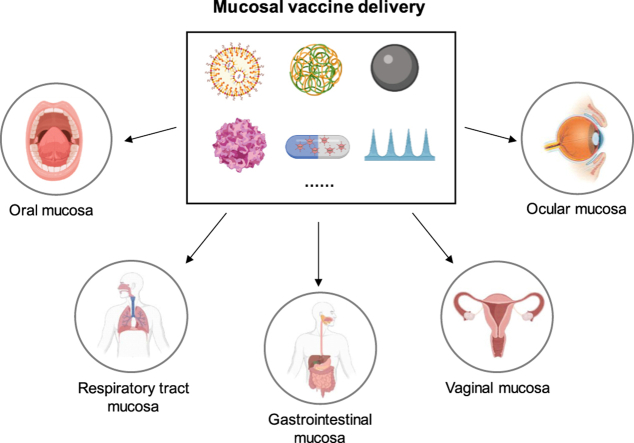
Abstract
Mucosal vaccines can effectively induce an immune response at the mucosal site and form the first line of defense against microbial invasion. The induced mucosal immunity includes the proliferation of effector T cells and the production of IgG and IgA antibodies, thereby effectively blocking microbial infection and transmission. However, after a long period of development, the transformation of mucosal vaccines into clinical use is still relatively slow. To date, fewer than ten mucosal vaccines have been approved. Only seven mucosal vaccines against coronavirus disease 2019 (COVID-19) are under investigation in clinical trials. A representative vaccine is the adenovirus type-5 vectored COVID-19 vaccine (Ad5-nCoV) developed by Chen and coworkers, which is currently in phase III clinical trials. The reason for the limited progress of mucosal vaccines may be the complicated mucosal barriers. Therefore, this review summarizes the characteristics of mucosal barriers and highlights strategies to overcome these barriers for effective mucosal vaccine delivery.
Key words: Mucosal vaccine, Mucosal barrier, Mucosal immune response, Vaccine delivery, Nanocarriers
Graphical abstract
The characteristics of different mucosal barriers are summarized, and strategies to overcome specific barriers to achieve effective mucosal vaccine delivery are highlighted.
1. Introduction
Mucosal vaccines are applied directly to the mucosal surface, such as the sublingual tract, digestive tract, respiratory tract, and urogenital mucosa, to induce an immune response. Compared with vaccines injected intramuscularly or subcutaneously, mucosal vaccines can effectively mimic the natural infection of microorganisms, stimulate the lymphatic tissues under the mucosa to recruit various immune cells and secrete antibodies1,2, which directly block the path by which microorganisms invade the host. Therefore, inducing mucosal immunity is the best way to prevent infection by pathogenic microorganisms that invade the body through the mucosa. In addition, a “homing” phenomenon exists in mucosal immunity3. Specifically, under the mediation of specific homing receptors4, 5, 6, most of mature immune cells will home to the mucosal propria or epithelium of the sensitized site for an immune response, and the rest will reach other mucosal site to form an extensive common mucosal immune network. This means that T cells and B cells that are activated at one mucosa site can migrate to other distal mucosal sites to produce a similar immune response7,8.
Thanks to these advantages, mucosal vaccines have attracted widespread attention. However, only a very small number of mucosal vaccines are approved for use worldwide, mainly include live attenuated, inactivated and adenovirus vector vaccines (Table 1). Some vaccines (e.g., rotavirus vaccine) have been temporarily suspended due to their toxicity9, which shows that there are still many difficulties in the design and development of mucosal vaccines. Currently, seven COVID-19-related mucosal vaccines have entered clinical evaluation (Table 2), including two live attenuated vaccines and five adenovirus vector vaccines. The fastest progress has been achieved for an adenovirus vector vaccine (Ad5-nCoV) developed by Chen and coworkers10, which has been demonstrated to trigger the same level of immune effect as intramuscular injection at a dose of 1/5, providing great encouragement to mucosal vaccine development.
Table 1.
Licensed mucosal vaccines.
| Type of vaccine | Name | Antigen | Formulation | Disease | Administration | Approved time | Approved |
|---|---|---|---|---|---|---|---|
| Live attenuated122 | OPV (b/m/tOPv) | Poliovirus | Aqueous | Poliomyelitis123 | Oral | 1961 | FDA |
| Subunit vaccine | Dukoral® | Vibrio cholerae124 rCTB | Aqueous | Cholera | Oral | 2003 | Canada |
| Live attenuated | Fluenz™/FluMist®125 | lnfluenza A and influenza B viruses | Spray | Influenza | Nasal | 2003 | FDA |
| Live reassortant126 | RotaTeq® | Rotavirus | Aqueous | Infant diarrhea | Oral | 2006 | FDA |
| Live attenuated | Rotarix | RIX4414 strain127 | Aqueous | Infant diarrhea | Oral | 2008 | FDA |
| Inactivated | Euvichol128 Shanchol |
Vibrio cholerae | Aqueous | Cholera | Oral | 2013 | WHO |
| Live attenuated | Vivotif®129 | Salmonella typhimurium | Capsule | Acute gastroenteritis | Oral | 2013 | FDA |
| Adenovirus vector vaccine130 | Adenovirus Type 4 and Type 7 Vaccine | Adenovirus Type 4 and Type 7 | / | Febrile acute respiratory disease | Oral | 2011 | FDA |
| Live attenuated | Vaxchora™ | Vibrio cholerae131 | Aqueous | Cholera | Oral | 2015 | FDA |
Table 2.
The candidates of COVID-19 mucosal vaccine in clinical.
| Type of vaccine | Name | Antigen | Carrier | Administration | Status | NCT |
|---|---|---|---|---|---|---|
| Ad-vectored vaccine | Ad5-nCoV | Spike protein | Ad | Inhale | Phase Ⅲ | NCT04540419 |
| Ad-vectored vaccine | hAd5-S-Fusion | Spike protein | Oral capsule | p.o. | Phase Ⅰ/Ⅱ | NCT04845191 |
| Live attenuated vaccine | DelNS1-2019-nCoV-RBDOPT1 | RBD domain of S protein | Influenza virus (CA4-DelNS1) | Inhale | Phase II | ChiCTR2000039715 |
| Live attenuated vaccine | COVI-VAC | SARS-CoV-2 | / | Inhale | Phase Ⅰ | NCT04619628 |
| Ad-vectored vaccine | AdCOVID | RBD domain of S protein | Ad5 | Inhale | Phase Ⅰ | NCT04679909 |
| BBV154 | Spike protein | Ad | i.n. | Phase Ⅰ | NCT04751682 | |
| ChAdOx1 nCOV-19 | Spike protein | Ad | i.n. | Phase Ⅰ | NCT04816019 |
Ad, adenovirus; p.o., oral administration; i.n., intranasal administration.
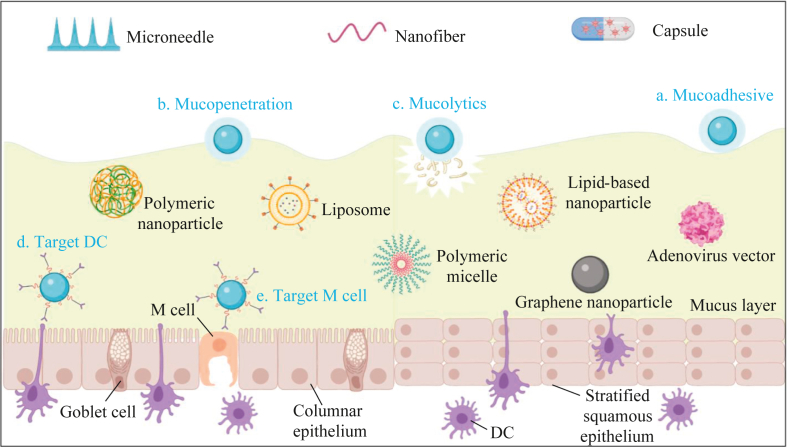
In addition to viral vectors, several biodegradable and safer nonviral vectors are also used for mucosal vaccine delivery. These nonviral vectors are usually designed to protect antigen components from degradation, increase the residence time of antigens in mucosal sites and increase the uptake of antigens by antigen-presenting cells (APCs), etc. (Fig. 1). Various mucosal vaccines based on different vectors are undergoing clinical evaluation or clinical research (Table 3). Strategies and delivery systems that target different cell types and promote the penetration of vaccines through the mucosal (including mucoadhesive, mucopenetration and mucolytics) have been proposed and summarized previously11, 12, 13, 14, 15, 16. Due to the significant differences in the structure and physiological environment of specific mucosae, there are different requirements for vaccine delivery. However, few articles have summarized in detail the vector design requirements for breaking through different mucosae. Therefore, we provide a detailed summary of the existing delivery strategies for overcoming different mucosal barriers.
Figure 1.
Carriers for mucosal vaccine delivery. Various carriers were designed to improve the mucosal delivery efficiency of vaccines. As shown in a‒c, to penetrate the mucus barrier, nanoparticles (NPs) for mucoadhesion, mucopenetration and mucolytics were designed. To improve the efficiency of antigen uptake (as shown in d and e), dendritic cell (DC)- and microfold cell (M cell)-targeting NPs were designed.
Table 3.
Advanced strategies for mucosal vaccine delivery.
| Barrier | Type of vaccine | Carrier | Antigen/epitope | Disease | Ref./NCT |
|---|---|---|---|---|---|
| Oral mucosa | Subunit vaccine | Mucoadhesive wafers | HIV gp140 protein | HIV | 69 |
| Microneedle arrays | HBsAg | HBV | 132 | ||
| SIMPL tablet | OVA | / | 67 | ||
| / | Nanofibrous mucoadhesive films | / | / | 68 | |
| Gastrointestinal mucosa | Inactivated vaccine | Chitosan and alginate delivery carriers | HEV71 | Hand-foot-and-mouth disease | 133 |
| Attenuated vaccine | Albumin–chitosan matrix microsphere | Typhoid Vi® antigen | Typhoid | 134 | |
| Ad-vectored vaccine | Adenovirus type-4 | Hemagglutinin from H5N1 virus | Influenza | 72 | |
| Adenovirus type-5 | Hemagglutinin from H1N1 virus | Influenza | NCT02918006, Phase Ⅱ | ||
| Adenovirus type-5 | Spike protein | COVID-19 | NCT04845191, Phase I/Ⅱ | ||
| Adenovirus type-5 | Spike protein and nucleocapsid | COVID-19 | NCT04732468, Phase I | ||
| Chimpanzee Adenovirus | Hepatitis B virus | Hepatitis B | NCT04297917, Phase Ⅰ | ||
| Subunit vaccine | Pollen grains or ragweed pollen | OVA | / | 135,136 | |
| Flagellin and mannosamine coated poly (anhydride) NPs | OVA | / | 137 | ||
| Porous silica NPs | BSA | / | 138 | ||
| CPP-rich PEGylated NPs | Recombination urease subunit B (rUreB) | Helicobacter pylori infection | 77 | ||
| PMMMA-PLGA | Surface immunogenic protein (SIP) from group B Streptococcus (GBS) | Streptococcus agalactiae infection | 139 | ||
| DNA vaccine | Poly (lactide-co-glycolide) microparticle | A rotavirus VP6 DNA | Infant diarrhea | 140 | |
| Chitosan NPs | LTB (L)、STXB (S) and CTXB (C) | Diarrhea | 141 | ||
| PLGA NPs | Hepatitis B virus (HBV) HBsAg | Hepatitis B | 140,142 | ||
| Poly(d,l-lactide-co-glycolide) (PLG) microparticle | gp160 | HIV | 143 | ||
| Respiratory tract mucosa | Ad-vectored vaccine | Adenovirus vector | RBD domain of Spike protein | COVID-19 | NCT04679909, Phase I |
| Adenovirus vector | Spike protein | COVID-19 | NCT04751682, Phase I | ||
| Adenovirus vector | 85A antigen | Tuberculosis | NCT04121494, Phase I | ||
| Adenovirus vector | Spike protein | COVID-19 | NCT04552366, Phase I | ||
| Adenovirus vector | Spike protein | COVID-19 | NCT04816019, Phase Ⅰ | ||
| Adenovirus vector | Influenza Vietnam 1194 Hemagglutinin | H5N1 Influenza | NCT01443936, Phase Ⅰ | ||
| Adenovirus vector | Spike protein | COVID-19 | NCT05043259, Phase Ⅰ/Ⅱ | ||
| Recombinant virus | CaP nanoshell | Recombinant dengue virus | Dengue | 144 | |
| Subunit vaccine | Polysaccharidic lapidated NPs | OVA | / | 145 | |
| Bacterium-like particle | RSV fusion (F) protein | RSV | 146 | ||
| N-Trimethyl chitosan NPs | OVA | / | 147 | ||
| Poly (γ-glutamic acid) NPs | OVA | / | 148 | ||
| PCL-PEI and PCL-PEG polymeric hybrid micelle | Citra conic anhydride-modified OVA | / | 149 | ||
| Adeno-associated virus type 12 | Influenza A nucleoprotein | Influenza | 150 | ||
| Nanoemulsion | N-Acetyl-neuroaminyllactose-binding hemagglutinin protein | Helicobacter pylori infection | 151 | ||
| Porous maltodextrin-based lipid core NPs | Toxoplasma gondii antigens | Toxoplasma gondii infection | 152 | ||
| Nanogel | Clostridium botulinum type-A neurotoxin BoHc/A | BoNT | 153 | ||
| Hybrid NPs | BSA | / | 154 | ||
| Thermal-sensitive hydrogel | Shigella flexneri outer membrane vesicles (OMV), split H5N1 antigen | Influenza | 155,156 | ||
| DNA vaccine | Mannosylated protamine sulphate | Model DNA: anti-GRP DNA | / | 157 | |
| mRNA vaccine | PEG12KL4 | Luciferase mRNA | RSV infection | 97 | |
| Cationic cyclodextrin-polyethylenimine 2k conjugate (CP 2k) | gp120 | HIV | 99 | ||
| Hyperbranched poly (beta amino esters) (hPBAEs) | Luciferase | / | 102 | ||
| Vaginal mucosa | Inactivated vaccine | Polystyrene nanospheres | HIV-1 | HIV | 158 |
| Subunit vaccine | Calcium phosphate (CAP) NPs | HSV-2 protein | HSV | 159 | |
| Poly-acrylic acid (Carbopol) gel | HIV-1 CN54 gp140 | HIV | 160, 161, 162 | ||
| Hydroxyethylcellulose (HEC) gel | HIV-1 CN54 gp140 | HIV | 163 | ||
| Thermosensitive poloxamer | HIV-1 CN54 gp140 | HIV | 164,165 | ||
| Liposome-loaded HEC gelling rods | HIV-1 CN54 gp140 | HIV | 108,166 | ||
| Liposome-loaded microneedle array | HSV-2 gD | HSV | 29 | ||
| Ad-vectored vaccine | Ad | HIV-1 gp140CF | HIV | 167,168 | |
| rAd5 | HIV gag | HIV | 169 | ||
| Recombinant vaccine | Pseudovirion | Recombinant HPV-SIV gag | HPV | 170 | |
| Recombinant virus | HIV gp160 and gag192-208 | HIV | 35 | ||
| Recombinant virus | HIV gag p24 | HIV | 171 | ||
| VLP vaccine | Virus-like NPs | HIV-1 gag | HIV | 172 | |
| Virus-like NPs | HIV-1 gag p55 | HIV | 173 |
Ad, adenovirus; ALG, alginate; CMC, carboxymethylcellulose; COPD, chronic obstructive pulmonary disease; ETEC, Escherichia coli; ETSD, enhanced T-cell stimulation domain; LAIVs, live attenuated influenza vaccines; MVA, modified vaccinia ankara; PMMMA-PLGA, Poly[(methyl methacrylate)-co-(methyl acrylate)-co-(methacrylic acid)]-poly(d,l-lactide-co-glycolide); rPA, recombinant protein; RSV, respiratory syncytial virus; UTI, urinary tract infection.
