Abstract
The Escherichia coli chemoreceptor array is a supramolecular assembly that displays extraordinary signaling capabilities for which the mechanistic basis is only partly understood. The array has a conserved architecture in which transmembrane receptors, organized as trimers of dimers, are connected at their cytoplasmic tips by hexameric rings of alternating kinase CheA and scaffolding protein CheW molecules (CheA-CheW rings). These intermolecular interactions can produce highly cooperative kinase responses to changes in receptor ligand occupancy. An uncharacterized feature of E. coli arrays is the possible presence of hexameric CheW rings, formed by interactions of CheW molecules with the members of receptor trimers that are not directly bound to CheA-CheW rings. Here, we developed an in vivo cysteine-directed crosslinking assay to detect such CheW rings and explored the requirements for their formation and their participation in array assembly. We found that CheW ring formation varied greatly with cellular CheW abundance, strictly depended on the presence of receptors capable of a trimer-of-dimers arrangement, and could occur in the absence of CheA. Nevertheless, crosslinking studies of a CheA~CheW fusion protein incapable of forming CheW oligomers demonstrated that CheW rings were not essential for assembly of CheA-containing arrays. Investigation of the signaling properties of arrays containing variable amounts of CheW rings with in vivo fluorescence resonance energy transfer (FRET)-based kinase assays revealed that CheW rings enhanced the cooperativity and the sensitivity of the responses to attractants. We propose that six-membered CheW rings provide the additional interconnectivity required for optimal signaling and gradient-tracking performance by chemosensory arrays.
INTRODUCTION
Motile Escherichia coli cells monitor their chemical environment with extraordinary precision to seek favorable living conditions (1–4). Studies of the E. coli chemotaxis machinery have revealed fundamental principles of biological signal transduction [see reviews (5–8)], yet the molecular basis for E. coli’s remarkable gradient-tracking ability is only now coming to light. E. coli, as well as other chemotactic Bacteria and Archaea, contain supramolecular arrays comprising thousands of transmembrane receptor molecules and their cytoplasmic signaling protein partners. The chemoreceptor arrays of E. coli elicit locomotor responses to attractant and repellent concentration changes as small as one part in a thousand over more than five orders of magnitude (9, 10). In this study we document an architectural feature of the E. coli chemoreceptor array that plays a major, but previously unappreciated, role in its high-gain signaling behavior.
E. coli chemoreceptors are members of a receptor superfamily known as methyl-accepting chemotaxis proteins (MCPs) (11, 12). The periplasmic (extracellular) domains of MCP molecules contain binding sites for chemoeffector ligands; their cytoplasmic tips bind CheA, a signaling autokinase, and CheW, a small protein that couples CheA to receptor control (Fig. 1A). In the unliganded state, chemoreceptors stimulate CheA to autophosphorylate, which in turn donates phosphoryl groups to CheY, a response regulator whose phosphorylated form triggers clock-wise rotation of the flagellar motors and random changes in swimming direction. CheY~P signals are short-lived due to rapid dephosphorylation by a dedicated phosphatase, CheZ. Attractant ligands inhibit CheA and stanch the flow of phosphoryl groups to CheY, allowing for default counter-clockwise flagellar rotation and forward swimming episodes. MCP-specific adaptation enzymes (the methyltransferase CheR and the methylesterase CheB) subsequently adjust signaling sensitivity through reversible covalent modifications of specific residues in the receptor cytoplasmic domain. Methylation increases shift receptor output toward the kinase-ON state; methylation decreases shift receptor output toward the kinase-OFF state. This sensory adaptation system enables the cell to detect and respond to temporal changes in chemoeffector concentrations through a continuously updated “memory” of the recent chemical past in the form of receptor modification state (13).
Fig. 1. Molecular architecture of the E. coli chemoreceptor array.

(A and B) Side (A) and top-down (B) cross-section views of a core complex, the minimal structure-function unit of the chemoreceptor array. Core complexes consist of two receptor trimers-of-dimers (dark blue and grey), a homodimer of the kinase CheA, and two molecules of the scaffolding protein CheW (W, light blue) (14, 16). Each CheA subunit contains five domains, P1–P5, joined by flexible linkers (gray in one subunit, black in the other). In addition to the P3/P3’ CheA dimerization domain, other protein-protein interfaces important for core complex assembly and function are: CheA.P5•receptor (black squares); CheW•receptor (black rectangles); trimer contacts (black triangles); and CheA.P5•CheW interface 1 (black circles). Two receptor dimers (light gray) in each trimer have different interaction partners and positions in the core complex (17): AA dimers interact with the CheA.P5 domain; AW dimers interact with CheW. WW dimers (dark blue) make no direct contacts with CheA or CheW in the core complex. (C) Interface 2 interactions (red circles) between CheA.P5 in one core complex and CheW in another link core complexes to form a signaling team lattice unit. (D) Additional interface 2 interactions between signaling teams extend the hexagonal array geometry based on P5-W rings (24, 25). In this lattice model, the WW receptors line array holes that might accommodate hexagonal CheW rings, formed through interactions between CheW molecules that are not associated with CheA.
Core complexes, the smallest signaling-competent array units, contain six receptor homodimers, organized as two trimers of dimers, a CheA homodimer, and two CheW monomers (Fig. 1, A and B) (14–16). CheA protomers contain five domains interconnected by flexible linkers: P1 contains the site of autophosphorylation (His48); P2 binds response regulators CheB and CheY to facilitate their phosphotransfer interactions with the P1 domain; P3 dimerizes the molecule; P4 binds ATP and interacts with P1 during the autophosphorylation reaction; P5 interacts with its paralog CheW and the receptor cytoplasmic tip [reviewed in (7)] (Fig. 1B). Protein-protein interfaces for receptor trimer contacts, CheW-receptor interactions, and interface 1 interactions between the CheA.P5 domain and CheW are all critical for core complex function and assembly (Fig. 1B). Receptor trimers can contain receptors of different detection specificities, which facilitates integration of multiple stimulus inputs. The three receptor dimers in each trimer have different interaction partners and positions in the core complex (17). One dimer (designated AW) makes direct contact to CheW, which in turn binds CheA.P5; another dimer (AA) makes direct contact to P5, which in turn binds to CheW; the third dimer (WW) makes no direct contacts to CheA or CheW in the core complex, but can make contacts to CheW in arrays assembled in vitro at high CheW stoichiometry (15, 18).
CheA.P5 and CheW have a common protein fold consisting of two similar subdomains sandwiching a central groove that fits a receptor helix (19–22). Whereas CheA.P5 subdomain 1 and CheW subdomain 2 make interface 1 contacts, bridging receptor trimers within a core complex (15, 23) (Fig. 1B), CheA.P5 subdomain 2 and CheW subdomain 1 form a second weaker interface (interface 2) that links core complexes to one another in the array (18, 22, 24, 25) (Fig. 1C). Extensive interface 2 interactions among core complexes create extended arrays with hexagonally packed receptor trimers of dimers connected at their tips by P5-W rings (Fig. 1D). This chemoreceptor array geometry is conserved across many chemotactic bacteria, but with considerable variation in the types and stoichiometries of the cytoplasmic components (26–29).
Amino acid replacements at interface 2 surface residues can disrupt P5-W rings and arrays; the disconnected core complexes signal with very low cooperativity (30). However, E. coli arrays might also contain hexagonal CheW rings whose members bind to the WW receptor dimers that have no direct interactions with CheA or CheW in the core complexes (Fig. 1D) (17, 24, 25). Cryo-EM studies of E. coli arrays assembled in vitro (15, 18) and of Vibrio cholerae arrays ex vivo (31) have imaged CheW molecules in such array holes. Here, we confirmed the in vivo existence of CheW rings in E. coli arrays with crosslinking assays and determined the conditions required for their formation. We explored the role of CheW rings in chemotaxis and their contribution to detection sensitivity and signal cooperativity with in vivo fluorescence resonance energy transfer (FRET)-based CheA kinase assays. Our findings provide new insights into the mechanism of array formation in E. coli and, more generally, identify important network connections that augment the signaling behavior of chemoreceptor arrays.
RESULTS
A molecular model for hexameric CheW rings
E. coli core complexes form larger signaling teams and arrays through interface 2 interactions between CheW and CheA.P5 that mediate the formation of hexameric P5-W rings (Fig. 1C). Given that CheW and CheA.P5 have homologous structures (19–22), CheW rings might form through head-to-tail interactions between CheW subdomains 1 and 2 that resemble the interfaces found in P5-W rings (Fig. 2A). Based on this premise, we used the Thermotoga maritima P5-W ring structure (22) to facilitate the rigid-body positioning of six E. coli CheW monomers into an initial hexameric ring configuration. Although a solution NMR structure of E. coli CheW is available (21), due to its dynamic nature, substitution of this structure into the T. maritima P5-W ring produced poorly fitted interfaces between the CheW monomers, including several substantial steric clashes. Previous studies demonstrated that reliable models of E. coli CheW can be based on homology with T. maritima CheW (15, 32), so we, therefore, opted to use the ring-bound CheW conformation observed in the T. maritima P5-W ring as a template to construct a clash-free homology model of the intact E. coli CheW ring (see Materials and Methods). Using a series of all-atom molecular dynamics simulations, we further refined the model in the presence of an array-like scaffold of Tsr, the serine chemoreceptor. We first allowed thorough conformational sampling of the interface residues in response to their altered electrostatic environment, followed by an extensive equilibration of the overall structure, culminating in a stable, low-energy ring configuration (Fig. 2B).
