Abstract
Alzheimer’s disease (AD) is a progressive neurological condition. The rising prevalence of AD necessitates the rapid development of efficient therapy options. Despite substantial study, only a few medications are capable of delaying the disease. Several substances with pharmacological activity, derived from plants, have been shown to have positive benefits for the treatment of AD by targeting various enzymes, such as acetylcholinesterase (AChE), butyrylcholinesterase (BuChE), β-secretase, γ-secretase, and monoamine oxidases (MAOs), which are discussed as potential targets. Medicinal plants have already contributed a number of lead molecules to medicine development, with many of them currently undergoing clinical trials. A variety of medicinal plants have been shown to diminish the degenerative symptoms associated with AD, either in their raw form or as isolated compounds. The aim of this review was to provide a brief summary of AD and its current therapies, followed by a discussion of the natural compounds examined as therapeutic agents and the processes underlying the positive effects, particularly the management of AD.
Keywords: Alzheimer’s disease, natural compound, mechanism of enzyme, management, inhibition activity
1. Introduction
Alzheimer’s disease (AD) is a severe, chronic, and progressive neurological illness that causes memory and cognitive loss and eventually death [1]. Dementia has become a major public health problem in both developed and developing countries as a result of the aging population and its fast-rising incidence [2]. Aging, cholinergic pathways, environmental factors, head injury, genetic factors, mitochondrial dysfunction, and immune system dysfunction are some common causes of the development of AD [3]. The most prevalent form of dementia is AD, which is a progressive neurological condition [4]. The reported deaths from AD increased by more than 145% [5]. According to the most recent estimates, dementia prevalence will double in Europe by 2050 and triple globally. AD is pathologically defined by the presence of amyloid plaques, hyperphosphorylated tau proteins, and neurofibrillary tangles; however, oxidative–nitrative stress, endoplasmic reticulum stress, mitochondrial dysfunction, inflammatory cytokines, pro-apoptotic proteins, and altered neurotransmitter levels are all common etiological attributes in its pathogenesis. Rivastigmine, memantine, galantamine, and donepezil are Food and Drug Administration-approved medications for the treatment of symptoms associated with AD [6]. The cellular phase of AD occurs concurrently with the accumulation of amyloid, causing tau pathology to spread. Heritable variables account for 60–80% of the risk of AD [7]. A decrease in brain acetylcholine (ACh) levels is implicated in the pathophysiology of cognitive dysfunction occurring in AD. The inhibition of ACh catabolic enzymes, such as acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE), can contribute to an increase in ACh levels. It has been hypothesized that the inhibition of AChE and BuChE may contribute to countering the formation of Aβ plaques and, therefore, represents a disease-modifying strategy principle, but no conclusive evidence was obtained to confirm this hypothesis [8].
AChE inhibition is one of the most used treatment approaches; however, it only provides symptomatic alleviation and has a mild disease-modifying impact. Antioxidant and vitamin treatment, stem cell therapy, hormone therapy, selective phosphodiesterase (PDE) inhibitors, inhibition of β-secretase, γ-secretase and Aβ aggregation, suppression of tau hyperphosphorylation, and intracellular neurofibrillary tangles are examples of non-cholinergic therapeutic methods. In a number of preclinical and clinical investigations, medicinal plants have been found to have anti-AD efficacy [9,10]. Ethnobotany plays a significant role in the identification of anti-AD compounds from botanicals in China and the far east, but maybe less so in Europe. Traditional Chinese medicine has been employed in the treatment of AD in China. A variety of medicinal plants have been shown to diminish the degenerative characteristics associated with AD, either in their crude form or as isolated substances [1]. The consumption of bioactive compound-rich foods or the administration of bioactive compound extracts can have a preventive impact against a variety of pathophysiological diseases. Various sources of bioactive chemicals are employed in the treatment of AD. We have just covered the most frequent options.
It is reported that dietary supplements might help to heal the disorders. Nutraceuticals are food-based extracts of chemicals that offer health advantages. Nutraceuticals are ingested in concentrated forms such as tablets, capsules, and drinks, and they have no negative effects, even at large doses. To avoid the negative side effects of the currently available medications, researchers are concentrating their efforts on identifying natural bioactive chemicals found in foods that can be used to treat AD [11,12,13,14]. The consumption of bioactive compound-rich foods or the administration of bioactive compound extracts can have a preventive impact against a variety of pathophysiological diseases. Although there are other sources of bioactive chemicals used in the treatment of AD, we only included the most widely available. The impact of numerous bioactive chemicals found in widely consumed foods on AD has been reviewed and addressed in this section. The aim of this review is to evaluate the role of natural compounds and the mechanism of enzymes for the management of AD. An extensive literature review (by inclusion of natural compounds and target enzymes, and the exclusion of synthetic compounds) was carried out, and published articles from PubMed, Scifinder, Google Scholar, Clinical Trials.org, and the Alzheimer Association reports were thoroughly examined in order to combine information on the various ways to battle AD. Therefore, in this article, we focus on reviewing the potential target and small natural compounds targeting various molecular mechanisms for the management of AD.
2. Natural Compounds and Alzheimer’s Disease
Natural products and their molecular frameworks have a long history of serving as important starting points for medicinal chemistry and drug development [15]. Recent studies have discussed the many therapeutic properties of natural products, such as their ability to improve sleep [16], hypolipidemic activity and anticancer effects [17,18], protective effects against viral pneumonia and anti-inflammatory effects [19], anticancer and antioxidative effects [20,21], neuroprotective effects [22], antioxidative stress and anti-asthmatic effects [23,24,25], alleviating the effect of skin inflammation [26], and anti-Trypanosoma effects [27]. However, natural products can cause pulmonary and central nervous system (CNS) irritation [28], developmental toxicity [29,30], nephrotoxicity and hepatotoxicity [31], and allergic responses [32,33]. There are presently no effective drugs available to treat ND. In traditional medicine, ashwagandha is used to treat general debility, nervous weariness, insomnia, and memory loss [34]. In studies, these natural compounds have been shown to exhibit biological qualities, such as antioxidant, anti-inflammatory, and antiapoptotic effects. In vitro and in vivo studies have confirmed the use of natural products in a variety of preclinical models of ND. Phytoconstituents, such as polyphenolic antioxidants found in herbs, fruits, nuts, and vegetables, as well as marine and freshwater flora, are examples of natural products. These phytoconstituents have the ability to prevent several NDs, such as AD [35,36]. Consumption of these substances at adequate quantities may have promising benefits in the prevention of AD [37].
