Abstract
Background
Patients with KRAS-mutant cancers have limited treatment options. Here we present a phase I study of JNJ-74699157, an oral, selective, covalent inhibitor of the KRAS G12C isoform, in patients with advanced cancer harboring the KRAS G12C mutation.
Methods
Eligible patients (aged ≥18 years) who had previously received or were ineligible for standard treatment received JNJ-74699157 once daily on a 21-day cycle. Dose escalation was guided by a modified continual reassessment method.
Results
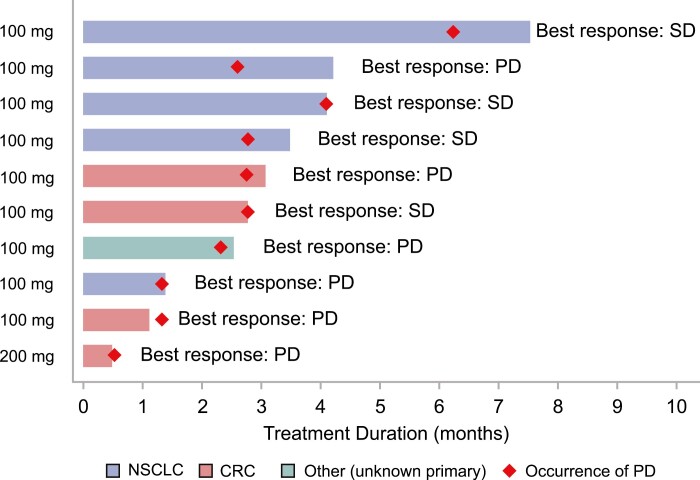
Ten patients (100 mg: 9 and 200 mg: 1) were enrolled. Tumor types included non–small cell lung cancer (n = 5), colorectal cancer (n = 4), and carcinoma of unknown primary site (n = 1). The median age was 65 (range: 36-74) years and median treatment duration was 2.91 (range: 0.5-7.5) months. Dose-limiting toxicities of grades 3–4 increased blood creatinine phosphokinase (CPK) were observed in 100 mg and 200 mg dose levels. The most common adverse event was increased blood CPK (6 patients). No significant clinical benefit was observed; the best response was stable disease in 4 patients (40%).
Conclusion
Based on dose-limiting skeletal muscle toxicities and the lack of efficacy at the 100 mg dose, further enrollment was stopped. The safety profile of JNJ-74699157 was not considered favorable for further clinical development.
ClinicalTrials.gov Identifier
Keywords: advanced solid tumors, JNJ-74699157, KRAS G12C mutation, pharmacokinetics, safety
Patients with KRAS-mutant cancers have limited treatment options. This article presents results of a phase I study of JNJ-74699157, an oral, selective, covalent inhibitor of the KRAS G12C isoform, in patients with advanced cancer harboring the KRAS G12C mutation.
Lessons Learned.
Inhibition of KRAS G12C is a potential therapeutic strategy for advanced stage KRAS G12C-mutant solid tumors.
In this phase I, multicenter, open-label study in advanced solid tumors harboring the KRAS G12C mutation, JNJ-74699157 monotherapy was associated with dose-limiting skeletal muscle toxicities and showed lack of efficacy at the maximum tolerated dose level of 100 mg.
In view of these findings, further enrollment was stopped and no future development of JNJ-74699157 is planned.
Discussion
KRAS, previously considered undruggable, has recently become tractable through the development of covalent inhibitors binding to the switch II pocket of KRAS G12C. To date, two KRAS G12C-specific inhibitors, sotorasib and adagrasib, have shown clinical benefit in patients with previously treated, advanced stage KRAS G12C-mutant solid tumors.1,2 JNJ-74699157 (formerly referred to as ARS-3248) covalently binds to the KRAS G12C-GDP complex near the switch II pocket by reacting with the mutant cysteine 12, thereby impeding downstream signaling of KRAS G12C.3-5 JNJ-74699157 is highly selective for the tumor-specific KRAS G12C protein, with no detectable activity against wild-type KRAS or other proteins.
This phase I study was conducted to determine the safety, pharmacokinetics, and preliminary antitumor activity of JNJ-74699157 monotherapy in adult patients with advanced solid tumors harboring the KRAS G12C mutation who previously received or were ineligible for standard treatment options.
