Abstract
Phytate (myo-inositol hexakisphosphate salts) can constitute a large fraction of the organic P in soils. As a more recalcitrant form of soil organic P, up to 51 million metric tons of phytate accumulate in soils annually, corresponding to ∼65% of the P fertilizer application. However, the availability of phytate is limited due to its strong binding to soils via its highly-phosphorylated inositol structure, with sorption capacity being ∼4 times that of orthophosphate in soils. Phosphorus (P) is one of the most limiting macronutrients for agricultural productivity. Given that phosphate rock is a finite resource, coupled with the increasing difficulty in its extraction and geopolitical fragility in supply, it is anticipated that both economic and environmental costs of P fertilizer will greatly increase. Therefore, optimizing the use of soil phytate-P can potentially enhance the economic and environmental sustainability of agriculture production. To increase phytate-P availability in the rhizosphere, plants and microbes have developed strategies to improve phytate solubility and mineralization by secreting mobilizing agents including organic acids and hydrolyzing enzymes including various phytases. Though we have some understanding of phytate availability and phytase activity in soils, the limiting steps for phytate-P acquisition by plants proposed two decades ago remain elusive. Besides, the relative contribution of plant- and microbe-derived phytases, including those from mycorrhizas, in improving phytate-P utilization is poorly understood. Hence, it is important to understand the processes that influence phytate-P acquisition by plants, thereby developing effective molecular biotechnologies to enhance the dynamics of phytate in soil. However, from a practical view, phytate-P acquisition by plants competes with soil P fixation, so the ability of plants to access stable phytate must be evaluated from both a plant and soil perspective. Here, we summarize information on phytate availability in soils and phytate-P acquisition by plants. In addition, agronomic approaches and biotechnological strategies to improve soil phytate-P utilization by plants are discussed, and questions that need further investigation are raised. The information helps to better improve phytate-P utilization by plants, thereby reducing P resource inputs and pollution risks to the wider environment.
Keywords: organic P, phytate and phytase, availability, transgenic plants, organic acids, Pteris vittata
1. Introduction
Phosphorus (P) is an essential and nonrenewable resource critical for agricultural production. On one hand, worldwide P reserves are limited and becoming harder to extract;1 on the other hand, P is often fixed strongly in soils, thereby becoming unavailable to plants.2 Due to the limited availability of P in many soils, excess fertilizer is applied to ensure optimal plant growth and crop yield annually.3 The excess P, together with its inefficient use by plants, leads to large accumulation of unavailable P in soils.4 Organic P (Po) is the dominant P fraction in many soils, typically accounting for ∼50%, but can be up to 95% of total P in some agricultural soils.5 This is because inorganic P (Pi) in fertilized soils is often transformed to Po through microbial and plant activities. This is particularly significant in systems with large carbon reserves such as pastures, while it is less in some overfertilized soils where microbial immobilization capacity is saturated.6,7 As such, improving the acquisition of Po by crops has attracted much attention to enhance agricultural production.8 Still, to use the finite P resources efficiently, a better understanding of soil Po availability and factors constraining its plant acquisition is necessary, which helps to improve agricultural production and environment quality.9
Phytate
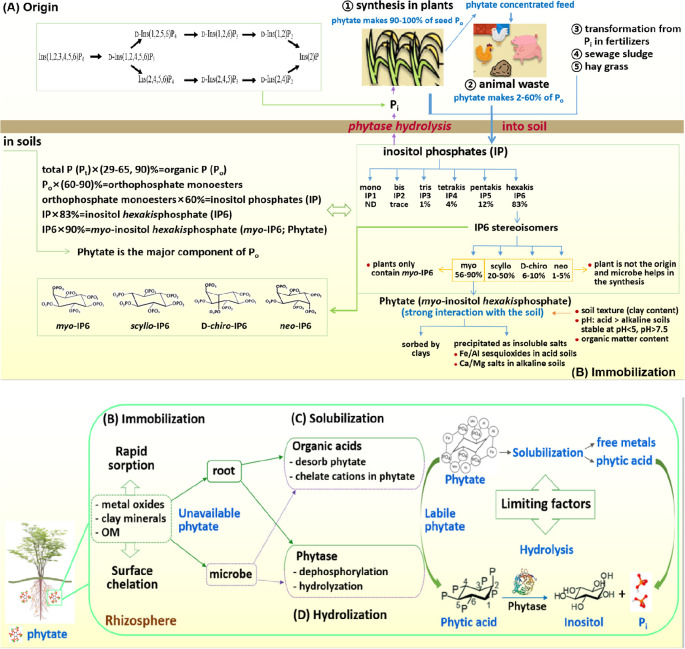
In agricultural soils, Po is mainly present as the highly-phosphorylated inositol phosphate (IP), which exists in six phosphorylation states with 1–6 phosphate groups (i.e., mono, bis, tris, tetrakis, pentakis, and hexakis; IP1–6) (Figure 1A).10 In soils, IP is generally found as hexakisphosphate (IP6; ∼83% of IP). As its P is only completely stripped during dephosphorylation, it is rare to find other phosphorylation states (IP1–5) in soils. The IP6 occurs in soils in four isomeric forms (i.e., myo, d-chiro, scyllo, and neo) but predominantly occurs as the myo isomer (∼56–90% of IP6) with small amounts of other stereoisomers (20–50% of scyllo, 6–10% of d-chiro, and 1–5% of neo) (Figure 1B).11,12
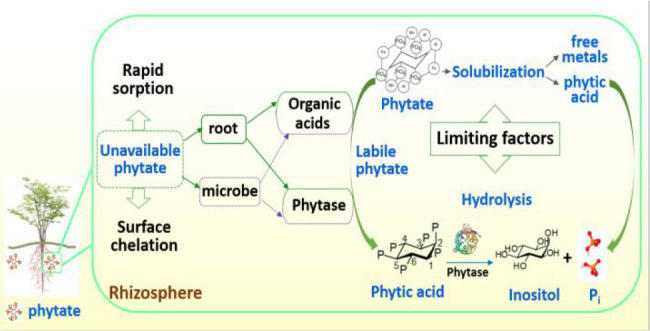
Figure 1.
Conceptual model of phytate cycle in the environment: (A) origin from plants and animal wastes, (B) immobilization by sorption or chelation, (C) mobilization by exuding organic acids, and (D) hydrolyzation by plant- and/or microbial phytase.
Phytate (myo-IP6), with six phosphate groups around its inositol ring, includes all metal derivatives of myo-inositol 1,2,3,4,5,6-hexakisphosphate.4 Phytate is synthesized by plants to serve as the primary storage form of phosphate (up to ∼90–100%) in plant seeds (Figure 1A). In soils, it can account for up to ∼50% of Po and ∼80% of IP (Figure 1B), thus being an important source for plant P nutrition.13
Due to its six orthophosphate moieties, phytate is highly reactive in soils, with a molecular weight of 660 g mol–1 and 12 hydrogen donors in its structure.4 With six phosphates on its inositol ring, phytate is not only bound to soils via sorption, surface complexation, and ternary phytate complexation (Figure 1B),14 but also becomes incorporated into organic matter (OM) structures via Fe/Al bridges.15 As such, large amounts of phytate can accumulate in soils and contribute to the soil Po pool,16,17 but with limited availability to plants.18
As the main P storage form in cereals and grains, phytate binds essential metal cations with low availability and is often introduced to soils via deposition of plant residues and manures from grain-fed animals, particularly monogastric animals, which lack phytase in their guts (Figure 1A). Phosphorus added to soils from the undigested phytate in animal manure poses potential pollution risks in areas of intensive animal production as it promotes eutrophication in aquatic systems, mostly surface waters like rivers, lakes, and oceans.19,20 Despite the prevalence of phytate in soils, the understanding of its solubility and availability is inconsistent due to difficulties in its extraction, separation, and detection.
Phytase
Although phytate is important in maintaining P supply to crops, the mechanisms associated with its solubilization in soils and acquisition by plants are poorly understood.21 As an essential macronutrient, P is taken up by plant roots as Pi.22,23 As Po, phytate must be hydrolyzed to release Pi into the soil solution before being taken up by plants.24 Plants can secrete different phosphatase enzymes that target different Po compounds, including phosphomonoesterase, phosphodiesterase, and phytase.25−27
Phytase (myo-inositol hexakisphosphate phosphohydrolase) is a class of phosphatase enzymes that specifically catalyze the hydrolysis of phytate to inositol, Pi, and free metals (Figure 1C,D).5 Phytate-P is released by phytase (EC 3.1.3.8, EC 3.1.3.72, and EC 3.1.3.26), often occurring at the 3, 5, and 6 phosphate positions. Phytase in the rhizosphere may originate from plant roots28,29 and/or soil microbes30 (Figure 1D). Their relative contributions to soil phytate hydrolysis are still unclear.31
To estimate phytate availability in soils, phytase-hydrolyzable P based on sequential extraction and enzyme hydrolysis has been used, which is the soluble Po that can be hydrolyzed by phytase to be used by plants.11,27 Soluble Po can be obtained using various extractants including H2O, NaHCO3, NaOAc, citrate, NaOH-EDTA, and HCl, which account for different processes and represent different solubilities.7,31,32 For example, water extracts estimate the Po that might be transferred in runoff,33 while NaHCO3 extracts estimate the Po that is readily mineralizable.31 Citrate extracts estimate the Po that is released by plant root exudates. This is because, among organic acids exuded by plants, citrate is the most abundant. Further, citrate shows greater extraction efficiency for Po than bicarbonate such as NaHCO3 (44–79% vs 1–9% of the Po).27,34,35 While NaOH-EDTA targets all phytate in soil as it can extract 71–90% of total soil P including phytate,36 HCl extracts recover minimal phytase-labile Po from soil.31
Crops that can utilize Po in soils require less external P inputs, thereby reducing nutrient loss and consumption of nonrenewable mineral P.37,38 Phytate can be hydrolyzed by phytase to enhance plant uptake, which is limited by the poor solubility of phytate and low activity of phytase in soils.39 Therefore, enhancing phytate solubility and phytase activity is critical to improve phytate availability for sustainable use of P in soils.40 Phytate can also become soluble after dissolution of OM that binds phytate.24
As such, this review aims to provide an overview of phytate availability in soils, especially the processes to improve phytate-P acquisition by plants. Understanding the mechanisms controlling phytate availability in soils helps to select plants/microbes that can exude organic acids/enzymes to enhance phytate-P utilization by crop plants.
2. Phytate in Soils
Phytate is stable in soils primarily due to its strong complexation by various metals and its strong binding to various components of soils.6,17 This section covers its origin, abundance, forms, solubilization, and availability in soils.
2.1. Origin, Abundance, and Forms
Origin
Phosphorus accumulation as phytate in soils can reach up to ∼51 million metric tons annually, corresponding to ∼65% of the P fertilizer.41 Soil phytate may come from plant tissues, monogastric animal manures, and microbial conversion from soil Pi. Phytate is synthesized by plants and microbes, with plants being the main source.11,42 In particular, plants accumulate large amounts of phytate in the grains and seeds, being up to 80% of total P and 90–100% of Po as a P reserve for seed germination (Figure 1A).43 Phytate also occurs in other tissues but in smaller concentrations, which participates in molecular signaling and biochemical reactions.44,45 In short, plant is an important source of soil phytate.3
Monogastric animals including poultry and swine cannot effectively utilize phytate-P in grain feed, and even ruminant animals like cattle and sheep are unable to mineralize all phytate-P, especially in high-phytate grain-based diets.44 Phytate accumulation in animal manures is attributed to several factors, including its high concentration in grain-based diets, complexation with metals, and rapid passage through the digestive tract.44 Therefore, phytate-rich animal manure is also an important source of phytate inputs to agricultural soils (Figure 1A).46,47
In addition, phytate can be transformed from immobilized Pi in soils (Figure 1A). George et al.7 showed that P fertilization increased phytase-hydrolyzable P, attributing to the continuous accumulation of soil P. Even in soils after more than a decade without P fertilization, phytase-hydrolyzable P is still significantly greater than unfertilized soils.7 The data indicate that phytate accumulation is associated not only with external inputs of phytate-rich substrates but also with soil immobilization and transformation from P fertilizers.4
Abundance
In soils, Po is abundant and typically accounts for 40–95% of total P, with phytate being the major fraction, accounting for up to 50–80% of the Po (Figure 1B).5,18 Phytate concentrations in soil depend on land use and soil properties and vary with extraction methods. For example, phytate-P concentrations range from 1.4–220 mg kg–1 in arable soils to 42–220 mg kg–1 in crop and pasture soils and to 153–1325 mg kg–1 in manures (Tables 1A, 1B, and S1A). The average phytate-P concentrations are 457, 1047, and 2277 mg kg–1 in swine, cattle, and poultry manure, accounting for ∼16–17% of total P (Table S1B). The phytase-hydrolyzable P concentrations range from 0.1–0.4 mg kg–1 in water extracts to 26–189 and 153–613 mg kg–1 in NaOH-EDTA extracts of pasture soils and cattle dung in Southern Chile (Table 1A), averaging ∼20% of total P or ∼40% of Po.48−50
Table 1A. Classification and Amount of P and Phytate in Soils: Total P (Pt), Organic P (Po), and Phytase-Hydrolyzable P (PPhy) Concentrations (mg kg–1) and Proportion (%) of the Po to Pt and PPhy to Pt or Po in Different Soils by Different Extraction and Analytical Methodsa.
| location | soil description | phytase-hydrolyzable P | % total P | % organic P | extraction and analytical method | ref | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| England and Wales | temperate lowland permanent pasture with high clay (22–68%) | 26–189 | ND–26 | NaOH-EDTA extraction, solution 31P NMR spectroscopy | Turner et al.161 | |||||
| Madagascar | rice humid tropical oxisols | ND–33 | 11–35, 22 | Turner162 | ||||||
| Southern Chile | dairy cattle dung | 153–613 | 9–14 | 44–73% | Fuentes163 | |||||
| feces | 1325 | Toor et al.164 | ||||||||
| Australia | pasture soils (n = 5) | 0.1–0.4 | 10.8–33.5 | H2O extraction, enzymatic hydrolysis | Turner et al.11 |
| location | soil description | dune age (years B.P.) | Pt | Po | total IP6 | % of Po | myo-IP6 | % of IP6 | extraction and analytical method | ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Haast, New Zealand | mineral soils along coastal dunes under lowland temperate rain forest | 290 | 178 | 128 | 56.7 | 44.4 | 34.9 | 61.6 | NaOH-EDTA extraction, solution 31P NMR spectroscopy, and spectral deconvolution | Turner165 |
| 392 | 196 | 140 | 65.8 | 46.9 | 42.3 | 64.3 | ||||
| 517 | 178 | 122 | 48.6 | 40.0 | 26.3 | 54.1 | ||||
| 787 | 210 | 139 | 49.9 | 36.0 | 24.9 | 49.9 | ||||
| 1826 | 126 | 99.0 | 51.1 | 51.6 | 27.2 | 53.3 | ||||
| 3384 | 115 | 86.4 | 40.0 | 46.3 | 19.9 | 49.7 | ||||
| 3903 | 85 | 66.9 | 29.7 | 44.4 | 17.0 | 57.1 | ||||
| 4422 | 97 | 70.0 | 36.2 | 51.7 | 19.5 | 54.0 | ||||
| 6500 | 82 | 63.2 | 26.4 | 41.8 | 12.7 | 48.0 |
| location | soil description | Pt | Po (%Pt) | Pphy | %Pt | %Po | extraction and analytical method | ref | ||
|---|---|---|---|---|---|---|---|---|---|---|
| western USA | semiarid arable soils (n = 11) | 220–1210 | 1.7–22.8 | 1.4–8.4 | 37–87 | NaHCO3 extraction, enzymatic hydrolysis, solution 31P NMR spectroscopy | Turner et al.166 | |||
| England and Wales | lowland permanent pasture soils (n = 29) | 376–1981 | 208–895 | 26–189 | 11–35 | NaOH-EDTA extraction, solution 31P NMR spectroscopy | Turner et al.161 | |||
| Gooding County in southern Idaho | loamy fine sand + solid manure | 440–970 | 71–170 (16–17) | 10.2–16.2 | 4.8–16.2 | Hansen et al.167 | ||||
| loamy fine sand + lagoon manure | 750–1000 | 55–130 (7.3–13) | 4.8–15 | |||||||
| Newport, ME (NS) | uncultivated soil (coarse-loamy, mixed, frigid, typic Haplorthod; 42% sand, 52% silt, and 6% clay) | 12 | 2.2 (H2O), 73 (NaHCO3), 239 (NaOH) | 0.7 (H2O), 64.2 (NaHCO3), 44.7 (NaOH) | 20–71 | sequential H2O, NaHCO3, and NaOH extraction, enzymatic hydrolysis | He et al.159 | |||
| Presque Isle, Maine | conventional cultivation practice (caribou sandy loam: fine-loamy, isotic, frigid Typic Haplorthods; 51% sand, 41% silt, and 8% clay) | 32.5 | 4.3 (H2O), 90 (NaHCO3), 249 (NaOH) | 1.9 (H2O), 83.3 (NaHCO3), 54.0 (NaOH) | 28–79 | |||||
| conventional cultivation practice with 10 yr swine manure application | 37 | 6.8 (H2O), 101 (NaHCO3), 330 (NaOH) | 1.0 (H2O), 42.3 (NaHCO3), 68.4 (NaOH) | 17–49 | ||||||
| New Zealand | pasture soils (n = 24) | 116–2746 | 46–991 (24–60) | 13–220 | 14–91 | NaOH-EDTA extraction, solution 31P NMR | McDowell et al.168 | |||
| Madagascar | rice Oxisols (n = 13) | 130–1380 | 22–393 (19–44) | trace–33.1 | 12.2–26 | Turner162 |
| location | soil description | Pt | Po (%Pt) | Pphy | %Pt | %Po | extraction and analytical method | ref | ||
|---|---|---|---|---|---|---|---|---|---|---|
| northern Alabama | fine loam (n = 1) | 9.4 (H2O), 9.1 (NaHCO3), 67.1 (NaOH), 2.1 (HCl) | 100 (H2O), 25.2 (NaHCO3), 45.4 (NaOH), 60 (HCl) | 9.7–16.4 (NaOH) | NaOH extraction, with or without enzymatic hydrolysis, solution 31P NMR | He et al.169 | ||||
| fine loam + poultry litter for 20 yr at rates of 1.36 mg ha–1 yr–1 (n = 1) | 32.2 (H2O), 82.2 (NaHCO3), 230 (NaOH), 34.6 (HCl) | 12.3 (H2O), 0 (NaHCO3), 37.6 (NaOH), 0 (HCl) | 0.9–5.2 (NaOH) | |||||||
| Delmarva Peninsula | poultry litter (PL) | 9988–12436 | 6103–7700 (61–62) | 5135–5968 | 48–51 | 77.5–84.1 | NaOH-EDTA extraction, solution 31P NMR | Hill and Cade-Menun170 | ||
| composted litter (CL): poultry litter and cow manure | 11372–16256 | 3550–11090 (31–68) | 3413–8412 | 21–52 | 75.9–96.1 | |||||
| crop soil (CS): soy or corn | 326–827 | 95–141 (17–29) | 42–53 | 6–13 | 37.6–44.2 | |||||
| ditch sediment (DS) | 49–687 | 14.2–86.7 (10–29) | 3–21 | 2–6 | 21.1–24.2 | |||||
| Pennsylvania | fine loam (n = 10) | 604–858 | 178–378 (28.2–60.9) | 31–111 | 4.93–16.9 | 17.4–40.1 | Dou et al.171 | |||
| fine loam + animal manure or spent mushroom compost (n = 10) | 808–4866 | 114–244 (6.91–19.4) | 53–106 | 1.57–6.76 | 22.6–43.4 | |||||
| Irish | nonbasaltic grassland soils (n = 4) | 616–2580 | 188–592 | 97–185 | 20–52 | Murphy et al.172 |
Numbers with underlines are the mean values.
