Abstract
Background and Purpose:
Social determinants of health (SDOH) have been previously associated with incident stroke. Although SDOH often cluster within individuals, few studies have examined associations between incident stroke and multiple SDOH within the same individual. The objective was to determine the individual and cumulative effects of SDOH on incident stroke.
Methods:
This study included 27,813 participants from the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study, a national, representative, prospective cohort of black and white adults aged ≥45 years. SDOH was the primary exposure. The main outcome was expert adjudicated incident stroke. Cox proportional hazards models examined associations between incident stroke and SDOH, individually and as a count of SDOH, adjusting for potential confounders.
Results:
The mean age was 64.7 years (SD 9.4) at baseline; 55.4% were women and 40.4% were blacks. Over a median follow up of 9.5 years (IQR 6.0, 11.5), we observed 1470 incident stroke events. Of 10 candidate SDOH, seven were associated with stroke (p<0.10): race, education, income, zip code poverty, health insurance, social isolation, and residence in one of the 10 lowest ranked states for public health infrastructure. A significant age interaction resulted in stratification at 75 years. In fully adjusted models, among individuals <75 years, risk of stroke rose as the number of SDOH increased (HR for one SDOH 1.26, 95% CI 1.02–1.55; two SDOH HR 1.38, 95% CI 1.12–1.71; ≥3 SDOH HR 1.51, 95% CI 1.21–1.89) compared to those without any SDOH. Among those ≥75 years, none of the observed effects reached statistical significance.
Conclusions:
Incremental increases in the number of SDOH were independently associated with higher incident stroke risk in adults aged <75 years, with no statistically significant effects observed in individuals ≥75 years. Targeting individuals with multiple SDOH may help reduce risk of stroke among vulnerable populations.
Keywords: Stoke, Social Determinants of Health, Risk Factors
Subject Terms: Cerebrovascular Disease/Stroke, Epidemiology, Risk Factors
Introduction
The incidence of stroke is higher in blacks than in whites in the US.1–5 Higher risk of stroke among blacks contributes to disparities in stroke mortality, with the greatest disparities observed in younger adults and diminishing after age 65.6–9 Racial disparities in incident stroke are well documented in US epidemiological cohort studies.1–9 However, although stroke risk factors have been identified, well-established stroke risk factors such as hypertension, diabetes, left ventricular hypertrophy, atrial fibrillation, cigarette smoking, and a history of heart disease explain only 50% of the excess stroke risk in blacks.8
National health disparities data for cardiovascular disease outcomes identified several social determinants of health (SDOH) that may help explain stroke disparities, including low education, low income, living in an impoverished area, residence in an area with relatively few healthcare services, Southeastern residence, living in rural areas, social isolation, and lacking health insurance.10–13 Studies investigating stroke risk factors beyond traditional Framingham stroke risk factors14, also reported that SDOH may explain existing stroke disparities. For example, the first National Health And Examination Survey found that living in the Southeast was significantly associated with increased stroke incidence.15 Similarly, the REasons for Geographic And Racial Differences in Stroke (REGARDS) study found that Southeastern residence over the life course was associated with higher risk for incident stroke.16 Generally, states in the Southeast have less investment in social safety nets and less healthful dietary practices.17 The Health and Retirement Study also found that low income during middle-age18 and social conditions in childhood predict incident strokes.19 Additionally, social isolation has been associated with 4-fold increased risk of stroke or transient ischemic attack in adults aged ≤65 years.20
Despite the documented increases in stroke risk for individuals exposed to these individual SDOH, little has been reported about differences in stroke incidence conferred by the existence of multiple SDOH in the same person. Most of the existing studies either evaluated effects of a limited number of SDOH (mostly focusing on socio-economic factors), or assessed individual associations between individual SDOH and stroke, or studied the combined effect of SDOH using indices that are not easily interpretable. To address these gaps, we used data from the REGARDS study to determine the relationship between an increasing number of SDOH in the same person and incident stroke. To do so, we created a count of SDOH that has intuitive interpretation and is easy to apply in clinical practice. We hypothesized that a greater number of SDOH would be significantly associated with a higher risk of incident stroke.
Methods
Initial contact for questions related to manuscript proposals and ancillary studies should be made through contact with REGARDSAdmin@uab.edu.
Study population and design
REGARDS is a population-based prospective cohort study of 30,239 black and white adults aged ≥45 years at baseline living across the 48 continental US states and the District of Columbia. REGARDS was designed to elucidate mechanisms leading to higher stroke mortality in the Southeastern US and among blacks, thus participants were recruited between 2003–2007 with oversampling in the “Stroke buckle” (coastal North Carolina (NC), South Carolina (SC) and some parts of Georgia (GA)) and the “Stroke belt” (remaining areas of NC, SC, and GA as well as Tennessee, Mississippi, Alabama, Louisiana, and Arkansas) and the other half from the rest of contiguous U.S.
Sociodemographic information, medical history, and stroke risk factors were collected during a 45-minute computer assisted telephone interview (CATI) at baseline. Participants also underwent in-home physical examinations, where physical measurements and electrocardiograms were obtained and biologic samples were collected. After the baseline survey, participants were contacted by phone every 6 months to assess their vital status and determine if they experienced a hospitalization. This process has been previously described in detail.5 This study was approved by the participating institutions’ Institutional Review Boards. All participants provided written informed consent.
Primary outcome: clinically adjudicated incident strokes
Incident stroke was based on expert adjudication after medical record review.9 A stroke event was defined as: 1) focal neurological deficit consistent with ischemia lasting for >24 hours and confirmed with medical records; 2) clinical strokes (focal or non-focal neurological deficit with diagnostic brain imaging regardless of symptom duration); and 3) expert adjudicated stroke deaths.
Primary exposure
Our selection of the 10 candidate SDOH was guided by the Healthy People 2020 framework,21 which consists of five major domains related to influences from economic, educational, social context, health and healthcare, and neighborhood and community factors.21 We selected SDOH to represent each of domain (Figure 1): economic domain (annual household income <$35,000 vs. ≥$35,000); education (<high school vs. high school graduate or more); social context (black vs. white race; seeing no friend or family member at least once a month vs. 1 or more; having nobody to care for you if you become seriously ill or disabled vs. having someone); healthcare (rural urban commuting area codes 9 and 10 vs all others, or residence in a county with poor availability of primary care physicians, defined as Health Professional Shortage Area [HPSA] vs. partial HPSA or not HPSA); neighborhood and community characteristics (living in a zip code with >25% of residents living below Federal poverty line, living in the worst ranked states for public health infrastructure, bottom 20% vs. all others).
