Abstract
Hypophosphatasia is an inherited metabolic disorder characterized by defective mineralization of bones and teeth with a wide variety of manifestations, ranging from stillbirth to dental symptoms alone. Recently, the prognosis of severe hypophosphatasia patients has been greatly improved by the introduction of enzyme replacement therapy. The typical dental manifestation is early exfoliation of primary teeth due to disturbed cementum formation, so dentures are recommended to ensure that important oral functions are acquired. Some studies have shown that enzyme replacement therapy improves dental mineralization, resulting in the stabilization of periodontal tissues and better growth of tooth roots. A nationwide Japanese survey revealed the common genetic and dental manifestations of patients with mild hypophosphatasia, which markedly differ from those of the severe forms. There may be many undiagnosed mild patients, so dentists should contribute to the early diagnosis by screening possible cases based on the typical finding of early exfoliation of primary teeth. Early diagnosis is important for patients to receive early intervention in both medical and dental fields. The establishment of fundamental dental therapy to solve the dental problems is still underway and is eagerly anticipated.
Abbreviations: HPP, hypophosphatasia; TNSALP, tissue-nonspecific alkaline phosphatase; ALP, alkaline phosphatase; ERT, enzyme replacement therapy; QOL, quality of life
Keywords: Hypophosphatasia, Primary teeth, Early exfoliation, Hypomineralization, Medical and dental collaboration
1. Introduction
Hypophosphatasia (HPP) is a metabolic disorder first reported by J. C. Rathbun in 1948 [1]. Early exfoliation of primary teeth was soon after reported as a characteristic clinical finding of this disorder [2]. HPP is a rare genetic disease characterized by defective mineralization of bones and teeth [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. Patients with HPP show a wide range of manifestations, ranging from stillbirths and severe skeletal hypomineralization with dental symptoms in severe cases to early exfoliation of primary teeth without bone symptoms in mildest cases. Dental manifestations, such as early exfoliation of primary teeth, sometimes lead to a diagnosis in mild HPP [20], [21], [22], [23], [24], [25], [26], [27]. Some patients also reach adulthood without being diagnosed with HPP, despite having dental symptoms [26]. If early exfoliation of primary teeth is recognized, dentists should promptly request that medical doctors conduct an examination for HPP. Fundamental treatment for HPP involving systemic problems is now available and has shown very impressive results [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]. Children with severe HPP who previously would not have achieved long-term survival before the introduction of the therapy can now survive and attend dental clinics. Thus, it has been recognized that patients with severe HPP show a variety of oral problems that are more complex than those of mild patients [38], [39], [40]. This review summarizes the general knowledge on HPP from a dental perspective, including recently reported notable findings.
2. Pathology and treatment of HPP
HPP is a rare inherited disease caused by inactivating mutations in the ALPL gene, which encodes tissue-nonspecific alkaline phosphatase (TNSALP) [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. More than 400 ALPL gene mutations have been reported in The ALPL Gene Variant Database (https://alplmutationdatabase.jku.at). Such mutations lead to a reduction of alkaline phosphatase (ALP) enzyme activity, which impairs bone mineralization [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. TNSALP, which is abundant in bone, liver, kidney, and developing teeth, produces inorganic phosphate by degrading inorganic pyrophosphate, a mineralization inhibitor, and the phosphate and calcium form hydroxyapatite, leading to the progression of mineralization [3], [4], [5], [7], [8], [10], [11], [13], [17]. In HPP patients, owing to the loss of TNSALP activity, inorganic pyrophosphate is not degraded and phosphate is not produced. As a result, calcium and phosphate cannot bind, and hydroxyapatite formation and outgrowth is disrupted, resulting in impaired bone mineralization.
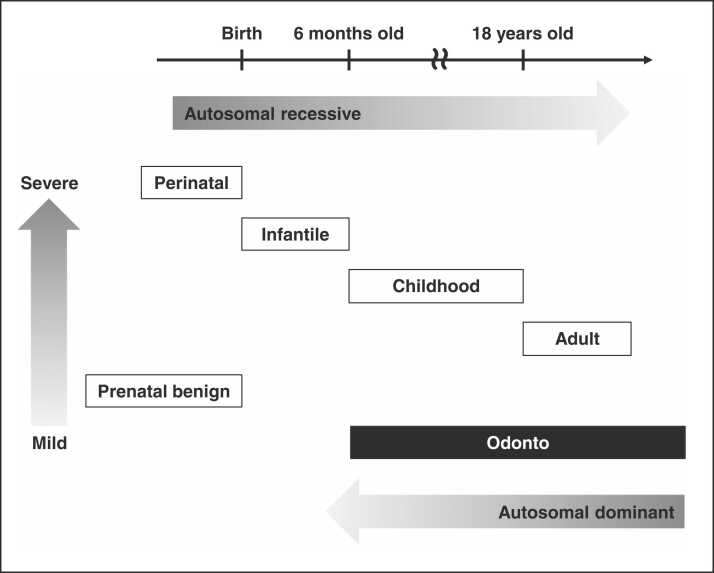
Diagnostic criteria for HPP in clinical practice guidelines define the primary symptoms as “bone mineralization disorder” and “premature loss of primary teeth” [41]. When a low serum ALP level is observed together with one or both of these main symptoms, HPP should be suspected. Then, gene testing should be performed for a definitive diagnosis. The phenotype of HPP is classified into six types based on the age at onset and severity of clinical features: perinatal, prenatal benign, infantile, childhood, adult, and odonto types (Table 1) [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [41]. In general, an earlier onset is associated with more severe systemic symptoms. Perinatal HPP is the most severe type with obvious symptoms at birth, whereas a child with infantile HPP presents with symptoms before 6 months of age. Perinatal HPP patients and half of infantile HPP patients have poor life prognoses without treatment because of respiratory complications [29]. Prenatal benign HPP, in which the prognosis is favorable despite the perinatal onset, has been documented recently [41], [42]. In childhood HPP, the first symptoms occur between 6 months and 18 years of age, while adult HPP is diagnosed after the age of 18 years. Odonto HPP is the mildest form, featuring only dental complications at any age, without skeletal bone symptoms. However, the clinical types of HPP are part of a continuous spectrum and laboratory data overlap between the different types [41].
Table 1.
Classification of HPP.
| Types | Period of diagnosis | Major symptoms | Inheritance patterns |
|---|---|---|---|
| Perinatal | Fetal to neonatal | Severe bone hypomineralization Respiratory disorder Convulsions |
Autosomal recessive |
| Prenatal benign | Bowed long bones | Autosomal recessive or autosomal dominant | |
| Infantile | < 6 months of age | Failure to thrive Hypercalcemia Premature craniosynostosis |
Autosomal recessive |
| Childhood | 6 months to 18 years old | Early exfoliation of primary teeth Short stature Bone pain |
Autosomal recessive or autosomal dominant |
| Adult | ≥ 18 years of age | Fracture Periodontitis |
Autosomal recessive or autosomal dominant |
| Odonto | Regardless of age | Early exfoliation of primary teeth Periodontitis |
Autosomal recessive or autosomal dominant |
The prevalence of life-threatening HPP (perinatal and infantile types) is reported to be 1/300,000 in Europe, 1/100,000 in North America, and 1/150,000 in Japan [13], [43], [44], [45], [46]. Therefore, it is estimated that approximately six to seven babies are born with life-threatening HPP every year in Japan, and that there are 100–200 affected patients. Severe forms of this disease are inherited in an autosomal recessive manner, and therefore the patients’ parents are typically carriers having one mutation in one allele and being asymptomatic [40], [47], [48] (Fig. 1). c.1559delT is a common mutation in severe HPP; this has been found only in the Japanese, with a prevalence of 1/480 in the general Japanese population, causing complete loss of ALP activity in patients with severe-form HPP [44], [45], [49], [50]. Mild HPP is expected to be more common because heterozygotes can express this condition; its prevalence in Europe is estimated to be 1/6370 [51]. The inheritance pattern of mild HPP is either autosomal recessive or autosomal dominant, and some autosomal dominant ALPL mutations have been reported to have a dominant-negative effect [52]. Odonto HPP is generally the mildest type with autosomal dominant inheritance. It is probably the most common form of HPP, although its prevalence in Japan remains to be elucidated [6], [13], [40], [48], [51].
Fig. 1.
