Abstract
Hyphomicrobium chloromethanicum CM2T, an aerobic methylotrophic member of the α subclass of the class proteobacteria, can grow with chloromethane as the sole carbon and energy source. H. chloromethanicum possesses an inducible enzyme system for utilization of chloromethane, in which two polypeptides (67-kDa CmuA and 35-kDa CmuB) are expressed. Previously, four genes, cmuA, cmuB, cmuC, and purU, were shown to be essential for growth of Methylobacterium chloromethanicum on chloromethane. The cmuA and cmuB genes were used as probes to identify homologs in H. chloromethanicum. A cmu gene cluster (9.5 kb) in H. chloromethanicum contained 10 open reading frames: folD (partial), pduX, orf153, orf207, orf225, cmuB, cmuC, cmuA, fmdB, and paaE (partial). CmuA from H. chloromethanicum (67 kDa) showed high identity to CmuA from M. chloromethanicum and contains an N-terminal methyltransferase domain and a C-terminal corrinoid-binding domain. CmuB from H. chloromethanicum is related to a family of methyl transfer proteins and to the CmuB methyltransferase from M. chloromethanicum. CmuC from H. chloromethanicum shows identity to CmuC from M. chloromethanicum and is a putative methyltransferase. folD codes for a methylene-tetrahydrofolate cyclohydrolase, which may be involved in the C1 transfer pathway for carbon assimilation and CO2 production, and paaE codes for a putative redox active protein. Molecular analyses and some preliminary biochemical data indicated that the chloromethane utilization pathway in H. chloromethanicum is similar to the corrinoid-dependent methyl transfer system in M. chloromethanicum. PCR primers were developed for successful amplification of cmuA genes from newly isolated chloromethane utilizers and enrichment cultures.
Chloromethane (CH3Cl) is a volatile organic compound with an average concentration in the atmosphere of 540 ppt (vol/vol) (22). Chloromethane is of environmental concern because it may be responsible for about 13% of the destruction of the stratospheric ozone layer (3). The primary sources of chloromethane are thought to be biological and nonbiological processes that occur in nature. The major sources of chloromethane to date include oceans, biomass burning, wood-rotting fungi, and salt marshes (20, 36). The main sink for chloromethane is thought to be the reaction with tropospheric and stratospheric hydroxyl radicals. Soils have also been shown to be a potentially significant sink for chloromethane (23).
Chloromethane can be cometabolized by bacteria, both by oxidation (35, 40) and by hydrolysis (21). In addition, several methylotrophic bacteria which are able to use chloromethane as a growth substrate have been characterized. These include the strictly anaerobic homoacetogenic bacterium Acetobacterium dehalogenans (31) and several aerobic methylotrophs of the genera Hyphomicrobium and Methylobacterium (6). Anoxic dehalogenation of chloromethane by A. dehalogenans has been shown to be catalyzed by enzymes that transfer the methyl group of chloromethane by means of a corrinoid protein to tetrahydrofolate to yield chloride and methyl tetrahydrofolate, an intermediate in the acetyl coenzyme A pathway (48). Doronina et al. (6) initially isolated eight strains from industrially contaminated Russian soils; however, 16S rRNA sequencing showed that only two distinct strains had been isolated, and these strains were recently designated Hyphomicrobium chloromethanicum CM2T and Methylobacterium chloromethanicum CM4T (29). Physiological and genetic studies exploring the mechanism of chloromethane metabolism in M. chloromethanicum CM4T recently suggested a pathway for chloromethane utilization (45, 46). It was shown that two polypeptides (67 and 35 kDa) were induced during growth on chloromethane (46). Growth yields and oxygen electrode stoichiometries suggested that chloromethane was completely oxidized to CO2 and that a total of six electrons were produced by this oxidation. Chloromethane-grown cells were also capable of dehalogenating bromomethane and iodomethane but not dichloromethane and higher chloroalkanes, such as chloroethane. This suggested that the enzyme(s) responsible for chloromethane degradation was specific for monohalomethanes. No growth was observed with bromomethane as the sole carbon and energy source, presumably due to the greater toxicity of this compound. Transposon mutagenesis was used to create Methylobacterium mutants unable to grow on chloromethane. Genes containing the transposon insertion were then cloned and sequenced, and the resulting information was used to develop biochemical assays. Based on the results, a pathway for chloromethane degradation was suggested (Fig. 1), which represents a novel catabolic pathway for aerobic methylotrophs (45).
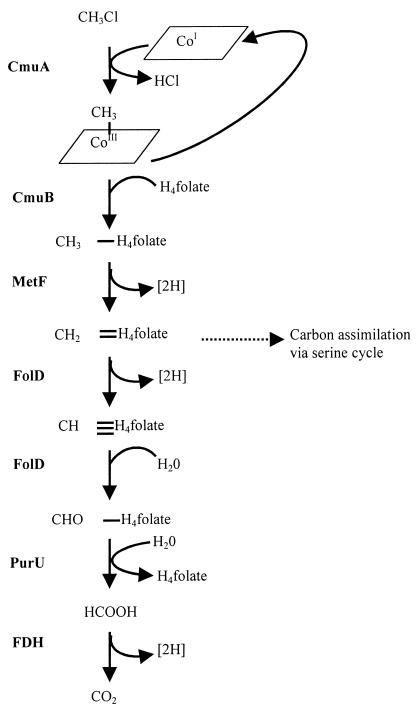
FIG. 1.
Proposed pathway for chloromethane metabolism in M. chloromethanicum CM4T. The pathway was modified from that of Vannelli et al. (45). CmuA, methyltransferase I; CmuB, methyltransferase II; MetF, putative 5,10-methylene-tetrahydrofolate reductase; FolD, putative 5,10-methylene-tetrahydrofolate dehydrogenase/5,10-methenyl-tetrahydrofolate cyclohydrolase; PurU, putative 10-formyl-tetrahydrofolate hydrolase; FDH, formate dehydrogenase; CoI, corrinoid protein acting as the primary methyl acceptor and thought to be part of CmuA; H4 folate, tetrahydrofolate.
The first step of this pathway involves CmuA, a 67-kDa polypeptide which has a methyltransferase domain and a corrinoid-binding domain. The methyltransferase domain transfers the methyl group of chloromethane to the Co atom of the enzyme-bound corrinoid group (methyltransferase I activity). A second polypeptide, CmuB, then transfers the methyl group to tetrahydrofolate, forming methyl tetrahydrofolate (methyltransferase II activity). This folate-linked methyl group is then progressively oxidized to formate and then to CO2 to provide reducing equivalents. Carbon assimilation presumably occurs at the level of methylene tetrahydrofolate, which can feed directly into the serine cycle. This pathway was postulated on the basis of physiological, genetic, and biochemical evidence. Four genes, cmuA, cmuB, cmuC, and purU, were shown to be essential for growth on chloromethane but not on other C1 substrates.
