Abstract
B-type RAF (BRAF)-V600E mutations in metastatic colorectal cancer (mCRC) have been described in up to 12% of the patients. This mutation confers a bad prognostic and poor response with standard chemotherapy. Unlike the scenario for BRAF mutant melanoma, successful BRAF blockade in mCRC has emerged as a complex path, primarily due to the complex underlying biology of mCRC. The BEACON trial has reshaped the therapeutic landscape of BRAF mCRC demonstrating the benefit of the BRAF inhibitor encorafenib in combination with the anti-epidermal growth factor receptor cetuximab. This paper aims to review the main features of BRAF mCRC as well as to review the development of targeted therapy and biomarkers in this specific population. Finally, a deep insight into the underlying biology and molecular classification of BRAF-V600E mCRC has also been performed. The words ‘BRAF-V600E mutation’, ‘colorectal cancer’, ‘BRAF inhibitors’, ‘consensus molecular subtypes’, ‘encorafenib’, and ‘cetuximab’ were used to identify the clinical trials from phase I to phase III related to the development of BRAF inhibitors in this population. A deep search among international meetings (American Society of Clinical Oncology and European Society of Medical Oncology) has been performed to incorporate the last trials presented. BRAF-V600E mCRC is a challenging disease, mostly because of its molecular biology. The BEACON trial has been the most important therapeutic change in the last decade. Nevertheless, new information regarding biomarkers or novel combinations including BRAF inhibitors plus immune checkpoint inhibitors are also promising.
Keywords: BRAF mutation, cetuximab, colorectal cancer, consensus molecular subtype, encorafenib, transcriptomic signatures
Introduction
Worldwide, colorectal cancer (CRC) is the third most common cancer and the second highest cause of cancer-related mortality in the United States with almost 150,000 new cases and around 53,000 deaths per year. CRC is a highly heterogeneous disease characterized by multiple genetic alterations with a range of prognoses, and with different responses to targeted agents.1,2 In recent years, substantial advances have been made regarding personalized treatments in metastatic CRC (mCRC). New agents targeting the B-type RAF (BRAF)-V600E mutation, HER2 amplification, the KRAS G12C mutation, and microsatellite instability (MSI) have all proved successful in certain sub-populations. For most patients with mCRC, cytotoxic chemotherapy with 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) and 5-fluorouracil, leucovorin, and irinotecan (FOLFIRI) in combination with monoclonal antibodies [anti-epidermal growth factor receptor (EGFR) or anti-vascular endothelial growth factor (VEGF)] remains the backbone of care, for upfront therapy at a minimum. The RAS/RAF/MEK/ERK pathway has been particularly implicated in the pathogenesis of mCRC. BRAF alterations are driver mutations leading to constitutive activation of the BRAF kinase and sustained MAPK/ERK signaling, resulting in increased cell proliferation, spread, and cancer cell survival. In the particular case of BRAF-V600E mCRC, outstanding advances have been made in the last few years despite that this mutation is associated with a poor prognosis and lack of response to standard chemotherapy compared to BRAF wild-type counterparts. Unlike the scenario for BRAF mutant melanoma, successful BRAF blockade in mCRC has emerged as a complex path, primarily due to the complex underlying biology of mCRC. Here, we review the most relevant clinical trials leading up to the first phase III trial, the BEACON trial, that successfully demonstrated the utility of BRAF blockade with encorafenib plus cetuximab in BRAF-V600E mCRC. We also review novel ongoing therapeutic approaches and potential predictive and prognostic biomarkers.
The molecular landscape of BRAF-V600E mutations in CRC
BRAF mutations in mCRC have been described in 8–12% of these patients, and exon 15 T1799A transversion resulting in a valine amino acid substitution, is the most frequent alteration (95% of these cases). This leads to constitutive activation of the BRAF kinase resulting in cancer progression. This mutation is associated with poor prognosis, with a median overall survival (OS) of 11 months, and poor response to standard chemotherapy.3–5 Colorectal tumors with BRAF-V600E mutation exhibit a well-defined phenotype; they are more frequent among older females, in right-sided mucinous tumors, and are associated with nodal and peritoneal metastases. From a molecular perspective, this mutation is nearly always mutually exclusive with KRAS mutations, while 30% present MSI.6,7 The BRAF-V600E mutation is associated with the CpG island methylation phenotype (CIMP) which leads to hypermethylation of DNA promotor regions and gene silencing. In the case of BRAF-V600E CRC, the CIMP phenotype is associated with MSI due to silencing of the MLH1 promoter gene caused by hypermethylation, leading to a sporadic MSI phenotype.6,8
Before the development of BRAF inhibitors, upfront treatment recommendations for BRAF-V600E mutant patients came from subgroups of several trials evaluating different chemotherapy regimens. The phase III TRIBE trial compared bevacizumab combined with either 5-fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) or FOLFIRI in the first-line setting. 9 In the subgroup analysis of the 28 BRAF-V600E mutant patients, the FOLFOXIRI plus the anti-VEGF bevacizumab was more active than FOLFIRI plus bevacizumab [median OS, 19.0 versus 10.7 months and median progression-free survival (PFS), 7.5 versus 5.5 months in the triple and double combinations, respectively]. However, these results were not confirmed either in the TRIBE-2 trial 10 or in a subsequent individual patient meta-analysis. 11 The TRIBE-2 trial randomized patients to receive first-line FOLFOX plus bevacizumab followed by FOLFIRI plus bevacizumab after disease progression, or FOLFOXIRI plus bevacizumab followed by the re-introduction of the same regimen after disease progression [PFS-2 hazard ratio (HR) 1.23, 95% confidence interval (CI), 0.72–2.09, p = 0.153; OS HR, 1.35, 95% CI, 0.79–2.30, p = 0.155]. Based on these data, there is currently insufficient evidence supporting the use of the triplet cytotoxic regimen over doublet chemotherapy in front-line treatment of BRAF-V600E-mutated mCRC, and the recommendation of FOLFOXIRI-bevacizumab should be individualized.
