Abstract
Introduction
Following the introduction of pertussis vaccination during infancy, the age-related demographics of pertussis epidemiology have changed.
Methods
To better understand the pertussis burden (defined here as number of cases and/or incidence rate [IR]) among older adults (OA; at least 50 years of age) in Europe, we collected data on the reported number of cases and IR in this population in Denmark, England and Scotland, Finland, Germany, the Netherlands, Norway and Sweden from 2010 to 2020. Additionally, we collected contextual epidemiological information on surveillance systems, case definitions, laboratory diagnostics and vaccination approaches.
Results
We observed large heterogeneity in the burden among OA between countries: annual IRs ranged from 0.4 (England, 2010) to 54.5 (Norway, 2011) per 100,000 population; 9% (Denmark, 2010) to 45% (England, 2017) of all reported cases occurred in OA. No clear impact of changes in contextual epidemiological information or common trends between countries could be observed, highlighting the need for standardised pertussis surveillance programmes across Europe. The epidemiological trends observed in OA were similar to those observed in 0–4-year-olds.
Conclusion
This analysis showed that B. pertussis continues to circulate among OA in Europe, suggesting that current vaccination strategies are insufficient to decrease the disease burden in all age groups. This may indicate that improved monitoring of pertussis in OA and booster vaccination throughout adulthood are necessary to control the total pertussis burden.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00668-y.
Keywords: Acellular pertussis vaccine, Adult vaccination, Booster vaccination, Epidemiology, Europe, Laboratory diagnostics, Older adults, Pertussis
Plain Language Summary
Whooping cough is an infectious, vaccine-preventable disease that is primarily serious in unvaccinated infants but can also affect adults (at least 50 years old). While vaccination is well established in children, many countries do not routinely vaccinate older adults. Moreover, whooping cough infections in older adults can be difficult to identify for healthcare professionals because of the atypical and mild nature of symptoms. Consequently, the extent of whooping cough occurrence in this population is underestimated. To better understand the extent of disease occurrence, we studied whooping cough infections in Denmark, England and Scotland, Finland, Germany, the Netherlands, Norway and Sweden from 2010 to 2020. Our study was based on the number of laboratory-confirmed cases reported to relevant institutions. We also assessed whether we could identify links between disease occurrence among older adults and contextual epidemiological information, such as disease monitoring systems, methods used for laboratory confirmation, vaccination schedules and vaccination coverage rates. Our study confirmed that whooping cough affects older adults and disease occurrence follows similar trends to those in 0- to 4-year-old children. Because the contextual epidemiological information differed over time and between countries, we could not establish links with disease occurrence in older adults. These data may provide further evidence to authorities that whooping cough among older adults would be better controlled and its burden more accurately estimated with a reinforced comprehensive approach around vaccination and monitoring. Because adults can also infect children who are not yet fully vaccinated, such an approach might help further control the disease in children.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00668-y.
Key Summary Points
| Why carry out this study? |
| Following the introduction of pertussis vaccination during infancy, the age-related demographics of pertussis epidemiology have changed. |
| We collected data on the pertussis burden (defined here as “the number of pertussis cases and/or incidence rate”) among older adults (OA) in Denmark, England and Scotland, Finland, Germany, the Netherlands, Norway and Sweden, from 2010 to 2020 as well as on contextual epidemiological information that may underlie changes in pertussis epidemiology. |
| What was learned from the study? |
| We observed that OA also carry a pertussis burden, which increased over the analytical period in several of the countries that we analysed, and that the proportion of reported cases that occurred in OA also increased over time in most countries. |
| The epidemiological trends observed in OA were similar to those observed in children 0–4 years of age and in the total population, which may indicate that better control of the burden among OA may be necessary to efficiently control the pertussis burden within a country. |
| To decrease the pertussis burden among OA, and thereby help protect the most vulnerable populations, a comprehensive strategy is needed, which should ideally comprise efforts that improve healthcare professionals’ awareness of pertussis infection among OA, increase the notification rate and implement booster vaccination throughout adulthood. |
Digital Features
This article is published with digital features, including a video, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.20066945.
Introduction
Pertussis, commonly known as whooping cough, is caused by the bacterium Bordetella pertussis [1]. It is a highly contagious disease, with an estimated basic reproduction number (R0) around 5.5 [2], which is comparable to the estimated R0 of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Delta variant [3]. Pertussis is characterized by coughing fits that may be accompanied by choking, vomiting and whooping [1, 4]. Before the introduction of pertussis vaccination, disease outbreaks followed a cyclical pattern, with peaks occurring every 3–5 years [1, 5].
To decrease the pertussis burden, whole-cell pertussis and acellular pertussis (aP) vaccines are available [6]. Because pertussis is considered a childhood disease with the most severe cases observed in unvaccinated infants [6], vaccination programmes are tailored to protect infants. However, pertussis can also cause disease in adults, with older age [4, 7] and underlying conditions [8] being risk factors for persistent disease (observed in more than 40% of those over 60 years old with pertussis [4]) and hospitalisation [9]. Currently, 15 European countries recommend booster vaccination against pertussis for all adults or for specific groups (e.g. pregnant women) [10]. However, while coverage of the childhood primary vaccination schedule is high, coverage of the adult booster(s) remains below target [1, 11].
In Europe alone, there is substantial variation in the reported pertussis incidence between countries in any given year; in 2018, the case notification rate across Europe ranged from no cases in Cyprus to 46.8 cases per 100,000 population in Norway [12]. Interpretation of the pertussis burden depends on several factors, including pertussis surveillance systems, case definitions, laboratory diagnostics (including the type of laboratory [state versus private] that performs the tests), healthcare reimbursement criteria, vaccination strategies and vaccination coverage rates [13]. These factors can differ between countries, as demonstrated by a comparative analysis of the pertussis burden in Europe between 2000 and 2013, which hinders reliability of comparisons [13].
Clinical pertussis case definitions have been proposed and adopted by several institutions, including the European Union Parliament and Council and the World Health Organization [12, 14, 15]. Despite some differences, they both define a clinical pertussis case as having more than 2 weeks of cough duration and paroxysms of coughing, whooping or post-tussive vomiting. Confirmation of a clinical case relies on laboratory diagnostics using isolation of B. pertussis (culture), detection of genomic sequences using polymerase chain reaction (PCR) or detection of anti-pertussis toxin (PT) antibodies (serology) [15, 16]. The reliability of laboratory diagnostical methods depends on the age of the patient (e.g. serology cannot be used in infants), time since symptom onset (the sensitivity of culture/PCR decreases with time) or pertussis vaccination (serology can give a false positive result in case of recent vaccination) and laboratory protocols (e.g. PCR target genes or serology targets [e.g. anti-PT IgG or IgA]) [17–20].
Initial diagnosis based on clinical symptoms is challenging because symptoms may differ between age groups [1, 14, 21]. Especially in adults, pertussis behaves atypically, and many cases remain undiagnosed [22]. This can be due to delayed health-seeking behaviour in adults who generally have mild symptoms and/or to unawareness of adult pertussis among healthcare professionals (HCPs) [1]. Consequently, the true pertussis burden is underestimated as has been demonstrated in seroprevalence studies [1]. Overall, it is clear that B. pertussis continues to circulate and surveillance data highlight changing demographics, with adults aged 50 years or more (“older adults” [OA]) now proportionally more affected than before [1]. This is likely attributable to waning immunity following vaccination or infection [23]. Additionally, infected adults may serve as a reservoir for infection of vulnerable populations [1].
There is a gap in our understanding of the true pertussis burden in OA [1, 24–26]. The overview presented here aims to describe the evolution of the pertussis burden (defined here as the number of cases and/or incidence rate [IR] per 100,000 population) and the pertussis surveillance systems, case definitions, diagnostical methods and vaccination strategies (referred to as contextual epidemiological information) in OA in eight European countries from 2010 to 2020, and to examine common trends across countries.
