Abstract
The post-acute COVID-19 syndrome (PACS) is characterized by the persistence of fluctuating symptoms over three months from the onset of the possible or confirmed COVID-19 acute phase. Current data suggests that at least 10% of people with previously documented infection may develop PACS, and up to 50–80% of prevalence is reported among survivors after hospital discharge. This viewpoint will discuss various aspects of PACS, particularly in older adults, with a specific hypothesis to describe PACS as the expression of a modified aging trajectory induced by SARS CoV-2. This hypothesis will be argued from biological, clinical and public health view, addressing three main questions: (i) does SARS-CoV-2-induced alterations in aging trajectories play a role in PACS?; (ii) do people with PACS face immuno-metabolic derangements that lead to increased susceptibility to age-related diseases?; (iii) is it possible to restore the healthy aging trajectory followed by the individual before pre-COVID?. A particular focus will be given to the well-being of people with PACS that could be assessed by the intrinsic capacity model and support the definition of the healthy aging trajectory.
1. Background
World Health Organization (WHO) recently acknowledged the long term effects of COVID-19 through a Delphi consensus clinical case definition: “Post COVID-19 condition in individuals with a history of probable or confirmed SARS- CoV-2 infection, may be present usually three months from the onset of COVID-19 with symptoms that last for at least two months and cannot be explained by an alternative diagnosis. Common symptoms include fatigue, shortness of breath, cognitive dysfunction but also others and generally have an impact on everyday functioning. Symptoms may be newly onset following initial recovery from an acute COVID-19 episode or persist from the initial illness. Symptoms may also fluctuate or relapse over time” (WHO Post COVID-19 definition, 2022).
This condition is also recognized with different names, including long covid, long-hauler syndrome, post-covid syndrome, or post-acute COVID-19 syndrome (PACS; used in this paper).
This definition intrinsically comprises three issues:
-
1)
Firstly, PACS should be discriminated against symptoms still present in an early recovery phase. Not by chance, PACS symptoms may occasionally occur in people who experience asymptomatic or mildly symptomatic acute phase conditions. Moreover, due to the relapsing/remitting nature of post-COVID symptoms, the following integrative classification has been proposed: potentially infection related-symptoms (up to 4–5 weeks), acute post-COVID symptoms (from week 5 to week 12), long post-COVID symptoms (from week 12 to week 24), and persistent post-COVID symptoms (lasting more than 24 weeks), the last two adequately defined as PACS (Fernández-de-Las-Peñas et al., 2021).
-
2)
Secondly, It is not a disease but rather a syndrome: a set of medical signs and symptoms correlated with each other, unable to singularly define the condition, and associated with a particular disease (Page, 2018). The construct “syndrome” well describes the multitudes of symptoms affecting different organ systems, grouped qualitatively as clusters in a non-exhaustive list of musculoskeletal, respiratory, neurocognitive, autonomic, gastrointestinal, psychological, sensory, and dermatological (Fernández-de-Las-Peñas et al., 2021).
-
3)
Thirdly, WHO operationalized this definition in an event that may alter everyday functioning, somehow suggesting a working definition based on quality-of-life impact. Interestingly, in the US, PACS can be considered a disability condition (CDC, 2022). A people with PACS have a disability if the person’s condition or any of its symptoms is a “physical or mental” impairment that “substantially limits” one or more major life activities. Disability is a hallmark of unhealthy aging, and therefore, the association between PACS and disability indicates that PACS may alter the aging trajectory.
Moreover, most people with PACS describe the constellation of cluster symptoms they experience as “feeling old”. The prevalence of PACS has been reported very differently according to diverse age groups, socioeconomic tiers, ethnicities, and geographical areas, varying from 10% of the people with previously documented infection (Wissler Gerdes et al., 2022) to as high as 50–80% among survivors after hospital discharge (Carfì et al., 2020, Garrigues et al., 2020, Carvalho-Schneider et al., 2021, Arnold et al., 2020, Nehme et al., 2021, Mandal et al., 2021, Tenforde et al., 2020, Stavem et al., 2020).
Prevalent diagnoses are important as many conditions may have been pre-existing. COVID could exacerbate some of these, but this is not part of the PACS phenotype. Only a few studies have analyzed incident diagnosis and symptoms in PACS.
In a large retrospective cohort study based on linked electronic health records (EHRs) data from 81 million patients, including 273,618 COVID-19 survivors, it was found that over 1 in 3 patients had one or more features of PACS recorded between 3 and 6 months after a diagnosis of COVID-19. The incidence of each feature was: abnormal breathing (18.7% during the first semester after the infection), fatigue/malaise (12.8%), chest/throat pain (12.6%), headache (8.7%), other pain (11.6%), abdominal symptoms (15.5%), myalgia (3.2%), cognitive symptoms (7.8%), and anxiety/depression (22.8%). The risk of long-COVID features was higher in patients who had experienced the most severe COVID-19 illness, and slightly higher among women and young adults. White and non-white patients seem equally affected (Taquet et al., 2021a). Thus, COVID-19 disease appears to have a different impact on men and women, and sex/gender differences in clinical outcomes and mortality rates of COVID-19 patients have been reported (Mussini et al., 2021, Meng et al., 2020). In this context, gender-sensitive research is needed, addressing the biological differences relevant to the disease and the gendered factors that can modulate, among others, the severity and long-term effects of COVID-19.
According to a recent UK survey, 1.3 million people living in private households in the UK (2.0% of the population) were estimated to suffer from PACS) (Prevalence of ongoing symptoms following coronavirus COVID-19 infection in the UK, 2022). This silent epidemic could qualify as a “hidden public health disaster” and media claims that PACS could become the most prominent chronic disease in some countries (Long COVID could become Finland’s largest chronic disease warns minister, 2022).
This viewpoint will concentrate on PACS in older adults, conventionally defined as people 50 + years old, with the specific hypothesis to describe PACS as the expression of a modified aging trajectory induced by SARS CoV-2.
For this purpose, three main questions will be addressed, and for each of them, biological, clinical and public health issues will be discussed.
The questions and chapters of this article are:
-
1.
Does PACS depict altered aging trajectories?
-
2.
Do people with PACS face immuno-metabolic derangements that increase susceptibility to age-related diseases?
-
3.
Is it possible to restore the healthy aging trajectory followed before the COVID-19 illness?
2. Does PACS depict altered aging trajectories?
The aging phenomenon can be approached by measuring the accumulation of un-repaired changes occurring over time at cellular, tissutal, and organ level. It depends on both internal adaptive mechanisms, as a response to those changes, and external factors, such as lifelong exposure to a variety of diverse infectious agents and to environmental stressors. Age has been recognized as a major driver of COVID-19 related mortality, particularly impacting the demographic structure of the population in countries with a higher proportion of older persons (Scortichini et al., 2020).
Moreover, older people who experience PACS may belong to the subset of individuals who survived acute COVID-19. Therefore, they may represent vulnerable patients but are still more robust than others who might have experienced the fatal consequences of the infection. This represents a selection bias with potential biological and clinical implications in the PACS geriatric cohorts.
