Abstract
Vitamin D deficiency is highly prevalent in athletes. Increased utilisation and storage depletion may be key contributing factor. We found a higher prevalence of vitamin D inadequacy (deficiency/ insufficiency) in power than endurance sport athletes, which may be related to vitamin D utilisation and reserve in skeletal muscles.
Keywords: Athletes, Vitamin D, Vitamin D deficiency, Musculoskeletal injury, Power sport, Supplementation
1. Introduction
Nutrition is an important element in sports medicine. Vitamin D is a fat-soluble sterol nutrient and hormone precursor required for many essential functions in overall health. It is synthesised in the skin, produced in the body upon 15–30 min of sunlight exposure, or absorbed from fatty fish, egg yolk, dairy products and/or fortified foods.
An individual's vitamin D status is defined by the serum 25(OH)D concentration.1, 2, 3 The Endocrine Society Committee (ESC) has defined concentration of ≥30 ng/mL (≥75 nmol/L) as sufficient, 21–29 ng/mL (52.5–72.5 nmol/L) as insufficient, and ≤20 ng/ml (≤50 nmol/L) as deficient.4 Concentration of 100 ng/mL may contribute to vitamin D toxicity.5 The recommended daily vitamin D intake for ages 18–70 is 1500–2000 IU6 or 600 IU7 according to the ESC and Institute of Medicine respectively. Actual dosage depends on age, geographical location, skin pigmentation, physical activity and season.
In the past decade, growing research interest into the role of vitamin D in sports medicine has unveiled a high prevalence of vitamin D deficiency in athletes.8 Low vitamin D levels have been demonstrated to have negative effects on muscle strength, power, and endurance; increase stress fractures and other musculoskeletal injuries; and affect acute muscle injuries and inflammation following high-intensity exercises.9 Nevertheless, currently there is no evaluation of vitamin D screening and supplementation protocol among elite athletes in the literature.
To address the problem of vitamin D deficiency in athletes, this perspective article will first provide a succinct background on the biochemistry and physiology of vitamin D, and then proceed to detail the evidence for vitamin D relevancy in athletes through pre-clinical and clinical lenses. We aim to provide biomedical scientists with a new perspective to investigate the causes of vitamin D deficiency in athletes; to athletes, coaches and sport nutritionists the knowledge on vitamin D source identification and optimal level maintenance; and to sports medicine practitioners and authoritative bodies more information on who and when to screen for vitamin D deficiency, and the possibility of supplementation. Knowledge exchange and collaborative effort among diverse experts will hopefully maximize the potential medical, social and economic benefits of treating vitamin D deficiency in athletes.
2. Vitamin D in athletes: Basic sciences
2.1. Factors contributing to vitamin D deficiency in athletes
Despite a presumably leaner physique and higher health consciousness in athletes, the high prevalence of vitamin D deficiency in athletes (56%10) as compared to the age-matched general population (24–40%11) is alarming. Vitamin D deficiency is supposedly caused by low bioavailability from sunlight and dietary intake,12 but to the best of our knowledge, there is no evidence to suggest a lower sun exposure nor dietary vitamin D intake in athletes when compared to non-athletes. On the other hand, a high prevalence of vitamin D deficiency has been documented in athletes who train outdoor and at latitudes favourable for sun exposure.13, 14, 15 Therefore, other factors may account for athletes’ unusual vulnerability to vitamin D deficiency. In particular, the role of vitamin D reserve and how exercise mobilises this reserve may be highly relevant.
2.2. General metabolic pathway
2.2.1. Regulation of circulating vitamin D levels
Major vitamin D metabolites include the precursor form (vitamin D2 and D3), circulating pre-hormone form (25(OH)D), bioactive form (1α,25(OH)2D) and excreted form (24,25(OH)2D).
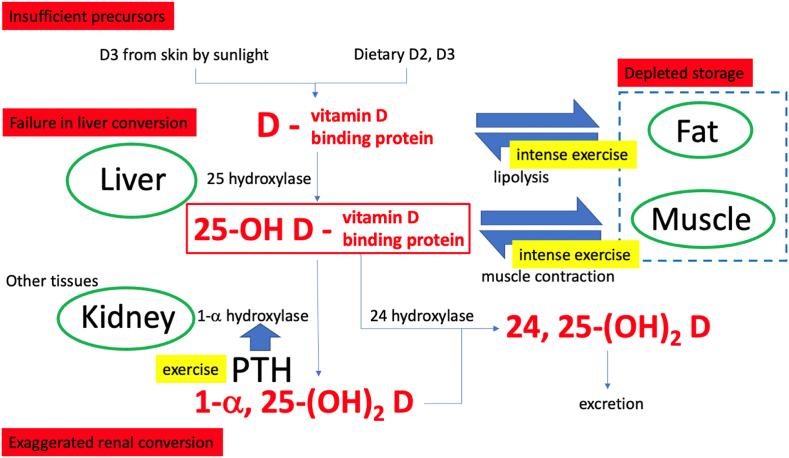
Three major enzymatic conversion steps are involved: 25-hydroxylation, 1α-hydroxylation and 24-hydroxylation.12 The main circulating form of vitamin D, 25(OH)D, is generated by 25-hydroxylation of vitamin D2 and D3 in the liver. 25(OH)D is then hydroxylated to 1α,25(OH)2D, the biologically active form of vitamin D, in the kidneys. Renal 1α-hydroxylation is tightly regulated by three hormones to maintain calcium and phosphate homoeostasis: the parathyroid hormone (PTH), fibroblast growth factor 23 (FGF23) and 1,25(OH)2D. PTH stimulates while FGF23 and 1,25(OH)2D inhibits the 1α-hydroxylase.12 Thus, the regulation of circulating 1α,25(OH)2D level is under a close negative feedback mechanism. Finally, vitamin D metabolites are cleared from the circulation via 24-hydroxylase-initiated catabolism, where biologically inactive 24,25(OH)2D is produced and excreted mainly through the bile (refer to Fig. 1).
Fig. 1.
Possible causes of vitamin D deficiency.
Traditionally, we refer to serum 25(OH)D concentration as the best biochemical marker of an individual's vitamin D status because of its relatively longer half-life than 1α,25(OH)2D.16 Majority of circulating 25(OH)D is bound to vitamin D binding protein (DBP). Though only free 25(OH)D is bioactive for renal conversion and responsible for subsequent biological effects of vitamin D, unresolved issues regarding their measurement and result interpretation hinder clinical application of bound/free form of 25(OH)D as marker for vitamin D status.17 Total circulating 25(OH)D (DBP-bound and free forms) is thus used as the measure to define vitamin D deficiency.
There are three sources of vitamin D and hence circulating 25(OH)D after hepatic conversion. The major source is endogenous synthesis in the skin, where upon exposure to ultraviolet B (UVB) radiation from the sun, 7-dehydrocholesterol, a liver-derived vitamin D precursor stored inside the plasma membrane of skin cells, eventually isomerizes to D3.12 Nutritional sources include fatty fish, fortified foodstuffs and supplements, where vitamin D2 and D3 can be obtained.18 Finally, despite less explored, the storage and release of D2, D3 and 25(OH)D from tissue reserve such as fat and muscle, may represent the third source of 25(OH)D in the circulation (refer to Fig. 1).
