Abstract
Background:
Glutamate, glutamine are involved in energy metabolism, and have been related to cardiometabolic disorders. However, their roles in the development of type-2 diabetes (T2D) remain unclear.
Aims:
To examine the effects of Mediterranean diet on associations between glutamine, glutamate, glutamine-to-glutamate ratio, and risk of new-onset T2D in a Spanish population at high risk for cardiovascular disease (CVD).
Methods:
The present study was built within the PREDIMED trial using a case-cohort design including 892 participants with 251 incident T2D cases and 641 non-cases. Participants (mean age 66.3 years; female 62.8%) were non diabetic and at high risk for CVD at baseline. Plasma levels of glutamine and glutamate were measured at baseline and after 1-year of intervention.
Results:
Higher glutamate levels at baseline were associated with increased risk of T2D with a hazard ratio (HR) of 2.78 (95% CI, 1.43–5.42, P for trend = 0.0002). In contrast, baseline levels of glutamine (HR: 0.64, 95% CI, 0.84–2.31; P for trend = 0.04) and glutamine-to-glutamate ratio (HR: 0.30, 95% CI, 0.16–0.57; P for trend = 0.0001) were inversely associated with T2D risk when comparing extreme quartiles. The two Mediterranean diets (MedDiet + EVOO and MedDiet + mixed nuts) did not alter levels of glutamine and glutamate after intervention for 1 year. However, MedDiet mitigate the positive association between higher baseline plasma glutamate and T2D risk (P for interaction = 0.01).
Conclusion:
Higher levels of glutamate and lower levels of glutamine were associated with increased risk of T2D in a Spanish population at high risk for CVD. Mediterranean diet might mitigate the association between the imbalance of glutamine and glutamate and T2D risk.
Keywords: Glutamine, Glutamate, Type-2 diabetes, Mediterranean diet
Graphical Abstract
Introduction
Globally, the prevalence of Type-2 Diabetes (T2D) is projected to affect 591.9 million people by 2035, which is equivalent to a 55% increase from 2013 [1]. In the US, an estimate of 30.3 million people had T2D in 2015 [2].The pathophysiology of T2D is beyond simple disorders in carbohydrate metabolism [3].Amino acid metabolites are also disturbed in conditions of insulin resistance and T2D. Unfavorable changes in energy metabolism accompanied by insulin resistance and dysfunctional pancreatic β-cell are present years before the disease is clinically diagnosed by elevated blood glucose levels [4,5]. Therefore, early diagnosis of T2D is extremely important as early intervention may delay or prevent the onset of complications.
Recent metabolomics technology provides new tools for identifying metabolites involved in metabolic pathways related toT2D. Imbalance of metabolic regulatory systems is the basis for many metabolic disorders, including diabetes. Elevated levels of branched chain amino acids (BCAAs) and aromatic amino acids (AAAs) have been associated with an increased risk of prediabetes and T2D [6,7].
A key function of the BCAAs is to provide nitrogen needed to maintain glutamate, alanine and glutamine pools in skeletal muscle [8]. Glutamine is the most abundant nonessential amino acid in human biology and is involved in regulation of pancreatic β-cell function and insulin secretion [9]. Previous studies have reported associations of glutamine and glutamate metabolites withT2D risk in healthy individuals and in subjects with metabolic syndrome [6,10]. Among participants who were free of diabetes mellitus and cardiovascular disease, plasmatic glutamate levels were associated with insulin resistance traits (glucose, insulin and HOMA), and with an increased risk of T2Dincidence [6].Glutamate was able to increase transamination of pyruvate to alanine and promote gluconeogenesis [11] which is commonly seen in obese individuals [12]. Glutamate is also a precursor to α-ketoglutarate, an intermediate in the Krebs cycle that has a crucial role in cellular energy metabolism [13].
The ability of glutamate and glutamine in predicting T2D risk in individuals at high risk of cardiovascular disease (CVD) remains uninvestigated [14]. This question is in need to be addressed as these high-risk individuals are those who will benefit the most from early diagnosis and lifestyle interventions to delay the onset of T2D.
Mediterranean diet has demonstrated protective effects in T2D through modifying metabolites related to T2D risk [15]. However, the underlying mechanisms of how Mediterranean diet influence on metabolites involved in energy metabolism is not fully understood. Therefore, in the present study, we examined the following hypotheses using a case-cohort study design nested within the PRE-vención con DIeta Mediterránea (PREDIMED) trial in nondiabetic participants at high risk for CVD : 1) baseline plasma levels of glutamate was positively associated with increased risk of incident T2D whereas, glutamine level is inversed associated with risk of incident T2D; 2) increases in these amino acids at 1 year are associated with a higher subsequent risk of T2D; (3) a Mediterranean style diet (MedDiet) can attenuate the association between glutamate, glutamine-to-glutamate ratio and T2D.
Method
Study population
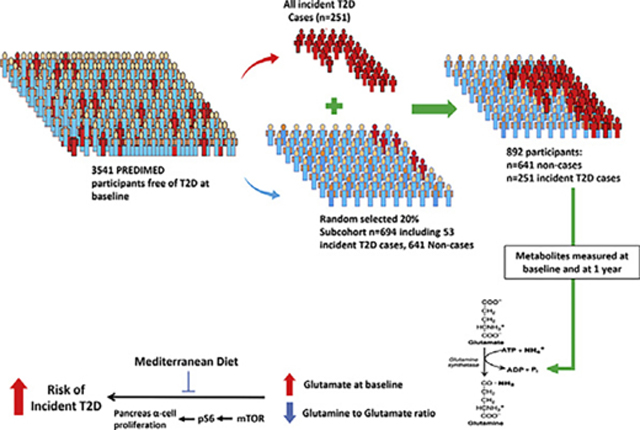
Design and protocol of the PREDIMED (www.predimed.es) study have been published in details elsewhere [16]. The PREDIMED study is an Spanish primary CVD prevention trial using a Mediterranean diet as the main intervention. In brief, 7447 participants were randomly allocated to three groups to examine the effects of two Mediterranean enriched diets with 1) extra virgin olive oil or 2) mixed nuts compared to a low-fat diet (control diet) on the primary prevention of CVD. Male and female participants between 55 and 80 years who were at high risk of CVD at baseline have been recruited and enrolled among 11 centers across Spain between 2003 and 2009. The study was stopped earlier in July 2011 when an interim analysis presented that there were significant benefits from to the two Mediterranean diets, Participants were considered to be at high CVD risk if they had either T2D or more than three of the following CVD risk factors including current smoking, hypertension, elevated low density lipoprotein cholesterol (LDL-C), decreased high density lipoprotein cholesterol (HDL-C), overweight/obesity, or family history of premature coronary heart disease. The primary endpoint of the PREDIMED trial was a composite of CVD events (non-fatal MI, stroke, or death from CVD) assessed after a median of 4.8-year of follow-up. 3541 participants were free of T2D at baseline We documented 273 incident T2D cases and plasma samples were available for 251 of those participants.
