Key Points
Question
Is the COVID-19 pandemic associated with a decrease in the number of cancer screening tests globally?
Findings
In this systematic review and meta-analysis of 39 publications, the screening types analyzed were associated with a significant overall decrease (−46.7%, −44.9%, and −51.8% for breast, colorectal, and cervical cancer screening, respectively) from January to October 2020. This decrease showed a U-shaped trend with a negative peak in April 2020 (−74.3% for mammography and −69.3% for colonoscopy and fecal occult blood test or fecal immunochemical test) and in March 2020 for Papanicolaou test or human papillomavirus test (−78.8%).
Meaning
COVID-19 pandemic measures were associated with widely reduced cancer screening services, which was possibly associated with delayed cancer diagnosis and increased cancer mortality.
Abstract
Importance
Public health services, including cancer screening tests, have been affected by the onset of the COVID-19 epidemic.
Objective
To investigate the pandemic’s association with cancer screening worldwide.
Data Sources
In this systematic review and meta-analysis, databases such as PubMed, ProQuest, and Scopus were searched comprehensively for articles published between January 1, 2020, and December 12, 2021.
Study Selection
Observational studies and articles that reported data from cancer registries that compared the number of screening tests performed before and during the pandemic for breast, cervical, and colorectal cancer were included.
Data Extraction and Synthesis
Two pairs of independent reviewers extracted data from the selected studies. The weighted average of the percentage variation was calculated between the 2 periods to assess the change in the number of cancer screening tests performed during the pandemic. Stratified analysis was performed by geographic area, period, and type of setting. The systematic review and meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.
Main Outcomes and Measures
The main outcome was the weighted average percentage variation in the number of screening tests performed between January and October 2020 compared with the previous period.
Results
The review comprised 39 publications. There was an overall decrease of −46.7% (95% CI, −55.5% to −37.8%) for breast cancer screening, −44.9% (95% CI, −53.8% to −36.1%) for colorectal cancer screening, and −51.8% (95% CI, −64.7% to −38.9%) for cervical cancer screening during the pandemic. For all 3 cancers, a U-shaped temporal trend was identified; for colorectal cancer, a significant decrease was still apparent after May 2020 (in June to October, the decrease was −23.4% [95% CI, −44.4% to −2.4%]). Differences by geographic area and screening setting were also identified.
Conclusions and Relevance
A summary estimate of the downscaling of cancer screening tests since the onset of the COVID-19 pandemic is provided in this systematic review and meta-analysis. This could be associated with an increase in the number of avoidable cancer deaths. Effective interventions are required to restore the capacity of screening services to the prepandemic level.
This systematic review and meta-analysis used databases, such as PubMed, ProQuest, and Scopus, to investigate the association between the COVID-19 pandemic and decreased rates of breast, colorectal, and cervical cancer screening globally.
Introduction
In 2020, according to GLOBOCAN estimates, 19.3 million new cancer cases and approximately 10 million cancer deaths occurred owing to breast, colorectal, and cervical cancer, which are the first, third, and seventh most prevalent cancers worldwide, respectively.1 Screening programs help to detect cancer at early stages or precursor lesions, leading to decreased mortality.
In the past few years, especially in low- to medium-income countries,2 cancer screening and prevention protocols have had a remarkable impact on public health. However, major events, such as natural disasters and epidemics, can negatively affect screening rates and access to health care infrastructures and facilities,3 increasing the burden of cancer mortality.
At the beginning of the COVID-19 pandemic, a suspension of many nonurgent medical services was imposed all over the world. Affected services concerned, for example, family planning and abortion4; HIV prevention, testing, and care5; cancer prevention; specialistic treatments such as delays or deferrals in oncologic surgery, systemic therapy, and radiotherapy; and visits for oncologic patients.6
Consequently, cancer screening programs have been temporarily interrupted in most countries. Although these measures may have led to a reduction in COVID-19 transmission,7 their association with public health must still be determined.8
Because the lockdown restrictions have been heterogeneous in their beginning date and duration, we performed a systematic review of studies that analyzed the variation in the total number of examinations for breast, cervical, and colorectal cancer screening performed since the beginning of the epidemic in comparison with the prepandemic period. Our aim was to quantify the global variation of cancer screening procedures during the lockdown period.
