Abstract
Hospital care of frail older adults is far from optimal. Although some geriatric models of care have been shown to improve outcomes, the effect size is small and models are difficult to fully implement, sustain and replicate. The two root causes for these shortcomings are competing interests (high revenue generating diseases, procedures and surgeries) and current hospital cultures (for example a culture of safety that emphasizes bed alarms and immobility rather than frequent ambulation). Geriatric hospitals would be hospitals completely dedicated to the care of frail older patients, a group which is most vulnerable to the negative consequences of a hospitalization. They would differ from a typical adult hospital because they could implement evidence based principles of successful geriatric models of care on a hospital wide basis, which would make them sustainable and allow for scaling up of proven outcomes. Innovative structural designs, unachievable in a typical adult hospital, would enhance mobility while maintaining safety. Financial viability and stability would be a challenge but should be feasible, likely through affiliation with larger health care systems with other hospitals because of cost savings associated with geriatric models of care (decreased length of stay, increased likelihood of discharge home, without increasing costs).
Keywords: hospital care, older adults, hospital culture, geriatric hospital, geriatric models of care
Introduction
Almost three decades ago, researchers identified that 32% of those over age 70 and approximately 50% of those above age 80 lose physical function (activities of daily living) during a hospitalization. The majority of these patients do not regain this lost function (Sager et al., 1996). Only 44% of patients 85 years and older and 58% of patients 75–84 years old return home after a hospitalization (Levant et al., 2015). Other negative consequences associated with hospitalization include delirium, falls, pressure ulcers, catheter-associated urinary tract infections (CAUTis) and adverse drug events (ADEs) (Creditor, 1993; Wald, 2017). In addition to the morbidity and mortality associated with these negative consequences (Creditor, 1993; Wald, 2017), all of these are associated with additional health care costs. Average 1-year health care costs were 2.5 times higher among general medical patients age 70+ with delirium compared to patients without delirium (Leslie et al., 2008). Average hospital costs were $20,327 higher among patients age 70+ who had delirium after elective surgery (Gou et al., 2021). Based on a meta-analysis, estimates of increased hospital costs per patient with a fall, pressure ulcer, CAUTI or ADE were $6,694, $14,506, $13,793, and $5,746, respectively. (Agency for Healthcare Research and Quality, 2017).
Several geriatric models of care have been developed to prevent these negative consequences and have been shown to improve outcomes such as increased likelihood of discharge home instead of a facility, decreased incidence of delirium, decreased mortality risk, shortened hospital length of stay (LOS) and lower or equal costs of hospital care. (Ellis et al., 2017; Van Creen et al., 2010; Baztán et al., 2009; Hshieh et al., 2015; Grigoryan et al., 2014; Kavalieratos et al., 2016). These models have their greatest impact on frail older patients (Gilbert et al., 2018; Gillick, 2014; Searle & Rockwood, 2018). Despite these positive outcomes, these models have not been widely implemented throughout adult hospitals (Clark, 2013; Palmer, 2018). Geriatric hospitals would be hospitals completely dedicated to the care of frail older patients, a group which is most vulnerable to the negative consequences of a hospitalization. They would differ from typical adult hospitals because they could implement evidence based principles of successful geriatric models of care on a hospital wide basis, which would make them sustainable and allow for scaling up of proven outcomes.
The aims of this article are to (1) review successful geriatric models of care, describe their positive outcomes and their limited size effect; (2) discuss why it is not possible to fully implement the principles of geriatric models in US adult hospitals; (3) delineate why geriatric hospitals would be the best option to implement these principles and how they would do this; and (4) discuss challenges of implementing this model of hospital care.
Current Geriatric Models of Care
In older adults, the combination of diseases, syndromes and psychosocial problems creates a clinical situation that is different from most single organ subspecialties, so that geriatric medicine developed assessments and interventions to address all of these. Assessments began with the Comprehensive Geriatric Assessment (CGA) (Matthews, 1984), followed by models of care such as Geriatric Evaluation and Management Units (GEMUs) (Rubenstein et al., 1984), Geriatric Special Care Units (Collard et al., 1985) and Acute Care of the Elderly (ACE) Units (Landefeld et al., 1995).
Subsequent models which pertain to hospitalized older adults, included delirium prevention (Inouye et al., 1999), and management models (Flaherty et al., 2003), orthopedic-geriatric models (Zuckerman et al., 1992; Khasraghi et al., 2005), palliative care (Carlson et al., 1988), trauma-geriatric models (Fallon et al., 2006) and geriatric emergency departments (Hogan et al., 2014).
The core principles of early geriatric models of care include CGA (which identifies geriatric syndromes and psychosocial problems in addition to medical conditions, especially among frail older adults), (Stuck et al., 1993) a geriatric-focused interdisciplinary team (for example, nurses, therapists, and social workers with special interest or training in geriatrics, as well as a geriatrician), and early discharge planning (Rubenstein et al., 1984; Collard et al., 1985; Landefeld et al., 1995). Later models have focused on specific syndromes or clinical scenarios for which frail older patients are particularly vulnerable (Inouye et al., 1999; Flaherty et al., 2003; Zuckerman et al., 1992; Khasraghi et al., 2005; Carlson et al., 1988; Fallon et al., 2006; Hogan et al., 2014).
Based on meta-analyses, several of these models have been shown to improve outcomes (Ellis et al., 2017; Van Creen et al., 2010; Baztán et al., 2009; Hshieh et al., 2015; Grigoryan et al., 2014; Kavalieratos et al., 2016). Table 1 describes the two most positive outcomes [defined as best odds ratios (ORs), relative risks (RRs) or standard mean differences (SMDs)] with other outcomes analyzed by meta-analyses reported under the last column as “significantly different” or “not significantly different.” CGA improves likelihood of being alive and living at home at discharge and at the end of the follow up period (Ellis et al., 2017). GEMU reduces institutionalization and functional decline (Van Creen et al., 2010). Various models of acute geriatric units, which include ACE Units, increase odds of living at home at discharge and 3 months after discharge (Baztán et al., 2009). They also decrease risk of functional decline during hospitalization, and reduce costs of hospital care (Baztán et al., 2009; Fox et al., 2012; Palmer, 2018). Delirium prevention studies reduce incident delirium and falls (Hshieh et al., 2015). Orthopedic-geriatric models reduce mortality during hospitalization and at 6–12 months follow up, as well as decrease hospital LOS (Grigoryan et al., 2014).
Table 1.