In the second-line setting, the phase III VELOUR trial, a prospective, randomized, double-blind study, evaluated the efficacy and safety of another anti-VEGF combination, comparing aflibercept plus FOLFIRI versus placebo plus FOLFIRI in patients with mCRC experiencing disease progression on or after completing an oxaliplatin-based regimen. Analysis of the 36 BRAF-V600E mutant CRC patients gave an OS of 10.3 months with FOLFIRI plus aflibercept. 12
Monoclonal antibodies targeting EGFR have also been tested in the BRAF-V600E population. The presence of BRAF-V600E mutations has been proposed to be a predictive marker for limited response to anti-EGFR therapies in mCRC patients.13–16 Furthermore, a meta-analysis including nine phase III trials that compared cetuximab or panitumumab, and involving 463 BRAF mutant patients, demonstrated that the addition of an anti-EGFR agent to standard therapy did not increase the benefit, for either PFS (HR, 0.88; 95% CI, 0.67–1.14; p = 0.33) or OS (HR, 0.91; 95% CI, 0.62–1.34; p = 0.63). 17 These findings support BRAF mutation assessment before initiation of treatment with anti-EGFR monoclonal antibodies. The phase III CRYSTAL trial evaluated the addition of cetuximab to FOLFIRI. Sub-analysis of BRAF-V600E patients showed that in this population the addition of cetuximab did not result in a statistically significant benefit in terms of PFS or OS. 18 Similar results were reported in a retrospective analysis of the FIRE-3 study, in which patients were randomly assigned to either FOLFIRI plus cetuximab or FOLFIRI plus bevacizumab. Analyses did not confirm an anti-EGFR benefit among BRAF-V600E mCRC patients. 19 Thus, currently, an anti-VEGF in combination with chemotherapy is preferred over chemotherapy plus an anti-EGFR for patients with BRAF-V600E-mutated CRC, for both the upfront and refractory settings. Guidelines on the use of anti-EGFR therapies currently mandate expanded RAS/BRAF testing and that patients with BRAF-V600E mutations should not receive an anti-EGFR either alone or in combination with chemotherapy. 20 While other treatments are recommended in the refractory CRC setting, such as trifluridine/tipiracil or regorafenib, there are no published analyses regarding the activity of these treatments in the BRAF-V600E subgroup.21,22 Promising preclinical data showed synthetically lethal activity of mitotic spindle poisons on BRAF-mutated and BRAF-like CRC models. 23 Based on these data, a phase II trial tested the activity of vinorelbine in patients with BRAF-V600E mCRC. However despite the encouraging preclinical data, the study did not show the signs of clinical activity among the 20 enrolled patients, with an overall response rate (ORR) of 0%, while median PFS and OS were 1.0 and 2.1 months, respectively. 24
Although V600E is the most frequent BRAF mutation in mCRC, several other mutations have been described. A landmark study categorized these mutations based on their oncogenic activity and their ability to activate the ERK pathway. 25 Three different classes of BRAF mutations were described. Class I mutations present with significantly increased kinase activity and operate as monomers. They include V600E, V660K, V600D, V600M, and V600R BRAF mutations. 26 Class II mutations require dimerization with other BRAF oncoproteins leading to a homodimer, are able to activate the ERK pathway without RAS activation, and have an intermediate degree of kinase activity. They include L597Q/R/S/V, G464V/E, G496A/V/R, K601 E/NT, and P367 L/S BRAF mutations. 27 Class III include those mutations with the lowest kinase activity, and function in a RAS-dependent manner. These mutations generate dimerization with cRAF, leading to increased ERK activity. They frequently co-occur with RAS mutations, and include D594G, D594N, G466E, and G466V. Non-V600E BRAF mutations occur less frequently, representing less than 5% of all BRAF mutations. Non-V600E mutations confer similar prognosis as RAS/BRAF wildtype and are more likely to occur with concomitant RAS mutations. Some reports suggest that non-V600E BRAF tumors might benefit from anti-EGFR therapies. 7
Transcriptomic classifications
Two subtypes of BRAF-V600E mCRC tumors have been described in terms of gene expression profile and regardless of MSI status, methylation patterns, PI3CA mutational status, sidedness, or gender. 5 BRAF-V600E mutant subtype 1 (BM1) represents 30% of all BRAF-V600E mutant CRC tumors and is characterized by KRAS/AKT pathway activation, mTOR/4EBP1 deregulation, and epithelial–mesenchymal transition. BM1 also exhibits a strong immune profile (IL2/STAT5/IL6/JAK/STAT3 pathway activation, enriched angiogenesis, and tumor necrosis factor-alpha signaling). The BM1 subtype has a poorer prognosis compared to BM2 subtypes, albeit non-significant, in terms of OS (HR, 1.61; 95% CI, 0.91–2.86; p = 0.106) and relapse-free survival (HR, 1.66; 95% CI, 0.95–2.92; p = 0.076). BM2 represents 70% of all BRAF-V600E mutant CRC tumors and is characterized by dysregulation of the cell cycle and cycle checkpoints. BM2 tumors are enriched in metabolic processes and display high cyclin-dependent kinase 1 and low cyclin-D1 levels.
Also based on transcriptomic classifications, the consensus molecular subtypes (CMS) establish four CRC subgroups. 28 Most BRAF-V600E tumors are included in the CMS1 subtype, which are hypermutated, MSI, and immune-infiltrated. These transcriptomic classifications may help explain differences in response to targeted treatments and identify the potential mechanisms of resistance.
The path toward successful BRAF blockade in CRC
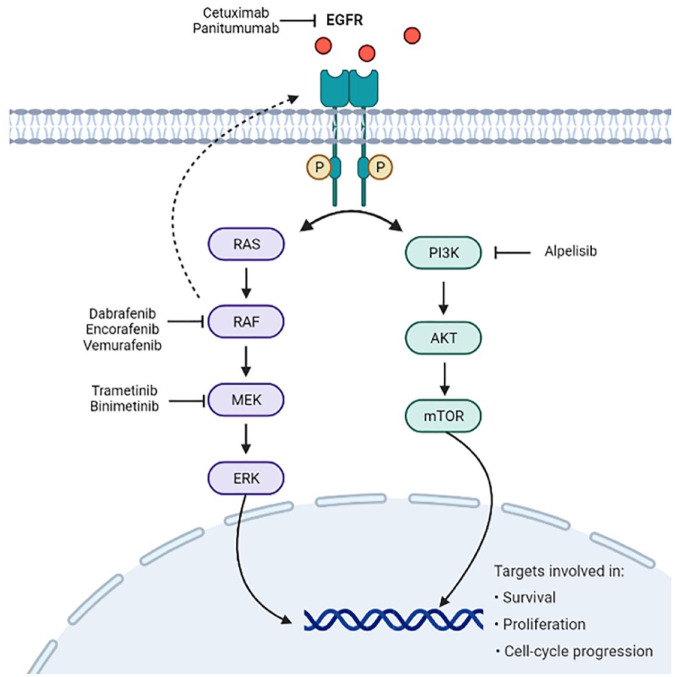
The historical evolution of the management of patients with BRAF-V600E mutant mCRC leading to current treatment recommendations and the main contributing studies are summarized in Table 1 and Figure 1 presents the therapeutic targets used in the treatment of this disease.
