Abstract
There is strong evidence from humans and animal models showing that abnormal functioning of the hypothalamic pituitary adrenal (HPA) axis and/or the inflammatory response system disrupts feedback regulation of both neuroendocrine and immune systems, contributing to disease. Stress is known to affect the physiology of pelvic organs and to disturb the HPA axis leading to chronic, painful, inflammatory disorders. A link between stress and disease has already been documented for many chronic conditions. Endometriosis is a complex chronic gynecological disease associated with severe pelvic pain and infertility that affects 10% of reproductive aged women. Patients report the negative impact of endometriosis symptoms on quality of life, work/study productivity, and personal relationships, which in turn cause high levels of psychological and emotional distress. The relationship between stress and endometriosis is not clear. Still, we have recently demonstrated that stress increases the size and severity of the lesions as well as inflammatory parameters in an animal model. Furthermore, the ‘controllability’ of stress influences the pathophysiology in this model, offering the possibility of using stress management techniques in patients. The crosstalk between stress-inflammation-pain through HPA axis activity indicates that stress relief should alleviate inflammation, and in turn, decrease painful responses. This opens up the opportunity of altering brain-body-brain pathways as potential new therapeutic option for endometriosis. The goal of this review is to gather the research evidence regarding the interaction between stress (psychological and physiological) and the development and progression of endometriosis on the exacerbation of its symptoms with the purpose of proposing new lines of emerging research and possible treatment modalities for this still incurable disease.
Keywords: endometriosis, stress, HPA, environment
Introduction
Endometriosis is a complex, chronic disease defined as the presence of lesions or cysts composed of endometrial-like glands and stroma commonly found in the peritoneal area, causing inflammation and the characteristic symptoms of endometriosis: pain (dysmenorrhea, dyspareunia, dyschezia) and infertility [1–5]. Endometriosis is a widespread gynecologic diagnosis, with an estimated prevalence of up to 10% among all pre-menopausal women [1]. It has been estimated that 176 million women are affected by endometriosis around the world, with an impact on health care that reaches 22 billion in the United States alone [2–4]. Many qualitative and quantitative studies have documented its negative impact on the patient’s quality of life in aspects related to physical health, mental health, relationships, reproduction, and work/study [5–8]. However, despite decades of research efforts, the etiology of endometriosis has not been fully elucidated as recently reviewed [9, 10]. Inadequate understanding of its pathogenesis results in a lack of non-invasive diagnostic markers and limited therapeutic options, none of which is curative.
Our own studies, and others, document that endometriosis is universally a debilitating disease with substantial detriment to quality of life, physical functioning and emotional wellbeing [6, 7, 11–13]. Patients, often teens and young adults, characterize endometriosis as the “disease of multiple losses” and the direct cause of high levels of psychological and emotional distress [6, 7, 14]. They report struggling for years without knowing the cause of their painful symptoms (diagnosis is delayed ~7 years in average) and are often frustrated by the chronicity, unpredictability, unresponsiveness to pharmacological treatment, and high recurrence rates following surgical treatment [15, 16]. Further, studies have demonstrated that endometriosis is such a disabling condition that it significantly compromises social relationships, sexuality, and mental health [17, 18]. All these elements factor in to cause a generalized feeling of having little or no control over their health and life in general, causing depression and anxiety [19–21]. In fact, the impact is thought to be so significant that it could be argued that not only should psychological support be available for endometriosis patients, but that psychological assessment should also become an integral part of their care [18].
A relationship between stress and disease has been documented for other chronic conditions such as cancer, inflammatory bowel disease, and multiple sclerosis, and also for chronic pelvic pain and mood disorders (anxiety, depression, post-traumatic stress syndrome). Normally, the brain responds to stressors by activation of the HPA axis, leading to eustress (positive stress). Chronic activation of the HPA axis by stressors, however, results in distress (negative stress) compromising the immune system, which in turn causes high levels of psychological and emotional distress. The relationship between stress and endometriosis is not clear, but we have demonstrated that stress increases the size and severity of the lesions as well as inflammatory parameters (e.g., colonic inflammation, infiltrating mast cells, inflammatory cells in the peritoneal fluid) in an animal model of endometriosis [22]. Furthermore, we also showed that the ‘controllability’ of stress influences the pathophysiology of endometriosis in the rat model [23], specifically causing a ~50% decrease in lesion size. Taken together, these data support the possibility of using stress management techniques in women with endometriosis as a treatment modality to help alleviate painful symptoms. The proposed crosstalk between stress-inflammation-pain through the HPA axis activity indicates that stress relief should reduce inflammation, which in turn should lead to decreased painful responses, and opens up the opportunity to alter brain-body-brain pathways as potential new therapeutic options for endometriosis.
Stress response mechanisms
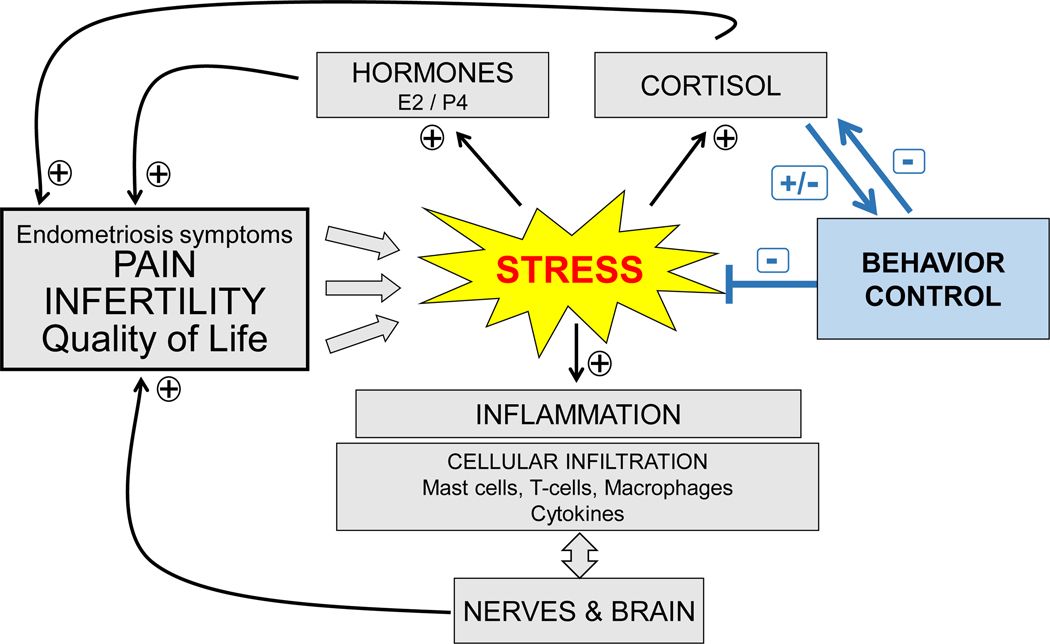
Stress, regardless of type (e.g., psychological, emotional, cognitive, physical), directly impacts pain processes by chronic activation of the HPA axis. The stress response is complex but has many interconnected mechanisms, including neuroendocrine, inflammatory, and nociception phenomena [24]. At the level of the hypothalamus, the stress signal activates corticotropin releasing hormone (CRH) production leading to the secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary gland [25, 26]. ACTH stimulates the production of glucocorticoids (cortisol) in the adrenal cortex. Cortisol has been shown to influence the activity of the immune system by suppressing cellular differentiation and proliferation, down-regulating gene transcription, and reducing cell adhesion [26, 27]. Different types of stressors—physical, emotional, psychological, social- can activate this axis via different mechanisms, resulting in different kinds of responses (eustress or distress) [28]. Chronic stress is thought to be associated with activation of cellular immunity, increased pro-inflammatory cytokine production, mast cell release of CRH and substance P, and hyperactivity of HPA (Figure 1).
Figure 1.
The proposed role of stress exacerbation on endometriosis pathology via activation of the hypothalamic-pituitary axis and potential impact of stress management via behavioral control.