Methods
Search Strategy and Selection Criteria
We conducted our systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.9 We developed the study question by following the patients or the PICO (population, intervention, comparison, and outcome) and study design framework.
This study is part of a larger project aimed at assessing the global effect of the COVID-19 pandemic on patients with cancer, including not only cancer screening but also cancer diagnosis, diagnostic tests for cancer, therapies for oncologic patients, and first visits and follow-up visits for oncology patients. The search string was unique for all these outcomes. We launched the search string on PubMed, ProQuest, and Scopus, without language restriction, for articles published between January 1, 2020, and December 12, 2021. The search string was made using Boolean operators (AND, OR) and Medical Subject Headings terms and their builder options, if relevant, to improve the search results. Searches were performed with the following terms: neoplasms, diagnosis, drug therapy, radiotherapy, surgery, therapy, diagnosis, epidemiology, prevention and control, early detection of cancer, COVID-19, and organization and administration.
We included observational studies and articles that reported data from cancer registries. We chose articles including a comparison between the periods before and after the beginning of the COVID-19 pandemic, with results indicated as number of screening tests performed, percentage variation between the 2 periods, or rate of screening. Studies were excluded if data on at least 1 of these comparisons were not available. We included only studies in which the 2 compared periods were explicitly stated. If the comparison was made between 2 or more pre–COVID-19 periods, we selected the closest period as a reference (eg, we considered 2019 if data for both 2019 and 2018 were available).
Data Collection and Quality Assessment
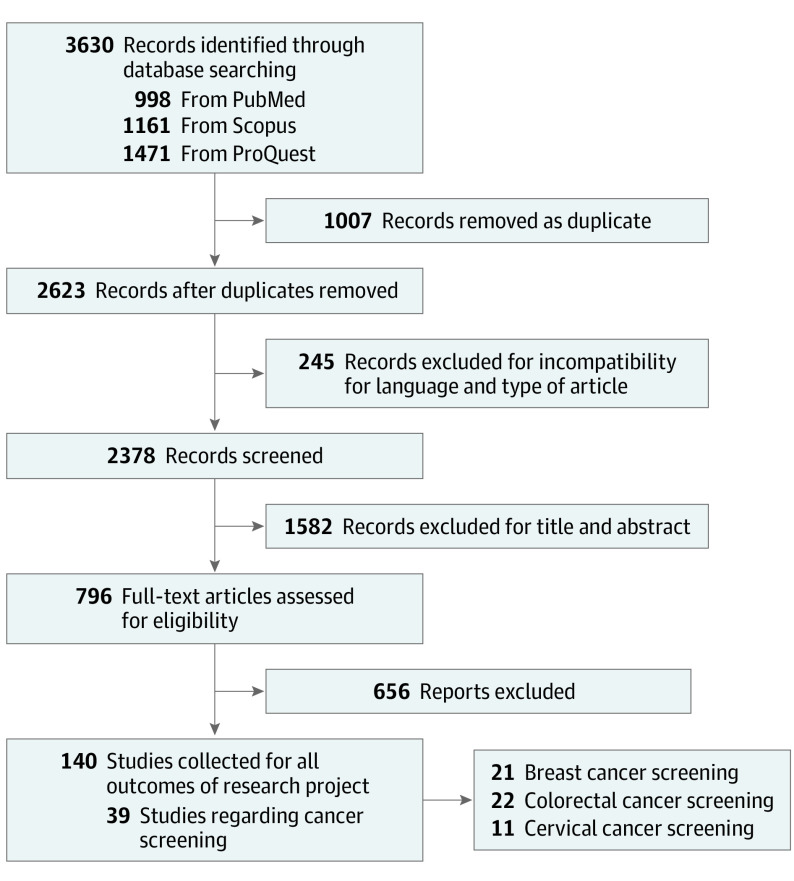
Our database search and study selection process are outlined in the flow diagram (Figure 1). We found a total of 3630 total articles: 998 articles on PubMed, 1471 on ProQuest, and 1161 on Scopus. We collected initial references in citation files, removed duplicates (1007 articles), and began our screening process of the remaining 2623 articles. We initially excluded articles in languages other than English, French, Spanish, or Italian, and also reviews, meta-analyses, or case reports (245 articles). After this process, studies were first reviewed by title and then by abstract. Finally, we examined the full text of each article against eligibility criteria, and we collected 140 studies for all the outcomes of the research project (screening, diagnosis, diagnostic tests, visits, and therapies). Results on cancer screening were reported in 39 articles. We decided to analyze the types of cancer screening recommended for the general population by the US Preventive Services Task Force.10,11,12 We excluded screening types performed for high-risk individuals (eg, lung cancer screening13) and those with lower-grade recommendation (eg, prostate cancer screening14). The title and abstract of the articles were independently reviewed by 2 pairs of reviewers (F.T. and G.C.; M.A. and L.A.) for inclusion in the first screening phase, followed by the full-text screening phase against eligibility criteria. Disagreements among reviewers in the initial abstract screening phase and full-text review were resolved through discussion with a fifth reviewer (P.B.).
Figure 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) Flow Diagram of Information Through the Various Phases of the Systematic Review.
