Abstract
Preeclampsia is a syndromic disease of the mother, fetus, and placenta. The main limitation in early and accurate diagnosis of preeclampsia is rooted in the heterogeneity of this syndrome as reflected by diverse molecular pathways, symptoms and clinical outcomes. Gaps in our knowledge preclude successful early diagnosis, personalized treatment and prevention. The advent of “omics” technologies and systems biology approaches enable addressing this problem by identifying the molecular pathways associated with the underlying mechanisms and clinical phenotypes of preeclampsia. Here, we provide a brief overview on how the field has progressed, focusing on studies utilizing state-of-the-art transcriptomics and proteomics methods. Moreover, we summarize our systems biology studies involving maternal blood proteomics and placental transcriptomics, which identified early maternal and placental disease pathways, and showed that their interaction influences the clinical presentation of preeclampsia. We also present an analysis of maternal blood proteomics data which revealed distinct molecular subclasses of preeclampsia and their molecular mechanisms. Maternal and placental disease pathways behind these subclasses are similar to those recently reported in studies on the placental transcriptome. These findings may promote the development of novel diagnostic tools for the distinct subtypes of preeclampsia syndrome, enabling early detection and personalized follow-up and tailored care of patients.
Keywords: class discovery, great obstetrical syndromes, high-dimensional biology, liquid biopsy, “omics” sciences, personalized medicine, prenatal diagnosis
The preeclampsia syndrome
Preeclampsia carries dire consequences for the mother and fetus, hence one of the main goals of prenatal follow-up is the early detection of the development of this syndrome. Its onset is multifactorial and can occur at various gestational ages and can display different grades of severity [1–6]. The current classification is based on the onset and the severity of symptoms; however, it does not accurately reflect the underlying pathophysiological processes. Based on this classification, we distinguish early-onset (<34 weeks) and late-onset (≥34 weeks), or preterm (<37 weeks) and term (≥37 weeks) preeclampsia [2]. Early-onset or preterm preeclampsia is more often complicated by fetal growth restriction (FGR) and more severe symptoms compared to late-onset or term preeclampsia [1,2,4,5].
In preterm preeclampsia, the extravillous trophoblast dysfunction and consequent impairment of spiral artery remodeling has a paramount importance in its pathogenesis. Decreased uteroplacental perfusion and ischemic stress lead to an imbalance in angiogenic and antiangiogenic factors, resulting in endothelial damage, systemic inflammation and multiorgan failure [3,7–20]. In term preeclampsia, the effect of various chronic stressors such as obesity, diabetes, kidney, metabolic or autoimmune diseases is more dominant [21–30] and maternal vascular and endothelial response may also be more sensitive to placental factors [31,32]. Genetic factors related to angiogenesis and immune interactions between the mother and the fetus are also key for the susceptibility to preeclampsia [33–41]. Due to these pathophysiological differences, early-onset or preterm preeclampsia can be more accurately predicted in the first trimester by a combination of maternal characteristics, biophysical and biochemical markers compared to late-onset preeclampsia [42]. Improved prediction can likely be achieved if the heterogeneous pathophysiological pathways and their specific biomarkers are identified.
Although considerable progress has been made in the understanding of preeclampsia using clinical epidemiology, astute observations by clinicians, and hypothesis-driven research, the advent of hypothesis-free research and post-genomic tools (also known as high dimensional biology or “omics” sciences) [43] enabled us to further tackle the complexity of the disease pathways and the heterogeneity of this severe syndrome.
High dimensional biology studies in preeclampsia
High-throughput “omics” techniques have revolutionized systems biology approaches to diseases from the molecular to the clinical levels. With current automation, “omics” properties of up to tens of thousands of samples can be stacked, and studies are not limited to only the set of markers that are known to be clinically relevant but novel disease biomarkers can be discovered. The evaluation of these data can be performed using multidimensional statistical and machine learning methods, which can work accurately and provide a proper picture of the studied disease only using a large number of samples and properly annotated databases. Another challenge for this field is the need for a universal platform that allows the evaluation of different “omics” data. Upon all these conditions present, these hypothesis-free examination methods allow us to find molecular patterns and to learn about pathological changes in their complexity at the systemic level. This way our findings will not be limited and biased by our presumptions or hypotheses [44].
Irrespective of the type of samples involved (e.g. placenta, blood) or type of molecular profiling (global, single-cell, cell-free, etc.), high-throughput experiments in preeclampsia can be broadly grouped in three types of applications [45]. The first application is called class comparison. It aims at comparing molecular profiles between cases with clinically defined phenotypes (e.g. all, early-onset or late-onset preeclampsia cases) versus controls. This method enables inferring pathways and biological processes perturbated in cases that are associated with the observed phenotypes and possibly also identifying therapeutic targets. The second type of application is class prediction. This uses discriminant analysis and machine learning methods to develop disease prediction models. The focus is on maximizing the prediction accuracy and parsimony rather than interpretation of revealed differences in molecular profiles. Unsurprisingly, the syndromic nature of preeclampsia, which is manifested by high heterogeneity in expression profiles, has brought challenges to both class-comparison and class-prediction applications, and hence the need for class discovery. The goal of this last type of application is to uncover disease subtypes using data-driven clustering of patient samples without assuming a particular number and pathology of disease subtypes. This approach is completely hypothesis-free and unbiased towards the diagnostic criteria [44], which is key since the categorization of patients based on the onset of clinical symptoms into a preset of two groups (i.e. early-onset vs late-onset) are prone to bias at several levels.
Placental transcriptomics in preeclampsia
The placenta, which represents inherent fetal characteristics and response to the intrauterine environment, has a central role in the pathophysiology of preeclampsia [3,4]. Therefore, it is not surprising that genome-wide profiling of the human placental transcriptome became the first unbiased approach in the study of normal maternal–placental–fetal physiology and the pathology in preeclampsia.
A recent comprehensive review [46] summarized human placental transcriptome studies from the cellular to tissue levels while addressing important aspects of study design in order to promote data sharing and meta-analyses. Yong and Chan summarized 179 studies since 2004 into four themes, with one focusing on pregnancy complications including preeclampsia.
Results provided by these transcriptomics studies not only improved our understanding of healthy placental development, but placenta-derived biomarkers secreted into the maternal circulation in preeclampsia (e.g. sFLT1, sEng) were discovered [11,12,47], and the biological processes and molecular pathways associated with clinical preeclampsia phenotypes were detected, providing clues into the underlying mechanisms of placental pathologies [46]. Due to limitations in placental sample collection and the late clinical onset of preeclampsia symptoms, most of these studies targeted the third trimester placental transcriptome, in which the molecular patterns representative of oxidative stress and inflammatory pathways were frequently seen. Of importance, one study [48] of first trimester placental tissues, left over from chorionic villus sampling, assessed the placental transcriptome of 4 women who later developed preeclampsia (2 preterm and 2 term) and 8 healthy controls. Despite the low sample size, the study showed that the dysregulation of genes involved in cell motility, immune modulation, and inflammation was already present at this early stage of gestation, however, gene dysregulation characteristic of hypoxia or ischemia were not found.
Another limitation of most studies was that they did or could not address the cellular heterogeneity of the placenta. This is an extremely heterogeneous organ with cell types of various origins and differing gene expression profiles [49,50]. Therefore, global or targeted expression studies using bulk tissues could not adequately dissect the pathological mechanisms, missing cell-level information and cellular interactions within this organ. As discussed later, a great advancement came with the rise of single cell transcriptomics studies, which solved this bottleneck and became prominent for the study of placental gene expression in healthy and diseased states [49,50].
Distinct placental gene modules are linked to fetal or maternal diseases in preterm preeclampsia
In one of the first “class comparison” microarray studies on third trimester placentas, we found that the transcriptome of women with severe preterm preeclampsia associated with the clinical presentation of “haemolysis, elevated liver enzymes, low platelet count” (HELLP) syndrome is similar to those women with preterm preeclampsia without HELLP syndrome [51]. Differentially expressed (DE) genes in preterm preeclampsia compared to controls were similar to those previously reported in this preeclampsia subtype [52–55], and many of the DE genes encoded proteins which had earlier been proposed as biomarkers for preeclampsia (e.g. FLT1, LEP, PAPPA2). Although similar biological processes, cellular compartments and signaling pathways were enriched in preterm preeclampsia, with or without the presence of HELLP syndrome, there was more engagement of the cytokine-cytokine receptor pathway in cases associated with HELLP syndrome, reflecting a more pronounced systemic maternal inflammatory response.
A further systems biology analysis of this dataset identified major gene co-expression network modules and their hub transcription regulatory genes in the third trimester placenta of women with preterm preeclampsia [56]. The largest module contained genes involved in fetal growth (CSH1, HSD11B2), and hub transcription regulatory genes (ESRRG, POU5F1, ZNF554) implicated in the regulation of trophoblast metabolism, stemness, differentiation and invasion [57,58]. Genes in the second largest module were associated with maternal blood pressure (e.g. FLT1), and their hub transcription regulatory genes (BCL6, BHLHE40, ARNT2) were implicated in the hypoxia response. In vitro functional experiments demonstrated that the trophoblastic overexpression of transcription factors BCL6 or ARNT2 sensitizes the trophoblast to hypoxia and leads to FLT1 overexpression upon hypoxic-ischemic stress. The expression of the “blood pressure module” biomarker genes was positively associated with the maternal vascular malperfusion score of the placenta, and the amounts of their secreted protein products (sFlt-1, sEng, leptin) started to increase in the maternal circulation after 12 weeks of gestation. These observations fit the overall concept that maternal vascular malperfusion in the first trimester leads to subsequent placental oxidative stress, increased placental expression of FLT1 and an anti-angiogenic state starting from late first, early second trimester [7,13,59]. Of interest, a set of transcription regulatory genes (e.g. BCL6, BHLHE40, JUNB) were DE in the placenta in preeclampsia in the opposite way as during villous trophoblast differentiation as revealed by our subsequent microarray study [60]. Five of these transcription regulatory genes are central members of the “blood pressure module”, suggesting links between disorders of trophoblast differentiation, maternal vascular malperfusion, placental oxidative stress, an anti-angiogenic state and preterm preeclampsia.
