Abstract
Metastasis is one of the major reasons for patient mortality in hepatocellular carcinoma (HCC), and the progression of HCC to a metastatic state depends on the local microenvironment. Hypoxia is a key condition affecting the microenvironment of HCC. Currently, various studies have shown that the expression of hypoxia-ainducible factor-1α (HIF-1α) is associated with the invasion and metastasis of HCC. High expression of HIF-1α often leads to poor prognosis in patients with HCC. In this review, the molecular structure of HIF-1α is described, and the expression pattern of HIF-1α in HCC under hypoxia, which is associated with metastasis and poor prognosis in HCC, is explained. The molecular mechanisms of HIF-1α function and the metastasis of HCC are further discussed. The modulation of HIF-1α can reduce sorafenib resistance and improve the prognosis of patients after TACE. Therefore, HIF-1α may be a critical target for inhibiting HCC metastasis in the future.
Keywords: hepatocellular carcinoma, tumor metastasis, HIF-1α, hypoxia, hypoxia-inducible factor-1α
1. Introduction
Hepatocellular carcinoma (HCC) is the most frequently diagnosed cancer worldwide and the fourth leading cause of cancer-related mortality (1). According to the Global Cancer Burden Data Report from the Global Cancer Observatory platform (https://gco.iarc.fr/projects), World Health Organization, there were 905,677 new cases of liver cancer in 2020, of which 72.5% occurred in Asia. The total deaths from liver cancer were 830,180 in 2020. Despite the advances in surgical excision and systematic treatment, the prognosis of patients with HCC remains poor (2). This may be due to the high rate of tumor recurrence and metastasis. Hypoxia is a hallmark of the solid tumor microenvironment. Activation of the transcription factor hypoxia-inducible factor (HIF) enables cancer cells to adapt to the hypoxic environment via the transactivation of downstream target genes (3). The human genome encodes three different HIF subtypes: HIF-1α, HIF-2α and HIF-3α (4). Recent studies have shown that the HIF-2α expression level in HCC is correlated with clinical progression and poor survival (5), which promotes HCC metastasis by promoting the epithelial-mesenchymal transition (EMT) pathway, promoting lncRNA NEAT1 activation and inducing stem cell factor (SCF) transcription (6–8). Moreover, HIF-1α plays an important role in HCC metastasis under hypoxic conditions (9), but the underlying mechanism remains unclear. This review focuses on the role of HIF-1α in tumor metastasis, including linked signaling pathways, their impact on the resistance to current first-line therapies, and their implication in future therapies. HIF-1α is expected to become a critical target for the treatment of HCC in the future.
2. The structure and biological function of HIF-1α
The significant sequelae of most solid tumors include hypoxia and necrosis. Abnormal tumor microvessels, blocked microcirculation, and impaired diffusion lead to insufficient or no oxygen supply in the tumor microenvironment (TME) (10). HIF is an important biosensor for the oxygen concentration in tumors. HIF is a heterodimer that consists of an O2-sensitive HIF-1α subunit and an O2-insensitive HIF-1β subunit (11).
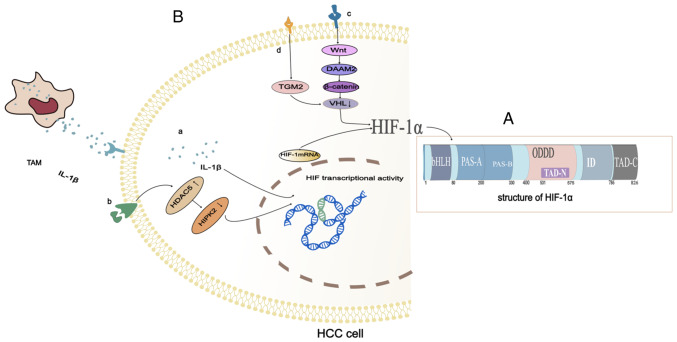
Hypoxia-induced gene expression mainly depends on the stability of the α subunit of HIF-1α, which is an oxygen-unstable subunit whose transcriptional activity is regulated by cellular oxygen tension (12). HIF-1α has two transactivation domains (TAD). The COOH terminal transactivation domain (C-TAD) is located in amino acid residues from 786 to 826 in humans. The C-TAD is recruited by co-activators, and the co-activators CBP and p300 regulate the transactivation of target genes (13). The NH2-terminal transactivation domain (N-TAD) is located in a region called the oxygen-dependent degradation domain (ODDD), which is positioned in amino acid residues from 400 to 600 (Fig. 1A). The ODDD is only recognized by von Hippel-Lindau tumor suppressor protein (pVHL) under normoxic conditions, and pVHL is required to mediate the degradation of HIF-1α in the ubiquitin-proteasome pathway. The ODDD contains two key proline residues that target hydroxylation under normoxic conditions. Under normal oxygen levels, HIF-1α undergoes hydroxylation of the proline. It is then recognized by the pVHL E3 ligase and degraded by the proteasome (14). In a hypoxic environment, an inadequate oxygen supply leads to impaired hydroxylation and degradation of the HIF-1α. This causes the HIF-1α to stabilize and the stable HIF-1α is then transferred to the nucleus. It acts as a heterodimer trans-activator with a nuclear protein called an aryl hydrocarbon receptor nuclear transposer (ARNT). The resulting heterodimer (HIF-1α/ARNT) specifically interacts with the hypoxia response element (HRE) to increase the transcription of genes involved in angiogenesis, erythropoiesis, and glycolysis (15). The HIF-1α and HIF-1β play critical roles in angiogenesis, proliferation, invasion, and cancer metabolism (16,17). Therefore, the ODDD controls the activity and stability of the α subunit, and the carboxy-terminal region of the HIF-1α represents the stability domain of the protein.
Figure 1.
Structure and synthetic pathway of HIF-1α. (A) Structure of HIF-1α. (B) (a) High expression of HDAC5 in hepatocellular carcinoma leads to silencing of HIPK2, which in turn induces the upregulation of HIF-1α. (b) M2-phenotype TAMs release IL-1β in the presence of persistent hypoxia and inflammation, which promotes HIF-1α synthesis. (c) DAAM can induce HIF-1α by reducing the expression of VHL in hepatoma cells. (d) Activated HSCs promote the upregulation of TGM2 in HCC cells through inflammatory signals, which can cause VHL depletion and lead to HIF-1α accumulation. TAMs, tumor-associated macrophages; IL-1β, interleukin-1β; HDAC5, histone deacetylase5; HIF-1α, hypoxia-inducible factor-1α; HIPK2, recombinant homeodomain interacting protein kinase 2; HSCs, hepatic stellate cells; VHL, Von Hippel-Lindau; TGM2, transglutaminase 2; DAAM, Dishevelled-associated activator of morphogenesis.
The HIF1A and HIF-1β genes are expressed in various tissues in humans. HIF1A mRNA expression was found to be stable in most tissue culture cell lines and was not affected by cellular oxygen tension (18,19). Hypoxia does not increase HIF1A because hypoxia stabilizes the HIF-1α protein primarily through post-translational modifications (20). In contrast to those observed in vitro, HIF1A mRNA levels were found to be significantly elevated in the brain, heart, kidney, lung, and skeletal muscle in vivo in response to hypoxia (21). The HIF-1α protein can be rapidly degraded by the ubiquitin-proteasome system under normal oxygen conditions (22,23). Therefore, the stability of HIF-1α is the main determinant of HIF-1α activity.
HIF-1α has been confirmed to be closely related to tumor metastasis, but the role of HIF-1α in the metastasis of primary HCC has not been systematically discussed. This review aims to comprehensively elaborate on the role of HIF-1α in the metastasis of HCC.