2. Mucosal immune response
Unlike systemic immunity, antigens that evoke mucosal immunity originate from the mucosal surface of various cavities and are captured by M cells or DCs in the mucosal inductive site17 and induce immune responses at the mucosal effector site. M cells and DCs have different mechanisms of antigen uptake. Specifically, in addition to having antigen phagocytosis similar to DC cells, M cells can also capture antigens by ingesting IgA-antigen complexes. An antigen can bind to IgA and induce a conformational change in the IgA molecule, enabling the antigen-secretory IgA (sIgA) complex to bind to M cells through specific receptors. In addition, M cells can also provide a transcellular antigen uptake pathway for DCs18. However, due to the lack of protein-degrading lysosomes in M cells, they do not have the ability to process antigens but instead transfer the captured antigens to nearby APCs (e.g., DCs and B cells)19,20. Only after undergoing this necessary APC processing can the captured antigens effectively induce antigen-specific immune responses. Nonetheless, in order to induce stronger mucosal immunity, researchers are not only interested in enhancing DCs uptake, but also targeting M cells with vector design to enhance the antigen uptake capacity of M cells11, 12, 13.
The production of sIgA is the main distinguishing feature compared with the systemic immune response. Concurrently, sIgA is the main effector of mucosal immunity, which can prevent viral invasion by combining with the virus21,22. Compared with neutralizing Abs (nAbs, the focus of vaccine development), sIgA has the potential advantages of preventing the spread of viruses through the mucosa23: sIgA (1) can prevent bacteria from adhering to the surface of epithelial cells; (2) can effectively neutralize harmful substances such as pathogens that enter the mucosal epithelium; and (3) can be discharged without the combination of complement and virus to form immune complexes. This immune complex can stimulate goblet cells to secrete mucus, which prevents the adhesion of microorganisms24. In contrast, nAbs bind to foreign pathogens and prevent them from entering target cells by blocking receptor binding or cellular uptake. Additionally, complement fixation of nAbs can also kill the virus23. However, the different escape mechanisms of viruses, including the arming of pathogens and the rapid mutation of surface glycoproteins expressed by viruses, limit the application of vaccines for inducing nAbs25, 26, 27. In this case, the induction of sIgA production through mucosal immunity is a promising measure to prevent viral spread.
3. Barriers to mucosal vaccine delivery
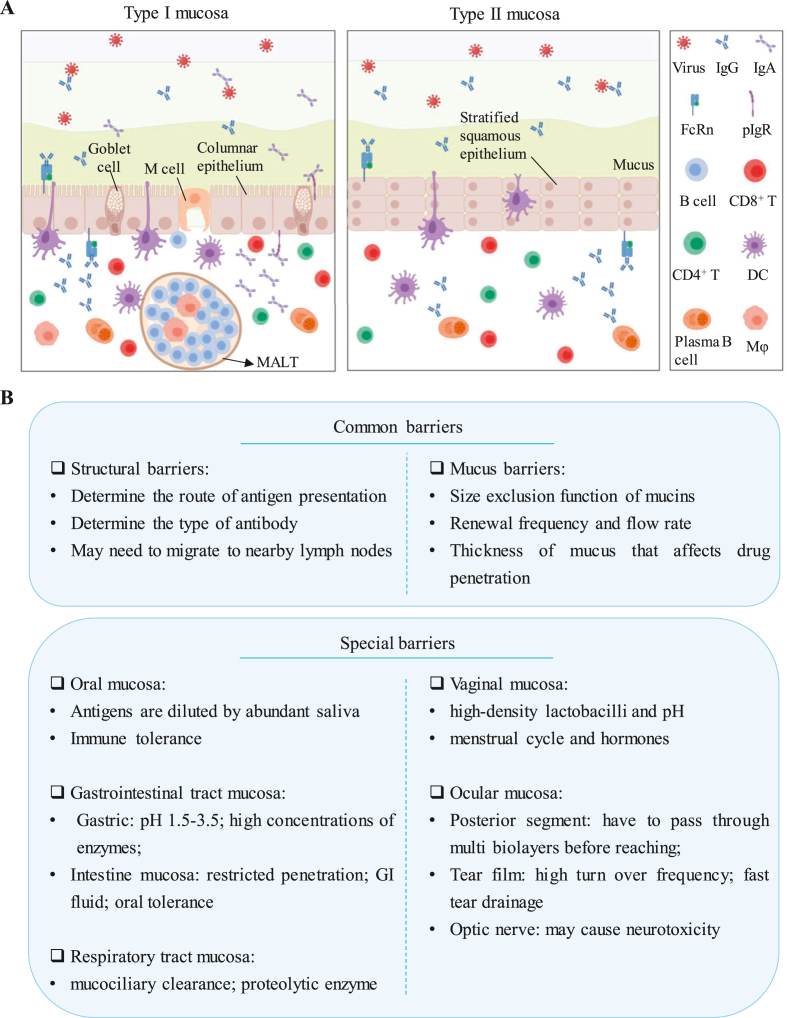
The mucosal delivery of various drugs must break through multilayer barriers. The factors that affect the mucosal delivery of drugs are summarized into the following categories (Fig. 2):
Figure 2.
Common features of type I and II mucosa and barriers for mucosal delivery. (A) Type I and type II mucosae are distributed in different tissues. There is a significant difference in the structural composition of the two, mainly in that the type I mucosa is composed of columnar epithelia, goblet cells, and M cells. It contains MALT and can secrete IgG and IgA. In contrast, the type II mucosa, which is composed of stratified squamous epithelium, does not have M cells, goblet cells or MALTs and cannot secrete IgA. (B) Common and special barriers limiting mucosal delivery.
3.1. Common barriers
3.1.1. Structural barriers
As shown in Fig. 2A, numerous characteristics clearly differ between type I mucosa (e.g., gastrointestinal, respiratory, and upper female reproductive tracts) and type II mucosa (e.g., corneal, oral, esophageal, and lower female reproductive tracts)23. Type I mucosa is typically covered with columnar epithelial cells, and the mucus layer is secreted by goblet cells28,29. More importantly, antigens are transported from the lumen to DCs through M cells and provide a place for antigen presentation in mucosa-associated lymphoid tissues (MALTs). In addition, the production of IgG and sIgA can be induced via transport by neonatal Fc receptor (FcRn)30 and polymeric immunoglobulin receptor (pIgR)31, respectively. However, type II mucosa with a multilayer squamous epithelium composed of multilayer keratinocytes lacks M cells, goblet cells, MALTs and polymeric immunoglobulin A (pIgA). Thus, other tissues (nearby glands) are needed for mucus secretion, and the DCs that take up the antigens must also migrate to adjacent lymph nodes. Furthermore, the absence of pIgA means that it can only induce an IgG response without a sIgA response.
3.1.2. Mucus barriers
The mucus layer is the first barrier to invaders entering the body through the mucosa16,24,32,33. Briefly, in the mucus layer, drug delivery is affected by the composition of the mucus (more attention is given to the structure of the mucin), renewal frequency, and flow rate. As an important part of mucus, high-molecular-weight mucins form a network with size exclusion function through hydrophobic interactions and chain entanglement, which implies that the pores formed by the mucin network demand customized drug sizes. Simultaneously, the mucus may fall off through mechanical action, or it may be degraded by certain enzymes and, thereby, undergo constant renewal.
In addition, the penetration efficiency of the drug is significantly affected by the thickness of the mucus34. The thickness of the mucus varies within and between different organs, which makes it difficult for vaccines to penetrate the mucus layer. For example, the thickness of the mucus layer in the cornea or conjunctiva is 1–7 μm, while it is approximately 10 μm in the trachea. In the same tissue, such as the digestive tract, the mucus layer gradually increases from 200 μm in the upper digestive tract to 800 μm in the lower digestive tract35, which is much thicker than the eyeball mucosa and the mucosae of other organ.
3.2. Special barriers
3.2.1. Barriers of the oral mucosal
There are some unique barriers to oral mucosal inoculation. In comparison to other mucosal tissues, there are no specialized epithelial-associated follicles and no existing organized lymphoid structures in oral mucosal tissues36. There are no signs of M cells in the oral mucosal tissue; in contrast, several dendritic cell subsets have been demonstrated to appear in epithelial tissue. DCs in the oral mucosa include38 (1) CD11b+CD11c‒ and CD11b+CD11c+ myeloid DCs, which are mostly located at the mucosal/submucosal interface; (2) B220+120G8+ plasma cell-like DCs, which are mainly present in submucosal tissues; and (3) CD207+ Langerhans cells (LCs), which are present only in the mucosa itself. When the antigen penetrates the mucus and reaches the epithelial tissue, it can be presented by LCs and trigger a specific immune response39. Moreover, there are three large salivary glands and hundreds of small salivary glands in the mouth, which will continuously secrete saliva (Fig. 2B). The saliva consists of 99% water, which leads to dilution of the antigen40. In addition, the oral cavity is the main site of sustenance ingestion and is constantly exposed to the external environment41,42. To avoid triggering an immune response against harmless microorganisms and bacteria, immune tolerance is triggered in the oral tissues41,42. Immune tolerance is triggered mainly by increased expression of IL-10, allergen-specific IgG4 and programmed cell death ligand 1 (PD-L1), together with decreased expression of IL-4, CD86 and CD8043.
3.2.2. Barriers of the gastrointestinal tract mucosa
To effectively induce mucosal immunity in the GI tract, various barriers need to be overcome by oral vaccines (Fig. 2B). First, the structure of the intestinal epithelium and mucous layer limit the penetration of antigenic substances. Second, the pH gradient of the GI tract for resisting foreign invaders (pH changes from 1.0 to 2.0 in the stomach, and increases to 2.5–6.0 in the duodenum and 7.0–8.0 in the colon) will also have a significant impact on the activity of antigen molecules32. Third, a high concentration of enzymes in the entire GI tract requires that the design of vaccines be strictly considered to avoid the degradation and denaturation of biomolecules. More importantly, vaccines that target the mucosa of the GI tract require higher antigen doses to induce an effective immune response. However, uptake of vaccine antigens by intestinal mucosa is relatively limited, which leads to an attenuated immune response. Against these barriers, nanocarriers are usually designed to protect antigens from extreme pH and proteases and to increase the uptake of antigens by targeting APCs44.
3.2.3. Barriers of the respiratory tract mucosa
To effectively induce the local immune response of the respiratory mucosa, an immunogenic vaccine must break through several mucosal barriers (Fig. 2B) and effectively transit to adjacent MALTs, such as nasal-associated lymphoid tissues (NALT) and bronchial-associated lymphoid tissues (BALT). The mucus layer comprising heavily glycosylated and sialylated mucins covers the respiratory tract and can efficiently block the foreign matter, including pathogens. However, this same layer also impedes the valid delivery of mucosal vaccines targeting the respiratory tract. The foreign matter trapped in the mucus layer will be removed from the organ cavity by the cilia to promote excretion of the mucus layer, which means that drugs adhering to the mucous must spread as quickly as possible. Otherwise, even if vaccines successfully adhere to the mucus layer, antigens may not reach the epithelial surface before being expelled by mucociliary clearance (MCC)45,46.
3.2.4. Barriers of the vaginal mucosa
Unlike other mucous membranes, the physiological state of the vaginal mucosa is significantly regulated by age and hormone levels, and thus, drug delivery to vaginal mucosa will be restricted by additional factors (Fig. 2B). As a type II mucosa, the vaginal mucosa is composed of uncornified and pluristratified epithelial cells with a thickness of 200–300 μm and lacks goblet cells47. In addition, lactobacilli were found uniquely in human vaginas with an abundance of more than 70%, while other mammals have only ∼1%. The high density of lactobacilli creates an acidic environment (pH 3.5–4.5), which effectively protects women from pathogens48. Factors such as hormones and microorganisms jointly regulate the formation of mucus, thereby affecting the delivery of drugs.
3.2.5. Barriers of ocular mucosa
The anatomical structure of the eye can be divided into different tissues, such as conjunctiva, cornea and vitreous body. Among these tissues, the conjunctiva and lacrimal glands contain IgA + plasma cells that secrete sIgA and various immune cells that produce cytokines and chemokines49, indicating their role as effector sites. In contrast, the cornea contains DCs and macrophages but no apparent lymphocytes50, which indicates that the cornea is an immune inductive site rather than an effective site. Different mucosae require tailored delivery strategies, and some drugs may have to reach the posterior segments of the eye, whereas there is no such need for ocular vaccines.
Eyedrops represent the most common drug formulation among all ocular drugs. However, the efficacy of eyedrop drugs is limited by rapid tear film turnover, quick tear drainage and the obstacle of multiple bilayers. The tear film contains multiple layers, including the mucus layer as the innermost layer. The outer mucus layer comprises secreted mucins, which can be reset quickly by blinking and tear film turnover. The inner layer is formed by epithelium-tethered mucins (glycocalyx) with lower clearance frequency51. Frequent instillation of eyedrops is needed because of the rapid reset of the mucus composition and the multilayers protecting the cornea. However, repeated instillation may result in worse patient compliance and ocular surface toxicity52.
