Abstract
Background
The number of lung transplantation procedures is rapidly increasing worldwide. Little is known about the effect of perioperative respiratory microbial colonization on pneumonia during lung transplantation. We evaluated the microbiome composition and incidence of early pneumonia in patients undergoing lung transplantation. We investigated factors related to post-transplant pneumonia (PTP) after lung transplantation.
Methods
A retrospective analysis of patients subjected to lung transplantation between May 2013 and December 2019 was performed. Perioperative microbial colonization, and its relationship with early pneumonia, were examined in specimens from bronchial washing, bronchoalveolar lavage, and sputum aspiration before and after surgery. One-year mortality, as the primary outcome, was analyzed using the Kaplan-Meier curve model.
Results
Among 76 patients who underwent lung transplantation, 34 donors (44.7%) and 28 recipients (36.8%) showed positive respiratory cultures with respect to preoperative respiratory colonization. A separate analysis of donors and recipients showed that 42 donors and 48 recipients were in respiratory non-colonized state, and 28 (53.8%) donors and 36 (69.2%) recipients survived 1 year after lung transplantation. Acinectobacter baumannii was the most common respiratory multidrug-resistant (MDR) pathogen. PTP was significantly lower in the survivor group (38.5% vs. 70.8%, P=0.009). Out of the recipients with preoperative respiratory colonization, 57.1% survived 1 year after lung transplantation. Patients with PTP had significantly higher 1-year mortality than those without PTP (P=0.009). Preoperative respiratory colonization of the recipients (P=0.010) and PTP patients (P=0.005) was associated with high 1-year mortality rate. Perioperative respiratory colonization of donors was not associated with the incidence of PTP and 1-year survival.
Conclusions
Perioperative colonization of recipients was a powerful predictive factor for PTP, which was associated with 1-year mortality in patients subjected to lung transplantation. Our results suggest that donor acceptance criteria may change to better address potential shortages in organ donation.
Keywords: Pneumonia, colonization, multidrug-resistant (MDR) bacteria, lung transplantation
Introduction
Lung transplantation is the choice of therapy for medically intractable end-stage lung disease and is now widely accepted as an effective treatment in selected individuals (1). The number of lung transplantation cases is growing rapidly globally; however, the sophisticated perioperative care required and poor long-term outcomes constitute important challenges in the field of lung transplantation (2); one of these challenges is acquired infection. Immunosuppression is required to prevent transplant rejection, therefore, lung transplant recipients are susceptible to infections by various microorganisms in the early postoperative period (3). Among these infections, pneumonia may be lethal for recipients in two ways. First, pneumonia itself can be fatal because of immunosuppression and delay in treatment due to infection by unusual organisms, opportunistic infections, and other complications (3). Second, direct injury to the transplanted graft due to pneumonia can breakdown the immune tolerance between the graft and host, leading to acute rejection and chronic allograft dysfunction (4,5). In this context, pneumonia after lung transplantation should be carefully addressed in terms of diagnosis, treatment, and immunosuppression.
Some respiratory multidrug-resistant (MDR) pathogen, especially Burkholderia Cepacia is a relative contraindication for lung transplant (6,7). To date, cystic fibrosis patients infected with Burkholderia cepacia are regarded as high risk for post-transplantation mortality (8). Considering that the proportion of patients requiring pre-transplantation intensive care unit (ICU) stay is increasing due to organ donor shortage and urgency-based organ allocation systems (9), preoperative respiratory microbial colonization and resistant organisms are expected to become more common. However, it is unclear which pathogens or pattern of resistance impacts post-transplantation pneumonia (PTP). Therefore, we aimed to assess the association between preoperative respiratory colonization and clinical outcomes after lung transplantation. In addition, we attempted to determine how MDR colonization affected other parameters of the lung transplantation outcome, including early PTP. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1724/rc).