Summary of severity and inheritance patterns in each type of HPP.
Prior to the recent introduction of ALP enzyme replacement therapy (ERT) using bone-targeting recombinant alkaline phosphatase (asfotase alfa), the management of HPP has been limited to the treatment of each symptom; however, ERT has had revolutionary effects on patients with severe HPP [28]. ERT was approved for manufacturing and marketing in Japan in 2015 [30], [33], and markedly improves the mineralization and survival rates in patients with severe HPP [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]. Asfotase alfa is generally administered subcutaneously three times a week at a dose of 1–3 mg/kg [37]; it significantly improves not only the survival rate but also the quality of life of patients with HPP [28], [29], [30], [31], [32], [33], [34], [35], [36], [37]. When patients are confirmed to have HPP with the expectation of a poor life prognosis, such as in patients with the perinatal and infantile types, ERT should always be administered [41]. Patients with other moderate to mild forms of HPP also have possible indications for ERT if their motor function and quality of life (QOL) are impaired because these impairments are expected to be alleviated by ERT. However, no clear criteria are currently available for recommending ERT based on the classification of HPP types. At present, the decision about the indication is made by evaluating the condition of each case in consideration of the expected effects and risks. Evidence on the effects of ERT on extraskeletal symptoms, such as convulsions and dental symptoms, should be clarified to establish criteria for the indications of ERT in the near future.
3. Dental manifestation of HPP
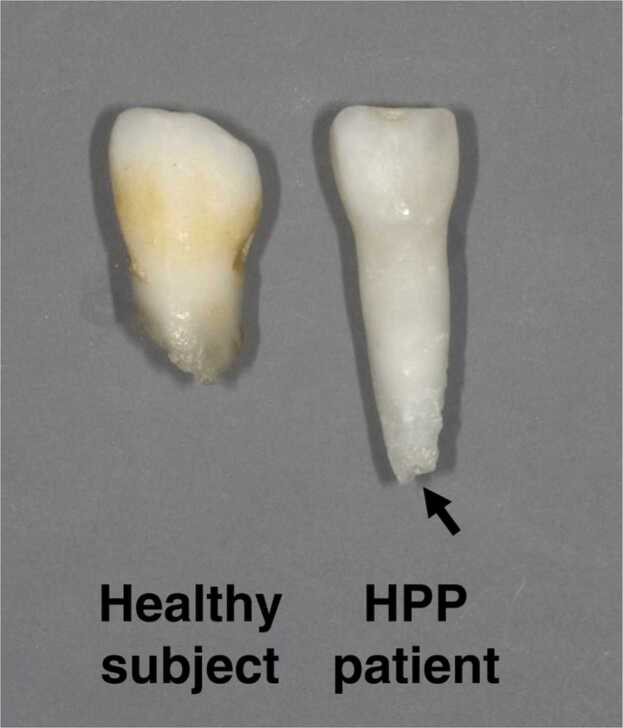
A typical dental finding of HPP is early exfoliation of primary teeth, most commonly affecting the anterior teeth, starting with the mandibular primary incisors [22]. Early exfoliation of primary teeth is observed between the ages of 1 and 4 years [22]. Generally, mandibular primary central incisors emerge into the oral cavity at around 6 months and completion of their roots is recognized at around 1.5 years of age [53]. Around age 4, the roots of the primary teeth start to resorb as the permanent teeth emerge into the oral cavity. The primary teeth are finally exfoliated under conditions of root resorption by their permanent successors emerging around the age of 6 (Fig. 2). However, in HPP patients, primary incisors are exfoliated prematurely between the ages of 1 and 4 years without inflammation [23], [54], [55], [56], [57]. The roots of early exfoliated teeth are under formation or not absorbed by the permanent successors, which is completely different from those replaced by the permanent successors in healthy children [22], [23], [24], [25], [27]. It has been speculated that the reason why primary anterior teeth rather than posterior teeth tend to be lost is because they have a single, short root, erupt earlier, and are more susceptible to lateral forces than posterior teeth [22].
Fig. 2.
Typical appearance of primary incisors when standard replacement with permanent teeth occurred in a healthy subject (left) and early exfoliation occurred in an HPP patient (right). The tooth root of the HPP patient is still developing (arrow).
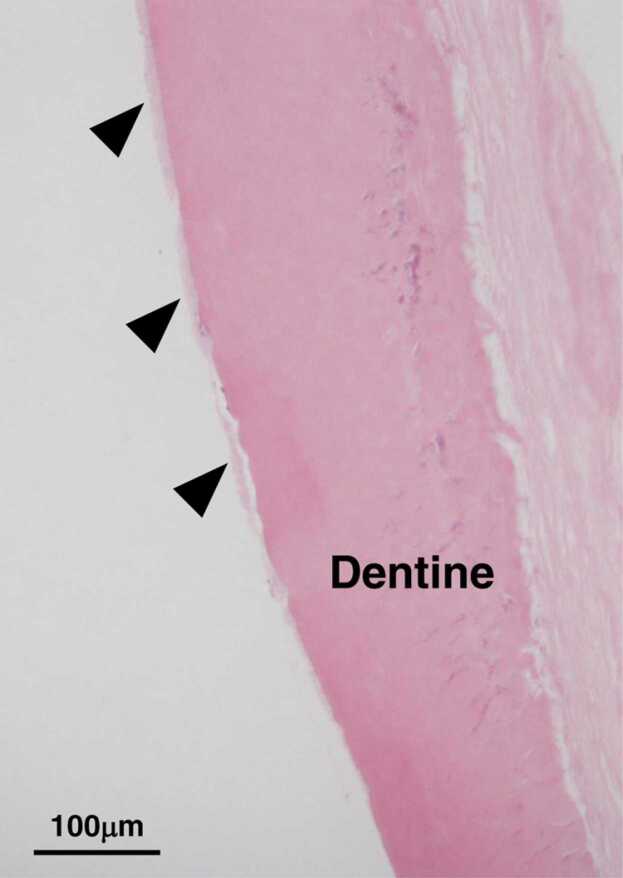
Early exfoliation of primary teeth is caused by poor mineralization of cementum due to low ALP activity (Fig. 3) [22], [25], [27], [53], [54], [55], [56]. Cementum of the teeth and alveolar bone are connected via the periodontal ligament [58]. In cases of HPP, this adhesion is insufficient due to cementum dysplasia [59], [60], resulting in even minor external force that causes mobility and eventually leads to exfoliation. Disturbed formation of cementum is also recognized in permanent teeth, with case reports describing that permanent teeth have been exfoliated [61], [62], [63], [64], [65], [66], [67]. The disturbed formation of cementum could confer susceptibility to not only early exfoliation of anterior teeth, but also ankylosis involving posterior teeth [23], [68]. The occlusal force gradually damages weak periodontal tissue and subsequently affects root adhesion to alveolar bone. In anterior teeth, the lateral occlusal force is stronger than the vertical force; this lateral force can promote tooth exfoliation. Conversely, the occlusal force acts vertically in the posterior region. Therefore, ankylosis may occur in molars but is less likely to occur in anterior teeth.
Fig. 3.
Histopathological appearance of decalcified section of an exfoliated primary incisor in an HPP patient (hematoxylin and eosin staining). Arrowheads indicate cementum. Disturbed cementum formation is observed.
The early exfoliation of primary incisors is a result of periodontitis, although periodontitis in primary dentition is rare [69], [70]. When dentists encounter periodontitis in children, they must suspect a background of systematic disease [71], [72]. However, the inflammation of periodontitis in HPP patients is much milder than that in other systematic diseases accompanied by periodontitis [23], [54], [55], [56], [57]. Deep periodontal pockets or tooth mobility is observed as a preliminary step prior to the exfoliation of affected teeth. The periodontal pockets of primary teeth are generally 1 mm in depth [73], but relatively deep periodontal pockets of 3 mm or more are identified in the primary teeth in HPP patients [25], [27]. In particular, the teeth adjacent to the exfoliated primary teeth often have a poor periodontal condition with mobility.
In addition to early exfoliation of primary teeth, other dental findings of HPP have been reported, such as early loss of permanent teeth, hypoplasia of enamel and dentine, reduced thickness of dentine, wide pulp chamber, thin and short roots, and dental caries [3], [24], [62].