Uncovering the molecular subclasses of preeclampsia by placental transcriptomics
Although the initial studies of the placental transcriptome accurately characterized the severe clinical subtype of preterm preeclampsia, the heterogeneity of cases and the underlying molecular subclasses were unknown until 2015. This hiatus was filled first by the class discovery studies on the placental transcriptome by Leavey et al. [61,62]. The authors conducted unsupervised analyses of placental transcriptomes to provide insights into the molecular taxonomy of preeclampsia. They identified five clusters among all cases and controls in the larger study: 1) the first included largely patients who delivered at term; 2) the second cluster was composed predominantly of patients with preterm preeclampsia; 3) the third cluster included a subset of patients with preeclampsia and other complications of pregnancy; 4) the fourth cluster consisted mostly of patients with spontaneous preterm delivery; and 5) the fifth cluster included women with placental chromosomal abnormalities with and without preeclampsia, due to the confined placental mosaicisms present in this group also detected by other studies [63,64].
The three major subclasses of preeclampsia identified in these studies are presented in Figure 1: 1) “canonical / placental preeclampsia”: The clinical characteristics consisted of preterm preeclampsia, with abnormal Doppler velocimetry (several vessels), and birthweight <50th centile, and included some patients with HELLP syndrome. Gene expression for sFlt-1 and endoglin was particularly high for this group of patients. This molecular phenotype was mostly characterized previously by the class comparison studies including ours [51]; 2) “maternal preeclampsia”: These represented a group of patients with preeclampsia mostly at term or near-term delivery with appropriate-for-gestational age (AGA) neonate and with known maternal risk factors, including nulliparity or prior hypertensive pregnancy. The placentas typically did not have any maternal vascular lesions; and 3) “immunological preeclampsia”: This group consisted of patients delivering between 30–37 weeks of gestation, low placental weights, small-for-gestational (SGA) age neonates, and a transcriptome enriched by the expression of genes involved in the immune response and poor maternal-fetal tolerance to the fetoplacental unit (e.g. CXCL-10).
Figure 1.
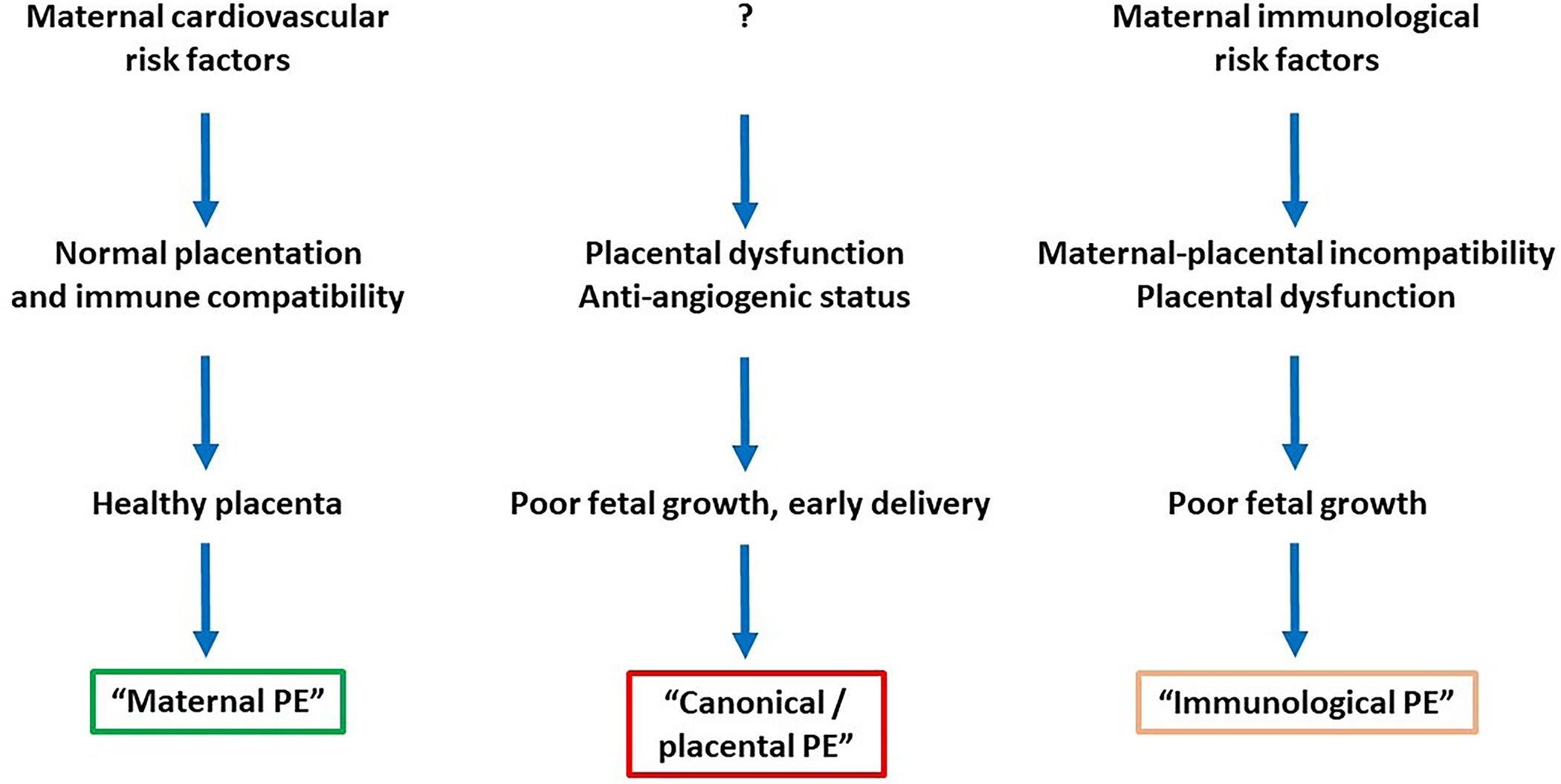
Molecular subclasses of preeclampsia derived from placental transcriptomics data. Major clinical and placental characteristics are depicted.
PE, preeclampsia.
The same authors reported subsequently that a high degree of concordance can be found between the results of gene expression clustering of the placentas and the histopathologic features of this fetal organ [65]. “Placental preeclampsia” was associated with maternal vascular lesions of underperfusion, while “immunological preeclampsia” was characterized by chronic inflammatory lesions of the placenta, intervillous thrombi, and maternal vascular lesions of malperfusion. In contrast, “maternal preeclampsia” typically had minimal placental histologic findings. In a subsequent study [66], “immunological preeclampsia” was associated with an enrichment in monocytes (positive for CD68) and neutrophils (positive for myeloperoxidase) in the intervillous space while “canonical preeclampsia” had a significantly less number of these cells.
It is important to note that the gene expression profiles of placentas with “placental preeclampsia” and “immunological preeclampsia” have also been observed in FGR without preeclampsia [67], indicating that the pattern of gene expression in the placenta is not sufficient to define the clinical phenotype. This suggests that placental disease could cause hypertension in a woman only if susceptible, and some women may be resistant to the hypertensive state induced by placental maldevelopment and/or dysfunction. Eventually, the maternal and fetal compartments may have a degree of independence, and preeclampsia could primarily be induced by either of these compartments or by their synergy or poor complementarity. As such, it transpired that the molecular investigations of maternal blood, which also reflects changes in the maternal compartment, is critical in depicting the interaction between the fetus and the maternal environment as both placental and maternal molecular factors determine the development of preeclampsia and its clinical phenotype.
Liquid biopsy of the placenta
Liquid biopsy is a fast-growing area in diagnostics and involves taking samples from body fluids (e.g. serum, plasma, urine) to derive information regarding the functional and molecular status of organs minimally invasively [68]. As such, liquid biopsy has become key for tumor diagnostics by monitoring circulating tumor cells and DNA [69]. Historically, the first attempts to non-invasively detect placental function in maternal blood can be linked to the systematic discovery and characterization of placenta-derived proteins and their investigation as potential biomarkers of placental function, pregnancy complications and fetal genetic disorders [70]. For example, the quantification of blood hCG, PP13/galectin-13, and PSG1 has become of importance for the detection of pregnancy or pregnancy complications including preeclampsia from maternal blood [71,72]. Since the discovery of cell-free fetal DNA (cffDNA) in the maternal circulation by Lo et al. in 1997 [73], the fast-evolving non-invasive prenatal diagnostics (NIPT) technologies have revolutionized prenatal screening of genetic defects based on the detection of cffDNA in small amounts of maternal blood [74]. Shortly after, the group of Lo et al. also identified circulating placental/fetal RNA (cpRNA) in the maternal circulation [75], and determined the earliest gestational age (4th week) at which these cpRNAs are present in maternal circulation. Their abundance increases with advancing gestation and reach 10–15% of total RNA in maternal circulation [76]. These discoveries have paved the way for the quantification of cpRNAs to non-invasively investigate the placental transcriptome and to predict pregnancy complications or monitor high-risk pregnancies without endangering the fetus [77,78]. In addition, various circulating microparticles are released from the syncytiotrophoblast during pregnancy into the maternal circulation, including exosomes, which contain various elements of placental origin, such as proteins, lipids, mRNAs, miRNAs. The molecular signatures of trophoblastic microparticles may provide important information about the condition of the placenta while non-placental microparticles including exosomes may reflect maternal health or disease states. In line with these, recent studies identified potential biomarkers of preeclampsia by examining the changes in the type, amount, and content of these exosomes [79–82].
