Abstract
Coronavirus pandemic has evidenced the importance of creating bioactive materials to mitigate viral infections, especially in healthcare settings and public places. Advances in antiviral coatings have led to materials with impressive antiviral performance; however, their application may face health and environmental challenges. Bio-inspired antimicrobial peptides (AMPs) are suitable building blocks for antimicrobial coatings due to their versatile design, scalability, and environmentally friendly features. This review presents the advances and opportunities on the AMPs to create virucidal coatings. The review first describes the fundamental characteristics of peptide structure and synthesis, highlighting the recent findings on AMPs and the role of peptide structure (α-helix, β-sheet, random, and cyclic peptides) on the virucidal mechanism. The following section presents the advances in AMPs coating on medical devices with a detailed description of the materials coated and the targeted pathogens. The use of peptides in vaccine formulations is also reported, emphasizing the molecular interaction of peptides with different viruses and the current clinical stage of each formulation. The role of several materials (metallic particles, inorganic materials, and synthetic polymers) in the design of antiviral coatings is also presented, discussing the advantages and the drawbacks of each material. The final section offers future directions and opportunities for using AMPs on antiviral coatings to prevent viral outbreaks.
Keywords: Peptides, Antimicrobial coatings, Antimicrobial peptides, Antiviral peptides
Graphical Abstract
1. Introduction
The pandemic of the new coronavirus disease 2019 (COVID-19) has raised a warning about the easy and rapid spread of viral infections that, despite all advances in human health care, can result in high morbidity and mortality. Methods that limit viral contamination have emerged as an alternative against several microorganisms, preventing new diseases from becoming a pandemic [1]. Social distancing is one of the most powerful alternatives to control the spread of viruses. However, the effectiveness of this method depends on cultural and social behavior, which proved to be one of the major challenges in mitigating coronavirus spread. For airborne transmission, other measures such as protective barriers against droplets and environmental and personal hygiene have been recommended, although they also depend on the effectiveness of antiviral products and social collaboration. Nanoengineered materials are suitable for the prevention of contamination not only for COVID-19, but also for other viruses that can still be discovered in the future [2].
Antimicrobial coatings are designed to inactivate or destroy microorganisms and can be used in the most diverse devices, whether biomedical or not. Inanimate surfaces can play a key role in the proliferation of infections in healthcare settings, even if they are constantly disinfected and considered sterile. Ellingson et al. [3] investigated the influence of antimicrobial coating application on the surfaces of patient rooms and common areas in three units of two urban hospitals. The authors found a 36% reduction in healthcare-associated infections compared to control, untreated units. In addition, tests with several strains of bacteria indicated a decrease in CFU (colony forming unit) of 79% and 75% for each hospital investigated. These findings reinforce those antimicrobial coatings as an option to prevent the dissemination of new diseases. One recent survey by Markets and Markets™ revealed that the worldwide market for antimicrobial coatings is estimated to have a compound annual growth rate of 10.7% between 2020 and 2025, growing from USD 3.3 billion in 2020 to around USD 5.6 billion in 2025 [4]. Suitable antimicrobial coatings should be able to inactivate or destroy the microorganism and be considered a non-toxic and environmentally friendly alternative. As these requirements are not usually met for traditional antimicrobial coatings, some of them, based on inorganic compounds that present cumulative behavior in the environment, other options should be sought [5].
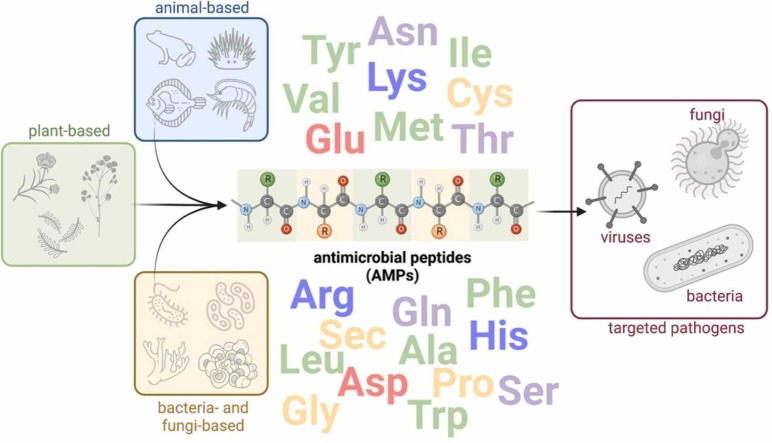
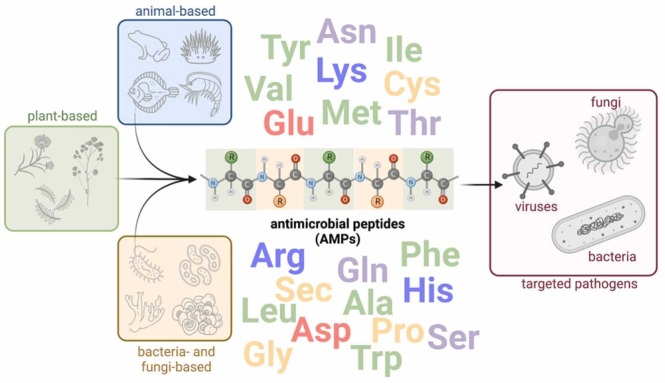
Peptide coatings have been widely investigated with a special focus on antimicrobial peptides (AMPs) and their ability to inhibit growth, inactivate and kill different microbes. AMPs are potent natural compounds found in the immune system of living organisms. These compounds can also be produced by synthetic routes, allowing the production of analogs with improved therapeutic properties. Most AMP coatings studies explore its ability to interact with negatively charged cell membranes. Other mechanisms of action, such as inhibition of protein synthesis and stimulation of the host's defense system, may also confer the virucidal capacity to several AMPs, contributing to its application in coatings to prevent the spread of viral diseases [6]. Fig. 1 summarizes the natural occurrence of AMPs and their application to inactivate various microbes.
Fig. 1.
Occurrence of antimicrobial peptides and their application to inactivate disease-related microorganisms.
Virucidal coatings have recently become an important matter, mostly focusing on metallic and inorganic materials, such as copper, silver, zinc, titanium dioxide (TiO2), as well as polymeric and organic materials, capable of inactivating viruses by contact. However, environmental or biocompatibility problems can be associated with these compounds. Also, the use of virucidal coatings may be limited by several factors, including non-universal processing methods, reduced efficacy against different viruses, long contact time for viral inactivation, and challenges associated with process scalability and cost. AMPs are an excellent alternative due to their therapeutic efficacy against a wide range of viruses, as well as the possibility of modifications that allow them to adjust their cost, biocompatibility, and selectivity features [7]. As this is a topic that is still little investigated, despite the current knowledge on the virucidal action of several AMPs, this review will focus on the state-of-the-art using peptides as antiviral therapeutic agents and their potential use as coating agents for biomedical devices.
2. Peptides
Peptides, commonly obtained by breaking down proteins, are molecules formed by the condensation of a short number of amino acids, differing from proteins in their smaller size. Their discovery dates from the end of the 19th and the beginning of the 20th century, gaining more attention in recent years with new technologies that have improved process efficiency and reduced costs on a large production scale. Peptides are usually synthesized in the solid phase (synthetic peptides) or by chemical or enzymatic digestion of proteins [8], [9].
Like proteins, peptides have essential functions in human metabolism and can be classified according to their mode of action as antioxidative, antihypertensive, antimicrobial, antithrombotic, opioid, immunomodulatory, or mineral binding [10]. Such features are directly linked to their potential as promising biomaterials, either in isolated form or identified from different sources, with applications in biomedical and food, among other fields [11]. In the food industry, peptides are often used as nutraceuticals in functional foods (i.e., substances that present physiological benefits, including nutritional, safety, and therapeutic effects) to improve health conditions [12]. In addition, they can contribute to preventing the oxidation and microbial degradation of food [10]. Studies also reveal that both animal- [13] and vegetable-derived [14] peptides can be used for these purposes. Several studies have evaluated the potential use of peptides as therapeutics as an alternative to undruggable targets [15] and small drug molecules due to their ability to bind smooth and flat proteins [16].
Peptides have also been considered as constituents of vaccines, either as active ingredients or adjuvants. One of the goals is to add small fragments of the pathogen in the form of peptides to the vaccines, which are not able to cause disease since they do not correspond to the entire pathogen, and at the same time, they can induce a controlled immune response with reduced risk of allergies and autoimmunity [17]. Chauhan et al. [18] studied a peptide-based vaccine against malaria infection, a disease caused by Plasmodium falciparum and which has worsened with the increased resistance of these microorganisms to the drugs in use. The authors found an excellent immune response in healthy individuals with the previous contact with P. falciparum. Wang et al. [19] evaluated peptide-based vaccines to control the spread of pulmonary chlamydial infections, specifically the psittacosis caused by Chlamydia psittaci. In vivo tests demonstrated a vaccine with high immunogenicity and robust immune protection against chlamydial infection.
The capacity of peptides as a cell-penetrating agent has been explored to target drug delivery [20] and theragnostic medicine, that is, for simultaneous diagnosis and treatment [21]. Lu et al. [22] evaluated the use of the synthetic peptide SP90 as a target system for delivering liposomal drugs to patients with breast cancer. The authors identified that SP90 can bind to several breast cancer cell lines, recognizing tumor tissues in patients affected by the disease without binding to normal human breast cells. Neoplastic cells are more negatively charged than healthy cells, becoming more susceptible to interacting with cationic peptides. These peptides allow liposomal drugs to be released on the target site more efficiently, with better release and penetration of the drug into the tumor, leading to higher drug accumulation at the intracellular target site and better therapeutic performance. In vivo results indicated a 3.2-fold reduction in tumor volume by administering the liposomal anticancer drug doxorubicin associated with SP90, compared to the non-targeting drug. The peptide properties also make these materials interesting for tumor-penetrating agents to inhibit the growth of cancer cells, and are suitable for antitumor therapy or clinical treatment. This target delivery approach may limit the side effects of conventional chemotherapy and radiotherapy treatments, improving the target concentration of the therapeutic and the penetration into the solid tumor parenchyma [23].
Peptides have also been anchored to biosensors to improve disease marker detection, helping in early diagnosis and treatment. The presence of peptides may also improve the antifouling performance of the biosensor, preventing the adsorption of unwanted elements that may hinder the biosensor performance [24]. Studies have also used peptides as radiolabeled components for medical images [25], [26], surfactants [27], [28], and catalysts [29], [30], to name few applications. For this review, antimicrobial activity is the main focus and, therefore, will be described in detail in the next sessions. We will also present, in session 3, the action of peptides as antiviral vaccine agents. Although this is probably not the main mechanism to be explored when using peptides in virucide coatings, it is still essential to provide a broad perspective of the specific abilities of peptides as antiviral agents.
3. Use of antiviral peptides as therapeutic or vaccine agents
Viruses can be divided into DNA and RNA viruses according to the genetic material they encapsulate. They can also be divided into enveloped, which have a lipid membrane around the viral particle, and non-enveloped ones. This feature directly influences the mechanism of viral entry into host cells and their resistance to antiviral agents. Generally, both viruses can enter host cells through receptor-mediated endocytosis, involving carbohydrates, proteins, or lipid molecules. In enveloped viruses, this process occurs by fusing both viral and cell membranes, whereas non-enveloped viruses invade the cell by penetration, and the viral genome is delivered to the cell's cytoplasm. In the lytic cycle, this process is followed by the viral genome replication, production of virions, and cell lysis, releasing a viral progeny that will infect other host cells [31].
The first antiviral drug was developed in 1963, and since then, some other antiviral therapeutics have been developed to prevent viral infections. Amantadine is one example of an antiviral drug currently used against RNA viruses for the treatment and prevention of Influenza A. Acyclovir is another example of an antiviral drug that is effective against several DNA viruses, such as types 1 and 2 of herpes simplex, shingles virus, Epstein–Barr virus, and cytomegalovirus. Vidarabine is also an antiviral drug used against the herpes virus. Additional examples of antiviral drugs include idoxuridine and trifluridine, for external use in case of herpes infections in the eyes (keratitis), and zidovudine, an antiretroviral drug clinically active against HIV-1. One interesting feature of these therapeutics is their possible use for more than one type of infectious disease, suggesting that approved antiviral drugs could be used off-label to treat multiple emerging viral infections [32], [33].
It is remarkable that, even in the face of the countless existing viral diseases (chickenpox, rubella, polio, smallpox, measles, among others), the existence of drug therapies capable of fighting them is still limited, and those that exist may cause strong side effects to patients, such as the case of zidovudine, associated with hematologic toxicity, anemia, neutropenia, headache and gastrointestinal intolerance [34]. This scenario, coupled with all the favorable aspects of AMPs, reinforces the importance of investigating peptides as antiviral therapeutic agents. The drugs mentioned above increase the cell resistance to the virus by suppressing the adsorption of the virus on the cell surface, preventing the viral diffusion into the cell, or causing the deproteinization of the viral particles [33]. Understanding these mechanisms at the molecular level is essential to the rational design of AMPs for therapeutic applications. The use of these peptides could also reduce drawbacks associated with increasing viral resistance and the emergence of new viral epidemics [35].
The use of peptides as a therapeutic agent was evaluated for the severe acute respiratory syndrome (SARS) caused by a coronavirus (CoV), aiming at different stages of the virus entry into the host cell. The spike (S) protein of the coronavirus, responsible for its entry into the cell, is divided into two portions: the S1 subunit, which has binding sites to the SARS-CoV cell receptor, angiotensin-converting enzyme 2 (ACE2), and the S2 subunit, responsible for the conformational changes that result in the fusion of the membrane and viral envelope. Ho et al. [36] obtained 14 small 12-residue peptides derived from S protein to assess their ability to inhibit the binding between S protein and the ACE2 receptor. The best result was obtained for the peptide SP-10 (STSQKSIVAYTM), with a secondary structure of β-sheet and half-maximal inhibitory concentration (IC50) of (1.88 ± 0.52) nM. This peptide also effectively blocked SARS-CoV S protein in Vero E6 cells in vitro. In turn, Yuan et al. [37] focused on blocking the SARS-CoV conformational change using a six-helix bundle peptide, inhibiting the viral fusion. Because the S2 subunit is composed of two regions of heptad repeats, HR1 and HR2, the authors developed 25 peptides that overlap these regions and can bind to HR1 or HR2, exposed after virus binding to the receptor, and block the conformational change of the virus. The HR1–1 peptide (NGIGVTQNVLYENQKQIANQFNKAISQIQESLTTTSTA), located in the HR1 region, and HR2–18 peptide (IQKEIDRLNEVAKNLNESLIDLQELGK), in the HR2 region, were the only ones that presented antiviral activity, with the 50% effective concentration (EC50) of 3.68 and 5.22 µM, respectively, according to in vitro assays with the wild-type SARS-CoV.