3. Inhibition of Acetylcholinesterase Activity Using Natural Compounds
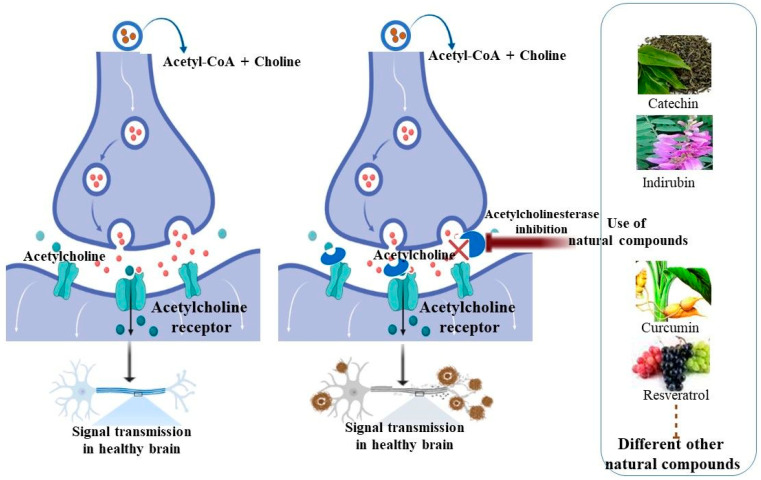
Acetylcholinesterase (AChE) is a serine hydrolase that hydrolyzes the neurotransmitter acetylcholine (ACh) into acetic acid and choline. The ellipsoidal structure of AChE has three binding sites: catalytic anionic (Ser200, Glu334, and His440), the aromatic gorge, and the peripheral anionic site (Tyr70, Asp72, Tyr121, Trp279, and Tyr334), where inhibitory chemicals engage. AChE inhibitors (AChEI) bind to this enzyme and prevent it from breaking down ACh, causing ACh to accumulate in nerve synapses and impair neurotransmission. Many medicinal compounds targeting AChE have been developed based on this mechanism of action [38,39,40,41]. As a result, using AChEI to treat symptoms associated with cholinergic imbalances in AD seemed a sensible strategy. AChE and the cholinergic system, on the other hand, appear to have broader impacts in AD. Many useful compounds that demonstrate a wide spectrum of pharmacological action against cholinesterase enzymes have been discovered through phytochemical research of various therapeutic plants [42]. Dihydroberberine and macelignan potently and effectively inhibited AChE with IC50 values of 1.18 and 4.16 µM, respectively [43]. Quercus suber cork and corkback ethanol–water extracts have been proven to be remarkable antioxidants with interesting AChE inhibitory activity [44]. Using the in vitro Ellman’s technique, extracts, fractions, and compounds from Calceolaria talcana and Calceolaria integrifolia showed substantial inhibitory effects on AChE activity. The most active samples were derived from the ethyl acetate extract, which inhibited AChE in a mixed-type manner (69.8 and 79.5% at 100 and 200 μg/mL, respectively) [45]. It was also reported that between 0 and 5 min, AChE inhibition increased as the time spent exposed to Malathion increased [46]. The edible component of the Garcinia parvifolia fruit has the potential to be a natural source of antioxidants and anti-AD agents [47]. Phytochemicals continue to enter clinical trials or give leads for the development of new therapeutic medicines [48]. The use of natural products or nutraceutical chemicals has emerged as a potential preventative therapy approach, as most medications focusing on specific targets have failed to establish a medical cure. Nutraceutical substances have the benefit of a multitarget strategy, tagging several biochemical locations in the human brain, as compared to the single-target action of most AD medications [49]. In the last decade, more than 200 potential therapeutic candidates have failed during clinical trials, indicating that the illness and its causes are likely to be complicated. Medicinal herbs and herbal therapies are gaining popularity as complementary and alternative interventions to create medication candidates for AD. Several scientific investigations have documented the use of numerous medicinal plants and their main phytochemicals in the treatment of AD [50,51]. The increasing collection of epidemiological and experimental research shows that eating fruits and vegetables protects the brain from the negative consequences of oxidative stress, neuroinflammation, and aging. These benefits are mediated by antioxidant, anti-inflammatory, and other beneficial phytochemical components present in plants [52]. However, it was also reported that consistent use of coffee, tea, and dark chocolate (cacao) may boost brain health and lower the incidence of age-related neurodegenerative disease (ND). Caffeine’s mode of action is based on the antagonism of several adenosine receptor subtypes. Theobromine and theophylline, which are downstream xanthine metabolites, may also contribute to the therapeutic benefits of coffee, tea, and cocoa on brain function [53]. Tea is said to have powerful antioxidant effects. Flavonoids, tannins, caffeine, polyphenols, boheic acid, theophylline, theobromine, anthocyanins, gallic acid, and ultimately epigallocatechin-3-gallate, which is regarded the most potent active element, are all abundant. Tea catechins, which are flavonoid phytochemicals that target common risk factors, including obesity, hyperlipidemia, hypertension, cardiovascular disease, and stroke, may help to reduce the risk of AD [54]. The effects and probable mechanisms of numerous widely eaten phytochemicals on neuropathology and AD outcomes are discussed in this study. We propose that frequently eating bioactive phytochemicals from a range of fruits and vegetables reduces age and insult-related neuropathology in AD, based on available data. This holistic approach to nutraceuticals paves the way for future research and clinical trials, which are expected to provide outcomes based on medical evidence. The molecular mechanism of AChE was described in Figure 1, along with the inhibition process of AChE using natural compounds.
Figure 1.
The inhibition process of AChE using natural compounds. AChE inhibitors such as natural compounds bind to the AChE enzyme and prevent the breaking down of ACh, causing ACh to accumulate in nerve synapses and impair neurotransmission.
4. Inhibition of BACE1 Activity Using Natural Compounds
In 1991, the amyloid hypothesis was proposed. It claimed that extracellular amyloid deposits are the primary cause of AD [55]. β-secretase (BACE1) was found to be responsible for the creation of β-amyloid (Aβ) observed in AD [56]. Aβ is a type I transmembrane protein with a large extracellular domain and a short cytoplasmic portion that is generated from an amyloid precursor protein (APP). As a result of alternative splicing, several distinct APP isoforms exist, ranging in length from 695 to 770 amino acid residues [57]. Neurons create a considerable quantity of APP. However, it is normally digested quite fast. APP may be cleaved by six distinct enzymes, namely, α-, β-, δ-, η- and θ-secretase and meprin β [58]. In AD, APP is cleaved alternatively in endosomal compartments by the successive action of the integral membrane β- and γ-secretase, releasing Aβ from the APP [59,60]. β-secretase divides APP, producing a 100 kDa soluble N-terminal APP ectodomain (APPs) and a 12 kDa membrane-tethered C-terminal fragment with 99 or 89 amino acid residues, depending on whether it cleaves at Asp1 or Glu11 of the APP. Under healthy settings, BACE1 mostly cleaves APP at the Glu11 location, resulting in the non-amyloidogenic form C89 and truncated Aβ production [61]. Verubecestat, lanabecestat, atabecestat, umibecestat, and elenbecestat are in II/III phase clinical trials as BACE1 inhibitors [61]. The IC50 values for these drugs were found to be 2.2 nM for verubecestat [62], 0.6 nM for lanabecestat [63], and 1.0–2.6 nM for atabecestat [64]. The reduction of Aβ in CSF depended on the daily dose and it was shown that verubecestat reduces Aβ in CSF by 50–75% at a 12 mg dose and 80–90% at a 40 mg dose [65]. Lanabecestat reduces 63% at a 15 mg dose and 79% at a 50 mg dose [66]; atabecestat reduces 50% at a 5 mg dose and 80–85% at a 30 mg dose [67]; and umibecestat reduces 95% at a 15 mg dose [68].