The effects of various geriatric models of care based on published meta-analyses (where available)
| Model of Care Author, yearTotal number of studies in meta-analysis | Two most positive outcomes a | Odds ratios (OR), Relative Risks (RR) or Standardized Mean Differences (SMD) with 95% Confidence Intervals (95% CI) Number of events, (%) in Intervention and Usual care/Control groups | Other outcomes that were significantly different from Usual care/Control group (+) or Not significantly different from Usual care/Control group (−) |
|---|---|---|---|
| Comprehensive geriatric assessment (CGA) Ellis, 201715 N = 29 studies |
Alive and living at home at end of follow up, 3–12 months
(n = 16 studies analyzed) Admission to a nursing home at end of follow up, 3–12 months (n = 14 studies analyzed) |
OR = 1.06 (1.01–1.10) Intervention: 2079/3498, 59.4% Usual care: 1852/3301, 56.1% OR =.80 (0.72–0.89) Intervention: 481/3224, 14.9% Usual care: 568/3061, 18.3% |
(+)
b
Alive and living at home (discharge) Admission to nursing home (discharge) Death or deterioration Cognitive function (−) Mortality at end of follow up, 3–12 months Dependence Death or dependence Activities of daily living Length of stay Readmission |
| Geriatric evaluation and management unit (GEMU) Van Craen, 201016 N = 7 studies |
Institutionalization at 12 months (4 studies
analyzed) Functional decline at hospital discharge (2 studies analyzed) |
RR = 0.78 (0.66–0.92) Intervention: 212/1423, 14.9% Control: 264/1438, 18.4% RR = 0.87 (0.77–0.99) Intervention: 306/1043, 29.3% Control: 342/1021, 33.5% |
(+) None (−) Mortality at 3 months Mortality at 6 months Mortality at 12 months Institutionalization at 3 months Institutionalization at 6 months Functional decline at 12 months Hospital readmission Length of stay |
| Acute geriatric units Baztan 200917 N = 11 studies |
Living at home at hospital discharge (5 studies
analyzed) Living at home 3 months after discharge (4 studies analyzed) |
OR = 1.30 (1.11–1.52) Intervention: 1229/1588, 77.4% Control: 1403/1962, 71.5% OR = 1.16 (0.99–1.37) Intervention: 972/1356, 71.7% Control: 992/1457, 68.1% |
(+) Functional decline at discharge Cost of admission (−) Admission to nursing home at discharge Admissiion to nursing home 3 months after discharge Case fatality Readmission at 3 months |
| Delirium prevention Hshieh 201518 N = 7 studies (4 medical, 3 surgical) |
Incident delirium (7 studies analyzed) Falls (4 studies analyzed) |
OR = 0.47 (0.39–0.58) Intervention: 129/1729, 7.5% Control: 301/2022, 14.9% OR = 0.38 (0.25–0.60) Intervention: 24/519, 4.6% Control: 95/519, 18.3% |
(+) None (−) Institutionalization Length of stay Change in functional status Change in cognitive status |
| Orthopedic- geriatric Gregoryan 201420 N = 18 studies |
Inpatient mortality (9 studies analyzed) Mortality at 6–12 months (11 studies analyzed) |
RR = 0.60 (0.43–0.84,) Intervention: 91/1694, 5.4% Control: 149/1639, 9.1% RR = 0.83 (0.74–0.94) Intervention: 348/2274, 15.3% Control: 703/4031, 17.4% |
(+) Length of stay (−) Time to surgery |
| Palliative care
c
Kavalieratos 201619 N = 43 included in review; 23 included in meta-analyis |
Quality of life at 1–3 months (15 studies analyzed; only 2/15
RCTs were hospital based) Symptom burden at 1–3 months (10 studies analyzed; only 1/10 RCTs was hospital based) |
SMD = 0.46 (0.08–0.83) SMD = −0.66 (−1.25 to −0.07) |
(+) Symptom burden at 4–6 months (−) Quality of life at 4–6 months Survival |
| Geriatric emergency department | No meta-analysis available | ||
| Trauma-geriatric | No meta-analysis available |
aFor the sake of space, only the two most positive outcomes [defined as best odds ratios (ORs), relative risks (RRs) or standard mean differences (SMDs)] for each meta-analysis are reported here, with other outcomes analyzed by meta-analyses reported under the last column, “significantly different” or “not significantly different.”
bMeta-analysis by Ellis did several analyses based on subgroups, for which this table does not have space. For example, Ward versus Team approach of CGA, targeting (needs-related vs. age-related criteria for admission to study); and timing of admission (for example, direct admission vs. stepdown vs. acute admission).
cFor palliative care, there were several different instruments used to evaluate quality of life and symptom burden, so pooled results were summarized as standardized mean differences (SMDs).
Table 1 also reports the total number (and percentage) of events for intervention and control groups in aggregate, for each meta-analysis. Despite these positive outcomes, there seems to be a limited size effect and not all outcomes were affected. Several reasons for this have been hypothesized by the authors of the meta-analyses: inclusion of patients for whom the outcome would not have happened anyway (lack of targeting highest risk patients), difficulty in implementation and compliance of all components of multicomponent models; financial and time constraints; inability to control for confounding factors; and complexities of care processes in hospitals beyond what is involved in the model (Ellis et al., 2017; Van Creen et al., 2010; Baztán et al., 2009; Hshieh et al., 2015; Grigoryan et al., 2014). The reason this is important is that a geriatric hospital based on principles in these geriatric models of care has the potential to address some of these problems. For example, a geriatric hospital may be better equipped to implement all components of these models and maintain compliance since the foundational goals of the hospital are to improve outcomes similar to these studies, for every patient.
Another reason in support of geriatric hospitals is that these models have not been widely implemented throughout adult hospitals (Clark, 2013; Palmer, 2018). Various reasons for low implementation rates of these models include the following: replication in the real world involves many more steps than a short-term study; not enough buy-in from hospital administration or front-line nurses and physicians; complexities and priorities of other services within hospitals; lack of leadership; lack of champions; shortage of geriatricians; market forces and ageism (Clark, 2013; Flaherty & Little, 2011; Inouye et al., 2006; Palmer, 2018; Rubin et al., 2011).
We propose that there are at least two root causes of why it is not possible to fully implement the principles of geriatric models in US adult hospitals: competing interests and too many different hospital cultures.
Competing Interests
Competing interests stem from several areas. Hospital Chief Financial Officers and other financial managers know that certain service lines generate more revenue than others, such oncology, cardiology or gastroenterology, or the surgical programs such as elective orthopedics and cardiothoracic surgery (Ellison, 2018; Butcher, 2102). Although leadership in hospitals may recognize the importance or even support geriatric-based concepts of care, these models have difficulty financially competing with higher revenue programs. (Palmer, 2018)
Another indirect source of competing interests comes as a result of frail older patients being cared for on different units throughout the hospital, rather than on one unit where staff can be trained with a focus on geriatric assessments and protocols. Although hospitals could train staff on cardiology units or neurology units for example, this makes trying to implement and maintain use of protocols and principles of geriatric models of care difficult. Another key component of models like the ACE Unit include a specialized environment that promotes mobility and safe patient self-care. Studies have shown more success of the ACE Unit model when it is located on one specialized unit with dedicated staff compared with ACE-like care for older patients on several different units (Flood et al., 2018). This idea of having more success with a geriatric model of care if it is done in one location is also evident from studies about orthopedic-geriatric models of care (Grigoryan et al., 2014).
Too Many Different Hospital Cultures
Hospitals have many different cultures of the way they care for a variety of patients and their acute illnesses. Cultures are important as they can create an environment to help employees know what is expected of them and what is important (Strasser et al., 2002). Although hospital cultures can have positive impact on the patient experience, patient safety and patient outcomes (Shaw, 2002), they can also lead to negative consequences for frail older patients who might not fit into that unit’s culture of care (Shaw, 2002; Khokher et al., 2009). For example, cardiology units and neurology units utilize important protocols that decrease variation and improve outcomes. However, if a frail older patient with a urinary tract infection and delirium is on one of these units, every 2 hour vital signs or neurological checks throughout the night (usual culture of care) could disrupt sleep leading to worsening of delirium.
Another hazard older patients face is that they are at risk of being considered a homogenous high-risk group for hospital associated adverse events, which creates potentially harmful blanket approaches. For example, bed alarms for fall risk, which limit mobility and disrupt sleep wake cycles, are implemented based on protocols which use older age as a major risk factor for falls (Growden et al., 2017). Another example is the use of physical restraints to prevent patients from pulling on lines, tubes or telemetry monitors before considering alternatives to restraints (Sullivan-Marx et al., 2003; Cosper et al., 2015; Tolson & Morley, 2012) or trying to discontinue what the patient is pulling on (Flaherty & Little, 2011).
Table 2 describes several other examples of typical hospital processes that have created a culture of how we care for all acutely ill adults. For frail older patients, these may directly or indirectly lead to negative consequences already discussed above. Some of these usual care processes include early morning blood draws, restricted diets, fasting protocols, hospital gowns and an expectation that older patients need discharge to a facility rather than home.
Table 2.
