Abstract
Purpose of review:
The intestinal enteroendocrine cells (EECs) are specialized hormone secreting cells that respond to both circulating and luminal cues. Collectively, EECs constitute the largest endocrine organ of the body and signal to a multitude of targets including locally to neighboring intestinal cells, enteric neurons, as well as systemically to other organs such as the pancreas and brain. To accomplish their wide range of downstream signaling effects, EECs secrete multiple hormones; however, the mechanisms that influence EEC development in the embryo and differentiation in adults are not well-defined.
Recent Findings:
This review highlights the recent discoveries in EEC differentiation and function while also discussing newly revealed roles of transcription factors and signaling networks involved in the allocation of EEC subtypes that were discovered using a combination of novel intestinal model systems and genetic sequencing. We also discuss the potential of these new experimental models that study the mechanisms regulating EEC function and development both to uncover novel therapeutic targets.
Summary:
Several EEC hormones are being used to treat various metabolic disorders such as type 2 diabetes and obesity. Therefore, understanding the signaling pathways and gene regulatory networks that facilitate EEC formation is paramount to the development of novel therapies.
Keywords: Nutrient Absorption, Metabolic Disorders, Pluripotent Stem Cells, Organoids, Cell-based Therapy
Introduction
The small intestine regulates nutrient digestion and facilitate caloric absorption. To carry out these roles, the intestine consists of organized crypt-villus structures that are maintained by intestinal stem cells that proliferate and differentiate into absorptive and secretory epithelial cell types [1, 2]. One of the most important secretory cell types are the enteroendocrine cells (EECs). EECs are essential for virtually all processes related to nutrient homeostasis, from the regulation of hunger, digestion and peristalsis to the breakdown of nutrients[3]. EECs make up ~1% of the intestinal epithelium that sense both luminal and circulating factors and in response secrete over 20 different biologically active peptides that signal to a variety of target cells. These peptides act as both endocrine hormones, regulating organs like the pancreas and brain, and as paracrine signals, influencing neighboring cells in the intestine and the enteric nervous system (ENS)[4]. The classic nomenclature defined EEC subtypes by the main hormone secreted by each cell; however, it has become evident that EECs can express multiple hormones simultaneously and may even switch hormone expression[5–7]. The precise mechanisms that regulate development of the multiple EEC cell types and how EEC hormone expression profiles change over time in response to nutrients is just beginning to be interrogated. Moreover, EECs differ molecularly and functionally in the different GI organs from the stomach to the colon. Although many questions remain unanswered, recent analysis of EECs at the single cell level have made important progress in building the foundation for the network of transcription factors involved in regulating EEC fate.
This review summarizes the recent findings on the biology and function of EECs. We focus on EEC physiology, the roles of novel transcription factors (TFs) and recent developments on the signaling pathways and luminal cues involved in EEC development. Lastly, we describe recent model systems that have helped provide a deeper understanding of both EEC development and function in controlling nutrient absorption. The most effective cure for T2D is bariatric surgery, which causes changes in many GI hormones, suggesting that EEC hormones play many unappreciated roles as metabolic regulators. Two such hormones, GIP and GLP-1 are currently in use for the treatment of T2D because of their ability to modulate insulin secretion from the pancreas. Therefore, a better understanding of EEC development and function in normal and pathological contexts is essential to fully unlock the therapeutic potential of this cell type to treat metabolic and digestive disorders.
EEC Physiology and Function
EECs are found throughout the intestinal tract along the crypt-villus axis where they can sense both luminal and basal cues that trigger hormone secretion. EEC hormones are packaged within secretory vesicles marked by ChromagraninA or Synaptophysin. When secreted, these hormones can act in an endocrine fashion and travel in the circulation to signal to distant organs such as the pancreas and the brain (Figure 1A). EECs can also act in a paracrine fashion and communicate with neighboring intestinal epithelial cells or to enteric neurons of the ENS, either by classical peptide signaling or by forming a glutamatergic synapse [4, 8]. The “gut-brain axis” involves both endocrine and paracrine acting factors to control physiological functions such as food intake, satiety, and inflammation. Two hormones, glucagon-like peptide 1 (GLP1) and gastric inhibitory peptide (GIP) are released soon after food ingestion and participate in glucose-dependent insulin secretion from pancreatic Beta cells. On the other hand, Ghrelin and Somatostatin are decretin hormones that decrease pancreatic insulin secretion. Ghrelin is also an orexigenic hormone that regulates appetite with some preliminary studies showing that colonic hormone INSL-5 also has an orexigenic function. A number of hormones also control gut motility (5-HT) and gastric emptying (CCK and PYY) as well as satiety (CCK and GLP-1) as they have been shown to remain active hours after food ingestion [3, 4, 9–11]. Somatostatin and CCK also have a role in the inhibition of gastric acid secretion by parietal cells in the stomach. GLP-2 has known roles in the enhancement of barrier function and increasing absorption and digestion but has recently been linked to have a role in increasing microvilli length in enterocytes [12]. Neurotensin (NTS) is secreted shortly after fat ingestion and stimulates mucosa growth under nutrient deprivation, but a recent study uncovered a novel role of NTS in the ISC. Rock et al. used a NTS knockout mouse model to show that loss of NTS led to impaired crypt proliferation. They also showed NTS to promote ERK1/2 signaling and drive cell cycle progression and crypt proliferation under nutrient rich conditions. On the other hand, under nutrient depleted conditions, NTS stimulated WNT/β-cat signaling promoting ISC gene signature and enhanced ISC function [13].
