Abstract
This current study review provides a brief review of a natural bee product known as propolis and its relevance toward combating SARS-CoV viruses. Propolis has been utilized in medicinal products for centuries due to its excellent biological properties. These include anti-oxidant, immunomodulatory, anti-inflammatory, anti-viral, anti-fungal, and bactericidal activities. Furthermore, studies on molecular simulations show that flavonoids in propolis may reduce viral replication. While further research is needed to validate this theory, it has been observed that COVID-19 patients receiving propolis show earlier viral clearance, enhanced symptom recovery, quicker discharge from hospitals, and a reduced mortality rate relative to other patients. As a result, it appears that propolis could probably be useful in the treatment of SARS-CoV-2-infected patients. Therefore, this review sought to explore the natural properties of propolis and further evaluated past studies that investigated propolis as an alternative product for the treatment of COVID-19 symptoms. In addition, the review also highlights the possible mode of propolis action as well as molecular simulations of propolis compounds that may interact with the SARS-CoV-2 virus. The activity of propolis compounds in decreasing the impact of COVID-19-related comorbidities, the possible roles of such compounds as COVID-19 vaccine adjuvants, and the use of nutraceuticals in COVID-19 treatment, instead of pharmaceuticals, has also been discussed.
Keywords: COVID-19, SARS-CoV-2, Vaccine, Propolis, Molecular simulation, Pharmacological properties, Antimicrobial activities
Introduction
The novel 2019 coronavirus (formally referred to as “SARS-CoV-2”) is one of several viruses that are notorious for causing various respiratory illnesses, particularly in human populations. The other viruses include influenza A, Middle East respiratory syndrome coronavirus (MERS-COV), and severe acute respiratory syndrome coronavirus (SARS-COV). Just like the other three indicated viruses (Abdelrahman et al. 2020; Mohammadbeigi et al. 2020), SARS-CoV-2 has also caused a global pandemic that started around December 2019 (Alhouri et al. 2020; Lu et al. 2020; Zhu et al. 2020; Ghosh et al. 2021; Meskini et al. 2021). By the first week of July 2021, the SARS-CoV-2, which causes a disease called COVID-19, was still ongoing with more than 185 million positive cases and over four million deaths having been recorded globally (Johns Hopkins University & Medicine Coronavirus Resource Center; https://coronavirus.jhu.edu/map.html). During that period, pharmaceutical companies had tested several vaccines and some have since been authorized for vaccinating citizens in different countries throughout the world. While SARS-CoV-2 vaccines are generally safe and effective (Polack et al. 2020; Anand and Stahel 2021), public acceptance among the general population and healthcare workers has been worryingly low in some countries (Kreps and Kriner 2021; Sallam 2021). Cited reasons for general vaccine hesitancy included cognitive, psychologic, socio-demographic, and cultural factors. In addition, the lack of sufficient infrastructure to receive and administer vaccines creates an impediment to vaccination programmes in rural and remote areas of countries, particularly in Africa (Nachega et al. 2021). Alternative treatment methods may help bridge the divide between modern treatments, traditional herbal medicines, and cultural beliefs.
One of the oldest known folk medicines is propolis, a wax-like resinous substance used by various nations since ancient times (Kuropatnicki et al. 2013; Toreti et al. 2013). Bees, especially the Western or European honeybee (Apis mellifera) and related subspecies, collect resins and pollen from various plants and combine these compounds with wax to produce propolis (Ahangari et al. 2018). Propolis serves many functions in bee colonies, including usage in sealing cracks or crevices as well as repairing combs in hives, protecting hives against weather elements, moisture control in hives, and providing antimicrobial protection for the larvae. In human and animal societies, propolis has been shown to exude many medicinal and therapeutic properties (Kuropatnicki et al. 2013; Toreti et al. 2013; Wagh, 2013; Mahmoud et al. 2016; Pasupuleti et al. 2017; Ahangari et al. 2018; Anjum et al. 2019; Zulhendri et al. 2021). Interestingly, such properties tend to vary depending on the chemical composition of propolis in line with plant and bee species occurring in the geographical source area (Toreti et al. 2013; Wagh 2013; Zulhendri et al. 2021). That around 3,000 propolis-related patents have been filed thus far (Toreti et al. 2013; de Carvalho Furtado et al. 2018) demonstrates the enormous potential that this compound holds in benefiting human populations, especially the treatment of novel diseases.
The current review specifically sought to address the roles that propolis can play as an anti-bacterial, anti-fungal, and anti-viral in human societies. Particularly, much emphasis was placed on the anti-viral properties of propolis, the possible mode of compound actions based on molecular simulations of propolis compounds that interact with the SARS-CoV-2 virus. Limitations on the use of propolis for medicinal purposes and its potential as a nutraceutical have also been discussed.
SARS-COV virus family and COVID-19
The ancestor of all coronaviruses is believed to have occurred approximately 8,000 BCE, even though several models inferred long-term evolution that was traced to the common ancestor ~ 55 million years ago (ref.). Coronaviridae is a positive sense, single-strand RNA genome family that infects animals including birds, amphibians, and mammals (Holmes 1999). The coronaviridae was identified as a new virus family in 1968, because of the intracellular budding and distinct site virion morphology (ref.). Characteristic features of their replication strategy, genome, DNA polymerase, and structural proteins were also considered for classifying members of the coronaviridae (Fig. 1).
Fig. 1.
Coronaviridae family classification. Genus: Alphacoronavirus: α coronavirus 1 (TGEV, FC: feline coronavirus, CC: canine coronavirus), HC 229E: human coronavirus 229E, HC NL63: human coronavirus NL63, MBC1: Miniopterus bat coronavirus 1, MBC HKU8: Miniopterus bat coronavirus HKU8, PEDV: Porcine epidemic diarrhea virus, RBC HKU2: Rhinolophus bat coronavirus HKU2, and SBC 512: Scotophilus bat coronavirus 512 (Decaro 2011). Genus Betacoronavirus; βcoronavirus 1 (BC:Bovine Coronavirus,HC OC43: Human coronavirus OC43), HC1: Hedgehog coronavirus 1, HC HKU1: Human coronavirus HKU1, MERS: Middle East respiratory syndrome-related coronavirus, MC: Murine coronavirus, PBC HKU5: Pipistrellus bat coronavirus HKU5, RBC HKU9: Rousettus bat coronavirus HKU9, SARS: Severe acute respiratory syndrome–related coronavirus (SARS-CoV, SARS-CoV-2), and TBC HKU4: Tylonycteris bat coronavirus HKU4 (Decaro 2011). Genus Gammacoronavirus;[18]: AC: Avian coronavirus, BWC SW1: Beluga whale coronavirus SW1, TCoV: Turkey coronavirus, BVCoVSW1: Beluga whale CoV SW1, and IPBNDCoV: Indo Pacific Botthe Nose Dolphins CoV (Decaro 2011). Genus Deltacoronavirus: BC HKU11: Bulbul coronavirus HKU11, PC HKU15: Porcine coronavirus HKU15, TCoV: Thrush CoV, WECoV: White Eye CoV, WCoV: Wigeeon CoV, NHCoV: Night Heron CoV, and CMCoV: Commen Moorhen CoV (Fan et al. 2019). FMNV: Fathead Minnow Nido Virus (Baird and Faisal 2016). PRRV: Porcine reproductive and respiratory syndrome (Lyoo 2015), EAV: Equine arteritis virus (Snijder et al. 1995). DKNV: Dak Nong virus (Warrilow et al. 2014). YHV: yellow head virus (Enjuanes et al. 2008)
The coronaviridae, arterviridae, mesoniviridae, and roniviridae are recognized as separate families within the sub-order cornidovirineae (Srivastava and Saxena 2020). Orthocoronavirinae and torovirinae are recognized as separate sub-families within the coronaviridae family (Decaro and Lorusso 2020; Li et al. 2020a), whereas the alphacoronavirus, betacoronavirus, gammacoronavirus, and deltacoronavirus are classified as members of the orthocoronavirinae sub-family (Zaim et al. 2020).
Currently, the coronaviridae family comprises two sub-families, six genera, 26 sub-genera, and 46 species. Further species descriptions are tentative or pending (Siddell et al. 2019; Igori et al. 2020). Human coronaviruses (CoV) are a huge family that are capable of causing diseases ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERS-CoV) and Severe Acute Respiratory Syndrome (SARS-CoV) (Liu et al. 2020). A novel coronavirus (nCoV) is a new strain within the coronaviridae family that had not been identified before, and it causes the coronavirus disease. The disease (named COVID-19) initially emerged in China around December 2019. Soon after, it was declared a global pandemic by the World Health Organization (WHO) on March 11, 2020 (Cascella et al. 2022).
COVID-19 is caused by the seventh known coronavirus that is capable of infecting humans, with the predecessors including 229E, NL63, OC43, HKU1, MERS-CoV, and the original SARS-CoV (Boni et al. 2020). Coronaviruses cause zoonotic diseases that can be transmitted between human and animals. Seemingly, MERS-CoV was transmitted from dromedary camels to humans, whereas SARS-CoV moved from civet cats to humans, and SARS-CoV-2 from bat to humans. Several known coronaviruses circulate in various animals that have not infected humans yet (Ye et al. 2020). Notably, the reports indicated the fatality rate of MERS is about 34%; however, the SARS-CoV is ~ 11–14% indicating that the death rate of MERS is much higher than the SARS-CoV (Mohammadi et al. 2022; Meskini et al. 2021).
Natural products in medical science
Historically, natural products are widely used to avoid and alleviate diseases (Bakkali et al. 2008; Saklani and Kutty 2008; Nahar and Sarker 2020). This is due to the fact that natural products tend to be reasonably priced, commonly available and rarely show undesirable side effects (Berretta et al. 2020). While natural products command significant interest in the fields of biomedical research and technology, researchers and physicians appear to be somewhat reluctant to explore their properties further, perhaps due to lack of knowledge on biomimicry (Scorza and Cavalheiro 2010; Scorza et al. 2020; Benyus 2021). Some researchers, however, observed extensive evidence supporting the use of propolis, a well-known bee product that serves as a bioactive food component used in dietary supplements for nutrition and health-related purposes (Shaha et al. 2018; Ali and Kunugi 2019, 2020b; Egawa et al. 2019; Boisard et al. 2020). Seemingly, propolis could potentially be used in the treatment for SARS-CoV-2 patients because it exudes specific and relevant characteristics including anti-inflammatory action, ability to reduce viral replication and immune system fortification (Ansorge et al. 2003; Shimizu et al. 2011; Machado et al. 2012; Chan et al. 2013; Hori et al. 2013).
Properties of propolis
Propolis is defined as a natural product that is sourced from plant resins and other plant exudates such as flower buds (Ali and Kunugi 2020a;b). Typically, plants protect themselves from pathogens by producing phytochemicals. Chemical substances such as terpenoids and phenols are present as phytochemicals, are collected by bees (Langenheim 1994; Cheng et al. 2007; Toreti et al. 2013). Propolis contains more than 300 phytochemical compounds (Kocot et al. 2018; Anjum et al. 2019; Ali and Kunugi, 2020a; Hashem 2020), and these include caffeic acid, myricetin, and quercetin—all of which are known to inhibit coronavirus activity in humans (Wang et al. 2018; Ali and Kunugi 2020b; Mani et al. 2020; Guler et al. 2021). However, it must be noted that the composition active components in propolis may vary at different geographical locations (Bankova 2005; Falcao et al. 2013; Toreti et al. 2013; Miguel et al. 2014; Ali and Kunugi 2020b; Hashem 2020). Nevertheless, honeybees can identify the anti-microbial characteristics of certain plants, and therefore, selectively collect and process flower material from chosen plants to produce propolis (Simone-Finstrom and Spivak 2010).
Overall, propolis possesses anti-cancer, anti-oxidant, immunomodulatory, anti-inflammatory, anti-bacterial, anti-fungal as well as antiviral properties (Berretta et al. 2012). As a result, it has been used widely for alleviating various diseases (Cowan 1999; Ramos and Miranda 2007; Bakkali et al. 2008; Saklani and Kutty 2008). Maruta and He proposed that propolis should be considered as an adjunct treatment for SARS-CoV-2 (Maruta and He 2020). This is mostly due to propolis being economical, commonly accessible, and hardly presenting unwanted side effects (Berretta et al. 2017).
Anti-microbial activities of propolis
Many studies have demonstrated the anti-microbial effects of propolis (Al-Ani et al. 2018a; Karadal et al. 2018). Certainly, propolis possesses anti-microbial properties including anti-bacterial, anti-fungal and anti-viral effects due to the presence of bioactive compounds (e.g., phenolics and flavonoids) present in its extracts (Uzel et al. 2005; Suleman et al. 2015; Al-Ani et al. 2018a; Karadal et al. 2018) (Fig. 2A). Moreover, there seems to be a correlation among the efficacy of propolis and the botanical source and geographical origin of its components (Al-Ani et al. 2018a). Propolis isolated from temperate zones seemingly possess stronger anti-microbial activity due to the higher content of phenolic compounds (46.45 ± 3.1 mg CAE/g—129.83 ± 5.9 mg CAE/g) and flavonoids (0.11 ± 0.01 mg QE/g -2.86 ± 0.2 mg QE/g) (Ramanauskien et al. 2009; Rahman and Richardson 2010; Al-Ani et al. 2018b; Karadal et al. 2018). It is, thus, not surprising that propolis samples with low phenolic contents are generally less effective towards Gram-negative bacteria (Almuhayawi 2020).
Fig. 2.
