Abstract
Bile acids participate in the intestinal emulsion, digestion, and absorption of lipids and fat-soluble vitamins. When present in high concentrations, as in cholestatic liver diseases, bile acids can damage cells and cause inflammation. After the discovery of bile acids receptors about two decades ago, bile acids are considered signaling molecules. Besides regulating bile acid, xenobiotic, and nutrient metabolism, bile acids and their receptors have shown immunomodulatory properties and have been proposed as therapeutic targets for inflammatory diseases of the liver. This review focuses on bile acid–related signaling pathways that affect inflammation in the liver and provides an overview of the preclinical and clinical applications of modulators of these pathways for the treatment of cholestatic and autoimmune liver diseases.
Keywords: Bile acids, Bile acid receptors, Inflammation, Liver, Cholestasis, FXR, TGR5
In humans, the primary bile acids cholic acid (CA) and chenodeoxycholic acid (CDCA) are synthesized in the liver from cholesterol and secreted as bile components into the duodenum. In the small intestine, bile acids aid in the absorption of fat, cholesterol, and fat-soluble vitamins and orchestrate bile acid, lipid, and energy metabolism by acting as ligands for bile acid receptors. The intestinal microbiota transforms the primary bile acids CA and CDCA into the secondary bile acids deoxycholic acid (DCA) and lithocholic acid (LCA), respectively. In mice, CDCA can also be converted to muricholic acid (MCA), which renders mouse bile more hydrophilic than human bile [1]. A small amount (~ 5%) of bile acids are lost in feces, whereas the remainder is reabsorbed either actively in the ileum (conjugated bile acids via the apical sodium–dependent bile acid transporter, ASBT) or passively in the colon (deconjugated bile acids) [2].
Upon reabsorption in the ileum, bile acids bind to the nuclear receptor farnesoid X receptor (FXR), which regulates the expression of genes involved in the uptake and efflux of bile acids to prevent their accumulation, and which cross-signals with other nuclear receptors to regulate bile acid, xenobiotic, and nutrient metabolism. Importantly, bile acid binding to intestinal FXR induces the production of fibroblast growth factor 19 (FGF19), which travels to the liver via the portal circulation along with the reabsorbed bile acids to inhibit hepatic bile acid synthesis, effectively providing a negative feedback mechanism to maintain bile acid pool homeostasis. FXR expressed in the liver further regulates bile acid synthesis and nutrient signaling [3].
Bile acids returning from the portal circulation and bile acids in the systemic circulation are taken up by hepatocyte Na+-dependent taurocholate cotransporting peptide (NTCP) and, together with the newly synthesized bile acids, are secreted into canaliculi via the bile salt export pump (BSEP), thereby completing their enterohepatic circulation [4] (Fig. 1).
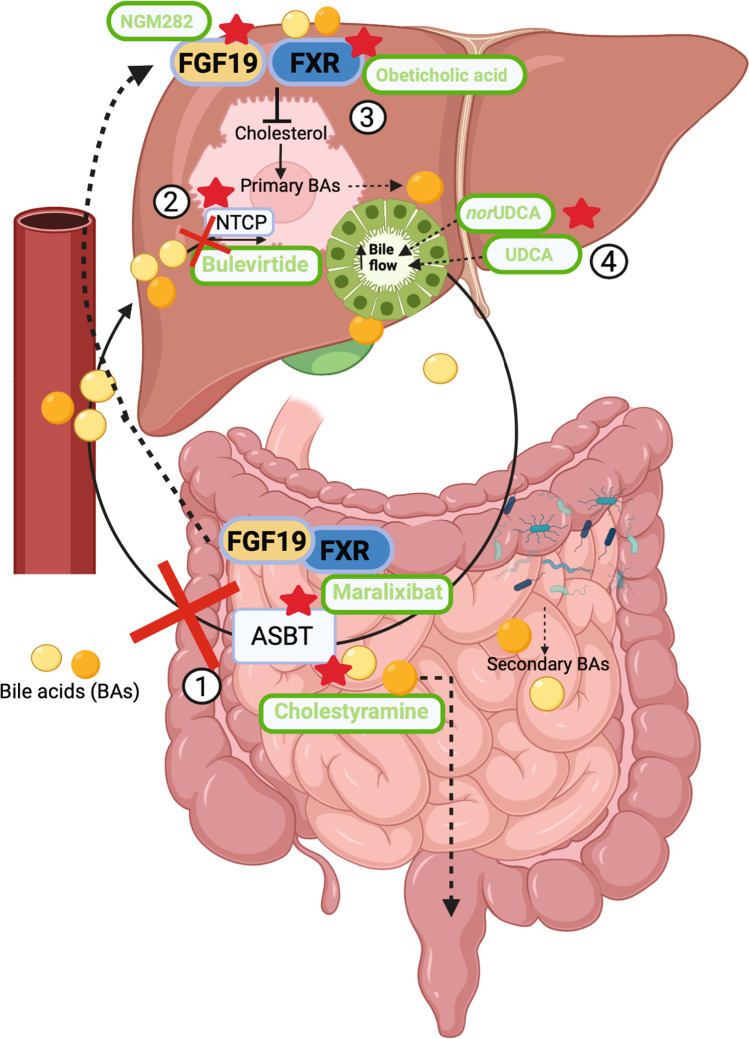
Fig. 1.
Bile acid homeostasis and bile acid–related treatments in cholestatic liver diseases. Bile acids are synthesized by hepatocytes in the liver from cholesterol and secreted into bile. After being
modified by the bile duct epithelium, the bile is secreted in the duodenum to accomplish digestive functions. Under the action of the intestinal microbiota, primary bile acids are modified into secondary bile acids. Bile acids are in large part re-absorbed by ileal ASBT to be returned to the liver via the portal circulation. Upon intestinal reabsorption, bile acids activate FXR-FGF19 that negatively regulates bile acids synthesis in the liver. The different steps of the enterohepatic circulation can potentially be targeted in cholestatic liver diseases to antagonize the effects of bile acids accumulation. In the figure are reported selected drugs that are in experimental trial or approved for (1) interrupting the enterohepatic circulation, (2) reducing bile acids uptake, (3) reducing bile acids synthesis, (4) increasing bile flow and decreasing bile acid hydrophobicity. ASBT: apical sodium–dependent bile acid transporter; BAs: bile acids; FXR: farnesoid X receptor; FGF19: fibroblast growth factor 19; nor-UDCA: nor-ursodeoxycholic acid; NTCP: Na+-dependent taurocholate cotransporting peptide; UDCA: ursodeoxycholic acid
Besides their well-studied roles in fat absorption and nutrient signaling, bile acids and their receptors contribute to the modulation of immunity. The interplay between bile acids and immunity is multifaceted and includes physicochemical interactions of bile acids with cells and immune-related pathways dependent or independent from interaction with bile acid receptors as well as interactions between bile acids and the gut microbiota. The scope of this review is to provide an overview of the bile acid–related signaling pathways that affect inflammation in the liver, and to review the preclinical and clinical evidence of how modulators of these pathways and receptors perform in the treatment of cholestatic and autoimmune liver diseases.
Bile acid accumulation and inflammation in cholestasis
In cholestatic diseases, elevated levels of bile acids within the liver cause injury and inflammation, which can progress to fibrosis and cirrhosis. The exact mechanisms of liver injury consequent to bile acid accumulation have not been fully clarified and are likely multifactorial and different in different types of cholestatic diseases. Bile acids are amphipathic molecules with different degrees of hydrophobicity, with ursodeoxycholic acid (UDCA) being the most hydrophilic and CA, CDCA, DCA, and LCA being progressively more hydrophobic [5].
Because of their hydrophobicity, based on early in vitro studies, it was proposed that bile acids could directly lyse hepatocyte cell membranes [6–8] or induce hepatocyte apoptosis [9]. However, these studies did not always recapitulate the cholestatic situation in vivo in terms of bile acid species and concentrations used to challenge hepatocytes. The mechanisms mediating bile acid–induced hepatocyte death are still debated [10] and different bile acid concentrations at different stages of cholestasis likely lead to different responses [11]. There is evidence supporting direct bile acid–mediated hepatocyte death, which is a pro-inflammatory event that likely propagates inflammation to other liver cell types. Hepatocyte necroptosis was observed in hepatocytes from PBC patients and after bile duct ligation in mice [12]. Another study suggested that hepatocyte death occurs primarily in the acute phase of cholestasis, 1–3 days after bile duct ligation in mice. In this situation, the pathogenetic sequence sees rupture of the hepatocyte apical membrane, entry of bile, and death of single cells followed by the death of surrounding hepatocytes (bile infarcts). This response was not observed in chronic cholestasis, either after 3 days from bile duct ligation or in Mdr2−/− mice, which chronically lack phospholipid secretion in bile [13]. Recent research suggests that increased bile acid levels in cholestatic conditions induce the secretion of cytokines by hepatocytes [14–17], which can recruit neutrophils to initiate the inflammatory response [16, 18] (Fig. 2). In these studies, bile acid–induced secretion of cytokines by hepatocytes was unrelated to cellular toxicity, apoptosis, or necrosis [14, 15], required bile acid uptake by hepatocytes via the bile acid importer NTCP [16], and was mediated via the nuclear factor of activated T-cells (NFAT) and toll-like receptor 9 (TLR9) [16, 17]. Consistently, NTCP deficiency, which prevents bile acid uptake by hepatocytes, does neither cause a hepatic phenotype in humans [19], nor in mice [20]. Instead, deficiency of the bile salt export pump BSEP (causing progressive familial intrahepatic cholestasis type 2, PFIC2) and FIC1 (causing PFIC1), in which bile acids enter hepatocytes but cannot be efficiently secreted into bile, causes severe liver injury [10, 21]. Unlike in hepatocytes, bile acid–induced cytokine secretion was not observed in liver non-parenchymal cells using the same bile acid concentrations [16]. Hepatic stellate cells (HSC) do not express NTCP and do not take up bile acids. Whereas no bile acid–induced apoptosis was observed in HSC, bile acids induced HSC proliferation [22], which promotes fibrosis (Fig. 2).
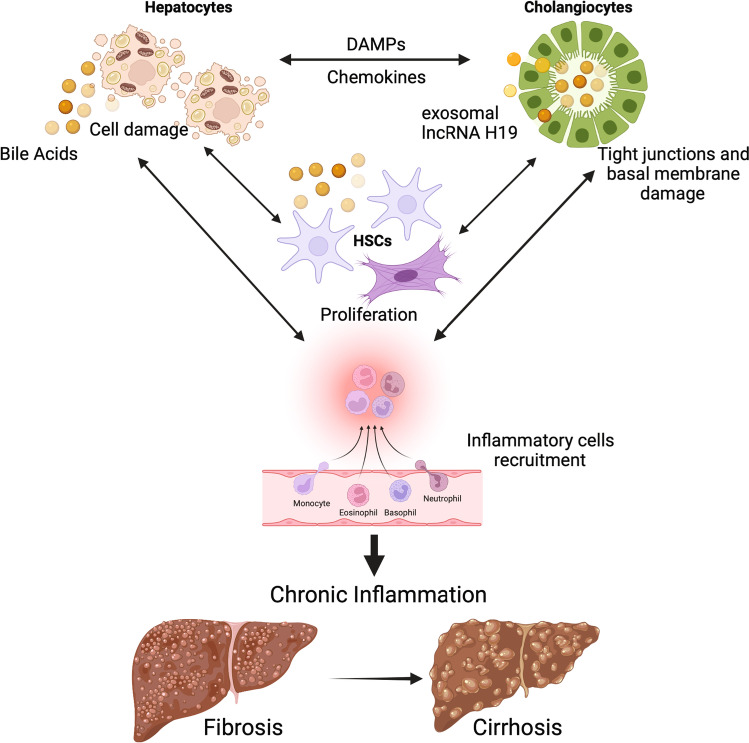
Fig. 2.
