Abstract
Simple Summary
MMP-13 is an enzyme that can digest and disrupt the tissue structures surrounding epithelial cells that line the surface of many internal organs, as well as the tissue structures surrounding endothelial cells that line the surface of blood vessels. The production of MMP-13 is tightly controlled in physiological conditions but is increased in various cancers and plays multiple roles in tumour progression and metastasis. This review summarises the current understanding of the regulation of MMP-13 production and discusses the actions of MMP-13 in cancer progression and metastasis.
Abstract
Matrix metalloproteinase-13 (MMP-13) is a member of the Matrix metalloproteinases (MMPs) family of endopeptidases. MMP-13 is produced in low amounts and is well-regulated during normal physiological conditions. Its expression and secretion are, however, increased in various cancers, where it plays multiple roles in tumour progression and metastasis. As an interstitial collagenase, MMP-13 can proteolytically cleave not only collagens I, II and III, but also a range of extracellular matrix proteins (ECMs). Its action causes ECM remodelling and often leads to the release of various sequestered growth and angiogenetic factors that promote tumour cell growth, invasion and angiogenesis. This review summarizes our current understanding of the regulation of MMP-13 expression and secretion and discusses the actions of MMP-13 in cancer progression and metastasis.
Keywords: MMP-13, tumour growth, cancer invasion, metastasis, angiogenesis
1. Introduction
Matrix metalloproteinases (MMPs) are a family of 28 (so far) zinc-dependent endopeptidases [1]. According to their substrate specificities, MMPs are divided into several subfamilies of collagenases (MMP-1, MMP-8, MMP-13 and MMP-18), matrilysins (MMP-7 and MMP-26), gelatinases (MMP-2 and MMP-9), stromelysins (MMP-3, MMP-10 and MMP-11), membrane-type MMPs (MT-MMPs), glycosylphosphatidylinositol-anchored MMPs (MMP-17 and MMP-25) and others (MMP-12, MMP19, MMP-20, MMP-21, MMP-22, MMP-23, MMP-27 and MMP-28) [2,3,4]. All MMP family members share a conventional structure of a catalytic domain and a pro-peptide domain. In all cases except MMP-7 and MMP-26, the catalytic domain of MMPs contains a zinc-binding motif HEXXHXXGXXH [5] and is linked to a hemopexin domain by a flexible hinge region. With the exception of MMP-23, whose cysteine residue is located in a different amino acid sequence [6], the MMP amino-terminal pro-peptide domain contains a consensus sequence PRCXXPD (also known as cysteine switch). MMPs are produced in low amounts, and this is well-regulated under normal physiological conditions by various factors, including endogenous MMP inhibitors and tissue inhibitors of MMPs (TIMPs) [7]. Some MMP family members are, however, overexpressed in pathological disorders, such as cancer [8,9,10]. They are considered to be the primary contributors to the degradation of extracellular matrix (ECM) in tumour cell invasion. MMP family members have the ability to cleave ECM molecules with a wide range of substrate specificities [11]. Most ECM components can be degraded by MMP-3, -7, -10 and -11, while other MMPs, such as MMP-1, -8 and -13, preferentially digest collagen I, II and III located near the cells.
MMP-13 is an interstitial collagenase (also known as collagenase 3) and is overexpressed in various cancers [12,13,14] and in cancer stromal cells [15]. As a collagenase, MMP-13 can cleave not only collagens I, II and III, but also a wide range of ECM components. The expression and secretion of MMP-13 are regulated at the transcriptional and cellular levels [14,16,17,18,19,20]. Considerable evidence has shown that MMP-13-mediated degradation and remodelling of ECM plays a very important role in cancer pathogenesis and metastasis.
2. MMP-13 Structure
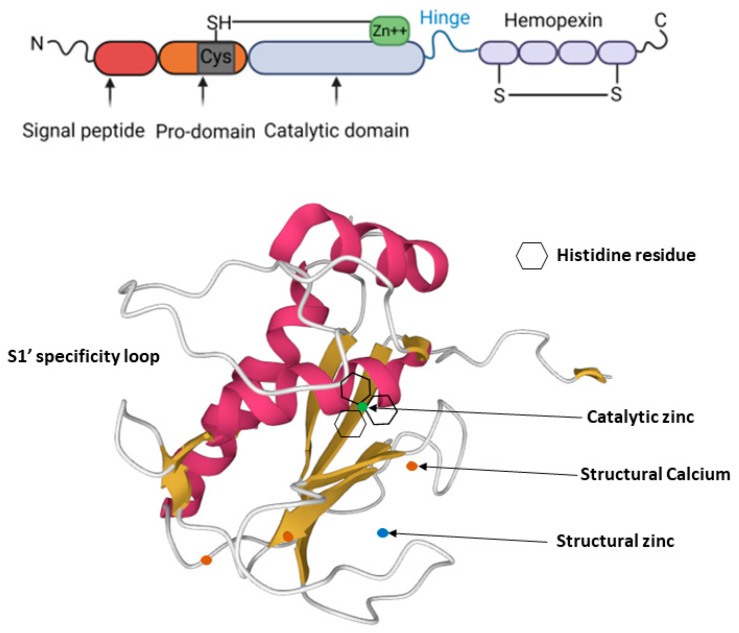
MMP-13 is a 53kDa protein that consists of four domains, namely, the N-terminal signal sequence, basic pro-domain, catalytic domain and C-terminal hemopexin-like domain (Figure 1). Its C-terminal hemopexin-like domain is linked to the catalytic domain by a flexible hinge region. Its signal peptide domain controls the movement of the newly synthesized molecule and guides it to the endoplasm reticulum, while its pro-domain, which contains a zinc-interacting thiol (SH) group, keeps MMP-13 as an inactive zymogen form of pro-MMP-13. The catalytic domain of MMP-13 is shielded by the pro-domain in the inactive pro-MMP-13, and this prevents substrate access [21]. The MMP-13 catalytic domain, which is highly conserved among MMPs, includes three α-helixes and five β-sheets connected by eight loops [22]. The highly conserved catalytic domain of MMP-13, like other MMP members, has an extended zinc-binding motif, which consists of three zinc-binding histidines and a glutamate, a second structural zinc ion and three structural calcium ions, which are essential for enzyme stability [23,24]. The MMP-13 C-terminal hemopexin-like domain consists of four β-propeller elements and functions primarily for substrate specificity [25], as well as for degradation of triple-helical collagens [26]. Activation of pro-MMP-13 is carried out by other MMPs, such as MT1-MMP or MMP-2, on the cell surface [27], in which the cysteine residue is pulled out by conformational change to generate a functional active site, and this, in turn, enables enzymes to remove the pro-domain completely [28].
Figure 1.
MMP-13 structure: MMP-13 consists of a highly conserved signal peptide, a pro-domain, a catalytic domain, a proline-rich hinge region and a C-terminal hemopexin-like domain (top panel). The cysteine residue (Cys 77) shown in grey within the pro-domain is linked to the catalytic zinc ion within the catalytic domain in pro-active MMP-13. The bottom panel shows the MMP-13 catalytic domain (PDB 2OW9). The structural zinc ion is in blue, the catalytic zinc ion is in green, and the three calcium ions are in orange. Three histidine residues, which bind to the catalytic zinc ion, are shown as hexagons.
MMP-13 substrate specificity is largely controlled by its S pockets in the catalytic domain. There are multiple S pockets sitting on two sides of the catalytic zinc ion: (1) on the left side are pockets without a prime: S1, S2, S3…Sn; (2) on the right side are pockets with a prime: S1′, S2′, S3′… Sn’ [29]. The substances or inhibitors, in correspondence with the specific pockets, are named P1, P2…. Pn and P1′, P2′…Pn’, respectively [30]. It is believed that the S1′ pocket is the key contributor to establish MMP binding specificity, possibly because it is the most variable in depth among all the pockets [31]. While all MMPs contain the S1′ pocket, the volume and shape of each S1′ pocket varies [31]. MMP-13 possesses an exceptionally large S1′ pocket made of residues 245–253 [20]. However, given that MMP-13, MMP-8 and MMP-1 are all collagenases, but MMP-1 has only a small shallow pocket, the S1′ pocket of these MMP members may not be the only determining factor for their collagenase activity.
3. Regulation of MMP-13 Expression and Secretion
Due to its destructive nature as a protease towards a wide range of ECM proteins, MMP-13 was initially thought to be absent or to lack steady production in normal tissues [32,33]. However, subsequent studies revealed that MMP-13 is expressed in human chondrocytes and other healthy human connective tissues, such as cartilage and developing bone [34]. MMP-13 is also detected in normal epithelial and neuronal cells [35]. However, the expression and secretion of MMP-13 in normal human tissues are low and are tightly controlled at multiple levels by multiple factors.
The promoter of the human MMP-13 gene contains several binding sites for transcription factors. This includes a PEA-3 binding site and an AP-1 consensus sequence [36]. The combination of PEA-3/AP-1 acts as a responsive unit to growth factors, oncogenes and tumour promoters [36]. The human MMP-13 promoter also contains an osteoblast-specific element (OSE-2) binding site, ACCACA, which can be bound by transcription factor Cbfal [37]. The more distal region of the MMP-13 promoter also contains a Transforming growth factor-beta inhibitory element (TIE) binding site [38]. A conserved forkhead response element (FHRE) consensus sequence for FOXO3a has also been reported in the MMP-13 promoters in humans, mice and rats [39]. Although the precise mechanisms of MMP-13 regulation at the transcriptional level remain largely unknown, the presence of multiple bindings sites in its promoter for several transcription factors clearly indicates the importance of MMP-13 regulation at the transcriptional level. Indeed, several transcription factors have been reported to regulate MMP-13 expression. For example, the binding of ETS variant transcription factor 4 (ETV4) to the AP-1 binding site in the MMP-13 promoter region induced MMP-13 expression in breast cancer [40]. The binding of Small leucine zipper protein (sLZIP) to MMP-13 promoter increased MMP-13 expression in prostate cancer cells [41].
Various hormones, cytokines and growth factors regulate MMP-13 expression in human tissues. Interleukin-1 (IL-1), Interleukin-6 (IL-6) and Tumour necrosis factor alpha (TNF-α) can induce MMP-13 expression in primary chondrocytes [42]. This process is reported to involve nuclear translocation of nuclear factor kappa B (NF-κB) [43]. Growth factors, such as insulin-like growth factors (IGF)-I and -II, can inhibit MMP-13 expression in chondrocytes [44], while transforming growth factor-β1 (TGF-β1) has been shown to induce MMP-13 expression in human KMST fibroblasts [45].