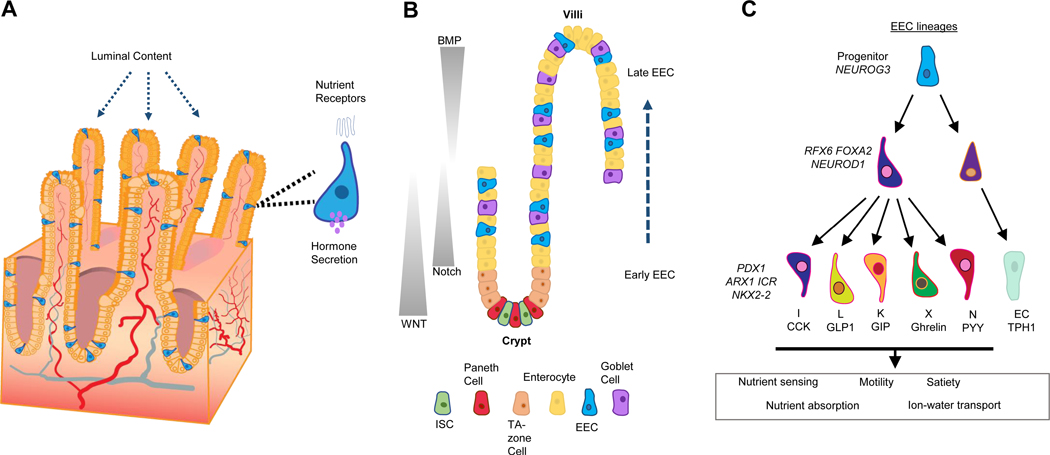
Figure 1:
A) Intestinal Enteroendocrine cells (EECs) sense luminal cues from nutrients and in response secrete hormones into the basal side that can communicate with neighboring cells, other segments of the small intestine and even distant organs. B) The differentiation of EECs in the small intestinal is influenced by signaling pathway gradients along the crypt-villus axis. They mature along the crypt to villus axis while changing gene expression leading to secretion of specific peptides in response to luminal cues. C) A time course of selected early vs intermediate vs late genes or TFs were listed.
EEC hormone secretion is largely mediated by G protein-coupled receptors (GPCRs) that express receptors for metabolites and nutrients such as lipids, monosaccharides, and free fatty acids. Initial activation of GPCR signaling results in Ca2+ release and activation of protein kinase C (PKC) signaling. Details of the cellular processes involved in hormone secretion have recently been reviewed elsewhere [11]. Traditionally, EEC nomenclature was based on individual hormone secretion (i.e. G cell- Gastrin). However, recent studies using single cell RNA-sequencing and cell lineage tracing have suggested that the system to define EEC “subtypes” may not be as accurate. Beumer et al. showed that EECs can express different hormones based on their location along the crypt-villus axis which suggests that EECs can secrete multiple hormones [6]. Recently, Goldspink et al. made a reporter for GLP-1 to t lineage trace L-cells and using a combination of RNA-seq and mass spectrometry, showed that L cells can secrete multiple hormones in response to nutrients. Additionally, they showed L-cells to exhibit depolarization and changes in calcium levels in response to G-protein-coupled receptors[14]. Another recent study used a Cck-eGFP transgenic line to sort EECs expressing Cck and showed that these cells were also actively expressing Glp1, Gip, Nts and Pyy suggesting there is an overlap in hormone expression in single EECs in mice [15, 16]. Lastly, human patient samples were used to show several populations of EECs expressing multiple hormones in the jejunum. Using high resolution microscopy this study reported that all hormones had some degree of co-localization including one population expressing GIP, CCK and GLP1/2, and several populations with 5HT overlapping with other hormones[16]. Taken together, these studies have demonstrated multiple hormones are expressed in individual EECs. However, the mechanisms determining hormone expression and regulation are not well understood.