Fig. 2. Structural model of the CheW ring.
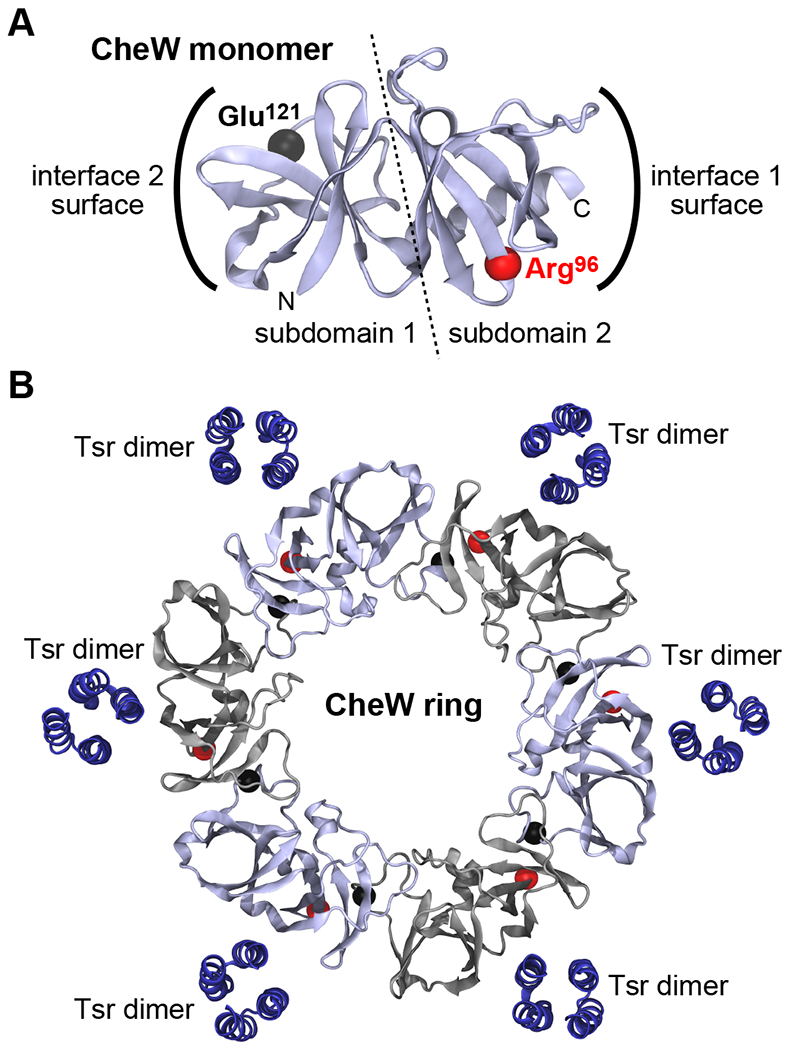
(A) Structure of the modeled E. coli CheW monomer. Spheres indicate the backbone α-carbon atoms of residues Arg96 and Glu121, which were replaced with cysteines for in vivo crosslinking analyses. (B) Model of a hexameric E. coli CheW ring. Spheres indicate the α-carbons of the cysteine reporter pairs located at the CheW-CheW interfaces (red, Arg96; black, Glu121). Alternating CheW molecules are shown in gray to accentuate the individual monomers in the ring. The last 10 CheW residues in each C-terminal helix were removed to better discern the packing interfaces. This model also includes the WW Tsr dimers proposed to line array holes to direct assembly of CheW rings.
To further assess the changes at the W•W interfaces during refinement, we aligned each W•W interface from the refined ring model with the one observed in the initial homology model (note, MODELLER generated a ring with identical interfaces), yielding an average backbone root-mean-square deviation (RMSD) of 2.5 +/− 0.3 A and an average all-atom RMSD of 4.0 +/− 0.2 Å for interface residues 40-70 and 115-145. An optimal superposition was obtained by alignment of both monomers forming the interface using the backbone atoms of residues 17-152. Therefore, the primary changes that took place at the W•W interfaces during refinement were at the side chain level, reflecting improvements in conformation and packing; however, some changes at the backbone level, especially in residues 116-130, were also observed.
In vivo detection of CheW rings by cysteine-directed crosslinking assays
We used our CheW ring model as a guide to search for sites where cysteine reporters could be engineered into CheW to monitor CheW ring formation in vivo. Accordingly, we screened hemagglutinin (HA)-tagged CheW proteins bearing cysteine replacement pairs at surface-exposed residues in subdomain 1 and subdomain 2 (Fig. 2A). Immunoblotting for HA revealed that 8 of the 86 tested CheW double-Cys proteins crosslinked into hexamers and lower oligomeric forms upon exposing cells to oxidizing conditions (Fig. S1A and Table S1). We never saw crosslinked products larger than CheW hexamers, indicating that this oligomeric form is the in vivo limit, presumably a closed ring. The lower-order CheW forms (pentamers to monomers) likely do not accurately reflect CheW ring contents in the array because they could conceivably arise in a variety of ways, including incomplete cross-linking reactions, partially filled CheW rings, and even nonspecific random collisions.
The cysteine replacement pairs able to form CheW hexamers did not affect CheW stability or function (Fig. S1, B and C), making them suitable crosslinking reporters for CheW rings.When mapped onto the E. coli CheW ring model (Fig. 2B), these 8 cysteine pairs were all within disulfide crosslinking range (β-β carbon distances ≤ 11 Å) (Table S1). Overall, our model of the CheW ring predicted the crosslinking behavior of 69/86 cysteine pairs (Table S1), implying that it accurately represents the structure of the W•W interface and the CheW ring in vivo. Most CheW reporters that failed to form hexameric crosslinking products also supported chemotaxis (Fig. S1D), indicating that those cysteine pairs had little effect on protein stability, but rather were not optimally positioned. In subsequent crosslinking assays we employed the R96C/E121C pair (Fig. 2, A and B), which displayed the best band resolution in gel immunoblots, thereby simplifying the detection and quantification of CheW hexamers, the parameter we used to assess CheW ring formation under different conditions. To simplify discussion of subsequent experiments with this CheW variant, we designate the HA-tagged cross-linking reporter protein for CheW ring formation as *CheW*.
CheW ring formation requires receptors, but not CheA
We investigated the array components required for CheW ring formation with cross-linking assays in strains producing chromosomally-encoded *CheW* from the native locus (Fig. 3A). Comparable amounts of CheW hexamers formed in a background expressing all array components (wild type) and in one expressing a form of CheA lacking cysteines, indicating that the three native CheA cysteine residues do not interfere with or participate in the crosslinking assay. Similarly, absence of the sensory adaptation enzymes CheR and CheB did not alter CheW ring formation (Fig. 3A). A strain lacking CheA produced higher amounts of CheW rings, whereas a strain lacking all chemoreceptors (Δmcp) produced no CheW rings (Fig. 3A). These results demonstrate that CheW rings were present in wild-type E. coli arrays (Fig. 3A) and that the presence of receptors, but not CheA, was essential for their formation. These findings moreover suggest that in the absence of CheA, receptors and CheW alone might build cellular arrays consisting of hexagonally arranged receptor trimers of dimers interconnected by hexameric CheW rings.
Fig. 3. In vivo requirements for CheW ring formation.

SDS-PAGE images These SDS-PAGE immunoblots are representative examples from 3 or more independent experiments. Bands labelled 1-6 denote monomeric to hexameric *CheW* cross-linking products. (A) Crosslinking products, detected with anti-HA antibodies, from isogenic strains expressing chromosomally-encoded, HA-tagged *CheW* Lane 1, wild type (strain UU3031); lane 2, cells expressing a form of CheA with no cysteine residues (strain UU3087); lane 3, cells lacking CheA (ΔcheA, strain UU3074); lane 4, cells lacking all chemoreceptors (Δmcp, strain UU3079); lane 5, cells lacking CheA and all chemoreceptors (strain UU3081); lane 6, cells lacking cheR and cheB (strain UU3070). (B) HA-tagged *CheW* crosslinking products, detected with anti HA antibodies, in strains UU2806 (ΔcheA, lanes 1-4) and UU3166 (cheA+, lanes 5-8) expressing variant Tsr receptors and *CheW*. Membranes were reblotted with an antibody specific for Tsr to confirm comparable cellular abundance of the receptor variants; total CheW provided the loading control. The Tsr-E402K receptor cannot form intact trimers of dimers, but can form dimers of dimers (37). (C) Effect of interface 2 lesions on *CheW* crosslinking products. Plasmid-encoded, HA-tagged *CheW* (wt) and *CheW*-X2 (X2) were detected with anti-HA antibodies, in strains UU2885 (cheA+, lanes 1-2); UU3111 (cheA-L545S, lanes 3-4); and UU1607 (ΔcheA, lanes 5-6) (Table S2). Membranes were reblotted with anti-CheA to confirm comparable CheA abundance in the appropriate strains; total CheW served as the loading control. CheAL and CheAS are translated from different in-frame start sites (49, 50). The X2 replacements (R117D/F122S) can affect CheW migration in polyacrylamide gels (38). (D) Total CheW and CheW hexamer fractions were quantified by densitometry scans of immunoblots like those shown in panels A-C. Histograms show values normalized to lane one in each panel. Error bars show the standard deviations for N=3 (A), N=3 (B), and N=4 (C) independent experiments.
