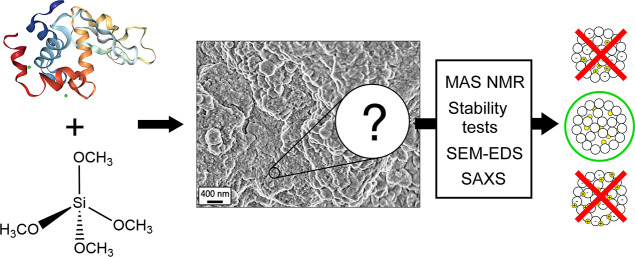
Abstract
Lysozyme is widely known to promote the formation of condensed silica networks from solutions containing silicic acid, in a reproducible and cost-effective way. However, little is known about the fate of the protein after the formation of the silica particles. Also, the relative arrangement of the different components in the resulting material is a matter of debate. In this study, we investigate the nature of the protein–silica interactions by means of solid-state nuclear magnetic resonance spectroscopy, small-angle X-ray scattering, and electron microscopy. We find that lysozyme and silica are in intimate contact and strongly interacting, but their interaction is neither covalent nor electrostatic: lysozyme is mostly trapped inside the silica by steric effects.
Introduction
Spherical silica is one of the most relevant contemporary industrial inorganic compounds, with uses that range across all areas of modern chemistry.1−3 With its large use, the sustainability and the environmental footprint of its production are to be considered. Bioinspired silica preparation (i.e., the formation of silica templated by biological macromolecules) has attracted an increasing interest being remarkably cost-effective,4 while tunability of the structure and reactivity of the silica-based composites is accessible through selection of biomolecules, offering a wide range of opportunities in several fields.5,6 Among the different bioinspired silica preparation strategies,7,8 the bioinspired synthesis proposed by Luckarift et al.9 is quite convenient. This synthesis uses lysozyme, a small prolate protein with a high positive surface charge, as a catalyst for the polycondensation of silicic acid. The latter is obtained from hydrolysis of silicic alkoxides such as tetramethylorthosilicate (TMOS). Therefore, the preparation is based on inexpensive10 and readily available reagents, is fast, can be performed under ambient conditions (i.e., room temperature and atmosphere), and yields a condensed silica network without thermal processing. Other inorganic oxides, such as titania, can be produced through a similar chemistry.9
Despite the significant interest in this approach, the templating effect of the protein on the silica aggregation mechanism is not completely understood. Lysozyme enhances the nanoparticle formation and affects the composite structure and composition,11 but a complete structural characterization of these silica–protein composites is still lacking. The reactivity of lysozyme, which is common to other highly positively charged proteins and polypeptides, is reasonably attributable to electrostatic interactions, which involve the positively charged side chains of lysozyme and the negatively charged monosilicic acid, oligomeric silica, and colloidal silica. Indeed, a recent crystal structure localized a hydrolyzed species of the titanium precursor at a positive patch on the protein surface.12 However, no such interaction could be observed for silicic acid.12 A recent study suggests that liquid–liquid phase separation (LLPS) is responsible for driving the polymerization; however, the LLPS is related to overall charge,13 and there are reports that lysozyme tends not to undergo LLPS at pH values around 7.14 It has been previously demonstrated that lysozyme catalyzes the silica colloid formation from silicon alkoxide solutions and dilute water glass solutions around neutral pH.7,15 In the Luckarift et al. work, it was demonstrated that the protein maintains its catalytic activity and is not removed by repeated washes.9 The latter indicates that the protein interacts strongly with silica. A recent study on both adsorption and co-precipitation experiments suggests that lysozyme neither alters the silica structure nor is trapped inside the silica.16 The same study states that partial unfolding of the enzyme can occur, and time-resolved SAXS data suggest that the structure of lysozyme is strongly distorted during the initial events of the composite formation and then regains its “native-like” shape at a later stage during the silica aggregation process.17 However, no evidence of structural distortion has been observed by solution nuclear magnetic resonance (NMR) spectroscopy.12
Lysozyme is often used as a model for interface interactions because it is easily obtainable, has intriguing biophysical properties (often proteins are negative, rather than positive18), and has a useful antimicrobial activity.19−24 Therefore, it is expected that an improved understanding of lysozyme–material interactions could provide hints for applications beyond the study of bioinspired mineralization.25−27
In the present work, we investigate this peculiar interaction occurring at the protein–material interface in the lysozyme–silica hybrid composite using solid-state NMR under magic-angle spinning (MAS), scanning electron microscopy (SEM), and small-angle X-ray scattering (SAXS). As these hybrid interfaces are non-crystalline in nature, MAS NMR spectroscopy is the ideal tool to unravel short- (1.5–5.0 Å) and medium-range (5.0–10.0 Å) atomic-level distances that define their structure.28
Solid-state NMR is indeed a critical methodology in the characterization of biomacromolecular interfaces, as demonstrated, for instance, in the characterization of bones,29−33 corals,34 and other biomaterials and bioinspired materials in general35−37 and silicic materials in particular.38−40
Results and Discussion
The protein immobilization was carried out according to the protocol previously reported by Luckarift et al.9 A sample containing particles with spherical shape was obtained (SEM micrographs in Figure S1a). Elemental analysis using energy-dispersive X-ray spectrometry (EDS, Figure S1b) shows that nitrogen (arising from the protein) and silicon (arising from the matrix) are both present in the sample.
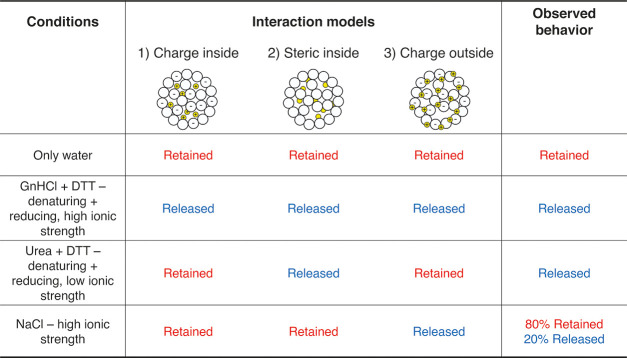
According to the previous literature,11,16,17 the possible relative arrangements of the protein and silica are:
-
(1)
charge inside: the protein is trapped inside the silica particles by electrostatic interactions,
-
(2)
steric inside: the protein is trapped inside the silica particles during their formation and cannot escape, while marginal interactions exist, and
-
(3)
charge outside: the protein is interacting with the exterior of the silica particles via (mainly) electrostatic interactions.
