rutledge S. J., Prescott D. J., Rodan G. A., Schmidt A. High fatty acid content in rabbit serum is responsible for the differentiation of osteoblasts into adipocyte-like cells. J Bone Miner Res. 1998;13:96–106. doi: 10.1359/jbmr.1998.13.1.96. [DOI] [PubMed] [Google Scholar]
- 30.Sacchetti B., Funari A., Remoli C., Giannicola G., Kogler G., Liedtke S., Cossu G., Serafini M., Sampaolesi M., Tagliafico E., Tenedini E., Saggio I., Robey P. G., Riminucci M., Bianco P. No identical “mesenchymal stem cells” at different times and sites: human committed progenitors of distinct origin and differentiation potential are incorporated as adventitial cells in microvessels. Stem Cell Reports. 2016;6:897–913. doi: 10.1016/j.stemcr.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robey P. “Mesenchymal stem cells”: fact or fiction, and implications in their therapeutic use. F1000Res. 2017;6 doi: 10.12688/f1000research.10955.1. F1000 Faculty Rev-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gronthos S., Mankani M., Brahim J., Robey P. G., Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura M., Gronthos S., Zhao M., Lu B., Fisher L. W., Robey P. G., Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seo B. M., Miura M., Gronthos S., Bartold P. M., Batouli S., Brahim J., Young M., Robey P. G., Wang C. Y., Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 35.Sherwood R. I., Christensen J. L., Conboy I. M., Conboy M. J., Rando T. A., Weissman I. L., Wagers A. J. Isolation of adult mouse myogenic progenitors: functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Liu X., Rui T., Zhang S., Ding Z. Heterogeneity of MSC: origin, molecular identities, and functionality. Stem Cells Int. 2019;2019:9281520. doi: 10.1155/2019/9281520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nemeth K., Mayer B., Sworder B. J., Kuznetsov S. A., Mezey E. A practical guide to culturing mouse and human bone marrow stromal cells. Curr Protoc Immunol. 2013;102:22F.12.1–22F.12.13. doi: 10.1002/0471142735.im22f12s102. [DOI] [PubMed] [Google Scholar]
- 38.Chu D. T., Phuong T. N. T., Tien N. L. B., Tran D. K., Thanh V. V., Quang T. L., Truong D. T., Pham V. H., Ngoc V. T. N., Chu-Dinh T., Kushekhar K. An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int J Mol Sci. 2020;21:708. doi: 10.3390/ijms21030708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman C. M., Curtin C., Barry F. P., O’Flatharta C., Murphy J. M. Mesenchymal stem cells and osteoarthritis: remedy or accomplice? Hum Gene Ther. 2010;21:1239–1250. doi: 10.1089/hum.2010.138. [DOI] [PubMed] [Google Scholar]
- 40.Caplan A. I., Hariri R. Body management: mesenchymal stem cells control the internal regenerator. Stem Cells Transl Med. 2015;4:695–701. doi: 10.5966/sctm.2014-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haniffa M. A., Collin M. P., Buckley C. D., Dazzi F. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica. 2009;94:258–263. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galipeau J., Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018;22:824–833. doi: 10.1016/j.stem.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satomura K., Derubeis A. R., Fedarko N. S. Ibaraki-O’Connor K., Kuznetsov S. A., Rowe D. W., Young M. F., Gehron Robey P. Receptor tyrosine kinase expression in human bone marrow stromal cells. J Cell Physiol. 1998;177:426–438. doi: 10.1002/(SICI)1097-4652(199812)177:3<426::AID-JCP6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 44.Rennerfeldt D. A., Raminhos J. S., Leff S. M., Manning P., Van Vliet K. J. Emergent heterogeneity in putative mesenchymal stem cell colonies: Single-cell time lapsed analysis. PLoS One. 2019;14:e0213452. doi: 10.1371/journal.pone.0213452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akintoye S. O., Lam T., Shi S., Brahim J., Collins M. T., Robey P. G. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–768. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 46.Kidwai F., Mui B. W. H., Arora D., Iqbal K., Hockaday M., de Castro Diaz L. F., Cherman N., Martin D., Myneni V. D., Ahmad M., Futrega K., Ali S., Merling R. K., Kaufman D. S., Lee J., Robey P. G. Lineage-specific differentiation of osteogenic progenitors from pluripotent stem cells reveals the FGF1-RUNX2 association in neural crest-derived osteoprogenitors. Stem Cells. 2020;38:1107–1123. doi: 10.1002/stem.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Debnath S., Yallowitz A. R., McCormick J., Lalani S., Zhang T., Xu R., Li N., Liu Y., Yang Y. S., Eiseman M., Shim J. H., Hameed M., Healey J. H., Bostrom M. P., Landau D. A., Greenblatt M. B. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562:133–139. doi: 10.1038/s41586-018-0554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sivaraj K. K., Jeong H. W., Dharmalingam B., Zeuschner D., Adams S., Potente M., Adams R. H. Regional specialization and fate specification of bone stromal cells in skeletal development. Cell Rep. 2021;36:109352. doi: 10.1016/j.celrep.2021.109352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ambrosi T. H., Sinha R., Steininger H. M., Hoover M. Y., Murphy M. P., Koepke L. S., Wang Y., Lu W. J., Morri M., Neff N. F., Weissman I. L., Longaker M. T., Chan C. K. Distinct skeletal stem cell types orchestrate long bone skeletogenesis. eLife. 2021;10:e66063. doi: 10.7554/eLife.66063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tormin A., Li O., Brune J. C., Walsh S., Schütz B., Ehinger M., Ditzel N., Kassem M., Scheding S. CD146 expression on primary nonhematopoietic bone marrow stem cells is correlated with in situ localization. Blood. 2011;117:5067–5077. doi: 10.1182/blood-2010-08-304287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boudin E., Fijalkowski I., Hendrickx G., Van Hul W. Genetic control of bone mass. Mol Cell Endocrinol. 2016;432:3–13. doi: 10.1016/j.mce.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 52.Ren J., Stroncek D. F., Zhao Y., Jin P., Castiello L., Civini S., Wang H., Feng J., Tran K., Kuznetsov S. A., Robey P. G., Sabatino M. Intra-subject variability in human bone marrow stromal cell (BMSC) replicative senescence: molecular changes associated with BMSC senescence. Stem cell research. 2013;11:1060–1073. doi: 10.1016/j.scr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fedarko N. S., Bianco P., Vetter U., Robey P. G. Human bone cell enzyme expression and cellular heterogeneity: correlation of alkaline phosphatase enzyme activity with cell cycle. J Cell Physiol. 1990;144:115–121. doi: 10.1002/jcp.1041440115. [DOI] [PubMed] [Google Scholar]
- 54.Galindo M., Kahler R. A., Teplyuk N. M., Stein J. L., Lian J. B., Stein G. S., Westendorf J. J., van Wijnen A. J. Cell cycle related modulations in Runx2 protein levels are independent of lymphocyte enhancer-binding factor 1 (Lef1) in proliferating osteoblasts. J Mol Histol. 2007;38:501–506. doi: 10.1007/s10735-007-9143-0. [DOI] [PubMed] [Google Scholar]
- 55.Oh J., Lee Y. D., Wagers A. J. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20:870–880. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ambrosi T. H., Goodnough L. H., Steininger H. M., Hoover M. Y., Kim E., Koepke L. S., Marecic O., Zhao L., Seita J., Bishop J. A., Gardner M. J., Chan C. K. F. Geriatric fragility fractures are associated with a human skeletal stem cell defect. Aging Cell. 2020;19:e13164. doi: 10.1111/acel.13164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sahin E., Depinho R. A. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ambrosi T. H., Marecic O., McArdle A., Sinha R., Gulati G. S., Tong X., Wang Y., Steininger H. M., Hoover M. Y., Koepke L. S., Murphy M. P., Sokol J., Seo E. Y., Tevlin R., Lopez M., Brewer R. E., Mascharak S., Lu L., Ajanaku O., Conley S. D., Seita J., Morri M., Neff N. F., Sahoo D., Yang F., Weissman I. L., Longaker M. T., Chan C. K. F. Aged skeletal stem cells generate an inflammatory degenerative niche. Nature. 2021;597:256–262. doi: 10.1038/s41586-021-03795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Josephson A. M., Bradaschia-Correa V., Lee S., Leclerc K., Patel K. S., Muinos Lopez E., Litwa H. P., Neibart S. S., Kadiyala M., Wong M. Z., Mizrahi M. M., Yim N. L., Ramme A. J., Egol K. A., Leucht P. Age-related inflammation triggers skeletal stem/progenitor cell dysfunction. Proc Natl Acad Sci U S A. 2019;116:6995–7004. doi: 10.1073/pnas.1810692116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sarkar T. J., Quarta M., Mukherjee S., Colville A., Paine P., Doan L., Tran C. M., Chu C. R., Horvath S., Qi L. S., Bhutani N., Rando T. A., Sebastiano V. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat Commun. 2020;11:1545. doi: 10.1038/s41467-020-15174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saul D., Monroe D. G., Rowsey J. L., Kosinsky R. L., Vos S. J., Doolittle M. L., Farr J. N., Khosla S. Modulation of fracture healing by the transient accumulation of senescent cells. eLife. 2021;10:e69958. doi: 10.7554/eLife.69958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cakouros D., Gronthos S. Epigenetic regulators of mesenchymal stem/stromal cell lineage determination. Curr Osteoporos Rep. 2020;18:597–605. doi: 10.1007/s11914-020-00616-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buisman S. C., de Haan G. Epigenetic changes as a target in aging haematopoietic stem cells and age-related malignancies. Cells. 2019;8:868. doi: 10.3390/cells8080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cakouros D., Gronthos S. The changing epigenetic landscape of mesenchymal stem/stromal cells during aging. Bone. 2020;137:115440. doi: 10.1016/j.bone.2020.115440. [DOI] [PubMed] [Google Scholar]
- 65.Tsai C. C., Hung S. C. Functional roles of pluripotency transcription factors in mesenchymal stem cells. Cell Cycle. 2012;11:3711–3712. doi: 10.4161/cc.22048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Z., Liu C., Xie Z., Song P., Zhao R. C., Guo L., Liu Z., Wu Y. Epigenetic dysregulation in mesenchymal stem cell aging and spontaneous differentiation. PLoS One. 2011;6:e20526. doi: 10.1371/journal.pone.0020526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.So A. Y., Jung J. W., Lee S., Kim H. S., Kang K. S. DNA methyltransferase controls stem cell aging by regulating BMI1 and EZH2 through microRNAs. PLoS One. 2011;6:e19503. doi: 10.1371/journal.pone.0019503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robey P. G., Kuznetsov S. A., Ren J., Klein H. G., Sabatino M., Stroncek D. F. Generation of clinical grade human bone marrow stromal cells for use in bone regeneration. Bone. 2015;70:87–92. doi: 10.1016/j.bone.2014.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mabuchi Y., Okawara C., Méndez-Ferrer S., Akazawa C. Cellular heterogeneity of mesenchymal stem/stromal cells in the bone marrow. Front Cell Dev Biol. 2021;9:689366. doi: 10.3389/fcell.2021.689366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Placzek M. R., Chung I. M., Macedo H. M., Ismail S., Mortera Blanco T., Lim M., Cha J. M., Fauzi I., Kang Y., Yeo D. C., Ma C. Y., Polak J. M., Panoskaltsis N., Mantalaris A. Stem cell bioprocessing: fundamentals and principles. J R Soc Interface. 