Transcription factors and Signaling Pathways controlling EEC Differentiation
Several transcription factors (TFs) are required for EEC formation. The basic helix-loop-helix TF NEUROG3 is the master regulator driving EEC fate and is required for the formation of all endocrine cells from the gastric antrum through the colon intestine in both mice and humans. However, loss of Neurog3 does not impact endocrine cells in the body of the stomach (the corpus), showing regional differences in transcriptional regulation of EECs [17]. Moreover, Neurog3 is not required for EECs in Zebrafish, instead EECs seem to require a closely related bHLH factor Neurod1 [18]. Neurog3 dose was recently shown as an important factor in directing EEC fate over other secretory lineages. Li et al used mutant Neurog3 reporter mice and discovered that the deletion of one Neurog3 allele resulted in a decrease of EECs and increase in goblet cell numbers and altered gene expression [19]. Loss of function studies in mice and humans have led to the identification of several TFs that act downstream of Neurog3 to regulate EEC formation, including Rfx6, Arx1, Nkx2–2, Pax4, Pax6, NeuroD1, Isl1 and Insm1 [20–25] (Figure 1C). From these studies, it was shown that some TFs are required for all EECs while others were shown to be required for subsets of EECs. Loss-of-function studies with these TFs have also helped shape the transcriptional network that is required for proper EEC differentiation by establishing which TFs are higher up in this network. Neurog3 is needed first to generate endocrine progenitors, other factors are required early on (Rfx6, NeuroD1, FoxA2) as they cause loss of multiple EEC lineages including GIP, GLP-1 and Ghrl. Other TFs are more lineage specific, suggesting they are required at the later stages of EEC differentiation (Pax4, Pax6, Insm1).
Two recent studies from the Clevers group combined the use of loss of function techniques in organoids with single cell RNA sequencing and lineage tracing to establish a more comprehensive network of TFs and regulators that control EEC development in mouse and humans [5, 7]. Gehart et al developed a novel bi-fluorescent reporter of Neurog3 in the mouse that tracks the age and lineage of EECs from the onset of Neurog3 transcription. This lineage tracing approach allowed for the isolation of EECs at various stages of differentiation and cataloged TFs that were expressed during early, intermediate and late stages of EEC development using single cell sequencing. Subsequent loss-of-function studies in organoids identified TFs that were required for EEC differentiation. In a second study, Beumer et al generated novel human intestinal organoid lines harboring fluorescent reporters within an ‘EEC-TAG Biobank’ and created a ‘human EEC atlas’ [Table 1]. This work identified key differences between murine and human EECs mainly focusing on genes expressed in human EECs but not in mouse EECs (MDK, FGF14, TAC3) and noted the expression of FGFR21 in human EECs. They validated these findings by adding exogenous FGF21 and noted an upregulation of multiple hormones. This study also provided evidence for the formation of a feedback loop facilitated by multiple hormones to control EEC hormone secretion. These studies have also proposed two novel transcription factors that may be involved in EEC hormone specification: Rfx6 and Mty1 [5]. Rfx6 mutations have been associated with Mitchell-Riley syndrome, an autosomal disorder that affects not only pancreatic development causing neonatal diabetes, but also intestinal mal-rotation and loss of several EEC lineages[26]. Rfx6 mutant mice were recently reported to lack several subtypes of EECs but showed an increase in mucosal 5-HT and enterochromaffin cells. This suggests that Rfx6 plays a role in directing EEC progenitors into specific lineages separating 5-HT-secreting cells from the other EEC lineages, and loss of Rfx6 causes a decrease of EEC progenitors [27].
Table 1–
Recent Toolkit of EEC Markers
Several reporters in both mouse (m−) and human (h−) were developed to examine EEC development. These various markers occur in a gradient of expression along the SI axis. These reporters have been used to map transcriptional networks for specific EEC subtypes as well as to further study hormone secretion in isolated lineages.
| Common EEC Markers | Function | Highest Expression | Reporters | References |
|---|---|---|---|---|
| Neurog3 | Master Transcription Factor Regulator | Pan SI | m-Chrono, m-EYFP, h-dTomato | 5,7,19 |
| Neurod1 | Pan-EEC Downstream Regulator | Pan SI | m-GFP | 23 |
| Chga | Pan-EEC Marker | Pan SI | h-tdTom, h-mNEON | 5 |
| Tph1 (5-HT) | Regulation of intestinal motility and hormone secretion | Pan SI | h-mClover | |
| Sst | Inhibitory regulator of hormone secretion and food intake | Pan SI | h-mNEON | |
| Gast | Regulation of acid secretion | Proximal | h-mNEON | |
| Ghr | Stimulator of food intake and hunger | Proximal | h-mNEON | |
| Mln | Regulation of gut motility | Proximal | h-mNEON | |
| Cck | Inhibitor of food intake; stimulates pancreas | Proximal/Medial | h-mNEON | |
| Gip | Incretin regulator; satiety regulator inhibitor | Proximal/Medial | h-mNEON | |
| Nts | Stimulator of acid secretion and biliary section | Distal | h-mNEON | |
| Gcg (Glp1) | Incretin regulator; delays gastric emptying; growth promotion | Distal | h-YFP, h-tdTomato | 5,14 |
In addition to TFs, there are several signaling pathways that play a role in the differentiation of EECs. Many studies have shown that counter-balancing gradients of WNT/BMP control the crypt-villus axis with WNT signaling being essential for maintaining the intestinal stem cell (ISC) niche and BMP promoting differentiation. The ISCs transition into the transit-amplifying zone where cells are exposed to either Wnt or Notch signaling which dictates their fate promoting proliferation and ultimately differentiation regulated through by BMP and Notch. Notch signaling drives the commitment of these TA cells into the absorptive lineage resulting in enterocytes [28, 29]. WNT, EGF, and MAPK signaling promote the maintenance of the stem cell niche and TA cells, however, inhibiting these signaling pathways has been previously shown to promote the differentiation of secretory progenitors, mainly leading to the EEC fate (Figure 1B) [30]. In addition to the pro-differentiation function of BMP, a recent study used EEC lineage tracing to show that a BMP gradient can EEC hormone switching along the crypt-villi axis [6]. At early stages of EEC differentiation, when cells are in the crypt in a low BMP environment, GLP-1 was highly expressed, however when these lineage-traced cells moved up the villus into a high BMP environment they switched to express Secretin and Neurotensin [6]. Another difference has emerged in EECs dependent on their crypt-villus location where EECs near the crypts were shown to acquire stem-cell like functions after injury and depletion of the Paneth cell population [31].
