Abstract
Background
A comprehensive analysis of peripheral immune cell phenotypes and tumor immune-gene expression profiles in locally advanced pancreatic cancer patients treated with neoadjuvant chemotherapy in a phase II clinical trial was carried out.
Methods
Patients were treated with neoadjuvant modified folinic acid, fluorouracil, irinotecan hydrochloride, oxaliplatin (mFOLFIRINOX) followed by surgery and adjuvant gemcitabine at the Asan Medical Center. Correlations between survival outcomes and baseline peripheral immune cells and their changes during preoperative chemotherapy were analyzed. Patients who had surgery were divided into two groups according to achievement of disease-free survival >10 months (achieved versus failed). Differential expression and pathway analysis of immune-related genes were carried out using the Nanostring platform, and immune cells within the tumor microenvironment were compared by immunohistochemistry.
Results
Forty-four patients were treated in the phase II clinical trial. Higher baseline CD14+CD11c+HLA-DR+ monocytes (P = 0.044) and lower Foxp3+CD4+ T cells (P = 0.02) were associated with poor progression-free survival of neoadjuvant mFOLFIRINOX. During the preoperative chemotherapy, PD-1 T cells significantly decreased (P = 0.0110). Differential expression and pathway analysis of immune-genes from the resected tumor after neoadjuvant treatment revealed transforming growth factor-β pathway enrichment and higher expression of MARCO (adjusted P < 0.05) associated with early recurrence. Enrichment of the Th1 pathway and higher peritumoral CD8+ T cells (P = 0.0103) were associated with durable disease-free survival from surgery (>10 months) following neoadjuvant mFOLFIRINOX.
Conclusions
Our results identify potential immune biomarkers for locally advanced pancreatic cancer and provide insights into pancreatic cancer immunity.
Key words: pancreatic cancer, TGF-β, peripheral immune phenotype, immune gene expression profile
Highlights
-
•
We performed immune profiling of locally advanced pancreatic cancer treated with neoadjuvant therapy in a phase II trial.
-
•
Proportion of programmed cell death protein 1-expressing peripheral CD8+ cells decreased after neoadjuvant chemotherapy.
-
•
Lower peripheral monocytes and higher regulatory T cells were associated with better progression-free survival.
-
•
Th1 pathway and higher peritumoral CD8+ T cells were associated with longer disease-free survival from surgery.
-
•
Transforming growth factor-β pathway and higher MARCO expression were associated with early recurrence after surgery.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive malignancy. The 5 years survival rate at the time of initial diagnosis is approximately 10% in the USA, with most patients presenting with unresectable or metastatic disease.1,2 PDAC is widely known as an immunologically cold tumor characterized by a dense desmoplastic reaction and an immunosuppressive tumor microenvironment (TME) with abundant infiltration of M2-like pro-tumor macrophages and regulatory T cells, and lacking infiltration of effector T cells.3,4 Several previous studies have shown an association between immunity and clinical outcomes in PDAC patients. A higher level of immunosuppressive macrophages and regulatory T cells was associated with a poor prognosis, while a higher level of effector T cells was associated with better outcomes.5 From a gene expression analysis, several immune-related genes associated with prognosis in PDAC patients who received surgical resection were suggested.6
Despite advances in immune biomarkers and therapeutic agents in the field of oncology, and efforts to characterize the immune responses in PDAC, there are no currently approved immune biomarkers or immunotherapies for the treatment of PDAC. Indeed, further studies of immune profiles in PDAC are needed to improve our understanding of the immunologic aspects of PDAC.
For borderline resectable and locally advanced PDAC, neoadjuvant modified FOLFIRINOX (folinic acid, fluorouracil, irinotecan hydrochloride, oxaliplatin; mFOLFIRINOX) is one of the standards of care with promising outcomes.7 Our previous phase II clinical trial of neoadjuvant mFOLFIRINOX (NCT02749136) for 44 patients with borderline resectable PDAC showed a median overall survival (OS) of 24.7 months [95% confidence interval (CI) 12.6-36.9 months], and 27 patients (61.4%) received surgical resection.8 Currently, there are few available data on the immune profiles of locally advanced PDAC treated with neoadjuvant mFOLFIRINOX.
This study is a comprehensive immune analysis of patients included in our previous phase II trial of neoadjuvant mFOLFIRINOX.8 Using peripheral immune profiling, we evaluated the association between peripheral immune cell composition and survival outcomes, as well as changes in the peripheral immune phenotype after systemic chemotherapy. We also carried out gene expression and immunohistochemical analyses of tumor sections to evaluate the relationship between immune cells in the TME and prognosis after the surgery in patients who had received neoadjuvant mFOLFIRINOX.
Materials and methods
Patients and study design
Histologically or cytologically confirmed PDAC patients with borderline resectable or locally advanced unresectable disease were enrolled in the prior phase II clinical trial.8 Details of the study design and outcomes were previously published elsewhere.8 Briefly, patients were treated with eight cycles of neoadjuvant mFOLFIRINOX (2400 mg/m2 fluorouracil as a continuous infusion for 46 h and 400 mg/m2 leucovorin, 85 mg/m2 oxaliplatin, and 150 mg/m2 irinotecan on day 1) every 2 weeks. Surgical resection followed by adjuvant gemcitabine was given for patients who achieved resectability.
Patients with adequate flow cytometry data from peripheral blood mononuclear cells (PBMCs) collected at baseline and/or after completion of neoadjuvant chemotherapy were included in the peripheral immune cell profiling. Among patients who received surgery, patients in whom the gene expression data passed quality control (QC) were included in the tumor immune profiling. As the median disease-free survival (DFS) was 10.4 months (95% CI 9.2-11.6 months),8 patients were divided into two groups according to whether DFS for >10 months was achieved (DFS10 achieved versus DFS10 failed). The expression of immune-related genes and the immune cell composition of the TME in the two groups were compared.