Examples of typical hospital processes that have created a culture of how we care for acutely ill older adults, and possible solutions for change
| Processes | Possible solutions for change |
|---|---|
| Admission processes | |
| Patients from home, facilities or clinics | Set up admission processes that avoid the ED, such as use of telemedicine or internet applications to communicate with patients and providers at home, facilities and clinics to expedite triage decisions |
| ED processes | |
| Use of typical gurneys, wait times, typical triage processes (prioritization), competing interests | Geriatric EDs are becoming more common |
| Other ideas | |
| Develop protocols/pathways for quicker admission processes With the presence of hospitalists, could some subset of patients be evaluated on various wards or locations within various wards, thus bypassing ED? | |
| Physician-centered routines or physician-based processes | |
| Tradition or routine of early morning rounds by physicians | Was once necessary for physicians who had outpatient clinic responsibilities |
| Use of hospitalists allows for changing the time of patient rounds | |
| This has potential for a better assessment (mid-morning, patients may be more alert and out of bed for functional assessment) | |
| Bed rest or other restricted mobility orders by physicians | Protocols for immediate baseline assessment and evaluation of mobility status; highest possible level ordered by interprofessional team member, usually therapist |
| Required physician order for physical therapy, occupational therapy or speech therapy | Develop protocols/pathways that a medical director of a floor could sign |
| Develop screening done by these therapists that allow them to improve efficiency of patients being seen | |
| This would need oversight and monitoring | |
| Mobility teams for daily ambulation instead of relying on therapists for daily ambulation/mobility | |
| Early morning blood tests Need for frequent blood tests | Adjust times to meet patients' needs |
| Examine and modify current internal medicine practices related to frequent blood tests (e.g. CBC every 6 hours for suspected bleeding; cardiac enzymes every 8 hours for suspected myocardial inschemia) | |
| Admission diet orders delayed or restricted diet ordered | Protocols for immediate baseline assessment and evaluation of nutrition and swallowing status; highest possible level ordered by interprofessional team member |
| Nurse processes | |
| Fasting (“NPO”) before procedures or surgeries | Individualize for patients and procedures |
| Critically analyze current routines | |
| Early recovery after surgery protocols already limit this practice | |
| Could geriatric hospitals develop further protocols for safely limiting current fasting procedures? | |
| Nurse:patient ratios and nurse assignments are typically based on having some patients with heavier or lighter care needs than others This can create variation from day to day | Although older patients are not a homogenous group, not having younger adult patients in the equation for nurse patient ratios may allow for more consistent determination of needs |
| This may also allow for developing new ideas to typical nurse:patient ratios | |
| For example, increasing nurse aides with more care responsibilities instead of registered nurses | |
| Routine vital signs and overutilization of vital signs | Use of “non-touch” technology to monitor RR and HR and T |
| Individualize vitals sign frequency | |
| Avoid middle-of-the-night vital signs when appropriate | |
| Routine medication times based on nurses' schedules and availability/prioritization of tasks | Individualize medication times to match what the patient does at home |
| Individualize patient care plans to allow self-administration of certain medications | |
| “Sitters” or one-to-one observers | Enhance training of these “care partners” who are the constant eyes and ears on the patient and who could implement mobility programs and other geriatric-based protocols |
| Bed alarms | Close constant observation |
| Wireless technology | |
| Direct patient care processes | |
| The bed as the center of the room The bed as the primary location for patient care | Develop patient rooms where bed is not the center |
| Develop or create ways to give care outside the bed. (e.g. IV treatments in a chair) | |
| Sleep schedules that are not similar to home and lack of healthy sleep environment | Night time sleep is a priority |
| Individualize sleep schedules | |
| Modify routine care to avoid sleep disruptions | |
| Routine meal times Suboptimal quality of meals, lack of preference for what older person might want | Individualize meal times to match what the patient does at home |
| Small kitchens where food is available and can be prepared anytime | |
| Expectation that families bring food from home | |
| Hospital gowns Gowns add to dehumanizing effect of the hospital and may add to sick behavior | Use of gowns that are more dignified |
| Use of a variety of gowns to give patients choice | |
| Discourage use of gowns; encourage use of patients' usual clothing | |
| Not allowing the family or significant others to be involved in direct patient care | Involve family as part of the health care team from admission |
| Would include some basic training and consent process | |
| Could be useful for basic activities such as feeding, bathing, toileting | |
| Adverse events (AEs) of older adults are diluted because hospital rates are based on all inpatients, most of whom are adults younger than age 65 years, who’s risk for AEs is very low (e.g. catheter-associated urinary tract infections, adverse drug events, falls with injury) | Although “higher” rates seem contradictory to improvement, having higher rates creates an importance to these AEs for which focused interventions targeted at a higher risk population can be done to lower rates |
| These lower rates could become new age-based goals for other adult hospitals | |
| Indirect patient care processes | |
| Transportation around the hospital | Use this as an opportunity |
| Getting outside of the hospital room could have health benefits | |
| Transporters could play an important role in this if they receive training in geriatric principles | |
| How patients are scheduled for procedures tests or surgeries Usually first come first serve, or no apparent process | Develop scheduling systems based on factors such as frailty or risk of delirium, especially if procedures absolutely require NPO |
| Division of labor for jobs in hospital | Consider education and training of any employees that have any type of contact with patients |
| For example, housekeeping employees could be trained on how to interact with people who have dementia or delirium | |
| Traffic into and out of the patients room is random and not monitored | Limited access to patient rooms; for example, housekeeping only during times when patient is not in room |
| Close monitoring through windows | |
| Having staff stay in a room is different than staff entering/leaving a room frequently | |
| Discharge processes | |
| Perception that discharge to post-acute care is common and expected | Many hospitals understand the benefit of starting discharge planning early |
| Could be done day one, with an emphasis on “our hospital’s goal is to help people return home after a hospitalization. Is that your goal?" | |
| Discharge follow up not standardized | Develop standardized protocols for follow up based on geriatric based goals identified in studies of geriatric models of care (such as function, return to home) |
| Develop systems that follow the patient for extended periods (e.g. 3 months, or even 1 year) | |
Abbreviations: ED, emergency department; IV, intravenous; CBC, complete blood count; NPO, latin for “nil per os” meaning nothing orally; RR, respiratory rate; HR, heart rate; T, temperature
One of the most important reasons that usual hospital culture is a barrier to implementing geriatric principles and models of care is that when a culture change is necessary, as is the case for geriatric models, it may be the most difficult part of a hospital to change. Culture was the number one response in a survey of senior health care executives when asked about major barriers for creating an effective patient experience. (Commins, 2013). The ACE Unit is an example of a geriatric model of care that requires a major cultural change (Palmer, 2018).
Geriatric Hospitals: Changing Typical Processes of Care and Developing Innovative Structural Designs
As long as these two root causes exist, (competing interests and cultures that distract focus away from needs of frail older patients), geriatric models of care and the principles upon which they are based will never be fully implemented and their full potential to have a positive impact will not be seen. Geriatric hospitals, dedicated solely to the care of frail older patients, would be different from adult hospitals because they could change typical processes of care on a hospital wide basis to fit the needs of frail older patients and develop innovative structural design, also on a hospital wide basis, in order to enhance mobility while maintaining safety.
Changing typical processes of care
Table 2 lists possible solutions for changing typical hospital processes. Geriatric hospitals, unlike adult hospitals, have the potential to widely implement these changes because all hospital processes would be planned and carried out with the needs of frail older patients in mind. A critical piece to carrying out these changes on a hospital wide basis is that job descriptions of everyone working in the hospital should include some type of responsibility related to the processes that help frail older adults return to their pre-illness functional level. This also requires that everyone directly involved in the care of the patient to know the patient’s baseline function before the acute illness. The goals of the interprofessional teams would become more clear and accurate, leading to better decisions about the inpatient treatment and discharge plans (Rosen et al., 2018).
Implementing these changes also need to be based on principles of successful geriatric models. For example, there would be an emphasis on goals similar to geriatric models of care such as prevention of delirium instead of lack of awareness of delirium (Hshieh et al., 2015) and returning home instead of an assumption that most patients need skilled nursing facilities after hospitalization (Tolson & Morley, 2012).
Geriatric hospitals must meet the highest standards of care while meeting the individual needs of frail older adults. Critical thinking encourages the use of best practices while allowing for individual care to avoid harm (Heffner & Rudy, 2008). This is an essential component of the modern movement toward patient centered (P4) care (Morley & Vellas, 2017). A geriatric hospital would allow for usual care processes to be scrutinized for risks versus benefits. Examples include early morning blood draws, vital signs that interrupt sleep, fasting protocols before surgery and frequent unnecessary blood sugars. (Gustafsson et al., 2019; Tóth et al., 2020; Warnock & Latifi. 2022).
A major challenge in the care of frail older patients is the ability of staff, especially nurses, to give adequate care for this vulnerable population, in the context of how inadequate nurse:patient ratios can affect patient outcomes (Driscoll et al., 2018). There are several reasons why geriatric hospitals should be able to handle this staffing challenge. First, successful geriatric models of care such as ACE Units, orthopedic-geriatric models and delirium prevention models have shown improved outcomes without a change in the nurse:patient ratios (Counsell et al., 2000; Bhattacharyya et al., 2013; Rubin et al., 2011). Second, interdisciplinary team work can make care more coordinated so there is less duplication and more efficiency (Bauer et al., 2009). The third reason is related to missed nursing care, the concept that required patient care during a nurse’s shift is omitted or delayed (Kalisch et al., 2009). While a geriatric hospital might not be able to increase nurse:patient ratios, it would be able to change many of the factors affecting missed nursing care among frail older patients. In one study, researchers identified lack of managers’ competence in establishing care guidelines, negative attitudes towards frail older adults, weakness in interdisciplinary care and lack of knowledge, skills, and experience of nurses in dealing with frail older patients as reasons for missed nursing care (Rezaei-Shassavarloo et al., 2021). These areas of concern are some of the strengths of many geriatric models of care (Ellis et al., 2017; Van Creen et al., 2010; Baztán et al., 2009; Hshieh et al., 2015; Grigoryan et al., 2014). Lastly, studies of geriatric models of care have shown higher satisfaction of staff, including nurses, compared to usual care (Counsell et al., 2000; Bhattacharyya et al., 2013; Rubin et al., 2011).