Figure 1: SDOH Guided by the Healthy People 2020 Conceptual Framework.
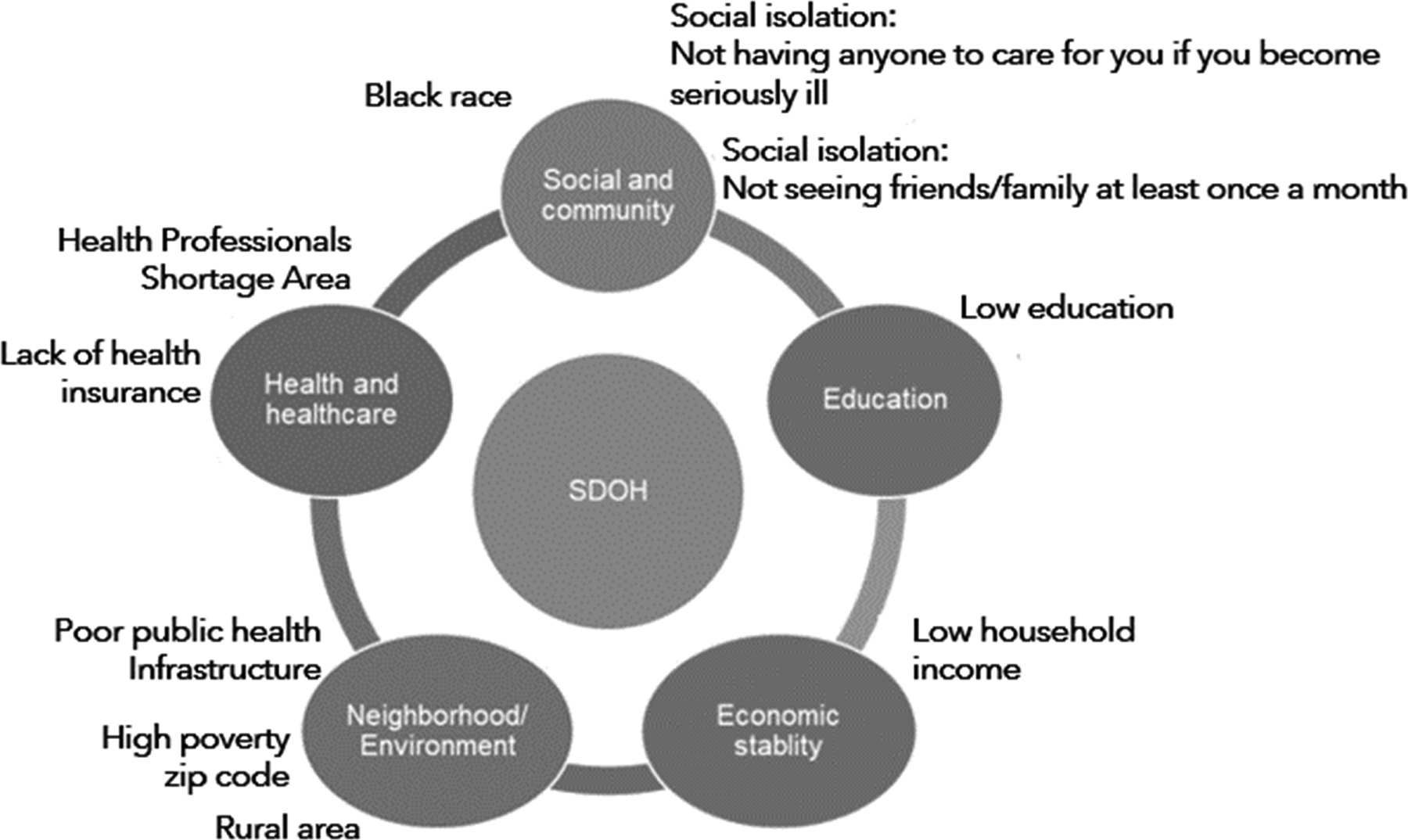
Black race, social isolation; low education, low annual household income, living in a rural area; living in a zip code with high poverty, living in a Health Professional Shortage Area, lack of health insurance, and living in a state with poor public infrastructure.
We created the public health infrastructure metric based on data from America’s Health Ranking (AHR)22 (Supplemental Table I). We examined state rankings from 1993–2002 (ten-year period prior to the beginning of REGARDS enrollment in 2003). For this analysis, nine states that fell into the bottom 20% of US states for public health infrastructure for at least 8 years (80% of the 10 year period) were considered to have poor public health infrastructure (Supplemental Table II).
Covariates Collected at Baseline
Demographic factors included the participant’s age at baseline, sex, and region. Medical conditions included self-reported history of heart disease; high blood pressure (antihypertensive medication use or blood pressure ≥140/90 mm Hg); dyslipidemia (self-report of a diagnosis, use of lipid-lowering medications, total cholesterol ≥200 mg/dL, high-density lipoprotein cholesterol <40 mg/dL, or low-density lipoprotein cholesterol >100 mg/dL); diabetes (use of hypoglycemic medications or fasting blood glucose ≥126 mg/dL or non-fasting glucose ≥200 mg/dL); atrial fibrillation (self-report or present on baseline ECG); and left ventricular hypertrophy (LVH) based on the baseline ECG using the Sokolow criteria.23 Use of medications included antihypertensives, statins, and insulin. Functional status was defined by the Physical Component Summary (PCS) and Mental Component Summary (MCS) scores from the Short Form-12.24 Health behaviors include cigarette smoking (current smoker vs. former or never), alcohol use (heavy drinking based on sex-specific National Institute of Alcohol Abuse And Alcoholism cut points vs. moderate or no consumption)25, physical activity (enough activity to work up a sweat on most days of the week vs. no physical activity), and Mediterranean diet adherence score (highest quartile vs. others).26 Physiological variables included body mass index (BMI), high sensitivity c-reactive protein (hsCRP), urinary albumin-to-creatinine ratio (ACR), and estimated glomerular filtration rate (eGFR) using the CKD-Epi equation.27
Statistical analyses
We examined bivariate associations between 10 dichotomized candidate SDOH and incident stroke in separate Cox proportional hazards models28, controlling for age at baseline and sex. SDOH that were significantly associated with stroke at the p<0.10 level and were retained for further analyses. We evaluated collinearity among SDOH using a variance inflation factor (VIF) threshold of 5.0.29 Using seven SDOH (p<0.10), we created a SDOH count variable: 0, 1, 2, and ≥3.
