Abstract
Portal hypertension is the cause of the clinical complications associated with cirrhosis. The primary complications of portal hypertension are ascites, acute variceal bleed, and hepatic encephalopathy. Hepatic venous pressure gradient measurement remains the gold standard test for diagnosing cirrhosis-related portal hypertension. Hepatic venous pressure gradient more than 10 mmHg is associated with an increased risk of complications and is termed clinically significant portal hypertension (CSPH). Clinical, laboratory, and imaging methods can also aid in diagnosing CSPH non-invasively. Recently, deep learning methods have been demonstrated to diagnose CSPH effectively. The management of portal hypertension is always individualized and is dependent on the etiology, the availability of therapies, and the degree of portal hypertension complications. In this review, we discuss the diagnosis and management of cirrhosis-related portal hypertension in detail. Also, we highlight the history of portal hypertension and future research areas in portal hypertension.
Keywords: history, ascites, acute kidney injury, vasoconstrictors, hemodynamics
Abbreviations: ACLF, acute-on-chronic liver failure; AKI, acute kidney injury; APRI, AST to platelet ratio; AST, aspartate transaminase; BB, Beta blocker; BRTO, balloon occluded retrograde transvenous obliteration; CKD, chronic kidney disease; CSPH, clinically significant portal hypertension; CT, computed tomography; GFR, glomerular filtration rate; GOV, gastrpoesopahegal varices; HE, hepatic encephalopathy; HRS, hepatorenal syndrome; HVPG, hepatic venous pressure gradient; ICG, indocyanine green; LOLA, l-ornithine l-aspartate; NAFLD, Non-alcoholic fatty liver disease; SBP, spontaneous bacterial peritonitis; SGLT2I, sodium glucose co-transporter 2 inhibitors; SSM, splenic stiffness measurement; TE, transient elastography; TIPS, transjugular intrahepatic portosystemic shunt; VITRO, von Willebrand factor to platelet counts
.
Portal hypertension is an increase in portal venous pressure above 5 mm Hg. Cirrhosis is the most common cause of portal hypertension. Non-alcoholic fatty liver disease (NAFLD), alcohol misuse, and viral hepatitis are the common causes of cirrhosis.1,2 In cirrhosis, a structural component driven by liver fibrosis and a dynamic component characterized by increased hepatic vascular tone leads to increased intrahepatic resistance, which in turn causes portal hypertension. Portal hypertension leads to the release of vasodilators such as nitric oxide (NO), which consequently leads to splanchnic vasodilation, decrease in effective arterial blood volume and blood pressure, activation of the renin-angiotensin-aldosterone system, and sodium and water retention. A hyperdynamic circulation ensues, further worsening portal pressure.
The main clinical complications due to these pathophysiological changes are:3
-
•
Ascites and associated complications such as hepatic hydrothorax, spontaneous bacterial peritonitis (SBP), and hepatorenal syndrome (HRS)
-
•
Variceal hemorrhage
-
•
Hepatic encephalopathy (HE)
The cause of portal hypertension can be pre-hepatic (portal vein thrombosis, idiopathic portal hypertension, or non-cirrhotic portal fibrosis) or post hepatic (Budd-Chiari syndrome and heart failure). In this review, we will focus on the diagnosis and management of portal hypertension, which develops as a result of cirrhosis.
History of Portal Hypertension
Andreas Vesalius was the first to demonstrate the pictorial graphic of the portal circulation. Rene Laennec coined the term cirrhosis in 1819. Didier Lebrec published the first trial on propranolol for portal hypertension. Since then, there has been significant progress in the management of portal hypertension. Some of the landmark years in the history of portal hypertension are described in Table 1.
Table 1.
History of Portal Hypertension.
| Andreas Vesalius in Latin, (Bruxelles, 1514— Zante, 1564)-Bleeding hemorrhoids and dilated portal veins-first picture of the portal venous system. |
| 1665, Marcello Malpighi and Francis Glisson: Anatomy of portal venous circulation |
| 1761, Giovan Battista Morgagni-GI hemorrhage from stomach in a patient with altered liver |
| 1819, Renè Laennec-coined the term cirrhosis |
| 1903, Rutherford Morssion: Omentopexy for ascites |
| 1906, Nicolas Augustin Gilbert and Maurice Villaret, introduced the term portal hypertension |
| 1930, K Westfal and 1947, LG Rowntree: Direct pressure on bleeding varices using balloon catheter |
| 1936, Clarence Crafoord and Paul Frenckner: Sclerotherapy for esophaegal varices. Later popularized by J Terblanche in 1979 |
| 1937, WP Thompson and AO Whipple demonstrated portal hypertension as a cause of varices in cirrhosis |
| 1937, Nicholas Eck: Side to side portocaval shunt |
| 1945, Allen O Whipple: Splenorenal shunts. |
| 1974, HH LeVeen L Peritoneo-venous shunt |
| 1950, RW Sengstaken and AH Blakemore balloon tamponade for bleeding esophageal varices. |
| 1967, Warren: Distal splenorenal shunt for bleed |
| 1969, Josep Rosch-Transjugular portal venography and radiologic portocaval shunt |
| 1980, Didier Lebrec: Propranolol for portal hypertension |
| 1982, R.F. Colapinto: Balloon dilated TIPS using Gruntzig catheter |
| 1983, Guido Banti: Splenomegaly is the cause of cirrhosis and anemia |
| 1984, E. Olson Transrenal-Vein Reflux Ethanol Sclerosis for gastric varices |
| 1986, JC Palmaz: Created stents for intrahepatic portocaval shunts |
| 1989, G. M Richter:Palmaz stents for TIPS |
| 1990, First BAVENO Consensus |
| 1992, GV Stiegmann and JS Goff: Ligation for esophaegal varices. |
| 1996, ZA Saeed: Multiband ligator for ligation of varices |
| 1996, H. Kanagawa coined the term BRTO for fundal varices obliteration |
Abbreviations: BRTO, balloon occluded retrograde transvenous obliteration; TIPS, transjugular intrahepatic portosystemic shunt.
Diagnosis of portal hypertension
In patients with compensated cirrhosis, the diagnosis of portal hypertension and risk stratification based on portal pressure is necessary for prognostication and to determine the therapeutic approach to prevent hepatic decompensation. We will discuss the hepatic venous pressure gradient (HVPG), the gold standard for the diagnosis of portal hypertension, and other non-invasive and invasive surrogates.
HVPG
HVPG is a surrogate for portal pressure and is the difference between the wedged hepatic venous pressure (WHVP) and the free hepatic venous pressure (FHVP). WHVP is a measure of hepatic sinusoidal pressure and is measured by extending a balloon catheter to the farthest branches of the hepatic vein. Here, occluding the vein with balloon inflation results in a column of blood with equal pressure to the preceding vascular territory, i.e., hepatic sinusoids. WHVP should be measured in triplicate for one minute without instability for 20 s. WHVP correlates well with portal pressure, especially in viral and alcohol-related cirrhosis. FHVP is a measure of systemic pressure and is subtracted from WHVP to obtain HVPG. FHVP should be measured within 2–3 cm of hepatic vein-inferior vena cava confluence.
Portal pressure is useful in the risk stratification of cirrhosis and has therapeutic implications. Clinically significant portal hypertension (CSPH) or HVPG ≥10 mmHg, is a key event in patients with compensated cirrhosis as it is associated with increased risk of decompensation, death, development of varices, and hepatocellular carcinoma.4 It is in patients with CSPH, non-selective beta-blockers (NSBBs) are indicated to increase decompensation-free survival. Despite the wide applicability of the concept of CSPH as a prognosticator in compensated cirrhosis, it is important to note that patients with NAFLD and PBC may decompensate at lower HVPGs. Severe portal hypertension (≥12 mm Hg) and very severe portal hypertension (≥16 mmHg and above) have been linked to worse outcomes including acute variceal hemorrhage, encephalopathy, ascites, and postsurgical decompensation.5,6 Changes in portal pressure have additional prognostic importance. A decrease in HVPG in response to NSBBs has been shown to prevent the first variceal hemorrhage, development of ascites and death. Changes, as small as 1 mmHg in HVPG, are associated with increased or decreased risk of decompensation and death. The demonstration of decrease in portal pressure is the principal end point used in studies to show the effect of portal pressure-lowering drugs. Though HVPG is accepted as the gold standard for portal pressure measurement, it has limitations. First, it correlates well with portal pressure in viral and alcohol-related cirrhosis (R = 0.92) but not as well in fatty liver disease (R = 0.61). Second, in 10% of patients with histologic cirrhosis, HVPG is normal. And lastly, HVPG is invasive, expensive, requires a specialized operator, and is available only in select centers, limiting its practical clinical utility. Thus, the diagnosis of portal hypertension and CSPH with non-invasive methods is of immense practical significance and is widely used clinically.
Non-invasive Tests in Patients With Compensated Cirrhosis
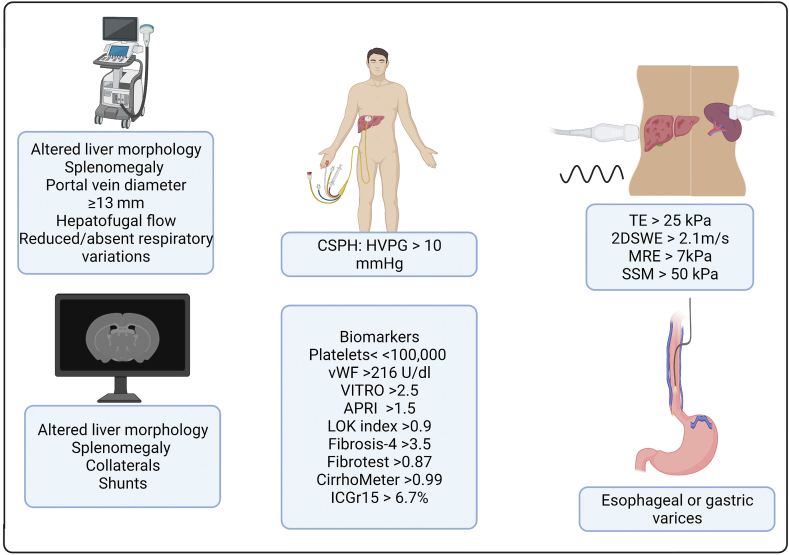
Spider nevi, splenomegaly, and visible abdominal portosystemic collaterals are clinical signs of CSPH. CSPH can also be diagnosed by the presence of porto-systemic collaterals or reversal of blood flow in the portal vein on imaging modalities. However, the absence of these features does not rule out CSPH.7 In the past decade, there has been significant progress in the development of non-invasive tests for the diagnosis of portal hypertension (Figure 1). Depending upon their availability, several elastographic and laboratory tests have been incorporated into clinical practice.
Figure 1.
Non-invasive methods for diagnosis of portal hypertension. HVPG, hepatic venous pressure gradient; APRI, AST to platelet ratio; ICGr, indocyanine green retention test; TE, transient elastography; SWE, shear wave elastography; MRE, magnetic resonance elastography; SSM, splenic stiffness measurement; CSPH, clinically significant portal hypertension; vWF, Von Willebrand factor.
