Abstract
Background:
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous chemicals with mechanisms of toxicity that include endocrine disruption. We examined associations of prenatal urinary PAH with spontaneous preterm birth (PTB) and gestational age (GA) at birth. We also assessed whether infant sex modifies the association of PAH exposure with spontaneous PTB and GA at birth.
Methods:
Participants included 1,677 non-smoking women from three cohorts (CANDLE, TIDES, and GAPPS) in the ECHO PATHWAYS Consortium. Twelve monohydroxylated-PAHs were measured in second trimester maternal urine. Seven metabolites with >60% overall detection were included in analyses: 1-hydroxynaphthalene [1-OH-NAP], 2-hydroxynaphthalene [2-OH-NAP], 2-hydroxyphenanthrene [2-OH-PHEN], 3-hydroxyphenanthrene [3-OH-PHEN], 1/9-hydroxyphenanthrene [1/9-OH-PHEN], 2/3/9-hydroxyfluorene [2/3/9-OH-FLUO], and 1-hydroxypyrene [1-OH-PYR]. Logistic and linear regression models were fit for spontaneous PTB and GA among births ≥34 weeks, respectively, with log10-transformed OH-PAH concentrations as the exposure, adjusted for specific gravity and suspected confounders. Effect modification by infant sex was assessed using interaction terms and marginal estimates.
Results:
Percent detection was highest for 2-OH-NAP (99.8%) and lowest for 1-OH-PYR (65.2%). Prevalence of spontaneous PTB was 5.5% (N = 92). Ten-fold higher 2-OH-NAP exposure was associated with 1.60-day (95% CI: −2.92, −0.28) earlier GA at birth. Remaining associations in the pooled population were null. Among females, we observed significant inverse associations between 1-OH-PYR and PTB (OR: 2.65 [95% CI: 1.39, 5.05]); and 2-OH-NAP with GA: −2.46 days [95% CI: −4.15, −0.77]). Among males, we observed an inverse association between 2/3/9-OH-FLUO and PTB (OR = 0.40 [95% CI: 0.17,0.98]). ORs for PTB were higher among females than males for 2-OH-PHEN (p = 0.02) and 1-OH-PYR (p = 0.02).
Discussion:
We observed inverse associations of 2-OH-NAP exposure with GA and null associations of remaining OH-PAHs with GA and PTB. Females may be more susceptible to spontaneous PTB or shorter GA following prenatal exposure to some OH-PAHs. This study is the first to assess sex-specific OH-PAH toxicity in relation to spontaneous PTB and GA.
Keywords: Polycyclic aromatic hydrocarbons (PAH), Maternal exposure, Gestational age, Preterm birth, Sex-specific associations
1. Introduction
Gestational age at birth is an important predictor of life course health (Zhang & Kramer, 2009, Crump et al., 2011). Preterm birth (PTB), defined as birth at <37 completed weeks of gestation, accounts for approximately 10 percent of births in the United States (Martin et al., 2019). PTB complications are the leading causes of death among children under five years of age (Liu et al., 2016) and are associated with neurodevelopmental impairments (Arpino et al., 2010), behavioral problems (Johnson & Marlow, 2011), and hearing and visual deficits (Arpino et al., 2010, Saigal & Doyle, 2008) among surviving infants. Estimates suggest there is a doubling of infant mortality for each decreasing gestational week prior to 38 weeks of gestational age (Reddy et al., 2011). Recent evidence also indicates that risk of neonatal complications is heterogeneous within the term period of 37–42 weeks of gestation; early-term births (37–38 6/7 weeks of completed gestation) are associated with a higher risk of infant mortality (Shapiro-Mendoza et al., 2008), childhood cognitive impairment (Yang et al., 2010), and respiratory morbidities (Parikh et al., 2014) compared with full-term births (39–10 6/7 weeks). While known demographic and lifestyle factors (e.g., maternal age, psychosocial stress, and tobacco use) as well as medical indications contribute to PTBs and early-term births, accumulating evidence suggests that environmental exposures may also affect the timing of parturition (Patel et al., 2014). Identification of modifiable environmental risk factors may contribute to public health strategies aimed at promoting full-term pregnancies.
Polycyclic aromatic hydrocarbons (PAHs) are a group of environmental contaminants produced by incomplete combustion. Several PAHs have mutagenic, teratogenic, and endocrine disrupting properties (IARC, 1983). Major sources of exposure include consumption of foods contaminated during processing and cooking, occupational hazards, ambient air pollution, and tobacco smoke. Exposure to PAHs is ubiquitous and well-documented in most populations, including pregnant individuals (Woodruff et al., 2011). A small number of epidemiological studies have demonstrated associations between maternal exposure to PAHs and PTB, but findings have been mixed and have notable limitations (Patel et al., 2014, Agarwal et al., 2018, Suter et al., 2019, Singh et al., 2008; Choi et al., 2008, Padula et al., 2014, Wilhelm et al., 2011). A secondary analysis of National Health and Nutrition Examination Surveys (NHANES) data found that a one standard deviation increase in urinary 1-hydroxypyrene was associated with 80% higher odds (95% CI: 1.1, 2.8) of prior PTB (Patel et al., 2014). However, the study relied on cross-sectional data and did not account for potential confounding by smoking. Three case-control studies with small sample sizes examined placental samples collected at birth and observed statistically significant higher amounts of benzo(b)fluoranthene (Suter et al., 2019, Singh et al., 2008), BaP (Agarwal et al., 2018, Suter et al., 2019), fluoranthene (Singh et al., 2008) and dibenz(a,h)anthracene (Suter et al., 2019) among preterm versus term births. Assignment of exposure using data from nearby ambient air monitors have demonstrated associations between proxy measures of trimester-specific PAH exposure and PTB, but these studies were unable to address dietary sources and were subject to exposure measurement error given the lack of spatial resolution from air monitors (Choi et al., 2008, Padula et al., 2014, Wilhelm et al., 2011). Prior studies examined a broad category of PTBs, including medically-indicated PTBs. Whereas causes of spontaneous PTB remain unknown, medically-indicated PTBs include a heterogenous set of conditions (e.g., infection, cervical incompetence, ischemic placental disease) that are typically not informative, in aggregate, of potential mechanisms underlying PAHs and their impacts on PTB. Further, humans are exposed to many PAHs simultaneously (Zoeller et al., 2012), yet studies on PAH exposure and gestational age at birth have evaluated single PAH chemicals in isolation. Co-exposures to multiple PAHs may elicit changes that are not observable in single-pollutant models (Zoeller et al., 2012). This study addresses prior challenges in PAH exposure ascertainment and analysis by measuring PAHs in maternal urine (thus quantifying PAHs from all sources) and including an aim to examine co-exposures to multiple PAHs. We also address limitations in sample size, generalizability, and confounding by using data from a large, geographically diverse, and well-characterized cohort. Finally, we restricted all PTB analyses to spontaneous PTB, which allows us to focus on potential mechanisms underlying PAHs and PTB.
Male and female fetuses respond differently to adverse environmental exposures. Sex-specific differences in gestational age at birth related to environmental exposures have been previously reported (Vatten and Skjærven, 2004, Al-Gubory, 2016). Sex specific differences in potential associations of PAHs with gestational age may also be driven by differences in circulating hormone levels that are important for labor induction (Li et al., 2020). This raises the possibility of sex-specific effects of PAH exposure on gestational age at birth.
Using a large, prospective multi-site study that combines several cohorts, we examined associations of seven urinary monohydroxylated PAH (OH-PAH) metabolites, collected during the second trimester in pregnancy, with spontaneous PTB and gestational age at birth. We also examined potential effect modification of these associations by offspring sex. To examine the potential effect of combined OH-PAH exposure on gestational age, we secondarily assessed OH-PAH mixtures in relation to gestational age at birth, again examining effect modification by offspring sex. To our knowledge, this is the first study to assess OH-PAHs and gestational age including term births with consideration of effect modification by offspring sex.
2. Methods
2.1. Study setting and study population
The current analysis included data from mother-child dyads enrolled in three pregnancy cohorts that constitute the ECHO PATHWAYS Consortium (https://deohs.washington.edu/echo/): the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study (Sontag-Padilla et al., 2015), The Infant Development and Environment Study (TIDES) (Swan et al., 2015, Barrett et al., 2014), and the Global Alliance to Prevent Prematurity and Stillbirth (GAPPS; https://www.gapps.org). Participants in the CANDLE cohort were recruited from the University of Tennessee Health Science Center in Memphis, TN between 2006 and 2011. Participants were eligible if they were 16 years of age or older, enrolled between 16 and 27 weeks of gestation, had medically uncomplicated pregnancies at enrollment, were able to speak and write in English, and planned to deliver at a study site. Participants in the TIDES cohort were recruited from the following institutions between 2010 and 2012: University of California, San Francisco (UCSF); University of Rochester Medical Center (URMC); University of Minnesota (UMN); and University of Washington/Seattle Children’s Hospital (UW/SCH). Participants were eligible for the TIDES cohort if they enrolled before 13 weeks of gestation, were 18 years of age or older, had medically uncomplicated pregnancies at enrollment, were able to speak and write in English, and planned to deliver at a study site. Participants in the GAPPS cohort were recruited throughout pregnancy from the following institutions between 2011 and 2014: University of Washington Medical Center (UWMC) and Swedish Medical Center in Seattle, WA and Yakima Valley Memorial Hospital in Yakima, WA. Participants were eligible to enroll in the GAPPS cohort if they were 18 years of age or older, English-speaking, and planned to deliver at a study site. All study participants provided informed consent and study protocols were approved by respective institutions where the studies were based.
For the current analysis, we included mother–child dyads from CANDLE, TIDES, and GAPPS cohorts with data available for both gestational age at birth and OH-PAH metabolites. We excluded participants with multiple pregnancies, stillbirths, or a history of smoking during pregnancy—defined as either self-reporting smoking behavior or having urinary cotinine levels of >200 ng/mL (Bramer & Kallungal, 2003, Schick et al., 2017). Active smokers were excluded due to evidence of strong associations of smoking with PAH exposure (Vu et al., 2015) and gestational age (Kyrklund-Blomberg & Cnattingius, 1998). After exclusions, 1,677 participants were eligible for analysis, including 867 (51.7%) participants from CANDLE, 597 (35.6%) participants from TIDES, and 213 (12.7%) participants from GAPPS. The ECHO-PATHWAYS study protocol was approved by the University of Washington Institutional Review Board (IRB).
2.2. Data collection
Prenatal data collection for all three cohorts occurred during routine prenatal period study visits, occurring approximately once per trimester. During these visits, participants completed health behavior and medical history questionnaires and provided spot urine samples. Birth and medical data were abstracted from medical records obtained from the participating clinics.
