Introduction
Epilepsy is the fourth most common neurological disorder. In 2015, the Centers for Disease Control estimated that there are at least 3.4 million people with epilepsy in the United States1. The cost to society of not optimizing the clinical care of these individuals is quite high. The annual direct medical cost of epilepsy in the U.S. is estimated to be at least $14B in today’s dollars2, although that number excludes most of the cost burden, such as community service costs and indirect costs from losses in quality of life and productivity. What’s more, costs for individuals with drug-resistant epilepsy (DRE) are as many as ten times greater than for those whose seizures are prevented by medication3.
30–40% of epilepsy patients have DRE, and therefore are surgical candidates4. Despite its potential to cure some types of epilepsy, surgery remains a vastly underutilized treatment, with only a small minority of candidates receiving surgical treatments. For example, the 2003 joint position paper from the American Academy of Neurology, American Association of Neurological Surgeons, and the American Epilepsy Society estimated that less than 3% of surgical candidates receive surgery, assuming 4000 epilepsy surgeries performed per year out of 150,000 potential surgical candidates5. Subsequent studies have demonstrated no change in epilepsy surgery utilization6–8. Moreover, the average time from diagnosis to surgery for a medically refractory patient is reported to be around 18 years, in data both from before and after the joint position paper’s recommendation that DRE patient should be referred to a comprehensive epilepsy center. DRE is defined as the failure of only two appropriately considered medications9. Alarmingly, contrary to these guidelines, more than 75% of DRE patients are not referred to an epilepsy specialist10. One of every 10,000 newly diagnosed people with epilepsy will die of sudden unexpected death in epilepsy (SUDEP)10, but sadly the SUDEP rate is 90-fold higher in those with DRE10. Thus, underutilization of epilepsy surgery is a public health crisis that requires proactive intervention.
Traditional resective epilepsy surgery can be curative in many cases but is often viewed incorrectly as dangerous9. Surprisingly, 60–75% of neurologists are not aligned with experts on best referral practices, with obstacles to referral including knowledge deficits regarding the definition of DRE, existing practice guidelines, indications and timing for epilepsy surgery referral, and understanding the numerous types of epilepsies that are amenable to surgery11. It may be helpful that several advances have become mainstream over the last decade that increase surgical options for patients with focal epilepsy, while being minimally invasive. These options include intracranial neuromodulation devices that can record from the brain, providing highly useful chronic and patient-specific data. In addition, there is growing evidence that intracranial neuromodulation is efficacious in the treatment of some primary generalized epilepsies. In light of this gap between what is possible and the surgical care actually received by the average DRE patient, this paper will review five paradigm shifts in epilepsy surgery that are useful to consider for optimizing treatment.
Paradigm Shift: Epilepsy surgery as network surgery
The need to shift to a network approach for epilepsy surgery in most American epilepsy centers stems from the deep-rooted tradition of considering primarily an electrical-anatomic, focus-oriented approach to epilepsy surgery. In the 1950s, Penfield and Jasper established surface electrocorticography (ECoG) as the mainstay for defining an “epileptogenic focus,”12 and Percival Bailey wrote that surgical eradication of focal seizure activity was comparable to eradicating a tumor.”13 In contrast, the stereo-electroencephalography philosophy originated by Talairach and Bancaud focuses on determining the regions of cortex generating the clinical manifestation of the seizure14 where the chronological occurrence of ictal clinical signs (semiology) is crucial for elucidating the “anatomo-electro-clinical” organization of seizures15. This approach facilitates the conception of seizures as an emergent property of brain networks and requires that epilepsy surgery address a patient’s network rather than solely one potential focus.
Resecting a critical seizure network node that may render a patient seizure free is always the first treatment of choice, but a network-oriented approach may best prepare the clinical team to make that assessment (Fig. 1). One downfall of approaching epilepsy surgery solely from the perspective of focus-hunting is that if a resection is performed and the patient is not seizure free, the interpretation is that one “didn’t get enough” or didn’t find “the right focus.” With an overall goal of epilepsy surgery is to take the seizure network offline, i.e. to prevent emergence of seizures by disrupting the critical nodes of seizure organization, the emphasis is shifted from resection and seizure freedom to modulation and improved quality of life, with the ultimate goal of arresting seizures indefinitely. Additionally, when the desired outcome of intracranial monitoring extends beyond whether or not a resection can be accomplished and considers how to take the network offline, the opportunity to use more than one therapeutic approach is presented16. This type of multi-modal surgical approach has been enabled by the advent of recent advances in epilepsy surgery in the United States: increased use of SEEG, the development of MRI-guided laser interstitial thermal therapy (LITT), and FDA approval of both responsive neurostimulation (RNS) and anterior nucleus of the thalamus (ANT) deep brain stimulation (DBS). For instance, LITT may be used in combination with RNS16. Others have reported the upfront combination of open craniotomy for partial resection of the epileptogenic zone with implantation of RNS, in cases where the epileptogenic zone encompasses eloquent cortex17. In the focus-oriented approach, these outcomes without seizure freedom often are referred to as “palliative.” Palliative means alleviating symptoms but not treating the underlying disease. Given the evidence that intracranial neuromodulation has a neuroplastic effect on brain circuitry18,19, and that multimodal therapy in multifocal epilepsy can take individual nodes offline, characterization of these therapies as palliative is incorrect and unhelpful. With a network-oriented approach, the goal of surgery is to reduce seizures as maximally as possible, where a clinically significant reduction in seizures after surgical therapy represents successful modulation of the seizure circuit. Whether seizure reduction in the absence of seizure freedom would improve the patient’s quality of life to an extent that justifies the full application of available surgical therapies is an important component of the presurgical discussion between the patient and multidisciplinary team. The practical implications of combining this philosophy with recent technological advances in surgical care is described in the following sections.
Figure 1.