Peripheral immune cell profiling during preoperative chemotherapy
Peripheral immune cell phenotypes were analyzed by two fluorescent-activated cell sorting panels; the immune cell panel and T-cell checkpoint panel (Supplementary Methods 1, available at https://doi.org/10.1016/j.esmoop.2022.100484). The peripheral immune cell profiles at baseline were analyzed according to survival outcome. Multivariate analyses including clinical characteristics which showed significant association with survival outcomes in the previous phase II clinical trial study were carried out by Cox proportional modeling with the backward selection method, with immune cell levels showing statistically significant association in univariate analysis.8 Peripheral immune cell phenotypes were compared in samples collected at baseline and after completion of mFOLFIRINOX treatment to evaluate the impact of cytotoxic chemotherapy on systemic immunity. Non-parametric bootstrapping with 1000 samplings was used to validate the paired comparison results.
Differential expression and pathway analysis of immune-related genes in resected pancreatic cancer specimens
Normalized expression levels of 579 immune-related genes were obtained from RNA samples collected from resected surgical specimens using the Nanostring nCounter platform Immunology Panel (Nanostring Technologies, Seattle, WA). Differential gene expression analyses were carried out to compare the immune-gene expression profiles of the two recurrence groups (DFS10 achieved versus DFS10 failed). Detailed methods of differential expression analysis are described in Supplementary Methods 2, available at https://doi.org/10.1016/j.esmoop.2022.100484. Gene set analysis was carried out by calculating the directed global significance score, and immune cell compositions in the tumor were estimated from the gene expression profile using the Nanostring Technologies immune cell score annotations (Supplementary Methods 3, available at https://doi.org/10.1016/j.esmoop.2022.100484).9
Clinical outcomes and analysis of MARCO expression from public dataset
To validate the differential expression of MARCO in the DFS10 failed group, MARCO gene expression was analyzed in pancreatic cancer tissue using RNA-sequencing data from public sources, and a correlation with survival was carried out. MARCO expression data and clinical features, including OS, were obtained from 174 pancreatic cancer patients in The Cancer Genome Atlas using the Human Protein Atlas, Pathology Atlas (https://www.proteinatlas.org/ENSG00000019169-MARCO/pathology/pancreatic+cancer).10
Immunohistochemistry analysis of resected pancreatic cancer specimens
To validate the differential expression of MARCO in the DFS10 failed group, immunohistochemistry (IHC) analysis was conducted. Along with MARCO, analysis of immune cells within the TME was carried out to evaluate the relationship between immune cell infiltration of the TME and clinical outcomes (Supplementary Methods 4, available at https://doi.org/10.1016/j.esmoop.2022.100484). Stained slides were scanned with the 3D Histech Panoramic 250 Flash II whole slide scanner (3DHISTECH, Budapest, Hungary) at ×20 magnification. Using the open-source software QuPath v0.2.3 (University of Edinburgh, Edinburgh, UK),11 immune cells within the peritumoral area (≤100 μm from tumor cells) and the total area (whole slide) were counted from slide images and compared between the two groups. Analysis methods using QuPath are described in Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2022.100484.
Statistical analysis
Categorical variables were compared by Fisher’s exact tests, and continuous variables were analyzed with Wilcoxon signed-rank tests or Mann–Whitney U tests, as appropriate. Progression-free survival (PFS) was defined as the time from the initiation of neoadjuvant mFOLFIRINOX to disease progression or death from any cause, whichever occurred first. OS was defined as the time from the initiation of neoadjuvant mFOLFIRINOX to death from any cause. DFS in patients who underwent surgical resection was defined as the time from the surgical resection to the confirmation of recurrence. Survival curves were estimated by the Kaplan–Meier method and compared by log-rank testing. For continuous variables, patients were dichotomized into two groups with median or quartile values as cut-off. Estimation of hazard ratios (HR) in terms of PFS and OS were carried out by Cox proportional modeling. A two-sided P value <0.05 was considered statistically significant. Statistical analyses and visualization were carried out with the R ver. 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) and GradphPad Prism v9.0 (GraphPad Software, San Diego, CA, RRID:SCR_002798).12
Results
Patients and study design
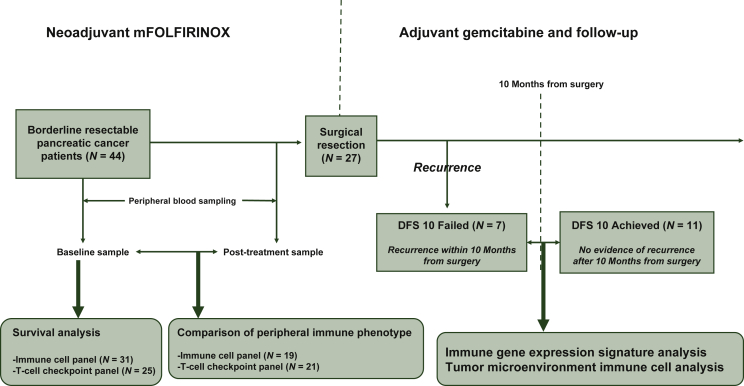
A total of 44 patients were enrolled in the phase II trial from May 2016 to March 2018 and were treated with neoadjuvant mFOLFIRINOX (Figure 1). With a median follow-up duration of 20.6 months (95% CI 19.7-21.6 months), the median OS was 24.7 months (95% CI 12.6-36.9 months), and the median PFS was 12.2 months (95% CI 8.9-15.5 months).8 Clinical characteristics of the patients are summarized in Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2022.100484. Patients with adequate flow cytometry data from baseline PBMC samples were included in the survival analysis. This included 31 patients with the immune cell panel data and 25 patients with T-cell checkpoint panel data. Patients with suitable matched flow cytometry data at baseline and after eight cycles of mFOLFIRINOX were included in a comparison of the peripheral immune phenotype before and after chemotherapy. This included 19 patients with the immune cell panel data and 21 patients with T-cell checkpoint panel data.
Figure 1.
Study outline.
DFS, disease-free survival; mFOLFIRINOX, modified folinic acid, fluorouracil, irinotecan hydrochloride, oxaliplatin.
Among 44 patients treated with neoadjuvant chemotherapy, 27 patients underwent surgical resection, and the median DFS was 10.4 months (95% CI 9.2-11.6 months). After QC of the Nanostring nCounter assay results, 18 samples with sufficient quality were included in the differential expression analysis (11 in the DFS10 achieved group and seven in the DFS10 failed group). Additional IHC analyses were carried out on the 18 patients to evaluate immune cell composition in the TME. Availability of biomarker data of patients treated in the phase II trial is summarized in Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2022.100484.
