Abstract
The condensation of aromatic dialdehydes with chiral diamines, such as 1,2-trans-diaminocyclohexane, leads to various enantiopure or meso-type macrocyclic Schiff bases, including [2 + 2], [3 + 3], [4 + 4], [6 + 6] and [8 + 8] condensation products. Unlike most cases of macrocycle synthesis, the [3 + 3] macrocycles of this type are sometimes obtained in high yields by direct condensation without a metal template. Macrocycles of other sizes from this family can often be selectively obtained in high yields by a suitable choice of metal template, solvent, or chirality of the building blocks. In particular, the application of a cadmium(II) template results in the expansion of the [2 + 2] macrocycles into giant [6 + 6] and [8 + 8] macrocycles. These imine macrocycles can be reduced to the corresponding macrocyclic amines which can act as hosts for the binding of multiple cations or multiple anions.
Keywords: macrocycles, chirality, imines, amines, metal binding, enantiomeric recognition
1. Introduction
The importance of macrocycles in chemistry and biology is well recognized. Among macrocyclic compounds, azamacrocycles constitute an important and diverse class of compounds, which have applications in catalysis, recognition, separation, and medical diagnostics. Perhaps the best-known compounds of the azamacrocyclic class are tetraaza macrocycles, such as porphyrins or 1,4,7,10-tetraazacyclododecane (cyclen) whose derivative 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) is widely used as a Gd(III) complex in magnetic resonance imaging. While tetraazamacrocycles are capable of binding a single metal ion, there are also much larger azamacrocycles containing, for example, 18 nitrogen atoms, which are capable of binding multiple metal ions.
The field of extended macrocycles and their complexes has already been the subject of several review articles, e.g., references [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. In this minireview, extended macrocyclic imines, which are the products of the condensation reactions of aromatic dialdehydes with chiral diamines, such as 1,2-trans-diaminocyclohexane (DACH) or 1,2-diphenylethylenediamine (DPEN), as well as the corresponding amine macrocycles, will be described. Particular emphasis will be placed on the ability of these macrocycles to bind guest molecules and their application in enantioselective catalysis. These extended macrocycles in their neutral or deprotonated form are able to bind multiple transition metal ions or large lanthanoid ions. The protonated amine macrocycles of this class are able to bind anions and the neutral forms of these enantiopure macrocycles are also used in enantioselective binding and recognition of chiral organic guest molecules. The properties resulting from the presence of chiral diamine centers, such as chiral recognition and self-recognition or chirality transfer, will also be briefly outlined.
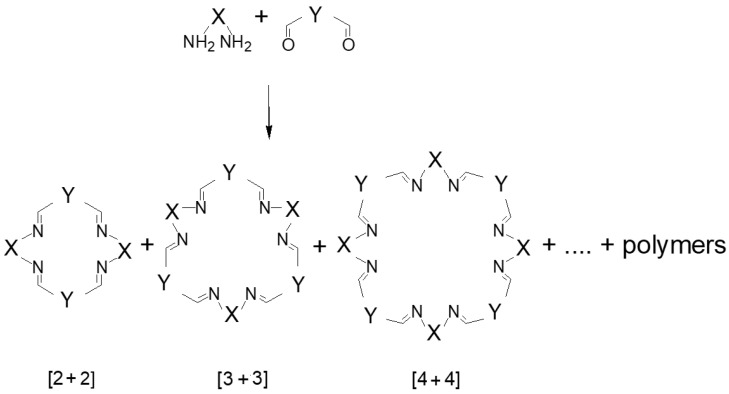
In general, the [n + n] condensation of diamines and dialdehydes may result in the formation of various macrocyclic products, such as [1 + 1], [2 + 2], [3 + 3], etc., macrocycles (Figure 1), as well as oligomeric and polymeric imines. In most cases (especially in the case of saturated substrates) such condensation reactions result in intractable mixtures of mostly polymeric products, and the isolation of pure macrocycles even under dilute conditions is not possible. Nevertheless, in the case of reactions of relatively rigid aromatic dialdehydes with chiral diamines, this type of condensation often leads to the successful preparation of [n + n] macrocyclic products in good yields. Sometimes macrocycles are practically the sole product of such a condensation reaction, and the yields are quantitative. Most typical n values in these reactions are 2, 3 or 4, but in the case of extended dialdehyde building blocks, where two aromatic moieties bearing aldehyde functionalities are connected by a sufficiently long link, [1 + 1] products are also possible.
Figure 1.
Some of the potential products of the [n + n] condensation of diamines and dialdehydes.
It should be noted that the formation of the imine bond is reversible [15,16,17,18,19,20,21,22] and that the formation of the various products presented in Figure 1 corresponds to dynamic covalent chemistry. Occasionally, the isolated pure imine macrocycle equilibrates back in solution into a mixture of products. The dynamic library of imines can be transformed into the corresponding mixture of amines by the reaction with reducing agents, such as sodium borohydride, and the corresponding “frozen” library of macrocycles can be separated into individual components. The preferred formation of any given imine [n + n] product may result from the geometric constraints encoded in the building blocks which lead to a preferred geometry of the final thermodynamic product in solution. The equilibrium of the system may also be shifted towards a particular product by its lowest solubility and crystallization. Finally, the equilibrium among the [n + n] imines may be shifted by metal ion templates towards the macrocycle which is best suited for metal complexation [23,24].
2. [2 + 2] Macrocycles
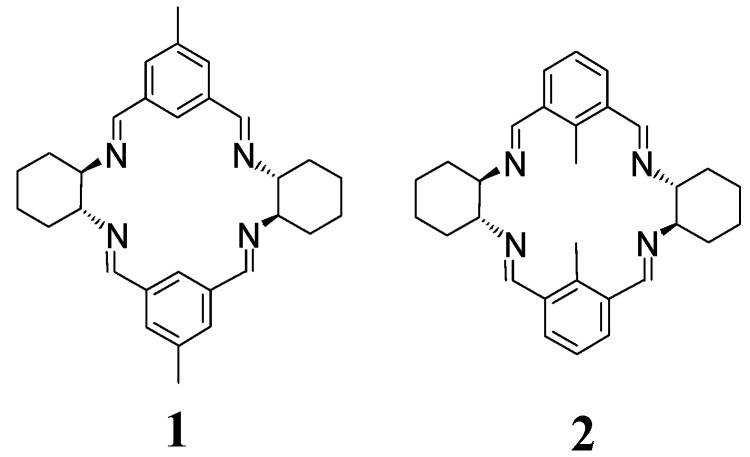
Condensation of isophthalaldehyde and its derivatives with enantiopure DACH leads typically to a mixture of [3 + 3] and [2 + 2] macrocyclic products where the larger [3 + 3] macrocycles are the dominant kinetic products, while the [2 + 2] macrocycles seem to be the thermodynamic products. Thus, prolonged reflux of [3 + 3] macrocycles in dichloromethane (DCM) resulted in the formation of smaller [2 + 2] macrocycles, such as 1 and 2 in quantitative yields (Figure 2) [25].
Figure 2.
[2 + 2] macrocycles obtained from the kinetic [3 + 3] products.
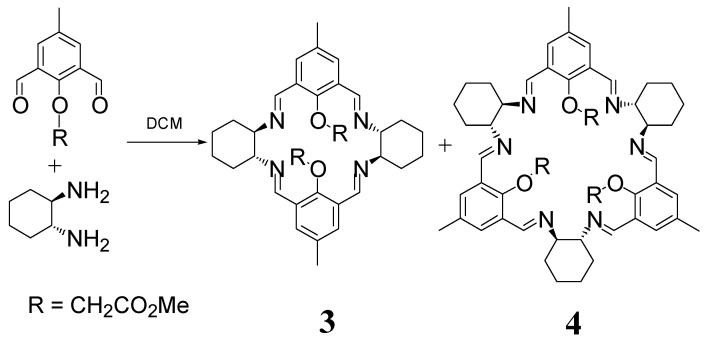
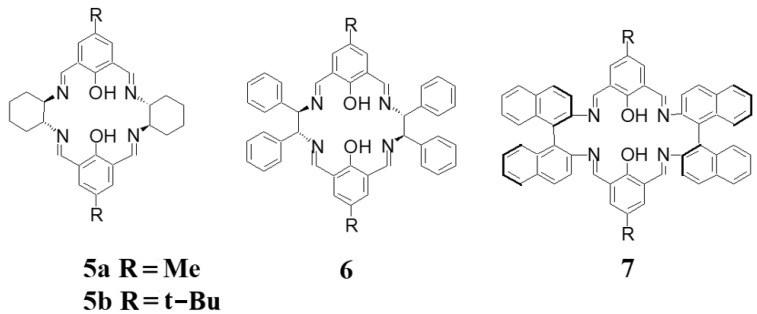
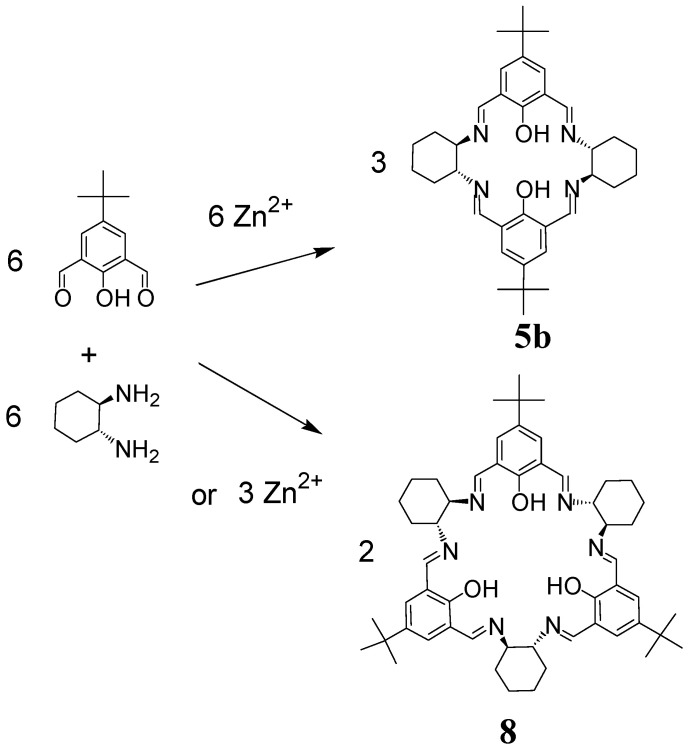
The [2 + 2] macrocycle 3 is also accompanying the main [3 + 3] macrocycle 4 (which can be isolated at only a 6% yield as a pure product) in the case of the condensation of DACH with an O-alkylated 2,6-diformylphenol (Figure 3) [26]. In contrast, the [2 + 2] macrocyclic imines 5–7 (Figure 4) derived from chiral diamines and 2,6-diformylphenols can be obtained in high (typically 50–90%) yields as dinuclear metal complexes of the deprotonated form of the macrocycles in condensation reactions templated by transition metal ions, such as zinc(II), copper(II) or nickel(II) [27,28,29,30,31,32,33,34].
Figure 3.
Formation of a mixture of [2 + 2] and [3 + 3] products.
Figure 4.
[2 + 2] diphenolic imines obtained in metal-templated condensations.
It should be noted that without a metal template the corresponding condensation reactions usually lead to [3 + 3] macrocycles (see Section 3). However, this is not the case when substrates used for 7 are reacted without a metal template. In this system mixture of imines is formed without templating ions. When the lead(II) ions are used as the template, the resulting dinuclear complexes can be reduced by sodium borohydride and after demetallation afford the corresponding free amine macrocycles. The dinuclear complexes of 5a, b and 6 are relatively flat and moderately twisted, while the conformation of the Cu(II) complex of 7 is considerably twisted [34]. Dinuclear zinc(II) complex of 5a, as well as dinuclear zinc(II) complex of analogous meso-type [2 + 2] macrocycle derived from racemic DACH, are fluorescent agents [30]. Moreover, these compounds induced apoptosis of cancer cells and the meso complex was found to be an efficient regulator of the cell cycle and anti-apoptosis genes. Dinuclear cobalt(II/III) complexes of 5a and 6 catalyzed asymmetric cyclopropanation of styrene with diazoacetate with high enantioselectivity reaching up to 94% [31] and the dinuclear copper(II) complexes of 5a,b and 6 and their amine counterparts were applied in enantioselective oxidative coupling of 2-naphthol [32]. The dinuclear zinc(II) complex of 6 was also studied as an enantioselective catalyst for the desymmetrization of meso diol to achieve a chiral product with 96% yield and 88% ee [33]. The dinuclear copper(II) complex of 7 was studied as an enantioselective catalyst in the asymmetric oxidative coupling of 2-naphthol to chiral 1,1′-bi-2-naphthol, which is an important chiral ligand (BINOL) [34].
In the case of the reaction of 2,6-diformyl-4-tert-butylphenol and enantiopure DACH, an unprecedented template effect was observed [27,28]. In this system, the size of the formed macrocycle depends on the stoichiometry of the applied metal template. By using equimolar amounts of dialdehyde, diamine and zinc(II) acetate (Figure 5), the dinuclear Zn(II) complex of the [2 + 2] macrocycle 5b is selectively obtained. On the other hand, by using just half of the equivalent of the same template metal salt the [3 + 3] macrocycle 8 is selectively obtained in the form of a trinuclear Zn(II) complex where the three metal ions are shared by two deprotonated macrocyclic units.
Figure 5.
Dependence of the size of the macrocycle on the amount of added template.
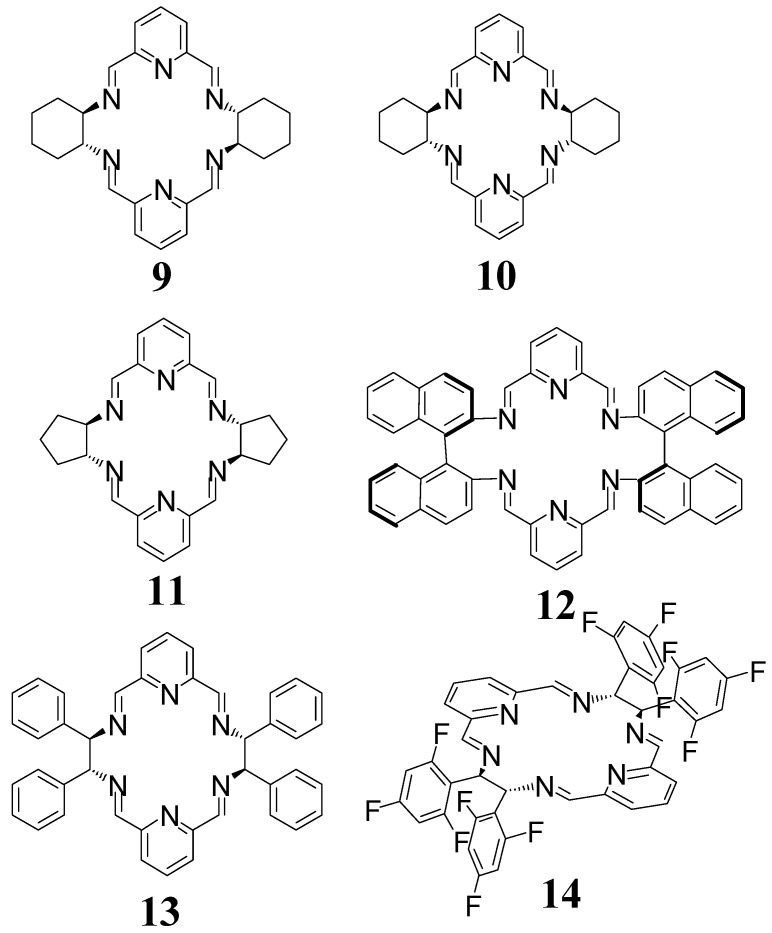
The condensations of 2,6-diformylpyridine (DFP) with enantiopure chiral diamines result in mixtures of macrocyclic products and the isolation of pure imine products is unsuccessful, partly due the reversible nature of the condensation reactions. For instance, the reaction of DFP with enantiopure DACH results in a mixture containing mostly the [3 + 3] and [2 + 2] imine macrocycles, but the pure product 9 (Figure 6) was not isolated from this mixture. This dynamic library can be “frozen” by reduction with sodium borohydride and the resulting amine [2 + 2] and [3 + 3] macrocycles can be separated by recrystallization [35]. On the other hand, the reaction DFP with the racemic form of DACH results in a mixture containing mainly isomeric meso-type [2 + 2] macrocycle 10 (Figure 6) and meso-type [4 + 4] macrocycle [36]. Pure imine 10 can be separated from this mixture as the least soluble product.
Figure 6.
Imine [2 + 2] macrocycles derived from 2,6-diformylpyridine.