Maternal blood transcriptomics as a prediction tool for preeclampsia
A comprehensive review [77] identified 24 studies between 2003–2014 which measured cpRNA in maternal whole peripheral blood or maternal plasma to predict and/or monitor preeclampsia. Multiple studies on cpRNAs showed congruent findings with placental transcriptomics studies in that many placenta-specific gene transcripts dysregulated in the placenta in preeclampsia were found similarly dysregulated in maternal circulation (e.g. CRH, FLT1, ENG upregulated, hPL, PP13 downregulated). Like placental transcriptomic changes in preeclampsia, alterations in maternal blood transcriptome in preeclampsia reflected disturbances with angiogenesis as well as hypoxia and oxidative stress response. There were considerable differences regarding cpRNA expression with the clinical phenotype of preeclampsia, as higher levels of specific cpRNA transcripts were observed in early-onset vs late-onset preeclampsia, and in more severe forms, especially those complicated by HELLP syndrome. This is in line with the larger gene expression changes and increased debris output by the placenta in these clinical forms [77]. In a recent large maternal blood cfRNA profiling study, the later onset of preeclampsia could be predicted in midtrimester with a sensitivity of 75% and a positive predictive value of 32.3% [83]. Of interest, by measuring panels of cpRNAs as early as in the first trimester, considerably good prediction models could be built for preeclampsia. Farina et al. found that the combination of endoglin, FLT1, and TGFβ1 transcripts had a detection rate of 72.3% at 5% false positive rate (FPR) at 10–14 weeks of gestation [84]. The same group showed that a panel of transcripts including FLT1 had a detection rate of 84% at 5% FPR at 15–20 weeks of gestation [85].
Since 2011, extracellular miRNAs have also received attention as potential biomarkers. Although their role in the pathophysiology of preeclampsia is still unclear, the altered expression of these nucleic acids has been observed. Their advantage over mRNAs is that they are shorter, have fewer species, and thus, more cost-effective in their analysis. In addition, miRNAs are more extracellularly stable, so they can be used as both prognostic tools and therapeutic targets in the future [86]. A recent study not only discovered and verified peripheral miRNAs as preeclampsia biomarkers in midtrimester, but also showed that the placenta contributes the most of the changes in miRNA pattern in preeclampsia, and that miR-155-5p - which negatively regulates NO synthase expression – has a central role in the pathogenesis [87].
In order to assess the maternal compartment as well and to reveal differences and similarities in the molecular basis of the two major clinical phenotypes at the time of diagnosis, we investigated maternal whole-blood transcriptome in early-onset and late-onset preeclampsia with microarrays [88]. This study uncovered common features of these two phenotypes including the dysregulation of genes involved in host defense (e.g. DEFA4, BPI), tight junctions (EMP1) and liver regeneration (ECT2). While DE genes in women with early-onset preeclampsia were involved in coagulation (SERPINI2), immune regulation (CD24, VSIG4), developmental process (H19) and inflammation (S100A10), those genes DE in late-onset preeclampsia were implicated in innate immunity (LTF, ELANE) and cell-to-cell recognition in the nervous system (CNTNAP3). A follow-up longitudinal transcriptomics study uncovered that mRNA whole blood signature of preeclampsia discovered at the time of diagnosis is also increased earlier in gestation at 22–28 weeks [89]. The combination of four genes from this signature, including an imprinted long non-protein coding RNA (H19), fibronectin 1 (FN1), tubulin beta-6 class V (TUBB6), and formyl peptide receptor 3 (FPR3), had a sensitivity of 85% and a specificity of 92% for the prediction of early-onset preeclampsia [89].
A major advancement in the field was the use of single-cell transcriptomics to dissect the cellular heterogeneity of normal term human placenta and to define individual cell-specific gene signatures [50,90]. This technology also enabled the reconstruction of the differentiation trajectory of normal trophoblast as well as the discovery of new cells in the placenta and the identification of cell type-specific molecular changes in the placenta of patients with preeclampsia. Of interest, the single-cell transcriptomics signature of extravillous trophoblasts was found to be increased in maternal blood of patients with early-onset preeclampsia compared to normal pregnant women at the time of disease [90]. Studies from our group suggested that increased RNA expression with early-onset preeclampsia is not limited to the extravillous trophoblasts, but the transcriptomics signatures of other placental cell types are also heightened. The rise in circulating RNA expression of placental signatures was identified at the time of disease as well as at earlier stages of gestation [89] suggesting, that the analysis of both maternal plasma cell-free and cellular RNA can be used to identify patients at risk to develop early-onset preeclampsia. The similarity of cpRNA- and cellular RNA-based findings was demonstrated not only when studying preeclampsia, but also across independent studies assessing changes with gestational age in normal pregnancies [91,92].
Maternal blood proteomics in preeclampsia
The study of proteomics yields essential molecular information regarding maternal and fetal health/disease states. A recent review [93] summarized 69 unbiased quantitative proteomics class comparison studies on preeclampsia since 2004 and proteins found to be DE in this syndrome, also taking into account of the continuous technical evolution to reach unified outcomes. Most of the studies targeted maternal serum/plasma, placenta, or urine proteomics, making it the largest compilation of quantitative proteomics data in preeclampsia. The total number of DE proteins in placenta, serum/plasma and urine were 912, 559 and, 132, respectively. After considering only those proteins which were described by more independent studies with inter-study agreement in control/preeclamptic ratio of protein abundance, they found a cluster of 18, 29 and 16 proteins consistently DE in preeclampsia in the placenta, serum/plasma and urine, respectively.
Of interest, among the 18 proteins with a robust up- or down-regulation in the placenta in preeclampsia at the time of the disease across 23 studies, Flt1 and PAPPA2 were also found, validating many findings of our group and others both at the RNA and protein levels, and underlining the up-regulation of the “blood pressure gene module” in the placenta in preeclampsia. Among the 29 proteins with a robust dysregulation in the serum/plasma in preeclampsia throughout gestation, sEng was consistently found to be up-regulated and PlGF to be down-regulated, proving the systemic anti-angiogenic state in preeclampsia with proteomics techniques. Moreover, 14 proteins in the maternal circulation, including sEng, PlGF, MMP7 and many immune-related proteins, were found to have the same directional change in the summarized studies as in our omics and ELISA studies, validating our findings discussed in the following sections.
First trimester proteomics profile of preterm and term preeclampsia
Initially, we performed a class comparison analysis with two-dimensional difference gel electrophoresis (2D-DIGE) proteomics of first trimester maternal blood which identified novel early maternal pathways of preeclampsia [56]. From the 26 proteins, 12 were DE in women who developed preterm preeclampsia, 7 were DE in women who developed term preeclampsia, and 7 were DE in both groups. The 19 DE proteins in women with subsequent preterm preeclampsia have a role in immune response, complement and coagulation cascades, lipid transport and metabolism, angiogenesis, blood pressure regulation, and ion transport, suggesting that these maternal pathways are already perturbed in the first trimester, in the clinically still silent phase of preterm preeclampsia [56]. Proteins enriched in term preeclampsia have identified pathways similar to those found in early-onset preeclampsia, but the detected changes were smaller in extent.
Subsequent studies identified molecular networks linking the 19 DE proteins detected in the maternal circulation in the first trimester with the 1409 DE genes found in the placenta of preterm preeclampsia patients [56], suggesting that the changes in the maternal proteome may have an effect on placental functions and gene expression. Indeed, we could validate these in silico findings by in vitro experiments, in which primary villous trophoblasts were cultured with first trimester maternal serum. The serum from the preterm preeclampsia group vs the healthy control group induced the up-regulation of many genes in villous trophoblasts, which were also up-regulated in the placenta in preterm preeclampsia patients and associated with blood pressure elevation (e.g. LEP, FLT1). Our data pointed to separate maternal and placental disease pathways and their interaction in the development of preeclampsia. Several maternal protein biomarkers we have identified early in gestation were already implicated by other studies in a later disease stage, when their dysregulation is more pronounced yet a limited connection between the maternal circulation and the placenta still exists [94]. This suggests the early activation of maternal disease pathways both in term and preterm preeclampsia, upstream of placental dysfunction, probably due to preexisting maternal diseases or perturbed maternal–fetal–placental immune interactions [95–97].
Plasma proteomic changes throughout gestation in early-onset and late-onset preeclampsia
To discover additional disease biomarkers and detect the dynamic changes in the maternal proteome throughout pregnancy, two longitudinal case control studies of 1125 plasma proteins via aptamer-based assays were conducted in women who developed early-onset or late-onset preeclampsia [98,99]. The best predictors for subsequent development of early-onset preeclampsia were: 1) high abundance of MMP7 and glycoprotein IIbIIIa complex at 16–22 weeks; and 2) low abundance of PlGF and VEGF-121, and elevated siglec-6 and activin-A at 22–28 weeks. At 22–28 weeks, the increased abundance in siglec-6, activin-A, and VEGF-121 differentiated women who subsequently developed early-onset preeclampsia from those who developed late-onset syndrome or had normal pregnancy. In agreement with earlier studies, the sensitivity of risk models was higher for early-onset preeclampsia with placental histology signs of maternal vascular malperfusion than for the entire early-onset preeclampsia group, potentially because these models are sensitive to the pathway of preeclampsia associated with the malperfusion of uteroplacental circulation. Biological processes dysregulated in preeclampsia included : 1) ‘cell adhesion’ and ‘response to hypoxia’ and seemed specific to early-onset preeclampsia; 2) ‘small molecule metabolic process’, ‘positive regulation of apoptotic process’ were specific to late-onset preeclampsia; and 3) ‘extracellular matrix organization’, ‘positive regulation of VEGFR signaling pathway’, and ‘positive regulation of cell adhesion’ were common for both phenotypes of this syndrome [98,99]. As implied from these and other proteomic discovery studies, an anti-angiogenic state, though in different extent, reflects the common pathway of preeclampsia in all phenotypes.
Uncovering the molecular subclasses of preeclampsia by maternal blood proteomics
Maternal blood proteomics class comparison studies are limited in the sense that groups are defined based on symptoms and signs of preeclampsia but not by underlying pathophysiology. In order to fill this gap and investigate molecular subclasses of preeclampsia, we performed two unsupervised class discovery studies by extending our previous maternal blood proteomics investigations [56,98,99].