CheW ring formation requires trimer-competent receptors in any modification state
The adaptation enzymes CheR and CheB, which add or remove methyl groups at conserved receptor glutamate residues, control the modification states and signaling properties of receptor molecules (5, 33). Tsr contains four canonical modification sites (Gln297, Glu304, Gln311 and Glu493) and one unorthodox site (Glu502) (34, 35). CheR-mediated methylation of Glu residues at modification sites shifts receptor output toward the kinase-ON state; Gln residues at modification sites mimic the signaling effects of methylation (36, 37). CheB-mediated demethylation of glutamyl methyl esters shifts output toward the kinase-OFF state.
We tested three Tsr variants (QEQEE, QQQQE, and EEEEE) to investigate the effect of receptor modification state and activity on CheW ring formation. In these experiments we expressed *CheW* in wild-type amounts from a derivative of regulatable plasmid pGP37 (Table S3) in hosts deleted for cheW and all receptor genes. The Tsr variants were expressed from a compatible, regulatable plasmid (pPA114) (38). The strains also carried a cheR-cheB deletion to preclude changes in Tsr modification state. We compared CheW ring contents in two versions of these strains, one containing wild-type CheA, and one lacking CheA (Fig. 3B). CheW ring formation was again higher in the strain lacking CheA, but receptor modification state seemed to have no effect on ring content (Fig. 3B). We conclude that receptor modification state, and hence activity state, do not influence CheW ring formation in either CheA-containing or CheA-free arrays. Consistent with this result, an OFF-shifting serine stimulus also had negligible effect on CheW ring content in CheA-containing arrays (Fig. S2A).
To determine if receptor trimer formation is required for CheW ring assembly, we used the experimental conditions described above to quantify CheW hexamers in cells expressing Tsr-E402K, a mutant receptor that cannot assemble complete trimers of dimers (39). Crosslinking assays in cheA+ and ΔcheA strains showed that CheW ring formation was substantially reduced in cells with Tsr-E402K receptors (Fig. 3B). These findings demonstrate that complete receptor trimers not only promoted core complex assembly, but also CheW ring formation.
Lesions at array interface 2 disrupt CheW ring formation
Core complexes in E. coli chemoreceptor arrays form signaling networks through interface 2 interactions between CheA.P5 subdomain 2 and CheW subdomain 1 (Fig. 1C) (30, 40). Amino acid replacements at critical interface 2 residues in CheA.P5 (Leu545, Val551, Tyr558) or CheW (Arg117, Phe122) disrupt array formation (30). We first asked whether the interface 2 surface of *CheW* was critical for ring formation, using a derivative of *CheW* that carried the “X2” interface 2 lesions R117D/F122S in CheW subdomain 1 (40). This *CheW* variant (*CheW*-X2) failed to form CheW rings, both in cells having or lacking wild-type CheA (Fig. 3, C and D). This result demonstrates a role for the interface 2 interaction surface of CheW in forming the proposed head-to-tail interactions needed for CheW ring assembly.
In these experiments, we noted that *CheW*-X2 formed crosslinked dimers, but no larger species, in both the absence and presence of CheA (Fig. 3C; Fig. S2B). Because CheW subdomains 1 and 2 are similar in structure, these crosslinked species could represent anomalous interactions of *CheW*-X2 molecules at their interface 1 or 2 surfaces, perhaps potentiated by their interface 2 defects. We tested this idea with CheW-X2 variants that carried only one of the ring reporter crosslinking sites. The R96C reporter failed to form crosslinked dimers, whereas the E121C reporter did (Fig. S2, C and D), indicating that the aberrant dimers represent head-to-head arrangements of CheW monomers crosslinked at their interface 2 surfaces.
Aberrant *CheW* dimers, presumably head-to-head forms, also arose in cells encoding CheA-L545S, which has a lesion at the interface 2 surface of the CheA.P5 domain. In this mutant CheA background, neither wild-type nor X2 versions of *CheW* formed hexamers, but both formed large amounts of a dimeric crosslinked species (Fig. 3C; Fig. S2B). Thus, the X2 lesion was not needed to obtain aberrant dimer forms of *CheW* and, moreover, an interface 2 lesion in CheA could potentiate aberrant dimers of *CheW* molecules that lack an interface 2 defect.
CheW ring formation scales with cellular CheW content
We investigated the effect of array component stoichiometry on CheW ring formation with *CheW* crosslinking assays in cells expressing variable amounts of CheW, CheA, or receptors. To determine the influence of CheW amounts on cellular CheW ring content, we used *CheW* plasmid pGP37RF to achieve low to native amounts and plasmid pGP48RF for higher amounts (Table S3). We surveyed CheW contents ranging from less than one to no more than 10-fold the native amount to avoid the detrimental signaling effects of higher CheW amounts (41). In cells with a full complement of receptors, we observed an approximately linear increase in CheW ring content over the CheW amounts tested, ranging from 0.04- to 22-fold the ring content of cells expressing native amounts of *CheW* (Fig. 4A and Table S4). In Tsr-only cells, ring content ranged from 0.09- to 23-fold (Fig. 4A and Table S4). In cells with all-receptor or Tsr-only arrays, the fraction of CheW molecules in hexamer-sized crosslinking products rose sharply below the native CheW amount, then less steeply up to ~9-fold the native amount (Fig. 4B and Table S4). The data fitted well to a Hill equation with an exponent of 1, suggesting that empty hexagonal holes in receptor arrays may be filled gradually by CheW molecules to produce six-membered CheW rings.
Fig. 4. Effects of array component stoichiometry on CheW ring content.

CheW hexamers were measured by *CheW* crosslinking in various strains producing either all chemoreceptors or only Tsr. Means and standard deviations for all values from 3 independent experiments (N=3) are plotted here and compiled in Table S4. Dashed lines are manual fits to the data; some data symbols are larger than their error bars. (A and B) Dependence of CheW ring content on cellular CheW abundance. The relative number of *CheW* hexamers (A) and the percentage of *CheW* molecules in hexamers (B) were measured in UU2885 (ΔcheW) cells expressing *CheW* from the IPTG-regulated plasmid pGP37RF (for low to native amounts) or pGP48RF (for higher amounts). Solid lines in (B) represent best fits to a Hill equation of the form (CheW-6max)(CheWH/(CheWH + KdH)), where Kd is the dissociation constant for the [6 CheW ↔ CheW-6] reaction, CheW-6max is the maximum amount of CheW in hexamers at very high CheW amounts, and H is the Hill coefficient, a measure of cooperativity. Hill coefficients were 0.9 for both line fits. (C) Effect of CheA and Tsr amounts on CheW ring formation. *CheW* hexamer contents in strain UU3074 (ΔcheA) expressing variable CheA amounts from plasmid pKJ9 and in strain UU3086 (Δ(mcp) expressing variable Tsr amounts from plasmid pRR53 (low to native amounts) or pJC3 (above-native amounts).
The effect of CheA amounts on CheW ring content was evaluated in a similar manner in a strain expressing a chromosomally-encoded *CheW* reporter. Variable CheA expression was provided by pKJ9, an isopropyl β-D-1 thiogalactopyranoside (IPTG)-inducible cheA plasmid (42). In the absence of CheA, CheW rings were maximal and declined with CheA expression, reaching 5-fold lower ring amounts at ~17-fold native CheA amount, the highest amount tested (Fig. 4C and Table S4).
The effect of receptor expression amount on CheW ring formation was assessed in a receptorless strain expressing chromosomally-encoded *CheW* and variable amounts of Tsr from IPTG-inducible plasmids: pRR53 (43) for low to wild-type amounts or pJC3 (38) for higher amounts. We observed modest increases in CheW ring formation at higher Tsr amounts. Consistent with an obligate requirement for trimer-proficient receptors, ring content was low at receptor amounts below wild-type, then rose at higher receptor amounts, saturating at about twice the native Tsr amount, which corresponds to the aggregate number of receptor molecules in a wild-type cell expressing all receptor types (Fig. 4C).
CheW ring content has little effect on the protein-protein interactions in native arrays
We evaluated core complex and interface 2 formation, two key events in array assembly, in Tsr-only and in all-receptor arrays that contained different CheW ring contents: “low” (0.09-fold, 0.04-fold); “native” (1-fold); and “high” (23-fold, 22-fold) (Table S5). To assess core complex formation, we measured crosslinking between cysteine reporter forms of CheA (L599C) and Tsr (L380C) (44). This assay detects interactions between the CheA.P5 domain and the receptor tip, which only occur if CheA is part of the core complex (44). In these experiments, we induced crosslinking with BMOE, a bifunctional thiol-reactive reagent, in a receptorless strain with CheA-L599C expressed from the chromosome and Tsr-L380C expressed from a derivative of plasmid pPA114. To manipulate CheW amounts, and hence CheW ring formation, the cells also carried a wild-type CheW expression plasmid, either pGP46 or pGP47. These experiments revealed comparable amounts of cross-linked CheA≈Tsr products, and hence core complex formation, at all three CheW amounts tested (Fig. 5A).
Fig. 5. Protein-protein interfaces and signaling properties of arrays with different CheW ring contents.