Animal models of stress
Animal models are of great value to study the fundamental etiological mechanisms of complex diseases that are challenging to study in humans. Experiments with humans are problematic to conduct due to ethical and practical considerations [29]. For endometriosis, in particular, evaluation and monitoring of disease presentation, progression, and response to therapies would require repeated surgeries since there are no specific and sensitive blood tests, and imaging diagnostic approaches are still under investigation. Also, person to person variability in genetic, environmental, dietary, and behavioral factors may confound results. Some of the advantages of using animal models are that studies can be conducted in a controlled manner, free from such confounding influences. Various experimental models of endometriosis have been developed, including those in monkeys, rabbits, mice, and rats [9, 30]. Non-human primates are considered the best model to study endometriosis: macaques and baboons are physiologically and anatomically similar to humans, they menstruate, and have been shown to have spontaneous endometriosis. Baboons, for instance, have been extensively used as a model to understand the early and progressive stages of endometriosis [31, 32]; however, their use in research is hampered by costs and limited access. Rodents are one of the most frequently used models for endometriosis research. A PubMed search using the keywords ‘endometriosis’ ‘animal model’ and ‘rat’ or ‘mice’ produced 633 hits, compared to 64 for ‘baboons.’ Rats are an appropriate choice to model endometriosis since they have similar reproductive physiology, endocrinology, and inflammatory systems to humans; further, the biological parameters activated during stress and healing are similar to those observed in humans. There are also many similarities in stress and endocrine responses, the HPA axis, and central pain pathways between rats and humans [33–35]. The most common rat model of endometriosis consists of autologous transplantation of uterine tissue either into the peritoneum or the omentum, which leads to the ectopic growth of vesicles or cysts with physiological and functional characteristics similar to those observed in women with endometriosis. The rat auto-transplant model has been extensively validated and shown to exhibit similar signs and symptoms to the human disease, including subfertility, hyperalgesia, innervation, and inflammation [36–39]. Endometriotic vesicles in rats are histologically similar, respond similarly to steroids, and activate similar inflammatory mechanisms to those that characterize the human disease [37, 38, 40–42]. We and others have reported that endometriotic lesions in this rat model have a global gene expression profile similar to the human disease, including inflammatory cytokines/receptors, tumor invasion/metastasis factors, angiogenic factors, and adhesion molecules [43, 44]. In addition, the rat model exhibits reduced fertility, impaired NK cell activity, and increased vaginal hyperalgesia [36, 39, 45, 46]. This model thus shares many of the end-points seen in patients (i.e., subfertility, hyperalgesia, innervation, inflammation), further validating its usefulness in pre-clinical and translational research.
One of the significant advantages of the rat model is that it has been one of the most frequently used species in stress-related research [47]. The HPA axis and the behaviors related to distress are well understood in rats, providing a significant advantage for their use for the study of stress in endometriosis. Different animal models of stress have been developed, with some reproducing physical stress and its associated neuroendocrine changes, while others reproduce psychological stress and related behavioral changes. Physical stress models can be subdivided into temperature fluctuation induced stress (immersion in cold water with no escape or cold environmental isolation), immobilization, electric foot shock, and forced swimming. Psychological stress models, on the other hand, include neonatal isolation, predatory stress, day-night light change, noise-induced stress, and water avoidance. One of the major disadvantages of both the physical and psychological stress models is the development of adaptation when chronic exposure is given. The HPA response undergoes desensitization or can become stable after repeated exposure of the same stressor. To prevent adaptation, exposing the individual to multiple stressors can be carried out, which will continue to elevate the corticosterone levels.
The role of the HPA axis in reproductive tissues and endometriosis
The HPA axis plays a significant role in regulating both the stress signaling as well as the immune response. During HPA axis activation, the release of CRH from the paraventricular nucleus of the hypothalamus leads to ACTH release from the anterior pituitary. ACTH will act upon the adrenal cortex leading to secretion of glucocorticoid (cortisol in humans and corticosterone in rodents) [48, 49]. On the other hand, the hypothalamic pituitary gonadal axis (HPG) is the main system that governs reproductive activities. Pulsatile release of gonadotropin-releasing hormone (GnRH) from the hypothalamic preoptic area and periventricular regions stimulates the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the anterior pituitary [50]. LH and FSH promote gonadal release of sex steroids such as testosterone, estradiol, and progesterone. The crosstalk between the HPA and HPG axes is bi-directional: activation of the HPA axis has suppressive effects in both male and female reproductive activities [51] and the CRH gene is directly regulated by estrogen [52]. Due to the intricate crosstalk between these two axes and their ultimate effects on regulating endometrial growth and the immune system, it is crucial to better understand the role of stress in endometriosis development and progression as well as symptom exacerbation.
One of the central regulators of the HPA axis signaling is CRH. Isolation of CRH by Vale in 1981 [53] led to the identification of the peptide within many peripheral tissues, including the gastrointestinal and reproductive tracts [54, 55]. The effects of CRH on the periphery are tissue-specific, having a role in intestinal permeability and motility as well as on uterine function [56–59], in addition to mediating behavioral, autonomic and visceral responses to stress [60]. Within female reproductive tissues, ovarian CRH regulates ovarian steroidogenesis via inhibition of estradiol and progesterone [61], an effect that is mediated by interleukin-1 (IL-1) [51, 61]. In the uterus, CRH induces the decidualization of endometrial stroma [62] and has a significant role in embryonic implantation and maintenance of pregnancy [63, 64]. Few studies have examined the levels of CRH, the CRH receptors, or the associated peptides in women with endometriosis. One study showed higher levels of CRH-binding protein in peritoneal fluid from women with endometriosis than in controls, suggesting possible changes also in circulating levels [65]. Others have reported that the mRNA and the protein of CRH, CRH receptors type 1 and 2, as well as urocortin (a neuropeptide that activates CRH receptors), are more strongly expressed within endometriotic lesions compared to endometrium from healthy women [66–68]. In addition to the reported changes in CRH, alterations in CRH receptor type 1 (CRHR1) have also been found in endometriotic tissue with decreased levels compared to controls throughout the menstrual cycle [69]. Using the auto-transplantation rat model of endometriosis we found increased CRH in endometriotic vesicles as compared to uterus [70], and a decrease in CRH immunoreactivity in the hippocampus [22], suggesting deregulation of the HPA axis including changes within brain structures that regulate its response [71].
Similar to CRH, the glucocorticoid receptor (GR) mRNA has been reported to be increased with a concomitant decrease in the progesterone receptor in women with endometriosis [72]. The glucocorticoid-regulated kinase (SGK1), which mediates cell responses to environmental stress and has been shown to have anti-apoptotic properties, is up-regulated in tissue from ovarian endometriosis [73]. Comparable to these findings, we have also reported an increase in GR within endometriotic vesicles versus normal uterine tissue in the rat model [70]. Intriguingly, we and others have found lower salivary cortisol levels [74, 75] as well as decreased total follicular cortisol in women with endometriosis compared to controls, which may contribute to some of the symptoms associated with endometriosis, including subfertility [76]. All these studies point towards a dysregulation of the HPA axis at multiple levels and outline a clear interaction between the stress systems and endometriosis, which we have systematically studied using the rat model. Together, the available human and animal data suggest a key role of stress and CRH signaling in the development, progression, and exacerbation of endometriosis that spans beyond the reproductive tissues.
The Impact of Stress on Endometriosis
Emotional distress has long been proposed to contribute to the exacerbation of many chronic inflammatory disorders, including endometriosis [77, 78]. Endometriosis, in particular, is known to be a source of considerable psychological stress because of its negative effects not only on physical health but also on the affective and personal life of patients [12, 79, 80]. Women with endometriosis have consistently reported reduced quality of life, high levels of perceived stress and anxiety, and depressive symptoms, which are higher than those reported by patients with other chronic inflammatory disorders [81–83]. Pain, especially when chronic in nature as is often seen in endometriosis, is also considered a major cause of physical, psychosocial, and emotional impairments [6, 84].
Although a relationship between stress and disease has been documented for many chronic conditions, including endometriosis, the exact mechanism(s) of this association are not well understood. Until recently, many unanswered questions remained regarding the role of stress in the prevalence, exacerbation, and management of endometriosis. Koninckx and colleagues were among the first to propose that psychological factors such as anxiety may be involved in the pathophysiology of endometriosis [85]. By using a primate animal model, D’Hooghe and colleagues [86] compared the prevalence rates of spontaneous endometriosis in baboons that were kept in captivity (likened to a chronic stress situation) and baboons living in the wild. They showed that spontaneous endometriosis was more prevalent in baboons that were kept in captivity over two years as compared to those recently captured or in captivity for less than two years. They concluded that the higher prevalence of endometriosis was due to captivity-associated stress. While other factors may play a role (age, more continuous menstrual cycles, captive diet), this study presented the first evidence that psychological stress may affect the prevalence of endometriosis. More recently, in our laboratory, we were able to demonstrate that stress given before the surgical induction of endometriosis resulted in larger lesions and increased inflammation [22]. Female Sprague-Dawley rats were subjected to swim stress prior to the surgical induction of endometriosis. Prior exposure to stress increased both the number and severity of vesicles found in animals with endometriosis. Stress also increased colonic inflammation, motility, myeloperoxidase levels and numbers of mast cells. Endometriosis, regardless of stress, produced a decrease in central CRF immunoreactivity, specifically in the CA3 sub-region of the hippocampus.