The chiral [2 + 2] imine 9 can be easily obtained in high yields (typically 40–80% for crystallized products) in the complexed form, when large metal ions, such as lanthanoid(III) or lead(II) ions are used as a template in the reaction of enantiopure DACH [37,38,39,40]. The application of these ions shifts the dynamic equilibrium selectively towards the [2 + 2] product. In most cases, the NMR spectra of the crude reaction mixtures containing these complexes of 9 indicate practically the quantitative formation of this macrocycle. A similar effect was observed in the case of formation of complexes of macrocycle 11 derived from the cyclopentane analog of DACH, that is trans-1,2-diaminocyclopentane (DACP) [41]. Another product derived from DFP is the macrocycle 12 based on the enantiopure 1,1′-binaphthyl-2,2′-diamine [42] and macrocycles 13 and 14 derived from DPEN or its fluorinated derivative [43,44,45] (Figure 6). The dysprosium complexes of macrocycles 13 and 14 exhibit exceptional Single Molecule Magnet (SMM) properties with record values of the energy barrier for the reorientation of the magnetization (Ueff) among air-stable SMMs known so far [44,45]. Interestingly, the application of racemic DACH in a similar templated condensation does not result in the complexes of meso-type macrocycle 10 (these complexes may be formed only as intermediate kinetic products) but leads to a racemic form of the complexes of chiral macrocycle 9 as the thermodynamic products.
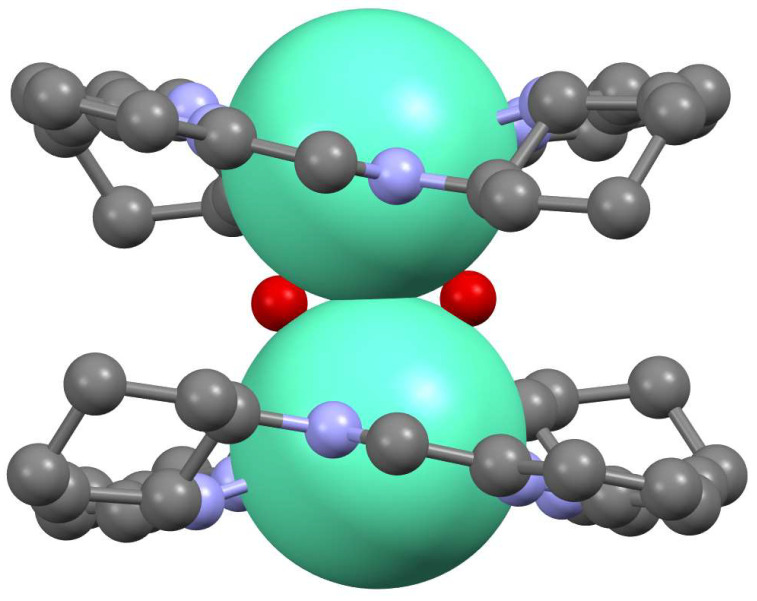
Macrocycles 9, 11–14 adopt helical conformations in their enantiopure lanthanoid(III) complexes. This conformation leads to interesting properties in these complexes related to their chiral nature, i.e., enantiomeric self-recognition, chirality transfer and enantioselective hydrolytic cleavage of DNA [38,39,40,46]. Two macrocyclic units of 9 containing lanthanoid(III) may be linked by hydroxo or fluorido bridges to form dinuclear complexes (Figure 7) [38,39,40]. These complexes are formed only when both macrocyclic units are derived from the monomeric complexes of the same chirality, i.e., the same direction of the helical twist. This corresponds to enantiomeric self-recognition, which is a narcissistic sorting of macrocyclic units with respect to their chirality. In similar heterodinuclear complexes, where one macrocyclic unit of 9 is linked to the macrocyclic unit of the achiral macrocycle derived from ethylenediamine, the achiral macrocycle adopts a defined direction of helical twist dictated by the direction of the helical twist of 9. This effect corresponds to chirality transfer between macrocyclic units.
Figure 7.
Side view of a dimeric complex where two Eu(III) ions residing in two macrocyclic units of 9 are linked by additional hydroxo bridges (metal ions are shown in spacefill representation, hydrogen atoms and additional anions are omitted for simplicity, Eu—green, N—blue, O—red). All the figures presenting molecular structures in this review were redrawn using the Mercury 2020.1 program and were based on the appropriate cif files deposited at the Cambridge Crystallographic Data Centre.
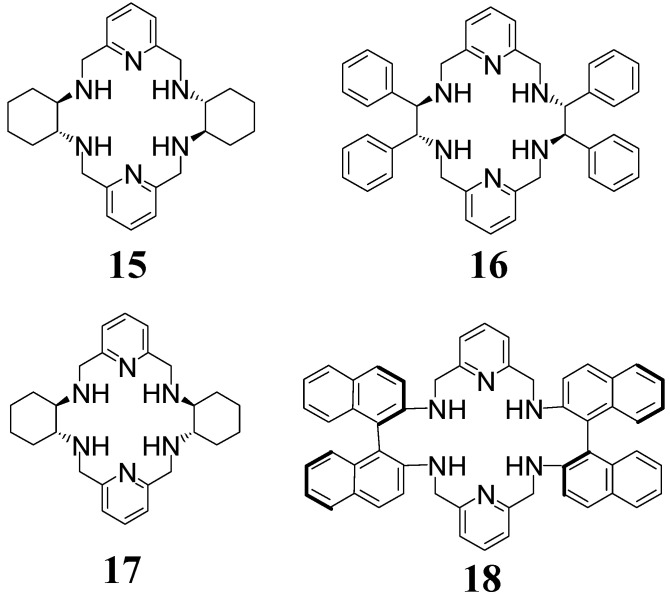
Lead(II) complexes of macrocycles 9, 10, 12, and 13 can be reduced and demetallated to give the corresponding free macrocyclic amines 15–18 (Figure 8). Unexpectedly, amine 18 does not bind lanthanoid(III) ions, unlike its imine counterpart. On the other hand, macrocycle 15 forms lanthanoid(III) complexes, which can undergo solvent [47] or anion-induced [48,49] helicity inversion. Macrocycle 15 and its derivatives, as well as macrocycles 16 and 18, can also be used as chiral solvating agents for the enantiodiscrimination of chiral carboxylic acids, such as ibuprofen. The determination of the enantiomeric excess of different carboxylic acids has been achieved on the basis of good splitting of the NMR signals for the enantiomers of the bound guest molecules [50,51,52,53]. Interestingly, in the gas phase, amine 15 is able to bind potassium cations, anions, such as carboxylates, but also to function as an acceptor of contact K+/anion pairs [54,55].
Figure 8.
[2 + 2] amines derived from 2,6-diformylpyridine.
The formation of [2 + 2] imines is preferred when enantiopure DACH is condensed with dialdehydes based on two benzaldehyde fragments that are connected by a link X (Figure 9) which enforces the bent conformation of the dialdehyde. Because of the resulting shape of the macrocycle, these products were called rhombimines (Figure 9) [56,57,58,59,60]. The reduced form of this kind of macrocycles, i.e., rhombamines was applied in NMR enantiodiscrimination of chiral carboxylic acids and their derivatives [61,62].
Figure 9.
Examples of rhombimine macrocycles.
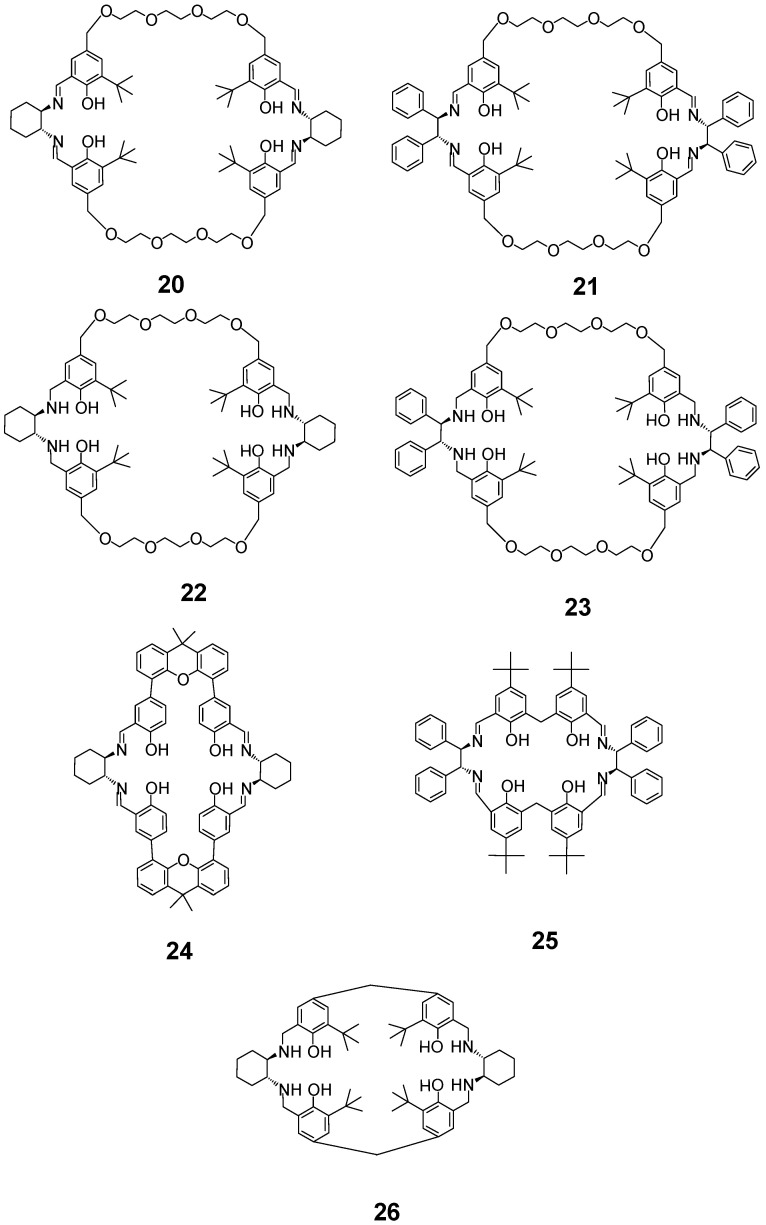
The condensation of enantiopure DACH or DPEN with aromatic dialdehydes containing two phenol fragments results in the formation of the extended [2 + 2] imines 20, 21, 24–26, which can also be transformed into their reduced amine forms 22 and 23 (Figure 10) [63,64,65,66,67,68,69,70]. These enantiopure ligands contain two compartments corresponding to the chiral salen environment (the parent macrocycle 26 was called calixsalen [63]). Transition metal complexes of acyclic salen-type ligands are well known to exhibit catalytic activity. Macrocycles 20–26 correspond to the “doubled” form of salen/salan ligands, and they adopt a folded conformation in their complexes. These macrocycles allow for the synergistic catalytic activity of two ligand-bound metal centers, as well as provide a kind of pocket for substrate binding. For example, in the dinuclear cobalt(III) complex of 20 one metal center may coordinate a hydroxide nucleophile, while the other metal center can activate epoxide. The energy-minimized structure of such a dinuclear complex shows a plausible intermediate in the catalytic cycle, where the epichlorohydrin molecule is bound in the center of the expanded macrocycle between the two cobalt(III) ions [64]. The extended tetraphenolic macrocycles 20–26 are predisposed to form dinuclear complexes with transition metals, such as cobalt(III), copper(II), vanadium(V), manganese(III) or titanium(IV), which exhibit interesting enantioselective catalytic activity in the kinetic resolution of epoxides [64], Henry reaction [65], aza-Henry reaction [70], Strecker reaction [67], O-acetylcyanation/cyanoformylation of aldehydes [66], asymmetric carbonylation of aldehydes [68], and epoxidation of alkenes [69]. The dinuclear copper(II) complex of another tetraphenolic [2 + 2] imine derived from DPEN was active as an enantioselective catalyst in the Henry reaction [71].
Figure 10.
Extended tetraphenolic [2 + 2] macrocycles.
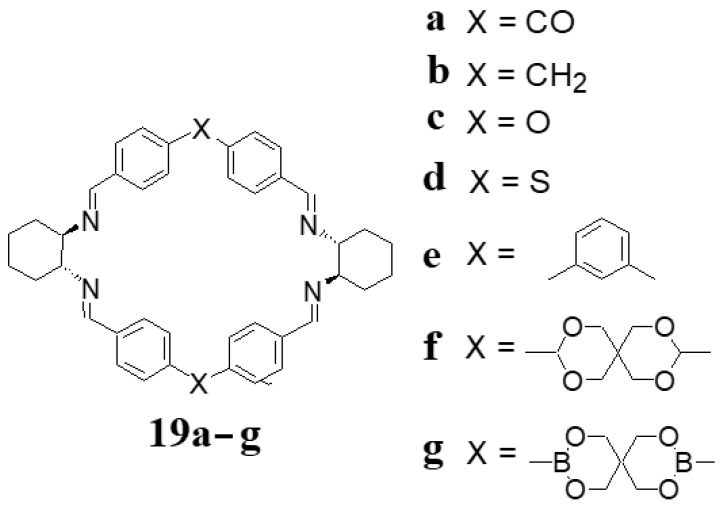
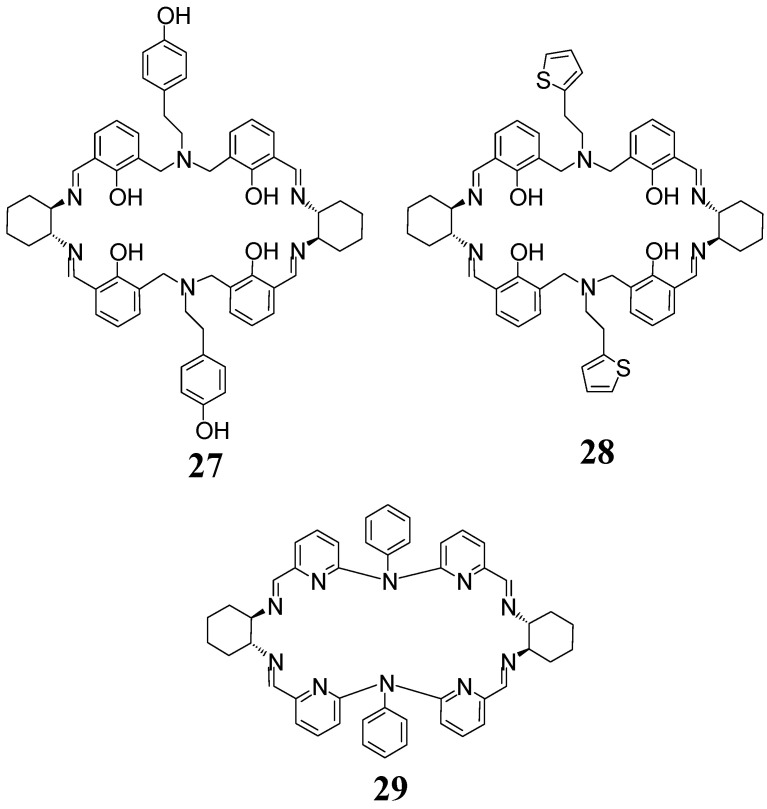
In contrast to the above tetraphenolic macrocycles obtained as free ligands, the extended tetraphenolic macrocycles 27 and 28 (Figure 11) were obtained only as metal complexes in condensation reactions templated by zinc(II) ions [72,73]. Similarly, the decaaza macrocycle 29 was obtained in template condensation [74]. In the latter case, an interesting influence of the kind of the metal template on the reaction of enantiopure DACH with the appropriate aromatic dialdehyde was observed. While the application of cadmium(II) salt resulted in the formation of the dinuclear Cd(II) complex of macrocycle 29, the application of copper(II) salt resulted in the formation of a mononuclear complex of the acyclic condensation product. The dinuclear Cu(II) complex was obtained, however, by transmetalation of the Cd(II) complex and the free macrocycle was obtained by the demetallation of the complexes with sodium sulfide via the formation of insoluble CuS.
Figure 11.
Extended [2 + 2] imines obtained in metal-templated condensations.
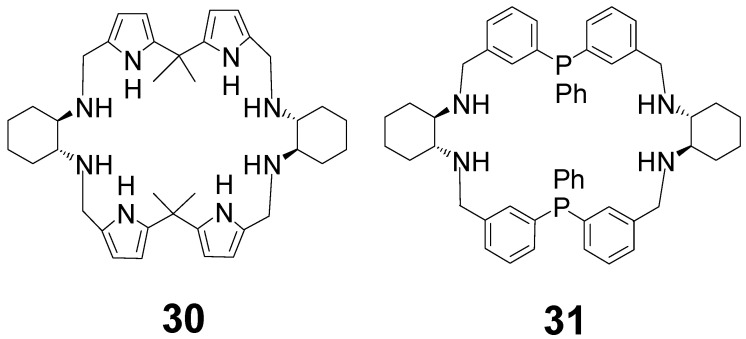
The imine precursor of the extended [2 + 2] amine 30 with four pyrrole fragments (Figure 12) was obtained by the condensation of DACH L-tartarate with dipyrrolic dicarbaldehyde in the presence of triethylamine with a 90% yield. This imine was reduced with sodium borohydride to give the amine macrocycle 30 [75]. The copper(II) complex of 30 was applied as an enantioselective catalyst in a Henry reaction with up to 95% ee values. The iron complex generated from the [2 + 2] amine 31 and triiron dodecacarbonyl was used in the enantioselective hydrogenation of ketones [76,77]. This N4P2 ligand contains two phosphine-type phosphorous donor atoms which allow the stabilization of the low oxidation state of iron in the active complex.