Methods
Study design and data collection
We retrospectively collected clinical data regarding donor-related parameters such as age, smoking, PaO2/FiO2 (PF) ratio, presence of chest X-ray infiltration, respiratory microbial culture data, and recipient-related parameters such as age, sex, body mass index (BMI), etiology of respiratory disease, pre-transplantation bridging status, antibiotic use, respiratory microbial culture data, and clinical outcome. In addition, outcome parameters such as early PTP, defined as the onset of pneumonia within 28 days after lung transplantation, postoperative ventilator-free day (VFD) within 28 days, postoperative ICU stay, and 1-year survival rate were collected. We reviewed all data based on the medical records.
Lung transplantation protocol
Lung transplantation was performed according to our institutional protocol. Preoperative and postoperative management, including immunosuppression, administration of prophylactic antibiotics, and supportive care, were standardized according to our established protocol. During perioperative care, antibiotic therapy was administered based on the results from sputum and blood bacterial cultures, or empirically when bacterial culture data were unavailable. Empirical antibiotics were chosen according to the guidelines for hospital-acquired pneumonia and conditionally modified based on the institutional microbiological pattern of resistance.
Definition of acquired pneumonia
Pneumonia was defined as infection of the pulmonary parenchyma caused by various organisms, with compatible laboratory findings and symptoms such as cough (with or without sputum), fever, chills, and respiratory difficulty. Laboratory findings included two or more of the following criteria: temperature ≥38.4 or <36 °C, white blood cell (WBC) count >11,000/mm3 or <4,000/mm3, and positive blood and sputum cultures (10). Positive bacterial cultures were defined as bacterial growth, in a qualitative manner. Imaging findings on chest radiography or computed tomography, such as consolidation and increased haziness, were defined as abnormal (11). Early post-transplantation pneumonia was based on the International Society for Heart and Lung Transplantation definition as pneumonia that developed within 30 days after transplantation surgery (12-15).
Respiratory colonization was defined as a positive culture from the sputum, bronchial washing fluid, or bronchoalveolar lavage fluid, without signs or symptoms for the host, and incompatible with the aforementioned criteria for pneumonia (16). MDR bacteria were defined as carbapenem-resistant Acinetobacter baumannii (CRAB), carbapenem-resistant Pseudomonas aeruginosa, and methicillin-resistant Staphylococcus aureus.
Adequacy of prophylactic antibiotics was ensured by testing in vitro their activity against the etiological pathogens, as identified based on diagnostic laboratory results in our hospital. When resistant bacteria were identified as a result of antibiotic susceptibility, antibiotic usage was determined according to the reported drug susceptibility testing (DST). When CRAB was detected, colistin was used for initial treatment, together with other antibiotics depending on sensitivity.
In addition, adequate prophylactic antibiotics were administered only to patients with positive culture for preoperative respiratory colonization, but not to those with negative culture. This finding suggested that not all patients with positive culture with preoperative respiratory colonization had pneumonia.
Microbiological samples
In transplant recipients, all respiratory samples were obtained during inpatient appointments via routine sputum tests or bronchial washing by flexible bronchoscopy prior to transplantation or within three days post-transplantation. In outpatients, a routine sputum culture test was performed to obtain respiratory samples. Respiratory samples from donors were collected with endotracheal suction or bronchial washing with flexible bronchoscopy prior to donor lung harvest. Respiratory samples were processed according to our laboratory protocol, and microbial cultures and susceptibility tests were performed simultaneously.
Statistical analysis
Categorical and continuous variables were expressed as numbers (percentages) and means ± standard deviations (SDs) or medians (interquartile ranges). Comparisons between the two groups were performed with the independent t-test and Chi-square test. Univariate logistic regression analysis to predict the risk of early PTP was performed to estimate the odds ratio (OR) and 95% confidence interval (CI). Kaplan-Meier curves with log-rank tests were used for analysis of 1-year mortality with respect to PTP. A multivariate Cox proportional hazard model with a stepwise regression selection method was used to identify the independent factors for 1-year mortality. After completing the univariate regression analysis, variables with a P≤0.25 were determined as eligible for use in multiple regression analysis. In addition, clinically reliable variables were included in multivariate analysis. Statistical significance was set at P≤0.05. All statistical analyses were performed using SPSS (version 23; IBM Corp).