Developing innovative structural designs
Innovation in structural design should follow two key principles. First, based on successful geriatric models of care (Van Craen et al., 2010; Baztan et al., 2009), an environment should be created that helps avoid time in bed and enhances and encourages mobility and self-care. Although we have known for almost a century about the dangers of bed rest (Asher, 1947), there has been little progress in developing interventions to address this problem (Brown et al., 2016). The bed continues to physically be the center of the patient room and from a culture of care perspective, the center of the care we give. There are several opportunities for innovation in this area. One option would be to remove the bed during non-sleeping hours by using a Murphy-type bed that folds up into the wall. An additional strategy would be to have an individualized specific goal for time out of bed, based on pre-illness time spent out of bed. Although ill patients need extra rest, what is lacking is an acceptable individualized goal for patients. While there are no clear data to guide physicians, a specific amount of time in bed and out of bed may be the first step towards developing research in this area. Even among hospitalized older adults in their 80s and 90s, individualized moderate-intensity resistance, balance, and walking exercises are feasible and effective (Martínez-Velilla et al., 2019).
Walking paths on units or walking programs, not just physical therapy, would be expected (Brown et al., 2016). Central dining areas, already a part of most ACE Units would also be needed (Palmer, 2018). For patients who do not prefer community or common dining, chairs and tables in the rooms can be used. Over-the-bed tables promote time in bed and increase risk of aspiration, so should not be a part of geriatric hospitals (Manganelli et al., 2014; Ishii et al., 2022).
The second key principle for innovation is the need to design environments with patient safety in mind. One major safety concern in hospitals is the risk of fall injury. Two examples of innovation here include dual stiffness floors which may reduce fall related injuries (Knoefel et al., 2013) and, since almost half of falls occur in and around the bathroom (Tzeng, 2010), use of automated sliding doors or replacing the doors with curtains, as long as privacy could be maintained, could make toileting easier and safer.
Another major safety concern for hospitals is related to the care of patients with delirium. These patients are very high risk for falls, sometimes require physical restraints or medications to control behaviors, can pull on lines or tubes necessary for care, and often need extra nursing time or even one-to-one sitters (Marcantonio, 2011). One innovative strategy is to design hospital rooms or units that allow for close observation without significantly increasing staff. This has been done using a model called the Delirium Room. The original model is an open 4-bed room, with dividing curtains for privacy. The care in the room is completely free of physical restraints, emphasizes non-pharmacological management of delirium, and utilizes patient care techs, trained to be active caregivers (instead of sitters), one of whom is always in the room. It has been shown to prevent loss of function and may have a positive effect on hospital LOS (Flaherty et al., 2010). This model has been replicated utilizing a 6-bed unit as part of an inpatient geriatrics program with a focus on older patients with any type of cognitive impairment (Flaherty et al., 2021). Another model of a Delirium Room in Singapore utilizes a central area within the room for activities and is also restraint free. It has been shown to improve function, decrease duration of delirium and decrease hospital LOS. (Chong et al., 2014).
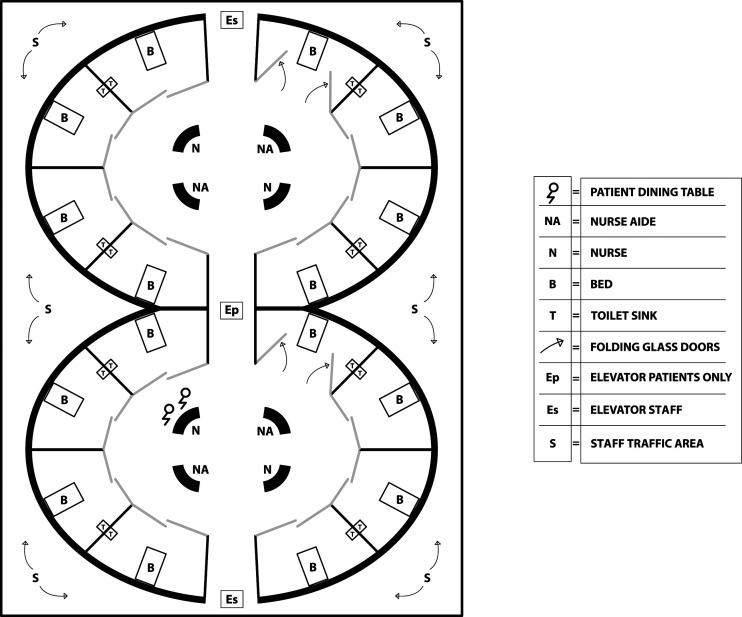
Other options for structural design to enhance close observation would be semicircular units which allow those outside the rooms, but not inside, to see into all of the rooms from a central location. An architectural example of semicircular units within a geriatric hospital is seen in Figure 1. This structural design of a more efficient layout may help improve factors associated with missed nursing care by decreasing time spent walking between patient rooms in long hallways and increase time spent directly observing patients (Rezaei-Shahsavarloo et al., 2021). This open design may also assist efforts and improve attitudes towards reducing restraints (Gunawardena & Smithard, 2019).
Figure 1.
Architectural example of semicircular units within a geriatric hospital.
Challenges
One of the first challenges geriatric hospitals may have is to figure out which older patients they should care for. As noted in the introduction, a geriatric hospital would have as its focus, the needs of frail older patients. The term frail is important for several reasons. It is a comprehensive term that encompasses most factors and characteristics associated with both the risk of negative consequences of hospital care and the likelihood of benefiting from principles of geriatric models of care (Gilbert et al., 2018; Gillick, 2014; Searle & Rockwood, 2018). Most studies on geriatric models of care used age instead of frailty as inclusion criteria, which allowed many older non-frail patients in, who would have had similar outcomes with usual care (Van Creen et al., 2010; Baztán et al., 2009; Hshieh et al., 2015; Grigoryan et al., 2014). Frailty is a well known entity (Fried et al., 2001) and many validated tools exist to identify this syndrome (Hewitt et al., 2018; Jørgensen & Brabrand, 2017; Warnier et al., 2016). Thus, it could be used as a screening criteria for which older adults with acute illnesses should be admitted to a geriatric hospital and which should be admitted to a typical adult hospital.
Another way to identify older patients who would benefit from geriatric hospitals is to screen for certain geriatric syndromes when older patients present with an acute illness. The most common geriatric syndromes among older patients coming to emergency departments in addition to frailty are dementia, delirium and falls (Carpenter & Mooijaart, 2020). The presence of these syndromes alone should not be reason enough for admission. However, the presence of both an acute illness justifying hospital care and geriatric syndromes could guide triage decisions about which patients need a geriatric hospital.
Another approach is to develop a list of certain conditions or illnesses that might benefit from principles of care based on geriatric models. However, there is no evidence that the medical treatment for some of the most common diagnoses such as heart failure, pneumonia or urinary tract infections (Agency for Healthcare Research and Quality, 2018) was any different in the geriatric models of care compared to usual care (Van Creen et al., 2010; Baztán et al., 2009; Hshieh et al., 2015; Grigoryan et al., 2014).
One final approach to consider is to use a higher age cutoff (e.g. age 80+) which would capture a population with enough high risk patients that positive outcomes could still be demonstrated (Sanford et al., 2020).
Another challenge is that geriatricians are in short supply. Thus it would be unlikely that there would be enough to cover all inpatient services. Specialized training for a new category of geriatric hospitalists may need to be developed (Sinvani et al., 2018).
Which services and which specialties geriatric hospitals require would need to be considered. Interventional radiology and surgical services such as orthopedics, trauma, and general surgery would likely be necessary (Mrdutt et al., 2019). Vascular surgery and urology services would be helpful (Drach & Griegling, 2003; Partridge et al., 2015). Based on the most common diagnoses for inpatient stays among patients age 75+ (Agency for Healthcare Research and Quality, 2018), neurology and common internal medicine subspecialties that adult hospitals have to offer would also be needed.
