Abstract
The new ionization technique termed vibrating sharp-edge spray ionization (cVSSI) has been coupled with corona discharge to investigate atmospheric pressure chemical ionization (APCI) capabilities. The optimized source was evaluated for its ability to enhance ion signal intensity, overcome matrix effects, and limit ion suppression. The results have been compared with state-of-the-art ESI source performance as well as a new APCI-like source. In methanol, the ion signal intensity increased 10 fold and >10 fold for cocaine and the suppressed analytes, respectively. The ability to overcome ion suppression was improved from 2-fold to 16-fold for theophylline and vitamin D2, respectively. For aqueous samples, ion signal levels increased by two orders of magnitude for all analytes. In both solvent systems, the signal-to-noise ratios also increased for all suppressed analytes. One example of the characterization of low-ionizing (by ESI or cVSSI alone) species in the presence of high-ionizing species by direct analysis from a cotton swab is presented. The work is discussed with respect to the advantages of cVSSI-APCI for direct, in-situ, and field analyses.
Keywords: ion suppression, new ionization sources, direct analysis, APCI
Graphical Abstract

INTRODUCTION
Over the last decade, tremendous growth has occurred in the development of ambient ionization sources for mass spectrometry.1–13 In part this has been motivated by a need to perform direct, in-situ, and field analysis work where minimal sample preparation is required and/or where instrumentation simplicity is beneficial.8, 14–19 A problem with minimal sample preparation is that analyte ion signals can be affected by contaminant and/or matrix compounds. These challenges are somewhat related to the effects observed for complex mixture analysis such as those encountered in ‘omics investigations where instead, each analyte becomes a potential source of chemical noise or an ion suppressor.20–34 A difference is that, for complex mixture analysis, an enhancement in the production of ions is desired for all compounds to ensure the greatest efficacy in compound detection and identification. In contrast, for many direct analyses, targeted ionization enhancement aimed at reducing matrix effects and ion suppression is desired. Addressing these issues occurs on many instrument component levels ranging from ion source to mass analyzer.
Matrix effects represent the influence that non-analyte components of the sample have on analyte signals.35, 36 In mass spectrometry (MS), these can often lead to the suppression of analyte ions. One of the earliest reportings of ion suppression came in 1993, when the mechanism of electrospray ionization (ESI) was examined.37 It was noted that the presence of other electrolytes dampened the signal of the desired analytes. This phenomenon has continued to be a problem in MS applications leading to issues such as a lack of detection of some compounds, decreased sensitivity toward analytes, or problems with analyte quantification.19, 33, 38–47 In mass spectrometry imaging (MSI)16, 32, 48–51, which can take on the form of direct analysis, ion suppression can lead to underreported regions of the sample image or lipid profiles where lower polarity head groups can be suppressed by higher polarity head groups.52
To overcome ion suppression, multiple approaches have been presented. For complex mixtures, one approach is to couple separations (one- and multi-dimensional) to reduce the interference of different components by temporally resolving the analyte mass spectra.53, 54 Notably, such methods have additional instrumentation auxiliary components and sample workup. Alone, some of these separation methods require significant time (≥50 minutes) and studies have shown that even with their incorporation into the experimental workflow, matrix effects are still present.41–46, 55 Additionally, the mixture complexity encountered in many studies exceeds the multi-dimensional peak capacity of the separate measurement techniques.56 Although effective for complex mixture analyses, the approaches mentioned above stand in stark contrast to the desired goal of no or limited sample preparation for direct, in-situ, or field analyses.
One ambient ionization source that has gained increased usage for direct analysis has been atmospheric pressure chemical ionization (APCI). The concept of APCI was originally demonstrated more than 40 years ago and was called atmospheric pressure ionization (API).57–60 An advantage of APCI is that it provides for the ionization of less polar compounds compared with ESI. While APCI shows benefits in accessing new classes of compounds, it has not been fully implemented in several direct analysis settings such as MSI.32, 48–51 This likely results from the physical requirements of the APCI source (size, heat, rigidity, and auxiliary components). Rather, most advancements in instrumentation development have focused on the alteration of ESI and matrix-assisted laser desorption ionization (MALDI) sources.17, 48, 49, 51
Recognizing the value of overcoming ion suppression for direct analyses, APCI-like sources have begun to be developed. In one study, laser ablation-APCI (LA-APCI) was developed for MSI by altering the commercial source.50 Further, studies have incorporated other liquid ionization sources such as nano-electrospray ionization (nESI)61, surface acoustic wave nebulization (SAWN)62, the MasSpec Pen63, and Ultrasonic Nebulization (USN)64, to allow for decreased signal dampening or increased ion signal compared with the original droplet generator. Given the simplicity of aerosolization for analyte introduction, it should be straightforward to adapt some for various direct analyses by APCI including MSI.
Capillary vibrating sharp-edge spray ionization (cVSSI) is a new, spray-based ionization method that relies on acousto-mechanical nebulization of liquid samples containing different analytes.65–68 cVSSI can be used with and without the application of voltage depending on the type of analysis desired. For example, some compounds are sensitive to redox reactions upon the application of voltage in ESI studies and therefore would benefit from a zero-voltage ionization source.68, 69 In some studies, field-enabled cVSSI has been shown to be comparable to, or better at, ionizing specific compounds compared with ESI. Additionally, this technique has demonstrated increased utility in proteomics and metabolomics investigations.67, 70, 71
In the study presented here, cVSSI is performed in the presence of a corona discharge emanating from a tungsten metal needle. This leads to an APCI-like process of ion formation that is somewhat similar to that described in previous studies.61–64 The cVSSI-APCI method is here examined to determine whether or not ion suppression and matrix effects are decreased for different samples making it amenable for direct analysis. Overall, the addition of the APCI needle increases the ion signal levels and the signal-to-noise ratios (S/N) for compounds in organic or aqueous environments and decreases ion suppression compared to ESI. Additionally, the increase in suppressed analyte ion signal levels is observed in both solvent compositions even in the presence of high-ionizing species such as cocaine or caffeine. These proof-of-principle studies present cVSSI-APCI as a potential low-cost and simple alternative ionization source for direct, in-situ, and field portable analysis work.