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) (17). The study was approved by Pusan National University Yangsan Hospital (PNUYH) Institutional Review Board (IRB No. 05-2021-037) and individual consent for this retrospective analysis was waived.
Results
Baseline characteristics and comparison of clinical outcomes between 1-year survivors and non-survivors
A total of 76 patients underwent lung transplantation between May 2013 and December 2019 at a tertiary teaching university hospital. Their baseline characteristics are presented in Table 1. No significant difference in donor-related parameters such as age, PF ratio, and total lung capacity (TLC) donor/recipient (DR) ratio was observed between the two groups. Regarding the recipient-related parameters, mean age was 55.2±10.8 years in the survivor group and 59.7±8.0 years in the non-survivor group (P=0.052). The profile of diagnostic etiology was not significantly different between groups, and the most common diagnostic etiology for lung transplantation was diffuse interstitial lung disease (DILD) in both groups. The adequacy of the initial prophylactic antibiotics was not significantly different between the two groups.
Table 1. Baseline characteristics and outcomes of 1-year survivors and non-survivors.
| Variables | Total (N=76) | Survivor (N=52) | Non-survivor (N=24) | P |
|---|---|---|---|---|
| Donor variables | ||||
| Age (years) | 40.1±12.6 | 39.7±12.3 | 41.1±13.5 | 0.665 |
| PF ratio | 426.6±120.0 | 434.1±109.0 | 407.5±145.3 | 0.403 |
| TLC DR ratio | 1.0±0.4 | 1.1±0.1 | 0.9±0.7 | 0.286 |
| Recipient variables | ||||
| Age (years) | 56.6±10.3 | 55.2±10.8 | 59.7±8.0 | 0.052 |
| Sex (M) | 48 (63.2) | 32 (61.5) | 16 (66.7) | 0.667 |
| BMI | 21.7±4.3 | 21.2±4.6 | 22.6±3.5 | 0.187 |
| Aetiology | 0.470 | |||
| DILD | 43 (56.6) | 29 (55.8) | 14 (58.3) | |
| COPD | 7 (9.2) | 6 (11.5) | 1 (4.2) | |
| Post-ARDS lung fibrosis | 13 (17.1) | 8 (15.4) | 5 (20.8) | |
| Pulmonary graft versus host disease | 7 (9.2) | 6 (11.5) | 1 (4.2) | |
| Bronchiectasis | 1 (1.3) | 0 | 1 (4.2) | |
| Others | 5 (6.6) | 3 (5.8) | 2 (8.3) | |
| BTT | 60 (78.9) | 39 (75.0) | 21 (87.5) | 0.214 |
| *Adequacy of prophylactic antibiotics | 21 (27.6) | 13 (25.0) | 8 (33.3) | 0.450 |
| Preoperative sputum colonisation | ||||
| Donor | 34 (44.7) | 24 (46.2) | 10 (41.7) | 0.715 |
| Recipient | 28 (36.8) | 16 (30.8) | 12 (50.0) | 0.106 |
| Clinical outcome | ||||
| Early PTP | 37 (48.7) | 20 (38.5) | 17 (70.8) | 0.009 |
| Postoperative VFD within 28 days | 14.5±9.8 | 15.4±9.7 | 12.6±9.9 | 0.264 |
| Postoperative ICU stay (days) | 20.7±17.6 | 15.3±12.6 | 32.3±21.4 | 0.001 |
Data is presented as mean ± standard deviations or number (%). *, adequacy of prophylactic antibiotics. Appropriate antimicrobial treatment is defined as the use of agents with in vitro activity against the etiologic pathogens based on our hospital’s diagnostic laboratory results (18). PF ratio, PaO2/FiO2 ratio; TLC DR ratio, total lung capacity donor/recipient ratio; BMI, body mass index; DILD, diffuse interstitial lung disease; COPD, chronic obstructive pulmonary disease; ARDS, acute respiratory distress syndrome; BTT, bridge to transplantation; PTP, post-transplant pneumonia; VFD, ventilator-free day, defined as days alive and free from mechanical ventilation; ICU, intensive care unit.