Two pairs of reviewers (F.T. and G.C.; M.A. and L.A.) perused the prepared checklist to extract from each article the following information: name of the first author, year of publication, country and type of setting (hospitals and private centers were considered clinic based; local and national cancer registries were considered population based), site of cancer, type of screening analyzed (mammography, research of blood in the stool with the fecal occult blood test or fecal immunochemical test, colonoscopy, Papanicolaou test, or human papillomavirus test), and absolute number or rate of screening tests performed in the analyzed period or percentage variation between intervals before and after the beginning of the pandemic. In particular, for colorectal cancer screening, we analyzed data from both types of screening (fecal occult blood test or fecal immunochemical test and colonoscopy) separately and together.
We performed an assessment of the quality and risk of bias of all the studies included, using 9 of the 10 items of the Critical Appraisal Skills Programme for qualitative research,15 for a maximum score of 10 points. Studies obtaining less than 7 points were considered inadequate and excluded (no article was excluded because of a low-quality score). eTables 1 through 3 in the Supplement list the articles included in the present analysis and their major characteristics and quality assessment.
Statistical Analyses
For each type of screening, we calculated the weighted average of the percentage variation between screening tests performed before and after the beginning of the COVID-19 pandemic. The weight was calculated with the natural logarithm of the number of daily events in the prepandemic period (ie, daily_screen_precovid obtained by dividing the number of screening tests in the prepandemic period by its duration in days: weight = ln [daily_screen_precovid]). We used the logarithm because of the great variability in the number of tests between studies. We used the absolute value to avoid negative weights.
Some studies did not provide the absolute number of screening tests performed in the prepandemic period, but only the measure of variation between the period being examined and the reference period. For these studies, to calculate the weight, we imputed a value of daily_screen_precovid as the mean value of this variable in the studies having the same type of setting and cancer screening.
We divided the pandemic period into 5 intervals (all referring to 2020) based on the beginning date of the COVID-19 period being examined in each article as follows: period 1 from January 1 to February 29; period 2 from March 1 to March 31; period 3 from April 1 to April 30; period 4 from May 1 to May 31; and period 5 from June 1 to October 31. This partition of the 5 periods was based on temporal distribution of the available observations along the pandemic period and on the trend of COVID-19 spread and pandemic measures in most countries.16 We did not include studies in which the pandemic period started before January 2020. We did not find studies in which the pandemic period of observation started after October 2020. Studies could contribute to more than 1 period, but not more than once in each period. Then we calculated weighted averages for each interval. Because each period analyzed was determined only by the starting date of the observation, for some studies it could include an interval longer than the predefined periods (eg, an observation that considered January 1 as the starting date and May 31 as the end date was included in the first period).
