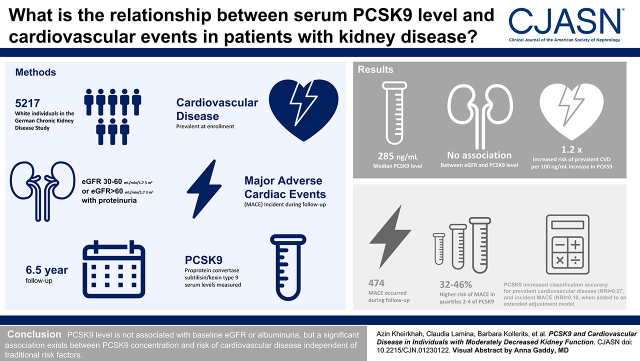
Visual Abstract
Keywords: chronic kidney disease, cardiovascular disease, PCSK9, prospective
Abstract
Background and objectives
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a key regulator of lipid homeostasis. Studies investigating the association between PCSK9 and cardiovascular disease in large cohorts of patients with CKD are limited.
Design, setting, participants, & measurements
The association of PCSK9 concentrations with prevalent and incident cardiovascular disease was investigated in 5138 White participants of the German Chronic Kidney Disease study with a median follow-up of 6.5 years. Inclusion criteria were eGFR of 30–60 or >60 ml/min per 1.73 m2 in the presence of overt proteinuria (urine albumin-creatinine ratio >300 mg/g or equivalent). Prevalent cardiovascular disease was defined as a history of nonfatal myocardial infarction, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, carotid arteries interventions, and stroke. Incident major adverse cardiovascular disease events included death from cardiovascular causes, acute nonfatal myocardial infarction, and nonfatal stroke.
Results
Median PCSK9 concentration in the cohort was 285 ng/ml (interquartile range, 231–346 ng/ml). There was no association between PCSK9 concentrations and baseline eGFR and albuminuria. With each 100-ng/ml increment of PCSK9, the odds for prevalent cardiovascular disease (n=1284) were 1.22-fold (95% confidence interval, 1.12 to 1.34; P<0.001) higher in a model with extended adjustment for major confounders. This association was stronger in nonstatin than statin users (P value for interaction =0.009). During follow-up, 474 individuals experienced a major adverse cardiovascular disease event, and participants in PCSK9 quartiles 2–4 had a 32%–47% higher risk compared with those in quartile 1 (P<0.05). Subgroup analysis revealed that this association was restricted to those participants who already had cardiovascular disease at baseline (all hazard ratios >1.75; P=0.01). In addition, PCSK9 showed a valuable gain in classification accuracy for both prevalent cardiovascular disease (net reclassification index =0.27; 95% confidence interval, 0.20 to 0.33) and incident major adverse cardiovascular disease events during follow-up (net reclassification index =0.10; 95% confidence interval, 0.01 to 0.21) when added to an extended adjustment model.
Conclusions
Our findings reveal no relation of PCSK9 with baseline eGFR and albuminuria but a significant association between higher PCSK9 concentrations and risk of cardiovascular disease independent of traditional risk factors, including LDL cholesterol levels.
Clinical Trial registry name and registration number: German Chronic Kidney Disease Study (GCKD), DRKS 00003971
Introduction
CKD remains a major health burden, with a prevalence of 9% worldwide (1). CKD-related mortalities account yearly for 1.2 million deaths globally, and an additional 1.4 million cardiovascular deaths have been reported to be due to impaired kidney function (1). CKD and cardiovascular disease are highly inter-related. One of the main causes of cardiovascular disease is the impaired lipid homeostasis. Patients with CKD have a higher risk for developing dyslipidemia due to impaired kidney function (2,3).
The role of proprotein convertase subtilisin/kexin type 9 (PCSK9) in lipid homeostasis was discovered less than two decades ago (4). It has meanwhile been well established that PCSK9 is a key regulator of serum LDL cholesterol levels. PCSK9 hinders the uptake of LDL cholesterol by targeting LDL receptors on the surface of hepatocytes for lysosomal degradation (5). PCSK9 inhibitors can decrease LDL cholesterol levels by 60% and strongly reduce the risk of cardiovascular events (6,7).
The existing data on PCSK9 concentrations in patients with CKD are very heterogeneous. Studies investigating whether PCSK9 concentrations are influenced by kidney function have mostly been small and revealed divergent results (8) (an overview is in Supplemental Table 1). The same holds true for PCSK9 concentrations and cardiovascular disease outcomes in patients with CKD (9–13) (Supplemental Table 2); most of the few studies conducted were either small and/or observed only a small number of cardiovascular disease events, which limits their conclusiveness.
In this study, we aimed to investigate (1) whether kidney function is associated with PCSK9 concentrations, (2) which other variables influence PCSK9 concentrations in individuals with CKD, and (3) whether PCSK9 concentrations are associated with cardiovascular disease outcomes. We performed these investigations in a large population of >5000 participants with moderate CKD and a prospective follow-up of up to 6.5 years.
Materials and Methods
German Chronic Kidney Disease Study Design
The German Chronic Kidney Disease (GCKD) study is an ongoing prospective cohort study including 5217 White participants with moderately severe CKD. A detailed description of the design and characteristics of the study has been published (14–16). In brief, inclusion criteria were age between 18 and 74 years and an eGFR of 30–60 ml/min per 1.73 m2 (Kidney Disease Improving Global Outcomes [KDIGO] stage G3, A1–A3) or an eGFR>60 ml/min per 1.73 m2 in the presence of overt proteinuria defined by a urine albumin-creatinine ratio (UACR) >300 mg/g or equivalent (KDIGO stage G1–G2, A3) under regular care by nephrologists. Additional information on inclusion/exclusion criteria and definitions of baseline variables are provided in Supplemental Material. The study was approved by the ethics committees of all participating institutions and was registered in the national registry for clinical studies. All data were collected and managed using Askimed as a cloud-based web platform (https://www.askimed.com).
Outcome Definitions
At baseline, prevalent cardiovascular disease was defined as a history of nonfatal myocardial infarction, coronary artery bypass grafting, percutaneous transluminal coronary angioplasty, interventions at the carotid arteries (carotid endarterectomy and/or carotid balloon angioplasty or stent implantation), and stroke.
Details on data collection for end points, time to event, and censoring are provided in Supplemental Material and have been described previously (14–16). Two incident major adverse cardiovascular event (MACE) groups were collected, namely three-point MACE and four-point MACE. Three-point MACE includes death from cardiovascular causes (myocardial infarction, coronary artery disease, sudden cardiac death, ischemic stroke) as well as acute nonfatal myocardial infarction (ST-elevation myocardial infarction [STEMI] and non–ST-elevation myocardial infarction [NSTEMI]) and nonfatal stroke. Four-point MACE was defined as three-point MACE but with the addition of fatal peripheral ischemia, amputation due to peripheral vascular disease, and surgical/percutaneous revascularization due to peripheral vascular disease. Incident events are defined as events that newly occur during the follow-up time of the study, irrespective of the occurrence of the event previously (prevalent events).