MMP-13 is normally secreted as an inactive pro-MMP-13 form by cells. Its activation is carried out through proteolytic cleavage of its pro-peptide domain by MT1-MMP and MMP-2 [27,46,47] (Figure 2). MMP-13 can also be activated by MMP-3 [27] and the major isoenzyme of human tumour-associated trypsinogen, trypsin-2 [48]. The activity of MMP-13 is controlled by TIMPs. Four TIMPs (TIMP1, TIMP2, TIMP3 and TIMP4) are known to exist in human tissues [49]. Each TIMP contains an N-terminal ‘wedge-shaped’ ridge domain, which binds to the MMP’s active site, and a C-terminal hemopexin interaction domain [50]. The function of TIMPs is to block substrate access to MMPs. In addition to the tight control of its expression and activity in normal physiological conditions, the secretion of MMP-13 to the outside of cells is also regulated by endocytosis. Low-density lipoprotein receptor-related protein 1 (LRP1) can bind to secreted MMP-13 (both pro- and activated forms) through its hemopexin domain and induce MMP-13 endocytosis and subsequent degradation in lysosomes in healthy human chondrocytes [34].
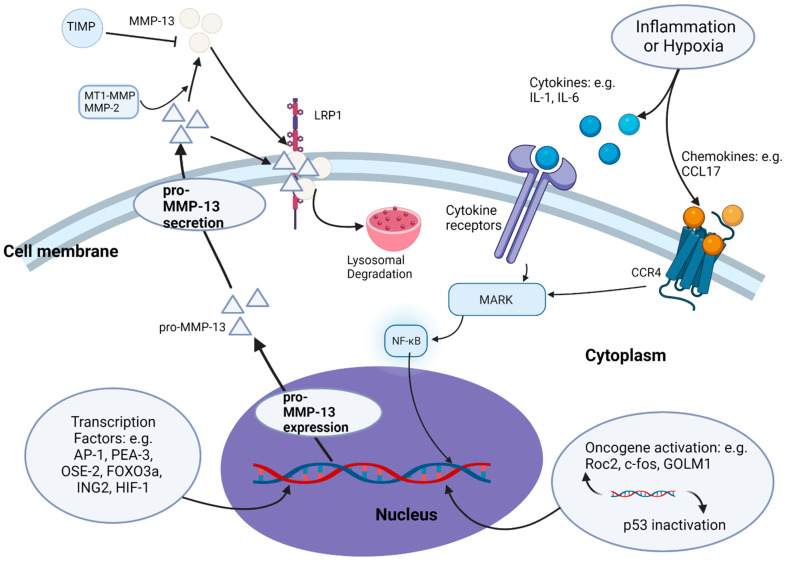
Figure 2.
Regulation of MMP-13 expression and secretion in pathophysiology: MMP-13 expression and secretion in physiological conditions are tightly controlled by transcription factors and TIMPs. Various transcription factors bind directly to the MMP-13 promoter region. In cancer, the tight control of MMP-13 expression is disrupted by intrinsic and extrinsic mechanisms. Intrinsic mechanisms include changes of expression of oncogenes (e.g., Ror2) and tumour suppressor genes (e.g., p53). Extrinsic mechanisms include hypoxia and inflammation-induced secretion of cytokines and chemokines that activate downstream MARK signalling and NF-κB nuclear translocation to regulate MMP-13 expression. MMP-13 secretion is regulated by endocytosis through endocytic receptor LRP1 and subsequent degradation in lysosomes.
In cancer, the tight control of MMP-13 production and activity is disrupted by intrinsic or extrinsic mechanisms. The intrinsic mechanisms include changes of expression of oncogenes (e.g., Ror2) or proto-oncogenes (e.g., c-fos) and tumour suppressor genes (e.g., p53), which directly activate MMP-13 expression [51]. Suppression of oncogene Ror2 expression downregulated MMP-13 expression in osteosarcoma SaOS-2 cells [52]. Suppression of oncogene Golgi membrane protein 1 (GOLM1) inhibited MMP-13 expression in breast cancer [53], while suppression of tumour suppressor p53 increased MMP-13 expression in squamous cell carcinomas [54]. The extrinsic mechanisms involved in MMP-13 regulation include hypoxia and inflammation. The hypoxic microenvironment inside a tumour, created by a restricted oxygen supply from the increasing tumour size, induces cell necrosis [55]. This triggers inflammation and attracts leukocytes to the area to produce cytokines such as IL-1, IL-6 and TNF-α [56]. As discussed above, cytokines such as IL-1, IL-6 and TNF-α, β are important MMP-13 expression enhancers [42,51,57]. The secretion of these cytokines leads to an increase in MMP-13 expression [58,59,60]. Tumour hypoxia can also trigger the expression of hypoxia-inducible transcription factor (HIF)-1, which directly promotes MMP-13 gene expression by binding to the MMP-13 promotor or indirectly through promotion of the expression of the growth factors or cytokines [61] that regulate MMP-13 expression.
Recent studies have also reported the regulation of MMP-13 expression in cancer by other effectors, such as chemokines and endogenous enzymes. The binding of chemokine CCL17 to its receptor CCR4 was shown to enhance MMP-13 expression in bladder cancer through the activation of extracellular signal-regulated kinase (ERK) 1/2 signalling [62], and in colorectal cancer through the activation of ERK/NF-κB signalling [63]. The upregulation of ERK/NF-κB signalling enhanced the binding of NF-κB to Inhibitor of growth 2 (ING2) promoter, leading to the activation of ING2, which subsequently increased MMP-13 expression in colon cancer [64]. Small ubiquitin related modifier (SUMO)-specific protease 2 (SENP2) altered SUMOylation of the MMP-13 promoter and enhanced MMP-13 expression in bladder cancer [65]. FTO, a demethylase for N6-methyladenosine modification, was also shown to upregulate MMP-13 expression in oesophageal squamous cell carcinoma [66]. Overall, the expression, secretion and activation of MMP-13 in cancer is regulated at multiple levels and by many molecules, including cytokines, growth factors and proteases.
4. MMP-13 Expression in Cancer
Given its powerful and destructive action toward ECM, it is not surprising that higher MMP-13 expression frequently occurs in cancer. MMP-13 overexpression in cancer was first reported in breast cancer [12] and has subsequently been observed in many other cancers, such as colorectal [67,68], prostate [69], oesophageal [70,71], thyroid [72] and gastric cancers [73], as well as multiple myeloma (MM) [74] (Table 1). Over 50% higher MMP-13 expression is seen in bladder and non-small cell lung cancers [75], particularly at the invading front of the tumours [14]. A high level of MMP-13 expression was not only detected in primary breast cancers but also in metastatic lymph nodes that are associated with cancer aggressiveness [13,76]. Moreover, even higher MMP-13 expression is seen in the stromal cells surrounding the tumour [15]. Aggressive cancers have been shown to express higher levels of MMP-13 than less aggressive ones in prostate [77], breast [78] and head and neck cancers (HNSCC) [14,79]. Higher MMP-13 expression is also associated with lymph node metastasis and poor prognosis in bladder and non-small cell lung cancers [75,80] and with poorer patient survival in breast, prostate and head and neck cancers [14,78,79]. Overall, MMP-13 overexpression occurs in various cancers, including many common cancer types, and is often associated with tumour aggressiveness, poorer prognosis and reduced patient survival.
Table 1.
MMP-13 expression in common cancer and its function and clinical significance.
| Cancer Type | Expression Level | Function and Clinical Significance | References |
|---|---|---|---|
| Breast cancer | Increased | Increased tumour growth, invasion and metastasis; potential diagnostic biomarker | [13] |
| Prostate cancer | Increased | Increased tumour differentiation, invasion and metastasis; diagnostic biomarker; poor prognosis | [69,81,82] |
| Bladder cancer | Increased | Increased tumour invasion and metastasis; poor prognosis | [75] |
| Colorectal cancer | Increased | Increased tumour growth, invasion and metastasis; poor prognosis | [47,68] |
| Oesophageal cancer | Increased | Promoted cancer aggressiveness; poor prognosis | [70,71] |
| Head and neck cancer | Increased | Increased tumour invasion and metastasis; poor prognosis | [14] |
| Lung cancer | Increased | Promoted lymph node metastasis; poor survival | [80] |
| Oesophageal cancer | Increased | Promoted cancer aggressiveness; poor prognosis | [70] |
| Gastric cancer | Increased | Increased tumour invasion and metastasis; poor prognosis | [73] |
| Thyroid cancer | Increased | Increased tumour invasion and metastasis; poor prognosis | [72] |
| Multiple Myeloma | Increased | Promoted tumour growth and MM-induced osteolysis; poor prognosis | [74] |
5. MMP-13 in Tumour Growth
The ECM contains multiple complex macromolecule components, such as collagens, proteoglycans and glycoproteins, and provides the scaffold support to tissues and organs [83]. ECM also helps to create an adequate environment for cell adhesion and tissue development. ECM generally consists of an architecture of fibrous polymers (e.g., collagens, elastins and resilins) [84] embedded in an undefined-shaped mixture of nonfibrous components (e.g., proteoglycans) [85]. ECM also includes basement membranes that are comprised of glycoproteins, such as laminin, fibronectin and entactin [86,87]. ECM component proteins are large multifunctional molecules with multiple domains. These domains are responsible for various functions, such as molecular recognition by cell surface receptors, predisposition to oligomerize and recognition by MMPs [88]. ECM serves as a general reservoir for growth factors and normally sequesters them in non-bioavailable forms. It also provides binding sites to cell surface adhesion molecules, such as integrins, for cell attachment and adhesion [89]. ECM degradation by MMPs such as MMP-13 releases sequestered growth factors, such as fibroblast growth factors (FGF) and TGF, which aid tumour cell proliferation [90]. MMP-mediated ECM degradation can also reveal the survival-associated hidden binding sites on ECM to enable ECM interaction with integrins on the tumour cell surface [91]. Although MMP-13 as a protease predominately degrades types I, II and III collagens, it can also cleave a range of other ECM components, such as gelatins [92], large tenascin C, fibronectin [93], aggrecan [94], fibrillin-1 [95], osteonectin [96] and perlecan. Increased expression of MMP-13 in tumour or stromal cells alters the collagen concentration in ECM and creates a more favourable environment for tumour growth [97]. MMP-13 can also deactivate non-matrix proteins, such as MCP-3 and SDF-1, by proteolytic actions [98,99,100] and reduce immune cell infiltration into the tumour and promote tumour growth [101].