An additional analysis was performed for specific geographic areas and for type of setting. We finally fitted ordinary least-squares linear model using Newton-Raphson (maximum likelihood) optimization with percentage change as dependent variables and terms for type of structure, geographic area, and period as independent variables. In the linear regression model, 2-sided P values were used, with P < .05 considered statistically significant.
Because a percentage reduction could not be lower than −100%, we limited the values of the 95% CIs to −100.0% in case they exceeded it. We considered the funnel plot and performed the Egger regression asymmetry test to assess publication bias.17
No ethics committee approval and no patient consent were necessary because the study was restricted to publicly available data. For all statistical analyses, we used Stata, version 16.1 (StataCorp). P values of differences of means are based on the t test, those of differences of proportions are based on z scores, and P tests of multivariate analyses are those derived from the regression generalized linear models for the respective variables.
Results
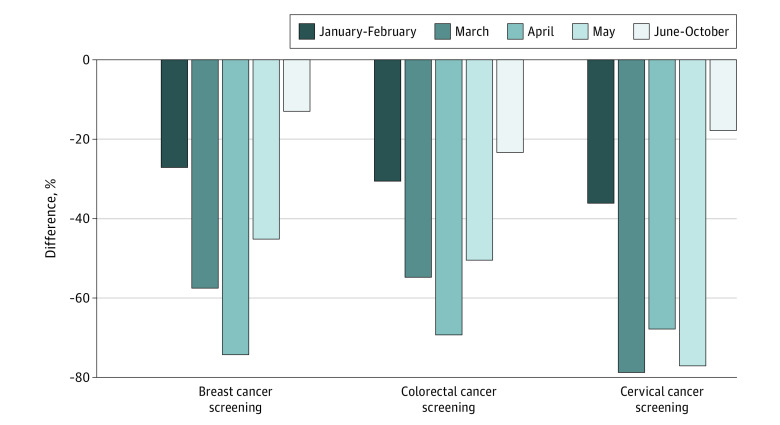
Data on race and ethnicity, as well as demographic information for the study population, were not available. eTable 4 in the Supplement shows the weighted average variation of screening tests performed by cancer type, and Figure 2 shows the corresponding temporal trend.
Figure 2. Weighted Average Variation of Screening Tests Performed From January to October 2020 Compared With the Prepandemic Period, by Cancer Type and Period.
Breast Cancer
The average variation of breast cancer screening from January to October 2020 was −46.7% (95% CI, −55.5% to −37.8%) compared with the pre–COVID-19 period. The maximum decrease in breast cancer screening occurred in April 2020 (−74.3% [95% CI, −91.8% to −56.7%]), whereas after June there was no significant reduction (−13.0% [95% CI, −33.0% to 7.1%]). The weighted average of the screening variation was −62.0% (95% CI, −75.1% to −49.0%) for clinic-based settings and −41.6% (95% CI, −53.2% to −30.0%) for population-based ones. The difference between the 2 types of structures was confirmed to be statistically significant in the linear regression analysis (adjusted difference, 18.9%; 95% CI, 2.0% to 35.9%; P = .03) (Table 1).
Table 1. Adjusted Difference Based on Multivariate Linear Analysis by Period, Geographic Area, and Study Setting for Breast Cancer Screening.