Elastography
Liver stiffness (LS) as measured by transient elastography (Fibroscan) is widely used in clinical practice and can aid in diagnosing CSPH. In a meta-analysis of 11 studies, the sensitivity of LS as measured by TE was 87.5% and the specificity was 85.3% for the diagnosis of CSPH with an area under the curve (AUROC) of 0.9.8 A cut-off of 21 kPa has more than 90% specificity to diagnose CSPH.9 A liver stiffness below 15 kPa and platelet count above 150,000 can definitely rule out CSPH in most etiologies. Based on Baveno VII criteria, in patients with virus-related, alcohol-related, and NAFLD (non-obese) compensated advanced chronic liver disease, LS measured by transient elastography above 25 kPa is sufficient to rule in CSPH.10 However, as demonstrated in the ANTICIPATE study, liver stiffness measured by transient elastography between 20 and 25 and platelet count below 150,000 would also be sufficient to rule in CSPH.11 However, in obese patients with NAFLD, liver stiffness cut off of 25 kPa has only a 62.8% positive predictive value in ruling in CSPH. For these patients, a new model incorporating body mass index and a nomogram to better predict CSPH has been proposed.12
Other elastographic techniques, such as two-dimensional shear wave elastography have an excellent sensitivity and specificity for diagnosing CSPH.13 However, their use is limited by considerable heterogeneity in cut-offs, techniques, and protocols for diagnosis at each center.13
Significant changes in splenic architecture have been utilized to assess the degree of portal hypertension.14 Spleen stiffness measurement (SSM) correlates well with the presence of esophageal varices and CSPH, as demonstrated recently in a meta-analysis of 32 studies.15 This is particularly helpful when liver stiffness is between 15 and 20 kPa.14 A spleen stiffness <21 kPa rules out CSPH while a value ≥ 50 kPa rules in CSPH.10 However, SSM requires a dedicated device and may not replace the well-validated liver stiffness measurement that is readily available at most major centers.
Common Laboratory Measures
In portal hypertension, the splenic sequestration of platelets and suppression of thrombopoietin by tumor necrosis factor leads to thrombocytopenia, which is the most common sign of portal hypertension.16 While a normal platelet count cannot be used to rule out the presence of portal hypertension.3 A platelet count <100,000/mL is strongly associated with the presence of CSPH.17 Several other serum biomarkers like aspartate transaminase to platelet ratio index, LOK index, fibrosis-4 score, and fibrotest, can accurately predict cirrhosis but are less sensitive to diagnose portal hypertension.18, 19, 20, 21 These tests should be used in combination with others, and relying solely on these blood tests to rule out CSPH is not currently recommended.
CirrhoMeter, a composite score of platelet count, prothrombin index, aspartate transaminase, α2-macroglobulin, hyaluronate, urea, age, and sex with a coefficient different from FibroMeter can also diagnose large esophageal varices, which in turn is a surrogate for CSPH.22 A CirrhoMeter score >0.99 would predict high-risk esophageal varices, and a score ≤0.21 would rule out high-risk varices.
Other Laboratory Tests
The ratio of von Willebrand factor to platelet counts (VITRO) has been studied as another non-invasive laboratory-based marker for portal hypertension. A VITRO ≥2.5 is a significant predictor of decompensation and mortality in patients with compensated cirrhosis irrespective of the etiology.23 However, in patients with hepatitis C-related compensated cirrhosis who achieve sustained virologic response, a VITRO score ≥2.1 increases the risk of decompensation. Indocyanine green (ICG), a water-soluble dye, is exclusively removed by the liver after an active uptake. ICG clearance correlates with the hepatocyte blood flow and function. ICG retention at 15 s (ICG-r15) ≥ 6.7% can rule in CSPH, while a value < 10% can rule out large esophageal varices and values ≥ 23% can predict long-term decompensation events.24,25
Endoscopic Techniques
As previously discussed, an HVPG of 10–12 mm Hg is necessary for the development of gastroesophageal varices. Thus in patients with cirrhosis, the diagnosis of varices establishes the presence of CSPH. Esophageal capsule endoscopy can help in the diagnosis of esophageal varices. However, capsule endoscopy is not economical and ubiquitously available.22
Imaging Modalities
Computed tomography (CT)-based liver surface nodularity (LSN) score is another imaging modality to detect CSPH.26 It is hypothesized that cirrhotic nodules visible on CT imaging progressively increase in size and number in cirrhosis and correlate with CSPH. The LSN score is the average distance between each pixel of the detected surface of the liver and a mathematically smoothened line derived from the detected surface that is designed to mimic a normal smooth liver surface. In the validation cohort of this study, an LSN score of 2.8 showed a positive predictive value of 86% for the detection of CSPH.26
The use of subharmonic aided pressure estimation (SHAPE), a form of contrast-enhanced ultrasonography, has shown promise in the diagnosis of portal hypertension in patients with chronic liver disease. In a small study, the SHAPE gradient between hepatic and portal vein has been shown to have a good agreement with HVPG (R = 0.82).27
Recently, artificial intelligence (AI) has been harnessed to diagnose CSPH. In a deep convolutional neural network (CNN) analysis of CT and magnetic resonance images of liver and spleen, the AUROC to diagnose CSPH was 0.9.28 This needs to be validated in further studies. Other examples are AI algorithms using platelet count, portal vein diameter, and splenic width to diagnose esophageal varices and 3D reconstruction of portal vein models and computational fluid dynamics from computed tomographic angiography to determine virtual HVPG.29, 30, 31 Further studies are needed to validate these findings.
Key message: HVPG is the gold standard test for the diagnosis of cirrhosis-related portal hypertension. LS measurement and platelet counts are the simplest non-invasive surrogates of portal hypertension commonly used in practice.
Management of acute complications of portal hypertension
In this section, we will discuss the management of acute, life-threatening complications or portal hypertension, namely variceal hemorrhage, hepatorenal syndrome-acute kidney injury (HRS-AKI) and HE.
Variceal Hemorrhage
Variceal hemorrhage, mainly from esophageal or gastric varices, is a life-threatening acute decompensating event associated with 10%–20% mortality at six weeks.32, 33, 34 The main goals in the management of acute variceal hemorrhage are to control bleeding and prevent early rebleeding and death. Varices outside the gastroesophageal region such as the rectum, duodenum, and at surgically created sites (e.g. stomal) are uncommon (<5% variceal bleeding) and are referred to as ectopic varices. The management of ectopic varices is determined by the anatomy of varices and availability of angiographic and/or endoscopic skills on a case-by-case basis.
As in any gastrointestinal bleeding, the initial steps involve adequate volume resuscitation with consideration to patient age, ongoing blood loss, cardiovascular disorders, and hemodynamic stability. Excessive volume resuscitation can increase portal pressure in variceal bleeding, which can worsen bleeding or cause early rebleeding.35 Unique to the management of variceal bleeding in cirrhosis is thus a cautious, restrictive transfusion strategy of 7–9 gm/dL, which is associated with improved survival.36 Elevated INR in cirrhosis is not an accurate reflection of bleeding tendency and does not warrant additional blood products.37,38 The use of fresh frozen plasma or factor VII transfusion to correct the elevated prothrombin time has shown to have no additional benefits in variceal bleeding and can be potentially harmful.34,39 No specific data are available to guide platelet or cryoprecipitate administration in the management of acute variceal bleeding.37
Bacterial infections are very common in patients with cirrhosis and gastrointestinal bleeding and are associated with poorer clinical outcomes, including 6-week mortality.40 Short-term (maximum 7 days) and early initiation of broad-spectrum antibiotics (such as ceftriaxone 1 gm IV every 24 h) is associated with decreased risk of rebleeding and death, especially in patients with advanced (Child C) cirrhosis.5,41 Local resistance patterns and anti-microbial policies should be considered in determining adequate antibiotic coverage.
The early initiation of vasoactive peptides (before endoscopy) is associated with improved outcomes in variceal hemorrhage.42 Somatostatin and its analogue octreotide, and terlipressin, a vasopressin analogue is the main vasoactive peptides used in the management of variceal hemorrhage. These should be continued for 2–5 days. A meta-analysis of 30 randomized controlled trials investigating the use of somatostatin, vasopressin, and their analogs in variceal hemorrhage found that their use is associated with improved survival, decreased transfusion requirements, improved control of bleeding, and shorter hospital stay.43 Somatostatin, octreotide, and terlipressin are comparable in efficacy and safety in the control of variceal hemorrhage defined by five-day treatment failure.44
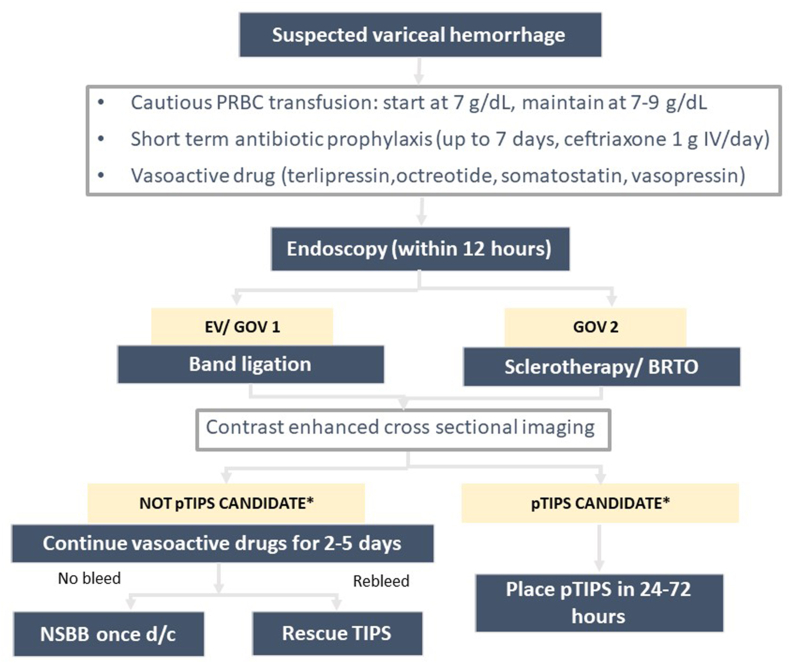
Upper GI endoscopy is definitive in diagnosing and managing suspected variceal hemorrhage.3 Patients with suspected variceal hemorrhage should undergo an endoscopy after hemodynamic resuscitation within 12 h. Prior to endoscopy, the infusion of 250 mg IV erythromycin to clear the stomach of blood is suggested. Intubation in patients with altered mental status should be considered with the goal of extubation as soon as the bleeding is controlled. Band ligation is the definitive therapy for esophageal varices and gastroesophageal varices (GOV) type 1. Sclerotherapy is recommended for bleeding from isolated gastric varices and GOV type 2. All patients should undergo contrast-enhanced cross-sectional imaging to exclude portal vein thrombosis and hepatocellular carcinoma. This imaging study will also be useful for planning pre-emptive transjugular intra-hepatic portosystemic shunt (pTIPS). Patients with Child-Pugh class C (<14 points) or Child-Pugh class B (>7 points) with active bleeding or HVPG >20 mmHg during variceal bleed have a high risk of rebleeding and should be considered for the placement of pTIPS in 24–72 h.10 pTIPS does not increase the risk of hepatic encephalopathy or worsening of ascites.45 ACLF, HE, and hyperbilirubinemia at admission should not be considered as a contraindication for pTIPS as per the recent guidelines10 (Figure 2).
Figure 2.
Management of acute variceal bleed. Patients with HVPG >20 mmHg or patients with a Child-Pugh score of 10–13 (Child class C) or Child-Pugh score of 7–9 (Class B) with active bleeding at endoscopy are high risk patients who may benefit from pTIPS. PRBC, packed red blood cells; EV, esophageal varices; GOV, gastrpoesopahegal varices; BRTO, balloon occluded retrograde transvenous obliteration; pTIPS, pre-emptive TIPS; TIPS, transjugular intrahepatic portosystemic shunt.