Prognostic implications of the baseline peripheral immune cell phenotype
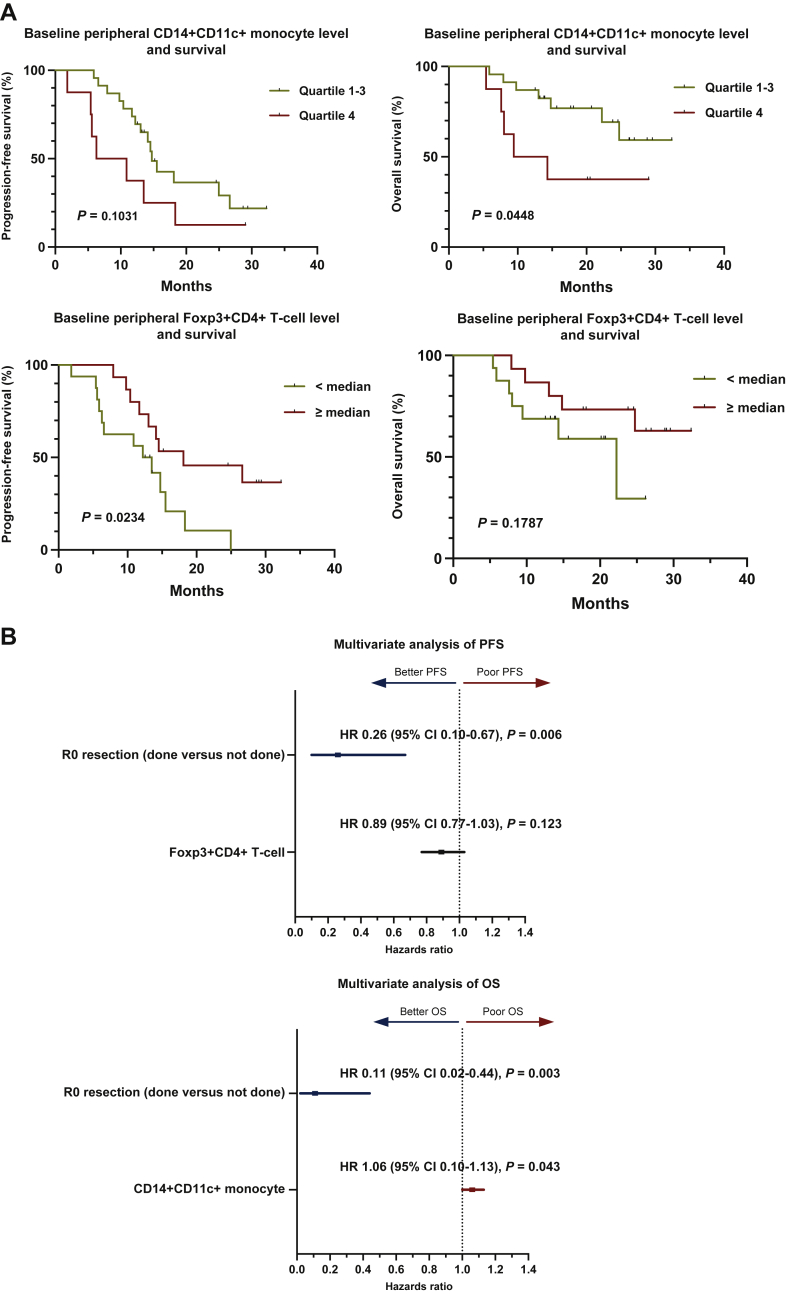
In terms of OS, only higher CD14+CD11c+human leukocyte antigen (HLA)-DR+ monocytes were significantly associated with poor survival with an HR of 1.07 (95% CI 1.01-1.13; P = 0.026, Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2022.100484). Higher CD14+CD11c+HLA-DR+ monocytes were also significantly associated with shorter survival (HR 1.06; 95% CI 1.00-1.12; P = 0.044), and higher Foxp3+CD4+ regulatory T cells were associated with better survival (HR, 0.8; 95% CI 0.72-0.97; P = 0.02) in terms of PFS. The assessment of the expression of the immune checkpoint molecules cytotoxic T lymphocyte-associated protein 4 (CTLA-4), Lag3, programmed cell death protein 1 (PD-1), and TIGIT on CD4+ and CD8+ T cells at baseline revealed that there were no significant associations between their expression and survival outcomes. Representative flow cytometry plots of CD14+CD11c+HLA-DR+ monocytes and Foxp3+CD4+ regulatory T cells are shown in Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2022.100484. Patients were dichotomized at the third quartile percentage of CD14+CD11c+HLA-DR+ monocyte and compared for PFS and OS (Figure 2A). Patients with higher monocyte levels showed significantly shorter OS (quartile 4 versus quartile 1-3, P = 0.0448), whereas no significant survival difference was shown in terms of PFS. Higher Foxp3+CD4+ regulatory T cell level showed significantly longer PFS when dichotomized at median level (P = 0.0234) while no significant difference was found in terms of OS.
Figure 2.
Analyses results of baseline peripheral immune cell levels with significant association with overall survival and progression-free survival in the univariate analyses with Cox proportional hazards model.
(A) Survival curves comparing survival outcomes of patients dichotomized at the median and third quartile of each peripheral immune cell level [CD14+CD11c+HLA-DR+ monocyte and progression-free survival (left, top; quartile 1-3 versus quartile 4, P = 0.1031), CD14+CD11c+HLA-DR+ monocyte and overall survival (right, top; quartile 1-3 versus quartile 4, P = 0.0448), Foxp3+CD4+ regulatory T cell and progression-free survival (left, bottom; median, P = 0.0234) and Foxp3+CD4+ regulatory T cell and overall survival (right, bottom; median, P = 0.1787)]. (B) Forest plot showing multivariate Cox proportional hazards modeling results with baseline peripheral immune cell levels with significant association in the univariate analyses and clinical characteristics [top; progression-free survival, R0 resection (HR 0.26, 95% CI 0.10-0.67, P = 0.006) and Foxp3+CD4+ T cell (HR 0.89, 95% CI 0.77-1.03, P = 0.123), bottom; overall survival, R0 resection (HR 0.11, 95% CI 0.02-0.44, P = 0.003) and CD14+CD11c+HLA-DR+ monocyte (HR 1.06, 95% CI 1.00-1.13, P = 0.043)].