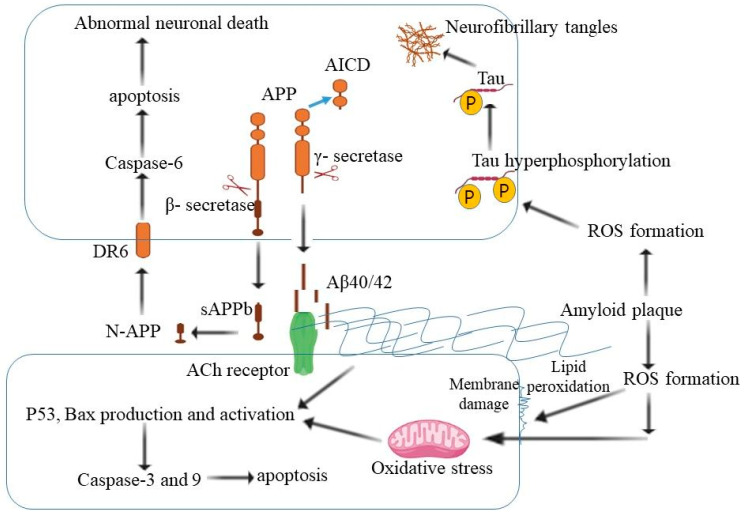
Natural products, particularly those used in traditional Chinese medicine, offer a safety advantage, since they have been used in humans for a long period [69]. Inhibiting BACE1 has been intensively researched as a possible AD disease-modifying medication. Clinical failures with BACE inhibitors have risen steadily. As a result, researchers are thinking about natural compounds as potent drug therapies for the management of AD-targeting BACE1. The natural compounds catechins may also aid people with AD by decreasing the formation of amyloid plaques and enhancing their cognitive ability [54]. To explore natural BACE1 inhibitors, isoflavones, including genistein, formononetin, glycitein, daidzein, and puerarin, were studied and found to be potent for AD management [70]. Compounds such as 2,2′,4′-trihydroxychalcone acid, quercetin, and myricetin have been demonstrated to efficiently inhibit BACE1 activity at lower dosages [71]. The compounds deoxyneocryptotanshinone, salvianolic acid A and salvianolic acid C were found to have good inhibition potential against BACE1, with IC50 values of 11.53 ± 1.13, 13.01 ± 0.32 and 9.18 ± 0.03 μM, respectively [72]. The natural compounds may be alternative agents that have β- and γ-secretase inhibition for the management of AD in the future. The molecular mechanism of β- and γ-secretase is described in Figure 2.
Figure 2.
The molecular mechanism of β- and γ-secretase for the processing of APP. The extracellular amyloid deposits are the primary cause of AD. The natural compounds may be alternative agents that have β- and γ-secretase inhibition for the management of AD (APP—amyloid precursor protein, AICD—APP intracellular domain, Aβ—β-amyloid, ROS—reactive oxygen species).
5. Inhibition of Monoamine Oxidase Activity Using Natural Compounds
Monoamine oxidases (MAOs) are flavoproteins that catalyze the oxidative deamination of biogenic and xenobiotic amines in the outer mitochondrial membrane. There are two isoforms of MAO in mammals (MAO-A and MAO-B), which may be identified by their substrate selectivity and susceptibility to certain inhibitors. Although both isoforms are found in most tissues, their presence in the CNS and their capacity to metabolize monoaminergic neurotransmitters have shifted the focus of MAO research to the adult brain’s functions. MAO activity has been linked to neurological and mental illnesses, as well as NDs [73]. Some inhibitors of the enzyme have showed promise in the treatment of a variety of NDs, such as Parkinson’s disease and AD. MAO inhibitors may be effective in regulating the outcome of stroke and other tissue damage linked with oxidative stress, since the process catalyzed by MAO creates hydrogen peroxide, which is a source of hydroxyl radicals [73,74,75]. MAO inhibitors might be used to treat AD [76]. While MAO-A inhibitors (e.g., chlorgyline, moclobemide, and lazabemide) are efficient antidepressants and anxiolytic medications, MAO-B inhibitors (e.g., l-deprenyl, pargyline, and rasagiline) are used to treat NDs such as Parkinson’s and AD. Natural products have become appealing targets for researchers, owing to the need for novel MAO inhibitors due to the negative effects of existing drugs. Many investigations have shown that flavonoid, xanthone, alkaloid, and coumarin derivatives from herbal sources have high MAO inhibitory action, making them ideal models for synthetic MAO inhibitors [77]. Curcumin and ellagic acid suppressed MAO activity; however, greater half-maximum inhibitory doses of curcumin (500.46 nM) and ellagic acid (412.24 nM) were needed when compared to the known MAO-B inhibitor selegiline. It has been discovered that curcumin and ellagic acid suppress MAO activity in both competitive and noncompetitive ways. These natural chemicals have the potential to be a source of MAO inhibitors, which are utilized in the treatment of Parkinson’s disease and other NDs [78]. Chelerythrine was reported to have an IC50 of 0.55 µM for inhibiting an isoform of recombinant human MAO-A. Chelerythrine was a reversible competitive MAO-A inhibitor (Ki = 0.22 µM) with a substantially higher potency than the marketed medication toloxatone, with an IC50 value of 1.10 µM [79]. The natural O-methylated flavonoid, with strong potency (IC50 33 nM; Ki 37.9 nM) and >292-fold selectivity against human MAO-A (vs. MAO-B), is a novel therapeutic lead for the treatment of NDs [80]. The other natural compounds, such as morin (IC50 = 16.2 µM), alizarin (IC50 = 8.16 µM), and fisetin (IC50 = 7.33 µM), were notable MAO inhibitors with MAO-A selectivity [80]. As compared to known drugs, natural products have fewer side effects and are efficient for the inhibition of these enzymes. Researchers are looking for natural products that have very good potential to inhibit these enzymes, which may be helpful for future treatment options.
Finally, there are certain known natural compounds listed in Table 1. These compounds were found to be suitable for the inhibition of targeted enzymes during in silico, in vitro and in vivo studies.
Table 1.
List of several natural compounds that have potential to inhibit AChE, BuChE, BACE1 and MAOs activity during in silico, in vitro, and in vivo studies.