Taken together, there could theoretically be four possible causes of low serum 25(OH)D: (1) insufficient source from sunlight exposure and diet, (2) pathological deregulation in liver and renal conversion, (3) increased utilisation that depletes the storage, and (4) difficulties in mobilisation from reserve (refer to Fig. 1). As mentioned before, the high prevalence of vitamin D deficiency in athletes is less likely to be attributed solely to insufficient sources from sunlight or diet. Defective liver modification or exaggerated renal conversion are rare and may only happen in certain diseases. While the excreted form (24,25(OH)2D) is highly correlated to the circulating pre-hormone form (25(OH)D),19 to the best of our knowledge, no evidence has demonstrated vitamin D deficiency due to exaggerated excretion. Therefore, though less investigated, increased utilisation and mobilisation of vitamin D reserve may be a key determinant for vitamin D deficiency in athletes.
2.2.2. Vitamin D storage in fat and muscle
There are two principal storage sites of vitamin D in human: adipose tissues and skeletal muscle.20 As vitamin D reserve, fat stores the highest concentration of vitamin D metabolites among all body tissues, skeletal muscle is second.20
Vitamin D2 and D3, being more lipophilic than other vitamin D metabolites, are the major storage forms in fat.20 Long-term vitamin D3 supplementation leads to a substantial accumulation in adipose tissues.21 What regulate the storage of vitamin D2 and D3 in adipose tissues remains unknown. It could be by simple passive diffusion with an equilibrium between vitamin D2 and D3 in serum and fat globules,22 or by active processes which are yet to be confirmed. Lipolysis may be a mechanism of mobilising this reserve for hepatic conversion,23 but the release appears to be slow21 and exercise-dependent,24 while the exact trafficking remains largely unknown. Fat may represent a site of sequestration where once up-taken, vitamin D2 and D3 do not readily contribute to the circulating 25(OH)D level, especially in obese subjects23 (refer to Fig. 1). On the other hand, 1α,25(OH)2D stimulates its own degradation25 and reduces adipocyte fat contents,26 it may be inferred that increased vitamin D utilisation also reduces the storage in fat.21
Alternatively, skeletal muscle cells uptake and accumulate 25(OH)D through the DBP-mediated megalin-cubulin membrane transport process.27 DBP has two specific, high affinity binding sites: one for vitamin D and its metabolites, another for actin.28 The cell membrane receptor megalin and its associated protein cubulin mediate extracellular uptake of DBP, which is retained in the cell by its specific binding to actin; such a retention provides high affinity binding sites for 25(OH)D.29 It has been demonstrated that exercise leads to an immediate transient increase in serum 25(OH)D level.30 Intense exercise may trigger a quick release of 25(OH)D from skeletal muscle (refer to Fig. 1), though further investigation is required to confirm this process. In short, skeletal muscle and adipose tissues play important roles as vitamin D reserve; the extent of exercise-induced vitamin D metabolites mobilisation varies.
2.3. Exercise-induced changes related to vitamin D metabolism
First, exercise indirectly activates renal hydroxylation of 25(OH)D to 1α,25(OH)2D, increasing vitamin D metabolite utilisation (refer to Fig. 1). Second, the increased 1,25(OH)2D level activates 24-hydroxylase conversion even in the pre-hormone forms (D2 and D3), increased excretion of vitamin D may further aggravate storage depletion on top of mobilising 25(OH)D reserve to circulation (refer to Fig. 1). Overall, intense exercise represents higher physiological demands for 25(OH)D and 1,25(OH)2D, which may be met by increased recruitment of circulating or muscle-stored 25(OH)D (fat storage unknown).
3. Vitamin D in athletes: Clinical implications
3.1. Assessment of athletes
3.1.1. Clinical feature of athletes with Vitamin D deficiency
Of particular relevance to sports medicine practitioners, the clinical feature of athletes with vitamin D deficiency serves as a guide to screen for further investigation and/or intervention. This include: (1) female athletes with amenorrhea (low energy availability,31,32 on hormonal contraceptives32), (2) jumping sport athletes with a history of previous stress fracture33,34 (high vitamin D utilisation, low reserve), (3) indoor sport athletes who require weight-maintenance,35 and (4) collegiate athletes with busy schedules32,34 (decreased vitamin D supplementation via food intake).
Although the majority of vitamin D deficiency cases are asymptomatic, some athletes may present with non-specific symptoms, such as muscular weakness (predominantly of the proximal limb muscles), chronic musculoskeletal pain, or fatigue.36 Physical examination is usually unremarkable.
3.1.2. Risk factors for Vitamin D deficiency in athletes
Intrinsic factors that place athletes at increased risks of vitamin D deficiency include young age (under 18 year-old),37 lean body mass,38 and darker pigmented skin.38 Extrinsic risk factors include winter and early spring seasons, >40°N latitude, and indoor sport activities.10 Noteworthy though, with prolonged restriction to home-training under COVID-19, outdoor sport athletes may also be at increased risk of vitamin D deficiency.
3.2. Investigation
3.2.1. Degree of vitamin D deficiency and the type of sport
In addition to the aforementioned intrinsic and extrinsic factors, significant difference in serum 25(OH)D concentration is observed between sport disciplines in the literature. Due to study heterogeneity, it was not feasible to adjust for the different sport types when investigating into athletes’ vitamin D status.8
To fill this gap, we have done a preliminary review by including relevant studies which would contribute to a diverse pool of sport activities (Table 1). Outcome is the prevalence of vitamin D inadequacy [insufficiency (21–29 ng/mL) or deficiency (≤20 ng/mL)] in different types of sport (endurance, power, water, and jumping sport) (Table 2). This information serves as a general concept for future investigators to save time and effort when trying to answer this particular question.
Table 1.
Characteristics of included studies.
| Data Collection |
Participants |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Study type | Country (latitude) | Season | Serum 25(OH)D measurement | Sport Activity | Sample size | Male (%) | Age [mean (SD)] (years) | Vitamin D statusa (n) | Vitamin D inadequacy (deficient/insufficient)b |
| Barcal et al.39 | Cohort | USA (43°N) | Fall, winter, spring | CLIA (DiaSorin Inc., USA) | Wrestling | 18 | 100 | 20.9 (2.0) | Fall (19): Deficient: 2, Insufficient: 12 Winter & spring (16): Deficient: 4, Insufficient: 11 |
93.8% |
| Bescos Garcia et al.40 | Cohort | Spain (41°N) | Spring | CLIA (DiaSorin Inc., USA) | Basketball | 21 | 100 | 25 (4.3) | Deficient: 12 Insufficient: 0 |
57.1% |
| Dubnov-Raz et al.41 | RCT | Israel (32°N) | Winter | NR | Swimming | 80 | NR | NR | Deficient/insufficient: 53 | 66.3% |
| Ducher et al.42 | Cohort | Australia (37°S) | Winter | CLIA (DiaSorin Inc., USA) | Ballet | 18 | 100 | 16 (2.8) | Deficient: 2 Insufficient: 7 |
50.0% |
| Guillaume et al.43 | Cross-sectional | France (46°N) | NR | NR | Swimming | 29 | 59 | NR | Deficient/insufficient: 13 | 44.8% |
| Geiker et al.44 | Cross-sectional | Denmark (56°N) | Spring | NR | Cycling | 29 | 100 | 26.5 (5.3) | Deficient/insufficient: 3 | 10.3% |
| Josh William et al.45 | Cohort | UK (>51°N) | Fall | CLIA | Swimming | 29 | NR | 21.0 (3.0) | Deficient: 3 Insufficient: 16 |
65.5% |
| Kim et al.46 | Cohort | South Korea (37°N) | NR | CLIA (Roche Diagnostics, Switzerland) | Volleyball | 52 | 100 | 23.8 (2.8) | Deficient: 14 Insufficient: 24 |
73.1% |
| Lovell et al.47 | Cross-sectional | Australia (35°S) | Spring | NR | Gymnastics | 18 | 0 | 13.6 (1.2) | Deficient: 1 Insufficient: 5 |
33.3% |
| Mielgo-Ayuso et al.48 | RCT | Spain (43°N) | Spring | HPLC TMS | Rowing | 36 | 100 | 27 (6) | Deficient: 4 Insufficient: 19 |
63.9% |
| Pollock et al.49 | Cross-sectional | UK (53°N) | Summer, winter | CLIA (DiaSorin Inc., USA) | Track and field | 63 | 51 | 24.9 (4.2) | Summer: Deficient: 8, Insufficient: 7 Winter: Deficient: 8, Insufficient: 6 |
22.2% |
| Umarov et al.50 | Case-control | Republic of Uzbekistan (41°N) | Summer, winter | ELISA (DIAsource, Belgium and LLC) | Swimming | 20 | 0 | 20.3 (0.6) | Summer: Deficient: 2, Insufficient: 16 Winter: Deficient: 6, Insufficient: 14 |
100% |
| Synchronised swimming | 20 | 0 | 21.1 (1.2) | Summer: Deficient: 0, Insufficient: 20 Winter: Deficient: 4, Insufficient: 16 |
100% | |||||
| Vitale et al.51 | Cohort | Italy (45°N) | Spring, summer, autumn, winter | CLIA (Siemens Healthcare Italy, Italy) | Alpine skiing | 152 | 59 | 24.1 (3.2) | Deficient: 45 Insufficient: 77 |
80.3% |
| Wentz et al.52 | Cross-sectional | USA (30°N) | NR | NR | Distance running | 59 | 0 | NR | Deficient: 3 Insufficient: 8 |
18.6% |
| Willis et al.53 | Cohort | USA (30°N) | NR | CLIA (DiaSorin Inc., USA) | Distance running | 19 | 47 | 28.3 (8.4) | Deficient: 2 Insufficient: 8 |
52.6% |
| Wolman et al.54 | Cohort | UK (52°N) | Summer, winter | CLIA (Roche Diagnostics, Switzerland) | Ballet | 19 | 32 | 26 (8.9) | Summer: Deficient: 2, Insufficient: 14 Winter: Deficient: 5, Insufficient: 14 |
100% |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; CLIA, Chemiluminescent immunoassay; ELISA, enzyme-linked immunosorbent assay; HPLC TMS, high-performance liquid chromatography tandem mass spectrometry; NR, not reported; RCT, randomized controlled trial.
