Abstract
Although the potential role of superior longitudinal fasciculus (SLF) in intellectual deficits and treatment response (TR) in patients with schizophrenia (SZ) has been previously described, little is known about the white-matter (WM) integrity of SLF subcomponents (SLF I, II, III, and arcuate fasciculus) and their particular relationships with the clinical presentations of the illness. This study examined the associations between fractional anisotropy (FA) of SLF subcomponents and intelligence level and 6-month treatment response (TR) of negative symptoms (NS) in patients with SZ. At baseline, 101 patients with SZ and 101 healthy controls (HCs) underwent structural magnetic resonance imaging. Voxel-wise group comparison analysis showed significant SLF FA reductions in patients with SZ compared with HCs. Voxel-wise correlation analyses revealed significant positive correlations of FAs of right SLF II with Korean–Wechsler Adult Intelligence Scale at baseline and the percentage reduction of negative syndrome subscale of the Positive and Negative Syndrome Scales at 6 months. These findings suggest that aberrance in WM microstructure in SLF II may be associated with intellectual deficits in patients with SZ and TR of NS, which may support the potential role of SLF II as a novel neuroimaging biomarker for clinical outcomes of the illness.
Subject terms: Schizophrenia, Schizophrenia
Introduction
Schizophrenia (SZ) is a debilitating psychiatric syndrome characterized by positive and negative symptoms (NS), and cognitive impairment. Pharmacological interventions, such as antipsychotic medications, are generally effective in treating positive symptoms; however, they have only limited effects on NS and cognitive impairment, which are the cardinal features of SZ largely responsible for long-term morbidity and poor occupational, social, and economic functioning in these patients1,2. Although often discussed separately, the negative and cognitive symptoms of SZ are increasingly being considered to originate in similar neural bases due to their shared features1. Previous studies3–5 have consistently reported widespread white-matter (WM) abnormalities and suggested the implications of disrupted neural networks (e.g., frontocortico-temporal, fronto-striatal, and fronto-temporal-parietal) on NS and cognitive deficits in SZ. More specifically, a recent meta-analysis6 of 59 individual studies found extensive alterations in WM bundles in the corpus callosum, thalamic radiations, inferior fronto-occipital fasciculus, uncinate fasciculus, cingulum, and superior longitudinal fasciculus (SLF) in patients with SZ.
The SLF is a major association fiber that encompasses the frontal, parietal, and temporal areas7. It has been implicated in patients with SZ largely because of its important role in cognitive functioning, including attention and memory8. For instance, a structural magnetic resonance imaging (MRI) study9 reported a significant association between the intelligence quotient and frontotemporal cortex in patients with first-episode psychosis, suggesting the involvement of cortical structures interconnected via the SLF in cognitive deficits presented in these patients. Additionally there is direct evidence that abnormalities in the SLF are associated with cognitive impairment in patients with SZ, and a previous diffusion tensor image (DTI) study10 demonstrated WM disruption in the SLF and its association with impaired verbal working memory tasks in patients with recent-onset SZ. Another recent DTI study11 reported a positive correlation between processing speed and fractional anisotropy (FA) values in the right SLF. Recent studies have also suggested a link between WM alterations in the SLF and NS in individuals at an ultra-high risk of psychosis12,13, those with first-episode psychosis14, and those with SZ15,16. Collectively, the findings from these studies indicate that SLF may be involved in the pathophysiology of cognition and NS in SZ.
Recent studies in literature have also proposed that SLFs may have implications in the clinical outcomes of SZ. In their DTI-tractography study14, Luck et al. reported that first-episode psychosis patients with poor outcomes showed greater disruptions in the frontotemporal WM microstructures (uncinate and SLF) than those with good outcomes. They speculated that smaller myelin alterations in patients with good outcomes, as reflected by higher FAs in these structures, might have facilitated a better response to antipsychotic medications. Another DTI study17 of patients with first-episode SZ demonstrated that improvement in disruptions in widespread WM tracts (indicated by decreased FAs and increased mean diffusivity [MD] and radial diffusivity [RD]), including the left SLF, correlated with improvement in psychosis and processing speed at 8 weeks from baseline. Thus, evidence from these studies suggests that WM disruptions in the SLF may be a key feature in predicting treatment response (TR) in patients with SZ, warranting further longitudinal studies with larger clinical cohorts to corroborate these findings.
Since the SLF connects a wide range of brain regions and is involved in a variety of associative and higher brain functions, researchers have suggested structural delineation of this large fiber system. Makris et al.18 discovered that the four subdivisions of the SLF observed in non-human primates, SLF I, SLF II, SLF III, and arcuate fasciculus (AF), could be identified in the human brain and tentatively proposed functional roles of each SLF subcomponent in humans. SLF I, which interconnects the medial/superior parietal regions with the dorsal premotor region, may contribute to higher levels of motor regulation. SLF II forms a major link between the prefrontal and parietal lobes, and may help these regions communicate visual information, thus contributing to the regulation of visuospatial attention. SLF III, which connects the rostral part of the inferior parietal lobule with the lateral inferior frontal lobe, is speculated to transfer somatosensory information such as language articulation. AF runs contiguously with SLF II and may help the prefrontal cortex to modulate audiospatial information. Subsequent multimodal studies19,20 have further contributed to our understanding of the functional roles of the SLF subcomponents in the human brain. However, there is a lack of studies investigating specific relationships between the structural aberrations of SLF subcomponents and their implications on the symptomatology of SZ.
Hence, this study sought to investigate the aberrant WM microstructure of SLF subcomponents and its association with intellectual deficits and TR of NS in patients with SZ. Our hypotheses were as follows:
There would be disrupted WM integrity in the SLF (observed as lower FAs) in patients with SZ at baseline, when compared to healthy controls (HCs).
In patients with SZ, intelligence level at baseline and 6-month TR of NS would be positively associated with FAs of SLF areas that showed significant differences between the two groups at baseline.
Distinct patterns of associations with intelligence level at baseline and 6-month TR of NS would be present in each SLF subcomponent, namely SLF I, II, III, and AF.