A video with the main findings of this study is available on the journal website; Supplementary file S1 contains summaries per country.
Summary of the contextual pertussis epidemiology in older adults across and in selected European countries (Denmark, England, Scotland, Finland, Germany, The Netherlands, Norway, and Sweden) (MP4 11173 kb)
Methods
Study Design
This was a descriptive overview of surveillance data from 2010 to 2020 (inclusive; or the longest follow-up period available) on the number of laboratory-confirmed cases, IR per 100,000 population and contextual epidemiological information for pertussis in OA in eight European countries: Denmark, England and Scotland, Finland, Germany, the Netherlands, Norway and Sweden. These countries were selected because pertussis was notifiable at the national level and/or because they had the most complete and comparable contextual epidemiological information available. In addition, we briefly discuss data on the number of pertussis cases and IR in Austria and Estonia (discussed separately because they did not have the same level of information available as the other countries).
This overview was an analysis of anonymised data from publicly accessible surveillance databases, annual epidemiological reports, published literature or governmental institutions; thus, ethics or regulatory approvals were not necessary.
Study Objectives
The objectives of this overview were:
To describe the reported number of pertussis cases, IR and contextual epidemiological information among OA per country over time, overall and stratified by age group (strata of 5 or 10 years from 50 years of age [YoA] to the highest age stratum, which differed between countries from ≥ 65 to ≥ 100 YoA)
To describe the proportion of the reported number of pertussis cases occurring in OA compared to the total population
To describe, across countries, how the reported number of pertussis cases, IR and contextual epidemiological information among OA may share common trends
Because children aged 0–4 years are most notably infected by pertussis, and adults may asymptomatically carry B. pertussis and infect (partly) unimmunised infants/children [1], we also explored trends in the reported number of pertussis cases and IR in children 0–4 YoA.
Data Collection
The following data were retrieved for 2010–2020:
Reported number of pertussis cases and IR among OA and all age groups per 100,000 population by calendar year. This includes age-stratified reported number of pertussis cases and IR for OA and data for children aged 0–4 years (when available). The data for all age groups are not presented in this article but were used for calculation of the proportion of cases that were reported among OA.
Pertussis surveillance: methods, case definitions and laboratory assays.
Pertussis vaccination: recommendations and coverage.
Data were collected from publicly accessible surveillance databases, annual epidemiological reports, published literature or governmental institutions. Data sources are summarised in Supplementary Table S1.
Data Analysis
All analyses were performed using Microsoft Excel.
We performed a descriptive analysis; a statistical comparison between countries was not considered suitable because of differences in contextual epidemiological information between/within countries throughout the follow-up period. If data on IRs were not available, the IR per 100,000 population was calculated by dividing the reported number of pertussis cases by the population at risk and multiplying by 100,000.
Results
Number of Pertussis Cases and Incidence Rate in Older Adults and Contextual Epidemiological Information per Country
Denmark
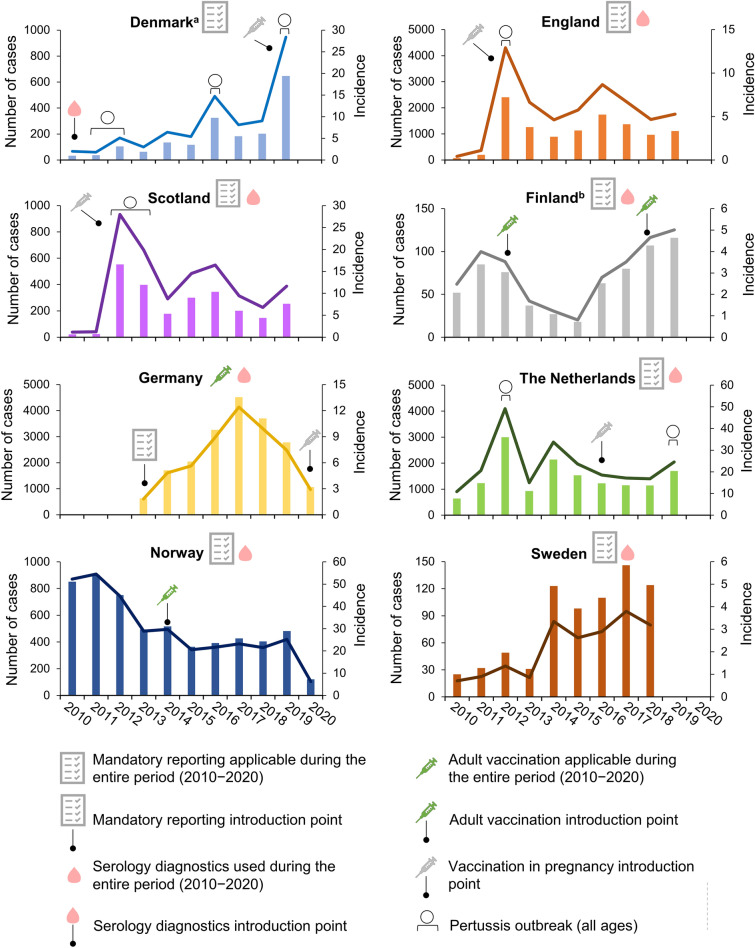
In 2010–2011, the pertussis IR in OA was 1.8 per 100,000 population, which corresponded to 37 cases [27]. From 2012 onwards, the IR gradually increased to 28.4 per 100,000 (647 cases) in 2019 [28, 29]. Pertussis outbreaks at the national level were reported for 2011–2012, 2016 and 2019 (Fig. 1) [29, 30]. Between 9% (2010) and 20% (2018) of all reported cases occurred in OA; the proportion of cases reported in OA was 9 percentage points (pp) higher at the end (2019) of the analytical period than at the start (2010) (Supplementary Fig. S1) [27–29].
Fig. 1.
Number of pertussis cases and incidence rate per 100,000 population in older adults from 2010 to 2020. aSerology diagnostics was introduced at the Statens Serum Institut in 2010; bin 2012, a booster dose was introduced for military personnel and healthcare professionals working with infants and in 2018 for the total population at 25 years of age; laboratories were obliged to report all the confirmed pertussis cases to the National Infectious Diseases Register, but surveillance was not mandatory for doctors. Bars represent number of cases (left y-axis; note the different scale between panels) and lines represent the incidence rate per 100,000 population (right y-axis; note the different scale between panels). For England, data from 2019 were provisional. For the Netherlands, data from 2018 and 2019 were provisional. In the Netherlands, vaccination in pregnancy was recommended since 2016 but only reimbursed from 2019 onwards. Data for outbreaks are at the national level (all age groups) and are based on the literature. Observed increases that were not explicitly described in the literature were not indicated as an outbreak on the figure. The increase observed in England and Scotland in 2016 was a cyclical peak [83]. In Germany, the number of pertussis cases was described to have reached “a worrying record” in 2016 [84]
Denmark used passive, population-based surveillance with mandatory, case-based reporting of laboratory-confirmed cases [27]. In OA with less than 3 weeks of symptom duration or with vaccination in the preceding 2 years, PCR diagnostics (and/or culture) was recommended [27, 31–33]. In other cases, serology was recommended (Table 1; Supplementary Table S2) [27, 31–33]. Pertussis diagnostical tests were primarily performed in state laboratories; general practitioner (GP) visits and laboratory tests were fully reimbursed (Table 1; Supplementary Table S3). There was no recommendation for pertussis vaccination in OA (Table 1; Supplementary Table S4) [27, 29, 34, 35].
Table 1.