These three arrangements would lead to a distinct outcome for different treatments of the sample (Scheme 1). We find that, upon denaturation with either guanidinium chloride (GnHCl) or urea and reduction with dithiothreitol [(2S,3S)-1,4-bis(sulfanyl)butane-2,3-diol, DTT], the protein is quantitatively released, regardless of the ionic strength, leaving the silica structure with a porosity on the nanometer scale (SEM micrographs in Figure S2a). The removal of the protein from the inorganic network is confirmed by EDS results (Figure S2b), showing the disappearance of the nitrogen signal with respect to the as-prepared sample. Washing with 1 mol dm–3 sodium chloride causes the release of about 1/5 of the protein, as observed from the UV absorbance of the supernatant and confirmed by the decrease in the amount of nitrogen with respect to silicon at the end of the washing step (SEM micrographs and EDS spectrum in Figure S3a,b, respectively). In this last case, the morphology of the sample remains almost unaltered if compared to the freeze-dried starting composite. These experimental observations appear to favor situation #2, at least for 80% of the protein, whereas the remaining 20% may fall in situation #3.
Scheme 1. Behavior of the Composite With Respect to Ionic Strength and Denaturation.
We have therefore proceeded with the solid-state NMR characterization (see details in the Supporting Information and Table S1). Observation of the protein resonances is possible through 13C MAS NMR spectra (Figure 1). These were first acquired for the freeze-dried sample. As typical for dry proteins, the resolution of the signals is low due to structural heterogeneity. However, upon rehydration, the spectral resolution increases because of the water that promotes local dynamics. This observation is rather common in solid-state NMR on passing from lyophilized to rehydrated biomolecules.41−54 The comparison with the spectrum of the sedimented lysozyme shows that the broader envelope of the composite spectrum encompasses the frequency range that is observed for the sedimented protein. Since the spread of the 13C NMR signals indicates the folding of the protein, we can infer that the fold of the protein is preserved.55,56 Even with a resulting broad spectrum, the distinctive features of the folded protein are observed, that is, features from the methyl-bearing side chains, which have shifts lower than 20 ppm when the hydrophobic core of the protein is intact, and in the overall distribution of the aliphatic region (65 to 5 ppm). This observation confirms previous reports by Mirau57 and by Ravera et al.56 and is in contrast with the idea that lysozyme loses its tertiary structure when the silica matrix is formed.16
Figure 1.
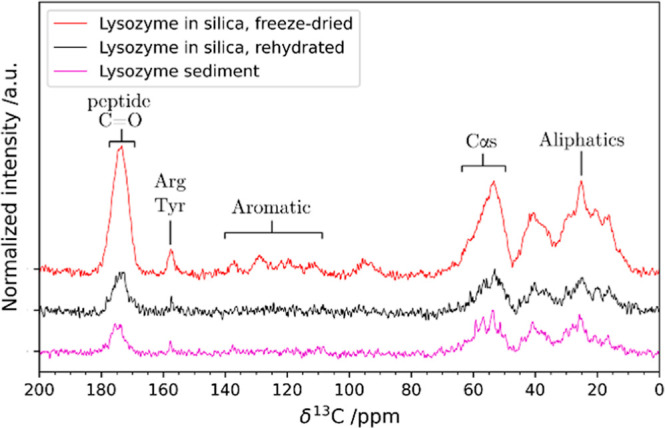
{1H}–13C CP MAS NMR spectra of the composite (red: freeze-dried and black: rehydrated) and of lysozyme sediment (magenta). The spectral regions where protein signals are usually observed are annotated on the spectra.
It is worth noting that the peak around 157 ppm can be attributed to either arginine or tyrosine Cζ. Since the lysozyme sequence features 11 arginine and 3 tyrosine residues, most of the intensity of this peak can be attributed to arginine residues.58 The intensity increase of the peak in the freeze-dried composite with respect to the protein sediment can be attributed to a lower mobility of the side chain in the composite with respect to the free protein. We also note that this peak is markedly narrowed upon rehydration and, considering the high arginine content, this behavior is similar to what was observed for arginine 15N lines in ubiquitin interacting with MCM41.54,59
Solid-state NMR also offers a unique view for the characterization of the silica matrix. The 29Si MAS NMR spectra show signals originating from siloxanes (Q4, around −110 ppm), single silanols (Q3, around −100 ppm), and geminal silanols (Q2, around −90 ppm), the latter being barely visible (Figure 2, refer to Scheme S1 for a summary of the observable species). The broad line widths are expected for amorphous silica as they are mainly affected by the dispersion of isotropic chemical shifts arising from a distribution of bond angles and distances.60 The results of the deconvolution are given in Table S2. The ratio Q4/Q3, which is indicative of the degree of condensation of the silica matrix, is 1.39, lying around the values observed for PL-12 templated bioinspired silica (1.54)61 and SBA-15 silica (1.60)62 and similar to the results obtained by Martelli et al., where similar preparation conditions were used.63 Notably, silica gel obtained under the same conditions but without the protein has a ratio of 0.68, and therefore, it is much less condensed (Figure S4, Table S3).
Figure 2.
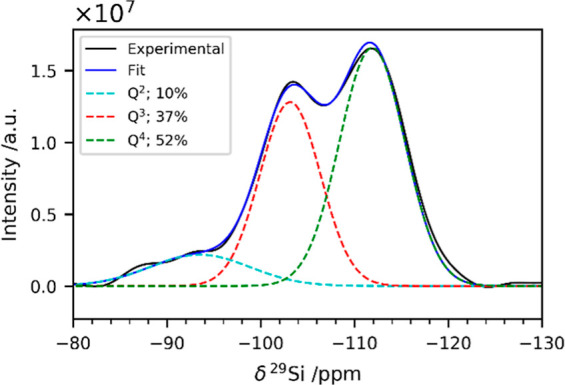
29Si direct-excitation MAS NMR spectra of the freeze-dried composite. The signals have been deconvoluted using three Gaussian peaks corresponding to Q2 (cyan), Q3 (red), and Q4 (green) sites.