4. Oral management of HPP
At present, no fundamental therapy is available to prevent early exfoliation of primary teeth. Notably, early exfoliation of primary teeth in HPP patients is derived not from periodontal inflammation but from the insufficient tooth attachment to alveolar bone due to cementum dysplasia [23], [54], [55], [56], [57]. However, periodontal inflammation is sometimes identified in patients with poor oral hygiene [38], [61], [62]. Therefore, guidance on adequate oral hygiene for patients and their parents/guardians to maintain good periodontal condition, as well as the regular management of periodontal condition in the clinic, is important.
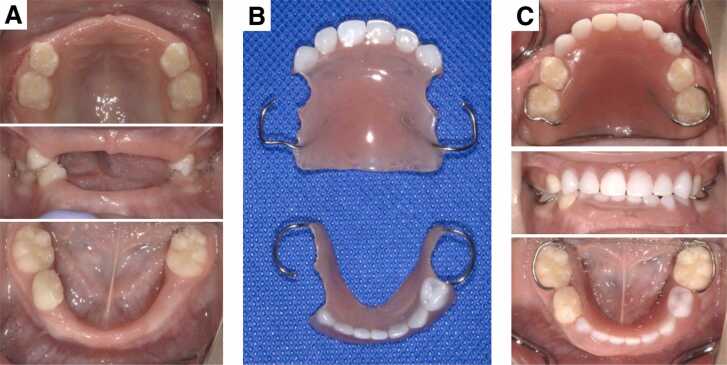
The application of partial dentures is also recommended for cases whose primary teeth have been exfoliated (Fig. 4). The period of primary dentition is important for the acquisition of masticatory and phonetic functions, and early loss of primary teeth influences these functions as well as having an aesthetic effect [74]. Such loss may also lead to abnormal swallowing habits, which induce malocclusion such as open bite [75]. Therefore, dentists should aim to apply partial dentures as early as possible at around the age of 3, when impressions can be taken [25]. A wire clasp should be selected and placed in the primary molar area where the periodontal tissue is stronger than in the anterior teeth. In patients using dentures for the first time, the clasp may be placed at the primary canine, but when the child is accustomed to dentures, the clasp should be cut so as not to interfere with the lateral growth of the jawbone. As the child grows, the dentures would gradually lose their fit, requiring adjustment or the production of new ones. In severe type HPP, the use of dentures may be difficult because of the condition of the alveolar bone and the remaining teeth, malocclusion, and intellectual disability [39].
Fig. 4.
Application of dentures in the case of a boy aged 3 years and 11 months with infantile HPP. A) Intraoral photographs before (A) and after (C) the application of partial dentures (B).
Recently, the use of a mouthguard in mixed dentition has been recommended to prevent strong pressure being applied to the teeth [76]. In particular, premature permanent teeth at the time of eruption have incomplete roots and are susceptible to external forces. To prevent dental trauma during anterior tooth replacement, such as in the early elementary school period, a mouthguard is applied especially during contact sports.
A few case reports on dental treatments for permanent dentition have been published, such as on prosthodontic rehabilitation, although no long-term follow-up was performed [77], [78], [79], [80], [81]. When HPP patients undergo prosthodontic rehabilitation, such as dental implants, it is important that sufficient examination of the periodontal condition be performed. In addition, it may be important to evaluate the conditions of alveolar bone where the implants are placed. In the future, it will be necessary to establish a fundamental treatment method by accumulating reports of treatment cases.
HPP patients often require orthodontic treatment due to insufficient space for permanent tooth eruption caused by a small jawbone and loss of space due to early loss of primary teeth [27], [38], [40], [62], [68]. When orthodontic treatment is performed in HPP patients, the weak attachment between the teeth and alveolar bone due to cementum dysplasia should be considered. Therefore, it is desirable to use a method that places less force on the teeth but, to the best of our knowledge, no reports of orthodontic treatment being used in HPP cases have yet been published.
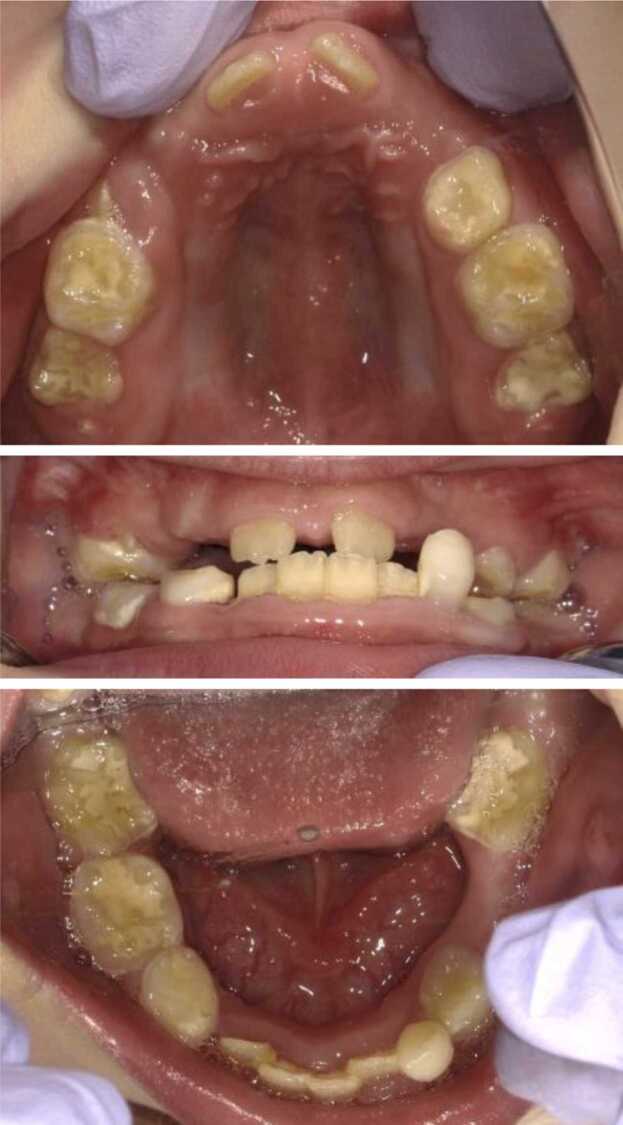
Recently, ERT has been initiated for HPP cases, and the dental manifestations of the severe form of this disease have gradually become apparent [40]. Severe HPP patients show hypomineralization of enamel and dentine, in addition to weak periodontal tissue, even when they are treated with ERT (Fig. 5, Fig. 6) [38], [39], [40]. A thin jawbone and malocclusion, such as an open bite or crowding caused by hypomineralization of jawbone, are also recognized. At present, it is difficult to understand the common features of severe HPP because of the low frequency of such cases and because most patients with severe HPP do not survive until the teeth have erupted prior to the start of ERT. However, dentists should make continuous efforts to establish appropriate treatment approaches in the near future.
Fig. 5.
Intraoral photographs of the case of a girl aged 8 years and 10 months with perinatal HPP treated by ERT. Enamel hypomineralization and multiple occlusal problems (mandibular prognathism, crowding, deep bite, high-arched palate, and V-shaped arch of maxilla) were recognized.
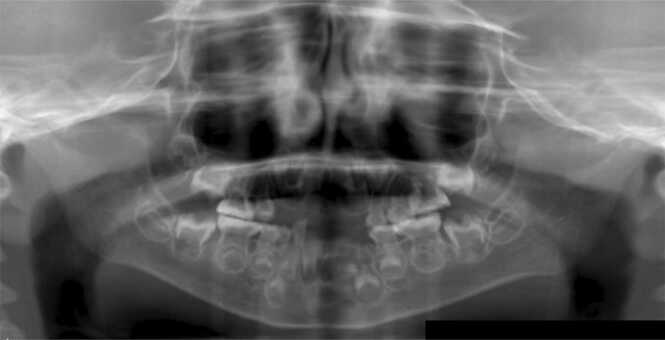
Fig. 6.
Orthopantomographic image of the case of a boy aged 6 years and 10 months with perinatal HPP treated by ERT. Severe hypomineralization of teeth was recognized.