3. Expression and prognosis of HIF-1α in hepatocellular carcinoma
Increased expression of HIF-1α in hepatocellular carcinoma
The progression of hepatocellular carcinoma (HCC) depends on its local microenvironment. Hypoxia and inflammation are two key factors affecting the microenvironment of HCC (24,25). Under moderate hypoxia, the stability of HIF-1α is increased and tumor-associated macrophages secrete more interleukin-1β (IL-1β). In the presence of persistent and severe hypoxia, the necrotic debris of HCC cells mediates the release of potent IL-1β from tumor-associated macrophages with an M2 phenotype via interferon-β/nuclear factor kappa-light-chain-enhancer containing Toll-like receptor 4/TIR domains that activate B-cell signaling. The IL-1β in HCC cells promotes HIF-1α synthesis through cyclooxygenase 2 (COX-2) (26). Inflammation and hypoxia interact to promote the development of tumors (Fig. 1B-a).
In addition to the interaction of hypoxia and inflammation, the increase in epigenetic regulators and the Dishevelled-associated activator of morphogenesis (DAAM) can also promote the high expression of HIF-1α in hepatoma cells. As an epigenetic regulator, histone deacetylase (HDAC) is essential for activating gene transcription under physiological conditions and is also involved in the occurrence and development of tumors. HDAC5 is a histone deacetylase with a high expression in HCC. An increase in HDAC5 leads to silencing of homeodomain-interacting protein kinase-2 (HIPK2), which induces the transcription of HIF-1α resulting in high expression of HIF-1α (27) (Fig. 1B-b). DAAM consisting of DAAM1 and DAAM2 mainly mediates the coordination of form-dependent actin remodeling (28). DAAM controls cell shape and polarized cell growth by regulating the actin cytoskeleton. In HCC cells, DAAM2 is a Wnt signal transduction effector that acts downstream of the Wnt ligand and upstream of β-catenin. DAAM2 reduces Von Hippel-Lindau (VHL) expression and thus upregulates HIF-1α (29) (Fig. 1B-c).
Pseudohypoxia also causes HIF-1α hyperexpression. Activated hepatic stellate cells promote epithelial-mesenchymal transformation (EMT) in HCC through pseudohypoxia promoted by transglutaminase 2 (TGM2) (30). Activated hepatic stellate cells upregulate TGM2 in HCC cells through inflammatory signaling (31). VHL induced by TGM2 under normoxic conditions is the key molecule of HIF-1α degradation (32). Under normoxic conditions, TGM2 can cause VHL consumption, resulting in HIF-1α accumulation and pseudohypoxia, thus promoting EMT in HCC cells (30) (Fig. 1B-d).
The accumulation of HIF-1α was also found to be related to its stability. Hydroxylation of proline residues by proline hydroxylase domain enzymes (PHDs) is a key step in the degradation of HIF-1α (33). Prolyl hydroxylase domain-containing protein 2 (PHD2) requires O2 and α-ketoglutarate as substrates for hydroxylation of HIF-1α. O-linked β-N-acetylglucosamine transferase (OGT) can stabilize HIF-1α by reducing the level of α-ketoglutarate (34). Recent results show that HAUSP (USP7) is a deubiquitinase of HIF-1α (35). HAUSP can undergo hypoxia-induced K63-linked polyubiquitination by HectH9 to enhances its ability to deubiquitinate HIF-1α and also serve as a scaffold of HIF-1α-induced gene transcription (36). Plasmacytoma variant translocation 1 (PVT1) is a long non-coding (lnc)RNA that has been found to play an oncogenic role in a variety of malignant tumors. Lysine acetyltransferase 2A (KAT2A) is a histone acetyltransferase. Research shows that lncRNA PVT1 stabilizes HIF-1α via KAT2A (37). Research has shown that the STAT3 protein binds to HIF-1α through competition with pVHL, thus stabilizing HIF-1α protein levels (38). GATA binding protein 3 interacts with the full-length and the N-terminal part of HIF-1α (aa 1–401) under hypoxia to inhibit ubiquitination of HIF-1α (39).
Relationship between HIF-1α expression and prognosis of HCC
The expression of HIF-1α in HCC tissues was found to be significantly higher than that in chronic hepatitis B and normal liver tissues, but there was no significant difference when compared with that in the cirrhotic liver (40,41). A study analyzing the clinicopathological features in patients with HCC showed that upregulation of HIF-1α mRNA expression was strongly associated with TNM stage III and Barcelona clinical hepatocellular carcinoma (BCLC) stage C. The survival analysis showed that HCC patients with high HIF-1α mRNA expression had reduced overall survival when compared with patients with low HIF-1α mRNA expression (42). HIF-1α expression was positively correlated with vascular invasion, TNM stage, tumor size, and portal vein tumor thromboembolism in HCC patients with cirrhosis. The patients with high HIF-1α expression on the basis of liver cirrhosis had reduced overall survival (OS) when compared with the patients with low HIF-1α expression (40). However, a meta-analysis involving 851 patients with HCC showed no association between HIF-1α protein expression and HCC envelope formation, cirrhosis, tumor size, or tumor differentiation, except for vascular invasion (43).
As a prognostic factor, high HIF-1α expression was found to be associated with poor disease-free survival and poor prognosis (44). A meta-analysis of 34 studies involving 3,578 patients showed that HIF-1α overexpression was associated with poor OS, disease-free survival (DFS), and relapse-free survival (RFS) in HCC patients. Overexpression of HIF-1α was also associated with clinicopathologic features including BCLC, intrahepatic metastasis, lymph node metastasis, tumor-to-lymph node metastasis (TNM), tumor differentiation, tumor number, tumor size, vascular invasion, and angiogenesis (5). Another meta-analysis involving 3,238 patients in 22 studies also showed poor prognosis in HCC patients presenting with overexpression of HIF-1α (45). However, other studies showed that HIF-1α protein expression was associated with tumor differentiation, and intrahepatic and extrahepatic metastases, but not with the presence of portal vein tumor embolism, prognosis, or hepatitis B surface antigen (HBsAg) status (41).
4. HIF-1α promotes metastasis of hepatocellular carcinoma
Metastasis is a major challenge in the treatment of cancers. The vast majority of cancer-related deaths are caused by metastatic conditions in vital organs (46). In a solid tumor, the abnormal new vascular system of the tumor and the increase in oxygen consumption by proliferating HCC cells are not balanced, and thereby there is always hypoxia in HCC tissue. Hypoxia promotes the invasion and migration of HCC, and HIF-1α is also upregulated in HCC (47). Our understanding of the molecular basis of metastasis has improved in recent years, and the hypoxia-induced transcription factor (HIF) remains one of the best indicators for monitoring metastasis (48).
Enhancement of HIF activity drives tumor progression by regulating the expression of hundreds of genes. These genes are closely associated with tumor progressions such as immune escape, glycolysis, cancer stem cell maintenance, angiogenesis, EMT, and cancer stem cell maintenance (Table I) (26,47,49–61). HIF signals affect almost every step of the cascade leading to tumor metastasis (48,62).
Table I.