Parameter values were measured at low, native, and high CheW ring contents, in all-receptor (open diamonds) or Tsr-only (filled squares) arrays, as defined in Table S4. Data represent means ± SD for N=3 or more independent experiments (Table S5). Error bars for some of the measurements are smaller than the data symbol. (A) Core complexes were assessed by BMOE-induced crosslinking between HA-tagged CheA-L599C and Tsr-L380C in strain UU3052 (N=3). Symbols show the fraction of CheA crosslinked to Tsr, normalized to the native value. CheA-CheW interface 2 interactions were assessed by BMOE-induced crosslinking between HA-tagged CheA-A546C and CheW-E27C in strain UU3010 bearing plasmids pPA114 and pGP46-E27C or pGP47-E27C (N=3). Symbols show the fraction of CheA crosslinked to CheW, normalized to the native value. Chemotaxis was evaluated by soft-agar colony sizes of UU3216 (Tsr-only; filled squares) or UU2871 (all-receptor; open diamonds) carrying plasmid pGP46 or pGP47 (N=3). Values are normalized to the performance of strain RP437 (native control). (B) Serine dose-response parameters in adaptation-deficient [Δ(cheRB)] strains UU2997 (Tsr-only; filled squares) and UU3025 (all-receptor; open diamonds) carrying plasmids pRZ30 and pGP46 or pGP47 (N=5). (C) Serine dose-response parameters in adaptation-proficient [(cheRB)+] strains UU2998 (Tsr-only; filled squares) and UU3026 (all-receptor; open diamonds) carrying plasmids pRZ30 and pGP46 or pGP47 (N=5).
To assess interface 2 interactions between CheA.P5 and CheW in arrays with different CheW ring contents we measured BMOE-induced crosslinking between CheA-A546C and CheW-E27C (30). These experiments were carried out in a receptorless strain expressing CheA-A546C from its native chromosomal locus and Tsr from plasmid pPA114. In that strain, CheW-E27C was expressed at variable amounts from derivatives of IPTG-inducible plasmids pGP46 or pGP47. As above, induction conditions were chosen to obtain arrays with low, wild-type, or high CheW ring content. We found similar amounts of CheA≈CheW crosslinks in arrays with low or wild-type ring amounts and a modest reduction at high CheW ring amounts (Fig. 5A). These experiments showed that CheW ring content has at most a two-fold effect on the extent of interface 2 connections in receptor arrays and consequently only a modest influence on array formation. These experiments demonstrated that both core complexes and higher-order receptor arrays were present at low CheW ring content, suggesting that arrays do form with empty holes available to bind CheW and form hexameric rings.
CheW rings enhance the cooperativity and sensitivity of chemoreceptor arrays
Adaptation-competent cells with Tsr-only or all-receptor arrays were chemotactic in semi-solid agar plate assays at all three tested CheW ring contents (Fig. 5A). Cells with low ring content exhibited partly compromised chemotaxis performance; cells with native or high ring contents had wild-type performance. To better assess the effect of CheW rings on signaling performance, we characterized serine dose-response behaviors of Tsr-only or all-receptor arrays with an in vivo FRET-based CheA kinase assay, which measures the extent of interaction between CheZ-CFP and phosphorylated CheY-YFP (45). We used Tsr-only and all-receptor hosts bearing chromosomal deletions of cheW and cheYZ that carried plasmid pGP46 or pGP47 to tune CheW ring contents. The cells also carried a compatible plasmid (pRZ30) that expressed the FRET reporter proteins. Serine responses were evaluated in adaptation-proficient (cheRB+) and adaptation-deficient (ΔcheRB) versions of these strains (Fig. 5B and 5C). In the adaptation-deficient backgrounds, serine response sensitivity (K1/2) and overall array kinase activity did not change appreciably with CheW ring content, whereas increased ring contents substantially augmented response cooperativity (Fig. 5B). In Tsr-only arrays, cooperativity was low at low CheW ring content (Hill=8) and several-fold higher at normal (Hill = 20) or high (Hill= 23) ring contents (Fig. 5B and Table S5). Similarly, cooperativity in all-receptor arrays was greatest at high CheW ring content (Fig. 5C and Table S5). In the adaptation-proficient strains, response cooperativities and kinase activities did not change appreciably with CheW ring content, whereas response sensitivity, defined as threshold attractant concentration that produces a kinase-control response, improved five-fold from low to high ring content (Fig. 5C and Table S5).
A CheA~CheW fusion protein assembles functional arrays that lack CheW rings
Several factors contribute to structural heterogeneity in native E. coli receptor arrays. Although the CheA and CheW proteins have a 1:1 stoichiometry, their cellular amounts are comparable to the aggregate number of receptor trimers (46), roughly in two-fold excess of the component stoichiometries in core complexes (14). CheW engages in binding interactions with the P5 domains of CheA molecules to recruit the kinase to nascent core complexes, but also binds directly to receptor dimers with comparable affinity (47, 48). Thus, some CheW molecules join core complexes, whereas others could fill array holes to form CheW rings.
We reasoned that it might be possible to limit the binding options for CheW by covalently joining it to CheA, thereby leading to a more structurally homogeneous array. An atomic model of the E. coli core complex (15) indicated that a linker ~65 Å in length might suffice to join the C-terminus of CheA.P5 to the N-terminus of CheW (Fig. 6A). Accordingly, we joined the cheA and cheW genes with four repeats of the coding sequence for a GGGGS linker and characterized the properties of the CheA-(GGGGS)x44-CheW fusion protein, hereafter designated CheA~CheW.
Fig. 6. Structure and function of a fused CheA~CheW protein.

(A) Atomic model (PDB 6S1K) of the E. coli core complex (15) showing how a flexible linker could join the C-terminus of the CheA.P5 domain to the N-terminus of CheW. In the model, these ends are separated by ~50 Å in one CheA subunit (shown) and by ~70 Å in the other (hidden on the back side). The length variability is due to flexibility of the P5 C-terminus, suggesting that a linker 60-70 Å in length might permit core complex assembly and function in a CheA~CheW fusion protein. (B) Colony sizes on tryptone soft agar of wild-type cells and strain UU2683 [Δ(cheAW); Tsr-only] expressing the CheA~CheW fusion from plasmid pGP74 in amounts comparable to chromosomally encoded CheA and CheW. The bottom two colonies also carry derivatives of compatible plasmid pGP46 to express CheW and CheW-X3 at native abundance. Strain UU2617 [(cheAW)+; Tsr-only] was the wild-type control.
The CheA~CheW protein, expressed either from a plasmid or the host chromosome, supported about 50% of the wild-type chemotaxis performance on soft agar plates (Fig. 6B; Fig. S3, A and B). Upon supplementation with wild-type CheW molecules, the performance of CheA~CheW cells closely resembled the wild-type control (Fig. 6B). However, a CheW-X3 variant (CheW-X2 with one additional change [E122K]) defective in interface 2 interactions (30) did not improve CheA~CheW performance (Fig. 6B), implying that the ancillary CheW must engage in an interface 2–dependent interaction, presumably with subdomain 2 of another CheW molecule, to produce the helping effect. An interface 1 lesion (K616E) (49) in the P5 component of the CheA~CheW fusion protein abrogated its ability to support chemotaxis (Fig. S3A), suggesting that CheA~CheW forms functional core complexes. Similarly, an interface 2 lesion in either P5 (L545S) or CheW (X3) of the CheA~CheW protein abolished chemotaxis (Fig. S3B), indicating that CheA~CheW forms functionally important connections between core complexes. Ancillary CheW molecules could not restore chemotaxis function to those CheA~CheW interface 2 mutants (Fig. S3B), demonstrating that the CheW helping effect only operated in arrays that had functional and networked CheA~CheW core complexes.
These qualitative performance tests cannot exclude the possibility that ancillary CheW molecules augment performance by replacing the CheW portion of the CheA~CheW fusion protein. To explore that issue, we first assessed cellular abundance and size of the CheA~CheW protein in gels immunoblotted with antisera directed against its CheA and CheW epitopes. All proteins reactive with a polyclonal CheA antiserum migrated at the position expected for full-length CheA~CheW fusion proteins (Fig. S4A). Moreover, a polyclonal CheW antiserum did not reveal CheW-sized species that might have been liberated by CheA~CheW proteolysis (Fig. S4A). Thus, cells expressing CheA~CheW from either a plasmid or the host chromosome did not contain any detectable degradation products of the fusion proteins. We conclude that the functional activities exhibited by CheA~CheW were carried out by the intact fusion protein.
We next identified cellular interaction partners of the CheA~CheW fusion protein with in vivo crosslinking assays diagnostic for interface 1 and interface 2 interactions (30, 49, 50). To simplify analysis of the crosslinking products, we introduced an HA epitope tag at the N-terminus of the CheA~CheW protein and eliminated the cheA gene’s internal cheAs translational start (51, 52). CheA~CheW proteins bearing an interface 1 (S630C) (44) or interface 2 (A546C) (30) reporter in the P5 domain failed to form BMOE-promoted crosslinking products with CheW molecules bearing a reporter site at the corresponding interface [Q44C for interface 1 (44); E27C for interface 2 (30)] (Fig. S4B). We also noted in these experiments that the CheA~CheW proteins with a single interface 1 or interface 2 reporter did not form crosslinked dimers, indicating that they did not engage in anomalous 1-1 or 2-2 interface interactions (Fig. S4B). By contrast, CheA~CheW proteins with both interface reporter sites produced one or more crosslinking products upon BMOE treatment (Fig. S4C). suggesting that their P5 reporter sites were accessible, but only to CheW reporter sites in CheA~CheW and not to the same reporter sites in an ectopically-expressed CheW protein (Fig. S4B).