Two other facultative methylotrophs capable of growth on both chloromethane and bromomethane as sole carbon and energy sources have been isolated. Strain IMB-1 was isolated from soil which had been fumigated with bromomethane (4, 32), while strain CC495 was isolated from topsoil in a pristine woodland site (5). These two strains were shown by 16S rRNA sequence analysis to be closely related to each other and to the genus Aminobacter (5, 18). Growth of IMB-1 on bromomethane was shown to be inducible (39). Growth was observed with bromomethane, chloromethane, and iodomethane as sole carbon and energy sources. Cells grown on bromomethane were capable of oxidizing chloromethane and vice versa, suggesting that a single inducible enzyme system was responsible for the oxidation of monohalomethanes. The physiology and biochemistry of chloromethane degradation by CC495 were investigated by Coulter et al. (5). Growth on chloromethane was inducible, and two polypeptides with apparent molecular masses of 67 and 29 kDa were expressed. The 67-kDa polypeptide was purified and identified as a halomethane: bisulfide/halide ion methyltransferase. This enzyme is a corrinoid protein, and its reported N-terminal sequence showed 81% identity to the N-terminal sequence of CmuA from M. chloromethanicum CM4T. A fifth isolate, strain MB2, isolated from a marine environment, was capable of growth on bromomethane as a sole carbon and energy source (14) but has not been characterized further.
Here we report on the sequence and an analysis of the cmu gene cluster from H. chloromethanicum CM2T. Biochemical evidence for degradation of chloromethane by CM2T is also presented. Development of specific PCR primers for detection of cmuA genes in isolates and enrichments is also described.
MATERIALS AND METHODS
Growth media and bacterial strains.
H. chloromethanicum CM2T, M. chloromethanicum CM4T, strain IMB-1, and isolates were routinely cultured on ammonium nitrate mineral salts (ANMS) medium, described by Whittenbury et al. (47), in 120-ml serum vials containing 50 ml of medium. For growth on chloromethane, vials were sealed with Teflon-coated butyl rubber stoppers (Owens Polyscience Ltd., Macclesfield, United Kingdom) and chloromethane was added directly to the headspace through each rubber stopper to a final concentration of 2% (vol/vol). During growth, sterile NaOH (5 M) was periodically added to maintain the pH at 6.8, and more chloromethane was added when required. Cultures were incubated at 30°C on an orbital shaker at 200 rpm. Plate cultures of chloromethane utilizers were grown on ANMS agar in sealed jars gassed with 2% chloromethane and incubated at 30°C. Colonies were generally visible after 3 or 4 days of incubation. H. chloromethanicum CM2T has been deposited in the All-Russian Collection of Microorganisms as strain VKM B-2176 and in the National Collections of Industrial, Food and Marine Bacteria as strain NCIMB13687. IMB-1 was obtained from Ronald Oremland, U.S. Geological Survey, Menlo Park, Calif.
Enrichment and isolation of chloromethane utilizers.
The strategy used for enrichment and isolation of chloromethane utilizers was a modification of that used by Doronina et al. (6). Soil samples (1 g) were mixed with 10 ml of ANMS medium, while aquatic samples were concentrated by centrifugation (10,000 × g for 15 min) or by filtration through a nitrocellulose filter (pore size, 0.2 μm; Millipore) and resuspended in 1% of the original volume with ANMS medium. One milliliter of each sample was then added to 50 ml of either ANMS medium or DM (6) supplemented with a vitamin solution (19) in a 120-ml serum bottle. For initial enrichments, either chloromethane, methanol, methylamine, or formate was used as the sole carbon and energy source. Cultures were grown at 30°C. Enrichments showing growth were then subcultured with 2% (vol/vol) chloromethane as the sole carbon and energy source. Chloromethane consumption was measured by removing 200-μl samples from the headspace and analyzing the chloromethane concentration by gas chromatography (GC).
Enrichments showing both growth and chloromethane disappearance after three subcultures were serially diluted, spread onto plates containing the appropriate medium, and incubated with 2% (vol/vol) chloromethane for 7 days. Individual colonies were then streaked onto plates and reincubated. Subsequent colonies were then used to inoculate liquid media. This process was repeated until pure cultures which were able to use chloromethane as a sole carbon and energy source were obtained.
SDS-PAGE analysis and N-terminal sequencing of a chloromethane-induced polypeptide.
Cells were harvested in the late exponential phase, washed twice with 25 mM morpholinepropanesulfonic acid (MOPS) (pH 7.0), and broken by four passages through a French press (American Instrument Company, Silver Spring, Md.) at 137 MPa. A cell extract was prepared by centrifugation to remove cell debris (10,000 × g, 30 min, 4°C). A soluble extract was prepared by centrifugation at 50,400 × g for 60 min at 4°C to remove the insoluble fraction. For sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), samples were diluted with an equal volume of SDS sample buffer (100 mM Tris-HCl [pH 6.8], 5% [vol/vol] 2-mercaptoethanol, 4% [wt/vol] SDS, 0.2% [wt/vol] bromophenol blue, 20% [vol/vol] glycerol) and boiled for 5 min before loading. Protein-containing samples were analyzed by SDS-PAGE by using the discontinuous buffer system of Laemmli (24). Gels were calibrated with Amersham Pharmacia Low Molecular Mass markers.
After electrophoresis, the polyacrylamide gel was soaked for 10 min in transfer buffer (10 mM CAPS [cyclohexylamino-1-propanesulfonic acid, pH 11.0], 10% [vol/vol] methanol). Proteins were electroblotted onto a polyvinylidene difluoride membrane in an X-Cell blot module (Novex). After transfer, the membrane was stained with 0.1% Coomassie blue R-250 in 50% (vol/vol) methanol for 5 min and then destained with several changes of 50% (vol/vol) methanol–10% (vol/vol) glacial acetic acid. The membrane was then rinsed in water for 5 min and air dried. N-terminal sequences were determined with a 476A protein sequencer (PE Applied Biosystems) by A. Moir (University of Sheffield).
DNA extraction.
DNA were extracted from H. chloromethanicum CM2T, M. chloromethanicum CM4T, IMB-1, and new isolates by using the method of Oakley and Murrell (34), as described previously for methanotrophs. Total DNA was extracted from enrichments by the method of Marmur (28).
Construction of partial libraries of DNA from H. chloromethanicum CM2T.
Genomic libraries were constructed by cloning DNA from H. chloromethanicum CM2T into the multicopy vector pBluescript II K/S digested with the appropriate restriction enzyme. H. chloromethanicum CM2T DNA was digested, and fragments of the required size were excised from gels, ligated into the cloning vector, and transformed into Escherichia coli. Fragments suitable for cloning were identified by probing Southern blots of H. chloromethanicum CM2T DNA with radioactively labelled probes for cmuA and cmuB from M. chloromethanicum CM4T, using methods described by Sambrook et al. (38).