Eyedrops must travel a long distance and pass through various ocular barriers to reach the back of the eye, leading to minimal drug levels in the posterior segment. Moreover, the blood‒retinal barrier (BRB) prevents effective drug and vaccine delivery via the systemic route to the eye. Direct injection into the eyeball (e.g., intravitreal) or periocular injection (e.g., subconjunctival) may address these problems53. Subconjunctival delivery penetrates the eye through transscleral diffusion54, and soluble drugs exhibit better penetration capability but worse sustained release than hydrophobic drugs. As a result, conventional subconjunctival delivery requires frequent injection55. Intravitreal (IVT) injections can directly deliver drugs to the back of the eye, but repeated injections are also required because of vitreous humor turnover. Repeated injections might increase the risk of retinal detachment, hemorrhage and endophthalmitis56. Moreover, direct delivery of drugs or vaccines to the retina may induce neurotoxicity, and the safety profile of drugs and vaccines should be seriously assessed.
4. Nanocarriers for mucosal vaccine delivery
Against the various barriers to mucosal vaccine delivery, a variety of excellent delivery systems have been designed to promote mucosal immune effects. An ideal mucosal delivery system should have the following characteristics: First, it must be safe and non-toxic. Second, antigen uptake and presentation can be successfully achieved. In mucosal immunity, M cells and DCs are the major players in the process of antigen uptake, so targeting M cells and DCs may be a potential strategy. Third, the mucus barrier is an important factor limiting vaccine uptake. Therefore, the structure of the mucus layer can be disrupted by vector design to facilitate vaccine uptake. Fourth, the design of the carrier must adequately protect the antigenic components from enzymatic or chemical degradation so that the vaccine components can be safely delivered to target cells. However, to truly obtain a mucosal vaccine with strong protection and durability, it is necessary to design the vaccine according to the characteristics of different mucosae, so as to realize the specific immune response of vaccine antigens in the mucosa. Therefore, we summarize the barrier characteristics of different mucosal membranes and enumerate existing representative work.
4.1. Oral mucosa
There are two main sites for oral mucosal inoculation: the sublingual and buccal mucosae (with upper and lower inner lip mucosa)57. As with other mucosal tissues, the surface of the oral mucosa is covered with mucus37. The buccal epithelial tissue which is 500–800 μm thick, contains 40–50 layers of cells58. The sublingual epithelial tissue, which is 100–200 μm thick, contains 8–12 layers of cells59. The oral mucosa allows rapid antigen passage because of its limited thickness. A study has shown that OVA antigen requires only approximately 20 min to cross the oral mucosa and then accumulates at the mucosal/submucosal junction. OVA is captured and processed by antigen-presenting cells at 1 h after administration59. The oral mucosa contains minimal amounts of enzymes (compared with the intestine) and possesses a neutral pH environment (compared with the stomach)60. In addition, antigens can be accessed directly by APCs or follow paracellular/pericellular pathways to avoid reaching the submucosal vasculature, thus avoiding first-pass effects61. It was also found that the risk of antigen redirection to the olfactory bulb observed after sublingual immunization is significantly lower than that after intranasal immunization62, indicating the safety of vaccination via the oral mucosa.
4.1.1. Advanced carriers for oral mucosal vaccine delivery
Current vaccines for oral mucosal inoculation recruit various forms of delivery systems to deliver the relevant antigens, such as virus-like particles, adenoviral vectors, polymers, proteins, and microneedles. Various properties of the delivery system have an impact on the antibody level induced by vaccines.
4.1.1.1. Microneedles
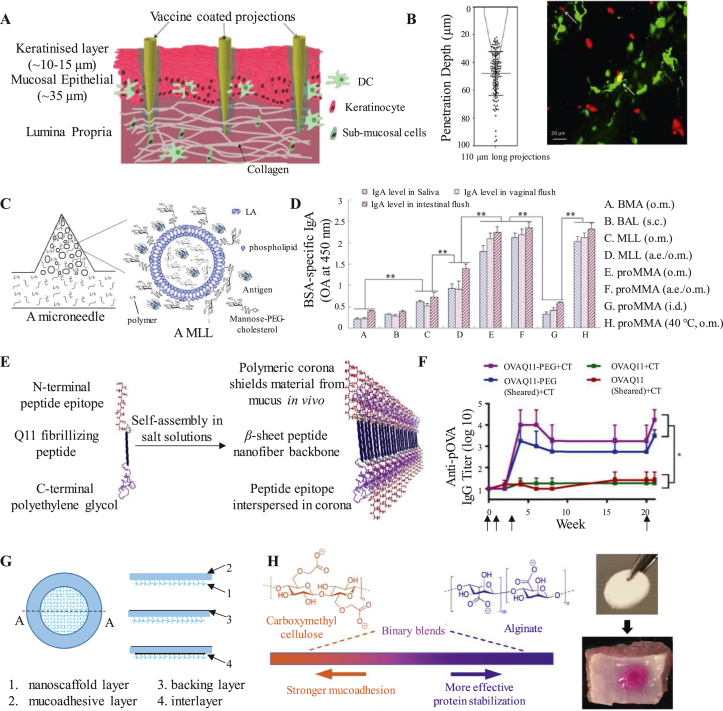
To deliver sufficient antigen to APCs deep in the epithelium and lamina propria, microneedle patches with various forms of antigens (e.g., DNA, peptides, etc.) are designed to breach the mucosal barrier through nanoneedle tips. For example, McNeilly et al. produced a microneedle array with a size of 16 mm2 (Nanopatch). This array contains 3364 protrusions, each with a length of 110 μm. This delivery system can directly deliver vaccines to a depth of 50 μm in the oral mucosa of mice, thereby facilitating antigen uptake by APCs63 (Fig. 3A and B). Similarly, Zhen et al. constructed microneedle arrays of proMLL fillers (proMMA) using mannose-PEG-cholesterol (MPC)/mannosylated lipid A-liposomes encapsulated with bovine serum albumin (BSA) as a model antigen (Fig. 3C). As a solid, proMMA is stable under storage conditions. In contrast, proMMA can dissolve in water rapidly and revert to MLL. Administration through the oral mucosal (o.m.) or oral mucosal administration under anesthesia (a.e./o.m.) significantly increased IgA levels in mouse saliva, intestinal and vaginal washings, which were not achieved by subcutaneous (s.c.) or intradermal (i.d.) immunization. In addition, storage at 40 °C for three days did not affect the activity of proMMA (Fig. 3D)64.
Figure 3.
Advanced strategies for oral mucosal delivery. (A and B) A microneedle array directly delivered the vaccine to a depth of 50 μm into the oral mucosa. Representative high ( × 40) magnification of a Nanopatch coated with a formulation containing DiD. Green: DCs, Red: DiD. Reprinted from Ref. 63. Copyright © 2014, Elsevier. (C and D) Microneedle arrays of proMLL fillers (proMMA) constructed with mannose-PEG-cholesterol (MPC)/mannosylated lipids A-liposomes. Reprinted from Ref. 64. Copyright © 2015, Elsevier. (E and F) Supramolecular nanofibers formed by eptide-polymer self-assembly. Reprinted from Ref. 66. Copyright © 2020, Elsevier. (G) A mucoadhesive film including the nanoscaffold layer, mucoadhesive layer, backing layer and interlayer. Reprinted from Ref. 68. Copyright © 2017, Elsevier. (H) Mucoadhesive wafers blended with carboxymethyl cellulose (CMC) and alginate (ALG) binary polymer. Reprinted from Ref. 69. Copyright © 2021, Elsevier.
4.1.1.2. Gels
To prolong the residence time of antigens at the mucosal sites, reduce antigen loss due to swallowing, White et al. used a thermoresponsive gel (TRG) combined with a trivalent inactivated poliovirus vaccine (IPV) for sublingual immunization65. TRG is in fluid-like liquid formation at 2–8 °C but transforms into a gel at physiological temperatures (∼37 °C) within seconds. This feature helps the retention of vaccine at the delivery site, thus allowing prolonged contact between the antigen and the mucosa65. As a result, TGR-assisted IPV induced higher levels of serum IgG and sIgA than IPV without TGR.
4.1.1.3. Polymer-based nanofibers
Another oral mucosal delivery system with enormous attention is nanofibers. In order to reduce antigens interaction with mucin, leading to a robust, long-lived antibody and T cell responses, Collier et al. developed an epitope-bearing peptide–polymer conjugate (OVAQ11-PEG) that has the ability to self-assemble into nanofibers (Fig. 3E)66. When used for sublingual immunization, OVAQ11-PEG can synergize with cholera toxin (CT) adjuvant to increase the titer of anti-pOVA IgG in mice (Fig. 3F). Furthermore, Collier et al.67 transformed peptide-polymer nanofibers into tablets (SIMPLs), which are heat-stable and easily-administrable. There was no obvious difference in the sublingual antibody responses after heating SIMPLs for 1 week at 45 °C compared to the conventional carrier vaccine.
Josef Mašek et al. have invented a mucoadhesive film based on nanofibers that enables prolonged release of NPs into submucosal tissue (Fig. 3G). This platform is composed of a variety of nanofibers with different functions, such as an electrospun nanofibrous reservoir layer, mucoadhesive film layer and protective backing layer. The electrospun nanofibrous reservoir layer could absorb a variety of antigens such as lipovirus bodies, virus-like particles and protein macromolecules. This mucoadhesive film could achieve prolonged antigen release by attaching to the oral mucosa68. This mucoadhesive film effectively ensures the local NPs concentration, which is beneficial to realize NPs-mediated delivery to take place. Furthermore, the platform effectively avoids the losses of NPs due to saliva washout.
4.1.1.4. Other delivery systems
Liquid formulations are the predominant form of sublingual vaccines, but are easily cleared during activities such as swallowing, speaking, and eating, resulting in poor immunogenicity. To break through this predicament, Wang et al.69 developed a biopolymer platform of mucoadhesive wafers blended with carboxymethyl cellulose (CMC) and alginate (ALG) binary polymer to enable effective sublingual delivery of lyophilized protein vaccines (Fig. 3H). The screened wafer is loaded with HIV gp140 protein and combined with an adjuvant (αGalSer) for sublingual inoculation of mice. A greater immune response was generated in immunized mice than in the control group.
Furthermore, virus-like particles are also commonly used oral mucosal delivery vehicles. For example, researchers found that sublingual administration of human papillomavirus-like particles could protect the genitalia from human papillomavirus pseudovirus attack with or without CT adjuvant70. Seth et al.71 developed a sublingual lyophilized (FD) formulation of VLPs carrying J8 peptide (J8-VLP). Through tracking of J8-VLPs in vivo after sublingual inoculation, J8-VLPs rapidly entered the circulation, triggering high serum IgG antibody levels along with high levels of IgA antibodies in saliva.
4.2. Gastrointestinal mucosa
In addition to the oral cavity, the gastrointestinal (GI) tract has other potential mucosal immune induction sites for oral vaccines, such as tonsils, Peyer's patch (PP) lymph nodes and submucosal lymphoid tissues. Although tonsils are the first lymphoid tissue to contact oral vaccines, most of the vaccines are designed for small intestinal PP lymph nodes because of the short residence time of vaccines in the upper GI tract. However, due to the complexity of the GI tract, the delivery of oral vaccines targeting the GI mucosa will be limited by a variety of obstacles.
4.2.1. Advanced carriers for gastrointestinal mucosal vaccine delivery
4.2.1.1. Adenoviral vectors
Replicable (live) vaccine vectors combine the improved efficacy of live attenuated vaccines with the safety of inactivated vaccines or subunit vaccines, which promotes its theoretical advantages in vaccine development. For example, the safety of an oral hemagglutinin-encoded vaccine based on adenovirus serotype 4 (Ad4-H5-Vtn, Fig. 4A) was evaluated in phase I studies (NCT01006798)72. The data showed that Ad4-H5-Vtn induced a cellular immune response with a promising safety profile. Kim et al. developed an influenza hemagglutinin vaccine based on an adenovirus vector73. To protect the adenovirus from degradation by stomach acid, they used radio-controlled capsules (RCCs) to deliver the adenovirus vaccines to either the jejunum or the ileum. Antigen-specific mucosal and systemic immune responses were both induced. However, compared with jejunum delivery, ileum delivery induced more antibody (e.g., IgG and IgA isotypes)-secreting cells and increased not only mucosal-homing B cells but also the number of vaccine responders.
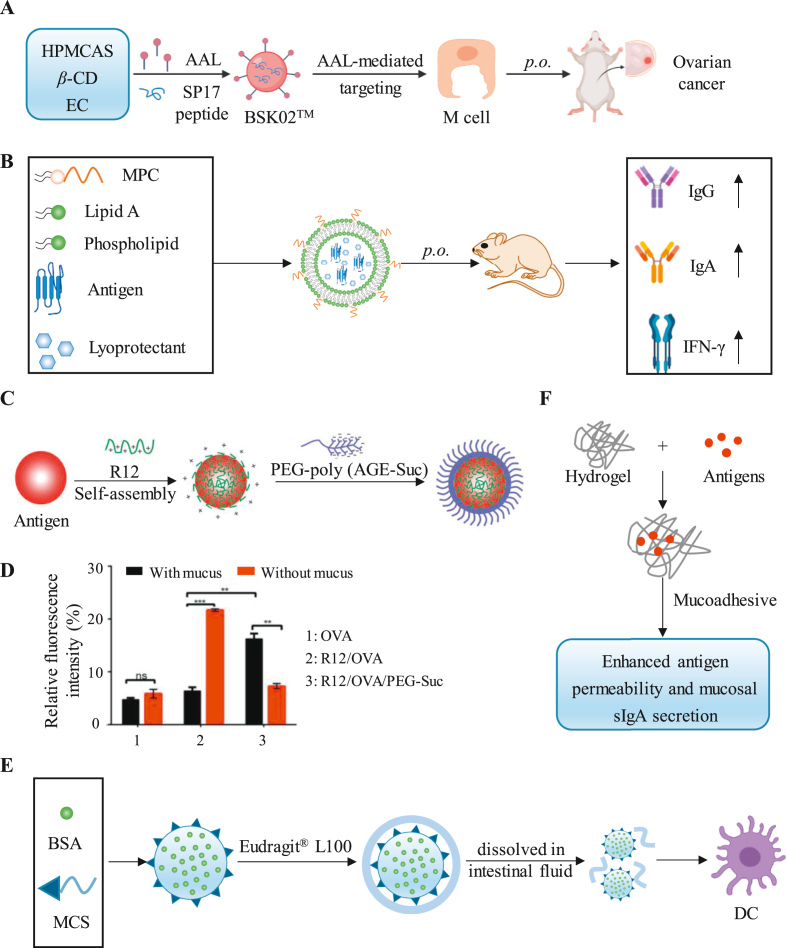
Figure 4.