CheA-S630C~CheW-Q44C yielded four crosslinking products, which we ascribe to intra- or inter-dimer P5-W crosslinks at interface 1 (Fig. S4C). One of those crosslinked CheA~CheW forms was monomeric and could only have arisen from an intra-subunit crosslink, perhaps within a CheA~CheW dimer in a core complex. Three other crosslinked forms were dimer-sized and might have arisen partly through interactions between CheA~CheW protomers before their assembly into core complexes (Fig. S4C). Clearly, the covalent connection between the P5 and W domains in the CheA~CheW protein greatly favored intramolecular interface 1 interactions and precluded intermolecular interactions with free CheW molecules.
A CheA~CheW protein bearing interface 2 reporter sites in P5 (A546C) and W (E27C) (30) formed crosslinked dimers and minor amounts of higher-order forms, but no smaller crosslinked products (Fig. S4C). These results indicate that the CheA~CheW interface 2 crosslinks occurred through intermolecular interaction of reporter sites in different core complexes, presumably ones connected by a P5-W ring. A CheA~CheW protein bearing a reporter site (L599C) in the CheA.P5 domain for a CheW-dependent interaction between CheA.P5 and the receptor tip (44) crosslinked well to Tsr reporter site L380C (44) (Fig. S4C). In all, these crosslinking studies confirmed that the CheA~CheW fusion protein made interface interactions typical of core complexes and signaling teams, but it did not interact at those same interfaces with ancillary CheW molecules. Thus, CheW improvement of CheA~CheW chemotaxis performance did not occur through CheW swapping, but rather by some other mechanism.
CheW rings augment response cooperativity of CheA~CheW receptor arrays
We expressed *CheW* in strains with plasmid- or chromosomally-encoded CheA~CheW and examined their ability to form CheW rings. Wild-type *CheW* made rings in cells that had a CheA~CheW protein with no interface 1 or 2 lesions, whereas the X2 variant of *CheW* did not (Fig. 7A). However, wild-type *CheW* failed to form rings or any crosslinked species in cells that carried an interface 2 lesion in either the CheW (X3) or P5 (L545S) portion of the CheA~CheW fusion protein (Fig. 7A). These findings show that rings of ancillary CheW molecules formed in the presence of CheA~CheW, but only if the fusion protein was competent in making interface 2 connections between core signaling units, and presumably in assembling higher-order arrays. The ability of ancillary CheW molecules to help CheA~CheW chemotaxis performance thus correlated with their ability to form hexameric rings in those cells.
Fig. 7. CheW ring formation and signaling in CheA~CheW strains.

(A) CheA~CheW and its interface 2 mutant variants (X3 and L545S) were expressed at native CheA amounts from plasmid pGP74 in ΔcheAW strain UU2683 (lanes 1-4) or from strains carrying the gene encoding cheA~cheW at the cheAW locus (lanes 5-8). The *CheW* reporter proteins were produced in native amounts from plasmid pGP37RF and its X2 derivative. Strain and plasmid combinations (see Tables S2 and S3) were: lane 1, UU2683/pGP74/pGP37RF; lane 2 UU2683/pGP74/pGP37RF-X2; lane 3, UU2683/pGP74-X3/pGP37RF; lane 4, UU2683/pGP74-L545S/pGP37RF; lane 5, UU3171/pGP37RF; lane 6, UU3171/pGP37RF-X2; lane 7, UU3173/pGP37RF; lane 8, UU3174/pGP37RF. (B) Serine dose-response parameters were determined in adaptation-deficient, Tsr-only strain UU3169 producing chromosomally-encoded CheA~CheW. In this strain, which also lacks CheY and CheZ, FRET reporters were expressed from plasmid pRZ30 and CheW from pGP46 or pGP46-X3. Data points are means ± SD for N=3 independent experiments.
We carried out FRET kinase assays on CheA~CheW cells expressing various amounts of CheW helper molecules: none, half the native CheW abundance, and the full native CheW abundance. The cells also lacked the sensory adaptation enzymes to maximize the cooperativity effects of CheW rings (see Fig. 5B). Wild-type CheW and CheW-X3 produced similar, modest reductions in serine sensitivity over the three CheW amounts (Fig. 7B). In contrast, wild-type CheW enhanced response cooperativity nearly three-fold over the tested range, whereas the X3 variant did not (Fig. 7B). These findings offer convincing evidence that arrays lacking any CheW rings signal with moderate cooperativity and that CheW rings alone can considerably enhance response cooperativity without compromising stimulus sensitivity.
DISCUSSION
CheW rings in E. coli receptor arrays
We found that CheW molecules with cysteine reporters at an interface 1 residue (R96C) and an interface 2 residue (E121C) formed oligomeric disulfide-crosslinked products under cellular oxidizing conditions. In cells that contained wild-type receptors, we observed *CheW* oligomers as large as hexamers, both in the presence and absence of the kinase CheA. We cannot say if those *CheW* hexamers are covalently closed rings, but we propose that they and smaller oligomeric products arise by sequential filling of hexagonal array holes delimited by six WW receptors (Fig. 8). Consistent with this interpretation, we never observed crosslinked products larger than a hexamer.
Fig. 8. Effects of mutant array components on CheW rings.

In CheA-containing arrays interface 2 lesions in either CheW (CheW-X2; X2=R117D/F122S) or the CheA.P5 domain (CheA-L545S) dispersed core complexes and prevented CheW ring formation. The trimer-defective Tsr-E402K receptor could not assemble core complexes or CheW rings. In CheA-free arrays, lesions that affected trimer stability (Tsr-E402K) or interface 2-like interactions between CheW molecules (CheW-X2), impaired CheW ring formation and disrupted the array. In the absence of an array lattice, the only *CheW* reporter species detected in cross-linking assays were *CheW* monomers and anomalous head-to-head dimers.
In CheA-containing arrays, *CheW* molecules that are not engaged in interface 1 or 2 interactions with CheA.P5 should be available to fill those hexagonal holes (Fig. 8, left). In the absence of CheA, all members of receptor trimers would have equivalent geometries, allowing binding interactions with CheW to seed formation of a ring-based array with hexagonal holes equivalent to, but more numerous than, those in CheA-containing arrays (Fig. 8, right). We suggest that these CheA-free arrays in E. coli have hexagonal geometry comparable to ones observed in cryo-EM images of Vibrio cholerae CheA-free arrays (31).
Trimer-proficient receptors were essential for CheW ring formation in both CheA-containing and CheA-free hosts (Fig. 8). Tsr-E402K, a receptor that cannot assemble intact trimers of dimers or core complexes (39), failed to support assembly of CheW hexamers (Fig. 3B), highlighting the importance of the trimer-of-dimer arrangement in array organization. In contrast, receptor activity state had no discernable effect on CheW ring formation in either CheA-containing or CheA-free arrays (Fig. 3B), consistent with a previous report indicating that overall array structure does not change with signaling state (53).
Interactions between CheW interfaces 1 and 2 assemble CheW rings. *CheW* molecules bearing replacements at critical interface 2 residues could not form rings regardless of CheA presence or absence (Fig. 3C; Fig. 8). Lesions at *CheW* interface 1 should also prevent ring formation, but we did not explicitly test that prediction in the current study. In CheA-containing cells, both CheW interfaces also contributed to array integrity through their interactions with the CheA.P5 domain. Interface 2 lesions in CheA.P5 also abrogated array assembly and CheW-ring formation (Fig. 3C; Fig. 8), presumably by disrupting the assembly of hexameric P5-W rings.
In CheA-free cells, CheW-CheW interactions with trimer-competent receptors were sufficient for ring formation (Fig. 3A; Fig. 3C), implying that CheW alone could promote array assembly with receptor trimers of dimers. In CheA-containing cells, by contrast, P5-W rings were evidently critical to array assembly (Fig. 3C; Fig. 8). This finding implies that interactions between CheW monomers and assembled core complexes cannot maintain array integrity in the absence of P5-W rings. Whether free CheW molecules interact with the WW receptors of isolated core complexes remains an open question. However, the stoichiometry of core signaling complexes assembled in nanodiscs indicates that core complex function does not depend on CheW molecules binding to the WW receptors (14).
In the absence of an array scaffold, *CheW* molecules gave rise to anomalous crosslinking products, apparently head-to-head dimers associated through their interface 2 surfaces (Fig. 3C; Fig. S2C). Such dimeric forms of *CheW* were much less prevalent in receptorless cells (Fig. 3A), suggesting that reversible interactions of *CheW* molecules with receptor dimers and/or with the interface 1 surface of CheA.P5, potentiated aberrant interactions between the interface 2 surfaces of *CheW* molecules. Considering that *CheW* behaved as a valid proxy for native CheW in all other respects, this anomalous interaction probably occurs between wild-type CheW molecules as well. The head-to-head CheW interaction is structurally analogous to a P5-P5 interaction noted in several CheA crystal structures (20, 23).