Overexpression of MMP-13 mediated by GOLM1, C1r and Leptin has been shown to increase tumour growth in breast cancer [53], cutaneous squamous cell carcinoma [102] and pancreatic cancer [103]. The inhibition of MMP-13 expression by hammerhead ribozyme suppresses squamous cell carcinoma tumour growth and reduces the number of proliferating cells within the tumours [104]. Oral administration of an MMP-13 inhibitor, CMPD-1, twice a day, markedly delayed the growth of breast tumours in syngeneic mice [105]. Suppression of MMP-13 expression by antisense ribozyme reduced squamous cell carcinoma growth and led to the inhibition of cell invasion and induction of cell apoptosis [104]. The inhibition of MMP-13 expression by interferon gamma (IFN-γ) via activation of ERK1,2 and STAT1 in human cutaneous SCC cells (UT-SCC-7) and Ras-transformed human epidermal keratinocytes (A-5 cells) reduced cell proliferation and induced apoptosis [106]. MMP-13 suppression, mediated by p53 in malignantly transformed squamous epithelial cells, displayed an initial anti-invasive effect and was followed by induction of cell death [54]. Together, these studies indicate that overexpression of MMP-13 in cancer, either by tumour cells or stromal cells in the tumour microenvironment, makes important contributions to tumour growth.
6. MMP-13 in Cancer Cell Invasion and Metastasis
Tumour cell infiltration into ECM is a critical early step during cancer invasion and metastasis. Degradation of ECM by proteases such as MMPs creates the pathway for tumour cell infiltration and plays a key role in this process. Each MMP family member has its own substrate specificities towards ECM components. For example, MMP-1 targets primarily collagen III, while MMP-3 and -10 preferentially degrade proteoglycans, fibronectin and laminin [107]. MMP-13 has a relatively broad target specificity and can degrade collagens I, II and III, as well as a range of other ECM components [93,94,95,108]. For example, MMP-13 can cleave ECM component Laminin-5, which is mostly expressed in the basement membrane and is responsible for static adhesion of the epidermis and dermis for hemidesmosome formation [109,110,111]. Laminin-5 cleavage can reveal cryptic sites and increase mobility of epithelial cancer cells in tumour cell invasion and tissue remodelling [112,113,114]. MMP-13 is also involved in the activation of other MMPs, such as MMP-2 and MMP-9, by cleavage of the inactive pro-MMP-2 and pro-MMP-9 forms [19,115]. Proteolytic activation of MMP-9 by MMP-13 occurs in osteoarthritic chondrocytes [116] and chronic periodontitis [117]. It is possible that such an MMP-13/MMP-9 activation cascade may also exist in cancer.
MMP-13 also contributes to Epithelial-to-mesenchymal transition (EMT) in the tumorigenesis of epithelial cancer [118]. EMT confers epithelial cells with the metastatic properties of increased mobility and invasion, as well as an ability to escape apoptosis [119]. A primary EMT inducer is TGF-β [120]. The release of active TGF-β is normally carried out through proteolytical cleavage of the TGF-β-complex by MMP-28 [121]. Similar TGF-β activation has been reported by MMP-13 with chondrocytes in matrix vesicles, where secreted MMP-13 activated latent TGF-β in the progress of mineralization of growth plate cartilage [122]. The inhibition of MMP-13 expression in breast cancer cells at the tumour–bone interface significantly reduced TGF-β signalling, leading to a decrease in tumour-induced osteolysis [123].
MMP-13 also participates in the process of tumour cell infiltration into the blood or lymphatic vessels during metastasis. The blood capillaries are composed of an endoluminal side formed by endothelial cells and an abluminal side containing a basement membrane and vascular smooth muscle cells [124]. MMPs assist tumour cell penetration into blood capillaries by degrading the vascular basement membrane. MMP-9 is the primary contributor to vascular basement membrane degradation [125]. As MMP-13 is capable of activating MMP-9 by cleavage of the inactive pro-MMP-9 form [115,126,127], its action on MMP-9 activation can therefore promote tumour cell infiltration into the blood/lymphatic vessels at primary tumour sites, as well as the extravasation of invaded tumour cells from blood/lymphatic vessels at remote organs. The discovery that the inhibition of MMP-13 expression in MC38 colon cancer cells decreased the number of tumour cells extravasated from the hepatic vasculature in an experimental metastasis model is in line with this possibility [128].
Bone is one of the preferential metastasis sites of cancers such as breast cancer [129]. The bone ECM is rich with type I collagens [130]. MMP-13 is believed to be the primary protease to degrade type I collagen and aids breast cancer bone metastasis [131,132]. A higher MMP-13 level occurs at the tumour–bone interface of breast cancer [133]. Soluble factors, such as IL-6, produced by breast cancer cells induce MMP-13 expression in osteoblasts [134]. Tumour cells also produce parathyroid hormone-related protein (PTHrP) to induce MMP-13 expression through the activation of protein kinase C (PKC)-ERK1/2 signalling [135]. Inflammatory cells or osteoblasts could also produce PTHrP to stimulate MMP-13 secretion to promote breast cancer bone metastasis [136]. MMP-13 is also detected at the MM and bone marrow interface, and its presence is shown to promote MM cell bone marrow infiltration [137] and induce osteoclast [138]. Injection of MMP-13-selective inhibitor Zn2+-chelating compound, which targets the catalytic domain of MMP-13 [139], significantly reduced the level of bone destruction and delayed MM growth in an immunocompetent syngeneic mouse model with multiple myeloma [74].
The arrival of tumour cells at distant organs, which is an alien microenvironment from the primary tumour sites, is often unfavourable for tumour cells to survive and grow. This can lead the tumour cell to die from apoptosis or enter a ‘silent’ mode without proliferation or death. The successful establishment of a metastasis at the distant organs/sites requires the build-up of a permissive environment, known as a pre-metastatic niche. Pre-metastatic niche formation is driven by many factors, including primary tumour-derived factors, tumour-mobilized bone marrow-derived cells (BMDC), hypoxia, ECM remodelling and exosomes [140]. The formation of a pre-metastatic niche is triggered by the release from the primary tumour of factors such as growth factors (e.g., TGF-α and -β) or cytokines (e.g., TNF-α). These, in turn, induce the expression and secretion of chemoattractants (e.g., S100 proteins) by the endothelium [141] and the production of fibronectin by fibroblasts at the niche site [142]. The expression of S100 can lead to the activation of NF-κB signalling [143] and the subsequent production of MMPs, including MMP-13, by stromal cells [144]. The structure of the new site’s intrinsic ECM is often less ideal for attachment, metabolism and migration of the recruited BMDC and immune cells. The production and action of the new MMPs, including MMP-13, cause ECM remodelling and aid the formation of the pre-metastatic niche. BMDCs and several immune cells are also recruited to the site to assist in the establishment of the pre-metastatic niche. These immune cells secrete inflammatory cytokines, growth factors and proangiogenic molecules to create a favourable local microenvironment for the extravasated tumour cells [145]. MMP-9 is responsible for the recruitment of BMDCs to the niche site via releasing soluble factors, such as Kit-ligand from BM stromal cells [146], and can be activated by MMP-13 through proteolytic cleavage of pro-MMP-9.
Lysl Oxidase (LOX) is another key regulator involved in the recruitment of BMDCs and is often released by primary tumours during hypoxia [147]. LOX cross-links collagen IV in the basement membrane and allows CD11b+ myeloid cells to adhere and release MMPs at the niche site [147]. The release of these MMPs further degrades collagen fibres and releases collagen IV peptides, which act as chemoattractants to aid recruitment of BMDCs to the niche site. As the inhibition of LOX by licofelone can reduce MMP-13 expression in human osteoarthritic chondrocytes [148], LOX-mediated MMP-13 expression can therefore contribute to pre-metastatic niche formation by aiding BMDC recruitment to niche sites.
Exosomes are small vesicles that contain proteins, mRNAs, microRNAs, small RNAs and DNA fragments [149]. Tumour-associated exosomes can assist pre-metastatic niche formation and aid tumour cell communication by transportation of regulatory molecules [150,151]. MMP-13 occurs in primary tumour cell-derived exosomes under hypoxic conditions [152]. It is later released into the circulation to modulate ECM components and helps the establishment of pre-metastasis sites [152]. Overall, MMP-13 makes important contributions to tumour cell invasion at primary tumour sites, as well as the establishment of tumour cells at remote organs in metastasis through the degradation and remodelling of ECM and the activation of other MMPs.
7. MMP-13 in Angiogenesis
Angiogenesis is an important process during cancer pathogenesis. It provides essential nutrients and a blood supply for sustained tumour growth and development [153]. The multiple-stepped process of angiogenesis consists of: (1) degradation of basement membrane and ECM around the blood vessels; (2) activation of endothelial cells for migration and proliferation; (3) transformation of endothelial cells into capillary tubes [154]. Angiogenesis is tightly regulated in normal tissues, but this tight control is disrupted in cancer [155] by a group of angiogenic factors released by endothelial cells, tumour cells, stromal cells and ECM [156,157,158,159,160]. These angiogenic factors can be either pro- or anti-angiogenic [161]. Pro-angiogenetic regulators include vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), TGF-α and -β, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), placental-derived growth factor and angiopoietin -1 and -2 [162], while anti-angiogenic regulators include angiostatin, endostatin, tumstatin, platelet factor-4, interleukin-12, thrombospondin-1, TIMPs and interferon-α, -β and -γ [154].
Many MMP family members are known to take part in the process of angiogenesis. Their primary action is to degrade ECM components. This leads to the release of ECM-bound angiogenic factors that enable endothelial cells to invade the tumour stroma leading to new blood vessel formation [163]. Interstitial collagens are the major proteins in the vascular tissue milieu [164]. The structural triple-helical, fibrillar collagen in vascular tissue is highly resistant to many proteolytic enzymes, such as trypsin, plasmin, extracellular serine proteases and many MMP members [165]. Only a limited number of MMPs can cleave the highly structured fibrillar collagens. MMP-2 and -9 can cleave fibrillar type I collagen and release ECM-bound angiogenic growth factors [88] to aid blood vessel formation and endothelial cell invasion [166,167]. MMP-13 is one of the proteases that can digest well-structured fibrillar collagens in ECM around the blood vessel and contribute to the release of ECM-bound angiogenic regulators [168]. MMP-13 has been shown to efficiently and specifically cleave interstitial collagens to initiate ECM remodelling and promote new blood vessel formation in the chorioallantoic membrane in a chicken embryo model [164]. MMP-13 expression in stromal fibroblasts was shown to enhance VEGF and VEGFR-2 concentrations in the tumour cell invasive areas around blood vessels and promote angiogenesis in skin carcinoma [169]. MMP-13-mediated release of VEGF-C increased cancer cell spreading through lymphatic vascular systems in paediatric multiple myeloma [170]. Higher MMP-13 expression correlates closely with a higher number of blood vessels in human head and neck cancer [168]. Overall, the expression and presence of MMP-13 in cancer and stromal cells is actively involved in promoting angiogenesis in tumour cell metastatic spreading.