Table 1B. Classification and Amount of P and Phytate in Soils: In the Whole Soil, NaOH-EDTA Extracts, and Bicarbonate Extracts of the 18 Western USA Soils and Phytase-hydrolyzable P (PPhy) Concentrations in Bicarbonate Extracts of 11 Western USA Soilsd.
| whole
soila |
NaOH-EDTA extractable Pb |
bicarbonate extractable Pb |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| soil origin | location and soil description | P | Po (%) | P (%) | Po (%) | P (%) | Po (%) | PPhy (%) | phytase used to determine phytate-P | ref |
| Western USA semiarid arable soils | Taunton | 568 | 18 (3) | 103 (18) | 21 (118) | 14.0 (2.5) | 1.7 (9.8) | 1.4 ± 0.32 (81) | phytase: myo-inositol hexakisphosphate 3-phosphohydrolase | Turner et al.166 |
| Warden | 1210 | 67 (6) | 175 (14) | 63 (94) | 19.3 (1.6) | 3.7 (5.6) | 1.9 ± 0.42 (50) | |||
| Amarillo | 251 | 68 (27) | 112 (45) | 42 (62) | 33.7 (13.4) | 4.1 (6.0) | 2.7 ± 0.26 (66) | |||
| Greenleaf | 1058 | 88 (8) | 205 (19) | 63 (72) | 17.7 (1.7) | 4.3 (4.9) | 2.3 ± 0.37 (53) | |||
| Portneuf (manured subsoil) | 1135 | 172 (15) | 255 (22) | 55 (32) | 110.5 (9.7) | 4.7 (2.8) | 3.8 ± 2.57 (81) | |||
| source: Aspergillus ficuum | ||||||||||
| Portneuf (manured) | 1070 | 158 (15) | 286 (27) | 92 (58) | 57.7 (5.4) | 3.3 (2.1) | 2.9 ± 0.28 (87) | |||
| Millville | 762 | 189 (25) | 224 (29) | 89 (47) | 11.9 (1.6) | 5.4 (2.9) | 2.6 ± 0.26 (48) | specified activity: 3.5 U mg–1 solid | ||
| Brinegar | 626 | 130 (21) | 214 (34) | 91 (71) | 40.2 (6.4) | 15.0 (11.6) | 6.5 ± 0.96 (44) | buffer: 2 M glycine-HCl, pH 2.5 | ||
| Palouse | 1000 | 189 (19) | 284 (28) | 144(76) | 53.3 (5.3) | 22.8 (12.0) | 8.4 ± 0.39 (37) | |||
| Labenzo | 1000 | 280 (28) | 323 (32) | 178 (63) | 38.4 (3.8) | 11.0 (3.9) | 6.9 ± 0.83 (62) | |||
| Wahpeton | 657 | 235 (36) | 272 (41) | 165 (70) | 31.9 (4.9) | 9.8 (4.2) | 4.6 ± 0.86 (47) | |||
| Olton | 220 | 44 (20) | 73 (33) | 29 (66) | 19.1 (8.7) | 1.8 (4.1) | ||||
| Declo | 827 | 119 (14) | 221 (27) | 90 (75) | 21.7 (2.6) | 2.5 (2.1) | ||||
| Portneuf (convc subsoil) | 970 | 147 (15) | 116 (12) | 34 (23) | 32.3 (3.3) | 2.2 (1.5) | ||||
| Williams | 439 | 119 (27) | 128 (29) | 74 (62) | 19.4 (4.4) | 4.3 (3.6) | ||||
| Portneuf (conv) | 966 | 193 (20) | 217 (22) | 79 (41) | 22.2 (2.3) | 3.7 (1.9) | ||||
| Roza | 729 | 91 (12) | 154 (21) | 61 (67) | 27.6 (3.8) | 5.3 (5.8) | ||||
| Portneuf (native) | 890 | 189 (21) | 192 (22) | 68 (36) | 39.2 (4.4) | 7.8 (4.1) | ||||
Values in parentheses are % of soil total Po to total P (Pt).
Values in parentheses are % of respective extractable P fraction to its concentration in whole soil.
conv – conventionally managed soils, Pt – total P, Po – organic P, PPhy – phytase-hydrolyzable P or phytate-P.
Values are means ± standard deviation of triplicate extracts. Values in parentheses are the proportion (%) of the Pt or Po.
The contribution of phytate to Po also varies greatly among soils. For example, phytate-P concentrations in 47 Australian soils are 1–356 mg kg–1, accounting for 0.4–38% of Po. For Scottish soils and Chilean Andisols, they are 56–460 and ∼674 mg kg–1, accounting for 24–58% and 42–67% of Po (Table 1C).51 In addition, phytate-rich animal manures (3413–8412 mg kg–1) have often been used as fertilizers, thereby increasing soil phytate content. For example, after 10 years of applying swine manure to soils with conventional cultivation in Maine, soil phytate-P reached 118 mg kg–1 (Table 1A).52 After 7 year of surface applications of 30 kg ha–1 dairy manure in Christiana soils with a permanent grass stand, soil plant-available P via Mehlich-3 is elevated by 78 mg kg–1, with phytase-hydrolyzable P making up 48–55% of the extractable P.53
Table 1C. Classification and Amount of P and Phytate in Soils: Pt, Po, Inositol-P (INP), Humic-P (HA-P), Fulvic-P (FA-P), and Specific P Fraction/Po Ratios in 15 Cultivated and Uncultivated (Native Grasslands) Chilean Volcanic Soils and 9 Representative Volcanic Soils under Grasslandsa.
| soil type | soil description and no. | Pt | Po (% Pt) | INP (% Po) | HA-P (% Po) | FA-P (% Po) | extraction method | ref |
|---|---|---|---|---|---|---|---|---|
| Chilean volcanic soils | cultivated (+P) | 1422–4011, 2582 | 870–3197 (42–80), 1618 (56) | (59–95, 61) | (5–41, 39) | hypobromide oxidation (Anderson, 1964) | Borie and Rubio51 | |
| uncultivated (-P, native grasslands) | 1150–3243, 1854 | 650–2375 (48–79), 1147 (62) | (43–81, 53) | (19–57, 47) | ||||
| Chilean volcanic soils under grasslands | Typic Distrandept 1 | 2348 | 1007 (43) | 499 (49) | 637 (63) | 370 (37) | ||
| 2 | 1925 | 1052 (55) | 705 (67) | 638 (61) | 414 (c) | |||
| 3 | 2697 | 1302 (49) | 612 (47) | 867 (66) | 435 (33) | |||
| 4 | 2327 | 1492 (64) | 987 (66) | 1041 (68) | 478 (32) | |||
| 5 | 2476 | 1450 (59) | 612 (42) | 965 (66) | 485 (33) | |||
| 6 | 3121 | 1310 (42) | 750 (57) | 841 (64) | 469 (36) | |||
| 7 | 2362 | 1208 (51) | 778 (64) | 793 (65) | 415 (34) | |||
| mean | 1925–3121, 2465 | 1007–1492 (42–64) | 499–987 (42–67) | 793–1041 (61–68) | 370–485 (32–49) | |||
| Typic Vitrandept 8 | 1849 | 1083 (59) | 709 (65) | 721 (66) | 362 (33) | |||
| 9 | 1107 | 654 (59) | 415 (63) | 333 (51) | 321 (49) |
Values in parentheses are % of Pt or Po; numbers with underlines are the mean values.
Forms
Of the six inositol phosphate esters (IP), i.e., mono-, bis-, tris-, tetrakis-, pentakis-, and hexakis-phosphates (IP1–6), IP6 is the predominant form, accounting for up to 83–100% of IP (Figure 1B).17 There are also four stereoisomeric forms of IP6, with the abundance being in the order of myo > scyllo > d-chiro > neo, representing 56–90, 20–50, 6–10, and 1–5% of IP6 (Figure 1B).11,12 Synthesized in plants, myo-IP6 or phytate is the principal form and the most common IP in soils, with lower order esters being rare.17 Since plants contain only the myo stereoisomer of IP6, with chemical epimerization of myo-IP6 being ruled out, microbes play a key role in synthesizing other IP6 stereoisomers in soils (Figure 1B).11
Due to its higher degree of phosphorylation with six phosphate groups on its inositol ring, phytate has a high charge density, thereby interacting strongly with soils.5 Phytate is bound to Fe/Al-oxides in acid soils and Ca/Mg minerals in alkaline soils.15,19 For example, phytate sorption onto goethite and ferrihydrite is greater than that of Pi (3.8–12.7 vs 2.4–4.6 μmol m2), and its binding to amorphous Al-oxide induces formation of stable Al-phytate precipitates (log K13–16 = 8.84–20.1; Table 2B).14,54 Besides minerals, phytate also binds strongly to OM.55
Table 2B. Stability Constants of Phytate-Metal Complexesb.
| cation | ionic strength(mol L–1) | medium | t (°C) | log K13 | log K14 | log K15 | log K16 | other i:j speciesa |
|---|---|---|---|---|---|---|---|---|
| Mg2+ | 0 | NaClO4 | 10 | 7.93 | 6.49 | 5.47 | 2:3, 2:4, 2:5, 3:2, 3:3, 3:4, 3:5 | |
| 0 | 25 | 7.82 | 6.66 | 6.03 | ||||
| 0.15 | 37 | 10.5 | 9.76 | 8.76 | 7.25 | 1:2 | ||
| Ca2+ | 0 | 10 | 7.67 | 6.34 | 5.31 | 2:3, 2:4, 2:5, 3:2, 3:3, 3:4, 3:5 | ||
| 0 | 25 | 7.64 | 5.82 | 5.41 | ||||
| 0.15 | NaClO4 | 37 | 8.3 | 8.4 | 7.4 | |||
| Cd2+ | 0.15 | NaClO4 | 37 | 9.7 | 8.76 | 7.53 | 6.92 | 1:2 |
| 0.15 | NaCl | 25 | 5.25 | 4.71 | 4.42 | 1:7, 2:4, 2:5, 2:6, 2:7, 3:4 | ||
| Cu2+ | 0 | 25 | 10.3 | 7.79 | 2:5 | |||
| 0.15 | NaClO4 | 37 | 13.5 | 12.2 | 9.07 | 5.73 | ||
| Zn2+ | 0.15 | 37 | 11.3 | 10.3 | 8.54 | 6.94 | ||
| Ni2+ | 0.15 | 37 | 8.78 | 8.44 | 7.20 | |||
| 0.10 | KCl | 36 | 7.27 | 5.96 | 5.18 | 5.05 | 1:0, 1:1, 1:2, 1:7 | |
| Co2+ | 0.15 | NaClO4 | 37 | 9.1 | 7.9 | 6.96 | 6.26 | 1:2, 1:7 |
| Hg2+ | 0.15 | NaCl | 25 | 15.6 | 15.9 | 16.3 | 16.5 | 1:0, 1:1, 1:2, 1:7, 2:0, 2:1, 2:2 |
| 0 | 25 | 14.7 | 15.1 | 15.5 | 15.7 | |||
| Mn2+ | 0.15 | NaClO4 | 37 | 8.78 | 8.44 | 7.2 | ||
| Fe2+ | 0.15 | 37 | 10.5 | 8.99 | 7.71 | 5.94 | 1:2 | |
| Fe3+ | 0.15 | 37 | 18.2 | 12.7 | 8.89 | 1:2 | ||
| Al3+ | 0.15 | 37 | 20.1 | 16.4 | 12.2 | 8.48 | 1:2 | |
| (CH3)2Sn2+ | 0 | 25 | 14.0 | 11.6 | 9.16 | 6.59 | 1:0, 1:1, 1:2, 1:7, 2:0–2:5, 3:0–3:5 | |
| (CH3)3Sn+ | 0.05 | 25 | 2.45 | 2:5, 3:4, 3:5, 4:6, 5:1 | ||||
| 0.075 | 25 | 3.25 |
Kij refers to the equilibrium: iMn+ + HjPhy(12–j)– = MiHjPhy(12–in–j)–.
Adapted from Crea et al.79
2.2. Sorption, Complexation, and Stability
Although phytate may be present in the soil solution, its direct uptake by plants has not been demonstrated.18,45 Thus, to contribute to plant P nutrition, soil phytate must first be dephosphorylated from phosphate ester (C–O–P), phosphoanhydride (P–O–P), or phosphonate (C–P) via phytase-mediated hydrolysis.54 However, it can only occur in the soil solution. Thus, its desorption from the solid phase is a prerequisite for its enzymatic hydrolysis by phytase. This section addresses its sorption, complexation, and stability in soils.
Sorption
Among Po compounds, phytate has the strongest affinity for soils, whose immobilization and fixation are stronger than IP1–5 and Pi.56 Its immobilization involves rapid sorption via surface complexation, which includes formation of phytate complexes with soil minerals14 and incorporation of phytate into OM structures via Fe/Al bridges.15
Since phytate has 12 ionizable protons, with pKa values being 1.1–12 for pK1–pK12 (Table 2A), phytate is a strong ligand due to its high anionic charge at −6 to −10 under pH 4–10.11 The six orthophosphate moieties and 12 replaceable protons in the phytate structure render its polyanionic property and strong ability to sorb onto soil solid phases and chelate with metal cations.19 Phytate sorbs to metal oxides, clay minerals, and OM, with sorption capacity being ∼4 times that of Pi.54 Depending on pH, phytate chelates metal cations to form sparingly-soluble precipitates, with Fe/Al complexes under acidic conditions and Ca/Mg complexes under alkaline conditions (Table 2B).57
Table 2A. Protonation Constants of Phytate in Different Media and Ionic Strengthsb.
| medium | ionic strength(mol L–1) | log K1a | log K2a | log K3 | log K4 | log K5 | log K6 | log K7 | log K8 | log K9 | log K10 | log K11 | log K12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (C2H5)4NI | 0.1 | 16.7 | 14.4 | 12.2 | 9.92 | 7.53 | 6.11 | 3.53 | |||||
| (n-C4H9)4NBr | >12 | >12 | >12 | 11.5 | 7.97 | 6.41 | 3.93 | 2.73 | 2 | <1.5 | <1.5 | <1.5 | |
| LiCl | 9.71 | 9.46 | 8.63 | 7.6 | 6.27 | 5 | 2.63 | ||||||
| NaNO3 | 9.48 | 9.98 | 9.53 | 8.2 | 6.49 | 5.17 | 3.02 | ||||||
| NaCl | 9.58 | 9.84 | 9.5 | 8.14 | 6.5 | 5.25 | 2.88 | ||||||
| KCl | 10.2 | 9.5 | 9.93 | 8.37 | 6.62 | 5.35 | 2.93 | ||||||
| CsCl | 10.4 | 10.3 | 10.1 | 8.62 | 6.53 | 5.16 | 3.18 | ||||||
| (CH3)4NCl | 0.15 | 10.8 | 10.5 | 10.3 | 8.79 | 6.9 | 5.72 | 3.1 | 1.9 | 1.9 | |||
| NaClO4 | 0.15 | 8.59 | 10.5 | 9.02 | 7.82 | 6.13 | 4.88 | 2.49 | 1.98 | ||||
| (C2H5)4NClO4 | 0.17 | >13 | >13 | 12.3 | 9.92 | 7.42 | 6.13 | 3.59 | 2 | 2.4 | 1 | <1 | <1 |
| KCl | 0.2 | 9.53 | 9.53 | 9.19 | 7.98 | 6.25 | 5.2 | 3.16 | 2.38 | 2.38 | 1.92 | 1.92 | 1.92 |
| (C2H5)4NI | 0.5 | 14.9 | 13.3 | 11.6 | 9.79 | 7.5 | 6.12 | 3.61 | |||||
| LiCl | 9.06 | 8.81 | 7.96 | 6.93 | 5.63 | 4.39 | 2.08 | ||||||
| NaNO3 | 8.73 | 9.39 | 8.82 | 7.57 | 5.88 | 4.59 | 2.6 | ||||||
| NaCl | 8.93 | 9.19 | 8.83 | 7.48 | 5.88 | 4.65 | 2.37 | ||||||
| KCl | 9.59 | 8.85 | 9.26 | 7.71 | 6.01 | 4.77 | 2.43 | ||||||
| CsCl | 9.79 | 9.54 | 9.51 | 7.93 | 5.78 | 4.51 | 2.49 | ||||||
| (C2H5)4NI | 1 | 13.6 | 12.5 | 11.1 | 9.71 | 7.5 | 6.16 | 3.72 | |||||
| LiCl | 8.83 | 8.57 | 7.69 | 6.67 | 5.4 | 4.15 | 1.92 | ||||||
| NaNO3 | 8.36 | 9.22 | 8.51 | 7.34 | 5.66 | 4.39 | 2.52 | ||||||
| NaCl | 8.69 | 8.95 | 8.56 | 7.21 | 5.65 | 4.42 | 2.22 | ||||||
| NaClO4 | 8.41 | 9.19 | 8.29 | 7.03 | 5.38 | 4.14 | 1.77 | 1.8 | |||||
| KCl | 9.35 | 8.61 | 8.99 | 7.45 | 5.77 | 4.54 | 2.28 | ||||||
| CsCl | 9.82 | 9.38 | 9.41 | 7.77 | 5.57 | 4.34 | 2.33 | ||||||
| LiCl | 3 | 8.6 | 8.34 | 7.34 | 6.35 | 5.18 | 3.95 | 2 | |||||
| NaCl | 8.47 | 8.71 | 8.21 | 6.89 | 5.43 | 4.22 | 2.3 | ||||||
| NaClO4 | 8.29 | 8.62 | 8.01 | 6.61 | 5.07 | 3.86 | 1.52 | 1.63 | |||||
| KCl | 9.13 | 8.38 | 8.64 | 7.13 | 5.56 | 4.34 | 2.36 | ||||||
| NaCl | 5 | 8.5 | 8.74 | 8.12 | 6.83 | 5.47 | 4.27 | 2.63 |
Predicated values in italics.
