Abstract
Background:
Metastasized pancreatic neuroendocrine tumors are the leading cause of death in patients with multiple endocrine neoplasia type 1. Aside from tumor size, prognostic factors of pancreatic neuroendocrine tumors are largely unknown. The present study aimed to assess whether the prognosis of patients with resected multiple endocrine neoplasia type 1-related nonfunctioning pancreatic neuroendocrine tumors differs from those with resected multiple endocrine neoplasia type 1-related insulinomas and assessed factors associated with prognosis.
Methods:
Patients who underwent resection of a multiple endocrine neoplasia type 1-related pancreatic neuroendocrine tumors between 1990 and 2016 were identified in 2 databases: the DutchMEN Study Group and the International MEN1 Insulinoma Study Group databases. Cox regression was performed to compare liver metastases-free survival of patients with a nonfunctioning pancreatic neuroendocrine tumors versus those with an insulinoma and to identify factors associated with liver metastases-free survival.
Results:
Out of 153 patients with multiple endocrine neoplasia type 1, 61 underwent resection for a nonfunctioning pancreatic neuroendocrine tumor and 92 for an insulinoma. Of the patients with resected lymph nodes, 56% (18/32) of nonfunctioning pancreatic neuroendocrine tumors had lymph node metastases compared to 10% (4/41) of insulinomas (P = .001). Estimated 10-year liver metastases-free survival was 63% (95% confidence interval 42%–76%) for nonfunctioning pancreatic neuroendocrine tumors and 87% (72%–91%) for insulinomas. After adjustment for size, World Health Organization tumor grade, and age, nonfunctioning pancreatic neuroendocrine tumors had an increased risk for liver metastases or death (hazard ratio 3.04 [1.47–6.30]). In pancreatic neuroendocrine tumors ≥2 cm, nonfunctioning pancreatic neuroendocrine tumors (2.99 [1.22–7.33]) and World Health Organization grade 2 (2.95 [1.02–8.50]) were associated with liver metastases-free survival.
Conclusion:
Patients with resected multiple endocrine neoplasia type 1-related nonfunctioning pancreatic neuroendocrine tumors had a significantly lower liver metastases-free survival than patients with insulinomas. Postoperative counseling and follow-up regimens should be tumor type specific and at least consider size and World Health Organization grade.
Introduction
Multiple endocrine neoplasia type 1 (MEN1) is an autosomal dominant inherited cancer syndrome caused by a germline mutation in the MEN1 tumor suppressor gene located on chromosome 11q13 encoding for the protein menin.1,2 The trait occurs in 2 to 3 per 100,000 persons.1 One of the hallmark manifestations of the disease is pancreatic neuroendocrine tumors (pNETs), which have a prevalence of 56%.3 Moreover, the age-related penetrance of pNETs gradually increases to over 80% by the age of 80 years.3,4 Metastasized pNETs are the leading cause of death in patients with MEN1 and significantly reduce life expectancy.3,5,6
Clinically, pNETs are classified as nonfunctioning or functioning tumors, depending on the presence of a distinct clinical syndrome caused by excessive hormone production. Nonfunctioning pancreatic neuroendocrine tumors (NF-pNETs) are the most prevalent pNETs, whereas insulinomas are the most frequently encountered functioning pNETs.7-10 Tumor formation occurs as a result of independent clonal events leading to a loss of heterozygosity of the wild-type MEN1 allele, which is observed in pNETs, microadenomas, and mono-hormonal endocrine cell clusters.11 Despite the assumed shared origin of pNETs, varying survival rates have been reported for patients with MEN1-related NF-pNETs and functioning pNETs.5,12,13
Although the majority of pNETs in MEN1 follow a relatively indolent natural course, a subgroup of pNETs metastasizes to the liver and subsequently leads to decreased survival.6,12,14-16 Therapy should be aimed at maintaining a good quality of life by relieving symptoms associated with excessive hormone production as well as preventing liver metastases.17 Besides tumor size as a predictor of liver metastases, prognostic factors of MEN1-related pNETs are largely unknown. It is generally assumed that insulinomas in MEN1 have a more favorable prognosis compared with NF-pNETs because of a relatively small tumor size, early symptomatology with subsequent treatment, or because of differences in grade.5,8,18 Patient counseling is inevitable in MEN1 daily clinical care considering the high, age-related prevalence of pNETs, intensive lifelong screening programs, and considerable operative morbidity in those referred for surgery.17,19 Nevertheless, the major unmet need for adequate patient counseling is insight in prognosis of MEN1-related pNETs.20,21 Knowledge of differences in prognostic factors will contribute to tailoring of surgical indications, timing and extent of surgery, and postoperative follow-up regimens in individual patients. Therefore, this study aimed to assess if patients with a resected MEN1-related NF-pNET have a different prognosis than those with a resected MEN1-related insulinoma. Furthermore, survival and factors associated with liver metastases-free survival were assessed to come to meaningful advice regarding postoperative counseling and follow-up specifically for NF-pNETs and insulinomas.
Methods
Study design and patient selection
For this observational study, patients with NF-pNETs and insulinomas were selected from the DutchMEN Study Group (DMSG) cohort.22 Considering the rarity of MEN1-related insulinomas, patients with a MEN1-related insulinoma were additionally identified from a MEN1 collaboration including European and North American hospitals. Patients were eligible if they had a resection for a NF-pNET or an insulinoma between 1990 and 2016 with histopathological neuroendocrine tumor confirmation and were followed for at least 1 year after surgery. MEN1 diagnosis was established according to the most recent practice guidelines.17 Patients operated on for a pNET before 1990 or with distant metastases at diagnosis were excluded. Patients with glucagonomas, vasoactive intestinal peptidomas, and somatostatinomas were not included considering their rarity (<2%).23 The study protocol was approved by the medical ethics committees or institutional review boards of all participating centers.
The DSMG database22
The DMSG database includes patients with MEN1 aged 16 years and older under treatment in 1 of the Dutch University Medical Centers. Patients were identified by review of the hospital diagnosis databases. Over 90% of the total Dutch MEN1 population is included in the database. Clinical and demographic data were collected every 3 months by standardized medical record review, according to a predefined protocol.
International MEN1 Insulinoma Study Group24
The collaboration includes the population-based database from the DMSG, the national database from the Groupe d’étude des Tumeurs Endocrines (GTE) from France, and 7 MEN1 expert centers including European and North American hospitals. Patients with a MEN1-related insulinoma were identified by review of the hospital databases using International Classification of Diseases codes.24 Clinical and demographic data were gathered by investigators from every hospital according to the same predefined protocol.
Clinical definitions
A pNET was considered as NF-pNET in case of positive histopathology or computed tomography (CT), magnetic resonance imaging (MRI), and/or endoscopic ultrasonography diagnostic of a pNET in combination with the absence of excessive hormone production provoking a distinct clinical tumor syndrome.16 The date of diagnosis was recorded as the date of the first positive imaging or the date of pathology.16,25
The presence of an insulinoma was based on a positive, 48- to 72-hour supervised fast test.26,27 If no 72-hour supervised fast test was performed, the insulinoma diagnosis was based on clinical criteria: symptoms or signs of hypoglycemia with concomitant biochemical endogenous hyperinsulinemic hypoglycemia according to clinical practice guidelines.26,27 The date of diagnosis was based on the date of the supervised fast test or the date of symptoms accompanied by endogenous hyperinsulinemic hypoglycemia. Patients with an insulinoma were analyzed in the insulinoma group, also in the presence of coexisting NF-pNETs.
Gastrinomas in MEN1 have a predominant duodenal origin and rarely occur in the pancreas.10,28,29 Therefore, patients with hypergastrinemia and a pNET were regarded as patients with a coexisting duodenal gastrinoma.
Multiple enucleations, a distal pancreatectomy plus enucleation, Whipple plus enucleation, and Whipple plus distal pancreatectomy were considered as combined resections.
Pathology
Pancreatic specimens were examined for the number of pNETs and size of the largest pNET. The size of the largest pNET was used for analysis. In patients with a resection for an insulinoma, positive immunohistochemistry for insulin classified the tumor as insulinoma. If insulin staining was negative or if detailed information on immunohistochemical staining was missing, the size of the largest pNET was used for analysis. Specimens were examined for lymph node metastases. Any peripancreatic lymph node harboring neuroendocrine tumor cells was considered as lymph node metastasis, regardless of hormone expression. If no lymph nodes were observed during the examination, it was assumed that no lymph nodes were resected. Tumors were examined for mitotic rate and Ki67 labeling. Tumor grade was classified according to the World Health Organization (WHO) 2017 classification: Grade 1 (G1): Ki67 labelling index (LI) <3 and mitosis <2 per 10 high power fields (HPF); G2: Ki67 LI 3–20 and/or mitoses 2–20/10 HPF; G3 Ki67 LI >20 and/or mitosis >20/10 HPF.30 In case of a contradiction between mitotic rate and Ki67 labeling, WHO grade was determined by the highest of both.31,32
For patients with multiple pancreatic resections for pNETs, tumor characteristics from the first resection were used for analysis. If the time between 2 resections was less than 3 months, characteristics of the largest tumor were obtained, since it is to be expected that the largest tumor was present at the time of the first operation and likely determines prognosis.