With respect to preoperative respiratory colonization, donors and recipients were analyzed separately (Figure 1). Overall, 34 donors (44.7%) and 28 recipients (36.8%) showed positive respiratory cultures. Among the recipients who received lungs from respiratory colonized donors (n=34), 24 (70.6%) survived 1 year after lung transplantation, and the respiratory colonization rate of the donors was not significantly different between survivors and non-survivors (P=0.715). In contrast, 16/28 (57.1%) recipients with preoperative respiratory colonization survived 1 year after lung transplantation. Preoperative respiratory colonization rates in recipients tended to be lower in survivors than in non-survivors (P=0.106). Among other clinical outcomes, the cumulative incidence of early PTP was significantly lower in the survivor group than in the non-survivor group (38.5% vs. 70.8%, P=0.009) and postoperative ICU stay was shorter in survivors than in non-survivors (15.3±12.6 vs. 32.3±21.4 days, P=0.001). However, there was no statistical difference in postoperative VFD within 28 days (15.4±9.7 vs. 12.6±9.9 days, P=0.264). Preoperative microbial community types in donors and recipients before and after surgery are presented in Table S1.
Figure 1.
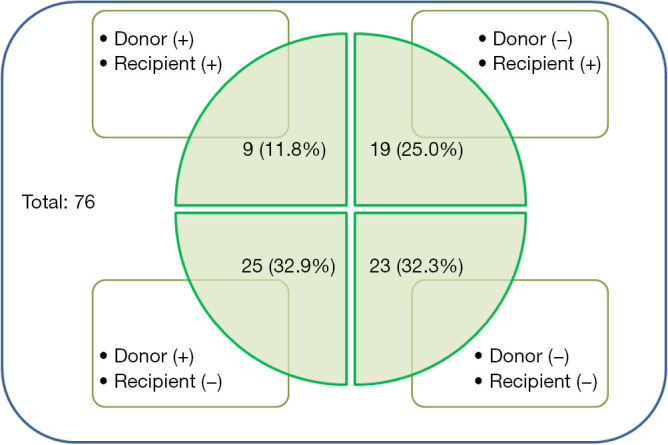
Perioperative respiratory culture results from the overall population (n=76).
Cox proportional hazard model for 1-year mortality
To assess predictors of 1-year mortality, age, BMI, preoperative respiratory colonization, bridge to lung transplantation (BTT), and early PTP were included in the Cox proportional hazard model (Table 2). Preoperative respiratory colonization of the recipients [hazards ratio (HR), 2.550; CI, 1.2–5.2; P=0.010] and PTP (HR, 3.239; CI, 1.4–7.3; P=0.005) were associated with high 1-year mortality rate. In addition, age (HR, 3.954; CI, 1.5–10.3; P=0.005) showed a trend toward increased 1-year mortality rate.
Table 2. Cox proportional hazard model for 1-year mortality.
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (CI) | P | HR (CI) | P | ||
| Recipient | |||||
| *Age | 3.523 (1.4–9.2) | 0.010 | 3.954 (1.5–10.3) | 0.005 | |
| BMI | 1.063 (1.0–1.2) | 0.146 | |||
| Preoperative sputum colonization | 1.917 (0.9–4.3) | 0.111 | 2.550 (1.2–5.2) | 0.010 | |
| BTT | 2.010 (0.6–6.7) | 0.258 | |||
| PTP | 3.067 (1.3–7.4) | 0.013 | 3.239 (1.4–7.3) | 0.005 | |
*, age was used as a qualitative variable with a breakpoint 55 yrs. HR, hazard ratio; CI, confidence interval; BMI, body mass index; BTT, bridge to transplantation; PTP, post-transplant pneumonia.
Baseline characteristics and outcome according to early PTP
Table 3 shows the differences in the baseline characteristics and outcomes according to early PTP. In donors, variables such as age, PF ratio, and TLC DR ratio were not significantly different between patients with and without PTP. However, in recipients, preoperative respiratory MDR colonization was significantly different between patients with and without PTP (35.1% vs. 15.4%, P=0.047). Other clinical outcomes, such as postoperative ICU stay, postoperative hospital of stay (HOS), and 1-year survival significantly differed between patients with PTP and those without PTP (postoperative ICU stay, 27.6±20.6 vs. 14.1±11.0 days, P=0.001; postoperative HOS, 76.0±50.9 vs. 50.9±31.7 days, P=0.011; and 1-year survival, 54.1% vs. 82.1%, P=0.009).
