Take Home Message
Photodynamic therapy for bacillus Calmette-Guérin–unresponsive bladder cancer with TLD-1433 appears safe with an acceptable pharmacokinetic profile. A signal for effectiveness was also seen in the phase 1b trial, thus warranting pursuit of a phase 2 trial.
Keywords: Bladder cancer, Non–muscle invasive bladder cancer, Bacillus Calmette-Guérin, BCG-unresponsive, Photosensitizer, Photodynamic compound, TLD-1433, TLC-3200, Photodynamic therapy, Carcinoma in situ
Abstract
Background
A phase 1b study of photosensitizer TLD-1433–mediated photodynamic therapy (PDT) was performed in bacillus Calmette-Guérin (BCG)-unresponsive non–muscle-invasive bladder cancer (NMIBC) patients.
Objective
The primary objectives were safety and tolerability of PDT, with secondary objectives of (1) pharmacokinetic (PK) properties of TLD-1433 and (2) efficacy, as evaluated by recurrence-free survival and complete response (CR) at 90 and 180 d for patients treated at the maximum recommended starting dose (0.35 mg/cm2 bladder surface area) and the therapeutic dose (0.70 mg/cm2).
Design, setting, and participants
Six BCG-unresponsive patients were enrolled in an open-label, single-arm, dose-escalating study of PDT. TLD-1433 was instilled intravesically for 60 min preoperatively. PDT was performed under general anesthesia using intravesically delivered irradiation of the bladder wall with green light (520 nm) to a dose of 90 J/cm2.
Outcome measurements and statistical analysis
Patients were followed by standard cystoscopy and cytology for up to 18 mo to assess time to recurrence.
Results and limitations
PDT was well tolerated by all patients. All patients experienced at least one grade ≤2 adverse event (AE). There were no patient deaths or light sensitivity reactions. The most common AE was moderate bladder irritability, which resolved within the first weeks after treatment. AEs were independent of the TLD-1433 dose. TLD-1433 was cleared in the urine and from the plasma within 24 and 72 h, respectively. Of three patients treated at the therapeutic dose, two achieved a CR at 180 d, which was durable at 18 mo. The other patient was diagnosed with metastatic disease at 138 d.
Conclusions
PDT with TLD-1433 appears safe for the treatment of BCG-unresponsive NMIBC. Early efficacy signals from full-dose photosensitizer are encouraging and warrant phase 2 trial investigation. The safety and PK results obtained support the potential for administration of consecutive PDT treatments as required.
Patient summary
Photodynamic therapy with TLD-1433 appears to be safe and effective for the treatment of bacillus Calmette-Guérin (BCG)-unresponsive bladder cancer.
1. Introduction
Patients with high-risk non–muscle-invasive bladder cancer (NMIBC; stage T1, high grade, and/ or carcinoma in situ [CIS]) have significant 5-yr risks of recurrence (up to 80%) and progression (up to 50%) if treated inadequately [1], [2]. Intravesical therapy with bacillus Calmette-Guérin (BCG) is used to reduce the risks of recurrence and progression; yet, many (20–30%) do not respond and up to 50% of responders eventually fail [3]. Progression to muscle invasion often requires cystectomy with significant mortality and morbidity [4]. While radical cystectomy remains the gold standard for the treatment of these BCG-unresponsive patients, there remains a strong incentive to develop and study new therapies.
Alternatives to cystectomy after BCG failure include the use of other intravesical agents and, more recently, systemic therapy with PD-1/PD-L1 checkpoint inhibitors [5], [6]. Generally, response rates have been low, so an effective alternative remains an important unmet clinical need.
Photodynamic therapy (PDT) with intravenous porfimer sodium (Photofrin) has been studied, but it has not been adopted widely due to therapy-associated morbidity, including urethral strictures, permanent incontinence, and prolonged skin photosensitivity (see the Supplementary material) [7]. Nevertheless, PDT remains attractive with well-studied mechanisms of action and treatment using standard urological techniques [8], [9]. Novel photosensitizers also have the potential to overcome the challenges posed by their original counterparts. Herein, we report a phase 1 study of TLD-1433, a novel ruthenium-based photosensitizer, in BCG-unresponsive NMIBC patients with a primary endpoint of safety and tolerability, a secondary endpoint of pharmacokinetics, and an exploratory endpoint of efficacy.