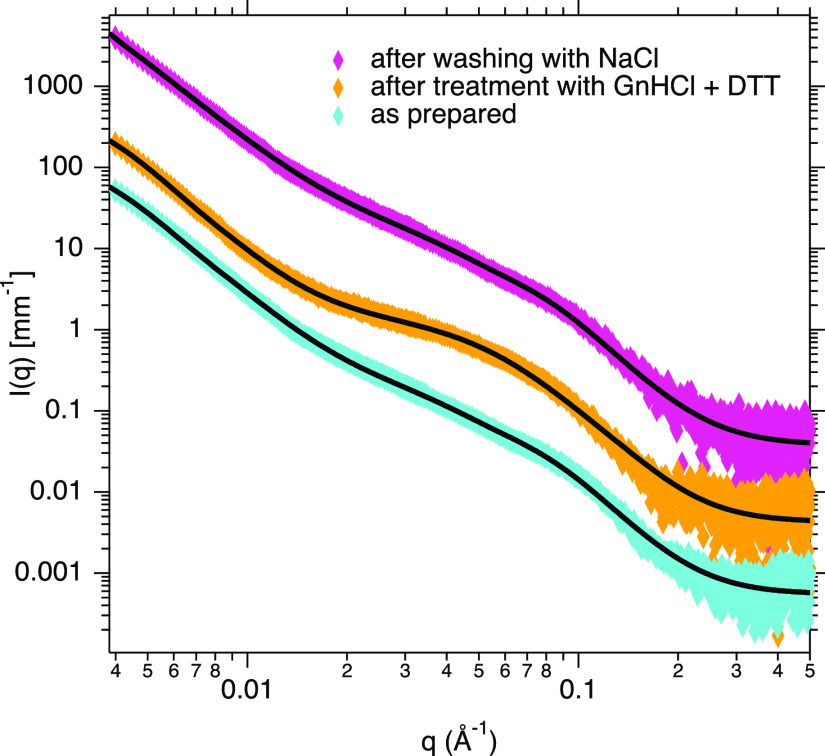
The spectrum of the composite treated with GnHCl and DTT (Figure S5, Table S4) shows an increase in the Q4/Q3 ratio from 1.39 to 1.72. This might be a consequence of the fact that lysozyme, when leaving the composite because of its denaturation, causes the detachment of the less condensed and thus more weakly bound parts of the silica. This is also consistent with the structural results obtained through SAXS (Figure 3).
Figure 3.
SAXS curves of the as-prepared composite (cyan), after treatment with GnHCl and DTT (orange) and after washing with NaCl (magenta) along with the best fit (black lines) using the Unified fit model. The experimental intensities are shifted along the y-axis for the sake of clarity (offset 1000 mm–1). The fitting results are reported in Table S5.
The scattering data were modeled using the Unified model approach.64 This approach describes scattering data as composed of multiple dimensional levels with different structural features. Each level is described by a Guinier and an associated Porod power-law regime (see Supporting Information). In particular, the exponential decay in the log–log plot (Guinier region) is directly connected to the average structural size of the scattering level, while the Porod power-law region reflects its fractal dimension. As apparent from the change in shape of the scattering profile after treatment with GnHCl and DTT, the SAXS data show a reduction of the mean radius of the silica primary units from 4 to 3 nm upon protein removal. This result agrees well with the formation of a less compact structure as already suggested by NMR and SEM data. On the contrary, no remarkable changes in the nanostructure of the silica matrix occur after washing with NaCl with a mean radius of the silica primary units of 4 nm as in the starting material.
A direct correlation of 13C MAS NMR signals from the protein to 29Si MAS NMR signals from the matrix would enable us to reveal an intimate contact between the two components.65,66 However, detection of such correlation is necessarily limited since both the 13C nuclei in the protein and the 29Si nuclei in silica are in natural abundance (1.1% 13C and 4.7% 29Si) in the complex, reducing the probability of a coupled 13C–29Si spin pair to 1 in 2000.65 Therefore, we have chosen to work on the comparison of the 1H traces and to discriminate among the protons that act as polarization sources for the heteronuclear sites. Hence, we have acquired 1H-X 2D correlation HETCOR spectra, with wPMLG67−691H homonuclear decoupling during the evolution of the indirect 1H dimension and cross-polarization (CP)70,71 for polarization transfer. To increase the sensitivity, all 2D NMR spectra were processed with multivariate curve resolution (see Supporting Information).72
The 1H NMR spectra that are obtained projecting the 2D HETCOR on the dry sample along the 29Si dimension show cross-polarization coming from at least three unique 1H sources. All the traces are rather broad, with features around 1.6, 4, and 7 ppm (a representative example is shown in Figure 4).
Figure 4.
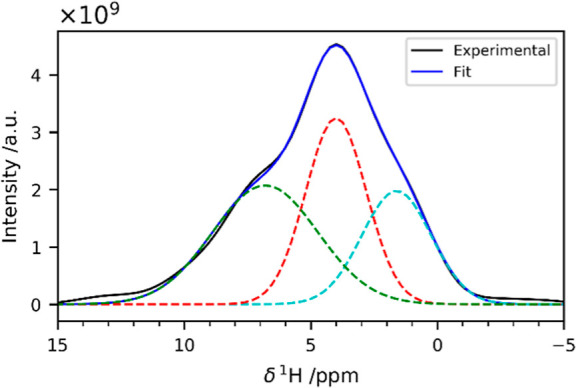
1H trace corresponding to the Q4 sites of the {1H}-29Si HETCOR acquired with 10 ms of CP contact time on the freeze-dried composite. The signal has been deconvolved with three Gaussian peaks centered at 1.6, 4.0 and 6.8 ppm.
The first two signals (1.6 and 4.0 ppm) are consistent with signals usually observed for silica: they can be attributed to silanols and physisorbed water, respectively.73,74 In biosilica, the feature around 1 ppm has also been ascribed to aliphatic protons from biomolecules.65 The feature around 7 ppm deserves more attention. This line is sometimes observed in 1H NMR spectra of silica and has been attributed to −OH2+ species or hydrogen-bonded silanols,73,75,76 or water molecules hydrogen-bonded to bridging oxygen atoms.74,77 In the specific case of biosilica, it may arise from the protein backbone and/or side chains, although this latter assignment remains elusive.65 In the spectrum of the gel obtained in the absence of lysozyme, this feature is completely absent (Figure S7). The fact that this peak, under our experimental conditions, is only observed in the presence of the protein implies that the interaction with the protein alters the behavior of the silica surface. One possibility for further sorting this out is to find out whether the carbon nuclei in the protein and the silicon nuclei in the silica matrix are getting polarized by the same proton source(s) or not. Therefore, the HETCOR experiments were repeated with 13C as the target nucleus, still on the dry sample. The 1H–13C correlation spectra are common for a protein sample where maximal transfer is observed at a shorter mixing time of 150 μs for the directly bonded 1H–13C spin pairs such as alpha protons (Hα)-alpha carbons (Cα) and at longer times for carbonyl (C′) carbons polarized by amide protons (HN) and Hα (Figure 5).
Figure 5.
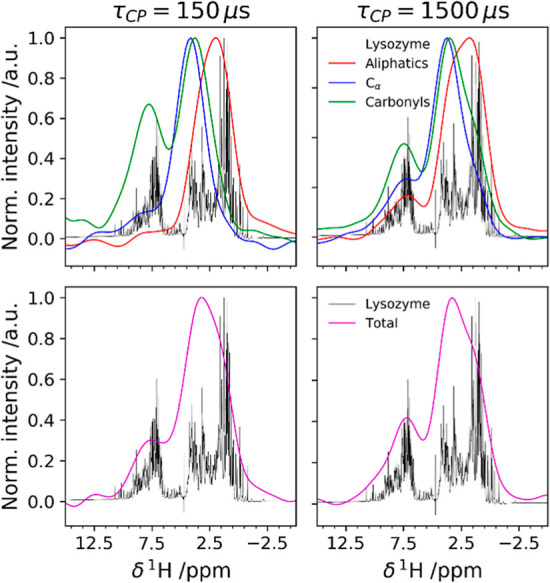
1H trace of the {1H}–13C HETCOR acquired on the freeze-dried composite with 150 μs (left) and 1500 μs (right) of CP contact time. In the top panels, the individual contributions of aliphatic (red), Cαs (blue), and C′s (green) are reported. The integration ranges for the three groups are shown in Figure S9. In the bottom panels, the signal is integrated over all the 13C species. The 1H spectrum of lysozyme in solution is shown in black in all the spectra.