| S.No. | Compound | Pub Chem ID | Properties | Work Type | Therapeutic Actions/Function | Reference |
|---|---|---|---|---|---|---|
| 1. | Apigenin | 5280443 | Antioxidant and antiinflammatory | in vitro | Decrease Aβ burden | [1,81] |
| in vivo (mouse model) | induced neurogenesis | |||||
| 2. | Dibenzo[1,4,5]thiadiazepine | 71358659 | antioxidant | in vitro (neuroblastoma cells) | neuroprotective and antioxidant properties | [82] |
| 3. | Berberine | 2353 | anti-inflammatory | in vitro (rat model) | inhibition of AChE | [83,84] |
| 4. | Catechin | 9064 | antioxidant | in vivo (rat model of AD) | inhibition of AChE | [85] |
| 5. | Genistein | 5280961 | Antioxidant and anti-inflammatory | in silico and in vitro (model of AD) | inhibition of human monoamine oxidase A and B | [86,87] |
| 6. | Hesperidin | 10621 | antioxidant and anti-inflammatory | in silico and in vivo (rat model of AD) | inhibition of BACE1 and Aβ aggregation | [88,89,90] |
| 7. | Morin | 5281670 | antioxidant, anti-inflammatory and neuroprotective | (MC65 cells) | BACE1, γ-secretase, Aβ fibrillogenesis, amyloid plaque, and tau hyperphosphorylation | [91,92] |
| 8. | Naringenin | 932 | anti-inflammatory | in vitro (rat model) | decrease inflammatory cytokines | [93] |
| 9. | Withanone | 21679027 | neuroprotective | in vivo (rat model of AD) | decrease Aβ fibril formation | [1,94] |
| 10. | Dehydroevodiamine | 9817839 | anti-inflammatory | rat brain slices against AD | inhibition of tau phosphorylation | [95] |
| 11. | Huperzine A | 449069 | neuroprotective | Alzheimer transgenic mouse model | reduces the level of Aβ | [96] |
| 12. | N-methylasimilobine | 197017 | Antioxidant | in vitro | inhibition of AChE | [97] |
| 13. | Isorhynchophylline | 3037048 | neuroprotective | rat model | restore Aβ–induced cognitive impairment | [98] |
| 14. | Palmatine | 19009 | anti-inflammatory and anti-neurodegenerative | in vitro, in vivo | inhibit tau aggregation | [99] |
| 15. | Sanguinarine | 5154 | Antitumor properties | in vitro | inhibition of AChE | [100] |
| 16. | Taspine | 215159 | anti-inflammatory | in vitro | inhibition of AChE | [101] |
| 17. | Indirubin | 10177 | antioxidant and anti-inflammatory | in silico | inhibition of AChE | [102,103] |
| 18. | Rutaecarpine | 65752 | anti-inflammatory | in silico | inhibition of Caspase 8 | [104] |
| 19. | Ajmalicine | 441975 | antihypertensive | in silico | inhibition of BACE1 | [105] |
| 20. | Resveratrol | 445154 | Antioxidant | in vitro and in vivo (AD models) | neuroprotective role in AD | [106] |
| 21. | Curcumin | 969516 | antioxidant, anticarcinogenic, anti-inflammatory, antiangiogenic | in vivo and in vitro | inhibition of AChE | [107] |
| 22. | Resveratrol | 445154 | Antioxidant | in vitro | inhibition of MAOA for AD treatment | [108] |
| 23. | Genistein | 5280961 | Antioxidant and anti-inflammatory | in vitro | anti-AD activities | [70] |
| 24. | Quercetin | 5280343 | Antioxidant | - | Anti-BACE1 Activity | [71] |
| 25. | Ellagic acid | 5281855 | antioxidant, antimutagenic, and anticancer properties | in vitro | MAO inhibitor for ND treatment | [78] |
| 26. | Chelerythrine | 2703 | anti-inflammatory | in vitro | MAO-A inhibitor | [79] |
6. Conclusions
The reviewed compounds have the ability to lessen the symptoms of AD. With the increasing average life expectancy, it is critical to find and create novel molecules easily capable of preventing AD. Several natural compounds and phytochemicals have shown promise in clinical research for AD management. Several medications appear to be useful for AD treatment in clinical studies. Natural substances in the early stages of study require more investigation to determine their medicinal potential for AD management. It is critical to recognize that alternative therapies for AD may be widely supported in medical research.
Abbreviations
| AD | Alzheimer’s disease |
| AChE | Acetylcholinesterase |
| BuChE | Butyrylcholinesterase |
| Ach | Acetylcholine |
| CNS | Central nervous system |
| ND | Neurodegenerative disease |
| Aβ | β-amyloid |
| APP | Amyloid precursor protein |
| MAOs | Monoamine oxidases |
Author Contributions
Conceptualization, S.W.; validation, X.K. and Z.C.; formal analysis, G.W.; writing—original draft preparation, J.Z.; writing—review and editing, J.Z. and J.W. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dey A., Bhattacharya R., Mukherjee A., Pandey D.K. Natural products against Alzheimer’s disease: Pharmaco-therapeutics and biotechnological interventions. Biotechnol. Adv. 2017;35:178–216. doi: 10.1016/j.biotechadv.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Noetzli M., Eap C.B. Pharmacodynamic, Pharmacokinetic and Pharmacogenetic Aspects of Drugs Used in the Treatment of Alzheimer’s Disease. Clin. Pharmacokinet. 2013;52:225–241. doi: 10.1007/s40262-013-0038-9. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong R.A. Review article What causes alzheimer’s disease? Folia Neuropathol. 2013;3:169–188. doi: 10.5114/fn.2013.37702. [DOI] [PubMed] [Google Scholar]
- 4.Scheltens P., Blennow K., Breteler M.M., de Strooper B., Frisoni G.B., Salloway S., van der Flier W.M. Alzheimer’s disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 5.Alzheimer’s Association 2021 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021;17:327–406. doi: 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- 6.Iqubal A., Rahman S.O., Ahmed M., Bansal P., Haider R., Iqubal M.K., Najmi A.K., Pottoo F.H., Haque S.E. Current Quest in Natural Bioactive Compounds for Alzheimer’s Disease: Multi-Targeted-Designed-Ligand Based Approach with Preclinical and Clinical Based Evidence. Curr. Drug Targets. 2021;22:685–720. doi: 10.2174/1389450121999201209201004. [DOI] [PubMed] [Google Scholar]
- 7.Scheltens P., De Strooper B., Kivipelto M., Holstege H., Chételat G., Teunissen C.E., Cummings J., van der Flier W.M. Alzheimer’s disease. Lancet. 2021;397:1577–1590. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marucci G., Buccioni M., Ben D.D., Lambertucci C., Volpini R., Amenta F. Efficacy of acetylcholinesterase inhibitors in Alzheimer’s disease. Neuropharmacology. 2021;190:108352. doi: 10.1016/j.neuropharm.2020.108352. [DOI] [PubMed] [Google Scholar]
- 9.Andrade S., Ramalho M.J., Loureiro J.A., Pereira M.D.C. Natural Compounds for Alzheimer’s Disease Therapy: A Systematic Review of Preclinical and Clinical Studies. Int. J. Mol. Sci. 2019;20:2313. doi: 10.3390/ijms20092313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur D., Behl T., Sehgal A., Singh S., Sharma N., Bungau S. Multifaceted Alzheimer’s Disease: Building a Roadmap for Advancement of Novel Therapies. Neurochem. Res. 2021;46:2832–2851. doi: 10.1007/s11064-021-03415-w. [DOI] [PubMed] [Google Scholar]