Using this rat animal model, we further demonstrated that stress given after the induction of endometriosis could also exacerbate the condition [87]. Two weeks after the induction of endometriosis, animals were exposed to a 10-day swim stress protocol. Again, stress significantly increased endometriotic vesicle size and increased inflammatory cell recruitment to the peritoneum. Intriguingly, stress also promoted nerve fiber growth, and expression of nerve growth factor, and its high-affinity receptor TrkA, in the uterus, suggesting that increased density of nerve fibers may play an important role in the pathogenesis of pain and tenderness [88–90]. Taken together, the results demonstrated that stress (prior or during) contributes to the development and severity of endometriosis in this animal model through mechanisms involving cell recruitment (e.g., mast cells, neutrophils, macrophages), the release of inflammatory mediators, nerve growth and deregulation of the hypothalamic-pituitary-axis.
Two additional studies carried out in the mouse syngeneic donor model of endometriosis further validate the detrimental impact of stress in this condition. Those mice exposed to 28 days of 2 hours of immobilization stress had accelerated development of endometriosis with increased generalized hyperalgesia [91]. Likewise, a different type of psychological stress (exposure to predator, in this case, a cat, for 24 hours every other day for 14 days) was also found to accelerate lesion growth alongside increased angiogenesis (shown by increased levels of VEGF and micro-vessel density) [92]. Importantly, our group was able to expand upon these findings to demonstrate that the ability to control stress could impact the condition in the rat model, suggesting a translational relevance [23]; Figure 1. Endometriosis was surgically induced, then two weeks later, the rats were subjected to either an uncontrollable or controllable swim stress protocol for ten days. The controllable arm consisted of the inclusion of a hidden platform in the watermaze that the rats could jump onto for the rest of the testing period. We ‘pair-matched’ each rat subjected to controllable stress with one subjected to uncontrollable stress so that both animals swam for the same length of time. Corticosterone levels and fecal pellet numbers were measured during the testing period as an indicator of stress. We observed that uncontrollable stress increased the number and size of the endometriotic cysts, and these animals had higher anxiety than those exposed to controllable stress. More colonic damage and increased colonic motility alongside increased uterine cell infiltration and motility were also observed, which was less evident with controllable stress. The level of stress controllability thus appears to modulate not only the pathophysiology of endometriosis but also behavioral responses, offering possibilities for therapeutic interventions.
It is possible that a chronic inflammatory response due to dysregulation of the HPA axis is related to the emotional distress caused by the symptoms reported by patients with endometriosis (e.g. incapacitating pain often refractory to treatments, inability to get pregnant, impact on study/work) [93]. The mechanisms underlying pain perception in women with endometriosis are largely unknown, and this knowledge is critical for identifying novel therapeutic targets. Ectopic growth of refluxed endometrium activates a vicious cycle of inflammation characterized by secretion of prostaglandins, growth factors (nerve growth factor-NGF, vascular endothelial growth factor-VEGF), neurotransmitters, and cytokines, into the peritoneal fluid that exacerbates the disease by increasing pain and promoting lesion survival [94]. The hyperalgesia these women suffer is unrelated to age, parity, menstrual phase, and even disease stage [46, 95–98]. Recent studies have documented both peripheral and central pain sensitization as mechanisms of endometriosis-associated pain (reviewed in [99]). At the peripheral level, nerve fibers (sensory afferent and autonomic efferent) have been shown to grow close to peritoneal lesions where there is the secretion of NGF and neurotransmitters that sensitize peripheral nerves to conduct pain signals. Central sensitization, documented in patients who are also afflicted by other concomitant pain syndromes such as migraines, irritable bowel syndrome, interstitial cystitis, fibromyalgia, and viscero-visceral hyperalgesia syndrome, may result from hyperactivation of neurons in the dorsal horn by chronic nociceptive stimuli caused by peritoneal and visceral lesions, leading to sustained pain signals to the brain [100]. Whether stress can lead to activation or modulation of peripheral and central pain mechanisms, to what degree, and by which mechanisms, is still under investigation.
In an effort to shed more light on the role of stress in modulating central and peripheral pain mechanisms we have begun to explore the contribution of opioids and their receptors to the nociceptive pathways in the rat model of endometriosis. Rats were exposed to psychological water avoidance stress (WAS) for seven consecutive days two weeks after the induction of endometriosis [101]. Nociception was measured using hot-plate tests and Von Frey filaments before surgery, before stress, and after stress. As expected from other studies, endometriosis by itself decreased the pain threshold in this model [101, 102]. Significantly fewer cells showing immunoreactivity for mu-opioid receptor were found in the dorsal lumbar region of the spinal cord, concomitant with increased enkephalin expression in the same region when compared to the Sham animals, suggesting dysregulation of the opioid pathways caused by endometriosis induction. Animals exposed to the WAS were significantly more anxious than those receiving no stress and had more vesicles of a higher grade of severity with increased mast cell infiltration, alongside more colonic damage. These animals developed significant hyperalgesia, similar to the effects of immobilization stress found in mice [90]. Interestingly, psychological stress appeared to have beneficial effects on abdominal allodynia, which could be a consequence of stress-induced analgesia that has been previously reported [103, 104].
Stress alleviation to improve endometriosis
In line with the beneficial effects our group has observed when using a controllable stress paradigm in the animal model, there is increasing evidence in the literature as to the link between coping strategies/interventions and positive outcomes in women with endometriosis. In a recent prospective study, endometriosis patients who were using positive coping strategies were found to adapt to stress better with less depression and associated pelvic pain [105]. Further, preliminary research found a significant reduction in pain and depression scores when physiotherapy and psychological interventions were carried out in women with endometriosis [106]. Normalization of cortisol levels was also observed [107]. In a small randomized sham-controlled trial of Japanese-style acupuncture significant associations were found between electrodermal measures and pelvic pain, quality of life and perceived stress [108]. These studies, albeit sparse, provide further evidence towards the therapeutic potential of mind-body interventions and complementary therapies in this condition.
Physical activity on a regular basis has already been shown to be protective in other chronic conditions that involve inflammatory processes, including diabetes and inflammatory bowel disease [109, 110]. Psychological stress and physical activity are thought to be reciprocally related, with significantly less unhealthy days reported by those adults who exercise as recommended [110]. The effects of physical activity on endometriosis and its progression are still unclear as very few studies have been conducted [112]. We studied the effect of a short period (7 days for 3 mins/day) of physical activity (swimming) on pain perception and spinal cord Mu opioid receptor (MOR) expression in the rat model of endometriosis [113]. We found that the average size of the vesicles was almost half that found in endometriosis animals left in the home cage, with significantly fewer vesicles developing (87.5% vs. 100% in exercise vs. no exercise respectively). Although the exercise did not ameliorate the hyperalgesia found in the endometriosis animals, it did improve allodynia. The decreased MOR levels observed in the dorsal lumbar region of the spinal cord of the endometriosis animals were normalized with exercise, alongside decreased levels of substance P, a neuropeptide involved in pain signaling [113]. This suggests that exercise might help counteract endometriosis pain by restoring MOR expression and, as a consequence, decreasing substance P expression. These results lead us to the intriguing possibility that exercise intervention might offer a potential new direction for therapy in endometriosis, however, these initial studies and more recent results by others [114] were confounded by the fact that swim exercise is, by its nature, a form of stress. Follow up studies with non-swimming voluntary exercise protocols are thus underway by our group to examine the impact of physical activity under non-stress conditions (i.e., voluntary wheel running). These have already yielded encouraging results whereby rats exposed to voluntary wheel running prior to endometriosis induction have significantly less vesicle development [115]. Additional studies exploring the impact of prolonged voluntary wheel running as an interventional therapy against stress exacerbation are also currently under investigation by our group.