Summary of surveillance systems, laboratory information and vaccination schedules in the included countries as available at the time of analysis
| Surveillance system | Laboratory confirmation required for case notification? | Laboratory testinga | Type of laboratory | Reimbursement | Recommended booster vaccinations (aP)b | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Passive | Mandatory reporting | PCR | Culture | Serology | State | Private | GP visit | Laboratory test | Childhood (1–9 YoA) |
Adolescence (10–18 YoA) |
Pregnancy | Other adulthood | ||
| DK | ✓ | ✓ | ✓ | ✓ | ✓ | From 2010 | ✓ | + | ✓ | ✓ | ✓ | × | 2019–2022c | × |
| EN + SC | *d | ✓ | ✓ | ✓ | *e | ✓ | ✓ | × | ✓ | ✓ | ✓ | ✓ | From 2012f | × |
| FI | ✓ | *g | ✓ | ✓ | + | ✓ | ✓ | ✓ | */† | ✓ | ✓ | ✓ | × | From 2012/2018h |
| DE | ✓ | From 2013 | × i | ✓ | *j | ✓ | × | ✓ | ✓ | ✓ | ✓ | ✓ | From 2020 | *k |
| NL | ✓ | ✓ | ✓ | ✓ | + e | ✓ | ✓ | ✓ | ✓ | †l | ✓ | × | From 2016m | × |
| NO | ✓ | ✓ | ✓ | ✓ | Raree | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | × | From 2014n |
| SE | *o | ✓ | ✓ | ✓ | *e | ✓ | ✓ | × | ✓ | ✓ | *p | *p | × | × |
If no year was added, the surveillance system, laboratory test or booster vaccination was applicable throughout the full period 2010–2020. All sources used to collect the data summarized in this table are listed in Supplementary Table S1. More details are provided in Supplementary Tables S2–S4
aP acellular pertussis vaccine, DE Germany, DK Denmark, EN + SC England and Scotland, FI Finland, GP general practitioner, NL the Netherlands, NO Norway, PCR polymerase chain reaction, SE Sweden, YoA years of age, * applicable, × not applicable, + optional, † partial
aThe choice between PCR and serology depends on time since symptom onset: PCR (culture) is mostly used until 2–3 weeks of symptom onset and serology after 2–3 weeks of symptom onset if the patient did not receive a pertussis vaccine in the preceding 1–3 years (depending on the country)
bAll countries recommended a primary childhood aP schedule consisting of 3 or 4 doses
cTemporary (from Nov 2019 to Mar 2022) booster free of charge following a pertussis outbreak
dEnhanced passive (i.e. additional details are collected following notification) for laboratory-confirmed cases
eCulture not in use as per reference [33]
fA temporary “vaccination in pregnancy” programme preceded the recommendation
gLaboratories were obliged to report all the confirmed pertussis cases to the National Infectious Diseases Register, but surveillance was not mandatory for doctors
hBooster for military personnel and healthcare professionals working with children introduced in 2012 and booster for all adults at 25 YoA introduced in 2018
iFor the purpose of this analysis, only laboratory-confirmed clinical cases are considered
jCulture in Germany is primarily performed in reference laboratories
kA booster for healthcare professionals, cocooning strategy and one booster in adulthood
lOut-of-pocket payment for a first pre-specified amount per year (e.g. 385€ for 2020–2021)
mVaccination in pregnancy was recommended but not reimbursed; vaccination in pregnancy was introduced in the national immunization programme in 2019
nDecennial booster
oEnhanced passive (i.e. additional details are collected following notification) for the age group 0–20 years
pInitially one booster dose at 10 YoA (from 2005 to 2011–2012) and later booster doses at 5–6 YoA (from 2007) and at 14–16 YoA (from 2016)
England and Scotland
The pertussis IR among OA in 2010 was 0.42 per 100,000 (76 cases) in England and 1.2 per 100,000 (22 cases) in Scotland, after which a sharp peak was observed that reflected a pertussis outbreak at the national level (and across Europe [36]). The IR in 2012 was 12.9 per 100,000 (2407 cases) in England and 28.0 per 100,000 (552 cases) in Scotland. In 2013–2014, the burden decreased, followed by another lower peak in 2016. The IR remained at a higher level after the 2012 outbreak compared to before (Fig. 1). Between 20% (England, 2010; Scotland, 2011) and 38% (Scotland, 2017) to 45% (England, 2017) of all reported pertussis cases occurred in OA; the proportion of cases reported in OA was 7 percentage points (pp) (Scotland) to 10 pp (England) higher at the end (2019) of the analytical period than at the start (2010) (Supplementary Fig. S1).
England and Scotland employed passive, population-based surveillance (enhanced passive surveillance for laboratory-confirmed cases); reporting was mandatory for laboratory-confirmed cases [37, 38]. Laboratory confirmation relied on PCR and culture if symptom duration was less than 3 weeks and on serology if cough symptom duration was more than 2 weeks and there was no vaccination in the preceding year (Table 1; Supplementary Table S2) [16, 33, 37, 38]. Testing was performed by state laboratories and was fully reimbursed, as were GP visits (Table 1; Supplementary Table S3) [39, 40]. There was no recommendation for pertussis vaccination in OA (Table 1; Supplementary Table S4) [41–45].
Finland
The pertussis IR among OA was 4.0 per 100,000 (85 cases) in 2011 [46]. It decreased to 0.8 per 100,000 (18 cases) in 2015, and steadily increased from 2016 onwards, with a maximum of 5.0 per 100,000 (116 cases) in 2019 [46]. No reports on national outbreaks were identified, but the observed peak in 2011–2012 coincided with an outbreak across Europe [36] (Fig. 1). Between 11% (2015) and 22% (2018) of all reported pertussis cases occurred in OA; the proportion of cases reported in OA was 6 pp higher at the end of the analytical period (2019) than at the start (2010) (Supplementary Fig. S1).
Pertussis surveillance was passive and population-based; laboratories were obliged to report all the confirmed pertussis cases to the National Infectious Diseases Register, but surveillance was not mandatory for doctors [47, 48]. For case confirmation, PCR with optional culture was used if cough duration was less than 3 weeks; serology was used if cough duration was less than 3 weeks (Table 1; Supplementary Table S2) [33, 48, 49]. Laboratory testing was reimbursed and performed in state/private laboratories. GP visits were fully/partially reimbursed, depending on the municipality (Table 1; Supplementary Table S3) [50]. Since 2012, booster doses were recommended for military personnel and HCPs working with infants and since 2018 for 25-year-olds (Table 1; Supplementary Table S4); the coverage for these boosters was unknown [51].
Germany
The pertussis burden in OA steadily increased from an IR of 1.8 per 100,000 (629 cases) in 2013 to 12.4 per 100,000 (4520 cases) in 2017 (data prior to 2013 were not included since mandatory reporting only started in 2013) [52]. From 2018, the burden appeared to decrease to an IR of 2.9 per 100,000 (1062 cases) in 2020 (Fig. 1). Social distancing and isolation during the COVID-19 pandemic likely influenced the data for 2020. Between 31% (2017) and 36% (2013/2015/2020) of all reported cases occurred in OA; the proportion of cases reported in OA was 0.4 pp higher at the end of the analytical period (2020) than at the start (2013) (Supplementary Fig. S1) [52].
Germany employed passive, population-based surveillance with case-based reporting, which became mandatory in 2013 [12, 22, 53]. Reporting included cases that were only clinically and/or epidemiologically confirmed; however, for the purpose of this analysis, only laboratory-confirmed clinical cases were considered. Laboratory confirmation included culture, PCR, and serology (the last of these only if no pertussis vaccination in the preceding 3 years) (Table 1; Supplementary Table S2) [16, 33, 54]. Testing was primarily performed in private laboratories (reference laboratories for culture) and GP visits and laboratory testing were fully reimbursed (Table 1; Supplementary Table S3). Booster doses were recommended for childcare workers and HCPs (since 2003), for adults in close contact with newborns/infants (i.e. cocooning strategy; since 2004) and for all adults (since 2007; a one-time booster, 10 years after the last pertussis vaccination) (Table 1; Supplementary Table S4) [55, 56]. The 10-year vaccination rate for the booster dose recommended for those aged 18 years old or over was estimated to be 32.4% in 2007–2016 (Supplementary Table S4) [56].