8. Concluding Remarks
As an interstitial collagenase that can cleave not only collagens but also a range of other ECM components, MMP-13 is overexpressed in various cancers and plays multiple roles in cancer development, progression and metastasis. Its proteolytic action leads to ECM remodelling and release of ECM-sequestered growth factors, cytokines and angiogenic factors that promote tumour cell proliferation, EMT, invasion and angiogenesis. MMP-13-mediated ECM remodelling and release of growth factors also aids the recruitment of immune cells to the pre-metastatic niche and helps the establishment of secondary metastasis sites in distant organs (Figure 3). Despite the critical involvement of MMP-13 in cancer progression and metastasis, the precise mechanisms of its regulation and actions are still not fully understood. Much also remains unknown about the possible coordination of MMP-13 action with other MMP family members during cancer pathogenesis. Little is known about whether MMP-13 appearance/overexpression in cancer and stromal cells influences the activity or function of cell surface molecules, such as cell adhesion and signalling proteins. Future research will help to gain further insights into the role and actions of MMP-13 in cancer and determine whether MMP-13 represents an effective therapeutic target for this disease.
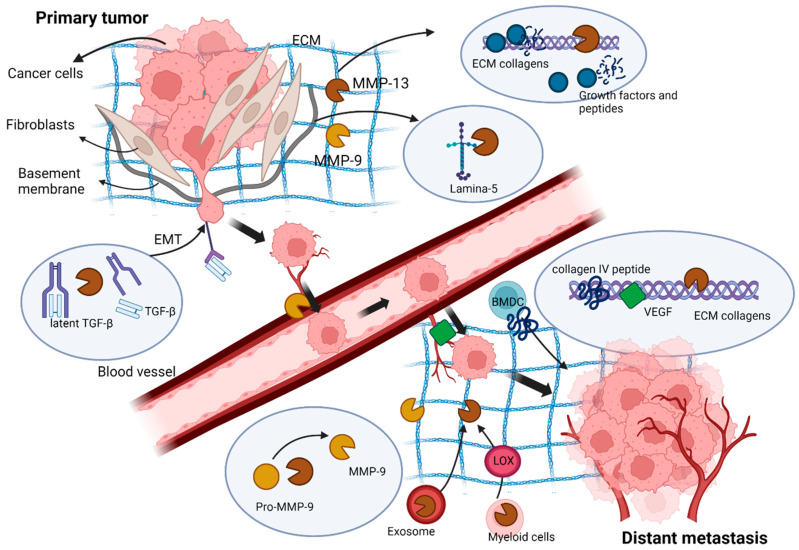
Figure 3.
The action of MMP-13 in cancer progression and metastasis: MMP-13, produced by cancer cells and stromal fibroblasts, cleaves ECM and leads to ECM remodelling and liberation of ECM-bound growth factors and angiogenic factors that promote tumour cell proliferation, invasion, EMT and angiogenesis at primary tumour sites. At distant metastasis sites, MMP-13-mediated ECM remodelling and release of growth factors and collagen IV peptides help the recruitment of immune cells to the pre-metastatic niche and the establishment of a favourable metastasis environment.
Abbreviations
| bFGF | Basic fibroblast growth factor |
| BMDC | Bone marrow-derived cells |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EMT | Epithelial-to-mesenchymal transition |
| ER | Extracellular signal-regulated kinase |
| ETV | ETS variant transcription factor 4 |
| FAK | Focal adhesion kinase |
| FHRE | Forkhead response element |
| FGF | Fibroblast growth factors |
| GOLM1 | Golgi membrane protein 1 |
| HIF | Hypoxia-inducible transcription factor |
| HNSCC | Squamous cell carcinoma of the head and neck |
| IL | Interleukin |
| IFN-γ | Interferon gamma |
| ING2 | Inhibitor of growth 2 |
| IGF | Insulin-like growth factor |
| IFN-β | Interferon β |
| LOX | Lysl Oxidase |
| MMP | Matrix metalloproteinase |
| NF-κB | Nuclear factor kappa B |
| MM | Multiple Myeloma |
| OSE-2 | Osteoblast-specific element |
| PDGF | Platelet-derived growth factor |
| PTHrP | parathyroid hormone-related protein |
| PKC | Protein kinase C |
| SH | Zinc-interacting thiol |
| SUMO | Small ubiquitin related modifier |
| SENP2 | SUMO-specific protease 2 |
| TGF-β | Transforming growth factor-β1 |
| TIMPs | Tissue inhibitors of MMPs |
| TIE | Transforming growth factor-beta inhibitory element |
| TNF-α | Tumour necrosis factor alpha |
| VEGF | Vascular endothelial growth factor |
Author Contributions
Conceptualization, S.L. and L.-G.Y.; writing—original draft preparation, S.L.; writing—review and editing, S.L., D.M.P. and L.-G.Y. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coussens L.M., Fingleton B., Matrisian L.M. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science. 2002;295:2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 2.Puente X.S., Sánchez L.M., Overall C.M., López-Otín C. Human and mouse proteases: A comparative genomic approach. Nat. Rev. Genet. 2003;4:544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- 3.Javaid M.A., Abdallah M.N., Ahmed A.S., Sheikh Z. Matrix metalloproteinases and their pathological upregulation in multiple sclerosis: An overview. Acta Neurol. Belg. 2013;113:381–390. doi: 10.1007/s13760-013-0239-x. [DOI] [PubMed] [Google Scholar]
- 4.Zhong Y., Lu Y.T., Sun Y., Shi Z.H., Li N.G., Tang Y.P., Duan J.A. Recent opportunities in matrix metalloproteinase inhibitor drug design for cancer. Expert Opin. Drug Discov. 2018;13:75–87. doi: 10.1080/17460441.2018.1398732. [DOI] [PubMed] [Google Scholar]
- 5.Dufour A., Sampson N.S., Zucker S., Cao J. Role of the hemopexin domain of matrix metalloproteinases in cell migration. J. Cell. Physiol. 2008;217:643–651. doi: 10.1002/jcp.21535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egeblad M., Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 7.Kapoor C., Vaidya S., Wadhwan V., Kaur G., Pathak A. Seesaw of matrix metalloproteinases (MMPs) J. Cancer Res. Ther. 2016;12:28–35. doi: 10.4103/0973-1482.157337. [DOI] [PubMed] [Google Scholar]
- 8.Sorsa T., Tervahartiala T., Leppilahti J., Hernandez M., Gamonal J., Tuomainen A.M., Lauhio A., Pussinen P.J., Mäntylä P. Collagenase-2 (MMP-8) as a point-of-care biomarker in periodontitis and cardiovascular diseases. Therapeutic response to non-antimicrobial properties of tetracyclines. Pharmacol. Res. 2011;63:108–113. doi: 10.1016/j.phrs.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Burrage P.S., Mix K.S., Brinckerhoff C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 10.Huang H. Matrix Metalloproteinase-9 (MMP-9) as a Cancer Biomarker and MMP-9 Biosensors: Recent Advances. Sensors. 2018;18:3249. doi: 10.3390/s18103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCawley L.J., Matrisian L.M. Matrix metalloproteinases: They’re not just for matrix anymore! Curr. Opin. Cell Biol. 2001;13:534–540. doi: 10.1016/S0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 12.Freije J.M., Díez-Itza I., Balbín M., Sánchez L.M., Blasco R., Tolivia J., López-Otín C. Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J. Biol. Chem. 1994;269:16766–16773. doi: 10.1016/S0021-9258(19)89457-7. [DOI] [PubMed] [Google Scholar]
- 13.Kotepui M., Punsawad C., Chupeerach C., Songsri A., Charoenkijkajorn L., Petmitr S. Differential expression of matrix metalloproteinase-13 in association with invasion of breast cancer. Contemp. Oncol. 2016;20:225–228. doi: 10.5114/wo.2016.61565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansson N., Airola K., Grénman R., Kariniemi A.L., Saarialho-Kere U., Kähäri V.M. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am. J. Pathol. 1997;151:499–508. [PMC free article] [PubMed] [Google Scholar]
- 15.Uría J.A., Ståhle-Bäckdahl M., Seiki M., Fueyo A., López-Otín C. Regulation of collagenase-3 expression in human breast carcinomas is mediated by stromal-epithelial cell interactions. Cancer Res. 1997;57:4882–4888. [PubMed] [Google Scholar]
- 16.Sun Y., Cheung J.M., Martel-Pelletier J., Pelletier J.P., Wenger L., Altman R.D., Howell D.S., Cheung H.S. Wild type and mutant p53 differentially regulate the gene expression of human collagenase-3 (hMMP-13) J. Biol. Chem. 2000;275:11327–11332. doi: 10.1074/jbc.275.15.11327. [DOI] [PubMed] [Google Scholar]
- 17.Pei Y., Harvey A., Yu X.P., Chandrasekhar S., Thirunavukkarasu K. Differential regulation of cytokine-induced MMP-1 and MMP-13 expression by p38 kinase inhibitors in human chondrosarcoma cells: Potential role of Runx2 in mediating p38 effects. Osteoarthr. Cartil. 2006;14:749–758. doi: 10.1016/j.joca.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 18.Yoon S.W., Chun J.S., Sung M.H., Kim J.Y., Poo H. alpha-MSH inhibits TNF-alpha-induced matrix metalloproteinase-13 expression by modulating p38 kinase and nuclear factor kappaB signaling in human chondrosarcoma HTB-94 cells. Osteoarthr. Cartil. 2008;16:115–124. doi: 10.1016/j.joca.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Cowell S., Knäuper V., Stewart M.L., D’Ortho M.P., Stanton H., Hembry R.M., López-Otín C., Reynolds J.J., Murphy G. Induction of matrix metalloproteinase activation cascades based on membrane-type 1 matrix metalloproteinase: Associated activation of gelatinase A, gelatinase B and collagenase 3. Pt 2Biochem. J. 1998;331:453–458. doi: 10.1042/bj3310453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leeman M.F., Curran S., Murray G.I. The structure, regulation, and function of human matrix metalloproteinase-13. Crit. Rev. Biochem. Mol. Biol. 2002;37:149–166. doi: 10.1080/10409230290771483. [DOI] [PubMed] [Google Scholar]
- 21.Van Wart H.E., Birkedal-Hansen H. The cysteine switch: A principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA. 1990;87:5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rangasamy L., Geronimo B.D., Ortín I., Coderch C., Zapico J.M., Ramos A., de Pascual-Teresa B. Molecular Imaging Probes Based on Matrix Metalloproteinase Inhibitors (MMPIs) Molecules. 2019;24:2982. doi: 10.3390/molecules24162982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whittaker M., Floyd C.D., Brown P., Gearing A.J. Design and therapeutic application of matrix metalloproteinase inhibitors. Chem. Rev. 1999;99:2735–2776. doi: 10.1021/cr9804543. [DOI] [PubMed] [Google Scholar]
- 24.Murphy G., Nagase H. Progress in matrix metalloproteinase research. Mol. Asp. Med. 2008;29:290–308. doi: 10.1016/j.mam.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy G., Knäuper V. Relating matrix metalloproteinase structure to function: Why the “hemopexin” domain? Matrix Biol. 1997;15:511–518. doi: 10.1016/S0945-053X(97)90025-1. [DOI] [PubMed] [Google Scholar]