PACS presentation, prognosis, and long-term outcomes may have unique features in older adults. Nevertheless, younger individuals have reported complaints related to PACS at a higher rate, possibly due to a pre-COVID healthier status. Indeed, the impact of age on the incidence of the syndrome is still under study (Vanichkachorn et al., 2021). However, pre-existing conditions, captured both by single diseases as well as the construct of multimorbidity, seem associated with PACS (Tenforde et al., 2020; Vanichkachorn et al., 2021; Tisminetzky et al., 2022). For example, patients with Alzheimer's disease experience a decreased capacity to return to the pre-COVID state, with a progression of their functional impairments (Alzheimer’s disease facts and figures, 2021, 2021; COVID-19 Associated with Long-Term Cognitive Dysfunction, 2022).
3. Biological interpretation
At the immunological level, aging is often described as the interplay between changes in the native immune system, mainly depicted by the construct of inflammaging (Franceschi et al., 2000), and those occurring in the adaptive immune system, represented by senescence of T-cells (Goronzy et al., 2015). In the context of COVID-19 disease in older persons, it is argued that the pre-existing reduction of the total number of naive T cells and increase of memory T cells may explain a higher risk of severe clinical presentation and worse outcomes (Fulop et al., 2018). The older person who survived the acute phase may experience an alteration of the immune-inflammatory homeostasis (Yanes et al., 2017). Persistent immune activation may be associated with ongoing symptoms following COVID-19 (Gibellini et al., 2022).
In a US study, persons who developed PACS presented higher levels of cytokine biomarkers, including tumor necrosis factor alfa and interferon-gamma-induced protein 10. There was also a trend toward more elevated interleukin-6 levels during the recovery (Peluso et al., 2021). It is noteworthy that the SARS CoV-2 infection may negatively impact several, although strongly interacting, hallmarks of aging (e.g., oxidative stress, metabolic derangement, mitochondria dysfunction, DNA damage) (Barzilai et al., 2020).
The virus-induced activation of innate and adaptive immune systems may represent an overwhelming challenge to this crucial defense mechanism, directly contributing to immunosenescence (Bektas et al., 2020, Borella et al., 2022, Gibellini et al., 2020). COVID-19 may predispose individuals to inflammaging through innate immunological response. Leukocytes can remain persistently infected by COVID-19, meaning that inflammation may persist after the initial infection and acute symptom resolution (Troyer et al., 2020, Arbour et al., 2000, Desforges et al., 2007).
The so-called cytokine storm activates inflammation so strongly that there could be an exhaustion of the capacity to produce an inflammatory reaction leading to immune paralysis (De Biasi et al., 2020). This diminishes the capacity of the individual to fully benefit from a robust immune response and, at the same time, predisposes to a chronic proinflammatory state (Bektas et al., 2020). However, whether blunting the cytokine storm by any type of intervention may prevent long-term consequences, as likely happens in those who can endogenously control the onset of inflammatory symptoms (De Biasi et al., 2020), the mechanisms leading to the development of inflammaging is unknown.
Inflammaging and immunosenescence progress in parallel. Increased production of inflammatory mediators, characteristic of inflamm-aging, contributes to the decrease of the adaptive immune response and, eventually, to immunosenescence. In contrast, the reduction of the adaptive immune response reinforces the stimulation of the innate immune response leading to inflammaging (Fulop et al., 2018).
The interplay between inflammaging and immunosenescence (Calabrese et al., 2015) may represent the basis of the immunopathogenesis of PACS, through the accumulation of senescent cells which acquire a senescence-associated secretory phenotype (SASP) (Fulop et al., 2018). It is hypothesized that this phenotypic change results from cellular stress secondary to SARS CoV-2 impairment cellular homeostatic mechanisms. The SASP can release cytokines, chemokines, proteases, reactive metabolites, growth factors, noncoding nucleotides (Coppé et al., 2008, Zhu et al., 2014, Iske et al., 2020). Even after the acute condition is over, the residual burden of senescent cells may cause chronic inflammation through the continuous production of SASP proteins. SASP can cause dysfunction and contribute to cognitive, metabolic, physical, and vascular dysfunction, tissue fibrosis, disease susceptibility and severity, and mortality (Khosla et al., 2020, Xu et al., 2015, Xu et al., 2018, Roos et al., 2016, Schafer et al., 2017, Tchkonia and Kirkland, 2018, Wang et al., 2020).
There are likely multiple ways to develop long COVID. These paths are not mutually exclusive and may even be dependent on one another.
With regards to the possible virological pathogenesis, it has been shown that a minority of people continued to have viral RNA detectable in nose for weeks. A few studies report detection of viral proteins and RNA in various tissues, by in situ methods months after infection (Gaebler et al., 2021, Su et al., 2022).
Reactivation of Epstein-Barr virus (EBV) has been indirectly inferred to correlate with PACS through antibody titer measurements (Gold et al., 2021). EBV viremia, but not CMV, was identified in 14% of tested patients, and positive SARS-CoV-2 RNAemia in 25% of patients, with few individuals positive for both one month after infection but they tended to wane 3 months after acute phase of the disease (Su et al., 2022).
Autoantibodies (autoAbs), especially those that neutralize type I interferons (IFNs), have also been reported to associate with immune dysfunction and COVID-19 and have been speculated to associate with PACS (Proal and VanElzakker, 2021), sharing a similar pattern of Systemic Lupus Erythematosus (Pisetsky and Lipsky, 2020).
4. Clinical interpretation
Several studies have shown that frailty predicts disease outcomes in patients admitted to hospital with COVID-19 better than either age or comorbidity (Petermann-Rocha et al., 2020).
Frailty is the accumulation of deficits that include a state of increased vulnerability and reduced endurance that are associated with higher risk of disability, mortality and other adverse health outcomes (Rockwood et al., 1999).
The aim of screening for frailty is to understand the acute manifestations of COVID-19 regarding the pre-existing health condition and to predict adverse outcomes during hospitalization or after discharge (Ensrud et al., 2018, Truog et al., 2020). Frailty screening may support medical and patients’ decision regarding the choice to hospitalize or rather opt to a palliative care in nursing/homes (Rajabali et al., 2016). Even in the case of hospital admission, frailty may guide the appropriateness of care intensification in intensive care units (ICU) being associated with poor outcomes or with the capacity to wean from mechanical ventilation in the recovery phase (Gilis et al., 2021, Blomaard et al., 2021).
There is a wide variety of frailty screening instruments that have been used in the context of COVID-19 pandemic. The National Institute for Health and Care Excellence (NICE) has opted for using the clinical frailty scale (CFS) as a screening tool in COVID-19 triage at the emergency department (NICE. COVID-19, 2022). The CFS considers the cognitive function, mobility, comorbidities, and functional status combined into a pictograph (Rockwood et al., 2005). NICE has arbitrarily chosen the degree of frailty in older adults with the CFS< 5 cut-off to consider hospitalization and critical care admission (NICE. COVID-19, 2022). It is crucial to understand that any frailty tool does not define futility because there is not yet a studied cut-off of any frailty instrument, which defines the patient who would benefit from ICU admission. Indeed, the frontier between “futility” and “worthiness” of ICU admission for old frail individuals is unclear (Hussien et al., 2021). It is surprising to observe that despite the large number of studies addressing the impact of frailty on adverse clinical outcomes of acute COVID-19, the opposite effect of COVID-19 on frailty onset and progression is largely unknown.
