Abstract
Background
We aimed to explore the association between sleep duration and sleep disorder variables obtained from the National Health and Nutrition Examination Survey (NHANES) and stroke.
Material/Methods
This cross-sectional study enrolled 10 442 participants from the United States. The outcome variable was stroke diagnosed by a doctor. Sleep disorder variables were assessed via self-report questionnaires and included sleep duration, the time required to fall asleep, frequency of sleep deprivation, frequency of early morning wakefulness, and frequency of middle of the night wakefulness. The odds ratio (OR) and 95% confidence intervals (CIs) were calculated by multivariate logistic regression models.
Results
Compared with participants with 6 to 7 h of sleep duration, participants with less than 6 h of sleep duration had an increased risk of stroke of 0.97 times (OR=1.97, 95% CI: 1.19–3.29). Participants with a sleep disorder had 0.71 times (OR=1.71, 95% CI: 1.04–2.82) the risk of stroke than those without a sleep disorder. Stroke was significantly associated with sleep deprivation 16 to 30 times a month (OR=1.99, 95% CI: 1.14–3.46), early morning wakefulness 16 to 30 times a month (OR=1.97, 95% CI: 1.20–3.25), and middle of the night wakefulness 16 to 30 times a month (OR=1.81, 95% CI: 1.05–3.09), compared with no sleep deprivation, early-morning wakefulness, or middle of the night wakefulness.
Conclusions
Short sleep duration and sleep disorder were associated with an increased risk of stroke, suggesting healthy sleep behaviors may reduce the risk of stroke. However, further studies are needed to confirm the causality and underlying mechanism.
Keywords: Nutrition Surveys, Sleep Wake Disorders, Stroke
Background
Stroke is a main cause of morbidity, disability, and death worldwide [1–3]. Due to the aging of the population, the incidence and prevalence of stroke are expected to rise significantly [1]. Worldwide, 86.5 per 100 000 people die of stroke each year [4]. Most of the survivors have varying degrees of symptoms, including dyskinesia, cognitive impairment, dysphagia, and lalopathy [5,6]. According to the data of the American Heart Association, the recurrence rate of stroke is estimated to be as high as 17% within 5 years [2]. Prevention and intervention of stroke in patients is of great importance; therefore, special attention should be given to the identification of risk factors for stroke.
The current research states that stroke is related to several risk factors, such as smoking, alcohol consumption, high blood pressure, cardiovascular disease, dyslipidemia, and diabetes [2,7]. Lifestyle changes or medication can be used to reduce or eliminate the impact of identified risk factors [8]. There is also increasing evidence that sleep duration and sleep disorders can affect the risk of stroke [9,10]. In addition, sleep-wake cycles and circadian disorders have been increasingly recognized as risk factors for stroke [11,12]. Therefore, the diagnosis and treatment of sleeping disorders should be considered as a prevention and intervention of stroke [13]. Although there have been many advances in determining various stroke-related risk factors based on sleeping, previous studies have mostly focused on sleep apnea syndrome and sleep duration, and other sleep quality-related indicators were rarely reported. Our present research further investigates the relationship between several sleep disorder variables and stroke.
Therefore, the purpose of this study was to explore the relationship between sleep disorder-related variables obtained from the National Health and Nutrition Examination Survey (NHANES), including sleep duration, the time required to fall asleep, frequency of sleep deprivation, frequency of early morning wakefulness, and frequency of middle of the night wakefulness, and stroke. We also stratified the ages of participants to explore the relationship between these sleep disorder-related variables and stroke in participants over 65 years old and those under 65 years old.
Material and Methods
Study Design and Population
We used 2-cycle cross-sectional surveys of NHANES conducted in 2005–2006 and 2007–2008 in this study. The NHANES is a major project of the National Center for Health Statistics (NCHS) in a large, nationally representative US population. There was a total of 20 479 participants included in the NHANES from 2005 to 2008. We excluded 9592 participants because it was uncertain whether they were patients with stroke. An additional 445 participants were excluded because of missing values in one or more of the sleep disorder-related variables required for the analyses. Finally, 10 442 participants with available information were included in the analyses. The flow chart of the selection process is shown in Figure 1. The NHANES protocols were approved by the NCHS Research Ethics Review Board, and written informed consent forms were provided by all participants. More information can be obtained at https://www.cdc.gov/nchs/nhanes/irba98.htm.
Figure 1.
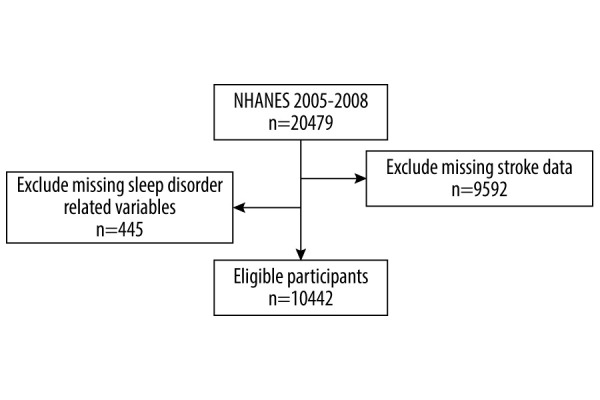
Flow chart of the eligible participants (Draw.io v. 13.9.9, JGraph Ltd.).
Outcome Variable
History of stroke was diagnosed by a positive answer to the question, “Has a doctor or other health professional ever told you that you had a stroke?” [14,15], which was consistent with methods used in the published literature [16,17].
Explanatory Variables
The sleep disorder-related variables were self-reported in a personal interview [18,19]. Sleep duration was assessed by the question “How much sleep do you usually get at night on weekdays or workdays?”; sleep duration was categorized as <6 h, 6 to 7 h, 7 to 8 h, or ≥8 h per day; the variable of time required to fall asleep was assessed by the question “How long does it usually take you to fall asleep at bedtime?”, with participants filling in the specific number of hours and minutes.
Whether the participant had a sleep disorder was assessed by the question “Have you ever been told by a doctor or other health professional that you have a sleep disorder?”, with responses given as yes/no. Frequency of sleep deprivation, frequency of early morning wakefulness, and frequency of middle of the night wakefulness were assessed by the questions “In the past month, how often did you not get enough sleep?”, “In the past month, how often did you wake up too early in the morning and were unable to get back to sleep?”, and “In the past month, how often did you wake up during the night and have trouble getting back to sleep?”, respectively. The categories of answers were never, 1 time a month, 2 to 4 times a month, 5 to 15 times a month, and 16 to 30 times a month.