Antimicrobial activities of the propolis active ingredients. A. Chemical structure of the active ingredients of propolis indicated by the colored circles. B. Normal bacterial cell. C. Antibacterial activities of the propolis ingredients causing leakage and shrinkage of the cell with disruptions in multiple organelles. D. Normal fungal cell. E. Accumulation and perforation of the propolis ingredients into the fungal cell causing cellular perturbation with disruption of their organelles. F. Normal viral cell. G. The propolis ingredients causing antiviral effect by inhibiting the viral spike proteins, neuraminidase and DNA polymerase prohibiting them to infect the host cell, viral replication and DNA replication respectively
Other factors that influence the efficacy of propolis against microbes include the type of microorganisms (strain) tested and concentration of propolis examined (Petruzzi et al. 2020). Notably, microbes have different resistance mechanisms to anti-microbial compounds (Reygaert 2018), and similarly, some microbes show stronger resistance to propolis than others (Almuhayawi 2020). In general, the concentration of an anti-microbial agent plays a pivotal role in resistance mechanisms of microbes (Li et al. 2017). For example, low concentrations (20,000–300,000 ppm) of propolis can be effective against Gram-positive bacteria (Petruzzi et al. 2020). Conversely, the growth of Gram-negative bacteria is mostly inhibited at higher concentrations of propolis. These findings suggest that the bacterium being investigated will require high amounts than the indicated studies of active compounds in propolis to initiate effective anti-microbial action.
Anti-bacterial
Both Gram-positive and Gram-negative bacteria have been investigated for their susceptibility towards propolis (Bankova et al. 2014). However, there is a consensus that Gram-negative bacteria are more resistant towards propolis than Gram positive bacteria (Campos et al. 2015; Przybyłek and Karpiński 2019; Petruzzi et al. 2020). For instance, propolis could inhibit Staphylococcus aureus (Gram-positive bacteria), but it exhibited limited action against Gram-negative Escherichia coli (Muli and Maingi 2007; Pobiega et al. 2019). In fact, the microbial inhibitory concentration (MIC) values in tests involving propolis are generally higher for Gram-negative bacteria (Al-Ani et al. 2018a). The resistance mechanism of Gram-negative bacteria could probably be attributed towards structural and biochemical differences in the cell wall composition (Ghasemi et al. 2017).
In general, Gram-negative bacteria are more resistant to antibiotics such as carbapenems, quinoles, cephalosporins, polymxins, and other β-lactam antibiotics that are commonly used to treat bacterial infections (Miller 2016; Breijyeh et al. 2020) or anti-microbial effects (Lee et al. 2007). The resistant mechanism of Gram-negative bacteria to propolis may be due to the efflux pumps on the bacterial cell wall that prevent the intracellular accumulation of propolis constituents (Petruzzi et al. 2020). Similarly, the production of hydrolytic enzymes that break down active compounds in propolis may contribute to the resistance mechanisms displayed by Gram-negative bacteria (Przybyłek and Karpiński 2019). Nonetheless, recent evidence shows that propolis may also be effective against Gram-negative bacteria, developments that further illustrate the versatility of propolis as an anti-microbial agent (Ramanauskien et al. 2009; Suleman et al. 2015; Karadal et al. 2018; Abdullah et al. 2019). However, there seems to be inconsistencies in the literature regarding the overall efficacy of propolis against Gram-negative bacteria. For example, several authors have reported that propolis is ineffective against the popular Gram-negative bacterium, E. coli (Ramanauskien et al. 2009; Gajger et al. 2017). Equally, other studies have demonstrated minimal to moderate susceptibility (Suleman et al. 2015; Karadal et al. 2018; Abdullah et al. 2019). However, other Gram-negative bacteria seem to show moderate to complete inhibition (Ramanauskien et al. 2009). At the other end, for Gram-positive bacteria, propolis is much more effective with species such as Staphylococcus aureus, Bacillus subtilis, Bifidobacteria spp., Streptococcus pyogenes, Streptococcus mutans, Streptococcus sobrinus, and Listeria monocytogenes, demonstrating high susceptibility (Fernandes Jr et al. 2005; Uzel et al. 2005; Rahman and Richardson 2010; Morawiec et al. 2015; Al-Ani et al. 2018a; Grecka et al. 2019). The species L. monocytogenes was initially reported to be resistant to propolis extracts, a finding that has since changed as the bacterium is susceptible (Temiz et al. 2011; Ristivojević et al. 2016; Karadal et al. 2018).
The anti-bacterial mechanism of propolis has been attributed to its constituent compounds including flavonoids(e.g., pinocembrin and galangin) and phenolics, (e.g., caffeic acid) (Rahman and Richardson 2010; Anjum et al. 2019; Gür et al. 2020). Typically, phenolic compounds can bind to various bacterial proteins and peptides effectively, resulting in the alteration of their 3D structures and functional inhibition (Bouarab-Chibane et al. 2019). The mechanism employed by propolis toward bacteria includes the inhibition of cell division, cell membrane collapse, enzymatic inactivation, the inhibition of protein synthesis (translation), and cell death through bacteriolysis (Fig. 2B, C). Using two Italian propolis samples, Bosio and co-workers reported that the key compounds that presented anti-bacterial activity against Staphylococcus pyogenes were galangin and pinocembrin (Bosio et al. 2000). Moreover, propolis exhibited anti-microbial activity against S. aureus through translation inhibition processes (Grecka et al. 2019). Conclusively, it has been observed that the antibacterial activities of propolis is comparable or better than commercially available antibiotics (Muli and Maingi 2007).
Anti-fungal
Anti-fungal effects of propolis have been reported in genera including Aspergillus, Furisia, Candida, Alternaría, Trichosporon, Rhodotorula, Saccharomyces, Cryptococcus, and Pseudoascus (Özcan 1999; Silici et al. 2005; Oliveira et al. 2006; Koc et al. 2007; Suleman et al. 2015; Ghosh et al. 2018; Bezerra et al. 2020). In one study, propolis moderately inhibited Furisia oxysporum spores probably due to the presence of phenolic acids and flavonoids (Petruzzi et al. 2020). These compounds act on F. oxysporum by making the cytoplasmic membrane permeable (Ahmed et al. 2008). Usually, this process will lead to the leakage of vital cellular components including proteins, nucleic acids and inorganic ions, which would induce cell death (Wang et al. 2021). Notably, propolis extracts in the study were highly effective to the fungal species than the bacterial counterparts (Petruzzi et al. 2020). Kambiz et al. (2013) reported that propolis extracts at a concentration of 0.125 g/L demonstrated effective anti-fungal activity against Aspergillus niger, which resulted in morphological changes on the cell membrane (Fig. 2D, E). In general, ethanol-based extracts of propolis tend to target cell membranes of fungi (Petruzzi et al. 2020).
Anti-fungal studies of propolis were generally focused on yeast-like fungi in the genus Candida, with most showing the anti-candidiasis effects of propolis (Yusoff et al. 2016; de Castro et al. 2013; Gucwa et al. 2018). Specifically, propolis extracts may inhibit the Candida switchover from yeast-like to hyphal growth, which effectively suppresses biofilm formation (de Castro et al. 2013; Tobaldini-Valerio et al. 2016). In such instances, the biofilm would aggregate as non-contiguous cells rather than pseudohyphae (Capoci et al. 2015). Elsewhere, propolis extracts exhibited higher anti-candidial activity compared to known anti-candida drugs such as azoles and amphotericin B (Gucwa et al. 2018). Another study hypothesized that chlorogenic acid, a derivative of caffeic acid, may disrupt the fungal cell membranes, leading to leakage of vital cytoplasmic contents (Sung and Lee 2010). Thus, there seems to be an inverse correlation between active compounds in propolis and anti-fungal activity (Fig. 2D, E). Phenolic compounds, on the other hand, could inhibit fungal growth by suppressing mitochondrial activity in the fungus (Gallucci et al. 2014).
Anti-viral
Besides anti-microbial activity against bacteria and fungi, propolis extracts have further demonstrated activity against viruses. For instance, such anti-viral effects were observed against viruses including herpes simplex virus (HSV), human rhinovirus, influenza A and B viruses, adenovirus, polio virus, rotavirus, human immune deficiency virus (HIV), human papillomavirus, vesicular stomatitis virus, Newcastle disease virus, and coronavirus (Debiaggi et al. 1990; Iljazović et al. 2006; Shimizu et al. 2008, 2011; Búfalo et al. 2009; da Silva et al. 2019; Kwon et al. 2020; Fiorini et al. 2021). In fact, current scientific evidence supports the effectiveness of propolis to inhibit and/or kill viruses (Fiorini et al. 2021).
Various compounds in propolis extracts show anti-viral properties against different viruses. Yildirim et al. (2016) showed that artepillin C and chrysin affect the replication of herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) by targeting the viral DNA polymerase (Shnitzler et al. 2008; Coelho et al. 2015; Fiorini et al. 2021) (Fig. 2F,G). Actually, propolis extracts have higher anti-viral activities against HSV-1 and HSV-2, as compared to acyclovir (Shimizu et al. 2011). The anti-viral activity of propolis against human rhinoviruses was putatively caused by gallic acid (Sithisarn et al. 2013; Khalil and Tazeddinova 2020; Kwon et al. 2020), whose mode of action relates to the inhibition of virus adsorption (Choi et al. 2010). Kai and co-workers similarly demonstrated the anti-viral activity of propolis against influenza virus strains, both in vitro and in vivo (Kai et al. 2014). The authors found that kaempferol inhibits viral growth by suppressing viral replication (Kai et al. 2014; Zhang et al. 2017a). Typically, kaempferol acts on the viral neuraminidase protein as described by Jeong et al. (2009) and Sithisarn et al. 2013 (Fig. 2F,G). Regarding HIV, Harish et al. (1997) reported that propolis could suppress HIV-1 replication processes probably due to the kaempferol. In the same way, propolis extracts at concentrations up to 66.6 µg/mL inhibited HIV activity in CD4+ cells and microglial cell cultures (Gekker et al. 2005). In addition, the compound acts by inhibiting the viral reverse transcriptase activity (Behbahani et al. 2014; Zakaryan et al. 2017) (Fig. 2F,G). Altogether, these anti-viral activity have the potential for effective and novel properties against a variety of viruses including coronavirus. Indeed, propolis acts against cutaneous warts with an efficacy of up to 75% (Zedan et al. 2009). In this instance, propolis acted as an immunomodulator to lessen warts.
The necessity of propolis in mitigating COVID-19
With over 231,091,474 confirmed cases (Worldometer 2021), the worldwide pandemic of COVID-19 has become a highly infectious disease that attributed to the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) pathogen (Goyal and Goyal 2020; Maaroufi 2020). Currently, 4,736,031 people have lost their lives due to the virus (Worldometer 2021) and the pandemic is recognized as having a significant impact on economic sectors involving human health, global finances as well as the environment (Cheval et al. 2020; Cullen et al. 2020; Gautam and Hens 2020; Hassan et al. 2020). It is believed that this virus is more deadly than other types of viruses causing common diseases (Vardeny et al. 2020). Around the world, several unusual measures have taken place, including the long-term closure of commercial businesses, educational institutions, and tourism sites (Yang and Wong 2020; Pokhrel and Chhetri 2021; Barua 2020). In an attempt to recover from disruptions caused by COVID-19 and mitigate against the impact of the pandemic, several biomedical health strategies have been implemented. These include the production and provision of vaccines (Koff et al. 2021; Onyeaka et al. 2021) as well as the implementation of numerous protective measures aimed at preventing the continuous spread of the disease (Pradhan et al. 2020). Although the production of vaccines is increasing, the demand for these vaccines is massive and many challenges remain (Koff et al. 2021; Onyeaka et al. 2021). Simultaneously, some preventive measures have limited efficacy (e.g., insert), which further lead to an increase in viral spreading (Corburn et al. 2020; Pereira et al. 2020; Setti et al. 2020; Lapolla et al. 2021). A number of alternative medicinal treatment regimens have been proposed and investigated, but most of these options need further validation and approval before being distributed and utilized (Sanders et al. 2020).
While a precise treatment plan for COVID-19 patients is presently lacking, consistent amendment of existing strategies is the utmost requirement in clinical treatment scenarios. Remedies including the use of blood transfusion-related technologies, convalescent plasma transfusion, anti-malarial agents, antibiotics, anti-viral therapy, and immunomodulatory agents have been approved and implemented in current medical practices (Li et al. 2020b). Translational research has concurrently been performed to develop a safe vaccine that can be used for combating SARS-CoV-2 infections globally (Moore and Klasse 2020). Furthermore, therapies involving plants and novel organic molecules sourced from different plants have crucially demonstrated repressive antiviral activity against SARS-CoV-2 (Serkedjieva et al. 1992; Calixto 2000; 2005; Maruta and He 2020; Orhan and Senol Deniz 2020).
Case studies on the usage of propolis against SARS-CoV-2 virus
Fiorini et al. 2021 (Fiorini et al. 2021) investigated the case of a 52 year-old woman who had tested positive for COVID-19. The patient suffered from common symptoms of COVID-19 including malaise, sore throat, and headache. She did not have any history of persistent illnesses. Blood tests yielded results that were within a usual reference range. As is generally recommended, the woman was advised to quarantine at home for 14 days. During that period, she consumed Brazilian green propolis at a dosage of 45 drops, thrice per day for the entire 14 days, along with healthy food and sufficient hydration. Eventually, the patient recovered from the viral infection by testing negative through the reverse transcription-polymerase chain reaction (RT-PCR) assay for SARS-CoV-2. Although the relevance of propolis and its therapeutic intervention is debatable as these results are based on a single case, it can still be argued that propolis may have offered some therapeutic benefits to the patient. Ali et al. (Ali and Kunugi 2021) theorized that among COVID-19 patients, propolis, and mixtures of honey associated with enhanced viral clearance and recovery from symptoms. In addition, the patients were overall discharged from healthcare facilities earlier than other patients and presented low mortality rates. However, since a combination of bee products were used, the performance of propolis in the treatment remains unclear. As such, the effect of individual bee products must be further investigated.
Possible mode of propolis action against SARS-COV viruses
Propolis contains various compounds that are responsible for different pharmacological properties. Among these, notable compounds are the hydroxycinnamic acids (p-coumaric acid, ferulic acid), phenols, and terpenoids. Additionally, there are also flavonoids such as quercetin, t-farnesol, caffeic acid fenethyl ester (CAPE), apigenin, luteolin, artepillin C, resveratrol, withanone, naringenin as well as caretonoids such as lutein (Chirumbolo 2011; Salonen et al. 2012; Kumazawa 2018).