Proprotein Convertase Subtilisin/Kexin Type 9 and Lipid Measurements
Serum samples were stored at −80°C. PCSK9 concentrations were quantified by a commercial human PCSK9 ELISA kit (R&D Systems, Minneapolis, MN). Intra- and interassay coefficients of variation were 4% and 7%, respectively (determined with four control samples measured on each plate).
Statistical Analyses
A detailed description of the applied statistical methods is provided in Supplemental Material. Briefly, variables that are independently associated with PCSK9 concentration were determined by linear regression analysis. Logistic regression analysis was done to evaluate the association between PCSK9 and prevalent cardiovascular disease using different adjustment models. For better interpretability and to check for possible nonlinear associations, odds ratio (ORs) and 95% confidence intervals (95% CIs) were not only given for each 100-ng/ml higher PCSK9 level but also for quartile groups of PCSK9 (quartile 1 as reference), and linearity has been examined by nonlinear penalized splines. Cox regression analysis for the first event on study was used to calculate hazard ratios (HRs) and their 95% CIs. The prospectively collected end points considered in this analysis refer to the first 6.5 (interquartile range [IQR], 6.5–6.5) years of follow-up (censoring date) on the basis of data export from October 10, 2020. For both logistic and Cox regression analyses, different models adjusted for various confounders were used, and details can be found in the tables, figures, and the Results section. We tested for interaction with statin use and sex due to the pronounced association of these two variables with PCSK9 concentrations. To assess whether PCSK9 concentrations contribute to a better risk classification of individuals in terms of prevalent cardiovascular disease and incident MACE, the continuous net reclassification index (NRI) was applied. We used the complete case approach for handling the missing data within our study.
All statistical analyses were conducted using R 3.5.2. (Vienna, Austria; https://www.r-project.org/), and P values of 0.05 were considered statistically significant.
Results
Baseline Characteristics
The median PCSK9 concentration among all 5138 participants was 285 ng/ml (IQR, 231–346 ng/ml). Table 1 represents baseline characteristics of the participants of the entire cohort and stratified by quartiles of PCSK9 concentrations; 48% of the participants received statins. With increasing quartiles of PCSK9, we observed a higher frequency of participants with comorbid conditions, including diabetes mellitus and cardiovascular diseases. Also, total cholesterol, HDL cholesterol, triglycerides, and lipoprotein(a) concentrations were significantly higher across PCSK9 quartiles.
Table 1.
Baseline characteristics of participants in the German Chronic Kidney Disease study and in stratified groups on the basis of quartiles of proprotein convertase subtilisin/kexin type 9 serum concentrations
| Baseline Characteristics | Proprotein Convertase Subtilisin/Kexin Type 9 Quartiles | ||||
|---|---|---|---|---|---|
| Total | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| PCSK9 range, ng/ml | 55–847 | <231 | 231–285 | 285–346 | >346 |
| PCSK9, mean±SD | 294±87 | 194±30 | 258±15 | 313±18 | 411±58 |
| PCSK9, median [25th–75th percentile] | 285 [231–346] | 201 [178–217] | 258 [245–270] | 312 [298–328] | 395 [367–439] |
| No. of participants, n | 5138 | 1285 | 1284 | 1284 | 1285 |
| Demographics | |||||
| Age, yr | 60±12 | 59±13 | 60±12 | 61±12 | 60±12 |
| Women, n (%) | 2048 (40) | 417 (33) | 488 (38) | 566 (44) | 577 (45) |
| Body mass index, kg/m2 | 29.8±5.9 | 29.0±5.7 | 29.6±6.0 | 29.9±6.0 | 30.7±5.9 |
| Current smokers, n (%) | 812 (16) | 183 (14) | 219 (17) | 193 (15) | 217 (17) |
| Medical history, n (%) | |||||
| Diabetes mellitus | 1827 (36) | 355 (28) | 440 (34) | 466 (36) | 566 (44) |
| Hypertension | 4946 (96) | 1218 (95) | 1236 (96) | 1234 (96) | 1258 (98) |
| Cardiovascular disease | 1322 (26)a | 233 (18) | 288 (22) | 361 (28) | 440 (34) |
| Medications, n (%) | |||||
| Statin treatment | 2440 (48) | 302 (24) | 478 (37) | 692 (54) | 968 (75) |
| Laboratory values | |||||
| Total cholesterol, mg/dl | 211±53 | 205±46 | 211±46.6 | 212±53 | 217±63 |
| Triglyceride, mg/dl | 199±126 | 178±110 | 191±110 | 200±123 | 227±151 |
| HDL cholesterol, mg/dl | 52±18 | 51±18 | 51±17 | 53±18 | 54±19 |
| LDL cholesterol, mg/dl | 118±44 | 117±39.5 | 121±39 | 118±43 | 117±51 |
| Lipoprotein(a), mg/dl | 24.6±30.4 | 20.8±25.2 | 23.2±27.4 | 25.0±30.4 | 29.6±36.8 |
| hs-CRP, mg/L | 4.7±7.8 | 4.2±5.9 | 5.1±7.8 | 4.8±8.7 | 4.8±8.5 |
| Serum albumin, g/dl | 3.8±0.4 | 3.9±0.4 | 3.8±0.4 | 3.8±0.4 | 3.8±0.5 |
| eGFR, ml/min per 1.73 m2 | 49±18 | 50±18 | 50±18 | 49±18 | 49±18 |
| UACR, mg/g | 429±958 | 367±765 | 359±736 | 457±1090 | 532±1158 |
Data are presented as mean±SD for continuous variables and n (percentage) for categorical measures. eGFR was calculated on the basis of the Chronic Kidney Disease Epidemiology Collaboration equation. In some participants, we had missing data: UACR in 2%; body mass index in 1%; total, LDL, and HDL cholesterol and triglycerides in 0.7%; eGFR, serum albumin, and hsCRP in 0.5%; and current smoking in 0.3%. PCSK9, proprotein convertase subtilisin/kexin type 9; hs-CRP, high-sensitivity C-reactive protein; UACR, urine albumin-creatinine ratio.
Because of a few missing values, this number is slightly higher than 1289 of the 5037 participants with PCSK9 measurements and information on age, sex, eGFR, and UACR.
