Abstract
Objective
To evaluate the effectiveness and safety of viscosupplementation for pain and function in patients with knee osteoarthritis.
Design
Systematic review and meta-analysis of randomised trials.
Data sources
Searches were conducted of Medline, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases from inception to 11 September 2021. Unpublished trials were identified from the grey literature and trial registries.
Eligibility criteria for study selection
Randomised trials comparing viscosupplementation with placebo or no intervention for knee osteoarthritis treatment.
Main outcome measures
The prespecified primary outcome was pain intensity. Secondary outcomes were function and serious adverse events. Pain and function were analysed as standardised mean differences (SMDs). The prespecified minimal clinically important between group difference was −0.37 SMD. Serious adverse events were analysed as relative risks.
Methods
Two reviewers independently extracted relevant data and assessed the risk of bias of trials using the Cochrane risk of bias tool. The predefined main analysis was based only on large, placebo controlled trials with ≥100 participants per group. Summary results were obtained through a random effects meta-analysis model. Cumulative meta-analysis and trial sequential analysis under a random effects model were also performed.
Results
169 trials provided data on 21 163 randomised participants. Evidence of small study effects and publication biases was observed for pain and function (Egger’s tests with P<0.001 and asymmetric funnel plots). Twenty four large, placebo controlled trials (8997 randomised participants) included in the main analysis of pain indicated that viscosupplementation was associated with a small reduction in pain intensity compared with placebo (SMD −0.08, 95% confidence interval −0.15 to −0.02), with the lower bound of the 95% confidence interval excluding the minimal clinically important between group difference. This effect corresponds to a difference in pain scores of −2.0 mm (95% confidence interval −3.8 to −0.5 mm) on a 100 mm visual analogue scale. Trial sequential analysis for pain indicated that since 2009 there has been conclusive evidence of clinical equivalence between viscosupplementation and placebo. Similar conclusions were obtained for function. Based on 15 large, placebo controlled trials on 6462 randomised participants, viscosupplementation was associated with a statistically significant higher risk of serious adverse events than placebo (relative risk 1.49, 95% confidence interval 1.12 to 1.98).
Conclusion
Strong conclusive evidence indicates that viscosupplementation leads to a small reduction in knee osteoarthritis pain compared with placebo, but the difference is less than the minimal clinically important between group difference. Strong conclusive evidence indicates that viscosupplementation is also associated with an increased risk of serious adverse events compared with placebo. The findings do not support broad use of viscosupplementation for the treatment of knee osteoarthritis.
Systematic review registration
PROSPERO CRD42021236894.
Introduction
Knee osteoarthritis is a chronic degenerative disease that involves inflammation and structural changes of the joints, resulting in joint pain and physical functional limitations.1 2 This condition is a leading cause of disability among older people, with over 560 million people living with knee osteoarthritis worldwide.3 Intra-articular hyaluronic acid (also known as viscosupplementation)4 5 is frequently used to treat knee osteoarthritis symptoms, but the effectiveness and safety of this treatment have remained controversial6 since the first clinical trial in the early 1970s.7
The latest national and international guidelines vary in their recommendations, but most discourage the use of intra-articular hyaluronic acid derivatives.8 In England, because the National Institute for Health and Care Excellence clinical guidelines recommends against the use of viscosupplementation, this treatment is given a low priority for funding by the NHS and is only considered in exceptional circumstances.9 However, healthcare systems in other countries offer viscosupplementation treatment to patients with knee osteoarthritis.10 11 For example, in the US, Medicare and commercial insurance companies cover viscosupplementation and use has grown considerably from 2012 to 201812—one in every seven patients with knee osteoarthritis receive injections of hyaluronic acid derivatives as first line treatment.13 Based on data from Medicare,12 14 expenditure on viscosupplementation treatment was estimated to be $287m (£233m; €273m) in 2012 and $325m in 2018. Evidence shows that approximately 28% of that amount was spent on treating large joint infections after viscosupplementation injections.12
This systematic review aimed to provide updated evidence on the clinical benefits and safety of viscosupplementation to treat patients with knee osteoarthritis using data from 50 years of randomised trials.
Methods
The reporting of the present systematic review was guided by the PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines.15 The study was registered in PROSPERO (CRD42021236894).
Trial selection
We included randomised or quasi-randomised controlled trials that had at least 75% of participants with clinically or radiologically confirmed knee osteoarthritis, and that reported at least one outcome of interest (pain, function, or serious adverse events). The intervention of interest was any intra-articular hyaluronic acid preparation or a hyaluronic acid derivative. Control interventions of interest were placebo (saline or preparations with negligible concentrations of hyaluronic acid) or no intervention. Web appendix 1 shows trial selection details.
Data sources
We searched Medline, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) databases for relevant trials (from database inception to 11 September 2021). We also identified eligible trials from theses or dissertations, personal communications, books, pamphlets, conference abstracts, trial registries, manufacturers’ reports, and regulatory documents. Details of the search strategy and data sources are described in web appendices 2 and 3. No language restriction was applied.
Data collection
Data extraction was performed by two of eight independent reviewers. Discrepancies were solved by consensus or consultation with a third reviewer. We extracted year of publication, sample size, methodological characteristics, trial duration (time from randomisation to end of follow-up), and characteristics of the viscosupplementation preparation, including molecular weight, structure, cycles, dosage, frequency of administration and treatment duration (see web appendix 4 for details).
Outcomes
The prespecified primary outcome was pain intensity. Secondary outcomes were function and serious adverse events. Continuous outcomes were analysed as standardised mean differences (SMDs). An SMD <0 indicates a better outcome for viscosupplementation than control. We back transformed pooled SMDs to a 100 mm visual analogue scale assuming a standard deviation of 25 mm (see web appendix 5 for details). Web appendix 6 describes the rationale for the choice of the minimal clinically important between group difference of −0.37.
If pain and function outcomes were reported for more than one time point, we extracted results closest to three months after the last injection (web appendix 4).16 17 If two or more measurement tools were used, we referred to a previously described hierarchy of outcome measures18 19 and extracted data for the one ranked highest on this list (web appendix 7). Serious adverse events were reported as events resulting in hospital admission, prolongation of hospital stay, persistent or major disability, congenital abnormality of offspring, life threatening events, or death.20
Risk-of-bias assessment
We assessed the risk of bias of trials included in our main analysis (large, placebo controlled trials) using the Cochrane collaboration tool 2.0 (web appendix 8).21 All other trials were assessed using the Cochrane collaboration tool 1.0.22 Assessments were performed by a pair of reviewers working independently. Inconsistencies were resolved by consensus or discussion with a third reviewer.
Data analysis
We summarised pain and function using SMDs,23 24 25 26 and serious adverse events using relative risks. Summary estimates with 95% confidence intervals were obtained with the DerSimonian-Laird random effects model.27 Web appendices 4 and 9 describe the approaches used to deal with multiarm trials and approximation of standard deviations, respectively. The prespecified main analysis was based only on large, placebo controlled trials (mean ≥100 participants in each group).28 We used this prespecified cutoff of ≥100 participants in each group on average because such trials are less likely to be influenced by small study effects.25 28
We conducted prespecified subgroup analyses based on methodological, clinical, and publication characteristics24 26 using random effects meta-regression models with the DerSimonian-Laird estimator. Statistical heterogeneity was interpreted on the basis of the between trial variance (τ2; see web appendix 10 for guidance on τ2 interpretation).24 29 We investigated the association between trial size and treatment effects using funnel plots accompanied by tests of asymmetry. For SMDs, funnel plot asymmetry was tested using Egger’s test, and for relative risks, Harbord’s test was used.