Environmental enrichment (EE) is classically defined as a “combination of inanimate and social stimulation” that has been proven to have neural effects in animal models by improving learning and memory, and increasing neurotransmitters and beneficial growth factors [116–118]. In rats, EE involves a socially integrated lifestyle, physical and cognitive stimulation, toys, and nesting materials that promote exploration and novelty, food supplementation, larger enclosures, and sensory and visual enhancement [119]. Physical activity is one of the main components used in environmental enrichment (EE) protocols in rodents, but EE can also be carried out without sources of exercise (e.g., running wheels). EE can also combat the deleterious effects of neurodegenerative disorders and stress [120]. In animal models, EE has been shown to improve symptomatology of brain injury, brain ischemia, Alzheimer’s disease, and Huntington’s disease, and to diminish symptoms of chronic stress, depression, anxiety, and inflammatory pain [118–121]. In light of this, we recently used EE in the rat model of endometriosis to explore if it may counteract the effects of physiological stress on disease progression. In these experiments, animals were exposed to EE: larger enclosure, 4 animals per cage (vs. 2) and toys/nesting changed on a weekly basis for 8 consecutive weeks. After surgical induction of endometriosis, we allowed it to progress for 60 days maintaining the EE conditions or no EE throughout. We observed that exposure to EE reduced endometriosis vesicle number by 30% compared to the group with no EE and there was also a 60% reduction in the size of the vesicles that developed. These observations were paralleled by a dampening of CRH and glucocorticoid mRNA expression within the vesicles of rats that received EE compared to the standard housing group [70]. These data indicate that non-pharmacological, non-surgical interventions that produce a significant reduction in disease progression deserve further attention for effective translation into the clinical scenario for the benefit of women.
Future work: from bench to bedside
Our group has provided evidence to show that in women with endometriosis-related pain, the HPA responses are impaired, as shown by lower basal salivary cortisol levels compared to healthy women, in agreement with reports by Friggi Sebe Petrelluzzi and colleagues [107] and by Vincent et al. [13]. Moreover, cortisol levels were negatively correlated with symptoms (dyspareunia, infertility), and anxiety state scores were elevated [77]. Low cortisol levels have been reported to have negative side effects on the body’s reaction to stress by promoting prolonged inflammatory responses, which may impact the symptomatology of women with endometriosis. Furthermore, dysregulation of the HPA axis has been proposed as a hallmark for chronic pain [121]. Pain is the most characteristic symptom presented by the majority of women with this disease, likely contributing as a significant source of stress. Our results add to a growing body of evidence reporting a link between chronicity of disease, stress, and altered HPA axis activity in women with endometriosis.
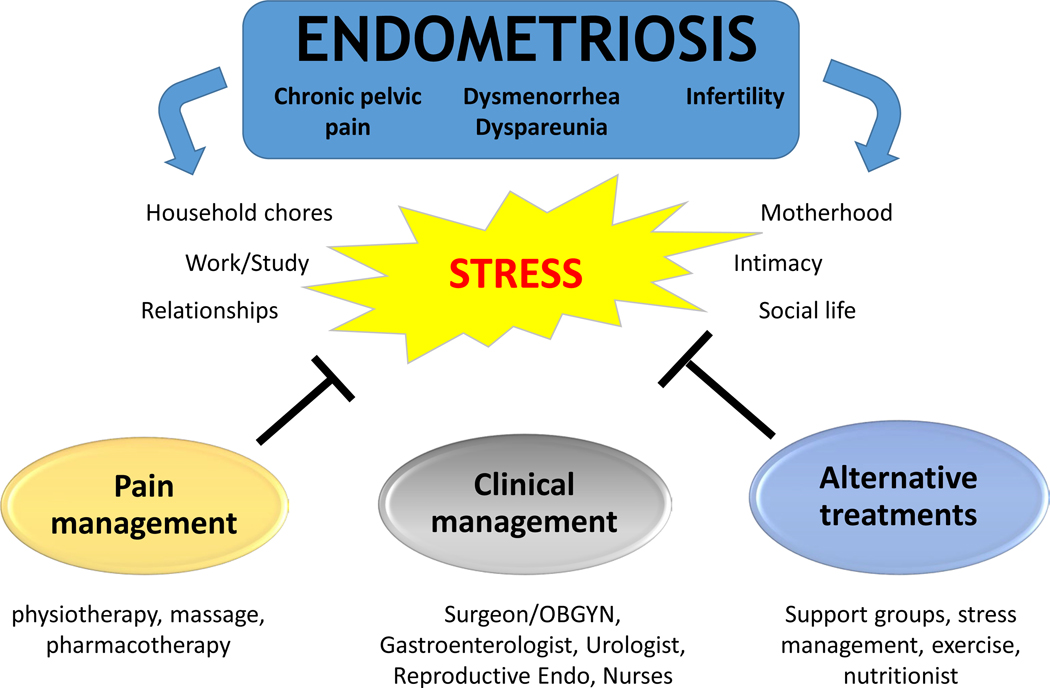
Prior research in the field of complementary and integrative medicine has found the beneficial effects of stress-coping strategies (yoga, tai-chi, support groups) on chronic pain conditions [122–125], inflammation, immune and brain function, as well as anxiety, depression, and stress levels [126–129]. Disease processes exacerbated by stress can be interrupted by psychological and stress reduction interventions, including social coping, psychotherapy, exercise, relaxation, meditation, and yoga [130–132]. Because chronic pelvic pain, a hallmark of endometriosis, is associated with multiple factors (social, biological, psychological), its clinical management is challenging. Despite multiple interventions involving hormonal therapies and surgery, many women remain in pain. In the case of endometriosis, there has been minimal investigation regarding integrative medicine alternatives [only two published randomized clinical trials (RCT)] [107, 133]. As a result, patients remain at a loss with respect to alternative options for managing symptoms when conventional therapies fail. We propose that to address this gap, therapies should build upon prior findings that women with endometriosis have abnormal HPA responses [74, 75], that stress-management produces less inflammation and decreases lesion size in the rat model of endometriosis [23, 87], and, importantly, that environmental enrichment effectively reduces lesion development in the rodent model [70]. In line with a recent meta-analysis exploring the association between endometriosis and psychological stress [134], establishing novel multi-modal paradigms that take into account psychological, behavioral, and stress-reduction interventions, as an integral part of patient-centered clinical management might provide much needed pain relief and improve quality of life for women with endometriosis (Figure 2).
Figure 2:
Relationship between stress and endometriosis, showing how a multidisciplinary approach that includes multiple tactics, including complementary medicine, could help manage the disease.
Conclusions
New therapeutic lines for endometriosis should focus on novel, integrative and patient-centered approaches for management of endometriosis’ painful symptoms, with the potential to be used as a complementary medicine approach in other inflammatory and chronic painful conditions. Interdisciplinary approaches must consider integrating behavioral interventions that target the underlying pathologic processes of this painful, chronic, and enigmatic disease, with the goal of reducing pain, improving quality of life, inflammation, and mental health in women with endometriosis. The potential to produce significant positive outcomes in endometriosis patients, shifting the field towards the use of non-pharmacological, non-surgical adjuvant treatment approaches for managing pain and inflammation, will translate to novel less-invasive alternatives for all women suffering from this incurable disease.
Based on our encouraging results of the EE intervention in the rat model of endometriosis, we now propose that the next step is to translate it to humans. While there are many studies using mind-body practices and alternative medicine modalities independently for inflammatory and painful disorders, we are proposing to use a combined multi-level systematic approach (social support, novelty and open, natural environments), based on what has proven effective in the endometriosis rat model. A translated EE intervention was recently shown to be effective in acute stroke patients, who benefitted from increased physical, social, and cognitive levels, and decreased adverse events compared to the standard of care counterparts [134–138]. Still, very few studies of multi-modal interventions involving features of EE have been conducted in humans [139, 140], and none in pain or inflammatory disorders despite ample evidence from animal models alluding to its anti-stress and anti-inflammatory effects. The mechanisms by which EE provides its benefits are hypothesized to be at multiple levels, with strong evidence showing modulation of immune pathways to produce anti-inflammatory effects [141, 142]. As recognized for many years, endometriosis is a very complex disease with no single etiology, involving changes in molecular and cellular pathways, adhesion molecules, inflammatory cells and mediators that can impact the peritoneal environment promoting appropriate conditions for ectopic endometrial cell survival [reviewed by Lagana, 143]. Many theories abound with increasing support for genetic and epigenetic changes, the role of stem cells, hormones and even ion channels [144], thus an integrative management strategy such as EE is probably necessary for optimal outcomes.