Outcomes
Primary outcomes were the occurrence of pNET-related liver metastases during follow-up and overall survival. A composite endpoint (pNET liver metastases and/or overall survival) was computed. Liver metastases were defined as (1) pathologically proven or (2) radiologically confirmed. If at least 2 consecutive CT/MRI reports described lesions suspicious for liver metastases, radiology was documented as positive. Pre- and postoperative assessment of the liver was performed according to local availability of imaging modalities and considered conventional imaging (CT or MRI) during the study period. Intraoperative assessment of the liver was guided by the individual surgeon’s preference and might have included bimanual palpation or intraoperative ultrasonography. The most likely cause of liver metastases was determined by multidisciplinary team discussion. Causes of death were captured from medical records. Deaths caused by MEN1 manifestations and MEN1-related therapy were considered as MEN1-related. Other causes of death were regarded as non-MEN1-related.3
Statistical analysis
Continuous variables were reported as median (range or interquartile range [IQR]) and categorical variables as counts (proportions). Mann-Whitney U tests were used for comparison of continuous variables, and categorical variables were compared using χ2 or Fisher exact tests. Follow-up time started at the date of surgery and ended at the date of (1) diagnosis of pNET-related liver metastases or (2) death or (3) last follow-up (ie, date of last visit or Jan 1st 2018). Kaplan-Meier curves were plotted and survival probability estimates were obtained.33 The log-rank test was used for univariable survival comparison. Pancreatic neuroendocrine tumor size was dichotomized to <2 cm and ≥2 cm.25,34
Univariable and multivariable Cox proportional hazard regression analyses were performed with the time to pNET-related liver metastases or death as outcome. Considering the relatively low number of outcomes, 4 covariates could be included in the multivariable analysis, which were selected based on clinical reasoning and previous literature.4,25,31 Besides pNET functionality (NF-pNET versus insulinoma), pNET size in mm, WHO grade (G2/G3 versus G1), and age in years were included in the model.4,25,31 A stratified Cox model was performed for pNETs <2 cm and ≥2 cm. Crude and adjusted hazard ratios (HR) and corresponding 95% confidence intervals (95% CI) were calculated. Cox proportional hazard regression assumptions were formally tested and graphically assessed using scaled Schoenfeld residuals; the assumptions were not violated. Tied events were handled using the exact method. A sensitivity analysis was performed including pNET functionality (NF-pNET versus insulinoma), pNET size in mm, and lymph node status (metastases versus none resected versus no metastases).
In addition, univariable Kaplan-Meier and/or Cox proportional hazard regression were performed to assess the influence of age at surgery (in years), sex (female versus male), pNET size in mm, pNET size (≥2 cm vs <2 cm), pNET functionality (NF-pNET versus insulinoma), WHO grade (G2/G3 versus G1), lymph node status (lymph node metastases versus no lymph node metastases versus no lymph nodes resected), and time from diagnosis until surgery (in years) on time to liver metastases or death. The latter analyses were additionally performed for the subgroups of NF-pNETs and insulinomas. In addition, these analyses were performed with pNET size arbitrarily categorized into <2 cm, 2 to 3 cm, and ≥3 cm.
Missing data were encountered for variables used in the Cox regression, and these were considered as missing at random and, therefore, imputed using multiple imputation with the iterative Markov chain Monte Carlo method creating 40 datasets.35,36 Variables listed in Table I were used as predictor variables for multiple imputation, together with the primary outcome (known in all patients) and the Nelson-Aalen estimator.37,38 For multiple imputation of time-to-event data, the event and the censoring time should be taken into account. The cumulative baseline hazard at the time of the event or censoring is often unknown but can be approximated by the Nelson-Aalen estimator.38 HRs with corresponding 95% CIs were pooled using Rubin’s rules.39
Table I.
Demographic, preoperative, surgical, and histopathological characteristics
| Characteristic | Overall (n = 153) | NF-pNET (n = 61) | Insulinoma (n = 92) | P value |
|---|---|---|---|---|
| Age at pNET diagnosis in y, median [range] | 36 [6–82] | 40 [15–73] | 32 [6–82] | .002 |
| Missing data (%) | 1 (1%) | 0 (0%) | 1 (1%) | |
| Age at surgery in y, median [range] | 38 [6–82] | 41 [20–73] | 34 [6–82] | .002 |
| Time from diagnosis until surgery in y, median [range] | 0.5 [0–15] | 0.5 [0–15] | 0.3 [0–15] | .006 |
| Missing data (%) | 1 (1%) | 0 | 1 (1%) | |
| Sex (%) | ||||
| Male | 66 (43%) | 34 (56%) | 32 (35%) | .010 |
| Female | 87 (57%) | 27 (44%) | 60 (65%) | |
| Size largest pNET on preoperative imaging in mm, median [range] | 20 [4–98] | 27 [8–86] | 20 [4–98] | .009 |
| Missing data (%) | 18 (12%) | 5 (8%) | 13 (14%) | |
| Number of pNETs on preoperative imaging (%) | ||||
| 0 | 5 (3%) | 4 (7%) | 1 (1%) | .221 |
| 1 | 71 (46%) | 29 (48%) | 42 (48%) | |
| 2 | 29 (19%) | 9 (15%) | 20 (23%) | |
| ≥3 | 42 (28%) | 18 (30%) | 24 (28%) | |
| Missing data (%) | 6 (4%) | 1 (1%) | 5 (5%) | |
| Suspected lymph node metastases on preoperative conventional imaging (%) | 5 (3%) | 5 (8%) | 0 (0%) | .009 |
| Missing data (%) | 1 (1%) | 0 | 1 (1%) | |
| Type of resection (%) | ||||
| Enucleation | 23 (15%) | 9 (15%) | 14 (15%) | - |
| Multiple enucleations | 6 (4%) | 2 (3%) | 4 (4%) | |
| Distal pancreatectomy | 71 (46%) | 33 (54%) | 38 (41%) | |
| Distal pancreatectomy and enucleation | 27 (18%) | 2 (3%) | 25 (27%) | |
| Whipple or PPPD | 10 (7%) | 5 (8%) | 5 (5%) | |
| Whipple/PPPD and enucleation | 2 (1%) | 0 (0%) | 2 (2%) | |
| Whipple/PPPD and distal pancreatectomy | 5 (3%) | 3 (5%) | 2 (2%) | |
| Pancreatic body resection | 1 (1%) | 1 (2%) | 0 (0%) | |
| Total pancreatectomy | 8 (5%) | 6 (10%) | 2 (2%) | |
| Combined resection (%) | 40 (26.1%) | 7 (12%) | 33 (36%) | .001 |
| Number of pNETs in the resection specimen (%) | ||||
| 1 | 39 (26%) | 18 (30%) | 21 (24%) | .100 |
| 2 | 27 (18%) | 15 (25%) | 12 (14%) | |
| ≥3 | 83 (56%) | 28 (46%) | 55 (63%) | |
| Missing data (%) | 3 (2%) | 0 (0%) | 4 (4%) | |
| Size largest pNET pathology in mm, median [range] | 20 [3–120] | 25 [3–120] | 20 [5–110] | .045 |
| Missing data (%) | 13 (8%) | 3 (5%) | 10 (11%) | |
| Lymph node metastases (%) | ||||
| Lymph node metastases | 22 (14%) | 18 (30%) | 4 (4%) | < .001 |
| No lymph node metastases | 51 (33%) | 14 (23%) | 37 (40%) | |
| No lymph nodes resected | 61 (40%) | 29 (48%) | 32 (35%) | |
| Missing data (%) | 19 (12%) | 0 (0%) | 19 (21%) | |
| WHO grade | ||||
| G1 | 87 (78%) | 45 (79%) | 42 (76%) | .539 |
| G2 | 24 (21%) | 11 (19%) | 13 (24%) | |
| G3 | 1 (1%) | 1 (2%) | 0 | |
| Missing data (%) | 41 (27%) | 4 (7%) | 37 (40%) |
PPPD, pylorus-preserving pancreatoduodenectomy.