PCR.
PCR primers designed to amplify cmuA, the methyltransferase enzyme that is responsible for the initial dehalogenation of chloromethane, were designed either manually by alignment of cmuA gene sequences or with the CODEHOP program (37), accessed on the World Wide Web (http://blocks.fhcrc.org/codehop.html). PCR amplifications were performed in 50-μl (total volume) mixtures in 0.5-ml microcentrifuge tubes by using a Hybaid Touchdown thermal cycling system (30). For nested PCR, 0.5 μl of the initial PCR mixture was used as the template for the second PCR.
DNA sequencing and analysis.
DNA sequencing was performed by cycle sequencing with a Dye Terminator kit (PE Applied Biosystems, Warrington, United Kingdom), and DNA sequences were analyzed with a model 373A automated DNA sequencing system (PE Applied Biosystems). DNA sequences and derived amino acid sequences were analyzed by using the Genetics Computer Group (GCG) Wisconsin package, version 8.0.1-Unix. Similarity searches were performed by using the gapped BLAST (Basic Local Alignment Search Tool) program (1) and public protein and gene databases (http://www.ncbi.nlm.nih.gov).
Biochemical assays for chloromethane degradation. (i) Conversion of chloromethane to methyl tetrahydrofolate and HCl.
The method used to study conversion of chloromethane to methyl tetrahydrofolate and HCl was that described by Vannelli et al. (45). Cell extract was prepared as described above. Oxygen was removed from solutions by degassing with N2-H2 (95:5, vol/vol). All enzyme reactions and measurements were performed under the same atmosphere. Assays were performed at 30°C by using 3-ml (total volume) mixtures in 12.4-ml serum flasks sealed with gas-tight rubber stoppers. Each reaction mixture comprised 1 mg of cell extract in a solution containing 100 mM Tris-SO4 (pH 7.8), 5 mM dithiothreitol, 2.4 mM tetrahydrofolate, and 1 mM titanium(III) citrate. The reaction was started by adding 2.2 μmol of chloromethane through the rubber stopper. Methyl tetrahydrofolate production was measured on the basis of separation from the reaction mixture by using high-performance liquid chromatography performed with a C8 reversed-phase column and with 0.175% (wt/vol) H3PO4 in H2O containing 10.5% (vol/vol) methanol as the eluent. The compound was detected spectophotometrically at 320 nm. Chloromethane consumption was measured by removing 50-μl samples from the headspace and analyzing the chloromethane concentration by GC.
(ii) Conversion of chloromethane to iodomethane.
The method used to study conversion of chloromethane to iodomethane was a modification of the method described by Coulter et al. (5). Reactions (total volume, 1 ml) were performed at 30°C in 10-ml crimp cap vials sealed with grey butyl rubber stoppers. Each reaction mixture comprised 0.5 to 2.0 mg of cell extract in a solution containing 50 mM phosphate buffer (pH 7.0), 5 mM dithiothreitol, and 3 mM KI. Reactions were started by adding 1 μmol of chloromethane through the rubber stoppers. Activation, when performed, was carried out in the same buffer without KI, incubated for 1 h at 30°C. The vial was then uncapped and allowed to stand for 1 h. Chloromethane consumption and iodomethane production were measured by GC of 50 to 200 μl of headspace gas.
Determination of halomethanes by GC.
Samples of headspace gas (50 to 200 μl) were injected into a GCD gas chromatograph (Pye Unicam Ltd., Cambridge, United Kingdom) fitted with a Porapak Q column (Phase Separations Ltd., Deeside, United Kingdom) at 200°C. A flame ionization detector was used to detect products, and the peak areas were determined with a 3390A integrator (Hewlett-Packard, Berkshire, United Kingdom). The gas chromatograph was calibrated by using samples of the headspace gases above standard solutions containing known halomethane concentrations equilibrated at 30°C.
Nucleotide sequence accession numbers.
The sequence of the cmu gene cluster of H. chloromethanicum CM2T has been deposited in the GenBank database under accession number AF281259. The sequences of the cmuA clones determined in this study have been deposited in the GenBank database under accession numbers AF307140 to AF307143.
RESULTS
SDS-PAGE analysis of cell extracts of H. chloromethanicum CM2T.
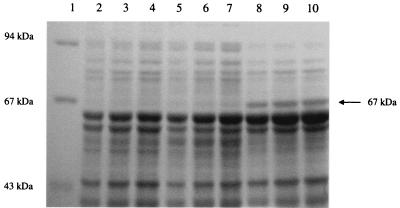
Chloromethane metabolism in CM2T was found to be inducible, and analysis of cell extracts by SDS-PAGE revealed polypeptides with apparent molecular masses of 25, 33, and 67 kDa, which were induced in chloromethane-grown cells but not in methanol-grown cells. The 25-kDa polypeptide was induced in methanol-grown cells which had been acid shocked at pH 5.0 for 12 h, indicating that it was probably involved in the stress response of this organism to low pH. The position of the 67-kDa chloromethane-induced polypeptide is shown in Fig. 2. The N-terminal sequence of the 67-kDa protein was determined to be MTQVPKMTSRERLFAAV and was shown to have significant identity to the N-terminal sequences of CmuA from M. chloromethanicum CM4T (46) and the halomethane:bisulfide/halide ion methyltransferase isolated from strain CC495 (5). Eleven of the 17 amino acid residues in the N-terminal sequences were conserved (MTSRERLFAAV) in all three sequences.
FIG. 2.
SDS–8% PAGE of cell extract from H. chloromethanicum CM2T. Lane 1, molecular mass markers; lanes 2 through 4, methanol-grown cells; lanes 5 through 7, acid-shocked cells; lanes 8 through 10, chloromethane-grown cells. Triplicate lanes contained three different batches of cells grown under the conditions indicated above.
Biochemical evidence for degradation of chloromethane by CM2T.
Growth yields on chloromethane and methanol (12.6 and 11.3 g mol of C−1, respectively) were the same and were at the upper end of the growth yields for serine pathway bacteria grown on methanol (13). This rules out a monooxygenase mechanism of dehalogenation, since breakdown of chloromethane catalyzed by a monooxygenase to produce formaldehyde would produce 4 reducing equivalents, 2 of which would be consumed by the monooxygenase. The net yield on chloromethane would be 2 reducing equivalents, compared to 6 reducing equivalents on methanol, so the growth yield on chloromethane would be approximately one-third that on methanol.
The oxidation rate of chloromethane was not affected by the addition of cyclopropanol, a potent inhibitor of pyrroloquinoline quinone-linked enzymes (10), which completely inhibited oxidation of methanol. This demonstrates that methanol is not an intermediate in chloromethane oxidation which rules out hydrolysis as the dehalogenation mechanism. This conclusion is supported by the fact that methanol oxidation rates were lower in chloromethane-grown cells (30 nmol min−1 mg [dry weight]−1) than in methanol-grown cells (45 nmol min−1 mg−1 [dry weight]).
