Abstract
Considering the high impact that severe Coronavirus disease 2019 (COVID-19) cases still pose on public health and their complex pharmacological management, the search for new therapeutic alternatives is essential. Mesenchymal stromal cells (MSCs) could be promising candidates as they present important immunomodulatory and anti-inflammatory properties that can combat the acute severe respiratory distress syndrome (ARDS) and the cytokine storm occurring in COVID-19, two processes that are mainly driven by an immunological misbalance. In this review, we provide a comprehensive overview of the intricate inflammatory process derived from the immune dysregulation that occurs in COVID-19, discussing the potential that the cytokines and growth factors that constitute the MSC-derived secretome present to treat the disease. Moreover, we revise the latest clinical progress made in the field, discussing the most important findings of the clinical trials conducted to date, which follow 2 different approaches: MSC-based cell therapy or the administration of the secretome by itself, as a cell-free therapy.
Abbreviations: COVID-19, Coronavirus Disease of 2019; MSCs, mesenchymal stromal cells; ARDS, acute respiratory distress syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; NIH, national institutes of health; EVs, extracellular vesicles; FDA, food and drug administration; IL-1β, interleukin-1β; IL-2, interleukin-2; IL-10, interleukin-10; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α; IP-10, inducible protein 10; GM-CSF, granulocyte macrophage-colony stimulating factor; MCP-1, monocyte chemoattractant protein-1; CRP, C-reactive protein; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; IL-6, interleukin-6; IL-1, interleukin-1; TNF, tumor necrosis factor; ACE2, angiotensin converting enzyme 2; S, protein spike; AT2, type 2 alveolar cells of the lungs; FGF, fibroblast growth factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor; NK, natural; DCs, dendritic cells; BALF, bronchoalveolar lavage fluid; IL-7, interleukin 7; G-CSF, granulocyte colony-stimulating factor; NETs, Neutrophil Extracellular Traps; RF, respiratory failure; ROS, reactive oxygen species; CD, cluster of differentiation; TLR, toll-like receptors; LPS, lipopolysaccharide; IDO, indoleamine 2,3-dioxygenase; NO, nitric oxide; STAT3, signal transducer activators of transcription-3; IL1Ra, Interleukin-1 receptor antagonist; TSG-6, TNF-α-stimulating gene 6; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-kappa B; IL1-α, interleukin 1-α; IL1-β, interleukin 1-β; IL-7, interleukin-7; IL-8, interleukin 8; IL-9, interleukin; miRNA, microRNA; PGE2, prostaglandin E2; TGF-β1, transforming growth factor-β1; HGF, hepatocyte growth factor; KGF, keratinocyte growth factor; Fas-L, Fas ligands; PD-1, programmed cell death protein 1; PD-L1, programmed death ligand-1; cAMP, cyclic adenosine monophosphate; IL-2R, IL-2 receptor; FOXP3 +, forkhead box P3 +; Treg, regulatory T cell; Gal-1, galectin-1; Gal-3, galectin-3; Gal-9, galectin-9; HLA, human leukocyte antigen; MHC, major histocompatibility complex; IL-4, interleukin-4; GvHD, acute graft-versus-host disease; UC-MSCs, umbilical cord derived MSCs; ICU, intensive care unit; PLX-PAD, placental expanded; MIS-C, multisystem inflammatory syndrome in children; BM-MSCs, bone marrow derived MSCs; AT-MSCs, Adipose Tissue derived Mesenchymal Stromal Cells; Allo-MSCs, Allogeneic Mesenchymal Stromal Cells; WJ-MSCs, Wharton’s Jelly–derived Mesenchymal Stromal Cells; HB-AT-MSCs, Hope Biosciences autologous adipose-derived mesenchymal stromal cells; DW-MSCs, Daewoong Pharmaceutical’s Mesenchymal Stromal Cells; DSCs, Placenta-derived decidua stromal cells; PL-MSCs, Placental-derived Mesenchymal Stromal Cells, MB-MSCs, Menstrual blood-derived Mesenchymal Stromal Cells; IV, intravenous; Ld, Low dose; Hd, High dose; IMV, Invasive mechanical ventilation; adm, administration; bw, Body weight; Allo, allogeneic; INH, inhalation; NVs, nanovesicles
Keywords: Mesenchymal stromal cells, COVID-19, SARS-CoV-2, Cytokine storm, Immunomodulation, Secretome
1. Introduction
As of June 2022, there have been over 526 million confirmed cases of Coronavirus disease 2019 (COVID-19) worldwide, with more than 6,2 million deaths [1]. Fortunately, the development of effective prevention measures is limiting the effects of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, slowly changing the course of the pandemic. The first line of prevention is vaccination. Vaccines are having a significant effect on case numbers and hospitalizations [1]. For those patients who are not eligible to vaccination – immunocompromised individuals who might have an inadequate immune response to COVID-19 vaccination, or patients that cannot be fully vaccinated due to a history of severe adverse reaction to a COVID-19 vaccine or any of its components – pre-exposure prophylaxis drugs can be used. These consists of Anti-SARS-CoV-2 monoclonal antibodies such as tixagevimab or cilgavimab [2]. In this line, other option is post-exposure prophylaxis, in which recently exposed people take a short course of medication to prevent infection. It is also based in monoclonal antibodies such as the combinations of bamlanivimab plus etesevimab and casirivimab plus imdevimab. However, currently, organisms such as the National Institutes of Health (NIH) recommend against the use of such post-exposure prophylaxis, since the current predominant variant Omicron and its subvariants are not susceptible to these agents [2].
These prevention measures – especially vaccines – have significantly reduced the severity of the disease in high-income countries. However, uncertainties exist regarding the duration of the protection and their efficacy towards emerging SARS-CoV-2 variants. Moreover, the limited global access to vaccines, together with the individuals that are not eligible to them, leads to the vulnerability of a great part of the population [1], [3]. This vast group of individuals still presents a high risk of suffering from critical COVID-19.
To date, multiple therapeutic options are available for the management of the disease. To stablish the treatment line, it is important to consider that there are two main processes that drive the pathogenesis of COVID-19. The early stage is characterized by the replication of SARS-CoV-2, whereas later in the clinical course, the disease seems to be driven by a dysregulated immune / inflammatory response to the virus, which results in tissue damage. Therefore, whereas therapies targeting SARS-CoV-2 would have the greatest effect in the early COVID-19, immunosuppressive / anti-inflammatory therapies come in handy in the later stages of the disease [2].
Regarding nonhospitalized adults with mild to moderate COVID-19 who are at high risk of disease progression, the preferred therapies are the antivirals ritonavir-boosted nirmatrelvir (paxlovid) and remdesivir. Alternatively, bebtelovimab and molnupiravir are recommended. In the case of patients who have been discharged from hospital but still are in need for supplemental oxygen, the use of dexamethasone is indicated, and its combination with remdesivir can be considered [2]. The clinical management of hospitalized adult patients will depend on the severity of the disease and the oxygen requirements. For individuals that do not require supplemental oxygen but who are at risk of disease progression, the use of remdesivir is recommended. When oxygen supply is needed, options include the use of remdesivir, dexamethasone or their combination. For patients with rapidly increasing oxygen needs and systemic inflammation, the addition of drugs such as tozilizumab, baricitinib or sarilumab can be considered [2].
However, it has to be considered that – especially in the most severe cases – the efficacy of these treatments might be limited. Moreover, many of these drugs present weak safety profiles with important side effects. Therefore, the search for new therapeutic alternatives is still essential. This fact is supported by the high number of pharmaceutical companies and laboratories conducting clinical trials to test drugs – both, new compounds and drugs already approved for other applications – for the treatment of COVID-19.
As above mentioned, patients suffering from severe COVID-19, present a dysregulated immune response with the subsequent inflammatory status. In this line, mesenchymal stromal cells (MSCs) emerge as a promising new avenue for the clinical management of COVID-19 [4], [5]. MSCs are non-hematopoietic precursor cells present in all mammalian supportive stromal tissues that present a regenerative and immunomodulatory function, which is mainly driven by the great range of bioactive factors they release – directly or by means of extracellular vesicles (EVs) –. These factors are able to regulate the exacerbated immune responses, limiting inflammation and promoting tissue regeneration [4], [6]. Recently, the United States Food and Drug Administration (FDA) authorized the compassionate use of MSC in the most severe cases of COVID-19, which present a poor prognosis [5]. In this review, the potential beneficial effects that MSCs could present to fight the inflammatory processes occurring in the most severe COVID-19 cases will be analyzed in depth. Moreover, the latest clinical progress made in the field will be revised, discussing the most important findings of the clinical trials conducted to date with MSCs or their derived products.
2. COVID-19: clinics and underlying mechanisms
Attending to its severity, COVID-19 cases can be classified as non-severe – including asymptomatic and mild affections –, moderate, severe or critical. The mildest cases are characterized by symptoms including fever, cough, sore throat, malaise, headache, muscle pain, nausea, vomiting, diarrhea, anosmia and ageusia. In moderate cases, patients suffer from shortness of breath, dyspnea and abnormal chest imaging. These symptoms are aggravated in severe COVID-19 cases, which are defined by oxygen saturation < 90%, signs of pneumonia and signs of severe respiratory distress. Finally, critical COVID-19 is characterized by acute respiratory distress syndrome (ARDS), which is clinically defined by the acute onset of hypoxemic respiratory failure (RF) that cannot be explained by the presence of heart failure or fluid overload. In the patient, ARDS leads to pulmonary edema, arterial hypoxia and dysfunction in the air exchange function [7]. Additional criteria that defines the critical cases are sepsis, septic shock and conditions that require mechanical ventilation or vasopressor therapy [2]. Critical patients can express increased levels of cytokines, which is known as a cytokine storm. In this regard, multiple studies have shown that COVID-19 patients present increased levels of interleukin-1β (IL-1β), interleukin-2 (IL-2), interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), inducible protein 10 (IP-10), granulocyte macrophage-colony stimulating factor (GM-CSF) or monocyte chemoattractant protein-1 (MCP-1). The levels of these cytokines are correlated with the severity of the disease [8], [9], [10]. In addition, it should be noted that the presence of the virus in the lungs also increases the risk of secondary infections [11].
For diagnosis and severity evaluation, the most widely performed clinical tests are imaging techniques and the assessment of different biomarkers. Regarding the former, chest X rays and computed tomography are frequently employed to determine the presence of abnormalities, being the most common of them bilateral multifocal opacities [12]. Common laboratory biomarkers are also valuable to determine the clinical course of COVID-19. The systematic inflammatory response observed in COVID-19, together with the use of hepatotoxic drugs can increase the serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), which indicate an abnormal liver function and hepatocyte damage [13]. The production of C-reactive protein (CRP) – synthesized by hepatocytes in response to infection or tissue inflammation – is stimulated by interleukin-6 (IL-6), interleukin-1 (IL-1) and TNF [14]. Therefore, the elevation of these cytokines in COVID-19 patients increase the CRP levels, making it one of the most effective and sensitive biomarkers to predict the disease severity and progression [15], [16]. The presence of increased D-dimer has been also associated with poor prognosis in COVID-19 patients during hospitalization. Indeed, several metanalyses showed that high levels of D-dimer have a dreadful impact on the mortality [17], [18]. Similarly, in response to infection of SARS-CoV-2, IL-1β, IL-6 and IFN-γ stimulate the production of ferritin [19]. Elevated ferritin levels are therefore indicators of severity of the disease [20], [21], [22]. Moreover, high levels of lactate dehydrogenase (LDH) have been associated to severe COVID-19 cases and it can be employed as a negative predictor of survival [23], [24]. In this line, an increase in troponin indicates cardiac injury in COVID-19 patients and has been associated to the risk of mortality [25].
2.1. The SARS-CoV-2 derived inflammatory process
To initiate the host cell infection process SARS-CoV-2 binds to the cell surface angiotensin converting enzyme 2 (ACE2) receptor through protein spike (S) [26]. The ACE2 receptor is expressed primarily in the lower respiratory tract, mainly in type 2 alveolar cells of the lungs (AT2). Therefore, the respiratory system is the most affected in the disease. Despite in a lower extent, multiple other systems can also be affected by SARS-CoV-2, since ACE2 receptors – which under physiological conditions contribute to the regulation of blood pressure – are also expressed in the heart, kidneys, stomach, bladder, esophagus, intestine and oral cavity [27], [28].