CI, confidence interval; HLA, human leukocyte antigen; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
Multivariate analyses were carried out, and among location of primary tumor (head versus body and tail), best response to mFOLFIRINOX (complete response or partial response versus stable disease or partial disease), R0 resection (done versus not done), and baseline CD14+CD11c+HLA-DR+ monocyte levels, R0 resection and baseline monocyte levels were included in the final model in terms of OS. Location of primary tumor, R0 resection, baseline CD14+CD11c+HLA-DR+ monocyte, and Foxp3+CD4+ regulatory T-cell levels were analyzed for PFS, and R0 resection and baseline regulatory T-cell levels were included in the final model. Receiving R0 resection was significantly associated with both better OS (HR 0.11, 95% CI 0.02-0.44, P = 0.003) and PFS (HR 0.26, 95% CI 0.10-0.67, P = 0.006) (Figure 2B). Higher CD14+CD11c+HLA-DR+ monocyte level was significantly associated with poor OS (HR 1.06, 95% CI 1.00-1.13, P = 0.043) while Foxp3+CD4+ regulatory T-cell level showed a trend towards better PFS (HR 0.89, 95% CI 0.77-1.03, P = 0.123).
Changes in peripheral T-cell checkpoint expression during mFOLFIRINOX
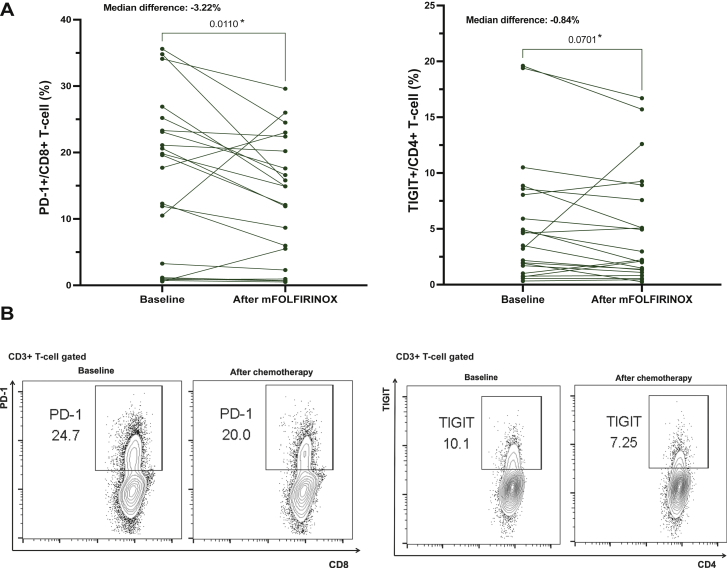
Changes in the peripheral immune phenotype after preoperative mFOLFIRINOX were analyzed to evaluate the effect of cytotoxic chemotherapy on peripheral immune profiles. PD-1+CD8+ T cells significantly decreased after eight cycles of mFOLFIRINOX, with a median difference of 3.22% and a P value of 0.0136 (Figure 3). No differences were seen in the immune cell composition using the immune cell panel or in the expression of other checkpoints (CTLA-4, Lag-3; Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2022.100484). Non-parametric bootstrapping results with 1000 repeated samplings also showed a significant decrease of PD-1+CD8+ T-cell level after chemotherapy [mean of bootstrapped median difference estimates −3.273% (95% CI −6.132 to −0.144), P = 0.047, Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2022.100484.]. TIGIT+CD4+ T cells also decreased following preoperative mFOLFIRINOX, although not statistically significant (median difference 0.84%, P = 0.0701).
Figure 3.
Changes in peripheral T-cell checkpoint expression after eight cycles of preoperative chemotherapy.
(A) Paired comparisons of the proportion of PD-1+CD8+ T cells (left; median difference = −3.22%, P = 0.0110) and TIGIT+CD4+ T cells (right; median difference = −0.84%, P = 0.0701) at baseline and after eight cycles of mFOLFIRINOX. (B) Flow cytometry dot plots comparing the proportion of checkpoint-expressing T cells (left, PD-1+CD8+ T cells; rights, TIGIT+CD4+ T cells). ∗P < 0.10.
mFOLFIRINOX, modified folinic acid, fluorouracil, irinotecan hydrochloride, oxaliplatin; PD-1, programmed cell death protein 1.