2.3. Gestational age and spontaneous preterm birth
Gestational age was calculated for CANDLE participants based on reported last menstrual period (LMP) and confirmed with ultrasound dating. If there was a discrepancy between the two, ultrasound dating was used for final determination of gestational age. Gestational age at delivery was calculated for TIDES and GAPPS participants based on ultrasound, or if unavailable, self-reported LMP. PTB was defined as <37 completed weeks of gestation. Birth indications were abstracted from medical records. Abstractions were performed by trained study staff for CANDLE participants and by study coordinators for TIDES participants (except the University of Minnesota site, which used clinicians). For GAPPS participants, indications were abstracted by research coordinators and then reviewed and signed by the site physician principal investigator. We decided a priori to restrict the PTB outcome to spontaneous PTB, defined as either idiopathic preterm births or births resulting from preterm premature rupture of membranes (PPROM). The gestational age at birth analysis was restricted to births occurring on or after 34 weeks of gestational age due to different recruitment strategies between the three cohorts. Specifically, CANDLE and TIDES primarily recruited women with healthy pregnancies whereas GAPPS included all pregnancies. We excluded births occurring before 34 weeks because this population represented a small percentage of the dataset (2% and primarily from the GAPPS cohort) and tends to be associated with severe medical indications, which would potentially lead to bias in outcome assessment.
2.4. Maternal urinary PAH metabolite analysis
The primary exposure of interest was maternal PAH levels, characterized using OH-PAH metabolites obtained through spot urine samples collected during the second trimester of pregnancy. After collection, samples were stored at −80 degrees C in study biorepositories until the time of analysis at the Wadsworth Laboratory located at the New York State Department of Health. Concentrations of the following 12 OH-PAH metabolites were assessed: Two metabolites of naphthalene (1-hydroxynaphthalene [1-OH-NAP], 2-hydroxynaphthalene [2-OH-NAP]), four metabolites of phenanthrene (2-hydroxyphenanthrene [2-OH-PHEN], 3-hydroxyphenanthrene [3-OH-PHEN], 4-hydroxyphenanthrene [4-phen], combined 1/9-hydroxyphenanthrene [1/9-OH-PHEN]), combined 2/3/9-hydroxyfluorene (2/3/9-OH-FLUO), 1-hydroxypyrene (1-OH-PYR), 3-hydroxybenzo[c]phenanthrene (3-bcp), two metabolites of hydroxychrysene (1-hydroxychrysene [1-OH-CHRY], 6-hydroxychrysene [6-OH-CHRY]), and 1-hydroxybenz[a]anthracene (1-OH-BAA).
Specific laboratory methods for analyzing OH-PAH metabolites have been previously described (Guo et al., 2012). Briefly, urine samples (500 μL) were fortified with 10 ng each of an isotopically-labeled internal standard mixture and mixed with 1 mL of 0.5 M ammonium acetate buffer containing 200 units/mL of β-glucuronidase/sulfatase enzyme (MP Biomedicals, LLC, Solon, OH, USA). The samples were mixed and incubated overnight at 37 °C, then diluted with 2 mL of HPLC-grade water, extracted with 7 mL of 80% pentane: 20% toluene by shaking on a reciprocating shaker for one hour, and centrifuged at 3600g for 20 min. The supernatant was transferred into a new glass tube for instrumental analysis. The chromatographic separation of OH-PAH metabolites was conducted using a Waters Acquity I-Class UPLC system (Waters; Milford, MA, USA) connected with an Acquity UPLC BEH C18 column (50 × 2.1 mm, 1.7 μm, Waters; Milford, MA, USA). Identification and quantification of OH-PAH metabolites was performed on an ABSCIEX 5500 triple quadrupole mass spectrometer (Applied Biosystems; Foster City, CA, USA). Quality assurance protocols included analysis of two Standard Reference Materials (SRM 3672, SRM 3673) containing certified values for several OH-PAH metabolites. Recoveries of analytes in SRMs ranged from 79 to 109%. A 13-point standard calibration curve (0.02–200 ng/mL) was prepared prior to the injection of samples. Calibration standards were injected periodically throughout the sample run to ensure instrument stability. The limits of detection (LOD) were metabolite- and cohort-specific and ranged from 0.017 to 0.48 ng/mL. The seven OH-PAH metabolites showing concentrations above the corresponding LODs in ≥60% of the pooled study population were included in final analyses: 1-OH-NAP, 2-OH-NAP, 2-OH-PHEN, 3-OH-PHEN, 1/9-OH-PHEN, 2/3/9-OH-FLUO, and 1-OH-PYR.
2.5. Covariates
Covariates included urinary specific gravity (SG, continuous), maternal age (years, continuous), site (Memphis, San Francisco, Minnesota, Rochester, Seattle-TIDES, Seattle-GAPPS, and Yakima), race (White, Black/African American, Asian, Other), ethnicity (Non-Hispanic White, Hispanic/Latinx), urinary cotinine (ng/mL, continuous), education (<high school, high school graduate, graduated college or technical school, some graduate work), annual household income (<$20,000, $20,000–45,000, $45,000–75,000, >$75,000), season of offspring birth (winter, spring, summer, autumn), OH-PAH analysis batch number (1,2,3), infant sex (male, female), alcohol use during pregnancy (any, none), pre-pregnancy body mass index (BMI, continuous), parity (nulliparous, parous), and prior preterm birth (yes, no). SG and maternal cotinine were measured from urine samples obtained during the second trimester visit. Immediately after urine collection, SG was recorded using a hand-held refractometer to measure urine dilution. Continuous maternal cotinine was included as a measure of passive smoking exposure. (Active smokers were excluded from analysis). Season of birth and infant sex were obtained from medical record abstraction. All other covariates were obtained from questionnaires completed by participants during prenatal visits. Race was included as a proxy for the influence of racist practices and structural inequities related to adverse environmental exposures and birth outcomes. The following groups were included in the “other” category for race, given small sample sizes: Native American/Other Pacific Islander (<1% of study population), American Indian/Alaskan Native (<1% of study population), multiple race (2.4% of study population), and self-reported other race (3.9% of study population). For the PTB analysis, the “other” category additionally included participants identifying as Asian due to small sample sizes in that group.
2.6. Statistical analysis
Demographic and behavioral characteristics of mother-offspring dyads were summarized overall and by cohort. The distribution of OH-PAH metabolites was characterized by cohort and child sex by examining the limit of detection (LOD), percent detection, median, interquartile range, geometric mean and confidence interval (CI). Correlations between log-transformed, SG-adjusted OH-PAHs were also summarized.
After excluding individuals with medically-indicated PTB (N = 74) and missing covariate data (N = 64), the analytic dataset for the spontaneous PTB analysis included 1,603 mother–child dyads and 92 spontaneous PTBs. Separate multivariable logistic regression models for each PAH metabolite were fit for spontaneous PTB to estimate adjusted odds ratios (ORs) and 95% CIs associated with a 10-fold higher maternal OH-PAH exposure. We chose to report a 10-fold change in OH-PAH exposure based on the overall and interquartile range of raw OH-PAH metabolites, which suggests a 10-fold change is sufficiently represented in the data for all seven metabolites (Supplementary Table 2). All regression models included log10-transformed, unadjusted OH-PAH metabolites as the exposure and SG as a covariate to account for urinary dilution. OH-PAH values below the LOD were imputed with the LOD/√2. Covariates were selected based on a Directed Acyclic Graph constructed from prior literature. Model 1 included the following covariates with strong evidence for a relationship between OH-PAH and gestational age at birth: SG, maternal age, study site, race, urinary cotinine (smoke exposure), education, income, season of birth, batch number, and infant sex. The full model (Model 2) included all Model 1 covariates plus additional hypothesized confounders and precision variables: alcohol use during pregnancy, pre-pregnancy BMI, parity (precision variable) and prior PTB (precision variable). To assess the extent to which restriction to spontaneous PTB may have influenced results, we conducted a sensitivity analysis examining all PTBs as the outcome. To examine potential interaction by offspring sex, we added an interaction term including sex and the OH-PAH metabolite of interest in each model and presented marginal estimates from the interaction models. The p-value for the interaction term was used to determine whether results significantly differed by sex at the alpha = 0.05 level.
Variables with high levels of missingness and considered appropriate for imputation (i.e., satisfies missing at random assumption, predictable by other variables in imputation model) were imputed using Multiple Imputation by Chained Equations (MICE) (Van Buuren & Groothuis-Oudshoorn, 2011). Ten imputations with 50 iterations were performed. Cotinine (7.6% missing), income (5.0% missing), and pre-pregnancy BMI (1.4% missing) were imputed. The following variables were included as predictors in the imputation models: gestational age, maternal education, study site, race, ethnicity, maternal age, prior preterm birth, parity, pre-pregnancy BMI, income, alcohol use during pregnancy, urinary cotinine, season of offspring birth, and seven OH-PAH metabolites. Model checking included comparison of imputed covariates to observed covariates and examination of trace plots to observe trends in the mean and standard deviation of the imputed values.
After excluding participants who gave birth prior to 34 weeks of gestational age (N = 38), the analytic dataset for the gestational age analysis included 1,639 mother–child dyads. The gestational age analysis was the same as the PTB analysis with respect to exposure characterization and covariate inclusion. We fit multivariable linear regression models with continuous gestational age (days) as the outcome to obtain the mean difference of gestational age at birth associated with 10-fold higher maternal OH-PAH exposure. An interaction term with log10-PAH and infant sex were added to the main gestational age models to assess effect modification by infant sex. Similar to the PTB analysis, we assessed statistical significance of the interaction terms at the α = 0.05 level and presented marginal estimates from the interaction models. To account for potential site-specific effects, a “leave-one-site-out” sensitivity analysis was conducted in which the main spontaneous PTB and gestational age analyses were repeated with one site removed in each iteration. An analogous leave-one-cohort-out sensitivity analysis was conducted to examine cohort-specific effects.
Weighted quantile sum regression (WQSR) (Carrico et al., 2015) was used to evaluate additive associations between the decile-transformed maternal exposure to combined OH-PAHs (OH-PAH mixtures) and gestational age at birth. WQS was chosen as a statistical method for examining mixture effects because it yields an interpretable coefficient representing the overall directional mixture effect as well as an indication of the relative contribution of OH-PAH metabolites to the identified association. Mixture associations were examined separately in the positive and negative (inverse) directions, and model estimates were determined using 1000 bootstrap iterations. Models were run without splitting the data into training and validation datasets to increase statistical power. To avoid the concomitant increased Type I error rate accompanying WQS regressions run without splitting the data, a modified permutation test method (Loftus et al., 2021) was applied to models yielding mixture coefficient 95% CIs that did not overlap the null so as to obtain a “confirmatory p-value” for the mixture coefficient with a nominal Type I error rate. Final statistical significance was based on this confirmatory permutation test p-value, with a confirmatory p-value < 0.05 suggesting statistical significance regardless of CIs obtained from the WQSR (because they are likely anti-conservative). WQS analyses used SG-adjusted OH-PAHs as the exposure and Model 2 (full model) covariates. Models were first run with the full dataset, then on sex-stratified data to examine effect modification by offspring sex. Mixture associations with preterm birth were not explored as there is currently no permutation test method for logistic WQS regression, which is necessary to preserve adequate power and a nominal Type I error rate. All analyses were conducted in R version 3.6.3 (R Core Team, 2020) using version 3.0.4 of the ‘gWQS’ package (Renzetti et al., 2021) and significance was assessed at an α level of 0.05.