P values of < .05 were considered statistically significant. Statistical analysis was performed using SPSS version 25.0 (IBM Corp, Armonk, NY), R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria) with ‘survival’ and ‘Mice’ packages, and Graphpad Prism version 7.02 (GraphPad Software, Inc, San Diego, CA).
Results
A total of 153 patients underwent resection for a pNET, 61 for a NF-pNET and 92 for an insulinoma (Fig 1). Baseline characteristics are shown in Table I. Patients with NF-pNETs were older at diagnosis, older at surgery, more often male, had larger tumors on imaging, more often with suspected lymph node metastases on imaging, and a longer time between diagnosis and surgery. Twenty-six patients (41%) in the NF-pNET group and 15 patients (16%) in the insulinoma group were operated on more than 1 year after diagnosis. Combined resections were more often performed for insulinomas. All insulinoma patients, except 1, were cured from hyperinsulinemic hypoglycemia immediately postoperative.
Fig 1.
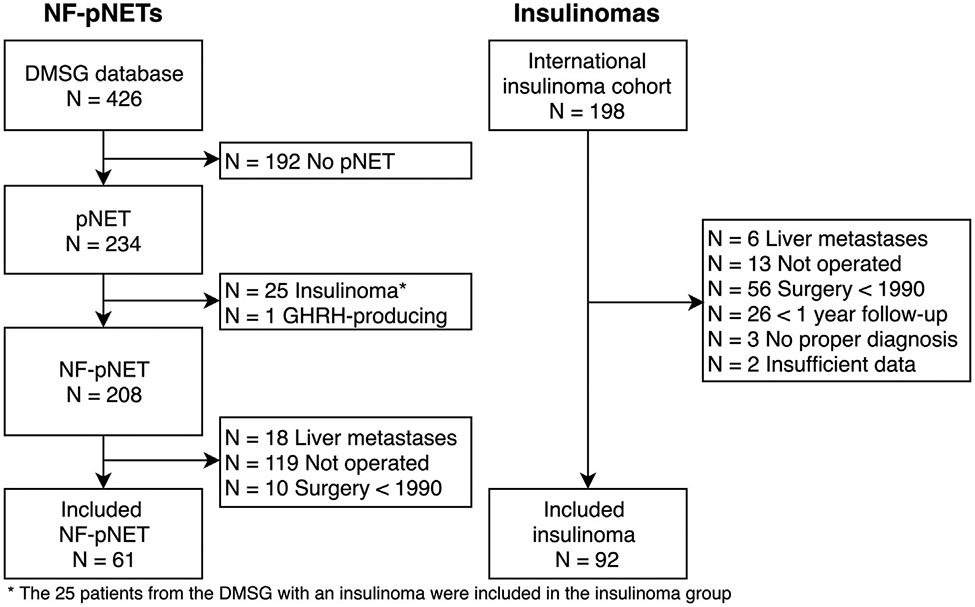
Flowchart of patient inclusion. GHRH, growth hormone-releasing hormone.
Pathology
Median size of the largest pNET in the surgical specimen was larger for NF-pNETs compared with insulinomas (median 25 mm [IQR 15–35 mm] vs 20 mm [IQR 15–25 mm], respectively; Table I). Tumor size, lymph node status, and WHO grade were missing in 8%, 12%, and 27%, respectively. Thirty-eight NF-pNETs (64%; 38/59) and 47 insulinomas (57%; 47/82) were larger than 2 cm. Multiple insulin immunopositive pNETs were observed in 24 (30%) patients with insulinomas. Three patients in the insulinoma group had negative insulin immunohistochemistry, all of whom were cured.
Of the 73 patients with lymph nodes resected, lymph nodes were tumor positive in 18 patients with NF-pNETs (56%; 18/32) compared to 4 (10%; 4/41) with insulinomas (P < 0.001; Table I). Lymph node metastases were more often observed in patients with a pNET ≥2 cm (17/44, 39%) compared with pNET <2 cm (4/25, 16%) (P = .050); 3 patients with lymph node metastases had missing tumor size. Of the 44 patients with resected lymph nodes and a pNET ≥2 cm, metastatic lymph nodes were observed in 15 patients (63%; 15/24) with NF-pNETs compared with 2 patients (10%; 2/20) with insulinomas (P = .001). No differences in WHO grade (Ki-67 and/or mitosis) were observed between NF-pNETs and insulinomas. In 1 patient, the NF-pNET was considered a well-differentiated WHO G3 tumor.
Long-term outcomes
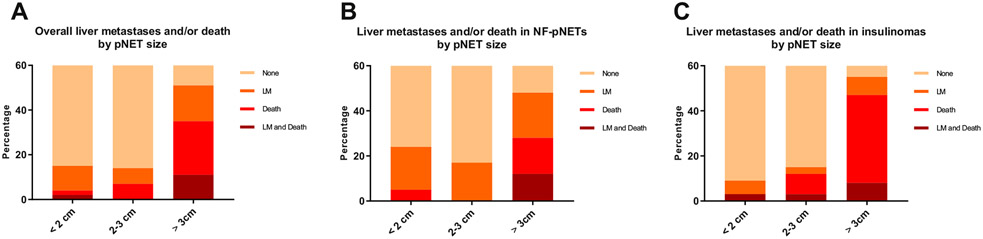
Long-term outcomes are summarized in Supplementary Table I. After a median follow-up of 8.8 years (range 0.3–25.3 years), 37 patients (24%) had developed liver metastases or died, which occurred more often in patients with NF-pNETs compared to insulinomas (22/61 (36%) vs 15/92 (16%); P = .005). No differences were observed regarding follow-up time between the NF-pNETs and insulinoma group. Liver metastases were observed in 15 patients (25%) with a NF-pNET and 6 (7%) with an insulinoma. Two of the 6 patients with a resected insulinoma had recurrent hypoglycemia at the time of liver metastases diagnosis. Median time from surgery until the development of liver metastases or death was significantly shorter for NF-pNETs (5.3 vs 9.5 years; P = .036). The development of subsequent liver metastases or death occurred after 5 and 10 years in 24 (65%) and 11 patients (30%), respectively. Causes of death are listed in Supplementary Table II. The percentage of liver metastases, death, or both correlated with tumor size (Fig 2). The proportion of patients with NF-pNETs developing liver metastases was higher for all tumor sizes (<2, 2–3, and ≥3 cm) compared to patients with an insulinoma, whereas patients with a resected insulinoma tended to die more often without pNET-related liver metastases.
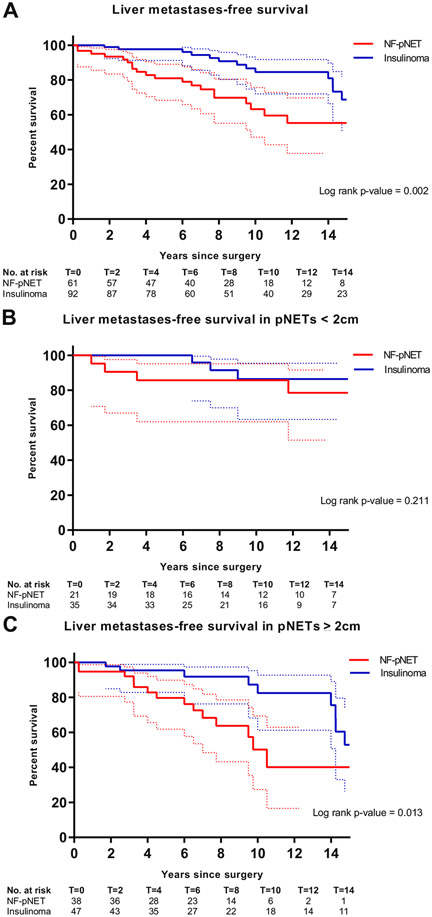
Fig 2.
(A) LMFS of patients with resected MEN1-related pancreatic neuroendocrine tumors. (B) LMFS of patients with resected MEN1-related pancreatic neuroendocrine tumors <2 cm. (C) LMFS of patients with resected MEN1-related pancreatic neuroendocrine tumors ≥2 cm.
Liver metastases-free survival after resection of NF-pNETs versus insulinomas
Patients with a resected NF-pNET had a significantly reduced liver metastases-free survival (LMFS) compared to those with a resected insulinoma (log-rank P value .002) (Fig 3). Ten-year LMFS probability estimates were 63% (95% CI 42%–76%) for NF-pNETs vs 87% (95% CI 72%–91%) for insulinomas. Of the 60 patients with pNETs <2 cm, 9 (15%) developed liver metastases or died; no survival differences were observed between patients with NF-pNETs and insulinomas (Fig 3, B). In contrast, 28 of the 93 patients (30%) with a pNET ≥2 cm developed liver metastases or died, and LMFS was significantly lower for patients with NF-pNETs ≥2 cm compared to insulinomas ≥2 cm (P = .011; Fig 3, C).