5. Nationwide Japanese dental survey of HPP
In a nationwide Japanese dental survey, the oral manifestations of 52 patients diagnosed with HPP were analyzed using the information submitted by dental hospitals and clinics [40]. The most common phenotype reported was the odonto type, since the survey covers dental institutes. Mean serum ALP values in odonto patients were significantly higher than those in non-odonto patients. ERT was administered to 50 % of all cases and to all cases of severe HPP. This survey revealed prominent differences in genetic and dental findings between odonto and non-odonto types. Autosomal dominant and autosomal recessive inheritance patterns were detected in 89 % of odonto and 96 % of non-odonto patients. The ALPL c.1559delT mutation, associated with extremely low ALP activity, was found in approximately 70 % of cases. Regarding dental manifestations, all patients classified as odonto type showed early exfoliation of the primary teeth, which was significantly higher than the rate in patients classified as non-odonto type (100 % vs. 56 %). This means that HPP patients sometimes do not exhibit dental manifestations whether they do or do not receive ERT. Tooth hypomineralization was detected in 42 % of non-odonto patients, but not in any odonto ones. For many with occlusal problems, such as an open bite or a high-arched palate, who are considered to have severe HPP, these changes can lead to difficulty in swallowing and mastication. Total dental management, including of persistent occlusal problems, is important especially for perinatal and infantile types, depending on the severity of HPP. Genetic and dental manifestations of patients with odonto and non-odonto HPP are significantly different, and these differences should be considered during clinical approaches for patients with HPP.
Notably, in the previous study, two cases belonging to the odonto group shifted to the childhood group because of the emergence of skeletal bone symptoms with aging. Most of the cases in the odonto group had heterozygous mutations, and thus it is reasonable to speculate that patients with heterozygous mutations have odonto HPP, whereas those with compound heterozygous mutations are likely to shift toward childhood HPP as they grow. In odonto patients with compound heterozygous mutation, who we predict may shift to childhood HPP as they grow, careful monitoring could be important. In other words, genetic analysis is important not only for a definitive diagnosis of the condition but also perhaps for a prediction of the prognosis. The importance of proactively diagnosing odonto HPP at an early stage and keeping it under observation during the developmental period by pediatricians with medical and dental collaboration is suggested.
6. Dental effects of ERT in HPP
TNSALP knockout mice (Akp2−/−) phenocopy the infantile type of HPP, including growth failure, skeletal hypomineralization, epileptic seizures, and lethality by 2 weeks after birth [82], [83]. These model mice display impaired formation of cementum and alveolar bone, which is consistent with the dental phenotype associated with HPP in humans [84], [85]. The dental effects of ERT in model mice were reported. Akp2−/− mice injected with recombinant TNSALP showed complete mineralization of all incisor tooth tissues, all molar dentine, and the surrounding alveolar bone [86]. This therapy also improved HPP-related defects in the cementum [84], enamel [87], and dentine [88]. Because ERT requires repeated injections, which place a burden on patients, research has recently been undertaken on gene therapy with only a single injection [89], [90], [91], [92], [93] Gene therapy can improve the general condition, and also induce significant alveolar bone formation and moderate improvement of cementum formation in model mice [85]. However, gene therapy did not fully recover cementum formation to the level found in the wild type.
Some studies on humans have suggested that ERT may improve these problems [38], [39], [94], [95], [96], [97], [98]. Cementum formation in the primary incisors was detected in perinatal HPP treated with ERT [38], [39]. ERT in childhood HPP resulted in improved tooth and mandibular bone mineralization, with notably good effects on teeth under the formation process. Acceleration of the mineralization of roots associated with erupting teeth leads to stabilization of the periodontal condition [94]. ERT in infantile HPP patients demonstrated stable periodontal conditions of primary molars that erupted after the initiation of ERT, due to improved alveolar bone and tooth mineralization [95]. Another paper reported that none of the patients receiving ERT suffered from premature mobility of previously stable primary teeth. In some cases, the teeth with Grade I mobility became stable during the treatment, and no mobility was observed in those teeth during the follow-up. Thus, the process of premature loss of primary teeth in children receiving ERT was stabilized [96]. After providing ERT for odonto HPP to prevent premature exfoliation, no tooth mobility was observed [97]. Infantile HPP cases prematurely lost significantly fewer teeth to HPP than childhood HPP cases who started ERT at a later age. The oral health of children with early-onset infantile hypophosphatasia may be improved by the early and continuous administration of ERT, compared with the initiation of therapy later in childhood [98]. However, in those treated with ERT, hypomineralization and exfoliation of their primary teeth were reported [38], [39], while another report described the exfoliation of primary teeth after the initiation of ERT [97]. It should be considered that the effects of ERT on teeth could be limited because they are not tissues that can remodel like bone. Specifically, primary teeth start mineralization during the fetal stage [53], and thus it is difficult to expect positive effects of ERT, which is initiated after birth. However, ERT could be more effective on permanent teeth and jawbones.
When assessing the dental manifestations of HPP and the dental effect of ERT, objective measurement methods are essential. Thus, a method for evaluating alveolar bone hypomineralization in pediatric HPP was established using orthopantomography [99]. Specifically, this previous study was performed to develop a method for quantitatively evaluating alveolar bone hypomineralization using orthopantomographic images. Alveolar bone density was defined according to the pixel values and corrected by brightness shown by an indicator applied to the orthopantomographic device. Images of 200 healthy subjects aged 2–15 years were classified into five age groups. Then, orthopantomographic images of 17 patients with HPP were evaluated. The corrected pixel values of three-quarters of the patients with odonto HPP were lower than the mean value of the healthy group. One-third of patients treated with ERT showed higher corrected pixel values than the healthy group. These results suggest that odonto HPP without skeletal problems is occasionally accompanied by hypomineralization of alveolar bone and that alveolar bone hypomineralization in patients with severe HPP may be improved by ERT. When HPP patients undergo prosthodontic rehabilitation, such as dental implants or orthodontic treatment, the status of alveolar bone is important. This evaluation method may be useful for dental diagnosis in the future.
7. Importance of early detection of HPP in the dentistry field
To detect HPP at an early stage in the field of dentistry, it is important to raise awareness of this disease among general dentists and to establish a diagnostic system based on medical/dental collaboration. In cases of severe HPP, the disease is diagnosed in the medical field before or immediately after birth and follow-up is initiated. Then, when the primary teeth emerge into the oral cavity, the patients are referred to a dentist and oral management begins. However, there are potentially still many cases of HPP that have not yet been diagnosed because the systemic symptoms do not affect daily life or there are no subjective symptoms at all in mild cases, such as childhood and odonto types [26], [100], [101], [102], [103], [104].
HPP is a progressive disease in some patients, so even odonto HPP patients with only early primary tooth loss could exhibit systemic problems, such as decreased motor function or bone pain, as they grow up [26]. In addition, cases of HPP diagnosed as adult type often have a history of early primary tooth loss [105], [106], [107], [108], which could have been classified into the odonto type if they had been diagnosed at the time when only dental symptoms were present. Notably, adult HPP cases are sometimes misdiagnosed as “osteoporosis” and have been reported to have received bisphosphonates, which can worsen the symptoms of HPP [109], [110]. For these reasons, it is important that suspected cases of HPP be screened in the dental field as early as possible, leading to early diagnosis in the medical field, so that growth and development can be managed and treatment can be received at an appropriate time.
Although the possibility of encountering HPP cases in daily clinical practice may be low, there are children who can be saved if a deeper understanding of HPP is developed among dentists. When encountering cases of early primary tooth loss, it is important to refer them to pediatricians who specialize in bone diseases. In particular, the root shape of the exfoliated tooth is one of the clues to screen possible HPP cases, so if cases of primary tooth loss are encountered, it is important to ask the parents/guardians to bring the primary tooth to enable analysis of this tooth. In addition, HPP cases detected in the dentistry field are always of the mild form, but these include not only the odonto type but also the childhood or adult type, occasionally with the presence of physical symptoms [27], [39]. From this perspective, early diagnoses with the finding of early exfoliation of primary teeth are very important.
HPP is recognized as an extremely rare disease, and therefore dentists often do not suspect it when they encounter cases of primary tooth loss. Moreover, comments from the parents that a tooth has fallen out due to dental trauma can often be an obstacle to recognizing HPP cases. Meanwhile, if dentists ask pediatricians to examine suspected cases of HPP, pediatricians who do not specialize in bone diseases may not consider the possibility of this rare disease. Furthermore, it is not fully recognized that the standard ALP values for pediatric patients are much higher than those for adults, so even if the test data show low values, it is possible that these are not recognized as abnormally low values [27]. In addition, the ALP activity test was converted from the Japan Society of Clinical Chemistry (JSCC) method to the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) method in April 2020. The ALP values in the IFCC method are approximately one-third of those in the JSCC method, so we expect that low values could be more easily recognized from this change.