Other signaling pathways are involved in regulating EECs. For example, RHOA signaling was recently reported to regulate both the number of EECs and Glp-1 secretion in mouse intestine. Using intestinal endocrine fluorescent reporters (Glp1-Venus, Neurog3- RFP) this study reported that inhibition of RHOA signaling led to an increase in the number of GLP1 secreting cells in vivo and in organoids isolated from mouse small intestine as well as Glp-1 secreted in the media. They also compare normal chow vs a high fat diet that caused an increased Glp-1 secreting cells along with a higher tolerance for glucose in vivo [32]. In addition to signaling pathways, multiple studies have shown that GI hormones to signal neighboring EECs in a paracrine fashion affecting EEC lineages and hormone secretion. A recent study showed G-protein coupled bile acid receptor 1 (GPBAR1) to be a selective regulator of intestinal L-cells with GPBAR1 agonist L3740 increasing L-cell density and Glp-1 secretion in both mouse and human intestinal organoids. They further showed that serotonin signaling through 5-HT4 receptor mimicked the effects of L3740 uncovering a mechanism that facilitates L-cell differentiation through Glp-1 paracrine signaling and is mediated by 5-HT. [33]. Incretins also have the ability to regulate secretion of other hormones along the GI tract, where ex vivo rat colons were infused with CCK and GIP peptides to examine their effects in the secretion of other peptides. This study found that GIP triggered an increase in both Glp-1 and PYY secretion from the colon. Since GIP is only produced in the duodenum, this data suggested that GIP secretion from the proximal gut may regulate hormone secretion in the colon and possibly other regions of the small intestine[34].
EECs are nutrient-sensing and as such their response to dietary changes can also affect both hormone secretion and the proportions and numbers of EEC subtypes [35]. Multiple studies in mice and humans have noted that obesity greatly affects the intestinal endocrine system[36, 37], affecting both overall EEC numbers and specific hormone levels. Moreover, specific short-term diet changes such as a high fat diet or a high fiber diet can also affect overall EEC numbers and the secretion of particular hormones[38–40]. A recent study used an obesogenic diet mouse model and performed single cell RNA-sequencing to show the diet’s effect in the ISC and specific epithelial populations. They showed that an obesogenic diet improves ISC and progenitor proliferation and differentiation enhancing cell turnover. More importantly, under the obesogenic diet, they identified a population of cells that expressed both endocrine progenitor and ISC markers which are reminiscent of Lgr5+ intestinal stem cells. Lastly, the obesogenic diet also led to an increase in plasma levels of Glp-1 and a decrease in plasma 5-HT and Ghrl [41].
New Systems for studying EECs and Implications Towards Human Health
Due to the rare number of EECs, researchers have used technologies developed over the past decade to generate novel tools that enabled in-depth analysis of EECs. One of the most important advances in studying the intestine has been the development of organoid model systems. Organoids can be generated either by isolation and propagation of the tissue-resident intestinal stem cells in the crypts or by the directed differentiation of pluripotent stem cells into an intestinal fate [42–44], although iPSC-derived human organoids have also been generated to represent the development of the entire GI tract from esophagus to colon [45–47]. Furthermore, PSC-derived organoids possess a more complex structure that includes epithelium, mesenchyme and can be engineered to include an enteric nervous system[48]. There are advantages and disadvantages to both model systems, both crypt-derived and PSC-derived intestinal organoids have proven to be useful model systems for the study of EECs. For example, murine crypt-derived organoid cultures were used as an in vitro model to demonstrate that an inhibition of Wnt, Notch, and EGF pathways promotes EEC differentiation [14, 30, 32]. New human in vitro systems offer a tremendous opportunity to study EEC development and function. Since EECs are rare, it is often challenging to study their biology. However, by targeting specific TFs or signaling pathways, several studies have used genetic r signaling pathway manipulation to increase human EEC numbers. For example, the generation of a NEUROG3-inducible system allowed for production of all EEC subtypes in PSC-derived intestinal organoids[49]; increasing the relative proportion of EECs from 1% to up to 25% of the intestinal epithelium and enabling functional hormone secretion studies. This work further showed that EEC hormone production was temporally linked to the onset of EEC differentiation, similar to what was described by Gehart et al in the mouse. This construct has since been added to human crypt derived cultures to establish a flexible in vitro model for functional studies of EECs development and function[50].