- 11.Trottier G., Boström P.J., Lawrentschuk N., Fleshner N.E. Nutraceuticals and prostate cancer prevention: A current review. Nat. Rev. Urol. 2009;7:21–30. doi: 10.1038/nrurol.2009.234. [DOI] [PubMed] [Google Scholar]
- 12.Zeisel S.H. Regulation of “Nutraceuticals”. Science. 1999;285:1853–1855. doi: 10.1126/science.285.5435.1853. [DOI] [PubMed] [Google Scholar]
- 13.Sadhukhan P., Saha S., Dutta S., Mahalanobish S., Sil P.C. Nutraceuticals: An emerging therapeutic approach against the pathogenesis of Alzheimer’s disease. Pharmacol. Res. 2018;129:100–114. doi: 10.1016/j.phrs.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad S.S., Khalid M., Kamal M.A., Younis K. Study of Nutraceuticals and Phytochemicals for the Management of Alzheimer’s Disease: A Review. Curr. Neuropharmacol. 2021;19:1. doi: 10.2174/1570159X19666210215122333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues T., Reker D., Schneider P., Schneider G. Counting on natural products for drug design. Nat. Chem. 2016;8:531–541. doi: 10.1038/nchem.2479. [DOI] [PubMed] [Google Scholar]
- 16.Woo J., Yang H., Yoon M., Gadhe C.G., Pae A.N., Cho S., Lee S.C.A.C.J. 3-Carene, a Phytoncide from Pine Tree Has a Sleep-enhancing Effect by Targeting the GABAA-benzodiazepine Receptors. Exp. Neurobiol. 2019;28:593–601. doi: 10.5607/en.2019.28.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallianou I., Hadzopoulou-Cladaras M. Camphene, a Plant Derived Monoterpene, Exerts Its Hypolipidemic Action by Affecting SREBP-1 and MTP Expression. PLoS ONE. 2016;11:e0147117. doi: 10.1371/journal.pone.0147117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girola N., Figueiredo C.R., Farias C.F., Azevedo R.A., Ferreira A.K., Teixeira S.F., Capello T.M., Martins E.G., Matsuo A.L., Travassos L.R., et al. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem. Biophys. Res. Commun. 2015;467:928–934. doi: 10.1016/j.bbrc.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 19.Lenis-Rojas O.A., Robalo M.P., Tomaz A.I., Carvalho A., Fernandes A.R., Marques F., Folgueira M., Yáñez J., Vázquez-García D., Torres M.L., et al. RuII(p-cymene) Compounds as Effective and Selective Anticancer Candidates with No Toxicity In Vivo. Inorg. Chem. 2018;57:13150–13166. doi: 10.1021/acs.inorgchem.8b01270. [DOI] [PubMed] [Google Scholar]
- 20.Hou J., Zhang Y., Zhu Y., Zhou B., Ren C., Liang S., Guo Y. α-Pinene Induces Apoptotic Cell Death via Caspase Activation in Human Ovarian Cancer Cells. Med. Sci. Monit. 2019;25:6631–6638. doi: 10.12659/MSM.916419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aydin E., Türkez H., Taşdemir Ş. Anticancer and Antioxidant Properties of Terpinolene in Rat Brain Cells. Arch. Ind. Hyg. Toxicol. 2013;64:415–424. doi: 10.2478/10004-1254-64-2013-2365. [DOI] [PubMed] [Google Scholar]
- 22.Shin M., Liu Q.F., Choi B., Shin C., Lee B., Yuan C., Song Y.J., Yun H.S., Lee I.-S., Koo B.-S., et al. Neuroprotective Effects of Limonene (+) against Aβ42-Induced Neurotoxicity in a Drosophila Model of Alzheimer’s Disease. Biol. Pharm. Bull. 2020;43:409–417. doi: 10.1248/bpb.b19-00495. [DOI] [PubMed] [Google Scholar]
- 23.Sabogal-Guáqueta A.M., Hobbie F., Keerthi A., Oun A., Kortholt A., Boddeke E., Dolga A. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed. Pharmacother. 2019;118:109295. doi: 10.1016/j.biopha.2019.109295. [DOI] [PubMed] [Google Scholar]
- 24.Islam A., Hellman B., Nyberg F., Amir N., Jayaraj R., Petroainu G., Adem A. Myrcene Attenuates Renal Inflammation and Oxidative Stress in the Adrenalectomized Rat Model. Molecules. 2020;25:4492. doi: 10.3390/molecules25194492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Y., Luan J., Jiang R.P., Liu J., Ma Y. Myrcene exerts anti-asthmatic activity in neonatal rats via modulating the matrix remodeling. Int. J. Immunopathol. Pharmacol. 2020;34:2058738420954948. doi: 10.1177/2058738420954948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A., Agarwal K., Singh M., Saxena A., Yadav P., Maurya A.K., Yadav A., Tandon S., Chanda D., Bawankule D.U. Essential oil from waste leaves of Curcuma longa L. alleviates skin inflammation. Inflammopharmacology. 2018;26:1245–1255. doi: 10.1007/s10787-018-0447-3. [DOI] [PubMed] [Google Scholar]
- 27.Baldissera M.D., Grando T.H., Souza C.F., Gressler L.T., Stefani L.M., da Silva A.S., Monteiro S.G. In vitro and in vivo action of terpinen-4-ol, γ-terpinene, and α-terpinene against Trypanosoma evansi. Exp. Parasitol. 2016;162:43–48. doi: 10.1016/j.exppara.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Falk A., Löf A., Hagberg M., Hjelm E.W., Wang Z. Human exposure to 3-carene by inhalation: Toxicokinetics, effects on pulmonary function and occurrence of irritative and CNS symptoms. Toxicol. Appl. Pharmacol. 1991;110:198–205. doi: 10.1016/S0041-008X(05)80002-X. [DOI] [PubMed] [Google Scholar]
- 29.Schlumpf M., Durrer S., Faass O., Ehnes C., Fuetsch M., Gaille C., Henseler M., Hofkamp L., Maerkel K., Reolon S., et al. Developmental toxicity of UV filters and environmental exposure: A review. Int. J. Androl. 2008;31:144–151. doi: 10.1111/j.1365-2605.2007.00856.x. [DOI] [PubMed] [Google Scholar]
- 30.Dörsam B., Wu C.-F., Efferth T., Kaina B., Fahrer J. The eucalyptus oil ingredient 1,8-cineol induces oxidative DNA damage. Arch. Toxicol. 2014;89:797–805. doi: 10.1007/s00204-014-1281-z. [DOI] [PubMed] [Google Scholar]
- 31.Ravichandran C., Badgujar P.C., Gundev P., Upadhyay A. Review of toxicological assessment of d-limonene, a food and cosmetics additive. Food Chem. Toxicol. 2018;120:668–680. doi: 10.1016/j.fct.2018.07.052. [DOI] [PubMed] [Google Scholar]
- 32.Audrain H., Kenward C., Lovell C., Green C., Ormerod A., Sansom J., Chowdhury M., Cooper S., Johnston G., Wilkinson S.M., et al. Allergy to oxidized limonene and linalool is frequent in the U.K. Br. J. Dermatol. 2014;171:292–297. doi: 10.1111/bjd.13037. [DOI] [PubMed] [Google Scholar]
- 33.De Groot A.C., Schmidt E. Tea tree oil: Contact allergy and chemical composition. Contact Dermat. 2016;75:129–143. doi: 10.1111/cod.12591. [DOI] [PubMed] [Google Scholar]
- 34.Kuboyama T., Tohda C., Komatsu K. Effects of Ashwagandha (Roots of Withania somnifera) on Neurodegenerative Diseases. Biol. Pharm. Bull. 2014;37:892–897. doi: 10.1248/bpb.b14-00022. [DOI] [PubMed] [Google Scholar]