The rationale that COVID-19, as an acute stressor, may induce or worsen frailty is very suggestive. Moreover, given the interplay between inflammaging and immunosenescence at the basis of the immunopathogenesis of PACS, we may also suggest that PACS itself may also perpetrate frailty with potential implication in the short and long effect in aging trajectories. In this context, treating frailty may serve as a clinically meaningful intervention in the management of PACS. Nevertheless, in the context of an event which is strongly connected with an acute stressor, this construct should also be linked with a measure which captures the nature of the stressor itself. This new construct is resilience.
Resilience is the capacity to reach a new health equilibrium after exposure to stress, It depicts a dynamic trajectory over time in which post-stress health status may be the same or different in comparison to the initial one and homeostasis may be reached at a different speed (Whitson et al., 2016). Ferrucci et al. underlined the need to describe resilience as complementary to frailty in aging trajectories. Resilience at a young age is capable of compensating damage. During the course of life, damage accumulates and resilience is overwhelmed. Unopposed damage accumulation leads to frailty and eventually to death (Ferrucci et al., 2020).
The operational definition of resilience, in its physical or rather psychological components, is elusive and resilient people are difficult to identify. Moreover COVD-19 stressor may be related to both “COVID disease” or “COVID crises” with its burden of suffering in terms of psychosocial implication, with an imbalance between the physical or the psychological resilience domains. Thus, it is unlikely that a single tool can be used in this setting.
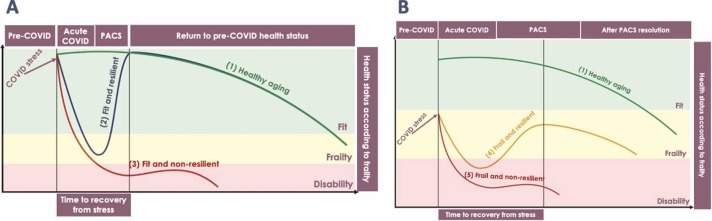
Fig. 1 conceptualizes an interaction model of frailty and resilience into four different phenotypes, namely “fit and resilient”, “fit and non-resilient”, “frail and resilient” and “frail and non-resilient” in comparison to a healthy aging trajectory. Fig. 1 A shows the impact of COVID stress in initially fit individuals. The first scenario describes Fit and resilient people who are able to regain the pre-COVID-19 health status in a relatively short period of time (Fig. 1A-2). These individuals may be transiently frail in the acute disease phase and may experience transient PACS. Eventually they will be able to recover and return to a healthy aging trajectory.
Fig. 1.
conceptualizes an interaction model of frailty and resilience into four different phenotypes, namely “fit and resilient”, “fit and non-resilient”, “frail and resilient” and “frail and non-resilient” in comparison to a healthy aging trajectory. Fig. 1A shows the impact of COVID stress in initially fit individuals. The first scenario describes Fit and resilient people who are able to regain the pre-COVID health status in a relatively short period of time (Fig. 1A-2). The Fit and non-resilient phenotype represents the subset of individuals in which the acute COVID stress leads rapidly to disability with dramatic change in the initial optimal health condition (Fig. 1A-3). Fig. 1B shows the impact of COVID stress in initially frail individuals. The Frail and resilient phenotype comprises individuals who were frail before COVID onset, but with preserved resilience (Fig. 1B-4). The last scenario depicts frail and non-resilient phenotype, represents the most vulnerable individuals in which COVID stress makes them rapidly develop irreversible disability (Fig. 1B-5). Abbrevations: PACS – post acute COVID-19 syndrome.
The Fit and non-resilient phenotype represents the subset of individuals in which the acute COVID-19 stress leads rapidly to disability (e.g. severe acute respiratory distress syndrome - ARDS with ICU admission) with dramatic change in the initial optimal health condition (Fig. 1A-3). These individuals will eventually remain in a permanent disability condition with or without PACS (e.g. persistent mild or moderate dyspnea with pulmonary fibrosis).
The Frail and resilient phenotype comprises individuals who were frail before COVID-19 onset, but with preserved resilience (Fig. 1B-4). COVID-19 stress deteriorates their frailty level and upon recovery they gain a new equilibrium but have lower probability to return to the pre-COVID health status (e.g. a person with obesity and diabetes that was already frail, but with an optimal control of his/her chronic conditions who will develop decompensated diabetes with metabolic complications). Interestingly, the interaction between frailty and resilience makes people in this phenotype take more time to recover.
The last scenario, depicted by frail and non-resilient phenotype, represents the most vulnerable individuals. COVID-19 stress makes them rapidly develop irreversible disability (Fig. 1B-5). They will experience long lasting PACS with potential severe impact on life expectancy and quality of life (e.g. a person with chronic obstructive pulmonary disease that after COVID-19 requires permanent oxygen support).
All together, these models describe that resilience is strongly associated with PACS. Apparently, PACS reversibility depends on the capacity to restore resilience.
5. Public health interpretation
PACS clinical manifestations depicts an increased burden of age-related conditions. A large observational study, including 47790 people who were admitted to hospital for severe COVID-19 disease, showed that they were more likely to have a new onset of diabetes, cardiovascular and respiratory disease in comparison to age-matched controls without previous history of COVID-19 (Ayoubkhani et al., 2021).
Ecological studies have not yet been performed to address if these age-related conditions occurring at an earlier age than in the general population who did not experience COVID-19.
This debate fueled controversy in the interpretation of aging in other chronic disease conditions including HIV. Some differences can be noticed because unlike HIV, people with PACS have a much larger age spectrum closer to the general population.
PACS may depict either an accelerated or an accentuated aging trajectory.
‘Accelerated aging’ refers to a fit/frail and non-resilient phenotype (Fig. 1). These individuals accumulate over time age-related conditions at a faster rate relative to the age-related changes that would be expected in the general population.
‘Accentuated aging’ refers to the frail and resilient phenotype (Fig. 1) in which the homeostasis is reached during PACS, and the subsequent year-on-year increases in the risk of the age-related conditions is the same as those observed in the general population. In this scenario, the trajectory is lower but still parallel to the healthy aging trajectory. A combination of accelerated and accentuated aging would also be possible (not depicted in the figure).
In a public health perspective, the interaction between frailty and resilience may inform future intervention and resources should be designed according to the projection of aging curves described in people with PACS.
6. Do people with PACS face immuno-metabolic derangements that lead to increased susceptibility to age-related diseases?
6.1. Biological intetrpretation
Metaflammation conceptualizes the inflammation which accompanies metabolic diseases associated with nutrient excess. Metaflammation might precede and contribute to inflammaging and metabolic age-related diseases could be considered manifestations of the acceleration of ageing. Inflammaging and metaflammation largely share the same molecular mechanisms, which have been highly preserved in the evolutionary process.
The gut microbiota has a central role in metaflammation and inflammaging, mediated by tryptophan metabolism. In detail, kynurenine (Kyn) metabolism can be described as a homeostatic process balancing kynurenine to tryptophan and quinolinic-to-kynurenic acid ratios. The former has been associated with obesity and the latter with inflammatory states in the general population; interestingly, both appear to be increased in PACS.
In the COVID-19 arena, several metabolomic studies have been conducted (Fraser et al., 2020, Shen et al., 2020) showing that Trp-Kyn pathway is altered in COVID-19 patients, leading to a decrease in Trp levels and an increase in Kyn and its metabolites (Thomas et al., 2020, Lawler et al., 2021, Lionetto et al., 2021).
Altered kynurenine pathway, was identified in patients with COVID-19 and correlated to IL-6 levels during the acute phase of the disease. Enhanced inflammation further leads to an increase in IDO-1 activity followed by enhanced degradation of Trp into Kyn and its metabolites (Vyavahare et al., 2021).