Variables Associated with Proprotein Convertase Subtilisin/Kexin Type 9 Levels at Baseline Investigation
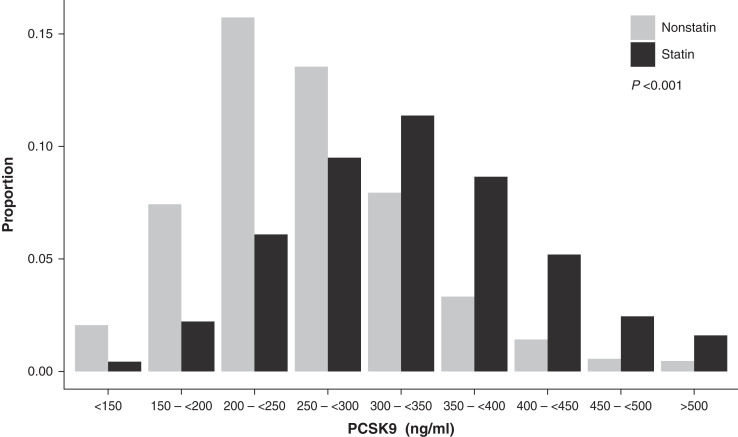
Figure 1 shows the distribution of PCSK9 concentrations in the GCKD study. Participants on statin treatment had significantly higher PCSK9 levels when compared with those not on statin treatment: median of 323 (IQR, 266–383) ng/ml versus 254 (IQR, 212–303) ng/ml, respectively (P<0.001). Table 2 shows the variables independently associated with PCSK9 concentrations in an adjusted linear regression model in the entire GCKD population. Statins showed the strongest association, with 70 ng/ml higher PCSK9 concentrations in statin-treated compared with nonstatin-treated participants. Higher LDL cholesterol, women, and diabetes mellitus were associated with higher PCSK9 concentrations. Each of the other variables explained <0.5% of the entire variance or 3% (<0.005/0.2019) of the total explained variance of the model. Kidney function parameters, such as eGFR or UACR as well as high-sensitivity C-reactive protein, were not associated with PCSK9 concentrations.
Figure 1.
Distribution of serum proprotein convertase subtilisin/kexin type 9 (PCSK9) concentrations in the German Chronic Kidney Disease (GCKD) study. The bar plot represents the PCSK9 serum concentrations, comparing participants under statin treatment (n=2440) with the nonstatin-treated individuals (n=2698) in the GCKD study population. Individuals treated with statins have significantly higher levels of PCSK9 than untreated individuals.
Table 2.
Clinical characteristics associated with serum proprotein convertase subtilisin/kexin type 9 (PCSK9) concentration
| Variable (Increment) | Adjusted Difference in PCSK9 Concentration, ng/ml [95% Confidence Interval]a | Proportional Marginal Variance Decompositionb [95% Confidence Interval]a | P Value |
|---|---|---|---|
| Statin treatment | 70.0 [74.9 to 65.1] | 0.1480 [0.1313 to 0.1662] | <0.001 |
| LDL cholesterol, 10 mg/dl | 2.6 [2.0 to 3.1] | 0.0167 [0.0111 to 0.0235] | <0.001 |
| Women | 21.5 [16.6 to 26.4] | 0.0162 [0.0099 to 0.0228] | <0.001 |
| Diabetes mellitus | 13.6 [8.6 to 18.7] | 0.0054 [0.0020 to 0.0099] | <0.001 |
| HDL cholesterol, 10 mg/dl | 3.1 [1.8 to 4.5] | 0.0037 [0.0010 to 0.0081] | <0.001 |
| Body mass index, 1 kg/m2 | 0.92 [0.52 to 1.33] | 0.0036 [0.0012 to 0.0073] | <0.001 |
| Cardiovascular disease | 11.3 [5.8 to 16.7] | 0.0028 [0.0007 to 0.0061] | <0.001 |
| Age, 10 yr | −3.7 [–5.8 to –1.6] | 0.0026 [0.0006 to 0.0055] | <0.001 |
| Current smoking | 5.3 [2.2 to 8.4] | 0.0022 [0.0004 to 0.0050] | <0.001 |
| Lipoprotein(a),a 10 mg/dl | 1.09 [0.35 to 1.82] | 0.0006 [0.0000 to 0.0030] | 0.07 |
| UACR,b 100 mg/g | 0.30 [0.06 to 0.54] | 0.0001 [0.0000 to 0.0018] | 0.42 |
| eGFR, 10 ml/min per 1.73 m2 | 0.41 [–0.89 to 1.70] | 4.16E-05 [0.0000 to 0.0016] | 0.65 |
| hs-CRP,b 1 mg/L | 0.23 [–5.32 to 0.52] | 8.69E-06 [0.0000 to 0.0009] | 0.83 |
| Σ = 0.2019 (total R2) |
A multivariable regression analysis was performed to identify variables that are significantly associated with PCSK9 concentrations in the entire population at the baseline investigation. All variables were included in one single model and are ordered by their relative importance to the model given by the proportional marginal variance decomposition (pmvd) metric. Estimates for the continuous variables are provided for a clinically relevant increment. UACR, urine albumin-creatinine ratio; hs-CRP, high-sensitivity C-reactive protein.
For variables that were skewed, β-estimates were taken from the untransformed model, whereas their relative importance (pmvd) and P values were calculated from the log-transformed model.
pmvd is defined as the relative importance of all included variables on proprotein convertase subtilisin/kexin type 9 concentrations.
Additionally, Supplemental Figure 1 represents PCSK9 concentrations across KDIGO risk categories on the basis of eGFR and UACR (17). There are only slight differences in PCSK9 concentrations among different categories, except in participants with nephrotic-range albuminuria (defined by UACR >2220 mg/g [17]) who had higher mean PCSK9 concentrations (306±80 compared with 293±78 ng/ml in non-nephrotic–range albuminuria participants; P=0.02, adjusted for major confounders).
Association of Proprotein Convertase Subtilisin/Kexin Type 9 Concentrations with Prevalent Cardiovascular Disease
At baseline, 1289 of 5037 participants with PCSK9 measurements and information on age, sex, eGFR, and UACR had already experienced a cardiovascular event. In a logistic model adjusted for these variables, each 100-ng/ml higher PCSK9 was associated with a higher odds for prevalent cardiovascular disease (OR, 1.56; 95% CI, 1.44 to 1.69; P<0.001) (Supplemental Table 3). Nonlinear P spline supported the linear association between PCSK9 and prevalent cardiovascular disease (Supplemental Figure 2A). Additional adjustment for statin use attenuated the estimate to 1.21. Further inclusion of diabetes mellitus, hypertension, smoking status, LDL cholesterol, HDL cholesterol, lipoprotein(a), and high-sensitivity C-reactive protein had no major effect on the still significant association. We therefore tested for an interaction with statin treatment and observed a significant interaction in all models, which was most pronounced for the extended adjusted model 3 (P for interaction =0.009). We performed further analyses stratifying for statin treatment and observed a significantly higher odds with every 100-ng/ml higher serum PCSK9 in both groups. However, this was weaker in statin-treated compared with nonstatin-treated individuals (OR, 1.16; 95% CI, 1.04 to 1.29; P=0.008 versus OR, 1.38; 95% CI, 1.18 to 1.62; P<0.001) (Supplemental Table 3). This was in line with P-spline analyses (Supplemental Figure 2, B and C). There was no interaction with sex (P for interaction =0.27).