| Characteristics | Coefficient (95% CI) |
|---|---|
| Period (2020) | |
| January and February | 0 [Reference] |
| March | −28.1 (−49.4 to −6.8) |
| April | −53.8 (−75.4 to −32.2) |
| May | −23.1 (−45.0 to −1.2) |
| June to October | 9.7 (−14.1 to 33.9) |
| Geographic area | |
| North America | 0 [Reference] |
| South America | −2.8 (−20.9 to 15.3) |
| Europe | −35.6 (−65.9 to −5.3) |
| Study setting | |
| Clinic based | 0 [Reference] |
| Population based | 18.9 (2.0 to 35.9) |
We divided the geographic areas for breast cancer screening according to the distribution of the countries analyzed in each study: North America (United States and Canada), South America (Brazil, Mexico, Peru, and Uruguay), and Europe (Italy, France, and Turkey). In North America, the number of breast cancer screening tests decreased by −44.6% (95% CI, −57.4% to −31.8%) during the COVID-19 period. In this geographic area, the decrease was −19.4% (95% CI, −55.1% to 16.2%) in January and February, −49.9% (95% CI, −66.5% to −33.2%) in March, −86.7% (95% CI, −100.0% to −73.5%) in April, −37.8% (95% CI, −62.3% to −13.4%) in May, and 0.8% (95% CI, −14.1% to 15.7%) from June to October 2020. Europe presented an average variation of −67.7% (95% CI, −100.0% to −9.3%), and South America presented an average variation of −51.1% (95% CI, −67.5% to −34.7%). As shown in Table 1, the decrease during April was significant (−53.8%; 95% CI, −75.4% to −32.2%; P < .001), using January and February as the reference; studies from Europe also reported a larger decrease compared with those from North America (−35.6%; 95% CI, −65.9% to −5.3%; P = .02).
Colorectal Cancer
The overall decrease in the number of colorectal cancer screening examinations performed throughout the period from January to October 2020 compared with the pre–COVID-19 period was equal to −44.9% (95% CI, −53.8% to −36.1%). In particular, the decrease for colonoscopy was −52.5% (95% CI, −66.3% to −38.8%), and the decrease for fecal occult blood test or fecal immunochemical test was −37.8% (95% CI, −49.9% to −25.8%). The weighted average variation was −51.5% (95% CI, −69.5% to −33.6%) in clinic-based settings and −43.7% (95% CI, −54.9% to −32.6%) in population-based ones.
Compared with the prepandemic period, the global evolution by period showed the greatest decrease in April 2020 (−69.3% [95% CI, −100.0% to −36.9%]) and only a partial recovery during the last months of the analysis (−23.4% [95% CI, −44.4% to −2.4%]) from June to October (eTable 4 in the Supplement).
We analyzed the 3 main geographic areas, including North America (United States and Canada), Asia (India, China, Japan, Taiwan, South Korea, and Singapore), and Europe (Italy, France, and the United Kingdom). The weighted average variation during the entire COVID-19 period was −45.1% (95% CI, −59.3% to −31.0%) for North America, −34.6% (95% CI, −58.1% to −11.1%) for Asia, and −52.4% (95% CI, −69.4% to −35.3%) for Europe. For North America, the greatest decrease was in April (−83.0% [95% CI, −100.0% to −55.8%]), whereas in Asia the most remarkable decrease in screening rates was observed in January and February (−36.2% [95% CI, −77.9% to −5.6%]). In linear regression analysis, April had a greater decrease compared with January and February (−38.3%; 95% CI, −63.6% to −12.9%; P = .003), whereas there was no statistically significant difference between geographic areas (Table 2).
Table 2. Adjusted Difference Based on Multivariate Linear Analysis by Period, Geographic Area, and Study Setting for Colorectal Cancer Screening.
| Characteristics | Coefficient (95% CI) |
|---|---|
| Period (2020) | |
| January and February | 0 [Reference] |
| March | −18.2 (−42.6 to 6.3) |
| April | −38.3 (−63.6 to −12.9) |
| May | −17.1 (−45.3 to 11.1) |
| June to October | 13.5 (−13.3 to 40.2) |
| Geographic area | |
| North America | 0 [Reference] |
| Asia | 12.2 (−13.9 to 38.4) |
| Europe | −12.3 (−30.3 to 5.7) |
| Study setting | |
| Clinic based | 0 [Reference] |
| Population based | 0.1 (−24.4 to 24.6) |
Cervical Cancer
For cervical cancer screening, the weighted average variation was equal to −51.8% (95% CI, −64.7% to −38.9%). The maximum decrease observed among the 5 periods was in March (−78.8% [95% CI, −99.3% to −58.3%]). In the linear regression model (Table 3), a significant decrease was observed in March compared with January and February (−38.9%; 95% CI, −75.2% to −2.5%; P = .04). Clinic-based studies showed a greater reduction than the population-based ones: −51.7% (95% CI, −77.3% to −26.1%) and −51.9% (95% CI, −67.8% to −35.9%), respectively. In North America (United States and Canada), the weighted average variation was −44.7% (95% CI, −73.8% to −15.7%), whereas in South America (Brazil, Mexico, Peru, Uruguay, and Puerto Rico), it was −62.4% (95% CI, −73.3% to −51.5%).