Table 3. Baseline characteristics and outcomes according to early PTP.
| Variables | Total (N=76) | With PTP (N=37) | Without PTP (N=39) | P |
|---|---|---|---|---|
| Donor variables | ||||
| Age | 40.1±12.6 | 40.6±12.3 | 39.6±13.0 | 0.726 |
| PF ratio | 426.6±120.0 | 422.5±145.0 | 430.4±92.7 | 0.784 |
| TLC DR ratio | 1.0±0.4 | 1.0±0.4 | 1.0±0.4 | 0.902 |
| Recipient variables | ||||
| Age | 56.6±10.3 | 55.4±11.9 | 57.8±8.4 | 0.318 |
| Sex (M) | 48 (63.2) | 24 (64.9) | 24 (61.5) | 0.764 |
| BMI | 21.7±4.3 | 22.1±4.2 | 21.3±4.4 | 0.407 |
| Aetiology | 0.828 | |||
| DILD | 43 (56.6) | 21 (56.8) | 22 (56.4) | |
| COPD | 7 (9.2) | 4 (10.8) | 3 (7.7) | |
| Post-ARDS lung fibrosis | 13 (17.1) | 5 (13.5) | 8 (20.5) | |
| Pulmonary graft versus host disease | 7 (9.2) | 4 (10.8) | 3 (7.7) | |
| Bronchiectasis | 1 (1.3) | 1 (2.7) | 0 | |
| Others | 2 (5.4) | 3 (7.7) | ||
| BTT | 60 (78.9) | 30 (81.1) | 30 (76.9) | 0.657 |
| *Adequacy of prophylactic antibiotics | 21 (27.6) | 10 (27.0) | 11 (28.2) | 0.909 |
| Preoperative respiratory colonisation | ||||
| Positive culture in donor | 34 (44.7) | 19 (51.4) | 15 (38.5) | 0.259 |
| Positive culture in recipient | 28 (36.8) | 16 (43.2) | 12 (30.8) | 0.260 |
| **MDR pathogen in recipient | 19 (25.0) | 13 (35.1) | 6 (15.4) | 0.047 |
| Clinical outcome | ||||
| Postoperative ICU stay (days) | 20.7±17.6 | 27.6±20.6 | 14.1±11.0 | 0.001 |
| Postoperative HOS (days) | 63.1±43.7 | 76.0±50.9 | 50.9±31.7 | 0.011 |
| 1-year survival | 52 (68.4) | 20 (54.1) | 32 (82.1) | 0.009 |
Data is presented as mean ± standard deviations or number (%). *, adequacy of prophylactic antibiotics. Appropriate antimicrobial treatment is defined as the use of agents with in vitro activity against the etiologic pathogens based on our hospital diagnostic laboratory results (18); **, MDR pathogen was included methicillin-resistant staphylococcus, carbapenem-resistant Acinetobacter baumannii, and carbapenem-resistant Pseudomonas aeruginosa. PTP, post-transplant pneumonia; PF ratio, PaO2/FiO2 ratio; TLC DR ratio, total lung capacity donor/recipient ratio; BMI, body mass index; DILD, diffuse interstitial lung disease; COPD, chronic obstructive pulmonary disease; ARDS, acute respiratory distress syndrome; BTT, bridge to transplantation; MDR, multidrug resistant; ICU, intensive care unit; HOS, hospital of stay.