2. Patients and methods
2.1. Study design
A phase 1b, open-label, single-arm, dose-escalating [10], single-center clinical study was conducted at Princess Margaret Cancer Centre, University Health Network (UHN), in Toronto, Canada. Ethics approval was provided by the Research Ethics Board of UHN. Patients with BCG-unresponsive NMIBC were recruited between March 2017 and February 2018 (see the Supplementary material and Supplementary Fig. 1 for details of study design).
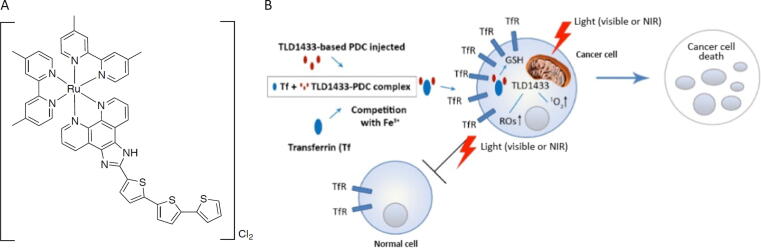
The primary objective of the study was to evaluate the safety and tolerability of TLD-1433 when activated with diffused green laser light generated by the study device (TLC-3200). TLD-1433, a novel photosensitizer, is a ruthenium-based, water-soluble molecule with unique properties including bladder tumor selectivity (Fig. 1A). With light activation, it generates cytotoxic singlet oxygen and radical oxygen species, leading to cell death (Fig. 1B and the Supplementary material). PDT is also known to induce an antitumor cascade of immune signaling [11], [12].
Fig. 1.
(A) Study drug TLD-1433 and (B) its mechanism of action. NIR = near infrared; PDC = photodynamic compound.
2.2. Endpoints
The primary endpoint was measured by adverse events (AEs) of grade ≥3 that did not resolve within 180 d of study treatment, with AEs coded according to the NCI Common Terminology Criteria for Adverse Events v4.03 [13]. The secondary objective of TLD-1433 pharmacokinetics was evaluated by measurements in plasma prior to and at 1, 4, 8, 24, and 72 h, and in urine samples at 8, 24, and 72 h after TLD-1433 administration. TLD-1433 concentration was determined by inductively coupled plasma mass spectrometry. The area under the curve (“AUC0-t”) was calculated with the linear trapezoidal method [14].
An exploratory endpoint of efficacy was defined as a complete response (CR) rate in CIS patients and high-grade recurrence-free survival (RFS) in papillary disease, as assessed by cystoscopy and urine cytology at 3, 6, and 12 mo after treatment. Recurrence was defined as any biopsy-confirmed new high-grade tumor growth. Progression was defined as NMIBC stage progression (ie, CIS to T1), progression to muscle-invasive disease (T2), or development of extravesical or metastatic disease.
2.3. Patients and interventions
Eligible patients had resected high-risk NMIBC (stage Ta, T1, or CIS) who were unresponsive to BCG, and who were not candidates for or refused radical cystectomy. All visible papillary disease was completely resected, but residual CIS was allowed. Potential study patients were also screened 2 wk prior to enrollment with cystoscopic evaluation, to ensure that the bladder was free of persistent or recurrent papillary disease after resection. A computed tomography (CT) scan of the chest, abdomen, and pelvis was performed to rule out upper tract or metastatic disease prior to enrollment in the trial.
A 3-d voiding diary was used to estimate a tolerable bladder volume to retain the study drug. TLD-1433 was instilled intravesically, with a dwell time of 1 h prior to light irradiance under general anesthetic. Based on preclinical studies, the human equivalent dose (maximal starting dose) was determined to be 0.35 mg/cm2 bladder surface (see the Supplementary material). The dose delivered to each patient was based on the calculated bladder surface area at the instilled volume estimated to fully efface the bladder lumen. After three patients had been treated at this dose and followed without significant safety concerns, the independent Data Safety Monitoring Board authorized a second group of three patients to be treated at the therapeutic dose (0.70 mg/cm2). Additional dose escalations were not pursued to avoid the excessive bladder toxicity seen in historical bladder PDT studies.