Assays developed by Vannelli et al. (45) were performed with cell extract to investigate the possibility that CM2T has a mechanism of chloromethane metabolism similar to that of M. chloromethanicum CM4T or isolate CC495. The assay for conversion of chloromethane to methyl tetrahydrofolate and HCl (45) by CM2T produced the same results as the assay performed with cell extract from CM4T; there were significant levels of chloromethane disappearance and methyl tetrahydrofolate production in an extract from chloromethane-grown cells, but there was no significant activity in an extract from methanol-grown cells (results not shown). The assay for conversion of chloromethane to iodomethane (5) by chloromethane-grown CM2T showed that conversion of chloromethane to iodomethane occurred at a low but measurable rate (0.44 nmol min−1 mg−1), while an extract from methanol-grown cells did not show any activity within the limit of detection (approximately 0.1 nmol min−1 mg−1). This rate is much lower than the rate reported for isolate CC495, 2.88 nmol min−1 mg−1 (5). This lower specific activity may be a reflection of structural differences in the enzymes involved, or there may be unknown conditions and/or cofactors that improve the activity of CM2T.
Southern hybridization to identify putative chloromethane utilization genes in CM2T.
H. chloromethanicum CM2T Southern blots were hybridized with the cmuA, cmuB, and metF genes from M. chloromethanicum CM4T. The cmuA and cmuB probes showed hybridization, while metF did not show any hybridization, even at low stringency. Restriction fragments of H. chloromethanicum CM2T chromosomal DNA were identified for cloning and were cloned by preparing partial clone libraries with inserts having the correct sizes. These libraries were screened with the cmuA and cmuB probes from M. chloromethanicum CM4T, and positive clones were selected for further analysis by restriction digestion. The following three clones were selected for sequencing: pCM2, pCAW1, and pCAW2.
Sequence analysis of the chloromethane utilization genes.
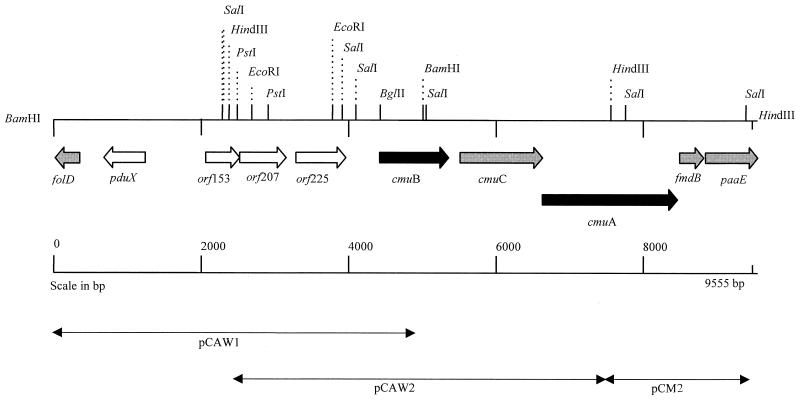
Sequence analysis of the three clones (pCAW1, pCAW2, and pCM2) indicated that a number of key chloromethane utilization genes in H. chloromethanicum CM2T occur in a cluster that is around 9.5 kb long (Fig. 3). Results of National Center for Biotechnology Information database BLAST searches revealed that 8 of 10 open reading frames showed identity to polypeptides from prokaryotes and archaea (Table 1).
FIG. 3.
Schematic representation of the methyltransferase gene cluster in H. chloromethanicum CM2T. Gene orientations and positions are shown. Genes shown by genetic analysis of M. chloromethanicum CM4T to be involved in methyl chloride dehalogenation are indicated by black arrows. Genes indicated by shaded arrows are predicted to have functions needed in a likely complete chloromethane catabolism pathway. Other open reading frames are indicated by open arrows. pCAW1 is a 5-kb BamHI fragment, pCAW2 is a 5.1-kb HindIII fragment, and pCM1 is a 2-kb HindIII fragment.
TABLE 1.
Summary of H. chloromethanicum CM2T methyltransferase genes and adjacent open reading frames
| Gene or open reading frame | Length of polypeptide (amino acids) | Gene start end position (bp) | Inferred function | Sequence comparison for representative protein hit (% identity)a |
|---|---|---|---|---|
| folD | >85 | 203–<start C | Methylene tetrahydrofolate cyclohydrolase/ dehydrogenase | P. phosphoreum (40), A. fulgidus (39), E. coli FolD (22) |
| pduX | 185 | 1239–682 C | Propandiol dehydratase | S. enterica serovar Typhimurium PduX (39%) |
| orf153 | 153 | 2060–2521 | No match | |
| orf207 | 207 | 2521–3144 | Porin precursor | N. denitrificans (37%) |
| orf225 | 225 | 3275–3952 | No match | |
| cmuB | 311 | 4413–5348 | Methyltransferase | M. chloromethanicum CM4T CmuB (57) |
| cmuC | 370 | 5501–6613 | Methyltransferase | M. chloromethanicum CM4T CmuC (36) and orf414 (34) |
| cmuA | 612 | 6610–8448 | Methyltransferase and corrinoid protein | M. chloromethanicum CM4T CmuA (80) |
| fmdB | 110 | 8476–8808 | Transcriptional regulator | M. methylotrophus FmdB (23) |
| paaE | >240 | 8833–<9556 | Reductase | E. coli PaaE (33), P. putida TdnB (32) |
| end |
Levels of protein identity were determined by performing a National Center for Biotechnology Information BLAST database search analysis.
The deduced N-terminal sequence of the cmuA gene is identical to that of the 67-kDa chloromethane-specific polypeptide from H. chloromethanicum CM2T. The predicted CmuA protein is 612 amino acids long and has a calculated Mr of 66,900 (estimated to be 67,000 by SDS-PAGE). The CM2T cmuA gene exhibits considerable identity at the DNA level (75%) and the amino acid level (80%) to the cmuA gene of M. chloromethanicum CM4T. It also has the same structure, an N-terminal methyltransferase domain and a C-terminal corrinoid-binding region. The N-terminal sequence also suggests that it is similar to the halomethane:bisulphide/halide ion methyltransferase from CC495, which is of similar size and has been shown to possess methyltransferase activity and to be a corrinoid-binding protein (5). The methyltransferase and corrinoid-binding domains of CmuA align most closely with MtbA and MtmC, respectively, from Methanosarcina barkeri, which are involved in metabolism of methylamine (Fig. 4 and 5). It seems that as in CM4T, the cmuA gene is a fusion of two genes, which in methanogens occur as separate genes encoding polypeptides involved in corrinoid binding and methyl transfer.
FIG. 4.
