Abstract
Objectives
This research examined main and moderating effects of global depressive symptoms upon in-the-moment associations of pain and affect among individuals with knee osteoarthritis (OA). Effects of depression on short-term change in pain and affect were also examined.
Method
Older adults with physician-confirmed OA (N = 325) completed a baseline interview tapping global depressive symptoms, followed by an experience sampling protocol that captured momentary pain and affect 4 times daily for 7 days. Multilevel models controlling demographics and health conditions examined main and moderating effects of depression on momentary associations of pain with positive affect (PA) and negative affect (NA). Similar methods addressed short-term change in pain and affect. Auxiliary analyses explored broad associations of depressive symptoms with person-level averages and variability in pain and affect.
Results
Global depression predicted current pain, PA, and NA, as well as change in pain and affect over a 3- to 8-h period. Furthermore, both in the moment and over short periods, the association of pain and NA was stronger among persons higher in depressive symptoms. No moderating effect for the PA–pain association was found. Depressive symptoms were also associated with variability in pain and affect, particularly NA.
Discussion
Results confirm previous work on the relation of chronic pain with both global depressive symptoms and short-term affect. This research further demonstrates a unique moderating role of depression on the association of momentary pain with NA and suggests that the causal path may be stronger from pain to affect than vice versa.
Keywords: Arthritis, Emotional well-being, Mood states
Osteoarthritis (OA) is among the most common and most debilitating health problems of later life (Barbour et al., 2013; Hootman et al., 2012). The pain and functional limitations that characterize this disorder place individuals at increased risk of negative psychological outcomes (van Baar et al., 1998; Davis et al., 1992; Salaffi et al., 1991), including depression (Agustini et al., 2020; Parmelee et al., 2007; Rathbun et al., 2020; Veronese et al., 2017). Longitudinal analyses provide evidence of a reciprocal relationship between pain and depression in which each influences the severity of the other both generally (Chou, 2007; Kroenke et al., 2011; Schieir et al., 2009) and among persons with OA specifically (Hawker et al., 2011).
Elaborating on the established relationship between global pain and affect, researchers have utilized both experimental and microlongitudinal designs to better understand the daily experience of individuals with chronic pain. In laboratory research, induced depressed mood resulted in greater pain unpleasantness (Berna et al., 2010; Loggia et al., 2008) and both higher pain ratings and lower pain tolerance (Tang et al., 2008) compared to neutral conditions. Similarly, induction of positive mood led to lower pain ratings and greater pain tolerance compared to persons experiencing induced depressed mood (Tang et al., 2008). Studies utilizing experience sampling (ESM; also called ecological momentary assessment) and other multiple repeated measures methods document a similar pain–affect relationship at weekly (Zautra et al., 2005), daily (Affleck et al.,1992; Katana et al., 2020), and momentary levels (Graham-Engeland et al., 2016; Smith & Parmelee, 2016; Vendrig & Lousberg, 1997).
There is evidence that short-term, within-person affective processes may differ for depressed and nondepressed individuals. On average, individuals with depression show greater daily negative affect (NA) and lower daily positive affect (PA) compared to nondepressed persons (Bijlsma et al., 2011; Lamers et al., 2018). Additionally, patterns of variability in daily affect differ among depressed versus nondepressed adults of all ages (Bijlsma et al., 2011; Hall et al., 1991; Lawton et al., 1996), reflecting generally greater variability in NA among depressed versus nondepressed individuals. These differences may help explain how depressive symptomatology relates to pain. In individuals with rheumatoid arthritis (RA) or OA, depression has been associated with greater weekly and momentary pain in complex ways. For example, Smith and Zautra (2008) found that the relationships of global depression and anxiety to weekly arthritis pain were partially mediated by weekly NA and PA. On the momentary level, however, Graham-Engeland et al. (2016) failed to find a mediating effect of affect on the depression–pain association. They suggested that these differential findings may stem from differences in observational time frames, that is, momentary affect captures a phenomenon that is more distinct from depressive symptomatology and more directly influenced by situational factors than weekly or even daily affect.
As arthritis pain can be considered a stressor, research has examined the effects of depression on pain and affect through stress and coping mechanisms. According to the kindling hypothesis, depressive episodes can lower the stress threshold and decrease individuals’ capacity to manage daily stressors, including chronic pain (Conner et al., 2006; Kendler et al., 2000). Studies of fibromyalgia (Tennen et al., 2006) and RA (Conner et al., 2006) indicate that individuals with and without a history of depression show comparable levels of average daily pain. However, in both these studies, previously depressed persons exhibited stronger pain–affect associations than never-depressed individuals. On higher pain days, previously depressed individuals showed steeper declines in affect, were more likely to cope by venting their emotions, and felt less effective in their coping.
As these studies demonstrate, ESM research on pain and affect has examined pain as both a predictor and an outcome. However, previous studies are largely cross-sectional and are therefore unable to determine the directionality of these pain–affect associations. In supplemental analyses examining lagged associations from one day to the next, Conner et al. (2006) found that pain was a stronger predictor of subsequent appraisals, coping, and affect than vice versa. Gil et al. (2004) also suggested that, although bidirectional relationships were observed, pain may be the more powerful predictor in the pain–affect cycle. This is supported by Katana et al.’s (2020) finding that daily pain was associated with next-day NA but not vice versa. Similarly, Graham-Engeland et al. (2016) reported that affect did not predict within-day lagged pain or pain-related restrictions; however, they noted that results should be interpreted cautiously due to problems with the density of their data. In contrast, at the weekly level, Zautra et al. (2005) found that NA predicted greater subsequent pain.
Although these studies are informative, the impact of depression on the dynamic associations between pain and affect remains unclear. The current study therefore examined main and interactive effects of depressive symptoms on the associations between momentary pain and affect among individuals with symptomatic OA. Research reviewed above suggests differences in the within-person pain–affect association for depressed and nondepressed individuals. Hence, we chose a moderation model to examine how the linkage of pain and affect differs as a function of global depressive symptoms. However, only two studies to our knowledge (Graham-Engeland et al., 2016; Mak & Schneider, 2020) have examined the impact of depression on the momentary associations of pain and affect. Additionally, the directionality of these effects is not yet clear. To address this gap, we examined contemporaneous and lagged associations with both momentary pain and affect as outcomes. Finally, the everyday manifestations of pain, affect, and fatigue have been shown to differ as a function of the specific chronic pain syndrome (Zautra et al., 2007). Although previous studies of momentary affect and pain have examined a range of chronic pain conditions, few have focused on OA pain specifically in a within-day model.