Educational activities to raise awareness of HPP among those involved in pediatric oral health have been performed throughout Japan. Recently, the establishment of a system for the early diagnosis of HPP in the dentistry field by adding items to check the early exfoliation of primary teeth in governmental dental examinations at the ages of 1.5 and 3 years old has been progressing in Japan. In fact, not only the odonto type but also children with growth and development problems, such as those with the infantile and child types, can now be screened in the dentistry field [27].
8. Future perspectives
We are undertaking ongoing clinical research in the form of a multicenter study for the quantitative evaluation of dental manifestations in HPP patients to examine the dental effects of asfotase alfa [97]. On the basis of accumulated evidence from similar studies, criteria for the introduction of ERT for mild HPP, such as childhood and odonto types, can be discussed while considering the expected benefits and potential risks in the near future [35], [41]. The main disadvantage of systematic treatment by ERT is that it requires repeated injections, which places a burden on patients. Meanwhile, it is possible to limit the period of ERT for mild HPP depending on the results of clinical studies, if it is performed to resolve dental problems. In mild forms of HPP, asfotase alfa may be reduced or stopped; however, evaluation of the effects of this on the therapeutic outcome is insufficient, and there is little evidence to support this [41]. In the dental field, it has been suggested that there may be a need for a dosing period that takes into account the growth of teeth and jawbone, especially for mild forms of HPP.
Asfotase alfa was approved in Japan in 2015, ahead of the rest of the world, and children with perinatal HPP who were saved by ERT since that time are now in the stage of mixed dentition, in which permanent teeth are starting to erupt [30], [38]. Dentists thus have no prior experience of dental management of permanent dentition in perinatal HPP treated with ERT. These children are expected to have various dental problems, such as poor periodontal status, tooth hypomineralization, occlusion, and obstructed feeding and swallowing [38], which should be clarified and for which appropriate treatment approaches should be established. As for cases with severe HPP, it is necessary to accumulate cases to develop better dental management for these children to improve their QOL.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Footnotes
Scientific field of dental science: Pediatric dentistry
References
- 1.Rathbun J.C. Hypophosphatasia; a new developmental anomaly. Am J Dis Child. 1948;75(6):822–831. doi: 10.1001/archpedi.1948.02030020840003. [DOI] [PubMed] [Google Scholar]
- 2.Sobel E.H., Clark L.C., Jr., Fox R.P., Robinow M. Rickets, deficiency of alkaline phosphatase activity and premature loss of teeth in childhood. Pediatrics. 1953;11(4):309–322. [PubMed] [Google Scholar]
- 3.Mornet E. Hypophosphatasia. Orphanet J Rare Dis. 2007;2:40. doi: 10.1186/1750-1172-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mornet E. Hypophosphatasia. Best Pr Res Clin Rheuma. 2008;22(1):113–127. doi: 10.1016/j.berh.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Millán J.L., Plotkin H. Hypophosphatasia - pathophysiology and treatment. Actual Osteol. 2012;8(3):164–182. [PMC free article] [PubMed] [Google Scholar]
- 6.Whyte M.P., Zhang F., Wenkert D., McAlister W.H., Mack K.E., Benigno M.C., et al. Hypophosphatasia: validation and expansion of the clinical nosology for children from 25 years experience with 173 pediatric patients. Bone. 2015;75:229–239. doi: 10.1016/j.bone.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi M.L. Hypophosphatasia: an overview of the disease and its treatment. Osteoporos Int. 2015;26(12):2743–2757. doi: 10.1007/s00198-015-3272-1. [DOI] [PubMed] [Google Scholar]
- 8.Whyte M.P. Hypophosphatasia - aetiology, nosology, pathogenesis, diagnosis and treatment. Nat Rev Endocrinol. 2016;12(4):233–246. doi: 10.1038/nrendo.2016.14. [DOI] [PubMed] [Google Scholar]
- 9.Whyte M.P., Wenkert D., Zhang F. Hypophosphatasia: natural history study of 101 affected children investigated at one research center. Bone. 2016;93:125–138. doi: 10.1016/j.bone.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Linglart A., Biosse-Duplan M. Hypophosphatasia. Curr Osteoporos Rep. 2016;14(3):95–105. doi: 10.1007/s11914-016-0309-0. [DOI] [PubMed] [Google Scholar]
- 11.Millán J.L., Whyte M.P. Alkaline phosphatase and hypophosphatasia. Calcif Tissue Int. 2016;98(4):398–416. doi: 10.1007/s00223-015-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whyte M.P. Hypophosphatasia: An overview for 2017. Bone. 2017;102:15–25. doi: 10.1016/j.bone.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Mornet E. Hypophosphatasia. Metabolism. 2018;82:142–155. doi: 10.1016/j.metabol.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Choida V., Bubbear J.S. Update on the management of hypophosphatasia. Ther Adv Musculoskelet Dis. 2019;11:1–8. doi: 10.1177/1759720X19863997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szabo S.M., Tomazos I.C., Petryk A., Powell L.C., Donato B.M.K., Zarate Y.A., et al. Frequency and age at occurrence of clinical manifestations of disease in patients with hypophosphatasia: a systematic literature review. Orphanet J Rare Dis. 2019;14(1):85. doi: 10.1186/s13023-019-1062-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bangura A., Wright L., Shuler T. Hypophosphatasia: current literature for pathophysiology, clinical manifestations, diagnosis, and treatment. Cureus. 2020;12(6):8594. doi: 10.7759/cureus.8594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salles J.P. Hypophosphatasia: Biological and clinical aspects, avenues for therapy. Clin Biochem Rev. 2020;41(1):13–27. doi: 10.33176/AACB-19-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tournis S., Yavropoulou M.P., Polyzos S.A., Doulgeraki A. Hypophosphatasia. J Clin Med. 2021;10(23):5676. doi: 10.3390/jcm10235676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villa-Suárez J.M., García-Fontana C., Andújar-Vera F., González-Salvatierra S., de Haro-Muñoz T., Contreras-Bolívar V., et al. Hypophosphatasia: A unique disorder of bone mineralization. Int J Mol Sci. 2021;22(9):4303. doi: 10.3390/ijms22094303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reibel A., Manière M.C., Clauss F., Droz D., Alembik Y., Mornet E., et al. Orodental phenotype and genotype findings in all subtypes of hypophosphatasia. Orphanet J Rare Dis. 2009;4:6. doi: 10.1186/1750-1172-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haliloglu B., Guran T., Atay Z., Abali S., Mornet E., Bereket A., et al. Infantile loss of teeth: odontohypophosphatasia or childhood hypophosphatasia. Eur J Pedia. 2013;172(6):851–853. doi: 10.1007/s00431-012-1868-4. [DOI] [PubMed] [Google Scholar]
- 22.Okawa R., Nakano K., Matsumoto M., Kawabata K., Ooshima T. Oral manifestations of patients with hypophosphatasia. Ped Dent J. 2012;22(2):155–162. [Google Scholar]
- 23.Hollis A., Arundel P., High A., Balmer R. Current concepts in hypophosphatasia: case report and literature review. Int J Paediatr Dent. 2013;23(3):153–159. doi: 10.1111/j.1365-263X.2012.01239.x. [DOI] [PubMed] [Google Scholar]
- 24.Bloch-Zupan A. Hypophosphatasia: diagnosis and clinical signs - a dental surgeon perspective. Int J Paediatr Dent. 2016;26:426–438. doi: 10.1111/ipd.12232. [DOI] [PubMed] [Google Scholar]