Alignment of the methyltransferase domain of CmuA from H. chloromethanicum CM2T with CmuA from M. chloromethanicum CM4T and MtbA and MtsA from M. barkeri. CmuA from H. chloromethanicum CM2T was aligned with CmuA from M. chloromethanicum CM4T (accession no. AJ011316) and with MtbA (U38918) and MtsA (U36337) from M. barkeri. Aligned sequences in GCG's MSF format were downloaded into the BoxShade 3.21 vs program, which highlighted similar residues (shaded boxes) and identical residues (black boxes). Asterisks indicate the putative zinc-binding motif identified in MtbA from M. barkeri (25).
FIG. 5.
Alignment of the corrinoid domain of CmuA from H. chloromethanicum CM2T with CmuA from M. chloromethanicum CM4T, MtmC from M. barkeri, and MetH from E. coli. CmuA from H. chloromethanicum CM2T was aligned with CmuA from M. chloromethanicum CM4T (accession no. AJ011316), MtmC from M. barkeri (AF013713), and MetH from E. coli (P13009). Aligned sequences in GCG's MSF format were downloaded into the BoxShade 3.21 vs program, which highlighted similar residues (shaded boxes) and identical residues (black boxes). Asterisks indicate the D-x-H-x2-G-x41–42-SxL-x24–28-GG motif, a motif consisting of conserved residues involved in cobalamin binding in methionine synthases and mutases (27).
The cmuB gene encodes a 311-amino-acid polypeptide and shows 63% identity to the M. chloromethanicum CM4T cmuB sequence at the DNA level and 57% identify at the amino acid level. The translated CmuB polypeptide also shows homology (25% identity) to MtrH sequences from methanogens (Fig. 6). This polypeptide is part of the methyltetrahydromethanopterin:coenzyme M methyltransferase complex and is thought to catalyze methyl transfer from the pterin to the corrinoid group of MtrA (17). CmuB also shows homology to methyl tetrahydrofolate-binding region of methionine synthase from Escherichia coli (7).
FIG. 6.
Alignment of CmuB from H. chloromethanicum CM2T with CmuB from M. chloromethanicum CM4T, MtrH from M. barkeri, and MetH from E. coli. CmuB from H. chloromethanicum CM2T was aligned with CmuB from M. chloromethanicum CM4T (accession no. AJ011317), M. barkeri MtrH (AJ132817), and E. coli MetH (P13009). Aligned sequences in GCG's MSF format were downloaded into the BoxShade 3.21 vs program, which highlighted similar residues (shaded boxes) and identical residues (black boxes). Asterisks indicate a sequence pattern that is present in all eight MtrH-related sequences and in all nine MetH-related sequences, G-[EA]-x2-[TNG]-x47–57-[LIM]-x16–20-[DN]-[RSA]-x8-[GA]-x10–15-[NA]-S-x19–24-[AL]-x10–18-[GA]-x9–23-G-x6–8-D-x19–27-[KRA]-x8–10-[GA]-x-[HAS]-N (Swissprot release 38; EMBL release 55)(41).
The cmuC gene encodes a 370-amino-acid polypeptide and shows 55% identity at the DNA level to the M. chloromethanicum CM4T sequence and 36% identity at the amino acid level. The function of CmuC is not known, although CmuC has been shown to be essential for growth of M. chloromethanicum CM4T on chloromethane.
The partial open reading frame folD codes for a methylene tetrahydrofolate cyclohydrolase/dehydrogenase, which exhibits 39% identity to an archaeal protein from Archaeoglobus fulgidus, the FolD polypeptide from E. coli, and a putative FolD polypeptide from M. chloromethanicum CM4T. Other open reading frames possibly involved in chloromethane degradation in H. chloromethanicum CM2T include fmdB which codes for a 110-amino-acid putative transcriptional regulator which shows 23% identity to FmdB from Methylophilus methylotrophus. The function of FmdB is not known, but FmdB may act as a regulator of the formidase enzyme FmdA (49). Another open reading frame codes for a 185-amino-acid sequence, PduX, which exhibits identity to a PduX propandiol dehydratase from Salmonella enterica serovar Typhimurium. Partial open reading frame paaE is potentially involved in electron transfer, since it shows 33% identity to paaE from E. coli (9) and tdnB from Pseudomonas putida (11), which are involved in electron transfer. PaaE contains a putative flavin mononucleotide-flavin adenine dinucleotide-binding motif, -R-x-Y-S, and a -A-G-S-G-I-A-P sequence, which is similar to the NAD-ribose-binding motif -G-G-x-G-x-x-P-(33). This electron transfer protein was also expected to posses a [2Fe-2S] binding site, but no potential site was found and a binding site presumably present in the C-terminal region, which was not cloned. Open reading frame orf207 showed identity to a region encoding a porin precursor from Neisseria denitrificans.
An obvious difference between H. chloromethanicum CM2T and M. chloromethanicum CM4T is that in CM2T the cmu genes are linked on a single chromosomal fragment, while in CM4T they are encoded in two separate clusters within the genome.
Development of cmuA-specific PCR primers.
Once the chloromethane utilization genes had been cloned from both M. chloromethanicum CM4T and H. chloromethanicum CM2T, the cmuA gene was selected as a marker for chloromethane utilizers because CmuA binds chloromethane and catalyzes the initial dehalogenation of this compound. CmuB, which is also involved in the degradation pathway, was not considered to be suitable as chloromethane is not the initial substrate for this enzyme.
PCR primers were designed for regions of cmuA, which are conserved in CM2T and CM4T. Since only two gene sequences were available, the unique structure of the gene was considered when primers were designed. The cmuA gene contains a 5′ methyltransferase domain and a 3′ corrinoid-binding domain; therefore, the forward primer was located in the methyltransferase domain and the reverse primer was located in the corrinoid-binding domain. As cmuA appears to be the only gene with this structure, this was expected to increase the specificity of the PCR. If primers were designed to amplify only part of a single domain, there would be the possibility that other methyltransferase or corrinoid-binding genes might be amplified rather than cmuA. Primers were tested with CM2T and CM4T as positive controls and then used to amplify partial cmuA sequences from other chloromethane-utilizing isolates.