We carried out random effects trial sequential analyses to assess whether the combined number of patients across all large placebo controlled trials offered sufficient statistical power to produce definitive conclusions about the effectiveness and safety of viscosupplementation or whether additional trials were needed (web appendix 11).30 Analyses were performed using Stata 14 (StataCorp) and R 3.6.3 (www.r-project.org). Statistical significance was set at P<0.05 (two sided) for all tests. P<0.005 was considered to indicate strong evidence against the null hypothesis.31 32
Patient and public involvement
Patients and members of the public were not involved in this study owing to limited resources and the covid-19 pandemic. Nevertheless, we intend to engage the public in disseminating our results, including social media engagement, newsletters, and conferences.
Results
All trials on viscosupplementation
Figure 1 shows the selection process. We identified 169 trials (involving 21 163 randomised participants) that reported at least one outcome of interest to our systematic review. Web appendix 12 shows the list of trials. Between 1972 and 2021, the publication rate of new trials on viscosupplementation averaged four per year, doubling after 2012 (four trials per year before 2012 v eight trials per year after 2012; web appendix 13).
Fig 1.
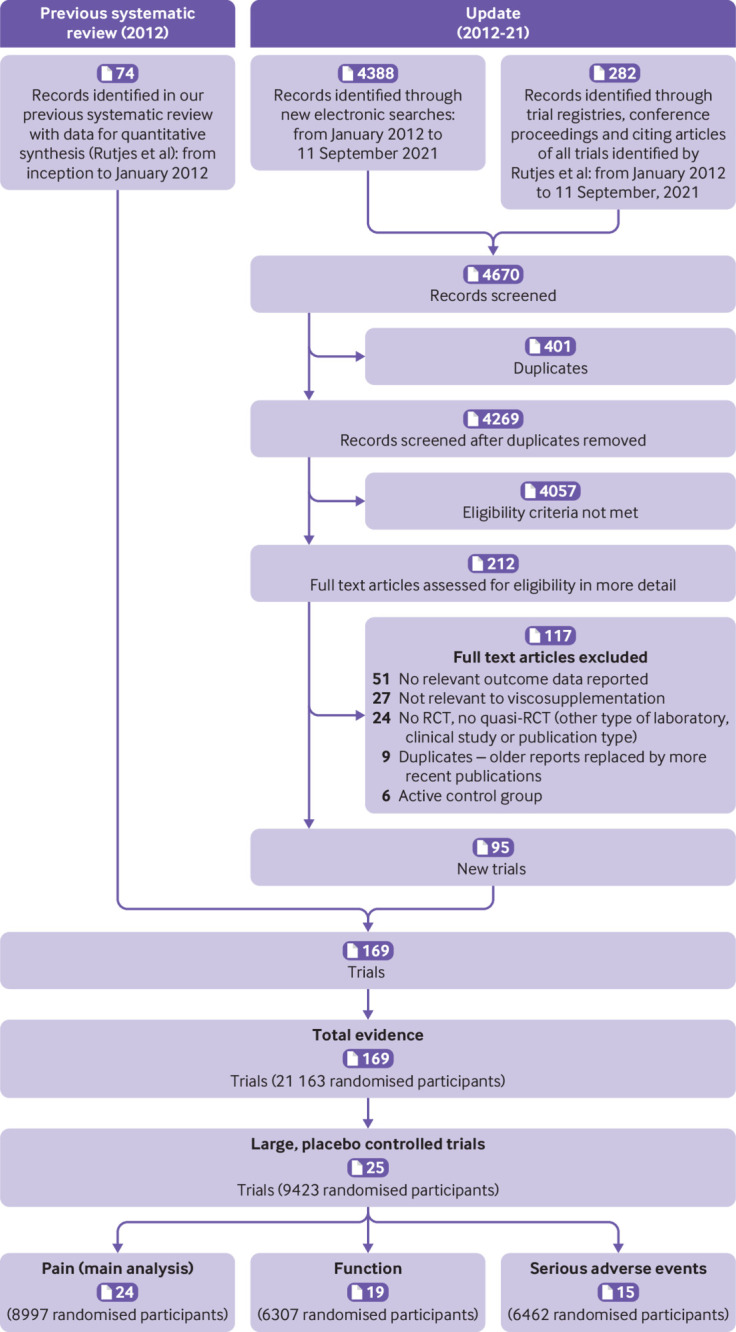
Flowchart showing steps in the selection of relevant trials. RCT=randomised controlled trial
The median total sample size was 92 participants (interquartile range 50-165). The median total trial follow-up was 24 weeks (8.6-27). The median average age of participants was 61 (58-64), whereas the median percentage of women was 62 (54-71). The clinical benefit of viscosupplementation was examined most frequently through open label trials as an add-on to standard treatment compared with standard treatment alone (93, 55%), followed by placebo controlled trials (76, 45%).
Risk of bias, publication bias and small study effects
Across all trials, a low risk of selection bias was found in 20 trials (12%, n=169), a low risk of performance bias in 27 (16%, n=169), a low risk of detection bias for pain in 52 (31%, n=165), a low risk of detection bias for function in 30 (23%, n=132), a low risk of attrition bias for pain in 82 (50%, n=165), and a low risk of attrition bias for function in 69 (52%, n=132; web appendix 12).
Web appendix 14 presents the summary estimates of pain intensity considering all trials (n=165 trials, 20 729 randomised participants). Web appendix 15 provides the corresponding results for function. Evidence was found of extreme between trial heterogeneity, with trials indicating treatment effects that ranged from extremely beneficial to clinically worse effects of viscosupplementation on pain intensity compared with the control group. Unequivocal evidence was found of funnel plot asymmetry (P<0.001, web appendix 16), indicating small study effects. 32 33 A similar pattern of funnel plot asymmetry was detected for function (web appendix 17).
Characteristics of large placebo controlled trials in main analyses
Of the 169 identified trials, 25 were large, placebo controlled trials (table 1),34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 which randomised 9423 participants (mean age 62 years, 59% women, mean disease duration 5.2 years). Twenty four trials reported data for pain intensity (main analysis), 19 trials reported data for function, and 15 trials reported data for serious adverse events. According to Cochrane’s risk of bias tool 2.0, a low risk of bias was found in the randomisation process in 13 trials (52%, n=25), a low risk of bias owing to deviations from intended interventions in 20 (80%, n=25), a low risk of bias due to missing outcome data in 18 trials that reported pain (75%, n=24) and in 14 that reported function (74%, n=19); a low risk of bias in the measurement of outcome in 23 trials that reported pain (96%, n=24) and in 19 that reported function (100%, n=19); a low risk of bias in the selection of reported results in 16 trials that reported pain (67%, n=24) and in 13 that reported function (68%, n=19). Details about the risk-of-bias assessment for the 25 large, placebo controlled trials are found in web appendix 12. The median follow-up time after the last injection was 13 weeks (interquartile range 12-16) for pain, and 12 weeks (10-13) for function. The median number of injections administered per cycle was 3 (interquartile range 1-5) for both pain intensity and function outcomes.
Table 1.