The present study was built within the PREDIMED trial using a case-cohort design [17].
Consistent with the case-cohort design, in addition to 251 cases of incident T2D, we included a random sample of 20% of PREDIMED participants who were free of diabetes at baseline known as the “subcohort participants” (n = 694). These “subcohort participants” which consists of 53 overlapping incident T2D cases and 641 “noncases” which were randomly selected using a computer-generated random number sequence. Together, 892 participants were included in the study (Supplemental Fig. 1). After 1 year of follow-up, 663 participants (505 noncases and 158 cases) had follow-up samples. With this sample size, we had 80% power to detect an OR of 1.25 at a false positive rate of 0.05 and 80% power to detect an OR of 1.45 even at the conservative Bonferroni correction threshold of 0.05/300 (~300 metabolites identified). The case-control study design with random sampling of the subcohort permit to measure the metabolites in a subsample rather than the entire study population and allows us to generalize the findings to the full cohort.
The PREDIMED trial was approved by the Institutional Review Boards at participating institutions across Spain. The present study was approved by the Institutional Review Boards at Harvard T.H. Chan School of Public Health and all participating institutions across Spain.
Case ascertainment
The primary endpoint of the present study was incidence of T2D, which was a secondary endpoint of the PREDIMED trial. Diagnosis of T2D incidence was based on the American Diabetes Association criteria including fasting plasma glucose concentrations ≥ 7.0 mmol/L or 2 h plasma glucose concentrations ≥ 11.1 mmol/L after an oral dose of 75-g glucose or recent use of an oral/insulin medication. Participants’ medical records were reviewed annually by physicians and investigators who were blinded to the intervention groups. When new-onset diabetes cases were identified on the basis of medical records or on a glucose test during routine biochemical analyses (conducted at least once per year), reports were sent to the PREDIMED Clinical Events Committee who were blind to the allocation group. The new diabetes cases would be confirmed only when a second test could be repeated within the following 3 months using the same criteria.
Covariate assessment
Lifestyle variables, medical records, medication, and family history of disease were collected by questionnaires at baseline and yearly during the follow-up. Anthropometric measurements and blood pressure were measured on sites by trained personnel. Physical activity was assessed using the validated Spanish version of the Minnesota Leisure-Time Physical Activity questionnaire [18]. Diet was assessed using a 137-items validated semi-quantitative food frequency questionnaire during an in-person interview with trained dietitians [19]. Energy and nutrient intakes were estimated by the Spanish food composition tables [20]. Adherence to the Mediterranean diet was assessed by a 14-items food questionnaire [21].
Metabolites profiling
Prior each blood draw, participants were required to fast overnight. Fasting plasma samples were collected at baseline and year-1, using EDTA tube and stored at −80 °C condition. Metabolites profiling was measured at the Broad Institute. Liquid chromatography-tandem mass spectrometry (LC-MS) on a system comprised of a Shimadzu Nexera X2 U-HPLC (Shimadzu Corp, Marlborough, MA) coupled to a Q Exactive hybrid quadrupole orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA) were used for metabolites profiling. Detailed mass spectrometry setting was published previously [22]. Authentic reference standards were used for metabolite identification.
Statistics
Baseline characteristics between cases and non-cases are presented as mean ± standard deviations (SDs) for continuous variables, and as n (%) for categorical variables. We used t-tests for comparing continuous variables and chi-squared tests for categorical variables.
Rank-based inverse normal transformations were applied to approximate a normal distribution of metabolites levels. General linear model was used to examine the associated between food intake and quartile of glutamine-to-glutamate ratio at baseline. Cox regression models with Barlow weights [to account for over-represented cases in the case-cohort study] were used to estimate Hazard Ratios (HRs) and their 95% Confidence Intervals (CIs) for T2D incidence. Non-cases in the sub-cohort were weighted inversely proportionally to the sampling fraction [17]. We calculated HR and their 95% CIs for T2D by quartiles of the amino acids and also for each SD as a continuous variable. Follow-up time was calculated from the date of enrollment to the date of diagnosis of T2D or the date of the last visit or the end of the follow-up period for participants without type 2 diabetes (December 1, 2010).
We fitted crude models adjusting for age (years). All models were stratified by intervention group (MedDiet + EVOO, MedDiet + nuts, low-fat control) and recruitment center. Multivariable-adjusted models were additionally adjusted for sex (male, female), BMI (kg/m2), smoking status (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day), dyslipidemia (yes, no), hypertension (yes, no). In a secondary analysis, we further adjusted for baseline levels of total BCAAs and fasting glucose levels as blood glucose was likely to be a confounder and/or an intermediate link in the causal pathway between glutamine-cycling pathway and risk of T2D. Quartile cut-off point for amino acids were generated based on the distribution of amino acids among participants without diabetes. Median value of metabolite in each quartile was assigned and analyzed as a continuous variable to test the linear trend across quartiles. To examine the associations between 1-year changes in glutamate, glutamine, Gln/Glu and risk of T2D, we used the same multivariable adjusted Cox regression models with additional adjustment for baseline metabolites levels. We conducted joint analyses and interaction tests for glutamine, glutamate, Gln/Glu at baseline and the intervention groups (MedDiet + EVOO and MedDiet + nuts vs control group). Likelihood ratio test was used to assess the significance of interaction between levels of amino acids and intervention groups. Participants with lowest risk were set as reference group based on the direction of association between baseline plasma levels of amino acids and risk of T2D.
We also examined how changes in individual amino acid levels at 1 year associated with risk of T2D. For each individual metabolite, we first calculated the changes in the individual amino acid level between baseline and 1 year and then normalized the differences using the inverses normal transformation. We used general linear model to assess the changes in glutamine and glutamate levels after one year in response to the dietary interventions. Models were adjusted for age (years), sex (male, female), body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day), dyslipidemia, hypertension, and baseline metabolites levels. We used robust estimates of the variance to correct for potential intra-cluster correlation, and adjusted models for propensity scores that has been built with 30 baseline variables to estimate the probability of assignment to each of the intervention groups [16]. All statistical analyses were performed by SAS (v9.4, SAS Institutes, Cary, NC).