In the first study, cases (n=82) and controls (n=82) were selected from a Hungarian cohort (n=2,545). Blood sampling was performed at 11–14th weeks. Cases included 22 women with subsequent early-onset and 60 women with subsequent late-onset preeclampsia, while controls were selected by matching gestational age at blood draw. Blood samples were analyzed by a mass spectrometry based targeted proteomics approach (MRM, multiple reaction monitoring, Biognosys AG, Switzerland) for 59 protein biomarkers either identified by us [56] (n=25) or retrieved from the literature (n=34), based on their biological plausibility for known disease pathways of preeclampsia in the second half of pregnancy. In addition, current biochemical and biophysical preeclampsia biomarkers were also assessed. In order to identify disease subclasses, consensus clustering was performed, a robust method for discovering clusters [100]. We performed consensus clustering with 1000x resampling using unsupervised k-means clustering to ensure cluster stability and optimal cluster numbers. Consensus matrix contained the probability of that an element pair was included in a common cluster during resampling. Using a subset of proteins showed that the 82 patients stably clustered into 4 distinct subclasses based on their proteomics profiles (Figure 2). Cluster 1 contained the most early-onset and SGA cases, a high number of cases with chronic hypertension, the most abnormal Doppler indices, and a protein profile consistent with vascular injury, oxidative stress, an anti-angiogenic status, matching the “placental” subclass. Cluster 2 contained a considerable number of early-onset cases, with a high prevalence of maternal metabolic problems (high BMI, chronic hypertension, and diabetes), the highest first trimester mean arterial pressure (MAP), and a molecular profile consistent with pro-inflammatory and vascular changes, matching a novel subclass which we coined “metabolic” subclass. Cluster 3 were all late-onset cases with maternal metabolic problems (high BMI, chronic hypertension, and diabetes), a high prevalence of previous preeclampsia cases, and a protein profile consistent with systemic pro-inflammatory changes, matching the “immunological” subclass. Cluster 4 cases were almost all late-onset, women with the highest percentage of nulliparity and with a protein profile least different from controls, matching the mildest “maternal” subclass (Table 1/Figure 3). Of note, 75% of the tested 59 proteins were validated to be DE by this study, including 76% of the biomarkers (19/25) we described previously [56]. Although the samples were obtained from women with two ethnic backgrounds (Caucasian and roma), we did not find significant ethnic disparity in the different subclasses of preeclampsia.
Figure 2.
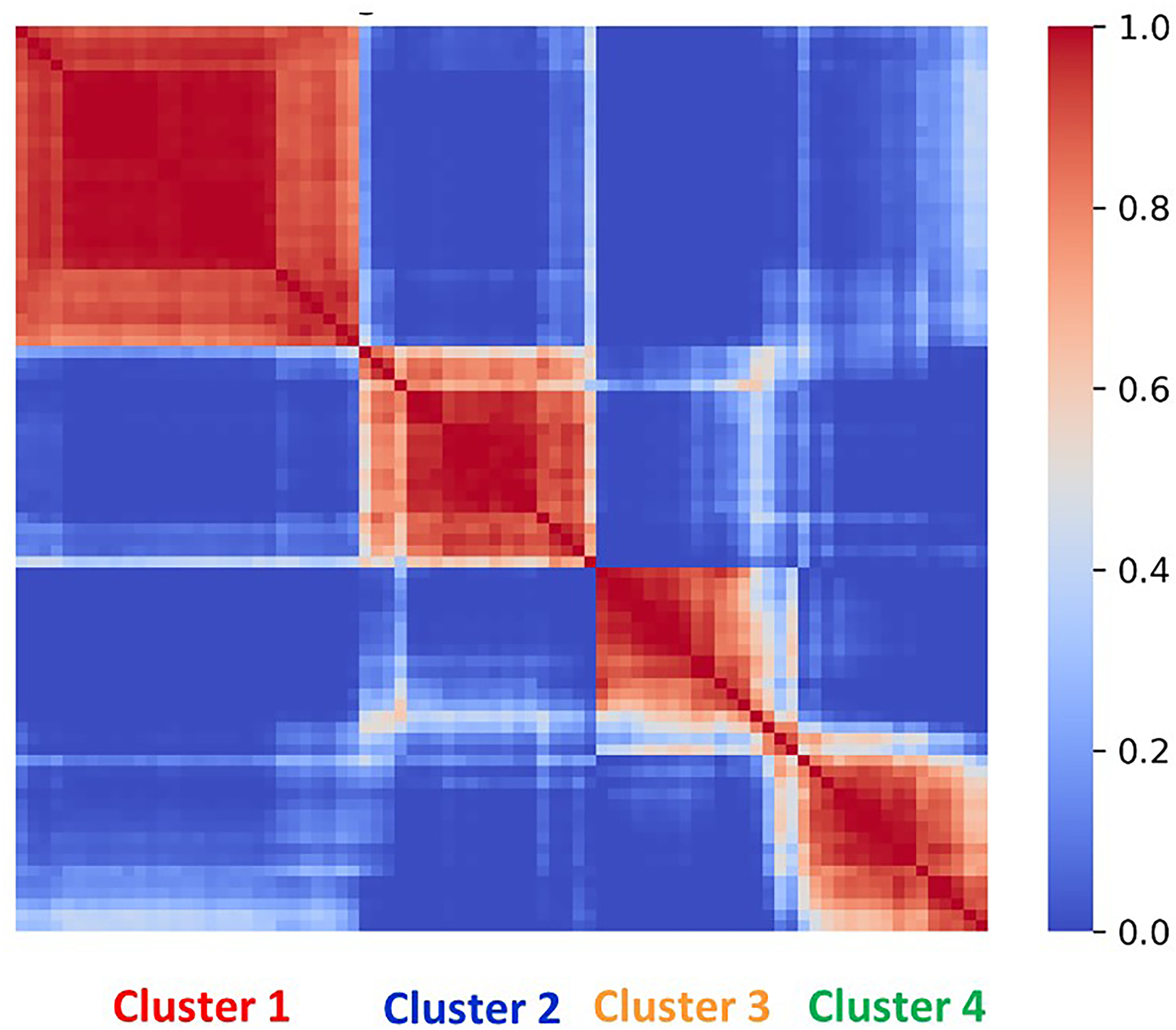
Consensus matrix of preeclampsia patients. The consensus matrix, represented in a heatmap, shows the probability of different patients with preeclampsia to appear in the same cluster. The 82 patients stably clustered into four molecular groups. The color spectrum depicted on the bar indicates clustering similarity.
Table 1.
Patient characteristics in the four molecular clusters.
| Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | |
|---|---|---|---|---|
| Patient number | n=29 | n=20 | n=16 | n=17 |
| Early-onset cases | 7 (24%) | 3 (15%) | 0 (0%) | 1 (6%) |
| Late-onset cases | 22 (76%) | 17 (85%) | 16 (100%) | 16 (94%) |
| BW percentile* | 79% | 89% | 100% | 86% |
| SGA cases | 9 (31%) | 3 (15%) | 2 (15%) | 3 (18%) |
| Nulliparity | 22 (76%) | 13 (65%) | 11 (69%) | 14 (82%) |
| Diabetes | 7% | 15% | 13% | 6% |
| BMI | 26 | 29 | 30 | 25 |
| Chr. hypertension | 28% | 25% | 31% | 6% |
| History of PE | 7% | 0% | 19% | 0% |
| Smoking | 7% | 10% | 0% | 18% |
| PIGF* | 66% | 90% | 90% | 103% |
| PAPP-A* | 59% | 72% | 62% | 81% |
| Mean Doppler PI* | 116% | 109% | 109% | 112% |
| First trim. MAP* | 113% | 118% | 116% | 109% |
Bold, colored numbers indicate statistically significant difference from controls. Asterisks denote percentage of control mean. BMI, body mass index. BW, birthweight; MAP, mean arterial pressure; PE, preeclampsia; PI, pulsatility index; PAPP-A, pregnancy-associated plasma protein A; PlGF, placental growth factor; SGA, small for gestational age.
Figure 3.
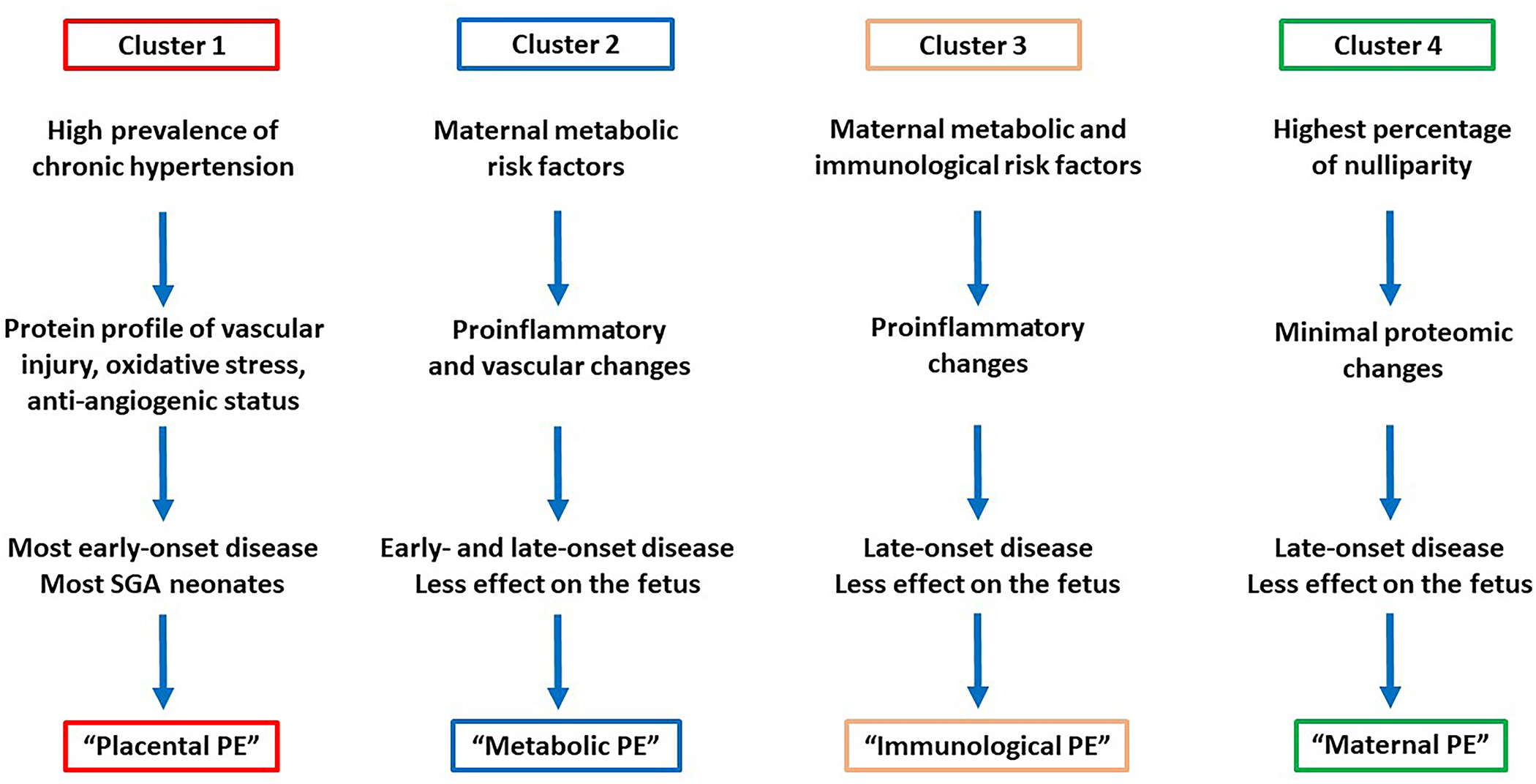
Molecular subclasses of preeclampsia derived from maternal blood proteomics data. Major clinical and placental characteristics are depicted.