Characteristics of the 25 large, placebo controlled trials*
| Author (year) | No of randomised participants | Instrument | Reported SAEs | Publication status | Funding independent of industry | Molecular weight | Structure | No of injections (cycles)† | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Visco | Placebo | Pain | Function | ||||||||
| Shichikawa (1983)34 | 114 | 114 | Global pain (VAS) | — | No | Published | No | Unclear | Non-cross linked | 5 (1) | |
| Puhl (1993)35 | 102 | 107 | Global pain (VAS) | Lequesne index | No | Published | No | Low | Non-cross linked | 5 (1) | |
| Lohmander (1996)36 | 120 | 120 | Global pain (VAS) | Lequesne index | No | Published | No | Low | Non-cross linked | 5 (1) | |
| Altman and Moskowitz (1998)37 | 164 | 168 | Pain on walking (VAS) | WOMAC function | Yes | Published | No | Low | Non-cross linked | 5 (1) | |
| Brandt (2001)38‡ | 114 | 112 | — | — | Yes | Published | No | Intermediate | Non-cross linked | 3 (1) | |
| Seikagaku [UK] (2001)39 | 116 | 115 | Lesquene index | Lequesne index | No | Unpublished | No | Low | Non-cross linked | 5 (1) | |
| Jubb (2003)40 | 208 | 200 | Pain on walking (VAS) | — | Yes | Published | No | Low | Non-cross linked | 3 (3) | |
| Altman (2004)41 | 173 | 174 | WOMAC pain | WOMAC function | Yes | Published | No | High | Cross linked | 1 (1) | |
| Day (2004)42 | 116 | 124 | WOMAC pain | WOMAC function | No | Published | No | Low | Non-cross linked | 5 (1) | |
| Pham (2004)43 | 131 | 85 | Global pain (VAS) | Lequesne index | No | Published | No | Intermediate | Unclear | 3 (3) | |
| Altman (2009)44 | 293 | 295 | Pain on walking (VAS) | WOMAC function | Yes | Published | No | Intermediate | Non-cross linked | 3 (1) | |
| Baltzer (2009)45 | 135 | 107 | Global pain (VAS) | WOMAC function | No | Published | Yes | Low | Non-cross linked | 3 (1) | |
| Chevalier (2010)46 | 124 | 129 | WOMAC pain | WOMAC function | Yes | Published | No | High | Cross linked | 1 (1) | |
| Jørgensen (2010)47 | 167 | 170 | Pain on walking (VAS) | Lequesne index | No | Published | No | Low | Non-cross linked | 5 (1) | |
| Huang (2011)48 | 100 | 100 | Pain on walking (VAS) | WOMAC function | Yes | Published | No | Low | Non-cross linked | 5 (1) | |
| Strand (2012)49 | 251 | 128 | WOMAC pain | WOMAC function | Yes | Published | No | Unclear | Cross linked | 1 (1) | |
| NCT00988091 (2012)50 | 298 | 298 | Pain on walking (VAS) | WOMAC function | Yes | Unpublished | No | Unclear | Unclear | 1 (1) | |
| Arden (2014)51 | 108 | 110 | WOMAC pain | WOMAC function | No | Published | No | High | Cross linked | 1 (1) | |
| NCT01372475 (2015)52 | 400 | 400 | WOMAC pain | — | No | Unpublished | No | Unclear | Non-cross linked | 2 (1) | |
| NCT01934218 (2017)53§ | 404 | 410 | Pain on walking (VAS) | — | Yes | Unpublished | No | Unclear | Cross linked | 1 (1) | |
| Hangody (2017)54 | 150 | 69 | WOMAC pain | WOMAC function | Yes | Published | No | Intermediate | Cross linked | 1 (1) | |
| Petterson and Plantcher (2018)55 | 184 | 185 | Patient global assessment (VAS) | WOMAC function | Yes | Published | No | Intermediate | Cross linked | 1 (1) | |
| NCT02495857 (2018)56¶ | 400 | 199 | WOMAC pain | WOMAC function | Yes | Unpublished | No | Intermediate | Non-cross linked | 3 (1) | |
| Ke (2021)57 | 220 | 220 | WOMAC pain | — | Yes | Published | No | High | Cross linked | 1 (1) | |
| Migliore (2021)58 | 347 | 345 | Global pain (VAS) | Lequesne index | Yes | Published | No | High and low | Unclear | 1 (1) | |
When available, molecular weight was categorised as low (<1500 kDa), intermediate (≥1500 and <6000 kDa) and high (≥6000 kDa).
SAE=serious adverse event; WOMAC=Western Ontario and McMaster Universities Arthritis Index; VAS=visual analogue scale.
In all 25 large, placebo controlled trials, 100% of the 9424 randomised participants had clinically or radiologically confirmed osteoarthritis of the knee. There were no quasi randomised trials among the 25 large trials.
Number of injections per cycle.
Pain and function only reported for a subgroup of patients, but serious adverse events reported for all randomised participants.
Only partially published as a subgroup analysis in Takamura et al.60
Trial NCT02495857 was a three arm trial (200 participants in viscosupplementation group 1, 200 participants in viscosupplementation group 2, and 199 in the placebo group). Pain and function estimates were not reported for viscosupplementation group 1, resulting in a total of 399 participants for efficacy estimates and 599 for serious adverse events (data from all three groups reported). Among the 25 large, placebo controlled trials, trial NCT02495857 was the only trial requiring combination of viscosupplementation groups. The remaining large, placebo controlled trials had one viscosupplementation group only.
Primary outcome: pain intensity
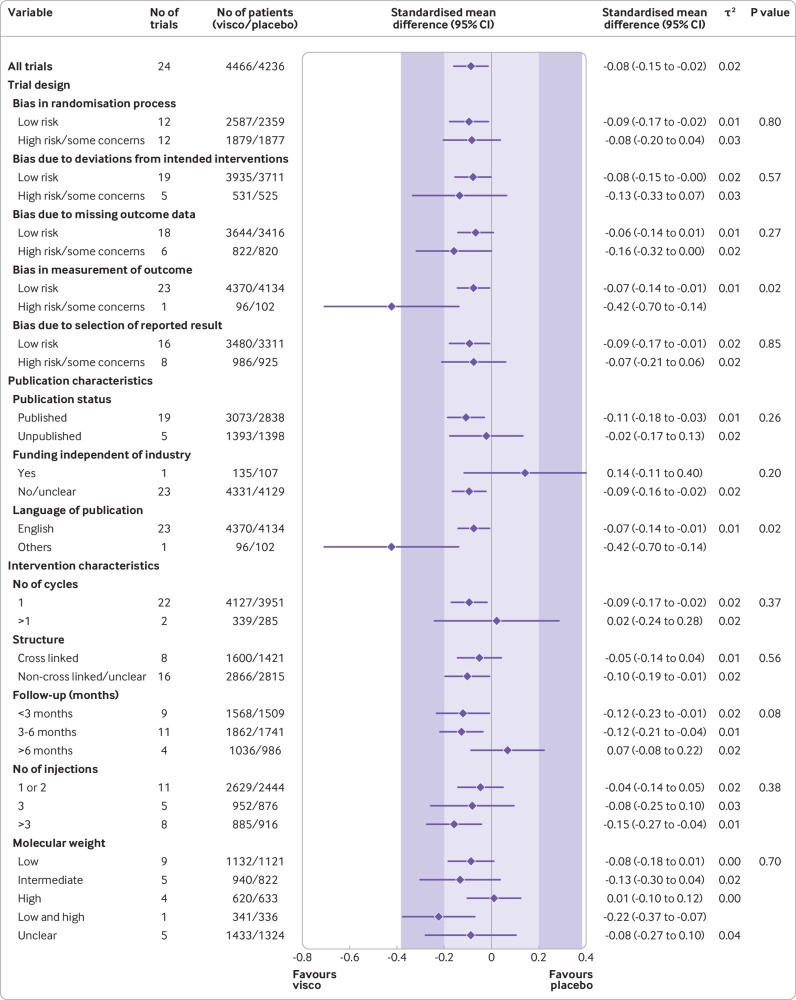
Summary estimates based on 24 large, placebo controlled trials (8997 randomised patients) indicated a small, non-clinically relevant reduction in pain intensity by viscosupplementation compared with placebo (SMD −0.08, 95% confidence interval −0.15 to −0.02, P=0.02, τ2=0.02; fig 2 and web appendix 18). This effect corresponds to a pain intensity reduction of −2.0 mm (95% confidence interval −3.8 to −0.5) on a 100 mm visual analogue scale compared with placebo. Web appendix 19 presents further considerations about the magnitude of the summary effect.