Since previous studies suggested age differences in the relationship between SDOH and stroke,30 we examined interactions between SDOH count and age.
We described baseline characteristics across groups defined by SDOH counts for each age stratum. We then estimated Kaplan Meier survival functions for groups defined by SDOH counts separately by age groups, and calculated incidence rates of stroke per 1,000 person-years with 95% CIs.
To determine if the SDOH count had an independent effect on incident stroke, we fitted minimally adjusted Cox proportional hazards models adjusting for age and sex for each age stratum. We then fitted fully adjusted Cox models that included potential confounders captured at baseline.
We conducted additional sensitivity analyses: a) exploring alternative National Heart, Lung, and Blood Institute (NHLBI) definitions of the Stoke Buckle31, b) adding exposure to tobacco smoke to multivariable models, c) adding Dietary Approaches to Stop Hypertension (DASH) diet scores to the healthy diet variable (Supplemental Table III).
To minimize bias due to missing data, we performed multiple imputations by chained equations.32 Analyses were conducted in SAS version 9.4 and R 3.4.1.
Results
The analytic sample included 27,813 adults (Supplemental Figure I).
Seven SDOH were associated with stroke (p<0.10) and retained for further analysis (Table 1). VIF values were <1.3, confirming no collinearity among retained SDOH.
Table 1.
Minimally Adjusted Associations of Each SDOH with Stroke Outcomes
| SDOH | HR (95% CI)† | p |
|---|---|---|
| Black race | 1.34 (1.20, 1.48)* | <.0001 |
| Low education | 1.32 (1.14, 1.52)* | 0.001 |
| Low annual household income | 1.59 (1.41, 1.79)* | <.0001 |
| Zip code poverty | 1.25 (1.10, 1.42)* | 0.001 |
| Residence in Health Professional Shortage Areas | 1.01 (0.91, 1.12) | 0.921 |
| Residence in the worst ranked states for public health infrastructure | 1.17 (1.05, 1.30)* | 0.003 |
| Lack of health insurance | 1.46 (1.16, 1.84)* | 0.002 |
| Rural residence | 1.02 (0.74, 1.42) | 0.902 |
| Social isolation (Not seeing friends or family members at least once a month) | 1.25 (1.00, 1.60)* | 0.054 |
| Social isolation (Not having anyone to care for you if become seriously ill or disabled) | 1.02 (0.88, 1.19) | 0.775 |
p<0.10
Adjusted for age and gender
The mean age of the sample was 64.7 years (SD 9.4) at baseline; 55.4% were women and 40.4% were black (Table 2).
Table 2.
Participants’ Characteristics by Age Strata and Number of Social Determinants of Health (SDOH)
| <75 years | ≥75 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of SDOH | 0 | 1 | 2 | 3 + | 0 | 1 | 2 | 3 + | |
| N | 4,361 | 6,289 | 4,667 | 5,182 | 566 | 1,156 | 1,004 | 1,139 | |
| N (%) or Mean (SD) or Median [IQR] | |||||||||
| Social Determinants of Health: | |||||||||
| Black race | 0 (0.0) | 1968 (31.3) | 2496 (53.5) | 4341 (83.8) | 0 (0.0) | 146 (12.6) | 382 (38.0) | 898 (78.8) | |
| Low education | 0 (0.0) | 79 (1.3) | 370 (7.9) | 1754 (33.8) | 0 (0.0) | 18 (1.6) | 119 (11.9) | 614 (53.9) | |
| Low annual household income | 0 (0.0) | 1609 (25.6) | 2890 (61.9) | 4279 (82.6) | 0 (0.0) | 657 (56.8) | 821 (81.8) | 972 (85.3) | |
| Zip code poverty | 0 (0.0) | 238 (3.8) | 923 (19.8) | 2976 (57.4) | 0 (0.0) | 25 (2.2) | 150 (14.9) | 673 (59.1) | |
| Lack of health insurance | 0 (0.0) | 80 (1.3) | 308 (6.6) | 1270 (24.5) | 0 (0.0) | 2 (0.2) | 10 (1.0) | 44 (3.9) | |
| Residence in the states with poor public health infrastructure | 0 (0.0) | 2145 (34.1) | 2061 (44.2) | 3148 (60.7) | 0 (0.0) | 274 (23.7) | 456 (45.4) | 630 (55.3) | |
| Not seeing friends or family members at least once a month | 0 (0.0) | 170 (2.7) | 286 (6.1) | 477 (9.2) | 0 (0.0) | 34 (2.9) | 70 (7.0) | 113 (9.9) | |
| Demographic Factors: | |||||||||
| Female | 1951 (44.7) | 3257 (51.8) | 2779 (59.5) | 3311 (63.9) | 173 (30.6) | 543 (47.0) | 556 (55.4) | 688 (60.4) | |
| Age | 60.8 (7.1) | 61.7 (7.1) | 62.1 (7.2) | 61.8 (7.2) | 79.3 (3.9) | 79.3 (3.7) | 79.3 (3.8) | 79.6 (4.0)* | |
| Southeast region residence | 1750 (40.1) | 3533 (56.2) | 2860 (61.3) | 3494 (67.4) | 190 (33.6) | 516 (44.6) | 546 (54.4) | 650 (57.1) | |
| Medical Conditions: | |||||||||
| History of heart disease | 548 (12.6) | 907 (14.4) | 718 (15.4) | 809 (15.6) | 179 (31.6) | 340 (29.4) | 266 (26.5) | 252 (22.1) | |
| Hypertension | 1828 (41.9) | 3365 (53.5) | 2810 (60.2) | 3579 (69.1) | 332 (58.7) | 733 (63.4) | 694 (69.1) | 838 (73.6) | |