Various components in propolis have been shown to interact with the SARS-CoV-2 virus. For example, Kumar et al. (Kumar et al. 2020) presented models showing the interaction between CAPEs and the SARS-CoV-2 protease to cause an inhibitory effect on the virus. Similarly, in vitro assays involving quercetin showed that it inhibited proteases in SARS-CoV-1 and MERS-CoV (Bachevski et al. 2020). CAPE further inhibits NF-kB (nuclear factor of kappa chains of B cells) molecules, which are responsible for controlling the transcription and regulation of immune response mechanism to viral antigens. This eventually increases the production of lymphocytes and antibodies, in accordance with in vitro studies. CAPE has also been shown to possess anti-inflammatory activity, reduces cytokine production and inhibits PAK1 (activated protein kinase) by blocking the ACE1 (angiotensin converting enzyme1) and the virus's main protease enzyme called Mpro. In addition, CAPE inhibits type II transmembrane serine proteases (TMPRSS2), whose main function is to facilitate viral entry into the cell. Elsewhere, CAPE seems to inhibit pulmonary fibrosis that is produced by coronaviruses (Maruta and He 2020). Quercetin, on the other hand, could have an inhibitory effect on SARS-CoV-2 by inhibiting the viral polymerase and further prevent replication when in combination with vitamin C. Additionally, quercetin and zinc are capable of blocking viral reproduction (Bachevski et al. 2020; Biancatelli et al. 2020) (Fig. 3).
Fig. 3.
Molecular mechanisms of propolis for the anti-SARS-COV-2 activities. Depicts the major pathways that propolis derived components interacts and inhibits the SARS-CoV-2 to the host cell, their replication and pathophysiological consequences. SARS-CoV-2 spike protein binds to the ACE2 receptor of the target host cell and gets activated by TMPRSS2. Following the binding, the viral endocytosis occurs followed by the activation of the PAK1 that reduces the adaptive immune response and antibody production against the virus. PAK1 also stimulates CCL2 production causing lungs fibrosis. Concurrently, the viral infection also induces NF-kB activation, causing pro-inflammatory cytokines overproduction. Propolis active ingredients downregulate and ACE2 and TMPRSS2 that restrict the virus entry to the cell. Furthermore, they reduce the PAK1 activation, increasing the antibodies against SARS-CoV-2. Also, the CAPE inhibits the NF-kB causing monocyte/macrophage immodulation, reducing pro-inflammatory cytokine overproduction
Phenolic compounds in propolis seemingly stimulate the production of interferons, which act on different viruses including avian influenza, HSV, Newcastle, and HIV-1 (Amoros et al. 1992; Harish et al. 1997; Shimizu et al. 2008; Fan et al. 2011). In addition to antiviral activities, kaempferol also has anti-inflammatory properties, which emerge through the decrease of tumor necrosis factor-alpha and cytokine production. It may also reduce the expression of TMPRSS2 and inhibit viral attachment to the main ACE2 receptor on human T cells. Crucially, kaempferol also acts on the main protease Mpro of the SARS-COV viruses (Schwarz et al. 2014; Solnier and Fladerer 2020) (Fig. 3).
Hesperetin is one of the most active polyphenols in propolis that also occurs in citrus juice (Lu et al. 2004; Muhammad et al. 2019). Notably, it has affinity for the ACE2 receptor, whereby after attachment, it will interfere with viral entry into the host cell and also interact with the viral enzymes Mpro and/or 3 CL pro (Bellavite and Donzelli 2020; Haggag et al. 2020). On the other hand, the flavonoid naringenin exhibits antiviral effects by interfering with viral entry into the cell, and thereby preventing its replication and addition to inhibiting proteases as well as acting on ion channels (Alberca et al. 2020; Clementi et al. 2021). Resveratrol and pterostilbene have antiviral properties against HIV-1, influenza, dengue virus, Zika virus as well as MERS-CoV and SARS-CoV-2 (Chan et al. 2017). In vitro studies involving the two compounds indicate effective antiviral properties by directly intervening in viral replication and decreasing expression of the ACE2 and DDP4 receptor proteins, which are required for viral entry into the host cell (Chan et al. 2017; Mattio et al. 2020; ter Ellen et al. 2021). In summary, the antiviral mechanisms of these compounds are responsible for the inhibition of viral proteases, some of which are considered as part of therapeutic strategies in clinical trials with lopinavir-ritonavir (Hung et al. 2020) (Fig. 3).
Molecular simulations of propolis compounds interacting with SARS-CoV-2
Compounds extracted from natural products are of great interest in the treatment of various diseases. To this end, several authors investigated the efficiency of natural products as potential treatments against the SARS-CoV-2 viral infection using different approaches (Merarchi et al. 2021; Romeo et al. 2021). Among the natural products used as anti-SARS-CoV-2, compounds extracted from propolis have been reported to be very promising (Berretta et al. 2020). Experimental testing of all propolis compounds against SARS-CoV-2 will surely be time and resource consuming. Therefore, computational studies have been performed to quickly identify and assess the performance of some compounds in binding and inhibiting active sites of the SARS-CoV-2 virus. Most computational assessments were performed using molecular docking to calculate the binding energies of various compounds at the active sites of SARS-CoV-2 (Berretta et al. 2020).
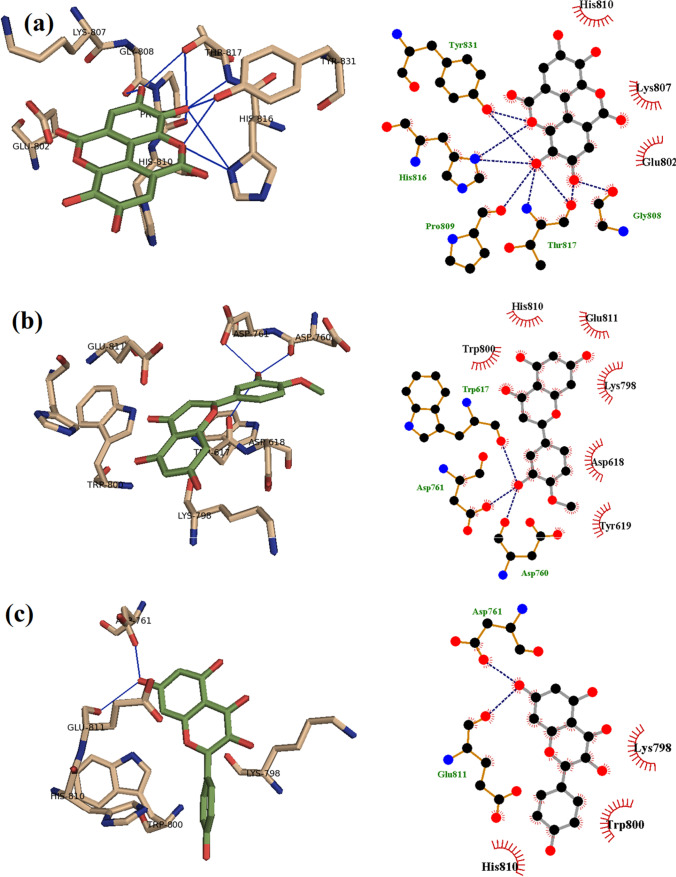
Ellagic acid extracted from honey and propolis has been found to form strong hydrogen bonds with Tyr831, Gly808, Thr817, His816, and Pro809 residues of the RNA-dependent RNA-polymerase in SARS-CoV-2 (Shaldam et al. 2021) (Fig. 4). Using molecular docking, it has been found that ellagic acid, p-coumaric acid, quercetin, and kaempferol strongly bind to the SARS-CoV-2 RNA-dependent RNA-polymerase. Specifically, ellagic acid has a highest binding score against the SARS-CoV-2 RNA-dependent RNA-polymerase, whereas kaempferol has showed the highest binding score against the main protease in SARS-CoV-2. In another study, Sahlan and co-workers performed molecular docking simulations to identify compounds in Sulawesi propolis that can inhibit the main protease in SARS-CoV-2 (Sahlan et al. 2019). They found that glyasperin A and broussoflavonol F can bind to the main protease strongly. Thus, the two compounds were identified as potential candidates for treatment against COVID-19 (Sahlan et al. 2019). Furthermore, extracts from Sulawesi propolis were further reported to be potential inhibitors of ACE2 through the molecular docking approach. Glyasperin A, sulabiroins A, broussoflavonol F, and isorhamnetin specifically showed the potential to inhibit ACE2 and SARS-CoV-2, with binding scores higher than 9.2 kcal/mol (Khayrani et al. 2021). Kumar et al. (Kumar et al. 2020) investigated the performance of aferine A and anone as anti-SARS-CoV-2 compounds. They found that both compounds can bind and inhibit the transmembrane protease serine 2 (TMPRSS2) (Kumar et al. 2020). Chrysin, another compound extracted from honey bee propolis, was also reported to interact with ACE2 and the SARS-CoV-2 spike protein through the molecular docking approach (Basu et al. 2020).
Fig. 4.
Binding of (a) ellagic acid, (b) hesperetin, and (c) kaempferol with SARS-CoV-2 RNA-dependent RNA polymerase, highlighting explicit hydrogen bonds established. Reproduced from (Shaldam et al. 2021)
Although molecular docking simulations and screenings are necessary for the initial assessment of compounds’ binding energies, further investigations are still needed to confirm the efficiency of identified compounds at the molecular level. Evidence gathered from the literature suggests that molecular dynamic simulations with proper choice of force fields have not been applied when assessing potential anti-SARS-CoV-2 compounds extracted from propolis. Thus, studies on molecular dynamic simulations with careful choice of fitted force fields will be awaited keenly. To achieve further accuracy, it may be worthwhile to examine dynamic interactions between compounds and viral active sites using combined quantum mechanical and molecular mechanical (QM/MM) simulations. QM/MM and molecular dynamic simulations with accurate force fields would be an ideal approach in providing reliable information regarding the binding efficiency of compounds extracted from propolis to SARS-CoV-2.
Propolis and comorbidities in COVID-19 patients
Comorbidities are key points of attention in COVID-19 treatment regimes. The death of many patients with comorbidities were attributed to multiple organ failure, shock, acute respiratory distress syndrome, and arrhythmias. Nevertheless, special attention is required to help patients with comorbidities in the treatment of COVID-19 (Wang et al. 2020).
Potentially, propolis raises the tolerance against COVID-19, which causes an exaggerated inflammatory process especially in the lungs. For instance, patients having comorbidities including hypertension, chronic kidney disease, dyslipidemia, chronic lung disease, allergic rhinitis, carcinoma, cardiovascular disease, diabetes, obesity, liver disease, and malignant tumors lack the tolerance towards COVID-19 that significantly attribute towards high mortality rate (Korish and Arafa, 2011). Besides, the aging process brings to the fore many issues related to comorbidities and leads to a destabilized immune system with reduced tolerance towards diseases. Cardioprotective properties of propolis has been linked to the in vitro reduction of calcium ion concentration-induced oxidation of low-density lipoprotein (LDL) (Claus et al. 2000). Propolis extracts reduce the activity of activated macrophages and expression of the matrix metalloproteinase-9 (MMP-9) gene that is involved in extracellular matrix (ECM) protein degradation that plays role in the pathogenicity during atherosclerosis (Galis et al. 1994). Polyphenols in propolis exhibited an inhibitory effect against platelet aggregation, thus, anticoagulation activity (Zhang et al. 2017b). Further, the antihypertensive effect of propolis has been proposed through the reduced tyrosine hydroxylase activity, a feat that will eradicate the over-activation of the sympathetic nervous system (Gogebakan et al. 2012), and lessens oxidative stress via lower expression of malondialdehyde (MDA) (Selamoglu Talas 2014).
The effectiveness of propolis in treating pre-diabetic and diabetic patients was achieved by decreasing the blood uric acid levels (Fukuda et al. 2015), and improving the antioxidant status (Zhao et al. 2016; Gao et al. 2018). The effect of propolis in treating chronic kidney disease entails the significant reduction of proteinuria and the urinary level of the inflammation marker monocyte chemoattractant protein-1 (MCP-1) and high-sensitivity C-reactive protein (hs-CRP) (Silveira et al. 2019; Silveira et al. 2020). Considering these findings, the World Health Organization (WHO) reported that propolis can be used alongside key drugs or vaccine adjuncts to treat infections as a risk-free product (Berretta et al. 2020).
Propolis is also known as a natural blocker for PAKl, the vital facilitator of the inflammatory process instigated by COVID-19. Therefore, it reduces or somehow eliminates the overproduction of pro-inflammatory cytokines such as interleukin 6, interleukin 1, and tumor necrosis factor (IL-6, IL-1, and TNF). Naggar et al. proposed that breathing aerosolized propolis may help with the prophylaxis against seasonal allergies, asthma as well as the treatment of comorbidities including diabetes, cardiovascular diseases, and obesity—with the comorbidities being conditions that triggers to more severe difficulties within COVID-19 (Al Naggar et al. 2021).
Propolis has the potential to be vaccine adjuvant
The COVID-19 pandemic has renewed research interest in propolis products as a potential adjuvant for vaccines worldwide. Data from pre-clinical investigations of propolis in vitro and in vivo (Sforcin 2016) suggests that propolis aids immuno-regulation of pro-inflammatory cytokines, which as a result, reduce the risk of a “cytokine storm syndrome”—a major mortality factor in advanced COVID-19 infections (Bachiega et al. 2012; Bufalo et al. 2014). Some proof-of-concept experiments showed that propolis stimulates the generation of reactive oxygen species (ROS), as well as enhancing the fungicidal (Murad et al. 2002) and bactericidal (Orsi et al. 2005) activity of these cytokines cells. Thus, propolis also possesses the potential to activate the mechanisms utilized in killing microorganisms. Standardized propolis products with consistent bioactive components are now available and sold commercially. These products are extensively used in traditional herbal medicine and widely consumed as health and immune system boosters in many countries (Kuropatnicki et al. 2013; Silva-Carvalho et al. 2015). For example, numerous companies in Brazil produce and sell propolis products such as throat sprays and extracts.