Fig 2.
Main and subgroup analyses for pain. Results are based on 24 large, placebo controlled trials, including 8997 randomised participants. The shaded areas represent the areas of clinical equivalence (darker areas represent the minimal clinically important difference of 0.37, lighter areas represent the more stringent 0.2 margin of equivalence). P denotes two tailed P values for interaction (two subgroups only) or trend tests for interaction (three or more subgroups). For the molecular weight categories, the P value was based on a simple interaction test because one trial examined a preparation made of high and low molecular weight hyaluronic acids. Cycles: patients are usually given a single injection or a course of two to six injections; one cycle refers to one such course of treatment. Number of participants analysed (shown for each subgroup) might be smaller than number of randomised participants. A τ2 of up to 0.04 was prespecified to represent low heterogeneity, 0.09 to represent moderate, and 0.16 to represent high statistical heterogeneity among trial estimates.29 visco=viscosupplementation
Summary estimates by subgroups indicated that viscosupplementation was associated with treatment effects less pronounced than the minimal clinically important between group difference of 0.37 standard deviation units compared with placebo for most subgroups (fig 2). Web appendix 20 presents further considerations about subgroup estimates.
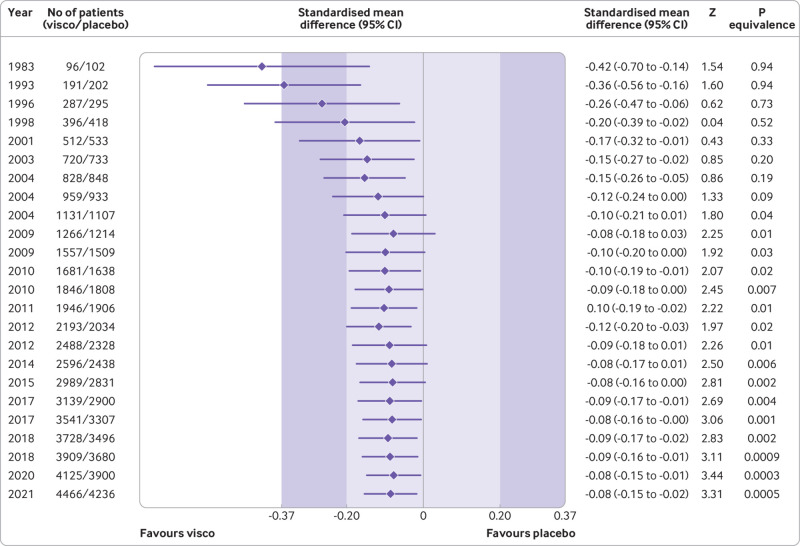
A cumulative meta-analysis indicates that summary estimates reduced from an SMD of −0.42 in 1983 to an SMD of −0.10 by 2009, which was less than the minimal clinically important between group difference. From the end of 2012 onwards, the combined estimates and their 95% confidence intervals remained stable and entirely within the −0.20 and +0.20 equivalence margins (fig 3).
Fig 3.
Cumulative pooled analysis for knee pain based on large, placebo controlled trials (n=24 trials, 8997 patients randomised). The shaded areas represent the areas of clinical equivalence (darker areas represent the minimal clinically important difference of 0.37, lighter areas represent the more stringent 0.2 margin of equivalence). Results are for random effects model. Over the years, between trial variance estimates (τ2) varied between 0 and 0.02, suggesting low heterogeneity. P values for equivalence are based on two one sided tests. The number of participants analysed might be smaller than the number of randomised participants
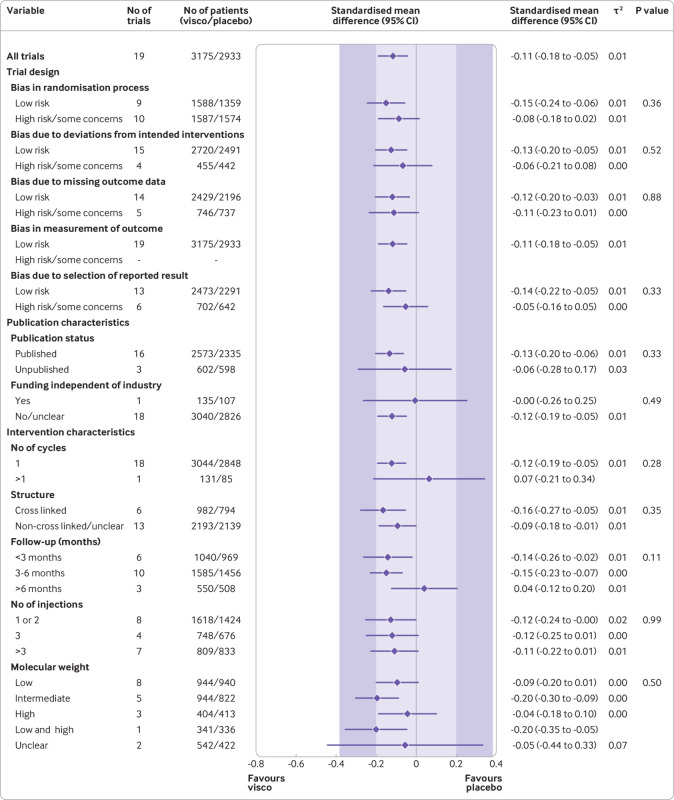
Secondary outcome: function
Summary estimates based on 19 large, placebo controlled trials (6307 randomised participants) indicated a small, non-clinically relevant improvement in function levels by viscosupplementation (SMD −0.11, 95% confidence interval −0.18 to −0.05, P=0.001, τ2=0.01; fig 4 and web appendix 21). Summary estimates by subgroups showed that viscosupplementation was associated with treatment effects less pronounced than the minimal clinically important between group difference of 0.37 standard deviation units compared with placebo (fig 4) for all subgroups. Cumulative meta-analysis results for knee function mirrored the cumulative meta-analysis for pain (web appendix 22).
Fig 4.
Main and subgroup analyses for function. Results are based on 19 large, placebo controlled trials, including 6307 randomised participants. The shaded areas represent the areas of clinical equivalence (darker areas represent the minimal clinically important difference of 0.37, lighter areas represent the more stringent 0.2 margin of equivalence). P denotes two tailed P values for interaction (two subgroups only) or trend tests for interaction (three or more subgroups). For the molecular weight categories, the P value was based on a simple interaction test because one trial examined a preparation made of high and low molecular weight hyaluronic acids. Cycles: patients are usually given a single injection or a course of two to six injections; one cycle refers to one such course of treatment. Number of participants analysed (shown for each subgroup) might be smaller than number of randomised participants. A τ2 of up to 0.04 was prespecified to represent low heterogeneity, 0.09 to represent moderate, and 0.16 to represent high statistical heterogeneity among trial estimates.29 visco=viscosupplementation
Secondary outcome: serious adverse events
A prespecified analysis based on 15 large, placebo controlled trials (6462 randomised participants) indicated that viscosupplementation was associated with a statistically significant risk of serious adverse events compared with placebo (relative risk 1.49, 95% confidence interval 1.12 to 1.98, P=0.003, τ2=0; web appendix 23). Overall, 3.7% of patients receiving viscosupplementation and 2.5% receiving placebo experienced a serious adverse event. Web appendix 24 shows the types of events reported by trial.