Defined by serum 25-hydroxyvitamin D levels. Deficient: ≤20 ng/ml (≤50 nmol/l); insufficient: 21–29 ng/ml (52–74 nmol/l).
Defined by serum 25-hydroxyvitamin D levels. Deficient: ≤20 ng/ml (≤50 nmol/l); insufficient: 21–29 ng/ml (52–74 nmol/l).
Table 2.
Prevalence of vitamin D inadequacy in different types of sport.
| Type of sport (biophysical effect) | Sport activity | Included studiesa | Sample size (total) | Vitamin D inadequacy (deficient/insufficient)b |
|---|---|---|---|---|
| Endurance (high-cardiac remodelling) | Cycling, Rowing, Distance running | Guillaume et al.,43 Mielgo-Ayuso et al.,48 Wentz et al.,52 Willis et al.53 |
143 | 32.9%; n = 47 |
| Power (low-cardiac remodelling) | Alpine skiing, Track and field, Wrestling | Barcal et al.,39 Pollock et al.,49 Vitale et al.51 |
231 | 65.4%; n = 151 |
| Jumping sport (high-impact) | Basketball, Ballet, Gymnastics, Track and field, Volleyball | Bescos Garcia et al.,40 Ducher et al.,42 Kim et al.,46 Lovell et al.,47 Pollock et al.,49 Wolman et al.54 |
191 | 51.3%; n = 98 |
| Water sport (low-impact) | Swimming, Synchronised swimming | Dubnov-Raz et al.,41 Geiker et al.,44 Josh et al.,45 Umarov et al.50 |
178 | 68.5%; n = 122 |
For study characteristics, refer to Table 1.
Defined by serum 25-hydroxyvitamin D levels. Deficient: ≤20 ng/ml (≤50 nmol/l); insufficient: 21–29 ng/ml (52–74 nmol/l).
To the best of our knowledge, only indoor and outdoor sport is recognised in the literature which demonstrates a statistically significant association with vitamin D status.55 We furthered our investigation into the relationship between degree of vitamin D deficiency and type of sport base on two sets of evidence-based criteria – cardiovascular effects (endurance and power sport) and impact of weight-bearing (water and jumping sport). Cardiac-remodelling is greater in endurance sport (higher heart rate and cardiac output, lower blood pressure during exercise) than power sport. Jumping sport entails greater bone stimulation by mechanical forces than water sport.
Result in Table 2 suggests that vitamin D inadequacy is more prevalent in power sport (65.4%) than endurance sport (32.9%). Same association is found in a single study done on basketball, cross-country, soccer and track and field athletes, where track and field (power sport) athletes demonstrated a higher degree of vitamin D deficiency than cross-country (endurance sport) athletes.33 The higher prevalence of vitamin D inadequacy in power than endurance sport athletes may be related to vitamin D utilisation and reserve in skeletal muscles.
On the other hand, the correlation between impact of weight-bearing and vitamin D inadequacy in athletes is inconclusive. Table 2 shows a higher prevalence of vitamin D inadequacy in water sport (68.5%) than jumping sport (44.4%), but Constantini et al.56 demonstrated that vitamin D deficiency was more prevalent in dancers (94%) and basketball players (94%) than swimmers (33%), and Williams et al.33 and Constantini et al.56 reported the highest prevalence of vitamin D deficiency in track and field athletes, basketball players and dancers. These findings are inconsistent with our result in Table 2.
Of note, different sports have different effects on the body systems, physical and mental demands, and rates of impairing vitamin D status. Current meta-analyses can only minimize heterogeneity by stratification, adjusting for latitudes and season of serum 25(OH)D measurement. The populations being studied were also very diverse in terms of nationalities and living/training latitudes. Readers should interpret any findings with caution.
3.2.2. Timing of vitamin D measurement and the type of sport
There are two approaches to decide on the timing of serum 25(OH)D measurement in athletes. The traditional approach is to assess serum 25(OH)D concentrations with reference to seasonal variations. Serum 25(OH)D level should be assessed at least twice yearly in any athletes screened ‘at-risk’ – once in the early spring for the nadir, once in the late summer for a peak level.57 Supplementation may be tailored according to athlete's serum 25(OH)D basal level and predicted seasonal variations throughout a competitive sporting season. Another approach is to base the assessment on actual training intensity and the schedule of a competitive sporting season, which would be more specific to each type of sport. Evidence from the literature favours the former approach.58, 59, 60, 61, 62 Specifically, Vitale et al. reported that despite the physical effort spent, vitamin D follows a classical season-associated rhythm with a peak in summer and a nadir in winter for professional skiers.51 Nevertheless, either way provides information of paramount importance to enable a more individualized supplementation regimen for athletes.
3.3. Management
To address vitamin D deficiency in athletes, three treatment modalities exist: (1) sunlight, (2) artificial ultraviolet B radiation, or (3) supplementation. All have their potential risks and benefits. Benefits of all treatment modalities outweigh their potential risks and greatly outweigh the risk of no treatment.63
3.3.1. Effects of Vitamin D supplementation to athletes
Vitamin D supplementation increases the serum 25(OH)D concentration in athletes. Fairbairn et al.64 reported a significantly increased serum 25(OH)D concentration in supplemented professional rugby players compared to placebo (32 nmol/L difference between groups at 11–12 weeks). Jung et al.65 reported a significant increase in serum 25(OH)D concentrations in the vitamin D group (96.0 ± 3.77 nmol/L) of collegiate taekwondo athletes after 4 weeks of supplementation, but no changes in the placebo group.