Effects of bile acid accumulation in the liver. Bile acid accumulation in cholestatic conditions can activate different signaling pathways in different cell types of the liver. Damaged hepatocytes can initiate an inflammatory response by secreting chemokines and DAMPs that activate other cells (i.e., cholangiocytes, HSCs, and inflammatory cells). High levels of bile acids can also directly disrupt tight junctions and basal membrane of bile ducts, leading to activation of cholangiocytes and perpetuation of the inflammatory/fibrotic response with proliferation and activation of HSCs. Persistent chronic inflammation and fibrosis can progress into cirrhosis. DAMPs: damage-associated molecular patterns; HSCs: hepatic stellate cells; lncRNA H19: long non-coding RNA H19
Central to cholestatic diseases is cholangitis, suggesting either direct or indirect bile acid–related damage to cholangiocytes. Cholangiocytes lining bile ducts are routinely exposed to high concentrations of bile acids in bile without sustaining injury. This can likely be attributed to (1) bile acid micellization with cholesterol and phospholipids in bile; (2) the capability of cholangiocytes to secrete bicarbonate in the lumen [23] that, in the presence of glycoproteins and other mucus-like components, may form a glycocalyx on the cholangiocyte luminal side; evidence so far is limited to in vitro studies, but this bicarbonate shield (or umbrella) may protect against bile acid–induced injury [24]; (3) the expression of FXR and its target genes that may be orchestrated to prevent intracellular bile acid accumulation during cholestasis [25]. It was proposed that free biliary bile acids (i.e., not micellized) can damage cholangiocyte membranes [26]. PFIC3, caused by deficiency of multidrug resistance protein 3 (MDR3), a transporter that facilitates phospholipids secretion in the canaliculus [21], is modelled by Mdr2−/− mice, which feature sclerosing cholangitis. In Mdr2−/− mice, however, cholangiocyte death is a late pathogenic event, suggesting it may not be caused by bile acid cytotoxicity. This study suggested that bile acids damage cholangiocyte tight junctions and basement membranes first, leading to bile leakage in the periductal area, which initiates the inflammatory and fibrotic response (Fig. 2). Cholangiocyte death would occur after the insurgence of fibrosis, which may deprive cholangiocytes from their blood supply [27]. In bile duct–ligated mice, cholangiocyte proliferation and periportal fibrosis occur after hepatocyte death [12]. Interestingly, cholangiocytes are capable of secreting inflammatory mediators to induce neutrophil activation in response to stimuli such as pathogen-associated molecular patterns (PAMPs) [28–33]. Whether induction of cytokine and chemokine expression occurs in cholangiocytes directly in response to bile acids in cholestatic conditions is still debated due to conflicting results [16, 34]. A series of studies showed that TCA stimulated cholangiocyte proliferation [35] and that cholangiocyte expression of exosomal lncRNA H19 in response to cholestatic injury promoted HSC activation and proliferation [36], as well as macrophage activation [37] to increase cholestatic liver injury (Fig. 2). Cholangiocyte mitochondrial damage was observed in isolated bile duct units in response to unconjugated, but not conjugated bile acids [38]. However, bile contains almost exclusively conjugated bile acids [39], and the authors did not observe cholangiocyte damage when perfusing isolated rat livers with unconjugated bile acids [38]. Patients with rare mutations impairing bile acid conjugation show signs of biliary ductular reaction and cholangiopathy, although inconsistently [27].
In summary, there is evidence supporting direct cytotoxic effects of bile acids on hepatocytes, especially in the acute phase of cholestatic injury, whereas damage to cholangiocytes seems primarily directed at the tight junction and basement membrane. Hepatocyte death likely initiates an inflammatory response that affects other liver cell types, which in turn further amplify inflammation. Independently from cell death, there is evidence supporting a role for bile acids in the induction of pro-inflammatory responses in hepatocytes, as well as in the proliferation of cholangiocytes and HSC. Interestingly, different signaling pathways were described in different cell types, highlighting the multifactoriality of the response to bile acid overload that ultimately results in cholestatic liver injury (Fig. 2).
Bile acid receptors and inflammation in cholestatic and autoimmune liver diseases
Immune modulation by bile acid has long been hypothesized. Early studies investigated whether elevated serum bile acids in cholestatic liver diseases could be responsible for infectious complications and endotoxemia by directly suppressing the immune response. Accordingly, numerous in vitro studies were carried out to assess the effect of bile acids on immune cell function. Lymphocyte proliferation, immunoglobulin production, and cytokine secretion were suppressed by bile acids [40–44]. Decreased cytokine release by monocytes upon bile acid stimulation was reported by several [43, 45, 46], but not all [47] studies. The phagocytic function of the Kupffer cell was also reported to be decreased by bile acids [48, 49].
On the other hand, a recent study found that bile acids act as damage-associated molecular patterns (DAMPs) that can activate the NOD-, LRR- and pyrin domain–containing protein 3 (NLRP3) inflammasome in macrophages by promoting intracellular calcium influx. In this study, the promotion of inflammasome activation by bile acids was synergistic with LPS and, in vivo, cholestasis aggravated LPS-induced sepsis [50].
The mechanisms underlying the immune-modulating effects of bile acids were studied only recently, after it was discovered that most of the biological actions of bile acid are mediated through the modulation of bile acid receptors. In the next sections, we review the role of bile acid receptors (FXR, TGR5, and PXR) in modulating inflammation and the preclinical and clinical evidence assessing their utility in the treatment of liver diseases.
FXR
Farnesoid X receptor (FXR, NR1H4) is a nuclear receptor central to nutritional homeostasis. Its major endogenous ligands are bile acids, with CDCA > DCA > LCA > CA in order of FXR activation potency [51, 52]. The mouse bile acid muricholic acid (MCA), which is derived from CDCA by the enzyme Cyp2c70, is a FXR antagonist [53]. The use of Cyp2c70−/− mice with a human-like bile acid pool is thus recommended in preclinical studies testing FXR modulators [1]. Of note, being a nuclear receptor, FXR activation requires cellular entry of bile acids. In response to ligand activation, FXR regulates the fed state response by modulating the expression of genes involved in (a) bile acid homeostasis (to maintain the bile acid pool size by regulating the amount of newly synthesized bile acids), (b) glucose homeostasis (to reduce postprandial glycemia by limiting hepatic glucose generation), and (c) lipid metabolism (to reduce hepatic fatty acid generation, storage, and release) [54]. A favorable effect of FXR agonism in liver diseases is thought to arise from the reduction of bile acid synthesis and accumulation and increase in bile acid and xenobiotic modification and secretion as well as decrease in hepatic lipogenesis [55]. Besides the well-known role as nutritional homeostat, FXR is also involved in the inflammatory response, which is the focus of this section. As for tissue distribution, the highest mRNA expression of NR1H4 is found in the liver [56, 57]. Here, NR1H4 is mostly expressed by cholangiocytes and hepatocytes and, to a lower extent, by Ito cells, Kupffer cells, and T cells. Other cell types that highly express NR1H4 are enterocytes in the intestine and cells lining the collecting duct system in the kidney [56, 57]. Importantly, in the mouse and under physiological conditions, FXR seems to be basally active in the intestine, but not in the liver. However, in the liver, FXR becomes strongly activated under cholestatic conditions [58].
FXR and inflammation
As for other nuclear receptors involved in nutrient metabolism [59], the expression and activation of FXR are repressed during inflammation [60–63]. Since FXR activation is generally anti-inflammatory, FXR repression during inflammation could allow for the inflammatory response to be amplified.
Several members of the nuclear receptor superfamily, including FXR, repress pro-inflammatory genes by regulating the transcription factors that control the expression of these genes, mainly NF-κB and AP-1, in a process known as transrepression [64]. NF-κB is a family of transcription factors that regulates the transcription of an array of pro-inflammatory genes [65]. FXR activation was shown to antagonize NF-κB activation in vitro in hepatocytes, macrophages, enterocytes, other cell types, and in vivo in liver tissue [63, 66–70]. NF-κB inhibition by FXR can also occur via small heterodimer partner (SHP) activation [67]. SHP, an atypical nuclear receptor and a FXR target gene, also prevents AP-1 binding to inflammatory genes [71] and downregulates the expression of the chemokine CCL2 [72]. Furthermore, in cholestasis, loss of SHP was linked to increased lncRNA H19 [73], which is pro-inflammatory. As another mechanism, in a study in hepatocytes, the inhibition of NF-κB signaling by the FXR agonist obeticholic acid (OCA) was dependent on the induction of cytochrome P450 epoxygenases, the enzymes responsible for the synthesis of anti-inflammatory eicosanoids [70]. As discussed in the previous section, hepatocytes responded to bile acids with induction of cytokines. These effects were observed in absence of NF-κB stimulation and were FXR-independent [14, 16]. Interestingly, post-translational modifications of FXR can affect the signaling pathways it modulates. FXR sumoylation is promoted by FXR agonism. SUMOylated FXR transrepresses NF-κB signaling without affecting classical FXR target genes such as SHP. On the contrary, FXR acetylation, which is constitutively active in obesity, promotes hepatic inflammation by inhibiting FXR sumoylation [74]. An FXR modulator that represses inflammation via NF-κB without inducing other classical FXR target genes was developed, demonstrating that gene-selective FXR modulation is possible [75]. FXR activation thus decreases inflammation by repressing NF-κB and AP-1 via several mechanisms (Fig. 3).
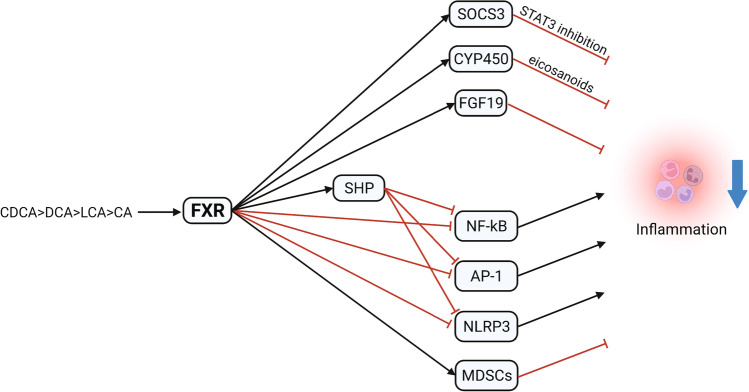
Fig. 3.
Effect of FXR activation on liver inflammation. Activation arrows are indicated in black and inhibition arrows in red. AP-1: activator protein 1; CA: cholic acid; CDCA: chenodeoxycholic acid; CYP450: cytochrome P450 family 7 subfamily A member 1; DCA: deoxycholic acid; FGF19: fibroblast growth factor 19; FXR: farnesoid X receptor; LCA: lithocholic acid; MDSCs: myeloid-derived suppressor cells; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3: NOD-, LRR- and pyrin domain–containing protein 3; SHP: small heterodimer partner; SOCS3: suppressor of cytokine signaling 3
In addition to repressing NF-κB signaling, FXR affects inflammasome activation. Inflammasomes are multiprotein complexes that control the inflammatory response and are assembled in response to PAMPs and DAMPs [65]. The FXR target gene SHP was shown to repress NLRP3 formation by inhibiting NLRP3 binding to ASC [76]. Additionally, it was observed that FXR could inhibit NLRP3 by directly interacting with NLRP3 and caspase 1 [50]. In mouse models of alcoholic liver disease, FXR agonism increased NLRP3 ubiquitination, which was associated with decreased steatosis and inflammation [77]. Thus, FXR negatively regulates the NLRP3 inflammasome (Fig. 3).
Several studies utilizing mouse models of different liver diseases have assessed the effectiveness of FXR agonism at decreasing hepatic inflammation and shed light on additional anti-inflammatory mechanisms, in addition to negative regulation of NF-κB and NLRP3 (Fig. 3). In mouse models of LPS-induced liver injury, FXR agonism decreased LPS-induced hepatic inflammation [78, 79]. Xu et al. [80] showed that the anti-inflammatory effects of FXR agonism in LPS-induced liver injury were mediated by increased expression of suppressor of cytokine signaling 3 (SOCS3), which downregulates cytokine-STAT3 signaling. Of note, STAT3 signaling is involved in tumorigenesis [81]. Accordingly, ageing Fxr−/− mice are prone to liver inflammation and spontaneous tumor development [82–85]. FXR is downregulated in hepatocellular carcinoma and cholangiocarcinoma [86, 87], whereas there is experimental evidence suggesting that FXR activation reduces the carcinogenic potential in both types of cancers (the reader is referred to recent, comprehensive reviews on this topic, [88, 89]). Fxr−/− mice are also more susceptible to autoimmune hepatitis induced by concanavalin A (Con A) and FXR agonism in wild-type mice attenuated liver damage. It was found that FXR in NKT cells activates SHP-mediated inhibition of osteopontin production [90]. Again in models of immune-mediated liver injury, induced by alpha-galactosylceramide (alpha-GalCer) or Con A, FXR agonism reduced inflammation and simultaneously promoted the hepatic accumulation, function, and homing of immune-suppressive granulocytic myeloid–derived suppressor cells (MDSCs) [91].