EXPERIMENTAL
Materials and reagents.
High performance liquid chromatography (HPLC)-grade methanol and water were purchased from Fisher Scientific (Waltham, MA, United States). Caffeine, vitamin D2, theophylline, and cocaine (1 mg/mL in methanol) were purchased from Sigma-Aldrich (St. Louis, MO, United States). Thymine was purchased from Alfa Aesar (Ward Hill, MA, United States). Stock solutions were made according to Table S1 in the Supporting Information section. Solution mixtures were made according to descriptions provided in Table S2. Table 1 provides the molecular weights and observed ions for all analytes used in this study and Figure S1 shows their structure.
Table 1:
Molecules investigated by cVSSI-APCI-MS and HESI-MS
| Analyte a | Monoisotopic Mass b | Observed Ions c | |
|---|---|---|---|
| 1 | Thymine | 126.04 | [M+H] + |
| 2 | Theophylline | 180.06 | [M+H] + |
| 3 | Caffeine | 194.08 | [M+H] + |
| [M+Na] + | |||
| 4 | Cocaine | 303.15 | [M+H] + |
| 5 | Vitamin D2 | 396.34 | [M+H] + |
Analyte common name
Monoisotopic mass obtained from ChemAxon
Ions observed in mass spectra
Mass Spectrometry (MS) Instrumentation.
For all experiments, apart from the sensitivity studies and the direct analysis demonstration, a linear ion trap mass spectrometer (LTQ XL, Fisher Scientific, Waltham, MA, USA) was used. The scan range was set from 120 to 400 mass-to-charge (m/z). All experiments were performed in positive ion mode using a maximum injection time of 10 ms and 1 microscan. The AGC target setting for a full MS scan was set at 1.0×104. The capillary temperature was set at 275 °C for all experiments.
For heated electrospray ionization (HESI) experiments, the sheath gas flow rate, the auxiliary gas flow rate, and the sweep gas flow rate were set at 15, 7, and 2 (arbitrary units), respectively. The spray voltage was varied between 2.0 kV and 5.0 kV. The capillary and tube lens voltages were set at 20 V and 50 V, respectively. The flow rate was set at 20 μL/min using a 500 μL Hamilton syringe.
For sensitivity studies and the direct analysis demonstration, an orbitrap (QExactive, Fisher Scientific, Waltham, MA, USA) was used. The scan range was set from 100 to 400 m/z. Experiments were performed in positive ion mode using a maximum injection time of 100 ms and 1 microscan. The AGC target for a full MS was 1.0 × 106. For HESI experiments, the sheath gas flow rate, the auxiliary gas flow rate, and the sweep gas flow rate were set at 10, 8, and 1(arbitrary units), respectively. The spray voltage was set at 4.0 kV. The lens voltage was set at 30 V. The flow rate was set at 10 μL/min using a 500 μL Hamilton syringe.
cVSSI-APCI setup.
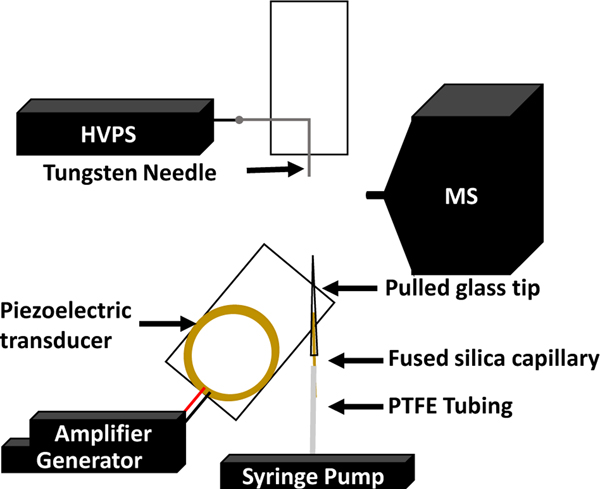
Figure 1 shows a schematic of the cVSSI-APCI setup. The cVSSI glass tips were fabricated using a laser puller (Sutter Instruments, Novato, CA, USA) to create an emitter tip with I.D. values of ~70 to ~115 μm, as shown in Figure S2A–C. A 4-cm piece of fused-silica capillary (360 μm O.D. and 250 μm I.D.) was inserted to within 1 cm of the tapered end of the glass tip and glued in place using epoxy glue. The remainder of the cVSSI device was fabricated as described in previous work.68 A piezoelectric transducer was glued to a microscope glass slide (No. 1) purchased from Azer Scientific (Morgantown, PA, United States). The glass emitter tip with the capillary was then glued onto the microscope slide using glass glue at a 60° angle from the edge to locate the tip directly over the center of the device. Sample solutions were introduced to the device utilizing plastic tubing (PTFE #30 Thin Wall) purchased from Cole Parmer (Vernon Hills, IL, United States). The tubing was connected to a 1 cc plastic syringe at one end and to the capillary insert at the other (Figure 1). The sample flow rate (10 to 80 μL/min) was controlled using a syringe pump (KD Scientific, Holliston, MA, USA). Droplet plumes were then generated using an amplifier (Krohn-Hite, Brockton, MA, USA) that was controlled by a waveform generator (Tektronix, Beaverton, OH, USA) as shown in Figure 1. The generator supplies a sine wave voltage function with a frequency between ~95 and 97 kHz having an amplitude ranging between 95 and 97 mVpp. With an amplification of 100 × this provides ~10 Vpp to the transducer. The total power of the system is ~110 mW. The average droplet diameter at a flow rate of 10 μL/min is 23 ± 9 μm, as shown in Figure S2D–F.
Figure 1.