Results
We examined associations of baseline glutamine, glutamate, and their ratio (Gln/Glu) with incident T2D in 892 participants from the PREDIMED study. Participants had a mean age of 66 years, BMI of 30 kg/m2 and majority of them had dyslipidemia and hypertension (Table 1). Participants with T2D were more likely to be obese (30.8 ± 3.3), hypertensive (96%), current smoker (25.1%) with higher baseline glucose level (118.6 ± 18.0 mg/dL). The proportion of women was lower in diabetic participants. Individuals with T2D had a slightly greater energy intake and lower consumption of vegetables, fish, and legume when compared with participants without T2D. Higher Gln/Glu was associated with greater fruit consumption (P for trend, 0.03), and lower consumption of meat and cereal (P for trend < 0.05 for both).
Table 1.
Baseline participant characteristics by quartile of glutamine-to-glutamate ratio.
| Non-cases | Cases | P value | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P valuea | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | 641 | 251 | 227 | 257 | 221 | 187 | ||
| Age (y) | 66.6 (5.8) | 66.4 (5.7) | 0.88 | 66.3 ± 5.9 | 66.1 ± 5.7 | 66.6 ± 5.7 | 67.2 ± 5.7 | 0.18 |
| BMI (kg/m2) | 29.8 (3.6) | 30.8 (3.3) | 0.24 | 30.8 ± 3.6 | 30.4 ± 3.3 | 30.0 ± 3.4 | 28.8 ± 3.6 | <0.001 |
| Sex (% women) | 62.8 | 55.0 | 0.03 | 132(58.1) | 131 (51.0) | 138 (62.4) | 145 (81.5) | <0.001 |
| Fasting glucose (mg/dL) | 99.7 (15.2) | 118.6 (18.0) | <0.01 | 102.(19.8) | 107.9 (17.9) | 104.1 (15.2) | 97.0 (14.7) | <0.001 |
| Hypertension, n (%) | 577 (90) | 241(96) | <0.01 | 206 (90.7) | 241 (93.8) | 200 (90.5) | 171 (91.4) | 0.54 |
| Dyslipidemia, n (%) | 552 (86) | 200 (80) | <0.01 | 184(81.1) | 216 (84.0) | 191 (86.4) | 161 (86.1) | 0.39 |
| Physical activity, METs/d | 238(235) | 249 (233) | 0.90 | 223.7 (247.1) | 242.8 (247.1) | 262.2 (257.1) | 233.4(197.1) | 0.35 |
| Smoking (%) | 0.06 | |||||||
| Never | 61.5 | 52.6 | 141 (62.1) | 130 (50.1) | 128 (57.9) | 127 (67.9) | 0.01 | |
| Former | 22.5 | 22.3 | 45 (19.8) | 75 (29.2) | 50 (22.6) | 30 (16.0) | ||
| Current | 16.1 | 25.1 | 41 (18.0) | 52 (20.2) | 43 (19.5) | 30 (16.0) | ||
| Intervention group (%) | ||||||||
| MedDiet + EVOO | 30.9 | 29.9 | 71 (31.3) | 79 (30.7) | 62 (28.1) | 61 (32.6) | 0.39 | |
| MedDiet + nuts | 37.2 | 33.9 | 81 (35.7) | 92 (35.8) | 81 (36.6) | 70 (37.5) | ||
| Control | 31.8 | 36.3 | 75 (33.0) | 86 (33.5) | 78 (35.3) | 56 (29.9) | ||
| Energy intake (kcal/day) Food intake (g/day) | 2289 (565) | 2327 (622) | 0.06 | 2315(550) | 2366 (596) | 2277 (586) | 2215 (585) | 0.04 |
| Vegetables | 333 (143) | 294(117) | 0.0002 | 336(145) | 316 (133) | 318 (128) | 318 (144) | 0.2 |
| Fruits | 360 (191) | 336 (174) | 0.09 | 319(174) | 367 (208) | 373 (177) | 356 (178) | 0.03 |
| Meat | 133 (54) | 135 (52) | 0.42 | 140 (52) | 138 (55) | 129(53) | 126 (54) | 0.003 |
| Fish | 101(49) | 97 (42) | 0.005 | 101 (52) | 102 (46) | 98 (43) | 98 (49) | 0.36 |
| Dairy | 374 (222) | 360 (229) | 0.55 | 368 (221) | 350 (211) | 385 (224) | 383 (242) | 0.27 |
| Nuts | 11 (13) | 12(14) | 0.24 | 11 (13) | 12(14) | 11 (14) | 10 (13) | 0.47 |
| Olive oil | 38 (16) | 39 (17) | 0.28 | 37 (16) | 39 (16) | 39 (16) | 37 (15) | 0.69 |
| Cereal | 227 (101) | 242 (110) | 0.09 | 240(101) | 242 (100) | 223 (108) | 217 (106) | <0.001 |
| Legume | 21 (14) | 18 (9) | <0.001 | 22 (13) | 19(11) | 20 (9) | 21 (17) | 0.07 |
Values are mean (SD) or percentage.
BMI, body mass index; MET, metabolic equivalent; MedDiet, Mediterranean diet intervention group; EVOO, Extra-virgin olive oil.
P value for the association of variables with quartile groups of glutamine-to-glutamate ratio.
Association of baseline metabolites with risk of incident T2D
Associations between glutamine, glutamate, Gln/Glu at baseline and risk of incident T2D are presented in Table 2. Higher glutamine levels at baseline were associated with lower risk of incident T2D when comparing extreme quantiles (model 2, HR: 0.64, 95% CI, 0.84–2.31, P for trend = 0.04). In contrast, higher glutamate levels were associated with increased T2D risk (model 2, P for trend = 0.0002). Participants who were in the top quartile of glutamate levels at baseline had significantly higher risk of incident T2D, with a HR of 2.78 (95% CI: 1.43–5.42) when compared to those who were in the bottom quartile. The Gln/Glu was strongly and inversely associated with risk of incident T2D [15]. Participants in the highest quartile had about 70% lower T2D risk (HR: 0.31, 95% CI: 0.23–0.64, P for trend <0.0001) compared with those in the bottom quartile.
Table 2.