Results
Demographic and clinical characteristics of the study participants
Table 1 displays the demographic and clinical profiles of patients with SZ and HCs. There were no significant differences in sex or age between the two groups. The years of education and total intracranial volume were significantly lower in patients with SZ than in HCs. The mean age of patients with SZ was 37.0 ± 11.5 years, and the median duration of illness was 10 months. Full-scale intelligence quotient (FSIQ) and all subtest scores of Korean–Wechsler adult intelligence scale (K-WAIS) except “information” were significantly lower in patients with SZ than in HCs. Table 2 describes the severity of clinical symptoms in patients with SZ at baseline and 6 months, assessed using the Positive and Negative Syndrome Scale (PANSS). No significant differences in demographic and clinical characteristics were found between patients with (n = 78) and without (n = 23) K-WAIS assessments.
Table 1.
Demographic and clinical characteristics of the study participants.
| Patients with SZ (N = 101) |
HCs (N = 101) |
Statistics | p | |
|---|---|---|---|---|
| Sex | ||||
| Male, n (%) | 35 (34.7) | 42 (41.6) | χ2 = 1.45 | 0.311 |
| Female, n (%) | 66 (65.3) | 59 (58.4) | ||
| Age (years), mean ± SD | 37.0 ± 11.5 | 37.9 ± 8.7 | t = 0.63 | 0.521 |
| Education (years), mean ± SD | 13.0 ± 2.4 | 16.7 ± 2.5 | t = 10.40 | <0.001 |
| Duration of illness (months), mean ± SD | 46.3 ± 72.2 | |||
| Intracranial volume (mL), mean ± SD | 1466.9 ± 139.7 | 1543.5 ± 130.4 | t = 4.01 | <0.001 |
| Antipsychotic medications at MRI scan (mg/day), mean ± SDa | 435.3 ± 256.5 | |||
| Antipsychotic medications at 6 months (mg/day), mean ± SDa | 715.0 ± 1634.1 | |||
| Duration of antipsychotic medication before scan of antipsychotic-naive or free participants (days), mean ± SD | 6.8 ± 7.2 | |||
| Types of antipsychotics | ||||
| Paliperidone (n) | 32 | |||
| Olanzapine (n) | 2 | |||
| Risperidone (n) | 27 | |||
| Amisulpride (n) | 32 | |||
| Aripiprazole (n) | 8 | |||
| Full-scale intelligence quotient, mean ± SD | 88.6 ± 18.5 | 114.9 ± 6.3 | t = 4.23 | <0.001 |
| Verbal intelligence quotient | 94.7 ± 16.2 | 117.0 ± 9.0 | t = 7.41 | <0.001 |
| Information | 9.3 ± 3.3 | 11.1 ± 2.5 | t = 1.40 | 0.166 |
| Digit span | 8.5 ± 3.4 | 11.6 ± 1.7 | t = 2.27 | 0.026 |
| Vocabulary | 9.2 ± 3.3 | 12.8 ± 1.1 | t = 6.99 | <0.001 |
| Arithmetic | 8.0 ± 2.9 | 12.1 ± 2.6 | t = 2.93 | 0.004 |
| Comprehension | 9.9 ± 3.3 | 13.7 ± 0.9 | t = 6.74 | <0.001 |
| Similarity | 9.4 ± 3.4 | 13.9 ± 1.6 | t = 2.73 | 0.008 |
| Performance intelligence quotient | 89.7 ± 17.5 | 110.7 ± 7.6 | t = 7.72 | <0.001 |
| Picture completion | 8.4 ± 2.9 | 10.1 ± 2.0 | t = 4.51 | <0.001 |
| Picture arrangement | 8.9 ± 2.9 | 11.0 ± 1.4 | t = 2.02 | 0.049 |
| Block design | 8.6 ± 3.9 | 13.7 ± 1.6 | t = 5.59 | <0.001 |
| Object assembly | 9.2 ± 3.8 | 12.6 ± 2.1 | t = 3.21 | 0.008 |
| Digit symbol | 9.0 ± 3.0 | 12.2 ± 1.2 | t = 2.54 | 0.014 |
SZ schizophrenia, HCs healthy controls, SD standard deviation, MRI magnetic resonance imaging.
aChlorpromazine equivalent doses were calculated according to Gardner et al.61.
Table 2.
Positive and Negative Syndrome Scale at baseline and at 6 months in patients with schizophrenia.
| Mean ± SD | PANSS score at baseline (N = 101) | PANSS score at 6 months (N = 95) |
|---|---|---|
| PANSS composite score | 116.5 ± 26.7 | 53.3 ± 12.3 |
| Positive scale | 29.4 ± 7.5 | 11.1 ± 3.9 |
| Negative scale | 27.2 ± 8.3 | 14.4 ± 4.0 |
| General psychopathology scale | 59.9 ± 13.4 | 27.8 ± 5.9 |
SD standard deviation, PANSS Positive and Negative Syndrome Scale.
Voxel-wise comparison of DTI indices of SLF between patients with SZ and HCs
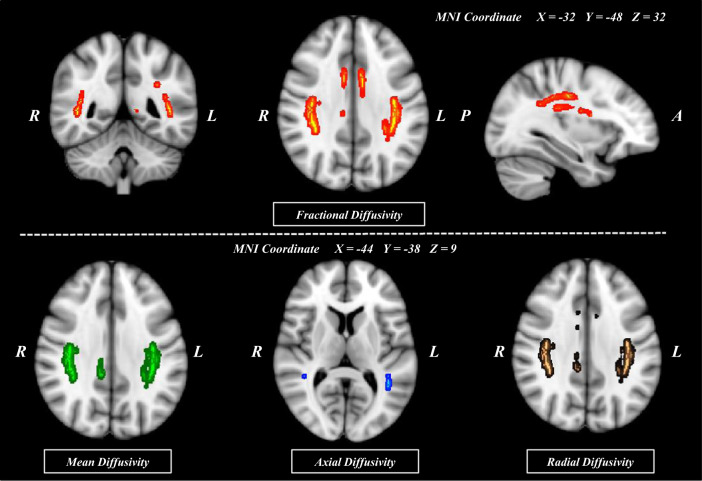
Figure 1 shows the voxel-wise comparison analyses for SLF FAs, along with MDs, axial diffusivity (AD)s, and RDs, between patients with SZ and HCs. FAs were significantly lower in patients with SZ for voxels in extensive areas of the SLF (p < 0.002; family-wise error [FWE] corrected). MD, AD, and RD were significantly higher in patients with SZ than in HCs (p < 0.003, p < 0.009, and p < 0.002, respectively; FWE corrected). Inspection of the areas with respect to the regions of interest (ROI) mask for SLF subcomponents demonstrated that voxels of significant between-group FA differences were identified in all eight SLF subcomponents. When controlling for age, sex, and years of education as covariates, voxel-wise comparison analyses demonstrated lower FAs for all SLF subcomponents in patients with SZ (p < 0.002; FWE corrected). The significance of the results remained the same after the exclusion of 14 patients with SZ who were on antipsychotic medications at the time of enrollment. For a comprehensive analysis of the SLF, repeated measures analysis of variance (RM-ANOVA) was performed in addition to voxel-wise analysis (Supplementary Methods and Results).