Schematic diagram of the cVSSI-APCI system setup. This includes the waveform generator, RF amplifier, high-voltage power supply, and syringe pump. Separate components are labeled.
Electrical breakdown (corona discharge) was introduced with the application of a high voltage to a tungsten needle. Applied voltages were varied from 0 to +3.0 kV of DC voltage. The tungsten needle was taped to a microscope glass slide to maintain position and was located 2 mm in front of and 2 mm to the right of the mass spectrometer inlet as shown in Figure 1.
Data analysis.
All data were analyzed using the XCalibur software suite (Thermo Scientific, Waltham, MA, USA). Ion chronograms and mass spectra were converted into text files and analyzed with Excel (Microsoft, Redmond, WA USA).
RESULTS AND DISCUSSION
cVSSI-APCI for samples in organic solvent.
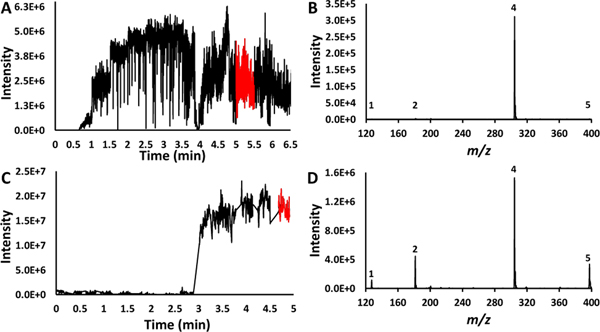
To conduct the cVSSI-APCI experiments, a solution containing a mixture of thymine, theophylline, cocaine, and Vitamin D2 (each 200 μM) in methanol was examined. To provide a comparison with a state-of-the-art commercial ionization source, analyses of the same mixture were performed using HESI. To determine the onset and optimum DC voltages utilized for the cVSSI-APCI (applied to the Tungsten needle) and the HESI analyses of this mixture, voltage ramp experiments were performed as shown in Figure 2. For HESI (Figure 2A) experiments, the voltage was increased from 2.0 kV to 5.0 kV at a rate of 0.25 kV every 30 seconds. The greatest ion signal level for the analytes, excluding chemical noise, occurred at ~5 minutes in the ion chronogram corresponding to a voltage of 4.5 kV, as shown in Figure S3A. Prior to this voltage, none of the suppressed analytes (thymine, theophylline, or Vitamin D2) were present in their highest abundance. Although the remaining three analytes were observed at higher voltages, compared with the ions corresponding to cocaine, the dataset features remained at very low intensities (<1% of the cocaine peak as shown in Figure 2B).
Figure 2.
Ion chronograms and mass spectra for the methanol mixture sample. Panel A shows the ion chronogram recorded over a 6.5-minute time frame during which the voltage applied to the HESI needle was ramped (see text for details). Panel B shows the mass spectrum recorded at the voltage setting for which maximum intensities were observed for the lower-intensity ions. The corresponding time frame for recording this spectrum is shown as a red trace in the ion chronogram (Panel A). Panel C shows the ion chronogram recorded over a 5-minute time frame during which the voltage applied to the APCI needle was ramped (see text for details) for the cVSSI-APCI setup. Panel D shows the mass spectrum recorded at the voltage setting for which maximum intensities were observed for the lower-intensity ions. The corresponding time frame for recording this spectrum is shown as a red trace in the ion chronogram (Panel C). The peak labels in panel B and D correspond with the numbered molecules in Table 1.
When cVSSI-APCI was utilized for the same sample, the voltage was increased from 0 to +3.0 kV at a step size of 0.25 kV. Discreet ion signals were observed in the mass spectrum for all analytes at +1.75 kV (3 minutes on the ion chronogram as shown in Figure 2C). The straight lines connecting regions of the ion chronogram are where data collection was paused to increase the voltage applied (dialed externally) to the needle. Prior to +1.75 kV, only cocaine ions were observed in high abundance, with thymine not even being observed until that voltage, as shown in Figure S3B. The optimum voltage providing the best ion intensities for all analytes was determined to be +3.0 kV. This voltage was utilized to compare the ion intensity with APCI turned on and with it turned off, as shown in Figure S4. At this voltage, all analytes are present with an intensity value of ≥105 (Figure 2D). These results are similar to those reported for the nESI-APCI studies.61 Compared to the HESI experiments (Figure 2B), the cVSSI-APCI combination increased the number of cocaine ions by a factor of 10 (Figure 2D) and dramatically increased the ion signal levels for thymine, theophylline, and Vitamin D2 by factors of 103, 102, and 102, respectively. The signal-to-noise (S/N) ratio was then analyzed for this mixture and for the typically suppressed analytes; the S/N was ~13 (Vitamin D2) to 38 (thymine) times higher for cVSSI-APCI compared to HESI. The only case in which the S/N decreased for cVSSI-APCI was cocaine which had a S/N about half that of HESI. The signal intensity and S/N ratios for both HESI and cVSSI-APCI are shown in Table S3.
From the observations of the cVSSI-APCI experiments, a question arises as to the behavior of the lower-signal ions in the absence of the cocaine. That is, is the same onset voltage observed and/or is there a greater enhancement in the absence of the high-ionizing cocaine molecule? Experiments were performed exactly as described above for an identical sample mixture with the exception that the cocaine molecule was removed. The intensity at each voltage for these conditions is shown in Figure S5. Figure S6 in the Supplementary Information section shows the results of this work. Here, all analytes were present with higher ion intensities than in the presence of cocaine (Figure 2B, Figure S6B). Figure S6B, D also shows that increased ion signals were observed for the cVSSI-APCI combination compared with the HESI source. Overall, for HESI experiments, S/N ratios were observed to be 2 to 5 times lower than those observed with cVSSI-APCI, with the signal intensity and S/N ratios shown in Table S4. Noticeably, the ratios of thymine and Vitamin D2 to theophylline remained similar to those observed in the cVSSI-APCI mass spectrum for the cocaine-containing sample. This could be an indicator that ionization of the compounds in solution does indeed proceed via an APCI-associated mechanism. That is, the number of molecules liberated by cVSSI is constant for a given sample concentration and the molecules are subsequently ionized by gas-phase proton transfer.