The Netherlands
A sharp increase in the pertussis burden among OA was observed between 2010 and 2012, which reflects a national pertussis outbreak in the Netherlands (and across Europe [36]) [57]. In 2012, an IR of 49.1 per 100,000 (3002 cases) was reported. From 2012 onwards, the burden gradually decreased to an IR of 16.8 per 100,000 (1144 cases) in 2018, with an increase to 24.5 per 100,000 (1697 cases) in 2019 (Fig. 1) [58–60]. Between 17% (2010/2011) and 27% (2013/2019) of all reported pertussis cases occurred in OA; the proportion of cases reported in OA was 10 pp higher at the end (2019) of the analytical period than at the start (2010) (Supplementary Fig. S1) [58].
The Netherlands used passive, population-based surveillance with mandatory, case-based reporting for laboratory-confirmed cases [59, 61, 62]. If symptom duration was less than 3 weeks, diagnostics employed PCR (optional culture), while serology was used in other cases (Table 1; Supplementary Table S2) [16, 33, 59, 61, 62]. Both state and private laboratories performed pertussis diagnostics. GP visits were usually reimbursed, but patients needed to pay an annual “own risk” for certain healthcare costs, including laboratory tests (Table 1; Supplementary Table S3) [63]. There was no recommendation for pertussis vaccination in OA (Table 1; Supplementary Table S4) [58, 59].
Norway
The pertussis burden among OA started at the highest level, with an IR of 54.5 per 100,000 (902 cases) in 2011 [64]. The IR gradually decreased from 2012 onwards, reaching the lowest level (IR 6.2 per 100,000; 121 cases) in 2020 (data collected in November; likely influenced by sanitary measures taken during the COVID-19 pandemic) (Fig. 1). The IR was 25.2 per 100,000 (482 cases) in 2019. Between 15% (2020) and 24% (2010) of all reported pertussis cases occurred in OA; the proportion of cases reported in OA was 9 pp lower at the end (2020) of the analytical period than at the start (2010) (Supplementary Fig. S1) [64].
The pertussis surveillance system in Norway was passive and population-based with case-based, mandatory reporting of laboratory-confirmed cases [65, 66]. Laboratory confirmation relied on PCR, 0-sample for antibody “parsera” (i.e. two paired samples taken with a sufficiently large time interval in which an increasing antibody trend indicates infection) and rarely on culture. Culture was used if symptom duration was less than 2 weeks. PCR and antibody test (possibly culture) were used if symptom duration was 2–4 weeks and serology was used if symptom duration was more than 4 weeks (Table 1; Supplementary Table S2) [16, 33, 65, 66]. Testing was performed by state and private laboratories, and both GP visits and testing were fully reimbursed (Table 1; Supplementary Table S3) [67]. Since 2014, a decennial booster is recommended from 15 YoA, which is paid out-of-pocket (Table 1; Supplementary Table S4) [68–70].
Sweden
The pertussis burden among OA appeared similar between 2010 (IR 0.71 per 100,000; 25 cases) and 2013 (IR 0.86 per 100,000; 31 cases) [71, 72]. From 2014 onwards, the burden increased to a maximum IR of 3.8 per 100,000 (146 cases) in 2017 (Fig. 1). Between 10% (2010) and 19% (2011/2017) of all reported cases occurred in OA; the proportion of cases reported in OA was 7 pp higher at the end of the analytical period (2018) than at the start (2010) (Supplementary Fig. S1) [71, 72].
Sweden employed enhanced passive (for 0–20-year-olds)/passive surveillance and mandatory, case-based reporting of laboratory-confirmed cases. Culture, PCR and serology were used for case confirmation (Table 1; Supplementary Table S2) [16, 33, 71]. Testing was performed in state laboratories and was reimbursed, as were GP visits (Table 1; Supplementary Table S3). There was no recommendation for pertussis vaccination in OA (Table 1; Supplementary Table S4) [71, 73].
Other Countries
Data on the number of pertussis cases and IR in OA were also collected for Estonia and are presented in Supplementary Fig. S2.
Number of Pertussis Cases and Incidence Rate in Older Adults, and Contextual Epidemiological Information Across Countries
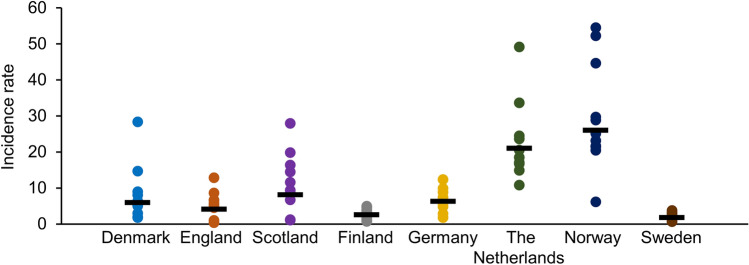
The heterogeneity in the pertussis burden in OA between countries is also apparent in Fig. 2. Annual incidence rates ranged from 0.4 (England, 2010) to 54.5 (Norway, 2011 [64]) per 100,000 population. The geometric mean IR was highest in Norway (26.1 per 100,000), followed by the Netherlands (21.1 per 100,000), Scotland (8.1 per 100,000), Germany (6.3 per 100,000), Denmark (6.0 per 100,000), England (4.1 per 100,000), Finland (2.6 per 100,000) and Sweden (1.8 per 100,000).
Fig. 2.
Incidence rate per 100,000 population in older adults in all countries from 2010 to 2020. For Germany, data were available from 2013 to 2020. For Norway, data were available until November 2020. For Sweden, data were available until 2018 (included). For other countries, data were available from 2010 to 2019 (included). For England, data from 2019 were provisional. For the Netherlands, data from 2018 and 2019 were provisional. Dots represent individual data points; geometric means are indicated with —
The number of cases and contextual epidemiological information across countries are described in Supplementary Fig. S3 [27–29, 46, 52, 58, 64, 71] and Supplementary text S1 [12, 16, 22, 27, 29, 31–35, 37–45, 47–51, 53–56, 58, 59, 61–63, 65–71, 73], respectively.
Number of Pertussis Cases and Incidence Rate in Different Age Strata in Older Adults and in 0–4-Year-Olds
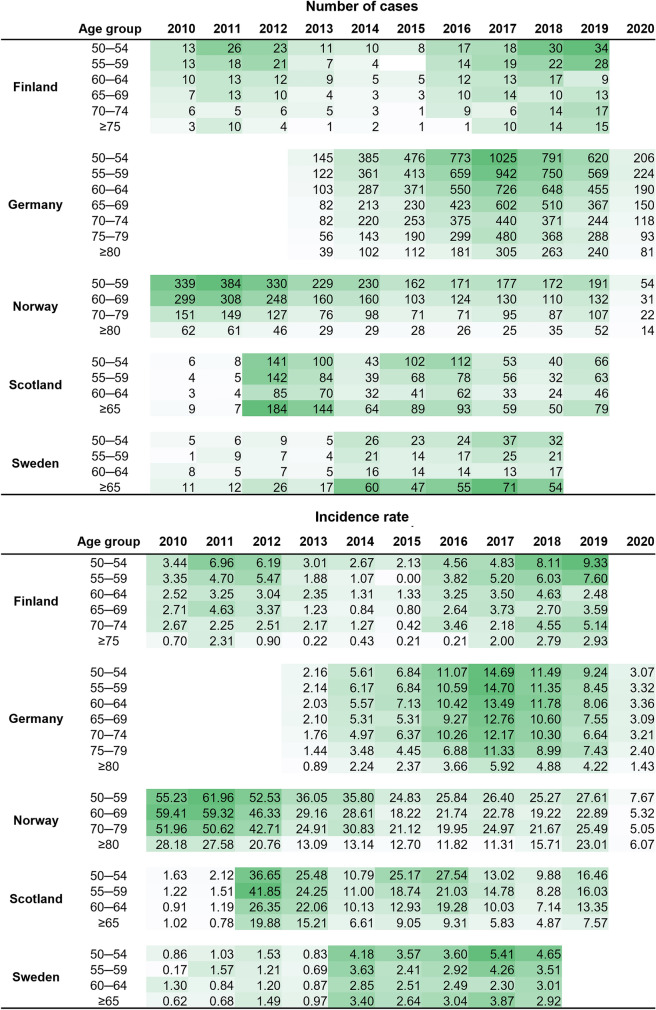
Age-stratified data were available for Finland, Germany, Norway, Scotland and Sweden. Overall, the highest reported IRs per year were observed among the youngest age strata (50–60 YoA) (Fig. 3) [46, 52, 64, 71]. Data were also available for Austria and a similar overall trend was observed (Supplementary Fig. S4).