Fig 3.
(A) Occurrence of liver metastases or death stratified by pNET size and pNET functionality in total cohort. (B) Occurrence of liver metastases or death stratified by pNET size for patients with a resected NF-pNET. (C) Occurrence of liver metastases or death stratified by pNET size for patients with a resected insulinoma.
After adjusting for age at surgery, pNET size, and WHO grade, patients with a resected NF-pNET had a significantly increased risk for liver metastases or death compared to patients with a resected insulinoma (HR 3.04 [95% CI 1.47–6.30]; Table II). In addition, pNET size per mm increase (HR 1.01 [95% CI 1.001–1.02]) was independent of pNET type, WHO grade, and age at surgery associated with LMFS. Sensitivity analysis showed similar HRs for pNET functionality and size when adjusted for lymph node status (Supplementary Table III).
Table II.
Multivariable analysis for factors associated with liver metastases or death
| Characteristic | Multivariable analysis (adjusted HR) |
|
|---|---|---|
| HR | 95% CI | |
| Age at surgery (per y) | 1.02 | 0.99–1.05 |
| Size largest pNET (per mm) | 1.01 | 1.001–1.02 |
| Tumor functionality | ||
| Insulinoma | 1 | Ref cat |
| NF-pNET | 3.04 | 1.47–6.30 |
| WHO grade | ||
| G1 | 1 | Ref cat |
| G2/G3 | 2.09 | 0.89–4.90 |
Data reported after multiple imputation.
Multivariable analysis included all factors listed above. cat, category; Ref, reference.
Stratified by pNET size <2 and ≥2 cm, no factors were associated with LMFS in pNETs <2 cm (Table III). For pNETs ≥2 cm, NF-pNETs were associated with LMFS after adjusting for age at surgery and WHO grade (HR 2.95 [95% CI 1.18–6.67]). In addition, patients with WHO G2 tumors had an increased risk for liver metastases or death (HR 2.52 [95% CI 1.16–5.47]).
Table III.
Univariable and multivariable analysis for factors associated with liver metastases or death stratified for pNETs <2 and ≥2 cm
| Characteristic | No of patients | LM/death | Univariable analysis (crude HR) |
Multivariable analysis (adjusted HR) |
|||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | ||||
| pNET <2 cm | Age at surgery (per y) | 60 | 9 | 1.03 | 0.98–1.08 | 1.03 | 0.98–1.08 |
| Tumor functionality | |||||||
| Insulinoma | 38 | 3 | 1 | Ref cat | 1 | Ref cat | |
| NF-pNET | 22 | 6 | 2.44 | 0.58–10.30 | 2.19 | 0.52–9.28 | |
| pNET ≥2 cm | Age at surgery (per y) | 93 | 28 | 1.02 | 0.99–1.06 | 1.01 | 0.98–1.05 |
| Tumor functionality | |||||||
| Insulinoma | 54 | 12 | 1 | Ref cat | 1 | Ref cat | |
| NF-pNET | 39 | 16 | 3.11 | 1.39–6.98 | 2.92 | 1.28–6.67 | |
| WHO grade | |||||||
| G1 | 64 | 15 | 1 | Ref cat | 1 | Ref cat | |
| G2/G3 | 29 | 13 | 2.13 | 0.998–4.55 | 2.52 | 1.16– 5.47 | |
Data reported after multiple imputation.
Multivariable analysis includes age and tumor functionality; for pNETs ≥2 cm, WHO grade was additionally included. cat, category; Ref, reference.
Prognostic factors for LMFS
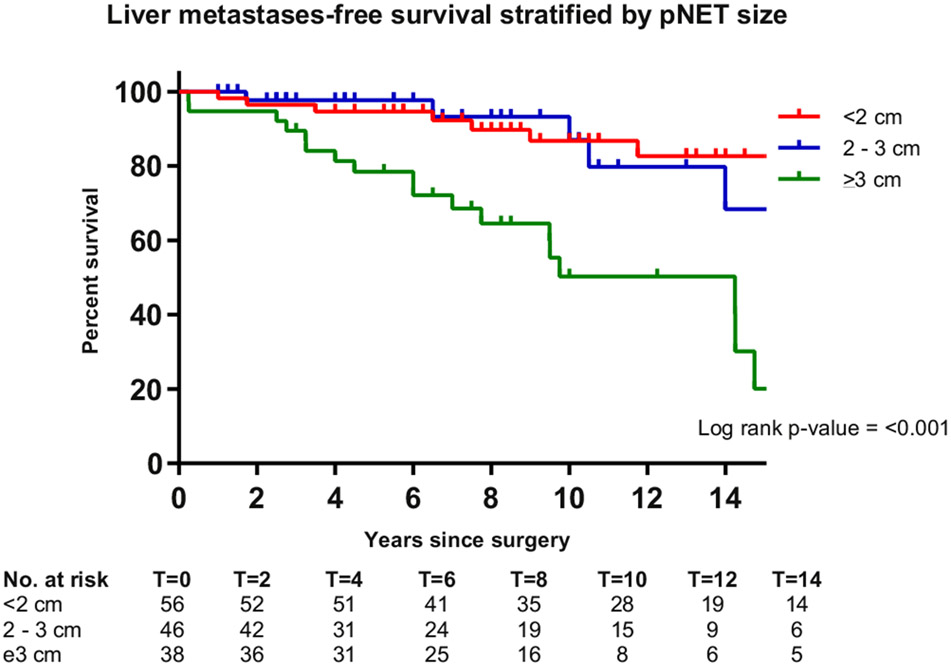
Estimated LMFS probabilities and factors associated with LMFS are summarized in Table IV. Patients who were older at surgery, had a NF-pNETs versus an insulinoma, had larger pNETs, a pNET ≥2 cm vs <2 cm, a WHO G2/G3 versus G1 tumor, lymph node metastases versus no lymph node metastases, and a longer delay from diagnosis until surgery had higher probabilities of liver metastases or death. Ten-year LMFS probability estimates were more than 80% for patients with an insulinoma (87%), pNET <2 cm (87%), G1 tumor (80%), and no lymph node metastases (81%). By contrast, for patients with a NF-pNET (63%), pNET ≥2 cm (42%) and G2/G3 tumor (42%), and lymph node metastases (51%), LMFS probability estimates were lower. Additional analysis revealed that patients with a resected pNET ≥3 cm as compared to those with a resected pNET <2 cm had a significantly decreased LMFS (HR 4.80 [95% CI 2.09–11.02]; Fig 4, Table V). No differences were observed for those with a pNET 2 to 3 cm compared to those with a pNET <2 cm. Estimated 5- and 10-year LMFS probabilities were 92% and 80% for patients operated on before 2003 and 90% and 75% for patients operated on from 2003 onwards.
Table IV.