Covariates
Demographic and sociodemographic characteristics were collected: sex (male, female); age (<65 and ≥65 years); race (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, and other race [including multi-racial]); marital status (married, widowed, divorced, separated, never married, and living with a partner); education level (less than 9th graduate, 9–11 grade, high school graduate or equivalent, some college, and college graduate or above); and family poverty income ratio (PIR, a ratio of family income to poverty threshold). Health status variables were collected: body mass index (BMI, calculated by weight and height, kg/m2), waist circumference (cm), and pulse (30 s pulse multiplied by 2), which were collected by trained health technicians in the Mobile Examination Center. Self-reported history of chronic diseases, including hypertension, congestive heart failure (CHF), coronary heart disease (CHD), angina pectoris, heart attack, and diabetes, was assessed by the question “Has a doctor or other health professional ever told you that you had ____?” Medication information was also collected, including angiotensin-converting enzyme inhibitors, beta-blockers, and antidepressants.
Platelet count, serum total cholesterol, and high-density lipoprotein were laboratory data included in the NHANES. The blood samples were collected, processed, and stored by the Mobile Testing Center laboratory, then the samples were transported to the analytical laboratory for analysis. Total cholesterol was measured enzymatically in serum using the Roche Hitachi 717 and 912. The high-density lipoprotein cholesterol reference method used heparin-manganese to precipitate high-density lipoprotein cholesterol and the Abell-Kendall method to measure cholesterol. Platelet counts were evaluated using automated hematology analyzing devices.
Statistical Analysis
The description of participant characteristics was given as the relative cases and frequency for categorical variables (n [weighted%]), the mean, and the corresponding standard error (SE) for continuous variables of a normal distribution. According to the categorization and distribution of the variables and data, the chi-square test or the t test was used to test the differences. Missing values were processed by multiple imputations. We used a univariate logistic regression model to screen out potential confounders for stroke. Three logistic regression models were used to complete multivariate logistic regression analyses to explore the associations between sleep disorder-related variables and stroke, and the odds ratio (OR) and 95% confidence interval (CI) were calculated. Model 1 was the crude model; Model 2 adjusted for age, sex, BMI, marital status, family PIR, and education level; Model 3 adjusted for age, sex, BMI, marital status, race, education level, family PIR, waist circumference, hypertension, CHF, CHD, angina pectoris, heart attack, platelet count, diabetes, beta-blockers, and antidepressants. We also performed a sensitivity analysis, using sleep disorder-related variables as independent variables and stroke as the dependent variable, comparing the results of multivariate logistic regression between the data without imputation and the imputation. Lastly, we stratified age to explore the association between sleep disorder-related variables and stroke in people 65 years or older and younger than 65 years old. P<0.05 was considered statistically different, and all statistical analysis was completed by using SAS version 9.4 (SAS Institute, Cary, NC, USA) and Python software version 3.6.8 (Python Software Foundation, DE, USA).
Results
Characteristics of the Study Population
A total of 10 442 participants were included for analysis, including 48.04% men and 51.96% women, with an average age of 46.28 years. There were 402 participants with stroke and 10 040 without stroke. The characteristics of the study population are shown in Table 1. In the 7.31% of participants with sleep disorders, and the average time required to fall asleep was 21.40 min. Most participants never had early morning wakefulness (43.77%) and middle of the night wakefulness (35.62%) in the past month, and the frequency of sleep deprivation was mostly 2 to 4 times (28.97%) per month.
Table 1.
Screening for confounders associated with stroke.
| Variables | Total [n=10,442 (weighted %)] | With stroke (n=402) | Without stroke (n=10,040) | Statistics | P |
|---|---|---|---|---|---|
| Gender, n (%) | χ2=5.46 | 0.019 | |||
| Male | 5,042 (48.04) | 190 (40.64) | 4,852 (48.25) | ||
| Female | 5,400 (51.96) | 212 (59.36) | 5,188 (51.75) | ||
| Age, mean (S.E.) | 46.28 (0.42) | 64.90 (1.17) | 45.74 (0.40) | t=16.36 | <0.001 |
| BMI, mean (S.E.) | 28.50 (0.14) | 29.73 (0.37) | 28.47 (0.14) | t=3.23 | 0.003 |
| Race, n (%) | χ2=16.23 | 0.003 | |||
| Mexican American | 1,966 (8.21) | 36 (3.58) | 1,930 (8.35) | ||
| Other Hispanic | 793 (4.19) | 18 (2.12) | 775 (4.25) | ||
| Non-Hispanic white | 4,997 (70.56) | 231 (74.96) | 4,766 (70.44) | ||