Fig. 3.
Age-stratified number of pertussis cases and incidence rate per 100,000 population in older adults in countries with data available from 2010 to 2020. Data per country are formatted according to a graded colour scale with the lowest value in white and the highest value in dark green
When comparing IRs between 0–4-year-olds and OA, the IR was generally higher in children, but similar trends could be observed between both groups (Supplementary Fig. S5).
Discussion
With this analysis, we provided an overview of the reported pertussis epidemiology in OA in eight European countries from 2010 to 2020. The reported pertussis incidence among OA was heterogeneous across countries, which had been reported before for all age groups [12, 13].
Some countries experienced an increase in the reported pertussis burden among OA over time, a trend that was most apparent in Denmark, England and Scotland, Sweden and Germany. A decrease in the pertussis burden over time was most apparent in Norway, where the IR nearly halved between 2010 and 2019, and in Finland, where the IR decreased markedly between 2012 and 2016 (from 4.0 to 0.8 per 100,000 population). This may be attributed to the introduction of a decennial booster for adults in 2014 in Norway and a single adult booster dose in Finland in 2012; however, only small percentages of OA receive these booster doses. Therefore, other factors may be involved and/or the decrease may reflect increasing underdiagnosis or underreporting. When stratifying IR and number of cases among OA by age, the burden decreased with increasing age, a trend that had been reported before in England [74, 75]. This may suggest that the frequency of underdiagnosis or underreporting increases with age in OA, which may appear counterintuitive since patients at extreme ends of life are most vulnerable to severe pertussis infection [7]. Alternatively, the trend may reflect the milder clinical presentation of infections in healthy patients from older age strata, leading to underreporting of pertussis cases in this population [1, 2], or the higher B. pertussis circulation among patients from younger age strata who more frequently encounter potential sources of infection (e.g. grandparents taking care of their grandchildren or working OA). Data on patients’ ages, severity of infection, or B. pertussis colonisation were not available in our analysis.
We observed similar trends in the IR between children and OA, suggesting that both populations are similarly affected by changes in B. pertussis circulation. Furthermore, the pertussis outbreaks that were reported at the national level (all ages) were reflected in the burden in OA. Coverage for the primary childhood vaccination schedule is high in most countries; however, immunity, whether from vaccination or infection, wanes over time [1, 5]. Therefore, routine vaccination throughout life may be required to control the pertussis burden in the total population [1, 23]. This is particularly important in countries using aP vaccines since whole-cell pertussis vaccines could provide a more effective and a longer duration of protection than aP vaccines [76].
The heterogeneity in the reported pertussis burden across countries reflects the heterogeneity in pertussis surveillance and vaccination [13]. Several factors can theoretically affect the reported pertussis burden, e.g. improved laboratory testing [23] such as the introduction of serology diagnostics [27]. It has been suggested that serology may be particularly efficient for case confirmation in adults, since they may seek medical attention at a later stage of the disease, as a result of mildness of symptoms, when serology diagnostics is most accurate [27]. After the introduction of serology diagnostics in Denmark, a shift occurred in the main method used for pertussis diagnosis in OA from PCR in 2010 to serology in 2013 [27]; on the basis of our results, PCR became the dominant method again from 2016 (note that the most suitable method depends on time since symptom onset, data which were not available in our review). Similarly, the use of serology diagnostics (commercial kits) also led to increased detection of pertussis in Norway [33, 77]. Also, changes in adult booster recommendations could influence the pertussis burden. One adult booster was already recommended in Germany in 2007 and was introduced in Finland in 2018; decennial boosters were introduced in Norway in 2014. None of these boosters were specifically targeted toward OA. Because the coverage rates for these boosters are currently unknown, we cannot draw conclusions regarding their impact on the pertussis burden in OA. Furthermore, the pertussis outbreak across Europe in 2012 may have raised awareness among HCPs, which could explain the trend towards a higher number of reported cases after compared to before this outbreak. Increases or decreases in the pertussis burden may also reflect the cyclical nature of pertussis disease, which was observed before the introduction of pertussis vaccination [1, 5]. While the current cyclicity of pertussis disease is unclear, outbreaks are still happening in countries with effective childhood vaccination programmes, suggesting a shift in cases from infants and young children to the adult population [1, 2]. Together, this demonstrates the complexity of interpreting the reported data related to the pertussis burden and attributing changes in the burden to changes in contextual epidemiological information.
This complexity is also apparent when attempts are made to compare IRs between countries (Fig. 3). For example, when comparing the mean IR between Scandinavian countries included in our analysis, it is noteworthy that the mean IR in Norway is approximately tenfold higher than the mean IR in Finland and Sweden. This difference exists despite reporting of cases being mandatory in all three countries, adult boosters being recommended in both Finland and Norway (although they are not targeted to OA and differ in frequency and year of introduction) and GP visits and laboratory testing being fully/partially reimbursement in all three countries. Our descriptive analysis lacks information that is needed to accurately define the source(s) of this difference (e.g. data on OA vaccination coverage rates are missing). National decisions are needed to improve the reporting of vaccination coverage in adults.
Different factors contribute to this complexity: (1) the heterogeneity of e.g. vaccination schedules between countries and over time [13, 23], (2) incomplete data (e.g. vaccination coverage) and (3) lacking data (e.g. surveillance system performance, laboratory diagnostical method used for case confirmation and time since symptom onset, vaccination status of confirmed cases, HCP awareness of adult pertussis, and disease severity). Moreover, comparisons of IRs between countries may be hampered because of lacking age-standardised IRs.
The extent of underdiagnosis and underreporting of pertussis cases, and consequently the underestimation of pertussis infections in OA, can be estimated by seroprevalence studies [77–81]. We did not include seroprevalence data in this review; however, two seroprevalence studies in Europe have demonstrated that notification rates underestimated pertussis infections by a factor of 685 in 3–79-year-olds in the Netherlands and a factor of 915 in those 20 years of age or older in Estonia [78, 80]. A recent study in those at least 40 years of age or at least 50 years old from 18 European countries (including Denmark, Finland, Norway, Sweden and the UK) demonstrated that 0.0–9.7% of participants had anti-pertussis antibody levels indicative of recent exposure (at least 100 international units/mL) following correction for assay sensitivity and specificity, with levels in most countries ranging between 2.7% and 5.8% [77]. This is higher than the notification rates observed in our analysis. Notification data have been described as a weak source of information on the incidence of pertussis infection in OA [2]. Possible reasons for limited health-seeking behaviour, underreporting and underdiagnosis of OA pertussis include the mild and atypical nature of symptoms in adults [21], passive surveillance systems [82], the lacking awareness of adult pertussis among HCPs [1] and the narrow time frame during which laboratory tests are sufficiently sensitive [19]. Together with homogenisation of the laboratory diagnostical and surveillance methods in Europe, increasing awareness on adult pertussis among HCPs, health policy makers and the public could improve diagnosis, reporting and estimation of the pertussis burden [1, 13].