Correcting low serum vitamin D levels reduces the risk of stress fractures,66 which are common in certain sport disciplines such as basketball, baseball, athletics, rowing, soccer, aerobics, and classical ballet.67 Very recent studies suggested that the prevalence of stress fractures decreased when athletes are supplemented daily with 800 IU 25(OH)D and 2000 mg calcium.34 High-risk collegiate athletes also demonstrated a statistically significant decrease in stress fracture rate from 7.51% to 1.65% with vitamin D3 supplementation.33
In addition, vitamin D supplementation may play an important role in the prevention of skeletal muscle injuries following exercise with eccentric muscle contraction in athletes.68 Żebrowska et al. reported that three weeks of vitamin D supplementation had a positive effect on serum 25(OH)D levels, and caused a marked decrease in post-exercise biomarkers (troponin, myoglobin, creatine kinase and lactic dehydrogenase) levels of ultra-marathon runners.
On the other hand, Han et al.69 and Farrokhyar et al.70 reported in their meta-analyses that there was no significant overall effect of vitamin D3 intervention on muscle strength, despite achieving sufficiency in 25(OH)D concentrations. Fairbairn et al.64 found that vitamin D supplementation had little impact on professional rugby players’ physical performance outcomes despite a significant improvement in vitamin D status. Of note, though vitamin D supplementation has no significant direct effect on athletic performance, potential participation time lost from musculoskeletal injuries due to vitamin D inadequacy will indirectly affect performance.
Regarding vitamin D supplementation and immunity, Jung et al.71 concluded from their randomized controlled trial that vitamin D3 supplementation may be effective in reducing the symptoms of upper respiratory tract infection during winter training in vitamin D insufficient taekwondo athletes. Pereira et al.72 reported in a recent systematic review and meta-analysis, that vitamin D deficiency was not associated with a higher chance of infection by COVID-19, but there is a positive association between vitamin D deficiency and the severity of the disease. A hypothesis now under scientific consideration is that taking vitamin supplements to raise serum 25(OH)D concentrations could quickly reduce the risk and/or severity of COVID-19.73
The target serum 25(OH)D levels should be above 40 ng/mL for fracture prevention, while optimal musculoskeletal benefits occur at levels above the current definition of sufficiency (>30 ng/mL), with no reported sports health benefits above 50 ng/mL.74 However, there is no optimal vitamin D supplementation regimen reported in the literature for serum 25(OH)D maintenance.75,76
3.3.2. Recommendations on supplementation
To the best of our knowledge, there is no evaluation of vitamin D screening and supplementation protocol among elite athletes reported in the literature. The following discussion aims to help sports medicine practitioners caring for a wide-variety of athletes make appropriate recommendations to avoid excessive, inadequate, or unnecessary supplementation.
Regarding the type of prescription, we recommend oral vitamin D3 (cholecalciferol), which may be more effective in restoring vitamin D levels than combined vitamin D3/D2 (ergocalciferol) therapy,77 since vitamin D3 has a superior bioavailability compared to vitamin D2 as a result of stronger association with vitamin D binding protein.78,79
Regarding the dosage and timing of administration, current evidence suggests that 2000–6000 IU of supplemental vitamin D3 can be consumed daily,9 a continuous consumption of <2000 IU may lead to sufficiency in vitamin D concentrations during spring and summer, and maintain sufficiency throughout the wintertime.70 However, as we believe the increased vitamin D utilisation in athletes may play a significant role in vitamin D deficiency, the administration of vitamin D supplementation to athletes who have a low basal vitamin D level would be too simplistic. The vitamin D requirements of these professionals may vary depending on the duration, intensity, type of training, and the body composition for vitamin D storage. Potentially this explains why thus far the vitamin D guidelines and guidance papers published have heterogeneous and partially opposed opinions and recommendations regarding vitamin D requirements, as the increased utilisation leading to depletion of vitamin D had not been taken into consideration. Supplementing the athletes with vitamin D when the levels are not depleted may be of limited value and may increase the risk of vitamin D toxicity. To establish the appropriate timing and dosage of vitamin D supplement, the athletes' vitamin D deficiency status should be guided by the levels after different training intensities for the identification of the ‘window period’ of vitamin D deficiency post-exercise.
There are a few additional considerations. First, although intense exercise may lead to a transient deficiency of vitamin D, exercise remains crucial to maintain the serum levels of vitamin D. The highest concentration of vitamin D metabolites is stored in fat, while long-term vitamin D supplementation leads to a substantial accumulation in adipose tissues; exercise stimulates lipid mobilisation from adipose tissue, potentially playing a crucial role in releasing vitamin D ‘trapped’ in adipocytes. This would explain why higher activity levels have been associated with higher serum vitamin D levels. Second, the lipolytic response of adipose tissue to exercise is impaired in obese subjects, which should be taken into consideration when determining the effective dose for vitamin D supplementation in them. Third, despite the increased UVB radiation from sunlight, it is recommended to supplement athletes training at altitude with up to 4000 IU/day of vitamin D, especially in winter months.70,80 Fourth, official recommendations should not limit the responsibility of clinicians to prevent or treat vitamin D deficiency in athletes. Current recommended daily intake values were not designed for and are not effective in preventing or treating vitamin D deficiency; instead, they are guidelines to prevent particular metabolic bone diseases.63 Finally, it is important that the clinician recognizes that vitamin D supplementation requirements are highly individualized due to their dependence on diet, endogenous synthesis, and storage.
3.3.3. Vitamin D toxicity
Theoretically, prolonged and disproportionate consumption of vitamin D supplement could induce hyperphosphatemia, hypercalcemia and hypercalciuria, which may impair organ function.81 Nevertheless, vitamin D toxicity which presents as asymptomatic hypercalcaemia, is exceedingly rare.82 Cranney et al. looked into twenty-two trials that assessed the adverse events associated with vitamin D supplementation: biochemical abnormalities such as hypercalcemia and hypercalciuria were most frequently reported, but the rates between vitamin D and placebo groups were not significant nor clinically relevant.83 Of note, most trials of higher vitamin D doses did not assess long-term adverse effects, extreme vitamin D supplementation may impair organ function (calcium and phosphorus dysregulation) even in hypovitaminosis D.82 Overall, there is fair evidence that adults tolerate vitamin D at doses above current dietary reference intake levels. From a safety perspective, the evidence supports higher doses of vitamin D supplementation to athletes.
4. Vitamin D in athletes: Future research theme
4.1. Relationship between training and vitamin D utilisation
The mechanism and exact relationship between training, vitamin D bioavailability and reserve depletion remain largely unknown. We suggest the following investigations to check on their correlation:
-
(1)
Investigation of exercise-induced changes in vitamin D metabolites to prove an increased utilisation. We postulate that all the excreted forms will increase, especially 24,25(OH)2D, which is the excreted form of active vitamin D.
-
(2)
Investigation to monitor changes in vitamin D storage in muscle and fat following prolonged physical activities to prove a depletion of storage.
-
(3)
Comparison of vitamin D deficiency status in athletes with different training intensities.
4.2. Degree of vitamin D deficiency and the type of sport
No observational studies have been done on this association. There were large inter-study heterogeneity in the data which we included (Table 1) for our preliminary review (Table 2). We may follow-up with a cross-sectional study on our local athletes at the Hong Kong Sports Institute. We postulate that the prevalence of vitamin D deficiency will be higher in power than endurance sport.