3. Results
3.1. Descriptive statistics
Most participants were between the ages of 18 and 29 (N = 827, 49.3%) or 30 and 39 (N = 762, 45.4%) years and were high school (N = 540, 32.2%) or college graduates (N = 570, 34.0%). Participants in the CANDLE cohort were more likely to be younger, Black/African American, high school graduates or less, and have lower income compared with participants in the TIDES and GAPPS cohorts (Table 1). The prevalence of overall PTB was highest in the TIDES cohort (N = 62, 10.4%), followed by CANDLE (N = 84, 9.7%) and GAPPS (N = 20, 9.4%).
Table 1.
Selected characteristics of study participants overall and by cohort.
| All (N = 1677) N (%) | CANDLE (N = 867) N (%) | TIDES (N = 597) N (%) | GAPPS (N = 213) N (%) | |
|---|---|---|---|---|
|
| ||||
| Site, | ||||
| Missing = 0 | ||||
| Memphis | 867 (51.7) | 867 (100) | ||
| San Francisco | 157 (9.4) | – | 157 (26.3) | |
| Minnesota | 162 (9.7) | – | 162 (27.1) | – |
| Rochester | 139 (8.3) | – | 139 (23.3) | – |
| Seattle - TIDES | 139 (8.3) | – | 139 (23.3) | – |
| Seattle - GAPPS | 96 (5.7) | – | – | 96 (45.1) |
| Yakima | 117 (7.0) | – | – | 117 (54.9) |
| Age, | ||||
| Missing = 20 (1.2%) | ||||
| <18 | 11 (<1) | 11 (1.3) | 0 (0) | 0 (0) |
| 18–29 | 827 (49.3) | 547 (63.1) | 200 (33.5) | 80 (37.6) |
| 30–39 | 762 (45.4) | 283 (32.6) | 360 (60.3) | 119 (55.9) |
| ≥40 | 57 (3.4) | 8 (1.0) | 37 (6.2) | 12 (3.8) |
| Race | ||||
| Missing = 17 (1.0%) | ||||
| White | 871 (51.9) | 266 (30.7) | 432 (72.4) | 173 (81.2) |
| Black/African American | 618 (36.9) | 550 (63.4) | 64 (10.7) | 4 (1.9) |
| Asian | 51 (3.0) | 9 (1.0) | 35 (5.9) | 7 (3.3) |
| Other | 120 (7.2) | 42 (4.8) | 55 (9.2) | 23 (10.8) |
| Ethnicity, | ||||
| Missing = 9 (<1%) | ||||
| Hispanic or Latinx | 94 (5.6%) | 14 (1.6) | 51 (8.5) | 29 (13.6) |
| Not Hispanic or Latinx | 1574 (93.9%) | 853 (98.4) | 538 (90.1) | 183 (85.9) |
| Education, | ||||
| Missing = 8 (<1%) | ||||
| <High School | 112 (6.7) | 73 (8.4) | 32 (5.4) | 7 (3.3) |
| High school graduate | 540 (32.2) | 397 (45.8) | 89 (14.9) | 54 (25.4) |
| Graduated college or technical school | 570 (34.0) | 278 (32.1) | 200 (33.5) | 92 (43.2) |
| Some graduate work, graduate/professional degree | 447 (26.7) | 118 (13.6) | 270 (45.2) | 59 (27.7) |
| Household Income, | ||||
| Missing = 84 (5.0%) | ||||
| <$20 k | 383 (22.8) | 254 (29.3) | 109 (18.3) | 20 (9.4) |
| $20 k-45 k | 308 (18.4) | 231 (26.6) | 50 (8.3) | 27 (12.7) |
| $45 k-75 k | 328 (19.6) | 175 (20.2) | 107 (17.9) | 46 (21.6) |
| >$75 k | 574 (34.2) | 153 (17.6) | 311 (52.1) | 110 (51.6) |
| Pre-pregnancy BMI, | ||||
| Missing = 23 (1.4%) | ||||
| Underweight (<18.5) | 50 (3.0) | 37 (4.3) | 7 (1.2) | 6 (2.8) |
| Normal (18.5–24.9) | 728 (43.4) | 328 (37.8) | 310 (51.9) | 90 (42.3) |
| Overweight (25.0–29.9) | 405 (24.2) | 203 (23.4) | 142 (23.8) | 60 (28.2) |
| Obese (>=30.0) | 471 (28.1) | 295 (34.0) | 128 (21.4) | 48 (22.5) |
| Infant Sex, | ||||
| Missing = 0 | ||||
| Male | 809 (48.2) | 422 (48.7) | 282 (47.2) | 105 (49.3) |
| Season of birth, Missing = 18 (1.1%) | ||||
| Autumn | 480 (28.6) | 246 (28.4) | 182 (30.5) | 52 (24.4) |
| Spring | 359 (21.4) | 179 (20.6) | 122 (20.4) | 58 (27.2) |
| Summer | 452 (27.0) | 236 (27.2) | 164 (27.5) | 52 (24.4) |
| Winter | 368 (21.9) | 188 (21.7) | 129 (21.6) | 51 (23.9) |
| Alcohol use during pregnancy | ||||
| Missing = 4 (<1%) | ||||
| Yes | 159 (9.5) | 62 (7.2) | 75 (12.6) | 22 (10.3) |
| Parity | ||||
| Missing = 16 (<1%) | ||||
| Nulliparous | 741 (44.2) | 349 (40.3) | 321 (53.8) | 71 (33.3) |
| Prior Preterm Birth | ||||
| Missing = 0 | ||||
| Yes | 134 (8.0) | 65 (7.5) | 59 (9.9) | 10 (4.7) |
| Preterm Birth | ||||
| Yes | 166 (9.9) | 84 (9.7) | 62 (10.4) | 20 (9.4) |
| Spontaneous PTB | 92 (5.5%) | 52 (6.0%) | 21 (3.5%) | 19 (8.9%) |
| Medically indicated PTB | 74 (4.4%) | 32 (3.7%) | 41 (6.9%) | 1 (<1% %) |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
|
| ||||
| Gestational Age at Birth (weeks) | ||||
| Mean (SD) | 39.0 (2.0) | 38.9 (1.9) | 39.3 (19) | 38.8 (2.6) |
| Raw cotinine value (ng/mL) | ||||
| Missing = 127 | ||||
| Mean (SD) | 2.21 (9.6) | 2.87 (10.1) | 1.59 (9.6) | 0.06 (0.19) |
| Specific gravity | ||||
| Missing = 26 (1.6%) | ||||
| Mean (SD) | 1.017 (0.01) | 1.017 (0.01) | 1.014 (0.01) | 1.028 (0.01) |
Abbreviations: CANDLE, Conditions Affecting Neurodevelopment and Learning in Early Childhood; TIDES, The Infant Development and Environment Study; GAPPS, Global Alliance to Prevent Prematurity and Stillbirth; BMI, body mass index; SD, standard deviation.
The mean gestational age at birth in the CANDLE, TIDES, and GAPPS cohort was 38.9 (SD = 1.9), 39.3 (SD = 1.9), and 38.8 (SD = 2.6) weeks, respectively, after excluding those born before 34 weeks. Percent detection and OH-PAH levels varied considerably across metabolites and cohorts (Table 2), but not across child sex (Supplementary Table 1). Overall percent detection was highest for 2-OH-NAP (99.8%) and lowest for 1-OH-PYR (65.2%). In the TIDES cohort, we observed 39.8% and 36.0% detection for 2/3/9-OH-FLUO and 1-OH-PYR, while CANDLE had 73.0% and 88.5% detection for these metabolites, respectively. CANDLE participants had higher geometric median and mean levels for all PAH metabolites compared with participants in TIDES and GAPPS. Pearson correlation coefficients were strongest between metabolites of the phenanthrene parent structure (2-OH-PHEN and 3-OH-PHEN: 0.80; 1/9 OH-PHEN and 3-OH-PHEN: 0.61; 1/9-OH-PHEN and 2-OH-PHEN: 0.59) while correlations between the remaining pairs of metabolites ranged from 0.31 (1-OH-NAP and 2-OH-PHEN) to 0.55 (2/3/9-FLUO and 3-OH-PHEN) (Supplementary Fig. 1).
Table 2.
Characterization of specific gravity-adjusted maternal urinary OH-PAH concentrationsa, overall and by cohort.