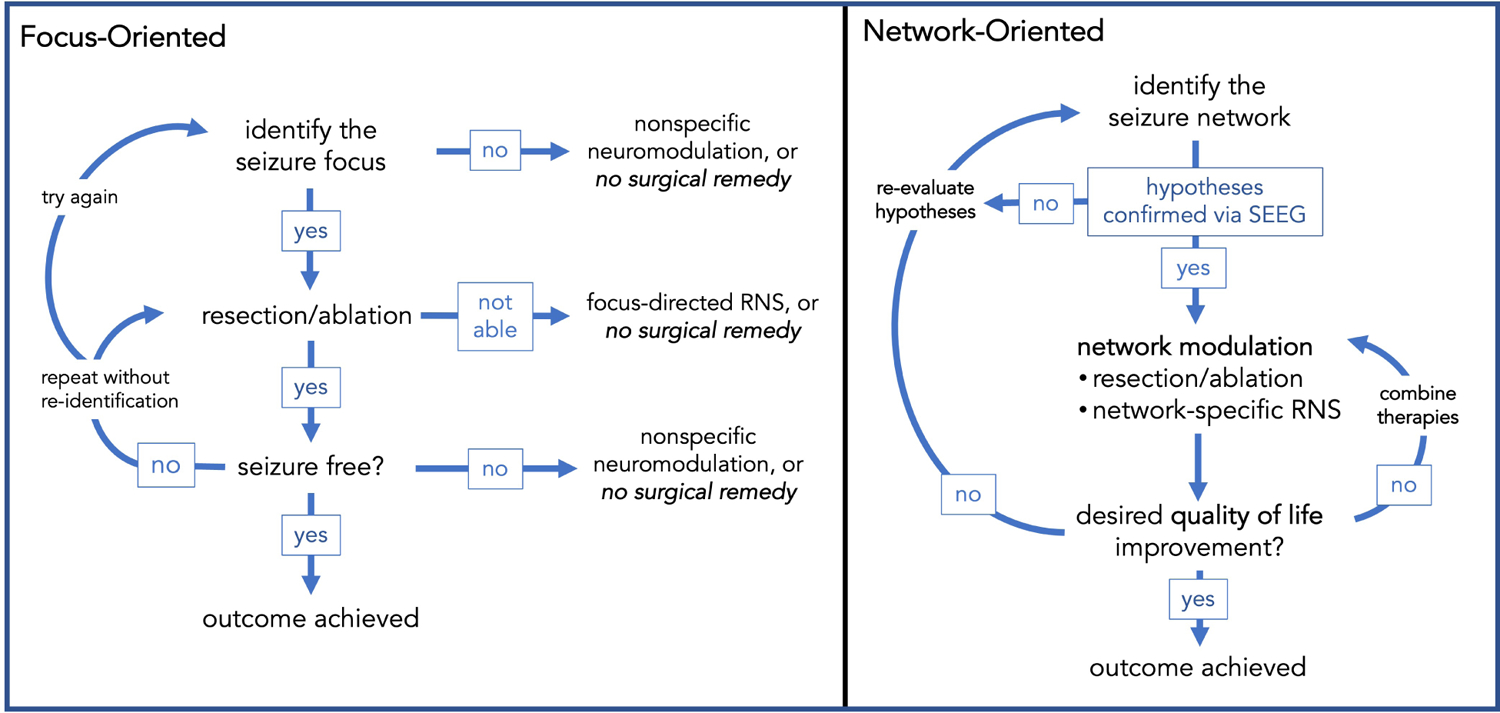
A network-oriented surgical approach increases opportunities for therapeutic success.
Paradigm Shift: Beyond seizure freedom – quality of life
Curing a patient’s epilepsy through resection of the seizure onset zone traditionally has been considered the only goal of epilepsy surgery. Given that patients with poorly localized focal epilepsy, focal epilepsy arising from eloquent cortex, and patients with primary generalized epilepsy are not candidates for resection, there is growing awareness that intracranial neuromodulation can produce meaningful quality of life improvements. The first FDA-approved intracranial neuromodulation device for epilepsy was the Responsive Neurostimulation System (RNS). The RNS system is a completely cranial implant, consisting of a programmable onboard processor with four recording channels coupled to two bi-directional leads capable of both recording and stimulating, as well as storing a subset of information for offline analysis. Since approval in 2013, several publications have described the long-term outcomes of patients who participated in both the feasibility and pivotal clinical trials of RNS therapy. Nair and colleagues20 reported outcomes from162 patients who participated in these trials and completed 9 years of follow up. The median percent reduction at the end of 3 years was 58%, improving to 75% by the end of 9 years of treatment. Importantly, 35% experienced ≥90% seizure reduction and 21% were seizure-free in the last 6 months of follow-up. A separate publication tackled the question of whether the timing of clinical improvements in RNS therapy has accelerated in the field’s post-trial experience. Based on a multi-center retrospective analysis, the answer appears to be yes: a median 75% seizure reduction was found at 2 years, and 82% reduction was achieved at ≥3 years21. The percentages of patients experiencing >90% seizure reduction or no seizures in the last 6 months of follow-up also were similar to that previously reported at 9 years.
Deep brain stimulation (DBS) of the anterior nucleus of the thalamus (ANT), due to its involvement in common seizure propagating circuitry, was proposed by Cooper and Upton for suppression of epileptiform discharges within the limbic system22. In 1987, they reported significant seizure control in 4 of 6 patients with drug-resistant complex partial seizures who underwent bilateral ANT stimulation23. ANT-DBS was FDA-approved in 2018, with outcomes having been followed out 10 years. At 7 years, median seizure frequency percent reduction from baseline was 75%24. It is important to note that patients in the pivotal trials for DBS and RNS were highly refractory, averaging an approximately 20-year history of epilepsy, 20–50 disabling seizures a month at baseline, and having failed multiple other epilepsy treatments20,25.
The benefit vs. risk profile of intracranial neuromodulation is impressive. No intraparenchymal hemorrhages were reported in either pivotal trial25,26. The infection rate with RNS was 3% and with DBS was 10%, the latter’s higher rate likely being secondary to the additional incisions and surgical site needed for DBS pulse generator placement in the chest. Remarkably, the SUDEP rate decreased by two-thirds for each therapy (~3 per 1000 patient years), compared to the expected rate in the DRE population (~9 per 1000 patient years). In addition to reduced seizure burden, low morbidity, and the prevention of mortality, these therapies produce measurable improvements in quality of life. Mean quality of life scores were significantly improved at 1 year for RNS patients, and these improvements were maintained through at least 9 years of treatment20. A separate study of patients who completed the randomized control trial found no significant cognitive declines for any neuropsychological measure, while improvements were found in the Boston Naming Test and Rey Auditory Verbal Learning tests, in patients with neocortical and mesial temporal seizure onset zones, respectively27. For DBS patients, improvements in quality of life at 5 years remained stable at 7 years, where 43% of subjects experienced a clinically meaningful improvement24.
Paradigm Shift: Surgical treatment of primary generalized epilepsies
Given this favorable risk-benefit profile, attention in the intracranial neuromodulation field has expanded to primary generalized epilepsies, for which there currently is no FDA-approved surgical intervention. Likewise, the role of thalamic nuclei in generalized epilepsies has been a longstanding area of focus in both animal and human models, since the work of Hunter and Jasper, who showed that seizures could be induced by electrical stimulation of the thalamus28. Subsequently, Monnier et al. showed that medial thalamic stimulation could desynchronize cortical electroencephalography (EEG)29. In the 1980s, Velasco and colleagues explored the centromedian nucleus (CM) as a DBS target for idiopathic generalized epilepsy (IGE), reporting excellent results30–32. Subsequent feasibility studies and case series demonstrated equivocal findings, until a clinical trial by Valentin et al. redemonstrated significant therapeutic benefit in IGE patients33,34. Recently, the University of Melbourne group reported results from a prospective, double-blind, randomized study of continuous, cycling stimulation of CM-DBS, in patients with Lennox Gastaut Syndrome (LGS)35. The DBS device used in that study was not sensing-enabled, but subjects demonstrated significantly reduced electrographic activity on 24-hour ambulatory EEG at the end of the 3-month blinded stimulation phase.