Two primers, 979f (TGCGATATCGACTGGACG) and 1646r (GTCATCACCGACATGCCG) (the number in each designation refers to the position of the first base of the primer in the cmuA gene), were designed for two stretches of sequence which were identical in the CM2T and CM4T sequences. These primers were then used to amplify the cmuA sequence from CM2T and CM4T (positive controls), from IMB-1, and from new chloromethane-utilizing Hyphomicrobium isolates. Six new isolates were identified as members of Hyphomicrobium species on the basis of morphology. CM2T, CM4T, and the Hyphomicrobium isolates all gave a product of the correct size (668 bp), but no product was obtained with IMB-1. Therefore, new degenerate primers were designed by using CODEHOP (37). Four primer sets were designed and tested with DNA from CM2T, CM4T, and IMB-1 and with DNA from several non-chloromethane-utilizing bacteria (E. coli, Methylobacterium extorquens AM1, and Methylosinus trichosporium OB3b) as negative controls. Primers 929f (AACTAGCTGCTGAGGTTGGCTAYAAYGGNGG) and 1669r (CAACGTATACGGTGGAGGAGTTNGTCATNAC) amplified a product of the correct size (741 bp) from CM2T, CM4T, IMB-1, and the new Hyphomicrobium isolates, and the product was confirmed to be cmuA by sequencing. However, it should be noted that only primer 1669r was specific for cmuA when it was used as a probe. These primers were also used to amplify cmuA sequences from a soil enrichment culture. After cloning and sequencing of PCR products, three different cmuA sequences were detected. The derived amino acid sequence of clone 2 was 99.5% identical to the H. chloromethanicum CmuA sequence, the clone 3 sequence was identical to the IMB-1 CmuA sequence, and the clone 1 sequence was a novel sequence not previously detected that exhibited 91% identity to the H. chloromethanicum CmuA sequence.
DISCUSSION
Our results strongly suggest that H. chloromethanicum CM2T metabolizes chloromethane by using a pathway similar to the corrinoid-dependent pathway (Fig. 1) identified in M. chloromethanicum CM4T (41, 45). Biochemical assays revealed production of methyl tetrahydrofolate from chloromethane in H. chloromethanicum CM2T cell extracts, and the polypeptide profiles provided strong evidence that CM2T and CM4T have similar, if not identical, pathways of chloromethane metabolism. The sizes of the chloromethane-induced polypeptides of CM2T (33 and 67 kDa) are similar to the sizes of the polypeptides detected in M. chloromethanicum CM4T and strain CC495. M. chloromethanicum CM4T expressed two polypeptides (35 and 65 kDa) during growth on chloromethane (45), while CC495 synthesized 29- and 67-kDa polypeptides during growth on chloromethane (5).
The results of cloning and sequencing of the cmuA, cmuB, and cmuC genes from H. chloromethanicum CM2T further support the view that H. chloromethanicum CM2T and M. chloromethanicum CM4T have similar pathways for chloromethane metabolism. In these pathways, the N-terminal domain of CmuA acts as a methyltransferase I enzyme, transferring the methyl group from chloromethane to a corrinoid group attached to the C-terminal domain of this protein. CmuB then acts as a methyltransferase II enzyme, transferring the methyl group to tetrahydrofolate. The corrinoid group of CmuA cycles between the active Co(I) state, capable of nucleophilic attack on chloromethane, and the methylated Co(III) state. The function of CmuC in this pathway is not known for either CM2T or CM4T, although transposon mutants of CM4T with the cmuC gene disabled cannot use chloromethane as a growth substrate but can still dehalogenate it. As CmuC apparently is not needed for dehalogenation, its role must be in a later step in the metabolism of chloromethane.
Alignment of CmuA with homologous polypeptides provides some insight into the likely mechanism of catalysis by this enzyme. Alignment of the methyltransferase domain of CmuA with MtbA from M. barkeri, a protein involved in transfer of the methyl group from a methylated corrinoid protein to coenzyme M (2, 15, 25), and with MtsA from M. barkeri, the methyltransferase subunit of methylthiol:coenzyme M methyltransferase (42, 43), is shown in Fig. 4. MtsA is known to catalyze methyl transfer from the corrinoid subunit to coenzyme M and is also thought to transfer methyl groups from methylated thiols to the corrinoid subunit. Little information about the methyltransferase reaction of CmuA can be deduced from the alignments since the amino acid residues responsible for activities such as substrate binding have not been identified in homologous methyltransferases. However, given the low overall identity of these polypeptides, it seems likely that conserved residues are important for methyltransferase activity. There is, however, one property of the methyltransferase domain that is suggested by this alignment. MtbA, the most closely related protein to have been purified, is a zinc-containing enzyme which is thought to bind zinc with the conserved sequence TVLHICG (25, 44, 50). The CmuA sequences of CM2T and CM4T have these conserved histidine and cysteine residues, which given the low overall sequence identity of MtbA and CmuA, may be functionally important, and these findings suggest that CmuA may also bind zinc.
Alignment of the C-terminal corrinoid-binding domain of CmuA with the sequences of other corrinoid-binding proteins allows tentative identification of the corrinoid-binding site (Fig. 5). A consensus sequence for B12-binding motifs has been identified in a subset of B12-dependent enzymes (DxHxxG- x41–42-SxL-x24–28-GG), including methionine synthase, mutases, and enzymes involved in methyl transfer in methanogens (27). Studies of the corrinoid-binding region of methionine synthase from E. coli, whose structure has been determined, have provided a rationale for this consensus sequence (7). In MetH, His-759 is the lower axial ligand for the cobalt of the corrinoid group, which is bound in the “base off-His on” form. This residue has been shown to be essential for enzyme activity (27). Importantly, in the CM2T CmuA sequence Gln-501 is equivalent to His-759 from MetH, implying that this glutamine residue is the lower axial ligand of the corrinoid group. A glutamine residue has previously been suggested to be the lower axial ligand in CM4T CmuA (45). Apart from CmuA, no corrinoid proteins that contain this glutamine residue in place of histidine have been identified. However, glutamine has been shown to be the lower axial ligand for the porphyrinoid nickel atom of methyl coenzyme reductase of Methanobacterium thermoautotrophicum (8).
CmuB from H. chloromethanicum CM2T exhibits high identity to CmuB from M. chloromethanicum CM4T and to subunit H of methyl-tetrahydromethanopterin:coenzyme M methyltransferase (MtrH) from M. barkeri (17) and M. thermoautotrophicum (12, 16). Alignment (Fig. 6) of CmuB from H. chloromethanicum CM2T and M. chloromethanicum CM4T with MtrH from M. barkeri and MetH from E. coli (7) showed that the CmuB polypeptides contain the conserved motifs present in all eight MtrH-related methyltransferase sequences and in all nine MetH-related methyltransferase sequences (41). Although MtrH and CmuB show sequence similarity and potentially belong to the same methyltransferase family, they exhibit different substrate specificities.
The product of the partial folD gene at the 5′ end of the cluster from H. chloromethanicum CM2T shows 40% identity to the GTP cyclohydrolase II from Photobacterium phosphoreum, which is involved in riboflavin biosynthesis (26), and 22% identity to the 5,10-methylene tetrahydrofolate dehydrogenase/cyclohydrolase (FolD) from E. coli. As methyl tetrahydrofolate is thought to be a product of chloromethane dehalogenation in H. chloromethanicum CM2T, a FolD protein would be an essential enzyme in the metabolism of chloromethane to formate.