For easier interpretability, the association between PCSK9 concentrations and odds for cardiovascular disease is also shown by PCSK9 quartiles (Table 3, Supplemental Figure 3A). The estimates were steadily higher from quartiles 2 to 4 and remained significant in the adjusted model in quartiles 3 and 4, with 36% and 51% higher odds, respectively. Because of a significant interaction between PCSK9 quartiles and statin treatment (P for interaction <0.001), we stratified further analysis for statin treatment. We still observed a higher odds in the upper two quartiles of PCSK9 in the nonstatin users (Table 3, Supplemental Figure 3B) but no longer observed an association in statin users (Table 3, Supplemental Figure 3C).
Table 3.
Associations of serum proprotein convertase subtilisin/kexin type 9 concentrations with prevalent cardiovascular disease
| Proprotein Convertase Subtilisin/Kexin Type 9 Quartiles | Adjustment Model 1 | Adjustment Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| N/Na | %a | Odds Ratio [95% Confidence Interval] | P Value | N/Na | %a | Odds Ratio [95% Confidence Interval] | P Value | |
| All GCKD study participants (1289 of 5037 participants with events) | ||||||||
| Quartile 1 | 225/1261 | 18 | 222/1250 | 18 | ||||
| Quartile 2 | 284/1262 | 23 | 1.38 [1.12 to 1.69] | 0.002 | 284/1258 | 23 | 1.12 [0.90 to 1.40] | 0.29 |
| Quartile 3 | 356/1263 | 28 | 1.94 [1.59 to 2.37] | <0.001 | 355/1258 | 28 | 1.36 [1.10 to 1.69] | 0.006 |
| Quartile 4 | 424/1251 | 34 | 2.76 [2.27 to 3.37] | <0.001 | 423/1244 | 34 | 1.51 [1.21 to 1.89] | <0.001 |
| Participants without statin treatmentb (336 of 2646 participants with events) | ||||||||
| Quartile 1 | 106/964 | 11 | 104/955 | 11 | ||||
| Quartile 2 | 90/788 | 11 | 1.12 [0.82 to 1.52] | 0.49 | 90/785 | 11 | 1.06 [0.77 to 1.45] | 0.73 |
| Quartile 3 | 97/584 | 17 | 1.98 [1.44 to 2.70] | <0.001 | 97/583 | 17 | 2.06 [1.49 to 2.84] | <0.001 |
| Quartile 4 | 43/310 | 14 | 1.64 [1.09 to 2.42] | 0.02 | 43/307 | 14 | 1.63 [1.07 to 2.44] | 0.02 |
| Participants with statin treatmentb (953 of 2391 participants with events) | ||||||||
| Quartile 1 | 119/297 | 40 | 118/295 | 40 | ||||
| Quartile 2 | 194/474 | 41 | 1.06 [0.78 to 1.44] | 0.72 | 194/473 | 41 | 1.05 [0.76 to 1.44] | 0.78 |
| Quartile 3 | 259/679 | 38 | 0.98 [0.73 to 1.31] | 0.87 | 258/675 | 38 | 0.97 [0.72 to 1.32] | 0.85 |
| Quartile 4 | 381/941 | 40 | 1.30 [0.98 to 1.73] | 0.07 | 380/937 | 41 | 1.29 [0.96 to 1.74] | 0.09 |
This association is presented for each proprotein convertase subtilisin/kexin type 9 quartile in the total GCKD population and stratified on the basis of statin treatment. The presented data are results from logistic regression analyses (odds ratio [95% confidence interval]) for the basic and fully adjusted models: Model 1 and Model 3, respectively. Model 2 is presented as a forest plot in Supplemental Material (Supplemental Figure 3). Model 1 was adjusted for age, sex, eGFR, and urine albumin-creatinine ratio. Model 3 was adjusted for age, sex, eGFR, urine albumin-creatinine ratio, statin treatment, HDL cholesterol, lipoprotein(a), high-sensitivity C-reactive protein, diabetes mellitus, hypertension, smoking, and LDL cholesterol. GCKD, German Chronic Kidney Disease.
N/N (percentage) is the number of participants with events/total number of participants (percentage of participants with events).
Because of statin stratification, these groups do not include statin treatment as confounders.
Association of Proprotein Convertase Subtilisin/Kexin Type 9 with Time to First Major Adverse Cardiovascular Event
During a median follow-up of 6.5 years, 474 participants experienced a three-point MACE, and 653 experienced a four-point MACE. There was no indication for violation of the proportional hazards assumption. The Cox regression analysis with extended adjustment revealed no significant linear association between PCSK9 concentrations and outcomes (P splines in Supplemental Figure 4, Supplemental Tables 4 and 5). Hence, we performed an analysis on the basis of quartiles of PCSK9 concentrations, with quartile 1 as the reference group. Table 4 and Supplemental Figure 5 show in the extended adjusted model 3 that participants in quartiles 2–4 had between 32% and 47% higher risk for three-point MACE when compared with quartile 1. To evaluate whether the association with the first MACE during the follow-up is influenced by an already prevalent cardiovascular disease, we stratified the analysis by prevalent cardiovascular disease status at baseline. This revealed a strong association of PCSK9 concentrations with first three-point MACE on study in participants with baseline cardiovascular disease, with a 76%–79% higher risk in the upper three quartiles (Table 4). We detected no significant association in participants without prevalent cardiovascular disease (Table 4).
Table 4.