Given that at least 20% of IGE patients are refractory to pharmacological treatment36 (~35% of those with juvenile myoclonic epilepsy are refractory37) and evidence that the CM participates in the early propagation of generalized seizures, our group hypothesized that bilateral CM responsive neurostimulation with the RNS system would be effective in IGE. fMRI studies have demonstrated increased signal in the thalamus both prior to38 and at the onset of39 generalized spike wave discharges (GSWD). Likewise, iEEG recordings from externalized DBS leads implanted in primary generalized epilepsy patients in previous studies have demonstrated that GSWD are present in the thalamus simultaneous with onset in the cortex40,41. We reported the first use of bilateral CM RNS in a patient with IGE, a 19-yr-old female, diagnosed with eyelid myoclonia with absences42. iEEG recordings during the baseline pre-stimulation period revealed a multitude of transient (2–5 s duration) bilateral 3–5 Hz spike-wave discharges in the CM region, recapitulating the morphology and spectral signature of presurgical scalp EEG ictal discharges (Fig.2). After one year of RNS therapy, the patient stopped taking all medications, and at 2 years she continued to report a nearly 90% reduction in seizures, which manifest only as brief episodes of eyelid myoclonia, without loss of consciousness. The first 4 adult patients in our IGE CM-region RNS case series have all exhibited significant seizure reduction and improved quality of life (Table 1). Bilateral CM-RNS also has been reported in a pediatric patient with primary generalized epilepsy, resulting in complete resolution of daily absence seizure activity and 75% reduction in convulsive seizures43.
Figure 2. Simultaneous scalp and intracranial EEG recordings in an individual with IGE.
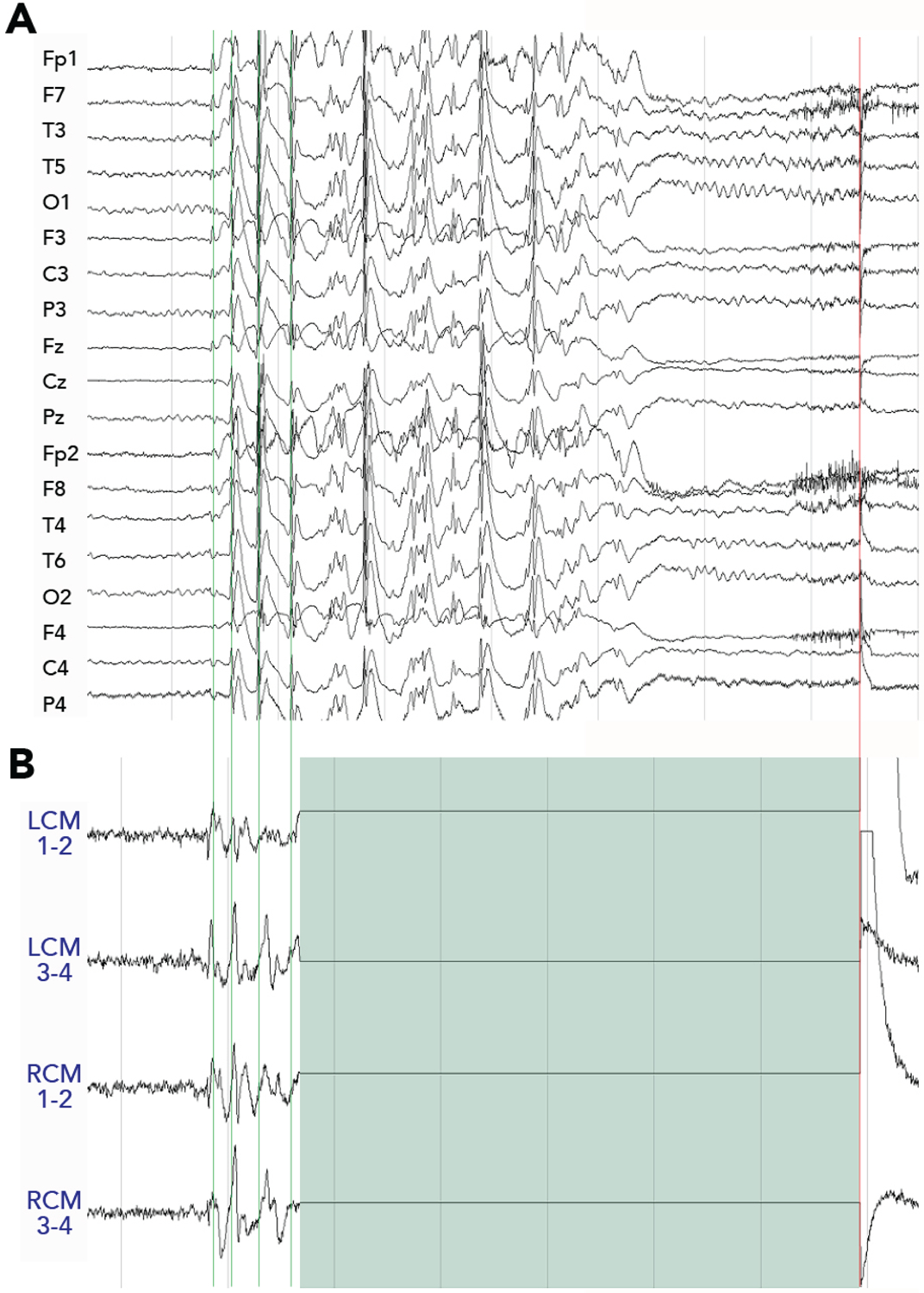
Simultaneous scalp EEG (A) and intracranial thalamic EEG recordings on the 4 channels of the RNS device (B), during a generalized discharge, exhibit a similar pattern of GSWDs. Responsive stimulation (green block) is seen to suppress GSWDs in this example. LCM = left centromedian; RCM = right centromedian.
Table 1.
First cohort of IGE patients with 2-year follow up after bilateral CM-region RNS
| Case | Age | Months implanted | Sex | Seizure type | #AEDs | Seizure frequency | Engel Score | Seizure severity | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| seizure onset | RNS implant | trialed | at RNS implant | at MRFU | Pre-RNS | Post-RNS | Pre-RNS | Post-RNS | |||||
| 1 | 11 | 19 | 33 | F | Absence with eyelid myoclonia | 6 | 2 | 0 | 60/d | 6/d | IB | 4 | 2 |
| 2 | 11 | 22 | 27 | M | Absence, GTC | 9 | 4 | 2 | 3/wk, 1/mo | <1/mo, <1/yr | IIA | 5 | 2 |
| 3 | 16 | 21 | 25 | F | Absence, GTC | 3 | 1 | 1 | 3/wk, 2–4/m | <1/mo, <1/yr | IIIA | 5 | 2 |
| 4 | 17 | 31 | 24 | F | Myoclonic, Absence, GTC | 5 | 2 | 2 | 1/d, 1/wk, 1/year | <1/d, <1/wk, <1/yr | IC | 4 | 1 |
The IGE experience demonstrates that it is important to think beyond the Engel score, which was created to assess outcomes relative to seizure freedom following surgical resection. The scale does not capture extensive quality of life changes that can occur in intracranial neuromodulation patients without seizure freedom, such as a reduction in emergency room visits and hospitalizations, work/school days missed, and improvements in behavioral and developmental indices in younger patients. A multicenter, single-blind, randomized, sham stimulation-controlled pivotal study has been initiated with the goal of validating responsive thalamic stimulation as a surgical option for primary generalized seizures (NCT05147571).
Paradigm Shift: Value of chronic intracranial data
The advent of recording-enabled intracranial neuromodulation systems has opened an entirely new realm of clinical care in epilepsy, that of evaluating and responding to data obtained from chronic iEEG recordings. The value of this data so far is evident in at least three domains: seizure forecasting, medication management, and biomarker detection.