Table 3. Adjusted Difference Based on Multivariate Linear Analysis by Period, Geographic Area, and Study Setting for Cervical Cancer Screening.
| Characteristics | Coefficient (95% CI) |
|---|---|
| Period (2020) | |
| January and February | 0 [Reference] |
| March | −38.9 (−75.2 to −2.5) |
| April | −21.2 (−55.1 to 12.6) |
| May | −31.0 (−68.9 to 6.9) |
| June to October | 25.9 (−8.7 to 56.6) |
| Geographic area | |
| North America | 0 [Reference] |
| South America | −11.6 (−34.5 to 11.3) |
| Study setting | |
| Clinic based | 0 [Reference] |
| Population based | −5.1 (−29.8 to 19.7) |
Discussion
This study investigated the association of the COVID-19 pandemic measures with cancer screening by systematic review. Against a global pattern showing an increasing trend for colorectal cancer screening and a slight decrease for cervical cancer screening and mammography from 2000 to 2015,18 our study found a marked association with a decrease in the number of tests performed for the 3 cancer types (−46.7%, −44.9%, and −51.8% for breast, colorectal, and cervical cancer screening, respectively) in all geographic areas considered during the early phase of the COVID-19 pandemic. Beyond the decision to interrupt all cancer screening programs, the main factors that may have caused this reduction were stay-at-home orders, people's fear of the infection, avoidance of nonurgent medical treatment and care, limited access to in-person medical examinations, and the reorganization of hospital departments.6 The greater decrease for the Papanicolaou test or human papillomavirus test and colonoscopies may be attributable to more invasive techniques used to diagnose these tumors compared with mammography for breast cancer and fecal occult blood test or fecal immunochemical test for colorectal cancer.
We identified a similar pattern by period for breast and colorectal cancer screening, with a U-shaped trend having a negative peak in April 2020 and an attenuation in May. This trend was largely attributable to the lockdown measures in numerous countries and territories worldwide. As illustrated through the stringency index of the Oxford COVID-19 Government Response Tracker,16 in April 2020 most of the countries had implemented COVID-19 measures. All 3 types of cancer screening showed the least reduction from June to October.
A significant feature of the present study is the analysis of different geographic areas; Europe presented a larger decrease in mammograms compared with North America (Table 1), which could be associated with the fact that in the United States, they are mostly performed through private health insurance,18 whereas in Europe, they are usually provided as part of the public health care system. Conversely, the decrease was similar for colorectal cancer screening between the 2 regions. The restrictions that occurred in Europe could therefore have had a greater association with the cancer screening procedures that imply an actual attendance to the health care structure providing the service. Our results indicated that invasive colorectal cancer screenings (colonoscopies) had a larger decrease than noninvasive ones, such as fecal occult blood test or fecal immunochemical test (−52.5% vs 37.8%, respectively), which were more easily performed as a colonoscopy alternative.19 Unlike the global decrease in colorectal cancer screening, Asia presented the most remarkable decrease in January and February; however, the variability of COVID-19 epidemiology and restrictive measures between Asian countries20,21 complicates the interpretation of this matter.
