Abstract
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, is associated with significant morbidity and mortality due to pneumonia, acute respiratory distress syndrome (ARDS) and multiorgan failure. Liver injury has been reported as a non-pulmonary manifestation of COVID-19 but characterization of liver test abnormalities and their association with clinical outcomes is incomplete. We conducted a retrospective cohort study of 1827 patients with confirmed COVID-19 who were hospitalized within the Yale-New Haven Health System (YNHHS) between March 14, 2020 and April 23, 2020. Clinical characteristics, liver tests (AST, ALT, ALP, TBIL, albumin) at three time points (pre-infection baseline, admission, peak hospitalization), and hospitalization outcomes (severe COVID-19, ICU admission, mechanical ventilation, death) were analyzed. Abnormal liver tests were commonly observed in hospitalized patients with COVID-19, both at admission (AST 66.9%, ALT 41.6%, ALP 13.5%, TBIL 4.3%) and peak hospitalization (AST 83.4%, ALT 61.6%, ALP 22.7%, TBIL 16.1%). Most patients with abnormal liver tests at admission had minimal elevations 1-2x ULN (AST 63.7%, ALT 63.5%, ALP 80.0%, TBIL 75.7%). A significant proportion of these patients had abnormal liver tests pre-hospitalization (AST 25.9%, ALT 38.0%, ALP 56.8%, TBIL 44.4%). Multivariate analysis revealed an association between abnormal liver tests and severe COVID-19, including ICU admission, mechanical ventilation, and death; associations with age, male gender, BMI, and diabetes mellitus were also observed. Medications used in COVID-19 treatment (lopinavir/ritonavir, hydroxychloroquine, remdesivir, and tocilizumab) were associated with peak hospitalization liver transaminase elevations >5x ULN.
Conclusion:
Abnormal liver tests occur in most hospitalized patients with COVID-19 and may be associated with poorer clinical outcomes.
Keywords: SARS-CoV-2, coronavirus, liver function tests, liver injury, hepatitis
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first described in December 2019 in patients with severe pneumonia in Wuhan, China [1]. The World Health Organization declared COVID-19 a global pandemic in March 2020 [2]. Although the initial burden of disease was predominantly found in China [1,3,4,5,6], the United States (U.S.) has reported the most cases of COVID-19 and COVID-19-related death globally since March 26, 2020 and April 11, 2020, respectively [7]. This infection is estimated to have resulted in over 11 million cases and 500,000 deaths globally, including 3 million cases and 130,000 deaths in the U.S. as of July 8, 2020 [7].
While patients with COVID-19 typically present with fever and respiratory symptoms consistent with pneumonia [1,8], SARS-CoV-2 has been associated with multiple extrapulmonary effects [1], including gastrointestinal and hepatic manifestations [9–22]. Previous studies have identified increased liver tests in 14-78% of affected individuals, primarily serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) [16–18]. Although the prevalence and severity of abnormal liver tests have been described [19], their relationship to baseline liver tests prior to COVID-19 hospitalization remains poorly understood [20]. Furthermore, although studies from China have reported associations between abnormal liver tests and hospitalization length of stay [14], risk for progression to severe COVID-19 [16–18], and mortality [21], there are limited data about these relationships in U.S. cohorts [11, 23]. This study aims to characterize liver test abnormalities and their association with clinical outcomes in hospitalized patients with COVID-19 in a major U.S. hospital network.
Methods:
Study design and participants
Study inclusion was limited to patients admitted to five hospitals (Yale-New Haven Hospital, Bridgeport Hospital, Greenwich Hospital, Lawrence and Memorial Hospital, Westerly Hospital) within the Yale New Haven Health System (YNHHS) between March 14, 2020 and April 23, 2020 who tested positive for SARS-CoV-2 via nasopharyngeal swab polymerase chain reaction (PCR). De-identified patient data were obtained by query of the Epic Systems electronic health record (EHR) through the YNHHS Joint Data Analytics Team and Yale Center for Medical Informatics and deemed exempt from regulatory requirements applicable to human subjects research by the Yale Human Research Protection Program. Demographic information was obtained, including age at admission, sex, body mass index (BMI), and diabetes mellitus (DM) based on ICD-10 code. Three values for liver tests were obtained: pre-hospitalization, admission, and peak value during hospitalization. Liver tests included serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (TBIL), and albumin. Liver test abnormalities were defined as per YNHHS laboratory reference range standards: AST >33 U/L, ALT > 34 U/L, ALP >122 U/L, TBIL >1.2 mg/dL, albumin <3.5 mg/dL. Medications used for the treatment of COVID-19 were assessed, including lopinavir/ritonavir (n=136), hydroxychloroquine (n=1469), remdesivir (n=46), and tocilizumab (n=772). Of 1827 patients with confirmed COVID-19 during the study period, 96% completed liver tests at admission or during the hospitalization: AST (1756), ALT (1753), ALP (1754), TBIL (1747), albumin (1746). Clinical characteristics data included oxygen saturation by pulse oximetry (%), fraction of inspired oxygen (FiO2) supplemental oxygen requirement, oxygen flow (L/min), ICU admission, respiratory failure requiring mechanical ventilation, use of vasopressors or extracorporeal membrane oxygenation (ECMO), and death. Severe COVID-19 was defined as ICU admission, respiratory failure requiring mechanical ventilation, use of vasopressor therapy or ECMO, or death. Death was assessed at end of study period on April 23, 2020.