Since 1) our study included the largest patient population among preeclampsia proteomics studies, 2) it had more cases than the first preeclampsia clustering study on the placental transcriptome [61], 3) it included an unbiased selection of all cases from a well-characterized cohort, 4) we used a robust bioinformatics pipeline to identify clusters, and 5) subclass-specific traits reflected clinical phenotypes and previously defined patient clusters, we believe that this study was adequate to identify the four subclasses of preeclampsia. Nevertheless, in order to validate these findings in an ethnically separate population, we reanalyzed our proteomics data on 1125 plasma proteins collected longitudinally throughout pregnancy from 199 pregnant women selected from a longitudinal cohort (Detroit, USA) [98,99]. This analysis supported the existence of 4 preeclampsia subclasses throughout pregnancy, with similar molecular profiles and patient phenotypes as discovered by the MRM proteomics study. Moreover, we could refine the molecular subclass profiles and reveal dysregulated molecular pathways by the assessment of longitudinal changes of >1000 proteins.
Summary and conclusions
Preeclampsia is a heterogeneous syndrome with multiple subtypes, and can be investigated with “omics” and bioinformatics approaches. Class discovery placental transcriptomics studies earlier revealed 3 molecular subtypes, so-called, “canonical/placental”, “immunological”, and “maternal” preeclampsia. However, these transcriptomic signatures could also be detected in FGR without preeclampsia, suggesting that placental gene expression patterns are not sufficient to define the clinical phenotype. As such, molecular investigations of maternal blood, which also reflects changes in the maternal compartment, may be much more useful in detecting both placental and maternal molecular factors that determine the development of preeclampsia and its clinical phenotypes.
Our proteomics investigations of maternal blood either in the first trimester or longitudinally throughout gestation in two ethnic populations both revealed 4 distinct patient clusters in preeclampsia, supporting the existence of the “placental”, “immunological” and “maternal” subclasses, and the presence of a novel “metabolic” subclass. It became clear that PlGF, previously used as a gold standard biomarker, is only effective for the prediction of “placental” preeclampsia, the only subclass where the characteristic drop in PlGF levels was observed. In this subgroup preventive aspirin therapy is especially effective [42,101,102]. Another important conclusion is that the molecular subclasses do not determine certain clinical phenotypes, which must be the complex interplay of maternal, placental, fetal, and environmental factors. Eventually, our data support the concept on that the maternal and fetal compartments have a degree of independence, and three different disease origins may exist: 1) the placental compartment, 2) the maternal compartment, and 3) the synergy or poor complementarity of these two compartments. Of importance, placental transcriptomics studies have found 3 preeclampsia subclasses at the end of pregnancy, while our studies showed that 4 distinct subclasses and their distinct disease pathways exist in the first trimester. This may be due to that two originating subclasses like “placental” and “metabolic” reach a similar end-stage and become indistinguishable viewed from the third trimester placenta. These findings are paramount for our improved understanding of the early pathways of preeclampsia, and may promote the development of novel diagnostic tools, enabling the early detection and follow-up of patients as well as their tailored therapies with aspirin or other potential preventive treatments under testing [29,103–106].
Acknowledgments:
We thank Magdalena Bober, Claudia Escher, Oliver Rinner (Biognosys AG) for their excellent contribution to the proteomics study, Sinuhe Hahn (University of Basel), Peter Zavodszky, Akos Szodenyi (Research Centre for Natural Sciences) for helpful discussions and advice. We also thank Maureen McGerty (Wayne State University) for editorial support.
Funding:
Proteomics study was funded by the Hungarian Ministry for National Economy, Grant GINOP-2.1.7-15-2016-00415. Review writing was funded in part by the Hungarian Academy of Sciences, Momentum Grant LP2014-7/2014; the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the FIEK_16-1-2016-0005, K124862, K128262, 2020-1.1.2-PIACI-KFI-2021-00273, funding schemes; the Perinatology Research Branch, Division of Obstetrics and Maternal Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS); and with Federal funds from NICHD/NIH/DHHS under contract No. HHSN275201300006C. ALT was supported by the Wayne State University School of Medicine Perinatal Initiative.
Footnotes
Conflicts of Interest: No potential conflict of interest was reported by the authors except NGT, ALT, ZP and RR, who are inventors of a patent on early biomarkers of preeclampsia. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. RR has contributed to this work as part of his official duties as an employee of the United States Federal Government.
References
- [1].Ness RB, Roberts JM, Heterogeneous causes constituting the single syndrome of preeclampsia: A hypothesis and its implications, Am. J. Obstet. Gynecol. 175 (1996) 1365–1370. 10.1016/S0002-9378(96)70056-X. [DOI] [PubMed] [Google Scholar]
- [2].von Dadelszen P, Magee LA, Roberts JM, Subclassification of Preeclampsia, Hypertens. Pregnancy. 22 (2003) 143–148. 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- [3].Brosens I, Pijnenborg R, Vercruysse L, Romero R, The “Great Obstetrical Syndromes” are associated with disorders of deep placentation, Am. J. Obstet. Gynecol. 204 (2011) 193–201. 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R, Pre-eclampsia part 1: current understanding of its pathophysiology, Nat. Rev. Nephrol. 10 (2014) 466–480. 10.1038/nrneph.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jung E, Romero R, Yeo L, Gomez-Lopez N, Chaemsaithong P, Jaovisidha A, Gotsch F, Erez O, The etiology of preeclampsia, Am. J. Obstet. Gynecol. 226 (2022) S844–S866. 10.1016/j.ajog.2021.11.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Myatt L, Roberts JM, Preeclampsia: Syndrome or Disease?, Curr. Hypertens. Rep. 17 (2015) 83. 10.1007/s11906-015-0595-4. [DOI] [PubMed] [Google Scholar]
- [7].Burton GJ, Woods AW, Jauniaux E, Kingdom JCP, Rheological and Physiological Consequences of Conversion of the Maternal Spiral Arteries for Uteroplacental Blood Flow during Human Pregnancy, Placenta. 30 (2009) 473–482. 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B, The frequency and severity of placental findings in women with preeclampsia are gestational age dependent, Am. J. Obstet. Gynecol. 189 (2003) 1173–1177. 10.1067/S0002-9378(03)00576-3. [DOI] [PubMed] [Google Scholar]
- [9].Ogge G, Chaiworapongsa T, Romero R, Hussein Y, Kusanovic JP, Yeo L, Kim CJ, Hassan SS, Placental lesions associated with maternal underperfusion are more frequent in early-onset than in late-onset preeclampsia, J. Perinat. Med. 39 (2011). 10.1515/jpm.2011.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Matthiesen L, Berg G, Ernerudh J, Ekerfelt C, Jonsson Y, Sharma S, Immunology of Preeclampsia, in: Markert UR (Ed.), Chem. Immunol. Allergy, KARGER, Basel, 2005: pp. 49–61. 10.1159/000087912. [DOI] [PubMed] [Google Scholar]
- [11].Maynard SE, Min J-Y, Merchan J, Lim K-H, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA, Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia, J. Clin. Invest. 111 (2003) 649–658. 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim K-H, Yuan H-T, Libermann TA, Stillman IE, Roberts D, D’Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA, Soluble endoglin contributes to the pathogenesis of preeclampsia, Nat. Med. 12 (2006) 642–649. 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- [13].Cindrova-Davies T, Gabor Than Award Lecture 2008: pre-eclampsia - from placental oxidative stress to maternal endothelial dysfunction, Placenta. 30 Suppl A (2009) S55–65. 10.1016/j.placenta.2008.11.020. [DOI] [PubMed] [Google Scholar]
- [14].Kumar A, Begum N, Prasad S, Agarwal S, Sharma S, IL-10, TNF-α & IFN-γ: Potential early biomarkers for preeclampsia, Cell. Immunol. 283 (2013) 70–74. 10.1016/j.cellimm.2013.06.012. [DOI] [PubMed] [Google Scholar]
- [15].Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK, Preeclampsia: An endothelial cell disorder, Am. J. Obstet. Gynecol. 161 (1989) 1200–1204. 10.1016/0002-9378(89)90665-0. [DOI] [PubMed] [Google Scholar]