A total of 10 patients (100 mg, n = 9 and 200 mg, n = 1) were enrolled between September, 05 2019 and July, 13 2020, and all patients discontinued treatment due to disease progression. Two patients were initially enrolled at 100 mg, and after the first patient completed the dose-limiting toxicity (DLT) period without DLT, the Study Evaluation Team (SET) recommended escalating the dose to 200 mg. One patient was subsequently enrolled at 200 mg, and the patient experienced dose-limiting skeletal muscle toxicity (grade 4 increased blood creatinine phosphokinase [CPK]), which led to the further enrollment of 7 additional patients at 100 mg. In total, 3 patients (2 in the 100 mg and 1 in the 200 mg groups) reported 6 AEs that were considered a DLT. Both patients who experienced DLTs in the 100 mg cohort were managed with treatment interruption, followed by a dose reduction to 50 mg. These DLT improved to a level allowing re-initiation of JNJ-74699157 treatment within 7 days in both cases. The patient in the 200 mg cohort who experienced a DLT was managed with a suspension of therapy, but the treatment was never reinitiated because of disease progression.
All 10 patients reported at least 1 treatment-emergent adverse event (TEAE) and at least 1 treatment-related AE (Table 1). One patient died due to grade 5 hepatic failure secondary to disease progression within 30 days of the last study agent administration. One other patient died from disease progression 129 days after study treatment cessation. No TEAEs led to study agent discontinuation, and no clinically significant ECG changes were reported.
Table 1.
Safety summary (safety analysis set).
| Events, n | JNJ-74699157 100 mg (n = 9) | JNJ-74699157 200 mg (n = 1) |
|---|---|---|
| Patients with ≥1 TEAEs | 9 | 1 |
| Most common TEAEs (≥2 patients) | ||
| Increased blood CPK | 5 | 1 |
| Back pain | 3 | 0 |
| Hyperglycemia | 3 | 0 |
| Increased AST | 2 | 1 |
| Myalgia | 2 | 1 |
| Muscle spasms | 2 | 0 |
| Muscular weakness | 2 | 0 |
| Musculoskeletal pain | 2 | 0 |
| Increased blood ALP | 2 | 0 |
| Increased GGT | 2 | 0 |
| Decreased appetite | 2 | 0 |
| Asthenia | 2 | 0 |
| Fatigue | 2 | 0 |
| Grade ≥3 TEAEs | 5 | 1 |
| Increased blood CPK | 4 | 1 |
| Increased AST | 1 | 1 |
| Back pain | 1 | 0 |
| Hypokalemia | 1 | 0 |
| Hypophosphatemia | 1 | 0 |
| Hepatic failure | 1 | 0 |
| Large intestinal obstruction | 0 | 1 |
| Grade ≥3 related TEAEs | 4 | 1 |
| Increased blood CPK | 4 | 1 |
| Increased AST | 1 | 1 |
| Serious TEAEs | 3 | 1 |
| Increased blood CPK | 2 | 0 |
| Hepatic failurea | 1 | 0 |
| Large intestinal obstruction | 0 | 1 |
| TEAEs leading to death a | 1 | 0 |
Grade 5 serious TEAE of hepatic failure secondary to disease progression.
Abbreviations: ALP, alkaline phosphatase; AST, aspartate aminotransferase; CPK, creatine phosphokinase; GGT, Gamma-glutamyl transferase; TEAE, treatment emergent adverse event.
No patient achieved an objective response. Four of 10 patients had a best overall response of stable disease, and the remaining 6 patients had a best overall response of progressive disease. Only 2 patients were on treatment for >3 months (125 days and 190 days).
Trial Information
| Disease | Advanced cancer/solid tumor only |
| Stage of Disease/Treatment | Metastatic/advanced |
| Prior Therapy | More than 2 prior regimens |
| Type of Study | Phase I, modified continual reassessment method using a Bayesian logistic regression model with escalation with overdose control principle |
| Primary Endpoints | Maximum tolerated dose, recommended phase II dose |
| Secondary Endpoints | Pharmacodynamics, safety |
| Investigator’s Analysis | Due to dose-limiting skeletal muscle toxicities and the lack of efficacy at the maximum tolerated dose of 100 mg, further enrollment was stopped |
Additional Details of Endpoints or Study Design
This was a phase I, multicenter, open-label study (NCT04006301) of JNJ-74699157 monotherapy conducted at 6 sites in France and the United States. The study consisted of 2 parts: a dose-escalation phase (Part 1) to determine the maximum-tolerated dose (MTD) and/or the recommended phase II dose (RP2D), and a dose-expansion phase (Part 2) to determine the safety and preliminary antitumor activity of JNJ-74699157 at the RP2D regimen. In Part 1, the starting dose of 100 mg once daily was administered orally on consecutive days of a 21-day cycle. After the starting dose, subsequent doses were selected by the SET guided by a modified continual reassessment method using a Bayesian logistic regression model with escalation with overdose control principle6,7 and based on the review of all available data including PK, pharmacodynamics, safety, and preliminary antitumor activity. Treatment was administered until unequivocal disease progression, unacceptable toxicity, withdrawal of consent, or investigator or sponsor decision. Study completion was defined as death or completion of the last scheduled study assessment.