2009;6:209–232. doi: 10.1098/rsif.2008.0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wilson A., Webster A., Genever P. Nomenclature and heterogeneity: consequences for the use of mesenchymal stem cells in regenerative medicine. Regen Med. 2019;14:595–611. doi: 10.2217/rme-2018-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu S., de Castro L. F., Jin P., Civini S., Ren J., Reems J. A., Cancelas J., Nayak R., Shaw G., O’Brien T., McKenna D. H., Armant M., Silberstein L., Gee A. P., Hei D. J., Hematti P., Kuznetsov S. A., Robey P. G., Stroncek D. F. Manufacturing differences affect human bone marrow stromal cell characteristics and function: comparison of production methods and products from multiple centers. Sci Rep. 2017;7:46731. doi: 10.1038/srep46731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iaquinta M. R., Mazzoni E., D’Manfrini M., Agostino A., Trevisiol L., Nocini R., Trombelli L., Barbanti-Brodano G., Martini F., Tognon M. Innovative biomaterials for bone regrowth. Int J Mol Sci. 2019;20:618. doi: 10.3390/ijms20030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang C., Zhang L., Liu L., Lv L., Gao L., Liu N., Wang X., Ye J. Mechanical behavior of a titanium alloy scaffold mimicking trabecular structure. J Orthop Surg Res. 2020;15:40. doi: 10.1186/s13018-019-1489-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Barbanti Brodano G., Mazzoni E., Tognon M., Griffoni C., Manfrini M. Human mesenchymal stem cells and biomaterials interaction: a promising synergy to improve spine fusion. Eur Spine J. 2012;21(Suppl 1):S3–9. doi: 10.1007/s00586-012-2233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hay S. B., Ferchen K., Chetal K., Grimes H. L., Salomonis N. The Human Cell Atlas bone marrow single-cell interactive web portal. Exp Hematol. 2018;68:51–61. doi: 10.1016/j.exphem.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zheng S., Papalexi E., Butler A., Stephenson W., Satija R. Molecular transitions in early progenitors during human cord blood hematopoiesis. Mol Syst Biol. 2018;14:e8041. doi: 10.15252/msb.20178041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dahlin J. S., Hamey F. K., Pijuan-Sala B., Shepherd M., Lau W. W. Y., Nestorowa S., Weinreb C., Wolock S., Hannah R., Diamanti E., Kent D. G., Göttgens B., Wilson N. K. A single-cell hematopoietic landscape resolves 8 lineage trajectories and defects in Kit mutant mice. Blood. 2018;131:e1–e11. doi: 10.1182/blood-2017-12-821413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chan C. K. F., Gulati G. S., Sinha R., Tompkins J. V., Lopez M., Carter A. C., Ransom R. C., Reinisch A., Wearda T., Murphy M., Brewer R. E., Koepke L. S., Marecic O., Manjunath A., Seo E. Y., Leavitt T., Lu W. J., Nguyen A., Conley S. D., Salhotra A., Ambrosi T. H., Borrelli M. R., Siebel T., Chan K., Schallmoser K., Seita J., Sahoo D., Goodnough H., Bishop J., Gardner M., Majeti R., Wan D. C., Goodman S., Weissman I. L., Chang H. Y., Longaker M. T. Identification of the human skeletal stem cell. Cell. 2018;175:43–56.e21. doi: 10.1016/j.cell.2018.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tikhonova A. N., Dolgalev I., Hu H., Sivaraj K. K., Hoxha E., Cuesta-Domínguez Á., Pinho S., Akhmetzyanova I., Gao J., Witkowski M., Guillamot M., Gutkin M. C., Zhang Y., Marier C., Diefenbach C., Kousteni S., Heguy A., Zhong H., Fooksman D. R., Butler J. M., Economides A., Frenette P. S., Adams R. H., Satija R., Tsirigos A., Aifantis I. The bone marrow microenvironment at single-cell resolution. Nature. 2019;569:222–228. doi: 10.1038/s41586-019-1104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baryawno N., Przybylski D., Kowalczyk M. S., Kfoury Y., Severe N., Gustafsson K., Kokkaliaris K. D., Mercier F., Tabaka M., Hofree M., Dionne D., Papazian A., Lee D., Ashenberg O., Subramanian A., Vaishnav E. D., Rozenblatt-Rosen O., Regev A., Scadden D. T. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. Cell. 2019;177:1915–1932.e16. doi: 10.1016/j.cell.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolock S. L., Krishnan I., Tenen D. E., Matkins V., Camacho V., Patel S., Agarwal P., Bhatia R., Tenen D. G., Klein A. M., Welner R. S. Mapping distinct bone marrow niche populations and their differentiation paths. Cell Rep. 2019;28:302–311.e5. doi: 10.1016/j.celrep.2019.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu S., Stroncek D. F., Zhao Y., Chen V., Shi R., Chen J., Ren J., Liu H., Bae H. J., Highfill S. L., Jin P. Single cell sequencing reveals gene expression signatures associated with bone marrow stromal cell subpopulations and time in culture. J Transl Med. 2019;17:23. doi: 10.1186/s12967-018-1766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baldarelli R. M., Hill D. P., Blake J. A., Adachi J., Furuno M., Bradt D., Corbani L. E., Cousins S., Frazer K. S., Qi D., Yang L., Ramachandran S., Reed D., Zhu Y., Kasukawa T., Ringwald M., King B. L., Maltais L. J., McKenzie L. M., Schriml L. M., Maglott D., Church D. M., Pruitt K., Eppig J. T., Richardson J. E., Kadin J. A., Bult C. J. Connecting sequence and biology in the laboratory mouse. Genome Res. 2003;13:1505–1519. doi: 10.1101/gr.991003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sun M., Schwalb B., Schulz D., Pirkl N., Etzold S., Lariviàre L., Maier K. C., Seizl M., Tresch A., Cramer P. Comparative dynamic transcriptome analysis (cDTA) reveals mutual feedback between mRNA synthesis and degradation. Genome Res. 2012;22:1350–1359. doi: 10.1101/gr.130161.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tung P. Y., Blischak J. D., Hsiao C. J., Knowles D. A., Burnett J. E., Pritchard J. K., Gilad Y. Batch effects and the effective design of single-cell gene expression studies. Sci Rep. 2017;7:39921. doi: 10.1038/srep39921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen J., Cheung F., Shi R., Zhou H., Lu W. CHI Consortium. PBMC fixation and processing for Chromium single-cell RNA sequencing. J Transl Med. 2018;16:198. doi: 10.1186/s12967-018-1578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Banfi A., Muraglia A., Dozin B., Mastrogiacomo M., Cancedda R., Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 89.Post S., Abdallah B. M., Bentzon J. F., Kassem M. Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone. 2008;43:32–39. doi: 10.1016/j.bone.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 90.Elsafadi M., Manikandan M., Atteya M., Hashmi J. A., Iqbal Z., Aldahmash A., Alfayez M., Kassem M., Mahmood A. Characterization of cellular and molecular heterogeneity of bone marrow stromal cells. Stem Cells Int. 2016;2016:9378081. doi: 10.1155/2016/9378081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiang Y., Wu C., Wu J., Quan W., Cheng C., Zhou J., Chen L., Xiang L., Li F., Zhang K., Ran Q., Zhang Y., Li Z. In vitro expansion affects the response of human bone marrow stromal cells to irradiation. Stem Cell Res Ther. 2019;10:82. doi: 10.1186/s13287-019-1191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oetjen K. A., Lindblad K. E., Goswami M., Gui G., Dagur P. K., Lai C., Dillon L. W., McCoy J. P., Hourigan C. S. Human bone marrow assessment by single-cell RNA sequencing, mass cytometry, and flow cytometry. JCI insight. 2018;3:e124928. doi: 10.1172/jci.insight.124928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wolock S. L., Krishnan I., Tenen D. E., Matkins V., Camacho V., Patel S., Agarwal P., Bhatia R., Tenen D. G., Klein A. M., Welner R. S. Mapping distinct bone marrow niche populations and their differentiation paths. Cell Rep. 2019;28:302–311.e5. doi: 10.1016/j.celrep.2019.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]