Preclinical studies
FXR agonism has been proposed as a therapeutic intervention for cholestatic liver diseases because of its potential to prevent the accumulation of bile acids by regulating bile acid synthesis and transporters. During cholestasis, the expression of bile acid transporters is modulated to prevent the accumulation of bile acids in hepatocytes. This was evident in a mouse model of ANIT-induced cholestasis, where wild-type mice had a lower hepatic expression of the bile acid uptake transporter Ostβ and higher expression of the bile acid efflux transporter Bsep. Fxr−/− mice lacked this response, suggesting FXR-dependency, and were more susceptible to liver injury [92]. Conversely, in rats with bile duct ligation and ANIT-induced cholestasis, FXR agonism decreased expression of bile acid synthesis genes and increased expression of genes related to canalicular bile acid transport such as Bsep, Mrp2, and Mdr2, which was associated with improved serum liver enzymes, markers of inflammation, liver damage, and decreased bile duct proliferation [93]. However, evidence for a positive role of FXR agonism in animal models of cholestasis is conflicting. Bsep upregulation by FXR agonism in a bile duct ligation model was also reported in another study; however, FXR agonism aggravated liver injury. Here, Bsep upregulation was regarded as counter-productive, as it would further promote bile duct obstruction by facilitating bile acid efflux from the hepatocyte [94]. Furthermore, another study reported decreased liver injury in bile duct–ligated Fxr−/− mice [95].
Clinical studies
The FXR activators that have undergone or are undergoing clinical trial are the steroidal FXR agonist obeticholic acid (OCA) and the non-steroidal FXR agonists cilofexor, tropifexor, and MET409.
So far, numerous clinical trials for OCA in PBC patients with insufficient response to UDCA [96–102] have shown improvements in liver enzymes. Consequently, OCA is recommended as a second-line treatment in addition to UDCA for PBC patients with insufficient response to UDCA, and as a first-line treatment for patients who are intolerant to UDCA [103]. A large international placebo-controlled phase 4 trial is ongoing to further assess the effectiveness of OCA in PBC (NCT02308111), as well as a phase 3 study assessing OCA plus bezafibrate in PBC (NCT04594694). Mechanistically, OCA increased the transport of bile acids from hepatocytes to canaliculi in UDCA-treated PBC patients [104]. Importantly, the FDA advises against the prescription of OCA to PBC patients with advanced cirrhosis, as a result of 25 reports of liver decompensation or failure associated with OCA use. Most of these incidents involved patients with compensated cirrhosis, mostly with portal hypertension, or patients with decompensated cirrhosis. Liver decompensation occurred 10 days to 10 months after initiation of OCA [105]. In eligible patients, the recommended starting dose is 5 mg, which can be titrated to 10 mg after 3 months if OCA is well-tolerated. It is further recommended to monitor liver function before and after initiating OCA therapy [103].
In PSC, OCA brought a reduction in ALP in a phase 2 trial [106]; however, further clinical trials are not in the pipeline at this time [107]. OCA is also being tested in patients with pediatric biliary atresia (EudraCT 2014–004,693-42). Preliminary data showed that tropifexor improved γGT and ALT in PBC patients [108]. Cilofexor, another non-steroidal FXR agonist, decreased serum ALP, γGT, AST, ALT, and bile acid levels in PSC [109] and PBC patients, according to preliminary data [110]. A phase 3 clinical trial for cilofexor in PSC is underway (NCT03890120).
Regardless of the liver disease treated, the most common side effects of FXR agonists were dose-dependent pruritus, fatigue, and increased LDL:HDL ratio, which could increase atherosclerotic risk. The addition of statins to OCA mitigated the latter side effect [111]. Assessment of the interactions of antipruritus drugs with OCA is underway (NCT05133830). These side effects seem target-specific, as they occurred with different FXR agonists.
FXR modulation is a promising therapeutic avenue for various liver diseases, as highlighted by the approval of OCA for PBC and the numerous clinical trials in progress. However, the side effects of FXR agonists pose a challenge and the long-term efficacy still needs to be characterized. The working mechanisms of FXR modulation in the various liver diseases also need to be further elucidated.
FGF19
Bile acid signaling is not restricted to the liver. Actually, a major part takes place in the intestine. An important hormone to fully understand the effects of (pan and intestinal) FXR activation by bile acids is FGF19, as it is mainly produced in the ileum in response to FXR activation by bile acids. After traveling from the ileum to the liver via the enterohepatic circulation, FGF19 exerts its effects through binding to the fibroblast growth factor receptor 4 (FGFR4) and its coreceptor β-klotho (KLB), which are mostly co-expressed in the liver [112].
In hepatocytes, FGF19 downregulates bile acid synthesis by inhibiting the major bile acid synthesis enzyme cholesterol 7-a-hydroxylase (CYP7A1) [113], a property that can be exploited to prevent bile acid overload-related liver injury. Because chronic FGF19 overexpression was shown to induce hepatocellular carcinoma in mice by activating STAT3 signaling [114, 115], a nontumorigenic FGF19 analogue (NGM282, also known as M70 and aldafermin) that does not activate STAT3 was developed [116]. NGM282 suppressed bile acid synthesis in humans [116] and reduced serum levels of hydrophobic bile acids in patients with NASH and PSC [117]. In mouse models of cholestasis induced by bile duct ligation or ANIT, NGM282 decreased the bile acid pool size and diminished liver injury [118]. In Mdr2−/− mice, FGF19 and NGM282 decreased liver injury, inflammation, and fibrosis [119]. A different FGF19 analogue lacking tumorigenic properties, FGF19-M52, similarly protected Mdr2−/− mice from cholestatic injury [120]. Moreover, constitutive activation of intestinal FXR in mice reduced the bile acid pool size and attenuated cholestatic injury caused by Mdr2 deficiency, bile duct ligation, and ANIT treatment [121]. Consequently, NGM282 was tested in humans for the treatment of cholestatic disorders. In PBC patients with inadequate response to UDCA, NGM282 decreased ALP, GGT, ALT, AST, and IgM levels after 28 days of treatment. Diarrhea was the most commonly reported side effect [122]. In PSC patients, NGM282 treatment for 12 weeks improved fibrosis biomarkers; however, it did not improve ALP levels, the primary endpoint [123]. NGM is not planning to pursue further clinical trials of NGM282 for PSC and PBC at this time [124].
TGR5
TGR5 (GPBAR1) is a G-protein-coupled receptor that is ubiquitously expressed. The highest mRNA expression of GPBAR1 is found in the gallbladder and in monocytes [56, 125]. In the liver, GPBAR1 is expressed by cholangiocytes, both intrahepatic [126] and extrahepatic [127], Kupffer cells [128], sinusoidal endothelial cells [129], activated hepatic stem cells [130], NTK cells [131], and hepatocytes [132]. In the intestine, GPBAR1 is mostly expressed by intestinal endocrine cells in the colon and goblet and Paneth cells in the small intestine [56, 133].
Known endogenous TGR5 ligands are bile acids, with potency for TGR5 activation LCA > DCA > CDCA > CA [134, 135]. Therefore, secondary bile acids produced by the gut microbiota are the preferred ligands for TGR5. Because TGR5 is expressed at the plasma membrane, unlike FXR, which is a nuclear receptor, TGR5 activation does not require bile acid entry into the cell. TGR5 can regulate various signaling pathways such as NF-κB, AKT, and ERK, among others [136]. TGR5 is also well-known as a regulator of energy and glucose metabolism [137].
As mentioned above, TGR5 is expressed in cholangiocytes, where it can be localized in the primary cilium, at the apical membrane and in intracellular vesicles, and its stimulation can cause opposite downstream effects depending on the subcellular location. TGR5 in cilia may come in contact with bile acids routinely, whereas TGR5 located on the apical membrane may be shielded from bile acids by a bicarbonate-rich apical glycocalyx [138]. Tgr5−/− mice had decreased biliary proliferation in response to cholestasis and TGR5 agonists induced cholangiocyte proliferation [139]. Increased TGR5-mediated cell proliferation could potentially promote cholangiocarcinoma progression, also based on the observation that TGR5 is overexpressed in cholangiocarcinoma tissue [139, 140]. In gallbladder cholangiocytes, TGR5 colocalizes with cystic fibrosis transmembrane conductance regulator (CFTR) [127]. TGR5 activation increases intracellular cAMP levels and stimulates chloride secretion via CFTR [127], which could contribute to bicarbonate-rich fluid secretion [141]. Decreased bile flow in Tgr5−/− mice and increased bile flow by TGR5 agonism were reported; however, it is not known whether these were CFTR-dependent [142]. Finally, TGR5 agonism increases cholangiocyte barrier function by stabilizing junctional adhesion molecule A (JAM-A) [143]. Although mutations leading to decreased TGR5 were identified in PSC patients, their rarity does not suggest these as a causative factor in PSC [144].
TGR5 and inflammation
TGR5 was first described as a bile acid receptor in monocytes and macrophages, where it suppressed their function in response to bile acids [134]. In macrophages, TGR5-mediated inhibition of cytokine production occurred through stabilization of the alternative (non-inflammatory) macrophage phenotype via CREB recruitment to the CRE on the promoter of the anti-inflammatory gene IL-10 [145–147]. TGR5 activation was further shown to downregulate cytokine production by inhibiting the NF-κB pathway in several cell types (Fig. 4), among which dendritic cells [148], NTK cells [131], endothelial cells [149], and macrophages and Kupffer cells [150–155]. In macrophages, NF-κB was inhibited by TGR5-dependent increased c-Fos phosphorylation [150], or by repressed phosphorylation of IκBα [151, 152]. Inhibition of macrophage chemokine production by TGR5 activation also occurred through activation of the AKT-mTOR complex 1, which promoted the translation of the C/EBPβ isoform LIP, which could blunt NF-κB activation [156]. In macrophages, TGR5 was also found to interfere with the β-catenin destruction complex to increase β-catenin levels, resulting in inhibition of the TLR4-NF-κB pathway via PI3K/Akt signaling [154] (Fig. 4).
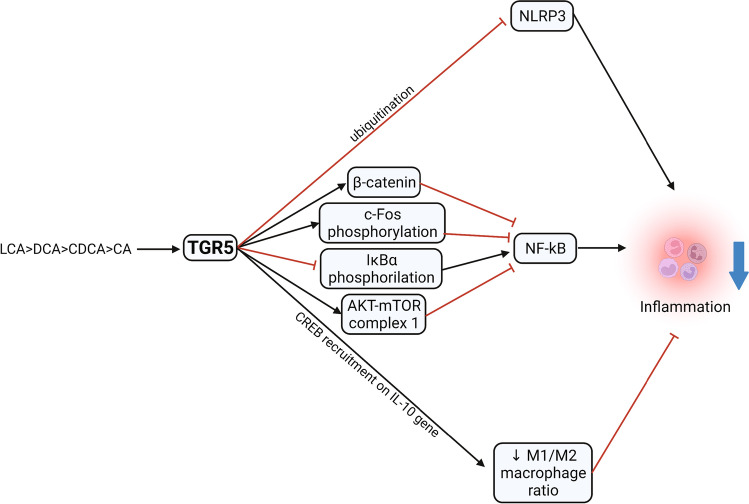
Fig. 4.
Effect of TGR5 activation on liver inflammation. Activation arrows are indicated in black and inhibition arrows in red. AKT-mTOR: protein kinase B—mammalian target of rapamycin signaling pathway; CA: cholic acid; CDCA: chenodeoxycholic acid; DCA: deoxycholic acid; IκBα: nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; LCA: lithocholic acid; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3: NOD-, LRR- and pyrin domain–containing protein 3; TGR5: takeda-G-protein-receptor-5
Like FXR, TGR5 is involved in inflammasome regulation (Fig. 4). TGR5 activation by bile acids inhibited the NLRP3 inflammasome by increasing its ubiquitination, thereby reducing inflammation in vitro and in vivo [157–159]. In liver failure, raised serum bile acids correlate with infections and mortality. It was recently reported that the serum bile acid composition of patients with liver failure promotes TGR5 activation and reduces the pro-inflammatory response of monocyte in response to bacterial challenge. The patients with a TGR5-activating serum bile acid composition were at increased risk for a fatal outcome [160].
Preclinical studies
Tgr5−/− mice are more susceptible to LPS-induced liver inflammation [151], which on the contrary is improved by TGR5 agonist treatment in wild-type mice [151]. Tgr5−/− mice, which have a smaller, more hydrophobic bile acid pool [142], also exhibit more severe liver injury and inflammation after partial hepatectomy, which could be attenuated by cholestyramine treatment (a bile acid–binding resin that interrupts the enterohepatic circulation of bile acids) and Kupffer cell depletion [161].
Tgr5−/− mice are also more susceptible to cholestatic damage caused by bile duct ligation, showing increased inflammatory cell infiltration [153, 154, 161], whereas TGR5 activation in wild-type mice protected against liver injury by decreasing the NF-κB pro-inflammatory response as well as oxidative stress [153]. Despite the positive effects of TGR5 activation in cholangiocytes detailed above, a TGR5 agonist alone did not improve the liver phenotype of Mdr2−/− mice, and neither did an FXR agonist alone. However, dual TGR5/FXR agonist treatment reduced hepatic inflammation and fibrosis, probably by reducing bile acid synthesis in a FXR-dependent manner [162]. The lack of efficacy of the TGR5 agonist was likely due to the downregulation of TGR5 in the Mdr2−/− mouse. TGR5 downregulation in biliary epithelial cells from Mdr2−/− mice, a PSC model, was associated with a pro-inflammatory phenotype, which was reversed by TGR5 overexpression. TGR5 is also downregulated in the PSC liver [163].