Statistical analysis
Demographic characteristics and baseline admission liver tests were compared by severity group using Student’s t-test for the mean and the non-parametric Wilcoxon rank sum test for the median of continuous variables and chi-square test for categorical variables. The distribution of liver tests was reported as within the normal range, 1-2 times the upper limit of normal (ULN), 2-5 times ULN, and over 5 times ULN at three points in time, including pre-hospitalization, hospital admission, and peak value during hospitalization. These results were summarized using count and percentage and compared by using generalized linear models that account for the correlation among lab values measured on the same patients across three time points. The association between demographics and the presence of an abnormal liver test on admission was examined using Student’s t-test for the mean and the non-parametric Wilcoxon rank sum test for the median of continuous variables and chi-square test for categorical variables. The comparison of liver tests, stratified by severity, at both pre-hospitalization and admission time points was performed using the Wilcoxon signed rank test. Clinical outcomes were modeled using liver test results at admission and at their peak during hospitalization using univariate logistic regression. Multivariate logistic regression was used to adjust for age, gender, BMI, diabetes status, liver tests, and medications. P-values less than or equal to 0.05 were considered statistically significant. All analyses were completed in SAS (version 9.4; Cary, NC) and R (version 3.6.2).
Results:
Clinical characteristics of the YNHHS cohort are summarized in Table 1. Of 1827 patients in this cohort, the mean age was 65 years (range 1-103), 53.0% were male, the mean BMI was 29.8 (42.5% obese), and 39.0% of patients had diabetes mellitus. On hospital admission, abnormal liver tests were commonly observed (AST 66.9%, ALT 41.6%, ALP 13.5%, TBIL 4.3%, albumin 56.7%). Severe COVID-19 infection was more likely to be seen in patients with increased age, male gender, higher BMI, and abnormal liver tests. The prevalence and severity of abnormal liver tests are summarized in Table 2. Abnormal liver tests were commonly observed in hospitalized patients with COVID-19 both pre-hospitalization (AST 20.3%, ALT 19.1%, ALP 13.4%, TBIL 4.1%, albumin 27.0%) and peak hospitalization (AST 83.4%, ALT 61.6%, ALP 22.7%, TBIL 16.1%, albumin 86.6%). Most patients with abnormal liver tests had minimal elevations pre-hospitalization or at admission, but an important subset of patients experienced more extreme elevations of liver transaminases (>5x ULN) during hospitalization (AST 16.6%, ALT 20.6%), although this was less commonly observed with other liver tests (ALP 1.5%, TBIL 3.9%). Baseline pre-hospitalization values were available in greater than 70% of patients with abnormal liver tests at admission (Table 3) and were abnormal in a significant proportion (AST 25.9%, ALT 38.0%, ALP 56.8%, TBIL 44.4%, albumin 27.1%). Predictors of abnormal liver tests at hospital admission are summarized in Table 4, including age, male gender, BMI, diabetes mellitus, and pre-hospitalization abnormal liver tests. Predictors of peak hospitalization abnormal liver tests >5x ULN are summarized in Table 5, including age, male gender, BMI, diabetes mellitus, and medications (lopinavir/ritonavir, hydroxychloroquine, remdesivir, and tocilizumab). The association between admission and peak hospitalization liver tests and clinical outcomes (ICU admission, mechanical ventilation, and death) are summarized in Table 6. On multivariate analysis, male gender, BMI, diabetes mellitus, and abnormal liver tests were associated with ICU stay, mechanical ventilation, and death. The relationship between pre-hospitalization and admission liver tests in patients with severe and non-severe COVID-19 is illustrated in Figure 1. A significant increase in transaminases and significant decrease in albumin were observed regardless of severity; a significant increase in total bilirubin was seen in patients with severe COVID-19. After exclusion of patients with abnormal liver tests prior to hospital admission, key associations between abnormal liver tests at admission and severe COVID-19, as well as between abnormal liver tests at peak hospitalization and clinical outcomes (ICU admission, mechanical ventilation) were sustained on multivariate analysis (Supplementary Tables 1–3).
Table 1:
Hospital admission liver tests of 1827 patients with COVID-19
| Characteristic | Total (n=1827) | Non-severe COVID-19 (n=1175) | Severe COVID-19 (n=652) | p-value |
|---|---|---|---|---|
|
| ||||
| Age, mean (SD), years | 64.6 (18.2) | 63.4 (18.5) | 66.7 (17.5) | <0.001 |
|
| ||||
| Male gender, n (%) | 969 (53.0%) | 574 (48.9%) | 395 (60.6%) | <0.001 |
|
| ||||
| BMI, mean (SD) | 29.8 (7.8) | 29.2 (7.3) | 30.7 (8.5) | <0.001 |
|
| ||||
| Diabetes mellitus, n (%) | 712 (39.0%) | 412 (35.1%) | 300 (46.0%) | <0.001 |
|
| ||||
| Obesity, n (%) | 748 (42.5%) | 455 (40.4%) | 293 (46.1%) | 0.019 |
|
| ||||
| AST, median (range), U/L | 42 (8-3054) | 38 (8-1399) | 51 (12-3054) | <0.001 |
| AST abnormal (>33 U/L) | 1158 (66.9%) | 667 (60.7%) | 491 (77.8%) | <0.001 |
|
| ||||
| ALT, median (IQR), U/L | 29 (5-1831) | 28 (5-1831) | 31 (6-1441) | <0.001 |
| ALT, abnormal (>34 U/L) | 726 (41.6%) | 440 (39.7%) | 286 (44.8%) | 0.035 |
|
| ||||
| ALP, median (IQR), U/L | 76 (20-919) | 76 (20-903) | 76 (28-919) | 0.79 |
| ALP abnormal (>122 U/L) | 237 (13.5%) | 140 (12.6%) | 97 (15.2%) | 0.13 |
|
| ||||
| TBIL, median (IQR), mg/dL | 0.5 (0.1-8.9) | 0.4 (0.1-8.0) | 0.5 (0.1-8.9) | <0.001 |
| TBIL, abnormal (>1.2 mg/dL) | 74 (4.3%) | 33 (3.0%) | 41 (6.5%) | <0.001 |
|
| ||||
| Albumin, median (IQR) mg/dL | 3.5 (1.2-5.4) | 3.5 (1.3-5.4) | 3.3 (1.2-4.8) | <0.001 |
| Albumin, abnormal (<3.5 mg/dL) | 990 (56.7%) | 584 (52.6%) | 406 (63.8%) | <0.001 |
SD = standard deviation; BMI = body mass index; AST = aspartate aminotransferase; ALT = alanine aminotransferase; IQR = interquartile range; ALP = alkaline phosphatase; TBIL = total bilirubin
Severe COVID-19 defined as: death OR ICU stay OR use of mechanical ventilation OR vasopressor requirement, OR ECMO requirement.