Table 1.
Main clinical trials and outcomes of targeted therapy in BRAF-V600E mutant mCRC.
| Clinical trial | Phase | Setting | Patients and treatment | Outcomes | References | ||
|---|---|---|---|---|---|---|---|
| ORR (%) | Median PFS (months) | Median OS (months) | |||||
| Monotherapy | |||||||
| Vemurafenib | I | Pts refractory BRAF-V600E mCRC | 21 pts vemurafenib 960 mg BID | 5 | 3.7 | NA | Kopetz et al. 29 |
| Encorafenib | I | Pts refractory BRAF-V600E mCRC | 18 pts encorafenib 300 or 450 mg | 0 | 4 | NA | Gomez-Roca et al. 30 |
| Vemurafenib | II | All solid tumors BRAF-V600 mut | 10 pts refractory BRAF-V600E mCRC vemurafenib 960 mg BID | 0 | 4.5 | 9.3 | Hyman et al. 31 |
| Dual therapy | |||||||
| Vemurafenib–cetuximab | II | All solid tumors BRAF-V600 mut | 27 pts refractory BRAF-V600E mCRC vemurafenib 960 mg BID + cetuximab 400 mg | 4 | 3.7 | 7.1 | Hyman et al. 31 |
| Vemurafenib–panitumumab | I | Pts refractory BRAF-V600E mCRC | 15 pts panitumumab 6 mg/kg + vemurafenib 960 mg BID | 13 | 3.2 | 7.6 | Yaeger et al. 32 |
| Dabrafenib–trametinib | I/II | Pts refractory BRAF-V600E mCRC | 43 pts dabrafenib 150 mg BID + trametinib 2mg QD | 12 | 3.5 | NA | Corcoran et al. 33 |
| Dabrafenib–panitumumab | I | Pts refractory BRAF-V600E mCRC | 10 pts dabrafenib 150 mg BID + panitumumab 6 mg/kg | 10 | 3.5 | 13.2 | Corcoran et al. 34 |
| Encorafenib-cetuximab | Ib/II | Pts refractory BRAF-V600E mCRC | 50 pts encorafenib 200 mg QD + cetuximab 400 mg | 22 | 4.2 | NA | Tabernero et al. 35 |
| Binimetinib–encorafenib | Ib/II | Pts refractory BRAF-V600E mCRC | 11 pts encorafenib 450 mg QD + binimetinib 45 mg BID | 18 | 11 | NA | Sullivat et al. 36 |
| Encorafenib–cetuximab | III | Pts with BRAF-V600E mCRC ⩾ 2 prior regimens | 220 pts encorafenib 300 mg QD + cetuximab 400mg | 20 | 4.2 | 8.4 | Tabernero et al. 37 |
| Triple therapy | |||||||
| Encorafenib–cetuximab–alpelisib | Ib/II | Pts refractory BRAF-V600E mCRC | 52 pts encorafenib 200 mg QD + cetuximab 400 mg + alpelisib 300 mg QD | 27 | 5.4 | 15.2 | Tabernero et al. 35 |
| Dabrafenib–panitumumab–trametinib | I | Pts refractory BRAF-V600E mCRC | 91 pts dabrafenib 150 mg BID + panitumumab 6 mg/kg + trametinib 1.5 mg QD | 21 | 4.2 | 9.1 | Corcoran et al. 34 |
| Encorafenib–cetuximab–binimetinib | III | Pts with BRAF-V600E mCRC ⩾ 2 prior regimens | 224 pts ENC 300 mg QD + cetuximab 400 mg iv + binimetinib 45 mg QD | 26 | 4.3 | 9 | Tabernero et al. 37 |
| Encorafenib–cetuximab–binimetinib | II | Previously untreated BRAF-V600E mCRC pts | 41 pts ENC 300 mg QD + binimetinib 45 mg BID + cetuximab 400 mg | 50 | 4.9 | NA | Grothey et al. 38 |
| Vemurafenib–irinotecan–cetuximab | II | Pts refractory BRAF-V600E mCRC | 49 pts irinotecan 180 mg/m2 Q2W + vemurafenib 960 mg QD + cetuximab 500 mg/m2 Q2W | 17 | 4.2 | 9.6 | Kopetz et al. 39 |
BID, twice daily; C, cetuximab; ENC, encorafenib; iv, intravenous; mCRC, metastatic colorectal cancer; mOS, median overall survival; mPFS, median progression-free survival; mut, mutation; NA, non-available; ORR, overall response rate; pts, patients; QD, once daily; Q2W, once every 2 weeks.
Figure 1.
Therapeutic targets used in the treatment of BRAF-V600E-mutated mCRC.
mCRC: metastatic colorectal cancer.
Initial steps using BRAF inhibitors as monotherapy
After the impressive results seen with BRAF inhibitors in BRAF-V600E mutant melanoma patients, this quickly led to the investigation of the potential role of BRAF inhibition in CRC. The results were unexpectedly poor. In the extension cohort that included mCRC in the phase I trial, 21 BRAF-V600E mCRC patients with central confirmation by Taq polymerase chain reaction (PCR) were treated with vemurafenib (PLX4032). Only one patient (5%) experienced an objective tumor response, while seven cases of stable disease (SD) lasting at last 8 weeks were reported. 29 Vemurafenib was generally well tolerated, with three patients presenting dose-limiting toxicities (DLT) of grade 3 rash and grade 3 nausea. The clinical activity reported in previously treated BRAF-V600E-mutated mCRC was noticeably more modest than that seen in melanoma, suggesting that BRAF activation in mCRC is more complex and requires more in-depth molecular understanding than in melanoma.
Encorafenib (LGX818) is a second-generation highly selective ATP-competitive small molecule RAF kinase inhibitor. Encorafenib monotherapy was evaluated in patients with BRAF-V600E mutant refractory mCRC during the dose-expansion part of study CLGX818X2101. 30 Modest clinical activity was observed. A total of 18 patients with mCRC were treated, with an ORR of 5.6% and a disease control rate (DCR) of 67%. Three patients had DLT, one patient presented arthralgia and myalgia, one had insomnia and myalgia, and a third patient had bone pain and vomiting.