To further investigate the utility of the new cVSSI-APCI source in examining analyte compounds in mixtures, it is instructive to consider the relative ability to overcome matrix effects. One approach involves the determination of the ability to overcome matrix effects as indicated by Equation 1.
| 1)47 |
In Equation 1, A and B represent the solutions in which the matrix component is not and is present, respectively. The ability to overcome matrix effects can then be used to calculate the ion suppression according to Equation 2.
| 2) |
This was determined by analyzing the 200 μM samples of individual thymine, theophylline, and Vitamin D2, recording their intensity, and then conducting the same experiment for these samples with 200 μM cocaine added. Ideally, the matrix effects value is close to 100 as this indicates an ability to overcome matrix effects, leaving the ion suppression to be a low value. Having a matrix effects value exactly equal to 100 indicates that there are 0 effects whether they be ion suppression or ion enhancement. Using Equation 2 and the data for the solution containing the thymine, theophylline, and Vitamin D2, the values of ion suppression for HESI and cVSSI-APCI have been calculated and are shown in Table 2. The zoomed-in region of the mass spectra for cVSSI-APCI is shown in Figure S7. Overall, the highest value of ion suppression with cVSSI-APCI is 21% whereas for HESI the lowest ion suppression value is 71%. This indicates that we have a significant improvement in overcoming ion suppression compared to HESI.
Table 2:
Matrix effects and ion suppression percentage for analytes 1, 3, and 5 for cVSSI-APCI-MS and ESI-MS.
| Analyte | Matrix Effects (%) a | Ion Suppression (%) b | ||
|---|---|---|---|---|
| cVSSI-APCI | HESI | cVSSI-APCI | HESI | |
| Thymine | 86 | 24 | 14 | 76 |
| Theophylline | 79 | 24 | 21 | 76 |
| Vitamin D2 | 94 | 29 | 6 | 71 |
Calculated using equation 1
Calculated using equation 2
Extension of the cVSSI-APCI setup to other solvent systems.
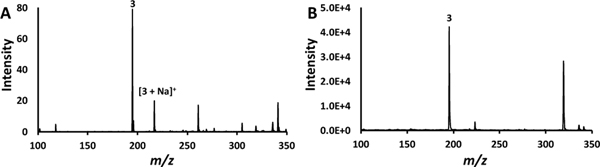
The results of the cVSSI-APCI work presented above were compared in a limited fashion to another technique that produces a droplet plume without the use of a high voltage. The recent work which coupled SAWN with APCI to demonstrate enhanced ionization served for this comparison.62 Indeed, a comparison to the current study is warranted as cVSSI also offers a low-power, low-cost alternative to generate microdroplets without the aid of an electric field. For the comparative experiments, a 5 μΜ solution of caffeine in 50/50 methanol/water is used to match that utilized in the SAWN-APCI experiments.62 Figure 3 shows the mass spectra that are obtained under conditions in which the DC voltage is not applied (3A) and is applied (3B) to the Tungsten needle.
Figure 3.
Mass spectra of 5 μM caffeine. Panel A shows the mass spectrum obtained when only cVSSI is activated. Panel B show the mass spectrum obtained when both cVSSI and APCI are activated. Dataset features are labeled with the compound number designation in Table 1.
In the absence of the corona discharge, cVSSI alone produces ions. The protonated caffeine peak exhibits a relative abundance of 100% while the sodiated peak exhibits a relative abundance of 30%. For the SAWN experiments which utilized the same mass analyzer type, these same ions were observed where the protonated and sodiated ions exhibit relative abundances of 33% and 100%, respectively. When voltage was applied to the APCI needle, there was an increase in signal for both methods, with the protonated species being the species with 100% relative abundance for both cVSSI-APCI and SAWN-APCI. Indeed, both techniques provide an enhancement of ~500 fold compared to when APCI is not functioning.
Having demonstrated that lower-signal species can be enhanced with the coupling of cVSSI and APCI for methanol solutions, a question arises as to whether it is possible to enhance the ionization of some low-signal compounds from aqueous samples. Here, the question is whether the greater surface tension of water droplets could result in efficient liberation of compounds that would subsequently be ionized by APCI. An aqueous mixture of thymine, theophylline, and caffeine (200 μM) was utilized. As with the analysis in methanol, the sample was analyzed using both HESI and cVSSI-APCI employing the same voltage ramps.
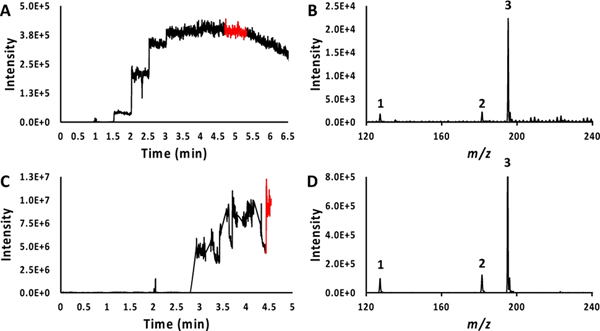
For the voltage-dependent HESI experiments, the same optimum voltage of 4.5 kV (Figure 4A, Figure S8A) was determined as in the study of the methanol mixture. That is, at this voltage, the greatest ion intensities (relative to that of caffeine) for the low-ionizing species were observed. The mass spectrum recorded at the optimum voltage showed that thymine and theophylline were observed at significantly lower intensity values than caffeine (Figure 4B).