| Non-Hispanic black | 2,267 (11.44) | 107 (14.57) | 2,160 (11.35) | ||
| Other race (including multi-raciala) | 419 (5.59) | 10 (4.76) | 409 (5.61) | ||
| Marital status, n (%) | χ2=138.70 | <0.001 | |||
| Married | 5,573 (57.23) | 195 (49.91) | 5,378 (57.45) | ||
| Widowed | 929 (5.92) | 106 (23.27) | 823 (5.41) | ||
| Divorced | 1,070 (9.94) | 51 (12.45) | 1,019 (9.87) | ||
| Separated | 346 (2.43) | 10 (2.19) | 336 (2.43) | ||
| Never married | 1,737 (16.77) | 28 (7.05) | 1,709 (17.05) | ||
| Living with partner | 787 (7.71) | 12 (5.13) | 775 (7.79) | ||
| Education level, n (%) | χ2=39.19 | <0.001 | |||
| Less than 9th grade | 1,339 (6.45) | 83 (14.36) | 1,256 (6.22) | ||
| 9–11th grade | 1,736 (12.36) | 77 (14.78) | 1,659 (12.29) | ||
| High school grade/GED or equivalent | 2,521 (25.09) | 105 (29.50) | 2,416 (24.97) | ||
| Some college | 2,819 (30.22) | 89 (25.21) | 2,730 (30.37) | ||
| College graduate or above | 2,027 (25.88) | 48 (16.15) | 1,979 (26.16) | ||
| Family PIR, mean (S.E.) | 3.06 (0.05) | 2.35 (0.11) | 3.08 (0.05) | t=−9.09 | <0.001 |
| Waist circumference, mean (S.E.) | 97.64 (0.40) | 103.67 (0.91) | 97.47 (0.41) | t=6.81 | <0.001 |
| Hypertension, n (%) | χ2=221.24 | <0.001 | |||
| Yes | 3,432 (29.24) | 305 (73.13) | 3,127 (27.95) | ||
| No | 7,010 (70.76) | 97 (26.87) | 6,913 (72.05) | ||
| CHF, n (%) | χ2=590.92 | <0.001 | |||
| Yes | 338 (2.19) | 70 (15.80) | 268 (1.79) | ||
| No | 10,104 (97.81) | 332 (84.20) | 9,772 (98.21) | ||
| CHD, n (%) | χ2=213.56 | <0.001 | |||
| Yes | 405 (3.11) | 67 (18.26) | 338 (2.66) | ||
| No | 10,037 (96.89) | 335 (81.74) | 9,702 (97.34) | ||
| Angina pectoris, n (%) | χ2=128.60 | <0.001 | |||
| Yes | 286 (2.18) | 50 (11.36) | 236 (1.91) | ||
| No | 10,156 (97.82) | 352 (88.64) | 9,804 (98.09) | ||
| Heart attack, n (%) | χ2=415.86 | <0.001 | |||
| Yes | 433 (3.16) | 83 (18.66) | 350 (2.71) | ||
| No | 10,009 (96.84) | 319 (81.34) | 9,690 (97.29) | ||
| Pulse, mean (S.E.) | 73.20 (0.20) | 73.61 (0.64) | 73.19 (0.20) | t=0.63 | 0.531 |
| Platelet count, mean (S.E.) | 274.00 (1.29) | 263.91 (4.77) | 274.30 (1.27) | t=−2.33 | 0.026 |
| TC, mean (S.E.) | 5.13 (0.01) | 4.97 (0.09) | 5.13 (0.02) | t=−1.62 | 0.115 |
| HDL-cholesterol, mean (S.E.) | 1.38 (0.01) | 1.33 (0.03) | 1.38 (0.01) | t=−1.98 | 0.057 |
| Diabetes, n (%) | χ2=202.50 | <0.001 | |||
| Yes | 1,198 (8.05) | 141 (30.93) | 1,057 (7.38) | ||
| No | 9,244 (91.95) | 261 (69.07) | 8,983 (92.62) | ||
| ACEi, n (%) | χ2=0.53 | 0.466 | |||
| Yes | 59 (0.48) | 2 (0.29) | 57 (0.49) | ||
| No | 10,383 (99.52) | 400 (99.71) | 9,983 (99.51) | ||
| Beta-blockers, n (%) | χ2=238.51 | <0.001 | |||
| Yes | 1,228 (10.00) | 145 (33.94) | 1,083 (9.30) | ||
| No | 9,214 (90.00) | 257 (66.06) | 8,957 (90.70) | ||
| Antidepressants, n (%) | χ2=58.47 | <0.001 | |||
| Yes | 1,005 (11.50) | 89 (25.51) | 916 (11.09) | ||
| No | 9,437 (88.50) | 313 (74.49) | 9,124 (88.91) | ||
| Sleep duration, n (%) | χ2=22.00 | <0.001 | |||
| <6 h | 1,655 (13.79) | 84 (22.63) | 1,569 (13.53) | ||
| 6–7 h | 2,410 (23.14) | 75 (17.64) | 2,334 (23.30) | ||
| 7–8 h | 2,790 (30.10) | 68 (20.91) | 2,722 (30.37) | ||
| ≥8 h | 3,587 (32.97) | 172 (38.82) | 3,415 (32.80) | ||
| Sleep disorder, n (%) | χ2=23.11 | <0.001 | |||
| Yes | 758 (7.31) | 62 (16.33) | 696 (7.05) | ||
| No | 9,684 (92.69) | 340 (83.67) | 9,344 (92.95) | ||
| Time required to fall asleep, min, mean (S.E.) | 21.40 (0.34) | 26.08 (1.32) | 21.26 (0.34) | t=3.66 | <0.001 |
| Frequency of sleep deprivation, n (%) | χ2=22.20 | <0.001 | |||
| Never | 3,440 (26.15) | 147 (32.88) | 3,293 (25.95) | ||
| One time a month | 1,821 (18.93) | 57 (14.06) | 1,764 (19.08) | ||
| 2–4 times a month | 2,692 (28.97) | 96 (26.76) | 2,596 (29.03) | ||
| 5–15 times a month | 1,463 (15.92) | 36 (9.50) | 1,427 (16.10) | ||
| 16–30 times a month | 1,026 (10.04) | 66 (16.80) | 960 (9.84) | ||
| Frequency of early-morning wakefulness, n (%) | χ2=36.03 | <0.001 | |||
| Never | 4,770 (43.77) | 158 (36.91) | 4,612 (43.97) | ||
| One time a month | 1,865 (19.73) | 57 (15.41) | 1,808 (19.85) | ||
| 2–4 times a month | 2,002 (19.82) | 79 (19.80) | 1,923 (19.82) | ||
| 5–15 times a month | 1,091 (10.57) | 48 (12.78) | 1,043 (10.51) | ||
| 16–30 times a month | 714 (6.11) | 60 (15.09) | 654 (5.85) | ||
| Frequency of midnight wakefulness, n (%) | χ2=28.13 | <0.001 | |||
| Never | 4,046 (35.62) | 127 (29.34) | 3,919 (35.80) | ||
| One time a month | 1,936 (20.33) | 65 (17.30) | 1,871 (20.42) | ||
| 2–4 times a month | 2,389 (23.68) | 91 (21.79) | 2,298 (23.74) | ||
| 5–15 times a month | 1,268 (13.09) | 58 (16.02) | 1,210 (13.00) | ||
| 16–30 times a month | 803 (7.28) | 61 (15.55) | 742 (7.04) |
S.E – standard error; BMI – body mass index; GED – general equivalent diploma; PIR – poverty income ratio; CHF – congestive heart failure; CHD – coronary heart disease; TC – serum total cholesterol; HDL – high-density lipoprotein; ACEi – angiotensin-converting enzyme inhibitors.