The current study is thus, to our knowledge, the first to investigate how depressive symptoms influence momentary associations between pain and affect in individuals with OA. Based on research just reviewed, we hypothesized, first, that depression would intensify the momentary linkage of pain and affect such that more depressed individuals would display stronger in-the-moment associations than would those reporting fewer depressive symptoms. We further posited that the temporal linkage of momentary pain and affect would flow more from pain to affect—that is, that current pain would be a stronger predictor of future within-day affect than vice versa—and that this linkage, too, would be stronger among persons higher in depressive symptoms. Finally, we hypothesized that all these effects would be stronger for NA than for PA.
Method
Sample
Three hundred twenty-five persons with physician-diagnosed OA of the knee comprise the current sample. Participants were recruited from diverse sources in the western Alabama and Long Island, New York areas. Sample sources included university-based general medical clinics, rheumatology practices, federally qualified health centers, community service networks for older adults, public service announcements in diverse media, and commercially prepared mailing lists. Inclusion criteria were age 45 years or older, physician confirmation of knee OA, no other disabling or life-threatening health conditions, cognitive capacity to complete the study protocol (Short Portable Mental Status Questionnaire score ≥6; Pfeiffer, 1975), and ability to complete in-person and telephonic interviews in English. Of 347 persons enrolled in the study, 93.7% completed at least half of the 28 ESM telephone calls (described below); the remaining 22 were excluded from the analysis. For the active sample of 325, mean number of completed calls was 22.7 (SD = 2.3); median and mode were both 23.
Demographic characteristics of the final sample, along with descriptive statistics for all other study variables, are given in Table 1. The sample, split roughly equally between the two study sites, was predominantly female and well educated. Because the aims of the larger project focused on racial/ethnic differences in dynamics examined here (i.e., the association of OA pain with everyday quality of life and emotional well-being), African Americans (AAs) were overrepresented vis-à-vis the general population.
Table 1.
Sample Characteristics and Primary Study Variables (N = 325)
| Variable (range in this sample) | Mean or N | SD or % |
|---|---|---|
| Alabama | 178 | 54.8% |
| New York | 147 | 45.2% |
| Age, years (48, 97) | 63.9 | 8.9 |
| Male | 76 | 23.4% |
| Female | 249 | 76.6% |
| African American | 143 | 44.0% |
| Non-Hispanic White | 182 | 56.0% |
| Coupled | 178 | 54.8% |
| Single | 147 | 45.2% |
| Grade 11 or less | 30 | 9.3% |
| High school/GED | 73 | 22.6% |
| Technical school/vocational training | 35 | 10.8% |
| One to four years of college | 61 | 18.9% |
| College graduate | 56 | 17.3% |
| Graduate/professional degree | 68 | 21.1% |
| Health problems other than OA (0, 13) | 3.25 | 2.63 |
| OA in joints other than knee (0, 6) | 2.49 | 1.96 |
| OA in both knees | 207 | 63.7% |
| Momentary positive affecta (1.00, 5.00) | 3.37 | 0.80 |
| Momentary negative affecta (1.00, 4.00) | 1.24 | 0.47 |
| Momentary paina (1.00, 5.00) | 2.19 | 1.06 |
| Depressive symptoms (0, 54) | 10.87 | 10.26 |
Note: OA = osteoarthritis.
aAssessed at the first call of experience sampling protocol; see text for details.
Procedures
All procedures were approved by institutional review boards at study sites. Recruitment differed slightly as a function of referral source but in general, potential participants first underwent telephone screening to confirm eligibility. Eligible, interested participants were mailed consent forms and a set of self-administered questionnaires. An in-person interview was scheduled for a week or so after packet mailout, to complete the consent process and collect additional study measures.
At the conclusion of the interview, each participant was given materials for and instructed in the ESM procedure. All participants received accelerometers to collect data (not reported here) on activity levels; those who did not have a cellular telephone were given one for the duration of the ESM protocol. Beginning the day after the interview, participants received four brief (<10 min) telephone calls daily for 1 week. Calls were randomized within 4-h blocks over a 12-h period, with the stipulation that calls occur at least a half-hour apart, in order to capture short-term variation over the course of a full day. Three attempts were made over a 15-min period before calls were considered missed. Persons who missed a full day of calls were requested to complete an extra day of the ESM procedure to afford as many data points as possible (N = 6).
Measures
Demographic characteristics, used as covariates in current analyses, included age in years; sex; race (AA vs. non-Hispanic White); marital status, coded dichotomously as married/cohabiting or not; and education (6-point scale, “less than high school” through “graduate/professional degree”). Site (Alabama vs. New York) was also included.
Health status characteristics, also assessed as potential covariates, were (a) physical health conditions experienced over the past year, for example, hypertension, stroke, bronchitis, identified via a 30-item checklist. (b) Joint count tapped the presence of OA in six joints other than the knee, with a possible seventh “other” category. Participants also indicated whether they experienced (c) OA in one or both knees.
Depressive symptoms (hereafter also called simply depression) were tapped with the 20-item Center for Epidemiologic Studies—Depression scale (CES-D; Radloff, 1977). Cronbach’s alpha for the current sample was 0.904.
The ESM protocol yielded three momentary measures for current analyses. All questions asked participants how they felt “when the phone rang” for the current call. Momentary pain was rated on a 5-point labeled scale (“no pain at all” to “extreme pain”). Momentary affect was assessed using the Philadelphia Geriatric Center Affect Scales (Lawton et al., 1996). Ten items, all rated on 5-point scales (“not at all” to “extremely”), yielded composite scales computed by averaging items representing PA (energetic, warm, interested, happy, content) and NA (annoyed, depressed, irritated, worried, sad). Cronbach’s alphas for this sample, computed using each participant’s data for the first ESM call, were 0.719 for PA and 0.757 for NA.
Analytic Plan
Preliminary analyses identified demographic and health correlates of depression and momentary pain and affect to permit their control in primary analyses.
Cross-sectional associations among momentary affect and pain (i.e., at the same phone call) were examined in a series of hierarchical multilevel models that nested the 28 ESM calls (Level 1) within participants (Level 2). Three analyses treated each of the momentary variables (PA, NA, pain) separately as outcome. For each, a null or empty model was first run to estimate appropriateness of the multilevel procedure, that is, the ratio of within- to between-person variance. Covariates were then added to the equation (Model 2). In Model 3, positive and negative affect were predicted by momentary pain and person-level depression; pain was predicted by PA, NA, and depression. Model 4 included centered interaction terms to assess moderating effects of depression on the affect–pain association. For each model, incremental improvement in explained variance was tested using likelihood ratio tests (LRTs), which compare change in the log-likelihood function (−2LL) across nested models and are distributed as chi-square.