| Dyslipidemia | 2497 (57.3) | 3602 (57.3) | 2616 (56.1) | 2822 (54.5)* | 347 (61.3) | 687 (59.4) | 579 (57.7) | 626 (55.0)* | |
| Diabetes | 468 (10.7) | 1090 (17.3) | 1088 (23.3) | 1585 (30.6) | 71 (12.5) | 172 (14.9) | 213 (21.2) | 319 (28.0) | |
| Atrial fibrillation | 252 (5.8) | 441 (7.0) | 371 (7.9) | 428 (8.3) | 92 (16.3) | 159 (13.8) | 118 (11.8) | 115 (10.1) | |
| Left ventricular hypertrophy | 52 (1.2) | 141 (2.2) | 141 (3.0) | 265 (5.1) | 27 (4.8) | 38 (3.3) | 57 (5.7) | 66 (5.8) | |
| Use of Medications: | |||||||||
| Antihypertensives | 1866 (42.8) | 3321 (52.8) | 2805 (60.1) | 3399 (65.6) | 389 (68.7) | 818 (70.8) | 728 (72.5) | 877 (77.0) | |
| Statins | 1337 (30.7) | 1883 (29.9) | 1359 (29.1) | 1412 (27.2) | 238 (42.0) | 443 (38.3) | 330 (32.9) | 369 (32.4) | |
| Insulin | 86 (2.0) | 245 (3.9) | 277 (5.9) | 481 (9.3) | 16 (2.8) | 29 (2.5) | 46 (4.6) | 94 (8.3) | |
| Functional Status: | |||||||||
| SF-12 physical component score | 53.1 [47.3, 55.9] | 51.7 [44.2, 55.5] | 49.7 [39.7, 54.7] | 46.9 [36.3, 53.2] | 51.4 [44.1, 55.3] | 48.7 [39.4, 54.2] | 46.5 [37.9, 53.0] | 45.6 [35.9, 52.4] | |
| SF-12 mental component score | 56.9 [53.3, 58.8] | 56.9 [52.9, 59.3] | 56.6 [50.9, 59.4] | 55.3 [47.2, 58.9] | 57.9 [55.2, 60.0] | 57.8 [54.0, 60.2] | 57.2 [52.7, 59.9] | 55.9 [49.5, 59.9] | |
| Health Behaviors | |||||||||
| Current cigarette smoking | 438 (10.0) | 830 (13.2) | 809 (17.3) | 1219 (23.5) | 17 (3.0) | 60 (5.2) | 82 (8.2) | 79 (6.9) | |
| Risky alcohol consumption | 265 (6.1) | 288 (4.6) | 167 (3.6) | 150 (2.9) | 35 (6.2) | 34 (2.9) | 21 (2.1) | 16 (1.4) | |
| Physical activity | 3176 (72.8) | 4374 (69.6) | 3043 (65.2) | 3206 (61.9) | 371 (65.5) | 701 (60.6) | 561 (55.9) | 600 (52.7) | |
| High adherence to Mediterranean diet | 987 (22.6) | 1390 (22.1) | 807 (17.3) | 660 (12.7) | 156 (27.6) | 275 (23.8) | 185 (18.4) | 132 (11.6) | |
| Physiological Factors: | |||||||||
| Body mass index | 28.4 (5.3) | 29.3 (5.9) | 30.3 (6.6) | 31.2 (7.1) | 26.5 (4.1) | 27.0 (4.6) | 27.4 (5.2) | 28.5 (5.7) | |
| Systolic blood pressure | 122.8 (14.7) | 125.6 (15.5) | 127.2 (16.23) | 130.5 (17.3) | 128.9 (14.9) | 130.3 (17.8) | 131.9 (16.8) | 133.2 (18.4) | |
| Diastolic blood pressure | 75.6 (8.9) | 76.6 (9.4) | 77.1 (9.4) | 78.8 (10.3) | 73.0 (9.3) | 74.0 (9.8) | 74.5 (9.6) | 75.0 (10.1) | |
| Total cholesterol | 192.7 (37.5) | 193.0 (39.3) | 194.5 (40.7) | 194.1 (42.2)* | 178.5 (35.2) | 183.7 (37.7) | 187.1 (38.6) | 189.9 (41.5) | |
| High density lipoprotein cholesterol | 48.0 [39.0, 60.0] | 49.0 [40.0, 61.0] | 49.0 [40.0, 61.0] | 50.0 [41.0, 61.0] | 48.0 [39.0, 59.0] | 49.0 [39.0, 60.0] | 50.0 [40.0, 62.0] | 51.0 [42.0, 63.0] | |
| C-reactive protein ≥3 | 1207 (27.7) | 2241 (35.6) | 1963 (42.1) | 2484 (47.9) | 173 (30.6) | 389 (33.7) | 356 (35.5) | 440 (38.6) | |
| Urinary Albumin/ Creatinine ratio≥30 (mg/g) | 357 (8.2) | 632 (10.1) | 631 (13.5) | 934 (18.0) | 95 (16.8) | 207 (17.9) | 215 (21.4) | 281 (24.7) | |
| Estimated GFR | 90.1 [78.6, 97.3] | 90.2 [77.5, 99.1] | 90.9 [76.4, 102.1] | 93.8 [78.4, 107.6] | 72.7 [60.4, 82.9] | 72.3 [59.9, 83.4] | 72.9 [57.0, 84.5] | 72.6* [56.4, 85.3] | |
p>0.05, no asterisk denotes statistical significance
Note. p-values correspond to ANOVA, Kruskal-Wallis test, Pearson’s chi-squared or Fisher’s exact test, depending on variable’s distribution
We observed statistically significant age interactions at age 75 years (p=0.41, p=0.06, and p=0.01 for 1, 2, and ≥3 SDOH, respectively), and stratified subsequent analyses by age <75 and ≥75 years. From the clinical perspective, age ≥75 years is a potent risk factor for stroke, as stroke rates are highest among adults 75+.33,34
The younger cohort (<75 years) included 83.4% of participants. Of these, 55.9% were women and 41.6% were blacks. The older cohort (≥75 years) included 52.6% women and 34.7% blacks. In both age strata, individuals with a greater number of SDOH were more likely to be black women, have low annual income, live in an impoverished neighborhood, and reside in the states with poor public health infrastructure. Individuals with a greater number of SDOH were more likely to have a history of hypertension or diabetes, taking antihypertensive medications and insulin. Compared to the younger cohort, the older cohort had a smaller percentage of Stroke Belt or Buckle residents, and a greater proportion with a history of heart disease, atrial fibrillation, LVH, antihypertensive medication use, and statin use.