Owing to its immunoregulatory potential, propolis has been considered as an ideal adjuvant for many candidate vaccines, including COVID-19 vaccines. To increase immunogenicity and ensure long-term protection of vaccines, vaccines must readily associate with selected adjuvants (El Ashry and Ahmad 2012). However, due to their reported toxicity and reactogenicity, all available adjuvants have one or more adverse effects in patients. Some of the adverse effects include inflammation, pain, swelling, necrosis, ulcers, sterile abscesses, and systemic reactions such as nausea, pyrexia, allergy, eosinophilia, anaphylaxis, toxicity, adjuvant arthritis, and autoimmune diseases (Berretta et al. 2020). Ideal adjuvants should possess some ultimate characteristics such as being cost-effective, biodegradable, biologically inert, ability to boost cellular and humoral immune responses, and having a long shelf-life (Fan et al. 2015). Despite this, previous studies suggested considering the benefits of adjuvants and their adverse effects before use (Ma et al. 2011; Patel, 2016). New adjuvants have been approved for human use, but these require stricter regulation than those utilized in the veterinary field (Mojarab et al. 2020).
Since clinical investigations of propolis suggest that it possesses immunoregulatory, antiviral, and anti-inflammatory activities, it is therefore scientifically logical to also analyze propolis for the treatment of COVID-19 and as a potential adjuvant for COVID-19 vaccines. Adjuvant properties of propolis may enhance the individuals’ immune response. The synergistic interactions between current antiviral drugs and propolis components should be clinically evaluated. We envisage that propolis may benefit COVID-19-vaccinated individuals by affecting cell entry, the viral life cycle, effecting an anti-inflammatory action, controlling the cytokine storm, and by boosting the production of antibody and cell-mediated immunity.
Limitations of using propolis
The limited number of reports and comparative studies that contain both biological activity and chemical composition of propolis from different climatic zones is a significant gap in propolis research (Katekhaye et al. 2019). Furthermore, propolis research and publications have been concentrated in a small number of countries. The majority of research has focused on propolis from Brazil and Europe. There have been, however, a few reports on propolis from Africa, New Zealand, and South Asia.
Further, the motivation for performing clinical trials frequently appears to be lacking or misdirected. This is most likely due to a lack of coordination among research initiatives as well as defined research objectives. The biological activities of propolis are being randomly tested by different research organizations, with little or no relevance to chemical composition. In addition, the small number of clinical studies is discouraging. Considering that propolis is recognized as having antibacterial properties in virtually all climatic zones, it is remarkable that so few clinical trials have been conducted. Propolis can reverse antibacterial resistance and lead to lower antibiotic doses through synergistic mechanisms, as well as reduce the likelihood of antibiotic resistance developing (Wojtyczka et al. 2013). These findings, however, must still be backed by clinical studies.
Another limitation in propolis research is inconsistencies in biological assessments. The antibacterial activity of propolis is the most essential of its many biological functions, especially in light of the growing problem of bacterial resistance to many treatments. Although the indicated findings in this review article are encouraging, the assessment approaches utilized in different studies vary greatly in terms of their concepts, analysis methods and research output. It is quite difficult to compare the outcomes of various procedures (Katekhaye et al. 2019). The antibacterial action of propolis is most typically measured using four approaches, namely, bio-autography, agar diffusion, agar dilution, and serial dilution assay (Seidel et al. 2008). Agar diffusion and bio-autography assays are typically presented as measurements in millimeters (mm), whereas broth dilution assays are published as minimum inhibition concentration (MIC) (Seidel et al. 2008). Aside from this fundamental difference, each approach has its own set of limitations. With propolis having a highly complex mixture of compounds with variable polarity, the diffusion method is not suitable for comparing propolis samples because results are directly influenced by the solubility of constituents in the agar media (Seidel et al. 2008). In addition, the choice of microorganisms to test against is also a challenge. Conclusion?
In today’s era of modern techniques like liquid chromatography-evaporative light scattering detector (LC-ELSD), liquid chromatography-mass spectrometry (LC–MS/MS), liquid chromatography-nuclear magnetic resonance (LC-NMR), gas chromatography-mass spectrometry (GC–MS), high-performance thin-layer chromatographic (HPTLC), and other analytical techniques, it is difficult to apply a single standardized approach to propolis research due to the diverse chemical structures, molecular weight, and polarity of constituents (Katekhaye et al. 2019). This has led to inconsistencies in analytical investigations, and really, a limitation since it is technically challenging to combine data based on multiple analytical techniques and principles to detect certain types of biological components (Ni et al. 2007). For instance, the GC–MS reference library pool focuses on small-molecular-weight primary metabolites such as organic and aliphatic acids, sugars, and amino acids. LC–MS, on the other hand, can detect large, hydrophobic secondary metabolites including alkaloids, terpenoids, and phenols (Liu et al. 2017). Thus, when the total content of these constituents is reported and quantified using a reference library pool, there may be differences in the results from the two techniques. To overcome this constraint, the analysis of propolis based on a variety of approaches is necessary.
Future prospects (using nutraceuticals instead of pharmaceuticals)
Natural products, such as propolis, have long been employed as substitutes for pharmaceuticals in the medical field (Silva-Carvalho et al. 2015). Due to their synergistic effects, the complex mixture of propolis components may exude more health advantages than would be apparent by evaluating the individual impacts of components. The current method for approving pharmaceuticals has some limitations and these include the long lead time and significant expenditure required to discover new alternatives. In addition, time is also required to test pharmaceutical drugs for safety and effectiveness, and receive clearance for their usage after 5–10 years (Berretta et al. 2020). Potentially valuable material may never become available due to the enormous expenses required during this process and the likelihood that this lengthy procedure may not result in a product that will compensate for the investment required. Another issue is that modern medicine may be highly expensive. As a result, proper healthcare may not be available to everyone who requires it (Berretta et al. 2020).
Natural products can have numerous active ingredients, but modern pharmaceuticals typically have only one or a handful. Propolis, for example, contains hundreds of constituents (Berretta et al. 2020), many of which have the potential to help treat various forms of disease or have different mechanisms of action against a specific disease and its repercussions (de Mendonca et al. 2015). These potentials have not truly been investigated. According to Sadhana et al. (2017), Indian propolis has a high nutraceutical value and is free of pesticides and heavy metals. The propolis extract was standardized using an innovative high-performance thin-layer chromatographic (HPTLC) method that targeted markers including caffeic acid phenethyl ester, caffeic acid, galangin, luteolin, curcumin, apigenin, pinocembrin, and quercetin. Therefore, the indicated analytical approach can be used to screen propolis quality in the future.
Conclusion
The COVID-19 pandemic instigated by SARS-CoV-2 appears to be one of the deadliest that has occurred in the recent past. This pandemic is characterized by millions of human deaths and disrupted virtually all economic sectors across the globe. Preventive measures have been introduced worldwide to combat the spread of the SARS-CoV-2 virus along with special efforts dedicated to the swift production and provision of vaccines. However, vaccines are not universally accessible, and therefore, making it crucial to explore alternative options that can assist in reducing the spread of infections and COVID-19. To this end, some researchers are now advocating the use of a natural product known as propolis as part of the treatment regimen. Active compounds in propolis (including phenols, flavonoids, and terpenoids) are known to possess various pharmacological activities against various human diseases. Key among these functions are the antibacterial, antifungal, and antiviral properties. These properties are effected when active compounds interfere with, among other roles, viral entry into host cells and inhibit viral polymerase. The current study reviewed possible modes of propolis action when effecting various roles as well as being a possible adjunct in COVID-19 treatments. While special efforts are dedicated toward studying the properties of propolis in traditional medicine, further research is however still required to completely understand the underlying molecular mechanisms of propolis when inhibiting different microbes. It is currently predicted that the COVID-19 pandemic will last a few more years, and therefore, the use of alternative medicine including propolis may feature strongly in minimizing the spread of COVID-19 and act as an adjunct to treatment regimens.
Acknowledgements
We are thankful to Dr. Swagata Ghosh, Assistant Professor of English, Kumaraguru College of Arts and Science, Coimbatore, India, for editing this manuscript.
Author contribution
Conceptualization, methodology, S. G., Z.T.A.S., M. M., H.O.; visualization, investigation, S.G., M.M., A.M., L.G., M.M.; software, supervision, S.G., M.H.D., M.R.R., S.A., S.Z.A.N.; writing—original draft, S.G., N.T. A.S., S.M.A.; validation, writing—review & editing, M.H.D., S.G., M.F.M
Data availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelrahman Z, Li MY, Wang XS (2020) Comparative review of SARS-CoV-2, SARS-CoV, MERS-CoV, and influenza A respiratory viruses. Front Immunol 11:2309. [DOI] [PMC free article] [PubMed]
- Abdullah NA, Ja'afar F, Yasin HM, Taha H, Petalcorin MIR, Mamit MH, Kusrini E, Usman A. Physicochemical analyses, antioxidant, antibacterial, and toxicity of propolis particles produced by stingless bee Heterotrigona itama found in Brunei Darussalam. Heliyon. 2019;5(9):e02476–e02476. doi: 10.1016/j.heliyon.2019.e02476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahangari Z, Naseri M, Vatandoost F. Propolis: chemical composition and its applications in endodontics. Iran Endod J. 2018;13:285–292. doi: 10.22037/iej.v13i3.20994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SD, Mohanad AK, Zaid NH. Study antifungal activity of ethanol extract propolis against Fusarium oxysporum fungi. J Res Diyala Human. 2008;24:87–98. [Google Scholar]
- Al-Ani I, Zimmermann S, Reichling J, Wink M. Antimicrobial activities of European propolis collected from various geographic origins alone and in combination with antibiotics. Medicines. 2018;5:1–17. doi: 10.3390/medicines5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ani I, Zimmermann S, Reichling J, Wink M. Antimicrobial activities of European propolis collected from various geographic origins alone and in combination with antibiotics. Medicines (basel, Switzerland) 2018;5(1):2. doi: 10.3390/medicines5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Naggar Y, Yahya G, Al-Kahtani S, Stangaciu S. Back to ancient remedy: could inhalation of aerosolised-honey and propolis tincture protect against the COVID-19 pandemic? J Apitherapy. 2021;8(2):1–5. [Google Scholar]
- Alberca RW, Teixeira FME, Beserra DR, De Oliveira EA, Andrade MMD, Pietrobon AJ, Sato MN (2020) Perspective: the potential effects of naringenin in COVID-19. Front Immunol 11:570919 [DOI] [PMC free article] [PubMed]
- Alhouri AL, Salloum A, Harfouch RM, Ghosh S. Possible side effects of using detergents during the Covid19 pandemic in Syria. Annals Clinical Cases. 2020;1:071–074. [Google Scholar]
- Ali AM, Kunugi H. Bee honey protects astrocytes against oxidative stress: a preliminary in vitro investigation. Neuropsychopharmacol Rep. 2019;39:312–314. doi: 10.1002/npr2.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AM, Kunugi H (2020a) Apitherapy for age-related skeletal muscle dysfunction (Sarcopenia): a review on the effects of royal jelly, propolis, and bee pollen. Foods 9(10):1362 [DOI] [PMC free article] [PubMed]