Like coronaviruses, several other viruses have an envelope in their structure and enter the host cell by membrane fusion. This mechanism applies to the smallpox virus, influenza virus, HIV, flavivirus (dengue, ebola, Zika), and arbovirus. Developing ways to inhibit typical stages of these viruses' life cycle may be more effective than focusing on individual viruses or specific target proteins, making it possible to obtain broad-spectrum antivirals [38]. In the human immunodeficiency virus type 1 (HIV-1) case, the envelope is formed by the glycoproteins gp160. This unit is divided into the gp120 subunit, responsible for binding to the CD4 receptor, and the gp41 subunit, which undergoes a conformational change, fusing the cell membrane. Conventional HIV-1 drugs act by inhibiting one of the two viral-specific enzymes (reverse transcriptase or protease). This feature brings disadvantages such as patient intolerance or the development of virological failure due to incomplete viral suppression, or the emergence of resistant virus strains. Kilby et al. [39] demonstrated that T20, a synthetic peptide with 36 amino acids, can inhibit the conformational changes of gp41, both in vitro and in vivo. HIV-1 disease was the first to have an approved commercial peptide antiviral from T20, enfuvirtide (Fuzeon™), in 2003 [40].
Another enveloped virus, the Influenza virus, has been widely studied to obtain peptides that can be used for the treatment or prophylaxis. Still, under the clinical trial, flufirvitide (FF-3) is a 16-amino acid peptide derived from one hemagglutinin subunit (VEDTKIDLWSYNAELL), the envelope glycoprotein responsible for the Influenza virus entry into the cell. Unlike the peptides presented above, FF-3 does not appear to interact with the subunit responsible for binding to the receptor or the one responsible for the cell membrane fusion. FF-3 interacts directly with the cell membrane and affects fusion indirectly [41]. A similar feature is observed for peptides against the Arenaviruses family, including several viruses that cause different diseases characterized by hemorrhagic fever (e.g., Lassa virus, Junin virus, Guanarito virus, and Sabia virus). The study used a model arenavirus, the Pichinde virus, to obtain the peptide AVP-p (LNLFKKTINGLISDSLVIR) derived from the glycoprotein envelope's expression region (GP2). The IC50 was 7.0 µM with complete inhibition of viral plaque formation at about 20 µM. The associated mechanism for this peptide involves membrane destabilization, triggered by fusion protein binding and endocytosis [42]. Table 1 lists several peptide drugs under clinical studies against enveloped viruses and the associated peptide action mechanism.
Table 1.
Peptide drugs under preclinical or clinical studies against enveloped viruses.
| Peptide drug | Virus pathogen | Mechanism of action | Current clinical phase | Reference |
|---|---|---|---|---|
| Hepalatide | HBV | Surface antigen (HbsAg) blockers | Phase II clinical trial | Chaudhuri et al. [43] |
| IM862 / SCV-07 | HCV | Immune stimulation on Th1 cells | Phase II clinical trial | Jenssen [44] |
| Adaptavir | HIV | Entry inhibition | Phase II clinical trial | Stanley and Yamamoto [45] |
| HBV | ||||
| Aviptadil | SARS-CoV 2 | Interleukin-6 inhibition | Phase III clinical trial | Yamamoto et al. [46] |
| Sifuvirtide | HIV | Fusion inhibition | Phase III clinical trial | Cao et al. [47] |
| Zadaxin (Thymosin α 1) | HBV | Immune response stimulation | FDA approved | Naylor [48] |
| HCV | ||||
| Boceprevir / Telaprevir | HCV | Protease inhibition | FDA/EMA approved | Schinazi et al. [49] |
| Hepcludex ™ (bulevirtide) | HDV | Entry inhibition | FDA/EMA approved | Kang and Syed [50] |
*HDV: Hepatitis D virus; HBV: Hepatitis B virus; SARS-CoV 2: coronavirus of severe acute respiratory syndrome 2; FDA: The United States Food and Drug Administration; EMA: European Medicines Agency
The use of peptides against non-enveloped viruses is limited, possibly because most AMPs act during the viral fusion to the cell membrane, exclusive of enveloped viruses. Few studies report the use of peptides as a therapy for diseases caused by non-enveloped viruses. Huang et al. [51] evaluated the effect of the Epinecidin-1 (Epi-1) peptide on the foot-mouth disease virus (FMDV), a non-enveloped virus belonging to the Picornaviridiae family. The authors reported an EC50 of (0.6 ± 0.1) µg/mL with remarkable inhibition of virus adsorption to host cells, moderate inhibition of the post-adsorption step, and little influence on the cells before contact with the virus. These findings indicate that Epi-1 can inactivate the viral particles or suppress their replication, reducing infection by FMDV. Another research group developed a 6-amino acid peptide (LVLQTM) for therapeutic use against two types of non-enveloped viruses: human rhinoviruses (HRVs) and human enterovirus 71 (EV-71). In both cases, the peptide inhibited the activity of protease 2 A, which plays several essential roles during viral replication, by coupling in the active site of this enzyme. The in vitro assay with A549 cells and the in vivo assay with mice demonstrated viral replication inhibition [52], [53]. Di Biase et al. [54] proposed the antiviral use of the lactoferricin peptide (bLfcin), composed of 25 amino acids, derived from bovine lactoferrin protein (bLf), which has already presented antiviral results against different enveloped viruses (HSV types 1 and 2, HIV, HCV, among others). The authors evaluated the effect of bLfcin against Adenoviruses and found that this peptide can inhibit the antigen synthesis of the virus by binding to negatively charged cell glycosaminoglycans. The antiviral action of bLfcin occurs by competing with viruses for these binding sites on the cell surface.
Despite the extensive development of antiviral drugs in recent decades, the investigation of new antiviral compounds is of interest to prevent and control the spread of viral diseases or even contribute to designing more effective vaccines. The ongoing research is necessary because even viral diseases that do not have specific treatment, such as measles, polio, and chickenpox, have already vaccines developed and widely used for their prevention. In this context, peptide-based vaccines may be an alternative to prevent other viral diseases. Namvar et al. [55] proposed a peptide-based vaccine to treat existing infections caused by human papillomavirus (HPV), which in the most dangerous types (HPV types 16, 18, 31, and 45) are associated with cervical cancer cases in women. The vaccines currently used for HPV have a prophylactic role; that is, the vaccine is ineffective in patients who already have the virus, which can take 10–20 years to manifest. The authors proposed obtaining peptides based on the early proteins E5 and E7 of HPV, which play an essential role in the cell cycle, replication, and transcription of the viral genome. This strategy enables stimulating the immune system against cancer development associated with high-risk types of HPV. The authors found that the E5-type 18 (SPATAFTVYVFCFLL) and E7-type 45 (TLQEIVLHLEPQNELDPVDLL) peptides presented the best results for inducing the production of IFN-γ, an essential cytokine in intracellular immunity against HPV infection.
Immunoinformatic approaches have also been used to determine potential vaccine peptides against viruses. Khan et al. [56] sought to determine suitable peptides for protective vaccination against the Ebola virus (EBOV), an enveloped virus of the Filoviridae family, responsible for causing fatal hemorrhagic fevers in humans and non-human primates, with a mortality rate that can reach up to 90%. Prasasty et al. [57], in turn, intended to establish potential peptides for vaccines against the Zika virus (ZIKV), an also enveloped virus in the Flaviviridae family, and that can cause Zika disease, fever, and other severe problems, such as microcephaly, and Guillain-Barré syndrome. Both studies reported the identification of peptides that can target both T cells, due to the high effectiveness of CD8 + T cells in producing cellular immune responses, and B cells, responsible for the humoral immune response. Atsmon et al. [58] also presented the results of a clinical trial in humans of the peptide-based vaccine Multimeric-001, developed to protect against Influenza A and B virus strains, regardless of mutations. The success of such a vaccine can completely change the influenza vaccination scenario, either by reducing the need for annual vaccinations or improving the immune response in more vulnerable people. The authors describe that the vaccine is well-tolerated, without significant side effects, and that although it does not contain any influenza viruses or viral proteins, the vaccine can induce specific immunity, mediated by the complement mechanism. Firbas et al. [59] also presented the clinical trial results for a peptide-based vaccine (IC41) against HCV. The treatment was also well-tolerated with reports of minor local reactions. Immunogenicity has been proven for groups that received different doses of IC41, with the highest rates of immune response and greater robustness obtained for the groups with the highest doses and vaccines.
Traditional therapies or vaccines present disadvantages that make peptides a critical factor in treating and preventing viral diseases. Conventional viral drugs are rare and, when they exist, can present limited efficacy or cause serious side effects. Regarding vaccines, the use of the attenuated or inactivated pathogen itself can result in additional safety concerns and processing costs, as well as social resistance against vaccination. The constant genetic mutations of a virus can also reduce the efficiency of vaccines worldwide. Overall, peptides make it possible to eliminate such limitations by promoting a more effective, specific, and long-lasting immune response, with less cost and side effects [56].
Besides the application of peptides as agents for therapies and vaccines, there is also room for using them as antimicrobial, including virucidal agents.
4. Antimicrobial activity of peptides
AMPs are amphiphilic compounds that are positively ionizable and can be isolated from several eukaryotic organisms, including insects, plants, amphibians, mammals, fish, and birds. These peptides are produced as part of organisms' first line of defense constitutively or induced by infectious stimuli. AMPs can bind to microbes through non-specific interactions with lipid membranes, damaging or destabilizing them and promoting rapid inactivation with less chance of developing resistance [60], [61].
Regarding their molecular conformation, AMPs are usually divided according to their structure into α-helix, β-sheet, unstructured (i.e., those that do not present any spatial conformation), or cyclic peptides. The class of α-helix AMPs is one of the most studied. These peptides usually present alanine, leucine, and lysine residues that stabilize the α-helix, which have random conformation in aqueous solutions and helical conformation after contact with cell membranes. β-sheet AMPs present in their structure proline, glycine, tryptophan, arginine, or histidine with about two to ten cysteine residues forming up to five disulfide bridges. Unstructured AMPs are flexible or extended, generally linear, and composed of rare amino acids. Cyclic peptides acquire this structure, in general, by forming one or more disulfide bonds [62], leading to high resistance against different microorganisms, such as bacteria, viruses, fungi, and protozoa. Table 2 presents a collection of studies that investigated the antimicrobial activity of AMPs against various microorganisms.
Table 2.
The activity of antimicrobial peptides against different microbe groups. AMPs were grouped according to their origin in nature (plants, animals, bacteria, fungi, others).
| Peptide | Peptide sequence | Type of microbe | Species | Inhibitory concentration | Reference |
|---|---|---|---|---|---|
| Plants | |||||
| DYDD | Asp-Tyr-Asp-Asp | Bacteria | E. coli | 18,075 µg/mL¹ | Liu et al. [63] |
| M. albicans | 4519 µg/mL¹ | ||||
| DDDY | Asp-Asp-Asp-Tyr | P. aeruginosa | 36,150 µg/mL¹ | ||
| CMC-based AMPMs | Not reported | Bacteria | S. aureus | 8500 µg/mL¹ | Zhang et al.[64] |
| Acacia catechu extract | Not reported | Virus |
Dengue virus (DENV) |
0.18 µg/mL | Panya et al.[65] |
| Leg1 | RIKTVTSFDLPALRFLKL | Bacteria | E. coli | 130 µg/mL¹ | Heymich et al.[66] |
| B. subtilis | 30 µg/mL¹ | ||||
| Leg2 | RIKTVTSFDLPALRWLKL | E. coli | 130 µg/mL¹ | ||
| B. subtilis | 30 µg/mL¹ | ||||
| Capsicum annuum L peptides | LRDSSNSKPASDAAP-QHVTLTSNR | Fungi | F. lateritium | – | Dos Santos et al.[67] |
| F. oxysporum | – | ||||
| F. solani | – | ||||
| Kalata B1 | Not reported | Virus | Human immunodeficiency virus (HIV) | – | Nawae et al.[68] |
| Yodha | SMLLLFFLGTISLSLCQ-DDQERC | Virus | Zika virus (ZIKV) | 0.052 µg/mL² | Lee et al. [69] |
| Animals | |||||
| Ovotransferrin-p12 | AGLAPYKLKPIA | Bacteria | E. coli | 32 µg/mL¹ | Ma et al. [70] |
| S. aureus | 32 µg/mL¹ | ||||
| Mouse cathelicidin | ISRLAGLLRKGGEKIGEKLK-KIGQKIKNFFQKLVPQPE | Virus | Enterovirus 71 (EV71) | – | Yu et al. [71] |
| Nk-lysin | EGVKSKLNIVCNEIGLLKSL-CRKFVNSHIW | Protozoa | P. dicentrarchi | – | Lama et al. [72] |
| Virus | Spring viremia of carp virus (SVCV) | 24.69 µg/mL² | Falco et al. [73] |
||
| Thanatin(S) | GSKKPVPIIYCNRRSGKC-QRM | Fungi | G. cichoracearum | – | Wu et al. [74] |
| Urumin | IPLRGAFINGRWDSQCHRF-SNGAIACA | Virus | H1 hemagglutinin (HA)-bearing human influenza A virus | 11.25 µg/mL² | Holthausen et al.[75] |
| Melittin | NH2-GIGAVLKVLTTGLPAL-ISWIKRKRQQ-CONH2 | Bacteria | S. enteritidis | 32 µg/mL¹ | Moridi et al.[76] |
| Bac8c | RIWVIWRR-NH2 | Fungi | C. albicans | 7.46 µg/mL ¹ | Lee and Lee [77] |
| M. furfur | 29.59 µg/mL ¹ | ||||
| Bacteria | |||||
| Gramicidin A | VGALAVVVWLWLWLW | Bacteria | E. coli | > 128 µg/mL¹ | Meikle et al.[78] |
| S. aureus | > 128 µg/mL¹ | ||||
| Fengycin | Not reported | Fungi | R. variabilis | 6.59 µg/mL¹ | Kulimushi et al.[79] |
| Surfactin analog (SLP5) | Palmityl-L-Glu-L-Val-D-Leu-L-Asp-D-Leu | Virus | Porcine epidemic diarrhea virus (PEDV) | – | Yuan et al.[80] |
| Actinonin | Not reported | Bacteria | S. Typhimurium | 0.768 µg/mL¹ | Jung et al.[81] |
| V. vulnificus | 0.192 µg/mL¹ | ||||
| Fungi | |||||
| Alamethicin | UPUAUAQUVUGLUP-VUUEQFol | Bacteria | E. coli | > 128 µg/mL¹ | Meikle et al.[78] |
| S. aureus | 32 µg/mL¹ | ||||
| SU2 | KREHGQHCEF | Virus | HIV | 50 µg/mL² | Wang et al.[82] |
| Others | |||||
| TGH1 | TWEMVSGKKKNGVVLMIK | Bacteria | V. parahaemolyticus | 12.5 µg/mL¹ | Yang et al.[83] |
| EC1–17KV | GWWRRTVKKVRNAVRKV | Fungi | C. albicans | 64 µg/mL¹ | Ma L et al.[84] |
| C. neoformans | 16 µg/mL¹ | ||||
¹MIC: Minimal inhibitory concentration; ²IC50: Half-maximal inhibitory concentration
As previously mentioned, the antimicrobial property of peptides can be used to prevent the proliferation of unwanted microorganisms in food to improve shelf-life. Um et al. [85] developed food-grade gels based on anionic biopolymers (alginate and pectin) in order to enrich cationic peptides from milk hydrolysates to improve the antimicrobial activity of these peptides. As a result, 60:40 pectin:alginate mixtures loaded with peptides present antimicrobial effects against eight types of bacterial strains, whereas the unfractionated hydrolyzate had an effect against only one of them. Velivelli et al. [86] also explored the antimicrobial activity of peptides formulations sprayed in crops to prevent gray mold disease caused by the fungus Botrytis cinerea. The authors found that both tomato and tobacco plants treated with NCR044, a peptide obtained from the legume Medicago truncatula, significantly reduced the gray mold disease in the crops and their post-harvest products.