Patients received intravesical irradiation/PDT under general anesthesia. Patients restricted fluid intake for 12 h prior to TLD-1433 instillation to minimize urine production during treatment. The bladder was drained and washed with sterile water to remove excess drug immediately prior to light treatment (Supplementary Fig. 2). The bladder was then distended with sterile water to efface folds in the bladder mucosa that might shield the bladder wall from laser light. Next, the TLC-3200 laser fiber device was inserted through a cystoscope. The 400 µm laser fiber with an 800 µm isotropic spherical diffuser tip was inserted through the device that was visually positioned in the geographic center of the bladder with pelvic ultrasound verification. The cystoscope was locked in position with a holder. Green laser light (520 nm) was delivered to a dose of 90 J/cm2 over approximately 1 h, depending on bladder volume and hence surface area. The laser source was also able to monitor irradiance using sensors built into the TLC-3200 laser fiber holder and thus providing real-time irradiance data to ensure intraoperative safety (ie, to optimize the dose of laser light delivered and thus prevent overtreatment).
Patients were monitored for 8 h after the procedure and discharged the same day.
2.4. Follow-up
Follow-up included collection of voided urine cytology on days 30, 60, and 90 and cystoscopy with cytology every 3–18 mo. Recurrence was assessed and confirmed via bladder biopsy or transurethral resection of bladder tumor in the setting of a visible bladder lesion or a positive urine cytology. Random bladder biopsies in the setting of a positive cytology with negative cystoscopy, however, were not mandated by the trial protocol and were at the discretion of the treating physician. Post-treatment axial imaging, in the form of CT scans of the chest, abdomen, and pelvis, was performed according to local practice (every 6–12 mo). AEs were rated on a scale from 1 (mild) to 5 (death).
3. Results
A total of six patients were approached and enrolled in the trial. The characteristics of these patients are listed in Table 1. Two out of the six patients were treated with other agents in the BCG-unresponsive setting prior to enrolling on the PDT trial. Specifically, one patient received both pembrolizumab and vicinium before being treated with PDT. The second patient received mitomycin C intravesical monotherapy prior to enrolling in this trial. All six patients attended follow-up visits on post-PDT days 1, 3, 7, 30, 60, and 90 (100%), and five patients completed follow-up on day 180 (83.3%).
Table 1.
Patient demographics and treatment bladder volumes
| Subject | Dose (mg/cm2) | Age a (yr) | Sex | Race | BMI (kg/m2) | Karnofsky performance status | Bladder cancer stage and grade at study entry | Number of prior BCG instills | Number of tumors (and location) | Average bladder volume based on voiding diary (ml) | Study drug volume instilled (ml) | Recurrence: in field or out of field |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 001 | 0.35 | 55 | M | Asian | 23.8 | 90% | T1HG + CIS | 11 | 1 (R UO) | 59.1 | 60 | Out of field |
| 002 | 0.35 | 54 | F | White | 23.5 | 90% | T1HG + CIS | 9 | 2 (L UO, L post wall) | 140.5 | 200 | In field |
| 003a | 0.35 | 62 | M | White | 37.5 | 90% | CIS | 24 | 1 (post to R UO) | 344.6 | 350 | In field and out of field |
| 004 | 0.70 | 79 | M | White | 27 | 100% | T1HG + CIS | 24 | 3 (R BN, L lateral wall, L BN) | 232.6 | 200 | In field and out of field |
| 005 | 0.70 | 58 | M | White | 35.1 | 100% | T1HG | 12 | 2 (L lateral wall, ant wall) | 215.7 | 200 | No recurrence |
| 006b | 0.70 | 68 | F | White | 24.4 | 100% | CIS | 18 | 3 (R dome, post wall, R lateral wall) | 179.9 | 180 | No recurrence |
ant = anterior; BCG = bacillus Calmette-Guérin; BMI = body mass index; BN = bladder neck; CIS = Carcinoma in situ; F = female; L = left; M = male; post = posterior; R = right; UO = ureteric orifice.
Received prior pembrolizumab and vicinium.
Received prior mitomycin C.
3.1. Safety and tolerability
The primary endpoint of safety and tolerability was achieved, with no serious AEs observed in any of the six patients. All AEs were of grade ≤2 (Table 2). The most common AE reported was lower urinary tract symptoms. All patients experienced mild to moderate storage symptoms requiring either anticholinergic or beta-3 agonist support. AEs improved significantly within the first 90 d of treatment, and most (95%) resolved completely within 180 d. There were no deaths related to treatment, and cystectomy related to treatment toxicity was not required. Additionally, TLD-1433 did not result in systemic photoreactions or photosensitivity, indicating low levels of systemic absorption.