Differential expression and pathway analysis of immune-related genes in surgical specimens according to DFS
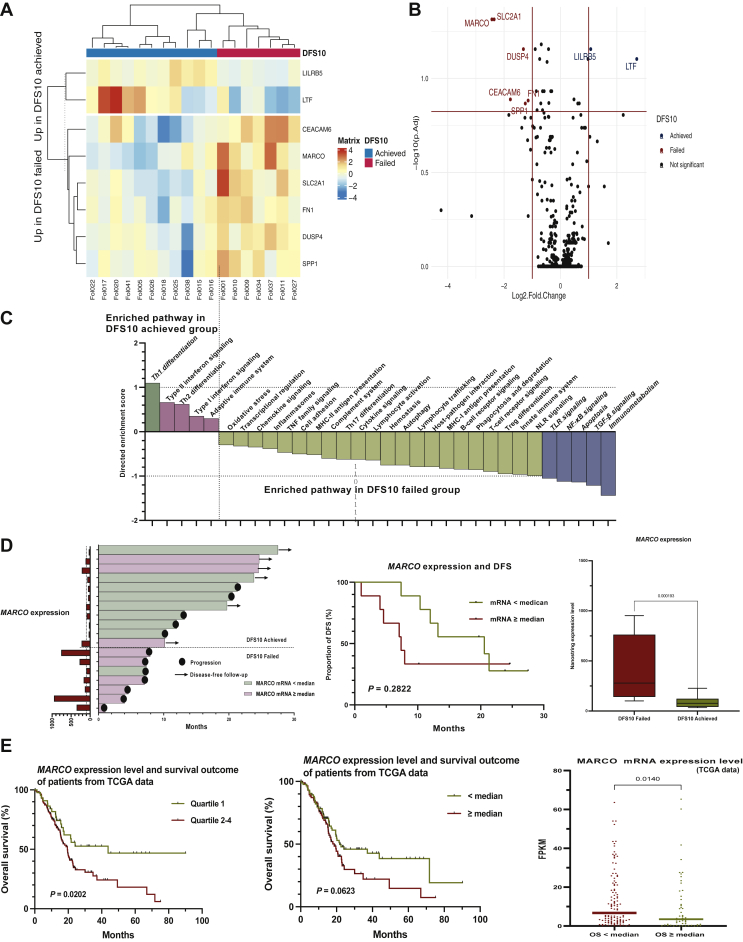
There were no statistically significant differences in clinical parameters between the two groups according to achievement of DFS longer than 10 months (DFS10 achieved vs. DFS10 failed), including the R0 resection rate, major vessel resection, or pathologic stage. Also, the proportion of patients who received adjuvant gemcitabine did not show a statistically significant difference between the two groups (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2022.100484). Eight genes were differentially expressed (absolute log2FC >1.0, adjusted P < 0.15; Figure 4A and B) in the two groups. The eight genes and the results of the differential expression analysis are described in Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2022.100484. Gene set analysis using the directed global significance score (Nanostring Technologies) was carried out on the immune-related gene expression data (Figure 4C). In the DFS10 achieved group, genes associated with Th1 differentiation were enriched, with a directed global significance score of 1.0996, whereas genes involved in transforming growth factor-β (TGF-β) signaling, and immunometabolism were enriched in the DFS10 failed group, with directed global significance scores of −1.2129 and −1.4371, respectively. The directed global significance scores of all gene sets are described in Supplementary Table S7, available at https://doi.org/10.1016/j.esmoop.2022.100484.
Figure 4.
Differential expression analysis of immune-related genes according to recurrence group (DFS10 achieved versus DFS10 failed).
(A) Heatmap of unsupervised clustering of patients according to the top eight genes with significant differential expression (log2 fold change >1.0 or <−1.0 and adjusted P < 0.15). (B) Volcano plot visualizing genes with significant differential expression (log2 fold change >1.0 or <−1.0 and adjusted P < 0.15). (C) Waterfall plot of the directed enrichment score from a gene set analysis of the Nanostring annotation. Pathways with a directed enrichment score of >1.0 (Th1 differentiation, enriched in the DFS10 achieved group) or <−1.0 (immunometabolism, TGF-β signaling, NF-κB signaling, TLR signaling, and apoptosis) are shown in colored bars. (D) MARCO expression level and clinical outcomes. Swimmer’s plot visualizing the MARCO expression level (left), DFS comparison of survival curves between patients dichotomized at the median MARCO expression level (middle, P = 0.2822), and nanostring expression level of MARCO (right; log2 fold change =−2.43307, P = 0.000193, adjusted P = 0.048545). (E) Analysis of the MARCO expression level and clinical outcomes in a public dataset [The Cancer Genome Atlas (TCGA)] from the Human Protein Atlas. Survival comparison of patients dichotomized at the first quartile (left; quartile 1 versus quartile 2-4, P = 0.0202) and median MARCO expression level (middle, P = 0.0632) and comparison of the MARCO expression level according to patient group, dichotomized at the median overall survival (right, P = 0.0140).
DFS, disease-free survival; FPKM, fragments per kilobase of exon per million mapped fragments; MHC, major histocompatibility complex; NF-κB, nuclear factor kappa B; NLR, NOD-like receptor; OS, overall survival; TGF-β, transforming growth factor-β; TLR, toll-like receptor; TNF, tumor necrosis factor.
Of note, MARCO was highly expressed in the DFS10 failed group, with an absolute log2FC of −2.43307 and a P value of 0.000193 (adjusted P = 0.048545). To evaluate the prognostic implication of MARCO expression, we carried out a survival analysis according to the MARCO expression level. Most patients in the DFS10 achieved group had a MARCO expression level of less than the median, and patients with a MARCO expression level higher than the median showed poor DFS, although this was not statistically significant (P = 0.2822; Figure 4D).
MARCO expression is associated with poor survival outcomes in pancreatic cancer patients from public data
We carried out further analysis of the relationship between MARCO expression and survival of pancreatic cancer patients using publicly available data from the Human Protein Atlas (Figure 4E).10 Patients with higher MARCO expression showed significantly poor OS (quartile 1 versus quartile 2-4, P = 0.02002) and patients with shorter survival had significantly higher levels of MARCO expression when dichotomized at median OS (P = 0.0140). The clinical characteristics of the cohort are described in Supplementary Table S8, available at https://doi.org/10.1016/j.esmoop.2022.100484.