Fig. 1. Comparison of the regions showing significant changes in the fractional anisotropy, mean diffusivity, axial diffusivity, and radial diffusivity values between patients with schizophrenia and healthy controls (N = 101 for both).
Voxels demonstrating significantly (threshold-free cluster enhancement, p = 0.05; family-wise error corrected) decreased fractional anisotropy values in patients with schizophrenia as compared to healthy controls are shown in red-yellow color. Significantly increased mean diffusivity, axial diffusivity, and radial diffusivity values in patients with schizophrenia as compared with healthy controls are shown in green, blue, and brown, respectively. The number of permutations was 1000. (MNI, Montreal Neurologic Institute; R, right; L, left; P, posterior; A, anterior).
WM microstructures of SLF subcomponents and their associations with intelligence level in patients with SZ
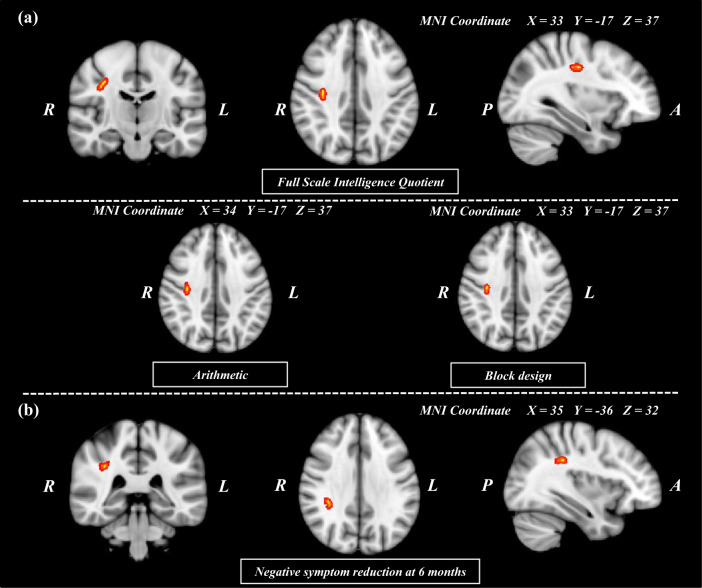
Voxel-wise correlation analyses showed positive correlations between FSIQ and FAs in areas within the right SLF II in patients with SZ (p < 0.004; FWE corrected). The significance of the results remained the same after controlling for age, sex, years of education, and antipsychotic medication use at baseline as covariates. No significant correlation was observed in HCs. Exploratory voxel-wise correlation analyses for K-WAIS subtest scores showed that FAs of right SLF II were positively correlated with “digit span”, “vocabulary,” “arithmetic,” “comprehension,” “similarities,” “picture arrangement,” and “block design” subscales in patients with SZ (Fig. 2a). Multivariate multiple stepwise linear regression analysis (F = 14.12, p < 0.001) showed that the FAs of the right SLF II were independently correlated with “arithmetic” (β = 0.016, 95% CI = 0.002–0.029, p = 0.024) in working memory dimension and “block design” (β = 0.012, 95% CI = < 0.001–0.024, p = 0.045) in visuospatial dimension.
Fig. 2. Regions showing positive correlation between fractional anisotropy values and intelligence quotient scores and negative symptom reduction at 6 months in patients with schizophrenia.
Voxels demonstrating regions showing significant (threshold-free cluster enhancement, p = 0.05; family-wise error corrected) positive correlation between the fractional anisotropy values and the intelligence quotient (a) and treatment response (b) in patients with schizophrenia are depicted by red-yellow circles. The number of permutations was 1000. (MNI, Montreal Neurologic Institute; R, right; L, left; P, posterior; A, anterior).
WM microstructures of SLF subcomponents and their associations with 6-month TR of NS in patients with SZ
Figure 2b depicts voxel-wise correlation analyses showing positive correlations between the 6-month TR of NS and FAs of areas within the right SLF II in patients with SZ (p < 0.04; FWE corrected). Pearson correlation analysis also demonstrated a significant positive correlation between the 6-month TR of NS and the FAs of the right SLF II (r = 0.420, p < 0.001; Supplementary Fig. 2). The significance of the results remained the same after excluding 4 patients who received long-acting injectable antipsychotics at 6 months and controlling for age, sex, years of education, and antipsychotic medication at 6 months as covariates. No significant associations were demonstrated for the total PANSS scores and the positive and general psychopathology subscales.
Multivariate multiple stepwise linear regression analysis (F = 11.09, p < 0.001) showed that the 6-month TR of NS was explained by the FAs of the right SLF II (β = 276.16, 95% CI = 107.07–445.26, p = 0.002), as well as sex (β = 21.90, 95% CI = 7.63–36.18, p = 0.003) and baseline PANSS scores (β = 0.58, 95% CI = 0.32–0.84, p = 0.003), indicating a significant predictive power of FAs of the right SLF II for TR of NS.
Discussion
To our knowledge, this is the first study to investigate the aberrant WM microstructure of SLF subcomponents and its association with intelligence level and TR in patients with SZ. In this study, patients with SZ showed significantly lower FAs in all SLF subcomponents in both hemispheres than HCs. We also demonstrated positive correlations in patients with SZ between FAs of right SLF II and FSIQ, as well as K-WAIS subtests in working memory (“arithmetic”) and perceptual organization (“block design”) and TR of NS at 6 months.