The basket trial evaluating vemurafenib for non-melanoma tumors with a BRAF-V600E mutation was a first-in-kind clinical trial to recruit patients based on the presence of a molecular alteration rather than on a specific indication. In total, 122 patients with BRAF-V600E mutant non-melanoma tumors received single-agent vemurafenib. The mCRC cohort included 10 patients, none of whom had a clinical response. 31 The most common adverse events (AEs) across all patients receiving vemurafenib monotherapy were rash (68% of patients), fatigue (56%), and arthralgia (40%).
Addition of an anti-EGFR to a BRAF inhibitor improves clinical outcomes
In vitro experiments demonstrated that inhibition at a single node in the MAPK pathway in BRAF-V600E mutant CRC cell lines resulted in increased EGFR phosphorylation by adaptative feedback, thus increasing the resistance to the BRAF inhibitor. 40 Interestingly, anti-EGFR therapy rendered these cell lines sensitive to the BRAF inhibitor. 41 In light of the poor clinical results with single agent vemurafenib and this intriguing preclinical evidence, the mCRC cohort of the basket study was amended to include a vemurafenib–cetuximab combination. A total of 27 patients with mCRC BRAF-V600E received vemurafenib in combination with cetuximab. 31 One patient had a partial response (PR) giving an ORR of 4%, and 69% of patients presented SD. Median PFS and OS were 3.7 months (95% CI, 1.8–5.1) and 7.1 months (95% CI, 4.4 to not reached), respectively. Patients included in this trial were heavily pretreated having received a median of two lines of previous therapy, ranging from one to six prior lines. Another study evaluated the combination of panitumumab and vemurafenib in 15 BRAF-V600E-mutated pretreated patients with mCRC. In all, 10 patients experienced tumor regression, with PR in two patients and SD lasting over 6 months in two patients. 32 Four patients (20%) presented grade 3 or 4 alkaline phosphatase elevations, and one patient (7%) presented neutropenia. Given the success of the dabrafenib and trametinib (a MEK inhibitor) combination in BRAF mutant melanoma, combined BRAF plus MEK inhibition was hypothesized to be a promising approach in BRAF-V600E mutant CRC. This was implemented using a combination of dabrafenib plus trametinib, and 43 patients were treated. 33 Of them, five patients (12%) achieved a response, including one complete response (CR) lasting for 36 months. Left ventricular ejection fraction decrease occurred in eight patients (19%), including two grade 3 events, and led to dose reduction in five patients (12%) and treatment discontinuation in one patient (2%). Another trial explored the use of dabrafenib plus the anti-EGFR monoclonal antibody panitumumab with or without trametinib. 34 CR or PR was achieved with dual EGFR/BRAF blockade in 2 out of 20 (10%) BRAF-V600E mutant mCRC patients with a median PFS of 3.5 months. The addition of a MEK inhibitor trametinib to the dual EGFR/BRAF blockade improved outcomes, with responses in 9 of 35 patients (26%) and a median PFS of 4.1 months. While both dual and triple blockade showed promising activity, the combination of trametinib and panitumumab gave no responses, whereas toxicity was increased. For patients who received dabrafenib plus panitumumab, no DLT was observed, with grade 3–4 hypokalemia being the main AE. Patients who received trametinib plus panitumumab in the absence of dabrafenib presented significant dermatologic toxicity (18% grade 3–4 dermatitis acneiform).
Promising results were observed in a dose escalation trial with encorafenib and cetuximab in 26 patients with BRAF-V600E mutant CRC exploring the combination of encorafenib and cetuximab with or without alpelisib, a phosphoinositide 3kinase (PI3K) inhibitor. In the phase II dose-expansion part of the study, for the 50 patients treated with the encorafenib plus cetuximab combination, median PFS was 4.2 months (95% CI, 3.4–5.4) and the ORR was 22% (95% CI, 12–36). 35 Grade 3–4 AEs presenting in more than 10% of patients receiving doublet treatment were anemia (6%), hyperglycemia (2%), and increased lipase (18%). The combination of binimetinib with encorafenib as dual or triple combination therapy was investigated in a dose-finding phase Ib/II study of binimetinib in combination with encorafenib in patients with BRAF-V600E mutant solid tumors. In all, 11 patients were enrolled in a phase II BRAF mutant CRC cohort, which had an ORR of 18% (95% CI, 2–52) and a DCR of 64% (95% CI, 31–89). The most frequently reported grade 3 or 4 AEs during the phase II part of the study for the overall population were increased alkaline phosphatase (9% of patients). 36
The development of the triplet combination
In vitro evidence suggested activation of the PI3K/AKT pathway as another possible mechanism of resistance to BRAF-V600E inhibitors. 42 To overcome this, the previously mentioned phase Ib/II study investigated the BRAF inhibitor encorafenib and the anti-EGFR antibody cetuximab with or without the PI3Ka inhibitor alpelisib (BYL719) in patients with advanced BRAF-V600E mutant mCRC. The phase Ib study did not identify a maximum tolerated dose for either combination. Based on the general tolerability of the triplet, the phase II encorafenib dose was chosen for both arms. In the phase II part, patients with advanced BRAF-mutated CRC failing at least one prior line of therapy were randomized 1:1 to doublet [encorafenib 200 mg once daily (QD) and cetuximab per label] or triplet (encorafenib, cetuximab, and alpelisib 300 mg QD) therapy. A total of 102 patients were randomized (triplet, n = 52; doublet, n = 50). A comparison of the triplet versus the doublet in terms of efficacy showed an HR of 0.69 (95% CI, 0.43–1.11; p = 0.064) with a median PFS of 5.4 months (95% CI, 4.1–7.2) and 4.2 months (95% CI, 3.4–5.4), respectively, and confirmed ORRs of 27% and 22%, respectively. With 35 events, an interim OS analysis (triplet versus doublet) demonstrated an HR of 1.21 (95% CI, 0.61–2.39). Grade 3 or 4 AEs in the triplet arm were anemia (17%), hyperglycemia (13%), and increased lipase (8%).