Differences Between Participants with Stroke and without Stroke
Comparing the differences between participants with and without stroke, the results showed that the 2 groups were significantly different in sex (P=0.019), age (P<0.001), BMI (P=0.003), race (P=0.003), marital status (P<0.001), education level (P<0.001), family PIR (P<0.001), waist circumference (P<0.001), hypertension (P<0.001), CHF (P<0.001), CHD (P<0.001), angina pectoris (P<0.001), heart attack (P<0.001), platelet count (P=0.026), diabetes (P<0.001), beta-blockers (P<0.001), antidepressants (P<0.001), sleep duration (P<0.001), sleep disorder (P<0.001), the time required to fall asleep (P<0.001), frequency of sleep deprivation (P<0.001), frequency of early-morning wakefulness (P<0.001), and frequency of middle of the night wakefulness (P<0.001). These factors were stroke confounders (Table 1).
Association Between Sleep Disorder-Related Variables and Stroke
The association between sleep disorder-related variables and stroke using logistic regression models is shown in Table 2. Sleep duration of less than 6 h increased the risk of stroke by 0.95 times (OR=1.95, 95% CI: 1.19–3.19), compared with 6 to 7 h of sleep duration after adjusting for age, sex, BMI, marital status, race, education level, family PIR, waist circumference, hypertension, CHF, CHD, angina pectoris, heart attack, platelet count, diabetes, beta-blockers, and antidepressants. Time required to fall asleep was also a statistically significant risk of stroke (OR=1.01, 95% CI: 1.01–1.01) in Model 3. Participants with a sleep disorder had an increased risk of stroke of 0.66 times (OR=1.66, 95% CI: 1.03–2.69) compared with those without a sleep disorder. Participants who had 16 to 30 times of sleep deprivation, early morning wakefulness, and middle of the night wakefulness a month were 0.90 times (OR=1.90, 95% CI: 1.06–3.40), 1.07 times (OR=2.07, 95% CI: 1.24–3.46), and 0.78 times (OR=1.78, 95% CI: 1.01–3.18), respectively, more likely to have a stroke than participants who had never had these symptoms. In sensitivity analyses, the association between sleep disorder-related variables and stroke was conducted through a logistic regression model using unimputed data. The results showed that the association was still strong (Table 3).
Table 2.
Association between sleep disorder-related variables and stroke.
| Variables | Model 1 | P | Model 2 | P | Model 3 | P |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Sleep duration | ||||||
| <6 h | 2.21 (1.42–3.45) | 0.001 | 2.07 (1.28–3.35) | 0.005 | 1.95 (1.19–3.19) | 0.006 |
| 6–7 h | Ref | Ref | Ref | |||
| 7–8 h | 0.91 (0.60–1.39) | 0.650 | 1.01 (0.68–1.50) | 0.953 | 1.09 (0.73–1.63) | 0.657 |
| ≥8 h | 1.56 (1.05–2.34) | 0.030 | 1.25 (0.82–1.90) | 0.294 | 1.31 (0.86–1.99) | 0.199 |
| Sleep disorder | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 2.57 (1.69–3.92) | <.001 | 2.33 (1.41–3.84) | 0.002 | 1.66 (1.03–2.69) | 0.031 |
| Time required to fall asleep | 1.01 (1.01–1.02) | <.001 | 1.01 (1.01–1.02) | 0.002 | 1.01 (1.01–1.01) | 0.046 |
| Frequency of sleep deprivation | ||||||
| Never | Ref | Ref | Ref | |||
| One time a month | 0.58 (0.37–0.92) | 0.023 | 0.89 (0.57–1.41) | 0.613 | 0.83 (0.51–1.35) | 0.438 |
| 2–4 times a month | 0.73 (0.53–1.01) | 0.050 | 1.38 (0.98–1.94) | 0.067 | 1.25 (0.88–1.79) | 0.198 |
| 5–15 times a month | 0.47 (0.28–0.78) | 0.005 | 0.97 (0.57–1.66) | 0.913 | 0.80 (0.45–1.42) | 0.420 |
| 16–30 times a month | 1.35 (0.86–2.11) | 0.186 | 2.47 (1.45–4.19) | 0.002 | 1.90 (1.06–3.40) | 0.024 |
| Frequency of early-morning wakefulness | ||||||
| Never | Ref | Ref | Ref | |||
| One time a month | 0.93 (0.61–1.40) | 0.702 | 0.95 (0.63–1.44) | 0.818 | 0.92 (0.61–1.38) | 0.677 |
| 2–4 times a month | 1.19 (0.82–1.73) | 0.349 | 1.22 (0.79–1.88) | 0.356 | 1.06 (0.69–1.65) | 0.775 |
| 5–15 times a month | 1.45 (0.93–2.27) | 0.101 | 1.36 (0.85–2.17) | 0.200 | 1.14 (0.68–1.92) | 0.603 |
| 16–30 times a month | 3.07 (2.03–4.67) | <.001 | 2.53 (1.56–4.09) | 0.001 | 2.07 (1.24–3.46) | 0.004 |
| Frequency of midnight wakefulness | ||||||
| Never | Ref | Ref | Ref | |||
| One time a month | 1.03 (0.68–1.57) | 0.874 | 1.14 (0.74–1.76) | 0.545 | 1.07 (0.71–1.60) | 0.741 |
| 2–4 times a month | 1.12 (0.78–1.60) | 0.521 | 1.15 (0.78–1.70) | 0.479 | 0.98 (0.67–1.44) | 0.905 |
| 5–15 times a month | 1.50 (1.10–2.07) | 0.014 | 1.49 (1.04–2.12) | 0.030 | 1.22 (0.82–1.80) | 0.309 |
| 16–30 times a month | 2.70 (1.71–4.25) | <.001 | 2.34 (1.34–4.10) | 0.004 | 1.78 (1.01–3.18) | 0.043 |
OR – odds ratio; CI – confidence interval; Ref – reference. Model 1: unadjusted model; Model 2: adjustment of age, gender, BMI, marital status, family PIR, and education level; Model 3: adjustment of age, gender, BMI, marital status, race, education level, family PIR, waist circumference, hypertension, CHF, CHD, angina pectoris, heart attack, platelet count, diabetes, beta-blockers, and antidepressants.