- 35.Corona J.C. Natural Compounds for the Management of Parkinson’s Disease and Attention-Deficit/Hyperactivity Disorder. BioMed Res. Int. 2018;2018:1–12. doi: 10.1155/2018/4067597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rehman M.U., Wali A.F., Ahmad A., Shakeel S., Rasool S., Ali R., Rashid S.M., Madkhali H., Ganaie M.A., Khan R. Neuroprotective Strategies for Neurological Disorders by Natural Products: An update. Curr. Neuropharmacol. 2019;17:247–267. doi: 10.2174/1570159X16666180911124605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Araújo F.F., de Paulo Farias D., Neri-Numa I.A., Pastore G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2020;338:127535. doi: 10.1016/j.foodchem.2020.127535. [DOI] [PubMed] [Google Scholar]
- 38.Silman I., Sussman J.L. Acetylcholinesterase: How is structure related to function? Chem. Interactions. 2008;175:3–10. doi: 10.1016/j.cbi.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 39.Lane R.M., Kivipelto M., Greig N.H. Acetylcholinesterase and Its Inhibition in Alzheimer Disease. Clin. Neuropharmacol. 2004;27:141–149. doi: 10.1097/00002826-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Lv M., Xu H. Acetylcholinesterase: A Primary Target for Drugs and Insecticides. Mini-Rev. Med. Chem. 2017;17:1665–1676. doi: 10.2174/1389557517666170120153930. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad S.S., Younis K., Philippe J., Aschner M., Khan H. Strategic approaches to target the enzymes using natural compounds for the management of Alzheimer’s disease: A review. CNS Neurol. Disord. Drug Targets. 2021;21:610–620. doi: 10.2174/1871527320666210811160007. [DOI] [PubMed] [Google Scholar]
- 42.Shah A.A., Dar T.A., Dar P.A., Ganie S.A., Kamal M.A. A Current Perspective on the Inhibition of Cholinesterase by Natural and Synthetic Inhibitors. Curr. Drug Metab. 2017;18:96–111. doi: 10.2174/1389200218666161123122734. [DOI] [PubMed] [Google Scholar]
- 43.Lee J.P., Kang M.-G., Lee J.Y., Oh J.M., Baek S.C., Leem H.H., Park D., Cho M.-L., Kim H. Potent inhibition of acetylcholinesterase by sargachromanol I from Sargassum siliquastrum and by selected natural compounds. Bioorganic Chem. 2019;89:103043. doi: 10.1016/j.bioorg.2019.103043. [DOI] [PubMed] [Google Scholar]
- 44.Ferreira J., Santos S., Pereira H. In Vitro Screening for Acetylcholinesterase Inhibition and Antioxidant Activity of Quercus suber Cork and Corkback Extracts. Evid. Based Complement. Altern. Med. 2020;2020:1–8. doi: 10.1155/2020/3825629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cespedes C.L., Muñoz E., Salazar J.R., Yamaguchi L., Werner E., Alarcon J., Kubo I. Inhibition of cholinesterase activity by extracts, fractions and compounds from Calceolaria talcana and C. integrifolia (Calceolariaceae: Scrophulariaceae) Food Chem. Toxicol. 2013;62:919–926. doi: 10.1016/j.fct.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 46.Krstić D.Z., Čolović M., Kralj M.B., Franko M., Krinulović K., Trebše P., Vasić V. Inhibition of AChE by malathion and some structurally similar compounds. J. Enzym. Inhib. Med. Chem. 2008;23:562–573. doi: 10.1080/14756360701632031. [DOI] [PubMed] [Google Scholar]
- 47.Hassan S.H.A., Fry J.R., Abu Bakar M.F. Phytochemicals Content, Antioxidant Activity and Acetylcholinesterase Inhibition Properties of IndigenousGarcinia parvifoliaFruit. BioMed Res. Int. 2013;2013:1–7. doi: 10.1155/2013/138950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmad S.S., Waheed T., Rozeen S., Mahmood S., Kamal M.A. Therapeutic Study of Phytochemicals Against Cancer and Alzheimer’s Disease Management. Curr. Drug Metab. 2020;20:1006–1013. doi: 10.2174/1389200221666200103092719. [DOI] [PubMed] [Google Scholar]
- 49.Calfio C., Gonzalez A., Singh S.K., Rojo L.E., Maccioni R.B. The Emerging Role of Nutraceuticals and Phytochemicals in the Prevention and Treatment of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020;77:33–51. doi: 10.3233/JAD-200443. [DOI] [PubMed] [Google Scholar]
- 50.Gregory J., Vengalasetti Y., Bredesen D., Rao R. Neuroprotective Herbs for the Management of Alzheimer’s Disease. Biomolecules. 2021;11:543. doi: 10.3390/biom11040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karthika C., Appu A.P., Akter R., Rahman H., Tagde P., Ashraf G.M., Abdel-Daim M.M., Hassan S.S.U., Abid A., Bungau S. Potential innovation against Alzheimer’s disorder: A tricomponent combination of natural antioxidants (vitamin E, quercetin, and basil oil) and the development of its intranasal delivery. Environ. Sci. Pollut. Res. 2022;29:10950–10965. doi: 10.1007/s11356-021-17830-7. [DOI] [PubMed] [Google Scholar]
- 52.Hartman R.E. Effects and mechanisms of actions of phytochemicals on Alzheimer rsquo s disease neuropathology. Front. Biosci. 2018;10:300–333. doi: 10.2741/e824. [DOI] [PubMed] [Google Scholar]
- 53.Camandola S., Plick N., Mattson M.P. Impact of Coffee and Cacao Purine Metabolites on Neuroplasticity and Neurodegenerative Disease. Neurochem. Res. 2018;44:214–227. doi: 10.1007/s11064-018-2492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernando W.M., Somaratne G., Goozee K.G., Williams S., Singh H., Martins R.N. Diabetes and Alzheimer’s Disease: Can Tea Phytochemicals Play a Role in Prevention? J. Alzheimer’s Dis. 2017;59:481–501. doi: 10.3233/JAD-161200. [DOI] [PubMed] [Google Scholar]
- 55.Hardy J., Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-V. [DOI] [PubMed] [Google Scholar]
- 56.Taylor H.A., Przemylska L., Clavane E.M., Meakin P.J. BACE1: More than just a beta-secretase. Obes. Rev. 2022;23:e13430. doi: 10.1111/obr.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.König G., Mönning U., Czech C., Prior R., Banati R., Schreiter-Gasser U., Bauer J., Masters C., Beyreuther K. Identification and differential expression of a novel alternative splice isoform of the beta A4 amyloid precursor protein (APP) mRNA in leukocytes and brain microglial cells. J. Biol. Chem. 1992;267:10804–10809. doi: 10.1016/S0021-9258(19)50090-4. [DOI] [PubMed] [Google Scholar]
- 58.Norstrom E. Metabolic processing of the amyloid precursor protein––New pieces of the Alzheimer’s puzzle. Discov. Med. 2017;23:269–276. [PubMed] [Google Scholar]
- 59.Sathya M., Premkumar P., Karthick C., Moorthi P., Jayachandran K., Anusuyadevi M. BACE1 in Alzheimer’s disease. Clin. Chim. Acta. 2012;414:171–178. doi: 10.1016/j.cca.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 60.He X., Zhu G., Koelsch G., Rodgers K.K., Zhang X.C., Tang J. Biochemical and Structural Characterization of the Interaction of Memapsin 2 (β-Secretase) Cytosolic Domain with the VHS Domain of GGA Proteins. Biochemistry. 2003;42:12174–12180. doi: 10.1021/bi035199h. [DOI] [PubMed] [Google Scholar]
- 61.Hrabinova M., Pejchal J., Kucera T., Juna D., Schmidt M., Soukup O. Is It the Twilight of BACE1 Inhibitors? Curr. Neuropharmacol. 2020;19:61–77. doi: 10.2174/1570159X18666200503023323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kennedy M.E., Wang W., Song L., Lee J., Zhang L., Wong G., Wang L., Parker E. Measuring human β-secretase (BACE1) activity using homogeneous time-resolved fluorescence. Anal. Biochem. 2003;319:49–55. doi: 10.1016/S0003-2697(03)00253-7. [DOI] [PubMed] [Google Scholar]