In PACS, Trp-Kyn catabolic pathway has been identified as a potential causal mechanism in musculoskeletal and neurocognitive complications. In detail, it has been involved in muscle loss which represents the organ tissue damage associated with frailty (Vyavahare et al., 2021). In a longitudinal study including 128 patients with mild to moderate COVID-19, KYN pathway remained activated 8–12 months after acute phase of the disease and was associated with lower cognitive performance after adjustment for comorbidities and disease severity (Cysique et al., 2022).
In this regard, PACS represents a unique setting where to explore Kyn pathways at the crossroad between metabolism and inflammation.
6.2. Clinical interpretation
At a clinical level, metaflammation in people with PACS can be captured by Non-alcoholic fatty liver disease (NAFLD) (Byrne and Targher, 2015, Chitturi and Farrell, 2007) which carries a high risk of cardiovascular complications and mortality. More recently, Eslam and colleagues suggested a name change from NAFLD to MAFLD that stands for Metabolic (dysfunction)-associated fatty liver disease. This new construct focuses on “positive” criteria that strength the association with metabolic risk factors rather than exclusionary criteria (absence of significant alcohol intake (Eslam et al., 2020). Recent studies have proposed that MAFLD is a hepatic manifestation of a multisystem disorder, which is heterogeneous in its underlying causes, presentation, course, and outcomes (Lonardo et al., 2021, Lonardo, 2021).
In a recent study comprising 235 patients assessed for PACS, the prevalence of MAFLD was 55.3% at the time of PACS diagnosis and 37.3% on hospital admission (p < 0.001). MAFLD was independently associated with insulin resistance, body mass index, and metabolic syndrome. Given the high prevalence of MAFLD, the authors argued that it may represent a specific PACS-cluster phenotype, with potential long-term metabolic and cardiovascular health implications (Milic et al., 2022).
Interestingly, a high prevalence of loss of both lean and fat mass but increased liver fat accumulation has also been described using CT imaging after ICU admission (Gualtieri et al., 2020). An observational study showed that low muscle quality and ectopic fat accumulation were associated with invasive mechanical ventilation and death (Besutti et al., 2021). Additionally, visceral adipose tissue volume was related to baseline inflammation, as measured by C-reactive protein levels (Besutti et al., 2021). This phenomenon is not limited to the acute phase of the disease. We may therefore argue that sarcopenic obesity also represents one of the metabolic manifestations of PACS.
A randomized controlled clinical trial conducted in 76 PACS patients with post-COVID-19 sarcopenia compared the clinical and psychological effects of low or high intensity aerobic training with resistance. The former was more effective in improving the clinical (muscle strength) and psychological (quality of life) measures than the latter in post-COVID-19 sarcopenia (Nambi et al., 2022).
6.3. Public health interpretation
Given the high prevalence of PACS, various health system responses have been developed. PACS clinics are generally organized as multidisciplinary centers where, geriatricians, internal medicine or infectious disease specialists eviews and manages patients.
A recent survey in 47 diverse mixes of academic tertiary and community hospitals, across the U.S, characterized post-discharge care delivery for PASC across a large network of U.S. academic and community hospitals. First-time patients’ evaluation in PASC clinics often received a range of testing, such as spirometry, six-minute walking test, chest X-ray or CT, cognitive assessment, mental health assessment, physical function assessment and quality of life. Most PACS clinic referrals (70%) relied on physician or patient/family requests; 39% of hospitals used specific criteria for referral; and only 21% of hospitals referred all hospitalized COVID patients (Valley et al., 2022).
The following are two examples of PACS clinics in the US and Italy.
The model developed at the Mayo clinic divides care into a coordinated 5-levels approach (Wissler Gerdes et al., 2022). The first focuses on diagnosis and treatment of new onset of acute medical pathologies associated with COVID-19 and its complication. The second level is the individualized rehabilitation program, often supervised by physical and occupational therapists. Medications may be used to control symptoms such as tachycardia, insomnia, and cough. The third level of treatment is psychosocial support focused on symptoms of anxiety often associated with post-traumatic stress disorder. The final two levels take care of more difficult to treat conditions including dysautonomia symptoms and subjective impaired cognition. Coping strategies are promoted to support patients to manage their condition best through the recovery process, which often takes 6–18 months.
Modena PACS clinic is a multidisciplinary tertiary care center which was established in July 2020 (Rapporto, 2022). People who were discharged from hospital or referred from general physicians are screened for PACS three months after COVID-19 and thereafter, if PACS is diagnosed every 6 months.
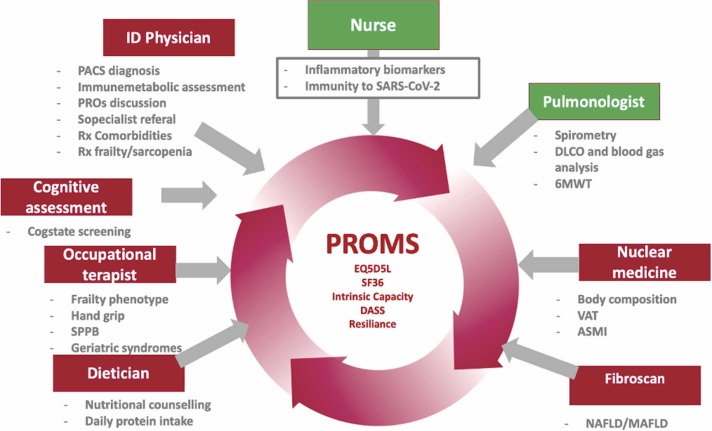
This model of care is mainly based on the inclusion of geriatric principles into PACS care. It comprises screening for frailty and delivery of a comprehensive geriatric assessment (CGA). It is a multidimensional, interdisciplinary diagnostic process used to determine the medical, psychosocial, and functional capabilities of older adults. This framework involves a strong participation of the patient itself and its community. Modena PACS clinic offers a holistic patient centered approach indicating healthy living/ageing as the ultimate goal ( Fig. 2).
Fig. 2.
Modena PACS Clinic: Multidisciplinary and multidimensional assessment and health care model. Abbrevations: 6MWT – 6 min walking test; ASMI – appendicular skeleteal muscle index; DASS (questionnaire) – depression, anxiety and stress scale; EQ5D5L (questionnaire) – EuroQoL-5 dimension-5 level; NAFLD/MAFLD – non alcoholic fatty liver disease/metabolically associated fatty liver disease; PACS - post-acute COVID-19 syndrome; PROMs – patient-related outcome measures; SF-36 (questionnaire) – 36 item short form health survey; SPPB – short physical performance battery; VAT – visceral adipose tissue.
Telemedicine has also been used to monitor people with PACS. It offers the possibility of redesigning previous models of care and gives new research opportunities regarding data collection, patient empowerment, diagnosis, and therapy provision. Our group recently conducted a participatory research and action project which included 50 patients, aged> 50 years with PACS, who were daily monitored with a vocal assistant device (Google Nest). The study proved a high level of patient satisfaction and contributed to empower people to improve diet and physical activity (Caselgrandi et al., 2021).