Table 3.
Sensitivity analysis.
| Variables | Model 3 | P |
|---|---|---|
| OR (95% CI) | ||
| Sleep duration | ||
| <6 h | 1.98 (1.18–3.32) | 0.007 |
| 6–7 h | Ref | |
| 7–8 h | 1.05 (0.65–1.71) | 0.829 |
| ≥8 h | 1.19 (0.76–1.87) | 0.438 |
| Sleep disorder | ||
| No | Ref | |
| Yes | 1.60 (1.03–2.47) | 0.028 |
| Time required to fall asleep | 1.01 (1.01–1.02) | 0.013 |
| Frequency of sleep deprivation | ||
| Never | Ref | |
| One time a month | 0.91 (0.55–1.49) | 0.685 |
| 2–4 times a month | 1.32 (0.84–2.07) | 0.206 |
| 5–15 times a month | 0.79 (0.42–1.48) | 0.447 |
| 16–30 times a month | 2.33 (1.28–4.22) | 0.004 |
| Frequency of early-morning wakefulness | ||
| Never | Ref | |
| One time a month | 0.91 (0.56–1.47) | 0.680 |
| 2–4 times a month | 1.12 (0.64–1.99) | 0.674 |
| 5–15 times a month | 1.09 (0.64–1.85) | 0.734 |
| 16–30 times a month | 2.44 (1.30–4.59) | 0.004 |
| Frequency of midnight wakefulness | ||
| Never | Ref | |
| One time a month | 0.96 (0.61–1.51) | 0.860 |
| 2–4 times a month | 1.10 (0.70–1.72) | 0.671 |
| 5–15 times a month | 1.31 (0.83–2.04) | 0.225 |
| 16–30 times a month | 2.16 (1.13–4.13) | 0.015 |
OR – odds ratio; CI – confidence interval; Ref – reference. Model 3: adjustment of age, gender, BMI, marital status, race, education level, family PIR, waist circumference, hypertension, CHF, CHD, angina pectoris, heart attack, platelet count, diabetes, beta-blockers, and antidepressants.
Association Between Sleep Disorder-Related Variables and Stroke by Age Stratification
In participants younger than 65 years old, those with sleep duration less than 6 h (OR=3.54, 95% CI: 1.68–7.46), sleep disorder (OR=2.53, 95% CI: 1.24–5.19), 16 to 30 times a month of sleep deprivation (OR=2.01, 95% CI: 1.07–3.78), and early morning wakefulness (OR=2.50, 95% CI: 1.24–5.02) had an associated higher risk of stroke than those with sleep duration 6 to 7 h, without sleep disorder, without sleep deprivation, and early morning wakefulness monthly in Model 3 (Table 4). In participants aged 65 years or older, those with middle of the night wakefulness 1 time a month (OR=1.59, 95% CI: 1.15–2.19) and 16 to 30 times a month (OR=1.80, 95% CI: 0.98–3.29) had an associated higher risk of stroke than those without middle of the night wakefulness (Model 3 in Table 5).
Table 4.
Association between sleep disorder-related variables and stroke in participants <65 years of age.
| Variables | Model 1 | P | Model 2 | P | Model 3 | P |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Sleep duration | ||||||
| <6 h | 4.61 (2.39–8.89) | <0.001 | 3.81 (1.86–7.81) | <0.001 | 3.54 (1.68–7.46) | <0.001 |
| 6–7 h | Ref | Ref | Ref | |||
| 7–8 h | 1.21 (0.57–2.60) | 0.604 | 1.31 (0.62–2.77) | 0.470 | 1.50 (0.68–3.31) | 0.296 |
| ≥8 h | 1.67 (0.76–3.66) | 0.183 | 1.72 (0.81–3.66) | 0.145 | 1.72 (0.78–3.81) | 0.163 |
| Sleep disorder | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 4.92 (2.63–9.23) | <0.001 | 3.83 (1.95–7.52) | <0.001 | 2.53 (1.24–5.19) | 0.008 |
| Time required to fall asleep | 1.02 (1.01–1.03) | <0.001 | 1.01 (1.01–1.02) | 0.015 | 1.01 (1.00–1.02) | 0.096 |
| Frequency of sleep deprivation | ||||||
| Never | Ref | Ref | Ref | |||
| One time a month | 0.52 (0.21–1.27) | 0.136 | 0.61 (0.25–1.52) | 0.270 | 0.54 (0.19–1.48) | 0.210 |
| 2–4 times a month | 1.07 (0.57–2.04) | 0.819 | 1.39 (0.74–2.59) | 0.282 | 1.18 (0.64–2.16) | 0.579 |
| 5–15 times a month | 0.57 (0.27–1.20) | 0.125 | 0.72 (0.35–1.46) | 0.340 | 0.60 (0.27–1.32) | 0.184 |
| 16–30 times a month | 2.65 (1.47–4.78) | <0.001 | 2.90 (1.62–5.20) | <0.001 | 2.01 (1.07–3.78) | 0.024 |
| Frequency of early-morning wakefulness | ||||||
| Never | Ref | Ref | Ref | |||
| One time a month | 1.04 (0.47–2.30) | 0.922 | 0.95 (0.43–2.10) | 0.901 | 0.87 (0.39–1.96) | 0.728 |
| 2–4 times a month | 1.56 (0.87–2.80) | 0.123 | 1.38 (0.76–2.52) | 0.272 | 1.12 (0.60–2.11) | 0.712 |
| 5–15 times a month | 1.84 (0.96–3.55) | 0.057 | 1.34 (0.68–2.62) | 0.382 | 1.14 (0.56–2.28) | 0.710 |
| 16–30 times a month | 5.53 (3.07–9.99) | <0.001 | 3.45 (1.82–6.53) | <0.001 | 2.50 (1.24–5.02) | 0.007 |
| Frequency of midnight wakefulness | ||||||
| Never | Ref | Ref | Ref | |||
| One time a month | 0.77 (0.32–1.87) | 0.551 | 0.74 (0.30–1.82) | 0.502 | 0.63 (0.25–1.55) | 0.293 |
| 2–4 times a month | 1.22 (0.67–2.24) | 0.498 | 1.10 (0.62–1.95) | 0.728 | 0.93 (0.52–1.66) | 0.802 |
| 5–15 times a month | 1.78 (1.08–2.93) | 0.018 | 1.45 (0.87–2.42) | 0.138 | 1.17 (0.70–1.96) | 0.533 |
| 16–30 times a month | 3.81 (1.81–7.99) | <0.001 | 2.56 (1.16–5.69) | 0.016 | 1.68 (0.75–3.74) | 0.189 |
OR – odds ratio; CI – confidence interval; Ref – reference. Model 1: unadjusted model; Model 2: adjustment of age, gender, BMI, marital status, family PIR, and education level; Model 3: adjustment of age, gender, BMI, marital status, race, education level, family PIR, waist circumference, hypertension, CHF, CHD, angina pectoris, heart attack, platelet count, diabetes, beta-blockers, and antidepressants.