Over a median follow up of 9.5 years, we observed 1,470 incident strokes. Stroke incidence was lower in the younger cohort (<75 years), compared to the older cohort (≥75 years) (Figure 2). In the younger cohort, the incidence of stroke increased with each additional SDOH. Stroke incidence was 2 times larger for individuals with ≥3 SDOH compared to those without SDOH. In the older cohort, the incidence of stroke was 1.3 times higher for individuals with ≥3 SDOH compared with those with no SDOH. The absolute difference in stroke incidence between individuals without SDOH vs. those with ≥3 SDOH was 3.42 per 1000 person-years for the younger group and 3.72 for the older group.
Figure 2.
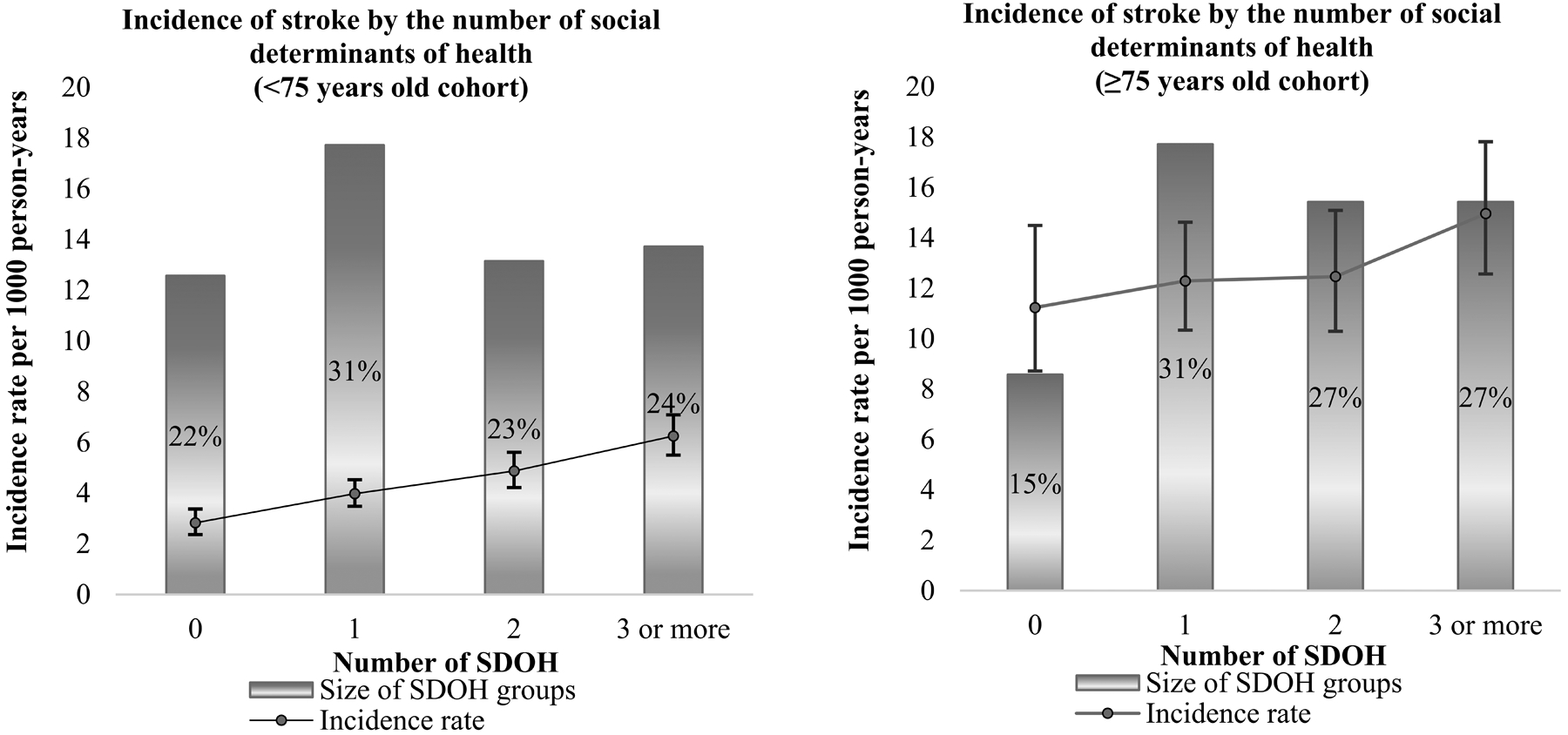
Age Adjusted Incidence of Stroke per 1,000 Person-years
Kaplan Meier curves are shown in Figure 3. In the younger cohort, participants with a greater number of SDOH had a greater probability of incident stroke. The log-rank test did not show statistically significant differences among survival functions in the older cohort (p=0.19).
Figure 3.