- Ali AM, Kunugi H (2020b) Apitherapy for Parkinson's disease: a focus on the effects of propolis and royal jelly. Oxid Med Cell Longev. 10.1155/2020/1727142 [DOI] [PMC free article] [PubMed]
- Ali AM, Kunugi H. Propolis, bee honey, and their components protect against coronavirus disease 2019 (COVID-19): a review of in silico, in vitro, and clinical studies. Molecules. 2021;26(5):232. doi: 10.3390/molecules26051232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almuhayawi MS. Propolis as a novel antibacterial agent. Saudi J Biol Sci. 2020;27:3079–3086. doi: 10.1016/j.sjbs.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoros M, Simoes CMO, Girre L, Sauvager F, Cormier M. Synergistic effect of flavones and flavonols against herpes-simplex virus type-1 in cell-culture - comparison with the antiviral activity of propolis. J Nat Prod. 1992;55:1732–1740. doi: 10.1021/np50090a003. [DOI] [PubMed] [Google Scholar]
- Anand P, Stahel VP. Review the safety of Covid-19 mRNA vaccines: a review. Patient Saf Surg. 2021;15(1):1–9. doi: 10.1186/s13037-021-00291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjum SI, Ullah A, Khan KA, Attaullah M, Khan H, Ali H, Bashir MA, Tahir M, Ansari MJ, Ghramh HA, Adgaba N, Dash CK. Composition and functional properties of propolis (bee glue): a review. Saudi J Biol Sci. 2019;26:1695–1703. doi: 10.1016/j.sjbs.2018.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansorge S, Reinhold D, Lendeckel U. Propolis and some of its constituents down-regulate DNA synthesis and inflammatory cytokine production but induce TGF-beta 1 production of human immune cells. Zeitschrift Fur Naturforschung Section C-a Journal of Biosciences. 2003;58:580–589. doi: 10.1016/j.sjbs.2018.08.013. [DOI] [PubMed] [Google Scholar]
- Bachevski D, Damevska K, Simeonovski V, Dimova M (2020) Back to the basics: propolis and COVID-19. Dermatol Therapy 33(4):e1380. 10.1111/dth.13780 [DOI] [PMC free article] [PubMed]
- Bachiega TF, Orsatti CL, Pagliarone AC, Sforcin JM. The effects of propolis and its isolated compounds on cytokine production by murine macrophages. Phytother Res. 2012;26:1308–1313. doi: 10.1002/ptr.3731. [DOI] [PubMed] [Google Scholar]
- Baird A, Faisal M. Fathead minnow nidovirus infects spotfin shiner Cyprinella spiloptera and golden shiner Notemigonus crysoleucas. Dis Aquat Org. 2016;119:37–44. doi: 10.3354/dao02970. [DOI] [PubMed] [Google Scholar]
- Bakkali F, Averbeck S, Averbeck D, Waomar M. Biological effects of essential oils - a review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- Bankova V. Recent trends and important developments in propolis research. Evid Based Complementary Alternative Med. 2005;2:29–32. doi: 10.1093/ecam/neh059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankova V, Popova M, Trusheva B. Propolis volatile compounds : chemical diversity and biological activity : a review. Chem Cent J. 2014;8:1–8. doi: 10.1186/1752-153X-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barua S (2020) COVID-19 Pandemic and World Trade: Some Analytical Notes. SSRN Electron J 1–35. 10.2139/ssrn.3577627
- Basu A, Sarkar A, Maulik U (2020) Molecular docking study of potential phytochemicals and their effects on the complex of SARS-CoV2 spike protein and human ACE2. Sci Rep 10:17699. 10.1038/s41598-020-74715-4 [DOI] [PMC free article] [PubMed]
- Behbahani M, Sayedipour S, Pourazar A, Shanehsazzadeh M. In vitro anti-HIV-1 activities of kaempferol and kaempferol-7-O-glucoside isolated from Securigera securidaca. Res Pharm Sci. 2014;9:463–469. [PMC free article] [PubMed] [Google Scholar]
- Bellavite P, Donzelli A. Hesperidin and SARS-CoV-2: new Light on the Healthy Function of Citrus Fruits. Antioxidants. 2020;9(8):42. doi: 10.3390/antiox9080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyus J (2021)What do you mean by the term biomimicry? A conversation with Janine Benyus, author of biomimicry: innovation inspired by in nature
- Berretta AA, Arruda CFM, Baptista N, Nascimento A, Marquele- Oliveira F, Hori J, Barud H, Damaso B, Ramos C, Ferreira R, Bastos J. "Functional properties of Brazilian Propolis: from chemical composition until the market. In: Waisundara V, editor. Superfood and Functional Food - An Overview of Their Processing and Utilization. London: Intech Open; 2017. pp. 55–98. [Google Scholar]
- Berretta AA, Nascimento AP, Bueno PCP, Vaz MMDLL, Marchetti JM. Propolis standardized extract (EPP-AF (R)), an innovative chemically and biologically reproducible pharmaceutical compound for treating wounds. Int J Biol Sci. 2012;8:512–521. doi: 10.7150/ijbs.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta AA, Silveira MAD, Capcha JMC, De Jong D (2020) Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed Pharmacother 131.110622. 10.1016/j.biopha.2020.110622 [DOI] [PMC free article] [PubMed]
- Bezerra CRF, Borges KRA, Alves RDNS, Teles AM, Rodrigues IVP, Da Silva MACN, Nascimento MDDSB, De Barros Bezerra GF. Highly efficient antibiofilm and antifungal activity of green propolis against Candida species in dentistry materials. PLoS ONE. 2020;15:1–14. doi: 10.1371/journal.pone.0228828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biancatelli RMLC, Berrill M, Catravas JD, Marik PE (2020) Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 Related Disease (COVID-19). Front Immunol. 10.3389/fimmu.2020.01451 [DOI] [PMC free article] [PubMed]
- Boisard S, Shahali Y, Aumond MC, Derbre S, Blanchard P, Dadar M, Le Ray AM, Richomme P. Anti-AGE activity of poplar-type propolis: mechanism of action of main phenolic compounds. Int J Food Sci Technol. 2020;55:453–460. doi: 10.1111/ijfs.14284. [DOI] [Google Scholar]
- Boni MF, Lemey P, Jiang X, Lam TT-Y, Perry BW, Castoe TA, Rambaut A, Robertson DL. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat Microbiol. 2020;5:1408–1417. doi: 10.1038/s41564-020-0771-4. [DOI] [PubMed] [Google Scholar]
- Bosio K, Avanzini C, D'avolio A, Ozino O, Savoia D. In vitro activity of propolis against Streptococcus pyogenes. Lett Appl Microbiol. 2000;31:174–177. doi: 10.1046/j.1365-2672.2000.00785.x. [DOI] [PubMed] [Google Scholar]
- Bouarab-Chibane L, Forquet V, Lantéri P, Clément Y, Léonard-Akkari L, Oulahal N, Degraeve P, Bordes C (2019) Antibacterial properties of polyphenols: characterization and QSAR (Quantitative structure-activity relationship) models. Front Microbiol 10:829. 10.3389/fmicb.2019.00829 [DOI] [PMC free article] [PubMed]
- Breijyeh Z, Jubeh B, Karaman R. Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25:1–23. doi: 10.3390/molecules25061340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufalo MC, Bordon-Graciani AP, Conti BJ, Golim MD, Sforcin JM. The immunomodulatory effect of propolis on receptors expression, cytokine production and fungicidal activity of human monocytes. J Pharm Pharmacol. 2014;66:1497–1504. doi: 10.1111/jphp.12279. [DOI] [PubMed] [Google Scholar]
- Búfalo MC, Figueiredo AS, De Sousa JPB, Candeias JMG, Bastos JK, Sforcin JM. Anti-poliovirus activity of Baccharis dracunculifolia and propolis by cell viability determination and real-time PCR. J Appl Microbiol. 2009;107:1669–1680. doi: 10.1111/j.1365-2672.2009.04354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calixto JB. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents) Braz J Med Biol Res. 2000;33:179–189. doi: 10.1590/S0100-879X2000000200004. [DOI] [PubMed] [Google Scholar]
- Calixto JB. Twenty-five years of research on medicinal plants in Latin America - a personal view. J Ethnopharmacol. 2005;100:131–134. doi: 10.1016/j.jep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Campos JF, Dos Santos UP, Dos Santos Da Rocha P, Damião MJ, Perrella Balestieri JB, Lima Cardoso CA, Paredes-Gamero EJ, Estevinho LM, Souza KDP, Dos Santos EL (2015) Antimicrobial, antioxidant, anti-inflammatory and cytoxic activities of propolis from stingless bee Tetragonisca fiebrigi (Jataí). Evidence-based Complementary and Alternative Med. 10.1155/2015/296186 [DOI] [PMC free article] [PubMed]
- Capoci IRG, Bonfim-Mendonça PDS, Arita GS, Pereira RRDA, Consolaro MEL, Bruschi ML, Negri M, Svidzinski TIE. Propolis is an efficient fungicide and inhibitor of biofilm production by vaginal Candida albicans. Evidence-Based Complementary Alternative Med. 2015;2015:1–9. doi: 10.1155/2015/287693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella M, Rajnik M, Aleem A, Dulebohn S, Di Napoli R (2022) Features, evaluation, and treatment of coronavirus (COVID-19). StatPearls [PubMed]
- Chan CN, Trinite B, Levy DN (2017) Potent inhibition of HIV-1 replication in resting CD4 T cells by resveratrol and pterostilbene. Antimicrobial Agents and Chemotherapy 61(9):e00408-17. 10.1128/AAC.00408-17 [DOI] [PMC free article] [PubMed]
- Chan GCF, Cheung KW, Sze DMY. The immunomodulatory and anticancer properties of propolis. Clin Rev Allergy Immunol. 2013;44:262–273. doi: 10.1007/s12016-012-8322-2. [DOI] [PubMed] [Google Scholar]
- Cheng AX, Lou YG, Mao YB, Lu S, Wang LJ, Chen XY. Plant terpenoids: biosynthesis and ecological functions. J Integr Plant Biol. 2007;49:179–186. doi: 10.1111/j.1744-7909.2007.00395.x. [DOI] [Google Scholar]
- Cheval S, Adamescu CM, Georgiadis T, Herrnegger M, Piticar A, Legates DR (2020) Observed and potential impacts of the COVID-19 pandemic on the environment. Int J Environ Res Public Health 17(11):4140. 10.3390/ijerph17114140 [DOI] [PMC free article] [PubMed]
- Chirumbolo S. Propolis as anti-inflammatory and anti-allergic compounds: which role for flavonoids? Int Immunopharmacol. 2011;11:1386–1387. doi: 10.1016/j.intimp.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Song JH, Bhatt LR, Baek SH. Anti-human rhinovirus activity of gallic acid possessing antioxidant capacity. Phytother Res. 2010;24:1292–1296. doi: 10.1002/ptr.3101. [DOI] [PubMed] [Google Scholar]
- Claus R, Kinscherf R, Gehrke C, Bonaterra G, Basnet P, Metz J, Deigner H-P. Antiapoptotic effects of propolis extract and propol on human macrophages exposed to minimally modified low density lipoprotein. Arzneimittelforschung. 2000;50:373–379. doi: 10.1055/s-0031-1300216. [DOI] [PubMed] [Google Scholar]
- Clementi N, Scagnolari C, D'amore A, Palombi F, Criscuolo E, Frasca F, Pierangeli A, Mancini N, Antonelli G, Clementi M, Carpaneto A, Filippini A (2021) Naringenin is a powerful inhibitor of SARS-CoV-2 infection in vitro. Pharmacol Res 163:105255. 10.1016/j.phrs.2020.105255 [DOI] [PMC free article] [PubMed]
- Coelho RG, Mendonça RZ, De Senna Vilar K, Figueiredo CA, Badari JC, Taniwaki N, Namiyama G, De Oliveira MI, Curti SP, Evelyn Silva P, Negri G (2015) Antiviral action of hydromethanolic extract of geopropolis from Scaptotrigona postica against antiherpes simplex virus (HSV-1). Evidence-based Complementary and Alternative Medicine 2015. 10.1155/2015/296086 [DOI] [PMC free article] [PubMed]
- Corburn J, Vlahov D, Mberu B, Riley L, Caiaffa WT, Rashid SF, Ko A, Patel S, Jukur S, Martinez-Herrera E, Jayasinghe S, Agarwal S, Nguendo-Yongsi B, Weru J, Ouma S, Edmundo K, Oni T, Ayad H. Slum health: arresting COVID-19 and improving well-being in urban informal settlements. J Urban Health Bull New York Acad Med. 2020;97:348–357. doi: 10.1007/s11524-020-00438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen W, Gulati G, Kelly BD. Mental health in the COVID-19 pandemic COMMENT. Qjm-an Int J Med. 2020;113:311–312. doi: 10.1093/qjmed/hcaa110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva CCF, Salatino A, Da Motta LB, Negri G, Salatino MLF. Chemical characterization, antioxidant and anti-HIV activities of a Brazilian propolis from Ceará state. Rev Bras. 2019;29:309–318. [Google Scholar]
- De Carvalho Furtado Jr JH, Rocha Valadas LA, Mendonca KS, De Oliveira Filho RD, Gadelha LMU, De Mello Fiallos N, Neto EMR, De Mello Fiallos AC, De Franca Fonteles M.M. Propolis and its dental applications: a technological prospection. Recent Pat Biotechnol. 2018;12:288–296. doi: 10.2174/2211550107666180815114855. [DOI] [PubMed] [Google Scholar]
- De Castro PA, Bom VLP, Brown NA, De Almeida RSC, Ramalho LNZ, Savoldi M, Goldman MHS, Berretta AA, Goldman GH. Identification of the cell targets important for propolis-induced cell death in Candida albicans. Fungal Genet Biol. 2013;60:74–86. doi: 10.1016/j.fgb.2013.07.001. [DOI] [PubMed] [Google Scholar]
- De Mendonca ICG, Porto ICCD, Do Nascimento TG, De Souza NS, Oliveira JMD, Arruda RED, Mousinho KC, Dos Santos AF, Basilio ID, Parolia A, Barreto FS. Brazilian red propolis: phytochemical screening, antioxidant activity and effect against cancer cells. Bmc Complementary and Alternative Medicine. 2015;15(1):1–12. doi: 10.1186/s12906-015-0888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiaggi M, Tateo F, Pagani L, Luini M, Romero E. Effects of propolis flavonoids on virus infectivity and replication. Microbiologica. 1990;13:207–213. [PubMed] [Google Scholar]
- Decaro N (2011) Alphacoronavirus‡: Coronaviridae. The Springer Index of Viruses 371–33. 10.1007/978-0-387-95919-1_56