Table 2.
CTCAE v4.03 adverse events by patient
| Condition/patient | 001-001 | 001-002 | 001-003 | 001-004 | 001-005 | 001-006 |
|---|---|---|---|---|---|---|
| Bladder spasms | 2—Moderate (resolved on day 6); 1—mild on day 91 (resolved on day 91) | 2—Moderate (ongoing at end of study) | Nil | Nil | 1—Mild (resolved on day 2) | Nil |
| Constipation | 1—Mild (resolved on day 5) | 1—Mild (resolved on day 6) | Nil | 1—Mild (resolved on day 3) | Nil | Nil |
| Urge incontinence | 2—Moderate (resolved on day 6) | Nil | Nil | Nil | 2—Moderate (resolved on day 17) | 2—Moderate (ongoing on day 90) |
| Fatigue | 2—Moderate (onset day 11, ongoing at end of study) | 1—Mild (ongoing at end of study) | Nil | Nil | Nil | 1—Mild (ongoing on day 60) |
| Urinary frequency | Nil | 1—Mild (resolved on day 22) | 1—Mild (resolved on day 6) | 2—Moderate (ongoing at end of study) | 2—Moderate (resolved on day 17) | 2—Moderate (ongoing on day 60) |
| Hematuria | Nil | 1—Mild (onset on day 61, resolved on day 168) | Nil | 1—Mild (ongoing at end of study) | 1—Mild (resolved on day 17) | 1—Mild (resolved on day 26) |
| Pain | Pelvic: 1—mild (ongoing at end of study) | Joint: 2—moderate (onset on day 13, resolved on day 57) | Eye: 1—mild (resolved on day 1) | Right flank pain: 1—mild (onset on day 2, resolved on day 14) | Urinary tract: 1—mild (resolved on day 17) | Urinary tract: 2—moderate (resolved on day 35) |
| Low back: 1—mild (onset on day 61, ongoing at end of study) | Back pain: 2—moderate (onset on day 127, ongoing at end of study) | |||||
| Pelvic: 2—moderate (resolved on day 6) | Urinary tract: 1—mild (ongoing at end of study) | |||||
| Penile discomfort | 1—Mild (onset on day 79, resolved on day 84) | Nil | 1—Mild (resolved on day 5) | Nil | Nil | Nil |
| Urinary urgency | Nil | Nil | Nil | 2—Moderate (ongoing at end of study) | 2—Moderate (resolved on day 17) | Nil |
| 1—Mild (onset on day 38, resolved on day 40) | ||||||
| Other | Nil | Nil | Nocturia: 1—mild (onset on day 170, ongoing at end of study) | Nil | Dry skin: 1—mild (onset on day 79), 2—moderate (ongoing on day 180) | Diarrhea: 1—mild (onset on day 43, resolved on day 57) |
CTCAE = NCI Common Terminology Criteria for Adverse Events.
3.2. Pharmacokinetics
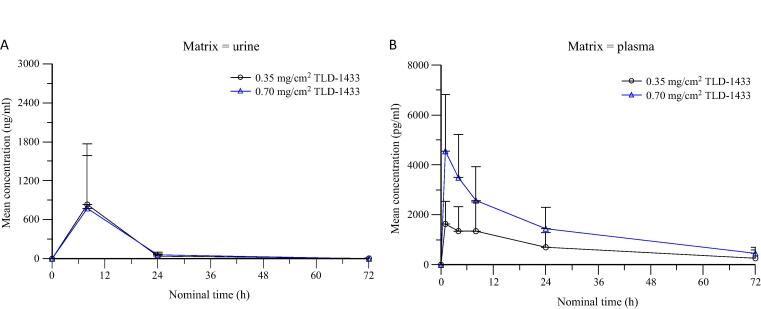
In patients treated with TLD-1433 at 0.35 mg/cm2, the average amounts of drug detected in the urine at 24 and 72 h were 39.4 and 5.3 ng/ml, respectively (Fig. 2A). In those treated with the therapeutic dose (0.70 mg/cm2), the average amounts were 60.1 and 0.6 ng/ml, respectively. Peak of mean concentration profiles were observed at approximately 8 h after treatment. No measurable concentrations were observed after 72 h following administration.
Fig. 2.
Pharmacokinetic concentration of TLD-1433 in (A) urine and (B) plasma over time. Data points represent average TLD-1433 concentrations per milliliter of sample (mean ± standard deviation).