In case of failure to control bleeding or refractory bleeding despite pharmacologic and endoscopic therapy, tamponade with Sengastaken-Blakemore or Minnesota tube can be considered as a bridge to more definitive therapies such as TIPS. Balloon occluded retrograde transvenous obliteration (BRTO) can be considered for GOV2, isolated gastric varices or ectopic varices depending on variceal anatomy and availability of local expertise.
Key message: Endoscopy should be performed after initial hemodynamic resuscitation and pharmacotherapy with vasoactive peptides.
HRS
HRS is a form of kidney injury due to a decrease in renal blood flow occurring in patients with cirrhosis and ascites.46 HRS portends poor survival and represents a state of further decompensation in patients with decompensated cirrhosis (history of uncomplicated ascites, variceal hemorrhage or encephalopathy).47,48 HRS can be classified as HRS-AKI and HRS-non-acute kidney injury. HRS-AKI is a rapidly developing AKI defined as an increase in serum creatine by ≥ 0.3 mg/dl within two days or ≥50% from baseline value and/or decrease in urinary output ≤0.5 ml/kg in ≥6 h in patients with cirrhosis and ascites with no other evident cause for acute renal injury such as shock or nephrotoxins.46 Liver transplantation is the definitive therapy for HRS. Pharmacologic therapy aiming at HRS reversal, i.e., improvement in serum creatinine, has been typically used to bridge liver transplantation. Vasoconstrictors and albumin are the primary pharmacological agents for the treatment of HRS.46,48 Vasoactive peptides -terlipressin, octreotide, and noradrenaline are three vasoconstrictors used in the treatment of HRS. Albumin is an essential adjunct to vasoconstrictor therapy that acts as a plasma expander, improves cardiac index, binds to nitrous oxide and other deleterious cytokines, and reduces plasma renin and aldosterone level.49 Vasoactive peptides for HRS should be initiated early as the most significant positive predictor of response to therapy is lower baseline creatinine.50,51
Terlipressin is the most investigated drug for HRS and is the preferred first-line treatment for HRS-AKI.47 Multiple clinical trials and metanalyses have demonstrated the efficacy of terlipressin and albumin in HRS reversal (reduction in serum creatinine to < 1.5 mg/dL).52 Terlipressin is associated with improved overall short-term survival, and responders to terlipressin have improved survival compared to non-responders.53,54 A recent landmark trial that compared terlipressin to placebo demonstrated higher efficacy of terlipressin in improving renal function.55 However, serious, adverse events, including respiratory failure, were noted in the terlipressin arm compared to placebo, probably due to a higher dose of albumin used in the trial.55,56 In addition, terlipressin in patients with a high model for end-stage liver disease (MELD) scores is known to cause ischemic adverse events which may be life-threatening.56,57 Norepinephrine can be used as an alternative to terlipressin to increase the mean arterial pressure. Octreotide is used in combination with midodrine, an alpha agonist that increases blood pressure and renal perfusion. Though octreotide/midodrine is administered via the oral/subcutaneous route in a non-intensive care setting and has a favorable safety profile, it is less effective in reversing HRS than terlipressin58.
Key message: The early recognition of HRS-AKI and initiation of treatment is critical in reversing HRS-AKI. Terlipressin is the recommended drug of choice. Octreotide/midodrine and noradrenaline are alternatives to terlipressin.
HE
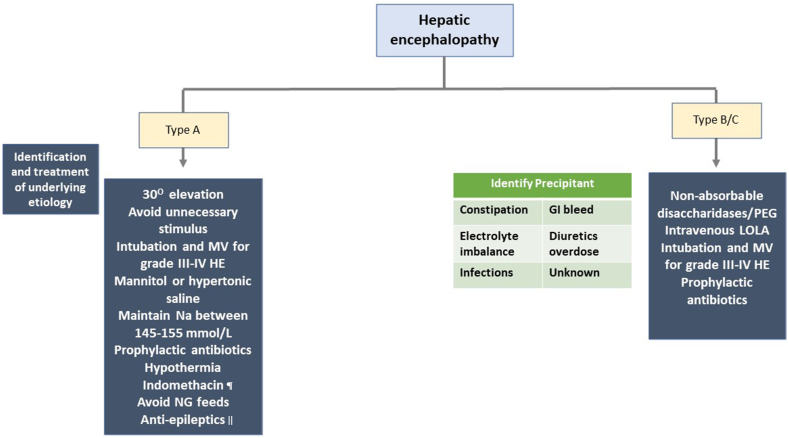
HE is defined based on the underlying disease (as type A-acute liver failure; B- shunting; C- cirrhosis), grade (using West-Haven criteria), time course (as episodic; recurrent; persistent), and the presence or absence of precipitating factor.59 The incidence of covert HE increases gradually as the disease progresses, reaching up to 80%, while overt HE is seen in up to 40% of patients with cirrhosis during illness.59 Therefore, identifying and correcting the precipitant of HE is the most important aspect in the management of HE. Non-absorbable disaccharidases are the first-line therapy for overt HE. A recent study has demonstrated that the addition of polyethylene glycol may lead to early and sustained improvement in HE with improved survival.60 Currently, rifaximin and intravenous l-ornithine l-aspartate (LOLA) are not recommended for overt HE treatment. However, few trials have demonstrated the efficacy of these drugs in overt HE management.61,62 The management of HE is depicted in Figure 3.
Figure 3.
Management of hepatic encephalopathy. ¶ A bolus intravenous indomethacin (0.5 mg/kg) may be considered for raised intracranial hypertension and cerebral hyperemia, which does not respond to mannitol and hypertonic saline. ǁ For patients with increased intracranial pressures and progressive encephalopathy, an electroencephalogram is suggested to evaluate seizure activity and start antiepileptics accordingly. MV, mechanical ventilation; HE, hepatic encephalopathy; Na, sodium; NG, nasogastric; PEG, polyethylene glycol; LOLA, l-ornithine l-Aspartate.
Key message: Identifying the precipitant of HE is the key to management.
Management of chronic complications of portal hypertension
In cirrhosis, portal hypertension can lead to chronic complications such as the development of varices, ascites, and HE. The main goals of management are to prevent recurrent variceal bleeds, control ascites, prevent further decompensation (refractory ascites, HRS or SBP), and prevent exacerbations of hepatic encephalopathy.
Varices
Primary Prophylaxis
Beta-blockers (BBs) and endoscopic variceal ligation are the mainstay of therapy to prevent the first variceal bleed.3 BBs are economical and can prevent bacterial translocation and SBP.63 BBs can also prevent decompensation (ascites) in patients with compensated cirrhosis and CSPH.64 Furthermore, BB therapy may prevent the progression of portal hypertensive gastropathy, while variceal ligation is deemed to be associated with accentuation of portal hypertensive gastropathy.65 The major concern with BB is the need for life-long therapy and regular monitoring to prevent hypotension. Carvedilol is preferred over traditional BB due to its multiple benefits. Carvedilol is 2–4 times more potent than propranolol.66 Carvedilol has intrinsic antioxidant properties and prevents vascular smooth muscle cell proliferation. Beta-2 blockade leads to vasoconstriction in the splanchnic circulation and due to intrinsic α1-adrenoceptor blockade causes precapillary vasodilatation leading to a reduction in resistance in hepatic and porto-systemic collaterals. Due to its low β1 blockade, the decrease in heart rate and cardiac output is less pronounced. Carvedilol reaches a peak plasma concentration within 60–120 min unless consumed with food when the absorption is delayed by another 60–120 min.67 Nearly 98% of the drug is plasma protein-bound in circulation. The terminal half-life of carvedilol is 7–10 h. Carvedilol is cleared by oxidative metabolism, and the conjugates of these metabolites are excreted in feces. Only 16% of conjugates are eliminated through the renal route. Carvedilol is also effective in delaying the progression of small to large esophageal varices.68 The recommended target to prevent variceal bleeds is a 25% reduction in heart rate or a 55–60/minute resting heart rate. The addition of ivabradine to BB can improve the hemodynamics and achieve the target heart rate with a reduced incidence of kidney injury and encephalopathy.69 However, the role of ivabradine in preventing variceal bleed needs further studies.
Nearly 20% of variceal bleeds are due to gastric varices. GOV-1 needs to be managed similarly to esophageal varices. The management of acute gastric variceal bleed from GOV-2 and isolated gastric varices (IGV-1) is complicated, and the mortality in these patients is higher.70 For GOV-2 and IGV-1, BB and endoscopic cyanoacrylate obliteration are the mainstay of therapy to prevent bleeds.3,71 BB is the recommended therapy for the primary prevention of GV bleed.47,70 However, few studies have demonstrated endoscopic cyanoacrylate obliteration to be superior to BB.71 The data to support BRTO/TIPS for primary prevention is sparse. Recently, a study from Korea demonstrated that both BRTO and endoscopic obliteration are equally effective in preventing gastric variceal bleeds than no treatment.72 However, this retrospective study had several concerns. Less than 30% of patients in each group were treated with BB. The study also included patients with GOV-1 for BRTO and endoscopic obliteration. The study had significant deviations from the current standard of care recommended by the American Association for the Study of the Liver Disease (AASLD) and the European Association for the Study of the Liver (EASL).5,47
Key message: Patients with small high-risk varices (red signs) or small varices in Child-Pugh Class C require primary prophylaxis with BB. In contrast, those with medium to large varices can be treated with BB or EVL. BB can prevent the decompensation and progression of varices. BB and endoscopic obliteration are equally effective for primary prophylaxis against GV bleed.
Secondary Prophylaxis
Surgical portosystemic shunting was the only effective modality in the early 1950s to prevent recurrent variceal bleeds in patients with cirrhosis. However, shunt surgery was associated with high morbidity and mortality. Propanolol was the first drug demonstrated to be effective in preventing recurrent bleeds from esophageal varices.73 Since then, BBs have become the standard of care for preventing recurrent variceal bleeds. Reduction in HVPG by ≥ 20% from baseline or reduction to <12 mmHg leads to reduced incidence of variceal rebleeding, ascites, SBP, HRS, and hepatic encephalopathy.74 Endoscopic variceal ligation with BB (propranolol and nadolol) is the recommended therapy to prevent recurrent variceal bleeds.3 A small trial has compared carvedilol + EVL vs. propranolol + EVL for secondary prevention of AVB.75 A higher number of patients in the carvedilol group achieved HVPG response though the incidence of recurrent bleed was similar in both groups.75 BBs are also associated with reduced waitlist mortality in patients with refractory ascites and variceal bleeds.76 However, the safety of beta-blockers in patients with advanced cirrhosis is still contentious.
TIPS is currently recommended for patients who fail EVL + NSBB; however, a recent meta-analysis has demonstrated significant mortality benefit with early TIPS defined as placement of the stent within five days after variceal bleed.77 In addition, early TIPS is also associated with a reduction in the incidence of recurrent variceal bleed (at 1-year) without the added risk of hepatic encephalopathy.77 Likewise, pTIPS has also been demonstrated to be safe and effective in preventing recurrent variceal bleed and improving mortality in patients with cirrhosis who are at high risk of bleeding (as discussed above).78 TIPS is also documented to be safe in patients with ACLF. A recent multicenter study demonstrated the safety of pTIPS in patients with ACLF identified by EASL criteria.79 pTIPS was associated with improved survival and reduced rebleeding rates.79 However, this retrospective study had several drawbacks.79,80 Only 6% of patients with ACLF underwent pTIPS. Patients with lower MELD scores and ACLF grades were chosen for TIPS, and the study failed to assess the hemodynamic response to pTIPS in patients with ACLF. Hence, further data are required to assess the safety of pTIPS in patients with ACLF.