Several biomedical applications also explore the antimicrobial properties of different peptides. Bacitracin is an example of a commercially available peptide produced by Bacillus subtilis and is known for its antibiotic effect. Chan et al. [87] evaluated the topical use of peptide-based formulations on surgical skin incisions after cardiac surgery as a prophylactic measure to prevent sternal wound infections, considered one of the most severe issues of this medical procedure. Sousa et al. [88] assessed the antimicrobial activity and the immunomodulatory potential of synthetic peptides, both pure and associated with the ciprofloxacin antibiotic, aiming at the application in revascularization of the dental pulp, a therapy used in the trauma of immature permanent teeth. The developed AMPs were efficient against Staphylococcus aureus and Enterococcus faecalis bacteria strains, commonly associated with dental infections, especially the peptide IDR-1002, which presented in conjunction with ciprofloxacin promising immunomodulatory results. He et al. [89] also studied the anticancer cell activity of the LHH1 peptide (AFALIAGALYRIFHRR), obtained from the genome of bacteria Lactobacillus casei HZ1. The authors found that LHH1, an AMP highly effective against Gram-positive bacteria, presented high anticancer activity. Although its inhibitory effect against cancer cells C666–1, MGC803, and HCT116 (IC50 = 18.27, 31.55, and 40.83 μM, respectively) is lower than those obtained for the positive study control, melittin (IC50 = 9.62, 7.08 and 12.17 μM, respectively), the hemolytic and cytotoxic properties are significantly superior for AMPs, both essential features for the safe use of LHH1 as a therapeutic agent. Additional clinical applications of AMPs involve their use in vaccines to prevent diseases that plague the world population, mainly due to the safety in the production process, good immune target, and few side effects. Huang et al. [90] reported the use of the AMP epinecidin (Epi-1) in a vaccine against the Japanese encephalitis virus (JEV). Epi-1 was administered alone and co-injected with inactivated JEV, resulting in modulation of the immune response in both cases and superior to the traditional formalin-inactivated JEV vaccine. Li et al. [91] also developed a vaccine from a mixture of synthetic AMP, KLKL5KLK, and combined DNA, aiming at immunization against Mycobacterium tuberculosis. The mixed vaccine resulted in greater protection than the combined DNA or the BCG vaccines (Bacillus Calmette Guérin), indicating that the AMP used is an excellent adjuvant capable of improving and prolonging the immune response against M. tuberculosis.
The antimicrobial action of peptides is an ongoing subject and has already been evaluated by several authors for different types of microbes. For example, AMPs, which tend to form amphipathic structures, can interact with the cell wall of bacteria and fungi, destroying their structure. After the cell wall, these peptides can bind to the phospholipid bilayer of microbial membranes by electrostatic interactions. In contrast, the hydrophobic terminals of AMPs interact with the hydrophobic core of the bilayer membrane, changing their structure and causing a loss of membrane integrity. Also, AMPs hydrophilic terminals connect with each other, forming channels through which intracellular material leaks and, consequently, causes cell death [63], [84]. Cell membrane integrity tests are performed with propidium iodide (PI), a fluorescent dye that binds to DNA but cannot enter living cells. When the cell membrane is compromised, PI can access the cell’s inner structure, emitting fluorescence [70].
The literature also reports the antimicrobial activity of plant-derived AMPs. Liu et al. [63] evaluated the mechanism of antibacterial action of two types of peptides extracted from the orchid variety Dendrobium aphyllum. The peptides Asp-Tyr-Asp-Asp (DYDD) and Asp-Asp-Asp-Tyr (DDDY) with the same amino acid composition but different sequences were evaluated against Escherichia coli, Pseudomonas aeruginosa, and Monilia albicans. Fluorescence tests indicate that both DYDD and DDDY damage the outer membranes of these bacteria, with different levels of membrane permeability according to the species. DYDD is also effective against E. coli membranes, whereas DDDY protects from P. aeruginosa and M. albicans. The same trend is observed for the cytoplasmic membrane; however, in this case, none of the peptides is effective against M. albicans. Another factor that helps to understand the bactericidal mechanism and the membrane permeability is the K+ ions, which are important to maintain the balance of the osmotic pressure of the cell and proteins. These ions only cross the membranes with the assistance of transmembrane transporters under normal circumstances. In the study, the bacteria strains tested presented increased leakage of both K+ ions and proteins, corroborating the damage to their membranes. The authors concluded that DYDD interacts through electrostatic forces with the cell membranes, forming pores that leak cell compounds, leading to cell death. DDDY, on the other hand, binds through van der Waals forces to membrane moieties and causes cell membrane fragmentation. Velivelli et al. [86] evaluated the antifungal mechanism of the peptide NCR044 (MQRVKNMTETLKFVYILILFIFIFLVLMVCDS), obtained from legumes of M. truncatula, on the plant fungal pathogen B. cinerea, since this material had already reported positive results against the human fungal pathogen Candida albicans. The authors observed the permeabilization of the plasma membrane in 30 min, with a peak reached in 120 min. However, although NCR044 does not cause sufficient permeabilization to promote cell lysis, spores resume their growth at the end of the peptide treatment, acting as a potent inhibitor of the fungus germination. The authors also evaluated reactive oxygen species (ROS) production by spores and germlings, leading to cell death due to apoptosis or necrosis. ROS production was detected only in germlings, indicating oxidative stress by ROS in these structures rather than in spores.
Animal-based peptides have also been investigated for antimicrobial applications. Ma et al. [70] evaluated the bactericidal action of an egg-derived peptide from the hydrolysate of ovotransferrin, called OVTp12. When treated with this peptide, the S. aureus strain reduces from 96% to 5.4% of cells after contact with twice the minimal inhibitory concentration. The authors also found that in just 60 min, the outer bacterial membrane had already been damaged, indicating a rapid effect of the peptide on the membrane integrity. In addition, the increase in fluorescence due to the concentration of OVTp12 suggests that the cytoplasmic membranes were also disrupted. Ma L et al. [84] also used several peptides from EeCentrocin 1, a component of the sea urchin Echinus esculentus. Besides visualizing the bactericidal effect in transmission electron microscopy analysis, indicating the presence of pores in the cell wall and membrane, leaks of cellular components, and loss of the bacterial structure of P. aeruginosa, the authors also evaluated the antimicrobial effect against C. albicans. As in the case of bacteria, the disruption of the cell membrane and an increase in its permeability were also observed in fungi strain, indicating the insertion of the peptide in the lipid bilayer. The presence of the peptides inhibited the germination of the blastopore and the formation of the germ tube of C. albicans, compromising the formation of hyphae by negatively regulating their gene expression. The alternation between yeasts, pseudohyphae, and hyphae was interrupted, reducing the virulence of the fungus.
Whilst most studies previously reported on bacteria and fungus present the contact and releasing killing mechanism from AMP action on their outer membranes, some specific peptides can act on a virus itself or its host, a fundamental piece for its replication. When the action of the peptides is on the virus, they can be considered virucidal since they have as their main target the proteins of these microorganisms. Moreover, the various steps in the virus life cycle can be the target of the AMPs virucidal action, such as the virus’ entry, synthesis, or assembly, with emphasis on inhibiting entry as an excellent antiviral strategy [1]. Despite the different virucidal targets, it is challenging to relate the virucidal capacity of peptides to their secondary structures. As the mechanism involved in the virucidal action of the peptide is specific to each case, this has been the subject of studies by several authors [61] and remains a big field to be explored.
Panya et al. [65] obtained peptides from the extract of the medicinal plant Acacia catechu and evaluated their activity against the dengue virus (DENV). When contacting the peptides with DENV in the pretreatment, coinfection, and post-infection, the authors concluded that the virucidal action occurs predominantly in the early steps of viral entry. Although in the mentioned study the virucidal mechanism has not been elucidated, the same research group has previously obtained peptides specifically designed for the hydrophobic pocket of the DENV envelope I protein, a region that directly affects the entry of the virus into the host cells. By impairing the protein-protein interaction on the virus surface through peptides that mimic part of the protein's structure, the stability of the virus can be affected, inhibiting its entry into the host cell [92], [93].
Yuan et al. [80] developed ten lipopeptides analogous to surfactin, produced by the bacterium Bacillus subtilis, to evaluate the AMPs effect on the porcine epidemic diarrhea virus (PEDV). In a previous report, the research group established that surfactin's viral activity occurs by inhibiting the fusion between viral and cell membranes, a fundamental step for enveloped viruses to invade host cells, but with limited application due to high surfactin cytotoxicity [94]. Among the compounds developed, SLP5 was the most promising surfactin analog, with equivalent antiviral effect and a substantial reduction in hemolytic activity. Compared to surfactin, SLP5 has a linear structure, one additional carboxyl group in the C-terminal of the peptide, and two fewer hydrophobic amino acids, which reduces the cost of synthesis and has little effect on antiviral activity.
Another study involves antiviral peptides derived from L. vannamei hemocyanin against the white spot syndrome virus (WSSV), a pathogen that significantly affects several shrimp species. The animals were challenged with WSSV and, by contact, five peptides derived from hemocyanin were identified in the shrimp sera, emphasizing LvHcL48, documented as the most sensitive and most strongly expressed under WSSV infection. In vitro and in vivo assays indicate that the presence of LvHcL48 can attenuate WSSV gene transcription and inhibit its replication through direct interaction with the virus' abundant envelope protein, VP28, disrupting intact viruses and, consequently, mitigating its replication [95].
Falco et al. [73] also verified the influence of the membrane fusion step on the virucidal activity of NK-lysine peptides (Nkl71–100) obtained from turbot (Scophthalmus maximus) against spring viremia of carp virus (SVCV). The authors evaluated the interaction between Nkl71–100 and phosphatidylserine (PS), a compound found in membranes, directly linked to the virus-cell interaction. The biophysical characterization demonstrated that Nkl71–100 enters the PS membrane bilayer, leading to cell leakage. Tests with SVCV indicated that the peptide interacts with PS, inhibiting viral binding in host cells and fusing viral and cell membranes. Because PS is essential for replicating several viruses, Nkl71–100 can find many antiviral applications. Holthausen et al. [75] studied the action of the frog peptide urumin against H1 hemagglutinin-bearing human influenza A virus (H1 HA), demonstrating the high specificity of urumin for a protein on the H1 HA cell surface. Antiviral peptides act on influenza viruses in one of three ways: (i) interference with viral polymerase and viral replication inhibition; (ii) rupture of the membrane by electrostatic interactions; (iii) and interaction with hemagglutinin (HA) to block viral fusion and entry. In this case, urumin does not follow any of these expected mechanisms, confirming its specificity when the peptide binds to the conserved stalk region of the H1 HA, leading to viral destruction through the combination of conformational changes in HA and electrostatic forces on the membrane. Another frog-derived peptide, Yodha, was evaluated against the Zika virus (ZIKV) by Lee et al. [69]. This peptide inhibits ZIKV and prevents its entry into host cells due to the physical disruption of viral particles by contact with the peptide, as observed with urumin. However, different from the specificity observed in that report, Yodha, in its different enantiomeric forms, is effective against the ZIKV and DENV strains. The disruption of the viral particles occurred by inserting the peptide into the viral lipid bilayer through the hydrophobic N-terminals.
Table 3 presents other recent studies that evaluated the virucidal mechanism of peptides according to their secondary structure, evidencing the difficulty of predicting the antimicrobial mechanism based on the molecular conformation. Regardless of the mechanism involved, several peptides can mitigate the proliferation of bacteria, fungi, and viruses to the greatest interest of this review. These findings indicate that several therapeutic or infection prevention applications can be explored based on AMPs.
Table 3.