Figure 4:
Ion chronograms and mass spectra for the water mixture sample. Panel A shows the ion chronogram recorded over 6.5-minute time frame during which the voltage applied to the HESI needle was ramped (see text for details). Panel B shows the mass spectrum recorded at the voltage setting for which maximum intensities were observed for the lower-intensity ions. The corresponding time frame for recording this spectrum is shown as a red trace in the ion chronogram (Panel A). Panel C shows the ion chronogram recorded over 5-minute time frame during which the voltage applied to the APCI needle was ramped (see text for details) for the cVSSI-APCI setup. Panel D shows the mass spectrum recorded at the voltage setting for which maximum intensities were observed for the lower-intensity ions. The corresponding time frame for recording this spectrum is shown as a red trace in the ion chronogram (Panel C). The peak labels in panel B and D correspond with the numbered molecules in Table 1.
When the aqueous mixture was examined using the cVSSI-APCI setup, the optimum voltage was again determined to be +3.0 kV (Figure 4C, Figure S8B). The mass spectrum in Figure 4D shows that the same ion intensity ratios of thymine and theophylline to caffeine are obtained as those from the HESI experiments, with each being about 10% of the caffeine peak. This suggests that there is not a dramatic increase in the ionization efficiency of several of the lower-signal compounds relative to the highest-signal species as observed for the methanol sample. That said, the signal levels observed for all ions in the cVSSI-APCI datasets were ~2 orders of magnitude higher than observed with HESI. However, the S/N increase is less, ranging from ~3 (caffeine) to ~13 (theophylline) fold higher for cVSSI-APCI, as shown in Table S5. Given that some of the noise represents chemical noise or signal noise, it can be argued that the cVSSI-APCI setup does indeed increase the signal levels of some ions relative to others and that this is important for experiments requiring high ion utilization.
Sensitivity considerations for the cVSSI-APCI approach.
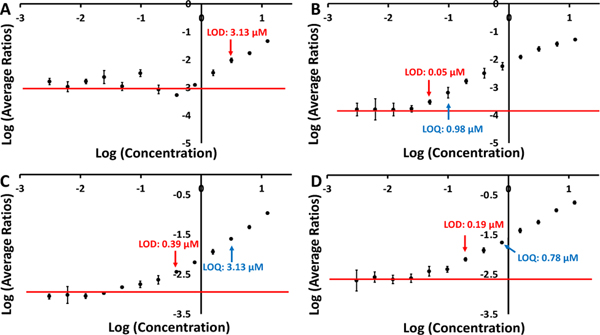
Having observed the dramatic signal level increases of low-ionizing compounds in methanol solutions, it is instructive to examine the relative sensitivity of the cVSSI-APCI and HESI sources when a sample contains a high-ionizing species. Because Vitamin D2 showed the lowest amount of ion suppression, this compound was not included in these experiments. To accomplish sensitivity comparisons, serial dilutions of two methanol solutions, one containing thymine and the other containing theophylline, were prepared. The concentrations of thymine and theophylline initiated from 12.5 μM and were varied using serial two-fold dilutions while the concentration of the high-ionizing caffeine was maintained at 200 μM in all samples. For both HESI and cVSSI-APCI, triplicate runs were obtained for all samples. To generate the standard curves, the average intensity of the analyte was normalized to the average intensity of caffeine. The triplicate runs occurred once the spray was stabilized for each ionization source. Figure 5 shows the average intensities (relative to caffeine’s average intensity) as a function of concentration for the different analytes. The red line on the curve indicates the average of the non-linear points of the curve. A limit of detection (LOD) and a limit of quantitation (LOQ) were determined for thymine analyzed by cVSSI-APCI and both theophylline curves while only the LOD was determined for the analysis of thymine with HESI. The LOD and LOQ were determined based on the location on the curve where the y-value was 3 and 10 times, respectively, the standard deviation of the non-linear region plus the average.
Figure 5.
Standard curves for thymine (A, B) and theophylline (C, D). The left and right panels show the data that were recorded for the HESI and cVSSI-APCI ionization sources, respectively. Data are reported as a ratio of the average intensity of [M+H]+ ions of the analytes and caffeine. The red line indicates the average of the non-linear region of the curve.
The calibration curve for thymine ions produced by HESI (Figure 5A) shows that the LOD is 3.13 μM and the LOQ is higher than any concentration on the curve. In comparison, when cVSSI-APCI was used, the LOD was determined to be 0.05 μM and the LOQ was 0.10 μM (Figure 5B). The use of cVSSI-APCI leads to an LOD that is ~64 times lower and an LOQ at least ~128 times lower than that of HESI.
The data for theophylline allows for the determination of the LOD and LOQ for both HESI and cVSSI-APCI (Figure 5C and 5D, respectively). The respective LOD and LOQ for cVSSI-APCI were 0.19 and 0.78 μM. For HESI, the respective LOD and LOQ were 0.39 and 3.13 μM. This indicates that the use of cVSSI-APCI leads to an LOD that is ~2 times lower than for HESI and an LOQ that is ~4 times lower. In nearly all respects related to utility, cVSSI-APCI outperformed HESI.
Direct analysis with cVSSI-APCI.
To demonstrate the ability to use the cVSSI-APCI approach for direct analyses, experiments monitored the ionization of compounds directly from two cotton swabs each containing a combination of a low ionizing species (Vitamin D2 or thymine) and a high-ionizing species (cocaine). The analytes were deposited by dispensing 100 μL of the 200 μM solutions onto the separate cotton swabs with subsequent drying (3 days). For direct MS analysis, 200 μL of methanol was deposited on the cotton swab and the swab was positioned in front of the mass spectrometer inlet as shown in Figure S9. A vibrating sharp-edge spray ionization (VSSI) device comprised only of the edge of a vibrating microscope slide was then applied by hand to the surface of the cotton swab. In the absence of the DC voltage, the application of the VSSI device immediately produced a plume eminating from the cotton swab. [M+H]+ cocaine ions were generated from this plume as shown in Figure S10A (thymine) and Figure S11A (Vitamin D2). Upon application of the DC voltage and corona discharge, the lower-ionizing species are easily observed (Figures S10B and S11B).