Figure 12.
Extended [2 + 2] amine ligands for enantioselective catalysis.
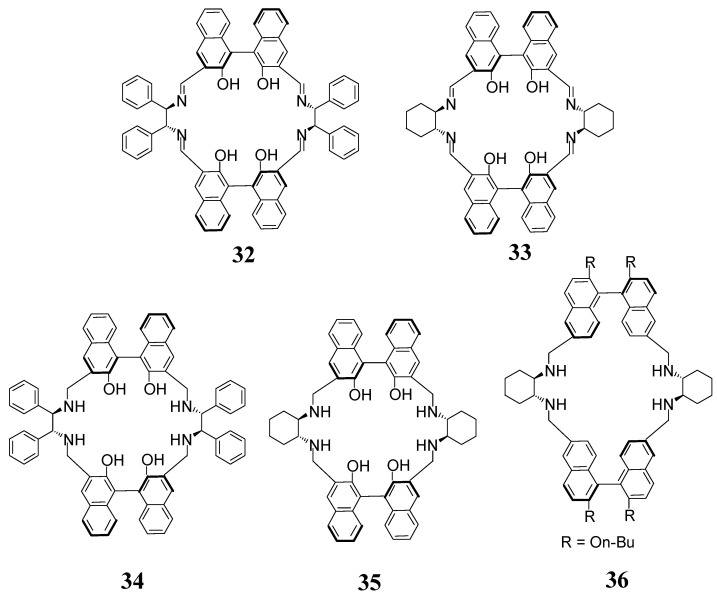
The enantiopure [2 + 2] imine and amine macrocycles 32–35 (Figure 13) have two chiral centers—chiral diamine fragment and chiral 1,1′-bi-2-naphthol (BINOL) fragment. These compounds were successfully used in enantioselective fluorescent recognition of mandelic acid and other chiral acids [78,79,80]. Macrocycle 35 was also used as a fluorescent probe selectively recognizing the mercury(II) cations [81]. Similar macrocyclic amine 36 acts as a selective fluorescent sensor for the detection of zinc(II) ions [82] as well as for the combined recognition of copper(II) ions and amino acids [83].
Figure 13.
[2 + 2] macrocycles used as fluorescent detectors of guests.
3. [3 + 3] Macrocycles
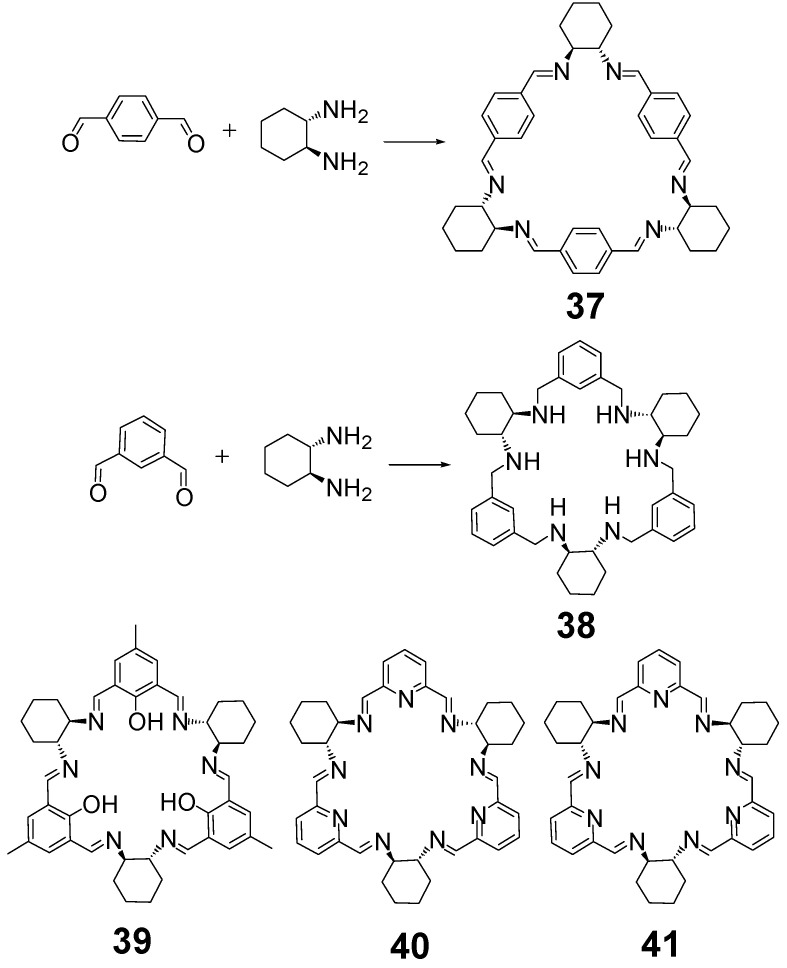
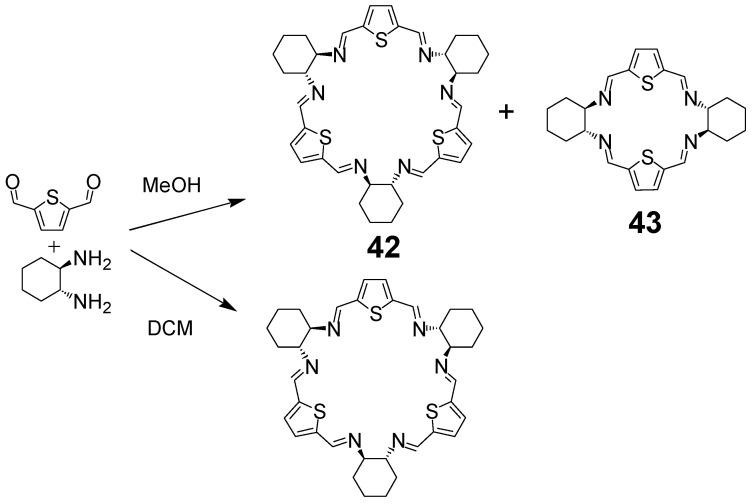
The angle formed by the amine groups of DACH in combination with the linear alignment of aldehyde groups of 1,4-terephthalaldehyde leads to the preferred [3 + 3] condensation product 37 of triangular shape (Figure 14), which was discovered in 2000 by Gawroński et al. and was called trianglimine [84]. This macrocyclic crude product can be obtained in quantitative yield simply by mixing substrates in dichloromethane at room temperature and subsequent evaporation of the solvent, while the yield after recrystallization was ca. 90%. This strong preference for the formation of [3 + 3] products is also observed in reactions of DACH with other linear dialdehydes (with aldehyde groups rigidly positioned at 180 degrees) leading to a rich family of trianglimines. A similar reaction of DACH with 1,3-isophthalaldehyde or its derivatives leads to [3 + 3] products called isotrianglimines, such as macrocycle 4 (Figure 3) or macrocycle 38 (Figure 14). As was already mentioned above, in the case of the condensation of isophthalaldehydes or DFP with enantiopure DACH, the [3 + 3] products 38 and 40 (Figure 14), respectively, are accompanied by [2 + 2] macrocyclic products. A similar situation was observed in the case of thiophene derivatives (Figure 15) where both macrocycles 42 and 43 were formed when the reaction was run in methanol. In contrast, the [3 + 3] macrocycle 42 was the sole product when the reaction was run in dichloromethane [25]. The introduction of the phenol group also influences the condensation reaction, the hydroxyl derivative of isophthalaldehyde, that is 2,6-diformyl-4-methylphenol, reacts with enantiopure DACH in acetonitrile to give macrocycle 39 as the sole product in practically quantitative yield (Figure 14) [85]. These and related macrocycles are called calixsalens and the phenolic macrocycles derived from dihydroxyisophthalaldehydes are called resorcisalens.
Figure 14.
Examples of [3 + 3] imines derived from DACH.
Figure 15.
Macrocycles from 2,6-diformylthiophene.
The rich family of trianglimines, isotrianglimines, calixsalens and resorcisalens has recently been the subject of an excellent review [8] and will not be discussed in detail. Only a few selected examples with special emphasis on metal derivatives of [3 + 3] imines and amines will be presented here.
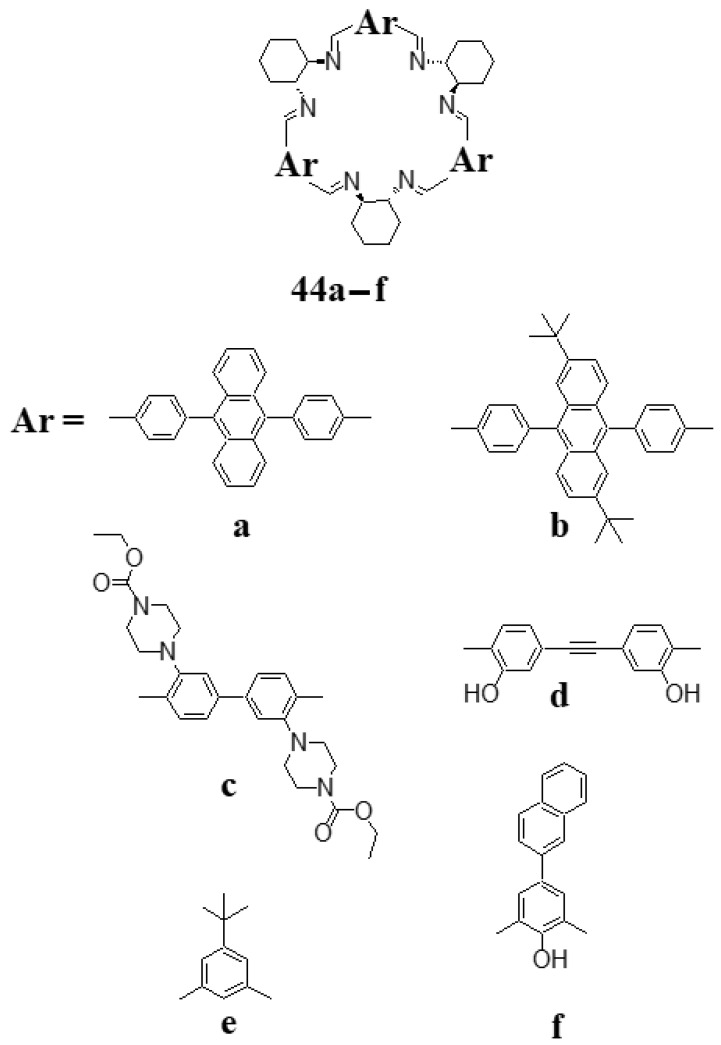
The application of extended rigid linear aromatic dialdehydes leads to expanded triangular [3 + 3] molecules, such as macrocycles 44a–d (Figure 16). In a recent paper [86] Grajewski et al. described the synthesis of the extended trianglimine 44a and its amine counterpart, as well as the corresponding endoperoxide derivatives of these macrocycles. Additionally, the reversible cycloaddition of singlet oxygen to anthracene fragments of 44a without degradation of the macrocyclic system was demonstrated. Olson et al. demonstrated luminescent properties of extended trianglimines, such as 44c and the corresponding trianglamines [87]. Both theoretical DFT optimized structures and experimental X-ray structures indicate that in this and similar imines the six large substituents appended to the biphenyl legs of the trianglimine macrocycles adopt an alternating conformation. In this conformation, these substituents partly close the space above and below the mean trianglimine plane, which leads to the formation of a kind of container molecule.
Figure 16.
Examples of macrocyclic [3 + 3] imines derived from DACH.
The [3 + 3] imines, such as 8, 39, 38, 44e and 44f exhibit rich supramolecular chemistry. They can act as hosts for small organic guest molecules, form dimers and capsules or self-associate into larger structures. The formation of various noncovalent aggregates of these macrocycles was demonstrated by Kwit, Janiak and others for solid, liquid, and gas phases [88,89,90,91,92,93,94,95].
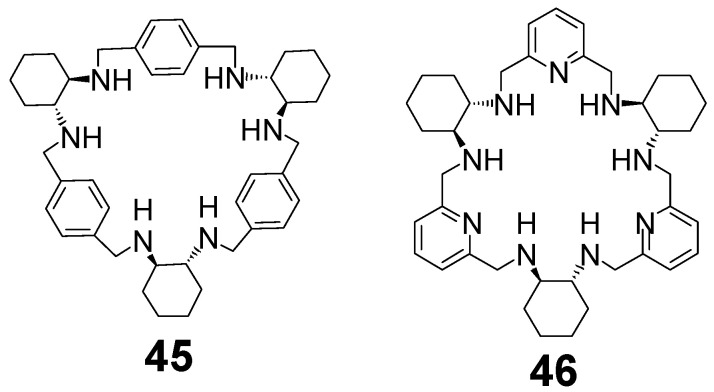
The reduced form of trianglimine 37 is trianglamine 45 (Figure 17) This macrocycle is able to bind zinc(II) ions and this enantiopure complex generated in situ is an active catalyst in the enantioselective hydrosilylation of imines [96] and ketones. This trianglamine as well as its derivative, where methylene bridges are substituted with additional phenyl rings, functions as an efficient chiral solvating agent in the enantiodiscrimination of chiral carboxylic acids [97,98]. Calixsalene 39 and its derivatives were used in enantioselective recognition of carboxylic acids on the basis of NMR [99,100]. The expanded macrocycle 44d has three chiral salen-like compartments and is predisposed to form trinuclear complexes [101].
Figure 17.
[3 + 3] amine macrocycles.
While the metal complexes of [3 + 3] imine 40 derived from DACH and DFP were not isolated in a pure form, the condensation of enantiopure DACH in the presence of cadmium chloride shifts the equilibrium to a dinuclear complex of this imine, which can be reduced and demetallated to the corresponding amine 46 (Figure 17) [102]. Similarly, when racemic DACH is used in this condensation reaction, the cadmium(II) template shifts the equilibrium of imine products into a heterochiral [3 + 3] imine 41, which can subsequently be reduced to heterochiral amine [103]. The amine 46 is able to bind two or three transition metal ions [104,105] or a single lanthanoid(III) ion [35,106,107]. In the latter type of complex, the large macrocycle wraps around the metal ion to form a double-helical conformation (Figure 18). A rare process of helicity inversion of 46 between the kinetic and thermodynamic complexation product was observed for these complexes. A similar process was observed in the case of complexes of heterochiral RRRRSS analog of 46 derived from imine 41 [108].
Figure 18.
Structures of trinuclear copper(II) (left) and mononuclear ytterbium(II) (right) complexes of macrocycle 46. Metal ions are shown in spacefill representation, hydrogen atoms and additional anions omitted for simplicity.
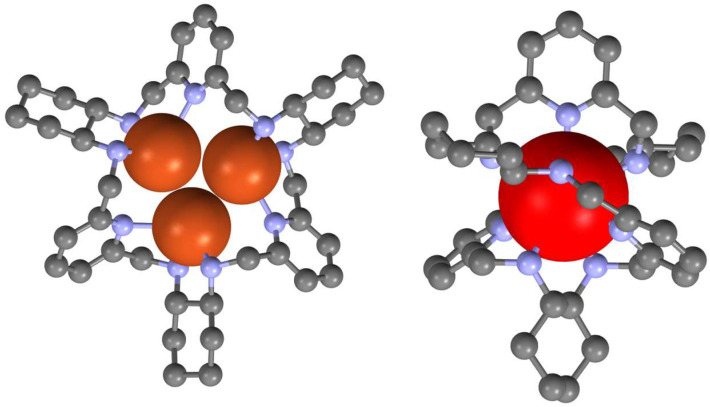
The reactions of [3 + 3] triphenolic calixsalen macrocycles, such as 8 and 39 or similar imines with metal salts do not lead to complexes of these macrocycles but to complexes of the contracted [2 + 2] forms or acyclic ligands [23,24,109]. For instance, the reaction of 8 or 39 with an excess of zinc(II) acetate leads to dinuclear complexes of 5b and 5a, respectively. In contrast, when 8 is reacted with zinc(II) acetate in a 2:3 molar ratio, a metal-organic cage complex of deprotonated macrocycle [Zn382] is formed (Figure 19) [27,28]. This barrel-shaped molecule has an empty interior. This cavity may be occupied by solvent molecules or gas molecules which results in remarkable gas sorption properties for some crystalline forms. Moreover, this chiral complex exhibits enantioselective binding of small guest molecules, such as 2-butanol (Figure 19). The ability to bind guest molecules and the enantiopure nature of [Zn382] was the basis of its application in the enantioseparations of chiral organic compounds by using gas chromatography [110] or capillary electrochromatography [111,112].