As above mentioned, the respiratory system is the most affected by SARS-CoV-2. Lung tissue inflammation and functional injury are characteristic in severe and critical COVID-19 patients. It is also important to mention that as a result of such an intense inflammatory reaction, pulmonary fibrosis appears frequently, with the subsequent increase of factors such as fibroblast growth factor (FGF) and transforming growth factor β1 (TGF-β1) [29], [30], [31], [32].
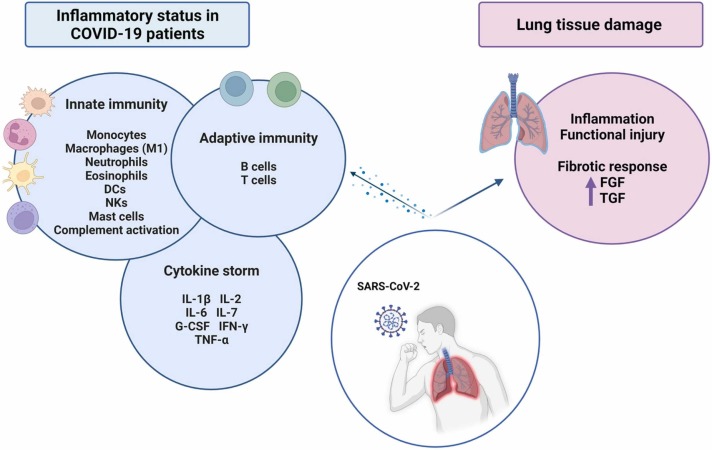
The inflammatory process occurring in COVID-19 patients is driven by the immune system activation as a response to the viral infection ( Fig. 1) [33]. As the first line of defense, innate immune cells including monocytes, macrophages, neutrophils, eosinophils, dendritic cells (DCs), natural killer cells (NKs) and mast cells increase their numbers with the aim to limit the viral replication and activate the adaptive immunity [34]. High levels of neutrophils, activated mast cells, NKs and proinflammatory M1 macrophages in the bronchoalveolar lavage fluid (BALF) of COVID-19 patients have been associated to the recruitment of other immune cells and correlate to the disease severity [35], [36], [37], [38], [39], [40]. In these patients, it has also been described that neutrophil activation can lead to the secretion of a DNA complex, forming the so-called Neutrophil Extracellular Traps (NETs). Those NETs can trap different pathogens including fungi, bacteria and viruses, thus protecting the host from the infection [41], [42]. The activation of immune cells such as macrophages triggers the release of pro-inflammatory cytokines – process known as cytokine storm – which importantly contributes to the establishment of the inflammatory state. Among them, as IL-1β, IL-2, IL-6, interleukin 7 (IL-7), granulocyte colony-stimulating factor (G-CSF), IFN-γ or TNF-α are remarkable. Importantly, these cytokines reach the bloodstream causing damage to multiple organs [43]. Overall, this exacerbated immune/inflammatory response increases the production of reactive oxygen species (ROS). ROS disrupt redox homeostasis and provoke oxidative stress and inflammation, damaging lung tissue and leading to ARDS [44]. Moreover, an intense activation of the complement system has also been observed in severe cases of the disease [45].
Fig. 1.
Inflammatory status and pathogenesis of severe COVID-19. SARS-Cov-2 infection can evoke an immune response overactivation, with the subsequent inflammatory status, in which diverse cytokines and growth factors play an important role. List of abbreviations: SARS-CoV-2 – Severe Acute Respiratory Syndrome Coronavirus 2; FGF – Fibroblast Growth Factor; TFG – Transforming Growth Factor; DCs – Dendritic Cells; NKs – Natural Killer Cells; IL-1β – Interleukin-1β; IL-2 – Interleukin-2; IL-6 – Interleukin-6; IL-7 – Interleukin-7; G-CSF – Granulocyte Colony-Stimulating Factor, IFN-γ – Interferon-γ; TNF-α – Tumor Necrosis Factor-α.
The innate response activates the adaptive immune system, which is essential for regulating and clearing the viral infection. B cells, CD4+ and CD8+ T cells of adaptive immune system in COVID-19 patients contribute to the inflammatory process and pathogenesis of SARS-CoV-2 infection. CD8+ T cells attack and kill virus-infected cells and CD4+ T cells are responsible for cytokine production to promote immune cell recruitment, thus reinforcing the inflammatory loop. Autopsies of COVID-19 patients reveal an accumulation of monocytes and T cells in the lungs, accompanied by low levels of hyperactive T cells in the peripheral blood [46]. These observations suggest that T cells are attracted away from the blood into the most damaged sites to control the infection.
3. MSCs: towards reducing the immune over-activation and inflammatory status
As above mentioned, MSCs are able to regulate innate and adaptive immune responses. This immunomodulatory capacity makes them excellent candidates for the treatment of not only COVID-19, but many other immune-mediated inflammatory diseases. To understand the therapeutic mechanism of these cells, it is necessary to mention that MSCs change from a pro-inflammatory to an anti-inflammatory phenotype depending on their local microenvironment. This phenotype change is mediated by the activation of toll-like receptors (TLR) expressed on the surface of MSCs, which recognize cytokines present in the surrounding medium [44]. MSCs mainly express TLR3 and TLR4; however, the degree of expression of these receptors depends on the source of MSCs [47]. Inflammatory cytokines such as IFN-γ and TNF-α activate TLR3, which induces a shift towards an anti-inflammatory phenotype – also known as MSC2 phenotype –. On the contrary, some factors such as lipopolysaccharide (LPS) activate TLR4 and induce a proinflammatory – or MSC1 – phenotype [44].
The immunomodulatory activity of MSCs is mediated by different mechanisms ( Fig. 2). Among them, their paracrine function is considered to be the major responsible for this effect [48]. MSCs are able to release a plethora of bioactive molecules – including interleukins, enzymes and growth factors – that can be directly released (soluble factors) or secreted through EVs. These factors and EVs compose the so-called MSC-derived secretome. It is interesting to note that as well as carrying immunomodulatory factors, EVs can induce immunomodulation by other mechanisms. In this regard, apoptotic bodies – a type of EVs ranging from 50 to 4000 nm and formed by apoptotic MSC membrane blebbing – have recently been considered to play a role in immunoregulation. Upon apoptotic body efferocytosis – process by which macrophages engulf the particles – the M2 anti-inflammatory phenotype of macrophages is activated [49]. Thus, efferocytosis has been related to an increased production of immunomodulatory factors such as IL-10 and indoleamine 2,3-dioxygenase (IDO) by macrophages, and to a reduction of their secretion of TNF-α and nitric oxide (NO) [6], [49].
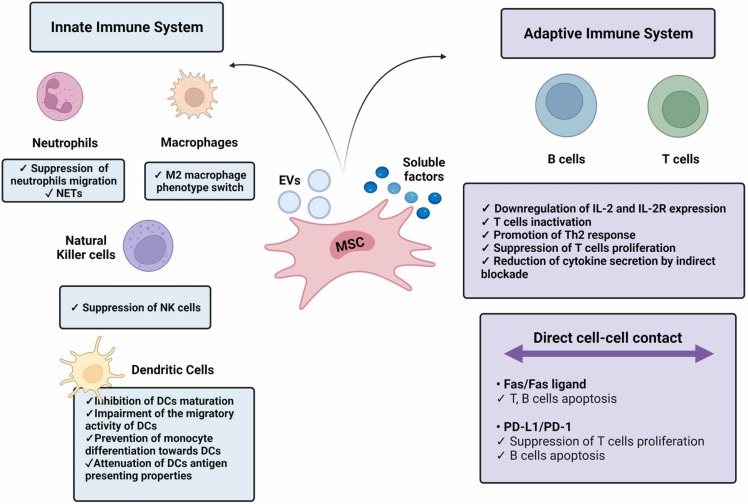
Fig. 2.
Therapeutic activity of mesenchymal stromal cells (MSCs). The MSC-mediated immunomodulation is mainly driven by direct cell-cell contacts as well as by soluble factors and extracellular vesicles (EVs) they release. List of abbreviations: NETs – Neutrophil Extracellular Traps; NK – Natural Killer; DCs – Dendritic Cells; IL-2 – Interleukin 2; IL-2R – Interleukin-2 receptor; PD-1 – Programmed cell death protein 1; PD-L1 – Programmed Death Ligand-1.
Another mechanism for MSC-immunomodulation is the direct cell-cell contact. MSCs present different surface ligands that can interact with immune cells, modulating their response [50].
3.1. Immunomodulatory effects of MSCs on the innate immune system
MSCs regulate cells within the innate immune system, which is key for alleviating the inflammatory process that occurs in severe and critical COVID-19 patients. Importantly, different factors released by MSCs regulate the response of macrophages. For instance, prostaglandin E2 (PGE2) induces a M2 anti-inflammatory macrophage phenotype switch due to the activation of signal transducer activators of transcription-3 (STAT3) [51]. STAT3 also promotes IL-10 production, an anti-inflammatory cytokine that favors immunoregulation [52]. Likewise, IDO is another soluble factor released by MSCs. This enzyme is responsible for the metabolism of tryptophan and contributes to the polarization of macrophages towards an M2 phenotype [53]. Interleukin-1 receptor antagonist (IL-1Ra) produced by MSCs inhibits IL-1 binding to its receptor. Therefore, it blocks the inflammatory effects of this cytokine – present in the cytokine storm evoked in COVID-19 –, including the promotion of the inflammatory M1 macrophage phenotype [54]. Lastly, TNF-α-stimulating gene 6 (TSG-6) produced by MSCs also limits the macrophage secretion of factors involved in the SARS-CoV-2-derived cytokine storm, such as TNF-α, while also promotes the macrophage anti-inflammatory M2 phenotype [55]. TSG-6 is also capable of inhibiting neutrophil migration [56].
Additionally, MSCs secrete different factors that control the response of DCs. TSG-6 suppresses DCs maturation by the inactivation of mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB). In this regard, IL1-Ra secreted by MSCs has been proven to attenuate the antigen presenting properties of DCs [57]. Furthermore, MSC-derived EVs can contain specific microRNA (miRNA) that also contribute to the inhibition of DCs maturation [52]. Human leukocyte antigen-G (HLA-G), a non-classical major histocompatibility complex (MHC) class I molecule [58], which is expressed as 7 different isoforms, from which HLA-G1 to G4 are membrane bound and HLA-G5 to G7 are soluble forms. These molecules are able to prevent the differentiation of monocytes to DCs by blocking the secretion of cytokines such as IFN-γ, TNF-α, interleukin 1-α (IL1-α), interleukin 1-β (IL1-β), IL-6, IL-7, interleukin 8 (IL-8), interleukin (IL-9) or GM-CSF [59]. It has also been described that HLA-G can induce the production of tolerant DCs. These molecules can also interact with other immune cells including NK cells, by binding to their inhibitory receptors [60].
Other factors produced by MSCs that are involved in NK regulation include IDO and PGE2, which synergistically act with TGF-β1 and hepatocyte growth factor (HGF) to inhibit the secretion of the inflammatory cytokine IL-2, which plays a role in NK activation [52].
3.2. Immunomodulatory effects of MSCs on the adaptive immune system
MSC-mediated immunomodulation has also a direct impact on the cells of the adaptive immune system. As previously mentioned, this is a key feature, since adaptive cell overactivation in severe and critical COVID-19 patients promotes the release of multiple pro-inflammatory mediators that aggravate the ongoing cytokine storm. By means of direct cell-cell contacts, MSCs are able to modulate both, T and B cell responses. For instance, Fas ligands (Fas-L) present on MSCs bind to Fas receptors on T cells, leading to their apoptosis [61]. This activity occurs by the downstream activation of the Fas-associated domain and caspases [62], [63]. In another example, programmed death ligand-1 (PD-L1) present in MSCs bind to PD-1 receptors on T cells causing the suppression of their proliferation, as well as their cytotoxic degranulation [62]. Similarly, Fas-L and PDL-1 interactions with B cells promote their apoptosis [52].