It has been proposed that EE causes ‘eustress,’ the type of stressor that provides hope and a feeling of fulfillment [145]. Eustress has positive effects on quality of life, psychological coping, and mental health, creating an overall increased level of resiliency to challenges [146, 147]. Independently, features of EE such as large, open spaces for therapy, use of multi-sensorial equipment, active engagement in novel activities, and enhanced social interactions have been used in the clinic [148, 149]. We propose that a multi-level structured program to be used as adjuvant to standard care could be effective in alleviating pain and quality of life in women with endometriosis, as well as other inflammatory, painful disease associated with chronic stress. Using an integrative, patient-centered methodology, this innovative approach could be adapted and developed into an EE intervention program consisting of activities mimicking and integrating the three hallmarks of EE: social support, novelty, and open environments. Whether the EE intervention promotes cognitive stimulation, stress management, and immune modulation, as well as providing symptom relief and improvements in quality of life for women with endometriosis, would need to be studied using rigorous RCTs. This approach has the potential to produce significant positive outcomes in patients, thus shifting the field towards the use of nonpharmacological, non-surgical adjuvant treatment approaches for managing pain and inflammation.
Acknowledgments and Funding Information
We acknowledge the tireless contribution and input of the many trainees and technical staff over the years without which this work would not be possible, especially Myrella L. Cruz and Leslie Rivera-Lopez. These studies were supported in part by R15AT006373 (CBA, IF), K07AT008027 (ATR), R15AT009915 (CBA), and R21HD098481 (IF, ATR, CBA) from the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Footnotes
Declarations
Conflict of Interest: Disclosures: No conflicts of interest, financial or otherwise are declared by the authors.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- [1].Signorello LB, Harlow BL, Cramer DW, et al. Epidemiologic determinants of endometriosis: a hospital-based case-control study. Ann Epidemiol 1997; 7: 267–741. [DOI] [PubMed] [Google Scholar]
- [2].Simoens S, Hummelshoj L, Dunselman G, et al. Endometriosis Cost Assessment (the EndoCost Study): A Cost-of-Illness Study Protocol. Gynecol Obstet Invest 2011; 71: 170–176. [DOI] [PubMed] [Google Scholar]
- [3].Simoens S, Meuleman C, D’Hooghe T. Non-health-care costs associated with endometriosis. Hum Reprod. Epub ahead of print 2011. DOI: der215 [pii] 10.1093/humrep/der215. [DOI] [PubMed] [Google Scholar]
- [4].Simoens S, Hummelshoj L, D’Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update 2007; 13: 395–404. [DOI] [PubMed] [Google Scholar]
- [5].Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011; 96: 366–373 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fourquet J, Baez L, Figueroa M, et al. Quantification of the impact of endometriosis symptoms on health-related quality of life and work productivity. Fertil Steril 2011; 96: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fourquet J, Gao X, Zavala D, et al. Patients’ report on how endometriosis affects health, work, and daily life. Fertil Steril 2010; 93: 2424–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tripoli TM, Sato H, Sartori MG, et al. Evaluation of quality of life and sexual satisfaction in women suffering from chronic pelvic pain with or without endometriosis. J Sex Med 2011; 8: 497–503. [DOI] [PubMed] [Google Scholar]
- [9].Lagana AS, Garzon S, Franchi M, et al. Translational animal models for endometriosis research: a long and windy road. Annals of translational medicine 2018; 6: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zondervan KT, Becker CM, Koga K, et al. Endometriosis. Nat Rev Dis Prim 2018; 4: 9. [DOI] [PubMed] [Google Scholar]
- [11].Facchin F, Barbara G, Dridi D, et al. Mental health in women with endometriosis: searching for predictors of psychological distress. Hum Reprod 2017; 32: 1855–1861. [DOI] [PubMed] [Google Scholar]
- [12].Facchin F, Saita E, Barbara G, et al. ‘Free butterflies will come out of these deep wounds’: A grounded theory of how endometriosis affects women’s psychological health. J Health Psychol 2018; 23: 538–549. [DOI] [PubMed] [Google Scholar]
- [13].Vincent K, Warnaby C, Stagg CJ, et al. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain 2011; 152: 1966–1975. [DOI] [PubMed] [Google Scholar]
- [14].Jones G, Jenkinson C, Kennedy S. Evaluating the responsiveness of the Endometriosis Health Profile Questionnaire: the EHP-30. Qual Life Res 2004; 13: 705–713. [DOI] [PubMed] [Google Scholar]
- [15].Seear K.The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc Sci Med 2009; 69: 1220–1227. [DOI] [PubMed] [Google Scholar]
- [16].Ding XM, Lang JH. Diagnostic delay in women with endometriosis. Chin Med Sci J 2005; 20: 209. [PubMed] [Google Scholar]
- [17].Vitale SG, La Rosa VL, Rapisarda AMC, et al. Impact of endometriosis on quality of life and psychological well-being. J Psychosom Obstet Gynaecol 2017. Dec;38(4):317–319 [DOI] [PubMed] [Google Scholar]
- [18].Lagana AS, La Rosa VL, Rapisarda AMC, et al. Anxiety and depression in patients with endometriosis: impact and management challenges. Int J Womens Health 2017. May 16;9:323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Denny E. I never know from one day to another how I will feel: pain and uncertainty in women with endometriosis. Qual Health Res 2009; 19: 985–995. [DOI] [PubMed] [Google Scholar]
- [20].Lemaire GS. More than just menstrual cramps: symptoms and uncertainty among women with endometriosis. J Obstet Gynecol neonatal Nurs JOGNN 2004; 33: 71–79. [DOI] [PubMed] [Google Scholar]
- [21].Cox H, Henderson L, Andersen N, et al. Focus group study of endometriosis: struggle, loss and the medical merry-go-round. Int J Nurs Pr 2003; 9: 2–9. [DOI] [PubMed] [Google Scholar]
- [22].Cuevas M, Flores I, Thompson KJ, et al. Stress exacerbates endometriosis manifestations and inflammatory parameters in an animal model. Reprod Sci 2012; 19: 851–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Appleyard CB, Cruz ML, Hernandez S, et al. Stress management affects outcomes in the pathophysiology of an endometriosis model. Reprod Sci 2015; 22: 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Selye H, Horava A. Stress Research. Science 1953; 117: 509. [DOI] [PubMed] [Google Scholar]
- [25].Bomholt SF, Harbuz MS, Blackburn-Munro G, et al. Involvement and role of the hypothalamo-pituitary-adrenal (HPA) stress axis in animal models of chronic pain and inflammation. Stress 2004; 7: 1–14. [DOI] [PubMed] [Google Scholar]
- [26].Woo KY. Exploring the Effects of Pain and Stress on Wound Healing. Wound Care J 2012; 25: 38–44. [DOI] [PubMed] [Google Scholar]
- [27].Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol 2007; 6: 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Engelmann M, Ebner K, Landgraf R, et al. Effects of Morris water maze testing on the neuroendocrine stress response and intrahypothalamic release of vasopressin and oxytocin in the rat. Horm Behav 2006; 50: 496–501. [DOI] [PubMed] [Google Scholar]
- [29].Grummer R.Translational animal models to study endometriosis-associated infertility. Semin Reprod Med 2013; 31: 125–132. [DOI] [PubMed] [Google Scholar]
- [30].Simitsidellis I, Gibson DA, Saunders PTK. Animal models of endometriosis: Replicating the aetiology and symptoms of the human disorder. Best Pract Res Clin Endocrinol Metab 2018; 32: 257–269. [DOI] [PubMed] [Google Scholar]
- [31].Fazleabas AT. A baboon model for inducing endometriosis. Methods Mol Med 2006; 121: 95–99. [DOI] [PubMed] [Google Scholar]
- [32].D’Hooghe TM, Debrock S, Kyama CM, et al. Baboon model for fundamental and preclinical research in endometriosis. Gynecol Obstet Invest 2004; 57: 43–46. [PubMed] [Google Scholar]