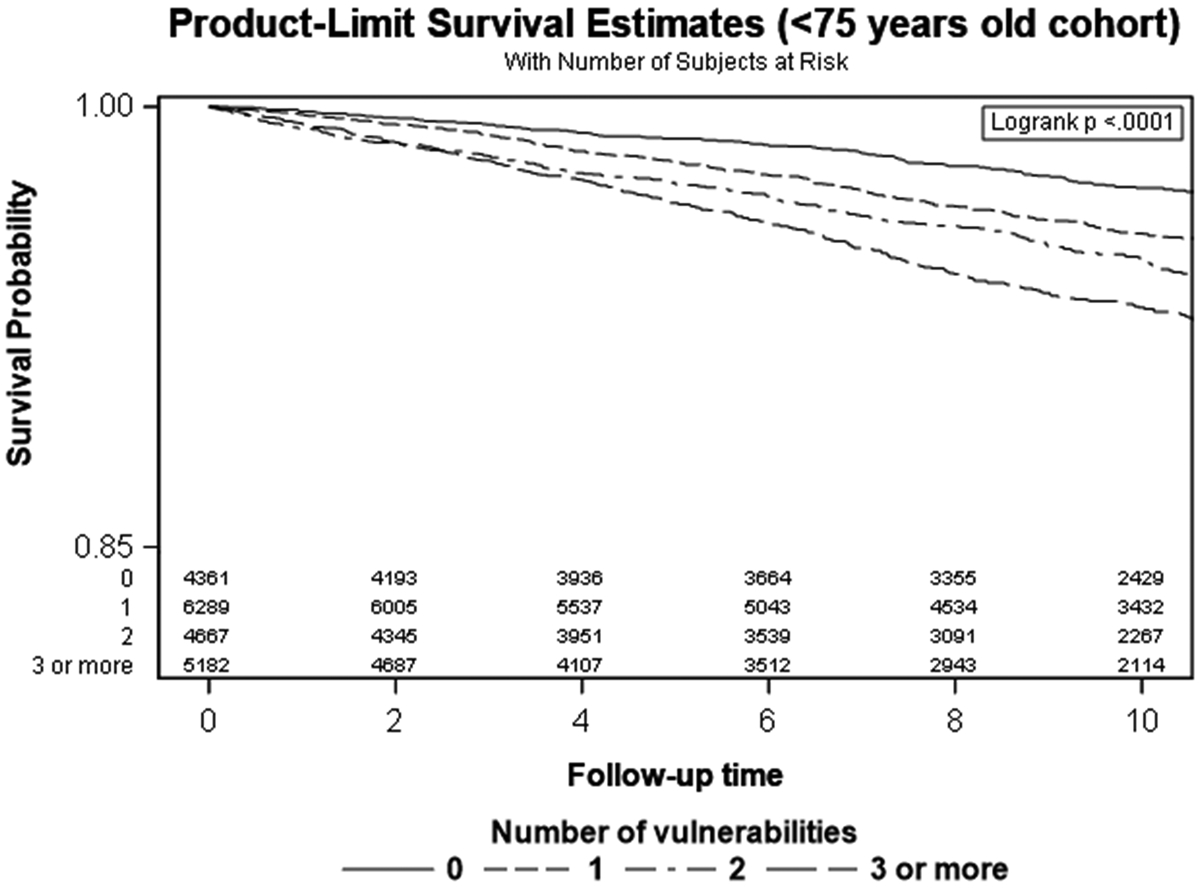

Kaplan-Meier Curves by Age Strata and Number of SDOH and Incident Stroke
In minimally adjusted Cox proportional hazards models, for the younger cohort, the risk of incident stroke gradually rose as the number of SDOH increased (Table 3). Compared to adults without any SDOH, the HR for incident stroke was 1.44 (95% CI 1.07–1.78) for individuals with 1 SDOH, 1.82 (95% CI 1.48–2.24) for those with 2 SDOH, and 2.38 (95% CI 1.94–2.92) for those with ≥3 SDOH, respectively. The upward trend of incident stroke risk was significant in the older cohort, but only the HR for ≥3 SDOH reached statistical significance (HR 1.40, 95% CI 1.02–1.92).
Table 3.
Number of SDOH and Associations with Stroke by Age Strata
| <75 years | ≥75 years | |||
|---|---|---|---|---|
| Minimally adjusted† | Fully adjusted‡ | Minimally adjusted† | Fully adjusted‡ | |
| Number of SDOH | HR (95% CI) | |||
| None | (reference) | (reference) | ||
| 1 | 1.44 (1.07, 1.78)* | 1.26 (1.02, 1.55)* | 1.13 (0.82, 1.55) | 0.92 (0.66, 1.26) |
| 2 | 1.82 (1.48, 2.24)* | 1.38 (1.12, 1.71)* | 1.15 (0.83, 1.60) | 0.94 (0.66, 1.33) |
| 3+ | 2.38 (1.94, 2.92)* | 1.51 (1.21, 1.89)* | 1.40 (1.02, 1.92)* | 0.97 (0.69, 1.35) |
| p for trend | <.0001 | 0.0002 | 0.03 | 0.97 |
In fully adjusted Cox models, observed effects from the minimally adjusted models were attenuated across both age groups, but the upward risk trend persisted among those <75 years of age (p for trend <0.001). Compared to having no SDOH, having 1 SDOH was associated with an adjusted HR of 1.26 (95% CI 1.02–1.55), 2 SDOH were associated with a HR of 1.38 (95% CI 1.12–1.71), and ≥3 SDOH were associated with a HR of 1.51 (95% CI 1.211.89). In the older cohort, none of the observed effects reached statistical significance.
Sensitivity analyses show that in fully adjusted models, results for the <75 year old cohort remained unchanged. For the ≥75 year old cohort, results remained insignificant for all levels of the exposure (Supplemental Table IV).
Discussion
We observed statistically significant associations between seven SDOH and increased risk of incident stroke. Importantly, consistent with existing stroke literature30, we observed statistically significant interactions between age and stroke risk factors. That is, when selected SDOH were operationalized as a count reflecting the combined burden of SDOH within the same individual, we found that each incremental increase in SDOH was significantly associated with an increased risk of stroke for individuals younger than 75, with stroke incidence nearly two and a half times higher for those with three or more SDOH compared with those without SDOH; these excess risks remained significant after adjusting for a host of individual characteristics including well known stroke risk factors. Consistent with the existing literature, the effect did not reach statistical significance in older individuals,30 and selective survival may be one of the most likely explanation of this important finding.
Our study contributes to the literature on SDOH and incident stroke in several ways. To date, many previous studies focused on either individual-level30,35,36 or area-level SDOH.36–39 We defined SDOH as a combination of individual-level as well as neighborhood-level factors, and evaluated their combined effect on incident stroke. Moreover, many prior studies explored relationships between SDOH and incident stroke, limiting definitions of SDOH to only SES factors.8,18,30,37 We expanded the list of SDOH to represent five major domains of SDOH including economic stability, education, social and community context, healthcare, neighborhood and the built environment.21
Although some studies have assessed associations between multiple SDOH within individuals and incidence of stroke, the SDOH in these studies were combined in composite indices,37,40–42 such as the Social Deprivation Index (SDI).40 The SDI used similar variables to the SDOH count in our study. Butler, et al,40 found that the SDI was positively associated with poor health outcomes, and this finding persisted after adjustment for other measures of healthcare access. The SDI was more strongly associated with health outcomes than poverty alone.40 However, the SDI may not be as interpretable as a count of SDOH, which was used in our study. Furthermore, operationalizing SDOH as a count is easier to calculate and interpret, which is appealing in clinical settings.