Associations of serum proprotein convertase subtilisin/kexin type 9 concentrations with incident cardiovascular events
| Proprotein Convertase Subtilisin/Kexin Type 9 Quartiles | Adjustment Model 1 | Adjustment Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| N/Na | %a | Odds Ratio [95% Confidence Interval] | P Value | N/Na | %a | Odds Ratio [95% Confidence Interval] | P Value | |
| All GCKD study participants (474 of 5038 participants with events) | ||||||||
| Quartile 1 | 86/1261 | 7 | 86/1250 | 7 | ||||
| Quartile 2 | 126/1262 | 10 | 1.54 [1.17 to 2.03] | 0.002 | 126/1258 | 10 | 1.38 [1.04 to 1.82] | 0.02 |
| Quartile 3 | 132/1263 | 10 | 1.63 [1.24 to 2.15] | <0.001 | 132/1258 | 10 | 1.47 [1.11 to 1.95] | 0.007 |
| Quartile 4 | 130/1252 | 10 | 1.64 [1.25 to 2.16] | <0.001 | 129/1244 | 10 | 1.32 [0.98 to 1.77] | 0.07 |
| Participants with cardiovascular disease at baselineb (247 of 1289 participants with events) | ||||||||
| Quartile 1 | 28/225 | 12 | 28/222 | 13 | ||||
| Quartile 2 | 63/284 | 22 | 2.02 [1.29 to 3.16] | 0.002 | 63/284 | 22 | 1.79 [1.14 to 2.82] | 0.01 |
| Quartile 3 | 71/356 | 20 | 1.86 [1.20 to 2.89] | 0.005 | 71/355 | 20 | 1.79 [1.15 to 2.80] | 0.01 |
| Quartile 4 | 85/424 | 20 | 1.85 [1.21 to 2.85] | 0.005 | 85/423 | 20 | 1.76 [1.13 to 2.76] | 0.01 |
| Participants without cardiovascular disease at baselineb (227 of 3748 participants with events) | ||||||||
| Quartile 1 | 58/1036 | 6 | 58/1028 | 6 | ||||
| Quartile 2 | 63/978 | 6 | 1.19 [0.83 to 1.70] | 0.34 | 63/974 | 6 | 1.14 [0.79 to 1.64] | 0.48 |
| Quartile 3 | 61/907 | 7 | 1.26 [0.88 to 1.81] | 0.21 | 61/903 | 7 | 1.31 [0.90 to 1.91] | 0.16 |
| Quartile 4 | 45/827 | 5 | 1.04 [0.70 to 1.54] | 0.85 | 44/821 | 5 | 0.98 [0.64 to 1.52] | 0.94 |
The presented data show the specific association between proprotein convertase subtilisin/kexin type 9 and incident three-point major adverse cardiovascular event during a median follow-up of 6.5 years in all GCKD participants and stratified on the basis of the presence of cardiovascular disease prevalence at baseline. Results of Cox regression analyses are presented (hazard ratio [95% confidence interval]) for the basic and fully adjusted models: Model 1and Model 3, respectively. Model 2 is shown for all GCKD in Supplemental Material (Supplemental Table 6). Model 1 was adjusted for age, sex, eGFR, and urine albumin-creatinine ratio. Model 3 was adjusted for age, sex, eGFR, urine albumin-creatinine ratio, statin treatment, HDL cholesterol, lipoprotein(a), high-sensitivity C-reactive protein, diabetes mellitus, hypertension, smoking, baseline cardiovascular diseases, and LDL cholesterol. GCKD, German Chronic Kidney Disease.
N/N (percentage) is the number of participants with events/total number of participants (percentage of participants with events).
Because of stratification on the basis of cardiovascular disease prevalence, these groups do not include baseline cardiovascular disease as confounders.
We found a very similar pattern for four-point MACE as for three-point MACE (Supplemental Figures 6–8). Furthermore, using measured systolic and diastolic BP instead of the hypertension variable revealed similar results (data not shown).
The subdistribution HRs for three-point MACE and four-point MACE were comparable with the cause-specific HRs (Supplemental Tables 6 and 7).
Additional Analyses
Because PCSK9 was only significantly associated with incident MACE in participants with baseline cardiovascular disease, we further explored whether recurrent clinical events caused the observed association. Supplemental Table 8 lists the frequency counts of clinical events that are included in a three-point MACE. Exclusion of 29 participants with recurrent stroke events and 67 individuals with recurrent myocardial infarctions led to a slight attenuation of the risk for developing three-point MACE in participants with cardiovascular disease at baseline (Supplemental Table 9).
Finally, we analyzed whether the addition of PCSK9 to the analyses on the basis of continuous NRI showed a significant gain in classification accuracy of individuals in terms of prevalent and incident outcomes (Supplemental Table 10). For prevalent cardiovascular disease, when PCSK9 quartiles were added to the extended adjusted model 3, overall NRI was 0.27 (95% CI, 0.20 to 0.33), NRI for cases was 0.18 (95% CI, 0.13 to 0.23), and NRI for controls was 0.08 (95% CI, 0.05 to 0.12). For incident three-point MACE, overall NRI was 0.10 (95% CI, 0.01 to 0.21); for cases, NRI was 0.31 (95% CI, −0.27 to 0.55), and for controls, NRI was −0.21 (95% CI, −0.38 to 0.34).
Discussion
To the best of our knowledge, this is the largest prospective study that has examined PCSK9 concentrations and outcomes in a cohort of high-risk participants with moderate CKD. The main findings are as follows. (1) PCSK9 concentrations are not associated with kidney function parameters, such as eGFR or UACR (except a slight increase in participants with nephrotic-range albuminuria). (2) The odds for prevalent cardiovascular disease at baseline were significantly higher with higher baseline PCSK9 concentrations. This association was stronger in participants without statin treatment compared with those treated with statins. (3) The risk for the first three-point MACE and four-point MACE during the prospective follow-up was significantly higher for participants with PCSK9 concentrations in the upper three quartiles when compared with the first quartile. This finding was restricted to participants with prevalent cardiovascular disease at baseline.
The main parameters associated with higher PCSK9 concentrations are statin treatment (+70 ng/ml), women (+21.5 ng/ml), diabetes mellitus (+13.6 ng/ml), and LDL cholesterol (+2.6 ng/ml per 10 mg/dl higher LDL cholesterol). It is known that PCSK9 reduces the hepatic uptake of LDL cholesterol by increasing the endosomal and lysosomal degradation of LDL receptors. Statin treatment results in a decreased intracellular cholesterol concentration, which activates the sterol regulatory element-binding protein-2 with a coexpression of LDL receptors and PCSK9 (18). Therefore, statin therapy with subsequent increase in PCSK9 can result in a negative feedback mechanism with a decreased efficacy of statins. This argues for a combined and very efficient therapy of statins with PCSK9 inhibitors when the LDL cholesterol targets cannot be achieved with statins alone (19). In such a case, not only is the further expression of LDL receptors by statin improved, but also the recycling of the LDL receptor is less interrupted by the PCSK9 inhibitor therapy.