Incident type 2 diabetes by baseline plasma amino acid levels in the PREDIMED trial, 2003–2010: Observed event rates and hazard ratios.a
| Levels at baseline | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
|---|---|---|---|---|---|
| Incidence Cases: 251 cases, 641 non-cases | |||||
|
| |||||
| Glutamine | HR (95% CI) | ||||
|
| |||||
| Cases | 59 | 85 | 54 | 53 | |
| Model 1 | 1.00 (ref) | 1.25 (0.84, 1.86) | 0.94 (0.61, 1.44) | 0.85 (0.55, 1.30) | 0.27 |
| Model 2 | 1.00 (ref) | 1.14(0.68, 1.93) | 0.80 (0.47, 1.36) | 0.64(0.36, 1.12) | 0.04 |
| Model 3 | 1.00 (ref) | 1.02 (0.60, 1.73) | 0.69 (0.41, 1.19) | 0.48 (0.26, 0.87) | 0.004 |
| Model 4 | 1.00 (ref) | 1.02 (0.51,2.02) | 1.02 (0.54, 1.92) | 0.77 (0.41,1.48) | 0.43 |
|
| |||||
| Glutamate | HR (95% CI) | ||||
|
| |||||
| Cases | 25 | 62 | 108 | 56 | |
| Model 1 | 1.00 (ref) | 2.35 (1.40, 3.95) | 4.13 (2.59, 6.98) | 2.45 (1.44, 4.16) | <0.0001 |
| Model 2 | 1.00 (ref) | 2.24(1.22,4.11) | 4.34 (2.42, 7.78) | 2.78 (1.43, 5.41) | 0.0002 |
| Model 3 | 1.00 (ref) | 2.06 (1.10, 3.85) | 3.91 (2.15, 7.08) | 2.32(1.17, 4.61) | 0.003 |
| Model 4 | 1.00 (ref) | 4.07 (1.50, 11.1) | 8.31 (3.40, 20.3) | 6.26 (2.21, 17.7) | <0.0001 |
|
| |||||
| Glutamine-to-glutamate ratio | HR (95% CI) | ||||
|
| |||||
| Cases | 67 | 98 | 59 | 27 | |
| Model 1 | 1.00 (ref) | 1.40 (0.95, 2.06) | 0.78 (0.51, 1.18) | 0.39 (0.23, 0.64) | <0.0001 |
| Model 2 | 1.00 (ref) | 1.18 (0.72, 1.93) | 0.69 (0.40, 1.20) | 0.31 (0.16, 0.57) | 0.001 |
| Model 3 | 1.00 (ref) | 1.22 (0.74, 1.99) | 0.72 (0.41, 1.25) | 0.34(0.18, 0.64) | 0.0006 |
| Model 4 | 1.00 (ref) | 0.99 (0.54, 1.82) | 0.52 (0.26, 1.04) | 0.26 (0.10, 0.66) | 0.001 |
Model 1: adjusted for age.
Model 2:Model 1 + sex (male, female), body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day), dyslipidemia (yes, no) and hypertension (yes, no). Analysis was stratified by recruitment center and intervention group (MedDiet + EVOO, MedDiet + nuts, low-fat control).
Model 3: Model 2 + baseline levels of BCAAs. Analysis was stratified by recruitment center and intervention group (MedDiet + EVOO, MedDiet + nuts, low fat control).
Model 4: Model 2 + baseline levels of glucose. Analysis was stratified by recruitment center and intervention group (MedDiet + EVOO, MedDiet + nuts, low fat control).
Inverse normal transformation was applied to raw values.
After we further adjusted for baseline BCAAs levels in model 3, the associations between glutamine, glutamate, Gln/Glu and incident T2D risk remained statistically significant. The association between baseline glutamine and incident T2D risk was attenuated after model (model 2) was further adjusted for baseline glucose (model 4).Whereas associations between glutamate, Gln/Glu and risk of incident T2D remained statistical significant.
Fig. 1 shows the HRs for joint effects of the intervention and baseline plasma levels of Gln/Glu, glutamine, and glutamate (dichotomised at their median) on T2D risk. Lower level of baseline glutamine was associated with increased risk of T2D (Fig. 1a). Participants in upper quartiles of glutamate levels had higher risk of T2D in both of the control group (HR 2.68 for Q3–4 vs Q1–Q2, 95% CI: 1.57–4.61) and the MedDiet group (HR 1.65 for Q3–4 vs Q1–Q2, 95% CI: 1.07–2.56) (Fig. 1b).The MedDiet attenuated the positive association between higher baseline plasma glutamate levels and T2D risk (P for interaction = 0.01). We also observed a higher risk of T2D in participants in lower quantiles of baseline Gln/Glu in the control group (HR 2.5 for Q1–2 vs Q3–Q4, 95% CI: 1.44–4.33) and the MedDiet group (HR 1.86 for Q1–2 vs Q3–Q4, 95% CI: 1.20–2.89) (Fig. 1c). The association between baseline plasma Gln/Glu and risk of T2D was attenuated in response to the MedDiet. There was significant interaction (P for interaction = 0.03) between the intervention (MedDiet) and baseline Gln/Glu.
Figure 1.