In mouse models of acute immune-mediated hepatitis, Tgr5−/− mice had a more severe liver injury, while a TGR5 agonist improved liver damage in wild-type mice by promoting a shift of NKT cells to a regulatory, IL-10 producing NKT cell subset [131].
In polycystic liver disease, antagonism rather than agonism of TGR5 could be beneficial. TGR5 was found to be overexpressed in cystic cholangiocytes, leading to increased cell proliferation and cyst growth. Deletion of Tgr5 significantly reduced hepatic cystic areas in a mouse model of polycystic liver disease, whereas TGR5 agonists stimulated cyst growth in vitro [164, 165]. TGR5 antagonism may also be beneficial in cholangiocarcinoma. TGR5 expression is upregulated in cholangiocarcinoma and TGR5 agonism increased cell proliferation and migration in vitro and cholangiocarcinoma growth in vivo [140, 166].
Clinical studies
Given that TGR5 is expressed ubiquitously, systemic side effects are likely to occur. Although these were not studied in humans, mouse models provide hints as to which side effects may occur when modulating TGR5. TGR5 activation stimulated gallbladder filling and delayed emptying by promoting smooth muscle relaxation in mice [142], a side effect that could increase the risk of cholelithiasis and cholecystitis. TGR5 activation in endothelial cells increased the generation of the vasodilatory mediators nitric oxide [129, 149, 167] and hydrogen sulfide [168], while it inhibited the secretion of endothelin-1, a vasoconstrictor [169]. These effects may be exploited for reducing portal pressure, as demonstrated in a mouse model of hepatic cirrhosis [167]. However, they may result in peripheral arterial vasodilation, leading to blood pressure drops at therapeutic dosages, as reported in dogs, but not in rats [170]. Furthermore, in mice, overexpression of TGR5 in sensory nerves or activation by bile acids or TGR5 agonist administration induced pruritus and analgesia [171]. In light of the induction of cholangiocyte proliferation upon TGR5 activation [139], there is also a potential risk of cholangiocarcinoma development. TGR5 agonists that deactivate rapidly after exerting effects in the intestine were designed [170]; however, intestine-restricted action may not be desirable for the treatment of cholestatic and autoimmune liver diseases. The TGR5 agonist SB-756050 was studied in 51 type 2 diabetes patients. The compound was well-tolerated and there were no safety issues reported. A comprehensive description of side effects, however, is lacking [172].
Thanks to its anti-inflammatory properties, TGR5 agonism is an attractive treatment for autoimmune liver diseases, as shown by encouraging results in pre-clinical studies. Further studies are needed to characterize the levels of TGR5 expression in the context of liver diseases, which may determine the success of TGR5 agonism. TGR5 antagonism, rather than agonism, could be helpful in polycystic liver disease to reduce cyst growth and in cholangiocarcinoma to reduce cell proliferation and resistance to apoptosis. Unfortunately, clinical application of TGR5 modulators is hampered by the potential systemic side effects that stem from its ubiquitous expression, and translation of preclinical results to humans is thus largely undetermined.
PXR
The pregnane-activated receptor (PXR, NR1I1) is a nuclear receptor highly and primarily expressed in intestinal enterocytes and liver hepatocytes [173]. PXR can be activated by numerous and structurally diverse ligands such as xenobiotics and natural and synthetic steroids, including the secondary bile acid lithocholic acid (LCA) [174]. PXR signaling is well-known to modulate the expression of drug-metabolizing enzymes and transporters (DMET) to facilitate xenobiotics metabolism, transport, and clearance, a function that is shared with the constitutive androstane receptor (CAR, NR1I3) [175, 176]. Besides DMET regulation, PXR is also involved in energy homeostasis [177], bile acid metabolism, and regulation of inflammation.
PXR is positively regulated by FXR [178] and the two receptors work synergistically to ensure bile acid homeostasis. Like FXR, PXR activation represses hepatic CYP7A1, the rate-limiting bile acid synthesis enzyme. PXR activation further promotes the expression of hepatocyte OATP2 (which can facilitate bile acid uptake by hepatocytes), CYP3A11 and SULT2A1 (which transform bile acids to promote their detoxification and excretion), and MRP2 (which promotes their canalicular transport) [174, 179–181]. These properties were hypothesized to counteract cholestatic injury. Accordingly, Pxr−/− mice were more susceptible to LCA feeding [180] and cholestasis induced by bile duct ligation [182], and PXR agonist treatment reduced liver damage induced by both LCA and CA feeding [174, 180, 183] and by bile duct ligation [182] in wild-type mice.
Similar to other nuclear receptors, PXR expression is decreased by NF-κB activation, through interaction with RXRα, which heterodimerizes with PXR [184, 185]. A recent study showed that PXR can suppress both NF-κB and AP-1 signaling, thereby reducing the expression of inflammatory mediators. Accordingly, treatment with a PXR agonist repressed CCl4-induced expression of chemokine genes Ccl2 and Cxcl2 and reduced hepatic neutrophil infiltration and necrosis in mice [186]. Additionally, a role for PXR in the negative regulation of TLR4 in the intestine has emerged [187, 188].
However, there are potential adverse effects of PXR activation, specifically in metabolic health parameters. Studies in healthy volunteers showed that PXR activation by rifampin increased blood pressure, serum LDL, and total cholesterol, and worsened postprandial glucose tolerance [189–191]. Additionally, PXR was linked to chemoresistance in hepatocellular carcinoma by increasing the expression of DMET, as well as by inhibiting apoptosis [192]. Target-specific PXR activation that would circumvent adverse metabolic health effects, perhaps in combination with other treatments, could be useful for the treatment of liver diseases, however much remains to be explored.
Bile acid–related treatments in cholestatic liver diseases
For cholestatic liver diseases, bile acid–related treatments aim at (a) reducing hepatic bile acid accumulation, (b) reducing bile acid toxicity, (c) promoting bile flow, and (d) reducing inflammation. As for (a) reducing hepatic bile acid accumulation, an approach is to reduce the bile acid pool size by interrupting the bile acid enterohepatic circulation (Fig. 1). This can be achieved by bile acid–binding resins (e.g., cholestyramine, which is used for cholestatic pruritus and hypercholesterolemia) or by inhibition of intestinal ASBT, as these treatments increase the fecal loss of bile acids. This treatment strategy is similar to partial external biliary diversion performed in children with PFIC and Alagille disease [193]. ASBT inhibitors improved cholestatic injury in mice [194, 195] and cholestatic pruritus in PBC patients [196]. The ASBT inhibitor maralixibat was recently approved for the treatment of cholestatic pruritus in patients with Alagille syndrome [197], whereas trials for other cholestatic disorders are ongoing [198, 199]. The most common side effects were diarrhea and abdominal pain. The deficiency of fat-soluble vitamins, which require bile acids for intestinal absorption, was also reported [196, 197]. Besides bile acid–binding resins and ASBT inhibition, inhibition of hepatic NTCP may reduce bile acid uptake by hepatocytes. Because hepatocyte NTCP is the entry receptor for hepatitis D virus (HDV), the NTCP inhibitor bulevirtide was recently approved in Europe for the treatment of chronic HDV infection in HDV RNA positive patients with compensated liver disease. In mouse models of cholestatic liver damage, bulevirtide attenuated liver injury by reducing biliary bile acid output and increasing biliary lipid output [200, 201]. Reduction of hepatic synthesis of bile acids can be achieved by agonists of FXR and PXR and recombinant FGF19, as discussed in the previous sections (Fig. 1). The FXR agonist OCA is approved for PBC patients, as discussed above. As for (b) reducing bile acid toxicity and (c) promoting bile flow, biliary bile acid composition can be modulated by UDCA, TUDCA, and norUDCA, which render bile less hydrophobic and thus less cytotoxic, and by NTCP inhibition, which increases the phospholipids/bile acid ratio [200, 201] (Fig. 1). (T)UDCA and norUDCA further promote bicarbonate-rich bile flow [202]. In particular, norUDCA escapes hepatic conjugation and can be thereby reabsorbed passively by the biliary epithelium, to be returned to hepatocytes for re-secretion (cholehepatic shunting). Both re-secretion by hepatocytes and bicarbonate secretion into bile upon norUDCA reabsorption can contribute to increased bile flow [203]. UDCA is approved for PBC, cholestasis of pregnancy, and cholesterol gallstone dissolution. UDCA is also used for PFIC3, cystic fibrosis–related liver disease (CFLD), and PSC, although long-term efficacy is uncertain due to the lack of large clinical trials [204]. norUDCA as a treatment for PSC is being evaluated in a phase 3 study (NCT03872921) after promising results in a phase 2 study [205]. As for (d) reducing inflammation, besides anti-inflammatory and immune-modulatory agents [206], bile acid–related targets include hepatic FXR, PXR, and TGR5 agonists, as discussed in the previous sections. Interestingly, immunomodulatory effects of norUDCA were recently demonstrated in Mdr2−/− mice and mice infected with non-cytolytic lymphocytic choriomeningitis virus (LCMV), a model of non-cholestatic liver injury. By modulating mTORC1 activity in CD8+ cells, norUDCA impaired the activation-induced metabolic reprogramming of CD8+ cells and significantly alleviated hepatic inflammation [207]. UDCA also has anti-inflammatory actions, reviewed elsewhere [204]. In addition to cholestatic diseases, targeting FXR, FGF19, and TGR5 signaling have also shown important therapeutic benefits for the treatment of NASH [208].
The most benefit from these treatments could likely be obtained by combining several approaches, depending on the type of cholestasis. Choosing the right treatment at the appropriate time is also important. It was proposed that drugs reducing bile acid synthesis are best used early, when the hepatic adaptations to cholestasis, which include suppression of bile acid synthesis, are not yet established [11].
Bile acids shape the gut microbiome by providing feeding substrate and by exerting antimicrobial activities. In turn, the gut microbiome shapes the bile acid pool composition by carrying out enzymatic activities (e.g., deconjugation and dehydroxylation) that modify primary bile acids, resulting in bile acids that have different affinities to bile acid receptors and can thus influence bile acid receptors signaling [209]. The gut-liver axis has been implicated in the pathophysiology of several liver diseases and the gut microbiota is altered in several liver diseases. Therefore, modulation of the gut microbiota is a potential therapeutic approach and can be achieved by dietary changes, prebiotics, probiotics, antibiotics, as well as by fecal microbiota transplantation and bacteriophages [210]. The relevance of the gut microbiota in liver diseases has been recently reviewed [211].
Conclusion
Bile acids and their receptors modulate inflammation in the context of liver diseases. On the one hand, bile acid accumulation in cholestasis causes hepatocellular damage and is pro-inflammatory (Fig. 2). On the other hand, activation of bile acid receptors by bile acids exerts anti-inflammatory actions by repressing NF-κB signaling and the NLRP3 inflammasome, among other pathways (Figs. 3 and 4). Additional anti-inflammatory effects obtained by modulating bile acid receptors arise from their roles in regulating bile acid homeostasis, which have the potential to attenuate cholestasis, as observed in preclinical studies. Clinical studies lead to the approval of several bile acid receptor and bile acid–related treatments, such as obeticholic acid, UDCA, and the ASBT inhibitor maralixibat. A large number of clinical trials are ongoing, especially for FXR agonists and recombinant FGF19 (Fig. 1). However, the clinical use of bile acid receptors modulators and other bile acid–related treatments is hampered by their (potential, long-term) side effects, which stem from ubiquitous bile acid receptors expression, breadth of target signaling pathways, or both. This is a challenge especially for TGR5 modulators. The first steps to circumvent these challenges are underway, with the development of pathway-specific modulators. A combination treatment with nuclear receptor ligands and bile acids with different therapeutic effects may also be of interest. Another aspect that should be taken into account when designing clinical trials for cholestatic liver diseases is the anatomical heterogeneity of the disease process and the “ascending” pathogenesis of cholestatic liver diseases, as discussed by Jansen et al. [11]. Along with clinical trials, preclinical studies remain essential to further characterize the downstream effects of bile acid receptors modulation and to elucidate the working mechanisms in various liver diseases.