Adapted from Crea et al.79
Phytate sorption occurs through its phosphate groups, which react with metal oxides via ligand exchange through surface H2O and OH groups, forming inner-sphere complexes.58 Strong sorption of phytate has been demonstrated with calcite,59 Illite, kaolinite, and montmorillonite,60 goethite,61,62 hematite,63 ferrihydrite,64 aluminum hydroxides,65 and gibbsite,66 especially at low pHs, as phytate sorption decreased with increasing pH. For example, phytate sorption on goethite and hematite decreased from 94% to 47% or from 0.95 to 0.38 μmol m–2 with pH increasing from 3 to 10.62,63 Similarly, phytate sorption on ferrihydrite decreased by 25–61% with pH increasing from 5 to 9, with P1,3 and P2 phosphate functional groups showing preferential affinities at pH 5 and 8.5.64 Moreover, the mechanism for phytate sorption is via formation of amorphous Fe-phytate precipitates on ferrihydrite surfaces.67 However, phytate sorption onto gibbsite increases (0.47–0.52 μmol m–2) with increasing temperature (4–55 °C) at pH 6, while it decreases (0.41–0.33 μmol m–2) at pH 10 as the temperature is raised.66 Phytate sorption onto soil minerals increases its negative charge, making it more reactive.68
Complexation
Complexation with metal cations occurs by ligand exchange and/or surface complexation, by which OH2 or OH groups are replaced by the PO4 anion.15 Complexation can occur via one phosphate group, between two phosphate groups of a molecule, or between phosphate groups of different phytate molecules.19 Phytate complexation with Fe3+ is stronger than Ca2+, so Fe-phytate is more stable than Ca-phytate, with their stability constants (log K13–15) at 8.89–18.2 and 8.3–8.4 (Table 2B). As such, Ca-phytate can be transformed to Fe-phytate in soils over time.69 Besides, phytate incorporation increases the stability of Fe oxyhydroxide via inhibiting its transformation. For example, 10 months of aging at 22 °C or 60 h of hydrothermal treatment at 70 °C fails to transform the phytate-coprecipitated ferrihydrite (∼60% is Fe-phytate) into hematite or goethite.70 The data indicate that the strong complexation of phytate suppresses Fe polymerization and crystallization.70 In the presence of Ca, phytate can form soluble complexes (Ca1- or Ca2-phytate) or insoluble precipitates (Ca3-phytate) at all pH values.54 Higher reactivity of phytate than Pi and other Po compounds suggests that phytate undergoes strong immobilization, limiting it from being hydrolyzed by phytase, resulting in its low availability and high accumulation in soils.71
Besides soil minerals and metal cations, phytate also binds to OM via Fe/Al-bridges. Coupled with Fe/Al, its sorption capacity exceeds 1.3 mM phytate-P mM–1 Fe/Al.72 However, without Fe/Al, OM shows limited binding capacity for phytate, similar to Pi.73 The data indicate that Fe/Al helps OM to sorb phytate (Figure 1B). Further, extraction with 1 M NaOH fails to liberate phytate from OM,74 as it takes hydrolysis with 6 M HCl at 100 °C to release phytate from OM.75 The data indicate incorporation of phytate into the Fe/Al-OM complex. As such, phytate bound to the Fe/Al-OM complex behaves differently from those bound to OM or Fe/Al-oxides.5
Stability
Phytate stability in soils is controlled by many factors including OM, clay type, clay content, pH, and metal oxides.4,61 For example, peat soils contain greater amounts of phytate than sandy soils due to their greater OM content.76 Clay type affects phytate sorption strength as phytate is more strongly sorbed to Illite than kaolinite.68 pH impacts phytate sorption by soils, with more being accumulated in acid soils than alkaline soils. For example, after 24 h of reaction at pH 4.5, 2.12 μmol m–2 phytate is sorbed by ferrihydrite.77 However, the amount sorbed is reduced by half at pH 6.5.54 This is because phytate can complex with Fe, Al, Ca, and/or Mg, which is pH-dependent, being stable at pH < 5 (sorbs to Fe/Al minerals) and > 7.5 (precipitates with Ca).11,58
Besides, phytate stability varies with metal oxides, especially amorphous Fe and Al.78 For example, phytate is sorbed onto goethite via four of the 6-phosphate groups, with the remaining two being free.68 This explains the 3:2 sorption ratio between phytate and P in soils.54 The large number of phosphate groups involved in phytate sorption leads to its stability with goethite, even in the presence of citrate and bicarbonate.61 Unlike goethite, phytate sorption onto ferrihydrite occurs via two phosphate groups, showing less stability than onto goethite, with its desorption increasing with increasing pH.77 In addition, phytate stability is metal-dependent, with Al3+ > Fe3+ > Mg2+ > Fe2+ > Ca2+.79 Their corresponding stability constants (log K13–15) are 12.2–20.1, 8.89–18.2, 8.76–10.5, 7.71–10.5, and 8.3–8.4 in the NaClO4 solution at 37 °C (Table 2B). These complexes are soluble only at pH < 2, as they are insoluble at mid-range pH values as Fe4/Al4-phytate (pH = 5–7) or Ca6/Mg6-phytate (pH > 7.5) complexes.54,80
3. Phytate-P Utilization by Plants
Phytate plays two roles in plants: serving as a reserve for P, inositol and minerals, and controlling P homeostasis.45 Phytate is only available to plants after its solubilization and hydrolysis via phytase, with the released P diffusing to rhizosphere solution.7,81 However, phytate is strongly bound to soils, so the concentrations of soluble phytate-P in the soil solution are typically very low (4–14.3 μg L–1).82 Therefore, plants and their associated microbes have developed strategies to solubilize and/or hydrolyze phytate to increase its availability.
3.1. Phytate Solubilization by Organic Acids
The accumulation of phytate in soils compared to other P-esters is attributed to its strong affinity for soils. The availability of soil phytate is low, hindering its interaction with phytase, thereby reducing its enzymatic cleavage of phytate ester bonds and the mineralization of its inositol ring.83 Desorption and solubilization are two ways to increase phytate access by phytase.5 In soils, P can be desorbed or solubilized by protons, organic acids, and phenolic acids, with organic acids being the primary factor in solubilizing sparingly-available P (Tables 3A, 3B, and S2).84,85
Table 3A. Summary of Known Plant to Mobilize Soil P.
| plant family/species |
location and soil P concn (mg kg–1) |
% total carboxylates |
soil mobilized Pa (mg kg–1) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fabaceae | location | Pt | bicarb.-extr.b P | total carboxylates (μmol g–1 root dw)c | malonic | malic | citric | malonic | malic | citric | ref | |||||
| chickpea (Cicerarietinum) | Mullewa | 83–97 | 17–19 | 40–65 | 70–79 | 7–20 | 8–12 | 1.6d | 1.25d | 2.0d | Wouterlood et al.91 | |||||
| Merredin | 82–108 | 11–24 | 100–310 | 63–82 | 10–19 | 7–22 | ||||||||||
| Esperance | 133–275 | 24–54 | 17–120 | 61–84 | 8–23 | 3–18 | ||||||||||
| Cicer arietinum | Heera | Northam | 158 | 5 | 30–70 | 50–91 | 30–41 | trace | 0.4 | 0.75 | 0.1 | |||||
| Tyson | Nyabing | 66 | 4 | 90–99 | 20–42 | 1.6 | 1.9 | 1.2 | ||||||||
| white lupin (Lupinusalbus) | Bindoon | – | – | 237 | – | 88.5 | 11.5 | – | – | – | Veneklaas et al.35 | |||||
| Merredin | 213 | 12 | 88 | |||||||||||||
| Pingrup | 282 | 41.9 | 58.1 | |||||||||||||
| Mingenew | 180 | 85.9 | 14.1 | |||||||||||||
| Nyabing | 109 | 66.7 | 33.3 | |||||||||||||
| Scadden | 92.8 | 29.4 | 70.6 | |||||||||||||
| |
organic acid species and % total carboxylates |
pH |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| plant species | total carboxylates concn (μmol g–1 root dw) | malic | citric | malonic | initial pH 6.7 | ref | ||||||||||
| 3 μM P | 300 μM P | 3 μM P | 300 μM P | 3 μM P | 300 μM P | 3 μM P | 300 μM P | 3 μM P | 300 μM P | Pearse et al.173 | ||||||
| Triticum aestivum | 5.00 | 3.33 | 90 | 93 | 10 | 6.31 | 4.04 | 4.09 | ||||||||
| Brassica napus | 3.33 | 8.00 | 95.4 | 98.7 | 2.8 | – | 4.35 | 4.70 | ||||||||
| Vicia faba | 6.66 | 5.00 | 62.8 | 68.4 | 38.5 | 32.3 | 6.52 | 6.78 | ||||||||
| Lens culinaris | 5.00 | 5.16 | – | – | 12.3 | 55.4 | 87.7 | 44.3 | 6.44 | 6.57 | ||||||
| Cicer arietinum | 55.0 | 30.0 | 3.1 | 4.8 | 40 | 27.8 | 56.9 | 65.8 | 6.26 | 6.09 | ||||||
| Pisum sativum | 25.0 | 9.3 | 4.8 | 34.9 | 95.4 | 63.1 | 6.26 | 6.37 | ||||||||
| Lupinus luteus | 51.6 | 18.0 | 10.8 | 13.8 | 90.7 | 87.7 | 4.70 | 5.17 | ||||||||
| L. albus | 49.1 | 20.0 | 38.8 | 44.8 | 58.6 | 55.7 | 5.22 | 5.39 | ||||||||
| L. atlanticus | 31.7 | 9.0 | 20.3 | 36.0 | 78.6 | 63.4 | 5.57 | 6.04 | ||||||||
| L. angustifolius | 28.3 | 14.2 | 28.5 | 36.9 | 72.7 | 67.1 | 5.57 | 5.70 | ||||||||
| L. mutabilis | 23.3 | 11.7 | 30.5 | 44.6 | 70.4 | 56.3 | 5.83 | 5.78 | ||||||||
| L. pilosus | 19.7 | 15.0 | 12.3 | 15.1 | 89.2 | 85.5 | 0.5 | 4.26 | 4.70 | |||||||
| L. cosentinii | 16.7 | 14.7 | 12.3 | 13.8 | 88.4 | 86.3 | 5.22 | 5.39 | ||||||||
| |
|
cultivars
and % total organic acids |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| plant species | location and soil P
concn (mg kg–1) |
total organic acids (μmol g–1 root dw) |
Sona |
Kaniva |
Tyson |
|||||||||||
| Fabaceae | location | Pt | bicarb.-extr.b P | Sona | Kaniva | Tyson | malonic | malic | citric | malonic | malic | citric | malonic | malic | citric | ref |
| chickpea (Cicerarietinum) | Bindoon | 193 | 11.3 | 110 | 200 | 191 | 83 | 1 | 7 | 86.4 | 4.5 | 9.1 | 81 | 4.8 | 14.3 | Veneklaas et al.35 |
| Merredin | 57 | 6.0 | 11 | 36.4 | 45.5 | 54 | <1 | 39 | 75 | – | 25 | 60 | – | 40 | ||
| Pingrup | 67 | 6.3 | 59 | 127 | 138 | 76 | <1 | 22 | 64.3 | 7.1 | 28.6 | 67.1 | 4.6 | 28.3 | ||
| Mingenew | 116 | 9.0 | 37 | 168 | 228 | 67 | 2 | 28 | 97.3 | – | 2.7 | 84 | 12 | 4 | ||
| Northam | 113 | 6.0 | 120 | – | – | 63 | – | 37 | – | |||||||
| Nyabing | 40 | 4.3 | 100 | 144 | 167 | 80 | – | 20 | 87.3 | 6.3 | 6.3 | 87 | 6.5 | 6.5 | ||
| Hyden | 43 | 2.7 | 15 | – | – | 82 | – | 17 | – | |||||||
| Scadden | 18 | 5.3 | 122 | 133 | 150 | 81 | 4 | 15 | 65.8 | 4.8 | 29.5 | 63.6 | 36.4 | |||
| Mullewa | 94 | 8.7 | 162 | – | – | 80 | 3 | 17 | – | |||||||
| Morowa | 91 | 10.3 | 52 | – | – | 78 | 3 | 19 | – | |||||||
| Proteaceae | organic acid species | concn (mg L–1in 100 mL soil leachates | % total organic acids | ref | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Banksia integrifolia | citric | 12.6 | 50 | Grierson174 | ||||||||||||
| maleic | 2.88 | 11 | ||||||||||||||
| malic | 4.58 | 18 | ||||||||||||||
| aconitic | 4.34 | 17 | ||||||||||||||
| fumaric | 1 | 4 |
The P-mobilizing capacity of carboxylates on soils was analyzed by extracting 3 g soil with 30 mL of 0.5 mM citrate, malate, or malonate.
bicarb.-extr. = bicarbonate-extractable; extr. = extractable. Bicarbonate-extractable P is extracted with 0.5 M sodium bicarbonate at pH 8.5.89
Mobilized P was extracted from a soil with total and bicarbonate-extractable P at 66 and 4 mg kg–1.91
Table 3B. Summary of Microbially-Secreted Organic Acids to Mobilize Soil P.
| microbe species | mobilized P |
||||
|---|---|---|---|---|---|
| strain | organic acid species | exptl conditions | mg L–1 | pH | ref |
| D 5/23 Pantoea agglomerans | succinate, hydroxyglutarate, adipate, lactate, ketogluconate | 200 μg P mL–1 as Ca3(PO4)2, 28 °C 7 d | 62.8 | 5.93 | Deubel and Merbach175 |
| PsIA12 Pseudomonas fluorescens | succinate, lactate, malate, ketogluconate, galacturonate, citrate | 44.1 | 4.77 | ||
| CC 322 Azospirillum sp. | gluconate, succinate, 2-ketoglutarate, ketogluconate | 83.4 | 6.19 | ||
| Mac 27 Azotobacter chroococcum | citrate, malate, fumarate, succinate, lactate | 98.1 | 4.84 | ||
| Msx 9 Azotobacter chroococcum | citrate, fumarate, malate, lactate, succinate | 65.9 | 5.82 | ||
| ER 3 | fumarate, isocitrate, lactate, malonate | 75.5 | 5.32 | ||
| ER 10 | lactate, gluconate, malonate, citrate | 36.2 | 5.72 | ||
| citric | 200 mg L–1 P as Ca3(PO4)2, 28 °C 7 d | 236 mg g–1 | |||
| succinic | 178 | ||||
| lactic | 126 | ||||
| citric | 2 g soil +100 mL 5 g L–1 carboxylic acid, pH 7, 24 h | 250 mg kg–1 | |||
| oxalic | 175 | ||||
| gluconic | 50 | ||||
| succinic | 25 | ||||
| A. calcoaceticus YC-5a | oxalic, malic, lactic, tartaric | 5 g L–1 Ca3(PO4)2, 28 °C, 7 d | 518 ± 17.3 | 3.92 ± 0.02 | Ren et al.176 |
| E. agglomerans KMC-7 | oxalic, lactic, citric, succinic | 435 ± 15.6 | 4.13 ± 0.01 | ||
| microbe species | |||||
|---|---|---|---|---|---|
| fungi | organic acid species | ref | |||
| Aspergillus flavus, A. candidus, A. niger, A. terreus, A. wentii, Fusarium oxysporum, Penicillium sp., Trichoderma isridae, Ttrichoderma sp. | lactic, maleic, malic, acetic, tartaric, citric, fumaric, gluconic | Akintokun et al.177 | |||
| Penicillium oxalicum | malic, gluconic, oxalic | Shin et al.178 | |||
| Aspergillus flavus, A. niger, P. canescens | oxalic, citric, gluconic, succinic | Maliha et al.179 | |||
| Penicillium rugulosum | citric, gluconic | Reyes et al.180 | |||
| A. niger | succinic | Vazquez et al.181 | |||
| Penicicllium variabile | gluconic | Fenice et al.182 | |||
| oxalic, lactic, glycolic, citric, succinic, tartaric | Whitelaw183 |
Organic acids contain carboxylate groups that can mobilize phytate via three mechanisms. First, carboxylates can desorb P anions from soil through ligand exchange by replacing P with a carboxylate anion. Specifically, tribasic citrate releases more P than dibasic oxalate due to its greater number of carboxyl groups, with closer pK2 value (4.76 vs 4.28) to soil pH (4.5–9.5), leading to rapid degradation of oxalate.86 Second, carboxylates can solubilize Fe and Al via H+, thereby destroying P sorption sites. Third, they can solubilize OM that binds to P via Fe/Al-bridges, with P being solubilized as the OM-Fe/Al-P complex.15
Since the interaction of phytate-P with soils is like Pi, similar reactions may solubilize phytate-P in soils but with more mechanisms being involved: 1) chelation of metals bound in metal-phytate complexes to release P and 2) chelation of metals to form complexes, which sorb to soils to prevent microbial degradation of organic acids, resulting in their long-lasting effect to improve phytate solubilization in soils.24 Organic acids in the soil solution can be quickly degraded by microbes, whereas sorption onto soil hinders their degradation. For example, 70% of citrate added to soil is degraded after 10 d, but its sorption onto Fe/Al-hydroxide reduces its degradation by 50–90%.87 The presence of organic acids in the soil solution is necessary for phytate solubilization in soils,84,88 and the effects of organic acids on phytate-P acquisition by plants were summarized by Gerke.24
Phytate solubilization is essential for phytate-P acquisition by plants. Under P deficiency conditions, plant roots alter soil chemistry by releasing organic acids (Tables 3A and S2).85 The typical organic acids exuded by plants include citrate, oxalate, malonate, gluconate, and acetate. In the rhizosphere, phytate solubilization and hydrolysis, and the subsequent P acquisition by plants are greater than bulk soil due to its greater organic acid concentrations.84 The concentrations of organic acids in the bulk soil solution are generally <50 μM, but they can be in the range of 92.8–282, 15–50, and 45.4–228 μmol g–1 root dw in white lupin and chickpea, with citrate (63–88%) and malonic (60–81%) being predominant (Table 3A).35,89
Many plants exude organic acids, with those being effective including rape, chickpea, and lupin.35 For example, cluster-forming plant species, such as white lupin and yellow lupin, excrete citrate to enhance P uptake under P deficiency.90 In addition, organic acids such as citrate from legumes and malate from chickpea can solubilize phytate-P in soils, showing greater phytate-P acquisition compared to plants with limited exudates such as sunflower or wheat.57,91,92
However, plants like pea and chickpea are unable to access phytate in sand culture despite their ability to release organic acids into the rhizosphere.93 Similarly, organic acids in the rhizosphere can not induce a significant difference in P acquisition from insoluble P by white lupin, implying that there is no simple relation between exudation of organic acids and available P in soil. It is possible that plant roots exude a basal level of organic acids into the rhizosphere. Plants increase the exudation of organic acids considerably when soil solution P availability is limiting (<1–2 μM), which often occurs in soils with a strong ability to bind P or nutrient-poor soil with sparingly-available P as Fe/Al-phosphate.91,94 Therefore, further work is needed to establish the relationship between the concentrations of organic acids in the rhizosphere and the amount of phytate-P that can be taken up by plants in different soils.
3.2. Phytate Hydrolysis by Phytase
Phytate Hydrolysis
Phytate hydrolysis is mediated by phytase, which is classified according to its catalytic mechanism as belonging to histidine acid phosphatase (HAP), purple acid phosphatase (PAP), Cys phosphatase, or β-propeller phosphatase,95 with HAP and PAP being more prevalent. Each group consists of several phosphatases, but only a few of them have phytase activity.96 HAPs originate mainly from plants and show specific activity toward phytate. Their catalytic hydrolysis is via a N-terminal RHGXRXP motif and a C-terminal HD motif position to form an active site.95 Unlike HAPs, PAPs originate from both plants and microbes and can hydrolyze various Po forms besides phytate.97 They are metallohydrolases that bind two metal cations in the active center. One of the cations is usually FeIII, while other metals can be Zn, Mn, or FeII, which are responsible for PAP’s color.98
Phytase activity in soils is affected by soil pH and its sorption.8 Phytases show optimal activity toward phytate at 2.5–8.0 (Tables 4A and 4B) and then decline with increasing pH; thus, normally it is higher in acidic soils than alkaline soils.99 Besides, phytase activity is inhibited due to its sorption onto soil minerals such as montmorillonite.100
Table 4A. Summary of Known Plant Phytases to Hydrolyze Mobilized Phytate.
| phytase
activity |
||||||||
|---|---|---|---|---|---|---|---|---|
| plant species and fraction | Ua mg–1 | μKat mg–1 | pH optim | temp (°C) | temp optim (°C) | Km (μM) | molecular wt (kDa) | ref |
| buttercup squash | – | – | 4.8 | 48 | – | – | 67 | Goel and Sharma184 |
| scallion leaves | 5.5 | 51 | – | 200 | – | Phillippy185 | ||
| sunflower | 5.2 | 55 | – | 290 | – | Agostini and Ida186 | ||
| tomato roots | 4.3 | 45 | – | 38 | 164 | Li et al.187 | ||
| 205 | 3.44 | 4.3 | – | 50 | – | – | ||
| Lilium longiflorum | 0.066 | 0.001 | 8.0 | 55–60 | 7.2 | 88 | Scott and Loewus188 | |
| maize roots | 5.7 | 0.1 | 5.0 | 40 | 71 | Hubel and Beck189 | ||
| Typha latifolia pollen | – | – | 8 | – | 17 | – | Hara et al.190 | |
| rye | – | – | 6.0 | 45 | 300 | 67 | Greiner et al.191 | |
| spelt | 262 | 4.38 | 6.0 | 45 | 400 | 68 | Konietzny et al.192 | |
| scallion (Alliumfistulosum) | 500 | 8.35 | 5.5 | 51 | 200 | 72 | Phillippy185 | |
| maize seedlings | – | – | 4.8 | 55 | 117 | 76 | Laboure et al.193 | |
| plant species | substrate | activity (EUb×10–5) | ref | |||||
|---|---|---|---|---|---|---|---|---|
| cereals (wheat, pearl millet, sorghum), legumes (mung, moth, cluster bean), oil seed crops (groundnut, sesame, mustard) | P-deficient | 29.3 | Yadaf and Tarafdar194 | |||||
| phytinc (250 mg L–1) | 29.1 | |||||||
| phytin (mg L–1) | ||||||||
| wheat (Triticumaestivum) | 50 | 2.32 | ||||||
| 100 | 3.87 | |||||||
| 150 | 5.25 | |||||||
| 200 | 7.89 | |||||||
| 250 | 9.35 | |||||||
| 300 | 9.82 | |||||||
| 500 | 9.87 |
| plant species | activity (EUb×10–5) | incubation condition | P released (mg L–1) | ref | ||||
|---|---|---|---|---|---|---|---|---|
| sorghum (Sorghum bicolor SSG-1000) | 65 | 5.4 × 10–5 EU, 500 mg kg–1 P as phytin, 2 wks | 68 | Tarafdar et al.109 | ||||
| cowpea (Vigna unguiculata RC-19) | 69 | 10.8 × 10–5 EU, 500 mg kg–1 P as phytin, 7 d | 136 | |||||
| mung bean (Phaseolus radiatus K-851) | 67 |
| phytase
activity |
||||||||
|---|---|---|---|---|---|---|---|---|
| plant species and fraction | P-fed plants | No-P plants | ref | |||||
| wheat whole root extract (soluble; mU g–1 root fw) | 4.4 ± 1.1 | 23.9 ± 1.2 | Celi and Barberis54 | |||||
| wheat total intact root (mU g–1 root fw) | 12.1 ± 4.0 | |||||||
| wheat external-root solution (mU g–1 root fw h–1) | <0.3 | <0.3 | ||||||
One unit (U, μmol mg–1) of phytase activity is the amount of phytase required to hydrolyze sodium phytate to produce 1 μmol P per min at 37 °C and pH 5.5.203,204
One EU corresponds to the amount of enzyme required to hydrolyze 1 μmol of p-nitrophenyl phosphate s–1 at pH 5.4, 35 °C.194
Phytin: Ca/Mg-phytate salts.