The combinations of dabrafenib plus panitumumab, dabrafenib and trametinib plus panitumumab, and trametinib plus panitumumab were also explored. Analyses showed an improved response for the triple therapy compared to either doublet, albeit with an increase in some AEs, notably dermatologic and grade 3–4 diarrhea relative to the doublet regimens. 34 Combinations of targeted therapies with irinotecan, such as cetuximab plus vemurafenib and irinotecan, or irinotecan and cetuximab with or without vemurafenib, have been tested with very modest efficacy results.39,43 In the phase II S1406 trial, patients with refractory BRAF-V600E mCRC were randomized to receive irinotecan plus cetuximab with or without vemurafenib. In this trial, MSI tumors were not excluded because at the time, immune checkpoints inhibitors had not yet received Food and Drug Administration (FDA) approval in this population. 39 Median PFS was 4.2 and 2.0 months in the experimental and control arms, respectively, and the ORR was 17 versus 4% (p = 0.05), with a DCR of 65 versus 21% (p = 0.001), respectively. In all, 21 patients in the control arm (42%) crossed over to the experimental regimen after disease progression. OS was not significantly different between the two arms (HR, 0.77; 95% CI, 0.50–1.18; p = 0.23). PFS following crossover in this cohort was 5.4 months, with an ORR and DCR of 19% and 76%, respectively.
This investigation into a deeper suppression of the BRAF pathway using a triplet blockade combination ultimately led to the development of the BEACON trial. The BEACON trial was an open-label, global, randomized phase III trial for patients with BRAF-V600E mCRC who had progressed on at least one previous line. 37 It is the largest trial including BRAF-V600E mCRC published to date. Patients were randomly assigned 1:1:1 to receive triplet (224 patients, encorafenib plus cetuximab plus binimetinib), doublet (220 patients, encorafenib plus cetuximab), or control treatment (221 patients, irinotecan-based chemotherapy plus cetuximab). Primary endpoints were OS and independently reviewed ORR comparing the triplet to control treatment. Updated data demonstrated a median OS of 9.3 months (95% CI, 8.2–10.8) for the triplet compared to 5.9 months (95% CI, 5.1–7.1) in the control group (HR, 0.65%; 95% CI, 0.47–0.75). 37 Median OS for the doublet was 9.3 months (95% CI, 8.0–11.3; HR versus control was 0.61, 95% CI, 0.48–0.77). Confirmed ORRs were 26.8% (95% CI, 21.1–33.1) for the triplet, 19.5% (95% CI, 14.5–25.4) for the doublet, and 1.8% (95% CI, 0.5–4.6) for control. The study was not powered to compare the triple therapy versus doublet treatment. The toxicity profile demonstrated that treatment was globally well-tolerated and consistent with previously reported data, with grade ⩾3 AEs in 66%, 57%, and 64% for triplet, doublet, and control, respectively. These results led to FDA and European Medicines Agency (EMA) approval for the doublet combination of encorafenib and cetuximab for patients with mCRC with BRAF-V600E mutation who have already progressed on at least one prior treatment regimen. Consequently, the BEACON trial has completely reshaped the therapeutic landscape of BRAF-V600E mCRC and is currently a new standard of care in this population.
MAPK reactivation as the main mechanism of resistance
Given the modest clinical activity with BRAF inhibitors as monotherapy, mechanisms of primary resistance are suspected. In vitro studies, showing the MAPK pathway as a driver of resistance, are confirmed by in vivo studies demonstrating that BRAF inhibitors result in increased EGFR phosphorylation. This increases the insensitivity to the BRAF inhibitor, explaining the mechanisms of primary resistance to a single MAPK node blockade. Interestingly, anti-EGFR therapy rendered these cell lines sensitive to the BRAF inhibitor.40,41 BRAF inhibitors combined with EGFR inhibitors resulted in synergistic inhibition of tumor growth in BRAF-V600E-mutated CRC xenograft models.40,41 Nevertheless, although these combinations induce tumor regression, acquired resistance invariably appears, leading to tumor progression. Most of the trials reviewed here included per protocol analyses of paired biopsies and plasma samples. However, since most patients presented with surgically unresectable disease and given the disease aggressivity with metastases frequently not suitable for biopsy, tumor tissue is not always available for analysis. Matched biopsies before and at the time of progression from eight patients included in several trials evaluating different combinations of BRAF and EGFR inhibitors, revealed genetic amplification of wild-type RAS as a recurrent mechanism of resistance, leading to increased receptor tyrosine kinase-dependent activation. Thus, inhibiting EGFR and RAF dimers offers a potential strategy to overcome resistance in BRAF-V600E mutant CRC. 44
The trial exploring the combination of dabrafenib plus panitumumab with or without trametinib included serial circulating tumor DNA (ctDNA) determination before, during, and at progression using digital PCR BEAMing (Beads, Emulsion, and Magnetics). Plasma levels of BRAF-V600E correlate with tumor response. 34 Almost half of the patients (48%) showed emergence of KRAS or NRAS mutations in ctDNA at the time of disease progression. Similarly, when ctDNA was evaluated following treatment with encorafenib and cetuximab with or without alpelisib, samples collected during acquired resistance showed MAPK activation (KRAS mutations or amplifications). 45 The phase S1406 trial evaluating irinotecan plus cetuximab with or without vemurafenib also collected plasma samples. The BRAF-V600E mutant allele fraction declined in 87% of patients after treatment initiation, whereas no patients in the chemotherapy arm had BRAF-V600E mutation allele fraction decrease. Plasma analysis upon progression showed one acquired KRAS mutation without other identifiable genomic mechanisms of resistance. 39
In a recent publication, genomic profiling, tumor mutational burden (TMB), and BM transcriptional subtype classification were evaluated as a mechanism of resistance among a small cohort of patients with BRAF-V600E/microsatellite stable (MSS) mCRC who received encorafenib with cetuximab, with or without binimetinib. There were no differences between BM or genomic profiling subtypes. The results suggested that high TMB (cutoff six mutations per megabase) limited the benefit from EGFR/BRAF blockade. However, the sample size was modest and these results require prospective validation. 46
Finally, clonal expansion of MET gene amplification subclone during panitumumab and vemurafenib treatment thought to cause tumoral progression has also been described. Interestingly, acquired MET amplification was overcome by combining BRAF and MET inhibition with subsequent rapid tumoral response. 47 Based on the reviewed evidence, the majority of acquired mechanisms of resistance are associated with MAPK pathway reactivation via alternative pathways.