Abbreviations
- ANIT
Alpha-naphthylisothiocyanate
- alpha-GalCer
Alpha-galactosylceramide
- AP-1
Activator protein 1
- ASBT
Apical sodium–dependent bile acid transporter
- ASC
Apoptosis-associated speck-like protein containing a CARD
- BSEP
Bile salt export pump
- CA
Cholic acid
- CAR
Constitutive androstane receptor
- CDCA
Chenodeoxycholic acid
- CCL2
C-C motif chemokine ligand 2
- C/EBPβ
CCAAT/enhancer-binding protein β
- CFTR
Cystic fibrosis transmembrane conductance regulator
- CFLD
Cystic fibrosis–related liver disease
- Con A
Concanavalin A
- CRE
CAMP response element
- CREB
CAMP response element-binding protein
- CYP3A11
Cytochrome P450 3A11
- CYP450
Cytochrome P450 family 7 subfamily A member 1
- CYP7A1
Cholesterol 7-a-hydroxylase
- Cxcl2
X-X-C motif chemokine ligand 2
- DAMPs
Damage-associated molecular patterns
- DCA
Deoxycholic acid
- DMET
Drug metabolizing enzymes and transporters
- FGF19
Fibroblast growth factor 19
- FGFR4
Fibroblast growth factor receptor 4
- FIC1
Familial intrahepatic cholestasis 1
- FXR
Farnesoid X receptor
- HSC
Hepatic stellate cells
- HDV
Hepatitis D virus
- IκBα
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha
- JAM-A
Junctional adhesion molecule A
- KLB
Coreceptor β-klotho
- LCA
Lithocholic acid
- LIP
Liver-inhibitory protein
- lncRNA H19
Long non-coding RNA H19
- LPS
Lipopolysaccharide
- MCA
Muricholic acid
- MDR2
Multidrug resistance protein 2
- MDR3
Multidrug resistance protein 3
- MDSCs
Myeloid-derived suppressor cells
- MRP2
Multidrug resistance–associated protein 2
- mTOR complex 1
Mammalian target of rapamycin complex 1
- NASH
Non-alcoholic steatohepatitis
- NTCP
Na+-dependent taurocholate cotransporting peptide
- NFAT
Nuclear factor of activated T-cells
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRP3
NOD-, LRR-, and pyrin domain–containing protein 3
- norUDCA
Nor-ursodeoxycholic acid
- NR1H4
Nuclear receptor subfamily 1 group H member 4
- OATP2
Organic anion–transporting polypeptide 2
- OCA
Obeticholic acid
- PAMPs
Pathogen-associated molecular patterns
- PBC
Primary biliary cholangitis
- PFIC1
Progressive familial intrahepatic cholestasis type 1
- PFIC2
Progressive familial intrahepatic cholestasis type 2
- PFIC3
Progressive familial intrahepatic cholestasis type 2
- PSC
Primary sclerosing cholangitis
- PXR
Pregnane X receptor
- SHP
Small heterodimer partner
- SOCS3
Suppressor of cytokine signaling 3
- STAT3
Signal transducer and activator of transcription 3
- SULT2A1
Sulfotransferase family 2A member 1
- TCA
Tauro-cholic acid
- TGR5
Takeda-G-protein-receptor-5
- TLR9
Toll-like receptor 9
- TUDCA
Tauro-ursodeoxycholic acid
- UDCA
Ursodeoxycholic acid
Funding
This work was supported in part by the National Institutes of Health under the award NIH RO1 DK096096 and the Yale Liver Center award NIH P30 DK034989.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
This article is a contribution to the special issue on: Tolerance and autoimmunity in the liver—Guest Editors: Christoph Schramm, Ansgar Lohse & Ye Oo
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.De Boer JF, Verkade E, Mulder NL, et al. A human-like bile acid pool induced by deletion of hepatic Cyp2c70 modulates effects of FXR activation in mice. J Lipid Res. 2020;61:291–305. doi: 10.1194/jlr.RA119000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawson PA, Karpen SJ. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56:1085–1099. doi: 10.1194/jlr.R054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang JYL. Bile acids: regulation of synthesis. J Lipid Res. 2009;50:1955–1966. doi: 10.1194/jlr.R900010-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann AF, Hagey LR. Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci. 2008;65:2461–2483. doi: 10.1007/s00018-008-7568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719–730. doi: 10.1016/S0022-2275(20)38331-0. [DOI] [PubMed] [Google Scholar]
- 6.Attili AF, Angelico M, Cantafora A, et al. Bile acid-induced liver toxicity: relation to the hydrophobic-hydrophilic balance of bile acids. Med Hypotheses. 1986;19:57–69. doi: 10.1016/0306-9877(86)90137-4. [DOI] [PubMed] [Google Scholar]
- 7.Schölmerich J, Becher M-S, Schmidt K, et al. Influence of hydroxylation and conjugation of bile salts on their membrane-damaging properties-studies on isolated hepatocytes and lipid membrane vesicles. Hepatology. 1984;4:661–666. doi: 10.1002/hep.1840040416. [DOI] [PubMed] [Google Scholar]
- 8.Galle PR, Theilmann L, Raedsch R, et al. Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology. 1990;12:486–491. doi: 10.1002/hep.1840120307. [DOI] [PubMed] [Google Scholar]
- 9.Patel T, Bronk SF, Gores GJ. Increases of intracellular magnesium promote glycodeoxycholate-induced apoptosis in rat hepatocytes. J Clin Invest. 1994;94:2183–2192. doi: 10.1172/JCI117579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fickert P, Wagner M. Biliary bile acids in hepatobiliary injury – what is the link? J Hepatol. 2017;67:619–631. doi: 10.1016/j.jhep.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 11.Jansen PLM, Ghallab A, Vartak N, et al. The ascending pathophysiology of cholestatic liver disease. Hepatology. 2017;65:722–738. doi: 10.1002/hep.28965. [DOI] [PubMed] [Google Scholar]
- 12.Afonso MB, Rodrigues PMP, Simão AL, et al. Activation of necroptosis in human and experimental cholestasis. Cell Death Dis. 2016;7:e2390–e2390. doi: 10.1038/cddis.2016.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghallab A, Hofmann U, Sezgin S, et al. Bile microinfarcts in cholestasis are initiated by rupture of the apical hepatocyte membrane and cause shunting of bile to sinusoidal blood. Hepatology. 2019;69:666–683. doi: 10.1002/hep.30213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes. Am J Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Hong J, Rockwell CE, et al. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012;32:58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai S-Y, Ouyang X, Chen Y, et al. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight. 2017;2:1–13. doi: 10.1172/jci.insight.90780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai S-Y, Yu D, Soroka CJ, et al. Hepatic NFAT signaling regulates the expression of inflammatory cytokines in cholestasis. J Hepatol. 2021;74:550–559. doi: 10.1016/j.jhep.2020.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gujral JS, Liu J, Farhood A, et al. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Liver Physiol. 2004;286:G499–G507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- 19.Zou T-T, Zhu Y, Wan C-M, Liao Q. Clinical features of sodium-taurocholate cotransporting polypeptide deficiency in pediatric patients case series and literature review. Transl Pediatr. 2021;10:1045–1054. doi: 10.21037/tp-20-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slijepcevic D, Kaufman C, Wichers CGK, et al. Impaired uptake of conjugated bile acids and hepatitis b virus pres1-binding in na + -taurocholate cotransporting polypeptide knockout mice. Hepatology. 2015;62:207–219. doi: 10.1002/hep.27694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felzen A, Verkade HJ. The spectrum of progressive familial intrahepatic cholestasis diseases: update on pathophysiology and emerging treatments. Eur J Med Genet. 2021;64:104317. doi: 10.1016/j.ejmg.2021.104317. [DOI] [PubMed] [Google Scholar]
- 22.Svegliati-Baroni G, Ridolfi F, Hannivoort R, et al. Bile acids induce hepatic stellate cell proliferation via activation of the epidermal growth factor receptor. Gastroenterology. 2005;128:1042–1055. doi: 10.1053/j.gastro.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Strazzabosco M, Joplin R, Zsembery A, et al. Na(+)-dependent and -independent Cl-/HCO3- exchange mediate cellular HCO3- transport in cultured human intrahepatic bile duct cells. Hepatology. 1997;25:976–985. doi: 10.1002/hep.510250431. [DOI] [PubMed] [Google Scholar]
- 24.Hohenester S, de Buy M, Wenniger L, Paulusma CC, et al. A biliary HCO 3 − umbrella constitutes a protective mechanism against bile acid-induced injury in human cholangiocytes. Hepatology. 2012;55:173–183. doi: 10.1002/hep.24691. [DOI] [PubMed] [Google Scholar]
- 25.Xia X, Francis H, Glaser S, et al. Bile acid interactions with cholangiocytes. World J Gastroenterol. 2006;12:3553–3563. doi: 10.3748/wjg.v12.i22.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smit JJM, Groen K, Mel CAAM, et al. Homozygous disruption of the murine MDR2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 27.Fickert P, Fuchsbichler A, Wagner M, et al. Regurgitation of bile acids from leaky bile ducts causes sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2004;127:261–274. doi: 10.1053/j.gastro.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Harada K, Chiba M, Okamura A, et al. Monocyte chemoattractant protein-1 derived from biliary innate immunity contributes to hepatic fibrogenesis. J Clin Pathol. 2011;64:660–665. doi: 10.1136/jclinpath-2011-200040. [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama T, Komori A, Nakamura M, et al. Human intrahepatic biliary epithelial cells function in innate immunity by producing IL-6 and IL-8 via the TLR4-NF-kappaB and -MAPK signaling pathways. Liver Int. 2006;26:467–476. doi: 10.1111/j.1478-3231.2006.01254.x. [DOI] [PubMed] [Google Scholar]
- 30.Strazzabosco M, Fiorotto R, Cadamuro M, et al. Pathophysiologic implications of innate immunity and autoinflammation in the biliary epithelium. Biochim Biophys Acta - Mol Basis Dis. 2018;1864:1374–1379. doi: 10.1016/j.bbadis.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazaridis KN, Strazzabosco M, LaRusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Yang M, Ramachandran A, Yan H-M, et al. Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol Lett. 2014;224:186–195. doi: 10.1016/j.toxlet.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savard CE, Blinman TA, Choi H-S, et al. Expression of cytokine and chemokine mRNA and secretion of tumor necrosis factor-α by gallbladder epithelial cells: response to bacterial lipopolysaccharides. BMC Gastroenterol. 2002;2:23. doi: 10.1186/1471-230X-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamireau T, Zoltowska M, Levy E, et al. Effects of bile acids on biliary epithelial cells: proliferation, cytotoxicity, and cytokine secretion. Life Sci. 2003;72:1401–1411. doi: 10.1016/S0024-3205(02)02408-6. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Aoki H, Yang J, et al. The role of sphingosine 1-phosphate receptor 2 in bile-acid-induced cholangiocyte proliferation and cholestasis-induced liver injury in mice. Hepatology. 2017;65:2005–2018. doi: 10.1002/hep.29076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao Y, Liu R, Li X, et al. Long noncoding RNA H19 contributes to cholangiocyte proliferation and cholestatic liver fibrosis in biliary atresia. Hepatology. 2019;70:1658–1673. doi: 10.1002/hep.30698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Liu R, Wang Y, et al. Cholangiocyte-derived exosomal lncRNA H19 promotes macrophage activation and hepatic inflammation under cholestatic conditions. Cells. 2020;9:190. doi: 10.3390/cells9010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benedetti A, Alvaro D, Bassotti C, et al. Cytotoxicity of bile salts against biliary epithelium: a study in isolated bile ductule fragments and isolated perfused rat liver. Hepatology. 1997;26:9–21. doi: 10.1002/hep.510260102. [DOI] [PubMed] [Google Scholar]
- 39.Matoba N, Une M, Hoshita T. Identification of unconjugated bile acids in human bile. J Lipid Res. 1990;27:1154–1162. doi: 10.1016/S0022-2275(20)38751-4. [DOI] [PubMed] [Google Scholar]
- 40.Keane RM, Gadacz TR, Munster AM, et al. Impairment of human lymphocyte function by bile salts. Surgery. 1984;95:439–443. [PubMed] [Google Scholar]
- 41.Gianni L, Di Padova F, Zuin M, Podda M. Bile acid-induced inhibition of the lymphoproliferative response to phytohemagglutinin and pokeweed mitogen: an in vitro study. Gastroenterology. 1980;78:231–235. doi: 10.1016/0016-5085(80)90570-3. [DOI] [PubMed] [Google Scholar]
- 42.Yoshikawa M, Tsujii T, Matsumura K, et al. Immunomodulatory effects of ursodeoxycholic acid on immune responses. Hepatology. 1992;16:358–364. doi: 10.1002/hep.1840160213. [DOI] [PubMed] [Google Scholar]