- [33].Joëls M, Karst H, Sarabdjitsingh RA. The stressed brain of humans and rodents. Acta Physiol (Oxf) 2018; 223: e13066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol 2016; 6: 603–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Da Silva JT, Seminowicz DA. Neuroimaging of pain in animal models: a review of recent literature. Pain reports 2019; 4: e732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Grummer R.Animal models in endometriosis research. Hum Reprod Update 2006; 12: 641–649. [DOI] [PubMed] [Google Scholar]
- [37].Sharpe-Timms KL. Using rats as a research model for the study of endometriosis. Ann N Y Acad Sci 2002; 955: 312–318,396–406. [DOI] [PubMed] [Google Scholar]
- [38].Uchiide I, Ihara T, Sugamata M. Pathological evaluation of the rat endometriosis model. Fertil Steril 2002; 78: 782–786. [DOI] [PubMed] [Google Scholar]
- [39].Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril 1985; 44: 684–694. [PubMed] [Google Scholar]
- [40].Nothnick WB, Curry TE, Vernon MW. Immunomodulation of rat endometriotic implant growth and protein production. Am J Reprod Immunol 1994; 31: 151–162. [DOI] [PubMed] [Google Scholar]
- [41].Witz CA. Cell adhesion molecules and endometriosis. Semin Reprod Med 2003; 21: 173–182. [DOI] [PubMed] [Google Scholar]
- [42].Rojas-Cartagena C, Flores I, Appleyard CB. Role of tumor necrosis factor receptors in an animal model of acute colitis. Cytokine 2005; 32: 85–93. [DOI] [PubMed] [Google Scholar]
- [43].Konno R, Fujiwara H, Netsu S, et al. Gene expression profiling of the rat endometriosis model. Am J Reprod Immunol 2007; 58: 330–343. [DOI] [PubMed] [Google Scholar]
- [44].Flores I, Rivera E, Ruiz LA, et al. Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease. Fertil Steril 2007; 87: 1180–1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Moon CE, Bertero MC, Curry TE, et al. The presence of luteinized unruptured follicle syndrome and altered folliculogenesis in rats with surgically induced endometriosis. Am J Obstet Gynecol 1993; 169: 676–682. [DOI] [PubMed] [Google Scholar]
- [46].Cason AM, Samuelsen CL, Berkley KJ. Estrous changes in vaginal nociception in a rat model of endometriosis. Horm Behav 2003; 44: 123–131. [DOI] [PubMed] [Google Scholar]
- [47].Sutanto W, de Kloet ER. The use of various animal models in the study of stress and stress-related phenomena. Lab Anim 1994; 28: 293–306. [DOI] [PubMed] [Google Scholar]
- [48].Kudielka BM, Wust S. Human models in acute and chronic stress: assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress 2010; 13: 1–14. [DOI] [PubMed] [Google Scholar]
- [49].Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci 2012; 1261:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Chappel SC, Barraclough CA. Plasma concentration changes in LH and FSH following electrochemical stimulation of the medial preoptic are or dorsal anterior hypothalamic area of estrogen- or androgen-sterilized rats. Biol Reprod 1976; 15: 661–669. [DOI] [PubMed] [Google Scholar]
- [51].Kalantaridou SN, Zoumakis E, Makrigiannakis A, et al. Corticotropin-releasing hormone, stress and human reproduction: An update. J Reprod Immunol 2010; 85: 33–39. [DOI] [PubMed] [Google Scholar]
- [52].Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimophism of the stress response and immune/inflammatory reaction. J Clin Invest 1993; 92: 1896–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vale W, Spiess J, Rivier C, et al. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science (80- ) 1981; 213: 1394–1397. [DOI] [PubMed] [Google Scholar]
- [54].Florio P, Rossi M, Sigurdardottir M, et al. Paracrine regulation of endometrial function: interaction between progesterone and corticotropin-releasing factor (CRF) and activin A. Steroids 2003; 68: 801–807. [DOI] [PubMed] [Google Scholar]
- [55].Buckinx R, Adriaensen D, Nassauw LV, et al. Corticotrophin-releasing factor, related peptides, and receptors in the normal and inflamed gastrointestinal tract. Front Neurosci 2011; 5: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhang LM, Wang YK, Hui N, et al. Corticotropin-releasing hormone acts on CRH-R1 to inhibit the spontaneous contractility of non-labouring human myometrium at term. Life Sci 2008; 83: 620–624. [DOI] [PubMed] [Google Scholar]
- [57].Cong B, Zhang L, Gao L, et al. Reduced expression of CRH receptor type 1 in upper segment human myometrium during labour. Reprod Biol Endocrinol 2009; 7: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Overman EL, Rivier JE, Moeser AJ. CRF induces intestinal epithelial barrier injury via the release of mast cell proteases and TNF-alpha. PLoS One 2012; 7: e39935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nozu T, Tsuchiya Y, Kumei S, et al. Peripheral corticotropin-releasing factor (CRF) induces stimulation of gastric contractions in freely moving conscious rats: role of CRF receptor types 1 and 2. Neurogastroenterol Motil 2013; 25: 190–197. [DOI] [PubMed] [Google Scholar]
- [60].Gareau MG, Silva MA, Perdue MH. Pathophysiological mechanisms of stress-induced intestinal damage. Curr Mol Med 2008; 8: 274–281. [DOI] [PubMed] [Google Scholar]
- [61].Ghizzoni L, Mastorakos G, Vottero A, et al. Corticotropin-releasing hormone (CRH) inhibits steroid biosynthesis by cultured human granulosa-lutein cells in a CRH and interleukin-1 receptor-mediated fashion. Endocrinology 1997; 138: 4806–4811. [DOI] [PubMed] [Google Scholar]
- [62].Ferrari A, Petraglia F, Gurpide E. Corticotropin releasing factor decidualizes human endometrial stromal cells in vitro. Interaction with progestin. J Steroid Biochem Mol Biol 1995; 54: 251–255. [DOI] [PubMed] [Google Scholar]
- [63].Makrigiannakis A, Zoumakis E, Kalantaridou S, et al. Corticotropin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat Immunol 2001; 2: 1018–1024. [DOI] [PubMed] [Google Scholar]
- [64].Kalantaridou SN, Zoumakis E, Makrigiannakis A, et al. The role of corticotropin-releasing hormone in blastocyst implantation and early fetal immunotolerance. Horm Metab Res = Horm und Stoffwechselforsch = Horm Metab 2007; 39: 474–477. [DOI] [PubMed] [Google Scholar]
- [65].Florio P, Luisi S, Vigano P, et al. Healthy women and patients with endometriosis show high concentrations of inhibin A, inhibin B, and activin A in peritoneal fluid throughout the menstrual cycle. Hum Reprod 1998; 13: 2606–2611. [DOI] [PubMed] [Google Scholar]
- [66].Vergetaki A, Jeschke U, Vrekoussis T, et al. Differential expression of CRH, UCN, CRHR1 and CRHR2 in eutopic and ectopic endometrium of women with endometriosis. PLoS One 2013; 8: e62313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Carrarelli P, Luddi A, Funghi L, et al. Urocortin and corticotrophin-releasing hormone receptor type 2 mRNA are highly expressed in deep infiltrating endometriotic lesions. Reprod Biomed Online 2016; 33: 476–483. [DOI] [PubMed] [Google Scholar]
- [68].Pergialiotis V, Tagkou NM, Tsimpiktsioglou A, et al. Urocortin Expression in Endometriosis: A Systematic Review. Int J Fertil Steril 2019; 13: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Novembri R, Carrarelli P, Toti P, et al. Urocortin 2 and urocortin 3 in endometriosis: evidence for a possible role in inflammatory response. Mol Hum Reprod 2011; 17: 587–593. [DOI] [PubMed] [Google Scholar]
- [70].Torres-Reveron A, Rivera LL, Flores I, et al. Environmental Manipulations as an Effective Alternative Treatment to Reduce Endometriosis Progression. Reprod Sci 2018; 25: 1336–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 1991; 12: 118–134. [DOI] [PubMed] [Google Scholar]
- [72].Grandi G, Mueller M, Bersinger N, et al. Progestin suppressed inflammation and cell viability of tumor necrosis factor-alpha-stimulated endometriotic stromal cells. Am J Reprod Immunol 2016; 76: 292–298. [DOI] [PubMed] [Google Scholar]
- [73].Monsivais D, Dyson MT, Yin P, et al. Estrogen receptor beta regulates endometriotic cell survival through serum and glucocorticoid-regulated kinase activation. Fertil Steril 2016; 105: 1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Petrelluzzi KFS, Garcia MC, Petta CA, et al. Salivary cortisol concentrations, stress and quality of life in women with endometriosis and chronic pelvic pain. Stress 2008; 11: 390–397. [DOI] [PubMed] [Google Scholar]