| OH-PAH Metabolite | N | LOD | Percent Detection | 50th (25th, 75th) | Geometric mean (95% CI) |
|---|---|---|---|---|---|
| 1-OH-Naphthalene | |||||
| Overall | 1,677 | 87.2 | 0.61 (0.27, 1.38) | 0.55 (0.51, 0.60) | |
| CANDLE | 867 | 0.02 | 100 | 1.02 (0.54, 2.08) | 1.20 (1.11, 1.29) |
| TIDES | 597 | 0.04 | 64.7 | 0.25 (0.04, 0.61) | 0.20 (0.17, 0.22) |
| GAPPS | 213 | 0.017 | 98.1 | 0.39 (0.23, 0.67) | 0.42 (0.37, 0.48) |
| 2-OH-Naphthalene | |||||
| Overall | 1,677 | 99.8 | 3.66 (2.02, 6.64) | 3.61 (3.45, 3.78) | |
| CANDLE | 867 | 0.025 | 99.8 | 4.90 (2.86, 7.90) | 4.89 (4.62, 5.18) |
| TIDES | 597 | 0.017 | 99.7 | 2.46 (1.29, 4.72) | 2.48 (2.29, 2.68) |
| GAPPS | 213 | 0.018 | 100.0 | 3.01 (1.70, 5.17) | 2.96 (2.62, 3.34) |
| 2/3/9-OH-Fluorene | |||||
| Overall | 1,677 | 73.0 | 0.66 (0.36, 1.16) | 0.63 (0.61, 0.66) | |
| CANDLE | 867 | 0.12 | 96.3 | 0.89 (0.59, 1.43) | 0.93 (0.89, 0.98) |
| TIDES | 597 | 0.48 | 30.8 | 0.53 (0.34, 0.93) | 0.59 (0.55, 0.62) |
| GAPPS | 213 | 0.017 | 96.2 | 0.14 (0.10, 0.20) | 0.15 (0.13, 0.16) |
| 1/9-OH-Phenanthrene | |||||
| Overall | 1,677 | 86.6 | 0.18 (0.07, 0.39) | 0.16 (0.15, 0.17) | |
| CANDLE | 867 | 0.08 | 83.4 | 0.34 (0.18, 0.57) | 0.28 (0.26, 0.30) |
| TIDES | 597 | 0.007 | 89.1 | 0.10 (0.05, 0.19) | 0.09 (0.08, 0.10) |
| GAPPS | 213 | 0.017 | 92.5 | 0.10 (0.06, 0.15) | 0.10 (0.09, 0.10) |
| 2-OH-Phenanthrene | |||||
| Overall | 1,677 | 88.1 | 0.07 (0.04, 0.12) | 0.07 (0.07, 0.07) | |
| CANDLE | 867 | 0.03 | 85.7 | 0.09 (0.06, 0.13) | 0.09 (0.09, 0.10) |
| TIDES | 597 | 0.003 | 98.2 | 0.06 (0.03, 0.09) | 0.06 (0.05, 0.06) |
| GAPPS | 213 | 0.017 | 70.0 | 0.04 (0.03, 0.07) | 0.05 (0.04, 0.05) |
| 3-OH-Phenanthrene | |||||
| Overall | 1,677 | 88.0 | 0.07 (0.04, 0.11) | 0.07 (0.07, 0.07) | |
| CANDLE | 867 | 0.03 | 85.6 | 0.09 (0.06, 0.14) | 0.10 (0.09, 0.10) |
| TIDES | 597 | 0.003 | 97.2 | 0.05 (0.03, 0.08) | 0.05 (0.04, 0.05) |
| GAPPS | 213 | 0.018 | 72.3 | 0.04 (0.03, 0.06) | 0.05 (0.05, 0.05) |
| 1-OH-Pyrene | |||||
| Overall | 1,677 | 65.2 | 0.09 (0.02, 0.20) | 0.07 (0.07, 0.08) | |
| CANDLE | 867 | 0.03 | 88.5 | 0.14 (0.08, 0.23) | 0.14 (0.13, 0.15) |
| TIDES | 597 | 0.009 | 36.0 | 0.02 (0.01, 0.14) | 0.03 (0.03, 0.03) |
| GAPPS | 213 | 0.02 | 52.6 | 0.05 (0.02, 0.07) | 0.04 (0.04, 0.05) |
Abbreviations: OH-PAH, monohydroxy-polycyclic aromatic hydrocarbon; LOD, limit of detection; CI, confidence interval; CANDLE, Conditions Affecting Neurodevelopment and Learning in Early Childhood; TIDES, The Infant Development and Environment Study; GAPPS, Global Alliance to Prevent Prematurity and Stillbirth.
All OH-PAH values are expressed in ng/mL.
3.2. Spontaneous PTB
In fully-adjusted models, we observed no statistically significant association between a 10-fold higher maternal OH-PAH exposure and spontaneous PTB (Table 3). There was suggestive evidence of a positive association between 1-OH-PYR and spontaneous PTB (OR: 1.60, 95% CI: 0.97, 2.63). To assess the extent to which restriction to spontaneous PTB may have influenced results and to produce estimates comparable to prior literature, we conducted a sensitivity analysis including all PTBs as an outcome. Findings from these sensitivity analyses had wide confidence intervals and no results were statistically significant (Supplementary Fig. 2). Leave-one-site-out analyses suggest results did not substantially change when excluding sites. Associations for 1-OH-PYR shifted from marginally significant to statistically significant when excluding Memphis or the Seattle GAPPS sites from analyses (Supplementary Fig. 3). Similarly, in the leave-one-cohort out analyses associations for 1-OH-PYR and spontaneous PTB became statistically significant when excluding CANDLE or GAPPS (Supplementary Fig. 4).
Table 3.
Odds ratios (95% confidence intervals) for spontaneous preterm birth associated with 10-fold higher urinary OH-PAH metabolites.a
| PAH Metaboliteb | Crudec | Model 1d | Model 2e |
|---|---|---|---|
| 1-OH-Naphthalene | 1.01 (0.99, 1.03) | 1.04 (0.69, 1.55) | 1.07 (0.71, 1.61) |
| 2-OH-Naphthalene | 1.00 (0.97, 1.02) | 0.70 (0.39, 1.23) | 0.69 (0.38, 1.24) |
| 2/3/9-OH-Fluorene | 0.98 (0.95, 1.01) | 0.58 (0.27, 1.27) | 0.55 (0.25, 1.22) |
| 1/9-OH-Phenanthrene | 0.99 (0.97, 1.01) | 0.80 (0.50, 1.29) | 0.81 (0.51, 1.31) |
| 2-OH-Phenanthrene | 1.01 (0.97, 1.04) | 1.00 (0.47, 2.09) | 1.03 (0.49, 2.18) |
| 3-OH-Phenanthrene | 1.02 (0.98, 1.06) | 1.25 (0.59, 2.66) | 1.17 (0.54, 2.50) |
| 1-OH-Pyrene | 1.03 (1.01, 1.05) | 1.60 (0.97, 2.63) | 1.60 (0.97, 2.63) |
Abbreviations: OH-PAH, monohydroxy-polycyclic aromatic hydrocarbon.
Analytic dataset included 1,603 mother-child pairs, including 92 spontaneous PTBs.
PAH compounds were measured in ng/mL and log10-transformed.
Adjusted for specific gravity.
Adjusted for maternal age, site, race, cotinine measurements, education, income, urinaiy specific gravity, infant sex, season of birth, and batch number.
Adjusted for model 1 covariates plus alcohol use during pregnancy, pre-pregnancy BMI, parity, and prior preterm birth.
In models examining potential interaction between log10PAH and infant sex on spontaneous PTB, we observed statistically significant multiplicative interaction for 2-OH-PHEN (p = 0.02) and 1-OH-PYR (p = 0.02) (Fig. 1). Marginal estimates suggested positive associations of maternal PAH metabolites (2-OH-PHEN, 1-OH-PYR) with odds of spontaneous PTB among females and inverse (2-OH-PHEN) or no (1-OH-PYR) association among males (Fig. 1), although a statistically significant association was observed only for 1-OH-PYR among females. Among females, 10-fold higher maternal exposure to 1-OH-PYR was associated with 2.65-fold higher odds (95% CI: 1.39, 5.05) of spontaneous PTB. The same exposure among males was null (OR: 1.07, 95% CI: 0.59, 1.95). We also observed a statistically significant lower odds of spontaneous PTB among males associated with maternal exposure to 2/3/9-OH-FLUO (OR: 0.40, 95% CI: 0.17, 0.98). Remaining interaction p-values and marginal associations were null.
Fig. 1.
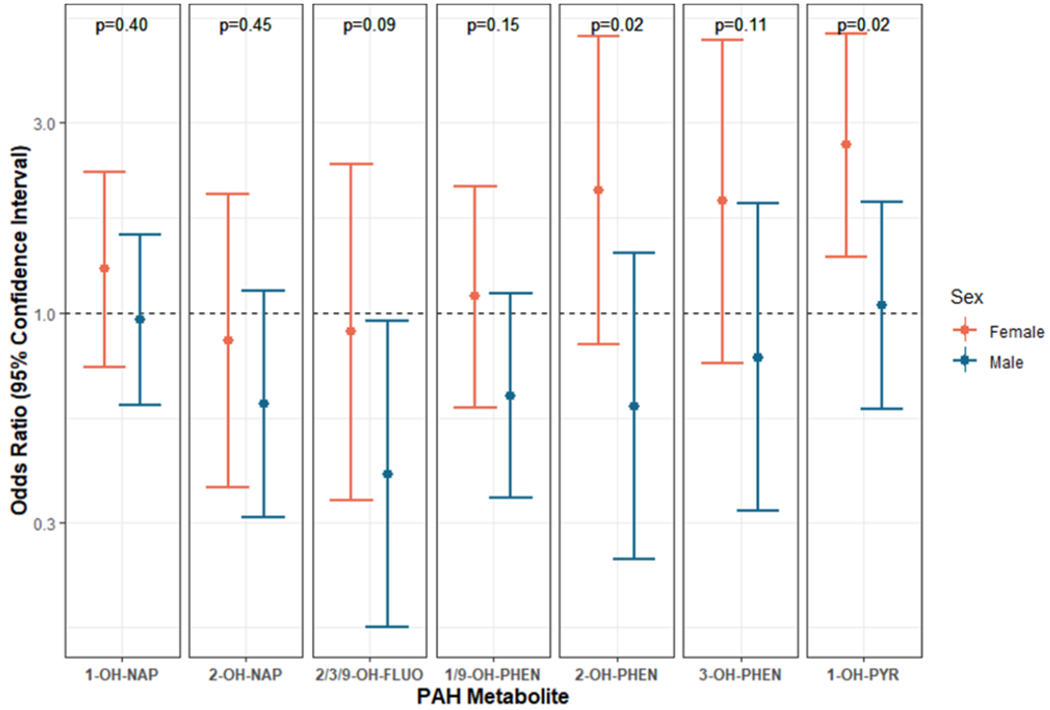
Marginal male and female log-odds ratios and 95% confidence intervals of spontaneous preterm birth associated with 10-fold higher maternal OH-PAH (ng/mL)a,b. Abbreviations: OH-PAH; monohydroxy-polycyclic aromatic hydrocarbon; 1-OH-nap, 1-hydroxynaphthalene; 2-OH-nap, 2-hydroxynaphthalene; 1/9-OH-PHEN, combined 1/9-hydroxyphenanthrene; 2-OH-PHEN, 2-hydroxyphenanthrene; 3-OH-PHEN, 3-hydroxyphenanthrene; 2/3/9-OH-FLUO, combined 2/3/9-hydroxyfluorene; 1-OH-PYR, 1-hydroxypyrene.
Adjusted for full model covariates: Maternal age, site, race, ethnicity, cotinine measurements, education, income, urinary specific gravity, infant sex, season of birth, batch number, alcohol during pregnancy, pre-pregnancy BMI, parity, and prior preterm birth
P-values presented above each pair of sex-specific marginal coefficients reflect the exposure by sex interaction term coefficient p-values
Plot is on the log10-scale.