The percentage variation for cervical and breast cancer screening was greater in South America compared with North America (−62.4% vs −44.7% and −51.1% vs −44.6%, respectively); however, we cannot assume that it will result in worse consequences for Latin America owing to its weaker health care system before the pandemic.22
An important aspect of our review was the consistency of results across a wide spectrum of health care settings. The association between the epidemic and cancer screening was greater in studies based in hospitals or other clinic settings than in population-based studies, especially for breast cancer screening (−62.0% vs −41.6%; P = .03), which could be explained by clinic-based studies’ considering only the decrease in the number of patients who actually attended the screening test vs population-based studies’ including also patients who did not participate in screening programs in the prepandemic period. There was no evidence of publication bias either qualitatively according to funnel plot asymmetry or quantitatively with the Egger regression test.
The aim of identifying early neoplastic lesions in the case of breast cancer and precursor lesions in the case of colorectal and cervical cancer was to decrease the incidence of invasive cancer.23 The most concerning potential effect of a decrease in cancer screening is an increase in cancer mortality, as estimated in the United States.24 The interruption of cancer screening could delay diagnosis of tumors, causing a shift to more advanced stages at diagnosis. Furthermore, this could be associated with increased avoidable cancer deaths, aggravate the patients’ discomfort and disease burden, and be associated with increased workload for medical personnel and increased costs for the health care system.8,25,26 In addition, a portion of the postponed screening tests could be impossible to recover because during the suspension some patients exceeded the maximum age to be included in screening programs.
Limitations
Our review has some limitations. First, considerable heterogeneity between countries was present in terms of screening protocols,27 services’ accessibility and participation of the target population, lockdown measures, and incidence of COVID-19 and its temporal trend; all of these factors could not be considered in our statistical analysis. Second, the attribution of an observation to 1 of the 5 periods was based only on its beginning date, which might lead to nondifferential misclassification. Future studies on variations in screening numbers will need to be performed to clarify long-term implications and adopt adequate public health strategies. Third, in some studies we had to impute the number of daily events to calculate the weight.
Conclusions
The results of our systematic review and meta-analysis suggest that there is an association between the COVID-19 pandemic and a reduction in breast, colorectal, and cervical cancer screening, with a U-shaped temporal pattern. Although for breast and cervical cancer screening, the data suggest a recovery after May 2020, the decrease in colorectal cancer screening persisted until October 2020. This is expected to be associated with an increase of avoidable advanced cancers and cancer-related deaths. Further investigation regarding cancer diagnosis and cancer treatment during the COVID-19 pandemic is required.
eTable 1. Characteristics of Breast Cancer Screening Studies
eTable 2. Characteristics of Colorectal Cancer Screening Studies
eTable 3. Characteristics of Cervical Cancer Screening Studies
eTable 4. Weighted Average Variation of Screening Tests Performed From January to October 2020 Compared With the Prepandemic, Divided by Cancer Type, and by Periods and Overall Interval
eReferences.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Canfell K, Kim JJ, Brisson M, et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):591-603. doi: 10.1016/S0140-6736(20)30157-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortiz AP, Gierbolini-Bermúdez A, Ramos-Cartagena JM, et al. Cervical cancer screening among Medicaid patients during natural disasters and the COVID-19 pandemic in Puerto Rico, 2016 to 2020. JAMA Netw Open. 2021;4(10):e2128806. doi: 10.1001/jamanetworkopen.2021.28806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma KA, Zangmo R, Kumari A, Roy KK, Bharti J. Family planning and abortion services in COVID 19 pandemic. Taiwan J Obstet Gynecol. 2020;59(6):808-811. doi: 10.1016/j.tjog.