The activity of AMPs against different microbe groups. AMPs were grouped according to their molecular structure.
| Peptide | Peptide sequence | Structure | Virus | Proposed virucide mechanism | Reference |
|---|---|---|---|---|---|
| C5A | SWLRDIWDWICEVLSDFK | α-helix | Hepatitis C virus (HCV) | Destabilize viral structural integrity and have viral membranolytic activity | Cheng et al. [96] |
| CPXV012 | QEGISRFKICPYHWYKQHMSLLFRRYYHKLDSII | α-helix | Enveloped viruses (poxviruses, HSV-1, hepatitis B virus, HIV-1, and Rift Valley fever virus) | Interact with phosphatidylserine in the viral envelope | Luteijn et al. [97] |
| CRAMP | ISRLAGLLRKGGEKIGEKLKKIGQKIKNFFQKLVPQPE | α-helix | EV71 | Downregulate the expression of viral receptors in the cells | Yu et al. [71] |
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | α-helix | EV71 | Inhibit viral binding to host | Yu et al. [71] |
| pFPs | GLFGAIAGFIKNGWKGMIKG | α-helix | Influenza A virus (IAV) | Inhibit the early step of viral entry | Wu et al. [98] |
| S-pep7B | Ace-VVTRRXLHR XFDTLA | α-helix | Herpes Simplex Virus - 1 (HSV-1) | Block processive DNA synthesis | Guan et al. [99] |
| Grifonin-1 | Cha-SC-Chg-R-Chg-RSGSY-Cha-DN-Chg-R-Chg-(D)Cys-CONH2² | β-sheet | HIV-1 | Inhibit viral entry step by binding to viral glycoproteins | Micewicz et al. [100] |
| Human β-defensin | GIGDPVTCLKSGAICHPVFCPRRYKQIGTCGLPGTKCCKKP | β-sheet | Middle East respiratory syndrome - coronavirus (MERS-CoV) | Stimulate antiviral innate and adaptive immune responses by enhanced delivery of fused antigens to APCs in vivo | Kim J et al. [101] |
| HIP | Check reference | β-sheet | HCV | Inhibit viral entry step by targeting cell receptor | Khachatoorian et al. [102] |
| CP11 | CGWIYWNV | Cyclic peptide | Hepatitis E virus (HEV) | Inhibit the interaction between viral protein and host cell, blocking virus release | Anang et al. [103] |
| HCV15R | CR-Nal-RV-(D)-P-Cha-HRYRC-CONH2 | Cyclic peptide | HCV | Inhibit viral entry step by targeting cell receptor | Khachatoorian et al. [102] |
| Cyclic D, L-α-peptides | Check reference | Cyclic peptide | HCV | Inhibit a post-binding step during viral entry into the target cell | Montero et al. [104] |
¹APC: Antigen-presenting cell; ² Cha: (L)-Cyclohexylalanine, Chg: (L)-Cyclohexyl-glycine, (D)Cys: (D)-Cysteine
The mechanisms for AMPs action against viruses are much more specific and dependent on the target, and hence, more challenging to extract a general rule such as a given sequence. In comparison, the studies for other pathogens such as bacteria and fungus show that most of the action for the latter is by disrupting the outer shell/membrane of these organisms, in a more contact or release killing mechanism. The information of action against viruses describes several mechanisms of action, from preventing the entrance of the virus into the host cells to turning the virus transcription process difficult. In this sense, the action of virucidal peptides can result from a much more specifically designed sequence, which may also turn the application specificity more robust.
The next session will explore the commonly used strategies and materials for producing virucide coatings before discussing the use of peptides as coating agents.
5. Strategies used for virucide coatings
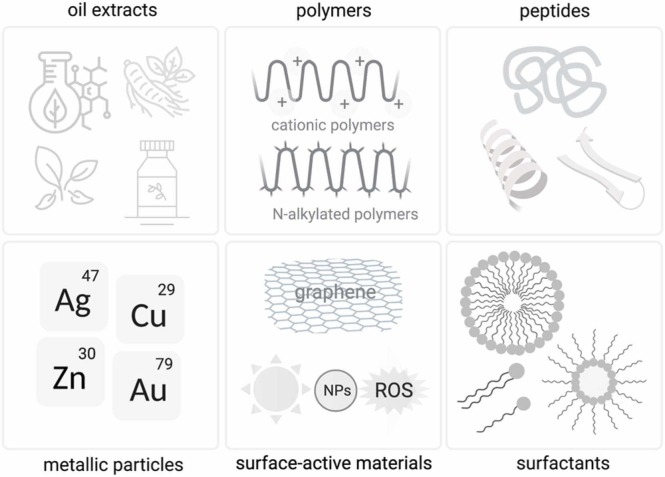
The SARS-CoV-2 pandemic evidenced the challenges in designing and implementing new drugs and vaccines to prevent viral outbreaks. Despite the several applications involving antibacterial materials, approaches to mitigate the virus spread outside the body have been little explored, mainly because of the reduced capacity of viral transmission by indirect contact. However, the high viability of SARS-CoV-2 on inanimate surfaces, which can last from 2 h to 9 days, reinforced the importance of eradicating their virions on surfaces, mainly using virucidal coatings [7]. Antiviral surfaces and coatings can be of natural or artificial origin, prepared by physical-chemical modification of surfaces, or even bioinspired, acting against viruses directly, indirectly, or by inactivating moieties on the surface of viral particles [108]. These materials include plant extracts, metallic ions/oxides, and polymers, as indicated in Fig. 2 [7], [105].
Fig. 2.
Materials used to produce antiviral coatings.
Pyankov et al. [106] evaluated the antiviral activity of natural oils extracted from Tea Trees and Eucalyptus against the influenza virus captured by the surface of conventional filters in heating, ventilation, and air conditioning systems. The authors found that both oils lead to antiviral activity under the conditions studied, contributing to filtration efficiency and reducing the risk of re-aerosolization of pathogens back into the ambient. Catel-Ferreira et al. [107] also used natural material, polyphenol (a secondary metabolite produced by plants), as an antiviral agent for coatings of commercial non-woven cellulose layers (Kimberly-Clark®). When applied to the commercial surgical mask, such as cellulose layers, these compounds work as efficient virucidal cleaning wipes and a better and more efficient antiviral filter, replacing the original inner layer. Natural compounds can be interesting virucidal agents because they are environmentally friendly, biodegradable, and present low to medium toxicity, enabling their use on public surfaces. These materials also have well-defined and relatively simple extraction methods, which facilitates their scaling. The major disadvantage of natural compounds is the low availability of certain plants, making their extracts more expensive [108].
Artificial coatings are the most studied materials for antiviral applications, covering the different materials mentioned above. Metallic and inorganic materials deserve to be highlighted because of their antimicrobial properties known since ancient times. Common metallic-based materials include copper, silver, zinc, and others less explored, such as gold [7]. The advantages of metallic compounds include superior stability, well-developed preparation technologies, and a controllable substrate/coating interface [108]. Copper action was evaluated by Hutasoit et al. [109]. The authors deposited a copper-based coating by spray to an in-use steel surface, widely used in access doors in public places. The coating was effective against SARS-CoV-2, inactivating up to 96% of the virus in 2 h. The coating performance was higher than the 49% inactivation obtained for the non-coating stainless steel. This formulation can be applied to any existing steel surface, eliminating the need to prepare copper surfaces. Silver, copper oxide (CuO), and zinc oxide (ZnO) nanoparticle coatings were compared using substrates like glass, a flat solid, and porous filter media such as FFP3 filter and fiberglass filter. Particle-based coatings were applied using the flame aerosol deposition technique. The best result against SARS-CoV-2 was obtained for the silver coatings, with a 98% reduction in viral load in 2 h, followed by copper with 76% reduction, and zinc with little significant action on the stability of the virus [110]. The major drawbacks of metallic-based materials are the high costs for some metals and the environmental and health issues associated with their application and disposal [108].
Surface-reactive materials can exert direct action on viruses, such as the metal ions mentioned above, or indirect action, in which free radicals are generated when in contact with moisture or light, capable of inactivating such microorganisms. For example, TiO2 reacts with moisture in visible light to form hydrogen peroxide, a potent antimicrobial agent [108]. Park et al. [111] evaluated the efficiency of flat glass surfaces coated with fluorinated TiO2 against human noroviruses and other surrogates (bacteriophage MS2, feline calicivirus, and murine norovirus). The authors found that oxidative damage can reduce the viral load in all cases studied. The fluorination of TiO2 accelerated the release of hydroxyl radicals from the surface, increasing the exposure of viruses to oxidizing agents and eliminating them quickly. This material also promotes singlet oxygen production, which reacts with proteins, effectively inactivating the viruses. Graphene was also evaluated as an antiviral coating for polypropylene mask air filters. Laboratory tests simulated the contact of people wearing a 3-layer mask (middle layer with functionalized graphene) with aerosol droplets from the speech/cough/sneeze of other people with high, moderate, low, or null viral loads of SARS-CoV-2. The results indicated that the viral RNA titers in the incident spray loaded with virus and in the outer layer of the mask filter were similar and high, whereas the middle layer (with functionalized graphene) and the inner layer were null. These results illustrate the efficacy of graphene in mitigating viral transmission [112]. Zhong et al. [113] recently also reported the development of nonwoven masks containing a graphene layer that presents self-cleaning properties. Besides creating a superhydrophobic surface, the authors report that the few-graphene layer also presents a photothermal response when exposed to sunlight which can inactivate viral particles and offer additional protection against pathogens.
Polymeric and organic materials are also used in antiviral coatings. Several polymeric coatings take advantage of the positive charge in the polycations to attract the negative charge inherent to viral particles, interacting with their genetic material or protein structure to inactivate them [7]. N-alkylated polyethylenimines (N-alkylated PEIs) comprise a class of polycations widely used against the most diverse types of viruses. N,N-dodecyl methyl polyethylenimines present excellent antiviral capacity as a coating on glass or polyethylene slides against influenza virus [114], [115], poliovirus, and rotavirus [116], HSV-1 and HSV-2 [117] and HIV [118]. N, N-dodecyl methyl polyurethane is another polycation evaluated for the same purpose. This material was applied as a coating on polyethylene slides and tested against influenza virus (enveloped) and poliovirus (non-enveloped), inactivating only the enveloped virus. These findings suggest that the elimination of polioviruses by N-alkylated PEIs occurs through an adsorptive disinfection mechanism, without involving the interaction with the viruses and consequent inactivation, as occurs with enveloped influenza virus. In the case of the polyurethane derivative, polioviruses cannot chemically interact, maintaining their viral activity [119].
Surfactants have also been studied as components of antiviral coatings. Cano-Vicent et al. [120] developed face masks made of polymeric non-woven fabrics coated with solidified hand soap, aiming to take advantage of the disinfection property of this compound commonly used with water. The product developed is efficient against SARS-CoV-2, eliminating 100% of the viruses after 1 min of contact. No cytotoxic effects were reported when the product was used as an inner layer of a 5-layer mask. Colnago et al. [121] used a widely available dishwasher detergent to produce films that can be applied to inanimate surfaces to promote a virucidal action. Tests against avian coronavirus on plastic surfaces indicated that this coating mitigates the viral activity on treated surfaces compared to untreated ones, remaining stable and active for hours or days. For both the hand soap and the dishwashing detergent, the virucidal action is associated with the presence of surfactants capable of denaturing both the envelope and the viral capsid proteins.
Given the above, the development of antimicrobial and, more specifically, antiviral coatings have driven a substantial interest in the academic setting. Advances in this topic can reduce pathogen dissemination. However, the need to meet environmental and safety criteria and create materials that become globally available during an infectious disease outbreak reinforces the importance of investigating the performance of antiviral materials, including promising yet little explored AMPs.
6. Use of peptides in active coatings for biomedical devices
Surfaces capable of controlling cellular responses, such as cell proliferation, dissemination, differentiation, and attachment, are of interest for biomedical applications. Such control is necessary for implantable devices, biosensors, cell culture tools, drug delivery systems, as well as regenerative medicine [122]. The use of peptides as coating agents in biomedical devices has gained increased attention in materials science. Peptides are generally immobilized on the substrate surface by physical or chemical methods. Physical methods include the direct adsorption of peptides on the device's surface or the self-assembly deposition by the layer-by-layer (LbL) technique. Functional films are deposited on the substrate by alternate adsorption of polycations and polyanion species. Another approach is the incorporation of peptides onto a material, such as a polymer. Chemically, the immobilization of peptides can occur via covalent bonds between the substrate and the peptides [123].
Several studies report using devices coated with peptides to target applications that include cell adhesion, antimicrobial activity, and selective attachment of peptides onto cells. Mohanty et al. [124] developed peptide-coated nanoparticles to improve their diffusive transport through solid tumors for therapeutic applications. The synthetic peptide P4, with CKPGDGGPC sequence, improves the transport (more rapid) and the uptake of nanoparticles in tumors in in vitro tests, compared to both the negative control and other traditional coatings, such as poly(ethylene) glycol (PEG).
Peptide cell adhesion is also relevant for designing biomedical implants, such as dental and medical applications. The attachment of cells to the surface of the materials usually occurs by the adsorption of cell proteins on the device surface, which start to dictate the superficial characteristics of the material. Functional coatings can lead to specific cell responses to control the interactions between the cells and the implant, as is the case of peptides [122]. Clauder et al. [125] were concerned with the insufficient endothelization of cardiovascular devices after surgical interventions to implant prosthetic valves or stents. Recent studies have evaluated the use of biopolymers, such as polycaprolactone (PCL), to prepare these devices due to their biodegradable features. However, their use may be limited by their low capacity for cell adhesion, which can lead to thrombosis or intimal hyperplasia, leading to implant failure. The use of peptide coatings on the surface can improve cell-surface interaction, serving as a scaffold and a cell activation modulator. In this context, the authors developed a mussel-based peptide coating surface with integrin and proteoglycan binding sites to improve endothelization. This coating led to enhanced cell adhesion compared to other materials, such as hydrophilized PCL-co-lactide, glass, and polystyrene, in addition to the survival of endothelial cells in static and fluidic conditions.
The application of peptide coatings on surfaces was also evaluated in TiO2 implants, widely used due to their biocompatibility, corrosion resistance, and the ability to anchor proteins. The success of the bone implant is directly linked to its interaction with cell membranes, which can be improved, for example, by adding peptides that can facilitate cell adhesion. The peptide DapSer, with the sequence PSFSWGGGQQQQQ, presents a high affinity to TiO2, spontaneously adsorbing to the implant surface. In vivo tests demonstrated that uncoated implants had cracks and deformations, cracked bone matrix around the implant, and osseointegration affected by a narrow gap. Coated implants had the opposite behavior, probably due to the high focal adhesion of cells on the peptide coating, stimulating contact osteogenesis [126].
Neuroprosthetic biosensors, that is, implants with electrodes that can replace certain nervous functions, are another biomedical device that demands high levels of surface specificity. The laminin-derived peptide, CAS-IKVAV-S, was used to coat polyimide electrodes functionalized with vinyl or amino groups. In general, polyimide electrodes induce damage to neural tissues and consequent inflammation and immune system reactions, encapsulating or impairing axonal regrowth. Both coated electrodes presented morphological and chemical changes that favor neuronal adhesion, reducing the contamination of fibroblasts on the substrates [127].
The immobilization of peptides in dental implants was explored by Fischer et al. [128], aiming to prevent peri-implantitis, a disease that affects about 20% of implanted patients. Another laminin-derived peptide, LamLG3, induced the formation of a hemidesmosome, a transmembrane link between the teeth and the gingiva that prolongs the implant durability by leading to better healing of the soft tissues around the biomaterial. Chemical immobilization techniques were evaluated, with superior biological effects for the non-chemoselective (silanization) than the chemoselective technique (click chemistry).