This overview aimed to improve understanding of the pertussis burden among OA in Europe, by assessing available epidemiological data in individual countries and putting this into context of their pertussis vaccination/surveillance strategies over a 10-year period. This analysis has some limitations. As a result of data availability, the overview was constricted to countries in North-western Europe and included no countries from Southern Europe. Additionally, it was difficult to collect precise data on all epidemiological factors from included countries, and pertussis epidemiology could not be formally compared between countries because of differences in surveillance systems, diagnostic methods and vaccination approaches. The lack of data on the severity of pertussis cases was a further limitation of this descriptive analysis. Finally, no direct impact of the changes in vaccination approaches on the number of pertussis cases and the incidence rates in OA could be assessed across age groups because of the heterogeneity and lack of standardisation of the available data, the limited collection of precise data for all countries and the lack of coverage data for booster doses.
Conclusions
Our analysis suggested that B. pertussis continues to circulate among OA in Europe. This may indicate that current vaccination strategies are insufficient to decrease the disease burden in all age groups, especially if vaccination coverage is not high enough and/or not all age groups are vaccinated. A comprehensive strategy consisting of efforts that improve HCPs’ awareness of adult pertussis and increase the notification rate as well as implementation of booster vaccination throughout adulthood is needed to accurately estimate and decrease the pertussis burden in OA.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
GlaxoSmithKline Biologicals SA funded this work, was involved in all stages of the study conduct and analysis and took charge of all costs associated with the development and journal’s Rapid Service Fee of this manuscript.
Medical Writing, Editorial, and Other Assistance
The authors thank Adrienne Guignard and Lauriane Harrington for contributions to this study. The authors thank the Modis platform for writing and editorial assistance, manuscript coordination, and design support (on behalf of GSK). Lotte Mathé provided medical writing support, Ivana Lesnjak coordinated manuscript development, and Gil Costa provided design support.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization: Daria Shamarina, Enas Bahar, Piyali Mukherjee, and Yan Sergerie. Formal analysis: Daria Shamarina, Enas Bahar, and Piyali Mukherjee. Investigation: Enas Bahar and Piyali Mukherjee. Methodology: Daria Shamarina, Enas Bahar, and Piyali Mukherjee. Validation: Enas Bahar. Writing – original draft: Enas Bahar. All authors had full access to the study data and take responsibility for data integrity and analysis accuracy. All authors contributed to the writing (review and editing) of the manuscript and approved the final manuscript as submitted.
Disclosures
Daria Shamarina, Piyali Mukherjee, and Yan Sergerie are employees of the GSK groups of companies. Piyali Mukherjee and Yan Sergerie hold shares in the GSK group of companies. Enas Bahar is an employee of Modis Life Sciences C/O GSK. All authors have no non-financial interest to declare.
Compliance with Ethics Guidelines
This overview was an analysis of anonymized data from publicly accessible surveillance databases, annual epidemiological reports, published literature, or governmental institutions; thus, ethics or regulatory approvals were not necessary.
Data Availability
The data that support the findings of this work are available from the corresponding author on reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Enas Bahar, Email: enas.x.bahar@gsk.com.
Daria Shamarina, Email: daria.x.shamarina@gsk.com.
Yan Sergerie, Email: yan.x.sergerie@gsk.com.
Piyali Mukherjee, Email: piyali.x.mukherjee@gsk.com.
References
- 1.Kandeil W, Atanasov P, Avramioti D, Fu J, Demarteau N, Li X. The burden of pertussis in older adults: what is the role of vaccination? A systematic literature review. Expert Rev Vaccines. 2019;18:439–455. doi: 10.1080/14760584.2019.1588727. [DOI] [PubMed] [Google Scholar]
- 2.Kretzschmar M, Teunis PFM, Pebody RG. Incidence and reproduction numbers of pertussis: estimates from serological and social contact data in five European countries. PLoS Med. 2010;7:e1000291. doi: 10.1371/journal.pmed.1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Rocklov J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med. 2021;28:tabb124. doi: 10.1093/jtm/taab124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riffelmann M, Littmann M, Hulsse C, Hellenbrand W, Wirsing von Konig CH. Pertussis: not only a disease of childhood. Dtsch Arztebl Int. 2008;105:623–8. [DOI] [PMC free article] [PubMed]
- 5.Guiso N, Meade BD, Wirsing von König CH. Pertussis vaccines: the first hundred years. Vaccine. 2020;38:1271–6. [DOI] [PubMed]
- 6.World Health Organization Pertussis vaccines: WHO position paper—September 2015. Wkly Epidemiol Rec. 2015;90:433–458. [PubMed] [Google Scholar]
- 7.Mbayei SA, Faulkner A, Miner C, et al. Severe pertussis infections in the United States, 2011–2015. Clin Infect Dis. 2019;69:218–226. doi: 10.1093/cid/ciy889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins VA, Savic M, Kandeil W. Pertussis in high-risk groups: an overview of the past quarter-century. Hum Vaccin Immunother. 2020;16:2609–2617. doi: 10.1080/21645515.2020.1738168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke MF, Rasiah K, Copland J, et al. The pertussis epidemic: informing strategies for prevention of severe disease. Epidemiol Infect. 2013;141:463–471. doi: 10.1017/S095026881200091X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Centre for Disease Prevention and Control (ECDC). Recommended immunisations for pertussis. https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=3&SelectedCountryIdByDisease=-1. Accessed 12 May 2022.
- 11.Choi JH, Correia de Sousa J, Fletcher M, et al. Improving vaccination rates in older adults and at-risk groups: focus on pertussis. Aging Clin Exp Res. 2022;34:1–8. [DOI] [PMC free article] [PubMed]
- 12.European Centre for Disease Prevention and Control (ECDC). Pertussis—Annual Epidemiological Report for 2018. https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2018_pertussis.pdf. Accessed 12 May 2022.
- 13.Heininger U, Andre P, Chlibek R, et al. Comparative epidemiologic characteristics of pertussis in 10 central and eastern European countries, 2000–2013. PLoS One. 2016;11:e0155949. doi: 10.1371/journal.pone.0155949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherry JD, Tan T, Wirsing von Konig CH, et al. Clinical definitions of pertussis: summary of a Global Pertussis Initiative roundtable meeting, February 2011. Clin Infect Dis. 2012;54:1756–64. [DOI] [PMC free article] [PubMed]
- 15.EU case definition Pertussis. Commission Decision of 28 April 2008 amending Decision 2002/253/EC laying down case definitions for reporting communicable diseases to the Community network under Decision No. 2119/98/EC of the European Parliament and of the Council, Official Journal of the European Union. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018D0945&from=EN#page=32. Accessed 12 May 2022.
- 16.World Health Organization (WHO). Vaccine-preventable diseases—surveillance standards: pertussis. https://www.who.int/publications/m/item/vaccine-preventable-diseases-surveillance-standards-pertussis. Accessed 12 May 2022.
- 17.Guiso N, Berbers G, Fry NK, et al. What to do and what not to do in serological diagnosis of pertussis: recommendations from EU reference laboratories. Eur J Clin Microbiol Infect Dis. 2011;30:307–312. doi: 10.1007/s10096-010-1104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO). Laboratory manual for the diagnosis of whooping cough caused by Bordetella pertussis/Bordetella parapertussis. https://apps.who.int/iris/bitstream/handle/10665/127891/WHO_IVB_14.03_eng.pdf?sequence=1&isAllowed=y. Accessed 12 May 2022.