Previously, DTI studies10,21–24 have consistently reported reduced FAs in the tracts connecting the frontal cortex with the temporal and parietal cortices in patients with SZ, suggesting widespread WM disintegration during the course of illness. Our findings of lower FAs in all SLF subcomponents of patients with SZ, as compared to HCs, are consistent with those of previous DTI studies24–26 that reported WM disruptions in the SLF as a whole. However, there is a lack of studies specifically investigating the structural aberrations of the WM tracts in each SLF subcomponent in patients with SZ. DTI studies of healthy individuals20 have suggested distinct functions of the SLF subcomponents in accordance with their respective brain regions. SLF I originates from the superior parietal lobe and terminates within the supplementary motor and premotor areas in the frontal lobe, thus serving a function in proprioception and motor movements. SLF II connects the posterolateral parietal lobe to the dorsolateral prefrontal cortex and has been suggested to be involved primarily in visuospatial awareness and attention. SLF III originates from the supramarginal gyrus, terminates within the dorsal prefrontal cortex, and plays a role in somatosensory input, fine movements, phonetics, and language articulation. Lastly, the AF connects the temporal, frontal, and parietal lobes and is known to play a major role in speech processing. Our findings of WM alterations across all SLF subcomponents in patients with SZ may have some implications for the wide spectrum of symptomatology of this illness. These include (1) disorders of self-awareness in the context of dysfunctional proprioception;18,27 (2) abnormal visuospatial processing, which has been suggested to contribute to the development of positive symptoms;28 and (3) reduced ability to comprehend language, which may be associated with positive formal thought disorders29, verbal hallucinations, and delusions30,31. However, it is noteworthy that these arguments remain largely speculative and are beyond the scope of our study, warranting further studies to shed light on the specific associations between the disturbances in WM microstructures of SLF subcomponents and the various symptoms of SZ.
The positive correlation noted between FAs of the right SLF II and FSIQ in patients with SZ corroborates previous studies32–34 that reported an association between SLF disruptions and cognitive functions. In our study, voxel-wise correlation analyses and exploratory multiple linear regression for K-WAIS subtests also revealed that FAs of right SLF II were associated independently with “arithmetic” and “block design” subtests. This finding is consistent with that of a recent DTI study35 on healthy individuals, which reported significant associations between SLF FAs and working memory and perceptual organization. Although the specific role of each SLF subcomponent in intelligence remains uncertain, evidence supports the involvement of SLF II in executive function, including working memory. In their study using voxel-based lesion-symptom mapping, Kinoshita et al.36 proposed that both the frontal and parietal terminations of SLF II (along with SLF I) could be more specifically associated with spatial working memory. It can be speculated that disrupted microstructures in SLF II, a major domain of the dorsal pathway of attention20, may contribute to working memory deficits in patients with SZ. This is supported by our finding of significant correlations between FAs of the right SLF II and “arithmetic” subtest scores, which typically represent working memory functioning. Moreover, the positive correlations found between FAs of the right SLF II and “block design” subtest scores, which correspond to perceptual organization37, may be interpreted as further evidence supporting the notion that SLF II, especially in the non-dominant hemisphere, subserves the function of visuospatial awareness38,39. Notably, the region in the right SLF II, which showed a significant correlation with “arithmetic” subtest scores, extended over the WM regions from the precentral (frontal lobe) to the postcentral and supramarginal gyri (parietal lobe). On the other hand, such a result was shown only in the WM near the precentral gyrus for subtest scores of “block design”. This may be relevant in understanding the potential role of SLF II with respect to parietal and frontal lobe involvement in working memory performance, as discussed in previous studies40. Thus, these findings suggest the involvement of SLF II in the cognitive deficits obesrved in patients with SZ, specifically in working memory and perceptual organization.
Our study also demonstrated a positive correlation between FAs of the right SLF II and TR of NS at 6 months in patients with SZ. Findings from previous studies have generally supported the notion that WM alterations are associated with clinical outcomes in patients with SZ. For instance, Kochunov et al.41 reported that the WM regional vulnerability index, a measure of agreement between FA values at the individual level and the expected pattern from the ENIGMA study, correlated with processing speed and negative symptoms. This indicates a heightened risk of treatment resistance among patients with SZ who exhibit more severe WM impairments. A recent DTI study42 in patients with first-episode SZ reported that those with poor outcomes (<50% reduction in PANSS scores at 1 year) showed significantly different patterns of baseline FAs in various WM tracts, including the right SLF, as compared to those with good outcomes. This provides further evidence that WM microstructures may be relevant in the prediction of clinical prognosis in patients with SZ. WM abnormalities displayed as a pattern of decreased FAs and increased RDs and MDs, similar to that observed in the right SLF II of patients with SZ in our study, might be interpreted as a result of demyelination43, although the precise biological processes underlying this finding should be further investigated. In vitro and in vivo studies44–46 have suggested that atypical antipsychotics target the development of oligodendrocytes by promoting their proliferation and differentiation, thus potentially facilitating the reversal of myelin deficits that may contribute to the pathogenesis of SZ47. Hence, it can be proposed that the beneficial effects of atypical antipsychotics on oligodendrocytes are lost in patients with SZ with more pronounced WM demyelination in the right SLF II, resulting in a poorer TR to antipsychotics. Furthermore, it may be relevant to address the findings of previous DTI studies12,48 that reported a significant positive correlation between changes in SLF FAs and those in NS in individuals at an ultra-high risk of psychosis. In the aforementioned studies, Krakauer et al. proposed that aberrant WM maturation process in SLF could be responsible for the SZ symptomatology, particularly NS. Although differing in directionality, our findings support the argument that the SLF may be involved in the development of NS in SZ.
The results of this study may expand our knowledge regarding the role of the SLF, particularly SLF II, in cognitive impairment and NS in patients with SZ. The strengths of our study include a relatively large cohort, high follow-up rate, and the use of voxel-wise permutation analyses with secondary analyses to corroborate our findings.
This study had several limitations. First, all patients with SZ underwent DTI after the initiation of antipsychotic treatment. Although majority of them were drug-naive or drug-free (86.1%) and the mean duration of antipsychotic treatment before DTI was relatively short (6.8 ± 7.2 days), it is unclear whether the effect of antipsychotic drugs was negligible on the WM structures. Although post hoc subgroup analyses for drug-naive patients with SZ showed the same significant result as the original analysis, future investigations with a larger number of drug-naive patients are required to exclude the confounding effect of antipsychotic medication. Second, although all patients in this study were provided with fairly consistent treatment, it is necessary to consider that possible individual differences in treatment, such as supportive psychotherapy, might have confounded treatment outcomes. Third, our results from voxel-wise analyses should be interpreted with caution, due to potential sources of type I errors uncontrolled for multiple corrections, for example multiple time points of clinical assessments. Fourth, while the focus of this study was to investigate the WM microstructure of SLF subcomponents, a whole brain analysis may be necessary for a more comprehensive understanding of the neural correlates of cognitive deficits and NS in patients with SZ. Finally, future longitudinal studies with DTI data at multiple time points are warranted to investigate structural changes in the SLF during the course of SZ.