A refined model for assembly of the E. coli chemosensory array
Our in vivo crosslinking results substantiate prior imaging and structural studies predicting the existence of CheW rings in E. coli receptor arrays (24, 25). Those landmark studies showed that an array architecture comprised of trimer-based core signaling units networked by hexagonal P5-W rings contains empty hexagonal spaces that might accommodate hexameric rings of CheW molecules (Fig. 9). We found that both ring content and the fraction of CheW monomers in rings scaled with cellular CheW content (Fig. 4, A and B), properties best explained by a gradual filling of array holes with CheW hexamers. Although we cannot exclude the possibility that hexamer assembly itself is a cooperative process, the data of Fig. 4B fit well to a Hill equation with an exponent of 1, consistent with non-cooperative filling.
Fig. 9. Refined model of chemoreceptor array formation.

Symbols and colors correspond to those used in Fig. 1 and Fig. 8. Nascent arrays contain “holes” bordered by WW receptors (blue circles) from adjoining core complexes that accommodate free CheW molecules, which populate those holes, forming hexameric CheW rings. High cellular CheW contents maximized CheW ring-filled holes in the array and substantially enhanced response cooperativity and sensitivity.
The cellular stoichiometry of other array components had less influence on the extent of CheW ring formation. CheA at native and higher amounts reduced ring formation compared to cells with no CheA (Fig. 4C). Because CheA alone cannot be incorporated into arrays without CheW (44), disruption of CheW ring formation through a direct effect of CheA on array structure seems unlikely. Conceivably, CheA inhibits incorporation of CheW into rings by promoting competing P5•W interface 1 interactions (22, 50, 54, 55). Although receptor trimers of dimers were essential for CheW ring formation (Fig. 3A), varying receptor amounts had little effect on CheW ring content (Fig. 4C), presumably because CheW rings assemble in array holes created by networked core complexes, and receptors were the limiting component in core complex assembly (Fig. S4) (46). Thus, CheW ring content gradually increased with receptor amounts and plateaued above native receptor amounts when core complexes became abundant but less CheW was available to form rings (Fig. 4C).
Sources of signal cooperativity in chemoreceptor arrays
By manipulating cellular CheW amounts in adaptation-deficient cells, we detected about a three-fold increase in cooperativity when arrays transitioned from low to high CheW ring content (Fig. 5B). Sourjik and Berg noted a similar correlation between CheW amounts and response cooperativity in an earlier study (10), an effect that we now attribute to variations in CheW ring content. As another means of controlling CheW ring content, we used a CheA~CheW fusion protein to assemble arrays that were only networked by P5-W rings. Expression of monomeric CheW in those cells allowed us to more precisely control CheW ring contents in their arrays (Fig. 7), because free CheW molecules failed to interact with the CheA~CheW fusion protein in core complexes (Fig. S4C). Both of these approaches revealed that cells with higher CheW ring contents had improved chemotaxis (Fig. 5A; Fig. 6B) and more sensitive, more cooperative attractant responses (Fig. 5B; Fig. 7B). These results demonstrate that array performance arises from two sorts of network connections, P5-W rings that join core complexes into larger signaling teams and CheW rings that form in array holes delimited by the WW receptors of core complexes (Fig. 1; Fig. 9). Interface 2 lesions in CheW disrupt both sorts of network connections, whereas interface 2 lesions in the CheA.P5 domain directly prevent assembly of P5-W rings, which in turn are essential for array integrity and concomitant assembly of CheW rings.
Allosteric models of the E. coli chemotaxis system based on the MWC Monod-Wyman-Changeaux model have provided a theoretical understanding of the interplay between array interconnectivity, activity state, response cooperativity, and detection sensitivity (10, 56, 57). Such models show that array connectivity both increases response sensitivity at low activity state and response cooperativity at high activity state. Thus, in cells lacking the sensory adaptation enzymes, where all receptors have uniform, highly active modification states, their homogeneous arrays produce less sensitive, but highly cooperative attractant responses (10) through extensive interface 2–mediated networking interactions (30, 40). In such arrays, CheW rings contributed substantially to the overall response cooperativity, increasing Hill coefficients more than two-fold at native CheW amounts (Fig. 5B; Fig. 7B). Conversely, in adaptation-proficient cells, receptors have heterogeneous modification states and their arrays produced lower kinase activity (see Fig. 5B and 5C). The attractant responses of these arrays were less cooperative but more sensitive than those of homogeneous receptor arrays. In adaptation-proficient cells array connections between core signaling complexes improve attractant detection sensitivity approximately 10-fold (38). Under these conditions, CheW rings further lowered response thresholds several-fold (Fig. 5B; Fig. 7B). Thus, CheW rings provide additional allosteric and/or structural connections in chemosensory arrays that augment gradient-tracking performance.
There are two likely mechanisms by which CheW rings could enhance array cooperativity; they are not mutually exclusive. Arrays lacking CheW rings (Fig. 10A), like those assembled with the CheA~CheW fusion protein, nevertheless exhibit substantial signal cooperativity, indicating that P5-W rings alone create allosteric connections between core complexes. Perhaps a hexameric CheW ring augments array performance by providing a static structural framework for the allosteric connections within core complexes, enabling them to more effectively communicate with their neighbors (Fig. 10B). For example, a WW receptor might interact more tightly with the other members of a trimer of dimers when it is stabilized through a binding interaction to CheW. The stabilized trimer in turn might more effectively transmit stimulus-induced changes through the core complex to its connected neighbors.
Fig. 10. Possible mechanisms of CheW ring-enhanced array performance.

Cartoon symbols and colors follow the conventions used in previous figures. Black circles indicate ligand-bound receptors; red octagons indicate attractant-inhibited CheA molecules; Dimmed core units indicate the effective size of a cooperative signaling team. For simplicity, we consider only array signaling triggered by ligand-binding to an AW receptor. AA receptors probably act in similar fashion, but WW receptors might not. (A) Limited signal spread in arrays lacking CheW rings. (B) Two possible mechanisms for enhanced signaling in arrays with CheW rings. Static rings could provide a structural framework that enhances allosteric interactions within and between core complexes. Dynamic rings could create additional pathways for propagating stimulus-induced conformational changes between core signaling units. (C) Model of a sparse-CheA array. In this type of array, which is common in other bacteria (for example, V. cholerae) (29, 31), scaffolding protein rings are critical for lattice integrity. Allosteric control of dispersed kinase molecules would most likely proceed through a dynamic ring mechanism.
Alternatively, the CheW ring and WW receptors might augment response cooperativity by creating new pathways for propagating stimulus-induced conformational changes between core complexes (Fig. 10B). In this scenario, the WW receptors play key roles in transmitting conformational changes from one core complex to another through the CheW ring. Structural coupling between the receptors in a trimer could enable the AA and AW members, which control CheA through direct core complex contacts, to respond to allosteric inputs from the WW member.
Architecture and operation of chemoreceptor arrays in other bacteria
Rings of adapter proteins like CheW may play particularly important signaling roles in bacteria such as V. cholerae (31, 58), Bacillus subtilis (59) or Sinorhizobium melliloti (60), whose receptor arrays contain relatively CheA molecules. Under sparse-CheA conditions, adapter protein rings would be the principal source of structural integrity of the receptor array and its allosteric control of embedded kinase molecules. It is easy to imagine how CheW rings could dictate the architecture and operation of a sparse-CheA array: Each CheA homodimer could form a signaling complex in normal fashion, but its P5 domains might incorporate into the array by joining nascent CheW rings to form hexameric P5-W rings with only a single P5 member (Fig. 10C). Cooperative control of CheA activity in such arrays would depend mainly on the allosteric connections between dispersed core complexes provided by WW receptors and CheW rings. Studies of response cooperativity in sparse-CheA arrays should shed light on these mechanistic speculations.
Although E. coli cells lacking CheA can form receptor arrays based on hexameric CheW rings, such arrays are evidently rare in wild-type cells, where CheA and CheW molecules are equally abundant. It seems likely that E. coli would form arrays dominated by CheW rings and WW receptors under artificially imposed sparse-CheA conditions. Such arrays could provide valuable models for exploring mechanisms of CheA control by W-ring CheW rings networked with receptors. Moreover, owing to their regular geometry, arrays of CheW rings offer good subjects for structural studies of receptor-CheW contacts and the conformations of receptor trimers in different output states.
MATERIALS AND METHODS
Bacterial strains and plasmids
Strains used were derivatives of E. coli K-12 strain RP437 (61). Their relevant genotypes are given in Table S2. Plasmid-borne mutations were transferred to their native chromosomal locus by two-step homologous recombination (62).
The plasmids used in this work are listed in Table S3. Mutant plasmids were constructed with the Agilent QuikChange II site-directed mutagenesis kit and confirmed by sequencing the entire coding region of the targeted gene(s). A synthetic gene encoding a CheA-(GGGGS)4 - CheW fusion protein was purchased from Integrated DNA Technologies and cloned into pKG116, yielding pGP74, which was used in subsequent cheA~cheW plasmid and strain constructions.
Media and growth conditions
Cell cultures were grown at 30°C in tryptone broth (TB) (10 g/L tryptone, 5 g/l NaCl). Soft agar chemotaxis assays were performed in TB containing 2.5g/L agar. Plasmid-containing cells were grown in TB media containing ampicillin (50 μg/mL) and/or chloramphenicol (12.5 μg/mL) to ensure plasmid maintenance. Expression of plasmid-borne genes was induced with 0.6 μM sodium salicylate and/or 5-100 μM IPTG (isopropyl β-D-1-thiogalactopyranoside).