3.3. Gestational age at birth
In the fully-adjusted model, we observed that 10-fold higher maternal concentration of 2-OH-NAP was associated with 1.60 days earlier gestational age at birth (95% CI: −2.92, −0.28). Associations for all other OH-PAH metabolites were null at the 95% confidence level (Table 4). Results from the leave-one-site-out sensitivity analysis suggest similar results across iterations of site exclusions (Supplementary Fig. 5), though estimates shifted in the null direction for 2-OH-NAP when removing Rochester, San Francisco, or the Seattle GAPPS sites. The leave-one-cohort-out analysis yielded some shifts in estimates associated with cohort exclusions (Supplementary Fig. 6). Estimates for 1-OH-NAP, 3-OH-PHEN, and 1-OH-PYR were significantly associated with later gestational age at birth when excluding the TIDES cohort, but not when excluding CANDLE or GAPPS.
Table 4.
Mean change in gestational age at birth (days) associated with 10-fold higher urinary OH-PAH metabolites.a
| OH-PAH Metaboliteb | Crudec | Model 1d | Model 2e |
|---|---|---|---|
| 1-OH-Naphthalene | 0.04 (−0.69, 0.77) | 0.51 (−0.40, 1.41) | 0.29 (−0.60, 1.18) |
| 2-OH-Naphthalene | −1.92 (−3.13, −0.70) | −1.46 (−2.79, −0.12) | −1.60 (−2.92, −0.28) |
| 2/3/9-OH-Fluorene | 0.10 (−1.19, 1.39) | 1.33 (−0.34, 2.99) | 1.23 (−0.41, 2.86) |
| 1/9-OH-Phenanthrene | 0.16 (−0.81, 1.12) | 0.79 (−0.28, 1.86) | 0.63 (−0.42, 1.68) |
| 2-OH-Phenanthrene | 0.23 (−1.26, −1.72) | 1.21 (−0.40, 2.83) | 0.93 (−0.67, 2.53) |
| 3-OH-Phenanthrene | 0.12 (−1.41, 1.65) | 1.16 (−0.52, 2.84) | 0.92 (−0.74, 2.58) |
| 1-OH-Pyrene | −0.74 (−1.55, 0.07) | 0.30 (−0.79, 1.39) | 0.24 (−0.83, 1.31) |
Abbreviations: OH-PAH; monohydroxy-polycyclic aromatic hydrocarbon; ng/mL.
Analytic dataset included 1,639 mother–child pairs.
OH-PAH compounds were measured in ng/mL and log10-transformed.
Adjusted for specific gravity.
Adjusted for maternal age, site, race, ethnicity, cotinine measurements, education, income, urinary specific gravity, infant sex, season of birth, and batch number.
Adjusted for model 1 covariates plus alcohol use during pregnancy, pre-pregnancy BMI, parity, and prior preterm birth.
In models examining potential interaction between log10PAH and infant sex on gestational age at birth, we did not observe statistically significant interaction on the additive scale (Fig. 2). However, we observed two significant marginal estimates. Among females, 10-fold higher maternal exposure to 2-OH-NAP was associated with 2.46 days earlier gestational age at birth (−4.15, −0.77). The same association among males was in the same direction but smaller in magnitude and not statistically significant (mean difference in gestational age at birth: −0.95 days, 95% CI: −2.49, 0.60). Among males, 10-fold higher maternal exposure to 2/3/9-OH-FLUO was associated with 1.96 days later gestational age at birth (95% CI: 0.05, 3.87). Associations were null for females with the same exposure (mean difference in gestational age at birth: 0.37 days, 95% CI: −1.62, 2.36). Remaining marginal associations and interaction p-values were null.
Fig. 2.
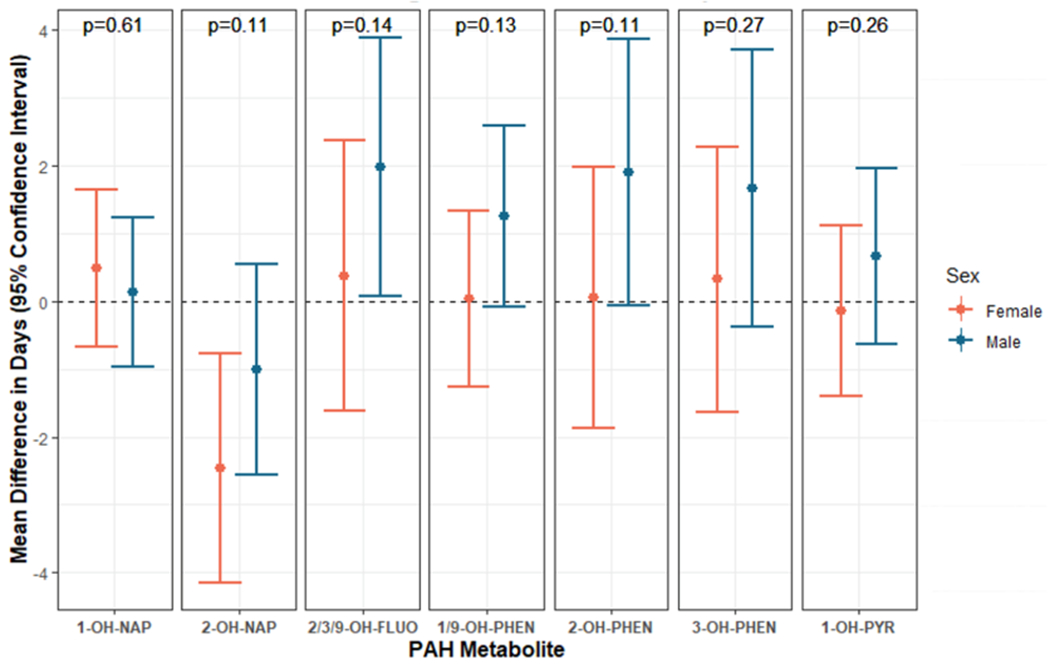
Marginal male and female mean difference in gestational age at birth (days) and 95% confidence intervals associated with 10-fold higher maternal OH-PAH (ng/mL)a,b,c, Abbreviations: OH-PAH; monohydroxy-polycyclic aromatic hydrocarbon; 1-OH-nap, 1-hydroxynaphthalene; 2-OH-nap, 2-hydroxynaphthalene; 1/9-OH-PHEN, combined 1/9-hydroxyphenanthrene; 2-OH-PHEN, 2-hydroxyphenanthrene; 3-OH-PHEN, 3-hydroxyphenanthrene; 2/3/9-OH-FLUO, combined 2/3/9-hydroxyfluorene; 1-OH-PYR, 1-hydroxypyrene.
Adjusted for full model covariates: Maternal age, site, race, ethnicity, cotinine measurements, education, income, urinary specific gravity, infant sex, season of birth, batch number, alcohol during pregnancy, pre-pregnancy BMI, parity, and prior preterm birth
P-values presented above each pair of sex-specific marginal coefficients reflect the exposure by sex interaction term coefficient p-values.
Plot is on the log10-scale.
3.4. Weighted quantile sum regression for OH-PAH mixtures and gestational age at birth
A 10-fold increase in each component of the OH-PAH mixture was associated with a 3.3-day (95% CI: −5.9, −0.80) lower gestational age at birth, primarily driven by maternal exposure to 2-OH-NAP (mixture weight = 77%) and 1-OH-PYR (20%) (Fig. 3). However, the permutation test p-value suggested this association was not statistically significant (p = 0.12). Among females, a 10-fold increase in the PAH mixture was associated with a 4.6-day (95% CI: 0.20, 9.0) higher gestational age at birth, for which 1-OH-NAP had the greatest weight (54%), followed by 2/3/9-OH-FLUO (25%) and 3-OH-PHEN (15%). However, the permutation test p-value suggested this association was not statistically significant (p = 0.16).
Fig. 3.
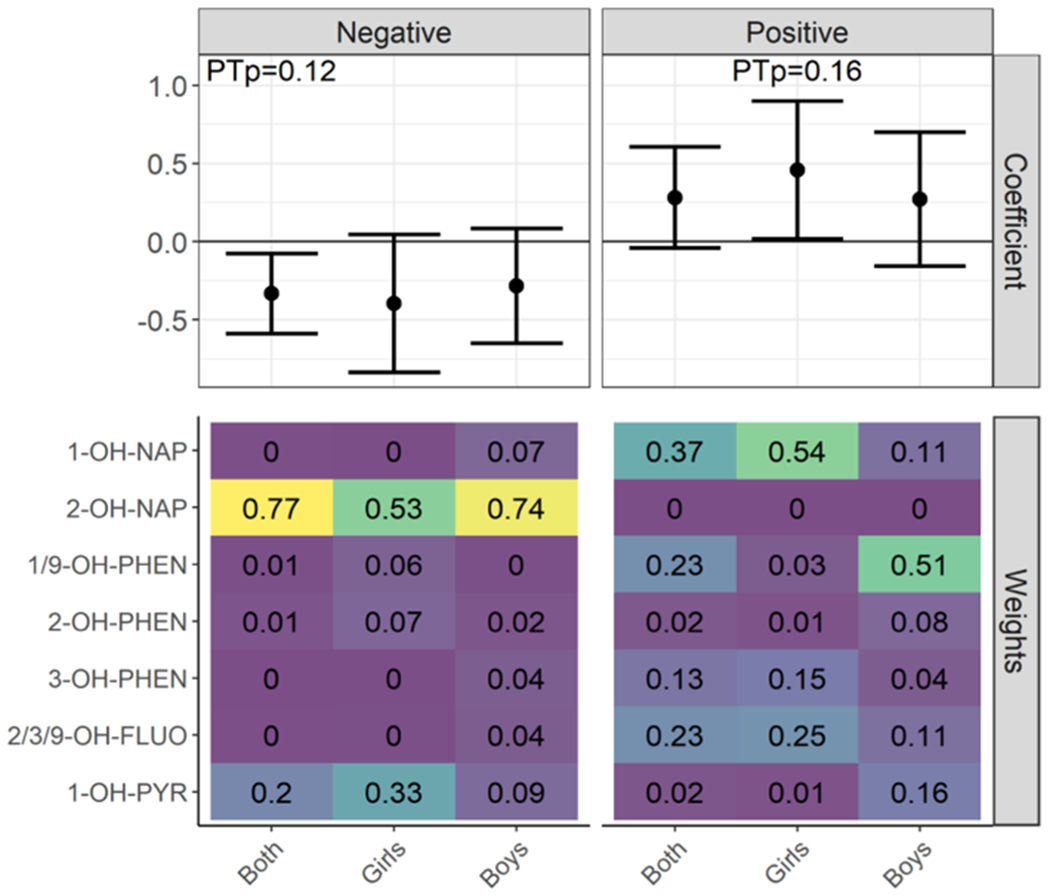
Weighted Quantile Sum Regression Results for OH-PAH Mixtures and Gestational Age at Birtha,b, Abbreviations: OH-PAH; monohydroxy-polycyclic aromatic hydrocarbon; PTp, permutation test p-value; 1-OH-nap, 1-hydroxynaphthalene; 2-OH-nap, 2-hydroxynaphthalene; 1/9-OH-PHEN, combined 1/9-hydroxyphenanthrene; 2-OH-PHEN, 2-hydroxyphenanthrene; 3-OH-PHEN, 3-hydroxyphenanthrene; 2/3/9-OH-FLUO, combined 2/3/9-hydroxyfluorene; 1-OH-PYR, 1-hydroxypyrene.