Figure 19.
Metal-organic cage based on two macrocyclic units of 8. Zinc atoms (violet) and (S)-2-butanol are in spacefill representation.
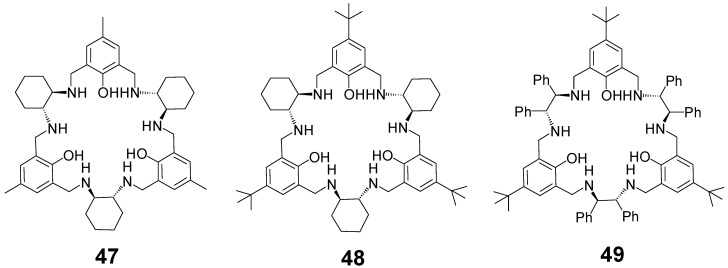
In contrast to the [3 + 3] calixsalene imine macrocycles, such as 39, their amine counterparts, such as 47–49 (Figure 20) easily form trinuclear complexes with transition metals [113,114]. In zinc(II) complexes of this type, a synergistic enantioselective effect of the three metal centers was observed in catalytic asymmetric aldol and Henry reactions [115]. These macrocycles are also able to bind one, two or three larger lanthanoid(III) ions in their cavities [116,117,118,119,120]. In these complexes, a trinuclear lanthanoid dihydroxo cluster is bound in the center of the macrocycle. The metal ions in these lanthanoid(III) complexes are additionally linked by phenoxo, as well as hydroxo bridges, which are associated with their magnetic properties.
Figure 20.
Amine counterparts of calixsalenes.
The chiral [3 + 3] imine 50 (Figure 21) is obtained in template condensation, where two types of metal ions are used simultaneously, thus three smaller transition metal ions, such as zinc(II) are bound in the three salen-type compartments, while the larger lanthanoid(III) ions occupy the central O6 cavity formed by deprotonated phenolic groups [121]. The Er(III) complex of this type exhibits remarkable single molecule magnet properties.
Figure 21.
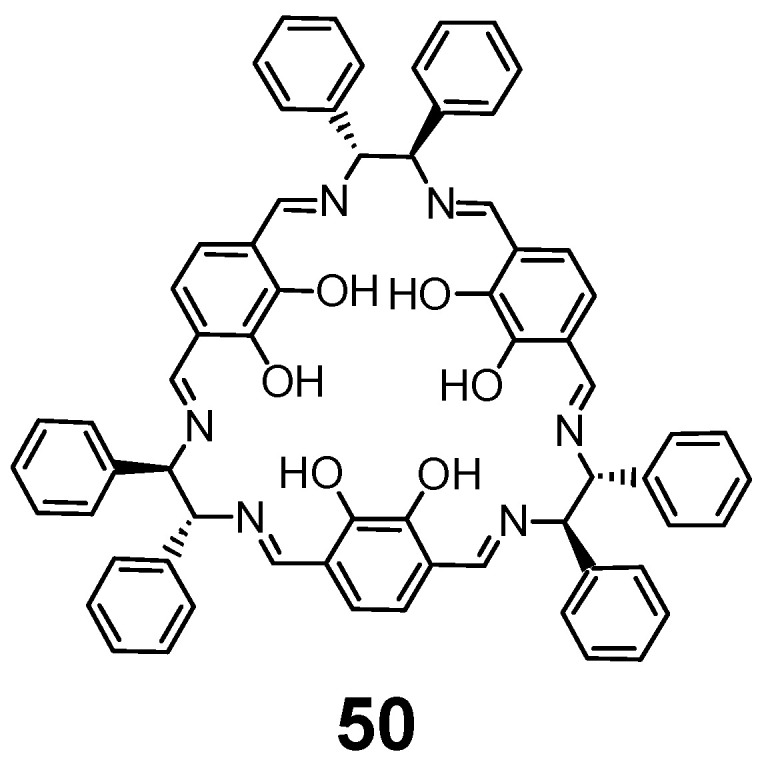
[3 + 3] macrocycle that is obtained in template condensation as a Zn3Er complex.
4. [4 + 4] Macrocycles
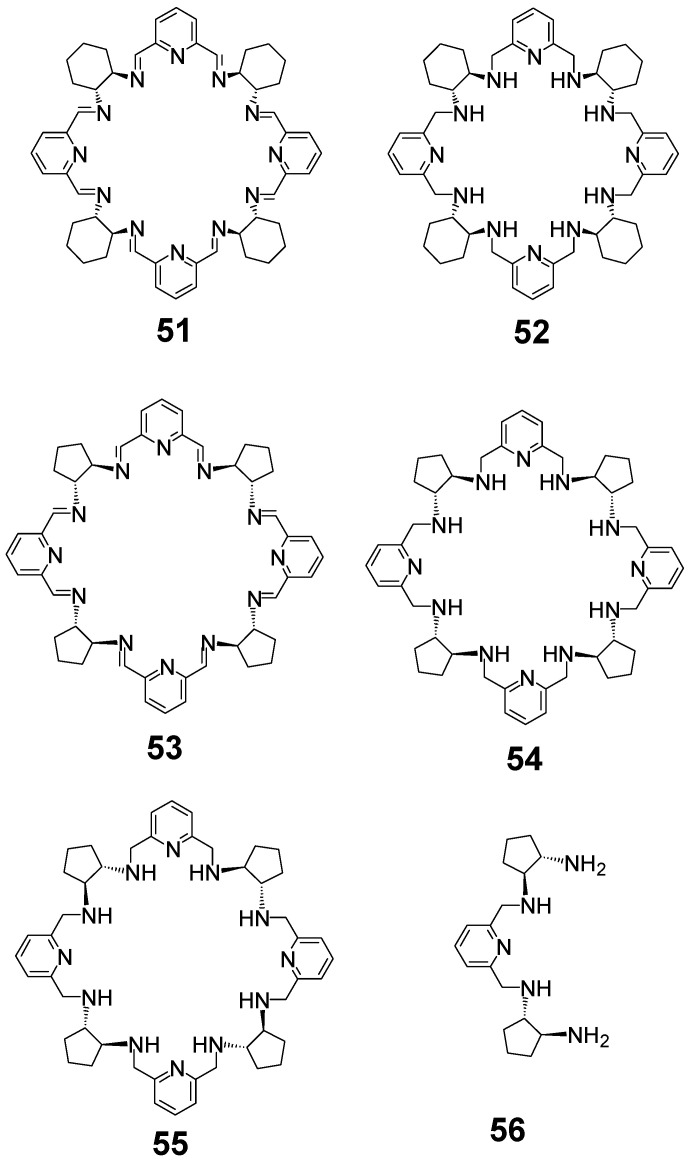
The condensation of racemic DACH with DFP results in a mixture consisting mainly of meso-type achiral [2 + 2] macrocycle 10 and [4 + 4] macrocycle 51 of the alternating RRSSRRSS chirality of the cyclohexane fragments (Figure 22). The latter macrocycle can be separated and then reduced to the corresponding amine 52 [36]. In a similar reaction involving racemic DACP, only the [2 + 2] macrocycle is formed in methanol, but a mixture of macrocycles is formed in benzene, from which solvent meso-type achiral [4 + 4] imine 53 can be isolated and converted into a corresponding [4 + 4] amine 54 [122]. The crystal structure of 51 indicates a benzene guest molecule that is held in the center of the macrocycle via CH-π interactions (Figure 23). Amine 55 (all—(S) enantiomer), which is a homochiral isomer of amine 54, may be isolated in small (5.6 %) yields by using gel permeation chromatography from the mixture of macrocyclic amines which are obtained by the reduction of the mixture of imines resulting from the condensation of enantiopure DACP and DFP (the main product being the [3 + 3] macrocycle). Alternatively, amine 55 may be obtained in high (52.2 %) yield in step-wise synthesis from the intermediate 56 and DFP [123]. Apart from meso amine 54 of RRSSRRSS chirality of diaminocyclohexane fragments and its homochiral isomer 55 of SSSSSSSS chirality, other isomers of RRRRSSSS (achiral) and RRRRRRSS (enantiopure) chirality may be obtained in step-wise synthesis via protection/deprotection strategy of linear intermediates [124].
Figure 22.
[4 + 4] macrocycles derived from DFP.
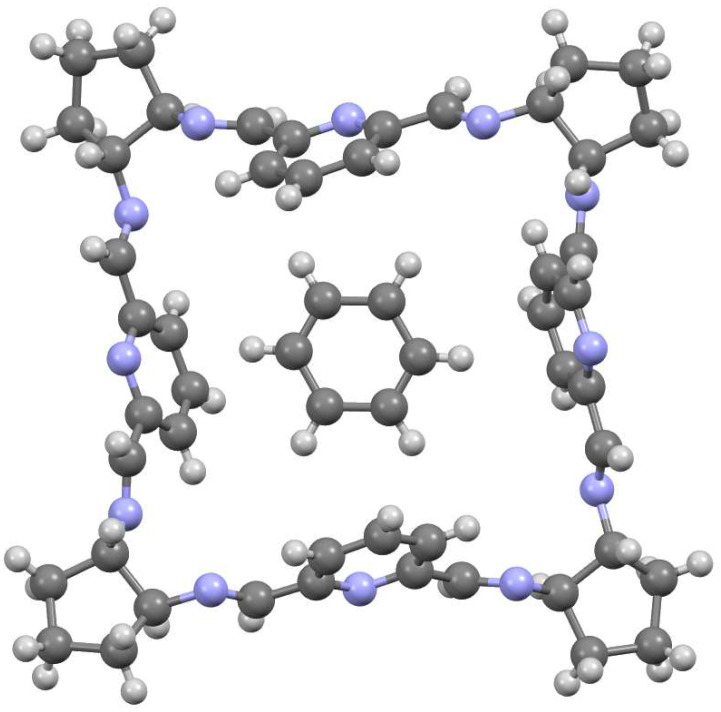
Figure 23.
Molecular structure of 51 with benzene guest molecule.
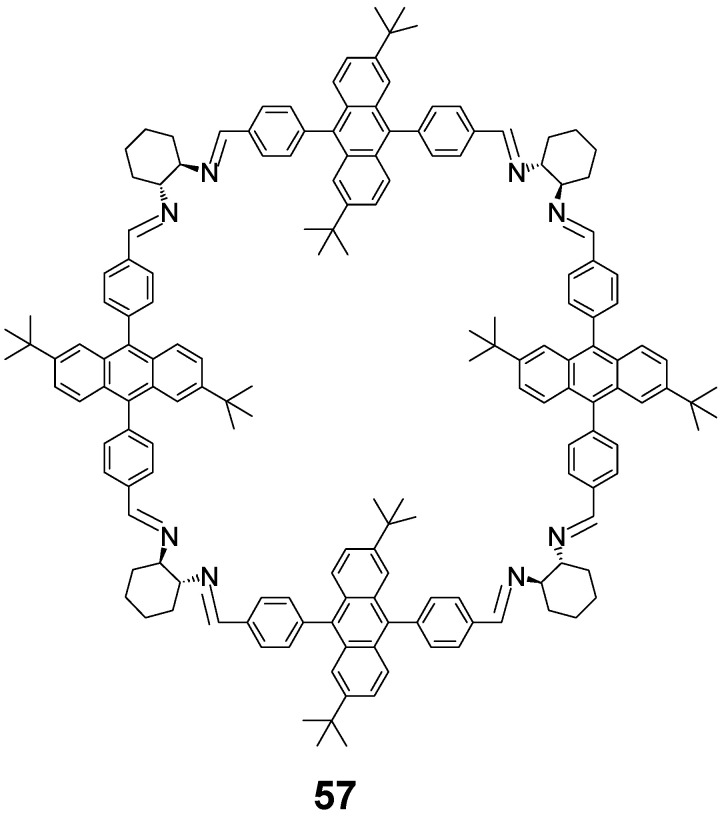
The reaction of an extended linear dialdehyde with DACH in boiling toluene and in the presence of p-toluenesulfonic acid results in a mixture of [3 + 3] imine 44b and [4 + 4] imine 57 (Figure 24). Heating the mixture of these macrocycles in p-xylene shifts the equilibrium completely towards the large macrocycle 57 [125]. The condensation of the substrates directly in p-xylene also led to 57 as the main product. These effects were not based on applying simply different reaction temperatures, which indicates a real template role of this solvent. The plausible template role of p-xylene, which is retained in the final product, may be based on the preorganization of substrates and/or stabilization of the [4 + 4] structure via CH-π and π-π interactions. The theoretical DFT structure of 57 suggests that the interior of this giant macrocycle is partly occupied by tert-butyl substituted 9,10-diphenylanthracene fragments. On the other hand, in the calculated structure of the amine counterpart of 57, these fragments are perpendicular to the mean macrocycle plane. In this way, a nano-sized square box is formed with a distance between the anthracene units equal to 2.14 nm. Another interesting feature of 57 is the exceptionally strong amplitude of its electronic circular dichroism spectra.
Figure 24.
Giant [4 + 4] macrocycle 57 obtained in p-xylene-templated reaction.
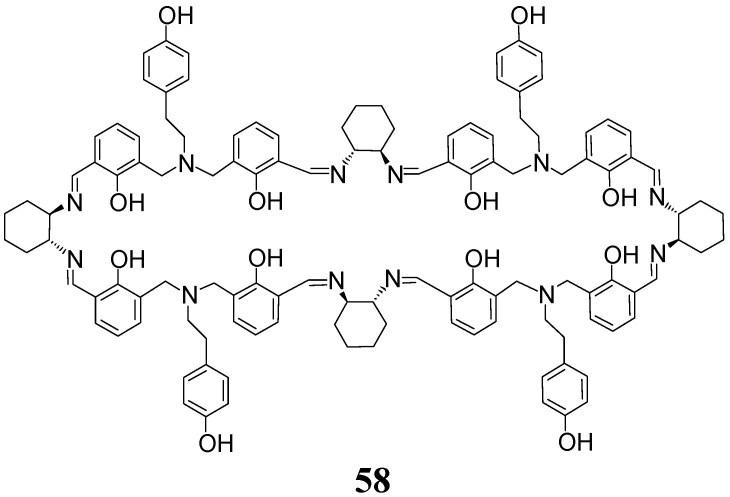
A tetranuclear zinc(II) complex of [4 + 4] macrocycle 58 (Figure 25) can be obtained in a template reaction by using the same enantiopure DACH, the same aldehyde substrates and the same templating cation, which were used for the synthesis of dinuclear zinc(II) complex of the [2 + 2] macrocycle 27 [72]. In this system, an interesting secondary template effect of counterions is observed. Thus, the application of zinc(II) nitrate as a templating salt led to [2 + 2] imine, while the application of zinc(II) chloride as a templating salt led to [4 + 4] imine. This result indicates that not only the kind of the metal cation but also the kind of counter-anion may shift the equilibrium of imine condensation products towards a specific macrocycle.
Figure 25.
[4 + 4] imine obtained in anion-dependent template condensation with zinc(II).