New scenarios and future approaches for managing BRAF-V600E mCRC
The promising preliminary efficacy data from the safety lead-in part of the BEACON trial supported the step toward first-line treatment with encorafenib, binimetinib, and cetuximab in patients with BRAF-V600E mutant mCRC. This triplet treatment was explored in the phase II single-arm ANCHOR-CRC trial which included 40 patients. 38 The ORR was 50% with a DCR of 85%, and median PFS was 4.9 months (95% CI, 4.4–8.1). Grade 3 or higher AEs occurred in 68% of patients; the most common grade ⩾3 AEs were diarrhea (15%), anemia (2%), and nausea (7%). Considering the outcomes with chemotherapy plus anti-VEGF in the setting of upfront therapy, the results from the ANCHOR trial were not as good as expected. Currently, the BREAKWATER trial in BRAF-V600E mutant CRC is evaluating the role of the combination of cetuximab and chemotherapy with encorafenib in the first-line setting. This phase III randomized study has three arms: encorafenib plus cetuximab; FOLFIRI or FOLFOX plus encorafenib and cetuximab; or the investigator’s choice of standard chemotherapy with or without bevacizumab.
Different strategies are being investigated to improve the current results for this challenging population. New strategies to overcome resistances include immune checkpoint inhibitors or novel molecules such as ERK inhibitors or SHP2 inhibitors. Based on the immunogenic biological landscape of BRAF mutant mCRC, most current approaches included immune checkpoint inhibitors combinations. Trials have evaluated the combination of a BRAF inhibitor with a MEK inhibitor plus an immune checkpoint inhibitor. Results are available for two clinical trials. The first was a phase II trial evaluating the combination of dabrafenib–trametinib plus the anti-PD-1 spartalizumab, 48 giving an ORR of 33% and a DCR 76%. This trial included 21 patients regardless of their MSI status (4 MSS and 17 MSI). Among them, five patients had prior therapy with BRAF inhibitors and/or immunotherapy. The second is a phase I/II trial evaluating encorafenib plus cetuximab plus the anti-PD-1 nivolumab. 49 A total of 26 patients have been included, all of them are MSS; the ORR was 45%, DCR was 95%, with a median PFS of 7.3 months (95% CI, 5.6 to not reached), and median OS of 11.4 months (95% CI, 7.7 to not reached). An ongoing multi-arm trial (NCT04294160), in previously treated (with or without previous BRAF inhibitors) BRAF-V600E mCRC patients, incorporates various combinations of the BRAF inhibitor dabrafenib with novel molecules: spartalizumab (anti-PD1), LTT462 (ERK inhibitor), TNO155 (SHP2 inhibitor), and LXH254 (BRAF/cRAF inhibitor). Further knowledge about mechanisms of resistance to target therapy will help to develop novel approaches to treat those patients. The role of BRAF inhibitors in terms of the detection of the BRAF-V600E mutation in ctDNA is being investigated in the adjuvant setting in the ACT-3 trial (NCT04259944). Patients who are ctDNA positive for the BRAF-V600E mutation after completion of 3–6 months of adjuvant treatment are considered as ‘molecularly metastatic’ and are randomized to surveillance or encorafenib plus binimetinib plus cetuximab.
Further research is also needed to identify the predictive biomarkers. In a small cohort of 23 patients treated with a BRAF inhibitor plus an anti-EGFR with or without a MEK inhibitor, RNF43 somatic mutations were enriched in responders to BRAF inhibitor combination therapies, suggesting that differential activation of the WTN/B-catenin pathway might underlie differential sensitivity to BRAF inhibitors. All but one patient with a BRAF mutant tumor harboring RNF43 achieved clinical benefit (CR, PR, or >6 months SD) with encorafenib plus cetuximab with or without binimetinib. 50 Regarding prognostic biomarkers, the BRAF mutant allele fraction in plasma was confirmed as a robust prognostic factor, regardless of the treatment.50,51 Finally, previous bevacizumab treatment has also been suggested as a potential predictive biomarker. In the BEACON trial, OS among patients who received the triplet was lower among patients who had previously received bevacizumab compared with patients who did not receive previous anti-VEGF (HR, 1.74, 95% CI, 1.21–2.49). These results were not observed among patients who received encorafenib–cetuximab. 52
Conclusions
In recent years, the therapeutic landscape of BRAF-V600E mCRC tumors has been completely reshaped, notably following the outcome of the BEACON trial. Prior to the development of BRAF inhibitors, standard chemotherapy has changed minimally giving only modest clinical outcomes. Subgroup analysis from several trials confirmed the benefit of adding an anti-VEGF drug; however, trials were not specific to the BRAF-V600E population. Despite the clinical improvement achieved with the combination of chemotherapy plus anti-VEGF, survival remains poor.
In stark contrast to the success observed in melanoma, BRAF inhibitors as monotherapy including vemurafenib, dabrafenib, and encorafenib have shown no activity in BRAF-V600E mCRC. Preclinical evidence pointed to rebound upregulation of EGFR as a critical component for successful BRAF inhibition. Dual blockade of both EGFR and BRAF resulted in synergistic inhibition of tumor growth in BRAF-V600E mutant CRC murine models, leading to new clinical combinations including an anti-EGFR, demonstrating more robust clinical activity. Subsequent strategies added a third MAPK pathway inhibitor, implementing MEK, ERK, or PI3CA blockade. The BEACON trial, which is the largest trial ever presented in the BRAF-V600E mCRC population, confirmed the benefit of the combination of encorafenib and cetuximab with or without binimetinib, over irinotecan-based chemotherapy. However, updated survival results demonstrated no differences in PFS or in OS for either the triplet or the doublet, despite the higher response rate among patients in the triplet arm. Based on this absence of differences but a better toxicity profile, both the FDA- and the EMA-approved encorafenib–cetuximab as a new standard of care for refractory BRAF-V600E mCRC. Of note, the higher response rate observed in the triplet arm of the BEACON trial was not associated with a PFS or OS improvement compared with the doublet. Interestingly, specific populations appear to benefit from triplet blockade; however, further prospective research is needed to identify the nature of these patient populations.
For upfront therapy, MSI status may help to guide treatment. Considering the results of the triplet in first line, the ANCHOR trial suggested that upfront targeted therapy was not active as expected for BRAF-V600E/MSS tumors. In an attempt to enhance the activity, the BREAKWATER trial is currently evaluating encorafenib–cetuximab combined with chemotherapy as upfront therapy. On the other hand, for patients with BRAF-V600E/MSI tumors, representing up to 30% of BRAF patients, the KEYNOTE-177 study demonstrated the outstanding effect of pembrolizumab as upfront therapy. 53 This benefit was further confirmed in the refractory setting with both pembrolizumab and nivolumab plus ipilimumab.54,55 Therefore, pembrolizumab may be the first-line therapy choice for patients with BRAF-V600E mutant MSI CRC.