A representative high-resolution 1H NMR spectrum of lysozyme in solution is given alongside with the 1H trace from the {1H}–13C HETCOR. We observe that, at low contact times, 13C receives magnetization from the closest protein protons and, at longer times, from more distant protons on neighboring bonded atoms (e.g., blue trace shows higher polarization of Cα by HN at 1500 μs). In the {1H}–29Si HETCOR (integration regions are shown in Figure S7), Q429Si sites receive magnetization mainly from silanols, whereas Q329Si sites also receive magnetization from the protons at higher chemical shift values. When longer mixing times are used, the contribution from this proton pool to the Q4 sites increases (Figure S9). The proton pool at a higher chemical shift is broad and overlaps with the HN proton pool as obtained from the {1H}–13C HETCOR (see Figure S10, Table S8). If D2O is used for rehydration, the 1H peak corresponding to amides is reduced (due to D/H exchange) in the traces of the 13C NMR spectra but not abolished. Interestingly, the higher-frequency proton pool as detected from the {1H}–29Si HETCOR sharpens, and the overlap to the HN proton from the {1H}–13C HETCOR becomes more apparent (Figure 6).
Figure 6.
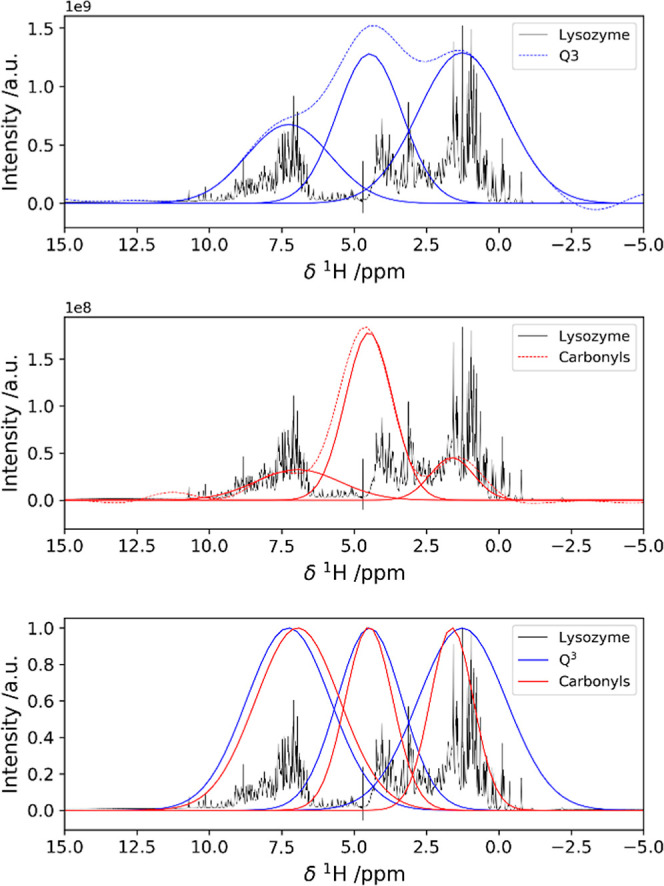
Overlap among the 1H polarization sources for 29Si and 13C HETCOR spectra, revealing the close contact between the protein and the silica matrix. 1H trace of the {1H}–29Si HETCOR acquired with 500 μs of CP contact time on the sample rehydrated with D2O. The signal is deconvoluted as a sum of three Gaussian peaks (top). 1H trace of the {1H}–13C HETCOR acquired with 150 μs of CP contact time on the sample rehydrated with D2O. The signal is deconvoluted as a sum of three Gaussian peaks (middle). For clarity of representation, the two sets of Gaussian peaks used for the deconvolution of the {1H}–29Si and the {1H}–13C HETCOR 1H traces are shown together and reported to the same height to highlight the similarity (bottom).
These observations suggest that aromatic, arginine, or lysine side chains or backbone amides are proximate to surface groups in silica. Even under the assumption that the protons at higher chemical shift values only belong to hydrogen-bonded silanols, the intensity of high-chemical shift proton peaks in the {1H}–29Si HETCOR implies that both H-bonded silanol protons and backbone amide protons are close to Q4 species on the silica surface.
However, other peaks at lower frequency in the 1H dimension, which would be given by Q4–Kε signals, are not observed, unlike other silica–peptide preparations, where clear indication of covalent interaction has been provided.34 This is consistent with our experimental observation that the denatured protein is detached from the material, which would not be the case in the presence of a covalent bond.
Conclusions
In conclusion, lysozyme remains in tight contact with the surface sites of the condensed silica. These interactions do not alter the rigid globular fold of lysozyme appreciably. Chemically induced denaturation is necessary to remove the protein from the composite, pointing again to strong interactions between lysozyme and the inorganic material. Lysozyme is apparently held inside the silica via occluding steric effects rather than by charge–charge interactions (Scheme 1, #2).
Acknowledgments
This work has been supported by the Fondazione Cassa di Risparmio di Firenze, by the Italian Ministero dell’Istruzione, dell’Università e della Ricerca, through the “Progetto Dipartimenti di Eccellenza 2018–2022” to the Department of Chemistry “Ugo Schiff” of the University of Florence. F.B., G.F., E.F., and E.R. acknowledge the Italian Ministry of Education, University and Research (MIUR), and European Social Fund (ESF) for the PON R&I 2014–2020 program, actions IV.4 “Doctorates and research contracts on Innovation topics” and IV.6 “Research contracts on green issues”. GF and EF acknowledge partial financial support from Consorzio per lo sviluppo dei Sistemi a Grande Interface (CSGI). The authors acknowledge the support and the use of resources of Instruct-ERIC, a landmark ESFRI project, and specifically the CERM/CIRMMP Italy center.
Glossary
Abbreviations
- TMOS
tetramethylorthosilicate
- FESEM
field-emission scanning electron microscope
- EDS
energy-dispersive X-ray spectroscopy
- wPMLG
windowed phase-modulated Lee-Goldberg
- CP
cross-polarization
- HETCOR
heteronuclear correlation experiment
- CPMG
Carr–Purcell–Meiboom–Gill sequence
- DTT
dithiothreitol
- GnHCl
guanidinium chloride
- MCR
multivariate curve resolution
- MAS
magic angle spinning
- NMR
nuclear magnetic resonance
- SAXS
small-angle X-ray scattering
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.langmuir.2c00836.