Advanced strategies for gastrointestinal mucosal delivery. (A) The microparticles targeted by M cells by Aleuria aurantia lectin (AAL) induced an antigen-specific antitumor response after oral administration. (B) Multivalent liposomes targeting APCs with mannose effectively induce the secretion of IgG and IgA. (C and D) NPs composed of polyarginine and PEG-Sue improve mucosal penetration and epithelial absorption. Reprinted from Ref. 77. Copyright © 2018, John Wiley and Sons. (E) A mannosylated chitosan nanoparticle (MCS NP) coated with Eudragit® L100 delivers antigens to APCs in the PP. (F) pH-responsive hydrogel microparticles (MPs) were used for the delivery of water-soluble antigens. Reprinted from Ref. 80. Copyright © 2019, American Chemical Society.
4.2.1.2. Lipid-based vehicles
Lipid-based nanoparticles (LNPs) are widely used as oral drug delivery systems74. They are usually able to effectively wrap the drug inside the particles and protect the drug from the external environment. For example, to prevent antigen and adjuvant degradation under acidic conditions in the stomach, a composite microparticle formulation was described by Chiriva-Internati's group75. Aleuria aurantia lectin (AAL, a high-affinity ligand targeting M cells) was added to the composition to impart microparticles with M cell-targeting ability (Fig. 4A). After oral administration of microparticles with an immunodominant peptide epitope from sperm protein 17 (SP17), SP17-specific antitumor effects were induced.
To develop subunit vaccines capable of targeting APCs, Wang et al. designed a multivalent liposome that has the ability to target APCs through the mannose-PEG1000-cholesterol conjugate (MPC)76. The system is composed of an emulsifier dissolved in the oil phase (O) with MPC, soy phosphatidylcholine, stearylamine and monophosphoryl lipid A, and the water phase (W) with sucrose and BSA. In addition, the O/W emulsions containing BSA (as a model antigen) administered to mice via oral mucosal administration induced an effective immune response, as evidenced by the secretion of IgG in the sera and IgA in the saliva, intestine and vagina (Fig. 4B).
4.2.1.3. Polymer-based nanoparticles
To effectively improve mucus permeation and transepithelial uptake, Zhang et al. synthesized an anionic and hydrophilic PEG derivative (PEG-Suc) coated on NPs enriched with the cell-penetrating peptide (CPP) poly-l-arginine (Fig. 4C)77. By analyzing the uptake of NPs by cells in the presence or absence of the mucus layer secreted by E12 cells, the results confirmed that the internalization of R12/OVA/PEG-Suc (18.24%) was significantly higher than that of R12/OVA and FITC-OVA (7.51% and 4.37%) in the presence of mucus (Fig. 4D). The mucus penetrating ability of the NPs may be derived from the slight negative surface charge generated by PEG-Suc. At the same time, PEG can enable NPs to effectively penetrate the mucus layer and promote the transport of antigens to the submucosal APCs.
Since APCs overexpress mannose receptors, Xu et al.78 encapsulated BSA, a model protein vaccine, into mannosylated chitosan nanoparticles (MCS NPs) for targeting APCs. Subsequent coating with Eudragit® L100 of the MCS NPs (MCS/BSA/Eud) protected the antigen from the harsh acid environment (Fig. 4E). Since Eudragit® L100 is an enteric coating polymer, MCS/BSA/Eud NPs are dissolved by intestinal fluid after entering the intestine, thereby exposing MCS/BSA NPs. This auxiliary coating retained the ability of MCS NPs to target DCs in the PP through mannose and finally effectively induced systemic IgG antibodies and mucosal IgA reactions.
4.2.1.4. Other strategies for GI mucosal immunity
To promote the penetration of hydrophilic antigens into the intestinal mucosa, Chen et al.79,80 designed pH-responsive bacterial nanocellulose/polyacrylic acid (BNC/PAA) hydrogel microparticles (MPs) entrapped with antigens (Fig. 4F). As an example, FITC-OVA-coated MPs promoted penetration into the PP in the small intestine by promoting the paracellular transport of hydrophilic OVA antigens. Furthermore, they confirmed that orally administered OVA and cholera toxin B (CTB)-loaded MPs generated more serum antigen-specific IgG and mucosal IgA in the intestinal washes compared with intramuscular administration.
4.3. Respiratory tract mucosa
The respiratory tract, which is composed of the upper respiratory tract (URT) and lower respiratory tract (LRT)81, is constantly exposed to various bacteria and viruses, which makes it an underlying route of entry for several viral infections, such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), influenza, measles, mumps, rubella, and smallpox81. A majority of viruses that enter the host through the respiratory tract mucosa have been found to replicate most actively in URT (consisting of nasal, pharyngeal and larynx). When pathogens enter the LRT (consisting of the lung, trachea and bronchi), they are likely to cause pulmonary inflammation. Therefore, inducing respiratory mucosal immunity is of great significance to the prevention of infectious diseases.
4.3.1. Advanced vectors for respiratory tract mucosal vaccine delivery
To effectively overcome the barriers of the respiratory mucosa and reach adjacent MALT, nanocarriers are used to simulate the size and characteristics of respiratory viruses, with the aim of inducing humoral and cellular immune responses and improving the effectiveness of various vaccines. There are many types of nanocarriers for respiratory mucosa delivery, such as adenovirus, lipid NPs, protein- or peptide-based nanocarriers, and inorganic nanocarriers. These nanocarriers usually have similar basic characteristics. For example, the size of NPs is generally between 20 and 200 nm (average diameter of most respiratory viruses), and they have a positive charge to enhance adhesion to nasal mucous and avoid elimination by cilia82,83. Since nanocarriers are important for vaccines to overcome mucosal barriers, the vaccine delivery vehicles in respiratory mucosa were summarized.
4.3.1.1. Adenovirus-based vaccines
Several adenovirus-based vaccines have been developed against COVID-19 over the past two years84, 85, 86, all of which confirmed that the ad-vectored vaccine encoding the antigen protein of SARS-CoV-2 protected mice from infection. Chen et al. developed the first aerosolized inhaled adenovirus type-5 vector-based vaccine that possesses the potential to prevent COVID-19 (Ad5-nCoV) worldwide10,87. They demonstrated that one dose of Ad5-nCoV, which is 1/5 the dosage of an intramuscular vaccine, could induce a strong cellular response, achieving protection of the URT and LRT against SARS-CoV-2 infection. In comparison to intramuscular injection, mucosal vaccination induces not only pathogen-specific systemic immunity but also mucosal immunity. Moreover, the mucosal vaccine shows an important advantage that protects against SARS-CoV-2 replication in URT, which further blocks person-to-person transmission. Vaccine inoculation by intramuscular injection does not exhibit this property.
4.3.1.2. Lipid-based nanocarriers
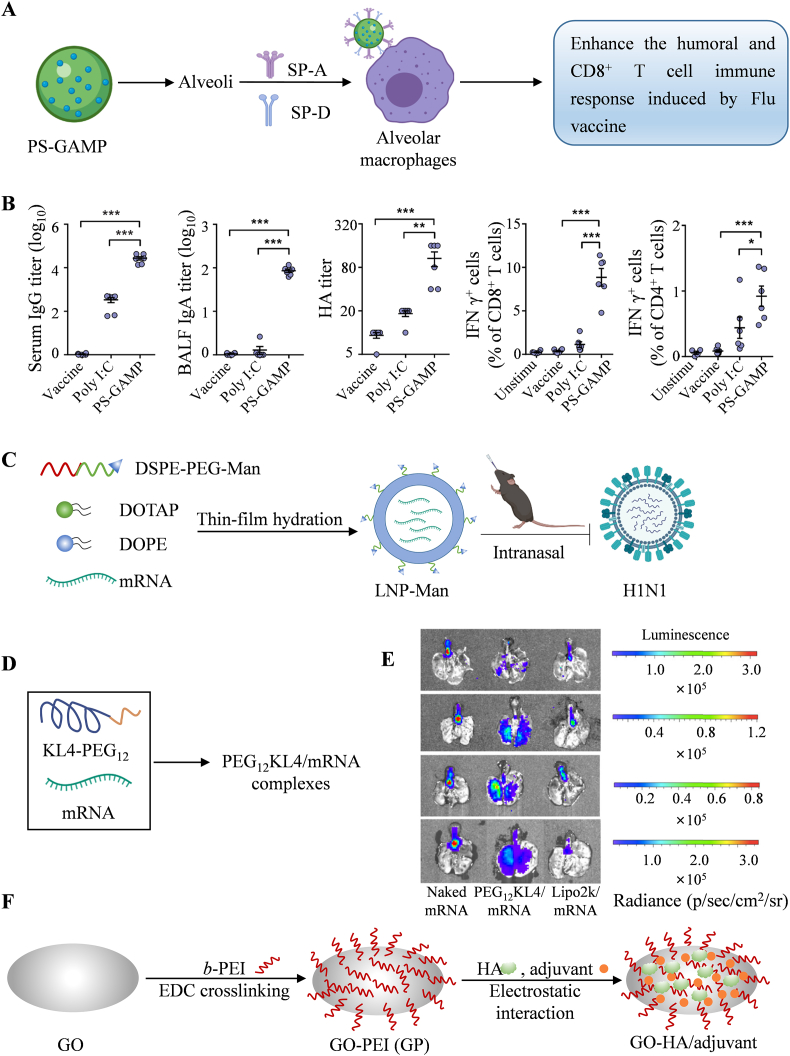
To deliver STING agonists into the cytoplasm of alveolar epithelial cells (AECs) without disrupting the integrity of the lung surfactant (PS) layer, Wu et al. screened a series of biomimetic liposomes that are modified by PS to encapsulate 2′,3′-cyclic guanosine monophosphate-adenosine monophosphate (cGAMP, an agonist of STING)88 (Fig. 5A). Augmentation of the recruitment and differentiation of DCs as well as activation of CD8+ T cells and humoral immune responses to the influenza vaccine were observed after intranasal immunization with the combination of PS-GAMP and influenza vaccine (Fig. 5B). Irvine et al. developed an interbilayer-crosslinked multilamellar vesicle (ICMV)89 and further used it for encapsulation of antigens and two Toll-like receptor (TLR) agonists (poly I:C and monophosphoryl lipid A)90. After immunization via the intratracheal route, ICMVs primed 13-fold more T cells than equivalent protein- or peptide-based soluble vaccines and generated long-lived T cells that were biased toward an effector memory (Tem) phenotype in the lung and distal mucosae (for example, vaginal). Notably, Tem cells play an important role in protection against therapeutic tumors and prophylactic viruses. Additionally, Jin et al. demonstrated that mannose-conjugated cationic lipid nanoparticle (LNP-Man) is more efficient than LNP in delivering mRNA encoding hemagglutinin (HA) antigens against the H1N1 influenza virus intranasally91 (Fig. 5C). Humoral and cellular immune responses were elicited in C57BL/6 mice that were immunized through intranasal administration.
Figure 5.
Advanced strategies for respiratory tract mucosal delivery. (A and B) Screening PS-modified biomimetic liposomes (PS-GAMP) to enhance the effect of the influenza vaccine. Reprinted from Ref. 88. Copyright © 2020, The American Association for the Advancement of Science. (C) A mannose-conjugated cationic lipid nanoparticle (LNP-Man) for influenza mRNA delivery. (D and E) PEG12KL4 for mRNA delivery. Reprinted from Ref. 97. Copyright © 2019, Elsevier. (F) Polyethylene (PEI)-functionalized graphene oxide (GO) NPs were used for HA antigen delivery. Reprinted from Ref. 103. Copyright © 2021, National Academy of Sciences.
4.3.1.3. Peptide-based nanocarriers
Peptide-based nanocarriers are also used in the development of mucosal vaccines. Chong and Collier et al. demonstrated a nanofiber that consists of an epitope from influenza acid polymerase (PA) and β-sheet forming peptide Q11 (a self-assembling peptide)92. After intranasal immunization, the nanofibers showed enhanced uptake by DCs in lung-draining mediastinal lymph nodes and further generated a stronger CD8+ T cell response compared with subcutaneous immunizations. Moreover, the soluble antigen peptide of the nanofibers can be easily replaced with other antigens, such as Eα93, OVA94,95 and PADRE96, and a similar immune effect can be achieved after administration via the same route. To develop a platform for robust mRNA transfection in the airways, Lam et al. introduced novel mRNA inhalation preparations carrying PEG12KL4, in which the cationic KL4 peptide was chemically linked to the linear PEG of 12-mers97 (Fig. 5D). Pulmonary delivery of the PEG12KL4/luciferase mRNA complex resulted in luciferase expression in the deep regions of mouse lungs (Fig. 5E). This mRNA delivery capability was not compromised after formulation into dry powders by spray drying (SD) and spray freeze drying (SFD) techniques. These results demonstrate the application potential of PEG12KL4 in mRNA inhalation vaccines. In addition, various strategies have been used to deliver mRNA into the respiratory mucosa to induce mucosal immunity98, 99, 100, 101, 102.
4.3.1.4. Other carriers
Additionally, many types of vectors are used in the development of vaccines, which help to induce respiratory tract mucosal immunity. For example, Wang et al. developed an influenza vaccine nanoplatform (GO-PEI, Fig. 5F) based on graphene oxide (GO) NPs functionalized by polyethylene (PEI)103. Combined with recombinant influenza hemagglutinin (HA), the influenza GO-PEI NPs (HA/GO-PEI) were found to elicit powerful humor and cellular immune responses, thereby protecting the intranasally immunized mice from influenza virus challenge.
To assist inactivated virus (WIV) vaccines in breaking through the barrier of the respiratory tract mucosa, Gao et al.104 introduced a chitosan (CS)-functionalized iron oxide nanozyme (IONzyme), which actually increased the adhesion of WIV to the mucosa and further triggered an 8.9-fold increase in IgA production indicative of strong adaptive mucosal immunity and thereby successfully protected immunized mice from the virus.
4.4. Vaginal mucosa
The lower reproductive tract, composed of the vagina and cervix, is a unique place for pathogens to enter and spread between individuals. The pathogen-specific immune response of the female reproductive tract is an important strategy to protect the health of women and control sexually transmitted infections. Compared with other mucosal delivery methods, vaginal delivery seems to be hindered by more factors. Thus, vaccine inoculation through other mucosal sites is preferred over vaginal mucosal inoculation when aiming to induce mucosal immunity in the lower reproductive tract, although the vaginal mucosa is certified to be a more effective inoculation site105. However, many outstanding researchers have developed potential vaginal mucosal delivery systems for the delivery of various drugs47,106,107, including vaccines.