4.3. An update on the vitamin D3 supplementation guideline
Although the current 2000 IU tolerable upper limit (TUL) may be appropriate for young children, such limits in older children, adolescents and adults have both the effects of limiting effective treatment of vitamin D deficiency and impairing dose-appropriate interventional research. To support a revision of the official guidelines, more large-scale randomized controlled trials which study into the adverse effects of long-term high-dose vitamin D3 supplementation are warranted. Current epidemiological and open trials may not be sufficient to prompt government and medical bodies into reviewing the TUL and recommended daily allowance (RDA).
5. Conclusion
Vitamin D deficiency is highly prevalent in athletes. Increased utilisation and storage depletion may be an important contributing factor for the high prevalence of vitamin D inadequacy in power sport (65.4%) compared to endurance sport (32.9%) athletes. Clinical feature of athletes at risk of vitamin D deficiency include: (1) female athletes with amenorrhea, (2) jumping sport athletes with a history of previous stress fracture, (3) indoor sport athletes who require weight-maintenance, and (4) collegiate athletes with busy schedules. Ideally, serum 25(OH)D level should be assessed at least twice yearly in any athletes screened ‘at-risk’ – once in the early spring for the nadir and once in the late summer for a peak level. Supplemental oral vitamin D3 (cholecalciferol) may be tailored according to athlete's serum 25(OH)D basal levels and predicted seasonal variation throughout a competitive sporting season. Prescription at doses higher than the official recommendations are acceptable from a safety perspective.
Funding/Support statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors, and no material support of any kind was received.
Acknowledgements
Not Applicable.
Contributor Information
Tina Shuk-Tin Ip, Email: tinaipshuktin@hku.hk.
Sai-Chuen Fu, Email: Bruma@cuhk.edu.hk.
Michael Tim-Yun Ong, Email: michael.ong@cuhk.edu.hk.
Patrick Shu-Hang Yung, Email: patrickyung@cuhk.edu.hk.
References
- 1.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., et al. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clinic Endocrinol Metabol. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. https://doi-org.eproxy.lib.hku.hk/10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Catharine Ross A., Taylor C.L., Yaktine A.L., editors. Institute of Medicine of the National Academies. Dietary Reference Intakes For Calcium And Vitamin D. Washington, DC, USA: The National Academy of Sciences; 2011. pp. 96–97. chapter 3. [PubMed] [Google Scholar]
- 3.Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. https://doi-org.eproxy.lib.hku.hk/10.1056/NEJMra070553 [DOI] [PubMed] [Google Scholar]
- 4.Ross A.C. The 2011 report on dietary reference intakes for calcium and vitamin D. Publ Health Nutr. 2011;14(5):938–939. doi: 10.1017/S1368980011000565. https://doi-org.eproxy.lib.hku.hk/10.1017/S1368980011000565 [DOI] [PubMed] [Google Scholar]
- 5.Holick M.F. Vitamin D is not as toxic as was once thought: a historical and an up-to-date perspective. Mayo Clin Proc. 2015;90(5):561–564. doi: 10.1016/j.mayocp.2015.03.015. https://doi-org.eproxy.lib.hku.hk/10.1016/j.mayocp.2015.03.015 [DOI] [PubMed] [Google Scholar]
- 6.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., et al. Endocrine Society Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clinic Endocrinol Metabol. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. https://doi-org.eproxy.lib.hku.hk/10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
- 7.Catharine Ross A., Taylor C.L., Yaktine A.L., editors. Institute of Medicine of the National Academies. Dietary Reference Intakes For Calcium And Vitamin D. The National Academy of Sciences; Washington, DC, USA: 2011. :363. chapter 5. [PubMed] [Google Scholar]
- 8.Farrokhyar F., Tabasinejad R., Dao D., et al. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports Med. 2015;45(3):365–378. doi: 10.1007/s40279-014-0267-6. https://doi-org.eproxy.lib.hku.hk/10.1007/s40279-014-0267-6 [DOI] [PubMed] [Google Scholar]
- 9.Yoon S., Kwon O., Kim J. Vitamin D in athletes: focus on physical performance and musculoskeletal injuries. Phys Activity Nutri. 2021;25(2):20–25. doi: 10.20463/pan.2021.0011. https://doi-org.eproxy.lib.hku.hk/10.20463/pan.2021.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrokhyar F., Tabasinejad R., Dao D., et al. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports Med. 2015;45(3):365–378. doi: 10.1007/s40279-014-0267-6. https://doi-org.eproxy.lib.hku.hk/10.1007/s40279-014-0267-6 [DOI] [PubMed] [Google Scholar]
- 11.Amrein K., Scherkl M., Hoffmann M., et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74(11):1498–1513. doi: 10.1038/s41430-020-0558-y. https://doi-org.eproxy.lib.hku.hk/10.1038/s41430-020-0558-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bikle D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–329. doi: 10.1016/j.chembiol.2013.12.016. https://doi-org.eproxy.lib.hku.hk/10.1016/j.chembiol.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allison R.J., Close G.L., Farooq A., et al. Severely vitamin D-deficient athletes present smaller hearts than sufficient athletes. Europe j Preventive Cardiol. 2015;22(4):535–542. doi: 10.1177/2047487313518473. https://doi-org.eproxy.lib.hku.hk/10.1177/2047487313518473 [DOI] [PubMed] [Google Scholar]
- 14.Galan F., Ribas J., Sánchez-Martinez P.M., Calero T., Sánchez A.B., Muñoz A. Serum 25-hydroxyvitamin D in early autumn to ensure vitamin D sufficiency in mid-winter in professional football players. Clin Nutr. 2012;31(1):132–136. doi: 10.1016/j.clnu.2011.07.008. https://doi-org.eproxy.lib.hku.hk/10.1016/j.clnu.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 15.Hamilton B., Grantham J., Racinais S., Chalabi H. Vitamin D deficiency is endemic in Middle Eastern sportsmen. Publ Health Nutr. 2010;13(10):1528–1534. doi: 10.1017/S136898000999320X. https://doi-org.eproxy.lib.hku.hk/10.1017/S136898000999320X [DOI] [PubMed] [Google Scholar]
- 16.Herrmann M., Farrell C.L., Pusceddu I., Fabregat-Cabello N., Cavalier E. Assessment of vitamin D status - a changing landscape. Clin Chem Lab Med. 2017;55(1):3–26. doi: 10.1515/cclm-2016-0264. https://doi-org.eproxy.lib.hku.hk/10.1515/cclm-2016-0264 [DOI] [PubMed] [Google Scholar]
- 17.Fraser W.D., Tang J., Dutton J.J., Schoenmakers I. Vitamin D measurement, the debates continue, new analytes have emerged, developments have variable outcomes. Calcif Tissue Int. 2020;106(1):3–13. doi: 10.1007/s00223-019-00620-2. https://doi-org.eproxy.lib.hku.hk/10.1007/s00223-019-00620-2 [DOI] [PubMed] [Google Scholar]