Our finding of a graded effect of multiple SDOH on the risk of incident stroke especially in younger individuals is concordant with a recent REGARDS study on a treatment disparity in cardiovascular disease prevention. Schroff, et al43, observed that among individuals with an indication for statins, only 45% of those with 4+ SDOH were taking statins compared to 65% of individuals without any SDOH.43 These findings support the value of examining multiple SDOH to identify particularly high-risk individuals for effective population health management.
Although eradicating SDOH may be beyond the scope of individual physicians or health systems, our study adds to understanding the crucial role of SDOH in impacting health outcomes. Durable policy solutions are needed to eliminate the deleterious influences of SDOH on health outcomes, especially for adults younger than 75 years. Ample evidence shows that there is need for continued improvement in the prevention of and control of stroke risk factors such as behavioral interventions to help people to quit smoking, eat a healthy diet, and be physically active.33 While prevention and better control of stroke risk factors cannot entirely overcome the influence of the cumulative burden of SDOH, considering these factors may substantially reduce overall risks. In our study, adults with ≥3 SDOH had minimally adjusted HR 2.38 that reduced to 1.51 after accounting for physiologic risk factors. While most physicians likely already recognize that certain demographic groups face poor health outcomes, our study elucidates the magnitude of this residual risk, which has not yet been reported.
Our study’s strengths include the large, national biracial sample, rich baseline data, and rigorously adjudicated stroke outcomes. Limitations include the use of some self-reported variables, which may introduce a risk of recall bias. Tobacco exposure information was limited to cigarette smoking at baseline. Additional SDOH such as perceived discrimination or environmental factors were not available in our dataset. The reliance of REGARDS procedures on reaching participants may have missed some stroke events, but we have shown that the magnitude of this limitation is similar to that in other epidemiology studies.42,44,45
Conclusions
We found that among adults <75 years, as the number of SDOH within the same individual increased, the incidence of stroke increased and was nearly two and a half times higher in those with ≥3 SDOH compared to those with no SDOH. After controlling for potential confounders, stroke risk remained 50% higher among those with ≥3 SDOH compared to those without any SDOH. Individuals with multiple SDOH such as black women living in impoverished neighborhoods in the Southeast may be excellent targets for focused interventions to reduce incidence of strokes. Healthcare professionals should consider heightening their vigilance to prevent the development of risk factors and achieve improved physiologic risk factor control in persons with multiple SDOH. Elimination of disparities in the incidence of stroke may require societal interventions targeted at SDOH, especially in younger individuals.
Supplementary Material
Acknowledgements:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of the manuscript but were not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS Investigators and Institutions can be found at http://www.regardsstudy.org.
Sources of Funding
This research project is supported by cooperative agreement U01 NS041588 co-funded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service.
Disclosures
Monika M. Safford, MD and April P. Carson, PhD receive grant funding from Amgen Inc. for unrelated investigator-initiated research.
Non-standard Abbreviations and Acronyms:
- REGARDS
REasons for Geographic And Racial Differences in Stroke
- SDOH
Social Determinants Of Health
- DASH
Dietary Approaches to Stop Hypertension
References
- 1.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 2.El-Saed A, Kuller LH, Newman AB, Lopez O, Costantino J, McTigue K, Cushman M, Kronmal R. Geographic variations in stroke incidence and mortality among older populations in four US communities. Stroke. 2006;37:1975–1979. [DOI] [PubMed] [Google Scholar]
- 3.Sacco RL, Boden-Albala B, Gan R, Chen X, Kargman DE, Shea S, Paik MC, Hauser WA. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147:259–268. [DOI] [PubMed] [Google Scholar]
- 4.Kleindorfer DO, Khoury J, Moomaw CJ, Alwell K, Woo D, Flaherty ML, Khatri P, Adeoye O, Ferioli S, Broderick JP, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke. 2010;41:1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 6.Chong JY, Sacco RL. Epidemiology of stroke in young adults: race/ethnic differences. J Thromb Thrombolysis. 2005;20:77–83. [DOI] [PubMed] [Google Scholar]
- 7.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, Woo D, Szaflarski J, Gebel J, Moomaw C, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004;35:426–431. [DOI] [PubMed] [Google Scholar]
- 8.Howard G, Cushman M, Kissela BM, Kleindorfer DO, McClure LA, Safford MM, Rhodes JD, Soliman EZ, Moy CS, Judd SE, et al. Traditional risk factors as the underlying cause of racial disparities in stroke: lessons from the half-full (empty?) glass. Stroke. 2011;42:3369–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.2018 National Healthcare Quality and Disparities Report. Department of Health and Human Services. https://www.ahrq.gov/research/findings/nhqrdr/nhqdr18/index.html. Accessed 18 May, 2020.
- 11.AHRQ. 2015 National Healthcare Quality and Disparities Report and 5th Anniversary Update on the National Quality Strategy. Rockville, MD: AHRQ; 2016. [Google Scholar]
- 12.CDC. CDC Health Disparities and Inequalities Report - United States, 2013. Atlanta, GA: 2013. November 2 [Google Scholar]
- 13.Institute of Medicine. Guidance for the National Healthcare Disparities Report. Washington, DC: National Academy Press; 2002. [PubMed] [Google Scholar]
- 14.Romero J, Wolf P. Epidemiology of Stroke Legacy of Framingham Heart Study. Global Health. 2013;8:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillum RF, Ingram DD. Relation between residence in the southeast region of the United States and stroke incidence. The NHANES I Epidemiologic Followup Study. Am J Epidemiol. 1996;144:665–673. [DOI] [PubMed] [Google Scholar]
- 16.Howard VJ, McClure LA, Glymour MM, Cunningham SA, Kleindorfer DO, Crowe M, Wadley VG, Peace F, Howard G, Lackland DT. Effect of duration and age at exposure to the Stroke Belt on incident stroke in adulthood. Neurology. 2013;80:1655–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Judd SE, Gutierrez OM, Newby PK, Howard G, Howard VJ, Locher JL, Kissela BM, Shikany JM. Dietary patterns are associated with incident stroke and contribute to excess risk of stroke in black Americans. Stroke. 2013;44:3305–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avendano M, Glymour MM. Stroke disparities in older Americans: is wealth a more powerful indicator of risk than income and education? Stroke. 2008;39:1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glymour MM, Avendano M, Haas S, Berkman LF. Lifecourse of Social Conditions and Racial Disparities in Incidence of First Stroke. Ann Epidemiol. 2008;18:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salaycik KJ, Kelly-Hayes M, Beiser A, Nguyen AH, Brady SM, Kase CS, Wolf PA. Depressive symptoms and risk of stroke: the Framingham Study. Stroke. 2007;38:16–21. [DOI] [PubMed] [Google Scholar]
- 21.Healthy People 2020: Social Determinants of Health. Office of Disease Prevention and Health Promotion. https://www.healthypeople.gov/2020/topics-objectives/topic/social-determinants-of-health. Accessed 10 Aug, 2018.