One aspect to be considered with regard to the relative ion signal levels observed in Figures S10B and S11B is the degree to which each compound is extracted/dissolved into the solvent. Solvent extraction efficiency for compounds dried on paper carrier substrate has long been examined for rapid/direct analysis by ESI.72,73 Such concerns have carried over to ambient ionization by paperspray ionization where different solvents and substrate material have been examined.74,75 Extraction efficiency has also been examined using desorption electrospray ionization for direct analyses.76 To demonstrate that this is also a concern for the proof-of-principle cotton swab analysis mentioned above, separate experiments employing aqueous solvent have been carried out. Here, pure water has been used to wet two cotton swabs each containing the dried cocaine and one of the other analyte compounds (thymine or Vitamin D2). The peak intensities of the water-extracted ions relative to the [M+H]+ cocaine ions are shown in Figure S12. Compared to [M+H]+ cocaine ions, the relative [M+H]+ thymine and Vitamin D2 ion intensity increases and decreases by ∼3-fold and 80-fold, respectively, in the transition from methanol to water extraction. This simple demonstration presents possibilities for tailoring direct analyses experiments performed with the VSSI setup by matching extraction solvent with target compound type.
The direct analysis experiment demonstrated with Figures S10 and S11 is admittedly simplified; real world analyses often contain samples exhibiting more complex mixtures. That said, these proof-of-principle experiments demonstrate a dramatic ability to obtain ion signal levels of lower-ionizing species relative to higher-ionizing compounds produced from the plume alone. Overall, these experiments begin to demonstrate how the approach might be implemented for direct, in-situ, or field analyses.
Distinguishing features of the cVSSI-APCI approach for field, in-situ, or direct analysis.
Considering the presence of other APCI-like ionization sources,61–64 it is instructive to review some of the salient features that make cVSSI-APCI especially suited for field, in-situ, or direct analysis. First, the nebulization process provided by cVSSI is unique in that the high acoutic streaming velocity produced by the vibrating sharp edge provides a uniquely efficient method for nebulization. This results in very low power requirements (~100 mW). For future experiments, this provides the ability to use only a small waveform driver to conduct such experiments. A second advantage is the portability of the cVSSI probe. The cotton swab experiments demonstrate that the small device can access many different sampling situations in a hand-held fashion. This includes the direct sampling directly from liquid or wetted surfaces. The fine droplets produced by cVSSI are another added feature of the methodology. Release of compounds from such droplets allows efficient ionization even at relatively low DC voltages. Finally, the plume production by cVSSI is extremely robust and the same device can generate plumes across a wide range in solent flow rates. For example, Figure S13 shows the mass spectra produced by the cVSSI-APCI approach when employing a flow rate of 80 μL/min. This flow rate is already in the microbore liquid chromatgraphy (LC) separation range and is approaching narrowbore LC flow rates. This is very encouraging considering the push to develop field-portable LC-MS systems.77
CONCLUSIONS
The combination of cVSSI with APCI is here demonstrated to improve the ionization efficiency of low-signal species that are in mixtures containing high-signal molecules. Overall, in the presence of cocaine in methanol, three analytes (thymine, theophylline, and Vitamin D2) show ion signal level improvements of ~2 to ~3 orders of magnitude when produced by cVSSI-APCI as compared to the state-of-the-art HESI source. Ion suppression was also shown to decrease by ~8 to ~15-fold when using cVSSI-APCI. In comparison, the signal levels of all ions were increased by ~3 orders of magnitude in the aqueous samples. Additionally, there was a significant increase in S/N levels for these compounds indicating a suppression of chemical noise (matrix effects). The ionization enhancement of low-signal compounds in the presence of high-ionization species in methanol was also examined as a function of compound concentration. Overall, it was determined that sensitivity enhancements of at least 8-fold could be achieved for thymine with the cVSSI-APCI source compared with the HESI source. Finally, proof-of-principle experiments demonstrate the utility of this method for direct analysis by examining analytes directly from a cotton swab; the ionization enhancement was retained for the direct analysis. These ionization enhancement gains open the door for analysis of complex mixtures having less polar analytes, such as biological samples of lipids. The lack of rigidity of the system also allows for advancement in the fields of direct, in-situ, or field analyses.
Supplementary Material
ACKNOWLEDGMENT
We are grateful for financial support of this work from the National Institutes of Health R01GM135432.
Conflict of Interest
S.J.V, and P.L. have co-founded a start-up company, Invibragen Inc., to commercialize technologies involving vibrating sharp-edge spray ionization (VSSI).