- 63.Eketjäll S., Janson J., Kaspersson K., Bogstedt A., Jeppsson F., Fälting J., Haeberlein S.B., Kugler A.R., Alexander R.C., Cebers G. AZD3293: A Novel, Orally Active BACE1 Inhibitor with High Potency and Permeability and Markedly Slow Off-Rate Kinetics. J. Alzheimer’s Dis. 2016;50:1109–1123. doi: 10.3233/JAD-150834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neumann U., Ufer M., Jacobson L., Rouzade-Dominguez M., Huledal G., Kolly C., Lüönd R.M., Machauer R., Veenstra S.J., Hurth K., et al. The BACE -1 inhibitor CNP 520 for prevention trials in Alzheimer’s disease. EMBO Mol. Med. 2018;10:e9316. doi: 10.15252/emmm.201809316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kennedy M.E., Stamford A.W., Chen X., Cox K., Cumming J.N., Dockendorf M.F., Egan M., Ereshefsky L., Hodgson R.A., Hyde L.A., et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer’s disease patients. Sci. Transl. Med. 2016;8:363ra150. doi: 10.1126/scitranslmed.aad9704. [DOI] [PubMed] [Google Scholar]
- 66.Sakamoto K., Matsuki S., Matsuguma K., Yoshihara T., Uchida N., Azuma F., Russell M., Hughes G., Haeberlein S.B., Alexander R.C., et al. BACE1 Inhibitor Lanabecestat (AZD3293) in a Phase 1 Study of Healthy Japanese Subjects: Pharmacokinetics and Effects on Plasma and Cerebrospinal Fluid Aβ Peptides. J. Clin. Pharmacol. 2017;57:1460–1471. doi: 10.1002/jcph.950. [DOI] [PubMed] [Google Scholar]
- 67.Timmers M., Van Broeck B., Ramael S., Slemmon J., De Waepenaert K., Russu A., Bogert J., Stieltjes H., Shaw L.M., Engelborghs S., et al. Profiling the dynamics of CSF and plasma Aβ reduction after treatment with JNJ-54861911, a potent oral BACE inhibitor. Alzheimer’s Dementia: Transl. Res. Clin. Interv. 2016;2:202–212. doi: 10.1016/j.trci.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopez C.L., Tariot P.N., Caputo A., Langbaum J.B., Liu F., Riviere M., Langlois C., Rouzade-Dominguez M., Zalesak M., Hendrix S., et al. The Alzheimer’s Prevention Initiative Generation Program: Study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer’s disease. Alzheimer’s Dementia: Transl. Res. Clin. Interv. 2019;5:216–227. doi: 10.1016/j.trci.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang C. Natural compounds that modulate BACE1-processing of amyloid-beta precursor protein in Alzheimer’s disease. Discov. Med. 2012;14:189–197. [PubMed] [Google Scholar]
- 70.Youn K., Park J.-H., Lee S., Lee S., Lee J., Yun E.-Y., Jeong W.-S., Jun M. BACE1 Inhibition by Genistein: Biological Evaluation, Kinetic Analysis, and Molecular Docking Simulation. J. Med. Food. 2018;21:416–420. doi: 10.1089/jmf.2017.4068. [DOI] [PubMed] [Google Scholar]
- 71.Naushad M., Durairajan S.S.K., Bera A.K., Senapati S., Li M. Natural Compounds with Anti-BACE1 Activity as Promising Therapeutic Drugs for Treating Alzheimer’s Disease. Planta Med. 2019;85:1316–1325. doi: 10.1055/a-1019-9819. [DOI] [PubMed] [Google Scholar]
- 72.Yu T., Paudel P., Seong S.H., Kim J.A., Jung H.A., Choi J.S. Computational insights into β-site amyloid precursor protein enzyme 1 (BACE1) inhibition by tanshinones and salvianolic acids from Salvia miltiorrhiza via molecular docking simulations. Comput. Biol. Chem. 2018;74:273–285. doi: 10.1016/j.compbiolchem.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 73.Youdim M.B.H., Edmondson D., Tipton K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 74.Shih J.C., Chen K., Ridd M.J. MONOAMINE OXIDASE: From Genes to Behavior. Annu. Rev. Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tipton K.F., Boyce S., O’Sullivan J., Davey G.P., Healy J. Monoamine Oxidases: Certainties and Uncertainties. Curr. Med. Chem. 2004;11:1965–1982. doi: 10.2174/0929867043364810. [DOI] [PubMed] [Google Scholar]
- 76.Kumar B., Dwivedi A.R., Sarkar B., Gupta S.K., Krishnamurthy S., Mantha A.K., Parkash J., Kumar V. 4,6-Diphenylpyrimidine Derivatives as Dual Inhibitors of Monoamine Oxidase and Acetylcholinesterase for the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2018;10:252–265. doi: 10.1021/acschemneuro.8b00220. [DOI] [PubMed] [Google Scholar]
- 77.Orhan I.E. Potential of Natural Products of Herbal Origin as Monoamine Oxidase Inhibitors. Curr. Pharm. Des. 2015;22:268–276. doi: 10.2174/1381612822666151112150612. [DOI] [PubMed] [Google Scholar]
- 78.Juvekar A.R., Khatri D. Kinetics of inhibition of monoamine oxidase using curcumin and ellagic acid. Pharmacogn. Mag. 2016;12:116–120. doi: 10.4103/0973-1296.182168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baek S.C., Ryu H.W., Kang M.-G., Lee H., Park D., Cho M.-L., Oh S.-R., Kim H. Selective inhibition of monoamine oxidase A by chelerythrine, an isoquinoline alkaloid. Bioorganic Med. Chem. Lett. 2018;28:2403–2407. doi: 10.1016/j.bmcl.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 80.Chaurasiya N.D., Midiwo J., Pandey P., Bwire R.N., Doerksen R.J., Muhammad I., Tekwani B.L. Selective Interactions of O-Methylated Flavonoid Natural Products with Human Monoamine Oxidase-A and -B. Molecules. 2020;25:5358. doi: 10.3390/molecules25225358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Santos G., Giraldez-Alvarez L.D., Avila-Rodríguez M., Capani F., Galembeck E., Neto A.G., Barreto G.E., Andrade B. SUR1 Receptor Interaction with Hesperidin and Linarin Predicts Possible Mechanisms of Action of Valeriana officinalis in Parkinson. Front. Aging Neurosci. 2016;8:97. doi: 10.3389/fnagi.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.González-Muñoz G.C., Arce M.P., Pérez C., Romero A., Villarroya M., Lopez M.G., Conde S., Rodríguez-Franco M.I. Dibenzo[1,4,5]thiadiazepine: A hardly-known heterocyclic system with neuroprotective properties of potential usefulness in the treatment of neurodegenerative diseases. Eur. J. Med. Chem. 2014;81:350–358. doi: 10.1016/j.ejmech.2014.04.075. [DOI] [PubMed] [Google Scholar]
- 83.Kaufmann D., Kaur Dogra A., Tahrani A., Herrmann F., Wink M. Extracts from Traditional Chinese Medicinal Plants Inhibit Acetylcholinesterase, a Known Alzheimer’s Disease Target. Molecules. 2016;21:1161. doi: 10.3390/molecules21091161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Singh A.K., Singh S.K., Nandi M.K., Mishra G., Maurya A., Rai A., Rai G.K., Awasthi R., Sharma B., Kulkarni G.T. Berberine: A Plant-derived Alkaloid with Therapeutic Potential to Combat Alzheimer’s disease. Central Nerv. Syst. Agents Med. Chem. 2019;19:154–170. doi: 10.2174/1871524919666190820160053. [DOI] [PubMed] [Google Scholar]