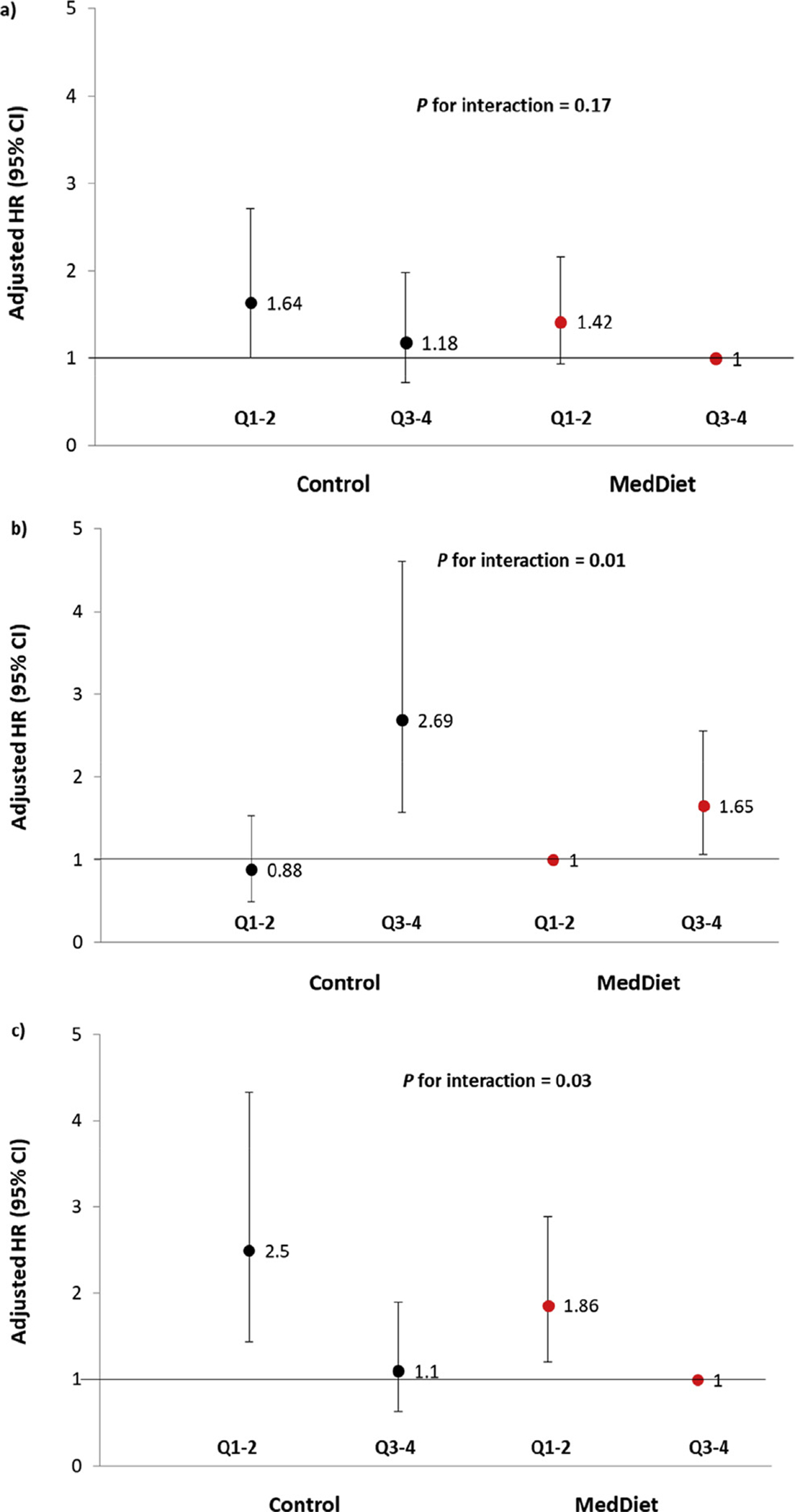
Multivariate-adjusted HRs (95% CIs) of incident T2D and quartiles of a) glutamine, b) glutamate, c) glutamine-to-glutamate ratio (GLN/GLU) at baseline stratified by intervention group (MedDiet versus control group). Control group is in solid black. MedDiet group is in solid red. An inverse normal transformation was applied to raw values of glutamine, glutamate and their ratio. Model was adjusted for age (years), sex (male/female), smoking status (never, former, or current smoker), body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (METs min/day), dyslipidemia (yes, no), hypertension (yes, no), metabolites (continuous) and stratified by intervention group (MedDiet + EVOO, MedDiet + nuts, and control diet); P for interaction with 2 degrees of freedom between MedDiet intervention groups (combined intervention groups vs. control: binary, yes/no) and baseline glutamine-to-glutamate ratio. Based on the association between metabolites and T2D risk, participants who were on the MedDiet and with higher quantiles (Q3–4) of baseline glutamine and Gln/Glu were set as the reference. Participants who were on MedDiet and with lower quantiles (Q1–2) of glutamate at baseline were set as the reference. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
One year changes in metabolites and the effect of dietary intervention on risk of incident T2D
Glutamine, glutamate, and Gln/Glu did not change significantly in response to the intervention diets after one year (Fig. 2). Overall, the associations of 1-year changes in glutamine, glutamate with risk of incident T2D were non-significant (Table 3). In contrast with our findings for baseline levels, changes in Glu/Gln were positively associated with the risk of incident T2D in age-adjusted model. However, the association was attenuated after further adjustment for additional anthropometric, lifestyle variables and baseline levels of Gln/Glu. We did not find any association between changes in these metabolites and risk of T2D nor did we find any effect of the intervention on these metabolites after one year.
Figure 2.
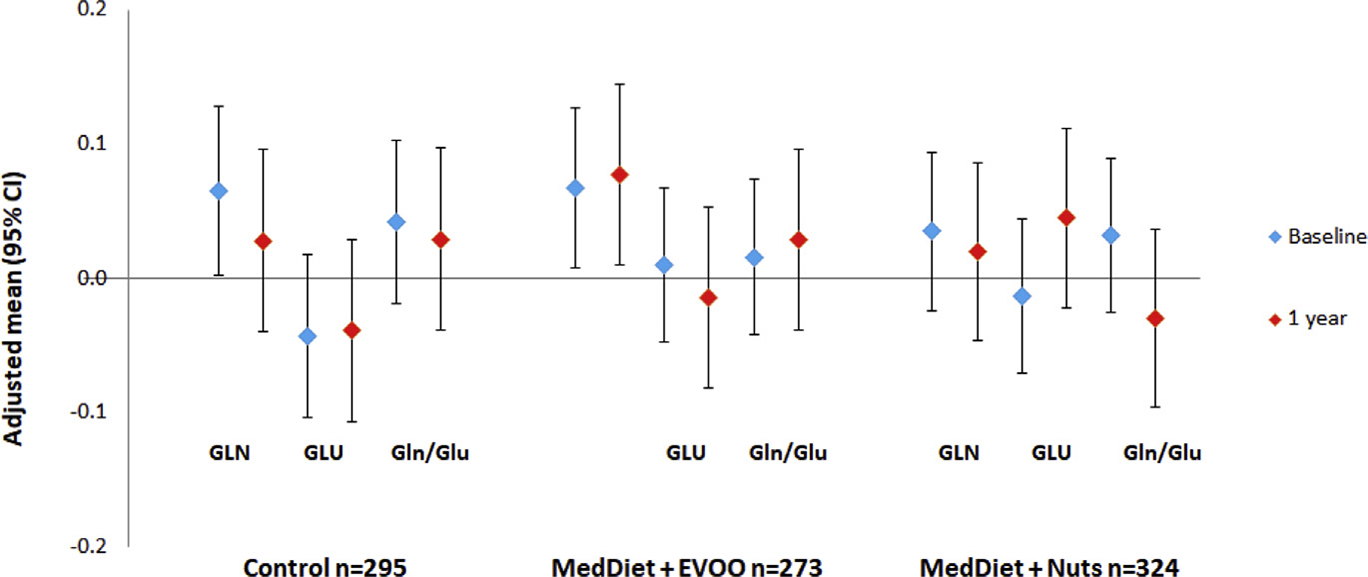
Glutamine (GLN), glutamate (GLU), glutamine-to-glutamate ratio (Gln/Glu) at baseline and changes after 1 year of intervention. Values were adjusted for age (years), sex (male, female), body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day), dyslipidemia, hypertension, and baseline metabolites levels. Blue diamond: baseline metabolites levels, red diamond: metabolites levels at 1 year Sample size was from baseline. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 3.