The use of iPSC-derived tissues allows for effective modeling of genes that impact EEC formation. This can be done by deriving iPSC lines from patients or by genetic manipulation of existing pluripotent stem cell lines. Genetic techniques that have been used include RNA interference, CRISPR-based editing technologies, or expressing mutant proteins, all of which have shed light on EEC functions, morphology and response to stimuli. For example, like in mice, patients with loss-of-function mutations in NEUROG3 have neonatal diabetes and malabsorptive diarrhea requiring total parental nutrition (TPN) for survival. However, some patients with NEUROG3 are not born with diabetes and mild forms of diarrhea. To identify the molecular basis for the range in phenotypes, Zhang et al. used human PSCs to model the pancreatic and intestinal phenotypes of the disease-causing mutations in NEUROG3 and overlaid that with biochemical structure-function studies [51]. From this they identified which mutations were null, hypomorphic and gain-of-function. In a follow up study, NEUROG3-deficient human and mouse intestinal tissues were used to identify how loss of EECs caused profound malabsorption [52]. In this study, the researchers showed that EECs are required to maintain normal ion transport in the small intestine, which was regulating ion-coupled absorption of glucose and dipeptides. Moreover, the EEC hormone PYY was able to restore ion-coupled nutrient absorption in vitro and rescued nutrient absorption and improved survival in Neurog3-deficient mice. This study implicated the use of PYY being clinically adopted for patients suffering from intestinal malabsorption.
One important role of intestinal EEC-secreted hormones is the regulation of glucose homeostasis through the incretin hormones GIP and GLP-1. These hormones augment insulin secretion in response to glucose. Moreover, GIP and GLP-1 have been shown to promote beta-cell proliferation. Because of this, incretin hormones have been exploited as a therapy to improve beta cell function in patients with type 2 diabetes (T2D) [53, 54]. In particular, GLP-1 analogues, GLP-1 receptor agonists or inhibitors of the GLP-1 cleaving enzyme DPP4, were all shown to reduce blood glucose levels in patients with T2D [55]. Additionally, EEC-secreted hormones have been implicated for treatment of obesity, as hormone levels, including GLP-1 and PYY, are inversely correlated to BMI [56, 57]. However, the most effective treatment for obesity and T2D is gastric bypass/bariatric surgery. Most patients who have undergone bariatric surgery experience a rapid reversal of T2D, long before weight loss. While the exact role of EECs in reversal of T2D is not clear, it seems reasonable that the alteration of the proximal-distal nutrient exposure in the small intestine[11]. A particular study investigated the effects in density and levels of gene expression in EEC hormones in obese patients with T2D after RYGB. They compared T2D patients and BMI and age matching patients after RYGB and took biopsies after 10 months. Both groups experienced a decrease in Ghrelin, Secretin and GIP and an upregulation in Glp1. However, PYY was the only hormone that showed different results between the groups, where the density of PYY-positive cells was seen only in the control group, highlighting the effects of T2D in L-cells which may not fully recover even after RYGB [58]. An additional study reported ketone body levels in patients after RYGB. The functional effects of this were studied in mice in a prolonged high fat diet which lead to a significant inhibition of GLP-1 secretion, suggesting RYGB can remove the inhibitory role of ketone bodies in EEC-hormone secretion[59].
Conclusions and Outlook
Recent work on EECs have highlighted their essential roles in small intestinal homeostasis and complex development, including the requirements of novel TFs and signaling pathways. However, further work is required to understand the molecular basis for how individual EEC-subtypes are specified, especially along the proximal-distal axis of the GI tract. Furthermore, the increased use of patient derived biopsies and iPSCs have the potential to drive patient-specific studies of drugs that act via EECs to control nutrient homeostasis. Some previous limitations of in vitro model systems to study how luminal factors impact these hormones have been overcome recently with the development of new engineered fluidic systems that allow for easy control of luminal contents [60]. Further studies profiling EECs interactions with the surrounding niche cells, the ENS, and the gut microbiome are necessary to further improve tissue-in-a-dish models of intestinal development and physiology to properly replicate EEC-hormone development, differentiation and secretion. With a better knowledge of EEC dynamics and improved human systems, identification of new and improved drugs that target absorptive and metabolic processes should be forthcoming.