Chemotaxis assays
Individual colonies were transferred with sterile toothpicks to TB soft agar and plates were incubated at 32.5°C for 7 h before imaging.
Disulfide crosslinking assays for CheW ring formation
Cells were grown to OD600 ~0.5 and a 1.5-mL aliquot was withdrawn, washed twice with 1 mL of PBS (50 mM NaH2PO4, pH 7.4). At each washing step, cells were centrifuged at 6,000 × g for 5 min at room temperature. After incubating 1 mL of washed cells at 35°C for 10 min, disulfide formation was promoted by the addition of 5 μL of a 60 mM Cu2+-phenanthroline solution (60 mM CuSO4, 200 mM 1,10-phenanthroline, 50 mM NaH2PO4, pH 7.4) to yield a final concentration of 300 μM Cu2+. Samples were kept at 35°C for 10 min, after which the reaction was stopped by adding EDTA to a final concentration of 10 mM. Cells were pelleted by centrifugation at 21,000 × g for 5 min, and then lysed by resuspension in 100 μL of 1X TruPAGE™ LDS Sample Buffer (Sigma-Aldrich). Protein lysates were separated in an 8-12% TruPAGE™ gradient gel (Sigma-Aldrich) using TruPAGE™ Tris-MOPS SDS Express Running Buffer (Sigma-Aldrich). CheW-oligomers were detected by western blot using a polyclonal anti-HA antibody (ThermoFisher, catalog no. PA1-985) and a Cy5-labeled secondary antibody (ThermoFisher, catalog no. A10523). CheA and Tsr proteins were detected in similar fashion with appropriate polyclonal rabbit antibodies. Immunoblots were imaged under fluorescence mode in a Typhoon FLA 9500 scanner (GE Healthcare) and bands were quantified with ImageQuant software (GE Healthcare).
BMOE crosslinking assays
Cells were grown and prepared as described above for disulfide crosslinking and reactions were initiated by adding bismaleimidoethane (BMOE) to a final concentration of 200 μM. Reactions proceeded for 30 sec at 30°C and were then stopped by blocking unreacted thiols with 10 mM N-ethylmaleimide. Cells were collected by centrifugation at 21,000 × g for 5 min and then lysed in 50 μL of Laemmli buffer (63). Protein samples were separated by conventional Tris-glycine SDS-PAGE and crosslinked species were visualized by HA immunoblotting.
In vivo FRET-based kinase assays
Cell preparation, flow cell assembly, stimulus protocol, FRET instrumentation, and data analysis have been described previously (9, 45, 64). Data were fitted to a multisite Hill equation,1 – [Ser]H/([Ser]H + K1/2H), where K1/2 is the concentration of serine that inhibits 50% of the kinase activity and H, the Hill coefficient, reflects the cooperativity of the response. Total CheA kinase activity was calculated as the larger of the FRET changes elicited by a saturating serine stimulus or by 3 mM KCN (64).
Protein modelling and structural display.
Atomic coordinates for the E. coli CheW ring were derived via template-based homology modeling using MODELLER v9.23 (65). A structural template was constructed from PDB 4JPB (22) by replacing the CheA.P5 domains with aligned copies of CheW and a previously published CheW multi-sequence alignment (32). The resulting side chain conformations were subsequently refined using SCWRL4 (66). To provide an array-like receptor scaffold for bounding the CheW ring, a portion of the cytoplasmic tip of E. coli Tsr (residues 366-415) was placed around each monomer using PDB 6S1K (15). The model was solvated with TIP3P water and 150 mM NaCl using VMD v1.9.4 (67) and subjected to a 2000-step conjugant-gradient energy minimization followed by a 10-ns equilibration simulation with protein backbone restraints.
To allow for conformational sampling of the W•W interfaces, a 75-ns simulation was conducted with weak harmonic restraints on the protein alpha carbons, leaving CheW residues 116-130 completely unrestrained. The interfaces were then symmetrized to provide a homogenous model for further relaxation and equilibration of the entire ring structure. The interface selected to provide the symmetrization template was the one with the shortest aggregate Cβ-Cβ distances between the eight pairs of residues experimentally shown to form CheW hexamers. Note, however, no external restraints were used to enforce specific distances between any experimentally identified residue pairs during symmetrization or at any other stage of the refinement process. A final, 75-ns simulation was conducted with positional restraints on the Tsr alpha carbons only to allow the overall ring structure to equilibrate, resulting in the presented model (Fig. 2B) from which the reported cross-linking distances were measured. All simulations used NAMD v2.13 (68) and the CHARMM36 force field (69) and were carried out in the NPT ensemble (pressure = 1 atm, temperature = 310 K) with values for general simulation parameters as previously described (15). Images were generated with VMD.
Supplementary Material
Acknowledgements:
We thank Peter Ames, Caralyn Flack, and Khoosheh Gosink for editorial improvements to the manuscript and Fred Adler for guidance on statistical anslyses of our data.
Funding:
This work was supported by U.S. Public Health Service research grant GM19559 (to JSP) from the National Institute of General Medical Sciences and U.K. Biotechnology and Biological Sciences Research Council grant BB/S003339/1 (to CKC). The Protein-DNA Core Facility at the University of Utah receives support from National Cancer Institute grant CA42014 to the Huntsman Cancer Institute.
Footnotes
Competing interests:
The authors declare that they have no competing interests.
Data and materials availability:
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. All strains and plasmids created or used in this work are available upon request to the corresponding author.
REFERENCES AND NOTES
- 1.Wong-Ng J, Celani A, Vergassola M, Exploring the function of bacterial chemotaxis. Curr. Opin. Microbiol 45, 16–21 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Waite AJ, Frankel NW, Emonet T, Behavioral variability and phenotypic diversity in bacterial chemotaxis. Annu Rev Biophys 47, 595–616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tu Y, Quantitative modeling of bacterial chemotaxis: signal amplification and accurate adaptation. Annu Rev Biophys 42, 337–359 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sourjik V, Wingreen NS, Responding to chemical gradients: bacterial chemotaxis. Curr. Opin. Cell Biol 24, 262–268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hazelbauer GL, Falke JJ, Parkinson JS, Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends. Biochem. Sci 33, 9–19 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parkinson JS, Hazelbauer GL, Falke JJ, Signaling and sensory adaptation in Escherichia coli chemoreceptors: 2015 update. Trends Microbiol. 23, 257–266 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muok AR, Briegel A, Crane BR, Regulation of the chemotaxis histidine kinase CheA: A structural perspective. Biochim Biophys Acta Biomembr 1862, 183030 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bi S, Sourjik V, Stimulus sensing and signal processing in bacterial chemotaxis. Curr. Opin. Microbiol 45, 22–29 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Sourjik V, Berg HC, Receptor sensitivity in bacterial chemotaxis. Proc. Natl. Acad. Sci. USA 99, 123–127 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sourjik V, Berg HC, Functional interactions between receptors in bacterial chemotaxis. Nature 428, 437–441 (2004). [DOI] [PubMed] [Google Scholar]
- 11.Alexander RP, Zhulin IB, Evolutionary genomics reveals conserved structural determinants of signaling and adaptation in microbial chemoreceptors. Proc. Natl. Acad. Sci. USA 104, 2885–2890 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salah Ud-Din AIM, Roujeinikova A, Methyl-accepting chemotaxis proteins: a core sensing element in prokaryotes and archaea. Cell Mol Life Sci 74, 3293–3303 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vladimirov N, Sourjik V, Chemotaxis: how bacteria use memory. Biol. Chem 390, 1097–1104 (2009). [DOI] [PubMed] [Google Scholar]
- 14.Li M, Hazelbauer GL, Core unit of chemotaxis signaling complexes. Proc. Natl. Acad. Sci. USA 108, 9390–9395 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassidy CK et al. , Structure and dynamics of the E. coli chemotaxis core signaling complex by cryo-electron tomography and molecular simulations. Nat. Commun. Biol 3, 24 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burt A et al. , Complete structure of the chemosensory array core signalling unit in an E. coli minicell strain. Nat Commun 11, 743 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang W et al. , In situ conformational changes of the Escherichia coli serine chemoreceptor in different signaling states. mBio 10, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassidy CK et al. , CryoEM and computer simulations reveal a novel kinase conformational switch in bacterial chemotaxis signaling. eLife 4, 08419 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griswold IJ et al. , The solution structure and interactions of CheW from Thermotoga maritima. Nat. Struct. Biol 9, 121–125. (2002). [DOI] [PubMed] [Google Scholar]
- 20.Bilwes AM, Alex LA, Crane BR, Simon MI, Structure of CheA, a signal-transducing histidine kinase. Cell 96, 131–141 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Hu Y, Fu W, Xia B, Jin C, Solution structure of the bacterial chemotaxis adaptor protein CheW from Escherichia coli. Biochem Biophys Res Commun 360, 863–867 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Li X et al. , The 3.2 Å resolution structure of a receptor: CheA:CheW signaling complex defines overlapping binding sites and key residue interactions within bacterial chemosensory arrays. Biochemistry 52, 3852–3865 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park SY et al. , Reconstruction of the chemotaxis receptor-kinase assembly. Nat Struct Mol Biol 13, 400–407 (2006). [DOI] [PubMed] [Google Scholar]