Despite the meaningful clinical activity observed in the BEACON trial and other trials evaluating BRAF inhibitors, not all patients responded and some responses are relatively short. This disparity in response highlights BRAF-V600E heterogeneity that has been confirmed through transcriptomic signatures. Given the immunogenic nature of BRAF-V600E mutant tumors, most are classified as CMS1 (MSI immune); studies have evaluated the combination of BRAF inhibitors plus immune checkpoint inhibitors with outstanding results, even among patients previously treated with either immunotherapy or BRAF inhibitors. Despite these important advances, BRAF-V600E mCRC remains a clinically challenging disease.56–58 Current studies are evaluating several combinations with BRAF inhibitors such as ERK1/2 inhibitors or SHP2 inhibitors that can overcome or delay acquired resistance. Considering these clinical advances in the BRAF-V600E field, future research should focus on identifying predictive and prognostic biomarkers as well as new strategies to overcome mechanisms of resistance.
Footnotes
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Javier Ros: Conceptualization; Writing – original draft; Writing – review & editing.
Nadia Saoudi: Conceptualization; Investigation; Writing – original draft; Writing – review & editing.
Iosune Baraibar: Conceptualization; Writing – original draft; Writing – review & editing.
Francesc Salva: Writing – original draft; Writing – review & editing.
Josep Tabernero: Writing – review & editing.
Elena Elez: Writing – review & editing.
ORCID iD: Javier Ros  https://orcid.org/0000-0002-8137-5415
https://orcid.org/0000-0002-8137-5415
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
Contributor Information
Javier Ros, Department of Medical Oncology, Vall d’Hebron Institute of Oncology, Barcelona, Spain; Medical Oncology, Department of Precision Medicine, Università degli Studi della Campania Luigi Vanvitelli, Naples, Italy.
Nadia Saoudi, Department of Medical Oncology, Vall d’Hebron Institute of Oncology, Barcelona, Spain.
Iosune Baraibar, Department of Medical Oncology, Vall d’Hebron Institute of Oncology, Barcelona, Spain.
Francesc Salva, Department of Medical Oncology, Vall d’Hebron Institute of Oncology, Barcelona, Spain.
Josep Tabernero, Department of Medical Oncology, Vall d’Hebron Institute of Oncology, Barcelona, Spain.
Elena Elez, Department of Medical Oncology, Vall d’Hebron Institute of Oncology, Centro Cellex, Carrer de Natzaret, 115-117, 08035 Barcelona, Spain.
References
- 1. Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020; 70: 145–164. [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 3. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002; 417: 949–954. [DOI] [PubMed] [Google Scholar]
- 4. De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol 2010; 11: 753–762. [DOI] [PubMed] [Google Scholar]
- 5. Barras D, Missiaglia E, Wirapati P, et al. BRAF V600E mutant colorectal cancer subtypes based on gene expression. Clin Cancer Res 2017; 23: 104–115. [DOI] [PubMed] [Google Scholar]
- 6. Clarke CN, Kopetz ES. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: clinical characteristics, clinical behavior, and response to targeted therapies. J Gastrointest Oncol 2015; 6: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones JC, Renfro LA, Al-Shamsi HO, et al. Non-V600 BRAF mutations define a clinically distinct molecular subtype of metastatic colorectal cancer. J Clin Oncol 2017; 35: 2624–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weisenberger DJ, Siegmund KD, Campan M, et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 2006; 38: 787–793. [DOI] [PubMed] [Google Scholar]
- 9. Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 2015; 16: 1306–1315. [DOI] [PubMed] [Google Scholar]
- 10. Cremolini C, Antoniotti C, Rossini D, et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol 2020; 21: 497–507. [DOI] [PubMed] [Google Scholar]
- 11. Cremolini C, Antoniotti C, Stein A, et al. Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol. Epub ahead of print 20 August 2020. DOI: 10.1200/JCO.20.01225. [DOI] [PubMed] [Google Scholar]
- 12. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012; 30: 3499–3506. [DOI] [PubMed] [Google Scholar]
- 13. Van Cutsem E, Lenz H-J, Köhne C-H, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015; 33: 692–700. [DOI] [PubMed] [Google Scholar]
- 14. Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS Studies. Clin Cancer Res 2014; 20: 5322–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol 2008; 26: 5705–5712. [DOI] [PubMed] [Google Scholar]
- 16. Loree JM, Dowers A, Tu D, et al. Expanded low allele frequency RAS and BRAF V600E testing in metastatic colorectal cancer as predictive biomarkers for cetuximab in the Randomized CO.17 trial. Clin Cancer Res 2021; 27: 52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pietrantonio F, Petrelli F, Coinu A, et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer 2015; 51: 587–594. [DOI] [PubMed] [Google Scholar]
- 18. Van Cutsem E, Köhne CH, Láng I, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011; 29: 2011–2019. [DOI] [PubMed] [Google Scholar]
- 19. Stintzing S, Miller-Phillips L, Modest DP, et al. Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: analysis of the FIRE-3 (AIO KRK-0306) study. Eur J Cancer 2017; 79: 50–60. [DOI] [PubMed] [Google Scholar]
- 20. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016; 27: 1386–1422. [DOI] [PubMed] [Google Scholar]
- 21. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. New Engl J Med 2015; 372: 1909–1919. [DOI] [PubMed] [Google Scholar]
- 22. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013; 381: 303–312. [DOI] [PubMed] [Google Scholar]
- 23. Vecchione L, Gambino V, Raaijmakers J, et al. A vulnerability of a subset of colon cancers with potential clinical utility. Cell 2016; 165: 317–330. [DOI] [PubMed] [Google Scholar]
- 24. Cremolini C, Pietrantonio F, Tomasello G, et al. Vinorelbine in BRAF V600E mutated metastatic colorectal cancer: a prospective multicentre phase II clinical study. ESMO Open 2017; 2: e000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004; 116: 855–867. [DOI] [PubMed] [Google Scholar]