5. [6 + 6] and [8 + 8] Macrocycles
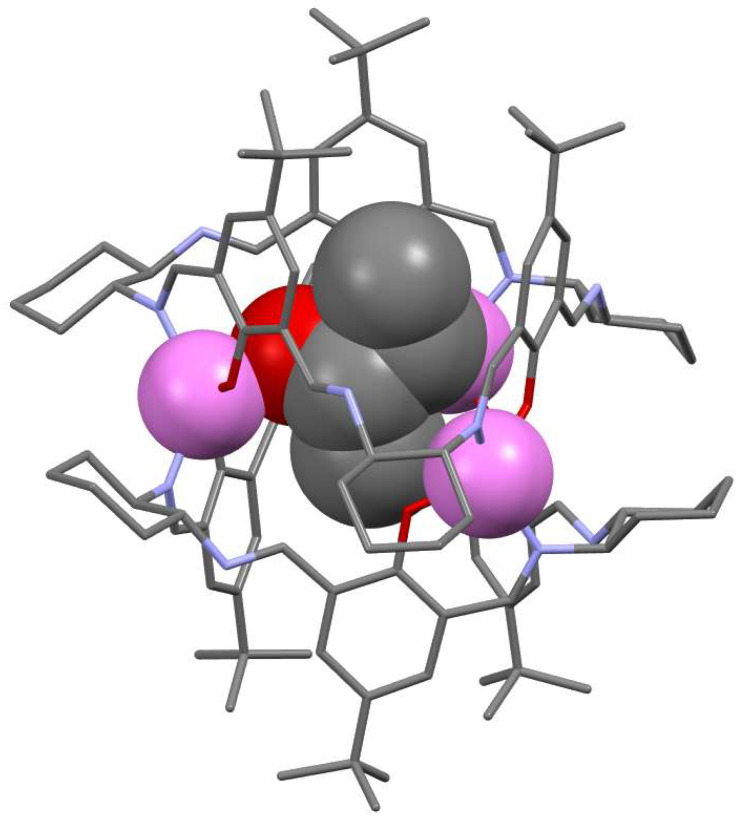
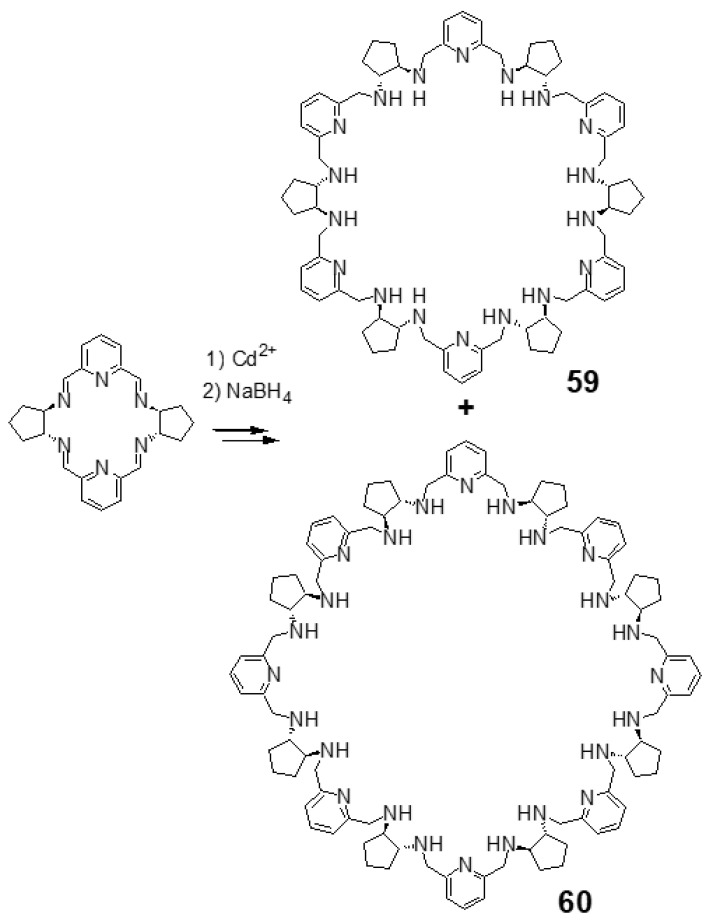
The reaction of meso-type [2 + 2] imine 10 with cadmium(II) chloride, followed by reduction with sodium borohydride leads to a giant [6 + 6] macrocyclic amine 59 as the main product and [8 + 8] amine 60 as the minor product (Figure 26) [122,126]. The X-ray crystal structure of the protonated 59 indicates a multiply folded macrocycle of a globular shape, which is a host molecule for four chloride anions and two solvent molecules (water or acetonitrile) embedded in the macrocycle (Figure 27). Similarly, the protonated form of the larger [8 + 8] macrocycle adopts globular multiply folded conformation and binds anion guest in the center (Figure 28). The neutral macrocycle 59 is predisposed for the binding of six transition metal cations, e.g., zinc(II) (Figure 28) and it adopts various conformations in these complexes [127]. It can also form a trinuclear zinc(II) complex, where the sections of the macrocycle wrap around the metal centers and the complex binds the chloride anion guest in the center (Figure 28).
Figure 26.
Expansion of the [2 + 2] macrocycle into giant meso-type macrocycles.
Figure 27.
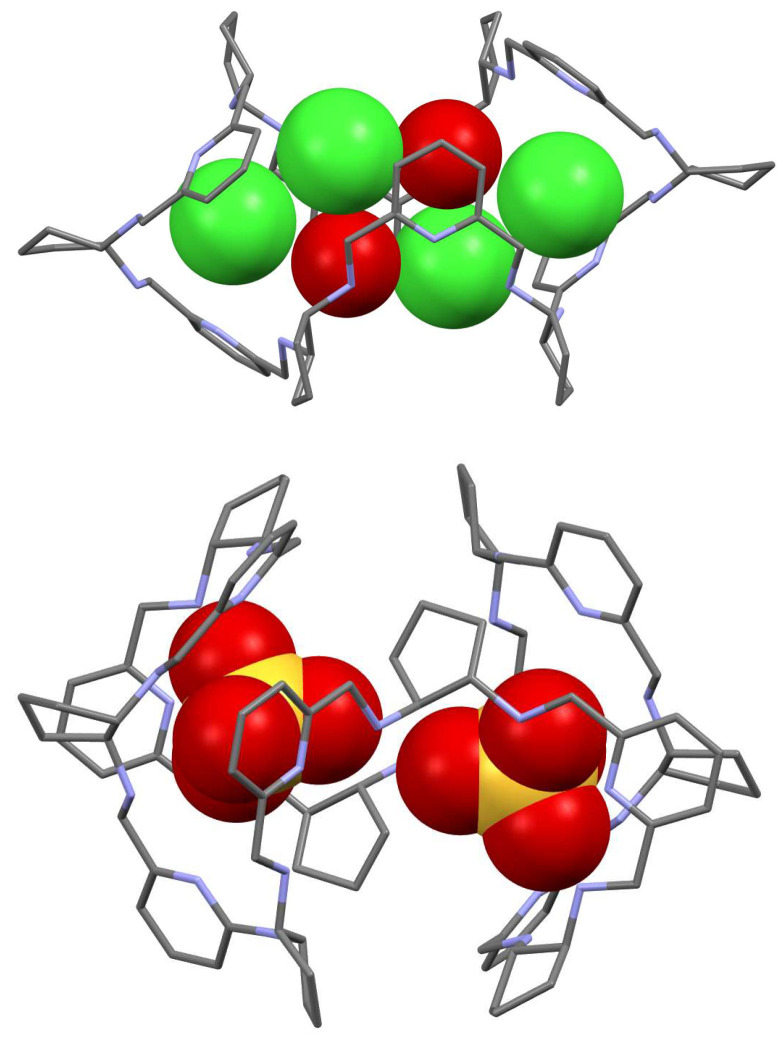
Top—structure of protonated [6 + 6] amine 59 embracing two chloride anions and two water molecules. Bottom—structure of protonated [8 + 8] amine 60 embracing two sulfate anions (chlorides, sulfates and water molecules are shown in spacefill representation, hydrogen atoms are omitted).
Figure 28.
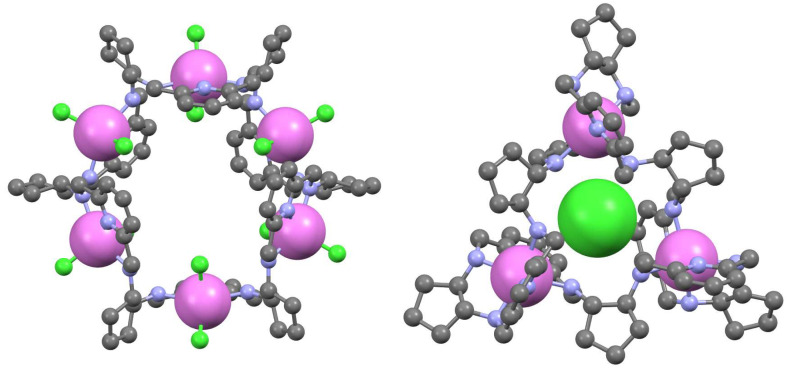
Molecular structures of the hexanuclear (left) and trinuclear (right) zinc(II) complexes of macrocycle 59. Metal ions (violet) and the central chloride guest anion (green) are shown in spacefill representation, hydrogen atoms are omitted.
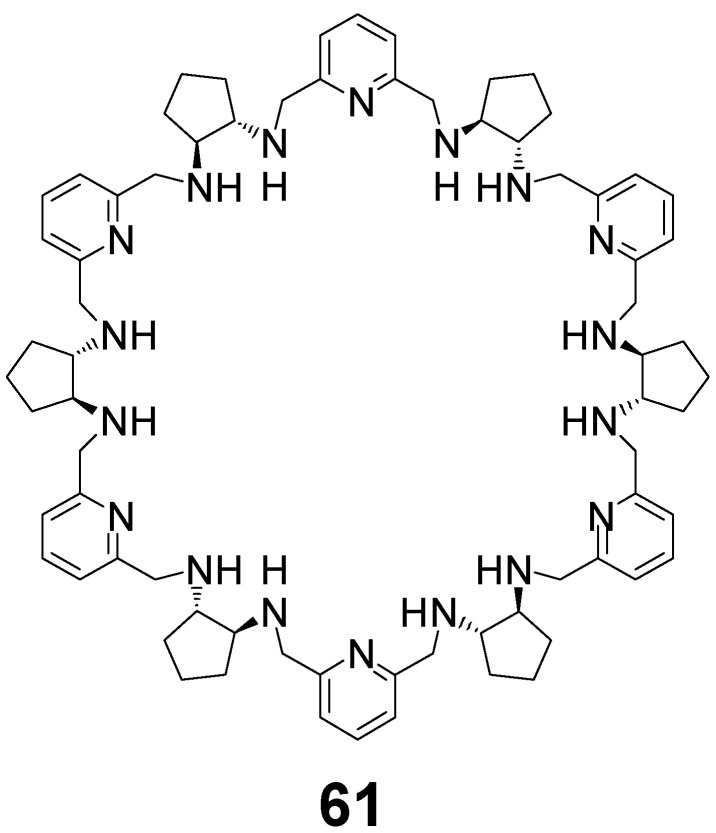
The direct condensation of enantiopure DACP with DFP followed by reduction with sodium borohydride leads to homochiral [6 + 6] amine 61 in trace amounts only (Figure 29) together with traces of [5 + 5] and [7 + 7] macrocycles. Macrocycle 61 can be isolated, however, in 10% yield by using gel permeation chromatography as a minor product accompanying the formation of homochiral [4 + 4] macrocycle 55 from the intermediate 56.
Figure 29.
[6 + 6] homochiral macrocycle derived from DACP.
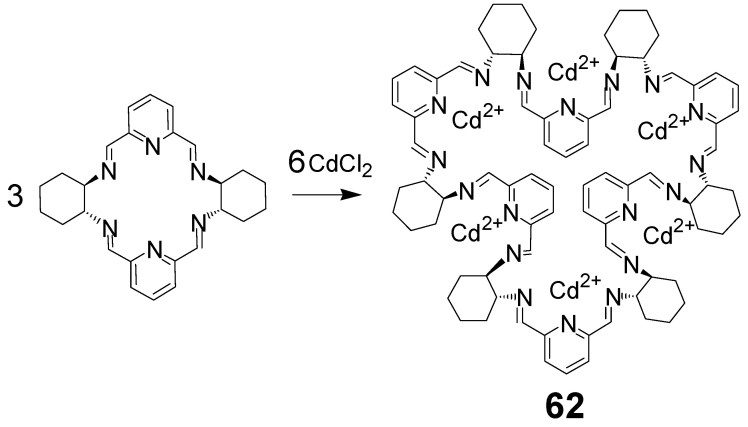
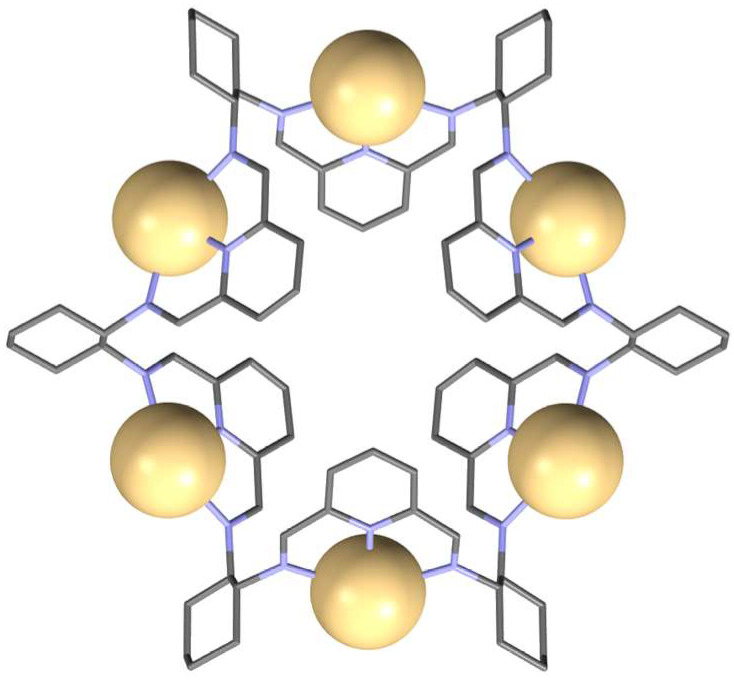
The X-ray crystal structures of the protonated 59 and its hexanuclear metal complexes show characteristic multiple folding of the macrocycle leading to the formation of six loops containing three nitrogen donor atoms each. This suggests that ring expansion of the [2 + 2] macrocycle under the influence of the templating Cd(II) ions is based on the reversible breaking of one of the imine bonds of the smaller macrocycle and reassembling of the linear fragments with the formation of six N3 sections for the coordination of six metal ions. Indeed, this hypothesis was confirmed in the case of an analogous reaction involving DACH instead of DACP derivatives [128]. In this case, the intermediate hexanuclear Cd(II) complex of the [6 + 6] imine macrocycle 62 was isolated and its X-ray crystal structure was determined (Figure 30 and Figure 31).
Figure 30.
Macrocycle expansion reaction triggered by cadmium(II) template.
Figure 31.
Molecular structure of hexanuclear Cd(II) complex of 62 (Cd atoms in spacefill representation, hydrogen atoms and chloride anions are omitted).
6. Macrocycles Containing Different Diamine Fragments
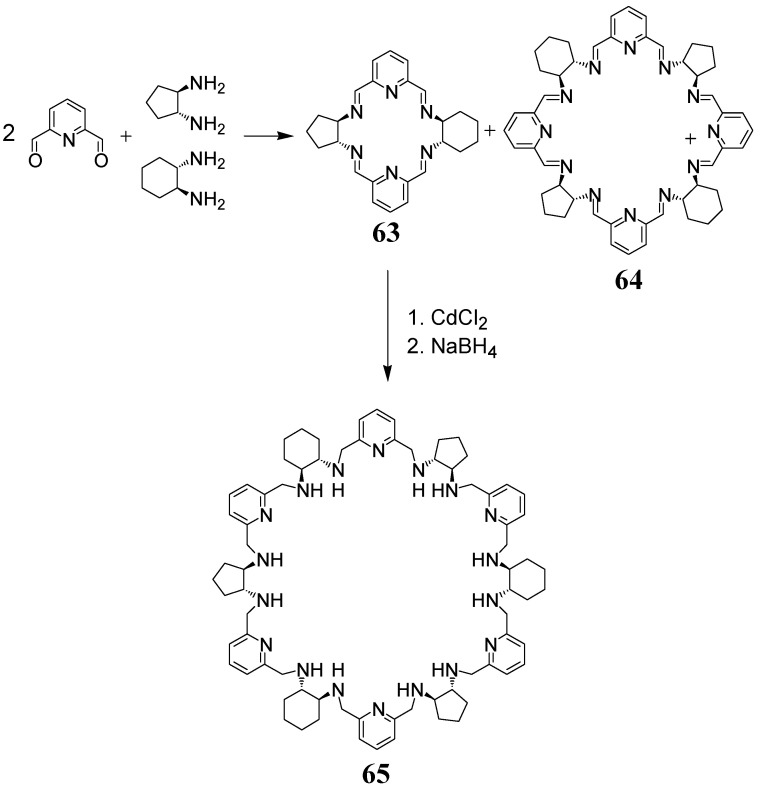
The condensation of DFP with an equimolar mixture of two enantiopure diamines of opposite chirality, e.g., trans-(1S, 2S)- diaminocyclohexane and its cyclopentane analog trans-(1R, 2R)- diaminocyclopentane in methanol results in the isolation of [2 + 1 + 1] macrocycle 63 (Figure 32) [129]. In contrast, the same condensation in benzene allows for the isolation of a larger [4 + 2 + 2] macrocycle 64 (Figure 32). The above equimolar mixture of diamines may be regarded as a quasi-racemic analog of racemic DACH, while the macrocycles 63 and 64 may be regarded as analogs of the meso-type macrocycles 10 and 51, respectively. It should be mentioned that the former two macrocycles are chiral and they can be obtained as pure enantiomers. This is because cyclohexane and cyclopentane rings are not equivalent, hence these macrocycles do not have the improper Sn symmetry axes present in the meso-type macrocycles. Similarly, a large [6 + 3 + 3] macrocycle 65 (Figure 32), which can be obtained in macrocycle expansion reaction of 63 under the template action of cadmium(II) ions followed by reduction to the amine form, is an enantiopure chiral analog of meso-type macrocycle 59 but has no S6 symmetry axis.