Apart from direct cell-cell contacts, multiple factors in the MSC-derived secretome are able to regulate T and B cells. One of these factors is PGE2, which acts through several pathways of action [64]. PGE2 promotes the production of cyclic adenosine monophosphate (cAMP), which exerts a regulatory effect on T cells. cAMP downregulates IL-2 and its receptor (IL-2R) expression, which are involved in T cell activation. Moreover, PGE2 inhibits T cell responses by negatively regulating the phosphatidylinositol hydrolysis and the diacylglycerol and inositol phosphate production [61]. This prostaglandin is also involved in T cell polarization. Indeed, it promotes the CD4+, CD25+, forkhead box P3+ (FOXP3+) regulatory T cell (Treg) responses, thus leading to the suppression of hyperactivated T cells [65].
IDO is one of the main effectors in the immunomodulation mediated by MSCs since it reduces tryptophan levels and produces several toxic kynurenine metabolites [66]. The depletion of tryptophan causes inhibition of the proliferation of T cells, as well as their anergy, while the generated toxic metabolites exert a cytotoxic action on effector T cells and favor the differentiation of Tregs [67].
NO produced by MSCs also contributes to suppressing the T cell responses by inhibiting their proliferation and production of inflammatory cytokines [61], [67]. Another relevant factors that mediate MSC-driven immunomodulation are galectins. Galectin 1 (Gal-1) and galectin 3 (Gal-3) have been demonstrated to suppress the proliferation of T cells. Moreover, galectin 9 (Gal-9) is significantly induced by inflammatory stimuli and demonstrated anti-proliferative effects on T and B cells [68], [69]. Additionally, one important molecule involved in this process is HLA-G, which inhibits the proliferation of hyperactive T cells in the presence of IDO and interleukin 10 (IL-10) [52].
4. Key effects and advantages of MSCs for the COVID-19 treatment
As described in the previous section, MSCs are able to exert an immunomodulatory effect, regulating the immune misbalance responsible for the inflammatory process that occurs in the COVID-19 patients.
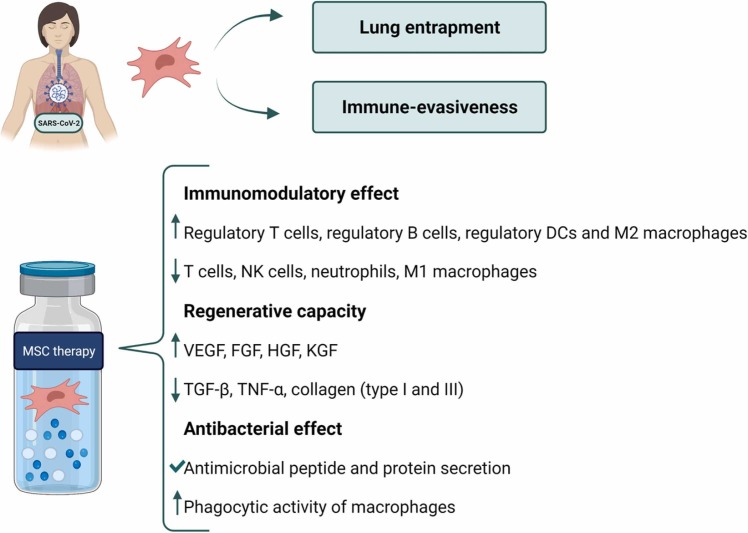
In addition to this immunomodulation, MSCs present other effects that can contribute to the resolution of COVID-19 ( Fig. 3). One of them is their regenerative capacity. The pathogenic inflammatory process results in an intense tissue damage, characterized by the compromised integrity of the lung alveolar capillary membrane. This contributes to pulmonary edema, lung tissue degeneration and fibrosis. This damage can be alleviated by the regenerative activity that MSCs present. These cells are able to release a plethora of regenerative factors in response to damage signals that promote tissue repair [52]. Among them, growth factors such as vascular endothelial growth factor (VEGF), FGF, HGF or keratinocyte growth factor (KGF) are remarkable [70]. Furthermore, MSCs are able to reduce the levels of pro-fibrotic factors in the lung microenvironment, which contributes to the prevention of pulmonary fibrosis. Interestingly, MSC-derived exosomes play a role in this effect, since they have been demonstrated to reduce the levels of TGF-β, TNF-α, type I collagen, type III collagen, hydroxyproline and serum ceruloplasmin in lung tissues. This leads to the reduction of endothelial cell apoptosis and myofibroblast growth, and to the promotion of alveolar epithelial cell regeneration. As well as having a regenerative effect on lung tissue, MSCs exert this effect in other damaged organs including kidneys and the intestine by through mucosal repair and epithelial regeneration [70].
Fig. 3.
Therapeutic potential of MSCs to combat COVID-19. List of abbreviations: MSC – Mesenchymal Stromal Cells; SARS-CoV-2 – Severe Acute Respiratory Syndrome Coronavirus 2; DCs – Dendritic Cells; NK – Natural Killer; VEGF – Vascular Endothelial Growth Factor; FGF – Fibroblast Growth Factor; HGF – Hepatocyte Growth Factor; KGF – Keratinocyte Growth Factor; TGF-β – Transforming Growth Factor-β; TNF-α – Tumor Necrosis Factor-α.
Furthermore, MSCs have antibacterial properties, which contribute to limit the risk of secondary infections that often occur in COVID-19 patients. They can mediate this effect through the secretion of antimicrobial peptides and proteins. Moreover, MSCs can also promote the elimination of bacteria by stimulating the phagocytic activity of macrophages [71], [72], [73].
In the particular case of COVID-19 treatment, the therapeutic activity of MSCs is potentiated by some key advantages that are inherent to the therapy (Fig. 3). One of them is the immune-evasiveness of these cells, which significantly enhances their permanence. MSCs lack MHC class II receptors unless they are strongly stimulated by inflammatory cytokines such as IFN-γ. In addition, they lack expression of costimulatory molecules such as CD40, CD80, CD83, CD86 and CD154 [74]. Moreover, after intravenous (IV) administration, the majority of MSCs get trapped in the microvasculature of the lung, which represents an important advantage in COVID-19 patients [75]. It has been observed that when accumulated in the lungs, MSC-derived secretion of immunomodulatory factors – including interleukin IDO, PGE2, IL-10, IL-4, TGF-α or NO – contributes to the resolution of inflammation [71], [72], [75].
5. Clinical progress in the use of MSCs for the treatment of COVID-19
Over the last decades, advances in preclinical studies with MSCs have led to a significant increase in the number of clinical trials performed with these cells for the treatment of multiple disorders. Clinical trials conducted so far have reported the safety of the approach [72], [76]. This has led to the pioneering approval of the first MSC-based cell therapies. To date, there are 10 MSC-based approved products over the globe for different applications. In particular, there are approved 2 for the treatment of graft-versus-host disease (GvHD), 2 for Crohn’s fistulas, 1 for subcutaneous tissues defects, 1 for amyotrophic lateral sclerosis, 1 for knee articular cartilage defects, 1 for spinal cord injury, 1 for critical limb ischemia and 1 for acute myocardial infarction [76], [77], [78], [79], [80], [81]. In Europe, the only approved product is Alofisel, used for the treatment of complex perianal fistulas derived from Chron’s disease. Other examples include Temcell HS in Japan and Prochymal, (Remestemcel-L) in Canada, both for the treatment of GvHD [79], [80], [82].
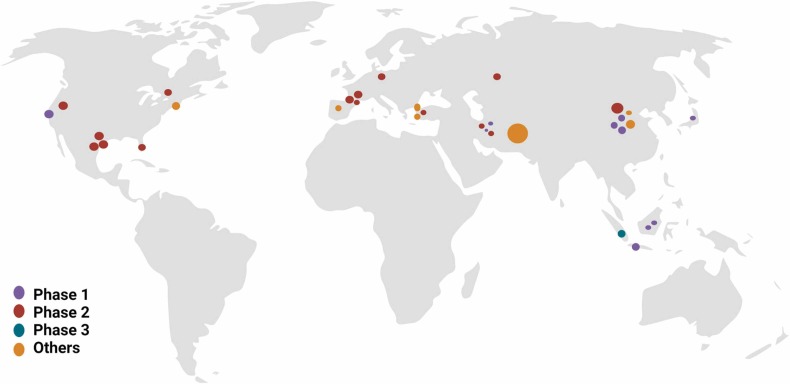
Currently several clinical trials are taking place to study if MSCs represent a real therapeutic approach to treat patients suffering from severe or critical COVID-19 ( Fig. 4). Since the beginning of the pandemic, the results of various clinical studies and case reports investigating the therapeutic potential of MSCs and their derived products to fight the SARS-CoV2 infection have already been published. It is important to note that these studies have gathered more than 1600 patients. The overall results of these studies have confirmed that the use of MSCs is safe, as no major adverse effects directly related to them have been reported [11], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93]. In general, published clinical trials and case reports follow similar approaches, administering MSCs as primary or adjunctive therapy in COVID-19 patients. Moreover, some studies have left the cell therapy aside to administer only isolated components of the MSC-derived secretome. In the following lines, we revise the clinical trials conducted to date for both approaches.
Fig. 4.
Ongoing clinical trials focused on MSC-based therapies for the treatment of COVID-19.
5.1. Clinical trials based on MSC cell therapy
As of June 2022, there are 97 clinical trials employing MSCs to combat COVID-19 registered on the international database clinicaltrials.gov, and 9 more clinical trials registered on clinicaltrialsregister.eu. The trials completed to date are shown in Table 1. In most cases, 1 × 106 cells / kg of body weight (bw) were administered; however, doses can vary significantly from study to study, as can be seen in Table 1. Regarding the administration route, MSCs were delivered IV in all cases, and the frequency of administration varies with MSCs infused once, twice, three or four times depending on the study and patient clinical condition. Some of the most remarkable findings in the published literature are presented in the following lines.
Table 1.
Clinical trials employing MSCs for the treatment of COVID-19.