Early PTP-related risk factors and Kaplan-Meier curve for 1-year mortality due to onset of early PTP
Univariate analysis was performed to investigate the clinical parameters related to early PTP. Out of all patients (n=76), 37 (48.7%) were diagnosed with early PTP. In univariate analysis, there were no differences in donor-related parameters such as age, PF ratio, and TLC DR ratio, and recipient-related parameters such as sex, BMI, etiology, BTT, and adequacy of antibiotics. In donors, preoperative respiratory colonized rates were not different between patients with and without early PTP. However, in recipients, 16 patients developed PTP (43.2%). In particular, MDR pathogens in recipients were identified in 13 patients with PTP (35.1%) as opposed to six patients without PTP (15.4%) (P=0.047). Multivariate analysis based on age, preoperative MDR colonization of recipients, preoperative MDR colonization of donors, BTT, and appropriateness of prophylactic antibiotics determined that only preoperative MDR colonization in recipients was significantly associated with PTP (P=0.052) (Table 4). Multivariate analysis based on the univariate analysis confirmed that preoperative MDR colonization in recipients was significantly associated with PTP (P=0.018). Kaplan-Meier survival curve analysis showed that patients with PTP had significantly higher 1-year mortality than those without PTP (X2=6.849, P=0.009) (Figure 2).
Table 4. Binary logistic regression analysis for risk factors of early postoperative pneumonia.
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Recipient | |||||
| *Age | 0.974 (0.4–2.3) | 0.951 | |||
| Preoperative SC MDR | 2.979 (1.0–9.0) | 0.052 | 3.284 (1.2–8.8) | 0.018 | |
| Donor | |||||
| Preoperative SC MDR | 1.550 (0.5–4.9) | 0.455 | |||
| BTT | 1.286 (0.4–3.9) | 0.657 | |||
| **Appropriateness of prophylactic antibiotics | 0.943 (0.3–2.6) | 0.909 | |||
*, age was used as a qualitative variable with a breakpoint 55 yrs; **, adequacy of prophylactic antibiotics. Appropriate antimicrobial treatment is defined as the use of agents with in vitro activity against the etiologic pathogens based on our hospital diagnostic laboratory results (18). BMI, body mass index; SC, sputum culture; MDR, multidrug resistance; BTT, bridge to transplantation; OR, odds ratio.
Figure 2.
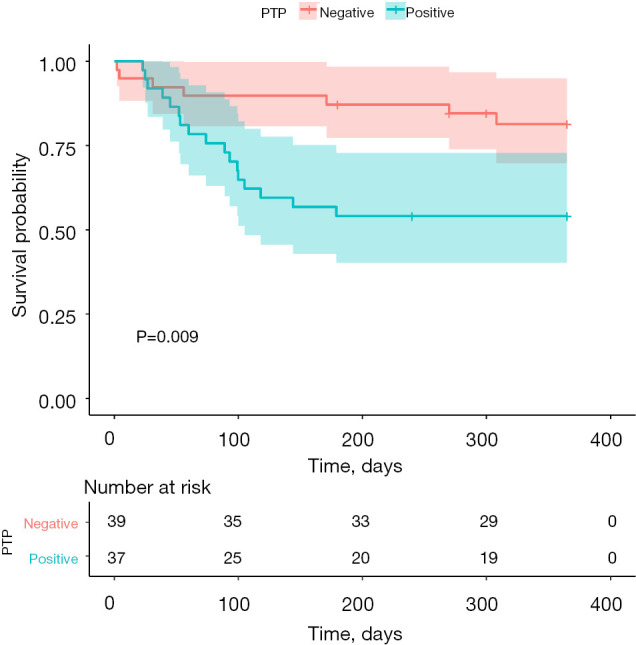
Post-transplant pneumonia (PTP) was significantly associated with 1-year mortality in patients with lung transplantation. Kaplan-Meier analysis. χ2=6.849, P=0.009.
Discussion
The current study showed that preoperative respiratory colonization in recipients was significantly associated with 1-year mortality. In addition, preoperative respiratory colonization was an important factor related to the onset of early PTP, a significant risk factor for 1-year mortality. The 1-year mortality rate markedly differed between patients with and without PTP. In addition, PTP was a very important factor affecting both acute rejection and chronic rejection of the transplant and reflected the success of transplantation (19,20).