- [16].Hahn S, Giaglis S, Hoesli I, Hasler P, Neutrophil NETs in reproduction: from infertility to preeclampsia and the possibility of fetal loss, Front. Immunol. 3 (2012). 10.3389/fimmu.2012.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Umapathy A, Chamley LW, James JL, Reconciling the distinct roles of angiogenic/anti-angiogenic factors in the placenta and maternal circulation of normal and pathological pregnancies, Angiogenesis. 23 (2020) 105–117. 10.1007/s10456-019-09694-w. [DOI] [PubMed] [Google Scholar]
- [18].Staff AC, Fjeldstad HE, Fosheim IK, Moe K, Turowski G, Johnsen GM, Alnaes-Katjavivi P, Sugulle M, Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia, Am. J. Obstet. Gynecol. 226 (2022) S895–S906. 10.1016/j.ajog.2020.09.026. [DOI] [PubMed] [Google Scholar]
- [19].James JL, Saghian R, Perwick R, Clark AR, Trophoblast plugs: impact on utero-placental haemodynamics and spiral artery remodelling, Hum. Reprod. Oxf. Engl. 33 (2018) 1430–1441. 10.1093/humrep/dey225. [DOI] [PubMed] [Google Scholar]
- [20].Blois SM, Dechend R, Barrientos G, Staff AC, A potential pathophysiological role for galectins and the renin-angiotensin system in preeclampsia, Cell. Mol. Life Sci. CMLS. 72 (2015) 39–50. 10.1007/s00018-014-1713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Verlohren S, Melchiorre K, Khalil A, Thilaganathan B, Uterine artery Doppler, birth weight and timing of onset of pre-eclampsia: providing insights into the dual etiology of late-onset pre-eclampsia: UtA Doppler, birth weight and pre-eclampsia, Ultrasound Obstet. Gynecol. 44 (2014) 293–298. 10.1002/uog.13310. [DOI] [PubMed] [Google Scholar]
- [22].Soto E, Romero R, Kusanovic JP, Ogge G, Hussein Y, Yeo L, Hassan SS, Kim CJ, Chaiworapongsa T, Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion, J. Matern. Fetal Neonatal Med. 25 (2012) 498–507. 10.3109/14767058.2011.591461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vatten LJ, Eskild A, Nilsen TIL, Jeansson S, Jenum PA, Staff AC, Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia, Am. J. Obstet. Gynecol. 196 (2007) 239.e1–239.e6. 10.1016/j.ajog.2006.10.909. [DOI] [PubMed] [Google Scholar]
- [24].Crispi F, Llurba E, Domínguez C, Martín-Gallán P, Cabero L, Gratacós E, Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction, Ultrasound Obstet. Gynecol. 31 (2008) 303–309. 10.1002/uog.5184. [DOI] [PubMed] [Google Scholar]
- [25].Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA, A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate, J. Matern. Fetal Neonatal Med. 21 (2008) 9–23. 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Redman CW, Sargent IL, Staff AC, IFPA Senior Award Lecture: Making sense of pre-eclampsia – Two placental causes of preeclampsia?, Placenta. 35 (2014) S20–S25. 10.1016/j.placenta.2013.12.008. [DOI] [PubMed] [Google Scholar]
- [27].Hahn S, Lapaire O, Than NG, Biomarker development for presymptomatic molecular diagnosis of preeclampsia: feasible, useful or even unnecessary?, Expert Rev. Mol. Diagn 15 (2015) 617–629. 10.1586/14737159.2015.1025757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Robillard P-Y, Dekker G, Scioscia M, Saito S, Progress in the understanding of the pathophysiology of immunologic maladaptation related to early-onset preeclampsia and metabolic syndrome related to late-onset preeclampsia, Am. J. Obstet. Gynecol. 226 (2022) S867–S875. 10.1016/j.ajog.2021.11.019. [DOI] [PubMed] [Google Scholar]
- [29].Hürter H, Vontelin van Breda S, Vokalova L, Brandl M, Baumann M, Hösli I, Huhn EA, De Geyter C, Rossi SW, Lapaire O, Prevention of pre-eclampsia after infertility treatment: Preconceptional minimalisation of risk factors, Best Pract. Res. Clin. Endocrinol. Metab. 33 (2019) 127–132. 10.1016/j.beem.2019.05.001. [DOI] [PubMed] [Google Scholar]
- [30].Tamás P, Early and late preeclampsia are characterized by high cardiac output, but in the presence of fetal growth restriction, cardiac output is low: insights from a prospective study, Am. J. Obstet. Gynecol. 219 (2018) 627. 10.1016/j.ajog.2018.07.029. [DOI] [PubMed] [Google Scholar]
- [31].Scioscia M, Karumanchi SA, Goldman-Wohl D, Robillard P-Y, Endothelial dysfunction and metabolic syndrome in preeclampsia: an alternative viewpoint, J. Reprod. Immunol. 108 (2015) 42–47. 10.1016/j.jri.2015.01.009. [DOI] [PubMed] [Google Scholar]
- [32].Stark MJ, Dierkx L, Clifton VL, Wright IMR, Alterations in the maternal peripheral microvascular response in pregnancies complicated by preeclampsia and the impact of fetal sex, J. Soc. Gynecol. Investig. 13 (2006) 573–578. 10.1016/j.jsgi.2006.06.006. [DOI] [PubMed] [Google Scholar]
- [33].van Dijk M, Oudejans C, (Epi)genetics of pregnancy-associated diseases, Front. Genet. 4 (2013). 10.3389/fgene.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Johnson MP, Brennecke SP, East CE, Dyer TD, Roten LT, Proffitt JM, Melton PE, Fenstad MH, Aalto-Viljakainen T, Makikallio K, Heinonen S, Kajantie E, Kere J, Laivuori H, for the FINNPEC Study Group, Austgulen R, Blangero J, Moses EK, Pouta A, Kivinen K, Ekholm E, Hietala R, Sainio S, Saisto T, Uotila J, Klemetti M, Inkeri Lokki A, Georgiadis L, Huovari E, Kortelainen E, Leminen S, Lahdesmaki A, Mehtala S, Salmen C, Genetic dissection of the pre-eclampsia susceptibility locus on chromosome 2q22 reveals shared novel risk factors for cardiovascular disease, Mol. Hum. Reprod. 19 (2013) 423–437. 10.1093/molehr/gat011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lau SY, Guild S-J, Barrett CJ, Chen Q, McCowan L, Jordan V, Chamley LW, Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis, Am. J. Reprod. Immunol. N. Y. N 1989. 70 (2013) 412–427. 10.1111/aji.12138. [DOI] [PubMed] [Google Scholar]
- [36].Dunk CE, van Dijk M, Choudhury R, Wright TJ, Cox B, Leavey K, Harris LK, Jones RL, Lye SJ, Functional Evaluation of STOX1 (STORKHEAD-BOX PROTEIN 1) in Placentation, Preeclampsia, and Preterm Birth, Hypertens. Dallas Tex 1979. 77 (2021) 475–490. 10.1161/HYPERTENSIONAHA.120.15619. [DOI] [PubMed] [Google Scholar]
- [37].Miralles F, Collinot H, Boumerdassi Y, Ducat A, Duché A, Renault G, Marchiol C, Lagoutte I, Bertholle C, Andrieu M, Jacques S, Méhats C, Vaiman D, Long-term cardiovascular disorders in the STOX1 mouse model of preeclampsia, Sci. Rep. 9 (2019) 11918. 10.1038/s41598-019-48427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yong HEJ, Murthi P, Brennecke SP, Moses EK, Genetic Approaches in Preeclampsia, Methods Mol. Biol. Clifton NJ. 1710 (2018) 53–72. 10.1007/978-1-4939-7498-6_5. [DOI] [PubMed] [Google Scholar]
- [39].Burton GJ, Redman CW, Roberts JM, Moffett A, Pre-eclampsia: pathophysiology and clinical implications, BMJ. 366 (2019) l2381. 10.1136/bmj.l2381. [DOI] [PubMed] [Google Scholar]
- [40].Hahn S, Hasler P, Vokalova L, van Breda SV, Than NG, Hoesli IM, Lapaire O, Rossi SW, Feto-Maternal Microchimerism: The Pre-eclampsia Conundrum, Front. Immunol. 10 (2019) 659. 10.3389/fimmu.2019.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jiménez KM, Morel A, Parada-Niño L, Alejandra González-Rodriguez M, Flórez S, Bolívar-Salazar D, Becerra-Bayona S, Aguirre-García A, Gómez-Murcia T, Fernanda Castillo L, Carlosama C, Ardila J, Vaiman D, Serrano N, Laissue P, Identifying new potential genetic biomarkers for HELLP syndrome using massive parallel sequencing, Pregnancy Hypertens. 22 (2020) 181–190. 10.1016/j.preghy.2020.09.003. [DOI] [PubMed] [Google Scholar]
- [42].Poon LC, Magee LA, Verlohren S, Shennan A, von Dadelszen P, Sheiner E, Hadar E, Visser G, Da Silva Costa F, Kapur A, McAuliffe F, Nazareth A, Tahlak M, Kihara AB, Divakar H, McIntyre HD, Berghella V, Yang H, Romero R, Nicolaides KH, Melamed N, Hod M, A literature review and best practice advice for second and third trimester risk stratification, monitoring, and management of pre-eclampsia: Compiled by the Pregnancy and Non-Communicable Diseases Committee of FIGO (the International Federation of Gynecology and Obstetrics), Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 154 Suppl 1 (2021) 3–31. 10.1002/ijgo.13763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Loscalzo J, Barabasi A-L, Systems biology and the future of medicine: Systems biology and the future of medicine, Wiley Interdiscip. Rev. Syst. Biol. Med. 3 (2011) 619–627. 10.1002/wsbm.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Robinson SW, Fernandes M, Husi H, Current advances in systems and integrative biology, Comput. Struct. Biotechnol. J. 11 (2014) 35–46. 10.1016/j.csbj.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tarca AL, Romero R, Draghici S, Analysis of microarray experiments of gene expression profiling, Am. J. Obstet. Gynecol. 195 (2006) 373–388. 10.1016/j.ajog.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yong HEJ, Chan S-Y, Current approaches and developments in transcript profiling of the human placenta, Hum. Reprod. Update. 26 (2020) 799–840. 10.1093/humupd/dmaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Palmer KR, Tong S, Kaitu’u-Lino TJ, Placental-specific sFLT-1: role in pre-eclamptic pathophysiology and its translational possibilities for clinical prediction and diagnosis, Mol. Hum. Reprod. 23 (2017) 69–78. 10.1093/molehr/gaw077. [DOI] [PubMed] [Google Scholar]