Trial sequential analysis
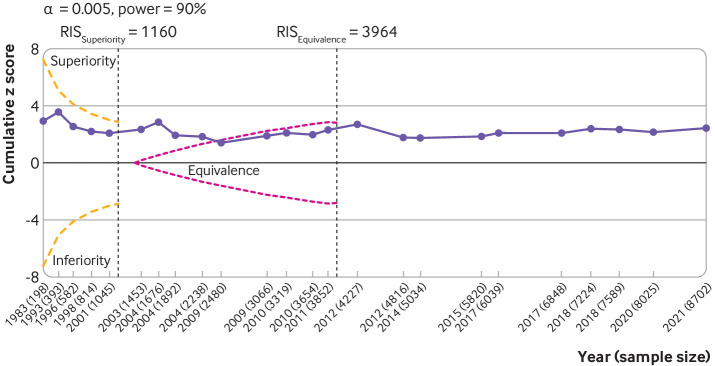
Trial sequential analysis for knee osteoarthritis pain based on the 24 large, placebo controlled trials (8997 randomised participants) indicated that the cumulative z score did not cross the monitoring boundary for superiority (fig 5). Large trials published or conducted after the required information size was reached did not change the results. Trial sequential analysis conducted against the equivalence boundary of 0.20 SMD unitsshowed that the accumulated evidence crossed the boundary for equivalence in 2009. Analogous results were observed for function (web appendix 25).
Fig 5.
Trial sequential analysis for pain. Results are based on 24 large, placebo controlled trials (8997 randomised participants). Cumulative z scores were calculated under a random effects model. RIS (required information size; vertical lines) detects a minimal clinically important difference of −0.37 with 90% of power at α level of 0.005. O'Brien-Fleming monitoring boundaries are represented by dashed orange lines. The inner wedges (futility boundaries) are shown in pink and represent limits to the equivalence region considering the 0.2 equivalence margin. Circles denote cumulative z score for each additional trial added to the analysis. Between trial variation was accounted for using diversity (D2) index adjusted sample sizes. A D2 of 50% was assumed. Number of participants analysed (shown by year) might be smaller than number of randomised participants
Trial sequential analysis focused on serious adverse events (15 large, placebo controlled trials, 6462 randomised participants) revealed that the cumulative z score crossed the monitoring boundaries in 2018 (z score=2.77, P=0.006) before the required information size was reached (fig 6), indicating that the significantly higher rate of serious adverse events in patients receiving viscosupplementation compared with those receiving placebo is a robust finding.
Fig 6.
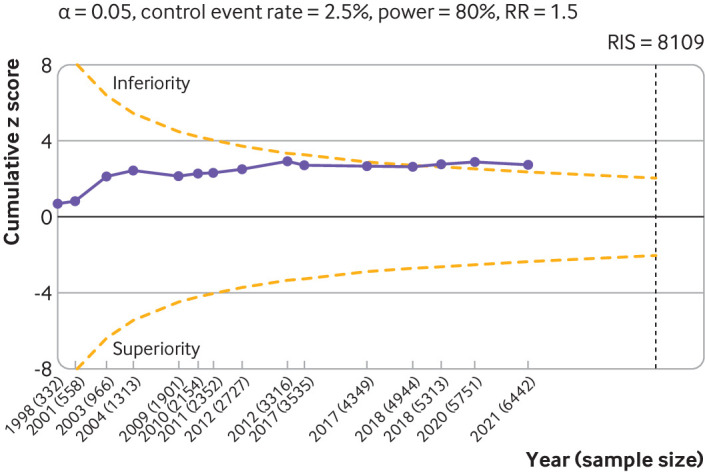
Trial sequential analysis of 15 large placebo controlled trials for serious adverse events. The analysis was based on 6462 randomised participants. Given the weak association between viscosupplementation and pain reduction in knee osteoarthritis, any increase in risk of serious adverse events caused by viscosupplementation compared with placebo can be considered a clinically important increase. Cumulative z scores are calculated under a random effects model. RIS (required information size) was calculated as the sample size that gives a trial 80% power to detect a 50% relative risk (RR) increase of serious adverse events, assuming a control event rate of 2.5% and a two sided α=0.05. O’Brien-Fleming monitoring boundaries are represented by dashed orange lines. Circles denote the z score for each additional trial. A D2 of 25% was assumed. Non-peer reviewed reports had their disclosure year defined as the earliest year in which the document was first officially created (when available within the file), year of online publication (eg, results first posted date on ClinicalTrials.gov), or the date on which the material was made available for review. Number of participants analysed (shown by year) might be smaller than number of randomised participants
Discussion
Principal findings
This systematic review identified 169 trials on 21 163 randomised patients with knee osteoarthritis. The main analysis of the primary outcome was based on 24 large, placebo controlled trials that included 8997 randomised patients with knee osteoarthritis. We found that viscosupplementation was significantly associated with a small reduction in pain intensity compared with placebo, but the difference was less than the minimal clinically important between group difference. Since 2009 strong evidence has shown that the pain reduction associated with viscosupplementation is clinically equivalent to the pain reduction associated with placebo when the equivalence margin is 0.2 SMD units (or a margin of 5 mm on a 100 mm visual analogue scale). Similar results were observed for function. We also found that viscosupplementation is associated with a higher incidence of serious adverse events compared with placebo.
The largest review on viscosupplementation for knee osteoarthritis was published in 2012 and analysed 89 trials with 12 667 patients.25 Our review includes 80 additional trials (representing an increase of 8496 participants), a cumulative meta-analysis, and trial sequential analysis. In contrast to the 2012 review,25 our review shows conclusive high quality evidence from randomised trials on the association between viscosupplementation and potential serious harmful effects, and the absence of clinically relevant benefits.
Overall, at least four major lessons can be drawn from 50 years of randomised evidence on viscosupplementation for knee osteoarthritis. Firstly, there was a dramatic increase in the number of trials after 2009, when sufficient evidence was already available to refute the benefits of viscosupplementation beyond those obtained with placebo. Secondly, the number of trials published or conducted after 2012 far outpaced the field’s capacity to find, appraise, and distil the evidence. As a result, many trials we identified never made their way into any systematic review.59 Although a large review conducted before this review reported a clinically non-relevant effect of viscosupplementation with a narrow 95% confidence interval, at least nine additional large, placebo controlled trials were registered and conducted after this review was published.25
Thirdly, we found evidence for the non-publication of many adequately powered, industry funded trials. Examples include large trials on viscosupplementation for knee osteoarthritis, but they were never fully published (ClinicalTrials.com identifier NCT01934218, n=814; NCT01372475, n=800; and NCT00988091, n=596; recruitment for these trials completed in 2016, 2013, and 2011, respectively). These trials, which tested different viscosupplementation formulations with distinct biochemical properties, reported similar or worse treatment effects on osteoarthritis pain than placebo. Only a subgroup analysis of one trial (NCT01934218) has been published, which is indicative of selective reporting.60 At the time of writing, at least 12 other unpublished trials were known to be completed, but their results were not retrievable (>3000 patients randomised in total). Additionally, we could not include the data of three unpublished, industry sponsored, placebo controlled trials (two of which are large trials), which were previously included in the 2012 systematic review25 because formal approval was not given by the companies.