TIPS is more effective than cyanoacrylate injection to prevent GV rebleeding, especially in patients with high portal pressure gradients.81,82 However, TIPS is associated with an increased risk of hepatic encephalopathy and liver failure due to shunting of blood away from the liver. BRTO may be more effective than TIPS in preventing rebleeding from gastric varices and is associated with improved survival.83 A study is underway comparing endoscopic cyanoacrylate obliteration against BRTO (ClinicalTrials.gov Identifier: NCT02468206). Nearly 20% of patients do not have gastro- (spleno) renal shunts making them less amenable to BRTO.84 Endoscopic ultrasonography (EUS) guided coiling with or without glue is also effective in preventing rebleeding.85 However, the expertise to perform these expensive radiological procedures are not available at all centers. Endoscopic obliteration with or without BB is recommended therapy to prevent GV rebleeding in the absence of expertise for TIPS and BRTO.5,86,87
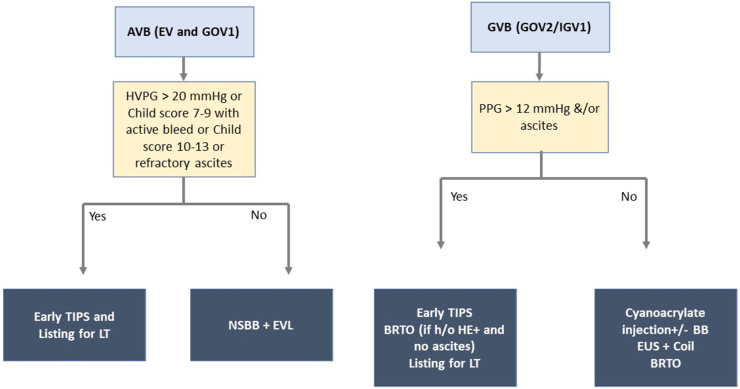
Key message: Endoscopic obliteration with BB or BRTO/TIPS is effective for preventing rebleeding from fundal varices. EUS guided coiling can be performed in patients without shunts (Figure 4).
Figure 4.
Secondary prophylaxis to prevent rebleeds. AVB, acute variceal bleed; EV, esophageal varices; GOV, gastroesophageal varices; HVPG, hepatic venous pressure gradient; TIPS, transjugular intrahepatic portosystemic shunt; LT, liver transplantation; NSBB, non-selective beta-blocker; EVL, endoscopic variceal ligation; GVB, gastric variceal bleed; PPG, portal pressure gradient; BRTO, balloon occluded retrograde transvenous oblit- eration; HE, hepatic encephalopathy; EUS, endoscopic ultrasonography.
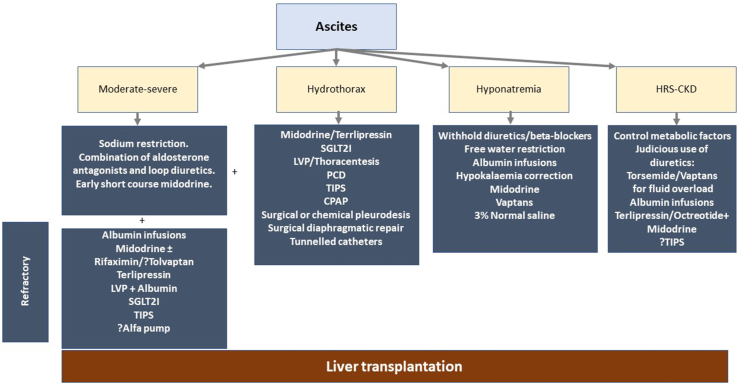
Ascites (Figure 5)
Figure 5.
Management of ascites and its complications. LVP, large volume paracentesis; SGLT2I, sodium glucose co-transporter 2 inhibitors; TIPS, transjugular intrahepatic portosystemic shunt; PCD, percutaneous drainage; CPAP, continuous positive airway pressure; HRS, hepatorenal syn- drome; CKD, chronic kidney disease.
Ascites is the most common decompensation in the natural history of cirrhosis.47,88 Dietary sodium restriction (5 g salt or 90 mmol of sodium) and oral diuretics are the first-line treatment for uncomplicated ascites. A combination of aldosterone antagonists and loop diuretics is preferred over sequential treatment with aldosterone antagonists followed by loop diuretic.89 Combination therapy is well tolerated and is associated with better control of ascites than sequential therapy.89 Early introduction of short-course midodrine can also lead to better control of ascites with lesser incidence diuretic associated complications.90
Refractory Ascites
Diagnosis and medical management
A mean weight loss of <0.8 kg over 4 days and urinary sodium less than sodium intake in a patient with cirrhosis on intensive diuretic therapy (furosemide 160 mg/day and spironolactone 400 mg/day) for at least one week is termed as diuretic resistant ascites.47 Conversely, any patient developing adverse events due to diuretics limiting the further use of diuretics are labeled as diuretic refractory ascites. Hepatocellular carcinoma, portal vein thrombosis, and infection should be ruled out before labeling refractory ascites. Most patients are diuretic intolerant ascites.91 Furthermore, a full dose of 160 mg of furosemide and 400 mg of spironolactone is rarely tolerated.90,92 The impaired tolerance, especially in Asian populations, is attributable to sarcopenia and a higher incidence of diuretic-induced complications like acute kidney injury, dyselectrolytemia, or encephalopathy.90,93
The incidence of tuberculosis is relatively high in Asian countries.94 It is suggested to perform adenosine deaminase level testing and tuberculosis nucleic acid testing for all cirrhotic patients before labeling refractory ascites.93 Recent reports suggest that patients with an ascitic fluid total protein content of >2 g/dl should be evaluated for tubercular ascites.95
Large volume paracentesis and paracentesis-induced circulatory dysfunction: Liver transplantation remains the definitive therapy for patients with refractory ascites. However, liver transplantation is not feasible due to organ donor shortage and the financial burden.96 Large-volume paracentesis with albumin is the recommended therapy to prevent paracentesis-induced circulatory dysfunction. Recently, a network meta-analysis has demonstrated midodrine to be superior to albumin in preventing paracentesis-induced circulatory dysfunction.97,98 Due to compromised cardiac performance, beta-blockers are contraindicated in patients with refractory ascites and those undergoing large-volume paracentesis.99, 100, 101
Vasoconstrictors and albumin: Midodrine, an alpha1 agonist, improves urinary sodium excretion and urinary volume and has a beneficial effect in patients with refractory ascites.102 The addition of rifaximin in patients with refractory ascites may improve hemodynamics by reducing endotoxemia.103 Tolvaptan is also reported to improve ascites control and prolong survival.104 However, tolvaptan depletes vitamin-K-dependent clotting factors and inhibits platelet aggregation increasing the risk of bleeds, hematomas, and ecchymosis.105 Moreover, tolvaptan has a black box warning due to the risk of liver injury.106 Hence tolvaptan is suggested only for patients with refractory hyponatremia in grade 3 ascites and/or refractory ascites for a short duration.48
Terlipressin has been shown to increase the GFR, urinary sodium excretion and contain the vasodilatory and antinatriuretic systems in cirrhotics with ascites.53 Terlipressin administration is beneficial in patients with refractory ascites, especially those with AKI.107,108 Terlipressin administration for a brief period of 3 weeks can improve ascites control and renal function and is suggested as an excellent non-transplant therapy.53
Long-term albumin administration in patients with refractory ascites improves survival. In addition, long-term albumin administration is associated with a lesser incidence of hospital admissions for overt hepatic encephalopathy, ascites, SBP, and non-SBP infections.109 However, the cost of albumin therapy deters one from recommending it, especially in lower socioeconomic countries. A short-term albumin therapy for up to 4–8 weeks may be beneficial in outpatients to overcome the hemodynamic abnormalities.90
TIPS: TIPS in carefully selected patients can prolong the transplant-free survival and may be preferred over repeated large volume paracentesis.110,111 Polytetrafluoroethylene covered stents have patency rates of 90% at two years and are preferred over bare-metal stents.111 Age <70 years, serum bilirubin <3 mg/dl and MELD score <18 with no history of hepatic encephalopathy are excellent candidates for TIPS.
Newer therapies: The ALFA pump system consists of a subcutaneously implanted battery-powered programmable pump connected to catheters that transfer ascites from the peritoneal cavity to the bladder, from which it is eliminated.112 Alfa pump is not available worldwide, and the currently available data are limited to suggest its use.48 Furthermore, alfa pump is associated with higher adverse events.112 Long-term abdominal drains are a palliative option for patients with malignant ascites and those with end-stage liver disease.113,114
Dapagliflozin, empagliflozin, and canagliflozin are three drugs that target Sodium-Glucose Cotransporter 2 (SGLT2) in the proximal tubule of the nephron, promoting increased excretion of both sodium and glucose in the urine.115 The inhibition of SGLT2 in the proximal tubule promotes glycosuria and natriuresis and attenuates renin secretion, thereby improving glycemic control, reducing salt and water retention.115,116 Few studies have reported significant improvement in ascites with SGLT2 inhibitors.116,117 Two major drawbacks with SGLT2I are increased frequency of urinary infections and questionable role in diuretic intolerant ascites.115,118 However, further research is required to ascertain the role of SGLT2I in patients with cirrhosis and refractory ascites.
Key message: TIPS, large volume paracentesis, and midodrine are suitable alternatives to liver transplantation for the management of ascites and its associated complications.
Hydrothorax
Hydrothorax is common in patients with ascites. The management of hepatic hydrothorax is step-wise.119 Apart from diuretics and salt restriction, vasoconstrictors such as oral midodrine and intravenous terlipressin can be used to control hepatic hydrothorax.53,119 Large-volume paracentesis and thoracentesis can improve pulmonary function immediately.120,121 Alfa pump is a useful measure in controlling ascites and enhancing the quality of life of cirrhotic patients with refractory ascites.119 Extensive trials with alfa pump should be evaluated in patients with ascites and hydrothorax. Continuous positive airway pressure (>5 cmH20) prevents the reaccumulation of fluid in pleural space. TIPS is an excellent therapeutic option for patients with favorable MELD (<15) and is associated with a response rate of nearly 75%.122 Although effective, the percutaneous drainage of pleural effusion is associated with infectious complications. Percutaneous drainage with small catheters can be used in hospitalized patients planned for liver transplantation. Approximately 400–500 ml of fluid is drained every 4–6 hourly to prevent respiratory distress. The surgical repair of diaphragmatic defects, surgical or chemical pleurodesis (with talc or tetracycline), have been tried with variable results.123 However, surgical procedures are poorly tolerated and are associated with higher complication rates. Indwelling catheters are recommended for palliative care.123
Hyponatremia
Hypervolemia is the most common cause of hyponatremia in patients with cirrhosis.124 The first step in the management of hyponatremia is to stop diuretics after a thorough clinical examination for features of hypervolemia. Free water restriction (<1000 ml/day) is recommended only for patients with serum sodium <125 meq/dl. Human albumin solution can increase serum sodium levels in patients with cirrhosis.125,126 Midodrine and vaptans are beneficial in treating hyponatremia.105,127 However, the data are limited. Tolvaptan is more effective in treating hyponatremia and is preferred for patients with refractory hyponatremia in grade 3 ascites for a short duration.48,128, 129, 130 Hypertonic saline should be avoided as it may exaggerate ascites. However, in patients with symptomatic hyponatremia with serum sodium <120 mEq/L or serum sodium <110 mEq/L, the cautious use of hypertonic saline is suggested with a target to increase serum sodium by ≤ 8 mEq per day.131 The plasma sodium concentration is determined by the ratio of sodium and potassium content to total body water.132 Therefore, hypokalemia correction may improve hyponatremia.