In the planned Part 2 dose-expansion phase, the RP2D of JNJ-74699157 determined in Part 1 was to be administered to a larger sample of patients in separate cohorts with NSCLC and other solid tumors harboring the KRAS G12C mutation. Based on findings in Part 1, the dose-expansion phase was not initiated.
The study protocol and amendments were reviewed by Independent Ethics Committees and Institutional Review Boards, and the study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Clinical Practices and applicable regulatory requirements. All patients provided written informed consent before participating in the study.
Patients
Eligible patients were aged ≥18 years with solid tumor malignancies harboring the KRAS G12C mutation as determined by local testing of tumor tissue or blood and had previously received or were ineligible for standard treatment options. Patients were also required to have evaluable or measurable disease according to the response evaluation criteria in solid tumors (RECIST) version 1.1, Eastern Cooperative Oncology Group (ECOG) performance status grade of 0 or 1, hematology and clinical chemistry laboratory values within specified ranges, including CPK ≤2.5X ULN, left ventricular ejection fraction within institutional limits, and QTc ≤470 ms. In addition, patients with NSCLC were required to have received prior platinum-containing chemotherapy, and an anti-programmed death/programmed death-ligand 1 (PD1/PDL1) antibody, and patients with CRC were required to have previously received at least 2 prior lines of therapy, including a fluoropyrimidine, oxaliplatin, and irinotecan.
Patients who had symptomatic brain metastases or known leptomeningeal disease, prior treatment with KRAS G12C inhibitor, prior anticancer therapy or unresolved toxicities from prior anticancer therapies, or received radiotherapy within 14 days before study treatment initiation (except palliative radiotherapy for pain) were excluded. Also excluded were patients with the history of myopathy or myositis unless the condition had resolved, or a history of clinically significant cardiovascular disease.
Study Assessments
Safety
Safety evaluations were based on TEAEs, clinical laboratory test results, cardiac monitoring, including electrocardiograms (ECGs), echocardiograms or multigated acquisition scans, and Holter monitoring; vital sign measurements, physical examination findings, and assessment of ECOG performance status score. Toxicities were graded for severity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0. Patients were followed for 30 days after the last dose of study treatment for TEAE assessment.
The dose-limiting toxicities (DLTs) were assessed during the first cycle (21 days). Non-hematologic toxicities of grade ≥3 except for lab abnormalities were considered DLT with exceptions for grade 3 asthenia, fever, or constipation lasting <7 days or grade 3 nausea, vomiting, or diarrhea lasting <3 days despite the best supportive care. Lab abnormality criteria for DLT included chemistry abnormalities (other than gamma-glutamyl transpeptidase [GGT], alkaline phosphatase [ALP], creatine phosphokinase [CPK], lipase or amylase) grade 3 for >3 days despite best supportive care or fulfilling Hy’s law criteria,8 or grade 4; grade 3 cardiac troponin; CPK grade ≥3 with grade ≥2 muscle weakness, myalgia, or myopathy, or grade 4; grade ≥3 lipase or amylase if associated with clinical or radiological evidence of pancreatitis. Isolated grade 3 or 4 GGT or ALP elevation associated with liver metastases was not a DLT. Hematological toxicities meeting DLT criteria included febrile neutropenia of any grade; grade 4 neutropenia for >3 days; grade 3 platelet count decreased associated with grade ≥2 bleeding or grade 4; grade 4 hemoglobin count decreased; or any hematological toxicity of grade 5. For patients who experienced toxicity that met the criteria of DLT, administration of additional study drug was held until management and resolution of the toxicity.