Table 2:
Pre-hospitalization vs. admission vs. peak hospitalization liver tests in patients with COVID-19
| Liver Test | Pre-Hospitalization | Admission | Peak Hospitalization |
|---|---|---|---|
|
| |||
| AST, U/L | n=1288 | n=1730 | n=1756 |
| Normal | 1027 (79.7%) | 572 (33.1%) | 291 (16.6%) |
| Abnormal | 261 (20.3%) | 1158 (66.9%) | 1465 (83.4%) |
| 1-2x ULN | 199 (76.2%) | 738 (63.7%) | 657 (44.8%) |
| 2-5x ULN | 53 (20.3%) | 351 (30.3%) | 565 (38.6%) |
| >5x ULN | 9 (3.4%) | 69 (6.0%) | 243 (16.6%) |
|
| |||
| ALT, U/L | n=1362 | n=1747 | n=1753 |
| Normal | 1102 (80.9%) | 1021 (58.4%) | 673 (38.4%) |
| Abnormal | 260 (19.1%) | 726 (41.6%) | 1080 (61.6%) |
| 1-2x ULN | 205 (78.9%) | 461 (63.5%) | 450 (41.7%) |
| 2-5x ULN | 49 (18.8%) | 222 (30.6%) | 408 (37.8%) |
| >5x ULN | 6 (2.3%) | 43 (5.9%) | 222 (20.6%) |
|
| |||
| ALP, U/L | N=1374 | N=1752 | N=1754 |
| Normal | 1190 (86.6%) | 1515 (86.5%) | 1355 (77.3%) |
| Abnormal | 184 (13.4%) | 237 (13.5%) | 399 (22.7%) |
| 1-2x ULN | 161 (87.5%) | 191 (80.6%) | 315 (78.9%) |
| 2-5x ULN | 20 (10.9%) | 42 (17.7%) | 78 (19.5%) |
| >5x ULN | 3 (1.6%) | 4 (1.7%) | 6 (1.5%) |
|
| |||
| TBIL, mg/dL | N=1343 | N=1729 | N=1747 |
| Normal | 1288 (95.9%) | 1655 (95.7%) | 1465 (83.9%) |
| Abnormal | 55 (4.1%) | 74 (4.3%) | 282 (16.1%) |
| 1-2x ULN | 47 (85.4%) | 56 (75.7%) | 201 (71.3%) |
| 2-5x ULN | 5 (9.1%) | 14 (18.9%) | 70 (24.8%) |
| >5x ULN | 3 (5.5%) | 4 (5.4%) | 11 (3.9%) |
|
| |||
| Albumin, mg/dL | N=1370 | N=1746 | N=1746 |
| Normal | 998 (73.0%) | 756 (43.3%) | 234 (13.4%) |
| Abnormal | 370 (27.0%) | 990 (56.7%) | 1512 (86.6%) |
AST = aspartate aminotransferase; ULN = upper limit of normal; ALT = alanine aminotransferase; ALP = alkaline phosphatase; TBIL = total bilirubin
Table 3:
Pre-hospitalization liver tests in patients with abnormal liver tests at hospital admission
| Abnormal test at admission | Pre-hospitalization liver test |
|---|---|
|
| |
| AST (n=768) | Normal 569 (74.1%) |
| Abnormal 199 (25.9%) | |
| 1-2x ULN=145 (72.9%) | |
| 2-5x ULN=45 (22.6%) | |
| >5x ULN=9 (4.0%) | |
|
| |
| ALT (n=473) | Normal 293 (62.0%) |
| Abnormal 180 (38.0%) | |
| 1-2x ULN=136 (75.6%) | |
| 2-5x ULN=40 (22.2%) | |