Experimental details for NMR, SEM, and SAXS, SEM micrographs, chemical shifts of 1H and 29Si species, parameters from the Gaussian deconvolution, 29Si direct excitation solid-state NMR spectra, experimental SAXS data, and 1H trace of the {1H }-29Si HETCOR and {1H }-13C HETCOR (PDF)
Author Contributions
The article was written through contributions of all authors, and all authors have given approval to the final version of the article.
The authors declare no competing financial interest.
Dedication
This work honors the memory of Shimon Vega, a profound scientist and a dedicated mentor. E.R. and G.G. acknowledge the immense contribution of Shimon Vega to their scientific career and training.
Supplementary Material
References
- Brown P. R. High-Performance Liquid Chromatography. Past Developments, Present Status, and Future Trends. Anal. Chem. 1990, 62, 995A–1008A. 10.1021/ac00218a721. [DOI] [Google Scholar]
- Narayanan R.; El-Sayed M. A. Effect of Catalysis on the Stability of Metallic Nanoparticles: Suzuki Reaction Catalyzed by PVP-Palladium Nanoparticles. J. Am. Chem. Soc. 2003, 125, 8340–8347. 10.1021/ja035044x. [DOI] [PubMed] [Google Scholar]
- Pagliaro M.Silica-Based Materials for Advanced Chemical Applications; Royal Society of Chemistry, 2009. [Google Scholar]
- Curley R.; Holmes J. D.; Flynn E. J. Can Sustainable, Monodisperse, Spherical Silica Be Produced from Biomolecules? A Review. Appl. Nanosci. 2021, 11, 1777–1804. 10.1007/s13204-021-01869-6. [DOI] [Google Scholar]
- Sumper M.; Brunner E. Learning from Diatoms: Nature’s Tools for the Production of Nanostructured Silica. Adv. Funct. Mater. 2006, 16, 17–26. 10.1002/adfm.200500616. [DOI] [Google Scholar]
- Wang Y.; Cai J.; Jiang Y.; Jiang X.; Zhang D. Preparation of Biosilica Structures from Frustules of Diatoms and Their Applications: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2013, 97, 453–460. 10.1007/s00253-012-4568-0. [DOI] [PubMed] [Google Scholar]
- Coradin T.; Livage J. Aqueous Silicates in Biological Sol–Gel Applications: New Perspectives for Old Precursors. Acc. Chem. Res. 2007, 40, 819–826. 10.1021/ar068129m. [DOI] [PubMed] [Google Scholar]
- Abdelhamid M. A. A.; Pack S. P. Biomimetic and Bioinspired Silicifications: Recent Advances for Biomaterial Design and Applications. Acta Biomater. 2021, 120, 38–56. 10.1016/j.actbio.2020.05.017. [DOI] [PubMed] [Google Scholar]
- Luckarift H. R.; Dickerson M. B.; Sandhage K. H.; Spain J. C. Rapid, Room-Temperature Synthesis of Antibacterial Bionanocomposites of Lysozyme with Amorphous Silica or Titania. Small 2006, 2, 640–643. 10.1002/smll.200500376. [DOI] [PubMed] [Google Scholar]
- Cheng C.-Y.; Wang M.-Y.; Suen S.-Y. Eco-Friendly Polylactic Acid/Rice Husk Ash Mixed Matrix Membrane for Efficient Purification of Lysozyme from Chicken Egg White. J. Taiwan Inst. Chem. Eng. 2020, 111, 11–23. 10.1016/j.jtice.2020.05.008. [DOI] [Google Scholar]
- Shiomi T.; Tsunoda T.; Kawai A.; Mizukami F.; Sakaguchi K. Biomimetic Synthesis of Lysozyme–Silica Hybrid Hollow Particles Using Sonochemical Treatment: Influence of PH and Lysozyme Concentration on Morphology. Chem. Mater. 2007, 19, 4486–4493. 10.1021/cm071011v. [DOI] [Google Scholar]
- Gigli L.; Ravera E.; Calderone V.; Luchinat C. On the Mechanism of Bioinspired Formation of Inorganic Oxides: Structural Evidence of the Electrostatic Nature of the Interaction between a Mononuclear Inorganic Precursor and Lysozyme. Biomolecules 2021, 11, 43. 10.3390/biom11010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H.; Bendikov T.; Gal A. Phase Separation of Oppositely Charged Polymers Regulates Bioinspired Silicification. Angew. Chem. Int. Ed. 2022, 61, e202115930 10.1002/anie.202115930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne P.; Mitra R. K. Excipients Do Regulate Phase Separation in Lysozyme and Thus Also Its Hydration. J. Phys. Chem. Lett. 2022, 13, 931–938. 10.1021/acs.jpclett.1c03449. [DOI] [PubMed] [Google Scholar]
- Coradin T.; Durupthy O.; Livage J. Interactions of Amino-Containing Peptides with Sodium Silicate and Colloidal Silica: A Biomimetic Approach of Silicification. Langmuir 2002, 18, 2331–2336. 10.1021/la011106q. [DOI] [Google Scholar]
- van den Heuvel D. B.; Stawski T. M.; Tobler D. J.; Wirth R.; Peacock C. L.; Benning L. G. Formation of Silica-Lysozyme Composites Through Co-Precipitation and Adsorption. Front. Mater. Sci. 2018, 5, 19. 10.3389/fmats.2018.00019. [DOI] [Google Scholar]
- Stawski T. M.; van den Heuvel D. B.; Besselink R.; Tobler D. J.; Benning L. G. Mechanism of Silica–Lysozyme Composite Formation Unravelled by in Situ Fast SAXS. Beilstein J. Nanotechnol. 2019, 10, 182–197. 10.3762/bjnano.10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraga J.; Mackiewicz P.; Mackiewicz D.; Kowalczuk M.; Biecek P.; Polak N.; Smolarczyk K.; Dudek M. R.; Cebrat S. The Relationships between the Isoelectric Point and: Length of Proteins, Taxonomy and Ecology of Organisms. BMC Genom. 2007, 8, 163. 10.1186/1471-2164-8-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertegel A. A.; Siegel R. W.; Dordick J. S. Silica Nanoparticle Size Influences the Structure and Enzymatic Activity of Adsorbed Lysozyme. Langmuir 2004, 20, 6800–6807. 10.1021/la0497200. [DOI] [PubMed] [Google Scholar]