In addition to biocompatibility, cell adhesion, and good healing properties, biomedical devices must not cause the onset of infections in the patient. Thus, apart from cell adhesion, functional peptide coatings can be designed to promote antimicrobial protection by inactivating or destroying pathogenic microorganisms, as described in the previous section. Table 4 presents studies involving AMPs-coated material and the antimicrobial activity related to specific pathogens.
Table 4.
Antimicrobial peptides applied in functional coatings against different microbe groups and their antimicrobial activities.
| Peptide | Material | Antimicrobial activity | Pathogen | Reference |
|---|---|---|---|---|
| Tet-124-G-BrPh-DOPA-G | Polystyrene | Partial depletion of bacteria growth due to cell membrane disruption | E. coli | Corrales-Ureña et al. [129] |
| Tet-124-G-BrPh-G | ||||
| KLR | Polystyrene | 100% bacterial killing | E. coli | Majhi et al. [130] |
| S. aureus | ||||
| Chain201D | EG4 SAMs previously activated by CDI | Binding and Killing |
E. coli | Monteiro et al. [131] |
| Contact killing | S. aureus | |||
| KYE28 | Poly(ethyl acrylate-co-methacrylic acid) | Contact killing | E. coli | Nyström et al. [132] |
| KRWWKWWR | Silicone | Growth inhibition | E. coli | Low et al. [133] |
| S. aureus | ||||
| P. aeruginosa | ||||
| Melittin | Titanium | Disruption of cell wall | MRSA | Zarghami et al. [134] |
| VRSA | ||||
| Nisin Z | HDPE | Inhibition of bacteria, adhesion, and biocidal property | S. epidermidis | Paris et al. [135] |
| SHAP1-GS-Cys | Slide glass | > 96% killing activity | E. coli | Jeong et al. [136] |
| Latex glove | S. aureus | |||
| BrEK | Gold nanorods | Disrupt bacteria due to attachment to the cell membrane | MRSA | Sheng et al. [137] |
| GL13K | Collagen | Killing by contact | E. coli | Ye et al. [138] |
| S. gordonii | ||||
| 6mer-HNP1 | Spider silk | Inhibition of bacteria adhesion | E. coli | Franco et al. [139] |
| MRSA | ||||
| β-Peptide | Polyethylene | Reduction in biofilm growth | C. albicans | Raman et al. [140] |
| Indolicidin | Gold | Inhibition of biofilm formation and damage of preformed mature biofilms | C. albicans | Alteriis et al. [141] |
| Caspofungin | Titanium | Inhibition of adhesion and proliferation due to interaction with cell wall | C. albicans | Akhavan et al. [142] |
*MRSA: Methicillin-resistant S. aureus; VRSA: Vancomycin-resistant S. aureus; HDPE: High-density polyethylene
In general, the antimicrobial action of peptides present in coatings occurs by preventing the microbes from anchoring or killing the bacteria that attach to the surface [143]. One of the most tested materials in this context is titanium. Zhang et al. [144] designed vancomycin peptide coatings using the electrospinning technique. In vitro assays evidenced that this coating is not cytotoxic and can inhibit the growth of S. aureus bacteria. Besides preventing cell death in-vivo, vancomycin-coated implants also promote the formation of both normal muscle and new bone tissues, recruiting less inflammatory cells to the implant region. Gevrek et al. [143] reported the need for a priming layer on titanium implants before AMPs deposition. The authors applied a copolymer-based film with reactive thiol handles over the metallic implant, which can conjugate with E6 AMP (RRWRIVVIRVRRC) peptide derived from cathelicidin. These coatings attracted both Gram-negative (P. aeruginosa) and Gram-positive (S. aureus) bacteria, killing them by contact and inhibiting their adhesion to the surface. Buckholtz et al. [145] also verified the need to apply an alkene-thiol interfacial pre-layer for covalent immobilization of AMP Inverse-CysHHC10 in bioceramic composite of calcium aluminum oxide (CaAlO) and hydroxyapatite (HA). The authors observed increased cell permeability and cell death of E. coli bacteria by contact with the AMP functionalized surface and inhibiting bacteria growth in culture. Titanium-based biomaterials were also studied by Tan et al. [146] to prepare corneal implants. The authors described that the bactericidal action of immobilized AMP SESB2V ([(RGRKVVRR)2 K]2KK) against P. aeruginosa and S. aureus was 80 times greater than the soluble peptide. However, the action of SESB2V is more effective against S. aureus bacteria than P. aeruginosa, possibly because the latter can infiltrate different inflammatory cell tissues close to the implant. Still, the action on S. aureus is advantageous because this is one of the main bacteria that cause inflammation in ocular implants.
Besides many studies focused on using AMP-based coatings in medical implants, these materials are suitable for several other applications, including wall paints in healthcare settings, clinical and surgical materials, and bioactive barriers in personal protective equipment (PPEs). Despite the disinfection and sterilization procedures, the spread of microbes through these devices is prevalent, which becomes an issue in healthcare applications. Jeong et al. [136] developed a simple and cost-effective method to immobilize the AMP SHAP1 (APKAMKLLKKLLKLQKKGI) on glass slide surfaces and latex gloves, which are widely used in medical care. The solid supports were functionalized with poly (2,4,6,8-tetravinyl-2,4,6,8-tetramethyl cyclotetrasiloxane) by an initiated chemical vapor deposition (iCVD), followed by SHAP1 binding to the surface by a cysteine residue (Cys). The authors observed that the orientation of the peptide affects the antimicrobial activity surface, with the best results observed for SHAP1-GS-Cys, which were killed by contact with more than 96% of the E. coli and S. aureus strains.
Textile materials are also prone to the spread of microorganisms as textile fibers are formed by a rich content of nutrients that, under adequate conditions, lead to the spread of pathogens. Due to their broad application in clinical apparatus, textiles can become a key factor for disease transmission through contact with patients and clinical staff. AMPs deposition on textiles can be crucial for impairing the spread of a given disease [147]. Cotton is one of the most studied substrates for AMPs functionalization because of its broad application in medical textiles. Scapin et al. [148] evaluated the antibacterial activity of AMP-functionalized cotton fabrics after repeated washes and friction cycles. Three synthetic peptides analogous to H-Orn-Orn-W-W-NH2 were chemically bound to cotton, which effectively inhibits the growth of S. epidermidis bacteria. In addition, even after a 30 min treatment with UV light for sterilization, this peptide coating maintains its antimicrobial performance. Orlandin et al. [149] also studied the antimicrobial activity of cotton-peptide conjugates by producing peptides directly on the cotton substrate. The authors investigated the peptides bound to palmitic acid (Pal) with the sequence Pal-X-A-D-A-X, with X varying among K, H, and R. Under physiological conditions, these amino acids present positive residues, binding negatively charged bacterial membranes and causing cell death. Peptide variants containing the His demonstrate an antibacterial effect against S. aureus and E. coli. This formulation also leads to the best textile coloring results with a lower degree of yellowing and a higher degree of whiteness, which favors appropriate staining without undesired color changes.
Nogueira et al. [147] evaluated the conjugation of peptides in polypropylene (PP), a textile-non-textile commonly used in hospital garments. PP was grafted with L-cysteine (L-Cys), generating the compound PP-g-SH, subjected to electrospinning, and later cross-linked with the Cys-LC-LL-37 AMP. Tests with S. aureus and P. aeruginosa indicated that the cross-linked nanofibers led to a CFU reduction of 99.9999% and 97.41% for the two bacterial strains tested, even after 25 washing cycles. PP, PP-g-SH, and PP + Cys-LC-LL-37 samples presented low or no reduction in CFU. These findings demonstrate that the presence of thiol groups on the surface, associated with the L-Cys residue, does not exert significative bacteriostatic activity (PP-g-SH), but they are essential for AMP attachment and consequent bactericidal activity. Aside from the clinical setting, wool is another fabric that leads to the spread of microorganisms, resulting in fiber damage and skin irritation. Two AMPs were applied to the wool fibers to promote antimicrobial features: the natural peptide Cecropin-B, found in pupae of Hyalophora cecropia, and the synthetic peptide [Ala5] -Tritrp7, derived from tritrpticin. These peptides were properly immobilized on the wool fibers after 1-hour contact, and, even after five washing cycles, they remained attached to fibers. This functionalization does not cause fiber yellowing, a common issue in applying other antimicrobial agents. The antibacterial action against S. aureus and K. pneumoniae strains resulted in a bacteriostatic effect, but not bactericidal, with bacterial inhibitions ranging from 66.74% to 88.65% [150].
This section highlighted several studies involving the antibacterial activity of peptide coatings, especially in biomedical devices. Despite the advances in antimicrobial coatings, the lack of studies focused on the antiviral action of AMPs coatings, as well as the emergence of viral-related outbreaks, reinforces the importance of investigating AMPs coatings to prevent virus spread.
7. Future perspectives
The emergence of contagious diseases has increased the demand for therapeutics and vaccines, evidencing their inherent challenges, including costs and time. In the case of viral diseases, drugs do not eliminate the pathogens directly, only preventing them from spreading among the population. Since viral pathogens depend on the host's cell machinery to replicate, few targets remain available for the action of drugs in the viral replication cycle.
The use of antiviral surfaces and coatings differs slightly from those widely investigated for antibacterial use, especially due to the difference in size between these microorganisms, with viruses ranging from 20 to 300 nm, except for some filoviruses that reach up to 1400 nm, whereas bacteria reaching size in the scale of a few micrometers [108]. During the coronavirus pandemic, new scientific approaches have been described to mitigate coronavirus spread, providing insights to create advanced antimicrobial coatings. As an enveloped RNA virus, SARS-COV-2 is extremely sensitive to simple disinfection methods, having the viral membrane damaged or destroyed by contact with detergents and disinfectants or by the action of specific sterilization methods such as temperature and UV-light. Peptide-based materials that bind to viral particles are also suitable for preventing the pathogen's interaction with the host cells, preventing viral transmission. These methods can also be tested against other viruses and adapted to large-scale use, creating alternatives to prevent potential viral outbreaks.
In this review, several antiviral agents and coatings were presented. Despite their efficacy on viral inactivation on surfaces, most of these materials present relevant drawbacks, from safety and environmental issues to process scalability, limiting their application in everyday life. Most methods are not universal, and their effectiveness depends on the virus. Furthermore, prolonged contact time with the pathogen may be necessary to control the viral transmission [7]. AMPs, known for their adhesive, selective, and antimicrobial properties will be increasingly explored to prevent pathogens transmission. As shown in this review, the mechanisms for AMPs action against viruses are diverse and much more specific, dependent on the target, and hence, more challenging to extract a general rule such as a given sequence. The possibility of producing specifically designed amino acid sequences and their relatively affordable processing make these peptides suitable candidates for the design of advanced agents. Upcoming studies that apply high-throughput methods for preparing peptide-based materials can improve the understanding of how individual modifications on amino acid sequences might affect the virucidal properties of AMPs, creating on-demand virucidal agents.
CRediT authorship contribution statement
Emmanuelle Freitas: Investigation, Methodology, Writing – original draft, Supervision. Rogério Bataglioli: Conceptualization, Writing – review & editing. Josephine Oshodi: Investigation. Marisa Beppu: Project administration, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the Coordination for the Improvement of Higher Level Personnel - Brazil (CAPES) - Finance Code 001 and PNPD/FEQ, for supporting this project. Figures created with BioRender.com.
Data Availability
No data was used for the research described in the article.
References
- 1.Agarwal G., Gabrani R. Antiviral peptides: identification and validation. Int. J. Pept. Res. Ther. 2020;27:149–168. doi: 10.1007/s10989-020-10072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zamora-Ledezma C., Clavijo D.F.C., Medina E., Sinche F., Vispo N.S., Dahoumane S.A., Alexis F. Biomedical science to tackle the COVID-19 pandemic: current status and future perspectives. Molecules. 2020;25 doi: 10.3390/molecules25204620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellingson K.D., Pogreba-Brown K., Gerba C.P., Elliott S.P. Impact of a novel antimicrobial surface coating on health care-associated infections and environmental bioburden at 2 Urban Hospitals. Clin. Infect. Dis. 2020;71:1807–1813. doi: 10.1093/cid/ciz1077. [DOI] [PubMed] [Google Scholar]
- 4.MarketsandMarkets, Antimicrobial Coatings Market by Type (Silver, Copper, Titanium dioxide), Application (Medical & Healthcare, Foods & Beverages, Building & Construction, HVAC system, Protective Clothing, Transportation), & Region - Global Forecast to 2025, (2020). 〈https://www.marketsandmarkets.com/Market-Reports/antimicrobial-coatings-market-1297.html〉.