Using iEEG recorded in 37 individuals with focal epilepsy implanted with RNS for up to 10 years, Baud et al.44 first showed that interictal epileptiform activity oscillates with subject-specific multidien (multi-day) periodicity, in addition to the well-known circadian rhythms. Seizures were found to occur preferentially during the rising phase of these multidien rhythms of interictal activity. In a follow up study of 222 individuals with focal epilepsy implanted with RNS, it was reported that 60% of individuals exhibited multidien seizure cycles45 and 89% had a circadian cycle. This type of individualized data has the potential to greatly impact patients, given that self-reported and electrographic seizures occurred during the days-long rising phase of interictal activity, regardless of the length of the multidien period. Thus, it eventually should be possible to forecast the risk of seizure at any given timepoint to the patient, and additionally to use this information to adjust medication timing to increase efficacy and reduce side effects.
The ability to get an objective read-out on medication efficacy and effects of medication adjustments in the patient’s real-world environment is unprecedented46. Quraishi et al. recently demonstrated that in RNS patients with stable detection settings, rates of interictal epileptiform and ictal detections predicted whether a new antiseizure drug would be efficacious, within the first 1–2 weeks47. Given that most individuals with epilepsy are bothered constantly by physical or psychological effects of antiseizure medications48, the ability alone to track changes to medication, including medication withdrawal, can render RNS therapy worthwhile. The availability of this objective iEEG data does create the need for increased effort on the part of epilepsy practitioners. For example, gaging medication response by patient seizure report alone in an individual with RNS without looking at the data would be akin to flying an airplane without looking at the instruments.
Chronic iEEG recordings also are providing new opportunities to assess response to RNS therapy itself. Using RNS recordings from individuals with mesial temporal lobe implantations, Desai et al. showed that the interictal spike rate was a strong differentiator of upper versus lower quartile clinical responders. Interictal spike rate was positively correlated with seizure rates at 7 years of therapy, suggesting that it could be used as a control signal to adapt stimulation delivered in a closed loop system. Kokkinos et al. made the first discovery of putative ictal electrophysiological biomarkers that indicate and potentially predict therapeutic response in individual paients18. By visually inspecting the spectral content of >5000 RNS recordings that captured putative seizures, distinct categories of electrographic seizure pattern modulation (ESPM) were detected that were always present in responders and never present in non-responders. In some cases, these ESPMs were observed in RNS recordings prior to patient-reported seizure reduction, suggesting their potential utilization in predicting therapeutic response. Subsequently, Khambhati et al. identified another type of potential treatment response biomarker19. By assessing interictal network reorganization during RNS therapy, they found that clinical seizure reduction was associated with changes in frequency-dependent functional connectivity within, between, and outside seizure foci. Since the extent of this reorganization scaled with seizure reduction and emerged within the first year of treatment, this network measure also may contribute to future strategies for prediction of therapeutic response.
In addition to the RNS System, a second sensing-enabled system with the ability for chronic recording of local field potentials is available clinically, the Medtronic Percept. The FDA-approved functionality of this system currently is limited to following the recording of peaks in the magnitude of the local field potential in the frequency domain (without ability for closed-loop stimulation), but other systems have been used in several pre-clinical studies of sensing-enabled DBS that explored the electrophysiology of seizure circuits in the time-frequency domain49–52. Use of the investigational Medtronic Summit RC+S research device, which can provide continuous iEEG (up to 1000Hz) from any of four contacts on four leads, was recently reported for the optimization of automated seizure detection using ANT recordings, in individuals with bilateral ANT and hippocampus DBS leads53. This work presages opportunities that will emerge to characterize and modulate seizure networks, as device technologies evolve to include wireless data streaming capabilities and increasing numbers of recording and stimulating channels.
Paradigm Shift: Goals and strategies of diagnostic epilepsy surgery
The increased use of intracranial neuromodulation of both cortical and subcortical regions has expanded the scope of diagnostic epilepsy surgery. Given the safety profile of intracranial neuromodulation, the different avenues it offers for potential improvement in quality of life, and its potential use in multifocal and generalized epilepsies, intracranial monitoring surgery, specifically stereo-electroencephalography (SEEG), has evolved to test a broader range of hypotheses about seizure onset zones and networks. SEEG typically is more versatile for hypothesis testing than subdural grid implantation (unless the phase 1 data is overwhelmingly concordant with a surface lesion)54,55. The ability to offer a surgical treatment that does not require choosing which brain region to resect has created the need to expand hypothesis testing about the seizure network in ways that inform clinical decisions involving intracranial neuromodulation (Table 2). If there is the potential for RNS therapy, one must consider whether sensing should occur in the same location as stimulation. For instance, the author’s practice now incorporates thalamic monitoring during SEEG, in cases where an eventual recommendation for RNS seems more likely than resection alone, and when it’s not clear whether there may be early thalamic involvement in the seizure onset. As one example, a 31-year-old man presented with a 30-year history of seizures having semiologies that suggested posterior frontal (left hand dystonia and left arm tonic posturing, head drops) and anterior frontal (hypermotor, integrated motor activity) onsets. His MRI showed bilateral extended polymicrogyria, and his ictal EEG showed diffuse onset. With this information, it was not clear whether the patient would be best suited for cortical RNS, or where cortical leads would best be placed, versus potentially undergoing bilateral thalamic RNS. The SEEG implantation included leads targeting the centromedian nucleus bilaterally, to test the hypothesis that the thalamus was involved very early in seizure onset and thus would be appropriate to serve as a location for both seizure detection and stimulation. Leads were implanted using the same trajectory and orientation in which they would be used if implanted therapeutically, in order to simulate recordings that would be captured by an RNS system in which the leads were implanted in a transfrontal approach (Fig. 3). In each seizure recorded, the onsets recorded from thalamic contacts were temporally indistinguishable from those recorded on cortical contacts (Fig. 4A), resulting in a recommendation for thalamic RNS. After implantation, seizures were detected readily (Fig. 4B). Using the thalamic signal to trigger stimulation, in cases such as this one, allows stimulation to affect widespread cortical networks, without having to choose a sub-territory of cortex for detection and stimulation, as would be required with the placement of cortical leads. At 14 months of therapy, this patient’s seizure frequency had been stably reduced from daily to weekly, with concomitant reduction in seizure severity.
Table 2.
Clinical scenarios in which thalamic implantation during SEEG informs surgical decisions
| Clinical scenario | SEEG implantation | Seizure onset zone interpretation | Surgical options informed by SEEG |
|---|---|---|---|
| 1 | standard | bilateral localized | bilateral region-specific RNS, bilateral ANT DBS |
| + thalamic | above with ETI | above + bilateral thalamic RNS | |
| above without ETI | bilateral cortical RNS, bilateral ANT DBS | ||
| 2 | standard | unilateral broad onset | unilateral cortical RNS |
| + thalamic | above with ETI | above + unilateral thalamo-cortical RNS | |
| above without ETI | unilateral cortical RNS | ||
| 3 | standard | bilateral multifocal onset | bilateral cortical RNS, bilateral ANT DBS |
| + thalamic | above with ETI | above | |
| above without ETI | bilateral thalamic RNS, bilateral ANT DBS | ||
| 4 | standard | primary generalized | bilateral thalamic RNS, bilateral ANT DBS |
| + thalamic | CM>ANT at onset | bilateral CM RNS | |
| ANT>CM at onset | bilateral ANT DBS |
Bold text denotes changes in management informed by recording from the thalamus during intracranial monitoring
ETI = early thalamic involvement
Figure 3. Thalamic implantation during SEEG.