LMFS and factors associated with LMFS
| Subgroup | Characteristic | LMFS* |
Univariable Cox regression† |
||||
|---|---|---|---|---|---|---|---|
| 5-y (%) | 10-y (%) | Log rank P value | LM/death | Crude HR | 95% CI | ||
| Overall | Age at surgery (per y) | - | - | NA | 37 of 153 | 1.03 | 1.01 – 1.06 |
| Sex | 87 | 77 | .491 | 18 of 66 | 1 | Ref cat | |
| Male | |||||||
| Female | 94 | 77 | 19 of 87 | 0.80 | 0.42–1.52 | ||
| pNET functionality | |||||||
| Insulinoma | 98 | 87 | .002 | 15 of 92 | 1 | Ref cat | |
| NF-pNET | 81 | 63 | 22 of 61 | 2.72 | 1.41–5.28 | ||
| Size continuous (per mm) | - | - | NA | 37 of 153 | 1.02 | 1.01 – 1.03 | |
| pNET 2 cm | |||||||
| <2 cm | 95 | 87 | .003 | 9 of 60 | 1 | Ref cat | |
| ≥2 cm | 88 | 70 | 28 of 93 | 2.97 | 1.36 – 6.48 | ||
| WHO grade | |||||||
| G1 | 93 | 80 | .002 | 21 of 109 | 1 | Ref cat | |
| G2/G3 | 83 | 42 | 16 of 44 | 2.20 | 1.02–4.76 | ||
| Lymph node status | |||||||
| No metastases | 91 | 81 | .144 | 12 of 60 | 1 | Ref cat | |
| None resected | 90 | 76 | 16 of 69 | 1.35 | 0.61–2.99 | ||
| Metastases | 86 | 51 | 9 of 24 | 2.77 | 1.12–6.85 | ||
| Y from diagnosis until surgery | - | - | NA | 37 of 153 | 1.12 | 1.01–1.24 | |
| NF-pNET | Age at surgery (per y) | - | - | NA | 22 of 61 | 1.04 | 0.995–1.08 |
| Sex | |||||||
| Male | 80 | 68 | .752 | 12 of 34 | 1 | Ref cat | |
| Female | 84 | 58 | 10 of 27 | 1.15 | 0.85–1.51 | ||
| Size continuous (per mm) | - | - | NA | 22 of 61 | 1.02 | 1.01–1.04 | |
| pNET 2 cm | |||||||
| <2 cm | 86 | 86 | .058 | 6 of 22 | 1 | Ref cat | |
| ≥2 cm | 80 | 50 | 16 of 39 | 2.37 | 0.89–6.34 | ||
| WHO grade | |||||||
| G1 | 86 | 73 | .046 | 14 of 47 | 1 | Ref cat | |
| G2/G3 | 74 | 24 | 8 of 14 | 2.99 | 1.19–7.54 | ||
| Lymph node status | |||||||
| No metastases | 77 | 77 | .360 | 5 of 14 | 1 | Ref cat | |
| None resected | 83 | 70 | 8 of 29 | 0.63 | 0.21–1.96 | ||
| Metastases | 82 | 44 | 9 of 18 | 1.28 | 0.42–3.94 | ||
| Y from diagnosis until surgery | - | - | NA | 22 of 61 | 1.01 | 0.88–1.16 | |
| Insulinoma | Age at surgery (per y) | - | - | NA | 15 of 92 | 1.02 | 0.99–1.06 |
| Sex | |||||||
| Male | 97 | 90 | .784 | 6 of 32 | 1 | Ref cat | |
| Female | 98 | 85 | 9 of 60 | 0.83 | 0.60–1.18 | ||
| Size continuous (per mm) | - | - | NA | 15 of 92 | 1.01 | 0.998–1.03 | |
| pNET 2 cm | |||||||
| <2 cm | 100 | 86 | .068 | 3 of 40 | 1 | Ref cat | |
| ≥2 cm | 95 | 87 | 12 of 52 | 3.43 | 0.98–12.01 | ||
| WHO grade | |||||||
| G1 | 100 | 90 | .003 | 7 of 62 | 1 | Ref cat | |
| G2/G3 | 91 | 68 | 8 of 30 | 2.60 | 0.92–7.29 | ||
| Lymph node status | 97 | 83 | .385 | 7 of 46 | 1 | Ref cat | |
| No metastases | |||||||
| None resected | 97 | 82 | 8 of 40 | 1.91 | 0.66–5.54 | ||
| Metastases | 100 | - | 0 of 6 | NA | NA | ||
| Y from diagnosis until surgery | - | - | NA | 15 of 92 | 1.25 | 1.05–1.49 | |
cat, category; NA, not applicable; Ref, reference.
Estimated survival percentages are based on the patients with complete data.
Data presented after multiple imputation.
Fig 4.
LMFS of patients with resected MEN1-related pancreatic neuroendocrine tumors stratified by tumor size (<2 cm, 2–3 cm, and ≥3 cm).
Table V.
Association between tumor size and LMFS
| Size | LMFS* |
Univariable Cox regression† |
||||
|---|---|---|---|---|---|---|
| 5-y (%) | 10-y (%) | Log rank P value | LM/death | Crude HR | 95% CI | |
| Overall | ||||||
| <2 cm | 95 | 87 | < .001 | 9 of 62 | 1 | Ref cat |
| 2–3 cm | 98 | 93 | 7 of 50 | 1.46 | 0.50–4.27 | |
| ≥3 cm | 78 | 50 | 21 of 41 | 4.80 | 2.09–11.02 | |
| NF-pNET subgroup | ||||||
| <2 cm | 86 | 86 | .012 | 5 of 21 | 1 | Ref cat |
| 2–3 cm | 100 | 86 | 3 of 13 | 1.36 | 0.29–6.28 | |
| ≥ 3 cm | 71 | 33 | 14 of 27 | 3.21 | 1.14–9.06 | |
| Insulinoma subgroup | ||||||
| <2 cm | 100 | 86 | .046 | 3 of 40 | 1 | Ref cat |
| 2–3 cm | 97 | 97 | 4 of 37 | 1.95 | 0.44–8.68 | |
| ≥ 3 cm | 92 | 73 | 8 of 15 | 5.47 | 1.45–20.63 | |
cat, category; Ref, reference.
Estimated survival percentages are based on the patients with complete data.
Data presented after multiple imputation.
Prognostic factors for LMFS in NF-pNETs
Within the patients with a resected NF-pNET, 10-year LMFS probability estimates were 50% for those with a NF-pNET ≥2 cm, 24% for those with a WHO G2/G3 tumor, and 44% for those with lymph node metastases. Size in mm (HR 1.02 [95% CI 1.01–1.04]) and WHO G2/G3 versus G1 (HR 2.99 [95% CI 1.19–7.54]) were associated with LMFS. Of the patients with a NF-pNET ≥2 cm graded as G2/G3, estimated 10-year LMFS was 23% compared with 84% for patients with a G1 NF-pNET <2 cm (Fig 5).
Fig 5.
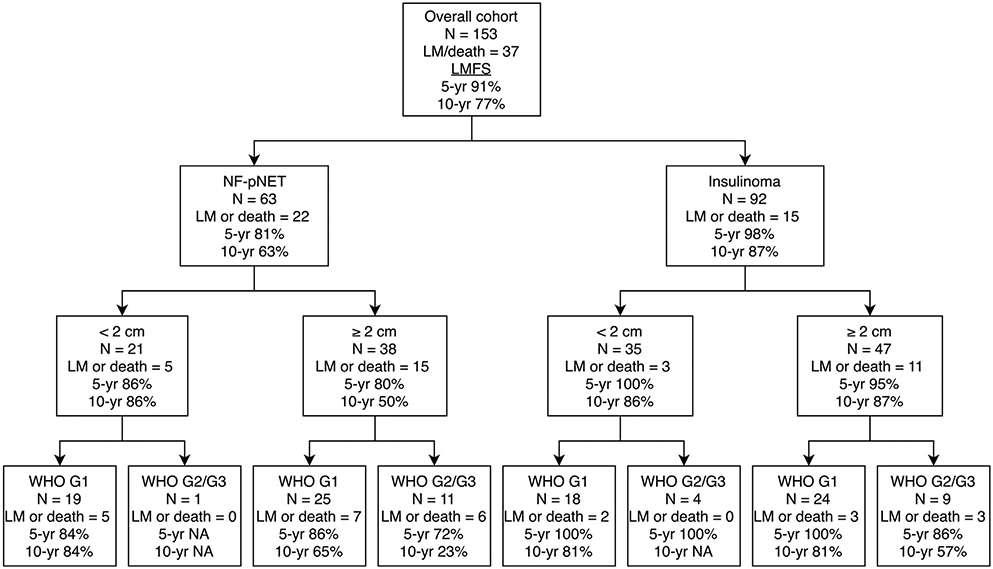
LMFS according to pathology. n, number.
Prognostic factors for LMFS in insulinomas
A longer time from diagnosis until surgery was associated with liver metastases or death in patients with a resected insulinoma. The CIs of other factors, such as age at surgery, size in mm, presence of a pNET ≥2 cm, and WHO G2/G3, crossed unity. Point estimates and 95% CI of these factors had similar direction and magnitude as within the NF-pNET group. Of the patients with complete data, those with an insulinoma ≥2 cm, which was G2/G3, had an estimated 10-year LMFS of 57%.
Discussion
This study shows that patients with a resected MEN1-related NF-pNET had a reduced LMFS compared to those with a resected MEN1-related insulinoma, irrespective of the age of surgery and the size and WHO grade of the tumor. These observations suggest differences in underlying tumor origin, development, or biology of MEN1-related pNETs. Postoperative counseling and monitoring of patients during follow-up should, therefore, be tumor-type specific and at least include tumor size and WHO grade.
Previous studies hypothesized that patients with MEN1-related insulinomas have favorable prognosis because of small tumor size, early symptomatology with subsequent treatment, or because of differences in grade.5,8,19 Indeed, in this study, patients with insulinomas were younger, had a shorter time from diagnosis to surgery, and had smaller pNETs than patients with NF-pNETs. No differences in WHO grade were observed between NF-pNETs and insulinomas. Nevertheless, when adjusted for age at surgery, size, and WHO grade, the risk of liver metastases or death was tripled for patients with a resected NF-pNET compared to those with a resected insulinoma. This indicates that the pathology of NF-pNETs likely is more aggressive.