- [75].Quiñones M, Urrutia R, Torres-Reverón A, et al. Anxiety, coping skills and hypothalamus-pituitary-adrenal (HPA) axis in patients with endometriosis. J Reprod Biol Heal 2015; 3: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Smith MP, Keay SD, Margo FC, et al. Total cortisol levels are reduced in the periovulatory follicle of infertile women with minimal-mild endometriosis. Am J Reprod Immunol 2002; 47: 52–56. [DOI] [PubMed] [Google Scholar]
- [77].Gluck O, Maricic M. Infusion therapies in rheumatic practice. J Clin Rheumatol 2000; 6: 294–299. [DOI] [PubMed] [Google Scholar]
- [78].Reber SO. Stress and animal models of inflammatory bowel disease--an update on the role of the hypothalamo-pituitary-adrenal axis. Psychoneuroendocrinology 2012; 37: 1–19. [DOI] [PubMed] [Google Scholar]
- [79].Sepulcri Rde P, do Amaral VF. Depressive symptoms, anxiety, and quality of life in women with pelvic endometriosis. Eur J Obstet Gynecol Reprod Biol 2009; 142: 53–56. [DOI] [PubMed] [Google Scholar]
- [80].Luisi S, Pinzauti S, Regini C, et al. Serum markers for the noninvasive diagnosis of endometriosis. Womens Health (Lond Engl) 2015; 11: 603–610. [DOI] [PubMed] [Google Scholar]
- [81].Barnack JL, Chrisler JC. The experience of chronic illness in women: a comparison between women with endometriosis and women with chronic migraine headaches. Women Health 2007; 46: 115–133. [DOI] [PubMed] [Google Scholar]
- [82].Low WY, Edelmann RJ, Sutton C. A psychological profile of endometriosis patients in comparison to patients with pelvic pain of other origins. J Psychosom Res 1993; 37: 111–116. [DOI] [PubMed] [Google Scholar]
- [83].Siedentopf F, Tariverdian N, Rucke M, et al. Immune status, psychosocial distress and reduced quality of life in infertile patients with endometriosis. Am J Reprod Immunol 2008; 60: 449–461. [DOI] [PubMed] [Google Scholar]
- [84].Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril 2011; 96: 366–373 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Koninckx PR. Pelvic endometriosis: a consequence of stress? Contrib Gynecol Obstet 1987; 16: 56–59. [PubMed] [Google Scholar]
- [86].D’Hooghe TM, Bambra CS, Raeymaekers BM, et al. The cycle pregnancy rate is normal in baboons with stage I endometriosis but decreased in primates with stage II and stage III-IV disease. Fertil Steril 1996; 66: 809–813. [PubMed] [Google Scholar]
- [87].Cuevas M, Cruz ML, Ramirez AE, et al. Stress During Development of Experimental Endometriosis Influences Nerve Growth and Disease Progression. Reprod Sci 2018; Mar;25(3):347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wang G, Tokushige N, Markham R, et al. Rich innervation of deep infiltrating endometriosis. Hum Reprod 2009; 24: 827–834. [DOI] [PubMed] [Google Scholar]
- [89].Noel JC, Anaf V, Borghese B, et al. The steroidogenic factor-1 protein is not expressed in various forms of endometriosis but is strongly present in ovarian cortical or medullary mesenchymatous cells adjacent to endometriotic foci. Fertil Steril 2011; 95: 2655–2657. [DOI] [PubMed] [Google Scholar]
- [90].Alvarez P, Bogen O, Levine JD. Role of nociceptor estrogen receptor GPR30 in a rat model of endometriosis pain. Pain 2014; 155: 2680–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Long Q, Liu X, Qi Q, et al. Chronic stress accelerates the development of endometriosis in mouse through adrenergic receptor beta2. Hum Reprod 2016; 31: 2506–2519. [DOI] [PubMed] [Google Scholar]
- [92].Guo S-W, Zhang Q, Liu X. Social psychogenic stress promotes the development of endometriosis in mouse. Reprod Biomed Online 2017; 34: 225–239. [DOI] [PubMed] [Google Scholar]
- [93].Morotti M, Vincent K, Becker CM. Mechanisms of pain in endometriosis. Eur J Obstet Gynecol Reprod Biol 2017; 209: 8–13. [DOI] [PubMed] [Google Scholar]
- [94].Coxon L, Horne AW, Vincent K. Pathophysiology of endometriosis-associated pain: A review of pelvic and central nervous system mechanisms. Best Pract Res Clin Obstet Gynaecol 2018; 1–15. [DOI] [PubMed] [Google Scholar]
- [95].Lu Y, Nie J, Liu X, et al. Trichostatin A, a histone deacetylase inhibitor, reduces lesion growth and hyperalgesia in experimentally induced endometriosis in mice. Hum Reprod 2010; 25: 1014–25. [DOI] [PubMed] [Google Scholar]
- [96].McAllister SL, Dmitrieva N, Berkley KJ. Sprouted innervation into uterine transplants contributes to the development of hyperalgesia in a rat model of endometriosis. PLoS One 2012; 7: e31758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Nagabukuro H, Berkley KJ. Influence of endometriosis on visceromotor and cardiovascular responses induced by vaginal distention in the rat. Pain 2008; 132: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Berkley KJ, Cason A, Jacobs H, et al. Vaginal hyperalgesia in a rat model of endometriosis. Neurosci Lett 2001; 306: 185–188. [DOI] [PubMed] [Google Scholar]
- [99].Zheng P, Zhang W, Leng J, et al. Research on central sensitization of endometriosis-associated pain: a systematic review of the literature. J Pain Res 2019; 12: 1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bajaj P, Bajaj P, Madsen H, Arendt-Nielsen L. Endometriosis is associated with central sensitization: a psychophysical controlled study. J Pain. 2003;4(7):372–380 [DOI] [PubMed] [Google Scholar]
- [101].Hernandez S, Cruz ML, Seguinot II, et al. Impact of Psychological Stress on Pain Perception in an Animal Model of Endometriosis. Reprod Sci 2017; Oct;24(10):1371–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Zhao T, Liu X, Zhen X, et al. Levo-tetrahydropalmatine retards the growth of ectopic endometrial implants and alleviates generalized hyperalgesia in experimentally induced endometriosis in rats. Reprod Sci 2011; 18: 28–45. [DOI] [PubMed] [Google Scholar]
- [103].Larauche M, Moussaoui N, Biraud M, et al. Brain corticotropin-releasing factor signaling: Involvement in acute stress-induced visceral analgesia in male rats. Neurogastroenterol Motil 2019; 31: e13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Tobaldini G, Andersen EOL, Polato JJ, et al. Pain and stress: functional evidence that supra-spinal mechanisms involved in pain-induced analgesia mediate stress-induced analgesia. Behav Pharmacol. Epub ahead of print December 2019. DOI: 10.1097/FBP.0000000000000529. [DOI] [PubMed] [Google Scholar]
- [105].Donatti L, Ramos DG, de Andres MP, et al. Patients with endometriosis using positive coping strategies have less depression, stress and pelvic pain. Einstein (Sao Paulo) 2017; 15: 65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lorencatto C, Petta CA, Navarro MJ, et al. Depression in women with endometriosis with and without chronic pelvic pain. Acta Obs Gynecol Scand 2006; 85: 88–92. [DOI] [PubMed] [Google Scholar]
- [107].Friggi Sebe Petrelluzzi K, Garcia MC, Petta CA, et al. Physical therapy and psychological intervention normalize cortisol levels and improve vitality in women with endometriosis. J Psychosom Obstet Gynaecol 2012; 33: 191–198. [DOI] [PubMed] [Google Scholar]
- [108].Ahn AC, Schnyer R, Conboy L, et al. Electrodermal measures of Jing-Well points and their clinical relevance in endometriosis-related chronic pelvic pain. J Altern Complement Med 2009; 15: 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011; 43: 1334–1359. [DOI] [PubMed] [Google Scholar]
- [110].Cheifetz AS, Gianotti R, Luber R, et al. Complementary and Alternative Medicines Used by Patients With Inflammatory Bowel Diseases. Gastroenterology 2017; 152: 415–429.e15. [DOI] [PubMed] [Google Scholar]
- [111].Brown DW, Balluz LS, Heath GW, et al. Associations between recommended levels of physical activity and health-related quality of life. Findings from the 2001 Behavioral Risk Factor Surveillance System (BRFSS) survey. Prev Med (Baltim) 2003; 37: 520–528. [DOI] [PubMed] [Google Scholar]