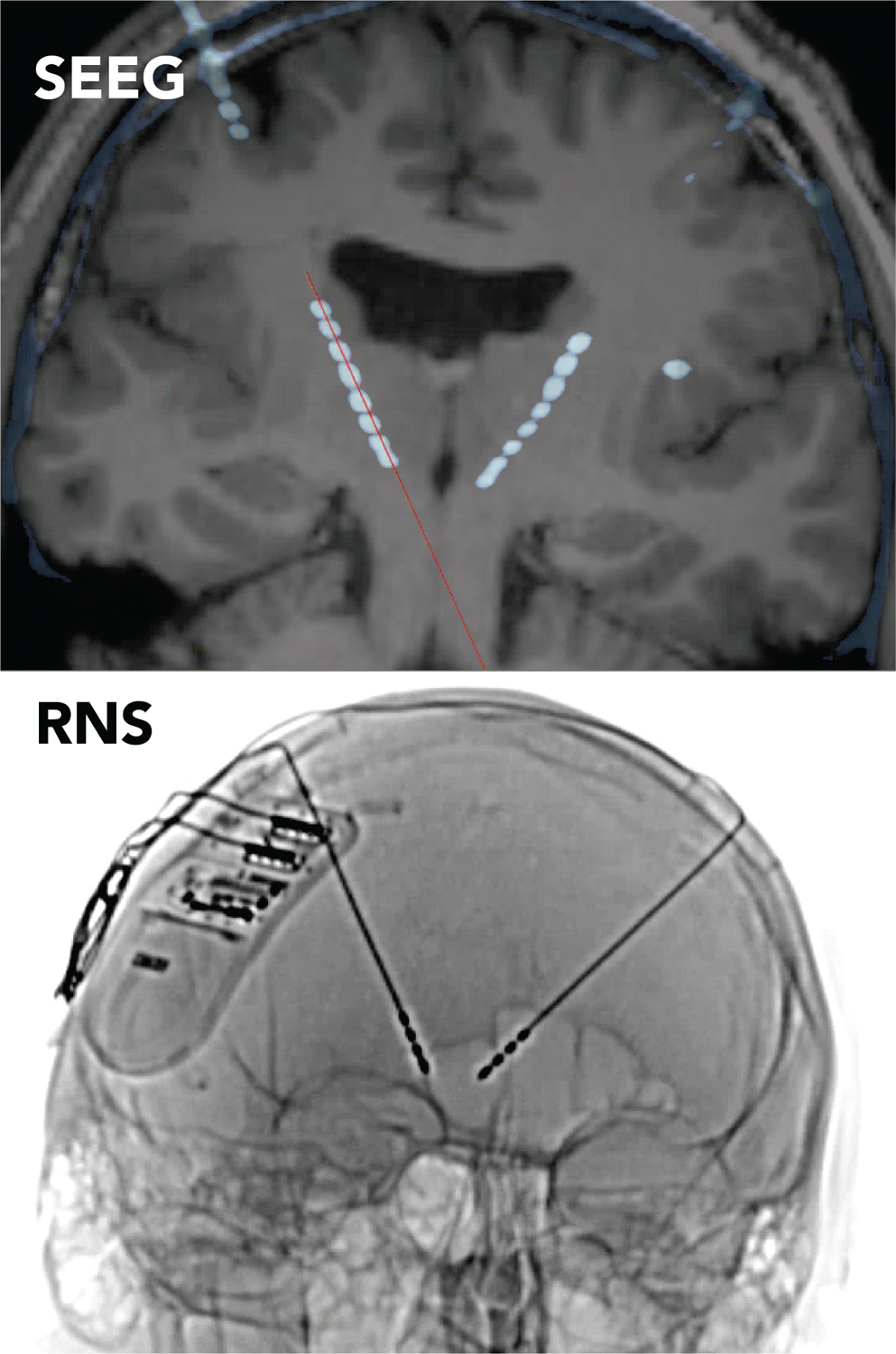
Post-SEEG implantation CT fused to the pre-operative brain MRI, demonstrating the position of the fronto-thalamic SEEG leads (top). The orientation and SEEG lead trajectories chosen for this patient were the same as that planned for use with transfrontal implantation of bilateral thalamic leads for RNS (bottom).
Figure 4. Thalamic recordings during SEEG.
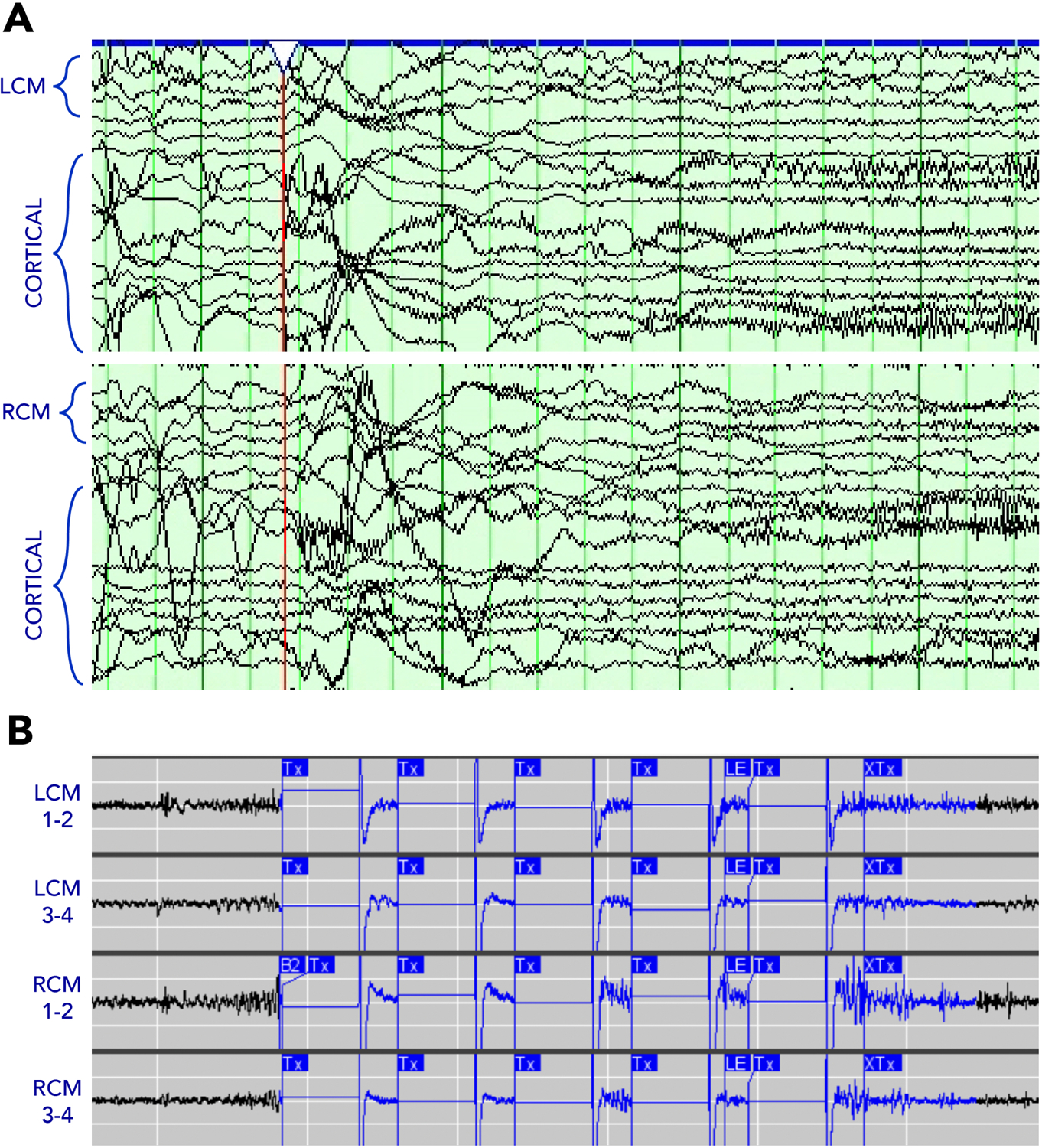
The thalamic contacts (LCM, left centromedian; RCM, right centromedian) are active simultaneous with the cortical contacts at seizure onset. Similar low-voltage fast activity subsequently was detected on the device and programmed to trigger stimulation.