Key Points.
Enteroendocrine cell-secreted hormones can act in act in an endocrine fashion and travel in the circulation to signal to distant organs such as the pancreas and the brain, or in a paracrine fashion and communicate with neighboring intestinal epithelial cells or to enteric neurons.
Neurog3 drives the endocrine fate and is necessary for all EECs, other downstream transcription factors are necessary for certain subsets of EECs.
EECs differentiate as they move up the crypt-villus axis which has an established WNT-BMP gradient that affects which hormone EECs secrete.
Incretin hormones (GIP, GLP-1) are popular targets for T2D therapy due to their role in promoting Beta cell function and differentiation.
Acknowledgments
Financial Support and Sponsorship:
JMW, JGS and JRE are supported by grants from the NIH (P01 HD093363, UG3 DK119982) the Shipley Foundation, and the Allen Foundation.
Footnotes
Conflicts of Interest: None.
References:
Papers of particular interest, published within 18 months of review have been highlighted as:
*of special interest
**of outstanding interest
- 1.Haber AL, Biton M, Rogel N, et al. , A single-cell survey of the small intestinal epithelium. Nature, 2017. 551(7680): p. 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spence JR, Lauf R, and Shroyer NF, Vertebrate intestinal endoderm development. Dev Dyn, 2011. 240(3): p. 501–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCauley HA, Enteroendocrine Regulation of Nutrient Absorption. J Nutr, 2020. 150(1): p. 10–21. [DOI] [PubMed] [Google Scholar]
- 4.Latorre R, Sternini C, De Giorgio R, Greenwood-Van B, Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil, 2016. 28(5): p. 620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gehart H, Van Es JH, Hamer K, et al. , Identification of Enteroendocrine Regulators by Real-Time Single-Cell Differentiation Mapping. Cell, 2019. 176(5): p. 1158–1173.e16. **They described generated a single cell RNA-seq dataset of EECs at different stages during differentiation.
- 6.Beumer J, Artegiani B, Post Y, et al. , Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat Cell Biol, 2018. 20(8): p. 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beumer J, Puschoff J, Bauza-Martinez J, et al. , High-Resolution mRNA and Secretome Atlas of Human Enteroendocrine Cells. Cell, 2020. 181(6): p. 1291–1306.e19. ** They generated a Human single cell RNA-seq atlas along with several EEC reporters.
- 8.Kaelberer MM, Buchanan KL, Klein ME., et al. , A gut-brain neural circuit for nutrient sensory transduction. Science, 2018. 361(6408). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nauck MA and Meier JJ, Incretin hormones: Their role in health and disease. Diabetes Obes Metab, 2018. 20 Suppl 1: p. 5–21. [DOI] [PubMed] [Google Scholar]
- 10.Xie C, Jones KL, Rayner CK, Wu T, Enteroendocrine Hormone Secretion and Metabolic Control: Importance of the Region of the Gut Stimulation. Pharmaceutics, 2020. 12(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gribble FM and Reimann F, Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol, 2019. 15(4): p. 226–237. [DOI] [PubMed] [Google Scholar]
- 12.Markovic MA and Brubaker PL, The roles of glucagon-like peptide-2 and the intestinal epithelial insulin-like growth factor-1 receptor in regulating microvillus length. Sci Rep, 2019. 9(1): p. 13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rock SA, Jiang K, Wu Y, et al. , Neurotensin Regulates Proliferation and Stem Cell Function in the Small Intestine in a Nutrient-Dependent Manner. Cell Mol Gastroenterol Hepatol, 2021. *They identified a novel role for NTS in the intestinal stem cell niche.
- 14. Goldspink DA, Lu VB, Miedzybrodzka EL, et al. , Labeling and Characterization of Human GLP-1-Secreting L-cells in Primary Ileal Organoid Culture. Cell Rep, 2020. 31(13): p. 107833. * They generated a novel reporter.