A parallel set of multilevel models tested lagged associations among momentary variables by substituting affect or pain at the next ESM call as outcome in the same hierarchical process just described. Thus, for example, current PA and NA were used to predict pain at the next momentary assessment. To adjust for autocorrelation, current status was entered into the equation for each next-call outcome, for example, for PA at the next call, current PA was included as a covariate, yielding an estimate of change in outcome over the lag period. These analyses were allowed to cross days, for example, data from the last call of Day 2 were used to predict the first call of Day 3.
Where significant interactive effects were observed, the effect was plotted as simple slopes computed using utilities available at www.quantpsy.org (Preacher et al., 2006).
All models were replicated using raw and person-centered variables. The two sets of analyses yielded identical patterns for effects of interest; we present only results for the raw predictors here.
A final, auxiliary analysis examined the linkage of depression with momentary pain and affect more broadly, in terms of the association of depressive symptoms with both mean levels of affect and pain as well as their variability (SDs) across the 28 data points.
Results
Covariates
Ordinary least squares regression of CES-D scores onto demographic (age, sex, race, marital status, education, site) and health (number of health conditions other than OA, OA in one or both knees, count of other joints affected by OA) variables yielded a significant equation, R = 0.477, adj. R2 = 0.227, F (9, 311) = 10.152, p < .001, driven by health conditions (β = 0.276, p < .002), age (β = −0.255, p < .001), and education (β = −0.113, p < .05); race was a marginal predictor (β = −0.095, p < .08).
In multilevel models, health conditions were a significant predictor of all three momentary variables: PA b = −0.059, SE = 0.016, p < .001; NA b = 0.039, SE = 0.009, p < .001; and pain b = 0.050, SE = 0.017, p < .004. Age similarly predicted all three: PA b = −0.013, SE = 0.004, p < .002; NA b = −0.006, SE = 0.002, p < .004; and pain b = −0.014, SE = 0.004, p < .002. Education was associated only with momentary positive affect, b = 0.070, SE = 0.023, p < .002, and joint count only with momentary pain, b = 0.116, SE = 0.023, p < .001.
Based on these findings, all subsequent analyses controlled effects of age, education, health conditions, and number of joints affected by OA. Race was only marginally associated with one outcome; marital status and study site showed no independent linkages with any of the primary variables. Those three covariates were therefore dropped from further analyses.
Contemporaneous Associations of Depression With Momentary Pain and Affect
A series of multilevel models examined the main effects of depression on momentary pain, PA, and NA, as well as interactions between depression and pain (predicting affect) and depression and affect (predicting pain).
For momentary positive affect, an initial null model confirmed sufficient within-person variance to use a multilevel approach, intraclass correlation (ICC) = 0.645, indicating that 35.5% of variance occurred within individuals, −2LL = 11,978.98. In Model 1, covariates explained significant variance in PA, LRT = 115.694, df = 4, p < .001, reflecting a negative influence of health conditions, and positive associations for age and education (upper portion of Table 2). Adding momentary pain and person-level depression significantly improved prediction of PA, Model 2 LRT = 120.225, df = 2, p < .001. Both predictors were negatively associated with momentary PA. Momentary pain did not interact with depression, Model 3 LRT < 1, n.s.
Table 2.
Contemporaneous Associations Among Momentary OA Pain and Affect as a Function of Global Depressive Symptoms
| Model 1 | Model 2 | Model 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| b | SE | p | b | SE | p | b | SE | p | |
| Positive affect | |||||||||
| Age | 0.013 | 0.004 | .001 | 0.005 | 0.004 | .212 | 0.005 | 0.004 | .213 |
| Education | 0.070 | 0.023 | .003 | 0.050 | 0.021 | .017 | 0.050 | 0.021 | .016 |
| Health conditions | −0.065 | 0.016 | <.001 | −0.027 | 0.015 | .068 | −0.027 | 0.015 | .069 |
| Joint count | 0.004 | 0.021 | .834 | 0.025 | 0.019 | .176 | 0.025 | 0.019 | .179 |
| Pain | −0.048 | 0.007 | <.001 | −0.042 | 0.012 | <.001 | |||
| Depression | −0.029 | 0.004 | <.001 | −0.028 | 0.004 | <.001 | |||
| Pain × Depression | <−0.001 | 0.001 | .486 | ||||||
| Negative affect | |||||||||
| Age | −0.006 | 0.001 | .006 | 0.0005 | 0.002 | .799 | 0.001 | 0.002 | .720 |
| Education | 0.006 | 0.013 | .632 | 0.021 | 0.010 | .034 | 0.016 | 0.010 | .093 |
| Health conditions | 0.039 | 0.009 | <.001 | 0.011 | 0.007 | .142 | 0.010 | 0.007 | .152 |
| Joint count | 0.012 | 0.012 | .287 | −0.007 | 0.009 | .440 | −0.005 | 0.009 | .555 |
| Pain | 0.067 | 0.005 | <.001 | −0.008 | 0.008 | .334 | |||
| Depression | 0.021 | 0.002 | <.001 | 0.008 | 0.002 | <.001 | |||
| Pain × Depression | 0.005 | <0.001 | <.001 | ||||||
| Pain | |||||||||
| Age | −0.015 | 0.004 | .001 | −0.007 | 0.004 | .102 | −0.007 | 0.004 | .109 |
| Education | −0.028 | 0.025 | .267 | −0.012 | 0.022 | .597 | −0.015 | 0.022 | .494 |
| Health conditions | 0.047 | 0.017 | .006 | 0.007 | 0.016 | .665 | 0.004 | 0.016 | .785 |
| Joint count | 0.118 | 0.022 | <.001 | 0.103 | 0.020 | <.001 | 0.103 | 0.020 | <.001 |
| Positive affect | −0.058 | 0.018 | .002 | −0.062 | 0.027 | .024 | |||
| Negative affect | 0.288 | 0.025 | <.001 | 0.138 | 0.045 | .002 | |||
| Depression | 0.021 | 0.004 | <.001 | 0.011 | 0.007 | .124 | |||
| PA × Depression | <−0.001 | 0.002 | .913 | ||||||
| NA × Depression | 0.007 | 0.002 | <.001 |
Notes: OA = osteoarthritis; PA = positive affect; NA = negative affect. See text for details of the analysis.