Other centers have explored the value of including the thalamus in diagnostic SEEG surgery. The Marseille group reported very early involvement of the thalamus in 4 patients and delayed involvement in 7 patients, among 13 patients with temporal lobe epilepsy in whom an electrode contact had entered the thalamus through an extended cortical trajectory56. Likewise, the University of Alabama group reported data from eleven patients undergoing SEEG for suspected temporal lobe epilepsy, who were implanted in the ANT. Seizure onset was reported to be preceded by a decrease in the mean power spectral density in both the thalamus and seizure onset zone.57 These investigators also observed early ictal recruitment of the midline thalamus in three cases of mesial temporal lobe epilepsy, where stimulation of either the thalamus or hippocampus induced similar habitual seizures58. These results demonstrating the variable participation of thalamic nuclei in cases of temporal lobe epilepsy indicate the utility of mapping the involvement of potential thalamic nodes in an individual’s seizure network, prior to including the thalamus in an intracranial neuromodulation strategy. Yu et al. additionally demonstrated that high-frequency stimulation of the ANT during SEEG can desynchronize epileptic networks in a position-specific manner, implying that thalamic stimulation mapping may be useful for guiding clinically optimal lead placement59.
Finally, it is important to note that although less frequent in epilepsy surgery prior to the approval of ANT-DBS, implantation of the thalamus and basal ganglia is an everyday occurrence in movement disorders DBS surgery. Thus, there is a known safety profile for inserting leads in these subcortical structures60, most importantly an approximately 1% chance of symptomatic hemorrhage61. This risk is not different than the general risk of hemorrhage in SEEG procedures62. Indeed, the safety of modifying the trajectory of one electrode planned for clinical sampling to extend to the thalamus, which obviates the need to implant an additional electrode for thalamic sampling, was recently described57. The event most likely to affect the clinical assessment may be a temporary lesion effect that can occur with thalamic implantation that could prevent the patient from having a seizure during the intracranial monitoring admission34.
Summary
Approaches to the evaluation and surgical treatment of individuals with epilepsy are evolving, especially with regard to epilepsy networks, quality of life, primary generalized epilepsies, the utility of chronic intracranial recordings, and goals of diagnostic surgery. These paradigm shifts may facilitate closure of the surgical treatment gap in drug-resistant epilepsy.
Key Points.
Epilepsy surgery is underutilized.
Responsive neurostimulation is FDA-approved for focal epilepsy and is highly efficacious.
Strategies and goals for diagnostic intracranial monitoring surgery have expanded.
The role of the thalamus is different epilepsies is emerging.
Generalized epilepsy may be a surgical target for brain stimulation.
Synopsis.
Advances in device technology have created greater flexibility in treating seizures as emergent properties of networks that exist on a local to global continuum. All patients with drug-resistant epilepsy are potential surgical candidates, given that intracranial neuromodulation through deep brain stimulation (DBS) and responsive neurostimulation (RNS) can reduce seizures and improve quality of life, even in multifocal and generalized epilepsies. To achieve this goal, indications and strategies for diagnostic epilepsy surgery are evolving. This paper describes the state-of-the-art in epilepsy surgery and related changes in how we define indications for diagnostic and therapeutic surgical intervention.
Clinics Care Points.
Intracranial neuromodulation can reduce seizure frequency by 75%.
Thalamic implantation in SEEG may inform intracranial neuromodulation treatment strategy.
Implantation of the thalamus is safe.
Responsive neurostimulation of the thalamus may be effective in treating IGE.
Disclosures
The author has current funding support from the NIH via U01NS117836, R01NS110424, U01NS121471, and R01NS119483.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.MM Z, R K. National and State Estimates of the Numbers of Adults and Children with Active Epilepsy — United States, 2015.; 2017:66:821–825. 10.15585/mmwr.mm6631a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon D, Frick KD, Carr DA, Austin JK. Economic impact of epilepsy in the United States. Epilepsia. 2009;50(10):2186–2191. doi: 10.1111/j.1528-1167.2009.02159.x [DOI] [PubMed] [Google Scholar]
- 3.Manjunath R, Paradis PE, Parisé H, et al. Burden of uncontrolled epilepsy in patients requiring an emergency room visit or hospitalization. Neurology. 2012;79(18):1908–1916. doi: 10.1212/wnl.0b013e318271f77e [DOI] [PubMed] [Google Scholar]
- 4.Kwan P, Brodie MJ. Early Identification of Refractory Epilepsy. New Engl J Medicine. 2000;342:314–319. doi: 10.1056/nejm200002033420503 [DOI] [PubMed] [Google Scholar]
- 5.Engel J, Wiebe S, French J, et al. Practice Parameter: Temporal Lobe and Localized Neocortical Resections for Epilepsy. Epilepsia. 2003;44(6):741–751. doi: 10.1046/j.1528-1157.2003.48202.x [DOI] [PubMed] [Google Scholar]
- 6.Kwon C, Blank L, Mu L, Jetté N. Trends in lobectomy/amygdalohippocampectomy over time and the impact of hospital surgical volume on hospitalization outcomes: A population-based study. Epilepsia. 2020;61(10):2173–2182. doi: 10.1111/epi.16664 [DOI] [PubMed] [Google Scholar]
- 7.Englot DJ, Ouyang D, Wang DD, Rolston JD, Garcia PA, Chang EF. Relationship between hospital surgical volume, lobectomy rates, and adverse perioperative events at US epilepsy centers: Clinical article. J Neurosurg. 2013;118(1):169–174. doi: 10.3171/2012.9.jns12776 [DOI] [PubMed] [Google Scholar]
- 8.Rolston JD, Englot DJ, Knowlton RC, Chang EF. Rate and complications of adult epilepsy surgery in North America: Analysis of multiple databases. Epilepsy Res. 2016;124:55–62. doi: 10.1016/j.eplepsyres.2016.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haneef Z, Stern J, Dewar S, Engel J. Referral pattern for epilepsy surgery after evidence-based recommendations. Neurology. 2010;75(8):699–704. doi: 10.1212/wnl.0b013e3181eee457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epilepsies C on the PHD of the, Policy B on HS, Medicine I of. Epilepsy Across the Spectrum. Published online 2012. doi: 10.17226/13379 [DOI] [Google Scholar]