- [48].Founds SA, Conley YP, Lyons-Weiler JF, Jeyabalan A, Hogge WA, Conrad KP, Altered global gene expression in first trimester placentas of women destined to develop preeclampsia, Placenta. 30 (2009) 15–24. 10.1016/j.placenta.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li H, Huang Q, Liu Y, Garmire LX, Single cell transcriptome research in human placenta, Reprod. Camb. Engl. 160 (2020) R155–R167. 10.1530/REP-20-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pavličev M, Wagner GP, Chavan AR, Owens K, Maziarz J, Dunn-Fletcher C, Kallapur SG, Muglia L, Jones H, Single-cell transcriptomics of the human placenta: inferring the cell communication network of the maternal-fetal interface, Genome Res. 27 (2017) 349–361. 10.1101/gr.207597.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Várkonyi T, Nagy B, Füle T, Tarca AL, Karászi K, Schönléber J, Hupuczi P, Mihalik N, Kovalszky I, Rigó J, Meiri H, Papp Z, Romero R, Than NG, Microarray profiling reveals that placental transcriptomes of early-onset HELLP syndrome and preeclampsia are similar, Placenta. 32 Suppl (2011) S21–29. 10.1016/j.placenta.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nishizawa H, Pryor-Koishi K, Kato T, Kowa H, Kurahashi H, Udagawa Y, Microarray analysis of differentially expressed fetal genes in placental tissue derived from early and late onset severe pre-eclampsia, Placenta. 28 (2007) 487–497. 10.1016/j.placenta.2006.05.010. [DOI] [PubMed] [Google Scholar]
- [53].Enquobahrie DA, Meller M, Rice K, Psaty BM, Siscovick DS, Williams MA, Differential placental gene expression in preeclampsia, Am. J. Obstet. Gynecol. 199 (2008) 566.e1–11. 10.1016/j.ajog.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sitras V, Paulssen RH, Grønaas H, Leirvik J, Hanssen TA, Vårtun A, Acharya G, Differential placental gene expression in severe preeclampsia, Placenta. 30 (2009) 424–433. 10.1016/j.placenta.2009.01.012. [DOI] [PubMed] [Google Scholar]
- [55].Winn VD, Gormley M, Paquet AC, Kjaer-Sorensen K, Kramer A, Rumer KK, Haimov-Kochman R, Yeh R-F, Overgaard MT, Varki A, Oxvig C, Fisher SJ, Severe preeclampsia-related changes in gene expression at the maternal-fetal interface include sialic acid-binding immunoglobulin-like lectin-6 and pappalysin-2, Endocrinology. 150 (2009) 452–462. 10.1210/en.2008-0990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Than NG, Romero R, Tarca AL, Kekesi KA, Xu Y, Xu Z, Juhasz K, Bhatti G, Leavitt RJ, Gelencser Z, Palhalmi J, Chung TH, Gyorffy BA, Orosz L, Demeter A, Szecsi A, Hunyadi-Gulyas E, Darula Z, Simor A, Eder K, Szabo S, Topping V, El-Azzamy H, LaJeunesse C, Balogh A, Szalai G, Land S, Torok O, Dong Z, Kovalszky I, Falus A, Meiri H, Draghici S, Hassan SS, Chaiworapongsa T, Krispin M, Knöfler M, Erez O, Burton GJ, Kim CJ, Juhasz G, Papp Z, Integrated Systems Biology Approach Identifies Novel Maternal and Placental Pathways of Preeclampsia, Front. Immunol. 9 (2018) 1661. 10.3389/fimmu.2018.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Knöfler M, Pollheimer J, Human placental trophoblast invasion and differentiation: a particular focus on Wnt signaling, Front. Genet. 4 (2013) 190. 10.3389/fgene.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].E Davies J, Pollheimer J, Yong HEJ, Kokkinos MI, Kalionis B, Knöfler M, Murthi P, Epithelial-mesenchymal transition during extravillous trophoblast differentiation, Cell Adhes. Migr. 10 (2016) 310–321. 10.1080/19336918.2016.1170258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Hansson SR, Nääv Å, Erlandsson L, Oxidative stress in preeclampsia and the role of free fetal hemoglobin, Front. Physiol. 5 (2015). 10.3389/fphys.2014.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Szilagyi A, Gelencser Z, Romero R, Xu Y, Kiraly P, Demeter A, Palhalmi J, Gyorffy BA, Juhasz K, Hupuczi P, Kekesi KA, Meinhardt G, Papp Z, Draghici S, Erez O, Tarca AL, Knöfler M, Than NG, Placenta-Specific Genes, Their Regulation During Villous Trophoblast Differentiation and Dysregulation in Preterm Preeclampsia, Int. J. Mol. Sci. 21 (2020) E628. 10.3390/ijms21020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Leavey K, Bainbridge SA, Cox BJ, Large scale aggregate microarray analysis reveals three distinct molecular subclasses of human preeclampsia, PloS One. 10 (2015) e0116508. 10.1371/journal.pone.0116508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Leavey K, Benton SJ, Grynspan D, Kingdom JC, Bainbridge SA, Cox BJ, Unsupervised Placental Gene Expression Profiling Identifies Clinically Relevant Subclasses of Human Preeclampsia, Hypertens. Dallas Tex 1979. 68 (2016) 137–147. 10.1161/HYPERTENSIONAHA.116.07293. [DOI] [PubMed] [Google Scholar]
- [63].Yuen RKC, Robinson WP, Review: A high capacity of the human placenta for genetic and epigenetic variation: implications for assessing pregnancy outcome, Placenta. 32 Suppl 2 (2011) S136–141. 10.1016/j.placenta.2011.01.003. [DOI] [PubMed] [Google Scholar]
- [64].Coorens THH, Oliver TRW, Sanghvi R, Sovio U, Cook E, Vento-Tormo R, Haniffa M, Young MD, Rahbari R, Sebire N, Campbell PJ, Charnock-Jones DS, Smith GCS, Behjati S, Inherent mosaicism and extensive mutation of human placentas, Nature. 592 (2021) 80–85. 10.1038/s41586-021-03345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Benton SJ, Leavey K, Grynspan D, Cox BJ, Bainbridge SA, The clinical heterogeneity of preeclampsia is related to both placental gene expression and placental histopathology, Am. J. Obstet. Gynecol. 219 (2018) 604.e1–604.e25. 10.1016/j.ajog.2018.09.036. [DOI] [PubMed] [Google Scholar]
- [66].Leavey K, Grynspan D, Cox BJ, Both “canonical” and “immunological” preeclampsia subtypes demonstrate changes in placental immune cell composition, Placenta. 83 (2019) 53–56. 10.1016/j.placenta.2019.06.384. [DOI] [PubMed] [Google Scholar]
- [67].Gibbs I, Leavey K, Benton SJ, Grynspan D, Bainbridge SA, Cox BJ, Placental transcriptional and histologic subtypes of normotensive fetal growth restriction are comparable to preeclampsia, Am. J. Obstet. Gynecol. 220 (2019) 110.e1–110.e21. 10.1016/j.ajog.2018.10.003. [DOI] [PubMed] [Google Scholar]
- [68].Michela B, Liquid Biopsy: A Family of Possible Diagnostic Tools, Diagn. Basel Switz. 11 (2021) 1391. 10.3390/diagnostics11081391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A, Liquid biopsy: monitoring cancer-genetics in the blood, Nat. Rev. Clin. Oncol. 10 (2013) 472–484. 10.1038/nrclinonc.2013.110. [DOI] [PubMed] [Google Scholar]
- [70].Than GN, Bohn H, Szabó DG, Advances in pregnancy-related protein research: functional and clinical applications, CRC Press Inc., 1993. [Google Scholar]
- [71].Cole LA, hCG, the wonder of today’s science, Reprod. Biol. Endocrinol. RBE. 10 (2012) 24. 10.1186/1477-7827-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Than NG, Balogh A, Romero R, Kárpáti E, Erez O, Szilágyi A, Kovalszky I, Sammar M, Gizurarson S, Matkó J, Závodszky P, Papp Z, Meiri H, Placental Protein 13 (PP13) - A Placental Immunoregulatory Galectin Protecting Pregnancy, Front. Immunol. 5 (2014) 348. 10.3389/fimmu.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, Wainscoat JS, Presence of fetal DNA in maternal plasma and serum, Lancet Lond. Engl. 350 (1997) 485–487. 10.1016/S0140-6736(97)02174-0. [DOI] [PubMed] [Google Scholar]
- [74].Lo YMD, Noninvasive prenatal testing: Advancing through a virtuous circle of science, technology and clinical applications, Prenat. Diagn. 41 (2021) 1190–1192. 10.1002/pd.5978. [DOI] [PubMed] [Google Scholar]
- [75].Poon LL, Leung TN, Lau TK, Lo YM, Presence of fetal RNA in maternal plasma, Clin. Chem. 46 (2000) 1832–1834. [PubMed] [Google Scholar]
- [76].Chiu RWK, Lui W, Cheung M, Kumta N, Farina A, Banzola I, Grotti S, Rizzo N, Haines CJ, Lo YMD, Time profile of appearance and disappearance of circulating placenta-derived mRNA in maternal plasma, Clin. Chem. 52 (2006) 313–316. 10.1373/clinchem.2005.059691. [DOI] [PubMed] [Google Scholar]
- [77].Whitehead CL, Walker SP, Tong S, Measuring circulating placental RNAs to non-invasively assess the placental transcriptome and to predict pregnancy complications, Prenat. Diagn. 36 (2016) 997–1008. 10.1002/pd.4934. [DOI] [PubMed] [Google Scholar]