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Supporting Information (PDF)
Contains S/N ratios, structures, glass tips, droplet measurements, intensities, ion production, ion suppression, experiment schematics, high flow rate spectra
REFERENCES
- (1).Shelley JT; Badal SP; Engelhard C; Hayen H.Anal. Bioanal. Chem. 2018, 410, 4061–4076. [DOI] [PubMed] [Google Scholar]
- (2).Hsu CC; Dorrestein PC Curr. Opin. Biotechnol 2015, 31, 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Covey TR; Thomson BA; Schneider BB Mass Spectrom. Rev 2009, 28, 870–897. [DOI] [PubMed] [Google Scholar]
- (4).Harris GA; Nyadong L; Fernandez FM Analyst 2008, 133, 1297–1301. [DOI] [PubMed] [Google Scholar]
- (5).Li Y; Cao Y; Guo Y.Chin. J. Chem 2020, 38, 25–38. [Google Scholar]
- (6).Rankin-Turner S; Heaney LM Anal. Sci. Adv 2021, 2, 193–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).El-Aneed A; Cohen A; Banoub J.Appl. Spectrosc. Rev 2009, 44, 210–230. [Google Scholar]
- (8).Beneito-Cambra M; Moreno-González D; García-Reyes JF; Bouza M; Gilbert-López B; Molina-Díaz A.TrAC Trends Analyt. Chem 2020, 132, No. 116046. [Google Scholar]
- (9).Peacock PM; Zhang W-J; Trimpin S.Anal. Chem 2017, 89, 372–388. [DOI] [PubMed] [Google Scholar]
- (10).Trimpin S; Inutan ED; Herath TN; McEwen CN Mol. Cell. Proteomics 2010, 9, 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Trimpin S.Rapid Commun. Mass Spectrom 2019, 33, 96–120. [DOI] [PubMed] [Google Scholar]
- (12).Zhang J; Rector J; Lin JQ; Young JH; Sans M; Katta N; Giese N; Yu W; Nagi C; Suliburk J; Liu J; Bensussan A; DeHoog RJ; Garza K; Ludolph B; Sorace A; Syed A; Zahedivash A; Milner T; Eberlin L.Sci. Transl. Med 2017, 9, No. eaan3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Mason TJ; Bettenhausen HM; Chaparro JM; Uchanski ME; Prenni JE Hortic. Res 2021, 8, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Winter GT; Wilhide JA; LaCourse WR J. Am. Soc. Mass Spectrom 2015, 26, 1502–1507. [DOI] [PubMed] [Google Scholar]
- (15).Evans-Nguyen KM; Gerling J; Brown H; Miranda M; Windom A; Speer J.Analyst 2016, 141, 3811–3820. [DOI] [PubMed] [Google Scholar]
- (16).Sampson JS; Hawkridge AM; Muddiman DC J. Am. Soc. Mass Spectrom 2008, 19, 1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Banerjee S.J. Biosci 2018, 43, 731−738. [PubMed] [Google Scholar]
- (18).Ferreira CR; Yannell KE; Jarmusch AK; Pirro V; Ouyang Z; Cooks RG Clin. Chem 2016, 62, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Kondyli A; Schrader W.Rapid Commun. Mass Spectrom 2020, 34, No. e8676. [DOI] [PubMed] [Google Scholar]
- (20).Wysocki VH; Resing KA; Zhang Q; Cheng G.Methods 2005, 35, 211–222. [DOI] [PubMed] [Google Scholar]
- (21).Sobott F; Robinson CV Curr. Opin. Struct. Biol 2002, 12, 729–734. [DOI] [PubMed] [Google Scholar]
- (22).Idle JR; Gonzalez FJ Cell Metab. 2007, 6, 348–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Manchester M; Anand A.Adv. Virus Res 2017, 98, 57–81. [DOI] [PubMed] [Google Scholar]
- (24).Clendinen CS; Monge ME; Fernández FM Analyst 2017, 142, 3101–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Holčapek M.Anal. Bioanal. Chem 2015, 407, 4971–4972. [DOI] [PubMed] [Google Scholar]
- (26).Vaz FDRM; Pras-Raves M; Bootsma AH; van Kampen AHC J. Inherit. Metab. Dis 2015, 38, 41–52. [DOI] [PubMed] [Google Scholar]
- (27).Marshall AG; Rodgers RP Proc. Natl. Acad. Sci. U.S.A 2008, 105, 18090–18095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Garg N; Kapono CA; Lim YW; Koyama N; Vermeij MJA; Conrad D; Rohwer F; Dorrestein PC Int. J. Mass Spectrom 2015, 377, 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Han X; Aslanian A; Yates JR III Curr. Opin. Chem. Biol 2008, 12, 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Nakamura T; Kuromitsu J; Oda Y.J. Proteome Res 2008, 7, 1007–1011. [DOI] [PubMed] [Google Scholar]
- (31).Stults JT; Arnott D.Meth. Enzymol 2005, 402, 245–289. [DOI] [PubMed] [Google Scholar]
- (32).Goto-Inoue N; Hayasaka T; Zaima N; Setou M.Biochim. Biophys. Acta 2011, 1811, 961–969. [DOI] [PubMed] [Google Scholar]
- (33).Chamberlain CA; Rubio VY; Garrett TJ Metabolomics 2019, 15, 135. [DOI] [PubMed] [Google Scholar]
- (34).Byrdwell WC Lipids 2001, 36, 327–346. [DOI] [PubMed] [Google Scholar]
- (35).Bergeron A; Garofolo F.Bioanalysis 2013, 5, 2331–2332. [DOI] [PubMed] [Google Scholar]
- (36).Buhrman DL; Price PI; Rudewiczcor PJ J. Am. Soc. Mass Spectrom 1996, 7, 1099–1105. [DOI] [PubMed] [Google Scholar]
- (37).Kebarle P; Tang L.Anal. Chem 1993, 65, 972A–986A. [Google Scholar]
- (38).Rodgers RP; Mapolelo MM; Robbins WK; Chacón- Patiño ML; Putman JC; Niles SF; Rowland SM; Marshall AG Faraday Discuss. 2019, 218, 29–51. [DOI] [PubMed] [Google Scholar]
- (39).Khoury S; El Banna N; Tfaili S; Chaminade P.Anal. Bioanal. Chem 2016, 408, 1453–1465. [DOI] [PubMed] [Google Scholar]