Table 4B. Summary of Microbially-Secreted Phytase to Hydrolyze Mobilized Phytate.
| specific activitya |
residual
activity after 24 h (nKat g–1 soil) |
phytate-hydrolyzing capacity |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| microbe species | U mg–1 | μKat mg–1 | Vmax(nKat mL–1) | soil type | initial activity | pH | solution phase | solid phase | soil type | incubation condition | P released (mg kg–1soil) | ref | ||
| Aspergillus niger | 282 | 4.7 | 0.112 | spodosol | 10.2 | 5.5 | 2.17 | 2.41 | alfisol-1 | water suspension (1:10 w:v), phytase (120 nKat g–1 soil), 24 h | 0.05 | George et al.32 | ||
| alfisol | 0.96 | 3.62 | alfisol-2 | 9.45 | ||||||||||
| oxisol | 0.24 | 0.72 | spodosol | 3.15 | ||||||||||
| spodosol | 7.5 | 9.40 | 0 | vertisol | 5.4 | |||||||||
| alfisol | 0.84 | 0.24 | ||||||||||||
| oxisol | 2.89 | 0 | ||||||||||||
| Peniophora lycii | 120 | 2 | 0.102 | spodosol | 5.5 | 9.40 | 0 | |||||||
| alfisol | 0.99 | 0.72 | ||||||||||||
| oxisol | 8.68 | 0 | alfisol-1 | 6.75 | ||||||||||
| spodosol | 7.5 | 8.19 | 0 | alfisol-2 | 21.6 | |||||||||
| alfisol | 0.87 | 0.24 | spodosol | 14.9 | ||||||||||
| oxisol | 8.44 | 0 | vertisol | 15.3 | ||||||||||
| stability |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| microbe species | class of phytase | specific activity | pH optim | temp optim (°C) | pIb | Kmc (μM) | Mwd (kDa) | pH | % activity | temp (°C) | half-life (min) | P (mM) | % activity | ref |
| Aspergillus ficuum | 2.5 | 60 | 14.6 | 2 | 65.5 | Kim et al.195 | ||||||||
| 70 | 0.5 | 4 | 42.0 | |||||||||||
| 80 | 0.2 | 6 | 12.6 | |||||||||||
| Bacillus amyloquefaciens DS11 | 7 | 4 | 80 | 70 | 1532 | 2 | 96.6 | |||||||
| 6 | 100 | 80 | 42 | 4 | 96.6 | |||||||||
| 8 | 60 | 90 | 10 | 6 | 88.2 | |||||||||
| pH | % activity (Tris-HCl) | time (min) | % activity(95 °C) | Ca (mM) | % activity(95 °C) | |||||||||
| Bacillus subtilis 168 | 35 U mL–1 | 6.6–7 | 55 | 44–47 | 5.5 | 96.6 | 15 | 43 | 1 | 47 | Tye et al.196 | |||
| 7.0 | 76.6 | 30 | 23 | 5 | 31 | |||||||||
| 36.9 U mg–1 | ||||||||||||||
| 8.5 | 3.33 | |||||||||||||
| Bacillus licheniformis | 28 U mL–1 | 6.1–7 | 65 | 5.5 | 97 | 15 | 61 | 1 | 60 | |||||
| 23.6 U mg–1 | 7.0 | 86.6 | 30 | 40 | ||||||||||
| pH | % activity (55 °C) | temp(°C) | % activity(pH 7) | metal(2 mM) | % activity(pH 7.5) | |||||||||
| Bacillus subtilis VTT E-68013 | PhyC | 7 | 55 | 6.5 | 43 | 5.5 | 28.6 | 37 | 58.5 | Ca2+ | 42 | Kerovuo et al.197 | ||
| Zn2+ | 15.6 | |||||||||||||
| 7.0 | 85.7 | 55 | 85.7 | Ni2+ | 11.6 | |||||||||
| Mn2+ | 10.7 | |||||||||||||
| 8.5 | 11.4 | 75 | 15.7 | Mg2+ | 1.7 | |||||||||
| Cu2+ | 1.4 | |||||||||||||
| no CaCl2 | pH | % activity(no CaCl2) | temp(°C) | % activity(no CaCl2) | metal | % activity(no CaCl2) | ||||||||
| Bacillus sp. KHU-10 | 36 U mg–1 | 6.5–8.5 | 30–40 | 6.8 | 50 | 44 | 4.5 | 25 | 30 | 99.8 | Cd2+ | 0 | Choi et al.198 | |
| 5.5 | 42.5 | 40 | 99.6 | Cr3+ | 45 | |||||||||
| in 10 mM CaCl2 | 6.5 | 85 | 50 | 35 | Cu2+ | 59 | ||||||||
| 7.5 | 100 | 60 | 0 | Hg2+ | 43 | |||||||||
| 6.0–9.5 | 60 | 8.5 | 97.5 | Mn2+ | 17 | |||||||||
| 9.5 | 77.5 | Co2+ | 60 | |||||||||||
| stability |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| microbe species | class of phytase | specific activity | pH optim | temp optim (°C) | pIb | Kmc (μM) | Mwd (kDa) | pH | % activity (37 °C) | temp (°C) | % activity (pH 7) | protease (0.1g L–1) | % activity (37 °C) | ref |
| Citrobacter braakii YH-15 | P2 | 3457 U mg–1 | 4 | 50 | 460 | 47 | 3 | 86 | 30 | 56 | papain | 85 | Kim et al.199 | |
| 4 | 84 | 40 | 78 | |||||||||||
| 5 | 68 | 45 | 84 | elastase | 80 | |||||||||
| 57.5 μKat mg–1 | ||||||||||||||
| 6 | 56 | 50 | 100 | |||||||||||
| 7 | 46 | 55 | 68 | pancreatin | 70 | |||||||||
| 8 | 13 | 60 | 34 | |||||||||||
| Escherichia coil ATCC 33965 | 1800 U mg–1 | 4.5 | 60 | 6.3 | 540 | 44.7 | 2 | 46.3 | 30 | 33.2 | pepsin (pH 2.5) | 80 | Golovan et al.200 | |
| 3 | 66.6 | 40 | 48.1 | |||||||||||
| 4 | 87 | 50 | 79.7 | pancreatic proteases (pH 7) | 38 | |||||||||
| 30.1 μKat mg–1 | 5 | 92.5 | 60 | 100 | ||||||||||
| 6.5 | 790 | 6 | 42.6 | 70 | 79.7 | intestinal fluid | 40 | |||||||
| 7 | 2.78 | 80 | 6.64 | |||||||||||
| enzyme
activity (EU × 10–7) |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| microbe species | intracellular (g–1 fungal mat) | extracellular (mL–1 filtrate) | incubation condition | P released (mg L–1) | ref | |||||||||
| A. niger | 441 ± 38 | 43.5 ± 2.1 | 5.4 × 10–5 EU, 500 mg kg–1 P as phytin, 2 wks | 185 | Tarafdar et al.109 | |||||||||
| A. terreus | 485 ± 17 | 44.7 ± 1.5 | ||||||||||||
| 10.8 × 10–5 EU, 500 mg kg–1 P as phytin, 7 d | 365 | |||||||||||||
| A. rugulosus | 433 ± 46 | 41.8 ± 2.2 | ||||||||||||
| specific activitya |
temp (°C) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| microbe species | U mg–1 | μKat mg–1 | pH optim | optim | maxim | pIb | Kmc (μM) | Mwd (kDa) | ref | |||||
| Aspergillus niger | 216 | 3.6 | 5.0 | 58 | 70 | 4.94–5.01 | 800 | 48.4–66.4 | Ullah and Sethumadhavan201 Lassen et al.202 Vats and Banerjee134 | |||||
| Peniophora lycii | 1374 | 22.9 | 5.5 | 58 | 70 | 3.61–4.37 | 800 | 44.6–72 | ||||||
Specific activity determined against phytate (myo-inositol hexakisphosphate).
pI: Isoelectric point.
Km: Michaelis constant, indicating affinity.
Mw: Molecular weight.
Plant Phytase
Plant phytase is associated with various cellular functions, including energy metabolism, nutrient transport, metabolic regulation, and protein activation.101 However, it is the extracellular phytase released from the roots that is of particular importance for phytate hydrolysis in soils.45 Plant extracellular phytase is induced under P deficiency conditions, which either remains associated with root cell walls or is released directly into the rhizosphere to catalyze phytate hydrolysis.30,102 For example, by exuding phytase into the rhizosphere, 1.7 μg P g–1 d–1 is released via phytate hydrolysis, facilitating phytate-P utilization by wheat.102 Similarly, the arsenic-hyperaccumulator Pteris vittata (Chinese brake fern) can grow in Murashige and Skoog media supplied with phytate as the sole source of P.29,94 After 40 days of growth, P. vittata takes up similar amounts of P grown in media with phytate or Pi, with tissue P concentrations being 2351 and 2208 mg kg–1. In comparison, other plants including angiosperms (Lactuca sativa, Trifolium subterraneum, and Allium schoenoprasum) and pteridophytes (Pteris ensiformis and Thelypteris kunthii) fail to grow with phytate as the sole source of P.29 The authors show the phytase activity in P. vittata roots at 0.018 U mg–1 (3 × 10–4 μkat mg–1). However, for most plants, they do not show phytase activity in the roots as most of the phytate is stored in the seeds.43
More recently, Sun et al.45 identified a novel root-specific phytase PvPHY1 from P. vittata via prokaryotic expression, which can hydrolyze phytate, showing activity analysis at 37 °C and pH 5.5. Unlike typical plants such as rice and A. thaliana, expression of PvPHY1 in P. vittata roots is greater than the fronds, which is consistent with the 7-fold stronger phytase activity in the roots than the fronds at 19.2 and 2.9 μmol P g–1 protein min–1. Besides, expressing PvPHY1 in tobacco plants enhances its growth by 0.7–1.1 g plant–1 and P concentration by 10–50% under low- and adequate-P conditions.45 Further, PvPHY1-expressed tobacco shows 25–32% less intracellular phytate and 30–56% more P in the roots, likely due to phytase-mediated hydrolysis of phytate within the roots.45 However, PvPHY1 expressed into tobacco plants fails to use phytate in the media, which is probably due to its inability to exude root phytase into the growth media. In comparison, PvPHY1 in P. vittata roots can help to use extracellular phytate in the media. In short, P. vittata can grow on media with phytate as the sole source of P likely because it can exude phytase into the media to hydrolyze phytate for its uptake.
Though root extracellular phytase can help plants to obtain P from phytase-hydrolyzable phytate under sterile media,103 phytase shows limited ability in soils.104 This is because both phytase and phytate are readily sorbed by soils, with phytase activity being reduced by 95%.104,105 This is consistent with wheat and pasture species growing in soils, which can use P from readily-hydrolyzable monoester (glucose-6-phosphate) and diester (ribonucleic acid) Po substrates, but show limited capacity to acquire P from phytate (227–238 vs 74 μg P shoot–1).103 This is especially true in soils with high OM content and/or a history of P fertilizer applications.11
Microbial Phytase
Both plant roots and microbes possess phytase activity. However, the accumulation of phytate in soils indicates that phytate is resistant to mineralization compared to other Po such as glucose 1-phosphate, nucleic acids, and phospholipids.18 Phytate hydrolysis and subsequent plant P uptake have been assessed based on the depletion of phytase-hydrolyzable phytate in the rhizosphere.106 However, there are conflicting results regarding the ability and relative contribution of root- and microbe-derived extracellular phytases to hydrolyze phytate in soils.107,108
For example, soil microbial phytase shows a greater ability to hydrolyze phytate than those from plant roots, i.e., 41.8–43.5 EU × 107 mL–1 filtrate for Aspergillus niger (A. terreus and A. rugulosus) vs 0.65–0.69 EU × 107 mL–1 filtrate for Sorghum bicolor (cowpea and mung bean) (Table 4B).109 In addition, it is speculated that significant extracellular phytase activity from plants is rare, and soil phytase activity is mainly attributed to microbes. However, Belinque et al.110 show that phytate-P acquisition by several plants is not improved with microbial inoculation, rather it is improved by plant-derived phytase. Besides, plant-derived phytase hydrolyzes phytate at a high rate, making acquisition of phytate-P similar to Pi.110 This result is consistent with Tarafdar and Claassen,111 suggesting that root phytase activity may be sufficient to hydrolyze phytate in the rhizosphere. On the other hand, microbial phytase may be important for microbial P turnover in soil.
The role of soil phytase in phytate hydrolysis was reviewed by Quiquampoix and Mousain,112 but the role of microbes in phytate-P acquisition by plants is not well understood. The close relation between phytase activity in the rhizosphere and P acquisition by plants does not address the question whether the enzyme comes from plants or microbes.106 Interestingly, recent research demonstrates that arbuscular mycorrhizal fungi show less ability to produce phytases than saprophytic fungi, but they compensate for this by recruiting hyphosphere bacteria that are able to produce phytase.113 In fact, in some circumstances, these bacteria migrate to phytate hotspots along the fungal hyphae.114 As such, the contribution of plant and microbial phytase in improving plant phytate-P acquisition needs further elucidation, especially in different soils.
Besides, phytase activity toward phytate is determined by both soil properties and microbial populations.105 For example, in two soils with comparable pools of phytase-hydrolyzable phytate (12.5–17.0 mg P kg–1), transgenic subterranean clover expressing phytase depletes ∼80% of phytate in a Spodosol soil with low ability for P retention, whereas only a small amount of phytate is depleted from an Alfisol with a greater P sorption capacity.107 In addition, the fact that phytate depletion in soils is similar for all plants (control and transgenic) and unplanted controls indicates that the ability of a plant to obtain phytate-P is independent of plant species. Further, depending on soil type, it is more likely a function of microbial activity.8 Nevertheless, this study highlights the potential contribution of phytate to plant P nutrition and the importance of microbial activity.
3.3. The Limiting Steps
Being the most abundant but also the most recalcitrant Po in soils, phytate has the potential to contribute to plant P nutrition. Two hypotheses have been proposed regarding the limited acquisition of phytate-P by plants: 1) limited solubility of phytate due to its strong binding in soils and 2) low activity of phytase in soils makes phytate-P unavailable to plant roots.5,24 As such, both phytate solubility and phytase activity are the limiting steps in plant acquisition of phytate-P.
It is generally known that soil phytate is relatively unavailable to plants, but findings are often inconsistent. In sand culture, Adams and Pate115 show that both white lupin and narrow leaf lupin take up Pi and phytate-P at a similar rate, indicating little phytate sorption by sand and little limitation of P acquisition by a low phytase activity. Further, Lessl et al.29 show that phytase from As-hyperaccumulator P. vittata roots can retain 93–98% of activity after being mixed with soils for a day, thereby helping phytate hydrolysis in the media and P utilization by P. vittata. Soil phytase is mostly effective in sand with low concentrations of organic matter, low microbial growth, and/or low sorption capacity.115 The results agree with Tarafdar and Jungk116 and Lung and Lim,108 but are in contrast to Hayes et al.18 and George et al.107
In P-fixing soil, both transgenic and nontransgenic lupin plants take up less phytate-P than Pi,84 although phytate application increases plant P uptake. The results suggest that, in both plants, phytate-P acquisition is limited by phytate sorption onto soils, not phytase activity. Tarafdar and Claassen117 and Lung and Lim108 also conclude that phytate solubility is the limiting step in phytate-P acquisition by plants.
However, others show that phytase activity is the limiting step for phytate-P acquisition by plants.40,118 Richardson et al.103 find that wheat grown under sterile conditions with soluble phytate but not phytase activity is unable to use phytate as a P source. Similar results were reported for grasses and clovers.18 Therefore, low plant phytase activity is a critical factor limiting phytate-P use under sterile conditions. On the other hand, some studies demonstrate phytate-P use by plants under nonsterile conditions, which may be attributed to microbial phytase in the rhizosphere.119 This hypothesis is supported by increased plant P acquisition via microbial inoculation and microbial enzyme addition.18,120,121 The results suggest that phytase activity on the root surface is the limiting step in phytate-P acquisition by plants, but this is only demonstrated in low-sorption capacity media such as agar.107,122 Besides, the experiments fail to show the mechanism of how soil extracellular phytase improves phytate-P nutrition for plants.
As such, there is no agreement regarding the limiting step in phytate-P acquisition by plants. The possible reasons for the conflicting results may be due to the following: 1) phytate is often complexed with multivalent metals with low availability, whereas most experiments use sodium phytate with high availability; 2) variations among plant species with inherent phytase activity and therefore the ability to use phytate-P; 3) variations in the strength of phytate sorption in different soils, so that even plants with extracellular phytase cannot use phytate in all soils; and 4) substrates contain substances that may detach metals from phytate-metal complexes.
4. Strategies to Improve Phytate-P Acquisition by Plants
Factors affecting phytate availability, phytase activity, and phytate–phytase interaction determine the acquisition of phytate-P by plants. There are three main ways to help plants acquire phytate-P: 1) accelerating solution P depletion by plant uptake to increase phytate desorption from the rhizosphere; 2) improving phytate solubilization into the soil solution to increase its availability to phytase; and 3) increasing phytase activity to enhance phytate hydrolysis in the soil solution.
Plants can adapt to soils with limited available P via changing root features by forming longer root hairs and large roots, both increasing root surface area. This may be feasible only when soil solution P is not too low (>1–2 μM).24 If soil solution P is too low, the diffusive flux of P to the root surface can not satisfy the P demand by plants. Under these conditions, plants and the associated microbes have developed strategies to increase rhizosphere P by secreting exudates (organic acids) and hydrolyzing enzymes (phytase).