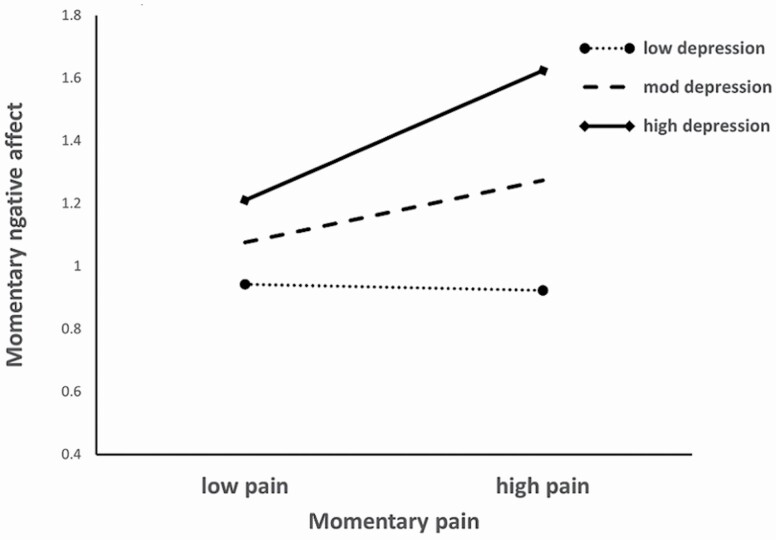
Parallel analysis of momentary negative affect yielded an ICC of 0.509, again recommending a nested model, −2LL = 7,462.58. Covariates enhanced the model, LRT = 131.90, df = 4, p < .001, reflecting a negative association of age with NA and a positive one of health conditions (middle portion of Table 2). Model 2, including momentary pain and person-level depression, explained significant additional variance in momentary NA, LRT = 297.45, df = 2, p < .001. Both predictors were positively related to momentary NA. In contrast to PA, adding the Pain × Depression interaction term significantly improved the NA model, Model 3 LRT =132.75, df = 1, p < .001. As Figure 1 indicates, depression intensifies the momentary linkage of pain with NA.
Figure 1.
Moderating effects of depression on the association of momentary pain with momentary negative affect.
For momentary pain, the null model yielded an ICC of 0.471, −2LL = 18,547.81. A significant effect of covariates, LRT = 207.20, df = 4, p < .001, was driven by the positive linkages of age, health conditions, and joint count with pain (lower portion of Table 2). Model 2 also significantly increased explained variance, LRT = 229.44, df = 3, p < .001, reflecting logical associations of momentary pain with PA and NA as well as global depressive symptoms. Entry of interaction terms again produced significant change, Model 3 RT = 16.89, df = 2, p < .001, reflecting a modifying effect of depression on the momentary relation of pain to NA. The effect mirrors that for the analysis with NA as the outcome, that is, the pain–affect linkage is stronger among persons higher in depression.
Lagged Associations of Depression With Momentary Pain and Affect
The second set of analyses replicated those just described but used lagged models to explore potential causal flow among momentary pain and affect. Specifically, we examined pain and affect at the current moment as predictors of change in affect and pain, respectively, over the period between ESM calls. Because null models and those containing covariates essentially replicated those reported above, we present here only Models 2 (main effects) and 3 (moderating effects of depression). The results of the three analyses are summarized in Table 3.
Table 3.
Lagged Associations Among Momentary Osteoarthritis Pain and Affect as a Function of Global Depressive Symptoms
| Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|
| b | SE | p | b | SE | p | |
| Positive affect at next call | ||||||
| Age | 0.003 | 0.003 | .250 | 0.003 | 0.003 | .252 |
| Education | 0.037 | 0.016 | .021 | 0.038 | 0.016 | .020 |
| Health conditions | −0.021 | 0.011 | .069 | −0.021 | 0.011 | .070 |
| Joint count | 0.013 | 0.014 | .363 | 0.013 | 0.014 | .369 |
| Current positive affect | 0.242 | 0.012 | <.001 | 0.242 | 0.012 | <.001 |
| Current pain | −0.001 | 0.008 | .886 | 0.004 | 0.013 | .760 |
| Depression | −0.023 | 0.003 | <.001 | −0.023 | 0.003 | <.001 |
| Pain × depression | <−0.001 | 0.001 | .608 | |||
| Negative affect at next call | ||||||
| Age | 0.0003 | 0.001 | .839 | <0.001 | 0.001 | .799 |
| Education | 0.016 | 0.007 | .034 | 0.014 | 0.007 | .048 |
| Health conditions | 0.009 | 0.005 | .099 | 0.009 | 0.005 | .102 |
| Joint count | −0.003 | 0.007 | .664 | −0.002 | 0.007 | .719 |
| Current negative affect | 0.298 | 0.012 | <.001 | 0.296 | 0.012 | <.001 |
| Current pain | 0.014 | 0.006 | .016 | −0.006 | 0.009 | .471 |
| Depression | 0.016 | 0.001 | .<.001 | 0.013 | 0.002 | <.001 |
| Pain × depression | 0.001 | <0.001 | .002 | |||
| Pain at next call | ||||||
| Age | −0.006 | 0.003 | .073 | −0.005 | 0.003 | .078 |
| Education | −0.003 | 0.017 | .855 | −.005 | 0.017 | .780 |
| Health conditions | 0.010 | 0.012 | .411 | 0.009 | 0.012 | .447 |
| Joint count | 0.078 | 0.015 | <.001 | 0.079 | 0.015 | <.001 |
| Current pain | 0.252 | 0.012 | <.001 | 0.252 | 0.012 | .<.001 |
| Current positive affect | 0.049 | 0.019 | .010 | 0.079 | 0.028 | .005 |
| Current negative affect | 0.050 | 0.027 | .066 | 0.054 | 0.048 | .265 |
| Depression | 0.023 | 0.003 | <.001 | 0.030 | 0.007 | <.001 |
| PA × depression | −0.002 | 0.002 | .137 | |||
| NA × depression | <−0.001 | 0.002 | .859 |
Notes: PA = positive affect; NA = negative affect. See text for details of the analysis.
For future (next call) PA, Model 2 yielded a significant positive association with education and a marginal negative one with health conditions, −2LL = 9,463.91. Of central interest, future PA was also significantly associated with current PA and global depression, but not with current pain. Additional of the Pain × Depression interaction term did not improve the model, Model 3 LRT < 1, n.s.