- 11.Samanta D, Ostendorf AP, Willis E, et al. Underutilization of epilepsy surgery: Part I: A scoping review of barriers. Epilepsy Behav. 2021;117:107837. doi: 10.1016/j.yebeh.2021.107837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.W P, H J. Epilepsy and the Functional Anatomy of the Human Brain. Little, Brown; 1954. [Google Scholar]
- 13.Bailey P, Gibbs FA. THE SURGICAL TREATMENT OF PSYCHOMOTOR EPILEPSY. J Amer Med Assoc. 1951;145(6):365–370. doi: 10.1001/jama.1951.02920240001001 [DOI] [PubMed] [Google Scholar]
- 14.Kahane P, Landré E, Minotti L, Francione S, Ryvlin P. The Bancaud and Talairach view on the epileptogenic zone: a working hypothesis. Epileptic Disord Int Epilepsy J Videotape. 2006;8 Suppl 2:S16–26. [PubMed] [Google Scholar]
- 15.Bonini F, McGonigal A, Trébuchon A, et al. Frontal lobe seizures: From clinical semiology to localization. Epilepsia. 2014;55(2):264–277. doi: 10.1111/epi.12490 [DOI] [PubMed] [Google Scholar]
- 16.Richardson RM. Decision Making in Epilepsy Surgery. Neurosurg Clin N Am. 2020;31(3):471–479. doi: 10.1016/j.nec.2020.03.014 [DOI] [PubMed] [Google Scholar]
- 17.Ma BB, Fields MC, Knowlton RC, et al. Responsive neurostimulation for regional neocortical epilepsy. Epilepsia. 2020;61(1):96–106. doi: 10.1111/epi.16409 [DOI] [PubMed] [Google Scholar]
- 18.Kokkinos V, Sisterson ND, Wozny TA, Richardson RM. Association of Closed-Loop Brain Stimulation Neurophysiological Features With Seizure Control Among Patients With Focal Epilepsy. Jama Neurol. 2019;76(7):800–808. doi: 10.1001/jamaneurol.2019.0658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khambhati AN, Shafi A, Rao VR, Chang EF. Long-term brain network reorganization predicts responsive neurostimulation outcomes for focal epilepsy. Sci Transl Med. 2021;13(608):eabf6588. doi: 10.1126/scitranslmed.abf6588 [DOI] [PubMed] [Google Scholar]
- 20.Nair DR, Laxer KD, Weber PB, et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology. 2020;95(9). doi: 10.1212/wnl.0000000000010154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Razavi B, Rao VR, Lin C, et al. Real-world experience with direct brain-responsive neurostimulation for focal onset seizures. Epilepsia. 2020;61(8):1749–1757. doi: 10.1111/epi.16593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Upton AR, Cooper IS, Springman M, Amin I. Suppression of seizures and psychosis of limbic system origin by chronic stimulation of anterior nucleus of the thalamus. Int J Neurol. 1985;19–20:223–230. [PubMed] [Google Scholar]
- 23.UPTON ARM, AMIN I, GARNETT S, SPRINGMAN M, NAHMIAS C, COOPER IS. Evoked Metabolic Responses in the LimbicStriate System Produced by Stimulation of Anterior Thalamic Nucleus in Man. Pacing Clin Electrophysiol. 1987;10(1):217–225. doi: 10.1111/j.1540-8159.1987.tb05952.x [DOI] [PubMed] [Google Scholar]
- 24.Salanova V, Sperling MR, Gross RE, et al. The SANTÉ study at 10 years of follow-up: Effectiveness, safety, and sudden unexpected death in epilepsy. Epilepsia. 2021;62(6):1306–1317. doi: 10.1111/epi.16895 [DOI] [PubMed] [Google Scholar]
- 25.Salanova V, Witt T, Worth R, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84(10):1017–1025. doi: 10.1212/wnl.0000000000001334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heck CN, King-Stephens D, Massey AD, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: Final results of the RNS System Pivotal trial. Epilepsia. 2014;55(3):432–441. doi: 10.1111/epi.12534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loring DW, Kapur R, Meador KJ, Morrell MJ. Differential neuropsychological outcomes following targeted responsive neurostimulation for partial-onset epilepsy. Epilepsia. 2015;56(11):1836–1844. doi: 10.1111/epi.13191 [DOI] [PubMed] [Google Scholar]
- 28.HUNTER J, JASPER HH. Effects of thalamic stimulation in unanaesthetised animals; the arrest reaction and petit mal-like seizures, activation patterns and generalized convulsions. Electroen Clin Neuro. 1949;1(3):305–324. doi: 10.1016/0013-4694(49)90043-7 [DOI] [PubMed] [Google Scholar]
- 29.Monnier M, Kalberer M, Krupp P. Functional antagonism between diffuse reticular and intralaminary recruiting projections in the medial thalamus. Exp Neurol. 1960;2(3):271–289. doi: 10.1016/0014-4886(60)90014-5 [DOI] [PubMed] [Google Scholar]
- 30.Velasco F, Velasco M, Ogarrio C, Fanghanel G. Electrical Stimulation of the Centromedian Thalamic Nucleus in the Treatment of Convulsive Seizures: A Preliminary Report. Epilepsia. 1987;28(4):421–430. doi: 10.1111/j.1528-1157.1987.tb03668.x [DOI] [PubMed] [Google Scholar]
- 31.Velasco F, Velasco AL, Velasco M, Jiménez F, Carrillo-Ruiz JD, Castro G. Operative Neuromodulation. Acta Neurochir Suppl. 2007;97(Pt 2):337–342. doi: 10.1007/978-3-211-33081-4_38 [DOI] [PubMed] [Google Scholar]
- 32.Velasco F, Velasco M, Velasco AL, Jimenez F, Marquez I, Rise M. Electrical Stimulation of the Centromedian Thalamic Nucleus in Control of Seizures: Long-Term Studies. Epilepsia. 1995;36(1):63–71. doi: 10.1111/j.1528-1157.1995.tb01667.x [DOI] [PubMed] [Google Scholar]
- 33.Fisher RS, Uematsu S, Krauss GL, et al. Placebo-Controlled Pilot Study of Centromedian Thalamic Stimulation in Treatment of Intractable Seizures. Epilepsia. 1992;33(5):841–851. doi: 10.1111/j.1528-1157.1992.tb02192.x [DOI] [PubMed] [Google Scholar]
- 34.Valentín A, Navarrete EG, Chelvarajah R, et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia. 2013;54(10):1823–1833. doi: 10.1111/epi.12352 [DOI] [PubMed] [Google Scholar]
- 35.Dalic LJ, Warren AEL, Bulluss KJ, et al. DBS of thalamic centromedian nucleus for Lennox–Gastaut syndrome (ESTEL trial). Ann Neurol. Published online 2021. doi: 10.1002/ana.26280 [DOI] [PubMed] [Google Scholar]
- 36.Panayiotopoulos CP. A Clinical Guide to Epileptic Syndromes and their Treatment. Published online 2010:377–421. doi: 10.1007/978-1-84628-644-5_13 [DOI] [Google Scholar]
- 37.Stevelink R, Koeleman BPC, Sander JW, Jansen FE, Braun KPJ. Refractory juvenile myoclonic epilepsy: a meta-analysis of prevalence and risk factors. Eur J Neurol. 2019;26(6):856–864. doi: 10.1111/ene.13811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moeller F, Siebner HR, Wolff S, et al. Changes in activity of striato–thalamo–cortical network precede generalized spike wave discharges. Neuroimage. 2008;39(4):1839–1849. doi: 10.1016/j.neuroimage.2007.10.058 [DOI] [PubMed] [Google Scholar]