Pharmacokinetics
Plasma samples were collected at multiple time points and were analyzed to determine concentrations of JNJ-74699157 and the active and equipotent metabolite, JNJ-74964487, using a validated, specific, and sensitive method. Patients were required to fast for 2 h before and 2 h after the dose of study agent on days when a trough PK sample was taken or no PK samples were drawn, or 6 h before and 4 h after the dose on serial PK sampling days. Based on the individual plasma concentration–time data, PK parameters were derived from the bioanalytical results using the nominal sampling times.
Efficacy
Imaging assessments using computed tomography (CT), or magnetic resonance imaging (MRI) of the chest, abdomen, and pelvis were performed every 2 cycles (6 weeks). Response to treatment was assessed by the investigator according to RECIST version 1.1. Evaluations were conducted until disease progression, the start of a new anticancer therapy, withdrawal of consent from study participation, or the end of the study.
Statistical analysis
Descriptive statistics were used to summarize the safety and PK parameters.
Drug Information
| Generic/working name | JNJ-74699157 |
| Trade name | NA |
| Company name | Janssen |
| Drug type | Small molecule |
| Drug class | KRAS G12C inhibitor |
| Dose | 100 and 200 mg per |
| Route | oral (po) |
| Schedule of administration | Once daily |
Dose Escalation Table
| Dose level | Dose of Drug: JNJ-74699157 | Number enrolled | Number evaluable for toxicity |
|---|---|---|---|
| 1 | 100 mg | 9 | 9 |
| 2 | 200 mg | 1 | 1 |
| Total | 10 | 10 |
Patient Characteristics
| Number of patients, male | 4 |
| Number of patients, female | 6 |
| Stage | IV: n = 8 IVA: n = 2 |
| Age | Median (range): 65 (36-74) years |
| Number of prior systemic therapies | Median (range): 5 (1-7) |
| Performance status: ECOG | 0–6 1–4 2–0 3–0 Unknown–0 |
| Other |
Prior cancer-related treatment
Radiotherapy, n = 5 Surgery, n = 8 Systemic therapy, n = 10 No. of lines of prior systemic therapy One, n = 1 Two: n = 0 Three or more, n = 9. |
| Cancer types or histologic subtypes | Non–small cell lung cancer, 5; colorectal cancer, 4; carcinoma of unknown primary site, 1 |
Primary Assessment Method
| Number of patients screened | 10 |
| Number of patients enrolled | 10 |
| Number of patients evaluable for toxicity | 10 |
| Number of patients evaluated for efficacy | 10 |
| Evaluation method | RECIST 1.1 |
| Response assessment CR | n = 0 (0%) |
| Response assessment PR | n = 0 (0%) |
| Response assessment SD | n = 4 (40%) |
| Response assessment PD | n = 6 (60%) |
| Median duration of treatment | 2.9 months |
Adverse Events
The most commonly reported TEAE was increased blood CPK (6 patients) (Table 1). Other frequently reported skeletal muscle-related toxicities were myalgia (3 patients), muscle weakness, muscle spasms, and increased AST (2 patients each). Overall, 6 patients reported one or more TEAEs of grade ≥3 severity, which included increased blood CPK (5 patients; all treatment-related), increased AST (2 patients; all treatment-related), back pain, hypokalemia, hypophosphatemia, large intestinal obstruction, and hepatic failure (1 patient each).
Serious Adverse Events
| Event | Grade | Attribution |
|---|---|---|
| Increased blood CPK | 4 | Probable |
| Increased blood CPK | 2 | Unrelated |
| Hepatic failure* | 5 | Unrelated |
| Large intestinal obstruction | 3 | Unrelated |
Grade 5 serious TEAE of hepatic failure secondary to disease progression.