Tumor size and WHO grade were associated with LMFS, also after adjusting for pNET type and age. Although size has been extensively studied and translated in clinical decision making, the present study observed that size–on a continuous scale–was associated with long-term outcomes and might therefore be used for postoperative counseling.12,25,34,40 In line with tumor size on a continuous scale being associated with LMFS, subsequent analyses revealed that especially patients with a resected pNET of at least 3 cm had the highest chance of subsequent liver metastases or death, regardless of pNET functionality. Although a randomized controlled trial is ideally demanded to determine whether surgery has added value over watchful waiting, based on these observations one might hypothesize that a MEN1-related pNET should ideally be resected before the 3cm cutoff is reached. These observations further underscore the importance of accurate size estimations.41 It has previously been observed within the DMSG database that patients with a resected WHO G2 NF-pNET larger than 2 cm had the highest risk of developing liver metastases.31 In the present study, WHO G2 or G3 tumors posed a 2.5 times increased risk for LMFS compared to G1 tumors in pNETs of 2 cm or larger. Although a number of patients with NF-pNETs was included in the previous DMSG study,31 the present analysis showed that WHO grade is associated with postoperative LMFS also in patients with resected MEN1-related insulinomas, irrespective of age and size. In line, patients with a resected WHO G2 of 2 cm had a reduced 10-year LMFS compared to those with G1 tumor of at least 2 cm (23% vs 65% for NF-pNETs and 57% vs 81% for insulinomas, respectively). Considering the relatively low number of outcomes, multivariable analysis was restricted to age at surgery, size, functionality, and WHO grade. Nevertheless, in univariable analysis, time from diagnosis until surgery was associated with LMFS, specifically in patients with a resected insulinoma. Despite that some CIs (barely) crossed unity, within the subgroups of patients with a resected NF-pNET and insulinoma, higher age at surgery, larger tumors, a pNET of 2 cm or larger, and WHO G2 or G3 increased the risk of liver metastases or death and could, therefore, be used for postoperative counseling.
Mutations in the interacting domains of menin, which affect transcriptional regulation–JunD and CHES1, have been reported to be associated with the prognosis of patients with MEN1-related pNETs but have not been validated successfully.12,16,42,43 More recently, tumor-based transcription factors ARX and PDX1 have been identified as enhancer signatures resembling a distinct alpha (ARX positive) or beta cell (PDX1 positive) subtype differentiation in MEN1-related pNETs. These subtypes subsequently affect prognosis and imply differences in cell lineages of origin responsible for the development of distinct subtypes of pNETs, which justify the present clinical observations.44,45 Liver metastases were reported almost exclusively in patients with ARX positive tumors, whereas patients with PDX1 positive tumors had a generally low risk.44 Although these immunohistochemical markers were not studied in the present study, one might reason that a higher proportion of NF-pNETs will harbor a true alpha cell differentiation, whereas insulinomas will generally resemble a beta cell differentiation. A small subgroup of insulinomas–which developed liver metastases–possibly harbors an alpha cell differentiation. This should be investigated in future studies in MEN1-related pNETs within a large and international cohort with surgical specimen collection and adequate follow-up. In addition, unraveling these differences in tumor biology might lead to subtype specific size cutoffs for operative resection.
Apart from long-term outcomes, pathological characteristics might reflect the more aggressive behavior of NF-pNETs because of early spread to regional lymph nodes. Within the 73 patients with lymph nodes removed, metastatic lymph nodes were more often observed in NF-pNETs compared to insulinomas (56% vs 10%) and in those with a pNET of 2 cm or larger (39% vs 16%). Patients with lymph node metastases had an almost 3 times higher risk of liver metastases or death than patients without lymph node metastases, which is supported by 10-year LMFS probabilities of 51% in the entire cohort and 44% in the subgroup of NF-pNETs. Although lymph nodes were resected in only 73 patients, it is unlikely that this has influenced the observations, since LMFS was similar between those with tumor negative lymph nodes and those without lymphadenectomy. In at least 40% of patients, no lymph nodes were resected, which might reflect the absence of guideline recommendations regarding lymph node resections in MEN1.17,40 European Neuroendocrine Tumor Society guidelines recommend routine dissection of lymph nodes in noninsulinoma pNETs.40 Current data might substantiate these recommendations also for patients with MEN1, since only 3 patients with insulinomas had positive lymph nodes compared to 18 patients with NF-pNETs. Nevertheless, only 5 patients (3%)–all in the NF-pNET group–had suspected lymph node metastases on preoperative conventional imaging. 68Ga labelled positron emission tomography (PET)/CT might overcome the limitations of conventional imaging in this matter.46,47 Nevertheless, the diagnostic, prognostic, and therapeutic implications of pNET-related lymph node metastases in patients with MEN1 should be investigated in future studies.
The major strength of the present study is that it represents the largest cohort of patients with resected MEN1-related pNETs to date. Histopathological data were available by including surgically treated patients, which has provided the unique opportunity to adjust for and study tumor size and grade. Patients were included over a recent period where MEN1 patients are screened and followed according to guidelines.17 Missing data were retrieved as far as possible and otherwise handled using multiple imputation–generating a sufficient number of datasets–which is currently considered as the best available statistical method.35,48,49 In addition, several statistical analyses, including Kaplan-Meier and Cox proportional hazard regression, were conducted to derive statistically sound conclusions. Despite the low prevalence of MEN1 and relatively low event rate, even multivariable analyses were performed. Nevertheless, a larger study population would enable more extensive multivariable analyses. Data from patients undergoing surgery for NF-pNETs in centers not included in the DMSG were not available, which is the main limitation of the present study. Inclusion of those patients could have led to a more homogeneous cohort. In addition, by including only patients undergoing surgery, the question remains as to whether the results are generalizable for patients not being exposed to operative resection. Patients with other MEN1-related duodenopancreatic neuroendocrine tumors (dpNETs), such as gastrinomas or rare functioning pNETs were not included. Furthermore, determining the origin of liver metastases is challenging considering the multifocality of dpNETs in MEN1, eg, only 2 of the 6 patients with a resected insulinoma had recurrent hypoglycemia at the time of liver metastases. The exact number of resected and metastatic lymph nodes was unknown, and therefore, patients were grouped into metastases, no metastases, or no lymph nodes resected regardless of the number of lymph nodes analyzed. Imaging (ie, presence of liver metastases) and histopathological specimens (ie, WHO grade) were not centrally collected and reassessed for the purpose of this study.
Pre and postoperative localization of insulinomas is challenging in the presence of diffuse background adenomatosis in MEN1. In the preoperative setting, 6SGa-DOTA-Exendin-4 PET/CT can successfully localize insulinomas in MEN1.50 Postoperatively, immunohistochemistry is still the most widely available and used method to identify the insulinoma. Most sporadically occurring insulinomas express insulin.51 Nevertheless, in MEN1, multiple pNETs might show immunoreactivity for insulin, and insulinomas might be negative for insulin.51,52 Insulin negative insulinomas were observed in 3, all of whom were biochemically cured, and multiple insulin immunopositive pNETs were encountered in 24. Insulinomas show positive immunohistochemistry signals specifically for PDX1.44 Therefore, PDX1 might be additionally used or replace insulin immunohistochemistry to overcome limitations encountered in clinical practice.
The differences in prognosis after surgery for MEN1-related pNETs is of direct clinical importance for postoperative patient counseling and monitoring during follow-up, regarding intensity and use of diagnostic modalities, to optimize care in MEN1. Based on the present data, at least tumor functionality, tumor size, and grade should be taken into account during postoperative MEN1 care. Patients with resected insulinomas–especially if small and WHO G1–can be counseled about the low likelihood of metastases, and the aim of the follow-up should be the detection and follow-up of new (NF)-pNETs. The follow-up of MEN1-related NF-pNETs should focus on identifying metastatic disease. Regardless of functionality, those with WHO G2 or G3 or large tumors have an increased risk. In patients with an increased risk of liver metastases, 68Ga labelled PET/CT might be used to identify metastatic disease to enable timely initiation of adjuvant therapy. Furthermore, regardless of tumor origin, patients should be counseled about the risk of recurrence 5 or l0 years after surgery, which additionally underscores long-term follow-up of patients with resected MEN1-related pNETs. Whether these observations will alter the currently accepted 2 cm criterion should be investigated in future studies. Additional studies should investigate the optimal surgical strategy and determine the added value of routine lymphadenectomy for the individual MEN1 patient, taking long-term oncological outcomes, survival, future occurrence of clinically relevant dpNETs, and postoperative complications as well as pancreatic function and quality of life into account.