Adjusted for full model covariates: Maternal age, site, race, ethnicity, cotinine measurements, education, income, urinary specific gravity, infant sex, season of birth, batch number, alcohol during pregnancy, pre-pregnancy BMI, parity, and prior preterm birth
PTp denotes the confirmatory p-value obtained from the permutation test for those coefficients with 95% CI estimates from the original WQSR model that did not overlap with zero.
4. Discussion
Using prospective, pooled data from three geographically diverse cohorts, we examined associations of second trimester maternal urinary OH-PAH biomarkers with spontaneous PTB risk. We also examined associations with gestational age at birth among pregnancies ≥ 34 weeks. In main analyses, higher maternal urinary concentrations of 2-OH-NAP were associated with earlier GA at birth among pregnancies ≥ 34 weeks. Remaining associations were null. We also observed some suggestive sex-specific associations of OH-PAH metabolites with spontaneous PTB. Women with higher maternal urinary concentrations of 1-OH-PYR who were carrying female fetuses, but not male fetuses, appeared to have higher odds of PTB. For the gestational age analysis, 2-OH-NAP exposure among women carrying female fetuses was associated with earlier GA at birth whereas 2/3/9-OH-FLUO exposure among women carrying male fetuses was associated with later GA at birth.
Comparison of our primary PTB results to prior studies is somewhat limited because we restricted outcomes to spontaneous PTB. Interestingly, our observations of positive association of 1-OH-PYR with PTB risk among females replicated reports from an NHANES study that one standard deviation increase in urinary biomarkers of 1-OH-PYR was associated with 80% higher odds (95% CI: 1.1, 2.8) of PTB. Compared with our findings (10-fold change related to 96% increase in odds of spontaneous PTB), the observed association in the NHANES study was likely an overestimate because they did not adjust for maternal smoking history, a major confounder in PAH and PTB. Further, temporality was problematic in that study because PAH exposure was measured after PTB for individuals in this study. Three studies that adjusted for maternal smoking found that among infants born preterm, placental benzo(b)fluoranthene (Suter et al., 2019, Singh et al., 2008), BaP (Agarwal et al., 2018, Suter et al., 2019), fluoranthene (Singh et al., 2008) and dibenz(a,h)anthracene (Suter et al., 2019) were significantly higher at birth compared to placentals from babies born at term. Associations were null for other PAHs observed, which may be due to small sample sizes of the prior studies, ranging from 42 to 84 total subjects and 22–29 preterm deliveries. Two of these studies examined naphthalene, phenanthrene, fluorene, and pyrene, but comparability with our study is limited because they assessed parent compounds instead of PAH metabolites. In a traffic-associated air pollutant study, researchers found that an interquartile range increase of summed naphthalene was associated with 29% higher odds of PTB (95% CI: 1.14, 1.45) (Wilhelm et al., 2011). We did not observe similar associations in our study, although 2-OH-NAP was associated with shorter gestational age among infants born after 34 weeks of gestation. While there is some suggestion of higher risk of PTB associated with several PAH metabolites in these studies, comparability to the present study is limited because prior studies used different PAH exposure ascertainment (primarily placenta measurements or air monitoring), assessed overall PTB rather than spontaneous PTB, and used a limited set of adjustment variables compared to those considered in the current study.
Prenatal exposure to PAHs may impact risk of PTB through changes in uterine physiology associated with labor induction (Arrowsmith, 2020, Laknaur et al., 2016, Barhoumi et al., 2006). One study found that compared to controls, pregnant rats exposed to benzo(a)pyrene (BaP) experienced higher rates of PTB and had higher levels of inflammatory biomarkers (inflammatory cytokines IL-1β, IL-8, and TNFα) and increased expression of genes related to uterine contractions (connexin 43, cox-2, and prostaglandin receptor 2α) (Laknaur et al., 2016). In uterine cells, exposure to BaP has been associated with alterations in oxytocin-induced calcium oscillations, which can contribute to PTB (Barhoumi et al., 2006). PAH exposure has also been linked to higher levels of oxidative stress biomarkers in the placenta (Agarwal et al., 2018), which have been implicated in spontaneous PTB (Moore et al., 2018, Elshenawy et al., 2020). PAH exposure has also been associated with epigenetic changes (e.g., DNA methylation) in the placenta (Suter et al., 2019, Le Vee et al., 2014) that have been related to both spontaneous PTB (Wang et al., 2019) and overall PTB (Toure et al., 2018).
Prior studies suggest PTB and early-term birth may share mechanisms such as placental inflammation and ischemia (McElrath et al., 2008, Brown et al., 2015, Delnord and Zeitlin, 2019), but risk factors for early-term birth are relatively understudied. Our findings suggest higher maternal urinary concentrations of some OH-PAH metabolites, particularly 2-OH-NAP, may be a risk factor for shorter gestation when examining the late-preterm and term period. This observation mirrors prior toxicology findings demonstrating that naphthalene metabolism leads to downstream production of quinones (Preuss et al., 2003), which are associated with formation of free radical species and oxidative stress (Kelly et al., 2019). Interestingly, associations for 1-OH-NAP and gestational age at birth were null despite having the same parent chemical structure as 2-OH-NAP. Differences may be due to slightly different metabolic pathways of toxicity in the formation of 1,4-napthoquinone and 1,2-naphthoquinone by 1-OH-NAP and 2-OH-NAP, respectively (Preuss et al., 2003, Buckpitt et al., 1995). Alternatively, observed differences may be related to the sources of exposure for 1-OH-NAP versus 2-OH-NAP. 1-OH-NAP is a metabolite of both carbaryl and naphthalene exposure (Meeker et al., 2007) whereas 2-OH-NAP is only from naphthalene exposure; differences in upstream sources of exposure to these metabolites may have unanticipated effects on subsequent metabolism. Future research is needed to understand 1-OH-NAP and 2-OH-NAP metabolic pathways and their relationship to fetal toxicity.
While our observation that 10-fold higher 2-OH-NAP is associated with 1.6 days earlier gestational age at birth may appear small, such a difference may have important impacts at the population level, as many more infants would be born preterm, leading to increased morbidity. Further, there could be major impacts on the population level when considering other sets of chemicals in addition to those presently examined. The potential significance of this shortening of gestational age on fetal growth, development, and programming, and eventually on the life course health of the offspring needs further research.
We observed statistically significant multiplicative interaction between sex and two OH-PAH metabolites, 1-OH-PYR and 2-OH-PHEN, on PTB. The direction of marginal estimates suggested that higher PAH exposure is associated with higher odds of spontaneous PTB among women carrying female fetuses. We observed some weak evidence for additive interaction by sex for PAH and gestational age, with male estimates generally in the positive direction and female estimates primarily in the null or inverse direction. The consistent trend across both analyses raises the possibility of sex-specific differences in biological effects of PAH metabolites, although it is important to note many interaction p-values were null. Prior research suggests the placenta mediates fetal programming in a sex-specific manner (Strakovsky & Schantz, 2018) and females experience more inflammation and DNA methylation in the placenta in response to maternal environmental stressors (Gabory et al., 2013). While this relationship has not been explored for PAHs, sex-specific differences in epigenetic changes in the placenta in response to endocrine-disrupting chemicals are well-documented (Clifton, 2010). In addition, sex-specific effects of PAHs on gestational age may be mediated by differences in circulating estrogens (e.g., 17β-estradiol, estriol, estrone) associated with exposure to endocrine disruptors (Li et al., 2020). Accumulating evidence suggests changes in circulating hormone levels in response to endocrine-disruptor exposure may be time- and sex-dependent (Li et al., 2020, Sathyanarayana et al., 2014). Hormones with estrogenic effects are important for regulating labor and birth through effects on uterine physiology and expression of oxytocin receptors (Weiss, 2000). One study found individuals who were pregnant with female fetuses were more sensitive to estriol from bisphenol A (BPA) exposure during the second trimester compared with females carrying male fetuses (Li et al., 2020). A population-based cohort study examining birth outcomes observed that exposure to BPA and two BPA analogs were associated with shorter gestational age and reduced growth parameters among females but not males (Yang et al., 2021). Animal studies have demonstrated similar relationships in which exposure to BPA was associated with higher risk of PTB in females compared with males (Bui et al., 1986).
This is the first study to examine OH-PAH mixtures in relation to continuous gestational age at birth. Our primary hypothesis tested an inverse association between mixtures of OH-PAHs and gestational age at birth. Metabolites with the largest weights in the inverse direction tended to have weights closer to zero in the positive direction (2-OH-NAP) and vice versa (1-OH-NAP, 1/9-OH-PHEN, 2/3/9-OH-FLUO). Our observation of an association for the pooled population in the inverse direction, driven by 2-OH-NAP, is consistent with our gestational age results using the single metabolite models. However, these were not significant using the confirmatory p-values. While sex-stratified estimates suggested a positive trend among females, no estimates were statistically significant using the permutation test. Overall, there was weak evidence to suggest co-exposures to the seven PAH metabolites examined here are positively or inversely associated with gestational age at birth. It is possible that if only one or a small fraction of the mixture components have a true effect on the outcome, this will be more readily detected using individual linear regressions rather than a mixture exposure model like WQSR. This may explain the statistically significant associations observed in the individual models but not the mixture models.