4.1. Plant and Microbial Traits
Plant Genotypes
Organic acids and phytases exuded by plant roots vary across and within different species, which helps to select genotypes to improve phytate solubilization and hydrolysis.93,94,123
The most effective organic acids to solubilize phytate include those containing carboxylate groups, especially citrate and to a lesser extent oxalate,24 which can exude 25–187 and 26–210 μmol g–1 root dw (Table 3A). There is genetic variation across different plant species and intraspecific variation among different cultivars of a plant species. For example, white lupin from acidic and alkaline soils exhibits different root exudation and capacity to access Ca-phytate.93 The composition and concentration of root exudates also vary among chickpea cultivars, with their concentrations in lateral roots increasing with plant growth.91,123 Likewise, different abilities in plant root exudation are identified in pigeon pea cultivars.124 In addition, cluster roots can help plants to efficiently uptake P by releasing organic acids. In a conventional single root, ∼80–90% of its soluble P diffuses away, while the cluster roots can take up most of that soluble P.24
Phytases from different origins have different physicochemical and biochemical properties, which affect their mobility and ability to hydrolyze phytate in soils. Studies show the activities of extracellular phytase vary in different plants. For example, tobacco exudes phytase of the purple-acid-phosphatase class, which is responsible for Na-phytate utilization. The phytase shows a high affinity for Na-phytate (Km = 14.7 μM) with specific activity at 6.03 μkat mg–1 and a Vmax value at 7.2 μkat mg–1.125 George et al.104 screened a range of wheat lines and identified considerable variation in extracellular phytase exudation among genotypes. Though relationships exist between root-exuded phytase activities and the ability to utilize phytate substrate in vitro, no clear relationships are demonstrated between extracellular phytase activities with P nutrition or plant growth when grown in soils.126
The data suggest that the variability in phytase activities among plants either has little effect on P nutrition of soil-grown plants or that the basal levels of phytase activities among plants are similar in their ability to hydrolyze phytate. However, it is more likely that the differences in plant-exuded phytase are masked by a much greater contribution of microbial-derived phytase.105 Clarifying the capacity and condition of effective root exudation of organic acids and/or phytase benefits crop growth by increasing phytate solubilization.
Microbial Species
Root inoculation with microbes that produce organic acids helps to improve phytate solubility, thereby enhancing phytate-P acquisition by plants. Specifically, evidence shows that the symbioses of red clover -with arbuscular mycorrhizal fungi (AMF; Glomus versiforme) increase P solubilization in soils compared with nonmycorrhizal control plants, with AMF contributing 55–64% to shoot P uptake.127 The data indicate that AMF hyphae play a main role in increasing soil P similar to the roots,128 and it is critical to recruit phytate-solubilizing microbes to allow access to phytase in soils.129
Microbes that can secrete phytase have been identified via screening studies based on their abilities in utilizing phytate, homologue sequences, and protein databases.4 The methodologies for screening phytase-producing microbes have been reviewed by Hill and Richardson,130 which include both phytase positive and negative individuals. The methods for screening phytase-producing microbes (medium with phytate as the sole P source) in some cases select microbes that can solubilize (via organic acids) and/or hydrolyze (via phytase) phytate. The ability of isolated microbes in improving phytate availability has been identified. In one case, 39% Pseudomonas are negative for phytate utilization, but they become positive after citrate addition to the medium, suggesting these isolates can produce phytase to hydrolyze phytate, but their ability is hindered by limited phytate availability in soils.130
To improve phytate solubility, plant inoculants, e.g., Pseudomonas spp.,131Citrobacter sp.,132 and Pantoea sp.133 that can secrete organic acids into the rhizosphere have been found. For example, in vitro experiments show that Ca-phytate hydrolysis by phytase is improved in the presence of microbial organic acids, due to either Ca2+-mediated phytase activation or solubilization via divalent metal chelation.57,83 To increase phytate hydrolysis, plants are often inoculated with phytase-producing microbes. For example, pasture plants inoculated with phytase-producing Pseudomonas spp. increase their shoot P by 3.9-fold over control plants.120 Recombinant Pseudomonas fluorescens CHA0 and P. putida KT2440 that overexpressed Citrobacter braakii appA (HAP-like phytase) improve phytate-P utilization of mung beans by 1.2–1.5-fold.131
Microbial phytases from different microbes are different in activity but are more abundant and with higher activities than plant phytases.134 However, microbial phytase activity in soils has not been clearly linked to P nutrition. This is because microbes tend to secrete intracellular phytases, which do not play a role in extracellular phytate hydrolysis, instead being more related to cell metabolic functions. Despite this, phytase activity is often interpreted as an expression of microbial community metabolic requirements under P deficiency.135 Besides, independent of the methodology, the environmental conditions and colony structure also affect the microbial ability to solubilize and hydrolyze phytate. For example, bioaggregates of microbes can improve P release from Al-phytate precipitates.136
At present, the understanding of the role of microbes in phytate solubilization and hydrolysis, and plant P nutrition is complex and incomprehensive.119 Nevertheless, due to the large amount of phytate in soil and its potential contribution to plant P nutrition,95 much research shows AMF’s roles in improving soil phytate solubility. Specifically, they change the bacterial community structure and enhance phytate mineralization by carrying bacteria along their extraradical hyphae.113,114 As such, biotechnologies using AMF’s phytase enzymes to increase phytate bioavailability are desirable.
Plant Intercropping
Certain plants can be used in agriculture via intercropping to increase phytate availability by optimizing plants’ contribution in modifying the soil P cycle. Their interactions in the rhizosphere are evident when plants with roots exuding phytase are intercropped with plants whose roots exude organic acids.137 The benefit is greater with intimate interaction between phytase and organic acids when the roots are intermingled. For example, wheat when intercropped with white lupin shows improved phytate-P uptake and growth compared with a wheat monoculture, attributing to the ability of wheat roots to acquire more phytate-P, which is freed up by citrate from white lupin cluster roots.138 Similarly, positive effects are apparent when wheat is intercropped with chickpea or pigeon pea is intercropped with rice or sorghum.139
Changes in Plant Root Traits
Several key morphological and physiological traits associated with P-uptake efficiency have been identified. In addition to plant and microbe strategies, agronomic practices can also improve phytate-P acquisition via facilitating root growth, enabling greater access to soil phytate, and ameliorating soil acidity and subsoil compaction. For example, breeding desirable root traits including rapid root growth, extensive root branching, and long dense root hairs are feasible by identifying specific genes.105
Though it is known that these morphological features can increase phytate availability in soils, there are few successful attempts to increase the efficiency of phytate-P use by crops.105 This is largely due to the complexity of plant P-acquisition mechanisms and their responses to different environments. Further, the difficulty in identifying and selecting specific root traits in plant populations to increase P uptake, and compensatory effects of alternative mechanisms for a given environment make it difficult to implement.140
Despite these difficulties, it is possible to select enhanced specific P-acquisition processes such as selecting organic acids and/or phytase-producing genotypes to increase phytate utilization by plants or developing more phytate-efficient plants by manipulating desired traits through molecular biotechnologies.
4.2. Soil Management
Besides plant and microbial factors, phytate mobilization and mineralization are influenced by soil conditions, including pH, temperature, redox state, moisture, nutrients, and vegetation type.141 Generally, mobilization is increased under anaerobic conditions and reduced with increasing labile Pi and organic C.142 Mineralization is positively correlated with pH and temperature,143,144 while its responses to the redox state and moisture are conflicted.145
Phytase shows the highest activity at optima pH, which ranges from 2.2 in yeast (Pichia farinosa) to 5.6 in Rhizopus oligosporus and 7.5 in Bacillus subtilis and mung bean (Table 4A).112 However, pH optima can be changed within 1–2 units when phytase enters into soils. This is because at high pH, electrostatic interactions between the negatively-charged phytase and clay are repulsive. This prevents phytase adsorption, so it is free to diffuse into the soil solution and performs better activity.112 Phytate mineralization is also affected by soil pH.143,146 In 50 different British soils, phytate mineralization rates increase with soil pH from 3.9 to 7.1.143 However, phytate mineralization only increases significantly as soil pH is at 6.5 compared to 5.0–6.0.146 Moreover, phytate mineralization increases with exchangeable Ca concentration, indicating that soils developed from limestone parent material favor mineralization.143 This can be attributed to the fact that Ca improves soil structure through aggregation and promotes microbial activity.142
Temperature affects phytate mineralization by influencing microbial growth and phytase activity.142 Phytase activity peaks at 45–57 °C for Bacillus subtilis, while it decreases considerably at 80 °C and stops at 90 °C. Particularly, Aspergillus fumigatus and A. niger phytase are denatured at 50 °C (Table 4A, 4B).112,147 However, phytase from As-hyperaccumulator P. vittata shows activity after being heated at 100 °C for 10 min, indicating its extreme heat-tolerance.29 Normally, phytate mobilization and mineralization increase at temperature > 30 °C. Therefore, tropical forest soils with consistent temperature show greater mineralization than temperate forest, where phytate concentration tends to increase in winter and decrease in spring.142
The role of the redox state in phytate mineralization is complex, so the findings are inconsistent. Mineralization can occur under both aerobic and anaerobic conditions.148 For example, Dick and Tabatabai149 found greater mineralization under aerobic conditions, while Brannon and Sommers145 reported higher mineralization under anaerobic conditions. The soil redox state affects phytate mineralization via affecting microbial populations, which are active in producing phytase.
Moisture is essential for phytase production and microbe survival. Phytase activity is positively correlated with soil moisture, and optimal hydrolysis of phytate is observed at 100% saturation.142,150 For example, phytate mineralization is increased more during the wet season than the dry season; this is because moisture and nutrients stimulate microbial growth and their access to phytate.151 Nevertheless, the correlation between flooding and mineralization is complex, so the significance of moisture in phytate mineralization remains uncertain.142
In short, many factors affect phytate mobilization and mineralization in soils. They are inter-related, making the outcome difficult to predict.
4.3. Genetic Engineering
Genetic engineering can be used for plants producing limited organic acids or extracellular phytases. Plants including subterranean clover, potato, A. thaliana, and tobacco-expressed microbial phytases can release extracellular phytase to utilize phytate-P.107,131,152,153 For example, the transgenic expression of Medicago truncatula phytase gene (MtPHY1) in A. thaliana increases its root phytase activity by 12–16 fold, thereby increasing phytate-P acquisition and plant growth by 4.1–5.5 and 3.1–4.0 fold, respectively.153 Besides, the secreted phytase and the associated gene have been characterized in the proteoid roots of white lupin.154
In addition to microbial phytases, plant phytase has been expressed in plants with limited phytase secretion. For example, expressing genes encoding extracellular phytase from Indian mustard into A. thaliana improves its phytase expression and secretion from lateral roots.155 However, tobacco after expressing P. vittata phytase PvPHY1 shows different results. Though tobacco P accumulation is increased by 10–50% and its growth is enhanced by 3.5–3.9 g plant–1, tobacco plants fail to use phytate in the media.45 The data indicate that, though phytase is probably exuded into growth media by P. vittata, thereby enabling its growth with phytate-P,29 tobacco expressing PvPHY1 fails to exude phytase into the media.45 More research is needed to understand the controlling factors to make PvPHY1 extracellular phytase.
Transgenic plants with extracellular phytase can hydrolyze phytate to enhance plant P nutrition and better growth under P-deprived conditions (Table S3)4,152,156 and sand or sterile media.29,107,131 For these experiments, plants are often grown in agar media using Na-phytate as a P source. For example, tobacco plants expressing A. niger phytase (phyA; ex::phyA) show increased extracellular phytase activity and accumulate 3.7-fold more phytate-P than control plants grown in sterile agar.157 Moreover, the expressed phytase in tobacco from B. subtilis phytase (168phyA) has a higher Km than the native enzyme, maintaining unchanged thermostability and catalytic activity at 2.3 U mg–1 protein (0.038 μkat mg–1) in agar.158
However, compared to phytate in soils, which often binds to multivalent metals, Na-phytate is much more soluble, so the above results may not apply to soils. As such, when grown in soils, transgenic plants often show limited ability to access phytate-P. For example, the phytate-P utilization of transgenic tobacco overexpressing A. niger phytase (phyA) in soil conditions is similar to wild-type plants.122,157 Even in soils with greater phytase-hydrolyzable phytate and greater extracellular phytase activity, subterranean clover does not show significant advantages in P nutrition and plant growth.7 Further, tobacco grown in sand at pH 6 accumulates more P when supplemented with Mg-phytate than less-soluble Ca-phytate,108 indicating the importance of phytate solubility during phytase hydrolysis. Therefore, although extracellular secretion of phytase is increased, poor availability of the phytate substrate due to its sorption by soil still constrains its activity in soils.
5. Conclusions and Recommendations for Future Research
Phosphorus is an essential nutrient for plant growth, and its management in soil is critical to ensure sustainable agriculture while protecting the environment.1 Although soils often contain a large amount of P, only a small proportion is available to plants. For many soils, the use of P fertilizer and manure results in considerable Po accumulation, especially in the form of phytate, which is relatively unavailable to plants. This review summarizes the following: 1) the origin, abundance, forms, solubility, and availability of phytate in soils; 2) limiting steps for phytate-P utilization by plants; and 3) strategies to improve phytate-P utilization by plants. The strategies include native traits to enhance the ability of plants and microbes to secrete organic acids and/or phytase. This can be achieved by selecting specific plant genotype or microbe species, plant intercropping, and genetic engineering. Genetic engineering can develop plants with increased phytase extracellular secretion by expressing microbial or plant phytase genes. Further, information regarding the limiting steps in phytate-P plant utilization, roles of OM-associated phytate, AMF, and manure phytase, and issues during practical application remains poorly understood and needs further study.
5.1. Limiting Steps in Phytate-P Acquisition by Plants
It is unclear whether soil phytate availability and/or phytase activity is the limiting step for phytate hydrolysis and its plant utilization, so efforts to understand the associated mechanisms are needed.
In terms of phytate availability, besides phytase-hydrolyzable phytate, there are other types of phytate in soils. Research shows that phytase-hydrolyzable phytate is not correlated with the growth or P acquisition of subterranean clover after expressing A. niger phytase phyA.107,122 The limited P acquisition by plants in the presence of phytase indicates that water-soluble phytate not phytase-hydrolyzable phytate may be the phytate pool available to plants. However, water-soluble phytate is a smaller portion of phytase-hydrolyzable phytate in soils (0.7–1.9 vs 42.3–83.3 mg P kg–1) (Table 1A) and animal manures (417 vs 708–1629 mg P kg–1) (Table S1B).50,159 As such, more attention should be paid to plant available phytate to clarify what constrains its access to phytase, thereby limiting its plant utilization. Besides, studies on extraction methods for available phytate and its predictability for plant-availability are needed.
In terms of phytase activity, besides increasing native phytase activity and genetic modification to increased phytase expression and extracellular secretion, the efficiency and performance of phytase once entering soils need more attention. Research shows that, with increased native phytase activity and transgenic-expressing extracellular phytase, soil phytate-P utilization is still limited,32 suggesting reduced phytase activity in soils. Therefore, phytase catalytic adaptation to environmental conditions (e.g., soil texture, pH, temperature, and metal cations) to reduce inactivation, and the associated mechanisms need further investigations.
5.2. Further Efforts and Practical Issues
OM-Associated Phytate
Similar to orthophosphate, phytate binds not only to soil minerals but also to OM via Fe/Al bridges. As such, OM-metal-phytate complexes may be transported to the rhizosphere for hydrolysis and P uptake by plants.17 However, experimental data on P availability of OM-associated phytate are old, so there is limited information on the interaction between phytate and OM.74,75 More recently, Celi and Barberis16 proposed hydrogen and covalent bonding as the mechanism, but this has not been tested.
Phytate Utilization in Manures
Given the worldwide scarcity in phosphate rock, a raw material for producing P fertilizer, it is necessary to utilize phytate in animal manures. During the production of animal feeds, phytase is added to facilitate phytate utilization. Since animal feeds are often rich in phytate, even with added phytase, manures with high phytate enter soils as amendments. As such, approaches to enhance the agronomic use of manure-derived phytate are needed. This way, manure phytate can be hydrolyzed to P before being applied to soil where it becomes poorly available. Another method is to increase phytate availability to plants. Coupling phytate reduction in manure and phytate uptake by plants helps to reduce P runoff and contamination of waters.
Plant and Microbial Processes
The organic acids and phytase in the rhizosphere arise from both plant roots and microbes, but their relative importance in contributing P acquisition is unclear.160 Phytate utilization by plants is often based on experiments using sterile media, with results using nonsterile media being variable.4,18 The continued discovery of widespread phytate-utilizing microbes and phytase-releasing plants may help to use recalcitrant phytate in soils.29,45 Thus, contributions of phytase from plants and microbes and their efficiency in different environments need further research.
In addition, soil microbes are an integral component of the soil P cycle, so they play important roles in phytate transformation and hydrolysis. Still, the relative importance of microbial processes to use phytate and the interaction of different microbes (e.g., AMF and bacteria) with plant roots in facilitating phytate-P utilization need further elucidation.
Practical Application
From an application perspective, there are issues and challenges regarding these agronomic, plant, microbe, and molecular strategies to effectively utilize soil phytate.
For phytate solubilization mediated by organic acids, the challenge is whether it can be exploited to better intercept soluble phytate in competition with its fixation in soils. For phytase-mediated hydrolysis, when soils are limed to elevated pH, the benefit of phytase exudation may be reduced due to decreased phytase activity under alkaline conditions (optima at pH 2.5 and 5.0) and phytate precipitation with metal cations such as Ca and Mg. Besides, phytate and phytase are readily sorbed by soils, so the relationship between the concentrations of organic acids and activities of phytase with the amount of phytate-P that can be taken up by plants needs to be established. In this case, correlation indexes based on soil parameters, organic acid-dependent solubilization, phytase-dependent hydrolysis, and plant availability of different phytates can be incorporated into mathematical models to better evaluate phytate utilization potential by plants.
For agronomic practices to improve plant phytate-P acquisition, whether these options are practical for different agricultural systems remains to be determined. For genetic modification, transgenic plants need to be evaluated for their ability to access insoluble phytate in soils, which is often associated with metals and/or OM.
In short, plant- and microbe-based approaches have the potential to increase phytate-P utilization by plants. This is particularly relevant for organic farming where the use of soluble-P fertilizers is restricted by industry rules. Therefore, more research is needed for effective phytate-P acquisition by plants via developing plants that can secrete organic acids and/or synthesize phytase, which resist sorption to soils or retain activity when sorbed onto soils.
Acknowledgments
This work was supported in part by the National Key Research and Development Program (2018YFC1800504), the National Natural Science Foundation of China (41867066, 41907129), the Yunnan Outstanding Foreign Expert Project (YNQR-GDWG-2018-017), and the Yunnan Innovation Team Project (202005AE160017).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.2c00099.