Dose-Limiting Toxicities
| Dose level | Dose of drug: JNJ-74699157 | Number enrolled | Number evaluable for toxicity | Number with a dose limiting toxicity | Dose limiting toxicity information |
|---|---|---|---|---|---|
| 1 | 100 mg | 9 | 9 | 2 | 2 Grade 4 increased blood CPK |
| 2 | 200 mg | 1 | 1 | 1 | Grade 4 increased blood CPK` |
Pharmacokinetics/Pharmacodynamics
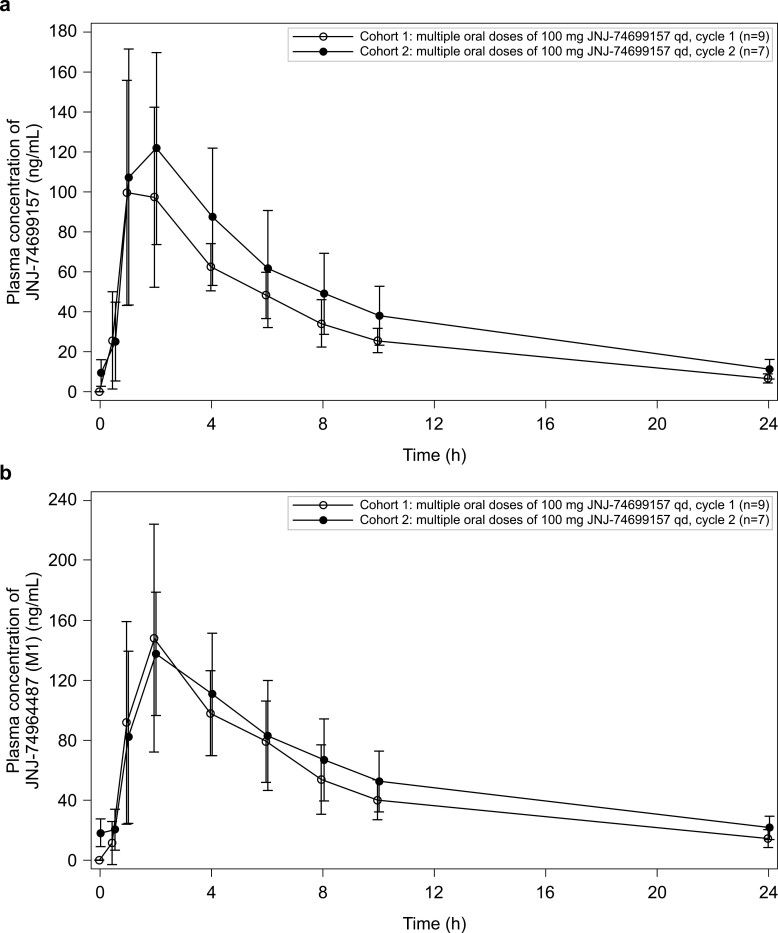
After a single oral dose of 100 mg JNJ-74699157, maximum plasma concentrations (Cmax) for JNJ-74699157 and JNJ-74964487 were reached at approximately 1–2 h post-dose and 2-4 h post-dose, respectively, and declined in a mono-exponential manner. Following multiple oral, once daily dosing of 100 mg JNJ-74699157, mean Cmax and area under the curve (AUC) of JNJ-74699157 at Cycle 2 Day 1 were slightly higher than at Cycle 1 Day 1, with accumulation ratios of 1.34 for Cmax and 1.35 for AUC (Table 2). For JNJ-74964487, mean Cmax was comparable between Cycle 2 Day 1 and Cycle 1 Day 1 and mean AUC accumulated with a ratio of 1.26. The elimination half-life (t1/2) of JNJ-74699157 was similar at Cycle 1 Day 1 (6.8 h) and Cycle 2 Day 1 (7.7 h), and the t1/2 of JNJ-74964487 was slightly longer than JNJ-74699157 (Table 2). Mean Cmax of JNJ-74964487 was 34% higher than JNJ-74699157 after the first 100 mg dose but was comparable after multiple once-daily dosing. Overall exposure to JNJ-74964487 was 59% higher than JNJ-74699157 based on mean AUC from time 0 to infinity (AUC∞) after the first dose and 35% higher based on mean AUC∞ after multiple once-daily 100 mg doses. For the patient who received 200 mg dose at Cycle 1 Day 1, individual Cmax and AUC from time 0 to 24 h (AUC24h) of JNJ-74699157 were approximately 2- to 4-fold higher and of JNJ-74964487 were 2- to 3-fold higher than the mean Cmax and AUC24h observed at Cycle 1 Day 1 following the first dose of 100 mg JNJ-74699157. The shape of the JNJ-74699157 and JNJ-74964487 plasma concentration time profiles were similar for the 100 mg and 200 mg dose (Figures 1 and 2). Based on limited data, there was a trend toward higher plasma concentrations (both Cmax and AUC) of JNJ-74699157 and JNJ-74964487 in patients who experienced grade 3 or 4 CPK increases.
Table 2.