In conclusion, we demonstrated disrupted WM integrity (measured as lower FAs) of all SLF subcomponents in patients with SZ, as well as associations between altered WM microstructure of SLF II and poorer intellectual functioning and TR of NS in patients with SZ. These findings suggest that SLF, particularly SLF II, may contribute to cognitive deficits in patients with SZ, and indicate a potential role of SLF II as a neuroimaging biomarker for clinical outcomes of NS.
Methods
Participants
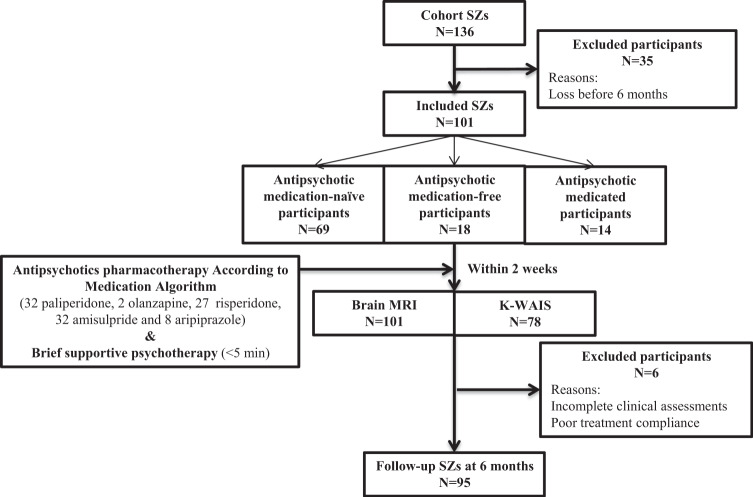
Figure 3 depicts a flowchart of the enrollment of participants in the present study. Participants in the CHA SZ cohort study were recruited from patients with SZ who received either inpatient or outpatient treatment at the Department of Psychiatry, CHA Bundang Medical Center, Seongnam, Republic of Korea, between February 2014 and September 2018. Patients with SZ were enrolled after having been diagnosed with SZ by experienced psychiatrists, in accordance with the Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR) and the structured clinical interview for DSM-IV-TR Axis I Disorders49. A total of 136 patients with SZ were recruited for the CHA SZ cohort study and underwent good-quality MR diffusion imaging at baseline.
Fig. 3. Flow-chart showing participants included in this analysis.
Flow-chart showing recruitment of patients with schizophrenia in this study (SZ, schizophrenia; MRI, magnetic resonance imaging; K-WAIS, Korean–Wechsler Adult Intelligence Scale).
One-hundred one of the 136 patients with SZ, who had continued pharmacological treatment for at least 6 months, were included in this study. At baseline, 69 of 101 patients with SZ (68.3%) were antipsychotic-naive, 18 (17.8%) were free of antipsychotics for at least 6 months, and the remaining 14 (13.9%) were on antipsychotics at the time of enrollment. Pharmacological treatment for all patients with SZ was provided in accordance with the Korean Medication Algorithm for SZ50 or Clinical Practice Guidelines: Treatment of SZ51. Psychological interventions provided to study participants were confined to brief supportive psychotherapy (<5 min). Six patients were excluded from the correlation analyses for TR of NS at 6 months due to incomplete clinical assessments.
Healthy individuals were recruited from the local community by using online and print advertisements. In this study, 101 individuals with no personal or first-relative family history of psychiatric disorders were included as HCs. The exclusion criteria for study enrollment were as follows: (1) current or past history of substance use disorders; (2) neurological diseases; (3) intellectual disability; and (4) head trauma with loss of consciousness. Left-handed individuals were also excluded based on the Edinburgh Handedness Inventory52.
All study procedures were approved by the Institutional Review Board of CHA Bundang Medical Center and adhered to the latest version of the Declaration of Helsinki and principles of Good Clinical Practice. (Approval number: 2019-05-030-009) All study participants and parents/guardians of minor participants (<18 years of age) provided written informed consent following a thorough explanation of the study procedures.
Clinical assessments
The intelligence level was assessed by trained clinical psychologists using the K-WAIS53 in patients with SZ and HCs. A detailed summary of K-WAIS scores in the two groups, as well as subtest scores for verbal IQ (information, digit span, vocabulary, arithmetic, comprehension, and similarities) and performance IQ (picture completion, picture arrangement, block design, object assembly, and digit symbols) are shown in Table 1.
The severity of clinical symptoms in patients with SZ was assessed by experienced psychiatrists using the PANSS54 at baseline and at 6 months. TR of NS was defined as the percentage reduction in the PANSS negative syndrome subscale from baseline to 6 months. In accordance with recent suggestions by Leucht et al.55 to use 0–6 scaling system instead of 1–7, “PANSS negative syndrome subscale at baseline −7” was used for the denominator of the fraction presented below.
Neuroimaging data acquisition
MRI scanning was performed for all study participants using a 3.0 T GE Signa HDxt scanner (GE Healthcare, Milwaukee, WI, USA) with an eight-channel phase-array head coil at CHA Bundang Medical Center, CHA University. MRI scans were acquired within two weeks of the initiation of antipsychotic medications for all antipsychotic-naive/free patients with SZ. The mean duration of pharmacotherapy before MRI of the drug-naive or drug-free participants was 5.09 ± 5.73 days. An echo-planar imaging (EPI) sequence was utilized for diffusion-weighted images with the following parameters: repetition time of 17,000 ms, echo time of 108 ms, field of view of 24 cm, 144 × 144 matrix, 1.7 mm slice thickness, and voxel size of 1.67 × 1.67 × 1.7 mm3. The eddy current effects were minimized by applying the double-echo option. To reduce the impact of EPI spatial distortions, an eight-channel coil and an array of spatial sensitivity encoding techniques (ASSET, GE Healthcare) with a sensitivity encoding speed-up factor of two were used. Seventy axial slices parallel to the anterior commissure–posterior commissure line covering the entire brain in 51 directions with a b-value of 900 s/mm2 and eight baseline scans with a b-value of 0 s/mm2 were acquired. From the diffusion-weighted images, the DTIs were approximated using the least-squares method (approximate scan time: 17 min). The average quality metrics of diffusion-weighted images from all participants, computed using the quality assessment method described by Roalf et al.56, were found to range in the “good” and “excellent” groups.