- 15.Egerod KL, Engelstoff MS, Grunddal KV, et al. , A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology, 2012. 153(12): p. 5782–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fazio Coles TE, Fothergill LJ, Hunne B, et al. , Quantitation and chemical coding of enteroendocrine cell populations in the human jejunum. Cell Tissue Res, 2020. 379(1): p. 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenny M, Uhl C, Roche C, et al. , Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J, 2002. 21(23): p. 6338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flasse LC, Pirson JL, Stern DG, et al. , Ascl1b and Neurod1, instead of Neurog3, control pancreatic endocrine cell fate in zebrafish. BMC Biol, 2013. 11: p. 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li HJ, Ray SK, Kucukural A, et al. , Reduced Neurog3 Gene Dosage Shifts Enteroendocrine Progenitor Toward Goblet Cell Lineage in the Mouse Intestine. Cell Mol Gastroenterol Hepatol, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du A, McCracken KW, Walp ER, et al. , Arx is required for normal enteroendocrine cell development in mice and humans. Dev Biol, 2012. 365(1): p. 175–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desai S, Loomis Z, Pugh-Bernard A, et al. , Nkx2.2 regulates cell fate choice in the enteroendocrine cell lineages of the intestine. Dev Biol, 2008. 313(1): p. 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson LI, et al. , Pax 4 and 6 regulate gastrointestinal endocrine cell development. Mech Dev, 1998. 79(1–2): p. 153–9. [DOI] [PubMed] [Google Scholar]
- 23.Li HJ, St-Onge L, Hougaard DM, et al. , Intestinal Neurod1 expression impairs paneth cell differentiation and promotes enteroendocrine lineage specification. Sci Rep, 2019. 9(1): p. 19489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soyer J, Flasse L, Raffelsberger W, et al. , Rfx6 is an Ngn3-dependent winged helix transcription factor required for pancreatic islet cell development. Development, 2010. 137(2): p. 203–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terry NA, Walp ER, Lee RA, et al. , Impaired enteroendocrine development in intestinal-specific Islet1 mouse mutants causes impaired glucose homeostasis. Am J Physiol Gastrointest Liver Physiol, 2014. 307(10): p. G979–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Concepcion JP, Reh CS, Daniels M, et al. , Neonatal diabetes, gallbladder agenesis, duodenal atresia, and intestinal malrotation caused by a novel homozygous mutation in RFX6. Pediatr Diabetes, 2014. 15(1): p. 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Piccand J, Vagne C, Blot F, et al. , Rfx6 promotes the differentiation of peptide-secreting enteroendocrine cells while repressing genetic programs controlling serotonin production. Mol Metab, 2019. 29: p. 24–39. **They showed a new role for Rfx6 in EEC lineage regulation and differentiation and showed co-expression of Rfx6 and several hormones.
- 28.van Neerven SM and Vermeulen L, Balancing signals in the intestinal niche. EMBO J, 2017. 36(4): p. 389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shyer AE, Huycke TR, Lee C, et al. , Bending gradients: how the intestinal stem cell gets its home. Cell, 2015. 161(3): p. 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basak O, Beumer J, Wiebrands K, et al. , Induced Quiescence of Lgr5+ Stem Cells in Intestinal Organoids Enables Differentiation of Hormone-Producing Enteroendocrine Cells. Cell Stem Cell, 2017. 20(2): p. 177–190.e4. [DOI] [PubMed] [Google Scholar]
- 31.van Es JH, Wiebrands K, Lopez-Iglesias C, et al. , Enteroendocrine and tuft cells support Lgr5 stem cells on Paneth cell depletion. Proc Natl Acad Sci U S A, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen N, Frimurer TM, Pedersen MT, et al. , Inhibiting RHOA Signaling in Mice Increases Glucose Tolerance and Numbers of Enteroendocrine and Other Secretory Cells in the Intestine. Gastroenterology, 2018. 155(4): p. 1164–1176.e2. [DOI] [PubMed] [Google Scholar]
- 33.Lund ML, Sorrentino G, Egerod KL, et al. , L-Cell Differentiation Is Induced by Bile Acids Through GPBAR1 and Paracrine GLP-1 and Serotonin Signaling. Diabetes, 2020. 69(4): p. 614–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Modvig IM, Christiansen CB, Rehfeld JF, et al. , CCK-1 and CCK-2 receptor agonism do not stimulate GLP-1 and neurotensin secretion in the isolated perfused rat small intestine or GLP-1 and PYY secretion in the rat colon. Physiol Rep, 2020. 8(2): p. e14352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gribble FM and Reimann F, Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annu Rev Physiol, 2016. 78: p. 277–99. [DOI] [PubMed] [Google Scholar]
- 36.Wölnerhanssen BK, Moran AW, Burdyga G, et al. , Deregulation of transcription factors controlling intestinal epithelial cell differentiation; a predisposing factor for reduced enteroendocrine cell number in morbidly obese individuals. Sci Rep, 2017. 7(1): p. 8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richards P, Pais R, Habib AM, et al. , High fat diet impairs the function of glucagon-like peptide-1 producing L-cells. Peptides, 2016. 77: p. 21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye L, Mueller O, Bagwell J, et al. , High fat diet induces microbiota-dependent silencing of enteroendocrine cells. Elife, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Jacobs JP, Lagishetty V, et al. , High-protein diet improves sensitivity to cholecystokinin and shifts the cecal microbiome without altering brain inflammation in diet-induced obesity in rats. Am J Physiol Regul Integr Comp Physiol, 2017. 313(4): p. R473–R486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reimer RA, Pelletier X, Carabin IG, et al. , Increased plasma PYY levels following supplementation with the functional fiber PolyGlycopleX in healthy adults. Eur J Clin Nutr, 2010. 64(10): p. 1186–91. [DOI] [PubMed] [Google Scholar]
- 41. Aliluev A, Tritschler S, Sterr M, et al. , Diet-induced alteration of intestinal stem cell function underlies obesity and prediabetes in mice. Nat Metab, 2021. 3(9): p. 1202–1216. **They showed EECs to de-differentiate to progenitors and supporting the ISC niche under a high fat diet.