| Principal investigator or sponsor | Patient condition | Number of participants | Study type | Cell source | MSC dosing | Ref. | |
|---|---|---|---|---|---|---|---|
| Cell-based therapies | |||||||
| Leng et al., | Pneumonia | 10 | Serie of cases | UC-MSCs | 1 × 106 UC-MSCs/kg, | [11] | |
| Sánchez-Guijo et al., | Pneumonia under IMV | 13 | Retrospective revision | AT-MSCs | 0,98 × 106 Allo AT-MSCs/kg, (days 1, 3, 5) | [83] | |
| Sadeghi et al., | ARDS | 10 | phase 1, 2 | DSCs | 1 × 106 cells/kg, | [84] | |
| Adas et al., | Pneumonia and/or multiple organ failure | 30 | phase 1, 2 | WJ-MSCs | 3 × 106 cells/kg (days 0, 3, 6) | [85], [103] | |
| Karyana et al., | – | 9 | Phase 1 | MSCs | Ld: 5 × 107 Allo-MSCs, Hd: 1 × 108 Allo-MSCs, | [104] | |
| Hadisoebroto Dilogo et al., | – | 40 | phase 1 | UC-MSCs | 1 × 106 UC-MSCs/kg BW | [86], [105] | |
| Lanzoni et al., | ARDS | 24 | phase 1, 2a | UC-MSCs | 100 × 106 UC-MSCs, (days 0, 3) | [87], [106] | |
| Saleh et al., | – | 5 | phase 1 | WJ-MSCs | 150 × 106 WJ-MSCs/injection (days 0, 3, 6) | [88], [107] | |
| Meng et al., | – | 18 | phase 1 | UC-MSCs | 4 × 107 UC-MSCs (days 0,3, 6) | [89] | |
| Shi et al., | Lung damage | 100 | phase 2 | UC-MSCs | 4 × 107 (days 0, 3, 6) | [108], [109] | |
| Hashemian et al. | ARDS | 11 | phase 1 | UC-MSCs and PL-MSCs | 200 × 106 cells/infusion of UC-MSCs (6 cases) or placental MSCs (5 cases) (3 iv every other day) | [110] | |
| Shu et al., | – | 41 | – | UC-MSCs | 2 × 106 cells/kg | [103] | |
| Xiaowei Xu et al., | – | 44 | phase 1 | MB-MSCs | 9 × 107 Allo MB-MSCs (days 1, 3, 5) | [93] | |
| Central Hospital, Nancy, France | ARDS | 30 | phase 2 | WJ-MSCs | WJ-MSCs, day 0: 1 × 106 MSCs/kg, day 3: 0,5 × 106 MSCs/kg, day 5: 0,5 106 MSCs/kg | [111] | |
| Kanuni Sultan Suleyman Training and Research Hospital | Pneumonia | 21 | – | MSCs | 2 × 106/kg (days 0,2, 4) | [112] | |
| SBÜ Dr. Sadi Konuk Eğitim ve Araştırma Hastanesi | Pneumonia | 30 | – | MSCs | MSCs transplantation (3 infusions with 30 days intervals) | [113] | |
| Hope Biosciences Stem Cell Research Foundation | – | 56 | phase 2 | HB-AT-MSCs | HB-AT-MSCs (5 iv infusions) | [114] | |
| Hope Biosciences Stem Cell Research Foundation | – | 55 | phase 2 | HB-AT-MSCs | Group 1: 200 × 106 cells/dose, Group 2: 100 × 106 cells/dose, Group 3: 50 × 106 cells/dose, (weeks 0,2,6,10 and 14), |
[115] | |
| Institute of Biophysics and Cell Engineering of National Academy of Sciences of Belarus | Pneumonia (viral, interstitial) | 32 | phase 1, 2 | Allo pooled olfactory mucosa-MSCs | – | [116] | |
| Hope Biosciences Stem Cell Research Foundation | – | 53 | phase 2 | HB-AT-MSC | HB-AT-MSCs 100 × 106 cells/dose (days 0,3,7 and 10) | [117] | |
| Rohto Pharmaceutical Co., Ltd. | – | 6 | phase 1 | AT-MSCs (ADR-001) | 1 × 108 AT-MSCs/week (4 adm) | [118] | |
| Ina-Respond | – | 9 | phase 1 | DW-MSCs | Ld group: 5 × 107 cells, Hd group: 1 × 108 cells. |
[119] | |
| UNICEF | Cytokine release syndrome, critical illness, ARDS | 600 | – | BM-MSCs | 2 × 106 cells/kg. Administration alone or in combination with other novel therapies. | [120] | |
| Assistance Publique - Hôpitaux de Paris | ARDS | 47 | phase 1, 2 | UC-MSCs | 1 × 106 cells/kg, (days 1,3, 5) |
[121] | |
| Ottawa Hospital Research Institute | ARDS | 15 | phase1, 2 | UC-MSCs | Panel 1: 25 × 106 cells/unit dose, panel 2: 50 × 106 cells/unit dose, panel 3: 90 × 106 cells/unit dose. (3 consecutive days) |
[122] | |
| CHRU NANCY | ARDS | 30 | phase 2 | WJ-MSCs | 2 × 106 cells/kg | [123] | |
| Secretome-based therapies | |||||||
| Sengupta et al., | Severe pneumonia | 24 | Cohort study | ExoFlo™ (derivated exosomes of BM-MSCs) | 15 mL ExoFlo™ (unspecified exosome number) | IV | [96] |
| State-Financed Health Facility "Samara Regional Medical Center Dinasty" | Pneumonia | 30 | Phase 1, 2 | Exosomes | EXO1 inh: 3 mL exosomes (0,5–2 ×1010); EXO 2 inh: 3 mL exosomes (0,5–2 ×1010). (2 Inh/day, 10 days) | INH | [97] |
| Indonesia University | – | 40 | Phase 3 | Secretome-MSCs | – | IV | [98] |
| Thomas Advanced Medical LLC | – | 40 | Phase 1 | UC-MSCs secretome (PrimePro™) | PrimePro™ (unspecified exosome number) | IV | [99] |
| Direct Biologics, LLC | ARDS and/or viral pneumonia | 120 | Phase 2 | BM-MSCs derived EVs (DB-001, ExoFlo) | Dose 1: 10 mL ExoFlo (800 billion EVs), Dose 2: 15 mL ExoFlo (1,2 trillion EVs). | IV | [95] |
| Ruijin Hospital | – | 24 | Phase 1 | Allo AT-MSCs-derived exosomes (MSCs-Exo) | MSCs-Exo (2 ×108 NVs/3 mL) (5 times, at days 1,2,3,4 and 5) |
INH | [100] |
| Avicenna Research Institute | Cytokine storm | 29 | Phase 1, 2 | Allo human MB-MSCs secretome | Allo MB-MSCs secretome (days 1,2,3,4 and 5) |
IV | [101] |
List of abbreviations: COVID-19 – Coronavirus disease 2019, ARDS – Acute Respiratory Distress Syndrome, SARS-CoV-2 – Severe Acute Respiratory Syndrome Coronavirus 2, UC-MSCs – Umbilical Cord-derived Mesenchymal Stromal Cells, AT-MSCs – Adipose Tissue-derived Mesenchymal Stromal Cells, Allo-MSCs – Allogeneic Mesenchymal Stromal Cells, WJ-MSCs – Wharton’s Jelly derived Mesenchymal Stromal Cells, HB-AT-MSCs - Hope Biosciences autologous adipose derived mesenchymal stromal cells, DW-MSCs – Daewoong Pharmaceutical’s Mesenchymal Stromal Cells, DSCs – Placenta derived decidua stromal cells, PL-MSCs – Placental derived Mesenchymal Stromal Cells, MB-MSCs – Menstrual blood derived Mesenchymal Stromal Cells, BM-MSCs – Bone Marrow derived Mesenchymal Stromal Cells, IV – intravenous, Ld – Low dose, Hd – High dose, IMV – Invasive mechanical ventilation, adm – administration, bw – Body weight, EVs - Extracellular Vesicles, INH – inhalation, adm – administration, NVs – nanovesicles.
Leng et al. conducted a clinical trial in 7 severe COVID-19 patients, who were administered IV 1 × 106 umbilical cord MSCs (UC-MSCs)/kg bw and were followed for 14 days. In all the MSC-treated patients, all symptoms resolved within 2–4 days after treatment and no short-term adverse effects were observed. In particular, chest computed tomography images showed that pneumonia infiltration decreased significantly, while the levels of CRP decreased. Moreover, MSCs restored the peripheral blood number of T and NK cells, decreased the levels of TNF-α and increased IL-10 and VEGF. Overall, this led to a reduced inflammation and promoted lung tissue regeneration [80], [92]. This study also demonstrated that MSCs do not express ACE2 receptors, which means that they are immune to SARS-CoV-2, thus reinforcing that they may be a feasible and convenient therapeutic agent to treat COVID-19 [80].
Other interesting study was the performed by Sadeghi et al., in which 10 patients with COVID-19-induced ARDS were administrated IV 1–2 times a dose of 1 × 106 placenta-derived MSCs/kg bw. An 80% of the patients recovered and left the intensive care unit (ICU) within a median of 6 days. They concluded that MSC-therapy is safe and capable of increasing oxygenation, clearing pulmonary infiltrates and reducing the peripheral blood inflammatory cytokine levels [82]. The study conducted by Lanzoni et al. supports these results by showing that the IV UC-MSCs administration importantly decreased the plasma levels of a set of inflammatory cytokines involved in cytokine storm including IFN-γ, IL-6 and TNF-α. It is important to highlight that UC-MSCs treatment was associated with a significant improvement of patient survival (91% vs 42% for the control group that received vehicle solution infusions) [85], [93].
Moving on to studies with a greater number of patients, Shi et al., conducted a phase 2 clinical trial to evaluate the efficacy and safety of UC-MSCs to treat severe COVID-19 patients with lung damage, based on a previous phase 1 trial they had previously performed [87]. They studied 100 COVID-19 patients with lung damage who received 3 IV infusions of 4 × 107 UC-MSCs on days 0, 3 and 6. Compared to placebo, UC-MSCs administration exerted a significant improvement in the lung lesion volume at day 28, the end-point of the study. Overall, their results conclude that MSC-based cell treatment is safe and effective [88]. In addition, the long-term safety and effectiveness of the treatment were studied in a 1-year follow-up, collecting data at 3-month intervals. Results demonstrated that at month 3 UC-MSC-treated patients still presented an improvement in whole lung lesions and the incidence of symptoms was lower [89].
Recently, two commercial companies have published press releases to display preliminary results of MSC-based therapy in severe COVID-19 patients. One of them is Pluristem, whose study is a retrospective case report of 8 critically ill patients on invasive mechanical ventilation, suffering from ARDS due to COVID-19. Patients were treated with PLacental eXpanded (PLX)-PAD, which contains placenta-derived MSC-like cells that have regenerative and immunomodulatory properties. PLX-PAD cells are termed MSC-like cells since they present typical MSC membrane markers but their capacity to differentiate in vitro into cells of the mesodermal lineage is limited. The intervention was an intramuscular injection of PLX-PAD (300 ×106 cells) in 1 or 2 administrations (300 ×106 cells each) at an interval of 8 or 11 days according to medical discretion. Improvement in several variables such as CRP, positive end-expiratory pressure and PaO2/FiO2 was observed following (PLX)-PAD treatment [94].
The other company is Mesoblast, who conducted a study on 2 pediatric patients with multisystem inflammatory syndrome in children (MIS-C). MIS-C is a serious postinfectious immune dysregulation associated to COVID-19, which can lead to hemodynamic instability, severe and life-threatening cardiovascular dysfunction, shock and multisystemic organ failure. Mesoblast proposed the used of Remestemcel-L, which, as mentioned before, has previously been approved to treat GvHD. Remestemcel-L is based on culture-expanded allogeneic bone marrow MSCs (BM-MSCs) of unrelated adult donors. Both children received 2 IV infusions of Remestemcel-L (2 ×106 cells/kg bw) separated by 48 h. Remestemcel-L contributed to improvements in myocardial and endothelial function and reduced cardiac and systemic inflammation [98], [99]. Overall, clinical trials have demonstrated that treating severe COVID-19 with MSCs is safe, effective and well-tolerated, constituting a promising therapy.
5.2. Clinical trials based on the MSC-derived secretome
Clinical trials following a cell-free approach based on the MSC-derived secretome have also been performed. Sengupta et al. conducted a prospective, open-label, non-randomized cohort study in 24 COVID-19 patients suffering from severe pneumonia. Patients were IV administered exosomes derived from BM-MSCs (ExoFlo™). Results demonstrated the safety of the approach, since no adverse effects related to the therapy were observed. In addition, there was an improvement in the condition of these patients, allowing to restore the oxygenation levels. In particular, 71% of the patients recovered after ExoFlo treatment and were discharged from the hospital at an average of 5–6 days after infusion; whereas 14% remained critically ill but stable. They observed mean reductions of CRP, ferritin and D-dimer were 77%, 43% and 42% respectively on day 5 post-treatment. Unfortunately, the remaining 16% of patients died, being the survival rate of the study of 83% [95].
Currently, there are other clinical trials ongoing applying the MSC-derived secretome to treat severe COVID-19. These studies have been registered in clinicaltrials.gov, but their results have not been published yet. All the registered clinical trials based on the MSC-derived secretome have been gathered in Table 1 [95], [96], [97], [98], [99], [100], [101].
In this cell-free approach, an alternative administration route is being explored: the inhalation route. The inhalation route is possible because of the small size of EVs and soluble factors produced by MSCs and represents an interesting alternative to the IV injection since it presents certain advantages. It is less invasive and enables a direct pulmonary delivery promoting a fast therapeutic effect [102].
6. Conclusions
Despite vaccination is undoubtedly one of the best options to fight against COVID-19, the limited global accessibility together with the yet unknown duration of the acquired protection, make inevitable the continuous transmission of the virus. The therapeutic tools approved to date to fight SARS-CoV-2, are often insufficient to treat the complications occurring in severe and critical COVID-19 cases. Furthermore, many of these treatments present weak safety profiles with important adverse effects [2].
Thus, the need to develop alternative therapies to combat severe and critical COVID-19 cases is still essential. MSCs could be excellent candidates because of their unique immunomodulatory properties. Thanks to this activity, MSCs are able regulate the immune response, diminish the cytokine storm and thus, limit the inflammatory status these patients suffer. Moreover, MSCs also contribute to tissue regeneration, counteracting the fibrotic damage caused by the virus in different organs. Clinical trials published to date provide very important preliminar information as they support the safety and efficacy of MSCs-based therapies – both, cell and secretome based treatments –. Overall, MSC-based therapies have been shown to improve respiratory function and to increase oxygenation levels, establishing them as suitable candidates for treating COVID-19 patients with a serious prognosis [11], [93], [94], [97], [98].