4.4.1. Advanced vectors for vaginal mucosal vaccine delivery
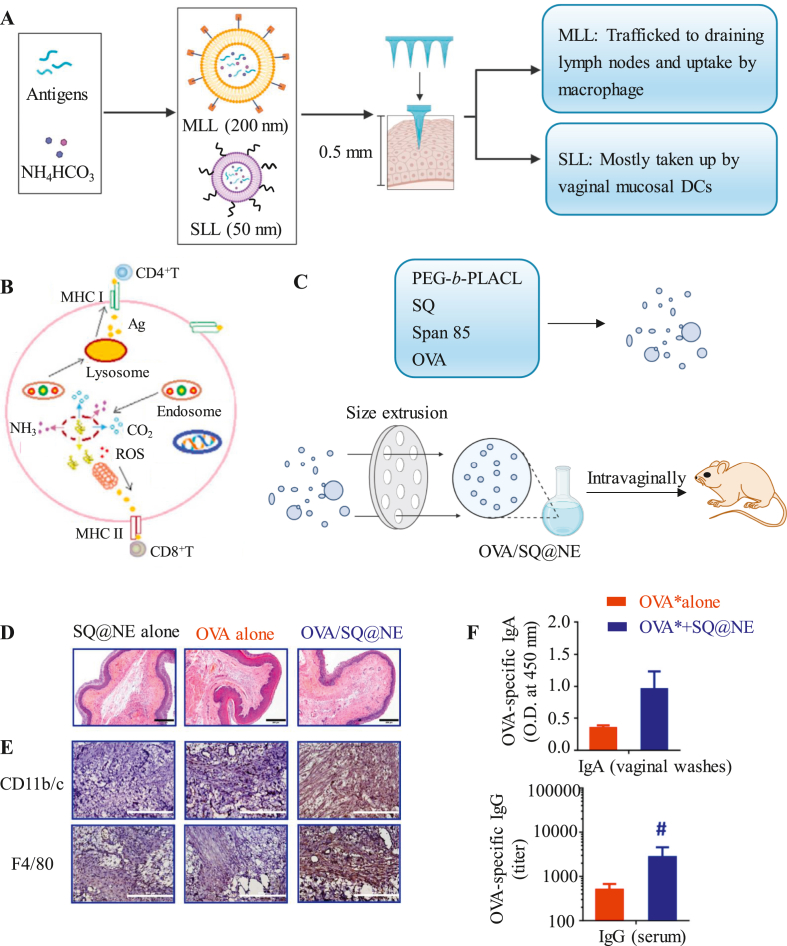
To effectively induce vaginal mucosal immunity, microneedles or nanoemulsions are used to improve the immune effect of vaccines. Wang et al.108 synthesized a microneedle array (proSMMA) composed of two types of liposomes, mannosylated lipid A-liposomes (MLLs) and stealth lipid A-liposomes (SLLs), which were both loaded with antigens and NH4HCO3 and had sizes of 200 and 50 nm, respectively (Fig. 6A). The proSMMA revert to two types of liposomes when they are rehydrated, which leads to vaginal mucosal DC uptake of MLLs and SLLs followed by direct drainage into the lymph nodes. Their results showed that under the action of lysosomes, NH4HCO3 reacted with CO2 and NH3 and further generated ROS (Fig. 6B). These reactions effectively promoted the mixed Th1/Th2-type response. Mice vaccinated with proSMMAs via vaginal mucosa patching were protected from the virus. Huang et al. developed a squalene-assisted PEG-b-PLACL nanoemulsion (∼200 nm) containing OVA antigens (OVA/SQ@NE)109,110 (Fig. 6C). Administered intravaginally into Balb/c mice, the retention time of protein antigens in the genital tract is prolonged, which further results in increased infiltration of CD11b/c+ cells and F4/80+ macrophages in the vagina (Fig. 6D) and increased secretion of antigen-specific serum IgG and mucosal IgA (Fig. 6E), suggesting strong antigen-specific cellular and humoral immune responses.
Figure 6.
Advanced strategies for vaginal mucosal delivery. (A and B) A microneedle array (proSMMA) was designed for vaccine delivery to the vaginal mucosa. Reprinted from Ref. 108. Copyright © 2017, Elsevier. (C) A squalene-assisted PEG-b-PLACL nanoemulsion was used to induce an antigen-specific vaginal mucosal immune response. CD11b/c+ and F4/80+ cells in vaginal mucosa were stained by (D) HE and (E) IHC after OVA/SQ@NE immunization. (F) OVA-specific IgG titers in serum and OVA-specific IgA levels in vaginal washes. Reprinted from Ref. 109. Copyright © 2021, Elsevier.
In addition, other drug delivery systems targeting the vaginal mucosa are also promising for future vaccine development. For example, propylene glycol-embodying deformable liposomes (FDL, ∼185 nm) effectively improve the permeability of fibronectin (FN) through the mucosa of the approach channel because propylene glycol, as an edge activator, can significantly increase the fluidity and flexibility of the phospholipid bilayer, thereby enhancing the permeability of the mucous membrane111.
4.5. Ocular mucosa
The eye, one of the most delicate organs in the human body, is where optesthesia occurs. When exposed to the external environment, the ocular mucosa could be a route of potential entry for various pathogens, including infectious pathogens. SARS-CoV-2 is reported to invade the ocular mucosa112. Moreover, SARS-CoV-2 RNA was detected in the conjunctival scrapings and tears of COVID-19 patients, indicating that SARS-CoV-2 could be transmitted through ocular secretions113. These findings suggest that the valid induction of ocular mucosal immunity might contribute to controlling the pandemic. However, drug delivery or vaccination through the ocular mucosa remains challenging.
4.5.1. Advanced strategies for ocular mucosa delivery
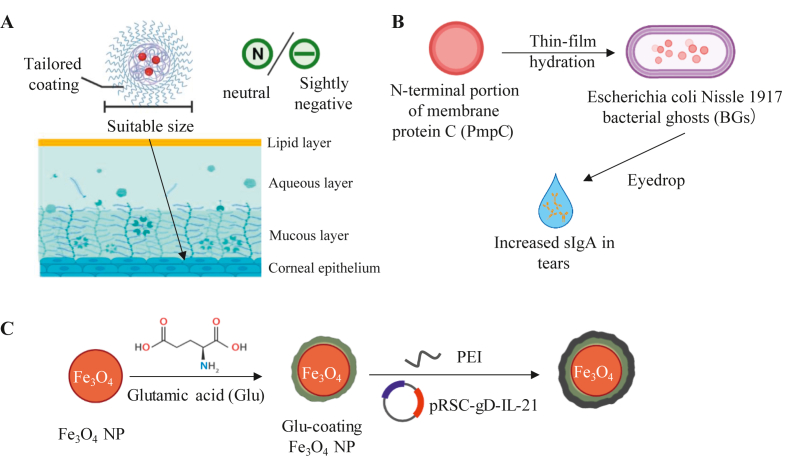
To elevate the penetration ability and retention time of eyedrop-formulation drugs, the physicochemical properties of NPs must be modified. Size, surface charge and surface coating are the three nanoparticle properties most focused on by researchers (Fig. 7A). NPs which have smaller sizes than the mucus meshwork pore show better penetration ability. The mucus layers are negatively charged due to the presence of carboxyl and sulfate groups on the mucin proteoglycans. Thus, NPs with a neutral or slightly negative surface charge could prevent electrostatic interactions and achieve proven penetration114. Polymeric coating of NPs could change the surface properties of NPs, reduce nonspecific binding to the NPs and improve the penetration capacity even further. Xu et al.115 demonstrated that a dense PEG coating on the surface of PLGA NPs (a dense brush PEG conformation) contributes to significant penetration. The fast penetration through the tear film reaching the cornea prevents the NPs from being eliminated by the quick tear film turnover and fast tear drainage, thus prolonging the retention time.
Figure 7.
Advanced strategies for ocular mucosal delivery. (A) The size, surface charge and surface coating of NPs have a decisive impact on the delivery abilities of eyedrop vaccines. (B) Membrane protein encapsulated in bacterial ghosts (BGs) could induce the production of Chlamydia trachomatis (Ct)-specific sIgA in tears by eyedrop inoculation. (C) The Fe3O4 NPs coated with glutamic acid, DNA vaccine pRSC-gD-IL-21 and PEI were developed against HSV-1 infection.
Regarding the injection formulation of drugs and vaccines targeting the ocular mucosa, the utilization of NPs remains effective. Soluble drugs exhibit great transscleral diffusion after subconjunctival injection; however, rapid elimination of the drug occurs. Drug encapsulated in PLGA NPs has been demonstrated to have sustained release for more than a week116, reducing the injection frequency. Another commonly used injection method is intravitreal injection. The vitreous is composed of a delicate mesh network of gel-forming collagen fibrils and hyaluronan molecules. Specific characteristics are needed for NPs to diffuse in the vitreous. NPs with particle sizes larger than 1000 nm would be trapped in the vitreous gel, while NPs with particle sizes smaller than 500 nm show excellent diffusion patterns117. The surface charge and coating of NPs are also important for drug delivery53.
To date, several ocular drugs for humans have been approved for clinical use, but fewer attempts at ocular vaccination have been reported. Among those vaccines, eyedrops are the main drug formulation. Barisani et al. used bacterial ghosts to construct a subunit vaccine against Chlamydia trachomatis (Ct), successfully inducing mice to produce specific IgG and sIgA118 (Fig. 7B). Gu et al. studied an ocular vaccine against herpes stromal keratitis (HSK)119. Glutamic acid-coated Fe3O4 NPs combine with the DNA plasmid expressing the HSV glycoprotein D (gD) antigen and interleukin-21 (IL-21) (Fig. 7C). This vaccine shows great transfection efficacy and induces not only mucosal but also systematic cellular immunity in mice. Wechsler et al. and Yasuo et al. constructed subunit vaccines (glycoprotein) and DNA vaccines (DNA plasmids) against HSV, respectively120,121. These two vaccines both require subconjunctival injection. Research on injection vaccines targeting the ocular mucosa is limited by the need for anesthesia before injection, precision of the injection and side effects following injection.
5. Summary and prospects
Mucosal vaccines exhibit promising potential in the treatment of some diseases as well as the prevention and interruption of disease transmission. However, since the first mucosal vaccine OPV was approved by the FDA in 1961, the development of mucosal vaccines has been slow and limited to traditional dosage forms, such as inactivated vaccines and attenuated vaccines. The specific barriers of different mucous membranes may be responsible for this limitation.
Since the first step toward successful mucosal vaccines is the effective uptake of antigen by APCs, a variety of advanced strategies have been designed to promote antigen delivery. Targeting M cells, DCs, etc. is considered an effective way to promote antigen presentation. However, no M cells exist in the type II mucosa, and methods including M cell targeting are not applicable in addition to the traditional APC strategy. Moreover, a mucus layer appears on the surface of various mucosae, and many mucosal drug delivery strategies focus on the breakthrough of the mucous layer. At present, many attempts to use NPs for mucoadhesion, mucopenetration and mucolytics have been conducted. Notably, different parts of the mucosa possess their own unique environmental and structural characteristics. Therefore, the design of drugs should fully consider the characteristics of the specific mucosa.
The COVID-19 outbreak in 2019 has attracted increased attention to the development of various types of vaccines, such as mRNA vaccines and mucosal vaccines. The mucosal vaccine represented by Ad5-nCoV has been developed and is promising to fill the gap in COVID-19 mucosal vaccines. However, most types of mucosal vaccines are developing slowly. As summarized in this review, numerous mucosal vaccines based on subunits and mRNA have been demonstrated to be valid against various diseases. We believe that more types of mucosal vaccines will be approved for use in the near future.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (52130301, 31870996 and 32071378), Guangdong Provincial Pearl River Talents Program (2017GC010713 and 2017GC010482, China), the Science and Technology Program of Guangzhou (202103030004, China). Figure images were created with BioRender.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences
Contributor Information
Xiaojiao Du, Email: duxjz@scut.edu.cn.
Jun Wang, Email: mcjwang@scut.edu.cn.