- 25.Okawa R., Kitaoka T., Saga K., Ozono K., Nakano K. Report of two dental patients diagnosed with hypophosphatasia. J Clin Case Rep. 2016;06:2. [Google Scholar]
- 26.Mori M., DeArmey S.L., Weber T.J., Kishnani P.S. Case series: Odontohypophosphatasia or missed diagnosis of childhood/adult-onset hypophosphatasia? Call for a long-term follow-up of premature loss of primary teeth. Bone Rep. 2016;5:228–232. doi: 10.1016/j.bonr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okawa R., Kadota T., Matayoshi S., Nakano K. Dental manifestations leading to the diagnosis of hypophosphatasia in two children. J Dent Child (Chic) 2020;87(3):179–183. [PubMed] [Google Scholar]
- 28.Whyte M.P., Greenberg C.R., Salman N.J., Bober M.B., McAlister W.H., Wenkert D., et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med. 2012;366(10):904–913. doi: 10.1056/NEJMoa1106173. [DOI] [PubMed] [Google Scholar]
- 29.Whyte M.P., Rockman-Greenberg C., Ozono K., Riese R., Moseley S., Melian A., et al. Asfotase alfa treatment improves survival for perinatal and infantile hypophosphatasia. J Clin Endocrinol Metab. 2016;101(1):334–342. doi: 10.1210/jc.2015-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okazaki Y., Kitajima H., Mochizuki N., Kitaoka T., Michigami T., Ozono K. Lethal hypophosphatasia successfully treated with enzyme replacement from day 1 after birth. Eur J Pedia. 2016;175:433–437. doi: 10.1007/s00431-015-2641-2. [DOI] [PubMed] [Google Scholar]
- 31.Whyte M.P., Madson K.L., Phillips D., Reeves A.L., McAlister W.H., Yakimoski A., et al. Asfotase alfa therapy for children with hypophosphatasia. JCI Insight. 2016;1:85971. doi: 10.1172/jci.insight.85971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Orimo H. Pathophysiology of hypophosphatasia and the potential role of asfotase alfa. Ther Clin Risk Manag. 2016;12:777–786. doi: 10.2147/TCRM.S87956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitaoka T., Tajima T., Nagasaki K., Kikuchi T., Yamamoto K., Michigami T., et al. Safety and efficacy of treatment with asfotase alfa in patients with hypophosphatasia: Results from a Japanese clinical trial. Clin Endocrinol. 2017;87:10–19. doi: 10.1111/cen.13343. [DOI] [PubMed] [Google Scholar]
- 34.Kishnani P.S., Rush E.T., Arundel P., Bishop N., Dahir K., Fraser W., et al. Monitoring guidance for patients with hypophosphatasia treated with asfotase alfa. Mol Genet Metab. 2017;122(1–2):4–17. doi: 10.1016/j.ymgme.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Whyte M.P. Hypophosphatasia: Enzyme replacement therapy brings new opportunities and new challenges. J Bone Min Res. 2017;32(4):667–675. doi: 10.1002/jbmr.3075. [DOI] [PubMed] [Google Scholar]
- 36.Bowden S.A., Foster B.L. Profile of asfotase alfa in the treatment of hypophosphatasia: design, development, and place in therapy. Drug Des Devel Ther. 2018;12:3147–3161. doi: 10.2147/DDDT.S154922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whyte M.P., Simmons J.H., Moseley S., Fujita K.P., Bishop N., Salman N.J., et al. Asfotase alfa for infants and young children with hypophosphatasia: 7 year outcomes of a single-arm, open-label, phase 2 extension trial. Lancet Diabetes Endocrinol. 2019;7(2):93–105. doi: 10.1016/S2213-8587(18)30307-3. [DOI] [PubMed] [Google Scholar]
- 38.Okawa R., Kokomoto K., Yamaura-Miyazaki N., Michigami T., Nakano K. Oral findings in patient with lethal hypophosphatasia with enzyme replacement therapy. Ped Dent J. 2017;27(3):153–156. [Google Scholar]
- 39.Okawa R., Miura J., Kokomoto K., Nakano K. Evaluation of avulsed primary incisor in 3-year-old girl with hypophosphatasia who received enzyme replacement therapy. Ped Dent J. 2018;28(3):136–140. [Google Scholar]
- 40.Okawa R., Kokomoto K., Kitaoka T., Kubota T., Watanabe A., Taketani T., et al. Japanese nationwide survey of hypophosphatasia reveals prominent differences in genetic and dental findings between odonto and non-odonto types. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0222931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michigami T., Ohata Y., Fujiwara M., Mochizuki H., Adachi M., Kitaoka T., et al. Clinical practice guidelines for hypophosphatasia. Clin Pedia Endocrinol. 2020;29(1):9–24. doi: 10.1297/cpe.29.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenkert D., McAlister W.H., Coburn S.P., Zerega J.A., Ryan L.M., Ericson K.L., et al. Hypophosphatasia: nonlethal disease despite skeletal presentation in utero (17 new cases and literature review) J Bone Min Res. 2011;26:2389–2398. doi: 10.1002/jbmr.454. [DOI] [PubMed] [Google Scholar]
- 43.Fraser D. Hypophosphatasia. Am J Med. 1957;22(5):730–746. doi: 10.1016/0002-9343(57)90124-9. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe A., Karasugi T., Sawai H., Naing B.T., Ikegawa S., Orimo H., et al. Prevalence of c.1559delT in ALPL, a common mutation resulting in the perinatal (lethal) form of hypophosphatasia in Japanese and effects of the mutation on heterozygous carriers. J Hum Genet. 2011;56(2):166–168. doi: 10.1038/jhg.2010.161. [DOI] [PubMed] [Google Scholar]
- 45.Ozono K., Michigami T. Hypophosphatasia now draws more attention of both clinicians and researchers: a commentary on Prevalence of c. 1559delT in ALPL, a common mutation resulting in the perinatal (lethal) form of hypophosphatasias in Japanese and effects of the mutation on heterozygous carriers. J Hum Genet. 2011;56(3):174–176. doi: 10.1038/jhg.2011.6. [DOI] [PubMed] [Google Scholar]
- 46.Taketani T., Onigata K., Kobayashi H., Mushimoto Y., Fukuda S., Yamaguchi S. Clinical and genetic aspects of hypophosphatasia in Japanese patients. Arch Dis Child. 2014;99(3):211–215. doi: 10.1136/archdischild-2013-305037. [DOI] [PubMed] [Google Scholar]
- 47.Mornet E., Taillandier A., Domingues C., Dufour A., Benaloun E., Lavaud N., et al. Hypophosphatasia: a genetic-based nosology and new insights in genotype-phenotype correlation. Eur J Hum Genet. 2021;29(2):289–299. doi: 10.1038/s41431-020-00732-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michigami T., Tachikawa K., Yamazaki M., Kawai M., Kubota T., Ozono K. Hypophosphatasia in Japan: ALPL mutation analysis in 98 unrelated patients. Calcif Tissue Int. 2020;106(3):221–231. doi: 10.1007/s00223-019-00626-w. [DOI] [PubMed] [Google Scholar]
- 49.Michigami T., Uchihashi T., Suzuki A., Tachikawa K., Nakajima S., Ozono K. Common mutations F310L and T1559del in the tissue-nonspecific alkaline phosphatase gene are related to distinct phenotypes in Japanese patients with hypophosphatasia. Eur J Pedia. 2005;164(5):277–282. doi: 10.1007/s00431-004-1612-9. [DOI] [PubMed] [Google Scholar]
- 50.Orimo H., Goseki-Sone M., Inoue M., Tsubakio Y., Sakiyama T., Shimada T. Importance of deletion of T at nucleotide 1559 in the tissue-nonspecific alkaline phosphatase gene in Japanese patients with hypophosphatasia. J Bone Min Metab. 2002;20(1):28–33. doi: 10.1007/s774-002-8443-4. [DOI] [PubMed] [Google Scholar]
- 51.Mornet E., Yvard A., Taillandier A., Fauvert D., Simon-Bouy B. A molecular-based estimation of the prevalence of hypophosphatasia in the European population. Ann Hum Genet. 2011;75(3):439–445. doi: 10.1111/j.1469-1809.2011.00642.x. [DOI] [PubMed] [Google Scholar]
- 52.Fauvert D., Brun-Heath I., Lia-Baldini A.S., Bellazi L., Taillandier A., Serre J.L., et al. Mild forms of hypophosphatasia mostly result from dominant negative effect of severe alleles or from compound heterozygosity for severe and moderate alleles. BMC Med Genet. 2009;10:51. doi: 10.1186/1471-2350-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schour I., Massler M. The development of the human dentition. J Am Dent Assoc. 1941;28:1153–1160. [Google Scholar]