- 85.Howes M.-J.R., Simmonds M.S. The role of phytochemicals as micronutrients in health and disease. Curr. Opin. Clin. Nutr. Metab. Care. 2014;17:558–566. doi: 10.1097/MCO.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 86.Larit F., Elokely K.M., Chaurasiya N.D., Benyahia S., Nael M.A., Leon F., Abu-Darwish M.S., Efferth T., Wang Y.-H., Belouahem-Abed D., et al. Inhibition of human monoamine oxidase A and B by flavonoids isolated from two Algerian medicinal plants. Phytomedicine. 2017;40:27–36. doi: 10.1016/j.phymed.2017.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sharifi-Rad J., Quispe C., Imran M., Rauf A., Nadeem M., Gondal T.A., Ahmad B., Atif M., Mubarak M.S., Sytar O., et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxidative Med. Cell. Longev. 2021;2021:1–36. doi: 10.1155/2021/3268136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Menze E.T., Tadros M.G., Abdel-Tawab A.M., Khalifa A. Potential neuroprotective effects of hesperidin on 3-nitropropionic acid-induced neurotoxicity in rats. NeuroToxicology. 2012;33:1265–1275. doi: 10.1016/j.neuro.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 89.Chakraborty S., Bandyopadhyay J., Chakraborty S., Basu S. Multi-target screening mines hesperidin as a multi-potent inhibitor: Implication in Alzheimer’s disease therapeutics. Eur. J. Med. Chem. 2016;121:810–822. doi: 10.1016/j.ejmech.2016.03.057. [DOI] [PubMed] [Google Scholar]
- 90.Parhiz H., Roohbakhsh A., Soltani F., Rezaee R., Iranshahi M. Antioxidant and Anti-Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of their Molecular Mechanisms and Experimental Models. Phytotherapy Res. 2014;29:323–331. doi: 10.1002/ptr.5256. [DOI] [PubMed] [Google Scholar]
- 91.Carmona V., Martín-Aragón S., Goldberg J., Schubert D., Bermejo-Bescós P. Several targets involved in Alzheimer’s disease amyloidogenesis are affected by morin and isoquercitrin. Nutr. Neurosci. 2018;23:575–590. doi: 10.1080/1028415X.2018.1534793. [DOI] [PubMed] [Google Scholar]
- 92.Rajput S.A., Wang X.-Q., Yan H.-C. Morin hydrate: A comprehensive review on novel natural dietary bioactive compound with versatile biological and pharmacological potential. Biomed. Pharmacother. 2021;138:111511. doi: 10.1016/j.biopha.2021.111511. [DOI] [PubMed] [Google Scholar]
- 93.Shal B., Ding W., Ali H., Kim Y.S., Khan S. Anti-neuroinflammatory Potential of Natural Products in Attenuation of Alzheimer’s Disease. Front. Pharmacol. 2018;9:548. doi: 10.3389/fphar.2018.00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dar N.J., Hamid A., Ahmad M. Pharmacologic overview of Withania somnifera, the Indian Ginseng. Exp. 2015;72:4445–4460. doi: 10.1007/s00018-015-2012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fang J., Liu R., Tian Q., Hong X.-P., Wang S.-H., Cao F.-Y., Pan X.-P., Wang J.-Z. Dehydroevodiamine attenuates calyculin A-induced tau hyperphos-phorylation in rat brain slices. Acta Pharmacol. Sin. 2007;28:1717–1723. doi: 10.1111/j.1745-7254.2007.00655.x. [DOI] [PubMed] [Google Scholar]
- 96.Wang C.-Y., Zheng W., Wang T., Xie J.-W., Wang S.-L., Zhao B.-L., Teng W.-P., Wang Z.-Y. Huperzine A Activates Wnt/β-Catenin Signaling and Enhances the Nonamyloidogenic Pathway in an Alzheimer Transgenic Mouse Model. Neuropsychopharmacology. 2011;36:1073–1089. doi: 10.1038/npp.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang Z.-D., Zhang X., Du J., Ma Z.-J., Guo F., Li S., Yao X.-J. An aporphine alkaloid from Nelumbo nucifera as an acetylcholinesterase inhibitor and the primary investigation for structure–activity correlations. Nat. Prod. Res. 2012;26:387–392. doi: 10.1080/14786419.2010.487188. [DOI] [PubMed] [Google Scholar]
- 98.Xian Y.-F., Mao Q.-Q., Wu J.C., Su Z.-R., Chen J.-N., Lai X.-P., Ip S.-P., Lin Z.-X. Isorhynchophylline Treatment Improves the Amyloid-β-Induced Cognitive Impairment in Rats via Inhibition of Neuronal Apoptosis and Tau Protein Hyperphosphorylation. J. Alzheimer’s Dis. 2014;39:331–346. doi: 10.3233/JAD-131457. [DOI] [PubMed] [Google Scholar]
- 99.Haj E., Losev Y., KrishnaKumar V.G., Pichinuk E., Engel H., Raveh A., Gazit E., Segal D. Integrating in vitro and in silico approaches to evaluate the “dual functionality” of palmatine chloride in inhibiting and disassembling Tau-derived VQIVYK peptide fibrils. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2018;1862:1565–1575. doi: 10.1016/j.bbagen.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 100.López S., Bastida J., Viladomat F., Codina C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002;71:2521–2529. doi: 10.1016/S0024-3205(02)02034-9. [DOI] [PubMed] [Google Scholar]
- 101.Rollinger J.M., Schuster D., Baier E., Ellmerer E.P., Langer T., Stuppner H. Taspine: Bioactivity-Guided Isolation and Molecular Ligand−Target Insight of a Potent Acetylcholinesterase Inhibitor from Magnolia x soulangiana. J. Nat. Prod. 2006;69:1341–1346. doi: 10.1021/np060268p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ahmad S., Khan M., Ahmad K., Lim J.-H., Shaikh S., Lee E.-J., Choi I. Biocomputational Screening of Natural Compounds against Acetylcholinesterase. Molecules. 2021;26:2641. doi: 10.3390/molecules26092641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Qi T., Li H., Li S. Indirubin improves antioxidant and anti-inflammatory functions in lipopolysaccharide-challenged mice. Oncotarget. 2017;8:36658–36663. doi: 10.18632/oncotarget.17560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahmad S.S., Sinha M., Ahmad K., Khalid M., Choi I. Study of Caspase 8 Inhibition for the Management of Alzheimer’s Disease: A Molecular Docking and Dynamics Simulation. Molecules. 2020;25:2071. doi: 10.3390/molecules25092071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ahmad S.S., Akhtar S., Rizvi S.M.D., Kamal M.A., Sayeed U., Khan M.K.A., Siddiqui M.H., Arif J.M. Screening and Elucidation of Selected Natural Compounds for Anti- Alzheimer’s Potential Targeting BACE-1 Enzyme: A Case Computational Study. Curr. Comput. Aided-Drug Des. 2017;13:311–318. doi: 10.2174/1573409913666170414123825. [DOI] [PubMed] [Google Scholar]
- 106.Kou X., Chen N. Resveratrol as a Natural Autophagy Regulator for Prevention and Treatment of Alzheimer’s Disease. Nutrients. 2017;9:927. doi: 10.3390/nu9090927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang M., Taghibiglou C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017;58:1003–1016. doi: 10.3233/JAD-170188. [DOI] [PubMed] [Google Scholar]
- 108.Ji H.-F., Zhang H.-Y. Multipotent natural agents to combat Alzheimer’s disease. Functional spectrum and structural features. Acta Pharmacol. Sin. 2008;29:143–151. doi: 10.1111/j.1745-7254.2008.00752.x. [DOI] [PubMed] [Google Scholar]