- Decaro N, Lorusso A. Novel human coronavirus (SARS-CoV-2): a lesson from animal coronaviruses. Vet Microbiol. 2020;244:108693. doi: 10.1016/j.vetmic.2020.108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa T, Ohno Y, Yokoyama S, Yokokawa T, Tsuda S, Goto K, Hayashi T. The protective effect of Brazilian propolis against glycation stress in mouse skeletal muscle. Foods. 2019;8(10):39. doi: 10.3390/foods8100439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ashry ESH, Ahmad TA. The use of propolis as vaccine's adjuvant. Vaccine. 2012;31:31–39. doi: 10.1016/j.vaccine.2012.10.095. [DOI] [PubMed] [Google Scholar]
- Enjuanes L, Gorbalenya A, De Groot RJ, Cowley JA, Ziebuhr J, Snijder EJ (2008) Enveloped, Positive-Strand RNA Viruses (Nidovirales). Encyclopedia of Virology 256-266. 10.1016/B978-0-12-814515-9.00775-X
- Falcao SI, Vale N, Gomes P, Domingues MRM, Freire C, Cardoso SM, Vilas-Boas M. Phenolic profiling of Portuguese propolis by LC-MS spectrometry: uncommon propolis rich in flavonoid glycosides. Phytochem Anal. 2013;24:309–318. doi: 10.1002/pca.2412. [DOI] [PubMed] [Google Scholar]
- Fan Y, Zhao K, Shi Z-L, Zhou P. Bat Coronaviruses in China Viruses. 2019;11:210. doi: 10.3390/v11030210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan YP, Guo LW, Hou WF, Guo C, Zhang WM, Ma X, Ma L, Song XP (2015) The adjuvant activity of epimedium polysaccharide-propolis flavone liposome on enhancing immune responses to inactivated porcine circovirus vaccine in mice. Evidence-Based Complementary and Alternative Medicine 2015:-9. 10.1155/2015/972083 [DOI] [PMC free article] [PubMed]
- Fan YP, Liu JG, Wang DY, Hu YL, Yang SJ, Wang JM, Guo LW, Zhao XN, Wang HL, Jiang Y. Epimedium polysaccharide and propolis flavone can synergistically inhibit the cellular infectivity of NDV and improve the curative effect of ND in chicken. Int J Biol Macromol. 2011;48:439–444. doi: 10.1016/j.ijbiomac.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Fernandes Jr A, Balestrin EC, Betoni JEC, De Oliveira Orsi R, De Souza Da Cunha MDLR, Montelli AC (2005) Propolis: anti-Staphylococcus aureus activity and synergism with antimicrobial drugs. Memorias do Instituto Oswaldo Cruz, Rio de Janeiro 100:563–566. 10.1590/S0074-02762005000500018 [DOI] [PubMed]
- Fiorini AC, Scorza CA, De Almeida ACG, Fonseca MCM, Finsterer J, Fonseca FLA, Scorza FA. Antiviral activity of Brazilian green propolis extract against sars-cov-2 (Severe acute respiratory syndrome-coronavirus 2) infection: Case report and review. Clinics. 2021;76:1–4. doi: 10.6061/clinics/2021/e2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Fukui M, Tanaka M, Senmaru T, Iwase H, Yamazaki M, Aoi W, Inui T, Nakamura N, Marunaka Y. Effect of Brazilian green propolis in patients with type 2 diabetes: A double-blind randomized placebo-controlled study. Biomedical Reports. 2015;3:355–360. doi: 10.3892/br.2015.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajger IT, Kosalec I, Bojic M, Kosalec I, Scecec S, Vlainic T, Vlainic J. The components responsible for the antimicrobial activity of propolis from continental and mediterranean regions in Croatia. Food Microbiol Saf. 2017;35:376–385. doi: 10.17221/103/2017-CJFS. [DOI] [Google Scholar]
- Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Investig. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallucci MN, Carezzano ME, Oliva MM, Demo MS, Pizzolitto RP, Zunino MP, Zygadlo JA, Dambolena JS. In vitro activity of natural phenolic compounds against fluconazole-resistant Candida species: a quantitative structure-activity relationship analysis. J Appl Microbiol. 2014;116:795–804. doi: 10.1111/jam.12432. [DOI] [PubMed] [Google Scholar]
- Gao W, Pu L, Wei J, Yao Z, Wang Y, Shi T, Zhao L, Jiao C, Guo C. Serum antioxidant parameters are significantly increased in patients with type 2 diabetes mellitus after consumption of Chinese propolis: a randomized controlled trial based on fasting serum glucose level. Diabetes Therapy. 2018;9:101–111. doi: 10.1007/s13300-017-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam S, Hens L. COVID-19: impact by and on the environment, health and economy. Environ Dev Sustain. 2020;22:4953–4954. doi: 10.1007/s10668-020-00818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekker G, Hu S, Spivak M, Lokensgard JR, Peterson PK. Anti-HIV-1 activity of propolis in CD4+ lymphocyte and microglial cell cultures. J Ethnopharmacol. 2005;102:158–163. doi: 10.1016/j.jep.2005.05.045. [DOI] [PubMed] [Google Scholar]
- Ghasemi FS, Eshraghi SS, Andalibi F, Hooshyar H, Kalantar N, Samadi A, Fatahi-Bafghi M. Anti-bacterial effect of propolis extract in oil against different bacteria. Zahedan J Res Med Sci. 2017;19:e7225–e7225. doi: 10.5812/zjrms.7225. [DOI] [Google Scholar]
- Ghosh S, Bornman C, Zafer MM. Antimicrobial resistance threats in the emerging COVID-19 pandemic: where do we stand? J Infect Public Health. 2021;14:555–560. doi: 10.1016/j.jiph.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Mcarthur RL, Guo ZC, Mckerchar R, Donkor K, Xu JP, Lausen C, Cheeptham N (2018) Evidence for anti-Pseudogymnoascus destructans (Pd) activity of propolis Antibiotics 7(1):1–12. 10.3390/antibiotics7010002 [DOI] [PMC free article] [PubMed]
- Gogebakan A, Talas ZS, Ozdemir I, Sahna E. Role of propolis on tyrosine hydroxylase activity and blood pressure in nitric oxide synthase-inhibited hypertensive rats. Clin Exp Hypertens. 2012;34:424–428. doi: 10.3109/10641963.2012.665542. [DOI] [PubMed] [Google Scholar]
- Goyal B, Goyal D. Targeting the dimerization of the main protease of coronaviruses: a potential broad-spectrum therapeutic strategy. ACS Comb Sci. 2020;22:297–305. doi: 10.1021/acscombsci.0c00058. [DOI] [PubMed] [Google Scholar]
- Grecka K, Kuś PM, Okińczyc P, Worobo RW, Walkusz J, Szweda P. The anti-staphylococcal potential of ethanolic Polish propolis extracts. Molecules. 2019;24:1–24. doi: 10.3390/molecules24091732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gucwa K, Kusznierewicz B, Milewski S, Van Dijck P, Szweda P (2018) Antifungal activity and synergism with Azoles of Polish propolis. Pathogens 7(2)56. 10.3390/pathogens7020056 [DOI] [PMC free article] [PubMed]
- Guler HI, Tatar G, Yildiz O, Belduz AO, Kolayli S. Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by molecular docking study. Arch Microbiol. 2021;203:3557–3564. doi: 10.1007/s00203-021-02351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gür N, Bayrak N, Topdemir A. Determination of antimicrobial activity and some biochemical properties of honey and propolis in Turkish markets. Prog Nutr. 2020;22:e2020040–e2020040. [Google Scholar]
- Haggag YA, El-Ashmawy NE, Okasha KM (2020) Is hesperidin essential for prophylaxis and treatment of COVID-19 Infection? J Glob 144. 10.1016/j.mehy.2020.109957 [DOI] [PMC free article] [PubMed]
- Harish Z, Rubinstein A, Golodner M, Elmaliah M, Mizrachi Y. Suppression of HIV-1 replication by propolis and its immunoregulatory effect. Drugs Exp Clin Res. 1997;23:89–96. [PubMed] [Google Scholar]
- Hashem HE. IN Silico Approach of some selected honey constituents as SARS-CoV-2 main protease (COVID-19) inhibitors. Eurasian J Med Oncol. 2020;4:196–200. [Google Scholar]
- Hassan MM, El Zowalaty ME, Khan SA, Islam A, Nayem MRK, Järhult JD. Role of environmental temperature on the attack rate and case fatality rate of coronavirus disease 2019 (COVID-19) Pandemic. Infect Ecol Epidemiol. 2020;10:1792620–1792620. doi: 10.1080/20008686.2020.1792620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KV (1999) Coronaviruses (Coronaviridae). Encyclopedia of Virology 291
- Hori JI, Zamboni DS, Carrao DB, Goldman GH, Berretta AA (2013) The Inhibition of Inflammasome by Brazilian Propolis (EPP-AF). Evidence-Based Complementary and Alternative Medicine 2013. 10.1155/2013/418508
- Hung IFN, Lung KC, Tso EYK, Liu R, Chung TWH, Chu MY, Ng YY, Lo J, Chan J, Tam AR, Shum HP, Chan V, Wu AKL, Sin KM, Leung WS, Law WL, Lung DC, Sin S, Yeung P, Yip CCY, Zhang RR, Fung AYF, Yan EYW, Leung KH, Ip JD, Chu AWH, Chan WM, Ng ACK, Lee R, Fung K, Yeung A, Wu TC, Chan JWM, Yan WW, Chan WM, Chan JFW, Lie AKW, Tsang OTY, Cheng VCC, Que TL, Lau CS, Chan KH, To KKW, Yuen KY. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igori D, Lim S, Kwon S-Y, Cho HS, Park JM, Kim H-S, Lee H-J, Lee S-H, Moon JS. Complete genome sequence and genome organization of achyranthes virus A, a novel member of the genus Potyvirus. Adv Virol. 2020;165:2695–2698. doi: 10.1007/s00705-020-04778-1. [DOI] [PubMed] [Google Scholar]
- Iljazović E, Ljuca D, Sahimpasić A, Avdić S. Efficacy in treatment of cervical HRHPV infection by combination of beta interferon, and herbal therapy in woman with different cervical lesions. Bosn J Basic Med Sci. 2006;6:79–84. doi: 10.17305/bjbms.2006.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JC, Kim MS, Kim TH, et al. Kaempferol induces cell death through Erk and Akt-dependent down-regulation of Xiap and Survivin in human Glioma cells. Neurochem Res. 2009;34:991–1001. doi: 10.1007/s11064-008-9868. [DOI] [PubMed] [Google Scholar]
- Kai H, Obuchi M, Yoshida H, Watanabe W, Tsutsumi S, Park YK, Matsuno K, Yasukawa K, Kurokawa M. In vitro and in vivo anti-influenza virus activities of flavonoids and related compounds as components of Brazilian propolis (AF-08) J Funct Foods. 2014;8:214–223. doi: 10.1016/j.jff.2014.03.019. [DOI] [Google Scholar]
- Kambiz D, Kamaleh G, Behnam H, Mitra S. Antifungal activity of Satureja hortensis alcoholic extract against Aspergillus and Candida species. J Med Plants Res. 2013;7(30):2271–2274. doi: 10.5897/JMPR12.659. [DOI] [Google Scholar]
- Karadal F, Onmaz NE, Abay S, Yildirim Y, Al S, Tatyuz I, Akcay A. Original article a study of antibacterial and antioxidant activities of bee products : propolis, pollen and honey samples. Ethiop J Heal Dev. 2018;32:116–122. [Google Scholar]
- Katekhaye S, Fearnley H, Fearnley J, Paradkar A. Gaps in propolis research: challenges posed to commercialization and the need for an holistic approach. J Apic Res. 2019;58:604–616. doi: 10.1080/00218839.2019.1614273. [DOI] [Google Scholar]
- Khalil A, Tazeddinova D. The upshot of Polyphenolic compounds on immunity amid COVID-19 pandemic and other emerging communicable diseases: an appraisal. Nat Prod Bioprospect. 2020;10:411–429. doi: 10.1007/s13659-020-00271-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayrani AC, Irdiani R, Aditama R, Pratami DK, Lischer K, Ansari MJ, Chinnathambi A, Alharbi SA, Almoallim HS, Sahlan M (2021) Evaluating the potency of Sulawesi propolis compounds as ACE-2 inhibitors through molecular docking for COVID-19 drug discovery preliminary study. J King Saud Univ Sci 33(2):101297. 10.1016/j.jksus.2020.101297 [DOI] [PMC free article] [PubMed]
- Koc AN, Silici S, Mutlu-Sariguzel F, Sagdic O. Antifungal activity of propolis in four different fruit juices. Food Technol Biotechnol. 2007;45:57–61. [Google Scholar]
- Kocot J, Kielczykowska M, Luchowska-Kocot D, Kurzepa J, Musik I (2018) Antioxidant potential of propolis, bee pollen, and royal jelly: possible medical application. Oxid Med Cell Longev 2018:1–29.ges. 10.1155/2018/7074209 [DOI] [PMC free article] [PubMed]
- Koff WC, Schenkelberg T, Williams T, Baric RS, Mcdermott A, Cameron CM, Cameron MJ, Friemann MB, Neumann G, Kawaoka Y, Kelvin AA, Ross TM, Schultz-Cherry S, Mastro TD, Priddy FH, Moore KA, Ostrowsky JT, Osterholm MT, Goudsmit J (2021) Development and deployment of COVID-19 vaccines for those most vulnerable. Sci Transl Med 13(579). 10.1126/scitranslmed.abd1525 [DOI] [PubMed]
- Korish AA, Arafa MM. Propolis derivatives inhibit the systemic inflammatory response and protect hepatic and neuronal cells in acute septic shock. Braz J Infect Dis. 2011;15:332–338. doi: 10.1016/S1413-8670(11)70201-X. [DOI] [PubMed] [Google Scholar]
- Kreps SE, Kriner DL. Factors influencing Covid-19 vaccine acceptance across subgroups in the United States: Evidence from a conjoint experiment. Vaccine. 2021;39:3250–3258. doi: 10.1016/j.vaccine.2021.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Dhanjal JK, Bhargava P, Kaul A, Wang J, Zhang H, Kaul SC, Wadhwa R, Sundar D. Withanone and Withaferin-A are predicted to interact with transmembrane protease serine 2 (TMPRSS2) and block entry of SARS-CoV-2 into cells. J Biomol Struct Dyn. 2020;40(1):1–13. doi: 10.1080/07391102.2020.1775704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa S (2018) Bioactive compounds in bee propolis for drug discovery. In: AIP Conference Proceedings. AIP Publishing LLC. 10.1063/1.5023948
- Kuropatnicki AK, Szliszka E, Krol W (2013) Historical aspects of propolis research in modern times. Evidence-Based Complementary and Alternative Medicine. 10.1155/2013/964149 [DOI] [PMC free article] [PubMed]
- Kwon MJ, Shin HM, Perumalsamy H, Wang X, Ahn YJ. Antiviral effects and possible mechanisms of action of constituents from Brazilian propolis and related compounds. J Apic Res. 2020;59:413–425. doi: 10.1080/00218839.2019.1695715. [DOI] [Google Scholar]