Molecular basis of HIF-1α promoting HCC metastasis.
| Phenotype | Target (Refs.) |
|---|---|
| Immune escape | ENTPD2, 5′-AMP (49), MDSCs, IL-6 (50) |
| pDCs, eADO, ADORA1 (51) | |
| Tumor-associated macrophages, IL-1β (26) | |
| IL-1β, PDL1 (52) | |
| Glycolysis | UPK1A (53) |
| lncRNA RAET1K, miR-100-5p (54) | |
| lncRNA NPSR1-AS1, MAPK/ERK (55) | |
| YAP (47) | |
| EMT, angiogenesis | LOXL2, VM formation (56) |
| VASP, MMP2, MMP9 (57) | |
| ROS, NOX4 (58) | |
| E-cadherin, Snai1, SIP1 (59) | |
| miR-1273F, Wnt/β-catenin (60) | |
| Stemness | SENP1, CD24, CD44, CD133 (61) |
HIF-1α, hypoxia-inducible factor-1α; ENTPD2, ectonucleoside tri-phosphate diphosphohydrolase 2; MDSCs, myeloid-derived suppressor cells; IL, interleukin; pDCs, plasmacytoid dendritic cells; eADO, extracellular adenosine; ADORA1, adenosine A1 receptor; PDL1, programmed death-ligand 1; UPK1A, uroplakin 1A; YAP, Yes-associated protein; LOXL2, lysyl oxidases like 2; VM, vasculogenic mimicry; VASP, vasodilator-stimulated phosphoprotein; MMP, matrix metalloproteinase; ROS, reactive oxygen species; NOX4, oxidase 4; SENP1, Sentrin-specific protease 1.
HIF-1α promotes metastasis by inducing immune escape in HCC
Changes in the development of immunosuppressive mechanisms by tumor cells play a crucial role in tumor development, which allows them to escape the host's immune system, thereby enhancing their survival and proliferative, migratory, and invasive capabilities (63). Hypoxia, which is associated with an imbalance in rapid tumor growth and an insufficient blood supply, is a common change that occurs in the microenvironment of solid tumors (64). Previous studies have found that HIF-1α can increase the number of myeloid-derived suppressor cells (MDSCs) (49,50) and plasmacytoid dendritic cells (pDCs) (51), and can induce IL-1β (26,52,65) expression in the TME leading to immune escape.
MDSCs possess immunosuppressive activities, which allow cancers to escape immune surveillance and become non-responsive to immune checkpoint blockade. HIF-1α induces ectonucleoside triphosphate diphosphohydrolase 2 (ENTPD2) in cancer cells to increase the extracellular level of 5′-AMP, which maintains MDSC undifferentiation in the tumor stroma (49). MDSCs enhance tumor cell stemness, increase angiogenesis, and advance the metastatic process by promoting EMT though IL-6 secretion (50). pDCs play immunosuppressive roles in the TME. HIF-1α was found to transcriptionally upregulate the expression of extracellular adenosine (eADO), and eADO was found to significantly enhance pDC recruitment into tumors via the adenosine A1 receptor (ADORA1) leading to immunosuppression (51). Tumor-associated macrophages, the primary proinflammatory cells within tumors, secrete more IL-1β under hypoxic conditions due to increased stability of HIF-1α (26). Another study demonstrated that IL-1β could increase the expression of solute carrier family 7 member 11, which facilitated HCC metastasis through colony stimulating factor 1-induced tumour-associated macrophage and MDSC infiltration in tumors (52).
HIF-1α promotes metastasis by inducing glycolysis in HCC
The glycolytic phenotype destroys normal tissues and promotes the secretion of proteolytic enzymes such as cathepsin B or metalloproteinases through lactate and H+-mediated extracellular environmental acidification, thereby promoting metastasis and invasion (66). As a result, glycolysis plays an important role in tumor invasion and metastasis. Recently, it was found that HIF-1α can enhance the process of glycolysis in HCC cells. HIF-1α directly binds to the hypoxia response element (HRE) in the promoter region of uroplakin 1A (UPK1A), leading to UPK1A being upregulated under hypoxia, thereby increasing the glycolysis of HCC cells (53). HIF-1α activates transcription of lncRNA RAET1K through miR-100-5p to enhance hypoxia-induced glycolysis in HCC cells (54). In addition, hypoxia-inducible lncRNA NPSR1-AS1 promotes the proliferation and glycolysis of HCC cells by regulating the MAPK/ERK pathway (55). Yes-associated protein (YAP) binds to HIF-1α and sustains HIF-1α protein stability to promote HCC cell glycolysis under hypoxic stress (47).
HIF-1 α promotes metastasis by inducing EMT and increasing the expression of factors related to angiogenesis and metastasis
EMT is a highly conserved cellular program during which epithelial cells lose their polarized organization and acquire migratory and invasive capabilities, which has been recognized as a pro-metastatic cellular event that promotes tumor cell invasion and malignant tumor progression (67). HIF-1α induces EMT to promote metastasis through many pathways. i) Lysyl oxidases like 2 (LOXL2) is a member of the lysyl oxidase family, whose main function is to catalyze the covalent cross-linkages of collagen and elastin in the extracellular matrix. HIF-1α was found to induce EMT, HCC cell migration, invasion and vasculogenic mimicry (VM) formation by regulating LOXL2 (56). ii) Vasodilator-stimulated phosphoprotein (VASP) is a regulator of the actin cytoskeleton and cell migration. HIF-1α induces VASP overexpression by directly binding two HREs in the VASP promoter region, and promotes the expression of EMT, MMP2 and MMP9 (57). iii) Hypoxia increases the production of reactive oxygen species (ROS) by inducing nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (NOX4). ROS can induce the GLI1-dependent EMT process and promote the invasion and metastasis of HCC (58). iv) Epithelial (E)-cadherin controls proliferation and cell polarization through intercellular adhesion and is essential for maintaining epithelial structure and homeostasis (68). E-cadherin activation inhibits metastasis at multiple stages, including CTC accumulation in the primary tumor and extravasation of tumor cells in the vascular system (69). HIF-1α inhibits transcription of E-cadherin by upregulating Snai1 and SIP1, a transcriptional inhibitor of E-cadherin (70), and activates EMT in HCC cells and promotes HCC invasion and metastasis (59). v) HIF-1α downregulates the Wnt/β-catenin pathway and enhances EMT in HCC cells by inducing expression of miR-1273F in exosomes (60).
HIF-1α promotes metastasis by inducing HCC stem cells
Cancer stem cells (CSCs) are a small subset of tumor cells with the capability to influence self-renewal, differentiation, and tumorigenesis. As far as we know, the inflammatory microenvironment in HCC leads to the proliferation of stem cells with genetic or epigenetic alterations, facilitating their transformation from normal liver stem cells to liver cancer stem cells (LCSCs) (71). LCSCs play critical roles in regulating HCC stemness, self-renewal, tumorigenicity, metastasis (72).
Accumulating evidence has demonstrated that CSCs are a primary cause of metastasis due to their role in new tumor initiation at local or distant sites (73–75). The generation of CSCs is closely related to the EMT mechanism (76), and β-catenin is one of the markers of EMT. Research has found that the Notch and Wnt/β-catenin signaling pathways promote the stemness characteristics of LCSCs. Expression of transcription factors involving EMT (such as Snail) and stemness (such as Sox2 and Nanog) can be decreased by blocking the function of Wnt/β-catenin and/or Notch (77). As mentioned above, stabilization of HIF-1α in hypoxic environments induces the EMT process and thus also modulates HCC stemness. Recent research has found that hypoxia enhances HIF-1α stability and transcriptional activity by promoting HIF-1α deSUMOylation of sentrin-specific protease 1 (SENP1), and HIF-1α promotes HCC stem cell by increasing the expression levels of CD24, CD44 and CD133 (61).