Tract-based spatial statistics analysis
Tract-based spatial statistics (TBSS, version 1.2) in the Functional MRI of the Brain (FMRIB) Software Library (FSL, version 6.0, Oxford, UK, http://www.fmrib.ox.ac.jk/fsl) were used for the statistical analysis of FAs according to the standard procedure57. The FSL of the FA images was used to proceed with the nonlinear regression and skeletonization stages to obtain ADs, RDs, and MDs, as well as to estimate the projection vectors onto the mean FA skeleton from each individual participant. Nonlinear distortion and skeleton projection can also be applied to other types of images. DTI preprocessing, including skull stripping using a brain extraction tool and eddy current correction, was performed using FSL. FA images were constructed by fitting a tensor model to the raw diffusion data58. The FMRIB nonlinear image registration tool was used to align the FA data of all participants in the standard space (Montreal Neurologic Institute 152 standard). All transformed FA images were combined and applied to the original FA map, resulting in a standard-space version. All adjusted images of FAs were averaged to generate an image of the mean FA, which was thinned (skeletonized) and used only at the center of the WM tract to produce a mean FA skeleton. To include only major fiber bundles, the threshold was set at FA > 0.2 (TBSS default). Voxel-wise permutation-based nonparametric inference was performed on the skeletonized FA data using FSL Randomize. Independent t-test and general linear model regression analysis were performed with 1000 permutations, and the significance level was set at p < 0.05, and corrected for the FWE rate. To avoid making an arbitrary choice of the cluster-forming threshold, threshold-free cluster enhancement with multiple comparison correction was used, while preserving the sensitivity benefits of cluster-wise correction.
Generation of masks of SLF and subcomponents
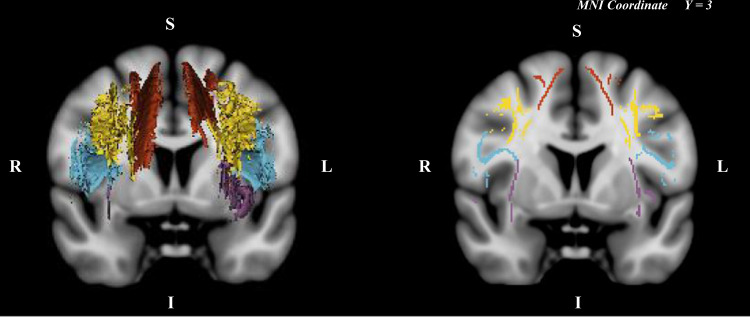
A mask representing the voxels that correspond to the SLF was manually drawn by experienced researchers (SJ and HJ) on anatomically defined areas on the mean FA skeleton map using 3D Slicer (version 4.8.1.). SLF subcomponent masks (SLF I, SLF II, SLF III, and AF) were drawn using the freehand paint tool (intraclass correlation coefficient > 0.95 for all subcomponents). The branches of the SLF were isolated according to well-validated definitions in previous studies59,60 (Fig. 4). AF was defined as fibers that did not pass through the anterior–posterior commissure line at the temporal lobe of the axial plane. On the coronal axis, the area passing through the superior frontal gyrus was defined as SLF I, the area passing through the middle frontal gyrus as SLF II, and the area passing through the inferior frontal/precentral gyrus as SLF III.
Fig. 4. Manually edited SLF subcomponents.
Regions of interest in both hemispheres extracted using 3D Slicer Red = superior longitudinal fasciculus I; Yellow = superior longitudinal fasciculus II; Blue = superior longitudinal fasciculus III; Purple = arcuate fasciculus (MNI, Montreal Neurologic Institute; R, right; L, left; S, superior; I, inferior).
Statistical analysis
Independent t-tests for continuous variables and chi-squared tests for categorical variables were applied to compare the demographic and clinical characteristics between patients with SZ and HCs. FAs of the SLF were compared between patients with SZ and HCs using voxel-wise comparison analysis in the TBSS. The same comparison analyses were performed for other DTI indices (MDs, ADs, and RDs). A brain mask was generated to specifically include SLF voxels that showed significant FA differences between the patients with SZ and HCs. Voxel-wise correlation analyses were performed for each group to investigate the correlations of FAs in the area in the mask with the FSIQ. Exploratory voxel-wise correlation analyses were additionally performed to investigate the associations of the K-WAIS subtest scores with the FAs of the areas that showed significant results. The same method was applied to TR of NS in patients with SZ. Corresponding SLF subcomponents for areas that showed significant results were then identified by inspections with respect to the ROI mask for the SLF subcomponents.
To further explore the nature of the group differences in WM integrity of manually edited SLF subcomponents between patients with SZ and HCs in addition to voxel-wise analysis, RM-ANOVA was performed (see Supplementary Methods and Results). In addition, to examine the independence of the associations of each K-WAIS subtest score with SLF FAs, multivariate multiple stepwise linear regression analysis was performed. Shapiro–Wilk tests were performed to confirm the normality of the variables. The FAs of the SLF voxels that showed significant correlations with the FSIQ scores were set as dependent variables, and the K-WAIS subtest scores were set as independent variables for the equation. Multivariate multiple stepwise linear regression analysis was performed to investigate the predictive value of FAs in areas that showed a significant association with the TR of NS. The 6-month TR of NS was set as the dependent variable. The FAs of the areas with significant associations as well as age, sex, duration of illness, and baseline PANSS scores were set as explanatory variables. All statistical analyses except for voxel-wise analyses were performed using Statistical Product and Service Solutions, version 26 (IBM Corp., Armonk, NY, USA).
Supplementary information
Acknowledgements
We are grateful to all of those who participated in this study. We acknowledge the assistance of Hye-Yeon Jung in drawing manually labeled SLF ROIs. This research was supported by the Basic Science Research Program of the National Research Foundation of Korea, funded by the Ministry of Science and ICT (grant number NRF-2019M3C7A1032262). This study was also funded in part by the Healthcare AI Convergence Research & Development Program through the National IT Industry Promotion Agency of Korea (NIPA), funded by the Ministry of Science and ICT (grant number S0316-21-1002). Both sets of funding were secured by S.H. Lee.