Fourthly, based on the trial sequential analysis, between 2009 and 2021 alone, more than 12 000 patients were subjected to intra-articular injections in viscosupplementation trials, which raises ethical concerns. Based on our trial sequential analysis, a sufficient number of patients have been accrued to confidently conclude that viscosupplementation is not only ineffective compared with placebo but might also be seriously harmful and therefore should be used cautiously in any ongoing trial.
Strengths and limitations
To our knowledge, this is the largest collection of randomised trials on viscosupplementation for knee osteoarthritis, representing a twofold increase in the number of trials compared with a comprehensive meta-analysis conducted previously.25 59 61 Unlike most previous systematic reviews, which have applied more restricted search methods and inclusion criteria,59 61 our international search for viscosupplementation trials was broad and not limited by publication status or language of publication. The predefined minimal clinically important between group difference of 9 on a 100 mm visual analogue scale used in this review can be considered conservative because some have reported minimal clinically important between group differences >20 mm.62 63 64 Our analyses of the association between viscosupplementation and clinical outcomes based on large, placebo controlled trials significantly decreases the risk of biases influencing our conclusions.
This study has several limitations. The findings represent summary estimates and do not exclude the possibility that selected osteoarthritis patient populations could benefit from viscosupplementation. Results from the meta-regression models are considered exploratory and should be interpreted with caution given the multiplicity of tests.
Our results corroborate previous concerns about the safety profile of viscosupplementation.25 However, to strengthen the notion of causality, the biological plausibility of the safety signals observed in our review should be established through an individual patient data meta-analysis with careful readjudication and classification of all serious adverse events. Patients included in randomised trials tend to have fewer comorbidities and use fewer drugs than patients commonly seen in real clinical settings.65 66 Although we found conclusive evidence of an increased risk of serious adverse events with viscosupplementation in trial populations, it is plausible that this risk could be more pronounced in a more fragile patient population, commonly seen in clinical settings outside of clinical trials.65 66 Therefore, large, properly conducted observational studies or phase IV post marketing surveillance trials would provide useful evidence.
Most trials included in our trial sequential analyses of effectiveness only reported results from intention to treat analyses, which can dilute treatment effects.67 Ideally, intention-to-treat analyses and per protocol analyses should be conducted separately, and equivalence be shown for both types of analysis.68 69 However, only two trials included in our trial sequential analyses of pain and function reported results from per protocol analyses, and so we were unable to perform both types of analysis. Given the strength of the evidence established in the trial sequential analysis, it seems unlikely that additional per protocol analyses would change our conclusions.
Conclusions
Strong conclusive evidence indicates that, among patients with knee osteoarthritis, viscosupplementation is associated with a clinically irrelevant reduction in pain intensity and with an increased risk of serious adverse events compared with placebo. Our findings do not support the broad use of viscosupplementation for the treatment of knee osteoarthritis.
What is already known on this topic
Intra-articular injections of hyaluronic derivatives (viscosupplementation) have been used to treat knee osteoarthritis for over 50 years
The effectiveness and safety of this treatment are still a topic of debate
Emerging evidence indicates that treatment effects could be smaller than previously reported
What this study adds
Strong conclusive evidence indicates that viscosupplementation leads to a small reduction in knee osteoarthritis pain compared with placebo, but the difference is less than the minimal clinically important between group difference
Strong conclusive evidence also indicates that viscosupplementation increases the risk of serious adverse events compared with placebo
The findings do not support broad use of viscosupplementation to treat knee osteoarthritis
Acknowledgments
We thank Martina Rudnicki, Institute of Ophthalmology, University College London, UK; Gülen Hatemi, Istanbul University Cerrahpasa Medical School, Turkey; Lars G Hemkens, Department of Clinical Research, University Hospital Basel, University of Basel, Switzerland; and Melodi Koşaner Kließ, Program in Health Technology Assessment, University of Glasgow, UK for their translations and data extractions of non-English trials. None of these people was compensated. Written permission has been obtained for all names mentioned.
Web extra.
Extra material supplied by authors
Web appendix: Web appendix
Contributors: TVP and PJ contributed equally to this work. BRdC and PJ conceived the idea for the review. BRdC, PJ, and TVP designed, undertook the literature search, and coordinated the study. AA, CAH, DX, LY, PB, and PS gave crucial intellectual input and provided critical revision for the initial protocol and database building. PB and PS contributed to the implementation of the study. TVP, PJ, AA, CAH, DX, LY, PB, PS, and BRdC acquired data, screened records, extracted data, and assessed risk of bias. TVP coded the statistical analysis, figures, and appendix in collaboration with PJ and BRdc. TVP, PJ, and BRdC analysed and interpreted the data. TVP, PJ, and BRdC wrote the first draft of the manuscript. All authors gave crucial feedback on the revised report and approved the final version of the manuscript. BRdC and PJ obtained funding. TVP, PJ, and BRdC are the guarantors of this manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by the Arthritis Society (grant No YIO-17-0164) and by St Michael’s Hospital Foundation. PJ is a tier 1 Canada research chair in clinical epidemiology of chronic diseases. This research was completed, in part, with funding from the Canada Research Chairs Programme. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the National Institute for Health Research. PB is supported by the Arthritis Society Postdoctoral Fellowship Award with funding reference No 20-0000000016. TVP was funded by the Chevening Scholarship Programme (Foreign and Commonwealth Office, UK). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the Arthritis Society, Canada Research Chairs Programme, National Institute for Health Research, Chevening Scholarship Program for the submitted work. PJ serves as unpaid member of the steering group of trials funded by Appili Therapeutics, Abbot Vascular, and Terumo; he has received research grants to the institution from Appili Therapeutics, and honorariums to the institution for participation in advisory boards or consulting from Amgen, Ava and Fresenius, but has not received personal payments by any pharmaceutical company or device manufacturer. All other authors report no financial relationships with any organisations that might have an interest in the submitted work in the previous three years. All authors report no other relationships or activities that could appear to have influenced the submitted work.
The lead author (BRdC) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: The results will be disseminated to clinicians, patient advocacy groups, and patient organisations, and also through press release and social media.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
This is a systematic review and meta-analysis and does not require ethical approval.