- Langenheim JH. Higher-plant terpenoids - a phytocentric overview of their ecological roles. J Chem Ecol. 1994;20:1223–1280. doi: 10.1007/BF02059809. [DOI] [PubMed] [Google Scholar]
- Lapolla P, Mingoli A, Lee R. Deaths from COVID-19 in healthcare workers in Italy-What can we learn? Infect Control Hosp Epidemiol. 2021;42:364–365. doi: 10.1017/ice.2020.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Jeong SH, Cha SS, Lee SH. A lack of drugs for antibiotic-resistant Gram-negative bacteria. Nat Rev Drug Discovery. 2007;6:938–939. doi: 10.1038/nrd2201-c1. [DOI] [Google Scholar]
- Li H, Liu S-M, Yu X-H, Tang S-L, Tang C-K. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020;55:105951. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xie SY, Ahmed S, Wang FN, Gu YF, Zhang CN, Chai XM, Wu YL, Cai JX, Cheng GY (2017) Antimicrobial activity and resistance: influencing factors. Front Pharmacol 8. 10.3389/fphar.2017.00364 [DOI] [PMC free article] [PubMed]
- Li Y, Liu SL, Zhang SC, Ju Q, Zhang SQ, Yang YM, Wang HY (2020b) Current treatment approaches for COVID-19 and the clinical value of transfusion-related technologies. Transfus Apher Sci 59(5):102839. 10.1016/j.transci.2020.102839 [DOI] [PMC free article] [PubMed]
- Liu J, Liu Y, Wang Y, Abozeid A, Zu Y-G, Tang Z-H. The integration of GC–MS and LC–MS to assay the metabolomics profiling in Panax ginseng and Panax quinquefolius reveals a tissue- and species-specific connectivity of primary metabolites and ginsenosides accumulation. J Pharm Biomed Anal. 2017;135:176–185. doi: 10.1016/j.jpba.2016.12.026. [DOI] [PubMed] [Google Scholar]
- Liu T, Huang P, Liu H, Huang L, Lei M, Xu W, Hu X, Chen J, Liu B (2020) Spectrum of chest CT findings in a familial cluster of COVID-19 infection. Radiology: Cardiothoracic Imaging 2: e200025 [DOI] [PMC free article] [PubMed]
- Lu RJ, Zhao X, Li J, Niu PH, Yang B, Wu HL, Wang WL, Song H, Huang BY, Zhu N, Bi YH, Ma XJ, Zhan FX, Wang L, Hu T, Zhou H, Hu ZH, Zhou WM, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan JY, Xie ZH, Ma JM, Liu WJ, Wang DY, Xu WB, Holmes EC, Gao GF, Wu GZ, Chen WJ, Shi WF, Tan WJ. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wu C, Yuan Z. Determination of hesperetin, cinnamic acid and nicotinic acid in propolis with micellar electrokinetic capillary chromatography. Fitoterapia. 2004;75:267–276. doi: 10.1016/j.fitote.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Lyoo YS. Porcine reproductive and respiratory syndrome virus vaccine does not fit in classical vaccinology. Clin Exp Vaccine Res. 2015;4:159–165. doi: 10.7774/cevr.2015.4.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Guo ZH, Shen ZQ, Wang JL, Hu YL, Wang DY. The immune enhancement of propolis adjuvant on inactivated porcine parvovirus vaccine in guinea pig. Cell Immunol. 2011;270:13–18. doi: 10.1016/j.cellimm.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Maaroufi H (2020) The spike protein S1 subunit of SARS-CoV-2 contains an LxxIxE-like Motif that is known to recruit the host PP2A-B56 phosphatase. bioRxiv, 2020.2004.2001.020941
- Machado JL, Assuncao AKM, Da Silva MCP, Dos Reis AS, Costa GC, Arruda DD, Rocha BA, Vaz MMDLL, Paes AMD, Guerra RNM, Berretta AA, Do Nascimento FRF (2012) Brazilian green propolis: anti-inflammatory property by an immunomodulatory activity. Evidence-Based Complementary and Alternative Medicine 2012:157652. 10.1155/2012/157652 [DOI] [PMC free article] [PubMed]
- Mahmoud UT, Cheng HW, Applegate TJ. Functions of propolis as a natural feed additive in poultry. Worlds Poult Sci J. 2016;72:37–47. doi: 10.1017/S0043933915002731. [DOI] [Google Scholar]
- Mani JS, Johnson JB, Steel JC, Broszczak DA, Neilsen PM, Walsh KB, Naiker M (2020) Natural product-derived phytochemicals as potential agents against coronaviruses: a review. Virus Res 15:284. 10.1016/j.virusres.2020.197989 [DOI] [PMC free article] [PubMed]
- Maruta H, He H. PAK1-blockers: potential therapeutics against COVID-19. Med Drug Discov. 2020;6:100039. doi: 10.1016/j.medidd.2020.100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattio LM, Catinella G, Pinto A, Dallavalle S (2020) Natural and nature-inspired stilbenoids as antiviral agents. Eur J Med Chem 202:12541. 10.1016/j.ejmech.2020.112541 [DOI] [PMC free article] [PubMed]
- Merarchi M, Dudha N, Das BC, Garg M. Natural products and phytochemicals as potential anti-SARS-CoV-2 drugs. Phytotherapy Res. 2021;35(10):5384–5396. doi: 10.1002/ptr.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meskini M, Rezghi Rami M, Maroofi P, Ghosh S, Siadat SD, Sheikhpour M. An overview on the epidemiology and immunology of COVID-19. J Infect Public Health. 2021;14(10):1284–1298. doi: 10.1016/j.jiph.2021.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel MG, Nunes S, Dandlen SA, Cavaco AM, Antunes MD. Phenols, flavonoids and antioxidant activity of aqueous and methanolic extracts of propolis (Apis mellifera L.) from Algarve South Portugal. Food Sci Technol. 2014;34:16–23. doi: 10.1590/S0101-20612014000100002. [DOI] [Google Scholar]
- Miller SI. Antibiotic resistance and regulation of the Gram-negative bacterial outer membrane barrier by host innate immune molecules. mBio. 2016;7:e01541–01516. doi: 10.1128/mBio.01541-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Meskini M, Do Nascimento Pinto AL. 2019 Novel coronavirus (COVID-19) overview. Z Gesundh Wiss. 2022;30(1):167–175. doi: 10.1007/s10389-020-01258-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadbeigi M, Koshkohi SA, Meskini M. An overview on wearing the face mask to avoid transmission of coronavirus disease 2019. Rev Med Microbiol. 2020;31(4):221–33. doi: 10.1097/MRM.0000000000000218. [DOI] [Google Scholar]
- Mojarab S, Shahbazzadeh D, Moghbeli M, Eshraghi Y, Bagheri KP, Rahimi R, Savoji MA, Mahdavi M (2020) Immune responses to HIV-1 polytope vaccine candidate formulated in aqueous and alcoholic extracts of Propolis: Comparable immune responses to Alum and Freund adjuvants. Microb Pathog 140:03932. 10.1016/j.micpath.2019.103932 [DOI] [PubMed]
- Moore JP, Klasse PJ (2020) COVID-19 vaccines: "Warp Speed" needs mind melds, not warped minds. J Virol 4(17):e01083–20 [DOI] [PMC free article] [PubMed]
- Morawiec T, Mertas A, Wojtyczka RD, Niedzielska I, Dziedzic A, Bubiłek-Bogacz A, Sender J, Wróbel J, Tanasiewicz M, Wesołowski P, Król W. The assessment of oral microflora exposed to 3% ethanolic extract of brazilian green propolis preparation used for hygiene maintenance following minor oral surgeries. Biomed Res Int. 2015;2015:1–10. doi: 10.1155/2015/869575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad T, Ikram M, Ullah R, Rehman SU, Kim MO. Hesperetin, a Citrus flavonoid, attenuates lps-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients. 2019;11:648. doi: 10.3390/nu11030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muli EM, Maingi JM. Antibacterial activity of Apis mellifera L. propolis collected in three regions of Kenya. J Venom Anim Toxins Incl Trop Dis. 2007;13:655–663. doi: 10.1590/S1678-91992007000300008. [DOI] [Google Scholar]
- Murad JM, Calvi SA, Soares AMVC, Bankova V, Sforcin JM. Effects of propolis from Brazil and Bulgaria on fungicidal activity of macrophages against Paracoccidioides brasiliensis. J Ethnopharmacol. 2002;79:331–334. doi: 10.1016/S0378-8741(01)00404-4. [DOI] [PubMed] [Google Scholar]
- Nachega JB, Sam-Agudu NA, Mellors JW, Zumla A, Mofenson LM. Scaling up Covid-19 vaccination in Africa - lessons from the HIV Pandemic. New England Journal of Medicine. 2021;385(3):196–198. doi: 10.1056/NEJMp2103313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahar L, Sarker SD. Chapter One - Medicinal natural products—an introduction. In: Sarker SD, Nahar L, editors. Annual Reports in Medicinal Chemistry. Academic Press; 2020. pp. 1–44. [Google Scholar]
- Ni Y, Su M, Qiu Y, Chen M, Liu Y, Zhao A, Jia W. Metabolic profiling using combined GC-MS and LC-MS provides a systems understanding of aristolochic acid-induced nephrotoxicity in rat. FEBS Lett. 2007;581:707–711. doi: 10.1016/j.febslet.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Oliveira ACP, Shinobu CS, Longhini R, Franco SL, Svidzinski TIE. Antifungal activity of propolis extract against yeasts isolated from onychomycosis lesions. Memórias Do Instituto Oswaldo Cruz, Rio De Janeiro. 2006;101:493–497. doi: 10.1590/S0074-02762006000500002. [DOI] [PubMed] [Google Scholar]
- Onyeaka H, Al-Sharify ZT, Ghadhban MY, Al-Najjar SZ. A review on the advancements in the development of vaccines to combat coronavirus disease 2019. Clin Exp Vaccine Res. 2021;10:6–12. doi: 10.7774/cevr.2021.10.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orhan IE, Senol Deniz FS. Natural products as potential leads against coronaviruses: could they be encouraging structural models against SARS-CoV-2? Nat Prod Bioprospect. 2020;10:171–186. doi: 10.1007/s13659-020-00250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsi RO, Sforcin JM, Funari SRC, Bankova N. Effects of Brazilian and Bulgarian propolis on bactericidal activity of macrophages against Salmonella Typhimurium. Int Immunopharmacol. 2005;5:359–368. doi: 10.1016/j.intimp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Özcan M. Antifungal properties of propolis. Grasas Aceites. 1999;50:395–398. doi: 10.3989/gya.1999.v50.i5.685. [DOI] [Google Scholar]
- Pasupuleti VR, Sammugam L, Ramesh N, Gan SH (2017) Honey, propolis, and royal jelly: a comprehensive review of their biological actions and health benefits. Oxid Med Cell Longev 2017:1259510. 10.1155/2017/1259510 [DOI] [PMC free article] [PubMed]
- Patel S. Emerging adjuvant therapy for cancer: propolis and its constituents. J Diet Suppl. 2016;13:245–268. doi: 10.3109/19390211.2015.1008614. [DOI] [PubMed] [Google Scholar]
- Pereira RJ, Do Nascimento GNL, Gratao LHA, Pimenta RS. The risk of COVID-19 transmission in favelas and slums in Brazil. Public Health. 2020;183:42–43. doi: 10.1016/j.puhe.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzi L, Corbo MR, Campaniello D, Speranza B, Sinigaglia M, Bevilacqua A. Antifungal and antibacterial effect of propolis: a comparative hit for food-bourne Pseudomonas, enterobacteriaceae and fungi. Foods. 2020;9:559–569. doi: 10.3390/foods9050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobiega K, Kraśniewska K, Derewiaka D, Gniewosz M. Comparison of the antimicrobial activity of propolis extracts obtained by means of various extraction methods. J Food Sci Technol. 2019;56:5386–5395. doi: 10.1007/s13197-019-04009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokhrel S, Chhetri R. A Literature review on Impact of COVID-19 pandemic on teaching and learning. High Educ Future. 2021;8:133–141. doi: 10.1177/2347631120983481. [DOI] [Google Scholar]
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Marc GP, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC, Grp CCT. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan D, Biswasroy P, Naik PK, Ghosh G, Rath G. A review of current interventions for COVID-19 prevention. Arch Med Res. 2020;51:363–374. doi: 10.1016/j.arcmed.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyłek I, Karpiński TM. Antibacterial properties of propolis (bee glue) Molecules. 2019;24:159–160. doi: 10.3390/molecules24112047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Richardson A. Antibacterial activity of propolis and honey against Staphylococcus aureus and Escherichia coli. Afr J Microbiol Res. 2010;4:1872–1878. [Google Scholar]
- Ramanauskien K, Inkeniene AM, Savickas A, Masteikova R, Brusokas V. Analysis of the antimicrobial activity of propolis and lysozyme in semisolid emulsion systems. Acta Poloniae Pharmaceutica - Natural Drugs. 2009;66:681–688. [PubMed] [Google Scholar]
- Ramos AFN, Miranda JL. Propolis: a review of its anti-inflammatory and healing actions. J Venom Anim Toxins Incl Trop Dis. 2007;13:697–710. doi: 10.1590/S1678-91992007000400002. [DOI] [Google Scholar]
- Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. Aims Microbiology. 2018;4:482–501. doi: 10.3934/microbiol.2018.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristivojević P, Dimkić I, Trifković J, Berić T, Vovk I, Milojković-Opsenica D, Stankovic S. Antimicrobial activity of Serbian Propolis evaluated by means of MIC, HPTLC, bioautography and chemometrics. PLoS One. 2016;11(6):1–15. doi: 10.1371/journal.pone.0157097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo I, Mesiti F, Lupia A, Alcaro S. Current updates on naturally occurring compounds recognizing SARS-CoV-2 druggable targets. Molecules. 2021;26(3):632. doi: 10.3390/molecules26030632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlan M, Devina A, Pratami DK, Situmorang H, Farida S, Munim A, Kusumoputro B, Yohda M, Faried A, Gozan M, Ledyawati M. Anti-inflammatory activity of Tetragronula species from Indonesia. Saudi J Biol Sci. 2019;26:1531–1538. doi: 10.1016/j.sjbs.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklani A, Kutty SK. Plant-derived compounds in clinical trials. Drug Discov Today. 2008;13:161–171. doi: 10.1016/j.drudis.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines. 2021;9(2):60. doi: 10.3390/vaccines9020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen A, Saarnio S, Julkunen-Tiitto R. Phenolic compounds of propolis from the boreal coniferous zone. J Apic Sci. 2012;56:13–22. [Google Scholar]