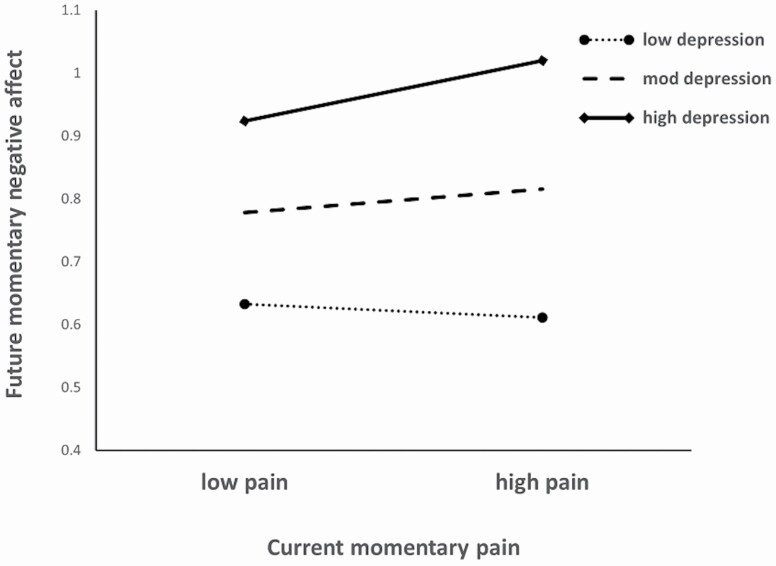
Future NA was significantly positively associated with education and marginally with health conditions, −2LL = 5,415.46. Current NA and pain, as well as depressive symptoms, also explained significant variance in future NA; all coefficients were positive. Model 3, testing the interaction of pain with depression upon future NA, was a significant improvement, LRT = 9.00, df = 1, p < .003. As Figure 2 indicates, the pattern was similar to but less pronounced than that for the contemporaneous pain–NA relationship depicted in Figure 1.
Figure 2.
Moderating effects of depression on the association of current pain with future negative affect.
The model for pain at the next call, −2LL = 14,851.61, displayed a significant positive effect of joint count and a marginal one of age (lower portion of Table 3). Current pain and PA, along with person-level depression, were also significant contributors; previous NA was marginal. Interactions of depression with current PA and NA did not improve prediction of next-call pain, Model 3 LRT = 2.23, df = 2, n.s.
To test robustness of these effects, we repeated all lagged analyses using the second call after current, for example, PA as a predictor of pain roughly 6–8 h later. Patterns of associations paralleled those for the shorter time lag with one minor exception. Specifically, unlike the shorter time lag, there was no significant relation of current pain to pain two calls previously. All other associations mirrored patterns of significance presented in Table 3. Details of these analyses are available on request.
Association of Depression With Summary Indices of Momentary Pain and Affect
A final, auxiliary analysis examined the broader association of depression with momentary pain and affect, in terms of the correlation of CES-D scores with within-person 28-call average and variability (SD) in momentary pain, NA, and PA. Depressive symptoms were significantly correlated with all three mean indices, pain r = 0.490, NA r = 0.626, PA r = –0.519, all ps < .001. Comparison of coefficients indicated that the three correlations did not differ from one another (Lee & Preacher, 2013). Depression was similarly associated with greater variability in all momentary measures, pain r = 0.397, NA r = 0.570, PA r = 0.296, all ps < .001. Comparison of coefficients indicated that depression was more strongly associated with variability in NA than with SDs for pain, z = 3.364, p < .001, or for PA, z = −5.318, p < .001. The differential correlation for pain SD versus PA SD was not significant, z = −1.687, p > .09.
Discussion
Taken together, these findings form a compelling illustration of how depressive symptoms are related to the everyday experience of OA pain and its impact on short-term affective states. Both global depressive symptoms at baseline and current PA and NA were related to momentary pain; conversely, current affect was associated with both depression and pain at that moment. With one exception, these patterns were replicated with respect to change in pain and affect from one call to the next. Global depression at baseline predicted short-term change in both affect and pain. Similarly, both PA and NA in the moment were logically associated with greater pain at the next call, and momentary pain predicted increased next-call NA. However, current pain did not predict change in positive affect.
These patterns confirm the well-documented association of depressive symptoms with pain both in the laboratory (see review by Othman et al., 2021) and in vivo in microlongitudinal work over varying time intervals (Smith & Zautra, 2008; Graham-Engeland et al., 2016; Mak & Schneider, 2020). They also bolster existing evidence suggesting that momentary pain is more closely associated with negative than with positive affect (Finan et al., 2013), and that this association may be exacerbated by current or previous depression (Conner et al., 2006; Tennen et al., 2006). Auxiliary analyses using summary measures confirm the linkage of global depressive symptoms with increased general pain and affective tone (i.e., 28-point means) and with within-day variability in pain and affect. Whereas this relationship was comparable for mean levels of pain and affect, depressive symptoms at baseline were more strongly linked with variability in NA than in PA or momentary pain. This replicates others’ findings that diagnosed depressive disorders are characterized not by the persistence of anhedonia and negative mood but by broad fluctuations specifically in NA (Bijlsma et al., 2011; Hall et al., 1991; Lawton et al., 1996). These more pronounced “ups and downs” in NA vis-à-vis less depressed individuals may contribute to the overall emotional impact of pain. In fact, they may be one factor underlying the stronger association of momentary pain and NA among more depressed individuals.
Our findings extend existing knowledge by demonstrating the role of global depressive symptoms as a moderator of the association of momentary pain with affective states both contemporaneously and over relatively brief periods of time. Notably, this effect was stronger for NA than for PA. That is, although both PA and NA were contemporaneously associated with momentary OA pain, the linkage was moderated by depression only for NA. That pattern is maintained for lagged relationships, suggesting that more depressed individuals are more likely to experience a “hangover” of pain on momentary affect over a period of at least a few hours. Interestingly, the reverse temporal pattern did not emerge, that is, current NA was only weakly associated with short-term increases in pain and did not interact with depressive symptoms to amplify pain over the next few hours. This is in contrast to more traditional work indicating not only that global depression at baseline evaluation predicts greater pain over time but that the relationship may be bidirectional (Chou, 2007; Hawker et al., 2011; Kroenke et al., 2011; Schieir et al., 2009). On the other hand, it confirms the work of Conners et al. (2006) and others regarding lagged associations over periods of hours or days. This differentiation again underscores the importance of examining these associations “in everyday life” rather than solely through global self-reports.
In contrast to NA, positive affect was less complexly related to momentary OA pain. To be sure, contemporaneous linkages were observed, reflecting lower PA at times when pain is more intense. Additionally, global depressive symptoms were associated with maintenance of PA over periods of several hours. However, depression did not color the PA–pain association either contemporaneously or over time.
Several limitations must be considered in interpreting these results. Perhaps most important conceptually is the fact that we did not control NA in analyses of the association of PA with pain and of pain with PA. There has been considerable discussion of the role of PA in buffering pain and its association with NA (Finan & Garland, 2015; Zautra et al., 2001). Because of the complexity of our central focus on the role of global depression, we did not test that effect directly in the current analyses. However, we did enter both PA and NA in models predicting pain, effectively replicating the approach of Graham-Engeland et al. (2016) and confirming the independent relations of both with momentary pain. An important direction for future analyses is to continue exploration of the complex short-term linkages among pain, PA, and NA, and how those linkages are influenced by more global indicators of personality and emotional well-being.