In addition, the MDR ratio of preoperative respiratory colonization was higher in patients with PTP than in patients without PTP. Traditionally, infection with MDR is the most common cause of chronic rejection (occurring as bronchiolitis obliterans syndrome) and the highest risk factor affecting long-term outcomes (21,22). The most common MDR organism in this study was Acinetobacter baumannii (AB), a gram-negative coccobacillus. MDR-AB is a serious ICU-acquired pathogen in various countries (23) that can survive in dry environments for several weeks and is characterized by various resistance mechanisms (22). Because of these properties, CRAB is considered a poorly resolvable pathogen in the ICU; it is an important challenge in the treatment of transplantation patients, because it is associated with high probability of pneumonia after transplantation. Here, we showed that MDR is not related to one-year mortality, but only to early PTP, suggesting that active treatment is required.
Considering these results, preoperative respiratory colonization in recipients is a powerful factor that can cause early PTP, increase the length of postoperative ICU, and increase 1-year mortality in lung transplantation patients.
Another interesting point of this study is that perioperative sputum colonization in donors was not associated with the incidence of PTP and 1-year survival, even when MDR was detected, indicating that the risk of microbial transmission from donor lung colonization is unlikely. A previous study showed that the rate of microbial transmission through the transplantation process is low, consistent with our findings (24). Therefore, microbial colonization must be considered during donor’s adequacy assessment, and when detected in donor lungs, it can be overcome through the use of appropriate antibiotics and lung management (18,24,25).
With regard to transplantation, the main difference between the lungs and other organs is that the lungs directly communicate with the external environment through the tracheobronchial tree. In this context, acquired infection in lung transplantation patients with immunosuppression may be a fundamental challenge for prognosis. Therefore, preoperative microbial colonization in donor or recipient lungs, which may be a reason for disqualification of donation or relative contraindication for lung transplantation, is an important concern.
There were some limitations in this study. Since this is a retrospective study conducted only at a single center, it is difficult to generalize our results. Second, selection bias may be prominent because only patients with lung transplantation were included, whereas other solid organ transplantation patients were excluded. As mentioned earlier, the CRAB colonization rate was higher in our study than in the Western ICU. Therefore, it is difficult to extrapolate our results to other lung transplantation centers, and these data should be cautiously interpreted. Despite these limitations, our study helps the understanding impact of preoperative respiratory pathogen on early lung transplant outcome.
Conclusions
The most important factor for PTP was recipient microbial colonization, but not donor colonization. PTP was significantly associated with 1-year mortality. Additionally, the proportion of MDR was higher in the PTP group than in the non-PTP group. This finding suggests that in order to improve the survival rate, recipient colonization must be well managed to prevent pneumonia.
Supplementary
The article’s supplementary files as
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding: This work was supported by a 2-Year Research Grant of Pusan National University.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Pusan National University Yangsan Hospital (PNUYH) Institutional Review Board (IRB No. 05-2021-037) and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1724/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1724/dss
Peer Review File: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1724/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-1724/coif). The authors have no conflicts of interest to declare.
References
- 1.Weill D. Lung transplantation: indications and contraindications. J Thorac Dis 2018;10:4574-87. 10.21037/jtd.2018.06.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sureshbabu A, Fleming T, Mohanakumar T. Autoantibodies in lung transplantation. Transpl Int 2020;33:41-9. 10.1111/tri.13487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nosotti M, Tarsia P, Morlacchi LC. Infections after lung transplantation. J Thorac Dis 2018;10:3849-68. 10.21037/jtd.2018.05.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauthier JM, Hachem RR, Kreisel D. Update on Chronic Lung Allograft Dysfunction. Curr Transplant Rep 2016;3:185-91. 10.1007/s40472-016-0112-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kroshus TJ, Kshettry VR, Savik K, et al. Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. J Thorac Cardiovasc Surg 1997;114:195-202. 10.1016/S0022-5223(97)70144-2 [DOI] [PubMed] [Google Scholar]