HRS-Chronic Kidney Disease
As cirrhosis disease progresses, renal plasma flow and glomerular filtration rate gradually decline in patients with refractory ascites. CKD is in cirrhosis is associated with poor outcomes both pre-and post-transplant.133,134 If the eGFR is less than 60 mL/min/1.73 m2 for more than three months in the absence of structural renal disease, then the patient is labeled as HRS-CKD.135 It is critical to differentiate HRS-CKD (functional CKD) and structural CKD as the treatment is different.136 However, the differentiation is only by the absence of abnormal urine analysis and normal renal structure.136 Furthermore, the cut-off of 60 ml is also affected by age and gender and may interfere with the diagnosis of HRS-CKD in elderly patients with cirrhosis. Further studies should assess novel biomarkers to identify HRS-CKD correctly. Patients who do not respond to terlipressin, patients with higher MELD, baseline serum cystatin, albuminuria, and those who develop recurrent AKI episodes progress to HRS-CKD.137, 138, 139
There are no clear guidelines on HRS-CKD management due to a lack of literature. Diuretic therapy should be avoided in patients with functional renal failure. If deemed necessary for fluid overload, torsemide is preferred due to lower renal clearance. Vaptans have good efficacy but should be used carefully in HRS-CKD with refractory ascites.128 Terlipressin has excellent efficacy in HRS-CKD; however, the risk of recurrence is high and patients need to be maintained on long-term therapy.53 Oral midodrine is used at some centers though currently, there is a lack of literature on its efficacy in patients with HRS-CKD. TIPS for HRS is still considered experimental due to the higher incidence of hepatic encephalopathy.140 However, a recent study demonstrated a significant reduction in in-patient mortality for patients who underwent TIPS for HRS compared to those undergoing dialysis.141 Fluid overload may be overcome with renal replacement therapy, but the optimal timing is unclear.48 Patients with GFR <60 ml/min for >90 days and subsequent GFR <30 ml/min or requirement for dialysis are candidates for simultaneous liver-kidney transplant (SLKT). Metabolic diseases (such as hyperoxaluria, atypical hemolytic uremic syndrome, familial non-neuropathic systemic amyloidosis, and methylmalonic aciduria) with CKD, which can be corrected with liver transplant, are also candidates for SLKT.142 On the contrary, for patients with AKI, the persistence of GFR <25 ml/min for >6 weeks and/or dialysis dependence are candidates for SLKT.142
HE
The presence of HE is independently associated with a significant increase in the risk of in-hospital and short-term mortality.143 Current therapeutic options for the treatment of HE are non-absorbable disaccharides such as lactulose and non-absorbable antibiotics such as rifaximin. Other treatment options such as branched-chain amino acids, probiotics, and LOLA have also been considered. In a randomized trial of patients who had recovered from a recent episode of HE, lactulose vs. placebo for 20 months showed a higher proportion of those in placebo group experiencing recurrence.144
Rifaximin was shown to be effective in a study of 299 patients with 2 or more episodes of HE within 6 months. These patients were randomized to receive rifaximin or a placebo. Rifaximin significantly decreased the risk of developing HE with 22.1% in the treatment group compared with 46% in the placebo group. The use of rifaximin decreased the risk of hospitalization (13.6% vs 22.6% P = 0.01).145 Currently, rifaximin is recommended in addition to lactulose to prevent HE after the second episode of overt hepatic encephalopathy.146 Norfloxacin is the recommended drug of choice for SBP prophylaxis; however, it is unclear if patients on norfloxacin should also receive rifaximin to prevent HE.47 A recent study demonstrated that norfloxacin alone effectively reduced the incidence of HE and bacterial infections in patients with ACLF.147 However, the study reported a higher incidence of fungal infections in such patients.
Probiotics are not currently FDA-approved for the treatment of hepatic encephalopathy. However, a study reported improved recovery and reductions in the development of overt hepatic encephalopathy with VSL#3.148
Zinc deficiency is common in patients with cirrhosis. If this is confirmed in a patient with a history of HE, supplementation can be considered, but its role in the management of HE without underlying zinc deficiency is uncertain.146
Lastly, LOLA can be considered as an additional agent for the prevention of recurrent HE; however, it is not currently available in the US.146,149 For patients with persistent HE, BRTO can be performed if there are portosystemic shunts demonstrated on CT scan.150 For further details on the prevention and management of HE, readers are suggested to refer the review article by Sahney et al151
Future directions
Large population-based studies are required to assess the incidence of portal hypertension. EUS guided portal pressure measurements in small trials have been shown to be safe and accurate for diagnosing portal hypertension.152 However, further studies are required to evaluate its safety and compare it with HVPG. Carvedilol with EVL may be superior to traditional beta-blockers; however, large randomized controlled trials are needed to ascertain the same. Given the relative rarity of gastric varices compared with esophageal varices, and less robust data regarding endoscopic and clinical predictors of bleeding risk, demonstration of benefit with specific approaches to primary prophylaxis is lacking. There is a significant lack of literature on the management of gastric varices, which needs further well-controlled studies in a homogenous population. Simvastatin has pleiotropic effects and can lower portal hypertension.153 However, the risk of rhabdomyolysis in advanced cirrhosis is a significant concern. Recent studies suggest that decompensation in NAFLD may occur with lower HVPG and the recent BAVENO guidelines suggest incorporating etiology-based treatment for patients with cirrhosis.6 Obeticholic acid, a farnesoid X receptor agonist, is relatively safe and effective for NASH.154,155 Obeticholic acid in preclinical models has been shown to improve portal hypertension.156 Whether this translates into clinical utility is unknown. In patients with ascites, a weight loss of 0.5–1 kg/day is recommended. However, the amount of weight loss required for ascertaining appropriate diuresis in patients with ascites and hydrothorax is unknown. Some of the research areas in portal hypertension are mentioned in Table 2.
Table 2.
Future Research Areas.
| Population-based study to determine the incidence of portal hypertension. |
| EUS guided portal pressure measurement |
| Biomarkers of CKD in cirrhosis |
| Optimal timing for dialysis in AKI/CKD. |
| Utility of alfa-pump in clinical practice. |
| Target weight loss required for ascertaining appropriate diuresis in patients with ascites and hydrothorax. |
| Role of Artificial Intelligence in the diagnosis of portal hypertension |
| Simvastatin and Obeticholic acid for prevention of variceal bleeding. |
| Transient elastography for determining response to beta-blockers. |
Abbreviations: AKI, acute kidney injury; CKD, chronic kidney disease; EUS, endoscopic ultrasonography.
Credit authorship contribution statement
AVK made the study concept and design; Initial drafting, compilation, and critical revision by AVK, AR, and AM. All members approved the final draft.
Conflicts of interest
The authors have none to declare.
Funding
None.
References
- 1.Idalsoaga F., Kulkarni A.V., Mousa O.Y., Arrese M., Arab J.P. Non-alcoholic fatty liver disease and alcohol-related liver disease: two intertwined entities. Front Med (Lausanne) 2020;7:448. doi: 10.3389/fmed.2020.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulkarni A.V., Duvvuru N.R. Management of hepatitis B and C in special population. World J Gastroenterol. 2021;27:6861–6873. doi: 10.3748/wjg.v27.i40.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Franchis R. Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743–752. doi: 10.1016/j.jhep.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Ripoll C., Groszmann R., Garcia-Tsao G., et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481–488. doi: 10.1053/j.gastro.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Tsao G., Abraldes J.G., Berzigotti A., Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 6.Bassegoda O., Olivas P., Turco L., et al. Decompensation in advanced nonalcoholic fatty liver disease may occur at lower hepatic venous pressure gradient levels than in patients with viral disease. Clin Gastroenterol Hepatol. 2021;(S1542–3565(21)):01136–01138. doi: 10.1016/j.cgh.2021.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Elmahdy A.M., Berzigotti A. Non-invasive measurement of portal pressure. Curr Hepatol Rep. 2019;18:20–27. [Google Scholar]
- 8.You M.W., Kim K.W., Pyo J., et al. A meta-analysis for the diagnostic performance of transient elastography for clinically significant portal hypertension. Ultrasound Med Biol. 2017;43:59–68. doi: 10.1016/j.ultrasmedbio.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Bureau C., Metivier S., Peron J.M., et al. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther. 2008;27:1261–1268. doi: 10.1111/j.1365-2036.2008.03701.x. [DOI] [PubMed] [Google Scholar]
- 10.de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022 Apr;76(4):959-974. doi: 10.1016/j.jhep.2021.12.022. Epub 2021 Dec 30. PMID: 35120736. [DOI] [PMC free article] [PubMed]
- 11.Abraldes J.G., Bureau C., Stefanescu H., et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: the "Anticipate” study. Hepatology. 2016;64:2173–2184. doi: 10.1002/hep.28824. [DOI] [PubMed] [Google Scholar]
- 12.Pons M., Augustin S., Scheiner B., et al. Noninvasive diagnosis of portal hypertension in patients with compensated advanced chronic liver disease. Am J Gastroenterol. 2021;116:723–732. doi: 10.14309/ajg.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 13.Suh C.H., Kim K.W., Park S.H., et al. Shear wave elastography as a quantitative biomarker of clinically significant portal hypertension: a systematic review and meta-analysis. AJR Am J Roentgenol. 2018;210:W185. doi: 10.2214/AJR.17.18367. w95. [DOI] [PubMed] [Google Scholar]