Figure 32.
Macrocycles containing DACH and DACP rings.
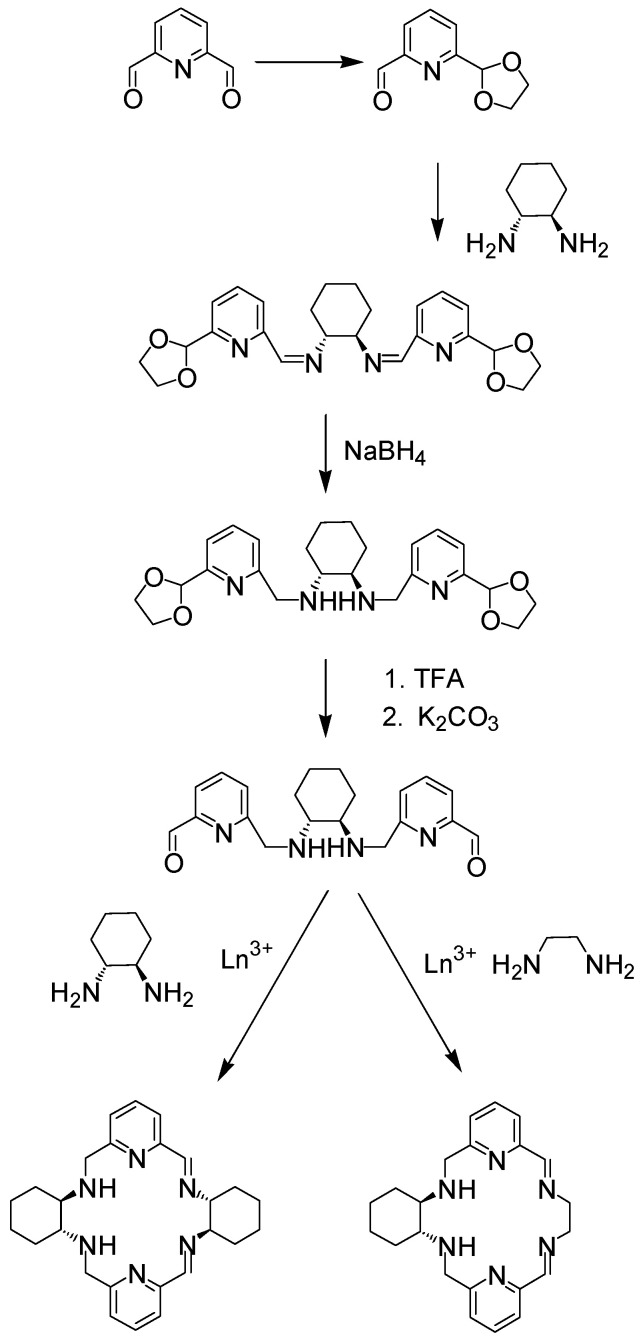
Mixed imine/amine unsymmetrical [2 + 2] or [2 + 1 + 1] macrocycles can be obtained as lanthanoid(III) complexes in a multi-step synthesis involving protection/deprotection reactions (Figure 33) [130].
Figure 33.
Step-wise synthesis of unsymmetrical imine/amine macrocycles.
7. Conclusions
The [n + n] condensation of aromatic dialdehydes with chiral diamines is in many cases, a remarkably simple reaction that leads to defined imine macrocycles of various sizes and shapes. These expanded macrocycles, as well as their reduced amine counterparts, typically possess multiple donor atoms which predispose them to bind multiple metal ions. The cooperative catalytic activity of these ions combined with the enantiopure nature of the complexes of chiral macrocycles may lead to efficient enantioselective catalytic systems. The binding of multiple metal ions in close vicinity within the core of a large macrocycle may also result in magnetic interactions among these ions leading to new magnetic materials. The large macrocycles of this type often have the tendency to form supramolecular systems. In particular, they can function as hosts for the enantioselective binding of chiral organic guest molecules. This recognition process may find applications in the analysis and separation of enantiomers. The simplicity of the synthesis of the [n + n] imines and amines allows rather rational planning of new macrocycles of this type. Nevertheless, the final size of such condensation products depends not only on the kind of the used substrates, but sometimes it depends on the unexpected influence of the metal template, solvent, time and temperature of the condensation reaction, or chirality of the diamine. The effectiveness of this approach to the synthesis of macrocycles and the richness of the structures obtained so far undoubtedly indicates that in the future many new elaborate macrocyclic systems of this type will be obtained. In particular, further development of enantiopure multimetallic catalysts based on [n + n] macrocycles are desirable. Future progress in this field can be based on fine-tuning the chiral environment around the catalytic metal center, introducing additional steric hindrance and trying various metal centers. More advanced heterometallic macrocyclic complexes for enantioselective catalysis may be envisaged, in which the macrocycle will embrace different metal ions. The cooperative action of different metal centers with different chemical characters may potentially lead to new synergistic reactivity, different from that of the corresponding homometallic complexes. Yet another design that may lead to new catalytic effects may be based on mononuclear metal complexes of large [n + n] macrocycles. In such complexes, only part of the large macrocyclic cavity would be occupied by a catalytic metal ion, leaving the rest of the macrocyclic cavity for enantioselective binding of the guest substrate molecule. Moreover, in such a mononuclear complex of a large macrocyclic amine, there is an additional possibility for the cooperative action of Lewis acids and Brønsted base/acids in the catalytic cycle, similarly as has been observed in many metalloenzymes. Thus, the metal ion will act as a Lewis acid, while the part of the large macrocyclic amine that is not engaged in metal binding will act as a Brønsted base (or Brønsted acid if this part is partially protonated). Another prospective goal is to develop very large, shape-persistent [n + n] macrocycles of this kind for gas storage applications. For example, the introduction of additional basic substituents within the macrocycle may result in the preferential fixation of carbon dioxide.
Abbreviations
| DACH | 1,2-trans-diaminocyclohexane |
| DPEN | 1,2-diphenylethylenediamine |
| DCAP | trans-1,2-diaminocyclopentane |
| DFP | 2,6-diformylpyridine |
| SMM | Single Molecule Magnet |
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lindoy L.F., Park K.-M., Lee S.S. Metals, macrocycles and molecular assemblies-macrocyclic complexes in metallo-supramolecular chemistry. Chem. Soc. Rev. 2013;42:1713–1727. doi: 10.1039/C2CS35218D. [DOI] [PubMed] [Google Scholar]
- 2.Liu Z., Nalluri S.K.M., Stoddart J.F. Surveying macrocyclic chemistry: From flexible crown ethers to rigid cyclophanes. Chem. Soc. Rev. 2017;46:2459–2478. doi: 10.1039/C7CS00185A. [DOI] [PubMed] [Google Scholar]
- 3.Rezaeivala M., Keypour H. Schiff base and non-Schiff base macrocyclic ligands and complexes incorporating the pyridine moiety–The first 50 years. Coord. Chem. Rev. 2014;280:203–253. doi: 10.1016/j.ccr.2014.06.007. [DOI] [Google Scholar]
- 4.Marti-Centelles V., Pandey M.D., Burguete M.I., Luis S.V. Macrocyclization Reactions: The Importance of Conformational, Configurational, and Template-Induced Preorganization. Chem. Rev. 2015;115:8736–8834. doi: 10.1021/acs.chemrev.5b00056. [DOI] [PubMed] [Google Scholar]
- 5.Alfonso I. Chiral Molecular Receptors Based on Trans-Cyclohexane-1,2-diamine. Curr. Org. Synth. 2010;7:1–23. doi: 10.2174/157017910790820364. [DOI] [Google Scholar]
- 6.Borisova N.E., Reshetova M.D., Ustynyuk Y.A. Metal-Free Methods in the Synthesis of Macrocyclic Schiff Bases. Chem. Rev. 2007;107:46–79. doi: 10.1021/cr0683616. [DOI] [PubMed] [Google Scholar]
- 7.Grajewski J. Recent Advances in the Synthesis and Applications of Nitrogen-Containing Macrocycles. Molecules. 2022;27:1004. doi: 10.3390/molecules27031004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwit M., Grajewski J., Skowronek P., Zgorzelak M., Gawroński J. One-Step Construction of the Shape Persistent, Chiral but Symmetrical Polyimine Macrocycles. Chem. Rec. 2019;19:213–237. doi: 10.1002/tcr.201800052. [DOI] [PubMed] [Google Scholar]
- 9.Tashiro S., Shionoya M. Novel Porous Crystals with Macrocycle-Based Well-Defined Molecular Recognition Sites. Acc. Chem. Res. 2020;53:632–643. doi: 10.1021/acs.accounts.9b00566. [DOI] [PubMed] [Google Scholar]
- 10.Xia D., Wang P., Ji X., Khashab N.M., Sessler J.L., Huang F. Functional Supramolecular Polymeric Networks: The Marriage of Covalent Polymers and Macrocycle-Based Host–Guest Interactions. Chem. Rev. 2020;120:6070–6123. doi: 10.1021/acs.chemrev.9b00839. [DOI] [PubMed] [Google Scholar]
- 11.He Q., Vargas-Zúñiga G.I., Kim S.H., Kim S.K., Sessler J.L. Macrocycles as Ion Pair Receptors. Chem. Rev. 2019;119:9753–9835. doi: 10.1021/acs.chemrev.8b00734. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhry M.T., Akine S., MacLachlan M.J. Contemporary macrocycles for discrete polymetallic complexes: Precise control over structure and function. Chem. Soc. Rev. 2021;50:10713–10732. doi: 10.1039/D1CS00225B. [DOI] [PubMed] [Google Scholar]
- 13.Wagner-Wysiecka E., Łukasik N., Biernat J.F., Luboch E. Azo group(s) in selected macrocyclic compounds. J. Incl. Phenom. Macrocycl. Chem. 2018;90:189–257. doi: 10.1007/s10847-017-0779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nath B.D., Takaishi K., Ema T. Macrocyclic multinuclear metal complexes acting as catalysts for organic synthesis. Catal. Sci. Technol. 2020;10:12–34. doi: 10.1039/C9CY01894H. [DOI] [Google Scholar]
- 15.Belowich M.E., Stoddart J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012;41:2003–2024. doi: 10.1039/c2cs15305j. [DOI] [PubMed] [Google Scholar]
- 16.Ciaccia M., Di Stefano S. Mechanisms of imine exchange reactions in organic solvents. Org. Biomol. Chem. 2015;13:646–654. doi: 10.1039/C4OB02110J. [DOI] [PubMed] [Google Scholar]
- 17.Dhers S., Holub J., Lehn J.-M. Coevolution and ratiometric behaviour in metal cation-driven dynamic covalent systems. Chem. Sci. 2017;8:2125–2130. doi: 10.1039/C6SC04662B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein J.M., Saggiomo V., Reck L., Lüning U., Sanders J.K.M. Dynamic combinatorial libraries for the recognition of heavy metal ions. Org. Biomol. Chem. 2012;10:60–66. doi: 10.1039/C1OB05976A. [DOI] [PubMed] [Google Scholar]
- 19.Kołodziejski M., Stefankiewicz A.R., Lehn J.-M. Dynamic polyimine macrobicyclic cryptands-self-sorting with component selection. Chem. Sci. 2019;10:1836–1843. doi: 10.1039/C8SC04598D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matache M., Bogdan E., Hădade N.D. Selective Host Molecules Obtained by Dynamic Adaptive Chemistry. Chem.—Eur. J. 2014;20:2106–2131. doi: 10.1002/chem.201303504. [DOI] [PubMed] [Google Scholar]
- 21.Rue N.M., Sun J., Warmuth R. Polyimine Container Molecules and Nanocapsules. Isr. J. Chem. 2011;51:743–768. doi: 10.1002/ijch.201100064. [DOI] [Google Scholar]
- 22.Ziach K., Jurczak J. Controlling and Measuring the Equilibration of Dynamic Combinatorial Libraries of Imines. Org. Lett. 2008;10:5159–5162. doi: 10.1021/ol802121s. [DOI] [PubMed] [Google Scholar]
- 23.Gao J., Reibenspies J.H., Zingaro R.A., Woolley F.R., Martell A.E., Clearfield A. Novel chiral “calixsalen” macrocycle and chiral Robson-type macrocyclic complexes. Inorg. Chem. 2005;44:232–241. doi: 10.1021/ic049181m. [DOI] [PubMed] [Google Scholar]
- 24.Korupoju S.R., Mangayarkarasi N., Ameerunisha S., Valente E.J., Zacharias P.S. Formation of dinuclear macrocyclic and mononuclear acyclic complexes of a new trinucleating hexaaza triphenolic Schiff base macrocycle: Structure and NLO properties. J. Chem. Soc. Dalton Trans. 2000;16:2845–2852. doi: 10.1039/b002700f. [DOI] [Google Scholar]
- 25.Kuhnert N., Rossignolo G.M., Lopez-Periago A. The synthesis of trianglimines: On the scope and limitations of the 3 + 3 cyclocondensation reaction between (1R,2R)diaminocyclohexane and aromatic dicarboxaldehydes. Org. Biomol. Chem. 2003;1:1157–1170. doi: 10.1039/b212102f. [DOI] [PubMed] [Google Scholar]
- 26.Kuhnert N., Lopez-Periago A., Rossignolo G.M. The synthesis and conformation of oxygenated trianglimine macrocycles. Org. Biomol. Chem. 2005;3:524–537. doi: 10.1039/b414747m. [DOI] [PubMed] [Google Scholar]
- 27.Janczak J., Prochowicz D., Lewiński J., Fairen-Jimenez D., Bereta T., Lisowski J. Trinuclear Cage-Like ZnII Macrocyclic Complexes: Enantiomeric Recognition and Gas Adsorption Properties. Chem.—Eur. J. 2016;22:598–609. doi: 10.1002/chem.201503479. [DOI] [PubMed] [Google Scholar]
- 28.Sarnicka A., Starynowicz P., Lisowski J. Controlling the macrocycle size by the stoichiometry of the applied template ion. Chem. Commun. 2012;48:2237–2239. doi: 10.1039/c2cc16673a. [DOI] [PubMed] [Google Scholar]
- 29.Byun J.C., Lee N.H., Mun D.H., Park K.M. Synthesis and characterization of dinuclear copper(II) complexes, Cu-2( 20 -DCHDC) (L-a)(2) (L-a = N-3(-), NCS- or S2O32-) with tetraazadiphenol macrocyclic ligand having cyclohexane rings. Inorg. Chem. Commun. 2010;13:1156–1159. doi: 10.1016/j.inoche.2010.06.035. [DOI] [Google Scholar]
- 30.Gao J., Liu Y.-G., Zhou Y., Boxer L.M., Woolley F.R., Zingaro R.A. Artificial Zinc(II) Complexes Regulate Cell Cycle and Apoptosis-Related Genes in Tumor Cell Lines. ChemBioChem. 2007;8:332–340. doi: 10.1002/cbic.200600299. [DOI] [PubMed] [Google Scholar]
- 31.Gao J., Woolley F.R., Zingaro R.A. Catalytic asymmetric cyclopropanation at a chiral platform. Org. Biomol. Chem. 2005;3:2126–2128. doi: 10.1039/b503971a. [DOI] [PubMed] [Google Scholar]
- 32.Gao J., Reibenspies J.H., Martell A.E. Structurally Defined Catalysts for Enantioselective Oxidative Coupling Reactions. Angew. Chem. Int. Ed. 2003;42:6008–6012. doi: 10.1002/anie.200351978. [DOI] [PubMed] [Google Scholar]
- 33.Chinnaraja E., Arunachalam R., Samanta J., Natarajan R., Subramanian P.S. Desymmetrization of meso diols using enantiopure zinc (II) dimers: Synthesis and chiroptical properties. Appl. Organomet. Chem. 2019;33:e4827. doi: 10.1002/aoc.4827. [DOI] [Google Scholar]
- 34.Chinnaraja E., Arunachalam R., Pillai R.S., Peuronen A., Rissanen K., Subramanian P.S. One-pot synthesis of [2 + 2]-helicate-like macrocycle and 2 + 4-μ4-oxo tetranuclear open frame complexes: Chiroptical properties and asymmetric oxidative coupling of 2-naphthols. Appl. Organomet. Chem. 2020;34:e5666. doi: 10.1002/aoc.5666. [DOI] [Google Scholar]
- 35.Gregoliński J., Starynowicz P., Hua K.T., Lunkley J.L., Muller G., Lisowski J. Helical Lanthanide(III) Complexes with Chiral Nonaaza Macrocycle. J. Am. Chem. Soc. 2008;130:17761–17773. doi: 10.1021/ja805033j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregoliński J., Lisowski J., Lis T. New 2 + 2, 3 + 3 and 4 + 4 macrocycles derived from 1,2-diaminocyclohexane and 2,6-diformylpyridine. Org. Biomol. Chem. 2005;3:3161–3166. doi: 10.1039/b505909g. [DOI] [PubMed] [Google Scholar]