- 42.Sato T, Vries RG, Snippert HJ, et al. , Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature, 2009. 459(7244): p. 262–5. [DOI] [PubMed] [Google Scholar]
- 43.Spence JR, Mayhew CN, Rankin SA, et al. , Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature, 2011. 470(7332): p. 105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sato T, Stange DE, Ferrante M, et al. , Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology, 2011. 141(5): p. 1762–72. [DOI] [PubMed] [Google Scholar]
- 45.McCracken KW, Cata EM, Crawford CM, et al. , Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature, 2014. 516(7531): p. 400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trisno SL, Philo KED, McCracken KW, et al. , Esophageal Organoids from Human Pluripotent Stem Cells Delineate Sox2 Functions during Esophageal Specification. Cell Stem Cell, 2018. 23(4): p. 501–515.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Múnera JO, Sundaram N, Rankin SA, et al. , Differentiation of Human Pluripotent Stem Cells into Colonic Organoids via Transient Activation of BMP Signaling. Cell Stem Cell, 2017. 21(1): p. 51–64.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Workman MJ, Mahe MM, Trisno S, et al. , Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med, 2017. 23(1): p. 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinagoga KL, McCauley HA, Munera JO, et al. , Deriving functional human enteroendocrine cells from pluripotent stem cells. Development, 2018. 145(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang-Graham AL, Danhof HA, Engevik MA, et al. , Human Intestinal Enteroids With Inducible Neurogenin-3 Expression as a Novel Model of Gut Hormone Secretion. Cell Mol Gastroenterol Hepatol, 2019. 8(2): p. 209–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang X, McGrath PS, Salomone J, et al. , A Comprehensive Structure-Function Study of Neurogenin3 Disease-Causing Alleles during Human Pancreas and Intestinal Organoid Development. Dev Cell, 2019. 50(3): p. 367–380.e7. *They showed different degrees of phenotypes based on the location of the Neurog3 mutation also comparing pancreas and intestine.
- 52. McCauley HA, Matthis AL, Enriquez JR, et al. , Enteroendocrine cells couple nutrient sensing to nutrient absorption by regulating ion transport. Nat Commun, 2020. 11(1): p. 4791. *They showed EEC to have a role in nutrient absorption and were able to rescue phenotypes by adding specific hormones confirming EECs’ involvement in nutrient absorption.
- 53.Tasyurek HM, Altumbas HA, Balci MK, Sanlioglu S, Incretins: their physiology and application in the treatment of diabetes mellitus. Diabetes Metab Res Rev, 2014. 30(5): p. 354–71. [DOI] [PubMed] [Google Scholar]
- 54.Petersen N, Reimann F, Bartfeld S, et al. , Generation of L cells in mouse and human small intestine organoids. Diabetes, 2014. 63(2): p. 410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gilbert MP and Pratley RE, GLP-1 Analogs and DPP-4 Inhibitors in Type 2 Diabetes Therapy: Review of Head-to-Head Clinical Trials. Front Endocrinol (Lausanne), 2020. 11: p. 178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batterham RL, Cohen MA, Ellis SM, et al. , Inhibition of food intake in obese subjects by peptide YY3–36. N Engl J Med, 2003. 349(10): p. 941–8. [DOI] [PubMed] [Google Scholar]
- 57.Færch K, Torekov SS, Vistisen D, et al. , GLP-1 Response to Oral Glucose Is Reduced in Prediabetes, Screen-Detected Type 2 Diabetes, and Obesity and Influenced by Sex: The ADDITION-PRO Study. Diabetes, 2015. 64(7): p. 2513–25. [DOI] [PubMed] [Google Scholar]
- 58.Rhee NA, et al. , Effect of Roux-en-Y gastric bypass on the distribution and hormone expression of small-intestinal enteroendocrine cells in obese patients with type 2 diabetes. Diabetologia, 2015. 58(10): p. 2254–8. [DOI] [PubMed] [Google Scholar]
- 59.Wallenius V, Wahlgren CD, Pedersen J, et al. , Suppression of enteroendocrine cell glucagon-like peptide (GLP)-1 release by fat-induced small intestinal ketogenesis: a mechanism targeted by Roux-en-Y gastric bypass surgery but not by preoperative very-low-calorie diet. Gut, 2020. 69(8): p. 1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nikolaev M, Mitrofanova O, Broguiere N, et al. , Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature, 2020. 585(7826): p. 574–578. [DOI] [PubMed] [Google Scholar]