Data availability statement
The guarantor (BRdC) is willing to examine all requests for the full dataset after a period of two years from the date of this publication. The corresponding author should be contacted at bruno.dacosta@utoronto.ca
References
- 1. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019;393:1745-59. 10.1016/S0140-6736(19)30417-9 [DOI] [PubMed] [Google Scholar]
- 2. Sharma L. Osteoarthritis of the knee. N Engl J Med 2021;384:51-9. 10.1056/NEJMcp1903768 [DOI] [PubMed] [Google Scholar]
- 3. Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020;29-30:100587. 10.1016/j.eclinm.2020.100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jones IA, Togashi R, Wilson ML, Heckmann N, Vangsness CT, Jr. Intra-articular treatment options for knee osteoarthritis. Nat Rev Rheumatol 2019;15:77-90. 10.1038/s41584-018-0123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rydell N, Balazs EA. Effect of intra-articular injection of hyaluronic acid on the clinical symptoms of osteoarthritis and on granulation tissue formation. Clin Orthop Relat Res 1971;80:25-32. 10.1097/00003086-197110000-00006 [DOI] [PubMed] [Google Scholar]
- 6. McAlindon TE, Bannuru RR. Osteoarthritis: is viscosupplementation really so unsafe for knee OA? Nat Rev Rheumatol 2012;8:635-6. 10.1038/nrrheum.2012.152 [DOI] [PubMed] [Google Scholar]
- 7. Balazs EA. Viscosupplementation for treatment of osteoarthritis: from initial discovery to current status and results. Surg Technol Int 2004;12:278-89. [PubMed] [Google Scholar]
- 8. Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA 2021;325:568-78. 10.1001/jama.2020.22171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NICE. Osteoarthritis: care and management. Clinical guideline [CG177].https://www.nice.org.uk/guidance/cg177/chapter/Recommendations#pharmacological-management%202014 (accessed May 8, 2022)
- 10. Migliore A, Integlia D, Pompilio G, Di Giuseppe F, Aru C, Brown T. Cost-effectiveness and budget impact analysis of viscosupplementation with hylan G-F 20 for knee and hip osteoarthritis. Clinicoecon Outcomes Res 2019;11:453-64. 10.2147/CEOR.S194669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mordin M, Parrish W, Masaquel C, Bisson B, Copley-Merriman C. Intra-articular hyaluronic acid for osteoarthritis of the knee in the united states: a systematic review of economic evaluations. Clin Med Insights Arthritis Musculoskelet Disord 2021;14:11795441211047284. 10.1177/11795441211047284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu KY, Acuña AJ, Samuel LT, Grits D, Kamath AF. Hyaluronic acid injections for knee osteoarthritis: has utilization among medicare beneficiaries changed between 2012 and 2018? J Bone Joint Surg Am 2022;104:e43. 10.2106/JBJS.21.00832 [DOI] [PubMed] [Google Scholar]
- 13. Dysart S, Utkina K, Stong L, et al. Insights from real-world analysis of treatment patterns in patients with newly diagnosed knee osteoarthritis. Am Health Drug Benefits 2021;14:56-62. [PMC free article] [PubMed] [Google Scholar]
- 14. Schmajuk G, Bozic KJ, Yazdany J. Using Medicare data to understand low-value health care: the case of intra-articular hyaluronic acid injections. JAMA Intern Med 2014;174:1702-4. 10.1001/jamainternmed.2014.3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W65-94. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 16. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2006;2006:CD005321. 10.1002/14651858.CD005321.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hunter DJ. Viscosupplementation for osteoarthritis of the knee. N Engl J Med 2015;372:1040-7. 10.1056/NEJMct1215534 [DOI] [PubMed] [Google Scholar]
- 18. Jüni P, Reichenbach S, Dieppe P. Osteoarthritis: rational approach to treating the individual. Best Pract Res Clin Rheumatol 2006;20:721-40. 10.1016/j.berh.2006.05.002 [DOI] [PubMed] [Google Scholar]
- 19. Reichenbach S, Sterchi R, Scherer M, et al. Meta-analysis: chondroitin for osteoarthritis of the knee or hip. Ann Intern Med 2007;146:580-90. 10.7326/0003-4819-146-8-200704170-00009 [DOI] [PubMed] [Google Scholar]
- 20.European Comission. Clinical investigations. Serious adverse event reporting under directives 90/385/EEC and 93/42/EEC. Brussels, Belgium: European Commission; 2015. https://ec.europa.eu/docsroom/documents/16477/attachments/1/translations/en/renditions/pdf (accessed Sep 1, 2021).
- 21. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Altman DG, Gøtzsche PC, et al. Cochrane Bias Methods Group. Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. da Costa BR, Pereira TV, Saadat P, et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ 2021;375:n2321. 10.1136/bmj.n2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jüni P, Hari R, Rutjes AW, et al. Intra-articular corticosteroid for knee osteoarthritis. Cochrane Database Syst Rev 2015;(10):CD005328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med 2012;157:180-91. 10.7326/0003-4819-157-3-201208070-00473 [DOI] [PubMed] [Google Scholar]
- 26.Wandel S, Jüni P, Tendal B, Nüesch E, Villiger PM, Welton NJ et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ 2010; 341:c4675:c4675. [DOI] [PMC free article] [PubMed]
- 27. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 28. Nüesch E, Trelle S, Reichenbach S, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 2010;341:c3515. 10.1136/bmj.c3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. da Costa BR, Juni P. Systematic reviews and meta-analyses of randomized trials: principles and pitfalls. Eur Heart J 2014;35:3336-45. 10.1093/eurheartj/ehu424 [DOI] [PubMed] [Google Scholar]
- 30. Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta-analysis. BMC Med Res Methodol 2017;17:39. 10.1186/s12874-017-0315-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ioannidis JPA. The proposal to lower P value thresholds to .005. JAMA 2018;319:1429-30. 10.1001/jama.2018.1536 [DOI] [PubMed] [Google Scholar]
- 32. Sterne JA, Davey Smith G. Sifting the evidence-what’s wrong with significance tests? BMJ 2001;322:226-31. 10.1136/bmj.322.7280.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shichikawa K, Maeda A, Ogawa N. [Clinical evaluation of sodium hyaluronate in the treatment of osteoarthritis of the knee]. Ryumachi 1983;23:280-90. [PubMed] [Google Scholar]
- 35. Puhl W, Bernau A, Greiling H, et al. Intra-articular sodium hyaluronate in osteoarthritis of the knee: a multicenter, double-blind study. Osteoarthritis Cartilage 1993;1:233-41. 10.1016/S1063-4584(05)80329-2 [DOI] [PubMed] [Google Scholar]
- 36. Lohmander LS, Dalén N, Englund G, et al. Hyaluronan Multicentre Trial Group . Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: a randomised, double blind, placebo controlled multicentre trial. Ann Rheum Dis 1996;55:424-31. 10.1136/ard.55.7.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Altman RD, Moskowitz R, Hyalgan Study Group . Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. J Rheumatol 1998;25:2203-12. [PubMed] [Google Scholar]
- 38. Brandt KD, Block JA, Michalski JP, Moreland LW, Caldwell JR, Lavin PT, ORTHOVISC Study Group . Efficacy and safety of intraarticular sodium hyaluronate in knee osteoarthritis. Clin Orthop Relat Res 2001;(385):130-43. 10.1097/00003086-200104000-00021 [DOI] [PubMed] [Google Scholar]
- 39.Seikagaku Corporation. Summary of safety and effectiveness data: Sodiumhyaluronate. Bethesda, MD. U.S. Food and Drug Administration; 2001. [UK]. https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170016B.pdf (accessed Oct 21, 2021).