If a geriatric hospital has intensive care unit (ICU) beds, their associated outcomes should be carefully assessed. One study showed that a higher number of ICU beds increased use of mechanical ventilation among patients with dementia, but did not improve outcomes (Teno et al., 2016). The potential to overuse ICUs would have to be balanced with the negative aspect of not having them for older patients who become critically ill and would require transfer to another hospital with ICU services.
Financial viability and stability would be a challenge but should be feasible. It is likely that geriatric hospitals will have to be affiliated with a health care system that has other hospitals, rather than be independent entities. Although geriatric models of care do not generate revenue, they could help with cost savings. For example, frail older patients compared to non-frail older patients have longer average LOS and more often are discharged to post-acute care facilities (Simpson et al., 2018; McIsaac et al., 2016; Fuertes-Guiró & Velasco, 2020; Evans et al., 2014). If geriatric hospitals can target this population and concentrate efforts in one location, they could scale up the outcomes associated with successful geriatric models of care such as decreasing LOS and improving likelihood of returning home, without increasing costs (Grigoryan et al., 2014; Palmer, 2018; Zaubler et al., 2013).
Over utilization of tests and treatments puts frail older patients at particular risk for harm (American Geriatrics Society, 2021; Chalmers et al., 2021; Hajjar et al., 2005; Kouladjian et al., 2014; Wald, 2017) and increases the costs of medical care (Chalmers et al., 2021). Geriatric hospitals would offer the best opportunity to address this problem because they could more easily monitor utilization and develop standards for frail patients since all of the patients are frail. For example, hospital wide standards could be developed concerning appropriate use of percutaneous gastric feeding tubes for patients with advanced dementia (American Geriatrics Society, 2021). A strong palliative care program would be necessary since they have been shown to reduce costs while improving patient quality of life and symptom burden (Kavalieratos et al., 2016; Morrison et al., 2008).
Reimbursement is moving towards value-based care (Pay for Performance) (Zaresani & Scott, 2021). Geriatric hospitals, if modeled after successful geriatric models, should be able to do this better than typical adult hospitals. Also, health care systems that participate as an Accountable Care Organization with bundled payments may find geriatric hospitals beneficial since some of the cost savings with geriatric models of care can occur after discharge, for example related to increased likelihood of discharge home instead of a facility and reduced readmission rates (Deschodt et al., 2013; Flood et al., 2013; Navathe et al., 2021; Palmer, 2018).
Choosing the right size for geriatric hospitals may be a challenge, but starting smaller would probably be prudent. In 2019, there were over 6000 registered hospitals in the US. More than half of the hospitals had less than 100 beds, and one-third had less than 50 beds. This suggests that small hospitals can be financially viable (Michas, 2021).
Setting New Standards
Geriatric hospitals have the potential to set standards or benchmarks for many of the outcomes mentioned above that geriatric models have examined (such as percentage of patients returning home or delirium incidence). They also have the potential to set either age-based or frailty-based goals, as opposed to adult hospitals, that lump all patients into quality data. This lumping “dilutes” the actual rates for older patients for areas such as infections (CAUTIs, hospital acquired pneumonia, central line infections), DVTs/PEs, pressure ulcers, 30 day readmissions and mortality (Agency for Healthcare Research and Quality, 2017). Data like this may encourage adult hospitals to use data from geriatric hospitals as benchmarks for older patients in their hospital.
Conclusion
Although several successful models of care exist for hospitalized older adults, their effect size is small and the models are difficult to fully implement, sustain and replicate, due to competing interests and current hospital cultures. In a geriatric hospital, every single hospital process, and in turn hospital-wide culture, would be based on the needs of frail older adults, with an acute awareness of the potential risks of negative outcomes associated with hospitalization. Key structural designs would improve mobility while maintaining safety. Although there are challenges to the development, management and success of a geriatric hospital, these challenges present opportunities for innovative solutions which would help advance the standards of hospital care of frail older adults.
Acknowledgments
The authors would like to thank Dr. Muriel Gillick whose advice and review of the paper were extremely helpful. We are also grateful to her for her original article 20 years ago on this topic. We would also like to thank Mengyang Zhang for her architectural drawing of the semicircular units in proposed Geriatric Hospitals.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Joseph H. Flaherty https://orcid.org/0000-0003-4338-8810
References
- Agency for Healthcare Research and Quality (2017). Estimating the additional hospital inpatient cost and mortality associated with selected hospital-acquired conditions. https://www.ahrq.gov/hai/pfp/haccost2017-results.html [Google Scholar]
- Agency for Healthcare Research and Quality (2018). HCUP fast stats - most common diagnoses for inpatient stays. https://www.hcup-us.ahrq.gov/faststats/NationalDiagnosesServlet?year1=2018&characteristic1=25&included1=1&year2=&characteristic2=0&included2=1&expansionInfoState=hide&dataTablesState=hide&definitionsState=hide&exportState=hide [PubMed] [Google Scholar]
- American Geriatrics Society . (2021). Choosing wisely: Ten things clinicians and patients should question. https://www.choosingwisely.org/societies/american-geriatrics-society/ [Google Scholar]
- Asher R. A. (1947). The dangers of going to bed. British Medical Journal, 2(4536), 967–968. 10.1136/bmj.2.4536.967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M., Fitzgerald L., Haesler E., Manfrin M. (2009). Hospital discharge planning for frail older people and their family. Are we delivering best practice? A review of the evidence. Journal of Clinical Nursing, 18(18), 2539–2546. 10.1111/j.1365-2702.2008.02685.x [DOI] [PubMed] [Google Scholar]
- Baztán J. J., Suárez-García F. M., López-Arrieta J., Rodríguez-Mañas L., Rodríguez-Artalejo F. (2009). Effectiveness of acute geriatric units on functional decline, living at home, and case fatality among older patients admitted to hospital for acute medical disorders: Meta-analysis. BMJ (Clinical Research Ed, 338(jan22 2), b50. 10.1136/bmj.b50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya R., Agrawal Y., Elphick H., Blundell C. (2013). A unique orthogeriatric model: A step forward in improving the quality of care for hip fracture patients. International Journal of Surgery, 11(10), 1083–1086. 10.1016/j.ijsu.2013.09.018 [DOI] [PubMed] [Google Scholar]
- Brown C. J., Foley K. T., Lowman J. D., Jr, MacLennan P. A., Razjouyan J., Najafi B., Locher J., Allman R. M. (2016). Comparison of posthospitalization function and community mobility in hospital mobility program and usual care patients: A randomized clinical trial. JAMA Internal Medicine, 176(7), 921–927. 10.1001/jamainternmed.2016.1870 [DOI] [PubMed] [Google Scholar]
- Butcher L. (2012). Hospital service line organization: Innovation in approaches and strategy. Modern Healthcare Research Insights. https://www.modernhealthcare.com/assets/pdf/CH81353810.PDF (Accessed on 17 April 2022). [Google Scholar]
- Carlson R. W., Devich L., Frank R. R. (1988). Development of a comprehensive supportive care team for the hopelessly ill on a university hospital medical service. JAMA, 259(3), 378–383. 10.1001/jama.1988.03720030038030 [DOI] [PubMed] [Google Scholar]