Incident T2D by 1-year changes in plasma amino acid levels in the PREDIMED trial, 2003–2010: Observed event rates and hazard ratios.a
| Changes at year 1 | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend |
|---|---|---|---|---|---|
| Incidence Cases: 158 cases, 505 non-cases | |||||
|
| |||||
| Glutamine | HR (95% Cl) | ||||
|
| |||||
| Cases | 31 | 58 | 44 | 48 | |
| Model 1 | 1.00 (ref) | 1.33 (0.80,2.20) | 1.06 (0.62,1.79) | 1.17 (0.71,1.95) | 0.82 |
| Model 2 | 1.00 (ref) | 1.12 (0.59,2.13) | 0.82 (0.41,1.62) | 0.94(0.47,1.87) | 0.64 |
| Model 3 | 1.00 (ref) | 0.94(0.48, 1.85) | 0.73 (0.36, 1.48) | 0.90 (0.45, 1.83) | 0.66 |
| Model 4 | 1.00 (ref) | 0.96 (0.47, 1.97) | 1.02 (0.52, 2.01) | 1.29 (0.65, 2.68) | 0.44 |
|
| |||||
| Glutamate | HR (95% Cl) | ||||
|
| |||||
| Cases | 54 | 47 | 55 | 25 | |
| Model 1 | 1.00 (ref) | 0.81 (0.52,1.29) | 1.00 (0.64,1.56) | 0.59 (0.34,1.02) | 0.14 |
| Model 2 | 1.00 (ref) | 1.00 (0.49,2.04) | 1.68 (0.84,3.34) | 0.68 (0.31,1.47) | 0.84 |
| Model 3 | 1.00 (ref) | 0.94(0.45, 1.94) | 1.69 (0.84, 3.4) | 0.76 (0.35, 1.64) | 0.90 |
| Model 4 | 1.00 (ref) | 1.28 (0.59, 2.77) | 1.79 (0.80, 4.01) | 1.05 (0.41, 2.68) | 0.52 |
|
| |||||
| Glutamine-to-glutamate ratio | HR (95% Cl) | ||||
|
| |||||
| Cases | 12 | 69 | 47 | 53 | |
| Model 1 | 1.00 (ref) | 4.88 (2.51,9.47) | 3.30 (1.66,6.58) | 3.63 (1.84,7.13) | 0.009 |
| Model 2 | 1.00 (ref) | 3.03 (1.37,6.70) | 1.26 (0.50,3.20) | 2.30 (0.99,5.33) | 0.41 |
| Model 3 | 1.00 (ref) | 3.07 (1.33, 6.78) | 1.08 (0.41, 2.84) | 2.38 (1.01, 5.63) | 0.38 |
| Model 4 | 1.00 (ref) | 3.67 (1.19, 11.3) | 1.83 (0.56, 5.97) | 2.37 (0.73, 7.68) | 0.70 |
Model 1: adjusted for age.
Model 2:Model 1 + sex (male, female), body mass index (kg/m2), smoking (never, current, former), leisure-time physical activity (metabolic equivalent tasks in minutes/day), dyslipidemia (yes, no), hypertension (yes, no) and baseline metabolites levels. Analysis was stratified by recruitment center and intervention group (MedDiet + EVOO, MedDiet + nuts, low fat control).
Model 3: Model 2 + baseline levels of BCAAs. Analysis was stratified by recruitment center and intervention group (MedDiet + EVOO, MedDiet + nuts, low fat control).
Model 4: Model 2 + baseline levels of glucose. Analysis was stratified by recruitment center and intervention group (MedDiet + EVOO, MedDiet + nuts, low fat control.
Inverse normal transformation was applied to raw values of 1-year changes in plasma metabolites.
Discussion
In this case-cohort study, we found that higher baseline plasma glutamate levels were associated with increased risk of incident T2D in a population at high risk of CVD. An imbalance between glutamine and glutamate may contribute to the development of T2D. We propose that abnormal glutamate homeostasis, could cause elevated extracellular glutamate concentrations that may participate in β-cell death, possibly in combination with increased FFA and glucose concentrations [13]. The two Mediterranean diets did not significantly change the levels of these metabolites after intervention for 1 year. However, our findings suggest that MedDiet could mitigate the adverse effects of T2D that is associated with imbalance between glutamine and glutamate. A unique aspect of our study is that we observed these prospective associations in a population at high risk for CVD in the context of a nutritional primary prevention study. In fact, these high-risk individuals are those who will benefit the most from early diagnosis and lifestyle interventions to delay the onset of T2D. These associations further support that glutamine-cycling pathways are prominently involved in the pathophysiology of T2D. The results reported herein add to the evidence base of using metabolomics signatures of glutamine and glutamate as possible future predictive tools of T2D risk in asymptomatic patients.7The metabolomics approach may enable timely and effective identification of T2D risk.
In a prospective cohort of 9369 Finish men, glutamine levels were inversely associated with risk of T2D after a follow-up of 4.7 years [10]. In 601 participants from the Framingham Heart Study (FHS) who were free of T2D at baseline, glutamine levels were also inversely associated with incident disease and other cardiometabolic risk factors over 12 years.6Evidence from clinical trials showed that supplementation of glutamine was beneficial in lowering blood glucose and improving other cardiometabolic risk factors in patients with T2D [23]. The proposed underlying mechanism from previous studies include that glutamine can stimulate insulin secretion by increasing the release of glucagon-like peptide (GLP-1), thus lowering blood glucose levels [24] and reducing postprandial insulin response [25]. In our study there was no significant association between glutamine levels and T2D risk. Differences in studies’ design and study populations can explain these discrepancies.
In contrast to the favorable effects of glutamine, abnormal glutamate homeostasis is often associated with increased oxidative stress and inflammation, which are commonly observed in people with obesity or diabetes [11]. Evidence from animal studies and clinical trials showed that elevated glutamate levels is likely to 1) increase the susceptibility of pancreatic β-cells to oxidative damage [26,27], 2) aggravate β-cell dysfunction and apoptosis by activating the glutamate receptor (N-methyl-D-aspartate receptor) inβ-cells [28], and 3) increase the activity of GAD65, one of the major antigens that triggers autoimmunity accelerating cell dysfunction and apoptosis [29]. Along with our findings, these data suggest that a dysregulation in glutamate homeostasis may contribute to the development of T2D. Glutamate is commonly present in protein-rich foods e.g. meats, poultry, seafood, dairy and offers a meaty savory taste known as “UMAMI” [30]. Previous studies showed that elderly tend to increase intake of glutamate enhanced foods [31,32]. Disregard of age, subjects with relatively poorer biochemical status preferred higher glutamate concentrations [33].It is plausible that the differences in plasma glutamate levels may partially reflect a dietary pattern with higher glutamate levels associated with a higher meat, poultry consumption and/or increased energy intake which may contribute to a greater T2D risk.