With respect to plasma concentrations, in patients treated with TLD-1433 at 0.35 mg/cm2, the average amounts of drug detected in plasma at 24 and 72 h were 704 and 266 pg/ml, respectively (Fig. 2B). In those treated with 0.70 mg/cm2, the average amounts of drug detected in plasma at 24 and 72 h were 1448 and 463 pg/ml, respectively. The plasma drug concentrations were several-fold lower than those observed in the urine.
3.3. Efficacy
The three patients treated with 0.35 mg/cm2 had evidence of persistent bladder cancer with positive cytology at 3 mo and recurrent disease without progression. Two of the three patients treated with the therapeutic dose of 0.70 mg/cm2 achieved a CR with negative urinary cytology and cystoscopy at 3 and 6 mo after treatment. One patient was diagnosed with metastatic disease at 138 d. The CRs were durable, and the patients remained disease free 18 mo after treatment. No low-grade recurrences were identified in any patients.
4. Discussion
Our phase 1b NMIBC clinical study for BCG-unresponsive patients provides several important findings. First, PDT with TLD-1433 was well tolerated by all patients at two dose levels but while using the same level of light activation. No patient discontinued study participation because of treatment-related AEs. There were no serious AEs (grade ≥3), and although transient moderate bladder irritability was reported by all six patients, these symptoms resolved within 90–180 d and were manageable with standard drug therapy. There were also no AEs attributed to the drug metabolism or excretion, as may typically be experienced by elderly patients receiving systemic drugs. The use of green laser light (penetration depth of 1.5 mm) to activate the photosensitizer TLD-1433 appears to have helped eliminate side effects and discomfort historically seen with red laser light activation, as reported in previous PDT studies (penetration to the detrusor muscle, increasing the risk of bladder fibrosis and contracture). There was no photosensitivity. The study device was equipped with light detectors inside the bladder, which were able to detect irradiance of the bladder wall, thus ensuring safe light delivery during treatment. Pharmacokinetic assays revealing nanomolar presence of the drug in urine and blood that was rapidly cleared (urine within 24 h and plasma within 72 h) provides additional reassurance of biosafety.
Importantly, two out of three patients receiving the therapeutic photosensitizer TLD-1433 dose (0.70 mg/cm2) were recurrence free at 3 mo, suggesting efficacy of the treatment. Even more encouraging was the durability of the therapy, with these two patients being free of disease at 18 mo, suggesting long-term therapeutic benefit with only a single treatment. Although the sample size is extremely small and the efficacy endpoint is purely exploratory in this phase 1 trial, the results are certainly encouraging, particularly because our allowance of residual CIS may also have contributed to the AE profile and affected the efficacy of PDT.
The remaining patient had persistently positive cytology and cystoscopy, and a positive bladder biopsy at 6 mo. Metastatic disease was detected at month 4 in this patient. Nevertheless, this patient is included in the local efficacy consideration evaluated by the rate of RFS at 90 and 180 d after treatment.
PDT with intravenous Photofrin has been studied previously for bladder cancer, but was not widely adopted due to high recurrence rates and therapy-associated morbidity, including urethral strictures, permanent incontinence, and prolonged skin photosensitivity [15], [16]. Nevertheless, PDT remains attractive for this population, with well-studied mechanisms of action and treatment with standard urological techniques. Our novel photosensitizing drug TLD-1433 offers a significant improvement over previous photosensitizers as a ruthenium-based, water-soluble molecule with unique properties, including bladder tumor selectivity [17], [18]. Upon light activation, TLD-1433 generates cytotoxic singlet oxygen and reactive oxygen species with a yield close to unity (number of singlet oxygen molecules produced for each photon of light absorbed by the drug) held spatially and temporally to the malignant cells, allowing efficient and effective cell death (Fig. 1B) [19], [20], [21]. As an intravesical instillation with low systemic absorption and rapid clearance, it is generally free of photosensitivity AEs.
The TLC-3200 system uses green light at 532 nm, which has a depth of penetration of 1.5 mm. This depth ensures adequate penetration of the bladder mucosa, but, unlike prior use of red light to achieve photoactivation, green light does not penetrate into the detrusor muscle, thus decreasing the risk of bladder fibrosis and contracture. With the lack of muscle absorption of TLD-1433 (administration is not intravenous), light activation of the photodynamic compound in the bladder musculature is avoided. The TLC-3200 laser fiber holder has accompanying light detectors deployed along the surface of the bladder urothelium. The detectors can measure both emitted and reflected light at the target of interest, thus ensuring adequate light delivery while preventing overtreatment of the urothelium.