- 24.Liu J et al. , Molecular architecture of chemoreceptor arrays revealed by cryoelectron tomography of Escherichia coli minicells. Proc. Natl. Acad. Sci. USA 109, E1481–1488 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Briegel A et al. , Bacterial chemoreceptor arrays are hexagonally packed trimers of receptor dimers networked by rings of kinase and coupling proteins. Proc. Natl. Acad. Sci. USA 109, 3766–3771 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briegel A et al. , Universal architecture of bacterial chemoreceptor arrays. Proc. Natl. Acad. Sci. USA 106, 17181–17186 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briegel A et al. , New insights into bacterial chemoreceptor array structure and assembly from electron cryotomography. Biochemistry 53, 1575–1585 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Briegel A et al. , Structural conservation of chemotaxis machinery across Archaea and Bacteria. Environmental microbiology reports 7, 414–419 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang W, Briegel A, Diversity of bacterial chemosensory arrays. Trends Microbiol 28, 68–80 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Piñas GE, Frank V, Vaknin A, Parkinson JS, The source of high signal cooperativity in bacterial chemosensory arrays. Proc. Natl. Acad. Sci. USA 113, 3335–3340 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang W, Alvarado A, Glatter T, Ringgaard S, Briegel A, Baseplate variability of Vibrio cholerae chemoreceptor arrays. Proc. Natl. Acad. Sci. USA 115, 13365–13370 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cashman DJ, Ortega DR, Zhulin IB, Baudry J, Homology modeling of the CheW coupling protein of the chemotaxis signaling complex. PLoS One 8, e70705 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hazelbauer GL, Lai WC, Bacterial chemoreceptors: providing enhanced features to two-component signaling. Curr. Opin. Microbiol 13, 124–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice MS, Dahlquist FW, Sites of deamidation and methylation in Tsr, a bacterial chemotaxis sensory transducer. J. Biol. Chem 266, 9746–9753 (1991). [PubMed] [Google Scholar]
- 35.Han XS, Parkinson JS, An unorthodox sensory adaptation site in the Escherichia coli serine chemoreceptor. J. Bacteriol 196, 641–649 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunten P, Koshland DE Jr., Tuning the responsiveness of a sensory receptor via covalent modification. J. Biol. Chem 266, 1491–1496 (1991). [PubMed] [Google Scholar]
- 37.Shimizu TS, Tu Y, Berg HC, A modular gradient-sensing network for chemotaxis in Escherichia coli revealed by responses to time-varying stimuli. Mol. Syst. Biol 6, 382 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ames P, Studdert CA, Reiser RH, Parkinson JS, Collaborative signaling by mixed chemoreceptor teams in Escherichia coli. Proc. Natl. Acad. Sci. USA 99, 7060–7065 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lai RZ, Gosink KK, Parkinson JS, Signaling consequences of structural lesions that alter the stability of chemoreceptor timers of dimers. J. Mol. Biol 429, 823–835 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank V, Pinas GE, Cohen H, Parkinson JS, Vaknin A, Networked chemoreceptors benefit bacterial chemotaxis performance. mBio 7, 01824–01816 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardozo MJ, Massazza DA, Parkinson JS, Studdert CA, Disruption of chemoreceptor signalling arrays by high levels of CheW, the receptor-kinase coupling protein. Mol. Microbiol 75, 1171–1181 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jahreis K, Morrison TB, Garzon A, Parkinson JS, Chemotactic signaling by an Escherichia coli CheA mutant that lacks the binding domain for phosphoacceptor partners. J. Bacteriol 186, 2664–2672 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Studdert CA, Parkinson JS, Insights into the organization and dynamics of bacterial chemoreceptor clusters through in vivo crosslinking studies. Proc. Natl. Acad. Sci. USA 102, 15623–15628 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piñas GE, DeSantis MD, Parkinson JS, Noncritical sgnaling role of a knase-receptor iteraction surface in the Escherichia coli chemosensory core complex. J. Mol. Biol 430, 1051–1064 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sourjik V, Vaknin A, Shimizu TS, Berg HC, In vivo measurement by FRET of pathway activity in bacterial chemotaxis. Methods Enzymol 423, 365–391 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Li M, Hazelbauer GL, Cellular stoichiometry of the components of the chemotaxis signaling complex. J. Bacteriol 186, 3687–3694 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gegner JA, Graham DR, Roth AF, Dahlquist FW, Assembly of an MCP receptor, CheW, and kinase CheA complex in the bacterial chemotaxis signal transduction pathway. Cell 70, 975–982 (1992). [DOI] [PubMed] [Google Scholar]
- 48.Boukhvalova M, VanBruggen R, Stewart RC, CheA kinase and chemoreceptor interaction surfaces on CheW. J. Biol. Chem 277, 23596–23603 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Piñas GE, Parkinson JS, Identification of a Kinase-Active CheA Conformation in Escherichia coli Chemoreceptor Signaling Complexes. J. Bacteriol 201, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Natale AM, Duplantis JL, Piasta KN, Falke JJ, Structure, function, and on-off switching of a core unit contact between CheA kinase and CheW adaptor protein in the bacterial chemosensory array: A disulfide mapping and mutagenesis study. Biochemistry 52, 7753–7765 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith RA, Parkinson JS, Overlapping genes at the cheA locus of Escherichia coli. Proc. Natl. Acad. Sci. USA 77, 5370–5374 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kofoid EC, Parkinson JS, Tandem translation starts in the cheA locus of Escherichia coli. J. Bacteriol 173, 2116–2119 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Briegel A, Beeby M, Thanbichler M, Jensen GJ, Activated chemoreceptor arrays remain intact and hexagonally packed. Mol. Microbiol 82, 748–757 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao J, Parkinson JS, Mutational analysis of the chemoreceptor-coupling domain of the Escherichia coli chemotaxis signaling kinase CheA. J. Bacteriol 188, 3299–3307 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boukhvalova MS, Dahlquist FW, Stewart RC, CheW binding interactions with CheA and Tar: Importance for chemotaxis signaling in Escherichia coli. J. Biol. Chem 277, 22251–22259 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Keymer JE, Endres RG, Skoge M, Meir Y, Wingreen NS, Chemosensing in Escherichia coli: two regimes of two-state receptors. Proc. Natl. Acad. Sci. USA 103, 1786–1791 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mello BA, Tu Y, Effects of adaptation in maintaining high sensitivity over a wide range of backgrounds for Escherichia coli chemotaxis. Biophys. J 92, 2329–2337 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alvarado A et al. , Coupling chemosensory array formation and localization. eLife 6, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cannistraro VJ, Glekas GD, Rao CV, Ordal GW, Cellular stoichiometry of the chemotaxis proteins in Bacillus subtilis. J. Bacteriol 193, 3220–3227 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arapov TD et al. , Cellular stoichiometry of chemotaxis proteins in Sinorhizobium meliloti. J. Bacteriol 202, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parkinson JS, Houts SE, Isolation and behavior of Escherichia coli deletion mutants lacking chemotaxis functions. J. Bacteriol 151, 106–113 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ames P, Zhou Q, Parkinson JS, Mutational analysis of the connector segment in the HAMP domain of Tsr, the Escherichia coli serine chemoreceptor. J. Bacteriol 190, 6676–6685 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Laemmli UK, Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970). [DOI] [PubMed] [Google Scholar]
- 64.Lai RZ, Parkinson JS, Functional suppression of HAMP domain signaling defects in the E. coli serine chemoreceptor. J. Mol. Biol 426, 3642–3655 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Webb B, Sali A, Comparative protein structure modeling using MODELLER. Curr. Protoc. Bioinformatics 2016, 5.6.1–5.6.37 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Krivov GG, Shapovalov MV, Dunbrack RL, Improved prediction of protein side-chain conformations with SCWRL4. Proteins: Structure, Function, and Bioinformatics 77, 778–795 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Humphrey W, Dalke A, Schulten K, VMD: Visual molecular dynamics. J. Mol. Graph 14, 33–38 (1996). [DOI] [PubMed] [Google Scholar]
- 68.Phillips JC et al. , Scalable molecular dynamics with NAMD. J. Computational Chem 26, 1781–1802 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bi S et al. , Discovery of novel chemoeffectors and rational design of Escherichia coli chemoreceptor specificity. Proc. Natl. Acad. Sci. USA 110, 16814–16819 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bibikov SI, Biran R, Rudd KE, Parkinson JS, A signal transducer for aerotaxis in Escherichia coli. J. Bacteriol 179, 4075–4079 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morrison TB, Parkinson JS, Liberation of an interaction domain from the phosphotransfer region of CheA, a signaling kinase of Escherichia coli. Proc. Natl. Acad. Sci. USA 91, 5485–5489 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang ACY, Cohen SN, Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J. Bacteriol 134, 1141–1156 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yen KM, Construction of cloning cartridges for development of expression vectors in Gram-negative bacteria. J. Bacteriol 173, 5328–5335. (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gosink KK, Buron-Barral M, Parkinson JS, Signaling interactions between the aerotaxis transducer Aer and heterologous chemoreceptors in Escherichia coli. J. Bacteriol 188, 3487–3493 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in the paper are present in the paper or the Supplementary Materials. All strains and plasmids created or used in this work are available upon request to the corresponding author.