Table 5.
Association between sleep disorder related variables and stroke in participants ≥65 years of age.
| Variables | Model 1 | P | Model 2 | P | Model 3 | P |
|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||
| Sleep duration | ||||||
| <6 h | 1.12 (0.71–1.77) | 0.623 | 1.03 (0.65–1.63) | 0.895 | 1.03 (0.62–1.73) | 0.894 |
| 6–7 h | Ref | Ref | Ref | |||
| 7–8 h | 0.80 (0.44–1.45) | 0.439 | 0.86 (0.47–1.58) | 0.617 | 0.90 (0.49–1.65) | 0.717 |
| ≥8 h | 1.01 (0.66–1.53) | 0.989 | 0.99 (0.63–1.56) | 0.977 | 1.04 (0.66–1.62) | 0.873 |
| Sleep disorder | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 1.14 (0.59–2.19) | 0.693 | 1.07 (0.47–2.43) | 0.862 | 0.85 (0.37–1.91) | 0.675 |
| Time required to fall asleep | 1.01 (1.01–1.02) | 0.037 | 1.01 (1.00–1.02) | 0.130 | 1.00 (0.99–1.01) | 0.539 |
| Frequency of sleep deprivation | ||||||
| Never | Ref | Ref | Ref | |||
| One time a month | 0.89 (0.56–1.42) | 0.619 | 1.07 (0.71–1.62) | 0.719 | 1.05 (0.69–1.60) | 0.808 |
| 2–4 times a month | 1.13 (0.78–1.64) | 0.513 | 1.32 (0.87–2.00) | 0.181 | 1.30 (0.84–1.99) | 0.218 |
| 5–15 times a month | 1.14 (0.60–2.18) | 0.675 | 1.30 (0.64–2.62) | 0.450 | 1.05 (0.48–2.29) | 0.893 |
| 16–30 times a month | 1.67 (0.82–3.43) | 0.144 | 1.73 (0.83–3.60) | 0.129 | 1.47 (0.64–3.35) | 0.343 |
| Frequency of early-morning wakefulness | ||||||
| Never | Ref | Ref | Ref | |||
| One time a month | 0.93 (0.59–1.47) | 0.746 | 0.98 (0.63–1.54) | 0.944 | 1.00 (0.65–1.55) | 0.995 |
| 2–4 times a month | 1.02 (0.60–1.73) | 0.943 | 1.07 (0.61–1.87) | 0.800 | 1.01 (0.58–1.77) | 0.956 |
| 5–15 times a month | 1.19 (0.62–2.28) | 0.581 | 1.31 (0.69–2.50) | 0.394 | 1.20 (0.60–2.44) | 0.591 |
| 16–30 times a month | 1.80 (1.01–3.21) | 0.039 | 1.68 (0.94–3.02) | 0.069 | 1.66 (0.90–3.05) | 0.092 |
| Frequency of midnight wakefulness | ||||||
| Never | Ref | Ref | Ref | |||
| One time a month | 1.38 (0.97–1.97) | 0.062 | 1.52 (1.09–2.14) | 0.011 | 1.59 (1.15–2.19) | 0.004 |
| 2–4 times a month | 0.99 (0.65–1.53) | 0.984 | 1.13 (0.72–1.77) | 0.581 | 1.02 (0.65–1.62) | 0.913 |
| 5–15 times a month | 1.25 (0.79–2.00) | 0.322 | 1.44 (0.86–2.39) | 0.148 | 1.29 (0.72–2.31) | 0.377 |
| 16–30 times a month | 1.91 (1.09–3.35) | 0.018 | 1.92 (1.08–3.42) | 0.020 | 1.80 (0.98–3.29) | 0.048 |
OR – odds ratio; CI – confidence interval; Ref – reference. Model 1: unadjusted model; Model 2: adjustment of age, gender, BMI, marital status, family PIR, and education level; Model 3: adjustment of age, gender, BMI, marital status, race, education level, family PIR, waist circumference, hypertension, CHF, CHD, angina pectoris, heart attack, platelet count, diabetes, beta-blockers, and antidepressant.
Discussion
Several studies have explored the association between sleep health and stroke and showed that sleep duration that is too short or too long and certain sleep disorders can increase the morbidity and mortality of stroke [9–12]. We aimed to explore the association between other less-reported sleep quality-related indicators and stroke. Therefore, the present study was based on the study population of NHANES and used a cross-sectional design to observe the sleep status of participants with and without a history of stroke, and then reveal the possible risk factors of stroke from the perspective of sleep. We found that sleep duration <6 h, sleep disorder, frequency of sleep deprivation, early morning wakefulness, and middle of the night wakefulness of 16 to 30 times a month were all associated with an increased risk of stroke. The finding of this study may provide a basis for prevention and treatment of stroke.