In conclusion, patients with resected MEN1-related NF-pNETs have a lower LMFS than those with insulinomas. These tumors should therefore be regarded as distinct entities of MEN1-related pNETs. Postoperative counseling and follow-up regimens should be subtype specific and, additionally at least, be guided by size and WHO grade.
Supplementary Material
Acknowledgments
The authors would like to thank the members from the DutchMEN Study Group (DMSG) and Groupe d’étude des Tumeurs Endocrines (GTE) for their support.
Funding/Support
This work was supported by an unrestricted grant from Ipsen Pharmaceutical. The funding source had no influence on the study question, design, data acquisition, statistical analysis, and interpretation of data.
Appendix
1. International MEN1 Insulinoma Study Group
Pierre Goudet, MD, PhD, on behalf of the French Endocrine Tumor Study Group (GTE)d,e, Nicolas Santucci, MDf,g,h, Detlef K. Bartsch, MD, PhDi, Jerena Manoharan, MDi, Nancy D. Perrier, MDj, Jonathan Zagzag, MD, PhDj, Maria Luisa Brandi, MD, PhDk, Francesca Giusti, MD, PhDk, Naris Nilubol, MDl, Laurent Brunaud, MD, PhDm, Jesse D. Pasternak, MD MPHn, Ralph Hsiao, BScn, Cord Sturgeon, MDo, Sneha Giri, MDo, Elfi B. Conemans, MD, PhDc,p,q, Lodewijk A. Brosens, MD, PhDq,r
dINSERM, CIC1432, Clinical Epidemiology Unit, Dijon, France;
eDijon-Bourgogne University Hospital, Clinical Investigation Center, Clinical Epidemiology/Clinical trial unit, Dijon, France;
fDijon-Bourgogne University Hospital, Department of Digestive and Endocrine Surgery, Dijon, France;
gINSERM U1231, EPICAD, Dijon, France;
hUniversity of Burgundy-Franche-Comte, UMR1231 Lipids, Nutrition, Cancer, epidemiology and clinical research in Digestive Oncology, Dijon, France;
iDepartment of Visceral, Thoracic and Vascular Surgery, Philipps University Marburg, Baldingerstrasse, Marburg, Germany;
jDepartment of Surgical Oncology, The University of Texas, MD Anderson Cancer Center, Houston, Texas, United States of America;
kDepartment of Surgery and Translational Medicine, University of Florence, Florence, Italy;
lSurgical Oncology Program, National Cancer Institute, National Institutes of Health, Bethesda, Maryland;
mDepartment of Digestive, Hepatobiliary and Endocrine Surgery, Université de Lorraine, Hôpital Brabois Adultes, CHU Nancy, France;
nDepartment of Surgery, University Health Network, Toronto, ON, Canada;
oDepartment of Surgery, Northwestern University, Chicago, Illinois, United States of America;
pDepartment of Endocrinology, Amsterdam University Medical Center location VUmc University Medical Center, Amsterdam, The Netherlands;
qDepartment of Pathology, University Medical Center Utrecht, Utrecht, the Netherlands;
rDepartment of Pathology, Radboud University Medical Center, Nijmegen, the Netherlands
Appendix
2. DutchMEN Surgery Study Group
Bert A. Bonsing, MD, PhDs, Casper H. van Eijck, MD, PhDt, Harry van Goor, MD, PhDu, Ruben H.J. de Kleine, MDv, Elisabeth J. Nieveen van Dijkum, MD, PhDw, Geert Kazemier, MD, PhDx, Cornelis H.C. Dejong, MD, PhDy,z
sDepartment of Surgery, Leiden University Medical Center, Leiden, the Netherlands;
tDepartment of Surgery, Erasmus Medical Center, Rotterdam, the Netherlands;
uDepartment of Surgery, Radboud University Medical Center, Nijmegen, the Netherlands;
vDepartment of Surgery, University of Groningen, University Medical Center Groningen, Groningen, the Netherlands;
wDepartment of Surgery, Cancer Center Amsterdam, Amsterdam University Medical Center location Academic Medical Center, Amsterdam, the Netherlands;
xDepartment of Surgery, Cancer Center Amsterdam, Amsterdam University Medical Center location VUmc University Medical Center, Amsterdam, the; Netherlands;
yDepartment of Surgery, Maastricht University Medical Center, NUTRIM School for Nutrition and Translational Research in Metabolism, Maastricht, the; Netherlands;
zDepartment of Surgery, Universitätsklinikum Aachen, Aachen, Germany
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at https://doi.org/10.1016/j.surg.2020.09.037.
Conflict of interest/Disclosure
The authors have nothing to disclose.
References
- 1.Chandrasekharappa SC, Guru SC, Manickam P, et al. Positional cloning of the gene for multiple endocrine neoplasia-type 1. Science. 1997;276:404–407. [DOI] [PubMed] [Google Scholar]
- 2.Lemmens I, Van de Ven WJ, Kas K, et al. Identification of the multiple endocrine neoplasia type 1 (MEN1) gene. The European Consortium on MEN1. Hum Mol Genet. 1997;6:1177–1183. [DOI] [PubMed] [Google Scholar]
- 3.de Laat JM, van der Luijt RB, Pieterman CRC, et al. MEN1 redefined, a clinical comparison of mutation-positive and mutation-negative patients. BMC Med. 2016;14:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triponez F, Dosseh D, Goudet P, et al. Epidemiology data on 108 MEN 1 patients from the GTE with isolated nonfunctioning tumors of the pancreas. Ann Surg. 2006;243:265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goudet P, Murat A, Binquet C, et al. Risk factors and causes of death in MEN1 disease. A GTE (Groupe d’Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg. 2010;34:249–255. [DOI] [PubMed] [Google Scholar]
- 6.Ito T, Igarashi H, Uehara H, Berna MJ, Jensen RT. Causes of death and prognostic factors in multiple endocrine neoplasia type 1: a prospective study: comparison of 106 MEN1/Zollinger-Ellison syndrome patients with 1613 literature MEN1 patients with or without pancreatic endocrine tumors. Medicine (Baltimore). 2013;92:135–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goudet P, Bonithon-Kopp C, Murat A, et al. Gender-related differences in MEN1 lesion occurrence and diagnosis: a cohort study of 734 cases from the Groupe d’etude des Tumeurs Endocrines. Eur J Endocrinol. 2011;165:97–105. [DOI] [PubMed] [Google Scholar]
- 8.Giudici F, Cavalli T, Giusti F, et al. Natural history of MEN1 GEP-NET: Single-center experience after a long follow-up. World J Surg. 2017;41:2312–2323. [DOI] [PubMed] [Google Scholar]
- 9.Donegan D, Singh Ospina N, Rodriguez-Gutierrez R, et al. Long-term outcomes in patients with multiple endocrine neoplasia type 1 and pancreaticoduodenal neuroendocrine tumours. Clin Endocrinol (Oxf). 2017;86:199–206. [DOI] [PubMed] [Google Scholar]
- 10.Pieterman CRC, Conemans EB, Dreijerink KMA, et al. Thoracic and duodenopancreatic neuroendocrine tumors in multiple endocrine neoplasia type 1: Natural history and function of menin in tumorigenesis. Endocr Relat Cancer. 2014;21:R121–R142. [DOI] [PubMed] [Google Scholar]
- 11.Perren A, Anlauf M, Henopp T, et al. Multiple endocrine neoplasia type 1 (MEN1): Loss of one MEN1 allele in tumors and monohormonal endocrine cell clusters but not in islet hyperplasia of the pancreas. J Clin Endocrinol Metab. 2007;92:1118–1128. [DOI] [PubMed] [Google Scholar]
- 12.Vinault S, Mariet A-S, Le Bras M, et al. Metastatic potential and survival of duodenal and pancreatic tumors in multiple endocrine neoplasia type 1. Ann Surg. 2018. Available from:. 10.1097/SLA.0000000000003162. [DOI] [PubMed] [Google Scholar]
- 13.Kouvaraki MA, Shapiro SE, Cote GJ, et al. Management of pancreatic endocrine tumors in multiple endocrine neoplasia type 1. World J Surg. 2006;30:643–653. [DOI] [PubMed] [Google Scholar]
- 14.Yates CJ, Newey PJ, Thakker RV. Challenges and controversies in management of pancreatic neuroendocrine tumours in patients with MEN1. Lancet Diabetes Endocrinol. 2015;3:895–905. [DOI] [PubMed] [Google Scholar]
- 15.Conemans EB, Nell S, Pieterman CRC, et al. Prognostic factors for survival of MEN1 patients with duodenopancreatic tumors metastatic to the liver: Results from the DMSG. Endocr Pract. 2017;23:641–648. [DOI] [PubMed] [Google Scholar]