- 19.van der Zee A, Schellekens JFP, Mooi FR. Laboratory diagnosis of pertussis. Clin Microbiol Rev. 2015;28:1005–1026. doi: 10.1128/CMR.00031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lind-Brandberg L, Welinder-Olsson C, Lagergard T, Taranger J, Trollfors B, Zackrisson G. Evaluation of PCR for diagnosis of Bordetella pertussis and Bordetella parapertussis infections. J Clin Microbiol. 1998;36:679–683. doi: 10.1128/JCM.36.3.679-683.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore A, Harnden A, Grant CC, Patel S, Irwin RS, Panel CEC. Clinically diagnosing pertussis-associated cough in adults and children: CHEST guideline and expert panel report. Chest. 2019;155:147–54 [DOI] [PMC free article] [PubMed]
- 22.Hellenbrand W, Beier D, Jensen E, et al. The epidemiology of pertussis in Germany: past and present. BMC Infect Dis. 2009;9:22. doi: 10.1186/1471-2334-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zepp F, Heininger U, Mertsola J, et al. Rationale for pertussis booster vaccination throughout life in Europe. Lancet Infect Dis. 2011;11:557–570. doi: 10.1016/S1473-3099(11)70007-X. [DOI] [PubMed] [Google Scholar]
- 24.Kilgore PE, Salim AM, Zervos MJ, Schmitt HJ. Pertussis: microbiology, disease, treatment, and prevention. Clin Microbiol Rev. 2016;29:449–486. doi: 10.1128/CMR.00083-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crowcroft NS. Pertussis vaccine is only modestly effective in adults: another piece in the pertussis puzzle. Clin Infect Dis. 2020;71:351–352. doi: 10.1093/cid/ciz822. [DOI] [PubMed] [Google Scholar]
- 26.Ridda I, Yin JK, King C, Raina MacIntyre C, McIntyre P. The importance of pertussis in older adults: a growing case for reviewing vaccination strategy in the elderly. Vaccine. 2012;30:6745–6752. doi: 10.1016/j.vaccine.2012.08.079. [DOI] [PubMed] [Google Scholar]
- 27.Dalby T, Andersen PH, Hoffmann S. Epidemiology of pertussis in Denmark, 1995 to 2013. Euro Surveill. 2016;21:30334. doi: 10.2807/1560-7917.ES.2016.21.36.30334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Statens Serum Institut (SSI). Whooping cough 2015—EPI-NEWS No 16—2016. https://en.ssi.dk/news/epi-news/2016/no-16---2016. Accessed 12 May 2022.
- 29.Statens Serum Institut (SSI). Whooping cough—2019 report on disease occurrence. https://en.ssi.dk/surveillance-and-preparedness/surveillance-in-denmark/annual-reports-on-disease-incidence/whooping-cough---2019-report-on-disease-occurrence#. Accessed 12 May 2022.
- 30.Statens Serum Institut (SSI). No 41—2012. Current whooping cough situation and updated recommendations for prophylaxis. https://en.ssi.dk/news/epi-news/2012/no-41---2012. Accessed 12 May 2022.
- 31.Statens Serum Institut (SSI). Diagnosis of pertussis—choice of correct method. https://www.ssi.dk/-/media/arkiv/dk/produkter-og-ydelser/diagnostik/diagnostisk-haandbog/diagnostik-af-kighoste---valg-af-korrekt-metode.pdf?la=da. Accessed 12 May 2022.
- 32.Statens Serum Institut (SSI). Annual reports on disease incidence. https://en.ssi.dk/surveillance-and-preparedness/surveillance-in-denmark/annual-reports-on-disease-incidence. Accessed 12 May 2022.
- 33.He Q, Barkoff AM, Mertsola J, et al. High heterogeneity in methods used for the laboratory confirmation of pertussis diagnosis among European countries, 2010: integration of epidemiological and laboratory surveillance must include standardisation of methodologies and quality assurance. Euro Surveill. 2012;17:20239. doi: 10.2807/ese.17.32.20239-en. [DOI] [PubMed] [Google Scholar]
- 34.Statens Serum Institut (SSI). The Danish Vaccination Program—Annual report 2016 version 2. https://en.ssi.dk/-/media/arkiv/indhold/dk-dansk/vaccination/boernevaccination/boernevaccinationsprogram_2016_v2_jan18. Accessed 12 May 2022.
- 35.Danish Ministry of Health. Pregnant women will still be able to get a free pertussis vaccination. https://sum.dk/nyheder/2021/juni/gravide-vil-fortsat-kunne-faa-en-gratis-kighostevaccination. Accessed 12 May 2022.
- 36.European Centre for Disease Prevention and Control (ECDC). Pertussis—Annual Epidemiological Report 2016 [2014 data]. https://www.ecdc.europa.eu/en/publications-data/pertussis-annual-epidemiological-report-2016-2014-data. Accessed 12 May 2022.
- 37.Public Health England (PHE). Guidelines for the public health management of pertussis in England. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/762766/Guidelines_for_the_Public_Health_management_of_Pertussis_in_England.pdf. Accessed 12 May 2022.
- 38.United Kingdom Government. Pertussis: guidance, data and analysis. https://www.gov.uk/government/collections/pertussis-guidance-data-and-analysis. Accessed 12 May 2022.
- 39.Public Health England (PHE). Pertussis brief for healthcare professionals. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/762782/Pertussis_brief_for_healthcare_professionals.pdf. Accessed 12 May 2022.
- 40.United Kingdom Government. Pertussis: background information on prevention and management. https://www.gov.uk/guidance/pertussis-clinical-and-public-health-management. Accessed 12 May 2022.
- 41.Public Health England (PHE). Historical vaccine development and introduction of routine vaccine programmes in the UK. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/816174/Vaccine_Timeline_2019.pdf. Accessed 12 May 2022.
- 42.Health Protection Agency. Health protection report—weekly report volume 5 number 50. https://webarchive.nationalarchives.gov.uk/20140714091638/http:/www.hpa.org.uk/hpr/archives/2011/hpr5011.pdf. Accessed 12 May 2022.
- 43.Public Health England (PHE). Vaccine update. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/805502/PHE_vaccineupdate_294_May19.pdf. Accessed 12 May 2022.
- 44.Public Health England (PHE). Vaccine update. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/653401/VU_271_october_2017.pdf. Accessed 12 May 2022.
- 45.Public Health England (PHE). Pertussis: the green book, chapter 24. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/514363/Pertussis_Green_Book_Chapter_24_Ap2016.pdf. Accessed 12 May 2022.
- 46.Finnish Institute for Health and Welfare (THL). Statistical database of the Communicable Diseases Register. https://sampo.thl.fi/pivot/prod/fi/ttr/shp/fact_shp?row=area-12260&column=time-12059&filter=reportgroup-12175. Accessed 12 May 2022.
- 47.Bhavsar A, Mertsola J, Poulsen A, Silfverdal S-A. Pertussis in infants in Nordic countries. Acta Paediatr. 2021;110:2040–2044. doi: 10.1111/apa.15800. [DOI] [PubMed] [Google Scholar]
- 48.Finnish Institute for Health and Welfare (THL). Notifiable diseases and microbes. https://thl.fi/fi/web/infektiotaudit-ja-rokotukset/seurantajarjestelmat-ja-rekisterit/tartuntatautirekisteri/ilmoitettavat-taudit-ja-mikrobit. Accessed 12 May 2022.
- 49.Finnish Institute for Health and Welfare (THL). Procedure for cases of pertussis. https://thl.fi/fi/web/infektiotaudit-ja-rokotukset/taudit-ja-torjunta/taudit-ja-taudinaiheuttajat-a-o/hinkuyska/toimenpideohje-hinkuyskatapauksiin. Accessed 12 May 2022.
- 50.Ministry of Social Affairs and Health. Health care payments. https://stm.fi/terveydenhuollon-maksut. Accessed 12 May 20212.
- 51.Finnish Institute for Health and Welfare (THL). Vaccination coverage. https://www.thl.fi/roko/rokotusrekisteri/atlas/public/atlas.html?show=infantbc. Accessed 12 May 2022.
- 52.Robert Koch Institut (RKI). SURVSTAT@RKI 2.0. https://survstat.rki.de/Content/Query/Create.aspx. Accessed 12 May 2022.