- 39.Benuzzi F, Mirandola L, Pugnaghi M, et al. Increased cortical BOLD signal anticipates generalized spike and wave discharges in adolescents and adults with idiopathic generalized epilepsies. Epilepsia. 2012;53(4):622–630. doi: 10.1111/j.1528-1167.2011.03385.x [DOI] [PubMed] [Google Scholar]
- 40.Velasco M, Velasco F, Velasco AL, Luján M, Mercado JV. Epileptiform EEG Activities of the Centromedian Thalamic Nuclei in Patients with Intractable Partial Motor, Complex Partial, and Generalized Seizures. Epilepsia. 1989;30(3):295–306. doi: 10.1111/j.1528-1157.1989.tb05301.x [DOI] [PubMed] [Google Scholar]
- 41.Martín-López D, Jiménez-Jiménez D, Cabañés-Martínez L, Selway RP, Valentín A, Alarcón G. The Role of Thalamus Versus Cortex in Epilepsy: Evidence from Human Ictal Centromedian Recordings in Patients Assessed for Deep Brain Stimulation. Int J Neural Syst. 2017;27(07):1750010. doi: 10.1142/s0129065717500101 [DOI] [PubMed] [Google Scholar]
- 42.Kokkinos V, Urban A, Sisterson ND, Li N, Corson D, Richardson RM. Responsive Neurostimulation of the Thalamus Improves Seizure Control in Idiopathic Generalized Epilepsy: A Case Report. Neurosurgery. 2020;87(5):E578–E583. doi: 10.1093/neuros/nyaa001 [DOI] [PubMed] [Google Scholar]
- 43.Welch WP, Hect JL, Abel TJ. Case Report: Responsive Neurostimulation of the Centromedian Thalamic Nucleus for the Detection and Treatment of Seizures in Pediatric Primary Generalized Epilepsy. Front Neurol. 2021;12:656585. doi: 10.3389/fneur.2021.656585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baud MO, Kleen JK, Mirro EA, et al. Multi-day rhythms modulate seizure risk in epilepsy. Nat Commun. 2018;9(1):88. doi: 10.1038/s41467-017-02577-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leguia MG, Andrzejak RG, Rummel C, et al. Seizure Cycles in Focal Epilepsy. Jama Neurol. 2021;78(4):454–463. doi: 10.1001/jamaneurol.2020.5370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skarpaas TL, Tcheng TK, Morrell MJ. Clinical and electrocorticographic response to antiepileptic drugs in patients treated with responsive stimulation. Epilepsy Behav. 2018;83:192–200. doi: 10.1016/j.yebeh.2018.04.003 [DOI] [PubMed] [Google Scholar]
- 47.Quraishi IH, Mercier MR, Skarpaas TL, Hirsch LJ. Early detection rate changes from a brain-responsive neurostimulation system predict efficacy of newly added antiseizure drugs. Epilepsia. 2020;61(1):138–148. doi: 10.1111/epi.16412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sajobi TT, Josephson CB, Sawatzky R, et al. Quality of Life in Epilepsy: Same questions, but different meaning to different people. Epilepsia. 2021;62(9):2094–2102. doi: 10.1111/epi.17012 [DOI] [PubMed] [Google Scholar]
- 49.Stypulkowski PH, Stanslaski SR, Jensen RM, Denison TJ, Giftakis JE. Brain Stimulation for Epilepsy – Local and Remote Modulation of Network Excitability. Brain Stimul. 2014;7(3):350–358. doi: 10.1016/j.brs.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 50.Stanslaski S, Afshar P, Cong P, et al. Design and Validation of a Fully Implantable, Chronic, Closed-Loop Neuromodulation Device with Concurrent Sensing and Stimulation. Ieee T Neur Sys Reh. 2012;20(4):410–421. doi: 10.1109/tnsre.2012.2183617 [DOI] [PubMed] [Google Scholar]
- 51.Lipski WJ, DeStefino VJ, Stanslaski SR, et al. Sensing-enabled hippocampal deep brain stimulation in idiopathic nonhuman primate epilepsy. J Neurophysiol. 2015;113(4):1051–1062. doi: 10.1152/jn.00619.2014 [DOI] [PubMed] [Google Scholar]
- 52.Wozny TA, Lipski WJ, Alhourani A, Kondylis ED, Antony A, Richardson RM. Effects of hippocampal low-frequency stimulation in idiopathic non-human primate epilepsy assessed via a remote-sensing-enabled neurostimulator. Exp Neurol. 2017;294:68–77. doi: 10.1016/j.expneurol.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 53.Gregg NM, Marks VS, Sladky V, et al. Anterior nucleus of the thalamus seizure detection in ambulatory humans. Epilepsia. 2021;62(10):e158–e164. doi: 10.1111/epi.17047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sokolov E, Sisterson ND, Hussein H, et al. Intracranial monitoring contributes to seizure freedom for temporal lobectomy patients with non-concordant pre-operative data. Epilepsia Open. Published online 2021. doi: 10.1002/epi4.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tandon N, Tong BA, Friedman ER, et al. Analysis of Morbidity and Outcomes Associated With Use of Subdural Grids vs Stereoelectroencephalography in Patients With Intractable Epilepsy. Jama Neurol. 2019;76(6):672–681. doi: 10.1001/jamaneurol.2019.0098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guye M, Régis J, Tamura M, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129(7):1917–1928. doi: 10.1093/brain/awl151 [DOI] [PubMed] [Google Scholar]
- 57.Pizarro D, Ilyas A, Chaitanya G, et al. Spectral organization of focal seizures within the thalamotemporal network. Ann Clin Transl Neur. 2019;6(9):1836–1848. doi: 10.1002/acn3.50880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romeo A, Roach ATI, Toth E, et al. Early ictal recruitment of midline thalamus in mesial temporal lobe epilepsy. Ann Clin Transl Neur. 2019;6(8):1552–1558. doi: 10.1002/acn3.50835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu T, Wang X, Li Y, et al. High-frequency stimulation of anterior nucleus of thalamus desynchronizes epileptic network in humans. Brain. 2018;141(9):2631–2643. doi: 10.1093/brain/awy187 [DOI] [PubMed] [Google Scholar]
- 60.Zhang K, Bhatia S, Oh MY, Cohen D, Angle C, Whiting D. Long-term results of thalamic deep brain stimulation for essential tremor: Clinical article. J Neurosurg. 2010;112(6):1271–1276. doi: 10.3171/2009.10.jns09371 [DOI] [PubMed] [Google Scholar]
- 61.Martin AJ, Starr PA, Ostrem JL, Larson PS. Hemorrhage Detection and Incidence during Magnetic Resonance-Guided Deep Brain Stimulator Implantations. Stereot Funct Neuros. 2017;95(5):307–314. doi: 10.1159/000479287 [DOI] [PubMed] [Google Scholar]
- 62.McGovern RA, Ruggieri P, Bulacio J, Najm I, Bingaman WE, Gonzalez-Martinez JA. Risk analysis of hemorrhage in stereo-electroencephalography procedures. Epilepsia. 2019;60(3):571–580. doi: 10.1111/epi.14668 [DOI] [PubMed] [Google Scholar]