Table S1, Po fractions and 25 EDTA-extractable phytase-hydrolyzable P; Table S2, summary of known plant secreted organic acids to mobilize soil P; and Table S3, transgenic plant or yeast, phytase gene source, and expressed phytase activity and properties (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Fang L.; Wang Q.; Li J. S.; Poon C. S.; Cheeseman C. R.; Donatello S.; Tsang D. C. W. Feasibility of wet-extraction of phosphorus from incinerated sewage sludge ash (ISSA) for phosphate fertilizer production: A critical review. Crit. Rev. Environ. Sci. Technol. 2021, 51 (9), 939–971. 10.1080/10643389.2020.1740545. [DOI] [Google Scholar]
- Stutter M. I.; Shand C. A.; George T. S.; Blackwell M. S. A.; Dixon L.; Bol R.; MacKay R. L.; Richardson A. E.; Condron L. M.; Haygarth P. M. Land use and soil factors affecting accumulation of phosphorus species in temperate soils. Geoderma 2015, 257–258, 29–39. 10.1016/j.geoderma.2015.03.020. [DOI] [Google Scholar]
- Sattari S. Z.; Bouwman A. F.; Giller K. E.; van Ittersum M. K. Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 6348–6353. 10.1073/pnas.1113675109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes-Blackburn D.; Jorquera M. A.; Greiner R.; Gianfreda L.; de la Luz Mora M. Phytases and phytase-labile organic phosphorus in manures and soils. Crit. Rev. Environ. Sci. Technol. 2013, 43 (9), 916–954. 10.1080/10643389.2011.627019. [DOI] [Google Scholar]
- Gerke J. Phytate (inositol hexakisphosphate) in soil and phosphate acquisition from inositol phosphates by higher plants. A Review. Plants 2015, 4 (2), 253–266. 10.3390/plants4020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condron L. M.; Turner B. L.; Cade-Menun B. J.. Chemistry and dynamics of soil organic phosphorus. Phosphorus: Agriculture and the Environment, Agronomy Monograph; American Society of Agronomy: Madison, USA, 2005; pp 87–121 10.2134/agronmonogr46.c4. [DOI] [Google Scholar]
- George T. S.; Simpson R. J.; Hadobas P. A.; Marshall D. J.; Richardson A. E. Accumulation and phosphatase-lability of organic phosphorus in fertilised pasture soils. Aust. J. Agr. Res. 2007, 58 (1), 47. 10.1071/AR06167. [DOI] [Google Scholar]
- Richardson A. E.; George T. S.; Jakobsen I.; Simpson R. J.. Plant utilization of inositol phosphates. In Inositol phosphates: linking agriculture and the environment; Turner B. L., Richardson A. E., Mullaney E. J., Eds.; CABI: Wallingford, UK, 2007; pp 242–260 10.1079/9781845931520.0242. [DOI] [Google Scholar]
- Godfray H. C. J.; Beddington J. R.; Crute I. R.; Haddad L.; Lawrence D.; Muir J. F.; Pretty J.; Robinson S.; Thomas S. M.; Toulmin C. Food security: the challenge of feeding. Science 2010, 327, 812–818. 10.1126/science.1185383. [DOI] [PubMed] [Google Scholar]
- Shears S.; Turner B. L.. Nomenclature and terminology of inositol phosphates: clarification and a glossary of terms. In Turner B. L., Richardson A. E., Mullaney E. J., Eds.; Inositol Phosphates: Linking Agriculture and the Environment; CAB International: Wallingford, UK, 2007; pp 1–7 10.1079/9781845931520.0001. [DOI] [Google Scholar]
- Turner B. L.; Paphazy M. J.; Haygarth P. M.; McKelvie I. D. Inositol phosphates in the environment. Philos. Trans. R. Soc., B 2002, 357 (1420), 449–469. 10.1098/rstb.2001.0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B. L.; Cheesman A. W.; Godage H. Y.; Riley A. M.; Potter B. V. Determination of neo- and D-chiro-inositol hexakisphosphate in soils by solution 31P NMR spectroscopy. Environ. Sci. Technol. 2012, 46 (9), 4994–5002. 10.1021/es204446z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner B. L.; Richardson A. E.; Mullaney E.. Inositol Phosphates: linking agriculture and the environment; CAB International: Wallingford, England, 2007. [Google Scholar]
- Yan Y.; Li W.; Yang J.; Zheng A.; Liu F.; Feng X.; Sparks D. L. Mechanism of myo-inositol hexakisphosphate sorption on amorphous aluminum hydroxide: spectroscopic evidence for rapid surface precipitation. Environ. Sci. Technol. 2014, 48 (12), 6735–6742. 10.1021/es500996p. [DOI] [PubMed] [Google Scholar]
- Gerke J. Humic (organic matter)-Al(Fe)-phosphate complexes: an underestimated phosphate form in soils and source of plant-available phosphate. Soil Sci. 2010, 175 (9), 417–425. 10.1097/SS.0b013e3181f1b4dd. [DOI] [Google Scholar]
- Celi L.; Barberis E.. Abiotic reaction of inositol phosphates in soil. In Inositol Phosphates: Linking Agriculture and the Environment ;Turner B. L., Richardson A. E., Mullaney E. J., Eds.; CABI:Wallingford, UK, 2007; pp 207–220 10.1079/9781845931520.0207. [DOI] [Google Scholar]
- Turner B. L.Inositol phosphates in soil: amounts, forms and significance of the phosphorylated inositol stereoisomers. Inositol phosphates: Linking agriculture and the environment; 2007; pp 196–206 10.1079/9781845931520.0186. [DOI] [Google Scholar]
- Hayes J. E.; Simpson R. J.; Richardson A. E. The growth and phosphorus utilisation of plants in sterile media when supplied with inositol hexaphosphate, glucose 1-phosphate or inorganic phosphate. Plant Soil 2000, 220 (1–2), 165–174. 10.1023/A:1004782324030. [DOI] [Google Scholar]
- Feil B. Phytic Acid. New Seeds 2001, 3 (3), 1–35. 10.1300/J153v03n03_01. [DOI] [Google Scholar]
- Lambers H.; Yuan L.; Liu X. Highlights of special issue on ″sustainable phosphorus use in agri-food system″. Front. Agr. Sci. Eng. 2019, 6 (4), 311–312. 10.15302/J-FASE-2019285. [DOI] [Google Scholar]
- Stutter M. I.; Richards S. Relationships between soil physicochemical, microbiological properties, and nutrient release in buffer soils compared to field soils. J. Environ. Qual. 2012, 41 (2), 400–410. 10.2134/jeq2010.0456. [DOI] [PubMed] [Google Scholar]
- Smith F. W.; Mudge S. R.; Rae A. L.; Glassop D. Phosphate transport in plants. Plant Soil 2003, 248, 71–83. 10.1023/A:1022376332180. [DOI] [Google Scholar]
- Raghothama K. G.; Karthikeyan A. S. Phosphate acquisition. Plant Soil 2005, 274, 37–49. 10.1007/s11104-004-2005-6. [DOI] [Google Scholar]
- Gerke J. The acquisition of phosphate by higher plants: Effect of carboxylate release by the roots. A critical review. J. Plant Nutr. Soil Sci. 2015, 178 (3), 351–364. 10.1002/jpln.201400590. [DOI] [Google Scholar]
- Turner B. L. Resource partitioning for soil phosphorus: a hypothesis. J. Ecol. 2008, 96 (4), 698–702. 10.1111/j.1365-2745.2008.01384.x. [DOI] [Google Scholar]
- Steidinger B. S.; Turner B. L.; Corrales A.; Dalling J. W.; Briones M. J. Variability in potential to exploit different soil organic phosphorus compounds among tropical montane tree species. Funct. Ecol. 2015, 29 (1), 121–130. 10.1111/1365-2435.12325. [DOI] [Google Scholar]
- Darch T.; Blackwell M. S.; Chadwick D.; Haygarth P. M.; Hawkins J. M.; Turner B. L. Assessment of bioavailable organic phosphorus in tropical forest soils by organic acid extraction and phosphatase hydrolysis. Geoderma 2016, 284, 93–102. 10.1016/j.geoderma.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes J. E.; Richardson A. E.; Simpson R. J. Phytase and acid phosphatase activities in extracts from roots of temperate pasture grass and legume seedlings. Aust. J. Plant Physiol. 1999, 26, 801–809. 10.1071/PP99065. [DOI] [Google Scholar]
- Lessl J. T.; Ma L. Q.; Rathinasabapathi B.; Guy C. Novel phytase from Pteris vittata resistant to arsenate, high temperature, and soil deactivation. Environ. Sci. Technol. 2013, 47 (5), 2204–2211. 10.1021/es3022073. [DOI] [PubMed] [Google Scholar]
- Richardson A. E.; Hadobas P. A. Soil isolates of Pseudomonas spp. that utilize inositol phosphates. Can. J. Microbiol. 1997, 43 (6), 509–516. 10.1139/m97-073. [DOI] [PubMed] [Google Scholar]
- Hayes J. E.; Richardson A. E.; Simpson R. J. Components of organic phosphorus in soil extracts that are hydrolysed by phytase and acid phosphatase. Biol. Fert. Soils 2000, 32 (4), 279–286. 10.1007/s003740000249. [DOI] [Google Scholar]
- George T. S.; Simpson R. J.; Gregory P. J.; Richardson A. E. Differential interaction of Aspergillus niger and Peniophora lycii phytases with soil particles affects the hydrolysis of inositol phosphates. Soil Biol. Biochem. 2007, 39 (3), 793–803. 10.1016/j.soilbio.2006.09.029. [DOI] [Google Scholar]
- Zhang H.; Dao T. H.; Basta N. T.; Dayton E. A.; Daniel T. C.. Remediation techniques for manure nutrient loaded soils. Animal Agriculture and the Environment: National Center for Manure and Animal Waste Management White Papers; Rice J. M., Caldwell D. F., Humenik F. J., Eds.; St. Joseph, Michigan: ASABE. Pub.: 2006; pp 482–504 10.13031/2013.20263. [DOI] [Google Scholar]
- Roelofs R. F. R.; Rengel Z.; Cawthray G. R.; Dixon K. W.; Lambers H. Exudation of carboxylates in Australian Proteaceae: chemical composition. Plant Cell Environ. 2001, 24 (9), 891–903. 10.1046/j.1365-3040.2001.00741.x. [DOI] [Google Scholar]
- Veneklaas E. J.; Stevens J.; Cawthray G. R.; Turner S.; Grigg A. M.; Lambers H. Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 2003, 248 (1–2), 187–197. 10.1023/A:1022367312851. [DOI] [Google Scholar]
- Cade-Menun B. J.; Preston C. M. A comparison of soil extraction procedures for 31P NMR spectroscopy. Soil Sci. 1996, 161, 770–785. 10.1097/00010694-199611000-00006. [DOI] [Google Scholar]
- Cordell D.; Drangert J. O.; White S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19 (2), 292–305. 10.1016/j.gloenvcha.2008.10.009. [DOI] [Google Scholar]
- Kleinman P.; Sharpley A.; Buda A.; McDowell R. W.; Allen A. Soil controls of phosphorus in runoff: Management barriers and opportunities. Can. J. Soil Sci. 2011, 91 (3), 329–338. 10.4141/cjss09106. [DOI] [Google Scholar]
- George T. S.; Quiquampoix H.; Simpson R.; Richardson A.. Interactions between phytases and soil constituents: Implications for the hydrolysis of inositol phosphates. In Turner B., Richardson A., Mullaney E., Eds.; Inositol Phosphates: Linking Agriculture and the Environment; CABI: Oxfordshire, UK, 2007; pp 221–241 10.1079/9781845931520.0221. [DOI] [Google Scholar]
- Giles C. D.; Hsu P. C.; Richardson A. E.; Hurst M. R. H.; Hill J. E. Plant assimilation of phosphorus from an insoluble organic form is improved by addition of an organic anion producing Pseudomonas sp. Soil Biol. Biochem. 2014, 68, 263–269. 10.1016/j.soilbio.2013.09.026. [DOI] [Google Scholar]
- Lott J. N. A.; Ockenden I.; Raboy V.; Batten G. D. Phytic acid and phosphorus in crop seeds and fruits: a global estimate. Seed Sci. Res. 2000, 10 (1), 11–33. 10.1017/S0960258500000039. [DOI] [Google Scholar]
- Lee Y. S.; Huang K.; Quiocho F. A.; O’Shea E. K. Molecular basis of cyclin-CDK-CKI regulation by reversible binding of an inositol pyrophosphate. Nat. Chem. Biol. 2008, 4 (1), 25–32. 10.1038/nchembio.2007.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn L.; Meyer A. S.; Rasmussen S. K. Phytate: impact on environment and human nutrition. A challenge for molecular breeding. J. Zhejiang Univ. Sci. B 2008, 9 (3), 165–191. 10.1631/jzus.B0710640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinch-Pedersen H.; Sorensen L. D.; Holm P. B. Engineering crop plants: getting a handle on phosphate. Trends Plant Sci. 2002, 7, 118–125. 10.1016/S1360-1385(01)02222-1. [DOI] [PubMed] [Google Scholar]
- Sun D.; Zhang W.; Feng H.; Li X.; Han R.; Turner B. L.; Qiu R.; Cao Y.; Ma L. Q. Novel phytase PvPHY1 from the As-hyperaccumulator Pteris vittata enhances P uptake and phytate hydrolysis, and inhibits As translocation in Plant. J. Hazard. Mater. 2022, 423, 127106. 10.1016/j.jhazmat.2021.127106. [DOI] [PubMed] [Google Scholar]
- Fuentes B.; Bolan N.; Naidu R.; Mora M. Phosphorus in organic waste soil systems. J. Soil Sci. Plant Nutr. 2006, 6, 64–83. 10.4067/S0718-27912006000200006. [DOI] [Google Scholar]
- Leytem A. B.; Thacker P. A.; Turner B. L. Phosphorus characterization in feces from broiler chicks fed low-phytate barley diets. J. Sci. Food Agr. 2007, 87 (8), 1495–1501. 10.1002/jsfa.2865. [DOI] [Google Scholar]
- Bünemann E. K. Enzyme additions as a tool to assess the potential bioavailability of organically bound nutrients. Soil Biol. Biochem. 2008, 40 (9), 2116–2129. 10.1016/j.soilbio.2008.03.001. [DOI] [Google Scholar]
- He Z. Q.; Olk D. C.; Honeycutt C. W.; Fortuna A. M. Enzymatically and ultraviolet-labile phosphorus in humic acid fractions from rice soils. Soil Sci. 2009, 174 (2), 81–87. 10.1097/SS.0b013e3181981dc5. [DOI] [Google Scholar]
- He Z. Q.; Waldrip H. W.; Honeycutt C. W.; Erich M. S.; Senwo Z. N. Enzymatic quantification of phytate in animal manure. Commu. Soil Sci. Plant Ana. 2009, 40 (1–6), 566–575. 10.1080/00103620802647116. [DOI] [Google Scholar]
- Borie F.; Rubio R. Total and organic phosphorus in Chilean volcanic soils. Gayana Bot. 2003, 60, 69–73. 10.4067/S0717-66432003000100011. [DOI] [Google Scholar]
- He Z.; Griffin T. S.; Honeycutt C. W. Enzymatic hydrolysis of organic phosphorus in swine manure and soil. J. Environ. Qual. 2004, 33 (1), 367. 10.2134/jeq2004.3670. [DOI] [PubMed] [Google Scholar]
- Dao T. Ligands and phytase hydrolysis of organic phosphorus in soils amended with dairy manure. Agron. J. 2004, 96, 1188–1195. 10.2134/agronj2004.1188. [DOI] [Google Scholar]
- Celi L.; Barberis E.. Abiotic stabilization of organic phosphorus in the environment. InOrganic Phosphorus in the Environment; Turner B. L., Frossard E., Baldwin D. S., Eds.; CABI: Oxfordshire, UK, 2005; pp 113–132 10.1079/9780851998220.0113. [DOI] [Google Scholar]
- Urrutia O.; Erro J.; Guardado I.; San Francisco S.; Mandado M.; Baigorri R.; Claude Yvin J.; Ma Garcia-Mina J. Physico-chemical characterization of humic-metal-phosphate complexes and their potential application to the manufacture of new types of phosphate-based fertilizers. J. Plant Nutr. Soil Sci. 2014, 177 (2), 128–136. 10.1002/jpln.201200651. [DOI] [Google Scholar]
- Berg A. S.; Joern B. C. Sorption dynamics of organic and inorganic phosphorus compounds in soil. J. Environ. Qual. 2006, 35 (5), 1855–1862. 10.2134/jeq2005.0420. [DOI] [PubMed] [Google Scholar]
- Dao T.Ligand effects on inositol phosphate solubility and bioavailability in animal manures. In Turner B. L., Richardson A. E., Mullaney E. J., Eds.; Inositol phosphates: Linking agriculture and the environment; CABI: Cambridge, MA, USA, 2007; pp 169–185 10.1079/9781845931520.0169. [DOI] [Google Scholar]
- Berry D. F.; Shang C.; Waltham Sajdak C. A.; Zelazny L. W. Measurement of phytase activity using tethered phytic acid as an artificial substrate: Methods development. Soil Biol. Biochem. 2007, 39 (1), 361–367. 10.1016/j.soilbio.2006.06.010. [DOI] [Google Scholar]
- Celi L.; Lamacchia S.; Barberis E. Interaction of inositol phosphate with calcite. Nutr. Cycling Agroecosyst. 2000, 57, 271–277. 10.1023/A:1009805501082. [DOI] [Google Scholar]
- Anderson G.; Arlidge E. Z. The adsorption of inositol phosphates and glycerophosphate by soil clays, clay minerals, and hydrated sesquioxides in acid media. Eur. J. Soil Biol. 1962, 13, 216–224. 10.1111/j.1365-2389.1962.tb00699.x. [DOI] [Google Scholar]
- Martin M.; Celi L.; Barberis E. Desorption and plant availability of myo-inositol hexaphosphate adsorbed on goethite. Soil Sci. 2004, 169 (2), 115–124. 10.1097/01.ss.0000117787.98510.9d. [DOI] [Google Scholar]
- Johnson B. B.; Quill E.; Angove M. J. An investigation of the mode of sorption of inositol hexaphosphate to goethite. J. Colloid Interface Sci. 2012, 367 (1), 436–442. 10.1016/j.jcis.2011.09.066. [DOI] [PubMed] [Google Scholar]
- Yan Y. P.; Wan B.; Liu F.; Tan W. F.; Liu M. M.; Feng X. H. Adsorption-desorption of myo-inositol hexakisphosphate on hematite. Soil Sci. 2014, 179 (10–11), 476–485. 10.1097/SS.0000000000000091. [DOI] [Google Scholar]
- Chen A.; Arai Y. Functional group specific phytic acid adsorption at the ferrihydrite-water interface. Environ. Sci. Technol. 2019, 53 (14), 8205–8215. 10.1021/acs.est.9b01511. [DOI] [PubMed] [Google Scholar]
- Guan X. H.; Shang C.; Zhu J.; Chen G. H. ATR-FTIR investigation on the complexation of myo-inositol hexaphosphate with aluminum hydroxide. J. Colloid Interface Sci. 2006, 293 (2), 296–302. 10.1016/j.jcis.2005.06.070. [DOI] [PubMed] [Google Scholar]
- Ruyter-Hooley M.; Larsson A. C.; Johnson B. B.; Antzutkin O. N.; Angove M. J. Surface complexation modeling of inositol hexaphosphate sorption onto gibbsite. J. Colloid Interface Sci. 2015, 440, 282–291. 10.1016/j.jcis.2014.10.065. [DOI] [PubMed] [Google Scholar]
- Wang X.; Hu Y.; Tang Y.; Yang P.; Feng X.; Xu W.; Zhu M. Phosphate and phytate adsorption and precipitation on ferrihydrite surfaces. Environ. Sci.: Nano 2017, 4 (11), 2193–2204. 10.1039/C7EN00705A. [DOI] [Google Scholar]
- Celi L.; Lamacchia S.; Marsan F. A.; Barberis E. Interaction of inositol hexaphosphate on clays: Adsorption and charging phenomena. Soil Sci. 1999, 164, 574–585. 10.1097/00010694-199908000-00005. [DOI] [Google Scholar]
- House W. A.; Denison F. H. Total phosphorus content of river sediments in relationship to calcium, iron and organic matter concentrations. Sci. Total Environ. 2002, 282–283, 341–351. 10.1016/S0048-9697(01)00923-8. [DOI] [PubMed] [Google Scholar]