PCSK9 is highly expressed in hepatocytes and, to a lesser extent, also in kidney cells (20). However, the physiologic role of PCSK9 in the kidney is not well understood. In this study, we did not find any association between PCSK9 and parameters such as UACR and eGFR. PCSK9 concentrations were only slightly elevated in participants having nephrotic-range proteinuria. The literature on the influence of kidney function on PCSK9 is rather heterogeneous, and Supplemental Table 1 lists 14 studies that have examined this association (13,21–24). The majority of reports on patients not on dialysis showed no correlation between PCSK9 levels, eGFR, and proteinuria. This is at first glance surprising considering that patients with CKD have manifold changes in their lipid parameters. Interestingly, a study by Haas et al. (25) examined 50 patients with nephrotic syndrome before and after remission of the disease. They observed a 14% reduction in PCSK9 when patients were on remission. This is in line with the slightly higher PCSK9 levels we observed in participants with nephrotic-range proteinuria. Furthermore, in nephrotic mouse models, Haas et al. (25) found that podocyte injury results in up to 24-fold increase in PCSK9 levels, and knockout of hepatic Pcsk9 decreases cholesterol and triglyceride concentrations by 40%–50%. Recently, increased PCSK9 expression in the cortical collecting duct of patients with FSGS was shown. The authors further observed that mice with selective deficiency of PCSK9 expression in the collecting duct failed to develop hypercholesterolemia after injection of nephrotoxic serum (26). These experimental data support the role of the hepatorenal axis, which becomes more evident in extreme situations in nephrotic syndrome, probably with the goal to maintain the oncotic pressure by production of macromolecules including lipoproteins (27). It might be postulated that in patients with pronounced proteinuria, PCSK9 might not only be involved in the development of hyperlipidemia but might also be correlated with kidney damage (28)—whether causally or as a consequence requires further data. Interestingly, a recent study in patients with CKD receiving PCSK9 inhibitors observed not only an LDL cholesterol reduction of 51% but also an improvement of proteinuria during 1 year of follow-up (29).
Since the discovery of PCSK9, it became apparent that it could be a potential risk factor for the development of cardiovascular disease. A recent meta-analysis including 22 prospectively observed cohorts with >28,000 participants revealed that high PCSK9 concentrations are associated with risk of MACE (30). These studies were mainly non-CKD cohorts. Data derived from CKD populations are rare (9,11,13,31,32) and heterogeneous in several ways; they included mild to moderate stages of kidney impairment, patients on dialysis or patients with transplants with a wide range of follow-up time, and a wide definition of outcomes. Most of the studies were either small or included only a small number of clinical events with a limited statistical power (Supplemental Table 2). It is therefore not surprising that most of these studies did not find an association with cardiovascular outcomes. With the study at hand, we provide data of a large study with >5000 participants, a large number of events, and a follow-up period of up to 6.5 years; 1289 already had cardiovascular disease at baseline, and PCSK9 concentrations were mainly predictive for cardiovascular disease in those who were not on statins. In statin-treated participants, PCSK9 was less strongly associated with prevalent cardiovascular disease, which might be explained by the fact that statin treatment indirectly influences PCSK9 concentration dramatically (+70 ng/ml). We have no information on the duration of statin therapy, but the measured PCSK9 values under statin treatment are probably not reflective of the major part of the earlier life prior to statin treatment, which may limit the predictive value of PCSK9 concentrations.
PCSK9 concentrations in the upper three quartiles were associated with a higher risk for the development of a new MACE on study during the prospective observation (Table 4). Interestingly, this higher risk was markedly more pronounced in participants who had already experienced an event before the enrollment into the study and was no longer significant in those without cardiovascular disease at baseline. This association was not influenced by recurring events and could rather be due to the high-risk profile of these participants.
Our findings suggest that PCSK9 is associated with cardiovascular disease beyond its regulation of LDL cholesterol homeostasis. The adjustment of the data even for LDL cholesterol had no influence on the observed association. In line with this, PCSK9 has been shown to have extrahepatic roles and was suggested to contribute to the pathogenesis and progression of atherosclerosis through its interaction with immune cells and induction of inflammation (33,34). Furthermore, there is growing evidence that inhibition of PCSK9 attenuates some viral and pathogenic infections and that PCSK9 affects the function of T cell and MHC-I receptors, which play a role in cancer and development of metastasis (35).
The main strength of this study is that it is by far the largest study in individuals with CKD with sufficient power to stratify participants by statin treatment and the presence of cardiovascular disease at baseline. Furthermore, it has a long median follow-up of 6.5 years with almost no loss to follow-up, a homogeneous study population, and a centralized assessment of the clinical outcomes. The study is limited by the observational design, which does not allow for clarifying causality or biologic mechanisms due to the potential of residual confounding. However, causality has already been demonstrated by genetic studies as well as therapies with PCSK9-lowering medications. Further limitations are the restriction to White individuals and participants in CKD stage G3 or A3. Therefore, findings might not be generalizable to other stages of CKD or other ethnicities.
The overall findings of our study suggest that higher baseline PCSK9 concentrations in participants with moderate CKD are associated with prevalent and incident cardiovascular disease. This observation would argue for a pronounced lipid-lowering therapy, especially in participants with prevalent cardiovascular disease. It remains to be seen whether in these participants, a targeted therapy against PCSK9 with PCSK9 inhibitors is superior compared with statins, which further increase PCSK9 concentrations. In addition, such controlled studies should also investigate whether the use of PCSK9 inhibitors in patients with impaired kidney function and pronounced proteinuria not only decreases lipid levels but also decreases cardiovascular events and the risk of CKD progression.
Disclosures
K.-U. Eckardt reports consultancy agreements with Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, Genzyme, Otsuka, Travere, and Vifor; research funding from Amgen, AstraZeneca, Bayer, Evotec, Fresenius, Genzyme, Shire, and Vifor; honoraria from Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, Genzyme, Otsuka, Travere, and Vifor; and serving on the editorial boards of BMJ and Kidney International. F. Kronenberg reports consultancy agreements with Amgen, Kaneka, and Novartis; honoraria from Amgen, Kaneka, and Novartis; and serving as Co-Editor of Atherosclerosis. J.F. Schachtl-Riess reports research funding from Dr. Legerlotz Stiftung. U.T. Schultheiss reports research funding from German Federal Ministry of Education and Research (BMBF) within the framework of the e:Med research and funding concept (BMBF grant 01ZX1912B). All remaining authors have nothing to disclose.
Funding
This study was supported by Austrian Research Fund grant W-1253. The GCKD study is supported by German Ministry of Education and Research grants 01ER 0804, 01ER 0818, 01ER 0819, 01ER 0820, and 01ER 0821; the KfH Foundation for Preventive Medicine; and corporate sponsors (http://www.gckd.org).
Supplementary Material
Acknowledgments
We are grateful for the willingness of the patients to participate in the GCKD study. The enormous effort of the study personnel of the various regional centers is highly appreciated. We thank the nephrologists who provide routine care for the participants and collaborate with the GCKD study (the list of nephrologists currently collaborating with the GCKD study is available at http://www.gckd.org). We appreciate the help of Lorenz M. Pammer with the forest plots’ codes in R.