Pharmacokinetic results of JNJ-74699157 and JNJ-74964487 (M1) after administration of 100 mg and 200 mg JNJ-74699157 once daily (Cycle 1 Day 1 and Cycle 2 Day 1) (pharmacokinetics data analysis set).
| Parameters | PK of JNJ-74699157 | PK of JNJ-74964487 (M1) | ||
|---|---|---|---|---|
|
JNJ-74699157
100 mg (n = 9) |
JNJ-74699157
200 mg (n = 1) |
JNJ-74699157
100 mg (n = 9) |
JNJ-74699157
200 mg (n = 1) |
|
| Cycle 1 Day 1, n | 8a | 1 | 8a | 1 |
| C max, ng/mL | 105(35.7) | 363 | 142 (54.7) | 384 |
| t max, median (range), h | 2.00 (1.00–2.00) | 2.00(-) | 2.00 (2.00– 4.00) | 2.00(-) |
| AUC24h, ng.h/mL | 723 (126) | 1627 | 1084 (303) | 2417 |
| AUClast, ng.h/mL | 723 (126) | 1627 | 1084 (303) | 2417 |
| AUC∞, ng.h/mL | 808 (149) | 1699 | 1294(411) | 2649 |
| t 1/2 h | 6.8 (0.8) | 5.7 | 8.6 (1.7) | 7.2 |
| CL/F, L/h | 128 (26.7) | 118 | - | - |
| Vd/F, L | 1246(159) | 974 | - | - |
| Ratio of Cmax, M1/JNJ-74699157 | - | - | 1.34(0.223) | 1.06 |
| Ratio of AUC24h, M1/JNJ-74699157 | - | - | 1.49(0.269) | 1.49 |
| Ratio of AUC∞, M1/JNJ-74699157 | - | - | 1.59(0.340) | 1.56 |
| Cycle 1 Day 2,n | 7b | - | 7b | - |
| C max, ng/mL | 134 (54.8) | - | 138 (40.9) | - |
| t max, median (range), h | 2.00 –1.00 - 2.00) | - | 2.00 (2.00–2.00) | - |
| AUCτ, ng.h/mL | 1057 (418) | - | 1376(507) | - |
| AUClast, ng.h/mL | 1057 (418) | - | 1376(507) | - |
| t 1/2, h | 7.7 (1.2) | - | 10.1(1.7) | - |
| CL/F, L/h | 107 (37.5) | - | - | - |
| Vd/F, L | 1194 (493) | - | - | - |
| Ratio of Cmax, M1/JNJ-74699157 | - | 1.10(0.320) | - | |
| Ratio of AUCτ, M1/JNJ-74699157 | - | 1.35(0.315) | - | |
| ARCmax | 1.34 (0.325) | - | 1.08 (0.265) | - |
| ARAUC | 1.35 (0.296) | - | 1.26(0.0742) | - |
Data are mean (SD) unless otherwise specified.
n = 7 for AUC∞, CL/F and Vd/F, and ratio of AUC∞, M1/JNJ-74699157.
n = 6 for ARCmax and ARAUC.
Abbreviations: ARAUC, accumulation ratio: the observed accumulation index determined after multiple dose administration; ARCmax, accumulation ratio based on Cmax; AUC∞, area under the concentration vs. time curve from the time of dosing extrapolated to infinity; AUC24h, area under the analyte concentration–time curve from time 0 to 24 h postdose; AUClast, area under the concentration vs. time curve from the time of dosing to the last observed plasma concentration; CL/F, total apparent oral clearance following extravascular dosing; Cmax, maximum observed plasma analyte concentration; t1/2, elimination half-life; tmax, sampling time to reach the maximum observed plasma analyte concentration; Vd/F, apparent volume of distribution during the terminal phase following extravascular administration.
Figure 1.
Overall anti-tumor activity of JNJ-74699157 based on best overall response (per RECIST v1.1) and duration of treatment. Abbreviations: CRC, colorectal adenocarcinoma; NSCLC, non–small cell lung cancer; PD, progressive disease; SD, stable disease.
Figure 2.
Plasma concentration–time profiles of JNJ-74699157 (a) and M1 (b) at Cycle 1 Day 1 (Cohort 1) and Cycle 2 Day 1 (Cohort 2) after administration of 100 mg dose once daily (Pharmacokinetics data analysis Set). Abbreviations: h, hours; qd, once daily.