- 43.Lacaille F, Paradis K. The immunosuppressive effect of ursodeoxycholic acid: a comparative in vitro study on human peripheral blood mononuclear cells. Hepatology. 1993;18:165–172. [PubMed] [Google Scholar]
- 44.Yamada K, Lim BO, Sugano M. Suppression of immunoglobulin production of rat lymphocytes by bile acids. Vitr Cell Dev Biol - Anim. 1993;29:840–841. doi: 10.1007/BF02631360. [DOI] [PubMed] [Google Scholar]
- 45.Greve JW, Gouma DJ, Buurman WA. Bile acids inhibit endotoxin-induced release of tumor necrosis factor by monocytes: Anin Vitro study. Hepatology. 1989;10:454–458. doi: 10.1002/hep.1840100409. [DOI] [PubMed] [Google Scholar]
- 46.Calmus Y, Guechot J, Podevin P, et al. Differential effects of chenodeoxycholic and ursodeoxycholic acids on interleukin 1, interleukin 6 and tumor necrosis factor–α production by monocytes. Hepatology. 1992;16:719–723. doi: 10.1002/hep.1840160317. [DOI] [PubMed] [Google Scholar]
- 47.Bergamini A, Dini L, Baiocchi L, et al. Bile acids with differing hydrophilic-hydrophobic properties do not influence cytokine production by human monocytes and murine Kupffer cells. Hepatology. 1997;25:927–933. doi: 10.1002/hep.510250423. [DOI] [PubMed] [Google Scholar]
- 48.Sung JJY, Go MYY. Reversible Kupffer cell suppression in biliary obstruction is caused by hydrophobic bile acids. J Hepatol. 1999;30:413–418. doi: 10.1016/S0168-8278(99)80099-3. [DOI] [PubMed] [Google Scholar]
- 49.Van Bossuyt H, Desmaretz C, Gaeta GB, Wisse E. The role of bile acids in the development of endotoxemia during obstructive jaundice in the rat. J Hepatol. 1990;10:274–279. doi: 10.1016/0168-8278(90)90132-B. [DOI] [PubMed] [Google Scholar]
- 50.Hao H, Cao L, Jiang C, et al. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab. 2017;25:856–867.e5. doi: 10.1016/j.cmet.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H, Chen J, Hollister K, et al. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543–553. doi: 10.1016/S1097-2765(00)80348-2. [DOI] [PubMed] [Google Scholar]
- 52.Parks DJ, Blanchard SG, Bledsoe RK, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science (80) 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 53.Sayin SI, Wahlström A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR ANTAGONIST. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 54.Panzitt K, Wagner M. FXR in liver physiology: multiple faces to regulate liver metabolism. Biochim Biophys Acta - Mol Basis Dis. 2021;1867:166133. doi: 10.1016/j.bbadis.2021.166133. [DOI] [PubMed] [Google Scholar]
- 55.Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uhlén M, Fagerberg L, Hallström BM et al (2015) Tissue-based map of the human proteome. Science 347(6220):1260419. 10.1126/SCIENCE.1260419 [DOI] [PubMed]
- 57.The Human Protein Atlas (2021) Cell type atlas - NR1H4. https://www.proteinatlas.org/ENSG00000012504-NR1H4/celltype. Accessed 16 Nov 2021
- 58.Houten SM, Volle DH, Cummins CL, et al. In vivo imaging of farnesoid X receptor activity reveals the ileum as the primary bile acid signaling tissue. Mol Endocrinol. 2007;21:1312–1323. doi: 10.1210/me.2007-0113. [DOI] [PubMed] [Google Scholar]
- 59.Beigneux AP, Moser AH, Shigenaga JK, et al. The acute phase response is associated with retinoid X receptor repression in rodent liver. J Biol Chem. 2000;275:16390–16399. doi: 10.1074/jbc.M000953200. [DOI] [PubMed] [Google Scholar]
- 60.Kim MS, Shigenaga J, Moser A, et al. Repression of farnesoid X receptor during the acute phase response. J Biol Chem. 2003;278:8988–8995. doi: 10.1074/jbc.M212633200. [DOI] [PubMed] [Google Scholar]
- 61.Gadaleta RM, Oldenburg B, Willemsen ECL, et al. Activation of bile salt nuclear receptor FXR is repressed by pro-inflammatory cytokines activating NF-κB signaling in the intestine. Biochim Biophys Acta - Mol Basis Dis. 2011;1812:851–858. doi: 10.1016/j.bbadis.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Renga B, Migliorati M, Mencarelli A, Fiorucci S. Reciprocal regulation of the bile acid-activated receptor FXR and the interferon-γ-STAT-1 pathway in macrophages. Biochim Biophys Acta - Mol Basis Dis. 2009;1792:564–573. doi: 10.1016/j.bbadis.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y-D, Chen W-D, Wang M, et al. Farnesoid X receptor antagonizes nuclear factor κB in hepatic inflammatory response. Hepatology. 2008;48:1632–1643. doi: 10.1002/hep.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pascual G, Glass CK. Nuclear receptors versus inflammation: mechanisms of transrepression. Trends Endocrinol Metab. 2006;17:321–327. doi: 10.1016/j.tem.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Liu T, Zhang L, Joo D, Sun S-C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gadaleta RM, van Erpecum KJ, Oldenburg B, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463–472. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 67.Li YTY, Swales KE, Thomas GJ, et al. Farnesoid X receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler Thromb Vasc Biol. 2007;27:2606–2611. doi: 10.1161/ATVBAHA.107.152694. [DOI] [PubMed] [Google Scholar]
- 68.Chen Y-H, Hu X-G, Zhou Y, et al. Obeticholic acid protects against lipopolysaccharide-induced fetal death and intrauterine growth restriction through its anti-inflammatory activity. J Immunol. 2016;197:4762–4770. doi: 10.4049/jimmunol.1601331. [DOI] [PubMed] [Google Scholar]
- 69.Zhang D-G, Zhang C, Wang J-X, et al. Obeticholic acid protects against carbon tetrachloride-induced acute liver injury and inflammation. Toxicol Appl Pharmacol. 2017;314:39–47. doi: 10.1016/j.taap.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 70.Gai Z, Visentin M, Gui T, et al. Effects of farnesoid X receptor activation on arachidonic acid metabolism, NF-kB signaling, and hepatic inflammation. Mol Pharmacol. 2018;94:802–811. doi: 10.1124/mol.117.111047. [DOI] [PubMed] [Google Scholar]
- 71.Fiorucci S, Antonelli E, Rizzo G, et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 72.Yang Z, Koehler AN, Wang L. A novel small molecule activator of nuclear receptor SHP inhibits HCC cell migration via suppressing Ccl2. Mol Cancer Ther. 2016;15:2294–2301. doi: 10.1158/1535-7163.MCT-16-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, Liu C, Barbier O, et al. Bcl2 is a critical regulator of bile acid homeostasis by dictating Shp and lncRNA H19 function. Sci Rep. 2016;6:20559. doi: 10.1038/srep20559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim D, Xiao Z, Kwon S, et al. A dysregulated acetyl/SUMO switch of FXR promotes hepatic inflammation in obesity. EMBO J. 2015;34:184–199. doi: 10.15252/embj.201489527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bijsmans ITGW, Guercini C, Ramos Pittol JM, et al. The glucocorticoid mometasone furoate is a novel FXR ligand that decreases inflammatory but not metabolic gene expression. Sci Rep. 2015;5:14086. doi: 10.1038/srep14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang C-S, Kim J-J, Kim TS, et al. Small heterodimer partner interacts with NLRP3 and negatively regulates activation of the NLRP3 inflammasome. Nat Commun. 2015;6:6115. doi: 10.1038/ncomms7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iracheta-Vellve A, Calenda CD, Petrasek J, et al. FXR and TGR5 agonists ameliorate liver injury, steatosis, and inflammation after binge or prolonged alcohol feeding in mice. Hepatol Commun. 2018;2:1379–1391. doi: 10.1002/hep4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yao J, Zhou C-S, Ma X, et al. FXR agonist GW4064 alleviates endotoxin-induced hepatic inflammation by repressing macrophage activation. World J Gastroenterol. 2014;20:14430–14441. doi: 10.3748/wjg.v20.i39.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu H, Lee T, Liao J. GW4064 attenuates lipopolysaccharide-induced hepatic inflammation and apoptosis through inhibition of the Toll-like receptor 4-mediated p38 mitogen-activated protein kinase signaling pathway in mice. Int J Mol Med. 2018;41:1455–1462. doi: 10.3892/ijmm.2018.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu Z, Huang G, Gong W, et al. FXR ligands protect against hepatocellular inflammation via SOCS3 induction. Cell Signal. 2012;24:1658–1664. doi: 10.1016/j.cellsig.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 81.He G, Karin M. NF-κB and STAT3 – key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li G, Zhu Y, Tawfik O, et al. Mechanisms of STAT3 activation in the liver of FXR knockout mice. Am J Physiol Liver Physiol. 2013;305:G829–G837. doi: 10.1152/ajpgi.00155.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang F, Huang X, Yi T, et al. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 84.Kim I, Morimura K, Shah Y, et al. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takahashi S, Tanaka N, Fukami T, et al. Role of farnesoid X receptor and bile acids in hepatic tumor development. Hepatol Commun. 2018;2:1567–1582. doi: 10.1002/hep4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martinez-Becerra P, Vaquero J, Romero MR, et al. No Correlation between the expression of FXR and genes involved in multidrug resistance phenotype of primary liver tumors. Mol Pharm. 2012;9:1693–1704. doi: 10.1021/mp300028a. [DOI] [PubMed] [Google Scholar]
- 87.Su H, Ma C, Liu J, et al. Downregulation of nuclear receptor FXR is associated with multiple malignant clinicopathological characteristics in human hepatocellular carcinoma. Am J Physiol Liver Physiol. 2012;303:G1245–G1253. doi: 10.1152/ajpgi.00439.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Girisa S, Henamayee S, Parama D, et al. Targeting farnesoid X receptor (FXR) for developing novel therapeutics against cancer. Mol Biomed. 2021;2:21. doi: 10.1186/s43556-021-00035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang X, Zhao W, Huang W. FXR and liver carcinogenesis. Acta Pharmacol Sin. 2015;36:37–43. doi: 10.1038/aps.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fiorucci S, Biagioli M, Zampella A, Distrutti E. Bile acids activated receptors regulate innate immunity. Front Immunol. 2018;9:1–17. doi: 10.3389/fimmu.2018.01853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang H, Liu Y, Bian Z, et al. The critical role of myeloid-derived suppressor cells and FXR activation in immune-mediated liver injury. J Autoimmun. 2014;53:55–66. doi: 10.1016/j.jaut.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 92.Cui YJ, Aleksunes LM, Tanaka Y, et al. Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice. Toxicol Sci. 2009;110:47–60. doi: 10.1093/toxsci/kfp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu Y, Binz J, Numerick MJ, et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Golen RF, Olthof PB, Lionarons DA, et al. FXR agonist obeticholic acid induces liver growth but exacerbates biliary injury in rats with obstructive cholestasis. Sci Rep. 2018;8:16529. doi: 10.1038/s41598-018-33070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stedman C, Liddle C, Coulter S, et al. Benefit of farnesoid X receptor inhibition in obstructive cholestasis. Proc Natl Acad Sci. 2006;103:11323–11328. doi: 10.1073/pnas.0604772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nevens F, Andreone P, Mazzella G, et al. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 97.Trauner M, Nevens F, Shiffman ML, et al. Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol Hepatol. 2019;4:445–453. doi: 10.1016/S2468-1253(19)30094-9. [DOI] [PubMed] [Google Scholar]
- 98.Bowlus CL, Pockros PJ, Kremer AE, et al. Long-term obeticholic acid therapy improves histological endpoints in patients with primary biliary cholangitis. Clin Gastroenterol Hepatol. 2020;18:1170–1178.e6. doi: 10.1016/j.cgh.2019.09.050. [DOI] [PubMed] [Google Scholar]
- 99.Harms MH, Hirschfield GM, Floreani A, et al. Obeticholic acid is associated with improvements in AST-to-platelet ratio index and GLOBE score in patients with primary biliary cholangitis. JHEP Reports. 2021;3:100191. doi: 10.1016/j.jhepr.2020.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hirschfield GM, Mason A, Luketic V, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology. 2015;148:751–761.e8. doi: 10.1053/j.gastro.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 101.Roberts SB, Ismail M, Kanagalingam G, et al. Real-world effectiveness of obeticholic acid in patients with primary biliary cholangitis. Hepatol Commun. 2020;4:1332–1345. doi: 10.1002/hep4.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.D’Amato D, De Vincentis A, Malinverno F, et al. Real-world experience with obeticholic acid in patients with primary biliary cholangitis. JHEP Reports. 2021;3:100248. doi: 10.1016/j.jhepr.2021.100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lindor KD, Bowlus CL, Boyer J, et al. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;69:hep.30145. doi: 10.1002/hep.30145. [DOI] [PubMed] [Google Scholar]