- 26.Bode W. A helping hand for collagenases: The haemopexin-like domain. Structure. 1995;3:527–530. doi: 10.1016/S0969-2126(01)00185-X. [DOI] [PubMed] [Google Scholar]
- 27.Knäuper V., Will H., López-Otin C., Smith B., Atkinson S.J., Stanton H., Hembry R.M., Murphy G. Cellular Mechanisms for Human Procollagenase-3 (MMP-13) Activation: Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J. Biol. Chem. 1996;271:17124–17131. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- 28.Grams F., Huber R., Kress L.F., Moroder L., Bode W. Activation of snake venom metalloproteinases by a cysteine switch-like mechanism. FEBS Lett. 1993;335:76–80. doi: 10.1016/0014-5793(93)80443-X. [DOI] [PubMed] [Google Scholar]
- 29.Gupta S.P., Patil V.M. Specificity of binding with matrix metalloproteinases. Exp. Suppl. 2012;103:35–56. doi: 10.1007/978-3-0348-0364-9_2. [DOI] [PubMed] [Google Scholar]
- 30.Liu J., Khalil R.A. Matrix Metalloproteinase Inhibitors as Investigational and Therapeutic Tools in Unrestrained Tissue Remodeling and Pathological Disorders. Prog. Mol. Biol. Transl. Sci. 2017;148:355–420. doi: 10.1016/bs.pmbts.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laronha H., Caldeira J. Structure and Function of Human Matrix Metalloproteinases. Cells. 2020;9:1076. doi: 10.3390/cells9051076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vincenti M.P., Brinckerhoff C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. Ther. 2002;4:157. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M., Sampson E.R., Jin H., Li J., Ke Q.H., Im H.-J., Chen D. MMP13 is a critical target gene during the progression of osteoarthritis. Arthritis Res. Ther. 2013;15:R5. doi: 10.1186/ar4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto K., Okano H., Miyagawa W., Visse R., Shitomi Y., Santamaria S., Dudhia J., Troeberg L., Strickland D.K., Hirohata S., et al. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016;56:57–73. doi: 10.1016/j.matbio.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui N., Hu M., Khalil R.A. Chapter One—Biochemical and Biological Attributes of Matrix Metalloproteinases. In: Khalil R.A., editor. Progress in Molecular Biology and Translational Science. Volume 147. Academic Press; Cambridge, MA, USA: 2017. pp. 1–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutman A., Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. Embo J. 1990;9:2241–2246. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiménez M.J., Balbín M., López J.M., Alvarez J., Komori T., López-Otín C. Collagenase 3 is a target of Cbfa1, a transcription factor of the runt gene family involved in bone formation. Mol. Cell. Biol. 1999;19:4431–4442. doi: 10.1128/MCB.19.6.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tardif G., Reboul P., Dupuis M., Geng C., Duval N., Pelletier J.P., Martel-Pelletier J. Transforming growth factor-beta induced collagenase-3 production in human osteoarthritic chondrocytes is triggered by Smad proteins: Cooperation between activator protein-1 and PEA-3 binding sites. J. Rheumatol. 2001;28:1631–1639. [PubMed] [Google Scholar]
- 39.Yu H., Fellows A., Foote K., Yang Z., Figg N., Littlewood T., Bennett M. FOXO3a (Forkhead Transcription Factor O Subfamily Member 3a) Links Vascular Smooth Muscle Cell Apoptosis, Matrix Breakdown, Atherosclerosis, and Vascular Remodeling Through a Novel Pathway Involving MMP13 (Matrix Metalloproteinase 13) Arter. Thromb. Vasc. Biol. 2018;38:555–565. doi: 10.1161/ATVBAHA.117.310502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumortier M., Ladam F., Damour I., Vacher S., Bièche I., Marchand N., de Launoit Y., Tulasne D., Chotteau-Lelièvre A. ETV4 transcription factor and MMP13 metalloprotease are interplaying actors of breast tumorigenesis. Breast Cancer Res. 2018;20:73. doi: 10.1186/s13058-018-0992-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S., Kang M., Ko J. Small leucine zipper protein promotes the metastasis of castration-resistant prostate cancer through transcriptional regulation of matrix metalloproteinase-13. Carcinogenesis. 2021;42:1089–1099. doi: 10.1093/carcin/bgab045. [DOI] [PubMed] [Google Scholar]
- 42.Borden P., Solymar D., Sucharczuk A., Lindman B., Cannon P., Heller R.A. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J. Biol. Chem. 1996;271:23577–23581. doi: 10.1074/jbc.271.38.23577. [DOI] [PubMed] [Google Scholar]
- 43.Jiang C., Xu M., Kuang X., Xiao J., Tan M., Xie Y., Xiao Y., Zhao F., Wu Y. Treponema pallidum flagellins stimulate MMP-9 and MMP-13 expression via TLR5 and MAPK/NF-κB signaling pathways in human epidermal keratinocytes. Exp. Cell. Res. 2017;361:46–55. doi: 10.1016/j.yexcr.2017.09.040. [DOI] [PubMed] [Google Scholar]
- 44.Delany A.M., Rydziel S., Canalis E. Autocrine down-regulation of collagenase-3 in rat bone cell cultures by insulin-like growth factors. Endocrinology. 1996;137:4665–4670. doi: 10.1210/endo.137.11.8895331. [DOI] [PubMed] [Google Scholar]
- 45.Uría J.A., Balbín M., López J.M., Alvarez J., Vizoso F., Takigawa M., López-Otín C. Collagenase-3 (MMP-13) expression in chondrosarcoma cells and its regulation by basic fibroblast growth factor. Am. J. Pathol. 1998;153:91–101. doi: 10.1016/S0002-9440(10)65549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Knäuper V., Bailey L., Worley J.R., Soloway P., Patterson M.L., Murphy G. Cellular activation of proMMP-13 by MT1-MMP depends on the C-terminal domain of MMP-13. FEBS Lett. 2002;532:127–130. doi: 10.1016/S0014-5793(02)03654-2. [DOI] [PubMed] [Google Scholar]
- 47.Leeman M.F., McKay J.A., Murray G.I. Matrix metalloproteinase 13 activity is associated with poor prognosis in colorectal cancer. J. Clin. Pathol. 2002;55:758–762. doi: 10.1136/jcp.55.10.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moilanen M., Sorsa T., Stenman M., Nyberg P., Lindy O., Vesterinen J., Paju A., Konttinen Y.T., Stenman U.H., Salo T. Tumor-associated trypsinogen-2 (trypsinogen-2) activates procollagenases (MMP-1, -8, -13) and stromelysin-1 (MMP-3) and degrades type I collagen. Biochemistry. 2003;42:5414–5420. doi: 10.1021/bi020582s. [DOI] [PubMed] [Google Scholar]
- 49.Gomez D.E., Alonso D.F., Yoshiji H., Thorgeirsson U.P. Tissue inhibitors of metalloproteinases: Structure, regulation and biological functions. Eur. J. Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- 50.Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011;12:233. doi: 10.1186/gb-2011-12-11-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gokulnath M., Swetha R., Thejaswini G., Shilpa P., Selvamurugan N. Transforming growth factor-β1 regulation of ATF-3, c-Jun and JunB proteins for activation of matrix metalloproteinase-13 gene in human breast cancer cells. Int. J. Biol. Macromol. 2017;94 Pt A:370–377. doi: 10.1016/j.ijbiomac.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 52.Enomoto M., Hayakawa S., Itsukushima S., Ren D.Y., Matsuo M., Tamada K., Oneyama C., Okada M., Takumi T., Nishita M., et al. Autonomous regulation of osteosarcoma cell invasiveness by Wnt5a/Ror2 signaling. Oncogene. 2009;28:3197–3208. doi: 10.1038/onc.2009.175. [DOI] [PubMed] [Google Scholar]
- 53.Zhang R., Zhu Z., Shen W., Li X., Dhoomun D.K., Tian Y. Golgi Membrane Protein 1 (GOLM1) Promotes Growth and Metastasis of Breast Cancer Cells via Regulating Matrix Metalloproteinase-13 (MMP13) Med. Sci. Monit. 2019;25:847–855. doi: 10.12659/MSM.911667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ala-aho R., Grenman R., Seth P., Kähäri V.-M. Adenoviral delivery of p53 gene suppresses expression of collagenase-3 (MMP-13) in squamous carcinoma cells. Oncogene. 2002;21:1187–1195. doi: 10.1038/sj.onc.1205198. [DOI] [PubMed] [Google Scholar]
- 55.Vaupel P., Multhoff G. Fatal Alliance of Hypoxia-/HIF-1α-Driven Microenvironmental Traits Promoting Cancer Progression. Adv. Exp. Med. Biol. 2020;1232:169–176. doi: 10.1007/978-3-030-34461-0_21. [DOI] [PubMed] [Google Scholar]
- 56.Furman D., Campisi J., Verdin E., Carrera-Bastos P., Targ S., Franceschi C., Ferrucci L., Gilroy D.W., Fasano A., Miller G.W., et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019;25:1822–1832. doi: 10.1038/s41591-019-0675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kusano K., Miyaura C., Inada M., Tamura T., Ito A., Nagase H., Kamoi K., Suda T. Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: Association of MMP induction with bone resorption. Endocrinology. 1998;139:1338–1345. doi: 10.1210/endo.139.3.5818. [DOI] [PubMed] [Google Scholar]
- 58.Mengshol J.A., Vincenti M.P., Coon C.I., Barchowsky A., Brinckerhoff C.E. Interleukin-1 induction of collagenase 3 (matrix metalloproteinase 13) gene expression in chondrocytes requires p38, c-Jun N-terminal kinase, and nuclear factor kappaB: Differential regulation of collagenase 1 and collagenase 3. Arthritis Rheum. 2000;43:801–811. doi: 10.1002/1529-0131(200004)43:4<801::AID-ANR10>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 59.Ibaragi S., Shimo T., Hassan N.M., Isowa S., Kurio N., Mandai H., Kodama S., Sasaki A. Induction of MMP-13 expression in bone-metastasizing cancer cells by type I collagen through integrin α1β1 and α2β1-p38 MAPK signaling. Anticancer Res. 2011;31:1307–1313. [PubMed] [Google Scholar]