The large number of clinical trials that are currently exploring the use of MSCs and their derived products for the treatment of COVID-19 demonstrate the feasibility of this approach [11], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90]. The knowledge that will be generated from them will surely shed light on the clinical translation of this therapy. Indeed, the FDA has pioneered the approval for the compassionate use of MSC in the most severe cases of SARS-Cov2 infection, which represents an important step forward towards making MSC-derived therapy a reality in the clinical practice, not only for the treatment of COVID-19, but also for the multiple immune-mediated diseases that nowadays lack of an effective therapy [5].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
A. Gonzalez-Pujana thanks the University of the Basque Country (UPV/EHU) for the postdoctoral grant (ESPDOC20/119). Authors thank Eusko Jaurlaritza (Grupos Consolidados, No ref: IT907–16). Figures were created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
Biographies
 Maria Rosselló Gelabert finished her degree in Pharmacy (2021) at the University of Basque Country, Spain. Currently, she is perusing her Ph.D. in Pharmaceutical Technology in the Development of Advanced Therapies, at the University of Basque Country, Spain. Her major research focuses on studying the therapeutic potential of Mesenchymal Stromal Cells (MSCs) and their secretome to combat immune-mediated inflammatory diseases (IMIDs). Currently, she is integrated into the laboratory of Professor Rosa Maria Hernandez at the Center for Research and Advanced Studies Lucio Lascaray, University of Basque Country, Spain.
Maria Rosselló Gelabert finished her degree in Pharmacy (2021) at the University of Basque Country, Spain. Currently, she is perusing her Ph.D. in Pharmaceutical Technology in the Development of Advanced Therapies, at the University of Basque Country, Spain. Her major research focuses on studying the therapeutic potential of Mesenchymal Stromal Cells (MSCs) and their secretome to combat immune-mediated inflammatory diseases (IMIDs). Currently, she is integrated into the laboratory of Professor Rosa Maria Hernandez at the Center for Research and Advanced Studies Lucio Lascaray, University of Basque Country, Spain.
 Ainhoa Gonzalez-Pujana is a post-doctoral researcher at the University of the Basque Country (UPV/EHU), Spain. She received her Ph.D. in Pharmaceutical Technology from UPV/EHU (2019), with her research in collaboration with Harvard University. Her Ph.D. received the Best Thesis Award by Bioaraba Health Research Institute, as well as by the SPLC-Controlled Release Society. Her research interest is focused on the development of mesenchymal stromal cell-based therapies for the treatment of immune-mediated inflammatory diseases.
Ainhoa Gonzalez-Pujana is a post-doctoral researcher at the University of the Basque Country (UPV/EHU), Spain. She received her Ph.D. in Pharmaceutical Technology from UPV/EHU (2019), with her research in collaboration with Harvard University. Her Ph.D. received the Best Thesis Award by Bioaraba Health Research Institute, as well as by the SPLC-Controlled Release Society. Her research interest is focused on the development of mesenchymal stromal cell-based therapies for the treatment of immune-mediated inflammatory diseases.
 Manoli Igartua received her PhD in Pharmaceutics from the University of the Basque Country (UPV/EHU), Spain in 1998. She is presently Full Professor of Pharmaceutics at the Faculty of Pharmacy (UPV/EHU). Her research focuses mainly on micro- and nano-encapsulation of biotechnology products for their application in various therapeutic fields such as tissue regeneration, vaccine development and diseases of the central nervous system. She has been involved in several research projects, funded by national and international institutions, and R&D contracts with pharmaceutical companies. She has completed research stages at the University of Aston in Birmingham (1996) and the University of Angers (1998). Since 2007 she has been a member of the Ciber-BBN Bioengineering, Biomaterials and Nano-medicine consortium, which depends on the Ministry of Health and Consumer Affairs of the Spanish Government.
Manoli Igartua received her PhD in Pharmaceutics from the University of the Basque Country (UPV/EHU), Spain in 1998. She is presently Full Professor of Pharmaceutics at the Faculty of Pharmacy (UPV/EHU). Her research focuses mainly on micro- and nano-encapsulation of biotechnology products for their application in various therapeutic fields such as tissue regeneration, vaccine development and diseases of the central nervous system. She has been involved in several research projects, funded by national and international institutions, and R&D contracts with pharmaceutical companies. She has completed research stages at the University of Aston in Birmingham (1996) and the University of Angers (1998). Since 2007 she has been a member of the Ciber-BBN Bioengineering, Biomaterials and Nano-medicine consortium, which depends on the Ministry of Health and Consumer Affairs of the Spanish Government.
 Edorta Santos-Vizcaino is a lecturer and researcher at the University of the Basque Country (UPV/EHU). He received his Ph.D. with international mention and Extraordinary Award from the UPV/EHU in collaboration with Harvard University and CIMA (University of Navarra). He also received the 2013/2015 SPLC-CRS Best Ph.D. Thesis Award. He has extensive experience in cell encapsulation and drug delivery systems. His research interests include the biomedical application of different biomaterials, mesenchymal stromal cells (MSCs), and extracellular vesicles to exert an immunomodulatory and regenerative effect in the treatment of immune-mediated inflammatory diseases (IMIDs) and chronic wound healing, among others.
Edorta Santos-Vizcaino is a lecturer and researcher at the University of the Basque Country (UPV/EHU). He received his Ph.D. with international mention and Extraordinary Award from the UPV/EHU in collaboration with Harvard University and CIMA (University of Navarra). He also received the 2013/2015 SPLC-CRS Best Ph.D. Thesis Award. He has extensive experience in cell encapsulation and drug delivery systems. His research interests include the biomedical application of different biomaterials, mesenchymal stromal cells (MSCs), and extracellular vesicles to exert an immunomodulatory and regenerative effect in the treatment of immune-mediated inflammatory diseases (IMIDs) and chronic wound healing, among others.
 Rosa Maria Hernández is Full Professor of Pharmaceutical Technology at the University of Basque Country, Spain. She received her Ph.D. (1992) from the University of Salamanca. She is Principal Investigator in several research projects and has supervised 21 Ph.D. thesis works and coauthored more than 200 scientific articles in high impact journals and 10 patents. Her research fields focus on several areas of drug delivery, including the micro- and nanoencapsulation of growth factors and peptides as an alternative therapy for neurodegenerative disorders and vaccine development. She is also interested in the development of cell-based and cell-free therapies for regenerative medicine.
Rosa Maria Hernández is Full Professor of Pharmaceutical Technology at the University of Basque Country, Spain. She received her Ph.D. (1992) from the University of Salamanca. She is Principal Investigator in several research projects and has supervised 21 Ph.D. thesis works and coauthored more than 200 scientific articles in high impact journals and 10 patents. Her research fields focus on several areas of drug delivery, including the micro- and nanoencapsulation of growth factors and peptides as an alternative therapy for neurodegenerative disorders and vaccine development. She is also interested in the development of cell-based and cell-free therapies for regenerative medicine.
References
- 1.Geneva: World Health Organization, "WHO Coronavirus (COVID-19) Dashboard [online database]," vol. 2022, (May 30,), 2022.
- 2.National Institutes of Health, "COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines." vol. 2022, (May 30,),. [PubMed]
- 3.Our World in Data, "Coronavirus (COVID-19) Vaccinations. Our World in Data; 2021," vol. 2022, (May 30,), May 30, 2022.
- 4.Barros I., Silva A., de Almeida L.P., Miranda C.O. Mesenchymal stromal cells to fight SARS-CoV-2: Taking advantage of a pleiotropic therapy. Cytokine amp; Growth Factor Rev. 2021;vol. 58:114–133. doi: 10.1016/j.cytogfr.2020.12.002. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Journal of Stem Cell Research & Therapeutics and (JSRT) Mesenchymal stem cells may be a credible alternative therapy to fight with COVID-19 pandemic. Tech. Rep. 2020;6(06/) [Google Scholar]
- 6.Gonzalez-Pujana A., Igartua M., Santos-Vizcaino E., Hernandez R.M. Mesenchymal stromal cell based therapies for the treatment of immune disorders: recent milestones and future challenges. Expert Opin. Drug Deliv. 2020;vol. 17(2):189. doi: 10.1080/17425247.2020.1714587. [DOI] [PubMed] [Google Scholar]
- 7.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S. Acute respiratory distress syndrome: the Berlin definition. JAMA: J. Am. Med. Assoc. 2012;vol. 307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 8.Zhu Z., Cai T., Fan L., Lou K., Hua X., Huang Z., Gao G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020;vol. 95:332–339. doi: 10.1016/j.ijid.2020.04.041. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;vol. 46(5):846–848. doi: 10.1007/s00134-020-05991-x. 〈https://link.springer.com/article/10.1007/s00134-020-05991-x〉 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Valle D.M., Kim-Schulze S., Huang H., Beckmann N.D., Nirenberg S., Wang B., Lavin Y., Swartz T.H., Madduri D., Stock A., Marron T.U., Xie H., Patel M., Tuballes K., Van Oekelen O., Rahman A., Kovatch P., Aberg J.A., Schadt E., Jagannath S., Mazumdar M., Charney A.W., Firpo-Betancourt A., Mendu D.R., Jhang J., Reich D., Sigel K., Cordon-Cardo C., Feldmann M., Parekh S., Merad M., Gnjatic S. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020;vol. 26(10):1636. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., Fan J., Wang W., Deng L., Shi H., Li H., Hu Z., Zhang F., Gao J., Liu H., Li X., Zhao Y., Yin K., He X., Gao Z., Wang Y., Yang B., Jin R., Stambler I., Lim L.W., Su H., Moskalev A., Cano A., Chakrabarti S., Min K., Ellison-Hughes G., Caruso C., Jin K., Zhao R.C. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;vol. 11(2) doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delvenne J. "Outbreak of multidrug-resistant tuberculosis in South Africa undetected by WHO-endorsed commercial tests: an observational study. Lancet Infect. Dis. 2019;vol. 20(4):425. doi: 10.1016/S1473-3099(18)30496-1. https://explore.openaire.eu/search/publication?articleId=od______1493::b58efecc73a58e06161921078a354cb5 (Available) [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim N., Hosri J., Bteich Y., Dib A., Abou A. Rached, “COVID-19 and Liver Dysfunction,” Curēus (Palo Alto. CA) 2022;vol. 14(1) doi: 10.7759/cureus.21302. https://www.ncbi.nlm.nih.gov/pubmed/35186564 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clyne B. Multitasking in emergency medicine. Acad. Emerg. Med. 2012;vol. 19(2):230–231. doi: 10.1111/j.1553-2712.2011.01265.x. https://api.istex.fr/ark:/67375/WNG-GZH0LXMD-N/fulltext.pdf (Available) [DOI] [PubMed] [Google Scholar]