- 104.Kjærgaard K, Frisch K, Sørensen M, et al. Obeticholic acid improves hepatic bile acid excretion in patients with primary biliary cholangitis. J Hepatol. 2021;74:58–65. doi: 10.1016/j.jhep.2020.07.028. [DOI] [PubMed] [Google Scholar]
- 105.U.S FDA (2021) 05–26–2021 FDA Drug Safety Communication. https://www.fda.gov/drugs/drug-safety-and-availability/due-risk-serious-liver-injury-fda-restricts-use-ocaliva-obeticholic-acid-primary-biliary-cholangitis
- 106.Kowdley KV, Vuppalanchi R, Levy C, et al. A randomized, placebo-controlled, phase II study of obeticholic acid for primary sclerosing cholangitis. J Hepatol. 2020;73:94–101. doi: 10.1016/j.jhep.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Intercept (2022) Our clinical development program. https://www.interceptpharma.com/our-research/pipeline/. Accessed 19 Mar 2022
- 108.Schramm C, Hirschfield G, Mason AL, et al. Early assessment of safety and efficacy of tropifexor, a potent non bile-acid FXR agonist, in patients with primary biliary cholangitis: an interim analysis of an ongoing phase 2 study. J Hepatol. 2018;68:S103. doi: 10.1016/S0168-8278(18)30426-4. [DOI] [Google Scholar]
- 109.Trauner M, Gulamhusein A, Hameed B, et al. The nonsteroidal farnesoid X receptor agonist cilofexor (GS-9674) improves markers of cholestasis and liver injury in patients with primary sclerosing cholangitis. Hepatology. 2019;70:788–801. doi: 10.1002/hep.30509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kowdley K, Minuk G, Pagadala M et al (2019) The nonsteroidal farnesoid X receptor (FXR) agonist cilofexor improves liver biochemistry in patients with primary biliary cholangitis (PBC): a phase 2, randomized, placebo-controlled trial. Hepatology 70(Suppl.1):1A. 10.1002/hep.30940
- 111.Pockros PJ, Fuchs M, Freilich B, et al. CONTROL: a randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int. 2019;39:2082–2093. doi: 10.1111/liv.14209. [DOI] [PubMed] [Google Scholar]
- 112.Gadaleta RM, Moschetta A. Dark and bright side of targeting fibroblast growth factor receptor 4 in the liver. J Hepatol. 2021;75:1440–1451. doi: 10.1016/j.jhep.2021.07.029. [DOI] [PubMed] [Google Scholar]
- 113.Inagaki T, Choi M, Moschetta A, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 114.Nicholes K, Guillet S, Tomlinson E, et al. A mouse model of hepatocellular carcinoma. Am J Pathol. 2002;160:2295–2307. doi: 10.1016/S0002-9440(10)61177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhou M, Yang H, Learned RM, et al. Non-cell-autonomous activation of IL-6/STAT3 signaling mediates FGF19-driven hepatocarcinogenesis. Nat Commun. 2017;8:15433. doi: 10.1038/ncomms15433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luo J, Ko B, Elliott M, et al. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med. 2014;6:1–12. doi: 10.1126/scitranslmed.3009098. [DOI] [PubMed] [Google Scholar]
- 117.Sanyal AJ, Ling L, Beuers U, et al. Potent suppression of hydrophobic bile acids by aldafermin, an FGF19 analogue, across metabolic and cholestatic liver diseases. JHEP Reports. 2021;3:100255. doi: 10.1016/j.jhepr.2021.100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Luo J, Ko B, Elliott M, et al. A nontumorigenic variant of FGF19 treats cholestatic liver diseases. Sci Transl Med. 2014;6:247ra100–247ra100. doi: 10.1126/scitranslmed.3009098. [DOI] [PubMed] [Google Scholar]
- 119.Zhou M, Learned RM, Rossi SJ, et al. Engineered fibroblast growth factor 19 reduces liver injury and resolves sclerosing cholangitis in Mdr2 -deficient mice. Hepatology. 2016;63:914–929. doi: 10.1002/hep.28257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gadaleta RM, Scialpi N, Peres C, et al. Suppression of hepatic bile acid synthesis by a non-tumorigenic FGF19 analogue protects mice from fibrosis and hepatocarcinogenesis. Sci Rep. 2018;8:17210. doi: 10.1038/s41598-018-35496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Modica S, Petruzzelli M, Bellafante E, et al. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology. 2012;142:355–365.e4. doi: 10.1053/j.gastro.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 122.Mayo MJ, Wigg AJ, Leggett BA, et al. <scp>NGM</scp> 282 for treatment of patients with primary biliary cholangitis: a multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Commun. 2018;2:1037–1050. doi: 10.1002/hep4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hirschfield GM, Chazouillères O, Drenth JP, et al. Effect of NGM282, an FGF19 analogue, in primary sclerosing cholangitis: a multicenter, randomized, double-blind, placebo-controlled phase II trial. J Hepatol. 2019;70:483–493. doi: 10.1016/j.jhep.2018.10.035. [DOI] [PubMed] [Google Scholar]
- 124.Bio N (2022) NGM Bio 2022 Strategic Priorities. https://ir.ngmbio.com/news-releases/news-release-details/ngm-bio-outlines-2022-strategic-priorities-across-its-portfolio. Accessed 19 Mar 2022
- 125.The Human Protein Atlas (2021) Tissue expression of GPBAR1. https://www.proteinatlas.org/ENSG00000179921-GPBAR1/tissue. Accessed 16 Nov 2021
- 126.Masyuk AI, Huang BQ, Radtke BN, et al. Ciliary subcellular localization of TGR5 determines the cholangiocyte functional response to bile acid signaling. Am J Physiol Liver Physiol. 2013;304:G1013–G1024. doi: 10.1152/ajpgi.00383.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Keitel V, Cupisti K, Ullmer C, et al. The membrane-bound bile acid receptor TGR5 is localized in the epithelium of human gallbladders. Hepatology. 2009;50:861–870. doi: 10.1002/hep.23032. [DOI] [PubMed] [Google Scholar]
- 128.Keitel V, Donner M, Winandy S, et al. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 129.Keitel V, Reinehr R, Gatsios P, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45:695–704. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 130.Sawitza I, Kordes C, Götze S, et al. Bile acids induce hepatic differentiation of mesenchymal stem cells. Sci Rep. 2015;5:13320. doi: 10.1038/srep13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Biagioli M, Carino A, Fiorucci C, et al. GPBAR1 functions as gatekeeper for liver NKT cells and provides counterregulatory signals in mouse models of immune-mediated hepatitis. Cell Mol Gastroenterol Hepatol. 2019;8:447–473. doi: 10.1016/j.jcmgh.2019.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Holter MM, Chirikjian MK, Briere DA, et al. Compound 18 improves glucose tolerance in a hepatocyte TGR5-dependent manner in mice. Nutrients. 2020;12:2124. doi: 10.3390/nu12072124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.The Human Protein Atlas (2021) Cell type atlas - GPBAR1. https://www.proteinatlas.org/ENSG00000179921-GPBAR1/celltype. Accessed 16 Nov 2021
- 134.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 135.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/S0006-291X(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 136.Guo C, Chen W-D, Wang Y-D. TGR5, not only a metabolic regulator. Front Physiol. 2016;7:1–9. doi: 10.3389/fphys.2016.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van Nierop FS, Scheltema MJ, Eggink HM, et al. Clinical relevance of the bile acid receptor TGR5 in metabolism. Lancet Diabetes Endocrinol. 2017;5:224–233. doi: 10.1016/S2213-8587(16)30155-3. [DOI] [PubMed] [Google Scholar]
- 138.de Buy M, Wenniger LJ, Hohenester S, Maroni L, et al. The cholangiocyte glycocalyx stabilizes the ‘biliary HCO3- umbrella’’: an integrated line of defense against toxic bile acids’. Dig Dis. 2015;33:397–407. doi: 10.1159/000371864. [DOI] [PubMed] [Google Scholar]
- 139.Reich M, Deutschmann K, Sommerfeld A, et al. TGR5 is essential for bile acid-dependent cholangiocyte proliferation in vivo and in vitro. Gut. 2016;65:487–501. doi: 10.1136/gutjnl-2015-309458. [DOI] [PubMed] [Google Scholar]
- 140.Erice O, Labiano I, Arbelaiz A, et al. Differential effects of FXR or TGR5 activation in cholangiocarcinoma progression. Biochim Biophys Acta - Mol Basis Dis. 2018;1864:1335–1344. doi: 10.1016/j.bbadis.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 141.Deutschmann K, Reich M, Klindt C, et al. Bile acid receptors in the biliary tree: TGR5 in physiology and disease. Biochim Biophys Acta - Mol Basis Dis. 2018;1864:1319–1325. doi: 10.1016/j.bbadis.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 142.Li T, Holmstrom SR, Kir S, et al. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol. 2011;25:1066–1071. doi: 10.1210/me.2010-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Merlen G, Kahale N, Ursic-Bedoya J, et al. TGR5-dependent hepatoprotection through the regulation of biliary epithelium barrier function. Gut. 2020;69:146–157. doi: 10.1136/gutjnl-2018-316975. [DOI] [PubMed] [Google Scholar]
- 144.Hov JR, Keitel V, Laerdahl JK, et al. Mutational characterization of the bile acid receptor TGR5 in primary sclerosing cholangitis. PLoS ONE. 2010;5:e12403. doi: 10.1371/journal.pone.0012403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Biagioli M, Carino A, Cipriani S, et al. The bile acid receptor GPBAR1 regulates the M1/M2 phenotype of intestinal macrophages and activation of GPBAR1 rescues mice from murine colitis. J Immunol. 2017;199:718–733. doi: 10.4049/jimmunol.1700183. [DOI] [PubMed] [Google Scholar]
- 146.Högenauer K, Arista L, Schmiedeberg N, et al. G-protein-coupled bile acid receptor 1 (GPBAR1, TGR5) agonists reduce the production of proinflammatory cytokines and stabilize the alternative macrophage phenotype. J Med Chem. 2014;57:10343–10354. doi: 10.1021/jm501052c. [DOI] [PubMed] [Google Scholar]
- 147.Haselow K, Bode JG, Wammers M, et al. Bile acids PKA-dependently induce a switch of the IL-10/IL-12 ratio and reduce proinflammatory capability of human macrophages. J Leukoc Biol. 2013;94:1253–1264. doi: 10.1189/jlb.0812396. [DOI] [PubMed] [Google Scholar]
- 148.Hu J, Wang C, Huang X, et al. Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling. Cell Rep. 2021;36:109726. doi: 10.1016/j.celrep.2021.109726. [DOI] [PubMed] [Google Scholar]
- 149.Kida T, Tsubosaka Y, Hori M, et al. Bile acid receptor TGR5 agonism induces NO production and reduces monocyte adhesion in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1663–1669. doi: 10.1161/ATVBAHA.113.301565. [DOI] [PubMed] [Google Scholar]
- 150.Yoneno K, Hisamatsu T, Shimamura K, et al. TGR5 signalling inhibits the production of pro-inflammatory cytokines by in vitro differentiated inflammatory and intestinal macrophages in Crohn’s disease. Immunology. 2013;139:19–29. doi: 10.1111/imm.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang Y-D, Chen W-D, Yu D, et al. The G-Protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology. 2011;54:1421–1432. doi: 10.1002/hep.24525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pols TWH, Nomura M, Harach T, et al. TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading. Cell Metab. 2011;14:747–757. doi: 10.1016/j.cmet.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang H, Luo F, Wei Y, et al. TGR5 protects against cholestatic liver disease via suppressing the NF-κB pathway and activating the Nrf2/HO-1 pathway. Ann Transl Med. 2021;9:1158–1158. doi: 10.21037/atm-21-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rao J, Yang C, Yang S, et al. Deficiency of TGR5 exacerbates immune-mediated cholestatic hepatic injury by stabilizing the β-catenin destruction complex. Int Immunol. 2020;32:321–334. doi: 10.1093/intimm/dxaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang L, Fu X, Gui T, et al. Effects of farnesiferol B on ischemia-reperfusion-induced renal damage, inflammation, and NF-κB signaling. Int J Mol Sci. 2019;20:6280. doi: 10.3390/ijms20246280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Perino A, Pols TWH, Nomura M, et al. TGR5 reduces macrophage migration through mTOR-induced C/EBPβ differential translation. J Clin Invest. 2014;124:5424–5436. doi: 10.1172/JCI76289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guo C, Xie S, Chi Z, et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45:802–816. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 158.Chen Y, Le TH, Du Q, et al. Genistein protects against DSS-induced colitis by inhibiting NLRP3 inflammasome via TGR5-cAMP signaling. Int Immunopharmacol. 2019;71:144–154. doi: 10.1016/j.intimp.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 159.Hu X, Yan J, Huang L, et al. INT-777 attenuates NLRP3-ASC inflammasome-mediated neuroinflammation via TGR5/cAMP/PKA signaling pathway after subarachnoid hemorrhage in rats. Brain Behav Immun. 2021;91:587–600. doi: 10.1016/j.bbi.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Leonhardt J, Haider RS, Sponholz C, et al. Circulating bile acids in liver failure activate TGR5 and induce monocyte dysfunction. Cell Mol Gastroenterol Hepatol. 2021;12:25–40. doi: 10.1016/j.jcmgh.2021.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Péan N, Doignon I, Garcin I, et al. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology. 2013;58:1451–1460. doi: 10.1002/hep.26463. [DOI] [PubMed] [Google Scholar]