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stover J, Kelly SL, Mudimu E, et al. The risks and benefits of providing HIV services during the COVID-19 pandemic. PLoS One. 2021;16(12):e0260820. doi: 10.1371/journal.pone.0260820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patt D, Gordan L, Diaz M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform. 2020;4:1059-1071. doi: 10.1200/CCI.20.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puricelli Perin DM, Christensen T, Burón A, et al. ; International Cancer Screening Network ICSN . Interruption of cancer screening services due to COVID-19 pandemic: lessons from previous disasters. Prev Med Rep. 2021;23:101399. doi: 10.1016/j.pmedr.2021.101399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yong JH, Mainprize JG, Yaffe MJ, et al. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. J Med Screen. 2021;28(2):100-107. doi: 10.1177/0969141320974711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siu AL; US Preventive Services Task Force . Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(4):279-296. doi: 10.7326/M15-2886 [DOI] [PubMed] [Google Scholar]
- 11.Curry SJ, Krist AH, Owens DK, et al. ; US Preventive Services Task Force . Screening for cervical cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;320(7):674-686. doi: 10.1001/jama.2018.10897 [DOI] [PubMed] [Google Scholar]
- 12.Davidson KW, Barry MJ, Mangione CM, et al. ; US Preventive Services Task Force . Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(19):1965-1977. doi: 10.1001/jama.2021.6238 [DOI] [PubMed] [Google Scholar]
- 13.Krist AH, Davidson KW, Mangione CM, et al. ; US Preventive Services Task Force . Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325(10):962-970. doi: 10.1001/jama.2021.1117 [DOI] [PubMed] [Google Scholar]
- 14.Grossman DC, Curry SJ, Owens DK, et al. ; US Preventive Services Task Force . Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(18):1901-1913. doi: 10.1001/jama.2018.3710 [DOI] [PubMed] [Google Scholar]
- 15.Oxford Centre for Triple Value Healthcare . Critical Appraisal Skills Programme (CASP). Accessed March 3, 2022. https://casp-uk.b-cdn.net/wp-content/uploads/2018/03/CASP-Qualitative-Checklist-2018_fillable_form.pdf
- 16.Hale T, Angrist N, Goldszmidt R, et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat Hum Behav. 2021;5(4):529-538. doi: 10.1038/s41562-021-01079-8 [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall IJ, Tangka FKL, Sabatino SA, Thompson TD, Graubard BI, Breen N. Patterns and trends in cancer screening in the United States. Prev Chronic Dis. 2018;15:E97. doi: 10.5888/pcd15.170465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazidimoradi A, Tiznobaik A, Salehiniya H. Impact of the COVID-19 pandemic on colorectal cancer screening: a systematic review. J Gastrointest Cancer. Published online August 18, 2021. doi: 10.1007/s12029-021-00679-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265-269. doi: 10.1038/s41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pai C, Bhaskar A, Rawoot V. Investigating the dynamics of COVID-19 pandemic in India under lockdown. Chaos Solitons Fractals. 2020;138:109988. doi: 10.1016/j.chaos.2020.109988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atun R, de Andrade LOM, Almeida G, et al. Health-system reform and universal health coverage in Latin America. Lancet. 2015;385(9974):1230-1247. doi: 10.1016/S0140-6736(14)61646-9 [DOI] [PubMed] [Google Scholar]
- 23.Loud JT, Murphy J. Cancer screening and early detection in the 21st century. Semin Oncol Nurs. 2017;33(2):121-128. doi: 10.1016/j.soncn.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma KP, Grosse SD, Maciosek MV, et al. Preventing breast, cervical, and colorectal cancer deaths: assessing the impact of increased screening. Prev Chronic Dis. 2020;17:E123. doi: 10.5888/pcd17.200039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Del Vecchio Blanco G, Calabrese E, Biancone L, Monteleone G, Paoluzi OA. The impact of COVID-19 pandemic in the colorectal cancer prevention. Int J Colorectal Dis. 2020;35(10):1951-1954. doi: 10.1007/s00384-020-03635-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023-1034. doi: 10.1016/S1470-2045(20)30388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ebell MH, Thai TN, Royalty KJ. Cancer screening recommendations: an international comparison of high income countries. Public Health Rev. 2018;39:7. doi: 10.1186/s40985-018-0080-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of Breast Cancer Screening Studies
eTable 2. Characteristics of Colorectal Cancer Screening Studies
eTable 3. Characteristics of Cervical Cancer Screening Studies
eTable 4. Weighted Average Variation of Screening Tests Performed From January to October 2020 Compared With the Prepandemic, Divided by Cancer Type, and by Periods and Overall Interval
eReferences.