Our findings on glutamine-to-glutamate ratio highlight the importance of the balance between these two gluconeogenic amino acids in relation to T2D risk. Glutamine and glutamate provide carbon for glucose production in kidney and liver [8] and glutamate is identified as one of the metabolic coupling factors that synergistically promote insulin secretion from pancreatic β-cell [34]. As the metabolism of glutamine and glutamate is exquisitely related to energy metabolism, the glutamine-to-glutamate ratio may reflect the overall status of energy metabolism. Glutamine, glutamate and BCAAs are correlated in metabolism. The branched-chain amino acid transaminase 1 (BCAT1) initiates the catabolism of BCAAs and uses glutamine as a nitrogen receiver; and leucine allosterically activating glutamate dehydrogenase that catalyzes the oxidative deamination of glutamate, which is present at a high concentration in the pancreatic β-cell [9]. The present study demonstrating that low glutamine levels and higher glutamate were associated with subsequent onset of T2D in addition to previous established metabolites markers such as BCAAs; suggest that the chronically imbalance of amino acids metabolism contribute to the progression of T2D. It has been recently proposed β-cell neogenesis from α-cell can be a new pathway of potential therapeutic significance [35]. Amino acids, especially glutamine can serve as an energy source and play a major role in the regulation of α-cell mass in animal models [36,37]. Therefore we hypothesize that imbalance between glutamine and glutamate may potentially hinder α-cell proliferation thus limit the regeneration of β-cell.
The two Mediterranean diets did not significantly change the metabolite levels after intervention for 1 year. Given that study participants were at high risk for CVD at baseline, which presumably already had an unfavorable metabolite profile, this could contribute to the lack of associations observed between 1-year changes in metabolites and T2D risk after adjusting for baseline metabolites levels. Also, the time period of 1 year may not be sufficient to have a significant change in metabolites to influence the risk of T2D. Considering that the PREDIMED intervention was not designed to modify amino acid intake, these results are not unexpected. These results suggest that the Mediterranean diet interventions may not directly influence glutamate levels at least in a short-time period, but may counteract their adverse effect on risk of T2D through other protective pathways and mechanisms [38]. The effects of olive oil and nuts on changes of glutamate may vary due to the different bioactive compounds in these foods.
One limitation of the present study is that these results may not be generalized to other populations, as the study subjects lived in a Mediterranean area and were at high risk of CVD. Another limitation is that we used LC-MS platform for metabolite profiling and applied inversenormal transformation to express the results, and this approach may hamper a direct clinical translation for each metabolite trait. The strengths of our study include the control of potential confounders by comprehensive data recording and monitoring of intervention compliance; using repeated measure of metabolites to examine the changes of metabolite s in response to dietary intervention. In addition, the case-cohort design maximized the efficiency of metabolite profiling, allowing us to generalize these findings to all PREDIMED participants.
Conclusion
In conclusion, we provide evidence of associations between glutamate glutamine-to-glutamate ratio and risk of incident T2D in a population at high-risk for CVD. Our findings support a role for these metabolites in the pathophysiology of T2D and the need to explore their potential predictive ability of disease risk. A Mediterranean-style dietary pattern appeared to modify risk of T2D associated with glutamate and the overall balance between glutamine and glutamate.
Supplementary Material
Highlights.
Type 2 diabetes (T2D) is associated with abnormal skeletal muscle energy metabolism.
Glutamine is the most abundant amino acid plays a key role in energy metabolism.
High levels of glutamate, low levels of glutamine associated with increased risk for T2D.
The imbalance between glutamine and glutamate can be predictive of T2D incidence.
Mediterranean Diet might mitigate the delirious association between glutamate and T2D incidence.
Acknowledgments
We express our gratitude to the participants for making this study possible. We also gratefully acknowledge the clinical coordinators and nurses for their assistance.
Dr. Marta Guasch-Ferré was supported by a post-doctoral fellowship granted by the Lilly Foundation European Association of Diabetes (EASD) through the Institut d’Investigacions Sanitàries Pere i Virgili (IISPV), Tarragona, Spain.
Dr. Christopher Papandreou was supported by a post-doctoral fellowship granted by the Autonomous Government of Catalonia (PERIS 2016–2020 Incorporació de Científics I Tecnòlegs, SLT002/0016/00428).
Sources of support
This study was supported by research grant NIDDK-R01DK 102896 from the National Institutes of Health.
ABBREVIATIONS/ACRONYMS USED
- EVOO
Extra virgin olive oil
- MedDiet
mediterranean diet
Footnotes
Conflicts of interest
Dr. Emilio Ros has received funds for research through his institution and personal fees for lecture presentation from the California Walnut Commission and is a nonpaid member of its Scientific Advisory Committee.Dr. Jordi Salas-Salvadó has have received grants from the International Nut and Dried Fruit Foundation and is a non-paid member of the scientific advisory board of the International Nut and Dried Fruit Foundation.
Liu, Zheng, Ruiz-Canela, Toledo, Clish, Liang, Corella, Estruch, Fito, Gómez-Gracia, Arós, Lapetra, Fiol, Serra-Majem, Papandreou, Martínez-González, Hu, Salas-Salvadó have no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.numecd.2019.06.005.
This trial is registered at http://www.controlled-trials.com, ISRCTN35739639.