It is not clear if fmdB and paaE have any involvement in chloromethane degradation. No homologous genes were cloned in M. chloromethanicum CM4T, but the close proximity of these genes to the cmu genes implies that they may be linked to chloromethane metabolism. FmdB may play a role as a transcriptional regulator, similar to the role proposed for FmdB from M. methylotrophus (49). PaaE, which is likely to be an electron transfer protein, could conceivably act as an electron donor in reactivation of the corrinoid subunit of CmuA.
ACKNOWLEDGMENTS
We acknowledge financial support provided by the Natural Environment Research Council (grant GR9/2192), by studentships for C. Woodall and C. McAnulla, and by INTAS (grant 94-3122).
We thank Don Kelly (University of Warwick) for useful comments on the manuscript.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J H, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burke S A, Lo S L, Krzycki J A. Clustered genes encoding the methyltransferases of methanogenesis from monomethylamine. J Bacteriol. 1998;180:3432–3440. doi: 10.1128/jb.180.13.3432-3440.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler J H. Better budgets for methyl halides? Nature. 2000;403:260–261. doi: 10.1038/35002232. [DOI] [PubMed] [Google Scholar]
- 4.Connell Hancock T L, Costello A M, Lidstrom M E, Oremland R S. Strain IMB-1, a novel bacterium for the removal of methyl bromide in fumigated agricultural soils. Appl Environ Microbiol. 1998;64:2899–2905. doi: 10.1128/aem.64.8.2899-2905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coulter C, Hamilton J T G, McRoberts W C, Kulakov L, Larkin M J, Harper D B. Halomethane:bisulfide/halide ion methyltransferase, an unusual corrinoid enzyme of environmental significance isolated from an aerobic methylotroph using chloromethane as the sole carbon source. Appl Environ Microbiol. 1999;65:4301–4312. doi: 10.1128/aem.65.10.4301-4312.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doronina N V, Sokolov A P, Trotsenko Y A. Isolation and initial characterization of aerobic chloromethane-utilizing bacteria. FEMS Microbiol Lett. 1996;142:179–183. [Google Scholar]
- 7.Drennan C L, Huang S, Drummond J T, Matthews R G, Ludwig M L. How a protein binds B12: a 3.0 Å X-ray structure of B12-binding domains of methionine synthase. Science. 1994;266:1669–1674. doi: 10.1126/science.7992050. [DOI] [PubMed] [Google Scholar]
- 8.Ermler U, Grabarse W, Shima S, Goubeaud M, Thauer R K. Crystal structure of methyl coenzyme M reductase: the key enzyme of biological methane formation. Science. 1997;278:1457–1462. doi: 10.1126/science.278.5342.1457. [DOI] [PubMed] [Google Scholar]
- 9.Ferrandez A, Garcia J L, Diaz E. Genetic characterization and expression in heterologous hosts of the 3-(3-hydroxyphenyl)propionate catabolic pathway of Escherichia coli K-12. J Bacteriol. 1997;179:2573–2581. doi: 10.1128/jb.179.8.2573-2581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank J, Van Krimpen S H, Verwiel P E J, Jongejan J A, Mulder A C, Duine J A. On the mechanism of inhibition of methanol dehydrogenase by cyclopropane-derived inhibitors. Eur J Biochem. 1989;184:187–195. doi: 10.1111/j.1432-1033.1989.tb15006.x. [DOI] [PubMed] [Google Scholar]
- 11.Fukumori F, Saint C P. Nucleotide sequences and regulational analysis of genes involved in conversion of aniline to catechol in Pseudomonas putida UCC22(pTDN1) J Bacteriol. 1997;179:399–408. doi: 10.1128/jb.179.2.399-408.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gärtner P, Ecker A, Fischer R, Linder D, Fuchs G, Thauer R K. Purification and properties of N5-methyltetrahydromethanopterin:coenzyme M methyltransferase from Methanobacterium thermoautotrophicum. Eur J Biochem. 1993;213:537–545. doi: 10.1111/j.1432-1033.1993.tb17792.x. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg I, Rock J S, Ben-Bassat A, Mateles R I. Bacterial yields on methanol, methylamine, formaldehyde and formate. Biotechnol Bioeng. 1976;18:1657–1668. doi: 10.1002/bit.260181202. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin K D, Schaefer J K, Oremland R S. Bacterial oxidation of dibromomethane and methyl bromide in natural waters and enrichment cultures. Appl Environ Microbiol. 1998;64:4629–4636. doi: 10.1128/aem.64.12.4629-4636.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harms U, Thauer R K. Methylcobalamin:coenzyme M methyltransferase isoenzymes MtaA and MtbA from Methanosarcina barkeri. Cloning, sequencing and differential transcription of the encoding genes, and functional overexpression of the mtaA gene in Escherichia coli. Eur J Biochem. 1996;235:653–659. doi: 10.1111/j.1432-1033.1996.00653.x. [DOI] [PubMed] [Google Scholar]
- 16.Harms U, Weiss D S, Gärtner P, Linder D, Thauer R K. The energy conserving N5-methyltetrahydromethanopterin:coenzyme M methyltransferase complex from Methanobacterium thermoautotrophicum is composed of eight different subunits. Eur J Biochem. 1995;228:640–648. doi: 10.1111/j.1432-1033.1995.0640m.x. [DOI] [PubMed] [Google Scholar]
- 17.Hippler B, Thauer R K. The energy conserving methyltetrahydromethanopterin:coenzyme M methyltransferase complex from methanogenic archaea: function of the subunit MtrH. FEBS Lett. 1999;449:165–168. doi: 10.1016/s0014-5793(99)00429-9. [DOI] [PubMed] [Google Scholar]
- 18.Kämpfer P, Müller C, Mau M, Neef A, Auling G, Busse H J, Osborn A M, Stolz A. Description of Pseudaminobacter gen. nov. with two new species, Pseudaminobacter salicylatoxidans sp. nov. and Pseudaminobacter defluvii sp. nov. Int J Syst Bacteriol. 1999;49:887–897. doi: 10.1099/00207713-49-2-887. [DOI] [PubMed] [Google Scholar]
- 19.Kanagawa T, Dazai M, Fukuoka S. Degradation of organo-phosphorus wastes. 2. Degradation of O,O-dimethyl phosphorodithioate by Thiobacillus thioparus TK-1 and Pseudomonas AK-2. Agric Biol Chem. 1982;46:2571–2578. [Google Scholar]
- 20.Keene W C, Khalil M A K, Erickson D J, McCulloch A, Graedel T E, Lobert J M, Aucott M L, Gong S L, Harper D B, Kleiman G, Midgley P M, Moore R M, Seuzaret C, Sturges W T, Benkovitz C M, Koropalov V, Barrie L A, Li Y F. Composite global emissions of reactive chlorine from anthropogenic and natural sources: reactive chlorine emissions inventory. J Geophys Res. 1999;104:8429–8440. [Google Scholar]