There are limitations to this study. First, extrapolating OH-PAH exposure based on a single urinary measurement may lead to misclassification. The half-life of PAH metabolites ranges from 2.5 to 6.1 h, and one study found that PAH concentrations return to baseline levels within 24–48 h after exposure (Li et al., 2012). A measurement from a single point in time may not capture the entire exposure window of interest (second trimester) and varies according to factors such as metabolism. Previous studies examining repeated measures of OH-PAHs in pregnant individuals have observed low to moderate intraclass correlation coefficients across trimesters of pregnancy (range: 0.06–0.62) (Cathey et al., 2018; Yang et al., 2017), suggesting multiple measures are ideal for estimating prenatal PAH exposure. Despite the potential measurement error, the current study adds to prior literature examining biomarkers of PAH exposure and GA at birth, which also used single measures of PAH but had small sample sizes (Agarwal et al., 2018; Singh et al., 2008; Suter et al., 2019). The extent to which urinary OH-PAHs accurately represent inhalation sources of PAHs is also somewhat unclear and may depend on the molecular weight of the PAH metabolite and sampling methods (Aquilina et al., 2010; Nethery et al., 2012; Polanska et al., 2014). The principal advantage of using urinary biomarkers compared to airborne measures is representation of non-airborne sources of PAH, particularly diet (Zhang et al., 2014). Diet is a major contributor of PAH exposure in most populations, and some studies estimate diet contributes as much as >90% of overall PAH exposure in non-smokers (Polachova et al., 2020, Suzuki and Yoshinaga, 2017). Another limitation to this study is examination of seven PAH metabolites individually, which raises the possibility that some associations may arise due to chance. We did not conduct any statistical corrections for multiple comparisons because we believed that each analysis was testing an independent hypothesis. Third, heterogeneity in laboratory methods (e.g., varying LODs by cohort) and sociodemographic characteristics across cohorts raises the concern that some results may be driven by site- or cohort-specific effects. We attempted to address this concern by 1) adjusting for study site in all analyses and 2) comparing main analyses to models that iteratively excluded a single site or cohort from the study population. Results changed slightly in leave-one-site-out sensitivity analyses, but the direction of association was consistent throughout different combinations of sites. Associations appeared less adverse when removing TIDES from the spontaneous PTB and gestational age analyses. The differences in associations after removing the TIDES cohort may be due to particularly low detection of OH-PAH metabolites among the TIDES participants. It is possible that cohort-specific measurement error associated with <LOD imputations was linked to unmeasured confounders and thus introduced bias. The challenges of pooling cohort data are outweighed by several advantages including having a large, diverse sample population with participants from seven study sites spanning urban and rural US regions.
Given the sparse literature on maternal PAH exposure and birth outcomes, we did not construct an a priori hypothesis about a critical window for PTB. Although the decision to examine second trimester data was driven by data availability, we believe the second trimester is a potentially important window of interest. Studies assessing risk of PTB have identified the second trimester as a vulnerable period for exposure to environmental exposures such as PM2.5, traffic-related pollutants, and ozone (Sheridan et al., 2019, Wang et al., 2018). To our knowledge, no studies have examined repeated measures of OH-PAH within a trimester of pregnancy, so it is challenging to assess the extent to which a single measure captures the entire second trimester. We hypothesize that correlation coefficients would be higher when examining multiple measures within versus across trimesters, given the seasonality of PAH exposure (Liu et al., 2017) as well as prior evidence of fair to substantial agreement observed for PAH measures collected over 2–3 consecutive months in females of reproductive age (Yang et al., 2017), but this would need to be confirmed in future studies.
Approximately 10% and 26.5% of all U.S. infants are born preterm and early term, respectively (3), and both conditions have been associated with several adverse neonatal and life course outcomes (Liu et al., 2016, Shapiro-Mendoza et al., 2008, Sengupta et al., 2013). Identifying modifiable environmental determinants of PTB and early-term births may have substantial public health impact. While we observed mostly null results in pooled analyses, sex-specific effects were observed for a number of metabolites, suggesting that females may be more vulnerable than males to adverse effects of maternal OH-PAH exposure and that sex-specific effects of PAH exposure is a potential area of interest for future research.
Supplementary Material
Acknowledgements
ECHO PATHWAYS is funded by NIH (UG3/UH3OD023271, P30ES007033). The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study was funded by the Urban Child Institute. The TIDES study was funded by NIH R01ES016863 and National Institute of Environmental Health Sciences (NIEHS) Intramural Funding (ZIA10331): Reproductive outcomes and oxidative stress in TIDES (ROOST). Dr. Kannan analyzed OH-PAH metabolites in TIDES with support from the New York University ECHO Cohort Center (UG3/UH3OD023305 (PI:Leonardo Trasande)). Research including Global Alliance to Prevent Prematurity and Stillbirth (GAPPS) participants was conducted using specimens and data collected and stored on behalf of the GAPPS Repository. We are grateful for the participation of families enrolled in the CANDLE, TIDES, and GAPPS cohort, as well as the dedication of CANDLE, TIDES, and GAPPS research staff and investigators. This manuscript has been reviewed by PATHWAYS for scientific content and consistency of data interpretation with previous PATHWAYS publications.
Abbreviations:
- OH-PAH
Monohydroxylated polycyclic aromatic hydrocarbons
- PTB
Preterm Birth
- GA
gestational age
- CANDLE
Conditions Affecting Neurocognitive Development and Learning in Early Childhood
- TIDES
The Infant Development and Environment Study
- GAPPS
Global Alliance to Prevent Prematurity and Stillbirth
- 1-OH-NAP
1-hydroxynaphthalene
- 2-OH-NAP
2-hydroxynaphthalene
- 2-OH-PHEN
2-hydroxyphenanthrene
- 3-OH-PHEN
3-hydroxyphenanthrene
- 1/9-OH-PHEN
combined 1- and 9-hydroxyphenanthrene
- 2/3/9-OH-FLUO
combined 2-, 3-, and 9-hydroxyfluorene
- 1-OH-PYR
1-hydroxypyrene
- OR
Odd’s Ratio
- CI
Confidence Interval
Footnotes
CRediT authorship contribution statement
Sophia L. Freije: Conceptualization, Methodology, Formal analysis, Writing – original draft, Visualization. Daniel A. Enquobahrie: Methodology, Writing – review & editing. Drew B. Day: Methodology, Formal analysis, Writing – review & editing. Christine Loftus: Methodology, Writing – review & editing. Adam A. Szpiro: Methodology, Writing – review & editing. Catherine J. Karr: Methodology, Writing – review & editing, Funding acquisition. Leonardo Trasande: Writing – review & editing, Funding acquisition. Linda G. Kahn: Writing – review & editing. Emily Barrett: Writing – review & editing. Kurunthachalam Kannan: Investigation, Writing – review & editing. Nicole R. Bush: Writing – review & editing. Kaja Z. LeWinn: Writing – review & editing. Shanna Swan: Writing – review & editing, Funding acquisition. W. Alex Mason: Writing – review & editing. Morgan Robinson: Investigation, Writing – review & editing. Sheela Sathyanarayana: Conceptualization, Methodology, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2022.107246.
References
- Agarwal P, Singh L, Anand M, et al. , 2018. Association between placental polycyclic aromatic hydrocarbons (PAHs), oxidative stress, and preterm delivery: A case–control study. Arch. Environ. Contam. Toxicol 74 (2), 218–227. [DOI] [PubMed] [Google Scholar]
- Al-Gubory KH, 2016. Multiple exposures to environmental pollutants and oxidative stress: Is there a sex specific risk of developmental complications for fetuses? Embry Today. 108 (4), 351–364. [DOI] [PubMed] [Google Scholar]
- Aquilina NJ, Delgado-Saborit JM, Meddings C, et al. , 2010. Environmental and biological monitoring of exposures to PAHs and ETS in the general population. Environ. Int 36 (7), 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpino C, Compagnone E, Montanaro ML, et al. , 2010. Preterm birth and neurodevelopmental outcome: a review. Childs Nerv. Syst 26 (9), 1139–1149. [DOI] [PubMed] [Google Scholar]
- Arrowsmith S, 2020. Oxytocin and vasopressin signaling and myometrial contraction. Curr. Opin. Physiol 13, 62–70. [Google Scholar]
- Barhoumi R, Awooda I, Mouneimne Y, et al. , 2006. Effects of benzo-a-pyrene on oxytocin-induced Ca2+ oscillations in myometrial cells. Toxicol. Lett 165 (2), 133–141. [DOI] [PubMed] [Google Scholar]
- Barrett ES, Sathyanarayana S, Janssen S, et al. , 2014. Environmental health attitudes and behaviors: findings from a large pregnancy cohort study. Eur. J. Obstet. Gynecol 176, 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramer SL, Kallungal BA, 2003. Clinical considerations in study designs that use cotinine as a biomarker. J. Biomark 8 (3–4), 187–203. [DOI] [PubMed] [Google Scholar]
- Brown H, Speechley K, Macnab J, et al. , 2015. Biological determinants of spontaneous late preterm and early term birth: a retrospective cohort study. Int. J. Obstet. Gynecol 122 (4), 491–499. [DOI] [PubMed] [Google Scholar]
- Buckpitt A, Chang AM, Winkle LV, et al. , 1995. Relationship of cytochrome P450 activity to Clara cell cytotoxicity. IV. Metabolism of naphthalene and naphthalene oxide in microdissected airways from mice, rats, and hamsters. Molecular Pharmacol. 47 (1), 74–81. [PubMed] [Google Scholar]
- Bui Q, Tran M, West W, 1986. A comparative study of the reproductive effects of methadone and benzo [a] pyrene in the pregnant and pseudopregnant rat. Toxicol. 42 (2–3), 195–204. [DOI] [PubMed] [Google Scholar]
- Carrico C, Gennings C, Wheeler DC, et al. , 2015. Characterization of weighted quantile sum regression for highly correlated data in a risk analysis setting. J. Agric. Biol. Environ. Stat 20 (1), 100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathey A, Ferguson KK, McElrath TF, et al. , 2018. Distribution and predictors of urinary polycyclic aromatic hydrocarbon metabolites in two pregnancy cohort studies. Environ. Pollut 232, 556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Rauh V, Garfinkel R, et al. , 2008. Prenatal exposure to airborne polycyclic aromatic hydrocarbons and risk of intrauterine growth restriction. Environ. Health Perspect 116 (5), 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton VL, 2010. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta 31, S33–S39. [DOI] [PubMed] [Google Scholar]
- Crump C, Sundquist K, Sundquist J, et al. , 2011. Gestational age at birth and mortality in young adulthood. JAMA 306 (11), 1233–1240. [DOI] [PubMed] [Google Scholar]
- Delnord M, Zeitlin J, 2019. Epidemiology of late preterm and early term births – An international perspective. Semin Fetal Neonatal Med. 24 (1), 3–10. [DOI] [PubMed] [Google Scholar]