- 16.Pieterman CRC, de Laat JM, Twisk JWR, et al. Long-term natural course of small nonfunctional pancreatic neuroendocrine tumors in MEN1-results from the Dutch MEN1 Study Group. J Clin Endocrinol Metab. 2017;102:3795–3805. [DOI] [PubMed] [Google Scholar]
- 17.Thakker RV, Newey PJ, Walls GV, et al. , and the Endocrine Society. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012;97:2990–3011. [DOI] [PubMed] [Google Scholar]
- 18.Cougard P, Goudet P, Peix JL, et al. Insulinomas in multiple endocrine neoplasia type 1. Report of a series of 44 cases by the multiple endocrine neoplasia study group. Ann Chir. 2000;125:118–123. [DOI] [PubMed] [Google Scholar]
- 19.Nell S, Borel Rinkes IHM, Verkooijen HM, et al. , and DMSG. Early and late complications after surgery for MEN1-related nonfunctioning pancreatic neuroendocrine tumors. Ann Surg. 2018;267:352–356. [DOI] [PubMed] [Google Scholar]
- 20.Jensen RT, Bodei L, Capdevila J, et al. , and the ENETS 2016 Munich Advisory Board Participants. Unmet needs in functional and nonfunctional pancreatic neuroendocrine neoplasms. Neuroendocrinology. 2019;108:26–36. [DOI] [PubMed] [Google Scholar]
- 21.Pieterman CR, Sadowski SM, Maxwell JE, et al. MEN1-related PanNETs: identifying unmet clinical needs and future directives. Endocr Relat Cancer. 2020;27:T9–T25. [DOI] [PubMed] [Google Scholar]
- 22.van Beek D-J, van Leeuwaarde RS, Pieterman CR, Vriens MR, Valk GD. “Quality in, quality out”, a stepwise approach to EBM for rare diseases promoted by MEN1. Endocr Connect. 2018;7:260–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lévy-Bohbot N, Merle C, Goudet P, et al. and the Groupe des Tumeurs Endocrines. Prevalence, characteristics and prognosis of MEN 1-associated glucagonomas, VIPomas, and somatostatinomas: Study from the GTE (Groupe des Tumeurs Endocrines) registry. Gastroenterol Clin Biol. 2004;28:1075–1081. [DOI] [PubMed] [Google Scholar]
- 24.van Beek DJ, Nell S, Verkooijen HM, et al. , and the International MEN1 Insulinoma Study Group. Surgery for multiple endocrine neoplasia type 1-related insulinoma: long-term outcomes in a large international cohort. Br J Surg. 2020;107:1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nell S, Verkooijen HM, Pieterman CRC, et al. Management of MEN1 related nonfunctioning pancreatic NETs: A shifting paradigm: Results from the DutchMEN1 Study Group. Ann Surg. 2018;267:1155–1160. [DOI] [PubMed] [Google Scholar]
- 26.Service FJ. Hypoglycemic disorders. N Engl J Med. 1995;332:1144–1152. [DOI] [PubMed] [Google Scholar]
- 27.Cryer PE, Axelrod L, Grossman AB, et al. , and the Endocrine Society. Evaluation and management of adult hypoglycemic disorders: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2009;94:709–728. [DOI] [PubMed] [Google Scholar]
- 28.Pipeleers-Marichal M, Somers G, Willems G, et al. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med. 1990;322:723–727. [DOI] [PubMed] [Google Scholar]
- 29.van Beek D-J, Nell S, Pieterman CRC, et al. Prognostic factors and survival in MEN1 patients with gastrinomas: Results from the DutchMEN study group (DMSG). J Surg Oncol. 2019;120:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lloyd RV, Osamura YR, Kloppel G, Rosai J. WHO Classification of Tumours of Endocrine Organs. Geneva (Switzerland): World Health Organization; 2017. [Google Scholar]
- 31.Conemans EB, Brosens LAA, Raicu-Ionita GM, et al. Prognostic value of WHO grade in pancreatic neuro-endocrine tumors in Multiple Endocrine Neoplasia type 1: Results from the DutchMEN1 study group. Pancreatology. 2017;17:766–772. [DOI] [PubMed] [Google Scholar]
- 32.McCall CM, Shi C, Cornish TC, et al. Grading of well-differentiated pancreatic neuroendocrine tumors is improved by the inclusion of both Ki67 proliferative index and mitotic rate. Am J Surg Pathol. 2013;37:1671–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 34.Triponez F, Sadowski SM, Pattou F, et al. Long-term follow-up of MEN1 patients who do not have initial surgery for small ≤2 cm nonfunctioning pancreatic neuroendocrine tumors, an AFCE and GTE study: Association Francophone de Chirurgie Endocrinienne & Groupe d’Etude des Tumeurs Endocrines. Ann Surg. 2018;268:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: Potential and pitfalls. BMJ. 2009;339:157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8:206–213. [DOI] [PubMed] [Google Scholar]
- 37.Moons KGM, Donders RART, Stijnen T, Harrell FE Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59:1092–1101. [DOI] [PubMed] [Google Scholar]
- 38.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med. 2009;28:1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken (NJ): John Wiley & Sons, Inc; 1987. [Google Scholar]
- 40.Falconi M, Eriksson B, Kaltsas G, et al. and the Vienna Consensus Conference participants. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and nonfunctional pancreatic neuroendocrine tumors. Neuroendocrinology. 2016;103:153–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Beek D-J, Verkooijen HM, Nell S, et al. Reliability and agreement of radiological and pathological tumor size in patients with MEN1-related pancreatic neuroendocrine tumors: Results from a population-based cohort. Neuroendocrinology. 2020. Available from: 10.1159/000510514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thevenon J, Bourredjem A, Faivre L, et al. Higher risk of death among MEN1 patients with mutations in the JunD interacting domain: A groupe d’étude des tumeurs endocrines (GTE) cohort study. Hum Mol Genet. 2013;22:1940–1948. [DOI] [PubMed] [Google Scholar]
- 43.Bartsch DK, Slater EP, Albers M, et al. Higher risk of aggressive pancreatic neuroendocrine tumors in MEN1 patients with men1 mutations affecting the CHES1 interacting MENIN domain. J Clin Endocrinol Metab. 2014;99:E2387–E2391. [DOI] [PubMed] [Google Scholar]
- 44.Cejas P, Drier Y, Dreijerink KMA, et al. Enhancer signatures stratify and predict outcomes of non-functional pancreatic neuroendocrine tumors. Nat Med. 2019;25:1260–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan CS, Laddha SV, Lewis PW, et al. ATRX, DAXX or MEN1 mutant pancreatic neuroendocrine tumors are a distinct alpha-cell signature subgroup. Nat Commun. 2018;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadowski SM, Millo C, Cottle-Delisle C, et al. Results of (68)Gallium-DOTATATE PET/CT scanning in patients with multiple endocrine neoplasia type 1. J Am Coll Surg. 2015;221:509–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Treijen MJC, van Beek D-J, van Leeuwaarde RS, Vriens MR, Valk GD. Diagnosing nonfunctional pancreatic NETs in MEN1: The evidence base. J Endocr Soc. 2018;2:1067–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Janssen KJM, Donders ART, Harrell FE, et al. Missing covariate data in medical research: To impute is better than to ignore. J Clin Epidemiol. 2010;63:717–727. [DOI] [PubMed] [Google Scholar]
- 49.van der Heijden GJ, Donders AR, Stijnen T, Moons KG. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: A clinical example. J Clin Epidemiol. 2006;59:1102–1109. [DOI] [PubMed] [Google Scholar]
- 50.Antwi K, Nicolas G, Fani M, et al. Ga-Exendin-4 PET/CT detects insulinomas in patients with endogenous hyperinsulinemic hypoglycemia in MEN-1. J Clin Endocrinol Metab. 2019;104:5843–5852. [DOI] [PubMed] [Google Scholar]
- 51.Anlauf M, Schlenger R, Perren A, et al. Microadenomatosis of the endocrine pancreas in patients with and without the multiple endocrine neoplasia type 1 syndrome. Am J Surg Pathol. 2006;30:560–574. [DOI] [PubMed] [Google Scholar]
- 52.Andreassen M, Ilett E, Wiese D, et al. Surgical management, pre-operative tumor localization and histopathology of 80 patients operated for insulinoma. J Clin Endocrinol Metab. 2019;104:6129–6138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.