- 6.Coiffard B, Prud'Homme E, Hraiech S, et al. Worldwide clinical practices in perioperative antibiotic therapy for lung transplantation. BMC Pulm Med 2020;20:109. 10.1186/s12890-020-1151-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lund LH, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016;35:1158-69. 10.1016/j.healun.2016.08.017 [DOI] [PubMed] [Google Scholar]
- 8.Murray S, Charbeneau J, Marshall BC, et al. Impact of burkholderia infection on lung transplantation in cystic fibrosis. Am J Respir Crit Care Med 2008;178:363-71. 10.1164/rccm.200712-1834OC [DOI] [PubMed] [Google Scholar]
- 9.Kotloff RM, Thabut G. Lung transplantation. Am J Respir Crit Care Med 2011;184:159-71. 10.1164/rccm.201101-0134CI [DOI] [PubMed] [Google Scholar]
- 10.Luna CM, Blanzaco D, Niederman MS, et al. Resolution of ventilator-associated pneumonia: prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit Care Med 2003;31:676-82. 10.1097/01.CCM.0000055380.86458.1E [DOI] [PubMed] [Google Scholar]
- 11.Mackenzie G. The definition and classification of pneumonia. Pneumonia (Nathan) 2016;8:14. 10.1186/s41479-016-0012-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016;35:1170-84. 10.1016/j.healun.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 13.Husain S, Mooney ML, Danziger-Isakov L, et al. A 2010 working formulation for the standardization of definitions of infections in cardiothoracic transplant recipients. J Heart Lung Transplant 2011;30:361-74. 10.1016/j.healun.2011.01.701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka S, Geneve C, Tebano G, et al. Morbidity and mortality related to pneumonia and TRACHEOBRONCHITIS in ICU after lung transplantation. BMC Pulm Med 2018;18:43. 10.1186/s12890-018-0605-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguilar-Guisado M, Givaldá J, Ussetti P, et al. Pneumonia after lung transplantation in the RESITRA Cohort: a multicenter prospective study. Am J Transplant 2007;7:1989-96. 10.1111/j.1600-6143.2007.01882.x [DOI] [PubMed] [Google Scholar]
- 16.Robinson J. Colonization and infection of the respiratory tract: What do we know? Paediatr Child Health 2004;9:21-4. 10.1093/pch/9.1.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Helsinki Declaration of the World Medical Association (WMA) . Ethical principles of medical research involving human subjects. Pol Merkur Lekarski 2014;36:298-301. [PubMed] [Google Scholar]
- 18.Bonde PN, Patel ND, Borja MC, et al. Impact of donor lung organisms on post-lung transplant pneumonia. J Heart Lung Transplant 2006;25:99-105. 10.1016/j.healun.2005.06.026 [DOI] [PubMed] [Google Scholar]
- 19.Gregson AL. Infectious Triggers of Chronic Lung Allograft Dysfunction. Curr Infect Dis Rep 2016;18:21. 10.1007/s11908-016-0529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin-Gandul C, Mueller NJ, Pascual M, et al. The Impact of Infection on Chronic Allograft Dysfunction and Allograft Survival After Solid Organ Transplantation. Am J Transplant 2015;15:3024-40. 10.1111/ajt.13486 [DOI] [PubMed] [Google Scholar]
- 21.Lee KH, Jeong SJ, Kim SY, et al. Effects of Multidrug-resistant Bacteria in Donor Lower Respiratory Tract on Early Posttransplant Pneumonia in Lung Transplant Recipients Without Pretransplant Infection. Transplantation 2020;104:e98-e106. 10.1097/TP.0000000000003102 [DOI] [PubMed] [Google Scholar]
- 22.Shoham S, Shah PD. Impact of multidrug-resistant organisms on patients considered for lung transplantation. Infect Dis Clin North Am 2013;27:343-58. 10.1016/j.idc.2013.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang CI, Song JH. Antimicrobial resistance in Asia: current epidemiology and clinical implications. Infect Chemother 2013;45:22-31. 10.3947/ic.2013.45.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konishi Y, Miyoshi K, Kurosaki T, et al. Airway bacteria of the recipient but not the donor are relevant to post-lung transplant pneumonia. Gen Thorac Cardiovasc Surg 2020;68:833-40. 10.1007/s11748-019-01273-6 [DOI] [PubMed] [Google Scholar]
- 25.Ciulli F, Tamm M, Dennis C, et al. Donor-transmitted bacterial infection in heart-lung transplantation. Transplant Proc 1993;25:1155-6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