References
- [1].Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103:137–49. [DOI] [PubMed] [Google Scholar]
- [2].Centers for Disease Control and Prevention. National diabetes statistics report. 2017. Atlanta, GA. [Google Scholar]
- [3].Gonzalez-Franquesa A, Burkart AM, Isganaitis E, Patti ME. What have metabolomics approaches taught us about type 2 diabetes? Curr Diabetes Rep 2016;16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Martin BC, Warram JH, Rosner B, Rich SS, Soeldner JS, Krolewski AS. Familial clustering of insulin sensitivity. Diabetes 1992;41:850–4. [DOI] [PubMed] [Google Scholar]
- [6].Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012;125:2222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R. Glutamine and glutamate–their central role in cell metabolism and function. Cell Biochem Funct 2003;21:1–9. [DOI] [PubMed] [Google Scholar]
- [9].Haber EP, Procopio J, Carvalho CR, Carpinelli AR, Newsholme P, Curi R. New insights into fatty acid modulation of pancreatic beta-cell function. Int Rev Cytol 2006;248:1–41. [DOI] [PubMed] [Google Scholar]
- [10].Stancakova A, Civelek M, Saleem NK, Soininen P, Kangas AJ, Cederberg H, et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes 2012;61:1895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabol 2009;9:311–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chevalier S, Burgess SC, Malloy CR, Gougeon R, Marliss EB, Morais JA. The greater contribution of gluconeogenesis to glucose production in obesity is related to increased whole-body protein catabolism. Diabetes 2006;55:675. [DOI] [PubMed] [Google Scholar]
- [13].Davalli AM, Perego C, Folli FB. The potential role of glutamate in the current diabetes epidemic. Acta Diabetol 2012;49:167–83. [DOI] [PubMed] [Google Scholar]
- [14].Guasch-Ferre M, Hruby A, Toledo E, Clish CB, Martinez-Gonzalez MA, Salas-Salvado J, et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 2016;39:833–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ruiz-Canela M, Guasch-Ferre M, Toledo E, Clish CB, Razquin C, Liang L, et al. Plasma branched chain/aromatic amino acids, enriched Mediterranean diet and risk of type 2 diabetes: case-cohort study within the PREDIMED Trial. Diabetologia 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Retraction and republication: primary prevention of cardiovascular disease with a mediterranean diet. N Engl J Med 2013;368: 1279e90. N Engl J Med. 2018;378:2441–2442. [DOI] [PubMed] [Google Scholar]
- [17].Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999;52:1165–72. [DOI] [PubMed] [Google Scholar]
- [18].Elosua R, Marrugat J, Molina L, Pons S, Pujol E. Validation of the Minnesota leisure time physical activity questionnaire in Spanish men. The MARATHOM investigators. Am J Epidemiol 1994;139:1197–209. [DOI] [PubMed] [Google Scholar]
- [19].Fernandez-Ballart JD, Pinol JL, Zazpe I, Corella D, Carrasco P, Toledo E, et al. Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain. Br J Nutr 2010;103:1808–16. [DOI] [PubMed] [Google Scholar]
- [20].Mataix Verdú José, Luis García Diz, Mañas Almendros Mariano, EMdV y, González JL. Tablas de composición de alimentos. 4th ed. Granada: Universidad de Granada; 2003. [Google Scholar]
- [21].Schroder H, Fito M, Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr 2011;141:1140–5. [DOI] [PubMed] [Google Scholar]
- [22].Guasch-Ferre M, Zheng Y, Ruiz-Canela M, Hruby A, Martinez-Gonzalez MA, Clish CB, et al. Plasma acylcarnitines and risk of cardiovascular disease: effect of Mediterranean diet interventions. Am J Clin Nutr 2016;103:1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mansour A, Mohajeri-Tehrani MR, Qorbani M, Heshmat R, Larijani B, Hosseini S. Effect of glutamine supplementation on cardiovascular risk factors in patients with type 2 diabetes. Nutrition 2015;31:119–26. [DOI] [PubMed] [Google Scholar]
- [24].Samocha-Bonet D, Wong O, Synnott EL, Piyaratna N, Douglas A, Gribble FM, et al. Glutamine reduces postprandial glycemia and augments the glucagon-like peptide-1 response in type 2 diabetes patients. J Nutr 2011;141:1233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Greenfield JR, Farooqi IS, Keogh JM, Henning E, Habib AM, Blackwood A, et al. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr 2009;89:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Albrecht P, Lewerenz J, Dittmer S, Noack R, Maher P, Methner A. Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target. CNS Neurol Disord - Drug Targets 2010;9:373–82. [DOI] [PubMed] [Google Scholar]
- [27].Oresic M, Simell S, Sysi-Aho M, Nanto-Salonen K, Seppanen-Laakso T, Parikka V, et al. Dysregulation of lipid and amino acid metabolism precedes islet autoimmunity in children who later progress to type 1 diabetes. J Exp Med 2008;205:2975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Huang XT, Li C, Peng XP, Guo J, Yue SJ, Liu W, et al. An excessive increase in glutamate contributes to glucose-toxicity in beta-cells via activation of pancreatic NMDA receptors in rodent diabetes. Sci Rep 2017;7:44120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Velloso LA, Kampe O, Hallberg A, Christmanson L, Betsholtz C, Karlsson FA. Demonstration of GAD-65 as the main immunogenic isoform of glutamate decarboxylase in type 1 diabetes and determination of autoantibodies using a radioligand produced by eukaryotic expression. J Clin Investig 1993;91:2084–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bellisle F. Glutamate and the UMAMI taste: sensory, metabolic, nutritional and behavioural considerations. A review of the literature published in the last 10 years. Neurosci Biobehav Rev 1999;23:423–38. [DOI] [PubMed] [Google Scholar]
- [31].Bellisle F, Monneuse MO, Chabert M, Larue-Achagiotis C, Lanteaume MT, Louis-Sylvestre J. Monosodium glutamate as a palatability enhancer in the European diet. Physiol Behav 1991;49:869–73. [DOI] [PubMed] [Google Scholar]
- [32].Schiffman SS, Warwick ZS. Effect of flavor enhancement of foods for the elderly on nutritional status: food intake, biochemical indices, and anthropometric measures. Physiol Behav 1993;53:395–402. [DOI] [PubMed] [Google Scholar]
- [33].Murphy C Flavor preference for monosodium glutamate and casein hydrolysate in young and elderly persons. In: Kawamura Y, Kare MR, editors. Umami, a basic taste. New York: Marcel Dekker; 1987. p. 139–51. [Google Scholar]
- [34].Wollheim CB. Beta-cell mitochondria in the regulation of insulin secretion: a new culprit in type II diabetes. Diabetologia 2000;43:265–77. [DOI] [PubMed] [Google Scholar]
- [35].Chung C-H, Levine F Adult pancreatic alpha-cells: a new source of cells for beta-cell regeneration. Rev Diabet Stud 2010;7:124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Solloway MJ, Madjidi A, Gu C, Eastham-Anderson J, Clarke HJ, Kljavin N, et al. Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of alpha-cell mass. Cell Rep 2015;12:495–510. [DOI] [PubMed] [Google Scholar]
- [37].Hayashi Y, Seino Y. Regulation of amino acid metabolism and alpha-cell proliferation by glucagon. J Diabetes Investing 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Martinez-Gonzalez MA, Salas-Salvado J, Estruch R, Corella D, Fito M, Ros E. Benefits of the mediterranean diet: insights from the PREDIMED study. Prog Cardiovasc Dis 2015;58:50–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