There have been many studies to explore the association between sleep duration and stroke. The results of a multicenter prospective cohort study using the data from the Sleep Heart Health Study database found that sleep duration <6 h was a risk factor for stroke with the adjustment of age, sex, race, smoking, alcohol use, BMI, hypertension, diabetes, apnea-hypopnea index, and benzodiazepine use [20], which was consistent with our results. Titova et al suggested that long sleep duration was associated with an increased risk of total stroke and ischemic stroke, while short sleep duration was associated with an increased risk of cerebral hemorrhage from a prospective study that included 79 881 individuals in Sweden [21]. A meta-analysis of 16 prospective studies enrolling 528 653 participants showed that short sleep duration was significantly associated with non-fatal stroke in people in non-Asian countries [10]. The results of these studies suggested that sleep duration may have different manifestations for different subtypes of stroke; therefore, further studies are needed to explore whether the difference exists. The current research on the mechanism between sleep duration and stroke is mostly focused on short sleep duration. Studies have found that too short of a monitored or self-reported sleep duration does not activate an individual’s hormonal response to regulate appetite and energy balance (such as the levels of leptin and ghrelin), increasing energy intake, reducing energy consumption, and leading to obesity and dyslipidemia [22–24]. Short sleep duration could affect blood sugar metabolism and increase the risk of diabetes, which is not conducive to the blood sugar control of diabetic patients [25,26]. Short sleep duration can increase the systolic and diastolic blood pressure and elevate nocturnal blood pressure [25,27]. Obesity, dyslipidemia, diabetes, and hypertension mentioned above were all risk factors for stroke [24].
In this study, we found that after adjusting for potential confounders, participants with severe sleep disorders (specifically, sleep deprivation, early morning wakefulness, and middle of the night wakefulness 16–30 times a month) had a stroke risk of 0.81 to 0.99 times higher than those who never had sleep deprivation, early morning wakefulness, and middle of the night wakefulness. It was suggested that these 3 factors that reflected a severe sleep disorder may be risk factors for stroke. Most studies on the relationship between sleep and stroke have focused on some sleep-related diseases, such as sleep apnea syndrome and restless leg syndrome [28]; other types of sleep problems were rarely reported. The present study involved self-reported variables of whether a participant had a sleep disorder, the frequency of sleep deprivation, frequency of early morning wakefulness, and frequency of middle of the night wakefulness, which could evaluate the sleep disorder of the included participants in a more in-depth and detailed manner. Sleep disorders could cause dyslipidemia, including the level of total cholesterol, triglycerides, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol changes, leading to a lipid disorder promoting the deposition of fat in the arterial endothelium, causing atherosclerosis [29,30]. Also, stroke is considered to be highly related to atherosclerosis [31].
In the subgroup analysis stratified by age, we found that participants under the age of 65 years had increased risk of stroke with sleep duration less than 6 h, sleep disorder, 16 to 30 times a month of sleep deprivation, and early morning wakefulness, and in participants 65 years and older, stroke was significantly associated with middle of the night wakefulness 1 time a month and 16 to 30 times a month. These results suggested that in middle-aged people and relatively young people, poor sleep may be more serious and may increase the risk of stroke. In the elderly participants over 65, poor sleep quality may be a common and persistent problem, and attention should be paid to middle of the night wakefulness. We adjusted for beta-blockers and antidepressants in the multivariate model, and the results suggested that clinicians may need to combine the medication of the elderly when performing sleep intervention in patients over 65 years old.
Based on the results of this study, it is necessary to screen the sleep duration and sleep disorder-related variables in the high-risk stroke population. A pre-existing sleep disorder can increase the risk of stroke, and intervening may focus on interventions for cardiovascular, metabolic, and inflammatory changes [32]. Stroke, in turn, promotes the development of sleep disorders. Sleep screening of stroke survivors is also necessary to help prevent subsequent strokes. After a stroke, sleep disorders can be treated by helping patients maintain adequate sleep-wake patterns [32,33]. Sleep is key to the recovery of neurological functions, including physical, occupational, and speech. Sleep screening can be monitored more accurately by polysomnography [34].
However, our study had a few limitations. First, stroke reported by the participants in this study was derived from self-reports, and especially for those with cognitive impairment, there may have been information bias due to the limitation of the NHANES. However, the NHANES questionnaire is conducted by trained professionals, and self-reported stroke through the questionnaire could be used to assess the prevalence of stroke with a certain degree of reliability in epidemiological studies [35]. Second, since the stroke data was self-reported, it was not possible to classify the stroke type and stroke location, and therefore the association with sleep disorder-related variables could not be investigated separately. However, it is worth noting that our study results were consistent with those of other studies regarding the association between stroke type and sleep duration [10,21]. Third, the sleep disorder-related variables were also self-reported; however, early studies have shown that self-reported sleep duration was consistent with the sleep duration obtained through actigraphy monitoring [13,36]. Fourth, this study was a cross-sectional study, and there was a certain limitation in determining the causal relationship between sleep-related variables and stroke. Finally, only the living stroke patients who could participate in the investigation were included in our study, and those who died of stroke were not in the scope of the investigation, and therefore there may be self-selection bias.
Conclusions
In conclusion, self-reported stroke was higher for participants with short (<6 h) sleep duration, severe sleep deprivation, severe early morning wakefulness, and severe middle of the night wakefulness compared with that of participants with 6 to 7 h sleep duration and no sleep deprivation, early morning wakefulness, or middle of the night wakefulness. In participants aged 65 years or older, there was no association between sleep duration and observed sleep disorder-related variables and stroke. These results suggest that interventions for healthy sleep in the population may be beneficial to reduce the risk of stroke, especially for individuals under the age of 65 years.