The funding source had no involvement in study design, data collection, analysis and interpretation of data, or preparation of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Contributor Information
Collaborators: GCKD Investigators, Kai-Uwe Eckardt, Heike Meiselbach, Markus P. Schneider, Mario Schiffer, Hans-Ulrich Prokosch, Barbara Bärthlein, Andreas Beck, André Reis, Arif B. Ekici, Susanne Becker, Dinah Becker-Grosspitsch, Ulrike Alberth-Schmidt, Birgit Hausknecht, Anke Weigel, Gerd Walz, Anna Köttgen, Ulla T. Schultheiß, Fruzsina Kotsis, Simone Meder, Erna Mitsch, Ursula Reinhard, Jürgen Floege, Turgay Saritas, Elke Schaeffner, Seema Baid-Agrawal, Kerstin Theisen, Hermann Haller, Jan Menne, Martin Zeier, Claudia Sommerer, Johanna Theilinger, Gunter Wolf, Martin Busch, Rainer Paul, Thomas Sitter, Christoph Wanner, Vera Krane, Antje Börner-Klein, Britta Bauer, Florian Kronenberg, Julia Raschenberger, Barbara Kollerits, Lukas Forer, Sebastian Schönherr, Hansi Weissensteiner, Peter Oefner, Wolfram Gronwald, Matthias Schmid, and Jennifer Nadal
Author Contributions
K.-U. Eckardt and F. Kronenberg conceptualized the study; L. Forer, B. Kollerits, F. Kotsis, F. Kronenberg, C. Lamina, U.T. Schultheiss, and P. Sekula were responsible for data curation; L. Forer, A. Kheirkhah, F. Kronenberg, and C. Lamina were responsible for investigation; A. Kheirkhah, B. Kollerits, C. Lamina, and J.F. Schachtl-Riess were responsible for formal analysis; A. Kheirkhah, F. Kronenberg, J.F. Schachtl-Riess, U.T. Schultheiss, and P. Sekula were responsible for methodology; F. Kronenberg was responsible for project administration; K.-U. Eckardt and F. Kronenberg were responsible for resources; L. Forer and F. Kronenberg were responsible for software; F. Kotsis, F. Kronenberg, and U.T. Schultheiss were responsible for validation; A. Kheirkhah was responsible for visualization; K.-U. Eckardt and F. Kronenberg were responsible for funding acquisition; F. Kronenberg provided supervision; A. Kheirkhah, B. Kollerits, and F. Kronenberg wrote the original draft; and K.-U. Eckardt, L. Forer, A. Kheirkhah, B. Kollerits, F. Kotsis, F. Kronenberg, C. Lamina, J.F. Schachtl-Riess, U.T. Schultheiss, and P. Sekula reviewed and edited the manuscript.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.01230122/-/DCSupplemental.
Supplemental Material. Materials and methods.
Supplemental Table 1. Literature on PCSK9 and kidney function.
Supplemental Table 2. Literature on PCSK9 in patients with CKD and cardiovascular outcomes.
Supplemental Table 3. Association of PCSK9 with prevalent cardiovascular disease in the total GCKD population and stratified on the basis of statin treatment.
Supplemental Table 4. Association of PCSK9 with incident three-point MACE during a median follow-up of 6.5 years in all GCKD participants and stratified on the basis of statin treatment.
Supplemental Table 5. Association of PCSK9 with incident four-point MACE during a median follow-up of 6.5 years in all GCKD participants and stratified on the basis of statin treatment.
Supplemental Table 6. Association of PCSK9 quartiles with incident three-point MACE during a median follow-up of 6.5 years on the basis of subdistribution HR adjustment models.
Supplemental Table 7. Association of PCSK9 quartiles with incident four-point MACE during a median follow-up of 6.5 years on the basis of subdistribution HR adjustment models.
Supplemental Table 8. Frequency counts of clinical incident three-point MACEs (only the first event occurred has been counted).
Supplemental Table 9. Association of PCSK9 quartiles with incident three-point MACE when excluding recurrent events (model 3).
Supplemental Table 10. Continuous net reclassification index on the basis of PCSK9 quartiles calculated for model 3.
Supplemental Figure 1. PCSK9 concentrations across Kidney Disease Improving Global Outcomes risk categories2 on the basis of eGFR and urine albumin-creatinine ratio.
Supplemental Figure 2. Nonlinear P splines for prevalent cardiovascular disease.
Supplemental Figure 3. Association of PCSK9 with prevalent cardiovascular disease presented for each PCSK9 quartile.
Supplemental Figure 4. Nonlinear P splines demonstrating the association between PCSK9 and incident three-point MACE.
Supplemental Figure 5. Association of PCSK9 with incident three-point MACE during a median follow-up of 6.5 years presented for each PCSK9 quartile.
Supplemental Figure 6. Nonlinear P splines demonstrating the association between PCSK9 and incident four-point MACE.
Supplemental Figure 7. Association of PCSK9 with incident four-point MACE during a median follow-up of 6.5 years presented for each PCSK9 quartile.
Supplemental Figure 8. Association of PCSK9 concentrations with incident four-point MACE during a median follow-up of 6.5 years.