- 54.Beumer J., 3rd, Trowbridge H.O., Silverman S., Jr., Eisenberg E. Childhood hypophosphatasia and the premature loss of teeth. A clinical and laboratory study of seven cases. Oral Surg Oral Med Oral Pathol. 1973;35(5):631–640. doi: 10.1016/0030-4220(73)90028-5. [DOI] [PubMed] [Google Scholar]
- 55.Kjellman M., Oldfelt V., Nordenram A., Olow-Nordenram M. Five cases of hypophosphatasia with dental findings. Int J Oral Surg. 1973;2(4):152–158. doi: 10.1016/s0300-9785(73)80032-8. [DOI] [PubMed] [Google Scholar]
- 56.Baab D.A., Page R.C., Morton T. Studies of a family manifesting premature exfoliation of deciduous teeth. J Periodo. 1985;56(7):403–409. doi: 10.1902/jop.1985.56.7.403. [DOI] [PubMed] [Google Scholar]
- 57.Feeney C., Stanford N., Lee S., Barry S. Hypophosphatasia and the importance of the general dental practitioner - a case series and discussion of upcoming treatments. Br Dent J. 2018;224(12):937–943. doi: 10.1038/sj.bdj.2018.441. [DOI] [PubMed] [Google Scholar]
- 58.Beertsen W., McCulloch C.A., Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000. 1997;13:20–40. doi: 10.1111/j.1600-0757.1997.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 59.Foster B.L., Nagatomo K.J., Nociti FH Jr, Jr., Fong H., Dunn D., Tran A.B., et al. Central role of pyrophosphate in acellular cementum formation. PLoS One. 2012;7(6):38393. doi: 10.1371/journal.pone.0038393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van den Bos T., Handoko G., Niehof A., Ryan L.M., Coburn S.P., Whyte M.P., et al. Cementum and dentin in hypophosphatasia. J Dent Res. 2005;84(11):1021–1025. doi: 10.1177/154405910508401110. [DOI] [PubMed] [Google Scholar]
- 61.el-Labban N.G., Lee K.W., Rule D. Permanent teeth in hypophosphatasia: light and electron microscopic study. J Oral Pathol Med. 1991;20(7):352–360. doi: 10.1111/j.1600-0714.1991.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 62.Okawa R., Miura J., Kokomoto K., Kubota T., Kitaoka T., Ozono K., et al. Early exfoliation of permanent tooth in patient with hypophosphatasia. Ped Dent J. 2017;27(3):173–178. [Google Scholar]
- 63.Eberle F., Hartenfels S., Pralle H., Käbisch A. Adult hypophosphatasia without apparent skeletal disease: “odontohypophosphatasia” in four heterozygote members of a family. Klin Woche. 1984;62:371–376. doi: 10.1007/BF01716257. [DOI] [PubMed] [Google Scholar]
- 64.Macfarlane J.D., Swart J.G. Dental aspects of hypophosphatasia: a case report, family study, and literature review. Oral Surg Oral Med Oral Pathol. 1989;67:521–526. doi: 10.1016/0030-4220(89)90266-1. [DOI] [PubMed] [Google Scholar]
- 65.Watanabe H., Umeda M., Seki T., Ishikawa I. Clinical and laboratory studies of severe periodontal disease in an adolescent associated with hypophosphatasia. A case report. J Periodo. 1993;64:174–180. doi: 10.1902/jop.1993.64.3.174. [DOI] [PubMed] [Google Scholar]
- 66.Olsson A., Matsson L., Blomquist H.K., Larsson A., Sjödin B. Hypophosphatasia affecting the permanent dentition. J Oral Pathol Med. 1996;25(6):343–347. doi: 10.1111/j.1600-0714.1996.tb00274.x. [DOI] [PubMed] [Google Scholar]
- 67.Wang Z.Y., Zhang K., Zheng G.S., Qiao W., Su Y.X. Current concepts in odontohypophosphatasia form of hypophosphatasia and report of two cases. BMC Oral Health. 2016;16:70. doi: 10.1186/s12903-016-0266-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamada M., Okawa R., Matayoshi S., Ogaya Y., Nomura R., Uzawa N., et al. Ankylosed primary molar in a Japanese child with hypophosphatasia. Dent J. 2020;9(1):3. doi: 10.3390/dj9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saxby M.S. Juvenile periodontitis: an epidemiological study in the west Midlands of the United Kingdom. J Clin Periodo. 1987;14:594–598. doi: 10.1111/j.1600-051x.1987.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 70.Albandar J.M., Tinoco E.M. Global epidemiology of periodontal diseases in children and young persons. Periodontol 2000. 2002;29:153–176. doi: 10.1034/j.1600-0757.2002.290108.x. [DOI] [PubMed] [Google Scholar]
- 71.Albandar J.M., Rams T.E. Risk factors for periodontitis in children and young persons. Periodontol 2000. 2002;29:207–222. doi: 10.1034/j.1600-0757.2002.290110.x. [DOI] [PubMed] [Google Scholar]
- 72.Oh T.J., Eber R., Wang H.L. Periodontal diseases in the child and adolescent. Clin Periodo. 2002;29(5):400–410. doi: 10.1034/j.1600-051x.2002.290504.x. [DOI] [PubMed] [Google Scholar]
- 73.Gomes-Filho I.S., Miranda D.A., Trindade S.C., de Souza Teles Santos C.A., de Freitas C.O., da Cruz S.S., et al. Relationship among gender, race, age, gingival width, and probing depth in primary teeth. J Periodo. 2006;77(6):1032–1042. doi: 10.1902/jop.2006.050198. [DOI] [PubMed] [Google Scholar]
- 74.Nadelman P., Bedran N., Magno M.B., Masterson D., de Castro A.C.R., Maia L.C. Premature loss of primary anterior teeth and its consequences to primary dental arch and speech pattern: a systematic review and meta-analysis. Int J Paediatr Dent. 2020;30(6):687–712. doi: 10.1111/ipd.12644. [DOI] [PubMed] [Google Scholar]
- 75.Kydd W.L., Akamine J.S., Mendel R.A., Kraus B.S. Tongue and lip forces exerted during deglutiton in subjects with and without an anterior open bite. J Dent Res. 1963;42:858–866. doi: 10.1177/00220345630420031801. [DOI] [PubMed] [Google Scholar]
- 76.Kadota T., Okawa R., Otsugu M., Ohata J., Hanaoka I., Nakano K. Mouthguards for a childhood hypophosphatasia patient to protect periodontal tissue of immature permanent teeth – case report. Ped Dent J. 2021;31(1):117–122. [Google Scholar]
- 77.Bağiş B., Baltacioğlu E., Aydoğan E., Tamam E. Prosthetic rehabilitation of hypophosphatasia: a case report. Cases J. 2008;2:7626. doi: 10.1186/1757-1626-2-7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lynch C.D., Ziada H.M., Buckley L.A., O’Sullivan V.R., Aherne T., Aherne S. Prosthodontic rehabilitation of hypophosphatasia using dental implants: a review of the literature and two case reports. J Oral Rehabil. 2009;36(6):462–468. doi: 10.1111/j.1365-2842.2009.01948.x. [DOI] [PubMed] [Google Scholar]
- 79.Grewal P.S., Gupta K.P. Prosthetic rehabilitation of a young patient with Hypophosphatasia - a review and case report. Conte Clin Dent. 2012;3(1):74–77. doi: 10.4103/0976-237X.94551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suvarna G.S., Nadiger R.K., Guttal S.S., Shetty O. Prosthetic rehabilitation of hypophosphatasia with precision attachment retained unconventional partial denture: a case report. J Clin Diagn Res. 2014;8(12):ZD08–ZD10. doi: 10.7860/JCDR/2014/9446.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang Y., Liu Z., Wei L., Taylor T.D., Xiao H. Prosthodontic rehabilitation of a patient with hypophosphatasia using dental implants: a case report with seven years follow-up. J Prosthodont. 2021;30(9):742–746. doi: 10.1111/jopr.13419. [DOI] [PubMed] [Google Scholar]
- 82.Narisawa S., Fröhlander N., Millán J.L. Inactivation of two mouse alkaline phosphatase genes and established of a model of infantile hypophosphatasia. Dev Dyn. 1997;208:432–446. doi: 10.1002/(SICI)1097-0177(199703)208:3<432::AID-AJA13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 83.Fedde K.N., Blair L., Silverstein J., Coburn S.P., Ryan L.M., Weinstein R.S., et al. Alkaline phosphatase knock-out mice recapitulate the metabolic and skeletal defects of infantile hypophosphatasia. J Bone Min Res. 1999;14(12):2015–2026. doi: 10.1359/jbmr.1999.14.12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McKee M.D., Nakano Y., Masica D.L., Gray J.J., Lemire I., Heft R., et al. Enzyme replacement therapy prevents dental defects in a model of hypophosphatasia. J Dent Res. 2011;90(4):470–476. doi: 10.1177/0022034510393517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okawa R., Iijima O., Kishino M., Okawa H., Toyosawa S., Sugano-Tajima H., et al. Gene therapy improves dental manifestations in hypophosphatasia model mice. J Periodontal Res. 2017;52(3):471–478. doi: 10.1111/jre.12412. [DOI] [PubMed] [Google Scholar]