7. Is it possible to restore healthy aging trajectory in a pre-COVID condition?
7.1. Biological interpretation
The geroscience hypothesis suggests that targeting aging mechanisms, so-called hallmarks of aging, rather than targeting a single age-related disease, can increase life expectancy (Khosla et al., 2020, Tchkonia et al., 2021, Wissler Gerdes et al., 2020). Such an approach implies that at the basis of pathogenetic mechanisms of multiple diseases there are aging pathways related to oxidative stress, metabolic derangement, and unrepaired DNA damage in which infections may act as a trigger.
Geroscience interventions may affect the relationship between SARS-CoV- 2 and aging mechanisms offering a new therapeutic option for PACS. COVID may act as an acute stressor, which pushes the individual’s function below the disability/dependence threshold, where the individual may remain, in the case of PACS, after recovery from the stressor. These intervention may enhance the individual’s ability to recover back to independence (Newman et al., 2016) through improvement of sarcopenia and frailty.
A variety of candidate drugs have emerged from basic and translational research that may target aging processes. Some of these drugs so called senotherapeutics (senolytics and senomorphics), are already in clinical use for other purposes, such as metformin and rapamycin, jointly with exercise, and dietary interventions (Wissler Gerdes et al., 2022).
Metformin has been shown to impact several aging related mechanisms, including reducing reactive oxygen species (ROS) and DNA damage, decreasing senescent cell burden, and activating AMPK, so inhibiting effects of increased mTOR activity (Barzilai et al., 2016, Barzilai et al., 2012, Algire et al., 2012). There are indications of links between metformin use and decreased severity of acute COVID-19 infection (Tamura et al., 2021, Blanc et al., 2021, Yang et al., 2021).
Rapamycin and its analogs, inhibit mTOR (Blagosklonny, 2017, Lamming et al., 2013) and hyperfunction of the immune system, specifically by expanding regulatory T lymphocytes (Treg) (Blagosklonny, 2020). Furthermore, as an mTOR inhibitor, rapamycin might temper the overall immune response to viral infections and decrease cytokine storm (Blagosklonny, 2020, Mannick et al., 2018, Wang et al., 2014).
7.2. Clinical interpretation
The hypothesis of PACS as an aging trajectory modifier implies a particular focus on reconditioning and rehabilitation that may promote the reaching of a new homeostasis equilibrium.
In this perspective, WHO has built the conceptual framework “healthy aging” This construct has been issued to promote a positive approach to aging that relies on reserves and preserved capacities in an individual, regardless of the presence of multimorbidity, frailty, or disability.
In 2015, the World Report on Ageing and Health attempted to combine clinical and public health outcomes for aging by defining healthy aging as the process of developing and maintaining the functional ability that enables well-being in older age (World Health Organization, 2022). This construct derives from the relationship of two entities: “intrinsic capacity”, that is the composite of all cognitive and physical functioning of the individual, and “the environment”. Intrinsic capacity is a multidimensional entity that can be described by five functional domains: locomotion, cognition, psychology, vitality, and sensory (World Health Organization, 2022).
Healthy aging should not be considered in competition with frailty, but perhaps as the two faces of the same coin, sharing a common biological background (World Health Organization, 2022, Cesari et al., 2018). In detail, the intrinsic capacity construct might be considered an evolution of the frailty concept, considering the continuum of the aging process and the opportunities offered by novel (and next-to-come) technologies (ICOPE guidelines, 2022). The WHO Guidelines on Integrated Care for Older People (ICOPE) offers evidence-based recommendations for effective interventions to prevent or slow declines in physical and mental capacities in older people. In addition, these guidelines introduce a mobile aging app (mAging) that can promote healthy lifestyles (ICOPE guidelines, 2022).
A common example of apps to promote lifestyles is represented by fitness tracking devices able to count steps and estimate calories, collecting personalized data to help guide health behaviors and wellness (Wang et al., 2020). Technology also allows new tools for PROs data collection. For example, Ecological Momentary Assessment (EMA) collects PROs in real-time by sending instant messages to the users. This may make data more reliable and be a step forward to integrate PROs in evaluating health and well-being.
Intrinsic capacity has seldom been used to assess well-being in the general population and not yet in PACS. This approach will be used in an international (Italy and Israel) multicentre prospective cohort study called: “Assessment of well-being state in PACS”.
The goal is to apply machine learning to build a data-driven index taking into account the background of the intrinsic capacity construct that negatively correlates with frailty and positively correlates with health-related quality of life and resilience. The dataset will comprise a multidimensional set of variables coming from the medical visit, patient-reported outcomes, and physiological data obtained by activity loggers (a triaxial accelerometer).
A total of 300 patients will be evaluated to assess intrinsic capacity domains at two-time points, respectively, at 12 and 18 months after hospital discharge. A data-driven approach will train machine learning algorithms to predict frailty, quality of life, and resilience of patients with PACS.
The index will be collected longitudinally by asking cohort participants to wear the accelerometers continuously for seven consecutive days, two times during the study period. This tool assesses mobility, including step count, need to rest after vigorous physical activity, hours spent standing and sleep duration, and quality of sleep (measured with numbers of REM phases and numbers of awakening per night).
In this context, machine learning may offer a unique opportunity for ‘P4 medicine’ (Preventive, Predictive, Personalized, and Participatory), moving it from reactive to proactive medicine. Ultimately, the objective is to maximize well-being for each individual than to treat disease (Hood and Friend, 2011).
7.3. Public health interpretation
Public health interventions in the prevention and treatment of PACS are still missing. A recent study from Israel investigated the effectiveness of COVID-19 vaccines against PACS. A total of 951 infected and 2437 uninfected individuals were asked to answer a PACS symptoms questionnaire. After adjusting for follow-up time and baseline symptoms, those who received two doses of vaccines were less likely than unvaccinated individuals to report fatigue (−64%), headache (−54%), weakness (−57%), and persistent muscle pain (−68%). Those who received two doses were no more likely to report any of these symptoms than individuals reporting no previous SARS-CoV-2 infection (Kuodi et al., 2021). Furthermore, PACS can occur post-vaccination, but the rates are consistently lower than unvaccinated individuals, suggestiong that COVID-19 vaccination may have a protective effect against PACS (Kuodi et al., 2021, Bergwerk et al., 2021, Taquet et al., 2021b, Antonelli et al., 2022).
8. Conclusions
PACS is a condition of growing interest for both clinical and public health reasons. It occurs even in individuals with mild or asymptomatic acute SARS COV2 infection and likely driven by a vicious cycle of inflammaging and immunosenescence. This altered immune-metabolic condition may affect aging trajectories captured by the relationship between frailty and resilience. Its clinical impact is an accentuated aging phenomenon that affects quality of life and daily living.
Metabolic-associated fatty liver disease affecting people with PACS is a clinical surrogate of meta-flammation and needs dedicated multidisciplinary patient-centered care models. A specific treatment for PACS is not yet available. While waiting for the availability of geroscience approaches, current interventions remain focused on healthy lifestyle, including the promotion of physical exercise and dietary interventions. The development of safe and efficient vaccines for COVID-19 may potentially prevent and restore the healthy aging trajectory in the future.
Funding
This study is supported by a Gilead Sciences Inc. unrestricted grant.
CRediT authorship contribution statement
GG and JM conceptualized, designed and wrote primary draft of the manuscript. GG, JM, MC, CM and AC did supervision of the final version of the manuscript. All the authors contributed to discussion and revised the manuscript.