5. HIF-1α: A new target for the treatment of HCC
Targeting HIF can reduce the resistance to sorafenib
Sorafenib, a kinase inhibitor drug, exerts anti-angiogenic and anti-metastatic effects by dose-dependent inhibition of HIF-1α and VEGF protein expression (78). However, the anti-angiogenic activity by continuous sorafenib therapy can lead to tumor starvation and intratumor hypoxia, which may facilitate the generation of resistant cell clones adapted to hypoxia and nutrient deficiency (79). Sustained sorafenib treatment increases intratumoral hypoxia, which stabilizes the HIF-1α protein, thereby reducing the sensitivity to sorafenib. Liang et al (80) suggested that hypoxia induced by continuous sorafenib treatment could lead to sorafenib resistance in HCC through activation of HIF-1α and nuclear factor (NF)-κB. It was also found that the β-2-adrenergic receptor (ADRB2) signal destroys the beclin1/VPS34/Atg14 complex in an Akt-dependent manner, which negatively regulates autophagy and leads to stability of HIF-1α, reprogramming of glucose metabolism in HCC cells, and resistance to sorafenib (81). A significant correlation between the hypoxic microenvironment and sorafenib resistance was demonstrated, suggesting that targeting HIF is a promising approach to improve therapeutic efficiency.
Targeting of HIF-1α can improve the efficacy of sorafenib. The combination of sorafenib and curcumin analog EF24 inhibited the hypoxic resistance to sorafenib by promoting the proteasome degradation of the VHL-dependent HIF-1α in HCC cells, resulting in the inhibition of target genes MDR1 and GLUT-1, and a decrease in the activities of VEGF and NF-κB (80). The use of natural compounds has also shown positive effects in improving sorafenib treatment. Genistein, a natural isoflavone, was found to enhance the antitumor effect of sorafenib in sorafenib-resistant HCC cells and HCC xenograft mouse models by downregulating HIF-1α, thereby inactivating Glut-1 and HK2 to inhibit and sensitize aerobic glycolysis in HCC cells, resulting in mitochondrial apoptosis (82). Simvastatin that belongs to a group of HMG-CoA reductase inhibitors, inhibits the HIF-1α/PPAR-γ/PKM2 axis by hindering PKM2-mediated glycolysis, resulting in decreased proliferation and increased apoptosis of HCC cells, and re-sensitization of HCC cells to sorafenib (83).
Targeting HIF can improve the prognosis of patients after the TACE
Transarterial chemoembolization (TACE) is considered to be one of the most effective palliative care for patients with unresectable HCC, and the BCLC staging system has defined it as the standard of care for patients with mid-stage HCC (84). Previous research has shown that the overexpression of HIF-1α reduces the effectiveness of TACE against HCC (85). The expression of the HIF-1α protein in HCC tissues was found to be increased after TACE surgery. To adapt to the hypoxic environment, HIF-1α stimulates the expression of COX-2 protein in HCC cells and promotes the EMT process, thus enhancing the invasion and metastasis of HCC, and this may lead to poor prognosis of HCC patients after TACE treatment (86). Therefore, improving the hypoxic state after TACE or reducing the expression of HIF-1α may improve the prognosis of these patients.
Targeting HIF can reduce immune escape
The innate and adaptive immune systems play an important role in anticancer immune surveillance. Immune checkpoint inhibitors (ICIs) have proven that an effective immune response is able to eliminate tumor cells (87). Currently, preclinical evidence and clinical use of immunotherapy in HCC includes the use of programmed cell death-1 (PD-1), programmed death-ligand 1 (PDL1) and cytotoxic T lymphocyte-associated antigen 4 (CTLA4) inhibitors, such as nivolumab, atezolizumab and ipilimumab (88). Although immunotherapy brings hope to the treatment of HCC, its efficacy is still limited, which may be related to the complex TME in HCC (89). Research has revealed the increased HIF-1α levels in the hypoxic TME causing the downregulation of T-cell activity and induction of PD-L1 on the surface of tumor cells, resulting in immune suppression and increased cell proliferation of tumor cells resulting in immune escape (90). Therefore, inhibition of HIF-1α expression represents a promising novel strategy in cancer immunotherapy (91).
New concepts for targeting HIF-1α
Studies have shown that HIPK2, USP22, and NF-κB can regulate the expression of HIF-1α. Some drugs, such as metformin, heme alkali, dandelion polysaccharide regulate HIF-1α and inhibit the proliferation and metastasis of hepatoma cells. This provides some novel ideas for the treatment of HCC.
Serine/threonine homologous domain interaction protein kinase 2 (HIPK2) is often downregulated in HCC tissues. HIPK2 directly binds to HIF-1α and stimulates HIF-1α ubiquitination to degrade proteasomes. The downregulation of HIPK2 can enhance the angiogenesis of HCC by activating the HIF-1α pathway and further promote tumor growth and metastasis. The downregulation of HIPK2 protein expression in HCC patients is associated with poor overall survival (92). Ubiquitin-specific proteinase 22 (USP22) can stabilize HIF-1α by deubiquitination. Promoting hypoxia can induce HCC stem cells and glycolysis (93). As the direct target genes of HIF-1α, USP22 and TP53 can be upregulated by HIF-1α transcription in hypoxia. The upregulation of TP53 inhibited the upregulation of USP22. In HCC cells with TP53 mutation, USP22 and HIF-1α form a positive feedback loop promoting HCC stem cells. With the loss of function mutation at TP53 and USP22 and/or HIF-1α, HCC patients with high expression tend to have poor prognoses. The lipoprotein complex targeting the USP22 highly induced tumor inhibition and enhanced the sensitivity to sorafenib in tumor-bearing mice. USP22 promotes hypoxia-induced HCC stem cells through the TP53-inactivated HIF-1α/USP22 positive feedback loop. USP22 is a promising target for HCC treatment (94). A study has assessed the temporal expression of HIF-1α and NF-κB subunits in HCC under short-term and long-term hypoxia and found that NF-κB regulates HIF-1α in hypoxic HCC (95). The NF-κB subunits, p50 and p65 were found to enhance HIF-1α transcription, while c-Rel downstream miRNAs (including miR-199a-5p and miR-93) and Dicer1 promoted HIF-1α degradation (96). Targeting this regulator temporarily in the early hypoxic microenvironment may be a new therapeutic option for early cancer intervention.
Metformin-mediated HIF-1α inactivation leads to a decrease in PFKFB3 expression, which further inhibits phospho-fructose kinase-1 (PFK1) activity. Metformin significantly inhibits HCC cell proliferation by blocking the PFK1 glycolytic flux. The HIF-1α/PFKFB3/pfk1 regulatory axis is an important determinant of glucose metabolism reprogramming in HCC, which may provide an effective therapeutic target to inhibit the HCC (97).
Heme base is a benzo phenanthridine alkaloid, which can inhibit HIF-1α signal transduction and EMT marker expression, Snail translocation, Smad and PI3K-AKT pathway activation. One study found that the heme base is a promising drug candidate that will target HIF-1α/TGF-β signal transduction to improve treatment in patients with HCC (98).
Dandelion polysaccharide (DP) is an α-type polysaccharide derived from the root of the dandelion. It consists of glucose, galactose, arabinose, arabinose rhamnose, and glucuronic acid (99,100). It was found that dandelion polysaccharide inhibited HIF-1α expression by regulating the PI3K/Akt signaling pathway and exhibited an anti-HCC cell proliferation, anti-metastasis, and angiogenesis role (101).