- Carpenter C. R., Mooijaart S. P. (2020). Geriatric Screeners 2.0: Time for a paradigm shift in emergency department vulnerability research. Journal of the American Geriatrics Society, 68(7), 1402–1405. 10.1111/jgs.16502 [DOI] [PubMed] [Google Scholar]
- Chalmers K., Smith P., Garber J., Gopinath V., Elshaug A. G., Saini V. (2021). Assessment of overuse of medical tests and treatments at US hospitals using medicare claims. JAMA Netw Open, 4(4), e218075. 10.1001/jamanetworkopen.2021.8075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong M. S., Chan M., Tay L., Ding Y. Y. (2014). Outcomes of an innovative model of acute delirium care: The geriatric monitoring unit (GMU). Clinical Interventions in Aging, 9, 603–612. 10.2147/CIA.S60259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C.(2013) If ACE units are so great, why aren’t they everywhere? Health Leaders Media. https://www.healthleadersmedia.com/clinical-care/if-ace-units-are-so-great-why-arent-they-everywhere [Google Scholar]
- Collard A. F., Bachman S. S., Beatrice D. F. (1985). Acute care delivery for the geriatric patient: An innovative approach. QRB. Quality Review Bulletin, 11(6), 180–185. [PubMed] [Google Scholar]
- Commins J. (2013). Changing a stubborn hospital culture. HealthLeaders Media. http://healthleadersmedia.com/page-1/LED-298351/Changing-a-Stubborn-Hospital-Culture [Google Scholar]
- Cosper P., Morelock V., Provine B. (2015). Please release me: Restraint reduction initiative in a health care system. Journal of Nursing Care Quality, 30(1), 16–23. http://doi.org.10.1097/NCQ.0000000000000074 [DOI] [PubMed] [Google Scholar]
- Counsell S. R., Holder C. M., Liebenauer L. L., Palmer R. M., Fortinsky R. H., Kresevic D. M., Landefeld C. S., Allen K. R., Covinsky K. E. (2000). Effects of a multicomponent intervention on functional outcomes and process of care in hospitalized older patients: A randomized controlled trial of acute care for elders (ACE) in a community hospital. Journal of the American Geriatrics Society, 48(12), 1572–1581. 10.1111/j.1532-5415.2000.tb03866.x [DOI] [PubMed] [Google Scholar]
- Creditor M. C. (1993). Hazards of hospitalization of the elderly. Annals of Internal Medicine, 118(3), 219–223. 10.7326/0003-4819-118-3-199302010-00011 [DOI] [PubMed] [Google Scholar]
- Deschodt M., Flamaing J., Haentjens P., Boonen S., Milisen K. (2013). Impact of geriatric consultation teams on clinical outcome in acute hospitals: A systematic review and meta-analysis. BMC Medicine, 11(1), 1–13. 10.1186/1741-7015-11-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drach G.W., Griebling T.L. (2003). Geriatric urology. Journal of the American Geriatrics Society, 51(7), S355–S358. 10.1046/j.1365-2389.2003.51348.x [DOI] [PubMed] [Google Scholar]
- Driscoll A., Grant M. J., Carroll D., Dalton S., Deaton C., Jones I., Lehwaldt D., McKee G., Munyombwe T., Astin F. (2018). The effect of nurse-to-patient ratios on nurse-sensitive patient outcomes in acute specialist units: A systematic review and meta-analysis. European Journal of Cardiovascular Nursing, 17(1), 6–22. 10.1177/1474515117721561 [DOI] [PubMed] [Google Scholar]
- Ellis G., Gardner M., Tsiachristas A., Langhorne P., Burke O., Harwood R. H., Conroy S. P., Kircher T., Somme D., Saltvedt I., Wald H., O'Neill D., Robinson D., Shepperd S. (2017). Comprehensive geriatric assessment for older adults admitted to hospital. The Cochrane database of systematic reviews, 2017(9), CD006211. 10.1002/14651858.CD006211.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison A. (2018). These 10 physician specialties generate the most revenue for hospitals. Becker’s hospital CFO report. https://www.beckershospitalreview.com/finance/10-physician-specialties-that-generate-the-most-revenue-for-hospitals.html (Accessed on 17 April 2022). [Google Scholar]
- Evans S. J., Sayers M., Mitnitski A., Rockwood K. (2014). The risk of adverse outcomes in hospitalized older patients in relation to a frailty index based on a comprehensive geriatric assessment. Age and Ageing, 43(1), 127–132. 10.1093/ageing/aft156 [DOI] [PubMed] [Google Scholar]
- Fallon W. F., Jr, Rader E., Zyzanski S., Mancuso C., Martin B., Breedlove L., DeGolia P., Allen K., Campbell J. (2006). Geriatric outcomes are improved by a geriatric trauma consultation service. The Journal of Trauma, 61(5), 1040–1046. 10.1097/01.ta.0000238652.48008.59 [DOI] [PubMed] [Google Scholar]
- Flaherty J. H., Bloomstone J. A., Sande E. V., Brantley A., Semien G. A. (2021). An inpatient geriatrics program with a focus on any type of cognitive impairment reduces mortality. The Journal of Nutrition, 26(1), 103–109. 10.1007/s12603-021-1709-0 [DOI] [PubMed] [Google Scholar]
- Flaherty J. H., Little M. O. (2011). Matching the environment to patients with delirium: Lessons learned from the delirium room, A restraint-free environment for older hospitalized adults with delirium. Journal of the American Geriatrics Society, 59(Supple.2), 295–300. 10.1111/j.1532-5415.2011.03678.x [DOI] [PubMed] [Google Scholar]
- Flaherty J. H., Steele D. K., Chibnall J. T., Vasudevan V. N., Bassil N., Vegi S. (2010). An ACE unit with a delirium room may improve function and equalize length of stay among older delirious medical inpatients. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 65(12), 1387–1392. 10.1093/gerona/glq136 [DOI] [PubMed] [Google Scholar]
- Flaherty J. H., Tariq S. H., Raghavan S., Bakshi S., Moinuddin A., Morley J. E. (2003). A model for managing delirious older inpatients. Journal of the American Geriatrics Society, 51(7), 1031–1035. 10.1046/j.1365-2389.2003.51320.x [DOI] [PubMed] [Google Scholar]
- Flood K. L., Booth K., Vickers J., Simmons E., James D. H., Biswal S., Deaver J., White M. L., Bowman E. H. (2018). Acute care for elders (ACE) team model of care: A clinical overview. Geriatrics (Basel, Switzerland), 3(3), 50. 10.3390/geriatrics3030050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood K. L., MacLenan P. A., McGrew D., Green D., Dodd C., Brown C. J. (2013). Effects of an acute care for elders unit on costs and 30-day readmissions. JAMA Internal Medicine, 173(11), 981–987. https://doi.org.10.1001/jamainternmed.2013.524 [DOI] [PubMed] [Google Scholar]
- Fox M. T., Persaud M., Maimets I., O'Brien K., Brooks D., Tregunno D., Schraa E. (2012). Effectiveness of acute geriatric unit care using acute care for elders components: A systematic review and meta‐analysis. Journal of the American Geriatrics Society, 60(12), 2237–2245. 10.1111/jgs.12028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried L. P., Tangen C. M., Walston J., Newman A. B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W. J., Burke G., McBurnie M. A. (2001). Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 56(3), M146–M157. 10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- Fuertes-Guiró F., Viteri Velasco E. (2020). The impact of frailty on the economic evaluation of geriatric surgery: Hospital costs and opportunity costs based on meta-analysis. Journal of Medical Economics, 23(8), 819–830. 10.1080/13696998.2020.1764965 [DOI] [PubMed] [Google Scholar]
- Gilbert T., Neuburger J., Kraindler J., Keeble E., Smith P., Ariti C., Arora S., Street A., Parker S., Roberts H.C., Bardsley M., Conroy S. (2018). Development and validation of a hospital frailty risk Score focusing on older people in acute care settings using electronic hospital records: An observational study. Lancet, 391(10132), 1775–1782. 10.1016/S0140-6736(18)30668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillick M. R. (2014). When frail elderly adults get sick: Alternatives to hospitalization. Annals of Internal Medicine, 160(3), 201–202. 10.7326/M13-1793 [DOI] [PubMed] [Google Scholar]