- 40. Jubb RW, Piva S, Beinat L, Dacre J, Gishen P. A one-year, randomised, placebo (saline) controlled clinical trial of 500-730 kDa sodium hyaluronate (Hyalgan) on the radiological change in osteoarthritis of the knee. Int J Clin Pract 2003;57:467-74. [PubMed] [Google Scholar]
- 41. Altman RD, Akermark C, Beaulieu AD, Schnitzer T, Durolane International Study Group . Efficacy and safety of a single intra-articular injection of non-animal stabilized hyaluronic acid (NASHA) in patients with osteoarthritis of the knee. Osteoarthritis Cartilage 2004;12:642-9. 10.1016/j.joca.2004.04.010 [DOI] [PubMed] [Google Scholar]
- 42. Day R, Brooks P, Conaghan PG, Petersen M, Multicenter Trial Group . A double blind, randomized, multicenter, parallel group study of the effectiveness and tolerance of intraarticular hyaluronan in osteoarthritis of the knee. J Rheumatol 2004;31:775-82. [PubMed] [Google Scholar]
- 43. Pham T, Le Henanff A, Ravaud P, Dieppe P, Paolozzi L, Dougados M. Evaluation of the symptomatic and structural efficacy of a new hyaluronic acid compound, NRD101, in comparison with diacerein and placebo in a 1 year randomised controlled study in symptomatic knee osteoarthritis. Ann Rheum Dis 2004;63:1611-7. 10.1136/ard.2003.019703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Altman RD, Rosen JE, Bloch DA, Hatoum HT, Korner P. A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA for treatment of painful osteoarthritis of the knee, with an open-label safety extension (the FLEXX trial). Semin Arthritis Rheum 2009;39:1-9. 10.1016/j.semarthrit.2009.04.001 [DOI] [PubMed] [Google Scholar]
- 45. Baltzer AW, Moser C, Jansen SA, Krauspe R. Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthritis Cartilage 2009;17:152-60. 10.1016/j.joca.2008.06.014 [DOI] [PubMed] [Google Scholar]
- 46. Chevalier X, Jerosch J, Goupille P, et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann Rheum Dis 2010;69:113-9. 10.1136/ard.2008.094623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jørgensen A, Stengaard-Pedersen K, Simonsen O, et al. Intra-articular hyaluronan is without clinical effect in knee osteoarthritis: a multicentre, randomised, placebo-controlled, double-blind study of 337 patients followed for 1 year. Ann Rheum Dis 2010;69:1097-102. 10.1136/ard.2009.118042 [DOI] [PubMed] [Google Scholar]
- 48. Huang TL, Chang CC, Lee CH, Chen SC, Lai CH, Tsai CL. Intra-articular injections of sodium hyaluronate (Hyalgan®) in osteoarthritis of the knee. a randomized, controlled, double-blind, multicenter trial in the Asian population. BMC Musculoskelet Disord 2011;12:221. 10.1186/1471-2474-12-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Strand V, Baraf HSB, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage 2012;20:350-6. 10.1016/j.joca.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 50.Ferring Pharmaceuticals. A 26 week, double-blind, randomized, placebo-controlled study of the efficacy and safety of single intra-articular injection 1.2% sodium hyaluronate for treatment of painful osteoarthritis of the knee, with optional 26-week open-label safety extension). https://clinicaltrials.gov/ct2/show/NCT00988091 (accessed 1 Sep 2021).
- 51. Arden NK, Åkermark C, Andersson M, Todman MG, Altman RD. A randomized saline-controlled trial of NASHA hyaluronic acid for knee osteoarthritis. Curr Med Res Opin 2014;30:279-86. 10.1185/03007995.2013.855631 [DOI] [PubMed] [Google Scholar]
- 52.Fidia Farmaceutici A. Multi-centre, double-blind, randomized, placebo controlled study to evaluate the safety and effectiveness of a new viscoelastic hydrogel (Hymovis) in the treatment of knee oa with an open-label extension. https://clinicaltrials.gov/ct2/show/NCT01372475; https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150010b.pdf (accessed 21 Oct 2021).
- 53.Seikagaku Corporation. A Multi-Center, Randomized, Double-Blind, Phosphate Buffered Saline-Controlled Study to Evaluate Effectiveness and Safety of a Single Intra-Articular Injection of Gel-One? for the Treatment of Osteoarthritis of the Knee With Open-Label Safety Extension. https://clinicaltrials gov/ct2/show/NCT01934218 and https://www.accessdata.fda.gov/cdrh_docs/pdf8/P080020S020B.pdf (accessed Oct 21, 2021).
- 54. Hangody L, Szody R, Lukasik P, et al. Intraarticular injection of a cross-linked sodium hyaluronate combined with triamcinolone hexacetonide (Cingal) to provide symptomatic relief of osteoarthritis of the knee: a randomized, double-blind, placebo-controlled multicenter clinical trial. Cartilage 2018;9:276-83. 10.1177/1947603517703732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Petterson SC, Plancher KD. Single intra-articular injection of lightly cross-linked hyaluronic acid reduces knee pain in symptomatic knee osteoarthritis: a multicenter, double-blind, randomized, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc 2019;27:1992-2002. 10.1007/s00167-018-5114-0 [DOI] [PubMed] [Google Scholar]
- 56.Teva Pharmaceuticals. A double-blind, randomized, study of the effectiveness and safety of hyaluronate injectable viscosupplement for treatment of osteoarthritis of the knee. https://clinicaltrials.gov/ct2/show/NCT02495857 and https://www.accessdata.fda.gov/cdrh_docs/pdf17/P170016B.pdf (accessed 21 Oct 2021).
- 57. Ke Y, Jiang W, Xu Y, et al. Efficacy and safety of a single intra-articular injection of 6 ml Hylan G-F 20 compared to placebo in Chinese patients with symptomatic knee osteoarthritis : C-SOUND study, a 26-week multicenter double-blind randomized placebo-controlled trial in China. BMC Musculoskelet Disord 2021;22:428. 10.1186/s12891-021-04252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Migliore A, Blicharski T, Plebanski R, et al. Knee osteoarthritis pain management with an innovative high and low molecular weight hyaluronic acid formulation (HA-HL): a randomized clinical trial. Rheumatol Ther 2021;8:1617-36. 10.1007/s40744-021-00363-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xing D, Wang B, Zhang W, et al. Intra-articular hyaluronic acid injection in treating knee osteoarthritis: assessing risk of bias in systematic reviews with ROBIS tool. Int J Rheum Dis 2017;20:1658-73. 10.1111/1756-185X.13192 [DOI] [PubMed] [Google Scholar]
- 60. Takamura J, Seo T, Strand V. A single intra-articular injection of gel-200 for treatment of symptomatic osteoarthritis of the knee is more effective than phosphate buffered saline at 6 months: a subgroup analysis of a multicenter, randomized controlled trial. Cartilage 2019;10:417-22. 10.1177/1947603518768015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xing D, Wang B, Liu Q, et al. Intra-articular hyaluronic acid in treating knee osteoarthritis: a PRISMA-compliant systematic review of overlapping meta-analysis. Sci Rep 2016;6:32790. 10.1038/srep32790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol 2000;27:2635-41. [PubMed] [Google Scholar]
- 63. Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Eur J Pain 2004;8:283-91. 10.1016/j.ejpain.2003.09.004 [DOI] [PubMed] [Google Scholar]
- 64. Quintana JM, Escobar A, Bilbao A, Arostegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after hip joint replacement. Osteoarthritis Cartilage 2005;13:1076-83. 10.1016/j.joca.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 65. Rogers JR, Liu C, Hripcsak G, Cheung YK, Weng C. Comparison of clinical characteristics between clinical trial participants and nonparticipants using electronic health record data. JAMA Netw Open 2021;4:e214732. 10.1001/jamanetworkopen.2021.4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019;27:1578-89. 10.1016/j.joca.2019.06.011 [DOI] [PubMed] [Google Scholar]
- 67. Detry MA, Lewis RJ. The intention-to-treat principle: how to assess the true effect of choosing a medical treatment. JAMA 2014;312:85-6. 10.1001/jama.2014.7523 [DOI] [PubMed] [Google Scholar]
- 68. Jones B, Jarvis P, Lewis JA, Ebbutt AF. Trials to assess equivalence: the importance of rigorous methods. BMJ 1996;313:36-9. 10.1136/bmj.313.7048.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Walker E, Nowacki AS. Understanding equivalence and noninferiority testing. J Gen Intern Med 2011;26:192-6. 10.1007/s11606-010-1513-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Web appendix
Data Availability Statement
The guarantor (BRdC) is willing to examine all requests for the full dataset after a period of two years from the date of this publication. The corresponding author should be contacted at bruno.dacosta@utoronto.ca