Conflict of interest
GG and CM received research grant and speaker honorarium from Gilead, ViiV, MERCK and Jansen. GG and CM attended advisory boards of Gilead, ViiV and MERCK. Other authors did not report conflicts of interest.
References
- 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. United States; 2021; 17(3):327–406. [DOI] [PubMed]
- Algire C., Moiseeva O., Deschênes-Simard X., et al. Metformin reduces endogenous reactive oxygen species and associated DNA damage. Cancer Prev. Res (Philos. ). U. S. 2012;5(4):536–543. doi: 10.1158/1940-6207.CAPR-11-0536. [DOI] [PubMed] [Google Scholar]
- Antonelli M., Penfold R.S., Merino J., et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022;22(1):43–55. doi: 10.1016/S1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour N., Day R., Newcombe J., Talbot P.J. Neuroinvasion by human respiratory coronaviruses. J. Virol. 2000;74(19):8913–8921. doi: 10.1128/jvi.74.19.8913-8921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D.T., Hamilton F.W., Milne A., et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. 2020;76(4):399–401. doi: 10.1136/thoraxjnl-2020-216086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoubkhani D., Khunti K., Nafilyan V., et al. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N., Huffman D.M., Muzumdar R.H., Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61(6):1315–1322. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N., Crandall J.P., Kritchevsky S.B., Espeland M.A. Metformin as a Tool to Target Aging. Cell Metab. 2016;23(6):1060–1065. doi: 10.1016/j.cmet.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilai N., Appleby J.C., Austad S.N., et al. Geroscience in the Age of COVID-19. Aging Dis. 2020:725–729. doi: 10.14336/AD.2020.0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bektas A., Schurman S.H., Franceschi C., Ferrucci L. A public health perspective of aging: Do hyper-inflammatory syndromes such as COVID-19, SARS, ARDS, cytokine storm syndrome, and post-ICU syndrome accelerate short- And long-term inflammaging? Immun Ageing. Immun. Ageing. 2020;17(1):1–10. doi: 10.1186/s12979-020-00196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwerk M., Gonen T., Lustig Y., et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besutti G., Pellegrini M., Ottone M., et al. The impact of chest CT body composition parameters on clinical outcomes in COVID-19 patients. PLoS One. 2021;16(5 May):1–16. doi: 10.1371/journal.pone.0251768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny M.V. From rapalogs to anti-aging formula. Oncotarget. 2017;8(22):35492–35507. doi: 10.18632/oncotarget.18033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny M.V. From causes of aging to death from COVID-19. Aging (Albany NY) 2020;12(11):10004–10021. doi: 10.18632/aging.103493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc F., Waechter C., Vogel T., et al. Therapeutic prevention of COVID-19 in elderly: a case-control study. GeroScience. 2021;43(5):2333–2343. doi: 10.1007/s11357-021-00397-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomaard L.C., Linden C.M.J., van der, Bol J.M. van der, et al. Frailty is associated with in-hospital mortality in older hospitalised COVID-19 patients in the Netherlands: the COVID-OLD study. Age Ageing. 2021;50(3):631–640. doi: 10.1093/ageing/afab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borella R., De Biasi S., Paolini A., et al. Metabolic reprograming shapes neutrophil functions in severe COVID-19. Eur. J. Immunol. 2022;52(3):484–502. doi: 10.1002/eji.202149481. [DOI] [PubMed] [Google Scholar]
- Byrne C.D., Targher G. NAFLD: a multisystem disease. J. Hepatol. [Internet]. Eur. Assoc. Study Liver. 2015;62(S1):S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J., Dhawan G., Kapoor R., Iavicoli I., Calabrese V. What is hormesis and its relevance to healthy aging and longevity? Biogerontology. 2015;16(6):693–707. doi: 10.1007/s10522-015-9601-0. [DOI] [PubMed] [Google Scholar]
- Carfì A., Bernabei R., Landi F. Group for the GAC-19 P-ACS. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Schneider C., Laurent E., Lemaignen A., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol Infect. 2021;27(2):258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselgrandi A., Milic J., Motta F. et al. Voice assistance to develop participatory research and action to improve health trajectories of people with PACS. Poster ADLH-28. 23rd International Workshop on Co-morbidities in HIV and COVID; 6–9 December 2021, virtual meeting.
- CDC, Post COVID conditions. Available at; https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html Last access: 19 Febbruary 2022.
- Cesari M., Araujo de Carvalho I., Amuthavalli, Thiyagarajan J. Evidence for the domains supporting the construct of intrinsic capacity. J. Gerontol. A Biol. Sci. Med Sci. 2018;73(12):1653–1660. doi: 10.1093/gerona/gly011. [DOI] [PubMed] [Google Scholar]
- Chitturi S., Farrell G.C. Fatty liver now, diabetes and heart attack later? The liver as a barometer of metabolic health. J. Gastroenterol. Hepatol. Aust. 2007:967–969. doi: 10.1111/j.1440-1746.2007.04995.x. [DOI] [PubMed] [Google Scholar]
- Coppé J.-P., Patil C.K., Rodier F., et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6(12):2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID-19 Associated with Long-Term Cognitive Dysfunction , Acceleration of Alzheimer’s Symptoms, press realease of the The Alzheimer’s Association International Conference. Available at: https://www.alz.org/aaic/releases_2021/covid-19-cognitive-impact.asp; (Accessed 19 February 2022).
- Cysique L.A., Bracken S.G., Allen-Davidan Y. et al. Quinolinic acid is a biomarker of COVID-19 associated cognitive impairment, Abs. No. 634. CROI 2022, Virtual Meeting.