- Gou R. Y., Hshieh T. T., Marcantonio E. R., Cooper Z., Jones R. N., Travison T. G., Fong T. G., Abdeen A., Lange J., Earp B., Schmitt E. M., Leslie D. L., Inouye S. K. (2021). One-year medicare costs associated with delirium in older patients undergoing major elective surgery. JAMA Surgery, 156(5), 430–442. 10.1001/jamasurg.2020.7260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan K. V., Javedan H., Rudolph J. L. (2014). Orthogeriatric care models and outcomes in hip fracture patients: A systematic review and meta-analysis. Journal of Orthopaedic Trauma, 28(3), Article e49–e55. 10.1097/BOT.0b013e3182a5a045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Growdon M. E., Shorr R. I., Inouye S. K. (2017). The tension between promoting mobility and preventing falls in the hospital. JAMA Internal Medicine, 177(6), 759–760. 10.1001/jamainternmed.2017.0840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunawardena R., Smithard D. G. (2019). The attitudes towards the use of restraint and restrictive intervention amongst healthcare staff on acute medical and frailty wards—a brief literature review. Geriatrics, 4(3), 50. 10.3390/geriatrics4030050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson U. O., Scott M. J., Hubner M., Nygren J., Demartines N., Francis N., Ljungqvist O., Young-Fadok T. M., Hill A. G., Soop M., de Boer H. D., Urman R. D., Chang G. J., Fichera A., Kessler H., Grass F., Whang E. E., Fawcett W. J., Carli F. (2019). Guidelines for perioperative care in elective colorectal surgery: Enhanced recovery after surgery (ERAS®) Society recommendations: 2018. World Journal of Surgery, 43(3), 659–695. 10.1007/s00268-018-4844-y [DOI] [PubMed] [Google Scholar]
- Hajjar E. R., Hanlon J. T., Sloane R. J., Lindblad C. I., Pieper C. F., Ruby C. M., Branch L. C., Schmader K. E. (2005). Unnecessary drug use in frail older people at hospital discharge. Journal of the American Geriatrics Society, 53(9), 1518–1523. 10.1111/j.1532-5415.2005.53523.x [DOI] [PubMed] [Google Scholar]
- Heffner S., Rudy S. (2008). Critical thinking: what does it mean in the care of elderly hospitalized patients? Critical care nursing quarterly, 31(1), 73–78. 10.1097/01.CNQ.0000306400.09777.68 [DOI] [PubMed] [Google Scholar]
- Hewitt J., Long S., Carter B., Bach S., McCarthy K., Clegg A. (2018). The prevalence of frailty and its association with clinical outcomes in general surgery: A systematic review and meta-analysis. Age and Ageing, 47(6), 793–800. 10.1093/ageing/afy110 [DOI] [PubMed] [Google Scholar]
- Hogan T. M., Olade T. O., Carpenter C. R. (2014). A profile of acute care in an aging America: Snowball sample identification and characterization of United States geriatric emergency departments in 2013. Academic Emergency Medicine Official Journal of the Society for Academic Emergency Medicine, 21(3), 337–346. 10.1111/acem.12332 [DOI] [PubMed] [Google Scholar]
- Hshieh T. T., Yue J., Oh E., Puelle M., Dowal S., Travison T., Inouye S. K. (2015). Effectiveness of multicomponent nonpharmacological delirium interventions: A meta-analysis. JAMA Internal Medicine, 175(4), 512–520. 10.1001/jamainternmed.2014.7779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S. K., Baker D. I., Fugal P., Bradley E. H., Dissemination Project H. E. L. P. (2006). Dissemination of the hospital elder life program: Implementation, adaptation, and successes. Journal of the American Geriatrics Society, 54(10), 1492–1499. 10.1111/j.1532-5415.2006.00869.x [DOI] [PubMed] [Google Scholar]
- Inouye S. K., Bogardus S. T., Jr, Charpentier P. A., Leo-Summers L., Acampora D., Holford T. R., Cooney L. M., Jr (1999). A multicomponent intervention to prevent delirium in hospitalized older patients. The New England Journal of Medicine, 340(9), 669–676. https://doi-org.foyer.swmed.edu/10.1056/NEJM199903043400901 [DOI] [PubMed] [Google Scholar]
- Ishii M., Nakagawa K., Yoshimi K., Okumura T., Hasegawa S., Yamaguchi K., Nakane A., Tamai T., Nagasawa Y., Yoshizawa A., Tohara H. (2022). Time spent away from bed to maintain swallowing function in older adults. Gerontology, 1–10, 1–10. 10.1159/000522499 [DOI] [PubMed] [Google Scholar]
- Jørgensen R., Brabrand M. (2017). Screening of the frail patient in the emergency department: A systematic review. European Journal of Internal Medicine, 45, 71–73. 10.1016/j.ejim.2017.09.036 [DOI] [PubMed] [Google Scholar]
- Kalisch B. J., Landstrom G. L., Hinshaw A. S. (2009). Missed nursing care: A concept analysis. Journal of Advanced Nursing, 65(7), 1509–1517. 10.1111/j.1365-2648.2009.05027.x [DOI] [PubMed] [Google Scholar]
- Kavalieratos D., Corbelli J., Zhang D., Dionne-Odom J. N., Ernecoff N. C., Hanmer J., Hoydich Z. P., Ikejiani D. Z., Klein-Fedyshin M., Zimmermann C., Morton S. C., Arnold R. M., Heller L., Schenker Y. (2016). Association between palliative care and patient and caregiver outcomes: A systematic review and meta-analysis. JAMA, 316(20), 2104–2114. 10.1001/jama.2016.16840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasraghi F. A., Christmas C., Lee E. J., Mears S. C., Wenz J. F. (2005). Effectiveness of a multidisciplinary team approach to hip fracture management. Journal of Surgical Orthopaedic Advances, 14(1), 27–31. [PubMed] [Google Scholar]
- Khokher P., Bourgeault I. L., Sainsaulieu I. (2009). Work culture within the hospital context in Canada: Professional versus unit influences. Journal of Health Organization and Management, 23(3), 332–345. 10.1108/14777260910966753 [DOI] [PubMed] [Google Scholar]
- Knoefel F., Patrick L., Taylor J., Goubran R. (2013). Dual-stiffness flooring: Can it reduce fracture rates associated with falls? Journal of the American Medical Directors Association, 14(4), 303–305. 10.1016/j.jamda.2012.12.077 [DOI] [PubMed] [Google Scholar]
- Kouladjian L., Gnjidic D., Chen T. F., Mangoni A. A., Hilmer S. N. (2014). Drug burden index in older adults: Theoretical and practical issues. Clinical Interventions in Aging, 9, 1503–1515. 10.2147/CIA.S66660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landefeld C. S., Palmer R. M., Kresevic D. M., Fortinsky R. H., Kowal J. (1995). A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. The New England Journal of Medicine, 332(20), 1338–1344. 10.1056/NEJM199505183322006 [DOI] [PubMed] [Google Scholar]
- Leslie D. L., Marcantonio E. R., Zhang Y., Leo-Summers L., Inouye S. K. (2008). One-year health care costs associated with delirium in the elderly population. Arch Intern Med, 168(1), 27–32. 10.1001/archinternmed.2007.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levant S., Chari K., DeFrances C. J. (2015). Hospitalizations for patients aged 85 and over in the United States, 2000-2010. NCHS data brief (pp. 1–8). [PubMed] [Google Scholar]
- Manganelli J., Threatt A., Brooks J. O., Healy S., Merino J., Yanik P., Walker I., Green K. (2014). Examination of how and why over-the-bed tables are used: Use cases and needs from healthcare providers. Health Environments Research and Design Journal, 7(2), 104–126. 10.1177/193758671400700207 [DOI] [PubMed] [Google Scholar]
- Marcantonio E. R. (2011). Delirium. Annals of Internal Medicine, 154(11), ITC6. 10.7326/0003-4819-154-11-201106070-01006 [DOI] [PubMed] [Google Scholar]
- Martínez-Velilla N., Casas-Herrero A., Zambom-Ferraresi F., Sáez de Asteasu M. L., Lucia A., Galbete A., García-Baztán A., Alonso-Renedo J., González-Glaría B., Gonzalo-Lázaro M., Apezteguía Iráizoz I., Gutiérrez-Valencia M., Rodríguez-Mañas L., Izquierdo M. (2019). Effect of Exercise Intervention on Functional Decline in Very Elderly Patients During Acute Hospitalization: A Randomized Clinical Trial. JAMA internal medicine, 179(1), 28–36. 10.1001/jamainternmed.2018.4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D.A. (1984). Dr. Marjory warren and the origin of British geriatrics. Journal of the American Geriatrics Society, 32(4), 253–258. 10.1111/j.1532-5415.1984.tb02017.x [DOI] [PubMed] [Google Scholar]
- McIsaac D. I., Beaule P. E., Bryson G. L., Van Walraven C. (2016). The impact of frailty on outcomes and healthcare resource usage after total joint arthroplasty: A population-based cohort study. The Bone & Joint Journal, 98(6), 799–805. 10.1302/0301-620X.98B6.37124 [DOI] [PubMed] [Google Scholar]
- Michas F. (2021). Number of hospitals in the United States by number of beds 2019. Statista. https://www.statista.com/statistics/459779/total-hospital-numbers-in-the-us-by-number-of-beds/ [Google Scholar]
- Morley J. E., Vellas B. (2017). Patient-centered (P4) medicine and the older person. Journal of the American Medical Directors Association, 18(6), 455–459. 10.1016/j.jamda.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Morrison R. S., Penrod J. D., Cassel J. B., Caust-Ellenbogen M., Litke A., Spragens L. (2008). Palliative care leadership centers’ outcomes group cost savings associated with US hospital palliative care consultation programs. Archives of Internal Medicine, 168(16), 1783–1790. http://doi.org.10.1001/archinte.168.16.1783 [DOI] [PubMed] [Google Scholar]