6. Prospects
Hypoxia is a typical microenvironmental condition in almost all solid tumors, including HCC (102). HIF-1α is highly expressed in a hypoxic environment and is associated with the metastasis and prognosis of HCC (64). This phenomenon has aroused research attention to the role of HIF-1α in promoting tumor progression. This review summarizes the role of HIF-1α in the metastasis and prognosis of HCC. Here, we revealed the mechanisms of HIF-1α in promoting the development of HCC and developing clinical drug resistance, as well as its predictive effect on the postoperative recurrence of HCC, which has important clinical significance for the treatment and prognosis of HCC. Detection of the HIF-1α expression level can be considered in the routine assessment of HCC, which is of great significance for the treatment and prognosis of HCC.
However, researchers are still faced with challenges when they switch from laboratory discovery to clinical application by targeting HIF-1α to inhibit the metastasis and development of HCC. Moreover, there are few experimental studies on inhibiting HIF-1α clinically in the treatment of HCC. In an open study, patients with HCC who participated in clinical trials were given intravenous (i.v.) infusion of drugs to prove the mechanism of the inhibition of HIF-1α by reducing HIF-1α mRNA (nct02564614). The study results have not been published yet. One observational study on the comparison of HIF-1α levels (nct00866957) in patients with various HCC treatments is being carried out by Dr Laura Kulik of the Feinberg School of Medicine at Northwestern University (Chicago, IL, USA).
Therefore, there is an urgent need for further studies involving multiple centers, higher sample size, and a long-time follow-up for the treatment and control of metastatic HCC by targeting HIF-1α. It is believed that it will become a clinical reality in the near future.
Acknowledgements
Not applicable.
Funding Statement
This study was supported by the Natural Science Foundation of Beijing, China (grant nos. 7212171 and 7212172) and the Pilot Project of Public Welfare Development and Reform of Beijing Municipal Medical Research Institutes (2019–6).
Availability of data and materials
Not applicable.
Authors' contributions
HC, WL and XL conceived and designed the review. HC, HY and JC were involved in the collection and collation of references. HY and JC put forward meaningful suggestions for the revision of the article and participated in the revision of the manuscript. HC and WL designed the figures. HC wrote the manuscript. Data authentication is not applicable. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Liu X, Zhang X, Peng Z, Li C, Wang Z, Wang C, Deng Z, Wu B, Cui Y, Wang Z, et al. Deubiquitylase OTUD6B Governs pVHL stability in an enzyme-independent manner and suppresses hepatocellular carcinoma metastasis. Adv Sci (Weinh) 2020;7:1902040. doi: 10.1002/advs.201902040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Li L, Zhao K, Lin Q, Li H, Xue X, Ge W, He H, Liu D, Xie H, et al. A novel LncRNA HITT forms a regulatory loop with HIF-1α to modulate angiogenesis and tumor growth. Cell Death Differ. 2020;27:1431–1446. doi: 10.1038/s41418-019-0449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo Y, Xiao Z, Yang L, Gao Y, Zhu Q, Hu L, Huang D, Xu Q. Hypoxia-inducible factors in hepatocellular carcinoma (Review) Oncol Rep. 2020;43:3–15. doi: 10.3892/or.2019.7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Méndez-Blanco C, Fernández-Palanca P, Fondevila F, González-Gallego J, Mauriz JL. Prognostic and clinicopathological significance of hypoxia-inducible factors 1α and 2α in hepatocellular carcinoma: A systematic review with meta-analysis. Ther Adv Med Oncol. 2021;13:1758835920987071. doi: 10.1177/1758835920987071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mu H, Yu G, Li H, Wang M, Cui Y, Zhang T, Song T, Liu C. Mild chronic hypoxia-induced HIF-2α interacts with c-MYC through competition with HIF-1α to induce hepatocellular carcinoma cell proliferation. Cell Oncol (Dordr) 2021;44:1151–1166. doi: 10.1007/s13402-021-00625-w. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Dong J, Jia L, Zhao T, Lang M, Li Z, Lan C, Li X, Hao J, Wang H, et al. HIF-2-dependent expression of stem cell factor promotes metastasis in hepatocellular carcinoma. Cancer Lett. 2017;393:113–124. doi: 10.1016/j.canlet.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Chen J, Huang J, Li Z, Gong Y, Zou B, Liu X, Ding L, Li P, Zhu Z, et al. HIF-2α upregulation mediated by hypoxia promotes NAFLD-HCC progression by activating lipid synthesis via the PI3K-AKT-mTOR pathway. Aging (Albany NY) 2019;11:10839–10860. doi: 10.18632/aging.102488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian H, Huang P, Zhao Z, Tang W, Xia J. HIF-1α plays a role in the chemotactic migration of hepatocarcinoma cells through the modulation of CXCL6 expression. Cell Physiol Biochem. 2014;34:1536–1546. doi: 10.1159/000366357. [DOI] [PubMed] [Google Scholar]
- 10.Albadari N, Deng S, Li W. The transcriptional factors HIF-1 and HIF-2 and their novel inhibitors in cancer therapy. Expert Opin Drug Discov. 2019;14:667–682. doi: 10.1080/17460441.2019.1613370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272:19253–19260. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- 13.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Lou T. Hypoxia inducible factors in hepatocellular carcinoma. Oncotarget. 2017;8:46691–46703. doi: 10.18632/oncotarget.17358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hur E, Kim HH, Choi SM, Kim JH, Yim S, Kwon HJ, Choi Y, Kim DK, Lee MO, Park H. Reduction of hypoxia-induced transcription through the repression of hypoxia-inducible factor-1alpha/aryl hydrocarbon receptor nuclear translocator DNA binding by the 90-kDa heat-shock protein inhibitor radicicol. Mol Pharmacol. 2002;62:975–982. doi: 10.1124/mol.62.5.975. [DOI] [PubMed] [Google Scholar]
- 16.Luo D, Wang Z, Wu J, Jiang C, Wu J. The role of hypoxia inducible factor-1 in hepatocellular carcinoma. Biomed Res Int. 2014;2014:409272. doi: 10.1155/2014/409272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mossenta M, Busato D, Dal Bo M, Toffoli G. Glucose metabolism and oxidative stress in hepatocellular carcinoma: Role and possible implications in novel therapeutic strategies. Cancers (Basel) 2020;12:1668. doi: 10.3390/cancers12061668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271:17771–17778. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- 19.Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271:32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 20.Fu C, An N, Liu J, A J, Zhang B, Liu M, Zhang Z, Fu L, Tian X, Wang D, Dong JT. The transcription factor ZFHX3 is crucial for the angiogenic function of hypoxia-inducible factor 1α in liver cancer cells. J Biol Chem. 2020;295:7060–7074. doi: 10.1074/jbc.RA119.012131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiener CM, Booth G, Semenza GL. In vivo expression of mRNAs encoding hypoxia-inducible factor 1. Biochem Biophys Res Commun. 1996;225:485–488. doi: 10.1006/bbrc.1996.1199. [DOI] [PubMed] [Google Scholar]