Assessment, Analysis, and Discussion
| Completion | Study terminated before completion |
| Reason terminated | Toxicity |
| Investigator’s Assessment | Due to dose-limiting skeletal muscle toxicities and the lack of efficacy at the maximum tolerated dose of 100 mg, further enrollment was stopped. |
Here we report the results of the phase I, multicenter, open-label study investigating the safety, PK, and preliminary antitumor activity of JNJ-74699157 monotherapy in adult patients with advanced solid tumor malignancies harboring the KRAS G12C mutation who had previously received or were ineligible for standard treatment options.
The predominant safety finding in the study was dose-limiting skeletal muscle toxicity, as manifested by blood CPK increase (in some cases with associated AST increase) and muscle weakness, myalgia, or muscle spasms. This toxicity was anticipated based on nonclinical toxicology studies, although the occurrence of high-grade toxicity was not expected at comparatively low doses of JNJ-74699157. Although blood CPK increases and associated muscle symptoms resolved quickly after treatment interruption, these toxicities were the main cause of treatment interruption and sole cause of dose reduction in the study. Blood CPK increases led to hospitalization for observation in 2 patients, and CPK increases reoccurred in some patients when JNJ-74699157 was resumed at the same dose.
Although CPK increases have been observed with several MEK inhibitors,[9] KRAS G12C is a tumor-specific target and JNJ-75699157 does not inhibit MAPK signaling in KRAS wild-type cells. Hence the muscle toxicity observed here is likely an off-target drug effect unrelated to inhibition of MAPK signaling. In vitro cysteine profiling in a tumor cell line did not identify proteins other than KRAS G12C that were significantly covalently modified by JNJ-74699157 (unpublished data). Investigation of the basis for the skeletal muscle toxicity is ongoing.
JNJ-74699157 appears to have a different toxicity profile from other novel KRAS G12C inhibitors, such as sotorasib and adagrasib, although the comparison is limited by the small sample size in this study and differences in maximum delivered dose. The most frequent treatment-related AEs (>10%) reported for sotorasib in the phase I study were diarrhea, AST increased, and ALT increased.1,2 Myalgia, regardless of causality, was reported in 10% of patients, but blood CPK increase was reported in only 3.9%, and all events were grade 1. The most frequent treatment-related AEs (>10%) with adagrasib in the KRYSTAL-1 study were nausea, diarrhea, vomiting, fatigue, increased ALT, increased AST, increased blood creatinine, decreased appetite, QTc prolongation, and anemia. Fewer than 10% of patients, if any, had treatment-related increased CPK, according to the reporting cut-off used. It would therefore appear that skeletal muscle toxicity is not a class effect of KRAS G12C inhibitors and may be a specific off-target toxicity of JNJ-74699157.
This study was limited by its small sample size (10 patients) and the inability to complete the primary objective of determining a RP2D and the secondary and exploratory objectives of the dose-expansion phase.
In conclusion, given the inability to escalate the dose significantly higher due to dose-limiting skeletal muscle toxicities and the lack of efficacy observed at the maximum tolerated dose level of 100 mg, further enrollment in the study was stopped. Of note, the dose levels tested were below predicted efficacious ranges. No future development of JNJ-74699157 is planned.
Acknowledgments
We thank the members of Araxes Pharma LLC, and its affiliate Kumquat Biosciences, for providing expertise relating to research, discovery, and development of certain small molecule compounds that inhibit the G12C mutant form of K-Ras. Writing assistance was provided by Ramji Narayanan, M. Pharm., CMPP (SIRO Clinpharm Pvt. Ltd.) funded by Janssen Global Services, LLC, and additional editorial support for this manuscript was provided by Tracy Cao, Ph.D., CMPP (Janssen Global Services, LLC).
Contributor Information
Judy Wang, Florida Cancer Specialists/Sarah Cannon Research Institute, Sarasota, FL, USA.
Patricia Martin-Romano, Institut Gustave Roussy, Villejuif, France.
Philippe Cassier, Medical Oncology, Centre Léon Bérard, Lyon, France.
Melissa Johnson, Sarah Cannon Cancer Research Institute/Tennessee Oncology, PLLC, Nashville, TN, USA.
Eric Haura, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, USA.
Laurie Lenox, Janssen Research & Development, Spring House, PA, USA.
Yue Guo, Janssen Research & Development, Spring House, PA, USA.
Nibedita Bandyopadhyay, Janssen Research & Development, Raritan, NJ, USA.
Michael Russell, Janssen Research & Development, Spring House, PA, USA.