Author contributions
Mengwen Huang collected relevant research articles and completed the manuscript. Miaomiao Zhang and Hongbin Zhu made additions and revisions to the manuscript. Xiaojiao Du and Jun Wang provided the idea and revised the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Mora J.R., von Andrian U.H. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008;1:96–109. doi: 10.1038/mi.2007.14. [DOI] [PubMed] [Google Scholar]
- 2.Yamamoto M., Rennert P., McGhee J.R., Kweon M.N., Yamamoto S., Dohi T. Alternate mucosal immune system: organized Peyer's patches are not required for IgA responses in the gastrointestinal tract. J Immunol. 2000;164:5184–5191. doi: 10.4049/jimmunol.164.10.5184. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg P., Johansen F.E. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 4.Schleier L., Wiendl M., Heidbreder K., Binder M.T., Atreya R., Rath T., et al. Non-classical monocyte homing to the gut via alpha4beta7 integrin mediates macrophage-dependent intestinal wound healing. Gut. 2020;69:252–263. doi: 10.1136/gutjnl-2018-316772. [DOI] [PubMed] [Google Scholar]
- 5.Fujimori H., Miura S., Koseki S., Hokari R., Komoto S., Hara Y., et al. Intravital observation of adhesion of lamina propria lymphocytes to microvessels of small intestine in mice. Gastroenterology. 2002;122:734–744. doi: 10.1053/gast.2002.31899. [DOI] [PubMed] [Google Scholar]
- 6.Rosen S.D. Ligands for l-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 7.Iijima H., Takahashi I., Kiyono H. Mucosal immune network in the gut for the control of infectious diseases. Rev Med Virol. 2001;11:117–133. doi: 10.1002/rmv.307. [DOI] [PubMed] [Google Scholar]
- 8.Kiyono H., Fukuyama S. NALT-versus Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy T.V., Gargiullo P.M., Massoudi M.S., Nelson D.B., Jumaan A.O., Okoro C.A., et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344:564–572. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 10.Wu S., Huang J., Zhang Z., Wu J., Zhang J., Hu H., et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021;12:1654–1664. doi: 10.1016/S1473-3099(21)00396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam M.A., Firdous J., Badruddoza A.Z.M., Reesor E., Azad M., Hasan A., et al. M cell targeting engineered biomaterials for effective vaccination. Biomaterials. 2019;192:75–94. doi: 10.1016/j.biomaterials.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 12.Garinot M., Fievez V., Pourcelle V., Stoffelbach F., des Rieux A., Plapied L., et al. PEGylated PLGA-based nanoparticles targeting M cells for oral vaccination. J Control Release. 2007;120:195–204. doi: 10.1016/j.jconrel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Ueno H., Klechevsky E., Schmitt N., Ni L., Flamar A.L., Zurawski S., et al. Targeting human dendritic cell subsets for improved vaccines. Semin Immunol. 2011;23:21–27. doi: 10.1016/j.smim.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang X., Chen X., Lei T., Qin L., Zhou Y., Hu C., et al. The construction of in vitro nasal cavity-mimic M-cell model, design of M cell-targeting nanoparticles and evaluation of mucosal vaccination by nasal administration. Acta Pharm Sin B. 2020;10:1094–1105. doi: 10.1016/j.apsb.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai S.K., Wang Y.Y., Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdulkarim M., Sharma P.K., Gumbleton M. Self-emulsifying drug delivery system: mucus permeation and innovative quantification technologies. Adv Drug Deliv Rev. 2019;142:62–74. doi: 10.1016/j.addr.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Rios D., Wood M.B., Li J., Chassaing B., Gewirtz A.T., Williams I.R. Antigen sampling by intestinal M cells is the principal pathway initiating mucosal IgA production to commensal enteric bacteria. Mucosal Immunol. 2016;9:907–916. doi: 10.1038/mi.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schulz O., Pabst O. Antigen sampling in the small intestine. Trends Immunol. 2013;34:155–161. doi: 10.1016/j.it.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Man A.L., Prieto-Garcia M.E., Nicoletti C. Improving M cell mediated transport across mucosal barriers: do certain bacteria hold the keys?. Immunology. 2004;113:15–22. doi: 10.1111/j.1365-2567.2004.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komban R.J., Stromberg A., Biram A., Cervin J., Lebrero-Fernandez C., Mabbott N., et al. Activated Peyer's patch B cells sample antigen directly from M cells in the subepithelial dome. Nat Commun. 2019;10:2423. doi: 10.1038/s41467-019-10144-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pabst O., Cerovic V., Hornef M. Secretory IgA in the coordination of establishment and maintenance of the microbiota. Trends Immunol. 2016;37:287–296. doi: 10.1016/j.it.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Macpherson A.J., Yilmaz B., Limenitakis J.P., Ganal-Vonarburg S.C. IgA function in relation to the intestinal microbiota. Annu Rev Immunol. 2018;36:359–381. doi: 10.1146/annurev-immunol-042617-053238. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki A. Exploiting mucosal immunity for antiviral vaccines. Annu Rev Immunol. 2016;34:575–608. doi: 10.1146/annurev-immunol-032414-112315. [DOI] [PubMed] [Google Scholar]
- 24.Garcia-Diaz M., Birch D., Wan F., Nielsen H.M. The role of mucus as an invisible cloak to transepithelial drug delivery by nanoparticles. Adv Drug Deliv Rev. 2018;124:107–124. doi: 10.1016/j.addr.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Hangartner L., Zinkernagel R.M., Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- 26.Para M.F., Goldstein L., Spear P.G. Similarities and differences in the Fc-binding glycoprotein (gE) of herpes simplex virus types 1 and 2 and tentative mapping of the viral gene for this glycoprotein. J Virol. 1982;41:137–144. doi: 10.1128/jvi.41.1.137-144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awasthi S., Huang J., Shaw C., Friedman H.M. Blocking herpes simplex virus 2 glycoprotein E immune evasion as an approach to enhance efficacy of a trivalent subunit antigen vaccine for genital herpes. J Virol. 2014;88:8421–8432. doi: 10.1128/JVI.01130-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johansson M.E., Hansson G.C. Is the intestinal goblet cell a major immune cell?. Cell Host Microbe. 2014;15:251–252. doi: 10.1016/j.chom.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y.S., Ho S.B. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rath T., Baker K., Pyzik M., Blumberg R.S. Regulation of immune responses by the neonatal fc receptor and its therapeutic implications. Front Immunol. 2014;5:664. doi: 10.3389/fimmu.2014.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woof J.M., Kerr M.A. The function of immunoglobulin A in immunity. J Pathol. 2006;208:270–282. doi: 10.1002/path.1877. [DOI] [PubMed] [Google Scholar]
- 32.Murgia X., Loretz B., Hartwig O., Hittinger M., Lehr C.M. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv Drug Deliv Rev. 2018;124:82–97. doi: 10.1016/j.addr.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Bandi S.P., Bhatnagar S., Venuganti V.V.K. Advanced materials for drug delivery across mucosal barriers. Acta Biomater. 2021;119:13–29. doi: 10.1016/j.actbio.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 34.Bansil R., Turner B.S. The biology of mucus: composition, synthesis and organization. Adv Drug Deliv Rev. 2018;124:3–15. doi: 10.1016/j.addr.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Atuma C., Strugala V., Allen A., Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280:G922–G929. doi: 10.1152/ajpgi.2001.280.5.G922. [DOI] [PubMed] [Google Scholar]
- 36.Song J.H., Kim J.I., Kwon H.J., Shim D.H., Parajuli N., Cuburu N., et al. CCR7-CCL19/CCL21-regulated dendritic cells are responsible for effectiveness of sublingual vaccination. J Immunol. 2009;182:6851–6860. doi: 10.4049/jimmunol.0803568. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Q., Chen Z., Paul P.K., Lu Y., Wu W., Qi J. Oral delivery of proteins and peptides: challenges, status quo and future perspectives. Acta Pharm Sin B. 2021;11:2416–2448. doi: 10.1016/j.apsb.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mascarell L., Lombardi V., Louise A., Saint-Lu N., Chabre H., Moussu H., et al. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J Allergy Clin Immunol. 2008;122:603–609. doi: 10.1016/j.jaci.2008.06.034. e5. [DOI] [PubMed] [Google Scholar]
- 39.Nagai Y., Shiraishi D., Tanaka Y., Nagasawa Y., Ohwada S., Shimauchi H., et al. Transportation of sublingual antigens across sublingual ductal epithelial cells to the ductal antigen-presenting cells in mice. Clin Exp Allergy. 2015;45:677–686. doi: 10.1111/cea.12329. [DOI] [PubMed] [Google Scholar]
- 40.Vila T., Rizk A.M., Sultan A.S., Jabra-Rizk M.A. The power of saliva: antimicrobial and beyond. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1008058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kildsgaard J., Brimnes J., Jacobi H., Lund K. Sublingual immunotherapy in sensitized mice. Ann Allergy Asthma Immunol. 2007;98:366–372. doi: 10.1016/S1081-1206(10)60884-8. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed O.G., Lane A.P. Is sublingual immunotherapy an effective therapy for allergic rhinitis?. Laryngoscope. 2020;130:2301–2302. doi: 10.1002/lary.28574. [DOI] [PubMed] [Google Scholar]
- 43.Piconi S., Trabattoni D., Rainone V., Borgonovo L., Passerini S., Rizzardini G., et al. Immunological effects of sublingual immunotherapy: clinical efficacy is associated with modulation of programmed cell death ligand 1, IL-10, and IgG4. J Immunol. 2010;185:7723–7730. doi: 10.4049/jimmunol.1002465. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y., Li M., Du G., Chen X., Sun X. Advancedoral vaccine delivery strategies for improving the immunity. Adv Drug Deliv Rev. 2021;177 doi: 10.1016/j.addr.2021.113928. [DOI] [PubMed] [Google Scholar]
- 45.Zanin M., Baviskar P., Webster R., Webby R. The interaction between respiratory pathogens and mucus. Cell Host Microbe. 2016;19:159–168. doi: 10.1016/j.chom.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace L.E., Liu M., van Kuppeveld F.J.M., de Vries E., de Haan C.A.M. Respiratory mucus as a virus-host range determinant. Trends Microbiol. 2021;11:983–992. doi: 10.1016/j.tim.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caramella C.M., Rossi S., Ferrari F., Bonferoni M.C., Sandri G. Mucoadhesive and thermogelling systems for vaginal drug delivery. Adv Drug Deliv Rev. 2015;92:39–52. doi: 10.1016/j.addr.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 48.Miller E.A., Beasley D.E., Dunn R.R., Archie E.A. Lactobacilli dominance and vaginal pH: why is the human vaginal microbiome unique?. Front Microbiol. 2016;7:1936. doi: 10.3389/fmicb.2016.01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Knop E., Knop N. The role of eye-associated lymphoid tissue in corneal immune protection. J Anat. 2005;206:271–285. doi: 10.1111/j.1469-7580.2005.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamrah P., Huq S.O., Liu Y., Zhang Q., Dana M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukocyte Biol. 2003;74:172–178. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 51.Hodges R.R., Dartt D.A. Tear film mucins: front line defenders of the ocular surface; comparison with airway and gastrointestinal tract mucins. Exp Eye Res. 2013;117:62–78. doi: 10.1016/j.exer.2013.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gelderblom H., Verweij J., Nooter K., Sparreboom A., Cremophor E.L. The drawbacks and advantages of vehicle selection for drug formulation. Eur J Cancer. 2001;37:1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- 53.Meng T., Kulkarni V., Simmers R., Brar V., Xu Q. Therapeutic implications of nanomedicine for ocular drug delivery. Drug Discov Today. 2019;24:1524–1538. doi: 10.1016/j.drudis.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leibowitz H.M., Kupferman A. Periocular Injection of Corticosteroids: an experimental evaluation of its role in the treatment of corneal inflammation. Arch Ophthalmol. 1977;95:311–314. doi: 10.1001/archopht.1977.04450020112019. [DOI] [PubMed] [Google Scholar]
- 55.Guilbert E., Bullet J., Sandali O., Basli E., Laroche L., Borderie V.M. Long-term rejection incidence and reversibility after penetrating and lamellar keratoplasty. Am J Ophthalmol. 2013;155:560–569. doi: 10.1016/j.ajo.2012.09.027. e2. [DOI] [PubMed] [Google Scholar]
- 56.Duvvuri S., Majumdar S., Mitra A.K. Drug delivery to the retina: challenges and opportunities. Expet Opin Biol Ther. 2003;3:45–56. doi: 10.1517/14712598.3.1.45. [DOI] [PubMed] [Google Scholar]
- 57.Paris A.L., Colomb E., Verrier B., Anjuere F., Monge C. Sublingual vaccination and delivery systems. J Control Release. 2021;332:553–562. doi: 10.1016/j.jconrel.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 58.Lam J.K.W., Cheung C.C.K., Chow M.Y.T., Harrop E., Lapwood S., Barclay S.I.G., et al. Transmucosal drug administration as an alternative route in palliative and end-of-life care during the COVID-19 pandemic. Adv Drug Deliv Rev. 2020;160:234–243. doi: 10.1016/j.addr.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kraan H., Vrieling H., Czerkinsky C., Jiskoot W., Kersten G., Amorij J.P. Buccal and sublingual vaccine delivery. J Control Release. 2014;190:580–592. doi: 10.1016/j.jconrel.2014.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aframian D.J., Davidowitz T., Benoliel R. The distribution of oral mucosal pH values in healthy saliva secretors. Oral Dis. 2006;12:420–423. doi: 10.1111/j.1601-0825.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- 61.Sudhakar Y., Kuotsu K., Bandyopadhyay A.K. Buccal bioadhesive drug delivery―a promising option for orally less efficient drugs. J Control Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 62.Hervouet C., Luci C., Bekri S., Juhel T., Bihl F., Braud V.M., et al. Antigen-bearing dendritic cells from the sublingual mucosa recirculate to distant systemic lymphoid organs to prime mucosal CD8 T cells. Mucosal Immunol. 2014;7:280–291. doi: 10.1038/mi.2013.45. [DOI] [PubMed] [Google Scholar]
- 63.McNeilly C.L., Crichton M.L., Primiero C.A., Frazer I.H., Roberts M.S., Kendall M.A. Microprojection arrays to immunise at mucosal surfaces. J Control Release. 2014;196:252–260. doi: 10.1016/j.jconrel.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 64.Zhen Y., Wang N., Gao Z., Ma X., Wei B., Deng Y., et al. Multifunctional liposomes constituting microneedles induced robust systemic and mucosal immunoresponses against the loaded antigens via oral mucosal vaccination. Vaccine. 2015;33:4330–4340. doi: 10.1016/j.vaccine.2015.03.081. [DOI] [PubMed] [Google Scholar]