IHC analysis of the TME
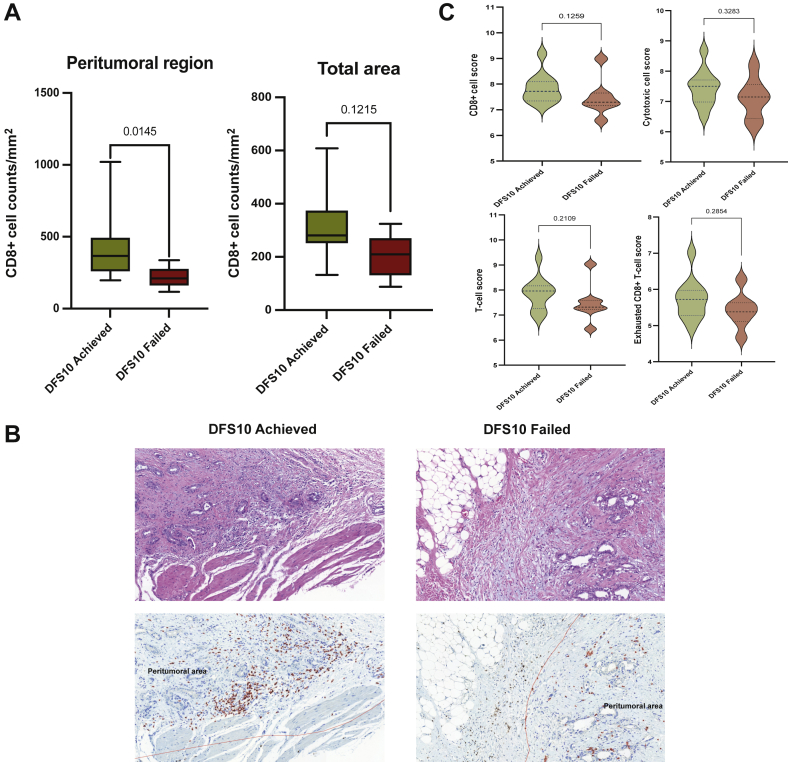
To verify the differential expression of MARCO in protein level, we carried out IHC analysis. There were, however, no significant differences of MARCO-positive immune cells between the two DFS10 groups for comparisons, peritumoral region (P > 0.9999), and total area (P = 0.4043) (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2022.100484). From the IHC analysis comparing immune cell components in the TME between the two groups, peritumoral CD8+ cell counts per mm2 were significantly higher in the DFS10 achieved group compared with the DFS10 failed group (P = 0.0145), whereas CD8+ T-cell counts of the total area did not show a statistically significant difference (P = 0.1215; Figure 5A and B). CD68+, CD163+, and MPO+ cells were not significantly different between the two groups in either the peritumoral or total area (Supplementary Figure S4, available at https://doi.org/10.1016/j.esmoop.2022.100484). The median Nanostring immune cell scores of CD8+ cells, cytotoxic cells, exhausted CD8+ T cells, and total T cells were higher in the DFS10 achieved group, but statistical significance was not reached (Figure 5C). Other immune cell scores were not different between the two groups (Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2022.100484).
Figure 5.
Comparison of immune cells in the tumor microenvironment according to the recurrence group (DFS10 achieved versus DFS10 failed).
(A) Comparison of CD8+ T-cell infiltration in the peritumoral region (left, P = 0.0145) and the total region (right, P = 0.1215). (B) Representative histopathologic and immunostained slides comparing CD8+ T-cell infiltration between the two groups (left: DFS10 achieved; right: DFS10 failed). A greater number of CD8+ cells (red staining) infiltration seen within the peritumoral region (marked with red line) in the DFS10 achieved group (left) compared with the DFS10 failed group (right). (C) Immune cell score estimated using the Nanostring annotation. CD8+ cell score (top left, P = 0.1259), cytotoxic cell score (top right, P = 0.3283), T-cell score (bottom left, P = 0.2109), and exhausted CD8+ T-cell score (bottom right, P = 0.2854).
DFS, disease-free survival.
Discussion
Our study evaluated the association between the peripheral immune profiles and clinical outcomes of locally advanced PDAC treated with neoadjuvant mFOLFIRINOX and surgery. Baseline peripheral CD14+CD11c+HLA-DR+ monocytes and Foxp3+ regulatory T cells were significantly associated with survival outcomes. These cell types may therefore serve as prognostic biomarkers. Conversely, chemotherapy affected the peripheral immune phenotype, with patients showing decreased PD-1-expressing CD8+ T cells after eight cycles of mFOLFIRINOX.
Higher levels of peripheral monocytes were associated with poor survival with respect to both OS and PFS. Several previous studies support the correlation between high peripheral monocyte levels and adverse outcomes in PDAC.13,14 Yet, higher peripheral monocyte levels and survival outcomes in PDAC patients treated with neoadjuvant chemotherapy and surgery have not been addressed in previous studies. Higher peripheral monocyte levels may reflect the abundant trafficking of circulating monocytes into the tumor, leading to differentiation into pro-tumor macrophages, which are well known to be a poor prognostic factor in PDAC.4,15 Foxp3+CD4+ regulatory T cells were significantly associated with better PFS, which is contrary to a previous study of the peripheral immune profile of unresectable PDAC patients which showed association of higher peripheral CD4+CD25+CD127− regulatory T-cell population and poor OS.16 However, a recent study with colon cancer patients showed that a specific subpopulation of regulatory T cells with a CD45RA−Foxp3low phenotype was associated with better outcomes.17 Quantification of Foxp3 expression level and deeper phenotyping of regulatory T-cell population of patients are needed for better explanation of the role of Foxp3+ regulatory T cells in PDAC.
During eight cycles of mFOLFIRINOX, the proportion of PD-1+CD8+ T cells significantly decreased. Although PD-1 expression alone does not suggest exhaustion status of CD8+ T cells, the significant decrease of PD-1+CD8+ T cells may be associated with a decrease in exhausted CD8+ T cells in the peripheral blood. From a previous study investigating the peripheral immune profiles of PDAC patients, the proportion of PD-1+CD8+ T cells was unchanged before and after surgery.18 Activation of naive CD8+ T cells via immunogenic cell death caused by chemotherapy may lead to proliferation of new effector CD8+ T cells which could partly explain the decrease in the PD-1+CD8+ cell population in our results.19 Peripheral immune profiling of non-small-cell lung cancer patients treated with platinum-based chemotherapy showed increased proliferation of effector subsets of CD8+ T cells in the peripheral blood, and a preclinical study in a mouse colon cancer model showed lower levels of PD-1+CD8+ T cells in the TME after FOLFOX treatment compared with the control group.20,21
Our study characterizes the immune profiles of resected PDAC and evaluates the effect of preoperative chemotherapy, as well as their association with clinical outcomes in locally advanced PDAC. Early recurrence of PDAC after surgery may be due to residual tumor cells or undetected micrometastases with rapid growth and resistance to adjuvant chemotherapy. Indeed, the immunologic features of the early recurrence group (DFS10 failed group), including high MARCO expression, enriched the expression of genes associated with TGF-β signaling, and lower peritumoral infiltration by CD8+ T cells may be associated with aggressive tumor behavior and resistance to cytotoxic chemotherapy. Only the patients who received surgery, who have better prognosis compared with those who did not, were included in the immune-related gene expression analysis and the results may not represent the whole population of locally advanced PDAC patients. These results, however, may serve as potential prognostic biomarkers in patients with locally advanced PDAC treated with neoadjuvant chemotherapy and who had surgical resection.