- Cao L.; Chen W.-Q.; Zhou L.-J.; Wang Y.-Y.; Liu Y.; Jiang F.-L. Regulation of the Enzymatic Activities of Lysozyme by the Surface Ligands of Ultrasmall Gold Nanoclusters: The Role of Hydrophobic Interactions. Langmuir 2021, 37, 13787–13797. 10.1021/acs.langmuir.1c02719. [DOI] [PubMed] [Google Scholar]
- Golubeva O. Y.; Ulyanova N. Y.; Vladimirova E. V.; Shamova O. V. Comparison of the Antimicrobial and Hemolytic Activities of Various Forms of Silver (Ions, Nanoparticles, Bioconjugates) Stabilized in a Zeolite Matrix. Langmuir 2021, 37, 12356–12364. 10.1021/acs.langmuir.1c01899. [DOI] [PubMed] [Google Scholar]
- Wang M.; Zhu Y. Defect Induced Charge Redistribution and Enhanced Adsorption of Lysozyme on Hydroxyapatite for Efficient Antibacterial Activity. Langmuir 2021, 37, 10786–10796. 10.1021/acs.langmuir.1c01666. [DOI] [PubMed] [Google Scholar]
- Vranckx C.; Lambricht L.; Préat V.; Cornu O.; Dupont-Gillain C.; vander Straeten A. Layer-by-Layer Nanoarchitectonics Using Protein–Polyelectrolyte Complexes toward a Generalizable Tool for Protein Surface Immobilization. Langmuir 2022, 38, 5579–5589. 10.1021/acs.langmuir.2c00191. [DOI] [PubMed] [Google Scholar]
- Uddin M. J.; Liyanage S.; Warzywoda J.; Abidi N.; Gill H. S. Role of Sporopollenin Shell Interfacial Properties in Protein Adsorption. Langmuir 2022, 38, 2763–2776. 10.1021/acs.langmuir.1c02682. [DOI] [PubMed] [Google Scholar]
- Yoo S. I.; Yang M.; Brender J. R.; Subramanian V.; Sun K.; Joo N. E.; Jeong S.-H.; Ramamoorthy A.; Kotov N. A. Inhibition of Amyloid Peptide Fibrillation by Inorganic Nanoparticles: Functional Similarities with Proteins. Angew. Chem. Int. Ed. 2011, 50, 5110–5115. 10.1002/anie.201007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigolato F.; Arosio P. The Role of Surfaces on Amyloid Formation. Biophys. Chem. 2021, 270, 106533. 10.1016/j.bpc.2020.106533. [DOI] [PubMed] [Google Scholar]
- Prabhu M. P. T.; Sarkar N. Inhibitory Effects of Carbon Quantum Dots towards Hen Egg White Lysozyme Amyloidogenesis through Formation of a Stable Protein Complex. Biophys. Chem. 2022, 280, 106714. 10.1016/j.bpc.2021.106714. [DOI] [PubMed] [Google Scholar]
- Ravera E.; Martelli T.; Geiger Y.; Fragai M.; Goobes G.; Luchinat C. Biosilica and Bioinspired Silica Studied by Solid-State NMR. Coord. Chem. Rev. 2016, 327–328, 110–122. 10.1016/j.ccr.2016.06.003. [DOI] [Google Scholar]
- Wilson E. E.; Awonusi A.; Morris M. D.; Kohn D. H.; Tecklenburg M. M. J.; Beck L. W. Three Structural Roles for Water in Bone Observed by Solid-State NMR. Biophys. J. 2006, 90, 3722–3731. 10.1529/biophysj.105.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P.; Xu J.; Sahar N.; Morris M. D.; Kohn D. H.; Ramamoorthy A. Time-Resolved Dehydration-Induced Structural Changes in an Intact Bovine Cortical Bone Revealed by Solid-State NMR Spectroscopy. J. Am. Chem. Soc. 2009, 131, 17064–17065. 10.1021/ja9081028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh C.; Rai R. K.; Kayastha A. M.; Sinha N. Ultra Fast Magic Angle Spinning Solid—State NMR Spectroscopy of Intact Bone. Magn. Reson. Chem. 2016, 54, 132–135. 10.1002/mrc.4331. [DOI] [PubMed] [Google Scholar]
- Mroue K. H.; Viswan A.; Sinha N.; Ramamoorthy A.. Solid-State NMR Spectroscopy: The Magic Wand to View Bone at Nanoscopic Resolution. In Annual Reports on NMR Spectroscopy; Webb G. A., Ed.; Academic Press, 2017; Vol. 92, Chapter 6, pp 365–413. [Google Scholar]
- Nanda R.; Hazan S.; Sauer K.; Aladin V.; Keinan-Adamsky K.; Corzilius B.; Shahar R.; Zaslansky P.; Goobes G. Molecular Differences in Collagen Organization and in Organic-Inorganic Interfacial Structure of Bones with and without Osteocytes. Acta Biomater. 2022, 144, 195–209. 10.1016/j.actbio.2022.03.032. [DOI] [PubMed] [Google Scholar]
- Gavriel R.; Nadav-Tsubery M.; Glick Y.; Yarmolenko A.; Kofman R.; Keinan-Adamsky K.; Berman A.; Mass T.; Goobes G. The Coral Protein CARP3 Acts from a Disordered Mineral Surface Film to Divert Aragonite Crystallization in Favor of Mg-Calcite. Adv. Funct. Mater. 2018, 28, 1707321. 10.1002/adfm.201707321. [DOI] [Google Scholar]
- Du Y.-P.; Chang H.-H.; Yang S.-Y.; Huang S.-J.; Tsai Y.-J.; Huang J. J.-T.; Chan J. C. C. Study of Binding Interaction between Pif80 Protein Fragment and Aragonite. Sci. Rep. 2016, 6, 30883. 10.1038/srep30883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberga I.; Li R.; Duer M. J. Collagen Structure-Function Relationships from Solid-State NMR Spectroscopy. Acc. Chem. Res. 2018, 51, 1621–1629. 10.1021/acs.accounts.8b00092. [DOI] [PubMed] [Google Scholar]
- Mohanram H.; Georges T.; Pervushin K.; Azaïs T.; Miserez A. Self-Assembly of a Barnacle Cement Protein (MrCP20) into Adhesive Nanofibrils with Concomitant Regulation of CaCO3 Polymorphism. Chem. Mater. 2021, 33, 9715–9724. 10.1021/acs.chemmater.1c03477. [DOI] [Google Scholar]
- Brunner E.; Lutz K.. Solid-State NMR in Biomimetic Silica Formation and Silica Biomineralization. In Handbook of Biomineralization; Bäuerlein E., Ed.; Wiley-VCH Verlag GmbH, 2007; pp 19–38. [Google Scholar]