- Elshenawy S, Pinney SE, Stuart T, et al. , 2020. The metabolomic signature of the placenta in spontaneous preterm birth. Int. J. Mol. Sci 21 (3), 1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabory A, Roseboom TJ, Moore T, et al. , 2013. Placental contribution to the origins of sexual dimorphism in health and diseases: Sex chromosomes and epigenetics. Biol. Sex Differ 4 (1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Huo X, Wu K, et al. , 2012. Carcinogenic polycyclic aromatic hydrocarbons in umbilical cord blood of human neonates from Guiyu, China. Sci. Total Environ 427–428, 35–40. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer, 2021. Polynuclear aromatic compounds, part 1: chemical, environmental, and experimental data. IARC Monographs on the evaluation of the carcinogenic risk of chemicals to humans, vol. 32. Published December 1983. (accessed June 1 2021). [PubMed] [Google Scholar]
- Johnson S, Marlow N, 2011. Preterm birth and childhood psychiatric disorders. Pediatr. Res 69, 11R–18R. [DOI] [PubMed] [Google Scholar]
- Kelly RA, Leedale J, Clleja D, et al. , 2019. Modelling changes in glutathione homeostasis as a function of quinone redox metabolism. Sci. Rep 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyrklund-Blomberg NB, Cnattingius S, 1998. Preterm birth and maternal smoking: Risks related to gestational age and onset of delivery. Am. J. Obstet. Gynecol 179 (4), 1051–1055. [DOI] [PubMed] [Google Scholar]
- Laknaur A, Foster T-L, Bobb LE, et al. , 2016. Altered expression of histone deacetylases, inflammatory cytokines and contractile-associated factors in uterine myometrium of Long Evans rats gestationally exposed to benzo[a ]pyrene: Effect of benzo(a)pyrene on gestation and uterine molecular biology. J. Appl. Toxicol 36 (6), 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Vee M, Kolasa E, Jouan E, et al. , 2014. Differentiation of human placental BeWo cells by the environmental contaminant benzo(a)pyrene. Chem. Biol. Interact 210, 1–11. [DOI] [PubMed] [Google Scholar]
- Li Z, Romanoff L, Bartell S, et al. , 2012. Excretion profiles and half-Lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem. Res. Toxicol 25 (7), 1452–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang W, Zhao H, et al. , 2020. Trimester-specific, gender-specific, and low-dose effects associated with non-monotonic relationships of bisphenol A on estrone, 17β-estradiol and estriol. Environ. Int 134, 105304. [DOI] [PubMed] [Google Scholar]
- Liu B, Xue Z, Zhu X, et al. , 2017. Long-term trends (1990–2014), health risks, and sources of atmospheric polycyclic aromatic hydrocarbons (PAHs) in the U.S. Environ. Pollut 220 (B), 1171–1179. [DOI] [PubMed] [Google Scholar]
- Liu L, Oza S, Hogan D, et al. , 2016. Global, regional, and national causes of under-5 mortality in 2000–15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 388 (10063), 3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus CT, Bush NR, Day DB, et al. , 2021. Exposure to prenatal phthalate mixtures and neurodevelopment in the Conditions Affecting Neurocognitive Development and Learning in Early childhood (CANDLE) study. Environ. Int 150, 106409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Osterman MJK, et al. , 2019. Births: final data for 2018. Natl. Vital Statistics Rep 68 (13), 1–47. [PubMed] [Google Scholar]
- McElrath TF, Hecht JL, Dammann O, et al. , 2008. Pregnancy disorders that lead to delivery before the 28th week of gestation: An epidemiologic approach to classification. Am. J. Epidemiol 168 (9), 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Barr DB, Serdar B, et al. , 2007. Utility of urinary 1-naphthol and 2-naphthol levels to assess environmental carbaryl and naphthalene exposure in an epidemiology study. J. Exp. Sci. Environ. Epidemiol 17, 314–320. [DOI] [PubMed] [Google Scholar]
- Moore TA, Ahmad IM, Zimmerman MC, 2018. Oxidative stress and preterm birth: An integrative review. Biol. Res. Nurs 20 (5), 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nethery E, Wheeler AJ, Fisher M, et al. , 2012. Urinary polycyclic aromatic hydrocarbons as a biomarker of exposure to PAHs in air: A pilot study among pregnant women. J. Expo. Sci. Environ. Epidemiol 22, 70–81. [DOI] [PubMed] [Google Scholar]
- Padula AM, Noth EM, Hammond SK, et al. , 2014. Exposure to airborne polycyclic aromatic hydrocarbons during pregnancy and risk of preterm birth. Environ. Res 135, 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh LI, Reddy UM, Männistö T, et al. , 2014. Neonatal outcomes in early term birth. Am. J. Obstet. Gynecol 211 (3), 265.e1–265.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel CJ, Yang T, Hu Z, et al. , 2014. Investigation of maternal environmental exposures in association with self-reported preterm birth. Reprod. Toxicol 45, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polachova A, Gramblicka T, Parizek O, et al. , 2020. Estimation of human exposure to polycyclic aromatic hydrocarbons (PAHs) based on the dietary and outdoor atmospheric monitoring in the Czech Republic. Environ. Res 182, 108977. [DOI] [PubMed] [Google Scholar]
- Polanska K, Hanke W, Dettbarn G, et al. , 2014. The determination of polycyclic aromatic hydrocarbons in the urine of non-smoking Polish pregnant women. Sci. Total 487, 102–109. [DOI] [PubMed] [Google Scholar]
- Preuss R, Angerer J, Drexler H, 2003. Naphthalene—an environmental and occupational toxicant. Int. Arch. Occupat. Environ. Health 76, 556–576. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. [Google Scholar]
- Reddy UM, Bettegowda VR, Dias T, et al. , 2011. Term pregnancy: a period of heterogeneous risk for infant mortality. Obstet. Gynecol 117 (6), 1279–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzetti S, Curtin P, Just AC, 2021. gWQS: Generalized Weighted Quantile Sum Regression. R package version 3.0.4 https://CRAN.R-project.org/package=gWQS. [Google Scholar]
- Saigal S, Doyle LW, 2008. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 371 (9608), 261–269. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Barrett E, Butts S, et al. , 2014. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reprod. 147 (4), 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick SF, Blount BC, Jacob P, et al. , 2017. Biomarkers of exposure to new and emerging tobacco delivery products. Am. J. Physiol. Lung Cell. Mol. Physiol 313 (3), L425–L452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Carrion V, Shelton J, et al. , 2013. Adverse neonatal outcomes associated with early-term birth. JAMA Pediatr. 167 (11), 1053. [DOI] [PubMed] [Google Scholar]
- Shapiro-Mendoza CK, Tomashek KM, Kotelchuck M, et al. , 2008. Effect of late-preterm birth and maternal medical conditions on newborn morbidity risk. Pediatr. 121 (2), e223–e232. [DOI] [PubMed] [Google Scholar]
- Sheridan P, Ilango S, Bruckner TA, et al. , 2019. Ambient find particulate matter and preterm birth in California: Identification of critical exposure windows. Am. J. Epidemiol 188 (9), 1608–1615. [DOI] [PubMed] [Google Scholar]
- Singh VK, Singh J, Anand M, et al. , 2008. Comparison of polycyclic aromatic hydrocarbon levels in placental tissues of Indian women with full- and preterm deliveries. Int. J. Hyg. Environ. Health 211 (5–6), 639–647. [DOI] [PubMed] [Google Scholar]
- Sontag-Padilla L, Burns R, Shih R, et al. , 2015. The urban child institute CANDLE study: Methodological overview and baseline sample description. RAND corporation. http://www.rand.org/pubs/research_reports/RR1336.html. [Google Scholar]
- Strakovsky RS, Schantz SL, 2018. Impacts of bisphenol A (BPA) and phthalate exposures on epigenetic outcomes in the human placenta. Environ. Epigenet 4 (3), dvy022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter MA, Aagaard KM, Coarfa C, et al. , 2019. Association between elevated placental polycyclic aromatic hydrocarbons (PAHs) and PAH-DNA adducts from Superfund sites in Harris County, and increased risk of preterm birth (PTB). Biochem. Biophys. Res. Commun 516 (2), 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Yoshinaga J, 2017. Inhalation and dietary exposure to polycyclic aromatic hydrocarbons and urinary 1-hydroxypyrene in non-smoking university students. Int. Arch. Occup. Environ. Health 81 (1), 115–121. [DOI] [PubMed] [Google Scholar]
- Swan SH, Sathyanarayana S, Barrett ES, et al. , 2015. First trimester phthalate exposure and anogenital distance in newborns. Hum. Reprod 30 (4), 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toure MD, ElRayes W, Barnes-Josiah D, et al. , 2018. Epigenetic modifications of human placenta associated with preterm birth. J. Matern. Fetal. Neonatal. Med 31 (4), 530–541. [DOI] [PubMed] [Google Scholar]
- Van Buuren S, Groothuis-Oudshoorn K, 2011. MICE: Multivariate imputation by chained equations in R. J. Stat. Softw 45 (3), 1–67. [Google Scholar]
- Vatten LJ, Skjærven R, 2004. Offspring sex and pregnancy outcome by length of gestation. Early Hum. Dev 76 (1), 47–54. [DOI] [PubMed] [Google Scholar]
- Vu AT, Taylor KM, Holman MR, et al. , 2015. Polycyclic aromatic hydrocarbons in the mainstream smoke of popular U.S. cigarettes. Chem. Res. Toxicol 28 (8), 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Benmarhnia T, Zhang H, et al. , 2018. Identifying windows of susceptibility for maternal exposure to ambient air pollution and preterm birth. Environ. Int 121 (1), 317–324. [DOI] [PubMed] [Google Scholar]
- Wang X-M, Tian F-Y, Fan L-J, et al. , 2019. Comparison of DNA methylation profiles associated with spontaneous preterm birth in placenta and cord blood. BMC Med. Genom 12 (1), 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, 2000. Endocrinology of parturition. J. Clin. Endocrinol. Metab 85 (12), 4421–4425. [DOI] [PubMed] [Google Scholar]
- Wilhelm M, Ghosh JK, Su J, et al. , 2011. Traffic-related air toxics and preterm birth: a population-based case-control study in Los Angeles county, California. Environ Health 10 (1), 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Zota AR, Schwartz JM, 2011. Environmental chemicals in pregnant women in the United States: NHANES 2003–2004. Environ. Health Perspect 119 (6), 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Platt RW, Kramer MS, 2010. Variation in child cognitive ability by week of gestation among healthy term births. Am. J. Epidemiol 171 (4), 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Lin B-G, Zhou B, et al. , 2021. Sex-specific associations of prenatal exposure to bisphenol A and its alternatives with fetal growth parameters and gestational age. Environ. Int 146, 106305. [DOI] [PubMed] [Google Scholar]
- Yang P, Sun H, Gong YJ, et al. , 2017. Repeated measures of urinary polycyclic aromatic hydrocarbon metabolites in relation to altered reproductive hormones: A cross-sectional study in China. Int. J. Hyg. Environ. Health 220 (8), 1340–1346. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ding J, Shen G, et al. , 2014. Dietary and inhalation exposure to polycyclic aromatic hydrocarbons and urinary excretion of monohydroxy metabolites–A controlled case study in Beijing, China. Environ. Pollut 184, 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kramer MS, 2009. Variations in mortality and morbidity by gestational age among infants born at term. J. Pediatr 154 (3), 358–362.e1. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Brown TR, Doan LL, et al. , 2012. Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinol. 153 (9), 4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