Author contributions
J.L.: conceptualization, formal analysis, data curation, and writing the original draft. J.-S.O.: conceptualization, formal analysis, data curation, and writing the original draft. C.I.P.: supervision, validation. M.B.: supervision, validation. G.S.: supervision, validation. S.J.: conceptualization, formal analysis, data curation, writing—original draft, supervision, and validation. S-H.L.: conceptualization, formal analysis, data curation, writing—original draft, supervision, and validation.
Data availability
The data that would be necessary to interpret, replicate and build upon the methods or findings reported in this article are available on request from the corresponding author S.J. The data are not publicly available because of ethical restrictions that protect patients’ privacy and consent.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Joonho Lee, Jong-Soo Oh.
Change history
7/7/2022
A Correction to this paper has been published: 10.1038/s41537-022-00266-4
Contributor Information
Sra Jung, Email: srajung89@gmail.com.
Sang-Hyuk Lee, Email: drshlee@cha.ac.kr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41537-022-00253-9.
References
- 1.Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr. Dis. Treat. 2020;16:519–534. doi: 10.2147/NDT.S225643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aleman A, et al. Treatment of negative symptoms: Where do we stand, and where do we go? Schizophr. Res. 2017;186:55–62. doi: 10.1016/j.schres.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Kelly S, et al. Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol. Psychiatry. 2018;23:1261–1269. doi: 10.1038/mp.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochunov P, et al. Association of white matter with core cognitive deficits in patients with schizophrenia. JAMA Psychiatry. 2017;74:958–966. doi: 10.1001/jamapsychiatry.2017.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bopp MHA, et al. White matter integrity and symptom dimensions of schizophrenia: a diffusion tensor imaging study. Schizophr. Res. 2017;184:59–68. doi: 10.1016/j.schres.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 6.Vitolo E, et al. White matter and schizophrenia: A meta-analysis of voxel-based morphometry and diffusion tensor imaging studies. Psychiatry Res. Neuroimaging. 2017;270:8–21. doi: 10.1016/j.pscychresns.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Kawamura K, Naito J. Corticocortical projections to the prefrontal cortex in the rhesus monkey investigated with horseradish peroxidase techniques. Neurosci. Res. 1984;1:89–103. doi: 10.1016/S0168-0102(84)80007-3. [DOI] [PubMed] [Google Scholar]
- 8.Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM. Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct. Funct. 2014;219:269–281. doi: 10.1007/s00429-012-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutierrez-Galve L, et al. Changes in the frontotemporal cortex and cognitive correlates in first-episode psychosis. Biol. Psychiatry. 2010;68:51–60. doi: 10.1016/j.biopsych.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karlsgodt KH, et al. Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent-onset schizophrenia. Biol. Psychiatry. 2008;63:512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Peng X, et al. Reduced white matter integrity associated with cognitive deficits in patients with drug-naive first-episode schizophrenia revealed by diffusion tensor imaging. Am. J. Transl. Res. 2020;12:4410–4421. [PMC free article] [PubMed] [Google Scholar]
- 12.Kristensen TD, et al. Changes in negative symptoms are linked to white matter changes in superior longitudinal fasciculus in individuals at ultra-high risk for psychosis. Schizophr. Res. 2021;237:192–201. doi: 10.1016/j.schres.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 13.DeRosse P, et al. Adding insult to injury: childhood and adolescent risk factors for psychosis predict lower fractional anisotropy in the superior longitudinal fasciculus in healthy adults. Psychiatry Res. 2014;224:296–302. doi: 10.1016/j.pscychresns.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luck D, et al. Fronto-temporal disconnectivity and clinical short-term outcome in first episode psychosis: a DTI-tractography study. J. Psychiatr. Res. 2011;45:369–377. doi: 10.1016/j.jpsychires.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Chuang JY, et al. Brain structural signatures of negative symptoms in depression and schizophrenia. Front. Psychiatry. 2014;5:116. doi: 10.3389/fpsyt.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JS, Han K, Lee SK, Seok JH, Kim JJ. Altered structural connectivity and trait anhedonia in patients with schizophrenia. Neurosci. Lett. 2014;579:7–11. doi: 10.1016/j.neulet.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Zeng B, et al. Abnormal white matter microstructure in drug-naive first episode schizophrenia patients before and after eight weeks of antipsychotic treatment. Schizophr. Res. 2016;172:1–8. doi: 10.1016/j.schres.2016.01.051. [DOI] [PubMed] [Google Scholar]
- 18.Makris N, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 19.Vassal F, et al. Combined DTI tractography and functional MRI study of the language connectome in healthy volunteers: extensive mapping of white matter fascicles and cortical activations. PLoS ONE. 2016;11:e0152614. doi: 10.1371/journal.pone.0152614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima R, Kinoshita M, Shinohara H, Nakada M. The superior longitudinal fascicle: reconsidering the fronto-parietal neural network based on anatomy and function. Brain Imaging Behav. 2020;14:2817–2830. doi: 10.1007/s11682-019-00187-4. [DOI] [PubMed] [Google Scholar]
- 21.Chawla N, Deep R, Khandelwal SK, Garg A. Reduced integrity of superior longitudinal fasciculus and arcuate fasciculus as a marker for auditory hallucinations in schizophrenia: A DTI tractography study. Asian J. Psychiatr. 2019;44:179–186. doi: 10.1016/j.ajp.2019.07.043. [DOI] [PubMed] [Google Scholar]
- 22.Epstein KA, et al. White matter abnormalities and cognitive impairment in early-onset schizophrenia-spectrum disorders. J. Am. Acad. Child. Adolesc. Psychiatry. 2014;53:362-372 e361-362. doi: 10.1016/j.jaac.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davenport ND, Karatekin C, White T, Lim KO. Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Res. 2010;181:193–198. doi: 10.1016/j.pscychresns.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shergill SS, et al. A diffusion tensor imaging study of fasciculi in schizophrenia. Am. J. Psychiatry. 2007;164:467–473. doi: 10.1176/ajp.2007.164.3.467. [DOI] [PubMed] [Google Scholar]