- 14.Colecchia A., Montrone L., Scaioli E., et al. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology. 2012;143:646–654. doi: 10.1053/j.gastro.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 15.Hu X., Huang X., Hou J., Ding L., Su C., Meng F. Diagnostic accuracy of spleen stiffness to evaluate portal hypertension and esophageal varices in chronic liver disease: a systematic review and meta-analysis. Eur Radiol. 2021;31:2392–2404. doi: 10.1007/s00330-020-07223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell O., Feldman D.M., Diakow M., Sigal S.H. The pathophysiology of thrombocytopenia in chronic liver disease. Hepat Med. 2016;8:39–50. doi: 10.2147/HMER.S74612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berzigotti A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67:399–411. doi: 10.1016/j.jhep.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Procopet B., Cristea V.M., Robic M.A., et al. Serum tests, liver stiffness and artificial neural networks for diagnosing cirrhosis and portal hypertension. Dig Liver Dis. 2015;47:411–416. doi: 10.1016/j.dld.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Ravaioli F., Montagnani M., Lisotti A., Festi D., Mazzella G., Azzaroli F. Noninvasive assessment of portal hypertension in advanced chronic liver disease: an update. Gastroenterol Res Pract. 2018;2018:4202091. doi: 10.1155/2018/4202091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng H., Qi X., Guo X. Diagnostic accuracy of APRI, AAR, FIB-4, FI, king, lok, forns, and FibroIndex scores in predicting the presence of esophageal varices in liver cirrhosis: a systematic review and meta-analysis. Medicine (Baltim) 2015;94 doi: 10.1097/MD.0000000000001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thabut D., Imbert-Bismut F., Cazals-Hatem D., et al. Relationship between the Fibrotest and portal hypertension in patients with liver disease. Aliment Pharmacol Ther. 2007;26:359–368. doi: 10.1111/j.1365-2036.2007.03378.x. [DOI] [PubMed] [Google Scholar]
- 22.Calès P., Sacher-Huvelin S., Valla D., et al. Large oesophageal varice screening by a sequential algorithm using a cirrhosis blood test and optionally capsule endoscopy. Liver Int. 2018;38:84–93. doi: 10.1111/liv.13497. [DOI] [PubMed] [Google Scholar]
- 23.Schwarzer R., Reiberger T., Mandorfer M., et al. The von Willebrand Factor antigen to platelet ratio (VITRO) score predicts hepatic decompensation and mortality in cirrhosis. J Gastroenterol. 2020;55:533–542. doi: 10.1007/s00535-019-01656-9. [DOI] [PubMed] [Google Scholar]
- 24.Lisotti A., Azzaroli F., Buonfiglioli F., et al. Indocyanine green retention test as a noninvasive marker of portal hypertension and esophageal varices in compensated liver cirrhosis. Hepatology. 2014;59:643–650. doi: 10.1002/hep.26700. [DOI] [PubMed] [Google Scholar]
- 25.Lisotti A., Azzaroli F., Cucchetti A., et al. Relationship between indocyanine green retention test, decompensation and survival in patients with Child-Pugh A cirrhosis and portal hypertension. Liver Int. 2016;36:1313–1321. doi: 10.1111/liv.13070. [DOI] [PubMed] [Google Scholar]
- 26.Sartoris R., Rautou P.E., Elkrief L., et al. Quantification of liver surface nodularity at CT: utility for detection of portal hypertension. Radiology. 2018;289:698–707. doi: 10.1148/radiol.2018181131. [DOI] [PubMed] [Google Scholar]
- 27.Eisenbrey J.R., Dave J.K., Halldorsdottir V.G., et al. Chronic liver disease: noninvasive subharmonic aided pressure estimation of hepatic venous pressure gradient. Radiology. 2013;268:581–588. doi: 10.1148/radiol.13121769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Ning Z., Örmeci N., et al. Deep convolutional neural network-aided detection of portal hypertension in patients with cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2998–3007. doi: 10.1016/j.cgh.2020.03.034. e5. [DOI] [PubMed] [Google Scholar]
- 29.Hong W.-D., Ji Y.-F., Wang D., Chen T.-Z., Zhu Q.-H. Use of artificial neural network to predict esophageal varices in patients with HBV related cirrhosis. Hepat Mon. 2011;11:544–547. [PMC free article] [PubMed] [Google Scholar]
- 30.Qi X., Li Z., Huang J., et al. Virtual portal pressure gradient from anatomic CT angiography. Gut. 2015;64:1004–1005. doi: 10.1136/gutjnl-2014-308543. [DOI] [PubMed] [Google Scholar]
- 31.Fang C., An J., Bruno A., et al. Consensus recommendations of three-dimensional visualization for diagnosis and management of liver diseases. Hepatol Int. 2020;14:437–453. doi: 10.1007/s12072-020-10052-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.D'Amico G., Garcia-Tsao G., Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Reverter E., Tandon P., Augustin S., et al. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology. 2014;146:412–419. doi: 10.1053/j.gastro.2013.10.018. e3. [DOI] [PubMed] [Google Scholar]
- 34.Mohanty A., Kapuria D., Canakis A., et al. Fresh frozen plasma transfusion in acute variceal haemorrhage: results from a multicentre cohort study. Liver Int. 2021;41:1901–1908. doi: 10.1111/liv.14936. [DOI] [PubMed] [Google Scholar]
- 35.Boyer J.L., Chatterjee C., Iber F.L., Basu A.K. Effect of plasma-volume expansion on portal hypertension. N Engl J Med. 1966;275:750–755. doi: 10.1056/NEJM196610062751403. [DOI] [PubMed] [Google Scholar]
- 36.Villanueva C., Colomo A., Bosch A., et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 37.O'Leary J.G., Greenberg C.S., Patton H.M., Caldwell S.H. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157:34–43. doi: 10.1053/j.gastro.2019.03.070. e1. [DOI] [PubMed] [Google Scholar]
- 38.Intagliata N.M., Argo C.K., Stine J.G., et al. Concepts and controversies in haemostasis and thrombosis associated with liver disease: proceedings of the 7th international coagulation in liver disease conference. Thromb Haemostasis. 2018;118:1491–1506. doi: 10.1055/s-0038-1666861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bosch J., Thabut D., Albillos A., et al. Recombinant factor VIIa for variceal bleeding in patients with advanced cirrhosis: a randomized, controlled trial. Hepatology. 2008;47:1604–1614. doi: 10.1002/hep.22216. [DOI] [PubMed] [Google Scholar]
- 40.Goulis J., Armonis A., Patch D., Sabin C., Greenslade L., Burroughs A.K. Bacterial infection is independently associated with failure to control bleeding in cirrhotic patients with gastrointestinal hemorrhage. Hepatology. 1998;27:1207–1212. doi: 10.1002/hep.510270504. [DOI] [PubMed] [Google Scholar]
- 41.Tandon P., Abraldes J.G., Keough A., et al. Risk of bacterial infection in patients with cirrhosis and acute variceal hemorrhage, based on child-pugh class, and effects of antibiotics. Clin Gastroenterol Hepatol. 2015;13:1189–1196. doi: 10.1016/j.cgh.2014.11.019. e2. [DOI] [PubMed] [Google Scholar]
- 42.Avgerinos A., Nevens F., Raptis S., Fevery J. Early administration of somatostatin and efficacy of sclerotherapy in acute oesophageal variceal bleeds: the European Acute Bleeding Oesophageal Variceal Episodes (ABOVE) randomised trial. Lancet. 1997;350:1495–1499. doi: 10.1016/s0140-6736(97)05099-x. [DOI] [PubMed] [Google Scholar]
- 43.Wells M., Chande N., Adams P., et al. Meta-analysis: vasoactive medications for the management of acute variceal bleeds. Aliment Pharmacol Ther. 2012;35:1267–1278. doi: 10.1111/j.1365-2036.2012.05088.x. [DOI] [PubMed] [Google Scholar]
- 44.Seo Y.S., Park S.Y., Kim M.Y., et al. Lack of difference among terlipressin, somatostatin, and octreotide in the control of acute gastroesophageal variceal hemorrhage. Hepatology. 2014;60:954–963. doi: 10.1002/hep.27006. [DOI] [PubMed] [Google Scholar]
- 45.Manning C., Elzubeir A., Alam S. The role of pre-emptive Transjugular Intrahepatic Portosystemic Shunt in acute variceal bleeding: a literature review. Ther Adv Chronic Dis. 2021;12 doi: 10.1177/2040622321995771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angeli P., Garcia-Tsao G., Nadim M.K., Parikh C.R. News in pathophysiology, definition and classification of hepatorenal syndrome: a step beyond the International Club of Ascites (ICA) consensus document. J Hepatol. 2019;71:811–822. doi: 10.1016/j.jhep.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 47.EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 48.Biggins S.W., Angeli P., Garcia-Tsao G., et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American association for the study of liver diseases. Hepatology. 2021;74:1014–1048. doi: 10.1002/hep.31884. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Martinez R., Caraceni P., Bernardi M., Gines P., Arroyo V., Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58:1836–1846. doi: 10.1002/hep.26338. [DOI] [PubMed] [Google Scholar]
- 50.Piano S., Schmidt H.H., Ariza X., et al. Association between grade of acute on chronic liver failure and response to terlipressin and albumin in patients with hepatorenal syndrome. Clin Gastroenterol Hepatol. 2018;16:1792–1800. doi: 10.1016/j.cgh.2018.01.035. e3. [DOI] [PubMed] [Google Scholar]
- 51.Kulkarni A., Sowmya T., Sharma M., et al. IDDF2020-ABS-0192 Terlipressin non-response predicts mortality in acute-on-chronic liver failure-a prospective cohort study. Gut. 2020;69(Suppl 2):A87–A88. [Google Scholar]
- 52.Allegretti A.S., Israelsen M., Krag A., et al. Terlipressin versus placebo or no intervention for people with cirrhosis and hepatorenal syndrome. Cochrane Database Syst Rev. 2017;6 doi: 10.1002/14651858.CD005162.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kulkarni A.V., Arab J.P., Premkumar M., et al. Terlipressin has stood the test of time: clinical overview in 2020 and future perspectives. Liver Int. 2020;40:2888–2905. doi: 10.1111/liv.14703. [DOI] [PubMed] [Google Scholar]
- 54.Gluud L.L., Christensen K., Christensen E., Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology. 2010;51:576–584. doi: 10.1002/hep.23286. [DOI] [PubMed] [Google Scholar]
- 55.Wong F., Pappas S.C., Curry M.P., et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N Engl J Med. 2021;384:818–828. doi: 10.1056/NEJMoa2008290. [DOI] [PubMed] [Google Scholar]
- 56.Kulkarni A., T.R S., Tevethia H., et al. Research Square; 2021. Safety and Efficacy of Terlipressin in Acute-On-Chronic Liver Failure with Acute Kidney Injury – A Prospective Cohort Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kulkarni A.V., Kumar P., Rao N.P., Reddy N. Terlipressin-induced ischaemic skin necrosis. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2019-233089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cavallin M., Kamath P.S., Merli M., et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology. 2015;62:567–574. doi: 10.1002/hep.27709. [DOI] [PubMed] [Google Scholar]
- 59.Vilstrup H., Amodio P., Bajaj J., et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American association for the study of liver diseases and the European association for the study of the liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 60.Ahmed S., Premkumar M., Dhiman R.K., et al. Combined PEG3350 plus lactulose results in early resolution of hepatic encephalopathy and improved 28-day survival in acute-on-chronic liver failure. J Clin Gastroenterol. 2022;56:e11–e19. doi: 10.1097/MCG.0000000000001450. [DOI] [PubMed] [Google Scholar]
- 61.Sharma B.C., Sharma P., Lunia M.K., Srivastava S., Goyal R., Sarin S.K. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108:1458–1463. doi: 10.1038/ajg.2013.219. [DOI] [PubMed] [Google Scholar]
- 62.Jain A., Sharma B.C., Mahajan B., et al. L-ornithine L-aspartate in acute treatment of severe hepatic encephalopathy: a double-blind randomized controlled trial. Hepatology. 2022;75:1194–1203. doi: 10.1002/hep.32255. [DOI] [PubMed] [Google Scholar]
- 63.Robertson M., Hayes P. Primary prophylaxis of variceal bleeding. Hepatol Int. 2018;12:1–5. doi: 10.1007/s12072-018-9846-1. [DOI] [PubMed] [Google Scholar]
- 64.Villanueva C., Albillos A., Genescà J., et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597–1608. doi: 10.1016/S0140-6736(18)31875-0. [DOI] [PubMed] [Google Scholar]