- 5.Antunes L., Faustino G., Mouro C., Vaz J., Gouveia I.C. Bioactive microsphere-based coating for biomedical-textiles with encapsulated antimicrobial peptides (AMPs) Cienc. Tecnol. Mater. 2014;26:118–125. doi: 10.1016/j.ctmat.2015.03.006. [DOI] [Google Scholar]
- 6.Barbosa M., Costa F., Monteiro C., Duarte F., Martins M.C.L., Gomes P. Antimicrobial coatings prepared from Dhvar-5-click-grafted chitosan powders. Acta Biomater. 2019;84:242–256. doi: 10.1016/j.actbio.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Imani S.M., Ladouceur L., Marshall T., Maclachlan R., Soleymani L., Didar T.F. Antimicrobial nanomaterials and coatings: current mechanisms and future perspectives to control the spread of viruses including SARS-CoV-2. ACS Nano. 2020;14:12341–12369. doi: 10.1021/acsnano.0c05937. [DOI] [PubMed] [Google Scholar]
- 8.Aguilar M.I., Purcell A.W. In: Encycl. Anal. Sci. Second ed. Worsfold P., Townshend A., Poole C., editors. Elsevier Ltd.; 2005. Peptides; pp. 29–36. [DOI] [Google Scholar]
- 9.Petrou C., Sarigiannis Y. Elsevier Ltd; 2018. Peptide Synthesis: Methods, Trends, and Challenges. [DOI] [Google Scholar]
- 10.Sánchez A., Vázquez A. Bioactive peptides: a review. Food Qual. Saf. 2017;1:29–46. doi: 10.1093/fqs/fyx006. [DOI] [Google Scholar]
- 11.Ganguly A., Sharma K., Majumder K. Elsevier Inc.; 2020. Peptides as Biopolymers-past, Present, and Future. [DOI] [Google Scholar]
- 12.Nasri H., Baradaran A., Shirzad H., Kopaei M.R. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014;5:1487–1499. [PMC free article] [PubMed] [Google Scholar]
- 13.Montiel-Aguilar L.J., Torres-Castillo J.A., Rodríguez-Servin R., López-Flores A.B., Aguirre-Arzola V.E., Méndez-Zamora G., Sinagawa-García S.R. Nutraceutical effects of bioactive peptides obtained from Pterophylla beltrani (Bolivar & Bolivar) protein isolates. J. Asia Pac. Entomol. 2020;23:756–761. doi: 10.1016/j.aspen.2020.06.006. [DOI] [Google Scholar]
- 14.Liu K., Du R., Chen F. Stability of the antioxidant peptide SeMet-Pro-Ser identified from selenized brown rice protein hydrolysates. Food Chem. 2020;319 doi: 10.1016/j.foodchem.2020.126540. [DOI] [PubMed] [Google Scholar]
- 15.Mitra M.S., DeMarco S., Holub B., Thiruneelakantapillai L., Thackaberry E.A. Development of peptide therapeutics: a nonclinical safety assessment perspective. Regul. Toxicol. Pharmacol. 2020;117 doi: 10.1016/j.yrtph.2020.104766. [DOI] [PubMed] [Google Scholar]
- 16.Berdan V.Y., Klauser P.C., Wang L. Covalent peptides and proteins for therapeutics. Bioorg. Med. Chem. 2021;29:1–6. doi: 10.1016/j.bmc.2020.115896. [DOI] [PubMed] [Google Scholar]
- 17.Nevagi R.J., Toth I., Skwarczynski M. In: Pept. Appl. Biomed. Biotechnol. Bioeng. Koutsopoulos S., editor. Elsevier Ltd; 2018. Peptide-based vaccines; pp. 327–358. [DOI] [Google Scholar]
- 18.Chauhan S., Kumar R., Khan N., Verma S., Sehgal R., Tripathi P.K., Farooq U. Designing peptide-based vaccine candidates for Plasmodium falciparum erythrocyte binding antigen 175. Biologicals. 2020;67:42–48. doi: 10.1016/j.biologicals.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Wang C., Li Y., Wang S., Yan X., Xiao J., Chen Y., Zheng K., Tan Y., Yu J., Lu C., Wu Y. Evaluation of a tandem Chlamydia psittaci Pgp3 multiepitope peptide vaccine against a pulmonary chlamydial challenge in mice. Microb. Pathog. 2020;147 doi: 10.1016/j.micpath.2020.104256. [DOI] [PubMed] [Google Scholar]
- 20.Deng X., Mai R., Zhang C., Yu D., Ren Y., Li G., Cheng B., Li L., Yu Z., Chen J. Discovery of novel cell-penetrating and tumor-targeting peptide-drug conjugate (PDC) for programmable delivery of paclitaxel and cancer treatment. Eur. J. Med. Chem. 2021;213 doi: 10.1016/j.ejmech.2020.113050. [DOI] [PubMed] [Google Scholar]
- 21.Chang C.H., Tsai I.C., Chiang C.J., Chao Y.P. A theranostic approach to breast cancer by a quantum dots- and magnetic nanoparticles-conjugated peptide. J. Taiwan Inst. Chem. Eng. 2019;97:88–95. doi: 10.1016/j.jtice.2019.02.013. [DOI] [Google Scholar]
- 22.Lu R.M., Chen M.S., Chang D.K., Chiu C.Y., Lin W.C., Yan S.L., Wang Y.P., Kuo Y.S., Yeh C.Y., Lo A., Wu H.C. Targeted drug delivery systems mediated by a novel peptide in breast cancer therapy and imaging. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang J., Wei Y., Yin H., Fang L., Chai D., Li H., Li H., Zhang Q., Zheng J. Modification of IL-24 by tumor penetrating peptide iRGD enhanced its antitumor efficacy against non-small cell lung cancer. Int. Immunopharmacol. 2019;70:125–134. doi: 10.1016/j.intimp.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 24.Chen M., Song Z., Han R., Li Y., Luo X. Low fouling electrochemical biosensors based on designed Y-shaped peptides with antifouling and recognizing branches for the detection of IgG in human serum. Biosens. Bioelectron. 2021;178 doi: 10.1016/j.bios.2021.113016. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen K.M., Kyneb M.H., Alstrup A.K.O., Jensen J.J., Bender D., Schønheyder H.C., Afzelius P., Nielsen O.L., Jensen S.B. 68Ga-labeled phage-display selected peptides as tracers for positron emission tomography imaging of Staphylococcus aureus biofilm-associated infections: Selection, radiolabelling and preliminary biological evaluation. Nucl. Med. Biol. 2016;43:593–605. doi: 10.1016/j.nucmedbio.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Rosestedt M., Velikyan I., Rosenström U., Estrada S., Åberg O., Weis J., Westerlund C., Ingvast S., Korsgren O., Nordeman P., Eriksson O. Radiolabelling and positron emission tomography imaging of a high-affinity peptide binder to collagen type 1. Nucl. Med. Biol. 2021;93:54–62. doi: 10.1016/j.nucmedbio.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Dilip H.N., Chakraborty D. Structural and dynamical properties of water in surfactant-like peptide-based nanotubes: effect of pore size, tube length and charge. J. Mol. Liq. 2021;323 doi: 10.1016/j.molliq.2020.115033. [DOI] [Google Scholar]
- 28.Sun W., Wang Y., Zhang W., Ying H., Wang P. Novel surfactant peptide for removal of biofilms. Colloids Surf. B Biointerfaces. 2018;172:180–186. doi: 10.1016/j.colsurfb.2018.08.029. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh S.Y., Tang Y., Crotti S., Stone E.A., Miller S.J. Catalytic enantioselective pyridine N-oxidation. J. Am. Chem. Soc. 2019;141:18624–18629. doi: 10.1021/jacs.9b10414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schnitzer T., Ganzoni R.L., Wennemers H. Impact of the β-turn hydrogen bond on the trans/cis ratio and the performance of the peptide catalyst H-DPro-Pro-Glu-NH2. Tetrahedron. 2020;76 doi: 10.1016/j.tet.2020.131184. [DOI] [Google Scholar]
- 31.Dimitrov D.S. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2004;2:109–122. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016;29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vardanyan R.S., Hruby V.J. In: Synth. Essent. Drugs. Vardanyan R.S., Hruby V.J., editors. Elsevier; 2006. Antiviral drugs; pp. 549–557. [DOI] [Google Scholar]
- 34.Rachlis A., Fanning M.M. Zidovudine toxicity. Drug Saf. 1993;8:312–320. doi: 10.2165/00002018-199308040-00005. [DOI] [PubMed] [Google Scholar]
- 35.Vilas Boas L.C.P., Campos M.L., Berlanda R.L.A., de Carvalho Neves N., Franco O.L. Antiviral peptides as promising therapeutic drugs. Cell. Mol. Life Sci. 2019;76:3525–3542. doi: 10.1007/s00018-019-03138-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho T.Y., Wu S.L., Chen J.C., Wei Y.C., Cheng S.E., Chang Y.H., Liu H.J., Hsiang C.Y. Design and biological activities of novel inhibitory peptides for SARS-CoV spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2006;69:70–76. doi: 10.1016/j.antiviral.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan K., Yi L., Chen J., Qu X., Qing T., Rao X., Jiang P., Hu J., Xiong Z., Nie Y., Shi X., Wang W., Ling C., Yin X., Fan K., Lai L., Ding M., Deng H. Suppression of SARS-CoV entry by peptides corresponding to heptad regions on spike glycoprotein. Biochem. Biophys. Res. Commun. 2004;319:746–752. doi: 10.1016/j.bbrc.2004.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sardar A., Lahiri A., Kamble M., Mallick A.I., Tarafdar P.K. Translation of mycobacterium survival strategy to develop a lipo-peptide based fusion inhibitor**. Angew. Chem. Int. Ed. 2021;60:6101–6106. doi: 10.1002/anie.202013848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kilby J.M., Hopkins S., Venetta T.M., Dimassimo B., Cloud G.A., Lee J.Y., Alldredge L., Hunter E., Lambert D., Bolognesi D., Matthews T., Johnson M.R., Nowak M.A., Shaw G.M., Saag M.S. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 40.Chew M.F., Poh K.S., Poh C.L. Peptides as therapeutic agents for dengue virus. Int. J. Med. Sci. 2017;14:1342–1359. doi: 10.7150/ijms.21875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Badani H. Tulane University; 2014. Understanding the Mechanism of Action of Flufirvitide-3 a Peptide based Inhibitor of Influenza Virus. [Google Scholar]
- 42.Spence J.S., Melnik L.I., Badani H., Wimley W.C., Garry R.F. Inhibition of arenavirus infection by a glycoprotein-derived peptide with a novel mechanism. J. Virol. 2014;88:8556–8564. doi: 10.1128/jvi.01133-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chaudhuri S., Symons J.A., Deval J. Innovation and trends in the development and approval of antiviral medicines: 1987–2017 and beyond. Antivir. Res. 2018;155:76–88. doi: 10.1016/j.antiviral.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenssen H. Therapeutic approaches using host defence peptides to tackle herpes virus infections. Viruses. 2009;1:939–964. doi: 10.3390/v1030939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.J. Stanley, N. Yamamoto, Novel prospective treatment options, trends basic ther. options HIV infect. - towar. a funct. cure, (2015). 〈 10.5772/60961〉. [DOI]
- 46.Yamamoto V., Bolanos J.F., Fiallos J., Strand S.E., Morris K., Shahrokhinia S., Cushing T.R., Hopp L., Tiwari A., Hariri R., Sokolov R., Wheeler C., Kaushik A., Elsayegh A., Eliashiv D., Hedrick R., Jafari B., Johnson J.P., Khorsandi M., Gonzalez N., Balakhani G., Lahiri S., Ghavidel K., Amaya M., Kloor H., Hussain N., Huang E., Cormier J., Wesson Ashford J., Wang J.C., Yaghobian S., Khorrami P., Shamloo B., Moon C., Shadi P., Kateb B. COVID-19: review of a 21st century pandemic from etiology to neuro-psychiatric implications. J. Alzheimer’s Dis. 2020;77:459–504. doi: 10.3233/JAD-200831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao P., Dou G., Cheng Y., Che J. The improved efficacy of Sifuvirtide compared with enfuvirtide might be related to its selectivity for the rigid biomembrane, as determined through surface plasmon resonance. PLoS One. 2017;12:1–12. doi: 10.1371/journal.pone.0171567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naylor P.H. Zadaxin (thymosin α1) for the treatment of viral hepatitis. Expert Opin. Investig. Drugs. 1999;8:281–287. doi: 10.1517/13543784.8.3.281. [DOI] [PubMed] [Google Scholar]
- 49.Schinazi R., Halfon P., Marcellin P., Asselah T. HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int. 2014;34:69–78. doi: 10.1111/liv.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kang C., Syed Y.Y. Bulevirtide: first approval. Drugs. 2020;80:1601–1605. doi: 10.1007/s40265-020-01400-1. [DOI] [PubMed] [Google Scholar]
- 51.Huang H.-N., Pan C.-Y., Chen J.-Y. Grouper (Epinephelus coioides) antimicrobial peptide epinecidin-1 exhibits antiviral activity against foot-and-mouth disease virus in vitro. Peptides. 2018;106:91–95. doi: 10.1016/j.peptides.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Falah N., Violot S., Decimo D., Berri F., Foucault-Grunenwald M.-L., Ohlmann T., Schuffenecker I., Morfin F., Lina B., Riteau B., Cortay J.-C. Ex vivo and in vivo inhibition of human rhinovirus replication by a new pseudosubstrate of viral 2A protease. J. Virol. 2012;86:691–704. doi: 10.1128/jvi.05263-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Falah N., Montserret R., Lelogeais V., Schuffenecker I., Lina B., Cortay J.C., Violot S. Blocking human enterovirus 71 replication by targeting viral 2A protease. J. Antimicrob. Chemother. 2012;67:2865–2869. doi: 10.1093/jac/dks304. [DOI] [PubMed] [Google Scholar]
- 54.Di Biase A.M., Pietrantoni A., Tinari A., Siciliano R., Valenti P., Antonini G., Seganti L., Superti F. Heparin-interacting sites of bovine lactoferrin are involved in anti-adenovirus activity. J. Med. Virol. 2003;69:495–502. doi: 10.1002/jmv.10337. [DOI] [PubMed] [Google Scholar]
- 55.Namvar A., Panahi H.A., Agi E., Bolhassani A. Development of HPV16,18,31,45 E5 and E7 peptides-based vaccines predicted by immunoinformatics tools. Biotechnol. Lett. 2020;42:403–418. doi: 10.1007/s10529-020-02792-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khan M.A., Hossain M.U., Rakib-Uz-Zaman S.M., Morshed M.N. Epitope-based peptide vaccine design and target site depiction against Ebola viruses: an immunoinformatics study. Scand. J. Immunol. 2015;82:25–34. doi: 10.1111/sji.12302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prasasty V.D., Grazzolie K., Rosmalena R., Yazid F., Ivan F.X., Sinaga E. Peptide-based subunit vaccine design of T-and b-cells multi-epitopes against zika virus using immunoinformatics approaches. Microorganisms. 2019;7 doi: 10.3390/microorganisms7080226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Atsmon J., Kate-Ilovitz E., Shaikevich D., Singer Y., Volokhov I., Haim K.Y., Ben-Yedidia T. Safety and immunogenicity of multimeric-001 - a novel universal influenza vaccine. J. Clin. Immunol. 2012;32:595–603. doi: 10.1007/s10875-011-9632-5. [DOI] [PubMed] [Google Scholar]