- 22.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 24.Bao MH, Wong CC. Hypoxia, metabolic reprogramming, and drug resistance in liver cancer. Cells. 2021;10:1715. doi: 10.3390/cells10071715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang YM, Kim SY, Seki E. Inflammation and liver cancer: Molecular mechanisms and therapeutic targets. Semin Liver Dis. 2019;39:26–42. doi: 10.1055/s-0038-1676806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Zhang Q, Lou Y, Fu Q, Chen Q, Wei T, Yang J, Tang J, Wang J, Chen Y, et al. Hypoxia-inducible factor-1α/interleukin-1β signaling enhances hepatoma epithelial-mesenchymal transition through macrophages in a hypoxic-inflammatory microenvironment. Hepatology. 2018;67:1872–1889. doi: 10.1002/hep.29681. [DOI] [PubMed] [Google Scholar]
- 27.Ye M, Fang Z, Gu H, Song R, Ye J, Li H, Wu Z, Zhou S, Li P, Cai X, et al. Histone deacetylase 5 promotes the migration and invasion of hepatocellular carcinoma via increasing the transcription of hypoxia-inducible factor-1α under hypoxia condition. Tumour Biol. 2017;39:1010428317705034. doi: 10.1177/1010428317705034. [DOI] [PubMed] [Google Scholar]
- 28.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/S0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 29.Fang X, Zhang D, Zhao W, Gao L, Wang L. Dishevelled associated activator of morphogenesis (DAAM) facilitates invasion of hepatocellular carcinoma by upregulating hypoxia-inducible factor 1α (HIF-1α) Expression. Med Sci Monit. 2020;26:e924670. doi: 10.12659/MSM.924670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma H, Xie L, Zhang L, Yin X, Jiang H, Xie X, Chen R, Lu H, Ren Z. Activated hepatic stellate cells promote epithelial-to-mesenchymal transition in hepatocellular carcinoma through transglutaminase 2-induced pseudohypoxia. Commun Biol. 2018;1:168. doi: 10.1038/s42003-018-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ientile R, Curro M, Caccamo D, Ferlazzo N, Gangemi C, Gugliandolo A. Transglutaminase 2 is involved in the inflammatory response through mechanisms linked to NF-kappa B/HIF-1 alpha pathways. Amino Acids. 2015;27:1630–1631. [Google Scholar]
- 32.Kim DS, Choi YB, Han BG, Park SY, Jeon Y, Kim DH, Ahn ER, Shin JE, Lee BI, Lee H, et al. Cancer cells promote survival through depletion of the von Hippel-Lindau tumor suppressor by protein crosslinking. Oncogene. 2011;30:4780–4790. doi: 10.1038/onc.2011.183. [DOI] [PubMed] [Google Scholar]
- 33.Chu Q, Gu X, Zheng Q, Zhu H. Regulatory mechanism of HIF-1α and its role in liver diseases: A narrative review. Ann Transl Med. 2022;10:109. doi: 10.21037/atm-21-4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Makwana V, Ryan P, Patel B, Dukie SA, Rudrawar S. Essential role of O-GlcNAcylation in stabilization of oncogenic factors. Biochim Biophys Acta Gen Subj. 2019;1863:1302–1317. doi: 10.1016/j.bbagen.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 35.Wu HT, Kuo YC, Hung JJ, Huang CH, Chen WY, Chou TY, Chen Y, Chen YJ, Chen YJ, Cheng WC, et al. K63-polyubiquitinated HAUSP deubiquitinates HIF-1α and dictates H3K56 acetylation promoting hypoxia-induced tumour progression. Nat Commun. 2016;7:13644. doi: 10.1038/ncomms13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu KJ. The role of miRNA biogenesis and DDX17 in tumorigenesis and cancer stemness. Biomed J. 2020;43:107–114. doi: 10.1016/j.bj.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Chen W, Lian J, Zhang H, Yu B, Zhang M, Wei F, Wu J, Jiang J, Jia Y, et al. The lncRNA PVT1 regulates nasopharyngeal carcinoma cell proliferation via activating the KAT2A acetyltransferase and stabilizing HIF-1α. Cell Death Differ. 2020;27:695–710. doi: 10.1038/s41418-019-0381-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung JE, Kim HS, Lee CS, Shin YJ, Kim YN, Kang GH, Kim TY, Juhnn YS, Kim SJ, Park JW, et al. STAT3 inhibits the degradation of HIF-1alpha by pVHL-mediated ubiquitination. Exp Mol Med. 2008;40:479–485. doi: 10.3858/emm.2008.40.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin MC, Lin JJ, Hsu CL, Juan HF, Lou PJ, Huang MC. GATA3 interacts with and stabilizes HIF-1α to enhance cancer cell invasiveness. Oncogene. 2017;36:4243–4252. doi: 10.1038/onc.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang D, Zhang X, Lu Y, Wang X, Zhu L. Hypoxia inducible factor 1α in hepatocellular carcinoma with cirrhosis: Association with prognosis. Pathol Res Pract. 2018;214:1987–1992. doi: 10.1016/j.prp.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Ding L, Chen XP, Wang HP. Expression and clinical significance of HIF-1a protein in hepatocellular carcinoma tissues. Zhonghua Gan Zang Bing Za Zhi. 2004;12:656–659. (In Chinese) [PubMed] [Google Scholar]
- 42.Cheng W, Cheng Z, Yang Z, Xing D, Zhang M. Upregulation of hypoxia-inducible factor 1α mRNA expression was associated with poor prognosis in patients with hepatocellular carcinoma. Onco Targets Ther. 2019;12:6285–6296. doi: 10.2147/OTT.S197077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao S, Yang S, Wu C, Wang Y, Jiang J, Lu Z. Protein expression of hypoxia-inducible factor-1 alpha and hepatocellular carcinoma: A systematic review with meta-analysis. Clin Res Hepatol Gastroenterol. 2014;38:598–603. doi: 10.1016/j.clinre.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Qian Y, Li Y, Ge Y, Song W, Fan H. Elevated LncRNA TRERNA1 correlated with activation of HIF-1α predicts poor prognosis in hepatocellular carcinoma. Pathol Res Pract. 2021;227:153612. doi: 10.1016/j.prp.2021.153612. [DOI] [PubMed] [Google Scholar]
- 45.Ding ZN, Dong ZR, Chen ZQ, Yang YF, Yan LJ, Li HC, Liu KX, Yao CY, Yan YC, Yang CC, Li T. Effects of hypoxia-inducible factor-1α and hypoxia-inducible factor-2α overexpression on hepatocellular carcinoma survival: A systematic review with meta-analysis. J Gastroenterol Hepatol. 2021;36:1487–1496. doi: 10.1111/jgh.15395. [DOI] [PubMed] [Google Scholar]
- 46.Chitty JL, Filipe EC, Lucas MC, Herrmann D, Cox TR, Timpson P. Recent advances in understanding the complexities of metastasis. F1000Res 7: F1000 Faculty Rev-1169. 2018 doi: 10.12688/f1000research.15064.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Li Y, Ma Y, Yang L, Wang T, Meng X, Zong Z, Sun X, Hua X, Li H. Yes-associated protein (YAP) binds to HIF-1α and sustains HIF-1α protein stability to promote hepatocellular carcinoma cell glycolysis under hypoxic stress. J Exp Clin Cancer Res. 2018;37:216. doi: 10.1186/s13046-018-0892-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schito L, Semenza GL. Hypoxia-inducible factors: Master regulators of cancer progression. Trends Cancer. 2016;2:758–770. doi: 10.1016/j.trecan.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 49.Chiu DK, Tse AP, Xu IM, Di Cui J, Lai RK, Li LL, Koh HY, Tsang FH, Wei LL, Wong CM, et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun. 2017;8:517. doi: 10.1038/s41467-017-00530-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79:4557–4566. doi: 10.1158/0008-5472.CAN-18-3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pang L, Ng KT, Liu J, Yeung WO, Zhu J, Chiu TS, Liu H, Chen Z, Lo CM, Man K. Plasmacytoid dendritic cells recruited by HIF-1α/eADO/ADORA1 signaling induce immunosuppression in hepatocellular carcinoma. Cancer Lett. 2021;522:80–92. doi: 10.1016/j.canlet.2021.09.022. [DOI] [PubMed] [Google Scholar]