- (40).Ruddy BM; Hendrickson CL; Rodgers RP; Marshall AG Energy Fuels 2018, 32, 2901–2907. [Google Scholar]
- (41).Matuszewski BK; Constanzer ML; Chavez-Eng CM Anal. Chem 1998, 70, 882–889. [DOI] [PubMed] [Google Scholar]
- (42).Matuszewski BK; Constanzer ML; Chavez-Eng CM Anal. Chem 2003, 75, 3019–3030. [DOI] [PubMed] [Google Scholar]
- (43).Mei H; Hsieh Y; Nardo C; Xu X; Wang S; Ng K; Korfmacher WA Rapid Commun. Mass Spectrom 2003, 17, 97–103. [DOI] [PubMed] [Google Scholar]
- (44).Niessen WMA; Manini P; Andreoli R.Mass Spectrom. Rev 2006, 25, 881–899. [DOI] [PubMed] [Google Scholar]
- (45).Desfontaine V; Capetti F; Nicoli R; Kuuranne T; Veuthey JL; Guillarme DJ Chromatogr., B Analyt. Technol. Biomed. Life Sci 2018, 1079, 51–61. [DOI] [PubMed] [Google Scholar]
- (46).Van Eeckhaut A; Lanckmans K; Sarre S; Smolders I; Michotte YJ Chromatogr., B Analyt. Technol. Biomed. Life Sci 2009, 877, 2198–2207. [DOI] [PubMed] [Google Scholar]
- (47).Jessome LL; Volmer DA LC GC N. Am 2006, 24, 498–510. [Google Scholar]
- (48).Boughton BA; Thinagaran D; Sarabia D; Bacic A; Roessner U.Phytochem. Rev 2016, 15, 445–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Buchberger AR; DeLaney K; Johnson J; Li L.Anal. Chem 2018, 90, 240–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Lorenz M; Ovchinnikova OS; Kertesz V; Van Berkel GJ Rapid Commun. Mass Spectrom 2013, 27, 1429–143 [DOI] [PubMed] [Google Scholar]
- (51).McDonnell LA; Heeren RM A. Mass Spectrom. Rev 2007, 26, 606–643 [DOI] [PubMed] [Google Scholar]
- (52).Knittelfelder OL; Weberhofer BP; Eichmann TO; Kohlwein SD; Rechberger GNJ Chromatogr., B Analyt. Technol. Biomed. Life Sci 2014, 951–952, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Kislinger T; Gramolini AO; MacLennan DH; Emili AJ Am. Soc. Mass Spectrom 2005, 16, 1207–1220. [DOI] [PubMed] [Google Scholar]
- (54).Washburn MP; Wolters D; Yates JR III Nat. Biotechnol 2001, 19, 242–247. [DOI] [PubMed] [Google Scholar]
- (55).Chambers E; Wagrowski-Diehl DM; Lu Z; Mazzeo JR J. Chromatogr., B Analyt. Technol. Biomed. Life Sci 2007, 852, 22–34. [DOI] [PubMed] [Google Scholar]
- (56).Valentine SJ; Kulchania M; Barnes CAS; Clemmer DE Int. J. Mass Spectrom 2001, 212, 97. [Google Scholar]
- (57).Carroll DI; Dzidic I; Stillwell RN; Haegele KD; Horning EC Anal. Chem 1975, 47, 2369–2373. [DOI] [PubMed] [Google Scholar]
- (58).Carroll DI; Dzidic I; Stillwell RN; Horning MG; Horning EC Anal. Chem 1974, 46, 706–710. [Google Scholar]
- (59).Kambara H; Kanomata I.Anal. Chem 1977, 49, 270–275. [Google Scholar]
- (60).Kambara H; Kanomata IJ Mass Spectrom. Soc. Jpn 1976, 24, 229–236. [Google Scholar]
- (61).Kulyk DS; Swiner DJ; Sahraeian T; Badu-Tawiah AK Anal. Chem 2019, 91, 11562–11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Song L; You Y; Evans-Nguyen T.Anal. Chem 2019, 91, 912–918. [DOI] [PubMed] [Google Scholar]
- (63).Feider CL; Gatmaitan AN; Hooper T; Chakraborty A; Gowda P; Buchanan E; Eberlin LS Anal. Chem 2021, 93, 7549–7556. [DOI] [PubMed] [Google Scholar]
- (64).Song L; You Y; Perdomo NR; Evans-Nguyen T.Anal. Chem 2020, 92, 11072–11079. [DOI] [PubMed] [Google Scholar]
- (65).Li C; Attanayake K; Valentine SJ; Li P.Anal. Chem 2020, 92, 2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Li X; Attanayake K; Valentine SJ; Li P.Rapid Commun. Mass Spectrom 2021, 35, No. e8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Majuta SN; DeBastiani A; Li P; Valentine SJ J. Am. Soc. Mass Spectrom 2021, 32, 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Ranganathan N; Li C; Suder T; Karanji AK; Li X; He Z; Valentine SJ; Li P.J. Am. Soc. Mass Spectrom 2019, 30, 824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Wleklinski M; Li Y; Bag S; Sarkar D; Narayanan R; Pradeep T; Cooks RG Anal. Chem 2015, 87, 6786–6793. [DOI] [PubMed] [Google Scholar]
- (70).Majuta SN; Li C; Jayasundara K; Kiani Karanji A; Attanayake K; Ranganathan N; Li P; Valentine SJ J. Am. Soc. Mass Spectrom 2019, 30, 1102–1114. [DOI] [PubMed] [Google Scholar]
- (71).Jayasundara KU; Li C; DeBastiani A; Sharif D; Li P; Valentine SJ J. Am. Soc. Mass Spectrom 2021, 32, 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Van Berkel GJ; Kertesz V.Anal. Chem 2009, 81, 9146–9152. [DOI] [PubMed] [Google Scholar]
- (73).Kertesz V; van Berkel GJ J. Mass Spectrom 2010, 45, 252–260. [DOI] [PubMed] [Google Scholar]
- (74).Riboni N; Quaranta A; Motwani HV; Österlund N; Gräslund A; Bianchi F; Ilag LL Sci. Rep 2019, 9, 10296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Bills BJ; Kinkade J; Ren G; Manicke NE Forensic Chem. 2018, 11, 15–22. [Google Scholar]
- (76).Chipuk JE; Brodbelt JS J. Am. Soc. Mass Spectrom 2009, 20, 584–592. [DOI] [PubMed] [Google Scholar]
- (77).Rahimi F; Chatzimichail S; Saifuddin A; Surman AJ; Taylor-Robinson SD; Salehi-Reyhani A.Chromatographia 2020, 83, 1165–1195. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.