- 162.Baghdasaryan A, Claudel T, Gumhold J, et al. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2 −/− ( Abcb4 −/− ) mouse cholangiopathy model by promoting biliary HCO 3− output. Hepatology. 2011;54:1303–1312. doi: 10.1002/hep.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Reich M, Spomer L, Klindt C, et al. Downregulation of TGR5 (GPBAR1) in biliary epithelial cells contributes to the pathogenesis of sclerosing cholangitis. J Hepatol. 2021;75:634–646. doi: 10.1016/j.jhep.2021.03.029. [DOI] [PubMed] [Google Scholar]
- 164.Masyuk TV, Masyuk AI, Lorenzo Pisarello M, et al. TGR5 contributes to hepatic cystogenesis in rodents with polycystic liver diseases through cyclic adenosine monophosphate/Gαs signaling. Hepatology. 2017;66:1197–1218. doi: 10.1002/hep.29284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Masyuk TV, Masyuk AI, LaRusso NF. TGR5 in the cholangiociliopathies. Dig Dis. 2015;33:420–425. doi: 10.1159/000371696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li A-D, Xie X-L, Qi W, et al. TGR5 promotes cholangiocarcinoma by interacting with mortalin. Exp Cell Res. 2020;389:111855. doi: 10.1016/j.yexcr.2020.111855. [DOI] [PubMed] [Google Scholar]
- 167.Renga B, Cipriani S, Carino A, et al. Reversal of endothelial dysfunction by GPBAR1 agonism in portal hypertension involves a AKT/FOXOA1 dependent regulation of H2S generation and endothelin-1. PLoS ONE. 2015;10:e0141082. doi: 10.1371/journal.pone.0141082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Renga B, Bucci M, Cipriani S, et al. Cystathionine γ-lyase, a H 2 S-generating enzyme, is a GPBAR1-regulated gene and contributes to vasodilation caused by secondary bile acids. Am J Physiol Circ Physiol. 2015;309:H114–H126. doi: 10.1152/ajpheart.00087.2015. [DOI] [PubMed] [Google Scholar]
- 169.Klindt C, Reich M, Hellwig B, et al. The G protein-coupled bile acid receptor TGR5 (Gpbar1) modulates endothelin-1 signaling in liver. Cells. 2019;8:1467. doi: 10.3390/cells8111467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fryer RM, Ng KJ, Mazurek SGN, et al. Correction to “G protein–coupled bile acid receptor 1 stimulation mediates arterial vasodilation through a KCa1.1 (BKCa)–dependent mechanism”. J Pharmacol Exp Ther. 2014;348:492–492. doi: 10.1124/jpet.114.03er14c. [DOI] [PubMed] [Google Scholar]
- 171.Alemi F, Kwon E, Poole DP, et al. The TGR5 receptor mediates bile acid–induced itch and analgesia. J Clin Invest. 2013;123:1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hodge RJ, Lin J, Vasist Johnson LS, et al. Safety, pharmacokinetics, and pharmacodynamic effects of a selective TGR5 agonist, SB-756050, in type 2 diabetes. Clin Pharmacol Drug Dev. 2013;2:213–222. doi: 10.1002/cpdd.34. [DOI] [PubMed] [Google Scholar]
- 173.The Human Protein Atlas (2021) Single cell type - NR1I2. https://www.proteinatlas.org/ENSG00000144852-NR1I2/single+cell+type. Accessed 4 Jan 2022
- 174.Staudinger JL, Goodwin B, Jones SA, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Oladimeji PO, Chen T. PXR: more than just a master xenobiotic receptor. Mol Pharmacol. 2018;93:119–127. doi: 10.1124/mol.117.110155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jonker JW, Liddle C, Downes M. FXR and PXR: potential therapeutic targets in cholestasis. J Steroid Biochem Mol Biol. 2012;130:147–158. doi: 10.1016/j.jsbmb.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mackowiak B, Hodge J, Stern S, Wang H. The roles of xenobiotic receptors: beyond chemical disposition. Drug Metab Dispos. 2018;46:1361–1371. doi: 10.1124/dmd.118.081042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jung D, Mangelsdorf DJ, Meyer UA. Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem. 2006;281:19081–19091. doi: 10.1074/jbc.M600116200. [DOI] [PubMed] [Google Scholar]
- 179.Kast HR, Goodwin B, Tarr PT, et al. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- 180.Xie W, Radominska-Pandya A, Shi Y, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wagner M, Halilbasic E, Marschall H-U, et al. CAR and PXR agonists stimulate hepatic bile acid and bilirubin detoxification and elimination pathways in mice. Hepatology. 2005;42:420–430. doi: 10.1002/hep.20784. [DOI] [PubMed] [Google Scholar]
- 182.Stedman CAM, Liddle C, Coulter SA, et al. Nuclear receptors constitutive androstane receptor and pregnane X receptor ameliorate cholestatic liver injury. Proc Natl Acad Sci. 2005;102:2063–2068. doi: 10.1073/pnas.0409794102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Teng S, Piquette-Miller M. Hepatoprotective role of PXR activation and MRP3 in cholic acid-induced cholestasis. Br J Pharmacol. 2007;151:367–376. doi: 10.1038/sj.bjp.0707235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gu X, Ke S, Liu D, et al. Role of NF-κB in regulation of PXR-mediated gene expression. J Biol Chem. 2006;281:17882–17889. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- 185.Zhou C, Tabb MM, Nelson EL, et al. Mutual repression between steroid and xenobiotic receptor and NF-κB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006;116:2280–2289. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Okamura M, Shizu R, Abe T, et al. PXR functionally interacts with NF-κB and AP-1 to downregulate the inflammation-induced expression of chemokine CXCL2 in mice. Cells. 2020;9:2296. doi: 10.3390/cells9102296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Qiu Z, Cervantes JL, Cicek BB, et al. Pregnane X receptor regulates pathogen-induced inflammation and host defense against an intracellular bacterial infection through Toll-like receptor 4. Sci Rep. 2016;6:31936. doi: 10.1038/srep31936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Venkatesh M, Mukherjee S, Wang H, et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41:296–310. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rysä J, Buler M, Savolainen MJ, et al. Pregnane X receptor agonists impair postprandial glucose tolerance. Clin Pharmacol Ther. 2013;93:556–563. doi: 10.1038/clpt.2013.48. [DOI] [PubMed] [Google Scholar]
- 190.Hassani-Nezhad-Gashti F, Salonurmi T, Hautajärvi H, et al. Pregnane X receptor activator rifampin increases blood pressure and stimulates plasma renin activity. Clin Pharmacol Ther. 2020;108:856–865. doi: 10.1002/cpt.1871. [DOI] [PubMed] [Google Scholar]
- 191.Karpale M, Käräjämäki AJ, Kummu O, et al. Activation of pregnane X receptor induces atherogenic lipids and PCSK9 by a SREBP2-mediated mechanism. Br J Pharmacol. 2021;178:2461–2481. doi: 10.1111/bph.15433. [DOI] [PubMed] [Google Scholar]
- 192.Bhagyaraj E, Ahuja N, Kumar S, et al. TGF-β induced chemoresistance in liver cancer is modulated by xenobiotic nuclear receptor PXR. Cell Cycle. 2019;18:3589–3602. doi: 10.1080/15384101.2019.1693120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yang H, Porte RJ, Verkade HJ, et al. Partial external biliary diversion in children with progressive familial intrahepatic cholestasis and alagille disease. J Pediatr Gastroenterol Nutr. 2009;49:216–221. doi: 10.1097/MPG.0b013e31819a4e3d. [DOI] [PubMed] [Google Scholar]
- 194.Baghdasaryan A, Fuchs CD, Österreicher CH, et al. Inhibition of intestinal bile acid absorption improves cholestatic liver and bile duct injury in a mouse model of sclerosing cholangitis. J Hepatol. 2016;64:674–681. doi: 10.1016/j.jhep.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 195.Miethke AG, Zhang W, Simmons J, et al. Pharmacological inhibition of apical sodium-dependent bile acid transporter changes bile composition and blocks progression of sclerosing cholangitis in multidrug resistance 2 knockout mice. Hepatology. 2016;63:512–523. doi: 10.1002/hep.27973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hegade VS, Kendrick SFW, Dobbins RL, et al. Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: a double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet. 2017;389:1114–1123. doi: 10.1016/S0140-6736(17)30319-7. [DOI] [PubMed] [Google Scholar]
- 197.Shirley M. Maralixibat: first approval. Drugs. 2022;82:71–76. doi: 10.1007/s40265-021-01649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Karpen SJ, Kelly D, Mack C, Stein P. Ileal bile acid transporter inhibition as an anticholestatic therapeutic target in biliary atresia and other cholestatic disorders. Hepatol Int. 2020;14:677–689. doi: 10.1007/s12072-020-10070-w. [DOI] [PubMed] [Google Scholar]
- 199.Trauner M, Fuchs CD, Halilbasic E, Paumgartner G. New therapeutic concepts in bile acid transport and signaling for management of cholestasis. Hepatology. 2017;65:1393–1404. doi: 10.1002/hep.28991. [DOI] [PubMed] [Google Scholar]
- 200.Slijepcevic D, Roscam Abbing RLP, Fuchs CD, et al. Na+-taurocholate cotransporting polypeptide inhibition has hepatoprotective effects in cholestasis in mice. Hepatology. 2018;68:1057–1069. doi: 10.1002/hep.29888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Roscam Abbing RLP, Slijepcevic D, Donkers JM, et al. Blocking sodium-taurocholate cotransporting polypeptide stimulates biliary cholesterol and phospholipid secretion in mice. Hepatology. 2020;71:247–258. doi: 10.1002/hep.30792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Beuers U. Drug insight: mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:318–328. doi: 10.1038/ncpgasthep0521. [DOI] [PubMed] [Google Scholar]
- 203.Yoon YB, Hagey LR, Hofmann AF, et al. Effect of side-chain shortening on the physiologic properties of bile acids: hepatic transport and effect on biliary secretion of 23-nor-ursodeoxycholate in rodents. Gastroenterology. 1986;90:837–852. doi: 10.1016/0016-5085(86)90859-0. [DOI] [PubMed] [Google Scholar]
- 204.Cabrera D, Arab JP, Arrese M. UDCA, NorUDCA, and TUDCA in liver diseases: a review of their mechanisms of action and clinical applications. In: Distrutti E, editor. Fiorucci S. Springer: Bile acids and their receptors; 2019. pp. 237–264. [DOI] [PubMed] [Google Scholar]
- 205.Fickert P, Hirschfield GM, Denk G, et al. norUrsodeoxycholic acid improves cholestasis in primary sclerosing cholangitis. J Hepatol. 2017;67:549–558. doi: 10.1016/j.jhep.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 206.Mazzetti M, Marconi G, Mancinelli M, et al. The management of cholestatic liver diseases: current therapies and emerging new possibilities. J Clin Med. 2021;10:1763. doi: 10.3390/jcm10081763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhu C, Boucheron N, Müller AC, et al. 24-Norursodeoxycholic acid reshapes immunometabolism in CD8+ T cells and alleviates hepatic inflammation. J Hepatol. 2021;75:1164–1176. doi: 10.1016/j.jhep.2021.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Li T, Chiang JYL. Bile acid-based therapies for non-alcoholic steatohepatitis and alcoholic liver disease. Hepatobiliary Surg Nutr. 2020;9:152–169. doi: 10.21037/hbsn.2019.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tannous BA, Teng J. Secreted blood reporters: insights and applications. Biotechnol Adv. 2011;29:997–1003. doi: 10.1016/j.biotechadv.2011.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Quigley EM, Gajula P. Recent advances in modulating the microbiome. F1000Research. 2020;9:46. doi: 10.12688/f1000research.20204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang R, Tang R, Li B, et al. Gut microbiome, liver immunology, and liver diseases. Cell Mol Immunol. 2021;18:4–17. doi: 10.1038/s41423-020-00592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]