BCG-unresponsive therapy is rapidly evolving, and a number of drugs have been evaluated as second-line treatment. Recently, the immune checkpoint inhibitor pembrolizumab was granted Food and Drug Administration approval for the management of BCG-unresponsive NMIBC [5], [6]. Pembrolizumab demonstrated a CR of 41% at 3 mo for patients with CIS, and a CR of 19% after 1 yr in a phase 2 clinical trial. Results for nadoferogene firadenovec (rAd-INFa/syn3), a nonreplicating adenovirus vector harboring recombinant IFN alpha2b with antitumor activity, have also been published recently, with 3- and 12-mo CR rates similar to those seen with pembrolizumab, but with an AE profile consistent with local therapy [22]. Finally, combination intravesical chemotherapy with gemcitabine and docetaxel has demonstrated a high-grade RFS rate of 50% at 2 yr in BCG-unresponsive patients with CIS and 58% in similar patients with papillary disease only [23]. This study, however, was retrospective in nature and was not designed as a registration trial, and thus may be subject to biases inherent in pooled, retrospective data.
5. Conclusions
Despite efforts to bring new treatment strategies forward for BCG-unresponsive NMIBC, a clear consensus for a standard treatment other than radical cystectomy has yet to be established. An effective therapy that provides high initial and durable responses remains an unmet need. Although limited by the small sample size inherent in phase 1 trials, we feel that the photosensitizer TLD-1433 and the delivery device TLC-3200 hold promise for the treatment of NMIBC. In this study, PDT was well tolerated and demonstrated safety and potential efficacy, thus warranting further study. To this end, a phase 2 clinical study with this technology is currently underway.
Author contributions: Girish S. Kulkarni had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kulkarni, Lilge, Dumoulin-White, Mandel, Jewett.
Acquisition of data: Kulkarni, Nesbitt, Jewett.
Analysis and interpretation of data: Kulkarni, Lilge, Nesbitt, Dumoulin-White, Mandel, Jewett.
Drafting of the manuscript: Kulkarni, Jewett.
Critical revision of the manuscript for important intellectual content: Lilge, Nesbitt, Dumoulin-white, Mandel.
Statistical analysis: Kulkarni, Lilge, Nesbitt, Dumoulin-White, Mandel, Jewett.
Obtaining funding: Dumoulin-White, Mandel.
Administrative, technical, or material support: Kulkarni, Lilge, Nesbitt, Dumoulin-White, Mandel, Jewett.
Supervision: Kulkarni, Lilge, Nesbitt.
Other: None.
Financial disclosures: Girish S. Kulkarni certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Roger Dumoulin-White: Director of Business Development at Theralase Technologies Inc. Arkady Mandel: Chief Scientific Officer at Theralase Technologies Inc. Lothar Lilge: medical and scientific advisory board for Theralase Technologies Inc. Michael Jewett: medical and scientific advisory board for Theralase Technologies Inc.
Funding/Support and role of the sponsor: Funding of this trial was provided by Theralase Technologies Inc.
Associate Editor: M. Carmen Mir
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2022.04.015.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Soukup V., Capoun O., Cohen D., et al. Risk stratification tools and prognostic models in non-muscle invasive bladder cancer: a critical assessment from the European Association of Urology Non-muscle-invasive Bladder Cancer Guidelines Panel. Eur Urol Focus. 2020;6:479–489. doi: 10.1016/j.euf.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Tang D.H., Chang S.S. Management of carcinoma in situ of the bladder: best practice and recent developments. Ther Adv Urol. 2015;7:351–364. doi: 10.1177/1756287215599694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Food and Drug Administration. BCG-unresponsive nonmuscle invasive bladder cancer: developing drugs and biologics for treatment guidance for industry. https://www.fda.gov/media/101468/download.
- 4.Tan W.S., Lamb B.W., Kelly J.D. Complications of radical cystectomy and orthotopic reconstruction. Adv Urol. 2015;2015:323157. doi: 10.1155/2015/323157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balar A., Kamat A., Kulkarni G.S., et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): an open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021;22:919–930. doi: 10.1016/S1470-2045(21)00147-9. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration. FDA approves pembrolizumab for BCG-unresponsive, high-risk non-muscle invasive bladder cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-bcg-unresponsive-high-risk-non-muscle-invasive-bladder-cancer.