- 18.Norman A.W. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clinic Nutri. 2008;88(2):491S–499S. doi: 10.1093/ajcn/88.2.491S. https://doi-org.eproxy.lib.hku.hk/10.1093/ajcn/88.2.491S [DOI] [PubMed] [Google Scholar]
- 19.Tang J., Jackson S., Walsh N.P., Greeves J., Fraser W.D., Facility team Bioanalytical. The dynamic relationships between the active and catabolic vitamin D metabolites, their ratios, and associations with PTH. Sci Rep. 2019;9(1):6974. doi: 10.1038/s41598-019-43462-6. https://doi-org.eproxy.lib.hku.hk/10.1038/s41598-019-43462-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mawer E.B., Backhouse J., Holman C.A., Lumb G.A., Stanbury S.W. The distribution and storage of vitamin D and its metabolites in human tissues. Clin Sci. 1972;43(3):413–431. doi: 10.1042/cs0430413. https://doi-org.eproxy.lib.hku.hk/10.1042/cs0430413 [DOI] [PubMed] [Google Scholar]
- 21.Martinaityte I., Kamycheva E., Didriksen A., Jakobsen J., Jorde R. Vitamin D stored in fat tissue during a 5-year intervention affects serum 25-hydroxyvitamin D levels the following year. J Clinic Endocrinol Metabol. 2017;102(10):3731–3738. doi: 10.1210/jc.2017-01187. https://doi-org.eproxy.lib.hku.hk/10.1210/jc.2017-01187 [DOI] [PubMed] [Google Scholar]
- 22.Drincic A.T., Armas L.A., Van Diest E.E., Heaney R.P. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity. 2012;20(7):1444–1448. doi: 10.1038/oby.2011.404. https://doi-org.eproxy.lib.hku.hk/10.1038/oby.2011.404 [DOI] [PubMed] [Google Scholar]
- 23.Di Nisio A., De Toni L., Sabovic I., et al. Impaired release of vitamin D in dysfunctional adipose tissue: new cues on vitamin D supplementation in obesity. J Clinic Endocrinol Metabol. 2017;102(7):2564–2574. doi: 10.1210/jc.2016-3591. https://doi-org.eproxy.lib.hku.hk/10.1210/jc.2016-3591 [DOI] [PubMed] [Google Scholar]
- 24.Hengist A., Perkin O., Gonzalez J.T., et al. Mobilising vitamin D from adipose tissue: the potential impact of exercise. Nutr Bull. 2019;44(1):25–35. doi: 10.1111/nbu.12369. https://doi-org.eproxy.lib.hku.hk/10.1111/nbu.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewison M., Zehnder D., Bland R., Stewart P.M. 1alpha-Hydroxylase and the action of vitamin D. J Mol Endocrinol. 2000;25(2):141–148. doi: 10.1677/jme.0.0250141. https://doi-org.eproxy.lib.hku.hk/10.1677/jme.0.0250141 [DOI] [PubMed] [Google Scholar]
- 26.Chang E., Kim Y. Vitamin D decreases adipocyte lipid storage and increases NAD-SIRT1 pathway in 3T3-L1 adipocytes. Nutrition. 2016;32(6):702–708. doi: 10.1016/j.nut.2015.12.032. https://doi-org.eproxy.lib.hku.hk/10.1016/j.nut.2015.12.032 [DOI] [PubMed] [Google Scholar]
- 27.Abboud M., Puglisi D.A., Davies B.N., et al. Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology. 2013;154(9):3022–3030. doi: 10.1210/en.2012-2245. https://doi-org.eproxy.lib.hku.hk/10.1210/en.2012-2245 [DOI] [PubMed] [Google Scholar]
- 28.Van Baelen H., Bouillon R., De Moor P. Vitamin D-binding protein (Gc-globulin) binds actin. J Biol Chem. 1980;255(6):2270–2272. [PubMed] [Google Scholar]
- 29.Mason R.S., Rybchyn M.S., Abboud M., Brennan-Speranza T.C., Fraser D.R. The role of skeletal muscle in maintaining vitamin D status in winter. Curr Develope Nutri. 2019;3(10):nzz087. doi: 10.1093/cdn/nzz087. https://doi-org.eproxy.lib.hku.hk/10.1093/cdn/nzz087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X., Cao Z.B., Taniguchi H., Tanisawa K., Higuchi M. Effect of an acute bout of endurance exercise on serum 25(OH)D concentrations in young adults. J Clinic Endocrinol Metabol. 2017;102(11):3937–3944. doi: 10.1210/jc.2017-00146. https://doi-org.eproxy.lib.hku.hk/10.1210/jc.2017-00146 [DOI] [PubMed] [Google Scholar]
- 31.Moss S.L., Randell R.K., Burgess D., et al. Assessment of energy availability and associated risk factors in professional female soccer players. Eur J Sport Sci. 2021;21(6):861–870. doi: 10.1080/17461391.2020.1788647. https://doi-org.eproxy.lib.hku.hk/10.1080/17461391.2020.1788647 [DOI] [PubMed] [Google Scholar]
- 32.Sheridan H.C., Parker L., Hammond K.M. Dietary supplements for consideration in elite female footballers. Eur J Sport Sci. 2022;22(5):733–744. doi: 10.1080/17461391.2021.1988149. https://doi-org.eproxy.lib.hku.hk/10.1080/17461391.2021.1988149 [DOI] [PubMed] [Google Scholar]
- 33.Williams K., Askew C., Mazoue C., Guy J., Torres-McGehee T.M., Jackson J.B., Iii Vitamin D3 supplementation and stress fractures in high-risk collegiate athletes - a pilot study. Orthop Res Rev. 2020;12:9–17. doi: 10.2147/ORR.S233387. https://doi-org.eproxy.lib.hku.hk/10.2147/ORR.S233387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knechtle B., Jastrzębski Z., Hill L., Nikolaidis P.T. Vitamin D and stress fractures in sport: preventive and therapeutic measures-A narrative review. Medicina. 2021;57(3):223. doi: 10.3390/medicina57030223. https://doi-org.eproxy.lib.hku.hk/10.3390/medicina57030223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson-Meyer D.E., Willis K.S. Vitamin D and athletes. Curr Sports Med Rep. 2010;9(4):220–226. doi: 10.1249/JSR.0b013e3181e7dd45. https://doi-org.eproxy.lib.hku.hk/10.1249/JSR.0b013e3181e7dd45 [DOI] [PubMed] [Google Scholar]
- 36.Erkal M.Z., Wilde J., Bilgin Y., et al. High prevalence of vitamin D deficiency, secondary hyperparathyroidism and generalized bone pain in Turkish immigrants in Germany: identification of risk factors. Osteoporos Int. 2006;17(8):1133–1140. doi: 10.1007/s00198-006-0069-2. https://doi-org.eproxy.lib.hku.hk/10.1007/s00198-006-0069-2 [DOI] [PubMed] [Google Scholar]
- 37.Tenforde A.S., Sayres L.C., Sainani K.L., Fredericson M. Evaluating the relationship of calcium and vitamin D in the prevention of stress fracture injuries in the young athlete: a review of the literature. PM & R. 2010;2(10):945–949. doi: 10.1016/j.pmrj.2010.05.006. https://doi-org.eproxy.lib.hku.hk/10.1016/j.pmrj.2010.05.006 [DOI] [PubMed] [Google Scholar]
- 38.Fields J.B., Payne D.C., Gallo S., Busteed D.R., Jones M.T. Vitamin D status differs by sex, sport-season, and skin pigmentation among elite collegiate basketball players. Sports. 2019;7(11):239. doi: 10.3390/sports7110239. https://doi-org.eproxy.lib.hku.hk/10.3390/sports7110239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barcal J.N., Thomas J.T., Hollis B.W., Austin K.J., Alexander B.M., Larson-Meyer D.E. Vitamin D and weight cycling: impact on injury, illness, and inflammation in collegiate wrestlers. Nutrients. 2016;8(12):775. doi: 10.3390/nu8120775. https://doi-org.eproxy.lib.hku.hk/10.3390/nu8120775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bescós García R., Rodríguez Guisado F.A. Low levels of vitamin D in professional basketball players after wintertime: relationship with dietary intake of vitamin D and calcium. Nutr Hosp. 2011;26(5):945–951. doi: 10.1590/S0212-16112011000500004. https://doi-org.eproxy.lib.hku.hk/10.1590/S0212-16112011000500004 [DOI] [PubMed] [Google Scholar]