- 59.Firbas C., Jilma B., Tauber E., Buerger V., Jelovcan S., Lingnau K., Buschle M., Frisch J., Klade C.S. Immunogenicity and safety of a novel therapeutic hepatitis C virus (HCV) peptide vaccine: a randomized, placebo controlled trial for dose optimization in 128 healthy subjects. Vaccine. 2006;24:4343–4353. doi: 10.1016/j.vaccine.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 60.Gaspar D., Salomé Veiga A., Castanho M.A.R.B. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013;4:1–16. doi: 10.3389/fmicb.2013.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jenssen H., Hamill P., Hancock R.E.W. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tornesello A.L., Borrelli A., Buonaguro L., Buonaguro F.M., Tornesello M.L. Antimicrobial peptides as anticancer agents: functional properties and biological activities. Molecules. 2020;25:1–25. doi: 10.3390/molecules25122850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu H., Zhang H., Wang Q., Li S., Liu Y., Ma L., Huang Y., Stephen Brennan C., Sun L. Mechanisms underlying the antimicrobial actions of the antimicrobial peptides Asp-Tyr-Asp-Asp and Asp-Asp-Asp-Tyr. Food Res. Int. 2021;139 doi: 10.1016/j.foodres.2020.109848. [DOI] [PubMed] [Google Scholar]
- 64.Zhang D., Liu H., Chen M., Wang Q., Feng J., Shu X., Li C., Li Y., Xie X., Shi Q. A series of carboxymethyl cellulose-based antimicrobial peptide mimics were synthesized for antimicrobial applications. Carbohydr. Polym. 2021;261 doi: 10.1016/j.carbpol.2021.117822. [DOI] [PubMed] [Google Scholar]
- 65.Panya A., Yongpitakwattana P., Budchart P., Sawasdee N., Krobthong S., Paemanee A., Roytrakul S., Rattanabunyong S., Choowongkomon K., Thai Yenchitsomanus P. Novel bioactive peptides demonstrating anti-dengue virus activity isolated from the Asian medicinal plant Acacia catechu. Chem. Biol. Drug Des. 2019;93:100–109. doi: 10.1111/cbdd.13400. [DOI] [PubMed] [Google Scholar]
- 66.Heymich M.L., Friedlein U., Trollmann M., Schwaiger K., Böckmann R.A., Pischetsrieder M. Generation of antimicrobial peptides Leg1 and Leg2 from chickpea storage protein, active against food spoilage bacteria and foodborne pathogens. Food Chem. 2021;347 doi: 10.1016/j.foodchem.2020.128917. [DOI] [PubMed] [Google Scholar]
- 67.de L., dos Santos A., Taveira G.B., de S., Ribeiro F.F., da L., Pereira S., de A., Carvalho O., Rodrigues R., Oliveira A.E.A., Machado O.L.T., da J., Araújo S., Vasconcelos I.M., Gomes V.M. Purification and characterization of peptides from Capsicum annuum fruits which are α-amylase inhibitors and exhibit high antimicrobial activity against fungi of agronomic importance. Protein Expr. Purif. 2017;132:97–107. doi: 10.1016/j.pep.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 68.Nawae W., Hannongbua S., Ruengjitchatchawalya M. Molecular dynamics exploration of poration and leaking caused by Kalata B1 in HIV-infected cell membrane compared to host and HIV membranes. Sci. Rep. 2017;7:1–13. doi: 10.1038/s41598-017-03745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee S.H., Kim E.H., O’neal J.T., Dale G., Holthausen D.J., Bowen J.R., Quicke K.M., Skountzou I., Gopal S., George S., Wrammert J., Suthar M.S., Jacob J. The amphibian peptide Yodha is virucidal for Zika and dengue viruses. Sci. Rep. 2021;11:1–13. doi: 10.1038/s41598-020-80596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma B., Guo Y., Fu X., Jin Y. Identification and antimicrobial mechanisms of a novel peptide derived from egg white ovotransferrin hydrolysates. LWT. 2020;131 doi: 10.1016/j.lwt.2020.109720. [DOI] [Google Scholar]
- 71.Yu J., Dai Y., Fu Y., Wang K., Yang Y., Li M., Xu W., Wei L. Cathelicidin antimicrobial peptides suppress EV71 infection via regulating antiviral response and inhibiting viral binding. Antivir. Res. 2021;187 doi: 10.1016/j.antiviral.2021.105021. [DOI] [PubMed] [Google Scholar]
- 72.Lama R., Pereiro P., Costa M.M., Encinar J.A., Medina-Gali R.M., Pérez L., Lamas J., Leiro J., Figueras A., Novoa B. Turbot (Scophthalmus maximus) Nk-lysin induces protection against the pathogenic parasite Philasterides dicentrarchi via membrane disruption. Fish Shellfish Immunol. 2018;82:190–199. doi: 10.1016/j.fsi.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 73.Falco A., Medina-Gali R.M., Antonio Poveda J., Bello-Perez M., Novoa B., Antonio Encinar J. Antiviral activity of a turbot (scophthalmus maximus) NK-Lysin peptide by inhibition of low-pH virus-induced membrane fusion. Mar. Drugs. 2019;17:1–20. doi: 10.3390/md17020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu T., Tang D., Chen W., Huang H., Wang R., Chen Y. Expression of antimicrobial peptides thanatin(S) in transgenic Arabidopsis enhanced resistance to phytopathogenic fungi and bacteria. Gene. 2013;527:235–242. doi: 10.1016/j.gene.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 75.Holthausen D.J., Lee S.H., Kumar V.T., Bouvier N.M., Krammer F., Ellebedy A.H., Wrammert J., Lowen A.C., George S., Pillai M.R., Jacob J. An amphibian host defense peptide is virucidal for human H1 hemagglutinin-bearing influenza viruses. Immunity. 2017;46:587–595. doi: 10.1016/j.immuni.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 76.Moridi K., Hemmaty M., Akbari Eidgahi M.R., Fathi Najafi M., Zare H., Ghazvini K., Neshani A. Construction, cloning, and expression of Melittin antimicrobial peptide using Pichia pastoris expression system. Gene Rep. 2020;21 doi: 10.1016/j.genrep.2020.100900. [DOI] [Google Scholar]
- 77.Lee W., Lee D.G. Fungicidal mechanisms of the antimicrobial peptide Bac8c. Biochim. Biophys. Acta Biomembr. 2015;1848:673–679. doi: 10.1016/j.bbamem.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 78.Meikle T.G., Dharmadana D., Hoffmann S.V., Jones N.C., Drummond C.J., Conn C.E. Analysis of the structure, loading and activity of six antimicrobial peptides encapsulated in cubic phase lipid nanoparticles. J. Colloid Interface Sci. 2021;587:90–100. doi: 10.1016/j.jcis.2020.11.124. [DOI] [PubMed] [Google Scholar]
- 79.Kulimushi P.Z., Arias A.A., Franzil L., Steels S., Ongena M. Stimulation of fengycin-type antifungal lipopeptides in Bacillus amyloliquefaciens in the presence of the maize fungal pathogen Rhizomucor variabilis. Front. Microbiol. 2017;8:1–12. doi: 10.3389/fmicb.2017.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuan L., Zhang S., Peng J., Li Y., Yang Q. Synthetic surfactin analogues have improved anti-PEDV properties. PLoS One. 2019;14:1–14. doi: 10.1371/journal.pone.0215227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jung D., Yum S.J., Jeong H.G. Characterization and evaluation of antimicrobial activity of actinonin against foodborne pathogens. Food Sci. Biotechnol. 2017;26:1649–1657. doi: 10.1007/s10068-017-0190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J., Wang H.X., Ng T.B. A peptide with HIV-1 reverse transcriptase inhibitory activity from the medicinal mushroom Russula paludosa. Peptides. 2007;28:560–565. doi: 10.1016/j.peptides.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 83.Yang S., Dong Y., Aweya J.J., Xie T., Zeng B., Zhang Y., Liu G.M. Antimicrobial activity and acting mechanism of Tegillarca granosa hemoglobin-derived peptide (TGH1) against Vibrio parahaemolyticus. Microb. Pathog. 2020;147 doi: 10.1016/j.micpath.2020.104302. [DOI] [PubMed] [Google Scholar]
- 84.Ma L., Ye X., Sun P., Xu P., Wang L., Liu Z., Huang X., Bai Z., Zhou C. Antimicrobial and antibiofilm activity of the EeCentrocin 1 derived peptide EC1-17KV via membrane disruption. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Um J., Manguy J., Anes J., Jacquier J.C., Hurley D., Dillon E.T., Wynne K., Fanning S., O’Sullivan M., Shields D.C. Enriching antimicrobial peptides from milk hydrolysates using pectin/alginate food-gels. Food Chem. 2021;352 doi: 10.1016/j.foodchem.2021.129220. [DOI] [PubMed] [Google Scholar]
- 86.Velivelli S.L.S., Czymmek K.J., Li H., Shaw J.B., Buchko G.W., Shah D.M. Antifungal symbiotic peptide NCR044 exhibits unique structure and multifaceted mechanisms of action that confer plant protection. Proc. Natl. Acad. Sci. U. S. A. 2020;117:16043–16054. doi: 10.1073/pnas.2003526117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chan J.L., Diaconescu A.C., Horvath K.A. Routine use of topical bacitracin to prevent sternal wound infections after cardiac surgery. Ann. Thorac. Surg. 2017;104:1496–1500. doi: 10.1016/j.athoracsur.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 88.Sousa M.G.C., Xavier P.D., de A.P., Cantuária C., Porcino R.A., Almeida J.A., Franco O.L., Rezende T.M.B. Host defense peptide IDR-1002 associated with ciprofloxacin as a new antimicrobial and immunomodulatory strategy for dental pulp revascularization therapy. Microb. Pathog. 2021;152 doi: 10.1016/j.micpath.2020.104634. [DOI] [PubMed] [Google Scholar]
- 89.He J.F., Jin D.X., Luo X.G., Zhang T.C. LHH1, a novel antimicrobial peptide with anti-cancer cell activity identified from Lactobacillus casei HZ1. AMB Express. 2020;10 doi: 10.1186/s13568-020-01139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang H.N., Pan C.Y., Rajanbabu V., Chan Y.L., Wu C.J., Chen J.Y. Modulation of immune responses by the antimicrobial peptide, epinecidin (Epi)-1, and establishment of an Epi-1-based inactivated vaccine. Biomaterials. 2011;32:3627–3636. doi: 10.1016/j.biomaterials.2011.01.061. [DOI] [PubMed] [Google Scholar]
- 91.Li M., Yu D.H., Cai H. The synthetic antimicrobial peptide KLKL5KLK enhances the protection and efficacy of the combined DNA vaccine against Mycobacterium tuberculosis. DNA Cell Biol. 2008;27:405–413. doi: 10.1089/dna.2007.0693. [DOI] [PubMed] [Google Scholar]
- 92.Panya A., Bangphoomi K., Choowongkomon K., Yenchitsomanus P.T. Peptide inhibitors against dengue virus infection. Chem. Biol. Drug Des. 2014;84:148–157. doi: 10.1111/cbdd.12309. [DOI] [PubMed] [Google Scholar]
- 93.Panya A., Sawasdee N., Junking M., Srisawat C., Choowongkomon K., Yenchitsomanus P.T. A peptide inhibitor derived from the conserved ectodomain region of DENV membrane (M) protein with activity against dengue virus infection. Chem. Biol. Drug Des. 2015;86:1093–1104. doi: 10.1111/cbdd.12576. [DOI] [PubMed] [Google Scholar]
- 94.Yuan L., Zhang S., Wang Y., Li Y., Wang X., Yang Q. Surfactin inhibits membrane fusion during invasion of epithelial cells by enveloped viruses. J. Virol. 2018;92:1–19. doi: 10.1128/jvi.00809-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhan S., Aweya J.J., Wang F., Yao D., Zhong M., Chen J., Li S., Zhang Y. Litopenaeus vannamei attenuates white spot syndrome virus replication by specific antiviral peptides generated from hemocyanin. Dev. Comp. Immunol. 2019;91:50–61. doi: 10.1016/j.dci.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 96.Cheng G., Montero A., Gastaminza P., Whitten-Bauer C., Wieland S.F., Isogawa M., Fredericksen B., Selvarajah S., Gallay P.A., Ghadiri M.R., Chisari F.V. A virocidal amphipathic α-helical peptide that inhibits hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. U. S. A. 2008;105:3088–3093. doi: 10.1073/pnas.0712380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luteijn R.D., Praest P., Thiele F., Sadasivam S.M., Singethan K., Drijfhout J.W., Bach C., De Boer S.M., Lebbink R.J., Tao S., Helfer M., Bach N.C., Protzer U., Costa A.I., Killian J.A., Drexler I., Wiertz E.J.H.J., Broad-Spectrum A. Antiviral peptide blocks infection. Cells. 2020;9:1989. doi: 10.3390/cells9091989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu W., Lin D., Shen X., Li F., Fang Y., Li K., Xun T., Yang G., Yang J., Liu S., He J. New influenza a virus entry inhibitors derived from the viral fusion peptides. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0138426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guan H., Nuth M., Lee V., Lin C., Mitchell C.H., Lu W., Scott R.W., Parker M.H., Kulp J.L., Reitz A.B., Ricciardi R.P. Herpes Simplex Virus-1 infection in human primary corneal epithelial cells is blocked by a stapled peptide that targets processive DNA synthesis. Ocul. Surf. 2021;19:313–321. doi: 10.1016/j.jtos.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Micewicz E.D., Cole A.L., Jung C.L., Luong H., Phillips M.L., Pratikhya P., Sharma S., Waring A.J., Cole A.M., Ruchala P. Grifonin-1: a small HIV-1 entry inhibitor derived from the algal lectin, griffithsin. PLoS One. 2010;5:1–11. doi: 10.1371/journal.pone.0014360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim J., Yang Y.L., Jang S.H., Jang Y.S. Human β-defensin 2 plays a regulatory role in innate antiviral immunity and is capable of potentiating the induction of antigen-specific immunity. Virol. J. 2018;15:1–12. doi: 10.1186/s12985-018-1035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Khachatoorian R., Ruchala P., Waring A., Jung C.L., Ganapathy E., Wheatley N., Sundberg C., Arumugaswami V., Dasgupta A., French S.W. Structural characterization of the HSP70 interaction domain of the hepatitis C viral protein NS5A. Virology. 2015;475:46–55. doi: 10.1016/j.virol.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Anang S., Kaushik N., Hingane S., Kumari A., Gupta J., Asthana S., Shalimar B., Nayak C.T., Ranjith-Kumar M., Surjit Potent inhibition of hepatitis E virus release by a cyclic peptide inhibitor of the interaction between viral open reading frame 3 protein and host tumor susceptibility gene 101. J. Virol. 2018;92:1–16. doi: 10.1128/jvi.00684-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Montero A., Gastaminza P., Law M., Cheng G., Chisari F.V., Ghadiri M.R. Self-assembling peptide nanotubes with antiviral activity against hepatitis C virus. Chem. Biol. 2011;18:1453–1462. doi: 10.1016/j.chembiol.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pemmada R., Zhu X., Dash M., Zhou Y., Ramakrishna S., Peng X., Thomas V., Jain S., Nanda H.S. Science-based strategies of antiviral coatings with viricidal properties for the COVID-19 like pandemics. Materials. 2020;13:1–18. doi: 10.3390/ma13184041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pyankov O.V., Usachev E.V., Pyankova O., Agranovski I.E. Inactivation of airborne influenza virus by tea tree and eucalyptus oils. Aerosol Sci. Technol. 2012;46:1295–1302. doi: 10.1080/02786826.2012.708948. [DOI] [Google Scholar]