- 52.He Q, Liu M, Huang W, Chen X, Zhang B, Zhang T, Wang Y, Liu D, Xie M, Ji X, et al. IL-1β-induced elevation of solute carrier family 7 member 11 promotes hepatocellular carcinoma metastasis through up-regulating programmed death ligand 1 and colony-stimulating factor 1. Hepatology. 2021;74:3174–3193. doi: 10.1002/hep.32062. [DOI] [PubMed] [Google Scholar]
- 53.Song Y, Wang H, Zou XJ, Zhang YX, Guo ZQ, Liu L, Wu DH, Zhang DY. Reciprocal regulation of HIF-1α and Uroplakin 1A promotes glycolysis and proliferation in Hepatocellular Carcinoma. J Cancer. 2020;11:6737–6747. doi: 10.7150/jca.48132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Y, Huang Y, Hu K, Zhang Z, Yang J, Wang Z. HIF1A activates the transcription of lncRNA RAET1K to modulate hypoxia-induced glycolysis in hepatocellular carcinoma cells via miR-100-5p. Cell Death Dis. 2020;11:176. doi: 10.1038/s41419-020-2366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.He H, Chen T, Mo H, Chen S, Liu Q, Guo C. Hypoxia-inducible long noncoding RNA NPSR1-AS1 promotes the proliferation and glycolysis of hepatocellular carcinoma cells by regulating the MAPK/ERK pathway. Biochem Biophys Res Commun. 2020;533:886–892. doi: 10.1016/j.bbrc.2020.09.076. [DOI] [PubMed] [Google Scholar]
- 56.Wang M, Zhao X, Zhu D, Liu T, Liang X, Liu F, Zhang Y, Dong X, Sun B. HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment. J Exp Clin Cancer Res. 2017;36:60. doi: 10.1186/s13046-017-0533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Z, Wang Y, Dou C, Xu M, Sun L, Wang L, Yao B, Li Q, Yang W, Tu K, Liu Q. Hypoxia-induced up-regulation of VASP promotes invasiveness and metastasis of hepatocellular carcinoma. Theranostics. 2018;8:4649–4663. doi: 10.7150/thno.26789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Z, Tu K, Wang Y, Yao B, Li Q, Wang L, Dou C, Liu Q, Zheng X. Hypoxia accelerates aggressiveness of hepatocellular carcinoma cells involving oxidative stress, epithelial-mesenchymal transition and non-canonical hedgehog signaling. Cell Physiol Biochem. 2017;44:1856–1868. doi: 10.1159/000485821. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Huang G, Li X, Zhang Y, Jiang Y, Shen J, Liu J, Wang Q, Zhu J, Feng X, et al. Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor-1α in hepatocellular carcinoma. BMC Cancer. 2013;13:108. doi: 10.1186/1471-2407-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu Y, Min Z, Zhou Zhihang, Linhong M, Tao R, Yan L, Song H. Hypoxia-induced exosomes promote hepatocellular carcinoma proliferation and metastasis via miR-1273f transfer. Exp Cell Res. 2019;385:111649. doi: 10.1016/j.yexcr.2019.111649. [DOI] [PubMed] [Google Scholar]
- 61.Cui CP, Wong CC, Kai AK, Ho DW, Lau EY, Tsui YM, Chan LK, Cheung TT, Chok KS, Chan ACY, et al. SENP1 promotes hypoxia-induced cancer stemness by HIF-1α deSUMOylation and SENP1/HIF-1α positive feedback loop. Gut. 2017;66:2149–2159. doi: 10.1136/gutjnl-2016-313264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science. 2016;352:175–180. doi: 10.1126/science.aaf4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Cao X. Immunosuppressive cells in tumor immune escape and metastasis. J Mol Med (Berl) 2016;94:509–522. doi: 10.1007/s00109-015-1376-x. [DOI] [PubMed] [Google Scholar]
- 64.Jing X, Yang F, Shao C, Wei K, Xie M, Shen H, Shu Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol Cancer. 2019;18:157. doi: 10.1186/s12943-019-1089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kiss M, Vande Walle L, Saavedra PHV, Lebegge E, Van Damme H, Murgaski A, Qian J, Ehling M, Pretto S, Bolli E, et al. IL1β promotes immune suppression in the tumor microenvironment independent of the inflammasome and gasdermin D. Cancer Immunol Res. 2021;9:309–323. doi: 10.1158/2326-6066.CIR-20-0431. [DOI] [PubMed] [Google Scholar]
- 66.Feng J, Li J, Wu L, Yu Q, Ji J, Wu J, Dai W, Guo C. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2020;39:126. doi: 10.1186/s13046-020-01629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yuan K, Xie K, Lan T, Xu L, Chen X, Li X, Liao M, Li J, Huang J, Zeng Y, Wu H. TXNDC12 promotes EMT and metastasis of hepatocellular carcinoma cells via activation of β-catenin. Cell Death Differ. 2020;27:1355–1368. doi: 10.1038/s41418-019-0421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Venhuizen JH, Jacobs FJC, Span PN, Zegers MM. P120 and E-cadherin: Double-edged swords in tumor metastasis. Semin Cancer Biol. 2020;60:107–120. doi: 10.1016/j.semcancer.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 69.Na TY, Schecterson L, Mendonsa AM, Gumbiner BM. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc Natl Acad Sci USA. 2020;117:5931–5937. doi: 10.1073/pnas.1918167117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Evans AJ, Russell RC, Roche O, Burry TN, Fish JE, Chow VW, Kim WY, Saravanan A, Maynard MA, Gervais ML, et al. VHL promotes E2 box-dependent E-cadherin transcription by HIF-mediated regulation of SIP1 and snail. Mol Cell Biol. 2007;27:157–169. doi: 10.1128/MCB.00892-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu Y, Zhang J, Zhang X, Zhou H, Liu G, Li Q. Cancer stem cells: A potential breakthrough in HCC-targeted therapy. Front Pharmacol. 2020;11:198. doi: 10.3389/fphar.2020.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu YC, Yeh CT, Lin KH. Cancer stem cell functions in hepatocellular carcinoma and comprehensive therapeutic strategies. Cells. 2020;9:1331. doi: 10.3390/cells9061331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168:670–691. doi: 10.1016/j.cell.2016.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang N, Wang S, Li MY, Hu BG, Liu LP, Yang SL, Yang S, Gong Z, Lai PBS, Chen GG. Cancer stem cells in hepatocellular carcinoma: An overview and promising therapeutic strategies. Ther Adv Med Oncol. 2018;10:1758835918816287. doi: 10.1177/1758835918816287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee TK, Guan XY, Ma S. Cancer stem cells in hepatocellular carcinoma-from origin to clinical implications. Nat Rev Gastroenterol Hepatol. 2022;19:26–44. doi: 10.1038/s41575-021-00508-3. [DOI] [PubMed] [Google Scholar]