In a differential expression analysis of immune-related genes, MARCO was preferentially expressed in patients who recurred within 10 months of surgery. A correlation between MARCO expression and poor clinical outcome was also observed by analysis of RNA-sequencing data from PDAC patients in a publicly available dataset. In the current study, however, we could not prove the significant differential expression in the protein level by IHC analysis. This discordant result may be explained if increased transcription of MARCO does not lead to expanded expression of MARCO protein in the immune cells of the TME; nonetheless there lacks evidence to support this idea. Lower yield of MARCO IHC staining in this study may hinder the discovery of differentially expressed MARCO protein.
MARCO is a scavenger receptor expressed by macrophages and functions as a pattern recognition receptor.22 Several preclinical and clinical studies in other types of cancers have demonstrated the pro-tumor role of MARCO, with inhibition of the receptor leading to tumor regression. In a study of human breast cancer and melanoma specimens, MARCO was expressed by immunosuppressive macrophages and associated with a gene signature related to epithelial–mesenchymal transition and metastasis.23 Moreover, in a mouse model, MARCO-expressing macrophages in the TME had immunosuppressive features, and inhibition of MARCO with a neutralizing antibody reprogrammed the macrophages from a pro-tumor phenotype to an antitumor phenotype, leading to inhibition of tumor growth and metastasis.23 In another preclinical study using a mouse melanoma model, inhibition of MARCO-expressing macrophages led to natural killer cell-mediated tumor cell killing via the tumor necrosis factor-related apoptosis-inducing ligand pathway.22
From the gene set analysis, the Th1 differentiation pathway was enriched in the DFS10 achieved group. This is biologically plausible, as Th1 CD4+ T cells are well known to be associated with favorable outcomes in several cancers.24 However, the TGF-β signaling pathway was enriched in the DFS10 failed group. This is also consistent with previous data, as the TGF-β signaling pathway is known to promote an immunosuppressive TME in PDAC, with stromal proliferation of cancer-associated fibroblasts and production of extracellular matrix.25, 26, 27 The combination of a TGF-β blocker with a PDAC vaccine altered the TME, promoted CD8+ T-cell infiltration, and led to better survival in a mouse model.28 Moreover, there are some attempts to target TGF-β pathways in treating PDAC patients including losartan, an angiotensin receptor blocker, and halofuginone, an antimalarial derivative.29 From a preclinical study of SPARC-null mice which shows higher TGF-β1 activity and aggressive tumor behavior, losartan administration slowed tumor growth and improved survival along with attenuation of TGF-β1-induced gene expression.30 A recent phase II clinical trial of locally advanced PDAC patients treated with losartan in combination with FOLFIRINOX demonstrated promising results with an R0 resection rate of 61% out of 49 patients treated, with a significant decrease of serum TGF-β and thrombospondin-1.31
Higher infiltration of CD8+ T cells in the peritumoral area of resected PDAC was associated with a DFS of >10 months. Similar results were observed in a previous study of the PDAC immune microenvironment.5,32 Higher CD8+ T cell counts in the peritumor region or the total tumor area were significantly associated with better outcomes, while the CD8+ T-cell count in the total area was not significantly different in the two groups. Similar results were shown in a study of PDAC specimens from patients who underwent curative resection, in which the proximity of cytotoxic T cells to the tumor was associated with better survival outcomes.32 Favorable clinical outcomes of tumors with higher CD8+ T cell infiltration of the peritumoral region after neoadjuvant chemotherapy, however, were not well evaluated in the previous studies. As CD8+ T cells are a major effector cell in antitumor immunity, it is presumed that higher infiltration of the peritumoral region by CD8+ T cells leads to better outcomes.
A strength of this study is that the clinical data and patient samples were obtained from a well-defined cohort from a prospective phase II clinical trial and included a comprehensive analysis of the immunologic features of the peripheral blood and resected tumor samples. There are, however, several limitations to this study, including the small number of cases and the absence of an external validation cohort. We carried out additional investigations including multivariate analyses, bootstrapping, and public data set analyses to compensate the small sample size of the study. The IHC analysis failed to validate the differential expression of MARCO. Neoadjuvant chemotherapy may induce desmoplastic reaction and matrix remodeling along with tumor cell death leading to alteration of the TME.33 This may explain the relatively lower yields of Nanostring results and immune cell IHC studies noticed in this study.
Conclusion
Our comprehensive analysis of the immune profiles of locally advanced PDAC patients treated in a phase II clinical trial of neoadjuvant mFOLFIRINOX and surgery may identify potential prognostic biomarkers. These results may provide better insights and real-world evidence for further investigation of PDAC immunity and the development of new therapeutics.
Acknowledgements
Not required.
Funding
This study was supported in part by the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea [grant number 2020IL0018]. The funding sources had no role in the study design, collection, and analysis of data, or writing of the report.
Disclosure
The authors have declared no conflicts of interest.
Ethics approval and consent to participate
All procedures in studies involving human participants were carried out in accordance with the ethical standards of the Institutional Review Board of Asan Medical Center, and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards (IRB approval number 2016-0010).
Supplementart data
References
- 1.Mizrahi J.D., Surana R., Valle J.W., Shroff R.T. Pancreatic cancer. Lancet. 2020;395(10242):2008–2020. doi: 10.1016/S0140-6736(20)30974-0. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Facciabene A., Motz G.T., Coukos G. T-regulatory cells: key players in tumor immune escape and angiogenesis. Cancer Res. 2012;72(9):2162–2171. doi: 10.1158/0008-5472.CAN-11-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karamitopoulou E. Tumour microenvironment of pancreatic cancer: immune landscape is dictated by molecular and histopathological features. Br J Cancer. 2019;121(1):5–14. doi: 10.1038/s41416-019-0479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ino Y., Yamazaki-Itoh R., Shimada K., et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108(4):914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Angelo A., Sobhani N., Roviello G., et al. Tumour infiltrating lymphocytes and immune-related genes as predictors of outcome in pancreatic adenocarcinoma. PLoS One. 2019;14(8) doi: 10.1371/journal.pone.0219566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network Pancreatic Adenocarcinoma (Version 1.2021) https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf Available at. Published 2021.