- Mrdutt M. M., Papaconstantinou H. T., Robinson B. D., Bird E. T., Isbell C. L. (2019). Preoperative frailty and surgical outcomes across diverse surgical subspecialties in a large health care system. Journal of the American College of Surgeons, 228(4), 482–490. 10.1016/j.jamcollsurg.2018.12.036 [DOI] [PubMed] [Google Scholar]
- Navathe A. S., Liao J. M., Wang E., Isidro U., Zhu J., Cousins D. S., Werner R. M. (2021). Association of patient outcomes with bundled payments among hospitalized patients attributed to accountable care organizations. JAMA Health Forum, 2(8), Article e212131–e212131. https://doi.org.10.1001/jamahealthforum.2021.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M. (2018). The acute care for elders unit model of care. Geriatrics (Basel, Switzerland), 3(3), 59. 10.3390/geriatrics3030059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge J. S., Fuller M., Harari D., Taylor P. R., Martin F. C., Dhesi J. K. (2015). Frailty and poor functional status are common in arterial vascular surgical patients and affect postoperative outcomes. International Journal of Surgery, 18, 57–63. 10.1016/j.ijsu.2015.04.037 [DOI] [PubMed] [Google Scholar]
- Rezaei-Shahsavarloo Z., Atashzadeh-Shoorideh F., Ebadi A., Gobbens R. J. (2021). Factors affecting missed nursing care in hospitalized frail older adults in the medical wards: A qualitative study. BMC Geriatrics, 21(1), 1–12. 10.1186/s12877-021-02524-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M. A., DiazGranados D., Dietz A. S., Benishek L. E., Thompson D., Pronovost P. J., Weaver S. J. (2018). Teamwork in healthcare: Key discoveries enabling safer, high-quality care. The American Psychologist, 73(4), 433–450. 10.1037/amp0000298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein L. Z., Josephson K. R., Wieland G. D., English P. A., Sayre J. A., Kane R. L. (1984). Effectiveness of a geriatric evaluation unit. A randomized clinical trial. The New England Journal of Medicine, 311(26), 1664–1670. 10.1056/NEJM198412273112604 [DOI] [PubMed] [Google Scholar]
- Rubin F. H., Neal K., Fenlon K., Hassan S., Inouye S. K. (2011). Sustainability and Scalability of the hospital elder life program at a community hospital. Journal of the American Geriatrics Society, 59(2), 359–365. 10.1111/j.1532-5415.2010.03243.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager M. A., Franke T., Inouye S. K., Landefeld C. S., Morgan T. M., Rudberg M. A., Sebens H., Winograd C. H. (1996). Functional outcomes of acute medical illness and hospitalization in older persons. Archives of Internal Medicine, 156(6), 645–652. 10.1001/archinte.1996.00440060067008 [DOI] [PubMed] [Google Scholar]
- Sanford A. M., Morley J. E., Berg-Weger M., Lundy J., Little M. O., Leonard K., Malmstrom T. K. (2020). High prevalence of geriatric syndromes in older adults. Plos One, 15(6), Article e0233857. 10.1371/journal.pone.0233857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle S. D., Rockwood K. (2018). What proportion of older adults in hospital are frail? Lancet, 391(10132), 1751–1752. 10.1016/S0140-6736(18)30907-3 [DOI] [PubMed] [Google Scholar]
- Shaw J. (2002). Tracking the merger: The human experience. Health Services Management Research, 15(4), 211–222. 10.1258/095148402320589019 [DOI] [PubMed] [Google Scholar]
- Simpson K. N., Seamon B. A., Hand B. N., Roldan C. O., Taber D. J., Moran W. P., Simpson A. N. (2018). Effect of frailty on resource use and cost for medicare patients. Journal of Comparative Effectiveness Research, 7(8), 817–825. 10.2217/cer-2018-0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinvani L., Carney M., Kozikowski A., Smilios C., Patel V., Qiu G., Zhang M., Babalola O., Kandov Y., Rosenberg D., Wolf-Klein G., Pekmezaris R. (2018). The role of geriatrician-hospitalists in the care of older adults: A retrospective cohort study. Archives of Gerontology and Geriatrics, 77, 31–37. 10.1016/j.archger.2018.03.006 [DOI] [PubMed] [Google Scholar]
- Strasser D. C., Smits S. J., Falconer J. A., Herrin J. S., Bowen S. E. (2002). The influence of hospital culture on rehabilitation team functioning in VA hospitals. Journal of rehabilitation research and development, 39(1), 115-125. [PubMed] [Google Scholar]
- Stuck A. E., Siu A. L., Wieland G. D., Rubenstein L. Z., Adams J. (1993). Comprehensive geriatric assessment: A meta-analysis of controlled trials. Lancet, 342(8878), 1032–1036. 10.1016/0140-6736(93)92884-V [DOI] [PubMed] [Google Scholar]
- Sullivan-Marx E., Strumpf N., Evans L, Capezuti E., Maislin G. (2003). Effects of an advanced practice nursing intervention with physical restraint use among hospitalized nursing home residents. The Gerontologist, 43(Special Issue I), 310. [Google Scholar]
- Teno J. M., Gozalo P., Khandelwal N., Curtis J. R., Meltzer D., Engelberg R., Mor V. (2016). Association of increasing use of mechanical ventilation among nursing home residents with advanced dementia and intensive care unit beds. JAMA Internal Medicine, 176(12), 1809–1816. 10.1001/jamainternmed.2016.5964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolson D., Morley J. E. (2012). Physical restraints: Abusive and harmful. Journal of the American Medical Directors Association, 13(4), 311–313. 10.1016/j.jamda.2012.02.004 [DOI] [PubMed] [Google Scholar]
- Tóth V., Meytlis M., Barnaby D. P., Bock K. R., Oppenheim M. I., Al-Abed Y., McGinn T., Davidson K. W., Becker L. B., Hirsch J. S., Zanos T. P. (2020). Let Sleeping Patients Lie, avoiding unnecessary overnight vitals monitoring using a clinically based deep-learning model. npj Digit. Med, 3(1), 149. 10.1038/s41746-020-00355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng H. M. (2010). Understanding the prevalence of inpatient falls associated with toileting in adult acute care settings. Journal of Nursing Care Quality, 25(1), 22–30. 10.1097/NCQ.0b013e3181afa321 [DOI] [PubMed] [Google Scholar]
- Van Craen K., Braes T., Wellens N., Denhaerynck K., Flamaing J., Moons P., Boonen S., Gosset C., Petermans J., Milisen K. (2010). The effectiveness of inpatient geriatric evaluation and management units: A systematic review and meta-analysis. Journal of the American Geriatrics Society, 58(1), 83–92. 10.1111/j.1532-5415.2009.02621.x [DOI] [PubMed] [Google Scholar]
- Wald H. L. (2017). The geometry of patient safety: Horizontal and vertical approaches to the hazards of hospitalization. Journal of the American Geriatrics Society, 65(12), 2559–2561. 10.1111/jgs.15049 [DOI] [PubMed] [Google Scholar]
- Warnier R. M. J., Van Rossum E., Van Velthuijsen E., Mulder W. J., Schols J. M. G. A., Kempen G. I. J. M. (2016). Validity, reliability and feasibility of tools to identify frail older patients in inpatient hospital care: A systematic review. The Journal of Nutrition, Health & Aging, 20(2), 218–230. 10.1007/s12603-015-0567-z [DOI] [PubMed] [Google Scholar]
- Warnock S., Latifi N. (2022). Inpatient management of type 2 diabetes. JAMA Internal Medicine, 182(5), 543–544. http://doi.org.10.1001/jamainternmed.2022.0410 [DOI] [PubMed] [Google Scholar]
- Zaresani A., Scott A. (2021). Is the evidence on the effectiveness of pay for performance schemes in healthcare changing? Evidence from a meta-regression analysis. BMC Health Services Research, 21(1), 1–10. https://doi.org.10.1186/s12913-021-06118-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaubler T. S., Murphy K., Rizzuto L., Santos R., Skotzko C., Giordano J., Bustami R., Inouye S. K. (2013). Quality improvement and cost savings with multicomponent delirium interventions: Replication of the hospital elder life program in a community hospital. Psychosomatics, 54(3), 219–226. 10.1016/j.psym.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Zuckerman J. D., Sakales S. R., Fabian D. R., Frankel V. H. (1992). Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program. Clinical Orthopaedics and Related Research 1992(274), 213–225. [PubMed] [Google Scholar]