- 41.Dubnov-Raz G., Livne N., Raz R., Cohen A.H., Constantini N.W. Vitamin D supplementation and physical performance in adolescent swimmers. Int J Sport Nutr Exerc Metabol. 2015;25(4):317–325. doi: 10.1123/ijsnem.2014-0180. https://doi-org.eproxy.lib.hku.hk/10.1123/ijsnem.2014-0180 [DOI] [PubMed] [Google Scholar]
- 42.Ducher G., Kukuljan S., Hill B., et al. Vitamin D status and musculoskeletal health in adolescent male ballet dancers a pilot study. J Dance Med Sci. 2011;15(3):99–107. [PubMed] [Google Scholar]
- 43.Guillaume G., Chappard D., Audran M. Evaluation of the bone status in high-level cyclists. J Clin Densitom. 2012;15(1):103–107. doi: 10.1016/j.jocd.2011.08.001. https://doi-org.eproxy.lib.hku.hk/10.1016/j.jocd.2011.08.001 [DOI] [PubMed] [Google Scholar]
- 44.Geiker N., Hansen M., Jakobsen J., et al. Vitamin D status and muscle function among adolescent and young swimmers. Int J Sport Nutr Exerc Metabol. 2017;27(5):399–407. doi: 10.1123/ijsnem.2016-0248. https://doi-org.eproxy.lib.hku.hk/10.1123/ijsnem.2016-0248 [DOI] [PubMed] [Google Scholar]
- 45.Newbury Josh William, Chessor Richard J., Evans Guy M., et al. The annual vitamin D status of world-class British swimmers following a standardised supplementation protocol for three years. 04 April 2022. PREPRINT (Version 1) available at Research Square [ [DOI]
- 46.Kim D.K., Park G., Kuo L.T., Park W.H. The relationship between vitamin D status and rotator cuff muscle strength in professional volleyball athletes. Nutrients. 2019;11(11):2768. doi: 10.3390/nu11112768. https://doi-org.eproxy.lib.hku.hk/10.3390/nu11112768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lovell G. Vitamin D status of females in an elite gymnastics program. Clin J Sport Med. 2008;18(2):159–161. doi: 10.1097/JSM.0b013e3181650eee. https://doi-org.eproxy.lib.hku.hk/10.1097/JSM.0b013e3181650eee [DOI] [PubMed] [Google Scholar]
- 48.Mielgo-Ayuso J., Calleja-González J., Urdampilleta A., et al. Effects of vitamin D supplementation on haematological values and muscle recovery in elite male traditional rowers. Nutrients. 2018;10(12):1968. doi: 10.3390/nu10121968. https://doi-org.eproxy.lib.hku.hk/10.3390/nu10121968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollock N., Dijkstra P., Chakraverty R., et al. Low 25(OH) vitamin D concentrations in international UK track and field athletes. S Afr J Sports Med. 2012;24(2):55–59. [Google Scholar]
- 50.Umarov J., Kerimov F., Toychiev A., Davis N., Osipova S. Association of the 25(OH) vitamin D status with upper respiratory tract infections morbidity in water sports elite athletes. J Sports Med Phys Fit. 2019;59(12):2058–2065. doi: 10.23736/S0022-4707.19.09834-7. https://doi-org.eproxy.lib.hku.hk/10.23736/S0022-4707.19.09834-7 [DOI] [PubMed] [Google Scholar]
- 51.Vitale J.A., Lombardi G., Cavaleri L., et al. Rates of insufficiency and deficiency of vitamin D levels in elite professional male and female skiers: a chronobiologic approach. Chronobiol Int. 2018;35(4):441–449. doi: 10.1080/07420528.2017.1410828. https://doi-org.eproxy.lib.hku.hk/10.1080/07420528.2017.1410828 [DOI] [PubMed] [Google Scholar]
- 52.Wentz L.M., Liu P.Y., Ilich J.Z., Haymes E.M. Female distance runners training in southeastern United States have adequate vitamin D status. Int J Sport Nutr Exerc Metabol. 2016;26(5):397–403. doi: 10.1123/ijsnem.2014-0177. https://doi-org.eproxy.lib.hku.hk/10.1123/ijsnem.2014-0177 [DOI] [PubMed] [Google Scholar]
- 53.Willis K.S., Smith D.T., Broughton K.S., Larson-Meyer D.E. Vitamin D status and biomarkers of inflammation in runners. Open Access J Sports Med. 2012;3:35–42. doi: 10.2147/OAJSM.S31022. https://doi-org.eproxy.lib.hku.hk/10.2147/OAJSM.S31022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolman R., Wyon M.A., Koutedakis Y., Nevill A.M., Eastell R., Allen N. Vitamin D status in professional ballet dancers: winter vs. summer. J Sci Med Sport. 2013;16(5):388–391. doi: 10.1016/j.jsams.2012.12.010. https://doi-org.eproxy.lib.hku.hk/10.1016/j.jsams.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 55.Peeling P., Fulton S.K., Binnie M., Goodman C. Training environment and Vitamin D status in athletes. Int J Sports Med. 2013;34(3):248–252. doi: 10.1055/s-0032-1321894. https://doi-org.eproxy.lib.hku.hk/10.1055/s-0032-1321894 [DOI] [PubMed] [Google Scholar]
- 56.Constantini N.W., Arieli R., Chodick G., Dubnov-Raz G. High prevalence of vitamin D insufficiency in athletes and dancers. Clin J Sport Med. 2010;20(5):368–371. doi: 10.1097/JSM.0b013e3181f207f2. https://doi-org.eproxy.lib.hku.hk/10.1097/JSM.0b013e3181f207f2 [DOI] [PubMed] [Google Scholar]
- 57.Holick M.F. The vitamin D epidemic and its health consequences. J Nutr. 2005;135(11) doi: 10.1093/jn/135.11.2739S. https://doi-org.eproxy.lib.hku.hk/10.1093/jn/135.11.2739S 2739S-48S. [DOI] [PubMed] [Google Scholar]
- 58.Valtueña J., Aparicio-Ugarriza R., Medina D., et al. Vitamin D status in Spanish elite team sport players. Nutrients. 2021;13(4):1311. doi: 10.3390/nu13041311. https://doi-org.eproxy.lib.hku.hk/10.3390/nu13041311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson-Barnes S.L., Hunt J., Williams E.L., et al. Seasonal variation in vitamin D status, bone health and athletic performance in competitive university student athletes: a longitudinal study. J Nutri Sci. 2020;9:e8. doi: 10.1017/jns.2020.1. https://doi-org.eproxy.lib.hku.hk/10.1017/jns.2020.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lombardi G., Vitale J.A., Logoluso S., et al. Circannual rhythm of plasmatic vitamin D levels and the association with markers of psychophysical stress in a cohort of Italian professional soccer players. Chronobiol Int. 2017;34(4):471–479. doi: 10.1080/07420528.2017.1297820. https://doi-org.eproxy.lib.hku.hk/10.1080/07420528.2017.1297820 [DOI] [PubMed] [Google Scholar]
- 61.Maruyama-Nagao A., Sakuraba K., Suzuki Y. Seasonal variations in vitamin D status in indoor and outdoor female athletes. Biomed Rep. 2016;5(1):113–117. doi: 10.3892/br.2016.671. https://doi-org.eproxy.lib.hku.hk/10.3892/br.2016.671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halliday T.M., Peterson N.J., Thomas J.J., Kleppinger K., Hollis B.W., Larson-Meyer D.E. Vitamin D status relative to diet, lifestyle, injury, and illness in college athletes. Med Sci Sports Exerc. 2011;43(2):335–343. doi: 10.1249/MSS.0b013e3181eb9d4d. https://doi-org.eproxy.lib.hku.hk/10.1249/MSS.0b013e3181eb9d4d [DOI] [PubMed] [Google Scholar]