- 60.Zeng L., Rong X.F., Li R.H., Wu X.Y. Icariin inhibits MMP-1, MMP-3 and MMP-13 expression through MAPK pathways in IL-1β-stimulated SW1353 chondrosarcoma cells. Mol. Med. Rep. 2017;15:2853–2858. doi: 10.3892/mmr.2017.6312. [DOI] [PubMed] [Google Scholar]
- 61.Zhang H., Yang Q., Lian X., Jiang P., Cui J. Hypoxia-Inducible Factor-1α (HIF-1α) Promotes Hypoxia-Induced Invasion and Metastasis in Ovarian Cancer by Targeting Matrix Metallopeptidase 13 (MMP13) Med. Sci. Monit. 2019;25:7202–7208. doi: 10.12659/MSM.916886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao H., Bo Q., Wang W., Wang R., Li Y., Chen S., Xia Y., Wang W., Wang Y., Zhu K., et al. CCL17-CCR4 axis promotes metastasis via ERK/MMP13 pathway in bladder cancer. J. Cell. Biochem. 2018;120:1979–1989. doi: 10.1002/jcb.27494. [DOI] [PubMed] [Google Scholar]
- 63.Ou B., Zhao J., Guan S., Feng H., Wangpu X., Zhu C., Zong Y., Ma J., Sun J., Shen X., et al. CCR4 promotes metastasis via ERK/NF-κB/MMP13 pathway and acts downstream of TNF-α in colorectal cancer. Oncotarget. 2016;7:47637–47649. doi: 10.18632/oncotarget.10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumamoto K., Fujita K., Kurotani R., Saito M., Unoki M., Hagiwara N., Shiga H., Bowman E.D., Yanaihara N., Okamura S., et al. ING2 is upregulated in colon cancer and increases invasion by enhanced MMP13 expression. Int. J. Cancer. 2009;125:1306–1315. doi: 10.1002/ijc.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan M., Gong H., Wang J., Tao L., Xu D., Bao E., Liu Z., Qiu J. SENP2 regulates MMP13 expression in a bladder cancer cell line through SUMOylation of TBL1/TBLR1. Sci. Rep. 2015;5:13996. doi: 10.1038/srep13996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu S., Huang M., Chen Z., Chen J., Chao Q., Yin X., Quan M. FTO promotes cell proliferation and migration in esophageal squamous cell carcinoma through up-regulation of MMP13. Exp. Cell Res. 2020;389:111894. doi: 10.1016/j.yexcr.2020.111894. [DOI] [PubMed] [Google Scholar]
- 67.Yamada T., Oshima T., Yoshihara K., Tamura S., Kanazawa A., Inagaki D., Yamamoto N., Sato T., Fujii S., Numata K., et al. Overexpression of MMP-13 Gene in Colorectal Cancer with Liver Metastasis. Anticancer Res. 2010;30:2693–2699. [PubMed] [Google Scholar]
- 68.Yang B., Gao J., Rao Z., Shen Q. Clinicopathological significance and prognostic value of MMP-13 expression in colorectal cancer. Scand. J. Clin. Lab. Investig. 2012;72:501–505. doi: 10.3109/00365513.2012.699638. [DOI] [PubMed] [Google Scholar]
- 69.Wang S.W., Tai H.C., Tang C.H., Lin L.W., Lin T.H., Chang A.C., Chen P.C., Chen Y.H., Wang P.C., Lai Y.W., et al. Melatonin impedes prostate cancer metastasis by suppressing MMP-13 expression. J. Cell. Physiol. 2021;236:3979–3990. doi: 10.1002/jcp.30150. [DOI] [PubMed] [Google Scholar]
- 70.Etoh T., Inoue H., Yoshikawa Y., Barnard G.F., Kitano S., Mori M. Increased expression of collagenase-3 (MMP-13) and MT1-MMP in oesophageal cancer is related to cancer aggressiveness. Gut. 2000;47:50–56. doi: 10.1136/gut.47.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiao X.L., Chen D., Wang J., Zhang K. Clinical significance of serum matrix metalloproteinase-13 levels in patients with esophageal squamous cell carcinoma (ESCC) Eur. Rev. Med. Pharmacol. Sci. 2014;18:509–515. [PubMed] [Google Scholar]
- 72.Wang J., Li X., Gao X., An S., Liu H., Liang J., Zhang K., Liu Z., Wang J., Chen Z. Expression of MMP-13 is associated with invasion and metastasis of papillary thyroid carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2013;17:427–435. [PubMed] [Google Scholar]
- 73.del Casar Lizcano J., LO G.S., Gava R., Santisteban D. Expression and clinical significance of collagenase-3 (MMP-13) in gastric cancer. Gastroenterol. Hepatol. 2003;26:1–7. doi: 10.1016/S0210-5705(03)70332-X. [DOI] [PubMed] [Google Scholar]
- 74.Lo C.H., Shay G., McGuire J.J., Li T., Shain K.H., Choi J.Y., Fuerst R., Roush W.R., Knapinska A.M., Fields G.B., et al. Host-Derived Matrix Metalloproteinase-13 Activity Promotes Multiple Myeloma-Induced Osteolysis and Reduces Overall Survival. Cancer Res. 2021;81:2415–2428. doi: 10.1158/0008-5472.CAN-20-2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boström P.J., Ravanti L., Reunanen N., Aaltonen V., Söderström K.O., Kähäri V.M., Laato M. Expression of collagenase-3 (matrix metalloproteinase-13) in transitional-cell carcinoma of the urinary bladder. Int. J. Cancer. 2000;88:417–423. doi: 10.1002/1097-0215(20001101)88:3<417::AID-IJC14>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 76.Kotepui M., Thawornkuno C., Chavalitshewinkoon-Petmitr P., Punyarit P., Petmitr S. Quantitative real-time RT-PCR of ITGA7, SVEP1, TNS1, LPHN3, SEMA3G, KLB and MMP13 mRNA expression in breast cancer. Asian Pac. J. Cancer Prev. 2012;13:5879–5882. doi: 10.7314/APJCP.2012.13.11.5879. [DOI] [PubMed] [Google Scholar]
- 77.Daja M.M., Niu X., Zhao Z., Brown J.M., Russell P.J. Characterization of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in prostate cancer cell lines. Prostate Cancer Prostatic Dis. 2003;6:15–26. doi: 10.1038/sj.pcan.4500609. [DOI] [PubMed] [Google Scholar]
- 78.Zhang B., Cao X., Liu Y., Cao W., Zhang F., Zhang S., Li H., Ning L., Fu L., Niu Y., et al. Tumor-derived matrix metalloproteinase-13 (MMP-13) correlates with poor prognoses of invasive breast cancer. BMC Cancer. 2008;8:83. doi: 10.1186/1471-2407-8-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luukkaa M., Vihinen P., Kronqvist P., Vahlberg T., Pyrhönen S., Kähäri V.M., Grénman R. Association between high collagenase-3 expression levels and poor prognosis in patients with head and neck cancer. Head Neck. 2006;28:225–234. doi: 10.1002/hed.20322. [DOI] [PubMed] [Google Scholar]
- 80.Jiang R., Yu S. Expression of MMP-13 and its correlation with prognosis in non-small cell lung cancer. Chin.-Ger. J. Clin. Oncol. 2008;7:142–144. doi: 10.1007/s10330-007-0176-3. [DOI] [Google Scholar]
- 81.Morgia G., Falsaperla M., Malaponte G., Madonia M., Indelicato M., Travali S., Mazzarino M.C. Matrix metalloproteinases as diagnostic (MMP-13) and prognostic (MMP-2, MMP-9) markers of prostate cancer. Urol. Res. 2005;33:44–50. doi: 10.1007/s00240-004-0440-8. [DOI] [PubMed] [Google Scholar]
- 82.Kalantari E., Abolhasani M., Roudi R., Farajollahi M.M., Farhad S., Madjd Z., Askarian-Amiri S., Mohsenzadegan M. Co-expression of TLR-9 and MMP-13 is associated with the degree of tumour differentiation in prostate cancer. Int. J. Exp. Pathol. 2019;100:123–132. doi: 10.1111/iep.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tanzer M.L. Current concepts of extracellular matrix. J. Orthop. Sci. 2006;11:326–331. doi: 10.1007/s00776-006-1012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tanzer M.L. Collagens and elastin: Structure and interactions. Curr. Opin. Cell Biol. 1989;1:968–973. doi: 10.1016/0955-0674(89)90067-7. [DOI] [PubMed] [Google Scholar]
- 85.Iozzo R.V. Matrix proteoglycans: From molecular design to cellular function. Annu. Rev. Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 86.Aumailley M., Smyth N. The role of laminins in basement membrane function. Pt 1J. Anat. 1998;193:1–21. doi: 10.1046/j.1469-7580.1998.19310001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wierzbicka-Patynowski I., Schwarzbauer J.E. The ins and outs of fibronectin matrix assembly. Pt 16J. Cell Sci. 2003;116:3269–3276. doi: 10.1242/jcs.00670. [DOI] [PubMed] [Google Scholar]
- 88.Mott J.D., Werb Z. Regulation of matrix biology by matrix metalloproteinases. Curr. Opin. Cell Biol. 2004;16:558–564. doi: 10.1016/j.ceb.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown N.H. Extracellular matrix in development: Insights from mechanisms conserved between invertebrates and vertebrates. Cold Spring Harb. Perspect. Biol. 2011;3:a005082. doi: 10.1101/cshperspect.a005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Agrez M., Chen A., Cone R.I., Pytela R., Sheppard D. The alpha v beta 6 integrin promotes proliferation of colon carcinoma cells through a unique region of the beta 6 cytoplasmic domain. J. Cell Biol. 1994;127:547–556. doi: 10.1083/jcb.127.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deryugina E.I., Quigley J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 92.Knäuper V., López-Otin C., Smith B., Knight G., Murphy G. Biochemical characterization of human collagenase-3. J. Biol. Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 93.Knäuper V., Cowell S., Smith B., López-Otin C., O’Shea M., Morris H., Zardi L., Murphy G. The role of the C-terminal domain of human collagenase-3 (MMP-13) in the activation of procollagenase-3, substrate specificity, and tissue inhibitor of metalloproteinase interaction. J. Biol. Chem. 1997;272:7608–7616. doi: 10.1074/jbc.272.12.7608. [DOI] [PubMed] [Google Scholar]
- 94.Fosang A.J., Last K., Knäuper V., Murphy G., Neame P.J. Degradation of cartilage aggrecan by collagenase-3 (MMP-13) FEBS Lett. 1996;380:17–20. doi: 10.1016/0014-5793(95)01539-6. [DOI] [PubMed] [Google Scholar]