It is encouraging that next-call relationships were, with the minor exception of the autocorrelation of pain over time, confirmed for a two-call lag, that is, over a period generally spanning 6–8 h. Note also that we included cross-day lags, covering a period of 12–16 h; this bolsters confidence that observed associations reflect more than simple autocorrelation of variables over time. Nonetheless, the robustness of these potential short-term causal dynamics needs substantiation.
Another crucial aspect of the momentary pain–affect experience that was not explored in our own (or, for that matter, most other investigators’) analysis is the role of activities and events in modulating both affect and the experience of pain. A long history of theory and research, beginning with Lewinsohn and Graf’s (1973) general work and extending to pain-specific studies (Bamonti & Fiske, 2021; Hausmann et al., 2014; Machado et al., 2008), illustrates the importance of pleasant experiences and activities for maintaining emotional well-being over diverse time periods. This is especially relevant for older adults with OA given clear evidence that having and maintaining favored leisure activities can buffer the depressive effects of OA pain (Neugebauer et al., 2003; Parmelee et al., 2007; Zimmer et al., 1997). The bulk of extant work has examined this at a global level, linking self-reports of general leisure activities to summary assessments of pain, depression, and general mood states over periods of weeks or months. To be sure, an emerging literature documents the association of physical activity with emotional well-being in both traditional (Lee & Russell, 2003; McAuley & Rudolph, 1995) and microlongitudinal contexts (Emerson et al., 2018). However, future research should examine immediate linkages among pain, affect, and activities—particularly, the type and quality of those activities—and how those linkages are associated with more global mental health.
These caveats aside, the current analyses confirm the dynamic linkage of pain and affect over short periods, and the role of more long-term depressive symptoms in moderating that linkage among older adults with OA. Building on previous research on the association of depression with OA pain (Agustini et al., 2020; Parmelee et al., 2007; Rathbun et al., 2020; Veronese et al., 2017), our data suggest that this association may be driven at least in part by the role of depression in exaggerating negative response to pain in everyday context. From a clinical perspective, these findings highlight the importance of therapeutic approaches that modulate affective responses to pain, for example, acceptance and commitment therapy and other mindfulness-based interventions. Such interventions may be especially important for older persons due to the documented role of depression in hastening not only OA-related functional decline (Hawker et al., 2011; Parmelee et al., 2013) but even radiographically assessed progression of the disease (Rathbun et al., 2017).
Acknowledgments
We gratefully acknowledge the assistance of Catherine Polster, Jessica Greenlee, and the many graduate and undergraduate students who assisted with data collection and management.
Contributor Information
Patricia A Parmelee, Alabama Research Institute on Aging and Department of Psychology, The University of Alabama, Tuscaloosa, Alabama, USA.
Emily A Behrens, Alabama Research Institute on Aging and Department of Psychology, The University of Alabama, Tuscaloosa, Alabama, USA.
Kyrsten Costlow Hill, Alabama Research Institute on Aging and Department of Psychology, The University of Alabama, Tuscaloosa, Alabama, USA.
Brian S Cox, Alabama Research Institute on Aging and Department of Psychology, The University of Alabama, Tuscaloosa, Alabama, USA.
Jason A DeCaro, Department of Anthropology, The University of Alabama, Tuscaloosa, Alabama, USA.
Francis J Keefe, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham, North Carolina, USA.
Dylan M Smith, Program in Public Health and Department of Family, Population, and Preventive Medicine, Stony Brook University, Stony Brook, New York, USA.
Funding
This work was supported by the National Institute on Aging grant R01 AG041655 (P. A. Parmelee and D. M. Smith, co-principal investigators).
Conflict of Interest
None declared.
Data Availability
Data and details of these analyses are available on request from the first author. This study was not preregistered.
References
- Affleck, G., Tennen, H., Urrows, S., & Higgins, P. (1992). Neuroticism and the pain–mood relation in rheumatoid arthritis: Insights from a prospective daily study. Journal of Consulting and Clinical Psychology, 60(1), 119–126. doi: 10.1037//0022-006x.60.1.119 [DOI] [PubMed] [Google Scholar]
- Agustini, B., Lotfaliany, M., Woods, R. L., McNeil, J. J., Nelson, M. R., Shah, R. C., Murray, A. M., Ernst, M. E., Reid, C. M., Tonkin, A., Lockery, J. E., Williams, L. J., Berk, M., & Mohebbi, M.; ASPREE Investigator Group . (2020). Patterns of association between depressive symptoms and chronic medical morbidities in older adults. Journal of the American Geriatrics Society, 68(8), 1834–1841. doi: 10.1111/jgs.16468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Baar, M. E., Dekker, J., Lemmens, J. A., Oostendorp, R. A., & Bijlsma, J. W. (1998). Pain and disability in patients with osteoarthritis of hip or knee: The relationship with articular, kinesiological, and psychological characteristics. The Journal of Rheumatology, 25(1), 125–133. [PubMed] [Google Scholar]
- Bamonti, P. M., & Fiske, A. (2021). Engaging in pleasant events explains the relation between physical disability and mental health outcomes in older adults. Aging & Mental Health, 25(2), 225–233. doi: 10.1080/13607863.2019.1683811 [DOI] [PubMed] [Google Scholar]
- Barbour, K. E., Helmick, C., Theis, K., Murphy, L.B., Hootman, J. M., & Brady, T. J. (2013). Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation—United States, 2010–2012. Morbidity & Mortality Weekly Report, 62(44), 869–873. doi:10.15585%2Fmmwr.mm6609e1 [PMC free article] [PubMed] [Google Scholar]
- Berna, C., Leknes, S., Holmes, E. A., Edwards, R. R., Goodwin, G. M., & Tracey, I. (2010). Induction of depressed mood disrupts emotion regulation neurocircuitry and enhances pain unpleasantness. Biological Psychiatry, 67(11), 1083–1090. doi: 10.1016/j.biopsych.2010.01.014 [DOI] [PubMed] [Google Scholar]