| >5x ULN=4 (2.2%) | |
|
| |
| ALP (n=176) | Normal 76 (43.2%) |
| Abnormal 100 (56.8%) | |
| 1-2x ULN=80 (80.0%) | |
| 2-5x ULN=18 (18.0%) | |
| >5x ULN=2 (2.0%) | |
|
| |
| TBIL (n=63) | Normal 35 (55.6%) |
| Abnormal 28 (44.4%) | |
| 1-2x ULN=21 (75.0%) | |
| 2-5x ULN=4 (14.2%) | |
| >5x ULN=3 (10.7%) | |
|
| |
| Albumin (n=719) | Normal 439 (61.1%) |
| Abnormal 280 (38.9%) | |
AST = aspartate aminotransferase; ULN = upper limit of normal; ALT = alanine aminotransferase; ALP = alkaline phosphatase; TBIL = total bilirubin
Table 4:
Predictors for abnormal liver tests at time of hospital admission in patients with COVID-19
| Characteristic | AST | ALT | ALP | TBIL | Albumin |
|---|---|---|---|---|---|
| Age, mean, years | 64.3 vs. 66.0 (NS) | 59.5 vs. 68.6 (<0.001) | 62.8 vs. 65.2 (NS) | 67.1 vs. 65.0 (NS) | 61.9 vs. 67.2 (<0.001) |
| Male gender, n (%) | 58.5 vs. 44.6% (<0.001) | 64.7 vs. 46.2% (<0.001) | 51.1 vs. 54.3% (NS) | 75.7 vs. 53.2% (<0.001) | 50.7 vs. 59.5% (0.016) |
| BMI (mean) | 30.0 vs. 29.3 (NS) | 30.8 vs. 29.1 (<0.001) | 28.4 vs. 30.0 (0.002) | 27.9 vs. 29.8 (0.037) | 30.3 vs. 29.4 (0.012) |
| Diabetes mellitus (%) | 38.4 vs. 41.8% (NS) | 33.1 vs. 44.2% (<0.001) | 40.1 vs. 39.5% (NS) | 32.4 vs. 39.8% (NS) | 40.1 vs. 39.1% (NS) |
| Preadmission AST Abnormal (%) | 23.1 vs. 11.9% (<0.001) | 32.9 vs. 13.4% (<0.001) | 34.3 vs. 17.9% (NS) | 36.5 vs. 19.5% (NS) | 21.0 vs. 19.5% (NS) |
| Preadmission ALT, U/L Abnormal (%) | 23.2 vs. 11.8% (<0.001) | 38.0 vs. 8.9% (<0.001) | 25.9 vs. 18.4% (NS) | 23.8 vs. 19.3% (NS) | 18.1 vs. 20.6% (NS) |
| Preadmission ALP, U/L Abnormal (%) | 13.4 vs. 13.1% (NS) | 13.8 vs. 13.1% (NS) | 56.8 vs. 6.6% (<0.001) | 20.6 vs. 13.0% (<0.001) | 9.9 vs. 15.9% (0.006) |
| Preadmission TBIL, mg/dL Abnormal (%) | 5.0 vs. 2.9% (NS) | 3.4 vs. 4.6% (NS) | 7.0 vs. 3.7% (0.015) | 44.4 vs. 2.1 (<0.001) | 5.1 vs 3.1% (NS) |
AST = aspartate aminotransferase; ALT = alanine aminotransferase; ALP = alkaline phosphatase; TBIL = total bilirubin; NS = not significant; BMI = body mass index
Table 5:
Predictors of abnormal liver tests greater than 5x ULN at time of peak liver test value during hospitalization