- 65.Lo G.H., Lai K.H., Cheng J.S., et al. The effects of endoscopic variceal ligation and propranolol on portal hypertensive gastropathy: a prospective, controlled trial. Gastrointest Endosc. 2001;53:579–584. doi: 10.1067/mge.2001.114062. [DOI] [PubMed] [Google Scholar]
- 66.Bañares R., Moitinho E., Piqueras B., et al. Carvedilol, a new nonselective beta-blocker with intrinsic anti- Alpha1-adrenergic activity, has a greater portal hypotensive effect than propranolol in patients with cirrhosis. Hepatology. 1999;30:79–83. doi: 10.1002/hep.510300124. [DOI] [PubMed] [Google Scholar]
- 67.Frishman W.H. Carvedilol. N Engl J Med. 1998;339:1759–1765. doi: 10.1056/NEJM199812103392407. [DOI] [PubMed] [Google Scholar]
- 68.Bhardwaj A., Kedarisetty C.K., Vashishtha C., et al. Carvedilol delays the progression of small oesophageal varices in patients with cirrhosis: a randomised placebo-controlled trial. Gut. 2017;66:1838–1843. doi: 10.1136/gutjnl-2016-311735. [DOI] [PubMed] [Google Scholar]
- 69.Premkumar M., Rangegowda D., Vyas T., et al. Carvedilol combined with ivabradine improves left ventricular diastolic dysfunction, clinical progression, and survival in cirrhosis. J Clin Gastroenterol. 2020;54:561–568. doi: 10.1097/MCG.0000000000001219. [DOI] [PubMed] [Google Scholar]
- 70.Goral V., Yılmaz N. Current approaches to the treatment of gastric varices: glue, coil application, TIPS, and BRTO. Medicina (Kaunas) 2019;55 doi: 10.3390/medicina55070335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mishra S.R., Sharma B.C., Kumar A., Sarin S.K. Primary prophylaxis of gastric variceal bleeding comparing cyanoacrylate injection and beta-blockers: a randomized controlled trial. J Hepatol. 2011;54:1161–1167. doi: 10.1016/j.jhep.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 72.Choe J.W., Yim H.J., Lee S.H., et al. Primary prophylaxis of gastric variceal bleeding: endoscopic obturation, radiologic intervention, or observation? Hepatol Int. 2021;15:934–945. doi: 10.1007/s12072-021-10154-1. [DOI] [PubMed] [Google Scholar]
- 73.Lebrec D., Nouel O., Corbic M., Benhamou J.P. Propranolol--a medical treatment for portal hypertension? Lancet. 1980;2:180–182. doi: 10.1016/s0140-6736(80)90063-x. [DOI] [PubMed] [Google Scholar]
- 74.Abraldes J.G., Tarantino I., Turnes J., Garcia-Pagan J.C., Rodés J., Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902–908. doi: 10.1053/jhep.2003.50133. [DOI] [PubMed] [Google Scholar]
- 75.Gupta V., Rawat R., Shalimar Saraya A. Carvedilol versus propranolol effect on hepatic venous pressure gradient at 1 month in patients with index variceal bleed: RCT. Hepatol Int. 2017;11:181–187. doi: 10.1007/s12072-016-9765-y. [DOI] [PubMed] [Google Scholar]
- 76.Leithead J.A., Rajoriya N., Tehami N., et al. Non-selective β-blockers are associated with improved survival in patients with ascites listed for liver transplantation. Gut. 2015;64:1111–1119. doi: 10.1136/gutjnl-2013-306502. [DOI] [PubMed] [Google Scholar]
- 77.Halabi S.A., Sawas T., Sadat B., et al. Early TIPS versus endoscopic therapy for secondary prophylaxis after management of acute esophageal variceal bleeding in cirrhotic patients: a meta-analysis of randomized controlled trials. J Gastroenterol Hepatol. 2016;31:1519–1526. doi: 10.1111/jgh.13303. [DOI] [PubMed] [Google Scholar]
- 78.Njei B., McCarty T.R., Laine L. Early transjugular intrahepatic portosystemic shunt in US patients hospitalized with acute esophageal variceal bleeding. J Gastroenterol Hepatol. 2017;32:852–858. doi: 10.1111/jgh.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trebicka J., Gu W., Ibáñez-Samaniego L., et al. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol. 2020;73:1082–1091. doi: 10.1016/j.jhep.2020.04.024. [DOI] [PubMed] [Google Scholar]
- 80.Elhence A., Kumar R., Shalimar Pre-emptive TIPS for acute variceal bleeding in acute-on-chronic liver failure: is there enough evidence for a routine recommendation? J Hepatol. 2020;73:976–977. doi: 10.1016/j.jhep.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Lo G.H., Liang H.L., Chen W.C., et al. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. 2007;39:679–685. doi: 10.1055/s-2007-966591. [DOI] [PubMed] [Google Scholar]
- 82.Tripathi D., Therapondos G., Jackson E., Redhead D.N., Hayes P.C. The role of the transjugular intrahepatic portosystemic stent shunt (TIPSS) in the management of bleeding gastric varices: clinical and haemodynamic correlations. Gut. 2002;51:270–274. doi: 10.1136/gut.51.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Paleti S., Nutalapati V., Fathallah J., Jeepalyam S., Rustagi T. Balloon-occluded retrograde transvenous obliteration (BRTO) versus transjugular intrahepatic portosystemic shunt (TIPS) for treatment of gastric varices because of portal hypertension: a systematic review and meta-analysis. J Clin Gastroenterol. 2020;54:655–660. doi: 10.1097/MCG.0000000000001275. [DOI] [PubMed] [Google Scholar]
- 84.Saad W.E. Vascular anatomy and the morphologic and hemodynamic classifications of gastric varices and spontaneous portosystemic shunts relevant to the BRTO procedure. Tech Vasc Intervent Radiol. 2013;16:60–100. doi: 10.1053/j.tvir.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 85.Mukkada R.J., Antony R., Chooracken M.J., et al. Endoscopic ultrasound-guided coil or glue injection in post-cyanoacrylate gastric variceal re-bleed. Indian J Gastroenterol. 2018;37:153–159. doi: 10.1007/s12664-018-0844-y. [DOI] [PubMed] [Google Scholar]
- 86.Hung H.H., Chang C.J., Hou M.C., et al. Efficacy of non-selective β-blockers as adjunct to endoscopic prophylactic treatment for gastric variceal bleeding: a randomized controlled trial. J Hepatol. 2012;56:1025–1032. doi: 10.1016/j.jhep.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 87.Mishra S.R., Chander Sharma B., Kumar A., Sarin S.K. Endoscopic cyanoacrylate injection versus beta-blocker for secondary prophylaxis of gastric variceal bleed: a randomised controlled trial. Gut. 2010;59:729–735. doi: 10.1136/gut.2009.192039. [DOI] [PubMed] [Google Scholar]
- 88.Kulkarni A.V., Kumar P., Sharma M., et al. Pathophysiology and prevention of paracentesis-induced circulatory dysfunction: a concise review. J Clin Transl Hepatol. 2020;8:42–48. doi: 10.14218/JCTH.2019.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Angeli P., Fasolato S., Mazza E., et al. Combined versus sequential diuretic treatment of ascites in non-azotaemic patients with cirrhosis: results of an open randomised clinical trial. Gut. 2010;59:98–104. doi: 10.1136/gut.2008.176495. [DOI] [PubMed] [Google Scholar]
- 90.Kulkarni A.V., Kumar P., Sharma M., et al. Midodrine improves the tolerability of diuretics in patients with acute-on-chronic liver failure-A pilot study. J Clin Exp Hepatol. 2021;11:573–578. doi: 10.1016/j.jceh.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Planas R., Montoliu S., Ballesté B., et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385–1394. doi: 10.1016/j.cgh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 92.Hanai T., Shiraki M., Miwa T., et al. Effect of loop diuretics on skeletal muscle depletion in patients with liver cirrhosis. Hepatol Res. 2019;49:82–95. doi: 10.1111/hepr.13244. [DOI] [PubMed] [Google Scholar]
- 93.Kulkarni A.V.P.M., Reddy D.N., Rao P.N. Clinical Liver Disease; 2022. The Challenges of Ascites Management—An Indian Perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bhatia V., Srivastava R., Reddy K.S., et al. Ending TB in Southeast Asia: current resources are not enough. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2019-002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mishra S., Taneja S., De A., et al. Tuberculosis in cirrhosis-A diagnostic and management conundrum. J Clin Exp Hepatol. 2022;12:278–286. doi: 10.1016/j.jceh.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kulkarni A.V., Kumar P., Sharma M., Menon B., Reddy D.N., Rao P.N. Letter to the editor: living donor liver transplantation or deceased donor liver transplantation in high model for end-stage liver disease score-which is better? Hepatology. 2021;73:2619–2620. doi: 10.1002/hep.31657. [DOI] [PubMed] [Google Scholar]
- 97.Kulkarni A.V., Kumar P., Singh S., et al. Prevention of paracentesis-induced circulatory dysfunction—a systematic review and network meta-analysis. Gastro Hep. 2020;2:92–101. [Google Scholar]
- 98.Kulkarni A.V., Padaki Rao N. Midodrine or albumin in paracentesis-induced circulatory dysfunction: author's reply. Gastro Hep. 2020;2:138–139. [Google Scholar]
- 99.Kimer N., Feineis M., Møller S., Bendtsen F. Beta-blockers in cirrhosis and refractory ascites: a retrospective cohort study and review of the literature. Scand J Gastroenterol. 2015;50:129–137. doi: 10.3109/00365521.2014.948053. [DOI] [PubMed] [Google Scholar]
- 100.Sersté T., Francoz C., Durand F., et al. Beta-blockers cause paracentesis-induced circulatory dysfunction in patients with cirrhosis and refractory ascites: a cross-over study. J Hepatol. 2011;55:794–799. doi: 10.1016/j.jhep.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 101.Giannelli V., Roux O., Laouénan C., et al. Impact of cardiac function, refractory ascites and beta blockers on the outcome of patients with cirrhosis listed for liver transplantation. J Hepatol. 2020;72:463–471. doi: 10.1016/j.jhep.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 102.Singh V., Dhungana S.P., Singh B., et al. Midodrine in patients with cirrhosis and refractory or recurrent ascites: a randomized pilot study. J Hepatol. 2012;56:348–354. doi: 10.1016/j.jhep.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 103.Hanafy A.S., Hassaneen A.M. Rifaximin and midodrine improve clinical outcome in refractory ascites including renal function, weight loss, and short-term survival. Eur J Gastroenterol Hepatol. 2016;28:1455–1461. doi: 10.1097/MEG.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 104.Bellos I., Kontzoglou K., Psyrri A., Pergialiotis V. Tolvaptan response improves overall survival in patients with refractory ascites: a meta-analysis. Dig Dis. 2020;38:320–328. doi: 10.1159/000503559. [DOI] [PubMed] [Google Scholar]
- 105.Dahl E., Gluud L.L., Kimer N., Krag A. Meta-analysis: the safety and efficacy of vaptans (tolvaptan, satavaptan and lixivaptan) in cirrhosis with ascites or hyponatraemia. Aliment Pharmacol Ther. 2012;36:619–626. doi: 10.1111/apt.12025. [DOI] [PubMed] [Google Scholar]
- 106.Watkins P.B., Lewis J.H., Kaplowitz N., et al. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf. 2015;38:1103–1113. doi: 10.1007/s40264-015-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gow P.J., Ardalan Z.S., Vasudevan A., Testro A.G., Ye B., Angus P.W. Outpatient terlipressin infusion for the treatment of refractory ascites. Am J Gastroenterol. 2016;111:1041–1042. doi: 10.1038/ajg.2016.168. [DOI] [PubMed] [Google Scholar]
- 108.Xing F., Li S., Zhang J.J., et al. [Observation of the therapeutic and characteristic effects of terlipressin on refractory cirrhotic ascites] Zhonghua Gan Zang Bing Za Zhi. 2019;27:982–988. doi: 10.3760/cma.j.issn.1007-3418.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 109.Di Pascoli M., Fasolato S., Piano S., Bolognesi M., Angeli P. Long-term administration of human albumin improves survival in patients with cirrhosis and refractory ascites. Liver Int. 2019;39:98–105. doi: 10.1111/liv.13968. [DOI] [PubMed] [Google Scholar]