- Bijlsma, L. M., Taylor-Clift, A., & Rottenberg, J. (2011). Emotional reactivity to daily events in major and minor depression. Journal of Abnormal Psychology, 120(1), 155–167. doi: 10.1037/a0021662 [DOI] [PubMed] [Google Scholar]
- Chou, K. L. (2007). Reciprocal relationship between pain and depression in older adults: Evidence from the English Longitudinal Study of Ageing. Journal of Affective Disorders, 102(1–3), 115–123. doi: 10.1016/j.jad.2006.12.013 [DOI] [PubMed] [Google Scholar]
- Conner, T. S., Tennen, H., Zautra, A. J., Affleck, G., Armeli, S., & Fifield, J. (2006). Coping with rheumatoid arthritis pain in daily life: Within-person analyses reveal hidden vulnerability for the formerly depressed. Pain, 126(1–3), 198–209. doi: 10.1016/j.pain.2006.06.033 [DOI] [PubMed] [Google Scholar]
- Davis, M. A., Ettinger, W. H., Neuhaus, J. M., Barclay, J. D., & Segal, M. R. (1992). Correlates of knee pain among US adults with and without radiographic knee osteoarthritis. The Journal of Rheumatology, 19(12), 1943–1949. [PubMed] [Google Scholar]
- Emerson, J. A., Dunsiger, S., & Williams, D. M. (2018). Reciprocal within-day associations between incidental affect and exercise: An EMA study. Psychology & Health, 33(1), 130–143. doi: 10.1080/08870446.2017.1341515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan, P. H., & Garland, E. L. (2015). The role of positive affect in pain and its treatment. The Clinical Journal of Pain, 31(2), 177–187. doi: 10.1097/AJP.0000000000000092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan, P. H., Quartana, P. J., & Smith, M. T. (2013). Positive and negative affect dimensions in chronic knee osteoarthritis: Effects on clinical and laboratory pain. Psychosomatic Medicine, 75(5), 463–470. doi: 10.1097/PSY.0b013e31828ef1d6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil, K. M., Carson, J. W., Porter, L. S., Scipio, C., Bediako, S. M., & Orringer, E. (2004). Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health Psychology, 23(3), 267–274. doi: 10.1037/0278-6133.23.3.267 [DOI] [PubMed] [Google Scholar]
- Graham-Engeland, J. E., Zawadzki, M. J., Slavish, D. C., & Smyth, J. M. (2016). Depressive symptoms and momentary mood predict momentary pain among rheumatoid arthritis patients. Annals of Behavioral Medicine, 50(1), 12–23. doi: 10.1007/s12160-015-9723-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, D. P.Jr, Sing, H. C., & Romanoski, A. J. (1991). Identification and characterization of greater mood variance in depression. The American Journal of Psychiatry, 148(10), 1341–1345. doi: 10.1176/ajp.148.10.1341 [DOI] [PubMed] [Google Scholar]
- Hausmann, L. R., Parks, A., Youk, A. O., & Kwoh, C. K. (2014). Reduction of bodily pain in response to an online positive activities intervention. The Journal of Pain, 15(5), 560–567. doi: 10.1016/j.jpain.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Hawker, G. A., Gignac, M. A., Badley, E., Davis, A. M., French, M. R., Li, Y., Perruccio, A. V., Power, J. D., Sale, J., & Lou, W. (2011). A longitudinal study to explain the pain–depression link in older adults with osteoarthritis. Arthritis Care & Research, 63(10), 1382–1390. doi: 10.1002/acr.20298 [DOI] [PubMed] [Google Scholar]
- Hootman, J. M., Helmick, C. G., & Brady, T. J. (2012). A public health approach to addressing arthritis in older adults: The most common cause of disability. American Journal of Public Health, 102(3), 426–433. doi: 10.2105/AJPH.2011.300423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katana, M., Röcke, C., & Allemand, M. (2020). Intra- and interindividual differences in the within-person coupling between daily pain and affect of older adults. Journal of Behavioral Medicine, 43(5), 707–722. doi: 10.1007/s10865-019-00099-0 [DOI] [PubMed] [Google Scholar]
- Kendler, K. S., Thornton, L. M., & Gardner, C. O. (2000). Stressful life events and previous episodes in the etiology of major depression in women: An evaluation of the “kindling” hypothesis. The American Journal of Psychiatry, 157(8), 1243–1251. doi: 10.1176/appi.ajp.157.8.1243 [DOI] [PubMed] [Google Scholar]
- Kroenke, K., Wu, J., Bair, M. J., Krebs, E. E., Damush, T. M., & Tu, W. (2011). Reciprocal relationship between pain and depression: A 12-month longitudinal analysis in primary care. The Journal of Pain, 12(9), 964–973. doi: 10.1016/j.jpain.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers, F., Swendsen, J., Cui, L., Husky, M., Johns, J., Zipunnikov, V., & Merikangas, K. R. (2018). Mood reactivity and affective dynamics in mood and anxiety disorders. Journal of Abnormal Psychology, 127(7), 659–669. doi: 10.1037/abn0000378 [DOI] [PubMed] [Google Scholar]
- Lawton, M. P., Parmelee, P. A., Katz, I. R., & Nesselroade, J. (1996). Affective states in normal and depressed older people. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 51(6), 309–316. doi: 10.1093/geronb/51b.6.p309 [DOI] [PubMed] [Google Scholar]
- Lee, I. A., & Preacher, K. J. (2013). Calculation for the test of the difference between two dependent correlations with one variable in common [Computer software].http://quantpsy.org
- Lee, C., & Russell, A. (2003). Effects of physical activity on emotional well-being among older Australian women: Cross-sectional and longitudinal analyses. Journal of Psychosomatic Research, 54(2), 155–160. doi: 10.1016/s0022-3999(02)00414-2 [DOI] [PubMed] [Google Scholar]
- Lewinsohn, P. M., & Graf, M. (1973). Pleasant activities and depression. Journal of Consulting and Clinical Psychology, 41(2), 261–268. doi: 10.1037/h0035142 [DOI] [PubMed] [Google Scholar]
- Loggia, M. L., Mogil, J. S., & Bushnell, M. C. (2008). Experimentally induced mood changes preferentially affect pain unpleasantness. The Journal of Pain, 9(9), 784–791. doi: 10.1016/j.jpain.2008.03.014 [DOI] [PubMed] [Google Scholar]
- Machado, G. P., Gignac, M. A., & Badley, E. M. (2008). Participation restrictions among older adults with osteoarthritis: A mediated model of physical symptoms, activity limitations, and depression. Arthritis and Rheumatism, 59(1), 129–135. doi: 10.1002/art.23259 [DOI] [PubMed] [Google Scholar]