| Characteristic | AST n = 243 vs. 1513 | ALT n = 222 vs. 1531 | ALP n = 6 vs. 1748 | TBIL n = 11 vs. 1736 |
|---|---|---|---|---|
| Age, mean, years | 58.6 vs. 65.9 (<0.001) | 57.2 vs 66.0 (<0.001) | 63.8 vs 64.9 (NS) | 62.2 vs 65.0 (NS) |
| Male gender, n (%) | 68.7 vs. 51.42% (<0.001) | 72.1 vs 51.2% (<0.001) | 16.7 vs. 54.0% (NS) | 63.6 vs. 53.9% (NS) |
| BMI (mean) | 30.9 vs. 29.6 (0.015) | 30.7 vs 29.6 (NS) | 25.8 vs. 29.8 (NS) | 29.7 vs. 29.8 (NS) |
| Diabetes mellitus (%) | 30.0 vs. 41.0% (0.001) | 26.6 vs 41.4% (<0.001) | 16.7 vs. 39.7% (NS) | 9.1% vs. 39.8% (NS) |
| Preadmission AST Abnormal (%) | 32.3 vs. 18.4% (<0.001) | 25.8 vs. 19.6% (0.033) | 60.0 vs 20.0% (0.016) | 55.6 vs 20.0% (0.003) |
| Preadmission ALT, U/L Abnormal (%) | 31.8 vs. 17.5% (<0.001) | 34.5 vs. 17.5% (<0.001) | 40.0 vs. 19.3% (NS) | 11.1 vs. 19.6% (NS) |
| Preadmission ALP, U/L Abnormal (%) | 11.6 vs. 13.6% (0.026) | 9.7 vs. 13.8% (NS) | 100 vs. 13.0% (<0.001) | 33.3 vs. 13.2% (0.003) |
| Preadmission TBIL, mg/dL Abnormal (%) | 7.0 vs. 3.8% (0.004) | 4.9 vs. 4.1% (NS) | 25.0 vs. 4.1% (0.018) | 66.7 vs. 3.8% (<0.001) |
| Preadmission albumin, mg/dL Abnormal (%) | 24.1 vs. 27.4 (NS) | 21.0 vs 27.8% (NS) | 80.0 vs. 26.8% (0.021) | 55.6 vs. 26.7% (NS) |
| Hydroxychloroquine use (%) | 91.8 vs. 84.4% (p=0.003) | 92.8 vs. 84.5% (p<0.001) | 66.7 vs. 85.5% (NS) | 81.9 vs. 85.5% (NS) |
| Lopinavir/Ritonavir use (%) | 12.4 vs. 7.3% (p=0.008) | 16.2 vs. 6.9% (p<0.001) | 33.3 vs. 8.0% (NS) | 9.1 vs. 8.1% (NS) |
| Remdesivir use (%) | 5.8 vs 2.7% (p=0.011) | 5.9 vs. 2.7% (p=0.013) | 0 vs. 3.2% (NS) | 0 vs 3.2% (NS) |
| Tocilizumab use (%) | 69.1 vs. 40.0% (p<0.001) | 72.1 vs. 40.0% (<0.001) | 33.3 vs. 44.1% (NS) | 36.4 s. 44.2% (NS) |
AST = aspartate aminotransferase; ALT = alanine aminotransferase; ALP = alkaline phosphatase; TBIL = total bilirubin; NS = not significant; BMI = body mass index
Table 6:
Association (odds ratio) between admission and peak hospitalization liver tests and clinical outcomes
| ICU admission (multivariate model) | Mechanical ventilation (multivariate model) | Death (multivariate model) | |
|---|---|---|---|
|
| |||
| Age (1-year increase) | 0.99 (p=NS) | 0.99 (p=NS) | 1.07 (p<0.001) |