- 110.Bai M., Qi X.S., Yang Z.P., Yang M., Fan D.M., Han G.H. TIPS improves liver transplantation-free survival in cirrhotic patients with refractory ascites: an updated meta-analysis. World J Gastroenterol. 2014;20:2704–2714. doi: 10.3748/wjg.v20.i10.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bureau C., Thabut D., Oberti F., et al. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152:157–163. doi: 10.1053/j.gastro.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 112.Bureau C., Adebayo D., Chalret de Rieu M., et al. Alfapump® system vs. large volume paracentesis for refractory ascites: a multicenter randomized controlled study. J Hepatol. 2017;67:940–949. doi: 10.1016/j.jhep.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 113.Macken L., Joshi D., Messenger J., et al. Palliative long-term abdominal drains in refractory ascites due to end-stage liver disease: a case series. Palliat Med. 2017;31:671–675. doi: 10.1177/0269216316671281. [DOI] [PubMed] [Google Scholar]
- 114.Macken L., Bremner S., Gage H., et al. Randomised clinical trial: palliative long-term abdominal drains vs large-volume paracentesis in refractory ascites due to cirrhosis. Aliment Pharmacol Ther. 2020;52:107–122. doi: 10.1111/apt.15802. [DOI] [PubMed] [Google Scholar]
- 115.Kumar K., Kulkarni A. Letter to the editor: sodium-Glucose cotransporter-2 inhibitors are not the magic Pills for control of ascites in cirrhosis and diabetes. Hepatology. 2021;73:865. doi: 10.1002/hep.31399. [DOI] [PubMed] [Google Scholar]
- 116.Montalvo-Gordon I., Chi-Cervera L.A., García-Tsao G. Sodium-glucose cotransporter 2 inhibitors ameliorate ascites and peripheral edema in patients with cirrhosis and diabetes. Hepatology. 2020;72:1880–1882. doi: 10.1002/hep.31270. [DOI] [PubMed] [Google Scholar]
- 117.Kalambokis G.N., Tsiakas I., Filippas-Ntekuan S., Christaki M., Despotis G., Milionis H. Empagliflozin eliminates refractory ascites and hepatic hydrothorax in a patient with primary biliary cirrhosis. Am J Gastroenterol. 2021;116:618–619. doi: 10.14309/ajg.0000000000000995. [DOI] [PubMed] [Google Scholar]
- 118.Nath P., Anand A.C. Hepatogenous diabetes: a primer. J Clin Exp Hepatol. 2021;11(5):603–615. doi: 10.1016/j.jceh.2021.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kulkarni A.V., Sharma M., Kumar P., Gupta R., Rao P.N. Letter to the editor: midodrine for hepatic hydrothorax. Hepatology. 2021;73:1236–1237. doi: 10.1002/hep.31513. [DOI] [PubMed] [Google Scholar]
- 120.Garbuzenko D.V., Arefyev N.O. Hepatic hydrothorax: an update and review of the literature. World J Hepatol. 2017;9:1197–1204. doi: 10.4254/wjh.v9.i31.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shojaee S., Khalid M., Kallingal G., Kang L., Rahman N. Repeat thoracentesis in hepatic hydrothorax and non-hepatic hydrothorax effusions: a case-control study. Respiration. 2018;96:330–337. doi: 10.1159/000490001. [DOI] [PubMed] [Google Scholar]
- 122.Dhanasekaran R., West J.K., Gonzales P.C., et al. Transjugular intrahepatic portosystemic shunt for symptomatic refractory hepatic hydrothorax in patients with cirrhosis. Am J Gastroenterol. 2010;105:635–641. doi: 10.1038/ajg.2009.634. [DOI] [PubMed] [Google Scholar]
- 123.Banini B.A., Alwatari Y., Stovall M., et al. Multidisciplinary management of hepatic hydrothorax in 2020: an evidence-based review and guidance. Hepatology. 2020;72:1851–1863. doi: 10.1002/hep.31434. [DOI] [PubMed] [Google Scholar]
- 124.Praharaj D.L., Anand A.C. Clinical implications, evaluation and management of Hyponatremia in cirrhosis. J Clin Exp Hepatol. 2022;12:575–594. doi: 10.1016/j.jceh.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.China L., Freemantle N., Forrest E., et al. Targeted albumin therapy does not improve short-term outcome in hyponatremic patients hospitalized with complications of cirrhosis: data from the ATTIRE trial. Am J Gastroenterol. 2021;116(11):2292–2295. doi: 10.14309/ajg.0000000000001488. [DOI] [PubMed] [Google Scholar]
- 126.Bajaj J.S., Tandon P., OʼLeary J.G., et al. The impact of albumin use on resolution of hyponatremia in hospitalized patients with cirrhosis. Am J Gastroenterol. 2018;113:1339. doi: 10.1038/s41395-018-0119-3. [DOI] [PubMed] [Google Scholar]
- 127.Patel S., Nguyen D.S., Rastogi A., Nguyen M.K., Nguyen M.K. Treatment of cirrhosis-associated hyponatremia with midodrine and octreotide. Front Med. 2017;4:17. doi: 10.3389/fmed.2017.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang X., Wang S.Z., Zheng J.F., et al. Clinical efficacy of tolvaptan for treatment of refractory ascites in liver cirrhosis patients. World J Gastroenterol. 2014;20:11400–11405. doi: 10.3748/wjg.v20.i32.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang S., Zhang X., Han T., et al. Tolvaptan treatment improves survival of cirrhotic patients with ascites and hyponatremia. BMC Gastroenterol. 2018;18:137. doi: 10.1186/s12876-018-0857-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hayashi M., Abe K., Fujita M., Okai K., Takahashi A., Ohira H. Association between the serum sodium levels and the response to tolvaptan in liver cirrhosis patients with ascites and hyponatremia. Intern Med. 2018;57:2451–2458. doi: 10.2169/internalmedicine.0629-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Attar B. Approach to hyponatremia in cirrhosis. Clin Liver Dis. 2019;13:98–101. doi: 10.1002/cld.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sterns R.H. Disorders of plasma sodium--causes, consequences, and correction. N Engl J Med. 2015;372:55–65. doi: 10.1056/NEJMra1404489. [DOI] [PubMed] [Google Scholar]
- 133.Wong F., Reddy K.R., O’Leary J.G., et al. Impact of chronic kidney disease on outcomes in cirrhosis. Liver Transplant. 2019;25:870–880. doi: 10.1002/lt.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tan H.K., Marquez M., Wong F., Renner E.L. Pretransplant type 2 hepatorenal syndrome is associated with persistently impaired renal function after liver transplantation. Transplantation. 2015;99:1441–1446. doi: 10.1097/TP.0000000000000557. [DOI] [PubMed] [Google Scholar]
- 135.Tariq R., Singal A.K. Management of hepatorenal syndrome: a review. J Clin Transl Hepatol. 2020;8:192–199. doi: 10.14218/JCTH.2020.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kumar R., Priyadarshi R.N., Anand U. Chronic renal dysfunction in cirrhosis: a new frontier in hepatology. World J Gastroenterol. 2021;27:990–1005. doi: 10.3748/wjg.v27.i11.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maiwall R., Pasupuleti S.S.R., Bihari C., et al. Incidence, risk factors, and outcomes of transition of acute kidney injury to chronic kidney disease in cirrhosis: a prospective cohort study. Hepatology. 2020;71:1009–1022. doi: 10.1002/hep.30859. [DOI] [PubMed] [Google Scholar]
- 138.Piano S., Gambino C., Vettore E., et al. Response to terlipressin and albumin is associated with improved liver transplant outcomes in patients with hepatorenal syndrome. Hepatology. 2021;73:1909–1919. doi: 10.1002/hep.31529. [DOI] [PubMed] [Google Scholar]
- 139.Maiwall R., Pasupuleti S.S.R., Jain P., Sarin S.K. Degree of portal and systemic hemodynamic alterations predict recurrent AKI and chronic kidney disease in patients with cirrhosis. Hepatol Commun. 2021;5:293–308. doi: 10.1002/hep4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tripathi D., Stanley A.J., Hayes P.C., et al. Transjugular intrahepatic portosystemic stent-shunt in the management of portal hypertension. Gut. 2020;69:1173–1192. doi: 10.1136/gutjnl-2019-320221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Charilaou P., Devani K., Petrosyan R., Reddy C., Pyrsopoulos N. Inpatient mortality benefit with transjugular intrahepatic portosystemic shunt for hospitalized hepatorenal syndrome patients. Dig Dis Sci. 2020;65:3378–3388. doi: 10.1007/s10620-020-06136-2. [DOI] [PubMed] [Google Scholar]
- 142.Singal A.K., Ong S., Satapathy S.K., Kamath P.S., Wiesner R.H. Simultaneous liver kidney transplantation. Transpl Int. 2019;32:343–352. doi: 10.1111/tri.13388. [DOI] [PubMed] [Google Scholar]
- 143.Bajaj J.S., O’Leary J.G., Tandon P., et al. Hepatic encephalopathy is associated with mortality in patients with cirrhosis independent of other extrahepatic organ failures. Clin Gastroenterol Hepatol. 2017;15:565–574. doi: 10.1016/j.cgh.2016.09.157. e4. [DOI] [PubMed] [Google Scholar]
- 144.Sharma B.C., Sharma P., Agrawal A., Sarin S.K. Secondary prophylaxis of hepatic encephalopathy: an open-label randomized controlled trial of lactulose versus placebo. Gastroenterology. 2009;137:885–891. doi: 10.1053/j.gastro.2009.05.056. 91.891. [DOI] [PubMed] [Google Scholar]
- 145.Bass N.M., Mullen K.D., Sanyal A., et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 146.Bajaj J.S., Lauridsen M., Tapper E.B., et al. Important unresolved questions in the management of hepatic encephalopathy: an ISHEN consensus. Am J Gastroenterol. 2020;115:989–1002. doi: 10.14309/ajg.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 147.Kulkarni A.V., Tirumalle S., Premkumar M., et al. Primary norfloxacin prophylaxis for APASL-defined acute-on-chronic liver failure: a placebo-controlled double-blind randomized trial. Am J Gastroenterol. 2022;117(4):607–616. doi: 10.14309/ajg.0000000000001611. [DOI] [PubMed] [Google Scholar]
- 148.Dalal R., McGee R.G., Riordan S.M., Webster A.C. Probiotics for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;2 doi: 10.1002/14651858.CD008716.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sidhu S.S., Sharma B.C., Goyal O., Kishore H., Kaur N. L-ornithine L-aspartate in bouts of overt hepatic encephalopathy. Hepatology. 2018;67:700–710. doi: 10.1002/hep.29410. [DOI] [PubMed] [Google Scholar]
- 150.Mukund A., Chalamarla L.K., Singla N., Shasthry S.M., Sarin S.K. Intractable hepatic encephalopathy in cirrhotic patients: mid-term efficacy of balloon-occluded retrograde portosystemic shunt obliteration. Eur Radiol. 2020;30:3462–3472. doi: 10.1007/s00330-019-06644-4. [DOI] [PubMed] [Google Scholar]
- 151.Sahney A., Wadhawan M. Encephalopathy in cirrhosis: prevention and management. J Clin Exp Hepatol. 2022;12:927–936. doi: 10.1016/j.jceh.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang W., Peng C., Zhang S., et al. EUS-guided portal pressure gradient measurement in patients with acute or subacute portal hypertension. Gastrointest Endosc. 2021;93:565–572. doi: 10.1016/j.gie.2020.06.065. [DOI] [PubMed] [Google Scholar]
- 153.Abraldes J.G., Albillos A., Bañares R., et al. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651–1658. doi: 10.1053/j.gastro.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 154.Kulkarni A.V., Tevethia H.V., Arab J.P., et al. Efficacy and safety of obeticholic acid in liver disease-A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2021;45:101675. doi: 10.1016/j.clinre.2021.101675. [DOI] [PubMed] [Google Scholar]
- 155.Sharma M., Premkumar M., Kulkarni A.V., Kumar P., Reddy D.N., Rao N.P. Drugs for non-alcoholic steatohepatitis (NASH): quest for the holy grail. J Clin Transl Hepatol. 2021;9:40–50. doi: 10.14218/JCTH.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Verbeke L., Farre R., Trebicka J., Komuta M., et al. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–2298. doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]