- 37.Lisowski J., Ripoli S., Di Bari L. Axial ligand exchange in chiral macrocyclic ytterbium(III) complexes. Inorg. Chem. 2004;43:1388–1394. doi: 10.1021/ic0353918. [DOI] [PubMed] [Google Scholar]
- 38.Lisowski J. Enantiomeric Self-Recognition in Homo- and Heterodinuclear Macrocyclic Lanthanide(III) Complexes. Inorg. Chem. 2011;50:5567–5576. doi: 10.1021/ic2001909. [DOI] [PubMed] [Google Scholar]
- 39.Starynowicz P., Lisowski J. Chirality transfer between hexaazamacrocycles in heterodinuclear rare earth complexes. Dalton Trans. 2019;48:8717–8724. doi: 10.1039/C9DT01318K. [DOI] [PubMed] [Google Scholar]
- 40.Ślepokura K., Cabreros T.A., Muller G., Lisowski J. Sorting Phenomena and Chirality Transfer in Fluoride-Bridged Macrocyclic Rare Earth Complexes. Inorg. Chem. 2021;60:18442–18454. doi: 10.1021/acs.inorgchem.1c03034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregoliński J., Ślepokura K. Monomeric and dimeric nitrate lanthanide(III) and yttrium(III) coordination compounds of (2 + 2) imine macrocycle derived from 2,6-diformylpyridine and trans-1,2-diaminocyclopentane. Polyhedron. 2020;181:114433. doi: 10.1016/j.poly.2020.114433. [DOI] [Google Scholar]
- 42.Paluch M., Gawryszewska P., Lis T., Lisowski J. Chiral macrocyclic lanthanide complexes derived from (R)-2,2′-diamino-1,1′-binaphthyl: X-ray crystal structure and luminescence studies. Polyhedron. 2010;29:3387–3393. doi: 10.1016/j.poly.2010.09.016. [DOI] [Google Scholar]
- 43.Mazurek J., Lisowski J. Chiral macrocyclic lanthanide complexes derived from (1R,2R)-1,2-diphenylethylenediamine and 2,6-diformylpyridine. Polyhedron. 2003;22:2877–2883. doi: 10.1016/S0277-5387(03)00406-6. [DOI] [Google Scholar]
- 44.Li Z.-H., Zhai Y.-Q., Chen W.-P., Ding Y.-S., Zheng Y.-Z., Li Z.H., Zhai Y.Q., Chen W.P., Ding Y.S., Zheng Y.Z. Air-Stable Hexagonal Bipyramidal Dysprosium(III) Single-Ion Magnets with Nearly Perfect D-6h Local Symmetry. Chem.—Eur. J. 2019;25:16219–16224. doi: 10.1002/chem.201904325. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Z.H., Zhao C., Feng T.T., Liu X.D., Ying X., Li X.L., Zhang Y.Q., Tang J.K. Air-Stable Chiral Single-Molecule Magnets with Record Anisotropy Barrier Exceeding 1800 K. J. Am. Chem. Soc. 2021;143:10077–10082. doi: 10.1021/jacs.1c05279. [DOI] [PubMed] [Google Scholar]
- 46.Krężel A., Lisowski J. Enantioselective cleavage of supercoiled plasmid DNA catalyzed by chiral macrocyclic lanthanide (III) complexes. J. Inorg. Biochem. 2012;107:1–5. doi: 10.1016/j.jinorgbio.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Gregoliński J., Ślepokura K., Lisowski J. Lanthanide complexes of the chiral hexaaza macrocycle and its meso-type isomer: Solvent-controlled helicity inversion. Inorg. Chem. 2007;46:7923–7934. doi: 10.1021/ic700831z. [DOI] [PubMed] [Google Scholar]
- 48.Gerus A., Ślepokura K., Lisowski J. Anion and Solvent Induced Chirality Inversion in Macrocyclic Lanthanide Complexes. Inorg. Chem. 2013;52:12450–12460. doi: 10.1021/ic401437r. [DOI] [PubMed] [Google Scholar]
- 49.Gerus A., Ślepokura K., Lisowski J. Carbonate-bridged dinuclear lanthanide(III) complexes of chiral macrocycle. Polyhedron. 2019;170:115–121. doi: 10.1016/j.poly.2019.05.005. [DOI] [Google Scholar]
- 50.Gospodarowicz K., Hołyńska M., Paluch M., Lisowski J. Novel chiral hexaazamacrocycles for the enantiodiscrimination of carboxylic acids. Tetrahedron. 2012;68:9930–9935. doi: 10.1016/j.tet.2012.09.093. [DOI] [Google Scholar]
- 51.González-Álvarez A., Alfonso I., Díaz P., García-España E., Gotor V. A highly enantioselective abiotic receptor for malate dianion in aqueous solution. Chem. Commun. 2006:1227–1229. doi: 10.1039/b517729d. [DOI] [PubMed] [Google Scholar]
- 52.Gonzalez-Alvarez A., Alfonso I., Diaz P., Garcia-Espana E., Gotor-Fernandez V., Gotor V. A simple helical macrocyclic polyazapyridinophane as a stereoselective receptor of biologically important dicarboxylates under physiological conditions. J. Org. Chem. 2008;73:374–382. doi: 10.1021/jo701636b. [DOI] [PubMed] [Google Scholar]
- 53.Busto E., Gonzalez-Alvarez A., Gotor-Fernandez V., Alfonso I., Gotor V. Optically active macrocyclic hexaazapyridinophanes decorated at the periphery: Synthesis and applications in the NMR enantiodiscrimination of carboxylic acids. Tetrahedron. 2010;66:6070–6077. doi: 10.1016/j.tet.2010.06.009. [DOI] [Google Scholar]
- 54.Fraschetti C., Filippi A., Crestoni M.E., Marcantoni E., Glucini M., Guarcini L., Montagna M., Guidoni L., Speranza M. Protonated Hexaazamacrocycles as Selective K+ Receptors. J. Am. Soc. Mass Spectrom. 2015;26:1186–1190. doi: 10.1007/s13361-015-1104-3. [DOI] [PubMed] [Google Scholar]
- 55.Fraschetti C., Filippi A., Crestoni M.E., Marcantoni E., Glucini M., Guarcini L., Montagna M., Guidoni L., Speranza M. Contact Ion Pairs on a Protonated Azamacrocycle: The Role of the Anion Basicity. J. Am. Soc. Mass Spectrom. 2016;27:615–621. doi: 10.1007/s13361-015-1327-3. [DOI] [PubMed] [Google Scholar]
- 56.Grajewski J., Piotrowska K., Zgorzelak M., Janiak A., Biniek-Antosiak K., Rychlewska U., Gawronski J. Introduction of axial chirality at a spiro carbon atom in the synthesis of pentaerythritol–imine macrocycles. Org. Biomol. Chem. 2018;16:981–987. doi: 10.1039/C7OB02672B. [DOI] [PubMed] [Google Scholar]
- 57.Zgorzelak M., Grajewski J. Axial chirality inversion at a spiro carbon leads to efficient synthesis of polyimine macrocycle. J. Mol. Struct. 2020;1202:127336–127341. doi: 10.1016/j.molstruc.2019.127336. [DOI] [Google Scholar]
- 58.Gawroński J., Brzostowska M., Kwit M., Plutecka A., Rychlewska U. Rhombimines-cyclic tetraimines of trans-1,2-diaminocyclohexane shaped by the diaryl ether structural motif. J. Org. Chem. 2005;70:10147–10150. doi: 10.1021/jo051687e. [DOI] [PubMed] [Google Scholar]
- 59.Gawroński J., Kwit M., Grajewski J., Gajewy J., Dlugokinska A. Structural constraints for the formation of macrocyclic rhombimines. Tetrahedron Asymmetry. 2007;18:2632–2637. doi: 10.1016/j.tetasy.2007.10.033. [DOI] [Google Scholar]
- 60.Grigoras M., Vacareanu L., Ivan T., Ailiesei G.L. Triphenylamine-based rhombimine macrocycles with solution interconvertable conformation. Org. Biomol. Chem. 2010;8:3638–3643. doi: 10.1039/c004999a. [DOI] [PubMed] [Google Scholar]
- 61.Tanaka K., Nakai Y., Takahashi H. Efficient NMR chiral discrimination of carboxylic acids using rhombamine macrocycles as chiral shift reagent. Tetrahedron Asymmetry. 2011;22:178–184. doi: 10.1016/j.tetasy.2011.01.013. [DOI] [Google Scholar]
- 62.Tanaka K., Iwashita T., Sasaki C., Takahashi H. Ring-expanded chiral rhombamine macrocycles for efficient NMR enantiodiscrimination of carboxylic acid derivatives. Tetrahedron Asymmetry. 2014;25:602–609. doi: 10.1016/j.tetasy.2014.03.009. [DOI] [Google Scholar]
- 63.Li Z.M., Jablonski C. Synthesis, characterization, and structure of macrocyclic mono- and C-2-symmetric, binuclear nickel calixsalen complexes. Inorg. Chem. 2000;39:2456–2461. doi: 10.1021/ic990935k. [DOI] [PubMed] [Google Scholar]
- 64.Sadhukhan A., Khan N.U.H., Roy T., Kureshy R.I., Abdi S.H., Bajaj H.C. Asymmetric Hydrolytic Kinetic Resolution with Recyclable Macrocyclic CoIII-Salen Complexes: A Practical Strategy in the Preparation of (R)-Mexiletine and (S)-Propranolol. Chem.—Eur. J. 2012;18:5256–5260. doi: 10.1002/chem.201103574. [DOI] [PubMed] [Google Scholar]
- 65.Kureshy R.I., Das A., Khan N.U., Abdi S.H., Bajaj H.C. Cu(II)-Macrocylic H-4 Salen Catalyzed Asymmetric Nitroaldol Reaction and Its Application in the Synthesis of alpha 1-Adrenergic Receptor Agonist (R)-Phenylephrine. Acs Catal. 2011;1:1529–1535. doi: 10.1021/cs2004467. [DOI] [Google Scholar]
- 66.Khan N.U.H., Sadhukhan A., Maity N.C., Kureshy R.I., Abdi S.H., Saravanan S., Bajaj H.C. Enantioselective O-acetylcyanation/cyanoformylation of aldehydes using catalysts with built-in crown ether-like motif in chiral macrocyclic V(V) salen complexes. Tetrahedron. 2011;67:7073–7080. doi: 10.1016/j.tet.2011.07.005. [DOI] [Google Scholar]
- 67.Saravanan S., Khan N.U.H., Bera P.K., Kureshy R.I., Abdi S.H., Kumari P., Bajaj H.C. Catalysis of Enantioselective Strecker Reaction in the Synthesis of D-Homophenylalanine Using Recyclable, Chiral, Macrocyclic MnIII-Salen Complexes. Chemcatchem. 2013;5:1374–1385. doi: 10.1002/cctc.201200700. [DOI] [Google Scholar]
- 68.Sadhukhan A., Choudhary M.K., Khan N.U.H., Kureshy R.I., Abdi S.H., Bajaj H.C. Asymmetric Cyanoethoxy Carbonylation Reaction of Aldehydes Catalyzed by a TiIV Macrocyclic Complex: An Efficient Synthetic Protocol for -Blocker and 1-Adrenergic Receptor Agonists. Chemcatchem. 2013;5:1441–1448. doi: 10.1002/cctc.201200617. [DOI] [Google Scholar]
- 69.Kureshy R.I., Roy T., Khan N.U., Abdi S.H., Sadhukhan A., Bajaj H.C. Reusable chiral macrocyclic Mn(III) salen complexes for enantioselective epoxidation of nonfunctionalized alkenes. J. Catal. 2012;286:41–50. doi: 10.1016/j.jcat.2011.10.011. [DOI] [Google Scholar]
- 70.Menapara T., Choudhary M.K., Tak R., Kureshy R.I., Khan N.U., Abdi S.H. Recyclable chiral Cu(II) macrocyclic salen complex catalyzed enantioselective aza-Henry reaction of isatin derived N-Boc ketimines and its application for the synthesis of beta-diamine. J. Mol. Catal. A Chem. 2016;421:161–166. doi: 10.1016/j.molcata.2016.05.021. [DOI] [Google Scholar]
- 71.Chinnaraja E., Arunachalam R., Choudhary M.K., Kureshy R.I., Subramanian P.S. Binuclear Cu(II) chiral complexes: Synthesis, characterization and application in enantioselective nitroaldol (Henry) reaction. Appl. Organomet. Chem. 2016;30:95–101. doi: 10.1002/aoc.3404. [DOI] [Google Scholar]
- 72.Hu Y., Zhang L., Chang F.F., Zhao P.C., Feng G.F., Zhang K., Huang W. Construction of Chiral 4 + 4 and 2 + 2 Schiff-Base Macrocyclic Zinc(II) Complexes Influenced by Counterions and Pendant Arms. Inorg. Chem. 2016;55:8260–8262. doi: 10.1021/acs.inorgchem.6b01493. [DOI] [PubMed] [Google Scholar]
- 73.Chang F.F., Li W.Q., Feng F.D., Huang W. Construction and Photoluminescent Properties of Schiff-Base Macrocyclic Mono-/Di-/Trinuclear Zn-II Complexes with the Common 2-Ethylthiophene Pendant Arm. Inorg. Chem. 2019;58:7812–7821. doi: 10.1021/acs.inorgchem.9b00454. [DOI] [PubMed] [Google Scholar]
- 74.Zhao P.C., Chang F.F., Feng F.D., Huang W. Transmetalation and Demetallization for Open-Oyster-like Non-Ionic Cd(II) Macrocycles. Inorg. Chem. 2020;59:7504–7511. doi: 10.1021/acs.inorgchem.0c00304. [DOI] [PubMed] [Google Scholar]
- 75.Gualandi A., Cerisoli L., Stoeckli-Evans H., Savoia D. Pyrrole Macrocyclic Ligands for Cu-Catalyzed Asymmetric Henry Reactions. J. Org. Chem. 2011;76:3399–3408. doi: 10.1021/jo200318b. [DOI] [PubMed] [Google Scholar]
- 76.Li Y.Y., Yu S.L., Wu X.F., Xiao J.L., Shen W.Y., Dong Z.R., Gao J.X. Iron Catalyzed Asymmetric Hydrogenation of Ketones. J. Am. Chem. Soc. 2014;136:4031–4039. doi: 10.1021/ja5003636. [DOI] [PubMed] [Google Scholar]
- 77.Yu S.L., Shen W.Y., Li Y.Y., Dong Z.R., Xu Y.Q., Li Q., Zhang J.N., Gao J.X. Iron-Catalyzed Highly Enantioselective Reduction of Aromatic Ketones with Chiral P2N4-Type Macrocycles. Adv. Synth. Catal. 2012;354:818–822. doi: 10.1002/adsc.201100733. [DOI] [Google Scholar]
- 78.Li Z.B., Lin J., Pu L. A cyclohexyl-1,2-diamine-derived bis(binaphthyl) macrocycle: Enhanced sensitivity and enantioselectivity in the fluorescent recognition of mandelic acid. Angew. Chem. Int. Ed. 2005;44:1690–1693. doi: 10.1002/anie.200462471. [DOI] [PubMed] [Google Scholar]
- 79.Li Z.B., Lin J., Sabat M., Hyacinth M., Pu L. Enantioselective fluorescent recognition of chiral acids by cyclohexane-1,2-diamine-based bisbinaphthyl molecules. J. Org. Chem. 2007;72:4905–4916. doi: 10.1021/jo0704715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin J., Zhang H.C., Pu L. Bisbinaphthyl macrocycle-based highly enantioselective fluorescent sensors for alpha-hydroxycarboxylic acids. Org. Lett. 2002;4:3297–3300. doi: 10.1021/ol026565c. [DOI] [PubMed] [Google Scholar]
- 81.Xu X.C., Trindle C.O., Zhang G.Q., Pu L. Fluorescent recognition of Hg2+ by a 1,1 ′-binaphthyl-based macrocycle: A highly selective off-on-off response. Chem. Commun. 2015;51:8469–8472. doi: 10.1039/C5CC02457A. [DOI] [PubMed] [Google Scholar]
- 82.Shen K., Yang X., Cheng Y.X., Zhu C.J. A highly selective ratiometric fluorescent chemosensor for Zn2+ ion based on a polyimine macrocycle. Tetrahedron. 2012;68:5719–5723. doi: 10.1016/j.tet.2012.05.045. [DOI] [Google Scholar]
- 83.Yang X., Liu X.C., Shen K., Zhu C.J., Cheng Y.X. A Chiral Perazamacrocyclic Fluorescent Sensor for Cascade Recognition of Cu(II) and the Unmodified alpha-Amino Acids in Protic Solutions. Org. Lett. 2011;13:3510–3513. doi: 10.1021/ol2013268. [DOI] [PubMed] [Google Scholar]