- Chen A.; Li Y.; Shang J.; Arai Y. Ferrihydrite transformation impacted by coprecipitation of phytic acid. Environ. Sci. Technol. 2020, 54 (14), 8837–8847. 10.1021/acs.est.0c02465. [DOI] [PubMed] [Google Scholar]
- McKercher R. B.; Anderson G. Organic phosphate sorption by neutral and basic soils. Soil Sci. Plant Anal. 1989, 20 (7–8), 723–732. 10.1080/00103628909368112. [DOI] [Google Scholar]
- Negrin M. A.; Gonzalez-Carcedo S.; Hernandez-Moreno J. M. P fractionation in sodium bicarbonate extracts of andic soils. Soil Biol. Biochem. 1995, 27, 761–766. 10.1016/0038-0717(94)00237-U. [DOI] [Google Scholar]
- Gerke J.; Herrmann R. Adsorption of orthophosphate to humic-Fe complexes and to amorphous Fe-oxide. J. Plant Nutr. Soil Sci. 1992, 155, 233–236. 10.1002/jpln.19921550313. [DOI] [Google Scholar]
- Moyer J.; Thomas R. L. Organic phosphorus and inositol phosphates in molecular size fractions of a soil organic matter extract. Soil Sci. Soc. Am. Proc. 1970, 34, 80–84. 10.2136/sssaj1970.03615995003400010024x. [DOI] [Google Scholar]
- Veinot R.; Thomas R. L. High molecular weight organic phosphorus complexes in soil organic matter: Inositol and metal content of various fractions. Soil Sci. Soc. Am. Proc. 1972, 36, 71–73. 10.2136/sssaj1972.03615995003600010016x. [DOI] [Google Scholar]
- Harrison A. F.Soil organic phosphorus. A review of world literature; CAB International: Oxford, 1987. [Google Scholar]
- Celi L.; Presta M.; Ajmore-Marsan F.; Barberis E. Effects of pH and electrolytes on inositol hexaphosphate interaction with goethite. Soil Sci. Soc. Am. J. 2001, 65, 753–760. 10.2136/sssaj2001.653753x. [DOI] [Google Scholar]
- Xu S.; Chen A.; Arai Y. Solution 31P NMR investigation of inositol hexakisphosphate surface complexes at the amorphous aluminum oxyhydroxide-water interface. Environ. Sci. Technol. 2021, 55 (21), 14628–14638. 10.1021/acs.est.1c04421. [DOI] [PubMed] [Google Scholar]
- Crea F.; De Stefano C.; Milea D.; Sammartano S. Formation and stability of phytate complexes in solution. Coordin. Chem. Rev. 2008, 252 (10–11), 1108–1120. 10.1016/j.ccr.2007.09.008. [DOI] [Google Scholar]
- Turner B. L.; Mahieu N.; Condron L. M.; Chen C. R. Quantification and bioavailability of scyllo-inositol hexakisphosphate in pasture soils. Soil Biol. Biochem. 2005, 37 (11), 2155–2158. 10.1016/j.soilbio.2005.03.005. [DOI] [Google Scholar]
- Mullaney E. J.; Ullah A. H. J. The term phytase comprises several different classes of enzymes. Biochem. Biop. Res. Co. 2003, 312 (1), 179–184. 10.1016/j.bbrc.2003.09.176. [DOI] [PubMed] [Google Scholar]
- Shand C. A.; Macklon A. E. S.; Edwards A. C.; Smith S. Inorganic and organic P in soil solutions from three upland soils. I. Effects of soil solution extraction conditions, soil type and season. Plant Soil 1994, 159, 255–264. 10.1007/BF00009288. [DOI] [Google Scholar]
- Tang J.; Leung A.; Leung C.; Lim B. L. Hydrolysis of precipitated phytate by three distinct families of phytases. Soil Biol. Biochem. 2006, 38 (6), 1316–1324. 10.1016/j.soilbio.2005.08.021. [DOI] [Google Scholar]
- Gerke J.; Römer W.; Beißner L. The quantitative effect of chemical phosphate mobilization by carboxylate anions on P uptake by a single root. II. The importance of soil and plant parameters for uptake of mobilized P. J. Plant Nutr. Soil Sci. 2000, 163 (2), 213–219. . [DOI] [Google Scholar]
- Richardson A. E.; Lynch J. P.; Ryan P. R.; Delhaize E.; Smith F. A.; Smith S. E.; Harvey P. R.; Ryan M. H.; Veneklaas E. J.; Lambers H.; Oberson A.; Culvenor R. A.; Simpson R. J. Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 2011, 349 (1–2), 121–156. 10.1007/s11104-011-0950-4. [DOI] [Google Scholar]
- Menezes-Blackburn D.; Paredes C.; Zhang H.; Giles C. D.; Darch T.; Stutter M.; George T. S.; Shand C.; Lumsdon D.; Cooper P.; Wendler R.; Brown L.; Blackwell M.; Wearing C.; Haygarth P. M. Organic acids regulation of chemical-microbial phosphorus transformations in soils. Environ. Sci. Technol. 2016, 50 (21), 11521–11531. 10.1021/acs.est.6b03017. [DOI] [PubMed] [Google Scholar]
- Boudot J. P. Relative efficiency of complexed aluminum, noncrystalline Al hydroxide, allophane and imogolite in retarding the biodegradation of citric acid. Geoderma 1992, 52, 29–39. 10.1016/0016-7061(92)90073-G. [DOI] [Google Scholar]
- Gerke J.; Beißner L.; Römer W. The quantitative effect of chemical phosphate mobilization by carboxylate anions on P uptake by a single root. I. The basic concept and determination of soil parameters. J. Plant Nutr. Soil Sci. 2000, 163 (2), 207–212. . [DOI] [Google Scholar]
- Nuruzzaman M.; Lambers H.; Bolland M. D. A.; Veneklaas E. J. Phosphorus benefits of different legume crops to subsequent wheat grown in different soils of Western Australia. Plant Soil 2005, 271 (1–2), 175–187. 10.1007/s11104-004-2386-6. [DOI] [Google Scholar]
- Römer W.; Kang D. K.; Egle K.; Gerke J.; Keller H. The acquisition of cadmium by Lupinus albus L., Lupinus angustifolius L. and Lolium multiflorum Lam. J. Plant Nutr. Soil Sci. 2000, 163, 623–628. . [DOI] [Google Scholar]
- Wouterlood M.; Cawthray G. R.; Scanlon T. T.; Lambers H.; Veneklaas E. J. Carboxylate concentrations in the rhizosphere of lateral roots of chickpea (Cicer arietinum) increase during plant development, but are not correlated with phosphorus status of soil or plants. New Phytol. 2004, 162 (3), 745–753. 10.1111/j.1469-8137.2004.01070.x. [DOI] [PubMed] [Google Scholar]
- Steffens D.; Leppin T.; Luschin-Ebengreuth N.; Yang Z. M.; Schubert S. Organic soil phosphorus considerably contributes to plant nutrition but is neglected by routine soil-testing methods. J. Plant Nutr. Soil Sci. 2010, 173 (5), 765–771. 10.1002/jpln.201000079. [DOI] [Google Scholar]
- Pearse S. J.; Venaklaas E. J.; Cawthray G.; Bolland M. D. A.; Lambers H. Rhizosphere processes do not explain variation in P acquisition from sparingly soluble forms among Lupinus albus accessions. Aust. J. Agr. Res. 2008, 59, 616–623. 10.1071/AR07404. [DOI] [Google Scholar]
- Liu X.; Fu J. W.; Guan D. X.; Cao Y.; Luo J.; Rathinasabapathi B.; Chen Y.; Ma L. Q. Arsenic induced phytate exudation, and promoted FeAsO4 dissolution and plant growth in As-hyperaccumulator Pteris vittata. Environ. Sci. Technol. 2016, 50 (17), 9070–9077. 10.1021/acs.est.6b00668. [DOI] [PubMed] [Google Scholar]
- Lei X.; Porres J.; Mullaney E.; Brinch-Pedersen H.. Phytase: source, structure and application. Industrial enzymes: Structure, function and applications; Springer: Dordrecht, the Netherlands, 2007; pp 505–529 10.1007/1-4020-5377-0_29. [DOI] [Google Scholar]
- Dionisio G.; Madsen C. K.; Holm P. B.; Welinder K. G.; Jorgensen M.; Stoger E.; Arcalis E.; Brinch-Pedersen H. Cloning and characterization of purple acid phosphatase phytases from wheat, barley, maize, and rice. Plant Physiol. 2011, 156 (3), 1087–1100. 10.1104/pp.110.164756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman C. E.; Grabau E. A novel phytase with sequence similarity to purple acid phosphatases is expressed in cotyledons of germinating soybean seedlings. Plant Physiol. 2001, 126, 1598–1608. 10.1104/pp.126.4.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A.; Borchers T.; Marcus K.; Meyer H. E.; Krebs B.; Spener F. Heterologous expression and characterization of recombinant purple acid phosphatase from red kidney bean. Arch. Biochem. Biophys. 2002, 401 (2), 164–172. 10.1016/S0003-9861(02)00046-2. [DOI] [PubMed] [Google Scholar]
- Mckelvie I. D.; Hart B. T.; Cardwell T. J.; Cattrall R. W. Use of immobilized 3-phytase and flow injection for the determination of phosphorus species in natural waters. Anal. Chim. Acta 1995, 316 (3), 277–289. 10.1016/0003-2670(95)00373-8. [DOI] [Google Scholar]
- Leprince F.; Quiquampoix H. Extracellular enzyme activity in soil: effect of pH and ionic strength on the interaction with montmorillonite of two acid phosphatases secreted by the ectomycorrhizal fungus Hebeloma cylindrosporum. Eur. J. Soil Sci. 1996, 47 (4), 511–522. 10.1111/j.1365-2389.1996.tb01851.x. [DOI] [Google Scholar]
- Duff S. M. G.; Sarath G.; Plaxton W. C. The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plantarum 1994, 90, 791–800. 10.1111/j.1399-3054.1994.tb02539.x. [DOI] [Google Scholar]
- Tarafdar J. C.; Claassen N. Organic phosphorus utilization by wheat plants under sterile conditions. Biol. Fert. Soils 2003, 39 (1), 25–29. 10.1007/s00374-003-0671-9. [DOI] [Google Scholar]
- Richardson A. E.; Hadobas P. A.; Hayes J. E. Acid phosphomonoeaterase and phytase activities of wheat roots and utilization of organic phosphorus substrates by seedings grown in sterile culture. Plant Cell Environ. 2000, 23, 397–405. 10.1046/j.1365-3040.2000.00557.x. [DOI] [Google Scholar]
- George T. S.; Gregory P. J.; Hocking P. J.; Richardson A. E. Variation in root-associated phosphatase activities in wheat contributes to the utilization of organic P substrates in vitro, but does not explain differences in the P-nutrition of plants when grown in soils. Environ. Exp. Bot. 2008, 64, 239–249. 10.1016/j.envexpbot.2008.05.002. [DOI] [Google Scholar]
- Richardson A. E.; Hocking P. J.; Simpson R. J.; George T. S. Plant mechanisms to optimize access to soil phosphorus. Crop Pasture Sci. 2009, 60 (2), 124–143. 10.1071/CP07125. [DOI] [Google Scholar]
- Chen C. R.; Condron L. M.; Davis M. R.; Sherlock R. R. Phosphorus dynamics in the rhizosphere of perennial ryegrass (Lolium perenne L.) and radiata pine (Pinus radiata D. Don.). Soil Biol. Biochem. 2002, 34, 487–499. 10.1016/S0038-0717(01)00207-3. [DOI] [Google Scholar]
- George T. S.; Richardson A. E.; Hadobas P. A.; Simpson R. J. Characterization of transgenic Trifolium subterraneum L. which expresses phyA and releases extracellular phytase: growth and P nutrition in laboratory media and soil. Plant Cell Environ. 2004, 27 (11), 1351–1361. 10.1111/j.1365-3040.2004.01225.x. [DOI] [Google Scholar]
- Lung S. C.; Lim B. L. Assimilation of phytate-phosphorus by the extracellular phytase activity of tobacco (Nicotiana tabacum) is affected by the availability of soluble phytate. Plant Soil 2006, 279 (1–2), 187–199. 10.1007/s11104-005-1009-1. [DOI] [Google Scholar]
- Tarafdar J. C.; Yadav R. S.; Meena S. C. Comparative efficiency of acid phosphatase originated from plant and fungal sources. J. Plant Nutr. Soil Sci. 2001, 164, 279–282. . [DOI] [Google Scholar]
- Belinque H.; Pucheu N.; Kerber N.; Rubio G. Utilization of organic phosphorus sources by oilseed rape, sunflower, and soybean. J. Plant Nutr. Soil Sci. 2015, 178 (2), 339–344. 10.1002/jpln.201400301. [DOI] [Google Scholar]
- Tarafdar J. C.; Claassen N. Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatases produced by plant roots and microorganisms. Biol. Fertil. Soils 1998, 5, 308–312. 10.1007/BF00262137. [DOI] [Google Scholar]
- Quiquampoix H.; Mousain D.. Enzymatic hydrolysis of organic phosphorus. InOrganic Phosphorus in the Environment; Turner B. L., Frossard E., Baldwin D. S., Eds.; CABI:Wallingford, UK, 2004; pp 89–112 10.1079/9780851998220.0089. [DOI] [Google Scholar]
- Zhang L.; Shi N.; Fan J.; Wang F.; George T. S.; Feng G. Arbuscular mycorrhizal fungi stimulate organic phosphate mobilization associated with changing bacterial community structure under field conditions. Environ. Microbiol. 2018, 20 (7), 2639–2651. 10.1111/1462-2920.14289. [DOI] [PubMed] [Google Scholar]
- Jiang F.; Zhang L.; Zhou J.; George T. S.; Feng G. Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytol. 2021, 230 (1), 304–315. 10.1111/nph.17081. [DOI] [PubMed] [Google Scholar]
- Adams M. A.; Pate J. S. Availability of organic and inorganic forms of phosphorus to lupins (Lupinus spp.). Plant Soil 1992, 145 (1), 107–113. 10.1007/BF00009546. [DOI] [Google Scholar]
- Tarafdar J. C.; Jungk A. Phosphatase activity in the rhizosphere and its relation to the depletion of soil organic phosphorus. Biol. Fertil. Soils 1987, 3, 199–204. 10.1007/BF00640630. [DOI] [Google Scholar]
- Tarafdar J. C.; Claassen N. Organic phosphorus compounds as a phosphorus source for higher plants through the activity of phosphatases produced by plant roots and microorganisms. Biol. Fert. Soils 1988, 5 (4), 308–312. 10.1007/BF00262137. [DOI] [Google Scholar]
- Giaveno C.; Celi L.; Richardson A. E.; Simpson R. J.; Barberis E. Interaction of phytases with minerals and availability of substrate affect the hydrolysis of inositol phosphates. Soil Biol. Biochem. 2010, 42 (3), 491–498. 10.1016/j.soilbio.2009.12.002. [DOI] [Google Scholar]
- Jorquera M. A.; Martínez O.; Maruyama F.; Marschner P.; de la Luz Mora M. Current and future biotechnological applications of bacterial phytases and phytase-producing bacteria. Microbes Environ. 2008, 23 (3), 182–191. 10.1264/jsme2.23.182. [DOI] [PubMed] [Google Scholar]
- Richardson A. E.; Hadobas P. A.; Hayes J. E.; O’hara C. P.; Simpson R. J. Utilization of phosphorus by pasture plants supplied with myo-inositol hexaphosphate is enhanced by the presence of soil micro-organisms. Plant Soil 2001, 229 (1), 47–56. 10.1023/A:1004871704173. [DOI] [Google Scholar]
- Richardson A. E. Prospects for using soil microorganisms to improve the acquisition of phosphorus by plants. Aust. J. Plant Physiol. 2001, 28, 897–906. 10.1071/PP01093. [DOI] [Google Scholar]
- George T. S.; Richardson A. E.; Smith J. B.; Hadobas P. A.; Simpson J. Limitations to the potential of transgenic Trifolium subterraneum L. plants that exude phytase, when grown in soils with a range of organic phosphorus content. Plant Soil 2005, 278 (1–2), 263–274. 10.1007/s11104-005-8699-2. [DOI] [Google Scholar]
- Wouterlood M.; Cawthray G. R.; Turner S.; Lambers H.; Veneklaas E. J. Rhizosphere carboxylate concentrations of chickpea are affected by genotype and soil type. Plant Soil 2004, 261, 1–10. 10.1023/B:PLSO.0000035568.28893.f6. [DOI] [Google Scholar]
- Ishikawa S.; Adu-Gyamfi J. J.; Nakamura T.; Yoshihara T.; Watanabe T.; Wagatsuma T. Genotypic variability in phosphorus solubilising activity of root exudates by pigeonpea grown in low-nutrient environments. Plant Soil 2002, 245, 71–81. 10.1023/A:1020659227650. [DOI] [Google Scholar]
- Lung S. C.; Leung A.; Kuang R.; Wang Y.; Leung P.; Lim B. L. Phytase activity in tobacco (Nicotiana tabacum) root exudates is exhibited by a purple acid phosphatase. Phytochemistry 2008, 69, 365–373. 10.1016/j.phytochem.2007.06.036. [DOI] [PubMed] [Google Scholar]
- Giles C. D.; Brown L. K.; Adu M. O.; Mezeli M. M.; Sandral G. A.; Simpson R. J.; Wendler R.; Shand C. A.; Menezes-Blackburn D.; Darch T.; Stutter M. I.; Lumsdon D. G.; Zhang H.; Blackwell M. S.; Wearing C.; Cooper P.; Haygarth P. M.; George T. S. Response-based selection of barley cultivars and legume species for complementarity: Root morphology and exudation in relation to nutrient source. Plant Sci. 2017, 255, 12–28. 10.1016/j.plantsci.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Yao Q.; Li X. I.; Feng G.; Christie P. Mobilization of sparingly soluble inorganic phosphates by the external mycelium of an arbuscular mycorrhizal fungus. Plant Soil 2001, 230, 279–285. 10.1023/A:1010367501363. [DOI] [Google Scholar]
- Jungk A. Root hairs and the acquisition of plant nutrients from soil. J. Plant Nutr. Soil Sci. 2001, 164 (2), 121–129. . [DOI] [Google Scholar]
- Zhang L.; Zhou J.; George T. S.; Limpens E.; Feng G. Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends Plant Sci. 2021, 1–10. 10.1016/j.tplants.2021.10.008. [DOI] [PubMed] [Google Scholar]
- Hill J. E.; Richardson A.. Isolation and assessment of microorganisms that utilize phytate. In Turner B. L., Ed.; Inositol phosphates: Linking agriculture and the environment; CAB International:Wallingford, England, 2007; pp 61–77 10.1079/9781845931520.0061. [DOI] [Google Scholar]
- Richardson A. E.; Hadobas P. A.; Hayes J. E. Extracellular secretion of Aspergillus phytase from Arabidopsis roots enables plants to obtain phosphorus from phytate. Plant J. 2001, 25, 641–649. 10.1046/j.1365-313x.2001.00998.x. [DOI] [PubMed] [Google Scholar]
- Patel K. J.; Vig S.; Naresh Kumar G.; Archana G. Effect of transgenic rhizobacteria overexpressing Citrobacter braakii appA on phytate-P availability to mung bean plants. J. Microbiol. Biotechnol. 2010, 20 (11), 1491–1499. 10.4014/jmb.1006.06016. [DOI] [PubMed] [Google Scholar]
- Patel K. J.; Singh A. K.; Nareshkumar G.; Archana G. Organic-acid-producing, phytate-mineralizing rhizobacteria and their effect on growth of pigeon pea (Cajanus cajan). Appl. Soil Ecol. 2010, 44, 252–261. 10.1016/j.apsoil.2010.01.002. [DOI] [Google Scholar]
- Vats P.; Banerjee U. C. Production studies and catalytic properties of phytases (myo-inositolhexakisphosphate phosphohydrolases): an overview. Enzyme Microb. Technol. 2004, 35 (1), 3–14. 10.1016/j.enzmictec.2004.03.010. [DOI] [Google Scholar]
- Caldwell B. A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49 (6), 637–644. 10.1016/j.pedobi.2005.06.003. [DOI] [Google Scholar]