- 65.White J.A., Blum J.S., Hosken N.A., Marshak J.O., Duncan L., Zhu C., et al. Serum and mucosal antibody responses to inactivated polio vaccine after sublingual immunization using a thermoresponsive gel delivery system. Hum Vaccin Immunother. 2014;10:3611–3621. doi: 10.4161/hv.32253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kelly S.H., Wu Y., Varadhan A.K., Curvino E.J., Chong A.S., Collier J.H. Enabling sublingual peptide immunization with molecular self-assemblies. Biomaterials. 2020;241 doi: 10.1016/j.biomaterials.2020.119903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kelly S.H., Opolot E.E., Wu Y., Cossette B., Varadhan A.K., Collier J.H. Tabletized supramolecular assemblies for sublingual peptide immunization. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Masek J., Lubasova D., Lukac R., Turanek-Knotigova P., Kulich P., Plockova J., et al. Multi-layered nanofibrous mucoadhesive films for buccal and sublingual administration of drug-delivery and vaccination nanoparticles―important step towards effective mucosal vaccines. J Control Release. 2017;249:183–195. doi: 10.1016/j.jconrel.2016.07.036. [DOI] [PubMed] [Google Scholar]
- 69.Hanson S.M., Singh S., Tabet A., Sastry K.J., Barry M., Wang C. Mucoadhesive wafers composed of binary polymer blends for sublingual delivery and preservation of protein vaccines. J Control Release. 2021;330:427–437. doi: 10.1016/j.jconrel.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 70.Çuburu N., Kweon M.N., Hervouet C., Cha H.R., Pang Y.Y.S., Holmgren J., et al. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J Immunol. 2009;183:7851–7859. doi: 10.4049/jimmunol.0803740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Seth A., Kong I.G., Lee S.H., Yang J.Y., Lee Y.S., Kim Y., et al. Modular virus-like particles for sublingual vaccination against group A streptococcus. Vaccine. 2016;34:6472–6480. doi: 10.1016/j.vaccine.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 72.Gurwith M., Lock M., Taylor E.M., Ishioka G., Alexander J., Mayall T., et al. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect Dis. 2013;13:238–250. doi: 10.1016/S1473-3099(12)70345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim L., Martinez C.J., Hodgson K.A., Trager G.R., Brandl J.R., Sandefer E.P., et al. Systemic and mucosal immune responses following oral adenoviral delivery of influenza vaccine to the human intestine by radio controlled capsule. Sci Rep. 2016;6 doi: 10.1038/srep37295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He H., Lu Y., Qi J., Zhu Q., Chen Z., Wu W. Adapting liposomes for oral drug delivery. Acta Pharm Sin B. 2019;9:36–48. doi: 10.1016/j.apsb.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mattila J.P., Mirandola L., Chiriva-Internati M. Development of a M cell-targeted microparticulate platform, BSK02, for oral immunization against the ovarian cancer antigen, sperm protein 17. J Biomed Mater Res B Appl Biomater. 2019;107:29–36. doi: 10.1002/jbm.b.34092. [DOI] [PubMed] [Google Scholar]
- 76.Wang N., Wang T., Zhang M., Chen R., Niu R., Deng Y. Mannose derivative and lipid A dually decorated cationic liposomes as an effective cold chain free oral mucosal vaccine adjuvant-delivery system. Eur J Pharm Biopharm. 2014;88:194–206. doi: 10.1016/j.ejpb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 77.Zhang Y., Li H., Wang Q., Hao X., Li H., Sun H., et al. Rationally designed self-assembling nanoparticles to overcome mucus and epithelium transport barriers for oral vaccines against Helicobacter pylori. Adv Funct Mater. 2018;28 [Google Scholar]
- 78.Xu B., Zhang W., Chen Y., Xu Y., Wang B., Zong L. Eudragit® L100-coated mannosylated chitosan nanoparticles for oral protein vaccine delivery. Int J Biol Macromol. 2018;113:534–542. doi: 10.1016/j.ijbiomac.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 79.Chen X.Y., Butt A.M., Mohd Amin M.C.I. Enhanced paracellular delivery of vaccine by hydrogel microparticles-mediated reversible tight junction opening for effective oral immunization. J Control Release. 2019;311–312:50–64. doi: 10.1016/j.jconrel.2019.08.031. [DOI] [PubMed] [Google Scholar]
- 80.Chen X.Y., Butt A.M., Mohd Amin M.C.I. Molecular evaluation of oral immunogenicity of hepatitis B antigen delivered by hydrogel microparticles. Mol Pharm. 2019;16:3853–3872. doi: 10.1021/acs.molpharmaceut.9b00483. [DOI] [PubMed] [Google Scholar]
- 81.Man W.H., de Steenhuijsen Piters W.A., Bogaert D. The microbiota of the respiratory tract: gatekeeper to respiratory health. Nat Rev Microbiol. 2017;15:259–270. doi: 10.1038/nrmicro.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fromen C.A., Robbins G.R., Shen T.W., Kai M.P., Ting J.P., DeSimone J.M. Controlled analysis of nanoparticle charge on mucosal and systemic antibody responses following pulmonary immunization. Proc Natl Acad Sci U S A. 2015;112:488–493. doi: 10.1073/pnas.1422923112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta N.K., Tomar P., Sharma V., Dixit V.K. Development and characterization of chitosan coated poly-(varepsilon-caprolactone) nanoparticulate system for effective immunization against influenza. Vaccine. 2011;29:9026–9037. doi: 10.1016/j.vaccine.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 84.Hassan A.O., Kafai N.M., Dmitriev I.P., Fox J.M., Smith B.K., Harvey I.B., et al. A single-dose intranasal Chad vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183:169–184. doi: 10.1016/j.cell.2020.08.026. e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feng L., Wang Q., Shan C., Yang C., Feng Y., Wu J., et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-CoV-2 challenge in rhesus macaques. Nat Commun. 2020;11:4207. doi: 10.1038/s41467-020-18077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu S., Zhong G., Zhang J., Shuai L., Zhang Z., Wen Z., et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat Commun. 2020;11:4081. doi: 10.1038/s41467-020-17972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang J., Li P., Yu Y., Fu Y., Jiang H., Lu M., et al. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science. 2020;367 doi: 10.1126/science.aau0810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moon J.J., Suh H., Bershteyn A., Stephan M.T., Liu H., Huang B., et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li A.V., Moon J.J., Abraham W., Suh H., Elkhader J., Seidman M.A., et al. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci Transl Med. 2013;5:204ra130. doi: 10.1126/scitranslmed.3006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhuang X., Qi Y., Wang M., Yu N., Nan F., Zhang H., et al. mRNA vaccines encoding the HA protein of influenza A H1N1 virus delivered by cationic lipid nanoparticles induce protective immune responses in mice. Vaccines (Basel) 2020;8:123. doi: 10.3390/vaccines8010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Si Y., Wen Y., Kelly S.H., Chong A.S., Collier J.H. Intranasal delivery of adjuvant-free peptide nanofibers elicits resident CD8(+) T cell responses. J Control Release. 2018;282:120–130. doi: 10.1016/j.jconrel.2018.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Si Y., Tian Q., Zhao F., Kelly S.H., Shores L.S., Camacho D.F., et al. Adjuvant-free nanofiber vaccine induces in situ lung dendritic cell activation and TH17 responses. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen J., Pompano R.R., Santiago F.W., Maillat L., Sciammas R., Sun T., et al. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials. 2013;34:8776–8785. doi: 10.1016/j.biomaterials.2013.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rudra J.S., Tian Y.F., Jung J.P., Collier J.H. A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci U S A. 2010;107:622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pompano R.R., Chen J., Verbus E.A., Han H., Fridman A., McNeely T., et al. Titrating T-cell epitopes within self-assembled vaccines optimizes CD4+ helper T cell and antibody outputs. Adv Healthc Mater. 2014;3:1898–1908. doi: 10.1002/adhm.201400137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qiu Y.S., Man R.C.H., Liao Q.Y., Kung K.L.K., Chow M.Y.T., Lam J.K.W. Effective mRNA pulmonary delivery by dry powder formulation of PEGylated synthetic KL4 peptide. J Control Release. 2019;314:102–115. doi: 10.1016/j.jconrel.2019.10.026. [DOI] [PubMed] [Google Scholar]
- 98.Mai Y., Guo J., Zhao Y., Ma S., Hou Y., Yang J. Intranasal delivery of cationic liposome-protamine complex mRNA vaccine elicits effective anti-tumor immunity. Cell Immunol. 2020;354 doi: 10.1016/j.cellimm.2020.104143. [DOI] [PubMed] [Google Scholar]
- 99.Li M., Zhao M.N., Fu Y., Li Y., Gong T., Zhang Z.R., et al. Enhanced intranasal delivery of mRNA vaccine by overcoming the nasal epithelial barrier via intra- and paracellular pathways. J Control Release. 2016;228:9–19. doi: 10.1016/j.jconrel.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 100.Li M., Li Y., Peng K., Wang Y., Gong T., Zhang Z.R., et al. Engineering intranasal mRNA vaccines to enhance lymph node trafficking and immune responses. Acta Biomater. 2017;64:237–248. doi: 10.1016/j.actbio.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 101.Dhaliwal H.K., Fan Y.F., Kim J., Amiji M.M. Intranasal delivery and transfection of mRNA therapeutics in the brain using cationic liposomes. Mol Pharm. 2020;17:1996–2005. doi: 10.1021/acs.molpharmaceut.0c00170. [DOI] [PubMed] [Google Scholar]
- 102.Patel A.K., Kaczmarek J.C., Bose S., Kauffman K.J., Mir F., Heartlein M.W., et al. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv Mater. 2019;31 doi: 10.1002/adma.201805116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dong C., Wang Y., Gonzalez G.X., Ma Y., Song Y., Wang S., et al. Intranasal vaccination with influenza HA/GO-PEI nanoparticles provides immune protection against homo- and heterologous strains. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2024998118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qin T., Ma S., Miao X., Tang Y., Huangfu D., Wang J., et al. Mucosal vaccination for influenza protection enhanced by catalytic immune-adjuvant. Adv Sci. 2020;7 doi: 10.1002/advs.202000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lindsay K.E., Vanover D., Thoresen M., King H., Xiao P., Badial P., et al. Aerosol delivery of synthetic mRNA to vaginal mucosa leads to durable expression of broadly neutralizing antibodies against HIV. Mol Ther. 2020;28:805–819. doi: 10.1016/j.ymthe.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ensign L.M., Cone R., Hanes J. Nanoparticle-based drug delivery to the vagina: a review. J Control Release. 2014;190:500–514. doi: 10.1016/j.jconrel.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang X., Liu S., Guan Y., Ding J., Ma C., Xie Z. Vaginal drug delivery approaches for localized management of cervical cancer. Adv Drug Deliv Rev. 2021;174:114–126. doi: 10.1016/j.addr.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 108.Wang N., Zhen Y., Jin Y., Wang X., Li N., Jiang S., et al. Combining different types of multifunctional liposomes loaded with ammonium bicarbonate to fabricate microneedle arrays as a vaginal mucosal vaccine adjuvant-dual delivery system (VADDS) J Control Release. 2017;246:12–29. doi: 10.1016/j.jconrel.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 109.Ho H.M., Huang C.Y., Cheng Y.J., Chen I.H., Liu S.J., Huang C.H., et al. Squalene nanoemulsion reinforces mucosal and immunological fingerprints following intravaginal delivery. Biomed Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111799. [DOI] [PubMed] [Google Scholar]
- 110.Huang C.H., Huang C.Y., Ho H.M., Lee C.H., Lai P.T., Wu S.C., et al. Nanoemulsion adjuvantation strategy of tumor-associated antigen therapy rephrases mucosal and immunotherapeutic signatures following intranasal vaccination. J Immunother Cancer. 2020;8 doi: 10.1136/jitc-2020-001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li W.Z., Hao X.L., Zhao N., Han W.X., Zhai X.F., Zhao Q., et al. Propylene glycol-embodying deformable liposomes as a novel drug delivery carrier for vaginal fibrauretine delivery applications. J Control Release. 2016;226:107–114. doi: 10.1016/j.jconrel.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 112.Seah I., Agrawal R. Can the coronavirus disease 2019 (COVID-19) Affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28:391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xia J., Tong J., Liu M., Shen Y., Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92:589–594. doi: 10.1002/jmv.25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu C., Jiang X., Gan Y., Yu M. Engineering nanoparticles to overcome the mucus barrier for drug delivery: design, evaluation and state-of-the-art. Medicine in Drug Discovery. 2021;12 [Google Scholar]
- 115.Suk J.S., Xu Q., Kim N., Hanes J., Ensign L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pan Q., Xu Q., Boylan N.J., Lamb N.W., Emmert D.G., Yang J.C., et al. Corticosteroid-loaded biodegradable nanoparticles for prevention of corneal allograft rejection in rats. J Control Release. 2015;201:32–40. doi: 10.1016/j.jconrel.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu Q., Boylan N.J., Suk J.S., Wang Y.Y., Nance E.A., Yang J.C., et al. Nanoparticle diffusion in, and microrheology of, the bovine vitreous ex vivo. J Control Release. 2013;167:76–84. doi: 10.1016/j.jconrel.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Inic-Kanada A., Stojanovic M., Schlacher S., Stein E., Belij-Rammerstorfer S., Marinkovic E., et al. Delivery of a chlamydial adhesin N-PmpC subunit vaccine to the ocular mucosa using particulate carriers. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu K., Dou J., Yu F., He X., Yuan X., Wang Y., et al. An ocular mucosal administration of nanoparticles containing DNA vaccine pRSC-gD-IL-21 confers protection against mucosal challenge with herpes simplex virus type 1 in mice. Vaccine. 2011;29:1455–1462. doi: 10.1016/j.vaccine.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 120.Nesburn A.B., Burke R.L., Ghiasi H., Slanina S., Bahri S., Wechsler S.L. Vaccine therapy for ocular herpes simplex virus (HSV) infection: periocular vaccination reduces spontaneous ocular HSV type 1 shedding in latently infected rabbits. J Virol. 1994;68:5084–5092. doi: 10.1128/jvi.68.8.5084-5092.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Inoue T., Inoue Y., Hayashi K., Shimomura Y., Fujisawa Y., Aono A., et al. Effect of herpes simplex virus-1 gD or gD-IL-2 DNA vaccine on herpetic keratitis. Cornea. 2002;21:S79–S85. doi: 10.1097/01.ico.0000263124.91639.4e. [DOI] [PubMed] [Google Scholar]