- 22.America’s Health Rankings. https://www.americashealthrankings.org. Accessed Jan 26, 2020.
- 23.O’Neal WT, Howard VJ, Kleindorfer D, Kissela B, Judd SE, McClure LA, Cushman M, Howard G, Soliman EZ. Interrelationship between electrocardiographic left ventricular hypertrophy, QT prolongation, and ischaemic stroke: the REasons for Geographic and Racial Differences in Stroke Study. Europace. 2016;18:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 25.Drinking Levels Defined. National Institute on Alcohol Abuse and Alcoholism. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Accessed 10 Aug, 2018.
- 26.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, et al. Retraction and Republication: Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N Engl J Med. 2018;378:2441–2442. [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox DR. Regression models and life-tables. Journal of the Royal Statistical Society: Series B (Methodological). 1972;34:187–202. [Google Scholar]
- 29.Freund RJ, Wilson WJ and Sa P. Regression analysis. Elsevier; 2006. [Google Scholar]
- 30.Avendano M, Kawachi I, Van Lenthe F, Boshuizen HC, Mackenbach JP, Van den Bos GA, Fay ME, Berkman LF. Socioeconomic status and stroke incidence in the US elderly: the role of risk factors in the EPESE study. Stroke. 2006;37:1368–73. [DOI] [PubMed] [Google Scholar]
- 31.National Heart, Lung, and Blood Institute Stroke Belt Initiative: Project Accomplishments and Lessons Learned. National Heart, Lung, and Blood Institute. https://www.nhlbi.nih.gov/files/docs/resources/heart/sb_spec.pdf. Accessed 18 May, 2020.
- 32.Buuren SV, Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in R. Journal of statistical software. 2010:1–68. [Google Scholar]
- 33.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 34.Howard G, Goff DC. Population shifts and the future of stroke: forecasts of the future burden of stroke. Ann N Y Acad Sci. 2012;1268:14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McFadden E, Luben R, Wareham N, Bingham S, Khaw KT. Social class, risk factors, and stroke incidence in men and women: a prospective study in the European prospective investigation into cancer in Norfolk cohort. Stroke. 2009;40:1070–7. [DOI] [PubMed] [Google Scholar]
- 36.Kuper H, Adami HO, Theorell T, Weiderpass E. The socioeconomic gradient in the incidence of stroke: a prospective study in middle-aged women in Sweden. Stroke. 2007;38:27–33. [DOI] [PubMed] [Google Scholar]
- 37.Bray BD, Paley L, Hoffman A, James M, Gompertz P, Wolfe CD, Hemingway H, Rudd AG, SSNAP Collaboration. Socioeconomic disparities in first stroke incidence, quality of care, and survival: a nationwide registry-based cohort study of 44 million adults in England. The Lancet Public Health. 2018;3:e185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlsson AC, Li X, Holzmann MJ, Ärnlöv J, Wändell P, Gasevic D, Sundquist J, Sundquist K. Neighborhood socioeconomic status at the age of 40 years and ischemic stroke before the age of 50 years: A nationwide cohort study from Sweden. International Journal of Stroke. 2017;12:815–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grimaud O, Béjot Y, Heritage Z, Vallée J, Durier J, Cadot E, Giroud M, Chauvin P. Incidence of stroke and socioeconomic neighborhood characteristics: an ecological analysis of Dijon stroke registry. Stroke. 2011;42:1201–6. [DOI] [PubMed] [Google Scholar]
- 40.Butler DC, Petterson S, Phillips RL, Bazemore AW. Measures of social deprivation that predict health care access and need within a rational area of primary care service delivery. Health Serv Res. 2013;48:539–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cesaroni G, Agabiti N, Forastiere F, Perucci CA. Socioeconomic differences in stroke incidence and prognosis under a universal healthcare system. Stroke. 2009;40:2812–9. [DOI] [PubMed] [Google Scholar]
- 42.Howard VJ, McClure LA, Kleindorfer DO, Cunningham SA, Thrift AG, Roux AV, Howard G. Neighborhood Socioeconomic Index And Stroke Incidence in a National Cohort of Blacks and Whites. Neurology. 2016;87:2340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroff P, Gamboa CM, Durant RW, Oikeh A, Richman JS, Safford MM. Vulnerabilities to Health Disparities and Statin Use in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) Study. J Am Heart Assoc. 2017;6:e005449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hlatky MA, Ray RM, Burwen DR, Margolis KL, Johnson KC, Kucharska-Newton A, Manson JE, Robinson JG, Safford MM, Allison M, et al. Use of Medicare Data to Identify Coronary Heart Disease Outcomes in the Women’s Health Initiative. Circ Cardiovasc Qual Outcomes. 2014;7:157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumamaru H, Judd SE, Curtis JR, Ramachandran R, Hardy NC, Rhodes JD, Safford MM, Kissela BM, Howard G, Jalbert JJ, et al. Validity of claims-based stroke algorithms in contemporary Medicare data: reasons for geographic and racial differences in stroke (REGARDS) study linked with medicare claims. Circ Cardiovasc Qual Outcomes. 2014;7:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zafarmand MH, Spanjer M, Nicolaou M, Wijnhoven H, van Schaik B, Uitterlinden AG, Snieder H, and Vrijkotte T. Influence of Dietary Approaches to Stop Hypertension-Type Diet, Known Genetic Variants and Their Interplay on Blood Pressure in Early Childhood: ABCD Study. Hypertension. 2020;75:59–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