- De Biasi S., Emilia R., Campi V., Meschiari M., Gibellini L. Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with Covid-19 pneumonia. Nat. Commun. 2020;11(1):3434. doi: 10.1038/s41467-020-17292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M., Miletti T.C., Gagnon M., Talbot P.J. Activation of human monocytes after infection by human coronavirus 229E. Virus Res. 2007;130(1–2):228–240. doi: 10.1016/j.virusres.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensrud K.E., Kats A.M., Schousboe J.T. Frailty phenotype and healthcare costs and utilization in older women. J. Am. Geriatr. Soc. 2018;66(7):1276–1283. doi: 10.1111/jgs.15381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslam M., Newsome P.N., Sarin S.K., et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J. Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- Fernández-de-Las-Peñas C., Palacios-Ceña D., Gómez-Mayordomo V., Cuadrado M.L., Florencio L.L. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): an Integrative Classification. Int J. Environ. Res Public Health. 2021;18(5) doi: 10.3390/ijerph18052621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L., Gonzalez-Freire M., Fabbri E. Measuring biological aging in humans: a quest. Aging Cell. 2020;19(2) doi: 10.1111/acel.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C., Bonafè M., Valensin S. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. U. S. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Fraser D.D., Slessarev M., Martin C.M. Metabolomics profiling of critically ill coronavirus disease 2019 patients: identification of diagnostic and prognostic biomarkers. Crit. care Explor. 2020;2(10) doi: 10.1097/CCE.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T., Larbi A., Dupuis G. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or Foes? Front Immunol. 2018;8:1960. doi: 10.3389/fimmu.2017.01960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaebler C., Wang Z., Lorenzi J.C.C., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591(7851):639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigues E., Janvier P., Kherabi Y., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J. Infect. 2020:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibellini L., De Biasi S., Paolini A., et al. Altered bioenergetics and mitochondrial dysfunction of monocytes in patients with COVID-19 pneumonia. EMBO Mol. Med. 2020;12(12) doi: 10.15252/emmm.202013001. (Available from) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibellini L., De Biasi S., Meschiari M. Plasma cytokine atlas reveals the importance of TH2 polarization and interferons in predicting COVID-19 severity and survival. frontiers in immunology. 2022;13:1664–3224. doi: 10.3389/fimmu.2022.842150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilis M., Chagrot N., Koeberle S., et al. Older adults with SARS-CoV-2 infection: utility of the clinical frailty scale to predict mortality. J. Med Virol. U. S. 2021;93(4):2453–2460. doi: 10.1002/jmv.26766. [DOI] [PubMed] [Google Scholar]
- Gold J.E., Okyay R.A., Licht W.E., Hurley D.J. Investigation of long COVID prevalence and its relationship to epstein-barr virus reactivation. Pathogens. 2021;10(6):763. doi: 10.3390/pathogens10060763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goronzy J.J., Fang F., Cavanagh M.M., Qi Q., Weyand C.M. Naive T cell maintenance and function in human aging. J. Immunol. 2015;194(9):4073–4080. doi: 10.4049/jimmunol.1500046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri P., Falcone C., Romano L., et al. Body composition findings by computed tomography in sars-cov-2 patients: Increased risk of muscle wasting in obesity. Int J. Mol. Sci. 2020;21(13):1–13. doi: 10.3390/ijms21134670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood L., Friend S.H. Predictive, personalized, preventive, participatory (P4) cancer medicine. Nat. Rev. Clin. Oncol. Nat. Publ. Group. 2011;8(3):184–187. doi: 10.1038/nrclinonc.2010.227. [DOI] [PubMed] [Google Scholar]
- Hussien H., Nastasa A., Apetrii M., Nistor I., Petrovic M., Covic A. Different aspects of frailty and COVID-19: points to consider in the current pandemic and future ones. BMC Geriatr. BMC Geriatr. 2021;21(1):1–11. doi: 10.1186/s12877-021-02316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICOPE guidelines , Available at: https://apps.who.int/iris/bitstream/handle/10665/258981/9789241550109-eng.pdf;jsessionid=05F0E68283FCFBBB5972AB52FF91D343?sequence=1; (Accessed 19 February 2022).
- Iske J., Seyda M., Heinbokel T. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat. Commun. 2020;11(1):4289. doi: 10.1038/s41467-020-18039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S., Farr J.N., Tchkonia T., Kirkland J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020;16(5):263–275. doi: 10.1038/s41574-020-0335-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuodi P., Gorelik Y., Zayyad H., et al. Association between vaccination status and reported incidence of post-acute COVID-19 symptoms in Israel: a cross-sectional study of patients tested between March 2020 and November 2021. medRxiv (pre print) Available from: https://www.medrxiv.org/content/early/2022/01/17/2022.01.05.22268800.
- Lamming D.W., Ye L., Sabatini D.M., Baur J.A. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J. Clin. Invest. 2013;123(3):980–989. doi: 10.1172/JCI64099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler N.G., Gray N., Kimhofer T. Systemic perturbations in amine and kynurenine metabolism associated with acute SARS-CoV-2 infection and inflammatory cytokine responses. J. Proteome Res. 2021;20(5):2796–2811. doi: 10.1021/acs.jproteome.1c00052. [DOI] [PubMed] [Google Scholar]
- Lionetto L., Ulivieri M., Capi M. Increased kynurenine-to-tryptophan ratio in the serum of patients infected with SARS-CoV2: An observational cohort study. Biochim Biophys. Acta Mol. basis Dis. 2021;1867(3) doi: 10.1016/j.bbadis.2020.166042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonardo A. Renaming NAFLD to MAFLD: could the LDE system assist in this transition? J. Clin. Med. 2021;10(3) doi: 10.3390/jcm10030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonardo A., Arab J.P., Arrese M. Perspectives on precision medicine approaches to NAFLD diagnosis and management. Adv. Ther. 2021;38(5):2130–2158. doi: 10.1007/s12325-021-01690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long COVID could become Finland’s largest chronic disease warns minister. Available at: https://www. reuters. com/article/us-health-coronavirus-finland-long-covid-idUSKBN2JH14W. Last access: 19 February 2022.
- Mandal S., Barnett J., Brill S.E. Long-COVID”: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax. 2021;76(4):396–398. doi: 10.1136/thoraxjnl-2020-215818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannick J.B., Morris M., Hockey H.-U.P. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med. 2018;10(449):eaaq1564. doi: 10.1126/scitranslmed.aaq1564. [DOI] [PubMed] [Google Scholar]
- Meng Y., Wu P., Lu W. Sex-specific clinical characteristics and prognosis of coronavirus disease-19 infection in Wuhan, China: a retrospective study of 168 severe patients. PLoS Pathog. 2020;16(4):1–13. doi: 10.1371/journal.ppat.1008520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milic J., Barbieri S., Gozzi L. Metabolic-associated fatty liver disease is highly prevalent in the postacute COVID syndrome. Open Forum Infect. Dis. 2022;9(3):ofac003. doi: 10.1093/ofid/ofac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussini C., Cozzi-Lepri A., Menozzi M. Better prognosis in females with severe COVID-19 pneumonia: possible role of inflammation as potential mediator. Clin. Microbiol Infect. 2021;27(8):1137–1144. doi: 10.1016/j.cmi.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambi G., Abdelbasset W.K., Alrawaili S.M. Comparative effectiveness study of low versus high-intensity aerobic training with resistance training in community-dwelling older men with post-COVID 19 sarcopenia: a randomized controlled trial. Clin. Rehabil. 2022;36(1):59–68. doi: 10.1177/02692155211036956. [DOI] [PubMed] [Google Scholar]
- Nehme M., Braillard O., Alcoba G. Vol. 174. COVID-19 Symptoms; Longitudinal: 2021. Evolution and Persistence in Outpatient Settings; pp. 723–725. (Ann Intern Med). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J.C., Milman S., Hashmi S.K. Strategies and Challenges in Clinical Trials Targeting Human Aging. J. Gerontol. Ser. A [Internet] 2016;71(11):1424–1434. doi: 10.1093/gerona/glw149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE. COVID-19 rapid guideline: critical care in adults UK: National Institute for Health and Care excellence; 2020. Available from: https://www.nice.org.uk/guidance/ng159/chapter/2-Admission-to-critical-ca re. (Accessed 21 February 2022). [PubMed]
- Page M. The British Medical Association Illustrated Medical Dictionary. 4th ed, Dorling Kindersley; London, UK: 2018. pp. 177–536. [Google Scholar]
- Peluso M.J., Lu S., Tang A.F. Markers of immune activation and inflammation in individuals with postacute sequelae of severe acute respiratory syndrome coronavirus 2 infection. J. Infect. Dis. 2021;224(11):1839–1848. doi: 10.1093/infdis/jiab490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann-Rocha F., Hanlon P., Gray S.R. Comparison of two different frailty measurements and risk of hospitalisation or death from COVID-19: findings from UK Biobank. BMC Med. 2020;18(1):355. doi: 10.1186/s12916-020-01822-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisetsky D.S., Lipsky P.E. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat. Rev. Rheuma. 2020;16(10):565–579. doi: 10.1038/s41584-020-0480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK : 3 February 2022 Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowin. (Accessed 19 February 2022).