- 122.Macklin G.R., O'Reilly K.M., Grassly N.C., Edmunds W.J., Mach O., Krishnan R.S.G., et al. Evolving epidemiology of poliovirus serotype 2 following withdrawal of the serotype 2 oral poliovirus vaccine. Science. 2020;368:401–405. doi: 10.1126/science.aba1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alfaro-Murillo J.A., Avila-Aguero M.L., Fitzpatrick M.C., Crystal C.J., Falleiros-Arlant L.H., Galvani A.P. The case for replacing live oral polio vaccine with inactivated vaccine in the Americas. Lancet. 2020;395:1163–1166. doi: 10.1016/S0140-6736(20)30213-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kang S.S., Yang J.S., Kim K.W., Yun C.H., Holmgren J., Czerkinsky C., et al. Anti-bacterial and anti-toxic immunity induced by a killed whole-cell-cholera toxin B subunit cholera vaccine is essential for protection against lethal bacterial infection in mouse pulmonary cholera model. Mucosal Immunol. 2013;6:826–837. doi: 10.1038/mi.2012.121. [DOI] [PubMed] [Google Scholar]
- 125.Carter N.J., Curran M.P. Live attenuated influenza vaccine (FluMist®; FluenzTM) a review of its use in the prevention of seasonal influenza in children and adults. Drugs. 2011;71:1591–1622. doi: 10.2165/11206860-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 126.Plosker G.L. Spotlight on pentavalent rotavirus vaccine (RotaTeq®) in the prevention of rotavirus gastroenteritis in Europe. BioDrugs. 2010;24:411–414. doi: 10.2165/11205070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 127.Nakagomi T., Nakagomi O. A critical review on a globally-licensed, live, orally-administrable, monovalent human rotavirus vaccine: Rotarix. Expet Opin Biol Ther. 2009;9:1073–1086. doi: 10.1517/14712590903103787. [DOI] [PubMed] [Google Scholar]
- 128.Baik Y.O., Choi S.K., Olveda R.M., Espos R.A., Ligsay A.D., Montellano M.B., et al. A randomized, non-inferiority trial comparing two bivalent killed, whole cell, oral cholera vaccines (Euvichol vs Shanchol) in the Philippines. Vaccine. 2015;33:6360–6365. doi: 10.1016/j.vaccine.2015.08.075. [DOI] [PubMed] [Google Scholar]
- 129.Levine M.M., Ferreccio C., Black R.E., Lagos R., Martin O.S., Blackwelder W.C. Ty21a live oral typhoid vaccine and prevention of paratyphoid fever caused by Salmonella enterica serovar paratyphi B. Clin Infect Dis. 2007;45:S24–S28. doi: 10.1086/518141. [DOI] [PubMed] [Google Scholar]
- 130.Choudhry A., Mathena J., Albano J.D., Yacovone M., Collins L. Safety evaluation of adenovirus type 4 and type 7 vaccine live, oral in military recruits. Vaccine. 2016;34:4558–4564. doi: 10.1016/j.vaccine.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 131.Saluja T., Mogasale V.V., Excler J.L., Kim J.H., Mogasale V. An overview of VaxchoraTM, a live attenuated oral cholera vaccine. Hum Vaccin Immunother. 2020;16:42–50. doi: 10.1080/21645515.2019.1644882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang T., Zhen Y., Ma X., Wei B., Li S., Wang N. Mannosylated and lipid A-incorporating cationic liposomes constituting microneedle arrays as an effective oral mucosal HBV vaccine applicable in the controlled temperature chain. Colloids Surf B. 2015;126:520–530. doi: 10.1016/j.colsurfb.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 133.Saeed M.I., Omar A.R., Hussein M.Z., Elkhidir I.M., Sekawi Z. Development of enhanced antibody response toward dual delivery of nano-adjuvant adsorbed human Enterovirus-71 vaccine encapsulated carrier. Hum Vaccin Immunother. 2015;11:2414–2424. doi: 10.1080/21645515.2015.1052918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Uddin A.N., Bejugam N.K., Gayakwad S.G., Akther P., D'Souza M.J. Oral delivery of gastro-resistant microencapsulated typhoid vaccine. J Drug Target. 2009;17:553–560. doi: 10.1080/10611860903067301. [DOI] [PubMed] [Google Scholar]
- 135.Atwe S.U., Ma Y., Gill H.S. Pollen grains for oral vaccination. J Control Release. 2014;194:45–52. doi: 10.1016/j.jconrel.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Uddin M.J., Gill H.S. From allergen to oral vaccine carrier: a new face of ragweed pollen. Int J Pharm. 2018;545:286–294. doi: 10.1016/j.ijpharm.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Salman H.H., Irache J.M., Gamazo C. Immunoadjuvant capacity of flagellin and mannosamine-coated poly(anhydride) nanoparticles in oral vaccination. Vaccine. 2009;27:4784–4790. doi: 10.1016/j.vaccine.2009.05.091. [DOI] [PubMed] [Google Scholar]
- 138.Wang T., Jiang H., Zhao Q., Wang S., Zou M., Cheng G. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: effect of silica architecture on immunological properties. Int J Pharm. 2012;436:351–358. doi: 10.1016/j.ijpharm.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 139.Zhang L., Zeng Z., Hu C., Bellis S.L., Yang W., Su Y., et al. Controlled and targeted release of antigens by intelligent shell for improving applicability of oral vaccines. Biomaterials. 2016;77:307–319. doi: 10.1016/j.biomaterials.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 140.Bian X., Xiao Y.T., Wu T., Yao M., Du L., Ren S., et al. Microvesicles and chemokines in tumor microenvironment: mediators of intercellular communications in tumor progression. Mol Cancer. 2019;18:50. doi: 10.1186/s12943-019-0973-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ghaffari Marandi B.H., Zolfaghari M.R., Kazemi R., Motamedi M.J., Amani J. Immunization against Vibrio cholerae, ETEC, and EHEC with chitosan nanoparticle containing LSC chimeric protein. Microb Pathog. 2019;134 doi: 10.1016/j.micpath.2019.103600. [DOI] [PubMed] [Google Scholar]
- 142.He X.W., Wang F., Jiang L., Li J., Liu S.K., Xiao Z.Y., et al. Induction of mucosal and systemic immune response by single-dose oral immunization with biodegradable microparticles containing DNA encoding HBsAg. J Gen Virol. 2005;86:601–610. doi: 10.1099/vir.0.80575-0. [DOI] [PubMed] [Google Scholar]
- 143.Kaneko H., Bednarek I., Wierzbicki A., Kiszka I., Dmochowski M., Wasik T.J., et al. Oral DNA vaccination promotes mucosal and systemic immune responses to HIV envelope glycoprotein. Virology. 2000;267:8–16. doi: 10.1006/viro.1999.0093. [DOI] [PubMed] [Google Scholar]
- 144.Wang X., Yang D., Li S., Xu X., Qin C.F., Tang R. Biomineralized vaccine nanohybrid for needle-free intranasal immunization. Biomaterials. 2016;106:286–294. doi: 10.1016/j.biomaterials.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 145.Bernocchi B., Carpentier R., Lantier I., Ducournau C., Dimier-Poisson I., Betbeder D. Mechanisms allowing protein delivery in nasal mucosa using NPL nanoparticles. J Control Release. 2016;232:42–50. doi: 10.1016/j.jconrel.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ascough S., Vlachantoni I., Kalyan M., Haijema B.J., Wallin-Weber S., Dijkstra-Tiekstra M., et al. Local and systemic immunity against respiratory syncytial virus induced by a novel intranasal vaccine a randomized, double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med. 2019;200:481–492. doi: 10.1164/rccm.201810-1921OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Slutter B., Jiskoot W. Dual role of CpG as immune modulator and physical crosslinker in ovalbumin loaded N-trimethyl chitosan (TMC) nanoparticles for nasal vaccination. J Control Release. 2010;148:117–121. doi: 10.1016/j.jconrel.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 148.Matsuo K., Koizumi H., Akashi M., Nakagawa S., Fujita T., Yamamoto A., et al. Intranasal immunization with poly(gamma-glutamic acid) nanoparticles entrapping antigenic proteins can induce potent tumor immunity. J Control Release. 2011;152:310–316. doi: 10.1016/j.jconrel.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 149.Li Y., Li M., Gong T., Zhang Z., Sun X. Antigen-loaded polymeric hybrid micelles elicit strong mucosal and systemic immune responses after intranasal administration. J Control Release. 2017;262:151–158. doi: 10.1016/j.jconrel.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 150.Quinn K., Quirion M.R., Lo C.Y., Misplon J.A., Epstein S.L., Chiorini J.A. Intranasal administration of adeno-associated virus type 12 (AAV12) leads to transduction of the nasal epithelia and can initiate transgene-specific immune response. Mol Ther. 2011;19:1990–1998. doi: 10.1038/mt.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang Y., Chen L., Sun H.W., Guo H., Song Z., You Y., et al. Epitope-loaded nanoemulsion delivery system with ability of extending antigen release elicits potent Th1 response for intranasal vaccine against Helicobacter pylori. J Nanobiotechnol. 2019;17:6. doi: 10.1186/s12951-019-0441-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dimier-Poisson I., Carpentier R., N'Guyen T.T., Dahmani F., Ducournau C., Betbeder D. Porous nanoparticles as delivery system of complex antigens for an effective vaccine against acute and chronic Toxoplasma gondii infection. Biomaterials. 2015;50:164–175. doi: 10.1016/j.biomaterials.2015.01.056. [DOI] [PubMed] [Google Scholar]
- 153.Nochi T., Yuki Y., Takahashi H., Sawada S., Mejima M., Kohda T., et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat Mater. 2010;9:572–578. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- 154.Sinani G., Sessevmez M., Koray Gok M., Ozgumus S., Okyar A., Oya Alpar H., et al. Nasal vaccination with poly(beta-amino ester)-poly(d,l-lactide-co-glycolide) hybrid nanoparticles. Int J Pharm. 2017;529:1–14. doi: 10.1016/j.ijpharm.2017.06.053. [DOI] [PubMed] [Google Scholar]
- 155.Pastor Y., Ting I., Martinez A.L., Irache J.M., Gamazo C. Intranasal delivery system of bacterial antigen using thermosensitive hydrogels based on a Pluronic‒Gantrez conjugate. Int J Pharm. 2020;579 doi: 10.1016/j.ijpharm.2020.119154. [DOI] [PubMed] [Google Scholar]
- 156.Wu Y., Wei W., Zhou M., Wang Y., Wu J., Ma G., et al. Thermal-sensitive hydrogel as adjuvant-free vaccine delivery system for H5N1 intranasal immunization. Biomaterials. 2012;33:2351–2360. doi: 10.1016/j.biomaterials.2011.11.068. [DOI] [PubMed] [Google Scholar]
- 157.Zeng Z., Dai S., Jiao Y., Jiang L., Zhao Y., Wang B., et al. Mannosylated protamine as a novel DNA vaccine carrier for effective induction of anti-tumor immune responses. Int J Pharm. 2016;506:394–406. doi: 10.1016/j.ijpharm.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 158.Kawamura M., Naito T., Ueno M., Akagi T., Hiraishi K., Takai I., et al. Induction of mucosal IgA following intravaginal administration of inactivated HIV-1-capturing nanospheres in mice. J Med Virol. 2002;66:291–298. doi: 10.1002/jmv.2144. [DOI] [PubMed] [Google Scholar]
- 159.He Q., Mitchell A., Morcol T., Bell S.J. Calcium phosphate nanoparticles induce mucosal immunity and protection against herpes simplex virus type 2. Clin Diagn Lab Immunol. 2002;9:1021–1024. doi: 10.1128/CDLI.9.5.1021-1024.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cranage M.P., Fraser C.A., Stevens Z., Huting J., Chang M., Jeffs S.A., et al. Repeated vaginal administration of trimeric HIV-1 clade C gp140 induces serum and mucosal antibody responses. Mucosal Immunol. 2010;3:57–68. doi: 10.1038/mi.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lewis D.J., Fraser C.A., Mahmoud A.N., Wiggins R.C., Woodrow M., Cope A., et al. Phase I randomised clinical trial of an HIV-1(CN54), clade C, trimeric envelope vaccine candidate delivered vaginally. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cranage M.P., Fraser C.A., Cope A., McKay P.F., Seaman M.S., Cole T., et al. Antibody responses after intravaginal immunisation with trimeric HIV-1 CN54 clade C gp140 in Carbopol gel are augmented by systemic priming or boosting with an adjuvanted formulation. Vaccine. 2011;29:1421–1430. doi: 10.1016/j.vaccine.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Curran R.M., Donnelly L., Morrow R.J., Fraser C., Andrews G., Cranage M., et al. Vaginal delivery of the recombinant HIV-1 clade-C trimeric gp140 envelope protein CN54gp140 within novel rheologically structured vehicles elicits specific immune responses. Vaccine. 2009;27:6791–6798. doi: 10.1016/j.vaccine.2009.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Park J.S., Oh Y.K., Kang M.J., Kim C.K. Enhanced mucosal and systemic immune responses following intravaginal immunization with human papillomavirus 16 L1 virus-like particle vaccine in thermosensitive mucoadhesive delivery systems. J Med Virol. 2003;70:633–641. doi: 10.1002/jmv.10442. [DOI] [PubMed] [Google Scholar]
- 165.Oh Y. Enhanced mucosal and systemic immune responses to a vaginal vaccine coadministered with RANTES-expressing plasmid DNA using in situ-gelling mucoadhesive delivery system. Vaccine. 2003;21:1980–1988. doi: 10.1016/s0264-410x(02)00779-x. [DOI] [PubMed] [Google Scholar]
- 166.Gupta P.N., Pattani A., Curran R.M., Kett V.L., Andrews G.P., Morrow R.J., et al. Development of liposome gel based formulations for intravaginal delivery of the recombinant HIV-1 envelope protein CN54gp140. Eur J Pharm Sci. 2012;46:315–322. doi: 10.1016/j.ejps.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 167.Weaver E.A., Nehete P.N., Nehete B.P., Yang G., Buchl S.J., Hanley P.W., et al. Comparison of systemic and mucosal immunization with helper-dependent adenoviruses for vaccination against mucosal challenge with SHIV. PLoS One. 2013;8 doi: 10.1371/journal.pone.0067574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Garulli B., Di Mario G., Stillitano M.G., Kawaoka Y., Castrucci M.R. Exploring mucosal immunization with a recombinant influenza virus carrying an HIV-polyepitope in mice with pre-existing immunity to influenza. Vaccine. 2014;32:2501–2506. doi: 10.1016/j.vaccine.2014.02.077. [DOI] [PubMed] [Google Scholar]
- 169.Li Z., Zhang M., Zhou C., Zhao X., Iijima N., Frankel F.R. Novel vaccination protocol with two live mucosal vectors elicits strong cell-mediated immunity in the vagina and protects against vaginal virus challenge. J Immunol. 2008;180:2504–2513. doi: 10.4049/jimmunol.180.4.2504. [DOI] [PubMed] [Google Scholar]
- 170.Gordon S.N., Kines R.C., Kutsyna G., Ma Z.M., Hryniewicz A., Roberts J.N., et al. Targeting the vaginal mucosa with human papillomavirus pseudovirion vaccines delivering simian immunodeficiency virus DNA. J Immunol. 2012;188:714–723. doi: 10.4049/jimmunol.1101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tan H.X., Wheatley A.K., Esterbauer R., Jegaskanda S., Glass J.J., Masopust D., et al. Induction of vaginal-resident HIV-specific CD8 T cells with mucosal prime-boost immunization. Mucosal Immunol. 2018;11:994–1007. doi: 10.1038/mi.2017.89. [DOI] [PubMed] [Google Scholar]
- 172.Vajdy M., Gardner J., Neidleman J., Cuadra L., Greer C., Perri S., et al. Human immunodeficiency virus type 1 gag-specific vaginal immunity and protection after local immunizations with sindbis virus-based replicon particles. J Infect Dis. 2001;184:1613–1616. doi: 10.1086/324581. [DOI] [PubMed] [Google Scholar]
- 173.Gupta S., Janani R., Bin Q., Luciw P., Greer C., Perri S., et al. Characterization of human immunodeficiency virus Gag-specific gamma interferon-expressing cells following protective mucosal immunization with alphavirus replicon particles. J Virol. 2005;79:7135–7145. doi: 10.1128/JVI.79.11.7135-7145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]