|
| |||
| Male | 1.66 (p<0.001) | 2.06 (p<0.001) | 1.71 (p=0.01) |
|
| |||
| BMI (1-point increase) | 1.03 (p<0.001) | 1.04 (p<0.001) | 1.03 (p=0.03) |
|
| |||
| Diabetes mellitus | 1.33 (p=0.04) | 1.47 (p=0.03) | 1.05 (p=NS) |
|
| |||
| Admission | |||
| Abnormal AST | 2.36 (p<0.001) | 3.09 (p<0.001) | 1.12 (p=NS) |
| Abnormal ALT | 0.85 (p=NS) | 0.82 (p=NS) | 1.01 (p=NS) |
| Abnormal ALP | 1.62 (p=0.03) | 1.74 (p=0.03) | 0.95 (p=NS) |
| Abnormal TBIL | 0.94 (p=NS) | 0.78 (p=NS) | 5.62 (p<0.001) |
| Abnormal albumin | 1.33 (p=0.06) | 1.54 (p=0.02) | 1.33 (p=NS) |
|
| |||
| Peak Hospitalization | |||
| Abnormal AST | 2.80 (p<0.001) | 5.87 (p=0.004) | 2.19 (p=0.02) |
| Abnormal ALT | 2.00 (p<0.001) | 2.70 (p<0.001) | 0.88 (p=NS) |
| Abnormal ALP | 2.24 (p<0.001) | 3.76 (p<0.001) | 1.09 (p=NS) |
| Abnormal TBIL | 2.10 (p<0.001) | 2.26 (p<0.001) | 3.41 (p<0.001) |
| Abnormal albumin | 2.95 (p<0.001) | 2.36 (p<0.001) | 1.00 (p=NS) |
BMI = body mass index, NS = not significant; AST = aspartate aminotransferase; ALT = alanine aminotransferase; ALP = alkaline phosphatase; TBIL = total bilirubin
Figure 1:
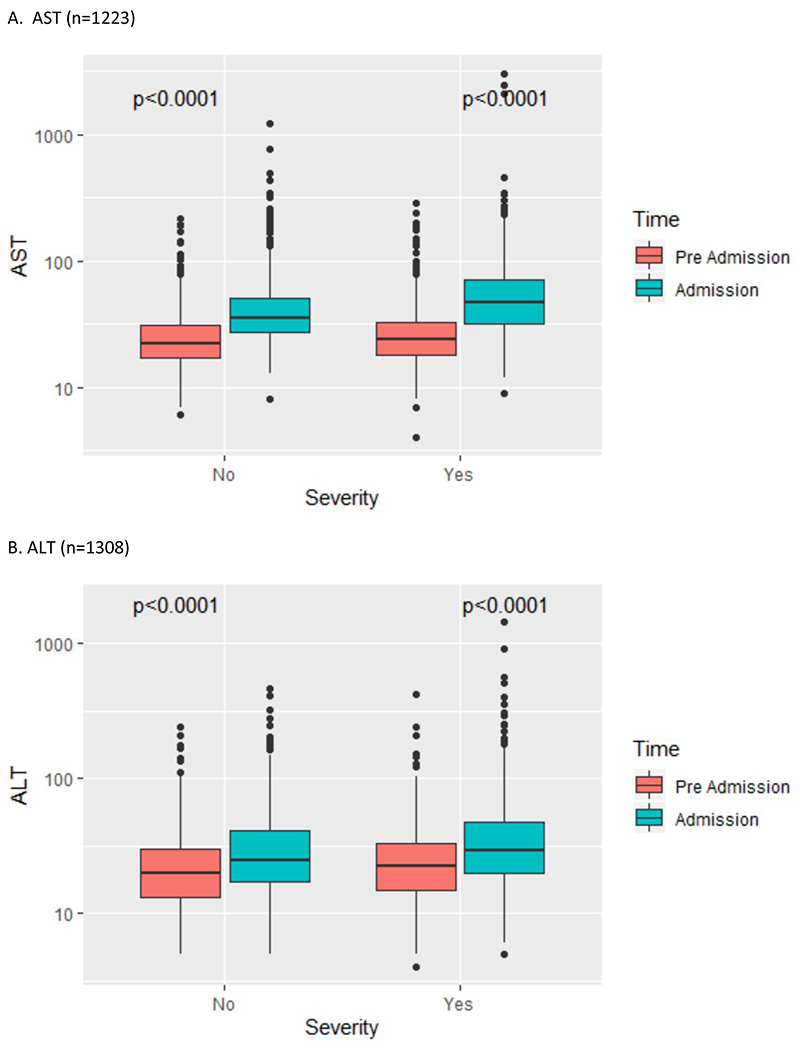
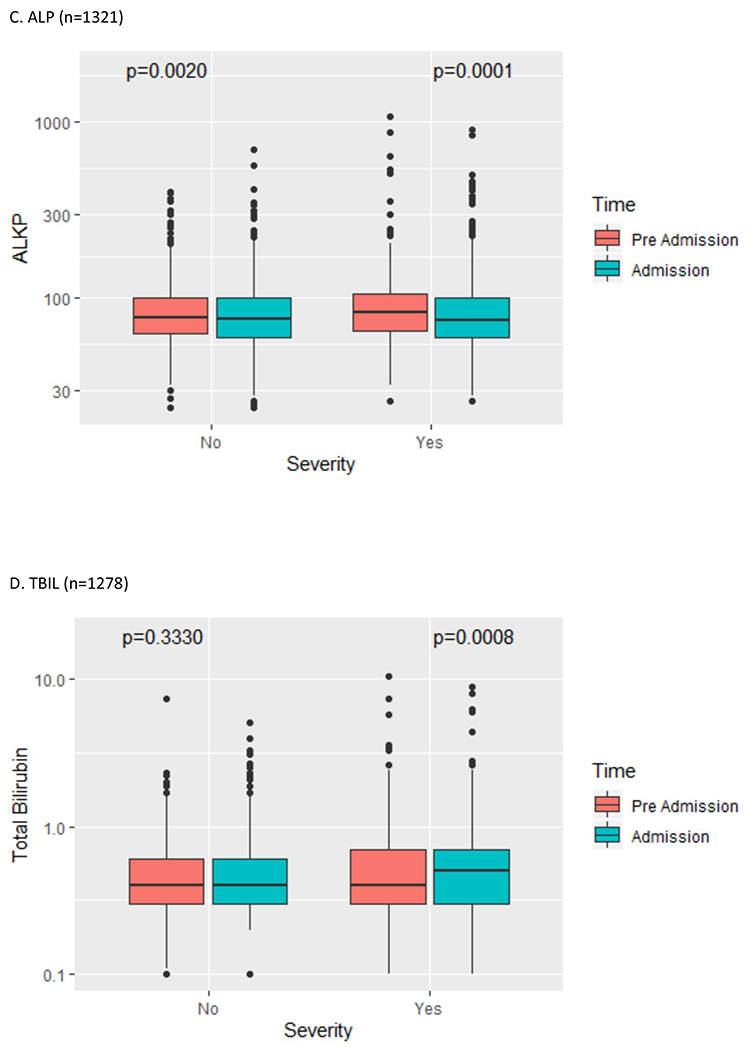
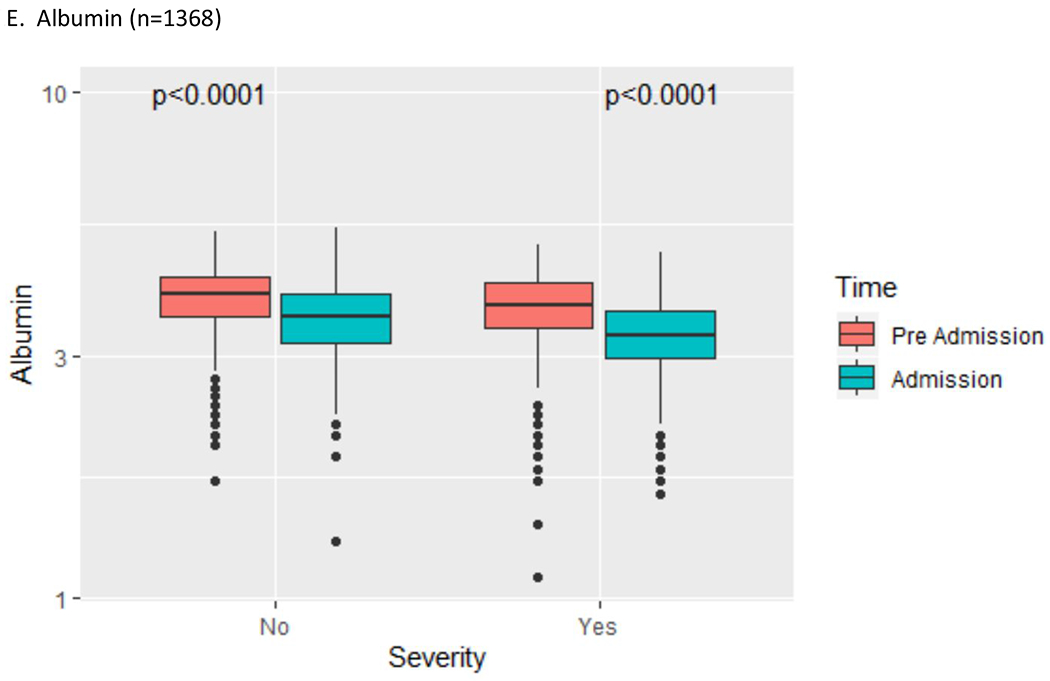
Liver test abnormality by COVID-19 severity (pre-hospital vs. admission liver tests)
AST = aspartate aminotransferase; ALT = alanine aminotransferase; ALP = alkaline phosphatase; TBIL = total bilirubin
Discussion:
This study represents one of the largest reports of liver tests and clinical outcomes to date in hospitalized patients with confirmed SARS-CoV-2 infection in the U.S. Although fever, cough, and shortness of breath have been identified as the most common symptoms in affected individuals, liver test elevation has been identified as one of a growing spectrum of non-pulmonary manifestations described in COVID-19, which may potentially be attributable to hepatic expression of the primary viral entry receptor, angiotensin converting enzyme II (ACE2) [24–25]. Based on a large systematic review and meta-analysis (17 studies, 2711 patients), liver test abnormalities are estimated to occur in approximately 15% of patients [26].