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.McDermott M, Brown DL, Chervin RD. Sleep disorders and the risk of stroke. Expert Rev Neurother. 2018;18:523–31. doi: 10.1080/14737175.2018.1489239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurd MD, Goel I, Sakai Y, Teramura Y. Current status of ischemic stroke treatment: From thrombolysis to potential regenerative medicine. Regen Ther. 2021;18:408–17. doi: 10.1016/j.reth.2021.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naghavi M, Abajobir AA, Abbafati C, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hankey GJ. Stroke. Lancet. 2017;389:641–54. doi: 10.1016/S0140-6736(16)30962-X. [DOI] [PubMed] [Google Scholar]
- 6.Cichon N, Wlodarczyk L, Saluk-Bijak J, et al. Novel advances to post-stroke aphasia pharmacology and rehabilitation. J Clin Med. 2021;10:3778. doi: 10.3390/jcm10173778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guzik A, Bushnell C. Stroke epidemiology and risk factor management. Continuum (Minneap Minn) 2017;23(1, Cerebrovascular Disease):15–39. doi: 10.1212/CON.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 8.Lip GYH, Gue Y, Zhang J, et al. Stroke prevention in atrial fibrillation. Trends Cardiovasc Med. 2021 doi: 10.1016/j.tcm.2021.10.001. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Mims KN, Kirsch D. Sleep and stroke. Sleep Med Clin. 2016;11:39–51. doi: 10.1016/j.jsmc.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 10.He Q, Sun H, Wu X, et al. Sleep duration and risk of stroke: A dose-response meta-analysis of prospective cohort studies. Sleep Med. 2017;32:66–74. doi: 10.1016/j.sleep.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb E, Landau E, Baxter H, et al. The bidirectional impact of sleep and circadian rhythm dysfunction in human ischaemic stroke: A systematic review. Sleep Med Rev. 2019;45:54–69. doi: 10.1016/j.smrv.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Duss SB, Seiler A, Schmidt MH, et al. The role of sleep in recovery following ischemic stroke: A review of human and animal data. Neurobiol Sleep Circadian Rhythms. 2016;2:94–105. doi: 10.1016/j.nbscr.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt M, Dekkers M, Baillieul S, et al. Measuring sleep, wakefulness, and circadian functions in neurologic disorders. Sleep Med Clin. 2021;16:661–71. doi: 10.1016/j.jsmc.2021.08.005. [DOI] [PubMed] [Google Scholar]
- 14.National Health Nutrition Examination Survey. 2005-2006 Data Documentation Codebook Frequencies. Medical Conditions (MCQ_D) Available from: https://wwwncdcgov/Nchs/Nhanes/2005-2006/MCQ_D.htm.
- 15.National Health Nutrition Examination Survey. 2007-2008 Data Documentation Codebook Frequencies. Medical Conditions (MCQ_E) Available from: https://wwwncdcgov/Nchs/Nhanes/2007-2008/MCQ_E.htm.
- 16.Ghozy S, Zayan AH, El-Qushayri AE, et al. Physical activity level and stroke risk in US population: A matched case-control study of 102,578 individuals. Ann Clin Transl Neurol. 2022;9(3):264–75. doi: 10.1002/acn3.51511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, He J, Shi W. Association between urinary environmental phenols and the prevalence of cardiovascular diseases in US adults. Environmental Science and Pollution Research. 2022 doi: 10.1007/s11356-021-18323-3. [DOI] [PubMed] [Google Scholar]
- 18.National Health Nutrition Examination Survey. 2005-2006 Data Documentation Codebook Frequencies. Sleep Disorders (SLQ_D) Available from: https://wwwncdcgov/Nchs/Nhanes/2005-2006/SLQ_D.htm.
- 19.National Health Nutrition Examination Survey. 2007-2008 Data Documentation Codebook Frequencies. Sleep Disorders (SLQ_E) Available from: https://wwwncdcgov/Nchs/Nhanes/2007-2008/SLQ_E.htm.
- 20.Zhao B, Wu Y, Jin X, et al. Objectively measured sleep characteristics and incidence of ischemic stroke: The sleep heart health study. Nat Sci Sleep. 2021;13:1485–94. doi: 10.2147/NSS.S313891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titova OE, Michaëlsson K, Larsson SC. Sleep duration and stroke: Prospective cohort study and mendelian randomization analysis. Stroke. 2020;51:3279–85. doi: 10.1161/STROKEAHA.120.029902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Primack C. Obesity and sleep. Nurs Clin North Am. 2021;56(4):565–72. doi: 10.1016/j.cnur.2021.07.012. [DOI] [PubMed] [Google Scholar]
- 23.St-Onge MP. Sleep-obesity relation: underlying mechanisms and consequences for treatment. Obes Rev. 2017;18(Suppl 1):34–39. doi: 10.1111/obr.12499. [DOI] [PubMed] [Google Scholar]
- 24.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: A systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–56. doi: 10.1016/j.sleep.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 25.St-Onge M, Grandner M, Brown D, et al. Sleep duration and quality: Impact on lifestyle behaviors and cardiometabolic health: A scientific statement from the American Heart Association. Circulation. 2016;134:e367–86. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu C, Liao B, Nie J, et al. The association between sleep duration and chronic diseases: A population-based cross-sectional study. Sleep Med. 2020;73:217–22. doi: 10.1016/j.sleep.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Silvani A. Sleep disorders, nocturnal blood pressure, and cardiovascular risk: A translational perspective. Auton Neurosci. 2019;218:31–42. doi: 10.1016/j.autneu.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Khot SP, Morgenstern LB. Sleep and stroke. Stroke. 2019;50:1612–17. doi: 10.1161/STROKEAHA.118.023553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki M, Shimamoto K, Sekiguchi H, et al. Arousal index as a marker of carotid artery atherosclerosis in patients with obstructive sleep apnea syndrome. Sleep Breath. 2019;23(1):87–94. doi: 10.1007/s11325-018-1664-0. [DOI] [PubMed] [Google Scholar]
- 30.Tobaldini E, Costantino G, Solbiati M, et al. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev. 2017;74(Pt B):321–29. doi: 10.1016/j.neubiorev.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Libby P, Buring JE, Badimon L, et al. Atherosclerosis. Nat Rev Dis Primers. 2019;5:56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 32.Pérez-Carbonell L, Bashir S. Narrative review of sleep and stroke. J Thorac Dis. 2020;12(Suppl 2):S176–90. doi: 10.21037/jtd-cus-2020-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hepburn M, Bollu PC, French B, Sahota P. Sleep medicine: Stroke and sleep. Mo Med. 2018;115(6):527–32. [PMC free article] [PubMed] [Google Scholar]
- 34.Markun LC, Sampat A. Clinician-focused overview and developments in polysomnography. Curr Sleep Med Rep. 2020;6(4):309–21. doi: 10.1007/s40675-020-00197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engstad T, Bønaa KH, Viitanen M. Validity of self-reported stroke: The Tromsø Study. Stroke. 2000;31:1602–7. doi: 10.1161/01.str.31.7.1602. [DOI] [PubMed] [Google Scholar]
- 36.Lockley S, Skene D, Arendt J. Comparison between subjective and actigraphic measurement of sleep and sleep rhythms. J Sleep Res. 1999;8(3):175–83. doi: 10.1046/j.1365-2869.1999.00155.x. [DOI] [PubMed] [Google Scholar]