- 25.Hubl D, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch. Gen. Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- 26.Kyriakopoulos M, Bargiotas T, Barker GJ, Frangou S. Diffusion tensor imaging in schizophrenia. Eur. Psychiatry. 2008;23:255–273. doi: 10.1016/j.eurpsy.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Arnfred SM, Raballo A, Morup M, Parnas J. Self-disorder and brain processing of proprioception in schizophrenia spectrum patients: a re-analysis. Psychopathology. 2015;48:60–64. doi: 10.1159/000366081. [DOI] [PubMed] [Google Scholar]
- 28.Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol. Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagels A, Kircher T, Grosvald M, Steines M, Straube B. Evidence for gesture-speech mismatch detection impairments in schizophrenia. Psychiatry Res. 2019;273:15–21. doi: 10.1016/j.psychres.2018.12.107. [DOI] [PubMed] [Google Scholar]
- 30.Catani M, et al. Altered integrity of perisylvian language pathways in schizophrenia: relationship to auditory hallucinations. Biol. Psychiatry. 2011;70:1143–1150. doi: 10.1016/j.biopsych.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Robbins M. The language of schizophrenia and the world of delusion. Int. J. Psychoanal. 2002;83:383–405. doi: 10.1516/ATTU-2M15-HX4F-5R2V. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Hu Y, Wang Y, Weng J, Chen F. Individual structural differences in left inferior parietal area are associated with schoolchildrens’ arithmetic scores. Front. Human Neurosci. 2013;7:844. doi: 10.3389/fnhum.2013.00844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matejko AA, Price GR, Mazzocco MM, Ansari D. Individual differences in left parietal white matter predict math scores on the Preliminary Scholastic Aptitude Test. Neuroimage. 2013;66:604–610. doi: 10.1016/j.neuroimage.2012.10.045. [DOI] [PubMed] [Google Scholar]
- 34.Moeller K, Willmes K, Klein E. A review on functional and structural brain connectivity in numerical cognition. Front. Hum. Neurosci. 2015;9:227. doi: 10.3389/fnhum.2015.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koshiyama D, et al. Association between the superior longitudinal fasciculus and perceptual organization and working memory: A diffusion tensor imaging study. Neurosci. Lett. 2020;738:135349. doi: 10.1016/j.neulet.2020.135349. [DOI] [PubMed] [Google Scholar]
- 36.Kinoshita M, et al. Chronic spatial working memory deficit associated with the superior longitudinal fasciculus: a study using voxel-based lesion-symptom mapping and intraoperative direct stimulation in right prefrontal glioma surgery. J. Neurosurg. 2016;125:1024–1032. doi: 10.3171/2015.10.JNS1591. [DOI] [PubMed] [Google Scholar]
- 37.Saklofske, D. H. et al. Clinical Interpretation of the WAIS-III and WMS-III. (Academic Press, 2003).
- 38.Nakajima R, et al. Damage of the right dorsal superior longitudinal fascicle by awake surgery for glioma causes persistent visuospatial dysfunction. Sci. Rep. 2017;7:17158. doi: 10.1038/s41598-017-17461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shulman GL, et al. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. J. Neurosci. 2010;30:3640–3651. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koenigs M, Barbey AK, Postle BR, Grafman J. Superior parietal cortex is critical for the manipulation of information in working memory. J. Neurosci. 2009;29:14980–14986. doi: 10.1523/JNEUROSCI.3706-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kochunov P, et al. White matter in schizophrenia treatment resistance. Am. J. Psychiatry. 2019;176:829–838. doi: 10.1176/appi.ajp.2019.18101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng J, et al. Pretreatment abnormalities in white matter integrity predict one-year clinical outcome in first episode schizophrenia. Schizophr. Res. 2021;228:241–248. doi: 10.1016/j.schres.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 43.Alexander AL, et al. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect. 2011;1:423–446. doi: 10.1089/brain.2011.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao L, et al. Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol. Psychiatry. 2008;13:697–708. doi: 10.1038/sj.mp.4002064. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, et al. Quetiapine alleviates the cuprizone-induced white matter pathology in the brain of C57BL/6 mouse. Schizophr. Res. 2008;106:182–191. doi: 10.1016/j.schres.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, et al. Quetiapine enhances oligodendrocyte regeneration and myelin repair after cuprizone-induced demyelination. Schizophr. Res. 2012;138:8–17. doi: 10.1016/j.schres.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Gouvea-Junqueira D, et al. Novel treatment strategies targeting myelin and oligodendrocyte dysfunction in schizophrenia. Front. Psychiatry. 2020;11:379. doi: 10.3389/fpsyt.2020.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krakauer K, et al. White matter maturation during 12 months in individuals at ultra-high-risk for psychosis. Acta Psychiatr. Scand. 2018;137:65–78. doi: 10.1111/acps.12835. [DOI] [PubMed] [Google Scholar]
- 49.First, M. B., Spitzer, R. L., Gibbon, M. & Williams, J. B. Structured Clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P, 2002).
- 50.Lee JS, et al. Korean medication algorithm for schizophrenia 2019, second revision: treatment of psychotic symptoms. Clin. Psychopharmacol. Neurosci. 2019;18:386–394. doi: 10.9758/cpn.2020.18.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canadian Psychiatric, A. Clinical practice guidelines. Treatment of schizophrenia. Can. J. Psychiatry. 2005;50:7S–57S. [PubMed] [Google Scholar]
- 52.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 53.Yeom, T., Park, Y., Oh, K., Kim, J. & Lee, Y. Korean Wechsler adult intelligence scale (K-WAIS) manual. (Seoul, Republic of Korea: Guidence 1992).
- 54.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 55.Leucht S, Kissling W, Davis JM. The PANSS should be rescaled. Schizophr. Bull. 2010;36:461–462. doi: 10.1093/schbul/sbq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roalf DR, et al. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage. 2016;125:903–919. doi: 10.1016/j.neuroimage.2015.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith SM, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 58.Smith SM. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48:82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 60.Fitzgerald J, et al. Abnormal fronto-parietal white matter organisation in the superior longitudinal fasciculus branches in autism spectrum disorders. Eur. J. Neurosci. 2018;47:652–661. doi: 10.1111/ejn.13655. [DOI] [PubMed] [Google Scholar]
- 61.Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am. J. Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that would be necessary to interpret, replicate and build upon the methods or findings reported in this article are available on request from the corresponding author S.J. The data are not publicly available because of ethical restrictions that protect patients’ privacy and consent.