- 26. Yao Z, Torres NM, Tao A, et al. BRAF mutants evade ERK-Dependent feedback by different mechanisms that determine their sensitivity to pharmacologic inhibition. Cancer Cell 2015; 28: 370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yaeger R, Kotani D, Mondaca S, et al. Response to anti-EGFR therapy in patients with BRAF non-V600-mutant metastatic colorectal cancer. Clin Cancer Res 2019; 25: 7089–7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015; 21: 1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kopetz S, Desai J, Chan E, et al. PLX4032 in metastatic colorectal cancer patients with mutant BRAF tumors. J Clin Oncol 2010; 28: 3534–3534. [Google Scholar]
- 30. Gomez-Roca CA, Delord J, Robert C, et al. Encorafenib (Lgx818), an oral braf inhibitor, in patients (Pts) with Braf V600E metastatic colorectal cancer (Mcrc): results of dose expansion in an open-label, phase 1 study. Ann Oncol 2014; 25: iv182. [Google Scholar]
- 31. Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. New Engl J Med 2015; 373: 726–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yaeger R, Cercek A, O’Reilly EM, et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res 2015; 21: 1313–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J Clin Oncol 2015; 33: 4023–4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Corcoran RB, André T, Atreya CE, et al. Combined BRAF, EGFR, and MEK inhibition in patients with BRAFV600E-mutant colorectal cancer. Cancer Discov 2018; 8: 428–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tabernero J, Geel RV, Guren TK, et al. Phase 2 results: encorafenib (ENCO) and cetuximab (CETUX) with or without alpelisib (ALP) in patients with advanced BRAF-mutant colorectal cancer (BRAFm CRC). J Clin Oncol 2016; 34: 3544–3544.27573652 [Google Scholar]
- 36. Sullivan RJ, Weber J, Patel S, et al. A phase Ib/II study of the BRAF inhibitor encorafenib plus the MEK inhibitor binimetinib in patients with BRAFV600E/K-mutant solid tumors. Clin Cancer Res 2020; 26: 5102–5112. [DOI] [PubMed] [Google Scholar]
- 37. Tabernero J, Grothey A, Van Cutsem E, et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol 2021; 39: 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Van Cutsem E, Taieb J, Yaeger R, et al. O-10 ANCHOR CRC: results from a single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E–mutant metastatic colorectal cancer. Ann Oncol 2021; 32: S222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kopetz S, Guthrie KA, Morris VK, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG S1406). J Clin Oncol 2021; 39: 285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prahallad A, Sun C, Huang S, et al. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 2012; 483: 100–103. [DOI] [PubMed] [Google Scholar]
- 41. Corcoran RB, Ebi H, Turke AB, et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov 2012; 2: 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mao M, Tian F, Mariadason JM, et al. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res 2013; 19: 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hong DS, Morris VK, El Osta B, et al. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov 2016; 6: 1352–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yaeger R, Yao Z, Hyman DM, et al. Mechanisms of acquired resistance to BRAF V600E inhibition in colon cancers converge on RAF dimerization and are sensitive to its inhibition. Cancer Res 2017; 77: 6513–6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Geel RMJM, Tabernero J, Elez E, et al. A phase Ib dose-escalation study of encorafenib and cetuximab with or without alpelisib in metastatic BRAF-mutant colorectal cancer. Cancer Discov 2017; 7: 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Randon G, Intini R, Cremolini C, et al. Tumour mutational burden predicts resistance to EGFR/BRAF blockade in BRAF-mutated microsatellite stable metastatic colorectal cancer. Eur J Cancer 2022; 161: 90–98. [DOI] [PubMed] [Google Scholar]
- 47. Pietrantonio F, Oddo D, Gloghini A, et al. MET-Driven resistance to dual EGFR and BRAF blockade may be overcome by switching from EGFR to MET inhibition in BRAF-mutated colorectal cancer. Cancer Discov 2016; 6: 963–971. [DOI] [PubMed] [Google Scholar]
- 48. Corcoran R, Giannakis M, Allen J, et al. SO-26 clinical efficacy of combined BRAF, MEK, and PD-1 inhibition in BRAFV600E colorectal cancer patients. Ann Oncol 2020; 31: S226–S227. [Google Scholar]
- 49. Morris VK, Parseghian CM, Escano M, et al. Phase I/II trial of encorafenib, cetuximab, and nivolumab in patients with microsatellite stable, BRAFV600E metastatic colorectal cancer. J Clin Oncol 2022; 40: 12.34752147 [Google Scholar]
- 50. Élez E, Ros J, Martini G, et al. LBA-3 integrated analysis of cell-free DNA (cfDNA) BRAF mutant allele fraction (MAF) and whole exome sequencing in BRAFV600E metastatic colorectal cancer (mCRC) treated with BRAF-antiEGFR ± MEK inhibitors. Ann Oncol 2021; 32: S226–S227. [Google Scholar]
- 51. Kopetz S, Murphy DA, Pu J, et al. Evaluation of baseline BRAF V600E mutation in circulating tumor DNA and efficacy response from the BEACON study. J Clin Oncol 2022; 40: 162–162. [Google Scholar]
- 52. Aderka D, Kopetz S, Grothey A, et al. SO-28 effect of prior bevacizumab treatment in BRAF V600E-mutant metastatic colorectal cancer: overall survival with encorafenib + cetuximab ± binimetinib in BEACON CRC. Ann Oncol 2021; 32: S214. [Google Scholar]
- 53. André T, Shiu K-K, Kim TW, et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. New Engl J Med 2020; 383: 2207–2218. [DOI] [PubMed] [Google Scholar]
- 54. Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 2018; 36: 773–779. [DOI] [PubMed] [Google Scholar]
- 55. Le DT, Kim TW, Van Cutsem E, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability–high/mismatch repair–deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol 2020; 38: 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ros Montañá J, Martini G, Baraibar I, et al. Patient and tumor characteristics as determinants of overall survival (OS) in BRAF V600 mutant (mt) metastatic colorectal cancer (mCRC) treated with doublet or triplet targeted therapy. J Clin Oncol 2020; 38: 4112. [Google Scholar]
- 57. Loupakis F, Intini R, Cremolini C, et al. A validated prognostic classifier for BRAF-mutated metastatic colorectal cancer: the ‘BRAF BeCool’ study. Eur J Cancer 2019; 118: 121–130. [DOI] [PubMed] [Google Scholar]
- 58. Ros J, Baraibar I, Sardo E, et al. BRAF, MEK and EGFR inhibition as treatment strategies in BRAF V600E metastatic colorectal cancer. Ther Adv Med Oncol 2021; 13: 1758835921992974. [DOI] [PMC free article] [PubMed] [Google Scholar]