Elizabeth Shearin, Janssen Research & Development, Spring House, PA, USA.
Josh Lauring, Janssen Research & Development, Spring House, PA, USA.
Laetitia Dahan, Centre Hospitalier Universitaire La Timone, Marseille, France.
Funding
The study was supported by Janssen Research & Development.
Conflict of Interest
Laurie Lenox, Nibedita Bandyopadhyay, Yue Guo, Elizabeth Shearin, Michael Russell, Josh Lauring: Janssen Research & Development (E) and Johnson & Johnson (OI); Judy S. Wang: Stemline/Menarini, Janssen Research & Development (C/A), AstraZeneca, Eisai (H); Janssen Research & Development, Taiho, Celgene, Teneobio, Prelude Therapeutics, LOXO Oncology, Forty Seven, Nurix, Novartis Pharmaceuticals, Aevi Genomics, Ignyta, AstraZeneca, Hutchinson MediPharma, BioNTech, Stemline/Menarini, GSK, Boehringer Ingelheim, Moderna, TopAlliance Biosciences, Macrogenics, Jacobio Pharmaceutics, Merck, Klus Pharma, Revolution Medicines, Kymab, Bicycle Therapeutics, Cyteir Therapeutics, Daiichi Sankyo Pharma, Qilu Puget Sound Biothera, Black Diamond Therapeutics, Xencor, Clovis Pharma, Ribon Therapeutics, Synthorx/Sanofi, Relay Therapeutics, StingThera, ORIC Pharmaceuticals, Artio Pharma, Genentech, Treadwell Therapeutics, Mabspace Biosciences, IGM Biosciences, Immuno-Gen, PureTech Health, Erasca, Bayer, Seven Eight Biopharma, BioTheryx, Samumed/Biosplice, Zymeworks, Immuno-Onc, Cullinan-Florentine (RF to institution); Patricia Martin-Romano: Roche, Ability Pharma, AstraZeneca (H); Philippe A. Cassier: Amgen, OSE immunotherapeutics, Janssen (H), Amgen, AbbVie, AstraZeneca, BMS, GSK, Lilly, Novartis, Roche, Taiho (RF to institution), Janssen (RF); Melissa Johnson: Amgen, AbbVie, AstraZeneca, Boehringer Ingelheim, Calithera Biosciences (C/A, paid to institution), Amgen, AbbVie, Acerta, Adaptimmune, Apexigen, Arcus Biosciences (RF to institution); Eric Haura: Protein-Protein Interactions as Biomarkers Patent (IP), Amgen; Ellipses Pharma; Janssen Oncology; Janssen Research & Development; Revolution Medicines (C/A), AstraZeneca, Genentech, Incyte, Janssen, Novartis, Revolution Medicines, Spectrum Pharmaceuticals (RF); Laetitia Dahan: Amgen, BMS, Servier, Oseus, Mylan (H).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Hong DS, Fakih MG, Strickler JH, et al. . KRASG12C inhibition with sotorasib in advanced solid tumors. N Engl J Med. 2020;383(13):1207-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jänne PA, Rybkin II, Spira AI, et al. . Krystal-1: activity and safety of adagrasib (MRTX849) in advanced/ metastatic non-small-cell lung cancer (NSCLC) harboring KRAS G12C mutation. Eur Journal Cancer. 2020;138:S1-S2. [Google Scholar]
- 3. Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM.. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503(7477):548-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lito P, Solomon M, Li LS, Hansen R, Rosen N.. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science. 2016;351(6273):604-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patricelli MP, Janes MR, Li LS, et al. . Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov. 2016;6(3):316-329. [DOI] [PubMed] [Google Scholar]
- 6. Babb J, Rogatko A, Zacks S.. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med. 1998;17(10):1103-1120. [DOI] [PubMed] [Google Scholar]
- 7. Neuenschwander B, Branson M, Gsponer T.. Critical aspects of the Bayesian approach to phase I cancer trials. Stat Med. 2008;27(13):2420-2439. [DOI] [PubMed] [Google Scholar]
- 8. Guidance for industry. Drug-induced liver injury: Premarketing clinical evaluation. Silver spring, MD: Food and drug administration. 2009. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm174090.pdf. Accessed August 30, 2021. [Google Scholar]
- 9. Zhao Y, Adjei AA.. The clinical development of MEK inhibitors. Nat Rev Clin Oncol. 2014;11(7):385-400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.