- 15.Ali A.M., Rostam H.M., Fatah M.H., Noori C.M., Ali K.M., Tawfeeq H.M. Serum troponin, D‐dimer, and CRP level in severe coronavirus (COVID‐19) patients. Immun. Inflam. amp; Dis. 2021;vol. 10(3) doi: 10.1002/iid3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malik P., Patel U., Mehta D., Patel N., Kelkar R., Akrmah M., Gabrilove J.L., Sacks H. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid. -Based Med. 2021;vol. 26(3):107–108. doi: 10.1136/bmjebm-2020-111536. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiss S., Gede N., Hegyi P., Németh D., Földi M., Dembrovszky F., Nagy B., Juhász M.F., Ocskay K., Zádori N., Molnár Z., Párniczky A., Hegyi P.J., Szakács Z., Pár G., Erőss B., Alizadeh H. Early changes in laboratory parameters are predictors of mortality and ICU admission in patients with COVID-19: a systematic review and meta-analysis. Med Microbiol Immunol. 2020;vol. 210(1):33–47. doi: 10.1007/s00430-020-00696-w. https://link.springer.com/article/10.1007/s00430-020-00696-w (Available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang L., Jin J., Luo W., Gan Y., Chen B., Li W. Risk factors for predicting mortality of COVID-19 patients: a systematic review and meta-analysis. PloS One. 2020;vol. 15(11) doi: 10.1371/journal.pone.0243124. https://www.ncbi.nlm.nih.gov/pubmed/33253244〉 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gómez-Pastora J., Weigand M., Kim J., Wu X., Strayer J., Palmer A.F., Zborowski M., Yazer M., Chalmers J.J. Hyperferritinemia in critically ill COVID-19 patients – is ferritin the product of inflammation or a pathogenic mediator. Clin. Chim. Acta. 2020;vol. 509:249–251. doi: 10.1016/j.cca.2020.06.033. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang J., Liang Z., Peng Y., Wei L., Liu Y., Hu Y., Peng P., Wang J., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;vol. 382(18):1708–1720. doi: 10.1056/NEJMoa2002032. https://nejm.org/doi/full/10.1056/NEJMoa2002032 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osamura R.Y., Magnani B. Memories of Gene Herbek, MD.(Letters to the Editor)(Letter to the editor) Arch. Pathol. amp; Lab. Med. (1976) 2021;vol. 145(2):130–131. https://search.proquest.com/docview/2499458650 (Available) [Google Scholar]
- 22.Cao P., Wu Y., Wu S., Wu T., Zhang Q., Zhang R., Wang Z., Zhang Y. vol. 26. 2021. Elevated serum ferritin level effectively discriminates severity illness and liver injury of coronavirus disease 2019 pneumonia; p. 207. (Biomarkers). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szarpak L., Ruetzler K., Safiejko K., Hampel M., Pruc M., Kanczuga L., Koda -, Filipiak K.J., Jaguszewski M.J. Lactate dehydrogenase level as a COVID-19 severity marker. Am. J. Emerg. Med. 2021;vol. 45:638–639. doi: 10.1016/j.ajem.2020.11.025. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han Y., Zhang H., Mu S., Wei W., Jin C., Xue Y., Tong C., Zha Y., Song Z., Gu G. “Lact. dehydrogenase, a Risk Factor Sev. COVID-19 Patients,”. 2020 doi: 10.18632/aging.103372. https://explore.openaire.eu/search/publication?articleId=od______9409::01e7efa71c3cd698b4f288f03e1e9d98 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun Hsin C., Ching K.C., Jun F.J., Lee Y., Wei J.C. Morbidity and mortality among adults experiencing homelessness hospitalized With COVID-19. J. Infect. Dis. 2021 doi: 10.1093/infdis/jiab261. https://www.ncbi.nlm.nih.gov/pubmed/34951649 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Y., Cao Q., Hong Z., Tan Y., Chen S., Jin H., Tan K., Wang D., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil. Med Res. 2020;vol. 7(1) doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osman M., Klopfenstein T., Belfeki N., Gendrin V., Zayet S. A comparative systematic review of COVID-19 and influenza. Viruses. 2021;vol. 13(3):452. doi: 10.3390/v13030452. https://www.ncbi.nlm.nih.gov/pubmed/33802155 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen Q., Li J., Zhang Z., Guo S., Wang Q., An X., Chang H. COVID-19: systemic pathology and its implications for therapy. Int. J. Biol. Sci. 2022;vol. 18(1):386. doi: 10.7150/ijbs.65911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghavami S., Yeganeh B., Zeki A.A., Shojaei S., Kenyon N.J., Ott S., Samali A., Patterson J., Alizadeh J., Moghadam A.R., Dixon I.M.C., Unruh H., Knight D.A., Post M., Klonisch T., Halayko A.J. Autophagy and the unfolded protein response promote profibrotic effects of TGF-β1 in human lung fibroblasts. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018;vol. 314(3):L493–L504. doi: 10.1152/ajplung.00372.2017. https://search.proquest.com/docview/1957462387 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidinger G., Hanselmayer G., Pieh S., Lackner B., Kaminski S., Ruhswurm I., Skorpik C. Effect of tenascin and fibronectin on the migration of human corneal fibroblast". J. Cataract Refract. Surg. 2003;vol. 29(2):354. doi: 10.1016/s0886-3350(02)01609-7. [DOI] [PubMed] [Google Scholar]
- 31.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;vol. 109 doi: 10.1016/j.jaut.2020.102433. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., Wu H., Lin Y., Zhang M., Zhang Q., Shi M., Liu Y., Zhou Y., Lan K., Chen Y. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes amp; Infect. 2020;vol. 9(1):761–770. doi: 10.1080/22221751.2020.1747363. https://www.tandfonline.com/doi/abs/10.1080/22221751.2020.1747363 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;vol. 184(4):861–880. doi: 10.1016/j.cell.2021.01.007. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chaolin Huang* Yeming Wang*, Xingwang Li* Lili Ren*, Jianping Zhao* Yi. Hu*, Li Zhang Guohui Fan, Jiuyang Xu Xiaoying Gu, Zhenshun Cheng Ting Yu, Jiaan Xia Yuan Wei, Wenjuan Wu Xuelei Xie, Wen Yin Hui Li, Min Liu Yan Xiao, Hong Gao Li. Guo, Jungang Xie Guangfa Wang, Rongmeng Jiang Zhancheng Gao, Qi Jin Jianwei Wang†, Bin Cao†. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;vol. 395 doi: 10.1016/S0140-6736(20)30183-5. https://pubmed.ncbi.nlm.nih.gov/31986264/ (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Z., Kang H., Li S., Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanism". J. Neurol. 2020;vol. 267(8):2179–2184. doi: 10.1007/s00415-020-09929-7. https://www.ncbi.nlm.nih.gov/pubmed/32458193 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 2020;vol. 26(6):842. doi: 10.1038/s41591-020-0901-9. [DOI] [PubMed] [Google Scholar]
- 37.Chua R.L., Lukassen S., Trump S., Hennig B.P., Wendisch D., Pott F., Debnath O., Thürmann L., Kurth F., Völker M.T., Kazmierski J., Timmermann B., Twardziok S., Schneider S., Machleidt F., Müller-Redetzky H., Maier M., Krannich A., Schmidt S., Balzer F., Liebig J., Loske J., Suttorp N., Eils J., Ishaque N., Liebert U.G., Von Kalle C., Hocke A., Witzenrath M., Goffinet C., Drosten C., Laudi S., Lehmann I., Conrad C., Sander L., Eils R. COVID-19 severity correlates with airway epithelium–immune cell interactions identified by single-cell analysis. Nat. Biotechnol. 2020;vol. 38(8):970. doi: 10.1038/s41587-020-0602-4. [DOI] [PubMed] [Google Scholar]
- 38.Wilk A.J., Rustagi A., Zhao N.Q., Roque J., Martínez-Colón G.J., Mckechnie J.L., Ivison G.T., Ranganath T., Vergara R., Hollis T., Simpson L.J., Grant P., Subramanian A., Rogers A.J., Blish C.A. A single-cell atlas of the peripheral immune response in patients with severe COVID-19. Nat. Med. 2020;vol. 26(7):1070. doi: 10.1038/s41591-020-0944-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. 2020;vol. 17(5):533. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., Hägglöf T., Oliveira T.Y., Viant C., Hurley A., Hoffmann H., Millard K.G., Kost R.G., Cipolla M., Gordon K., Bianchini F., Chen S.T., Ramos V., Patel R., Dizon J., Shimeliovich I., Mendoza P., Hartweger H., Nogueira L., Pack M., Horowitz J., Schmidt F., Weisblum Y., Michailidis E., Ashbrook A.W., Waltari E., Pak J.E., Huey-Tubman K.E., Koranda N., Hoffman P.R., West A.P., Rice C.M., Hatziioannou T., Bjorkman P.J., Bieniasz P.D., Caskey M., Nussenzweig M.C. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;vol. 584(7821):437. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borges L., Pithon-Curi T.C., Curi R., Hatanaka E. COVID-19 and neutrophils: the relationship between hyperinflammation and neutrophil extracellular traps. Mediat. Inflamm. 2020;vol. 2020:8829674. doi: 10.1155/2020/8829674. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenne C., Wong C.Y., Zemp F., McDonald B., Rahman M., Forsyth P., McFadden G., Kubes P. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host amp; Microbe. 2013;vol. 13(2):169–180. doi: 10.1016/j.chom.2013.01.005. (Available) [DOI] [PubMed] [Google Scholar]
- 43.Samudrala P.K., Kumar P., Choudhary K., Thakur N., Wadekar G.S., Dayaramani R., Agrawal M., Alexander A. Virology, pathogenesis, diagnosis and in-line treatment of COVID-19. Eur. J. Pharmacol. 2020;vol. 883 doi: 10.1016/j.ejphar.2020.173375. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waterman R.S., Tomchuck S.L., Henkle S.L., Betancourt A.M. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PloS One. 2010;vol. 5(4) doi: 10.1371/journal.pone.0010088. https://www.ncbi.nlm.nih.gov/pubmed/20436665 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nooijer A.H. d, Grondman I., Janssen N.A.F., Netea M.G., Willems L., Veerdonk F.L., van de E.J., Giamarellos-Bourboulis M., Jaeger H.I., Dijkstra H.L.M., Lemmers L. v, Emst K., Schraa C.W.M., Jacobs A.G., Hijmans T.J.G., Jansen F.B.M., Weren L.H.G., Fransman J.J.F., Gerretsen Maat, van de J.S., Nijman G., Moorlag S.J.C.F.M., Taks E.J.M., Debisarun A., Kouijzer I.J.E., Wertheim H.F.L., Hopman J., Rahamat-Langendoen J.C., Bleeker-Rovers C.P., Oever J. t, Crevel R. v, Hoogerwerf J.J., Mast Q. d, Hoeven H. v d, Pickkers P., Kox M., Frenzel T., Schouten J.A., Hemelaar P., Beunders R., Velde S. v d, Kooistra E.J., Waalders N.J.B., Klop-Riehl M., Toonen E.J.M., Joosten L.A.B. Complement Activation in the Disease Course of Coronavirus Disease 2019 and Its Effects on Clinical Outcomes. J. Infect. Dis. 2021;vol. 223(2):214–224. doi: 10.1093/infdis/jiaa646. https://www.narcis.nl/publication/RecordID/oai:repository.ubn.ru.nl:2066%2F232398 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lei Shi* Zhe Xu*, Jiyuan Zhang Yijin Wang*, Chao Zhang Lei Huang, Peng Zhao Shuhong Liu, Li Zhu Hongxia Liu, Changqing Bai Yanhong Tai, Jinwen Song Tingting Gao, Jinghui Dong Peng Xia, Fu-Sheng Wang Jingmin Zhao. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;vol. 8(420–22) doi: 10.1016/S2213-2600(20)30076-X. Available: https://doi.org/10.1016/ S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Najar M., Raicevic G., Fayyad-Kazan H., De Bruyn C., Bron D., Toungouz M., Lagneaux L. vol. 11. 2014. Bone marrow mesenchymal stromal cells induce proliferative, cytokinic and molecular changes during the T cell response: the importance of the IL-10/CD210 axis; pp. 442–452.https://link.springer.com/article/10.1007/s12015-014-9567-3 (Stem Cell Rev and Rep). (Available) [DOI] [PubMed] [Google Scholar]
- 48.Ferreira J.R., Teixeira G.Q., Santos S.G., Barbosa M.A., Almeida-Porada G., Gonçalves R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018;vol. 9 doi: 10.3389/fimmu.2018.02837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghahremani Piraghaj M., Soudi S., Ghanbarian H., Bolandi Z., Namaki S., Hashemi S.M. “Effect of efferocytosis of apoptotic mesenchymal stem cells (MSCs) on C57BL/6 peritoneal macrophages function,”. Life Sci. 2018;vol. 212:203–212. doi: 10.1016/j.lfs.2018.09.052. (Available) [DOI] [PubMed] [Google Scholar]
- 50.Juárez-Navarro K.J., Padilla-Camberos E., Díaz N.F., Miranda-Altamirano A., Díaz-Martínez N.E. Human mesenchymal stem cells: the present alternative for high-incidence diseases, even SARS-Cov-2. Stem Cells Int. 2020:8892189. doi: 10.1155/2020/8892189. 2020. Available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gur-Wahnon D., Borovsky Z., Beyth S., Liebergall M., Rachmilewitz J. Contact-dependent induction of regulatory antigen-presenting cells by human mesenchymal stem cells is mediated via STAT3 signaling. Exp. Hematol. 2007;vol. 35(3):426–433. doi: 10.1016/j.exphem.2006.11.001. https://www.clinicalkey.es/playcontent/1-s2.0-S0301472×0600693X (Available) [DOI] [PubMed] [Google Scholar]
- 52.Loke X.Y., Imran S.A.M., Tye G.J., Wan Safwani Wan Kamarul Zaman, Nordin F. Immunomodulation and regenerative capacity of MSCs for Long-COVID. Int. J. Mol. Sci. 2021;vol. 22(22):12421. doi: 10.3390/ijms222212421. https://search.proquest.com/docview/2602125126 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.François M., Romieu-Mourez R., Li M., Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 2012;vol. 20(1):187–195. doi: 10.1038/mt.2011.189. (Available) [DOI] [PubMed] [Google Scholar]