- 95.Ashworth J.L., Murphy G., Rock M.J., Sherratt M.J., Shapiro S.D., Shuttleworth C.A., Kielty C.M. Fibrillin degradation by matrix metalloproteinases: Implications for connective tissue remodelling. Pt 1Biochem. J. 1999;340:171–181. doi: 10.1042/bj3400171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sasaki T., Göhring W., Mann K., Maurer P., Hohenester E., Knäuper V., Murphy G., Timpl R. Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteinases increases its affinity for collagens. J. Biol. Chem. 1997;272:9237–9243. doi: 10.1074/jbc.272.14.9237. [DOI] [PubMed] [Google Scholar]
- 97.Abety A.N., Pach E., Giebeler N., Fromme J.E., Aramadhaka L.R., Mauch C., Fox J.W., Zigrino P. Loss of ADAM9 Leads to Modifications of the Extracellular Matrix Modulating Tumor Growth. Biomolecules. 2020;10:1290. doi: 10.3390/biom10091290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McQuibban G.A., Butler G.S., Gong J.-H., Bendall L., Power C., Clark-Lewis I., Overall C.M. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J. Biol. Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 99.McQuibban G.A., Gong J.-H., Tam E.M., McCulloch C.A., Clark-Lewis I., Overall C.M. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–1206. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 100.Deng S.-J., Bickett D.M., Mitchell J.L., Lambert M.H., Blackburn R.K., Carter H.L., Neugebauer J., Pahel G., Weiner M.P., Moss M.L. Substrate specificity of human collagenase 3 assessed using a phage-displayed peptide library. J. Biol. Chem. 2000;275:31422–31427. doi: 10.1074/jbc.M004538200. [DOI] [PubMed] [Google Scholar]
- 101.Hu J.-Y., Li G.-C., Wang W.-M., Zhu J.-G., Li Y.-F., Zhou G.-H., Sun Q.-B. Transfection of colorectal cancer cells with chemokine MCP-3 (monocyte chemotactic protein-3) gene retards tumor growth and inhibits tumor metastasis. World J. Gastroenterol. 2002;8:1067–1072. doi: 10.3748/wjg.v8.i6.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Viiklepp K., Nissinen L., Ojalill M., Riihilä P., Kallajoki M., Meri S., Heino J., Kähäri V.M. C1r Upregulates Production of Matrix Metalloproteinase-13 and Promotes Invasion of Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2021;142:1478–1488. doi: 10.1016/j.jid.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 103.Fan Y., Gan Y., Shen Y., Cai X., Song Y., Zhao F., Yao M., Gu J., Tu H. Leptin signaling enhances cell invasion and promotes the metastasis of human pancreatic cancer via increasing MMP-13 production. Oncotarget. 2015;6:16120–16134. doi: 10.18632/oncotarget.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ala-aho R., Ahonen M., George S.J., Heikkilä J., Grenman R., Kallajoki M., Kähäri V.-M. Targeted inhibition of human collagenase-3 (MMP-13) expression inhibits squamous cell carcinoma growth in vivo. Oncogene. 2004;23:5111–5123. doi: 10.1038/sj.onc.1207678. [DOI] [PubMed] [Google Scholar]
- 105.Shah M., Huang D., Blick T., Connor A., Reiter L.A., Hardink J.R., Lynch C.C., Waltham M., Thompson E.W. An MMP13-selective inhibitor delays primary tumor growth and the onset of tumor-associated osteolytic lesions in experimental models of breast cancer. PLoS ONE. 2012;7:e29615. doi: 10.1371/journal.pone.0029615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ala-aho R., Johansson N., Grenman R., Fusenig N.E., Lopez-Otin C., Kähäri V.-M. Inhibition of collagenase-3 (MMP-13) expression in transformed human keratinocytes by interferon-γ is associated with activation of extracellular signal-regulated kinase-1, 2 and STAT1. Oncogene. 2000;19:248–257. doi: 10.1038/sj.onc.1203306. [DOI] [PubMed] [Google Scholar]
- 107.Lu P., Takai K., Weaver V.M., Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Cold Spring Harb. Perspect. Biol. 2011;3:a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Whitelock J.M., Murdoch A.D., Iozzo R.V., Underwood P.A. The degradation of human endothelial cell-derived perlecan and release of bound basic fibroblast growth factor by stromelysin, collagenase, plasmin, and heparanases. J. Biol. Chem. 1996;271:10079–10086. doi: 10.1074/jbc.271.17.10079. [DOI] [PubMed] [Google Scholar]
- 109.Marinkovich M.P., Verrando P., Keene D.R., Meneguzzi G., Lunstrum G.P., Ortonne J.P., Burgeson R.E. Basement membrane proteins kalinin and nicein are structurally and immunologically identical. Lab. Investig. 1993;69:295–299. [PubMed] [Google Scholar]
- 110.Xia Y., Gil S.G., Carter W.G. Anchorage mediated by integrin alpha6beta4 to laminin 5 (epiligrin) regulates tyrosine phosphorylation of a membrane-associated 80-kD protein. J. Cell Biol. 1996;132:727–740. doi: 10.1083/jcb.132.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jones J.C., Kurpakus M.A., Cooper H.M., Quaranta V. A function for the integrin alpha 6 beta 4 in the hemidesmosome. Cell Regul. 1991;2:427–438. doi: 10.1091/mbc.2.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Giannelli G., Falk-Marzillier J., Schiraldi O., Stetler-Stevenson W.G., Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 113.Koshikawa N., Giannelli G., Cirulli V., Miyazaki K., Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J. Cell Biol. 2000;148:615–624. doi: 10.1083/jcb.148.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao X., Sun B., Li Y., Liu Y., Zhang D., Wang X., Gu Q., Zhao J., Dong X., Liu Z., et al. Dual effects of collagenase-3 on melanoma: Metastasis promotion and disruption of vasculogenic mimicry. Oncotarget. 2015;6:8890–8899. doi: 10.18632/oncotarget.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Knäuper V., Smith B., López-Otin C., Murphy G. Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13) Eur. J. Biochem. 1997;248:369–373. doi: 10.1111/j.1432-1033.1997.00369.x. [DOI] [PubMed] [Google Scholar]
- 116.Dreier R., Grässel S., Fuchs S., Schaumburger J., Bruckner P. Pro-MMP-9 is a specific macrophage product and is activated by osteoarthritic chondrocytes via MMP-3 or a MT1-MMP/MMP-13 cascade. Exp. Cell Res. 2004;297:303–312. doi: 10.1016/j.yexcr.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 117.Hernández Ríos M., Sorsa T., Obregón F., Tervahartiala T., Valenzuela M.A., Pozo P., Dutzan N., Lesaffre E., Molas M., Gamonal J. Proteolytic roles of matrix metalloproteinase (MMP)-13 during progression of chronic periodontitis: Initial evidence for MMP-13/MMP-9 activation cascade. J. Clin. Periodontol. 2009;36:1011–1017. doi: 10.1111/j.1600-051X.2009.01488.x. [DOI] [PubMed] [Google Scholar]
- 118.Roche J. The Epithelial-to-Mesenchymal Transition in Cancer. Cancers. 2018;10:52. doi: 10.3390/cancers10020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018;13:395–412. doi: 10.1146/annurev-pathol-020117-043854. [DOI] [PubMed] [Google Scholar]
- 120.Kim B.N., Ahn D.H., Kang N., Yeo C.D., Kim Y.K., Lee K.Y., Kim T.-J., Lee S.H., Park M.S., Yim H.W., et al. TGF-β induced EMT and stemness characteristics are associated with epigenetic regulation in lung cancer. Sci. Rep. 2020;10:10597. doi: 10.1038/s41598-020-67325-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Illman S.A., Lehti K., Keski-Oja J., Lohi J. Epilysin (MMP-28) induces TGF-beta mediated epithelial to mesenchymal transition in lung carcinoma cells. Pt 18J. Cell Sci. 2006;119:3856–3865. doi: 10.1242/jcs.03157. [DOI] [PubMed] [Google Scholar]
- 122.D’Angelo M., Billings P.C., Pacifici M., Leboy P.S., Kirsch T. Authentic matrix vesicles contain active metalloproteases (MMP). A role for matrix vesicle-associated MMP-13 in activation of transforming growth factor-beta. J. Biol. Chem. 2001;276:11347–11353. doi: 10.1074/jbc.M009725200. [DOI] [PubMed] [Google Scholar]
- 123.Nannuru K.C., Futakuchi M., Varney M.L., Vincent T.M., Marcusson E.G., Singh R.K. Matrix metalloproteinase (MMP)-13 regulates mammary tumor-induced osteolysis by activating MMP9 and transforming growth factor-beta signaling at the tumor-bone interface. Cancer Res. 2010;70:3494–3504. doi: 10.1158/0008-5472.CAN-09-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Banyard J., Bielenberg D.R. The role of EMT and MET in cancer dissemination. Connect. Tissue Res. 2015;56:403–413. doi: 10.3109/03008207.2015.1060970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Barillari G. The Impact of Matrix Metalloproteinase-9 on the Sequential Steps of the Metastatic Process. Int. J. Mol. Sci. 2020;21:4526. doi: 10.3390/ijms21124526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Folgueras A.R., Pendás A.M., Sánchez L.M., López-Otín C. Matrix metalloproteinases in cancer: From new functions to improved inhibition strategies. Int. J. Dev. Biol. 2004;48:411–424. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 127.Mondal S., Adhikari N., Banerjee S., Amin S.A., Jha T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur. J. Med. Chem. 2020;194:112260. doi: 10.1016/j.ejmech.2020.112260. [DOI] [PubMed] [Google Scholar]
- 128.Mendonsa A.M., VanSaun M.N., Ustione A., Piston D.W., Fingleton B.M., Gorden D.L. Host and tumor derived MMP13 regulate extravasation and establishment of colorectal metastases in the liver. Mol. Cancer. 2015;14:49. doi: 10.1186/s12943-014-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kolb A.D., Bussard K.M. The Bone Extracellular Matrix as an Ideal Milieu for Cancer Cell Metastases. Cancers. 2019;11:1020. doi: 10.3390/cancers11071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu P., Weaver V.M., Werb Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ohshiba T., Miyaura C., Inada M., Ito A. Role of RANKL-induced osteoclast formation and MMP-dependent matrix degradation in bone destruction by breast cancer metastasis. Br. J. Cancer. 2003;88:1318–1326. doi: 10.1038/sj.bjc.6600858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Page-McCaw A., Ewald A.J., Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lafleur M.A., Drew A.F., de Sousa E.L., Blick T., Bills M., Walker E.C., Williams E.D., Waltham M., Thompson E.W. Upregulation of matrix metalloproteinases (MMPs) in breast cancer xenografts: A major induction of stromal MMP-13. Int. J. Cancer. 2005;114:544–554. doi: 10.1002/ijc.20763. [DOI] [PubMed] [Google Scholar]