- 63.Cannell J.J., Hollis B.W., Zasloff M., Heaney R.P. Diagnosis and treatment of vitamin D deficiency. Expet Opin Pharmacother. 2008;9(1):107–118. doi: 10.1517/14656566.9.1.107. https://doi-org.eproxy.lib.hku.hk/10.1517/14656566.9.1.107 [DOI] [PubMed] [Google Scholar]
- 64.Fairbairn K.A., Ceelen I., Skeaff C.M., Cameron C.M., Perry T.L. Vitamin D3 supplementation does not improve sprint performance in professional rugby players: a randomized, placebo-controlled, double-blind intervention study. Int J Sport Nutr Exerc Metabol. 2018;28(1):1–9. doi: 10.1123/ijsnem.2017-0157. https://doi-org.eproxy.lib.hku.hk/10.1123/ijsnem.2017-0157 [DOI] [PubMed] [Google Scholar]
- 65.Jung H.C., Seo M.W., Lee S., Jung S.W., Song J.K. Correcting vitamin D insufficiency improves some but not all aspects of physical performance during winter training in taekwondo athletes. Int J Sport Nutr Exerc Metabol. 2018;28(6):635–643. doi: 10.1123/ijsnem.2017-0412. https://doi-org.eproxy.lib.hku.hk/10.1123/ijsnem.2017-0412 [DOI] [PubMed] [Google Scholar]
- 66.Millward D., Root A.D., Dubois J., et al. Association of serum vitamin D levels and stress fractures in collegiate athletes. Orthopaedic J Sport Med. 2020;8(12) doi: 10.1177/2325967120966967. https://doi-org.eproxy.lib.hku.hk/10.1177/2325967120966967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knechtle B., Jastrzębski Z., Hill L., Nikolaidis P.T. Vitamin D and stress fractures in sport: preventive and therapeutic measures-A narrative review. Medicina. 2021;57(3):223. doi: 10.3390/medicina57030223. https://doi-org.eproxy.lib.hku.hk/10.3390/medicina57030223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Żebrowska A., Sadowska-Krępa E., Stanula A., et al. The effect of vitamin D supplementation on serum total 25(OH) levels and biochemical markers of skeletal muscles in runners. Sports Nutr Rev J. 2020;17(1):18. doi: 10.1186/s12970-020-00347-8. https://doi-org.eproxy.lib.hku.hk/10.1186/s12970-020-00347-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han Q., Li X., Tan Q., Shao J., Yi M. Effects of vitamin D3 supplementation on serum 25(OH)D concentration and strength in athletes: a systematic review and meta-analysis of randomized controlled trials. Sports Nutr Rev J. 2019;16(1):55. doi: 10.1186/s12970-019-0323-6. https://doi-org.eproxy.lib.hku.hk/10.1186/s12970-019-0323-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Farrokhyar F., Sivakumar G., Savage K., et al. Effects of vitamin D supplementation on serum 25-hydroxyvitamin D concentrations and physical performance in athletes: a systematic review and meta-analysis of randomized controlled trials. Sports Med. 2017;47(11):2323–2339. doi: 10.1007/s40279-017-0749-4. https://doi-org.eproxy.lib.hku.hk/10.1007/s40279-017-0749-4 [DOI] [PubMed] [Google Scholar]
- 71.Jung H.C., Seo M.W., Lee S., Kim S.W., Song J.K. Vitamin D₃ supplementation reduces the symptoms of upper respiratory tract infection during winter training in vitamin D-insufficient taekwondo athletes: a randomized controlled trial. Int J Environ Res Publ Health. 2018;15(9):2003. doi: 10.3390/ijerph15092003. https://doi-org.eproxy.lib.hku.hk/10.3390/ijerph15092003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pereira M., Dantas Damascena A., Galvão Azevedo L.M., de Almeida Oliveira T., da Mota Santana J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2022;62(5):1308–1316. doi: 10.1080/10408398.2020.1841090. https://doi-org.eproxy.lib.hku.hk/10.1080/10408398.2020.1841090 [DOI] [PubMed] [Google Scholar]
- 73.Grant W.B., Lahore H., Rockwell M.S. The benefits of vitamin D supplementation for athletes: better performance and reduced risk of COVID-19. Nutrients. 2020;12(12):3741. doi: 10.3390/nu12123741. https://doi-org.eproxy.lib.hku.hk/10.3390/nu12123741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shuler F.D., Wingate M.K., Moore G.H., Giangarra C. Sports health benefits of vitamin d. Sports health. 2012;4(6):496–501. doi: 10.1177/1941738112461621. https://doi-org.eproxy.lib.hku.hk/10.1177/1941738112461621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bezuglov E., Tikhonova A., Zueva A., et al. Prevalence and treatment of vitamin D deficiency in young male Russian soccer players in winter. Nutrients. 2019;11(10):2405. doi: 10.3390/nu11102405. https://doi-org.eproxy.lib.hku.hk/10.3390/nu11102405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jastrzębska J., Skalska M., Radzimiński Ł., et al. Changes of 25(OH)D concentration, bone resorption markers and physical performance as an effect of sun exposure, supplementation of vitamin D and lockdown among young soccer players during a one-year training season. Nutrients. 2022;14(3):521. doi: 10.3390/nu14030521. https://doi-org.eproxy.lib.hku.hk/10.3390/nu14030521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weber A.E., Bolia I.K., Korber S., et al. Five-year surveillance of vitamin D levels in NCAA division I football players: risk factors for failed supplementation. Orthopaedic J Sport Med. 2021;9(1) doi: 10.1177/2325967120975100. https://doi-org.eproxy.lib.hku.hk/10.1177/2325967120975100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tripkovic L., Lambert H., Hart K., et al. Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clinic Nutri. 2012;95(6):1357–1364. doi: 10.3945/ajcn.111.031070. https://doi-org.eproxy.lib.hku.hk/10.3945/ajcn.111.031070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lehmann U., Hirche F., Stangl G.I., Hinz K., Westphal S., Dierkes J. Bioavailability of vitamin D(2) and D(3) in healthy volunteers, a randomized placebo-controlled trial. J Clinic Endocrinol Metabol. 2013;98(11):4339–4345. doi: 10.1210/jc.2012-4287. https://doi-org.eproxy.lib.hku.hk/10.1210/jc.2012-4287 [DOI] [PubMed] [Google Scholar]
- 80.Michalczyk M., Czuba M., Zydek G., Zając A., Langfort J. Dietary recommendations for cyclists during altitude training. Nutrients. 2016;8(6):377. doi: 10.3390/nu8060377. https://doi-org.eproxy.lib.hku.hk/10.3390/nu8060377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Razzaque M.S. Can adverse effects of excessive vitamin D supplementation occur without developing hypervitaminosis D? J Steroid Biochem Mol Biol. 2018;180:81–86. doi: 10.1016/j.jsbmb.2017.07.006. https://doi-org.eproxy.lib.hku.hk/10.1016/j.jsbmb.2017.07.006 [DOI] [PubMed] [Google Scholar]
- 82.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clinic Nutri. 1999;69(5):842–856. doi: 10.1093/ajcn/69.5.842. https://doi-org.eproxy.lib.hku.hk/10.1093/ajcn/69.5.842 [DOI] [PubMed] [Google Scholar]
- 83.Cranney A., Weiler H.A., O'Donnell S., Puil L. Summary of evidence-based review on vitamin D efficacy and safety in relation to bone health. Am J Clinic Nutri. 2008;88(2):513S–519S. doi: 10.1093/ajcn/88.2.513S. https://doi-org.eproxy.lib.hku.hk/10.1093/ajcn/88.2.513S [DOI] [PubMed] [Google Scholar]