- 54.Dana M.R., Yamada J., Streilein J.W. Topical interleukin 1 receptor antagonist promotes corneal transplant survival1. Transplantation. 1997;vol. 63(10):1501–1507. doi: 10.1097/00007890-199705270-00022. [DOI] [PubMed] [Google Scholar]
- 55.Qi Y., Jiang D., Sindrilaru A., Stegemann A., Schatz S., Treiber N., Rojewski M., Schrezenmeier H., Vander Beken S., Wlaschek M., Böhm M., Seitz A., Scholz N., Dürselen L., Brinckmann J., Ignatius A., Scharffetter-Kochanek K. TSG-6 released from intradermally injected mesenchymal stem cells accelerates wound healing and reduces tissue fibrosis in murine full-thickness skin wounds. J. Invest. Dermatol. 2014;vol. 134(2):526–537. doi: 10.1038/jid.2013.328. (Available) [DOI] [PubMed] [Google Scholar]
- 56.Hertsenberg A.J., Shojaati G., Funderburgh M.L., Mann M.M., Du Y., Funderburgh J.L. Corneal stromal stem cells reduce corneal scarring by mediating neutrophil infiltration after wounding. PloS One. 2017;vol. 12(3) doi: 10.1371/journal.pone.0171712. https://www.ncbi.nlm.nih.gov/pubmed/28257425 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Harrell C.R., Markovic B.S., Fellabaum C., Arsenijevic N., Djonov V., Volarevic V. vol. 46. 2020. The role of Interleukin 1 receptor antagonist in mesenchymal stem cell‐based tissue repair and regeneration; pp. 263–275.https://onlinelibrary.wiley.com/doi/abs/10.1002/biof.1587 (BioFactors (Oxford)). (Available) [DOI] [PubMed] [Google Scholar]
- 58.Yen B.L., Hwa H., Hsu P., Chen P., Wang L., Jiang S., Liu K., Sytwu H., Yen M. HLA-G expression in human Mesenchymal Stem Cells (MSCs) is related to unique methylation pattern in the proximal promoter as well as gene body DNA. Ijms. 2020;vol. 21(14) doi: 10.3390/ijms21145075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y., Yin Z., Zhang R., Yan K., Chen L., Chen F., Huang W., Lv B., Sun C., Jiang X. MSCs inhibit bone marrow-derived DC maturation and function through the release of TSG-6. Biochem. Biophys. Res. Commun. 2014;vol. 450(4):1409–1415. doi: 10.1016/j.bbrc.2014.07.001. (Available) [DOI] [PubMed] [Google Scholar]
- 60.Bu X., Zhong J., Li W., Cai S., Gao Y., Ping B. Immunomodulating functions of human leukocyte antigen-G and its role in graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Ann. Hematol. 2021;vol. 100(6):1391–1400. doi: 10.1007/s00277-021-04486-z. https://link.springer.com/article/10.1007/s00277-021-04486-z (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mallis P., Michalopoulos E., Chatzistamatiou T., Stavropoulos-Giokas C. Mesenchymal stromal cells as potential immunomodulatory players in severe acute respiratory distress syndrome induced by SARS-CoV-2 infection. World J. Stem Cells. 2020;vol. 12(8):731–751. doi: 10.4252/wjsc.v12.i8.731. https://search.proquest.com/docview/2444607179 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akiyama K., Chen C., Wang D., Xu X., Qu C., Yamaza T., Cai T., Chen W., Sun L., Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-Ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;vol. 10(5):544–555. doi: 10.1016/j.stem.2012.03.007. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galleu A., Riffo-Vasquez Y., Trento C., Lomas C., Dolcetti L., Cheung T.S., Von Bonin M., Barbieri L., Halai K., Ward S., Weng L., Chakraverty R., Lombardi G., Watt F.M., Orchard K., Marks D.I., Apperley J., Bornhauser M., Walczak H., Bennett C., Dazzi F. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 2017;vol. 9(416) doi: 10.1126/scitranslmed.aam7828. [DOI] [PubMed] [Google Scholar]
- 64.Christ B., Franquesa M., Najimi M., van der Laan Luc, J. W, Dahlke M.H. Cellular and molecular mechanisms of mesenchymal stem cell actions. Stem Cells Int. 2017:2489041–2489042. doi: 10.1155/2017/2489041. 2017. Available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yagi H., Soto-Gutierrez A., Parekkadan B., Kitagawa Y., Tompkins R.G., Kobayashi N., Yarmush M.L. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 2010;vol. 19(6–7):667–679. doi: 10.3727/096368910X508762. https://journals.sagepub.com/doi/full/10.3727/096368910×508762 (Available:) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson J.M., Rodriguez A., Chang D.T. “Foreign body reaction to biomaterials,”. Semin. Immunol. 2007;vol. 20(2):86–100. doi: 10.1016/j.smim.2007.11.004. https://www.clinicalkey.es/playcontent/1-s2.0-S1044532307000966 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yadav P., Vats R., Bano A., Bhardwaj R. Mesenchymal stem cell immunomodulation and regeneration therapeutics as an ameliorative approach for COVID-19 pandemics. Life Sci. 1973;vol. 263(2020) doi: 10.1016/j.lfs.2020.118588. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ungerer C., Quade-Lyssy P., Radeke H.H., Henschler R., Königs C., Köhl U., Seifried E., Schüttrumpf J. Galectin-9 is a suppressor of t and b cells and predicts the immune modulatory potential of mesenchymal stromal cell preparations. Stem Cells Dev. 2014;vol. 23(7):755. doi: 10.1089/scd.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gieseke F., Böhringer J., Bussolari R., Dominici M., Handgretinger R., Müller I. Human multipotent mesenchymal stromal cells use galectin-1 to inhibit immune effector cells. Blood. 2010;vol. 116(19):3770–3779. doi: 10.1182/blood-2010-02-270777. (Available) [DOI] [PubMed] [Google Scholar]
- 70.Li Z., Niu S., Guo B., Gao T., Wang L., Wang Y., Wang L., Tan Y., Wu J., Hao J. Stem cell therapy for COVID‐19, ARDS and pulmonary fibrosis. Cell Prolif. 2020;vol. 53(12) doi: 10.1111/cpr.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mei S.H.J., Haitsma J.J., Dos Santos C.C., Yupu Deng P.F.H., Lai A.S., Slutsky W.C., Liles, Stewart D.J. Mesenchymal Stem Cells Reduce Inflammation while Enhancing Bacterial Clearance and Improving Survival in Sepsis. Am. J. Respir. Crit. Care Med. 2010;vol. 182(8):1047–1057. doi: 10.1164/rccm.201001-0010OC. https://www.ncbi.nlm.nih.gov/pubmed/20558630 (Available) [DOI] [PubMed] [Google Scholar]
- 72.Krasnodembskaya A., Samarani G., Song Y., Zhuo H., Su X., Lee J., Gupta N., Petrini M., Matthay M.A. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am. J. Physiol. - Lung Cell. Mol. Physiol. 2012;vol. 302(10):1003–1013. doi: 10.1152/ajplung.00180.2011. http://ajplung.physiology.org/content/302/10/L1003 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harrell C.R., Jovicic N., Djonov V., Arsenijevic N., Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells (Basel, Switz. ) 2019;vol. 8(12):1605. doi: 10.3390/cells8121605. https://www.ncbi.nlm.nih.gov/pubmed/31835680 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Regmi S., Pathak S., Kim J.O., Yong C.S., Jeong J. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur. J. Cell Biol. 2019;vol. 98(5–8) doi: 10.1016/j.ejcb.2019.04.002. (Available) [DOI] [PubMed] [Google Scholar]
- 75.Mao Y., Xu J., Seeley E.J., Tang X., Xu L., Zhu Y., Song Y., Qu J. Adipose Tissue‐Derived Mesenchymal Stem Cells Attenuate Pulmonary Infection Caused by P seudomonas aeruginosa via Inhibiting Overproduction of Prostaglandin E. Stem Cells. 2015;vol. 33(7):2331. doi: 10.1002/stem.1996. [DOI] [PubMed] [Google Scholar]
- 76.Harrell C.R., Jovicic N., Djonov V., Arsenijevic N., Volarevic V. Mesenchymal stem cell-derived exosomes and other extracellular vesicles as new remedies in the therapy of inflammatory diseases. Cells (Basel, Switz. ) 2019;vol. 8(12):1605. doi: 10.3390/cells8121605. https://www.ncbi.nlm.nih.gov/pubmed/31835680 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Regmi S., Pathak S., Kim J.O., Yong C.S., Jeong J. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: Challenges, opportunities, and future perspectives. Eur. J. Cell Biol. 2019;vol. 98(5–8) doi: 10.1016/j.ejcb.2019.04.002. (Available) [DOI] [PubMed] [Google Scholar]
- 78.Mao Y., Xu J., Seeley E.J., Tang X., Xu L., Zhu Y., Song Y., Qu J. Adipose tissue‐derived mesenchymal stem cells attenuate pulmonary infection caused by P seudomonas aeruginosa via inhibiting overproduction of prostaglandin E 2. Stem Cells. 2015;vol. 33(7):2331. doi: 10.1002/stem.1996. [DOI] [PubMed] [Google Scholar]
- 79.Levy O., Kuai R., Siren E.M.J., Bhere D., Milton Y., Nissar N., De Biasio M., Heinelt M., Reeve B., Abdi R., Alturki M., Fallatah M., Almalik A., Alhasan A.H., Shah K., Karp J.M. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020;vol. 6(30) doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muthu S., Jeyaraman M., Kotner M.B., Jeyaraman N., Rajendran R.L., Sharma S., Khanna M., Rajendran S.N.S., Oh J.M., Gangadaran P., Ahn B. Evolution of mesenchymal stem cell therapy as an advanced therapeutic medicinal product (ATMP)—an indian perspective. Bioengineering. 2022;vol. 9(3) doi: 10.3390/bioengineering9030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sánchez-Guijo F., García-Arranz M., López-Parra M., Monedero P., Mata-Martínez C., Santos A., Sagredo V., Álvarez-Avello J., Guerrero J.E., Pérez-Calvo C., Sánchez-Hernández M., Del-Pozo J.L., Andreu E.J., Fernández-Santos M., Soria-Juan B., Hernández-Blasco L.M., Andreu E., Sempere J.M., Zapata A.G., Moraleda J.M., Soria B., Fernández-Avilés F., García-Olmo D., Prósper F. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;vol. 25 doi: 10.1016/j.eclinm.2020.100454. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pereira Chilima T.D., Moncaubeig F., Farid S.S. Impact of allogeneic stem cell manufacturing decisions on cost of goods, process robustness and reimbursement. Biochem. Eng. J. 2018;vol. 137:132–151. doi: 10.1016/j.bej.2018.04.017. (Available) [DOI] [Google Scholar]
- 83.Sánchez-Guijo F., García-Arranz M., López-Parra M., Monedero P., Mata-Martínez C., Santos A., Sagredo V., Álvarez-Avello J., Guerrero J.E., Pérez-Calvo C., Sánchez-Hernández M., Del-Pozo J.L., Andreu E.J., Fernández-Santos M., Soria-Juan B., Hernández-Blasco L.M., Andreu E., Sempere J.M., Zapata A.G., Moraleda J.M., Soria B., Fernández-Avilés F., García-Olmo D., Prósper F. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;vol. 25 doi: 10.1016/j.eclinm.2020.100454. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sadeghi B., Roshandel E., Pirsalehi A.A., Kazemi S., Sankanian G., Majidi M.M., Salimi M., Aghdami N.N., Sadrosadat H., Samadi Kochaksaraei S., Alaeddini F., Ringden O., Hajifathali A. Conquering the cytokine storm in COVID‐19–induced ARDS using placenta‐derived decidua stromal cells. J. Cell Mol. Med. 2021;vol. 25(22):10554. doi: 10.1111/jcmm.16986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Adas G., Cukurova Z., Yasar K.K., Yilmaz R., Isiksacan N., Kasapoglu P., Yesilbag Z., Koyuncu I., Karaoz E. The systematic effect of mesenchymal stem cell therapy in critical COVID-19 patients: a prospective double controlled trial. Cell Transplant. 2021;vol. 30 doi: 10.1177/09636897211024942. https://journals.sagepub.com/doi/full/10.1177/09636897211024942 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dilogo I.H., Aditianingsih D., Sugiarto A., Burhan E., Damayanti T., Sitompul P.A., Mariana N., Antarianto R.D., Liem I.K., Kispa T., Mujadid F.F., Novialdi N.N., Luviah E., Kurniawati T.T., Lubis A.M.T., Rahmatika D.D. Umbilical cord mesenchymal stromal cells as critical COVID-19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl. Med. 2021;vol. 10(9):1279. doi: 10.1002/sctm.21-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lanzoni G., Linetsky E., Correa D., Messinger Cayetano S., Alvarez R.A., Kouroupis D., Alvarez Gil A., Poggioli R., Ruiz P.P., Marttos A.C., Hirani K., Bell C.A., Kusack H., Rafkin L., Baidal D., Pastewski A., Gawri K., Leñero C., Mantero A.M.A., Metalonis S.W., Wang X., Roque L.L., Masters B., Kenyon N.S., Ginzburg E., Xu X., Tan J., Caplan A.I., Glassberg M.K., Alejandro R., Ricordi C. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 2021;vol. 10(5):660. doi: 10.1002/sctm.20-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mahshid Saleh, "Cell therapy in patients with COVID-19 using mesenchymal stem cells," vol. 2020, (March 5,), September 13, 2020.