References
- 1.GBD Chronic Kidney Disease Collaboration : Global, regional, and national burden of chronic kidney disease, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 395: 709–733, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwan BCH, Kronenberg F, Beddhu S, Cheung AK: Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol 18: 1246–1261, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Kronenberg F: HDL in CKD-The devil is in the detail. J Am Soc Nephrol 29: 1356–1371, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derré A, Villéger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C: Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 34: 154–156, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Zhang DW, Lagace TA, Garuti R, Zhao Z, McDonald M, Horton JD, Cohen JC, Hobbs HH: Binding of proprotein convertase subtilisin/kexin type 9 to epidermal growth factor-like repeat A of low density lipoprotein receptor decreases receptor recycling and increases degradation. J Biol Chem 282: 18602–18612, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ; ODYSSEY LONG TERM Investigators : Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 372: 1489–1499, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA; Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators : Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 372: 1500–1509, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Pavlakou P, Liberopoulos E, Dounousi E, Elisaf M: PCSK9 in chronic kidney disease. Int Urol Nephrol 49: 1015–1024, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Vlad CE, Foia L, Pavel-Tanasa M, Toma V, Florea L, Voroneanu L, Apetrii M, Dodi G, Covic A: Evaluation of cardiovascular events and progression to end-stage renal disease in patients with dyslipidemia and chronic kidney disease from the North-Eastern area of Romania. Int Urol Nephrol 54: 647–659, 2022 [DOI] [PubMed] [Google Scholar]
- 10.Strålberg T, Nordenskjöld A, Cao Y, Kublickiene K, Nilsson E: Proprotein convertase subtilisin/kexin type 9 and mortality in patients starting hemodialysis. Eur J Clin Invest 49: e13113, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Rasmussen LD, Bøttcher M, Ivarsen P, Jørgensen HS, Nyegaard M, Buttenschøn H, Gustafsen C, Glerup S, Bøtker HE, Svensson M, Winther S: Association between circulating proprotein convertase subtilisin/kexin type 9 levels and prognosis in patients with severe chronic kidney disease. Nephrol Dial Transplant 35: 632–639, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Kajingulu FM, Lepira FB, Nkodila AN, Makulo JR, Mokoli VM, Ekulu PM, Bukabau JB, Nlandu YM, Longo AL, Nseka NM, Sumaili EK: Circulating proprotein convertase subtilisin/kexin type 9 levels predict future cardiovascular event risks in hemodialyzed Black African patients. Rambam Maimonides Med J 12: e0020, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogacev KS, Heine GH, Silbernagel G, Kleber ME, Seiler S, Emrich I, Lennartz S, Werner C, Zawada AM, Fliser D, Böhm M, März W, Scharnagl H, Laufs U: PCSK9 plasma concentrations are independent of GFR and do not predict cardiovascular events in patients with decreased GFR. PLoS One 11: e0146920, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckardt KU, Bärthlein B, Baid-Agrawal S, Beck A, Busch M, Eitner F, Ekici AB, Floege J, Gefeller O, Haller H, Hilge R, Hilgers KF, Kielstein JT, Krane V, Köttgen A, Kronenberg F, Oefner P, Prokosch HU, Reis A, Schmid M, Schaeffner E, Schultheiss UT, Seuchter SA, Sitter T, Sommerer C, Walz G, Wanner C, Wolf G, Zeier M, Titze S: The German Chronic Kidney Disease (GCKD) study: Design and methods. Nephrol Dial Transplant 27: 1454–1460, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Titze S, Schmid M, Köttgen A, Busch M, Floege J, Wanner C, Kronenberg F, Eckardt KU; GCKD study investigators : Disease burden and risk profile in referred patients with moderate chronic kidney disease: Composition of the German Chronic Kidney Disease (GCKD) cohort. Nephrol Dial Transplant 30: 441–451, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Pammer LM, Lamina C, Schultheiss UT, Kotsis F, Kollerits B, Stockmann H, Lipovsek J, Meiselbach H, Busch M, Eckardt KU, Kronenberg F; GCKD Investigators : Association of the metabolic syndrome with mortality and major adverse cardiac events: A large chronic kidney disease cohort. J Intern Med 290: 1219–1232, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 18.Urban D, Pöss J, Böhm M, Laufs U: Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol 62: 1401–1408, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Dadu RT, Ballantyne CM: Lipid lowering with PCSK9 inhibitors. Nat Rev Cardiol 11: 563–575, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Seidah NG, Awan Z, Chrétien M, Mbikay M: PCSK9: A key modulator of cardiovascular health. Circ Res 114: 1022–1036, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Elewa U, Fernández-Fernández B, Mahillo-Fernández I, Martin-Cleary C, Sanz AB, Sanchez-Niño MD, Ortiz A: PCSK9 in diabetic kidney disease. Eur J Clin Invest 46: 779–786, 2016 [DOI] [PubMed] [Google Scholar]
- 22.Morena M, Le May C, Chenine L, Arnaud L, Dupuy AM, Pichelin M, Leray-Moragues H, Chalabi L, Canaud B, Cristol JP, Cariou B: Plasma PCSK9 concentrations during the course of nondiabetic chronic kidney disease: Relationship with glomerular filtration rate and lipid metabolism. J Clin Lipidol 11: 87–93, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Zhang HW, Zhao X, Xu RX, Guo YL, Zhu CG, Wu NQ, Cui CJ, Dong Q, Li JJ: Relationship between plasma proprotein convertase subtilisin/kexin type 9 and estimated glomerular filtration rate in the general Chinese population. Cardiorenal Med 8: 311–320, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Didas N, Thitisopee W, Porntadavity S, Jeenduang N: Arylesterase activity but not PCSK9 levels is associated with chronic kidney disease in type 2 diabetes. Int Urol Nephrol 52: 1725–1732, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Haas ME, Levenson AE, Sun X, Liao WH, Rutkowski JM, de Ferranti SD, Schumacher VA, Scherer PE, Salant DJ, Biddinger SB: The role of proprotein convertase subtilisin/kexin type 9 in nephrotic syndrome-associated hypercholesterolemia. Circulation 134: 61–72, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molina-Jijon E, Gambut S, Macé C, Avila-Casado C, Clement LC: Secretion of the epithelial sodium channel chaperone PCSK9 from the cortical collecting duct links sodium retention with hypercholesterolemia in nephrotic syndrome. Kidney Int 98: 1449–1460, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Appel GB, Blum CB, Chien S, Kunis CL, Appel AS: The hyperlipidemia of the nephrotic syndrome. Relation to plasma albumin concentration, oncotic pressure, and viscosity. N Engl J Med 312: 1544–1548, 1985 [DOI] [PubMed] [Google Scholar]
- 28.Artunc F: Kidney-derived PCSK9-a new driver of hyperlipidemia in nephrotic syndrome? Kidney Int 98: 1393–1395, 2020 [DOI] [PubMed] [Google Scholar]
- 29.Muñoz Ramos P, Gil Giraldo Y, Álvarez-Chiva V, Arroyo D, Sango Merino C, Moncho Francés F, Ocaña J, Reque J, Sánchez-Álvarez E, Górriz JL, Quiroga B: Proteinuria-lowering effects of proprotein convertase subtilisin/kexin type 9 inhibitors in chronic kidney disease patients: A real-world multicentric study. Metabolites 11: 760, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y, Chen W, Lu M, Wang Y: Association between circulating proprotein convertase subtilisin/kexin type 9 and major adverse cardiovascular events, stroke, and all-cause mortality: Systemic review and meta-analysis. Front Cardiovasc Med 8: 617249, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eisenga MF, Zelle DM, Sloan JH, Gaillard CAJM, Bakker SJL, Dullaart RPF: High serum PCSK9 is associated with increased risk of new-onset diabetes after transplantation in renal transplant recipients. Diabetes Care 40: 894–901, 2017 [DOI] [PubMed] [Google Scholar]
- 32.Hwang HS, Kim JS, Kim YG, Lee SY, Ahn SY, Lee HJ, Lee DY, Lee SH, Moon JY, Jeong KH: Circulating PCSK9 level and risk of cardiovascular events and death in hemodialysis patients. J Clin Med 9: E244, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luquero A, Badimon L, Borrell-Pages M: PCSK9 functions in atherosclerosis are not limited to plasmatic LDL-cholesterol regulation. Front Cardiovasc Med 8: 639727, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruscica M, Tokgözoğlu L, Corsini A, Sirtori CR: PCSK9 inhibition and inflammation: A narrative review. Atherosclerosis 288: 146–155, 2019 [DOI] [PubMed] [Google Scholar]
- 35.Seidah NG: The PCSK9 discovery, an inactive protease with varied functions in hypercholesterolemia, viral infections, and cancer. J Lipid Res 62: 100130, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.