The current report has several key findings. First, abnormal liver tests are very common in hospitalized patients with COVID-19, and possibly more common in U.S. patients than previously reported in China. Whereas the prevalence of abnormal liver tests in studies from China was estimated at 14.9% (14 studies, 2595 patients) [26], recent studies from the U.S. reveal higher estimates ranging between 40.0-67.5% in cohorts ranging from 12-1059 patients [27–30]. In this cohort, AST and ALT elevations were observed at admission in 66.9% and 41.6% of patients, respectively, and during hospitalization in 83.4% and 61.6% of patients, respectively. Although differences in baseline risk factors (e.g. fatty liver, alcohol, medications) and hospital management (e.g. COVID-19 treatment) may potentially account for some of this disparity, future studies are needed to clarify the extent to which such factors account for these differences. Second, the current study is consistent with prior observations that the pattern of liver injury is predominantly hepatocellular rather than cholestatic, although our study suggests that elevations in TBIL and ALP may be more common than previously reported. The ACE2 receptor is much more heavily expressed in cholangiocytes than in hepatocytes [31], so our findings may therefore suggest that the most common mechanism of liver damage is not due to a direct cytopathic effect of the SARS-CoV-2 virus. Third, we found that abnormal liver tests are usually minimally elevated (1-2x ULN), although more severe hepatitis (2-5x or >5x) may be observed. A previous study reported that 11.1% and 9.2% of AST and ALT elevations, respectively, were beyond 2x ULN at the time of hospital admission [16]. In contrast, our report reveals higher rates of abnormal liver transaminases >2x ULN at admission (36.3% AST, 36.5% ALT) and peak hospitalization (55.2% AST, 58.4% ALT), and >5x ULN at peak hospitalization (55.2% AST, 58.4% ALT), signaling that severe hepatitis is more common than previously described. Fourth, abnormal liver tests are pre-existing in a significant proportion of patients prior to hospitalization for COVID-19, particularly among patients with abnormal liver tests at hospital admission. We are not aware of previous U.S. studies that report pre-hospitalization liver tests. Fifth, this study reveals an association between medications used in the treatment of COVID-19 and abnormal liver transaminases during hospitalization, with the strongest relationship observed with tocilizumab. The use of pharmacotherapy for COVID-19 may represent a surrogate for disease severity and therefore this association may be confounded by indication, although a contribution of drug-induced liver injury cannot be excluded. Finally, abnormal liver tests during hospitalization are associated with worse outcomes in COVID-19 patients, with the strongest associations observed between peak liver tests (AST, ALT, TBIL, ALP, albumin) and ICU admission/mechanical ventilation, as well as peak liver tests (AST, TBIL) and death.
This study has several limitations, including retrospective observational cohort study design with inclusion restricted to patients with COVID-19 who were hospitalized within a single health system, and limited access to demographic, laboratory, imaging, and medication variables which may influence key clinical outcomes. Liver outcomes such as acute liver failure could not be assessed due to the absence of key variables to define severe coagulopathy. This report did not include a time variable to permit Cox regression and Kaplan-Meier survival analyses. However, this study provides robust new evidence characterizing the burden, severity, and pattern of abnormal liver tests, and associated clinical outcomes, in a major U.S. hospital network with 1827 hospitalized patients with confirmed SARS-CoV-2 infection. Future studies are needed to further elucidate the etiology and implications of liver test elevations in ambulatory and hospitalized patients. Although histopathology specimens from liver biopsies obtained in a single patient with SARS-CoV-2 infection revealed lobular and portal activity which may be compatible with viral infection [31], liver injury in affected patients may be attributable to a range of secondary effects of the systemic inflammatory response and cytokine storm associated with COVID-19, as well as alternative etiologies such as drug-induced liver injury, hepatic ischemia/shock, and alcoholic hepatitis [20]. Additional insights into the etiology and implications of liver injury may further strengthen evidence-based guidance in the care of patients with COVID-19.
Supplementary Material
Financial Support:
This study was supported by NIH grants P30 DK34989, P01 DK57751, R01 DK114041, and R01 DK112797.
Abbreviations:
- COVID-19
coronavirus-19 disease
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- U.S.
United States
- AST
serum aspartate aminotransferase
- ALT
serum alanine aminotransferase
- YNHHS
Yale-New Haven Health System
- PCR
polymerase chain reaction
- EHR
electronic health record
- BMI
body mass index
- DM
diabetes mellitus
- ALP
serum alkaline phosphatase
- TBIL
serum total bilirubin
- ULN
upper limit of normal
- OR
odds ratio
- ACE2
angiotensin converting enzyme II
Footnotes
Conflict of interest disclosure: MAH, YD, MMC, and MHN report no disclosures. JKL reports research contracts (to Yale University) from Allergan, Conatus, Genfit, Gilead, and Intercept.