- Jantschke A.; Koers E.; Mance D.; Weingarth M.; Brunner E.; Baldus M. Insight into the Supramolecular Architecture of Intact Diatom Biosilica from DNP-Supported Solid-State NMR Spectroscopy. Angew. Chem., Int. Ed. 2015, 54, 15069–15073. 10.1002/anie.201507327. [DOI] [PubMed] [Google Scholar]
- Brückner S. I.; Donets S.; Dianat A.; Bobeth M.; Gutiérrez R.; Cuniberti G.; Brunner E. Probing Silica–Biomolecule Interactions by Solid-State NMR and Molecular Dynamics Simulations. Langmuir 2016, 32, 11698–11705. 10.1021/acs.langmuir.6b03311. [DOI] [PubMed] [Google Scholar]
- Poole P. L.; Finney J. L. Hydration-Induced Conformational and Flexibility Changes in Lysozyme at Low Water Content. Int. J. Biol. Macromol. 1983, 5, 308–310. 10.1016/0141-8130(83)90047-8. [DOI] [Google Scholar]
- Huang T. H.; Bachovchin W. W.; Griffin R. G.; Dobson C. M. High-Resolution Nitrogen-15 Nuclear Magnetic Resonance Studies of .Alpha.-Lytic Protease in Solid State. Direct Comparison of Enzyme Structure in Solution and Solid States. Biochemistry 1984, 23, 5933–5937. 10.1021/bi00320a007. [DOI] [PubMed] [Google Scholar]
- Harbison G. S.; Smith S. O.; Pardoen J. A.; Courtin J. M. L.; Lugtenburg J.; Herzfeld J.; Mathies R. A.; Griffin R. G. Solid-State Carbon-13 NMR Detection of a Perturbed 6-s-Trans Chromophore in Bacteriorhodopsin. Biochemistry 1985, 24, 6955–6962. 10.1021/bi00345a031. [DOI] [PubMed] [Google Scholar]
- Kennedy S. D.; Bryant R. G. Structural Effects of Hydration: Studies of Lysozyme by 13C Solids Nmr. Biopolymers 1990, 29, 1801–1806. 10.1002/bip.360291411. [DOI] [PubMed] [Google Scholar]
- Gregory R. B.; Gangoda M.; Gilpin R. K.; Su W. The Influence of Hydration on the Conformation of Bovine Serum Albumin Studied by Solid-State 13C-Nmr Spectroscopy. Biopolymers 1993, 33, 1871–1876. 10.1002/bip.360331212. [DOI] [PubMed] [Google Scholar]
- Auger M.; McDermott A. E.; Robinson V.; Castelhano A. L.; Billedeau R. J.; Pliura D. H.; Krantz A.; Griffin R. G. Solid-State Carbon-13 NMR Study of a Transglutaminase-Inhibitor Adduct. Biochemistry 1993, 32, 3930–3934. 10.1021/bi00066a012. [DOI] [PubMed] [Google Scholar]
- Jakeman D. L.; Mitchell D. J.; Shuttleworth W. A.; Evans J. N. S. Effects of Sample Preparation Conditions on Biomolecular Solid-State NMR Lineshapes. J. Biomol. NMR 1998, 12, 417–421. 10.1023/a:1008305118426. [DOI] [PubMed] [Google Scholar]
- Pauli J.; van Rossum B.; Förster H.; de Groot H. J. M.; Oschkinat H. Sample Optimization and Identification of Signal Patterns of Amino Acid Side Chains in 2D RFDR Spectra of the α-Spectrin SH3 Domain. J. Magn. Reson. 2000, 143, 411–416. 10.1006/jmre.2000.2029. [DOI] [PubMed] [Google Scholar]
- Martin R. W.; Zilm K. W. Preparation of Protein Nanocrystals and Their Characterization by Solid State NMR. J. Magn. Reson. 2003, 165, 162–174. 10.1016/s1090-7807(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Seidel K.; Etzkorn M.; Heise H.; Becker S.; Baldus M. High-Resolution Solid-State NMR Studies on Uniformly [13C,15N]-Labeled Ubiquitin. Chembiochem 2005, 6, 1638–1647. 10.1002/cbic.200500085. [DOI] [PubMed] [Google Scholar]
- Krushelnitsky A.; Gogolev Y.; Golbik R.; Dahlquist F.; Reichert D. Comparison of the Internal Dynamics of Globular Proteins in the Microcrystalline and Rehydrated Lyophilized States. Biochim. Biophys. Acta, Proteins Proteomics 2006, 1764, 1639–1645. 10.1016/j.bbapap.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Fragai M.; Luchinat C.; Parigi G.; Ravera E. Practical Considerations over Spectral Quality in Solid State NMR Spectroscopy of Soluble Proteins. J. Biomol. NMR 2013, 57, 155–166. 10.1007/s10858-013-9776-0. [DOI] [PubMed] [Google Scholar]
- Ravera E. The Bigger They Are, the Harder They Fall: A Topical Review on Sedimented Solutes for Solid-State NMR. Concepts Magn. Reson. 2014, 43, 209–227. 10.1002/cmr.a.21318. [DOI] [Google Scholar]
- Adiram-Filiba N.; Schremer A.; Ohaion E.; Nadav-Tsubery M.; Lublin-Tennenbaum T.; Keinan-Adamsky K.; Goobes G. Ubiquitin Immobilized on Mesoporous MCM41 Silica Surfaces - Analysis by Solid-State NMR with Biophysical and Surface Characterization. Biointerphases 2017, 12, 02D414. 10.1116/1.4983273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marulanda D.; Tasayco M. L.; McDermott A.; Cataldi M.; Arriaran V.; Polenova T. Magic Angle Spinning Solid-State NMR Spectroscopy for Structural Studies of Protein Interfaces. Resonance Assignments of Differentially Enriched Escherichia Coli Thioredoxin Reassembled by Fragment Complementation. J. Am. Chem. Soc. 2004, 126, 16608–16620. 10.1021/ja0464589. [DOI] [PubMed] [Google Scholar]
- Ravera E.; Michaelis V. K.; Ong T.-C.; Keeler E. G.; Martelli T.; Fragai M.; Griffin R. G.; Luchinat C. Biosilica-Entrapped Enzymes Studied by Using Dynamic Nuclear-Polarization-Enhanced High-Field NMR Spectroscopy. ChemPhysChem 2015, 16, 2751–2754. 10.1002/cphc.201500549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirau P. A.Interfacial Structure Determination. In Bio-Inspired Nanotechnology: From Surface Analysis to Applications; Knecht M. R., Walsh T. R., Eds.; Springer: New York, NY, 2014; pp 95–125. [Google Scholar]