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19) A Review. Jama-J Am Med Assoc. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Schwarz S, Sauter D, Wang K, Zhang RH, Sun B, Karioti A, Bilia AR, Efferth T, Schwarz W. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014;80:177–182. doi: 10.1055/s-0033-1360277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorza CA, Goncalves VC, Scorza FA, Fiorini AC, De Almeida ACG, Fonseca MCM, Finsterer J. Propolis and coronavirus disease 2019 (COVID-19): lessons from nature. Complement Ther Clin Pract. 2020;41(2020):10127. doi: 10.1016/j.ctcp.2020.101227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorza FA, Cavalheiro EA. Biomimicry: applying design for nature to solve problems in epilepsy research. Epilepsy Behav. 2010;18:327–328. doi: 10.1016/j.yebeh.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Seidel V, Peyfoon E, Watson DG, Fearnley J. Comparative study of the antibacterial activity of propolis from different geographical and climatic zones. Phytother Res. 2008;22:1256–1263. doi: 10.1002/ptr.2480. [DOI] [PubMed] [Google Scholar]
- Selamoglu Talas Z. Propolis reduces oxidative stress in l-NAME-induced hypertension rats. Cell Biochemistry Function. 2014;32:150–154. doi: 10.1002/cbf.2986. [DOI] [PubMed] [Google Scholar]
- Serkedjieva J, Manolova N, Bankova V. Anti-influenza virus effect of some propolis constituents and their analogues (esters of substituted cinnamic acids) J Nat Prod. 1992;55:294–302. doi: 10.1021/np50081a003. [DOI] [PubMed] [Google Scholar]
- Setti L, Kirienko M, Dalto SC, Bonacina M, Bombardieri E. FDG-PET/CT findings highly suspicious for COVID-19 in an Italian case series of asymptomatic patients. Eur J Nucl Med Mol Imaging. 2020;47:1649–1656. doi: 10.1007/s00259-020-04819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforcin JM. Biological properties and therapeutic applications of propolis. Phytother Res. 2016;30:894–905. doi: 10.1002/ptr.5605. [DOI] [PubMed] [Google Scholar]
- Shaha A, Mizuguchi H, Kitamura Y, Fujino H, Yabumoto M, Takeda N, Fukui H. Effect of royal jelly and Brazilian green propolis on the signaling for histamine H-1 receptor and interleukin-9 gene expressions responsible for the pathogenesis of the allergic rhinitis. Biol Pharm Bull. 2018;41:1440–1447. doi: 10.1248/bpb.b18-00325. [DOI] [PubMed] [Google Scholar]
- Shaldam MA, Yahya G, Mohamed NH, Abdel-Daim MM, Al Naggar Y. In silico screening of potent bioactive compounds from honeybee products against COVID-19 target enzymes. Environ Sci Pollut Res. 2021;28(30):40507–40514. doi: 10.1007/s11356-021-14195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Hino A, Tsutsumi A, Yong KP, Watanabe W, Kurokawa M. Anti-influenza virus activity of propolis in vitro and its efficacy against influenza infection in mice. Antiviral Chem Chemother. 2008;19(1):7–13. doi: 10.1177/095632020801900102. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Takeshita Y, Takamori Y, Kai H, Sawamura R, Yoshida H, Watanabe W, Tsutsumi A, Park YK, Yasukawa K, Matsuno K, Shiraki K, Kurokawa M (2011) Efficacy of Brazilian Propolis against Herpes Simplex Virus Type 1 Infection in Mice and Their Modes of Antiherpetic Efficacies. Evidence-Based Complementary and Alternative Medicine. 10.1155/2011/976196 [DOI] [PMC free article] [PubMed]
- Shnitzler P, Nauner A, Nolkemper S, Zundel C, Nowack H, Sensch KH, Reichling J. Antiviral activity and mode of action of propolis extracts and selecetd compounds. Phytother Res. 2008;24:S20–S28. doi: 10.1002/ptr.2868. [DOI] [PubMed] [Google Scholar]
- Siddell SG, Walker PJ, Lefkowitz EJ, Mushegian AR, Adams MJ, Dutilh BE, Gorbalenya AE, Harrach B, Harrison RL, Junglen S. Additional changes to taxonomy ratified in a special vote by the International Committee on Taxonomy of Viruses (October 2018) Adv Virol. 2019;164:943–946. doi: 10.1007/s00705-018-04136-2. [DOI] [PubMed] [Google Scholar]
- Silici S, Koç NA, Ayangil D, Çankaya S. Antifungal activities of propolis collected by different races of honeybees against yeasts isolated from patients with superficial mycoses. J Pharmacol Sci. 2005;99:39–44. doi: 10.1254/jphs.FPE05002X. [DOI] [PubMed] [Google Scholar]
- Silva-Carvalho R, Baltazar F, Almeida-Aguiar C (2015) Propolis: a complex natural product with a plethora of biological activities that can be explored for drug development. Evidence-Based Complementary and Alternative Medicine. https://doi.org/10.1155/2015/206439 [DOI] [PMC free article] [PubMed]
- Silveira M, Teles F, Melo E, Borges V, Miranda F, Dutra F, Berretta A, Cezar R, Silva J, Santos H (2020) "Effects of Brazilian green propolis extract (epp-af) on inflammation in hemodialysis patients", in: Nephrology Dialysis Transplantation: OXFORD UNIV PRESS). 10.1093/ndt/gfaa142.P1574
- Silveira MAD, Teles F, Berretta AA, Sanches TR, Rodrigues CE, Seguro AC, Andrade L. Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: a randomized, double-blind, placebo-controlled trial. BMC nephrology. 2019;20:1–12. doi: 10.1186/s12882-019-1337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone-Finstrom M, Spivak M. Propolis and bee health: the natural history and significance of resin use by honey bees. Apidologie. 2010;41:295–311. doi: 10.1051/apido/2010016. [DOI] [Google Scholar]
- Sithisarn P, Michaelis M, Schubert-Zsilavecz M, Cinatl J. Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antiviral Res. 2013;97:41–48. doi: 10.1016/j.antiviral.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Snijder EJ, Wassenaar AL, Spaan WJ, Gorbalenya AE. The Arterivirus Nsp2 protease.: an unusual cysteine protease with primary structure similarities to both papain-like and chymotrypsin-like proteases (∗) J Biol Chem. 1995;270:16671–16676. doi: 10.1074/jbc.270.28.16671. [DOI] [PubMed] [Google Scholar]
- Solnier J, Fladerer JP. Flavonoids: a complementary approach to conventional therapy of COVID-19? Phytochem Rev. 2020;20(4):773–795. doi: 10.1007/s11101-020-09720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava N, Saxena SK. Prevention and control strategies for SARS-CoV-2 infection. In: Saxena SK, editor. Coronavirus Disease 2019 (COVID-19): Epidemiology, Pathogenesis, Diagnosis, and Therapeutics. Singapore: Springer Singapore; 2020. pp. 127–140. [Google Scholar]
- Suleman T, Van Vuuren S, Sandasi M, Viljoen AM. Antimicrobial activity and chemometric modelling of South African propolis. J Appl Microbiol. 2015;119:981–990. doi: 10.1111/jam.12906. [DOI] [PubMed] [Google Scholar]
- Sung WS, Lee DG. Antifungal action of chlorogenic acid against pathogenic fungi, mediated by membrane disruption. Pure Appl Chem. 2010;82:219–226. doi: 10.1351/PAC-CON-09-01-08. [DOI] [Google Scholar]
- Temiz A, Şener A, Özkök Tüylü A, Sorkun K, Salih B. Antibacterial activity of bee propolis samples from different geographical regions of Turkey against two foodborne pathogens, Salmonella Enteritidis and Listeria monocytogenes. Turk J Biol. 2011;35:503–511. [Google Scholar]
- Ter Ellen BM, Kumar ND, Bouma EM, Troost B, Van De Pol DPI, Van Der Ende-Metselaar HH, Apperloo L, Van Gosliga D, Van Den Berge M, Nawijn MC, Van Der Voort PHJ, Moser J, Rodenhuis-Zybert IA, Smit JM (2021) Resveratrol And pterostilbene potently inhibit SARS-CoV-2 replication in vitro. bioRxiv 2020.2009.2024.285940.
- Tobaldini-Valerio FK, Bonfim-Mendonça PS, Rosseto HC, Bruschi ML, Henriques M, Negri M, Silva S, Svidzinski TIE. Propolis: a potential natural product to fight Candida species infections. Future Microbiol. 2016;11:1035–1046. doi: 10.2217/fmb-2015-0016. [DOI] [PubMed] [Google Scholar]
- Toreti VC, Sato HH, Pastore GM, Park YK (2013) Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evidence-Based Complementary and Alternative Medicine 2013:697390. 10.1155/2013/697390 [DOI] [PMC free article] [PubMed]
- Uzel A, Sorkun K, Önçaǧ Ö, Çoǧulu D, Gençay Ö, Salih B. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol Res. 2005;160:189–195. doi: 10.1016/j.micres.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Vardeny O, Madjid M, Solomon SD. Applying the Lessons of Influenza to COVID-19 during a time of uncertainty. Circulation. 2020;141:1667–1669. doi: 10.1161/CIRCULATIONAHA.120.046837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagh VD. Propolis: a wonder bees product and its pharmacological potentials. Adv Pharmacol Sci. 2013;2013:308249. doi: 10.1155/2013/308249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang Z, Liu Z, Wang K, Xu W. Membrane disruption of Fusarium oxysporum f. sp. niveum induced by myriocin from Bacillus amyloliquefaciens LZN01. Microb Biotechnol. 2021;14:517–534. doi: 10.1111/1751-7915.13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sui X, Sui DJ, Yang P (2018) Flavonoid extract from propolis inhibits cardiac fibrosis triggered by myocardial infarction through upregulation of SIRT1. Evidence-Based Complementary and Alternative Medicine. 10.1155/2018/4957573 [DOI] [PMC free article] [PubMed]
- Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, Jiang B. Comorbidities and multi-organ injuries in the treatment of COVID-19. The Lancet. 2020;395:e52. doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrilow D, Watterson D, Hall RA, Davis SS, Weir R, Kurucz N, Whelan P, Allcock R, Hall-Mendelin S, O’brien, C.A. A new species of mesonivirus from the Northern Territory. Australia Plos One. 2014;9:e91103. doi: 10.1371/journal.pone.0091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtyczka RD, Dziedzic A, Idzik D, Kepa M, Kubina R, Kabala-Dzik A, Smolen-Dzirba J, Stojko J, Sajewicz M, Wasik TJ. Susceptibility of Staphylococcus aureus Clinical Isolates to Propolis Extract Alone or in Combination with Antimicrobial Drugs. Molecules. 2013;18:9623–9640. doi: 10.3390/molecules18089623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worldometer ( 2021) COVID-19 coronavirus pandemic
- Yang FNX, Wong IKA. The social crisis aftermath: tourist well-being during the COVID-19 outbreak. J Sustain Tour. 2020;29:859–878. doi: 10.1080/09669582.2020.1843047. [DOI] [Google Scholar]
- Ye Z-W, Yuan S, Yuen K-S, Fung S-Y, Chan C-P, Jin D-Y. Zoonotic origins of human coronaviruses. Int J Biol Sci. 2020;16:1686. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim A, Duran GG, Duran N, Jenedi K, Bolgul BS, Miraloglu M, Muz M (2016) Antiviral Activity of Hatay Propolis Against Replication of Herpes Simplex Virus Type 1 and Type 2. Medical science monitor. International Medical Journal of Experimental and Clinical Research 22:422–430. 10.12659/msm.897282 [DOI] [PMC free article] [PubMed]
- Yusoff NYN, Mohamad S, Abdullah HN, Rahman NA (2016) Antifungal activity of Malaysian honey and propolis extracts against pathogens implicated in denture stomatitis. AIP Conference Proceedings 1791:020006. 10.1063/1.4968861
- Zaim S, Chong JH, Sankaranarayanan V, Harky A. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45:100618. doi: 10.1016/j.cpcardiol.2020.100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakaryan H, Arabyan E, Oo A, Zandi K. Flavonoids: promising natural compounds against viral infections. Adv Virol. 2017;162:2539–2551. doi: 10.1007/s00705-017-3417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zedan H, Hofny ERM, Ismail SA. Propolis as an alternative treatment for cutaneous warts. Int J Dermatol. 2009;48:1246–1249. doi: 10.1111/j.1365-4632.2009.04184.x. [DOI] [PubMed] [Google Scholar]
- Zhang R, Ai X, Duan Y, Xue M, He W, Wang C, Xu T, Xu M, Liu B, Li C, Wang Z, Zhang R, Wang G, Tian S, Liu H. Kaempferol ameliorates H9N2 swine influenza virus-induced acute lung injury by inactivation of TLR4/MyD88-mediated NF-κB and MAPK signaling pathways. Biomed Pharmacother. 2017;89:660–672. doi: 10.1016/j.biopha.2017.02.081. [DOI] [PubMed] [Google Scholar]
- Zhang Y-X, Yang T-T, Xia L, Zhang W-F, Wang J-F, Wu Y-P (2017b) Inhibitory effect of Propolis on platelet aggregation in vitro. J Healthc Eng 2017:1-6. 10.1155/2017/3050895 [DOI] [PMC free article] [PubMed]
- Zhao L, Pu L, Wei J, Li J, Wu J, Xin Z, Gao W, Guo C. Brazilian green propolis improves antioxidant function in patients with type 2 diabetes mellitus. Int J Environ Res Public Health. 2016;13:498. doi: 10.3390/ijerph13050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Zhang DY, Wang WL, Li XW, Yang B, Song JD, Zhao X, Huang BY, Shi WF, Lu RJ, Niu PH, Zhan FX, Ma XJ, Wang DY, Xu WB, Wu GZ, Gao GGF, Tan WJ, Coronavirus CN. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulhendri F, Chandrasekaran K, Kowacz M, Ravalia M, Kripal K, Fearnley J, Perera CO. Antiviral, antibacterial, antifungal, and antiparasitic properties of propolis: a review. Foods. 2021;10(6):1360. doi: 10.3390/foods10061360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.