- 8.Yoo C., Lee S.S., Song K.B., et al. Neoadjuvant modified FOLFIRINOX followed by postoperative gemcitabine in borderline resectable pancreatic adenocarcinoma: a Phase 2 study for clinical and biomarker analysis. Br J Cancer. 2020;123(3):362–368. doi: 10.1038/s41416-020-0867-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nanostring Technologies nCounter Advanced Analysis 2.0 PlugIn for nSolver Software User Manual. https://www.nanostring.com/wp-content/uploads/2020/12/MAN-10030-03_nCounter_Advanced_Analysis_2.0_User_Manual.pdf Available at. Published 2018.
- 10.Uhlen M., Zhang C., Lee S., et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357(6352) doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 11.Bankhead P., Loughrey M.B., Fernández J.A., et al. QuPath: open source software for digital pathology image analysis. Sci Rep. 2017;7(1):16878. doi: 10.1038/s41598-017-17204-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. [computer program] [Google Scholar]
- 13.Sierzega M., Lenart M., Rutkowska M., et al. Preoperative neutrophil-lymphocyte and lymphocyte-monocyte ratios reflect immune cell population rearrangement in resectable pancreatic cancer. Ann Surg Oncol. 2017;24(3):808–815. doi: 10.1245/s10434-016-5634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xue P., Hang J., Huang W., et al. Validation of lymphocyte-to-monocyte ratio as a prognostic factor in advanced pancreatic cancer: an East Asian cohort study of 2 countries. Pancreas. 2017;46(8):1011–1017. doi: 10.1097/MPA.0000000000000891. [DOI] [PubMed] [Google Scholar]
- 15.Nixon A.B., Schalper K.A., Jacobs I., Potluri S., Wang I.M., Fleener C. Peripheral immune-based biomarkers in cancer immunotherapy: can we realize their predictive potential? J Immunother Cancer. 2019;7(1):325. doi: 10.1186/s40425-019-0799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C., Cheng H., Luo G., et al. Circulating regulatory T cell subsets predict overall survival of patients with unresectable pancreatic cancer. Int J Oncol. 2017;51(2):686–694. doi: 10.3892/ijo.2017.4032. [DOI] [PubMed] [Google Scholar]
- 17.Saito T., Nishikawa H., Wada H., et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22(6):679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 18.Shen T., Zhou L., Shen H., et al. Prognostic value of programmed cell death protein 1 expression on CD8+ T lymphocytes in pancreatic cancer. Sci Rep. 2017;7(1):7848. doi: 10.1038/s41598-017-08479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galluzzi L., Buqué A., Kepp O., Zitvogel L., Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17(2):97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 20.de Goeje P.L., Poncin M., Bezemer K., et al. Induction of peripheral effector CD8 T-cell proliferation by combination of paclitaxel, carboplatin, and bevacizumab in non-small cell lung cancer patients. Clin Cancer Res. 2019;25(7):2219–2227. doi: 10.1158/1078-0432.CCR-18-2243. [DOI] [PubMed] [Google Scholar]
- 21.Guan Y., Kraus S.G., Quaney M.J., Daniels M.A., Mitchem J.B., Teixeiro E. FOLFOX chemotherapy ameliorates CD8 T lymphocyte exhaustion and enhances checkpoint blockade efficacy in colorectal cancer. Front Oncol. 2020;10:586. doi: 10.3389/fonc.2020.00586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisinger S., Sarhan D., Boura V.F., et al. Targeting a scavenger receptor on tumor-associated macrophages activates tumor cell killing by natural killer cells. Proc Natl Acad Sci U S A. 2020;117(50):32005–32016. doi: 10.1073/pnas.2015343117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Georgoudaki A.M., Prokopec K.E., Boura V.F., et al. Reprogramming tumor-associated macrophages by antibody targeting inhibits cancer progression and metastasis. Cell Rep. 2016;15(9):2000–2011. doi: 10.1016/j.celrep.2016.04.084. [DOI] [PubMed] [Google Scholar]
- 24.Wörmann S.M., Diakopoulos K.N., Lesina M., Algül H. The immune network in pancreatic cancer development and progression. Oncogene. 2014;33(23):2956–2967. doi: 10.1038/onc.2013.257. [DOI] [PubMed] [Google Scholar]
- 25.Hosein A.N., Brekken R.A., Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol. 2020;17(8):487–505. doi: 10.1038/s41575-020-0300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho W.J., Jaffee E.M., Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17(9):527–540. doi: 10.1038/s41571-020-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Principe D.R., Doll J.A., Bauer J., et al. TGF-β: duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst. 2014;106(2):djt369. doi: 10.1093/jnci/djt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soares K.C., Rucki A.A., Kim V., et al. TGF-β blockade depletes T regulatory cells from metastatic pancreatic tumors in a vaccine dependent manner. Oncotarget. 2015;6(40):43005–43015. doi: 10.18632/oncotarget.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauge A., Rofstad E.K. Antifibrotic therapy to normalize the tumor microenvironment. J Transl Med. 2020;18(1):207. doi: 10.1186/s12967-020-02376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold S.A., Rivera L.B., Carbon J.G., et al. Losartan slows pancreatic tumor progression and extends survival of SPARC-null mice by abrogating aberrant TGFβ activation. PLoS One. 2012;7(2) doi: 10.1371/journal.pone.0031384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murphy J.E., Wo J.Y., Ryan D.P., et al. Total neoadjuvant therapy with FOLFIRINOX in combination with losartan followed by chemoradiotherapy for locally advanced pancreatic cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(7):1020–1027. doi: 10.1001/jamaoncol.2019.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carstens J.L., Correa de Sampaio P., Yang D., et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat Commun. 2017;8:15095. doi: 10.1038/ncomms15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox T.R. The matrix in cancer. Nat Rev Cancer. 2021;21(4):217–238. doi: 10.1038/s41568-020-00329-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.