- 21.Keuning S, Janssen D B, Witholt B. Purification and characterization of hydrolytic haloalkane dehalogenase from Xanthobacter autotrophicus GJ10. J Bacteriol. 1985;163:635–639. doi: 10.1128/jb.163.2.635-639.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khalil M A K, Moore R M, Harper D B, Lobert J M, Erickson D J, Koropalov V, Sturges W T, Keene W C. Natural emissions of chlorine-containing gases: reactive chlorine emissions inventory. J Geophys Res. 1999;104:8333–8346. [Google Scholar]
- 23.Khalil M A K, Rasmussen R A. Atmospheric methyl chloride. Atmos Environ. 1999;33:1305–1321. [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.LeClerc G M, Grahame D A. Methylcobamide:coenzyme M methyltransferase isoenzymes from Methanosarcina barkeri. J Biol Chem. 1996;271:18725–18731. doi: 10.1074/jbc.271.31.18725. [DOI] [PubMed] [Google Scholar]
- 26.Lee C Y, O'Kane D J, Meighen E A. Riboflavin synthesis genes are linked with the lux operon of Photobacterium phosphoreum. J Bacteriol. 1994;176:2100–2104. doi: 10.1128/jb.176.7.2100-2104.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig M L, Matthews R G. Structure-based perspectives on B12-dependent enzymes. Annu Rev Biochem. 1997;66:269–313. doi: 10.1146/annurev.biochem.66.1.269. [DOI] [PubMed] [Google Scholar]
- 28.Marmur J. A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 29.McDonald, I. R., N. V. Doronina, Y. A. Trotsenko, C. McAnulla, and J. C. Murrell.Hyphomicrobium chloromethanicum sp. nov. and Methylobacterium chloromethanicum sp. nov., chloromethane-utilising bacteria isolated from a polluted environment. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 30.McDonald I R, Kenna E M, Murrell J C. Detection of methanotrophic bacteria in environmental samples with the PCR. Appl Environ Microbiol. 1995;61:116–121. doi: 10.1128/aem.61.1.116-121.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Messmer M, Wohlfarth G, Diekert G. Methyl chloride metabolism of the strictly anaerobic methyl chloride-utilizing homoacetogen strain MC. Arch Microbiol. 1993;160:383–387. [Google Scholar]
- 32.Miller L G, Connell T L, Guidetti J R, Oremland R S. Bacterial oxidation of methyl bromide in fumigated agricultural soils. Appl Environ Microbiol. 1997;63:4346–4354. doi: 10.1128/aem.63.11.4346-4354.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakatsu C H, Straus N A, Wyndham R C. The nucleotide sequence of the TN5271 3-chlorobenzoate 3,4-dioxygenase genes (cbaAB) unites the class Ia oxygenases in a single lineage. Microbiology. 1995;141:485–495. doi: 10.1099/13500872-141-2-485. [DOI] [PubMed] [Google Scholar]
- 34.Oakley C J, Murrell J C. nifH genes in obligate methane oxidising bacteria. FEMS Microbiol Lett. 1988;49:53–57. [Google Scholar]
- 35.Rasche M E, Hyman M R, Arp D J. Biodegradation of halogenated hydrocarbon fumigants by nitrifying bacteria. Appl Environ Microbiol. 1990;56:2568–2571. doi: 10.1128/aem.56.8.2568-2571.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rhew R C, Miller B R, Weiss R F. Natural methyl bromide and methyl chloride emissions from coastal salt marshes. Nature. 2000;403:292–295. doi: 10.1038/35002043. [DOI] [PubMed] [Google Scholar]
- 37.Rose T M, Schultz E R, Henikoff J G, Pietrokovski S, McCallum C M, Henikoff S. Consensus-degenerate hybrid oligonucleotide primers for amplification of distantly related sequences. Nucleic Acids Res. 1998;26:1628–1635. doi: 10.1093/nar/26.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 39.Schaefer J K, Oremland R S. Oxidation of methyl halides by the facultative methylotroph strain IMB-1. Appl Environ Microbiol. 1999;65:5035–5041. doi: 10.1128/aem.65.11.5035-5041.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stirling D I, Dalton H. Properties of the methane mono-oxygenase from extracts of Methylosinus trichosporium OB3b and evidence for its similarity to the enzyme from Methylococcus capsulatus (Bath) Eur J Biochem. 1979;96:205–212. doi: 10.1111/j.1432-1033.1979.tb13030.x. [DOI] [PubMed] [Google Scholar]
- 41.Studer A, Vuilleumier S, Leisinger T. Properties of the methylcobalamin:H4folate methyltransferase involved in chloromethane utilization by Methylobacterium sp. strain CM4. Eur J Biochem. 1999;264:242–249. doi: 10.1046/j.1432-1327.1999.00629.x. [DOI] [PubMed] [Google Scholar]
- 42.Tallant T C, Krzycki J A. Coenzyme M methylase activity of the 480-kDa corrinoid protein from Methanosarcina barkeri. J Bacteriol. 1996;178:1295–1301. doi: 10.1128/jb.178.5.1295-1301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tallant T C, Krzycki J A. Methylthiol:coenzyme M methyltransferase from Methanosarcina barkeri, an enzyme of methanogenesis from dimethylsulfide and methylmercaptopropionate. J Bacteriol. 1997;179:6902–6911. doi: 10.1128/jb.179.22.6902-6911.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vallee B L, Auld D S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990;29:5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- 45.Vannelli T, Messmer M, Studer A, Vuilleumier S, Leisinger T. A corrinoid-dependent catabolic pathway for growth of a Methylobacterium strain with chloromethane. Proc Natl Acad Sci USA. 1999;96:4615–4620. doi: 10.1073/pnas.96.8.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vannelli T, Studer A, Kertesz M, Leisinger T. Chloromethane metabolism by Methylobacterium sp. strain CM4. Appl Environ Microbiol. 1998;64:1933–1936. doi: 10.1128/aem.64.5.1933-1936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whittenbury R, Phillips K C, Wilkinson J F. Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol. 1970;61:205–218. doi: 10.1099/00221287-61-2-205. [DOI] [PubMed] [Google Scholar]
- 48.Wohlfarth G, Diekert G. Anaerobic dehalogenases. Curr Opin Microbiol. 1997;8:290–295. doi: 10.1016/s0958-1669(97)80006-7. [DOI] [PubMed] [Google Scholar]
- 49.Wyborn N R, Mills J, Williams S G, Jones C W. Molecular characterisation of formamidase from Methylophilus methylotrophus. Eur J Biochem. 1996;240:31–38. doi: 10.1111/j.1432-1033.1996.0314h.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Z S, Peariso K, Penner-Hahn J E, Matthews R G. Identification of the zinc ligands in cobalamin-independent methionine synthase (MetE) from Escherichia coli. Biochemistry. 1999;38:15915–15926. doi: 10.1021/bi992062b. [DOI] [PubMed] [Google Scholar]