References:
- 1.Wu Z, McGoogan JM. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13): 1239–1242. [DOI] [PubMed] [Google Scholar]
- 2.WHO COVID timeline. Weblink: https://www.who.int/news-room/detail/27-04-2020-who-timeline---covid-19. Accessed May 12, 2020.
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020. April; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020. February; 395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutierrez-Ocampo E, Villamizar-Pena R, Holguin-Rivera Y, Escalera-Antezana JP et al. Clinical and laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Inf Dis. 2020. Mar 13 [Epub ahead of print] doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020. May; 8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johns Hopkins Coronavirus Resource Center. Weblink: https://coronavirus.jhu.edu. Accessed July 8, 2020.
- 8.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal A, Chen A, Ravindran N, To C, Thuluvath PJ. Gastrointestinal and liver manifestations of COVID-19. J Clin Exp Hepatol 2020. May-June; 10:263–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel KP, Patel PA, Vunnam RR, Hewlett AT, Jain R, Jing R, et al. Gastrointestinal, hepatobiliary, and pancreatic Manifestations of COVID-19. J Clinical Virol 2020. April 29 [Epub ahead of print]. doi: 10.1016/j.jcv.2020.104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Thompson CC, et al. Prevalence and characteristics of gastrointestinal symptoms in Patients with SARS-CoV-2 infection in the United States: a multicenter cohort study, Gastroenterology 2020. April 22 [Epub ahead of print]. doi: 10.1053/j.gastro.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee IC, Huo TI, Huang YH. Gastrointestinal and liver manifestations in patients with COVID-19. J Chin Med Assoc 2020. April 1 [Epub ahead of print]. doi: 10.1097/JCMA.0000000000000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong SH, Lui RN, Sung JJ. Covid-19 and the digestive system. J Gastroenterol Hepatol. 2020. May; 35:744–748. [DOI] [PubMed] [Google Scholar]
- 14.Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, et al. Clinical features of COVID-19-related liver damage. Clin Gastroenterol Hepatol 2020. April 10 [Epub ahead of print]. doi: 10.1016/j.cgh.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu J, Han B, Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology 2020. May; 158:1518–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: abnormal liver function tests. J Hepatol. 2020. Apr 13 [Epub ahead of print]. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng G, Zheng KI, Yan QQ, Rios RS, Targher G, Byrne CD, et al. COVID-19 and liver dysfunction: current insights and emergent therapeutic strategies. J Clin Transl Hepatol 2020. March; 8:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID-19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan city, China. Liver Int 2020. April 2 [Epub ahead of print]. doi: 10.1111/liv.14455. [DOI] [PubMed] [Google Scholar]
- 19.Sun J, Aghemo A, Forner A, Valenti L. COVID-19 and liver disease. Liver Int 2020. April 6 [Epub ahead of print]. doi: 10.1111/liv.14470. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 2020. May; 5:428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, et al. Longitudinal association between markers of liver injury and mortality in COVID-19 in China. Hepatology 2020. May 2 [Epub ahead of print]. doi: 10.1002/hep.31301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mao R, Liang J, Shen J, Ghosh S, Zhu LR, Yang H, et al. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol 2020. May; 5:426–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med 2020. Apr 17 [Epub ahead of print]. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during pathogenic human coronavirus infections. Liver International 2020. May; 40:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sultan S, Lim JK, Altayar O, Davitkov P, Feuerstein JD, Siddique SM, et al. AGA Institute rapid recommendations for gastrointestinal endoscopy during the COVID-19 pandemic. Gastroenterology 2020. April 1 [Epub ahead of print]. doi: 10.1053/j.gastro.2020.03.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, et al. AGA Institute rapid review of the GI and liver manifestations of COVID-19, meta-analysis of international data, and recommendations for the consultative management of patients with COVID-19. Gastroenterology 2020. May 11 [Epub ahead of print]. doi: 10.1053/j.gastro.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kujawski SA, Wong KK, Collins JP, Epstein L, Killerby ME, Midgley CM, et al. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med 2020. Apr 23 [Epub ahead of print]. doi: 10.1038/s41591-020-0877-5. [DOI] [PubMed] [Google Scholar]
- 28.Cholankeril G, Podboy A, Aivaliotis VI, Tarlow B, Pham EA, Spencer S, et al. High prevalence of concurrent gastrointestinal manifestations in patients with SARS-CoV-2: early experience from California. Gastroenterology 2020. Apr 4 [Epub ahead of print]. doi: 10.1053/j.gastro.2020.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajifathalian K, Krisko T, Mehta A, Kumar S, Schwartz R, Fortune B, et al. Gastrointestinal and hepatic manifestations of 2019 novel coronavirus disease in a large cohort of infected patients from New York: clinical implications. Gastroenterology 2020. May 1 [Epub ahead of print]. doi: 10.1053/j.gastro.2020.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh S, Khan A. Clinical characteristics and outcomes of COVID-19 among patients with pre-existing liver disease in the United States: a multi-center research network study. Gastroenterology 2020. April 28 [Epub ahead of print]. doi: 10.1053/j.gastro.2020.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z, Shi L, Wang Y, Zhang J. Pathological finding of COVID-19 associated with respiratory distress syndrome. Lancet Respir Med 2020. April; 8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