- Ulrich E. L.; Akutsu H.; Doreleijers J. F.; Harano Y.; Ioannidis Y. E.; Lin J.; Livny M.; Mading S.; Maziuk D.; Miller Z.; Nakatani E.; Schulte C. F.; Tolmie D. E.; Kent Wenger R.; Yao H.; Markley J. L. BioMagResBank. Nucleic Acids Res. 2007, 36, D402–D408. 10.1093/nar/gkm957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adiram-Filiba N.; Ohaion E.; Verner G.; Schremer A.; Nadav-Tsubery M.; Lublin-Tennenbaum T.; Keinan-Adamsky K.; Lucci M.; Luchinat C.; Ravera E.; Goobes G. Structure and Dynamics Perturbations in Ubiquitin Adsorbed or Entrapped in Silica Materials Are Related to Disparate Surface Chemistries Resolved by Solid-State NMR Spectroscopy. Biomacromolecules 2021, 22, 3718–3730. 10.1021/acs.biomac.1c00495. [DOI] [PubMed] [Google Scholar]
- Leonardelli S.; Facchini L.; Fretigny C.; Tougne P.; Legrand A. P. Silicon-29 NMR Study of Silica. J. Am. Chem. Soc. 1992, 114, 6412–6418. 10.1021/ja00042a018. [DOI] [Google Scholar]
- Geiger Y.; Gottlieb H. E.; Akbey Ü.; Oschkinat H.; Goobes G. Studying the Conformation of a Silaffin-Derived Pentalysine Peptide Embedded in Bioinspired Silica Using Solution and Dynamic Nuclear Polarization Magic-Angle Spinning NMR. J. Am. Chem. Soc. 2016, 138, 5561–5567. 10.1021/jacs.5b07809. [DOI] [PubMed] [Google Scholar]
- Lutz W.; Täschner D.; Kurzhals R.; Heidemann D.; Hübert C. Characterization of Silica Gels by 29Si MAS NMR and IR Spectroscopic Measurements. Z. Anorg. Allg. Chem. 2009, 635, 2191–2196. 10.1002/zaac.200900237. [DOI] [Google Scholar]
- Martelli T.; Ravera E.; Louka A.; Cerofolini L.; Hafner M.; Fragai M.; Becker C. F. W.; Luchinat C. Atomic Level Quality Assessment of Enzymes Encapsulated in Bio-Inspired Silica. Chem. Eur J. 2016, 22, 425–432. 10.1002/chem.201503613. [DOI] [PubMed] [Google Scholar]
- Beaucage G. Approximations Leading to a Unified Exponential/Power-Law Approach to Small-Angle Scattering. J. Appl. Crystallogr. 1995, 28, 717–728. 10.1107/s0021889895005292. [DOI] [Google Scholar]
- Christiansen S. C.; Hedin N.; Epping J. D.; Janicke M. T.; del Amo Y.; Demarest M.; Brzezinski M.; Chmelka B. F. Sensitivity Considerations in Polarization Transfer and Filtering Using Dipole–Dipole Couplings: Implications for Biomineral Systems. Solid State Nucl. Magn. Reson. 2006, 29, 170–182. 10.1016/j.ssnmr.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Wisser D.; Brückner S. I.; Wisser F. M.; Althoff-Ospelt G.; Getzschmann J.; Kaskel S.; Brunner E. 1H–13C–29Si Triple Resonance and REDOR Solid-State NMR—A Tool to Study Interactions between Biosilica and Organic Molecules in Diatom Cell Walls. Solid State Nucl. Magn. Reson. 2015, 66–67, 36–39. 10.1016/j.ssnmr.2014.12.007. [DOI] [PubMed] [Google Scholar]
- Vinogradov E.; Madhu P. K.; Vega S. High-Resolution Proton Solid-State NMR Spectroscopy by Phase-Modulated Lee–Goldburg Experiment. Chem. Phys. Lett. 1999, 314, 443–450. 10.1016/s0009-2614(99)01174-4. [DOI] [Google Scholar]
- Vinogradov E.; Madhu P. K.; Vega S. Proton Spectroscopy in Solid State Nuclear Magnetic Resonance with Windowed Phase Modulated Lee–Goldburg Decoupling Sequences. Chem. Phys. Lett. 2002, 354, 193–202. 10.1016/s0009-2614(02)00060-x. [DOI] [Google Scholar]
- Leskes M.; Thakur R. S.; Madhu P. K.; Kurur N. D.; Vega S. Bimodal Floquet Description of Heteronuclear Dipolar Decoupling in Solid-State Nuclear Magnetic Resonance. J. Chem. Phys. 2007, 127, 024501. 10.1063/1.2746039. [DOI] [PubMed] [Google Scholar]
- Pines A.; Gibby M. G.; Waugh J. S. Proton Enhanced NMR of Dilute Spins in Solids. J. Chem. Phys. 1973, 59, 569–590. 10.1063/1.1680061. [DOI] [Google Scholar]
- Marks D.; Vega S. A Theory for Cross-Polarization NMR of Nonspinning and Spinning Samples. J. Magn. Reson., Ser. A 1996, 118, 157–172. 10.1006/jmra.1996.0024. [DOI] [Google Scholar]
- Bruno F.; Francischello R.; Bellomo G.; Gigli L.; Flori A.; Menichetti L.; Tenori L.; Luchinat C.; Ravera E. Multivariate Curve Resolution for 2D Solid-State NMR Spectra. Anal. Chem. 2020, 92, 4451–4458. 10.1021/acs.analchem.9b05420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastikhin V. M.; Mudrakovsky I. L.; Nosov A. V. 1H N M R Magic Angle Spinning (MAS) Studies of Heterogeneous Catalysis. Prog. Nucl. Magn. Reson. Spectrosc. 1991, 23, 259–299. 10.1016/0079-6565(91)80006-n. [DOI] [Google Scholar]
- Kim H. N.; Lee S. K. Atomic Structure and Dehydration Mechanism of Amorphous Silica: Insights from 29Si and 1H Solid-State MAS NMR Study of SiO2 Nanoparticles. Geochim. Cosmochim. Acta 2013, 120, 39–64. 10.1016/j.gca.2013.05.047. [DOI] [Google Scholar]
- Vega A. J.; Scherer G. W. Study of Structural Evolution of Silica Gel Using 1H and 29Si NMR. J. Non-Cryst. Solids 1989, 111, 153–166. 10.1016/0022-3093(89)90276-7. [DOI] [Google Scholar]
- Yan C.; Kayser F.; Dieden R. Sensitivity Enhancement via Multiple Contacts in the {1H–29Si}–1H Cross Polarization Experiment: A Case Study of Modified Silica Nanoparticle Surfaces. RSC Adv. 2020, 10, 23016–23023. 10.1039/d0ra04995f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody G. D.; Mysen B. O.; Lee S. K. Structure vs. Composition: A Solid-State 1H and 29Si NMR Study of Quenched Glasses along the Na2O-SiO2-H2O Join. Geochim. Cosmochim. Acta 2005, 69, 2373–2384. 10.1016/j.gca.2004.11.012. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.