- 134.Morrison C., Mancini S., Cipollone J., Kappelhoff R., Roskelley C., Overall C. Microarray and proteomic analysis of breast cancer cell and osteoblast co-cultures: Role of osteoblast matrix metalloproteinase (MMP)-13 in bone metastasis. J. Biol. Chem. 2011;286:34271–34285. doi: 10.1074/jbc.M111.222513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ibaragi S., Shimo T., Iwamoto M., Hassan N.M., Kodama S., Isowa S., Sasaki A. Parathyroid hormone-related peptide regulates matrix metalloproteinase-13 gene expression in bone metastatic breast cancer cells. Anticancer Res. 2010;30:5029–5036. [PubMed] [Google Scholar]
- 136.Pivetta E., Scapolan M., Pecolo M., Wassermann B., Abu-Rumeileh I., Balestreri L., Borsatti E., Tripodo C., Colombatti A., Spessotto P. MMP-13 stimulates osteoclast differentiation and activation in tumour breast bone metastases. Breast Cancer Res. 2011;13:R105. doi: 10.1186/bcr3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fu J., Li S., Feng R., Ma H., Sabeh F., Roodman G.D., Mapara M., Weiss S.J., Lentzsch S. Matrix Metalloproteinase 13 (MMP13) Upregulation Is Essential for Multiple Myeloma Related Bone Lytic Lesion. Blood. 2012;120:4025. doi: 10.1182/blood.V120.21.4025.4025. [DOI] [Google Scholar]
- 138.Fu J., Li S., Feng R., Ma H., Sabeh F., Roodman G.D., Wang J., Robinson S., Guo X.E., Lund T., et al. Multiple myeloma-derived MMP-13 mediates osteoclast fusogenesis and osteolytic disease. J. Clin. Investig. 2016;126:1759–1772. doi: 10.1172/JCI80276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fuerst R., Yong Choi J., Knapinska A.M., Smith L., Cameron M.D., Ruiz C., Fields G.B., Roush W.R. Development of matrix metalloproteinase-13 inhibitors—A structure-activity/structure-property relationship study. Bioorg. Med. Chem. 2018;26:4984–4995. doi: 10.1016/j.bmc.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu Y., Cao X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell. 2016;30:668–681. doi: 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 141.Hiratsuka S., Watanabe A., Aburatani H., Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 142.Kaplan R.N., Riba R.D., Zacharoulis S., Bramley A.H., Vincent L., Costa C., MacDonald D.D., Jin D.K., Shido K., Kerns S.A., et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hiratsuka S., Watanabe A., Sakurai Y., Akashi-Takamura S., Ishibashi S., Miyake K., Shibuya M., Akira S., Aburatani H., Maru Y. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 144.Bond M., Fabunmi R.P., Baker A.H., Newby A.C. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: An absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/S0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- 145.Kitamura T., Qian B.-Z., Pollard J.W. Immune cell promotion of metastasis. Nat. Rev. Immunol. 2015;15:73–86. doi: 10.1038/nri3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Heissig B., Hattori K., Dias S., Friedrich M., Ferris B., Hackett N.R., Crystal R.G., Besmer P., Lyden D., Moore M.A., et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/S0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Erler J.T., Bennewith K.L., Cox T.R., Lang G., Bird D., Koong A., Le Q.-T., Giaccia A.J. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Boileau C., Pelletier J.P., Tardif G., Fahmi H., Laufer S., Lavigne M., Martel-Pelletier J. The regulation of human MMP-13 by licofelone, an inhibitor of cyclo-oxygenases and 5-lipoxygenase, in human osteoarthritic chondrocytes is mediated by the inhibition of the p38 MAP kinase signalling pathway. Ann. Rheum. Dis. 2005;64:891–898. doi: 10.1136/ard.2004.026906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang L., Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim. Biophys. Acta Rev. Cancer. 2019;1871:455–468. doi: 10.1016/j.bbcan.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu Y., Gu Y., Cao X. The exosomes in tumor immunity. Oncoimmunology. 2015;4:e1027472. doi: 10.1080/2162402X.2015.1027472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Costa-Silva B., Aiello N.M., Ocean A.J., Singh S., Zhang H., Thakur B.K., Becker A., Hoshino A., Mark M.T., Molina H., et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 2015;17:816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shan Y., You B., Shi S., Shi W., Zhang Z., Zhang Q., Gu M., Chen J., Bao L., Liu D., et al. Hypoxia-Induced Matrix Metalloproteinase-13 Expression in Exosomes from Nasopharyngeal Carcinoma Enhances Metastases. Cell Death Dis. 2018;9:382. doi: 10.1038/s41419-018-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shah A.A., Kamal M.A., Akhtar S. Tumor Angiogenesis and VEGFR-2: Mechanism, Pathways and Current Biological Therapeutic Interventions. Curr. Drug Metab. 2021;22:50–59. doi: 10.2174/1389200221666201019143252. [DOI] [PubMed] [Google Scholar]
- 154.Li T., Kang G., Wang T., Huang H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol. Lett. 2018;16:687–702. doi: 10.3892/ol.2018.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nishida N., Yano H., Nishida T., Kamura T., Kojiro M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006;2:213–219. doi: 10.2147/vhrm.2006.2.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Welti J., Loges S., Dimmeler S., Carmeliet P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J. Clin. Investig. 2013;123:3190–3200. doi: 10.1172/JCI70212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.de la Puente P., Muz B., Azab F., Azab A.K. Cell trafficking of endothelial progenitor cells in tumor progression. Clin. Cancer Res. 2013;19:3360–3368. doi: 10.1158/1078-0432.CCR-13-0462. [DOI] [PubMed] [Google Scholar]
- 158.Ferrara N., Adamis A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016;15:385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 159.Eelen G., de Zeeuw P., Simons M., Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015;116:1231–1244. doi: 10.1161/CIRCRESAHA.116.302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moschetta M., Mishima Y., Sahin I., Manier S., Glavey S., Vacca A., Roccaro A.M., Ghobrial I.M. Role of endothelial progenitor cells in cancer progression. Biochim. Biophys. Acta. 2014;1846:26–39. doi: 10.1016/j.bbcan.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 161.Liekens S., De Clercq E., Neyts J. Angiogenesis: Regulators and clinical applications. Biochem. Pharmacol. 2001;61:253–270. doi: 10.1016/S0006-2952(00)00529-3. [DOI] [PubMed] [Google Scholar]
- 162.Milkiewicz M., Ispanovic E., Doyle J.L., Haas T.L. Regulators of angiogenesis and strategies for their therapeutic manipulation. Int. J. Biochem. Cell Biol. 2006;38:333–357. doi: 10.1016/j.biocel.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 163.Quintero-Fabián S., Arreola R., Becerril-Villanueva E., Torres-Romero J.C., Arana-Argáez V., Lara-Riegos J., Ramírez-Camacho M.A., Alvarez-Sánchez M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019;9:1370. doi: 10.3389/fonc.2019.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zijlstra A., Aimes R.T., Zhu D., Regazzoni K., Kupriyanova T., Seandel M., Deryugina E.I., Quigley J.P. Collagenolysis-dependent angiogenesis mediated by matrix metalloproteinase-13 (collagenase-3) J. Biol. Chem. 2004;279:27633–27645. doi: 10.1074/jbc.M313617200. [DOI] [PubMed] [Google Scholar]
- 165.Perumal S., Antipova O., Orgel J.P. Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proc. Natl. Acad. Sci. USA. 2008;105:2824–2829. doi: 10.1073/pnas.0710588105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Seandel M., Noack-Kunnmann K., Zhu D., Aimes R.T., Quigley J.P. Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood. 2001;97:2323–2332. doi: 10.1182/blood.V97.8.2323. [DOI] [PubMed] [Google Scholar]
- 167.Rundhaug J.E. Matrix metalloproteinases and angiogenesis. J. Cell. Mol. Med. 2005;9:267–285. doi: 10.1111/j.1582-4934.2005.tb00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kudo Y., Iizuka S., Yoshida M., Tsunematsu T., Kondo T., Subarnbhesaj A., Deraz E.M., Siriwardena S.B., Tahara H., Ishimaru N., et al. Matrix metalloproteinase-13 (MMP-13) directly and indirectly promotes tumor angiogenesis. J. Biol. Chem. 2012;287:38716–38728. doi: 10.1074/jbc.M112.373159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lederle W., Hartenstein B., Meides A., Kunzelmann H., Werb Z., Angel P., Mueller M.M. MMP13 as a stromal mediator in controlling persistent angiogenesis in skin carcinoma. Carcinogenesis. 2010;31:1175–1184. doi: 10.1093/carcin/bgp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Xu L., Sun K., Xia M., Li X., Lu Y. MMP13 Regulates Aggressiveness of Pediatric Multiple Myeloma Through VEGF-C. Cell. Physiol. Biochem. 2015;36:509–516. doi: 10.1159/000430116. [DOI] [PubMed] [Google Scholar]