- 53.Sin MA, Zenke R, Ronckendorf R, Littmann M, Jorgensen P, Hellenbrand W. Pertussis outbreak in primary and secondary schools in Ludwigslust, Germany demonstrating the role of waning immunity. Pediatr Infect Dis J. 2009;28:242–244. doi: 10.1097/INF.0b013e31818a5d69. [DOI] [PubMed] [Google Scholar]
- 54.Robert Koch Institut (RKI). Falldefinitionen für die Gesundheitsbehörden der Länder, in denen zusätzlich zum IfSG eine Meldepflicht für weitere Krankheiten besteht (Ausgabe 2009). https://www.rki.de/DE/Content/Infekt/IfSG/Falldefinition/laenderverordnungen_falldefs.html. Accessed 12 May 2022.
- 55.Robert Koch Institut (RKI). Vaccination calendar (standard vaccinations). https://www.rki.de/DE/Content/Kommissionen/STIKO/Empfehlungen/Aktuelles/Impfkalender.pdf?__blob=publicationFile. Accessed 12 May 2022.
- 56.Rieck T, Matysiak-Klose D, Hellenbrand W, et al. [Compliance with adult measles and pertussis vaccination recommendations: analysis of data from the national monitoring system KV-Impfsurveillance]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2019;62:422–32. [DOI] [PubMed]
- 57.National Institute for Public Health and the Environment (RIVM). The state of infectious diseases in the Netherlands. https://www.rivm.nl/bibliotheek/rapporten/150002002.pdf. Accessed 12 May 2022.
- 58.National Institute for Public Health and the Environment (RIVM). The National Immunisation Programme in the Netherlands: Surveillance and developments in 2019–2020. https://www.rivm.nl/publicaties/national-immunisation-programme-in-netherlands-surveillance-and-developments-in-2019. Accessed 12 May 2022.
- 59.National Institute for Public Health and the Environment (RIVM). The National Immunisation Programme in the Netherlands: Surveillance and developments in 2018–2019. https://www.rivm.nl/publicaties/national-immunisation-programme-in-netherlands-surveillance-and-developments-in-2018. Accessed 12 May 2022.
- 60.Central Bureau for Statistics (CBS) Statline. Bevolking; geslacht, leeftijd en burgerlijke staat, 1 januari. https://opendata.cbs.nl/statline/#/CBS/nl/dataset/7461BEV/table?ts=1616172184222. Accessed 12 May 2022.
- 61.Heil J, Ter Waarbeek HLG, Hoebe C, et al. Pertussis surveillance and control: exploring variations and delays in testing, laboratory diagnostics and public health service notifications, the Netherlands, 2010 to 2013. Euro Surveill. 2017;22 [DOI] [PMC free article] [PubMed]
- 62.National Institute for Public Health and the Environment (RIVM). Kinkhoest richtlijn. https://lci.rivm.nl/richtlijnen/kinkhoest. Accessed 12 May 2022.
- 63.The Dutch Government. Wanneer betaal ik een eigen risico voor mijn zorg? https://www.rijksoverheid.nl/onderwerpen/zorgverzekering/vraag-en-antwoord/eigen-risico-zorgverzekering. Accessed 12 May 2022.
- 64.Norwegian Surveillance System for Communicable Diseases (MSIS). http://www.msis.no/. Accessed 12 May 2022.
- 65.Norwegian Institute of Public Health (FHI). Barnevaksinasjonsprogrammet. Rapport for perioden 2001–2010. https://www.fhi.no/publ/2012/barnevaksinasjonsprogrammet.-rappor/. Accessed 12 May 2022.
- 66.Norwegian Institute of Public Health (FHI). Notification criteria for diseases in MSIS. https://www.fhi.no/publ/2017/meldingskriterier-for-sykdommer-i-msis/. Accessed 12 May 2022.
- 67.Norwegian Institute of Public Health (FHI). Whooping cough (pertussis) - supervisor for health professionals. https://www.fhi.no/nettpub/smittevernveilederen/sykdommer-a-a/kikhoste-pertussis---veileder-for-h/. Accessed 12 May 2022.
- 68.Norwegian Institute of Public Health (FHI). When will your child be offered vaccines? https://www.fhi.no/en/id/vaccines/childhood-immunisation-programme/when-will-your-child-be-offered-vaccines/. Accessed 12 May 2022.
- 69.World Health Organization (WHO). WHO vaccine-preventable diseases: monitoring system. 2020 global summary—Coverage time series for Norway. https://apps.who.int/immunization_monitoring/globalsummary/coverages?c=NOR. Accessed 12 May 2022.
- 70.Norwegian Institute of Public Health (NIPH). Norhealth. https://www.norgeshelsa.no/norgeshelsa/. Accessed 12 May 2022.
- 71.The Public Health Agency of Sweden. Pertussis surveillance in Sweden—21st annual report. https://www.folkhalsomyndigheten.se/contentassets/cd49fff196f44e6a8db234ffb9da8b80/pertussis-surveillance-sweden-twenty-first-report-19071.pdf. Accessed 12 May 2022.
- 72.Statistics Sweden. The statistics database. https://www.statistikdatabasen.scb.se/pxweb/en/ssd/START__BE__BE0101__BE0101A/BefolkningR1860N/. Accessed 12 May 2022.
- 73.The Public Health Agency of Sweden. Pertussis surveillance in Sweden—Eighteen-year report. https://www.folkhalsomyndigheten.se/contentassets/dbd8cd9e157c47189d72dd8ad9f6c94b/pertussis-eighteen-year-report-16109.pdf. Accessed 12 May 2022.
- 74.Aris E, Harrington L, Bhavsar A, et al. Burden of pertussis in COPD: a retrospective database study in England. COPD. 2021;18:157–169. doi: 10.1080/15412555.2021.1899155. [DOI] [PubMed] [Google Scholar]
- 75.Aris E, Akpo EI, Bhavsar A, et al. Late breaking abstract—the burden of pertussis in adults with asthma: a retrospective database study in England. Eur Respir J. 2020;56:4926. [Google Scholar]
- 76.Chen Z, He Q. Immune persistence after pertussis vaccination. Hum Vaccin Immunother. 2017;13:744–756. doi: 10.1080/21645515.2016.1259780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berbers G, van Gageldonk P, Kassteele JV, et al. Circulation of pertussis and poor protection against diphtheria among middle-aged adults in 18 European countries. Nat Commun. 2021;12:2871. doi: 10.1038/s41467-021-23114-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jogi P, Oona M, Toompere K, Lutsar I. Estimated and reported incidence of pertussis in Estonian adults: a seroepidemiological study. Vaccine. 2015;33:4756–4761. doi: 10.1016/j.vaccine.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 79.de Greeff SC, de Melker HE, van Gageldonk PG, et al. Seroprevalence of pertussis in the Netherlands: evidence for increased circulation of Bordetella pertussis. PLoS One. 2010;5:e14183. doi: 10.1371/journal.pone.0014183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Melker HE, Versteegh FG, Schellekens JF, Teunis PF, Kretzschmar M. The incidence of Bordetella pertussis infections estimated in the population from a combination of serological surveys. J Infect. 2006;53:106–113. doi: 10.1016/j.jinf.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 81.Kristensen M, van Lier A, Eilers R, et al. Burden of four vaccine preventable diseases in older adults. Vaccine. 2016;34:942–949. doi: 10.1016/j.vaccine.2015.12.052. [DOI] [PubMed] [Google Scholar]
- 82.Centers for Disease Control and Prevention. Lesson 5: Public Health Surveillance. https://www.cdc.gov/csels/dsepd/ss1978/lesson5/appendixe.html. Accessed 12 May 2022.
- 83.Public Health England (PHE). Laboratory confirmed cases of pertussis (England): annual report for 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/797712/hpr1419_prtsss-ann.pdf. Accessed 12 May 2022.
- 84.Euroimmunblog. Whooping cough afflicts Germany more than ever. https://www.euroimmunblog.com/whooping-cough-afflicts-germany. Accessed 12 May 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this work are available from the corresponding author on reasonable request.