- Mak, H. W., & Schneider, S. (2020). Individual differences in momentary pain–affect coupling and their associations with mental health in patients with chronic pain. Journal of Psychosomatic Research, 138, 110227. doi: 10.1016/j.jpsychores.2020.110227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley, E., & Rudolph, D. (1995) Physical activity, aging, and psychological well-being. Journal of Aging and Physical Activity, 3(1), 67–98. doi: 10.1123/japa.3.1.67 [DOI] [Google Scholar]
- Neugebauer, A., Katz, P. P., & Pasch, L. A. (2003). Effect of valued activity disability, social comparisons, and satisfaction with ability on depressive symptoms in rheumatoid arthritis. Health Psychology, 22(3), 253–262. doi: 10.1037/0278-6133.22.3.253 [DOI] [PubMed] [Google Scholar]
- Othman, R., Jayakaran, P., Swain, N., Dassanayake, S., Tumilty, S., & Mani, R. (2021). Relationships between psychological, sleep, and physical activity measures and somatosensory function in people with peripheral joint pain: A systematic review and meta-analysis. Pain Practice, 21(2), 226–261. doi: 10.1111/papr.12943 [DOI] [PubMed] [Google Scholar]
- Parmelee, P. A., Harralson, T. L., McPherron, J. A., & Schumacher, H. R. (2013). The structure of affective symptomatology in older adults with osteoarthritis. International Journal of Geriatric Psychiatry, 28(4), 393–401. doi: 10.1002/gps.3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmelee, P. A., Harralson, T. L., Smith, L. A., & Schumacher, H. R. (2007). Necessary and discretionary activities in knee osteoarthritis: Do they mediate the pain–depression relationship? Pain Medicine (Malden, Mass.), 8(5), 449–461. doi: 10.1111/j.1526-4637.2007.00310.x [DOI] [PubMed] [Google Scholar]
- Pfeiffer, E. (1975). A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. Journal of the American Geriatrics Society, 23, 433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x [DOI] [PubMed] [Google Scholar]
- Preacher, K. J., Curran, P. J., & Bauer, D. J. (2006). Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics, 31, 437–448. doi:10.3102%2F10769986031004437 [Google Scholar]
- Radloff, L. S. (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psycholological Measurement, 1, 385–401. doi:10.1177%2F014662167700100306 [Google Scholar]
- Rathbun, A. M., Yau, M. S., Shardell, M.., Stuart, E. A., & Hochberg, M C. (2017). Depressive symptoms and structural disease progression in knee osteoarthritis: data from the Osteoarthritis Initiative. Clinical Rheumatology, 36, 155–163. doi:1007/s10067-016-3495-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbun, A. M., Shardell, M. D., Ryan, A. S., Yau, M. S., Gallo, J. J., Schuler, M. S., Stuart, E. A., & Hochberg, M. C. (2020). Association between disease progression and depression onset in persons with radiographic knee osteoarthritis. Rheumatology (Oxford, England), 59(11), 3390–3399. doi: 10.1093/rheumatology/keaa141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaffi, F., Cavalieri, F., Nolli, M., & Ferraccioli, G. (1991). Analysis of disability in knee osteoarthritis. Relationship with age and psychological variables but not with radiographic score. The Journal of Rheumatology, 18(10), 1581–1586. [PubMed] [Google Scholar]
- Schieir, O., Thombs, B. D., Hudson, M., Taillefer, S., Steele, R., Berkson, L., Bertrand, C., Couture, F., Fitzcharles, M. A., Gagné, M., Garfield, B., Gutkowski, A., Kang, H., Kapusta, M., Ligier, S., Mathieu, J. P., Ménard, H., Mercille, S., Starr, M., … Baron, M. (2009). Symptoms of depression predict the trajectory of pain among patients with early inflammatory arthritis: A path analysis approach to assessing change. The Journal of Rheumatology, 36(2), 231–239. doi: 10.3899/jrheum.080147 [DOI] [PubMed] [Google Scholar]
- Smith, D. M., & Parmelee, P. A. (2016). Within-day variability of fatigue and pain among African Americans and non-Hispanic Whites with osteoarthritis of the knee. Arthritis Care & Research, 68(1), 115–122. doi: 10.1002/acr.22690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, B. W., & Zautra, A. J. (2008). The effects of anxiety and depression on weekly pain in women with arthritis. Pain, 138(2), 354–361. doi: 10.1016/j.pain.2008.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, N. K. Y., Salkovskis, P. M., Hodges, A., Wright, K. J., Hanna, M., & Hester, J. (2008). Effects of mood on pain responses and pain tolerance: An experimental study in chronic back pain patients. Pain, 138(2), 392–401. doi: 10.1016/j.pain.2008.01.018 [DOI] [PubMed] [Google Scholar]
- Tennen, H., Affleck, G., & Zautra, A. (2006). Depression history and coping with chronic pain: A daily process analysis. Health Psychology, 25(3), 370–379. doi: 10.1037/0278-6133.25.3.370 [DOI] [PubMed] [Google Scholar]
- Vendrig, A. A., & Lousberg, R. (1997). Within-person relationships among pain intensity, mood and physical activity in chronic pain: A naturalistic approach. Pain, 73(1), 71–76. doi: 10.1016/s0304-3959(97)00075-4 [DOI] [PubMed] [Google Scholar]
- Veronese, N., Stubbs, B., Solmi, M., Smith, T. O., Noale, M., Cooper, C., & Maggi, S. (2017). Association between lower limb osteoarthritis and incidence of depressive symptoms: Data from the osteoarthritis initiative. Age and Ageing, 46(3), 470–476. doi: 10.1093/ageing/afw216 [DOI] [PubMed] [Google Scholar]
- Zautra, A. J., Fasman, R., Parish, B. P., & Davis, M. C. (2007). Daily fatigue in women with osteoarthritis, rheumatoid arthritis, and fibromyalgia. Pain, 128(1–2), 128–135. doi: 10.1016/j.pain.2006.09.004 [DOI] [PubMed] [Google Scholar]
- Zautra, A. J., Johnson, L. M., & Davis, M. C. (2005). Positive affect as a source of resilience for women in chronic pain. Journal of Consulting and Clinical Psychology, 73(2), 212–220. doi: 10.1037/0022-006X.73.2.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra, A., Smith, B., Affleck, G., & Tennen, H. (2001). Examinations of chronic pain and affect relationships: Applications of a dynamic model of affect. Journal of Consulting and Clinical Psychology, 69(5), 786–795. doi: 10.1037//0022-006x.69.5.786 [DOI] [PubMed] [Google Scholar]
- Zimmer, Z., Hickey, T., & Searle, M. S. (1997). The pattern of change in leisure activity behavior among older adults with arthritis. The Gerontologist, 37(3), 384–392. doi: 10.1093/geront/37.3.384 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and details of these analyses are available on request from the first author. This study was not preregistered.