- 86.Millán J.L., Narisawa S., Lemire I., Loisel T.P., Boileau G., Leonard P., et al. Enzyme replacement therapy for murine hypophosphatasia. J Bone Min Res. 2008;23(6):777–787. doi: 10.1359/JBMR.071213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yadav M.C., de Oliveira R.C., Foster B.L., Fong H., Cory E., Narisawa S., et al. Enzyme replacement prevents enamel defects in hypophosphatasia. J Bone Min Res. 2012;27:1722–1734. doi: 10.1002/jbmr.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Foster B.L., Nagatomo K.J., Tso H.W., Tran A.B., Nociti FH Jr, Jr., Narisawa S., et al. Tooth root dentin mineralization defects in a mouse model of hypophosphatasia. J Bone Min Res. 2013;28:271–282. doi: 10.1002/jbmr.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsumoto T., Miyake K., Yamamoto S., Orimo H., Miyake N., Odagaki Y., et al. Rescue of severe infantile hypophosphatasia mice by AAV-mediated sustained expression of soluble alkaline phosphatase. Hum Gene Ther. 2011;22:1355–1364. doi: 10.1089/hum.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamamoto S., Orimo H., Matsumoto T., Iijima O., Narisawa S., Maeda T., et al. Prolonged survival and phenotypic correction of Akp2–/– hypophosphatasia mice by lentiviral gene therapy. J Bone Min Res. 2011;26:135–142. doi: 10.1002/jbmr.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Iijima O., Miyake K., Watanabe A., Miyake N., Igarashi T., Kanokoda C., et al. Prevention of lethal murine hypophosphatasia by neonatal ex vivo gene therapy using lentivirally transduced bone marrow cells. Hum Gene Ther. 2015;26:801–812. doi: 10.1089/hum.2015.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sugano H., Matsumoto T., Miyake K., Watanabe A., Iijima O., Migita M., et al. Successful gene therapy in utero for lethal murine hypophosphatasia. Hum Gene Ther. 2012;23:399–406. doi: 10.1089/hum.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kinoshita Y., Mohamed F.F., Amadeu de Oliveira F., Narisawa S., Miyake K., Foster B.L., et al. Gene therapy using adeno-associated virus serotype 8 ENcoding TNAP-D10 improves the skeletal and dentoalveolar phenotypes in Alpl-/- Mice. J Bone Min Res. 2021;36(9):1835–1849. doi: 10.1002/jbmr.4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Okawa R., Kokomoto K., Nakano K. Dental effects of enzyme replacement therapy in case of childhood-type hypophosphatasia. BMC Oral Health. 2021;21(1):323. doi: 10.1186/s12903-021-01673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okawa R., Matayoshi S., Kariya R., Ogaya Y., Nomura R., Nakano K. Effects of enzyme replacement therapy for primary teeth in a patient with infantile hypophosphatasia. J Clin Pedia Dent. 2020;44(5):348–351. doi: 10.17796/1053-4625-44.5.9. [DOI] [PubMed] [Google Scholar]
- 96.Kiselnikova L., Vislobokova E., Voinova V. Dental manifestations of hypophosphatasia in children and the effects of enzyme replacement therapy on dental status: A series of clinical cases. Clin Case Rep. 2020;8(5):911–918. doi: 10.1002/ccr3.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Takagi M., Kato S., Muto T., Sano Y., Akiyama T., Takagi J., et al. Odontohypophosphatasia treated with asfotase alfa enzyme replacement therapy in a toddler: a case report. Clin Pedia Endocrinol. 2020;29(3):115–118. doi: 10.1297/cpe.29.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schroth R.J., Long C., Lee V.H.K., Alai-Towfigh H., Rockman-Greenberg C. Dental outcomes for children receiving asfotase alfa for hypophosphatasia. Bone. 2021;152 doi: 10.1016/j.bone.2021.116089. [DOI] [PubMed] [Google Scholar]
- 99.Okawa R., Nakamoto T., Matayoshi S., Nakano K., Kakimoto N. Evaluation of alveolar bone hypomineralization in pediatric hypophosphatasia using orthopantomography. Sci Rep. 2022;12(1):1211. doi: 10.1038/s41598-022-05171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galeano-Valle F., Vengoechea J., Galindo R.J. A rare mutation in hypophosphatasia: a case report of adult form and review of the literature. Arch Endocrinol Metab. 2019;63(1):89–93. doi: 10.20945/2359-3997000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Högler W., Langman C., Gomes da Silva H., Fang S., Linglart A., Ozono K., et al. Diagnostic delay is common among patients with hypophosphatasia: initial findings from a longitudinal, prospective, global registry. BMC Musculoskelet Disord. 2019;20(1):80. doi: 10.1186/s12891-019-2420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vogt M., Girschick H., Schweitzer T., Benoit C., Holl-Wieden A., Seefried L., et al. Pediatric hypophosphatasia: lessons learned from a retrospective single-center chart review of 50 children. Orphanet J Rare Dis. 2020;15(1):212. doi: 10.1186/s13023-020-01500-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schmidt T., Schmidt C., Amling M., Kramer J., Barvencik F. Prevalence of low alkaline phosphatase activity in laboratory assessment: Is hypophosphatasia an underdiagnosed disease? Orphanet J Rare Dis. 2021;16(1):452. doi: 10.1186/s13023-021-02084-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.İnci A., Ergin F.B.C., Yüce B.T., Çiftçi B., Demir E., Buyan N., et al. Hypophosphatasia: is it an underdiagnosed disease even by expert physicians? J Bone Min Metab. 2021;39(4):598–605. doi: 10.1007/s00774-020-01193-z. [DOI] [PubMed] [Google Scholar]
- 105.Berkseth K.E., Tebben P.J., Drake M.T., Hefferan T.E., Jewison D.E., Wermers R.A. Clinical spectrum of hypophosphatasia diagnosed in adults. Bone. 2013;54(1):21–27. doi: 10.1016/j.bone.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 106.Whyte M.P., Leelawattana R., Reinus W.R., Yang C., Mumm S., Novack D.V. Acute severe hypercalcemia after traumatic fractures and immobilization in hypophosphatasia complicated by chronic renal failure. J Clin Endocrinol Metab. 2013;98(12):4606–4612. doi: 10.1210/jc.2013-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Iqbal U., Anwar H., Chaudhary A., Alvi M., Freeth A. Recurrent metatarsal fractures in postmenopausal woman with low serum alkaline phosphatase: a rare diagnosis not to miss. J Invest Med High Impact Case Rep. 2017;5(3) doi: 10.1177/2324709617718851. 2324709617718851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shapiro J.R., Lewiecki E.M. Hypophosphatasia in adults: clinical assessment and treatment considerations. J Bone Min Res. 2017;32(10):1977–1980. doi: 10.1002/jbmr.3226. [DOI] [PubMed] [Google Scholar]
- 109.Sutton R.A., Mumm S., Coburn S.P., Ericson K.L., Whyte M.P. “Atypical femoral fractures” during bisphosphonate exposure in adult hypophosphatasia. J Bone Min Res. 2012;27(5):987–994. doi: 10.1002/jbmr.1565. [DOI] [PubMed] [Google Scholar]
- 110.Bianchi M.L., Bishop N.J., Guañabens N., Hofmann C., Jakob F., Roux C., et al. Hypophosphatasia in adolescents and adults: overview of diagnosis and treatment. Rare bone disease action group of the european calcified tissue society. Osteoporos Int. 2020;31(8):1445–1460. doi: 10.1007/s00198-020-05345-9. [DOI] [PubMed] [Google Scholar]