- [78].Nagy B, Csanádi Z, Póka R, A „szabad” nukleinsavak jelentősége a noninvazív diagnosztikában, Orv. Hetil. 157 (2016) 1900–1909. 10.1556/650.2016.30621. [DOI] [PubMed] [Google Scholar]
- [79].Nair S, Salomon C, Extracellular vesicles as critical mediators of maternal-fetal communication during pregnancy and their potential role in maternal metabolism, Placenta. 98 (2020) 60–68. 10.1016/j.placenta.2020.06.011. [DOI] [PubMed] [Google Scholar]
- [80].Salomon C, Guanzon D, Scholz-Romero K, Longo S, Correa P, Illanes SE, Rice GE, Placental Exosomes as Early Biomarker of Preeclampsia: Potential Role of Exosomal MicroRNAs Across Gestation, J. Clin. Endocrinol. Metab. 102 (2017) 3182–3194. 10.1210/jc.2017-00672. [DOI] [PubMed] [Google Scholar]
- [81].Tannetta D, Collett G, Vatish M, Redman C, Sargent I, Syncytiotrophoblast extracellular vesicles – Circulating biopsies reflecting placental health, Placenta. 52 (2017) 134–138. 10.1016/j.placenta.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].McElrath TF, Cantonwine DE, Gray KJ, Mirzakhani H, Doss RC, Khaja N, Khalid M, Page G, Brohman B, Zhang Z, Sarracino D, Rosenblatt KP, Late first trimester circulating microparticle proteins predict the risk of preeclampsia < 35 weeks and suggest phenotypic differences among affected cases, Sci. Rep. 10 (2020) 17353. 10.1038/s41598-020-74078-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Rasmussen M, Reddy M, Nolan R, Camunas-Soler J, Khodursky A, Scheller NM, Cantonwine DE, Engelbrechtsen L, Mi JD, Dutta A, Brundage T, Siddiqui F, Thao M, Gee EPS, La J, Baruch-Gravett C, Santillan MK, Deb S, Ame SM, Ali SM, Adkins M, DePristo MA, Lee M, Namsaraev E, Gybel-Brask DJ, Skibsted L, Litch JA, Santillan DA, Sazawal S, Tribe RM, Roberts JM, Jain M, Høgdall E, Holzman C, Quake SR, Elovitz MA, McElrath TF, RNA profiles reveal signatures of future health and disease in pregnancy, Nature. 601 (2022) 422–427. 10.1038/s41586-021-04249-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Farina A, Zucchini C, Sekizawa A, Purwosunu Y, de Sanctis P, Santarsiero G, Rizzo N, Morano D, Okai T, Performance of messenger RNAs circulating in maternal blood in the prediction of preeclampsia at 10–14 weeks, Am. J. Obstet. Gynecol. 203 (2010) 575.e1–7. 10.1016/j.ajog.2010.07.043. [DOI] [PubMed] [Google Scholar]
- [85].Purwosunu Y, Sekizawa A, Okazaki S, Farina A, Wibowo N, Nakamura M, Rizzo N, Saito H, Okai T, Prediction of preeclampsia by analysis of cell-free messenger RNA in maternal plasma, Am. J. Obstet. Gynecol. 200 (2009) 386.e1–7. 10.1016/j.ajog.2008.11.035. [DOI] [PubMed] [Google Scholar]
- [86].Lv Y, Lu C, Ji X, Miao Z, Long W, Ding H, Lv M, Roles of microRNAs in preeclampsia, J. Cell. Physiol. 234 (2019) 1052–1061. 10.1002/jcp.27291. [DOI] [PubMed] [Google Scholar]
- [87].Srinivasan S, Treacy R, Herrero T, Olsen R, Leonardo TR, Zhang X, DeHoff P, To C, Poling LG, Fernando A, Leon-Garcia S, Knepper K, Tran V, Meads M, Tasarz J, Vuppala A, Park S, Laurent CD, Bui T, Cheah PS, Overcash RT, Ramos GA, Roeder H, Ghiran I, Parast M, PAPR Study Consortium, Breakefield XO, Lueth AJ, Rust SR, Dufford MT, Fox AC, Hickok DE, Burchard J, Boniface JJ, Laurent LC, Discovery and Verification of Extracellular miRNA Biomarkers for Non-invasive Prediction of Pre-eclampsia in Asymptomatic Women, Cell Rep. Med. 1 (2020) 100013. 10.1016/j.xcrm.2020.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chaiworapongsa T, Romero R, Whitten A, Tarca AL, Bhatti G, Draghici S, Chaemsaithong P, Miranda J, Hassan SS, Differences and similarities in the transcriptional profile of peripheral whole blood in early and late-onset preeclampsia: insights into the molecular basis of the phenotype of preeclampsiaa, J. Perinat. Med. 41 (2013) 485–504. 10.1515/jpm-2013-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tarca AL, Romero R, Erez O, Gudicha DW, Than NG, Benshalom-Tirosh N, Pacora P, Hsu C-D, Chaiworapongsa T, Hassan SS, Gomez-Lopez N, Maternal whole blood mRNA signatures identify women at risk of early preeclampsia: a longitudinal study, J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 34 (2021) 3463–3474. 10.1080/14767058.2019.1685964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Tsang JCH, Vong JSL, Ji L, Poon LCY, Jiang P, Lui KO, Ni Y-B, To KF, Cheng YKY, Chiu RWK, Lo YMD, Integrative single-cell and cell-free plasma RNA transcriptomics elucidates placental cellular dynamics, Proc. Natl. Acad. Sci. 114 (2017) E7786–E7795. 10.1073/pnas.1710470114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Gomez-Lopez N, Romero R, Hassan SS, Bhatti G, Berry SM, Kusanovic JP, Pacora P, Tarca AL, The Cellular Transcriptome in the Maternal Circulation During Normal Pregnancy: A Longitudinal Study, Front. Immunol. 10 (2019) 2863. 10.3389/fimmu.2019.02863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ngo TTM, Moufarrej MN, Rasmussen M-LH, Camunas-Soler J, Pan W, Okamoto J, Neff NF, Liu K, Wong RJ, Downes K, Tibshirani R, Shaw GM, Skotte L, Stevenson DK, Biggio JR, Elovitz MA, Melbye M, Quake SR, Noninvasive blood tests for fetal development predict gestational age and preterm delivery, Science. 360 (2018) 1133–1136. 10.1126/science.aar3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Navajas R, Corrales F, Paradela A, Quantitative proteomics-based analyses performed on pre-eclampsia samples in the 2004–2020 period: a systematic review, Clin. Proteomics. 18 (2021) 6. 10.1186/s12014-021-09313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Jauniaux E, Watson AL, Hempstock J, Bao Y-P, Skepper JN, Burton GJ, Onset of Maternal Arterial Blood Flow and Placental Oxidative Stress, Am. J. Pathol. 157 (2000) 2111–2122. 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Moffett A, Loke C, Immunology of placentation in eutherian mammals, Nat. Rev. Immunol. 6 (2006) 584–594. 10.1038/nri1897. [DOI] [PubMed] [Google Scholar]
- [96].Saito S, Th17 cells and regulatory T cells: new light on pathophysiology of preeclampsia, Immunol. Cell Biol. 88 (2010) 615–617. 10.1038/icb.2010.68. [DOI] [PubMed] [Google Scholar]
- [97].Saito S, Sakai M, Th1/Th2 balance in preeclampsia, J. Reprod. Immunol. 59 (2003) 161–173. 10.1016/S0165-0378(03)00045-7. [DOI] [PubMed] [Google Scholar]
- [98].Tarca AL, Romero R, Benshalom-Tirosh N, Than NG, Gudicha DW, Done B, Pacora P, Chaiworapongsa T, Panaitescu B, Tirosh D, Gomez-Lopez N, Draghici S, Hassan SS, Erez O, The prediction of early preeclampsia: Results from a longitudinal proteomics study, PloS One. 14 (2019) e0217273. 10.1371/journal.pone.0217273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Erez O, Romero R, Maymon E, Chaemsaithong P, Done B, Pacora P, Panaitescu B, Chaiworapongsa T, Hassan SS, Tarca AL, The prediction of late-onset preeclampsia: Results from a longitudinal proteomics study, PloS One. 12 (2017) e0181468. 10.1371/journal.pone.0181468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Monti S, Consensus Clustering: A Resampling-Based Method for Class Discovery and Visualization of Gene Expression Microarray Data, Mach. Learn. 52 (2003) 91–118. 10.1023/A:1023949509487. [DOI] [Google Scholar]
- [101].Roberge S, Villa P, Nicolaides K, Giguère Y, Vainio M, Bakthi A, Ebrashy A, Bujold E, Early Administration of Low-Dose Aspirin for the Prevention of Preterm and Term Preeclampsia: A Systematic Review and Meta-Analysis, Fetal Diagn. Ther. 31 (2012) 141–146. 10.1159/000336662. [DOI] [PubMed] [Google Scholar]
- [102].Rolnik DL, Nicolaides KH, Poon LC, Prevention of preeclampsia with aspirin, Am. J. Obstet. Gynecol. 226 (2022) S1108–S1119. 10.1016/j.ajog.2020.08.045. [DOI] [PubMed] [Google Scholar]
- [103].Romero R, Erez O, Hüttemann M, Maymon E, Panaitescu B, Conde-Agudelo A, Pacora P, Yoon BH, Grossman LI, Metformin, the aspirin of the 21st century: its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity, Am. J. Obstet. Gynecol. 217 (2017) 282–302. 10.1016/j.ajog.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].de Alwis N, Binder NK, Beard S, Kaitu’u-Lino TJ, Tong S, Brownfoot F, Hannan NJ, Novel approaches to combat preeclampsia: from new drugs to innovative delivery, Placenta. 102 (2020) 10–16. 10.1016/j.placenta.2020.08.022. [DOI] [PubMed] [Google Scholar]
- [105].Morales-Prieto DM, Favaro RR, Markert UR, Placental miRNAs in feto-maternal communication mediated by extracellular vesicles, Placenta. 102 (2020) 27–33. 10.1016/j.placenta.2020.07.001. [DOI] [PubMed] [Google Scholar]
- [106].McLaughlin K, Hobson SR, Chandran AR, Agrawal S, Windrim RC, Parks WT, Bowman AW, Sovio U, Smith GC, Kingdom JC, Circulating maternal placental growth factor responses to low-molecular-weight heparin in pregnant patients at risk of placental dysfunction, Am. J. Obstet. Gynecol. 226 (2022) S1145–S1156.e1. 10.1016/j.ajog.2021.08.027. [DOI] [PubMed] [Google Scholar]