- 7.D’Hallewin M.A., Baert L. Long-term results of whole bladder wall photodynamic therapy for carcinoma in situ of the bladder. Urology. 1995;45:763–767. doi: 10.1016/S0090-4295(99)80080-6. [DOI] [PubMed] [Google Scholar]
- 8.Agostinis P., Berg K., Cengel K.A., et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61:250–281. doi: 10.3322/caac.20114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwiatkowski S., Knap B., Przystupski D., et al. Photodynamic therapy – mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 10.Hansen A.R., Graham D.M., Pond G.R., Siu L.L. Phase 1 trial design: is 3+3 the best? Cancer Control. 2014;21:200–208. doi: 10.1177/107327481402100304. [DOI] [PubMed] [Google Scholar]
- 11.Alzeibak R., Mishchenko T.A., Shilyagina N.Y., Balalaeva I.V., Vedunova M.V., Krysko D.V. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future [published correction appears in J Immunother Cancer 2021 Oct; 9(10):] J Immunother Cancer. 2021;9:e001926. doi: 10.1136/jitc-2020-001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meng Z., Zhou X., Xu J., et al. Light-triggered in situ gelation to enable robust photodynamic-immunotherapy by repeated stimulations. Adv Mater. 2019;31:e1900927. doi: 10.1002/adma.201900927. [DOI] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Cancer Therapy Evaluation Program (CTEP). https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.
- 14.Wojciech J. Searching for an optimal AUC estimation method: a never-ending task? J Pharmacokinet Pharmacodyn. 2014;41:655–673. doi: 10.1007/s10928-014-9392-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nseyo UO. Sequential whole bladder photodynamic therapy (WBPDT) in the management of superficial bladder cancer. Clinicaltrials.gov identifier: NCT00322699. https://clinicaltrials.gov/ct2/show/record/NCT00322699?view=record.
- 16.Mejia MC, Nseyo UO. Safety of three sequential whole bladder photodynamic therapy (WBPDT) treatments in the management of resistant bladder cancer. Photonic Therapeutics and Diagnostics V; 71611P. 2009. Progress in Biomedical Optics and Imaging—Proceedings of SPIE. 7161.
- 17.Kasper P., Lazic S., Forward S., Arenas Y., Mandel A., Lilge L. A ruthenium(II) based photosensitizer and transferrin complexes enhance photo-physical properties, cell uptake, and photodynamic therapy safety and efficacy. Photochem Photobiol Sci. 2016;15:481–495. doi: 10.1039/c5pp00450k. [DOI] [PubMed] [Google Scholar]
- 18.Monro S., Colón K.L., Yin H., et al. Transition metal complexes and photodynamic therapy from a tumor-centered approach: challenges, opportunities, and highlights from the development of TLD1433. Chem Rev. 2019;119:797–828. doi: 10.1021/acs.chemrev.8b00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi G., Monro S., Hennigar R., et al. Ru(II) dyads derived from α-oligothiophenes: a new class of potent and versatile photosensitizers for PDT. Coord Chem Rev. 2015;282–3:127–138. [Google Scholar]
- 20.Alberto M.E., Pirillo J., Russo R., Adamo C. Theoretical exploration of type I/type II dual photoreactivity of promising Ru(II) dyads for PDT approach. Inorg Chem. 2016;55:11185–11192. doi: 10.1021/acs.inorgchem.6b01782. [DOI] [PubMed] [Google Scholar]
- 21.Srikrishna G., Freeze H.H. Endogenous damage-associated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia. 2009;11:615–628. doi: 10.1593/neo.09284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boorjian S.A., Alemozaffar M., Konety B.R., et al. Intravesical nadofaragene firadenovec gene therapy for BCG-unresponsive non-muscle-invasive bladder cancer: a single-arm, open-label, repeat-dose clinical trial. Lancet Oncol. 2021;22:107–117. doi: 10.1016/S1470-2045(20)30540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinberg R.L., Thomas L.J., Brooks N., et al. Multi-institution evaluation of sequential gemcitabine and docetaxel as rescue therapy for nonmuscle invasive bladder cancer. J Urol. 2020;203:902–909. doi: 10.1097/JU.0000000000000688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.