- 107.Catel-Ferreira M., Tnani H., Hellio C., Cosette P., Lebrun L. Antiviral effects of polyphenols: development of bio-based cleaning wipes and filters. J. Virol. Methods. 2015;212:1–7. doi: 10.1016/j.jviromet.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 108.Sun Z., (Ken) Ostrikov K. Future antiviral surfaces: lessons from COVID-19 pandemic. Sustain. Mater. Technol. 2020;25 doi: 10.1016/j.susmat.2020.e00203. [DOI] [Google Scholar]
- 109.Hutasoit N., Kennedy B., Hamilton S., Luttick A., Rashid R.A.R., Palanisamy S. Sars-CoV-2 (COVID-19) inactivation capability of copper-coated touch surface fabricated by cold-spray technology. Manuf. Lett. 2020;25:93–97. doi: 10.1016/j.mfglet.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Merkl P., Long S., McInerney G.M., Sotiriou G.A. Antiviral activity of silver, copper oxide and zinc oxide nanoparticle coatings against sars-cov-2. Nanomaterials. 2021;11 doi: 10.3390/nano11051312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Park G.W., Cho M., Cates E.L., Lee D., Oh B.T., Vinjé J., Kim J.H. Fluorinated TiO2 as an ambient light-activated virucidal surface coating material for the control of human norovirus. J. Photochem. Photobiol. B Biol. 2014;140:315–320. doi: 10.1016/j.jphotobiol.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goswami M., Yadav A.K., Chauhan V., Singh N., Kumar S., Das A., Yadav V., Mandal A., Tiwari J.K., Siddiqui H., Ashiq M., Sathish N., Kumar S., Biswas D., Srivastava A.K. Facile development of graphene-based air filters mounted on a 3D printed mask for COVID-19. J. Sci. Adv. Mater. Devices. 2021;6:407–414. doi: 10.1016/j.jsamd.2021.05.003. [DOI] [Google Scholar]
- 113.Zhong H., Zhu Z., Lin J., Cheung C.F., Lu V.L., Yan F., Chan C.-Y., Li G. Reusable and recyclable graphene masks with outstanding superhydrophobic and photothermal performances. ACS Nano. 2020;14:6213–6221. doi: 10.1021/acsnano.0c02250. [DOI] [PubMed] [Google Scholar]
- 114.Hsu B.B., Wong S.Y., Hammond P.T., Chen J., Klibanov A.M. Mechanism of inactivation of influenza viruses by immobilized hydrophobic polycations. Proc. Natl. Acad. Sci. U. S. A. 2011;108:61–66. doi: 10.1073/pnas.1017012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu H., Elkin I., Chen J., Klibanov A.M. Why do some immobilized N-Alkylated polyethylenimines far surpass others in inactivating influenza viruses? Biomacromolecules. 2015;16:351–356. doi: 10.1021/bm5015427. [DOI] [PubMed] [Google Scholar]
- 116.Larson A.M., Hsu B.B., Rautaray D., Haldar J., Chen J., Klibanov A.M. Hydrophobic polycationic coatings disinfect poliovirus and rotavirus solutions. Biotechnol. Bioeng. 2011;108:720–723. doi: 10.1002/bit.22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Larson N., Ghandehari H. Polymeric conjugates for drug delivery. Chem. Mater. 2012;24:840–853. doi: 10.1021/cm2031569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gerrard S.E., Larson A.M., Klibanov A.M., Slater N.K.H., Hanson C.V., Abrams B.F., Morris M.K. Reducing infectivity of HIV upon exposure to surfaces coated with N,N-dodecyl, methyl-polyethylenimine. Biotechnol. Bioeng. 2013;110:2058–2062. doi: 10.1002/bit.24867. [DOI] [PubMed] [Google Scholar]
- 119.Park D., Larson A.M., Klibanov A.M., Wang Y. Antiviral and antibacterial polyurethanes of various modalities. Appl. Biochem. Biotechnol. 2013;169:1134–1146. doi: 10.1007/s12010-012-9999-7. [DOI] [PubMed] [Google Scholar]
- 120.Cano-Vicent A., Tuñón-Molina A., Martí M., Muramoto Y., Noda T., Takayama K., Serrano-Aroca Á. Antiviral face mask functionalized with solidified hand soap: low-cost infection prevention clothing against enveloped viruses such as SARS-CoV-2. ACS Omega. 2021 doi: 10.1021/acsomega.1c03511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Colnago L.A., Trevisol I.M., Rech D.V., Forato L.A., Mitre C.I.D.N., Leite J.P.G., Giglioti R., Okino C.H. Simple, low-cost and long-lasting film for virus inactivation using avian coronavirus model as challenge. Int. J. Environ. Res. Public Health. 2020;17:1–9. doi: 10.3390/ijerph17186456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koegler P., Pasic P., Gardiner J., Glattauer V., Kingshott P., Thissen H. Polymerizable peptide copolymer coatings for the control of biointerfacial interactions. Biomacromolecules. 2014;15:2265–2273. doi: 10.1021/bm500386y. [DOI] [PubMed] [Google Scholar]
- 123.Costa F., Gomes P., Martins M.C.L. Elsevier Ltd.; 2018. Antimicrobial Peptides (AMP) Biomaterial Coatings for Tissue Repair. [DOI] [Google Scholar]
- 124.Mohanty R.P., Liu X., Kim J.Y., Peng X., Bhandari S., Leal J., Arasappan D., Wylie D.C., Dong T., Ghosh D. Identification of peptide coatings that enhance diffusive transport of nanoparticles through the tumor microenvironment. Nanoscale. 2019;11:17664–17681. doi: 10.1039/c9nr05783h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Clauder F., Zitzmann F.D., Friebe S., Mayr S.G., Robitzki A.A., Beck-Sickinger A.G. Multifunctional coatings combining bioactive peptides and affinity-based cytokine delivery for enhanced integration of degradable vascular grafts. Biomater. Sci. 2020;8:1734–1747. doi: 10.1039/c9bm01801h. [DOI] [PubMed] [Google Scholar]
- 126.Gitelman Povimonsky A., Rapaport H. Peptide coating applied on the spot improves osseointegration of titanium implants. J. Mater. Chem. B. 2017;5:2096–2105. doi: 10.1039/c6tb03093a. [DOI] [PubMed] [Google Scholar]
- 127.Righi M., Puleo G.L., Tonazzini I., Giudetti G., Cecchini M., Micera S. Peptide-based coatings for flexible implantable neural interfaces. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-017-17877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fischer N.G., He J., Aparicio C. Surface immobilization chemistry of a laminin-derived peptide affects keratinocyte activity. Coatings. 2020;10 doi: 10.3390/COATINGS10060560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Corrales-Ureña Y.R., Souza-Schiaber Z., Lisboa-Filho P.N., Marquenet F., Michael Noeske P.L., Gätjen L., Rischka K. Functionalization of hydrophobic surfaces with antimicrobial peptides immobilized on a bio-interfactant layer. RSC Adv. 2020;10:376–386. doi: 10.1039/c9ra07380a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Majhi S., Arora A., Mishra A. Surface immobilization of a short antimicrobial peptide (AMP) as an antibacterial coating. Materialia. 2019;6 doi: 10.1016/j.mtla.2019.100350. [DOI] [Google Scholar]
- 131.Monteiro C., Costa F., Pirttilä A.M., Tejesvi M.V., Martins M.C.L. Prevention of urinary catheter-associated infections by coating antimicrobial peptides from crowberry endophytes. Sci. Rep. 2019;9:1–14. doi: 10.1038/s41598-019-47108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nyström L., Strömstedt A.A., Schmidtchen A., Malmsten M. Peptide-loaded microgels as antimicrobial and anti-inflammatory surface coatings. Biomacromolecules. 2018;19:3456–3466. doi: 10.1021/acs.biomac.8b00776. [DOI] [PubMed] [Google Scholar]
- 133.Le Low J., Kao P.H.-N., Tambyah P.A., Koh G.L.E., Ling H., Kline K.A., Cheow W.S., Leong S.S.J. Development of a polymer-based antimicrobial coating for efficacious urinary catheter protection. Biotechnol. Notes. 2021;2:1–10. doi: 10.1016/j.biotno.2020.12.001. [DOI] [Google Scholar]
- 134.Zarghami V., Ghorbani M., Pooshang Bagheri K., Shokrgozar M.A. Melittin antimicrobial peptide thin layer on bone implant chitosan-antibiotic coatings and their bactericidal properties. Mater. Chem. Phys. 2021;263 doi: 10.1016/j.matchemphys.2021.124432. [DOI] [Google Scholar]
- 135.Paris J.B., Seyer D., Jouenne T., Thébault P. Various methods to combine hyaluronic acid and antimicrobial peptides coatings and evaluation of their antibacterial behaviour. Int. J. Biol. Macromol. 2019;139:468–474. doi: 10.1016/j.ijbiomac.2019.07.188. [DOI] [PubMed] [Google Scholar]
- 136.Jeong G.M., Seong H., Im S.G., Sung B.H., Kim S.C., Jeong K.J. Coating of an antimicrobial peptide on solid substrate via initiated chemical vapor deposition. J. Ind. Eng. Chem. 2018;58:51–56. doi: 10.1016/j.jiec.2017.09.006. [DOI] [Google Scholar]
- 137.Sheng G., Ni J., Xing K., Fan L., Dai T., Yu J., Dai X., Chen R., Wu J., Li N., Chen J., Mao Z., Li L. Infection microenvironment-responsive multifunctional peptide coated gold nanorods for bimodal antibacterial applications. Colloids Interface Sci. Commun. 2021;41 doi: 10.1016/j.colcom.2021.100379. [DOI] [Google Scholar]
- 138.Ye Z., Zhu X., Mutreja I., Boda S.K., Fischer N.G., Zhang A., Lui C., Qi Y., Aparicio C. Biomimetic mineralized hybrid scaffolds with antimicrobial peptides. Bioact. Mater. 2021;6:2250–2260. doi: 10.1016/j.bioactmat.2020.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Franco A.R., Fernandes E.M., Rodrigues M.T., Rodrigues F.J., Gomes M.E., Leonor I.B., Kaplan D.L., Reis R.L. Antimicrobial coating of spider silk to prevent bacterial attachment on silk surgical sutures. Acta Biomater. 2019;99:236–246. doi: 10.1016/j.actbio.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 140.Raman N., Marchillo K., Lee M.R., Rodríguez López A.D.L., Andes D.R., Palecek S.P., Lynn D.M. Intraluminal release of an antifungal β-peptide enhances the antifungal and anti-biofilm activities of multilayer-coated catheters in a rat model of venous catheter infection. ACS Biomater. Sci. Eng. 2016;2:112–121. doi: 10.1021/acsbiomaterials.5b00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.de Alteriis E., Maselli V., Falanga A., Galdiero S., Di Lella F.M., Gesuele R., Guida M., Galdiero E. Efficiency of gold nanoparticles coated with the antimicrobial peptide indolicidin against biofilm formation and development of Candida spp. clinical isolates. Infect. Drug Resist. 2018;11:915–925. doi: 10.2147/IDR.S164262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Akhavan B., Michl T.D., Giles C., Ho K., Martin L., Sharifahmadian O., Wise S.G., Coad B.R., Kumar N., Griesser H.J., Bilek M.M. Plasma activated coatings with dual action against fungi and bacteria. Appl. Mater. Today. 2018;12:72–84. doi: 10.1016/j.apmt.2018.04.003. [DOI] [Google Scholar]
- 143.Gevrek T.N., Yu K., Kizhakkedathu J.N., Sanyal A. Thiol-reactive polymers for titanium interfaces: fabrication of antimicrobial coatings. ACS Appl. Polym. Mater. 2019;1:1308–1316. doi: 10.1021/acsapm.9b00117. [DOI] [Google Scholar]
- 144.Zhang L., Yan J., Yin Z., Tang C., Guo Y., Li D., Wei B., Xu Y., Gu Q., Wang L. Electrospun vancomycin-loaded coating on titanium implants for the prevention of implant-associated infections. Int. J. Nanomed. 2014;9:3027–3036. doi: 10.2147/IJN.S63991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Buckholtz G.A., Reger N.A., Anderton W.D., Schimoler P.J., Roudebush S.L., Meng W.S., Miller M.C., Gawalt E.S. Reducing Escherichia coli growth on a composite biomaterial by a surface immobilized antimicrobial peptide. Mater. Sci. Eng. C. 2016;65:126–134. doi: 10.1016/j.msec.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 146.Tan X.W., Goh T.W., Saraswathi P., Nyein C.L., Setiawan M., Riau A., Lakshminarayanan R., Liu S., Tan D., Beuerman R.W., Mehta J.S. Effectiveness of antimicrobial peptide immobilization for preventing perioperative cornea implant-associated bacterial infection. Antimicrob. Agents Chemother. 2014;58:5229–5238. doi: 10.1128/AAC.02859-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nogueira F., Teixeira P., Gouveia I.C. Electrospinning polypropylene with an amino acid as a strategy to bind the antimicrobial peptide Cys-LC-LL-37. J. Mater. Sci. 2018;53:4655–4664. doi: 10.1007/s10853-017-1841-8. [DOI] [Google Scholar]
- 148.Scapin S., Formaggio F., Glisenti A., Biondi B., Scocchi M., Benincasa M., Peggion C. Sustainable, site-specific linkage of antimicrobial peptides to cotton textiles. Macromol. Biosci. 2020;20:1–6. doi: 10.1002/mabi.202000199. [DOI] [PubMed] [Google Scholar]
- 149.Orlandin A., Dolcet P., Biondi B., Hilma G., Coman D., Oancea S., Formaggio F., Peggion C. Covalent graft of lipopeptides and peptide dendrimers to cellulose fibers. Coatings. 2019;9 doi: 10.3390/coatings9100606. [DOI] [Google Scholar]
- 150.Mouro C., Gouveia I.C. Antimicrobial functionalization of wool: assessment of the effect of Cecropin-B and [Ala5]-Tritrp7 antimicrobial peptides. J. Text. Inst. 2016;107:1575–1583. doi: 10.1080/00405000.2015.1130297. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.