- 84.Gawroński J., Kolbon H., Kwit M., Katrusiak A. Designing large triangular chiral macrocycles: Efficient 3 + 3 diamine-dialdehyde condensations based on conformational bias. J. Org. Chem. 2000;65:5768–5773. doi: 10.1021/jo000623v. [DOI] [PubMed] [Google Scholar]
- 85.Korupoju S.R., Zacharias P.S. New optically active hexaaza triphenolic macrocycles: Synthesis, molecular structure and crystal packing features. Chem. Commun. 1998;12:1267–1268. doi: 10.1039/a802201a. [DOI] [Google Scholar]
- 86.Grajewski J., Zgorzelak M., Janiak A., Taras-Goslinska K. Controlled, Sunlight-Driven Reversible Cycloaddition of Multiple Singlet Oxygen Molecules to Anthracene-Containing Trianglimine Macrocycles. Chempluschem. 2022;87:e202100510. doi: 10.1002/cplu.202100510. [DOI] [PubMed] [Google Scholar]
- 87.Wang Z., Nour H.F., Roch L.M., Guo M., Li W., Baldridge K.K., Sue A.C.H., Olson M.A. 3 + 3 Cyclocondensation of Disubstituted Biphenyl Dialdehydes: Access to Inherently Luminescent and Optically Active Hexa-substituted C-3-Symmetric and Asymmetric Trianglimine Macrocycles. J. Org. Chem. 2017;82:2472–2480. doi: 10.1021/acs.joc.6b02868. [DOI] [PubMed] [Google Scholar]
- 88.Janiak A., Gajewy J., Szymkowiak J., Gierczyk B., Kwit M. Specific Noncovalent Association of Truncated exo-Functionalized Triangular Homochiral Isotrianglimines through Head-to-Head, Tail-to-Tail, and Honeycomb Supramolecular Motifs. J. Org. Chem. 2022;87:2356–2366. doi: 10.1021/acs.joc.1c02238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Szymkowiak J., Warżajtis B., Rychlewska U., Kwit M. Consistent supramolecular assembly arising from a mixture of components-self-sorting and solid solutions of chiral oxygenated trianglimines. Crystengcomm. 2018;20:5200–5208. doi: 10.1039/C8CE01044G. [DOI] [Google Scholar]
- 90.Szymkowiak J., Warżajtis B., Rychlewska U., Kwit M. One-step Access to Resorcinsalens-Solvent-Dependent Synthesis, Tautomerism, Self-sorting and Supramolecular Architectures of Chiral Polyimine Analogues of Resorcinarene. Chem.—Eur. J. 2018;24:6041–6046. doi: 10.1002/chem.201800316. [DOI] [PubMed] [Google Scholar]
- 91.Troć A., Gajewy J., Danikiewicz W., Kwit M. Specific Noncovalent Association of Chiral Large-Ring Hexaimines: Ion Mobility Mass Spectrometry and PM7 Study. Chem.—Eur. J. 2016;22:13258–13264. doi: 10.1002/chem.201602515. [DOI] [PubMed] [Google Scholar]
- 92.Petryk M., Janiak A., Barbour L.J., Kwit M. Awkwardly-Shaped Dimers, Capsules and Tetramers: Molecular and Supramolecular Motifs in C5-Arylated Chiral Calixsalens. Eur. J. Org. Chem. 2018;16:1916–1923. doi: 10.1002/ejoc.201800314. [DOI] [Google Scholar]
- 93.Petryk M., Troć A., Gierczyk B., Danikiewicz W., Kwit M. Dynamic Formation of Noncovalent Calixsalen Aggregates. Chem.—Eur. J. 2015;21:10318–10321. doi: 10.1002/chem.201500945. [DOI] [PubMed] [Google Scholar]
- 94.Janiak A., Bardziński M., Gawroński J., Rychlewska U. From Cavities to Channels in Host:Guest Complexes of Bridged Trianglamine and Aliphatic Alcohols. Cryst. Growth Des. 2016;16:2779–2788. doi: 10.1021/acs.cgd.6b00100. [DOI] [Google Scholar]
- 95.Janiak A., Petryk M., Barbour L.J., Kwit M. Readily prepared inclusion forming chiral calixsalens. Org. Biomol. Chem. 2016;14:669–673. doi: 10.1039/C5OB02090E. [DOI] [PubMed] [Google Scholar]
- 96.Gajewy J., Gawroński J., Kwit M. Convenient, enantioselective hydrosilylation of imines in protic media catalyzed by a Zn-trianglamine complex. Org. Biomol. Chem. 2011;9:3863–3870. doi: 10.1039/c1ob05074e. [DOI] [PubMed] [Google Scholar]
- 97.Tanaka K., Fukuda N., Fujiwara T. Trianglamine as a new chiral shift reagent for secondary alcohols. Tetrahedron Asymmetry. 2007;18:2657–2661. doi: 10.1016/j.tetasy.2007.10.024. [DOI] [Google Scholar]
- 98.Gualandi A., Grilli S., Savoia D., Kwit M., Gawroński J. C-hexaphenyl-substituted trianglamine as a chiral solvating agent for carboxylic acids. Org. Biomol. Chem. 2011;9:4234–4241. doi: 10.1039/c0ob01192d. [DOI] [PubMed] [Google Scholar]
- 99.Tanaka K., Fukuda N. ‘Calixarene-like’ chiral amine macrocycles as novel chiral shift reagents for carboxylic acids. Tetrahedron Asymmetry. 2009;20:111–114. doi: 10.1016/j.tetasy.2008.11.018. [DOI] [Google Scholar]
- 100.Tanaka K., Tsuchitani T., Fukuda N., Masumoto A., Arakawa R. Highly enantioselective fluorescent recognition of mandelic acid derivatives by chiral salen macrocycles. Tetrahedron Asymmetry. 2012;23:205–208. doi: 10.1016/j.tetasy.2012.01.024. [DOI] [Google Scholar]
- 101.Kwit M., Zabicka B., Gawroński J. Synthesis of chiral large-ring triangular salen ligands and structural characterization of their complexes. Dalton Trans. 2009;34:6783–6789. doi: 10.1039/b909445h. [DOI] [PubMed] [Google Scholar]
- 102.Gonzalez-Alvarez A., Alfonso I., Lopez-Ortiz F., Aguirre A., Garcia-Granda S., Gotor V. Selective host amplification from a dynamic combinatorial library of oligoimines for the syntheses of different optically active polyazamacrocycles. Eur. J. Org. Chem. 2004;5:1117–1127. doi: 10.1002/ejoc.200300628. [DOI] [Google Scholar]
- 103.Gonzalez-Alvarez A., Alfonso I., Gotor V. Highly diastereoselective amplification from a dynamic combinatorial library of macrocyclic oligoimines. Chem. Commun. 2006;21:2224–2226. doi: 10.1039/B603203F. [DOI] [PubMed] [Google Scholar]
- 104.Gonzalez-Alvarez A., Alfonso I., Cano J., Diaz P., Gotor V., Gotor-Fernandez V., Garcia-Espana E., Garcia-Granda S., Jimenez H.R., Lloret F. A Ferromagnetic [Cu3(OH)2]4+ Cluster Formed inside a Tritopic Nonaazapyridinophane: Crystal Structure and Solution Studies. Angew. Chem. Int. Ed. 2009;48:6055–6058. doi: 10.1002/anie.200901888. [DOI] [PubMed] [Google Scholar]
- 105.Loffler M., Gregoliński J., Korabik M., Lis T., Lisowski J. Multinuclear Ni(II), Cu(II) and Zn(II) complexes of chiral macrocyclic nonaazamine. Dalton Trans. 2016;45:15586–15594. doi: 10.1039/C6DT02504H. [DOI] [PubMed] [Google Scholar]
- 106.Zhao C.Q., Ren J.S., Gregoliński J., Lisowski J., Qu X.G. Contrasting enantioselective DNA preference: Chiral helical macrocyclic lanthanide complex binding to DNA. Nucleic Acids Res. 2012;40:8186–8196. doi: 10.1093/nar/gks524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gregoliński J., Lisowski J. Helicity inversion in lanthanide(III) complexes with chiral nonaaza macrocyclic ligands. Angew. Chem. Int. Ed. 2006;45:6122–6126. doi: 10.1002/anie.200602464. [DOI] [PubMed] [Google Scholar]
- 108.Gregoliński J., Lis T., Cyganik M., Lisowski J. Lanthanide Complexes of the Heterochiral Nonaaza Macrocycle: Switching the Orientation of the Helix Axis. Inorg. Chem. 2008;47:11527–11534. doi: 10.1021/ic8005986. [DOI] [PubMed] [Google Scholar]
- 109.Jiang J.C., Chu Z.L., Huang W., Wang G., You X.Z. Dinuclear Cadmium(II), Zinc(II), and Manganese(II), Trinuclear Nickel(II), and Pentanuclear Copper(II) Complexes with Novel Macrocyclic and Acyclic Schiff-Base Ligands Having Enantiopure or Racemic Camphoric Diamine Components. Inorg. Chem. 2010;49:5897–5911. doi: 10.1021/ic100349d. [DOI] [PubMed] [Google Scholar]
- 110.Xie S.M., Fu N., Li L., Yuan B.Y., Zhang J.H., Li Y.X., Yuan L.M. Homochiral Metal-Organic Cage for Gas Chromatographic Separations. Anal. Chem. 2018;90:9182–9188. doi: 10.1021/acs.analchem.8b01670. [DOI] [PubMed] [Google Scholar]
- 111.He L.X., Tian C.R., Zhang J.H., Xu W., Peng B., Xie S.M., Zi M., Yuan L.M. Chiral metal-organic cages used as stationary phase for enantioseparations in capillary electrochromatography. Electrophoresis. 2020;41:104–111. doi: 10.1002/elps.201900294. [DOI] [PubMed] [Google Scholar]
- 112.Li Z.T., Mao Z.K., Zhou W., Chen Z.L. Incorporation of homochiral metal-organic cage into ionic liquid based monolithic column for capillary electrochromatography. Anal. Chim. Acta. 2020;1094:160–167. doi: 10.1016/j.aca.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 113.Kobyłka M.J., Janczak J., Lis T., Kowalik-Jankowska T., Klak J., Pietruszka M., Lisowski J. Trinuclear Cu(II) complexes of a chiral N6O3 amine. Dalton Trans. 2012;41:1503–1511. doi: 10.1039/C1DT11286D. [DOI] [PubMed] [Google Scholar]
- 114.Korupoju S.R., Mangayarkarasi N., Zacharias P.S., Mizuthani J., Nishihara H. Synthesis, structure, and DNA cleavage activity of new trinuclear Zn-3 and Zn-2Cu complexes of a chiral macrocycle: Structural correlation with the active center of P1 nuclease. Inorg. Chem. 2002;41:4099–4101. doi: 10.1021/ic0201102. [DOI] [PubMed] [Google Scholar]
- 115.Gao J., Zingaro R.A., Reibenspies J.H., Martell A.E. Direct observation of enantioselective synergism at trimetallic centers. Org. Lett. 2004;6:2453–2455. doi: 10.1021/ol049156k. [DOI] [PubMed] [Google Scholar]
- 116.Kobyłka M.J., Ślepokura K., Rodicio M.A., Paluch M., Lisowski J. Incorporation of Trinuclear Lanthanide(III) Hydroxo Bridged Clusters in Macrocyclic Frameworks. Inorg. Chem. 2013;52:12893–12903. doi: 10.1021/ic400508y. [DOI] [PubMed] [Google Scholar]
- 117.Bereta T., Mondal A., Ślepokura K., Peng Y., Powell A.K., Lisowski J. Trinuclear and Hexanuclear Lanthanide(III) Complexes of the Chiral 3 + 3 Macrocycle: X-ray Crystal Structures and Magnetic Properties. Inorg. Chem. 2019;58:4201–4213. doi: 10.1021/acs.inorgchem.8b03266. [DOI] [PubMed] [Google Scholar]
- 118.Lin S.Y., Guo Y.N., Guo Y., Zhao L., Zhang P., Ke H.S., Tang J.K. Macrocyclic ligand encapsulating dysprosium triangles: Axial ligands perturbed magnetic dynamics. Chem. Commun. 2012;48:6924–6926. doi: 10.1039/c2cc32827e. [DOI] [PubMed] [Google Scholar]
- 119.Lin S.Y., Wang C., Zhao L., Tang J.K. Enantioselective Self-Assembly of Triangular Dy-3 Clusters with Single Molecule Magnet Behavior. Chem. Asian J. 2014;9:3558–3564. doi: 10.1002/asia.201402670. [DOI] [PubMed] [Google Scholar]
- 120.Wydra K., Kobyłka M.J., Lis T., Ślepokura K., Lisowski J. Versatile Binding Modes of Chiral Macrocyclic Amine towards Rare Earth Ions. Eur. J. Inorg. Chem. 2020;2020:2096–2104. doi: 10.1002/ejic.202000247. [DOI] [Google Scholar]
- 121.Yamashita A., Watanabe A., Akine S., Nabeshima T., Nakano M., Yamamura T., Kajiwara T. Wheel-Shaped (ErZn3II)-Zn-III Single-Molecule Magnet: A Macrocyclic Approach to Designing Magnetic Anisotropy. Angew. Chem. Int. Ed. 2011;50:4016–4019. doi: 10.1002/anie.201008180. [DOI] [PubMed] [Google Scholar]
- 122.Gregoliński J., Ślepokura K., Paćkowski T., Lisowski J. Expansion of a 2 + 2 Macrocycle into a 6 + 6 Macrocycle: Template Effect of Cadmium(II) Org. Lett. 2014;16:4372–4375. doi: 10.1021/ol501602f. [DOI] [PubMed] [Google Scholar]
- 123.Gregoliński J., Ślepokura K., Bil A., Lisowski J. A New Synthetic Strategy Leading to Homochiral Macrocycles Derived from 2,6-Diformylpyridine and (1S,2S)-trans-1,2-Diaminocyclopentane. Eur. J. Org. Chem. 2020;2020:5714–5728. doi: 10.1002/ejoc.202000919. [DOI] [Google Scholar]
- 124.Fedorowicz D., Banach S., Koza P., Frydrych R., Ślepokura K., Gregoliński J. Controlling chirality in the synthesis of 4 + 4 diastereomeric amine macrocycles derived from trans-1,2-diaminocyclopentane and 2,6-diformylpyridine. Org. Biomol. Chem. 2022;20:1080–1094. doi: 10.1039/D1OB02410H. [DOI] [PubMed] [Google Scholar]
- 125.Zgorzelak M., Grajewski J., Gawroński J., Kwit M. Solvent-assisted synthesis of a shape-persistent chiral polyaza gigantocycle characterized by a very large internal cavity and extraordinarily high amplitude of the ECD exciton couplet. Chem. Commun. 2019;55:2301–2304. doi: 10.1039/C8CC10184A. [DOI] [PubMed] [Google Scholar]
- 126.Gregoliński J., Ślepokura K., Paćkowski T., Panek J., Stefanowicz P., Lisowski J. From 2 + 2 to 8 + 8 Condensation Products of Diamine and Dialdehyde: Giant Container-Shaped Macrocycles for Multiple Anion Binding. J. Org. Chem. 2016;81:5285–5294. doi: 10.1021/acs.joc.6b00531. [DOI] [PubMed] [Google Scholar]
- 127.Gregoliński J., Ślepokura K., Lisowski J. Hexanuclear and Trinuclear Metal Complexes of a Giant Octadecaaza Macrocycle. Inorg. Chem. 2017;56:12719–12727. doi: 10.1021/acs.inorgchem.7b01173. [DOI] [PubMed] [Google Scholar]
- 128.Paćkowski T., Gregoliński J., Ślepokura K., Lisowski J. 6 + 6 Macrocycles derived from 2,6-diformylpyridine and trans-1,2-diaminocyclohexane. Tetrahedron Lett. 2018;59:3669–3673. doi: 10.1016/j.tetlet.2018.08.059. [DOI] [Google Scholar]
- 129.Frydrych R., Ślepokura K., Bil A., Gregoliński J. Mixed Macrocycles Derived from 2,6-Diformylpyridine and Opposite Enantiomers of trans-1,2-Diaminocyclopentane and trans-1,2-Diaminocyclohexane. J. Org. Chem. 2019;84:5695–5711. doi: 10.1021/acs.joc.9b00614. [DOI] [PubMed] [Google Scholar]
- 130.Wolska K., Janczak J., Gawryszewska P., Lisowski J. Rare earth complexes of chiral unsymmetrical hexaazamacrocycles. Polyhedron. 2021;198:115057. doi: 10.1016/j.poly.2021.115057. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.