- 76.Du B, Shim JS. Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules. 2016;21:965. doi: 10.3390/molecules21070965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang R, Sun Q, Wang P, Liu M, Xiong S, Luo J, Huang H, Du Q, Geller DA, Cheng B. Notch and Wnt/β-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget. 2016;7:5754–5768. doi: 10.18632/oncotarget.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu LP, Ho RL, Chen GG, Lai PB. Sorafenib inhibits hypoxia-inducible factor-1α synthesis: Implications for antiangiogenic activity in hepatocellular carcinoma. Clin Cancer Res. 2012;18:5662–5671. doi: 10.1158/1078-0432.CCR-12-0552. [DOI] [PubMed] [Google Scholar]
- 79.Méndez-Blanco C, Fondevila F, García-Palomo A, González-Gallego J, Mauriz JL. Sorafenib resistance in hepatocarcinoma: Role of hypoxia-inducible factors. Exp Mol Med. 2018;50:1–9. doi: 10.1038/s12276-018-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liang Y, Zheng T, Song R, Wang J, Yin D, Wang L, Liu H, Tian L, Fang X, Meng X, et al. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel-Lindau tumor suppressor-dependent HIF-1α inhibition in hepatocellular carcinoma. Hepatology. 2013;57:1847–1857. doi: 10.1002/hep.26224. [DOI] [PubMed] [Google Scholar]
- 81.Wu FQ, Fang T, Yu LX, Lv GS, Lv HW, Liang D, Li T, Wang CZ, Tan YX, Ding J, et al. ADRB2 signaling promotes HCC progression and sorafenib resistance by inhibiting autophagic degradation of HIF1α. J Hepatol. 2016;65:314–324. doi: 10.1016/j.jhep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 82.Li S, Li J, Dai W, Zhang Q, Feng J, Wu L, Liu T, Yu Q, Xu S, Wang W, et al. Genistein suppresses aerobic glycolysis and induces hepatocellular carcinoma cell death. Br J Cancer. 2017;117:1518–1528. doi: 10.1038/bjc.2017.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feng J, Dai W, Mao Y, Wu L, Li J, Chen K, Yu Q, Kong R, Li S, Zhang J, et al. Simvastatin re-sensitizes hepatocellular carcinoma cells to sorafenib by inhibiting HIF-1α/PPAR-γ/PKM2-mediated glycolysis. J Exp Clin Cancer Res. 2020;39:24. doi: 10.1186/s13046-020-1528-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Song DS, Nam SW, Bae SH, Kim JD, Jang JW, Song MJ, Lee SW, Kim HY, Lee YJ, Chun HJ, et al. Outcome of transarterial chemoembolization-based multi-modal treatment in patients with unresectable hepatocellular carcinoma. World J Gastroenterol. 2015;21:2395–2404. doi: 10.3748/wjg.v21.i8.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu K, Min XL, Peng J, Yang K, Yang L, Zhang XM. The changes of HIF-1α and VEGF expression After TACE in patients with hepatocellular carcinoma. J Clin Med Res. 2016;8:297–302. doi: 10.14740/jocmr2496w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang M, Wang L, Chen J, Bai M, Zhou C, Liu S, Lin Q. Regulation of COX-2 expression and epithelial-to-mesenchymal transition by hypoxia-inducible factor-1α is associated with poor prognosis in hepatocellular carcinoma patients post TACE surgery. Int J Oncol. 2016;48:2144–2154. doi: 10.3892/ijo.2016.3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:525–543. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruiz de Galarreta M, Bresnahan E, Molina-Sánchez P, Lindblad KE, Maier B, Sia D, Puigvehi M, Miguela V, Casanova-Acebes M, Dhainaut M, et al. β-catenin activation promotes immune escape and resistance to Anti-PD-1 therapy in hepatocellular carcinoma. Cancer Discov. 2019;9:1124–1141. doi: 10.1158/2159-8290.CD-19-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kalantari Khandani N, Ghahremanloo A, Hashemy SI. Role of tumor microenvironment in the regulation of PD-L1: A novel role in resistance to cancer immunotherapy. J Cell Physiol. 2020;235:6496–6506. doi: 10.1002/jcp.29671. [DOI] [PubMed] [Google Scholar]
- 91.Deng Z, Teng YJ, Zhou Q, Ouyang ZG, Hu YX, Long HP, Hu MJ, Mei S, Lin FX, Dai XJ, et al. Shuyu pills inhibit immune escape and enhance chemosensitization in hepatocellular carcinoma. World J Gastrointest Oncol. 2021;13:1725–1740. doi: 10.4251/wjgo.v13.i11.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen P, Duan X, Li X, Li J, Ba Q, Wang H. HIPK2 suppresses tumor growth and progression of hepatocellular carcinoma through promoting the degradation of HIF-1α. Oncogene. 2020;39:2863–2876. doi: 10.1038/s41388-020-1190-y. [DOI] [PubMed] [Google Scholar]
- 93.Xu S, Ling S, Shan Q, Ye Q, Zhan Q, Jiang G, Zhuo J, Pan B, Wen X, Feng T, et al. Self-activated cascade-responsive sorafenib and USP22 shRNA Co-delivery system for synergetic hepatocellular carcinoma therapy. Adv Sci (Weinh) 2021;8:2003042. doi: 10.1002/advs.202003042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ling S, Shan Q, Zhan Q, Ye Q, Liu P, Xu S, He X, Ma J, Xiang J, Jiang G, et al. USP22 promotes hypoxia-induced hepatocellular carcinoma stemness by a HIF1α/USP22 positive feedback loop upon TP53 inactivation. Gut. 2020;69:1322–1334. doi: 10.1136/gutjnl-2019-319616. [DOI] [PubMed] [Google Scholar]
- 95.Korbecki J, Simińska D, Gąssowska-Dobrowolska M, Listos J, Gutowska I, Chlubek D, Baranowska-Bosiacka I. Chronic and cycling hypoxia: Drivers of cancer chronic inflammation through HIF-1 and NF-κB Activation: A review of the molecular mechanisms. Int J Mol Sci. 2021;22:10701. doi: 10.3390/ijms221910701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang Y, Zhu Y, Wang X, Gong J, Hu C, Guo B, Zhu B, Li Y. Temporal regulation of HIF-1 and NF-κB in hypoxic hepatocarcinoma cells. Oncotarget. 2015;6:9409–9419. doi: 10.18632/oncotarget.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu L, Zeng Z, Xia Q, Liu Z, Feng X, Chen J, Huang M, Chen L, Fang Z, Liu Q, et al. Metformin attenuates hepatoma cell proliferation by decreasing glycolytic flux through the HIF-1α/PFKFB3/PFK1 pathway. Life Sci. 2019;239:116966. doi: 10.1016/j.lfs.2019.116966. [DOI] [PubMed] [Google Scholar]
- 98.Su Q, Fan M, Wang J, Ullah A, Ghauri MA, Dai B, Zhan Y, Zhang D, Zhang Y. Sanguinarine inhibits epithelial-mesenchymal transition via targeting HIF-1α/TGF-β feed-forward loop in hepatocellular carcinoma. Cell Death Dis. 2019;10:939. doi: 10.1038/s41419-019-2173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen M, Wu J, Shi S, Chen Y, Wang H, Fan H, Wang S. Structure analysis of a heteropolysaccharide from Taraxacum mongolicum Hand.-Mazz. and anticomplementary activity of its sulfated derivatives. Carbohydr Polym. 2016;152:241–252. doi: 10.1016/j.carbpol.2016.06.110. [DOI] [PubMed] [Google Scholar]
- 100.Cai L, Wan D, Yi F, Luan L. Purification, preliminary characterization and hepatoprotective effects of polysaccharides from dandelion root. Molecules. 2017;22:1409. doi: 10.3390/molecules22091409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ren F, Wu K, Yang Y, Yang Y, Wang Y, Li J. Dandelion polysaccharide exerts anti-angiogenesis effect on hepatocellular carcinoma by regulating VEGF/HIF-1α expression. Front Pharmacol. 2020;11:460. doi: 10.3389/fphar.2020.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shao C, Yang F, Miao S, Liu W, Wang C, Shu Y, Shen H. Role of hypoxia-induced exosomes in tumor biology. Mol Cancer. 2018;17:120. doi: 10.1186/s12943-018-0869-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.