- [112].Bonocher CM, Montenegro ML, Rosa E Silva JC, et al. Endometriosis and physical exercises: a systematic review. Reprod Biol Endocrinol 2014; 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Hernandez S, Cruz M, Torres-Reveron A, et al. Impact of physical activity on pain perception in an animal model of endometriosis. J endometr 2015; 7: 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Montenegro ML, Bonocher CM, Meola J, et al. Effect of Physical Exercise on Endometriosis Experimentally Induced in Rats. Reprod Sci 2019; 26: 785–793. [DOI] [PubMed] [Google Scholar]
- [115].Cruz ML, Chompre G, Velazquez J, et al. Beneficial Effects of Voluntary Wheel Running in an Endometriosis Animal Model. FASEB J 2019; 33: 536.4. [Google Scholar]
- [116].van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci 2000; 1: 191–198. [DOI] [PubMed] [Google Scholar]
- [117].Hutchinson E, Avery A, Vandewoude S. Environmental enrichment for laboratory rodents. ILAR J 2005; 46: 148–161. [DOI] [PubMed] [Google Scholar]
- [118].Renoir T, Pang TYC, Mo C, et al. Differential effects of early environmental enrichment on emotionality related behaviours in Huntington’s disease transgenic mice. J Physiol 2013; 591: 41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Young J, Pionk T, Hiatt I, et al. Environmental enrichment aides in functional recovery following unilateral controlled cortical impact of the forelimb sensorimotor area however intranasal administration of nerve growth factor does not. Brain Res Bull 2015; 115: 17–22. [DOI] [PubMed] [Google Scholar]
- [120].Benaroya-Milshtein N, Hollander N, Apter A, et al. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur J Neurosci 2004; 20: 1341–1347. [DOI] [PubMed] [Google Scholar]
- [121].Chatzikokkinou P, Thorfinn J, Angelidis IK, et al. Spontaneous endometriosis in an umbilical skin lesion. Acta Dermatovenerol Alp Panonica Adriat 2009; 18: 126–130. [PubMed] [Google Scholar]
- [122].Segura-Jimenez V, Romero-Zurita A, Carbonell-Baeza A, et al. Effectiveness of Tai-Chi for decreasing acute pain in fibromyalgia patients. Int J Sports Med 2014; 35: 418–423. [DOI] [PubMed] [Google Scholar]
- [123].Shariff F, Carter J, Dow C, et al. Mind and body management strategies for chronic pain and rheumatoid arthritis. Qual Health Res 2009; 19: 1037–1049. [DOI] [PubMed] [Google Scholar]
- [124].Wang C.Tai Chi improves pain and functional status in adults with rheumatoid arthritis: results of a pilot single-blinded randomized controlled trial. Med Sport Sci 2008; 52: 218–229. [DOI] [PubMed] [Google Scholar]
- [125].Bushnell MC, Case LK, Ceko M, et al. Effect of environment on the long-term consequences of chronic pain. Pain 2015; 156 Suppl: S42–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Sharma P, Poojary G, Dwivedi SN, et al. Effect of Yoga-Based Intervention in Patients with Inflammatory Bowel Disease. Int J Yoga Therap 2015; 25: 101–112. [DOI] [PubMed] [Google Scholar]
- [127].Mostagi FQRC, Dias JM, Pereira LM, et al. Pilates versus general exercise effectiveness on pain and functionality in non-specific chronic low back pain subjects. J Bodyw Mov Ther 2015; 19: 636–645. [DOI] [PubMed] [Google Scholar]
- [128].Sadeghi Aval Shahr H, Saadat M, Kheirkhah M, et al. The effect of self-aromatherapy massage of the abdomen on the primary dysmenorrhoea. J Obstet Gynaecol 2015; 35: 382–385. [DOI] [PubMed] [Google Scholar]
- [129].Cheong YC, Smotra G, Williams AC de C. Non-surgical interventions for the management of chronic pelvic pain. Cochrane database Syst Rev 2014; CD008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Hsu Y-C, Tsai S-F, Yu L, et al. Long-term moderate exercise accelerates the recovery of stress-evoked cardiovascular responses. Stress 2016; 19: 125–132. [DOI] [PubMed] [Google Scholar]
- [131].Goyal M, Singh S, Sibinga EMS, et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med 2014; 174: 357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Berga SL, Loucks TL. Use of cognitive behavior therapy for functional hypothalamic amenorrhea. Ann N Y Acad Sci 2006; 1092: 114–129. [DOI] [PubMed] [Google Scholar]
- [133].Goncalves AV, Barros NF, Bahamondes L. The Practice of Hatha Yoga for the Treatment of Pain Associated with Endometriosis. J Altern Complement Med 2017; 23: 45–52. [DOI] [PubMed] [Google Scholar]
- [134].Brasil DL, Montagna E, Trevisan CM, et al. Psychological stress levels in women with endometriosis: systematic review and meta-analysis of observational studies. Minerva medica. 2019. 10.23736/S0026-4806.19.06350-X. [DOI] [PubMed] [Google Scholar]
- [135].Rosbergen ICM, Grimley RS, Hayward KS, et al. The effect of an enriched environment on activity levels in people with stroke in an acute stroke unit: protocol for a before-after pilot study. Pilot feasibility Stud 2016; 2: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Janssen H, Ada L, Karayanidis F, et al. Translating the use of an enriched environment poststroke from bench to bedside: study design and protocol used to test the feasibility of environmental enrichment on stroke patients in rehabilitation. Int J Stroke 2012; 7: 521–526. [DOI] [PubMed] [Google Scholar]
- [137].Rosbergen ICM, Brauer SG, Fitzhenry S, et al. Qualitative investigation of the perceptions and experiences of nursing and allied health professionals involved in the implementation of an enriched environment in an Australian acute stroke unit. BMJ Open 2017; 7: e018226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Rosbergen IC, Grimley RS, Hayward KS, et al. The impact of environmental enrichment in an acute stroke unit on how and when patients undertake activities. Clin Rehabil 2019; 33: 784–795. [DOI] [PubMed] [Google Scholar]
- [139].Clemenson GD, Stark CEL. Virtual Environmental Enrichment through Video Games Improves Hippocampal-Associated Memory. J Neurosci 2015; 35: 16116–16125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Woo CC, Donnelly JH, Steinberg-Epstein R, et al. Environmental enrichment as a therapy for autism: A clinical trial replication and extension. Behav Neurosci 2015; 129: 412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Singhal G, Jaehne EJ, Corrigan F, et al. Cellular and molecular mechanisms of immunomodulation in the brain through environmental enrichment. Front Cell Neurosci 2014; 8: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Gimenez-Llort L, Mate I, Manassra R, et al. Peripheral immune system and neuroimmune communication impairment in a mouse model of Alzheimer’s disease. Ann N Y Acad Sci 2012; 1262: 74–84. [DOI] [PubMed] [Google Scholar]
- [143].Lagana AS, Garzon S, Gotte M., et al. , The pathogenesis of endometriosis: molecular and cell biology insights. Int J Mol Sci 2019. Nov 10:20(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Riemma G, Lagana AS, Schiattarella A, et al. Ion channels in the pathogenesis of endometriosis: a cutting edge point of view. Int J Mol Sci 2020. Feb 21(3):1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Cao L, Liu X, Lin E-JD, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell 2010; 142: 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Le Fevre M, Kolt G, Matheny J. Eustress, distress and their interpretation in primary and secondary occupational stress management interventions: which way first? J Manag Psychol 2006; 21: 547–565. [Google Scholar]
- [147].Konkle ATM, Kentner AC, Baker SL, et al. Environmental-enrichment-related variations in behavioral, biochemical, and physiologic responses of Sprague-Dawley and Long Evans rats. J Am Assoc Lab Anim Sci 2010; 49: 427–436. [PMC free article] [PubMed] [Google Scholar]
- [148].Kilpatrick LA, Suyenobu BY, Smith SR, et al. Impact of Mindfulness-Based Stress Reduction training on intrinsic brain connectivity. Neuroimage 2011; 56: 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Steenbarger BN. The importance of Novelty in Psychotherapy. In: Clinical Strategies for Becoming a Master Psychotherapist. Academic Press, 2006, pp. 277–290. [Google Scholar]