- Proal A.D., VanElzakker M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabali N., Rolfson D., Bagshaw S.M. Assessment and utility of frailty measures in critical illness, cardiology, and cardiac surgery. Can. J. Cardiol. Engl. 2016;32(9):1157–1165. doi: 10.1016/j.cjca.2016.05.011. [DOI] [PubMed] [Google Scholar]
- Rapporto I.S.S. COVID-19. Indicazioni ad interim sui principi di gestione del long-COVID. n.15/2021, Istituto Superiore di Sanita’. Available at: https://www.iss.it/documents/20126/0/Rapporto+ISS+COVID-19+15_2021.pdf/a97f5be0–983b-efaa-2638–3cafc8380296?t=16. (Accessed 19 February 2022).
- Rockwood K., Stadnyk K., MacKnight C., McDowell I., Hebert R., Hogan D.B. A brief clinical instrument to classify frailty in elderly people. Lancet (Lond., Engl. ). Engl. 1999:205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- Rockwood K., Song X., MacKnight C. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173(5):489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos C.M., Zhang B., Palmer A.K. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973–977. doi: 10.1111/acel.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer M.J., White T.A., Iijima K. Cellular senescence mediates fibrotic pulmonary disease. Nat. Commun. 2017;8(1):14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scortichini M., Schneider dos Santos R., De’ Donato F. Excess mortality during the COVID-19 outbreak in Italy: a two-stage interrupted time-series analysis. Int J. Epidemiol. 2020;49(6):1909–1917. doi: 10.1093/ije/dyaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B., Yi X., Sun Y. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. 2020;182(1):59–72. doi: 10.1016/j.cell.2020.05.032. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavem K., Ghanima W., Olsen M.K., Gilboe H.M., Einvik G. Persistent symptoms 1.5-6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax. 2020;76(4):405–407. doi: 10.1136/thoraxjnl-2020-216377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y., Yuan D., Chen D.G. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881–895.e20. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura R.E., Said S.M., Freitas L.M., de, Rubio I.G.S. Outcome and death risk of diabetes patients with Covid-19 receiving pre-hospital and in-hospital metformin therapies. Diabetol. Metab. Syndr. 2021;13(1):76. doi: 10.1186/s13098-021-00695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Dercon Q., Harrison P.J. Six-month sequelae of post-vaccination SARS-CoV-2 infection: a retrospective cohort study of 10,024 breakthrough infections. medRxiv (pre print) 2021b; Available from: https://www.medrxiv.org/content/early/2021/11/08/2021.10.26.21265508. [DOI] [PMC free article] [PubMed]
- Taquet M., Dercon Q., Luciano S., Geddes J.R., Husain M., Harrison P.J. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18(9) doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T., Kirkland J.L. Aging, cell senescence, and chronic disease: emerging therapeutic strategies. JAMA. 2018;320(13):1319–1320. doi: 10.1001/jama.2018.12440. [DOI] [PubMed] [Google Scholar]
- Tchkonia T., Palmer A.K., Kirkland J.L. New Horizons: Novel Approaches to Enhance Healthspan Through Targeting Cellular Senescence and Related Aging Mechanisms. J. Clin. Endocrinol. Metab. 2021;106(3):e1481–e1487. doi: 10.1210/clinem/dgaa728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenforde M.W., Kim S.S., Lindsell C.J. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March-June 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69(30):993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T., Stefanoni D., Reisz J.A. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI Insight. 2020;5(14) doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisminetzky M., Delude C., Hebert T., Carr C., Goldberg R.J., Gurwitz J.H. Age, Multiple Chronic Conditions, and COVID-19: a literature review. J. Gerontol. A Biol. Sci. Med Sci. 2022;77(4):872–878. doi: 10.1093/gerona/glaa320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truog R.D., Mitchell C., Daley G.Q. The toughest triage - allocating ventilators in a pandemic. N Engl J Med. N. Engl. J. Med. U. S. 2020;382(21):1973–1975. doi: 10.1056/NEJMp2005689. [DOI] [PubMed] [Google Scholar]
- Valley T.S., Schutz A., Peltan I.D. Organization of outpatient care after COVID-19 hospitalization. Chest. 2022;S0012–3692(22) doi: 10.1016/j.chest.2022.01.034. 00197-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanichkachorn G., Newcomb R., Cowl C.T. Post-COVID-19 syndrome (Long Haul Syndrome): description of a Multidisciplinary Clinic at Mayo Clinic and Characteristics of the Initial Patient Cohort. Mayo Clin. Proc. 2021;96(7):1782–1791. doi: 10.1016/j.mayocp.2021.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyavahare S., Kumar S., Cantu N. Tryptophan-Kynurenine pathway in COVID-19-dependent musculoskeletal pathology: a minireview. deng zhenhan, Editor. Mediat. Inflamm. 2021;2021:2911578. doi: 10.1155/2021/2911578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Liu Z., Chen V.P. Transplanting cells from old but not young donors causes physical dysfunction in older recipients. Aging Cell. 2020;19(3) doi: 10.1111/acel.13106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C.-H., Chung F.-T., Lin S.-M. Adjuvant treatment with a mammalian target of rapamycin inhibitor, sirolimus, and steroids improves outcomes in patients with severe H1N1 pneumonia and acute respiratory failure. Crit. Care Med. 2014;42(2):313–321. doi: 10.1097/CCM.0b013e3182a2727d. [DOI] [PubMed] [Google Scholar]
- Whitson H.E., Duan-Porter W., Schmader K.E., Morey M.C., Cohen H.J., Colón-Emeric C.S. Physical resilience in older adults: systematic review and development of an emerging construct. J. Gerontol. A Biol. Sci. Med Sci. 2016;71(4):489–495. doi: 10.1093/gerona/glv202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Post COVID-19 definition, 2021 available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 Last access: 19 February 2022.
- Wissler Gerdes E.O., Zhu Y., Tchkonia T., Kirkland J.L. Discovery, development, and future application of senolytics: theories and predictions. FEBS J. 2020;287(12):2418–2427. doi: 10.1111/febs.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissler Gerdes E.O., Vanichkachorn G., Verdoorn B.P. Role of senescence in the chronic health consequences of COVID-19. Transl. Res. 2022;241:96–108. doi: 10.1016/j.trsl.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World report on ageing and health 2015. WHO. Available at: https://www.who.int/publications/i/item/9789241565042 (Accessed 22 February 2022).
- Xu M., Palmer A.K., Ding H. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife. 2015;4 doi: 10.7554/eLife.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Pirtskhalava T., Farr J.N. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018;24(8):1246–1256. doi: 10.1038/s41591-018-0092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanes R.E., Gustafson C.E., Weyand C.M., Goronzy J.J. Lymphocyte generation and population homeostasis throughout life. Semin Hematol. 2017;54(1):33–38. doi: 10.1053/j.seminhematol.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., Sun X., Zhang J., Zhang K. The effect of metformin on mortality and severity in COVID-19 patients with diabetes mellitus. Diabetes Res Clin. Pr. 2021;178 doi: 10.1016/j.diabres.2021.108977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Armstrong J.L., Tchkonia T., Kirkland J.L. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr. Opin. Clin. Nutr. Metab. Care. Engl. 2014;17(4):324–328. doi: 10.1097/MCO.0000000000000065. [DOI] [PubMed] [Google Scholar]