- 89.Meng F., Xu R., Wang S., Xu Z., Zhang C., Li Y., Yang T., Shi L., Fu J., Jiang T., Huang L., Zhao P., Yuan X., Fan X., Zhang J., Song J., Zhang D., Jiao Y., Liu L., Zhou C., Maeurer M., Zumla A., Shi M., Wang F. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Sig Transduct. Target Ther. 2020;vol. 5(1) doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi L., Huang H., Lu X., Yan X., Jiang X., Xu R., Wang S., Zhang C., Yuan X., Xu Z., Huang L., Fu J., Li Y., Zhang Y., Yao W., Liu T., Song J., Sun L., Yang F., Zhang X., Zhang B., Shi M., Meng F., Song Y., Yu Y., Wen J., Li Q., Mao Q., Maeurer M., Zumla A., Yao C., Xie W., Wang F. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Sig Transduct. Target Ther. 2021;vol. 6(1) doi: 10.1038/s41392-021-00488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shi L., Yuan X., Yao W., Wang S., Zhang C., Zhang B., Song J., Huang L., Xu Z., Fu J., Li Y., Xu R., Li T., Dong J., Cai J., Li G., Xie Y., Shi M., Li Y., Zhang Y., Xie W., Wang F. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. EBioMedicine. 2022;vol. 75 doi: 10.1016/j.ebiom.2021.103789. (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shu L., Niu C., Li R., Huang T., Wang Y., Huang M., Ji N., Zheng Y., Chen X., Shi L., Wu M., Deng K., Wei J., Wang X., Cao Y., Yan J., Feng G. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;vol. 11(1) doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu X., Jiang W.W., Chen L.L., Xu Z., Zhang Q., Zhu M., Ye P., Li H., Yu L., Zhou X., Zhou C., Chen X.L., Zheng X., Xu K., Cai H., Zheng S., Jiang W.W., Wu X., Li D., Chen L.L., Luo Q., Wang Y., Qu J., Li Y., Zheng W., Jiang Y.W., Tang L., Xiang C., Li L. Evaluation of the safety and efficacy of using human menstrual blood‐derived mesenchymal stromal cells in treating severe and critically ill COVID‐19 patients: an exploratory clinical trial. Clin. Transl. Med. 2021;vol. 11(2) doi: 10.1002/ctm2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barkama R., Mayo A., Paz A., Solopov A., Mann T., Vadasz Z., Appel T., Ofir R., Shani L., Sheleg M., Allen H., Shaked Nitzan R., Tsarfaty N., Gilad H., Birch T., Kachel E., Reinke P., Volk H., Zalts R., Raz Pasteur A. Placenta-derived cell therapy to treat patients with respiratory failure due to coronavirus disease 2019. Crit. Care Explor. 2020;vol. 2(9) doi: 10.1097/CCE.0000000000000207. https://search.proquest.com/docview/2446994577 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.L. Direct Biologics, "Extracellular Vesicle Infusion Treatment for COVID-19 Associated ARDS (EXIT-COVID19)," vol. 2022, (March 1,), January 14, 2022.
- 96.Sengupta V., Sengupta S., Lazo A., Woods P., Nolan A., Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;vol. 29(12):747. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.State-Financed Health FacilitySamara Regional Medical Center Dinasty, "Evaluation of Safety and Efficiency of Method of Exosome Inhalation in SARS-CoV-2 Associated Pneumonia. (COVID-19EXO).," vol. 2021, (December 20,), November 4, 2020.
- 98.I.U. Murdani abdullah, Mesenchymal Stem Cell Secretome In Severe Cases of COVID-19 vol. 2022, (February 24,), November 16, 2021.
- 99.L. Thomas Advanced Medical, Mesenchymal Stem Cells for the Treatment of COVID-19 vol. 2022, (March 1,), October 5, 2020.
- 100.Ruijin Hospital, A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia vol. 2020, (March 2,), September 7, 2020.
- 101.Avicenna Research Institute, Menstrual Blood Stem Cells in Severe Covid-19 vol. 2022, (March 2,), September 2, 2021.
- 102.Liu A., Zhang X., He H., Zhou L., Naito Y., Sugita S., Lee J. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin. Biol. Ther. 2020;vol. 20(2):125–140. doi: 10.1080/14712598.2020.1689954. https://www.tandfonline.com/doi/abs/10.1080/14712598.2020.1689954 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.SBÜ Dr. Sadi Konuk Eğitim ve Araştırma Hastanesi, "Clinical Use of Stem Cells for the Treatment of Covid-19," vol. 2022, (February 15,), May 25, 2021.
- 104.Karyana M., Djaharuddin I., Rif’ati L., Arif M., Choi M.K., Angginy N., Yoon A., Han J., Josh F., Arlinda D., Narulita A., Muchtar F., Bakri R.A., Irmansyah S. Safety of DW-MSC infusion in patients with low clinical risk COVID-19 infection: a randomized, double-blind, placebo-controlled trial. Stem Cell Res. amp; Ther. 2022;vol. 13(1):134. doi: 10.1186/s13287-022-02812-4. https://www.ncbi.nlm.nih.gov/pubmed/35365239 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Indonesia University, Administration of Allogenic UC-MSCs as Adjuvant Therapy for Critically-Ill COVID-19 Patients vol. 2020, (March 3,), July 7, 2020.
- 106.Camillo Ricordi, Use of UC-MSCs for COVID-19 Patients vol. 2022, (January 11,), December 6, 2021.
- 107.Saleh M., Vaezi A.A., Aliannejad R., Sohrabpour A.A., Kiaei S.Z.F., Shadnoush M., Siavashi V., Aghaghazvini L., Khoundabi B., Abdoli S., Chahardouli B., Seyhoun I., Alijani N., Verdi J. Cell therapy in patients with COVID-19 using Wharton’s jelly mesenchymal stem cells: a phase 1 clinical trial. Stem Cell Res. amp; Ther. 2021;vol. 12(1):1–410. doi: 10.1186/s13287-021-02483-7. https://search.proquest.com/docview/2553226394 (Available) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shi L., Yuan X., Yao W.Q., Wang S., Zhang C., Zhang B., Song J., Huang L., Xu Z., Fu J., Li Y., Xu R., Li T., Dong J., Cai J., Li G., Xie Y., Shi M., Li Y., Zhang Y., Xie W., Wang F. Human mesenchymal stem cells treatment for severe COVID-19: 1-year follow-up results of a randomized, double-blind, placebo-controlled trial. SSRN Electron. J. 2021 doi: 10.1016/j.ebiom.2021.103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beijing 302 Hospital, "Treatment With Human Umbilical Cord-derived Mesenchymal Stem Cells for Severe Coronavirus Disease 2019 (COVID-19)," vol. 2022, (March 1,), August 19, 2020.
- 110.Hashemian S.R.R., Aliannejad R., Zarrabi M., Soleimani M., Vosough M., Hosseini S., Hossieni H., Keshel S.H., Naderpour Z., Hajizadeh-Saffar E., Shajareh E., Jamaati H., Soufi-Zomorrod M., Khavandgar N., Alemi H., Karimi A., Pak N., Rouzbahani N.H., Nouri M., Sorouri M., Kashani L., Madani H., Aghdami N., Vasei M., Baharvand H. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;vol. 12(1) doi: 10.1186/s13287-021-02165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.N. Central Hospital France, "Efficacy of Infusions of MSC From Wharton Jelly in the SARS-CoV-2 (COVID-19) Related Acute Respiratory Distress Syndrome (MSC-COVID-19).," vol. 2021, (February 24,), November 5, 2021.
- 112.Kanuni Sultan Suleyman Training and Research Hospital, "Mesenchymal Stem Cells Therapy in Patients With COVID-19 Pneumonia," vol. 2022, (March 1,), January 20, 2021.
- 113.SBÜ Dr. Sadi Konuk Eğitim ve Araştırma Hastanesi, "A Proof of Concept Study for the DNA Repair Driven by the Mesenchymal Stem Cells in Critical COVID-19 Patients," vol. 2022, (March 1,), May 24, 2021.
- 114.Hope Biosciences Stem Cell Research Foundation, "A Clinical Trial to Determine the Safety and Efficacy of Hope Biosciences Autologous Mesenchymal Stem Cell Therapy (HB-adMSCs) to Provide Protection Against COVID-19," vol. 2022, (March 1,), July 7, 2021.
- 115.Hope Biosciences Stem Cell Research Foundation, "A Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Determine the Safety and Efficacy of Hope Biosciences Allogeneic Mesenchymal Stem Cell Therapy (HB-adMSCs) to Provide Protection Against COVID-19," vol. 2022, (March 1,), August 20, 2021.
- 116.Institute of Biophysics and Cell Engineering of National Academy of Sciences of Belarus, "Treatment of Covid-19 Associated Pneumonia With Allogenic Pooled Olfactory Mucosa-derived Mesenchymal Stem Cells," vol. 2022, (March 1,), August 23, 2021.
- 117.Hope Biosciences Stem Cell Research Foundation, "Efficacy and Safety Study of Allogeneic HB-adMSCs for the Treatment of COVID-19," vol. 2022, (March 1,), February 7, 2022.
- 118.L. Rohto Pharmaceutical Co., "An Exploratory Study of ADR-001 in Patients With Severe Pneumonia Caused by SARS-CoV-2 Infection (COVID-19)," vol. 2022, (March 1,), March 18, 2021.
- 119.Ina-Respond, "Therapeutic Study to Evaluate the Safety and Efficacy of DW-MSC in COVID-19 Patients (DW-MSC)," vol. 2022, (March 1,), January 27, 2021.
- 120.UNICEF, "Investigational Treatments for COVID-19 in Tertiary Care Hospital of Pakistan," vol. 2022, (February 20,), July 30, 2020.
- 121.Assistance Publique - Hôpitaux de Paris, "Cell Therapy Using Umbilical Cord-derived Mesenchymal Stromal Cells in SARS-CoV-2-related ARDS (STROMA-CoV2)," vol. 2022, (March 2,), February 18, 2022.
- 122.Ottawa Hospital Research Institute, "Cellular Immuno-Therapy for COVID-19 Acute Respiratory Distress Syndrome (CIRCA-19)," vol. 2022, (March 2,), April 26, 2021.
- 123.CHRU NANCY, "Efficacy of infusions of mesenchymal stem cells from Wharton jelly in the moderate to severe SARS-Cov-2 related acute respiratory distress syndrome (COVID-19): A Phase IIa double-blind randomized controlled trial," vol. 2022, (March 2,), September 1, 2021.