- Shang C.; Caldwell D. E.; Stewart J. W. B.; Tiessen H.; Huang P. M. Bioavailability of organic and inorganic phosphates adsorbed on short-range ordered aluminum precipitate. Microb. Ecol. 1996, 31 (1), 29–39. 10.1007/BF00175073. [DOI] [PubMed] [Google Scholar]
- Giles C. D.; Richardson A. E.; Cade-Menun B. J.; Mezeli M. M.; Brown L. K.; Menezes-Blackburn D.; Darch T.; Blackwell M. S.; Shand C. A.; Stutter M. I.; Wendler R.; Cooper P.; Lumsdon D. G.; Wearing C.; Zhang H.; Haygarth P. M.; George T. S. Phosphorus acquisition by citrate- and phytase-exuding Nicotiana tabacum plant mixtures depends on soil phosphorus availability and root intermingling. Physiol. Plantarum 2018, 163, 356–371. 10.1111/ppl.12718. [DOI] [PubMed] [Google Scholar]
- Kamh M.; Horst W. J.; Amer F.; Mostafa H.; Maier P. Mobilization of soil and fertilizer phosphate by cover crops. Plant Soil 1999, 211 (1), 19–27. 10.1023/A:1004543716488. [DOI] [Google Scholar]
- Li L.; Tang C.; Rengel Z.; Zhang F. Chickpea facilitates phosphorus uptake by intercropped wheat from an organic phosphorus source. Plant Soil 2003, 248, 297–303. 10.1023/A:1022389707051. [DOI] [Google Scholar]
- Wissuwa M. How do plants achieve tolerance to phosphorus deficiency? Small causes with big effects. Plant Physiol. 2003, 133 (4), 1947–1958. 10.1104/pp.103.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nannipieri P.; Giagnoni L.; Landi L.; Renella G.. Role of phosphatase enzymes in soil. In Phosphorus in Action. Soil Biology; Springer: Heidelberg, 2011; pp 215–243 10.1007/978-3-642-15271-9_9. [DOI] [Google Scholar]
- Arenberg M. R.; Arai Y. Uncertainties in soil physicochemical factors controlling phosphorus mineralization and immobilization processes. Adv. Agronomy 2019, 154, 153–200. 10.1016/bs.agron.2018.11.005. [DOI] [Google Scholar]
- Harrison A. F. Labile organic phosphorus mineralization in relationship to soil properties. Soil Biol. Biochem. 1982, 14, 343–351. 10.1016/0038-0717(82)90004-9. [DOI] [Google Scholar]
- Tiessen H.; Stewart J. W. B.; Cole C. V. Pathways of phosphorus transformations in soils of differing pedogenesis. Soil Sci. Soc. Am. J. 1984, 48, 853–858. 10.2136/sssaj1984.03615995004800040031x. [DOI] [Google Scholar]
- Brannon C. A.; Sommers L. E. Stability and mineralization of organic phosphorus incorporated into model humic polymers. Soil Biol. Biochem. 1985, 17, 221–227. 10.1016/0038-0717(85)90118-X. [DOI] [Google Scholar]
- Trasar-Cepeda M. C.; Carballas T.; Gil-Sotres F.; de Blas E. Liming and the phosphatase activity and mineralization of phosphorus in an andic soil. Soil Biol. Biochem. 1991, 23, 209–215. 10.1016/0038-0717(91)90054-N. [DOI] [Google Scholar]
- Powar V. K.; Jagannathan V. Purification and properties of phytate-specific phosphatase from Bacillus subtilis. J. Bacteriol. 1982, 151, 1102–1108. 10.1128/jb.151.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzumura M.; Kamatani A. Mineralization of inositol hexaphosphate in aerobic and anaerobic marine sediments: implications for the phosphorus cycle. Geochim. Cosmochim. Acta 1995, 59, 1021–1026. 10.1016/0016-7037(95)00006-2. [DOI] [Google Scholar]
- Dick W. A.; Tabatabai M. A. Hydrolysis of organic and inorganic phosphorus compounds added to soils. Geoderma 1978, 21, 175–182. 10.1016/0016-7061(78)90025-3. [DOI] [Google Scholar]
- Criquet S.; Ferre E.; Farnet A. M.; Le petit J. Annual dynamics of phosphatase activities in an evergreen oak litter: influence of biotic and abiotic factors. Soil Biol. Biochem. 2004, 36 (7), 1111–1118. 10.1016/j.soilbio.2004.02.021. [DOI] [Google Scholar]
- Devi N. B.; Yadava P. S. Seasonal dynamics in soil microbial biomass C, N and P in a mixed-oak forest ecosystem of Manipur, North-east India. Appl. Soil Ecol. 2006, 31 (3), 220–227. 10.1016/j.apsoil.2005.05.005. [DOI] [Google Scholar]
- Zimmermann P.; Zardi G.; Lehmann M.; Zeder C.; Amrhein N.; Frossard E.; Bucher M. Engineering the root-soil interface via targeted expression of a synthetic phytase gene in trichoblasts. Plant Biotechnol. J. 2003, 1, 353–360. 10.1046/j.1467-7652.2003.00033.x. [DOI] [PubMed] [Google Scholar]
- Xiao K.; Harrison M. J.; Wang Z. Y. Trasngenic expression of a novel Medicago truncatula phytase gene results in improved acquisition of organic phosphorus by Arabidopsis. Planta 2005, 222, 27–36. 10.1007/s00425-005-1511-y. [DOI] [PubMed] [Google Scholar]
- Miller S. S.; Liu J.; Allan D. L.; Menzhuber C. J.; Fedorova M.; Vance C. P. Molecular control of acid phosphatase secretion into the rhizosphere of proteoid roots from phosphorus-stressed white lupin. Plant Physiol. 2001, 127 (2), 594–606. 10.1104/pp.010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haran S.; Logendra S.; Seskar M.; Bratanova M.; Raskin I. Characterization of Arabidopsis acid phosphatase promoter and regulation of acid phosphatase expression. Plant Physiol. 2000, 124 (2), 615–626. 10.1104/pp.124.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge S. R.; Smith F. W.; Richardson A. E. Root-specific and phosphate-regulated expression of phytase under the control of a phosphate transporter promoter enables Arabidopsis to grow on phytate as a sole P source. Plant Sci. 2003, 165 (4), 871–878. 10.1016/S0168-9452(03)00286-3. [DOI] [Google Scholar]
- George T. S.; Simpson R. J.; Hadobas P. A.; Richardson A. E. Expression of a fungal phytase gene in Nicotiana tabacum improves phosphorus nutrition of plants grown in amended soils. Plant Biotechnol. J. 2005, 3 (1), 129–140. 10.1111/j.1467-7652.2004.00116.x. [DOI] [PubMed] [Google Scholar]
- Chan W. L.; Lung S. C.; Lim B. L. Properties of beta-propeller phytase expressed in transgenic tobacco. Protein Expres. Purif. 2006, 46 (1), 100–106. 10.1016/j.pep.2005.07.019. [DOI] [PubMed] [Google Scholar]
- He Z. Q.; Griffin T. S.; Honeycutt C. W. Evaluation of soil phosphorus transformations by sequential fractionation and phosphatase hydrolysis. Soil Sci. 2004, 169 (7), 515–527. 10.1097/01.ss.0000135164.14757.33. [DOI] [Google Scholar]
- Richardson A. E.; George T. S.; Jakobsen I.; Simpson R. J.. Plant access to inositol phosphates in soil. In Turner B. L., Richardson A. E., Mullaney E. J., Eds.; Inositol phosphates in the soil-plant-animal system: Linking agriculture and environment; Soil Science Society of America: Sun Valley, ID, 2005; pp 42–43. [Google Scholar]
- Turner B. L.; Mahieu N.; Condron L. M. Quantification of myo-inositol hexakisphosphate in alkaline soil extracts by solution 31P NMR spectroscopy and spectral deconvolution. Soil Sci. 2003, 168 (7), 469–478. 10.1097/01.ss.0000080332.10341.ed. [DOI] [Google Scholar]
- Turner B. L. Organic phosphorus in Madagascan rice soils. Geoderma 2006, 136 (1–2), 279–288. 10.1016/j.geoderma.2006.03.043. [DOI] [Google Scholar]
- Fuentes B.; Jorquera M. A.; Mora M. L. Dynamics of phosphorus and phytate-utilizing bacteria during aerobic degradation of dairy cattle dung. Chemosphere 2009, 74 (2), 325–331. 10.1016/j.chemosphere.2008.08.045. [DOI] [PubMed] [Google Scholar]
- Toor G. S.; Hunger S.; Peak J. D.; Sims J. T.; Sparks D. L. Advances in the characterization of phosphorus in organic wastes: environmental and agronomic applications. Adv. Agron. 2006, 89, 1–72. 10.1016/S0065-2113(05)89001-7. [DOI] [Google Scholar]
- Turner B. L.; Wells A.; Condron L. M. Soil organic phosphorus transformations along a coastal dune chronosequence under New Zealand temperate rain forest. Biogeochemistry 2014, 121 (3), 595–611. 10.1007/s10533-014-0025-8. [DOI] [Google Scholar]
- Turner B. L.; Cade-Menun B. J.; Westermann D. T. Organic phosphorus composition and potential bioavailability in semi-arid arable soils of the western United States. Soil Sci. Soc. Am. J. 2003, 67, 1168–1179. 10.2136/sssaj2003.1168. [DOI] [Google Scholar]
- Hansen J. C.; Cade-Menun B. J.; Strawn D. G. Phosphorus speciation in manure-amended alkaline soils. J. Environ. Qual. 2004, 33 (4), 1521–1527. 10.2134/jeq2004.1521. [DOI] [PubMed] [Google Scholar]
- McDowell R. W.; Condron L. M.; Stewart I.; Cave V. Chemical nature and diversity of phosphorus in New Zealand pasture soils using 31P nuclear magnetic resonance spectroscopy and sequential fractionation. Nutr. Cycl. Agroecosys. 2005, 72 (3), 241–254. 10.1007/s10705-005-2921-8. [DOI] [Google Scholar]
- He Z. Q.; Honeycutt C. W.; Cade-Menun B. J.; Senwo Z. N.; Tazisong I. A. Phosphorus in poultry litter and soil: enzymatic and nuclear magnetic resonance characterization. Soil Sci. Soc. Am. J. 2008, 72 (5), 1425–1433. 10.2136/sssaj2007.0407. [DOI] [Google Scholar]
- Hill J. E.; Cade-Menun B. J. Phosphorus-31 nuclear magnetic resonance spectroscopy transect study of poultry operations on the Delmarva Peninsula. J. Environ. Qual. 2009, 38 (1), 130–138. 10.2134/jeq2007.0587. [DOI] [PubMed] [Google Scholar]
- Dou Z.; Ramberg C. F.; Toth J. D.; Wang Y.; Sharpley A. N.; Boyd S. E.; Chen C. R.; Williams D.; Xu Z. H. Phosphorus speciation and sorption-desorption characteristics in heavily manured soils. Soil Sci. Soc. Am. J. 2009, 73 (1), 93–101. 10.2136/sssaj2007.0416. [DOI] [Google Scholar]
- Murphy P. N. C.; Bell A.; Turner B. L. Phosphorus speciation in temperate basaltic grassland soils by solution 31P NMR spectroscopy. Eur. J. Soil Sci. 2009, 60 (4), 638–651. 10.1111/j.1365-2389.2009.01148.x. [DOI] [Google Scholar]
- Pearse S. J.; Veneklaas E. J.; Cawthray G.; Bolland M. D.; Lambers H. Carboxylate composition of root exudates does not relate consistently to a crop species’ ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytol. 2007, 173 (1), 181–190. 10.1111/j.1469-8137.2006.01897.x. [DOI] [PubMed] [Google Scholar]
- Grierson P. F. Organic acids in the rhizosphere of Banksia integrifolia L.f. Plant Soil 1992, 144, 259–265. 10.1007/BF00012883. [DOI] [Google Scholar]
- Deubel A.; Merbach W.. Influence of microorganisms on phosphorus bioavailability in soils. Microorganisms in Soils: Roles in Genesis and Functions; Buscot F., Varma A., Eds.; Springer: Berlin Heidelberg, 2005; Vol. 3, pp 177–191 10.1007/3-540-26609-7_9. [DOI] [Google Scholar]
- Ren Y. X.; Zhu X. L.; Fan D. D.; Ma P.; Liang L. H. Inoculation of phosphate solubilizing bacteria for the improvement of lead accumulation by Brassica juncea. Environ. Technol. 2013, 34 (1–4), 463–469. 10.1080/09593330.2012.701234. [DOI] [PubMed] [Google Scholar]
- Akintokun A. K.; G.A A.; P.O A.; T.O.S P.; A.O B. Solubilization of insoluble phosphate by organic acid producing fungi isolated from Nigerian soil. Int. J. Soil Sci. 2007, 2, 301–307. 10.3923/ijss.2007.301.307. [DOI] [Google Scholar]
- Shin W.; Ryu J.; Kim Y.; Yang J.; Madhaiyan M.; Sa T.. Phosphate solubilization and growth promotion of maize (Zea mays L.) by the rhizosphere soil fungus Penicillium oxalicum. 18th World Congress of Soil Science. Philadelphia, Pennsylvania, USA; 2006.
- Rashid M.; Samina K.; Najm A.; Sadia A.; Farooq L. Organic acids production and phosphate solubilization by phosphate solubilizing microorganisms (PSM) under in vitro conditions. Pakistan J. Biological Sci. 2004, 7 (2), 187–196. 10.3923/pjbs.2004.187.196. [DOI] [Google Scholar]
- Reyes I.; Bernier L.; Simard R. R.; Antoun H. Solubilization of phosphate rocks and minerals by a wild-type strain and two UV-induced mutants of Penicillium rugulosum. Soil Biol. Biochem. 2001, 33 (12–13), 1741–1747. 10.1016/S0038-0717(01)00099-2. [DOI] [Google Scholar]
- Vazquez P.; Holguin G.; Puente M. E.; Lopez-Cortes A.; Bashan Y. Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol. Fertility Soils 2000, 30 (5–6), 460–468. 10.1007/s003740050024. [DOI] [Google Scholar]
- Fenice M.; Selbman L.; Federici F.; Vassilev N. Application of encapsulated Penicillium variabile P16 in solubilization of rock phosphate. Bioresour. Technol. 2000, 73, 157–162. 10.1016/S0960-8524(99)00150-9. [DOI] [Google Scholar]
- Whitelaw M. A. Growth promotion of plants inoculated with phosphate-solubilizing fungi. Adv. Agron. 1999, 69, 99–151. 10.1016/S0065-2113(08)60948-7. [DOI] [Google Scholar]
- Goel M.; Sharma C. B. Multiple forms of phytase in germinating cotyledons of Cucurbita maxima. Phytochemistry 1979, 18 (12), 1939–1942. 10.1016/S0031-9422(00)82707-7. [DOI] [Google Scholar]
- Phillippy B. Q. Purification and catalytic properties of a phytase from scallion (Allium fistulosum L.) leaves. J. Agr. Food Chem. 1998, 46 (9), 3491–3496. 10.1021/jf9803177. [DOI] [Google Scholar]
- Agostini J. D. S.; Ida E. I. Partially characterization and application of phytase extracted from germinated sunflower seeds. Pesqui. Agropecu. Bras. 2006, 41 (6), 1041–1047. 10.1590/S0100-204X2006000600021. [DOI] [Google Scholar]
- Li M.; Osaki M.; Honma M.; Tadano T. Purification and characterization of phytase induced in tomato roots under phosphorus-deficient conditions. Soil Sci. Plant Nut. 1997, 43 (1), 179–190. 10.1080/00380768.1997.10414726. [DOI] [Google Scholar]
- Scott J. J. Alkaline phytase activity in nonionic detergent extracts of legume seeds. Plant Physiol. 1991, 95 (4), 1298–1301. 10.1104/pp.95.4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel F.; Beck E. Maize root phytase—Purification, characterization, and localization of enzyme activity and its putative substrate. Plant Physiol. 1996, 112 (4), 1429–1436. 10.1104/pp.112.4.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara A.; Ebina S.; Kondo A.; Funaguma T. A new type of phytase from pollen of Typha latifolia L. J. Agric. Chem. Soc. Jpn. 1985, 49 (12), 3539–3544. 10.1080/00021369.1985.10867290. [DOI] [Google Scholar]
- Greiner R.; Konietzny U.; Jany K. D. Purification and properties of a phytase from rye. J. Food Biochem. 1998, 22 (2), 143–161. 10.1111/j.1745-4514.1998.tb00236.x. [DOI] [Google Scholar]
- Konietzny U.; Greiner R.; Jany K. D. Purification and characterization of a phytase from spelt. J. Food Biochem. 1994, 18 (3), 165–183. 10.1111/j.1745-4514.1994.tb00495.x. [DOI] [Google Scholar]
- Laboure A. M. Purification and characterization of a phytase (myo-inositol-hexakisphosphate phosphohydrolase) accumulated in maize (Zea mays) seedlings during germination. Biochem. J. 1993, 295 (2), 413–419. 10.1042/bj2950413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R.; Tarafdar J. C. Influence of organic and inorganic phosphorus supply on the maximum secretion of acid phosphatase by plants. Biol. Fert. Soils 2001, 34 (3), 140–143. 10.1007/s003740100376. [DOI] [Google Scholar]
- Kim Y. O.; Kim H. K.; Bae K. S.; Yu J. H.; Oh T. K. Purification and properties of a thermostable phytase from Bacillus sp. DS11. Enzyme Microb. Technol. 1998, 22 (1), 2–7. 10.1016/S0141-0229(97)00096-3. [DOI] [Google Scholar]
- Tye A.; Siu F.; Leung T.; Lim B. Molecular cloning and the biochemical characterization of two novel phytases from B. subtilis 168 and B. licheniformis. Appl. Microbiol. Biotechnol. 2002, 59 (2–3), 190–197. 10.1007/s00253-002-1033-5. [DOI] [PubMed] [Google Scholar]
- Kerovuo J.; Lappalainen I.; Reinikainen T. The metal dependence of Bacillus subtilis phytase. Biochem. Biophys. Res. Commun. 2000, 268 (2), 365–369. 10.1006/bbrc.2000.2131. [DOI] [PubMed] [Google Scholar]
- Choi Y. M. Isolation of phytase-producing Bacillus sp. KHU-10 and its phytase production. J. Microbiol. Biotechnol. 1999, 9, 223–226. [Google Scholar]
- Kim H. W.; Kim Y. O.; Lee J. H.; Kim K. K.; Kim Y. J. Isolation and characterization of a phytase with improved properties from Citrobacter braakii. Biotechnol. Lett. 2003, 25 (15), 1231–1234. 10.1023/A:1025020309596. [DOI] [PubMed] [Google Scholar]
- Golovan S.; Wang G. R.; Zhang J.; Forsberg C. W. Characterization and overproduction of the Escherichia coli appA encoded bifunctional enzyme that exhibits both phytase and acid phosphatase activities. Can. J. Microbiol. 1999, 46 (1), 59–71. 10.1139/w99-084. [DOI] [PubMed] [Google Scholar]
- Ullah A. H. J.; Sethumadhavan K. PhyA gene product of Aspergillus ficuum and Peniophora lycii produces dissimilar phytases. Biochem. Biophys. Res. Commun. 2003, 303 (2), 463–468. 10.1016/S0006-291X(03)00374-7. [DOI] [PubMed] [Google Scholar]
- Lassen S. F.; Breinholt J.; Ostergaard P. R.; Brugger R.; Bischoff A.; Wyss M.; Fuglsang C. C. Expression, gene cloning, and characterization of five novel phytases fromfour basidiomycete fungi: Peniophora lycii, Agrocybe pediades, a Ceriporia sp., and Trametes pubescens. Appl. Environ. Microb. 2001, 67 (10), 4701–4707. 10.1128/AEM.67.10.4701-4707.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. H.; An L. J.; Gao X. R.; Wang Y. J. Properties of A. ficuum AS3.324 phytase expressed in tobacco. Process Biochem. 2005, 40 (1), 213–216. 10.1016/j.procbio.2003.12.005. [DOI] [Google Scholar]
- Hamada A.; Yamaguchi K.; Ohnishi N.; Harada M.; Nikumaru S.; Honda H. High-level production of yeast (Schwanniomyces occidentalis) phytase in transgenic rice plants by a combination of signal sequence and codon modification of the phytase gene. Plant Biotechnol. J. 2005, 3 (1), 43–55. 10.1111/j.1467-7652.2004.00098.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.